Abstract
Introduction
Central nervous system leukemia (CNSL) is the most common extramedullary relapse site in patients with Philadelphia chromosome-positive (Ph-positive) acute lymphoblastic leukemia (ALL), with a poor prognosis and high relapse rate.
Methods
We characterized the clinical data of 21 Ph-positive B-ALL patients to analyze the efficacy and safety of ponatinib for patients with central nervous system relapsed Ph-positive ALL retrospectively.
Results
There were 11 males and 10 females in the cohort, and their median age was 45 (9-58) years old. The total CR (complete remission) rate was 90.5%. All 9 patients achieved CR in the ponatinib group, and 10 patients achieved CR in the dasatinib group (100% vs 83.3%, respectively; P = .486) and minimal residual disease-positive CR in the ponatinib group and dasatinib group (88.9% vs 58.3%, P = .178). The medium time after achieving CR was 5 and 8 weeks (P = .047). The total median overall survival (OS) was 31.1 months, and the 3-year OS was 49.0%. The median relapse-free survival (RFS) was 31.0 months, and the 3-year RFS was 45.2%. Patients in the ponatinib group showed a significantly longer OS than those patients in the dasatinib group with (medium OS not reached vs 27.6 months, P = .045) or without (medium OS not reached vs 27.6 months, P = .039) T315I mutations. The median RFS between the ponatinib group and the dasatinib group with T315I was not reached and 16.2 months, P = .065. The median RFS between the ponatinib group and the dasatinib group without T315I was not reached and 16.2 months, P = .036. No treatment-related deaths were observed during the therapy.
Conclusion
(1) Ph-positive CNSL patients seemed to have a high rate of response and postinduction MRD negativity with ponatinib and dasatinib, but ponatinib seemed to show a shorter time to achieve remission than dasatinib. (2) Ponatinib maintenance treatment might show superior survival for Ph-positive CNSL patients with or without the T315I mutation. (3) Ponatinib and dasatinib seemed to be both safe for the clinical application of Ph-positive CNSL.
Keywords: Philadelphia chromosome-positive, acute lymphoblastic leukemia, central nervous system leukemia, ponatinib, dasatinib
Introduction
Central nervous system leukemia (CNSL) involvement is a serious complication of acute leukemia. Malignant tumor cells derived from B cells and T cells are more likely to infiltrate the cerebrospinal fluid and meninges.1 A total of 5% to 10% of newly diagnosed adult patients also had CNSL. Moreover, patients not receiving central nervous system (CNS) preventive treatments had a higher relapse rate.2–4 In B-cell precursor lymphoblastic leukemia, certain cytogenetic abnormalities were associated with a higher incidence of CNSL, such as the E2A-PBX1 fusion gene caused by t(1;19) and the BCR-ABL1 fusion gene caused by t(9;22).5–7
Tyrosine kinase inhibitors (TKIs) can improve the prognosis of Philadelphia chromosome-positive (Ph-positive) acute lymphoblastic leukemia (ALL).8 However, some patients relapse in the CNS after treatment, which is caused by the low permeability of imatinib.9 CNSL appearing after the administration of CR seems to be associated with a high risk of hematological relapse and a significantly worse outcome. Second-generation dasatinib and third-generation ponatinib TKI have potent activity in Ph-positive ALL with wild-type and mutated BCR-ABL,10–13 and ponatinib is more effective in some mutations, including T315I.14,15 Dasatinib holds clinical potential in managing CNSL,16,17 and ponatinib is also effective in CNSL.18,19 However, the priority of dasatinib and ponatinib treatments has not been demonstrated. Therefore, we attempted to characterize the efficacy and safety of ponatinib and dasatinib for patients with CNS-relapsed Ph-positive ALL in our institution, and the results showed promising clinical activity.
Methods
Patients
Twenty-one Ph-positive B-ALL patients with CNS relapse from May 2014 to April 2021 who were treated outside of clinical trials were screened consecutively in this retrospective study. All of the patients fulfilled the diagnostic criteria according to morphology, immunology, cytogenetics, molecular biology, and gene mutation examinations. The following laboratory examinations were conducted: cerebrospinal fluid (CSF) examination, head computed tomography (CT) scan examination, and electroencephalogram (EEG). In all 21 patients, blast cells were observed in the cerebrospinal fluid, and this population of cells could be observed via flow cytometry (FCM). Seven head CT scans showed infiltration of ALL in the brain, and one magnetic resonance imaging (MRI) scan showed multiple infiltration changes. There were no obvious abnormalities in the 7 cases of EEG. Moreover, 9 patients in the ponatinib group were induced by ponatinib, and 12 patients in the dasatinib group were induced by dasatinib. The deadline for follow-up was December 31, 2021, and the median follow-up time was 22.0 (3.5-52.2) months. Our study was approved by the medical ethics committee of Lianyungang First People's Hospital (approval number: LW-20221231001-01; location: No. 6 Zhenhua East Road, Lianyungang City, Jiangsu, China; approval date: January 13, 2023). And our study was conducted in accordance with the Declaration of Helsinki. Verbal informed consent was obtained from all of the subjects and/or their legal guardians. The reporting of this study conforms to STROBE guidelines.20
Definitions
According to the National Comprehensive Cancer Network (NCCN) guidelines, combined with the patient's MICM classification, all of the patients fulfilled the diagnostic criteria of Ph-positive B-ALL. Leukemia cells were observed in the cerebrospinal fluid of all of the patients, excluding other diseases that cause CNS symptoms or changes in cerebrospinal fluid. CR was defined as the proportion of bone marrow (BM) blast cells < 5%, no blast cells in the peripheral blood, no extramedullary infiltration, no relapse within 4 weeks, and the restoration of hematopoietic function. Minimal residual disease (MRD) in the bone marrow and cerebrospinal fluid was assessed by using FCM and real-time fluorescence quantitative polymerase chain reaction (RT-qPCR), and MRD-negative was defined as a proportion of blast cells less than 0.01% and no expression of the BCR-ABL fusion gene in bone marrow, no lymphoblasts in cerebrospinal fluid regardless of WBC count (according to the achievement of CNS-1 status).21
Treatments
All of the patients received TKI plus chemotherapy after diagnosis and initial induction chemotherapy regimens, including the VDCP regimen (vincristine, daunorubicin, cyclophosphamide, and prednisone) and the hyper-CVAD regimen (cyclophosphamide, vincristine sulfate, doxorubicin hydrochloride, and dexamethasone). Seven patients accepted hematopoietic stem cell transplantation (HSCT) after achieving CR.
All of the patients received TKIs combined with systemic chemotherapy after CNS relapse. Ponatinib (45 mg at an oral daily dose) or dasatinib (140 mg at an oral daily dose) was used for patients with resistance or intolerance to previously approved TKIs; only ponatinib was used for patients with the BCR-ABL1 T315I mutation, and the dosage was adjusted according to the toxicities of TKIs. The systemic chemotherapy regimen was the hyper-CVAD regimen, after which patients received consolidation chemotherapy and regimens including high-dose methotrexate (1-3 g/m2) and/or high-dose cytarabine (1-3 g/m2 for 3 days). Intrathecal injections of methotrexate, cytarabine, and dexamethasone were given to patients twice a week to prevent CNSL until no blast cells were observed in the cerebrospinal fluid and then once a week for 4 to 6 weeks.
Statistical Analysis
GraphPad Prism 8.0 and SPSS 26.0 software were used to analyze the data and plots. The Kaplan‒Meier method was used to plot the survival curve. The Fisher's exact test and the Mann‒Whitney U-test were used to assessing categorical and continuous variables. The Kaplan‒Meier method and the log-rank (Mantel-Cox) test were used to assess overall survival (OS) and relapse-free survival (RFS). The former parameter was calculated as the time from the date of diagnosis to the date of the last follow-up or death of any cause. The latter parameter was calculated from the beginning of treatment until relapse. A P-value <.05 (2-tailed) was considered to be statistically significant.
Result
Characteristics of the Patients
There were 11 males and 10 females in the cohort, and the median age of these patients was 45 (9-58)-years old. Twelve patients had the P190 fusion protein, and 9 patients had the P210 fusion protein. The median time of CNS relapse was 7 (range: 1-35) months after achieving remission. The main manifestations of neurological symptoms included headache, dizziness, nausea, and vomiting, among other symptoms. Additionally, 2 patients with a T315I mutation received ponatinib therapy. Twenty patients received imatinib or nilotinib when diagnosed, and 1 patient received dasatinib and was initially switched to ponatinib after CNS relapse. Moreover, 7 patients relapsed after HSCT. The characteristics of the patients are summarized in Table 1. There was no significant difference at baseline between the 2 groups at the time of CNS relapse.
Table 1.
Clinical Characteristics of Ph-Positive ALL Patients.
| Ponatinib (n = 9) | Dasatinib (n = 12) | P | |
|---|---|---|---|
| Gender | .387 | ||
| Male | 6 | 5 | |
| Female | 3 | 7 | |
| Age (years) | 45 (17-58) | 46 (9-55) | .776 |
| BCR-ABL fusion gene | 1.000 | ||
| P190 | 5 | 7 | |
| P210 | 4 | 5 | |
| T315I mutation | 2 | 0 | .171 |
| Complex karyotype | 3 | 4 | 1.000 |
| Primary induction TKIs | .488 | ||
| Imatinib | 5 | 7 | |
| Nilotinib | 3 | 5 | |
| Dasatinib | 1 | 0 | |
| Relapse after HSCT | 3 | 4 | 1.000 |
| Relapse time (months) | 8 (1-21) | 6.5 (1-35) | .618 |
| Sites of relapse | 1.000 | ||
| CNS isolated | 5 | 6 | |
| BM + CNS | 4 | 6 |
Abbreviations: Ph-Positive ALL, Philadelphia chromosome-positive acute lymphoblastic leukemia; TKIs, tyrosine kinase inhibitors; HSCT, hematopoietic stem cell transplantation; CNS, central nervous system; BM, bone marrow.
Response to Treatment
In all 21 patients, 19 patients achieved CR after treatment, and the total CR rate was 90.5%. As summarized in Table 2, all 9 patients achieved CR in the ponatinib group, 10 patients achieved CR in the dasatinib group, and the median times after achieving CR were 5 and 8 weeks. There was no significant difference in the CR rate between the ponatinib group and the dasatinib group (P = .486). However, patients in the ponatinib group exhibited a significantly shorter duration of time to achieve CR than those patients in the dasatinib group (P = .047). MRD was assessed at 3 months after treatment, and the MRD-negative CR rates of patients in the ponatinib group and dasatinib group were 88.9% versus 58.3% (P = .178).
Table 2.
Response for Patients Treated by Ponatinib or Dasatinib.
| Ponatinib (n = 9) | Dasatinib (n = 12) | P | |
|---|---|---|---|
| CR (%) | 9 (100%) | 10 (83.3%) | .486 |
| Medium time after achieved CR (weeks) | 5 | 8 | .047 |
| MRD negative (%) | 8 (88.9%) | 7 (58.3%) | .178 |
Abbreviations: MRD, minimal residual disease; CR, complete remission.
Survival
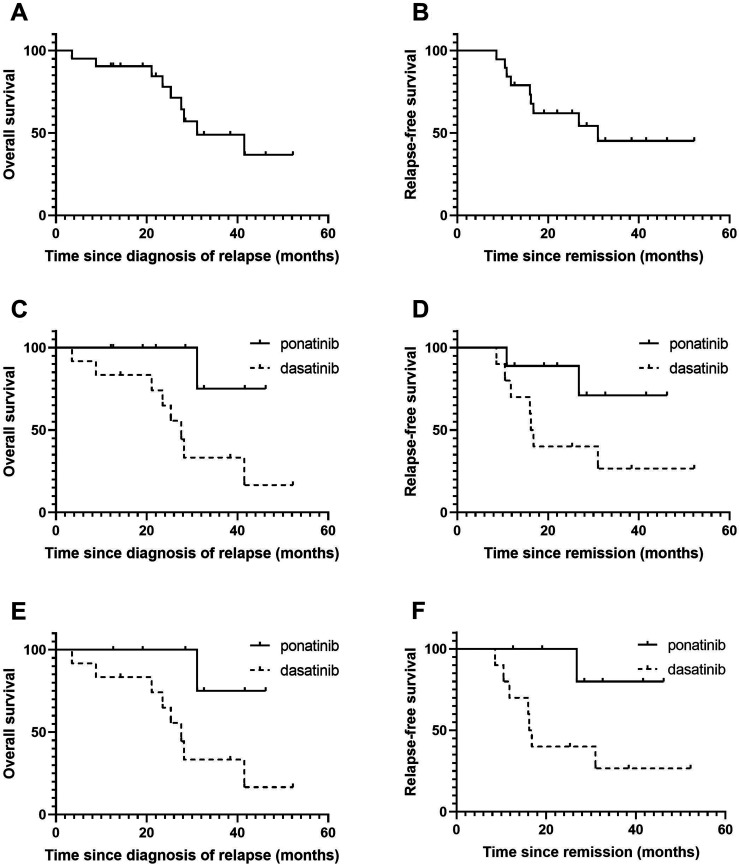
In the complete cohort of 21 patients, 8 patients underwent HSCT (4 patients in the ponatinib group and 4 patients in the dasatinib group). All of the patients received maintenance TKIs after transplantation. The total median OS was 31.1 months, and the 3-year OS was 49.0% (Figure 1A). In addition, the median OS of the ponatinib group and the dasatinib group did not reach 27.6 months, and the 3-year OS rates of the 2 groups were 75.0% and 33.3%, respectively (P = .045). Patients in the ponatinib group showed a significantly longer OS than those patients in the dasatinib group (Figure 1B). In 19 patients without the T315I mutation, the median OS of the ponatinib group was not reached, and the median OS of the dasatinib group was 27.6 months (P = .039). Patients in the ponatinib group also showed a significantly longer OS (Figure 1C). The cause of death of all of the patients was the disease itself.
Figure 1.
Survival of the entire study population. (A) Overall survival and (B) relapse-free survival of the entire study population of patients with Philadelphia chromosome-positive central nervous system leukemia (Ph-positive CNSL). (C) Overall survival and (D) relapse-free survival of patients who received ponatinib or dasatinib. (E) Overall survival and (F) relapse-free survival of patients without T315I mutation received ponatinib or dasatinib.
Among the 19 patients who achieved CR, 9 experienced relapses, 2 patients relapsed after ponatinib treatment, and 7 patients relapsed after dasatinib treatment; moreover, the median RFS was 31.0 months, and the 3-year RFS was 45.2% (Figure 1D). There were no statistically significant differences in the median RFS between the ponatinib group and the dasatinib group (not reached vs 16.2 months, P = .065, Figure 1E). However, except for 2 patients with the T315I mutation in the ponatinib group, there was a statistically significant difference between the ponatinib group and the dasatinib group (not reached vs 16.2 months, P = .036, Figure 1F).
Safety
We analyzed the grade 3 or 4 adverse events of the 2 groups, which are shown in Table 3. Hematological toxicity was similar between the 2 groups. The incidence of grade 3 to 4 infections was 22.2% (2/9) in the ponatinib group and 33.3% (4/12) in the dasatinib group, and 4 cases (1 case in the ponatinib group and 3 cases in the dasatinib group) of septic shock after treatments were observed; however, it was controlled after anti-infection treatment. One patient in the dasatinib group suffered a serious cardiac failure, which led to discontinuity during the maintenance treatment. There were no hemorrhage events caused by thrombocytopenia and other adverse events (such as renal failure, hepatic failure, and gastrointestinal and nervous system toxicity) occurred in these 21 patients. No treatment-related death was observed during the therapy.
Table 3.
Adverse Events of the 2 Groups.
| Toxicities (grade 3 or 4) | Ponatinib (n = 9) | Dasatinib (n = 12) | P |
|---|---|---|---|
| Infections | 2 | 4 | .659 |
| Cardiac failure | NA | 1 | 1.000 |
| Neutropenia | 1 | 3 | .603 |
| Anemia | 1 | NA | .429 |
| Thrombocytopenia | NA | 3 | .229 |
Discussion
In this study, we retrospectively analyzed the clinical data of 21 patients with Ph-positive CNSL and compared the efficacy and safety of ponatinib and dasatinib. Our conclusions were as follows: (1) Ph-positive CNSL patients had a high rate of response and negative postinduction MRD with ponatinib and dasatinib, but ponatinib showed a shorter time to achieve remission than dasatinib. (2) Ponatinib maintenance treatment showed superior survival for Ph-positive CNSL patients with or without the T315I mutation. (3) Ponatinib and dasatinib are both safe for the clinical application of Ph-positive CNSL.
Approximately 25% of adults with B-ALL have the Philadelphia chromosome or the BCR-ABL1 rearrangement, and the incidence increases with age.7 CNSL is the most common extramedullary relapse site of patients with Ph-positive B-ALL, with a poor prognosis and a high relapse rate.22 The frequency of CNSL in Ph-positive ALL ranges from 8% to 17%.23 Additionally, the BCR-ABL1 fusion gene encodes the P190 or P210 protein with tyrosinase activity, which is the key to the pathogenesis of leukemia and the target of this disease. By receiving treatment with TKIs and allo-HSCT, 90% of patients were shown to achieve CR, and the 5-year OS reached 60%.24 In our study, ponatinib and dasatinib were both safe and efficient for Ph-positive patients, and ponatinib seemed to be more valuable. Two patients with the T315I mutation received ponatinib, and one patient sustained relapse-free survival. As the frontline treatment option for Ph-positive ALL, ponatinib was also feasible in the treatment of CNSL. For patients without T315I, ponatinib remains a highly potent and safe regimen for frontline therapy. However, for those patients who cannot afford ponatinib, dasatinib can also be used as a clinical option.
There is no consensus on the duration of TKI maintenance and optimal conditions for stopping TKI maintenance treatment. As one of the most concerning immunotherapies, CAR-T therapy has achieved great success in the field of CNSL. Recently, one study of the effectiveness of CD19 CAR-T therapy in children with high-burden CNS refractory B-ALL showed that 9 of 12 (75%) patients who received CD19 CAR-T treatment achieved sustained remission.25 In adults. CD19 CAR-T-cell infusion resulted in an overall response rate of 87.5% in BM disease and a remission rate of 85.4% in CNSL; however, the median event-free survival was only 8.7 months.26 CD19 CAR T-cell therapy may provide a potential treatment option for CNSL patients, but TKI maintenance treatment is still needed to achieve long-term survival. Another study showed that CNSL relapse rapidly occurred in patients after ponatinib was reduced.18 Our results showed that TKI maintenance seemed to be necessary; however, prospective clinical trials are needed for further verification.
Another question to answer is whether HSCT is possible for sequential therapy when ponatinib maintenance treatment is in progress. A single-center retrospective study showed that a better OS can be achieved through ponatinib and bridged to HSCT in Ph-positive ALL patients,27 and another study also confirmed this conclusion.28 Ponatinib may induce GVL effects, thus leading to long-term survival.17 However, the phase 2 PACE trial of ponatinib in Ph-positive leukemia showed that the patients who did not undergo HSCT had a 73% 5-year overall survival, but this trial excluded CNS disease. In our cohort, 3/5 patients maintained relapse-free survival without HSCT, and 3/4 patients who underwent HSCT maintained relapse-free survival. HSCT did not demonstrate advantages (P = 1.000), but more clinical data are needed for verification.
The following information provides details on the limitations of the study. The small number of patients and the retrospective nature could have some influence on the results. Therefore, our results suggest the possibility of clinical treatment, rather than reaching definitive conclusions. Due to the low incidence rate of diseases, it is difficult for a single center to obtain large samples, so a larger-scale multicenter clinical trial was needed. Some cases suggested that the penetration of ponatinib to the CSF was extremely limited.29,30 Unfortunately, therapeutic drug in CSF was not monitored in these patients in our study, a further study is necessary to reach definitive conclusions.
Conclusions
Ph-positive ALL with CNS relapse is associated with a poor outcome. Ponatinib and dasatinib might be effective and safe for Ph-positive CNSL patients, and ponatinib maintenance treatment seemed to have a shorter time to achieve remission and superior survival for Ph-positive CNSL patients with or without T315I mutations. Due to the small sample dataset, data from large-sample, long-term follow-up, and prospective studies are needed for further verification.
Acknowledgments
The authors thank the patients. They also thank the support from the Kangda College of Nanjing Medical University and Soochow University.
Footnotes
Authors’ Contribution: YXZ and YZ designed the research, YXZ, YZ, LM, TJ, JPM, and LGX collected and analyzed the clinical data, and YXZ and YW made the summary. YXZ did the statistics and wrote this manuscript.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional review committee of First People's Hospital of Lianyungang (KY-20220907001-01) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Funding: The authors received the following financial support for the research, authorship, and/or publication of this article: This work is supported by funding from the Kangda College of Nanjing Medical University (grant KD2021KYJJZD033).
References
- 1.Larson RA. Managing CNS disease in adults with acute lymphoblastic leukemia. Leuk Lymphoma. 2018;59(1):3-13. [DOI] [PubMed] [Google Scholar]
- 2.Gokbuget N, Hoelzer D. Meningeosis leukaemica in adult acute lymphoblastic leukaemia. J Neurooncol. 1998;38(2–3):167-180. [DOI] [PubMed] [Google Scholar]
- 3.Lazarus HM, Richards SM, Chopra R, et al. Central nervous system involvement in adult acute lymphoblastic leukemia at diagnosis: results from the international ALL trial MRC UKALL XII/ECOG E2993. Blood. 2006;108(2):465-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kantarjian HM, Walters RS, Smith TL, et al. Identification of risk groups for development of central nervous system leukemia in adults with acute lymphocytic leukemia. Blood. 1988;72(5):1784-1789. [PubMed] [Google Scholar]
- 5.Alsadeq A, Schewe DM. Acute lymphoblastic leukemia of the central nervous system: on the role of PBX1. Haematologica. 2017;102(4):611-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jeha S, Pei D, Raimondi SC, et al. Increased risk for CNS relapse in pre-B cell leukemia with the t(1;19)/TCF3-PBX1. Leukemia. 2009;23(8):1406-1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanchez R, Ayala R, Alonso RA, et al. Clinical characteristics of patients with central nervous system relapse in BCR-ABL1-positive acute lymphoblastic leukemia: the importance of characterizing ABL1 mutations in cerebrospinal fluid. Ann Hematol. 2017;96(7):1069-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fielding AK, Rowe JM, Buck G, et al. UKALLXII/ECOG2993: addition of imatinib to a standard treatment regimen enhances long-term outcomes in Philadelphia-positive acute lymphoblastic leukemia. Blood. 2014;123(6):843-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leis JF, Stepan DE, Curtin PT, et al. Central nervous system failure in patients with chronic myelogenous leukemia lymphoid blast crisis and Philadelphia chromosome-positive acute lymphoblastic leukemia treated with imatinib (STI-571). Leukemia Lymphoma. 2009;45(4):695-698. [DOI] [PubMed] [Google Scholar]
- 10.Ravandi F, O’Brien SM, Cortes JE, et al. Long-term follow-up of a phase 2 study of chemotherapy plus dasatinib for the initial treatment of patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Cancer. 2015;121(23):4158-4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim DY, Joo YD, Lim SN, et al. Adult acute lymphoblastic leukemia working party of the Korean society of hematology. Nilotinib combined with multiagent chemotherapy for newly diagnosed Philadelphia-positive acute lymphoblastic leukemia. Blood. 2015;126(6):746-756. [DOI] [PubMed] [Google Scholar]
- 12.Cortes JE, Kim DW, Pinilla-Ibarz J, et al. A phase 2 trial of ponatinib in Philadelphia chromosome-positive leukemias. N Engl J Med. 2013;369(19):1783-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cortes JE, Kim DW, Pinilla-Ibarz J, et al. Ponatinib efficacy and safety in Philadelphia chromosome-positive leukemia: final 5-year results of the phase 2 PACE trial. Blood. 2018;132(4):393-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nicolini FE, Basak GW, Kim D, et al. Overall survival with ponatinib versus allogeneic stem cell transplantation in Philadelphia chromosome positive leukemias with the T315I mutation. Cancer-Am Cancer Soc. 2017;123(15):2875-2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cerveira N, Ferreira RB, Bizarro S, et al. Ponatinib induces a sustained deep molecular response in a chronic myeloid leukaemia patient with an early relapse with a T315I mutation following allogeneic hematopoietic stem cell transplantation: a case report. BMC Cancer. 2018;18(1):1229-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Porkka K, Koskenvesa P, Lundan T, et al. Dasatinib crosses the blood–brain barrier and is an efficient therapy for central nervous system Philadelphia chromosome-positive leukemia. Blood. 2008;112(4):1005-1012. [DOI] [PubMed] [Google Scholar]
- 17.Shen SH, Chen XJ, Cai JY, et al. Effect of dasatinib vs imatinib in the treatment of pediatric Philadelphia chromosome-positive acute lymphoblastic leukemia: a randomized clinical trial. JAMA Oncol. 2020;6(3):358-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He JB, Zhang X, Guo ZW, et al. Ponatinib therapy in recurrent Philadelphia chromosome-positive central nervous system leukemia with T315I mutation after allo-HSCT. Int J Cancer. 2020;147(4):1071-1077. [DOI] [PubMed] [Google Scholar]
- 19.Masuda K, Nakazato T, Nishiyama-Fujita T, et al. Successful treatment with ponatinib for central nervous system relapse of Philadelphia chromosome-positive B-cell acute lymphoblastic leukaemia. Intern Med J. 2019;49(10):1332-1334. [DOI] [PubMed] [Google Scholar]
- 20.von Elm E, Altman DG, Egger M, et al. The strengthening of the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573-577. [DOI] [PubMed] [Google Scholar]
- 21.Alvarnas JC, Brown PA, Aoun P, et al. Acute lymphoblastic leukemia, version 2.2015. J Natl Compr Canc Netw. 2015;13(10):1240-1279. [DOI] [PubMed] [Google Scholar]
- 22.Fielding AK, Richards SM, Chopra R, et al. Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL): an MRC UKALL12/ECOG2993 study. Blood. 2007;109(3):944-950. [DOI] [PubMed] [Google Scholar]
- 23.Gaur S, Torabi AR, Corral J. Isolated central nervous system relapse in two patients with BCR-ABL-positive acute leukemia while receiving a next-generation tyrosine kinase inhibitor. In Vivo. 2014;28(6):1149-1153. [PubMed] [Google Scholar]
- 24.Fielding AK. Current treatment of Philadelphia chromosome-positive acute lymphoblastic leukemia. Haematologica. 2010;95(1):8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tan Y, Pan J, Deng B, et al. Toxicity and effectiveness of CD19 CAR T therapy in children with high-burden central nervous system refractory B-ALL. Cancer Immunol Immunother. 2021;70(7):1979-1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qi YK, Zhao MF, Hu YX, et al. Efficacy and safety of CD19-specific CAR T cell-based therapy in B-cell acute lymphoblastic leukemia patients with CNSL. Blood. 2022;139(23):3376-3386. [DOI] [PubMed] [Google Scholar]
- 27.Shi T, Xie MX, Zhu LX, et al. T315i mutation exerts a dismal prognosis on adult BCR-ABL1-positive acute lymphoblastic leukemia, and salvage therapy with ponatinib or CAR-T cell and bridging to allogeneic hematopoietic stem cell transplantation can improve clinical outcomes. Ann Hematol. 2020;99(4):829-834. [DOI] [PubMed] [Google Scholar]
- 28.Leotta S, Markovic U, Pirosa MS, et al. The role of ponatinib in adult BCR-ABL1 positive acute lymphoblastic leukemia after allogeneic transplantation: a real-life retrospective multicenter study. Ann Hematol. 2021;100(7):1743-1753. [DOI] [PubMed] [Google Scholar]
- 29.Abid MB, De Mel S. Does ponatinib cross the blood–brain barrier? Br J Haematol. 2017;179(3):497-498. [DOI] [PubMed] [Google Scholar]
- 30.Tanimura K, Yamasaki K, Okuhiroet Y, et al. Monitoring ponatinib in a child with Philadelphia chromosome-positive acute lymphoblastic leukemia. Case Rep Oncol. 2021;14(1):24-28. [DOI] [PMC free article] [PubMed] [Google Scholar]