Abstract
Chemotherapy-induced peripheral neuropathy (CIPN)-mediated paresthesias are a common complication in cancer patients undergoing chemotherapy. There are currently no treatments available to prevent or reverse CIPN. Therefore, new therapeutic targets are urgently needed to develop more effective analgesics. However, the pathogenesis of CIPN remains unclear, and the prevention and treatment strategies of CIPN are still unresolved issues in medicine. More and more studies have demonstrated that mitochondrial dysfunction has become a major factor in promoting the development and maintenance of CIPN, and peroxisome proliferator-activated receptor gamma (PPARγ) coactivator 1α (PGC1α) plays a significant role in maintaining the mitochondrial function, protecting peripheral nerves, and alleviating CIPN. In this review, we highlight the core role of PGC1α in regulating oxidative stress and maintaining normal mitochondrial function and summarize recent advances in its therapeutic effects and mechanisms in CIPN and other forms of peripheral neuropathy. Emerging studies suggest that PGC1α activation may positively impact CIPN mitigation by modulating oxidative stress, mitochondrial dysfunction, and inflammation. Therefore, novel therapeutic strategies targeting PGC1α could be a potential therapeutic target in CIPN.
Keywords: chemotherapy-induced peripheral neuropathy, mitochondrial biogenesis, mitochondrial dysfunction, oxaliplatin, oxidative stress, paclitaxel, PGC1α
Introduction
Cancer is one of the top death causes across the globe, and despite the enormous efforts to implement novel chemotherapy strategies, the disease remains one of the major concerns worldwide. According to the International Agency for Research on Cancer (IARC), it is estimated that over 19.3 million new cancer cases were diagnosed in 2020, followed by 10.0 million deaths.1,2 With the rapid development of modern cancer diagnosis and treatment technology, cancer patients’ survival rate has increased year by year, and the survival period has been prolonged.3 Statistical analysis shows that the cancer mortality rate in the United States is decreasing at an annual rate of 1.5%, and the overall reduction in 2020 is 29% compared with 1991. Among them, the 5-year survival rate of prostate cancer and female breast cancer is as high as 90%.4 Consistently, the 5-year survival rate of cancer patients in China also showed a significant upward trend.5 Cancer is gradually changing from a ‘terminal illness’ to a chronic disease, and the goal of cancer treatment is changing from ‘survival’ to ‘quality survival’. Chemotherapy is the most extensively used approach for cancer treatment, while chemotherapy-induced peripheral neuropathy (CIPN) is the major side effect that hinders cancer treatment. Up to 80% of chemotherapy patients develop CIPN, and 40–60% of the patients have to terminate or delay the treatment.6
Chemotherapy-induced peripheral neuropathy
Many first-line cytotoxic agents inducing peripheral neuropathy have been used in solid tumors and hematological malignancies, including blockbuster anti-tumor agents, paclitaxel, lenalidomide, and oxaliplatin. CIPN severely limits the use of chemotherapy agents and is a common cause of chemotherapy discontinuation. Besides, immune checkpoint inhibitors such as programmed cell death protein 1 (PD-1) and cytotoxic-T-lymphocyte-antigen-4 (CTLA4) have recently been found to cause similar neurological side effects.7,8 CIPN symptoms initially manifested as typical ‘glove and stocking’ neuropathy, symmetrical numbness of the extremities, with pinning and burning paresthesias, and then progressed to sensory and motor disturbances, and eventually to muscle weakness, difficulty moving, burning sensations, and severe pain in the extremities.6 Some patients suffer CIPN even if their cancer has been cured, such as numbness or burning pain in the hands and feet for years, with major and permanent consequences for life barriers. However, CIPN does not respond well to conventional analgesics and is extremely difficult to treat. There is no specific drug that can prevent and treat CIPN in clinical practice.9 The American Society of Clinical Oncology (ASCO) acknowledged this dilemma, recommending only duloxetine for the relief of CIPN symptoms in its published clinical practice guidelines on the prevention and management of CIPN. The difficulties faced in the development of CIPN drugs mainly include (1) The pathogenic mechanism of CIPN is still unclear; (2) Disease factors such as cancer increase the complexity of CIPN treatment as cancer and CIPN can synergize on a common signaling pathway; (3) The prevention and treatment of CIPN should not affect the anticancer effect of chemotherapy. Therefore, elucidating the pathogenesis of CIPN and developing specific agents for CIPN prevention and treatment have very important scientific significance and clinical practical value. Prevention and treatment of CIPN represent a critical unmet medical need. This demand has also shifted from academic research to industrial investment. In August 2018, Pfizer, Eli Lilly, and AbbVie have jointly invested US$31 million to launch drug development for the prevention of peripheral neuropathy and cognitive impairment caused by chemotherapy.10 Therefore, the prevention and treatment of CIPN is a medical need that needs to be urgently met.
Mitochondrial dysfunction in CIPN
The pathogenesis of CIPN induced by chemotherapy mainly includes, (1) abnormal expression and activation of cell membrane receptors and ion channels, (2) changes in intracellular signal transduction, (3) mitochondrial damage and oxidative stress, (4) the activation of glial cells and the generation of neuroinflammation eventually lead to the sensitization of peripheral sensory neurons and the damage of neurons and nerve fibers.6 Among them, mitochondrial dysfunction is reported to be significant pathogenesis of CIPN, which corresponds to the clinical manifestations.11–15
Mitochondria, as highly active organelles, carry out their own ‘renewal and regeneration’ through biosynthesis, division/fusion, and autophagy to maintain their morphological and functional integrity. Disruption of mitochondrial homeostasis and subsequent mitochondrial dysfunction plays a key role in peripheral neuropathy caused by multiple pathological factors.16–19 Accumulating evidence indicates that many chemotherapeutic agents cause mitochondrial damage in the peripheral sensory nerves by disrupting mitochondrial structure and bioenergetics, increasing nitro-oxidative stress, and altering mitochondrial transport and fission, fusion, and mitophagy. The mitotoxicity theory of CIPN has proposed that the abnormal and dysfunctional mitochondria in sensory neurons are led to axonal growth defects and the loss of intraepidermal nerve fibers, which in turn increased spontaneous discharge and the sensitization of peripheral sensory neurons and the central nervous system that promote the establishment of chronic pain state.20 Mitochondrial damage has been reported to be involved in the pathological process of paclitaxel-induced CIPN. Flatters et al. found that mitochondrial swelling and vacuolization in both C-fibers and myelinated axons are the main neuropathological features for paclitaxel-induced painful peripheral neuropathy.21 Besides, a clinical study has demonstrated that mitochondrial swelling and vacuolization were observed in sensory axons by electron microscopy in sural nerve biopsies from CIPN patients induced by paclitaxel22 and docetaxel.23 This phenomenon has also been observed in C-fiber and A-fiber of oxaliplatin, and bortezomib-induced CIPN rats.24,25 In addition, paclitaxel-induced changes in neuronal mitochondria in C fiber and myelinated axons are correlated to paclitaxel-induced pain syndrome.21
Recent research shows that mitochondrial swelling and vacuolization in C-fibers and myelinated axons disrupt the maintenance of the proton gradient, impair mitochondrial ATP production, and result in severe energy insufficiency, and increased reactive oxygen species (ROS) production in neurons.26,27 Chemotherapy-evoked changes in bioenergetics are also associated with decreased ATP production.28,29 The maximal respiration and respiratory ability were significantly decreased in dorsal root ganglia (DRG) neurons of paclitaxel-induced CIPN rats. Duggett et al. found enhanced basal glycolysis and maximal glycolytic ATP production in peripheral sensory neurons during peak pain in absence of altered respiration or respiratory capacity, suggesting the energy supplement of sensory neurons switched from relying on oxidative phosphorylation through less efficient glycolysis.29 Moreover, the function of mitochondrial was also decreased in complex I-stimulated and complex II-stimulated respiration in sciatic nerves from paclitaxel-, oxaliplatin-, and bortezomib-induced CIPN rats.25,28 It has been reported that a complex III inhibitor, antimycin A significantly prevented the development of paclitaxel-induced CIPN, but had no therapeutic effect on CIPN.30 Mitochondrial DNA damage has been demonstrated to be a novel process for the induction of CIPN induced by chemotherapeutic agents.31 Mannelli et al. reported that the DNA oxidation product 8-Hydroxy-2’-deoxyguanosine (8-OH-dG) has increased in the sciatic nerve and spinal cord with a rat model of oxaliplatin-induced CIPN.32
The therapies targeting chemotherapy-induced mitochondrial oxidative stress have the potential to protect mitochondrial function and alleviate neuropathological damage. Antioxidant pharmacological strategies using ROS/RNS scavengers, superoxide dismutase (SOD) mimetics, and peroxynitrite decomposition catalysts have shown success in attenuating chemotherapy-induced neurotoxic effects in cellular and animal models, including n-tert-Butyl-a-phenylnitrone (PBN)-a global free-radical scavenger,33 4-hydroxy-2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPOL)-a nonselective nitroxyl antioxidant,34 the active metabolite of amifostine [N-2-mercaptoethyl]-1-3-diaminopropane (WR-1065), a ROS/RNS scavenger,35 and the SOD mimetic, polyamine-polycarboxylate-MnII complex 4,10-dimethyl-1,4,7,10 tetraazacyclododecane-1,7-diacetic acid MnII (MnL4).36 Other strategies attempt to protect against nerve damage by indirectly hindering mitochondrial oxidative stress. Meclizine, a histamine H1 receptor antagonist, has been reported to switch cells to glycolysis and pentose phosphate pathways to improve ATP production and neurite outgrowth in DRG neurons treated with cisplatin.37 Recent research has shown that oxidative stress can drive matrix metalloproteases 9 (MMP9) mitochondria translocation and induce mitochondria dysfunction,38 while intrathecal administration of the monoclonal antibody of MMP9 attenuated ROS production in the DRG and resulted in decreased IENF loss and paclitaxel-induced neuropathic pain in mice.39 Targeting the oxidative stress-sensitive poly (ADP-ribose) polymerase (PARP)/p53 pathway has been reported to prevent mitochondrial dysfunction and neural damage. Pifithrin-μ, a p53 inhibitor, has been reported to prevent mitochondrial damage in the DRG by preventing the accumulation of p53 in the mitochondria and protects against paclitaxel- and cisplatin-induced mechanical allodynia and loss of intraepidermal nerve fiber in mice.40,41 Therefore, promoting and repairing mitochondrial function may be a new strategy for the treatment of CIPN.
PGC1α, a master coactivator, triggers mitochondrial biogenesis in CIPN
Peroxisome proliferator-activated receptor gamma (PPARγ) coactivator 1α (PGC1α) was first discovered in brown adipose tissue as a PPARγ coactivator in response to cold stimulation.42 The major function of PGC1α is to regulate mitochondrial biosynthesis to promote aerobic metabolism and resist oxidative stress, and its expression level directly determines mitochondrial function and self-renewal level.43,44 PGC1α is highly expressed in multiple tissues, including brown adipose tissue, liver, heart, pancreas, skeletal muscle, and kidney,45,46 as well as in the nervous system, including the cerebral cortex, spinal cord, and DRG neurons, and mediates various neurological diseases.43,47 It has been reported that PGC1α regulates the expression of enzymes for ROS detoxifying, such as SOD1 and 2, catalase, and glutathione peroxidase-1.48 Besides, PGC1α acts as a coactivator with other transcription factors including the nuclear respiratory factor (NRF), transcriptional factor A mitochondrial (TFAM), and myocyte enhancer factor 2.49 The PGC1α-NRF1/2-TFAM axis plays an important role in the regulation of mitochondrial regeneration. PGC1α promotes the expression of TFAM through NRF1/2. TFAM is a key factor in initiating mitochondrial transcription, promoting mitochondrial DNA (mtDNA) replication, and regulating mitochondrial regeneration.50 In models of Parkinson’s disease, Alzheimer’s disease, and aging, the expression levels of TFAM and mtDNA are reduced and mitochondrial function is impaired, while overexpression of TFAM in mitochondria is neuroprotective against these neurodegenerative diseases.50 A recent study reveals that PGC1α inhibitor SR-18292 reverses the analgesic effect of ZLN005 and abolishes the analgesic effect of formoterol against CIPN.51
In sensory neurons, AMP-activated protein kinase (AMPK) mediates muscarinic ACh type 1 receptor (M1R) antagonists induced PGC1α and mitochondrial activity to promote neurite outgrowth from adult sensory neurons and to protect animal models against peripheral neuropathy induced by diabetes, the chemotherapeutic agents such as dichloroacetate and paclitaxel, or human immunodeficiency virus (HIV) envelope protein gp120.52,53 Furthermore, overexpression of PGC1α can increase the number and quality of mitochondria in DRG neurons of diabetic mice.54 PGC1α-mediated mitochondrial biogenesis has also been reported to be involved in the attenuation of neuropathic pain at the spinal level in peripheral nerve injury. Activation of PGC1α in the spinal cord attenuates established mechanical allodynia in rats with neuropathic pain.55 NRF2 attenuates chronic constriction injury-induced neuropathic pain via the induction of PGC1α-mediated mitochondrial biogenesis in the spinal cord.56 In addition, PGC1α also regulates a series of nuclear receptors including the thyroid hormone receptor, and the estrogen receptor.49
PGC1α acts as an oxidative stress regulator in CIPN
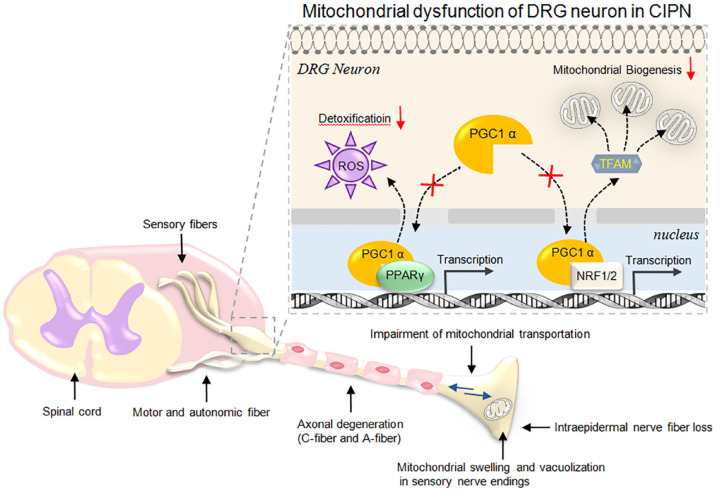
Intracellular ROS is mainly derived from mitochondrial and excessive production of ROS leads to mitochondrial dysfunction. ROS-induced oxidative stress and the subsequent injury of the myelin sheath, mitochondrial proteins, and antioxidant enzymes in peripheral neurons is a substantial initiator for CIPN.13 In vivo study has demonstrated that scavenge ROS inhibited the development of CIPN induced by paclitaxel and bortezomib.57,58 Elamipretide (SS-31), a mitochondria-targeted antioxidant, attenuated oxaliplatin-induced CIPN.59 MitoVitE attenuated the development of paclitaxel-induced mechanical hypersensitivity.60 Previous in vivo study has also demonstrated that ROS production was significantly increased in the spinal cord and lumbar DRG after chemotherapeutic agent treatment.59,61 Besides, ROS levels were also significantly increased in the superficial spinal and DRG neurons in vivo before the onset of paclitaxel-induced mechanical allodynia, suggesting ROS works as an initiating factor.62 However, antioxidant enzymes were also enhanced in the DRG and peripheral sensory nerves in CIPN animals.62 These data reveal that mitochondrial ROS increase is an initiating factor for the development and maintenance of CIPN (Figure 1).
Figure 1.
The peripheral neuropathological features and mitochondrial dysfunction of dorsal root ganglia (DRG) neurons in chemotherapy-induced peripheral neuropathy (CIPN).
The pathological characterizations of peripheral nerves in CIPN are indicated by arrows, including axonal degeneration, intraepidermal nerve fiber loss, mitochondrial swelling and vacuolization in sensory nerve endings, and impairment of mitochondrial transportation. The enlarged illustration on the upper right shows the central role of peroxisome proliferator-activated receptor gamma coactivator 1α (PGC1α) in mitochondrial dysfunction of DRG neurons in CIPN.
Recent research has shown that the administration of Ghrelin, an endogenous ligand for the growth hormone secretagogue receptor, decreases plasma oxidative stress, increases the expression of PGC1α and mitochondrial antioxidant proteins, alleviates mechanical and thermal hypersensitivity, and partially prevents neuronal loss of small unmyelinated intraepidermal nerve fibers. In addition, evodiamine, a plant-derived natural compound, has been reported to prevent paclitaxel-induced loss of mitochondrial membrane potential, increase PGC1α expression in DRG cells, and maintain mitochondrial anti-oxidant functions, and ameliorate paclitaxel-induced neuropathic pain.63 However, the mechanism of PGC1α in mitochondrial anti-oxidative stress in CIPN is still unclear and needs further exploration.
PGC1α acts as a therapeutic target in peripheral neuropathy
PGC1α is increasingly recognized as an important target in the prevention and regulation of CIPN and other forms of neuropathy. A series of literature have reported the potential of natural compounds or molecules to treat CIPN by modulating PGC1α expression or function. Resveratrol has been reported to reduce apoptosis by SIRT1/PGC1α signal pathway, prevent paclitaxel-induced mitochondrial damage, and improve the relevant pain symptoms.64 Evodiamine prevented paclitaxel-induced loss of mitochondrial membrane potential and paclitaxel-induced neuropathic pain via regulating PGC1α expression in DRG neurons.63 Ghrelin alleviates paclitaxel-induced CIPN by reducing oxidative stress and enhancing PGC1α expression in mice.65 Nuclear sirtuin 1 (SIRT1)/PGC1α signaling has been reported to be involved in the analgesic effect of translocator protein in a rat spinal nerve ligation model.66 SIRT1 activation alleviates oxidative damage and enhances PGC1α-NRF1/2-TFAM axis-mediated mitochondrial biogenesis in diabetic neuropathy rats.67–69 Furthermore, multiple lines of evidence suggest that PGC1α mediates the mitochondrial function promotion and neuroprotection effect of multiple upstream regulators. Berberine exposure augmented PGC1α-mediated mitochondrial biogenesis in DRG neurons in experimental diabetic neuropathy rats.70 Impaired AMPK signaling in DRG neurons is associated with PGC1α-mediated mitochondrial dysfunction and peripheral neuropathy in diabetes.71 Salvianolic acid A application and potassium voltage-gated channel subfamily B member 1 (Kv2.1) inhibition protect the peripheral nerve function in diabetic rats through the regulation of the AMPK/PGC1α pathway.72,73 Furthermore, overexpression of human TFAM in mice can prevent type 1 diabetes-induced nerve conduction slowdown, improve epidermal fiber loss, and alleviate mechanical hyperalgesia.74 As an upstream regulator of TFAM, knockout of PGC1α leads to mitochondrial damage, increases oxidative stress, leads to mitochondrial dysfunction, and exacerbates diabetic neuropathy.75 Therefore, promoting mitochondrial regeneration mediated by the PGC1α-NRF1/2-TFAM axis might be a potential approach for exploring the prevention and treatment of diabetic peripheral neuropathy.
In addition, several natural compounds and molecules targeting PGC1α, including resveratrol, evodiamine, berberine, and ghrelin have shown neuroprotective effects against CIPN and diabetic neuropathy in preclinical studies as discussed above, further clinical trials refer to these molecules are highly recommended. The pan-PPAR agonist bezafibrate, ferulic acid, and co-activation of PPARγ and PGC1α with N-(2-benzoylphenyl)-O-[2-(methyl-2-pyridinylamino)ethyl]-l-tyrosine (GW1929) and alpha-lipoic acid have shown strong therapeutic potential in animal models of degenerative disease like Huntington’s and Parkinson’s disease in the central nervous system, which can be further investigated in the treatment of peripheral neurodegenerative diseases.76–78 Several substances that have been proven to promote PGC1α expressions in human skeletal muscle, such as mitoquinone (mitoQ) and pyrroloquinoline quinone, were recommended to test their clinical potential in the treatment of peripheral neuropathy.79,80 Other substances including hydrogen gas and inorganic nitrate targeting the PGC1α pathway to promote mitochondrial function are also tested in the prevention and treatment of CIPN.81,82 Thus, modulation of the PGC1α-NRF1/2-TFAM axis in DRG neurons has the potential to promote mitochondrial regeneration or protect peripheral nerve fibers and prevent CIPN (Figure 1).
Other potential mitochondrial markers for CIPN diagnostics
To date, no specific biomarkers of mitochondrial dysfunction have been identified to determine the earliest changes or the severity of CIPN. There are indications that modulators of mitochondrial dysfunction may aid in CIPN diagnosis and predict the outcome of CIPN treatments. These modulators include 8-hydroxy-2’-deoxyguanosine (8-OHdG), mtDNA, and heat shock proteins (HSPs). 8-OHdG, the predominant production of free radical-induced oxidative lesions, has been widely used as a biomarker of oxidative stress and oxidative DNA damage in diseases.83 Preclinical studies have shown that paclitaxel induces a rise of 8-OHdG in DRG and amplified oxidative stress to lead the neuropathic pain in rats.84 These results suggested the potential diagnostic value of 8-OHdG as a biomarker in clinical trials.
Moreover, as transcription of mitochondrial genes and level of mitochondrial activity is often proportional to mtDNA copy number, circulating mtDNA has been wildly used as a biomarker for predicting mitochondrial dysfunction and diseases, including cardiovascular disease, metabolic diseases, and cancer.85,86 Recently preclinical studies assessed the potential of circulating mtDNA as a blood biomarker to predict the progression of CIPN. Paclitaxel and bortezomib-induced increases in mtDNA levels were synchronized with the peak of pain behavioral manifestations. Especially, the mtDNA content (determined by mtDNA/nDNA ratio) in blood was increased in the early phase of oxaliplatin-induced CINP, before the emergence of pain-like behaviors. These results suggested that circulating mtDNA in the blood may use as a potential biomarker to identify early stages of CIPN. Furthermore, mtDNA copy number has often been used to evaluate the treatment of CIPN targeting mitochondrial dysfunction in the peripheral and central nervous system in preclinical research.37,51 Together, these findings indicate that mtDNA may serve as a potential biomarker to predict and access the development stage of CIPN and its therapeutic effects.
In addition, several studies have also shown that HSP modulation is the underlying mechanism to prevent chemotherapy-induced neurotoxicity.87–89 Recent findings have reported that human Hsp27 prevents mitochondrial dysfunction, neurotoxicity, and subsequent painful behavior induced by paclitaxel or vincristine.90,91 These preclinical results suggest the potential of Hsp27 as a biomarker for the conservation of mitochondrial function and efficacy of CIPN treatment. However, studies on the conservation of mitochondrial function that refer to mitochondrial function are limited. Therefore, mechanism-based biomarkers, including PGC1α-modulators may potentially overcome this shortcoming. Taken together, these markers might be potential biomarkers in the clinical diagnosis for the stage and severity or efficacy of treatment in CIPN regarding mitochondrial dysfunction.
Conclusions and future perspective
In this review, we propose a scientific conclusion that PGC1α-mediated recovery of mitochondrial function might be a novel target for the treatment and prevention of CIPN. The ideas are clear and the theoretical basis is sufficient. As a transcriptional co-activator, PGC1α plays a key role in regulating oxidative stress and mitochondrial biosynthesis. Not limited to the traditional pain pathway, takes PGC1α as the target to study its mechanism of action in CIPN, which has important reference and guiding significance for elucidating the pathogenesis of CIPN, developing innovative drugs and clinical treatment for the prevention and treatment of CIPN. Therefore, targeting PGC1α is expected to be rapidly transformed and applied to fill the gap in the prevention and treatment of CIPN.
Regulation of PPARγ/PGC1α in sensory neurons activates mitochondrial transcription and achieves ‘purification and renewal’ of mitochondria. However, directly targeting PGC1α might cause severe side effects as PGC1α is majorly involved in non-shivering thermogenesis and temperature homeostasis in humans. Whereas indirectly regulating the expression level of PGC1α is an ideal means to interfere with mitochondrial regeneration. Besides, the upstream regulating and downstream responding signals of PGC1α-mediated mitochondrial regeneration also need to be further explored.
Last but not the least, several natural compounds and molecules targeting PGC1α, including resveratrol, evodiamine, berberine, and ghrelin have shown neuroprotective effects against CIPN and diabetic neuropathy in preclinical studies, further clinical trials refer to these molecules are highly recommended. The pathogenesis of CIPN discussed here is mainly derived from animal models of ‘pain induced by chemotherapy agents’ from preclinical studies. It does not fully represent the real situation of cancer patients treated with neurotoxic chemotherapy. Therefore, future clinical trials could also pave the way for the clinical translation of CIPN therapy targeting mitochondrial dysfunction.
Acknowledgments
None.
Footnotes
ORCID iD: Wuping Sun  https://orcid.org/0000-0003-2543-4369
https://orcid.org/0000-0003-2543-4369
Contributor Information
Mingzhu Zhai, Center for Medical Experiments (CME), University of Chinese Academy of Sciences-Shenzhen Hospital, Shenzhen, China; Yantian Hospital, Southern University of Science and Technology, Shenzhen, China.
Haibei Hu, Center for Medical Experiments (CME), University of Chinese Academy of Sciences-Shenzhen Hospital, Shenzhen, China.
Yi Zheng, Center for Medical Experiments (CME), University of Chinese Academy of Sciences-Shenzhen Hospital, Shenzhen, China.
Benqing Wu, Center for Medical Experiments (CME), University of Chinese Academy of Sciences-Shenzhen Hospital, Shenzhen 518016, China.
Wuping Sun, Department of Pain Medicine and Shenzhen Municipal Key Laboratory for Pain Medicine, Shenzhen Nanshan People’s Hospital and The 6th Affiliated Hospital of Shenzhen University Health Science Center, Shenzhen 518060, China.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: The paper has been read and approved by all authors. All authors approved the submission of this paper to ‘Therapeutic Advances in Neurological Disorders’ for publication. All authors confirmed that neither the manuscript submitted nor any part of it has been published or is being considered for publication elsewhere.
Author contributions: Mingzhu Zhai: Conceptualization; Data curation; Writing – original draft; Writing – review & editing.
Haibei Hu: Data curation; Writing – original draft; Writing – review & editing.
Yi Zheng: Visualization; Writing – original draft; Writing – review & editing.
Benqing Wu: Conceptualization; Funding acquisition; Supervision; Writing – original draft; Writing – review & editing.
Wuping Sun: Conceptualization; Funding acquisition; Project administration; Supervision; Writing – original draft; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the National Natural Science Foundation of China (Number 82171378), Shenzhen Municipal Science, Technology and Innovation Commission (Number JCYJ20210324112202006, JCYJ20220531094815034), and Medical and Health Science and Technology Plan Project of Shenzhen Yantian District Science and Technology Innovation Bureau (Number YTWS20220208).
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Availability of data and materials: Not applicable.
References
- 1. Ferlay J, Colombet M, Soerjomataram I, et al. Cancer statistics for the year 2020: an overview. Int J Cancer. Epub ahead of print 5 April 2021. DOI: 10.1002/ijc.33588. [DOI] [PubMed] [Google Scholar]
- 2. Dalakas MC, Semino-Mora C, Leon-Monzon M. Mitochondrial alterations with mitochondrial DNA depletion in the nerves of AIDS patients with peripheral neuropathy induced by 2’3’-dideoxycytidine (ddC). Lab Invest 2001; 81: 1537–1544. [DOI] [PubMed] [Google Scholar]
- 3. Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018; 391: 1023–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020; 70: 7–30. [DOI] [PubMed] [Google Scholar]
- 5. Guo M, Xu J, Du J. Trends in cervical cancer mortality in China from1989 to 2018: an age-period study and joinpoint analysis. BMC Public Health 2021; 21: 1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sisignano M, Baron R, Scholich K, et al. Mechanism-based treatment for chemotherapy-induced peripheral neuropathic pain. Nat Rev Neurol 2014; 10: 694–707. [DOI] [PubMed] [Google Scholar]
- 7. Kao JC, Liao B, Markovic SN, et al. Neurological complications associated with anti-programmed death 1 (PD-1) antibodies. JAMA Neurol 2017; 74: 1216–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Staff NP, Grisold A, Grisold W, et al. Chemotherapy-induced peripheral neuropathy: a current review. Ann Neurol 2017; 81: 772–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Loprinzi CL, Lacchetti C, Bleeker J, et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: ASCO guideline update. J Clin Oncol 2020; 38: 3325–3348. [DOI] [PubMed] [Google Scholar]
- 10. Terry M. Magnolia neurosciences launches with $31 million. Biospace, 13 August 2018, https://www.biospace.com/article/magnolia-neurosciences-launches-with-31-million/
- 11. Trecarichi A, Flatters SJL. Mitochondrial dysfunction in the pathogenesis of chemotherapy-induced peripheral neuropathy. Int Rev Neurobiol 2019; 145: 83–126. [DOI] [PubMed] [Google Scholar]
- 12. Canta A, Pozzi E, Carozzi VA. Mitochondrial dysfunction in chemotherapy-induced peripheral neuropathy (CIPN). Toxics 2015; 3: 198–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Areti A, Yerra VG, Naidu V, et al. Oxidative stress and nerve damage: role in chemotherapy induced peripheral neuropathy. Redox Biol 2014; 2: 289–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sahu K, Langeh U, Singh C, et al. Crosstalk between anticancer drugs and mitochondrial functions. Curr Res Pharmacol Drug Discov 2021; 2: 100047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zajaczkowska R, Kocot-Kepska M, Leppert W, et al. Mechanisms of chemotherapy-induced peripheral neuropathy. Int J Mol Sci 2019; 20: 1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Khuankaew C, Sawaddiruk P, Surinkaew P, et al. Possible roles of mitochondrial dysfunction in neuropathy. Int J Neurosci 2021; 131: 1019–1041. [DOI] [PubMed] [Google Scholar]
- 17. Ye D, Fairchild TJ, Vo L, et al. Painful diabetic peripheral neuropathy: role of oxidative stress and central sensitisation. Diabet Med 2022; 39: e14729. [DOI] [PubMed] [Google Scholar]
- 18. Dalakas MC. Peripheral neuropathy and antiretroviral drugs. J Peripher Nerv Syst 2001; 6: 14–20. [DOI] [PubMed] [Google Scholar]
- 19. Lewis W, Dalakas MC. Mitochondrial toxicity of antiviral drugs. Nat Med 1995; 1: 417–422. [DOI] [PubMed] [Google Scholar]
- 20. Doyle TM, Salvemini D. Mini-review: mitochondrial dysfunction and chemotherapy-induced neuropathic pain. Neurosci Lett 2021; 760: 136087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Flatters SJL, Bennett GJ. Studies of peripheral sensory nerves in paclitaxel-induced painful peripheral neuropathy: evidence for mitochondrial dysfunction. Pain 2006; 122: 245–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sahenk Z, Barohn R, New P, et al. Taxol neuropathy. Electrodiagnostic and sural nerve biopsy findings. Arch Neurol 1994; 51: 726–729. [DOI] [PubMed] [Google Scholar]
- 23. Fazio R, Quattrini A, Bolognesi A, et al. Docetaxel neuropathy: a distal axonopathy. Acta Neuropathol 1999; 98: 651–653. [DOI] [PubMed] [Google Scholar]
- 24. Xiao WH, Zheng H, Bennett GJ. Characterization of oxaliplatin-induced chronic painful peripheral neuropathy in the rat and comparison with the neuropathy induced by paclitaxel. Neuroscience 2012; 203: 194–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zheng H, Xiao WH, Bennett GJ. Mitotoxicity and bortezomib-induced chronic painful peripheral neuropathy. Exp Neurol 2012; 238: 225–234. [DOI] [PubMed] [Google Scholar]
- 26. Franco-Iborra S, Vila M, Perier C. Mitochondrial quality control in neurodegenerative diseases: focus on Parkinson’s disease and Huntington’s disease. Front Neurosci 2018; 12: 342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Garcia GC, Bartol TM, Phan S, et al. Mitochondrial morphology provides a mechanism for energy buffering at synapses. Sci Rep 2019; 9: 18306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zheng H, Xiao WH, Bennett GJ. Functional deficits in peripheral nerve mitochondria in rats with paclitaxel- and oxaliplatin-evoked painful peripheral neuropathy. Exp Neurol 2011; 232: 154–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Duggett NA, Griffiths LA, Flatters SJL. Paclitaxel-induced painful neuropathy is associated with changes in mitochondrial bioenergetics, glycolysis, and an energy deficit in dorsal root ganglia neurons. Pain 2017; 158: 1499–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Griffiths LA, Flatters SJ. Pharmacological modulation of the mitochondrial electron transport chain in paclitaxel-induced painful peripheral neuropathy. J Pain 2015; 16: 981–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Podratz JL, Knight AM, Ta LE, et al. Cisplatin induced mitochondrial DNA damage in dorsal root ganglion neurons. Neurobiol Dis 2011; 41: 661–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Di Cesare Mannelli L, Zanardelli M, Failli P, et al. Oxaliplatin-induced neuropathy: oxidative stress as pathological mechanism. J Pain 2012; 13: 276–284. [DOI] [PubMed] [Google Scholar]
- 33. Shim HS, Bae C, Wang J, et al. Peripheral and central oxidative stress in chemotherapy-induced neuropathic pain. Mol Pain 2019; 15: 1744806919840098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fidanboylu M, Griffiths LA, Flatters SJ. Global inhibition of reactive oxygen species (ROS) inhibits paclitaxel-induced painful peripheral neuropathy. PLoS ONE 2011; 6: e25212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Popović J, Klajn A, Paunesku T, et al. Neuroprotective role of selected antioxidant agents in preventing cisplatin-induced damage of human neurons in vitro. Cell Mol Neurobiol 2019; 39: 619–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Di Cesare Mannelli L, Zanardelli M, Landini I, et al. Effect of the SOD mimetic MnL4 on in vitro and in vivo oxaliplatin toxicity: possible aid in chemotherapy induced neuropathy. Free Radic Biol Med 2016; 93: 67–76. [DOI] [PubMed] [Google Scholar]
- 37. Gorgun MF, Zhuo M, Englander EW. Cisplatin toxicity in dorsal root ganglion neurons is relieved by meclizine via diminution of mitochondrial compromise and improved clearance of DNA damage. Mol Neurobiol 2017; 54: 7883–7895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jobin PG, Butler GS, Overall CM. New intracellular activities of matrix metalloproteinases shine in the moonlight. Biochim Biophys Acta Mol Cell Res 2017; 1864(Pt. A): 2043–2055. [DOI] [PubMed] [Google Scholar]
- 39. Tonello R, Lee SH, Berta T. Monoclonal antibody targeting the matrix metalloproteinase 9 prevents and reverses paclitaxel-induced peripheral neuropathy in mice. J Pain 2019; 20: 515–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Krukowski K, Nijboer CH, Huo X, et al. Prevention of chemotherapy-induced peripheral neuropathy by the small-molecule inhibitor pifithrin-μ. Pain 2015; 156: 2184–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Maj MA, Ma J, Krukowski KN, et al. Inhibition of mitochondrial p53 accumulation by PFT-mu prevents cisplatin-induced peripheral neuropathy. Front Mol Neurosci 2017; 10: 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Puigserver P, Wu Z, Park CW, et al. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 1998; 92: 829–839. [DOI] [PubMed] [Google Scholar]
- 43. McMeekin LJ, Fox SN, Boas SM, et al. Dysregulation of PGC-1alpha-dependent transcriptional programs in neurological and developmental disorders: therapeutic challenges and opportunities. Cells 2021; 10: 352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rius-Perez S, Torres-Cuevas I, Millan I, et al. PGC-1alpha, inflammation, and oxidative stress: an integrative view in metabolism. Oxid Med Cell Longev 2020; 2020: 1452696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Esterbauer H, Oberkofler H, Krempler F, et al. Human peroxisome proliferator activated receptor gamma coactivator 1 (PPARGC1) gene: cDNA sequence, genomic organization, chromosomal localization, and tissue expression. Genomics 1999; 62: 98–102. [DOI] [PubMed] [Google Scholar]
- 46. Sun W, Uchida K, Suzuki Y, et al. Lack of TRPV2 impairs thermogenesis in mouse brown adipose tissue. EMBO Rep 2016; 17: 383–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chaturvedi RK, Adhihetty P, Shukla S, et al. Impaired PGC-1alpha function in muscle in Huntington’s disease. Hum Mol Genet 2009; 18: 3048–3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. St-Pierre J, Drori S, Uldry M, et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell 2006; 127: 397–408. [DOI] [PubMed] [Google Scholar]
- 49. Puigserver P, Spiegelman BM. Peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1 alpha): transcriptional coactivator and metabolic regulator. Endocr Rev 2003; 24: 78–90. [DOI] [PubMed] [Google Scholar]
- 50. Kang I, Chu CT, Kaufman BA. The mitochondrial transcription factor TFAM in neurodegeneration: emerging evidence and mechanisms. FEBS Lett 2018; 592: 793–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chen N, Ge MM, Li DY, et al. β2-adrenoreceptor agonist ameliorates mechanical allodynia in paclitaxel-induced neuropathic pain via induction of mitochondrial biogenesis. Biomed Pharmacother 2021; 144: 112331. [DOI] [PubMed] [Google Scholar]
- 52. Calcutt NA, Smith DR, Frizzi K, et al. Selective antagonism of muscarinic receptors is neuroprotective in peripheral neuropathy. J Clin Invest 2017; 127: 608–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fernyhough P. Mitochondrial dysfunction in diabetic neuropathy: a series of unfortunate metabolic events. Curr Diab Rep 2015; 15: 89. [DOI] [PubMed] [Google Scholar]
- 54. Choi J, Chandrasekaran K, Inoue T, et al. PGC-1α regulation of mitochondrial degeneration in experimental diabetic neuropathy. Neurobiol Dis 2014; 64: 118–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhang LQ, Zhou YQ, Li JY, et al. 5-HT1F receptor agonist ameliorates mechanical allodynia in neuropathic pain via induction of mitochondrial biogenesis and suppression of neuroinflammation. Front Pharmacol 2022; 13: 834570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sun J, Li JY, Zhang LQ, et al. Nrf2 activation attenuates chronic constriction injury-induced neuropathic pain via induction of PGC-1α -mediated mitochondrial biogenesis in the spinal cord. Oxid Med Cell Longev 2021; 2021: 9577874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kim HK, Zhang YP, Gwak YS, et al. Phenyl N-tert-butylnitrone, a free radical scavenger, reduces mechanical allodynia in chemotherapy-induced neuropathic pain in rats. Anesthesiology 2010; 112: 432–439. [DOI] [PubMed] [Google Scholar]
- 58. Duggett NA, Flatters SJL. Characterization of a rat model of bortezomib-induced painful neuropathy. Br J Pharmacol 2017; 174: 4812–4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Toyama S, Shimoyama N, Ishida Y, et al. Characterization of acute and chronic neuropathies induced by oxaliplatin in mice and differential effects of a novel mitochondria-targeted antioxidant on the neuropathies. Anesthesiology 2014; 120: 459–473. [DOI] [PubMed] [Google Scholar]
- 60. McCormick B, Lowes DA, Colvin L, et al. MitoVitE, a mitochondria-targeted antioxidant, limits paclitaxel-induced oxidative stress and mitochondrial damage in vitro, and paclitaxel-induced mechanical hypersensitivity in a rat pain model. Br J Anaesth 2016; 117: 659–666. [DOI] [PubMed] [Google Scholar]
- 61. Doyle T, Chen Z, Muscoli C, et al. Targeting the overproduction of peroxynitrite for the prevention and reversal of paclitaxel-induced neuropathic pain. J Neurosci 2012; 32: 6149–6160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Duggett NA, Griffiths LA, McKenna OE, et al. Oxidative stress in the development, maintenance and resolution of paclitaxel-induced painful neuropathy. Neuroscience 2016; 333: 13–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wu P, Chen Y. Evodiamine ameliorates paclitaxel-induced neuropathic pain by inhibiting inflammation and maintaining mitochondrial anti-oxidant functions. Hum Cell 2019; 32: 251–259. [DOI] [PubMed] [Google Scholar]
- 64. Li X, Yang S, Wang L, et al. Resveratrol inhibits paclitaxel-induced neuropathic pain by the activation of PI3K/Akt and SIRT1/PGC1α pathway. J Pain Res 2019; 12: 879–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ishii N, Tsubouchi H, Miura A, et al. Ghrelin alleviates paclitaxel-induced peripheral neuropathy by reducing oxidative stress and enhancing mitochondrial anti-oxidant functions in mice. Eur J Pharmacol 2018; 819: 35–42. [DOI] [PubMed] [Google Scholar]
- 66. Hao C, Ma B, Gao N, et al. Translocator protein (TSPO) alleviates neuropathic pain by activating spinal autophagy and nuclear SIRT1/PGC-1α signaling in a Rat L5 SNL model. J Pain Res 2022; 15: 767–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bheereddy P, Yerra VG, Kalvala AK, et al. SIRT1 activation by polydatin alleviates oxidative damage and elevates mitochondrial biogenesis in experimental diabetic neuropathy. Cell Mol Neurobiol 2021; 41: 1563–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Chandrasekaran K, Muragundla A, Demarest TG, et al. mGluR2/3 activation of the SIRT1 axis preserves mitochondrial function in diabetic neuropathy. Ann Clin Transl Neurol 2017; 4: 844–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zhang Q, Song W, Zhao B, et al. Quercetin attenuates diabetic peripheral neuropathy by correcting mitochondrial abnormality via activation of AMPK/PGC-1α pathway in vivo and in vitro. Front Neurosci 2021; 15: 636172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Yerra VG, Kalvala AK, Sherkhane B, et al. Adenosine monophosphate-activated protein kinase modulation by berberine attenuates mitochondrial deficits and redox imbalance in experimental diabetic neuropathy. Neuropharmacology 2018; 131: 256–270. [DOI] [PubMed] [Google Scholar]
- 71. Roy Chowdhury SK, Smith DR, Saleh A, et al. Impaired adenosine monophosphate-activated protein kinase signalling in dorsal root ganglia neurons is linked to mitochondrial dysfunction and peripheral neuropathy in diabetes. Brain 2012; 135(Pt. 6): 1751–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Yu X, Zhang L, Yang X, et al. Salvianolic acid A protects the peripheral nerve function in diabetic rats through regulation of the AMPK-PGC1alpha-Sirt3 axis. Molecules 2012; 17: 11216–11228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Xu X, Xu X, Hao Y, et al. Antispasmodic drug drofenine as an inhibitor of Kv2.1 channel ameliorates peripheral neuropathy in diabetic mice. Iscience 2020; 23: 101617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Chandrasekaran K, Anjaneyulu M, Inoue T, et al. Mitochondrial transcription factor A regulation of mitochondrial degeneration in experimental diabetic neuropathy. Am J Physiol Endocrinol Metab 2015; 309: E132–E141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Chandrasekaran K, Anjaneyulu M, Choi J, et al. Role of mitochondria in diabetic peripheral neuropathy: influencing the NAD(+)-dependent SIRT1-PGC-1alpha-TFAM pathway. Int Rev Neurobiol 2019; 145: 177–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Chandra A, Sharma A, Calingasan NY, et al. Enhanced mitochondrial biogenesis ameliorates disease phenotype in a full-length mouse model of Huntington’s disease. Hum Mol Genet 2016; 25: 2269–2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Anis E, Zafeer MF, Firdaus F, et al. Ferulic acid reinstates mitochondrial dynamics through PGC1alpha expression modulation in 6-hydroxydopamine lesioned rats. Phytother Res 2020; 34: 214–226. [DOI] [PubMed] [Google Scholar]
- 78. Das NR, Vaidya B, Khare P, et al. Combination of peroxisome proliferator-activated receptor gamma (PPARgamma) agonist and PPAR gamma co-activator 1alpha (PGC-1alpha) activator ameliorates cognitive deficits, oxidative stress, and inflammation in rodent model of Parkinson’s disease. Curr Neurovasc Res 2021; 18: 497–507. [DOI] [PubMed] [Google Scholar]
- 79. Broome SC, Pham T, Braakhuis AJ, et al. MitoQ supplementation augments acute exercise-induced increases in muscle PGC1alpha mRNA and improves training-induced increases in peak power independent of mitochondrial content and function in untrained middle-aged men. Redox Biol 2022; 53: 102341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Hwang PS, Machek SB, Cardaci TD, et al. Effects of pyrroloquinoline quinone (PQQ) supplementation on aerobic exercise performance and indices of mitochondrial biogenesis in untrained men. J Am Coll Nutr 2020; 39: 547–556. [DOI] [PubMed] [Google Scholar]
- 81. Akagi J, Baba H. Hydrogen gas restores exhausted CD8+ T cells in patients with advanced colorectal cancer to improve prognosis. Oncol Rep 2019; 41: 301–311. [DOI] [PubMed] [Google Scholar]
- 82. Roberts LD, Ashmore T, McNally BD, et al. Inorganic nitrate mimics exercise-stimulated muscular fiber-type switching and myokine and gamma-aminobutyric acid release. Diabetes 2017; 66: 674–688. [DOI] [PubMed] [Google Scholar]
- 83. Da˛browska N, Wiczkowski A. Analytics of oxidative stress markers in the early diagnosis of oxygen DNA damage. Adv Clin Exp Med 2017; 26: 155–166. [DOI] [PubMed] [Google Scholar]
- 84. Miao H, Xu J, Xu D, et al. Nociceptive behavior induced by chemotherapeutic paclitaxel and beneficial role of antioxidative pathways. Physiol Res 2019; 68: 491–500. [DOI] [PubMed] [Google Scholar]
- 85. Malik AN, Czajka A. Is mitochondrial DNA content a potential biomarker of mitochondrial dysfunction. Mitochondrion 2013; 13: 481–492. [DOI] [PubMed] [Google Scholar]
- 86. Castellani CA, Longchamps RJ, Sun J, et al. Thinking outside the nucleus: mitochondrial DNA copy number in health and disease. Mitochondrion 2020; 53: 214–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Zhu J, Carozzi VA, Reed N, et al. Ethoxyquin provides neuroprotection against cisplatin-induced neurotoxicity. Sci Rep 2016; 6: 28861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Zhu J, Chen W, Mi R, et al. Ethoxyquin prevents chemotherapy-induced neurotoxicity via Hsp90 modulation. Ann Neurol 2013; 74: 893–904. [DOI] [PubMed] [Google Scholar]
- 89. Cetinkaya-Fisgin A, Zhu J, Luan X, et al. Development of EQ-6, a novel analogue of ethoxyquin to prevent chemotherapy-induced peripheral neuropathy. Neurotherapeutics 2021; 18: 2061–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Chine VB, Au NPB, Kumar G, et al. Targeting axon integrity to prevent chemotherapy-induced peripheral neuropathy. Mol Neurobiol 2019; 56: 3244–3259. [DOI] [PubMed] [Google Scholar]
- 91. Chine VB, Au NPB, Ma CHE. Therapeutic benefits of maintaining mitochondrial integrity and calcium homeostasis by forced expression of Hsp27 in chemotherapy-induced peripheral neuropathy. Neurobiol Dis 2019; 130: 104492. [DOI] [PubMed] [Google Scholar]