Abstract
Depression is a common mental illness, which is related to monoamine neurotransmitters and the dysfunction of the cholinergic, immune, glutamatergic, and neuroendocrine systems. The hypothesis of monoamine neurotransmitters is one of the commonly recognized pathogenic mechanisms of depression; however, the drugs designed based on this hypothesis have not achieved good clinical results. A recent study demonstrated that depression and inflammation were strongly correlated, and the activation of alpha7 nicotinic acetylcholine receptor (α7 nAChR)-mediated cholinergic anti-inflammatory pathway (CAP) in the cholinergic system exhibited good therapeutic effects against depression. Therefore, anti-inflammation might be a potential direction for the treatment of depression. Moreover, it is also necessary to further reveal the key role of inflammation and α7 nAChR in the pathogenesis of depression. This review focused on the correlations between inflammation and depression as well-discussed the crucial role of α7 nAChR in the CAP.
Keywords: Depression, Inflammation, α7 nAChR
Introduction
Depression is a common mental illness, which is clinically manifested as persistent depressed mood, loss of interest, and cognitive dysfunction, and the disease burden caused by depression ranks first among all mental illnesses [1, 2]. It has been predicted that by 2030, depression will surpass cardiovascular and cerebrovascular diseases and become the first largest disease, causing human death and disability [3]. Numerous studies showed that the pathogenesis of depression might be related to the low levels of monoamine neurotransmitters and the dysfunction of multiple systems in the body, such as the cholinergic, immune, glutamatergic, and neuroendocrine systems [4–15]. At present, the hypothesis of monoamine neurotransmitters is a commonly recognized pathogenic mechanism of depression [4]. Therefore, the pharmacological effects of currently available antidepressants, such as fluoxetine and venlafaxine, are mainly exerted by blocking the reuptake of monoamine neurotransmitters and increasing the levels of 5-hydroxytryptamine (5-HT), dopamine (DA), and norepinephrine (NE) in the synaptic cleft. However, the results of most double-blind trials showed that the disadvantages of these drugs were that they usually showed effects after 2–4 weeks of administration, and about 40% of patients with depression were non-responsive to these drugs [16, 17]. This suggested that the potential pathophysiology of depression was not well-understood, and the antidepressant therapies based on the hypothesis of monoamine neurotransmitters had certain limitations. Therefore, a more comprehensive elucidation of the pathogenesis of depression is urgently needed to develop more effective antidepressant drugs.
The cholinergic system is made up of enzymes-active substances (involved in manufacturing acetylcholine, ACh), cholinergic neurons or histiocytes (release ACh), and receptors (bind to ACh). The cholinergic system can organize into a network in the body, perform various complex functions [18], and take part in the regulation of learning, cognition, memory, and emotion [19–21]. As early as 1972, Davidson et al. [22] put forward the cholinergic hypothesis of depression and suggested that the occurrence of depression was closely related to the enhancement of cholinergic substance activity in the brain. For example, studies showed that depressive behavior could be induced by elevating central choline and ACh levels or blocking the activity of acetylcholinesterase (AchE) [7–9]. On the contrary, increasing the AchE activity in the hippocampus could reverse depression- and anxiety-like behavior in mice caused by physostigmine, an AchE inhibitor [23].
The functions performed by the cholinergic system are mediated by the cholinergic receptors, including the muscarinic ACh receptor and nicotinic ACh receptor [9]. Interestingly, in recent years, increasing studies have found that the activation of the CAP can exert an antidepressant effect, which is inconsistent with the above-mentioned cholinergic hypothesis for depression [24–26]. CAP is a neuroimmune regulatory pathway; when the central nervous system (CNS) is stimulated by immunity, it can activate the vagus nerve and urge the nerve endings to release ACh. The released ACh can activate α7 nAChR on the surface of various immune cells, such as macrophages and microglia, down-regulate the release of related inflammatory factors, and finally inhibit peripheral and central inflammatory reactions [27]. Therefore, the activation of α7 nAChR-mediated CAP might be a promising direction in antidepressant therapies. The correlations between depression and inflammation, which is mediated by α7 nAChR, should be urgently explored to identify novel antidepressant drugs. This review focused on these correlations and the crucial role of α7 nAChR in the CAP.
Relationship between inflammation and depression
Evidence of inflammation associated with depression
The correlation between the nervous and immune systems has been widely studied [24]. Numerous recent studies suggested that inflammation was closely related to the pathogenesis of some nervous system diseases, such as depression [10, 11]. For instance, a meta-analysis, including a series of clinical data, showed a correlation between depression and inflammation among children, adolescents, and adults [28], such as the elevated levels of C-reactive protein and interleukin-6 (IL-6) [29–31]. Other studies showed that the levels of proinflammatory cytokines in the peripheral nervous system and CNS tissue of patients with depression were higher as compared to those in healthy subjects [32], and the brain tissue obtained from the victims of depressive suicide exhibited elevated expression levels of interleukin-1beta (IL-1β), IL-6, and tumor necrosis factor (TNF) [33, 34]. In addition, the results of several clinical studies showed that the anti-inflammatory treatment was used to alleviate depression symptoms [35, 36]. For instance, the brain of depression animal models showed high levels of proinflammatory cytokines, and the intraventricular infusion of anti-inflammatory cytokine interleukin-4 (IL-4) might show antidepressant benefits by modifying central neurotransmitters [12, 37, 38]. With the deepening of research, researchers have gradually found that cerebral neuroinflammation is involved in the occurrence of depression, and peripheral immune factors serve as one of the trigger factors of cerebral neuroinflammation [39].
The occurrence and development of inflammation in depression: from peripheral inflammation to cerebral neuroinflammation
Inflammation is an immune response during infection or trauma. It is the coordinated activation of inflammatory cytokines-mediated signal cascade and is considered a healthy defense mechanism of the body [40–42]. Normal inflammation is necessary for the damages caused by infection, trauma, or neurodegenerative diseases and neuritis is also a method of restoring neural homeostasis. However, when the inflammatory mediators fail to inhibit the pro-inflammatory immune response, a persistent inflammatory state appears, damaging the neurons and the body. Therefore, it is necessary to control inflammation in a certain range to prevent its excessive production [43]. It has been reported that the structural and functional abnormalities of the enteric nervous system and the imbalance of intestinal flora can destroy the integrity of the intestinal barrier, which leads to the leaking of intestinal contents and/or inflammatory mediators into the peripheral blood circulation and triggers of excessive immune response [39, 44].
In peripheral inflammation, Toll-like receptors (TLRs), present on the surface of macrophages and dendritic cells (DCs) of the innate immune system, are recognized by the pathogen-associated molecular pattern (PAMP) or damage-associated molecular pattern (DAMP). This leads to increasing the local and systemic inflammatory activities [45], including triggering the inflammatory signal pathways, such as nuclear factors-kappa B (NF-κB) and mitogen-activated protein kinase (MAPK) signaling pathways, which lead to the overproduction of TNF, interleukin-1 (IL-1), IL-6, and interleukin-18 (IL-18) [46, 47]. These cellular signal transduction mediators can also communicate with the components of the adaptive immune system, such as T cells and B cells [48, 49]. In addition, cyclooxygenase activity can be enhanced by inflammatory cytokines, leading to an increase in prostaglandins levels [48], which further increases inflammation, forming a vicious circle [45]. As a result, the activation of the peripheral immune system can significantly increase proinflammatory cytokine levels.
Due to the existence of blood–brain barrier (BBB), the brain has been traditionally considered as an immune-privileged site; however, numerous studies have shown that peripheral inflammatory cytokines can enter the CNS via cellular, humoral, and neural pathways [46, 50]. BBB is mainly composed of endothelial cells and astrocytes of the capillary wall, and the destruction or absence of either component can affect its function and increase the probability of CNS inflammation [51]. For example, inflammatory factors, including tumor necrosis factor alpha (TNF-α), IL-1β, and IL-6, could impede the tight junctions of endothelial cells in the BBB in the animal models of inflammation [52, 53], increasing the permeability of the BBB, and make the entry of peripheral inflammatory cytokines and immune cells into the brain easier [54]. Furthermore, TNF-α and IL-1β receptors are expressed on endothelial cells, and their activation by inflammatory factors leads to the synthesis of nitric oxide (NO) and prostaglandins in the brain [55]; this further activates microglia and astrocytes, leading to neuroinflammation [50]. Microglia, known as resident immune cells in the CNS, controls the homeostasis of the CNS internal environment and can be divided into basal state microglia, inflammatory state microglia, and anti-inflammatory state microglia [56]. The activation of microglia induces the polarization of basal state microglia into inflammatory state microglia, which is closely associated with the activation of the NF-κB signaling pathway and NOD-like receptor protein 3 (NLRP3) inflammasome [57–59]. Microglia Kv1.3 channels have a crucial role in the activation of NLRP3 inflammasome and neuroinflammation. Kv1.3 is a delayed rectifier voltage-gated K channel, which is widely expressed in the CNS [60]. For example, Di Lucente et al. showed that microglia activation and IL-1β production could be prevented by Kv1.3 knockdown [61]. Moreover, in addition to microglia, astrocytes also play an important role in the immune system of the brain [62]. For example, astrocytes, a BBB component, are involved in regulating BBB permeability, synaptic transmission, and secretion of brain-derived neurotrophic factor (BDNF) [62, 63]. In addition, the release of proinflammatory cytokines from microglia can also activate astrocytes, causing secondary inflammatory reactions, and producing more inflammatory factors, thereby aggravating neurotoxicity, impairing neurogenesis and synaptic plasticity, and ultimately triggering depression and other related psychiatric diseases [57].
It is worth noting that the pathological changes of systemic inflammation-induced neuroinflammation are usually limited and are mainly concentrated in the cortex, hippocampus, amygdala, and other brain regions, which might be due to the diversity of neurons and glial cells in different brain regions [51, 64, 65]. Recently, Wang et al. [66] showed that the release of proinflammatory cytokines was positively correlated with the degree of connexin (Cx) 43 ubiquitination. The gap junction channel of Cx facilitates the communication between neighboring cells, and the role of glial cells in regulating neuroinflammation is mainly based on this function of Cx, which is lost upon the ubiquitination of Cx [67]. Therefore, repairing neuroinflammatory processes might inhibit the ubiquitination of Cx43, which was consistent with the experimental results of Huang et al. and Wang et al. [68, 69].
Other causes of cerebral neuroinflammation
Cerebral neuroinflammation, in addition to being associated with peripheral immunity, is also related to oxidative stress (OS) [70, 71], mitochondrial dysfunction [72, 73], energy metabolism disorders, nitroenergy system [74, 75], eating habits [76, 77], sleep quality [78], etc., to some extent.
OS is a series of highly reactive cytotoxic events induced by reactive oxygen species (ROS) [70]. ROS can destroy sensitive cellular target compounds, such as lipids, proteins, and DNA. When OS occurs, antioxidants in the body deplete, such as a reduction in glutathione (GSH), leading to the excessive accumulation of ROS. Due to the rich lipid contents in the membrane of brain cells, excessive ROS destroys the structure and function of the phospholipid bilayer of brain cells through lipid peroxide, alters the permeability of the BBB, and ultimately exacerbates neuroinflammation [71]. Therefore, antidepressant effects could theoretically be exerted by reducing OS and neuroinflammatory responses; this is in agreement with the reports of the study by Nouri et al. [79] and Mozafari et al. [73].
A mitochondrion is a key organelle that provides energy for cells and body and can regulate various cellular processes [80]. Similarly, triggering OS leads to mitochondrial dysfunction as well as deficiency in cell energy, which further aggravates mitochondrial damage, forming a vicious circle [72]. The resulting multiple DAMP, such as ROS and lipid oxide, can activate inflammatory signals by activating TLRs and different mechanisms, such as inflammasome formation, which can also change the permeability of the BBB and eventually exacerbate neuroinflammation [73].
l-Arginine can produce NO in brain under the action of nitric oxide synthase (NOS). This enzyme family consists of three subtypes, including inducible NOS (iNOS), endothelial NOS, and neuronal NOS [74]. A study by Beheshti et al. [75] has shown that NO is an activator of neuroinflammatory response. Therefore, nitrergic system are also related to neuroinflammation and play an antidepressant role by reducing the NO level in the body, which leads to the inhibition of NOS; this was consistent with the report of a study by Lorigooini et al. [81]. In addition, a study by Haj-Mirzaian et al. [74] showed that iNOS played a more prominent role in the depression-like behavior induced by amphetamine withdrawal.
Pathways of neuroinflammation affecting depression
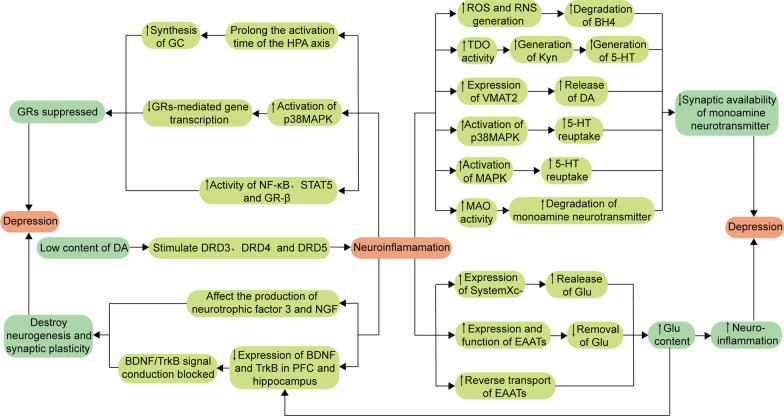
As mentioned above, the overactivation of microglia and astrocytes in the brain results in producing a large number of inflammatory mediators, thereby aggravating neuroinflammation. These inflammatory mediators and downstream signaling pathways can trigger depression by affecting monoamine neurotransmitters, glutamic acid (Glu), the hypothalamus pituitary adrenal (HPA) axis, and neurotrophic factors (NTF) in the body (Fig. 1) [12, 15, 34, 37, 38, 82–87].
Fig. 1.
Role of neuroinflammation in the pathogenesis of depression. Low levels of DA can promote the inflammatory reaction, and the produced inflammatory cytokines can increase the probability of depression by reducing the prominent availability of neurotransmitters, increasing neurotoxicity, inhibiting GRs activity, and destroying neurogenesis and synaptic plasticity. ↑: upregulate; ↓: downregulate; ROS reactive oxygen species, RNS reactive nitrogen species, BH4 tetrahydrobiopterin, TDO tryptophan 2,3-dioxygenase, Kyn kynurenine, 5-HT 5-hydroxytryptamine, VMAT2 vesicle monoamine transporter 2, DA dopamine, p38MAPK p38 mitogen-activated protein kinase, MAPK mitogen-activated protein kinase, MAO monoamine oxidase, DRD3 dopamine receptors 3, DRD4 dopamine receptors 4, DRD5 dopamine receptors 5, SystemXc− Cystine/Glu reverse transporter system, Glu glutamate, EAATS excitatory amino acid reuptake transporters, HPA hypothalamus pituitary adrenal, GC glucocorticoid, GRs glucocorticoid receptors, NF-κB nuclear factors-kappa B, STAT5 signal transducer and activator of transcription 5, NGF nerve growth factor, BDNF brain-derived neurotrophic factor, TrkB tropomyosin receptor kinase B, PFC prefrontal cortex
Effect of neuroinflammation on monoamine neurotransmitters
There is growing evidence, suggesting that inflammatory cytokines can affect the synaptic availability of monoamine neurotransmitters through mechanisms, involving inflammatory cytokines in modulating the synthesis, release, reuptake, and degradation of 5-HT, DA, and NE [34, 82]. This section focuses on the effects of inflammation on the synaptic availability of 5-HT.
Tetrahydrobiopterin (BH4) is a crucial cofactor of tryptophan (Trp), tyrosine, and phenylalanine hydroxylases, which are necessary for the production of 5-HT, DA, and NE. Inflammatory cytokines can exacerbate OS by promoting the formation of ROS and reactive nitrogen species, thereby inducing the degradation of BH4 and reducing the production of 5-HT, DA, and NE. 5-HT is a Trp metabolite, which is an essential amino acid with the lowest levels among the eight essential amino acids; therefore, Trp can easily become deficient in a malnutrition state [88]. Only 1% of the dietary intake of Trp is involved in the biosynthesis of protein, and the majority of the remaining Trp is converted to some bioactive metabolites via the indole, 5-HT, and kynurenine (Kyn) signaling pathways [89]. Through the 5-HT signaling pathway, Trp is converted to 5-hydroxytryptophan by Trp hydroxylase 1 or 2 and then decarboxylated by aromatic acid decarboxylase, forming 5-HT. Then, 5-HT is metabolized by monoamine oxidase (MAO), producing 5-hydroxyindoleacetic acid [90]. About 95% of free Trp is metabolized through the Kyn pathway. In this pathway, Trp, in a rate-limiting step, is first converted to Kyn by indoleamine 2,3-dioxygenase 1 and 2 (IDO1 and IDO2) and tryptophan 2,3-dioxygenase (TDO). Kyn has two major metabolic branches. (1) Kyn is preferentially converted to 3-hydroxykynurenine (3‑HK) or anthranilic acid, catalyzed by kynurenine mono‑oxygenase and kynureninase (Kynu), respectively. The 3-HK is then converted to 3-hydroxy anthranilic acid (3-HAA) and xanthurenic acid by Kynu and kynurenine aminotransferase (KAT), respectively, while 3-HAA is metabolized into picolinic acid and quinolinic acid (QA) by α-amino-β-carboxymuconate-ε-semialdehyde decarboxylase and non-enzymatic conversion, respectively. (2) The remaining Kyn is metabolized by the enzymatic action of KAT into kynurenic acid (Kyna) [91, 92]. Studies showed that inflammatory mediators, such as IL-1, TNF-α, and interferon-γ, could induce the hyperactivation of IDO, which caused more Trp metabolism through the Kyn pathway, thereby decreasing the 5-HT level in the brain [93–95]. According to studies, the Kyn pathway can produce 3-HK and QA, both of which are neurotoxic. For instance, QA cannot cross the BBB, acting as an agonist of the N-methyl-d-aspartic acid receptor (NMDAR), while the massive accumulation of QA in the CNS can promote the overactivation of NMDAR in neurons, including those in the striatum and hippocampus [96], thereby increasing the release of Glu and apoptosis rate of neurons [97]. In addition, Kyna can inhibit the release of dopamine, resulting in cognitive impairment [98, 99]. Interestingly, Kyna is also considered a neuroprotective metabolite, acting as an antagonist of α7 nAChR and NMDAR to reduce the release of Glu [100].
DA entry into the vesicle is mediated by monoamine transporter protein 2 (VMAT2) and subsequent release into the synapse. Studies have shown that pro-inflammatory cytokines can down-regulate the expression of VMAT2, thereby interfering with DA release and reducing the level of DA in synaptic cleft, such as IL-1β and TNF-α [101]. Monoamine neurotransmitter transport proteins translocate monoamine neurotransmitters released in the synaptic cleft back to the presynaptic membrane, decreasing synaptic cleft availability [102]. Proinflammatory mediators can regulate the expression and function of monoamine neurotransmitter transporter proteins, thereby altering their reuptake process [36]. For example, proinflammatory cytokines can trigger the p38MAPK signaling pathway, enhancing the expression and activity of 5-HT transporter proteins and decreasing the availability of 5-HT in the synaptic cleft. In addition, the activation of the MAPK signaling pathway can also increase the activity of DA transporter proteins and ultimately reduce the DA levels in the synaptic cleft [103]. Studies have shown that inflammation plays a key role in the degradation of monoamine neurotransmitters by MAO. For example, after lipopolysaccharide administration, the low levels of NE in the hippocampus and elevated levels of an NE metabolite 3-methoxy-4-hydroxyphenylglycol might be associated with elevated monoamine oxidase activity [104].
Notably, some monoamine neurotransmitters, such as dopamine, also play an essential role in regulating the brain’s immune responses by affecting inflammatory responses [105–107]. Kv1.3-induced K+ efflux can cause the activation of NLRP3 inflammasome and local fluctuations in extracellular K+ concentration, promoting the release of dopamine from proximal dopaminergic neurons [60]. The high levels of dopamine, ranging from 1 to 10 μM, in vivo binding to low-affinity dopamine receptors (DRD1 and DRD2) on microglia could exert anti-inflammatory effects by modulating the proinflammatory renin–angiotensin system [108, 109], and DRD1 could mediate the autophagic degradation of NLRP3 protein. The low levels of dopamine in the body, ranging from 20 to 500 nM, could selectively stimulate the high-affinity dopamine receptors (DRD3, DRD4, and DRD5), thereby inducing inflammatory responses [110].
Effect of neuroinflammation on the glutamatergic system
Glu is one of the most prominent excitatory neurotransmitters in the nervous system, acting on Glu receptors and transmitting excitatory signals. There are two types of Glu receptors: (1) ionic receptors, including NMDAR, α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor, and kainic acid receptor, which mediate the rapid action of postsynaptic potential; and (2) metabolic receptor, which mediates the slow-acting neurotransmission and plays an important role in cellular metabolism [87, 111]. The different Glu-activated receptors can regulate neuronal growth, migration, apoptosis, and synaptogenesis [112]. Therefore, the Glu system might play a crucial role in the pathogenesis of emotional disorders, such as the dysfunction of Glu neurotransmissions in the cerebral cortex and marginal regions, which are closely related to depression [83, 84].
The cystine/Glu reverse transporter system (SystemXc−), a transmembrane amino acid transporter system in CNS cells, allows the exchange of the extracellular cystine with intracellular Glu and regulates the extracellular Glu concentration. Intracellular cystine uptake can be reduced to cysteine, which is an essential precursor for conversion to the antioxidant GSH [113–115]. The depolarization of presynaptic neurons and subsequent influx of Ca2+ can induce the release of Glu stored in vesicles into the synaptic cleft, where it binds to the corresponding target receptors [116, 117]. On the other hand, the free or excessive Glu is immediately removed and detoxified by the excitatory amino acid reuptake transporters (EAATs), thereby preventing the “Glu spillover effect” [83]. In this process, EAATs are the special transporter proteins found on the synaptic surface of neurons and glial cells [118, 119]. Releasing the Glu in large amounts can induce the excessive activation of NMDAR both inside and outside the synapse, which reduces the expression of NTF, such as BDNF, causing excitotoxicity, synaptic damage, and neuronal degeneration [13, 14, 120, 121].
Numerous studies showed that inflammation could affect the release and reuptake process of Glu [116, 120, 122]. For example, the activation of NF-κB signaling increased the IL-1β levels, which in turn promoted the expression of SystemXc− and increased the release of Glu to the outside of the synapse through the vesicular pathway [123, 124]. The inflammatory cytokines-induced OS could also play a similar role [120]. In addition, inflammatory factors can also promote the Glu release through other non-vesicular pathways, such as impairing the expression and function of EAATs in astrocytes, which resulted in reducing the reuptake and clearance of synaptic Glu [116, 125]. Other studies indicated that inflammatory factors could also reverse EAATs, thereby further increasing the levels of Glu in the synaptic cleft [126, 127].
Effect of neuroinflammation on HPA axis
The HPA axis, an important component of the neuroendocrine system, can maintain the homeostasis of the stress response system and internal environment, producing substances, such as adrenocorticotropin-releasing hormone (CRH), adrenocorticotropic hormone (ACTH), cortisol (CORT), and glucocorticoids (GC). The GC receptors (GRs) are widely distributed in the CNS, such as the hypothalamus, pituitary, adrenal gland, and cortex margin [128]. Under normal conditions, the binding of GC to GRs acts as a negative feedback inhibitor on the HPA axis, regulating its activation status. Under depression conditions, the expression of GRs decreases, which weakens the inhibition of the HPA axis, leading to the excessive activation of the HPA axis, thereby increasing the levels of CRH, ACTH, CORT, and GC in the patients with depression and affecting the secretion in vivo [85, 86].
Studies showed that sustained stress can also stimulate the release of CORT from the HPA axis; CORT can disrupt the balance of gut microbiota, causing intestinal permeability [129]. Furthermore, the CORT-induced increase in TDO enzyme activity can over-activate the Kyn pathway, which leads to decreasing the production of 5-HT [130]. CORT also plays an important role in regulating CNS-related activities, such as learning, memory, and emotion; the overproduction of CORT might alter the function of the hippocampus, prefrontal cortex (PFC), etc. [131]. Under normal conditions, GC is a powerful anti-inflammatory molecule and suppresses the synthesis and efficacy of cytokines, thereby blocking numerous inflammatory pathways [117]. However, high GC levels can trigger microglia-induced neuroinflammation and impair the integrity of neuronal membranes by interfering with the neuronal repair function of BDNF and promoting neurotoxicity and atrophy in the hippocampus [132–134].
Notably, inflammation can significantly affect the neuroendocrine function through the HPA axis [132]. The continuous production of proinflammatory cytokines, such as IL-6 [128], can increase the activation time of the HPA axis, increase GC synthesis, inhibit GRs and downregulate their function, cause negative feedback regulation disorder, and finally cause the disorder of GC level regulation [135]. Further studies suggested that proinflammatory cytokines could disrupt the signaling pathways, such as NF-κB, p38MAPK, and signal transducer and activator of transcription 5 signaling pathways, resulting in the impaired function of GRs [136, 137]. For example, IL-1α can inhibit the GRs-mediated gene transcription by activating the p38MAPK signaling pathway [138]. In addition, GR-β as an inactive form of GRs, can be activated by proinflammatory cytokines, thereby inhibiting the function of GRs [139].
Effect of neuroinflammation on NTF
NTF plays an important role in maintaining the function of the peripheral and CNS and provides relevant nutritional support for regulating emotional behavior in the nervous system [140]. BDNF, the most common type of NTF, can alter synaptic plasticity, increase synaptic connections, and promote long-term potentiation [141], which has a significant effect on neuronal morphology and physiology. Numerous studies showed that BDNF could produce antidepressant-like effects in the PFC and hippocampus. Interestingly, BDNF, acting on the ventral tegmental area (VTA)–nucleus accumbens (NAc) signaling pathway, can induce a depression-like phenotype [142]. Numerous recent studies indicated that BDNF and/or tropomyosin receptor kinase B (TrkB) signaling pathways could play important roles in the rapid antidepressant effects of ketamine [143]. For example, BDNF might trigger a mammalian target of the rapamycin protein (mTOR) signaling pathway, causing synaptogenesis [144, 145]. In addition, BDNF could also bind to TrkB receptors, activating other signaling pathways, such as phosphatidylinositol 3-kinase (PI3K)/Akt and MAPK signaling pathways, which play a role in mood disorders and depression [146–148]. Furthermore, an increase in the serum levels of glial-derived neurotrophic factor and transforming growth factor-β could improve depressive behavior [149].
Studies suggested that several inflammatory cytokines could downregulate the expression levels of BDNF and TrkB in the PFC and hippocampus and inhibit the phosphorylation of TrkB, thereby blocking the BDNF/TrkB signaling pathway and ultimately inducing the apoptosis of neurons [87]. In addition, inflammatory cytokines can also induce the hyperactivation of IDO and promote the Trp metabolism through the Kyn pathway, leading to the production of neurotoxic metabolites, such as 3‑HK and QA [93–95]. Among them, the stimulation of NMDAR by QA can cause the excitatory toxicity of Glu and signaling cascade, thereby reducing the BDNF expression and ultimately disrupting neurogenesis and synaptic plasticity [150, 151]. Notably, inflammatory mediators can also affect NFT3 and nerve growth factor to varying degrees [152].
Correlations between depression and inflammation based on α7 nAChR
Overview of α7 nAChR
The α7 nAChR, belonging to the Cys-loop receptor family, is a homopentamer ligand-gated ion channel assembled from five α7 subunits [153]. The five subunits of α7 nAChR are arranged in a pentagonal shape, forming an ion channel in the center, which mainly controls the flow of Na+, K+, and Ca2+ ions in and out of cells. Cys-loop receptors have three common structures, including the extracellular domain (ECD), transmembrane domain (TMD), and intracellular domain (ICD) [154]. The ECD contains the binding site for the agonist. The TMD has four hydrophobic areas designated M1, M2, M3, and M4, among which, M2 forms the inner lining of the ion channel and has an affinity for cations due to containing more acidic amino acid residues [155]. As compared to ECD and TMD, the ICD has more complex functions and plays important roles in the localization, transport, and assembly of receptors, thereby affecting the conductance and desensitization of channels and regulating downstream signaling pathways [156–158]. Consequently, ICD has been recognized as a potential target for drug design [159]. Recently, the Noviello CM team introduced the structure and dynamic interconversion process of α7 nAChR in three states, including resting, activated, and desensitized states, and reported an ECD component, which might be related to the relative permeability of the receptor [160]. Notably, X-ray crystallography and freeze electron microscopy could not analyze the specific structure of ICD due to the flexibility of its structure [161, 162]. Bondarenko et al. combined the experimental structure limitations of nuclear magnetic resonance and electron spin resonance spectroscopy with Rosetta calculation to determine the full-length ICD structure of human α7 nAChR [154].
As one of the most abundant subtypes in the human brain, α7 nAChR is enriched in the hippocampus, PFC, VTA, NAc, locus coeruleus, hypothalamus, dorsal raphe nucleus, and other brain regions, and is related to various CNS functions and diseases [163, 164]. Numerous studies indicated that α7 nAChR was expressed in different locations in neuronal cells, including presynaptic membrane, postsynaptic membrane, and perisynaptic [24]. In addition, α7 nAChR was also expressed in various non-neuronal cells, such as macrophages/monocytes [165], lymphocytes, DCs [166], microglia [167], astrocytes [168, 169], endothelial cells, bronchial epithelial cells, and vascular smooth muscle cells [153].
α7 nAChR is closely related to learning, memory, neuroprotection, synaptic plasticity, movement, attention, and anxiety [56, 170–173]. The ligand-binding sites of α7 nAChR mainly include agonist/antagonist-binding sites and allosteric modulator-binding sites, which are activated by agonists and positive allosteric modulators (PAMs), respectively. The binding of agonists to the extracellular ligand-binding domain of α7 nAChR can cause the rapid opening of central ion channels within milliseconds. Due to the high permeability of α7 nAChR to Ca2+, a large Ca2+ influx depolarizes the presynaptic membrane and promotes the fusion of neurotransmitter-containing vesicles and presynaptic membrane, thereby further increasing the release of neurotransmitters, such as ACh, NE, DA, Glu, and γ-aminobutyric acid. At the postsynaptic membrane, a large Ca2+ influx acts on the downstream Ca2+-sensitive kinases, which triggers a series of signal transduction processes [174, 175]. Although α7 nAChR is a ligand-gated ion channel, it can also increase the intracellular cyclic adenosine monophosphate (cAMP) levels through adenylate cyclase 1, a common signaling pathway for G protein-coupled receptors [24]. Thus, the activated α7 nAChRs have ionotropic and metabotropic functions in neurons and immune cells, including microglia, and are involved in regulating the Ca2+ influx, neurotransmitter release, and intercellular signal transduction [171, 176]. Moreover, the activated α7 nAChRs are closely related to autophagy, necrosis, transcription, apoptosis, and inflammatory processes in the body [170, 177, 178]. For instance, Hung et al. found that α7 nAChR could bind to amyloid-beta (Aβ), leading to its internalization into the cytoplasm and further inhibition of Aβ-induced neurotoxicity through autophagy. On the other hand, Lc3 and melatonin could enhance autophagy by increasing the expression of α7 nAChR, thereby showing neuroprotective effects. Further studies showed that in microglia, the enhanced autophagy, induced by the activation of α7 nAChR, was mediated by activating the AMPK–mTOR–p70S6K signaling pathway [179]. Interestingly, Hou et al. indicated that in cardiomyocytes, the activation of Janus kinase 2 (JAK2) and PI3K could mediate the α7 nAChR activation-induced enhanced autophagy [180]. Thus, in different organs or cells, α7 nAChR activation-induced autophagy enhancement might act through different signaling pathways. In addition, Hua et al. also showed that the α7 nAChR agonist PNU-282987 could decrease the activation of caspase-3, increase the expression of anti-apoptotic protein B-cell lymphoma-2, and exert anti-apoptotic effects in microglia [181]. Numerous recent studies reported that activating the α7 nAChR could induce anti-inflammatory effects, which might be an effective way to treat depression, Alzheimer’s disease, and other CNS diseases [24].
Evidence of α7 nAChR associated with depression
Numerous studies have shown that the activated α7 nAChR plays an essential role in the pathogenesis of depression. For example, the α7 nAChR disorder, such as the depression-like phenotype in α7 knockout mice, can trigger depression [142]. α7 nAChR is encoded by Chrna7. Pu et al. showed that the composition of gut microbiota in mice with Chrna7 knocked out mice was abnormal, such as a decrease in the abundance of Muribaculum intestinale and an increase in those of Helicobacter ganmani and Lactobacillus animalis, showing depression-like phenotype. Furthermore, as compared to the control mice, the fecal microbiota transplantation into Chrna7 knockout mice resulted in systemic inflammation, downregulation of synaptophysin, and depression-like phenotype in the mice treated with an antibiotic mixture [182]. Zhang et al. also showed that the α7 nAChR knockout mice did not alter the BDNF/TrkB signaling pathway and synapsis in the hippocampus and PFC but increased the BDNF/TrkB signaling pathway in NAc, showing a depression-like phenotype. Interestingly, the bilateral infusion of TrkB antagonist ANA-12 with NAc could restore the increase in synapses in NAc and rapidly exert antidepressant effects, while fluoxetine could not show similar effects [142]. In contrast, antidepressant-like effects were exhibited in animals by agonism of α7 nAChR, such as α7 nAChR agonists DMXBA and PNU-282987 [183–186]. Further studies have shown that the activated α7 nAChR could mediate the release of DA and NE in the rat’s hippocampus and PFC [187, 188] but showed no effects on the uptake of 5-HT [189]. However, the α7 nAChR agonists have the disadvantages of insufficient selectivity, tendency to desensitize receptors, and lack of data related to clinical trials; these factors limit the application of α7 nAChR agonists in the treatment of depression [24, 174].
Therefore, studying the application of α7 nAChR PAMs in antidepressant treatment may be a promising direction. α7 nAChR PAMs were effective only in the presence of endogenous agonist ACh and could further enhance the agonistic effects of ACh on α7 nAChR. There are two types of α7 nAChR PAMs; type I PAMs do not affect receptors desensitization, while type II PAMs can delay receptors desensitization and reactivate desensitized receptors [190, 191]. Studies have shown that NS-1738 (type I PAMs), PNU-120596 (type II PAMs) and PAM-2 (type II PAMs) cannot exert significant antidepressant effects after 0.5 h or 1 h of administration, but can significantly improve depressive-like behavior after 7 days of administration, which still has some advantages over traditional antidepressants [163, 192–194]. Interestingly, PAM-2 induced more potent and durable antidepressant-like activity compared to NS-1738 and PNU-120596, indicating the importance of dosing cycle [163, 194]. In addition, Targowska-Duda et al. showed that PAM did not have a high affinity for human 5-HT, DA, and NE transporter proteins (< 1 μM) [163]. Notably, α7 nAChR PAMs can also specifically target α7 nAChR without affecting the physiological functions of other receptors, thereby showing fewer side effects than α7 nAChR agonists while exerting precise pharmacological effects. However, α7 nAChR PAMs also have the same disadvantage of lacking sufficient relevant clinical data [195, 196]. Therefore, the active state of α7 nAChR is closely related to depression, but requires extensive experiments to confirm. Table 1 lists the changes in depression-like behavior of animals when targeting α7 nAChR.
Table 1.
Effect of targeted α7 nAChR on depressive-like behavior
| Compound type | Compound name | Study subject | Intervention | Outcome | References |
|---|---|---|---|---|---|
| Male C57BL/6 | Knock-out α7 nAChR | Presence of depressive-like behavior | [142, 182] | ||
| α7 nAChR agonist | PNU282987 | Male Wistar rats | FST was performed after 24 h of administration (0.5 mg/kg) | Significantly improve depressive-like behavior | [183] |
| Male adult Sprague–Dawley rats | CUMS induced depression 4 weeks and FST was performed after 35 min of administration (1 mg/kg) | Significantly improve depressive-like behavior | [185] | ||
| C57BL/6 mice | Aβ1-42-induced depression, and FST was performed after 10 days of administration (1 mg/kg) | Significantly improve depressive-like behavior | [186] | ||
| DMXBA(GTS-21) | Male Kunming mice | CRS-induced depression, FST, TST, and SPT were performed after 11 days of continuous administration (4 mg/kg) | Significantly improve depressive-like behavior | [184] | |
| Type I PAMs | NS-1738 | Male Wistar rats | FST was performed after 24 h of administration (1 mg/kg) | Does not significantly improve depression-like behavior | [183] |
| Male C57BL/6J mice | FST and TST were performed after 30 min, 7 days and 14 days of administration (1 mg/kg, 10 mg/kg), respectively | Depression-like behavior was significantly improved after 7 days of administration (1 mg/kg, 10 mg/kg) | [163] | ||
| Type II PAMs | PNU-120596 | Male C57BL/6J mice | LPS was injected after 0.5 h of administration (4 mg/kg), and TST and SPT were performed after 26 h of LPS injection, and FST was performed after 28 h of LPS injection | LPS-induced depression-like behavior was blocked, but the antidepressant effect was reversed by MLA (3 mg/kg) | [192] |
| LPS was injected after 0.5 h of administration (4 mg/kg), and FST was performed after 28 h of LPS injection | LPS-induced depression-like behavior was blocked, but the antidepressant effect was reversed by MLA (3 mg/kg) | [193] | |||
| FST and TST were performed after 30 min, 7 days and 14 days of administration (1 mg/kg, 10 mg/kg), respectively | Depression-like behavior was significantly improved after 7 days of administration (1 mg/kg, 10 mg/kg) | [163] | |||
| PAM-2 | C57BL/6J mice | FST were performed after 1 h, 7 days, 14 days and 21 days of administration (1 mg/kg), respectively | Depression-like behavior was significantly improved after 7 days, 14 days, and 21 days of administration (1 mg/kg) | [194] | |
| Male C57BL | FST and TST were performed after 30 min, 7 days and 14 days of administration (0.5 mg/kg, 1 mg/kg), respectively | Depression-like behavior was significantly improved after 7 days and 14 days of administration (0.5 mg/kg, 1 mg/kg) | [163] |
FST forced swimming test, CUMS chronic unpredictable mild stress, Aβ1-42 amyloid-beta1-42, CRS chronic restraint stress, TST tail suspension test, SPT sucrose preference test, PAMs positive allosteric modulators, LPS lipopolysaccharide, MLA methylprednisolone citrate
Activation of α7 nAChR-mediated CAP for antidepressant effect and its mechanism
In recent years, the activation of α7 nAChR-mediated CAP in anti-depression therapy has attracted researchers’ attention [24–26]. It is well-known that the vagus nerve, which connects the brain and surrounding organs, plays an important role in CAP. After stimulation, it can activate α7nAChR, which is closely related to the inhibition of NF-κB activation [56, 197, 198]. NF-κB is a transcription factor, which coordinates the inflammatory response and regulates the expression levels of inflammatory genes [199]. The NF-κB-binding inhibitors of NF-κB (IκB) are normally present in the cytoplasm. Studies have shown that vagal stimulation can upregulate the α7 nAChR expression in hippocampal microglia and exert anti-inflammatory effects by inhibiting the nuclear translocation of NF-κB and phosphorylation of p65. However, the vagus nerve stimulation could not exert similar anti-inflammatory effects after vagotomy or injection of α7 nAChR antagonists or using α7nAChR(−/−) rats [198, 200]. Thus, the α7 nAChR/NF-κB signaling pathway might play a crucial role in the CAP. However, the mechanism of α7 nAChR, affecting the upstream and downstream pathways of NF-κB, requires further investigation.
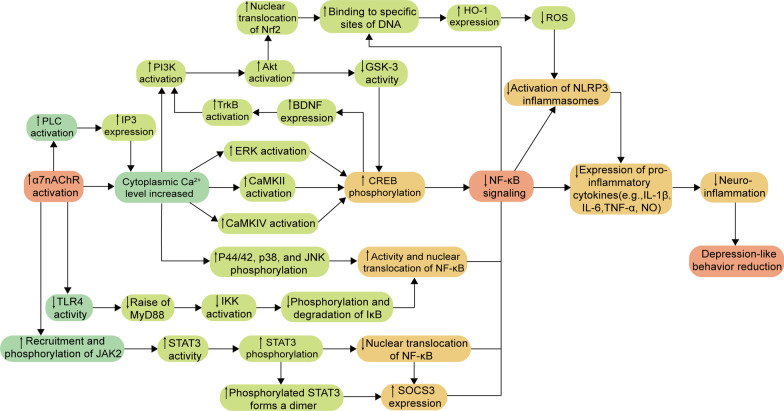
An in-depth study revealed that the activation of α7 nAChR exerted anti-inflammatory effects through the TLR4/NF-κB/NLRP3, JAK2/STAT3/NF-κB, and Ca2+-related signaling pathways [184, 192, 201–207]. In addition, activating the α7 nAChR can activate chronic stress-induced neuroinflammation to promote Tregs cell function. As a subset of CD4+ T cells, Tregs can protect the BBB, inhibit the infiltration of peripheral inflammatory cells and factors into the brain, and play an important role in maintaining immune homeostasis [208, 209]. For instance, Zhao et al. demonstrated that the treatment with α7 nAChR agonist DMXBA could reverse the chronic stress-induced increase in Tregs cells, thereby limiting the inflammatory response in the brain and attenuating the depression-like behavior in chronic restraint stress (CRS) mice [184]. The specific molecular mechanisms of α7 nAChR’s anti-inflammatory effects around NF-κB are summarized in the following sections (Fig. 2).
Fig. 2.
Molecular mechanisms of activation of α7 nAChR-mediated CAP. The activation of α7 nAChR could inhibit the expression of NF-κB through TLR4/NF-κB/NLRP3, JAK2/STAT3/NF-κB and Ca2+-related signaling pathways, reduce the production of inflammatory cytokines, reduce neuroinflammation, and finally play an antidepressant role. ↑: upregulate, ↓: downregulate, TLR4 Toll-like receptors 4, MyD88 myeloid differentiation factor 88, IKK inhibitor of kappa B kinase, IκB inhibitor of NF-κB, JAK2 Janus Kinase 2, STAT3 signal transduction and transcription activator 3, SOCS3 suppressor of cytokine signaling 3, NF-κB nuclear factors-kappa B, PLC phospholipase C, IP3 inositol 1,4,5-triphosphate, PI3K phosphatidylinositol 3-kinase, Akt protein kinase B, GSK-3 glycogen synthase kinase 3, BDNF brain-derived neurotrophic factor, TrkB tropomyosin receptor kinase B, ERK extracellular signal-regulated kinase, CaMKII Ca2+/calmodulin-dependent protein kinase II, CaMKIV Ca2+/calmodulin-dependent protein kinase IV, JNK c-Jun N-terminal kinase, Nrf2 nuclear transcription factor E2-related factor, HO-1 heme oxygenase-1, ROS reactive oxygen species, CREB cAMP-response element binding protein, NLRP3 NOD-like receptor protein 3, IL-1β interleukin-1β, IL-6 interleukin-6, TNF-α tumor necrosis factor-α, NO nitric oxide
Activation of α7 nAChR triggers TLR4/NF-κB/NLRP3
TLRs are significant receptors on the surface of microglia and play important roles in neuroinflammation-mediating signaling pathways [210]. TLRs can be activated by recognizing PAMP or DAMP, and the activated TLR4 can recruit the adaptor protein myeloid differentiation factor 88 (MyD88) [57, 58]. The stimulation by MyD88 and cytokines, such as TNF-α, can activate the inhibitor of kappa B kinase, which in turn phosphorylates and subsequently degrade IκB, thereby promoting the activation of NF-κB and its entry into the nucleus and increasing the transcriptional expression of inflammasome components and pro-IL-1β [43, 211]. NLRP3 inflammasome is a multi-protein assembly, which consists of NLRP3 (cytoplasmic sensor molecule), adapter protein caspase activation recruitment domain, and effector protein pro-caspase-1 [212]. Studies have shown that the activation of NLRP3 inflammasome is closely related to NF-κB [59] and generally requires the initiation and activation of two signals, including the initiation signal and activation signal. The initiation signal involves the activation of NF-κB induced by the activation of TLR, while in the activation signal, the sustained stimulation induces the inflammasome components to assemble into complete NLRP3 inflammasome and promotes the conversion of pro-caspase-1 into activated caspase-1, which subsequently cleaves the pro-IL-1β and pro-IL-18 into active forms, ultimately leading to an inflammatory response and induction of cytotoxicity [42, 212, 213]. In addition, studies have also shown that ROS can activate the NLRP3 inflammasome [214, 215].
The selective α7 nAChR partial agonist DMXBA could alleviate chronic stress-induced activation of the TLR4 signaling pathway and exert antidepressant-like behavior in mice; however, this effect was reversed after pretreatment with the selective α7 nAChR antagonist α-bungarotoxin (α-BGT), indicating that the protective effects of DMXBA were α7 nAChR-dependent. Interestingly, using α-BGT at the dose (1 μg/kg/d) did not reverse the inhibitory effects of DMXBA on the activation of microglia [184]. Deng et al. showed that the activation of α7 nAChR could significantly inhibit the expression of NLRP3 inflammasome; this result was consistent with those of a study by Fu [201, 202]. Furthermore, activating the α7 nAChR could also attenuate OS [216]. Therefore, the activation of α7 nAChR might exert anti-neuroinflammatory effects by inhibiting the TLR4/NF-κB/NLRP3 signaling pathway and reducing OS.
Activation of α7 nAChR triggers JAK2/STAT3/NF-κB
JAK2 is widely distributed in the cytoplasm of somatic cells and is important in activating immune cells. STAT3 is an essential transcription factor, which regulates the expression of downstream target genes associated with the differentiation and apoptosis of cells [217]. JAK2/STAT3 is the most crucial signaling pathway in the JAK–STAT family and is closely related to inflammation [218]. The recruited and phosphorylated JAK2 can activate STAT3 to induce the phosphorylation of STAT3, which then prevents the nuclear translocation of NF-κB [170]. In addition, the phosphorylated STAT3 can readily form dimers to enter the nucleus and bind to DNA, thereby positively regulating the transcription of suppressor of cytokine signaling 3, which leads to inhibiting NF-κB activation and reducing the production of inflammatory cytokines, such as TNF-α and IL-1β [170].
Numerous studies showed that the activated α7 nAChR could regulate other signal transduction pathways by promoting the JAK2/STAT3 signaling pathway and regulating the gene transcription in immune cells independent of ion influx [176, 192, 203]. For example, blocking the JAK2 phosphorylation using AG490 attenuated the inflammatory regulatory effects of α7 nAChR agonists; inhibiting the STAT3 phosphorylation also showed a similar effect [205]. Zhao et al. showed that the treatment with the α7 nAChR agonist DMXBA could significantly reverse the CRS-induced downregulation of STAT3 in the hippocampal nucleus, thereby restoring the central cholinergic signaling function [184]. Therefore, the activation of α7 nAChR might attenuate the inflammatory response by promoting the JAK2/STAT3 signaling pathway and ultimately inhibiting the activation of NF-κB.
Activation of α7 nAChR triggers Ca2+-related signaling pathway
The PI3K/Akt and extracellular signal-regulated kinase (ERK) signaling pathways play an important role in cell proliferation and maturation [219]. Ca2+/calmodulin-dependent protein kinase II (CaMKII) is associated with the enhancement of learning and memory as well as synaptic remodeling, while CaMKIV is involved in regulating the growth of DCs in cortical and hippocampal neurons [220–222]. A large Ca2+ influx can activate the PI3K/Akt, ERK, CaMKII, and CaMKIV signaling pathways [223–226], thereby increasing the phosphorylation of cAMP-response element binding protein (CREB) at residue Serine-133 and its subsequent BDNF expression [227–229]. The phosphorylated CREB can compete with CREB-binding protein to bind with NF-κB; this process is affected by the activity of glycogen synthase kinase-3beta (GSK-3β) and inhibits the transcription of NF-κB, thereby playing an anti-inflammatory role and promoting adult hippocampal neurogenesis [230–233]. Among them, the activated PI3K/Akt signaling pathway can facilitate the nuclear translocation of nuclear transcription factor E2-related factor (Nrf2), which increases the binding of Nrf2 to electrophilic response elements (EpRE) or antioxidant response elements at DNA-specific sites as well as the expression levels of antioxidant genes, such as heme oxidase-1 [234], thereby inhibiting the degree of OS and ultimately reducing the production of the proinflammatory cytokines TNF-α and IL-1β. In addition, studies have shown that PI3K/Akt can inhibit the GSK-3 activity, while GSK-3 can inhibit the CREB signaling pathway [235]. Notably, some studies indicated possible interactions between NF-κB and Nrf2; the NF-κB subunit p65 can negatively regulate Nrf2 and inhibit its interaction with EpRE [236]. Furthermore, an increase in the intracellular Ca2+ levels can also inhibit the activation of NF-κB and subsequent nuclear translocation by suppressing the phosphorylation of neuroinflammation-associated p44/42, p38, and c-Jun N-terminal kinase [237, 238]. This can ultimately reduce the production of inflammatory mediators, such as IL-6, TNF-α, and NO [239, 240].
Activating the α7 nAChR can trigger a large Ca2+ influx [206]. Moreover, the activated α7 nAChR in mice microglia can activate phospholipase C via Gαq, producing 1,4,5-triphosphate (IP3), which can bind to the IP3 receptor on the endoplasmic reticulum, inducing the release of Ca2+ from the endoplasmic reticulum [207]. Recently, Morioka et al. proposed that activating the α7 nAChR-mediated IP3 and Ca2+/CaMKII signaling pathways upregulated the expression of Glu/aspartate transporter protein and increased Glu uptake [24]. Therefore, the activation of α7 nAChR can trigger the process of massive Ca2+ influx and release from the endoplasmic reticulum, which might increase intracellular Ca2+ levels and promote a series of signaling pathways, ultimately inhibiting the inflammatory response.
Conclusions and prospects
This review article summarized the relationship between inflammation and depression as well as several pathways of neuroinflammation affecting depression, such as neuroinflammation can affect the synaptic availability of monoamine neurotransmitters and Glu, increase the activation time of the HPA axis, regulate the BDNF/TrkB signaling pathway in various brain regions. This, in turn, aggravates neurotoxicity and damages neurogenesis and synaptic plasticity, which finally induces neuronal apoptosis and triggers depression and other related psychiatric diseases. This review article also highlighted the crucial role of α7 nAChR in the CAP and suggested that its activation might exert anti-inflammatory effects by promoting Tregs function and through TLR4/NF-κB/NLRP3, JAK2/STAT3/NF-κB, and Ca2+-related signaling pathways, thereby alleviating depression-like behavior.
Depression is the result of multiple mechanisms; the recent drugs developed based on the hypothesis of monoamine neurotransmitters could not achieve favorable clinical outcomes. However, the importance of the inflammation hypothesis in the occurrence of depression has gradually been recognized, especially the activation of α7 nAChR-mediated CAP. Notably, the BBB should also be considered as a factor in developing the corresponding PAMs due to the large distribution of α7 nAChR within and outside the CNS. Therefore, based on the idea of activating the α7 nAChR-mediated CAP to exert antidepressant effects, developing the α7 nAChR type II PAMs, capable of passing through the BBB, might be a current research direction for developing new antidepressant drugs. Furthermore, the results should be confirmed in a large number of subsequent preclinical and clinical trials.
Acknowledgements
Not applicable.
Abbreviations
- α7 nAChR
Alpha7 nicotinic acetylcholine receptor
- CAP
Cholinergic anti-inflammatory pathway
- 5-HT
5-Hydroxytryptamine
- DA
Dopamine
- NE
Norepinephrine
- Ach
Acetylcholine
- AchE
Acetylcholinesterase
- CNS
Central nervous system
- IL-6
Interleukin-6
- IL-1β
Interleukin-1beta
- TNF
Tumor necrosis factor
- IL-4
Interleukin-4
- TLRs
Toll-like receptors
- DCs
Dendritic cells
- PAMP
Pathogen-associated molecular pattern
- DAMP
Damage-associated molecular pattern
- NF-κB
Nuclear factors-kappa B
- MAPK
Mitogen-activated protein kinase
- IL-1
Interleukin-1
- IL-18
Interleukin-18
- BBB
Blood–brain barrier
- TNF-α
Tumor necrosis factor alpha
- NO
Nitric oxide
- NLRP3
NOD-like receptor protein 3
- BDNF
Brain-derived neurotrophic factor
- Cx
Connexin
- OS
Oxidative stress
- ROS
Reactive oxygen species
- GSH
Glutathione
- NOS
Nitric oxide synthase
- iNOS
Inducible NOS
- Glu
Glutamic acid
- HPA
Hypothalamus pituitary adrenal
- NTF
Neurotrophic factors
- BH4
Tetrahydrobiopterin
- Trp
Tryptophan
- Kyn
Kynurenine
- MAO
Monoamine oxidase
- IDO
Indoleamine 2,3-dioxygenase
- TDO
Tryptophan 2,3-dioxygenase
- 3‑HK
3-Hydroxykynurenine
- Kynu
Kynureninase
- 3-HAA
3-Hydroxy anthranilic acid
- KAT
Kynurenine aminotransferase
- QA
Quinolinic acid
- Kyna
Kynurenic acid
- NMDAR
N-Methyl-d-aspartic acid receptor
- VMAT2
Monoamine transporter protein 2
- DRD
Dopamine receptors
- SystemXc−
Cystine/Glu reverse transporter system
- GSH
Glutathione
- EAATs
Excitatory amino acid reuptake transporters
- CRH
Adrenocorticotropin-releasing hormone
- ACTH
Adrenocorticotropic hormone
- CORT
Cortisol
- GC
Glucocorticoids
- GRs
GC receptors
- PFC
Prefrontal cortex
- VTA
Ventral tegmental area
- NAc
Nucleus accumbens
- TrkB
Tropomyosin receptor kinase B
- mTOR
Mammalian target of the rapamycin protein
- PI3K
Phosphatidylinositol 3-kinase
- ECD
Extracellular domain
- TMD
Transmembrane domain
- ICD
Intracellular domain
- PAMs
Positive allosteric modulators
- cAMP
Cyclic adenosine monophosphate
- Aβ
Amyloid-beta
- JAK2
Janus kinase 2
- IκB
Inhibitors of NF-κB
- CRS
Chronic restraint stress
- MyD88
Myeloid differentiation factor 88
- α-BGT
α-Bungarotoxin
- ERK
Extracellular signal-regulated kinase
- CaMK
Ca2+/calmodulin-dependent protein kinase
- CREB
CAMP-response element binding protein
- GSK-3β
Glycogen synthase kinase-3beta
- Nrf2
Nuclear transcription factor E2-related factor
- EpRE
Electrophilic response elements
- IP3
1,4,5-Triphosphate
Author contributions
HL and XZ drafted the manuscript. HL and PS designed the illustration. HL, JY, QJ, CP, TC, LX, JC, JT, RY, ZL and HS revised the manuscript and prepared the final draft. LZ, YW and YZ supervised the project and critically appraised the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by the central government guides the local science and technology development special fund (No. 2022ZYD0084), Key R&D projects (No. 2022YFS0607, 2022YFS0635, 2022YFS0630, 2022YFS0627), the Youth Science and Technology Innovation Research Team (No. 2021JDTD0008) of the Science and Technology Department of Sichuan province of China, the Science and Technology Innovation Team from Jiucheng Science and Technology Talent Cultivation Plan in Luzhou (No. 2019-1), Key Research and Development Projectors of Luzhou (No. 2021-SYF-26, 2022-SYF-85), the the Research Fund (No. 210027-01SZ, 200017-01SZ, 230008-01SZ, 230007-01SZ) of Sichuan Province, Sichuan Credit Pharmaceutical CO., Ltd., Central Nervous System Drug Key Laboratory of Sichuan Province, Chongqing Traditional Chinese Medicine Inheritance and Innovation Team Construction Project “Traditional Chinese Medicine New Drug and Safety Research Inheritance and Innovation Team” (No. 2022-8).
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable as no patients/participants were involved in this review.
Competing interests
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Huiyang Liu, Xiaomei Zhang and Peng Shi contributed equally to this work
Contributor Information
Huiyang Liu, Email: lhy15612688262021@163.com.
Xiaomei Zhang, Email: ZXM761@163.com.
Peng Shi, Email: shipengmg@foxmail.com.
Jiyuan Yuan, Email: 458303508@qq.com.
Qiang Jia, Email: jiaq@swmu.edu.cn.
Chao Pi, Email: pichao2016@163.com.
Tao Chen, Email: chentao1998666@163.com.
Linjin Xiong, Email: 13320953765@163.com.
Jinglin Chen, Email: Chenjl0729@163.com.
Jia Tang, Email: tangjia19992021@163.com.
Ruxu Yue, Email: 1209762960@qq.com.
Zerong Liu, Email: ronzelau@dingtalk.com.
Hongping Shen, Email: 707702127@qq.com.
Ying Zuo, Email: zuoying222abc@126.com.
Yumeng Wei, Email: weiyumeng-268@163.com.
Ling Zhao, Email: zhaoling-998@163.com.
References
- 1.Malhi GS, Mann JJ. Depression. Lancet. 2018;392:2299–2312. doi: 10.1016/S0140-6736(18)31948-2. [DOI] [PubMed] [Google Scholar]
- 2.Bland RC. Epidemiology of affective disorders: a review. Can J Psychiatry. 1997;42:367–377. doi: 10.1177/070674379704200403. [DOI] [PubMed] [Google Scholar]
- 3.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ménard C, Hodes GE, Russo SJ. Pathogenesis of depression: insights from human and rodent studies. Neuroscience. 2016;321:138–162. doi: 10.1016/j.neuroscience.2015.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu X, Ce Q, Jin L, Zheng J, Sun M, Tang X, et al. Deoiled sunflower seeds ameliorate depression by promoting the production of monoamine neurotransmitters and inhibiting oxidative stress. Food Funct. 2021;12:573–586. doi: 10.1039/D0FO01978J. [DOI] [PubMed] [Google Scholar]
- 6.Dong Z, Xie Q, Xu F, Shen X, Hao Y, Li J, et al. Neferine alleviates chronic stress-induced depression by regulating monoamine neurotransmitter secretion and gut microbiota structure. Front Pharmacol. 2022;13:974949. doi: 10.3389/fphar.2022.974949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steingard RJ, Yurgelun-Todd DA, Hennen J, Moore JC, Moore CM, Vakili K, et al. Increased orbitofrontal cortex levels of choline in depressed adolescents as detected by in vivo proton magnetic resonance spectroscopy. Biol Psychiatry. 2000;48:1053–1061. doi: 10.1016/S0006-3223(00)00942-2. [DOI] [PubMed] [Google Scholar]
- 8.Charles HC, Lazeyras F, Krishnan KR, Boyko OB, Payne M, Moore D. Brain choline in depression: in vivo detection of potential pharmacodynamic effects of antidepressant therapy using hydrogen localized spectroscopy. Prog Neuropsychopharmacol Biol Psychiatry. 1994;18:1121–1127. doi: 10.1016/0278-5846(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 9.Arias HR, Targowska-Duda KM, García-Colunga J, Ortells MO. Is the antidepressant activity of selective serotonin reuptake inhibitors mediated by nicotinic acetylcholine receptors? Molecules. 2021;26:2149. doi: 10.3390/molecules26082149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kiecolt-Glaser JK, Derry HM, Fagundes CP. Inflammation: depression fans the flames and feasts on the heat. Am J Psychiatry. 2015;172:1075–1091. doi: 10.1176/appi.ajp.2015.15020152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beurel E, Toups M, Nemeroff CB. The bidirectional relationship of depression and inflammation: double trouble. Neuron. 2020;107:234–256. doi: 10.1016/j.neuron.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duan C-M, Zhang J-R, Wan T-F, Wang Y, Chen H-S, Liu L. SRT2104 attenuates chronic unpredictable mild stress-induced depressive-like behaviors and imbalance between microglial M1 and M2 phenotypes in the mice. Behav Brain Res. 2020;378:112296. doi: 10.1016/j.bbr.2019.112296. [DOI] [PubMed] [Google Scholar]
- 13.Cui W, Ning Y, Hong W, Wang J, Liu Z, Li MD. Crosstalk between inflammation and glutamate system in depression: signaling pathway and molecular biomarkers for ketamine’s antidepressant effect. Mol Neurobiol. 2019;56:3484–3500. doi: 10.1007/s12035-018-1306-3. [DOI] [PubMed] [Google Scholar]
- 14.Thomas Broome S, Louangaphay K, Keay K, Leggio G, Musumeci G, Castorina A. Dopamine: an immune transmitter. Neural Regen Res. 2020;15:2173. doi: 10.4103/1673-5374.284976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim E-Y, Choi J-E, Kim M, Hong J, Park Y. N-3 PUFA have antidepressant-like effects via improvement of the HPA-axis and neurotransmission in rats exposed to combined stress. Mol Neurobiol. 2020;57:3860–3874. doi: 10.1007/s12035-020-01980-9. [DOI] [PubMed] [Google Scholar]
- 16.Duman RS. A silver bullet for the treatment of depression? Neuron. 2007;55:679–681. doi: 10.1016/j.neuron.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 17.MacQueen G, Santaguida P, Keshavarz H, Jaworska N, Levine M, Beyene J, et al. Systematic review of clinical practice guidelines for failed antidepressant treatment response in major depressive disorder, dysthymia, and subthreshold depression in adults. Can J Psychiatry. 2017;62:11–23. doi: 10.1177/0706743716664885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Halder N, Lal G. Cholinergic system and its therapeutic importance in inflammation and autoimmunity. Front Immunol. 2021;12:660342. doi: 10.3389/fimmu.2021.660342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ballinger EC, Ananth M, Talmage DA, Role LW. Basal forebrain cholinergic circuits and signaling in cognition and cognitive decline. Neuron. 2016;91:1199–1218. doi: 10.1016/j.neuron.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Picciotto MR, Higley MJ, Mineur YS. Acetylcholine as a neuromodulator: cholinergic signaling shapes nervous system function and behavior. Neuron. 2012;76:116–129. doi: 10.1016/j.neuron.2012.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ventura-Bort C, Wirkner J, Wendt J, Hamm AO, Weymar M. Establishment of emotional memories is mediated by vagal nerve activation: evidence from noninvasive taVNS. J Neurosci. 2021;41:7636–7648. doi: 10.1523/JNEUROSCI.2329-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davidson J. Cholinergic-adrenergic hypothesis of mania and depression. Lancet. 1972;2:1249. doi: 10.1016/S0140-6736(72)92297-0. [DOI] [PubMed] [Google Scholar]
- 23.Mineur YS, Obayemi A, Wigestrand MB, Fote GM, Calarco CA, Li AM, et al. Cholinergic signaling in the hippocampus regulates social stress resilience and anxiety- and depression-like behavior. Proc Natl Acad Sci USA. 2013;110:3573–3578. doi: 10.1073/pnas.1219731110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu ZQ, Zhang WJ, Su DF, Zhang GQ, Miao CY. Cellular responses and functions of α7 nicotinic acetylcholine receptor activation in the brain: a narrative review. Ann Transl Med. 2021;9(6):509. doi: 10.21037/atm-21-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu RT, Rowan-Nash AD, Sheehan AE, Walsh RFL, Sanzari CM, Korry BJ, et al. Reductions in anti-inflammatory gut bacteria are associated with depression in a sample of young adults. Brain Behav Immun. 2020;88:308–324. doi: 10.1016/j.bbi.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu C-H, Yang M-H, Zhang G-Z, Wang X-X, Li B, Li M, et al. Neural networks and the anti-inflammatory effect of transcutaneous auricular vagus nerve stimulation in depression. J Neuroinflamm. 2020;17:54. doi: 10.1186/s12974-020-01732-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han B, Li X, Hao J. The cholinergic anti-inflammatory pathway: an innovative treatment strategy for neurological diseases. Neurosci Biobehav Rev. 2017;77:358–368. doi: 10.1016/j.neubiorev.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 28.Colasanto M, Madigan S, Korczak DJ. Depression and inflammation among children and adolescents: a meta-analysis. J Affect Disord. 2020;277:940–948. doi: 10.1016/j.jad.2020.09.025. [DOI] [PubMed] [Google Scholar]
- 29.Haapakoski R, Mathieu J, Ebmeier KP, Alenius H, Kivimäki M. Cumulative meta-analysis of interleukins 6 and 1β, tumour necrosis factor α and C-reactive protein in patients with major depressive disorder. Brain Behav Immun. 2015;49:206–215. doi: 10.1016/j.bbi.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67:446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 31.Osimo EF, Baxter LJ, Lewis G, Jones PB, Khandaker GM. Prevalence of low-grade inflammation in depression: a systematic review and meta-analysis of CRP levels. Psychol Med. 2019;49:1958–1970. doi: 10.1017/S0033291719001454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Green C, Shen X, Stevenson AJ, Conole ELS, Harris MA, Barbu MC, et al. Structural brain correlates of serum and epigenetic markers of inflammation in major depressive disorder. Brain Behav Immun. 2021;92:39–48. doi: 10.1016/j.bbi.2020.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sakamoto S, Zhu X, Hasegawa Y, Karma S, Obayashi M, Alway E, et al. Inflamed brain: targeting immune changes and inflammation for treatment of depression. Psychiatry Clin Neurosci. 2021;75:304–311. doi: 10.1111/pcn.13286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016;16:22–34. doi: 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Köhler-Forsberg O, Lydholm CN, Hjorthøj C, Nordentoft M, Mors O, Benros ME. Efficacy of anti-inflammatory treatment on major depressive disorder or depressive symptoms: meta-analysis of clinical trials. Acta Psychiatr Scand. 2019;139:404–419. doi: 10.1111/acps.13016. [DOI] [PubMed] [Google Scholar]
- 36.Dionisie V, Filip GA, Manea MC, Manea M, Riga S. The anti-inflammatory role of SSRI and SNRI in the treatment of depression: a review of human and rodent research studies. Inflammopharmacology. 2021;29:75–90. doi: 10.1007/s10787-020-00777-5. [DOI] [PubMed] [Google Scholar]
- 37.Jiang C, Lin WJ, Sadahiro M, et al. VGF function in depression and antidepressant efficacy. Mol Psychiatry. 2018;23(7):1632–1642. doi: 10.1038/mp.2017.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park H-J, Shim H-S, An K, Starkweather A, Kim KS, Shim I. IL-4 inhibits IL-1β-induced depressive-like behavior and central neurotransmitter alterations. Mediat Inflamm. 2015;2015:941413. doi: 10.1155/2015/941413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Craig CF, Filippone RT, Stavely R, Bornstein JC, Apostolopoulos V, Nurgali K. Neuroinflammation as an etiological trigger for depression comorbid with inflammatory bowel disease. J Neuroinflamm. 2022;19(1):4. doi: 10.1186/s12974-021-02354-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Delgado I, Huet L, Dexpert S, Beau C, Forestier D, Ledaguenel P, et al. Depressive symptoms in obesity: relative contribution of low-grade inflammation and metabolic health. Psychoneuroendocrinology. 2018;91:55–61. doi: 10.1016/j.psyneuen.2018.02.030. [DOI] [PubMed] [Google Scholar]
- 41.Arteaga-Henríquez G, Simon MS, Burger B, Weidinger E, Wijkhuijs A, Arolt V, et al. Low-grade inflammation as a predictor of antidepressant and anti-inflammatory therapy response in MDD patients: a systematic review of the literature in combination with an analysis of experimental data collected in the EU-MOODINFLAME consortium. Front Psychiatry. 2019;10:458. doi: 10.3389/fpsyt.2019.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cardinal P, Monchaux de Oliveira C, Sauvant J, Foury A, Darnaudéry M, Vancassel S, et al. A new experimental design to study inflammation-related versus non-inflammation-related depression in mice. J Neuroinflamm. 2021;18:290. doi: 10.1186/s12974-021-02330-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tayab MA, Islam MN, Chowdhury KAA, Tasnim FM. Targeting neuroinflammation by polyphenols: a promising therapeutic approach against inflammation-associated depression. Biomed Pharmacother. 2022;147:112668. doi: 10.1016/j.biopha.2022.112668. [DOI] [PubMed] [Google Scholar]
- 44.Kronsten VT, Tranah TH, Pariante C, Shawcross DL. Gut-derived systemic inflammation as a driver of depression in chronic liver disease. J Hepatol. 2022;76:665–680. doi: 10.1016/j.jhep.2021.11.008. [DOI] [PubMed] [Google Scholar]
- 45.Roman M, Irwin MR. Novel neuroimmunologic therapeutics in depression: a clinical perspective on what we know so far. Brain Behav Immun. 2020;83:7–21. doi: 10.1016/j.bbi.2019.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jones BDM, Daskalakis ZJ, Carvalho AF, et al. Inflammation as a treatment target in mood disorders: review. BJPsych Open. 2020;6(4):e60. doi: 10.1192/bjo.2020.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Felger JC. Role of inflammation in depression and treatment implications. Handb Exp Pharmacol. 2019;250:255–286. doi: 10.1007/164_2018_166. [DOI] [PubMed] [Google Scholar]
- 48.Sokol CL, Luster AD. The chemokine system in innate immunity. Cold Spring Harb Perspect Biol. 2015;7:a016303. doi: 10.1101/cshperspect.a016303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jiang Z, Jiang JX, Zhang G-X. Macrophages: a double-edged sword in experimental autoimmune encephalomyelitis. Immunol Lett. 2014;160:17–22. doi: 10.1016/j.imlet.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brites D, Fernandes A. Neuroinflammation and depression: microglia activation, extracellular microvesicles and microRNA dysregulation. Front Cell Neurosci. 2015;9:476. doi: 10.3389/fncel.2015.00476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sun Y, Koyama Y, Shimada S. Inflammation from peripheral organs to the brain: how does systemic inflammation cause neuroinflammation? Front Aging Neurosci. 2022;14:903455. doi: 10.3389/fnagi.2022.903455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McKim DB, Weber MD, Niraula A, Sawicki CM, Liu X, Jarrett BL, et al. Microglial recruitment of IL-1β-producing monocytes to brain endothelium causes stress-induced anxiety. Mol Psychiatry. 2018;23:1421–1431. doi: 10.1038/mp.2017.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Han Y, Ding L, Cheng X, Zhao M, Zhao T, Guo L, et al. Hypoxia augments cerebral inflammation in a dextran sulfate sodium-induced colitis mouse model. Front Cell Neurosci. 2020;14:611764. doi: 10.3389/fncel.2020.611764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nedic Erjavec G, Sagud M, Nikolac Perkovic M, Svob Strac D, Konjevod M, Tudor L, et al. Depression: biological markers and treatment. Prog Neuropsychopharmacol Biol Psychiatry. 2021;105:110139. doi: 10.1016/j.pnpbp.2020.110139. [DOI] [PubMed] [Google Scholar]
- 55.Schiltz JC, Sawchenko PE. Signaling the brain in systemic inflammation: the role of perivascular cells. Front Biosci. 2003;8:s1321–1329. doi: 10.2741/1211. [DOI] [PubMed] [Google Scholar]
- 56.Corsi-Zuelli FMDG, Brognara F, Quirino GFDS, Hiroki CH, Fais RS, Del-Ben CM, et al. Neuroimmune interactions in schizophrenia: focus on vagus nerve stimulation and activation of the alpha-7 nicotinic acetylcholine receptor. Front Immunol. 2017;8:618. doi: 10.3389/fimmu.2017.00618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kwon HS, Koh S-H. Neuroinflammation in neurodegenerative disorders: the roles of microglia and astrocytes. Transl Neurodegener. 2020;9:42. doi: 10.1186/s40035-020-00221-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Troubat R, Barone P, Leman S, Desmidt T, Cressant A, Atanasova B, et al. Neuroinflammation and depression: a review. Eur J Neurosci. 2021;53:151–171. doi: 10.1111/ejn.14720. [DOI] [PubMed] [Google Scholar]
- 59.Sun Y, Zhou Y-Q, Liu Y-K, Zhang H-Q, Hou G-G, Meng Q-G, et al. Potential anti-neuroinflammatory NF-кB inhibitors based on 3,4-dihydronaphthalen-1(2H)-one derivatives. J Enzyme Inhib Med Chem. 2020;35:1631–1640. doi: 10.1080/14756366.2020.1804899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pike AF, Szabò I, Veerhuis R, Bubacco L. The potential convergence of NLRP3 inflammasome, potassium, and dopamine mechanisms in Parkinson’s disease. NPJ Parkinsons Dis. 2022;8:32. doi: 10.1038/s41531-022-00293-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Di Lucente J, Nguyen HM, Wulff H, Jin LW, Maezawa I. The voltage-gated potassium channel Kv1.3 is required for microglial pro-inflammatory activation in vivo. Glia. 2018;66(9):1881–1895. doi: 10.1002/glia.23457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hashioka S, Miyaoka T, Wake R, Furuya M, Horiguchi J. Glia: an important target for anti-inflammatory and antidepressant activity. Curr Drug Targets. 2013;14(11):1322–1328. doi: 10.2174/13894501113146660214. [DOI] [PubMed] [Google Scholar]
- 63.Jo WK, Zhang Y, Emrich HM, Dietrich DE. Glia in the cytokine-mediated onset of depression: fine tuning the immune response. Front Cell Neurosci. 2015;9:268. doi: 10.3389/fncel.2015.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Peppas S, Pansieri C, Piovani D, Danese S, Peyrin-Biroulet L, Tsantes AG, et al. The brain–gut axis: psychological functioning and inflammatory bowel diseases. J Clin Med. 2021;10:377. doi: 10.3390/jcm10030377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Han Y, Zhao T, Cheng X, Zhao M, Gong S-H, Zhao Y-Q, et al. Cortical inflammation is increased in a DSS-induced colitis mouse model. Neurosci Bull. 2018;34:1058–1066. doi: 10.1007/s12264-018-0288-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang H, Yang Y, Yang S, Ren S, Feng J, Liu Y, et al. Ginsenoside Rg1 ameliorates neuroinflammation via suppression of connexin43 ubiquitination to attenuate depression. Front Pharmacol. 2021;12:709019. doi: 10.3389/fphar.2021.709019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Roy S, Jiang JX, Li AF, Kim D. Connexin channel and its role in diabetic retinopathy. Prog Retin Eye Res. 2017;61:35–59. doi: 10.1016/j.preteyeres.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huang Y, Mao Z, Zhang Z, et al. Connexin43 contributes to inflammasome activation and lipopolysaccharide-initiated acute renal injury via modulation of intracellular oxidative status. Antioxid Redox Signal. 2019;31(16):1194–1212. doi: 10.1089/ars.2018.7636. [DOI] [PubMed] [Google Scholar]
- 69.Wang X, Feng L, Xin M, et al. Mechanisms underlying astrocytic connexin-43 autophagy degradation during cerebral ischemia injury and the effect on neuroinflammation and cell apoptosis. Biomed Pharmacother. 2020;127:110125. doi: 10.1016/j.biopha.2020.110125. [DOI] [PubMed] [Google Scholar]
- 70.Allen J, Romay-Tallon R, Brymer KJ, Caruncho HJ, Kalynchuk LE. Mitochondria and mood: mitochondrial dysfunction as a key player in the manifestation of depression. Front Neurosci. 2018;12:386. doi: 10.3389/fnins.2018.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Calingasan NY, Ho DJ, Wille EJ, Campagna MV, Ruan J, Dumont M, et al. Influence of mitochondrial enzyme deficiency on adult neurogenesis in mouse models of neurodegenerative diseases. Neuroscience. 2008;153:986–996. doi: 10.1016/j.neuroscience.2008.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Adzic M, Brkic Z, Bulajic S, Mitic M, Radojcic MB. Antidepressant action on mitochondrial dysfunction in psychiatric disorders. Drug Dev Res. 2016;77(7):400–406. doi: 10.1002/ddr.21332. [DOI] [PubMed] [Google Scholar]
- 73.Mozafari H, Amiri S, Mehr SE, Momeny M, Amini-Khoei H, Bijani S, et al. Minocycline attenuates depressive-like behaviors in mice treated with the low dose of intracerebroventricular streptozotocin; the role of mitochondrial function and neuroinflammation. Mol Biol Rep. 2020;47:6143–6153. doi: 10.1007/s11033-020-05696-w. [DOI] [PubMed] [Google Scholar]
- 74.Haj-Mirzaian A, Amiri S, Amini-Khoei H, Haj-Mirzaian A, Hashemiaghdam A, Ramezanzadeh K, et al. Involvement of NO/NMDA-R pathway in the behavioral despair induced by amphetamine withdrawal. Brain Res Bull. 2018;139:81–90. doi: 10.1016/j.brainresbull.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 75.Beheshti F, Hashemzehi M, Hosseini M, Marefati N, Memarpour S. Inducible nitric oxide synthase plays a role in depression- and anxiety-like behaviors chronically induced by lipopolysaccharide in rats: evidence from inflammation and oxidative stress. Behav Brain Res. 2020;392:112720. doi: 10.1016/j.bbr.2020.112720. [DOI] [PubMed] [Google Scholar]
- 76.Dutheil S, Ota KT, Wohleb ES, Rasmussen K, Duman RS. High-fat diet induced anxiety and anhedonia: impact on brain homeostasis and inflammation. Neuropsychopharmacology. 2016;41(7):1874–1887. doi: 10.1038/npp.2015.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Spielman LJ, Gibson DL, Klegeris A. Unhealthy gut, unhealthy brain: the role of the intestinal microbiota in neurodegenerative diseases. Neurochem Int. 2018;120:149–163. doi: 10.1016/j.neuint.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 78.Hurtado-Alvarado G, Domínguez-Salazar E, Pavon L, Velázquez-Moctezuma J, Gómez-González B. Blood–brain barrier disruption induced by chronic sleep loss: low-grade inflammation may be the link. J Immunol Res. 2016;2016:4576012. doi: 10.1155/2016/4576012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nouri A, Hashemzadeh F, Soltani A, Saghaei E, Amini-Khoei H. Progesterone exerts antidepressant-like effect in a mouse model of maternal separation stress through mitigation of neuroinflammatory response and oxidative stress. Pharm Biol. 2020;58:64–71. doi: 10.1080/13880209.2019.1702704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Moradi-Marjaneh R, Hassanian SM, Mehramiz M, Rezayi M, Ferns GA, Khazaei M, et al. Reactive oxygen species in colorectal cancer: the therapeutic impact and its potential roles in tumor progression via perturbation of cellular and physiological dysregulated pathways. J Cell Physiol. 2019;234:10072–10079. doi: 10.1002/jcp.27881. [DOI] [PubMed] [Google Scholar]
- 81.Lorigooini Z, Boroujeni SN, Sayyadi-Shahraki M, Rahimi-Madiseh M, Bijad E, Amini-Khoei H. Limonene through attenuation of neuroinflammation and nitrite level exerts antidepressant-like effect on mouse model of maternal separation stress. Behav Neurol. 2021;2021:8817309. doi: 10.1155/2021/8817309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Miller AH. Beyond depression: the expanding role of inflammation in psychiatric disorders. World Psychiatry. 2020;19(1):108–109. doi: 10.1002/wps.20723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Niciu MJ, Ionescu DF, Richards EM, Zarate CA. Glutamate and its receptors in the pathophysiology and treatment of major depressive disorder. J Neural Transm (Vienna) 2014;121:907–924. doi: 10.1007/s00702-013-1130-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Duman RS, Aghajanian GK. Synaptic dysfunction in depression: potential therapeutic targets. Science. 2012;338(6103):68–72. doi: 10.1126/science.1222939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ancelin ML, Scali J, Norton J, et al. Heterogeneity in HPA axis dysregulation and serotonergic vulnerability to depression. Psychoneuroendocrinology. 2017;77:90–94. doi: 10.1016/j.psyneuen.2016.11.016. [DOI] [PubMed] [Google Scholar]
- 86.Keller J, Gomez R, Williams G, Lembke A, Lazzeroni L, Murphy GM, et al. HPA axis in major depression: cortisol, clinical symptomatology and genetic variation predict cognition. Mol Psychiatry. 2017;22:527–536. doi: 10.1038/mp.2016.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang YT, Wang XL, Feng ST, Chen NH, Wang ZZ, Zhang Y. Novel rapid-acting glutamatergic modulators: targeting the synaptic plasticity in depression. Pharmacol Res. 2021;171:105761. doi: 10.1016/j.phrs.2021.105761. [DOI] [PubMed] [Google Scholar]
- 88.Kanova M, Kohout P. Tryptophan: a unique role in the critically ill. IJMS. 2021;22:11714. doi: 10.3390/ijms222111714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Melhem NJ, Taleb S. Tryptophan: from diet to cardiovascular diseases. IJMS. 2021;22:9904. doi: 10.3390/ijms22189904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Höglund E, Øverli Ø, Winberg S. Tryptophan metabolic pathways and brain serotonergic activity: a comparative review. Front Endocrinol (Lausanne) 2019;10:158. doi: 10.3389/fendo.2019.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Savitz J. The kynurenine pathway: a finger in every pie. Mol Psychiatry. 2020;25:131–147. doi: 10.1038/s41380-019-0414-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hsu C-N, Tain Y-L. Developmental programming and reprogramming of hypertension and kidney disease: impact of tryptophan metabolism. IJMS. 2020;21:8705. doi: 10.3390/ijms21228705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gałecki P, Talarowska M. Inflammatory theory of depression. Psychiatr Pol. 2018;52:437–447. doi: 10.12740/PP/76863. [DOI] [PubMed] [Google Scholar]
- 94.Halaris A. Inflammation and depression but where does the inflammation come from? Curr Opin Psychiatry. 2019;32:422–428. doi: 10.1097/YCO.0000000000000531. [DOI] [PubMed] [Google Scholar]
- 95.Dean J, Keshavan M. The neurobiology of depression: an integrated view. Asian J Psychiatr. 2017;27:101–111. doi: 10.1016/j.ajp.2017.01.025. [DOI] [PubMed] [Google Scholar]
- 96.St’astný F, Lisý V, Mares V, Lisá V, Balcar VJ, Santamaría A. Quinolinic acid induces NMDA receptor-mediated lipid peroxidation in rat brain microvessels. Redox Rep. 2004;9:229–233. doi: 10.1179/135100004225006001. [DOI] [PubMed] [Google Scholar]
- 97.Zhou Q, Sheng M. NMDA receptors in nervous system diseases. Neuropharmacology. 2013;74:69–75. doi: 10.1016/j.neuropharm.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 98.Fukuwatari T. Possibility of amino acid treatment to prevent the psychiatric disorders via modulation of the production of tryptophan metabolite kynurenic acid. Nutrients. 2020;12:1403. doi: 10.3390/nu12051403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pocivavsek A, Wu H-Q, Potter MC, Elmer GI, Pellicciari R, Schwarcz R. Fluctuations in endogenous kynurenic acid control hippocampal glutamate and memory. Neuropsychopharmacology. 2011;36:2357–2367. doi: 10.1038/npp.2011.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kruse JL, Cho JH-J, Olmstead R, Hwang L, Faull K, Eisenberger NI, et al. Kynurenine metabolism and inflammation-induced depressed mood: a human experimental study. Psychoneuroendocrinology. 2019;109:104371. doi: 10.1016/j.psyneuen.2019.104371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kazumori H, Ishihara S, Rumi MA, Ortega-Cava CF, Kadowaki Y, Kinoshita Y. Transforming growth factor-alpha directly augments histidine decarboxylase and vesicular monoamine transporter 2 production in rat enterochromaffin-like cells. Am J Physiol Gastrointest Liver Physiol. 2004;286(3):G508–G514. doi: 10.1152/ajpgi.00269.2003. [DOI] [PubMed] [Google Scholar]
- 102.Bermingham DP, Blakely RD. Kinase-dependent regulation of monoamine neurotransmitter transporters. Pharmacol Rev. 2016;68(4):888–953. doi: 10.1124/pr.115.012260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Feng Y, Lu Y. Immunomodulatory effects of dopamine in inflammatory diseases. Front Immunol. 2021;12:663102. doi: 10.3389/fimmu.2021.663102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Clarke M, Razmjou S, Prowse N, Dwyer Z, Litteljohn D, Pentz R, et al. Ketamine modulates hippocampal neurogenesis and pro-inflammatory cytokines but not stressor induced neurochemical changes. Neuropharmacology. 2017;112:210–220. doi: 10.1016/j.neuropharm.2016.04.021. [DOI] [PubMed] [Google Scholar]
- 105.Pacheco R. Targeting dopamine receptor D3 signalling in inflammation. Oncotarget. 2017;8:7224–7225. doi: 10.18632/oncotarget.14601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Elgueta D, Aymerich MS, Contreras F, Montoya A, Celorrio M, Rojo-Bustamante E, et al. Pharmacologic antagonism of dopamine receptor D3 attenuates neurodegeneration and motor impairment in a mouse model of Parkinson’s disease. Neuropharmacology. 2017;113:110–123. doi: 10.1016/j.neuropharm.2016.09.028. [DOI] [PubMed] [Google Scholar]
- 107.Elgueta D, Contreras F, Prado C, Montoya A, Ugalde V, Chovar O, et al. Dopamine receptor D3 expression is altered in CD4+ T-cells from Parkinson’s disease patients and its pharmacologic inhibition attenuates the motor impairment in a mouse model. Front Immunol. 2019;10:981. doi: 10.3389/fimmu.2019.00981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Torres-Rosas R, Yehia G, Peña G, Mishra P, del Rocio T-B, Moreno-Eutimio MA, et al. Dopamine mediates vagal modulation of the immune system by electroacupuncture. Nat Med. 2014;20:291–295. doi: 10.1038/nm.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dominguez-Meijide A, Rodriguez-Perez AI, Diaz-Ruiz C, Guerra MJ, Labandeira-Garcia JL. Dopamine modulates astroglial and microglial activity via glial renin-angiotensin system in cultures. Brain Behav Immun. 2017;62:277–290. doi: 10.1016/j.bbi.2017.02.013. [DOI] [PubMed] [Google Scholar]
- 110.Campos J, Pacheco R. Involvement of dopaminergic signaling in the cross talk between the renin-angiotensin system and inflammation. Semin Immunopathol. 2020;42:681–696. doi: 10.1007/s00281-020-00819-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Harrison PJ, Lyon L, Sartorius LJ, Burnet PWJ, Lane TA. The group II metabotropic glutamate receptor 3 (mGluR3, mGlu3, GRM3): expression, function and involvement in schizophrenia. J Psychopharmacol. 2008;22:308–322. doi: 10.1177/0269881108089818. [DOI] [PubMed] [Google Scholar]
- 112.Hashimoto K. Emerging role of glutamate in the pathophysiology of major depressive disorder. Brain Res Rev. 2009;61(2):105–123. doi: 10.1016/j.brainresrev.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 113.Lewerenz J, Hewett SJ, Huang Y, Lambros M, Gout PW, Kalivas PW, et al. The cystine/glutamate antiporter system x(c)(−) in health and disease: from molecular mechanisms to novel therapeutic opportunities. Antioxid Redox Signal. 2013;18:522–555. doi: 10.1089/ars.2011.4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nehser M, Dark J, Schweitzer D, Campbell M, Zwicker J, Hitt DM, et al. System Xc− antiporter inhibitors: azo-linked amino-naphthyl-sulfonate analogues of sulfasalazine. Neurochem Res. 2020;45:1375–1386. doi: 10.1007/s11064-019-02901-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lim JKM, Delaidelli A, Minaker SW, Zhang H-F, Colovic M, Yang H, et al. Cystine/glutamate antiporter xCT (SLC7A11) facilitates oncogenic RAS transformation by preserving intracellular redox balance. Proc Natl Acad Sci USA. 2019;116:9433–9442. doi: 10.1073/pnas.1821323116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Haroon E, Miller AH. Inflammation effects on glutamate as a pathway to neuroprogression in mood disorders. Mod Trends Pharmacopsychiatry. 2017;31:37–55. doi: 10.1159/000470805. [DOI] [PubMed] [Google Scholar]
- 117.Young K, Singh G. Biological mechanisms of cancer-induced depression. Front Psychiatry. 2018;9:299. doi: 10.3389/fpsyt.2018.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/S0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- 119.Nedergaard M, Takano T, Hansen AJ. Beyond the role of glutamate as a neurotransmitter. Nat Rev Neurosci. 2002;3:748–755. doi: 10.1038/nrn916. [DOI] [PubMed] [Google Scholar]
- 120.Haroon E, Miller AH. Inflammation effects on brain glutamate in depression: mechanistic considerations and treatment implications. Curr Top Behav Neurosci. 2017;31:173–198. doi: 10.1007/7854_2016_40. [DOI] [PubMed] [Google Scholar]
- 121.Nunes SOV, Vargas HO, Prado E, Barbosa DS, de Melo LP, Moylan S, et al. The shared role of oxidative stress and inflammation in major depressive disorder and nicotine dependence. Neurosci Biobehav Rev. 2013;37:1336–1345. doi: 10.1016/j.neubiorev.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 122.Haroon E, Miller AH, Sanacora G. Inflammation, glutamate, and glia: a trio of trouble in mood disorders. Neuropsychopharmacology. 2017;42:193–215. doi: 10.1038/npp.2016.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jackman NA, Uliasz TF, Hewett JA, Hewett SJ. Regulation of system x(c)(−)activity and expression in astrocytes by interleukin-1β: implications for hypoxic neuronal injury. Glia. 2010;58:1806–1815. doi: 10.1002/glia.21050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Malarkey EB, Parpura V. Mechanisms of glutamate release from astrocytes. Neurochem Int. 2008;52(1–2):142–154. doi: 10.1016/j.neuint.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kim YK, Na KS. Role of glutamate receptors and glial cells in the pathophysiology of treatment-resistant depression. Prog Neuropsychopharmacol Biol Psychiatry. 2016;70:117–126. doi: 10.1016/j.pnpbp.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 126.Zhou Y, Danbolt NC. Glutamate as a neurotransmitter in the healthy brain. J Neural Transm (Vienna) 2014;121:799–817. doi: 10.1007/s00702-014-1180-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Haroon E, Chen X, Li Z, Patel T, Woolwine BJ, Hu XP, et al. Increased inflammation and brain glutamate define a subtype of depression with decreased regional homogeneity, impaired network integrity, and anhedonia. Transl Psychiatry. 2018;8:189. doi: 10.1038/s41398-018-0241-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhou L, Wang T, Yu Y, Li M, Sun X, Song W, et al. The etiology of poststroke-depression: a hypothesis involving HPA axis. Biomed Pharmacother. 2022;151:113146. doi: 10.1016/j.biopha.2022.113146. [DOI] [PubMed] [Google Scholar]
- 129.Makris AP, Karianaki M, Tsamis KI, Paschou SA. The role of the gut-brain axis in depression: endocrine, neural, and immune pathways. Hormones (Athens) 2021;20:1–12. doi: 10.1007/s42000-020-00236-4. [DOI] [PubMed] [Google Scholar]
- 130.Hlavacova N, Li Y, Pehrson A, Sanchez C, Bermudez I, Csanova A, et al. Effects of vortioxetine on biomarkers associated with glutamatergic activity in an SSRI insensitive model of depression in female rats. Prog Neuropsychopharmacol Biol Psychiatry. 2018;82:332–338. doi: 10.1016/j.pnpbp.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 131.Shapero BG, Curley EE, Black CL, Alloy LB. The interactive association of proximal life stress and cumulative HPA axis functioning with depressive symptoms. Depress Anxiety. 2019;36:1089–1101. doi: 10.1002/da.22957. [DOI] [PubMed] [Google Scholar]
- 132.Dooley LN, Kuhlman KR, Robles TF, Eisenberger NI, Craske MG, Bower JE. The role of inflammation in core features of depression: insights from paradigms using exogenously-induced inflammation. Neurosci Biobehav Rev. 2018;94:219–237. doi: 10.1016/j.neubiorev.2018.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Leonard BE. Inflammation and depression: a causal or coincidental link to the pathophysiology? Acta Neuropsychiatr. 2018;30(1):1–16. doi: 10.1017/neu.2016.69. [DOI] [PubMed] [Google Scholar]
- 134.Cernackova A, Durackova Z, Trebaticka J, Mravec B. Neuroinflammation and depressive disorder: the role of the hypothalamus. J Clin Neurosci. 2020;75:5–10. doi: 10.1016/j.jocn.2020.03.005. [DOI] [PubMed] [Google Scholar]
- 135.Ahmad MH, Rizvi MA, Fatima M, Mondal AC. Pathophysiological implications of neuroinflammation mediated HPA axis dysregulation in the prognosis of cancer and depression. Mol Cell Endocrinol. 2021;520:111093. doi: 10.1016/j.mce.2020.111093. [DOI] [PubMed] [Google Scholar]
- 136.Pace TW, Hu F, Miller AH. Cytokine-effects on glucocorticoid receptor function: relevance to glucocorticoid resistance and the pathophysiology and treatment of major depression. Brain Behav Immun. 2007;21(1):9–19. doi: 10.1016/j.bbi.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pace TW, Miller AH. Cytokines and glucocorticoid receptor signaling. Relevance to major depression. Ann N Y Acad Sci. 2009;1179:86–105. doi: 10.1111/j.1749-6632.2009.04984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang X, Wu H, Miller AH. Interleukin 1alpha (IL-1alpha) induced activation of p38 mitogen-activated protein kinase inhibits glucocorticoid receptor function. Mol Psychiatry. 2004;9(1):65–75. doi: 10.1038/sj.mp.4001339. [DOI] [PubMed] [Google Scholar]
- 139.Kim Y-K, Na K-S, Myint A-M, Leonard BE. The role of pro-inflammatory cytokines in neuroinflammation, neurogenesis and the neuroendocrine system in major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2016;64:277–284. doi: 10.1016/j.pnpbp.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 140.Saltiel PF, Silvershein DI. Major depressive disorder: mechanism-based prescribing for personalized medicine. Neuropsychiatr Dis Treat. 2015;11:875–888. doi: 10.2147/NDT.S73261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zagrebelsky M, Korte M. Form follows function: BDNF and its involvement in sculpting the function and structure of synapses. Neuropharmacology. 2014;76(Pt C):628–638. doi: 10.1016/j.neuropharm.2013.05.029. [DOI] [PubMed] [Google Scholar]
- 142.Zhang J-C, Yao W, Ren Q, Yang C, Dong C, Ma M, et al. Depression-like phenotype by deletion of α7 nicotinic acetylcholine receptor: Role of BDNF-TrkB in nucleus accumbens. Sci Rep. 2016;6:36705. doi: 10.1038/srep36705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Conroy JN, Jhaveri DJ, Coulson EJ. Fast-Trk(B)ing the mechanism of antidepressants. Neuron. 2021;109:1593–1595. doi: 10.1016/j.neuron.2021.04.027. [DOI] [PubMed] [Google Scholar]
- 144.Gerhard DM, Pothula S, Liu R-J, Wu M, Li X-Y, Girgenti MJ, et al. GABA interneurons are the cellular trigger for ketamine’s rapid antidepressant actions. J Clin Invest. 2020;130:1336–1349. doi: 10.1172/JCI130808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Picard N, Takesian AE, Fagiolini M, Hensch TK. NMDA 2A receptors in parvalbumin cells mediate sex-specific rapid ketamine response on cortical activity. Mol Psychiatry. 2019;24:828–838. doi: 10.1038/s41380-018-0341-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bonni A, Brunet A, West AE, Datta SR, Takasu MA, Greenberg ME. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science. 1999;286(5443):1358–1362. doi: 10.1126/science.286.5443.1358. [DOI] [PubMed] [Google Scholar]
- 147.Björkholm C, Monteggia LM. BDNF—a key transducer of antidepressant effects. Neuropharmacology. 2016;102:72–79. doi: 10.1016/j.neuropharm.2015.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Castrén E, Monteggia LM. Brain-derived neurotrophic factor signaling in depression and antidepressant action. Biol Psychiatry. 2021;90:128–136. doi: 10.1016/j.biopsych.2021.05.008. [DOI] [PubMed] [Google Scholar]
- 149.Li J, He P, Zhang J, Li N. Orcinol glucoside improves the depressive-like behaviors of perimenopausal depression mice through modulating activity of hypothalamic-pituitary-adrenal/ovary axis and activating BDNF-TrkB-CREB signaling pathway. Phytother Res. 2021;35(10):5795–5807. doi: 10.1002/ptr.7237. [DOI] [PubMed] [Google Scholar]
- 150.Stanika RI, Pivovarova NB, Brantner CA, Watts CA, Winters CA, Andrews SB. Coupling diverse routes of calcium entry to mitochondrial dysfunction and glutamate excitotoxicity. Proc Natl Acad Sci USA. 2009;106:9854–9859. doi: 10.1073/pnas.0903546106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Leal GC, Bandeira ID, Correia-Melo FS, et al. Intravenous arketamine for treatment-resistant depression: open-label pilot study. Eur Arch Psychiatry Clin Neurosci. 2021;271(3):577–582. doi: 10.1007/s00406-020-01110-5. [DOI] [PubMed] [Google Scholar]
- 152.Hardingham GE, Bading H. Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders. Nat Rev Neurosci. 2010;11:682–696. doi: 10.1038/nrn2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wu J, Liu Q, Tang P, Mikkelsen JD, Shen J, Whiteaker P, et al. Heteromeric α7β2 nicotinic acetylcholine receptors in the brain. Trends Pharmacol Sci. 2016;37:562–574. doi: 10.1016/j.tips.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bondarenko V, Wells MM, Chen Q, Tillman TS, Singewald K, Lawless MJ, et al. Structures of highly flexible intracellular domain of human α7 nicotinic acetylcholine receptor. Nat Commun. 2022;13:793. doi: 10.1038/s41467-022-28400-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Unwin N. Acetylcholine receptor channel imaged in the open state. Nature. 1995;373:37–43. doi: 10.1038/373037a0. [DOI] [PubMed] [Google Scholar]
- 156.Li S, Li Z, Pei L, Le AD, Liu F. The α7nACh-NMDA receptor complex is involved in cue-induced reinstatement of nicotine seeking. J Exp Med. 2012;209:2141–2147. doi: 10.1084/jem.20121270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ivica J, Lape R, Jazbec V, Yu J, Zhu H, Gouaux E, et al. The intracellular domain of homomeric glycine receptors modulates agonist efficacy. J Biol Chem. 2021;296:100387. doi: 10.1074/jbc.RA119.012358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.King JR, Nordman JC, Bridges SP, Lin M-K, Kabbani N. Identification and characterization of a G protein-binding cluster in α7 nicotinic acetylcholine receptors. J Biol Chem. 2015;290:20060–20070. doi: 10.1074/jbc.M115.647040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Stokes C, Treinin M, Papke RL. Looking below the surface of nicotinic acetylcholine receptors. Trends Pharmacol Sci. 2015;36:514–523. doi: 10.1016/j.tips.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Noviello CM, Gharpure A, Mukhtasimova N, Cabuco R, Baxter L, Borek D, et al. Structure and gating mechanism of the α7 nicotinic acetylcholine receptor. Cell. 2021;184:2121–2134.e13. doi: 10.1016/j.cell.2021.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Basak S, Gicheru Y, Samanta A, Molugu SK, Huang W, Fuente MLD, et al. Cryo-EM structure of 5-HT3A receptor in its resting conformation. Nat Commun. 2018;9:514. doi: 10.1038/s41467-018-02997-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Polovinkin L, Hassaine G, Perot J, Neumann E, Jensen AA, Lefebvre SN, et al. Conformational transitions of the serotonin 5-HT3 receptor. Nature. 2018;563:275–279. doi: 10.1038/s41586-018-0672-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Targowska-Duda KM, Budzynska B, Michalak A, Wnorowski A, Loland CJ, Maj M, et al. Type I and type II positive allosteric modulators of α7 nicotinic acetylcholine receptors induce antidepressant-like activity in mice by a mechanism involving receptor potentiation but not neurotransmitter reuptake inhibition. Correlation with mTOR intracellular pathway activation. Eur Neuropsychopharmacol. 2021;52:31–47. doi: 10.1016/j.euroneuro.2021.06.006. [DOI] [PubMed] [Google Scholar]
- 164.Papke RL, Horenstein NA. Therapeutic targeting of α7 nicotinic acetylcholine receptors. Pharmacol Rev. 2021;73(3):1118–1149. doi: 10.1124/pharmrev.120.000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Yoshikawa H, Kurokawa M, Ozaki N, Nara K, Atou K, Takada E, et al. Nicotine inhibits the production of proinflammatory mediators in human monocytes by suppression of I-kappaB phosphorylation and nuclear factor-kappaB transcriptional activity through nicotinic acetylcholine receptor alpha7. Clin Exp Immunol. 2006;146:116–123. doi: 10.1111/j.1365-2249.2006.03169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Aicher A, Heeschen C, Mohaupt M, Cooke JP, Zeiher AM, Dimmeler S. Nicotine strongly activates dendritic cell-mediated adaptive immunity: potential role for progression of atherosclerotic lesions. Circulation. 2003;107:604–611. doi: 10.1161/01.CIR.0000047279.42427.6D. [DOI] [PubMed] [Google Scholar]
- 167.Shytle RD, Mori T, Townsend K, Vendrame M, Sun N, Zeng J, et al. Cholinergic modulation of microglial activation by alpha 7 nicotinic receptors. J Neurochem. 2004;89:337–343. doi: 10.1046/j.1471-4159.2004.02347.x. [DOI] [PubMed] [Google Scholar]
- 168.Sfera A, Cummings M, Osorio C. Non-neuronal acetylcholine: the missing link between sepsis, cancer, and delirium? Front Med (Lausanne) 2015;2:56. doi: 10.3389/fmed.2015.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kawashima K, Fujii T. Expression of non-neuronal acetylcholine in lymphocytes and its contribution to the regulation of immune function. Front Biosci. 2004;9:2063–2085. doi: 10.2741/1390. [DOI] [PubMed] [Google Scholar]
- 170.Egea J, Buendia I, Parada E, Navarro E, León R, Lopez MG. Anti-inflammatory role of microglial alpha7 nAChRs and its role in neuroprotection. Biochem Pharmacol. 2015;97:463–472. doi: 10.1016/j.bcp.2015.07.032. [DOI] [PubMed] [Google Scholar]
- 171.Shen J, Yakel JL. Nicotinic acetylcholine receptor-mediated calcium signaling in the nervous system. Acta Pharmacol Sin. 2009;30:673–680. doi: 10.1038/aps.2009.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Yakel JL. Nicotinic ACh receptors in the hippocampal circuit; functional expression and role in synaptic plasticity. J Physiol. 2014;592:4147–4153. doi: 10.1113/jphysiol.2014.273896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Nordman JC, Phillips WS, Kodama N, Clark SG, Del Negro CA, Kabbani N. Axon targeting of the alpha 7 nicotinic receptor in developing hippocampal neurons by Gprin1 regulates growth. J Neurochem. 2014;129:649–662. doi: 10.1111/jnc.12641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Piovesana R, Salazar Intriago MS, Dini L, Tata AM. Cholinergic modulation of neuroinflammation: focus on α7 nicotinic receptor. IJMS. 2021;22:4912. doi: 10.3390/ijms22094912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sun JL, Stokoe SA, Roberts JP, Sathler MF, Nip KA, Shou J, et al. Co-activation of selective nicotinic acetylcholine receptors is required to reverse beta amyloid-induced Ca2+ hyperexcitation. Neurobiol Aging. 2019;84:166–177. doi: 10.1016/j.neurobiolaging.2019.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ma KG, Qian YH. Alpha 7 nicotinic acetylcholine receptor and its effects on Alzheimer’s disease. Neuropeptides. 2019;73:96–106. doi: 10.1016/j.npep.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 177.Corradi J, Bouzat C. Understanding the bases of function and modulation of α7 nicotinic receptors: implications for drug discovery. Mol Pharmacol. 2016;90(3):288–299. doi: 10.1124/mol.116.104240. [DOI] [PubMed] [Google Scholar]
- 178.de Jonge WJ, Ulloa L. The alpha7 nicotinic acetylcholine receptor as a pharmacological target for inflammation. Br J Pharmacol. 2007;151(7):915–929. doi: 10.1038/sj.bjp.0707264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Shao B-Z, Ke P, Xu Z-Q, Wei W, Cheng M-H, Han B-Z, et al. Autophagy plays an important role in anti-inflammatory mechanisms stimulated by alpha7 nicotinic acetylcholine receptor. Front Immunol. 2017;8:553. doi: 10.3389/fimmu.2017.00553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Hou Z, Zhou Y, Yang H, Liu Y, Mao X, Qin X, et al. Alpha7 nicotinic acetylcholine receptor activation protects against myocardial reperfusion injury through modulation of autophagy. Biochem Biophys Res Commun. 2018;500:357–364. doi: 10.1016/j.bbrc.2018.04.077. [DOI] [PubMed] [Google Scholar]
- 181.Hua Y, Yang B, Chen Q, Zhang J, Hu J, Fan Y. Activation of α7 nicotinic acetylcholine receptor protects against 1-methyl-4-phenylpyridinium-induced astroglial apoptosis. Front Cell Neurosci. 2019;13:507. doi: 10.3389/fncel.2019.00507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Pu Y, Tan Y, Qu Y, Chang L, Wang S, Wei Y, et al. A role of the subdiaphragmatic vagus nerve in depression-like phenotypes in mice after fecal microbiota transplantation from Chrna7 knock-out mice with depression-like phenotypes. Brain Behav Immun. 2021;94:318–326. doi: 10.1016/j.bbi.2020.12.032. [DOI] [PubMed] [Google Scholar]
- 183.Marcus MM, Björkholm C, Malmerfelt A, et al. Alpha7 nicotinic acetylcholine receptor agonists and PAMs as adjunctive treatment in schizophrenia. An experimental study. Eur Neuropsychopharmacol. 2016;26(9):1401–1411. doi: 10.1016/j.euroneuro.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 184.Zhao D, Xu X, Pan L, Zhu W, Fu X, Guo L, et al. Pharmacologic activation of cholinergic alpha7 nicotinic receptors mitigates depressive-like behavior in a mouse model of chronic stress. J Neuroinflamm. 2017;14:234. doi: 10.1186/s12974-017-1007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Neves GA, Grace AA. α7 nicotinic receptor full agonist reverse basolateral amygdala hyperactivity and attenuation of dopaminergic neuron activity in rats exposed to chronic mild stress. Eur Neuropsychopharmacol. 2019;29:1343–1353. doi: 10.1016/j.euroneuro.2019.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Chang K-W, Zong H-F, Wang M, Rizvi MY, Neha SI, Yang W-N, et al. PNU282987 alleviates Aβ-induced anxiety and depressive-like behaviors through upregulation of α7nAChR by ERK-serotonin receptors pathway. Neurosci Lett. 2020;731:135118. doi: 10.1016/j.neulet.2020.135118. [DOI] [PubMed] [Google Scholar]
- 187.Barik J, Wonnacott S. Indirect modulation by α7 nicotinic acetylcholine receptors of noradrenaline release in rat hippocampal slices: interaction with glutamate and GABA systems and effect of nicotine withdrawal. Mol Pharmacol. 2006;69:618–628. doi: 10.1124/mol.105.018184. [DOI] [PubMed] [Google Scholar]
- 188.Livingstone PD, Srinivasan J, Kew JNC, Dawson LA, Gotti C, Moretti M, et al. alpha7 and non-alpha7 nicotinic acetylcholine receptors modulate dopamine release in vitro and in vivo in the rat prefrontal cortex. Eur J Neurosci. 2009;29:539–550. doi: 10.1111/j.1460-9568.2009.06613.x. [DOI] [PubMed] [Google Scholar]
- 189.Andreasen JT, Redrobe JP, Nielsen EØ. Combined α7 nicotinic acetylcholine receptor agonism and partial serotonin transporter inhibition produce antidepressant-like effects in the mouse forced swim and tail suspension tests: a comparison of SSR180711 and PNU-282987. Pharmacol Biochem Behav. 2012;100:624–629. doi: 10.1016/j.pbb.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 190.Williams DK, Wang J, Papke RL. Positive allosteric modulators as an approach to nicotinic acetylcholine receptor-targeted therapeutics: advantages and limitations. Biochem Pharmacol. 2011;82:915–930. doi: 10.1016/j.bcp.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Sun L, Yang T, Wei N, Lu W, Jiao W, Zhou Q, et al. Pharmacological characterization of JWX-A0108 as a novel type I positive allosteric modulator of α7 nAChR that can reverse acoustic gating deficits in a mouse prepulse inhibition model. Acta Pharmacol Sin. 2019;40:737–745. doi: 10.1038/s41401-018-0163-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Alzarea S, Rahman S. Effects of alpha-7 nicotinic allosteric modulator PNU 120596 on depressive-like behavior after lipopolysaccharide administration in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2018;86:218–228. doi: 10.1016/j.pnpbp.2018.05.018. [DOI] [PubMed] [Google Scholar]
- 193.Alzarea S, Rahman S. Alpha-7 nicotinic receptor allosteric modulator PNU120596 prevents lipopolysaccharide-induced anxiety, cognitive deficit and depression-like behaviors in mice. Behav Brain Res. 2019;366:19–28. doi: 10.1016/j.bbr.2019.03.019. [DOI] [PubMed] [Google Scholar]
- 194.Targowska-Duda KM, Feuerbach D, Biala G, Jozwiak K, Arias HR. Antidepressant activity in mice elicited by 3-furan-2-yl-N-p-tolyl-acrylamide, a positive allosteric modulator of the α7 nicotinic acetylcholine receptor. Neurosci Lett. 2014;569:126–130. doi: 10.1016/j.neulet.2014.03.067. [DOI] [PubMed] [Google Scholar]
- 195.Bertrand D, Lee C-HL, Flood D, Marger F, Donnelly-Roberts D. Therapeutic potential of α7 nicotinic acetylcholine receptors. Pharmacol Rev. 2015;67:1025–1073. doi: 10.1124/pr.113.008581. [DOI] [PubMed] [Google Scholar]
- 196.Yang T, Xiao T, Sun Q, Wang K. The current agonists and positive allosteric modulators of α7 nAChR for CNS indications in clinical trials. Acta Pharm Sin B. 2017;7:611–622. doi: 10.1016/j.apsb.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Jiang Y, Li L, Tan X, Liu B, Zhang Y, Li C. miR-210 mediates vagus nerve stimulation-induced antioxidant stress and anti-apoptosis reactions following cerebral ischemia/reperfusion injury in rats. J Neurochem. 2015;134:173–181. doi: 10.1111/jnc.13097. [DOI] [PubMed] [Google Scholar]
- 198.Wang J-Y, Zhang Y, Chen Y, Wang Y, Li S-Y, Wang Y-F, et al. Mechanisms underlying antidepressant effect of transcutaneous auricular vagus nerve stimulation on CUMS model rats based on hippocampal α7nAchR/NF-κB signal pathway. J Neuroinflamm. 2021;18:291. doi: 10.1186/s12974-021-02341-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Mitchell S, Vargas J, Hoffmann A. Signaling via the NFκB system. WIREs Mech Dis. 2016;8:227–241. doi: 10.1002/wsbm.1331. [DOI] [Google Scholar]
- 200.Zhao YX, He W, Jing XH, Liu JL, Rong PJ, Ben H, et al. Transcutaneous auricular vagus nerve stimulation protects endotoxemic rat from lipopolysaccharide-induced inflammation. Evid Based Complement Alternat Med. 2012;2012:627023. doi: 10.1155/2012/627023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Deng Y, Guo SL, Wei B, Gao XC, Zhou YC, Li JQ. Activation of nicotinic acetylcholine α7 receptor attenuates progression of monocrotaline-induced pulmonary hypertension in rats by downregulating the NLRP3 inflammasome. Front Pharmacol. 2019;10:128. doi: 10.3389/fphar.2019.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Fu H, Shen QR, Zhao Y, et al. Activating α7nAChR ameliorates abdominal aortic aneurysm through inhibiting pyroptosis mediated by NLRP3 inflammasome. Acta Pharmacol Sin. 2022;43(10):2585–2595. doi: 10.1038/s41401-022-00876-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Yang N-N, Yang J-W, Ye Y, Huang J, Wang L, Wang Y, et al. Electroacupuncture ameliorates intestinal inflammation by activating α7nAChR-mediated JAK2/STAT3 signaling pathway in postoperative ileus. Theranostics. 2021;11:4078–4089. doi: 10.7150/thno.52574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Pohanka M. Alpha7 nicotinic acetylcholine receptor is a target in pharmacology and toxicology. Int J Mol Sci. 2012;13:2219–2238. doi: 10.3390/ijms13022219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Kox M, van Velzen JF, Pompe JC, Hoedemaekers CW, van der Hoeven JG, Pickkers P. GTS-21 inhibits pro-inflammatory cytokine release independent of the Toll-like receptor stimulated via a transcriptional mechanism involving JAK2 activation. Biochem Pharmacol. 2009;78:863–872. doi: 10.1016/j.bcp.2009.06.096. [DOI] [PubMed] [Google Scholar]
- 206.Decker ER, Dani JA. Calcium permeability of the nicotinic acetylcholine receptor: the single-channel calcium influx is significant. J Neurosci. 1990;10:3413–3420. doi: 10.1523/JNEUROSCI.10-10-03413.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Suzuki T, Hide I, Matsubara A, Hama C, Harada K, Miyano K, et al. Microglial α7 nicotinic acetylcholine receptors drive a phospholipase C/IP3 pathway and modulate the cell activation toward a neuroprotective role. J Neurosci Res. 2006;83:1461–1470. doi: 10.1002/jnr.20850. [DOI] [PubMed] [Google Scholar]
- 208.Brea D, Agulla J, Rodríguez-Yáñez M, Barral D, Ramos-Cabrer P, Campos F, et al. Regulatory T cells modulate inflammation and reduce infarct volume in experimental brain ischaemia. J Cell Mol Med. 2014;18:1571–1579. doi: 10.1111/jcmm.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Li P, Gan Y, Sun B-L, Zhang F, Lu B, Gao Y, et al. Adoptive regulatory T-cell therapy protects against cerebral ischemia. Ann Neurol. 2013;74:458–471. doi: 10.1002/ana.23815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Zhou X, Yuan L, Zhao X, Hou C, Ma W, Yu H, et al. Genistein antagonizes inflammatory damage induced by β-amyloid peptide in microglia through TLR4 and NF-κB. Nutrition. 2014;30:90–95. doi: 10.1016/j.nut.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 211.O’Neill LAJ. The role of MyD88-like adapters in Toll-like receptor signal transduction. Biochem Soc Trans. 2003;31:643–647. doi: 10.1042/bst0310643. [DOI] [PubMed] [Google Scholar]
- 212.Sutterwala FS, Haasken S, Cassel SL. Mechanism of NLRP3 inflammasome activation. Ann N Y Acad Sci. 2014;1319:82–95. doi: 10.1111/nyas.12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Cain DW, Cidlowski JA. Immune regulation by glucocorticoids. Nat Rev Immunol. 2017;17:233–247. doi: 10.1038/nri.2017.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Hou Y, Wang Q, Han B, Chen Y, Qiao X, Wang L. CD36 promotes NLRP3 inflammasome activation via the mtROS pathway in renal tubular epithelial cells of diabetic kidneys. Cell Death Dis. 2021;12:523. doi: 10.1038/s41419-021-03813-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Song J-Q, Jiang L-Y, Fu C-P, Wu X, Liu Z-L, Xie L, et al. Heterozygous SOD2 deletion deteriorated chronic intermittent hypoxia-induced lung inflammation and vascular remodeling through mtROS-NLRP3 signaling pathway. Acta Pharmacol Sin. 2020;41:1197–1207. doi: 10.1038/s41401-019-0349-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Li D-J, Fu H, Tong J, Li Y-H, Qu L-F, Wang P, et al. Cholinergic anti-inflammatory pathway inhibits neointimal hyperplasia by suppressing inflammation and oxidative stress. Redox Biol. 2018;15:22–33. doi: 10.1016/j.redox.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Chen Q, Giedt M, Tang L, Harrison DA. Tools and methods for studying the Drosophila JAK/STAT pathway. Methods. 2014;68:160–172. doi: 10.1016/j.ymeth.2014.03.023. [DOI] [PubMed] [Google Scholar]
- 218.Cai B, Cai J, Luo Y, Chen C, Zhang S. The specific roles of JAK/STAT signaling pathway in sepsis. Inflammation. 2015;38:1599–1608. doi: 10.1007/s10753-015-0135-z. [DOI] [PubMed] [Google Scholar]
- 219.Li BS, Ma W, Zhang L, Barker JL, Stenger DA, Pant HC. Activation of phosphatidylinositol-3 kinase (PI-3K) and extracellular regulated kinases (Erk1/2) is involved in muscarinic receptor-mediated DNA synthesis in neural progenitor cells. J Neurosci. 2001;21:1569–1579. doi: 10.1523/JNEUROSCI.21-05-01569.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Giese KP, Fedorov NB, Filipkowski RK, Silva AJ. Autophosphorylation at Thr286 of the alpha calcium-calmodulin kinase II in LTP and learning. Science. 1998;279:870–873. doi: 10.1126/science.279.5352.870. [DOI] [PubMed] [Google Scholar]
- 221.Tai Y, Feng S, Ge R, Du W, Zhang X, He Z, et al. TRPC6 channels promote dendritic growth via the CaMKIV-CREB pathway. J Cell Sci. 2008;121:2301–2307. doi: 10.1242/jcs.026906. [DOI] [PubMed] [Google Scholar]
- 222.Nagendran T, Hardy LR. Calcium/calmodulin-dependent protein kinase IV mediates distinct features of basal and activity-dependent dendrite complexity. Neuroscience. 2011;199:548–562. doi: 10.1016/j.neuroscience.2011.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Danciu TE, Adam RM, Naruse K, Freeman MR, Hauschka PV. Calcium regulates the PI3K-Akt pathway in stretched osteoblasts. FEBS Lett. 2003;536:193–197. doi: 10.1016/S0014-5793(03)00055-3. [DOI] [PubMed] [Google Scholar]
- 224.Conus NM, Hemmings BA, Pearson RB. Differential regulation by calcium reveals distinct signaling requirements for the activation of Akt and p70S6k. J Biol Chem. 1998;273:4776–4782. doi: 10.1074/jbc.273.8.4776. [DOI] [PubMed] [Google Scholar]
- 225.Apáti A, Jánossy J, Brózik A, Magócsi M. Effects of intracellular calcium on cell survival and the MAPK pathway in a human hormone-dependent leukemia cell line (TF-1) Ann N Y Acad Sci. 2003;1010:70–73. doi: 10.1196/annals.1299.010. [DOI] [PubMed] [Google Scholar]
- 226.Fries GR, Saldana VA, Finnstein J, Rein T. Molecular pathways of major depressive disorder converge on the synapse. Mol Psychiatry. 2022;28:1–14. doi: 10.1038/s41380-022-01806-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Wu H, Lu D, Jiang H, et al. Simvastatin-mediated upregulation of VEGF and BDNF, activation of the PI3K/Akt pathway, and increase of neurogenesis are associated with therapeutic improvement after traumatic brain injury. J Neurotrauma. 2008;25(2):130–139. doi: 10.1089/neu.2007.0369. [DOI] [PubMed] [Google Scholar]
- 228.Kato S, Ding J, Du K. Differential activation of CREB by Akt1 and Akt2. Biochem Biophys Res Commun. 2007;354:1061–1066. doi: 10.1016/j.bbrc.2007.01.094. [DOI] [PubMed] [Google Scholar]
- 229.West AE, Griffith EC, Greenberg ME. Regulation of transcription factors by neuronal activity. Nat Rev Neurosci. 2002;3:921–931. doi: 10.1038/nrn987. [DOI] [PubMed] [Google Scholar]
- 230.Martin M, Rehani K, Jope RS, Michalek SM. Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat Immunol. 2005;6:777–784. doi: 10.1038/ni1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Wang H, Brown J, Gu Z, Garcia CA, Liang R, Alard P, et al. Convergence of the mammalian target of rapamycin complex 1- and glycogen synthase kinase 3-β-signaling pathways regulates the innate inflammatory response. J Immunol. 2011;186:5217–5226. doi: 10.4049/jimmunol.1002513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Moriguchi S, Shinoda Y, Yamamoto Y, Sasaki Y, Miyajima K, Tagashira H, et al. Stimulation of the sigma-1 receptor by DHEA enhances synaptic efficacy and neurogenesis in the hippocampal dentate gyrus of olfactory bulbectomized mice. PLoS ONE. 2013;8:e60863. doi: 10.1371/journal.pone.0060863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Shioda N, Han F, Morioka M, Fukunaga K. Bis(1-oxy-2-pyridinethiolato)oxovanadium(IV) enhances neurogenesis via phosphatidylinositol 3-kinase/Akt and extracellular signal regulated kinase activation in the hippocampal subgranular zone after mouse focal cerebral ischemia. Neuroscience. 2008;155:876–887. doi: 10.1016/j.neuroscience.2008.05.056. [DOI] [PubMed] [Google Scholar]
- 234.Ma Q. Role of Nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol. 2013;53:401–426. doi: 10.1146/annurev-pharmtox-011112-140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Marsden WN. Synaptic plasticity in depression: molecular, cellular and functional correlates. Prog Neuropsychopharmacol Biol Psychiatry. 2013;43:168–184. doi: 10.1016/j.pnpbp.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 236.Nair S, Doh ST, Chan JY, Kong A-N, Cai L. Regulatory potential for concerted modulation of Nrf2- and Nfkb1-mediated gene expression in inflammation and carcinogenesis. Br J Cancer. 2008;99:2070–2082. doi: 10.1038/sj.bjc.6604703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Koistinaho M, Koistinaho J. Role of p38 and p44/42 mitogen-activated protein kinases in microglia. Glia. 2002;40:175–183. doi: 10.1002/glia.10151. [DOI] [PubMed] [Google Scholar]
- 238.Youssef M, Ibrahim A, Akashi K, Hossain MS. PUFA-plasmalogens attenuate the LPS-induced nitric oxide production by inhibiting the NF-kB, p38 MAPK and JNK pathways in microglial cells. Neuroscience. 2019;397:18–30. doi: 10.1016/j.neuroscience.2018.11.030. [DOI] [PubMed] [Google Scholar]
- 239.Lee YB, Schrader JW, Kim SU. p38 map kinase regulates TNF-alpha production in human astrocytes and microglia by multiple mechanisms. Cytokine. 2000;12:874–880. doi: 10.1006/cyto.2000.0688. [DOI] [PubMed] [Google Scholar]
- 240.You P, Fu S, Yu K, Xia Y, Wu H, Yang Y, et al. Scutellarin suppresses neuroinflammation via the inhibition of the AKT/NF-κB and p38/JNK pathway in LPS-induced BV-2 microglial cells. Naunyn-Schmiedeberg’s Arch Pharmacol. 2018;391:743–751. doi: 10.1007/s00210-018-1503-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.