Abstract
Prenatal intrauterine exposures and postnatal caregiving environments may both shape the development of infant parasympathetic nervous system (PNS) activity. However, the relative contributions of prenatal and postnatal influences on infant respiratory sinus arrhythmia (RSA)–an index of PNS functioning–are relatively unknown. We examined whether prenatal and postnatal maternal emotion dysregulation, a transdiagnostic construct that spans mental health diagnoses, were independently related to infant RSA trajectories during a social stressor, the still-face paradigm. Our sample included 104 mothers and their 7-month-old infants. Maternal emotion dysregulation was measured with the Difficulties in Emotion Regulation Scale during the 3rd trimester of pregnancy and again at a 7-month postpartum laboratory visit. Infant RSA was recorded during the still-face paradigm. Only postnatal maternal emotion dysregulation was associated with infant RSA. Specifically, high postnatal emotion dysregulation was associated with a blunted (i.e., dampened reactivity and recovery) infant RSA response profile. Infant sex did not moderate the associations between maternal emotion dysregulation and infant RSA. Findings suggest that postnatal interventions to promote effective maternal emotion regulation may reduce risk for infants’ dysregulated psychophysiological stress responses.
Keywords: Emotion dysregulation, Prenatal, Postnatal, Infant RSA, Still-face paradigm
Introduction
Maternal distress during prenatal and early postnatal life has downstream effects on newborn and infant outcomes (O’Donnell et al., 2014; Van den Bergh et al., 2018). One hypothesized mechanism underlying this pathway is infants’ altered physiological stress reactivity and regulation (Davis et al., 2011). Relative to other stress response pathways (e.g., the hypothalamic–pituitary–adrenal axis; Davis et al., 2011), the parasympathetic nervous system (PNS) has received less empirical attention in infants. Respiratory sinus arrhythmia (RSA) is one marker of PNS functioning (Porges, 2007) that has been identified as a potential precipitant to mental health concerns (Beauchaine, 2001; Wagner & Waller, 2020). Understanding effects of maternal prenatal and postnatal distress on infant RSA may inform early interventions to reduce children’s risk for psychopathology. The goal of the present study was to examine the associations between maternal prenatal and postnatal distress and infant RSA at 7 months of age.
Emotion Dysregulation as a Marker of Maternal Distress
There is limited consensus in the literature regarding measurement of maternal distress, which poses a challenge for research examining associations between maternal distress and infant RSA during the perinatal period. Maternal distress is a complex construct that encompasses psychological, physiological, and behavioral components (Dunkel Schetter, 2011; Glover, 2011). Previous research either focused on one domain of maternal distress during the perinatal period (e.g., maternal mental health symptoms, number of stressful life events) or adopted a cumulative risk approach by tallying the number of risk factors (Bush et al., 2017; Gray et al., 2017; Suurland et al., 2018). In the current study, we focused on maternal emotion dysregulation, a transdiagnostic marker that spans mental health diagnoses (Beauchaine & Cicchetti, 2019), as an indicator of maternal distress.
Emotion dysregulation is defined as “a pattern of emotional experience and/or expression that interferes with appropriate goal-directed behavior” (Beauchaine, 2015, p. 876). It has been increasingly recognized as a transdiagnostic vulnerability that underlies many psychiatric diagnoses (e.g., anxiety, depression) and health-risk behaviors (e.g., suicide risk; Beauchaine & Cicchetti, 2019; Cole et al., 2017). Mothers with high emotion dysregulation tend to show less sensitive parenting, and their children display less optimal developmental outcomes (Leerkes et al., 2020; Ostlund et al., 2019). Improvements in maternal emotional regulation following intervention are associated with better developmental outcomes in children (e.g., Zimmer-Gembeck et al., 2019). This research on early to middle childhood (cf. Leerkes et al., 2020; Ostlund et al., 2019) provides an empirical basis for hypothesizing relations between maternal emotion dysregulation and infant outcomes.
Measuring Intraindividual Patterns of Change in Respiratory Sinus Arrhythmia
Another challenge for examining associations between maternal distress during the perinatal period and infant PNS functioning is a limited consensus regarding how to use infant RSA activity as an indicator of PNS functioning. RSA measures variability in heart rate across the respiratory cycle (i.e., the high frequency spectrum). Baseline RSA and RSA responses (which include task reactivity, or changes in RSA values from baseline to stress exposure, as well as task recovery, or changes in RSA from stress exposure to removal of a stressor) are two commonly reported measures. Baseline RSA typically is measured as the average RSA value during minimal or neutral external stress. According to polyvagal theory (Porges, 2007), high baseline RSA reflects an individual’s ability to maintain homeostasis under normal circumstances, which promotes adaptive social interactions and is considered a marker of the individual’s potential capacity for emotion regulation (Beauchaine, 2001). In infancy, high baseline RSA is generally considered adaptive and associated with greater emotional and behavioral flexibility to external events (Beauchaine, 2001). Children with high baseline RSA in infancy are found to have more optimal developmental outcomes in childhood, such as fewer oppositional defiant behaviors and more cognitive control (Feldman et al., 2014; Wagner & Waller, 2020). Conversely, low baseline RSA may reflect vulnerability to stressors and has been associated with risk for childhood internalizing and externalizing behavior problems (Skowron et al., 2014).
RSA responses, on the other hand, represent individuals’ efforts to cope and self-regulate following discrete stressors or emotionally evocative events (El-Sheikh et al., 2009; Porges, 2007). In the absence of strong sympathetic nervous system influence, decreases in RSA correspond to higher heart rate, which signifies shifting attentional and behavioral resources in response to threat or challenge; increases in RSA typically correspond to lower heart rate, which facilitates maintenance of the body’s homeostasis (Brooker & Buss, 2010; Porges, 2007). Smaller decreases in RSA from baseline to experimental challenges (i.e., less RSA reactivity) are related to more adjustment and cognitive problems, although results differ across samples (see Graziano & Derefinko, 2013 for a review). Healthy RSA responses also include returning to pre-stress, baseline levels after the stressor is removed (i.e., RSA recovery). Studies with young infants have found that a lack of RSA recovery to a social stressor is associated with low levels of attention, engagement, and positive affect, which may suggest poor emotion regulation capacity (Conradt & Ablow, 2010; Moore & Calkins, 2004; Suurland et al., 2018).
Although RSA is considered an indicator of physiological regulation that is responsive to chronic or acute challenges, RSA responses are typically measured with static approaches, such as change scores (i.e., subtracting the averaged RSA values across a challenging task from baseline RSA) or residualized change scores (i.e., regressing the averaged RSA values during a challenging task on baseline RSA). These traditional approaches to measuring RSA responses capture only interindividual differences and not intraindividual temporal changes that correspond with changes in task demands and emotions (Brooker & Buss, 2010). Individuals’ RSA levels can change on much smaller time scales (i.e., changes can occur within an episode), and thus task differences or across-episode aggregates may fail to detect meaningful differences among individuals (Brooker & Buss, 2010; Butler et al., 2006). Indeed, one meta-analysis showed that young children with a profile of initial RSA decreases and subsequent RSA increases during social interaction tasks were at lower risk for psychopathological symptoms (Shahrestani et al., 2014). Therefore, modeling intraindividual RSA reactivity and recovery patterns during a stressful task appear to be necessary for thoroughly understanding infants’ physiological regulation.
One widely used laboratory task to elicit and measure infant RSA activity is the Still-Face Paradigm (SFP; Haley & Stansbury, 2003; Tronick et al., 1978). During the SFP, mothers first play with their infants freely (i.e., Play episode). Then, mothers are instructed to stop interacting with their infants and maintain neutral expressions (i.e., Still-Face [SF] episode). This episode has been shown to elicit significant behavioral and physiological stress responses among infants (e.g., Moore & Calkins, 2004). Lastly, mothers are instructed to resume interactions with their infants (i.e., Reunion episode), providing an opportunity for infants to recover from distress. Typically, infants exhibit decreases in RSA during the SF episode and increases during the Reunion episode, although average RSA levels often do not return to levels observed during the Play episode (Conradt & Ablow, 2010; Jones-Mason et al., 2018; Moore & Calkins, 2004; Moore et al., 2009).
Unique Contributions of Prenatal and Postnatal Influences
Infant PNS development is thought to be shaped by both prenatal intrauterine influences and postnatal caregiving environments (Del Giudice et al., 2011). Although many studies suggest that associations between prenatal maternal distress and child behavioral outcomes are maintained even when controlling for postnatal influences (e.g., anxiety and depression; Davis et al., 2007; Kataja et al., 2019; O’Donnell et al., 2014), little is known about whether prenatal maternal emotion dysregulation exerts a unique effect on infant PNS functioning. Theoretically, prenatal distress may play a more influential role than postnatal distress on infants’ baseline RSA levels (i.e., the “starting point” of infant PNS). Specifically, prenatal exposures may exert programming effects on the developing fetal brain and autonomic nervous system (ANS) that shape later ANS activity (Wadhwa et al., 2009). Indeed, at least two studies have found that high levels of maternal prenatal psychopathology predicted low baseline RSA levels in newborns (Field et al., 2006; Propper, 2012).
Although infant baseline RSA also could be calibrated by postnatal experiences, this calibration takes time and may be indirect through infants’ RSA responses to stressful events. In other words, infant RSA responses to stressful events, compared to infant baseline RSA, may be more influenced by postnatal maternal distress. Infants’ physiological responses to environmental events are largely dyadic in nature. Infants rely on their parents and other caregivers to garner information about appropriate reactions to the immediate context by gaze or orienting toward the caregiver (Tronick & Beeghly, 2011). They also receive direct assistance from caregivers to regulate their physiological arousal (Haley & Stansbury, 2003). Elevated feelings of distress in caregivers may compromise their abilities to sensitively respond to the infant’s needs (Moore & Calkins, 2004). Indeed, maternal distress has been associated with compromised infant neurophysiological regulatory abilities during first years of life, as evidenced by smaller RSA changes to stressors and slower RSA recovery after removal of the stressors (Ostlund et al., 2017; Rash et al., 2016).
Sex differences.
Associations between prenatal maternal distress and child outcomes may be sex dependent. Sex differences in child outcomes following exposure to prenatal risks have received accumulating empirical support (Gray et al., 2017; Sandman et al., 2013; Van den Bergh et al., 2018). However, whether female or male offspring are more susceptible to prenatal exposure to maternal distress is not yet clear in the literature. For instance, some studies found that prenatal exposures to adverse intrauterine environments (Spinillo et al., 2009) and problematic maternal health behaviors (Willoughby et al., 2007) posed greater risk for male versus female infants’ neurodevelopment in the first two years of life. Conversely, other studies have found that prenatal maternal distress was related to more compromised childhood mental health outcomes in females versus males (O’Donnell et al., 2014; Sandman et al., 2013). One possible direction to advancing clarity of this disparate literature is to examine the biological mechanisms underpinning these sex-dependent effects (Van den Bergh et al., 2020). It could be that although males and females are at similar risk when exposed to prenatal stress, the biological pathways leading to later developmental problems may be different. For example, in older children, higher baseline RSA or heightened RSA reactivity has been found to associate with less aggression in males, but more aggression in females (Morales et al., 2015). The in utero risk exposure may have “programmed” male and female fetuses’ PNS in opposite directions, which subsequently lead to different outcomes.
Only one study to our knowledge, however, has examined sex differences in the association between prenatal maternal stress and infant RSA. Gray and colleagues (2017) reported that high levels of prenatal stress were associated with high overall (including baseline) RSA levels for boys. For girls, high levels of prenatal stress were associated with low overall RSA levels, which were generally thought to reflect less optimal PNS functioning. Although Gray et al. (2017) focused on perceptions of stress rather than maternal emotion dysregulation, their results suggest that prenatal exposure to maternal distress may be more strongly associated with lower baseline RSA levels among girls than boys.
The Present study
The goal of the present study was twofold. First, we examined the unique contributions of maternal prenatal and postnatal emotion dysregulation on infant RSA activity at 7 months postpartum (Aim 1). We hypothesized that maternal prenatal emotion dysregulation would be negatively associated with infant RSA levels during a baseline task and the play episode of the SFP and not associated with infant RSA reactivity and recovery to the SFP (H1a). Conversely, we hypothesized that maternal postnatal emotion dysregulation would be related to infant RSA reactivity and recovery patterns, but not baseline RSA levels or RSA during the play episode. Specifically, we expected that infants of mothers with high levels of postnatal emotion dysregulation would exhibit a blunted pattern of RSA reactivity and/or recovery. That is, these infants would show decreases in RSA during the SF episode followed by increases in RSA during the reunion episode (H1b).
Second, we tested whether the associations between maternal prenatal and postnatal emotion dysregulation and infant RSA outcomes differed by infant sex (Aim 2). We hypothesized that there would be sex-specific effects of prenatal and postnatal emotion dysregulation on infant RSA, although no specific sex differences were hypothesized given mixed findings in the literature (H2). All hypotheses were pre-registered on the Open Science Framework (https://osf.io/zpk3j).
Method
Participants
Mother-infant dyads were drawn from a prospective longitudinal study that spanned the 3rd trimester of pregnancy to 18 months postpartum. Pregnant women were recruited during prenatal care appointments at OB/GYN clinics affiliated with University of Utah via flyers, brochures, and social media posts. Women interested in participating first completed the Difficulties in Emotion Regulation Scale (DERS; Gratz & Roemer, 2004) and answered questions related to eligibility criteria (i.e., ages 18–40, ≥ 25 weeks gestation, no pregnancy complications, no substance use during pregnancy, anticipated singleton delivery, and planned delivery at a participating hospital). We intentionally oversampled women with high and low DERS scores in order to achieve a uniform distribution of emotion dysregulation in our sample. See (Lin et al., 2019) for additional information on recruitment.
A total of 162 pregnant women participated prenatally. At 7-months postpartum, 135 mothers provided data. Mothers either returned with their infants to the laboratory and completed questionnaires (N = 114) or completed questionnaires online only (N = 21). There were no systematic differences between mother-infant dyads who provided data at 7 months and those who dropped out from the study. Of the 114 infants who participated the laboratory visit with their mothers, RSA data were not available for 10 infants due to hardware problems or because infants were too distressed to complete the study. Therefore, the final sample included 104 infants and their mothers. Comparing our final sample (N = 104) and the excluded sample due to unavailable RSA data (N = 29), we found no significant differences in the following variables: infant age and sex, maternal education, maternal identity as a woman of color, and emotional dysregulation at prenatal and 7-month visits. However, mothers in the final sample reported higher household income than those who were excluded from final analyses, t(111) = 2.08, p = 0.040. Demographic information of the final sample is presented in Table 1.
Table 1.
Demographic Information
| n (%) | Mean (SD) | |
|---|---|---|
| Infant characteristics (at seven-month visit) | ||
| Age (days) | 6.5 months (0.8) | |
| Sex (male) | 54 (51.9) | |
| Race and Ethnicity | ||
| White, Hispanic/Latinx | 28 (26.9) | |
| White, non-Hispanic/Latinx | 49 (47.1) | |
| Asian | 3 (2.9) | |
| Hawaiian or Pacific Islander | 1 (1.0) | |
| Multiracial | 23 (22.1) | |
| Maternal Characteristics (at prenatal visit) | ||
| Age (years) | 28.94 years (4.69) | |
| Income | ||
| Less than $19,999 | 12 (11.7) | |
| $20,000 – $29,999 | 6 (5.8) | |
| $30,000 – $39,999 | 10 (9.6) | |
| $40,000– $49,999 | 6 (5.8) | |
| $50,000 – $79,999 | 35 (33.7) | |
| $80,000 – $99,999 | 11 (10.6) | |
| $100,000 and greater | 15 (14.4) | |
| Education | ||
| Less than 12th grade | 1 (1.0) | |
| High school graduate or equivalent | 14 (13.5) | |
| Junior college graduate, or some college, or technical school | 31 (29.8) | |
| College graduate | 36 (34.6) | |
| Any post graduate school | 21 (20.2) | |
| Race and Ethnicity | ||
| White, Hispanic/Latina | 29 (27.9) | |
| White, non-Hispanic/Latina | 53 (51.0) | |
| Asian | 11 (10.6) | |
| American Indian or Alaskan Native | 2 (2.0) | |
| Hawaiian or Pacific Islander | 1 (1.0) | |
| Multiracial | 8 (7.7) |
Due to missing data, the numbers of sample size in the second column do not consistently add to 104 (the full sample)
Procedure
During prenatal and 7-month assessments, mothers completed online questionnaires about themselves and their infants. At 7 months, mother-infant dyads also participated in several behavioral tasks in the laboratory during which their physiological data were collected. Experimenters attached heart rate and respiration monitoring equipment to both mothers and infants. Dyads then watched a 2-min Baby Einstein video while infants sat in their mothers’ laps. This baseline physiology assessment was used to examine infants’ RSA while in a neutral state (Conradt et al., 2013). Following baseline, infants were placed in a high chair and experimenters introduced the SFP (Haley & Stansbury, 2003; Tronick et al., 1978). Mothers were instructed to play with their infants as they typically would for 2 min (i.e., Play). Then, mothers were asked to turn their faces away for a moment and then turn back to face their infants with a neutral expression for 2 min (i.e., Still-Face). Mothers were then instructed to look away again for a moment and then return to interact with their infants in whatever manner they would like while remaining seated for another 2 min (i.e., Reunion). The procedure was stopped if the infant became too distressed or if the mother requested to end the procedure (n = 5 infants). Participants provided written informed consent before each portion of the study and were compensated up to $80 for prenatal participation and $75 for 7-month participation. All study procedures were approved by the Institutional Review Board at University of Utah.
Measures
Maternal emotion dysregulation.
The Difficulties in Emotion Regulation Scale (DERS; Gratz & Roemer, 2004) was used to measure maternal emotion dysregulation during the prenatal visit and at 7 months postpartum. At both time points, mothers rated the extent to which each of the 36 items applied to them using a 1–5 Likert scale (1 – almost never, 5 – almost always). Sample items included “When I’m upset, I have difficulty getting work done” and “When I’m upset, I believe there is nothing I can do to feel better.” The DERS assesses six specific aspects of emotion dysregulation, including nonacceptance of emotional responses, difficulty with goal-directed behavior, impulse control difficulties, lack of emotional awareness, limited access to emotion regulation strategies, and lack of emotional clarity. Total scores were computed to indicate participants’ overall level of emotion dysregulation at prenatal and 7-month visits, with higher scores representing more emotion dysregulation. The DERS demonstrated strong internal consistency for the current sample at the prenatal visit (Cronbach’s α = 0.96) and the 7-month visit (Cronbach’s α = 0.96).
Infant respiratory sinus arrhythmia (RSA).
Electrocardiogram (ECG) data were collected from infants using a two-lead configuration with spot electrodes placed on the right clavicle and left ribcage (Qu et al., 1986) using MindWare mobile devices (MindWare Technologies Ltd., Gahanna, OH; Biolab software version 3.1). RSA was defined as the high frequency band of the power spectrum waveform (0.24 – 1.04 Hz for infants) and was scored in 30-s epochs by trained research assistants using MindWare’s heart rate variability analysis software. This software automatically identifies peaks of the R wave within each QRS complex (the graphical depiction of ventricle depolarization, i.e., a heartbeat) and records the interval between adjacent R peaks (i.e., inter-beat intervals). The software also calculates whether the inter-beat intervals are within an expected range for an inter-beat interval series, as well as whether they are within expected deviations considering surrounding data. Trained research assistants reviewed the identified R peaks and made corrections when necessary (i.e., misidentified R peak). A 30-s epoch of data was considered missing if: (1) there were ≥ 5 s of missing or unusable data within the epoch, or (2) RSA values fell outside of the expected range of 1–10. After the RSA data were computed, they were double-checked by a senior investigator (SEC) when necessary.
Baseline RSA levels were computed by averaging scores obtained during the 2-min video baseline. Because the SFP was 6 min long, twelve RSA scores (i.e., 30-s epochs) were available for each infant. Scores were averaged across two epochs to capture RSA levels at each minute of the SFP, resulting in six RSA values during the SFP for each participant. Our decision to combine the two neighboring epochs was made after consultation with physiological methodologists at a MindWare Technologies workshop. We were encouraged to examine RSA in 60-s epochs to reliably capture variability in heart rate based on respiratory cycles. Additionally, this is the same approach used by Gray et al. (2017). By following their analytic approach, we hoped to compare our results against the only study that had examined sex differences in the association between prenatal exposure and infant RSA.
Analytic Plan
Missing data.
Of the 162 women enrolled in the study at the prenatal time point, prenatal and postnatal DERS had less than 5% of missingness on the item-level; therefore, participants’ responses on the DERS were imputed via mean imputation. Minute-level infant RSA values had a missing rate ranging from 1% (i.e., the first minute of baseline and the first minute of Still-Face) to 10.6% (i.e., the second minute of Reunion). To account for missingness, full information maximum likelihood was used in all multilevel models.
Statistical models.
Two linear regression models were used separately to test the associations between: (a) maternal prenatal emotion dysregulation and infant baseline RSA at 7 months postpartum as well as (b) maternal emotion dysregulation at 7 months and infant baseline RSA. Household annual income at the prenatal time point was included as a covariate because of its significant correlation with infant RSA at the first minute of the Reunion episode (see OSF pre-registration for detailed criteria for including covariates). The standardized coefficients from these two models were compared to evaluate the relative contribution of prenatal and postnatal effects. We modeled prenatal and postnatal effects separately because of the strong correlation between prenatal and postnatal maternal emotion dysregulation (r = 0.784, p < 0.001).
Multilevel models were used to test associations between maternal prenatal emotion dysregulation and infant RSA during the SFP (i.e., RSA levels during Play, RSA reactivity during the Still-Face, and RSA recovery during the Reunion episodes). Level 1 of the multilevel model captured the intraindividual temporal RSA changes during the SFP. Time was modeled with linear and quadratic terms to represent the reactivity and recovery patterns of infant RSA across the SFP. Interindividual differences were captured on Level 2 of the multilevel models with group-mean centered prenatal emotion dysregulation and household income. Models were built in a sequential manner. First, we tested whether there were intraindividual changes across the SFP. Equation (1) presents the combined equation (no Level 2 predictor):
| (1) |
Then we included centered prenatal maternal emotion dysregulation in Level 2 to examine the main effect of prenatal emotion dysregulation on infant RSA activity during the SFP task (Eq. (2)):
| (2) |
Next, the 2-way interaction terms were included to examine the differences in RSA reactivity and recovery () by prenatal emotion dysregulation (Eq. (3)):
| (3) |
Lastly, we included the 3-way interaction terms examining sex differences (Eq. (4)):
| (4) |
Three corresponding models were tested with postnatal emotion dysregulation predicting infant RSA. Models 2.1, 2.2, and 2.3 were the same as models 1.1, 1.2, and 1.3, respectively, with the exception that prenatal emotion dysregulation was replaced by maternal emotion dysregulation at 7 months postpartum. These models also were examined in a sequential manner.
The PROC MIXED statement in SAS 9.4 was used to test the above multilevel models. For each model, we increased the model complexity by subsequently estimating (1) random intercept only, (2) random intercept and random and effects, and (3) random effects of all parameters. Model fit indices were compared across these models to identify the best fitting model. Models 1.1, 1.2, 2.1, and 2.2 were used to test Aim 1. Models 1.3 and 2.3 were used to test Aim 2 regarding potential sex differences.
Results
Descriptive statistics and bivariate correlations among key study variables are presented in Table 2. Neither prenatal nor postnatal maternal emotion dysregulation were significantly associated with infant RSA during any of the SFP episodes in bivariate tests. Male and female infants did not differ in RSA levels (all ps > 0.498). Paired sample t-test indicated that DERS decreased from prenatal (M = 78.8, SD = 26.2) to postnatal visits (M = 71.1, SD = 23.2), t (99) = 4.21, p < 0.001.
Table 2.
Mean, Standard Deviations, and Bivariate Correlations of the Study Variables
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1.Baseline RSA | – | |||||||||||
| 2. P1 RSA | 0.43*** | – | ||||||||||
| 3. P2 RSA | 0.54*** | 0.73*** | – | |||||||||
| 4. SF1 RSA | 0.50*** | 0.44*** | 0.58*** | – | ||||||||
| 5. SF2 RSA | 0.49*** | 0.44*** | 0.56*** | 0.67*** | – | |||||||
| 6. R1 RSA | 0.59*** | 0.58*** | 0.70*** | 0.61*** | 0.74*** | – | ||||||
| 7. R2RSA | 0.51*** | 0.58*** | 0.59*** | 0.50*** | 0.62*** | 0.72*** | – | |||||
| 8. DERS0 | −0.03 | −0.07 | 0.02 | 0.05 | 0.09 | −0.04 | −0.08 | – | ||||
| 9. DERS7 | 0.03 | −0.10 | −0.02 | 0.09 | 0.12 | 0.04 | −0.08 | 0.78*** | – | |||
| 10. Infant Sex | −0.01 | −0.06 | −0.04 | 0.05 | 0.07 | 0.00 | −0.04 | 0.09 | 0.09 | – | ||
| 11. Age at 7 months | 0.01 | −0.01 | 0.05 | 0.15 | 0.06 | 0.02 | 0.03 | 0.02 | 0.08 | 0.09 | – | |
| 12. Household Income | 0.05 | −0.02 | −0.15 | −0.14 | −0.22* | −0.23* | −0.07 | −0.12 | −0.03 | −0.07 | −0.04 | – |
| n (N = 104) | 103 | 104 | 104 | 103 | 95 | 99 | 93 | 102 | 102 | 104 | 102 | 103 |
| Mean (SD) | 3.57 (0.93) | 3.59 (1.13) | 3.62 (1.07) | 3.39 (1.15) | 3.16 (1.14) | 3.23 (1.32) | 3.50 (1.35) | 78.8 (26.2) | 71.1 (23.2) | – | 195.3 (22.7) | – |
Correlations are calculated with raw values
Infant sex is 0/1 coded (0 indicates male and 1 indicates female)
Descriptive information of infant sex and household income of this sample is presented in Table 1
P1/P2 first/second minute of Play episode, SF1/SF2 first/second minute of Still-Face episode, R1/R2 first/second minute of Reunion episode, DERS0 prenatal maternal emotion dysregulation, DERS7 postnatal maternal emotion dysregulation
p < 0.05
p < 0.001
Baseline RSA
In the multiple regression models, neither prenatal (β = −0.015, p = 0.886) nor postnatal (, p = 0.746) emotion dysregulation was significantly associated with infant baseline RSA levels, which did not support hypothesis H1a.
Sex differences.
Interaction terms involving infant sex were not significant (all ps > 0.105) in prenatal and postnatal models. These results suggested that associations between maternal emotion dysregulation and baseline RSA did not vary by infant sex, which was contrary to hypothesis H2.
RSA during the SFP
Within-subject variance accounted for most of the variance (intraclass correlation coefficient = 59.8%), which supported our decision to use multilevel models to capture interindividual differences in the intraindividual temporal changes in infant RSA. Results from Model 0, which included only infant RSA activity across the SFP, are presented in Table 3. Both linear (, SE = 0.08, p = 0.002) and quadratic (, SE = 0.02, p = 0.011) time terms were significant, indicating significant changes in RSA levels across the SFP. Specifically, on average, RSA levels decreased during the SF episode and then increased during the Reunion episode (Table 3), suggesting that infants exhibited the expected stress response to the SFP.
Table 3.
Fixed and Random Effects of Maternal Emotion Dysregulation on Infant Respiratory Sinus Arrhythmia Responses during the Still-Face Paradigm
| Model 0 | Prenatal Model (Model 1.2) |
Postnatal Model (Model 2.2) |
||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Fixed Effect | B (SE) | p value | B (SE) | p value | B (SE) | p value |
| Intercept | 4.06 (0.30) | <0.001 | 3.92 (0.32) | <0.001 | 4.10 (0.30) | <0.001 |
| Income | −0.05 (0.04) | 0.166 | −0.03 (0.04) | 0.393 | −0.05 (0.04) | 0.145 |
| Time | −0.25 (0.08) | 0.002 | −0.26 (0.08) | 0.001 | −0.26 (0.08) | 0.002 |
| 0.04 (0.02) | 0.010 | 0.04 (0.02) | 0.008 | 0.04 (0.02) | 0.008 | |
| DERS | – | – | −0.003 (0.004) | 0.446 | −0.006 (0.005) | 0.200 |
| Time x DERS | – | – | 0.006 (0.003) | 0.060 | 0.007 (0.003) | 0.032 |
| – | – | −0.001 (0.001) | 0.056 | −0.001 (0.001) | 0.042 | |
| Random Effect | Var/Cov (SE) | p value | Var/Cov (SE) | p value | Var/Cov (SE) | p value |
| Intercept | 0.93 (0.18) | <0.001 | 0.94 (0.18) | <0.001 | 0.91 (0.18) | <0.001 |
| Time | 0.32 (0.09) | <0.001 | 0.29 (0.09) | <0.001 | 0.30 (0.09) | <0.001 |
| 0.01 (0.003) | <0.001 | 0.01 (0.003) | <0.001 | 0.01 (0.003) | <0.001 | |
| (Intercept, Time) | −0.25 (0.11) | 0.020 | −0.24 (0.10) | 0.019 | −0.23 (0.10) | 0.030 |
| (Intercept, ) | 0.05 (0.02) | 0.012 | 0.05 (0.02) | 0.013 | 0.05 (0.02) | 0.020 |
| () | −0.06 (0.02) | 0.001 | −0.05 (0.02) | <0.001 | −0.05 (0.02) | 0.002 |
| Level 1 error | 0.42 (0.03) | <0.001 | 0.41 (0.04) | <0.001 | 0.42 (0.04) | <0.001 |
| Number of parameters | 11 | 14 | 14 | |||
| Goodness of fit | ||||||
| −2 Log Likelihood | 1550.2 | 1507.5 | 1517.0 | |||
Data in boldface indicate significant fixed effects
DERS Difficulties with Emotion Regulation Scale, SE Standard error, Var/Cov Variance/Covariance
Following the pre-registered analytic plan, we evaluated fit indices across models to identify the best-fitting model. Models 1.2 and 2.2 were selected as best-fitting models. These models included 2-way interaction terms (i.e., ) as fixed effects, as well as intercept and two 2-way interaction terms as random effects. Table 3 presents parameter estimates and variance components for these two models.
Prenatal emotion dysregulation.
Results from Model 1.2 indicated that maternal prenatal emotion dysregulation was not associated significantly with temporal change patterns in RSA or with RSA levels during the Play episode (see Table 3).
Postnatal emotion dysregulation.
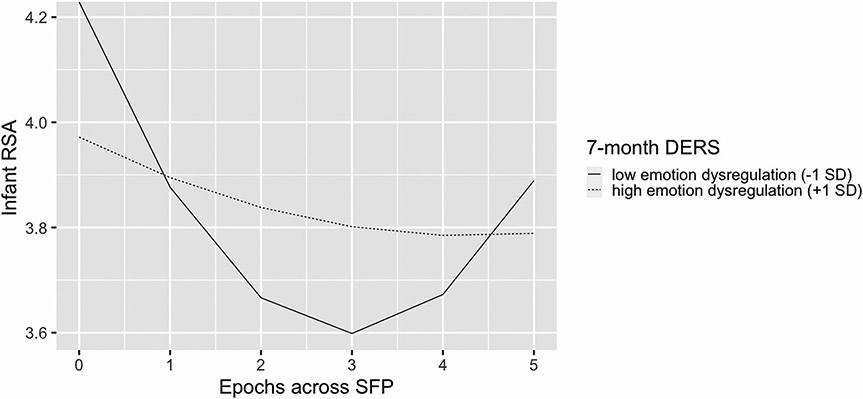
Results from Model 2.2 showed that maternal postnatal emotion dysregulation was not significantly related to RSA levels during the Play episode. In contrast, postnatal emotion dysregulation was associated with linear (, SE = 0.003, p = 0.032) and quadratic (, SE = 0.001, p = 0.042) changes in RSA during the SFP. Post-hoc analyses indicated that infants with less dysregulated mothers (as measured by one SD below the mean) demonstrated a significant decrease (, SE = 0.078, p < 0.001) followed by an increase (, SE = 0.015, p < 0.001) in RSA levels. In contrast, infants with highly dysregulated mothers (i.e., one SD above the mean) showed a flatter, more blunted pattern of RSA change across the SFP (Fig. 1). For infants of highly dysregulated mothers, their decreases (, SE = 0.078, p = 0.268) and subsequent increases in RSA (, SE = 0.015, p = 0.505) were not significant.
Fig. 1.
Respiratory sinus arrhythmia (RSA) changes across time for infants of mothers with different levels of postnatal emotion dysregulation. DERS = Difficulties in Emotion Regulation Scale; SD = standard deviation; SFP = Still-Face Paradigm. Epochs 0 and 1 are the Play episode of the SFP; Epochs 2 and 3 are the Still-Face of the SFP; Epochs 4 and 5 are the Reunion episode of the SFP
Sex differences
Model 1.3 did not fit the data significantly better than the more parsimonious Model 1.2, Δ −2 LL = 4.1, critical value χ2(Δdf = 6) = 12.59, p = 0.66. Similarly, Model 2.3 did not fit the data significantly better than the more parsimonious Model 2.2, Δ −2 LL = 4.6, critical value χ2(Δdf = 6) = 12.59, p = 0.60. Moreover, none of the interaction terms involving infant sex were significant in Model 1.3 or 2.3. Altogether, these results did not support our hypothesis (H2) that there would be sex differences in the associations between maternal emotion dysregulation and infants’ patterns of RSA during the SFP.
Discussion
Maternal emotion dysregulation is a useful construct for the field of maternal and infant mental health given its associations with maternal mental health symptoms, life stress, and parenting sensitivity as well as newborn and infant neurobehavioral outcomes (Leerkes et al., 2020; Lin et al., 2019; Ostlund et al., 2019). However, no study to our knowledge has examined associations between maternal emotion dysregulation and infant RSA, which may be an early-life marker of risk for psychopathology (Suurland et al., 2018; Wagner & Waller, 2020). To address this gap, we examined whether maternal prenatal and postnatal emotion dysregulation would uniquely predict infant baseline RSA levels and infant RSA responses to a stressful event. Overall, our results showed that maternal postnatal, but not prenatal, emotion dysregulation was related to a blunted (i.e., dampened reactivity and recovery) infant RSA pattern to a social stressor. No sex differences in these associations were observed.
Maternal Emotion Dysregulation and Infant Baseline RSA
We hypothesized that infant baseline RSA and RSA levels during non-stressful interpersonal interactions (i.e., play episode of the SFP) would be predicted by maternal prenatal emotion dysregulation. Contrary to this hypothesis, no significant associations were observed in the present study. Existing studies that reported the association between prenatal stress and infant PNS functioning have mostly focused on life stress, such as mothers’ adverse childhood experiences, perceived stress, stressful life events, and financial strain (e.g., Bush et al., 2017; Gray et al., 2017; Jones et al., 2019). Stress exposures during the prenatal period are theorized to become biologically embedded and “program” the fetus by altering its biophysiological structures and functions (Hamada & Matthews, 2019; Wadhwa et al., 2009). It is possible that maternal emotion dysregulation, the indicator of prenatal distress in the present study, may not have as strong of a “programming effect” as do other these other prenatal stressors.
Although emotion dysregulation underlies numerous mental health diagnoses and is associated with stressful life experiences (Beauchaine & Cicchetti, 2019; Lin et al., 2019), it is a broad construct that involves one’s ability to notice, accept, and cope with emotions. As such, emotion dysregulation is distinct from stress exposure. Many women who encounter high levels of stress may be well-equipped with skills and traits for coping with the feelings of distress, such as mindfulness (Braeken et al., 2017). Thus, it is likely that prenatal emotion dysregulation may interact with mothers’ coping strategies and social supports to shape infant PNS functioning.
Maternal Emotion Dysregulation and Infant RSA Responses to Stressful Events
Consistent with our hypothesis, maternal postnatal emotion dysregulation was related to dampened infant RSA reactivity and recovery over the course of the SFP. Infants of mothers with high levels of postnatal emotion dysregulation were not only less reactive to an attachment-related stressor (i.e., briefly losing the support of their attachment figure, the mother), they showed less recovery after the stressor was removed and dyadic interactions resumed. This pattern of dampened PNS activity was consistent with previous research documenting the influence of maternal mental health symptoms and low parenting sensitivity on infant RSA responses to stress (Moore et al., 2009; Ostlund et al., 2017). Less decreases in infant RSA levels (i.e., dampened PNS activity) in response to a stressor indicate lack of metabolic processes that are important for infants to cope with a challenging state. Moreover, dampened RSA recovery when the stressor is removed may prevent the infant from engaging in self-regulatory behaviors, which may have long-term negative impacts on infants’ development of regulatory skills. Additionally, infants with persistent dampened PNS activity, especially when mothers resume typical interactions, may be limited in their abilities to effectively use their mothers as sources of co-regulation (Busuito et al., 2019; Feldman et al., 2011). In the long run, dampened PNS responses to stressful events, especially when coupled with environmental adversities, may lead to elevated risk for psychopathology (Beauchaine & Cicchetti, 2019; El-Sheikh et al., 2009; Suurland et al., 2018). Granted, the lack of change in infant RSA during latter part of the SFP may not necessarily reflect less recovery in PNS activity for infants of highly dysregulated mothers. These infants also exhibited less RSA decreases during the Still Face episode SFP, which may have subsequently limited the amount of change in RSA levels that could be observed during reunion of mother–infant interactions.
The significant associations between postnatal maternal emotion dysregulation and infant RSA responses in our sample may suggest that infants’ postnatal experiences with caregivers play a critical role in shaping infants’ RSA responses to an attachment-related stressor. Mothers with high levels of emotion dysregulation may be physiologically dysregulated during stressful parenting tasks such that they may be less sensitive to distressed infants (Leerkes et al., 2020) and less in-sync with their infants’ nonverbal signals (Lotzin et al., 2015) than well-regulated mothers. As a result, dyadic mother–infant interactions may be less effective at providing infants with co-regulatory support upon reunion, indicated by less parasympathetic recovery. Repeated challenges with parasympathetic recovery may subsequently lead to attenuated PNS reactions to stressors, which is considered a less flexible stress response (Graziano & Derefinko, 2013).
Although the effects of prenatal emotion dysregulation on infant RSA responses were not significant, they were approaching significance level (see Model 1.2 results in Table 3). The results of the prenatal model paralleled those in the postnatal model in that infants of mothers with high prenatal emotion dysregulation also tended to show a pattern of blunted RSA responses. On one hand, this result is not surprising given the high correlation between prenatal and postnatal emotion dysregulation. However, it may also suggest that mothers’ emotion dysregulation levels during pregnancy influence infants’ physiological regulation. Future replication studies with a larger sample size may help clarify the unique effects of prenatal and/or postnatal emotion dysregulation on infant PNS functioning. A large sample size permits simultaneous inclusion of prenatal and postnatal emotion dysregulation in the same model to tease apart their relative contributions to infant RSA outcomes. Additionally, a larger sample would increase statistical power to detect small effect sizes that may have rendered some findings null in the present study.
In this study, maternal emotion dysregulation was used as a marker of maternal distress during the perinatal period and was measured by the DERS (Gratz & Roemer, 2004). Although the DERS was designed to measure dispositional tendencies by asking about typical emotion-related experiences, emotion dysregulation can vary from day to day (see e.g., Lavender et al., 2017) and change in response to significant life events, such as giving birth. In fact, we observed that mothers reported significantly less difficulties with emotion regulation from the third trimester to seven months postpartum. There are numerous neurobiological, psychological, and social factors that may have contributed to overall lower postnatal emotion dysregulation in our sample, and to our knowledge, few studies have examined changes in maternal emotion dysregulation across the perinatal period (c.f., Agako et al., 2021). We recommend researchers to examine how changes in maternal emotion dysregulation could influence young children’s early physiological and behavioral development.
Infant RSA responses to stress have been linked to infant attachment types that may have implications for adaptation and mental health across the life span (Groh & Narayan, 2019; Hill-Soderlund et al., 2008). Besides, infants are at higher risk for developing attachment disorganization when their mothers report greater emotion regulation difficulties (Leerkes et al., 2020). It is likely that infant RSA patterns may mediate the effect of maternal emotion dysregulation on the development of insecure attachments, an important question to be tested by future research.
No Observed Sex Differences
Evidence in the literature has been mixed regarding sex differences in the association between prenatal distress and child outcomes. The only study that has tested whether the effect of prenatal distress on infant PNS activity differed by infant sex reported a “sex-differentiated advantage” conferred to boys (Gray et al., 2017, p. 926). That is, high prenatal stress was related to high baseline RSA and high RSA levels across the course of the SFP (i.e., a heightened responsiveness for potential stressors) for boys, whereas an opposite pattern was found in girls. These findings were not observed in the present study. Given that the present study and that by Gray et al. (2017) are the only two that examined these sex differences, it would be remiss to draw conclusions from our results. Continued investigations of sex-differentiated effects of maternal distress during the perinatal period, particularly the effects on infant RSA, and their etiologies are greatly needed. Understanding whether male and female infants’ RSA are differentially influenced by exposure to maternal distress can advance our understanding of the ontogeny of mental and physical health problems. For instance, depression and cardiovascular disease are two RSA-related outcomes that exhibit sex differences in adults (Nolen-Hoeksema, 2001; Thayer & Lane, 2007).
Strengths, Limitations, and Future Directions
Our study has a number of strengths, including the longitudinal design; a sample of pregnant women with uniformly distributed emotion dysregulation scores; the examination of infant baseline, reactivity and recovery RSA levels; and the use of pre-registration to facilitate open science. However, our findings should be interpreted in light of limitations and in consideration of future directions. First, maternal emotion dysregulation was self-reported by participants. Although the DERS converges with physiological measures of emotion dysregulation in our own sample (Lin et al., 2019) as well as in others (Leerkes et al., 2020), women’s self-reported emotion dysregulation may be affected by their pregnancy and postpartum experiences. Future studies should include both behavioral observations and physiological assessments to obtain a more comprehensive profile of maternal emotion dysregulation during the prenatal and early postnatal periods.
Second, maternal postnatal emotion dysregulation and infant RSA were assessed at the same general time point (i.e., at the 7-month visit), and thus the directionality of effects cannot be unambiguously discerned with the current data. Although we interpreted our finding as indicating that maternal emotion dysregulation influenced infant RSA responses, the opposite relation also is possible. For example, low infant baseline RSA has been posited to undermine mothers’ parenting self-efficacy and exacerbate maternal depressive symptoms (Somers et al., 2021), which could possibly lead to higher maternal emotion dysregulation. A task for future research will be to shed light on the processes underlying the potential bidirectional processes underlying the link between maternal emotion dysregulation and infant PNS activity. In fact, the current literature points to the possibility of a dynamic process co-created by the mother-infant dyad on behavioral and physiological levels (Provenzi et al., 2018).
Third, we chose to use RSA as our outcome of interest given that RSA may be a valid peripheral biomarker of emotion dysregulation and the relative paucity of research on this physiological outcome among infants. However, some scholars have proposed that multiple infant stress response systems (e.g., PNS, sympathetic nervous system, central nervous system) interact to mediate the pathway between prenatal exposure and offspring long-term health and well-being (e.g., Rash et al., 2016; Suurland et al., 2018). Therefore, it is important for future research to include multiple indicators of different stress response systems, such as preejection period, electrodermal activity, and cortisol, and examine how prenatal and postnatal exposures contribute to the complex multisystem activity. Lastly, although our sample included a sizable proportion of Hispanic/Latinx infants (26.9%), the largest subgroup of infants was white and non-Hispanic/Latinx (47.1%). It is unclear whether our findings could be generalized to other samples with different racial and ethnic identities.
Conclusions
Our study is the first to evaluate discrete associations between maternal emotion dysregulation during the prenatal and postnatal periods and infant RSA, a marker of physiological regulation that emerges within the first few months of life. We found that infants of mothers who reported high postnatal emotion dysregulation exhibited a pattern of dampened infant RSA responsivity. Given the heightened risk for psychopathology among children with dampened RSA responses (Graziano & Derefinko, 2013), our findings provide additional support for interventions that promote mothers’ emotion regulation skills. Taken together, implementing interventions on maternal emotion dysregulation, especially in the first year after birth when the infant PNS is rapidly developing (Porges & Furman, 2011), may be particularly fruitful for preventing later psychopathology.
Acknowledgements
We would like to thank all of the families who generously donated their time to participate in our study. We would also like to thank Mike Varner and Bob Silver for their support of the BABY study and for providing their dedicated OBGYN Research Network staff to help with screening and recruitment. We thank Connie Hammen for her assistance with training and scoring the UCLA Life Stress Interview. We would also like to thank the University of Utah Vice President’s Clinical Translational Research Scholars program for their mentorship and grantsmanship assistance. Last but not the least, we thank Celine Saenz and Sarah Terrell for their hard work on recruitment, coordination, and study management.
Funding
This study was funded by the National Institute of Mental Health R01MH119070 and R21MH109777 (to S.C. and E.C.) and grants from the University of Utah Consortium for Families and Health Research and Interdisciplinary Research Pilot Program.
Footnotes
Availability of Data and Material Part of the data (i.e., infant physiological measure) that supports the findings of the study will be available on the National Institute of Mental Health (NIMH) Data Archive (https://nda.nih.gov/edit_collection.html?id=3240). Other parts of the data are available from the corresponding author upon reasonable request.
Code Availability The code of all multilevel models is available on Open Science Framework (osf.io/j4ye2).
Ethics Approval All study procedures were approved by the Institutional Review Board at the University of Utah (approval number: 00081198 and 00090356). The procedures used in this study adhere to the tenets of the Declaration of Helsinki.
Consent to Participate Participants provided a written informed consent before each time point of the study.
Consent for Publication Participants signed informed consent regarding publishing their data.
Conflict of Interest The authors declare no conflict of interests, financial or otherwise.
References
- Agako A, Donegan E, McCabe RE, Frey BN, Streiner D, & Green S (2021). The role of emotion dysregulation in cognitive behavioural group therapy for perinatal anxiety: Results from a randomized controlled trial and routine clinical care. Journal of Affective Disorders, 292(April), 517–525. 10.1016/j.jad.2021.05.084 [DOI] [PubMed] [Google Scholar]
- Beauchaine TP (2001). Vagal tone, development, and Gray’s motivational theory: Toward an integrated model of autonomic nervous system functioning in psychopathology. Development and Psychopathology, 13, 183–214. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP (2015). Future directions in emotion dysregulation and youth psychopathology. Journal of Clinical Child & Adolescent Psychology, 44(5), 875–896. 10.1080/15374416.2015.1038827 [DOI] [PubMed] [Google Scholar]
- Beauchaine TP, & Cicchetti D (2019). Emotion dysregulation and emerging psychopathology: A transdiagnostic, transdisciplinary perspective. Development and Psychopathology, 31, 799–804. 10.1017/S0954579419000671 [DOI] [PubMed] [Google Scholar]
- Braeken MAKA, Jones A, Otte RA, Nyklíček I, & Van den Bergh BRH (2017). Potential benefits of mindfulness during pregnancy on maternal autonomic nervous system function and infant development. Psychophysiology, 54, 279–288. 10.1111/psyp.12782 [DOI] [PubMed] [Google Scholar]
- Brooker RJ, & Buss KA (2010). Dynamic measures of RSA predict distress and regulation in toddlers. Developmental Psychobiology, 52(4), 372–382. 10.1002/dev.20432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush NR, Jones-Mason K, Coccia M, Caron Z, Alkon A, Thomas M, Coleman-Phox K, Wadhwa PD, Laraia BA, Adler NE, & Epel ES (2017). Effects of pre- and postnatal maternal stress on infant temperament and autonomic nervous system reactivity and regulation in a diverse, low-income population. Development and Psychopathology, 29(5), 1553–1571. 10.1017/S0954579417001237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busuito A, Quigley KM, Moore GA, Voegtline KM, & DiPietro JA (2019). In sync: Physiological correlates of behavioral synchrony in infants and mothers. Developmental Psychology, 55(5), 1034–1045. 10.1037/dev0000689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler EA, Wilhelm FH, & Gross JJ (2006). Respiratory sinus arrhythmia, emotion, and emotion regulation during social interaction. Psychophysiology, 43(6), 612–622. 10.1111/j.1469-8986.2006.00467.x [DOI] [PubMed] [Google Scholar]
- Cole PM, Hall SE, & Hajal NJ (2017). Emotion dysregulation as a vulnerability to psychopathology. In Beauchaine TP & Hinshaw SP (Eds.), Child and Adolescent Psychopathology (3rd ed., p. 346). Hoboken, NJ: John Wiley and Sons. [Google Scholar]
- Conradt E, & Ablow J (2010). Infant physiological response to the still-face paradigm: Contributions of maternal sensitivity and infants’ early regulatory behavior. Infant Behavior and Development, 33(3), 251–265. 10.1016/j.infbeh.2010.01.001 [DOI] [PubMed] [Google Scholar]
- Conradt E, Measelle J, & Ablow JC (2013). Poverty, problem behavior, and promise: Differential susceptibility among infants reared in poverty. Psychological Science, 24(3), 235–242. 10.1177/0956797612457381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EP, Glynn LM, Schetter CD, Hobel C, Chicz-Demet A, & Sandman CA (2007). Prenatal exposure to maternal depression and cortisol influences infant temperament. Journal of the American Academy of Child and Adolescent Psychiatry, 46(6), 737–746. 10.1097/chi.0b013e318047b775 [DOI] [PubMed] [Google Scholar]
- Davis EP, Glynn LM, Waffarn F, & Sandman CA (2011). Prenatal maternal stress programs infant stress regulation. Journal of Child Psychology and Psychiatry and Allied Disciplines, 52(2), 119–129. 10.1111/j.1469-7610.2010.02314.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Giudice M, Ellis BJ, & Shirtcliff EA (2011). The Adaptive Calibration Model of stress responsivity. Neuroscience and Biobehavioral Reviews, 35(7), 1562–1592. 10.1016/j.neubiorev.2010.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkel Schetter C (2011). Psychological science on pregnancy: Stress processes, biopsychosocial models, and emerging research issues. Annual Review of Psychology, 62, 531–558. 10.1146/annurev.psych.031809.130727 [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Kouros CD, Erath S, Cummings EM, Keller P, & Staton L (2009). Marital conflict and children’s externalizing behavior: Interactions between parasympathetic and sympathetic nervous system activity. Monographs of the Society for Research in Child Development, 74, 1–101. http://www.jstor.com/stable/25580856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R, Magori-Cohen R, Galili G, Singer M, & Louzoun Y (2011). Mother and infant coordinate heart rhythms through episodes of interaction synchrony. Infant Behavior and Development, 34(4), 569–577. 10.1016/j.infbeh.2011.06.008 [DOI] [PubMed] [Google Scholar]
- Feldman R, Rosenthal Z, & Eidelman AI (2014). Maternal-preterm skin-to-skin contact enhances child physiologic organization and cognitive control across the first 10 years of life. Biological Psychiatry, 75(1), 56–64. 10.1016/.biopsych.2013.08.012 [DOI] [PubMed] [Google Scholar]
- Field T, Diego M, & Hernandez-Reif M (2006). Prenatal depression effects on the fetus and newborn: A review. Infant Behavior and Development, 29, 445–455. 10.1016/j.infbeh.2006.03.003 [DOI] [PubMed] [Google Scholar]
- Glover V (2011). Annual research review: Prenatal stress and the origins of psychopathology: An evolutionary perspective. Journal of Child Psychology and Psychiatry and Allied Disciplines, 52(4), 356–367. 10.1111/j.1469-7610.2011.02371.x [DOI] [PubMed] [Google Scholar]
- Gratz KL, & Roemer L (2004). Multidimensional assessment of emotion regulation and dysregulation: Development, factor structure, and initial validation of the Difficulties in Emotion Regulation Scale. Journal of Psychopathology and Behavioral Assessment, 26(1), 41–54. 10.1023/B:J0BA.0000007455.08539.94 [DOI] [Google Scholar]
- Gray SAO, Jones CW, Theall KP, Glackin E, & Drury SS (2017). Thinking across generations: Unique contributions of maternal early life and prenatal stress to infant physiology. Journal of the American Academy of Child and Adolescent Psychiatry, 56(11), 922–929. 10.1016/j.jaac.2017.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano P, & Derefinko K (2013). Cardiac vagal control and children’s adaptive functioning: A meta-analysis. Biological Psychology, 94(1), 22–37. 10.1016/j.biopsycho.2013.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groh AM, & Narayan AJ (2019). Infant attachment insecurity and baseline physiological activity and physiological reactivity to interpersonal stress: A meta-analytic review. Child Development, 90(3), 679–693. 10.1111/cdev.13205 [DOI] [PubMed] [Google Scholar]
- Haley DW, & Stansbury K (2003). Infant stress and parent responsiveness:behavior during still-face and reunion. Child Development, 74(5), 1534–1546. http://www.jstor.com/stable/3696192 [DOI] [PubMed] [Google Scholar]
- Hamada H, & Matthews SG (2019). Prenatal programming of stress responsiveness and behaviours: Progress and perspectives. Journal of Neuroendocrinology, 31(3). 10.1111/jne.12674 [DOI] [PubMed] [Google Scholar]
- Hill-Soderlund AL, Mills-Koonce WR, Propper C, Calkins SD, Granger DA, Moore GA, Gariepy JL, & Cox MJ (2008). Parasympathetic and sympathetic responses to the strange situation in infants and mothers from avoidant and securely attached dyads. Developmental Psychobiology, 50(4), 361–376. 10.1002/dev.20302 [DOI] [PubMed] [Google Scholar]
- Jones-Mason K, Alkon A, Coccia M, & Bush NR (2018). Autonomic nervous system functioning assessed during the still-face paradigm: A meta-analysis and systematic review of methods, approach and findings. Developmental Review, 50, 113–139. 10.1016/j.dr.2018.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CW, Esteves KC, Gray SAO, Clarke TN, Callerame K, Theall KP, & Drury SS (2019). The transgenerational transmission of maternal adverse childhood experiences (ACEs): Insights from placental aging and infant autonomic nervous system reactivity. Psychoneuroendocrinology, 106, 20–27. 10.1016/j.psyneuen.2019.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataja E, Karlsson L, Parsons CE, Pelto J, & Pesonen H (2019). Maternal pre- and postnatal anxiety symptoms and infant attention disengagement from emotional faces. Journal of Affective Disorders, 243, 280–289. 10.1016/j.jad.2018.09.064 [DOI] [PubMed] [Google Scholar]
- Lavender JM, Tull MT, DiLillo D, Messman-Moore T, & Gratz KL (2017). Development and validation of a state-based measure of emotion dysregulation: The State Difficulties in Emotion Regulation Scale (S-DERS). Assessment, 24(2), 197–209. 10.1177/1073191115601218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leerkes EM, Su J, & Sommers SA (2020). Mothers’ self-reported emotion dysregulation: A potentially valid method in the field of infant mental health. Infant Mental Health Journal, 41(5), 642–650. 10.1002/imhj.21873 [DOI] [PubMed] [Google Scholar]
- Lin B, Kaliush PR, Conradt E, Terrell S, Neff D, Allen AK, Smid MC, Monk C, & Crowell SE (2019). Intergenerational transmission of emotion dysregulation: Part I. Psychopathology, self-injury, and parasympathetic responsivity among pregnant women. Development and Psychopathology, 31(3), 817–831. 10.1017/S0954579419000336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotzin A, Romer G, Schiborr J, Noga B, Schulte-Markwort M, & Ramsauer B (2015). Gaze synchrony between mothers with mood disorders and their infants: Maternal emotion dysregulation matters. PLoS ONE, 10(12), 1–23. 10.1371/journal.pone.0144417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore GA, & Calkins SD (2004). Infants’ vagal regulation in the still-face paradigm is related to dyadic coordination of mother-infant interaction. Developmental Psychology, 40(6), 1068–1080. 10.1037/0012-1649.40.6.1068 [DOI] [PubMed] [Google Scholar]
- Moore GA, Calkins SD, Hill-Soderlund AL, Mills-Koonce WR, Propper CB, & Cox MJ (2009). Mother-infant vagal regulation in the face-to-face still-face paradigm is moderated by maternal sensitivity. Child Development, 80(1), 209–223. [DOI] [PubMed] [Google Scholar]
- Morales S, Beekman C, Blandon AY, Stifter CA, & Buss KA (2015). Longitudinal associations between temperament and socioemotional outcomes in young children: The moderating role of RSA and gender. Developmental Psychobiology, 57(1), 105–119. 10.1002/dev.21267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolen-Hoeksema S (2001). Gender differences in depression. Current Directions in Psychological Science, 10(5), 173–176. [Google Scholar]
- O’Donnell KJ, Glover V, Barker ED, & O’Connor TG (2014). The persisting effect of maternal mood in pregnancy on childhood psychopathology. Development and Psychopathology, 26(2), 393–403. 10.1017/S0954579414000029 [DOI] [PubMed] [Google Scholar]
- Ostlund BD, Measelle JR, Laurent HK, Conradt E, & Ablow JC (2017). Shaping emotion regulation: Attunement, symptomatology, and stress recovery within mother–infant dyads. Developmental Psychobiology, 59(1), 15–25. 10.1002/dev.21448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund BD, Vlisides-Henry RD, Crowell SE, Raby KL, Terrell S, Brown MA, Tinajero R, Shakiba N, Monk C, Shakib JH, Buchi KF, & Conradt E (2019). Intergenerational transmission of emotion dysregulation: Part II. Developmental origins of newborn neurobehavior. Development and Psychopathology, 31, 833–846. 10.1017/S0954579419000440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW (2007). The polyvagal perspective. Biological Psychology, 74(2), 116–143. 10.1016/j.biopsycho.2006.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW, & Furman S (2011). The early developmetn of the autonomic nervous system provides a neural platform for social behaivor: A polyvagal perspective. Infant and Child Development, 20, 106–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Propper C (2012). The early development of vagal tone: Effects of poverty and elevated contextual risk. In Maholmes V & King RB (Eds.), The Oxford Handbook of Poverty and Child developmentevelopment (pp. 103–123). Oxford University Press. [Google Scholar]
- Provenzi L, di Minico GS, Giusti L, Guida E, & Müller M (2018). Disentangling the dyadic dance: Theoretical, methodological and outcomes systematic review of mother-infant dyadic processes. Frontiers in Psychology, 9, 1–22. 10.3389/fpsyg.2018.00348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu M, Zhang Y, Webster JG, & Tompkins WJ (1986). Motion artifact from spot and band electrodes during impedance cardiography. IEEE Transactions on Biomedical Engineering, 33(11), 1029–1036. 10.1109/TBME.1986.325869 [DOI] [PubMed] [Google Scholar]
- Rash JA, Thomas JC, Campbell TS, Letourneau N, Granger DA, Giesbrecht GF, Kaplan BJ, Field CJ, Dewey D, Bell RC, Bernier FP, Cantell M, Casey LM, Eliasziw M, Farmer A, Gagnon L, Goonewardene L, Johnston DW, Kooistra L, & Singhal N (2016). Developmental origins of infant stress reactivity profiles: A multi-system approach. Developmental Psychobiology, 58, 578–599. 10.1002/dev.21403 [DOI] [PubMed] [Google Scholar]
- Sandman CA, Glynn LM, & Davis EP (2013). Is there a viability – vulnerability tradeoff ? Sex differences in fetal programming. Journal of Psychosomatic Research, 75, 327–335. 10.1016/j.jpsychores.2013.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahrestani S, Stewart EM, Quintana DS, Hickie IB, & Guastella AJ (2014). Heart rate variability during social interactions in children with and without psychopathology: A meta-analysis. Journal of Child Psychology and Psychiatry, 55(9), 981–989. 10.1111/jcpp.12226 [DOI] [PubMed] [Google Scholar]
- Skowron EA, Cipriano-Essel E, Gatzke-Kopp LM, Teti DM, & Ammerman RT (2014). Early adversity, RSA, and inhibitory control: Evidence of children’s neurobiological sensitivity to social context. Developmental Psychobiology, 56(5), 964–978. 10.1002/dev.21175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers JA, Curci SG, & Luecken LJ (2021). Infant vagal tone and maternal depressive symptoms: A bottom-up perspective. Journal of Clinical Child and Adolescent Psychology, 50(1), 105–117. 10.1080/15374416.2019.1622122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinillo A, Montanari L, Gardella B, Roccio M, Stronati M, & Fazzi E (2009). Infant sex, obstetric risk factors, and 2-year neurodevelopmental outcome among preterm infants. Developmental Medicine and Child Neurology, 51(7), 518–525. 10.1111/j.1469-8749.2009.03273.x [DOI] [PubMed] [Google Scholar]
- Suurland J, van der Heijden KB, Huijbregts SCJ, van Goozen SHM, & Swaab H (2018). Infant parasympathetic and sympathetic activity during baseline, stress and recovery: Interactions with prenatal adversity predict physical aggression in toddlerhood. Journal of Abnormal Child Psychology, 46(4), 755–768. 10.1007/s10802-017-0337-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer JF, & Lane RD (2007). The role of vagal function in the risk for cardiovascular disease and mortality. Biological Psychology, 74(2), 224–242. 10.1016/j.biopsycho.2005.11.013 [DOI] [PubMed] [Google Scholar]
- Tronick EZ, Als H, Adamson L, Wise S, & Brazelton TB (1978). The infant’s response to entrapment between contradictory messages in face-to-face interaction. Journal of American Academy of Child Psychiatry, 17(1), 1–13. [DOI] [PubMed] [Google Scholar]
- Tronick EZ, & Beeghly M (2011). Infants’ meaning-making and the development of mental health problems. American Psychologist, 66(2), 107–119. 10.1037/a0021631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Bergh BRH, Dahnke R, & Mennes M (2018). Prenatal stress and the developing brain: Risks for neurodevelopmental disorders. Development and Psychopathology, 30, 743–762. 10.1017/S0954579418000342 [DOI] [PubMed] [Google Scholar]
- Van den Bergh BRH, van den Heuvel MI, Lahti M, Braeken M, de Rooij SR, Entringer S, Hoyer D, Roseboom T, Räikkönen K, King S, & Schwab M (2020). Prenatal developmental origins of behavior and mental health: The influence of maternal stress in pregnancy. Neuroscience and Biobehavioral Reviews, 117, 26–64. 10.1016/j.neubiorev.2017.07.003 [DOI] [PubMed] [Google Scholar]
- Wadhwa PD, Buss C, Entringer S, & Swanson JM (2009). Developmental origins of health and disease: Brief history of the approach and current focus on epigenetic mechanisms. Seminars in Reproductive Medicine, 27(5), 358–368. 10.1055/s-0029-1237424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner NJ, & Waller R (2020). Leveraging parasympathetic nervous system activity to study risk for psychopathology: The special case of callous-unemotional traits. Neuroscience and Biobehavioral Reviews, 118(April), 175–185. 10.1016/j.neubiorev.2020.07.029 [DOI] [PubMed] [Google Scholar]
- Willoughby M, Greenberg M, Blair C, & Stifter C (2007). Neurobehavioral consequences of prenatal exposure to smoking at 6 to 8 months of age. Infancy, 12(3), 273–301. 10.1111/j.1532-7078.2007.tb00244.x [DOI] [Google Scholar]
- Zimmer-Gembeck MJ, Kerin JL, Webb HJ, Gardner AA, Campbell SM, Swan K, & Timmer SG (2019). Improved perceptions of emotion regulation and reflective functioning in parents: Two additional positive outcomes of parent-child interaction therapy. Behavior Therapy, 50(2), 340–352. 10.1016/j.beth.2018.07.002 [DOI] [PubMed] [Google Scholar]