Abstract
Central nervous system (CNS) malignances include tumors of the brain and spinal cord. Taking into account the cell type where they originate from, there are almost 120 different types of CNS tumors. Benign tumors are not aggressive and normally do not invade other organs; however, they require surgical removal before they alter the surrounding brain functions. Primary malignant brain tumors commonly include astrocytomas, oligodendrogliomas, and ependimomas, where astrocytomas represent around 76%. The World Health Organization (WHO) has defined four histological grades of astrocytomas that range from the less aggressive tumors (grade I) to highly malignant tumors (grade IV). These grade IV tumors, also called glioblastoma (GBM), are the most aggressive of the primary malignant brain tumors. Patients with GBM have a median survival of 12 to 15 months. Current treatment for GBM includes surgery, radiotherapy and chemotherapy. Although there have been some advances in diagnosis and treatment, there is still no optimal treatment available for GBMs. In this review, we will discuss the approaches for GBM diagnosis and treatment, with a special emphasis to post-treatment imaging, and whether novel targeted therapies have impacted the survival of GBM patients. In addition, we will discuss clinical trials and the future of GBM diagnosis and treatment.
Keywords: Glioblastoma, GBM, diagnosis, treatment, clinical trials, radiotherapy, temozolomide
INTRODUCTION
Astrocytomas are the most common malignancies of the CNS. They have been classified by WHO into four distinct grades (I-IV) based primarily on histology, which also correlate with increasing grade of malignancy and decreasing prognosis. Patients with grade I tumors may occasionally be cured by surgical resection alone. Grade II tumors usually progress to grade III (also named anaplastic astrocytoma) or grade IV (also named glioblastoma, GBM) [1–2]. Anaplastic astrocytomas (grade III) require major surgical removal and aggressive adjuvant treatment with radiotherapy and/or chemotherapy [1]. GBMs (grade IV) are diagnosed in adults, with a peak incidence within 50 to 60 years of age. Most GBMs develop de novo; about 10% progress from grade II or grade III astrocytomas [1]. Because they do not respond well to conventional treatment, consisting of a combination of radiotherapy and chemotherapy, GBM has a poor prognosis [1, 3]. Patients with GBM exhibit rapid disease progression and, with few exceptions, the survival is less than one year after initial diagnosis [1].
GBM is the second most common primary brain tumor, accounting for 52% of all gliomas and 17% of all primary brain tumors [1]. The National Cancer Institute (NCI) estimates that the incidence of GBM is 2–3 per 100,000 adults per year, and accounts for approximately 14,000 deaths annually [4] According to the American Brain Tumor Association, around 700,000 people are living with brain tumors in the United States of America, and over 189,000 people die annually of CNS tumors, worldwide [4].
GBM CLINICAL PRESENTATION AND MOLECULAR BIOLOGY
GBM tumors usually originate in the cerebral white matter, grow quickly, and become very large before provoking symptoms in a patient [5]. At the time of diagnosis, about 50% are found in more than one lobe of a hemisphere or are found in bilateral hemispheres forming a butterfly appearance. These bilateral tumors generally develop in the frontal and parietal lobes and then cross the midline by infiltrating the corpus callosum to involve the contralateral side [6].
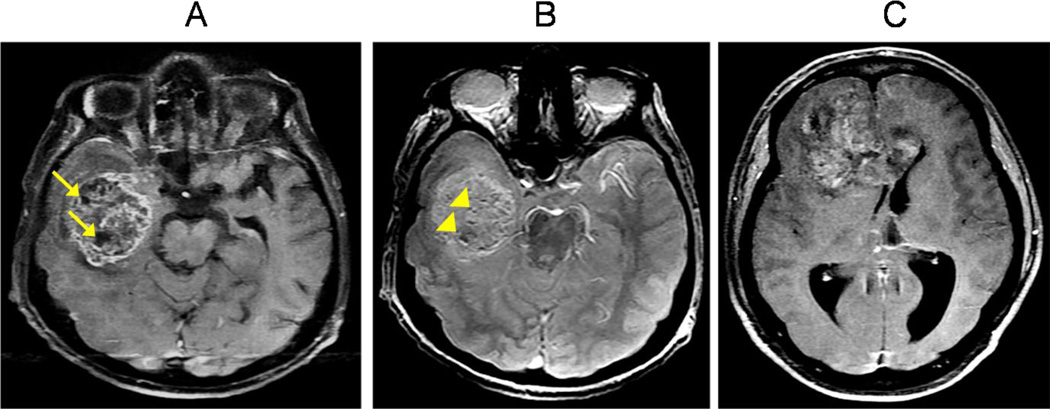
On microscope examination, GBMs are anaplastic, poorly differentiated, pleomorphic, astrocytic tumor cells with brisk nuclear atypia and mitotic activity. Histopathological features also include vascular thrombosis, microvascular proliferation and necrosis [6]. On the other hand, GBMs are poorly delineated macroscopically. They have a diffuse grey hypercellular rim with a yellowish center due to necrosis and myelin breakdown and, in many cases, have foci of necrosis or hemorrhage throughout (Figure 1A–C). Because of this poor delineation, total resection of GBMs is difficult and not always achievable [7].
Figure 1. Classic GBM appearances.
(A) T1W contrast-enhanced axial sequence shows an infiltrative mass centered at the right temporal white matter with heterogeneous enhancement and pockets of necrosis (arrows). (B) T2* axial sequence demonstrates multiple susceptibility foci within the mass, suggestive of microhemorrhages (arrowheads), commonly seen with GBMs. (C) T1W contrast-enhanced axial sequence shows a heterogeneously enhancing mass which involves both frontal lobes by crossing via the white matter tracts of the corpus callosum. This “butterfly glioma” pattern is observed in many GBMs.
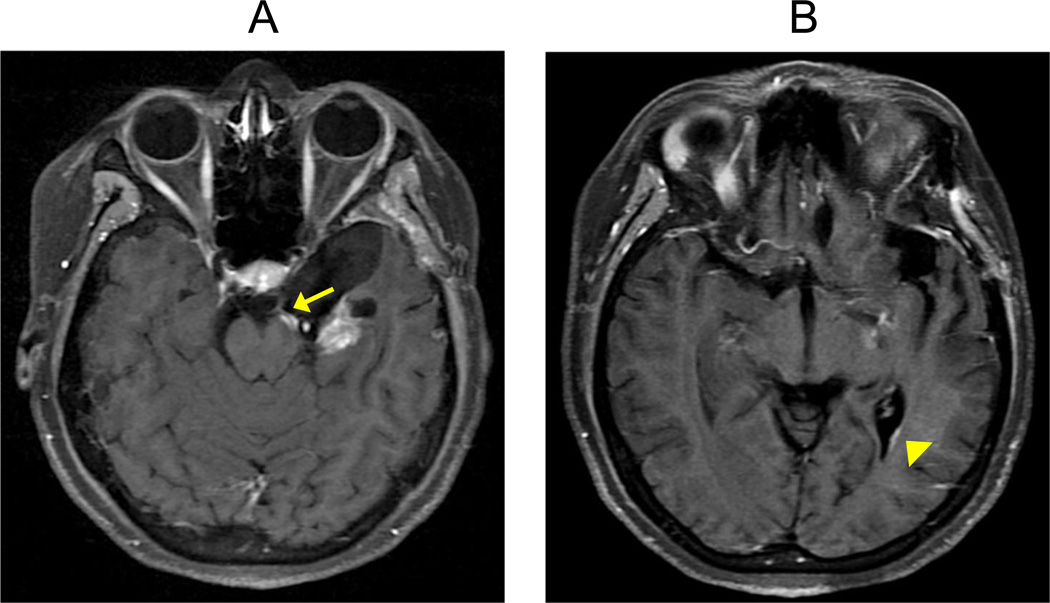
Although GBM uses several pathways to infiltrate other parts of the brain, the most common is through the white matter tracts, including the corpus callosum (Figure 2A). Other less common pathways include diffuse infiltration of the ependymal/subependymal linings of the ventricles and central spinal cord canal, and dissemination through the cerebrospinal fluid (CSF). In the latter, groups of malignant cells travel through the subarachnoid spaces of both the brain and spine with eventual distant seeding (Figure 2A–B). However, extra-cranial CSF dissemination to the spine (called drop metastases) or other organs is extremely rare. Studies of integrated genomic analysis on GBM tumors, available at The Cancer Genome Atlas (TCGA) data portal, identified four clinically relevant subtypes according to their genetic abnormalities [8]. These molecular subtypes are: (1) classical, manifesting high EGFR amplification, CDKN2A deletions and no TP53 alterations, (2) proneural, manifesting TP53 mutations, PDGFRA amplification and IDH1 mutations, (3) neural, manifesting expression of NELFL, GABRA1, SYT1 and SLC12A5 neuron markers, and (4) mesenchymal, manifesting NF1 and PTEN mutations and expression of CHI3L1 and MET markers [8]. Other molecular alterations described in GBMs include: chromosome 1p and 19q deletions, retinoblastoma gene (RB1) mutations, alterations in the pathways of PI3K/AKT, c-MYC, and mammalian target of rapamicyn (mTOR), and methylation alterations of the O6-methylguanine DNA methyltransferase (MGMT) gene [1, 8–12].
Figure 2. Routes of GBM tumor spread.
(A) Contrast-enhanced T1W axial sequence in a patient with GB which shows enhancement following the cisternal segment of the left oculomotor nerve/CNIII (arrow). The image suggests cerebrospinal fluid (CSF) tumor spread with cranial nerve involvement. (B) Contrast-enhancement T1W axial sequence in the same GBM patient demonstrates linear enhancement along the surface of the left lateral ventricle (arrowhead), suggestive of ependymal tumor spread.
Interestingly, GBMs exhibit numerous alterations in genes that encode ion channels, including upregulation of the big conductance K+ (gBK), and the voltage-gated chloride (Cl−) channels, ClC-3, among many others [13–14]. It has been hypothesized that overexpression of these ion channels alter the membrane potential, which is associated with progression through the checkpoints of the cell cycle phase and the concomitant promotion of cell growth and proliferation [13]. Another molecule frequently altered in GBMs are the microRNAs (miRNAs) [15], which are endogenous, short (of 19–24 nucleotides) non-protein-coding RNAs that regulate gene expression at the post-transcriptional level [16–17]. Several dysregulated miRNAs have been reported in all tumor types [18]; various identified to contribute to GBM initiation, progression, and tumor maintenance [15, 19–22]. Other studies have identified particular miRNA signatures that might differentiate GBMs from other brain tumors, including lower grade astrocytomas [15, 23–30]. Some miRNAs consistently identified as dysregulated in GBMs include: the upregulation of miR-21, miR-92b, miR-26a and miR-27a; and the downregulation of miR-7, miR-128, miR-34a and miR-124 [27, 31–32]. Given these findings, altered miRNAs assays have been proposed as useful diagnostic and prognostic markers for GBM, as well as other cancer types [25, 33–34]. Furthermore, targeting therapies to some of these dysregulated miRNAs in GBM are being envisioned [29, 31].
The origin of GBM, as with most brain tumors, is unknown. Risk factors that have been associated with GBM include: age, male gender, exposure to treatment radiation during childhood, personal history of prior cancers, and other conditions (i.e. HIV). As in many tumors, GBM has diverse cell heterogeneity in various stages of differentiation, including the presence of populations of cancer-stem cells (CSCs) [22, 35–38]. It is believed that these CSCs play a major role in tumor maintenance, angiogenesis, regression, and chemotherapy resistance [22, 35–37].
GBM DIAGNOSIS
The clinical presentation of most patients with GBM consists of the onset of persistent headaches, seizures, focal neurological deficits, or changes in their mental status. The initial diagnosis involves a detailed examination by a neurologist or neurosurgeon, including a mental status test, and brain imaging studies, such a computed tomographic (CT) scan and/or magnetic resonance imaging (MRI). For the histopathological diagnosis, a brain tumor tissue biopsy is required, which might include the assessment of the promoter methylation status of the DNA repair enzyme O(6)-methylguanine-DNA methyltransferase (MGMT) in many cases [39–40]. Results of the MGMT analysis will assist in deciding the patient’s response to chemotherapy [39–40]. Similarly, identification of IDH1 or IDH2 mutations by immunohistochemistry or real-time polymerase chain reaction (PCR) is of clinical/prognostic interest because of their association with increased overall survival [41–43]. IDH proteins are nicotinamide adenine dinucleotide phosphate (NADP)-dependent isocitrate dehydrogenases that catalyze the conversion of isocitrate to alpha-ketoglutarate [44]. IDH1 and IDH2 mutations appear to be an early event in the development of lower grade gliomas, so they are frequently present in grade II and III astrocytomas (70–90%), oligodendrogliomas (69–94%), oligoastrocytomas (78–100%), and secondary GBMs (82–88%) [45]. Primary GBMs have these mutations less frequently (0–5%) [45–46].
Non-Invasive Brain Imaging Diagnosis
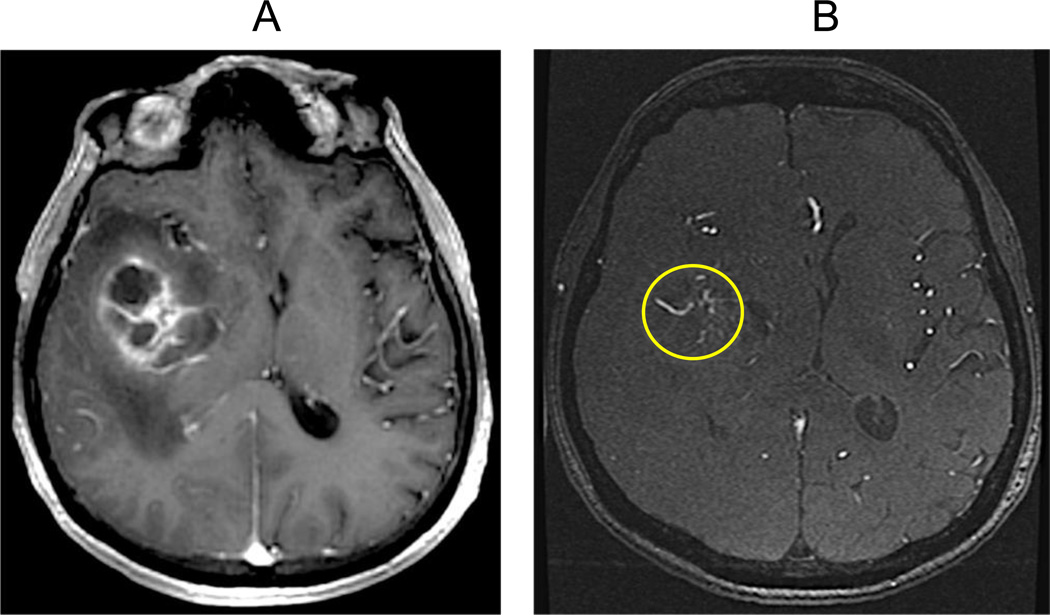
There are many imaging modalities and advanced imaging techniques available; however, the study of choice for the initial diagnosis and characterization of a brain tumor is the contrast-enhanced brain MRI [47]. This study provides important information regarding the extent of visible tumor, the peritumoral edema, and the meningeal/ependymal tumor involvement. Although contrast-enhanced CT is also used for tumor diagnosis and characterization, it is less sensitive and specific for tumor imaging as compared to the contrast-enhanced MRI [48]. CT and MRI images are focused on the hemispheric white matter in GBM, although extension beyond the white matter may be present at the time of diagnosis [47]. Most GBMs display a dominant mass with thick and irregular enhancing margins and central areas of non-enhancing necrosis [47–48]. A multifocal presentation (of more than one enhancing lesion) is less common, found in 12–15% of all GBMs [49]. Finding tumor hemorrhage is more common in GBM than lower-grade gliomas, and can be reliably identified using the T2* sequence, a gradient echo MRI sequence introduced in recent years that is now part of most brain MRI protocols. In this sequence, hemorrhagic foci within the tumor produce susceptibility artifacts, which appear dark, making them readily identifiable. Although calcifications can also produce susceptibility artifacts on T2*, they are less frequent in GBM [50]. Another common feature in MRI images of GBM is the finding of prominent vessels in the vicinity of the tumor mass. This characteristic demonstrates the presence of neovascularity, a phenomenon of new tumor-induced vascular channels [51–52] (Figure 3A–B).
Figure 3. Neovascularity.
(A) Contrast-enhanced T1W axial sequence shows a GBM with right basal ganglia involvement. (B) Source sequence from a magnetic resonance angiogram without IV contrast (TOF-MRA) at the same level as in A which demonstrates multiple tubular tortuous structures representing blood vessels within and in the direct vicinity of the mass (circle). This image pattern is consistent with new tumor-induced vascular channels (neovascularity).
The conventional images of GBM show an enhancing tumor surrounded by T2/FLAIR white matter hyperintensity [53–54]. Areas of enhancement represent macroscopic tumor, whereas the surrounding areas of non-enhancing T2/FLAIR hyperintensity represents either edema or microscopic tumor, or a combination of both [54–56]. This is one of the major limitations of conventional imaging. The surrounding areas with no abnormal enhancement or abnormal signal intensity could still contain tumor cells, manifesting the infiltrative nature of GBMs [47].
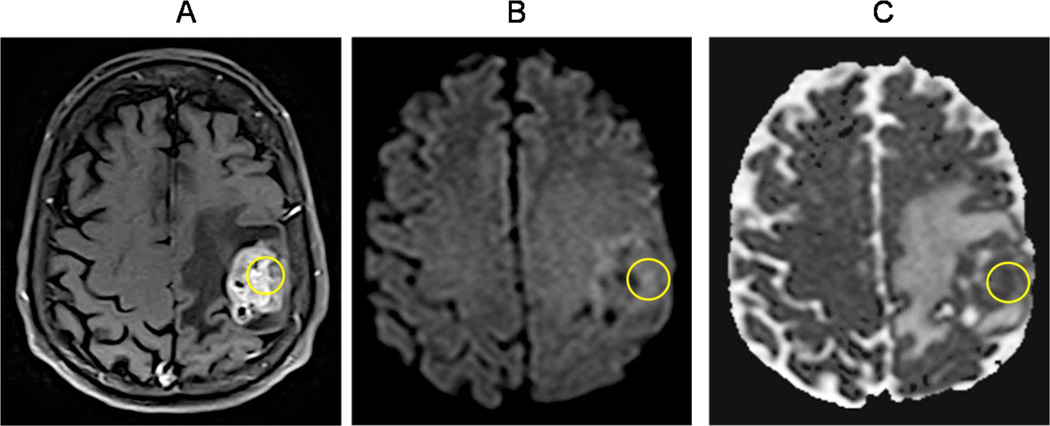
A technique gaining popularity for brain tumor diagnosis and post-treatment monitoring is the diffusion weighted imaging (DWI) [47, 57–58]. In tumors, water movement within the extracellular compartment is restricted due to tightly packed tumor cells; whereas, in areas of edema and necrosis, there is less restriction of extracellular water movement due to a lower cell density [47, 57–59]. DWI can detect this microscopic water movement both qualitatively and quantitatively, expressed as the degree of restricted diffusion, and the values are expressed as the apparent diffusion coefficient (ADC) to create ADC images [59]. A low ADC value indicates that molecular movement in the sampled tissue is restricted (dark signal) while a high ADC value indicates that the tissue has free diffusion (bright signal). Accordingly, the ADC values for GBM are generally lower than normal-appearing white matter. Although some reports indicate that DWI is unable to differentiate between astrocytoma grades; however, Rumboldt, et al. found that ADC values were significantly higher in pilocytic astrocytomas than in ependymomas and medulloblastomas [60]. Also, Pope, et al. found that increased ADC values correlated with a significant increased progression free survival (PFS) and overall survival (OS) in recurrent GBM patients treated with bevacizumab (BEV) [61]. These findings suggest that increase in ADC values in brain MRIs could be a useful tool to predict clinical outcomes with BEV treatment in patients with recurrent GBM [61]. Furthermore, specific genomic signatures have been found for GBM patients with high vs. low ADC values [62]. Since DWI detects the full extension of the GBM tumor, it now forms part of most brain MRI protocols [63] (Figure 4A–C).
Figure 4. Diffusion weighted imaging (DWI).
(A) Contrast-enhanced T1W axial sequence showing a left frontoparietal necrotic mass in the vicinity of the central sulcus, consistent with a GBM (circle). (B–C) Diffusion weighted image/DWI shows increased signal intensity with corresponding decreased signal intensity on the apparent diffusion coefficient/ADC map within areas of enhancing tumor seen in A (circles). This restricted diffusion pattern is seen in highly cellular tumors such as GBM.
A variation of the DWI technique is the diffusion tensor imaging (DTI) which adds information regarding the direction of the water diffusion within the tissues [59, 64–65]. DTI can also be used to assess the integrity of the white matter tracts [59, 64]. Assessment of the tracts is performed using a parameter known as fractional anisotropy (FA) [59, 64]. FA values close to 1.0 are expected in healthy highly-ordered and cohesive white matter tracts, and decreased in high grade tumors, such as GBM, due to white matter tract infiltration and disruption [59, 66–67]. At the present time, DTI can be used to construct a 3D image (tractography) of selected white matter tracts. This white matter tractography technique is mostly used as a surgical planning tool, rather than a diagnostic tool, providing the surgeon a 3D visual map of important white matter tracts that should be avoided during surgery [68].
Perfusion weighted imaging (PWI), a contrast-enhancing technique performed by CT or MRI, is used to estimate the relative cerebral blood volume (rCBV), which is a measurement of microvascular tissue density. rCBV is the most broadly used MR perfusion parameter in the clinical setting, and is typically elevated in the solid portions of the tumor [69–70], indicative of increased tumor vascularization and alterations in the brain-blood barrier (BBB) [69]. Although rCBV is a strong predictor of tumor aggressiveness and poor survival rates, it can also be elevated in oligodendrogliomas. Therefore, elevated rCBV is not always indicative of GBM [71–72]. Recently, Jabehdar-Maralani, et al. evaluated the prognostic value of dynamic susceptibility contrast (DSC) MR perfusion in older (≥65 years) vs. younger (<65 years) patients with GBM [73]. All rCBV parameters were significantly higher in elderly patients compared to younger patients. Also, high rCBV in elderly patients was independently associated with a shorter survival [73]. These findings suggest that rCBV values may be an imaging prognostic tool for elderly GBM patients [73].
Functional MRI (fMRI) is a technique that evaluates the activation of certain areas of the brain (while a patient performs a series of tasks) and subsequently maps them [74]. During brain activation, local changes in oxy and deoxyhemoglobin occur [74–75] allowing detection using a MRI technique named Blood-Oxygen-Level Dependent (BOLD). For surgical planning, this activation map is fused with anatomic volumetric sequences to be readily identified and potentially avoided during surgery. This allows for a more comprehensive resection without potentially affecting key functional brain areas [68].
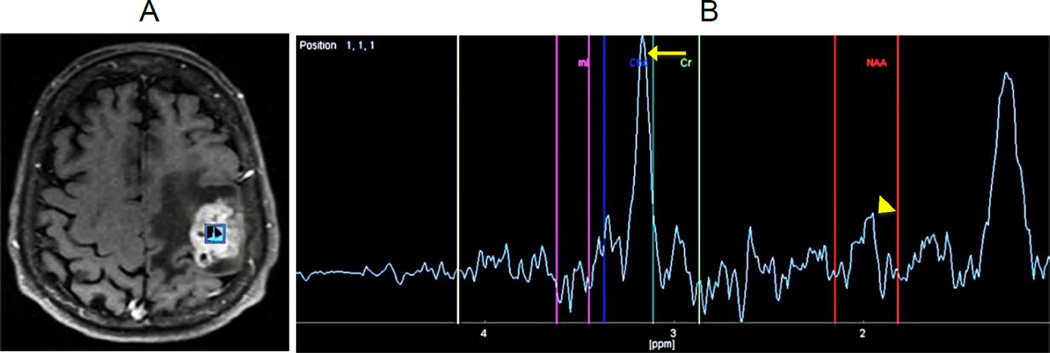
Proton magnetic resonance spectroscopy (1H-MRS) is a non-invasive neuroimaging technique that can detect brain metabolite abnormalities [56, 76]. Information on choline (Cho), N-acetylaspartate (NAA), creatine (Cr), glutamine, and glutamate (Glx) values within tumor areas is obtained and compared to their values in normal-appearing brain parenchyma [56, 76]; subsequently, qualitative patterns and quantitative metabolite values are analyzed [55–56]. In some cases, 1H-MRS is able to differentiate tumoral from non-tumoral tissue by the increase in the choline peak and Cho/Cr ratio within tumors. Since choline is a marker of cell membrane turnover, increased levels of this metabolite is found in most high grade neoplasms [55]. N-acetylaspartate (NAA) is a marker of neuronal viability; so, decreased levels may indicate tumor infiltration due to loss of viable neurons, as seen in high grade tumors such as GBM (Figure 5A–B).
Figure 5. Magnetic resonance spectroscopy (MRS).
(A) Proton magnetic resonance spectroscopy (H-MRS) was performed using a time of echo (TE) of 136 with single voxel measuring 10×10×10mm placed within the enhancing portion of a left frontoparietal mass. (B) The resulted spectrum is abnormal with a dominant Choline (Cho) peak, and a decreasing in N-acetylaspartate (NAA) to the baseline level. Cho/Cr ratio was elevated at 2.5. Choline peak elevation (arrow) indicates an increasing in the cellular membrane turnover typical of most high grade tumors. The NAA peak decreasing (arrowhead) indicates tumor infiltration with loss of viable neurons.
As previously discussed, contrast-enhanced brain MRI remains the study of choice in the initial imaging evaluation of brain tumors. Nevertheless, combining advanced imaging techniques (such as, MRS, PWI, DTI, and H-MRS) will increase the diagnostic accuracy [77–79]. These improved imaging techniques are now available because of advances in hardware, software and the availability of higher field MR scanners. 3-D high-resolution imaging is one example of an improvement, where 3D T1-weighted contrast-enhanced sequences have led to a more accurate tumor volume evaluation [50]. Another example of MR sequence improvement is the susceptibility weighted imaging (SWI), a 3D high-spatial resolution technique, with a higher sensitivity for hemorrhage detection compared to the conventional MRI and 2-D gradient echo T2* sequences [50]. Innovations during the last 10 years, has permitted the diagnosis of brain tumors to shift from a purely anatomical-based approach to a combined anatomical, physiological and molecular-based multidisciplinary approach.
Invasive Brain Biopsy
A brain tumor biopsy is a procedure where a tissue sample is obtained by a neurosurgeon, which is then sent to a pathologist in order to make a histological diagnosis. Although the vast majority of GBMs have a very characteristic appearance on contrast-enhanced MRI, there is still a 23% inaccuracy rate with cystic lesions appearing as GBMs [80]. Therefore, an invasive biopsy is required for an unequivocal diagnosis and classification.
The least invasive way to obtain a brain tumor tissue sample is by needle biopsy, either with a stereotactic frame or a frameless approach. For a frameless biopsy, an MRI or CT image is always used. When comparing the frame-based stereotactic CT or MRI guided biopsy versus a frameless-based system, the accuracy is similar [81]. The major disadvantage of a stereotactic biopsy is that the yield of the sample is very small. With a small sample, the correct diagnosis can vary up to 60% because of different cell populations within a tumor [82–83]. To overcome this, it is recommended to obtain samples at different areas of the tumor using the same cranial entry point [84–85]. The safety of this procedure is well documented [85]. In a series of 300 stereotactic CT-guided intraaxial brain biopsies, Bernstein, et al. reported a mortality rate of 1.7%, and neurological adverse events to be major in 1.3% cases and minor in 3.3%; implicating this procedure is safer than a craniotomy [86].
GBM TREATMENT
The gold standard for GBM treatment is surgical resection, followed by chemotherapy and/or radiotherapy (XRT). Although the surgical procedures for brain tumor resection have improved in the last decade, the overall survival (OS) of GBM patients has remained the same for the past 15 years. After surgery, first-line therapy for GBM is the use of the chemotherapy temozolomide (TMZ) [Temodar,Temodal] in combination with XRT. However, other chemotherapies and XRT modalities are now clinically available or in clinical trials for GBM treatment.
Tumor Resection
Besides obtaining sample tissue for histological diagnosis, tumor resection is beneficial because the maximal surgical reduction of tumor volume directly improves the outcomes of adjuvant therapies [87]. Several reports from randomized controlled trials (RTC) and retrospective analyses reveal an increased efficacy of chemotherapy and XRT when the tumor is removed vs. having only a biopsy [88–90]. Keles, et al. performed CT and/or MRI volumetric measurements in 92 GBM patients with a Karnofsky performance status (KPS) of >70 (able to carry on normal activity with or without effort). He found that by increasing the amount of tumor resection, i.e. <25%, 25–49%, 50–74%, 75–99% and 100%, patients had longer median time to progression (TTP), i.e. 14.1, 24, 31.9, 45.8 and 53.1 weeks, respectively; and longer median survival, i.e. 31.8, 56.6, 62.9, 88.5 and 93 weeks, respectively [89]. Vuorinen, et al. reported that patients having a craniotomy and surgical resection demonstrated an increased progression free survival (PFS), and the estimated survival time was 2.8 times longer (p=0.049) as compared to having only a stereotactic biopsy [88].
There are several ways to enhance the accuracy and efficacy of the surgical resection. One way is using image guidance or neuronavigation during tumor resection [91–93]. A major concern of the image-guided surgery is that the image obtained pre-operative may not reflect accurately the intra-operative findings as a consequence of tissue resection and loss of cerebrospinal fluid, a phenomenon called brain shift [94]. Using intra-operative MRI will better assist the extent of resection because it is based on real-time imaging rather than a pre-operative image. A randomized trial by Senft, et al. compared the use of intra-operative MRI vs. conventional microsurgical technique (control arm) using blinded radiologists to evaluate the pre-operative and post-operative imaging data [95]. They found that using intra-operative MRI achieved complete tumor resections in 23 out of 24 (96%) patients as compared to the control arm where only 17 out of 25 (68%) patients achieved complete tumor resection [95]. These findings prompt the need for additional studies to validate the efficacy of MRI-guided surgery over conventional surgery with pre-operative imaging [75, 96].
A major innovation of the last decade regarding tumor resection is the oral administration of a non-fluorescent prodrug 5-Aminolevulinic acid (5-ALA). Once inside cells, 5-ALA is converted to protoporphyrin IX, which emits red fluorescence under blue light [96]. Since it is incorporated inside cancer cells faster than inside normal brain cells, this allows for fluorescence-based identification of malignant cells and a more accurate resection of the tumor tissue [87, 97]. Best of all, fluorescence-guided resection of malignant gliomas with 5-ALA is less expensive and yields comparable results as using intra-operative MRIs [87, 98].
Several studies regarding the effectiveness of 5-ALA fluorescence-guided surgery have reported contrasting results [88, 99]. Stummer, et al. conducted a randomized study with 322 malignant glioma patients scheduled for surgical resection. 161 patients underwent fluorescence-based resection using 5-ALA and 161 had conventional microsurgery with white light. Complete tumor resection was achieved in 90 patients (65%) with 5-ALA use vs. 47 patients (36%) in the conventional group [88]. Furthermore, the PFS for the 5-ALA group was 6 months longer than the conventional group [88]. A meta-analysis of 10 studies focusing on safety/efficacy/outcomes with the use of 5-ALA fluorescent-guided surgery for high grade gliomas was conducted by Zhao, et al. [100]. Compared to image-guided surgery with conventional neuronavigation and contrast enhanced MRI, they found evidence that the use of 5-ALA - guided surgery is more effective in increasing diagnostic accuracy, improving extent of resection, enhancing quality of life and prolonging survival in high grade glioma patients [100]. Subsequently, Barone, et al. conducted a database meta-analysis on image-guided surgery for the resection of low grade and high grade gliomas with contrasting findings [92]. They concluded that there is insufficient evidence that any modality (intraoperative MRI, 5-ALA fluorescence or neuronavigation using DTI) increases the proportion of high grade glioma patients with a complete tumor resection on post-operative MRI [92]. Furthermore, they suggested that surgical resection using 5-ALA could lead to an increased frequency of early neurological deficits [92].
Chemotherapy
TMZ was first approved by the U.S. Food and Drug Administration (FDA) in 1999 for the treatment of refractory anaplastic astrocytoma, and then in 2005, for newly diagnosed GBM patients [101–103]. TMZ is an oral alkylating prodrug capable of crossing the BBB. When in circulation (at physiological pH) it is activated to 5-(3-dimethyl-1-triazenyl)imidazole-4-carboxamide (MTIC) [103–104]. The methyldiazonium ion formed by the breakdown of MTIC primarily methylates guanine residues in the DNA molecule, resulting in the formation of O6– and N7–methlyguanine, although methylation at the O3 position of adenine may also occur [104–105]. These methyl-DNA adducts induce nicks in the DNA, leading to cell cycle arrest and apoptosis [104]. The clinical response of TMZ was demonstrated in a phase III trial comparing XRT alone vs. XRT with concurrent TMZ, finding that the OS was increased in the chemotherapy arm (14.6 months) vs. the XRT only (12.1 months) [106].
The major drawback of TMZ-based therapy is that over 90% of GBM patients do not show any response to a second cycle of TMZ treatment (attributed to acquired resistance). In addition, a group of GBM patients exhibit an intrinsic TMZ resistance [104, 107], where one of the major mechanisms is the enhanced activity of the DNA repair enzyme, O6-methylguanine-DNA-methyltransferase (MGMT) [104]. The methyl group of the O6-methylguanine is removed by MGMT, counteracting the TMZ-induced methyl-DNA adducts [104]. Other mechanisms of TMZ resistance have been proposed, including activation of the mismatch DNA repair systems and the poly(ADP-ribose)polymerase (PARP) [104, 108–109]. To overcome TMZ resistance, several strategies are under investigation, mainly those that target MGMT [104, 110]. These therapies could improve the survival of GBM patients by improving the TMZ efficacy.
Epigenetic silencing of MGMT by promoter methylation decreases its expression and DNA repair cell capacity [40]. Tumor cells lacking MGMT activity are significantly more sensitive to TMZ treatment than cells with functional MGMT [39]. Because MGMT promoter is methylated in around 50% of GBM patients, the MGMT promoter methylation status is a good indicator of response to TMZ [39–40, 111]. In a randomized clinical study comparing chemoradiotherapy (TMZ+XRT) vs. XRT alone, a companion analysis of methylation-specific PCR was performed from DNA isolated from paraffin samples [112]. The entire study included 537 GBM patients, but only 307 paraffin samples were suitable for PCR, and the methylation status could be determined in only 206 of them. In the patients with MGMT promoter methylation, the combination TMZ+XRT treatment showed an OS of 21.7 months vs. 15.3 months for XRT only arm [112]. Similarly, patients with methylated MGMT and TMZ+XRT therapy had a median PFS of 10.3 months, as compared to 5.9 months for patients receiving XRT alone [112]. It became evident that MGMT promoter methylation is a good indication of TMZ response, and more recent studies found that it could also determine the effectiveness of other chemotherapeutic agents [113].
The use of Gliadel wafers is another therapeutic option for newly diagnosed GBM patients. Gliadel wafers are implantable biodegradable polymers containing the chemotherapeutic drug carmustine (BCNU) that gradually releases over a period of 2–3 weeks. BCNU is a β-chloro-nitrosourea alkylating agent that forms DNA inter-strand crosslinks, preventing the DNA replication and transcription processes [114]. Gliadel wafers are intended to be implanted in the surgical bed after tumor resection. A phase III, prospective, placebo-controlled, randomized trial comparing the use of Gliadel wafers vs. placebo wafers in 240 patients with malignant gliomas was completed [115]. After tumor resection, patients were randomized to receive either BCNU or placebo wafer, followed by external beam radiation. The median OS for those in the BCNU wafer group was 13.9 months compared to 11.6 months for the placebo wafer group (p= 0.03) [115].
Another targeted therapeutic option is bevacizumab (Avastin; Genentech, CA, USA), a humanized monoclonal antibody against the vascular endothelial growth factor (VEGF) signaling pathway [116–118]. VEGF plays an important role in the abnormal vascular proliferation, seen in many solid metastatic tumors and malignant gliomas, and its expression correlates with higher-grade malignancy and poor prognosis [119–120]. Bevacizumab binds to VEGF and prevents the interaction of VEGF with its receptor (VEGFR). As a consequence, the intracellular tyrosine kinase domain of the receptor and its downstream intracellular pathways are inactivated [120]. In 2009, the FDA accelerated the approval of Bevacizumab as a single agent for recurrent gliomas based on clinical trials demonstrating the benefits of the anti-VEGF therapy [121–122]. A multi-centric, open label, randomized study where 167 recurrent GBM patients were assigned to receive bevacizumab alone or combined with irinotecan had the treatment efficacy determined by MRI [120]. In the bevacizumab alone group the estimated 6-month PFS rate was 42.6% compared to 50.3% for the bevacizumab plus irinotecan group; the median OS were 9.2 months and 8.7 months, respectively [121]. Another single site study offered bevacizumab alone to 48 recurrent GBM patients, adding irinotecan at tumor progression, which found a 6-month PFS rate of 29%, and a median OS of 31 weeks [122]. However, the 19 patients treated with bevacizumab plus irinotecan at progression did not include objective radiographic responses as per Levin and Macdonald criteria (qualitative imaging assessments of tumor, normal brain structures, brain edema, and clinical assessment) [123–124].
Although clinicians may recommend bevacizumab in combination with TMZ for newly diagnosed GBM patients, there are reports showing no additional benefit of adding bevacizumab to the GBM standard of care (tumor resection, XRT+TMZ). A randomized phase II study evaluated TMZ+XRT with or without bevacizumab in 70 newly diagnosed GBM patients [124]. Although the PFS improved with the addition of bevacizumab, there was no significant improvement in the OS [125]. Unfortunately, the patients receiving bevacizumab experienced increased adverse effects, such as: hypertension, thromboembolic events, intestinal perforation, neutropenia, and decline in neurocognitive function, leading to a worse quality of life [125]. In an open phase II study for recurrent GBM, 13 patients received the combination of bevacizumab and temsirolimus (mTOR inhibitor) as second-line therapy after TMZ/XRT [126]. Although the combination of temsirolimus with bevacizumab did not show additional adverse effects, it did not demonstrate an increase in PFS beyond the use of bevacizumab alone; so, the trial was terminated [126].
Treatment attempts for GBM with other small molecule inhibitors that block the kinase domain of VEGF receptor (VEGFR) and other kinases have been investigated [127–128]. One such attempt was using cediranib, an oral panvascular endothelial growth factor receptor tyrosine kinase inhibitor that inhibits both DNA and RNA synthesis through DNA alkylation. Cediranib was evaluated in a phase III trial (clinical trial: NCT00777153) with 325 recurrent GBM patients that were randomly assigned (2:2:1) to receive cediranib monotherapy, cediranib plus lomustine, or lomustine plus placebo. The primary endpoint of this study was an improvement in PFS based on MRI assessments, but it was not observed with the combined therapy [129].
Amplification of the epidermal growth factor receptor (EGFR) gene can be present in around 30–50% of all GBMs. Because of this, EGFR has been proposed as a target for GBM therapy [1, 130]. EGFR amplification occurs by several molecular mechanisms including expression of several isoforms, truncations and overexpression of the oncogenic variant III of the receptor (EGFRvIII). EGFRvIII lacks exons 2–7 (coding 267 amino acids of the EGFR extracellular domain) that leads to a receptor unable to bind the ligand [131]. However, the EGFRvIII variant is constitutively active, which potentiates the downstream mitogenic and pro-invasive signaling pathways in GBM cells [131]. So far, tyrosine kinase EGFR inhibitors have been tested without significant activity in GBM, as evidenced by phase II trials with erlotinib (Tarceva) [132–133]. The North American Brain Tumor Consortium conducted a phase II study combining erlotinib with sorafenib in 18 recurrent GBM patients, which terminated early because the median PFS was only 8 weeks and there were no patients with PFS at 6 months [133]. Incidentally, sorafenib is a small molecule inhibitor of various tyrosine and serine/threonine protein kinases that induces cell death mainly by autophagy [134]. The same research group published more recent Phase I (N=22) and phase II (N=47) studies offering erlotinib with temsirolimus for patients with recurrent GBM [132]. Only 1 patient (6%) achieved a complete response and 1 (6%) a partial response. Evidently, this drug combination had a minimal antitumor activity in GBM, but it resulted more toxic and the maximum tolerated dosage was lower than expected.
Platelet derived growth factor receptor (PDGFR) is overexpressed in about 30% of GBM patients, for which the inhibition of PDGFR has been tested in the clinical setting; however, minimal response has been observed [135]. Although therapies targeting VEGF/VEGFR, EGFR, PDGFR, mTOR, PI3K, HDAC, and integrins, among others, have been or are being investigated in GBM, most have only shown modest results.
Radiotherapy
Post-operative whole-brain XRT has been the gold standard therapy for GBM patients for the past 30 years. Basically, the purpose of XRT is to induce DNA damage by the interaction of the ionizing radiation directly with the DNA or by the formation of free radicals that subsequently affect DNA synthesis and cause DNA breakdown [136–137]. The technology has improved in such a way that it allows the radiation beam to focus better at the tumor tissue and decrease damage to surrounding non-neoplastic areas [137]. At the present time, the linear particle accelerator (LINAC) is the most frequent technology for XRT [138]. The introduction of double focused mini and micro multileaf collimators in the LINAC instruments has greatly improved the effectiveness of the stereotactic XRT [138].
Recently, Yin, et al. performed meta-analysis studies to compare if there were differences in the OS of TMZ vs. XRT alone in elderly GBM patients [139–141]. Although, in patients with methylated tumors, TMZ was more beneficial than XRT alone in improving OS, no differences in the OS were observed for unmethylated tumors. However, a study performed by Stupp, et al. clearly demonstrated that XRT+TMZ had a positive impact in the improvement of the OS in GBM patients [106]. In this study, the OS of GBM patients treated with TMZ alone, XRT alone, and XRT+TMZ was 3–6 months, 12 months, and 14 months, respectively [106]. Another study also found an improved OS with TMZ+XRT rather than TMZ or XRT alone [106, 142].
RadioSurgery
Given the infiltrative nature of GBM and the worse prognosis of patients with diffuse tumor presentation, the alternative and benefits of high-dose focal radiation treatment such as stereotactic radiosurgery (SRS) has been extensively debated over the past two decades [143–144]. Stereotactic radiotherapy is usually administered as a single fraction or fractionated (i.e. fractionated stereotactic radiotherapy, FSRT) in which multiple fractions are given over a period of 2 to 4 weeks [145–147]. The most common technology used for focal radiation therapy is the Gamma Knife (Elekta, Stockholm, Sweden), which is available worldwide. The Gamma Knife surgery consists of delivering high-intensity cobalt radiation therapy to the tumor area [146, 148].
Retrospective studies have indicated some benefits of SRS while delivered with escalating doses for the treatment of GBM [144]. However, this treatment approach did not achieve a significantly different OS in patients as compared to standard-of-care (resection, XRT+TMZ). Nevertheless, there is still some controversy whether the application of SRS as a boost to the tumor prior to surgery may impact the OS of GBM patients [143, 149]. Further investigations are required to yield better conclusions and validate the use of SRS for GBM treatment [150].
While the Gamma Knife is dedicated to radiosurgery, LINACs are built for conventional fractionated XRT. Additional technologies with sophisticated beam accelerators and image-guidance tools have made it possible for the LINACs to become an important therapeutic tool for GBM treatment. The CyberKnife Radiosurgery System is a compact LINAC mounted onto a robotic arm that moves around the patient and irradiates the tumor from an abundant set of fixed positions, thereby mimicking the Gamma Knife technology [151].
Novo Tumor Treating Fields (TTF) Therapy
Novo Tumor Treating Fields (TTF) is a localized treatment that uses electric fields within the human body to disrupt rapidly diving cells, a feature of cancer cells. NovoTTF therapy is now considered a fourth treatment option for cancer, in addition to surgery, radiation therapy and chemotherapy. The FDA approved this device to be offered as monotherapy for recurrent GBM patients. It has a battery and a transducer array and is placed over the shaved scalp. Clinical studies have showed that the OS of patients treated solely with novoTTF was at least comparable with the best standard-of-care available for recurrent GBM patients [152]. Several clinical trials combining NovoTTF-based therapy, chemotherapy and/or radiotherapy are currently under investigation.
POST-TREATMENT IMAGING
Post-treatment imaging (PTI) is routinely used to determine possible residual or recurrent tumor and to assess treatment response. Several modalities of PTI are currently available, including conventional MRI imaging, DWI, MRS, and DTI. However, contrast-enhanced MRI remains the most frequent imaging modality used post-surgery or post-treatment, mainly to assess possible residual or recurrent tumor. It is recommended to have a contrast-enhanced MRI done preferably within the first 24 hours after surgery to avoid imaging alterations related to post-operative inflammation [55, 153]. This initial post-operative study serves to determine if there is residual tumor and will also serve as a baseline study to compare in case of the evolution of subsequent changes associated to XRT or chemotherapy treatment.
To assist in the interpretation of the post-operative contrast-enhanced MRI images, a basic understanding of the effects of chemotherapy and/or XRT on brain imaging is required. Depending on the parameters used, XRT causes damage to the vascular endothelium and oligodendrocytes, provoking radiation-induced necrotic degradation of brain tissue, which could be misinterpreted as tumor recurrence [55, 153]. Images showing an increase in contrast enhancement and/or increased extent of white matter signal abnormalities on follow-up studies, may suggest progression of disease and a need to change to another treatment modality. Sometimes, the perceived worsening in neuroimaging is actually pseudoprogression, a non-tumoral interval increase in enhancement and signal abnormality. This phenomenon usually occurs within the first 3 months of treatment, and is thought to be due to an active inflammation response against the tumor [153]. It is important to recognize this entity because it does not mandate a change in GBM treatment. In fact, in some cases, this evidence of pseudoprogression has been associated with an increase in patient survival [48]. The best way to distinguish between pseudoprogression and early disease progression is by having serial imaging performed after surgery every 3 months or so. By evaluating subsequent follow-up studies, if the enhancement and abnormal parenchymal T2 signal intensity decreases over time, it favors pseudoprogression; whereas, if the findings remain stable or increase, it favors progression of disease [48, 153].
When no improvement is observed on serial follow-up neuroimaging studies and actual tumor progression is suggested, TMZ chemotherapy is usually discontinued to begin a second line treatment regimen. Possible treatment alternatives include anti-VEGF therapy (bevacizumab or cediranib), which stabilizes the BBB and consequently produces a rapid decrease in contrast enhancement, a phenomenon called pseudoresponse [153]. Pseudoresponse is usually associated with a modest improvement in patient survival; but in some cases, it may actually contain tumor growth, manifested as a non-enhancing tumor component on the MRI. In light of this, contrast enhanced sequences should not be the only criteria used to assess the progression or improvement in disease status/burden, since non-enhancing tumor is commonly seen in GBM patients, particularly if on anti-VEGF therapy [153].
1H-MRS imaging has been used for post-treatment assessment with adequate results [154–155] (Figure 6A–B). Zeng, et al. describes that recurrent tumors have higher Cho/Cr and Cho/NAA values as compared to the results seen with radionecrosis [154]. Unfortunately, results have been inconsistent for 1H-MRS alone as a post-treatment imaging tool; therefore, combining MRS with other imaging modalities is recommended [48, 156].
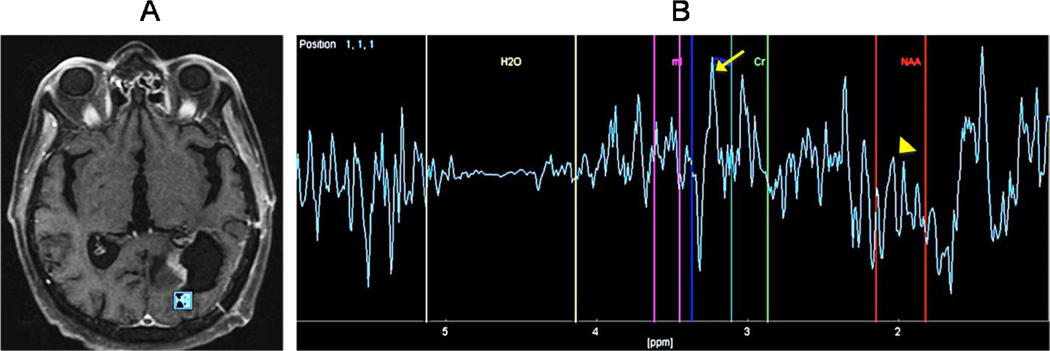
Figure 6. GBM status after post-surgical resection and chemoradiation.
(A) Proton magnetic resonance spectroscopy (H-MRS) was performed using a time of echo (TE) of 136 with single voxel measuring 10×10×10mm placed within a region of focal nodular enhancement at the posterior margin of resection. (B) The resulted spectrum is abnormal with a dominant Choline (Cho) peak (arrow) and a decreasing in the NAA (arrowhead) almost to the level of baseline. Cho/Cr ratio was elevated at 3.1. These findings are highly suggestive of recurrent tumor. These H-MRS images can help to differentiate recurrent tumor from radiation necrosis, which typically causes a decreasing in all peaks without elevation of the Choline peak.
PWI is yet another important tool in post-treatment evaluation in GBM [157–158]. Because high grade gliomas increase angiogenesis, it is manifested as an increase of the PWI (rCBV) parameter [158–159]. Therefore, rCBV is characteristically increased in disease progression, and decreased in non-tumoral treatment-related changes, such as radiation necrosis and pseudoprogression [160]. Bobek-Billewicz, et al. determined that PWI was able to distinguish between actual tumor recurrence and radiation injury with greater efficacy than both MRS and DWI [161]. As discussed for the diagnostic imaging modalities, sensitivity and specificity of post-treatment imaging is greatly increased when utilizing a multimodal approach, including both conventional and advanced techniques. However, the importance of serial imaging, with a carefully detailed tracking of changes over time, is critical for post-treatment evaluation in GBM patients, regardless of the imaging modalities used. The Major advantages and disadvantages of the GMB diagnosis and treatment modalities are summarized in the Table 1.
Table 1.
Major advantages and disadvantages of GMB diagnosis and treatment modalities.
| GBM Diagnosis Techniques | ||
|---|---|---|
| Technique | Advantages | Disadvantages |
| Molecular Markers |
|
|
| Non-Invasive Brain Imaging Diagnosis |
|
|
| Tumor Biopsy | Histologic analysis of the tumor remains the gold standard for the diagnosis of GBM and other tumor types. |
|
| GBM Treatment and Post-treatment Techniques | ||
|---|---|---|
| Technique | Advantages | Disadvantages |
| Tumor Resection |
|
|
| Chemotherapy |
|
|
| Radiotherapy |
|
|
| Intraoperative Radiotherapy |
Delivers more specifically a dose of radiation to the tumor bed intraoperatively. |
|
| Electric Fields |
|
|
| Post-treatment Imaging |
|
|
CURRENT CLINICAL TRIALS FOR GBM TREATMENT
Several clinical trials for GBM treatment are currently registered in the ClinicalTrials.gov database (https://clinicaltrials.gov). Some of them have been completed, and others are ongoing or have not started recruiting participants yet. The status of some relevant clinical trials for GBM treatment is summarized in the Table 2. Most of the clinical trials incorporate another novel chemotherapeutic agent with the standard TMZ+XRT. A few examples are: (1) varinostat, a planar polar compound that binds to the catalytic domain of the histone deacetylases (HDAC) and activates transcription of genes that control cell cycle progression and apoptosis [162] (clinical trial: NCT01110876); (2) bevacizunab (Avastin, approved by FDA in 2004), a recombinant humanized monoclonal antibody that produces angiogenesis inhibition by inhibiting vascular endothelial growth factor (VEGF) (clinical trial: NCT00590681); (3) dacomitinib, a potent and irreversible EGFR inhibitor showed to cross the BBB in preclinical models [163] (clinical trial: NCT01520870); and (4) ANG1005, a 19 amino acid long peptide vector conjugated to paclitaxel that are able to cross the BBB [164–165] (clinical trial: NCT01967810).
Table 2.
Examples of relevant completed and ongoing GBM Clinical Trials
| Clinical Trial Name | Identifier | Current Status | References |
|---|---|---|---|
| Phase II Study of Concurrent Radiation Therapy, Temozolomide, and Bevacizumab Followed by Bevacizumab/Everolimus in the First-line of Treatment of Patients With Glioblastoma Multiforme. |
NCT00805961 | Completed: bevacizumab and everolimus as part of first-line combined modality therapy (RT plus TMZ) for glioblastoma was feasible and efficacious. |
[169–171] |
| Phase II Study of Bevacizumab Plus Temodar and Tarceva After Radiation Therapy and Temodar in Patients With Newly Diagnosed Glioblastoma or Gliosarcoma Who Are Stable Following Radiation. |
NCT00525525 | Completed: the combination of bevacizumab, erlotinib, TMZ, and was well tolerated and improved progression-free survival however, did not improve the OS. |
[132–133] |
| Phase I / II Adaptive Randomized Trial of Vorinostat, Erlotinib and Temozolomide in Adults With Recurrent Glioblastoma Multiforme. |
NCT01110876 | Termined: Unanticipated Toxicities. | [162] |
| Phase II Avastin and Temozolomide Following Radiation and Chemotherapy for Newly Diagnosed Glioblastoma Multiforme. |
NCT00590681 | Completed: no results have been published yet. |
[116–128] |
| Phase II Study of Bevacizumab Plus Either Temozolomide or Etoposide for (GBM) Patients Who Have Failed Bevacizumab Plus Irinotecan. |
NCT00613028 | Completed: results have not been published yet. |
[172] |
| Phase I Trial of Vaccination With Autologous Dendritic Cells Pulsed With Lysate Derived From an Allogeneic Glioblastoma Stem-like Cell Line for Patients With Newly Diagnosed or Recurrent Glioblastoma. |
NCT02010606 | Currently recruiting participants. | [183] |
| Phase II Study of TRC102 in Combination With Temozolomide for Recurrent Glioblastoma |
NCT02395692 | Currently recruiting participants. | [168] |
| Phase III Trial of CCNU/Temozolomide (TMZ) Combination Therapy vs. Standard TMZ Therapy for Newly Diagnosed MGMT-methylated Glioblastoma Patients. |
NCT01149109 | Ongoing, but not recruiting participants. | [129, 166] |
| Phase II Pilot, Prospective, Open Label, Multicenter CT, to Evaluate the Safety and Efficacy of PF299804, a Pan-HER Irreversible Inhibitor, in Patients With Recurrent Glioblastoma With EGFR Amplification or Presence of EGFRvIII Mutation. |
NCT01520870 | Ongoing, but not recruiting participants. | [163] |
| Phase II, Open-Label, Multi-Center Study of ANG1005 (paclitaxel- peptide drug conjugate in Patients With Recurrent High-Grade Glioma. |
NCT01967810 | Ongoing, but not recruiting participants. | [164–165] |
| Phase I/II Randomized Prospective Trial for Newly Diagnosed GBM, With Upfront Gross Total Resection, Gliadel, Followed by TMZ With Concurrent Standard Radiation or GammaKnife for New GBM. |
NCT02085304 | Ongoing, but not recruiting participants. | [105, 115, 167] |
| Phase II Study of Methoxyamine (TRC102) in Combination With Temozolomide for Recurrent Glioblastoma. |
NCT02395692 | Currently recruiting participants. | [168] |
| Phase III Clinical Trial Evaluating DCVax®-L, Autologous Dendritic Cells Pulsed With Tumor Lysate Antigen For The Treatment Of Glioblastoma Multiforme (GBM). |
NCT00045968 | Ongoing, but not recruiting participants. | [185] |
| Phase I Trial of Combination of DNX-2401 (Formerly Named Delta-24- RGD) Oncolytic Adenovirus With a Short Course of Temozolomide for Treatment of Glioblastoma at First Recurrent. |
NCT01956734 | Ongoing, but not recruiting participants. | [179] |
| Phase 1b, Randomized, Multi-center, Open-label Study of a Conditionally Replicative Adenovirus (DNX-2401) and Interferon Gamma (IFN-γ) for Recurrent Glioblastoma or Gliosarcoma (TARGET-I). |
NCT02197169 | Currently recruiting participants. | [180] |
| Phase III, International, Randomized, Double-Blind, Controlled Study of Rindopepimut/GM-CSF With Adjuvant Temozolomide in Patients With Newly Diagnosed, Surgically Resected, EGFRvIII-positive Glioblastoma. |
NCT01480479 | Ongoing, but not recruiting participants. | [181] |
| Phase I Administration of HER2 Chimeric Antigen Receptor Expressing CMV-Specific Cytotoxic T Cells Ins Patients With Glioblastoma Multiforme (HERT-GBM). |
NCT01109095 | Ongoing, but not recruiting participants. | [186] |
Other interventional trials include the use of: (1) lomustine, which inhibits both DNA and RNA synthesis through DNA alkylation [166] (clinical trial: NCT01149109); (2) Gliadel (carmustine) wafers followed by TMZ with standard radiation or GammaKnife for newly diagnosed GBMs [105, 115, 167] (clinical trial: NCT02085304); and (3) Methoxyamine, (TRC-102) an orally bioavailable small molecule inhibitor that covalently binds to DNA damage, inhibits base excision repair, increases DNA strand breaks, and apoptosis in a phase II trial [168] (clinical trial NCT02395692).
Additional trials are using bevacizumab and TMZ together as baseline chemotherapy with the incorporation of other anticancer agents, such as: (1) everolimus, an mTOR inhibitor [169–171] (clinical trial: NCT00805961); (2) Tarceva (clinical trial: NCT00525525); (3) etoposide, a topoisomerase-II inhibitor [172] (clinical trial: NCT00613028); and (4) irinotecan, a topoisomerase I inhibitor [173–176] (multiple clinical trials registered with this drug), among others.
Immunotherapy, gene therapies, electric fields, and novel devices for radiation are also being proposed as possible therapeutic approaches. One example is DNX-2401, an oncolytic adenovirus that is injected inside the tumor shortly after diagnosis [177–178]. This viral protein has been modified to recognize specific proteins in the tumor cells and not affect the normal healthy cells; once inside the cancer cells, the viruses replicate rapidly and kill cancer cells [179] (clinical trial: NCT01956734). A clinical trial with DNX-2401 and interferon gamma [180] (NCT02197169) is also being studied.
Additional clinical trials of note include: (1) a randomized arm of a phase III study assessing the safety/efficacy of Rindopepimut (also known as CDX-110) in combination with TMZ [181] (clinical trial: NCT01480479), where CDX-110 is an experimental cancer vaccine that promotes anti-cancer effects in GBM patients expressing the EGFRvIII protein [181–182]; (2) a phase I study of a dendritic cell vaccine (ICT-107) for patients with either newly diagnosed or recurrent GBM [183] (NCT02010606), which uses the patient's own immune-stimulating dendritic cells (DC) to designed the vaccine and promote an immune response [184]; (3) a phase III clinical trial evaluating DCVax(R)-L in patients with newly diagnosed GBM and positive to EGFRνIII [185] (clinical trial: NCT00045968), where the DCVax(R)-L is designed from the patient's own tumor lysate, containing tumor antigens, and white blood cells, containing DC precursors, to induce a de novo immune response against brain cancer cells [185]. Another phase I clinical trial (NCT01109095) involves the use of T-cells attached to a Human Epidermal Growth Factor Receptor 2 (HER2) –Specific Chimeric Antigen Receptor (HER2-CAR) chimeric antibody [186], taking advantage of the fact that HER2 is expressed in up to 80% of GBM cells, and the CAR protein increases the potency of the T-cells to recognize and bind to Cytomegalovirus, a virus present in most humans [187].
CONCLUSIONS AND FUTURE DIRECTIONS
As in most cancers, the diagnosis of GBM is not feasible until major symptoms appear which in GBM are more than often devastating, life-threatening and the patients remain with variable types of impairments or disabilities. Furthermore, the standard-of-care treatment for GBM (tumor resection plus TMZ+XRT) has remained the same for the last 15 years, even though this regimen is definitely not optimal. The highly infiltrative and invasive nature of GBM continue to be major obstacles for complete tumor resection, and combining TMZ+XRT with novel chemotherapeutic agents have improved only slightly the OS and PFS of GBM patients. For GBM, it is evident that non-invasive screening tests for early detection and more effective therapeutic options are urgently needed. Several molecular biomarkers that discriminate between GBM and other brain tumors have been proposed, as well as, novel targets for therapy have been identified; however, all are still being investigated.
Over the past 20 years, new technologies for GBM diagnosis and treatment have been developed. The diagnosis of GBM has evolved from being always invasive with subjective histological analysis to the capability of rendering a diagnosis with very sophisticated contrast-enhanced MRI and H-MRS. The advances in these imaging modalities have allowed not only to differentiate GBMs from other brain tumors with high accuracy, but also to detect tumor extension. In addition, MRIs and 1H-MRS modalities are being used as post-treatment imaging tools to assess the extension of tumor resection, and to evaluate the effectiveness of the GBM treatment.
Regarding TMZ treatment, a major disadvantage is that GBM patients become rapidly resistant to a second intervention with TMZ. Because of this, the study of drug resistance mechanisms and the design of novel compounds to overcome such resistance are two areas of intense research [188–189]. Also, the design of drug delivery systems capable of crossing the BBB is an area of interest being investigated. Another obstacle for successful GBM treatment relate to the heterogeneous diversity of cancer cell subclones (including CSC) that are present in the tumor at diagnosis, not accounting for the genetic evolution that usually occur in cancer cell subclones after exposure to radiation and chemotherapy. Knowing this, therapies targeting various cell survival pathways in GBM, as well as in other cancers, have been proposed. To definitely impact the outcomes of patients with GBM, further investigation should be focused on the following proposed areas: (1) elucidation of the key molecular events; (2) definition of risk factors associated with the malignant transformation of astrocytes into astrocytomas and GBMs; (3) elucidation of the origin of CSC populations; (4) identification of less invasive molecular biomarkers for early tumor diagnosis; (5) identification of more specific molecular targets for therapy; (6) design of optimal drugs and drug carriers capable of crossing the BBB; and finally, (7) creation of better animal models imitating the origin and progression of astrocytomas in human beings, for eventual testing of novel chemotherapeutic agents.
Acknowledgments
We acknowledge Dr. Orlando de Jesus for the critical revision of the manuscript. This project was supported partially by institutional seed funds from the University of Puerto Rico Comprehensive Cancer Center (PEVM), PRCTRC (NCRR: U54 RR 026139-01A1), NIMHD: 8U54 MD 007587-03) and RCMI: NCRR (2G12-RR003051).
We wish to thank the editorial services of Dr. Deana Hallman from the Puerto Rico Clinical & Translational Research Consortium, which is supported by the National Institute on Minority Health and Health Disparities of the National Institutes of Health under Award Number 2U54MD007587.
Footnotes
CONFLICT OF INTEREST
We declare that we have no conflict of interest.
REFERENCES
- 1.Furnari FB, Fenton T, Bachoo RM, et al. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 2007;21:2683–2710. doi: 10.1101/gad.1596707. [DOI] [PubMed] [Google Scholar]
- 2.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shugg D, Allen BJ, Blizzard L, et al. Brain cancer incidence, mortality and case survival: observations from two Australian cancer registries. Int J Cancer. 1994;59:765–770. doi: 10.1002/ijc.2910590610. [DOI] [PubMed] [Google Scholar]
- 4.Ostrom QT, Gittleman H, Fulop J, et al. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2008–2012. Neuro Oncol. 2015;17(Suppl 4):iv1–iv62. doi: 10.1093/neuonc/nov189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adamson C, Kanu OO, Mehta AI, et al. Glioblastoma multiforme: a review of where we have been and where we are going. Expert Opin Investig Drugs. 2009;18:1061–1083. doi: 10.1517/13543780903052764. [DOI] [PubMed] [Google Scholar]
- 6.Raghavan R, Steart PV, Weller RO. Cell proliferation patterns in the diagnosis of astrocytomas, anaplastic astrocytomas and glioblastoma multiforme: a Ki-67 study. Neuropathol Appl Neurobiol. 1990;16:123–133. doi: 10.1111/j.1365-2990.1990.tb00941.x. [DOI] [PubMed] [Google Scholar]
- 7.Scott JN, Rewcastle NB, Brasher PM, et al. Which glioblastoma multiforme patient will become a long-term survivor? A population-based study. Ann Neurol. 1999;46:183–188. [PubMed] [Google Scholar]
- 8.Verhaak RG, Hoadley KA, Purdom E, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17:98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohgaki H, Kleihues P. Epidemiology and etiology of gliomas. Acta Neuropathol. 2005;109:93–108. doi: 10.1007/s00401-005-0991-y. [DOI] [PubMed] [Google Scholar]
- 10.Kim H, Huang W, Jiang X, et al. Integrative genome analysis reveals an oncomir/oncogene cluster regulating glioblastoma survivorship. Proc Natl Acad Sci U S A. 2010;107:2183–2188. doi: 10.1073/pnas.0909896107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saunus JM, Quinn MC, Patch AM, et al. Integrated genomic and transcriptomic analysis of human brain metastases identifies alterations of potential clinical significance. J Pathol. 2015 doi: 10.1002/path.4583. [DOI] [PubMed] [Google Scholar]
- 12.Wang J, Wang H, Li Z, et al. c-Myc is required for maintenance of glioma cancer stem cells. PLoS One. 2008;3:e3769. doi: 10.1371/journal.pone.0003769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Catacuzzeno L, Michelucci A, Sforna L, et al. Identification of key signaling molecules involved in the activation of the swelling-activated chloride current in human glioblastoma cells. J Membr Biol. 2014;247:45–55. doi: 10.1007/s00232-013-9609-9. [DOI] [PubMed] [Google Scholar]
- 14.Molenaar RJ. Ion channels in glioblastoma. ISRN Neurol. 2011;2011:590249. doi: 10.5402/2011/590249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rao SA, Santosh V, Somasundaram K. Genome-wide expression profiling identifies deregulated miRNAs in malignant astrocytoma. Mod Pathol. 2010;23:1404–1417. doi: 10.1038/modpathol.2010.135. [DOI] [PubMed] [Google Scholar]
- 16.Liu X, Fortin K, Mourelatos Z. MicroRNAs: biogenesis and molecular functions. Brain Pathol. 2008;18:113–121. doi: 10.1111/j.1750-3639.2007.00121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 18.Lu J, Getz G, Miska EA, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 19.Turner JD, Williamson R, Almefty KK, et al. The many roles of microRNAs in brain tumor biology. Neurosurg Focus. 2010;28:E3. doi: 10.3171/2009.10.FOCUS09207. [DOI] [PubMed] [Google Scholar]
- 20.Malzkorn B, Wolter M, Liesenberg F, et al. Identification and functional characterization of microRNAs involved in the malignant progression of gliomas. Brain Pathol. 2010;20:539–550. doi: 10.1111/j.1750-3639.2009.00328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gonzalez-Gomez P, Sanchez P, Mira H. MicroRNAs as regulators of neural stem cell-related pathways in glioblastoma multiforme. Mol Neurobiol. 2011;44:235–249. doi: 10.1007/s12035-011-8196-y. [DOI] [PubMed] [Google Scholar]
- 22.Yang HW, Xing H, Johnson MD. A major role for microRNAs in glioblastoma cancer stem-like cells. Arch Pharm Res. 2015;38:423–434. doi: 10.1007/s12272-015-0574-y. [DOI] [PubMed] [Google Scholar]
- 23.Manterola L, Guruceaga E, Gallego Perez-Larraya J, et al. A small noncoding RNA signature found in exosomes of GBM patient serum as a diagnostic tool. Neuro Oncol. 2014;16:520–527. doi: 10.1093/neuonc/not218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barbano R, Palumbo O, Pasculli B, et al. A miRNA signature for defining aggressive phenotype and prognosis in gliomas. PLoS One. 2014;9:e108950. doi: 10.1371/journal.pone.0108950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zadran S, Remacle F, Levine R. Surprisal analysis of Glioblastoma Multiform (GBM) microRNA dynamics unveils tumor specific phenotype. PLoS One. 2014;9:e108171. doi: 10.1371/journal.pone.0108171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li R, Gao K, Luo H, et al. Identification of intrinsic subtype-specific prognostic microRNAs in primary glioblastoma. J Exp Clin Cancer Res. 2014;33:9. doi: 10.1186/1756-9966-33-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Visani M, de Biase D, Marucci G, et al. Expression of 19 microRNAs in glioblastoma and comparison with other brain neoplasia of grades I-III. Mol Oncol. 2014;8:417–430. doi: 10.1016/j.molonc.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roth P, Wischhusen J, Happold C, et al. A specific miRNA signature in the peripheral blood of glioblastoma patients. J Neurochem. 2011;118:449–457. doi: 10.1111/j.1471-4159.2011.07307.x. [DOI] [PubMed] [Google Scholar]
- 29.Rivera-Diaz M, Miranda-Roman MA, Soto D, et al. MicroRNA-27a distinguishes glioblastoma multiforme from diffuse and anaplastic astrocytomas and has prognostic value. Am J Cancer Res. 2015;5:201–218. [PMC free article] [PubMed] [Google Scholar]
- 30.Guan Y, Mizoguchi M, Yoshimoto K, et al. MiRNA-196 is upregulated in glioblastoma but not in anaplastic astrocytoma and has prognostic significance. Clin Cancer Res. 2010;16:4289–4297. doi: 10.1158/1078-0432.CCR-10-0207. [DOI] [PubMed] [Google Scholar]
- 31.Genovese G, Ergun A, Shukla SA, et al. microRNA regulatory network inference identifies miR-34a as a novel regulator of TGF-beta signaling in glioblastoma. Cancer Discov. 2012;2:736–749. doi: 10.1158/2159-8290.CD-12-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nass D, Rosenwald S, Meiri E, et al. MiR-92b and miR-9/9* are specifically expressed in brain primary tumors and can be used to differentiate primary from metastatic brain tumors. Brain Pathol. 2009;19:375–383. doi: 10.1111/j.1750-3639.2008.00184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yue X, Lan F, Hu M, et al. Downregulation of serum microRNA-205 as a potential diagnostic and prognostic biomarker for human glioma. J Neurosurg. 2015:1–7. doi: 10.3171/2015.1.JNS141577. [DOI] [PubMed] [Google Scholar]
- 34.Wang Q, Li P, Li A, et al. Plasma specific miRNAs as predictive biomarkers for diagnosis and prognosis of glioma. J Exp Clin Cancer Res. 2012;31:97. doi: 10.1186/1756-9966-31-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lathia JD, Mack SC, Mulkearns-Hubert EE, et al. Cancer stem cells in glioblastoma. Genes Dev. 2015;29:1203–1217. doi: 10.1101/gad.261982.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brower JV, Clark PA, Lyon W, Kuo JS. MicroRNAs in cancer: glioblastoma and glioblastoma cancer stem cells. Neurochem Int. 2014;77:68–77. doi: 10.1016/j.neuint.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seton-Rogers S. Glioblastoma: Cancer stem cell knockout. Nat Rev Cancer. 2014;14:452–453. doi: 10.1038/nrc3771. [DOI] [PubMed] [Google Scholar]
- 38.Kamnasaran D. Stem cells and models of astrocytomas. Clin Invest Med. 2009;32:E166–E179. doi: 10.25011/cim.v32i2.6035. [DOI] [PubMed] [Google Scholar]
- 39.Gerson SL. Clinical relevance of MGMT in the treatment of cancer. J Clin Oncol. 2002;20:2388–2399. doi: 10.1200/JCO.2002.06.110. [DOI] [PubMed] [Google Scholar]
- 40.Hau P, Stupp R, Hegi ME. MGMT methylation status: the advent of stratified therapy in glioblastoma? Dis Markers. 2007;23:97–104. doi: 10.1155/2007/159242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tran AN, Lai A, Li S, et al. Increased sensitivity to radiochemotherapy in IDH1 mutant glioblastoma as demonstrated by serial quantitative MR volumetry. Neuro Oncol. 2014;16:414–420. doi: 10.1093/neuonc/not198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arita H, Narita Y, Matsushita Y, et al. Development of a robust and sensitive pyrosequencing assay for the detection of IDH1/2 mutations in gliomas. Brain Tumor Pathol. 2015;32:22–30. doi: 10.1007/s10014-014-0186-0. [DOI] [PubMed] [Google Scholar]
- 43.Zou P, Xu H, Chen P, et al. IDH1/IDH2 mutations define the prognosis and molecular profiles of patients with gliomas: a meta-analysis. PLoS One. 2013;8:e68782. doi: 10.1371/journal.pone.0068782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ye D, Xiong Y, Guan KL. The mechanisms of IDH mutations in tumorigenesis. Cell Res. 2012;22:1102–1104. doi: 10.1038/cr.2012.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360:765–773. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang C, Moore LM, Li X, et al. IDH1/2 mutations target a key hallmark of cancer by deregulating cellular metabolism in glioma. Neuro Oncol. 2013;15:1114–1126. doi: 10.1093/neuonc/not087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Osborn AG. Osborn's brain : imaging, pathology, and anatomy. xi. Salt Lake City, Utah: Amirsys Pub.; 2013. p. 1272. [Google Scholar]
- 48.Scarabino T, Popolizio T, Trojsi F, et al. Role of advanced MR imaging modalities in diagnosing cerebral gliomas. Radiol Med. 2009;114:448–460. doi: 10.1007/s11547-008-0351-9. [DOI] [PubMed] [Google Scholar]
- 49.Patil CG, Yi A, Elramsisy A, et al. Prognosis of patients with multifocal glioblastoma: a case-control study. J Neurosurg. 2012;117:705–711. doi: 10.3171/2012.7.JNS12147. [DOI] [PubMed] [Google Scholar]
- 50.Chavhan GB, Babyn PS, Thomas B, et al. Principles, techniques, and applications of T2*-based MR imaging and its special applications. Radiographics. 2009;29:1433–1449. doi: 10.1148/rg.295095034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hou BL, Bradbury M, Peck KK, et al. Effect of brain tumor neovasculature defined by rCBV on BOLD fMRI activation volume in the primary motor cortex. Neuroimage. 2006;32:489–497. doi: 10.1016/j.neuroimage.2006.04.188. [DOI] [PubMed] [Google Scholar]
- 52.Rees JH, Smirniotopoulos JG, Jones RV, Wong K. Glioblastoma multiforme: radiologic-pathologic correlation. Radiographics. 1996;16:1413–1438. doi: 10.1148/radiographics.16.6.8946545. quiz 1462–3. [DOI] [PubMed] [Google Scholar]
- 53.Kazi AZ, Joshi PC, Kelkar AB, et al. MRI evaluation of pathologies affecting the corpus callosum: A pictorial essay. Indian J Radiol Imaging. 2013;23:321–332. doi: 10.4103/0971-3026.125604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Renard D, Castelnovo G, Campello C, et al. Thalamic lesions: a radiological review. Behav Neurol. 2014;2014:154631. doi: 10.1155/2014/154631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brandao LA, Shiroishi MS, Law M. Brain tumors: a multimodality approach with diffusion-weighted imaging, diffusion tensor imaging, magnetic resonance spectroscopy, dynamic susceptibility contrast and dynamic contrast-enhanced magnetic resonance imaging. Magn Reson Imaging Clin N Am. 2013;21:199–239. doi: 10.1016/j.mric.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 56.Brandao LA, Castillo M. Adult brain tumors: clinical applications of magnetic resonance spectroscopy. Neuroimaging Clin N Am. 2013;23:527–555. doi: 10.1016/j.nic.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 57.Romano A, Calabria LF, Tavanti F, et al. Apparent diffusion coefficient obtained by magnetic resonance imaging as a prognostic marker in glioblastomas: correlation with MGMT promoter methylation status. Eur Radiol. 2013;23:513–520. doi: 10.1007/s00330-012-2601-4. [DOI] [PubMed] [Google Scholar]
- 58.Pope WB, Qiao XJ, Kim HJ, et al. Apparent diffusion coefficient histogram analysis stratifies progression-free and overall survival in patients with recurrent GBM treated with bevacizumab: a multi-center study. J Neurooncol. 2012;108:491–498. doi: 10.1007/s11060-012-0847-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ellingson BM, Bendszus M, Sorensen AG, Pope WB. Emerging techniques and technologies in brain tumor imaging. Neuro Oncol. 2014;16(Suppl 7):vii12–vii23. doi: 10.1093/neuonc/nou221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rumboldt Z, Camacho DL, Lake D, et al. Apparent diffusion coefficients for differentiation of cerebellar tumors in children. AJNR Am J Neuroradiol. 2006;27:1362–1369. [PMC free article] [PubMed] [Google Scholar]
- 61.Pope WB, Kim HJ, Huo J, et al. Recurrent glioblastoma multiforme: ADC histogram analysis predicts response to bevacizumab treatment. Radiology. 2009;252:182–189. doi: 10.1148/radiol.2521081534. [DOI] [PubMed] [Google Scholar]
- 62.Zinn PO, Hatami M, Colen RR. 138 Diffusion MRI ADC Mapping of Glioblastoma Edema/Tumor Invasion and Associated Gene Signatures. Neurosurgery. 2015;62(Suppl 1):210. [Google Scholar]
- 63.Rundle-Thiele D, Day B, Stringer B, et al. Using the apparent diffusion coefficient to identifying MGMT promoter methylation status early in glioblastoma: importance of analytical method. J Med Radiat Sci. 2015;62:92–98. doi: 10.1002/jmrs.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Woodworth DC, Pope WB, Liau LM, et al. Nonlinear distortion correction of diffusion MR images improves quantitative DTI measurements in glioblastoma. J Neurooncol. 2014;116:551–558. doi: 10.1007/s11060-013-1320-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Debnam JM, Schellingerhout D. Diffusion MR Imaging of the Brain in Patients with Cancer. Int J Mol Imaging. 2011;2011:714021. doi: 10.1155/2011/714021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ellingson BM, Kim E, Woodworth DC, et al. Diffusion MRI quality control and functional diffusion map results in ACRIN 6677/RTOG 0625: a multicenter, randomized, phase II trial of bevacizumab and chemotherapy in recurrent glioblastoma. Int J Oncol. 2015;46:1883–1892. doi: 10.3892/ijo.2015.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pollice S, Muto M, Scarabino T. Post-therapeutic imaging findings. Eur J Radiol. 2015;84:799–806. doi: 10.1016/j.ejrad.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 68.Mabray MC, Barajas RF, Jr, Cha S. Modern brain tumor imaging. Brain Tumor Res Treat. 2015;3:8–23. doi: 10.14791/btrt.2015.3.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Di Costanzo A, Pollice S, Trojsi F, et al. Role of perfusion-weighted imaging at 3 Tesla in the assessment of malignancy of cerebral gliomas. Radiol Med. 2008;113:134–143. doi: 10.1007/s11547-008-0232-2. [DOI] [PubMed] [Google Scholar]
- 70.D'Angelo V, Napolitano M, Gorgoglione L, et al. Surgical treatment of anterior callosal tumors. J Neurosurg Sci. 1997;41:117–122. [PubMed] [Google Scholar]
- 71.Di Costanzo A, Trojsi F, Giannatempo GM, et al. Spectroscopic, diffusion and perfusion magnetic resonance imaging at 3.0 Tesla in the delineation of glioblastomas: preliminary results. J Exp Clin Cancer Res. 2006;25:383–390. [PubMed] [Google Scholar]
- 72.Di Costanzo A, Scarabino T, Trojsi F, et al. Recurrent glioblastoma multiforme versus radiation injury: a multiparametric 3-T MR approach. Radiol Med. 2014;119:616–624. doi: 10.1007/s11547-013-0371-y. [DOI] [PubMed] [Google Scholar]
- 73.Jabehdar Maralani P, Melhem ER, Wang S, et al. Association of dynamic susceptibility contrast enhanced MR Perfusion parameters with prognosis in elderly patients with glioblastomas. Eur Radiol. 2015;25:2738–2744. doi: 10.1007/s00330-015-3640-4. [DOI] [PubMed] [Google Scholar]
- 74.Giesel FL, Wustenberg T, Bongers A, et al. [MR-based methods of the functional imaging of the central nervous system] Rofo. 2005;177:714–730. doi: 10.1055/s-2005-858108. [DOI] [PubMed] [Google Scholar]
- 75.Gasser T, Szelenyi A, Senft C, et al. Intraoperative MRI and functional mapping. Acta Neurochir Suppl. 2011;109:61–65. doi: 10.1007/978-3-211-99651-5_10. [DOI] [PubMed] [Google Scholar]
- 76.Di Costanzo A, Scarabino T, Trojsi F, et al. Proton MR spectroscopy of cerebral gliomas at 3 T: spatial heterogeneity, and tumour grade and extent. Eur Radiol. 2008;18:1727–1735. doi: 10.1007/s00330-008-0938-5. [DOI] [PubMed] [Google Scholar]
- 77.Essig M, Anzalone N, Combs SE, et al. MR imaging of neoplastic central nervous system lesions: review and recommendations for current practice. AJNR Am J Neuroradiol. 2012;33:803–817. doi: 10.3174/ajnr.A2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tsougos I, Svolos P, Kousi E, et al. Differentiation of glioblastoma multiforme from metastatic brain tumor using proton magnetic resonance spectroscopy, diffusion and perfusion metrics at 3 T. Cancer Imaging. 2012;12:423–436. doi: 10.1102/1470-7330.2012.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fink KR, Fink JR. Imaging of brain metastases. Surg Neurol Int. 2013;4:S209–S219. doi: 10.4103/2152-7806.111298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shukla-Dave A, Gupta RK, Roy R, et al. Prospective evaluation of in vivo proton MR spectroscopy in differentiation of similar appearing intracranial cystic lesions. Magnetic Resonance Imaging. 2001;19:103–110. doi: 10.1016/s0730-725x(01)00224-7. [DOI] [PubMed] [Google Scholar]
- 81.Woodworth G, McGirt MJ, Samdani A, et al. Accuracy of frameless and frame-based image-guided stereotactic brain biopsy in the diagnosis of glioma: comparison of biopsy and open resection specimen. Neurol Res. 2005;27:358–362. doi: 10.1179/016164105X40057. [DOI] [PubMed] [Google Scholar]
- 82.Kepes JJ. Pitfalls and problems in the histopathologic evaluation of stereotactic needle biopsy specimens. Neurosurg Clin N Am. 1994;5:19–33. [PubMed] [Google Scholar]
- 83.Jackson RJ, Fuller GN, Abi-Said D, et al. Limitations of stereotactic biopsy in the initial management of gliomas. Neuro Oncol. 2001;3:193–200. doi: 10.1093/neuonc/3.3.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Heper AO, Erden E, Savas A, et al. An analysis of stereotactic biopsy of brain tumors and nonneoplastic lesions: a prospective clinicopathologic study. Surg Neurol. 2005;64(Suppl 2):S82–S88. doi: 10.1016/j.surneu.2005.07.055. [DOI] [PubMed] [Google Scholar]
- 85.Young RM, Jamshidi A, Davis G, Sherman JH. Current trends in the surgical management and treatment of adult glioblastoma. Ann Transl Med. 2015;3:121. doi: 10.3978/j.issn.2305-5839.2015.05.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bernstein M, Parrent AG. Complications of CT-guided stereotactic biopsy of intra-axial brain lesions. J Neurosurg. 1994;81:165–168. doi: 10.3171/jns.1994.81.2.0165. [DOI] [PubMed] [Google Scholar]
- 87.Panciani PP, Fontanella M, Schatlo B, et al. Fluorescence and image guided resection in high grade glioma. Clin Neurol Neurosurg. 2012;114:37–41. doi: 10.1016/j.clineuro.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 88.Stummer W, Pichlmeier U, Meinel T, et al. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7:392–401. doi: 10.1016/S1470-2045(06)70665-9. [DOI] [PubMed] [Google Scholar]
- 89.Vuorinen V, Hinkka S, Farkkila M, Jaaskelainen J. Debulking or biopsy of malignant glioma in elderly people - a randomised study. Acta Neurochir (Wien) 2003;145:5–10. doi: 10.1007/s00701-002-1030-6. [DOI] [PubMed] [Google Scholar]
- 90.Keles GE, Anderson B, Berger MS. The effect of extent of resection on time to tumor progression and survival in patients with glioblastoma multiforme of the cerebral hemisphere. Surg Neurol. 1999;52:371–379. doi: 10.1016/s0090-3019(99)00103-2. [DOI] [PubMed] [Google Scholar]
- 91.Schulz C, Waldeck S, Mauer UM. Intraoperative image guidance in neurosurgery: development, current indications, and future trends. Radiol Res Pract. 2012;2012:197364. doi: 10.1155/2012/197364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Barone DG, Lawrie TA, Hart MG. Image guided surgery for the resection of brain tumours. Cochrane Database Syst Rev. 2014;1:CD009685. doi: 10.1002/14651858.CD009685.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pamir MN, Seifert V, K*r**s T. Intraoperative imaging. x. Wien; New York: SpringerWienNewYork; 2011. p. 272. [Google Scholar]
- 94.Roberts DW, Hartov A, Kennedy FE, et al. Intraoperative brain shift and deformation: a quantitative analysis of cortical displacement in 28 cases. Neurosurgery. 1998;43:749–758. doi: 10.1097/00006123-199810000-00010. discussion 758–60. [DOI] [PubMed] [Google Scholar]
- 95.Senft C, Bink A, Franz K, et al. Intraoperative MRI guidance and extent of resection in glioma surgery: a randomised, controlled trial. Lancet Oncol. 2011;12:997–1003. doi: 10.1016/S1470-2045(11)70196-6. [DOI] [PubMed] [Google Scholar]
- 96.Hall WA, Nimsky C, Truwit CL. Intraoperative MRI-guided neurosurgery. xv. New York: Thieme; 2011. p. 256. [Google Scholar]
- 97.Regula J, MacRobert AJ, Gorchein A, et al. Photosensitisation and photodynamic therapy of oesophageal, duodenal, and colorectal tumours using 5 aminolaevulinic acid induced protoporphyrin IX--a pilot study. Gut. 1995;36:67–75. doi: 10.1136/gut.36.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Aldave G, Tejada S, Pay E, et al. Prognostic value of residual fluorescent tissue in glioblastoma patients after gross total resection in 5-aminolevulinic Acid-guided surgery. Neurosurgery. 2013;72:915–920. doi: 10.1227/NEU.0b013e31828c3974. discussion 920–1. [DOI] [PubMed] [Google Scholar]
- 99.Moiyadi A, Syed P, Srivastava S. Fluorescence-guided surgery of malignant gliomas based on 5-aminolevulinic acid: paradigm shifts but not a panacea. Nat Rev Cancer. 2014;14:146. doi: 10.1038/nrc3566-c1. [DOI] [PubMed] [Google Scholar]
- 100.Zhao S, Wu J, Wang C, et al. Intraoperative fluorescence-guided resection of high-grade malignant gliomas using 5-aminolevulinic acid-induced porphyrins: a systematic review and meta-analysis of prospective studies. PLoS One. 2013;8:e63682. doi: 10.1371/journal.pone.0063682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Stevens MF, Hickman JA, Langdon SP, et al. Antitumor activity and pharmacokinetics in mice of 8-carbamoyl-3-methyl-imidazo[5,1-d]-1,2,3,5-tetrazin-4(3H)-one (CCRG 81045; M & B 39831), a novel drug with potential as an alternative to dacarbazine. Cancer Res. 1987;47:5846–5852. [PubMed] [Google Scholar]
- 102.Stevens MF, Newlands ES. From triazines and triazenes to temozolomide. Eur J Cancer. 1993;29A:1045–1047. doi: 10.1016/s0959-8049(05)80221-7. [DOI] [PubMed] [Google Scholar]
- 103.Friedman HS, Kerby T, Calvert H. Temozolomide and treatment of malignant glioma. Clin Cancer Res. 2000;6:2585–2597. [PubMed] [Google Scholar]
- 104.Zhang J, Stevens MF, Bradshaw TD. Temozolomide: mechanisms of action, repair and resistance. Curr Mol Pharmacol. 2012;5:102–114. doi: 10.2174/1874467211205010102. [DOI] [PubMed] [Google Scholar]
- 105.Liu L, Taverna P, Whitacre CM, et al. Pharmacologic disruption of base excision repair sensitizes mismatch repair-deficient and -proficient colon cancer cells to methylating agents. Clin Cancer Res. 1999;5:2908–2917. [PubMed] [Google Scholar]
- 106.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 107.Weller M, Stupp R, Reifenberger G, et al. MGMT promoter methylation in malignant gliomas: ready for personalized medicine? Nat Rev Neurol. 2010;6:39–51. doi: 10.1038/nrneurol.2009.197. [DOI] [PubMed] [Google Scholar]
- 108.Javle M, Curtin NJ. The potential for poly (ADP-ribose) polymerase inhibitors in cancer therapy. Ther Adv Med Oncol. 2011;3:257–267. doi: 10.1177/1758834011417039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liu X, Han EK, Anderson M, et al. Acquired resistance to combination treatment with temozolomide and ABT-888 is mediated by both base excision repair and homologous recombination DNA repair pathways. Mol Cancer Res. 2009;7:1686–1692. doi: 10.1158/1541-7786.MCR-09-0299. [DOI] [PubMed] [Google Scholar]
- 110.St-Coeur PD, Poitras JJ, Cuperlovic-Culf M, et al. Investigating a signature of temozolomide resistance in GBM cell lines using metabolomics. J Neurooncol. 2015 doi: 10.1007/s11060-015-1899-6. [DOI] [PubMed] [Google Scholar]
- 111.Everhard S, Kaloshi G, Criniere E, et al. MGMT methylation: a marker of response to temozolomide in low-grade gliomas. Ann Neurol. 2006;60:740–743. doi: 10.1002/ana.21044. [DOI] [PubMed] [Google Scholar]
- 112.Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 113.Grossman R, Burger P, Soudry E, et al. MGMT inactivation and clinical response in newly diagnosed GBM patients treated with Gliadel. J Clin Neurosci. 2015 doi: 10.1016/j.jocn.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 114.Wedge SR, Porteous JK, Newlands ES. 3-aminobenzamide and/or O6-benzylguanine evaluated as an adjuvant to temozolomide or BCNU treatment in cell lines of variable mismatch repair status and O6-alkylguanine-DNA alkyltransferase activity. Br J Cancer. 1996;74:1030–1036. doi: 10.1038/bjc.1996.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Westphal M, Hilt DC, Bortey E, et al. A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro Oncol. 2003;5:79–88. doi: 10.1215/S1522-8517-02-00023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Borgstrom P, Bourdon MA, Hillan KJ, et al. Neutralizing anti-vascular endothelial growth factor antibody completely inhibits angiogenesis and growth of human prostate carcinoma micro tumors in vivo. Prostate. 1998;35:1–10. doi: 10.1002/(sici)1097-0045(19980401)35:1<1::aid-pros1>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 117.Borgstrom P, Hillan KJ, Sriramarao P, Ferrara N. Complete inhibition of angiogenesis and growth of microtumors by anti-vascular endothelial growth factor neutralizing antibody: novel concepts of angiostatic therapy from intravital videomicroscopy. Cancer Res. 1996;56:4032–4039. [PubMed] [Google Scholar]
- 118.Ferrara N. From the discovery of vascular endothelial growth factor to the introduction of avastin in clinical trials - an interview with Napoleone Ferrara by Domenico Ribatti. Int J Dev Biol. 2011;55:383–388. doi: 10.1387/ijdb.103216dr. [DOI] [PubMed] [Google Scholar]
- 119.Borgstrom P, Gold DP, Hillan KJ, Ferrara N. Importance of VEGF for breast cancer angiogenesis in vivo: implications from intravital microscopy of combination treatments with an anti-VEGF neutralizing monoclonal antibody and doxorubicin. Anticancer Res. 1999;19:4203–4214. [PubMed] [Google Scholar]
- 120.Ferrara N, Hillan KJ, Novotny W. Bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody for cancer therapy. Biochem Biophys Res Commun. 2005;333:328–335. doi: 10.1016/j.bbrc.2005.05.132. [DOI] [PubMed] [Google Scholar]
- 121.Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27:4733–4740. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 122.Kreisl TN, Kim L, Moore K, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27:740–745. doi: 10.1200/JCO.2008.16.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Levin VA, Crafts DC, Norman DM, et al. Criteria for evaluating patients undergoing chemotherapy for malignant brain tumors. J Neurosurg. 1977;47:329–335. doi: 10.3171/jns.1977.47.3.0329. [DOI] [PubMed] [Google Scholar]
- 124.Macdonald DR, Cascino TL, Schold SC, Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 125.Gilbert MR, Dignam JJ, Armstrong TS, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370:699–708. doi: 10.1056/NEJMoa1308573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lassen U, Sorensen M, Gaziel TB, et al. Phase II study of bevacizumab and temsirolimus combination therapy for recurrent glioblastoma multiforme. Anticancer Res. 2013;33:1657–1660. [PubMed] [Google Scholar]
- 127.Barone A, Sengupta R, Warrington NM, et al. Combined VEGF and CXCR4 antagonism targets the GBM stem cell population and synergistically improves survival in an intracranial mouse model of glioblastoma. Oncotarget. 2014;5:9811–9822. doi: 10.18632/oncotarget.2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bota DA, Alexandru D, Keir ST, et al. Proteasome inhibition with bortezomib induces cell death in GBM stem-like cells and temozolomide-resistant glioma cell lines, but stimulates GBM stem-like cells' VEGF production and angiogenesis. J Neurosurg. 2013;119:1415–1423. doi: 10.3171/2013.7.JNS1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Batchelor TT, Mulholland P, Neyns B, et al. Phase III randomized trial comparing the efficacy of cediranib as monotherapy, and in combination with lomustine, versus lomustine alone in patients with recurrent glioblastoma. J Clin Oncol. 2013;31:3212–3218. doi: 10.1200/JCO.2012.47.2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ohgaki H, Kleihues P. Genetic pathways to primary and secondary glioblastoma. Am J Pathol. 2007;170:1445–1453. doi: 10.2353/ajpath.2007.070011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hatanpaa KJ, Burma S, Zhao D, Habib AA. Epidermal growth factor receptor in glioma: signal transduction, neuropathology, imaging, and radioresistance. Neoplasia. 2010;12:675–684. doi: 10.1593/neo.10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wen PY, Chang SM, Lamborn KR, et al. Phase I/II study of erlotinib and temsirolimus for patients with recurrent malignant gliomas: North American Brain Tumor Consortium trial 04-02. Neuro Oncol. 2014;16:567–578. doi: 10.1093/neuonc/not247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Peereboom DM, Ahluwalia MS, Ye X, et al. NABTT 0502: a phase II and pharmacokinetic study of erlotinib and sorafenib for patients with progressive or recurrent glioblastoma multiforme. Neuro Oncol. 2013;15:490–496. doi: 10.1093/neuonc/nos322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shimizu S, Takehara T, Hikita H, et al. Inhibition of autophagy potentiates the antitumor effect of the multikinase inhibitor sorafenib in hepatocellular carcinoma. Int J Cancer. 2012;131:548–557. doi: 10.1002/ijc.26374. [DOI] [PubMed] [Google Scholar]
- 135.Wen PY, Yung WK, Lamborn KR, et al. Phase I/II study of imatinib mesylate for recurrent malignant gliomas: North American Brain Tumor Consortium Study 99-08. Clin Cancer Res. 2006;12:4899–4907. doi: 10.1158/1078-0432.CCR-06-0773. [DOI] [PubMed] [Google Scholar]
- 136.Orton CG. Uses of therapeutic x rays in medicine. Health Phys. 1995;69:662–676. doi: 10.1097/00004032-199511000-00004. [DOI] [PubMed] [Google Scholar]
- 137.Baskar R, Lee KA, Yeo R, Yeoh KW. Cancer and radiation therapy: current advances and future directions. Int J Med Sci. 2012;9:193–199. doi: 10.7150/ijms.3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wen N, Li H, Song K, et al. Characteristics of a novel treatment system for linear accelerator-based stereotactic radiosurgery. J Appl Clin Med Phys. 2015;16:5313. doi: 10.1120/jacmp.v16i4.5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yin AA, Cai S, Dong Y, et al. A meta-analysis of temozolomide versus radiotherapy in elderly glioblastoma patients. J Neurooncol. 2014;116:315–324. doi: 10.1007/s11060-013-1294-0. [DOI] [PubMed] [Google Scholar]
- 140.Yin AA, Zhang LH, Cheng JX, et al. Radiotherapy plus concurrent or sequential temozolomide for glioblastoma in the elderly: a meta-analysis. PLoS One. 2013;8:e74242. doi: 10.1371/journal.pone.0074242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yin AA, Cheng JX, Zhang X, Liu BL. The treatment of glioblastomas: a systematic update on clinical Phase III trials. Crit Rev Oncol Hematol. 2013;87:265–282. doi: 10.1016/j.critrevonc.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 142.Teo M, Martin S, Owusu-Agyemang K, et al. A survival analysis of GBM patients in the West of Scotland pre- and post-introduction of the Stupp regime. Br J Neurosurg. 2014;28:351–355. doi: 10.3109/02688697.2013.847170. [DOI] [PubMed] [Google Scholar]
- 143.Hsieh PC, Chandler JP, Bhangoo S, et al. Adjuvant gamma knife stereotactic radiosurgery at the time of tumor progression potentially improves survival for patients with glioblastoma multiforme. Neurosurgery. 2005;57:684–692. discussion 684–92. [PubMed] [Google Scholar]
- 144.Nwokedi EC, DiBiase SJ, Jabbour S, et al. Gamma knife stereotactic radiosurgery for patients with glioblastoma multiforme. Neurosurgery. 2002;50:41–46. doi: 10.1097/00006123-200201000-00009. discussion 46–7. [DOI] [PubMed] [Google Scholar]
- 145.Leksell L. Stereotactic radiosurgery. J Neurol Neurosurg Psychiatry. 1983;46:797–803. doi: 10.1136/jnnp.46.9.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Leksell D, Lindquist CE. Ladislau Steiner, 1920–2013. J Neurosurg. 2013;119:785–788. doi: 10.3171/2013.4.JNS13564. [DOI] [PubMed] [Google Scholar]
- 147.Leksell DG. Stereotactic radiosurgery: current status and future trends. Stereotact Funct Neurosurg. 1993;61(Suppl 1):1–5. [PubMed] [Google Scholar]
- 148.Aristu JJ, Ciervide R, Guridi J, et al. [Stereotactic radiation therapy] An Sist Sanit Navar. 2009;32(Suppl 2):61–71. doi: 10.23938/ASSN.0176. [DOI] [PubMed] [Google Scholar]
- 149.Thumma SR, Elaimy AL, Daines N, et al. Long-term survival after gamma knife radiosurgery in a case of recurrent glioblastoma multiforme: a case report and review of the literature. Case Rep Med. 2012;2012:545492. doi: 10.1155/2012/545492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wright G, Hatfield P, Loughrey C, et al. Quantifying and improving the efficiency of Gamma Knife treatment plans for brain metastases: results of a 1-year audit. J Neurosurg. 2014;121(Suppl):44–50. doi: 10.3171/2014.7.GKS141415. [DOI] [PubMed] [Google Scholar]
- 151.Dieterich S, Gibbs IC. The CyberKnife in clinical use: current roles, future expectations. Front Radiat Ther Oncol. 2011;43:181–194. doi: 10.1159/000322423. [DOI] [PubMed] [Google Scholar]
- 152.Kirson ED, Gurvich Z, Schneiderman R, et al. Disruption of cancer cell replication by alternating electric fields. Cancer Res. 2004;64:3288–3295. doi: 10.1158/0008-5472.can-04-0083. [DOI] [PubMed] [Google Scholar]
- 153.Hygino da Cruz LC, Jr, Rodriguez I, Domingues RC, et al. Pseudoprogression and pseudoresponse: imaging challenges in the assessment of posttreatment glioma. AJNR Am J Neuroradiol. 2011;32:1978–1985. doi: 10.3174/ajnr.A2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zeng QS, Li CF, Liu H, et al. Distinction between recurrent glioma and radiation injury using magnetic resonance spectroscopy in combination with diffusion-weighted imaging. Int J Radiat Oncol Biol Phys. 2007;68:151–158. doi: 10.1016/j.ijrobp.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 155.Liu ZL, Zhou Q, Zeng QS, et al. Noninvasive evaluation of cerebral glioma grade by using diffusion-weighted imaging-guided single-voxel proton magnetic resonance spectroscopy. J Int Med Res. 2012;40:76–84. doi: 10.1177/147323001204000108. [DOI] [PubMed] [Google Scholar]
- 156.Drevelegas A. Imaging of brain tumors with histological correlations. x. Berlin: Springer; 2011. p. 432. [Google Scholar]
- 157.Fathi Kazerooni A, Mohseni M, Rezaei S, et al. Multi-parametric (ADC/PWI/T2-w) image fusion approach for accurate semi-automatic segmentation of tumorous regions in glioblastoma multiforme. MAGMA. 2015;28:13–22. doi: 10.1007/s10334-014-0442-7. [DOI] [PubMed] [Google Scholar]
- 158.Essig M, Giesel F, Stieltjes B, Weber MA. [Functional imaging for brain tumors (perfusion, DTI and MR spectroscopy)] Radiologe. 2007;47:513–519. doi: 10.1007/s00117-007-1518-4. [DOI] [PubMed] [Google Scholar]
- 159.Voglein J, Tuttenberg J, Weimer M, et al. Treatment monitoring in gliomas: comparison of dynamic susceptibility-weighted contrast-enhanced and spectroscopic MRI techniques for identifying treatment failure. Invest Radiol. 2011;46:390–400. doi: 10.1097/RLI.0b013e31820e1511. [DOI] [PubMed] [Google Scholar]
- 160.Mangla R, Singh G, Ziegelitz D, et al. Changes in relative cerebral blood volume 1 month after radiation-temozolomide therapy can help predict overall survival in patients with glioblastoma. Radiology. 2010;256:575–584. doi: 10.1148/radiol.10091440. [DOI] [PubMed] [Google Scholar]
- 161.Bobek-Billewicz B, Stasik-Pres G, Majchrzak H, Zarudzki L. Differentiation between brain tumor recurrence and radiation injury using perfusion, diffusion-weighted imaging and MR spectroscopy. Folia Neuropathol. 2010;48:81–92. [PubMed] [Google Scholar]
- 162.Dokmanovic M, Clarke C, Marks PA. Histone deacetylase inhibitors: overview and perspectives. Mol Cancer Res. 2007;5:981–989. doi: 10.1158/1541-7786.MCR-07-0324. [DOI] [PubMed] [Google Scholar]
- 163.Zahonero C, Aguilera P, Ramirez-Castillejo C, et al. Preclinical Test of Dacomitinib, an Irreversible EGFR Inhibitor, Confirms Its Effectiveness for Glioblastoma. Mol Cancer Ther. 2015;14:1548–1558. doi: 10.1158/1535-7163.MCT-14-0736. [DOI] [PubMed] [Google Scholar]
- 164.Thomas FC, Taskar K, Rudraraju V, et al. Uptake of ANG1005, a novel paclitaxel derivative, through the blood-brain barrier into brain and experimental brain metastases of breast cancer. Pharm Res. 2009;26:2486–2494. doi: 10.1007/s11095-009-9964-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bertrand Y, Currie JC, Poirier J, et al. Influence of glioma tumour microenvironment on the transport of ANG1005 via low-density lipoprotein receptor-related protein 1. Br J Cancer. 2011;105:1697–1707. doi: 10.1038/bjc.2011.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Glas M, Happold C, Rieger J, et al. Long-term survival of patients with glioblastoma treated with radiotherapy and lomustine plus temozolomide. J Clin Oncol. 2009;27:1257–1261. doi: 10.1200/JCO.2008.19.2195. [DOI] [PubMed] [Google Scholar]
- 167.Buckner JC, Ballman KV, Michalak JC, et al. Phase III trial of carmustine and cisplatin compared with carmustine alone and standard radiation therapy or accelerated radiation therapy in patients with glioblastoma multiforme: North Central Cancer Treatment Group 93-72-52 and Southwest Oncology Group 9503 Trials. J Clin Oncol. 2006;24:3871–3879. doi: 10.1200/JCO.2005.04.6979. [DOI] [PubMed] [Google Scholar]
- 168.Tang JB, Svilar D, Trivedi RN, et al. N-methylpurine DNA glycosylase and DNA polymerase beta modulate BER inhibitor potentiation of glioma cells to temozolomide. Neuro Oncol. 2011;13:471–486. doi: 10.1093/neuonc/nor011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Mason WP, Macneil M, Kavan P, et al. A phase I study of temozolomide and everolimus (RAD001) in patients with newly diagnosed and progressive glioblastoma either receiving or not receiving enzyme-inducing anticonvulsants: an NCIC CTG study. Invest New Drugs. 2012;30:2344–2351. doi: 10.1007/s10637-011-9775-5. [DOI] [PubMed] [Google Scholar]
- 170.Ma DJ, Galanis E, Anderson SK, et al. A phase II trial of everolimus, temozolomide, and radiotherapy in patients with newly diagnosed glioblastoma: NCCTG N057K. Neuro Oncol. 2015;17:1261–1269. doi: 10.1093/neuonc/nou328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hainsworth JD, Shih KC, Shepard GC, et al. Phase II study of concurrent radiation therapy, temozolomide, and bevacizumab followed by bevacizumab/everolimus as first-line treatment for patients with glioblastoma. Clin Adv Hematol Oncol. 2012;10:240–246. [PubMed] [Google Scholar]
- 172.Clarke JL, Molinaro AM, Phillips JJ, et al. A single-institution phase II trial of radiation, temozolomide, erlotinib, and bevacizumab for initial treatment of glioblastoma. Neuro Oncol. 2014;16:984–990. doi: 10.1093/neuonc/nou029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Peters KB, Lou E, Desjardins A, et al. Phase II Trial of Upfront Bevacizumab, Irinotecan, and Temozolomide for Unresectable Glioblastoma. Oncologist. 2015;20:727–728. doi: 10.1634/theoncologist.2015-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Reardon DA, Desjardins A, Peters KB, et al. Phase II study of carboplatin, irinotecan, and bevacizumab for bevacizumab naive, recurrent glioblastoma. J Neurooncol. 2012;107:155–164. doi: 10.1007/s11060-011-0722-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Reardon DA, Vredenburgh JJ, Coan A, et al. Phase I study of sunitinib and irinotecan for patients with recurrent malignant glioma. J Neurooncol. 2011;105:621–627. doi: 10.1007/s11060-011-0631-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Reardon DA, Desjardins A, Peters KB, et al. Phase 2 study of carboplatin, irinotecan, and bevacizumab for recurrent glioblastoma after progression on bevacizumab therapy. Cancer. 2011;117:5351–5358. doi: 10.1002/cncr.26188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Conrad C, Miller CR, Ji Y, et al. Delta24-hyCD adenovirus suppresses glioma growth in vivo by combining oncolysis and chemosensitization. Cancer Gene Ther. 2005;12:284–294. doi: 10.1038/sj.cgt.7700750. [DOI] [PubMed] [Google Scholar]
- 178.Fueyo J, Gomez-Manzano C, Alemany R, et al. A mutant oncolytic adenovirus targeting the Rb pathway produces anti-glioma effect in vivo. Oncogene. 2000;19:2–12. doi: 10.1038/sj.onc.1203251. [DOI] [PubMed] [Google Scholar]
- 179.Jiang H, Gomez-Manzano C, Lang FF, et al. Oncolytic adenovirus: preclinical and clinical studies in patients with human malignant gliomas. Curr Gene Ther. 2009;9:422–427. doi: 10.2174/156652309789753356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ehtesham M, Samoto K, Kabos P, et al. Treatment of intracranial glioma with in situ interferon-gamma and tumor necrosis factor-alpha gene transfer. Cancer Gene Ther. 2002;9:925–934. doi: 10.1038/sj.cgt.7700516. [DOI] [PubMed] [Google Scholar]
- 181.Swartz AM, Li QJ, Sampson JH. Rindopepimut: a promising immunotherapeutic for the treatment of glioblastoma multiforme. Immunotherapy. 2014;6:679–690. doi: 10.2217/imt.14.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Neagu MR, Reardon DA. Rindopepimut vaccine and bevacizumab combination therapy: improving survival rates in relapsed glioblastoma patients? Immunotherapy. 2015;7:603–606. doi: 10.2217/imt.15.39. [DOI] [PubMed] [Google Scholar]
- 183.Yang L, Guo G, Niu XY, Liu J. Dendritic Cell-Based Immunotherapy Treatment for Glioblastoma Multiforme. Biomed Res Int. 2015;2015:717530. doi: 10.1155/2015/717530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Phuphanich S, Wheeler CJ, Rudnick JD, et al. Phase I trial of a multi-epitope-pulsed dendritic cell vaccine for patients with newly diagnosed glioblastoma. Cancer Immunol Immunother. 2013;62:125–135. doi: 10.1007/s00262-012-1319-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Hdeib A, Sloan AE. Dendritic cell immunotherapy for solid tumors: evaluation of the DCVax(R) platform in the treatment of glioblastoma multiforme. CNS Oncol. 2015;4:63–69. doi: 10.2217/cns.14.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Nakazawa Y, Huye LE, Salsman VS, et al. PiggyBac-mediated cancer immunotherapy using EBV-specific cytotoxic T-cells expressing HER2-specific chimeric antigen receptor. Mol Ther. 2011;19:2133–2143. doi: 10.1038/mt.2011.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Sun M, Shi H, Liu C, et al. Construction and evaluation of a novel humanized HER2-specific chimeric receptor. Breast Cancer Res. 2014;16:R61. doi: 10.1186/bcr3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Babic I, Mischel PS. Discovery in context: leveraging multidimensional glioblastoma datasets to identify targetable regulatory networks. Cancer Discov. 2012;2:676–678. doi: 10.1158/2159-8290.CD-12-0309. [DOI] [PubMed] [Google Scholar]
- 189.Fan CH, Liu WL, Cao H, et al. O6-methylguanine DNA methyltransferase as a promising target for the treatment of temozolomide-resistant gliomas. Cell Death Dis. 2013;4:e876. doi: 10.1038/cddis.2013.388. [DOI] [PMC free article] [PubMed] [Google Scholar]