ABSTRACT
Adverse effects linked to antiretroviral therapy (ART) may contribute to poor adherence on the patient’s side. Consequently, human immunodeficiency virus (HIV) drug resistance mutations could emerge, negatively impacting the body’s immune system. Meanwhile, severe immunosuppression can lead to several conditions, including anemia. The cause of anemia in HIV infection is multifactorial, and can be mainly explained by deleterious direct effects of the virus on the bone marrow, and opportunistic infections such as Parvovirus B19. Other causes include blood loss resulting from neoplasms and gastrointestinal lesions. Moreover, anemia can also be caused by antiretroviral drugs. We report a case of persistent anemia after ART initiation, kidney injury and treatment failure following a lengthy period of non-adherence to ART. The anemia was classified as Pure Red Cell Aplasia (PRCA). With treatment modification, the anemia resolved and the patient attained virologic suppression. Lamivudine (3TC) was pointed out as the cause of PRCA, which resolved after its withdrawal from the ART regimen. This rare side effect should be investigated in patients on 3TC who present with recurrent anemia.
INTRODUCTION
Anemia in people living with human immunodeficiency virus (PLHIV) has been associated with poor HIV treatment responses, high viral loads (VL), low CD4 counts and increased risk of death [1, 2]. Several medicines have been implicated in developing adverse drug events, compromising the quality of life [3]. Thus, patients lose interest by stopping adhering to antiretroviral therapy (ART), resulting in poor treatment outcomes [3].
Besides, Nucleoside Reverse Transcriptase Inhibitors (NRTIs) such as zidovudine (AZT) and lamivudine (3TC) have the propensity to cause hematologic toxicity, including anemia [4].
We present a patient assessed with severe persistent anemia, multiple treatment interruptions, HIV drug resistance and treatment failure months after ART initiation.
CASE REPORT
This is a 44-year-old single and self-employed female who was initiated on tenofovir disoproxil fumarate (TDF), 3TC and efavirenz (EFV) as an outpatient in July 2012. Her baseline laboratory test results were as follows: CD4 count: 190 cells/mm3; hemoglobin (Hb): 10.8 g/dl; creatinine clearance: 63 ml/min and hepatitis B surface antigen (HBsAg): non-reactive. She neither had a history of alcohol intake nor cigarette smoking. She interrupted her ART 1 month after ART initiation. All efforts to retain her in care were unsuccessful.
Six years later, when she returned to care, she was not taking ART. She complained of chronic fatigue and was assessed with advanced HIV disease and kidney dysfunction. She was reinitiated on the same ART regimen (TDF + 3TC + EFV). At re-initiation of ART, her CD4 count was 33 cells/mm3, and her Hb was 9.6 g/dl. A gradual decline in her Hb level—from 9.6 g/dl to 6.3 g/dl—was observed over 19 months (February 2018 to September 2019). She neither had leucopenia nor thrombocytopenia; her CD4 count had dropped to 11 cells/mm3, and blood loss was excluded during this period. From February 2019 to January 2020, the HIV plasma viral loads were persistent at >1000 copies/ml despite sessions of enhanced adherence counseling. Her creatinine clearance dropped to 30 ml/min and TDF doses were adjusted accordingly.
In July 2019, a full blood count showed normocytic, normochromic anemia and reticulocytes <1%. Additionally, bone marrow aspirate analysis revealed histologic features suggestive of Pure Red Cell Aplasia (PRCA), whereas, polymerase chain reactions (PCR) on the bone marrow biopsy were negative for Parvovirus B19, Cytomegalovirus, Epstein Bar Virus and mycobacterium avium complex. The protein electrophoresis and immunofixation were negative for multiple myeloma. The HBsAg remained non-reactive, as well as the cryptococcal serum antigen and tuberculosis screenings, and the creatinine clearance was estimated at 36 ml/min.
The patient was initiated empirically on steroids in the oncology department for 7 months to control possible immunologic disorders commonly associated with anemia in PLHIV. During the course of the treatment, her Hb slightly improved to 8.5 g/dl. However, during the same period, she suffered from esophageal candidiasis, an acquired immunodeficiency syndrome (AIDS)-defining condition treated successfully with fluconazole.
In January 2020, she was referred to an HIV specialist who diagnosed her with HIV treatment failure and PRCA. Hence, 3TC was suspected to be the cause of PRCA. The steroids were discontinued, and the Sanger genotyping resistance testing (GRT) exposed these mutations: K70KE, M184V, V106VA, E138G, V179D, G190GA and P225H in the reverse transcriptase enzyme, but no mutations in the viral protease enzyme (Table 1). The HIV Drug Resistance Database of Stanford University version 9.0 was used to predict drug activity [5]. The genotyping results and drug toxicity profiles were used to guide the optimization of the ARV regimen.
Table 1.
Mutations detected at the HIV-1 GRT, corresponding penalty scores and drug susceptibilities following analysis with the Stanford HIV drug resistance database version 9.0
| ARVs class | Detected mutations | Penalty scores | Drug susceptibility | |
|---|---|---|---|---|
| NRTIs | K70KE, M184V, | AZT | −20 | Susceptible |
| TDF | 15 | Low-level resistance | ||
| ABC | 30 | Intermediate resistance | ||
| 3TC/FTC | 70 | High-level resistance | ||
| Non-NRTIs | V106VA, E138G, V179D, G190GA and P225H | ETR | 30 | Intermediate resistance |
| NVP EFV |
185 155 |
High-level resistance | ||
| Protease Inhibitors (PI) majors, accessories and other PI mutations | None | LPV/r | 0 | Susceptible |
| ATV/r | 0 | Susceptible | ||
| DRV/r | 0 | Susceptible | ||
End of February 2020, 3TC was withdrawn from the regimen, and a combination of drugs that pharmacokinetics does not affect kidney function was initiated; abacavir (ABC) + atazanavir/ritonavir (ATV/r) + dolutegravir (DTG). Over a period of 9 months, the patient’s (Fig. 2) creatinine clearance improved to >60 ml/min; her CD4 count increased to 154 cells/mm3, her plasma VL was maintained at <40 copies/ml of plasma (Fig. 1) and the Hb rose to >11.5g/dl (Fig. 2).
Figure 2.
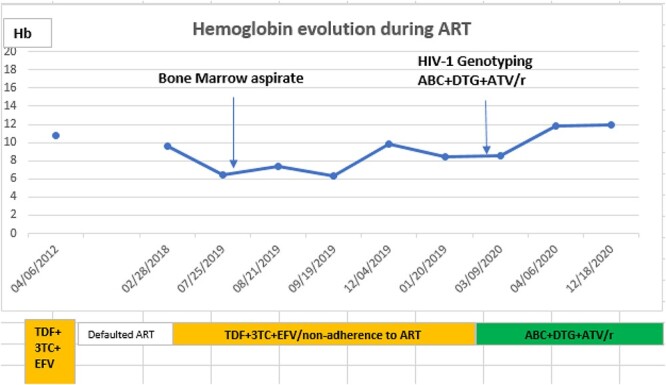
Hemoglobin (g/dl)' profile during ART: The initial patient’s Hb levels persistently below 10 g/dl while on 3TC containing ART regimen improved on ABC + DTG + ATV/r.
Figure 1.
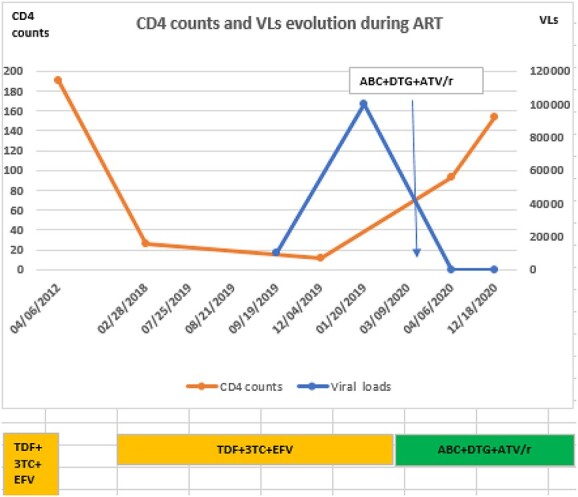
CD4 counts (cells/mm3) and VLs (copies/ml of plasma) evolution: A considerable drop in CD4 cell count and increase in VLs during the non-adherence time interval with the patient’s initial regimen (TDF + 3TC + EFV) was observed. Based on the findings, the initial regimen was switched to ABC + DTG + ATV/r, followed by improved immunologic status and virologic outcomes, as illustrated.
DISCUSSION
Although anemia is frequently seen in PLHIV, rare side effects of ARVs should be investigated and managed accordingly. HIV disease severity is one of the predisposing factors to anemia [5], and anemia increases mortality and morbidity in PLHIV [6]. The above patient-initiated ART in a state of severe immunosuppression with a CD4 count <200 cells/mm3. Her low baseline Hb was expected to be corrected after ART initiation and good adherence [7]. However, 1 month later, the patient discontinued ART for ˃5 years.
Bukenya et al. reported that fear of ART-related side effects was associated with non-adherence and treatment failure [8]. A decline in the patient’s general condition was believed to be attributed to ART side effects that led to treatment interruption. After a lengthy period off ART, the patient returned to care with a very low CD4 count.
ART was re-started with the initial regimen namely—TDF, 3TC and EFV. A combination of AZT and 3TC can potentially cause PRCA, a rare hematologic adverse event [4]. This line of thought led the clinicians who managed the patient to have a low index of suspicion toward 3TC as a single cause of anemia, despite its recurrence and histologic findings on the bone marrow biopsy. Besides, the slight improvement in Hb level while on steroids contributed to misleading the clinician. However, Nakamura et al. [9] argued that 3TC in the absence of AZT could induce anemia.
Only 7 months after treatment with steroids, an HIV-experienced clinician, guided by the patient’s medical history, suspected 3TC as the culprit. The PRCA could have resulted from HIV, multiple myeloma or opportunistic infections such as parvovirus B19 [10, 11]. However, the negative PCR results for opportunistic infections and protein electrophorese for multiple myeloma contributed to the diagnosis of the 3TC-induced PRCA.
Regarding renal function, a decline in creatinine clearance was observed in the same period—following ART re-initiation. TDF causes proximal tubular cell injury, which aggravates pre-existent kidney diseases, such as HIV-associated Nephropathy, a condition frequently observed in patients of African descent [12]. As such, TDF was substituted with ABC followed by improved creatinine clearance.
Overall, 3TC remained this patient’s sole cause of anemia, and its withdrawal from the ART regimen was corroborated by improved patient’s general condition and raised Hb levels. However, symptomatic anemia associated with 3TC was responsible for non-adherence, resulting in extensive HIV-1 reverse transcriptase mutations and treatment failure, which was addressed with a treatment switch.
CONCLUSION
Delayed work-up of the persistent symptomatic anemia negatively impacted the patient’s adherence to ART, and treatment failure ensued. We want to draw the reader’s attention to the rare and reversible drug toxicity profile of 3TC since earlier detection could have prevented non-adherence and the selection of mutations in the HIV-1 reverse transcriptase.
Supplementary Material
ACKNOWLEDGMENT
We acknowledge Dr Annemarie Wensing, MD, PhD of the University Medical Center Utrecht, Netherlands and Honorary Professor of the University of the Witwatersrand in Johannesburg, South Africa, for critical reading of the manuscript and the registered nurse Helena Ndjavera for her contribution to patient’s care.
Contributor Information
Mireille A M Kakubu, Ministry of Health and Social Services of Namibia, Windhoek, Namibia.
Tarisai Bikinesi, Ministry of Health and Social Services of Namibia, Windhoek, Namibia.
Patrick D M C Katoto, Centre for Tropical Disease and Global Health, School of Medicine, Catholic University of Bukavu, Bukavu, Democratic Republic of Congo; Cochrane South Africa, South African Medical Research Council, Francie Van Zijl Drive, Parow Valley, 7501 Cape Town, South Africa.
CONFLICTS OF INTEREST STATEMENT
None declared.
CONSENT
The patient provided written consent for publication of this case report.
ETHICAL APPROVAL
Standards ethics concerns were observed, and the ethical approval was obtained from the Research Department of Namibia.
GUARANTOR
Dr Mireille A Mpalang Kakubu is the corresponding author and responsible for the manuscript integrity, and attest that the information in this case report is true and verifiable.
REFERENCES
- 1. Noor RA, Abioye AI, Hertzmark E, Darling AM, Aboud S, Mugusi FM et al. Impaired Hematological status increases the risk of mortality among HIV-infected adults initiating antiretroviral therapy in Tanzania. J Nutr 2020;150:2375–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sah SK, Dahal P, Tamang GB, Mandal DK, Shah R, Pun SB. Prevalence and predictors of Anemia in HIV-infected persons in Nepal. HIV AIDS (Auckl) 2020;12:193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sherfa A, Haile D, Yihune M, Sako S. Incidence and predictors of adverse drug reaction (ADR) among adult HIV positive patients on anti-retroviral treatment in Arba Minch town public health facilities, southern Ethiopia: a retrospective cohort study, 2020. PLoS One 2021;16:e0251763-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Manickchund N, du Plessis C, John M-AA, Manzini TC, Gosnell BI, Moosa M-YS. A case series of emtricitabine-induced pure red cell aplasia. Southern African J HIV Med 2021;22(1):1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Harding BN, Whitney BM, Nance RM, Ruderman SA, Crane HM, Burkholder G et al. Anemia risk factors among people living with HIV across the United States in the current treatment era: a clinical cohort study. BMC Infect Dis 2020;20:238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gebremedhin KB, Haye TB. Factors associated with Anemia among people living with HIV/AIDS taking ART in Ethiopia. Adv Hematol 2019;2019:9614205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Quiros-Roldan E, Castelli F, Lanza P, Pezzoli C, Vezzoli M, Inflammation in HIVSG et al. The impact of antiretroviral therapy on iron homeostasis and inflammation markers in HIV-infected patients with mild anemia. J Transl Med 2017;15(1):256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bukenya D, Mayanja BN, Nakamanya S, Muhumuza R, Seeley J. What causes non-adherence among some individuals on long term antiretroviral therapy? Experiences of individuals with poor viral suppression in Uganda. AIDS Res Ther 2019;16:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nakamura K, Tateyama M, Tasato D, Haranaga S, Tamayose M, Yara S et al. Pure red cell aplasia induced by lamivudine without the influence of zidovudine in a patient infected with human immunodeficiency virus. Intern Med 2014;53:1705–8. [DOI] [PubMed] [Google Scholar]
- 10. Ito T, Nakaya A, Fujita S, Satake A, Nakanishi T, Azuma Y et al. Secondary pure red cell aplasia in multiple myeloma treated with lenalidomide. Leuk Res Rep 2018;10:4–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Niparuck P, Kanoksil W, Wacharapornin P, Chantrathammachart P, Boongird S. Etiologies and treatment burden in adult patients with pure red cell aplasia: a single-Center experience and review of literature. Anemia 2020;2020:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mtisi TJ, Ndhlovu CE, Maponga CC, Morse GD. Tenofovir-associated kidney disease in Africans: a systematic review. AIDS Res Ther 2019;16:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


