Abstract
With rapid advancements in diagnosis and treatment of malignancies, the gap between generalists and subspecialists continues to widen, particularly in cancers like lymphoma where the spectrum of disease varies from indolent to rapidly progressive. Prior to establishing with a hematologist/oncologist, patients must be accurately and comprehensively diagnosed and managed for lymphoma in the generalist setting. In the following manuscript, we review the common clinical presentations in which should raise concern for lymphoma. We summarize the literature regarding the role of laboratory studies including complete blood count and peripheral blood flow cytometry, the recommendations for lymph node sampling, the role and selection of imaging modalities, and ideal patient monitoring for high-risk clinical syndromes that may be encountered in lymphoma.
Keywords: lymphoma, primary care, workup
1 |. INTRODUCTION
Lymphoma is a heterogenous group of lymphoid neoplasms with marked differences in clinical course and response to treatment.1 Lymphomas are grouped by their postulated normal cell type of origin (B-cell vs. T-cell), and morphology (Hodgkin vs. non-Hodgkin) as well as the degree of cellular differentiation.2 With advancements in molecular medicine, more than 100 discrete lymphomas have been identified and grouped by genetic and morphologic characteristics.3 Under the most recent classification system, there is increasing emphasis on genetic and pathologic markers in characterizing distinct lymphomas and on using these data to drive clinical management.2,3 While lymphoma will ultimately be managed by a hematologist/oncologist in the United States, the initial lymphoma diagnosis and early clinical management is typically performed in a primary care or general hospital setting.
There is no “classic” presentation, standard workup, nor single test to diagnose or even definitively rule out lymphoma.4 When symptomatic, lymphoma commonly presents with fatigue, pain, palpable lymphadenopathy, and shortness of breath/cough,5 all of which are nonspecific findings with exceptionally broad differential diagnoses. However, lymphoma can also have a variety of atypical presentations, for example, extra-nodal lymphoma could present with GI symptoms, CNS symptoms, cutaneous symptoms, or other generalized or organ-specific symptoms.6–8 Despite this, lymphoma is a rare diagnosis with an incidence of 2.6 and 19.6 cases per 100 000 people per year for Hodgkin and non-Hodgkin lymphoma (NHL), respectively.9,10 Even for symptoms with more narrow differentials, such as lymphadenopathy, lymphoma remains an uncommon diagnosis. For example, in patients presenting to a generalist practice with lymphadenopathy, only approximately 4% of patients older than age 40 and less than 0.4% of patients under age 40 were ultimately diagnosed with any malignancy.11
To date, there is little guidance for primary care doctors once lymphoma has emerged as the most likely diagnosis and after other competing differentials have been effectively ruled out. Furthermore, in our experience, lymphoma is increasingly being diagnosed during the workup of incidental lymphadenopathy. Most articles that do address the workup of lymphoma focus narrowly on a single subset of lymphoma or stratify by Hodgkin versus non-HL, a distinction that is determined by tissue biopsy which is often unavailable at the time when lymphoma is suspected.12–17
This article reviews the current evidence regarding the diagnostic and early clinical management of probable lymphoma including the role of laboratory studies, such as complete blood count and peripheral blood flow cytometry (FC), recommendations for lymph node sampling, the role, and selection of imaging modalities, and patient monitoring for high-risk clinical syndromes that may be encountered in lymphoma.
1.1 |. Identification of probable lymphoma
1.1.1 |. Palpable lymphadenopathy
Lymphoma can be discovered by palpable lymphadenopathy.18 Importantly, in patients with lymphadenopathy, the risk of lymphoma is low. In patients presenting to primary care with lymphadenopathy, the overall incidence of any malignancy is 1.1%.19 While most cases have a benign etiology, internet searches by medically illiterate patients can lead to excessive worry that this symptom represents lymphoma.20
Briefly, the evaluation of palpable lymphadenopathy should include a history and physical exam targeted to the distribution (localized vs. generalized), location (which distinct lymph node group is involved if localized), and character of the nodes as well as the clinical history including the time course of lymphadenopathy.21–24 History should elicit localizing symptoms, other primary malignancy, specific exposures, such as cat scratches, tick bites, undercooked meat, high-risk sexual activity, injection drug use, travel to areas with high rates of endemic infection, or recent vaccinations.21 Vaccination against COVID-19 may lead to reactive axillary lymphadenopathy up to 10 weeks postvaccination.25 A history positive for any of the afore-mentioned items can reveal the etiology and lower the suspicion of lymphoma. The presence of constitutional symptoms including fever, drenching night sweats, and weight loss can be suggestive of lymphoma, but can also be seen with most infectious causes of lymphadenopathy.22 Comprehensive lymph exam should include palpation of all accessible lymph nodes (cervical, supraclavicular, occipital, preauricular, infraclavicular, axillary, pectoral, epitrochlear, inguinal, femoral, and popliteal), as well as palpation of the liver and spleen, and visualization of Waldeyer’s ring22 (Figure 1). Generalized adenopathy, nodes >1 cm in diameter, firm, rubbery, or hard consistency, fixation to adjacent tissue or “matted” fixation to adjacent nodes, splenomegaly, and non-tender nodes are most concerning for malignancy (not limited to lymphoma), whereas <1 cm, freely mobile, tender nodes are far more likely to be of infectious etiology.22
FIGURE 1.
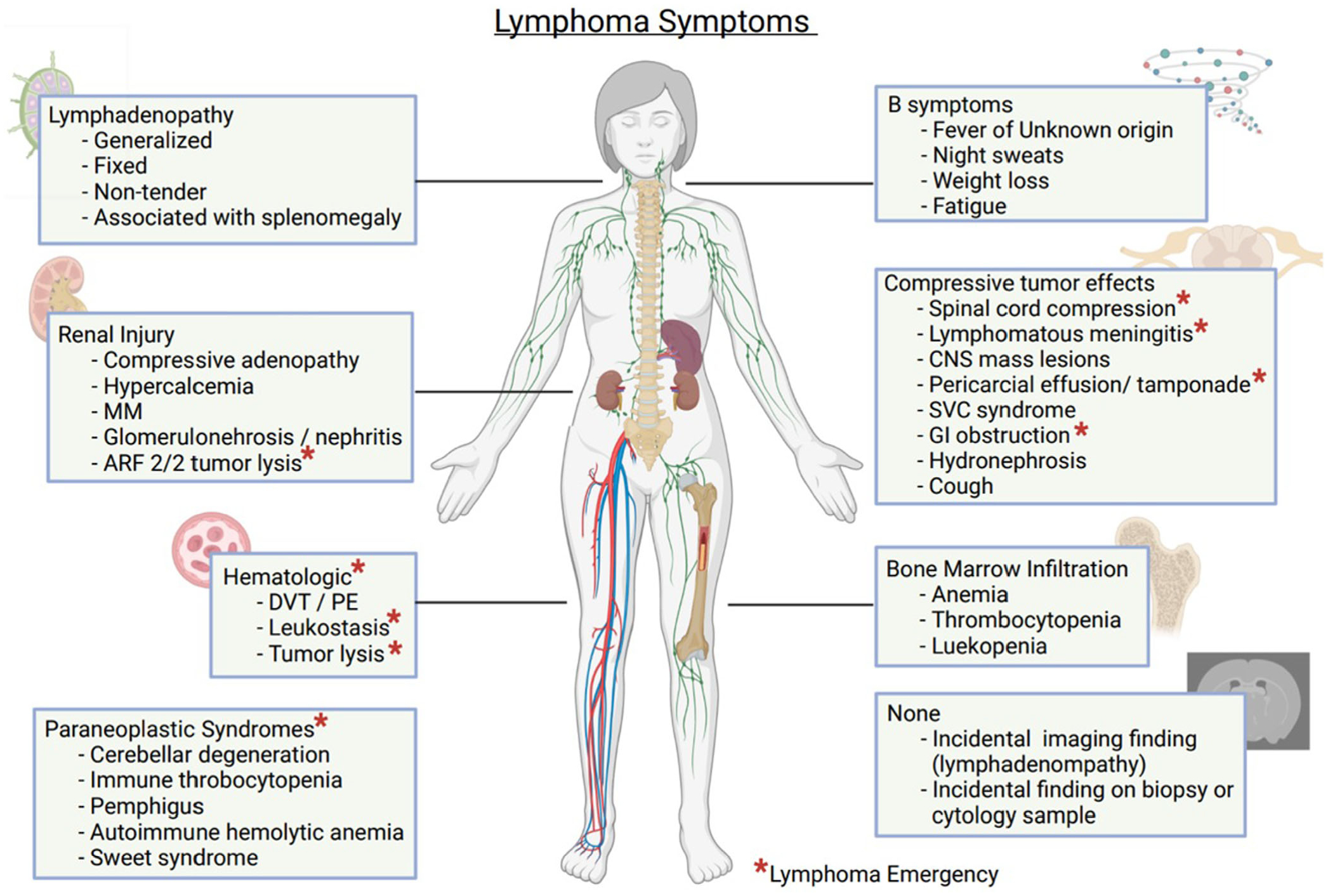
Lymphoma symptoms
Expectedly, studies have demonstrated that generalists are highly skilled at diagnosing and managing palpable lymphadenopathy and appropriately referring for biopsy when indicated.11 Since lymphadenopathy can be a sequalae of multiple infectious, immunologic, endocrine, drug-induced, and neoplastic processes, much have been written about best practices for workup of lymphadenopathy without an obvious cause,21–24 and these guidelines will not be discussed in detail in this review. The diagnostic approach for unexplained lymphadenopathy will vary significantly based on the physician’s overall clinical impression of the likelihood of malignancy as the cause and the presence of localized versus generalized lymphadenopathy (with a higher likelihood of malignancy and generalized lymphadenopathy being more concerning features that would prompt biopsy rather than watchful waiting).26
1.1.2 |. B-symptoms: Fever, drenching night sweats, and unexplained weight loss
Less frequently, lymphoma can be suspected in patients who complain of constitutional symptoms including fevers (unexplained temperatures >38°C in the last month), night sweats (recurrent and drenching in the last month), and weight loss (>10% of body weight within the last 6 months) without known adenopathy.27 In the context of lymphoma, these symptoms are known as “B-symptoms” as the presence of one or more of these symptoms corresponds with B staging in the Ann Arbor Staging System.27 To the best of our knowledge, there are no studies that characterize the positive predictive value of each B-symptom in isolation or in aggregate, in part due to the vast heterogeneity of distinct lymphomas.
In cases where patients present to a generalist with isolated B symptoms, initial physical exam should include a comprehensive lymph node exam, as lymphoma is on the differential for each independent symptom. If lymphadenopathy is found, the clinician should follow the guidelines for workup of lymphadenopathy. B-symptoms without lymphadenopathy or splenomegaly should not prompt further targeted workup for lymphoma. Instead, suggested workups for each symptom have been well established and are expected to uncover further evidence of lymphoma if present.28–30 For example, lymphoma may be suspected if imaging obtained during workup of fever of unknown origin (FUO) reveals diffuse lymphadenopathy. Likewise, if laboratory workup for unexplained weight loss reveals liver or kidney dysfunction this could prompt further workup and possible subsequent diagnosis of compressive adenopathy.31 A rare symptom that should trigger investigation is new-onset alcohol-related pain or intolerance.32 Thought to be due to vasodilation within the lymph-node capsule, this may be seen in up to 5% of cases of HL.
1.1.3 |. Incidental imaging finding of lymphadenopathy
In primary care and hospital medicine, localized or generalized adenopathy can be discovered incidentally on imaging ordered for a different indication.33,34 For example, a chest X-ray ordered for workup of cough could show a large mediastinal mass, or abdominal imaging obtained for workup of pain or for abnormal liver or kidney function could reveal compressive and/or diffuse adenopathy. In these cases, the radiologist may be able to assist with risk stratification of adenopathy and provide impressions that indicate a high or low concern for lymphoma. Depending on the radiologic interpretation and the overall clinical picture after further history, a subset of these cases will fall under the diagnostic category of “suspected” and should follow the steps below for further workup of suspected lymphoma.
1.1.4 |. Nonlymph node biopsy concerning for lymphoma
Lymphoma, while typically found in lymph nodes, can develop in other lymphatic tissues and spread throughout the body.35,36 As such, biopsies and cytology from sources other than lymph nodes may be the initial indication for lymphoma. Samples from head and neck masses, gastric mucosa, ascitic fluid, and pleural effusions may provide preliminary evidence of a monoclonal lymphoid population suggestive of lymphoma.37 If the initial sample is insufficient for final diagnosis, further workup and management is indicated.
1.2 |. Laboratory tests
Once the diagnosis of lymphoma is suspected, further evaluation includes laboratory testing, imaging, and biopsy. While not always in this order, laboratory testing is often the initial step. These are described in detail below and summarized in Table 1. Certain patterns of lab abnormalities are consistent with a diagnosis of lymphoma and can heighten suspicion for complications such as bone marrow involvement, compressive adenopathy, or advanced disease. Importantly, the absence of abnormalities on any of the following blood tests should not be used to definitively rule out a diagnosis of lymphoma.
TABLE 1.
Lab tests for probable lymphoma
| Lab test | Rationale | Abnormalities suggestive of lymphoma | Red flag results |
|---|---|---|---|
| Complete blood count (CBC) | Ensure trilineage normality, assess for gross abnormalities suggestive of bone marrow involvement | Anemia, thrombocytopenia, leukopenia/leukocytosis | Neutropenia, WBC > 100 K, severe anemia or thrombocytopenia |
| Comprehensive metabolic panel | Assess for the presence of hepatic or renal dysfunction suggestive of compressive or infiltrative disease | AKI, electrolyte abnormalities, hepatic or cholestatic injury patterns | Significant electrolyte abnormalities or evidence of significant organ injury |
| Phosphorus | Evaluate for evidence of tumor lysis. (Although phosphate levels in spontaneous TLS may be normal as opposed to phosphate levels in post-chemotherapy TLS) | Tumor lysis | Hyperphosphatemia with phosphorus levels >4.5 mg/dl in adults or > 25% increase from baseline. (In conjunction with elevated LDH, uric acid, potassium, ±AKI, as this is consistent with TLS) |
| LDH | Evaluate for evidence of rapid cellular turnover which may be associated with TLS. | Elevated LDH | In conjunction with elevated potassium, uric acid ± AKI, and hyperphosphatemia as this is consistent with TLS |
| Uric acid | To rule out tumor lysis syndrome | No clear association outside of TLS | Hyperuricemia with uric acid >8 mg/dl or 25% increase from baseline In conjunction with elevated LDH, potassium, ±AKI, and elevated phosphorus as this is consistent with TLS |
Abbreviations: AKI, acute kidney injury; ED, emergency department; LDH, lactate dehydrogenase; MAHA, microangiopathic hemolytic anemia; TLS, tumor lysis syndrome.
1.2.1 |. Complete blood count
Complete blood count (CBC) abnormalities can occur with multiple complications of lymphoma and should be obtained in the workup of probable lymphoma. However, many patients with lymphoma, even aggressive lymphoma, will have a normal CBC and this should not be considered evidence against a lymphoma diagnosis.38
When present, CBC abnormalities including anemia, thrombocytopenia, and leukopenia/lymphocytosis can suggest complications, such as bone marrow infiltration, anemia of chronic disease, iron or B12 deficiency, autoimmune hemolytic anemia, hypersplenism from splenic involvement, or diagnosis of small lymphocytic lymphoma. The presence of anemia in untreated lymphoma is estimated between 32%–45% and up to 57% of patients with anemia reported symptoms.39–42 Early identification of anemia can guide multiple aspects of management including early iron and B12 repletion and heightened suspicion for bone marrow involvement.40,43 Anemia is also an adverse prognostic factor in some types of lymphoma, with varying degrees of prognostic value by lymphoma subtypes.41,44,45
1.2.2 |. Comprehensive metabolic panel
There are multiple mechanisms by which lymphoma can cause electrolyte abnormalities. Hypercalcemia, while present in less than 15% of cases of lymphoma, is associated with decreased overall survival.46 The mechanisms of hypercalcemia in lymphoma may include bone invasion, paraneoplastic syndromes involving parathyroid-like-hormones, or prostaglandin release. In addition, tumor-associated macrophages or tumor cells themselves can form excess 1,25-dihidroxyvitamin D and clinicians should consider testing for this hormone specifically.47
Renal injury may further perturb the metabolic panel. Compressive adenopathy can directly cause renal dysfunction, although indirect causes are more common.48 Renal injury more typically occurs from hypercalcemia or from cellular release of monoclonal paraproteinemia, similar to how multiple myeloma causes renal injury.49,50 Lymphomas have also been associated with development of immunologically mediated glomerulonephrosis and glomerulonephritis.51,52 Aggressive lymphomas can present with tumor lysis syndrome (TLS), which can lead to acute renal failure and electrolyte abnormalities.53 Of note, there are also a handful of cases of non-HL presenting with acute renal failure due to lymphomatous infiltration of the kidneys, although the validity of this finding remains debated within the literature.54,55 Hepatic and/or cholestatic dysfunction can be seen in cases of compressive adenopathy, metastatic hepatic infiltration, paraneoplastic syndromes, and hemophagocytic syndrome.56
1.2.3 |. Lactate dehydrogenase, uric acid, and phosphate
Though not specific, lactate dehydrogenase (LDH) is commonly elevated in aggressive lymphoma and serves as an important prognostic factor for many types of lymphomas.57 Aside from this, LDH and uric acid levels should be checked to rule out spontaneous TLS (discussed in greater detail below). Interestingly, unlike post-chemotherapy TLS where high rates of cell death cause hyperuricemia and hyperphosphatemia, spontaneous TLS is often seen without hyperphosphatemia, possibly because phosphate released during high cell turnover is rapidly used in the synthesis of new cells.58,59
1.2.4 |. Peripheral blood FC
FC is a method of identifying and quantifying specific cell types by physical characteristics and surface antigen expression.60 When malignant cells are identified, the immunophenotype of the atypical cells aids in diagnosis, prognostication, and monitoring.60 The diagnostic yield of the test, however, varies greatly by sample source and malignancy subtype; atypical lymphocytes must be present in the sample tested and must be able to be identified by the flow cytometer. This presents challenges in lymphoma. For example, Reed-Sternberg cells (pathognomonic of classical HL) have historically been considered to be too big or too sparse to detect using FC,61 although recent advancements are bridging this gap.61,62 Apart from leukemic forms of lymphoma, it is rare to see circulating tumor cells.63 In a real-world study, only 15.4% of clinically suspected lymphoma cases were positive by FC of bone marrow and peripheral blood combined (peripheral blood samples were not analyzed independently).64 A single-center study reviewed 185 requests for peripheral blood FC performed over the course of 1 year and found that only 15.1% of those tests were positive for a monoclonal lymphoid population (all samples were B-cell clones). Of those, greater than 50% were chronic lymphocytic leukemia; the other 50% were not further characterized.65 Furthermore, 0 of the 18 tests performed for constitutional symptoms and only 2 of the 12 tests performed for lymphadenopathy/suspicious mass returned positive results.65 This study, among others, influenced the statement from the American Society of Clinical Pathology for the Choosing Wisely initiative that peripheral blood FC is not recommended for screening for hematologic malignancy in most cases.66 Specifically, it is of low yield in cases of mature neutrophilia, basophilia, erythrocytosis, thrombocytosis, isolated anemia, or isolated thrombocytopenia. Even in cases where morphologically abnormal cells (blasts or lymphoma cells) are seen on peripheral smear, the likelihood of obtaining diagnostic results from peripheral blood FC is extremely low.66
1.3 |. Imaging
In the workup of lymphoma, imaging is an important tool for diagnostic and prognostic purposes. Computerized tomography (CT) scan is the imaging modality historically used in the Ann Arbor staging system, the most common staging system used in clinical and research settings for prognostic indices, therapy selection, and outcomes reporting. Nearly, all lymphomas are metabolically active and therefore fluorodeoxyglucose (FDG) avid.67,68 Furthermore, they can be distinguished from other etiologies of FDG uptake, such as physiologic or patterns of infection or inflammation, by the distribution of avid nodes and/or CT characteristics.69,70 Subsequently, PET-CT has been widely adopted as the imaging modality of choice to stage and to monitor treatment response in lymphoma. Compared to CT alone, FDG PET-CT has superior accuracy for staging and increased specificity, particularly for extranodal disease.71 FDG PET-CT offers further advantage in the diagnosis and management of indolent lymphomas. Since FDG avidity is higher in more aggressive lymphomas, biopsy of sites with higher FDG uptake may increase diagnostic yield in cases of suspected transformation.72–74 It is important to note that not all lymphomas are FDG avid, and lack of FDG update on PET-CT does not rule out a diagnosis of lymphoma. To this end, multiple international working groups have crafted consensus statements to standardize PET-CT methods and interpretation.75
Regarding CT contrast, there is some evidence for benefit of contrast enhancement in the detection of abdominal or pelvic disease. Under ideal conditions, current recommendations from several radiology and nuclear medicine associations recommend low-dose non-contrast CT with PET scan, followed by high dose with contrast CT. However, in clinical practice, many patients have already had a recent high contrast CT prior to PET-CT, and the ICML working group currently recommends against repeat at the time of PET-CT in these cases, unless needed for other reasons such as clinical trial participation or radiation planning.
Molecular and pathologic diagnosis is vital in the interpretation of PET-CT results; recent research has shown significant variability in predictive value PET-CT findings in individual lymphoma subcategories, and data is overall lacking for NK and T-cell lymphomas.76–78 In some lymphomas, presence of bulky disease is a negative prognostic factor while in others the association is more complex or not yet clear. In Hodgkin lymphoma (HL) and in aggressive NHL, focal FDG uptake in the bone marrow is highly sensitive for involvement, and in some cases may spare the patient from bone marrow biopsy. However, in more indolent lymphomas, sensitivity for bone marrow involvement is low, and biopsy is required for staging. Since the brain has high physiologic FDG uptake, PET-CT may miss less severe cases of leptomeningeal disease. In cases where CNS involvement is suspected due to neurologic symptoms, MRI remains the preferred imaging modality.79
1.4 |. Biopsy
In lymphoma, tissue diagnosis is imperative. Solid tissue samples can be obtained via needle (fine needle aspiration [FNA] or core needle biopsy) with or without imaging guidance, or via surgical biopsy (incisional vs. excisional biopsy) (Table 2). The general principles regarding performing and obtaining an ideal (or even suitable) biopsy are to perform the least burdensome procedure (in terms of patient risk and healthcare system resources such as procedural spaces, personnel, time, and cost) that will provide the diagnostic yield required for timely and accurate diagnosis and management.
TABLE 2.
Tissue studies/biopsy for probable lymphoma
| Diagnostic study | Description | Limitations | Notes |
|---|---|---|---|
| Peripheral blood flow cytometry | Method of identifying and quantifying specific cell types by physical characteristics and surface antigen expression | Lymphoma cells are rarely detected in peripheral blood | Not recommended for screening for lymphoma under most circumstances, and unlikely to be diagnostic even in cases where blasts or lymphoma cells are seen on peripheral smear |
| Fine needle aspiration | Least invasive sampling technique, can be combined with ancillary testing such as IHC, FC, and cytogenetic tests to improve diagnostic sensitivity | Extremely high false negative rate, especially for HL. High rate of incorrect or incomplete subclassification |
Reasonable to consider in combination with ancillary testing if lymphoma is not the most likely diagnosis or if the biopsy site is difficult to safely access by more invasive techniques, though unlikely to provide subclassification/actionable lymphoma diagnosis and low NPV |
| Core needle biopsy | Less invasive and more readily available diagnostic technique than excisional biopsy and non-inferior or superior in some circumstances when combined with ancillary testing | Not recommended for diagnosis of HL due to exceptionally high false negative rate. Risk of insufficient sample for complete subclassification/actionable diagnosis, or insufficient sample for follow-up tests for research/clinical trial eligibility. |
Reasonable to consider in cases where NHL is suspected and robust ancillary testing is available, though subsequent surgical biopsy may be needed. |
| Surgical (excisional) biopsy | Gold standard sampling technique | Most invasive and least available technique, NPV < 100 | Recommended in all cases where HL is highly suspected or ancillary testing capabilities are limited |
Abbreviations: FC, flow cytometry; HL, Hodgkin lymphoma; IHC, immunohistochemistry; NPV, negative predictive value.
Many benign causes of lymphadenopathy have a morphologic pattern overlapping with lymphomas such as reactive follicular or paracortical/interfollicular hyperplasia or necrosis.80 Assessment of lymph node architecture via histology therefore remains essential for differentiating between many subtypes of lymphoma and benign lymphadenopathy.80 In addition, new genetic, pathologic, and immunologic markers are increasingly incorporated into diagnostic criteria, prognostic models, predictive factors, and disease-defining lesions, with some lymphomas now being diagnosed differently than just a few years ago.1 For all biopsy types, there is a concerningly high false negative rate for lymphoma, with a recent study noting a negative predictive value (NPV) as low as 54.3% for open surgical biopsy.81
Providers should monitor all patients with negative biopsy results for persistent signs and symptoms of lymphoma and pursue larger-volume biopsy if clinical suspicion for lymphoma remains high.4 In addition, FC may not accurately pick up T-cell clones and T-cell rear-rangement studies are often needed for diagnosis of T-cell lymphoma, though this is typically managed by the pathologist and not the ordering provider.82
1.5 |. Fine needle aspiration
Overall, FNA is unlikely to yield an actionable, accurate, or complete diagnosis of lymphoma, and is not recommended for a targeted workup of suspected lymphoma.4 Subspecialty guidelines for evaluation of head and neck masses (including lymphadenopathy) do however recommend FNA as the first line sampling method.83 This is in part because of the relative ease of sample access as well as the broad differential diagnosis of head and neck masses, most of which can be diagnosed by FNA alone. The accuracy of FNA for head and neck masses of all etiologies is high; a single institution review of all (2772) head and neck FNAs performed within a 10-year period reported an accuracy of 95.1% and a meta-analysis of 3459 FNA samples demonstrated an accuracy of 96.5%.84 Sub-group analysis of 542 lymph node FNA samples from a single institution and 782 FNA samples from a meta-analysis showed diagnostic accuracy of 94.5%, PPV of 98.8%, and NPV of 86.7%. However, the 151 aspirates of lymphoma included 39 false negatives and 3 nondiagnostic samples for a sensitivity of only 74%, and the authors specifically comment on limitations in subclassifying lymphomas and accurate diagnosis of low-grade lymphomas, which they reported requires open biopsy.84
Ultimately, FNA alone is not recommended for diagnosis or rule out of lymphoma due to low sensitivity and low NPV as well as a high rate of incorrect lymphoma subtyping.85 However, FNA combined with ancillary tests such as FC and immunohistochemistry (IHC) is reasonable to consider if lymphoma is lower on a differential diagnosis than other causes of localized lymphadenopathy (such as is the case in head and neck lymphadenopathy) or if the biopsy site cannot be safely accessed for more invasive biopsy techniques.4
1.5.1 |. Core needle biopsy
Core needle biopsy (CNB) is subject to many of the same limitations as FNA, although more tissue is obtained.4 With the rapid advancements in molecular diagnosis such as FC, IHC, and FISH/cytogenetics, some studies have reported reliable diagnosis of certain types of lymphoma with core biopsy combined with appropriate ancillary tests.81 For example, a recent review article reported diagnostic efficacy of image-guided CNB of 79%–97% with diagnostic reproducibility among hematopathologists from 87% to 93%.86 In some circumstances, CNB even may be superior to excisional biopsy, presumably for the ability to sample a more suspicious node that may not be amenable to excision.81 A recent prospective cohort studies comparing open surgical biopsy to doppler-assisted CNB found NPV of 54.3% for surgical biopsy compared to 84.5% for CNB when samples were compared to the gold standard of a subsequent biopsy demonstrating lymphoma.81 However, other studies have shown far lower diagnostic yield. A recent metanalysis of CNB for diagnosis of lymphoma in cervical lymphadenopathy found a rate of actionable lymphoma diagnoses as low as 30% (range 30%–96.3%)87 and a recent study of 457 biopsies (339 excisional and 118 CNB) found that only 56.8% of CNB samples contained adequate tissue compared to 96.8% of excisional biopsy samples.88
In this rapidly evolving field, the American Society for Clinical Pathology and the College of American Pathologists published guidelines for the laboratory workup of lymphoma in adults, which was affirmed as having value for hematologists by the American Society of Hematology.4 The role of CNB continues to evolve. These guidelines give a strong recommendation based on moderate evidence for excisional biopsy or CNB in patients with a high suspicion for lymphoma. This recommendation is written with a caveat that CNB must be used with careful consideration of the patient selection, technique, and available ancillary diagnostic methods. Specifically, the authors note variable diagnostic accuracy of CNB for lymphoma from 64%–98% and for subclassification of lymphoma from 68%–96%, with HL and follicular lymphoma being the least accurate, with accuracy as low as 8% for Grade 3 follicular lymphoma.89
Other considerations in the selection of CNB versus excisional biopsy include the possibility of insufficient tissue for complete ancillary testing at the time of diagnosis and/or for further testing on residual tissue for research and potential clinical trial eligibility.4 Regarding size of the needle used for CNB, while intuitively one would expect a larger needle gauge and higher number of passes/cores to provide higher diagnostic accuracy, no appropriate studies have been performed to address this, and the most recent guidelines refrain from making a recommendation on this.4
1.5.2 |. Incisional/excisional biopsy
Excisional biopsy remains the gold standard sampling method for suspected lymphomas, though, as above, some studies are showing equivalent diagnostic accuracy of CNB in certain situations. The joint ASCP, ACP guidelines that were deemed valuable by ASH specifically give a strong recommendation for surgical biopsy when feasible in a clinical setting where HL is suspected.4 This recommendation is based on studies showing high false negative rates for HL and lower diagnostic sensitivity of CNB for HL, for example, one study reported sensitivity as low as 50%.90
1.6 |. Lymphoma emergencies
Lymphoma embodies many heterogenous subtypes, each with varying clinical presentation. Often, lymphoma workup, diagnosis, specialist referral, and treatment plan can all be done in the outpatient setting. However, some lymphoma subtypes are high grade with an aggressive pace of disease. Prompt recognition of this subset is vital as medical emergencies related to aggressive lymphoma confer a worse prognosis.91 Recognition of potential emergencies by the generalist is key as they may be present during initial presentation and necessitate hospitalization and stat hematology/oncology consultation for intervention and expedited treatment.
High-grade lymphomas include Burkitt lymphoma, subsets of diffuse large B-cell lymphoma, lymphoblastic lymphoma, some mantle cell lymphomas, and peripheral T- and NK-cell lymphomas.92 They are associated with high tumor proliferation rate and extensive disease burden that can result in medical emergencies. Often, patients with aggressive lymphomas will describe profound B symptoms and rapidly enlarging lymph nodes.19 Compressive effects from bulky adenopathy may result in local damage that can involve any organ system. Other symptoms due to electrolyte derangements or paraneoplastic processes may be present as well. These emergent lymphoma presentations and the immediate interventions are described below and summarized in Table 3.
TABLE 3.
Lymphoma emergencies
| Hematologic emergency | Suggestive signs/symptoms | Initial management |
|---|---|---|
| Tumor lysis syndrome | Elevated lactate dehydrogenase (LDH), uric Acid, Potassium, ±phosphorus. AKI | Aggressive fluid resuscitation with isotonic fluids, management of hyperkalemia if indicated, immediate emergency room evaluation and hospitalization |
| Venous thromboembolism | Unilateral lower extremity swelling, erythema, pain. Sudden onset dyspnea/hypoxia. | If stable for outpatient management, low molecular weight heparin (LMWH), apixaban, rivaroxaban have the most evidence for anticoagulation in patients with malignancy |
| Paraneoplastic processes | Neurologic symptoms, dermatologic changes, fever, hematologic abnormalities, arthralgias, renal dysfunction, angioedema | Commensurate with the severity of presentation. Evidence of MAHA, severe neurologic involvement, rash and fever, severe AKI should be referred to the ED |
| Compressive or infiltrative tumor effects | Hepatic or renal dysfunction Evidence of neurovascular compromise including claudication, bowel or bladder dysfunction, saddle anesthesia, motor or sensory dysfunction. | Commensurate with the severity of presentation Severe hepatic or renal dysfunction, evidence of cauda equina syndrome, or rapidly progressive motor/sensory changes should be referred to the ED |
1.6.1 |. Compressive or infiltrative tumor effects
Compressive symptoms from lymphadenopathy include: spinal cord compression, lymphomatous meningitis, CNS mass lesions, airway obstruction, pericardial tamponade, SVC syndrome, GI obstruction, liver failure, hydronephrosis, or renal failure.93 All patients with metastatic spinal compression should undergo urgent evaluation for surgical decompression. If urgent surgical intervention is not possible, or available or there are delays to surgical decompression where tissue can be obtained, then immediate use of corticosteroids could be employed. Steroids are lymphodepleting and kill lymphoma tumor cells. Tumor cell death can relieve compressive symptoms but can also interfere with tissue diagnosis. Therefore, steroid administration should be avoided until biopsy is performed, whenever possible.
1.6.2 |. Tumor lysis syndrome
TLS is caused by high cell turn over. It most often presents after initiation of chemotherapy and is mitigated with preventative medications such as allopurinol. However, some tumor lysis may present spontaneously, prior to therapy initiation. Ongoing lysis results in accumulation of intracellular contents in the extracellular serum. Lab findings include elevated potassium, phosphorus, and uric acid and low calcium. Symptoms may include nausea, vomiting, diarrhea, arrhythmias, shortness of breath, congestive heart failure, arthralgias, lethargy, and cloudy urine. If untreated, these symptoms can progress to acute renal failure, seizures, muscle dystonia, arrhythmias, and death.94,95 Ultimately, patient with signs consistent with TLS as described above, should be referred for emergent hospitalization.
1.6.3 |. Paraneoplastic processes
Paraneoplastic processes can be the presenting symptoms for underlying lymphoma. Paraneoplastic symptoms associated with lymphoma include—cerebellar degeneration, immune thrombocytopenia, pemphigus, autoimmune hemolytic anemia.96 Other paraneoplastic syndromes that occur in lymphoma include sweet syndrome and other dermatologic manifestations.97 Sweet syndrome presents as sudden erythematous skin lesions, fever, leukocytosis, and neutrophilia. Sweet syndrome is commonly seen in hematologic conditions and the presence of sweet syndrome should prompt a lymphoma workup. Diagnosis of sweet syndrome is usually by biopsy which shows dense dermal neutrophilic infiltrate. Evidence of microangiopathic hemolytic anemia, sudden skin lesions, and fever should be referred to the ED.
1.6.4 |. Venous thromboembolism
While cancer in general increases the risk of thrombosis, patients with lymphoma have been found to be particularly at risk.98 The reported Incidence of Venous thromboembolism (VTE) in patients with lymphoma ranges from 5% to 59.5%.99 Treatment for VTE in patients with lymphoma is similar to other patients with malignancy. While low molecular weight heparin (LMWH) was traditionally used, direct oral anticoagulants (DOACs), such as apixaban and rivaroxaban have been shown to carry favorable efficacy profiles in patients with malignancy their use is becoming widely adopted in this space.100,101 These treatments can begin in the outpatient setting depending on the stability of the patient.
2 |. CONCLUSION
The workup of a patient with potential lymphoma can be challenging given the many uncertainties that surround the presentation and possible complications. Likewise, the clinical course of lymphoma ranges from asymptomatic and indolent to highlight morbid and fatal, thus, complicating the urgency of workup. Our aim of this manuscript is to highly the common presentations for lymphoma and reasonable diagnostic pathways that consider the operating characteristics of various diagnostic tools including peripheral blood FC, fine needle aspiration, core biopsy, and excisional biopsy. Notably, the majority of patients with lymphadenopathy presenting to primary care will ultimately be found to have an alternative diagnosis. B-symptoms without lymphadenopathy or splenomegaly should not prompt further targeted workup for lymphoma. Peripheral blood FC may be useful in patients with lymphocytosis but can lead to false negative findings in patients with predominantly nodal lymphoma. Fine needle aspiration is often insufficient for a complete diagnosis; however, core biopsy has a reasonable likelihood of leading to an accurate diagnosis. Excisional biopsy, if feasible, remains the gold standard for diagnosis.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1.Cazzola M Introduction to a review series: the 2016 revision of the WHO classification of tumors of hematopoietic and lymphoid tissues. Blood. 2016;127(20):2361–2364. [DOI] [PubMed] [Google Scholar]
- 2.Jiang M, Bennani NN, Feldman AL. Lymphoma classification update: T-cell lymphomas, Hodgkin lymphomas, and histiocytic/dendritic cell neoplasms. Expert Rev Hematol. 2017;10(3):239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kroft SH, Sever CE, Bagg A, et al. Laboratory workup of lymphoma in adults: Guideline from the American Society for Clinical Pathology and the College of American Pathologists. Arch Pathol Lab Med. 2021;145(3):269–290. [DOI] [PubMed] [Google Scholar]
- 5.Howell DA, Smith AG, Jack A, et al. Time-to-diagnosis and symptoms of myeloma, lymphomas and leukaemias: a report from the Haema-tological malignancy research network. BMC Hematol. 2013;13(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seymour JF. Extra-nodal lymphoma in rare localisations: bone, breast and testes. Hematol Oncol. 2013;31(Suppl 1):60–63. [DOI] [PubMed] [Google Scholar]
- 7.Morris SL. Skin lymphoma. Clin Oncol (R Coll Radiol). 2012;24(5): 371–385. [DOI] [PubMed] [Google Scholar]
- 8.Mikhaeel NG. Primary bone lymphoma. Clin Oncol (R Coll Radiol). 2012;24(5):366–370. [DOI] [PubMed] [Google Scholar]
- 9.Howlader N, Noone AM, Krapcho M, Miller D, Brest A, Yu M, eds. SEER Cancer Stat Facts, Hodgekin Lymphoma. Bethesda, MD: National Cancer Institute. Available from: https://seer.cancer.gov/statfacts/html/hodg.html. [Google Scholar]
- 10.Howlader N, Noone AM, Krapcho M, Miller D, Brest A, Yu M, eds. SEER Cancer Stat Facts Non Hodgekin Lymphoma. Bethesda, MD: National Cancer Institute. Available from: https://seer.cancer.gov/statfacts/html/nhl.html. [Google Scholar]
- 11.Fijten GH, Blijham GH. Unexplained lymphadenopathy in family practice. An evaluation of the probability of malignant causes and the effectiveness of physicians’ workup. J Fam Pract. 1988;27(4): 373–376. [DOI] [PubMed] [Google Scholar]
- 12.Iwamuro M Diagnosis of follicular lymphoma of the gastrointestinal tract: a better initial diagnostic workup. World J Gastroenterol. 2016; 22(4):1674–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsi ED, Horwitz SM, Carson KR, et al. Analysis of peripheral T-cell lymphoma diagnostic workup in the United States. Clin Lymphoma Myeloma Leuk. 2017;17(4):193–200. [DOI] [PubMed] [Google Scholar]
- 14.Arnold S, Freedman MWF, Aster MDJC. Clinical presentation and initial evaluation of non-Hodgkin lymphoma. In: Post T, ed. UpToDate. UpToDate; 2021. Accessed December 12, 2021. [Google Scholar]
- 15.Ann S, LaCasce MKN, Aster MPHJC. Clinical presentation and diagnosis of classic Hodgkin lymphoma in adults. In: Post T, ed. UpToDate. UpToDate; 2021. Accessed on December 29, 2021. [Google Scholar]
- 16.Ansell SM, Armitage J. Non-Hodgkin lymphoma: diagnosis and treatment. Mayo Clin Proc. 2005;80(8):1087–1097. [DOI] [PubMed] [Google Scholar]
- 17.Shankland KR, Armitage JO, Hancock BW. Non-Hodgkin lymphoma. Lancet. 2012;380(9844):848–857. [DOI] [PubMed] [Google Scholar]
- 18.Chau I, Kelleher MT, Cunningham D, et al. Rapid access multidisciplinary lymph node diagnostic clinic: analysis of 550 patients. Br J Cancer. 2003;88(3):354–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaddey HL, Riegel AM. Unexplained lymphadenopathy: evaluation and differential diagnosis. Am Fam Physician. 2016;94(11): 896–903. [PubMed] [Google Scholar]
- 20.McCarthy DM et al. What did you Google? Describing online health information search patterns of ED patients and their relationship with final diagnoses. West J Emerg Med. 2017;18(5):928–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferrer R Lymphadenopathy: differential diagnosis and evaluation. Am Fam Physician. 1998;58(6):1313–1320. [PubMed] [Google Scholar]
- 22.Freeman AM, Matto P. Adenopathy. StatPearls; 2021. [PubMed] [Google Scholar]
- 23.Libman H Generalized lymphadenopathy. J Gen Intern Med. 1987; 2(1):48–58. [DOI] [PubMed] [Google Scholar]
- 24.Habermann TM, Steensma DP. Lymphadenopathy. Mayo Clin Proc. 2000;75(7):723–732. [DOI] [PubMed] [Google Scholar]
- 25.El-Sayed MS et al. The incidence and duration of COVID-19 vaccine-related reactive lymphadenopathy on (18)F-FDG PET-CT. Clin Med (Lond). 2021;21(6):e633–e638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferrer R Evaluation of peripheral lymphadenopathy in adults. UpToDate. Post TW. UpToDate; 2022. (Accessed January 31, 2022). [Google Scholar]
- 27.Carbone PP, Kaplan HS, Musshoff K, Smithers DW, Tubiana M. Report of the committee on Hodgkin’s disease staging classification. Cancer Res. 1971;31(11):1860–1861. [PubMed] [Google Scholar]
- 28.Cunha BA, Lortholary O, Cunha CB. Fever of unknown origin: a clinical approach. Am J Med. 2015;128(10):1138.e1–1138.e15. [DOI] [PubMed] [Google Scholar]
- 29.Bryce C Persistent night sweats: diagnostic evaluation. Am Fam Physician. 2020;102(7):427–433. [PubMed] [Google Scholar]
- 30.Gaddey HL, Holder K. Unintentional weight loss in older adults. Am Fam Physician. 2014;89(9):718–722. [PubMed] [Google Scholar]
- 31.Metalidis C, Knockaert DC, Bobbaers H, Vanderschueren S. Involuntary weight loss. Does a negative baseline evaluation provide adequate reassurance? Eur J Intern Med. 2008;19(5):345–349. [DOI] [PubMed] [Google Scholar]
- 32.Bobrove AM. Alcohol-related pain and Hodgkin’s disease. West J Med. 1983;138(6):874–875. [PMC free article] [PubMed] [Google Scholar]
- 33.Chalian H, McAdams HP, Lee Y, et al. Mediastinal lymphadenopathy in the National Lung Screening Trial (NLST) is associated with interval lung cancer. Radiology. 2022;302(3):684–692. [DOI] [PubMed] [Google Scholar]
- 34.Lucey BC, Stuhlfaut JW, Soto JA. Mesenteric lymph nodes seen at imaging: causes and significance. Radiographics. 2005;25(2):351–365. [DOI] [PubMed] [Google Scholar]
- 35.Olszewska-Szopa M, Wrobel T. Gastrointestinal non-Hodgkin lymphomas. Adv Clin Exp Med. 2019;28(8):1119–1124. [DOI] [PubMed] [Google Scholar]
- 36.Chang CJ, Cheng JH, Lin MS, Dai YC, Hsiue TR. Eosinophilic pleural effusion as the first presentation of angioimmunoblastic T cell lymphoma. J Formos Med Assoc. 2007;106(2):156–160. [DOI] [PubMed] [Google Scholar]
- 37.Amir AR, Sheikh SS. Hodgkin’s lymphoma with concurrent systemic amyloidosis, presenting as acute renal failure, following lymphomatoid papulosis. J Nephrol. 2006;19(3):361–365. [PubMed] [Google Scholar]
- 38.Storck K, Brandstetter M, Keller U, Knopf A. Clinical presentation and characteristics of lymphoma in the head and neck region. Head Face Med. 2019;15(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ludwig H, van Belle S, Barrett-Lee P, et al. The European cancer Anaemia survey (ECAS): a large, multinational, prospective survey defining the prevalence, incidence, and treatment of anaemia in cancer patients. Eur J Cancer. 2004;40(15):2293–2306. [DOI] [PubMed] [Google Scholar]
- 40.Yasmeen T, Ali J, Khan K, Siddiqui N. Frequency and causes of anemia in lymphoma patients. Pak J Med Sci. 2019;35(1):61–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moullet I, Salles G, Ketterer N, et al. Frequency and significance of anemia in non-Hodgkin’s lymphoma patients. Ann Oncol. 1998;9(10): 1109–1115. [DOI] [PubMed] [Google Scholar]
- 42.Truong PT, Parhar T, Hart J, Alexander C, Wai ES. Population-based analysis of the frequency of anemia and its management before and during chemotherapy in patients with malignant lymphoma. Am J Clin Oncol. 2010;33(5):465–468. [DOI] [PubMed] [Google Scholar]
- 43.Zhou JC, Wu MQ, Peng ZM, Zhao WH, Bai ZJ. Clinical analysis of 20 patients with non-Hodgkin lymphoma and autoimmune hemolytic anemia: a retrospective study. Medicine (Baltimore). 2020;99(7): e19015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matsumoto K, Fujisawa S, Ando T, et al. Anemia associated with worse outcome in diffuse large B-cell lymphoma patients: a single-center retrospective study. Turk J Haematol. 2018;35(3):181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Essouma M, Soh DM, Temgoua MN, et al. Adult T-type lymphoblastic lymphoma presenting as hypercalcemic crisis and aplastic anemia: a case report. J Med Case Reports. 2019;13(1):305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vallet N, Ertault M, Delaye JB, et al. Hypercalcemia is associated with a poor prognosis in lymphoma a retrospective monocentric matched-control study and extensive review of published reported cases. Ann Hematol. 2020;99(2):229–239. [DOI] [PubMed] [Google Scholar]
- 47.Tebben PJ, Singh RJ, Kumar R. Vitamin D-mediated hypercalcemia: mechanisms, diagnosis, and treatment. Endocr Rev. 2016;37(5): 521–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Whiles BB, Duchene DA. Periureteral marginal zone lymphoma resulting in Hydronephrosis and flank pain in the absence of disseminated disease: Case report of two patients presenting with rare but important differential. J Endourol Case Rep. 2020;6(4):519–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 16–1988. An 83-year-old woman with anemia, oliguric renal failure, and past lymphoma. N Engl J Med. 1988;318(16):1047–1057. [DOI] [PubMed] [Google Scholar]
- 50.Coggins CH. Renal failure in lymphoma. Kidney Int. 1980;17(6):847–855. [DOI] [PubMed] [Google Scholar]
- 51.Da’as N, Polliack A, Cohen Y, et al. Kidney involvement and renal manifestations in non-Hodgkin’s lymphoma and lymphocytic leukemia: a retrospective study in 700 patients. Eur J Haematol. 2001; 67(3):158–164. [DOI] [PubMed] [Google Scholar]
- 52.Petzel RA, Brown DC, Staley NA, McMillen JJ, Sibley RK, Kjellstrand CM. Crescentic glomerulonephritis and renal failure associated with malignant lymphoma. Am J Clin Pathol. 1979;71(6):728–732. [DOI] [PubMed] [Google Scholar]
- 53.Kagu MB, Ahmed SG, Bukar AA. Pre-treatment tumour lysis syndrome and acute renal failure in adult Nigerians with Burkitt’s lymphoma: report of three cases and literature review. Afr J Med Med Sci. 2005;34(4):399–402. [PubMed] [Google Scholar]
- 54.Davies J, Healey DA, Wood KM, Jones K, Kanagasundaram NS. Acute renal failure due to mantle cell lymphoma--a case report and discussion of the literature. Clin Nephrol. 2007;67(6):394–396. [DOI] [PubMed] [Google Scholar]
- 55.Gianviti A, Boldrini R, Bosman C, Rizzoni G. Chronic renal failure due to kidney infiltration by Burkitt type lymphoma. Pediatr Nephrol. 1989;3(4):448–450. [DOI] [PubMed] [Google Scholar]
- 56.Bunchorntavakul C, Reddy KR. Hepatic manifestations of lymphoproliferative disorders. Clin Liver Dis. 2019;23(2):293–308. [DOI] [PubMed] [Google Scholar]
- 57.Jurisic V, Radenkovic S, Konjevic G. The actual role of LDH as tumor marker, biochemical and clinical aspects. Adv Exp Med Biol. 2015; 867:115–124. [DOI] [PubMed] [Google Scholar]
- 58.Patel V, Case R. Spontaneous tumor lysis syndrome in Blastoid-variant mantle cell lymphoma: considerations for the general internist. J Investig Med High Impact Case Rep. 2020;8:232470962 0944709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kjellstrand CM. Hyperuricemic Acute. Ren Fail. 1974;133(3):349. [PubMed] [Google Scholar]
- 60.McKinnon KM. Flow cytometry: an overview. Curr Protoc Immunol. 2018;120:5.1.1–5.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roshal M, Wood BL, Fromm JR. Flow cytometric detection of the classical hodgkin lymphoma: clinical and research applications. Adv Hematol. 2011;2011:387034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Martig DS, Fromm JR. A comparison and review of the flow cytometric findings in classic Hodgkin lymphoma, nodular lymphocyte predominant Hodgkin lymphoma, T cell/histiocyte rich large B cell lymphoma, and primary mediastinal large B cell lymphoma. Cytometry B Clin Cytom. 2022;102(1):14–25. [DOI] [PubMed] [Google Scholar]
- 63.Cirillo M, Craig AFM, Borchmann S, Kurtz DM. Liquid biopsy in lymphoma: molecular methods and clinical applications. Cancer Treat Rev. 2020;91:102106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Promsuwicha O et al. Utilization of flow cytometry for diagnosis of hematologic malignancies in Thailand: increasing trends and diagnostic yields in 7,982 samples. J Med Assoc Thai. 2014;97(12):1296–1301. [PubMed] [Google Scholar]
- 65.Lim HY, Hong FS. Maximising yield of peripheral blood flow cytometry for chronic lymphoproliferative disorders. Int J Lab Hematol. 2018;40(5):556–560. [DOI] [PubMed] [Google Scholar]
- 66.Pathology, A.S.F.C. Do not perform peripheral blood flow cytometry to screen for hematological malignancy in the settings of mature neutrophilia, basophilia, erythrocytosis, thrombocytosis, isolated anemia, or isolated thrombocytopenia. ChoosingWisely.org September 25, 2018. [cited 2022 January 31]; Available from: https://www.choosingwisely.org/clinician-lists/ascp-peripheral-blood-flow-cytometry-hematological-malignancy/. [Google Scholar]
- 67.Tsukamoto N, Kojima M, Hasegawa M, et al. The usefulness of (18) F-fluorodeoxyglucose positron emission tomography ((18)F-FDG-PET) and a comparison of (18)F-FDG-pet with (67)gallium scintigraphy in the evaluation of lymphoma: relation to histologic subtypes based on the World Health Organization classification. Cancer. 2007;110(3):652–659. [DOI] [PubMed] [Google Scholar]
- 68.Weiler-Sagie M, Bushelev O, Epelbaum R, et al. (18)F-FDG avidity in lymphoma readdressed: a study of 766 patients. J Nucl Med. 2010; 51(1):25–30. [DOI] [PubMed] [Google Scholar]
- 69.Barrington SF, O’Doherty MJ. Limitations of PET for imaging lymphoma. Eur J Nucl Med Mol Imaging. 2003;30(Suppl 1):S117–S127. [DOI] [PubMed] [Google Scholar]
- 70.Cook GJ, Wegner EA, Fogelman I. Pitfalls and artifacts in 18FDG PET and PET/CT oncologic imaging. Semin Nucl Med. 2004;34(2): 122–133. [DOI] [PubMed] [Google Scholar]
- 71.Cheson BD. Role of functional imaging in the management of lymphoma. J Clin Oncol. 2011;29(14):1844–1854. [DOI] [PubMed] [Google Scholar]
- 72.Bodet-Milin C, Touzeau C, Leux C, et al. Prognostic impact of 18F-fluoro-deoxyglucose positron emission tomography in untreated mantle cell lymphoma: a retrospective study from the GOELAMS group. Eur J Nucl Med Mol Imaging. 2010;37(9):1633–1642. [DOI] [PubMed] [Google Scholar]
- 73.Schoder H, Noy A, Gönen M, et al. Intensity of 18fluorodeoxyglucose uptake in positron emission tomography distinguishes between indolent and aggressive non-Hodgkin’s lymphoma. J Clin Oncol. 2005;23(21):4643–4651. [DOI] [PubMed] [Google Scholar]
- 74.Watanabe R, Tomita N, Takeuchi K, et al. SUVmax in FDG-PET at the biopsy site correlates with the proliferation potential of tumor cells in non-Hodgkin lymphoma. Leuk Lymphoma. 2010;51(2): 279–283. [DOI] [PubMed] [Google Scholar]
- 75.Barrington SF, Mikhaeel NG, Kostakoglu L, et al. Role of imaging in the staging and response assessment of lymphoma: consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J Clin Oncol. 2014;32(27):3048–3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zinzani PL. PET in T-cell lymphoma. Curr Hematol Malig Rep. 2011; 6(4):241–244. [DOI] [PubMed] [Google Scholar]
- 77.Otero HJ, Jagannathan JP, Prevedello LM, et al. CT and PET/CT findings of T-cell lymphoma. AJR Am J Roentgenol. 2009;193(2):349–358. [DOI] [PubMed] [Google Scholar]
- 78.Casulo C, Schöder H, Feeney J, et al. 18F-fluorodeoxyglucose positron emission tomography in the staging and prognosis of T cell lymphoma. Leuk Lymphoma. 2013;54(10):2163–2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.O’Doherty MJ, Barrington SF, Campbell M, Lowe J, Bradbeer CS. PET scanning and the human immunodeficiency virus-positive patient. J Nucl Med. 1997;38(10):1575–1583. [PubMed] [Google Scholar]
- 80.Weiss LM, O’Malley D. Benign lymphadenopathies. Mod Pathol. 2013;26(Suppl 1):S88–S96. [DOI] [PubMed] [Google Scholar]
- 81.Pugliese N, di Perna M, Cozzolino I, et al. Randomized comparison of power Doppler ultrasonography-guided core-needle biopsy with open surgical biopsy for the characterization of lymphadenopathies in patients with suspected lymphoma. Ann Hematol. 2017;96(4): 627–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gibbs JD, Ma S, Kim A, et al. Utility of flow cytometry and gene rear-rangement analysis in tissue and blood of patients with suspected cutaneous T-cell lymphoma. Oncol Rep. 2021;45(1):349–358. [DOI] [PubMed] [Google Scholar]
- 83.Pynnonen MA, Gillespie MB, Roman B, et al. Clinical practice guideline: evaluation of the neck mass in adults. Otolaryngol Head Neck Surg. 2017;157(2_suppl):S1–s30. [DOI] [PubMed] [Google Scholar]
- 84.Tandon S, Shahab R, Benton JI, Ghosh SK, Sheard J, Jones TM. Fine-needle aspiration cytology in a regional head and neck cancer center: comparison with a systematic review and meta-analysis. Head Neck. 2008;30(9):1246–1252. [DOI] [PubMed] [Google Scholar]
- 85.Roh JL, Lee YW, Kim JM. Clinical utility of fine-needle aspiration for diagnosis of head and neck lymphoma. Eur J Surg Oncol. 2008;34(7): 817–821. [DOI] [PubMed] [Google Scholar]
- 86.Seviar D, Yousuff M, Chia Z, Ramesar K, Newman J, Howlett DC. Image-guided core needle biopsy as the first-line diagnostic approach in lymphoproliferative disorders—a review of the current literature. Eur J Haematol. 2021;106(2):139–147. [DOI] [PubMed] [Google Scholar]
- 87.Warshavsky A, Rosen R, Perry C, et al. Core needle biopsy for diagnosing lymphoma in cervical lymphadenopathy: meta-analysis. Head Neck. 2020;42(10):3051–3060. [DOI] [PubMed] [Google Scholar]
- 88.Ye X, Tucker C, Gardner C, Redilla A, Uppal G, Binder AF. Assessment of the diagnostic accuracy of Core needle biopsies in the diagnosis of lymphoma. Blood. 2020;136(Supplement 1):12–13. [Google Scholar]
- 89.Farmer PL, Bailey DJ, Burns BF, Day A, LeBrun DP. The reliability of lymphoma diagnosis in small tissue samples is heavily influenced by lymphoma subtype. Am J Clin Pathol. 2007;128(3):474–480. [DOI] [PubMed] [Google Scholar]
- 90.Groneck L, Quaas A, Hallek M, Zander T, Weihrauch MR. Ultra-sound-guided core needle biopsies for workup of lymphadenopathy and lymphoma. Eur J Haematol. 2016;97(4):379–386. [DOI] [PubMed] [Google Scholar]
- 91.Kane E, Howell D, Smith A, et al. Emergency admission and survival from aggressive non-Hodgkin lymphoma: a report from the UK’s population-based haematological malignancy research network. Eur J Cancer. 2017;78:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.American Society of Hematology Self-Assessment Program. 7th ed. American Society of Hematology; 2019. [Google Scholar]
- 93.Halfdanarson TR, Hogan WJ, Madsen BE. Emergencies in hematology and oncology. Mayo Clin Proc. 2017;92(4):609–641. [DOI] [PubMed] [Google Scholar]
- 94.Jasek AM, Day HJ. Acute spontaneous tumor lysis syndrome. Am J Hematol. 1994;47(2):129–131. [DOI] [PubMed] [Google Scholar]
- 95.Hande KR, Garrow GC. Acute tumor lysis syndrome in patients with high-grade non-Hodgkin’s lymphoma. Am J Med. 1993;94(2): 133–139. [DOI] [PubMed] [Google Scholar]
- 96.Hagler KT, Lynch JW Jr. Paraneoplastic manifestations of lymphoma. Clin Lymphoma. 2004;5(1):29–36. [DOI] [PubMed] [Google Scholar]
- 97.Agrawal A, Arif SH, Kumarasan K, Janjua D. Sweet’s syndrome: an update. Curr. Pediatr Rev 2022;18:265–273. [DOI] [PubMed] [Google Scholar]
- 98.Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111(10):4902–4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yokoyama K Thrombosis in lymphoma patients and in myeloma patients. Keio J Med. 2015;64(3):37–43. [DOI] [PubMed] [Google Scholar]
- 100.Agnelli G, Becattini C, Meyer G, et al. Apixaban for the treatment of venous thromboembolism associated with cancer. N Engl J Med. 2020;382(17):1599–1607. [DOI] [PubMed] [Google Scholar]
- 101.Young AM, Marshall A, Thirlwall J, et al. Comparison of an Oral factor Xa inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism: results of a randomized trial (SELECT-D). J Clin Oncol. 2018;36(20):2017–2023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


