Abstract
Background
Attention deficit hyperactivity disorder (ADHD) is one of the most commonly diagnosed and treated psychiatric disorders in childhood. Typically, children and adolescents with ADHD find it difficult to pay attention and they are hyperactive and impulsive. Methylphenidate is the psychostimulant most often prescribed, but the evidence on benefits and harms is uncertain. This is an update of our comprehensive systematic review on benefits and harms published in 2015.
Objectives
To assess the beneficial and harmful effects of methylphenidate for children and adolescents with ADHD.
Search methods
We searched CENTRAL, MEDLINE, Embase, three other databases and two trials registers up to March 2022. In addition, we checked reference lists and requested published and unpublished data from manufacturers of methylphenidate.
Selection criteria
We included all randomised clinical trials (RCTs) comparing methylphenidate versus placebo or no intervention in children and adolescents aged 18 years and younger with a diagnosis of ADHD. The search was not limited by publication year or language, but trial inclusion required that 75% or more of participants had a normal intellectual quotient (IQ > 70). We assessed two primary outcomes, ADHD symptoms and serious adverse events, and three secondary outcomes, adverse events considered non‐serious, general behaviour, and quality of life.
Data collection and analysis
Two review authors independently conducted data extraction and risk of bias assessment for each trial. Six review authors including two review authors from the original publication participated in the update in 2022. We used standard Cochrane methodological procedures. Data from parallel‐group trials and first‐period data from cross‐over trials formed the basis of our primary analyses. We undertook separate analyses using end‐of‐last period data from cross‐over trials. We used Trial Sequential Analyses (TSA) to control for type I (5%) and type II (20%) errors, and we assessed and downgraded evidence according to the GRADE approach.
Main results
We included 212 trials (16,302 participants randomised); 55 parallel‐group trials (8104 participants randomised), and 156 cross‐over trials (8033 participants randomised) as well as one trial with a parallel phase (114 participants randomised) and a cross‐over phase (165 participants randomised). The mean age of participants was 9.8 years ranging from 3 to 18 years (two trials from 3 to 21 years). The male‐female ratio was 3:1. Most trials were carried out in high‐income countries, and 86/212 included trials (41%) were funded or partly funded by the pharmaceutical industry. Methylphenidate treatment duration ranged from 1 to 425 days, with a mean duration of 28.8 days. Trials compared methylphenidate with placebo (200 trials) and with no intervention (12 trials). Only 165/212 trials included usable data on one or more outcomes from 14,271 participants.
Of the 212 trials, we assessed 191 at high risk of bias and 21 at low risk of bias. If, however, deblinding of methylphenidate due to typical adverse events is considered, then all 212 trials were at high risk of bias.
Primary outcomes: methylphenidate versus placebo or no intervention may improve teacher‐rated ADHD symptoms (standardised mean difference (SMD) −0.74, 95% confidence interval (CI) −0.88 to −0.61; I² = 38%; 21 trials; 1728 participants; very low‐certainty evidence). This corresponds to a mean difference (MD) of −10.58 (95% CI −12.58 to −8.72) on the ADHD Rating Scale (ADHD‐RS; range 0 to 72 points). The minimal clinically relevant difference is considered to be a change of 6.6 points on the ADHD‐RS. Methylphenidate may not affect serious adverse events (risk ratio (RR) 0.80, 95% CI 0.39 to 1.67; I² = 0%; 26 trials, 3673 participants; very low‐certainty evidence). The TSA‐adjusted intervention effect was RR 0.91 (CI 0.31 to 2.68).
Secondary outcomes: methylphenidate may cause more adverse events considered non‐serious versus placebo or no intervention (RR 1.23, 95% CI 1.11 to 1.37; I² = 72%; 35 trials 5342 participants; very low‐certainty evidence). The TSA‐adjusted intervention effect was RR 1.22 (CI 1.08 to 1.43). Methylphenidate may improve teacher‐rated general behaviour versus placebo (SMD −0.62, 95% CI −0.91 to −0.33; I² = 68%; 7 trials 792 participants; very low‐certainty evidence), but may not affect quality of life (SMD 0.40, 95% CI −0.03 to 0.83; I² = 81%; 4 trials, 608 participants; very low‐certainty evidence).
Authors' conclusions
The majority of our conclusions from the 2015 version of this review still apply. Our updated meta‐analyses suggest that methylphenidate versus placebo or no‐intervention may improve teacher‐rated ADHD symptoms and general behaviour in children and adolescents with ADHD. There may be no effects on serious adverse events and quality of life. Methylphenidate may be associated with an increased risk of adverse events considered non‐serious, such as sleep problems and decreased appetite. However, the certainty of the evidence for all outcomes is very low and therefore the true magnitude of effects remain unclear.
Due to the frequency of non‐serious adverse events associated with methylphenidate, the blinding of participants and outcome assessors is particularly challenging. To accommodate this challenge, an active placebo should be sought and utilised. It may be difficult to find such a drug, but identifying a substance that could mimic the easily recognised adverse effects of methylphenidate would avert the unblinding that detrimentally affects current randomised trials.
Future systematic reviews should investigate the subgroups of patients with ADHD that may benefit most and least from methylphenidate. This could be done with individual participant data to investigate predictors and modifiers like age, comorbidity, and ADHD subtypes.
Plain language summary
Is methylphenidate an effective treatment for children and adolescents with attention deficit hyperactivity disorder (ADHD) and does it cause unwanted effects?
Key messages
‐ Methylphenidate might reduce hyperactivity and impulsivity and might help children to concentrate. Methylphenidate might also help to improve general behaviour, but does not seem to affect quality of life.
‐ Methylphenidate does not seem to increase the risk of serious (life‐threatening) unwanted effects when used for periods of up to six months. However, it is associated with an increased risk of non‐serious unwanted effects like sleeping problems and decreased appetite.
‐ Future studies should focus more on reporting unwanted effects and should take place over longer periods of time.
What is attention deficit hyperactivity disorder (ADHD)?
ADHD is one of the most commonly diagnosed and treated childhood psychiatric disorders. Children with ADHD find it hard to concentrate. They are often hyperactive (fidgety, unable to sit still for long periods) and impulsive (doing things without stopping to think). ADHD can make it difficult for children to do well at school, because they find it hard to follow instructions and to concentrate. Their behavioural problems can interfere with their ability to get on well with family and friends, and they often get into more trouble than other children.
How is ADHD treated?
Methylphenidate (for example, Ritalin) is the medication most often prescribed to children and adolescents with ADHD. Methylphenidate is a stimulant that helps to increase activity in parts of the brain, such as those involved with concentration. Methylphenidate can be taken as a tablet or given as a skin patch. It can be formulated to have an immediate effect, or be delivered slowly, over a period of hours. Methylphenidate may cause unwanted effects, such as headaches, stomachaches and problems sleeping. It sometimes causes serious unwanted effects like heart problems, hallucinations, or facial 'tics' (twitches).
What did we want to find out?
We wanted to find out if methylphenidate improves children's ADHD symptoms (attention, hyperactivity) based mainly on teachers' ratings using various scales, and whether it causes serious unwanted effects, like death, hospitalisation, or disability. We were also interested in less serious unwanted effects like sleep problems and loss of appetite, and its effects on children's general behaviour and quality of life.
What did we do?
We searched for studies that investigated the use of methylphenidate in children and adolescents with ADHD. Participants in the studies had to be aged 18 years or younger and have a diagnosis of ADHD. They could have other disorders or illnesses and be taking other medication or undergoing behavioural treatments. They had to have a normal IQ (intelligence quotient). Studies could compare methylphenidate with placebo (something designed to look and taste the same as methylphenidate but with no active ingredient) or no treatment. Participants had to be randomly chosen to receive methylphenidate or not. We compared and summarised the results of the studies and rated our confidence in the evidence, based on factors such as study methods and sizes.
What did we find?
We found 212 studies with 16,302 children or adolescents with ADHD. Most of the trials compared methylphenidate with placebo. Most studies were small with around 70 children, with an average age of 10 years (ages ranged from 3 to 18 years). Most studies were short, lasting an average of around a month; the shortest study lasted just one day and the longest 425 days. Most studies were in the USA.
Based on teachers' ratings, compared with placebo or no treatment, methylphenidate:
‐ may improve ADHD symptoms (21 studies, 1728 children)
‐ may make no difference to serious unwanted effects (26 studies, 3673 participants)
‐ may cause more non‐serious unwanted effects (35 studies, 5342 participants)
‐ may improve general behaviour (7 trials 792 participants)
‐ may not affect quality of life (4 trials, 608 participants)
Limitations of the evidence
Our confidence in the results of the review is limited for several reasons. It was often possible for people in the studies to know which treatment the children were taking, which could influence the results. The reporting of the results was not complete in many studies and for some outcomes the results varied across studies. Studies were small and they used different scales for measuring symptoms. And most of the studies only lasted for a short period of time, making it impossible to assess the long‐term effects of methylphenidate. Around 41% of studies were funded or partly funded by the pharmaceutical industry.
How up to date is this evidence?
This is an update of a review conducted in 2015. The evidence is current to March 2022.
Summary of findings
Summary of findings 1. Methylphenidate compared with placebo or no intervention for children and adolescents with ADHD.
| Methylphenidate compared with placebo or no intervention for ADHD | ||||||
| Patient or population: children and adolescents (up to and including 18 years of age) with ADHD Settings: outpatient clinic, inpatient hospital ward and summer school Intervention: methylphenidate Comparison: placebo or no intervention | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (trials) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo or no intervention | Methylphenidate | |||||
|
ADHD symptoms: all parallel‐group trials and first‐period cross‐over trials
ADHD Rating Scale (teacher‐rated) Average trial duration: 68.7 days |
Mean ADHD symptom score in the intervention groups corresponds to a mean difference of −10.58 (95% CI −12.58 to −8.72) on ADHD Rating Scale |
SMD −0.74 (−0.88 to −0.61) |
1728 (21 trials) |
⊕⊝⊝⊝ Very lowa,b | The analysis was conducted on a standardised scale with data from studies that used different teacher‐rated scales of symptoms (Conners' Teacher Rating Scale (CTRS), Strengths and Weaknesses of ADHD Symptoms and Normal Behaviour (SWAN) Scale, The Swanson, Nolan and Pelham (SNAP) Scale ‐ Teacher, Fremdbeurteilungsbogen für Hyperkinetische Störungen (FBB‐HKS)). We translated the effect size on to the ADHD Rating Scale from the SMD. | |
| Proportion of participants with one or more serious adverse events | Trial population | RR 0.80 (0.39 to 1.67) | 3673 (26 trials) |
⊕⊝⊝⊝ Verylowa,c | TSA RIS = 9349 TSA showed a RR of 0.91 (TSA‐adjusted Cl 0.31 to 2.68) |
|
| 8 per 1000 | 6 per 1000 (5 less to 5 more) | |||||
| Proportion of participants with one or more adverse events considered non‐serious | Trial population |
RR 1.23 (1.11 to 1.37) |
5342 (35 trials) |
⊕⊝⊝⊝ Verylowa,b | TSA RIS = 9139 TSA showed a RR of 1.22 (TSA‐adjusted Cl 1.08 to 1.43) | |
| 437 per 1000 | 538 per 1000 (348 less to 162 more) | |||||
| General behaviour: all parallel‐group trials and first‐period cross‐over trials General behaviour rating scales (teacher‐rated) | Mean general behaviour score in the intervention groups was 0.62 standard mean deviations lower (95% CI 0.91 lower to 0.33 lower) |
SMD −0.62 (−0.91 to −0.33) |
792 (7 trials) | ⊕⊝⊝⊝ Very lowa,b,d | ||
|
Quality of life (parent‐rated) |
Mean quality‐of‐life score in the intervention groups corresponds to a mean difference of 4.94 (95% CI −0.37 to 10.25) on the Child Health Questionnaire |
SMD 0.40 (−0.03 to 0.83) |
608 (4 trials) | ⊕⊝⊝⊝ Verylowa,b,c,e | ||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ADHD: attention deficit hyperactivity disorder; CI: confidence interval; RIS: required information size; RR: risk ratio; SMD: standardised mean difference; TSA: Trial Sequential Analysis | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded two levels due to high risk of bias (systematic errors causing overestimation of benefits and underestimation of harms) in several risk of bias domains, including lack of sufficient blinding and selective outcome reporting (many of the included trials did not report on this outcome). bDowngraded one level due to inconsistency: moderate statistical heterogeneity. cDowngraded two levels due to imprecision: wide confidence intervals and/or the accrued number of participants was below 50% of the diversity‐adjusted required information size (DARIS) in Trial Sequential Analysis. dDowngraded one level due to indirectness: children's general behaviour was assessed by different types of rating scales with different focus on behaviour. e Downgraded one level due to indirectness: children's quality of life was assessed by their parents.
Background
Description of the condition
Attention deficit hyperactivity disorder (ADHD) is one of the most commonly diagnosed and treated developmental psychiatric disorders (Scahill 2000). It is acknowledged to be a complex heterogenous neurodevelopmental condition with no known cure (Buitelaar 2022). Many clinicians and academics see pharmacological treatments as being effective and safe but there is “considerable individual variability” of treatment response, dose needed, and tolerability (Buitelaar 2022).
The prevalence of ADHD in children and adolescents is estimated to be 3% to 5% (Polanczyk 2007), depending on the classification system used, with boys two to four times more likely to be diagnosed than girls (Schmidt 2009). Individuals with ADHD exhibit difficulties with attentional and cognitive functions including problem‐solving, planning, maintaining flexibility and orientation, sustaining attention, inhibiting responses, and sustaining working memory (Pasini 2007; Sergeant 2003). They also experience difficulties in managing affects, for example, motivational delay and mood dysregulation (Castellanos 2006; Nigg 2005; Schmidt 2009). The diagnosis of ADHD has become more aligned between the American Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM‐5; APA 2013), and the World Health Organization’s (WHO) International Classification of Diseases (ICD), 11th Edition (ICD‐11; WHO 2019). The ICD‐11 was adopted in 2019, and came into effect in January 2022.
Both the DSM‐5 and the ICD‐11 base diagnoses on several inattentive and hyperactive‐impulsive symptoms being present before the age of 12 years, and causing impairment of functioning in several settings. There are also 'predominantly inattentive', 'predominantly hyperactive/impulsive' and 'combined' presentations in both systems (APA 2013; WHO 2019).
ADHD is increasingly recognised as a psychiatric disorder that extends into adulthood and occurs with high heterogeneity and comorbidity with other psychiatric disorders (Schmidt 2009). The Multimodal Treatment of Attention Deficit Hyperactivity Disorder (MTA) trial identified one or more co‐occurring conditions in almost 40% of participants (MTA 1999a). These included oppositional defiant disorder, conduct disorder, depression, anxiety, tics, learning difficulties and cognitive deficits (Jensen 2001; Kadesjö 2001). Some argue that ADHD should be “considered not only a neurodevelopmental disorder, but also a persistent and complex condition, with detrimental consequences for quality of life in adulthood” (Di Lorenzo 2021, p. 283).
Rising rates of ADHD diagnoses, possible harm to children resulting from drug treatment (Zito 2000), and variation in prevalence estimates are matters of increasing concern (Moffit 2007; Polanczyk 2014). The need for a validated diagnostic test to confirm the clinical diagnosis of ADHD has given rise to a debate about its validity as a diagnosis (Timimi 2004). Professional and national bodies have developed guidelines on assessment, diagnosis and treatment of ADHD in an attempt to ensure that high standards are maintained in diagnostic and therapeutic practice (American Academy of Pediatrics 2011; CADDRA 2011; NICE 2018; Pliszka 2007a; SIGN 2009). Psychosocial interventions, such as parent management training, are recommended in the first instance for younger children and for those with mild to moderate symptoms (American Academy of Pediatrics 2011; NICE 2018; Pliszka 2007a), whereas stimulants (given alone or in combination with psychosocial interventions) are recommended for children with more severe ADHD (American Academy of Pediatrics 2011; CADDRA 2011; NICE 2018).
Description of the intervention
Methylphenidate, lisdexamphetamine/dexamphetamine, atomoxetine (a non‐stimulant selective noradrenaline reuptake inhibitor) and guanfacine (an alpha‐2 agonist) are recommended medical treatments for children, aged five years and above, and adolescents with ADHD, when psychoeducation and environmental modification have been implemented and reviewed, according to the NICE guidelines 2018 (NICE 2018). Furthermore, research suggests that the combination of behaviour therapy (e.g. behavioural parent training, school consultation, direct contingency management) and pharmacotherapy might benefit children with ADHD (Gilmore 2001; MTA 1999a).
Globally, methylphenidate has been used for longer than 50 years for the treatment of children with ADHD (Kadesjö 2002; NICE 2018). It has been part of driving innovation in controlled‐release technologies and new formulations. However, it has also contributed to concerns of pharmaceutical cognitive enhancement as well as created debate on pharmaceutical sales techniques in medicine, driven by high and possibly still increasing prescription rates (Wenthur 2016). In Europe, around 3% to 5% of children and adolescents have a prescription for methylphenidate (Bachmann 2017; Hodgkins 2013; Schubert 2010; Trecenõ 2012; Zoëga 2011) and in the USA approximately 8% of children and adolescents under 15 years of age have a prescription of methylphenidate (Akinbami 2011). However, USA statistics reported a trend of reduction in 2019 (Drug Usage Statistics 2013‐2019).
Pharmacological treatment with methylphenidate of children and adolescents with ADHD is reported to have a beneficial effect of reducing the major symptoms of hyperactivity, impulsivity, and inattention. It is licensed for the treatment of children aged six years and older with ADHD (Kanjwal 2012), but is recommended by the NICE guideline as off‐label use from the age of five years (NICE 2018). Before starting medication for ADHD, a baseline assessment is necessary; the ADHD criteria must be reviewed, mental health and social circumstances considered and a review of physical health including a cardiovascular assessment with cardiological history, heart rate, and blood pressure should be conducted. If positive cardiovascular history or a co‐existing condition is being treated with a medicine that may pose an increased cardiac risk, electrocardiogram (ECG) is recommended (NICE 2018). Individual parent‐training programmes for parents and carers of children and young people with ADHD and symptoms of oppositional defiant disorder or conduct disorder must likewise be considered (NICE 2018).
Different releases (immediate, sustained, or extended‐release) and formulations (oral or transdermal) of methylphenidate are available and it is important to individualise the treatment to optimise effect and minimise adverse events (Childress 2019). Response of treatment is individual and intervention dose can vary significantly between children with some responding to relatively low dosages while others require larger doses to achieve the same effect (Stevenson 1989). Therefore, it is important that the dose of methylphenidate is titrated to an optimal level that maximises therapeutic benefits while producing minimal adverse events. Immediate‐release formulations of methylphenidate are usually initiated at 5 mg once or twice daily then titrated weekly by 5 mg to 10 mg daily, divided into two or three doses until effects are noted and adverse effects are tolerable. The dose can range from 5 mg to 60 mg methylphenidate, 1.4 mg/kg daily administered in two to three doses (BNF 2020; Pliszka 2007a). Under specialist supervision, the dose may be increased to 2.1 mg/kg daily in two to three doses (maximum 90 mg daily). Modified‐release formulations are initiated with 18 mg once daily and increased up to a maximum of 54 mg.
Immediate‐release methylphenidate has a bioavailability of 11% to 53% and an approximate duration of two to four hours with a peak blood concentration after two hours and a half‐life of two hours. Sustained‐release and extended‐release formulations of methylphenidate have a duration of action of three to eight hours and eight to 12 hours, respectively (Kimko 1999; NICE 2018).
Studies have indicated impairments in children's height and weight during treatment with methylphenidate (Schachar 1997a; Swanson 2004b; Swanson 2009). McCarthy and colleagues' study using the ‘German Health Interview and Examination Survey for Children and Adolescents’ (KiGGS) database found that methylphenidate use in boys with ADHD was associated with low body mass index (BMI) but were “unable to confirm that methylphenidate use is also associated with low height (≤3rd percentile) and changes in blood pressure” (McCarthy 2018).
Monitoring of height, weight, heart rate, blood pressure, and adverse events, as well as encouraging adherence for effective treatment, are suggested. Medication‐free periods are recommended to reassess the treatment efficacy on ADHD symptoms (Kidd 2000; NICE 2018). Adverse effects of methylphenidate are common and dose‐dependent (Rossi 2010; Storebø 2018b). In a large Cochrane Review of observational studies, more than half (51.3%) of participants being treated with methylphenidate experienced one or more adverse events considered non‐serious such as headache, sleep difficulties, abdominal pain, decreased appetite, anxiety, and sadness (Storebø 2018b). Furthermore, 16% discontinued methylphenidate due to ‘unknown’ reasons and another 6% due to adverse events considered non‐serious (Storebø 2018b).
Serious adverse events such as psychosis, mood disorders (Block 1998; Cherland 1999; MTA 1999a), serious cardiovascular events, and sudden unexplained death have also been reported (Cooper 2011; Habel 2011), but methylphenidate does not seem to increase serious adverse events in randomised clinical trials (Storebø 2015a). It must however be taken into consideration that this meta‐analysis was considerably underpowered and not able to draw firm conclusions (Storebø 2015a).
As a stimulant, methylphenidate carries the risk of addiction, and the nonmedical use has been reported to vary from 5% to 35% (Clemow 2014), with a peak risk at ages estimated to be between 16 and 19 years, and a new user rate of 0.7% to 0.8% per year (Austic 2015). Conversely, methylphenidate has been correlated with the reduction of harmful outcomes such as reducing emergency department visits (Dalsgaard 2015), reducing criminality (Lichtenstein 2012), reducing transport accidents (Chang 2017), and having a protective effect on abuse of other substances (Chang 2014).
How the intervention might work
The pharmacodynamics of methylphenidate have been extensively investigated in animal and human studies with brain imaging and chemistry studies, yet they remain uncertain. It is presumed that the effects of methylphenidate on ADHD symptoms are related to its effects on dopaminergic and noradrenergic neurotransmissions within the central nervous system (Engert 2008). Methylphenidate is assumed to act by inhibiting catecholamine reuptake, primarily as a dopamine‐norepinephrine re‐uptake inhibitor, modulating levels of dopamine and, to a lesser extent, levels of norepinephrine (Heal 2006; Iversen 2006).
Methylphenidate binds to and blocks dopamine and norepinephrine transporters (Heal 2006; Iversen 2006), and increased concentrations of dopamine and norepinephrine in the synaptic cleft lead to escalated neurotransmission. On average, methylphenidate elicits a 3 to 4 times increase in dopamine and norepinephrine in the striatum and prefrontal cortex (Hodgkins 2013), which is responsible for executive functions and produces effects such as increased alertness, reduced fatigue, and improved attention.
Methylphenidate is thought to activate self‐regulated control processes to ameliorate what are believed to be the core neurofunctional problems of ADHD (Barkley 1977a; Schulz 2012; Solanto 1998). Evidence suggests that symptom control is strongly related to functional improvement (Biederman 2003a; Cox 2004a; Swanson 2004a).
Studies indicate that methylphenidate is effective for treating both the core symptoms of ADHD (inattention, hyperactivity, and impulsivity) and aggression (Connor 2002), since children can manage their impulsivity better (Barkley 1981; Barkley 1989a; Shaw 2012). Barkley noted differences in response to methylphenidate between ADHD inattentive and combined subtypes: children with the inattentive subtype were judged to have a less favourable response to methylphenidate than those diagnosed with the combined presentation (Barkley 1991b). Some children and adolescents may become less responsive to methylphenidate treatment over time (Molina 2009). However, magnetic resonance imaging studies suggest that long‐term treatment with ADHD stimulants may decrease abnormalities in the brain structure and function found in patients with ADHD (Frodl 2012; Spencer 2013).
Why it is important to do this review
During the past 20 years, several systematic reviews and narrative reviews have investigated the efficacy of methylphenidate for ADHD (with or without meta‐analysis). Fifteen reviews have pooled results on methylphenidate treatment for children and adolescents with ADHD (Bloch 2009; Charach 2011; Charach 2013; Faraone 2002; Faraone 2006; Faraone 2009; Faraone 2010; Hanwella 2011; Kambeitz 2014; King 2006; Maia 2014; Punja 2013; Reichow 2013; Schachter 2001; Van der Oord 2008). However, none of these were conducted as Cochrane systematic reviews. Most of them did not adhere to the guidelines of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2022a), nor the Preferred Reporting Items for Systematic Reviews and Meta‐Analysis (PRISMA) guidelines (Liberati 2009; Moher 2015). None of these reviews had a peer‐reviewed protocol published before the analyses were conducted. Thirteen did not undertake subgroup analyses examining the effects of comorbidity on treatment effects (Bloch 2009; Charach 2011; Charach 2013; Faraone 2002; Faraone 2006; Faraone 2009; Faraone 2010; Hanwella 2011; Kambeitz 2014; Maia 2014; Punja 2013; Schachter 2001; Van der Oord 2008). Some did not control for treatment effects by ADHD subtype (Bloch 2009; Charach 2013; Faraone 2002; Hanwella 2011; Kambeitz 2014; King 2006; Maia 2014; Punja 2013; Schachter 2001; Van der Oord 2008). Others did not consider effects according to the dose of methylphenidate (Charach 2011; Charach 2013; Faraone 2006; Faraone 2009; Hanwella 2011; Kambeitz 2014; Maia 2014; Punja 2013; Reichow 2013; Van der Oord 2008). As for the outcomes, most meta‐analyses pooled data from parents, teachers and independent assessors (Bloch 2009; Charach 2011; Charach 2013; Hanwella 2011; Kambeitz 2014; King 2006; Reichow 2013), and did not separate outcome measures for inattention and hyperactivity/impulsivity (Bloch 2009; Charach 2013; Faraone 2002; Faraone 2006; Faraone 2009; Hanwella 2011; Kambeitz 2014; Van der Oord 2008). Moreover, most previous reviews only investigated the effects of methylphenidate on symptoms of ADHD; review authors did not present data on spontaneous adverse events (Charach 2013; Faraone 2002; Faraone 2006; Faraone 2009; Faraone 2010; Hanwella 2011; Kambeitz 2014; Maia 2014; Van der Oord 2008), nor on adverse events, as measured by rating scales (Bloch 2009; Charach 2013; Faraone 2002; Faraone 2006; Faraone 2009; Faraone 2010; Hanwella 2011; Kambeitz 2014; King 2006; Maia 2014; Punja 2013; Reichow 2013; Schachter 2001; Van der Oord 2008), and they did not try to explain why such information was not provided. Finally, these reviews did not systematically assess the risk of random errors, risk of bias, or trial quality (Bloch 2009; Charach 2011; Charach 2013; Faraone 2002; Faraone 2006; Faraone 2009; Faraone 2010; Hanwella 2011; Kambeitz 2014; King 2006; Van der Oord 2008). These shortcomings plus other methodological limitations including potential bias in excluding non‐English language publications (Charach 2013; Faraone 2010; Punja 2013; Van der Oord 2008), and not searching the principal major international databases nor reporting search terms clearly (Bloch 2009; Faraone 2002; Kambeitz 2014; Reichow 2013), may have compromised data collection, consequently calling the results of these previous meta‐analyses into question.
The first version of this systematic review was published in 2015 (Storebø 2015a). In this version, we reported that methylphenidate may improve teacher‐reported ADHD symptoms, teacher‐reported general behaviour, and parent‐reported quality of life among children and adolescents diagnosed with ADHD. We also underlined that the low quality of the evidence meant that we could not be certain of the magnitude of the effects. There was evidence that methylphenidate is associated with an increased risk of adverse events considered non‐serious, such as sleep problems and decreased appetite. We did not have evidence that methylphenidate increased the risk of serious adverse events, but this was unclear due to underreporting of serious adverse events (Storebø 2015a). We received many critical responses which were published as articles and letters to editors as well as blog comments. Six comments were received on the BMJ version of this review (Storebø 2015b). An editorial by Mina Fazel commenting on the article in the BMJ was also published alongside the review article (Fazel 2015). Mina Fazel recognised that our review was a comprehensive and rigorous Cochrane systematic review and meta‐analysis of the use of methylphenidate in young people with ADHD. She underlined the need for more research as she concluded: "The slow progress of ADHD research and limited evidence base for treatments are in stark contrast with the hallmarks of the disorder itself, with its high prevalence and broad symptomology" (Fazel 2015). A short version of the review was also published in JAMA in 2016 (Storebø 2016b), followed by a commenting editorial by Philip Shaw who concluded: "Sometimes in medicine, the best available data are imperfect. Such imperfections do not render the data unusable; rather, the limitations can be weighed by physicians and other health care professionals, and by families as they decide how best to help a child struggling with ADHD. Psychostimulants improve ADHD symptoms and quality of life. This meta‐analysis highlights the complexities in quantifying this benefit." (Shaw 2016 p. 1954). Philip Shaw wrote that in a meta‐analysis of methylphenidate for adults with ADHD (Epstein 2014), the trial biases were similar to those in our review and that the bias assessment seemed to be very subjective (Shaw 2016). The review by Epstein and colleagues (Epstein 2016), was withdrawn from the Cochrane Library on 26 May 2016 due to several methodological problems including erroneous risk of bias assessment (Boesen 2017; Storebø 2015b [pers comm]).
Several critical comments on our 2015 review from different authors were published in blog posts, articles and letters to editors (Hollis 2016; Banaschewski 2016a; Banaschewski 2016b; Hoekstra 2016; Romanos 2016). All these comments and our responses are listed with references in the 2015 published version of this review (Storebø 2015a). The critical points raised focused on our certainty assessment, including our use of the vested interest risk of bias domain, concerns that blinding may be affected by easily recognisable adverse events, concerns that we erroneously included too many non‐eligible trials (such as cross‐over trials and trials with add‐on treatment to methylphenidate), and that we had errors in the data extracted. We showed in several response articles and letters to editors that our trial selection was not flawed and that our data collection and interpretation of data in most aspects was systematic and sound (Storebø 2016a; Storebø 2016c; Storebø 2016d; Storebø 2016e; Storebø 2016f; Storebø 2018a). We answered all criticism, but in one case our response to a critical editorial (Gerlach 2017), in the Journal ADHD Attention Deficit and Hyperactivity Disorders was declined by the editor. In addition, we have argued that our assessment of quality and our conclusion were not misleading (Storebø 2016c; Storebø 2016d; Storebø 2016e; Storebø 2016f; Storebø 2018a). We agreed that minor errors were present in the review, yet we were still able to show that the effects were negligible and that these minor errors did not affect our conclusions (Storebø 2016c; Storebø 2016d; Storebø 2016e; Storebø 2016f; Storebø 2018a). We stated that the evidence for the use of methylphenidate in children and adolescents with ADHD was flawed (Storebø 2016c; Storebø 2016d; Storebø 2016e; Storebø 2016f; Storebø 2018a).
In 2018 an application for including methylphenidate on the 21st update of the WHO's List of Essential Medicines was rejected due to concerns regarding the quality of the evidence for benefits and harms (Storebø 2021). An extended research team made a comparable application in 2020 for the 22nd update of the list. The decision of the committee was — for the second time — not to include methylphenidate on the WHO Model List of Essential Medicines due to low quality of evidence, lack of data after 12 weeks, and adverse effects of concern (Pereira Ribeiro 2022). The committee also stressed that "evidence of the effectiveness and safety of methylphenidate in the treatment of ADHD of at least 52 weeks duration, outcomes of the revision of the of the Mental Health Gap Action Programme (mhGAP) Guideline for Mental, Neurological and Substance use Disorders, and evaluation of health system capacity to provide appropriate diagnostic, non‐pharmacological and pharmacological treatment and monitoring in low‐resource settings would be informative for any future consideration for inclusion of methylphenidate on the Model Lists" (WHO 2021 p. 538).
We have published an overview article where we found 24 eligible systematic reviews and meta‐analyses published after the 2015 version of the current review (Ribeiro 2021). The results showed that the evidence was uncertain due to the low quality of evidence. There was also an underreporting of adverse events in randomised clinical trials. We concluded that there is uncertain evidence to support that methylphenidate is beneficial in treating children and adolescents with ADHD. (Ribeiro 2021).
In October 2021 the European ADHD Guidelines Group (EAGG) published an overview article summarising the current evidence and identified methodological issues and gaps in the current evidence (Coghill 2021). The authors of this article were mostly the same authors that had published the many critical comments to our 2015 version of this review. They wrote in this article: "We have summarized the current evidence and identified several methodological issues and gaps in the current evidence that we believe are important for clinicians to consider when evaluating the evidence and making treatment decisions. These include understanding potential impact of bias such as inadequate blinding and selection bias on study outcomes; the relative lack of high‐quality data comparing different treatments and assessing long‐term effectiveness, adverse effects and safety for both pharmacological and non‐pharmacological treatments; and the problems associated with observational studies, including those based on large national registries and comparing treatments with each other" (Coghill 2021).
Combined, this indicates a need to update this systematic review on the benefits and harms of methylphenidate for children and adolescents with ADHD and that this should continue to be done until more solid evidence for the recommendation about the use of methylphenidate for children and adolescents with ADHD can be established. Given the mounting concerns regarding the increasing use of methylphenidate in children younger than six years, it is vital that researchers explore the risks versus benefits of treatment in this younger population (US FDA 2011). Although stimulant medications may have a favourable risk‐benefit profile, they might carry potential risks of both serious and non‐serious adverse events.
To expand our understanding of adverse events, particularly where these are rare or take time to become apparent, we felt it necessary to bolster the limited data from randomised clinical trials (RCTs) by including data from non‐randomised studies (Storebø 2015a). Our Cochrane systematic review from 2018 focused on the harms of methylphenidate treatment in children and adolescents with ADHD (Storebø 2018b). This review included 260 non‐randomised studies: four patient‐controlled studies, seven comparative cohort studies, 177 cohort studies, two cross‐sectional studies, and 70 patient reports, including over 2.2 million participants. In contrast to our 2015 review based on RCTs (Storebø 2015a), methylphenidate compared to no intervention significantly increased the risk of serious adverse events in comparative studies (risk ratio (RR) 1.36, 95% confidence interval (CI), 1.17 to 1.58; 2 trials; 72,005 participants). Serious adverse events included psychotic disorders, arrhythmia, seizures, and hypertension. Approximately half (51.2%) of participants experienced one or more non‐serious adverse event (95% CI 41.2 to 61.1%; 49 trials; 13,978 participants). These were sleep difficulties (17.9%), decreased appetite (31.1%), and abdominal pain (10.7%). Furthermore, 16.2% (95% CI 13.0 to 19.9%; 57 trials, 8340 participants) discontinued methylphenidate because of 'unknown' reasons and 6.20% (95% CI 4.90 to 8.00%; 37 trials; 7142 participants) because of non‐serious adverse events. We assessed most included studies as having critical risk of bias. The GRADE quality rating of the certainty of evidence was very low. Some studies indicated that methylphenidate can decrease children's normal growth rate (Schachar 1997b; Swanson 2004a; Swanson 2009). Given the unclear evidence in this field and the need for better data, we, therefore, conducted the present update of this systematic review of the benefits and harms of methylphenidate for children and adolescents with ADHD in RCTs while adhering to the Cochrane guidance (Higgins 2022a), and to the PRISMA guidelines (Liberati 2009; Moher 2015).
Objectives
To assess the beneficial and harmful effects of methylphenidate for children and adolescents with ADHD.
Methods
Criteria for considering studies for this review
Types of studies
RCTs of methylphenidate for the treatment of children and adolescents with ADHD. We included trials irrespective of language, publication year, publication type or publication status.
Types of participants
Children and adolescents aged 18 years and younger with a diagnosis of ADHD, according to the DSM‐III (APA 1980), DSM‐III‐R (APA 1987), DSM‐IV (APA 1994), and DSM‐5 (APA 2013), or with a diagnosis of hyperkinetic disorders according to the ICD‐9, ICD‐10 (WHO 1992), and ICD‐11 (WHO 2019) . We included participants with ADHD with or without comorbid conditions such as conduct or oppositional disorders, tics, depression, attachment disorders or anxiety disorders. Trials eligible for inclusion were those in which at least 75% of participants were aged 18 years or younger, and the mean age of the trial population was 18 years or younger. We also required that at least 75% of participants had a normal intellectual quotient (IQ > 70).
Types of interventions
Methylphenidate, administered at any dosage or in any formulation, versus placebo or no intervention.
We permitted co‐interventions if the experimental and control intervention groups received the co‐interventions similarly. In some trials that included co‐interventions in both groups, such as a behavioral intervention combined with methylphenidate versus a behavioral intervention, we considered these as methylphenidate versus no intervention. We did not permit polypharmacy as a co‐intervention in only one of the intervention groups.
Types of outcome measures
Primary outcomes
ADHD symptoms (attention, hyperactivity and impulsivity), measured over the short term (within six months) and over the long term (longer than six months) by psychometric instruments or by observations of behaviour, using, for example, Conners' Teacher Rating Scales (Conners 1998a; Conners 2008). Raters could be teachers, independent assessors, or parents. We chose to report the results of teacher‐rated outcomes as the primary outcome (see Results).
Number of serious adverse events. We defined a serious adverse event as any event that led to death, was life‐threatening, required inpatient hospitalisation or prolongation of existing hospitalisation, resulted in persistent or significant disability, or as any important medical event that may have jeopardised the patient's life or that required intervention for prevention. We considered all other adverse events to be considered non‐serious (ICH 1996).
Secondary outcomes
Non‐serious adverse events. We assessed all adverse events, including, for example, growth retardation and cardiological, neurological and gastrointestinal events, as described in ICH (International Conference on Harmonisation of technical requirements for registration of pharmaceuticals for human use) Harmonised Tripartite Guideline. Guideline for Good Clinical Practice E6(R1) (ICH 1996).
General behaviour in school and at home, as rated by psychometric instruments such as the Child Behaviour Checklist (CBCL; Achenbach 1991), measured over the short term (within six months) and over the long term (longer than six months). Raters could be teachers, independent assessors, or parents. We chose to report the results of teacher‐rated outcomes as primary outcomes (see Results).
Quality of life, as measured by psychometric instruments such as the Child Health Questionnaire (CHQ; Landgraf 1998). Raters could be teachers, the children, independent assessors, or parents.
Search methods for identification of studies
Electronic searches
We ran the first literature searches in October 2011 and updated them in November 2012, March 2014, between 26 February and 10 March 2015 and most recently 11 January 2021 and 25 March 2022. We searched the following sources.
Cochrane Central Register of Controlled Trials (CENTRAL; 2021, Issue 1; part of the Cochrane Library, which includes the Specialised Register of the Cochrane Developmental, Psychosocial and Learning Problems Group), searched 25 March 2022
MEDLINE Ovid (1946 to current), searched 25 March 2022
Embase Ovid (1980 to current), searched 25 March 2022
CINAHL EBSCOhost (Cumulative Index to Nursing and Allied Health Literature; 1980 to current), searched 25 March 2022
PsycINFO Ovid (1806 to current), searched 25 March 2022
Epistemonikos (www.epistemonikos.org/) searched 25 March 2022
Conference Proceedings Citation Index ‐ Science (CPCI‐S) and Conference Proceedings Citation Index ‐ Social Science & Humanities (CPCI‐SS&H) (Web of Science; 1990 to 25 March 2022)
ClinicalTrials.gov (ClinicalTrials.gov ), searched 25 March 2022
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP; who.int/ictrp/en), searched 25 March 2022
Networked Digital Library of Theses and Dissertations (NDLTD; ndltd.org), searched 29 November 2022)
DART Europe E‐Theses Portal (www.dart-europe.eu/basic-search.php), searched 28 November 2022)
Theses Canada (library-archives.canada.ca/eng/services/services-libraries/theses/Pages/theses-canada.aspx), searched 29 November 2022
Worldcat (worldcat.org), searched 28 November 2022
The search strategy for each database is shown in Appendix 1. We used a broad strategy to capture trials on efficacy and trials on adverse events. To overcome poor indexing and abstracting, we listed individual brand names within the search strategies. We did not limit searches by language, year of publication or type or status of the publication. We sought translation of relevant sections of non‐English language articles.
Searching other resources
To find additional relevant trials not identified by electronic searches, we checked the bibliographic references of identified review articles, meta‐analyses and a selection of included trials. Furthermore, we requested published and unpublished data from pharmaceutical companies manufacturing methylphenidate, including Takeda Pharmaceuticals, Medice (represented in Denmark by HB Pharma), Janssen‐Cilag, Novartis, Rhodes Pharmaceuticals, Ironshore Pharmaceuticals and Pfiizer (Appendix 2). We also requested data from unpublished trials from experts in the field.
Data collection and analysis
We conducted this review according to the recommendations provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2022a), and performed analyses using Review Manager 5 (RevMan 5; Review Manager 2020).
Selection of studies
In this update of the Storebø 2015a review, seven review authors (OJS, HEC, JPS, JPR, MROS, PDR, CMLH) worked together in groups of two and independently screened titles and abstracts of all publications obtained from the literature searches. We obtained full‐text papers for any abstract/title that might match our inclusion criteria and assessed them against our listed inclusion criteria. We discussed disagreements, and if we were unable to reach agreement or consensus, we consulted a third review author (OJS).
Data extraction and management
In this update of the Storebø 2015a review, working together in groups of two, six review authors extracted data (MROS, CMLH, JPR, JPS, MS, OJS). We resolved disagreements by discussion and we used an arbiter if required. When data were incomplete, or when data provided in published trial reports were unclear, we contacted trial authors to ask for clarification of missing information. We contacted the authors of all cross‐over trials to obtain first‐period data on ADHD symptoms.
We developed data extraction forms a priori. After performing data extraction pilots, we updated these forms to accommodate the extraction of more detailed data and to facilitate standardised approaches to data extraction among review authors. All data extractors used these extraction forms (see Appendix 3; Appendix 4).
Six review authors (MS, HEC, JPR, JPS, MROS and OJS) entered data into RevMan 5 (Review Manager 2020).
Assessment of risk of bias in included studies
For each included trial, data extractors (MROS, CMLH, JPR, JPS, MS, OJS) independently evaluated risk of bias domains (listed below), resolving disagreements by discussion. For each domain, we assigned each trial to one of the following three categories: low risk of bias, unclear (uncertain) risk of bias or high risk of bias, according to guidelines provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Given the risk of overestimation of beneficial intervention effects and underestimation of harmful intervention effects in RCTs with unclear or inadequate methodological quality (Kjaergard 2001; Lundh 2012; Lundh 2018; Moher 1998; Savović 2012a; Savović 2012b; Savovic 2018; Schulz 1995; Wood 2008), we assessed the influence of risk of bias on our results (see Subgroup analysis and investigation of heterogeneity). Risk of bias components were as follows: random sequence generation; allocation concealment; blinding of participants and personnel; blinding of outcome assessment; incomplete outcome data; selective reporting; and other potential sources of bias. We defined low risk of bias trials as trials that had low risk of bias in all domains. We considered trials with one or more unclear or high risk of bias domains as trials with high risk of bias.
Measures of treatment effect
We defined the treatment effect as an improvement in ADHD symptoms, general behaviour and quality of life.
Dichotomous data
We summarised dichotomous data as risk ratios (RRs) with 95% confidence intervals (CIs). We calculated the risk difference (RD).
Continuous data
If all trials used the same measure of a given continuous outcome in a meta‐analysis, we calculated mean differences (MDs) with 95% CIs. If trials used different measures, we calculated standardised mean differences (SMDs) with 95% CIs. If trials did not report means and standard deviations but did report other values (e.g. t‐tests, P values), we transformed these into standard deviations.
For primary analyses of teacher‐rated ADHD symptoms, teacher‐rated general behaviour and quality of life, we transformed SMDs into MDs on the following scales to assess whether results exceeded the minimal clinically relevant difference: ADHD Rating Scale (ADHD‐RS; DuPaul 1991a), Conners' Global Index (CGI; Conners 1998a), and Child Health Questionnaire (CHQ; Landgraf 1998). We transformed SMDs into MDs on the ADHD‐RS by using the SD 14.3 from Riggs 2011, on the CGI by using the SD 5.79 from Greenhill 2002, and on the CHQ by using the SD 12.35 from Newcorn 2008. We identified a minimal clinically relevant difference (MIREDIF) of 6.6 points on the ADHD‐RS, ranging from 0 to 72 points, based on a trial by Zhang 2005, and a MIREDIF of 7.0 points on the CHQ, ranging from 0 to 100 points, based on a trial by Rentz 2005. We could find no references describing a MIREDIF on the CGI (range 0 to 30 points).
Unit of analysis issues
Many ADHD trials use cross‐over methods. We aimed to obtain data from the first period of these trials and to pool these data with data from parallel‐group trials, as they are similar (Curtin 2002). We requested these data from trial authors if they were not available in the published report. When we were not able to obtain first‐period data from cross‐over trials, we established another group comprising only end‐of‐last‐period data. Our original intention was to adjust for the effect of the unit of analysis error in cross‐over trials by conducting a covariate analysis, but data were insufficient for this. As cross‐over trials are more prone to bias from carry‐over effects, period effects and unit of analysis errors (Curtin 2002), we conducted a subgroup analysis to compare these two groups. We tested for the possibility of a carry‐over effect and a period effect (Subgroup analysis and investigation of heterogeneity). We found similar treatment effects in the two groups and no significant subgroup differences. However, we noted considerable heterogeneity, and so we presented the results of the analyses separately (Effects of interventions). In a methods article, we investigated the risk of carry‐over effect and unit of analysis error due to period effects comparing parallel‐group trials, the first period of cross‐over trials and the end of the last period of cross‐over trials and found no signs of period effects or carry‐over effects in cross‐over trials assessing methylphenidate for children and adolescents with ADHD (Krogh 2019).
For dichotomous outcomes in cross‐over trials, we were unable to adjust the variance to account for the correlation coefficient as advised by Elbourne 2002 due to insufficient information or to estimate the RR using the marginal probabilities as recommended by Becker 1993. Consequently, we used end‐of‐last‐period data for estimating RRs. As these effect estimates are prone to potential bias, we performed a sensitivity analysis by removing these trials to assess the robustness of the pooled results.
We used endpoint data when these were reported or could be obtained from trial authors. However, when RCTs reported only 'change scores', we pooled these with scores from the end of intervention (da Costa 2013). We used only endpoint standard deviations in the trials with 'change scores'. We explored whether inclusion of change data affected the outcomes by performing a sensitivity analysis (see Sensitivity analysis).
Dealing with missing data
We obtained missing data by contacting trial authors. When we were not able to obtain missing data, we conducted analyses using available (incomplete) data. Although some trials reported that they used intention‐to‐treat (ITT) analyses, data were missing for many primary outcomes (Hollis 1999). We could not use 'best‐case scenario' and 'worst‐case scenario' analyses on our assessment of benefit as there were no dichotomous outcomes. Also, we decided not to use 'best‐case scenario' and 'worst‐case scenario' analyses in our assessment of adverse events because we evaluated these analyses to be imprecise due to the high number of trials not reporting adverse events, and due to the high number of dropouts in the trials reporting adverse events.
Assessment of heterogeneity
We identified three types of heterogeneity: clinical, methodological and statistical. Clinical heterogeneity reflects variability among participants, interventions and outcomes of trials; methodological heterogeneity reflects variability in the trial designs; and statistical heterogeneity reflects differences in effect estimates between trials. We assessed clinical heterogeneity by comparing differences in trial populations, interventions and outcomes, and we evaluated methodological heterogeneity by comparing the trial designs. We identified potential reasons for clinical and methodological heterogeneity by examining individual trial characteristics and subgroups. Furthermore, we observed statistical heterogeneity in trials both by visual inspection of a forest plot and by use of a standard Chi² test value with a significance level of α (alpha) = 0.1. We examined the I² statistic (Higgins 2003). We judged values between 0% and 40% to indicate little heterogeneity, between 30% and 60% to indicate moderate heterogeneity, between 50% and 90% to indicate substantial heterogeneity, and between 75% and 100% to indicate considerable heterogeneity (Deeks 2022).
Assessment of reporting biases
We followed the recommendations for reporting bias, including publication bias and outcome reporting bias, provided in the Cochrane Handbook for Systematic Reviews of Interventions (Page 2022). We drew funnel plots (estimated differences in treatment effects against their standard error) and performed Egger's statistical test for small‐study effects; asymmetry could be due to publication bias or could indicate genuine heterogeneity between small and large trials (Page 2022). We did not visually inspect the funnel plot if fewer than 10 trials were included in the meta‐analysis, in accordance with the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions (Page 2022). We compared results extracted from published journal reports to results obtained from other sources (including correspondence) as a direct test for publication bias.
Data synthesis
We performed statistical analyses as recommended by the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2022). We synthesised data statistically when clinical heterogeneity was not excessive (e.g. variability in participant characteristics was minimal). Furthermore, we included and analysed trials undertaken in any configuration or setting (e.g. in groups, at home, or at a centre).
We used the inverse variance method, which gives greater weight to larger trials, to generate more precise estimates. For some adverse events we combined dichotomous and continuous data using the generic inverse variance method. We synthesised data using change‐from‐baseline scores or endpoint data. If data were available for several intervals, we used the longest period assessed. We used the fixed‐effect and random‐effects models in all meta‐analyses, however, we reported the results of the random‐effects model when we included more than one trial in the meta‐analysis. This approach gives greater weight to smaller trials. Statistical significance did not change when we applied a fixed‐effect model (Jakobsen 2014). We performed separate meta‐analyses for three types of raters (teachers, independent assessors, and parents) for data from parallel‐group trials combined with data from the first period of cross‐over trials and data from the end of the last period from cross‐over trials.
ADHD symptom scales describe the severity of inattention, hyperactivity and impulsivity at home and at school; high scores indicate severe ADHD. We judged that, in spite of the diversity of psychometric instruments, they could be used for our outcomes, and we integrated different types of scales into the analyses. We used MDs if all trials used the same measure and SMDs when different trials used different outcome measures for the same construct.
When separate measures of hyperactivity, impulsivity and inattention were available, we used combined scores. When symptoms were measured and reported at different time points during the day (after ingestion of medication or placebo), we used the time point closest to noon.
Three types of raters, teachers, independent assessors and parents measured two outcomes — ADHD symptoms and general behaviour. We considered these data as showing different outcomes. We presented the results of teacher‐rated measures as the primary outcome because symptoms of ADHD are more readily detectable in the school setting (Hartman 2007).
For children weighing 25 kg or less, the maximum recommended dose of methylphenidate is 30 mg/day compared to 60 mg/day for children weighing more than 25 kg. After careful consideration, we renamed the high‐dose group as 'moderate/high' dose because doses are not always 'high' in heavier children. When trials reported data for different doses, we used data for the dose that we defined as moderate/high (> 20 mg/day) in our primary analyses.
We summarised adverse event data as RRs with 95% CIs for dichotomous outcomes. For the purposes of this review, we used only dichotomous outcomes that reflected the number of participants affected by the event per the total number of participants.
Diversity‐adjusted required information size and Trial Sequential Analysis
Trial Sequential Analysis is a method that combines the required information size (RIS) for a meta‐analysis with the threshold for statistical significance to quantify the statistical reliability of data in a cumulative meta‐analysis, with P value thresholds controlled for sparse data and repetitive testing of accumulating data (Brok 2008; Brok 2009; Thorlund 2009; Wetterslev 2008; Wetterslev 2017).
Comparable to the a priori sample size estimation provided in a single RCT, a meta‐analysis should include a RIS at least as large as the sample size of an adequately powered single trial to reduce the risk of random error. A Trial Sequential Analysis calculates the RIS in a meta‐analysis and provides trial sequential monitoring boundaries with an adjusted P value.
When new trials emerge, multiple analyses of accumulating data lead to repeated significance testing and hence introduce multiplicity. Use of conventional P values exacerbates the risk of random error (Berkey 1996; Lau 1995; Wetterslev 2017). Meta‐analyses not reaching the RIS are analysed with trial sequential monitoring boundaries analogous to interim monitoring boundaries in a single trial (Wetterslev 2008; Wetterslev 2017).
If a Trial Sequential Analysis does not result in significant findings (no Z‐curve crossing the trial sequential monitoring boundaries) before the RIS has been reached, the conclusion should be that more trials are needed to reject or accept an intervention effect that was used to calculate the required sample size, or when the cumulated Z‐curve enters the futility area, the anticipated intervention effect should be rejected.
For calculations with the Trial Sequential Analysis programme, we included trials with zero events by substituting 0.25 for zero (CTU 2022; Thorlund 2011).
For the outcomes 'total serious adverse events' and 'total non‐serious adverse events', we calculated the a priori diversity‐adjusted required information size (DARIS; i.e. number of participants in the meta‐analysis required to detect or reject a specific intervention effect) and performed a Trial Sequential Analysis for these outcomes based on the following assumptions (Brok 2008; Brok 2009; Thorlund 2009; Wetterslev 2008; Wetterslev 2009).
Proportion of participants in the control group with adverse events
Relative risk reduction of 20% (25% on 'total serious adverse events')
Type I error of 5%
Type II error of 20%
Observed diversity of the meta‐analysis
Subgroup analysis and investigation of heterogeneity
We performed the following subgroup analyses of teacher‐rated ADHD symptoms (primary outcome) to test the robustness of this estimate.
Age of participants (trials with participants aged 2 to 6 years compared to trials with participants aged 7 to 11 years compared to trials with participants aged 12 to 18 years)
Sex (boys compared to girls)
Comorbidity (children with comorbid disorders compared to children without comorbid disorders)
Type of ADHD (participants with predominantly inattentive subtype compared to participants with predominantly combined subtype)
After learning about other factors that may affect the effects of methylphenidate, we performed the following additional post hoc subgroup analyses on teacher‐rated ADHD symptoms to test the robustness of the estimate.
Types of scales (e.g. Conners' Teacher Rating Scale (CTRS; Conners 1998a), compared to Strengths and Weaknesses of ADHD Symptoms and Normal Behavior (SWAN) Scale (Swanson 2006)
Dose of methylphenidate (low dose (≤ 20 mg/day or ≤ 0.6 mg/kg/day) compared to moderate or high dose (> 20 mg/day or > 0.6 mg/kg/day)).
Duration of treatment (short‐term trials (≤ 6 months) compared to long‐term trials (> 6 months))
Trial design (parallel‐group trials compared to cross‐over trials (first‐period data and end‐of‐last‐period data))
Medication status before randomisation (medication naive (> 80% of included participants were medication naive) compared to not medication naive (< 20% of included participants were medication naive))
Risk of bias (trials at low risk of bias compared to trials at high risk of bias)
Enrichment trials. Enrichment trials (trials that excluded methylphenidate non‐responders, placebo responders, and/or participants who had adverse events due to the medication before randomisation) compared to trials without enrichment
Vested interest ((conflict of interest regarding funding) trials at either high or unclear risk of vested interest compared to trials at low risk of vested interest). Our assessment of vested interest for the individual studies can be seen in Table 2.
Type of control group (trials with placebo control group compared to trials with no‐intervention control group)
1. Vested interest of included studies.
| Study | Vested interest | Support for judgement |
| Abikoff 2009 | High | Funding: investigator‐initiated trial funded by a grant from Ortho‐McNeil Janssen Scientific Affairs to Dr Abikoffx Conflicts of interest: Drs Abikoff and Gallagher have a contract with Multi‐Health Systems to further develop the Children’s Organizational Skills Scale (COSS) used in this trial. Dr Abikoff has served on the ADHD Advisory Board of Shire Pharmaceuticals and of Novartis Pharmaceuticals. Dr Boorady has served on the ADHD Advisory Board and Speakers’ Bureau of Shire Pharmaceuticals. Other trial authors report no conflicts of interest |
| Ahmann 1993 | Low | Funding: trial was funded by Marshfield Clinic grants Conflict of interest: not declared |
| Arnold 2004 | High | Funding: trial was supported by the Celgene Corporation Conflicts of interest: Drs Arnold, Wigal and Bohan received research Funding from Celgene for the trial reported. Dr Wigal and Dr West are on the Advisory Panel and Speakers' Bureau for Novartis. Dr Arnold and Dr Bohan are on the Speakers' Bureau for Novartis. Dr Zeldis is Chief Medical Officer and Vice President of Medical Affairs at the Celgene Corporation. |
| Barkley 1989b | Low | Funding: trial was internally funded by the medical school Conflict of interest: not declared |
| Barkley 1991 | Low | Funding: research was supported by the National Institute of Mental Health (NIMH) Conflicts of interest: not declared |
| Barkley 2000 | Low | Funding: University of Massachusetts Medical School Conflict of interest: not declared |
| Barragán 2017 | High | Funding: trial was funded by Vifor Pharma Conflict of interest: trial authors affiliated with the medical industry |
| Bedard 2008 | Low | Funding: funding and operating grant from the Canadian Institute of Health Research and Funding from the Canada Research Chairs Programme Conflicts of interest: none |
| Bhat 2020 | High | Funding: this work was supported in part by a grant from the Fond de Recherche du Québec and the Canadian Institutes of Health Research. Weam Fageera is a recipient of a PhD scholarship from the Ministry of Education of Saudi Arabia. Conflicts of interest: authors affiliated with medical industry |
| Biederman 2003b | High | Funding: received from Novartis Conflict of interest: not declared |
| Bliznakova 2007 | Unclear | Funding: not declared Conflict of interest: not declared |
| Blum 2011 | High | Funding: trial was supported by an investigator‐initiated grant from Ortho McNeil Janssen Scientific Affairs, the manufacturer of OROS methylphenidate (Concerta) Conflict of interest: not declared |
| Borcherding 1990 | Unclear | Funding: not declared Conflicts of interest: not declared |
| Brams 2008 | High | Funding: sponsored by Novartis Pharmaceuticals Corporation Conflicts of interest: first trial author has been a speaker, consultant and advisory board member for Novartis and Shire |
| Brams 2012 | High | Funding: Novartis Pharmaceuticals Corporation, with the following involvement reported: design and conduct of the trial; collection, management, analysis and interpretation of data; and preparation, review and approval of the manuscript. All trial authors are employees or consultants or have received research grants from pharmaceutical companies. Conflicts of interest: all trial authors are employees or consultants or have received research grants from pharmaceutical companies. |
| Brown 1984a | Unclear | Funding: funded by National institute of Mental Health and National institutes of Health. Placebo and methylphenidate were supplied by CIBA‐GEIGY Corporation, Summit, New Jersey Conflicts of interest: not declared |
| Brown 1985 | Unclear | Funding: research supported by US Public Health Services Grant from the National Institute of Mental Health (NIMH), and by the Biomedical Research Award from the National Institutes of Health (NIH). Methylphenidate provided by CIBA‐GEIGY Corporation, Summit, New Jersey Conflicts of interest: not declared |
| Brown 1988 | Low | Funding: Biomedical Research Support Grant Program, Division of Research Resources, National Institutes of Health and Emory University Research Conflicts of interest: not declared |
| Brown 1991 | Unclear | Funding: Biomedical Research Support Grant Program, Division of Research Resources, National Institutes of Health, and by the Emory University Research Fund Conflicts of interest: not declared |
| Buitelaar 1995 | Unclear | Funding: not declared Conflicts of interest: no affiliations with pharmaceutical companies were declared |
| Bukstein 1998 | Unclear | Funding: no Funding declared Conflicts of interest: not declared |
| Butter 1983 | Low | Funding: the Scientific Development Group, Organon International BV, Oss, the Netherlands Conflicts of interest: none |
| Carlson 1995 | Unclear | Funding: not declared Conflict of interest: not declared |
| Carlson 2007 | High | Funding: research was funded by Eli Lilly and Company, Indianapolis, Indiana Conflicts of interest: Dr Carlson has received research support or has consulted with the following companies: Abbott Laboratories, Cephalon, Eli Lilly and Company, Janssen, McNeil, Otsuka and Shire Pharmaceuticals. Dr Dunn has received research support or has served on Speakers' Bureaus of the following companies: AstraZeneca, Eli Lilly and Company, National Institues of Health, Otsuka and Pfizer Pharmaceuticals. Drs Kelsey, Ruff, Ball and Allen and Ms Ahrbecker are employees and/or shareholders of Eli Lilly and Company. |
| Castellanos 1997 | Unclear | Funding: unclear Conflicts of interest: not declared |
| Chacko 2005 | High | Funding: during the conduct of this research, Dr Pelham was supported by grants from the National Institute of Mental Health (NIMH) (MH48157, MH47390, MH45576, MH50467, MH53554, MH62946), NIAAA (AA06267, AA11873), National Institute on Drug Abuse (NIDA) (DA05605, DA12414), National Institute of Neurological Disorders and Stroke (NINDS) (NS39087), National Institute for Environmental Studies (NIES) (ES05015) and National Institute of Child Health and Human Development (NICHHD) (HD42080) Conflicts of interest: several trial authors have affiliations with medical companies |
| Childress 2009 | High | Funding: Novartis Pharmaceuticals Corporation. Novartis Pharma has been helping with development of the manuscript. Conflicts of interest: several trial authors have received research support from, are speakers for, are consultants of, are on the Advisory Board, have served on the Speakers' Bureaus of or are employees of several pharmaceutical companies |
| Childress 2017 | High | Funding: this trial was supported by funds from Neos Therapeutics, Inc, PI. Conflicts of interest: Carolyn R Sikes is affiliated with Neos Therapeutics, Inc. |
| Childress 2020a | High | Funding: trial was funded by Purdue Pharma Conflict of interest: trial authors affiliated with medical industry |
| Childress 2020b | High | Funding: trial funded by Ironshore Pharmaceuticals Conflict of interest: trial authors affiliated with the medical industry |
| Childress 2020c | High | Funding: trial funded by Rhodes Pharmaceuticals LP. Conflict of interest: authors affiliated with medical industry |
| Chronis 2003 | High | Funding: supported by a grant from Shire‐Richwood Pharmaceuticals, Incorporated ‐ manufacturer of Adderall ‐ and from the National Institute of Mental Health (NIMH) Conflict of interest: not declared |
| Coghill 2007 | High | Funding: this work was supported by a local trust through a Tenovus Scotland initiative. Conflicts of interest: some trial authors have affiliations with different pharmaceutical companies |
| Coghill 2013 | High | Funding: Shire Development LLC Conflicts of interest: C Anderson, R Civil, N Higgins, A Lyne and L Squires are employees of Shire and own stock/stock options. Some trial authors have received compensation for serving as consultants or speakers, or they or the institutions they work for have received research support or royalties from different companies or organisations. |
| Connor 2000 | Low | Funding: supported by a UMMS (University of Massachusetts Medical School) Small Grants Project Award Conflicts of interest: not declared |
| Cook 1993 | Low | Funding: supported by the Medical Center Rehabilitation Hospital Foundation and the School of Medicine, University North Dakota; the Veterans Hospital; the Dakota Clinic; and The Neuropsychiatric Institute, Fargo, North Dakota Conflicts of interest: not declared |
| Corkum 2008 | Low | Funding: research was supported by a grant from the Izaak Walton Killam IWK Health Centre in Halifax, Nova Scotia Conflicts of interest: "none declared" |
| Corkum 2020 | Low | Funding: the Canadian Institutes of Health Research Conflicts of interest: there were no conflicts of interest of any trial investigator with the pharmaceutical or equipment manufacturers. |
| Cox 2006 | High | Funding: trial was supported by Funding from McNeil Pediatrics, a division of McNeil‐PPC Incorporated Conflicts of interest: none declared |
| CRIT124US02 | High | Funding: trial by Novartis Conflicts of interest: no information on investigators |
| Döpfner 2004 | High | Funding: trial was conducted and sponsored by MEDICE Arzneimittel Pütter GmbH & Co. KG as part of the drug approval process for Medikinet‐Retard Conflicts of interest: some trial authors have affiliations with medical companies |
| Douglas 1986 | Low | Funding: research was supported by Grant Number MA 6913, from the Medical Research Council of Canada Conflicts of interest: not declared |
| Douglas 1995 | Low | Funding: grants from the Medical Research Council of Canada and by William T Grant Foundation Faculty Scholar Program Conflicts of interest: none |
| DuPaul 1996 | Unclear | Funding: unclear Conflict of interest: no conflicts of interest declared |
| Duric 2012 | Low | Funding: the Child and Adolescent Psychiatry Department of Helse Fonna Hospital Haugesund, Helse Fonna Trust Haugesund, Norway Conflicts of interest: trial authors declare no potential conflicts of interests with regard to authorship or publication of this article. |
| Epstein 2011 | Low | Funding: National Institutes of Health (NIH) and National Institute of Mental Health (NIMH) Conflicts of interest: no evidence of conflicts of interest |
| Fabiano 2007 | Low | Funding: National Institute of Mental Health (NIMH) grant MH62946 Conflicts of interest: supported only by National Institutes |
| Findling 2006 | High | Funding: provided by Celltech Americas Incorporated, currently part of UCB (Union Chimique Belge) Conflicts of interest: Drs Hatch and DeCory and Miss Cameron were employees of Celltech at the time of this trial. Dr Findling received research support, acted as a consultant and/or served on a Speakers' Bureau for Abbott, AstraZeneca, Bristol‐Myers Squibb, Celltech‐Medeva, Forest, GlaxoSmithKline, Johnson & Johnson, Lilly, New River, Novartis, Otsuka, Pfizer, Sanofi‐Synthelabo, Shire, Solvay and Wyeth. Dr Quinn claims no competitive interests. Dr McDowell has consulted for Janssen‐Cilag and Lilly. |
| Findling 2007 | High | Funding: the Stanley Medical Research Institute Conflicts of interest: some trial authors have affiliations with pharmaceutical companies |
| Findling 2008 | High | Funding: Shire Development Incorporated, Wayne, Pennsylvania Conflicts of interest: some trial authors received research support, acted as consultants and/or served on a Speakers' Bureau for several pharmaceutical companies. |
| Findling 2010 | High | Funding: Shire Development Incorporated, which was involved in trial design, conduct and data analysis. The open‐label trial was industry‐sponsored. Conflicts of interest: Dr Findling has acted as consultant to, has served on Speakers' Bureaus of and/or has received research support from Abbott, Addrenex, AstraZeneca, Biovail, Bristol‐Myers Squibb, Eli Lilly, Forest Pharmaceuticals, GlaxoSmithKline, KemPharm, Johnson & Johnson, Lundbeck, Neuropharm, Novartis, Noven, Organon, Otsuka, Pfizer, Sanofi‐Aventis, Sepracor, Shire, Solvay, Supernus, Validus and Wyeth. Dr. Turnbow receives or has received research support, acted as a consultant and/or served on Speakers' Bureaus for Eli Lilly, Novartis US, Sanofi‐Aventis, Shire and UCB (Union Chimique Belge). Dr Burnside has acted as consultant to, has served on Speakers’ Bureaus of and/or has received research support from Eli Lilly, Johnson & Johnson, Shire and Wyeth. Dr Melmed has acted as consultant to, has served on Speakers' Bureaus of and/or has received research support from Bristol‐Myers, Eli Lilly, McNeil, Novartis and Shire. Drs Civil and Li are full‐time employees of Shire Development Incorporated. |
| Fine 1993 | High | Funding: CIBA‐GEIGY Canada Conflicts of interest: not declared |
| Firestone 1981 | Low | Funding: Ministry of Health Conflicts of interest: not stated |
| Fitzpatrick 1992a | Low | Funding: National Institute of Mental Health (NIMH) grant MH38118 Conflicts of interest: not declared |
| Flapper 2008 | Low | Funding: none (no funding was available). This double‐blind placebo‐controlled (DBPC) trial of methylphenidate was performed as a clinical treatment program as best clinical practice to determine the effects of methylphenidate and optimal dose compared with placebo. Conflicts of interest: no affiliations with pharmaceutical companies or similar declared. |
| NCT02039908 | Low | Funding: Florida International University Conflicts of interest: nothing declared for trial investigators |
| Forness 1992 | Low | Funding: National Institute of Mental Health (NIMH) grant MH38686 Conflicts of interest: no affiliations described |
| Froehlich 2011 | High | Funding: National Institute of Mental Health (NIMH) and Cincinnati Children’s Hospital Center for Education and Research Therapeutics Award Conflicts of interest: Dr Epstein receives Funding from Eli Lilly and Co. Dr Stein has received research support from Eli Lilly and Co., McNeil Pharmaceuticals, Novartis and Shire. He has served on a Speakers' Bureau for Novartis and has served as consultant to Novartis, Shire and Shinogi Pharmaceuticals. |
| Froehlich 2018 | High | Funding: data collection for the project was supported by the National Institute of Mental Health (Bethesda, MD) by R01MH074770 [Epstein] and K23MH083881 [Froehlich], while investigators’ time on the project was funded by National Institute of Mental Health K24MH064478 [Epstein], K23MH083027 [Brinkman], and R01MH070564 [Stein]). Conflicts of interest: trial authors are affiliated with the medical industry |
| Gadow 1990 | Unclear | Funding: Ciba Pharmaceutical Company supplied methylphenidate placebo Conflicts of interest: not declared |
| Gadow 1995 | Low | Funding: research grants from the Tourette Syndrome Association and the National Institute of Mental Health (NIMH) Conflicts of interest: not declared |
| Gadow 2007 | Low | Funding: this trial was supported in part by a research grant from the Tourette Syndrome Association Incorporated, and by Public Health Service (PHS) grant number MH45358 from the National Institute of Mental Health (NIMH). Conflicts of interest: trial authors have no financial relationships to disclose. |
| Gadow 2011 | Unclear | Funding: National Institute of Mental Health (NIMH) and the Tourette Syndrome Association Incorporated. CIBA Pharmaceutical Company supplied methylphenidate placebos. Novartis supplied immediate‐release methylphenidate. Conflicts of interest: "Kenneth D. Gadow is a shareholder in Checkmate Plus, publisher of the Child Symptom Inventory‐4" |
| Garfinkel 1983 | Low | Funding: Ontario Mental Health Foundation Conflicts of interest: none |
| Gonzalez‐Heydrich 2010 | High | Funding: supported by National Institute of Mental Health (NIMH) Grant, Number K23 MH066835 Conflicts of interest: 4 trial authors are involved in the pharmaceutical sector. |
| Gorman 2006 | Low | Funding: National Institute of Mental Health (NIMH) Conflicts of interest: trial authors have no financial relationships to declare |
| Green 2011 | Low | Funding: the Basil O’Connor Starter Scholar Research Award of the March of Dimes, NARSAD (National Alliance for Research in Schizophrenia and Affective Disorders) Young Investigator Award, the Marguerite Stolz Award from the Sackler Faculty of Medicine and the National Institute on Drug Abuse (NIDA) Conflicts of interest: trial authors have had no institutional or corporate/commercial relationships for the past 36 months that might pose a conflict of interest. |
| Greenhill 2002 | High | Funding: Celltech Pharmaceuticals Incorporated Conflicts of interest: Dr Greenhill is a consultant for Celltech‐Medeva and a member of its medical advisory board. Drs Findling and Swanson are consultants for Celltech‐Medeva. |
| Greenhill 2006 | High | Funding: Novartis Conflicts of interest: 2 trial authors are employed by Novartis. Only Roberta R Ball has no conflicts of interest. |
| Gruber 2007 | Low | Funding: this was not an industry‐supported trial. Conflicts of interest: trial authors have indicated no financial conflicts of interest. |
| Hale 2011 | Low | Funding: research part funded by the Neuropsychiatric Research Institute, Fargo, North Dakota, USA Conflicts of interest: trial authors disclose no conflicts of interest |
| Hawk 2018 | Low | Funding: supported by grants from the National Institute of Mental Health (NIMH) and from the National Institute on Drug Abuse (NIDA) Conflicts of interest: no conflicts declared |
| Heriot 2008 | Low | Funding: no funding to conduct the trial was received from any party. Conflicts of interest: none of the trial authors are affiliated with pharmaceutical companies. |
| Hicks 1985 | Low | Funding: National Institutes of Health (NIH) Conflicts of interest: not declared |
| Hoeppner 1997 | Unclear | Funding: not declared Conflicts of interest: not declared |
| Horn 1991 | Unclear | Funding: not declared Conflicts of interest: not declared |
| Huang 2021 | High | Funding: this work is supported by Orient Pharma Co, Ltd. Conflicts of interest: authors affiliated with medical industry |
| Ialongo 1994 | Unclear | Funding: not declared Conflicts of interest: not declared |
| Jacobi‐Polishook 2009 | Unclear | Funding: not declared Conflicts of interest: not declared |
| Jensen 1999 (MTA) | High | Funding: this trial was supported by several grants from the National Institute of Mental Health, Bethesda, Maryland. Conflicts of interest: several trial authors have affiliations with medical companies. |
| Johnston 1988 | Unclear | Funding: not declared. During the writing of this report, C Johnston was supported by a Doctoral Fellowship from the Social Sciences and Humanities Research Council of Canada. Conflicts of interest: not declared |
| Kaplan 1990 | Unclear | Funding: not declared Conflicts of interest: not declared |
| Kelly 1989 | Unclear | Funding: CIBA Geigy Pharmaceuticals provided placebos Conflicts of interest: not declared |
| Kent 1995 | Low | Funding: this work was supported by the John and Maxine Bendheim Fellowship and by the Leon Lowenstein Foundation. Conflicts of interest: not declared |
| Kent 1999 | High | Funding: Ms Kent was a summer medical student supported in part by the IWK Grace Research Foundation, Halifax, NovaScotia, and by the Pharmaceutical Manufacturers Association of Canada Studentship, Ottawa, Ontario Conflicts of interest: trial authors sponsored by Pharmaceutical Manufacturers’ Association of Canada Studentship |
| Klorman 1990 | Low | Funding: National Institute of Mental Health (NIMH) grant MH38118 Conflicts of interest: no corporate affiliations declared |
| Kolko 1999 | Unclear | Funding: not declared Conflicts of interest: not declared |
| Kollins 2006 (PATS) | High | Funding:
Conflicts of interest:
|
| Kollins 2021 | High | Funding: clinical research was funded by KemPharm, Inc. Funding for editorial and writing assistance in the form of proofreading, copyediting, and fact‐checking was provided by Corium, Inc. Conflicts of interest: authors affiliated with medical industry |
| Konrad 2004 | Low | Funding: the German Society for the Advancement of Scientific Research (DFG grant KFO112) Conflicts of interest: none declared |
| Konrad 2005 | Low | Funding: provided through a grant from the German Research Foundation (DFG grant: KFO112–TP5) Conflicts of interest: none declared |
| Kortekaas‐Rijlaarsdam 2017 | High | Funding: unclear, but Shire was a collaborator Conflicts of interest: the second trial author has some affiliation to the medical industry. |
| Kritchman 2019 | Low | Funding: Shalvata Mental Health Center Conflicts of interest: “The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.” |
| Leddy 2009 | High | Funding: not declared Conflicts of interest: Dr Waxmonsky has served on the Speakers' Board for Novartis, received an honorarium from Shire and received research support from Shire and Eli Lilly. Dr Erbe has received educational and research support from Genzyme Corporation. Dr Pelham was paid an honorarium by Shire Pharmaceuticals. |
| Lehmkuhl 2002 | High | Funding: Medice Arzneimittel Pütter GmbH & Co. KG, Kuhloweg 37, D‐58638 Iserlohn Conflicts of interest: Dr Doepfner is a consultant for Lilly, Medice, Novartis and Union Chimique Belge; serves on the Advisory Boards of Lilly, Medice, Shire, Novartis and Union Chimique Belge; participates as a member of the Speakers' Bureaus of Lilly, Medice, Janssen‐Cilag and Union Chimique Belge; and has research contracts with Lilly, Medice, Novartis, Union Chimique Belge, the German Research Foundation and the Federal Ministry of Health. Dr Lehmkuhl is on the Advisory Boards of Lilly and Medice. Dr Sinzig has no financial relationships to disclose. |
| Lijffijt 2006 | Unclear | Funding: not declared Conflicts of interest: none declared |
| Lin 2014 | High | Funding: Ely Lilly Conflicts of interest: 5 authors work for Lilly. |
| Lopez 2003 | High | Funding: Novartis Conflicts of interest: Dr Silva is a consultant and a member of the Speakers' Bureau for Novartis. Dr Lopez is a consultant for Eli Lilly, Novartis and Shire. He is also a member of the Speakers' Bureaus for Novartis and Shire. |
| Lufi 1997 | Unclear | Funding: not declared Conflits of interest: not declared |
| Lufi 2007 | Unclear | Funding: not declared Conflicts of interest: not declared |
| Manos 1999 | High | Funding: in part by from Shire Pharmaceutical Development Incorporated to Dr Faraone Conflicts of interest: trial authors acknowledge partial support to the second author from the National Institute on Drug Abuse (NIDA) (grants R01‐DA07957 and MCJ‐390592) and from the Maternal and Child Health Program, Health Resources and Service Administration, Department of Health and Human Services (grant 390715), and to the third author from the Stanley Foundation. |
| Martins 2004 | Unclear | Funding: methylphenidate and placebo pills were supplied by Novartis Pharmaceuticals (São Paulo, Brazil) at no cost and without restrictions. No additional funding was requested or received from Novartis or any other commercial entity. Conflicts of interest: trial authors have reported no conflicts of interest |
| Matthijssen 2019 | Low | Funding: The Netherlands Organization for Health Research and development (ZonMw, grant 836011014) Conflicts of interest: not declared |
| McBride 1988a | Unclear | Funding: not declared Conflicts of interest: not declared |
| McCracken 2016 | High | Funding: National Institute of Mental Health (NIMH) Research Center grant P50MH077248, “Translational Research to Enhance Cognitive Control” Conflicts of interest: trial authors affiliated with the medical industry |
| McGough 2006 | High | Funding: Shire US Inc Conflicts of interest: 2 medical writers acknowledged (Amy M Horton & Michelle Roberts) but were unclear about where they came from or what their role was in the publication. |
| McInnes 2007 | High | Funding: the Psychiatric Endowment Fund Conflicts of interest: trial authors had received Funding from Eli Lilly, Shire Pharmaceuticals, Janssen‐Ortho and McNeil Pharmaceuticals |
| Merrill 2021 | Unclear | Funding: not stated Conflicts of interest: trial authors declare that they have no conflict of interest |
| Moshe 2012 | Low | Funding: none Conflicts of interest: none declared |
| Muniz 2008 | High | Funding: "This study was funded by Novartis Pharmaceuticals Corporation and reports the following involvement: design and conduct of the study; collection, management, analysis, and interpretation of data; preparation, review, and approval of the manuscript" Conflicts of interest: Dr Muniz is an employee of Novartis Pharmaceuticals Corporation. He has no other relationships to disclose. Dr Brams reports the following relationships: serves as speaker, consultant and Advisory Board member for Novartis and Shire; receives grant research support from Novartis, Shire and Eli Lilly. Dr Mao reports the following relationships: speaker for Novartis, Eli Lilly, Bristol‐Myers Squibb, AstraZeneca and Shire; consultant for Eli Lilly, Novartis and Shire; receives grant research support from Novartis. Mr McCague is an employee of Novartis Pharmaceuticals Corporation. He has no other relationships to disclose. Ms Pestreich is an employee of Novartis Pharmaceuticals Corporation. She has no other relationships to disclose. Dr Silva reports the following relationships: none since 15 December 2006; before that, she was a speaker for Novartis, AstraZeneca and Janssen; received grant/research support from Novartis and Celgene. |
| Murray 2011 | High | Funding: Ortho‐McNeil Janssen Scientific Affairs, LLC Conflicts of interest: several trial authors had affiliations with pharmaceutical companies producing methylphenidate |
| Musten 1997 | Low | Funding: Health Canada grant Conflicts of interest: none declared |
| NCT00409708 | High | Funding: Novartis Conflicts of interest: no information on investigators |
| NCT02293655 | Unclear | Funding: Children's Hospital Medical Center, Cincinnati Conflicts of interest: not stated |
| NCT02536105 | High | Funding: Massachusetts General Hospital Conflicts of interest: trial investigators affiliated with the medical industry |
| Newcorn 2008 | High | Funding: Eli Lilly and Company Conflicts of interest: Dr Newcorn receives grant support from Eli Lilly and McNeil; is a consultant and/or advisor for Eli Lilly, McNeil, Shire, Novartis and Sanofi‐Aventis; and is a member of Speakers' Bureaus for Eli Lilly and Novartis. Dr Kratochvil receives grant support from Abbott, Cephalon, Eli Lilly, McNeil, Pfizer, Shire and Somerset; receives from Eli Lilly trial medication for an NIMH (National Institute of Mental Health)‐funded trial; is a consultant for Abbott, AstraZeneca, Eli Lilly and Pfizer; and is a member of the Eli Lilly Speakers' Bureau. Dr Casat receives research Funding from Eli Lilly, Novartis and Abbott, and serves on an advisory board for Eli Lilly. Dr Allen and Dr Ruff are employees and shareholders of Eli Lilly. Dr Michelson and Dr Moore are former employees of Eli Lilly. |
| Newcorn 2017a (flexible dose) | High | Funding: Shire Conflicts of interest: trial authors affiliated with pharmaceutical companies |
| Newcorn 2017b (forced dose) | High | Funding: Shire Conflicts of interest: trial authors heavily affiliated with pharmaceutical companies |
| Nikles 2006 | Low | Funding: the General Practice Evaluation Program, the Department of Health and Aged Care, Queensland Medical Laboratory, and the Royal Australian College of General Practitioners Conflicts of interest: trial authors have indicated that they have no financial relationships relevant to this article to disclose |
| Oesterheld 1998 | Low | Funding: University of South Dakota/USF‐Mini Grant Conflicts of interest: none declared |
| Overtoom 2003 | Low | Funding: Netherlands Organisation for Scientific Research (NWO) Grant 575‐63‐082 Conflicts of interest: not declared |
| Palumbo 2008 | High | Funding: NIH (National Institutes of Health) and NINDS (National Institute of Neurological Disorders and Stroke) Conflicts of interest: some trial authors are on the ADHD Advisory Board and the Speakers' Bureau of; are scientific consultants or principal or site investigators for; and/or have received educational or funding support from several pharmaceutical companies. |
| Pearson 2013 | Low | Funding: grant number MH072263 from National Institute of Mental Health (NIMH) Conflicts of interest: none declared |
| Pelham 1989 | Unclear | Funding: not declared Conflicts of interest: not declared |
| Pelham 1990a | Unclear | Funding: not declared Conflicts of interest: not declared |
| Pelham 1993a | Unclear | Funding: not declared Conflicts of interest: not declared |
| Pelham 1999 | High | Funding: grants from the Shire Richwood Pharmaceutical Company and National Institute of Mental Health (Grants MH53554, MH45576 and MH50467) Conflicts of interest: not declared |
| Pelham 2001a | High | Funding: ALZA Corporation, the manufacturers of Concerta Conflicts of interest: Dr Pelham is a member of the ALZA advisory committee on Concerta and its development. Drs Hoffman and Lock are members of the ALZA paediatric advisory board. |
| Pelham 2002 | High | Funding: NIMH (Grant MH48157) Conflicts of interest: Pelham served as an advisor for ALZA Corporation (see Pelham 2001a) |
| Pelham 2005 | High | Funding: Noven Pharmaceuticals. Furthermore, Dr Pelham was supported by grants from NIAAA, NIDA, NIMH and NINDS. Conflicts of interest: several trial authors have received consulting fees and research funding and have been consultants and/or served on the Speakers' Bureaus of several pharmaceutical companies in the past year. |
| Pelham 2011 | High | Funding: grant from Noven Pharmaceuticals Conflicts of interest: Dr Pelham has served as a consultant for Shire, McNeil, Noven, Celltech/Medeva, Novartis and Abbott Laboratories; has received honoraria from Shire and Janssen and research support from Shire, Alza, Eli Lilly, Noven and Cephalon; and holds common stock in Abbott Laboratories. Dr Waxmonsky has served on the Speakers' Bureau for Novartis and has received research support from Eli Lilly and Shire Incorporated. Dr Hoffman has served on the advisory board and Speakers’ Bureau for Shire Pharmaceuticals and on the Speakers' Bureau for McNeil. Dr Ballow has received research support from GlaxoSmithKline, Panacos, Boehringer Ingelheim, Pharmasset, Jacobus and Pharmena. Dr Schentag has served as a consultant for or received support from Noven, Wyeth, Daiichi, Targanta Therapeutics and Astellas. Dr Gonzalez is a full‐time employee of P’Kinetics International Incorporated. No other conflicts of interest are known. |
| Pelham 2014 | Low | Funding: grant from the National Institute of Mental Health (MH62946). Dr Pelham was funded by grants from the National Institutes of Health (MH62946, MH69614, MH53554, MH69434, MH65899, MH78051, MH062946, NS39087, AA11873, DA12414, HD42080) and the Institute of Education Sciences (L03000665A). Dr Fabiano was supported in part by a Ruth S Kirschstein National Research Service Award Predoctoral Fellowship (1F31MH064243‐01A1) and by the Department of Education, Institute of Education Sciences (R324J06024, R324B06045). Conflicts of interest: not declared |
| Perez‐Alvarez 2009 | Low | Funding: none. Research was part of the work day, participants were voluntary and no funding was needed to implement the trial Conflicts of interest: none. Investigators are staff members at institutions (affiliations) reported in the paper. |
| Pliszka 1990 | Low | Funding: National Institute of Mental Health (NIMH) Conflicts of interest: not declared |
| Pliszka 2000 | High | Funding: Shire Richwood Incorporated Conflicts of interest: Dr Browne is currently with Watson Pharmaceuticals, Corona, California |
| Pliszka 2007 | High | Funding: National Institute of Mental Health Grant R01 MH63986 Conflicts of interest: Pliszka received honoraria and research support from Shire and MacNeil and research support from Ely Lilly and Cephalon |
| Pliszka 2017 | High | Funding: Ironshore Pharmaceuticals Conflicts of interest: trial authors affiliated with the medical industry |
| Quinn 2004 | High | Funding: Celgene Conflicts of interest: all trial authors disclosed that they have past and present affiliations with the pharmaceutical industry. |
| Ramtvedt 2013 | High | Funding: the first phase was conducted as part of ordinary clinical practice at Neuropsychiatric Unit, Østfold Hospital Trust. The second and third phases, data analysis and preparation of manuscript were sponsored by South‐Eastern Norway Regional Health Authority, and also by Østfold Hospital Trust and National Resource Centre for ADHD, both under the umbrella of South‐Eastern Norway Regional Health Authority. Conflicts of interest: Henning Aabech is a member of the Strattera Advisory Board, Eli Lilly, Norway. |
| Rapport 1985 | Unclear | Funding: not declared Conflicts of interest: not declared |
| Rapport 1987 | Low | Funding: none, neither external nor internal. This project was supported in part by a Biomedical Research Support Grant (no. S07 RR05712), which was awarded to the first trial author by the Biomedical Research Support Grant Program, Division of Research Resources, National Institutes of Health. Conflicts of interest: not declared |
| Rapport 2008 | Low | Funding: none Conflicts of interest: no financial, corporate or commercial relationships to disclose |
| Reitman 2001 | Unclear | Funding: not declared Conflict of interest: none |
| Riggs 2011 | High | Funding: OROS methylphenidate and matching placebo were supplied to the Clinical Trials Network contract pharmacy (EMINENT Services Corporation) by McNeil Consumer and Specialty Pharmaceuticals (distributor for Concerta), at no cost. Principal investigators are not employed by the organisation sponsoring the trial. No agreement between principal investigators and trial sponsor (or its agents) restricts the principal investigator's rights to discuss or publish trial results after the trial is complete Conflicts of interest: some trial authors have received research support from, served on Speakers' Bureaus of or acted as consultants for pharmaceutical companies. |
| Rubinsten 2008 | Low | Funding: the research was completed while Dr Rubinsten was a post‐doctoral fellow at the Hospital for Sick Children (HSC), in Toronto, Canada, and was supported by the Rothschild Fellowship from Israel. It was undertaken, in part, through funding received from the Canadian Institutes of Health (CIHR: grant #MOP 64312), a CIHR post‐doctoral fellowship, and the Canada Research Chairs Program (RT). Conflicts of interest: not declared |
| Samuels 2006 | Unclear | Funding: not declared Conflicts of interest: not declared |
| Schachar 1997a | High | Funding: Medical Research Council of Canada, National Health Research Development Program of Canada and the Department of Psychiatry, The Hospital for Sick Children, Toronto. Placebo pills were provided by Ciba Geigy, Canada, Ltd Conflicts of interest: 2 trial authors have reported working as consultants for pharmaceutical companies, and 1 has furthermore received industry‐sponsored research grants. |
| Schachar 2008 | High | Funding: Purdue Pharma (Canada) Conflicts of interest: some trial authors are working for Purdue Pharma |
| Schrantee 2016 | Low | Funding: this trial was funded by faculty resources of the Academic Medical Center, University of Amsterdam, and by grant 11.32050.26 from the European Research Area Network Priority Medicines for Children (Sixth Framework Programme). Dr Rombouts was supported by Vici (Netherlands Organisation for Scientific Research), and Dr Andersen was supported by grant DA‐015403 from the National Institute on Drug Abuse Conflicts of interest: Dr Niessen reported being cofounder, shareholder, and part‐time scientific officer of Quantib BV. No other disclosures were reported. Through personal correspondence it was clarified that Dr Niessen did not facilitate any part of the trial, but was involved in the data‐analysis of MRI imaging sequence technique used (arterial spin labelling). |
| Schulz 2010 | High | Funding: Novartis Pharma GmbH, Germany. Trial aimed at showing efficacy of Ritalin LA with purpose of obtaining marketing authorisation Conflicts of interest: almost all trial authors have received grants, research support or other kinds of financial support from the medical industry. |
| Schwartz 2004 | High | Funding: grants from Le Fonds de la Recherche en Santé du Québec and the Canadian Institutes of Health Research Conflicts of interest: yes. Dr Joober is a principal investigator on a clinical trial not related to this trial that is sponsored by AstraZeneca Canada Incorporated, and receives no direct compensation for this trial. Dr Boivin has the following industry financial ties: The Litebook Company Ltd., Medicine Hat, Alberta, Canada; and Pulsar Informatics Inc., Vancouver, British Columbia, Canada. |
| Sharp 1999 | Unclear | Funding: not declared Conflicts of interest: not declared |
| Shiels 2009 | High | Funding: National Institute of Mental Health Conflicts of interest: "In the past 3 years, James G. Waxmonsky has served on the Speakers Bureau for Novartis, received honoraria from Scepter, and received research support from Eli Lilly" |
| Silva 2005a | High | Funding: Novartis Pharmaceuticals Corporation Conflicts of interest: all trial authors have been consultants, have received honoraria or have worked for Novartis. |
| Silva 2006 | High | Funding: Novartis Conflicts of interest: some trial authors have affiliations with medical companies |
| Silva 2008 | High | Funding: Novartis Conflicts of interest: some trial authors have affiliations with medical companies |
| Smith 1998 | Low | Funding: grants from the National Institute on Drug Abuse, the National Institute of Mental Health, the National Institute on Alcohol Abuse and Alcoholism and the National Institute of Child Health and Human Development Conflicts of interest: not declared |
| Smith 2004 | Unclear | Funding: not declared Conflicts of interest: not declared |
| Smithee 1998 | Low | Funding: National Institute of Mental Health (NIMH) Grant MH 38228; Rafael Klorman Conflicts of interest: not declared |
| Solanto 2009 | High | Funding: the National Institute of Mental Health Conflicts of interest: 3 trial authors have served or received grants from pharmaceutical companies in the past. |
| Soleimani 2017 | Low | Funding: Guilan University of Medical Sciences Conflicts of interest: none declared |
| Stein 1996 | Low | Funding: the work was supported by the Smart Family Foundation. Conflicts of interest: no affiliations with pharmaceutical companies stated |
| Stein 2003 | High | Funding: the National Institute of Mental Health, the General Clinical Research Center Program of the National Center for Research Resources and the National Institutes of Health, Department of Health and Human Services Conflicts of interest: Drs Stein, Robb, Conlon and Newcorn participate in the Speakers' Bureau for McNeil Consumer and Specialty Pharmaceuticals, and Drs Stein and Newcorn are members of the Concerta National Advisory Committee. |
| Stein 2011 | High | Funding: investigator‐initiated trial sponsored by Novartis Pharmaceuticals, with additional support provided by the University of Illinois at Chicago (UIC) Center for Clinical and Translational Science (CCTS) Conflicts of interest: some trial authors are affiliated with pharmaceutical companies |
| Stoner 1994 | Low | Funding: National Association of School Psychologists Conflicts of interest: not declared |
| Sumner 2010 | Unclear | Funding: it was not clear who sponsored the trial, but someone did (see authors' affiliations). Conflicts of interest: Calvin R Sumner is an employee of and an equity holder for the trial sponsor. Virginia S Haynes, PhD, is an employee of 3i Global (Basking Ridge, NJ) and a paid consultant for the trial sponsor. Martin H Teicher, MD, PhD, served as paid consultant and clinical investigator for the sponsor. Jeffrey H Newcorn, MD, serves as advisor and consultant for Lilly, Ortho‐McNeil Janssen, Schering‐Plough and Shire. He receives research support from Lilly, Ortho‐McNeil Janssen and Shire. |
| Sunohara 1999 | High | Funding: RESTRACOM graduate studentship for The Hospital for Sick Children Research Institute and Novartis Pharmaceuticals Conflicts of interest: not declared |
| Swanson 1998 | High | Funding: grant from Richwood Pharmaceutical Company Conflicts of interest: not declared |
| Swanson 1999 | High | Funding: ALZA Corporation, Palo Alto, California Conflicts of interest: not declared |
| Swanson 2002a | High | Funding: ALZA Corporation Conflicts of interest: not declared |
| Swanson 2002b | High | Funding: ALZA Corporation Conflicts of interest: not declared |
| Swanson 2004b | High | Funding: Celltech Pharmaceuticals Incorporated Conflicts of interest: some trial authors are consultants for pharmaceutical companies |
| Symons 2007 | Unclear | Funding: A McKnight Land‐Grant Professorship to the first author Conflicts of interest: this work was supported, in part, by a McKnight Land‐Grant Professorship to Frank Symons. |
| Szobot 2004 | High | Funding: research funds from Hospital de Clínicas de Porto Alegre, FAPERGS and NOVARTIS Conflicts of interest: not declared |
| Szobot 2008 | High | Funding: "The ADHD outpatient program receives research support from Bristol‐Myers Squibb, Eli‐Lilly, Janssen‐Cilag and Novartis" Conflicts of interest: trial authors are consultants and speakers for various companies |
| Tannock 1989 | Low | Funding: jointly funded by Ontario Mental Health Foundation (Grant No. 963‐86/88) and Health and Welfare Canada (Grant No. 6606‐3166‐42) Conflict of interest: not declared |
| Tannock 1992 | Low | Funding: grant from the Canadian Psychiatric Research Foundation and a post‐doctoral fellowship by the Ontario Mental Health Foundation Conflicts of interest: not declared |
| Tannock 1993 | Low | Funding: the Canadian Psychiatric Research Foundation and the Medical Research Council of Canada Conflicts of interest: not declared |
| Tannock 1995a | Low | Funding: Medical Research Council of Canada and Health and Welfare Canada Conflicts of interest: nothing to declare |
| Tannock 1995b | Low | Funding: in part, by the Ontario Mental Health Foundation and the National Health Research and Development Program, Health Canada Conflicts of interest: not declared |
| Tannock 2018 | Unclear | Funding: an operating grant from the Canadian Institutes of Health Research (Grant # MT 13366), and by the donation of placebo medication from Novartis Pharmaceuticals Conflict of interest: none declared |
| Taylor 1987 | High | Funding: partially funded by grant from CIBA Ltd., which provided medicine and placebo Conflicts of interest: Dr Schachar was supported during this period by a fellowship from the Medical Research Council of Canada. |
| Taylor 1993 | Unclear | Funding: not declared Conflicts of interest: not declared |
| Tervo 2002 | Unclear | Funding: not declared Conflicts of interest: no conflicts of interest have been disclosed |
| Tirosh 1993a | Unclear | Funding: none Conflicts of interest: not declared |
| Tirosh 1993b | Unclear | Funding: not declared Conflicts of interest: not declared |
| Tourette's Syndrome Study Group 2002 | Unclear | Funding: National Institute of Neurological Disorders and Stroke, the General Clinical Research Center, the National Center for Research Resources, the Tourette Syndrome Association Boeringer Ingelheim Inc. (particularly Dr Virgil Dias), for supplying clonidine and matching placebo; Bausch and Lomb, Inc., for supplying small gifts for our trial participants Conflicts of interest: none declared |
| Tucker 2009 | High | Funding: Novartis Pharmaceuticals Corporation Conflicts of interest: some trial authors were employed by Novartis (5 of 8 had a Novartis email address) |
| Ullmann 1985 | Unclear | Funding: National Institutes of Mental Health (NIMH). Ciba‐Geigy provided medication and placebo Conflicts of interest: not declared |
| Ullmann 1986 | Unclear | Funding: in part by a National Institute of Mental Health (NIMH) grant. Ciba‐Geigy provided medication and placebo Conflicts of interest: not declared |
| Urman 1995 | Low | Funding: in part by funds from the Medical Research Council of Canada and the Research Institute of the Hospital for Sick Children Conflicts of interest: not declared |
| Van der Meere 1999a | High | Funding: grants from the Sophia Foundation for Medical Research and Boehringer Ingelheim BV, the Netherlands Conflicts of interest: not declared |
| Wallace 1994 | Low | Funding: The Veterans Administration Medical Center, Vermont Conflicts of interest: not declared |
| Wallander 1987 | Low | Funding: in part by Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) grants and the University of Southern California Faculty Research and Innovation Fund Conflicts of interest: not declared |
| Waxmonsky 2008 | High | Funding: National Institute of Mental Health (NIMH) Grant MH62946 and a Klingenstein Third Generation Foundation Fellowship in Child and Adolescent Depression Research Conflicts of interest: several authors have affiliations with pharmaceutical companies |
| Weiss 2021 | High | Funding: Rhodes Pharmaceuticals, LP Conflict of interest: the trial authors are affiliated with the medical industry. |
| Whalen 1990 | Unclear | Funding: not declared Conflicts of interest: not declared |
| Wigal 2003 | High | Funding: Celltech Americas Incorporated Conflicts of interest: some trial authors are working for Celltech Americas Incorporated |
| Wigal 2004 | High | Funding: Celgene Corporation Conflicts of interest: Dr Wigal reports extensive disclosure. |
| Wigal 2011 | High | Funding: Ortho‐McNeil‐Janssen Scientific Affairs, LLC. Phase IV trial Conflicts of interest: several trial authors had affiliations with pharmaceutical companies producing methylphenidate |
| Wigal 2013 | High | Funding: trial received funds from NextWave Pharmaceutics (Belden and Berry are with NextWave) Conflicts of interest: all trial authors are affiliated with NextWave Pharmaceuticals. |
| Wigal 2014 | High | Funding: Rhodes Pharmaceuticals LP Conflicts of interest: several trial authors work for, or have received grant and research support or both from pharmaceutical companies |
| Wigal 2015 | High | Funding: Rhodes Pharmaceuticals […]. Medical writing assistance was provided by Linda Wagner, PharmD, from Excel Scientific Solutions and funded by Rhodes Pharmaceuticals LP Conflicts of interest: not declared |
| Wigal 2017 | High | Funding: the research was sponsored by NextWave Pharmaceuticals, a wholly owned subsidiary of Pfizer, Inc. Conflicts of interest: trial authors are affiliated with the medical industry |
| Wilens 2006b | High | Funding: McNeil Consumer and Specialty Pharmaceuticals Conflicts of interest: several trial authors have had commitments (e.g. speakers, consultants, advisors) with various pharmaceutical companies |
| Wilens 2008 | High | Funding: Shire Development Incorporated Conflicts of interest: several trial authors have affiliations with medical companies |
| Wilens 2010 | High | Funding: trial and medication/placebo were funded by a grant through Shire Pharmaceuticals. Shire had no role in design, collection, analysis, interpretation, writing or decision to submit Conflicts of interest: some trial authors have received research support from medical companies |
| Wilkison 1995 | Low | Funding: a University of Utah Biomedical Sciences research grant and a grant from the University Research Committee Conflicts of interest: no corporate affiliations described |
| Wodrich 1998 | Unclear | Funding: not declared Conflicts of interest: not declared |
| Wolraich 2001 | High | Funding: ALZA Corporation Conflicts of interest: trial authors are part of the Concerta Study Group |
| Zeiner 1999 | Low | Funding: the Norwegian Medical Research Council, the Norwegian Public Health Association and the Legacy of Haldis and Josef Andresen Conflicts of interest: not declared |
| Zeni 2009 | High | Funding: research grants from Conselho Nacional de Desenvolvimento Cientifico e Tecnologico (CNPq, Brazil) (Grant 471761=03‐6) and Hospital de Clinicas de Porto Alegre (GPPG 03‐325). Aripiprazole was provided by Bristol‐Myers Squibb without restriction. Conflicts of interest: stated, "this is an independent investigator trial"; however some study authors have affiliations with medical companies. |
ADHD: Attention deficit hyperactivity disorder; BV: besloten vennootschap (corresponding to a private limited liability company (LLC) in the USA); DFG: Deutsche Forschungsgemeinschaft; NIH: National Institutes of Health; Inc.: Incorporated; IWK: Izaak Walton Killam; LA: Long acting; Ldt.: Limited liability; LLC: Limited liability company; LP: Limited partnership; MRI: Magnetic resonance imaging; NIAAA: National Institute on Alcohol Abuse and Alcoholism; NIDA: National Institute on Drug Abuse; NIMH: National Institute of Mental Health; NINDS: National institute of Neurological Disorders and Stroke; OROS: osmotic‐release oral system; PI: Primary Investigator; ZonMw: Organisation for Health Research and Development in the Netherlands
Sensitivity analysis
We conducted sensitivity analyses to determine whether findings were sensitive to the following.
Decisions made during the review process, such as our assessment of clinical heterogeneity (listed below)
Combined 'change scores' and 'endpoint data' in the meta‐analyses
Random‐effects and fixed‐effect model meta‐an lyses
No sufficiently well‐designed method has been used to combine the results of trials at high risk of bias and trials at low risk of bias (Higgins 2022a). We performed sensitivity analyses by grouping together trials with similar classifications of bias, as described above, and investigated the impact on intervention effects.
We excluded the following trials from the sensitivity analyses.
IQ under 70 (Oesterheld 1998; Pearson 2013; Smith 1998; Taylor 1987)
Change scores (Carlson 2007; Findling 2008; Kollins 2021; McCracken 2016; Newcorn 2008; Newcorn 2017a (flexible dose); Newcorn 2017b (forced dose); Palumbo 2008; Tucker 2009)
Older than 18 years of age (Green 2011; Szobot 2008)
Summary of findings and assessment of the certainty of the evidence
We constructed a summary of findings table in which to document all review outcomes. Two review authors (HEC and OJS) assessed the evidence using the GRADE approach. The GRADE approach appraises the certainty of a body of evidence based on the extent to which one can be confident that an estimate of effect or association reflects the item being assessed. Considerations are due to within‐trial risk of bias; directness of the evidence; heterogeneity of the data; precision of effect estimates; and risk of publication bias (Guyatt 2011; Schünemann 2022). When possible, that is, when the MD or the RR was available, we used the results from the Trial Sequential Analysis as the rating for imprecision (Jakobsen 2014). We downgraded imprecision in GRADE by two levels if the accrued number of participants was below 50% of the DARIS, and one level if between 50% and 100% of the DARIS (Korang 2020). We did not downgrade for imprecision if the cumulative Z‐curve crossed the monitoring boundaries for benefit, harm, futility, or the DARIS (Korang 2020). We reported two primary outcomes (teacher‐rated ADHD symptoms and serious adverse events) and three secondary outcomes (non‐serious adverse events, teacher‐rated general behaviour, and quality of life) at end of treatment in Table 1.
Results
Description of studies
For more information, please see Characteristics of included studies, Characteristics of excluded studies, Characteristics of studies awaiting classification and Characteristics of ongoing studies, as well as Table 3 for an overview of study characteristics and Table 4 for an overview of key inclusion and exclusion criteria.
2. Key demographics of included studies.
aResearch unit at a hospital. bIn addition to bipolar disorder, which was an inclusion criterion for this trial. c2 participants also had drug or alcohol abuse. dParticipants were grouped to be with or without anxiety. e50% also suffered from psychosis. All participants had either bipolar disorder or borderline personality disorder. fAggressive conduct disorder. gDrug/alcohol use. hNeurofeedback was part of the intervention. iFor the review, we used data from arms with no behavioural intervention. jDifferent reporting across different articles, ranging between 39% and 63%. kDelayed‐release and extended‐release methylphenidate. lGiven 3 times a day as well as an experimental delivery, to evaluate the effect of applying an osmotically driven, continuous delivery system. mFor the parallel trial. nFor the cross‐over trial. oIn this cross‐over trial, children received methylphenidate twice for 7‐10 days, resulting in a full methylphenidate intervention period of 14‐20 days. pBetween 7‐21 days depending on group size. The mean intervention period considered 14 days for this review. qTwelve days for the 20 inpatients, 19 days for the 24 outpatients. rIt is only stated that this trial was not industry‐funded.
3. Key inclusion and exclusion criteria.
aSevere learning disability (defined by special education enrolment). bExceptions: obsessive‐compulsive disorder, conduct or oppositional disorder, overanxious disorder and specific developmental disorders. cOnly bipolar disorder, not major depressive disorder.
Results of the search
An overview of the searches can be seen in Figure 1.
1.
We carried out electronic searches over six periods. The four searches that took place from October 2011 to February 2015 are described in detail in the previous version of this review (Storebø 2015a). All searches from the previous version of the review resulted in 183 included trials (from 433 reports), one study (from one report) awaiting classification, and five ongoing trials (from six reports).
Searches up to January 2021 produced an additional 2244 records after duplicates were removed (3132 initial records). Searches up to March 2022 produced an additional 285 records after duplicates were removed (401 initial records). Searches for dissertations up to December 2022 resulted in an additional 100 records after duplicates were removed (237 initial records). We identified an additional 347 records after duplicates were removed (409 initial records) by reading through the reference lists of other reviews on ADHD and stimulant therapy published since 2015, and by corresponding with study authors of included studies and with pharmaceutical companies. We also looked through all included studies from all searches to look for protocols or trial registrations that were not already included, which resulted in 35 of the 347 records. We rescreened studies awaiting classification and ongoing studies from the previous version of the review (eight reports).
During 2021 we contacted authors of 24 of the new included trials twice for supplemental information and data; nine responded and we received data from seven trials. Additionally, we contacted two authors of studies included during the latest search in March 2022 only once but they did not respond in due time to be applied to this review.
For the searches in 2021 and 2022 we excluded non‐randomised studies during screening, focusing only on RCTs. From 3019 screened records we excluded 2574 clearly irrelevant reports on the basis of titles and abstract. We retrieved the full texts of the remaining 445 reports, which we assessed for eligibility. They were all accessible in English. We excluded 254 full‐text reports and identified two studies as awaiting classification (from 2 reports; see Characteristics of studies awaiting classification) and 16 trials as ongoing (from 17 reports; see Characteristics of ongoing studies). We included 172 reports of which 109 described 29 new RCTs, while 63 reports were added to the trials included in the previous version of the review. Additional information about all included trials can be found in the Characteristics of included studies tables.
Included studies
We included a total of 212 trials (from 607 reports) in this review (Figure 1). Of these, 55 are parallel‐group trials (from 222 reports) and 156 are cross‐over trials (from 359 reports). One study (24 reports) includes a parallel phase as well as a cross‐over phase, thus we used data from this study in the parallel trial analyses as well as the cross‐over trial analyses (Kollins 2006 (PATS)). A total of 165 trials (14,271 participants) provided usable data for the quantitative analyses. An overview of key demographics for all included trials can be seen in Table 3, and the distribution of key inclusion and exclusion criteria across the trials is available in Table 4 .
Included parallel‐group trials
Including Kollins 2006 (PATS), we included 56 parallel‐group trials described in 244 reports. Fifty trials (7895 participants) provided usable data for the quantitative analyses.
Duration
Most trials (n = 51) were short‐term (< 6 months in duration). Only four were long‐term trials (conducted for ≥ 6 months; Barragán 2017; Jensen 1999 (MTA); Perez‐Alvarez 2009; Schachar 1997a). The duration of one trial was unclear, but lasted somewhere between three to five months (Tannock 2018). The mean duration of the methylphenidate intervention across 56 trials was 67.1 days (range 1 to 425 days).
Location
Thirty‐five of the 56 trials (including Kollins 2006 (PATS)), were conducted in the USA. Three trials were conducted in the USA and Canada (Biederman 2003b; Jensen 1999 (MTA); Weiss 2021); one in the USA, Canada and Australia (Findling 2006); and one in the USA, Canada, Taiwan, Mexico and Puerto Rico (Lin 2014). Three trials each were conducted in Canada (Butter 1983; Schachar 1997a; Tannock 2018), and the Netherlands (Matthijssen 2019; Schrantee 2016; Van der Meere 1999a). Two trials each were conducted in Brazil (Martins 2004; Szobot 2004), Israel (Green 2011; Jacobi‐Polishook 2009). Single trials were conducted in Mexico (Barragán 2017), New Zealand (Heriot 2008), Germany (Lehmkuhl 2002), and Norway (Duric 2012). One trial was conducted in Germany, Sweden, Spain, Hungary, France, UK, Italy, Belgium, Poland, and the Netherlands (Coghill 2013).
The location of one trial was not clear (Firestone 1981).
Setting
All but the following 10 trials were conducted in outpatient clinics. One was carried out in an outpatient as well as inpatient setting (Green 2011), two were carried out in a naturalistic classroom setting (Biederman 2003b; Greenhill 2006), four in a laboratory classroom (Childress 2017; Childress 2020a; Childress 2020b; Kollins 2021), one in a research unit at a hospital (Schachar 1997a), and two provided no information on setting (Brown 1985; McCracken 2016).
Participants
The 56 trials included a total of 8218 participants with a boy‐to‐girl ratio of 3:1 (with the percentage of girls ranging from 0% to 41% (mean across studies 23.8%)). All participants were between 3 and 20 years of age (mean 9.9 years).
Thirty‐nine trials described the percentage of methylphenidate‐naive participants (range 0% to 100%; mean 51.6%).
Thirty‐seven trials described the proportion of participants with combined subtype ADHD (range 25% to 100%; mean 69.9%); 33 trials reported the proportion of participants with hyperactive subtype (range 0% to 56%; mean 4.5%) and 34 trials revealed the proportion with inattentive subtype (range 0% to 72.9%; mean 25.8%).
Six trials excluded children and adolescents with any psychiatric comorbidity (Findling 2010; Greenhill 2002; Greenhill 2006; Perez‐Alvarez 2009; Tucker 2009; Wigal 2017). Eleven trials clearly stated what comorbidities were or could be included, (Duric 2012; Green 2011; Jensen 1999 (MTA); Lehmkuhl 2002; Pliszka 2000; Riggs 2011; Schachar 1997a; Szobot 2004; Tourette's Syndrome Study Group 2002; Van der Meere 1999a; Wolraich 2001), and 21 trials excluded psychiatric comorbidities to some extent. Thirteen of the trials only allowed the psychiatric comorbidities oppositional defiant disorder, conduct disorder, socially aggressive, or disturbance in social behavior (Carlson 2007; Findling 2008; Heriot 2008; Horn 1991; Ialongo 1994; Lin 2014; Martins 2004; McCracken 2016; Newcorn 2008; Newcorn 2017a (flexible dose); Newcorn 2017b (forced dose); Palumbo 2008; Tannock 2018). One only included participants with oppositional defiant disorder or conduct disorder but there was no information on the presence of other psychiatric comorbidities (Connor 2000). For two trials there was no limit to psychiatric comorbidities (Coghill 2013; Kollins 2006 (PATS)). Two trials stated nothing about the inclusion or exclusion of comorbidities or their prevalence among participants (Butter 1983; NCT00409708). Opositional defiant disorder was the most commonly reported comorbidity (prevalence clearly reported for 19 trials, range 8.2% to 53%; mean 35.1%), followed by conduct disorder (prevalence clearly reported for 11 trials, range 2% to 32.3%; mean 11.2%). Three trials reported oppositional defiant disorder and conduct disorder together (range 57.6% to 100%) (Connor 2000; Martins 2004; Szobot 2004), therefore we could not use them for the calculated mean.
Nine trials specifically excluded participants taking other medications (Carlson 2007; Childress 2017; Heriot 2008; Ialongo 1994; Jacobi‐Polishook 2009; Kollins 2006 (PATS); Perez‐Alvarez 2009; Tucker 2009; Wigal 2017), and 35 trials specified the exclusion or inclusion of some medications. Four trials had comedication as part of the intervention (Carlson 2007; Connor 2000; McCracken 2016; Riggs 2011). No information on comedication was available for 16 of the trials.
Some form of co‐therapy was part of the intervention in 10 of the trials (Brown 1985; Firestone 1981; Heriot 2008; Horn 1991; Jensen 1999 (MTA); NCT00409708; Palumbo 2008; Perez‐Alvarez 2009; Riggs 2011; Tucker 2009). Two trials had a therapy phase prior to medication (Childress 2020c; Kollins 2006 (PATS)), and five trials specified limitations to therapy during the study (Biederman 2003b; Childress 2009; Greenhill 2006; Matthijssen 2019; NCT02293655). Nothing was stated about co‐therapy for the remaining 39 trials.
Interventions
Twenty‐nine trials used extended‐ and modified‐release methylphenidate. Two trials used immediate‐ and extended‐release methylphenidate (Findling 2006; Wolraich 2001). One trial used transdermal methylphenidate patches (Findling 2010), and one trial used both transdermal patches and extended‐release methylphenidate (Findling 2008). The type used in four trials was unclear (Butter 1983; Horn 1991; Ialongo 1994; Schrantee 2016). The remaining 19 trials used immediate‐release methylphenidate.
The method of reporting the dosage of methylphenidate varied considerably between trials, but the overall daily dose ranged from 5 mg to 68 mg with a mean reported total daily dose of 34.4 mg/day or 0.78 mg/kg/day. The average dose of any type of modified‐ or extended‐release methylphenidate was 44.2 mg, and the average dose of immediate‐release methylphenidate was 23.0 mg.
Forty‐eight trials used placebo as control, and eight used no intervention as control (Barragán 2017; Brown 1985; Duric 2012; Heriot 2008; Jensen 1999 (MTA); NCT00409708; Perez‐Alvarez 2009; Tucker 2009).
Eight trials used clonidine (Connor 2000; Palumbo 2008; Tourette's Syndrome Study Group 2002), omega 3/6 (Barragán 2017), atomoxetine (Carlson 2007), guanfacine (McCracken 2016), or lisdexamphetamine (Newcorn 2017a (flexible dose); Newcorn 2017b (forced dose)), as a co‐intervention in both the intervention and control groups. Three trials used parent training (Firestone 1981; Heriot 2008; Schachar 1997a), two used cognitive‐behavioural therapy (Brown 1985; Riggs 2011), and six used other behavioural therapies (Duric 2012; Horn 1991; Jensen 1999 (MTA); NCT00409708; Perez‐Alvarez 2009; Tucker 2009), as co‐interventions for the intervention and control groups. One trial used neurofeedback as co‐intervention in both the intervention and control group (Duric 2012).
Included cross‐over trials
We included 157 cross‐over trials (including Kollins 2006 (PATS)), described in 383 reports. Of these, 116 trials (6490 participants) provided usable data for the quantitative analyses
Seventy‐five trials were described in a single publication. Sixteen trials yielded five or more publications. One trial, that ran until 2020 is reported across the greatest number of publications per trial, with 27 publications all reporting preliminary data with a varying number of participants across reports (Bhat 2020).
Duration
Three cross‐over trials did not report duration (Kelly 1989; Sunohara 1999; Tannock 1993). The remaining 154 cross‐over trials had a duration of less than six months.
The duration of methylphenidate treatment, including all periods of methylphenidate at any dose for the individual participant, without counting the duration of the placebo intervention, ranged between 1 and 56 days with a mean of 15.2 days.
Location
A total of 109 trials were carried out in the USA; 21 in Canada; and one in both the USA and Canada (Quinn 2004). Six trials were conducted in Israel (Kritchman 2019; Lufi 1997; Lufi 2007; Moshe 2012; Tirosh 1993a; Tirosh 1993b); five in Germany (Bliznakova 2007; Döpfner 2004; Konrad 2004; Konrad 2005; Schulz 2010); five in the Netherlands (Buitelaar 1995; Flapper 2008; Kortekaas‐Rijlaarsdam 2017; Lijffijt 2006; Overtoom 2003); two each in the UK (Coghill 2007; Taylor 1987), Norway (Ramtvedt 2013; Zeiner 1999), and Brazil (Szobot 2008; Zeni 2009); and one each in Australia (Nikles 2006), Iran (Soleimani 2017), and Taiwan (Huang 2021). One trial did not specify the country of origin (Hicks 1985).
Setting
Twenty‐one trials were completed as a part of summer treatment programmes or summer schools. Nine trials were conducted in inpatient wards (Brown 1991; Carlson 1995; Gonzalez‐Heydrich 2010; Kent 1995; Konrad 2005; Pelham 1993a; Pelham 2002; Solanto 2009; Wallace 1994), and seven in both outpatient clinics and inpatient wards (Garfinkel 1983; Hicks 1985; Kaplan 1990; Kolko 1999; Konrad 2004; Tannock 1992; Wallander 1987). Sixteen trials were conducted in a laboratory classroom setting and one in a naturalistic school setting (Ullmann 1986). Six trials did not report the setting (Bliznakova 2007; CRIT124US02; Pliszka 2007; Stoner 1994; Ullmann 1985; Urman 1995). All remaining trials were conducted in outpatient clinics only.
Participants
The 157 cross‐over trials included a total of 8198 participants (range 1 to 430 per trial; mean 52.2). The percentage of girls ranged from 0% in 30 trials to 100% in one trial (CRIT124US02); (mean across the 149 studies that reported ratio; 18.7%, equivalent to a boy‐to‐girl ratio of 7:2). All participants were between 4 and 21 years of age (mean 9.7 years). Sixteen trials did not report average age; however, all of these trials reported age range (Ahmann 1993; Carlson 1995; Coghill 2007; Corkum 2008; Gadow 1990; Kent 1999; Klorman 1990; Leddy 2009; NCT02039908; Pelham 1989; Quinn 2004; Rapport 1987; Solanto 2009; Sumner 2010).
A total of 97 trials described the percentage of methylphenidate‐naive participants included (range 0% to 100%; mean 52.7%). In 29 trials, all participants were methylphenidate‐naive. Thirty trials only included participants previously treated with methylphenidate.
Eighty‐one trials described the proportion of participants with combined ADHD subtype (range 0% to 100%; mean 70.3%), 73 trials reported the proportion with hyperactive subtype (range 0% to 100%; mean 7.8%) and 74 trials reported the proportion with inattentive subtype (range 0% to 73.7%; mean 21.6%).
Fifteen trials excluded children with any psychiatric comorbdity (Flapper 2008; Garfinkel 1983; Huang 2021; Lufi 1997; Moshe 2012; Muniz 2008; Quinn 2004; Schachar 2008; Soleimani 2017; Swanson 1998; Swanson 2002a; Tirosh 1993a; Tirosh 1993b; Wilens 2008; Wilkison 1995). Forty‐six trials clearly stated what comorbidities were or could be included and 22 trials excluded psychiatric comorbidities to some extent. Thirty‐eight of the trials only allowed the psychiatric comorbidities oppositional defiant disorder, conduct disorder, socially aggressive or disturbance in social behaviour. For five trials there was no limit to psychiatric comorbidities (Cox 2006; Gadow 2011; Kent 1999; Kollins 2006 (PATS); Symons 2007). Thirty‐three stated nothing about the inclusion or exclusion of comorbidities or their prevalence among participants.
Opposotional defiant disorder was the most commonly reported comorbidity (prevalence clearly reported for 67 trials, range 1.36% to 100%; mean 43.2%), followed by conduct disorder (prevalence clearly reported for 56 trials, range 2.9% to 100%; mean 24%). Six trials reported oppositional defiant disorder and conduct disorder together (range 57.6 to 100%) therefore we could not use them for the calculated mean (Carlson 1995; Döpfner 2004; Gorman 2006; Hale 2011; Pelham 2011; Tannock 1995a). Six trials reported participants with Tourette's syndrome (range 2.7% to 100%; mean 67%; Castellanos 1997; Coghill 2007; Gadow 1995; Gadow 2007; Gadow 2011; Kent 1999). One trial only included participants with epilepsy (Gonzalez‐Heydrich 2010), one trial only included participants with cerebral palsy (Symons 2007), and two trials only included participants with bipolar disorder or borderline personality (Findling 2007; Zeni 2009). Twenty‐eight trials reported prevalence of participants with comorbid anxiety (range 2.7% 46% mean 20.8%).
Twenty‐seven trials specifically excluded participants taking other medications, and 48 trials specified the exclusion or inclusion of some medications. Six trials had comedication as part of the intervention (Carlson 1995; Findling 2007; Gonzalez‐Heydrich 2010; Kaplan 1990; Szobot 2008; Zeni 2009). No information on comedication was available for 82 of the trials.
Some form of co‐therapy was part of the intervention in five of the trials (Döpfner 2004; Fabiano 2007; Kolko 1999; Pelham 2014; Waxmonsky 2008). One trial had a therapy phase prior to medication (Kollins 2006 (PATS)), and six trials specified limitations to therapy during the study (Brams 2012; Froehlich 2018; Lufi 1997; Muniz 2008; Silva 2006; Silva 2008). The remaining 145 trials stated nothing about co‐therapy.
Interventions
Thirty‐two of the trials used extended‐ and modified‐release methylphenidate and 83 of the trials used immediate‐release methylphenidate. Nine trials used both immediate‐ and extended‐release methylphenidate (Döpfner 2004; Johnston 1988; Fitzpatrick 1992a; Pearson 2013; Pelham 1990a; Pelham 2001a; Schachar 2008; Swanson 2002b; Wigal 2003), four trials used two different types of extended‐release methylphenidate (Lopez 2003; Schulz 2010; Silva 2005a; Swanson 2004b), one trial used three different types of extended‐release methylphenidate (NCT02536105), and five trials used transdermal methylphenidate patches (McGough 2006; Pelham 2005; Pelham 2011; Wilens 2008; Wilens 2010). It was unclear what type of methylphenidate the remaining 28 trials used.
The method of reporting the dose of methylphenidate varied considerably between trials, and the dose administered to participants was unclear for 20 trials. Overall daily dose ranged from 4 mg to 72 mg, with a mean reported total daily dose of 24.6 mg or 0.77 mg/kg/day. Doses of immediate‐release methylphenidate ranged from 4 mg to 50 mg, with a mean reported total daily dose of 21.4 mg or 0.8 mg/kg. Doses of extended‐release methylphenidate ranged from 15 mg to 72 mg, with a mean reported total daily dose of 35.2 mg or 1.1 mg/kg. The duration of methylphenidate treatment ranged from 1 to 56 days, with an average duration of 15.2 days.
All trials used a placebo as a control.
In six trials, participants received some kind of comedication in both the intervention and control groups (antidepressant: Carlson 1995; divalproex sodium: Findling 2007; continuation of stable antiepileptic medication: Gonzalez‐Heydrich 2010; diphenhydramine 50 mg: Kaplan 1990; marijuana and/or cocaine: Szobot 2008; an antipsychotic: Zeni 2009).
In five trials some form of therapy was part of the intervention in both the intervention and control groups (Döpfner 2004; Fabiano 2007; Kolko 1999; Pelham 2014; Waxmonsky 2008).
Outcomes
Some psychometric ADHD instruments measured the total score for ADHD symptoms, whereas others assessed only specific symptom domains of ADHD (e.g. inattention, hyperactivity, impulsivity). We categorised all scales into five subgroups: ADHD symptoms; serious adverse events; non‐serious adverse events; general behaviour; and quality of life. Some psychometric instruments are abbreviated versions or revised versions, but all have been validated.
ADHD symptoms
Conners' questionnaires were the most frequently used measures of ADHD symptoms; more than 30 different versions measured core symptoms of ADHD (normative data are generally well intercorrelated in revised versions; Goyette 1978).
Table 5 presents the list of all measures that the included trials used to assess ADHD symptoms. This list primarily refers to original articles describing the psychometric properties of measurement scales, but in a few cases, we refer to trials describing the use of a specific measurement scale.
4. ADHD symptoms rating scales.
| Name of scale | Abbreviation | Reference |
| Abbreviated Conners’ Rating Scales, Parent (ACPRS) and Teacher (ACTRS), including Abbreviated Parent Rating Scale (APRS) and Teacher Rating Scale, Hyperkinesis Index and ADHD and Emotional Lability subscales |
ACRS | Conners 1997a |
| Abbreviated Symptom Questionnaire, including ASQ Teacher and ASQ Parent | ASQ | Conners 1995 |
| Academic Performance Rating Scale | APRS | DuPaul 1991a |
| The ADD/H Comprehensive Teacher Rating Scale | ACTeRS | Ullmann 1984 |
| ADHD/ODD Rating Scale, Parent‐ and Teacher‐Rated | ADHD‐RS | Barkley 1998 |
| ADHD Rating Scale, including ADHD Rating Scale Parent and Teacher Ratings | ADHD‐RS | DuPaul 1991a |
| ADHD Rating Scale‐IV, including ADHD Rating Scale‐IV Parent and Teacher Versions | ADHD‐RS‐IV | DuPaul 1991a |
| Brief Psychiatric Rating Scale for Children | BPRS | Gale 1986 |
| Child Attention Problems Rating Scale | CAP | Achenbach 1986 |
| Child Attention Profile | CAP | Barkley 1988b |
| Child Behavior Rating Form | NCBHF | Aman 1996 |
| Child Symptom Inventory | CSI | Gadow 1994 |
| Children’s Psychiatric Rating Scale | CPRS | Pfefferbaum‐Levine 1983 |
| Conners’ Abbreviated Hyperactivity Questionnaire | C‐HI | Conners 1997a |
| Conners’ Abbreviated Questionnaire | ASQ | Conners 1995 |
| Conners’ Abbreviated Parent Teacher Questionnaire | APTQ | Rowe 1997 |
| Conners’ Abbreviated Rating Scale | ABRS | Conners 1997a |
| Conners’ Abbreviated Symptom Questionnaire | ASQ | Conners 1995 |
| Conners Abbreviated Symptom Questionnaire for Parents | ASQ‐Parent | Conners 1995 |
| Conners’ Abbreviated Symptom Questionnaire for Teachers | ASQ‐Teacher | Conners 1997a |
| Conners’ Abbreviated Teacher Rating Scale | ABTRS | Conners 2001 |
| Conners’ ADHD/DSM‐IV Scales Adolescent | CADS‐A | Conners 1997b |
| Conners’ ADHD/DSM‐IV Scales Parent | CADS–P, CADS‐P DSM‐IV | Conners 1997a |
| Conners’ ADHD/DSM‐IV Scale Teacher, including Inattentive and Hyperactive‐Impulsive subscales | CADS‐T, CADS‐T DSM‐IV | Conners 1997a |
| Conners’ Rating Scale ‐ Revised, Parent and Teacher: Hyperactivity and Conduct Factors score | CPRS‐R and CTRS‐R | Goyette 1978 |
| Conners’ Hyperactivity Index, Parent and Teacher, including abbreviated versions | CPRS/CTRS‐Hyperactivity index | Conners 1997a |
| Conners’ Hyperkinesis Index | ‐ | Milich 1980 |
| Conners, Loney and Milich Scale | CLAM | Milich 1980 |
| Conners’ Parent and Teacher Rating Scale ‐ Revised, Short Form | CRS‐R:S | Conners 1997a |
| Conners’ Parent Rating Scale, including abbreviated versions | CPRS | Conners 1998b |
| Conners’ Parent Rating Scale ‐ Revised | CPRS‐R | Conners 1997a |
| Conners’ Parent Rating Scale ‐ Revised, Short Form | CPRS‐R:S | Conners 1997a |
| Conners’ Parent Rating Scale ‐ Revised, Long Version | CPRS‐R:L | Conners 1997a |
| Conners’ Rating Scale ‐ Revised | CRS‐R | Conners 1997a |
| Conners’ Short Form Rating Scale, Parent and Teacher | ‐ | Conners 1997a |
| Conners’ Teacher Rating Scale | CTRS | Conners 1998a |
| Conners’ Teacher Rating Scale ‐ Revised, Long Version | CTRS‐R:L | Conners 1998a |
| Diagnostic and Statistical Manual of Mental Disorders Total | DSM‐IV | APA 1994 |
| Diagnostiksystem für Psychische Störungen im Kindes ‐ und Jugendalter nach ICD‐10 und DSM‐IV Parental Questionnaire of ADHD symptoms |
DISYPS | Döpfner 2000 |
| Fremdbeurteilungsbogen für Hyperkinetische Störungen | FBB‐HKS | Döpfner 2008 |
| German Teacher’s report on ADHD symptoms | FBB‐HKS of the DISYPS | Döpfner 2000 |
| Hyperactivity Index of the Revised Conners Parent and Teacher Rating Scales | ‐ | Goyette 1978 |
| IOWA Conners Parent Rating Scale, including abbreviated versions | IOWA CPRS | Loney 1982 |
| IOWA Conners Teacher Rating Scale, including abbreviated versions | IOWA CTRS | Loney 1982 |
| IOWA Conners Teacher Rating Scale, Inattention/Overactivity (I/O) and Oppositional/Defiant (O/D) subscales | IOWA‐I/O and O/D subscales | Loney 1982 |
| IOWA Inattention/Overactivity and Aggression/Noncompliance scales ‐ Parent and Teacher rating | IOWA | Loney 1982 |
| Lehrer‐Fragenbogen von Steinhausen | LF | Steinhausen 1993 |
| Loney’s Time on Task Scale, Hyperactivity, Attention and Aggression subscales | TOTS | Fitzpatrick 1992b |
| Modified Conner Scale Parent and Teacher | ACR | Conners 1997a |
| Mothers’ Objective Method for Subgrouping | MOMS | Loney 1984 |
| Parent Symptom Checklist | PSC ADHD | Döpfner 2000 |
| Parental Account of Children’s Symptoms | PACS | Chen 2006 |
| Restricted Academic Situation Scale | RASS | Fischer 1998 |
| Schedule for Affective Disorders and Schizophrenia | K‐SADS/ K‐SADS‐E for diagnosis | Chambers 1985 |
| Swanson, Nolan, and Pelham ‐ IV SNAP‐ADHD Rating scale | SNAP‐ADHD | Swanson 1992 |
| Swanson, Nolan, and Pelham ‐ IV SNAP‐IV (Brazilian Version) | SNAP‐IV | Clark 1993; Clark 1996 |
| Swanson, Kotkin, Atkins, M‐Flynn, Pelham Scale (SKAMP combined, SKAMP attention, and SKAMP deportment) | SKAMP (SKAMP combined, SKAMP attention, and SKAMP deportment) | Wigal 1998; Murray 2009 |
| Teacher Self‐control Rating Scale | SCRS | Kendall 1979 |
| Turgay ‐ DSM‐IV Scale, Parent | T‐DSM‐IV Scale, Parent | Turgay 1994; Ercan 2001 |
| Turgay ‐ DSM‐IV Scale, Teacher | T‐DSM‐IV Scale, Teacher | Turgay 1994; Ercan 2001 |
| Teacher Hyperactivity Index | THI | Achenbach 1991b |
| Teacher Symptom Checklist | TSC | Döpfner 2000 |
| Vanderbilt ADHD Rating Scale | VADP(T)RS | Wolraich 2003 |
| Wender Utah Rating Scale | WURS | Ward 1993 |
| Wide Range Achievement Test | WRAT‐4 | Wilkinson 2006 |
| Wide Range Achievement Test Revised | WRAT‐R | Woodcock 2001 |
| ADD/H: Attention deficit disorder/with hyperactivity; ADHD: Attention deficit hyperactivity disorder; DSM‐IV:Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; ICD‐10:International Classification of Diseases, Tenth Edition; ODD: ODD | ||
Serious and non‐serious adverse events
Trials used rating scales or spontaneous reports to measure adverse events, or they were recorded by investigators at regular interviews or visits, or both. Some trials included physical examinations or paraclinical examinations, or both, such as blood testing, electrocardiogram, blood pressure reading, measurement of heart rate and assessment of weight and height. We recorded serious adverse events in accordance with the ICH classification (ICH 1996). However, when in doubt, we asked trial authors which classification or definition they had used in their trial.
Some trials combined all of the above modes of measurement; others used a single measure such as spontaneous reports or rating scales. Sixty‐eight trials employed rating scales; the Barkley Side Effect Rating Scale (SERS) was used most frequently (Barkley 1990).
Other scales used included the Significant Adverse Event Reviews Questionnaire (SAERS; Barkley 1990; Zeni 2009), the Pittsburgh Side Effect Rating Scale (PSERS; Pelham 1993b; Pelham 2005a) and Subject’s Treatment Emergent Symptom Scale (STESS; Guy 1976a).
For the purpose of measuring specific adverse events, some trials used rating scales such as Paediatric Sleep Questionnaire (PSQ; Chervin 2000), Sleep Disturbances Scale for Children (SDSC; Bruni 1996), Children’s Sleep Habits Questionnaire (CSHQ; Owens 2000), Childrens' Depression Rating Scale (CDRS‐R; Poznanski 1983), The Colombia Suicide Severity Rating Scale (C‐SSRS, Posner 2011), Young Mania Rating Scale (YMRS; Young 1978), Yale Global Tic Severity Scale (YGTSS; Leckman 1989), Tic Symptom Self Report Scale (TSSR; Leckman 1988), and the Massachusetts General Hospital (MGH) Abuse and Diversion Questionnaire (Wilens 2006a).
General behaviour
Trials used many different scales to assess general behaviour. These scales have different foci, such as aggression or oppositional behaviour, but all describe participants’ behaviour and the influence of methylphenidate. Higher scores on general behaviour symptom scales signify better outcomes.
Table 6 presents the list of all measures used to assess general behaviour. This list refers primarily to the original articles describing the psychometric properties of measurement scales used to measure general behaviour in the included trials. In a few cases, we refer to trials that describe the use of a specific measurement scale.
5. General behaviour rating scales.
| Name of scale | Abbreviation | Reference |
| Achenbach Child Behaviour Checklist | CBCL | Achenbach 1991a |
| Achenbach’s Teacher Report | ATRF | Achenbach 1991b; Achenbach 2001 |
| ADHD Rating Scale | ADHD‐RS | DuPaul 1991a |
| ADHD School Observation Code | ADHD‐SOC | Gadow 1996 |
| Barkley Scales, Disruptive Behavior Disorders Rating Scale | ‐ | Barkley 1991a |
| Before School Functioning Questionnaire | BSFQ | Faraone 2018 |
| Behavior Rating Inventory of Executive Function | BRIEF | Gioia 2000 |
| Child Attention Problems Scale | CAP | Barkley 1991 |
| Child Attention Profile | CAP | Barkley 1988b |
| Child Behavior Checklist | CBCL | Achenbach 1991a |
| Child Health Questionnaire | CHQ | Landgraf 1998 |
| Child and Adolescent Psychiatric Assessment, selected items | CAPA | Angold 1995 |
| Children’s Psychiatric Rating Scale | CPRS | Fish 1985 |
| Classroom Observation Code (Abikoff Classroom Observational System) | COC | Abikoff 1980 |
| Code for Observing Social Activity | COSA | Sprafkin 1986 |
| Conners' Child Behavior Scale | UC‐CCBS | Ladd 1996 |
| Conners Early Childhood Behavior—Parent Short Response scale | ‐ | Conners 2009 |
| Conners' Global Index Scale | CGI‐S | Conners 1998a |
| Conners’ Global Index ‐ Parent | CGI‐P | Conners 1997a |
| Conners' Global Index ‐ Teacher | CGI‐T | Conners 1998a |
| Conners', Loney and Milich Scale | CLAM | Milich 1980 |
| Conners’ Parent Questionnaire | CPQ | Conners 1995 |
| Conners’ Parent Rating Scale | CPRS | Conners 1998b |
| Conners’ Teacher Rating Scale | CTRS | Conners 1998a |
| Conners’ Teacher Rating Conduct Problems | ‐ | Miller 1997 |
| Disruptive Behavior Disorders Rating Scale, Parent‐ and Teacher‐Rated | DBS | Mendelsohn 1978 |
| Disruptive Behavior Disorders Rating Scale | DBD | Silva 2005b |
| Groninger Behaviour Observation Scale | GOO and GBO | Van der Meere 1999b |
| Groninger Behaviour Checklists, Parent and Teacher Versions of the abbreviated Groninger | GGGS and GGBS | Van der Meere 1999b |
| Hillside Behavior Rating Scale | HBRS | Gittleman‐Klein 1976 |
| Home Situations Questionnaire | HSQ | Barkley 1987 |
| Home Situations Questionnaire ‐ Revised | HSQ‐R | DuPaul 1992 |
| Humphrey’s Teacher Self‐Control Rating Scale | TSCRS | Humphrey 1982 |
| Hyperactivity Index from the Conners Revised Teacher Rating Scale | CTRS‐R‐Hyperactivity Index | Goyette 1978 |
| Impairment Rating Scale | IRS | Fabiano 2006 |
| Inpatient Global Rating Scale, Revised | IGRS | Conners 1985 |
| Inpatient Global Rating Scale, Somatic factor | IGRS‐S | Conners 1985 |
| IOWA Conners' Rating Scale, Oppositional/Defiant (O/D) subscales | IOWA‐O/D subscales | Loney 1982 |
| Nisonger Child Behavior Rating Form | NCBRF | Aman 1996 |
| Paired Associates Learning | PAL | Wechsler 1945 |
| Parent Global Assessment for Improvement | PGA | McGough 2006a |
| Parent Rating of Evening and Morning Behavior‐Revised, Morning | PREMP‐R AM | Sutton 2003 |
| Parent Rating of Evening and Morning Behavior‐Revised, Evening | PREMP‐R PM | Sutton 2003 |
| Peer Conflict Scale | PCS | Marsee 2007 |
| Personality Inventory for Children | PIC | Lachar 1986 |
| School Situations Questionnaire | SSQ | Barkley 1987 |
| School Situations Questionnaire ‐ Revised | SSQ‐R | DuPaul 1992 |
| Retrospective Modified Overt Aggression Scale | R‐MOAS | Bladder 2009 |
| Strengths and Weaknesses of ADHD Symptoms and Normal Behavior Scale, Parent and Teacher | SWAN | Swanson 2006; Polderman 2007 |
| Subjective Treatment Emergent Symptom Scale | STESS‐R | Guy 1976 |
| Swanson, Nolan and Pelham, Fourth Edition | SNAP‐IV | Bussing 2008 |
| Teachers Report Form | TRF | Achenbach 1991b |
| Telephone Interview Probe (Parent and Teacher) | TIP | Corkum 2007 |
| Vanderbilt ADHD rating scales: Vanderbilt ADHD Diagnostic Parent Rating Scale and Vanderbilt ADHD Diagnostic Teacher Rating Scale | VADPRS and VADTRS | Wolraich 2003 |
| Wahler, House and Stambaugh’s Ecobehavioral Assessment System | ECO | Wahler 1976 |
| The Weekly Parent Ratings of Evening and Morning Behaviour | WREMB‐R | Kelsey 2004 |
| Werry‐Weiss‐Peters Activity Rating Scale | WWP | Routh 1978 |
| Woodcock‐Johnson Achievement Battery | WJ‐III Ach | Woodcock 2001 |
| ADHD: attention deficit hyperactivity disorder | ||
Quality of life
Seven scales measured quality of life in relation to both ADHD and life in general. For all scales, higher values equated to better health. Only four could be used in meta‐analyses: Child Health Questionnaire (CHQ; Landgraf 1998); Children's Global Assessment Scale (CGAS; Shaffer 1983); Child Health and Illness Profile, Child Edition: Parent Report Form (CHIP‐CE: PRF; Riley 2004); and The Parent‐ and Child‐rated Revised Questionnaire for Children and Adolescents to record health‐related quality of life (KINDL‐R; Ravens‐Sieberer 1998).
See Table 7 for additional information on the types of rating scales used to assess the quality of life in the included trials.
6. Quality of life ratings scales.
| Name of scale | Abbreviation | Reference |
| ADHD Impact Module‐Child | AIM‐C | AIM‐C 2013 |
| Child Impact Scale and Home Impact Scale | CIS/HIS | Landgraf 2002 |
| Child Health and Illness Profile, Child Edition: Parent Report Form | CHIP‐CE:PRF | Riley 2004 |
| Child Health Questionnaire | CHQ‐P | Landgraf 1998 |
| Children's Global Assessment Scale | CGAS | Shaffer 1983 |
| Comprehensive Psychopathological Rating Scale | CPRS | Aasberg 1978 |
| Health Utilities Index ‐ 2 | HUI‐2 | Torrance 1982 |
| The parent‐ and child‐rated Revised questionnaire for Children and adolescents to record health‐related quality of life | KINDL‐R | Ravens‐Sieberer 1998 |
| ADHD: attention deficit hyperactivity disorder | ||
Excluded studies
In the previous review we excluded 691 full‐text reports for reasons reported in Storebø 2015a [https://revman.cochrane.org/#/700705021509502610/dashboard/htmlCompare/current/4.36.11?version1WithProductionChanges=false&version2WithProductionChanges=false#REF‐Storeb_x00f8_‐2015a]. For this update, we excluded an additional 254 full‐text reports, which were ineligible for the following reasons: ineligible study design (94 reports), ineligible intervention (40 reports), ineligible comparator (31 reports), study not executed (8 reports), irrelevant supplement (11 reports), ineligible population (13 reports), other reasons (1 report).
The remaining 47 trials (from 56 reports) initially seemed to meet our eligibility criteria but on closer inspection did not, because they examined the impact of methylphenidate on very specific domains that were outside the focus of this review such as motor co‐ordination, reaction time, memory tasks, and reading skills. We added these to the Characteristics of excluded studies [https://revman.cochrane.org/#/700705021509502610/dashboard/htmlCompare/current/4.36.11?version1WithProductionChanges=false&version2WithProductionChanges=false#CHARACTERISTICS_OF_EXCLUDED_STUDIES], which now lists 125 trials. For more information on these trials, please see Characteristics of excluded studies.
Studies awaiting classification
Two trials (Drtílková 1997; Wang 2020), are currently awaiting classification for various reasons. Drtílková 1997 required translation from the Czech language into English, which we were unable to procure within the time frame of working with the update of this review. The other trial Wang 2020 was unavailable to us with all institutional library IDs in our possession. We contacted the trial authors in an attempt to retrieve their report or further information on outcome data but did not receive any reply. For further details on each trial see Studies awaiting classification.
Ongoing trials
We included 16 ongoing trials assessing methylphenidate for children and adolescents with ADHD but for which outcome data have not yet been made available. Ten trials have a parallel design (ChiCTR1800014945; EUCTR2007‐004664‐46‐NL; EUCTR2008‐001291‐71‐DE; IRCT138804132000N2; IRCT201701131556N94; IRCT20190317043079N; NCT00414921; NCT00485550; NCT02807870; Verlaet 2017), and six have a cross‐over design (EUCTR2008‐004425‐42‐NL; EUCTR2020‐003660‐11‐NL; Müller 2021; NCT00141050; NCT00254878; NCT00446537). See Ongoing studies for further information regarding each trial.
Risk of bias in included studies
We assessed the risk of bias of each included trial using the Cochrane risk of bias tool (RoB 1; Higgins 2011). A summary of our assessment is displayed in Figure 2 and Figure 3. One trial, Kollins 2006 (PATS), includes a parallel as well as a cross‐over phase and accounts for a low risk of bias trial among both parallel and cross‐over trials. As shown, we assessed 13 of the 157 cross‐over trials (8.3%) and nine of the 56 included parallel‐group trials (16.1%) at low risk of bias in all domains apart from blinding. However, even the 21 trials (here, counting Kollins 2006 (PATS) as a single trial) likely had breaks in their blinding due to prevalent adverse events due to methylphenidate (see below). We assessed the remaining 191 trials (90.1%) at high risk of bias. Accordingly, we judged all 212 trials to be trials at high risk of bias.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included trial
We assessed nine of the 56 included parallel trials at low risk of bias in all bias domains (Childress 2020a; Jacobi‐Polishook 2009; Kollins 2006 (PATS); Lehmkuhl 2002; Pliszka 2017; Riggs 2011; Schrantee 2016; Tourette's Syndrome Study Group 2002; Weiss 2021). However, we still considered these trials at risk of deblinding due to prevalent adverse events (see below).
Thirteen of the 157 included cross‐over trials were at low risk of bias in all bias domains (Cook 1993; DuPaul 1996; Flapper 2008; Kollins 2006 (PATS); McGough 2006; Moshe 2012; Rapport 2008; Soleimani 2017; Stein 1996; Stein 2011; Waxmonsky 2008; Wilkison 1995; Zeni 2009). However, even these were considered at risk of deblinding due to prevalent adverse events (see below).
Allocation
Parallel trials
Random sequence generation
We considered 34 trials to be at low risk of bias for random sequence generation, four trials to be at high risk of bias (Connor 2000; Green 2011; Heriot 2008; Tannock 2018), and 18 at unclear risk of bias.
Allocation concealment
We considered 26 trials to be at low risk of bias for allocation concealment (often because medications and packaging were identical in appearance for blinding purposes) and two trials to be at high risk of bias (Barragán 2017; Green 2011). Twenty‐eight trials did not report allocation concealment in sufficient detail to allow us to make a judgement, so were at unclear risk.
Cross‐over trials
Random sequence generation
We considered 64 trials to be at low risk for random sequence generation, nine trials at high risk of bias (Carlson 1995; Fitzpatrick 1992a; Kaplan 1990; Kelly 1989; Manos 1999; McBride 1988a; Szobot 2008; Tirosh 1993b; Wigal 2014), and 84 trials at unclear risk of bias.
Allocation concealment
We considered 57 trials to be at low risk of bias for allocation concealment (often because medications and packaging were identical in appearance for blinding purposes), five trials at high risk of bias (Carlson 1995; Fitzpatrick 1992a; Szobot 2008; Ullmann 1985; Wigal 2014), and 95 trials did not sufficiently report allocation concealment so we judged them at unclear risk of bias.
Blinding
Parallel trials
We considered that 40 trials adequately described their method of blinding of participants and personnel so we judged them to be at low risk of bias. Ten trials gave insufficient information about their methods so we judged them to be at unclear risk of bias (Biederman 2003b; Childress 2009; Childress 2017; Childress 2020b; Childress 2020c; Findling 2010; Greenhill 2006; Lin 2014; Tucker 2009; Wigal 2017). Six trials were not blinded (Barragán 2017; Brown 1985; Duric 2012; Jensen 1999 (MTA); NCT00409708; Perez‐Alvarez 2009), so we judged them to be at high risk of bias.
We considered that 34 trials adequately described their method of blinding of outcome assessment so we judged them to be at low risk of bias. Seventeen trials gave insufficient information about their methods and were considered to be at unclear risk of bias. Six trials did not include blinded outcome assessors (Barragán 2017; Brown 1985; Duric 2012; Jensen 1999 (MTA); NCT00409708; Perez‐Alvarez 2009), so we judged them to be at high risk of bias.
Cross‐over trials
We judged that 118 trials adequately described their method of blinding of participants and personnel so we therefore judged them at low risk of bias. Thirty‐one trials were considered at unclear risk of bias. Eight trials were not blinded (Lopez 2003; Manos 1999; Pearson 2013; Pelham 2014; Ramtvedt 2013; Stein 2003; Ullmann 1985; Wigal 2013), so we judged them to be at high risk of bias.
We judged that 86 trials adequately described their method of blinding of outcome assessment and were therefore considered at low risk of bias, and 66 trials were considered at unclear risk of bias. Five trials did not include blinded outcome assessors (Cox 2006; Douglas 1986; Manos 1999; Wigal 2013; Wodrich 1998), so we judged them to be at high risk of bias.
Incomplete outcome data
Parallel trials
Thirty trials adequately addressed incomplete data and were considered at low risk of bias. Twenty trials did not so we judged them to be at high risk of bias. Six trials gave insufficient information for us to assess whether the method they used to handle missing data was likely to bias the estimate of effect (Childress 2020b; Coghill 2013; Firestone 1981; NCT02293655; Palumbo 2008; Wigal 2004), and we therefore considered them at unclear risk of bias.
Cross‐over trials
Eighty‐five trials adequately addressed incomplete data so we judged them to be at low risk of bias. Forty‐eight trials gave insufficient information to assess whether the method they used to handle missing data was likely to bias the estimate of effect so we judged them to be at unclear risk of bias. Twenty‐four trials had incomplete outcome data and were therefore considered at high risk of bias.
Selective reporting
Parallel trials
Thirty‐four trials reported all pre‐defined or otherwise expected outcomes so we judged them at low risk of bias. Five trials did not (Childress 2020b; Greenhill 2006; Lin 2014; NCT02293655; Wigal 2015), and these were considered at high risk of bias. In 17 trials it was unclear whether trial authors reported all pre‐defined or otherwise expected outcomes (Arnold 2004; Barragán 2017; Biederman 2003b; Brown 1985; Butter 1983; Findling 2006; Firestone 1981; Greenhill 2002; Heriot 2008; Horn 1991; Ialongo 1994; Schachar 1997a; Szobot 2004; Tannock 2018; Tucker 2009; Wigal 2004; Wolraich 2001), so we judged them at unclear risk of bias.
Cross‐over trials
Forty‐seven trials reported all pre‐defined or otherwise expected outcomes so we judged them at to be at low risk of bias. Thirteen trials did not so these were considered at high risk of bias (Castellanos 1997; Chacko 2005; CRIT124US02; Froehlich 2018; Gonzalez‐Heydrich 2010; Gorman 2006; Hawk 2018; Huang 2021; McInnes 2007; NCT02536105; Stein 2003; Taylor 1993; Wallace 1994). In 97 trials it was unclear whether trial authors reported all pre‐defined or otherwise expected outcomes so we judged them at unclear risk of bias.
Other potential sources of bias
We identified no other potential sources of bias for either parallel or cross‐over trials.
Effects of interventions
See: Table 1
Below, we present the results of meta‐analyses performed for the comparison methylphenidate versus placebo or no intervention for two primary outcomes (ADHD symptoms and serious adverse events) and three secondary outcomes (non‐serious adverse events, general behaviour, and quality of life). Twenty‐nine parallel‐group trials (50%) and 60 cross‐over trials (38.2%) excluded methylphenidate non‐responders, placebo responders or patients with methylphenidate adverse events before randomisation. The subgroup analyses on enrichment designs compared to no enrichment designs are described in each outcome section below. For a summary of key results, please see Table 1.
Primary outcomes
ADHD symptoms
We were able to combine data on ADHD symptoms from 47 parallel‐group trials and 82 cross‐over trials, of which five also provided first‐period data.
Teacher‐rated ADHD symptoms
Parallel‐group trials and cross‐over trials (end of first‐period data only)
A meta‐analysis showed a difference in effects between methylphenidate and placebo on teacher‐rated ADHD symptoms favouring methylphenidate (SMD −0.74, 95% CI −0.88 to −0.61; I² = 38%; 21 trials, 1728 participants; Analysis 1.1). The SMD of −0.74 for ADHD symptoms corresponds to a mean difference (MD) of −10.58 points (95% CI −12.58 to −8.72) on the ADHD Rating Scale (DuPaul 1991a). This is an effect above the minimal relevant difference (MIREDIF; Zhang 2005).
1.1. Analysis.

Comparison 1: Teacher‐rated ADHD symptoms, Outcome 1: All parallel‐group trials and first‐period cross‐over trials
-
Subgroup analyses
We found that types of scales used influenced the intervention effect of methylphenidate (test for subgroup differences: Chi² = 24.94, df = 10 (P = 0.005), I² = 59.9%; Analysis 1.2). The differences between the scale that influenced the effect most and least was more than SMD −0.5.
We found lower effect of methylphenidate in long‐term trials (SMD −0.47, 95% CI −0.72 to −0.22; 1 trial, 253 participants) compared to that of short‐term trials (SMD −0.77, 95% CI −0.91 to −0.64; I² = 30%; 20 trials, 1475 participants). Test for subgroup differences showed Chi² = 4.26, df = 1 (P = 0.04), I² = 76.5%; Analysis 1.3. The SMD effect of −0.47 for ADHD long‐term trials corresponds to an MD of −6.72 points (95% CI −10.3 to −3.15) on the ADHD‐RS (DuPaul 1991a). This is an effect just above the MIREDIF (Zhang 2005).
-
No evidence suggested that any of the following influenced the estimated intervention effect:
the risk of bias (test for subgroup differences: Chi² = 0.13, df = 1 (P = 0.71), I² = 0%; Analysis 1.1)
dose (test for subgroup differences: Chi² = 3.15, df = 2 (P = 0.21), I² = 36.5%; Analysis 1.4)
medication status before randomisation (test for subgroup differences: Chi² = 0.59, df = 1 (P = 0.44), I² = 0%; Analysis 1.5)
enrichment design (test for subgroup differences: Chi² = 0.07, df = 1 (P = 0.79), I² = 0%; Analysis 1.6)
trial design (parallel‐group trials compared to first‐period cross‐over trials, test for subgroup differences: Chi² = 0.71, df = 1 (P = 0.40), I² = 0%; Analysis 1.7)
vested interest (test for subgroup differences: Chi² = 2.64, df = 1 (P = 0.10), I² = 62.1%; Analysis 1.8) or
type of control group (test for subgroup differences: Chi² = 0.59, df = 1 (P = 0.44), I² = 0%; Analysis 1.9)
Inspection of the funnel plot in Figure 4 suggested potential bias (asymmetry), although we found no evidence of significant publication bias: Egger’s regression intercept (bias) was −0.2260 (two‐tailed, P = 0.81).
One of the trials in this meta‐analysis used change‐from‐baseline scores (Palumbo 2008), but removing this trial did not significantly change the estimate.
1.2. Analysis.

Comparison 1: Teacher‐rated ADHD symptoms, Outcome 2: Subgroup analysis: types of scales
1.3. Analysis.

Comparison 1: Teacher‐rated ADHD symptoms, Outcome 3: Subgroup analysis: duration of treatment
1.4. Analysis.

Comparison 1: Teacher‐rated ADHD symptoms, Outcome 4: Subgroup analysis: dose
1.5. Analysis.

Comparison 1: Teacher‐rated ADHD symptoms, Outcome 5: Subgroup analysis: medication status ‐ medication naive versus not medication naive
1.6. Analysis.

Comparison 1: Teacher‐rated ADHD symptoms, Outcome 6: Subgroup analysis: trials with enrichment design compared with trials without enrichment design
1.7. Analysis.

Comparison 1: Teacher‐rated ADHD symptoms, Outcome 7: Subgroup analysis: parallel‐group trials compared with first‐period cross‐over trials
1.8. Analysis.

Comparison 1: Teacher‐rated ADHD symptoms, Outcome 8: Subgroup analysis: vested interest
1.9. Analysis.

Comparison 1: Teacher‐rated ADHD symptoms, Outcome 9: Subgroup analysis: type of control group
4.

Funnel plot of comparison 1. Teacher‐rated ADHD symptoms, outcome: 1.8 All data at low and high risk of bias (parallel‐group and cross‐over trials)
We assessed the evidence to be of very low certainty (see GRADE assessment below). Therefore we are uncertain that the estimated effect accurately reflects the true effect, and the addition of more data could change the findings.
Cross‐over trials (end of last period)
Meta‐analysis suggested a difference in effect between methylphenidate and placebo on teacher‐rated ADHD symptoms favouring methylphenidate (SMD −0.88, 95% CI −1.01 to −0.75; I² = 82%; 64 trials, 6341 participants; Analysis 1.10).
1.10. Analysis.

Comparison 1: Teacher‐rated ADHD symptoms, Outcome 10: Cross‐over trial (endpoint data)
-
Subgroup analyses
The estimated intervention effect varied according to risk of bias (test for subgroup differences: Chi² = 4.76, df = 1 (P = 0.03), I² = 79.0%; Analysis 1.10), and dose of methylphenidate (test for subgroup differences: Chi² = 4.12, df = 1 (P = 0.04), I² = 75.7%; Analysis 1.11). Three of the trials included some participants with an IQ less than 70 (Pearson 2013; Smith 1998; Taylor 1987). Removing these trials from the analyses did not significantly change the results.
1.11. Analysis.

Comparison 1: Teacher‐rated ADHD symptoms, Outcome 11: Subgroup analysis: cross‐over trials (endpoint data): dose
Parallel‐group trials and cross‐over trials (end of first period) and cross‐over trials (end of last period)
Meta‐analysis suggested a difference in effects between methylphenidate and placebo on reduced teacher‐rated ADHD symptoms favouring methylphenidate (SMD −0.82, 95% CI −0.87 to −0.77; I² = 78%; 81 trials, 7564 participants; Analysis 1.12).
1.12. Analysis.

Comparison 1: Teacher‐rated ADHD symptoms, Outcome 12: Subgroup analysis: all parallel‐group trials and first‐period cross‐over trials compared with cross‐over trials (endpoint data)
-
Subgroup analyses
No evidence suggested that the intervention effect varied according to trial design (parallel and first period cross‐over compared to cross‐over trials end of last period; test for subgroup differences: Chi² = 3.41, df = 1 (P = 0.06), I² = 70.6%; Analysis 1.12).
No evidence suggested that the intervention effect varied according to the risk of bias assessment in subgroups (low risk of bias compared to high risk of bias; test for subgroup differences: Chi² = 2.13, df = 1 (P = 0.14), I² = 53.0%; Analysis 1.13).
Nor did it vary according to high or low risk of vested interest (test for subgroup differences: Chi² = 0.02, df = 1 (P = 0.89), I² = 0%, Analysis 1.14).
1.13. Analysis.

Comparison 1: Teacher‐rated ADHD symptoms, Outcome 13: All parallel‐group trials and cross‐over trials: risk of bias
1.14. Analysis.

Comparison 1: Teacher‐rated ADHD symptoms, Outcome 14: All parallel‐group trials and cross‐over trials: vested interest
Independent assessor‐rated ADHD symptoms
Most independent assessors were clinicians.
Parallel‐group trials and cross‐over trials (end first‐period data only)
A meta‐analysis suggested there was a difference in effect between methylphenidate and placebo on independent assessor‐rated ADHD symptoms favouring methylphenidate (SMD −1.10, 95% CI −1.44 to −0.77; I² = 95%; 22 trials, 3724 participants; Analysis 2.1). The SMD effect of −1.10 for ADHD symptoms corresponds to an MD of −15.7 points (95% CI −14.7 to −7.9) on the ADHD‐RS (DuPaul 1991a). This is a clinical effect above the MIREDIF (Zhang 2005). Five trials reported change from baseline scores (Findling 2008; McCracken 2016; Newcorn 2008; Newcorn 2017a (flexible dose); Newcorn 2017b (forced dose)), but removing these trials did not significantly change the estimate. Two trials were outliers as they reported unrealistically high effect sizes. These were: Kollins 2021 and Wigal 2017. Removing these trials showed a SMD effect of −0.62 (95% CI −0.79 to −0.46). The SMD effect of −0.62 for ADHD symptoms corresponds to an MD of −8.86 points (95% CI −11.3 to −6.6) on the ADHD‐RS (DuPaul 1991a), which is a clinical effect above the MIREDIF (Zhang 2005).
2.1. Analysis.

Comparison 2: Independent assessor‐rated ADHD symptoms, Outcome 1: All parallel‐group trials and first‐period cross‐over trials
-
Subgroup analyses
We found lower effect of methylphenidate in trials at low risk of bias (SMD −0.40, 95% CI −0.78 to −0.03; I² = 86%; 4 trials, 942 participants), compared to trials at high risk of bias (SMD −1.30, 95% CI −1.70 to −0.89; I² = 96%; 18 trials, 2782 participants; test for subgroup differences: Chi² = 9.97, df = 1 (P = 0.002), I² = 90.0%; Analysis 2.1). The SMD effect of −0.40 in trials at low risk of bias corresponds to a MD of only −5.7 points (95% CI −10.4 to −0.4) on the ADHD‐RS (DuPaul 1991a). This is an effect below the MIREDIF (Zhang 2005).
Types of scales used (test for subgroup differences: Chi² = 16.05, df = 3 (P = 0.001), I² = 81.3%; Analysis 2.2).The differences between the scales that influenced the effect most and least was more than SMD −2.0.
We found lower effect of methylphenidate in long‐term trials (SMD −0.35, 95% CI −0.61 to −0.08; 1 trial, 221 participants) compared to short‐term trials (SMD −1.15, 95% CI −1.50 to −0.80; I² = 95%; 21 trials, 3503 participants; test for subgroup differences: Chi² = 12.82, df = 1 (P = 0.0003), I² = 92.2%; Analysis 2.3). The SMD effect of −0.35 for ADHD long‐term trials corresponds to an MD of only −5 points (95% CI −8.7 to −1.1) on the ADHD‐RS (DuPaul 1991a), which is a clinical effect below the MIREDIF (Zhang 2005).
We found larger effect of methylphenidate at high doses (SMD −0.84, 95% CI −1.13 to −0.55; I² = 95%; 17 trials, 3005 participants) compared to lower doses (SMD −0.19, 95% CI −0.52 to 0.15; 1 trial, 138 participants; test for subgroup differences: Chi² = 12.95, df = 2 (P = 0.002), I² = 84.6%; Analysis 2.4. Four trials comprised a subgroup of unknown dose (Findling 2008; Findling 2010; Kollins 2021; Taylor 1987). Including this subgroup in the analysis did not significantly change the subgroup differences between doses.
We found larger effects of methylphenidate in trials with enrichment design (SMD −1.24, 95% CI −1.61 to −0.87; I² = 95%; 19 trials, 3245 participants) compared to trials without enrichment designs (SMD −0.22, 95% CI −0.62 to 0.17; I² = 70%; 3 trials, 479 participants; test for subgroup differences: Chi² = 13.65, df = 1 (P = 0.0002), I² = 92.7%; Analysis 2.5).
We found larger effects of methylphenidate in trials with placebo control group (SMD −1.22, 95% CI −1.58 to −0.85; I² = 95%; 20 trials, 3200 participants) compared to trials with no‐intervention control groups (SMD −0.14, 95% CI −0.52 to 0.23; I² = 79%; 2 trials, 524 participants; test for subgroup differences: Chi² = 16.00, df = 1 (P < 0.0001); I² = 93.8%; Analysis 2.6).
No evidence suggested that trial design (parallel‐group trials compared to first‐period cross‐over trials) influenced the estimated intervention effect (test for subgroup differences: Chi² = 3.38, df = 1 (P = 0.07), I² = 70.4%; Analysis 2.7). There were not enough data to conduct a test of vested interest.
2.2. Analysis.

Comparison 2: Independent assessor‐rated ADHD symptoms, Outcome 2: Subgroup analysis: types of scales
2.3. Analysis.

Comparison 2: Independent assessor‐rated ADHD symptoms, Outcome 3: Subgroup analysis: duration of treatment
2.4. Analysis.

Comparison 2: Independent assessor‐rated ADHD symptoms, Outcome 4: Subgroup analysis: dose
2.5. Analysis.

Comparison 2: Independent assessor‐rated ADHD symptoms, Outcome 5: Subgroup analysis: trials with enrichment design compared with trials without enrichment design
2.6. Analysis.

Comparison 2: Independent assessor‐rated ADHD symptoms, Outcome 6: Subgroup analysis: type of control group
2.7. Analysis.

Comparison 2: Independent assessor‐rated ADHD symptoms, Outcome 7: Subgroup analysis: parallel‐group trials compared with first‐period cross‐over trials
Cross‐over trials (end of last period)
A meta‐analysis suggested a difference in effect between methylphenidate and placebo on independent assessor‐rated ADHD symptoms favouring methylphenidate (SMD −0.97, 95% CI −1.11 to −0.83; I² = 71%; 22 trials, 3854 participants; Analysis 2.8).
2.8. Analysis.

Comparison 2: Independent assessor‐rated ADHD symptoms, Outcome 8: Cross‐over trials (endpoint data)
-
Subgroup analyses
We assessed all 22 trials to be at high risk of bias, therefore we could not conduct a subgroup analysis.
The estimated intervention effect varied according to dose of methylphenidate, with a seemingly increased effect of a high dose (SMD −1.07, 95% CI −1.27 to −0.86; 13 trials, 2051 participants; I² = 77%) compared to a low dose (SMD −0.72, 95% CI −0.86 to −0.58; I²= 61%; 17 trials, 3067 participants; test for subgroup differences: Chi² = 8.86, df = 2 (P = 0.01); I² = 77.4%; Analysis 2.9). One trial comprised a subgroup of unknown dose (NCT02536105). Including this subgroup in the analysis did not significantly change the subgroup differences between doses.
2.9. Analysis.

Comparison 2: Independent assessor‐rated ADHD symptoms, Outcome 9: Subgroup analysis: cross‐over trials (endpoint data): dose
Parallel‐group trials and cross‐over trials (end of first period) and cross‐over trials (end of last period)
A meta‐analysis suggested there was a difference in effect between methylphenidate and placebo on independent assessor‐rated ADHD symptoms favouring methylphenidate (SMD −0.99, 95% CI −1.18 to −0.80; I² = 92%; 42 trials, 7277 participants; Analysis 2.10).
2.10. Analysis.

Comparison 2: Independent assessor‐rated ADHD symptoms, Outcome 10: Subgroup analysis: all parallel‐group trials and first‐period cross‐over trials compared with cross‐over trials (endpoint data)
-
Subgroup analyses
The risk of bias assessment influenced the estimated intervention effect when combining parallel and first‐period cross‐over data with end‐of‐last‐period cross‐over data, with a seemingly lower effect in the subgroup of trials assessed to be at low risk of bias (SMD −0.40, 95% CI −0.78 to −0.03; I² = 86%; 4 trials, 942 participants), compared to those assessed to be at high risk of bias (SMD −1.06, 95% CI −1.25 to −0.86; I² = 92%; 38 trials, 6335 participants; test for subgroup differences: Chi² = 9.02, df = 1 (P = 0.003), I² = 88.9%; Analysis 2.11).
We did not find any subgroup difference on intervention effect between trials at high or unclear risk of vested interest compared to trials at low risk of vested interest (test for subgroup differences: Chi² = 0.02, df = 1 (P = 0.89), I² = 0%; Analysis 2.12).
No evidence suggested that the intervention effect varied according to trial design (parallel and first‐period cross‐over compared to cross‐over trials end of last period; test for subgroup differences: Chi² = 1.42, df = 1 (P = 0.23), I² = 29.5%; Analysis 2.10).
2.11. Analysis.

Comparison 2: Independent assessor‐rated ADHD symptoms, Outcome 11: All parallel‐group trials and cross‐over trials: risk of bias
2.12. Analysis.

Comparison 2: Independent assessor‐rated ADHD symptoms, Outcome 12: All parallel‐group trials and cross‐over trials: vested interest
Parent‐rated ADHD symptoms
Parallel‐group trials and cross‐over trials (end of first‐period data only)
A meta‐analysis suggested there is a difference in effects between methylphenidate and placebo in parent‐rated ADHD symptoms favouring methylphenidate (SMD −0.63, 95% CI −0.76 to −0.50; I² = 58%; 27 trials, 2927 participants; Analysis 3.1). The SMD effect of −0.63 for ADHD symptoms corresponds to an MD of −9.0 points (95% CI −10.9 to −7.0) on the ADHD‐RS (DuPaul 1991a). This is a clinical effect above the MIREDIF (Zhang 2005). Three trials in the meta‐analysis reported change from baseline scores (Carlson 2007; Newcorn 2008; Tucker 2009), but removing these trials did not significantly change the estimate.
3.1. Analysis.

Comparison 3: Parent‐rated ADHD symptoms, Outcome 1: All parallel‐group trials and first‐period cross‐over trials
-
Subgroup analyses
Types of scales (test for subgroup differences: Chi² = 27.14, df = 11 (P = 0.004), I² = 59.5%; Analysis 3.2). The difference between the scales that influenced the effect most and least was more than SMD −0.5.
-
No evidence suggested that the following influenced the intervention effect:
risk of bias assessment (test for subgroup differences: Chi² = 1.56, df = 1 (P = 0.21), I² = 36.0%; Analysis 3.1)
duration of treatment (test for subgroup differences: Chi² = 0.27, df = 1 (P = 0.60), I² = 0%; Analysis 3.3)
dose of methylphenidate (test for subgroup differences: Chi² = 0.54, df = 2 (P = 0.76), I² = 0%, Analysis 3.4)
medication status before randomisation (test for subgroup differences: Chi² = 0.51, df = 1 (P = 0.48), I² = 0%; Analysis 3.5)
enrichment design (test for subgroup differences: Chi² = 0.02, df = 1 (P = 0.88), I² = 0%; Analysis 3.6)
trial design (parallel group trials compared to first period cross‐over trials) (test for subgroup differences: Chi² = 0.03, df = 1 (P = 0.86), I² = 0%; Analysis 3.7)
type of control group (test for subgroup differences: Chi² = 0.10, df = 1 (P = 0.75), I² = 0%; Analysis 3.8)
There were not enough data to test the influence of vested interest on the effect estimate.
3.2. Analysis.

Comparison 3: Parent‐rated ADHD symptoms, Outcome 2: Subgroup analysis: types of scales
3.3. Analysis.

Comparison 3: Parent‐rated ADHD symptoms, Outcome 3: Subgroup analysis: duration of treatment
3.4. Analysis.

Comparison 3: Parent‐rated ADHD symptoms, Outcome 4: Subgroup analysis: dose
3.5. Analysis.

Comparison 3: Parent‐rated ADHD symptoms, Outcome 5: Subgroup analysis: medication status ‐ medication naive versus not medication naive
3.6. Analysis.

Comparison 3: Parent‐rated ADHD symptoms, Outcome 6: Subgroup analysis: trials with enrichment design compared with trials without enrichment design
3.7. Analysis.

Comparison 3: Parent‐rated ADHD symptoms, Outcome 7: Subgroup analysis: parallel‐group trials compared with first‐period cross‐over trials
3.8. Analysis.

Comparison 3: Parent‐rated ADHD symptoms, Outcome 8: Subgroup analysis: type of control group
Cross‐over trials (end of last period data)
A meta‐analysis suggested a difference in effect between methylphenidate and placebo in parent‐rated ADHD symptoms favouring methylphenidate (SMD −0.70, 95% CI −0.86 to −0.55; I² = 84%; 45 trials, 4971 participants; Analysis 3.9).
3.9. Analysis.

Comparison 3: Parent‐rated ADHD symptoms, Outcome 9: Cross‐over trials (endpoint data)
-
Subgroup analyses
-
This effect did not vary between:
assessed risk of bias ratings (test for subgroup differences: Chi² = 2.41, df = 1 (P = 0.12), I² = 58.5%; Analysis 3.9);
dose of methylphenidate (test for subgroup differences: Chi² = 3.81, df = 2 (P = 0.15), I² = 47.5%; Analysis 3.10); or
trial design (parallel and first‐period cross‐over compared to cross‐over trials, test for subgroup differences: Chi² = 0.58, df = 1 (P = 0.45), I² = 0%; Analysis 3.11).
Two trials included some participants with an IQ less than 70 (Pearson 2013; Taylor 1987), but removing these trials did not significantly change the estimate.
-
3.10. Analysis.

Comparison 3: Parent‐rated ADHD symptoms, Outcome 10: Subgroup analysis: cross‐over trials (endpoint data): dose
3.11. Analysis.

Comparison 3: Parent‐rated ADHD symptoms, Outcome 11: Subgroup analysis: all parallel‐group trials and first‐period cross‐over trials compared with cross‐over trials (endpoint data)
Parallel‐group trials and cross‐over trials (end of first period) and cross‐over trials (end last of last period)
When combining data from parallel‐group trials with endpoint data from cross‐over trials our meta‐analysis suggested a difference in effects between methylphenidate and placebo on reduced parent‐rated ADHD symptoms favouring methylphenidate (SMD −0.67, 95% CI −0.78 to −0.56; I² = 79%; 69 trials, 7838 participants; Analysis 3.11).
-
Subgroup analyses
-
No evidence suggested that the intervention effect varied according to:
the risk of bias assessment (test for subgroup differences: Chi² = 3.24, df = 1 (P = 0.07), I² = 69.1%; Analysis 3.12);
high or unclear and low risk of vested interest (test for subgroup differences: Chi² = 0.00, df = 1 (P = 0.95), I² = 0%; Analysis 3.13).
-
3.12. Analysis.

Comparison 3: Parent‐rated ADHD symptoms, Outcome 12: All parallel‐group trials and cross‐over trials: risk of bias
3.13. Analysis.

Comparison 3: Parent‐rated ADHD symptoms, Outcome 13: All parallel‐group trials and cross‐over trials: vested interest
Additional subgroup analyses
We tested for differences between raters (teachers, independent assessors and parents) and found no significant differences (test for subgroup differences: Chi² = 2.73, df = 2 (P = 0.26), I² = 26.7%; Analysis 4.1).
We found no evidence suggesting that age (test for subgroup differences: Chi² = 2.84, df = 2 (P = 0.24), I² = 29.6%; Analysis 4.2) or comorbidity influenced the intervention effect (test for subgroup differences: Chi² = 0.15, df = 1 (P = 0.70), I² = 0%; Analysis 4.3). However, the intervention effect was influenced by ADHD subtype, with a greater intervention effect noted for the inattentive subtype (SMD −1.31, 95% CI −1.61 to −1.01; 1 trial, 204 participants) compared to the combined subtype (SMD 0.65, 95% CI −1.30 to 2.60; I² = 99%; 2 trials, 559 participants; test for subgroup differences: Chi² = 3.79, df = 1 (P value = 0.05); I² = 73.6%; Analysis 4.4). This difference rested upon one single trial.
We found no evidence of a 'carry‐over effect' in the cross‐over trials. We conducted a subgroup analysis to investigate the difference between first‐period data and endpoint data from four cross‐over trials (372 participants), and we found no subgroup differences (test for subgroup differences: Chi² = 1.91, df = 1 (P = 0.17), I² = 47.6%; Analysis 4.5).
4.1. Analysis.

Comparison 4: Additional subgroup analyses of ADHD symptoms, Outcome 1: Parallel‐group trials and first‐period cross‐over trials: comparison of raters
4.2. Analysis.

Comparison 4: Additional subgroup analyses of ADHD symptoms, Outcome 2: Parallel‐group trials and first‐period cross‐over trials: age
4.3. Analysis.

Comparison 4: Additional subgroup analyses of ADHD symptoms, Outcome 3: Parallel‐group trials and first‐period cross‐over trials: comorbidity versus no comorbidity
4.4. Analysis.

Comparison 4: Additional subgroup analyses of ADHD symptoms, Outcome 4: Parallel‐group trials and first‐period cross‐over trials: subtypes ADHD: ADHD Rating Scale (parent‐, teacher‐ or independent assessor‐rated)
4.5. Analysis.

Comparison 4: Additional subgroup analyses of ADHD symptoms, Outcome 5: Cross‐over trials: first‐period data versus endpoint data (parent‐, independent assessor‐ and teacher‐rated)
Serious adverse events
We were only able to combine data on serious adverse events from 26 parallel‐group trials and 17 cross‐over trials.
Parallel‐group trials and cross‐over trials (first‐period data only)
Overall serious adverse events
There was no clear evidence of a difference between participants in the methylphenidate group versus those in the control group with regards to the proportion of participants with serious adverse events (RR 0.80, 95% CI 0.39 to 1.67; I² = 0%; 26 trials, 3673 participants; Analysis 5.1).
5.1. Analysis.

Comparison 5: Serious adverse events: parallel‐group trials and first‐period cross‐over trials, Outcome 1: Proportions of participants with serious adverse events (SAE)
We assessed the evidence to be of very low certainty (see GRADE assessment below). Therefore we are uncertain that the estimated effect accurately reflects the true effect, and the addition of more data could change the findings.
-
Trial Sequential Analysis
We conducted a Trial Sequential Analysis on the 'proportion of participants with serious adverse events’ outcome, involving 26 parallel‐group and first‐period cross‐over trials. We had planned to use a relative risk reduction of 20%, but the distance between the accrued information and the required information was too large, and the program failed to calculate and draw an interpretable figure. Therefore, we increased the relative risk reduction to 25%. We included trials with zero serious adverse events by substituting zero with a constant of 0.25 (Carlson 2007; Childress 2017; Childress 2020a; Childress 2020b; Childress 2020c; Green 2011; Huang 2021; Jacobi‐Polishook 2009; Kollins 2021; Matthijssen 2019; McCracken 2016; NCT00409708; Pliszka 2017; Schrantee 2016; Wigal 2017; Wolraich 2001). We calculated the DARIS on the basis of serious adverse events in the control group of 2%; a relative risk reduction or increase in the experimental group of 25%; type I error of 5%; type II error of 20% (80% power); and diversity (D²) of 0%. The DARIS was 9349 participants. The cumulative Z‐curve did not cross the conventional or trial sequential monitoring boundaries for benefit, harm, or futility (see Figure 5). As only less than 36% of the DARIS was accrued, risks of random type II error cannot be excluded. The Trial Sequential Analysis‐adjusted intervention effect was RR 0.91 (CI 0.31 to 2.68).
-
Specific serious adverse events:
nervous system: aggression (RR 0.50, 95% CI 0.05 to 5.49; 1 trial, 303 participants; Analysis 5.2)
nervous system: concussion (RR 0.34, 95% CI 0.01 to 8.17; 1 trial, 303 participants; Analysis 5.2)
nervous system: loss of consciousness (RR 0.33, 95% CI 0.01 to 8.02; 1 trial, 221 participants; Analysis 5.2)
nervous system: psychosis (RR 0.81, 95% CI 0.13 to 5.12; I² = 0%; 4 trials, 919 participants; Analysis 5.2)
nervous system: syncope (RR 1.39, 95% CI 0.23 to 8.47; I²= 0%; 3 trials, 741 participants; Analysis 5.2)
nervous system: suicidal ideation (RR 1.63, 95% CI 0.07 to 38.55; 6 trials, 1032 participants; Analysis 5.2)
nervous system: suicidal behaviour: no events; 2 trials, 233 participants; Analysis 5.2)
nervous system: oppositional behaviour/negativism (RR 0.17, 95% CI 0.01 to 4.04, 1 trial, 217 participants; Analysis 5.2)
nervous system: adjustment disorder (RR 0.78, 95% CI 0.03 to 18.91; 1 trial, 230 participants; Analysis 5.2)
digestive system: appendicitis (RR 2.11, 95% CI 0.22 to 20.04; I² = 0%; 2 trials, 414 participants; Analysis 5.3)
cardiovascular systems: haematoma (RR 0.33, 95% CI 0.01 to 8.02; 1 trial, 221 participants; Analysis 5.4)
cardiovascular systems: tachycardia (RR 3.10, 95% CI 0.13 to 73.14; 1 trial, 59 participants; Analysis 5.4)
respiratory system: bronchitis (RR 0.34, 95% CI 0.01 to 8.17; 1 trial, 303 participants; Analysis 5.5)
respiratory system: asthma (RR 3.02, 95% CI 0.12 to 73.54; 1 trial, 303 participants; Analysis 5.5)
urinary system: renal cyst (RR 1.49, 95% CI 0.06 to 36.27; 1 trial, 275 participants; Analysis 5.6)
urinary system: kidney infection (RR 3.02, 95% CI 0.12 to 73.54; 1 trial, 303 participants; Analysis 5.6)
skeletal and muscular system: clavicle fracture (RR 0.33, 95% CI 0.01 to 8.02; 1 trial, 221 participants; Analysis 5.7)
immune system: cyst rupture (RR 3.02, 95% CI 0.12 to 73.54; 1 trial, 303 participants; Analysis 5.8)
other: drug toxicity (RR 0.34, 95% CI 0.01 to 8.17; 1 trial, 303 participants; Analysis 5.9
other: overdose (RR 2.97, 95% CI 0.12 to 72.20; 1 trial, 221 participants; Analysis 5.9
5.
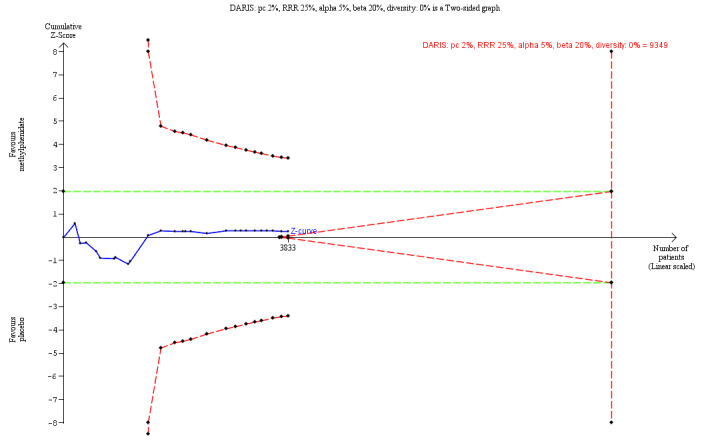
Trial Sequential Analysis: proportion of participants with one or more serious adverse events
5.2. Analysis.

Comparison 5: Serious adverse events: parallel‐group trials and first‐period cross‐over trials, Outcome 2: Nervous system (including psychiatry)
5.3. Analysis.

Comparison 5: Serious adverse events: parallel‐group trials and first‐period cross‐over trials, Outcome 3: Digestive system
5.4. Analysis.

Comparison 5: Serious adverse events: parallel‐group trials and first‐period cross‐over trials, Outcome 4: Cardiovascular systems
5.5. Analysis.

Comparison 5: Serious adverse events: parallel‐group trials and first‐period cross‐over trials, Outcome 5: Respiratory systems
5.6. Analysis.

Comparison 5: Serious adverse events: parallel‐group trials and first‐period cross‐over trials, Outcome 6: Urinary system
5.7. Analysis.

Comparison 5: Serious adverse events: parallel‐group trials and first‐period cross‐over trials, Outcome 7: Skeletal and muscular system (including pain)
5.8. Analysis.

Comparison 5: Serious adverse events: parallel‐group trials and first‐period cross‐over trials, Outcome 8: Immune system (including infections)
5.9. Analysis.

Comparison 5: Serious adverse events: parallel‐group trials and first‐period cross‐over trials, Outcome 9: Other
Cross‐over trials (end of last period data)
There were no clear differences between participants in the methylphenidate group and individuals in the control group regarding the proportion of participants with serious adverse events (RR 2.46, 95% CI 0.50 to 12.03; I² = 0%; 16 trials, 3323 participants; Analysis 6.1).
6.1. Analysis.

Comparison 6: Serious adverse events: cross‐over trials (endpoint data), Outcome 1: Proportion of participants with serious adverse events (SAE)
-
Specific serious adverse events:
nervous system: hallucinations (RR 1.33, 95% CI 0.06 to 30.42; 1 trial, 37 participants; Analysis 6.2)
nervous system: psychiatric disorders (RR 3.21, 95% CI 0.13 to 78.04; 1 trial, 267 participants; Analysis 6.2)
urinary system: proteinuria (RR 3.00, 95% CI 0.12 to 72.37; 1 trial, 136 participants; Analysis 6.3)
immune system: peritonsillar abscess (RR 2.93, 95% CI 0.12 to 71.32; 1 trial, 322 participants; Analysis 6.4)
immune system: oral bullae (RR 2.93, 95% CI 0.12 to 71.32; 1 trial, 322 participants; Analysis 6.4)
6.2. Analysis.

Comparison 6: Serious adverse events: cross‐over trials (endpoint data), Outcome 2: Nervous system (including psychiatry)
6.3. Analysis.

Comparison 6: Serious adverse events: cross‐over trials (endpoint data), Outcome 3: Urinary system
6.4. Analysis.

Comparison 6: Serious adverse events: cross‐over trials (endpoint data), Outcome 4: Immune system
Secondary outcomes
Adverse events considered non‐serious
We were able to combine data on non‐serious adverse events from 26 parallel‐group trials and 67 cross‐over trials in meta‐analyses. We assessed the evidence to be of very low certainty (see GRADE assessment below). Therefore we are uncertain that the estimated effect accurately reflects the true effect and the addition of more data could change the findings.
Parallel‐group trials and cross‐over trials (end of first‐period data only)
Overall adverse events considered non‐serious
Participants receiving methylphenidate were more likely to experience non‐serious adverse events overall (RR 1.23, 95% CI 1.11 to 1.37; I² = 72%; 35 trials, 5342 participants; Analysis 7.1). We observed substantial heterogeneity between trials: Tau² = 0.05; Chi² = 118.99, df = 33, (P < 0.00001); I² = 72%. This heterogeneity could be related to dose, as we observed differences between low‐dose and high‐dose methylphenidate trials (test for subgroup differences: Chi² = 18.52, df = 2 (P < 0.0001), I² = 89.2%; Analysis 7.2). Eight trials did not specify the dose they used. Including these trials in the dose subgroup analysis did not significantly alter the subgroup difference between methylphenidate doses.
7.1. Analysis.

Comparison 7: Non‐serious adverse events: parallel‐group trials and first‐period cross‐over trials, Outcome 1: Proportion of participants with non‐serious adverse events
7.2. Analysis.

Comparison 7: Non‐serious adverse events: parallel‐group trials and first‐period cross‐over trials, Outcome 2: Subgroup analysis: proportion of participants with non‐serious adverse events according to dose
-
Trial Sequential Analysis
We conducted a Trial Sequential Analysis on the 'proportion of participants with non‐serious adverse events’ outcome involving 35 parallel‐group and first‐period cross‐over trials. We included one trial with zero non‐serious adverse events by substituting zero with a constant of 0.25 (Jacobi‐Polishook 2009). We calculated the DARIS on the basis of adverse events in the control group of 44%; relative risk reduction in the intervention group of 20%; type I error of 5%; type II error of 20% (80% power); and diversity (D‐square) of 89.6%. The DARIS was 9139 participants. The cumulative Z‐curve (blue line) crossed the trial sequential monitoring boundaries for harm (red inward sloping line) after the 25th trial (Figure 6). Accordingly, risk of random error in the finding can be excluded according to the Lan‐DeMetz‐O’Brien‐Fleming monitoring boundary. The Trial Sequential Analysis‐adjusted intervention effect was RR 1.22 (CI 1.08 to 1.43).
Non‐serious adverse events included those affecting the nervous system (Analysis 7.3), the digestive system (Analysis 7.4), the cardiovascular system (Analysis 7.5), respiratory system (Analysis 7.6), the urinary system (Analysis 7.7), the skeletal and muscular system (Analysis 7.8), the immune system (Analysis 7.9), and the integumentary system (Analysis 7.10). Other reported adverse events included sleep variability (Analysis 7.11; Analysis 7.12), vital signs (Analysis 7.13), physical parameters (Analysis 7.14) and others including drug toxicity (Analysis 7.15).
-
Compared with those in the control group, participants in the methylphenidate may be more likely to report the following:
nervous system: headache (RR 1.33, 95% CI 1.04 to 1.70; I² = 32%; 32 trials, 5041 participants; Analysis 7.3)
nervous system: tension (RR 23.00, 95% CI 1.42 to 373.44; 1 trial, 60 participants; Analysis 7.3)
digestive system: a decrease in appetite (RR 3.35, 95% CI 2.49 to 4.50; I² = 48%; 30 trials, 5127 participants; Analysis 7.4)
digestive system: a decrease in weight (RR 5.44, 95% CI 2.47 to 11.98; I² = 0%; 11 trials, 2001 participants; Analysis 7.4)
physical parameters: having a lower body mass index (BMI) (SMD −1.00, 95% CI −1.26 to −0.73; I² = 65%; 3 trials, 810 participants; Analysis 7.14)
digestive system: having dry mouth (RR 3.79, 95% CI 1.26 to 11.39; I² = 0%; 4 trials, 1057 participants; Analysis 7.4)
cardiovascular system: pallor (RR 23.00, 95% CI 1.42 to 373.44; 1 trial, 60 participants; Analysis 7.5)
sleep variability: trouble sleeping or sleep problems (RR 1.62, 95% CI 1.18 to 2.21; I² = 0%; 15 trials, 2620 participants; Analysis 7.11)
sleep variability: insomnia (RR 1.90, 95% CI 1.12 to 3.22; I² = 49%; 15 trials, 2315 participants; Analysis 7.11)
sleep variability: having a lower sleep efficiency percentage (time spent asleep while in bed) after treatment discontinuation (MD 5.42, 95% CI 0.21 to 10.63; 1 trial, 48 participants; Analysis 7.12)
vital signs: having a higher diastolic blood pressure (MD 1.90, 95% CI 0.68 to 3.11; I² = 54%; 13 trials, 2032 participants; Analysis 7.13
vital signs: having a higher pulse (MD 3.86, 95% CI 2.09 to 5.63; I² = 65%; 13 trials, 2205 participants; Analysis 7.13).
other: excoriation (chronic skin‐picking) (RR 3.22, 95% CI 1.20 to 8.64; I² = 0%; 2 trials, 389 participants; Analysis 7.15)
6.
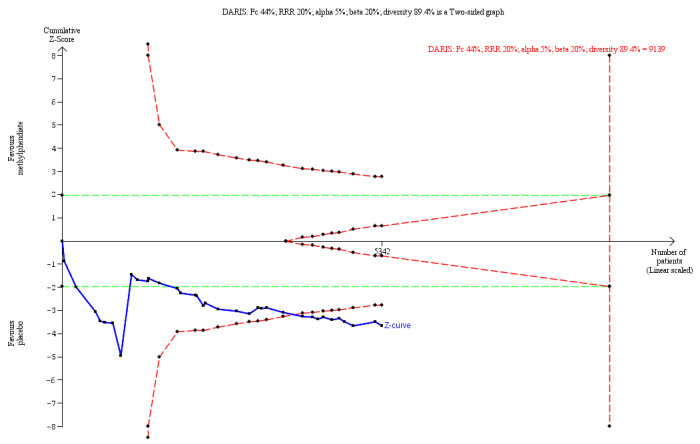
Trial Sequential Analysis: proportion of participants with one or more non‐serious adverse events
7.3. Analysis.

Comparison 7: Non‐serious adverse events: parallel‐group trials and first‐period cross‐over trials, Outcome 3: Nervous system (including psychiatry)
7.4. Analysis.

Comparison 7: Non‐serious adverse events: parallel‐group trials and first‐period cross‐over trials, Outcome 4: Digestive system
7.5. Analysis.

Comparison 7: Non‐serious adverse events: parallel‐group trials and first‐period cross‐over trials, Outcome 5: Cardiovascular system
7.6. Analysis.

Comparison 7: Non‐serious adverse events: parallel‐group trials and first‐period cross‐over trials, Outcome 6: Respiratory system
7.7. Analysis.

Comparison 7: Non‐serious adverse events: parallel‐group trials and first‐period cross‐over trials, Outcome 7: Urinary system
7.8. Analysis.

Comparison 7: Non‐serious adverse events: parallel‐group trials and first‐period cross‐over trials, Outcome 8: Skeletal and muscular systems (including pain)
7.9. Analysis.

Comparison 7: Non‐serious adverse events: parallel‐group trials and first‐period cross‐over trials, Outcome 9: Immune system (including infections)
7.10. Analysis.

Comparison 7: Non‐serious adverse events: parallel‐group trials and first‐period cross‐over trials, Outcome 10: Integumentary system
7.11. Analysis.

Comparison 7: Non‐serious adverse events: parallel‐group trials and first‐period cross‐over trials, Outcome 11: Sleep variability
7.12. Analysis.

Comparison 7: Non‐serious adverse events: parallel‐group trials and first‐period cross‐over trials, Outcome 12: Sleep variability continuous outcomes
7.13. Analysis.

Comparison 7: Non‐serious adverse events: parallel‐group trials and first‐period cross‐over trials, Outcome 13: Vital signs
7.14. Analysis.

Comparison 7: Non‐serious adverse events: parallel‐group trials and first‐period cross‐over trials, Outcome 14: Physical parameters
7.15. Analysis.

Comparison 7: Non‐serious adverse events: parallel‐group trials and first‐period cross‐over trials, Outcome 15: Other (including drug toxicity)
Cross‐over trials (end‐of‐last‐period data)
Overall adverse events considered non‐serious
Participants receiving methylphenidate were significantly more likely to experience adverse events considered non‐serious compared with the control group (RR 1.39, 95% CI 1.13 to 1.70; I² = 60%; 24 trials, 2696 participants; Analysis 8.1). In addition, we noted differences in the numbers of events reported in trials of low doses of methylphenidate compared to trials of high doses of methylphenidate as there were more events in the high‐dose group (test for subgroup differences: Chi² = 5.72, df = 2 (P = 0.026, I² = 65.0%; Analysis 8.2). Five trials did not specify the dose they used. Including these in the dose subgroup analysis did not alter the subgroup difference between methylphenidate doses.
8.1. Analysis.

Comparison 8: Non‐serious adverse events: cross‐over trials (endpoint data), Outcome 1: Proportion of participants with non‐serious events
8.2. Analysis.

Comparison 8: Non‐serious adverse events: cross‐over trials (endpoint data), Outcome 2: Subgroup analysis: total number of non‐serious adverse events according to dose
Categories of non‐serious adverse events included those affecting the nervous system (Analysis 8.3; Analysis 8.4), the digestive system (Analysis 8.5), the cardiovascular system (Analysis 8.6), the respiratory system (Analysis 8.7), the urinary system (Analysis 8.8), the skeletal and muscular system (Analysis 8.9; Analysis 8.10), the immune system (Analysis 8.11), and the integumentary system (Analysis 8.12). Other reported adverse events included effects on sleep variability (Analysis 8.13; Analysis 8.14), vital signs (Analysis 8.15), physical parameters (Analysis 8.16), and others including drug toxicity (Analysis 8.17).
8.3. Analysis.

Comparison 8: Non‐serious adverse events: cross‐over trials (endpoint data), Outcome 3: Nervous system (including psychiatry)
8.4. Analysis.

Comparison 8: Non‐serious adverse events: cross‐over trials (endpoint data), Outcome 4: Nervous system (including psychiatry) continuous outcomes
8.5. Analysis.

Comparison 8: Non‐serious adverse events: cross‐over trials (endpoint data), Outcome 5: Digestive system
8.6. Analysis.

Comparison 8: Non‐serious adverse events: cross‐over trials (endpoint data), Outcome 6: Cardiovascular system
8.7. Analysis.

Comparison 8: Non‐serious adverse events: cross‐over trials (endpoint data), Outcome 7: Respiratory system
8.8. Analysis.

Comparison 8: Non‐serious adverse events: cross‐over trials (endpoint data), Outcome 8: Urinary system
8.9. Analysis.

Comparison 8: Non‐serious adverse events: cross‐over trials (endpoint data), Outcome 9: Skeletal and muscular system
8.10. Analysis.

Comparison 8: Non‐serious adverse events: cross‐over trials (endpoint data), Outcome 10: Skeletal and muscular system continuous outcomes
8.11. Analysis.

Comparison 8: Non‐serious adverse events: cross‐over trials (endpoint data), Outcome 11: Immune system (including infections)
8.12. Analysis.

Comparison 8: Non‐serious adverse events: cross‐over trials (endpoint data), Outcome 12: Integumentary system
8.13. Analysis.

Comparison 8: Non‐serious adverse events: cross‐over trials (endpoint data), Outcome 13: Sleep variability continuous outcomes
8.14. Analysis.

Comparison 8: Non‐serious adverse events: cross‐over trials (endpoint data), Outcome 14: Sleep variability
8.15. Analysis.

Comparison 8: Non‐serious adverse events: cross‐over trials (endpoint data), Outcome 15: Vital signs
8.16. Analysis.

Comparison 8: Non‐serious adverse events: cross‐over trials (endpoint data), Outcome 16: Physical parameters
8.17. Analysis.

Comparison 8: Non‐serious adverse events: cross‐over trials (endpoint data), Outcome 17: Other (including drug toxicity)
Compared with the control group, participants in the methylphenidate group were less likely to report experiencing the following:
nervous system: anger (RR 0.45, 95% CI 0.26 to 0.77; I² = 0%; 3 trials, 264 participants; Analysis 8.3)
nervous system: behavioural complaints (RR 0.55, 95% CI 0.35 to 0.86; 1 trial, 82 participants; Analysis 8.3)
nervous system: daydreaming (RR 0.66, 95% CI 0.44 to 0.98; I² = 0%; 3 trials, 222 participants; Analysis 8.3)
digestive system: an increase in appetite (RR 0.20, 95% CI 0.08 to 0.50; 1 trial, 136 participants; Analysis 8.5)
sleep variability: a reduction in actigraphic sleep onset latency (time to transition from full wakefulness to sleep; MD 21.10, 95% CI 1.33 to 40.87; 1 trial, 52 participants; Analysis 8.13)
However, they were more likely to report the following:
nervous system: compulsive acts (RR 2.57, 95% CI 1.45 to 4.56; 1 trial, 90 participants; Analysis 8.3)
nervous system: headache (RR 1.25, 95% CI 1.06 to 1.48; I² = 0%; 43 trials, 5981 participants; Analysis 8.3)
nervous system: being overly meticulous (RR 40.77, 95% CI 2.35 to 706.72; 1 trial, 96 participants; Analysis 8.3)
nervous system: obsessive thinking (RR 2.35, 95% CI 1.53 to 3.62; 1 trial, 90 participants; Analysis 8.3)
nervous system: tics or nervous movements ((RR 1.23, 95% CI 1.02 to 1.50; I² = 3%; 24 trials, 3429 participants; Analysis 8.3)
nervous system: emotional lability (RR 9.25, 95% CI 2.24 to 38.22; 1 trial, 154 participants; Analysis 8.3)
nervous system: being prone to crying (RR 1.72, 95% CI 1.04 to 2.86; 1 trial, 1052 participants; Analysis 8.3)
digestive system: a decrease in appetite (RR 3.89, 95% CI 2.76 to 5.48; I² = 78%; 41 trials, 6091 participants; Analysis 8.5)
digestive system: nausea (RR 1.67, 95% CI 1.13 to 2.46; I² = 0%; 11 trials, 1182 participants; Analysis 8.5)
digestive system: stomach ache (RR 1.70, 95% CI 1.35 to 2.15; I² = 34%; 38 trials, 5803 participants; Analysis 8.5)
sleep variability: insomnia or sleep problems (RR 1.88, 95% CI 1.39 to 2.56; I² = 69%; 37 trials, 5499 participants; Analysis 8.14)
vital signs: increased pulse/heart rate (SMD 0.43, 95% CI 0.23 to 0.64; I² = 53%; 14 trials, 939 participants; Analysis 8.15).
skeletal and muscular system: somatic complaints (MD 0.85, 95% CI 0.79 to 0.91; 1 trial, 82 participants; Analysis 8.10)
General behaviour
We were able to include in our analyses data on general behaviour from 13 parallel‐group trials and from 21 cross‐over trials.
Teacher‐rated general behaviour
Parallel‐group trials and cross‐over trials (end‐of‐first‐period data only)
A meta‐analysis suggested a difference in effect between methylphenidate and placebo in teacher‐rated general behaviour favouring methylphenidate (SMD −0.62, 95% CI −0.91 to −0.33; I² = 68%; 7 trials, 792 participants; Analysis 9.1). The SMD effect of −0.62 for general behaviour corresponds to an MD of −3.58 points (95% CI −5.26 to −1.91) on the CGI (Conners 1998a). Due to a lack of MIREDIF, we do not know whether this is a clinical relevant difference.
9.1. Analysis.

Comparison 9: Teacher‐rated general behaviour, Outcome 1: All parallel‐group trials and first‐period cross‐over trials
We assessed the evidence to be of very low certainty (see GRADE assessment below). Therefore we are uncertain that the estimated effect accurately reflects the true effect and the addition of more data could change the findings.
-
Subgroup analyses
We were not able to test for subgroup differences based on the risk of bias as all seven trials were at high risk of bias (Analysis 9.1).
The intervention effect varied according to type of scale (test for subgroup differences: Chi² = 18.75, df = 5 (P = 0.002), I² = 73.3%; Analysis 9.2).
We found no evidence to suggest a difference in effects between doses (test for subgroup differences: Chi² = 0.29, df = 2 (P = 0.87), I² = 0%; Analysis 9.3).
We were not able to test for subgroup differences according to duration, as all trials were of short duration, that is, less than six months (Analysis 9.4), or according to trial design, as all trials in the analysis were parallel‐group trials (Analysis 9.5).
9.2. Analysis.

Comparison 9: Teacher‐rated general behaviour, Outcome 2: Subgroup analysis: types of scales
9.3. Analysis.

Comparison 9: Teacher‐rated general behaviour, Outcome 3: Subgroup analysis: dose
9.4. Analysis.

Comparison 9: Teacher‐rated general behaviour, Outcome 4: Subgroup analysis: duration of treatment
9.5. Analysis.

Comparison 9: Teacher‐rated general behaviour, Outcome 5: Subgroup analysis: parallel‐group trials versus first‐period cross‐over trials
Cross‐over trials (endpoint data)
Meta‐analysis suggested a difference in effects between methylphenidate and placebo in reduced teacher‐rated general behaviour favouring methylphenidate (SMD −0.75, 95% CI −0.87 to −0.63; I² = 5%; 16 trials, 1302 participants; Analysis 9.6).
9.6. Analysis.

Comparison 9: Teacher‐rated general behaviour, Outcome 6: Cross‐over trials (endpoint data)
-
Subgroup analyses
The intervention effect varied according to dose of methylphenidate favouring the high‐dose group (test for subgroup differences: Chi² = 5.64, df = 1 (P = 0.02), I² = 82.3%; Analysis 9.7).
9.7. Analysis.

Comparison 9: Teacher‐rated general behaviour, Outcome 7: Subgroup analysis: cross‐over trials (endpoint data): dose
Parallel‐group trials and cross‐over trials (endpoint data)
When combining data from parallel‐group trials with endpoint data from cross‐over trials our meta‐analysis similarly suggested a difference in effects between methylphenidate and placebo in reduced teacher‐rated general behaviour favouring methylphenidate (SMD −0.72, 95% CI −0.84 to −0.60; I²= 37%; 23 trials, 2094 participants; Analysis 9.8). The intervention effect did not vary according to high or low risk of vested interest (Analysis 9.9)
9.8. Analysis.

Comparison 9: Teacher‐rated general behaviour, Outcome 8: Subgroup analysis: all parallel‐group trials and first‐period cross‐over trials (teacher‐rated) versus cross‐over trials (endpoint data)
9.9. Analysis.

Comparison 9: Teacher‐rated general behaviour, Outcome 9: Subgroup analysis: all parallel‐group trials and cross‐over trials: vested interest
Independent assessor‐rated general behaviour
Parallel‐group trials and cross‐over trials (first‐period data only)
Meta‐analysis suggested a difference in effects between methylphenidate and placebo in reduced independent assessor‐rated general behaviour favouring methylphenidate (MD 1.10, 95% CI −1.01 to 3.21; 1 trial, 94 participants; Analysis 10.1).
10.1. Analysis.

Comparison 10: Independent assessor‐rated general behaviour, Outcome 1: All parallel‐group trials and first‐period cross‐over trials
-
Subgroup analyses
We found only one parallel‐group trial that provided data on independent assessor‐rated general behaviour, so we were unable to perform any subgroup analyses.
Cross‐over trials (endpoint data)
Meta‐analysis suggested a difference in effects between methylphenidate and placebo in reduced independent assessor‐rated general behaviour favouring methylphenidate (SMD −0.98, 95% CI −1.39 to −0.57; I² = 87%; 9 trials, 987 participants; Analysis 10.2).
10.2. Analysis.

Comparison 10: Independent assessor‐rated general behaviour, Outcome 2: Cross‐over trials (endpoint data)
-
Subgroup analyses
We were not able to test for subgroup differences based on the risk of bias as all trials were at high risk of bias.
The intervention effect did not vary according to dose of methylphenidate (test for subgroup differences: Chi² = 1.83, df = 1 (P = 0.18), I² = 45.3%; Analysis 10.3)
The intervention effect varied according to trial design (test for subgroup differences: Chi² = 16.36, df = 1 (P < 0.0001), I² = 93.9%; Analysis 10.4).
10.3. Analysis.

Comparison 10: Independent assessor‐rated general behaviour, Outcome 3: Subgroup analysis: general behaviour, cross‐over trials (endpoint data): dose
10.4. Analysis.

Comparison 10: Independent assessor‐rated general behaviour, Outcome 4: Subgroup analysis: all parallel‐group trials and first‐period cross‐over trials (independent assessor‐rated) compared with cross‐over trials (endpoint data)
Parallel‐group trials and cross‐over trials (endpoint data)
When combining data from parallel‐group trials with endpoint data from cross‐over trials our meta‐analysis similarly suggested a difference in effects between methylphenidate and placebo in reduced independent assessor‐rated general behaviour favouring methylphenidate (SMD −0.86, 95% CI −1.27 to −0.46; I² = 89; 10 trials, 1081 participants; Analysis 10.4).
-
Subgroup analyses
The intervention effect did not vary according to high or low risk of vested interest (test for subgroup differences: Chi² = 0.26, df = 1 (P = 0.61), I² = 0%; Analysis 10.5). One trial had unclear risk of vested interest (Merrill 2021) and including this trial in the subgroup analysis significantly changed the results (test for subgroup differences: Chi² = 51.05, df = 2 (P < 0.00001), I² = 96.1%; Analysis 10.5).
10.5. Analysis.

Comparison 10: Independent assessor‐rated general behaviour, Outcome 5: Subgroup analysis: all parallel‐group trials and cross‐over trials: vested interest
Parent‐rated general behaviour
Parallel‐group trials and cross‐over trials (first‐period data only)
Meta‐analysis suggested a difference in effects between methylphenidate and placebo in reduced parent‐rated general behaviour favouring methylphenidate (SMD −0.42, 95% CI −0.62 to −0.23; I² = 57%; 10 trials, 1376 participants; Analysis 11.1).
11.1. Analysis.

Comparison 11: Parent‐rated general behaviour, Outcome 1: All parallel‐group trials and first‐period cross‐over trials
-
Subgroup analyses
The intervention effect was significantly influenced by the type of scale (test for subgroup differences: Chi² = 15.13, df = 6 (P = 0.02), I² = 60.3%; Analysis 11.2).
We found no evidence to suggest that trial design influenced the intervention effect (test for subgroup differences: Chi² = 0.63, df = 1 (P = 0.43), I² = 0%; Analysis 11.3).
We found no evidence to suggest that risk of bias assessment influenced the intervention effect (test for subgroup differences: Chi² = 1.90, df = 1 (P = 0.17), I² = 47.5%; Analysis 10.1).
We were not able to test for subgroup differences according to duration, as all trials were of short duration, that is, less than six months (Analysis 11.4), or according to dose, as no trials reporting parent‐rated general behaviour used low‐dose methylphenidate (Analysis 11.5).
11.2. Analysis.

Comparison 11: Parent‐rated general behaviour, Outcome 2: Subgroup analysis: types of scales
11.3. Analysis.

Comparison 11: Parent‐rated general behaviour, Outcome 3: Subgroup analysis: parallel‐group trials compared with first‐period cross‐over trials
11.4. Analysis.

Comparison 11: Parent‐rated general behaviour, Outcome 4: Subgroup analysis: duration of treatment
11.5. Analysis.

Comparison 11: Parent‐rated general behaviour, Outcome 5: Subgroup analysis: dose
Cross‐over trials (endpoint data)
Meta‐analysis suggested a difference in effects between methylphenidate and placebo in reduced parent‐rated general behaviour favouring methylphenidate (SMD −0.84, 95% CI −1.05 to −0.63; I² = 0%; 6 trials, 384 participants; Analysis 11.6)
11.6. Analysis.

Comparison 11: Parent‐rated general behaviour, Outcome 6: Cross‐over trials (endpoint data)
-
Subgroup analyses
The intervention effect was not influenced by the dose of methylphenidate (test for subgroup differences: Chi² = 0.91, df = 1 (P = 0.34), I² = 0%; Analysis 11.7).
All trials were at high risk of bias therefore we could not conduct a subgroup analysis.
11.7. Analysis.

Comparison 11: Parent‐rated general behaviour, Outcome 7: Subgroup analysis: cross‐over trials (endpoint data): dose
Parallel‐group trials and cross‐over trials (endpoint data)
When combining data from parallel‐group trials with endpoint data from cross‐over trials our meta‐analysis similarly suggested a difference in effects between methylphenidate and placebo in reduced parent‐rated general behaviour favouring methylphenidate (SMD −0.56, 95% CI −0.74 to −0.39; I² = 59%; 16 trials, 1760 participants; Analysis 11.8).
11.8. Analysis.

Comparison 11: Parent‐rated general behaviour, Outcome 8: Subgroup analysis: all parallel‐group trials and first‐period cross‐over trials (parent‐rated) compared with cross‐over trials (endpoint data)
-
Subgroup analyses
The intervention effect varied according to trial design (test for subgroup differences: Chi² = 8.26, df = 1 (P = 0.004), I² = 87.9%; Analysis 11.8).
No evidence suggested a difference between subgroups of trials assessed with high, or low risk of bias (test for subgroup differences: Chi² = 3.33, df = 1 (P = 0.07), I² = 69.9%; Analysis 11.9).
No evidence suggested a difference between subgroups of trials with low and high risk of vested interest (test for subgroup differences: Chi² = 0.04, df = 1 (P = 0.83), I² = 0%; Analysis 11.10).
11.9. Analysis.

Comparison 11: Parent‐rated general behaviour, Outcome 9: All parallel‐group trials and cross‐over trials: risk of bias
11.10. Analysis.

Comparison 11: Parent‐rated general behaviour, Outcome 10: Subgroup analysis: all parallel‐group trials and cross‐over trials: vested interest
Additional subgroup analyses
Additional subgroup analysis suggested that the intervention effect varied according to raters (teacher, independent assessor and parents), with a higher intervention effect for teacher‐rated trials (SMD −0.62, 95% CI −0.91 to −0.33; I² = 68%; 7 trials, 792 participants) compared with parent‐rated trials (SMD −0.42, 95% CI −0.62 to −0.23; I² = 57%; 10 trials, 1376 participants), and independent assessor‐rated trials (SMD 0.21, 95% CI −0.20 to 0.61; 1 trial, 94 participants; test for subgroup differences: Chi² = 10.89, df = 2 (P = 0.004), I² = 81.6%; Analysis 12.1).
We found no evidence that comorbidity influences the intervention effect (test for subgroup differences: Chi² = 0.13, df = 1 (P = 0.72), I² = 0%; Analysis 12.2)
We found no evidence of a 'carry‐over effect' in the cross‐over trials (test for subgroup differences: Chi² = 2.14, df = 1 (P = 0.14), I² = 53.3%; Analysis 12.3).
No data were available for subgroup analyses by age, sex or ADHD subtype.
12.1. Analysis.

Comparison 12: Additional subgroup analyses of general behaviour, Outcome 1: Parallel‐group trials and first‐period cross‐over trials: comparisons of raters
12.2. Analysis.

Comparison 12: Additional subgroup analyses of general behaviour, Outcome 2: Parallel‐group trials and first‐period cross‐over trials: comorbidity versus no comorbidity
12.3. Analysis.

Comparison 12: Additional subgroup analyses of general behaviour, Outcome 3: Cross‐over trials: first‐period data versus endpoint data in the same trials (teacher‐, parent‐, and independent assessor‐rated)
Quality of life
We could include data on quality of life from only four parallel‐group trials in our analyses. We assessed the evidence to be of very low certainty (see GRADE assessment below).
There was no difference in effects between methylphenidate versus placebo in quality of life at end of treatment (SMD 0.40, 95% CI −0.03 to 0.83; I²= 81%; 4 trials, 608 participants; Analysis 13.1). The SMD of 0.40 for quality of life corresponds to an MD of 4.94 (95% CI −0.37 to 10.25) on the Child Health Questionnaire (CHQ; Landgraf 1998), which ranges from 0 to 100 points. This is below the MIREDIF of 7.0 points on CHQ (Rentz 2005).
13.1. Analysis.

Comparison 13: Quality of life: parallel‐group trials and first‐period cross‐over trials, Outcome 1: Subgroup analysis: types of scales
-
Subgroup analysis
All trials were at high risk of bias therefore we could not conduct a subgroup analysis.
It was not possible to investigate subgroup differences of vested interest bias as there were no trials reporting quality of life with low risk in all risk of bias domains.
There was no evidence indicating that the type of rating scale influenced the effect of the intervention (Analysis 13.1).
Additional sensitivity analysis
We tested whether a change from a random‐effects model meta‐analysis to a fixed‐effect model meta‐analysis changed our results. This was only the case in one analysis 'Socially withdrawn – decreased interaction with others (Analysis 8.3.20). The P value using a random‐effects model was P = 0.0002, which changed to P = 0.10 with a fixed‐effect model.
Discussion
Summary of main results
We included 55 parallel‐group trials and 156 cross‐over trials in this review and one trial with a parallel‐phase (114 participants randomised) and a cross‐over phase (165 participants randomised). Altogether, these trials randomised more than 16,000 participants and were reported in 614 publications. The majority compared methylphenidate with placebo in short‐term trials of less than six months' duration. The average trial duration in the 56 parallel trials was 67.1 days (range 1 to 425 days). Most were conducted in outpatient clinics in high‐income countries, particularly the USA. Participants' ages ranged from 3 to 18 years across most studies; in two studies ages ranged from 3 to 21 years (Green 2011; Szobot 2008). Both boys and girls were recruited, in a ratio of 3:1.
We considered 22 trials (9 parallel‐group trials and 13 cross‐over trials, including the two phases in Kollins 2006 (PATS)) to have an overall assessment at low risk of bias. We considered 191 trials to have an overall assessment at high risk of bias. We considered all trials to be of high risk of bias due to the risk of deblinding described elsewhere. This raises important concerns, which are discussed after a summary of the results.
Primary outcomes
ADHD symptoms
A meta‐analysis of data from parallel‐group trials combined with data from the first period of cross‐over trials suggests that methylphenidate may improve ADHD symptoms as reported by teachers. The SMD calculated modest improvement in ADHD symptoms on the ADHD‐RS scale (DuPaul 1991a), however, we judged the certainty of the evidence to be ‘very low’ (see Quality of the evidence).
We found that the types of scales used influenced the intervention effect of methylphenidate. The differences between scales ranged from 0.5 SMD to 2.0 SMD. We found lower effects of methylphenidate in long‐term trials compared to short‐term trials. There was no difference between the subgroups of trials using placebo compared to the trials using no intervention in the control group.
Serious adverse events
Methylphenidate does not appear to be associated with an increased occurrence of serious adverse events. However, data for this outcome were only available in 42 of the 212 included trials (20%) and we judged the certainty of the evidence to be ‘very low’ (see Quality of the evidence).
Secondary outcomes
Adverse events considered non‐serious
Amongst those in the methylphenidate‐exposed groups, 538 per 1000 experienced non‐serious adverse events, compared with 437 per 1000 in the control group. The most common non‐serious adverse events were sleep problems and decreased appetite.
We also judged the overall certainty of the evidence for this outcome to be ‘very low’, and as a result, we are uncertain of the magnitude of the harmful effects. Furthermore, for methodological reasons, we used only dichotomous outcomes reflecting the number of participants affected by the event per the total number of participants. As most participants reported more than one adverse event, the actual increase in risk of non‐serious adverse events may very well be higher than the 23% calculated.
General behaviour
Meta‐analyses of data from only seven parallel‐group trials indicated that methylphenidate was associated with an improvement in children’s general behaviour, as reported by teachers. We cannot state anything for sure about the clinical importance of this SMD value. Comparable findings emerged from meta‐analyses of cross‐over trials (endpoint data) as reported by teachers, and from meta‐analyses of nine cross‐over trials (endpoint data) as rated by independent assessors. We also judged this evidence to be of ‘very low’ certainty (see Quality of the evidence).
Quality of life
Meta‐analyses of data from only four parallel‐group trials indicated that methylphenidate was associated with no improvement in children’s quality of life as reported by parents and clinicians. We judged the certainty of the evidence to be ‘very low’ (see Quality of the evidence).
The very low certainty of the evidence, as assessed using the GRADE approach, undermines the confidence that can be placed in the magnitude of any effect. In particular, the prevalence of non‐serious adverse events raises questions about the effectiveness of blinding in these trials. If blinding was broken in just 20% or 30% of participants given methylphenidate, the resulting bias might well account for the small but statistically significant findings concerning the possible benefits of methylphenidate (Coghill 2021; Storebø 2015a).
Overall completeness and applicability of evidence
This review highlights two major issues concerning the overall completeness and applicability of the evidence of the benefits and harms of methylphenidate for children with ADHD: the dearth of trials conducted in children and adolescents in low‐ and middle‐income countries, and the lack of follow‐up beyond six months. Here, we focus on the impact on the applicability of findings of decisions taken as part of this review (choice of rater for assessing change in ADHD symptoms and quality of life, choice of dose and diagnosis), together with issues relating to rating scales, diagnostic criteria, choice of comparators and adverse events.
ADHD symptoms ‐ choice of teacher report
We chose to use teacher‐rated outcomes as the primary measure for both ADHD symptoms and general behaviour, although a number of trials used or relied on parent reports. Some researchers have argued that parent evaluations of ADHD symptoms may not be as reliable as those of other raters such as teachers of pre‐school children (Murray 2007), or college students (Lavigne 2012). For example, Caye 2017 suggests inconsistency in ratings between parents, and in the MTA trial (MTA 1999b), information provided by parents was not always thought to be strong (Efstratopoulou 2013). We tested the robustness of our decision by conducting subgroup analyses and found no significant differences between this score and those of other raters.
Importantly, we do not really know what a lower score on an ADHD symptom scale (like that reported in this review) means for a child’s quality of life and ability to live, learn and function with other people.
Short‐term versus long‐term effects
Based on a subgroup analysis comparing 20 short‐term trials of six months or less to a single long‐term trial of more than six months, we found that the treatment effect for teacher‐rated ADHD symptoms decreased over time (test for subgroup differences: P = 0.04). This was also the case for independent assessor ADHD symptoms (test for subgroup differences: P = 0.0003). However, this was not the case for parent‐rated ADHD symptoms, for which we found no significant differences between short‐term and long‐term duration (test for subgroup differences: P = 0.60). The power was limited in all three subgroup analyses.
We did not identify any trials that examined the effects of more extended exposure on children's general behaviour. Overall, evidence on the long‐term effects of methylphenidate for children and young people with ADHD is lacking, and it is possible that when used for longer periods, any beneficial effects may be diminished or offset by an increase in the risk of harm (Light 2015). Decisions to initiate and persist with treatment will need to weigh potential improvement in ADHD symptoms against adverse events, such as lack of sleep, since this may impact effects on quality of life and learning abilities. This review indicates that these important issues have not been studied sufficiently.
Quality of life
ADHD can exert a significant, negative impact on children’s quality of life, broadly defined. Yet only eight of the 212 included trials measured quality of life in relation both to ADHD and life in general, and it was only possible to synthesise data from four of these trials. In each case the assessments were made by parents, teachers or independent assessors, rather than by children themselves. These external assessors observed no beneficial effects of methylphenidate on quality of life. Children might well have had different views on their own quality of life, and the failure to include child‐reported ratings of quality of life is a significant limitation on the completeness of the evidence. Furthermore, observations of quality of life reported by parents, teachers and independent assessors may be subject to both systematic and random errors.
Dose – choice of moderate or high dose
For children weighing 25 kg or less, the maximum recommended dose of methylphenidate is 30 mg/day compared to 60 mg/day for children weighing more than 25 kg. After careful consideration, we renamed the high‐dose group as 'moderate/high' dose because doses are not always 'high' in heavier children.
Guidelines from the National Institute for Health and Care Excellence (NICE) recommend that methylphenidate can be increased to 0.7 mg/kg per dose up to three times a day, or a total daily dose of 2.1 mg/kg/day (NICE 2018). European guidelines recommend that dosage should begin at a low level of 0.2 mg/kg per dose up to three times a day and should increase according to response, to a ceiling of 0.7 mg/kg per dose (up to three times a day), or a total daily dose of 60 mg/day. (Taylor 2004).
In the parallel‐group trials included in this review, the overall daily dose ranged from 5 mg to 68 mg with a mean reported total daily dose of 34.4 mg/day or 0.78 mg/kg/day. The average dose of any type of modified‐ or extended‐release methylphenidate was 44.2 mg, and the average dose of immediate‐release methylphenidate was 23.0 mg.
However, many of the included trials were short‐term trials, involving medication‐naive children who consequently received lower doses. Furthermore, many of the cross‐over trials used only morning and midday doses to achieve a cross‐over for trial purposes, with no afternoon dose given. However, extended‐release methylphenidate is designed to reduce symptoms in the late afternoon too, so the average expected daily dose would be higher.
We performed subgroup analyses to test differences in the estimate of effect based on differences in dosage. These analyses revealed no differences between low doses (≤ 20 mg/day) compared to moderate/high doses (> 20 mg/day) of methylphenidate. Given the many adverse events that can result when this medication is used, evidence suggests that higher doses may not be needed.
Rating scales
This review included trials from several countries conducted between 1981 and 2022. Pioneers in ADHD research conduct trials in different countries, and psychometric instruments change with trends over time; this is reflected in the variety of rating scales used by investigators in the included trials. Scales based on the diagnostic criteria of the DSM and the ICD measure slightly different constructs. We found significant differences between scales measuring ADHD symptoms, but not between scales measuring general behaviour; we found fewer differences when we performed sensitivity analyses where we pooled subgroups of scales measuring the same ADHD subtype (e.g. scales measuring the inattentive subtype). All trials using subjective rating scales as proxy measures of outcomes are affected by these problems.
Diagnostic criteria
The concept of ADHD has evolved over many years from Sir George Still’s “defect of moral control” in 1902, to Tredgold’s 1908 “ostencephalitic behaviour disorder”, and Kramer’s “hyperkinetic disease of infancy” in 1932 (Lange 2010). Bradley 1937 first reported the positive effects of dextroamphetamine on hyperactive children in 1937 and methylphenidate came onto the market in 1954 (Lange 2010). “Minimal Brain Damage” and “Minimal Brain Dysfunction” were terms used to describe suspected but unproved damage or dysfunction (Lange 2010).
In 1968 the DSM‐II included the term “Hyperkinetic Reaction of Childhood” (APA 1968). In this the DSM‐II referred to a condition “characterized by overactivity, restlessness, distractibility, and short attention span, especially in young children; the behaviour usually diminishes by adolescence” (APA 1968, p. 50).
In the 1970s the emphasis shifted to inattention in the DSM‐III, while the International Classification of Diseases (ICD‐9) continued to focus on hyperactivity (WHO 1988). The DSM‐III introduced the term “Attention Deficit Disorder: with and without hyperactivity (APA 1980).
The DSM‐III‐R then introduced the term “Attention deficit hyperactivity disorder”, removing the subtypes, and focused on a single list of symptoms of inattention, hyperactivity and impulsivity, with a single cut‐off score (Lange 2010).
The DSM‐IV reintroduced subtypes: a predominantly inattentive type and a predominantly hyperactive‐impulsive type, and they added a combined type, with both inattentive and hyperactive‐impulsive symptoms (APA 1994). The DSM‐IV‐TR modified some descriptive text.
In 2013 the DSM‐5 was introduced with several changes, including being placed in the neurodevelopmental section and taking a view across the lifespan (APA 2013). The same 18 symptoms were continued; nine inattentive and nine hyperactive‐impulsive symptoms. At least six symptoms of one domain were required for a diagnosis. Subtypes were replaced with presentation specifiers; “predominantly inattentive presentation”, “predominantly hyperactive‐impulsive presentation” and “combined presentation”. Several symptoms are required in each setting, the age of onset has been changed to require that several inattentive or hyperactive‐impulsive symptoms should have been present before the age of 12 and examples are provided to the criteria to facilitate diagnosis across the lifespan (APA 2013). Comorbid diagnosis with autism is now permitted as well.
The ICD‐11 (WHO 2019), has become more closely aligned with the DSM‐5 (APA 2013), with the diagnosis now being called Attention Deficit Hyperactivity Disorder, based on several inattentive and hyperactive‐impulsive symptoms being present before the age of 12 and causing impairment of functioning in several settings. There are also “predominantly inattentive”, “predominantly hyperactive/impulsive” and “combined” presentations (WHO 2019).
The criteria of both the ICD‐11 (WHO 2019), and the DSM‐5 (APA 2013), encompass a broader spectrum of children with ADHD compared to the earlier criteria for hyperkinetic disorder in the ICD‐10 (WHO 1992) and the DSM‐IV‐TR (APA 2000).
Comparators
The majority of trials in this review compared methylphenidate with placebo, and we previously highlighted the problems surrounding blinding in these trials, due to the prevalence of non‐serious adverse events caused by methylphenidate. Trials that assess methylphenidate using an ‘active placebo’ (or 'nocebo tablets' ‐ tablets with a placebo‐like substance that causes similar adverse events as in the experimental drug arm), can strengthen double‐blinding and are thus recommended (Jakobsen 2013; Jakobsen 2014; Moncrieff 2004). We identified no such trials, and so far, no substance has yet been identified that has the necessary properties to act as a nocebo in trials of stimulants. The use of nocebo tablets for all conditions is ethically uncertain, and any decision to conduct nocebo tablet‐controlled trials in children would normally be deferred by the Food and Drug Administration (FDA) in the USA or the European Medicines Agency (EMA) regulators, until trials have been done safely in adults. If these show methylphenidate to be superior compared with nocebo in treating ADHD symptoms, a rationale would exist for conducting such trials in children. Laursen and colleagues have conducted a systematic review with the aim of investigating the difference between an active versus a standard placebo when these are compared with an experimental (drug) intervention (Laursen 2022). This difference in effects of the pharmaceutical intervention can be estimated by directly comparing the effect difference between the active and standard placebo intervention. Laursen 2022 included 21 trials with both an active placebo and a standard placebo control arm. The primary analysis showed no difference on patient‐reported outcomes between standard and active placebo in preclinical and clinical trials. However, an analysis including only trials at low risk of bias showed a difference of SMD −0.24 (95% CI −0.34 to −0.13). This means that a drug intervention compared with an active placebo (nocebo) control group will show a SMD between −0.34 to −0.13 lower effect than when the drug is compared with standard placebo (Laursen 2020; Laursen 2022).
Adverse events
Twenty‐four parallel‐group trials and 61 cross‐over trials excluded methylphenidate non‐responders, placebo responders or participants with methylphenidate adverse events before randomisation. Such designs are often named enrichment designs (Burnett 2021). We compared the intervention effect of methylphenidate in these trials with that in the remaining trials in subgroup analyses (Analysis 1.6; Analysis 2.5; Analysis 3.6), which found no differences in terms of the intervention effect of methylphenidate in teacher‐rated and parent‐rated ADHD symptoms. However, there were differences in the intervention effects of methylphenidate when we compared the independent‐rated ‘enrichment trials’ with the remaining trials.
Some of our included trials involved participants who were not medication‐naive before randomisation, which may have exaggerated the benefits of methylphenidate. They might have detected the physiological effects (for example, improved concentration or adverse events such as appetite suppression) through prior exposure to the effects of methylphenidate. To investigate this, we performed post hoc subgroup analyses and found that effects of methylphenidate were not different in trials involving medication‐naive participants (> 80% of included participants were medication‐naive) than in trials involving participants already familiar with methylphenidate before randomisation (< 20% of included participants were medication‐naive) for teacher‐rated ADHD symptoms (P = 0.44), and parent‐rated ADHD symptoms (P = 0.48). One might expect the issue of prior exposure to be of greatest concern in cross‐over trials. However, we found no differences between parallel‐group trials and cross‐over trials in teacher‐rated, independent‐rated or parent‐rated ADHD outcomes. Consequently, we believe that prior exposure is not a major concern when the effects of methylphenidate are assessed.
Our Cochrane systematic review from 2018, which focused on the harms from methylphenidate for children and adolescents with ADHD included 260 non‐randomised studies, with around 2.2 million participants (Storebø 2018b). We found that methylphenidate compared to no intervention significantly increased the risk of serious adverse events in comparative studies (RR 1.36, 95% CI, 1.17 to 1.58; 2 trials, 72,005 participants). Serious adverse events included psychotic disorders, arrhythmia, seizures, and hypertension. More than half of participants (51.2%) experienced one or more non‐serious adverse event (95% CI 41.2% to 61.1%; 49 trials, 13,978 participants). These included sleep difficulties (17.9%), decreased appetite (31.1%), and abdominal pain (10.7%). Furthermore, 16.2% (95% CI 13.0 to 19.9%; 57 trials, 8340 participants) discontinued methylphenidate because of “unknown” reasons and 6.20% (95% CI 4.90 to 8.00%; 37 trials, 7142 participants) because of non‐serious adverse events (Storebø 2018b).
Many claims have been made about significant increases in global rates of methylphenidate prescribing; this drug is usually prescribed for long‐term use and seldom with medication‐free periods. However, a recent paper reports that many children in primary care in the UK do not continue methylphenidate treatment for longer than six months (Raman 2015). Furthermore, the prevalence of ADHD diagnoses in the UK has decreased between 1998 and 2010 (Holden 2013). In the USA, however, almost 70% of children with ADHD, estimated at 6.4 million children, take medication (Visser 2014). This might mean that clinicians in the UK are more cautious about prescribing methylphenidate, while clinicians in the USA assume that evidence for the safe use of methylphenidate is sound.
Our assessment of the evidence does not deny that some patients may benefit from methylphenidate. However, despite more than 70 years of research in this field, we do not yet know how to identify those in whom the benefits outweigh the harms. Further research, possibly through individual patient data meta‐analyses or other new methodologies, is needed to identify such patient characteristics. This personalised medicine approach can be used for discovering predictors and moderators for treatment response (Buitelaar 2022).
Quality of the evidence
We assessed the certainty of the evidence that contributed to all outcomes using the GRADE approach. We downgraded all primary and secondary outcomes by two levels due to the high risk of bias. This was due to risk of bias in several domains, including loss of blinding (explained below) and selective outcome reporting. We rated the risk of outcome reporting bias for adverse events to be high, as we only managed to obtain data on the proportion of participants with total serious adverse events from 43 of the 212 included trials, and on proportions of participants with total non‐serious adverse events from 60 of the 212 included trials.
Except for results on serious adverse events, we additionally downgraded all other primary outcomes by one level due to inconsistency as a result of moderate statistical heterogeneity. We additionally downgraded the results on serious adverse events by two levels due to imprecision as a result of wide confidence intervals and because the acquired number of participants was below 50% of the DARIS in Trial Sequential Analysis. Finally, we additionally downgraded general behaviour and quality of life by one level each due to indirectness, considering the discrepancy in the use of rating scales and because the assessment was performed by the parents, respectively. As a result, we assessed the certainty of the evidence for each outcome to be very low, thus reflecting the uncertainty in the robustness of our estimates.
We initially rated 22 of the included trials at low risk of bias, but it is likely that these trials may, in fact, be trials at high risk of bias. This is because methylphenidate gives rise to common, easily recognisable adverse events. This can lead to loss of blinding and overestimation of benefits whilst underestimating the harms (Kjaergard 2001; Savović 2012b; Storebø 2015a; Wood 2008).
To ensure adequate blinding, it is therefore important for researchers to try to reduce the bias that may arise from this. This could include using separate assessors to measure adverse effects and efficacy, which could help maintain blinding of those assessing efficacy whilst allowing both adverse effects and efficacy to be measured. As we found no trials in which separate assessors evaluated adverse effects and efficacy and no trials that used active placebos, we cannot assess the extent of this bias. There is also increasing interest in finding a safe active placebo (a nocebo) that could mimic the adverse effects in the control group without acting as a stimulant. This too could improve blinding significantly. But the practicalities of identifying such a substance, testing the safety, and obtaining regulatory approval for its use, are likely to take many years to achieve. There are researchers working on this already, and increasing interest to find solutions to the important area of protecting blinding. The fact that the intervention effect of methylphenidate on ADHD symptoms did not differ significantly between trials at low risk of bias compared to trials at high risk of bias may be taken as an indication that deblinding has occurred among former trials. Also, the average duration of treatment was no longer than about two months. Therefore, we can conclude little about the benefits and harms of methylphenidate used for longer than six months. (Coghill 2021; Laursen 2020; Laursen 2022).
Potential biases in the review process
The present systematic review has many strengths. We developed a protocol for this review according to instructions provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2022a), and this protocol was published before we embarked on the review itself. We conducted extensive searches of relevant databases, and we requested published and unpublished data from pharmaceutical companies manufacturing methylphenidate, including Takeda Pharmaceuticals, Medice (represented in Denmark by HB Pharma), Janssen‐Cilag, Novartis, Rhodes Pharmaceuticals, Ironshore Pharmaceuticals and Pfiizer. Two review authors, working independently, selected trials for inclusion and extracted data. We resolved disagreements by discussion with team members. We assessed risk of bias in all trials according to the recommendations provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We conducted Trial Sequential Analyses to control the risk of type I errors and type II errors and to estimate how far we were from obtaining the DARIS to detect or reject a certain plausible intervention effect (CTU 2022). In the meta‐analyses on non‐serious adverse events, the Trial Sequential Analysis showed that observed intervention effects were not likely to be due to type I error and confirmed that sufficient data had been obtained.
Although we added new search terms to the strategy, we limited the search to the period since the previous search (2015 onwards), so it is possible that we did not capture pre‐2015 records containing the new search terms. However, we believe that we are unlikely to have missed any important trials because of our supplementary searches, which included identifying studies through reference checks of relevant reviews, and contacting pharmaceutical companies.
We excluded 126 trials described in 144 reports, which assessed the effects of methylphenidate on specialised outcomes (e.g. experimental, neurocognitive or functional outcomes) in children or adolescents with ADHD (see Characteristics of excluded studies). This raises the issue of bias in our review process as we did not write to these authors asking whether they collected data on other outcomes. This potential bias, however, is not likely to change our conclusions.
Agreements and disagreements with other studies or reviews
Over the past 20 years, several published systematic reviews and narrative reviews have examined the efficacy of methylphenidate for ADHD (with or without meta‐analysis). All of them described methylphenidate as being very helpful to children and adolescents with ADHD. However, they each had methodological shortcomings.
The 2015 version of this review contradicted earlier published reviews as we reported that methylphenidate may improve teacher‐reported ADHD symptoms, teacher‐reported general behaviour, and parent‐reported quality of life among children and adolescents diagnosed with ADHD, but the low quality of the evidence meant that we could not be certain of the true magnitude of these effects (Storebø 2015a). The 2015 version of this review provoked many critical responses, published in articles, letters to editors and blogs. We responded to all of the comments; for more details please see the section “Why it is important to do this review". An overview article found 24 eligible systematic reviews and meta‐analyses published after our 2015 review (Ribeiro 2021). The results from the overview also showed that the evidence was uncertain due to its low quality. Additionally, this overview highlighted the underreporting of adverse events in RCTs, and concluded that evidence supporting methylphenidate being beneficial in the treatment of children and adolescents with ADHD remains uncertain (Ribeiro 2021).
Our current updated review confirms our 2015 findings with 29 additional RCTs included (Storebø 2015a). These results differ from the large network meta‐analysis by Cortese and colleagues (Cortese 2018), in which the use of methylphenidate in children and adolescents was strongly supported by the evidence where they compared the efficacy and tolerability of methylphenidate for ADHD to placebo alongside other medications (Cortese 2018). We published a letter to the editor of the Lancet in which we highlighted several problems with their review, namely, the exclusion of many relevant trials in order to fulfil statistical and methodological assumptions that they made. While the pooled comparison for clinician‐rated effects of methylphenidate versus placebo for children was rated as "moderate quality of evidence", they also assessed all of the indirect comparisons as being of “low to very low quality of evidence”. Indirect evidence differentiates network meta‐analyses from conventional meta‐analyses. Given the decreased interpretability of the indirect comparisons, there are no novel findings in this network meta‐analysis (Faltinsen 2018a; Storebø 2018a). In sum, the part of the network meta‐analysis that is different from our review published in 2015 (Storebø 2015a), consists of evidence of low to very low certainty.
In a response to our letter, the authors confirmed that they had excluded 65% of the trials that we had included in our 2015 review. They excluded 51 trials that had under seven days of treatment, 38 cross‐over trials without pre‐crossover data or a washout, 18 trials with responders to previous treatment, and 14 trials where treatment was not monotherapy (Cipriani 2018). They did this because including these trials would have been a clear violation of their published protocol and would have compromised the transitivity of the network meta‐analyses (Cipriani 2018).
Catalá‐López and colleagues published a large systematic review with network meta‐analyses in 2017 (Catala‐Lopez 2017). They included 190 RCTs with a total of 26,114 children and adolescents with ADHD, and found that stimulant monotherapy was significantly more efficacious than placebo; however, all analyses were assessed in GRADE at “low or very low certainty”. The authors of the review concluded that stimulants may improve the symptoms of ADHD, but the strength of the underlying evidence remains uncertain (Catala‐Lopez 2017).
Padilha and colleagues published a network meta‐analysis investigating the benefits and harms of different types of ADHD medication (including methylphenidate) for children and adolescents with ADHD, including 48 trials with 4169 participants (Padilha 2018). The review found that there were beneficial effects of methylphenidate on the Clinical Global Impressions Improvement scale (CGI‐I) and that methylphenidate was more effective than the non‐stimulants atomoxetine and guanfacine (Padilha 2018). There are several methodological problems with this review which we commented on in a letter to the editor (Faltinsen 2019). The issues we raised were focused on selection bias (as the authors had excluded placebo‐controlled trials), the fact that authors judged the methodological quality of the included trials to be good, asserting that they were well designed, reported, and conducted, even when this clearly was not the case and that they did not include an overall assessment of certainty such as the GRADE system. They also included cross‐over trials without reporting the method as to how they pooled this data with that from parallel‐group trials and they failed to discuss other possible issues, such as carry‐over and period effects (Faltinsen 2019). Furthermore, they did not assess the transitivity assumption in their network meta‐analyses (Faltinsen 2018b; Faltinsen 2019).
A review by Cerrillo‐Urbina and colleagues investigating the benefits and harms of stimulants and non‐stimulants included 15 RCTs, with 4648 children or adolescents, or both, from 6 to 17 years of age diagnosed with ADHD (Cerrillo‐Urbina 2018). Only four trials assessed methylphenidate, all of which were conducted before 2013. The GRADE assessment of the evidence concerning the total score of ADHD symptoms was assessed to be “moderately high quality of evidence” for both stimulant and non‐stimulant medications. They downgraded the quality of evidence by one level due to a high degree of heterogeneity in the pooled results (I² > 75%) but did not downgrade it further for risk of bias or publication bias, even though they found that there was significant publication bias for all outcomes. It is striking that this review only included four trials on methylphenidate, whereas we found 184 trials in our 2015 review covering the same period (Storebø 2015a).
A network meta‐analysis by Li and colleagues found that methylphenidate was beneficial in the treatment of ADHD in children and adolescents (Li 2017). Methylphenidate was considered the second safest treatment compared to the other ADHD medications. The review included 62 trials in a meta‐analysis, which included 12,930 participants. They did not make any attempt to evaluate the risk of bias or the certainty of evidence, which significantly lowers the robustness and validity of this review.
The NICE guideline recommends methylphenidate as the first‐line pharmacological treatment for children over five and adolescents, "1.7.7: Offer methylphenidate (either short or long acting) as the first‐line pharmacological treatment for children aged 5 years and over and young people with ADHD" (NICE 2018). The NICE guideline committee concluded that methylphenidate and lisdexamphetamine provide clinically important benefits to patients with ADHD as compared to placebo and other drugs (NICE 2018). We found several methodological problems in the NICE ADHD guidelines as we believe they conducted an erroneous assessment of the certainty of the included studies. They assessed the quality of meta‐analysis to be “ high quality”, when it could be strongly argued that it was, in fact, “low quality”. In their assessment of the effect of methylphenidate, they included only 16 trials that focused solely on immediate and osmotic‐release methylphenidate in children and adolescents. We included 185 trials (175 of which were placebo‐controlled) in our 2015 review (Storebø 2015a). NICE did not adjust for multiple comparisons and did not discuss the concern that all the data arose from short‐term follow‐up (NICE 2018).
The American Academy of Pediatrics guideline was updated in 2019 based on patients’ age (Wolraich 2019). With regard to preschool children, the guideline recommends evidence‐based behavioural interventions (behavioural parent training or behavioural classroom interventions, or both) as the first‐choice treatment. Methylphenidate may be considered when a child has moderate to severe problems with functioning and if the behavioural treatment does not provide the necessary improvements. With regard to school children, the guideline strongly recommends pharmaceutical treatments (FDA‐approved medications for ADHD) together with behavioural interventions. Regarding adolescents, the guideline strongly recommends pharmaceutical treatment and if possible, evidence‐based behavioural interventions. The guideline states that there is a strong effect observed in the trials investigating the effects of stimulant medications (Wolraich 2019). For the comparison of pharmacological treatments versus placebo or usual care, the review only identified eight articles representing seven studies. The review concluded that there was limited additional evidence concerning FDA‐approved ADHD medications compared with placebo or usual care across all outcomes in this updated systematic evidence review. The conclusions regarding methylphenidate, therefore, seem overly positive. The risk of harm is considered as low and the benefits, in general, are described as outweighing the risks.
Our current updated review is in line with two recent Cochrane systematic reviews of methylphenidate in adults. These two reviews found low‐ or very low‐certainty evidence that methylphenidate, compared with placebo, improved ADHD symptoms (Boesen 2022; Candido 2021).
Authors' conclusions
Implications for practice.
Methylphenidate may improve attention deficit hyperactivity disorder (ADHD) symptoms and general behaviour in children and adolescents with ADHD aged 18 years and younger. We rated the evidence to be of very low certainty and, as a result, we cannot be certain about the magnitude of the effects from the meta‐analyses. The evidence is limited by the serious risk of bias in the included trials, underreporting of relevant outcome data, and a high level of statistical variation between the results of the trials. There is also very low‐certainty evidence that methylphenidate causes numerous adverse events. The risk of serious adverse events seems low, but data were only available from 43 of the 212 included trials. It is also problematic that only 93 of the 212 included trials reported on specific and overall non‐serious adverse events. Accordingly, we cannot rule out the possibility that non‐serious harms are more prevalent than reported in our review.
If methylphenidate treatment is considered, clinicians might need to use it for short periods, with careful monitoring of both benefits and harms, and cease its use if no evidence of clear improvement of symptoms is noted, or if harmful effects appear. A problem is that clinicians very often rely on their assessment of methylphenidate in their clinical evaluation. Arguments like "I know that this medication helps" can be problematic when they are based on anecdotal evidence and case reports. A new review found that clinicians had difficulties in assessing benefits or harms following treatment, with inaccuracies in both directions (Hoffmann 2017). Clinicians mostly underestimated instead of overestimated harms and overestimated rather than underestimated benefits. Inaccurate perceptions of the benefits and harms of treatments are likely to result in uncertain clinical management choices (Hoffmann 2017).
Implications for research.
This review highlights the urgent need for long‐term, high‐quality, and large randomised clinical trials (RCTs), at low risk of bias, to investigate the benefits and harms of methylphenidate treatment versus placebo in children and adolescents with ADHD. Such trials ought to be designed according to the SPIRIT (Standard Protocol Items: Recommendations for Intervention Trials) guidelines (Chan 2013), and reported in keeping with the CONSORT (Consolidated Standards of Reporting Trials) standards Moher 2010). Pre‐published protocols could help reduce the inconsistent measurement of benefits and harms caused by the use of many different rating scales and by lack of assessment of adverse events.
The important issue of protecting blinding of these trials needs to be addressed urgently. Immediate measures could be implemented to improve blinding. Having independent, blinded assessors monitor adverse effects, whilst separate, independent blinded assessors measure efficacy, is likely to reduce the risk of unblinding due to adverse effects. Active placebos need to be sought and are likely to be important in the future, but their development is still at the very early stages. Research in this field should be strongly supported, but it is likely to take many years before such substances can be used safely and ethically in research with children and adolescents. The prevalent use of cross‐over trials needs to be reconsidered as they usually only provide short‐term interventions, which can limit the assessment of benefits and harms. However, we were not able to identify major differences when comparing parallel‐group trials with cross‐over trials.
Future trials ought to publish depersonalised individual participant data and should report all outcomes, including adverse events, to ensure that future systematic reviews and meta‐analyses can access and use individual participant data. Only through meta‐analyses will we be able to assess differences between intervention effects according to age, sex, comorbidity, ADHD subtype, and dose. Reviews show that many different rating scales are used for children with ADHD. Consistent use of well‐validated scales is needed, as is a country‐wide adverse events reporting system, such as the Food and Drug Administration, to increase awareness of adverse events. In addition, the findings in this review clearly show the urgent need for large RCTs to investigate the efficacy of non‐pharmacological treatments. As with RCTs, systematic reviews of such trials assess average effects in groups of individuals. Such average effects may comprise strong benefits for a single participant or a few participants and no effect or negative effects for others. Despite more than 50 years of research in this field, we have no knowledge on how to identify patients who may obtain more benefits than harms. Individual patient data meta‐analyses are needed to identify such patient characteristics. Therefore, it would be extremely helpful for review authors to gain full access to anonymised individual participant data for inclusion in meta‐analyses examining these data (Gluud 2015). Patient subgroups may benefit from intervention if those with reduced rates of adverse events can be identified. This personalised medicine approach can be used for discovering predictors and moderators for treatment response. The use of biomarkers for both more precise diagnoses and for more precise assessment of treatment response is necessary in future RCTs. The use of enrichment designs will improve statistical power for biomarker analyses (Buitelaar 2022).
Feedback
Comments on the BMJ version of this review, 29 November 2016
Summary
Fazel M. Methylphenidate for ADHD. BMJ 2015;351:h5875. [DOI: 10.1136/bmj.h5875]. Available from bmj.com/content/351/bmj.h5875.long
Grant E. Re: Methylphenidate for attention‐deficit/hyperactivity disorder in children and adolescents: Cochrane systematic review with meta‐analyses and trial sequential analyses of randomised clinical trials [personal communication]. Response to: OJ Storebø, HB Krogh, E Ramstad, CR Moreira‐Maia, M Holmskov, M Skoog, et al. 27 November 2015. Available from bmj.com/content/351/bmj.h5203/rr
Kremer HJ. Re: Methylphenidate for attention‐deficit/hyperactivity disorder in children and adolescents: Cochrane systematic review with meta‐analyses and trial sequential analyses of randomised clinical trials [personal communication]. Response to: OJ Storebø, HB Krogh, E Ramstad, CR Moreira‐Maia, M Holmskov, M Skoog, et al. 27 November 2015. Available from bmj.com/content/351/bmj.h5203/rr-0
Chandrasekaran V, Mahadevan S. Re: Methylphenidate for attention‐deficit/hyperactivity disorder in children and adolescents: Cochrane systematic review with meta‐analyses and trial sequential analyses of randomised clinical trials [personal communication]. Response to: OJ Storebø, HB Krogh, E Ramstad, CR Moreira‐Maia, M Holmskov, M Skoog, et al. 29 November 2015. Available from bmj.com/content/351/bmj.h5203/rr-1
Büchter RB, Thomas S. Re: Methylphenidate for attention‐deficit/hyperactivity disorder in children and adolescents: Cochrane systematic review with meta‐analyses and trial sequential analyses of randomised clinical trials [personal communication]. Response to: OJ Storebø, HB Krogh, E Ramstad, CR Moreira‐Maia, M Holmskov, M Skoog, et al. 10 December 2015. Available from bmj.com/content/351/bmj.h5203/rr-3
Saripanidis S. Management and treatment of hyperactivity and ADHD, without methylphenidate [personal communication]. Response to: OJ Storebø, HB Krogh, E Ramstad, CR Moreira‐Maia, M Holmskov, M Skoog, et al. 27 December 2015. Available from bmj.com/content/351/bmj.h5203/rr-5
Banaschewski T, Buitelaar J, Chui CSL, Coghill D, Cortese S, Simonoff E, et al, on behalf of the European ADHD Guidelines Group. Are Methylphenidate Effects in Children with ADHD Really Uncertain? [personal communication]. Response to Storebø OJ, Krogh HB, Ramstad E, Moreira‐Maia CR, Holmskov M, Skoog M, et al. 27 July 2016. Available from bmj.com/content/351/bmj.h5203/rr-6
Reply
Storebø OJ, Gluud C. Re: Methylphenidate for attention‐deficit/hyperactivity disorder in children and adolescents: Cochrane systematic review with meta‐analyses and trial sequential analyses of randomised clinical trials [personal communication]. Response to: E Grant, HJ Kremer, V Chandrasekaran, S Mahadevan. 30 November 2015. Available from bmj.com/content/351/bmj.h5203/rr-2
Storebø OJ, Gluud C. Re: Methylphenidate for attention‐deficit/hyperactivity disorder in children and adolescents: Cochrane systematic review with meta‐analyses and trial sequential analyses of randomised clinical trials [personal communication]. Response to: RB Büchter, S Thomas. 22 December 2015. Available from bmj.com/content/351/bmj.h5203/rr-4
Storebø OJ, Zwi M, Gluud C. Re: Methylphenidate for attention‐deficit/hyperactivity disorder in children and adolescents: Cochrane systematic review with meta‐analyses and trial sequential analyses of randomised clinical trials [personal communication]. Response to: T Banaschewski, J Buitelaar, CSL Chui, D Coghill, S Cortese, E Simonoff, et al. 29 July 2016. Available from bmj.com/content/351/bmj.h5203/rr-9
Storebø OJ, Zwi M, Gluud C. Re: Methylphenidate for attention‐deficit/hyperactivity disorder in children and adolescents: Cochrane systematic review with meta‐analyses and trial sequential analyses of randomised clinical trials [personal communication]. Response to: T Banaschewski, J Buitelaar, CSL Chui, D Coghill, S Cortese, E Simonoff. 29 July 2016. Available from bmj.com/content/351/bmj.h5203/rr-10
Storebø OJ, Zwi M, Gluud C. Re: Methylphenidate for attention‐deficit/hyperactivity disorder in children and adolescents: Cochrane systematic review with meta‐analyses and trial sequential analyses of randomised clinical trials [personal communication]. Response to: T Banaschewski, J Buitelaar, CSL Chui, D Coghill, S Cortese, E Simonoff. 29 July 2016. Available from bmj.com/content/351/bmj.h5203/rr-11
Contributors
Contributor 1: Mina Fazel, National Institute for Health Research (NIHR) Postdoctoral Research Fellow, Department of Psychiatry, University of Oxford, Warneford Hospital, Oxford, UK; Consultant in Child and Adolescent Psychiatry, Department of Children’s Psychological Medicine, The Children’s Hospital, Oxford University Hospitals Foundation Trust, Oxford, UK. Contributor 2: Ellen Grant, Physician and Medical Gynaecologist, Retired, Kingston‐upon‐Thames, UK. Contributor 3: Hans‐Joachim Kremer, Medical Writer, Medical Writing Service, Alemannenstraße 101, 79117 Freiburg, Germany.Contributor 4: Venkatesh Chandrasekaran Assistant Professor of Pediatrics, Jawaharlal Institute of Postgraduate Medical Education & Research (JIPMER), Dhanvantri Nagar,Puducherry‐605006, India.Contributor 5: Subramanian Mahadevan, Senior Professor and Head of the Department, Department of Pediatrics, JIPMER, Dhanvantri Nagar,Puducherry‐605006, India.Contributor 6: Roland Brian Büchter Medical Writer, Institute for Quality and Efficiency in Health Care (IQWiG), Im Mediapark 8, 50670 Cologne, Germany.Contributor 7: Stefanie Thomas, Head of Quality Assurance Unit, IQWiG, Im Mediapark 8, 50670 Cologne, Germany. Contributor 8: Stavros Saripanidis, Consultant in Obstetrics and Gynaecology, Thessaloniki, Greece. Contributor 9: Tobias Banaschewski, Department of Child Adolescent Psychiatry and Psychotherapy, Central Institute of Mental Health, Mannheim, Germany. Contributor 10: Jan Buitelaar, Department of Cognitive Neuroscience, Donders Institute of Brain, Cognition and Behavior, Radboud University Medical Center, Nijmegen, the Netherlands; Karakter Child and Adolescent Psychiatry University Center, Nijmegen, the Netherlands. Contributor 11: Celine SL Chui, Department of Pharmacology and Pharmacy, University of Hong Kong, Hong Kong, China. Contributor 12: David Coghill, Departments of Paediatrics and Psychiatry, University of Melbourne, Victoria, Australia. Contributor 13: Samuele Cortese, Department of Psychology, University of Southampton, Southampton, UK; Clinical and Experimental Sciences, Faculty of Medicine, University of Southampton. Contributor 14: Emily Simonoff, Department of Child & Adolescent Psychiatry, Institute of Psychiatry Psychology & Neuroscience, King’s College London, London, UK. Contributor 15: Ian C. K. Wong, UCL School of Pharmacy, Faculty of Life Sciences, UCL, London, UK; Institute of Child Health, London, UK.
Author 1: Ole Jakob Storebø, Senior Researcher, Psychiatric Research Unit, Psychiatric Department, Region Zealand, Slagelse, Denmark; Department of Psychology, Faculty of Health Science, University of Southern Denmark. Author 2: Christian Gluud, Head of department, The Copenhagen Trial Unit, Centre for Clinical Intervention Research, Department 7812, Rigshospitalet, Copenhagen, Denmark. Author 3: Morris Zwi, Consultant Child & Adolescent Psychiatrist and Clinical Lead, Islington CAMHS, Whittington Health, London, UK.
Mental Elf Blog, 29 November 2016
Summary
Hollis C. Methylphenidate for ADHD: have Cochrane got it wrong this time? [personal communication]. Response to: Storebø OJ, Ramstad E, Krogh HB, Nilausen TD, Skoog M, Holmskov M, et al. 10 March 2016. Available from nationalelfservice.net/mental-health/adhd/methylphenidate-for-adhd-have-cochrane-got-it-wrong-this-time
Reply
Storebø OJ, Gluud C. Re: Methylphenidate for ADHD: have Cochrane got it wrong this time? [personal communication]. Response to: Chris Hollis. March 2016. Available from nationalelfservice.net/mental-health/adhd/methylphenidate-for-adhd-have-cochrane-got-it-wrong-this-time/#comment-1002564
Hollis C. Re: Methylphenidate for ADHD: have Cochrane got it wrong this time? [personal communication]. Response to: OJ Storebø, C Gluud. April 2016. Availabe from nationalelfservice.net/mental-health/adhd/methylphenidate-for-adhd-have-cochrane-got-it-wrong-this-time/#comment-1007912
Storebø OJ, Gluud C. Re: Methylphenidate for ADHD: have Cochrane got it wrong this time? [personal communication]. Response to: C Hollis. April 2016. Available from nationalelfservice.net/mental-health/adhd/methylphenidate-for-adhd-have-cochrane-got-it-wrong-this-time/#comment-1008187
Hollis C. Re: Methylphenidate for ADHD: have Cochrane got it wrong this time? [personal communication]. Response to: OJ Storebø, C Gluud. April 2016. Available from nationalelfservice.net/mental-health/adhd/methylphenidate-for-adhd-have-cochrane-got-it-wrong-this-time/#comment-1010366
Storebø OJ, Gluud C. Re: Methylphenidate for ADHD: have Cochrane got it wrong this time? [personal communication]. Response to: C Hollis. April 2016. Available from nationalelfservice.net/mental-health/adhd/methylphenidate-for-adhd-have-cochrane-got-it-wrong-this-time/#comment-1011280
Storebø OJ, Gluud C. Methylphenidate for ADHD: have Chris Hollis also got it wrong this time? [personal communication]. Response to: C Hollis. June 2016. Available from nationalelfservice.net/mental-health/adhd/methylphenidate-for-adhd-have-cochrane-got-it-wrong-this-time/#comment-1018081
Contributors
Contributor 1: Chris Hollis, Professor Child & Adolescent Psychiatry, Faculty of Medicine & Health Sciences, University of Nottingham, Nottingham, UK.
Author 1: Ole Jakob Storebø, Senior Researcher, Psychiatric Research Unit, Psychiatric Department, Region Zealand, Slagelse, Denmark; Department of Psychology, Faculty of Health Science, University of Southern Denmark. Author 2: Christian Gluud, Head of department, The Copenhagen Trial Unit, Centre for Clinical Intervention Research, Department 7812, Rigshospitalet, Copenhagen, Denmark.
Comments on the JAMA version of this review, 29 November 2016
Summary
Shaw P. Quantifying the benefits and risks of Methylphenidate as treatment for childhood attention‐deficit/hyperactivity disorder. JAMA. 2016;315(18):1953‐5. [DOI:10.1001/jama.2016.3427]. Available from jamanetwork.com/journals/jama/article-abstract/2520612
Romanos M, Reif A, Banaschewski T. Methylphenidate for attention‐deficit/hyperactivity disorder. JAMA. 2016;316(9):994‐5. [DOI:10.1001/jama.2016.10279]. Available from jamanetwork.com/journals/jama/article-abstract/2547744
Reply
Storebø OJ, Simonsen E, Gluud C. Methylphenidate for attention‐deficit/hyperactivity disorder — Reply. JAMA. 2016;316(9):995. [DOI:10.1001/jama.2016.10300]. Available from jamanetwork.com/journals/jama/article-abstract/2547750
Contributors
Contributor 1: Philip Shaw, Section on Neurobehavioral Clinical Research, Social and Behavioral Research Behavioral Research Branch, National Human Genome Research Institute, Bethesda, Maryland; National Institute of Mental Health, Bethesda, Maryland. Contributor 2: Marcel Romanos, Department of Child and Adolescent Psychiatry, Psychosomatics and Psychotherapy, University Hospital of Würzburg, Würzburg, Germany. Contributor 3: Andreas Reif, Department of Psychiatry, Psychosomatic Medicine and Psychotherapy, University Hospital Frankfurt, Frankfurt, Germany. Contributor 4: Tobias Banaschewski, Department of Child and Adolescent Psychiatry, Central Institute of Mental Health, Mannheim, Germany.
Author 1: Ole Jakob Storebø, Psychiatric Research Unit, Region Zealand, Denmark. Author 2: Erik Simonsen, Psychiatric Research Unit, Region Zealand, Denmark. Author 3: Christian Gluud, Copenhagen Trial Unit, Copenhagen, Denmark.
Other comments on this review published elsewhere, 29 November 2016
Summary
Mulder R, Hazell P, Rucklidge JJ, Malhi GS. Methylphenidate for attention‐deficit/hyperactivity disorder: too much of a good thing? Australian & New Zealand Journal of Psychiatry 2016;50(2):113–4. [DOI: 10.1177/0004867415626823] Available from anp.sagepub.com/search/results?fulltext=storebo&x=0&y=0&submit=yes&journal_set=spanp&src=selected&andorexactfulltext=and
Levy F. Methylphenidate for attention‐deficit/ hyperactivity disorder: the longest debate. Australian & New Zealand Journal of Psychiatry 2016;50(7):616–7. [DOI: 10.1177/0004867416643390] Available from anp.sagepub.com/content/50/7/616.full.pdf
Hoekstra PJ, Buitelaar JK. Is the evidence base of methylphenidate for children and adolescents with attention‐deficit/hyperactivity disorder flawed? European Child & Adolescent Psychiatry 2016;25(4):339–40. [DOI: 10.1007/s00787‐016‐0845‐2] Available from link.springer.com/article/10.1007/s00787-016-0845-2
Banaschewski T, Gerlach M, Becker K, Holtmann M, Döpfner M, Romanos M. The errors and misinterpretations in the Cochrane analysis by O. J. Storebo and colleagues on the efficacy and safety of methylphenidate for the treatment of children and adolescents with ADHD. Trust, but verify. Zeitschrift für Kinder‐ und Jugendpsychiatrie und Psychotherapie 2016;44:307‐14. [DOI: 10.1024/1422‐4917/a000433] Available from econtent.hogrefe.com/doi/abs/10.1024/1422-4917/a000433
Banaschewski T, Buitelaar J, Chui CSL, Coghill D, Cortese S, Simonoff E, et al. Methylphenidate for ADHD in children and adolescents: throwing the baby out with the bathwater. Evidence‐Based Mental Health 2016;19(4):97‐9. [DOI: 10.1136/eb‐2016‐102461 ] Available from ebmh.bmj.com/content/19/4/97.full
Reply
Storebø OJ, Simonsen E, Gluud C. The evidence base of methylphenidate for children and adolescents with attention‐deficit hyperactivity disorder is in fact flawed. European Child & Adolescent Psychiatry 2016;25(9):1037–8. [DOI:10.1007/s00787‐016‐0855‐0]. Available from link.springer.com/article/10.1007/s00787-016-0855-0
Storebø OJ, Zwi M, Moreira‐Maia CR, Skoog M, Camilla G*, Gillies D, et al. Response to “Trust, but verify” by Banaschewski et al. Zeitschrift für Kinder‐ und Jugendpsychiatrie und Psychotherapie 2016;44:334‐5. [DOI: 10.1024/1422‐4917/a000472]. Available from econtent.hogrefe.com/doi/abs/10.1024/1422-4917/a000472
Storebø OJ, Gluud C. Re: Trust, but verify. The errors and misinterpretations in the Cochrane analysis by O. J. Storebo and colleagues on the efficacy and safety of methylphenidate for the treatment of children and adolescents with ADHD [personal communication]. Response to: T BanaschewskI, M Gerlach, K Becker, M Holtmann, M Döpfner, M Romanos. 24 June 2016. Available from ncbi.nlm.nih.gov/pubmed/27270192#cm27270192_16360
Storebø OJ, Zwi M, Krogh HB, Moreira‐Maia CR, Holmskov M, Gillies D, et al. Evidence on methylphenidate in children and adolescents with ADHD is in fact of ‘very low quality'. Evidence‐Based Mental Health 2016;19(4):100‐2. Available from ebmh.bmj.com/content/19/4/100.full
Contributors
Contributor 1: Roger Mulder, Department of Psychological Medicine, University of Otago — Christchurch, Christchurch, New Zealand. Contributor 2: Philip Hazell, Discipline of Psychiatry, Sydney Medical School, University of Sydney, Sydney, NSW, Australia.Contributor 3: Julia J Rucklidge, University of Canterbury, Christchurch, New Zealand.Contributor 4: Gin S Malhi, Discipline of Psychiatry, Kolling Institute, Sydney Medical School, University of Sydney, Sydney, NSW, Australia; CADE Clinic, Department of Psychiatry, Royal North Shore Hospital, St Leonards, NSW, Australia.Contributor 5: Florence Levy, Child and Family East, Prince of Wales Hospital and School of Psychiatry, University of New South Wales, Sydney, NSW, Australia. Contributor 6: Pieter J Hoekstra, Department of Psychiatry, University of Groningen, University Medical Center Groningen, Groningen, the Netherlands.Contributor 7: Jan K Buitelaar, Department of Cognitive Neuroscience, Donders Institute for Brain, Cognition and Behaviour, Radboud University Medical Centre, Nijmegen, the Netherlands; Karakter Child and Adolescent Psychiatry University Centre, Nijmegen, the Netherlands. Contributor 8: Tobias Banaschewski, Zentralinstitut für Seelische Gesundheit Mannheim, Klinik für Psychiatrie und Psychotherapie des Kindes‐ und Jugendalters (KJP) / Department of Child & Adolescent Psychiatry and Psychotherapy, Central Institute of Mental Health, Mannheim, Germany. Contributor 9: Manfred Gerlach, Zentrum für Psychische Gesundheit, Klinik für Kinder‐ und Jugendpsychiatrie, Psychosomatik und Psychotherapie, Universitätsklinikum Würzburg / Centre for Mental Health, Department of Child & Adolescent Psychiatry, Psychosomatics and Psychotherapy, University Hospital Würzburg, Würzburg, Germany.Contributor 10: Katja Becker, Klinik für Kinder‐ und Jugendpsychiatrie, Psychosomatik und Psychotherapie, Universitätsklinikum Gießen und Marburg / Department of Child & Adolescent Psychiatry, Psychosomatics and Psychotherapy, University Hospital of Giessen and Marburg, Marburg, Germany.Contributor 11: Martin Holtmann, Klinik für Kinder‐ und Jugendpsychiatrie, Psychotherapie und Psychosomatik, LWL‐Universitätsklinik Hamm der Ruhr‐Universität Bochum / Department of Child & Adolescent Psychiatry, Psychotherapy and Psychosomatics, LWL University Hospital of Hamm of Ruhr University Bochum, Bochum, Germany.Contributor 12: Manfred Döpfner, Ausbildungsinstitut für Kinder‐ und Jugendlichenpsychotherapie (AKiP) am Klinikum der Universität zu Köln; Medizinische Fakultät, Klinik und Poliklinik für Psychiatrie, Psychosomatik und Psychotherapie des Kindes‐ und Jugendalters, Universität zu Köln / Institute of Child and Adolescent Psychiatry (AKiP), University Hospital of Cologne, Cologne, Germany; Medical School, Department of Child & Adolescent Psychiatry, Psychosomatics and Psychotherapy, University Hospital of Cologne, Cologne, Germany. Contributor 13: Marcel Romanos, Zentrum für Psychische Gesundheit, Klinik für Kinder‐ und Jugendpsychiatrie, Psychosomatik und Psychotherapie, Universitätsklinikum Würzburg / Centre for Mental Health, Department of Child & Adolescent Psychiatry, Psychosomatics and Psychotherapy, University Hospital Würzburg, Würzburg, Germany. Contributor 14: Celine SL Chui, Department of Pharmacology and Pharmacy, University of Hong Kong, Hong Kong, China.Contributor 15: David Coghill, Department of Paediatrics and Psychiatry Faculty of Medicine, Dentistry and Health Sciences, University of Melbourne, Victoria, Australia.Contributor 16: Samuele Cortese, Department of Psychology, University of Southampton, Southampton, UK; Faculty of Medicine, Clinical and Experimental Sciences, University of Southampton, UK.Contributor 17: Emily Simonoff, Department of Psychology, University of Southampton, Southampton, UK; Department of Child & Adolescent Psychiatry, Institute of Psychiatry Psychology & Neuroscience,King's College London, London, UK.Contributor 18: Ian CK Wong, Department of Pharmacology and Pharmacy, University of Hong Kong, Hong Kong, China; Research Department of Practice and Policy, UCL School of Pharmacy, London, UK.
Author 1: Ole Jakob Storebø, Psychiatric Research Unit, Region Zealand Psychiatry, Region Zealand, Denmark; Child and Adolescent Psychiatric Department, Region Zealand, Denmark; Department of Psychology, Faculty of Health Science, University of Southern Denmark, Odense, Denmark. Author 2: Erika Simonsen, Psychiatric Research Unit, Region Zealand Psychiatry, Region Zealand, Denmark; Institute of Clinical Medicine and Faculty of Health and Medical Sciences, Copenhagen University, Copenhagen, Denmark. Author 3: Christian Gluud, The Copenhagen Trial Unit, Centre for Clinical Intervention Research, Department 7812, Rigshospitalet, Copenhagen University Hospital, Copenhagen, Denmark; The Cochrane Hepato‐Biliary Group, Copenhagen Trial Unit, Centre for Clinical Intervention Research, Rigshospitalet, Copenhagen University Hospital, Copenhagen, Denmark. Author 4: Morris Zwi, Islington CAMHS, Whittington Health, London, UK. Author 5: Carlos R Moreira‐Maia, Federal University of Rio Grande do Sul, Porto Alegre, Brazil. Author 6: Maria Skoog, Copenhagen Trial Unit, Centre for Clinical Intervention Research, Rigshospitalet, Copenhagen University Hospital, Copenhagen, Denmark. Author 7: Camilla Groth, Pediatric Department, Herlev University Hospital, Herlev, Denmark. Author 8: Donna Gillies, Western Sydney Local Health District, Mental Health, Parramatta, Australia. Author 9: Helle B Krogh, Psychiatric Research Unit, Region Zealand Psychiatry, Slagelse, Denmark; Child and Adolescent Psychiatric Department, Region Zealand, Roskilde, Denmark. Author 10: Mathilde Holmskov, Psychiatric Research Unit, Region Zealand Psychiatry, Slagelse, Denmark; Child and Adolescent Psychiatric Department, Region Zealand, Roskilde, Denmark.
*Typo in article. Author's name should be reported as Groth C.
What's new
| Date | Event | Description |
|---|---|---|
| 27 March 2023 | New citation required but conclusions have not changed | Twenty‐nine new studies included in the review |
| 27 March 2023 | New search has been performed | Updated following a new search in January 2021 and a top‐up search in March 2022 |
History
Protocol first published: Issue 5, 2012 Review first published: Issue 12, 2015
| Date | Event | Description |
|---|---|---|
| 29 November 2016 | Feedback has been incorporated | This review was published in the Cochrane Library on 25 November 2015, with abridged versions appearing in the BMJ on 26 November 2015 and JAMA on 10 May 2016. These abridged reviews have received many comments in editorials, 'letters to the editor', articles, rapid responses, and blogs. Interestingly, however, no comment has been directed to the full review in the Cochrane Library. In order to inform readers of the Cochrane Library, we have provided the links to these comments, as well as review authors' responses, in the Feedback section below. |
Acknowledgements
We thank Trine Lacoppidan Kæstel, Research Librarian, at the Psychiatric Research Unit, Region Zealand, Denmark, for helping with searches and descriptions of measurement scales.
We thank Anne Fink for helping with the review.
We are grateful to the many authors who kindly responded to our requests for further information on the trials in which they were involved.
Thanks also to the Psychiatric Research Unit, Region Zealand Psychiatry, Roskilde, Denmark; Region Zealand Research Foundation, Denmark; and the Copenhagen Trial Unit, Centre for Clinical Intervention Research, The Capital Region, Copenhagen University Hospital — Rigshospitalet, Copenhagen, Denmark, for funding and enabling the review.
We also wish to warmly thank Geraldine McDonald (Co‐ordinating Editor), Joanne Duffield (Managing Editor), Sarah Davies (Assistant Managing Editor), and Margaret Anderson (Information Specialist) of Cochrane Developmental, Psychosocial and Learning Problems for providing help and support.
Cochrane Developmental, Psychosocial and Learning Problems supported the authors in the development of this update.
The following people conducted the editorial process for this article.
Sign‐off Editor (final editorial decision): Geraldine Macdonald, University of Bristol;
Managing Editor (provided editorial guidance to authors, edited the article): Joanne Duffield, Queen's University Belfast;
Deputy Managing Editor (conducted editorial policy checks and supported editorial team): Sarah Davies, University of Bristol;
Information Specialist (search review): Margaret Anderson, Queen's Univeristy Belfast; and
Copy Editor (copy editing and production): Denise Mitchell, Cochrane Evidence, Production and Methods Directorate.
Appendices
Appendix 1. Search strategies
| Database | Search strategy |
| Cochrane Central Register of Controlled Trials (CENTRAL; Cochrane Library) | #1 MeSH descriptor: [Methylphenidate] explode all trees #2 (methylphenidate):ti,ab,kw #3 adaphen or adhansia or addwize or aptensio or artige or attenta or biphentin or calocain or centredrin or concerta or cotempla #4 daytrana or dexmethylphenidat* or difumenil or elmifiten or equasym or focalin or foquest or inspiral or jornay or matoride or medikid or medikinet* #5 meridil or metadate or methyl phenidat* or methyl phenidylacetat* or methylfenid* or methylin or methylofenidan or methylphenid* or methyl phenid* #6 methyl phenidyl acetat* or methypatch or metidate or metilfenidat* or motiron* or MPH or omzin or penid* or phenid* or phenidyl hydrochlorid* or phenidylat* or plimasin #7 PMS‐methylphenid* or prohiper or quasym* or quilli* or relexxii or Richter Works or riphenidat* or ritalin* or rubifen or stimdat* or tifinidat or tradea or tranquilyn or tsentedrin #8 #1 or #2 or #3 or #4 or #5 or #6 or #7 #9 MeSH descriptor: [Child] explode all trees #10 MeSH descriptor: [Adolescent] explode all trees #11 MeSH descriptor: [Infant] explode all trees #12 (child* OR boy* OR girl* OR adolescen* OR teen* OR preschool OR pre school OR infant* OR baby OR babies OR toddler* OR school child* or youth*) #13 #9 or #10 or #11 #12 #14 #8 and #13 #15 MeSH descriptor: [Attention Deficit and Disruptive Behavior Disorders] explode all trees #16 adhd or addh or adhs or add #17 (((attention* or behav*) near/3 (defic* or dysfunc* or disorder*))):ti,ab,kw (Word variations have been searched) #18 ((impulsiv* or inattentiv* or inattention*)):ti,ab,kw (Word variations have been searched) #19 MeSH descriptor: [Hyperkinesis] explode all trees #20 (hyperkine*):ti,ab,kw (Word variations have been searched) #21 ((minimal near/3 brain near/3 (disorder* or dysfunct* or damage*))):ti,ab,kw (Word variations have been searched) #22 ((disrupt* near/3 disorder*) or (disrupt* near/3 behav*) or (defian* near/3 disorder*) or (defian* near/3 behav*)):ti,ab,kw (Word variations have been searched) #23 (hyperactiv*):ti,ab,kw #24 #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 #25 #14 and #24 #26 MeSH descriptor: [] explode all trees and with qualifier(s): [adverse effects ‐ AE, drug effects ‐ DE, chemically induced ‐ CI] #27 ((safe or safety or adverse or tolerability or toxicity or toxic or adrs or adr or tolerance or tolerate or harm or harms or harmful or complication* or risk or risks)):ti,ab,kw (Word variations have been searched) #28 (side next effect*):ti,ab,kw #29 (undesirable next effect*):ti,ab,kw (Word variations have been searched) #30 (treatment next emergent):ti,ab,kw (Word variations have been searched) #31 (unintended next event):ti,ab,kw (Word variations have been searched) #32 (unintended next effect):ti,ab,kw (Word variations have been searched) #33 #26 or #27 or #28 or #29 or #30 or #31 or #32 827410 #34 MeSH descriptor: [Mood Disorders] explode all trees #35 (depression or depressive):ti,ab,kw (Word variations have been searched) #36 MeSH descriptor: [Psychotic Disorders] explode all trees #37 (psychosis or (psychotic near/4 symptom*)):ti,ab,kw (Word variations have been searched) #38 MeSH descriptor: [Body Weight] explode all trees #39 MeSH descriptor: [Anorexia] explode all trees #40 ((loss or lose or losing or decreas* or reduc*) near/3 (weight or appetite)):ti,ab,kw (Word variations have been searched) #41 ((reduc* or retard* or inhibit* or deficit*) near/4 growth):ti,ab,kw (Word variations have been searched) 5140 #42 MeSH descriptor: [Hypertension] explode all trees #43 MeSH descriptor: [Heart Rate] explode all trees #44 MeSH descriptor: [Tachycardia] explode all trees #45 MeSH descriptor: [Death] explode all trees #46 (death):ti,ab,kw (Word variations have been searched) #47 MeSH descriptor: [Infertility] explode all trees #48 MeSH descriptor: [Carcinogens] explode all trees #49 ((increas* near/4 (heart rate or pulse or blood pressure))):ti,ab,kw (Word variations have been searched) #50 ((((loss or reduc*) near/4 fertility) or infertility)):ti,ab,kw (Word variations have been searched) #51 MeSH descriptor: [Neoplasms] explode all trees #52 (((risk near/2 cancer) or (cytogenetic near/2 effect*))):ti,ab,kw (Word variations have been searched) #53 #33 or #34 or #35 or #36 or #37 or #37 or #38 or #39 or #40 or #41 or #42 or #43 or #44 or #45 or #46 or #47 or #48 or #49 or #50 or #51 or #52 #54 #8 and #13 and #24 #55 #8 and #13 and #53 #56 #54 or #55 with Publication Year from 2015 to 2021, in Trials |
| Ovid MEDLINE | 1 exp "Attention Deficit and Disruptive Behavior Disorders"/ 2 adhd.mp. 3 addh.mp. 4 adhs.mp. 5 "add".mp. 6 (ad adj hd).mp. ( 7 ((attention* or behav*) adj3 (defic* or dysfunc* or disorder*)).mp. 8 ((disrupt* adj3 disorder*) or (disrupt* adj3 behav*) or (defian* adj3 disorder*) or (defian* adj3 behav*)).mp. 9 (impulsiv* or inattentiv* or inattention*).mp. 10 hyperactiv*.mp. 11 hyperkinesis*.mp. 12 exp Hyperkinesis/ 13 (minimal adj brain adj3 disorder*).mp. 14 (minimal adj brain adj3 dysfunction*).mp. 15 (minimal adj brain adj3 damage*).mp. 16 or/1‐15 17 randomized controlled trial.pt. 18 controlled clinical trial.pt. 19 randomized controlled trials.mp. 20 exp Randomized Controlled Trial/ 21 random allocation.mp. or Random Allocation/ 22 double blind method.mp. 23 single blind method.mp. 24 clinical trial.pt. 25 (clin* adj25 trial*).ab,ti. 26 ((singl* or doubl* or tripl* or trebl*) adj25 (blind* or mask* or dumm*)).mp. 27 exp Clinical Trial/ 28 placebos.mp. 29 "placebo*".ab,ti. 30 "random*".ab,ti. 31 comparative study.mp. 32 comparative trial.mp. 33 Evaluation Studies as Topic/ 34 exp Clinical Trials as Topic/ 35 follow up studies.mp. 36 prospective studies.mp. 37 (control* or prospectiv* or volunteer*).ab,ti. 38 or/17‐37 39 methylphenidate.mp. or exp Methylphenidate/ 40 adaphen.mp. 41 adhansia.mp. 42 addwize.mp. 43 aptensio.mp. 44 artige.mp. 45 attenta.mp. 46 biphentin.mp. 47 calocain.mp. 48 centedrin*.mp. 49 concerta*.mp. 50 cotempla.mp. 51 daytrana.mp. 52 dexmethylphenidat*.mp. 53 difumenil.mp. 54 elmifiten.mp. 55 equasym*.mp. 56 elmifiten*.mp. 57 focalin.mp. 58 focalin*.mp. 59 foquest.mp. 60 inspiral.mp. 61 jornay.mp. 62 matoride.mp. 63 medikid.mp. 64 medikinet*.mp. 65 meridil.mp. 66 metadate*.mp. 67 methyl phenidat*.mp. 68 methyl phenid*.mp. 69 methyl phenidylacetat*.mp. 70 methylfenid*.mp. 71 methylin*.mp. 72 methylofenid*.mp. 73 methylphenid*.mp. 74 methyl phenid*.mp. 75 methypatch.mp. 76 metidate.mp. 77 metilfenidat*.mp. 78 motiron*.mp. 79 MPH.mp. 80 omozin*.mp. 81 penid*.mp. 82 phenida.mp. 83 phenidyl hydrochlorid*.mp. 84 phenidylat*.mp. 85 phenidyl*.mp. 86 plimasin*.mp. 87 PMS‐methylphenid*.mp. 88 prohiper.mp. 89 quazym*.mp. 90 quilli*.mp. 91 relexxii.mp. 92 Richter Works.mp. 93 riphenidat*.mp. 94 ritalin*.mp. 95 rubifen*.mp. 96 stimdat*.mp. 97 tifinidat*.mp. 98 tradea.mp. 99 tranquilyn.mp. 100 tsentedrin*.mp. 101 or/39‐100 102 exp Child/ 103 exp Adolescent/ 104 exp Infant/ 105 (child* or boy* or girl* or adolescen* or teen* or preschool* or pre school or infant* or baby or babies or toddler* or school child* or youth* or young).mp. 106 or/102‐105 107 16 and 38 and 101 and 106 108 (ae or co or de).fs. 109 (((safe or safety or (side adj1 effect*) or undesirable) adj1 effect*) or (treatment adj1 emergent) or tolerability or tolerance or tolerate or toxicity or toxic or adrs or adr or harm or harms or harmful or complication* or risk or risks or (unintended adj1 event*)).mp. or (un‐intendend adj1 effect*).ab,ti. 110 (adverse adj2 (effect or effects or reaction or reactions or event or events or outcome or outcomes)).ab,ti. 111 Methylphenidate/ae, po, to 112 exp Mood Disorders/ 113 (depression or depressive).ab,ti. 114 exp Psychotic Disorders/ 115 (psychosis or (psychotic adj4 symptom*)).ab,ti. 116 exp body weight/ or anorexia/ 117 ((loss or lose or losing or reduc*) adj3 (weight or appetite)).ab,ti. 118 ((reduc* or retard* or inhibit* or deficit*) adj4 growth).ab,ti. 119 exp Hypertension/ 120 Heart Rate/ 121 Tachycardia/ 122 (increas* adj4 (heart rate or pulse or blood pressure)).ab,ti. 123 exp Death, Sudden/ 124 death.ab,ti. 125 exp Infertility/ 126 (((loss or reduc*) adj4 fertility) or infertility).ab,ti. 127 exp Carcinogens/ 128 exp Neoplasms/ 129 ((risk adj2 cancer) or (cytogenic adj2 effect*)).ab,ti. 130 or/108‐129 131 38 and 101 and 106 and 130 132 107 or 131 133 101 and 106 and 130 134 16 and 101 and 106 and 38 135 131 or 134 136 limit 135 to yr="2015 ‐Current" |
| Embase (Ovid) | 1 exp attention deficit disorder/ 2 adhd.ti,ab. 3 addh.ti,ab. 4 ADHS.ti,ab. 5 (ad adj HD).ti,ab. 6 "(ADD)".mp. 7 ((disrupt* adj3 disorder*) or (disrupt* adj3 behav*) or (defian* adj3 disorder*) or (defian* adj3 behav*)).ti,ab. 8 ((attention* or behav*) adj3 (defic* or dysfunc* or disorder*)).ti,ab. 9 (impulsiv* or inattentiv* or inattention*).ti,ab. 10 exp hyperactivity/ 11 hyperkinesia/ 12 "hyperactiv*".ti,ab. 13 "hyperkinesis*".ti,ab. 14 (minimal adj brain adj3 disorder*).ti,ab. 15 (minimal adj brain adj3 dysfunction*).ti,ab. 16 (minimal adj brain adj3 damage*).ti,ab. 17 or/1‐16 18 exp methylphenidate/ or methylphenidate.mp. 19 adaphen.mp. 20 adhansia.mp. 21 addwize.mp. 22 aptensio.mp. 23 artige.mp. 24 attenta.mp. 25 biphentin*.mp. 26 calocain.mp. 27 centedrin*.mp. 28 concerta*.mp. 29 cotempla.mp. 30 daytrana.mp. 31 dexmethylphenidat*.mp. 32 difumenil*.mp. 33 elmifiten*.mp. 34 equasym.mp. 35 focalin*.mp. 36 foquest.mp. 37 inspiral.mp. 38 jornay.mp. 39 matoride.mp. 40 medikid.mp. 41 medikinet*.mp. 42 meridil.mp. 43 metadate.mp. 44 methyl phenidat*.mp. 45 methyl phenidylacetat*.mp. 46 methylfenid*.mp. 47 methylin*.mp. 48 methylofenid*.mp. 49 methylphenid*.mp. 50 methyl phenidyl acetat*.mp. 51 methypatch.mp. 52 metidate.mp. 53 metilfenidat*.mp. 54 motiron.mp. 55 MPH.mp. 56 omozin*.mp. 57 penid*.mp. 58 phenidyl hydrochlorid.mp. 59 phenidyl hydrochlorid*.mp. 60 phenidyl*.mp. 61 plimasin*.mp. 62 PMS‐methylphenid*.mp. 63 quasym.mp. 64 quilli*.mp. 65 relexxii.mp. 66 Richter Works.mp. 67 riphenidat*.mp. 68 ritalin*.mp. 69 rubifen*.mp. 70 stimdat*.mp. 71 tifinidat*.mp. 72 tradea.mp. 73 tranquilyn.mp. 74 tsentedrin*.mp. 75 or/18‐62 76 exp child/ 77 exp adolescent/ 78 exp juvenile/ 79 exp infant/ 80 (child* or boy* or girl* or adolescen* or teen* or preschool or pre school or infant* or baby or babies or toddler* or school child* or young or youth*).mp. 81 76 or 77 or 78 or 79 or 80 82 clinical trial/ 83 randomized controlled trial/ 84 randomization/ 85 single blind procedure/ 86 double blind procedure/ 87 crossover procedure/ 88 placebo/ 89 prospective study/ 90 (randomi?ed controlled adj1 trial*).ti,tw. 91 rct.ti,tw. 92 randomly allocated.ti,tw. 93 allocated randomly.ti,tw. 94 random allocation.ti,tw. 95 (allocated adj2 random).ti,tw. 96 (single adj1 blind*).ti,tw. 97 (double adj1 blind*).ti,tw. 98 ((treble or triple) adj1 blind*).ti,tw. 99 "placebo*".ti,tw. 100 or/82‐99 101 17 and 75 and 81 and 100 102 (safe or safety or (side adj1 effect*) or (undesirable adj1 effect*) or (treatment adj1 emergent) or tolerability or tolerance or tolerate or toxicity or toxic or adrs or adr or harm or harms or harmful or complication* or risk or risks or (unintended adj1 event*) or (un‐intended adj1 effect*)).ab,ti. 103 (adverse adj2 (effect or effects or reaction or reactions or event or events or outcome or outcomes)).ab,ti. 104 exp adverse drug reaction/ 105 exp side effect/ 106 methylphenidate/ae, to [Adverse Drug Reaction, Drug Toxicity] 107 exp mood disorder/si [Side Effect] 108 (depression or depressive).ab,ti. 109 exp psychosis/si [Side Effect] 110 (psychosis or (psychotic adj4 symptom*)).ab,ti. 111 growth retardation/ 112 growth inhibition/ 113 ((loss or lose or losing or reduc*) adj3 (weight or appetite)).ab,ti. 114 ((reduc* or retard* or inhibit* or deficit*) adj4 growth).ab,ti. 115 hypertension/si [Side Effect] 116 heart rate/ 117 cardiovascular effect/ 118 tachycardia/si [Side Effect] 119 (increas* adj4 (heart rate or pulse or blood pressure)).ab,ti. (68994) 120 sudden death/ 121 death.ab,ti. 122 infertility/si [Side Effect] 123 (((loss or reduc*) adj4 fertility) or infertility).ab,ti. 124 cancer risk/ 125 drug carcinogenicity/ or carcinogenicity/ 126 chromosome aberration/si [Side Effect] 127 childhood cancer/si [Side Effect] 128 ((risk adj2 cancer) or (cytogenic adj2 effect*)).ab,ti. 129 or/102‐128 130 75 and 81 and 100 and 129 131 101 or 130 132 limit 131 to yr="2015 ‐Current" |
| CINAHL (Cumulative Index to Nursing and Allied Health Literature; EBSCOhost) | S1 (MH "Methylphenidate") S2 AB methylphenidate S3 TI methylphenidate S4 S2 OR S3 S5 TI ( adaphen or adhansia or addwize or aptensio or artige or attenta or biphentin ) OR AB ( adaphen or adhansia or addwize or aptensio or artige or attenta or biphentin ) S6 TI ( calocain or centedrin or concerta or cotempla or daytrana or dexmethylphenidat* or difumenil or elmifiten or equasym or focalin or foquest or inspiral or jornay or motoride ord medikid or medikinet* ) OR AB ( calocain or centedrin or concerta or cotempla or daytrana or dexmethylphenidat* or difumenil or elmifiten or equasym or focalin or foquest or inspiral or jornay or motoride ord medikid or medikinet* ) S7 TI ( meridil or metadate or methyl phenidat or methyl phenidylacetat* or methylphenid* or methylfenid* or methylin or methylofenidan or methylphenid* or methyl phenid* ) OR AB ( meridil or metadate or methyl phenidat or methyl phenidylacetat* or methylphenid* or methylfenid* or methylin or methylofenidan or methylphenid* or methyl phenid* ) S8 TI ( methylphenidyl acetat* or methypatch or metidate or metilfenidat* or motiron* or MPH or omzin* or penid* or phenid* or phenidyl hydrochlorid* or phenidylat* or plimasin* ) OR AB ( methylphenidyl acetat* or methypatch or metidate or metilfenidat* or motiron* or MPH or omzin* or penid* or phenid* or phenidyl hydrochlorid* or phenidylat* or plimasin* ) S9 TI ( PMS‐methylphenid* or prohiper or quasym* or quilli* or relexxii or Richter Works or riphenidat* or ritalin* or rubifen or stimdat* or tifinidat or tradea or tranquilyn or tsentedrin ) OR AB ( PMS‐methylphenid* or prohiper or quasym* or quilli* or relexxii or Richter Works or riphenidat* or ritalin* or rubifen or stimdat* or tifinidat or tradea or tranquilyn or tsentedrin ) S10 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 S11 (MH "Child+") OR (MH "Infant+") OR (MH "Adolescence+") S12 TI ( (child* OR boy* OR girl* OR adolescen* OR teen* OR preschool OR pre school OR infant* OR baby OR babies OR toddler* OR school child* or youth*) ) OR AB ( (child* OR boy* OR girl* OR adolescen* OR teen* OR preschool OR pre school OR infant* OR baby OR babies OR toddler* OR school child* or youth*) ) S13 S11 OR S12 S14 S10 AND S13 S15 (MH "Randomized Controlled Trials+") OR (MH "Double‐Blind Studies") OR (MH "Single‐Blind Studies") OR (MH "Random Assignment") OR (MH "Pretest‐Posttest Design+") OR (MH "Cluster Sample+") S16 TI randomised or randomized S17 AB random* S18 TI trial S19 (MH "Sample Size") AND AB ( assigned or allocated or control ) S20 (MH "Placebos") S21 PT Randomized Controlled Trials S22 PT randomized controlled trial S23 AB control W5 group S24 (MH "Crossover Design") OR (MH "Comparative Studies+") S25 AB cluster W3 RCT S26 (MH "Animals+") S27 (MH "Animal Studies") S28 TI animal model* S29 S26 OR S27 OR S28 S30 (MH "Human") S31 S29 NOT S30 S32 S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 S33 S32 NOT S31 S34 S14 AND S33 S35 S14 AND S33 |
| PsycINFO (Ovid) | 1 exp Attention Deficit Disorder/ 2 adhd.ab,ti. 3 addh.ab,ti. 4 ADHS.ab,ti. 5 (AD adj HD).ab,ti. 6 "ADD".ab,ti. 7 ((attention* or behav*) adj3 (defic* or dysfunc* or disorder*)).ab,ti. 8 ((disrupt* adj3 disorder*) or (disrupt* adj3 behav*) or (defian* adj3 disorder*) or (defian* adj3 behav*)).ab,ti. 9 (impulsiv* or inattentiv* or inattention*).ab,ti. 10 "hyperactiv*".ab,ti. 11 hyperkinesis.ab,ti. 12 exp HYPERKINESIS/ 13 (minimal adj brain adj3 disorder*).ab,ti. 14 (minimal adj brain adj3 dysfunction*).ab,ti. 15 (minimal adj brain adj3 damage*).ab,ti. 16 or/1‐15 17 exp Treatment Effectiveness Evaluation/ 18 exp "Treatment outcomes"/ 19 Placebo/ 20 Followup Studies/ 21 placebo*.ab,ti. 22 random*.ab,ti. 23 comparative stud*.ab,ti. 24 (clinical adj3 trial*).ab,ti. 25 (research adj3 design).ab,ti. 26 (evaluat* adj3 stud*).ab,ti. 27 (prospectiv* adj3 stud*).ab,ti. 28 ((singl* or doubl* or trebl* or tripl*) adj3 (blind* or mask*)).ab,ti. 29 or/17‐28 30 methylphenidate.mp. or exp METHYLPHENIDATE/ 31 adaphen.mp. 32 adhansia.mp. 33 addwize.mp. 34 aptensio.mp. 35 artige.mp. 36 attenta.mp. 37 biphentin.mp. 38 calocain.mp. 39 centedrin.mp. 40 concerta.mp. 41 cotempla.mp. 42 daytrana.mp. 43 dexmethylphenidat*.mp. 44 difumenil.mp. 45 elmifiten.mp. 46 equasym*.mp. 47 focalin*.mp. 48 foquest.mp. 49 inspiral.mp. 50 jornay.mp. 51 matoride.mp. 52 medikid.mp. 53 medikinet*.mp. 54 meridil.mp. 55 metadate*.mp. 56 methyl phenidat*.mp. 57 methyl phenidylacetat*.mp. 58 methylfenid*.mp. 59 methylin.mp. 60 methylofenidan.mp. 61 methylphenid*.mp. 62 methyl phenid*.mp. 63 methyl phenidyl acetat*.mp. 64 methypatch.mp. 65 metidate.mp. 66 metilfenidat*.mp. 67 motiron*.mp. 68 MPH.mp. 69 omozin.mp. 70 penid*.mp. 71 phenid*.mp. 72 phenidyl hydrochlorid*.mp. 73 phenidylat*.mp. 74 plimasin*.mp. 75 PMS‐methylphenid*.mp. 76 prohiper.mp. 77 quasym*.mp. 78 quilli*.mp. 79 relexxii.mp. 80 Richter works.mp. 81 riphenidat*.mp. 82 ritalin*.mp. 83 rubifen.mp. 84 stimdat*.mp. 85 tifinidat.mp. 86 tradea.mp. 87 tranquilyn.mp. 88 tsentedrin*.mp. 89 or/30‐88 90 (child* or boy* or girl* or adolescen* or teen* or preschool or pre school or infant* or baby or babies or toddler* or school child* or young or youth*).ab,ti. 91 16 and 29 and 89 and 90 92 exp "Side Effects (Drug)"/ 93 (safe or safety or (side adj1 effect*) or (undesirable adj1 effect*) or (treatment adj1 emergent) or tolerability or tolerance or tolerate or toxicity or toxic or adrs or adr or harm or harms or harmful or complication* or risk or risks or (unintended adj1 event*) or (un‐intended adj1 effect*)).ab,ti. 94 (adverse adj2 (effect or effects or reaction or reactions or event or events or outcome or outcomes)).ab,ti. 95 92 or 93 or 94 96 exp Major Depression/ 97 exp Affective Disorders/ 98 (depression or depressive).ab,ti. 99 exp Psychosis/ 100 (psychosis or (psychotic adj4 symptom*)).ab,ti. 101 exp Body Weight/ 102 exp Appetite Depressing Drugs/ 103 exp Appetite/ 104 ((loss or lose or losing or reduc*) adj3 (weight or appetite)).mp. [mp=title, abstract, heading word, table of contents, key concepts, original title, tests & measures, mesh] 105 ((reduc* or retard* or inhibit* or deficit*) adj4 growth).ab,ti. 106 exp Cardiovascular Disorders/ 107 exp Heart Rate/ 108 exp Heart Rate Affecting Drugs/ 109 (increas* adj4 (heart rate or pulse or blood pressure)).ab,ti. 110 exp "Death and Dying"/ 111 death.ab,ti. 112 exp Infertility/ 113 exp Fertility/ 114 (((loss or reduc*) adj4 fertility) or infertility).ab,ti. 115 exp Neoplasms/ 116 exp Carcinogens/ 117 ((risk adj2 cancer) or (cytogenic adj2 effect*)).ab,ti. 118 or/96‐117 119 29 and 89 and 90 and 118 120 91 or 119 121 limit 120 to yr="2015 ‐Current" |
| Epistemonikos | searched with a simple search: (title:((title:(attention deficit OR adhd OR hyperactivity OR hypekinetic) OR abstract:(attention deficit OR adhd OR hyperactivity OR hypekinetic)) AND (title:(methylphenidate OR ritalin OR MPH) OR abstract:(methylphenidate OR ritalin OR MPH)) AND ((title:children OR child OR young OR youth OR adolescent) OR abstract:(children OR child OR young OR youth OR adolescent))) 2015‐2021 Combined with search for ten published reviews where all included studies for each review where downloaded if possible. 1. Catala‐Lopez F, Hutton B, Nunez‐Beltran A, et al. The pharmacological and non‐pharmacological treatment of attention deficit hyperactivity disorder in children and adolescents: A systematic review with network meta‐analyses of randomised trials. PLOS ONE 2017;12:e0180355. doi: 10.1371/journal.pone.0180355 2. Cerrillo‐Urbina AJ, Garcia‐Hermoso A, Pardo‐Guijarro MJ, et al. The effects of long‐acting stimulant and nonstimulant medications in children and adolescents with attention‐deficit/hyperactivity disorder: A meta‐analysis of randomized controlled trials. J Child Adolesc Psychopharmacol 2018;28:494‐507. doi: 10.1089/cap.2017.0151 3. Cortese S, Adamo N, Del Giovane C, et al. Comparative efficacy and tolerability of medications for attention‐deficit hyperactivity disorder in children, adolescents, and adults: a systematic review and network meta‐analysis. Lancet Psychiatry 2018;5:727‐38. doi: 10.1016/s2215‐0366(18)30269‐4 4. Holmskov M, Storebø OJ, Moreira‐Maia CR, et al. Gastrointestinal adverse events during methylphenidate treatment of children and adolescents with attention deficit hyperactivity disorder: A systematic review with meta‐analysis and Trial Sequential Analysis of randomised clinical trials. PLOS ONE 2017;12:e0178187. doi: 10.1371/journal.pone.0178187 5. Kemper AR, Maslow GR, Hill S, et al. AHRQ Comparative Effectiveness Reviews Rockville (MD): Agency for Healthcare Research and Quality (US); 2018 [Available from: Available from: https://www.ncbi.nlm.nih.gov/books/NBK487761/. 6. Liu H, Feng W, Zhang D. Association of ADHD medications with the risk of cardiovascular diseases: a meta‐analysis. Eur Child Adolesc Psychiatry 2019;28:1283‐93. doi: 10.1007/s00787‐018‐1217‐x 7. Osland ST, Steeves TD, Pringsheim T. Pharmacological treatment for attention deficit hyperactivity disorder (ADHD) in children with comorbid tic disorders. Cochrane Database Syst Rev 2018;6:Cd007990. doi: 10.1002/14651858.CD007990.pub3 8. Punja S, Xu D, Schmid CH, et al. N‐of‐1 trials can be aggregated to generate group mean treatment effects: a systematic review and meta‐analysis. J Clin Epidemiol 2016;76:65‐75. doi: 10.1016/j.jclinepi.2016.03.026 9. Ramstad E, Storebø OJ, Gerner T, et al. Hallucinations and other psychotic symptoms in response to methylphenidate in children and adolescents with attention‐deficit/hyperactivity disorder: a Cochrane systematic review with meta‐analysis and trial sequential analysis. Scandinavian Journal of Child and Adolescent Psychiatry and Psychology 2018;6(1):52‐71. doi: https://doi.org/10.21307/sjcapp‐2018‐003 10. Solmi M, Fornaro M, Ostinelli EG, et al. Safety of 80 antidepressants, antipsychotics, anti‐attention‐deficit/hyperactivity medications and mood stabilizers in children and adolescents with psychiatric disorders: a large scale systematic meta‐review of 78 adverse effects. World Psychiatry 2020;19(2):214‐32. doi: https://doi.org/10.1002/wps.20765 |
| CPCI‐S (Conference Proceedings Citation Index ‐ Science); CPCI‐SSH (Conference Proceedings Citation Index ‐ Social Science & Humanities) (Web of Science) |
#2 AND #1 #2 TS=(child* or boy* or girl* or adolescen* or teen* or preschool* or "pre school*" or infant* or baby or babies or toddler* or "school child*" or schoolchild* or youth*) #1 TS=(Methylphenidate or Attenta or Biphentin or Calocain or Centedrin* or Concerta or Daytrana or Dexmethylphenidat* or Elmifiten or Equasym or Focalin or Medikid or Medikinet or Meridil or Metadate or "Methyl phenidat*" or "Methyl phenidylacetat" or Methylfenid or Methylin or Methylofenidan or Methylphenid* or "Methyl phenidyl acetat*" or Methypatch or Metilfenidato or Motiron or MPH or Penid or Omozin or Quazym or "Phenidyl hydrochlorid*" or Phenidylat* or Plimasin* or "PMS‐Methylphenid* " or "Richter Works" or Riphenidat* or Ritalin* or Rubifen or Stimdat* or Tifinidat or Tranquilyn or Tsentedrin*) |
| ClinicalTrials.gov | Advanced search: Methylphenidate OR concerta OR daytrana OR dexmethylphenidate OR equasym OR focalin OR medikinet OR MPH OR Ritalin Studies with results Age group: Child Studies With Results | Methylphenidate OR concerta OR daytrana OR dexmethylphenidate OR equasym OR focalin OR medikinet OR MPH OR Ritalin | Child Applied Filters: With Results Child (birth–17) |
| WHO ICTRP (World Health Organisation International Clinical Trials Registry Platform; who.int/ictrp/en) | Methylphenidate OR concerta OR daytrana OR dexmethylphenidate OR equasym OR focalin OR medikinet OR MPH OR Ritalin Children |
| NDLTD (Networked Digital Library of Theses and Dissertations; ndltd.org/) | methylphenidate AND child* AND (attention deficit or hyperactiv*) |
| DART‐Europe E‐theses Portal (https://www.dart-europe.org/basic-search.php) | methylphenidate AND child* |
| Thesis Canada (https://library-archives.canada.ca/eng/services/services-libraries/theses/Pages/theses-canada.aspx) | methylphenidate |
| Worldcat (https://www.worldcat.org/search?q=methylphenidate) | methylphenidate |
Appendix 2. Letter to pharmaceutical companies
Date: September 2021
The Cochrane Methylphenidate Group
Psychiatric Research Unit
Fælledvej 6
4200 Slagelse, Denmark
Ole Jakob Storebø
Email: ojst@regionsjaelland.dk
Phone: +45 24965917
To whom it might concern,
Regarding: Methylphenidate for attention deficit hyperactivity disorder (ADHD) in children and adolescents – a Cochrane systematic review.
On behalf of the Cochrane Methylphenidate Group we address you in order to request your assistance. We are presently updating our systematic review on the effects of methylphenidate for children and adolescents with ADHD, first version published in 2015 [1, 2]. We are entrusted the elaboration of this review by the Cochrane Developmental, Psychosocial and Learning Problems Group, and it has become relevant to update the review with new randomized clinical trials and data.
The Cochrane systematic review intends to include all relevant literature empirically describing both the positive and negative effects of the treatment. We believe the elaboration of this review is in common interest of patients, physicians and manufacturers of methylphenidate. The results from the first version of the review has been cited 202 times (Cochrane version), 129 (BMJ version), and applied into 7 guidelines. By updating the review, it will continue to be applicable to guide authorities, clinicians and researchers when it comes to considering the use of methylphenidate in the treatment for children and adolescents with ADHD.
For the original version of the review, we contacted authors of significant publications, experts in the field and pharmaceutical companies, asking for information on possible relevant clinical trials. We did this because the published literature only provides us with limited and possibly selective knowledge, as it is unlikely that all studies and data are available through these databases. In 2015 we received many additional possible relevant studies for screening. We are now using the same approach for the update of the Cochrane review and are hoping that you will be as forthcoming in assisting us in our work to include published as well as unpublished studies.
We hope you will assist us with providing data that are relevant for our review. As previously noted, we are interested in data regarding both positive and negative effects of methylphenidate from randomized clinical trials, regardless of the year the data was recorded or published.
It is important for us to point out that we are not investigating specific methylphenidate preparations but simply the effect of the active substance, methylphenidate. Thus, we will not refer to or recommend any specific methylphenidate preparation or drug company. However, as we did in 2015 we will state which companies we had been in contact with, and which of these who have assisted us with data.
If possible, we would be very pleased to meet a representative from your company.
Enclosed to this letter are a list of the currently included studies in our review.
We are hoping to hear from you. If you have any questions, please contact us.
With best wishes
On behalf of the study authors
Ole Jakob Storebø, Project coordinator, Ph.D, Research Manager at Center for Evidence Based Psychiatry, Psychiatric Research Unit and senior researcher at Department of Child and Adolescent Psychiatry, Region Zealand. Professor at Department of Psychology, University of Southern Denmark, Odense, Denmark. Phone: +45 24965917 E‐mail: ojst@regionsjaelland.dk
Erik Simonsen, Medical Director, PhD, Dr.h.c. Psychiatric Research Unit, Region Zealand. Professor at Department of Clinical Medicine, University of Copenhagen, Copenhagen, Denmark.
Christian Gluud, MD, Dr, Med, Sci, Head of Department, Copenhagen Trial Unit, Centre for Clinical Intervention Research, The Capital Region, Copenhagen University Hospital − Rigshospitalet, Denmark. Professor at Department of Regional Health Research, The Faculty of Health Sciences, University of Southern Denmark, Odense, Denmark.
1. Storebø, O.J.; Ramstad, E.; Krogh, H.B.; Nilausen, T.D.; Skoog, M.; Holmskov, M.; Rosendal, S.; Groth, C.; Magnusson, F.L.; Moreira‐Maia, C.R.; Gilles, D. et al. Methylphenidate for children and adolescents with attention deficit hyperac‐tivity disorder (ADHD). Cochrane Database Syst. Rev. 2015, CD009885, doi:10.1002/14651858.cd009885.pub2.
2. Cochrane Central Register of Controlled Trials (CENTRAL) part of the Cochrane Library, MEDLINE, PsycINFO, EMBASE, CINAHL, ISI Conference Proceedings Citation Index (Science, and Social Science and Humanities), Clinical Trials.gov, and International Clinical Trials Registry Platform (ICTRP)
Appendix 3. Data extraction sheet RCTs: parallel‐group trials
Version 09.04.2014
Source
| Trial ID (e.g. Plizska 2000) |
| Trial registry with ID Searchclinicaltrials.gov(from 2008 ‐) andwho.int/ictrp/en(from 2004 ‐) |
| Full citation |
| Form filled by |
| Author contact information |
| Other publications on same trial |
ID: identifier.
Eligibility
| Confirm eligibility | Yes No Awaiting assessment |
Correspondence
| Correspondence required |
Method
| Cluster‐randomised | Yes/no Intervention (n (number)) =, control (n) = |
| Location (e.g. hospital, out‐clinic) | ‐ |
| Summary (method) | Parallel trial with 2 arms:
|
Participants
| Summary (participants) | Number of participants screened Number of participants included Number of participants randomly assigned to methylphenidate and control Number of participants followed up in each arm: methylphenidate and control Number of withdrawals in each arm: Methylphenidate and control Diagnostic and Statistical Manual of Mental Disorders, Third Edition (DSM‐III) diagnosis of attention deficit hyperactivity disorder (ADHD) (combined (%), hyperactive‐impulsive (%), inattentive (%)) Age (years) (mean, range) IQ (mean, range) Sex (male, female) Methylphenidate naive (%/number) Ethnicity (white (%), African American (%), Asian (%), Hispanic (%), other (%)) Country Comorbidity (type %) Comedication (no/yes) Sociodemographics (e.g. double or single parent family, low, middle or upper class) Inclusion criteria Exclusion criteria |
Interventions
Participants were randomly assigned to type of (e.g. immediate‐release (IR), extended‐release (ER)) (dex‐) methylphenidate or control Methylphenidate dosage: Mean (standard deviation (SD)) Administration schedule: time points Duration of intervention Titration period: none/duration initiated before/after randomisation Treatment compliance
Outcome listing
| (Our outcomes according to our protocol: short, general description) ADHD symptoms Measure instrument (e.g. ADHD Rating Scale (ADHD‐RS); Swanson, Kotkin, Atkins, M‐Flynn and Pelham (SKAMP) Scale), parent‐/teacher‐/independent assessor‐rated, time point General behaviour Measure instrument (e.g. Child Behavior Checklist (CBCL)), parent‐/teacher‐/independent assessor‐rated, time point Quality of life Measure instrument, parent‐/teacher‐/independent assessor‐rated, time point Serious adverse events Type of outcome/adverse event, measure method/instrument, parent‐/teacher‐/independent assessor‐rated, time point Non‐serious adverse events Type of outcome/adverse event, measure method/instrument, parent‐/teacher‐/independent assessor‐rated, time point |
Outcomes (positive effects)
*e.g. copy of table from article*
Outcomes (adverse events)
*e.g. copy of table from article*
| Outcomes specified | Type of adverse events/responses | Total numbers | Mean | SD | Time point |
| Serious adverse events (temporal association, but not necessarily causal relationship) | |||||
| Serious adverse reaction (response to the drug) | |||||
| Non‐serious adverse events (temporal association) |
Risk of bias
| Item | Quote | Risk of bias (high, unclear, low) |
| Random sequence generation/generation of allocation sequence (selection bias) | ||
| Allocation concealment (selection bias) | ||
| Blinding of participants and personnel (performance bias) | ||
| Blinding of outcome assessment (detection bias) | ||
| Incomplete outcome data (intention‐to‐treat (ITT), imputation method) (attrition bias) | ||
| Selective outcome reporting (according to protocol?) | ||
| Vested interest | ||
| Other sources of bias | Authors’ affiliations (e.g. Novartis) Selection bias (e.g. titration after randomisation → exclusion of methylphenidate non‐responders or placebo responders) |
Notes
| Sample calculation Ethics approval Inclusion of methylphenidate responders only/exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate Any withdrawals due to adverse events Comments from trial authors Key conclusions of trial authors Comments from review authors Supplemental information/data received through personal email correspondence with trial authors in *month* 2014 |
Appendix 4. Data extraction sheet RCTs: cross‐over trials
Version 09.04.2014
Source
| Trial ID (e.g. Plizska 2000) | |
| Trial registry with ID Searchclinicaltrials.gov(from 2008 ‐) andwho.int/ictrp/en(from 2004 ‐) | |
| Full citation | |
| Form filled by | Date and name |
| Author contact information | |
| Other publications on same trial |
ID: identifier
Eligibility
| Confirm eligibility | Yes No Awaiting assessment |
Correspondence
| Correspondence required | Data for each intervention period |
Method
| Cluster‐randomised | Yes/No Intervention (number (n)) =, control (n) = |
| Location (e.g. hospital, out‐clinic) | |
| Ethics approval | Yes/No/No information |
| Summary (method) | Cross‐over trial with 2 interventions:
Phases |
Participants
| Summary (participants) | Number of participants screened Number of participants included Participants were randomly assigned to 1 of X possible drug condition orders Number of participants followed up Number of withdrawals Diagnostic and Statistical Manual of Mental Disorders (DSM) or International Classification of Diseases (ICD) diagnosis of attention deficit hyperactivity disorder (ADHD) (combined (%), hyperactive‐impulsive (%), inattentive (%)) Age (years) (mean, range) IQ (mean, range) Sex (male, female) Methylphenidate naive (%/number) Ethnicity (white (%), African‐american (%), Asian (%), Hispanic (%), other (%)) Country Comorbidity (type %) Comedication (no/yes) Sociodemographics (e.g. double‐ or single‐parent family, low, middle or upper class) Inclusion criteria Exclusion criteria |
Interventions
Participants were randomly assigned to 1 of X possible drug condition orders of methylphenidate and control Methylphenidate dosage: mean (standard deviation (SD)) Administration schedule: time points Duration of each medication condition Washout before trial initiation Medication‐free period between interventions Titration period: none/duration initiated before/after randomisation Treatment compliance
Outcome listing
| (Our outcomes according to our protocol: short, general description) ADHD symptoms Measure instrument (e.g. ADHD Rating Scale (ADHD‐RS), Swanson, Kotkin, Atkins, M‐Flynn and Pelham (SKAMP) scale), parent‐/teacher‐/independent assessor‐rated, time point General behaviour Measure instrument (e.g. Child Behavior Checklist (CBCL)), parent‐/teacher‐/independent assessor‐rated, time point Quality of life Measure instrument, parent‐/teacher‐/independent assessor‐rated, time point Serious adverse events Type of outcome/adverse event, measure method/instrument, parent‐ /teacher‐/independent assessor‐rated, time point Non‐serious adverse events Type of outcome/adverse event, measure method/instrument, parent‐ /teacher‐/independent assessor‐rated, time point |
Outcomes (positive effects)
*e.g. copy of table from article*
Outcomes (adverse events)
*e.g. copy of table from article*
| Outcomes specified | Types of adverse events/responses | Total numbers | Mean | SD | Time point |
| Serious adverse events (temporal association, but not necessarily causal relationship) | |||||
| Serious adverse reaction (response to the drug) | |||||
| Non‐serious adverse events (temporal association) |
Risk of bias
| Item | Quote | Risk of bias (high, unclear, low) |
| Random sequence generation/generation of allocation sequence (selection bias) | ||
| Allocation concealment (selection bias) | ||
| Blinding of participants and personnel (performance bias) | ||
| Blinding of outcome assessment (detection bias) | ||
| Incomplete outcome data (intention‐to‐treat (ITT), imputation method) (attrition bias) Exclusion of placebo responders etc.: methylphenidate non‐responders (after randomisation) |
||
| Selective outcome reporting (according to protocol?) | ||
| Vested interest | ||
| Other sources of bias | Authors’ affiliations (e.g. Novartis) Selection bias (e.g. titration after randomisation → exclusion of methylphenidate non‐responders or placebo responders) |
Notes
| Sample calculation Ethics approval Comments from trial authors Key conclusions of trial authors Comments from review authors Inclusion of methylphenidate responders only/exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate Any withdrawals due to adverse events Supplemental information/data received through personal email correspondence with trial authors in *month* 2014 |
Data and analyses
Comparison 1. Teacher‐rated ADHD symptoms.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 All parallel‐group trials and first‐period cross‐over trials | 21 | 1728 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.74 [‐0.88, ‐0.61] |
| 1.1.1 Low risk of bias | 3 | 206 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.67 [‐1.11, ‐0.22] |
| 1.1.2 High risk of bias | 18 | 1522 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.75 [‐0.90, ‐0.61] |
| 1.2 Subgroup analysis: types of scales | 20 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.2.1 Conners' Teacher Rating Scale (CTRS) | 9 | 470 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.63 [‐0.82, ‐0.45] |
| 1.2.2 Abbreviated Conners' Rating Scale (ACRS) ‐ Teacher | 2 | 105 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.75 [‐1.79, 0.29] |
| 1.2.3 Conners' Abbreviated Symptom Questionnaire for Teachers (ASQ‐Teacher) | 1 | 59 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.79, 0.23] |
| 1.2.4 IOWA Conners' Teacher Rating Scale (IOWA CTRS) ‐ hyperactivity | 2 | 193 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.08 [‐1.39, ‐0.77] |
| 1.2.5 The Swanson, Nolan, and Pelham (SNAP) Scale ‐ Teacher | 2 | 328 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.61 [‐0.96, ‐0.25] |
| 1.2.6 Teacher ratings of attention | 1 | 20 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.55 [‐1.45, 0.35] |
| 1.2.7 Teacher ratings of impulsivity | 1 | 20 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.83, 0.92] |
| 1.2.8 IOWA Conners' Teacher Rating Scale ‐ Inattention/Overactivity (IOWA‐I/O) | 2 | 197 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.03 [‐1.36, ‐0.69] |
| 1.2.9 Fremdbeurteilungsbogen für Hyperkinetische Störungen (FBB‐HKS) | 1 | 85 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.06 [‐1.52, ‐0.61] |
| 1.2.10 Conners’ ADHD/DSM‐IV Scales ‐ Teacher (CADS‐T) | 2 | 254 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.05 [‐1.31, ‐0.78] |
| 1.2.11 Strengths and Weaknesses of ADHD Symptoms and Normal Behaviour (SWAN) Scale | 1 | 64 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐0.82, 0.17] |
| 1.3 Subgroup analysis: duration of treatment | 21 | 1728 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.74 [‐0.88, ‐0.61] |
| 1.3.1 Short term (up to 6 months) | 20 | 1475 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.77 [‐0.91, ‐0.64] |
| 1.3.2 Long term (over 6 months) | 1 | 253 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐0.72, ‐0.22] |
| 1.4 Subgroup analysis: dose | 21 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.4.1 Low dose | 7 | 361 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐0.82, ‐0.39] |
| 1.4.2 High dose | 8 | 766 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.75 [‐1.00, ‐0.50] |
| 1.4.3 Unknown dose | 8 | 753 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.85 [‐1.02, ‐0.68] |
| 1.5 Subgroup analysis: medication status ‐ medication naive versus not medication naive | 11 | 882 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.76 [‐0.94, ‐0.59] |
| 1.5.1 Medication naive | 7 | 480 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.70 [‐0.88, ‐0.51] |
| 1.5.2 Not medication naive | 4 | 402 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.85 [‐1.20, ‐0.50] |
| 1.6 Subgroup analysis: trials with enrichment design compared with trials without enrichment design | 20 | 1679 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.75 [‐0.89, ‐0.62] |
| 1.6.1 Trials with enrichment design of all participants | 8 | 1072 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.74 [‐0.96, ‐0.53] |
| 1.6.2 Trials without enrichment design of all participants | 12 | 607 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.78 [‐0.96, ‐0.60] |
| 1.7 Subgroup analysis: parallel‐group trials compared with first‐period cross‐over trials | 21 | 1728 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.74 [‐0.88, ‐0.61] |
| 1.7.1 Parallel‐group trials | 19 | 1633 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.76 [‐0.90, ‐0.61] |
| 1.7.2 First‐period cross‐over trials | 2 | 95 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.57 [‐0.98, ‐0.15] |
| 1.8 Subgroup analysis: vested interest | 18 | 1460 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.74 [‐0.90, ‐0.58] |
| 1.8.1 Low risk of vested interest | 4 | 186 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.49 [‐0.79, ‐0.20] |
| 1.8.2 High risk of vested interest | 14 | 1274 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.78 [‐0.96, ‐0.60] |
| 1.9 Subgroup analysis: type of control group | 21 | 1728 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.74 [‐0.88, ‐0.61] |
| 1.9.1 Placebo control group | 17 | 1358 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.77 [‐0.92, ‐0.63] |
| 1.9.2 No‐intervention control group | 4 | 370 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.62 [‐0.99, ‐0.24] |
| 1.10 Cross‐over trial (endpoint data) | 64 | 6341 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.88 [‐1.01, ‐0.75] |
| 1.10.1 Low risk of bias | 7 | 733 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.62 [‐0.84, ‐0.40] |
| 1.10.2 High risk of bias | 57 | 5608 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.91 [‐1.06, ‐0.77] |
| 1.11 Subgroup analysis: cross‐over trials (endpoint data): dose | 58 | 7403 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.82 [‐0.93, ‐0.71] |
| 1.11.1 Low dose | 43 | 4530 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.72 [‐0.86, ‐0.59] |
| 1.11.2 High dose | 31 | 2873 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.96 [‐1.15, ‐0.77] |
| 1.12 Subgroup analysis: all parallel‐group trials and first‐period cross‐over trials compared with cross‐over trials (endpoint data) | 81 | 7564 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.82 [‐0.87, ‐0.77] |
| 1.12.1 All parallel‐group trials and first‐period cross‐over trials | 21 | 1728 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.74 [‐0.84, ‐0.64] |
| 1.12.2 Cross‐over trials (endpoint data) | 60 | 5836 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.85 [‐0.90, ‐0.79] |
| 1.13 All parallel‐group trials and cross‐over trials: risk of bias | 81 | 7564 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.85 [‐0.96, ‐0.74] |
| 1.13.1 Low risk of bias | 8 | 518 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.68 [‐0.91, ‐0.45] |
| 1.13.2 High risk of bias | 73 | 7046 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.87 [‐0.99, ‐0.75] |
| 1.14 All parallel‐group trials and cross‐over trials: vested interest | 77 | 7212 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.85 [‐0.96, ‐0.73] |
| 1.14.1 Low risk of vested interest | 23 | 1446 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.83 [‐1.08, ‐0.58] |
| 1.14.2 High risk of vested interest | 54 | 5766 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.85 [‐0.98, ‐0.72] |
Comparison 2. Independent assessor‐rated ADHD symptoms.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 All parallel‐group trials and first‐period cross‐over trials | 22 | 3724 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.10 [‐1.44, ‐0.77] |
| 2.1.1 Low risk of bias | 4 | 942 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐0.78, ‐0.03] |
| 2.1.2 High risk of bias | 18 | 2782 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.30 [‐1.70, ‐0.89] |
| 2.2 Subgroup analysis: types of scales | 22 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.2.1 Swanson, Kotkin, Agler, M‐Glynn and Pelham (SKAMP) Scale | 6 | 778 | Std. Mean Difference (IV, Random, 95% CI) | ‐2.79 [‐4.10, ‐1.47] |
| 2.2.2 ADHD Rating Scale (ADHD‐RS ) | 14 | 2802 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐0.81, ‐0.38] |
| 2.2.3 Swanson, Nolan and Pelham (SNAP) Scale | 1 | 221 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐0.61, ‐0.08] |
| 2.2.4 Unknown | 1 | 78 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.94 [‐1.41, ‐0.47] |
| 2.3 Subgroup analysis: duration of treatment | 22 | 3724 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.10 [‐1.44, ‐0.77] |
| 2.3.1 Short term (up to 6 months) | 21 | 3503 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.15 [‐1.50, ‐0.80] |
| 2.3.2 Long term (over 6 months) | 1 | 221 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐0.61, ‐0.08] |
| 2.4 Subgroup analysis: dose | 22 | 3724 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.10 [‐1.44, ‐0.77] |
| 2.4.1 Low dose | 1 | 138 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.52, 0.15] |
| 2.4.2 High dose | 17 | 3005 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.84 [‐1.13, ‐0.55] |
| 2.4.3 Unknown dose | 4 | 581 | Std. Mean Difference (IV, Random, 95% CI) | ‐2.57 [‐4.40, ‐0.74] |
| 2.5 Subgroup analysis: trials with enrichment design compared with trials without enrichment design | 22 | 3724 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.10 [‐1.44, ‐0.77] |
| 2.5.1 Trials with enrichment design of all participants | 19 | 3245 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.24 [‐1.61, ‐0.87] |
| 2.5.2 Trials without enrichment design of all participants | 3 | 479 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.62, 0.17] |
| 2.6 Subgroup analysis: type of control group | 22 | 3724 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.10 [‐1.44, ‐0.77] |
| 2.6.1 Placebo control group | 20 | 3200 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.22 [‐1.58, ‐0.85] |
| 2.6.2 No‐intervention control group | 2 | 524 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.52, 0.23] |
| 2.7 Subgroup analysis: parallel‐group trials compared with first‐period cross‐over trials | 22 | 3724 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.10 [‐1.44, ‐0.77] |
| 2.7.1 Parallel‐group trials | 19 | 3550 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.17 [‐1.54, ‐0.80] |
| 2.7.2 First‐period cross‐over trials | 3 | 174 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.72 [‐1.03, ‐0.41] |
| 2.8 Cross‐over trials (endpoint data) | 22 | 3854 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.97 [‐1.11, ‐0.83] |
| 2.8.1 High risk of bias | 22 | 3854 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.97 [‐1.11, ‐0.83] |
| 2.9 Subgroup analysis: cross‐over trials (endpoint data): dose | 22 | 5257 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.88 [‐1.00, ‐0.76] |
| 2.9.1 Low dose | 17 | 3067 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.72 [‐0.86, ‐0.58] |
| 2.9.2 High dose | 13 | 2051 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.07 [‐1.27, ‐0.86] |
| 2.9.3 Unknown dose | 1 | 139 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.03 [‐1.39, ‐0.68] |
| 2.10 Subgroup analysis: all parallel‐group trials and first‐period cross‐over trials compared with cross‐over trials (endpoint data) | 42 | 7277 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.99 [‐1.18, ‐0.80] |
| 2.10.1 All parallel‐group trials and first‐period cross‐over trials | 21 | 3586 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.15 [‐1.50, ‐0.81] |
| 2.10.2 Cross‐over trials (endpoint data) | 21 | 3691 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.93 [‐1.07, ‐0.78] |
| 2.11 All parallel‐group trials and cross‐over trials: risk of bias | 42 | 7277 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.99 [‐1.18, ‐0.80] |
| 2.11.1 Low risk of bias | 4 | 942 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐0.78, ‐0.03] |
| 2.11.2 High risk of bias | 38 | 6335 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.06 [‐1.25, ‐0.86] |
| 2.12 All parallel‐group trials and cross‐over trials: vested interest | 43 | 7414 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.98 [‐1.17, ‐0.80] |
| 2.12.1 Low risk of vested interest | 6 | 600 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.96 [‐1.43, ‐0.48] |
| 2.12.2 High risk or unclear risk of vested interest | 37 | 6814 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.99 [‐1.19, ‐0.79] |
Comparison 3. Parent‐rated ADHD symptoms.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 All parallel‐group trials and first‐period cross‐over trials | 27 | 2927 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.63 [‐0.76, ‐0.50] |
| 3.1.1 Low risk of bias | 6 | 702 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.49 [‐0.71, ‐0.26] |
| 3.1.2 High risk of bias | 21 | 2225 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐0.81, ‐0.51] |
| 3.2 Subgroup analysis: types of scales | 27 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.2.1 Conners' Parent Rating Scale (CPRS) | 8 | 800 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐0.80, ‐0.26] |
| 3.2.2 ADHD Rating Scale ‐ Fourth Edition (ADHD‐RS‐IV) | 5 | 753 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.37 [‐0.53, ‐0.21] |
| 3.2.3 Fremdbeurteilungsbogen für Hyperkinetische Störungen (FBB‐HKS) | 1 | 85 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.91 [‐1.36, ‐0.46] |
| 3.2.4 Conners’ ADHD/DSM‐IV Scales ‐ Parent (CADS‐P) | 2 | 195 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.12 [‐1.44, ‐0.81] |
| 3.2.5 CADS‐P Inattentive subscale | 1 | 109 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.78 [‐1.17, ‐0.39] |
| 3.2.6 CADS‐P Hyperactivity subscale | 1 | 109 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.93 [‐1.32, ‐0.53] |
| 3.2.7 Clinican's Manual for the Assesment of Disruptive Behavior Disorders Rating Scale for Parents (Barkley) | 1 | 41 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.82, 0.41] |
| 3.2.8 Abbreviated Conners' Rating Scale (ACRS) ‐ Parent | 2 | 121 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.62 [‐0.99, ‐0.25] |
| 3.2.9 Swanson, Nolan, and Pelham, Fourth Edition ‐ Parent (SNAP‐IV‐Parent) Scale | 4 | 390 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.59 [‐0.80, ‐0.39] |
| 3.2.10 Strengths and Weaknesses of ADHD Symptoms and Normal Behavior (SWAN) Scale | 1 | 86 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐0.86, 0.00] |
| 3.2.11 IOWA Conners' Rating Scale ‐ Inattention/Overactivity (IOWA‐I/O) | 3 | 352 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.78 [‐1.35, ‐0.21] |
| 3.2.12 Parent Vanderbilt ADHD Rating Scale | 1 | 97 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.67 [‐1.23, ‐0.11] |
| 3.3 Subgroup analysis: duration of treatment | 27 | 2927 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.63 [‐0.76, ‐0.50] |
| 3.3.1 Short term (up to 6 months) | 25 | 2605 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.63 [‐0.78, ‐0.49] |
| 3.3.2 Long term (over 6 months) | 2 | 322 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.56 [‐0.79, ‐0.34] |
| 3.4 Subgroup analysis: dose | 27 | 3075 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.61 [‐0.74, ‐0.48] |
| 3.4.1 Low dose | 4 | 254 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.51 [‐1.14, 0.13] |
| 3.4.2 High dose | 12 | 1650 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.58 [‐0.80, ‐0.37] |
| 3.4.3 Unknown dose | 13 | 1171 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.67 [‐0.83, ‐0.51] |
| 3.5 Subgroup analysis: medication status ‐ medication naive versus not medication naive | 11 | 992 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐0.87, ‐0.43] |
| 3.5.1 Medication naive | 7 | 544 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.58 [‐0.84, ‐0.32] |
| 3.5.2 Not medication naive | 4 | 448 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.75 [‐1.16, ‐0.35] |
| 3.6 Subgroup analysis: trials with enrichment design compared with trials without enrichment design | 25 | 2803 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.63 [‐0.77, ‐0.49] |
| 3.6.1 Trials with enrichment design of all participants | 13 | 2155 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.63 [‐0.78, ‐0.48] |
| 3.6.2 Trials without enrichment design of all participants | 12 | 648 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.61 [‐0.88, ‐0.34] |
| 3.7 Subgroup analysis: parallel‐group trials compared with first‐period cross‐over trials | 27 | 2927 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.63 [‐0.76, ‐0.50] |
| 3.7.1 Parallel‐group trials | 25 | 2874 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.62 [‐0.76, ‐0.49] |
| 3.7.2 First‐period cross‐over trials | 2 | 53 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.68 [‐1.25, ‐0.11] |
| 3.8 Subgroup analysis: type of control group | 27 | 2927 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.63 [‐0.76, ‐0.50] |
| 3.8.1 Placebo control group | 19 | 2263 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐0.80, ‐0.48] |
| 3.8.2 No‐intervention control group | 8 | 664 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.59 [‐0.84, ‐0.34] |
| 3.9 Cross‐over trials (endpoint data) | 45 | 4971 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.70 [‐0.86, ‐0.55] |
| 3.9.1 Low risk of bias | 8 | 853 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.56 [‐0.72, ‐0.40] |
| 3.9.2 High risk of bias | 37 | 4118 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.75 [‐0.94, ‐0.57] |
| 3.10 Subgroup analysis: cross‐over trials (endpoint data): dose | 45 | 6155 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.63 [‐0.76, ‐0.50] |
| 3.10.1 Low dose | 26 | 3242 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.58 [‐0.71, ‐0.45] |
| 3.10.2 High dose | 29 | 2508 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.75 [‐0.90, ‐0.60] |
| 3.10.3 Unknown dose | 3 | 405 | Std. Mean Difference (IV, Random, 95% CI) | 0.19 [‐1.55, 1.93] |
| 3.11 Subgroup analysis: all parallel‐group trials and first‐period cross‐over trials compared with cross‐over trials (endpoint data) | 69 | 7838 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.67 [‐0.78, ‐0.56] |
| 3.11.1 All parallel‐group trials and first‐period cross‐over trials | 27 | 2955 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.63 [‐0.76, ‐0.50] |
| 3.11.2 Cross‐over trials (endpoint data) | 43 | 4883 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.71 [‐0.86, ‐0.55] |
| 3.12 All parallel‐group trials and cross‐over trials: risk of bias | 69 | 7503 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.68 [‐0.79, ‐0.57] |
| 3.12.1 Low risk of bias | 12 | 1234 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐0.68, ‐0.39] |
| 3.12.2 High risk of bias | 57 | 6269 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.71 [‐0.84, ‐0.58] |
| 3.13 All parallel‐group trials and cross‐over trials: vested interest | 69 | 7503 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.68 [‐0.79, ‐0.57] |
| 3.13.1 Low risk of vested interest | 19 | 1187 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.69 [‐0.84, ‐0.54] |
| 3.13.2 High risk or unclear risk of vested interest | 50 | 6316 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.68 [‐0.82, ‐0.54] |
Comparison 4. Additional subgroup analyses of ADHD symptoms.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Parallel‐group trials and first‐period cross‐over trials: comparison of raters | 46 | 7965 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.73 [‐0.85, ‐0.60] |
| 4.1.1 Teacher‐rated | 21 | 1759 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.75 [‐0.90, ‐0.60] |
| 4.1.2 Independent assessor‐rated | 20 | 3601 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.88 [‐1.18, ‐0.58] |
| 4.1.3 Parent‐rated | 26 | 2605 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐0.77, ‐0.51] |
| 4.2 Parallel‐group trials and first‐period cross‐over trials: age | 13 | 2374 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.93 [‐1.35, ‐0.50] |
| 4.2.1 2 to 6 years | 2 | 153 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.50 [‐0.82, ‐0.18] |
| 4.2.2 7 to 11 years | 5 | 623 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.77 [‐3.24, ‐0.29] |
| 4.2.3 12 to 18 years | 6 | 1598 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.48 [‐0.77, ‐0.19] |
| 4.3 Parallel‐group trials and first‐period cross‐over trials: comorbidity versus no comorbidity | 29 | 3433 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.69 [‐0.84, ‐0.54] |
| 4.3.1 ADHD with comorbidity | 20 | 2128 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.67 [‐0.88, ‐0.47] |
| 4.3.2 ADHD without comorbidity | 9 | 1305 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.73 [‐0.91, ‐0.54] |
| 4.4 Parallel‐group trials and first‐period cross‐over trials: subtypes ADHD: ADHD Rating Scale (parent‐, teacher‐ or independent assessor‐rated) | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.4.1 Combined ADHD | 2 | 559 | Std. Mean Difference (IV, Random, 95% CI) | 0.65 [‐1.30, 2.60] |
| 4.4.2 Inattentive ADHD | 1 | 204 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.31 [‐1.61, ‐1.01] |
| 4.5 Cross‐over trials: first‐period data versus endpoint data (parent‐, independent assessor‐ and teacher‐rated) | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.5.1 First‐period data | 4 | 191 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐0.93, ‐0.34] |
| 4.5.2 Endpoint data | 4 | 372 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.91 [‐1.18, ‐0.65] |
Comparison 5. Serious adverse events: parallel‐group trials and first‐period cross‐over trials.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Proportions of participants with serious adverse events (SAE) | 26 | 3673 | Risk Ratio (IV, Random, 95% CI) | 0.80 [0.39, 1.67] |
| 5.2 Nervous system (including psychiatry) | 12 | 4199 | Risk Ratio (IV, Random, 95% CI) | 0.70 [0.29, 1.71] |
| 5.2.1 Aggression | 1 | 303 | Risk Ratio (IV, Random, 95% CI) | 0.50 [0.05, 5.49] |
| 5.2.2 Concussion | 1 | 303 | Risk Ratio (IV, Random, 95% CI) | 0.34 [0.01, 8.17] |
| 5.2.3 Loss of consciousness | 1 | 221 | Risk Ratio (IV, Random, 95% CI) | 0.33 [0.01, 8.02] |
| 5.2.4 Psychosis | 4 | 919 | Risk Ratio (IV, Random, 95% CI) | 0.81 [0.13, 5.12] |
| 5.2.5 Syncope | 3 | 741 | Risk Ratio (IV, Random, 95% CI) | 1.39 [0.23, 8.47] |
| 5.2.6 Suicidal ideation | 6 | 1032 | Risk Ratio (IV, Random, 95% CI) | 1.63 [0.07, 38.55] |
| 5.2.7 Suicidal behaviour | 2 | 0 | Risk Ratio (IV, Random, 95% CI) | Not estimable |
| 5.2.8 Oppositional behaviour/negativism | 1 | 217 | Risk Ratio (IV, Random, 95% CI) | 0.17 [0.01, 4.04] |
| 5.2.9 Adjustment disorder | 1 | 230 | Risk Ratio (IV, Random, 95% CI) | 0.78 [0.03, 18.91] |
| 5.3 Digestive system | 2 | 414 | Risk Ratio (IV, Random, 95% CI) | 2.11 [0.22, 20.04] |
| 5.3.1 Appendicitis | 2 | 414 | Risk Ratio (IV, Random, 95% CI) | 2.11 [0.22, 20.04] |
| 5.4 Cardiovascular systems | 2 | 280 | Risk Ratio (IV, Random, 95% CI) | 1.02 [0.11, 9.65] |
| 5.4.1 Haematoma | 1 | 221 | Risk Ratio (IV, Random, 95% CI) | 0.33 [0.01, 8.02] |
| 5.4.2 Tachycardia | 1 | 59 | Risk Ratio (IV, Random, 95% CI) | 3.10 [0.13, 73.14] |
| 5.5 Respiratory systems | 1 | 606 | Risk Ratio (IV, Random, 95% CI) | 1.01 [0.11, 9.62] |
| 5.5.1 Bronchitis | 1 | 303 | Risk Ratio (IV, Random, 95% CI) | 0.34 [0.01, 8.17] |
| 5.5.2 Asthma | 1 | 303 | Risk Ratio (IV, Random, 95% CI) | 3.02 [0.12, 73.54] |
| 5.6 Urinary system | 2 | 578 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.14 [0.23, 19.81] |
| 5.6.1 Renal cyst | 1 | 275 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.49 [0.06, 36.27] |
| 5.6.2 Kidney infection | 1 | 303 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.02 [0.12, 73.54] |
| 5.7 Skeletal and muscular system (including pain) | 1 | 221 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.02] |
| 5.7.1 Clavical fracture | 1 | 221 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.02] |
| 5.8 Immune system (including infections) | 1 | 303 | Risk Ratio (IV, Random, 95% CI) | 3.02 [0.12, 73.54] |
| 5.8.1 Cyst rupture | 1 | 303 | Risk Ratio (IV, Random, 95% CI) | 3.02 [0.12, 73.54] |
| 5.9 Other | 2 | 524 | Risk Ratio (IV, Random, 95% CI) | 1.00 [0.10, 9.55] |
| 5.9.1 Drug toxicity | 1 | 303 | Risk Ratio (IV, Random, 95% CI) | 0.34 [0.01, 8.17] |
| 5.9.2 Overdose | 1 | 221 | Risk Ratio (IV, Random, 95% CI) | 2.97 [0.12, 72.20] |
Comparison 6. Serious adverse events: cross‐over trials (endpoint data).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Proportion of participants with serious adverse events (SAE) | 16 | 3323 | Risk Ratio (IV, Random, 95% CI) | 2.46 [0.50, 12.03] |
| 6.2 Nervous system (including psychiatry) | 2 | 304 | Risk Ratio (IV, Random, 95% CI) | 2.05 [0.22, 19.14] |
| 6.2.1 Hallucinations | 1 | 37 | Risk Ratio (IV, Random, 95% CI) | 1.33 [0.06, 30.42] |
| 6.2.2 Psychiatric disorder | 1 | 267 | Risk Ratio (IV, Random, 95% CI) | 3.21 [0.13, 78.04] |
| 6.3 Urinary system | 1 | 136 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.00 [0.12, 72.37] |
| 6.3.1 Proteinuria | 1 | 136 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.00 [0.12, 72.37] |
| 6.4 Immune system | 1 | 644 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.93 [0.31, 27.99] |
| 6.4.1 Peritonsillar abscess | 1 | 322 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.93 [0.12, 71.32] |
| 6.4.2 Oral bullae | 1 | 322 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.93 [0.12, 71.32] |
Comparison 7. Non‐serious adverse events: parallel‐group trials and first‐period cross‐over trials.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Proportion of participants with non‐serious adverse events | 35 | 5342 | Risk Ratio (IV, Random, 95% CI) | 1.23 [1.11, 1.37] |
| 7.2 Subgroup analysis: proportion of participants with non‐serious adverse events according to dose | 32 | 4718 | Risk Ratio (IV, Random, 95% CI) | 1.24 [1.12, 1.37] |
| 7.2.1 Low dose | 3 | 289 | Risk Ratio (IV, Random, 95% CI) | 1.02 [0.97, 1.07] |
| 7.2.2 High dose | 22 | 3365 | Risk Ratio (IV, Random, 95% CI) | 1.23 [1.14, 1.32] |
| 7.2.3 Unknown dose | 8 | 1064 | Risk Ratio (IV, Random, 95% CI) | 1.37 [0.93, 2.02] |
| 7.3 Nervous system (including psychiatry) | 37 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 7.3.1 Affective | 4 | 456 | Risk Ratio (IV, Random, 95% CI) | 2.39 [0.48, 11.96] |
| 7.3.2 Aggression | 3 | 503 | Risk Ratio (IV, Random, 95% CI) | 1.23 [0.30, 5.08] |
| 7.3.3 Apathy | 1 | 59 | Risk Ratio (IV, Random, 95% CI) | 0.80 [0.19, 3.33] |
| 7.3.4 Confusion or confusional state | 3 | 823 | Risk Ratio (IV, Random, 95% CI) | 0.75 [0.17, 3.24] |
| 7.3.5 Depression | 1 | 59 | Risk Ratio (IV, Random, 95% CI) | 0.83 [0.22, 3.10] |
| 7.3.6 Dizziness | 11 | 2149 | Risk Ratio (IV, Random, 95% CI) | 1.77 [0.95, 3.30] |
| 7.3.7 Drowsiness | 4 | 811 | Risk Ratio (IV, Random, 95% CI) | 1.27 [0.82, 1.98] |
| 7.3.8 Emotional lability | 5 | 624 | Risk Ratio (IV, Random, 95% CI) | 1.24 [0.54, 2.85] |
| 7.3.9 Fatigue | 14 | 2228 | Risk Ratio (IV, Random, 95% CI) | 0.67 [0.44, 1.02] |
| 7.3.10 Headache | 32 | 5041 | Risk Ratio (IV, Random, 95% CI) | 1.33 [1.04, 1.70] |
| 7.3.11 Irritability | 20 | 3290 | Risk Ratio (IV, Random, 95% CI) | 1.05 [0.82, 1.34] |
| 7.3.12 Nervousness | 2 | 362 | Risk Ratio (IV, Random, 95% CI) | 2.52 [0.82, 7.76] |
| 7.3.13 Pain | 1 | 132 | Risk Ratio (IV, Random, 95% CI) | 1.91 [0.21, 17.60] |
| 7.3.14 Picking at skin or fingers, nail biting, lip or cheek chewing | 5 | 686 | Risk Ratio (IV, Random, 95% CI) | 0.97 [0.60, 1.57] |
| 7.3.15 Sad, tearful or depressed | 7 | 1015 | Risk Ratio (IV, Random, 95% CI) | 1.47 [0.95, 2.28] |
| 7.3.16 Thirst | 2 | 179 | Risk Ratio (IV, Random, 95% CI) | 3.00 [0.13, 71.82] |
| 7.3.17 Dull, tired, listless | 1 | 110 | Risk Ratio (IV, Random, 95% CI) | 1.70 [0.51, 5.68] |
| 7.3.18 Tics or nervous movements | 12 | 1556 | Risk Ratio (IV, Random, 95% CI) | 0.77 [0.27, 2.22] |
| 7.3.19 Worried or anxious | 3 | 596 | Risk Ratio (IV, Random, 95% CI) | 1.37 [0.84, 2.25] |
| 7.3.20 Feeling jittery | 1 | 86 | Risk Ratio (IV, Random, 95% CI) | 0.52 [0.05, 5.56] |
| 7.3.21 Malaise | 1 | 0 | Risk Ratio (IV, Random, 95% CI) | Not estimable |
| 7.3.22 Dysgeusia | 2 | 171 | Risk Ratio (IV, Random, 95% CI) | 0.79 [0.19, 3.30] |
| 7.3.23 Lethargy | 2 | 224 | Risk Ratio (IV, Random, 95% CI) | 0.69 [0.40, 1.17] |
| 7.3.24 Aphonia | 1 | 86 | Risk Ratio (IV, Random, 95% CI) | 3.14 [0.13, 74.98] |
| 7.3.25 Psychomotor hyperactivity | 3 | 522 | Risk Ratio (IV, Random, 95% CI) | 3.67 [0.61, 22.01] |
| 7.3.26 Syncope | 1 | 86 | Risk Ratio (IV, Random, 95% CI) | 0.35 [0.01, 8.33] |
| 7.3.27 Tremor | 3 | 231 | Risk Ratio (IV, Random, 95% CI) | 0.35 [0.01, 8.33] |
| 7.3.28 Social withdrawal | 1 | 110 | Risk Ratio (IV, Random, 95% CI) | 2.56 [0.24, 26.71] |
| 7.3.29 Sedation | 1 | 138 | Risk Ratio (IV, Random, 95% CI) | 1.30 [0.66, 2.53] |
| 7.3.30 Mood swings | 2 | 247 | Risk Ratio (IV, Random, 95% CI) | 2.53 [0.94, 6.82] |
| 7.3.31 Anger | 1 | 86 | Risk Ratio (IV, Random, 95% CI) | 0.35 [0.01, 8.33] |
| 7.3.32 Anxiety | 2 | 180 | Risk Ratio (IV, Random, 95% CI) | 0.64 [0.08, 5.08] |
| 7.3.33 Change in sustained attention | 1 | 86 | Risk Ratio (IV, Random, 95% CI) | 0.35 [0.01, 8.33] |
| 7.3.34 Emotional poverty | 2 | 175 | Risk Ratio (IV, Random, 95% CI) | 3.47 [0.37, 32.68] |
| 7.3.35 Trichotillomania | 1 | 85 | Risk Ratio (IV, Random, 95% CI) | 2.80 [0.12, 66.85] |
| 7.3.36 Stuttering | 1 | 94 | Risk Ratio (IV, Random, 95% CI) | 0.33 [0.01, 7.98] |
| 7.3.37 Emotional disorder | 2 | 174 | Risk Ratio (IV, Random, 95% CI) | 0.42 [0.02, 10.16] |
| 7.3.38 Negativism | 2 | 174 | Risk Ratio (IV, Random, 95% CI) | 3.83 [0.16, 91.41] |
| 7.3.39 Migraine | 1 | 0 | Risk Ratio (IV, Random, 95% CI) | Not estimable |
| 7.3.40 Tension | 1 | 60 | Risk Ratio (IV, Random, 95% CI) | 23.00 [1.42, 373.44] |
| 7.4 Digestive system | 34 | 22299 | Risk Ratio (IV, Random, 95% CI) | 1.81 [1.54, 2.14] |
| 7.4.1 Change in appetite | 1 | 94 | Risk Ratio (IV, Random, 95% CI) | 0.78 [0.32, 1.92] |
| 7.4.2 Decreased appetite | 30 | 5127 | Risk Ratio (IV, Random, 95% CI) | 3.35 [2.49, 4.50] |
| 7.4.3 Increased appetite | 2 | 265 | Risk Ratio (IV, Random, 95% CI) | 0.15 [0.02, 1.34] |
| 7.4.4 Change in weight | 1 | 94 | Risk Ratio (IV, Random, 95% CI) | 0.40 [0.08, 1.96] |
| 7.4.5 Increased weight | 1 | 0 | Risk Ratio (IV, Random, 95% CI) | Not estimable |
| 7.4.6 Decreased weight | 11 | 2001 | Risk Ratio (IV, Random, 95% CI) | 5.44 [2.47, 11.98] |
| 7.4.7 Dyspepsia | 5 | 390 | Risk Ratio (IV, Random, 95% CI) | 0.71 [0.16, 3.19] |
| 7.4.8 Upper abdominal pain | 10 | 1745 | Risk Ratio (IV, Random, 95% CI) | 1.25 [0.79, 1.96] |
| 7.4.9 Stomachache (abdominal pain) | 18 | 3069 | Risk Ratio (IV, Random, 95% CI) | 1.19 [0.94, 1.50] |
| 7.4.10 Vomiting | 20 | 3105 | Risk Ratio (IV, Random, 95% CI) | 1.26 [0.85, 1.86] |
| 7.4.11 Constipation | 1 | 0 | Risk Ratio (IV, Random, 95% CI) | Not estimable |
| 7.4.12 Oral pain | 2 | 171 | Risk Ratio (IV, Random, 95% CI) | 3.14 [0.13, 74.98] |
| 7.4.13 Retching | 1 | 0 | Risk Ratio (IV, Random, 95% CI) | Not estimable |
| 7.4.14 Diarrhoea | 8 | 1088 | Risk Ratio (IV, Random, 95% CI) | 0.83 [0.35, 1.94] |
| 7.4.15 Dry mouth | 4 | 1057 | Risk Ratio (IV, Random, 95% CI) | 3.79 [1.26, 11.39] |
| 7.4.16 Nausea | 19 | 3484 | Risk Ratio (IV, Random, 95% CI) | 1.40 [1.00, 1.95] |
| 7.4.17 Flatulence | 1 | 86 | Risk Ratio (IV, Random, 95% CI) | 0.35 [0.01, 8.33] |
| 7.4.18 Gastritis | 1 | 89 | Risk Ratio (IV, Random, 95% CI) | 3.83 [0.16, 91.40] |
| 7.4.19 Gastrointestinal concerns (unspecified) | 1 | 94 | Risk Ratio (IV, Random, 95% CI) | 1.00 [0.06, 15.52] |
| 7.4.20 Polydipsia | 1 | 0 | Risk Ratio (IV, Random, 95% CI) | Not estimable |
| 7.5 Cardiovascular system | 12 | 3047 | Risk Ratio (IV, Random, 95% CI) | 1.41 [0.81, 2.46] |
| 7.5.1 ECG: prolonged QT‐interval | 2 | 466 | Risk Ratio (IV, Random, 95% CI) | 0.81 [0.13, 5.00] |
| 7.5.2 ECG: tachycardia | 5 | 686 | Risk Ratio (IV, Random, 95% CI) | 0.66 [0.20, 2.22] |
| 7.5.3 Increased diastolic blood pressure | 1 | 119 | Risk Ratio (IV, Random, 95% CI) | 1.07 [0.43, 2.68] |
| 7.5.4 Increased systolic blood pressure | 1 | 86 | Risk Ratio (IV, Random, 95% CI) | 0.35 [0.01, 8.33] |
| 7.5.5 Increased heart rate | 2 | 422 | Risk Ratio (IV, Random, 95% CI) | 4.99 [0.86, 28.85] |
| 7.5.6 Supraventricular extrasystoles | 1 | 17 | Risk Ratio (IV, Random, 95% CI) | 3.00 [0.11, 84.55] |
| 7.5.7 Ventricular arrhythmia | 1 | 0 | Risk Ratio (IV, Random, 95% CI) | Not estimable |
| 7.5.8 Epistaxis | 3 | 303 | Risk Ratio (IV, Random, 95% CI) | 1.71 [0.29, 10.07] |
| 7.5.9 Increased blood pressure | 2 | 414 | Risk Ratio (IV, Random, 95% CI) | 2.72 [0.00, 7037727483218459000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000.00] |
| 7.5.10 Orthostatic hypotension | 1 | 329 | Risk Ratio (IV, Random, 95% CI) | 2.72 [0.00, 7037727483218459000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000000.00] |
| 7.5.11 Palpitations | 1 | 60 | Risk Ratio (IV, Random, 95% CI) | 11.00 [0.64, 190.54] |
| 7.5.12 Pallor | 1 | 60 | Risk Ratio (IV, Random, 95% CI) | 23.00 [1.42, 373.44] |
| 7.6 Respiratory system | 13 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 7.6.1 Pharyngolaryngeal pain | 1 | 303 | Risk Ratio (IV, Random, 95% CI) | 1.12 [0.59, 2.13] |
| 7.6.2 Upper respiratory tract infection | 3 | 421 | Risk Ratio (IV, Random, 95% CI) | 1.14 [0.56, 2.34] |
| 7.6.3 Bronchitis | 1 | 85 | Risk Ratio (IV, Random, 95% CI) | 0.31 [0.01, 7.43] |
| 7.6.4 Oropharyngeal pain | 1 | 86 | Risk Ratio (IV, Random, 95% CI) | 7.33 [0.39, 137.67] |
| 7.6.5 Rhinorrhoea | 1 | 86 | Risk Ratio (IV, Random, 95% CI) | 5.23 [0.26, 105.88] |
| 7.6.6 Allergic bronchitis | 1 | 86 | Risk Ratio (IV, Random, 95% CI) | 3.14 [0.13, 74.98] |
| 7.6.7 Sneezing | 1 | 86 | Risk Ratio (IV, Random, 95% CI) | 3.14 [0.13, 74.98] |
| 7.6.8 Nasal congestion | 3 | 565 | Risk Ratio (IV, Random, 95% CI) | 1.24 [0.62, 2.48] |
| 7.6.9 Cough | 9 | 1656 | Risk Ratio (IV, Random, 95% CI) | 1.31 [0.62, 2.78] |
| 7.6.10 Shortness of breath | 2 | 179 | Risk Ratio (IV, Random, 95% CI) | 3.00 [0.13, 71.82] |
| 7.7 Urinary system | 1 | 178 | Risk Ratio (M‐H, Random, 95% CI) | 3.83 [0.41, 36.08] |
| 7.7.1 Pollakiuria | 1 | 89 | Risk Ratio (M‐H, Random, 95% CI) | 3.83 [0.16, 91.40] |
| 7.7.2 Urinary incontinence | 1 | 89 | Risk Ratio (M‐H, Random, 95% CI) | 3.83 [0.16, 91.40] |
| 7.8 Skeletal and muscular systems (including pain) | 7 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 7.8.1 Arthralgia | 2 | 388 | Risk Ratio (IV, Random, 95% CI) | 0.67 [0.24, 1.84] |
| 7.8.2 Asthenia | 1 | 177 | Risk Ratio (IV, Random, 95% CI) | 0.21 [0.01, 4.25] |
| 7.8.3 Back pain | 3 | 474 | Risk Ratio (IV, Random, 95% CI) | 0.77 [0.38, 1.57] |
| 7.8.4 Myalgia | 3 | 474 | Risk Ratio (IV, Random, 95% CI) | 0.55 [0.22, 1.35] |
| 7.8.5 Toothache | 1 | 303 | Risk Ratio (IV, Random, 95% CI) | 1.01 [0.43, 2.35] |
| 7.8.6 Ligament strain | 1 | 85 | Risk Ratio (IV, Random, 95% CI) | 0.31 [0.01, 7.43] |
| 7.8.7 Muscle strain | 1 | 0 | Risk Ratio (IV, Random, 95% CI) | Not estimable |
| 7.8.8 Fractures | 1 | 94 | Risk Ratio (IV, Random, 95% CI) | 3.00 [0.13, 71.82] |
| 7.8.9 Muscle cramps | 1 | 94 | Risk Ratio (IV, Random, 95% CI) | 3.00 [0.13, 71.82] |
| 7.8.10 Pain in extremity | 1 | 89 | Risk Ratio (IV, Random, 95% CI) | 0.26 [0.01, 5.16] |
| 7.8.11 Pain | 1 | 132 | Risk Ratio (IV, Random, 95% CI) | 1.87 [0.22, 16.19] |
| 7.9 Immune system (including infections) | 17 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 7.9.1 Gastroenteritis | 5 | 606 | Risk Ratio (IV, Random, 95% CI) | 2.41 [0.66, 8.78] |
| 7.9.2 Influenza | 4 | 709 | Risk Ratio (IV, Random, 95% CI) | 0.55 [0.23, 1.30] |
| 7.9.3 Nasopharyngitis | 10 | 1623 | Risk Ratio (IV, Random, 95% CI) | 1.14 [0.76, 1.70] |
| 7.9.4 Otitis media | 3 | 271 | Risk Ratio (IV, Random, 95% CI) | 0.99 [0.15, 6.61] |
| 7.9.5 Pharyngitis | 5 | 614 | Risk Ratio (IV, Random, 95% CI) | 2.02 [0.55, 7.35] |
| 7.9.6 Pyrexia | 5 | 678 | Risk Ratio (IV, Random, 95% CI) | 0.68 [0.12, 3.81] |
| 7.9.7 Rhinitis | 1 | 132 | Risk Ratio (IV, Random, 95% CI) | 1.28 [0.43, 3.79] |
| 7.9.8 Upper respiratory tract infection ‐ not otherwise specified (NOS) | 12 | 1929 | Risk Ratio (IV, Random, 95% CI) | 0.95 [0.65, 1.39] |
| 7.9.9 Viral infection NOS | 5 | 1029 | Risk Ratio (IV, Random, 95% CI) | 0.97 [0.44, 2.14] |
| 7.9.10 Seasonal allergy | 1 | 86 | Risk Ratio (IV, Random, 95% CI) | 0.35 [0.01, 8.33] |
| 7.9.11 Streptococcal impetigo | 1 | 86 | Risk Ratio (IV, Random, 95% CI) | 0.35 [0.01, 8.33] |
| 7.9.12 Sinusitis | 1 | 89 | Risk Ratio (IV, Random, 95% CI) | 0.42 [0.02, 10.16] |
| 7.9.13 Cellulitis | 1 | 89 | Risk Ratio (IV, Random, 95% CI) | 0.42 [0.02, 10.16] |
| 7.9.14 Pleurisy | 1 | 275 | Risk Ratio (IV, Random, 95% CI) | 1.49 [0.06, 36.27] |
| 7.10 Integumentary system | 7 | 1455 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.37, 1.58] |
| 7.10.1 Purple spots | 1 | 94 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.13, 71.82] |
| 7.10.2 Skin disorder (rash) | 5 | 460 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.12, 4.96] |
| 7.10.3 Skin laceration | 3 | 474 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.18, 1.22] |
| 7.10.4 Burns second degree | 1 | 85 | Risk Ratio (M‐H, Random, 95% CI) | 2.80 [0.12, 66.85] |
| 7.10.5 Burns first degree | 1 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 3.14 [0.13, 74.98] |
| 7.10.6 Subcutaneous haematoma | 1 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 3.14 [0.13, 74.98] |
| 7.10.7 Periorbital haematoma | 1 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 7.10.8 Rash maculo‐papular | 1 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 7.11 Sleep variability | 29 | 7308 | Risk Ratio (IV, Random, 95% CI) | 1.57 [1.23, 2.02] |
| 7.11.1 Somnolence | 6 | 757 | Risk Ratio (IV, Random, 95% CI) | 0.87 [0.52, 1.46] |
| 7.11.2 Trouble sleeping or sleep problems | 15 | 2620 | Risk Ratio (IV, Random, 95% CI) | 1.62 [1.18, 2.21] |
| 7.11.3 Insomnia | 15 | 2315 | Risk Ratio (IV, Random, 95% CI) | 1.90 [1.12, 3.22] |
| 7.11.4 Initial insomnia | 5 | 917 | Risk Ratio (IV, Random, 95% CI) | 2.25 [0.89, 5.71] |
| 7.11.5 Middle insomnia | 2 | 171 | Risk Ratio (IV, Random, 95% CI) | 1.05 [0.07, 16.22] |
| 7.11.6 Sleep disorder | 2 | 528 | Risk Ratio (IV, Random, 95% CI) | 0.67 [0.22, 2.10] |
| 7.12 Sleep variability continuous outcomes | 2 | 943 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.01, 0.06] |
| 7.12.1 Sleep efficiency after treatment discontinuation (percentage) | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | 5.42 [0.21, 10.63] |
| 7.12.2 Sleep onset latency (min) | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | ‐20.16 [‐45.74, 5.42] |
| 7.12.3 Total sleep time (min) | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | 30.82 [‐7.73, 69.37] |
| 7.12.4 Total in‐bed time (min) | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | ‐2.07 [‐33.82, 29.68] |
| 7.12.5 Wake after sleep onset (min) | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | ‐7.89 [‐22.87, 7.09] |
| 7.12.6 Number of wake bouts | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | ‐0.66 [‐8.38, 7.06] |
| 7.12.7 Mean wake bout time (min) | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | ‐0.17 [‐0.47, 0.13] |
| 7.12.8 Interdaily stability | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.06, 0.12] |
| 7.12.9 Interdaily variability | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.01, 0.07] |
| 7.12.10 Amount of activity during the 5 hours with the lowest activity | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | ‐0.46 [‐1.62, 0.70] |
| 7.12.11 Amount of activity during the 10 hours with the highest activity | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | ‐0.59 [‐3.57, 2.39] |
| 7.12.12 Amplitude of sleep‐wake rhythm | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐2.87, 2.63] |
| 7.12.13 Pittsburgh Sleep Quality Index (PSQI) | 1 | 367 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.83, 1.03] |
| 7.13 Vital signs | 14 | 6407 | Mean Difference (IV, Random, 95% CI) | 2.12 [1.26, 2.98] |
| 7.13.1 Diastolic blood pressure (mmHg) | 13 | 2032 | Mean Difference (IV, Random, 95% CI) | 1.90 [0.68, 3.11] |
| 7.13.2 Systolic blood pressure (mmHg) | 13 | 2032 | Mean Difference (IV, Random, 95% CI) | 0.85 [‐0.20, 1.89] |
| 7.13.3 Pulse or heart rate (bpm) | 13 | 2205 | Mean Difference (IV, Random, 95% CI) | 3.86 [2.09, 5.63] |
| 7.13.4 ECG: changes in QTcB | 1 | 138 | Mean Difference (IV, Random, 95% CI) | ‐1.70 [‐7.94, 4.54] |
| 7.14 Physical parameters | 7 | 2425 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.97 [‐1.24, ‐0.70] |
| 7.14.1 Height | 1 | 215 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.35, 0.22] |
| 7.14.2 Weight | 7 | 1400 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.13 [‐1.40, ‐0.85] |
| 7.14.3 BMI | 3 | 810 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.00 [‐1.26, ‐0.73] |
| 7.15 Other (including drug toxicity) | 9 | 4145 | Risk Ratio (IV, Random, 95% CI) | 1.13 [0.74, 1.72] |
| 7.15.1 Accidental injury | 3 | 656 | Risk Ratio (IV, Random, 95% CI) | 0.99 [0.48, 2.07] |
| 7.15.2 Excoriation | 2 | 389 | Risk Ratio (IV, Random, 95% CI) | 3.22 [1.20, 8.64] |
| 7.15.3 Overdose | 1 | 221 | Risk Ratio (IV, Random, 95% CI) | 2.97 [0.12, 72.20] |
| 7.15.4 Arthropod‐bite | 2 | 171 | Risk Ratio (IV, Random, 95% CI) | 0.35 [0.01, 8.33] |
| 7.15.5 Contusion | 1 | 86 | Risk Ratio (IV, Random, 95% CI) | 0.12 [0.01, 2.10] |
| 7.15.6 Wound | 2 | 171 | Risk Ratio (IV, Random, 95% CI) | 0.73 [0.06, 9.22] |
| 7.15.7 Tinnitus | 1 | 86 | Risk Ratio (IV, Random, 95% CI) | 5.23 [0.26, 105.89] |
| 7.15.8 Dry eye | 2 | 171 | Risk Ratio (IV, Random, 95% CI) | 3.14 [0.13, 74.98] |
| 7.15.9 Excessive eye blinking | 1 | 86 | Risk Ratio (IV, Random, 95% CI) | 0.35 [0.01, 8.33] |
| 7.15.10 Occular hyperaemia | 1 | 86 | Risk Ratio (IV, Random, 95% CI) | 0.35 [0.01, 8.33] |
| 7.15.11 Visual impairment | 1 | 86 | Risk Ratio (IV, Random, 95% CI) | 3.14 [0.13, 74.98] |
| 7.15.12 Red eyes | 1 | 94 | Risk Ratio (IV, Random, 95% CI) | 0.33 [0.01, 7.98] |
| 7.15.13 Conjunctival abrasion | 1 | 86 | Risk Ratio (IV, Random, 95% CI) | 3.14 [0.13, 74.98] |
| 7.15.14 Radius fracture | 1 | 86 | Risk Ratio (IV, Random, 95% CI) | 3.14 [0.13, 74.98] |
| 7.15.15 Snake bite | 1 | 86 | Risk Ratio (IV, Random, 95% CI) | 3.14 [0.13, 74.98] |
| 7.15.16 Enuresis | 1 | 86 | Risk Ratio (IV, Random, 95% CI) | 0.35 [0.01, 8.33] |
| 7.15.17 Night sweats | 1 | 86 | Risk Ratio (IV, Random, 95% CI) | 0.35 [0.01, 8.33] |
| 7.15.18 Abnormal liver function test | 1 | 85 | Risk Ratio (IV, Random, 95% CI) | 0.31 [0.01, 7.43] |
| 7.15.19 Alopecia | 1 | 0 | Risk Ratio (IV, Random, 95% CI) | Not estimable |
| 7.15.20 Nail injury | 1 | 86 | Risk Ratio (IV, Random, 95% CI) | 0.35 [0.01, 8.33] |
| 7.15.21 Itching | 2 | 179 | Risk Ratio (IV, Random, 95% CI) | 5.00 [0.25, 101.43] |
| 7.15.22 Ear pain | 1 | 0 | Risk Ratio (IV, Random, 95% CI) | Not estimable |
| 7.15.23 Corneal injury | 1 | 89 | Risk Ratio (IV, Random, 95% CI) | 0.42 [0.02, 10.16] |
| 7.15.24 Wrist fracture | 1 | 329 | Risk Ratio (IV, Random, 95% CI) | 1.51 [0.06, 36.85] |
| 7.15.25 Dysmennorhea | 1 | 329 | Risk Ratio (IV, Random, 95% CI) | 1.51 [0.06, 36.85] |
| 7.15.26 Myopia | 1 | 0 | Risk Ratio (IV, Random, 95% CI) | Not estimable |
| 7.15.27 Other AEs unspecified | 1 | 60 | Risk Ratio (IV, Random, 95% CI) | 0.40 [0.08, 1.90] |
Comparison 8. Non‐serious adverse events: cross‐over trials (endpoint data).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Proportion of participants with non‐serious events | 24 | 2696 | Risk Ratio (IV, Random, 95% CI) | 1.39 [1.13, 1.70] |
| 8.2 Subgroup analysis: total number of non‐serious adverse events according to dose | 24 | 3483 | Risk Ratio (IV, Random, 95% CI) | 1.31 [1.12, 1.53] |
| 8.2.1 Low dose | 16 | 1539 | Risk Ratio (IV, Random, 95% CI) | 1.11 [0.94, 1.31] |
| 8.2.2 High dose | 12 | 1080 | Risk Ratio (IV, Random, 95% CI) | 1.57 [1.22, 2.01] |
| 8.2.3 Unknown dose | 5 | 864 | Risk Ratio (IV, Random, 95% CI) | 1.50 [0.88, 2.56] |
| 8.3 Nervous system (including psychiatry) | 52 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 8.3.1 Aggression | 3 | 743 | Risk Ratio (IV, Random, 95% CI) | 0.52 [0.17, 1.60] |
| 8.3.2 Agitation | 2 | 273 | Risk Ratio (IV, Random, 95% CI) | 1.93 [0.37, 10.16] |
| 8.3.3 Anger | 3 | 264 | Risk Ratio (IV, Random, 95% CI) | 0.45 [0.26, 0.77] |
| 8.3.4 Behavioural complaints | 1 | 82 | Risk Ratio (IV, Random, 95% CI) | 0.55 [0.35, 0.86] |
| 8.3.5 Buccal or lingual movements | 5 | 569 | Risk Ratio (IV, Random, 95% CI) | 1.12 [0.81, 1.55] |
| 8.3.6 Compulsive acts | 1 | 90 | Risk Ratio (IV, Random, 95% CI) | 2.57 [1.45, 4.56] |
| 8.3.7 Daydreaming | 3 | 222 | Risk Ratio (IV, Random, 95% CI) | 0.66 [0.44, 0.98] |
| 8.3.8 Dizziness | 12 | 2163 | Risk Ratio (IV, Random, 95% CI) | 1.06 [0.91, 1.23] |
| 8.3.9 Drowsiness: dull, tired, listless or sleepy | 24 | 3011 | Risk Ratio (IV, Random, 95% CI) | 0.98 [0.80, 1.20] |
| 8.3.10 Euphoria | 7 | 1457 | Risk Ratio (IV, Random, 95% CI) | 0.97 [0.72, 1.31] |
| 8.3.11 Headache | 43 | 5981 | Risk Ratio (IV, Random, 95% CI) | 1.25 [1.06, 1.48] |
| 8.3.12 Thirst | 1 | 211 | Risk Ratio (IV, Random, 95% CI) | 2.78 [0.11, 69.04] |
| 8.3.13 Irritability | 27 | 4110 | Risk Ratio (IV, Random, 95% CI) | 0.97 [0.74, 1.27] |
| 8.3.14 Nightmares | 11 | 1738 | Risk Ratio (IV, Random, 95% CI) | 1.00 [0.73, 1.35] |
| 8.3.15 Overly meticulous | 1 | 96 | Risk Ratio (IV, Random, 95% CI) | 40.77 [2.35, 706.72] |
| 8.3.16 Obsessive thinking | 1 | 90 | Risk Ratio (IV, Random, 95% CI) | 2.35 [1.53, 3.62] |
| 8.3.17 Picking at skin or fingers, nail biting, lip or cheek chewing | 18 | 2549 | Risk Ratio (IV, Random, 95% CI) | 1.04 [0.86, 1.25] |
| 8.3.18 Repetitive language | 1 | 48 | Risk Ratio (IV, Random, 95% CI) | 1.00 [0.32, 3.10] |
| 8.3.19 Sad, tearful or depressed | 26 | 3510 | Risk Ratio (IV, Random, 95% CI) | 1.15 [0.96, 1.37] |
| 8.3.20 Socially withdrawn ‐ decreased interaction with others | 15 | 2432 | Risk Ratio (IV, Random, 95% CI) | 1.36 [0.95, 1.95] |
| 8.3.21 Stares a lot | 11 | 2110 | Risk Ratio (IV, Random, 95% CI) | 1.04 [0.83, 1.31] |
| 8.3.22 Tics or nervous movements | 24 | 3429 | Risk Ratio (IV, Random, 95% CI) | 1.23 [1.02, 1.50] |
| 8.3.23 Unusual blinking | 1 | 48 | Risk Ratio (IV, Random, 95% CI) | 3.13 [0.12, 80.68] |
| 8.3.24 Worried or anxious | 23 | 3366 | Risk Ratio (IV, Random, 95% CI) | 0.85 [0.66, 1.11] |
| 8.3.25 Fatique | 1 | 211 | Risk Ratio (IV, Random, 95% CI) | 4.59 [0.22, 94.57] |
| 8.3.26 Emotional lability | 1 | 154 | Risk Ratio (IV, Random, 95% CI) | 9.25 [2.24, 38.22] |
| 8.3.27 Dysphoria | 1 | 154 | Risk Ratio (IV, Random, 95% CI) | 4.63 [0.23, 94.88] |
| 8.3.28 Moody | 1 | 211 | Risk Ratio (IV, Random, 95% CI) | 4.59 [0.22, 94.57] |
| 8.3.29 Uninterested | 1 | 1052 | Risk Ratio (IV, Random, 95% CI) | 1.20 [0.82, 1.75] |
| 8.3.30 Prone to crying | 1 | 1052 | Risk Ratio (IV, Random, 95% CI) | 1.72 [1.04, 2.86] |
| 8.4 Nervous system (including psychiatry) continuous outcomes | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐0.80 [‐3.56, 1.96] |
| 8.4.1 Anxiety | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐0.80 [‐3.56, 1.96] |
| 8.5 Digestive system | 48 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 8.5.1 Decreased appetite or loss of appetite | 41 | 6091 | Risk Ratio (IV, Random, 95% CI) | 3.89 [2.76, 5.48] |
| 8.5.2 Diarrhoea | 5 | 767 | Risk Ratio (IV, Random, 95% CI) | 0.95 [0.38, 2.34] |
| 8.5.3 Dry mouth | 6 | 496 | Risk Ratio (IV, Random, 95% CI) | 1.32 [0.59, 2.97] |
| 8.5.4 Dyspepsia | 1 | 62 | Risk Ratio (IV, Random, 95% CI) | 0.22 [0.02, 2.14] |
| 8.5.5 Nausea | 11 | 1182 | Risk Ratio (IV, Random, 95% CI) | 1.67 [1.13, 2.46] |
| 8.5.6 Increased appetite | 1 | 136 | Risk Ratio (IV, Random, 95% CI) | 0.20 [0.08, 0.50] |
| 8.5.7 Stomach ache (abdominal pain) | 38 | 5803 | Risk Ratio (IV, Random, 95% CI) | 1.70 [1.35, 2.15] |
| 8.5.8 Vomiting | 7 | 1278 | Risk Ratio (IV, Random, 95% CI) | 2.47 [0.82, 7.47] |
| 8.5.9 Upper abdominal pain | 1 | 107 | Risk Ratio (IV, Random, 95% CI) | 442413.39 [0.00, 294025957312946900000000000000000000000000000000000000000000000000000000000000000000000000000.00] |
| 8.5.10 Decreased weight | 2 | 365 | Risk Ratio (IV, Random, 95% CI) | 5.04 [0.59, 43.15] |
| 8.5.11 Gastrointestinal distress | 1 | 77 | Risk Ratio (IV, Random, 95% CI) | 8103.08 [0.00, 1011052702371868400000000000000000000000000000000000000000000000000000000.00] |
| 8.5.12 Constipation | 1 | 154 | Risk Ratio (IV, Random, 95% CI) | 4.63 [0.23, 94.88] |
| 8.5.13 Oropharyngeal pain | 1 | 211 | Risk Ratio (IV, Random, 95% CI) | 2.72 [0.00, 11653710777400273000000000000000000000000000000000000000000000000000000000000000000000000000000.00] |
| 8.5.14 Anorexia | 1 | 203 | Risk Ratio (IV, Random, 95% CI) | 2.97 [0.61, 14.37] |
| 8.6 Cardiovascular system | 2 | 730 | Risk Ratio (M‐H, Random, 95% CI) | 4.97 [1.09, 22.76] |
| 8.6.1 Epistaxis | 1 | 154 | Risk Ratio (M‐H, Random, 95% CI) | 2.78 [0.11, 67.14] |
| 8.6.2 Heart palpitations | 2 | 365 | Risk Ratio (M‐H, Random, 95% CI) | 5.65 [0.67, 47.90] |
| 8.6.3 Chest pain | 1 | 211 | Risk Ratio (M‐H, Random, 95% CI) | 6.43 [0.34, 123.02] |
| 8.7 Respiratory system | 2 | 673 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.36, 4.68] |
| 8.7.1 Nasal congestion | 1 | 154 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.06, 14.52] |
| 8.7.2 Strep throat/sore throat | 1 | 154 | Risk Ratio (M‐H, Random, 95% CI) | 2.78 [0.30, 26.09] |
| 8.7.3 Cough | 1 | 154 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.04, 5.00] |
| 8.7.4 Dyspnea | 1 | 211 | Risk Ratio (M‐H, Random, 95% CI) | 2.76 [0.11, 66.91] |
| 8.8 Urinary system | 1 | 136 | Risk Ratio (IV, Random, 95% CI) | 0.50 [0.05, 5.39] |
| 8.8.1 Urinary incontinence | 1 | 136 | Risk Ratio (IV, Random, 95% CI) | 0.50 [0.05, 5.39] |
| 8.9 Skeletal and muscular system | 2 | 673 | Risk Ratio (M‐H, Random, 95% CI) | 4.86 [0.83, 28.54] |
| 8.9.1 Ankle pain/strain | 1 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 8.9.2 Muscle strain/pain | 1 | 154 | Risk Ratio (M‐H, Random, 95% CI) | 12.04 [0.69, 210.03] |
| 8.9.3 Toe fracture | 1 | 154 | Risk Ratio (M‐H, Random, 95% CI) | 2.78 [0.11, 67.14] |
| 8.9.4 Asthenia | 1 | 211 | Risk Ratio (M‐H, Random, 95% CI) | 2.76 [0.11, 66.91] |
| 8.10 Skeletal and muscular system continuous outcomes | 1 | 82 | Mean Difference (IV, Random, 95% CI) | 0.85 [0.79, 0.91] |
| 8.10.1 Somatic complaints | 1 | 82 | Mean Difference (IV, Random, 95% CI) | 0.85 [0.79, 0.91] |
| 8.11 Immune system (including infections) | 9 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 8.11.1 Allergic rhinitis | 4 | 475 | Risk Ratio (IV, Random, 95% CI) | 1.38 [0.35, 5.51] |
| 8.11.2 Fever | 2 | 91 | Risk Ratio (IV, Random, 95% CI) | 1.39 [0.09, 20.56] |
| 8.11.3 Lymphadenitis | 2 | 296 | Risk Ratio (IV, Random, 95% CI) | 3.93 [0.44, 35.11] |
| 8.11.4 Pharyngolaryngeal pain | 1 | 160 | Risk Ratio (IV, Random, 95% CI) | 2.00 [0.19, 21.62] |
| 8.11.5 Pharyngitis | 4 | 754 | Risk Ratio (IV, Random, 95% CI) | 0.71 [0.19, 2.62] |
| 8.11.6 Upper respiratory tract infection | 7 | 1245 | Risk Ratio (IV, Random, 95% CI) | 1.21 [0.51, 2.86] |
| 8.11.7 Nasopharyngitis | 1 | 203 | Risk Ratio (IV, Random, 95% CI) | 0.79 [0.22, 2.87] |
| 8.11.8 Influenza | 1 | 154 | Risk Ratio (IV, Random, 95% CI) | 4.63 [0.23, 94.87] |
| 8.11.9 Mouth ulcers/bad breath | 1 | 154 | Risk Ratio (IV, Random, 95% CI) | 4.63 [0.23, 94.87] |
| 8.11.10 Urinary tract infection | 1 | 154 | Risk Ratio (IV, Random, 95% CI) | 2.78 [0.11, 67.14] |
| 8.11.11 Otitis media (ear pain) | 1 | 0 | Risk Ratio (IV, Random, 95% CI) | Not estimable |
| 8.12 Integumentary system | 4 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 8.12.1 Rash | 3 | 362 | Risk Ratio (IV, Random, 95% CI) | 1.49 [0.35, 6.37] |
| 8.12.2 Skin laceration | 1 | 167 | Risk Ratio (IV, Random, 95% CI) | 2.96 [0.12, 71.75] |
| 8.12.3 Cellulitis | 1 | 154 | Risk Ratio (IV, Random, 95% CI) | 2.78 [0.11, 67.14] |
| 8.13 Sleep variability continuous outcomes | 1 | 512 | Mean Difference (IV, Fixed, 95% CI) | ‐2.21 [‐5.23, 0.81] |
| 8.13.1 Actigraphic total sleep time | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | ‐29.80 [‐60.86, 1.26] |
| 8.13.2 Actigraphic sleep onset latency | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | 21.10 [1.33, 40.87] |
| 8.13.3 Actigraphic sleep efficiency | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | ‐3.30 [‐8.16, 1.56] |
| 8.13.4 Polysonmnographic total sleep time | 1 | 252 | Mean Difference (IV, Fixed, 95% CI) | ‐24.60 [‐52.20, 3.00] |
| 8.13.5 Polysomnographic sleep onset latency | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | 8.40 [‐7.11, 23.91] |
| 8.13.6 Polysomnographic sleep efficiency | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | ‐2.20 [‐6.34, 1.94] |
| 8.14 Sleep variability | 39 | 5810 | Risk Ratio (IV, Random, 95% CI) | 1.81 [1.34, 2.44] |
| 8.14.1 Insomnia or sleep problems | 37 | 5499 | Risk Ratio (IV, Random, 95% CI) | 1.88 [1.39, 2.56] |
| 8.14.2 Sleep efficiency (SEF) | 2 | 108 | Risk Ratio (IV, Random, 95% CI) | 0.48 [0.02, 14.28] |
| 8.14.3 Initial insomnia | 1 | 203 | Risk Ratio (IV, Random, 95% CI) | 0.59 [0.15, 2.42] |
| 8.15 Vital signs | 14 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.15.1 Diastolic blood pressure (mmHg) | 11 | 755 | Std. Mean Difference (IV, Random, 95% CI) | 0.15 [‐0.04, 0.34] |
| 8.15.2 Systolic blood pressure (mmHg) | 11 | 755 | Std. Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.15, 0.26] |
| 8.15.3 Pulse or heart rate (bpm) | 14 | 939 | Std. Mean Difference (IV, Random, 95% CI) | 0.43 [0.23, 0.64] |
| 8.16 Physical parameters | 6 | 576 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.20, 0.13] |
| 8.16.1 Height (cm) | 1 | 46 | Std. Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.38, 0.78] |
| 8.16.2 Weight | 6 | 530 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.23, 0.11] |
| 8.17 Other (including drug toxicity) | 2 | 1443 | Risk Ratio (M‐H, Random, 95% CI) | 3.25 [0.99, 10.62] |
| 8.17.1 Growth hormone deficiency | 1 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 8.17.2 Eye pain | 1 | 154 | Risk Ratio (M‐H, Random, 95% CI) | 2.78 [0.11, 67.14] |
| 8.17.3 Carious teeth | 1 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 8.17.4 Foreign body swallowed | 1 | 154 | Risk Ratio (M‐H, Random, 95% CI) | 2.78 [0.11, 67.14] |
| 8.17.5 Bug bites/bee stings | 1 | 154 | Risk Ratio (M‐H, Random, 95% CI) | 4.63 [0.23, 94.87] |
| 8.17.6 Sunburn | 1 | 154 | Risk Ratio (M‐H, Random, 95% CI) | 2.78 [0.11, 67.14] |
| 8.17.7 Finger laceration | 1 | 154 | Risk Ratio (M‐H, Random, 95% CI) | 2.78 [0.11, 67.14] |
| 8.17.8 Flat affect (lack of emotional expression) | 1 | 154 | Risk Ratio (M‐H, Random, 95% CI) | 4.63 [0.23, 94.87] |
| 8.17.9 Peripheral oedema | 1 | 211 | Risk Ratio (M‐H, Random, 95% CI) | 2.76 [0.11, 66.91] |
Comparison 9. Teacher‐rated general behaviour.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 9.1 All parallel‐group trials and first‐period cross‐over trials | 7 | 792 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.62 [‐0.91, ‐0.33] |
| 9.1.1 High risk of bias | 7 | 792 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.62 [‐0.91, ‐0.33] |
| 9.2 Subgroup analysis: types of scales | 7 | 792 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.62 [‐0.91, ‐0.33] |
| 9.2.1 Conners' Global Index ‐ Teacher (CGI‐T) | 1 | 314 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.91 [‐1.14, ‐0.68] |
| 9.2.2 Groninger Behaviour Observation Scale (GBOS) | 1 | 43 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.84 [‐1.46, ‐0.21] |
| 9.2.3 Conners' Teacher Rating Scale (CTRS‐RS) | 1 | 75 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.54, 0.37] |
| 9.2.4 Conners' Teacher Rating Scale ‐ Oppositional behaviour (CRS‐R) | 1 | 49 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.50, 0.62] |
| 9.2.5 Conners' Teacher Rating Scale ‐ Conduct problems | 1 | 25 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.67 [‐1.48, 0.14] |
| 9.2.6 IOWA Conners' Rating Scale ‐ Oppositional/Defiant (IOWA‐O/D) | 2 | 286 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.86 [‐1.12, ‐0.59] |
| 9.3 Subgroup analysis: dose | 7 | 820 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.61 [‐0.88, ‐0.34] |
| 9.3.1 Low dose | 2 | 71 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.67 [‐1.16, ‐0.19] |
| 9.3.2 High dose | 4 | 541 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐1.05, ‐0.27] |
| 9.3.3 Unknown dose | 2 | 208 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐1.29, 0.46] |
| 9.4 Subgroup analysis: duration of treatment | 7 | 792 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.62 [‐0.91, ‐0.33] |
| 9.4.1 Short term (up to 6 months) | 7 | 792 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.62 [‐0.91, ‐0.33] |
| 9.5 Subgroup analysis: parallel‐group trials versus first‐period cross‐over trials | 7 | 792 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.62 [‐0.91, ‐0.33] |
| 9.5.1 Parallel‐group trials | 7 | 792 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.62 [‐0.91, ‐0.33] |
| 9.6 Cross‐over trials (endpoint data) | 16 | 1302 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.75 [‐0.87, ‐0.63] |
| 9.6.1 High risk of bias | 16 | 1302 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.75 [‐0.87, ‐0.63] |
| 9.7 Subgroup analysis: cross‐over trials (endpoint data): dose | 16 | 2008 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.69 [‐0.78, ‐0.60] |
| 9.7.1 Low dose | 13 | 1104 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐0.72, ‐0.48] |
| 9.7.2 High dose | 12 | 904 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.82 [‐0.95, ‐0.68] |
| 9.8 Subgroup analysis: all parallel‐group trials and first‐period cross‐over trials (teacher‐rated) versus cross‐over trials (endpoint data) | 23 | 2094 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.72 [‐0.84, ‐0.60] |
| 9.8.1 All parallel‐group trials and first‐period cross‐over trials | 7 | 792 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.62 [‐0.91, ‐0.33] |
| 9.8.2 Cross‐over trials (endpoint data) | 16 | 1302 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.75 [‐0.87, ‐0.63] |
| 9.9 Subgroup analysis: all parallel‐group trials and cross‐over trials: vested interest | 23 | 2094 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.72 [‐0.84, ‐0.60] |
| 9.9.1 Low risk of vested interest | 6 | 509 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.67 [‐0.90, ‐0.43] |
| 9.9.2 High risk of vested interest | 17 | 1585 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.74 [‐0.89, ‐0.60] |
Comparison 10. Independent assessor‐rated general behaviour.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 10.1 All parallel‐group trials and first‐period cross‐over trials | 1 | 94 | Mean Difference (IV, Fixed, 95% CI) | 1.10 [‐1.01, 3.21] |
| 10.1.1 High risk of bias | 1 | 94 | Mean Difference (IV, Fixed, 95% CI) | 1.10 [‐1.01, 3.21] |
| 10.2 Cross‐over trials (endpoint data) | 9 | 987 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.98 [‐1.39, ‐0.57] |
| 10.2.1 High risk of bias | 9 | 987 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.98 [‐1.39, ‐0.57] |
| 10.3 Subgroup analysis: general behaviour, cross‐over trials (endpoint data): dose | 9 | 1319 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.02 [‐1.39, ‐0.64] |
| 10.3.1 Low dose | 9 | 987 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.82 [‐1.23, ‐0.41] |
| 10.3.2 High dose | 5 | 332 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.49 [‐2.37, ‐0.61] |
| 10.4 Subgroup analysis: all parallel‐group trials and first‐period cross‐over trials (independent assessor‐rated) compared with cross‐over trials (endpoint data) | 10 | 1081 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.86 [‐1.27, ‐0.46] |
| 10.4.1 Parallel‐group trials and first‐period cross‐over trials | 1 | 94 | Std. Mean Difference (IV, Random, 95% CI) | 0.21 [‐0.20, 0.61] |
| 10.4.2 Cross‐over trials (endpoint data) | 9 | 987 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.98 [‐1.39, ‐0.57] |
| 10.5 Subgroup analysis: all parallel‐group trials and cross‐over trials: vested interest | 9 | 1031 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.81 [‐1.24, ‐0.39] |
| 10.5.1 Low risk of vested interest | 2 | 190 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐1.36, 0.72] |
| 10.5.2 High risk of vested interest | 6 | 799 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.59 [‐0.77, ‐0.41] |
| 10.5.3 Unclear risk of vested interest | 1 | 42 | Std. Mean Difference (IV, Random, 95% CI) | ‐5.99 [‐7.46, ‐4.51] |
Comparison 11. Parent‐rated general behaviour.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 11.1 All parallel‐group trials and first‐period cross‐over trials | 10 | 1376 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.42 [‐0.62, ‐0.23] |
| 11.1.1 Low risk of bias | 3 | 386 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.64, 0.41] |
| 11.1.2 High risk of bias | 7 | 990 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.51 [‐0.69, ‐0.33] |
| 11.2 Subgroup analysis: types of scales | 10 | 1376 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.42 [‐0.62, ‐0.23] |
| 11.2.1 The Weekly Parent Ratings of Evening and Morning Behaviour (WPREMB) ‐ Revised | 2 | 167 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐1.32, 0.96] |
| 11.2.2 Conners' Global Index (CGI) ‐ Parent | 2 | 352 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐0.63, ‐0.20] |
| 11.2.3 Swanson, Nolan and Pelham, Fourth Edition ‐ Oppositional (SNAP‐IV‐Oppositional) | 1 | 15 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.86 [‐1.95, 0.23] |
| 11.2.4 IOWA Conners' Rating Scale ‐ Oppositional/Defiant (IOWA‐I/O) | 2 | 286 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.75 [‐1.01, ‐0.49] |
| 11.2.5 Conners' Early Childhood Behavior ‐ Parent Short Response Scale | 1 | 117 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐0.81, ‐0.07] |
| 11.2.6 Strengths and Dificulties Questionnaire (SDQ) | 1 | 85 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.46, 0.39] |
| 11.2.7 Behaviour Rating Inventory of Executive Function (BRIEF) | 1 | 354 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.38, 0.14] |
| 11.3 Subgroup analysis: parallel‐group trials compared with first‐period cross‐over trials | 10 | 1376 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.42 [‐0.62, ‐0.23] |
| 11.3.1 Parallel‐group trials | 9 | 1361 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐0.61, ‐0.21] |
| 11.3.2 First‐period cross‐over trials | 1 | 15 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.86 [‐1.95, 0.23] |
| 11.4 Subgroup analysis: duration of treatment | 10 | 1376 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.42 [‐0.62, ‐0.23] |
| 11.4.1 Short term (up to 6 months) | 10 | 1376 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.42 [‐0.62, ‐0.23] |
| 11.5 Subgroup analysis: dose | 10 | 1376 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.42 [‐0.62, ‐0.23] |
| 11.5.1 High dose | 7 | 1052 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.54, ‐0.10] |
| 11.5.2 Unknown dose | 3 | 324 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.71 [‐0.95, ‐0.47] |
| 11.6 Cross‐over trials (endpoint data) | 6 | 384 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.84 [‐1.05, ‐0.63] |
| 11.6.1 High risk of bias | 6 | 384 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.84 [‐1.05, ‐0.63] |
| 11.7 Subgroup analysis: cross‐over trials (endpoint data): dose | 6 | 550 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.75 [‐0.93, ‐0.56] |
| 11.7.1 Low dose | 5 | 248 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐0.93, ‐0.38] |
| 11.7.2 High dose | 4 | 302 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.83 [‐1.07, ‐0.60] |
| 11.8 Subgroup analysis: all parallel‐group trials and first‐period cross‐over trials (parent‐rated) compared with cross‐over trials (endpoint data) | 16 | 1760 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.56 [‐0.74, ‐0.39] |
| 11.8.1 All parallel‐group trials and first‐period cross‐over trials | 10 | 1376 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.42 [‐0.62, ‐0.23] |
| 11.8.2 Cross‐over trials (endpoint data) | 6 | 384 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.84 [‐1.05, ‐0.63] |
| 11.9 All parallel‐group trials and cross‐over trials: risk of bias | 16 | 1760 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.56 [‐0.74, ‐0.39] |
| 11.9.1 Low risk of bias | 3 | 386 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.64, 0.41] |
| 11.9.2 High risk of bias | 13 | 1374 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.63 [‐0.78, ‐0.47] |
| 11.10 Subgroup analysis: all parallel‐group trials and cross‐over trials: vested interest | 16 | 1760 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.56 [‐0.74, ‐0.39] |
| 11.10.1 Low risk of vested interest | 4 | 223 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.62 [‐1.14, ‐0.10] |
| 11.10.2 High risk of vested interest | 12 | 1537 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.56 [‐0.75, ‐0.38] |
Comparison 12. Additional subgroup analyses of general behaviour.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 12.1 Parallel‐group trials and first‐period cross‐over trials: comparisons of raters | 13 | 2262 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐0.64, ‐0.27] |
| 12.1.1 Teacher‐rated | 7 | 792 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.62 [‐0.91, ‐0.33] |
| 12.1.2 Independent assessor‐rated | 1 | 94 | Std. Mean Difference (IV, Random, 95% CI) | 0.21 [‐0.20, 0.61] |
| 12.1.3 Parent‐rated | 10 | 1376 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.42 [‐0.62, ‐0.23] |
| 12.2 Parallel‐group trials and first‐period cross‐over trials: comorbidity versus no comorbidity | 8 | 696 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐0.90, ‐0.40] |
| 12.2.1 ADHD with comorbidity | 6 | 265 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.59 [‐0.95, ‐0.23] |
| 12.2.2 ADHD without comorbidity | 2 | 431 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.70 [‐1.16, ‐0.24] |
| 12.3 Cross‐over trials: first‐period data versus endpoint data in the same trials (teacher‐, parent‐, and independent assessor‐rated) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 12.3.1 First‐period data | 1 | 16 | Mean Difference (IV, Random, 95% CI) | ‐0.81 [‐1.75, 0.13] |
| 12.3.2 Endpoint data | 1 | 14 | Mean Difference (IV, Random, 95% CI) | 0.14 [‐0.71, 1.00] |
Comparison 13. Quality of life: parallel‐group trials and first‐period cross‐over trials.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 13.1 Subgroup analysis: types of scales | 4 | 608 | Std. Mean Difference (IV, Random, 95% CI) | 0.40 [‐0.03, 0.83] |
| 13.1.1 Child Health Questionnaire (CHQ) | 1 | 257 | Std. Mean Difference (IV, Random, 95% CI) | 0.54 [0.25, 0.83] |
| 13.1.2 Children´s Global Assessment Scale (CGAS) | 1 | 36 | Std. Mean Difference (IV, Random, 95% CI) | 0.79 [0.10, 1.47] |
| 13.1.3 Child Health and Illness Profile, Child Edition: Parent Report Form (CHIP‐CE:PRF) | 1 | 221 | Std. Mean Difference (IV, Random, 95% CI) | 0.64 [0.37, 0.91] |
| 13.1.4 Parent & child reported Revised questionnaire for Children and adolescents to record health‐related quality of life (KINDL‐R) | 1 | 94 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.71, 0.11] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abikoff 2009.
| Study characteristics | ||
| Methods | 8‐week double‐blind, randomised, placebo‐controlled, cross‐over trial with 2 interventions
|
|
| Participants | Number of participants screened: not stated Number of participants included: 19 (15 boys, 4 girls) Number of participants followed up: 19 Number of withdrawals: none Diagnosis of ADHD: DSM‐IV (combined (42%), hyperactive‐impulsive (0%), inattentive (58%)) Age: mean 10.05 years (SD 1.62, range 8‐13) IQ: mean 107.1 (SD 14.3) Methylphenidate‐naive: 100% Ethnicity: not stated Country: USA Setting: outpatient clinic Comorbidity: ODD (26.3%), anxiety disorder (10.5%), dysthymic disorder (5.3%), CD (5.3%) Comedication: not stated Additional sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 2 possible drug condition orders of OROS‐MPH and placebo Mean MPH dosage: 48.3 mg (range 18 ± 54 mg); weight‐based final OROS‐MPH dose was 1.3 mg/kg Administration schedule: not stated Duration of each medication condition: 4 weeks: 2 weeks titration and 2 weeks optimal dose Washout before trial initiation: all were medication‐naive Medication‐free period between interventions: 2 days Titration period: 2 weeks after randomisation Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
|
|
| Notes | Sample calculation: no Ethics approval: trial protocol was reviewed and approved by the University’s institutional review board Comment from trial authors
Key conclusion of trial authors
Inclusion of MPH responders only/exclusion of MPH non‐responders/children who have previously experienced adverse events while taking MPH before randomisation: no Any withdrawals due to AEs: no Funding source: a grant from Ortho‐McNeil Janssen Scientific Affairs to Dr. Abikoff Email correspondence with trial authors: December 2013. No supplemental information provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Post‐treatment scores for all 19 trial children were obtained from parents. Because 1 child’s treatment was delayed and ran beyond the end of the school year, teacher data on 18 youngsters were analysed Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol published |
Ahmann 1993.
| Study characteristics | ||
| Methods | 4‐week double‐blind, placebo‐controlled, cross‐over trial, in which participants were randomly assigned to
|
|
| Participants | Number of participants screened: not stated Number of participants included: 234 Number of participants followed up: 206 Number of withdrawals: not stated, but it is described in the text that 4 children experienced severe AEs while taking MPH (Ritalin) and could not complete the protocol Regarding the 206 participants Diagnosis of ADHD: DSM‐III‐R (subtype not stated) Age: 5‐15 years IQ: > 70 Sex: 161 boys, 45 girls MPH‐naive: not stated Ethnicity: not stated Country: USA Setting: outpatient clinic Comorbidity: not stated Comedication: not stated Additional sociodemographics: none Inclusion criteria
In addition, ≥ 3 of the following criteria had to be met.
Children were divided into responders and non‐responders based on the following criteria.
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to different orders of LD‐MPH (0.3 mg/kg), HD‐MPH (0.5 mg/kg) or placebo Administration schedule: 3 times a day Duration of each medication condition: 7 consecutive days for both doses Washout before trial initiation: not stated Titration period: no Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
Non‐serious AEs
|
|
| Notes | Sample calculation: no Ethics approval: yes; protocol was approved by the Institutional Review Board at the Marshfield Medical Center Key conclusion of trial authors
Comment from review authors
Inclusion of MPH responders only/exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: yes, (n = 4) Funding source: Marshfield Clinic grants Email correspondence with trial authors: not able to find trial authors’ contact information; therefore not able to obtain supplemental information regarding trial design and data on ADD‐H Comprehensive Teacher Rating Scale, CTRS, CPRS |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned, but not described how and/or by whom |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind trial, identical appearing pills |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind trial, identical appearing pills |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 206 had sufficient data for analyses Selection bias: participants were divided into responders and non‐responders. However, data from this article pertain to AEs only and include both groups |
| Selective reporting (reporting bias) | Unclear risk | Not able to obtain protocol or other information |
Arnold 2004.
| Study characteristics | ||
| Methods | 7‐centre USA trial consisting of a 6‐week, open‐label, dose‐titration phase (Part A) and a 2‐week, double‐blind, randomised, parallel‐group, placebo‐controlled withdrawal trial (Part B) with 2 arms
|
|
| Participants | Number of patients screened: 116 Part A Number of participants included: 89 (72 boys, 17 girls) Number of participants followed up: 76 Number of withdrawals: 13 DSM‐IV diagnosis of ADHD (combined 80%) Age range: 6‐16 years IQ: not stated MPH‐naive: 71.9% Ethnicity: not stated Country: USA Comorbidity: not stated Comedication: not stated Additional sociodemographics: none Part B Number of participants included: 75 (61 boys, 14 girls) Number followed up in each arm: MPH 34, placebo 39 Number of withdrawals in each arm: MPH 1, placebo 1 DSM‐IV diagnosis of ADHD (combined (80%), hyperactive‐impulsive (0%), inattentive (20%)) Age range: 6‐16 years IQ: > 70 (mean not stated) MPH‐naive (MPH 82.9%, placebo 62.5%) Ethnicity: white (MPH 80%, placebo 75%), African American (MPH 14.3%, placebo 12.5%), Hispanic (MPH 5.7%, placebo 12.5%) Country: USA Setting: outpatient clinic and hospital Comorbidity: not stated Comedication: antihistamines, non‐steroidal anti‐inflammatory agents, multi‐vitamins, nasal decongestants or other analgesics or antipyretics (MPH 34.3%, placebo 40.0%) Additional sociodemographics: none No significant differences in baseline demographics were noted between the 2 groups. Thus, slightly more treatment‐naive participants were receiving d‐MPH than placebo Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Part B Participants were randomly assigned to d‐MPH at optimised dose or placebo Number randomised to each group: MPH 35, placebo 40 Mean MPH dosage: 68.6% of d‐MPH continuers and 79.5% of placebo participants were receiving 20 mg at end of Part B, mean (SD) not stated Administration schedule: 10 mg twice daily. Time points 7 am to 8 am and 11:30 am to 12 pm Duration of intervention: 2 weeks Titration period: 6 weeks, initiated before randomisation Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms (Part B)
Non‐serious AEs (Parts A and B)
|
|
| Notes |
Comments from trial authors
Key conclusion of trial authors
Inclusion of MPH responders only/exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: yes Any withdrawals due to AEs: no Funding source: trial was supported by the Celgene Corporation Email correspondence with trial authors: October 2013. Supplemental information regarding additional information was received. However, trial authors advised us to contact the sponsoring drug company for additional information. This process has been difficult, and no further communication was attempted to request additional information. (see Storebø 2015a) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was central, irrespective of whether the drug was pre‐packaged and pre‐randomised, or if it was bottled and labelled by an unblinded dispenser who had no contact with participants and kept the other staff blind |
| Allocation concealment (selection bias) | Low risk | Randomisation was central. "In all industry studies I have been involved with, either the drug was pre‐packaged and pre‐randomized or it was bottled and labeled by an unblinded dispenser who had no contact with patients and kept the other staff blind" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind. In Part B, participants/guardians and medical personnel were blinded to the drug. Also, d‐MPH was available in tablets, each identical in appearance to a matching placebo. trial drug (or placebo) was dispensed in bottles containing a weekly supply, labelled for use at “Home” and “School”, with the strength designated “A,” “B” or “C” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind. In Part B, participants/guardians and medical personnel were blinded to the drug |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT sample was used in analysis of efficacy parameters: participants who received d‐MPH and had a Part B baseline efficacy evaluation and ≥ 1 post‐baseline assessment Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol published |
Barkley 1989b.
| Study characteristics | ||
| Methods | Triple‐blind, randomised, cross‐over trial with 3 interventions
|
|
| Participants | Number of participants screened: not stated Number of participants included: 83 (71 boys, 12 girls) Number of participants followed up: 80 Number of withdrawals: 3 Diagnosis of ADHD: DSM‐III‐R (subtype distribution not stated) Age: mean 8.2 years (range 5‐13) IQ: mean 105.1 MPH‐naive: 85% Ethnicity: not stated Country: USA Setting: outpatient clinic Comorbidity: not stated Comedication: not stated Additional sociodemographics: mothers, married (n = 48), divorced (n = 13), unmarried or widowed (n = 13) Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 6 possible drug condition orders of 0.3 mg/kg MPH, 0.5 mg/kg MPH and placebo Mean MPH dosage: not stated Administration schedule: morning and noon Duration of each medication condition: 7‐10 days Washout before trial initiation: not stated Medication‐free period between interventions: not stated Titration period: none Treatment compliance: unused capsules returned to the clinic each week for adherence check No family was discontinued from the trial because of non‐compliance with the drug regimen, defined as more than 1 day of failure to take medication, or 2 missed capsules/week |
|
| Outcomes |
ADHD symptoms
General behaviour
Adverse effects
|
|
| Notes | Sample calculation: not stated Ethics approval: trial was approved by the Human Subjects Committee at the medical centre Comments from trial authors
Key conclusions of trial authors
Comment from review authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: yes; 3. One child discontinued because of nervous facial tics, headache and dizziness; a second as the result of excessive thinking and disjointed thinking (during HD‐MPH); and a third because of headache, dizziness and increased hyperactivity Funding source: trial was internally funded by the medical school Email correspondence with trial authors: July 2013. We obtained additional information regarding funding and ethics approval. Unfortunately, it was not possible to receive from the trial authors supplemental data on ADHD symptoms and general behaviour |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Trial used a completely counterbalanced design, with participants randomly assigned in relatively equal numbers to 1‐6 possible drug conditions |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both medication and placebo were crushed and placed within orange opaque gelatin capsules to disguise distinctive differences in flavour between medication and placebo and dose differences across conditions. Children and their parents and teachers, as well as the research assistant evaluating the children, were blinded to medication conditions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Children and their parents and teachers, as well as the research assistant evaluating the children, were blinded to medication conditions |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information on incomplete outcome data Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Low risk | No indication of selective reporting |
Barkley 1991.
| Study characteristics | ||
| Methods | Triple‐blind, placebo‐controlled, cross‐over design with participants randomly assigned to the following conditions
Each intervention period lasted 1 week |
|
| Participants | Number of participants screened: not stated Number of participants included: 40 Number of participants followed up: 40 (36 boys, 4 girls) Number of withdrawals: 0 Diagnosis of ADD: DSM‐III‐R (with hyperactivity: 58%; without hyperactivity: 42%) Age: mean 8.6 years (range 6‐12) IQ: 103.5 MPH‐naive: not stated Ethnicity: not stated Country: USA Setting: outpatient clinic Comorbidity: borderline and low internalising symptoms. No others stated Comedication: not stated Other sociodemographics: 54.8 on Hollingheads Two Factor Index Participants were divided into different categories :
Inclusion criteria
Additional criteria for children with combined ADHD
Additional criteria for children with ADD‐H
Differences regarding the 2 ADHD groups
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 3 possible drug condition orders of MPH (5 mg, 10 mg and 15 mg) and placebo Administration schedule: twice/d, morning and noon Duration of each medication condition: 1 week Washout before trial initiation: no Titration period: none, but highest dose was never given unless preceded by moderate dose Compliance: children were permitted to miss 1 day of medication over 7 days and still remain in the trial. No families were removed from this trial because of non‐compliance as defined in this way |
|
| Outcomes |
ADHD symptoms
Non‐serious AEs
|
|
| Notes | Sample calculation: no Ethics approval: trial was approved by the Institutional Review Board at the medical centre Key conclusion of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: yes; excluded those with history of adverse reactions, but included both naive and prior users of antipsychotics Any withdrawals due to AEs: no Funding source: NIMH Email correspondence with trial author: 18 January 2013. Dr. Barkley informed us that data from the trial on side effects, for example, are no longer available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned to 1 of 6 possible drug conditions |
| Allocation concealment (selection bias) | Low risk | Hospital pharmacy prepared placebo (lactose powder) and MPH by crushing and placing them into 6 orange opaque gelatin capsules |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Triple‐blind design |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Triple‐blind design |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
Barkley 2000.
| Study characteristics | ||
| Methods | Double‐blind, placebo‐controlled, within‐participant, cross‐over trial with 3 interventions
Phases: 5, but high doses of each stimulant always followed lower dose of the same stimulant |
|
| Participants | Number of participants screened: 46 Number of participants included: 38 Number of participants followed up: 35 (30 boys, 5 girls) Number of withdrawals: 2. One was a post hoc exclusion Diagnosis of ADHD: DSM‐IV (subtype not described) Age: mean 14 years (range 12‐17) IQ: mean 103.9 (range 80‐141) MPH‐naive: not stated Ethnicity: not stated Country: USA Setting: outpatient clinic Comorbidity: not stated Comedication: not stated Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 4 possible drug condition orders of 5 mg MPH followed by 10 mg MPH and 5 mg MAS (Adderall) followed by 10 mg Adderall and placebo
Mean MPH dosage: LD‐MPH 10 mg/d; HD‐MPH 20 mg/d Administration schedule: morning and midday Duration of each medication condition: 1 week Washout before trial initiation: none Medication‐free period between interventions: none Titration period: none, although 5 mg dose was given before 10 mg, initiated after randomisation Treatment compliance: parents were required to return all unused capsules, but nothing further was said about this |
|
| Outcomes |
ADHD symptoms
Non‐serious AEs
|
|
| Notes | Sample calculation: not described Ethics approval: yes Comments from trial authors
Key conclusion of trial authors
Comment from review authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: yes; excluded patients who had a history of AEs to stimulants Any withdrawals due to AEs: no Funding source: University of Massachusetts Medical School Email correspondence with trial authors: January 2014. We received additional information (see Krogh 2014a [pers comm]) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Email correspondence with trial author: “Randomization was done by me as best as I can recall” (Krogh 2014a [pers comm]) |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Opaque gelatin capsules were prepared by the pharmacist |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Completer analysis reported 11/46 teens LTFU, 15 parents LTFU, 33 English teachers and 31 Maths teachers LTFU Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Low risk | Email correspondence with trial author: "All planned analyses were done and all measures we collected as treatment endpoints were analyzed" (Krogh 2014a [pers comm]) |
Barragán 2017.
| Study characteristics | ||
| Methods | A 12‐month randomised, unblinded parallel‐trial with 3 arms:
|
|
| Participants | Number of participants screened: 107 Number of participants included: 60 for the 2 relevant groups (90 in all (60 boys, 30 girls)) Number of participants followed‐up: 49 for relevant groups (69 in all) Number of withdrawals: 11 from omega group and combined group (10 more in MPH group) Diagnosis of ADHD: DSM‐IV‐TR (51 (57%) combined type, 7 (8%) hyperactive‐impulsive type and 32 (36%) inattentive type) Age: mean 8.27 years (SD 1.74; range 6‐12) IQ: not stated MPH‐naive: 100% Ethnicity: not stated Country: Mexico Setting: outpatient Comorbidity: some were exclusion criteria Comedication: medication for chronic conditions specified as exclusion criteria Additional sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | 30 participants were randomly assigned to: 3 different groups, omega 3/6 twice daily, MPH or a combination Mean medication dosage: combined group: baseline: 0.49 mg/kg, month 1: 0.79 mg/kg, month 3: 0.8 mg/kg Administration schedule: not stated Duration of each medication condition: 12 months Washout before trial initiation: not stated Titration period: during the first 4 weeks Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
Non‐serious AEs
|
|
| Notes | Sample calculation: no Ethics approval: “[…] the trial was approved by the local ethical review board.” Comments from trial authors
Key conclusion of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH: no Any withdrawals due to AEs: yes, 2 (6 more in the MPH group) Funding source: Vifor Pharma Email correspondence with trial authors:contacted through personal email in August and October 2021, for information regarding participant data, but no answer received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised (unblinded) by means of an aleatorised table to receive MPH, omega‐3/6, or combination therapy with MPH + omega‐3/6. |
| Allocation concealment (selection bias) | High risk | None |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The main analyses were ITT analyses with LOCF for patients who dropped out Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): yes, withdrawals due to "no efficacy" |
| Selective reporting (reporting bias) | Unclear risk | No trial protocol available |
Bedard 2008.
| Study characteristics | ||
| Methods | 4‐day randomised, double‐blind, placebo‐controlled, cross‐over trial with 2 interventions in 2 groups
and
|
|
| Participants | Number of participants screened: not stated Number of participants included: 130 Number of participants followed up: 130 (110 boys, 20 girls) Number of withdrawals: 0 Diagnosis of ADHD: DSM‐IV (combined (63%), hyperactive‐impulsive (30%), inattentive (6%)) Age: mean 9 years (SD 1.46, range 6‐12) IQ: mean 104.11 MPH‐naive: 70% Ethnicity: white (90%) Country: Canada Setting: outpatient clinic Comorbidity: specific learning disorder (34%), CD (24%), ODD (26%), generalised anxiety disorder (17%), separation anxiety disorder (11%) Comedication: not stated Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 11 possible drug condition orders of MPH and placebo. Children weighing < 25 kg received 5 mg, 10 mg and 15 mg of MPH; children weighing ≥ 25 kg received 10 mg, 15 mg and 20 mg of MPH Mean MPH dosage: 0.28 mg/kg, 0.45 mg/kg, 0.61 mg/kg Administration schedule: not stated Duration of each medication condition: 1 day, 3 days of MPH in all Washout before trial initiation: none Medication‐free period between interventions: not stated Titration period: none Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
|
|
| Notes | Sample calculation: no Ethics approval: approved by the institutional ethics review board Comment from trial authors
Key conclusions of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: no Funding source: Funding and operating grant from the Canadian Institute of Health Research and funding from the Canada Research Chairs Programme Email correspondence with trial authors: December 2013. Not able to get supplemental information from trial authors |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Master randomisation tables were prepared by the research support pharmacist at the hospital by using simple randomisation with restrictions (high dose not to be given on the first possible drug day nor immediately following placebo; no directly ascending or descending dose order). Therefore, a balanced block 22 design was used |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Examiner, psychiatrist, participant and participant’s family were not informed about participant’s randomisation order or daily medication status until completion. Placebo and active medication were prepared by the hospital pharmacist and were powdered and packaged in an opaque capsule to prevent identification of contents by colour, taste or volume |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Trained clinicians, blinded to other aspects of the participant’s assessment, conducted interviews independently |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
Bhat 2020.
| Study characteristics | ||
| Methods | A 2‐week cross‐over trial with 2 arms:
Phases: 2 (baseline week and week 2 ) |
|
| Participants | Number of participants screened: 866 Number of participants included: 676 Number of participants followed‐up: 670 completed the 3 weeks. Maximum analysed are 575/573 (444 boys, 129 girls) Number of withdrawals: only information on 6 of 676 participants available Diagnosis of ADHD: DSM‐IV (percentage for 540 participants: 53.3% combined, 9.6% hyperactive‐impulsive and 37.0% inattentive type) Age: mean 9.03 (SD 1.81, 6‐12 years) (information for 575 participants) IQ: separated by DRD3 genotype group for 69 participants: 96.94 (13.27), for 236 participants: 97.07 (12.6), for 268 participants: 96.04 (13.42) (information for 573 participants) MPH‐naive: 222 (information for 573 participants): 62.9% (information for 540 participants). Ethnicity: 496 whites (information for 573 participants) Country: Canada Setting: outpatient Comorbidity: for 540 participants: CD (18.5%), ODD (41.4%), mood disorders (7.5%), anxiety disorders (42.6%) Comedication: not stated Additional sociodemographics: parental income: percentage low income (defined as ≤ CAD 30,000): 40.1%; marital status: percentage single mothers 43.2%; maternal education: percentage lower education 36.7%; maternal smoking during pregnancy: 38.3% Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 2 possible orders of MPH (Ritalin) 0.5 mg/kg/d and placebo twice/d. 346 allocated to placebo first, 330 allocated to MPH first Mean medication dosage: all participants received a fixed dosage at 0.5 mg/kg/d Administration schedule: twice/d, 0.25 mg/kg, morning and noon Duration of each medication: 1 week of MPH, 1 week of placebo Washout before trial initiation: washout during 1‐week baseline assessment week Medication‐free period between interventions: none Titration period: none Treatment compliance: at the end of each week of treatment, the blister packs were collected and medication adherence was checked. |
|
| Outcomes |
ADHD symptoms
Serious AEs
Non‐serious AEs
The outcome reporting used for this review was taken from Bhat 2020, with data from 526 participants. |
|
| Notes | Sample calculation: no Ethics approval: yes. approved by the Research Ethics Board of the Douglas Mental Health University Institute in Montreal, Canada. Comments from trial authors
Key conclusion of trial authors
Comments from review authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH: no Any withdrawals due to AEs: yes, unclear how many, but no fewer than 2 Funding source: this work was supported in part by a grant from the Fond de Recherche du Québec and the Canadian Institutes of Health Research. Weam Fageera is a recipient of a PhD scholarship from the Ministry of Education of Saudi Arabia. Email correspondence with trial authors: October 2013. We received some data from trial authors. We sent another email to ask for additional information but have not received a response. trial authors were contacted again through personal email in August, October and December 2021, for information regarding the flow of participants and first‐period data, but no answer was received. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Their order of administration was determined by counterbalanced random assignment, using a computer‐generated randomisation list prepared by a statistician not otherwise affiliated with the trial. |
| Allocation concealment (selection bias) | Low risk | Placebo and MPH were prepared individually in opaque gelatin capsules in weekly blister packs by a pharmacist not otherwise involved in the trial to maintain blind allocation of treatments |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | MPH and placebo were encapsulated into opaque gelatin capsules in weekly blister packs |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Both parents and teachers were aware that after 1 week of baseline observation (which served also as a washout period for children who were previously on medication), participants (in a blind order) received either 1 week of active medication (MPH 0.25 mg/kg twice/d) followed by 1 week of placebo or the reverse order to assess their response to medication in an unbiased fashion |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Flowchart in Naumova 2019 (secondary reference under Bhat 2020), claims 676 included and randomised participants, but the articles' outcomes are reported for a maximum of 575 participants with no information on withdrawals. Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Low risk | All protocol outcomes reported |
Biederman 2003b.
| Study characteristics | ||
| Methods | 15‐site, multi‐centre, double‐blind, randomised, 2‐week parallel trial with 2 arms:
Phases: 3. Pre‐randomisation (4 weeks' titration plus 1 week washout), randomisation, double‐blind treatment and open‐label extension |
|
| Participants | Number of participants screened: unknown Number of participants included: 164 (122 boys, 39 girls). 137 participants randomised Number of participants followed up: MPH 63, placebo 71 Number of withdrawals: MPH 3, placebo 0 Diagnosis of ADHD: DSM‐IV (combined (75.8%), hyperactive‐impulsive (1.2%), inattentive (18.6 %)) Age: mean 8.81 years (range 6‐14) IQ: not stated MPH‐naive: 94 (58.4%) Ethnicity: white (85.7%), African American (3.7%), Asian (1.2%), other (9.3%) Country: USA and Canada Setting: outpatient clinic (naturalistic school setting) Comorbidity: not stated Comedication: not stated Other sociodemographics: none. No significant differences in baseline demographics were noted between the 2 groups Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to ER‐MPH at optimised dose or placebo Number randomised to each group: MPH 66, placebo 71 Mean MPH dosage: not stated Administration schedule: once daily in the morning Duration of intervention: 2 weeks (mean: MPH 13.91, placebo 13.96) Titration period: 1 week before randomisation Treatment compliance: 130 completed treatment (MPH 61, placebo 69) |
|
| Outcomes |
ADHD symptoms
Non‐serious AEs
|
|
| Notes | Sample calculation: yes. A total of 128 participants (n = 64 per treatment group) were required for analysis of the primary efficacy variable, based on an effect size of 0.5 with a power of 80% and a two‐tailed α‐level of 0.05 Ethics approval: yes. An institutional review board approved this trial at each participating site Comment from trial authors
Key conclusion of trial authors
Comments from review authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: yes Any withdrawals due to AEs: yes (n = 3) Funding source: funding was received from Novartis Email correspondence with trial authors. April 2014. Emailed trial authors for additional information/data but have not received a response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed by Novartis Drug Supply Management, which used a validated system that automates random assignment of treatment groups to randomisation numbers |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not clear whether investigator was blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not clear whether investigator was blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | LOCF analysis of ITT population. ITT population included all participants who received the double‐blind trial drug, and from whom ≥ 1 Conners’ ADHD/DSM‐IV Scale, Teachers, was obtained Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): yes, 2 participants, due to unsatisfactory therapeutic effect |
| Selective reporting (reporting bias) | Unclear risk | No protocol was published. |
Bliznakova 2007.
| Study characteristics | ||
| Methods | 11‐day N‐of‐1 randomised, double‐blind, cross‐over trial with 2 interventions:
|
|
| Participants | Number of participants screened: 1 boy Number included: 1 Number of participants followed up: 1 Number of withdrawals: 1 Diagnosis of ADHD: ICD‐10 (predominantly hyperactive type) Age: 15 years IQ: not stated MPH‐naive: no Ethnicity: not stated Country: Germany Setting: not stated Comorbidity: not stated Comedication: not stated Other sociodemographics: 2 parents |
|
| Interventions | The participant was randomly assigned to MPH and placebo across 11 days Mean MPH dosage: not stated Administration schedule: not stated Duration of each medication condition: the condition was changed daily, but placebo was given for 6 days and MPH for 5 days Washout before trial initiation: not relevant Medication‐free period between interventions: none Titration period: none Treatment compliance: 100% according to Table 7 |
|
| Outcomes |
ADHD symptoms
Non‐serious AEs
|
|
| Notes | Sample calculation: yes Ethics approval: not stated Key conclusion of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: no Funding source: not stated Email correspondence with trial authors: December 2013. Emailed trial author to request information about missing data but received no response. Not possible to use data from this trial |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Low risk | No indication of selective reporting |
Blum 2011.
| Study characteristics | ||
| Methods | This double‐blind, placebo‐controlled, cross‐over trial with the child's clinically most effective dose as identified by a systematic open‐label titration procedure investigated whether components of attention and executive functioning improve when children with ADHD are treated with OROS‐MPH 2‐week, cross‐over trial with 2 interventions:
|
|
| Participants | Number of participants screened: 41 Number of participants included: 34 Number of participants followed up: 30 (24 boys, 6 girls) Number of withdrawals: 0 Diagnosis of ADHD: DSM‐IV‐TR (combined (100%)) Age: mean 8 years 6 months (range 6 years 5 months‐12 years 6 months) IQ: mean 97.8 (range 77‐132) MPH‐naive: number not stated Ethnicity: white (80%), African American (13.3%), other (6.7%) Country: USA Setting: outpatient clinic Comorbidity type: ODD (40%), specific learning difficulty (33.3%), anxiety (6.67%), dysthymia (3.3%) Comedication: not stated Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to OROS‐MPH and placebo in random order MPH dosage: 9 children treated with 18 mg, 13 with 36 mg and 8 with 54 mg of OROS‐MPH Administration schedule: not stated Duration of each medication condition: 1 week Washout before trial initiation: not stated Medication‐free period between interventions: not stated Titration period: 2‐ to 3‐week open‐label, multi‐dose‐titration protocol to determine the child’s optimal dose as recommended by practice guidelines Treatment compliance: 30 children completed the trial; however, compliance regarding trial medication is not stated |
|
| Outcomes |
ADHD symptoms
Non‐serious AEs
|
|
| Notes | Sample calculation: not stated Ethics approval: yes; approved by the Committee for the Protection of Human Subjects at The Children’s Hospital of Philadelphia Comment from trial authors
Key conclusion of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: yes Withdrawals due to AEs: 0 Funding source: supported by an investigator‐initiated grant from Ortho McNeil Janssen Scientific Affairs Email correspondence with trial authors: January 2014: emailed trial authors twice to request additional information but received no response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned and counterbalanced across participants |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Referred to as double‐blind but no information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Referred to as double‐blind but no information provided |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Completer analysis reported Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Low risk | Clinical trial ID: NCT00530257, no indication of selective reporting |
Borcherding 1990.
| Study characteristics | ||
| Methods | 11‐week, double‐blind, placebo‐controlled, cross‐over trial with 3 interventions:
|
|
| Participants | Number of participants screened: not stated Number of participants included: 46 (all boys) Number of participants followed up: 45 Number of withdrawals: 1 Diagnosis of ADHD: DSM‐III (subtype not stated) Age: mean 8.6 years (range 6‐12) IQ: mean 106.1 (range > 80) MPH‐naive: 13 had not received past stimulant treatment Ethnicity: white (72%), African American (22%), Asian/Hispanic (6%) Country: USA Setting: outpatient clinic Comorbidity: medically healthy Comedication: not stated Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of the possible drug condition orders of MPH, dextroamphetamine and placebo Mean MPH dosage: 1.3 mg/kg Administration schedule: twice daily, 9:00 am and 1:00 pm Duration of each medication condition: 3 weeks Washout before trial initiation: 2 weeks Medication‐free period between interventions: none Titration period: during the 3 weeks, LD was given week 1, intermediate dose week 2 and high dose week 3 Treatment compliance: not stated |
|
| Outcomes |
Non‐serious AEs
|
|
| Notes | Sample calculation: no Ethics approval: not stated Comment from trial authors
Key conclusions of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Withdrawals due to AEs: no (the 1 withdrawal due to AE happened while on dextroamphetamine) Funding source: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Double‐blind random fashion |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Oral medication in identical capsules was administered at 9:00 am and 1:00 pm |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants except 1 (who experienced AEs) completed the trial Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
Brams 2008.
| Study characteristics | ||
| Methods | Randomised, double‐blind, cross‐over, multi‐centre trial evaluating the efficacy of the following over an 8‐h laboratory classroom day in children with ADHD
Phases:
|
|
| Participants | Number of participants screened: 92 Number of participants included: 86 (53 boys, 33 girls). Participants were randomly assigned to 1 of 2 possible drug condition orders Number of participants followed up: 86 Number of withdrawals: 0 Diagnosis of ADHD: DSM‐IV (combined (87.2%), hyperactive‐impulsive (0%), inattentive (12.8%)) Age: mean 9.5 years (range 6‐12) IQ: > 70 MPH‐naive: 0% Ethnicity: white (48.8%), African American (24.4%), Asian (2.3%), Hispanic (23.3%) Country: USA Setting: multi‐centre, outpatient clinic (laboratory classroom) Comorbidity: not stated Comedication: no antidepressant or other antipsychotic medication Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 2 possible drug condition orders of once‐daily, ER‐d‐MPH 20 mg (Focalin XR (Novartis Pharmaceuticals Corporation) and placebo Mean MPH dosage: fixed dose of 20 mg Administration schedule: once daily in the morning Duration of each medication condition: 7 days Washout before trial initiation: 1 week before the trial Titration period: before trial participation, all participants were stabilised on a total daily dose or nearest equivalent dose of MPH 40 mg to 60 mg or d‐MPH 20 mg to 30 mg for ≥ 2 weeks before screening Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
Non‐serious AEs
|
|
| Notes | Sample calculation: yes Ethics approval: not stated Comments from trial authors
Key conclusions of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: yes; children with poor response or known sensitivity to MPH or d‐MPH were excluded Withdrawals due to AEs: no Funding source: Novartis Pharmaceuticals Corporation Email correspondence with trial authors: September 2013. We received an email from Dr. Brams, in which we were told that Novartis had control and ownership of trial data. Consequently, we had to contact the Public Affairs Department at Novartis to request the information (e.g. protocols) (Krogh 2013a [pers comm]) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation list was generated by the trial sponsor, who used an automated random assignment of treatment sequences to randomisation numbers in the specified ratio |
| Allocation concealment (selection bias) | Low risk | All trial medications and packaging were identical in appearance for blinding purposes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants, parents, trial centre personnel and those who assessed outcomes were blinded to trial treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants, parents, trial centre personnel and those who assessed outcomes were blinded to trial treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The safety population consisted of all participants who took ≥ 1 dose of trial medication. The efficacy population included all randomly assigned participants who provided valid efficacy measurements for both treatment periods Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Unclear risk | No published protocol |
Brams 2012.
| Study characteristics | ||
| Methods | Randomised, double‐blind, 3‐period × 3‐treatment cross‐over trial in a 12‐h laboratory classroom setting with 3 interventions
Each period lasted 7 days |
|
| Participants | Number of participants screened: not stated Number of participants included: 165 (57% boys, 43% girls) Number of participants followed up: 157 Number of withdrawals: 8 Diagnosis of ADHD: DSM‐IV (combined or predominantly hyperactive‐impulsive subtype) Age: mean 9.6 years (range 9.3 to 10.0) IQ: above normal MPH‐naive: 0% Ethnicity: white (38.2%), African American (31.5%), Hispanic (22.4%), other (7.9%) Country: USA Setting: outpatient clinic (laboratory classroom) Comorbidity: no significant medical illness Comedication: not stated Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 6 possible drug condition orders of 20 mg ER‐d‐MPH, 30 mg ER‐d‐MPH and placebo Administration schedule: once daily, morning Duration of each medication condition: 7 days Washout before trial initiation: 1 week Medication‐free period between interventions: no Titration period: none (fixed doses) Treatment compliance: no information |
|
| Outcomes |
ADHD symptoms
Serious AEs
1 participant experienced 2 serious AEs (peritonsillar abscess and oral bullae) while receiving 20 mg ER‐d‐MPH and was hospitalised for 6 days for the peritonsillar abscess. Serious AEs were considered not related to trial drug. Participant discontinued the trial for missed trial drug during hospitalisation Non‐serious AEs
|
|
| Notes | Sample calculation: no Ethics approval: yes Comments from trial authors
Key conclusions of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: yes; children with a poor prior response, or known sensitivity, to all MPH or d‐MPH products based on medical history were excluded Withdrawals due to AEs: no Funding source: Novartis Pharmaceuticals Corporation Email correspondence with trial authors: September 2013. Not possible to contact trial authors |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned to 1 of 6 treatment sequences. All participants were given the lowest available number from the randomisation numbers provided at each site. A randomisation list was produced by using a validated system that automated the random assignment of treatment sequences to randomisation numbers in the specified ratio |
| Allocation concealment (selection bias) | Low risk | Randomisation data were kept strictly confidential until the time of unblinding |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All trial medications and packaging were identical in appearance for blinding purposes. Participants, parents, trial centre personnel and those who assessed outcomes were blinded to trial treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All trial medications and packaging were identical in appearance for blinding purposes. Participants, parents, trial centre personnel and those who assessed outcomes were blinded to trial treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8 dropouts from the MPH group. The ITT population included all randomly assigned participants who took ≥ 1 dose of trial medication and had ≥ 1 post‐dose efficacy measurement. The safety population consisted of all participants who took ≥ 1 dose of trial medication Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Unclear risk | No published protocol |
Brown 1984a.
| Study characteristics | ||
| Methods | 4‐week cross‐over trial with 2 interventions:
Phases: 2 weeks of placebo and 2 weeks of MPH treatment with sequence according to randomisation |
|
| Participants | Number of participants screened: not stated Number of participants included: 11 (all boys) Number of participants followed up: 11 Number of withdrawals: 0 Diagnosis of ADHD: DSM‐III Age: mean 10 years, 5 months (range 9 years 1 month‐12 years 1 month) IQ: > 80 MPH‐naive: not stated Ethnicity: not stated Country: USA Setting: outpatient clinic Comorbidity: not stated Comedication: not stated Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 2 possible drug condition orders of 0.3 mg/kg MPH and placebo Mean MPH dosage: not stated Administration schedule: twice/d Duration of each medication condition: 2 weeks Washout before trial initiation: not stated Medication‐free period between interventions: time of day the pills were taken not stated Titration period: none Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
Non‐serious AEs
|
|
| Notes | Sample calculation: no Ethics approval: yes Comments from trial authors
Key conclusions of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: no Funding source: funded by NIMH and NIH. Placebo and MPH were supplied by CIBA‐GEIGY Corporation, Summit, New Jersey Email correspondence with trial authors: November 2013. We received additional information regarding ethics approval, sample calculation, etc., from trial authors. However, it was not possible to receive all requested data, as the trial author no longer possessed raw data from the trial. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The sequence of the 2 medication conditions was randomly assigned, but no information was provided on methods |
| Allocation concealment (selection bias) | Low risk | Triple blinding; dosage was administered twice daily in the form of opaque capsules packaged by hospital pharmacists to conceal the contents |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Child and parent, teacher and the physician were blinded to the child’s medication condition |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Physician was blinded to the child’s medication concealment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data provided on all 11 participants Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Low risk | No indication of selective reporting |
Brown 1985.
| Study characteristics | ||
| Methods | 12‐week, randomised, parallel trial
Cognitive training programme: individual, twice‐weekly, 1‐h sessions for a total of 24 sessions spanning a 3‐month period |
|
| Participants | Number of participants included: MPH + cognitive training 10, cognitive training 10 Number of participants followed up: MPH + cognitive training 10, cognitive training 10 Number of withdrawals: MPH + cognitive training 0, cognitive training 0 Diagnosis of ADHD: DSM‐III (types not stated) Age: mean 11.36 years (range 6.4‐11.9) IQ: 101.92 (range 91‐136) MPH‐naive: not stated Ethnicity: not stated Country: USA Setting: not stated Comorbidity: not stated Comedication: no. No child was receiving any psychopharmacological treatment Other sociodemographics: none. No significant differences in baseline demographics between the 2 groups Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to MPH + cognitive training or to cognitive training only Mean MPH dosage: 0.3 mg/kg (range 5 mg/d‐15 mg/d) Administration schedule: twice daily (morning and lunch) Duration of intervention: 12 weeks + 3 months (only with medication) Titration period: none Treatment compliance: not stated Cognitive training programme: individual, twice‐weekly, 1‐h sessions for a total of 24 sessions spanning a 3‐month period |
|
| Outcomes |
ADHD symptoms
|
|
| Notes | Sample calculation: no Ethics approval: no information Key conclusion of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: no Funding source: research supported by US Public Health Services Grant from the NIMH, and by the Biomedical Research Award from the NIH. MPH provided by CIBA‐GEIGY Corporation, Summit, New Jersey |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label MPH |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants followed up Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol/design published |
Brown 1988.
| Study characteristics | ||
| Methods | 8‐week, double‐blind, randomised, cross‐over trial with 4 interventions:
|
|
| Participants | Number of participants screened: not stated Number of participants included: 11 (all boys) Number of participants followed up: 11 Number of withdrawals: 0 Diagnosis of ADHD: DSM‐III (subtype not stated) Age: mean 13 years, 7 months (range 12 years and 10 months‐14 years and 10 months) IQ: full‐scale mean 92.91 (range 86‐104) MPH‐naive: not stated, but none of the participants had been treated with stimulants during the year preceding the trial Ethnicity: African American (100%) Country: USA Setting: outpatient clinic Comorbidity: CD, socialised aggressive (45%) Comedication: no Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to possible drug condition orders: Interventions (mean dosage)
Mean MPH dosage: MPH low (4.38 mg), medium (12.55 mg), high (21.28 mg) Administration schedule: twice daily; morning and noon Duration of each medication condition: 2 weeks Washout before trial initiation: none (but no stimulant treatment for the past year) Titration period: none Treatment compliance: compliance was determined to be satisfactory |
|
| Outcomes |
ADHD symptoms At the end of each 2‐week trial, parents and teachers completed the following rating scales
Non‐serious AEs
|
|
| Notes | Sample calculation: no Ethics approval: yes; Institutional Review Board (IRB) at Emory University Key conclusion of trial authors
Comment from trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Withdrawals due to AEs: no Funding source: Biomedical Research Support Grant Program, Division of Research Resources, NIH and Emory University Research Email correspondence with trial authors: October 2013. We received from trial authors additional information about ethics approval, planned outcomes and participants followed up. Unfortunately, it was not possible for trial authors to provide other data that we needed because the trial was conducted many years ago, and trial authors no longer had the data |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Drug order was randomly assigned across participants; no further description |
| Allocation concealment (selection bias) | Low risk | All medication was prepared in identical capsules by hospital pharmacists. Medication was dispensed in dated envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All medication was prepared in identical capsules by hospital pharmacists. Medication was dispensed in dated envelopes. Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Low risk | Researchers administered all measures that were proposed and reported these data in the published report |
Brown 1991.
| Study characteristics | ||
| Methods | Double‐blind, randomised, cross‐over trial with 2 interventions:
Phases:
|
|
| Participants | Trial consisted of 22 participants, but only 7 had ADD. As outcomes were reported separately for these 7 participants, we were able to include the trial Number of participants screened: 25 Number of participants included: 7/22 (all boys) Number of participants followed up: 22 Number of withdrawals: 0 Diagnosis: DSM‐III (CD, with 7 of 22 also diagnosed with ADD) Age: mean:15.8 years (range 12.9‐18.9) IQ: 96.22 (SD 15.12, range 80‐123) MPH‐naive: not stated Ethnicity: white (100%) Country: USA Setting: hospital Comorbidity: CD (100%) Comedication: occasional allergy medication was allowed Other sociodemographics: middle and upper‐middle class Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 24 possible drug condition orders of MPH (10 mg, 15 mg, 20 mg) and placebo (in counterbalanced order) Mean MPH dosage: 0.15 mg/kg, 0.22 mg/kg and 0.31 mg/kg Administration schedule: twice daily, 8:00 am and 12:00 pm Duration of each medication condition: 1 day each, 3 days of MPH in all Washout before trial initiation: not stated Titration period: 1 day before first phase to test tolerability Treatment compliance: 100% |
|
| Outcomes |
General behaviour
Non‐serious AEs
|
|
| Notes | Sample calculation: not stated Ethics approval: not stated Comments from trial authors
Key conclusions of trial authors
Comment from review authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: yes; initially participants received a 1‐day open trial of MPH. 3 participants were excluded because of intolerability Any withdrawals due to AEs: no Funding source: not stated Email correspondence with trial authors: unable to locate contact details for trial authors |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | All participants received each of the 4 doses in 1 of 24 possible randomly assigned sequences |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | MPH and placebo were packaged in coloured gelatin capsules by the hospital pharmacist to avoid detection of dose, visually or by taste |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Under double‐blinded conditions |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants followed up Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol published |
Buitelaar 1995.
| Study characteristics | ||
| Methods | Randomised, cross‐over trial with 3 interventions:
Phases:
First 32 participants were randomly assigned to interventions 1‐3 in the first treatment block, and to intervention 1 or 2 in the second treatment block. Next 20 participants were randomly assigned to intervention 2 or 3 in the first treatment block, and to intervention 2 or 3 in the second treatment block |
|
| Participants | Number of participants screened: not stated Number of participants included: 52 (46 boys, 6 girls); however, because of an incomplete block design, only 46 were treated with MPH and 31 were treated with placebo in first or second treatment block Number of participants followed up: 52 Number of withdrawals: 0 Diagnosis of ADHD: DSM‐III‐R (subtype not stated) Age: mean 9.3 years (range 6‐13) IQ: mean 94.2 MPH‐naive: 100% Ethnicity: not stated Country: the Netherlands Setting: outpatient clinic Comorbidity: CD (38%); depressive disorder, dysthymia or major depressive disorder (15%); anxiety disorder, overanxious disorder or avoidant disorder (42%); psychomotor epilepsy (2%) Comedication: antiepileptic medication (carbamazepine) at a fixed dosage (2%) Other sociodemographics: none. 20% were from families of high socioeconomic status, 50% of middle socioeconomic status and 30% of low socioeconomic status (on the Hollingshead Index). No significant difference in baseline characteristics were noted between groups of children treated with MPH, pindolol or placebo Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to possible drug condition orders of 40 mg pindolol, 20 mg MPH and placebo Fixed dosage: 10 mg MPH, twice daily (approximately 0.6 mg/kg/d) Administration schedule: morning and noon Duration of each medication condition: 4 weeks Washout before trial initiation: no (medication‐naive) Medication‐free period between interventions: 2 weeks Titration period: yes. After randomisation, during the first 3 days of a treatment period, participants received 1 morning dose (10 mg MPH, 20 mg pindolol or placebo). After completion of endpoint assessment, medication was tapered off (3 days with 1 morning dose) Treatment compliance: good to very good in 96% of children. 2 children had poor compliance under MPH treatment as the result of side effects |
|
| Outcomes |
ADHD symptoms
Parents and teachers completed ratings at baseline, at week 2 and at endpoint of each treatment period. The psychologist completed ratings at baseline and at endpoint of each treatment period. Furthermore, ACRS was rated 30 min after drug administration Non‐serious AEs
|
|
| Notes | Sample calculation: yes; for comparison of pindolol with MPH (50 participants) Ethics approval: yes; approved by the Committee for Research on Human Subjects of Utrecht University Hospital Comments from trial authors
Key conclusions of trial authors
Comments from review authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no; only medication‐naive participants Any withdrawals due to AEs: no; however, in 4 participants, dosages of MPH had to be adjusted in the first 2 weeks of the trial because of increased agitation, restlessness and insomnia. 2 participants remained on 5 mg of MPH for 4 weeks, whereas dosage for the other 2 participants could be gradually increased to 10 mg MPH in the last 2 weeks Funding source: not stated Email correspondence with trial authors: January to March 2014. Requested but did not receive from trial authors supplemental efficacy and safety data and information regarding randomisation, allocation concealment and blinding |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned, not further described |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, not further described. MPH and placebo were administered in identical‐looking tablets |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 participants from the MPH group had bad compliance but were included in the analyses, as an ITT analysis was planned Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no, but design of trial changed during the course of the trial because of AEs |
| Selective reporting (reporting bias) | Unclear risk | Protocol not identified |
Bukstein 1998.
| Study characteristics | ||
| Methods | Cross‐over trial with 3 interventions:
Phases: trial included 2 phases: a baseline phase and the medication trial itself. The baseline phase occurred during the first 2 weeks (9 days) of the programme, when the children were medication‐free. Each medication condition was administered for 7 days of the programme during the 21‐day trial |
|
| Participants | Number of participants screened: not stated Number of participants included: 18 Number of participants followed up: 18 (14 boys, 4 girls) Number of withdrawals: 0 DSM‐III‐R criteria for ADHD and ODD or CD Age: mean 9.4 years (range 6.1‐12.2) IQ: not stated MPH‐naive: not stated Ethnicity: white (17%), African American (83%) Country: USA Setting: outpatient clinic (summer school at clinic) Comorbidity: ODD (56%) and CD (44%) Comedication: no Other sociodemographics: participants were predominantly from lower socioeconomic classes, with an average Hollingshead Index of Social Status of 3.83 (SD 1.65, range 1 to 5). 13 (72%) of the participants' families were receiving public assistance. Only 4 of the children lived with both biological parents; 12 (67%) lived with their biological mother only. Inner city environment characterised by higher than average rates of poverty and community violence. No significant differences in baseline demographics between groups Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 6 possible drug condition orders of MPH (0.3 mg/kg or 0.6 mg/kg) and placebo Mean MPH dosage: not stated Administration schedule: 8:30 am, 11:45 AM and 3:00 pm Duration of each medication condition: 7 days Washout before trial initiation: 9 days Medication‐free period between interventions: none Titration period: none Treatment compliance: poor compliance with the weekend medication condition; most families missed ≥ 1 dose each weekend of the trial. Poor compliance with the 3‐dose regimen was so widespread that trial authors omitted from the trial all data on weekend doses |
|
| Outcomes |
ADHD symptoms
Non‐serious AE
|
|
| Notes |
Key conclusions of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: no Funding source: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Schedule for each condition was randomly assigned across the 5 weekdays to minimise programme effects; the only qualifying condition was that approximately half of the 7 days of each medication condition would occur during each half of the 21‐day trial |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Nurse and other Summer Treatment and Enrichment Program (STEP) staff, children and parents were blinded to dosages and schedules |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Each child's daily data were collected and entered by trained research associates, who were unaware of medication status |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All included in the analyses Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no. Number of non‐responders and responders was calculated but not used to exclude participants |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified |
Butter 1983.
| Study characteristics | ||
| Methods | 1‐week, double‐blind, parallel trial with 3 arms:
|
|
| Participants | Number of participants screened: not stated Number of participants included: 30 (all boys) Number of participants followed up: MPH 10, placebo 10 Diagnosis of ADHD: DSM‐III Age: mean not stated (range 6‐12) IQ: > 85 MPH‐naive: not stated Ethnicity: not stated Country: Canada Setting: outpatient clinic Comorbidity: not stated Comedication: not stated Other sociodemographics: no information about significant differences in baseline demographics between groups Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to adrenocorticotropic hormone, MPH or placebo Number of participants randomly assigned to each group: adrenocorticotropic hormone 10, MPH 10, placebo 10 Mean MPH dosage: 0.5 mg/kg Administration schedule: once daily, 7.30 am Duration of intervention: 1 week. 1 week drug‐free followed by 1 week of placebo treatment. After placebo washout, randomly assigned to adrenocorticotropic hormone, MPH or placebo Titration period: none Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
Non‐serious AE
EEG, haematology, blood chemistry and urinalysis were within normal limits before treatment and remained so after treatment |
|
| Notes | Sample calculation: not stated Ethics approval: not stated Key conclusion of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: no Funding source: the Scientific Development Group, Organon International B.V., Oss, the Netherlands |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | After placebo washout, treatment was assigned in a double‐blind and random manner |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Described as double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Neurologist assessing EEG blinded. Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified |
Carlson 1995.
| Study characteristics | ||
| Methods | Double‐blind, placebo‐controlled, repeat‐measures (across drug and dosage), cross‐over trial with 6 interventions:
Phases: each child received placebo, desipramine, each of the 3 doses of MPH (10 mg, 15 mg, 20 mg) and combined desipramine and MPH (at the same 3 doses) |
|
| Participants | Number of participants screened: not stated Number of participants included: 16 (all boys) Number of participants followed up: 16 Number of withdrawals: 0 Diagnosis of ADHD: DSM‐IV (combined (62.5%), inattentive (12.5%), “in partial remission” (25%)) Age: mean: not reported (range 7.9‐12.10 years) IQ: mean not stated (range overall 81‐121; range verbal 74‐113, range performance 73‐126) MPH‐naive: 4 (25%) Ethnicity: white (87.5%), African American (12.5%) Country: USA Setting: inpatient ward Comorbidity: yes; major depressive disorder (68.75%), dysthymic disorder (31.25%). “All had ODD, CD or both” Comedication: yes Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to different possible drug condition orders of MPH (10 mg, 15 mg and 20 mg), twice/d at 7:30 am and 11:30 am × 6 days, then 1‐day washout; the titrated therapeutic level (125 ng/mL to 225 ng/mL) of desipramine twice daily at 3:30 pm and 7: 30 pm x 3 weeks minimum before final measures taken; and placebo Mean MPH dosage: not stated Duration of each medication condition: 1 week Washout before trial initiation: 14 days Medication‐free period between intervention: 1 day Titration period: none Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
General behaviour
Non‐serious AEs
|
|
| Notes | Sample calculation: not stated Ethics approval: not stated Key conclusion of trial authors
Comment from review authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: no Funding source: not stated Email correspondence with trial authors. Emailed first trial author to ask for outcome data in mean and SD format. Trial authors replied in July 2014 to say that they were unable to help us |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not stated |
| Allocation concealment (selection bias) | High risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Medications were packaged in identical grey capsules (size 00) that were administered 4 times/d |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | For this protocol, all raters (including teachers, nurses, psychologist and physicians, except for the attending child psychiatrist, who controlled the desipramine dosage but did not rate the children), children and parents were blinded to all medication conditions |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): not stated |
| Selective reporting (reporting bias) | Unclear risk | Not protocol identified |
Carlson 2007.
| Study characteristics | ||
| Methods | 6‐week, multi‐centre, double‐blind, parallel, RCT with 2 arms:
RCT was preceded by a 4‐week, open‐label, atomoxetine and placebo phase, during which participants who had adequate response were removed |
|
| Participants | Number of participants screened: not stated Number of participants included: met inclusion criteria 25; phase 1 (4‐week open‐label atomoxetine and placebo phase) 24 (20 boys, 4 girls); phase 2 (6‐week, double‐blind RCT) 17 Number of participants followed up: MPH 8, placebo 7 Number of withdrawals: MPH 1, placebo 1 Diagnosis of ADHD: DSM‐IV (combined (79% in phase 1)) Age: phase 1 mean 9.6 years (range 6‐12) IQ: > 70 Stimulant‐naive: 4% Ethnicity: white (83%), other (17%) Country: USA Setting: outpatient clinic Comorbidity: ODD (50% in phase 1) Comedication: no Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to ER‐OROS‐MPH or placebo Co‐intervention: atomoxetine Number of participants randomly assigned: MPH 9, placebo 8 MPH mean dosage: 1.02 mg/kg Administration schedule: once/d Duration of intervention: 6 weeks Titration period for MPH: after randomisation to target dose of 1.08 mg/kg/d (max 1.2 mg/kg/d ) Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
General behaviour
Non‐serious AEs
|
|
| Notes | Sample calculation: included sample (25 participants) is too small in relation to the sample calculation (85 participants) Ethics approval: yes Comment from trial authors
Key conclusion of trial authors
Comment from review authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no, but exclusion of atomoxetine/placebo responders before trial phase 2 Withdrawals due to adverse events: yes, 2 Funding source: research was funded by Eli Lilly and Company, Indianapolis, Indiana Email correspondence with trial authors. June‐November 2013. We received from trial authors and sponsoring pharmaceutical company, supplemental information regarding IQ and blinding procedures, as well as data on weight, treatment‐emergent AEs and ECG |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned via an interactive voice response system to receive ER‐MPH or placebo |
| Allocation concealment (selection bias) | Low risk | Investigators and participants were blinded to the precise visit at which randomisation to blinded placebo or MPH occurred, as this visit is not identified in the investigator’s copy of the protocol, and as use of blinded trial drug begins at visit 2 and continues up to visit 8 |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Efficacy outcome measures were conducted on the ITT sample by using an LOCF method Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): yes, 1 participant discontinued due to perceived lack of efficacy |
| Selective reporting (reporting bias) | Low risk | No indication of selective reporting |
Castellanos 1997.
| Study characteristics | ||
| Methods | 9‐week, double‐blind, cross‐over trial with 3 interventions for 3 weeks each:
Trial was followed up by an open clinical follow‐up of 22 months |
|
| Participants | Number of participants screened: 64 Number of participants included: 22 (all boys) Number of participants followed up: 20 Number of withdrawals: 2 Diagnosis of ADHD: DSM‐III‐R Age: mean 9.4 years (range 6‐13) IQ: mean 98.8 MPH‐naive: not stated Ethnicity: white (80%), African American (10%), Asian (5%), Hispanic (5%) Country: USA Setting: outpatient clinic Comorbidity: Tourette’s disorder (95%), chronic motor tics (5%), CD (5%), ODD (30%), reading disorder (5%), overanxious disorder (5%), OCD (10%), enuresis (20%) Comedication: 4 received haloperidol Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of the possible drug condition orders of MPH and placebo. MPH was increased weekly. For body weight > 30 kg, weekly MPH doses were 15 mg/dose, 25 mg/dose and 45 mg/dose twice/d. For weight ≤ 30 kg, 12.5: 25 mg/dose and 45 mg/dose, twice/d Mean MPH dosage: main cohort 1.20 mg/kg, 2nd cohort 0.69 mg/kg, 3rd cohort 1.22 mg/kg Administration schedule: twice/d: breakfast and lunch Duration of each medication condition: 3 weeks. 1st cohort underwent weekly increases in stimulant doses described as low, medium and high. 2nd cohort underwent increase described as low, medium and medium, and the 3rd cohort as low, high and high Washout before trial initiation: minimum 4 weeks Medication‐free period between interventions: 20 h Titration period: during the first 3 weeks of intervention Treatment compliance: no information |
|
| Outcomes |
ADHD symptoms
Non‐serious AEs
|
|
| Notes | Sample calculation: no Ethics approval: approved by NIMH Institutional Review Board Comments from trial authors
Key conclusion of trial authors
Comment from review authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Withdrawals due to AEs: yes, 1 on placebo Funding source: not stated Email correspondence with trial authors: January 2014. Corresponding author was not able to supply us with further information |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly assigned |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Capsule formulation prepared by a pharmacy |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 2 dropouts; their data are not included in subsequent analyses. One of these was dropped from the trial because of acute exacerbation of tics, and 1 because of excessively disruptive behaviour Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | High risk | Yes; they seem to have reported only hyperactivity scores from CTRS |
Chacko 2005.
| Study characteristics | ||
| Methods | 6‐week within‐participant, randomised, placebo‐controlled, double‐blind, daily cross‐over trial with 3 interventions:
Phases: daily cross‐over between medication conditions. 2 weeks of baseline and adjustment period before 6‐week medication trial. Data from this paper pertain to 5‐ and 6‐year‐old children attending a summer treatment programme between 1987 and 1997 (Pelham 1996) |
|
| Participants | Number of participants screened: not stated Number of participants included: 36 (32 boys, 4 girls) Number of participants followed up: 36 Number of withdrawals: 0 Diagnosis of ADHD: DSM‐III‐R (n = 35, subtype not stated); DSM‐IV (n = 1, combined type) Age: mean 6.13 years (range 5‐6) IQ: mean 102 (SD 15.50) MPH‐naive: not reported Ethnicity: white (86%), other (14%) Country: USA Setting: outpatient clinic (summer treatment programme) Comorbidity: ODD (50%), CD (27.7%) Comedication: not stated Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned, daily, to 1 of 3 possible interventions: 0.3 mg/kg MPH, 0.6 mg/kg MPH and placebo Mean MPH dosage: not stated Administration schedule: twice/d 7:45 am and 11:45 am from Monday through Thursday Duration of each medication condition: daily shifted Washout before trial initiation: not stated Medication‐free period between interventions: maximum 18‐h gap between doses (11:45 am to 7:45 am the following day) and no medication Thursday to Sunday Titration period: before the 6‐week trial began, participants underwent a 2‐week baseline and adjustment period Treatment compliance: not stated |
|
| Outcomes |
Serious AEs
Non‐serious AEs
Side effects are reported as occurring: "none" (i.e. symptom was assessed and was found absent), "mild" (i.e. symptom was present but was not sufficient to cause concern among child, peers or adults), "moderate" (i.e. symptom caused impairment of functioning or social embarrassment to the degree that benefits of medication must be considerable to justify risks of continuing medication) or "severe" (i.e. symptom caused significant impairment of functioning or social embarrassment to the degree that the child should not continue to receive medication). Rating of "moderate" or "severe" signifies clinically significant side effects. Side effects are based on staff/parent subjective report on severity of the side effect. Children were considered to have significant side effects in a particular area if clinically significant side effects were reported within that area for ≥ 50% of observations in a given condition |
|
| Notes | Sample calculation: no information Ethics approval: no information Comments from trial authors
Key conclusion of trial authors
Comments from review authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: unclear; 2 weeks of adjustment period before the medication condition ‐ no information on how many were screened and how many participated during baseline weeks Withdrawals due to AEs: none Funding source: during the conduct of this research, Dr. Pelham was supported by grants from the NIMH (MH48157, MH47390, MH45576, MH50467, MH53554, MH62946), NIAAA (AA06267, AA11873), National Institute on Drug Abuse (NIDA) (DA05605, DA12414), National Institute of Neurological Disorders and Stroke (NINDS) (NS39087), National Institute for Environmental Studies (NIES) (ES05015) and National Institute of Child Health and Human Development (NICHHD) (HD42080) Email correspondence with trial authors: December 2013. We emailed trial authors to request additional information about trial sample and side effects. Trial authors replied to say that the data are no longer available. Data from this trial could not be used in meta‐analyses |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Active medication and placebo were disguised in opaque capsules and were dispensed in daily pill reminders. Each condition occurred one or two times per week, with the order of the conditions randomized on a daily basis. Data were averaged across days within conditions for each child" |
| Allocation concealment (selection bias) | Unclear risk | “Each condition occurred one or two times per week, with the order of the conditions randomized on a daily basis” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “Active medication and placebo were disguised in opaque capsules and were dispensed in daily pill reminders” Double‐blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double‐blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts Side effects were measured for 30 participants Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | High risk | Unclear how the population sample was selected from the group of attendees at these programmes over a 10‐year period |
Childress 2009.
| Study characteristics | ||
| Methods | 5‐week, multi‐centre, multiple‐setting, phase III, double‐blind, randomised, placebo‐controlled, parallel‐trial with 4 arms
1‐3 weeks titration and 2‐4 weeks maintenance period dependent on the allocated active drug group |
|
| Participants | Number of participants screened: 332 Number of participants included: 253 (163 boys, 90 girls) Number of participants followed up: ER‐d‐MPH 161 (10 mg, n = 56; 20 mg, n = 54; 30 mg, n = 51), placebo 57 Number of withdrawals: ER‐d‐MPH 27 (10 mg, n = 10; 20 mg, n = 8; 30 mg, n = 9), placebo 8 Diagnosis of ADHD: DSM‐IV (combined (73.9%), hyperactive‐impulsive (2.8%), inattentive (21.7%), missing data (1.6 %)) Age: mean 8.7 years (6‐12 years) IQ: not stated MPH‐naive: 69.2% Ethnicity: white (57.7%), African American (28.9%), Asian (0.8%), other (12.6%) Country: 34 centres in the USA Setting: outpatient clinic Comorbidity: respiratory, thoracic and mediastinal disorders (MPH 26.9%, placebo 38.1%), immune system disorders (MPH 22.5%, placebo 14.3%), nervous system disorders (MPH 18.7%, placebo 19.0%), surgical and medical procedures (MPH 15.4%, placebo 11.0%), infections and infestations (MPH 14.3%, placebo 17.5%) and skin and subcutaneous tissue disorders (MPH 11.0%, placebo 9.5%) Comedication: ≥ 1 concomitant medication or non‐drug therapy after start of trial (MPH 39.0%, placebo 44.4%). The most common concomitant medications were analgesics, antihistamines and allergy medications Other sociodemographics: none reported. Treatment groups were well balanced in relation to participant background characteristics, and baseline demographics were comparable among treatment groups Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to ER‐d‐MPH or placebo Fixed MPH dosage: 10 mg, 20 mg or 30 mg Number of participants randomly assigned: ER‐d‐MPH 188 (10 mg, n = 66; 20 mg, n = 62; 30 mg, n = 60), placebo 65 Administration schedule: once daily, morning Duration of intervention: 5 weeks Washout before trial initiation: up to 28 days (duration dependent on the half‐life of any previous psychotropic medication) Titration period: 1‐3 weeks, initiated after randomisation Treatment compliance: 86% completed |
|
| Outcomes |
ADHD symptoms
Non‐serious AEs
|
|
| Notes | Sample calculation: yes; 252 participants (63 per treatment group) Ethics approval: institutional review boards or ethical review committees at each centre approved the trial Comment from trial authors
Key conclusions of trial authors
Comment from review authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: yes, 6 all in the MPH groups Funding source: Novartis Pharmaceuticals Corporation. Novartis Pharma has been helping with development of the manuscript Email correspondence with trial authors in December 2013. We have contacted trial authors twice in an attempt to obtain supplemental information regarding blinding, allocation concealment and weight loss. We have not received a response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed by using a validated system that automated the assignment of treatment arms to randomisation numbers in the specified ratio |
| Allocation concealment (selection bias) | Low risk | Allocation concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, but no detailed description |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double‐blind, but no detailed description |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT population. LOCF Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Low risk | No indication of selective reporting |
Childress 2017.
| Study characteristics | ||
| Methods | A 1‐week parallel trial with 2 arms:
Phases: 5 (screening, washout, open‐label stepwise dose optimisation, dose stabilisation, and double‐blind parallel group treatment) |
|
| Participants | Number of participants screened: 87 entered open‐label phase Number of participants included: 83 Number of participants followed‐up: 80 (82 included in full analysis) (54 boys (65.9%), 28 girls (34.1%) Number of withdrawals: 3 Diagnosis of ADHD: DSM‐IV‐TR (65 (74.7%) combined type, 1 (1.1%) hyperactive‐impulsive type, 21 (24.1%) inattentive type) Age: mean 9.2 years (SD 1.75, range 6‐12) IQ: not stated MPH‐naive: 0% Ethnicity: Hispanic/Latino (n = 28, 34.1%), not Hispanic/Latino (n = 54 (65.9%). Race was reported: white (n = 65, 79.3%), black or African American (n = 10, 12.2%), Asian (n = 2, 2.4%), Native Hawaiian or other Pacific Islander (n = 1, 1.2%), and other (n = 4, 4.9%) Country: USA Setting: outpatient clinic, laboratory classroom setting Comorbidity: most were specified as exclusion criteria Comedication: exclusion criterion Additional sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | After the screening period, all participants went through a washout period of 3‐7 days, then an open‐label dose optimisation period of 4 weeks, then a 1‐week dose stabilisation week at their optimised dose (20‐60 mg). Participants were randomly assigned to: ER‐MPH ODT (at optimised dose between 20‐60 mg) or ODT placebo Number randomised to each group: intervention = 44, placebo = 41 Mean medication dosage: 34.3mg (SD 9.06) Administration schedule: once daily in the morning Duration of each medication: ER‐MPH ODT group: 42.6 days; placebo group: 35.5 days; ER‐MPH ODT plus 7 days placebo Washout before trial initiation: 3‐7 days before open‐label period depending on prior medications Titration period: 4 weeks before randomisation Treatment compliance: drug adherence was assessed at each visit from visits 3‐8 by recording pill counts of returned medication. Non‐adherence was defined as taking < 75% of the trial medication between 2 consecutive visits. No participants were excluded on lack of compliance |
|
| Outcomes |
ADHD symptoms
Serious AEs
Non‐serious AEs
|
|
| Notes | Sample calculation: yes, 84 Ethics approval: yes, “The study was approved by a central Institutional Review Board (IRB; Copernicus Group, Research Triangle Park, NC), and each site, if required, submitted the protocol and consent/assent forms to its local IRB” Comments from trial authors
Key conclusion of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH: yes Any withdrawals due to AEs: no Funding source: supported by funds from Neos Therapeutics, Inc Email correspondence with trial authors: trial authors were contacted for information regarding risk of bias through personal email in August and October 2021, but no answer was received. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial is referred to as randomised, however the method used to generate the allocation sequence is not described in sufficient detail to allow an assessment of whether it produced comparable groups. |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Several mentions of “double blind” and the “double blind phase” or “double blind classroom”, but no information on method or if the blinding was successful. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Protocol mentions blinding of outcome assessors, but the method is not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The 1 participant who was excluded from the full analysis set did not have a baseline SKAMP rating and also had a positive drug screen for amphetamine. 2 other participants were excluded from the per‐protocol set because they used prohibited medications. One had been assigned to placebo and the other to ER‐MPH ODT Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Low risk | Outcomes according to protocol/trial registration |
Childress 2020a.
| Study characteristics | ||
| Methods | A 1‐week parallel‐trial with 2 arms:
Phases: 4 (washout (3 days), open‐label dose‐optimisation (6 weeks), placebo‐controlled double‐blind trial (1 week), and follow‐up phone call (after 1 week)) |
|
| Participants | Number of participants screened: 156 included in open‐label phase (102 (65.4%) boys, 54 (34.6%) girls) Number of participants included: 148 randomised Number of participants followed‐up: 140; per protocol population: 112 Number of withdrawals: 8 Diagnosis of ADHD: DSM‐5 (131 (84.0%) combined, 0 hyperactive‐impulsive and 25 (16.0%) inattentive type) Age: mean 9.4 years SD 1.88, range 6‐12) IQ: > 80 MPH‐naive: not stated Ethnicity: Hispanic/Latino (n = 42), not Hispanic/Latino (n = 105) (of 147). Race: 1 Asian (n ‐ 1), black or African American (n = 58), white (n = 81), more than one race (n = 7) (of 147) Country: USA Setting: outpatient (classroom laboratory) Comorbidity: some comorbidity allowed under exclusion criteria 4. Prevalence not stated Comedication: not stated Additional sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants went through a 6‐week, open‐label dose‐optimisation phase before being randomised. Participants were randomly assigned to: ER‐MPH (PRC‐063) at optimised dose (either 25 35, 45, 55, 70, or 85 mg/d) or placebo for 1 week Number randomised to each group: 75 to MPH, 73 to placebo Mean medication dosage: for the 148 47.84 (SD: 15.12) mg/d Administration schedule: once/d Duration of each medication: 6 weeks of MPH for all participants, 1 more week for participants allocated to MPH otherwise 1 week placebo Washout before trial initiation: 3 days before open‐label Medication‐free period between interventions: none Titration period: 6 weeks Treatment compliance: 1 participant excluded due to noncompliance in week 1 |
|
| Outcomes |
ADHD symptoms
Serious AEs
Non‐serious AEs
The text reported no meaningful changes in haematology, urinalysis and chemistry, but no exact data were reported that could be used for analyses. |
|
| Notes | Sample calculation: no Ethics approval: yes. The trial was approved by an Institutional Review Board (Schulman IRB, Cincinnati, OH). Comments from trial authors
Key conclusion of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH: yes Any withdrawals due to AEs: no Funding source: Purdue Pharma Email correspondence with trial authors: August 2021. Supplemental information regarding risk of bias was received through personal email correspondence with the authors in August 2021.(Storm 2021 [pers comm]) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was applied centrally across all sites via an interactive voice/web response system and was stratified by individual dose level so that approximately half the participants within each dose level received ER‐MPH (PRC‐063) and half received placebo. |
| Allocation concealment (selection bias) | Low risk | Through the interactive voice/web response system randomisation, sites were instructed which bottles to provide to each patient through a unique bottle ID code. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Trial medication was packaged in bottles containing 10 capsules with a unique bottle ID code. The treatment assignment was not identified on the bottles, and the trial medication capsules (ER‐MPH (PRC‐063) and placebo) were indistinguishable. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Neither the participant nor any trial staff (including investigators, trial co‐ordinators and trained observers) were unblinded to treatment assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5.4% withdrawal rate, no imputed data Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Low risk | Outcome reporting according to protocol |
Childress 2020b.
| Study characteristics | ||
| Methods | A 1‐week parallel trial with 2 arms:
Phases: 4 (screening and wash‐out (up to 4 weeks), open‐label dose‐optimisation (6 weeks), double‐blind trial (1 week), and follow‐up call (after 2 weeks) |
|
| Participants | Number of participants screened: 161 included in open‐label phase Number of participants included: 155 randomised (85 (68.0%) boys, 40 (32.0%) girls of 125) Number of participants followed‐up: 117 Number of withdrawals: 38. Of these, 36 were due to exclusion (after randomisation) of a full trial site by the FDA due to concerns about data integrity. Diagnosis of ADHD: DSM‐5 (108 (86.4%) combined, 0 hyperactive‐impulsive and 17 (13.6%) inattentive type) Age: mean 9.4 years (SD 1.65, range 6‐12 years) IQ: not stated MPH‐naive: no Ethnicity: Hispanic/Latino (n = 49, 39.2%), non‐Hispanic/Latino (n = 76, 60.8%). Race: white (n = 99, 79.2%), black/African American (n = 15, 12.0%), Asian (n = 0), native Hawaiian/Pacific Islander (n = 2, 1.6%), other (n = 9, 7.2%) Country: USA Setting: outpatient; assessment on last trial day in laboratory classroom Comorbidity: not stated, but many were defined as exclusion criteria Comedication: specified list of allowed comedication not available for review authors Additional sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants went through a 6‐week open‐label dose‐optimisation phase before being randomised. Participants were randomly assigned to: continuation of optimised dose of ER‐MPH (HLD200) (20, 40, 60, 80 or 100 mg/d) or placebo for 1 week No. randomised to each group: 65 to ER‐MPH (HDL200), 54 to placebo Mean medication dosage: 66.2 mg/d Administration schedule: once/d in the evening (8:00 ± 1.5h) Duration of each medication: mean of 40.4 days of MPH during open‐label phase, plus 1 week MPH or placebo Washout before trial initiation: not stated Medication‐free period between interventions: no Titration period: 6 weeks, starting at 10 mg, increases/decreases of 10‐20 mg Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
Serious AEs
General behaviour
"PREMB‐R results were investigator‐rated summary scores derived from clinician‐administered parent interviews, with the parent PREMB‐R ratings based on the last 2 weekdays before the laboratory classroom day." (Childress 2020b p 4). "PREMB‐R AM and PREMB‐R PM at Visit 9 were assessed by using an analysis of covariance model with treatment as the main effect and trial centre and baseline score at baseline (Visit 2) as the covariates" (Childress 2020b p 5) Non‐serious AEs
|
|
| Notes | Sample calculation: no Ethics approval: yes, approved by each site’s Institutional Review Board Comments from trial authors
Key conclusion of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH: yes Any withdrawals due to AEs: no Funding source: Ironshore Pharmaceuticals Email correspondence with trial authors: September and October 2021. No supplemental information was gained through personal email correspondence with trial authors in September 2021 and no answer was received in October 2021. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, method not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | A full trial site was excluded: 36 participants. Beside that only 2 withdrawals from double‐blind phase (1.7%) Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | High risk | CSSR‐S is reported as a safety assessment, but no results are reported. |
Childress 2020c.
| Study characteristics | ||
| Methods | A 2‐week parallel‐trial with 2 arms:
Phases: 6 (screening and wash‐out (up to 4 weeks), behaviour‐management treatment (2‐4 weeks), eligibility confirmation (2 weeks), open‐label phase (6 weeks), double‐blind trial (2 weeks), and follow‐up phone call after 2 weeks) |
|
| Participants | Number of participants screened: 194, 128 entered open‐label phase Number of participants included: 90 entered double‐blind phase (68 (75.6%) boys, 22 (24.4%) girls) Number of participants followed‐up: 88 completed the double‐blind trial Number of withdrawals: 4 Diagnosis of ADHD: DSM‐5 (from safety population: placebo group: 88% combined type, 12% hyperactive/impulsive type; MPH group: 89.9% combined type, 8.4% hyperactive/impulsive type, 1.7% inattentive type) Age: mean 58.9 months, approximately 4.9 years (SD 6.1, range 48‐68 months) IQ: estimated IQ ≥ 80 MPH‐naive: not stated Ethnicity: white (n = 54, 60.0%), African American (n = 33, 36.7%), Asian (n = 1, 1.1%), other (n = 2, 2.2%), Hispanic or Latino (n = 11, 12.2%) Country: USA Setting: outpatient Comorbidity: not stated, but many comorbidities were specified as exclusion criteria Comedication: non‐sedating antihistamines, acetaminophen (paracetamol), ibuprofen, antibiotics for treatment of a minor illness, and vitamins were permitted during the trial. Additional sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants went through a 6‐week, open‐label, dose‐optimisation phase before being randomised. Participants were randomly assigned to: either continue MPH at optimised dose between 10‐40 mg or have placebo Number randomised to each group: 40 to MPH‐MLR, 50 to placebo Mean medication dosage: 27.5 mg Administration schedule: once/d Duration of each medication: 8 weeks MPH‐MLR for MPH group (6 weeks open label and 2 weeks randomised phase), 6 weeks for placebo group (open label phase) + 2 weeks placebo (randomised phase) Washout before trial initiation: a minimum of 3 days before open‐label, none before double‐blind trial Medication‐free period between interventions: no Titration period: 6 weeks Treatment compliance: not stated |
|
| Outcomes |
ADHD Symptoms
Serious AEs
General behaviour
Non‐serious AEs
Non‐serious AEs
|
|
| Notes | Sample calculation: yes Ethics approval: yes Comments from trial authors
Key conclusion of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH: yes; to participate in the double‐blind trial, participants must have completed the open‐label with good results Any withdrawals due to AEs: 1 Funding source: Rhodes Pharmaceuticals LP Email correspondence with trial authors: August and October 2021. Trial authors were contacted for information regarding risk of bias through personal email in August and October 2021, but no answer was received. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Children were randomised in a 1:1 ratio through a computer‐generated randomisation schedule to either continue their optimised dose or receive matching placebo |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, method not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data from 2.2% of participants Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Low risk | All protocol outcomes reported |
Chronis 2003.
| Study characteristics | ||
| Methods | 8‐week within‐participant, placebo‐controlled, randomised, cross‐over trial (summer treatment camp) with 3 interventions:
Phases: 2‐week baseline assessment followed by 6‐week medication trial |
|
| Participants | Number of participants screened: 48 Number of participants included: 21 (19 boys, 2 girls) Number of participants followed up: not stated Number of withdrawals: not stated Diagnosis of ADHD: DSM‐IV (subtype not stated) Age: mean 10.3 years (±1.9, range 6‐12) IQ: mean 109.9 (± 18.8) MPH‐naive: 14 children had received MPH before start of the trial Ethnicity: white (100%) Country: USA Setting: outpatient clinic (summer treatment programme) Comorbidity: learning problems (42.9%), ODD (66.7%), CD (23.8%) Comedication: not stated Other sociodemographics: (median family income = USD 35,000/year (< USD 15,000/year to > USD 100,000/year); 66.7% of parents married Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 7 possible drug condition orders of IR‐MPH, MAS (Adderall) and placebo
Mean MPH dosage: not stated Administration schedule: MPH once, twice or 3 times daily according to the randomisation procedure Duration of each medication condition: all participants received medication each Monday through Friday throughout a period of 6 weeks for a 24‐day clinical assessment period. Assessment period was divided into three, 8‐day segments. Within each segment, placebo occurred twice and each other condition occurred once, with the order of conditions randomly assigned on a daily basis Washout before trial initiation: not stated Medication‐free period between interventions: none Titration period: none Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
Non‐serious AEs
|
|
| Notes | Sample calculation: no Ethics approval: yes Comments from trial authors
Key conclusions of trial authors
Comments from review authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: no Funding source: a grant from Shire‐Richwood Pharmaceuticals, Incorporated ‐ manufacturer of Adderall ‐ and from the NIMH Email correspondence with trial authors: July 2014. Emailed trial authors twice to request additional information. Trial author was not able to provide us with additional data |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Randomized by day”, no description of how randomisation took place |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | To ensure blinding, placebo capsules were given at 11:30 am in the MAS conditions and for applicable doses in the other conditions. Active medication and placebo were disguised in opaque gelation capsules by a local pharmacy and were dispensed in daily pill reminders by the trial doctor. Furthermore, children were informed that they would be receiving 2 different kinds of medication to see how well they worked, and that some days they would receive inactive pills, but that they, their counsellors or teachers and their parents would not know what kind of pill they would get each day |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Children were informed that they would be receiving 2 different kinds of medication to see how well they worked, and that some days they would receive inactive pills, but that they, their counsellors or teachers and their parents would not know what kind of pill they would get each day |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention of dropout Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Coghill 2007.
| Study characteristics | ||
| Methods | 12‐week randomised, placebo‐controlled, double‐blind cross‐over trial with 3 interventions:
|
|
| Participants | Number of participants screened: not stated Number of participants included: 75 (all boys) Number of participants followed up: not stated Number of withdrawals: not stated Diagnosis of ADHD: DSM‐IV (combined type); ICD‐10 (hyperkinetic disorder) Age: mean not reported (range 7‐15 years) IQ: > 80 MPH‐naive: 100% Ethnicity: not stated Country: UK Setting: outpatient clinic Comorbidity: ODD (41.3%), CD (28%), depressive disorder (4%), generalised anxiety disorder (2.7%), separation anxiety disorder (4%), tic disorder (2.7%), social phobia (1.3%) Comedication: not stated Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 3 possible drug condition orders: 0.3 mg/kg/dose MPH or 0.6 mg/kg/dose MPH and placebo Administration schedule: twice daily Duration of each medication condition: 4 weeks Washout before trial initiation: no Titration period: none Treatment compliance: assessed by pill count and clinical enquiry but not further described |
|
| Outcomes |
ADHD symptoms
|
|
| Notes | Sample calculation: no Ethics approval: yes Key conclusions of trial authors
Comment from trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: no Funding source: supported by a local trust through a Tenovus Scotland initiative Email correspondence with trial authors in 2013. Emailed trial authors twice with a request for additional information regarding protocol and number of participants on which analyses were based. Have not received a reply |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by an independent clinical trials pharmacist (using a computer‐generated random number sequence with block design to ensure equal numbers in each treatment arm) |
| Allocation concealment (selection bias) | Low risk | Participants were randomly assigned by an independent clinical trials pharmacist |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Placebo‐controlled, double‐blind, no further information |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Clinical status was assessed by interview conducted by an experienced, blinded child and adolescent psychiatrist. Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No description about how many participants were included in analyses Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): not stated |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified |
Coghill 2013.
| Study characteristics | ||
| Methods | 7‐week, multi‐centre, double‐blind, parallel, dose‐optimised trial with 3 arms:
|
|
| Participants | Number of participants screened: not stated Number of participants included: 221 (181 boys, 40 girls) (To the MPH and placebo group, not the LDX group) Number of participants followed up: MPH 74, placebo 42 Number of withdrawals: MPH 38, placebo 68 Diagnosis of ADHD: DSM‐IV‐TR (combined (MPH 86.4%, placebo 79.1%), hyperactive‐impulsive (MPH 0.9%, placebo 6.4%), inattentive (MPH 12.7%, placebo 14.5%) Age: mean 10.9 years (range 6‐17) IQ: normal MPH‐naive: MPH 60 (54.1%), placebo 58 (52.7%) Ethnicity: white (MPH 96.4%, placebo 98.2%), African American (0%), Asian (0%), Hispanic/Latino (MPH 1.8%, placebo 0%) Countries: Germany, Sweden, Spain, Hungary, France, UK, Italy, Belgium, Poland, the Netherlands Setting: outpatient clinic Comorbidity: ODD (MPH 9.0%, placebo 7.3%), concomitant psychiatric diagnosis (MPH 26.1%, placebo 18.2%) Comedication: not stated Other sociodemographics: no significant differences in baseline demographics were noted between the 2 groups Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to OROS‐MPH or placebo Number of participants randomly assigned: MPH 112, placebo 111 Mean MPH dosage: 45.4 ± 12.7 mg/d (9.9% 18 mg, 19.8% 36 mg, 53.2% 54 mg) Administration schedule: once/d, 7:00 am Duration of intervention: 7 weeks Titration period: 4‐week stepwise dose‐optimisation period after randomisation. 3‐week dose‐maintenance period followed by 1‐week washout and safety follow‐up Treatment compliance: 2 discontinued in placebo group because of non‐compliance, 3 in MPH group |
|
| Outcomes |
ADHD symptoms
Quality of life
Non‐serious AEs
|
|
| Notes | Sample calculation: yes Ethics approval: approved by an independent ethics committee/institutional review board and regulatory agency at each centre (as appropriate) before trial initiation Comments from trial authors
Key conclusions of trial authors
Comment from review authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: yes Any withdrawals due to AEs: yes, 2 in the MPH group and 2 in the placebo group (as well as 5 in the LDX dimesylate group) Funding source: Shire Development LLC |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Interactive voice/web response system was used to allocate a unique randomisation number to each participant. Randomisation was stratified by country and age group (6 to 12 or 13 to 17 years of age) |
| Allocation concealment (selection bias) | Low risk | Interactive voice/web response system |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Trial drugs were over‐encapsulated and appeared identical |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Masking: double‐blinded (participant, caregiver, investigator, outcomes assessor) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | LOCF approach was used when efficacy assessments were incomplete for a participant owing to early withdrawal from the trial or for missing data. However, as the review authors cannot understand the relationship between the n of the full analysis set and the endpoint data, we assessed risk of bias as unclear Selection bias (e.g. titration before randomisation → exclusion): no |
| Selective reporting (reporting bias) | Low risk | Outcomes according to protocol |
Connor 2000.
| Study characteristics | ||
| Methods | 3‐month, randomised, blind, parallel‐group trial with 3 arms:
|
|
| Participants | Number of participants screened: 24 Number of participants included: 24 (MPH + clonidine 8 boys, 0 girls; clonidine + placebo 8 boys, 0 girls; MPH + placebo 8 boys, 0 girls) Number of participants randomly assigned: MPH + clonidine 8, placebo + clonidine 8, placebo + MPH 8 Number of participants followed up: MPH + clonidine 8, placebo + clonidine 6, placebo + MPH 7 Number of withdrawals: MPH + clonidine 0, placebo + clonidine 2, placebo + MPH 1 Diagnosis of ADHD: DSM‐III‐R (combined (100%)) Mean age: MPH + clonidine 10.1 years, placebo + clonidine 9.3 years, placebo + MPH 8.9 years IQ: > 70 MPH‐naive: 54% Ethnicity: white (11%), African American (1%), Asian (0%), Hispanic (0%), other (0%) Country: USA Setting: outpatient clinic Comorbidity: ODD or CD (100%) Comedication: not stated Other sociodemographics: no significant differences in baseline demographics were noted between groups Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to MPH + clonidine or placebo + clonidine MPH dose: 35.0 (5.67) mg/d Administration schedule: twice/d Duration of intervention: 12 weeks Titration period: 4 weeks after randomisation Treatment compliance: “All subjects were acceptably compliant with the protocol” |
|
| Outcomes |
General behaviour
Non‐serious AEs
|
|
| Notes | Intervention groups used: MPH + clonidine; control group: placebo + clonidine Ethics approval: yes Sample calculation: no Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: no (1 in the MPH‐only group, not included here) Funding source: supported by a UMMS (University of Massachusetts Medical School) Small Grants Project Award Email correspondence with trial authors: June 2013. We obtained additional information from trial authors, but it was not possible to receive supplemental data. We could not perform a meta‐analysis on any of the outcomes, as we did not have relevant data and transformation was not possible. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | 8 participants were randomly assigned to each group. 3 participants were previous MPH treatment failures and refused randomisation to the MPH‐alone trial arm. These 3 participants were partially randomly assigned to MPH and clonidine or to clonidine alone. All other children were fully randomly assigned |
| Allocation concealment (selection bias) | Low risk | After trial completion, the medication blind was broken. All medication capsules and placebo capsules were prepared by the UMMS (University of Massachusetts Medical School) Pharmacy in identical capsules to disguise taste and smell. All participants in all trial groups received an equal number of capsules per day. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All teachers, school nurses, parents, children and research assistants completing dependent measures were blinded to the child's treatment group for the trial duration. Completion of ECGs for only 2 clonidine treatment groups may have broken blinding for parent raters and for the child (but not for teacher raters nor for research assistants administering the Gordon Diagnostic System (GDS), who remained blinded as to whether the child had received an ECG). This is not relevant to us, as we are using data only on the 2 groups completing ECGs |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | As stated above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | LOCF Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Low risk | Reply from trial author on our request for the protocol: protocol described in the trial |
Cook 1993.
| Study characteristics | ||
| Methods | 6‐week, double‐blind, single cross‐over, placebo‐controlled trial conducted on a group of 15 participants with ADD and a comparison group of 10 age‐matched participants who did not have ADD ADD group was randomly assigned to 1 of 2 experimental groups:
|
|
| Participants |
ADD group Number of participants screened: not stated Number of participants included: 15 (all boys) Number of participants followed up: 15 Number of withdrawals: 0 Diagnosis of ADHD: DSM‐III (100% met DSM‐III criteria for ADD with hyperactivity) Age: mean 104.5 months (approximately 8.7 years) (range 6‐10 years) IQ: mean 110.5 (SD 7.15) MPH‐naive: 100% Ethnicity: not stated Country: USA Setting: outpatient clinic Comorbidity: central auditory processing disorder (80%) Comedication: not stated Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 2 possible drug condition orders of MPH and placebo Mean MPH dosage: 0.30 mg/kg (0.057) Administration schedule: not stated Duration of each medication condition: 3 weeks Washout before trial initiation: not relevant, 100% treatment‐naive Medication‐free period between interventions: minimum of 48 h between the 2 interventions Titration period: dose was titrated up to a maximum (MPH tablets or placebo tablets) over the first 3 weeks of the experimental period Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
|
|
| Notes | Sample calculation: no information Ethics approval: yes Comment from trial authors
Comment from review authors
Key conclusion of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: no Funding source: supported by the Medical Center Rehabilitation Hospital Foundation and the School of Medicine, University North Dakota; the Veterans Hospital; the Dakota Clinic; and The Neuropsychiatric Institute, Fargo, North Dakota Email correspondence with trial authors: September 2013. Emailed trial author requesting additional information about the trial and data. Dr. Cook referred to his Ph.D. dissertation, which we managed to get. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were assigned to groups by a table of random numbers known only to the pharmacist |
| Allocation concealment (selection bias) | Low risk | Drugs were coded and administered by a pharmacist, and clinical titration of dosage was done by the participant’s paediatrician; neither practitioner was involved in data collection |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Physician, audiologist, teachers and parents involved in behaviour ratings and participants themselves were blinded to assigned treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Physician, audiologist, teachers and parents involved in behaviour ratings and participants themselves were blinded to assigned treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Low risk | No indication of selective reporting |
Corkum 2008.
| Study characteristics | ||
| Methods | 3‐week, blind medication trial with cross‐over design, in which children were randomly assigned to the following:
|
|
| Participants | Number of participants included: 28 Number of participants followed‐up: 21 (15 boys, 6 girls) Number of withdrawals: 7 children excluded from final data analyses Diagnosis of ADHD: DSM‐IV (combined type (52%), hyperactive‐impulsive type (10%), inattentive type (38%)) Age: mean not reported (range 6 years 1 month‐12 years 1 month) IQ: no intellectual disability MPH‐naive: 100% Setting: outpatient clinic Country: Canada Ethnicity: all participants were white Comorbidity: learning disabilities (29%), ODD (10%), CD (0%), generalised anxiety disorder (0%), depression (0%) Comedication: not stated Other sociodemographics: predominantly middle‐class families Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 3 medication‐dosing schedules, including 1 week of baseline, placebo and low and moderate IR‐MPH (Ritalin) dose condition Children weighing ≤ 25 kg received 5‐mg and 10‐mg doses; children weighing > 25 kg received 10‐mg and 15‐mg doses. Children received medication 3 times/d (8:00 am, 12:00 pm, 4:00 pm) Duration of medication: 1 week of each dose Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
Non‐serious AEs
|
|
| Notes | Sample calculation: not stated Ethics approval: yes Key conclusion of trial authors
Comment from review authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: no Funding source: research was supported by a grant from the Izaak Walton Killam IWK Health Centre in Halifax, Nova Scotia Email correspondence with trial authors: August‐October 2013. We received supplemental information regarding additional data from trial authors, but we never received first‐period data from the cross‐over trial. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Baseline data from 1 week were collected, followed by a 3‐week medication trial with random assignment of children to 1 of 3 medication‐dosing schedules. The original, fully randomised schedule was modified after a pilot trial, conducted before the current trial, indicated that children receiving MD‐MPH before the LD‐MPH reported increased side effects and were more likely to stop taking the third dose (4:00 pm) of medication |
| Allocation concealment (selection bias) | Low risk | Pharmacy local to the ADHD clinic prepared both placebo and active medication, which were packaged into identical gelatin capsules |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Child, family, school personnel and trial investigators were unaware of the randomisation schedule. This information was made available only to the paediatrician and the pharmacist |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Child, family, school personnel and trial investigators were unaware of the randomisation schedule. This information was made available only to the paediatrician and the pharmacist |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 7 children excluded from final data analyses for the following reasons: actigraphic problems (i.e. data failure/loss of ActiGraph/refusal to wear ActiGraph) (n = 5), withdrawal of consent due to marital discord (n = 1) and decision to try alternative medication immediately before the start of the medication trial (n = 1) Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Low risk | Protocol received through correspondence with trial author. No indication of selective reporting. |
Corkum 2020.
| Study characteristics | ||
| Methods | A 4‐week cross‐over‐trial with 2 arms:
Phases: 2 (baseline followed by 4‐week, double‐blind trial) |
|
| Participants | Number of participants screened: 32 Number of participants included: 26 (23 boys, 3 girls) Number of participants followed up: 26 Number of withdrawals: 0 Diagnosis of ADHD: DSM‐IV‐TR (combined type: 17 (65.4%), inattentive type: 9 (34.6%)) Age: mean 8 years and 8 months (SD 24.5 months, range 6‐12 years) IQ: 95.6 (SD 31.6) MPH‐naive: 100% Ethnicity: white (n = 23), Latin American (n = 2), Aboriginal (n = 1) Country: Canada Setting: outpatient and sleep laboratory Comorbidity: learning disorder (n = 8, 30.8%) Comedication: not stated Additional sociodemographics: socioeconomic status was calculated using the Boyd‐NP scale and was in the average range (M 68.7, SD 23.2). Mean household annual income fell in the bracket of CAD 51,000 to CAD 60,000. Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 2 possible orders of placebo for 2 weeks and MPH (Biphentin) for 2 weeks. Children weighing < 20 kg received a 20 mg daily dose, children weighing 20‐30 kg were given 30 mg, and children weighing > 30 kg were given 40 mg. Number randomised to each group: not stated Mean medication dosage: not stated Administration schedule: once daily within 1 h of waking Duration of each medication: 2 weeks (data related to sleep and daytime behaviour were collected during the 1st and 3rd weeks of this trial (i.e. after the 1st week of each condition). The 2nd and 4th weeks were used in situations in which the first week of a condition was not considered typical by the parent, e.g. child was ill) Washout before trial initiation: not necessary as all participants were medication‐naive Medication‐free period between interventions: none Titration period: none Treatment compliance: not measured. Parents reported informally to the paediatrician that the children had been adherent |
|
| Outcomes |
ADHD symptoms
Non‐serious AEs
|
|
| Notes | Sample calculation: yes; "with an effect size (Cohen’s f) of 0.18 and power set at 0.95 and an alpha of 0.05, 26 participants were determined to be the minimal sample size needed” Ethics approval: yes Comments from trial authors
Key conclusion of trial authors
Comment from review authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH: no Any withdrawals due to AEs: no Funding source: Canadian Institutes of Health Research Email correspondence with trial authors: August and September 2021. Trial authors were contacted for information regarding main article, risk of bias and first‐period data through personal email in August and Semptember 2021, but no answer was received. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was conducted using blocking on an online calculator (randomised in blocks of 10) |
| Allocation concealment (selection bias) | Low risk | The randomisation schedule was conducted through a third‐party |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The pharmacists prepared the medication and placebo by placing these in identical gelatin capsules so that children and parents could not identify whether capsules contained medication or the placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The randomisation schedule was conducted through a third‐party, and the child, family, teachers, and trial staff were all blind to medication condition; only the trial paediatricians and pharmacists were aware of the medication status of the participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
Cox 2006.
| Study characteristics | ||
| Methods | Randomised, double‐blind, placebo‐controlled, cross‐over trial of adolescent drivers with ADHD who were assessed on a driving simulator after taking:
Phases: 3 (2 relevant phases) |
|
| Participants | Number of participants screened: not stated Number of participants included: 35 (19 boys, 16 girls) Number of participants followed up: 35 Number of withdrawals: 2 Diagnosis of ADHD: DSM‐IV (combined 21 (60%), hyperactive‐impulsive 2 (6%), inattentive 12 (34%)) Age: mean 17.8 years (range 16‐19) IQ: not stated MPH‐naive: not stated Ethnicity: not stated Country: USA Setting: outpatient clinic Comorbidity: agoraphobia (2.9%), CD and marijuana abuse (2.9%), OCD (2.9%), OCD and hypomania (2.9%), nicotine dependence (5.7%) Comedication: 2 were taking no medication, 21 were taking MPH and 12 were taking amphetamine formulations at the start of the trial Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 2 relevant drug condition orders of MPH and placebo Mean MPH dosage: 72 mg/d Administration schedule: 2 overlaid capsules/tablets in blister packs each day on awakening Duration of each medication condition: 17 days Washout before trial initiation: not stated Medication‐free period between interventions: 4‐21 days between OROS‐MPH and MAS Titration period: half dose (36 mg/d OROS‐MPH) days 1‐5, full dose (72 mg/d OROS‐MPH) days 6‐17 initiated after randomisation Treatment compliance: not stated. Pill counts were completed at each trial visit |
|
| Outcomes |
Non‐serious AEs
|
|
| Notes | Sample calculation: no Ethics approval: yes Comments from trial authors
Key conclusions of trial authors
Comments from review authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: yes; excluded if any history of AEs to stimulant medications Any withdrawals due to AEs: no Funding source: supported by funding from McNeil Pediatrics, a division of McNeil‐PPC Incorporated Email correspondence with trial authors: February 2014. We received additional information from trial authors. Data from the Self‐Reported Stimulant Drug Side Effects Rating Scale are no longer available (Krogh 2013b [pers comm]). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Using a random‐numbers table, each participant was assigned" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Treatments were provided in different forms: "Participants took 2 overlaid capsules/tablets in blister packs each day on awakening". Participants and research assistants were blinded to medication conditions |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "Participants were either tested on placebo or were not required to come in for testing". Participants and research assistants were blinded to medication condition |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Email correspondence with trial author: no dropouts and all were followed up (Krogh 2013b [pers comm]) Selection bias (e.g. titration after randomisation → exclusion): none described |
| Selective reporting (reporting bias) | Low risk | Email correspondence with trial author, who stated that all planned outcome measures and analyses are described in the papers (Krogh 2013b [pers comm]) |
CRIT124US02.
| Study characteristics | ||
| Methods | An 8‐week, cross‐over trial of LA‐MPH 20‐60 mg/d with 2 arms
Phases: unknown; mention of washout in Cortese 2018 (page 362 of appendix) |
|
| Participants | Number of participants screened: not stated Number of participants included: 109 (100% girls) Number of participants followed‐up: 83 Number of withdrawals: 26 Diagnosis of ADHD: not stated (55% combined type, 0.9% hyperactive‐impulsive type and 44% inattentive type) Age: mean 13.8 years (range 12‐17) IQ: not stated MPH‐naive: not stated Ethnicity: race: white (70%), black (20%), Asian (1%), other (9%) Country: USA Setting: not stated Comorbidity: not stated Comedication: not stated Additional sociodemographics: family history of ADHD: 38% no, 60% yes, 3% unknown Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 2 different medication orders of LA‐MPH 20‐60 mg/d or placebo for 4 weeks Number randomised to each group: not stated Mean medication dosage: not stated Administration schedule: once daily Duration of each medication: 4 weeks Washout before trial initiation: mention of washout in Cortese 2018 but not clear if before or in between interventions Medication‐free period between interventions: mention of washout in Cortese 2018 but not clear if before or in between interventions Titration period: not stated Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
Serious AEs
Non‐serious AEs
|
|
| Notes | Sample calculation: 108 Ethics approval: not stated Comments from review authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH: no Any withdrawals due to AEs: no Funding source: Novartis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All capsules were over‐encapsulated in a one‐colour capsule of the same size |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropout of > 20% of the participants Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): yes, 10 withdrawals due to lack of efficacy |
| Selective reporting (reporting bias) | High risk | The results for vital signs, ECG, laboratory evaluations and physical condition and body weight are not reported. |
Douglas 1986.
| Study characteristics | ||
| Methods | 5‐day cross‐over trial with 2 interventions:
Phases
Before the trial was undertaken, 16 drug and test order combinations for the morning test battery were ordered randomly. As participants entered the trial, they were assigned to these drug‐plus‐test order combinations until all 16 participants had been assigned. The order of the 2 tests in the afternoon battery (arithmetic and word discovery) was alternated over children, and each child received tests in the same order over 5 days of testing |
|
| Participants | Number of participants screened: not stated Number of participants included: 16 (15 boys, 1 girl) Number of participants followed up: 16 Number of withdrawals: not stated Diagnosis of ADHD: DSM‐III (subtypes not stated) Age: mean 9.2 years (range 6‐11.6) IQ: mean 103.19 (range 89‐125) MPH‐naive: 13 (81%) Ethnicity: not stated Country: Canada Setting: outpatient clinic Comorbidity: not stated Comedication: not stated Other sociodemographics: children’s families varied from I to V on the Hollingshead and Redlich Index (with most families falling within level III to IV (V being the poorest) Inclusion criteria
Participants were referred to the Hyperactivity Project at Montreal Children’s Hospital by paediatricians or school personnel. Referral was based on presence of the following symptoms:
Symptoms were of sufficient severity to prompt referring physicians to consider a trial on stimulant medication Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 4 possible orders of drug (D) or placebo (P): (1) PDDPP; (2) DPPDD; (3) DDPPD; (4) PPDDP. On days when participants were assigned to active medication, they received a capsule containing the quantity of medication closest to a calculated dose of 0.3 mg/kg for each dose (morning and afternoon) Mean MPH dosage: not stated Administration schedule: morning capsule administered approximately 1 h after breakfast and 45 min before morning test battery was administered. Second capsule, identical to the morning capsule, administered before child left for lunch and returned to school. Time between morning and afternoon capsule was approximately 3½ h Duration of each medication condition: 1 day each; in all 2 or 3 days, depending on order of drugs assigned Washout before trial initiation: 24 h before screening, 48 h before first testing day Titration period: none Treatment compliance: capsule administered by examiner |
|
| Outcomes |
ADHD symptoms
Score for the Index is based on the mean of item ratings on a 4‐point scale (0‐3) |
|
| Notes | Sample calculation: no Ethics approval: yes Key conclusions of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: not stated Funding source: supported by grant number MA 6913, from the Medical Research Council of Canada Email correspondence with trial authors: July 2014. Emailed trial authors to ask for additional information. Received information on ethics approval, but first trial author was not able to provide additional data or information on the trial |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Capsule was administered by examiner |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | In a few cases in which data were missing, scores from 1 or 2 days were used to compute means for drug and placebo conditions. No information on dropout rate Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol obtained |
Douglas 1995.
| Study characteristics | ||
| Methods | 8‐day, double‐blind, cross‐over trial in which participants were randomly assigned to different doses of MPH and placebo:
Phases
|
|
| Participants | Number of participants screened: not stated Number of participants included: 17 (16 boys, 1 girl) Number of participants followed up: not stated Number of withdrawals: not stated Diagnosis of ADHD: DSM‐III‐R. Also had to meet DSM‐III criteria for ADD‐H Age: mean 9 years 5 months (range 6 years 3 months‐11 years 9 months) IQ: mean 104.3 (range 89.0‐127) MPH‐naive: 53% Ethnicity: not stated Country: Canada Setting: treatment centre (hospital) or outpatient clinic Comorbidity: ODD (71%), CD (18%) Comedication: not stated Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 24 possible drug condition orders of MPH (0.3 mg/kg, 0.6 mg/kg, 0.9 mg/kg) and placebo Mean MPH dosage: 0.3 mg/kg: 9.71; 0.6 mg/kg: 19.42; 0.9 mg/kg: 29.14 Administration schedule: once daily Duration of each medication condition: 1 day; each child received each dosage twice during the trial (i.e. during first and second assessment weeks) Washout before trial initiation: in the case of children currently receiving stimulants, medication was discontinued ≥ 48 h before screening Titration period: none Treatment compliance: to ensure compliance, medications were administered at the laboratory |
|
| Outcomes |
Non‐serious AEs
|
|
| Notes | Sample calculation: no information Ethics approval: no information Key conclusion of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: no Funding source: grants from the Medical Research Council of Canada and by William T. Grant Foundation Faculty Scholar Program Email correspondence with trial authors: October 2013. Emailed last trial author twice to get supplemental information (protocol, ethics approval, data on side effects, etc.) but received no response. Therefore we included no data from this trial |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Drug order was determined by consecutive assignment to a randomly ordered list of 24 possible combinations of 4 medication levels for each of the 2 testing weeks |
| Allocation concealment (selection bias) | Low risk | Drug order was determined by consecutive assignment to a randomly ordered list of 24 possible combinations of 4 medication levels for each of the 2 testing weeks. To maximise blindness of examiners, all participants received a different drug order for assessment weeks 1 and 2 |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Medications containing active drug and placebo were prepared in identical opaque gelatin capsules and were administered in a double‐blind fashion |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | To maximise blindness of examiners, all participants received a different drug order during assessment weeks 1 and 2 |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated whether any participants were LTFU Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified |
DuPaul 1996.
| Study characteristics | ||
| Methods | 4‐week, double‐blind, placebo‐controlled, cross‐over trial in which participants were randomly assigned to 3 doses of MPH:
|
|
| Participants | Number of participants screened: not stated Number of participants included: 24 (19 boys, 5 girls) Number of participants followed up: 24 Number of withdrawals: 0 Diagnosis of ADHD: DSM‐III‐R (subtype not stated) Age: mean 11.09 years (range 9‐15) IQ: > 70 MPH‐naive: not stated Ethnicity: white (100%) Country: USA Setting: outpatient clinic Comorbidity: ODD (21%), CD (% not reported) Comedication: not stated Other sociodemographics: children were primarily from lower‐middle class and middle class families Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 6 possible drug condition orders of low‐ (0.16 mg/kg; SD 0.08), moderate‐ (0.29 mg/kg; SD 0.11 kg) and high‐dose (0.42 mg/kg; SD 0.14) MPH and placebo Administration schedule: twice/d, morning, noon Duration of each medication condition: 1 week Washout before trial initiation: not stated Titration period: none Treatment compliance: no participant was removed from the investigation for non‐compliance (e.g. > 1 day of failure to administer medication as scheduled) |
|
| Outcomes |
ADHD symptoms
Non‐serious AEs
|
|
| Notes | Sample calculation: no information Ethics approval: Human Subjects Research Board at the University of Massachusetts Medical Center Comments from trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: no Funding source: not stated Email correspondence with trial authors: January 2013‐August 2013. Emailed first trial author regarding additional information. Not able to get all data requested, as trial author no longer has these data |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Children were randomly assigned to 1 of 6 possible orders of MPH dosage |
| Allocation concealment (selection bias) | Low risk | Medication was prepared by the hospital pharmacy in increments of 5 mg and packaged within opaque gelatin capsules |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants, their parents and teachers and the research assistant in charge of collecting data were blinded to the order of medication |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants, their parents and teachers and the research assistant in charge of collecting data were blinded to the order of medication |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Low risk | No indication of reporting bias. Analyses on all dependent measures in the trial are reported in the article |
Duric 2012.
| Study characteristics | ||
| Methods | RCT, parallel with 3 arms:
Phases: 2 (3 months with treatment in 3 arms. 6 months' follow‐up with participants continuing on MPH) |
|
| Participants | Number of participants screened: 628 Number of participants included: 86 to relevant interventions (130 in all) Number of participants followed up: MPH 30 (23 boys, 7 girls), control 30 (22 boys, 8 girls) Number of withdrawals: MPH 14, control 12 Diagnosis of ADHD: ICD‐10 (subtype not stated) Age: MPH 11.2 (SD 2.8), control 11.4 (SD 3.1) IQ: mean 87 Methyphenidate‐naive: not stated Ethnicity: not stated Country: Norway Setting: outpatient clinic Comorbidity: not stated Comedication: not stated Other sociodemographics: no significant differences in baseline demographics were noted between the 2 groups Inclusion criteria
Exclusion criteria
|
|
| Interventions | Partcipants were randomly assigned to MPH and neurofeedback (intervention group) or to neurofeedback (control group) Number of participants randomly assigned: MPH 44, control 42 MPH dosage: 20 mg daily to 60 mg daily. No placebo pill Administration schedule: twice/d Duration of intervention: 11‐13 weeks (MPH continued the MPH interventions for 6 months) Titration period: not stated Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
|
|
| Notes | Ethics approval: yes Comments from trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: not stated Funding source: the Child and Adolescent Psychiatry Department of Helse Fonna Hospital Haugesund, Helse Fonna Trust Haugesund, Norway Email correspondence with trial authors: March‐June 2013. Emailed first trial author. All questions were answered. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Children with ADHD were randomly placed into 3 groups |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding, as it is not a 'pure' placebo group |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of parents |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Low risk | Outcomes reported according to protocol |
Döpfner 2004.
| Study characteristics | ||
| Methods | Randomised, double‐dummy, double‐blind, cross‐over, multi‐centre trial with 3 interventions:
Phases: trial was subdivided into 5 stages: pre‐screening, run‐in phase (duration: 1 workday), trial phases 1 and 2 (duration in each case: 4 workdays plus weekend) and trial phase 3 (duration: 4 workdays). Participants also received a behavioural therapy intervention and social skills training at school. |
|
| Participants | Number of participants screened: not stated Number of participants included: 82 Number of participants followed up: 79 (71 boys, 8 girls) Number of withdrawals: 3 Participants followed up Diagnosis of ADHD: DSM‐IV or ICD‐10 (combined (92.4%), hyperactive‐impulsive (0%), inattentive (7.6%)) Age: mean 10 years (range 6 to 16) IQ: 103 ± 10.4 MPH‐naive: 0% Ethnicity: not stated Country: Germany Setting: outpatient clinic Comorbidity: ODD/CD (44%) Comedication: not stated Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to different orders of IR‐MPH, ER‐MPH and placebo Mean MPH dosage: 22 mg ± 6 mg. Dosage was identical in MPH groups but did not exceed 1 mg/kg body weight. Thus, 9 (11%) participants received a daily dose of 10 mg, 54 (68%) received 20 mg, 14 (17%) received 30 mg and 2 (3 %) received 40 mg Administration schedule: 9:00 am and 1:00 pm Duration of each medication condition: 4 days, and for trial phase 1 + 2 (also weekends) Washout before trial initiation: none Medication‐free period between interventions: none Titration period: had to be oriented to the optimum individual dosage previously determined in clinical treatment trials initiated before randomisation Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
Non‐serious AEs
|
|
| Notes | Sample calculation: not stated Ethics approval: approved by local university ethics committees Comment from trial authors
Key conclusions of trial authors
Comments from review authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH: yes; included only MPH responders Any withdrawals due to AEs: no Funding source: conducted and sponsored by MEDICE Arzneimittel Pütter GmbH & Co. KG as part of the drug approval process for Medikinet‐Retard Email correspondence with trial authors. We received some data from trial authors in July 2013. We sent 2 additional emails to different trial authors to request data in July 2014 but received no answer. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Order in which participants were allocated to respective treatment arms was randomly assigned |
| Allocation concealment (selection bias) | Unclear risk | Order in which participants were allocated to respective treatment arms was randomly assigned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | To guarantee a double‐blind trial, the double‐dummy method was used (i.e. participants took the capsule once/d and the tablets twice daily). Only 1 of the 2 galenical forms contained the active substance; the other form contained placebo. In the placebo group, participants took both placebo capsules and placebo tablets |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol obtained |
Epstein 2011.
| Study characteristics | ||
| Methods | Cross‐over trial with 2 interventions 3 preliminary phases to determine optimal dose followed by 2‐phase trial of the following:
|
|
| Participants | Number of participants screened: not stated Number of participants included: not clear Number of participants followed up: 93 (numbers of boys and girls: not stated) Number of withdrawals: not clear Diagnosis of ADHD: DSM‐IV (combined (n = 45), hyperactive‐impulsive (n = 48), inattentive (n = 0)) Age: mean 8.1 years (range 7‐11) IQ: mean 105.58 MPH‐naive: 100% Ethnicity: white (75%), African American (22%), other (3%) Country: USA Setting: outpatient clinic Comorbidity: ODD (n = 34), CD (n = 4), anxiety disorders (n = 31), mood disorders (n = 2) Comedication: not stated Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to an optimal dose of MPH and placebo Mean MPH dosage: 1.13 mg/kg Administration schedule: testing 1‐4 h after medication ingestion Duration of each medication condition: 1 week Washout before trial initiation: no apparent washout Titration period: each of the 3 doses was trialled for 1 week before random assignment to identify an optimal dose Treatment compliance: not reported |
|
| Outcomes |
ADHD symptoms
Non‐serious AEs
|
|
| Notes | Sample calculation: not stated Ethics approval: not stated Comments from trial authors
Key conclusion of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: not stated Funding source: NIH and NIMH Email correspondence with trial authors: April 2014. We were able to obtain supplemental information regarding data (Storebø 2015b). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Double‐blind"; capsules were "identical" (p 2, p 1063) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Double‐blind", capsules were "identical" (p 2, p 1063) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not clear whether any participants were LTFU Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Low risk | No indication of selective reporting |
Fabiano 2007.
| Study characteristics | ||
| Methods | Randomised, double‐blind, within‐participant design, cross‐over trial with 2 interventions:
Phases: 0.15 mg/kg, 0.30 mg/kg and 0.60 mg/kg. Medication was randomly assigned for each child and varied daily during a 9‐week summer treatment programme |
|
| Participants | Number of participants screened: not stated Number of participants included: 48 (44 boys, 4 girls) Number of participants followed up: 47 Number of withdrawals: 1 Diagnosis of ADHD: DSM‐IV (subtype not stated) Age: mean 9.35 years (SD 1.98, range 5‐12) IQ: 106.33 (SD 14.61) MPH‐naive: not stated Ethnicity: white (79%); African American (12.5%); Hispanic, Native American or mixed race (8.5%) Country: USA Setting: outpatient clinic (summer treatment programme) Comorbidity: not stated Comedication: not stated Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 3 possible drug condition orders of IR‐MPH (0.15 mg/kg, 0.30 mg/kg and 0.60 mg/kg) and placebo Mean MPH dosage: 5.03 mg (range 2.5‐10), 10.8 mg (range 5‐20) and 21 mg (range 12.5‐30) Administration schedule: 3 times daily (7:45 am, 11:45 pm and 3:45 pm) Duration of each medication condition: varied daily Washout before trial initiation: none Titration period: none Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
General behaviour
Non‐serious AEs
|
|
| Notes | Sample calculation: no Ethics approval: yes Comments from trial authors
Comments of review authors
Inclusion of MPH responders only/exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: yes Any withdrawals due to AEs: yes, 1 (withdrawn by parents due to concern about side effects and not included in the analysis) Funding source: NIMH grant MH62946 Email correspondence with trial authors: April 2014. We obtained supplemental information regarding risk of bias. No further information was received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation and allocation concealment were completed with a random number generator by a researcher not involved in treatment |
| Allocation concealment (selection bias) | Low risk | Randomisation and allocation concealment were completed with a random number generator by a researcher not involved in treatment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Medication assessment procedure was double‐blinded" (p 201) "children, their parents, and all clinical staff members were blinded to medication condition" (p 202) Medication was prepared in opaque capsules by a pharmacist not otherwise involved in the trial. It was administered to children by research staff who were not involved in administration of behavioural treatment nor in daily activities |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Observers and raters were blinded to medication conditions |
| Incomplete outcome data (attrition bias) All outcomes | High risk | One child's parents elected to stop medication for the child after 2 days because of their concerns about possible side effects of the medication. This child was not included in the analyses Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol/design was published |
Findling 2006.
| Study characteristics | ||
| Methods | 3‐week, randomised, double‐blind, parallel trial with 3 arms:
|
|
| Participants | Number of participants screened: 346 Number of participants randomly assigned: 327 Number of participants included: 318; IR‐MPH 133, MR‐MPH (EqXL) 139, placebo 46 Number of participants followed up: IR‐MPH 120, MR‐MPH (EqXL) 120, placebo 39 (number in each arm is only per protocol population) Number of withdrawals: not stated Diagnosis of ADHD: DSM‐IV (combined (71%), hyperactive‐impulsive (6%), inattentive (23%)) Age: mean 9.5 years (range not reported) IQ: above 80 Sex: 252 boys, 66 girls MPH‐naive: 0% Ethnicity: white (86%), African Caribbean (5.2%), Asian (0.3%), Hispanic (1.6%), other (6.9%) Country: Australia, Canada, USA Setting: outpatient clinic Comorbidity: not stated Comedication: not stated Other sociodemographics: none. No significant differences in baseline demographics were noted between the 2 groups Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to IR‐MPH, ER‐MPH (EqXL) or placebo Mean MPH dosage: not stated Administration schedule: twice daily, morning and lunch Duration of intervention: 3 weeks Titration period: all participants were stable while taking MPH medication before randomisation Treatment compliance: not stated. Children with a previous total daily dose of 10 mg‐20 mg IR‐MPH or 20 mg ER‐MPH were randomly assigned to receive 10 mg IR‐MPH twice daily, 20 mg MR‐MPH (EqXL) once daily or placebo; children given a previous total daily dose of 25 mg‐40 mg IR‐MPH or > 20 mg to < 40 mg ER‐MPH were randomly assigned to receive 20 mg IR‐MPH twice daily, 40 mg MR‐MPH (EqXL) once daily or placebo; children given a previous total daily dose > 40 mg IR‐MPH or < 40 mg ER‐MPH were randomly assigned to receive 20 mg IR‐MPH twice daily, 60 mg MR‐MPH (EqXL) once daily or placebo |
|
| Outcomes |
ADHD symptoms
Serious AEs
General behaviour
Non‐serious AEs
|
|
| Notes | Sample calculation: yes Ethics approval: yes; independent ethics committee at each clinical site before trial initiation Key conclusion of trial authors
Comment from review authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: yes Any withdrawals due to AEs: yes, 14 placebo, 4 MPH‐IR, 3 Equasym Funding source: provided by Celltech Americas Incorporated, currently part of UCB (Union Chimique Belge) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised" but did not state how |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both MR‐MPH (EqXL) capsules and IR‐MPH tablets were over‐encapsulated in hard gelatin capsules identical to the placebo capsule |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Stated LOCF but primary efficacy population was the per protocol population, defined as participants who received trial treatment and had ≥ 1 efficacy measurement after the first dose Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol/design was published |
Findling 2007.
| Study characteristics | ||
| Methods | 4‐week, double‐blind, randomised, placebo‐controlled, cross‐over trial with 4 interventions (MPH at 3 different doses, in the morning and at midday):
Phases Participants were assigned to receive, at random, 1 of 6 possible dosing orders that included the following:
Schedules were designed in such a way that participants did not receive the 15 mg dose before the 10 mg dose, so in the event that a participant experienced AEs while taking a lower dose, the 15 mg dose was not administered |
|
| Participants | Number of participants screened: not stated Number of participants included: 20 Number of participants followed up: 16 (12 boys, 4 girls) Number of withdrawals: 4 Demographic data regarding the 16 who completed the trial: Diagnosis of ADHD: DSM‐IV (combined (94%), inattentive (6%)) Age: mean 10.43 years (range 5‐17) IQ: > 70 MPH‐naive: not stated Ethnicity: white (75%), African American (6%), Hispanic (19%) Country: USA Setting: outpatient clinic Comorbidity: bipolar (100%), ODD (50%), CD (25%), enuresis (12.5%), encopresis (12.5%) Comedication: 100% divalproex sodium. Some received clonidine for sleep at night Other sociodemographics: none. No statistically significant differences in distribution based on sex, ethnicity, age group, rate/proportion of comorbid ODD or comorbid CD were found between 6 dosing order groups Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 6 possible drug condition orders of MPH (5 mg twice/d, 10 mg twice/d, 15 mg twice/d) and placebo Mean MPH dosage: not provided, as the trial compares MPH at different doses Administration schedule: morning and midday Duration of each medication condition: 1 week Washout before trial initiation: not stated Medication‐free period between interventions: between lunchtime dose and morning dose the following day Titration period: none. Dosing schedules were designed in such a way that patients did not receive the 15 mg dose before the 10 mg dose, so in the event that a participant experienced AEs while on a lower dose, the 15 mg dose was not administered Treatment compliance: 1 of 20 screened participants was withdrawn from the trial because of poor compliance. Among the 16 who participated, no compliance issues were reported |
|
| Outcomes |
ADHD symptoms
General behaviour
Non‐serious AEs
|
|
| Notes | Sample calculation: yes Ethics approval: yes Comments from trial authors (limitations)
Key conclusion of trial authors
Comment from review authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: yes; 2 Funding source: Stanley Medical Research Institute Email correspondence with trial authors: November 2013. We wrote to the trial author twice to request a copy of the protocol and information about sample size and allocation concealment but have not received a response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random numbers table was generated and was used by a pharmacist to assign dose orders to participants. Counterbalancing was applied in such a way that as each dose order was used, its number was eliminated from the next dose order assignment. |
| Allocation concealment (selection bias) | Low risk | Placebo and MPH in identical capsules. No participants were discontinued from this trial because of broken integrity of the blind. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | At the conclusion of the 4‐week trial, trial physician, participant and participant's guardian determined a "best dose week", taking into consideration behaviour ratings and reports of any AEs. After all assessments had been completed, the trial blind was broken to reveal the dose that had been prescribed during the previously identified best dose week. No participants were discontinued from this trial because of broken integrity of the blind. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "After all of the assessments had been completed, the study blind was then broken to reveal the dose that had been prescribed during the previously identified 'best dose week'" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data present Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified |
Findling 2008.
| Study characteristics | ||
| Methods | 7‐week randomised, phase III, double‐blind, multi‐centre, parallel‐group, placebo‐controlled, naturalistic home and school trial with 3 arms:
5‐week titration phase, 2‐week maintenance phase |
|
| Participants | Number of participants screened: not stated Number of participants included: 282 (187 boys, 95 girls) Number of participants randomly assigned (and administered ≥ 1 dose of trial medication): MPH transdermal system 100, OROS‐MPH 94, placebo 88 Number of participants followed up: MPH transdermal system 71, OROS‐MPH 66, placebo 32 Number of withdrawals: MPH transdermal system 27, OROS‐MPH 25, placebo 53 Diagnosis of ADHD: DSM‐IV‐TR (combined (80.5%), hyperactive‐impulsive (1.4%), inattentive (17.0%), unclassified (1.1%)) Age: mean 8.8 years (range 6‐12). MPH transdermal system 8.9, OROS‐MPH 8.8, placebo 8.5 IQ: ≥ 80 MPH‐naive: 86% Ethnicity: white (77.3%), African American (14.5%), Asian (0.7%), Hispanic (not stated), other (7.4%) Country: USA Setting: outpatient clinic Comorbidity: not stated Comedication: not stated Other sociodemographics: no significant differences in baseline demographics (age, sex, ethnicity, ADHD subtype, prior ADHD medication use) among the 3 groups Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to MPH patch, OROS‐MPH or placebo Titration period: 5 weeks (after randomisation) of optimisation to 1 of 4 total daily dosage strength. OROS‐MPH: 18 mg, 27 mg, 36 mg and 54 mg. MPH transdermal system and placebo transdermal system: 10 mg, 15 mg, 20 mg and 30 mg over a 9‐h period in patches of 12.5 cm², 18.75 cm², 25 cm² and 37.5 cm², respectively Administration schedule: treatments were administered at approximately 7:00 am each morning; patches were applied to the hip area and were worn for approximately 9 h daily, different hip each day Mean patch wear time: 8.70 (0.51) to 9.46 (0.53) h Duration of intervention: 7 weeks Washout before trial initiation: up to 28 days if applicable Treatment compliance: mean compliance 97% to 99% during both trial phases |
|
| Outcomes |
ADHD symptoms
Non‐serious AEs
|
|
| Notes | Sample calculation: yes (258 participants) Ethics approval: yes Comments from trial authors
Key conclusions of trial authors
Inclusion of MPH responders only/exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: yes. An inclusion criterion is that participants are stimulant‐naive or stimulant‐responsive Any withdrawals due to AEs: yes; 11 Funding source: Shire Development Incorporated, Wayne, Pennsylvania Email correspondence with trial authors. June‐September 2013. We attempted to obtain supplemental efficacy and safety data from trial authors but without success |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers schedule |
| Allocation concealment (selection bias) | Low risk | Computer‐generated random numbers schedule |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, double‐dummy: participants received both a patch and a capsule to be administered each day. MPH and placebo capsules were over‐encapsulated to blind the identity of the capsule’s content |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT, LOCF Selection bias: yes; non‐responders excluded during the trial. Participants who did not reach an acceptable condition by the final dose‐optimisation visit (week 5) were withdrawn from the trial |
| Selective reporting (reporting bias) | Low risk | Outcomes reported according to protocol |
Findling 2010.
| Study characteristics | ||
| Methods | Phase IIIB, randomised, double‐blind, parallel‐group, placebo‐controlled, multi‐centre, dose‐optimisation trial evaluating the efficacy and safety of the following in adolescents 13‐17 years of age with ADHD:
Double‐blind RCT consisted of 4 experimental periods
Open‐label extension follow‐up consisted of an open‐label extension trial conducted to evaluate the safety and efficacy of the MPH transdermal system (10 mg, 15 mg, 20 mg or 30 mg/9‐h patches) for participants who completed all required trial visits; consisted of 3 experimental periods |
|
| Participants |
Double‐blind RCT Number of participants screened: not stated Number of participants included: 217 (162 boys, 55 girls) Number of participants randomly assigned: MPH 145, placebo 72 Number of participants followed up (ITT population): MPH 143, placebo 72 Number of withdrawals: MPH 2, placebo 0 Number that completed 7‐week dose‐optimisation/dose‐maintenance phase: MPH 95, placebo 72 Diagnosis of ADHD: DSM‐IV (types not stated) Age: mean 14.6 years (SD 1.3, range 13‐17) IQ: ≥ 80 MPH‐naive: 122 (56%) Ethnicity: white (77%), African American (18%), Asian (0.5%), other (4.5%) Country: USA Setting: outpatient clinic Comorbidity: 0% Comedication: not stated Other sociodemographics: no significant difference in baseline demographics were noted between the 2 groups Open‐label extension (safety measures) Number of participants included: 163 (previously taking MPH 110, placebo 53) Number of participants followed up: 162 (121 boys, 41 girls) Number of withdrawals: 1 Diagnosis of ADHD: DSM‐IV (types not stated) Age: 14.5 years (SD 1.24, range 13‐17) IQ: ≥ 80 MPH‐naive: 122 (56%) Ethnicity: white (78%), African American (17%), Asian (0.6%), other (4.4%) Country: USA Comorbidity: 0 % Comedication: not stated Other sociodemographics: none Inclusion criteria
Open‐label extension (6 months):
Exclusion criteria
Open‐label extension (6 months):
|
|
| Interventions | Participants were randomly assigned to MPH transdermal (patches) or placebo Mean MPH dosage at week 7: 10 mg (4.2%), 15 mg (16.7%), 20 mg (24.0%), 30 mg (55.2%); median exposure time: 48 days (range 4‐57) Administration schedule: single patch in the morning, once daily for 9 h Duration of intervention: 7 weeks Titration period: 5 weeks, initiated after randomisation Treatment compliance: 124 fulfilled the protocol. However, it is not stated in the article how compliance regarding the medication had to be assessed to fulfil the protocol Mean MPH dosage at month 6: 10 mg (5.6%), 15 mg (7.9%), 20 mg (32.6%), 30 mg (53.9%); median exposure time: 168 days (range 3‐200) Administration schedule: single patch in the morning, once daily for 9 h Duration of intervention: 6 months Titration period: 5 weeks of the 6 months Treatment compliance: not stated. 88 fulfilled the protocol. However, it is not stated in the article how compliance regarding the medication had to be assessed to fulfil the protocol |
|
| Outcomes |
7‐week parallel trial (Double‐blind RCT) ADHD symptoms
Serious AEs
Non‐serious AEs
The investigator determined the clinical significance of any physical examination, height or vital sign measurement that was outside the normal range. Clinically significant deviations from measurements recorded at screening were reported as AEs. A 12‐lead ECG evaluation was obtained at screening, at baseline, at week 5 and at the final double‐blind trial visit. Dermal skin reaction: Dermal Scale was used to evaluate observed skin findings: range 0‐7, where 0 shows no evidence of irritation Regarding 6‐month open‐label extension:
Changes noted between evaluation data at trial entry and data obtained at schedule visits deemed to be clinically significant by the investigator were considered an AE |
|
| Notes | Sample calculation: yes. By using 85% power to detect an effect size of 0.5 between active treatment and placebo at the significance level of 5%, it was estimated that 112 participants were needed for MPH transdermal system groups and 56 for placebo transdermal system groups. Assuming a 20% dropout rate, ~ 210 participants (MPH transdermal system 140, placebo transdermal system 70) were required for the trial Ethics approval: yes Comments from trial authors
Key conclusions of trial authors
Comments from review authors
Inclusion of MPH responders only/exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: yes, participants were excluded if they had a history of being non‐responsive to psychostimulant treatment Any withdrawals due to AEs: yes, 10 (2 in placebo group, 8 on MPH group) Fnding source: Shire Development Incorporated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were allocated the next sequential randomisation number and received treatment that corresponded with that randomised number as given by an interactive voice response system |
| Allocation concealment (selection bias) | Low risk | Randomisation schedule was produced by computer software that incorporated a standard procedure for generating random numbers |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, but no information on MPH and placebo blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Efficacy analyses were performed on the ITT population, defined as all randomly assigned participants who received ≥ 1 dose of trial medicine and ≥ 1 post‐baseline primary efficacy assessment. Safety population was defined as all randomly assigned participants who received ≥ 1 dose of trial medicine. Selection bias (e.g. titration after randomisation → exclusion): yes. No further dose titration was permitted for any participant after week 5, and participants who had not reached an acceptable response by the end of week 5 were withdrawn from the trial |
| Selective reporting (reporting bias) | Low risk | No indication of selective reporting. Protocol registered 12 July 2007. First participant consent was obtained 29 August 2007 for the open‐label trial |
Fine 1993.
| Study characteristics | ||
| Methods | 3‐week double‐blind, cross‐over trial with 2 interventions:
Phases: medical trial or typical clinical procedure, concluded with recommendation for treatment, follow‐up (6 weeks and 3 months) |
|
| Participants | Number of participants screened: 24 who were randomly assigned: 12 "typical clinical procedure", 12 "medical trial" Number of participants included: 12 (sex not stated). Participants in the medical trial group were randomly assigned to different possible drug condition orders Number of participants followed up: 12 Number of withdrawals: 0 Diagnosis of ADHD: DSM‐III‐R (subtype not stated) Age: mean 101.58 months (approximately 8.5 years) (range 6‐10 years) IQ: not stated MPH‐naive: 12 Ethnicity: not stated Country: Canada Setting: outpatient clinic Comorbidity: not stated Comedication: not stated Other sociodemographics: (socioeconomic status calculated using Blishen Index; lower score indicates higher socioeconomic status): medical trial: mean 3.42 (SD 0.90); typical clinical procedure: mean 3.50 (SD 1.88) Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants in the medical trial group were randomly assigned to different possible drug condition orders of 0.3 mg/kg and 0.6 mg/kg MPH and placebo Mean MPH dosage: not stated Administration schedule: twice/d Duration of each medication condition: "the different interventions were randomly assigned across days" Washout before trial initiation: not stated Medication‐free period between interventions: not stated Titration period: not stated Treatment compliance: missed pills mean 1.92 (SD 1.44). Furthermore, compliance was assessed by urine test. By 6‐week follow‐up, parent‐reported compliance was 83%; by 3 months, it was 73% |
|
| Outcomes |
ADHD symptoms
Non‐serious AEs
|
|
| Notes | Sample calculation: not stated Ethics approval: not stated Comments from trial authors
Key conclusions of trial authors
Comments from review authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: no Funding source: CIBA‐GEIGY Canada Email correspondence with trial authors: June 2014. Emailed trial authors for supplemental information and received the following response: "I did look at the questions, but unfortunately, given how long ago the study was conducted, I do not still have the data necessary to answer" (Krogh 2014b [pers comm]) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 24 children were matched in pairs on sex and age and then were randomly assigned to the medication trial or to the typical clinical procedure. Higher and lower doses of MPH and placebo were randomly assigned across days by the hospital pharmacy |
| Allocation concealment (selection bias) | Low risk | Higher and lower doses of MPH and placebo were randomly assigned across days by the hospital pharmacy. Active medication and placebo were packaged in capsule form to disguise taste and visual differences, and were dispensed in envelopes for daily use |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Parents, teacher, child and resident were blinded to the daily medication status of the child. "Active medication and placebo were packaged in capsule form to disguise taste and visual differences, and were dispensed in envelopes for daily use". All evaluations were conducted by psychiatric residents blinded to the hypotheses of the trial |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Parents, teacher, child and resident were blinded to the daily medication status of the child. All evaluations were conducted by psychiatric residents blinded to the hypotheses of the trial |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Across 12 children, ratings were available for an average of 2.58 placebo days, 1.33 LD‐MPH days and 1.75 HD‐MPH days. Ratings for each child were averaged across each of the 3 treatment conditions. Data for all participants were available for measures of missed pills and appointments. 1 participant was missing on measures of parent‐reported compliance at 6‐week follow‐up, and 2 participants at 3‐month follow‐up. 3 participants lacked data for the number of missing envelopes. 4 participants did not provide urine for analysis, and 4 were missing physician reports of compliance (6‐week and 3‐month follow‐ups). As the result of intervening school holidays, teacher reports of compliance are available for only 16 children (8 in each condition) at each follow‐up.
MPH non‐responders were not excluded. Did not state the method used to account for missing outcome data Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol was identified |
Firestone 1981.
| Study characteristics | ||
| Methods | 3‐month, randomised, double‐blind, placebo‐controlled, parallel trial, wherein participants were randomly assigned to 3 arms:
|
|
| Participants | Number of participants screened: 91 Number of participants included: not stated (includes boys and girls) Number of participants followed up: intervention 18, control 13 Number of withdrawals: not stated Diagnosis of ADHD: DSM‐III Age: mean 7.32 years (range 5‐9) MPH‐naive: not stated Ethnicity: not stated Country: not stated Setting: outpatient clinic Comorbidity: not stated Comedication: not stated IQ: 116 Other sociodemographics: all children living at home with ≥ 1 parent. No significant differences in age and IQ between treatment groups. No data on remaining parameters Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to MPH + parent training (intervention group) or to placebo + parent training (control group). After the titration period MPH was given only on school days Average MPH dosage: 22 mg/d (range 10 mg/d‐30 mg/d) Titration period: first 3‐4 weeks: MPH was titrated (after randomisation), starting with 5 mg twice/d (morning, noon), 7 days a week Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
|
|
| Notes | Ethics approval: no information Key conclusions of trial authors
Comment from review authors
Inclusion of MPH responders only/exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: yes, 4 participants responded adversely and were dropped from the trial Funding source: Ministry of Health |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Children were randomly assigned; no description of how |
| Allocation concealment (selection bias) | Unclear risk | No data |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Parents, teachers, therapists and those testing the children were unaware of medication conditions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Parents, teachers, therapists and those testing the children were unaware of medication conditions |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No data Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): none |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified |
Fitzpatrick 1992a.
| Study characteristics | ||
| Methods | Cross‐over trial with 4 interventions
Phases: 4 pharmaceutical conditions, each lasting 2 weeks (including weekends), with different dosage schedules based on weight of child and type of intervention |
|
| Participants | Number of participants screened: not stated Number of participants included: 19 (17 boys, 2 girls) Number of participants followed up: not stated Number of withdrawals: not stated Diagnosis of ADD: DSM‐III during Diagnostic Instrument for Childhood and Adolescence (ADD with hyperactivity 16/19) (ADD without hyperactivity 3/19) Age: mean 8.71 years (SD 1.33, range 6.9 to 11.5) IQ: 114.11 (SD 13.34) MPH‐naive: 18 Ethnicity: not stated Country: USA Setting: outpatient clinic Comorbidity: oppositional disorder (n = 12), oppositional + CD (n = 1), enuresis (n = 2), encopresis (n = 2), phobia (n = 1), overanxious (n = 1), adjustment disorder (n = 1) Comedication: not stated Other sociodemographics: middle class (Hollingshead 4‐factor mean 38.11, SD 13.18) Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 24 (3 MPH and 1 placebo) possible drug condition orders of ER‐MPH (SR), standard MPH (SA), MPH combination and placebo Mean MPH dosage: SRSA‐MPH 17.1 mg (0.56 mg/kg), SASR‐MPH 20 mg (0.67 mg/kg), combination 11.8 mg SA MPH + 20 mg SR MPH (0.38 mg/kg SA, 0.67 mg/kg SR) Administration schedule: ER‐MPH, mornings (8:00 am) daily, SA MPH morning (8:00 am) and noon daily Duration of each medication condition: 2 weeks Washout before trial initiation: not stated Medication‐free period between interventions: not stated Titration period: not stated Treatment compliance: parents phoned weekly to encourage compliance. School nurse contacted at the beginning of each individual's participation to promote co‐operation and to check on compliance |
|
| Outcomes |
ADHD symptoms
General behaviour
Non‐serious AEs
|
|
| Notes | Sample calculation: not stated Ethics approval: not stated Key conclusions of trial authors
Comment from review authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: not clear Any withdrawals due to AEs: not clear Funding source: National Institute of Mental Health (NIMH) grant MH38118 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Double‐blind trial consisting of 4 pharmacological conditions, each lasting 2 weeks and ordered according to a Latin square (i.e. not randomly assigned) |
| Allocation concealment (selection bias) | High risk | Not randomised |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind. Blindness was maintained by administering placebo tablets consisting of the vehicle for the SA and SR preparations, respectively, as appropriate for each condition |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Data for 6 participants on Paired Associate Learning Test excluded, as they could not read well, but this was not 1 of our outcomes Selection bias (e.g. titration after randomisation): no, but because of emergent side effects, reductions of 2.5 mg SA MPH per dose were performed blindly for 1 participant in the combined condition. Similarly, blinded (but sham) adjustments of SA placebo were made for 2 participants in the placebo phase and for 1 in the SR condition |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified |
Flapper 2008.
| Study characteristics | ||
| Methods | 4‐week, double‐blind, randomised, placebo‐controlled, cross‐over trial of MPH and placebo with weekly switches of 4 dosage levels; capsules of:
MPH‐sensitive children continued in an open‐label trial for 4 weeks |
|
| Participants | Number of participants screened: 80 Number of participants included: 30 (22 boys, 8 girls) Number of participants followed up: 30 Number of withdrawals: 0 Diagnosis of ADHD: DSM‐IV (combined (37%), hyperactive‐impulsive (7%), inattentive (57%)) Age: mean 105.2 months (approximately 8.8 years) (SD 25.1, range 7‐12 years) IQ: > 70 MPH‐naive: 100% Ethnicity: not stated Country: the Netherlands Setting: outpatient clinic Comorbidity: developmental co‐ordination disorder (100%) Comedication: not stated Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to possible drug orders of the 3 daily doses of MPH (0.5 mg/kg, 0.75 mg/kg, 1 mg/kg) and placebo Mean optimal dosage: 0.66 mg/kg/d (SD 0.22) Administration schedule: twice/d Duration of each medication condition: 1 week Washout before trial initiation: none. All were medication‐naive Medication‐free period between interventions: none Titration period: none Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
|
|
| Notes | Sample calculation: yes Ethics approval: procedures were performed in accordance with the ethical standards of the University Medical Centre, University of Groningen Comment from trial authors
Key conclusions of trial authors
Comment from review authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: no Funding source: none (no funding was available). This double‐blind placebo‐controlled trial of MPH was performed as a clinical treatment program as best clinical practice to determine the effects of MPH and optimal dose compared with placebo Email correspondence with trial authors: August 2013‐January 2014. We obtained supplemental information regarding participant demographics and efficacy data |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random order by pharmacist |
| Allocation concealment (selection bias) | Low risk | Medication codes were broken at time 2 (endpoint) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Parents, teachers, children and paediatrician were kept blinded to the child’s drug condition and dosage level |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Parents, teachers, children and paediatrician were kept blinded to the child’s drug condition and dosage level |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participant withdrew; no ITT was needed. 7 children whose ADHD symptoms were not sensitive enough to MPH (effect < 25%) were excluded from the 4‐week open‐label period. However, as we are using in this review only data from the preceding cross‐over period, exclusion of these children does not cause high risk of bias Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Low risk | No indication of reporting bias |
Forness 1992.
| Study characteristics | ||
| Methods | Double‐blind, placebo‐controlled, cross‐over trial with 2 interventions:
Phases: 4 |
|
| Participants | Number of participants screened: 82 Number of participants included: 71 (all boys) Number of participants followed up: CTRS (n = 47), CPRS (n = 69) Number of withdrawals: CTRS 33.8%, CPRS 12.6% Diagnosis of ADHD: DSM‐III‐R (hyperactive‐impulsive (100%)) Age: mean 9.3 years (range 7‐11) IQ: mean 106.2 (range 67‐137) MPH‐naive: 100% Ethnicity: ethnic minority (11.3%) Country: USA Setting: outpatient clinic Comorbidity: CD (30/71) Comedication: no; had no psychotropics for a month before Other sociodemographics: all participants were middle to upper class Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to different possible drug condition orders of 0.3, 0.6 and 1.0 mg/kg MPH (unless dosages would exceed 20 mg, so LD would be 0.15 mg/kg) 3 times/d and placebo Mean MPH dosage: not stated Administration schedule: 3 times/d – 7:30 am, 11:30 am, 3:30 pm Duration of each medication condition: 7 days Washout before trial initiation: not stated Titration period: none Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
|
|
| Notes | Sample calculation: not stated Ethics approval: not stated Key conclusion of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: unclear Funding source: NIMH grant MH38686 Email correspondence with trial authors. Emailed trial authors to ask for additional information but have not received a reply |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Reported as double‐blind but not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Reported as double‐blind but not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated Selection bias (e.g. titration after randomisation → exclusion): no, but high loss to follow‐up for teachers' scores on CTRS Washout period between medication conditions: unclear |
| Selective reporting (reporting bias) | Unclear risk | Not protocol identified |
Froehlich 2011.
| Study characteristics | ||
| Methods | Randomised, double‐blind, placebo‐controlled, cross‐over trial of multiple MPH doses in stimulant‐naive school‐aged children Phases: 3 active dosage weeks and 1 week of placebo |
|
| Participants | Number of participants screened: 162 Number of participants included: 105 Number of participants followed up: 89 (65 boys, 24 girls) Number of withdrawals: 16 Diagnosis of ADHD: DSM‐IV (combined (48%), hyperactive‐impulsive (0%), inattentive (52%)) Age: mean 8.13 years (range 7‐11) IQ: mean: 105.34 ± 12.65 MPH‐naive: 100% Ethnicity: white (79%), African American (18%), Hispanic (2%), other (1%) Country: USA Setting: outpatient clinic Comorbidity: anxiety disorder (17%), mood disorder (2%), disruptive behaviour disorder (36%) Comedication: not stated Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 6 possible drug condition orders of OROS‐MPH (18 mg, 27 mg, 36 mg for children ≤ 25 kg; 18 mg, 36 mg or 54 mg for children > 25 kg; sample mean maximum dose 1.57 mg/kg/d) and placebo Administration schedule: once daily Duration of each medication condition: 1 week Washout before trial initiation: drug‐naive Medication‐free period between interventions: not stated Titration period: none Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
|
|
| Notes | Sample calculation: yes Ethics approval: yes; Cincinnati Children’s Hospital Institutional Review Board Comments from trial authors
Key conclusions of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: no Funding source: NIMH and Cincinnati Children’s Hospital Center for Education and Research Therapeutics Award Email correspondence with trial author: October/November 2013. Contacted trial author to obtain mean and SD for ADHD symptoms. Never received additional data. Therefore no further email correspondence regarding allocation concealment, randomisation, etc. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned to different drug orders. No description about how |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Trial medication consisted of identical capsules filled with an inert white powder (placebo) or prescribed dose of Concerta over‐encapsulated to preserve double‐blind Double‐blind, but not specified who was blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Trial medication consisted of identical capsules filled with an inert white powder (placebo) or prescribed dose of Concerta over‐encapsulated to preserve double‐blind Double‐blind, but not specified who was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data provided on 89 participants who completed the trial Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Unclear risk | Not able to find protocol or trial identifier |
Froehlich 2018.
| Study characteristics | ||
| Methods | A 4‐week cross‐over trial with 4 arms
Phases: 1 |
|
| Participants | Number of participants screened: 194 met inclusion criteria Number of participants included: 171 (122 boys, 49 girls) Number of participants followed‐up: 168 (154 for sleep outcomes) Number of withdrawals: 3 (found from table on ClinicalTrials.gov) (14 further for sleep outcomes) Diagnosis of ADHD: DSM‐IV (45 combined, 0 hyperactive‐impulsive and 126 inattentive) Age: 8.4 (SD 1.3, range 7‐11) IQ: inattentive‐type: 107.8 (SD 13.3), combined‐type: 105.6 (SD 12) MPH‐naive: 100% Ethnicity: white (n = 139), black (n = 21), Hispanic/Latino (n = 4), other (n = 3) Country: USA Setting: outpatient Comorbidity: comorbid ODD, CD, depression, and anxiety disorders were allowed unless determined to be the primary cause of ADHD symptomatology or necessitating different treatment. Anxiety disorder = 2, mood disorder = 2, disruptive behavior disorder = 45 Comedication: no medication for psychological or psychiatric problems Additional sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 6 different medication orders, of 3 different medication weeks of OROS‐MPH (Concerta) at a high, medium and low dose, and 1 week of placebo. Number randomised to each group: not stated Mean medication dosage: 18 mg, 27 mg, 36 mg for children ≤ 25kg; 18 mg, 36 mg or 54 mg for children > 25kg; sample mean maximum dose = 1.57 mg/kg/d) Administration schedule: not stated Duration (of (each) medication): 3 weeks of OROS‐MPH (Concerta), 1 week at each dose, 1 week of placebo Washout before trial initiation: not applicable, all were MPH‐naive Medication‐free period between interventions: not stated Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
Data on ADHD symptoms were not reported in a way that could be used for this review. Trial authors were contacted, but no data were received. Serious AEs
Non‐serious AEs
|
|
| Notes | Sample calculation: no Ethics approval: institutional Review Board‐approved protocol Comments from trial authors
Key conclusion of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH: no Any withdrawals due to AEs: not stated Funding source: data collection for the project was supported by the NIMH (Bethesda, MD) by R01MH074770 [Epstein] and K23MH083881 [Froehlich], while investigators’ time on the project was funded by NIMH K24MH064478 [Epstein], K23MH083027 [Brinkman], and R01MH070564 [Stein]). Email correspondence with trial authors: August and November 2021 We received supplemental information regarding risk of bias through personal email correspondence with the trial authors in August 2021 (Storm 2021b [pers comm]). Inquiries for outcome data was also made but no answer was received in either September and November 2021. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list was used to randomise participants equally to one of 6 dosing schedules |
| Allocation concealment (selection bias) | Low risk | Trial medication consisted of identical capsules filled with either an inert white powder (placebo) or the prescribed dose of OROS‐MPH (Concerta_ over‐encapsulated to preserve double‐blind |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Trial pills were identical capsules filled with either an inert white powder (placebo) or the prescribed dose of MPH over‐encapsulated to preserve the blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Yes, outcome assessors were blind to allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 3 participants did not complete the medical trial. 17 missing data for sleep outcomes, LOCF (high risk of bias for this particular outcome) Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | High risk | Family satisfaction with medication trial not reported |
Gadow 1990.
| Study characteristics | ||
| Methods | 6‐week double‐blind, cross‐over trial in which participants received the following in random order:
Furthermore, a single case report from the trial is described |
|
| Participants | Number of participants screened: not stated Number of participants included: 11 (11 boys, 0 girls) Number of participants followed up: between 9 and 10, depending on ratings Number of withdrawals: 1‐2 Diagnosis of ADHD: DSM‐III (type not stated) Age: mean not reported (range 5.9‐11.9 years) Case report: 10 years of age IQ: > 75 Stimulant‐naive: 6 Ethnicity: not stated Country: USA Setting: outpatient clinic Comorbidity: disruptive behaviour disorder Comedication: not reported Other sociodemographics: children represented a full range of socioeconomic backgrounds Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 6 possible drug condition orders of LD‐MPH (0.3 mg/kg) and MD‐MPH (0.6 mg/kg) (upper limit 25 mg) and placebo Mean MPH dosage: not stated Administration schedule: twice/d, morning, noon, 3.5 h apart, 7 d/week Duration of each medication condition: 2 weeks Washout before trial initiation: yes Titration period: when MD was not preceded by LD condition, the child was gradually built up to MD. Data from these titration days were excluded from the analyses Treatment compliance: pill count, no further information |
|
| Outcomes |
ADHD symptoms
Non‐serious AEs
|
|
| Notes | Sample calculation: no information Ethics approval: no information Comment from trial authors
Key conclusion of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: no Funding source: Ciba Pharmaceutical Company supplied MPH placebo Email correspondence with trial authors: July 2013. Emailed first trial author twice to get additional information (funding, ethics approval, etc.) and data from the trial. Trial authors not able to provide us with additional data |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Dose schedules were assigned on a random basis |
| Allocation concealment (selection bias) | Low risk | Medication and placebo pills were identical and were dispensed to parents and school nurses in dated, sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Parents, teachers, observers, treating physicians and children were blinded to dose and order |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Parents, teachers, observers, treating physicians and children were blinded to dose and order |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | For classroom analyses, data on 10 boys were analysed, and for lunch room analyses, data on 9 boys were analysed Selection bias (e.g. titration before randomisation → exclusion): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified. Email sent to first trial author. No answer; therefore not able to get information |
Gadow 1995.
| Study characteristics | ||
| Methods | Randomised, placebo‐controlled, double‐blind, cross‐over trial with 2 interventions:
Phases
|
|
| Participants | Number of participants screened: not stated Number of participants included: 34 (31 boys, 3 girls) Number of participants followed up: RCT 34, minimal effective dose, after RCT 27; 12‐month follow‐up 30; 18‐month follow up 26; 24‐month follow‐up 26 Number of withdrawals: RCT: 0 Diagnosis of ADHD: DSM‐III‐R (subtypes not stated) Age: mean 8 years and 10 months (range 6.1 years‐11.9 years) IQ: mean 105.9 (SD 13.7, range no information) MPH‐naive: 24 (71%) Ethnicity: white (85%), African American (3%), Asian (3%), Hispanic (9%), other (0%) Country: USA Setting: outpatient clinic Comorbidity: data from only 21 children from Gadow 1995: tics (100%); anxiety or depressive disorder, or both (8/21; 38%); OCD (3/2; 14%); most of the children also had ODD or CD and academic problems Comedication: not during RCT. 4 children were treated with an anti‐tic medication in combination with MPH at some time during the course of follow‐up (neuroleptic 3, clonidine 1) Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 4 possible drug condition orders of doses: 0.1 mg/kg (mean 4.4 mg), 0.3 mg/kg (mean 9.0 mg) and 0.5 mg/kg (mean 14.0 mg) of MPH and placebo Upper dosage limit was 20 mg. When the 0.5‐mg/kg dose was not preceded by a LD condition, the child was gradually built up to MD. Build‐up days occasionally fell on scheduled school observation days. Observers were unaware of these days and observations were conducted as usual, but these data were excluded from the analyses Administration schedule: twice/d (or 3 times/d) at morning and noon, approximately 3.5 h apart, 7 d/week Duration of each medication condition: 2 weeks Washout before trial initiation: MPH 1 week, antipsychotic 3 weeks, clonidine 2 weeks Medication‐free period between interventions: none Titration period: none Treatment compliance: parents and nurses were asked to return unused medication envelopes, which allowed researchers to assess compliance. No further information was provided in the paper Regarding 24‐month follow‐up: total daily dose of MPH, minimal effective dose ‐ after RCT mean 16.5 mg (range 5 mg‐40 mg); second visit mean 28.5 mg (range 15 mg‐60 mg); third visit mean 29.2 mg (range 10 mg‐90 mg); and 4th visit mean 34.5 mg (range 15 mg‐92 mg) |
|
| Outcomes |
During 8‐week RCT ADHD symptoms
General behaviour
Non‐serious AEs
Physician evaluations
During 24‐month follow‐up Physician evaluations
Parent ratings
|
|
| Notes | Sample calculation: yes Ethics approval: no information Comments from trial authors
Key conclusions of trial authors
Comments from trial authors (limitations)
Comments from review authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no; children were not excluded from participation in the trial if they had prior experience with stimulant drug therapy, or if such therapy purportedly had exacerbated their tics Any withdrawals due to AEs: no Funding source: research grants from the Tourette Syndrome Association and the NIMH Email correspondence with trial authors: April 2013. We emailed trial authors for supplemental information regarding cross‐over data. Data were not available. Also no further data for the interventions were available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Dose schedules were counterbalanced and assigned on a random basis |
| Allocation concealment (selection bias) | Low risk | Medication and identically matching placebos were dispensed to parents and school nurses in dated, sealed envelopes at 2‐week intervals |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Parents, teachers, participants, observers and physicians were blinded to the identity of those conditions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Medication was administered under double‐blind conditions (i.e. no one involved in clinical management of the participant, data collection or interaction with the school knew the identity of treatment conditions) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Trial describes how many people were included in the different analyses Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified or received |
Gadow 2007.
| Study characteristics | ||
| Methods | Double‐blind, randomised, cross‐over trial with interventions:
Phases
|
|
| Participants | Number of participants screened: not stated Number of participants included: 71 (39 + 32, cohorts 1 and 2, respectively) Number of participants followed up: 71 (57 boys, 14 girls) Number of withdrawals: not stated Diagnosis of ADHD: DSM‐III‐R or DSM‐IV (subtype not stated) Age: mean: 8.9 ± 1.9 years (range 6‐12) IQ: mean 103.8 MPH‐naive: not stated Ethnicity: white (87%), African American (6%), Asian (1%), Hispanic (6%) Country: USA Setting: outpatient clinic Comorbidity: Tourette’s syndrome (96%), chronic motor tic disorder (4%), ODD (56%), CD (7%), overanxious or generalised anxiety (30%), simple phobia (7%), OCD (11%) Comedication: not stated Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 4 possible drug condition orders of placebo or IR‐MPH (0.1 mg/kg (mean 4.5 mg; SD 1.6), 0.3 mg/kg (mean 9.3 mg; SD 3.0) and 0.5 mg/kg (mean 14.3 mg; SD 3.3)), twice/d for 2 weeks, each under double‐blind conditions. Upper limit was 20 mg/d Administration schedule: twice daily, 3.5 h apart. Most days, a morning dose and a noon dose, 7 days/week Duration of each medication condition: 2 weeks Washout before trial initiation: minimum washout periods for children receiving medication at referral were as follows: 1 week for stimulants (n = 10), 3 weeks for neuroleptic or SSRI (n = 2) and 2 weeks for clonidine (n = 1) Medication‐free period between interventions: none Titration period: none Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
General behaviour
Non‐serious AEs
|
|
| Notes | Sample calculation: no Ethics approval: yes Comment from trial authors
Key conclusion of trial authors
Comments from review authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: no Funding source: this trial was supported in part by a research grant from the Tourette Syndrome Association Incorporated, and by Public Health Service (PHS) grant number MH45358 from NIMH Email correspondence with trial authors: April 2014. We obtained supplemental information from trial authors |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Dose schedules were counterbalanced and assigned on a random basis |
| Allocation concealment (selection bias) | Low risk | Medication was administered twice daily, approximately 3.5 h apart, 7 d/week, and was dispensed in dated, sealed envelopes at 2‐week intervals |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind conditions |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None required withdrawal of medication Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified |
Gadow 2011.
| Study characteristics | ||
| Methods | 8‐week, placebo‐controlled, cross‐over trial with 2 interventions:
|
|
| Participants | Number of participants screened: not stated Number of participants included: 54 (42 boys, 12 girls). Participants were randomly assigned to the different possible drug condition orders: ADHD without anxiety 37, ADHD with anxiety 17 Number of participants followed up: not stated Number of withdrawals: not stated Diagnosis of ADHD: DSM‐III‐R or DSM‐IV (subtype not stated) Age: ADHD without anxiety 8.9 years, ADHD with anxiety 9.1 years (range 7‐12) IQ: ADHD without anxiety mean 103.5, ADHD with anxiety mean 103.1 MPH‐naive: 48 (89%) Ethnicity: not stated Country: USA Setting: outpatient clinic Comorbidity: Tourette's (n = 52), chronic multiple tic disorder (n = 2), anxiety (n = 17), major depressive episode or dysthymia (n = 11), overanxious disorder or generalised anxiety disorder (n = 12), separation disorder (n = 6), social phobia (n = 1), ODD (n = 36), CD (n = 4) Comedication: not stated Other sociodemographics: ADHD without anxiety 37.1, ADHD with anxiety 35.4 (according to Hollingshead) Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to different possible drug condition orders of 0.1 mg/kg, 0.3 mg/kg and 0.5 mg/kg MPH and placebo Mean MPH dosage: 4.7 mg, 9.5 mg, 14.5 mg Administration schedule: "administered twice daily, approximately 3.5 hours apart, 7 days a week" Duration of each medication condition: 2 weeks Washout before trial initiation: not stated Medication‐free period between interventions: none Titration period: none Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
General behaviour
Non‐serious AEs
|
|
| Notes | Sample calculation: not stated Ethics approval: yes; approved by a university Institutional Review Board (IRB) Comments from trial authors
Key conclusions of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: no Funding source: NIMH and the Tourette Syndrome Association Incorporated. CIBA Pharmaceutical Company supplied MPH placebos. Novartis supplied IR‐MPH Email correspondence with trial authors: May 2015. Emailed trial authors requesting additional information but have not received a reply |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Dose schedules were counterbalanced and were assigned on a random basis |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "...dispensed in dated, sealed envelopes at two‐week intervals" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Unclear risk | "In order to increase sample homogeneity, an additional 3 children with diagnosed specific phobia (n = 2) or major depressive episode without comorbid anxiety disorder (n = 1) were excluded from data analyses" |
Garfinkel 1983.
| Study characteristics | ||
| Methods | Double‐blind, randomised, placebo‐controlled, cross‐over experiment with 4 arms:
Trial lasted 20 weeks; first and last 2 weeks were baseline periods during which no medication was given. Different interventions lasted 3 weeks each (Monday to Friday) with a 7‐day washout between changes in drugs |
|
| Participants | Number of participants screened: not stated Number of participants included: 12 (all boys) Number of participants followed up: 12 Number of withdrawals: 0 Diagnosis: DSM‐III Age: mean 7.3 years (range 5.9‐11.6) IQ: > 70 MPH‐naive: 50% Ethnicity: not stated Country: USA Setting: outpatient clinic and patient ward. 8 participants were day hospital patients and 4 were inpatients Comorbidity: no Comedication: no comedication during trial period Other sociodemographics: children presented with remarkably similar clinical, family and educational histories. None of the children had localising neurological signs or met criteria for other psychiatric diagnoses Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to the different possible drug condition orders of MPH, clomipramine, desipramine and placebo Mean MPH dosage: 18 mg/d Administration schedule: twice/d, morning and lunch Duration of each medication condition: 3 weeks Washout before trial initiation: 2 weeks before trial entry and 7 days between drug conditions Titration period: first week of the drug condition after randomisation Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
General behaviour
Non‐serious AEs
|
|
| Notes | Sample calculation: no information Ethics approval: no information Key conclusions of trial authors
Comment from review authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: no Funding source: Ontario Mental Health Foundation Email correspondence with trial authors. October 2013. We obtained supplemental information regarding number of dropouts and SD for CRS. We also wanted other data but were not able to obtain these, as the trial took place several years ago and the data are no longer available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Children were randomly given MPH, desipramine, clomipramine, placebo. A Latin square was followed to control for the order of presentation of drugs |
| Allocation concealment (selection bias) | Low risk | Children were randomly given MPH, desipramine, clomipramine, placebo. A Latin square was followed to control for the order of presentation of drugs |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants, parents, attending physicians, teachers, nursing staff and child care workers did not know which medication the child received, ensuring the double‐blind procedure. Medication was added to lactose powder and was placed in identical‐appearing gelatin capsules |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants, parents, attending physicians, teachers, nursing staff and child care workers did not know which medication the child received, ensuring the double‐blind procedure. Medication was added to lactose powder and was placed in identical‐appearing gelatin capsules |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Low risk | No indication of reporting bias |
Gonzalez‐Heydrich 2010.
| Study characteristics | ||
| Methods | Cross‐over trial with 2 interventions:
Phases: number of phases varied by dose group. For all dose groups, after completing first phase, participants were on a 1‐week washout period before cross‐over. Participants assigned to 1 of 3 maximum OROS‐MPH dose groups in randomly assigned order
Participants assigned to next group after 3 participants have successfully completed preceding group with no side effects |
|
| Participants | Number of participants screened: 40 Number of participants included: 33 (19 boys, 14 girls) Number of participants followed up: 33 Number of withdrawals: 0 Diagnosis of ADHD: DSM‐IV‐R (combined (51.1%), inattentive (48.5%)) Age: mean 10.5 years (SD 3.0, range 6.4‐17.5) IQ: mean 89.7 (SD 16.9, range 59‐123) MPH‐naive: not stated Ethnicity: not stated Country: USA Setting: hospital ward Comorbidity: epilepsy (100%), others (not stated) Comedication: yes, continuation of previous medication allowed. All participants were currently on a stable dose of antiepileptic medication. Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to different possible drug condition orders of MPH and placebo. Each child was given 5 mg of IR‐MPH in the morning and at noon for 1 day. If this dose was tolerated, 18 mg of OROS‐MPH was administered in the morning for the remaining 6 days of the 1st week. For group 1, maximum dose remained 18 mg, and MPH and placebo arms lasted 1 week. For group 2, a further 1 week of OROS‐MPH at a dose of 36 mg was given in the morning for 1 week; this group was also administered placebo for 2 weeks. For group 3, maximum dose was 54 mg in the morning, and each arm of the cross‐over lasted 3 weeks Mean MPH dosage: 11 participants < 1 mg/kg/d, 13 participants 1‐1.5 mg/kg/d, 9 participants 1.5‐2 mg/kg/d Administration schedule: 1 dose in the morning Duration of each medication condition: 1 week Washout before trial initiation: participants were not allowed to have taken ADHD medication within 2 weeks before the telephone interview screening Medication‐free period between interventions: yes; 1 week Titration period: no Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
Serious AEs
Non‐serious AEs
|
|
| Notes | Sample calculation: no Ethics approval: not stated Comment from trial authors
Key conclusions of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: yes Any withdrawals due to AEs: no Funding source: supported by NIMH grant, Number K23 MH066835 Email correspondence with trial authors: April 2014. We obtained supplemental efficacy data from trial authors. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation lists for each maximum‐dose group were prepared by a statistician and maintained by the research pharmacist |
| Allocation concealment (selection bias) | Low risk | Randomisation lists for each maximum‐dose group were prepared by a statistician and maintained by the research pharmacist |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were randomly assigned to take OROS‐MPH or placebo. Principal investigator was blind to medication status. In cases of seizure worsening... Data and Safety Monitoring Board (DSMB) and Institutional Review Board (IRB) were informed of these seizures. trial personnel, the DSMB and the IRB were not unblinded through this process, as the participant would not be exposed again to the same condition |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Principal investigator was blinded to medication status |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 5 participants discontinued treatment while taking placebo and 14 while taking OROS‐MPH; however, all were included in all analyses Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | High risk | All outcomes reported referred to parent‐ and teacher‐rated versions of ADHD‐RS‐IV, and to clinician‐rated ADHD Severity measured weeks 1 to 4 in the protocol. Barkley Total scores (although significantly different) were not reported, although individual effects were reported |
Gorman 2006.
| Study characteristics | ||
| Methods | Participants with ADHD took part in a randomly ordered, double‐blind, cross‐over clinical drug trial comprising 21 consecutive days of:
|
|
| Participants | Number of participants screened: not stated Number of participants included: 43 Number of participants followed up: 41 (21 boys, 20 girls) Number of withdrawals: 2 Diagnosis of ADHD: DSM‐IV (combined (n = 22), hyperactive‐impulsive (n = 0), inattentive (n = 19)) Age: mean 9.08 years (range 6.26‐12.55) IQ: mean 107.66 MPH‐naive: 37 (out of 41, 90%) Ethnicity: white (92.67%) Country: USA Setting: outpatient clinic Comorbidity: > 2 anxiety disorders (12.16%), lifetime affective disorder (2.46%), ODD or CD (49.28%) Comedication: 0% Other sociodemographics: mean 50.43 (range 22‐66) (Hollingsworth socioeconomic status) Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 dose of MPH and placebo Mean MPH dosage: final daily dose 33.12 mg ± 1.36 (SE), range 25‐50 mg/d (0.94 ± 0.02 mg/kg) Administration schedule: twice/d Duration of each medication condition: 21 days Washout before trial initiation: not stated Medication‐free period between interventions: from lunch to the following morning Titration period: 0.25 mg/kg twice/d (breakfast and lunch) on days 1‐2; 0.25 mg/kg twice/d, plus 0.125 mg/kg at 4:00 pm on days 3‐7; 0.3 mg/kg twice/d and 0.15 mg/kg at 4:00 pm on days 8 to 14; and 0.4 mg/kg twice/d, and 0.2 mg/kg at 4:00 pm on days 15‐21. All dosages were administered to the nearest 2.5 mg Titration: took place after randomisation Treatment compliance: not stated. 4 participants with ADHD had undergone previous trials of stimulant therapy ranging from 2 weeks to 7 months |
|
| Outcomes |
ADHD symptoms
Serious AEs
Non‐serious AEs
|
|
| Notes | Sample calculation: not described Ethics approval: yes Comment from trial authors
Key conclusions of trial authors
Comment from review authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: not stated Funding source: NIMH Email correspondence with trial authors: January 2014. We obtained supplemental information from trial authors. Trial authors do not have the files anymore; therefore we are not able to obtain all of the data requested |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Answer from trial author: "Table of random numbers were used to make the allocation process, such that numbers ending in an even digit corresponded to one order and those ending in an odd digit to another" (Krogh 2014c [pers comm] |
| Allocation concealment (selection bias) | Low risk | Answer from trial author: "Table of random numbers were used to make the allocation process, such that numbers ending in an even digit corresponded to one order and those ending in an odd digit to another" (Krogh 2014c [pers comm] |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo capsules, identical in appearance and taste to those containing MPH, and administered on the same schedule |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Second author blinded. Placebo capsules, identical in appearance and taste to those containing MPH, and administered on the same schedule |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | When parents reported serious side effects (n = 5), their children’s dosages were reduced, or planned increments were omitted. However, trial authors noted "eliminating participants who could not tolerate their assigned dosage might potentially skew the sample" Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | High risk | Answer from trial author: "Event related potentials and performance measures on a cognitive task were not reported (I didn't attempt to publish the ERP data because the analyses I conducted did not yield significant results) ‐ And since we do not look at this outcome, we see it as low" (Krogh 2014c [pers comm]) |
Green 2011.
| Study characteristics | ||
| Methods | 1‐day, randomised, parallel trial with 2 arms:
6‐month follow‐up of trial participants continuing MPH treatment |
|
| Participants | Number of participants screened: not stated Number of participants included: 34 (20 boys, 14 girls) Number of participants followed up: 34 Number of patients continuing MPH treatment beyond the 1‐day trial: 16 Number of participants followed up: 15 Number of withdrawals from extension trial: 1 Patients participating in the 1‐day trial Diagnosis of ADHD: DSM‐IV‐TR (combined (33.3%), hyperactive‐impulsive (not stated), inattentive (50%), not otherwise specified (17.7%)) Age: mean 11.1 years (range 5‐20) IQ: mean: 81.4 MPH‐naive: 61.8% Ethnicity: not stated Country: Israel Setting: hospital/outpatient clinic Comorbidity in total sample: velocardiofacial syndrome (100%), ODD (23.5%), specific phobia (26.5%), generalised anxiety disorder (11.8%), social phobia(11.8%), dysthymic disorder (8.8%) and separation anxiety (5.9%) Comorbidity in MPH group: congenital anomalies of the heart and great vessels (54.5%) Comedication: no other psychotropic medication during the trial Other sociodemographics: none. The 2 groups had similar baseline demographics Inclusion criteria
Exclusion criteria
|
|
| Interventions | RCT: participants were randomly assigned to MPH or placebo Number randomised to each group: MPH 22, placebo 12 Mean MPH dosage: 15.7 ± 5.6 mg (0.5 mg/kg) Administration schedule: once Duration of intervention: 1 day Titration period: no mention, none Washout before trial initiation: 3 days Treatment compliance: not stated Follow‐up: some participants continued MPH treatment beyond the RCT Mean MPH dosage: not stated Administration schedule: not stated Duration of treatment: 6 months Treatment compliance: 1 withdrew because of poor compliance |
|
| Outcomes |
Non‐serious AEs
|
|
| Notes | Sample calculation: not stated Ethics approval: trial protocol was approved by the Institutional Review Board of the Rabin Medical Center Comments from trial authors
Key conclusions of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: no Funding source: The Basil O’Connor Starter Scholar Research Award of the March of Dimes, NARSAD (National Alliance for Research in Schizophrenia and Affective Disorders) Young Investigator Award, the Marguerite Stolz Award from the Sackler Faculty of Medicine and the National Institute on Drug Abuse (NIDA) Email correspondence with trial authors: November 2013. We obtained supplemental information regarding ADHD diagnostic criteria from trial authors. Furthermore, we received safety data from the trial sample, excluding participants > 18 years or with IQ < 70 (or both), but we decided not to use these data in our analyses, as data were missing for 60% of the control group. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Participants were randomly assigned to drug or placebo in a 2:1 ratio, according to their order of recruitment |
| Allocation concealment (selection bias) | High risk | Participants were randomly assigned to drug or placebo in a 2:1 ratio, according to their order of recruitment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and their parents were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Low risk | No indication of selective reporting. Trial authors have confirmed that planned outcomes for the 1‐day RCT were measured and reported |
Greenhill 2002.
| Study characteristics | ||
| Methods | 3‐week, randomised, double‐blind, 32‐site, parallel trial with 2 arms:
|
|
| Participants | Number of participants screened: 507 Number of participants included: 321 (257 boys, 57 girls) Number of participants followed up: MPH 141, placebo 135 Number of withdrawals: MPH 17, placebo 28 Diagnosis of ADHD: DSM‐IV (combined subtype or predominantly hyperactive‐impulsive subtype) Age: mean 9 years (range 6‐15) IQ: > 80 ADHD treatment‐naive: 36% Ethnicity: white (71%), African American (15%), Hispanic (10%), other (4%) Country: USA Setting: outpatient clinic Comorbidity: none Comedication: concomitant use of clonidine, anticonvulsant drugs and medications known to affect BP and heart rate was not allowed Other sociodemographics: no significant differences in baseline demographics were noted between the 2 groups Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to MR‐MPH or placebo Number randomised to each group: MPH 158, placebo 163 Mean MPH dosage: 40.7 mg/d (1.28 mg/kg/d) Administration schedule: once daily Duration of intervention: 3 weeks Titration period: none Treatment compliance: medication counts showed satisfactory adherence in both groups |
|
| Outcomes |
General behaviour
Non‐serious AEs
|
|
| Notes | Sample calculation: no Ethics approval: yes; probably approved by an institutional review board Comments from trial authors
Key conclusion of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: yes; participants with a documented allergy or intolerance to MPH were excluded Any withdrawals due to AEs: yes, 2 Funding source: Celltech Pharmaceuticals Incorporated Email correspondence with trial authors: November and December 2013: not possible to get supplemental information regarding the trial through personal email correspondence with trial authors |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated "randomised". Stratification based on previous treatment before randomisation ensured equal distribution across the 2 treatment groups |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical appearing MR‐MPH and placebo capsules were packaged in blister cards |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Stated "double‐blinded" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 316 were included in the safety population, and 314 in the ITT efficacy population. All statistical summaries and analyses were conducted for the ITT population using the LOCF approach for children who withdrew prematurely Selection bias (e.g. titration after randomisation → exclusion): yes; exclusion of placebo responders |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
Greenhill 2006.
| Study characteristics | ||
| Methods | 7‐week multi‐centre, randomised, double‐blind, placebo‐controlled, parallel trial with 2 arms:
To compare the efficacy and safety of ER‐d‐MPH vs placebo in paediatric patients with ADHD Pre‐randomisation phase of up to 2 weeks followed by double‐blind treatment phase for 7 weeks (5 weeks of dose titration followed by 1 week of optimal constant dose) |
|
| Participants | Number of participants screened: not stated Number of participants included: 103 (66 boys, 37 girls) Number of participants followed up: MPH 48, placebo 37 (ITT analyses: MPH 52, placebo 45) Number of withdrawals: MPH 5, placebo 13 Diagnosis of ADHD: DSM‐IV (combined (83%), hyperactive‐impulsive (1.9%), inattentive (15.1%)) Age: mean MPH 9.6 years, placebo 10.4 years (range 6‐17) IQ: > 70 (age‐appropriate functioning levels academically) MPH‐naive: 40 Ethnicity: white (60%), African American (23.3%), other (16.5%) Country: USA Setting: classroom setting Comorbidity: no psychiatric comorbidity Comedication: not stated Other sociodemographics: no significant difference in baseline demographics was noted between the 2 groups. See Tables 1 and 2 Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to an ER formulation of d‐MPH or placebo. Permitted doses were 5 mg/d for the 1st week; 5 or 10 mg/d for the 2nd week; 5 mg/d, 10 mg/d or 15 mg/d for the 3rd week; 5 mg/d, 10 mg/d, 15 mg/d or 20 mg/d for the 4th week; and 5 mg/d, 10 mg/d, 15 mg/d, 20 mg/d or 30 mg/d for the 5th through 7th weeks Number randomised to each group: MPH 53, placebo 50 Mean final MPH dosage: 24.0 ± 7.1 mg/d Administration schedule: once daily in the mornings Duration of intervention: 5‐week titration period plus 2 weeks at a constant dose Titration period: initiated after randomisation Treatment compliance: at each trial visit, compliance was assessed by investigator and trial staff on the basis of pill count and participant report |
|
| Outcomes |
ADHD symptoms
Serious AEs
Quality of life
Non‐serious AEs
|
|
| Notes | Sample calculation: assumptions for sample size and power included treatment difference of 9.0 and SD of 13.5 Ethics approval: no information provided Comments from trial authors
Key conclusions of trial authors
Comments from review authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: yes; participants with history of poor response or intolerance to MPH were excluded Withdrawals due to AEs: MPH 0, placebo 1 Funding source: Novartis Email correspondence: July 2014. Wrote to Novartis to request additional information but have not received a reply |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further description provided |
| Allocation concealment (selection bias) | Unclear risk | No description provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, no further description provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double‐blind, no further description provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Trial used an ITT analysis. LOCF analysis was used to impute missing values for all final visit analyses Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | High risk | Trial authors defined baseline scores on Conners' ADHD/DSM‐IV Scales ‐ teacher‐rated, as inclusion criteria (different scores for age groups), but they informed efficacy as the difference from baseline to endpoint for all medicated individuals |
Gruber 2007.
| Study characteristics | ||
| Methods | 14‐day, double‐blind, placebo‐controlled, cross‐over trial with 2 interventions:
|
|
| Participants | Number of participants screened: not stated Number of participants included: 37 (31 boys, 6 girls) Number of participants followed up: 37 Number of withdrawals: 0 Diagnosis of ADHD: DSM‐IV (combined (70%), hyperactive‐impulsive (11%), inattentive (19%)) Age: mean 9.2 years (range 6‐12) IQ: mean 96.7 MPH‐naive: not stated Ethnicity: white (94%), other (6%) Country: Canada Setting: outpatient clinic Comorbidity: ODD (30%), CD (46%), major depressive disorder (5%), general anxiety disorder (3%) Comedication: no Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 2 possible drug condition orders of MPH and placebo Mean MPH dosage: 0.5 mg/kg/d Administration schedule: twice daily, morning and noon Duration of each medication condition: 7 days Washout before trial initiation: no Medication‐free period between interventions: no Titration period: none Treatment compliance: not stated |
|
| Outcomes |
General behaviour
Non‐serious AEs
|
|
| Notes | Ethics approval: trial was approved by the Research Ethics Board of Douglas Mental Health University Institute Key conclusion of trial authors
Comment from review authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: yes (see exclusion criteria) Any withdrawals due to AEs: no Funding source: this was not an industry‐supported trial Email correspondence with trial authors: February and March 2014. Emailed trial author twice but have not received a reply |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Order of administration (MPH or placebo) was determined by random assignment |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "MPH and placebo were prepared in identical coloured gelatin capsules by the hospital’s clinical pharmacist, who was not involved in the study in any other way. Capsules were sealed in individual, daily‐dose envelopes to help control accurate administration" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified |
Hale 2011.
| Study characteristics | ||
| Methods | Cross‐over trial with 2 interventions:
Phases: baseline, placebo, LD‐MPH and HD‐MPH for 4 weeks per medication phase |
|
| Participants | Number of participants screened: 65 Number of participants included: 56 (39 boys, 17 girls) Number of participants followed up: not stated Number of withdrawals: not stated Diagnosis of ADHD: DSM‐IV‐TR (combined (58.9%), hyperactive‐impulsive (7.1%), inattentive (33.9%)) Age: mean 120.84 months (approximately 10.1 years) (SD 30.85 months, range 6‐16 years) IQ: mean 99.56 (SD 6.84, n = 41) MPH‐naive: number not stated ("All participants were either medication‐naive or received an appropriate wash‐out period") Ethnicity: European American (82%), African American (18%) Country: USA Setting: outpatient clinic Comorbidity: specific learning disability (n = 13), ODD/CD (n = 11), anxiety/depression (n = 6) Comedication: not stated Other sociodemographics: middle class (n = 44), lower class (n = 12); urban (n = 36), suburban (n = 13), rural (n = 7) Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 6 possible drug condition orders of MPH (0.15 mg/kg, 0.30 mg/kg) and placebo Mean MPH dosage: not stated Administration schedule: twice daily Duration of each medication condition: 4 weeks Washout before trial initiation: 2 days Medication‐free period between interventions: no Titration period: none Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
General behaviour
Non‐serious AEs
|
|
| Notes | Sample calculation: no Ethics approval: not stated Comment from trial authors
Key conclusion of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: not stated Funding source: research part funded by the Neuropsychiatric Research Institute, Fargo, North Dakota, USA Email correspondence with trial authors: June‐August 2014. Emailed trial authors twice to request additional information but have not received a reply |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | All medications and placebos were prepared by the trial pharmacist, who randomly assigned children to 1 of 6 trial orders in placebo (P), low‐dose (L) and high‐dose (H) conditions (P‐L‐H, P‐H‐L, L‐P‐H, L‐H‐P, H‐L‐P, H‐P‐L) |
| Allocation concealment (selection bias) | Low risk | Research assistants, teacher, parents and participants were blinded to the order of conditions. Ground MPH tablet was placed in lactose‐filled opaque capsules for active drug conditions, with lactose included only for the placebo condition, and was administered twice per day |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Research assistants, teacher, parents and participants were blinded to the order of conditions. All medications and placebos were prepared by the trial pharmacist. Ground MPH tablet was placed in lactose‐filled opaque capsules for the active drug condition, with lactose included only for the placebo condition |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | After results were analysed, the order of conditions was revealed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Participants for whom data were missing or different instruments were used for MPH response were excluded Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified |
Hawk 2018.
| Study characteristics | ||
| Methods | A 3‐day cross‐over‐trial with 3 arms
Phases: 2 (1 day baseline day, then cross‐over‐trial) |
|
| Participants | Number of participants screened: not stated Number of participants included: 84 Number of participants followed‐up: 82 (74% boys, 26% girls). Trial authors provided data for 80 participants Number of withdrawals: 2 (AE: moderate motor ticks at the 0.3 mg/kg dose, which led to exclusion) Diagnosis of ADHD: DSM‐IV (subtype not stated) Age: mean 10.8 years (SD 1.1, range 9‐12) IQ: 103 (SD 14) MPH‐naive: 21% Ethnicity: white (81%), black (12%) other (7%) Country: USA Setting: outpatient clinic Comorbidity: ODD (44%), CD (27%) Comedication: no psychotropic medication Additional sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 6 possible drug condition orders different orders of LA‐MPH in 2 different doses (OROS‐MPH; Concerta) and placebo, receiving each drug for a single day.
Number randomised to each group: dosing order was counterbalanced among participants Mean medication dosage: doses ranged from 27‐90 mg (dose was capped at 90 mg for safety reasons). Mean low and high doses were 1.06 mg/kg (SD 0.12) and 2.02 mg/kg (SD 0.23), respectively Administration schedule: once daily, morning, 90 min before trial Duration of each medication: 1 day Washout before trial initiation: 24 h Medication‐free period between interventions: none Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
Serious AEs
Non‐serious AEs
|
|
| Notes | Sample calculation: no Ethics approval: yes; all procedures were approved by the University at Buffalo Children and Youth Institutional Review Board Comments from trial authors
Key conclusion of trial authors
Comments from review authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH: no Any withdrawals due to AEs: yes, 2 Funding source: supported by grants from the NIMH and from the National Institute on Drug Abuse (NIDA) Email correspondence with trial authors: August and October 2021. Trial authors supplied us with information regarding risk of bias and teacher ratings of inattention on the modified IOWA scale through personal email in August and October 2021 (Storm 2021c [pers comm]) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Nothing stated |
| Allocation concealment (selection bias) | Low risk | Meds were dispensed by a pharmacist who was otherwise uninvolved with trial procedures |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Medication and placebo were administered in identical opaque capsules by trial staff upon arrival to camp |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Ratings were made blind to treatment condition and were aggregated across classes within condition |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Data were reported for the 80 participants who were not withdrawn due to AEs. Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): yes, the high medication dose was withheld from 2 participants who experienced AE's at the low dose. These 2 participants were excluded from the analysis. |
| Selective reporting (reporting bias) | High risk | No trial protocol available While the Pittsburgh Side Effect Rating Scale, which inquires about common side effects seen with stimulants (rated none to severe) was used during the trial, no side effect data are available. |
Heriot 2008.
| Study characteristics | ||
| Methods | 3‐month, parallel trial with 4 arms
|
|
| Participants | Number of participants screened: 93 Number of participants included: 20‐26 (13 boys, 3 girls) (6 withdrew, but unclear whether before or after random assignment) Number of participants followed up: MPH + parent training 4, MPH + no training 4, placebo + training 4, placebo + no training 4 Number of withdrawals: not stated Diagnosis of ADHD: DSM‐IV (combined (25%), hyperactive‐impulsive (56%), inattentive (19%)) Age: mean 4.78 years (range 3‐6) IQ: mean 97 (range 80‐123) MPH‐naive: 100% Ethnicity: not stated Country: New Zealand Setting: outpatient clinic Comorbidity (type: ODD 5/31%) Comedication: no Other sociodemographics: No significant differences in baseline demographics were noted between the 4 intervention groups. Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to MPH + parent training, MPH + no parent training, placebo + parent training or placebo + no parent training Number randomised to each group: not stated Mean MPH dosage: 0.3 mg/kg Administration schedule: twice/d ‐ morning and lunchtime Duration of intervention: 3 months Titration period: dosage was built up over the 1st week Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
Non‐serious AEs
|
|
| Notes | Sample calculation: no Ethics approval: yes; obtained from the University of Waikato, Department of Psychology Ethical Review Committee and the Waikato Ethics Committee for Waikato Hospital Comments from trial authors
Key conclusion of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: unclear Funding source: no funding to conduct the trial was received from any party Email correspondence with trial authors: January, February and May 2014. Emailed trial author but have not received a reply |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Participants were allocated to conditions sequentially, using RAND function (SPSS) to generate the sequence. Each parent was randomly assigned to attend the training programme group or the support group. |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Throughout the period of data collection, participants and therapist were blinded to medication status |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Throughout the period of data collection, participants and therapist were blinded to medication status. All parents and teachers were blinded to the intervention |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only data for completing participants were reported Selection bias (e.g. titration after randomisation → exclusion): unclear. 4 were excluded after randomisation because of "treatment integrity problems" |
| Selective reporting (reporting bias) | Unclear risk | We were unable to identify a protocol |
Hicks 1985.
| Study characteristics | ||
| Methods | Double‐blind, cross‐over trial with 2 interventions:
|
|
| Participants | Number of participants screened: not stated Number of participants included: 44 Number of participants followed up: 44 (36 boys, 8 girls; 20 inpatients, 24 outpatients) Number of withdrawals: 0 Diagnosis of ADHD: DSM‐III (subtype not stated) Age: mean 8.4 years (SD 1.75, range not reported) IQ: mean 98.31 (SD 12.96) MPH‐naive: not stated Ethnicity: white (68.2%), African American (31.8%) Country: not stated Setting: outpatient clinic and patient ward Comorbidity: not stated Comedication: not stated Other sociodemographics: mean 3.59 (SD 1.29) (Hollingshead 2‐factor socioeconomic status index) Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to different orders of MPH (LD 0.3 mg/kg, HD 0.6 mg/kg) and placebo Mean MPH dosage: not stated Administration schedule: twice/d, morning and noon Duration of each medication condition: 12 for inpatients, 19 for outpatients Washout before trial initiation: not stated Medication‐free period between interventions: 68 h Titration period: none Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
Non‐serious AEs
|
|
| Notes | Sample calculation: not stated Ethics approval: yes Key conclusion of trial authors
Comment from review authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: no Funding source: NIH Email correspondence with trial authors: March 2014. We obtained from trial authors supplemental information regarding funding and ethics. Not able to obtain additional data as they are no longer available. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
Hoeppner 1997.
| Study characteristics | ||
| Methods | Double‐blind, cross‐over trial with 2 interventions:
4 orders
|
|
| Participants | Number of participants screened: 95 Number of participants included: 50 Number of participants followed up: 50 (39 boys, 11 girls) Number of withdrawals: none Diagnosis of ADHD: DSM‐III‐R Age: mean 9.6 years (range 6‐18.1) IQ: not stated MPH‐naive: not stated Ethnicity: not stated Country: USA Setting: outpatient clinic Comorbidity: not stated Comedication: not stated Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to different possible orders of HD‐MPH (0.3 mg/kg), LD‐MPH (0.15 mg/kg) and placebo (doses rounded up to the nearest 2.5 mg) Mean MPH dosage: not stated Administration schedule: twice/d, 8:00 am and 12:00 pm Duration of each medication condition: 1 week Washout before trial initiation: "received an appropriate wash‐out period" Medication‐free period between interventions: from lunchtime until next morning, 20 h Titration period: none Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
|
|
| Notes | Sample calculation: no Ethics approval: no information Comments from trial authors
Key conclusion of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: no Funding source: not declared Email correspondence with trial authors: March 2014. Emailed trial authors to request additional information but have received no reply |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Low risk | Pharmacists randomly assigned participants to 1 of 4 trial sequences |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo consisted of an opaque capsule filled with lactose; active drug consisted of the same lactose‐filled capsule, to which MPH was added |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Placebo consisted of an opaque capsule filled with lactose; active drug consisted of the same lactose‐filled capsule, to which MPH was added |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Low risk | No indication of reporting bias |
Horn 1991.
| Study characteristics | ||
| Methods | 12‐week, double‐blind, randomised, parallel trial with 6 arms:
9‐month follow‐up |
|
| Participants | Number of participants screened: 117 Number of participants included: 107 (83 boys, 24 girls) Number of withdrawals after randomisation: 18 Number followed up: at end of trial (12 weeks) 78, 9 months after termination of trial 71 Diagnosis of ADHD: DSM‐III‐R (subtype not stated) Age: mean 8.27 years (range 7‐11) IQ: > 70 MPH‐naive: not stated Ethnicity: white (84.9%), African American (9.4%), Hispanic (3.8%), Asian American (1.9%) Country: USA Setting: outpatient clinic Comorbidity: CD (7.5%), disruptive oppositional disorder (15%), ODD (43%) Comedication: not stated Other sociodemographics: mean yearly family income USD 25,019. No significant differences in baseline demographics were noted between treatment groups or between treatment groups and treatment dropouts in any of the following parameters: child's age, grade, sex, IQ; parental marital status, annual family income and maternal education and age. However, a significantly greater number of non‐white children were included in the placebo‐alone condition than in remaining treatment conditions or among treatments dropouts Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 6 treatment conditions, including placebo, LD‐MPH (0.4 mg/kg) and HD‐MPH (0.8 mg/kg) No of participants assigned to each group: 16 to each arm Administration schedule: daily Duration of intervention: 12 weeks Washout before trial initiation: 2 weeks before initial diagnostic evaluation Titration period: no Treatment compliance: 87.39% of parents in the non‐placebo medication conditions group reported anonymously that their child took the medications almost every day, whereas the remainder of families reported usage an average of 3 to 4 days a week. Stimulant medication was withdrawn immediately after post‐test assessments |
|
| Outcomes |
ADHD symptoms All dependent measures were administered at pre‐test, post‐test (within 1 week or 2 weeks of the end of the treatment phase of the trial) and follow‐up
AEs
|
|
| Notes | Sample calculation: no information Ethics approval: no information Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: yes, 3 Funding: not declared Email correspondence with trial authors: August 2013. Not possible to receive supplemental information or data through personal email correspondence with trial authors. They do not recommend inclusion of the trial in this review because of problems with the design and methods used at the time the trial was carried out (Ramstad 2013a [pers comm]) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 2 of 12 families were randomly assigned to each of the 6 treatment conditions. This procedure was repeated 8 times, so that by the end of the trial, 16 families were randomly assigned to each of the 6 treatment conditions, for a total of 96 families |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind. Note that assessors were blinded to treatment status at all times |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Analyses were conducted on 4 samples: (1) participants completing the entire trial and followed up at 9 months (n = 71), (2) participants completing the entire trial (n = 78), (3) participants completing the entire trial + participants completing ≥ 6 weeks (n = 90) and (4) all of the above + those who dropped out immediately after initiating treatment (n = 96). When no post‐test and/or follow‐up data were available for dropouts, pre‐test values or post‐test values, or both, were substituted. Given that analyses produced essentially the same results, only the analyses that include completers are reported Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified |
Huang 2021.
| Study characteristics | ||
| Methods | A 2‐week 2‐way cross‐over trial with 2 arms:
Phases: 3 (open‐label titration period (2–4 weeks), double‐blinded and placebo‐controlled 2‐way cross‐over treatment phase (4 weeks), and follow‐up phase (2 weeks)) |
|
| Participants | Number of participants screened: 110 participants entered the open‐label period Number of participants included: 103 randomised. Participants were randomly assigned to 1 of 2 possible drug condition orders. Number of participants followed up: 100 (ITT population: 99; 72.2% boys, 28.8% girls) Number of withdrawals: 5; 3 withdrew after randomisation, 2 withdrew after the 1st treatment periods (1 in each group) Diagnosis of ADHD: DSM‐5 (of the ITT population 75% were combined type and 25% were inattentive type) Age: mean: LA‐MPH, placebo: 9.16 years (SD 2.42); placebo, LA‐MPH: 8.96 years (SD 2.39) (range 6‐18) IQ: < 80 was an exclusion criterion, no further information MPH‐naive: not stated Ethnicity: not stated Country: Taiwan Setting: outpatient Comorbidity: no Comedication: concomitant medication was an exclusion criterion Additional sociodemographics: parental education level
Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 2 possible orders of 2 weeks of LA‐MPH (22 mg/d, 33 mg/d or 44 mg/d depending on optimal dose) and 2 weeks of placebo Number randomised to each group: LA‐MPH, placebo 50, placebo, LA‐MPH 50 Mean medication dosage: of the 99 who received 2 weeks of LA‐MPH: 30.56 mg/d (SD 8.40 mg/d, range 22–44 mg/d) and 0.92 mg/kg/d (SD 0.28 mg/kg/d, range 0.42–1.90 mg/kg/d) Administration schedule: once daily in the morning, 20 min after breakfast Duration (of (each) medication): LA‐MPH 2 weeks, placebo 2 weeks Washout before trial initiation: medication within the past 30 days was an exclusion criterion. There was minimum 1 week treatment at optimal dosage before randomisation. No washout period between open‐label and blinded trial period Medication‐free period between interventions: no washout period between interventions Titration period: 2‐4 weeks, where each participant was titrated to their optimal dosage of either 22 mg/d, 33 mg/d or 44 mg/d Participants who were unable to tolerate 22 mg of MPH were withdrawn. After optimisation, each participant had 1 additional week at their optimal dosage Treatment compliance: pill count and parental reporting. No information on adherence |
|
| Outcomes |
ADHD symptoms
Serious AEs
Non‐serious AEs
|
|
| Notes | Sample calculation: yes; “By assuming that the mean difference between MPH and the placebo in terms of the SNAP‐IV total score at 2 weeks was to be −6.0, the individual standard deviations (SDs) would have been 10.0. Based on such information, the total sample size was estimated using a one‐sided significance level of 2.5% and a power of 80%. If an 18% dropout rate is included, then we need to recruit at least 110 subjects to this trial to obtain a target sample size of 90 for evaluation.” Ethics approval: yes Comments from trial authors
Key conclusion of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH: yes, during an open‐label titration phase before randomisation Any withdrawals due to AEs: yes, 1 Funding source: this work is supported by Orient Pharma Co, Ltd. Email correspondence with trial authors: August and October 2021. We contacted the trial authors for information regarding risk of bias and first period data through personal email in August and October 2021, but no answer was received. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Based on a computer‐generated random sequence, the trial participants were randomly assigned into either the LA‐MPH or the placebo parts of the trial at a 1:1 ratio |
| Allocation concealment (selection bias) | Unclear risk | Nothing stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blinded, no mention of method “[…] methylphenidate gives rise to several easily recognizable AEs, one such being decreased appetite; such well‐known adverse effects may lead to a loss of blinding and thus influence rating of symptom, particularly when this is carried out by parents and assessors who have knowledge of reported adverse effects.” |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 5 participants withdrew during the double‐blind phase (2 of them were included in the ITT sample). Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | High risk | Conners' Continuous Performance Test (CPT‐II) performance in LA‐MPH vs. placebo is mentioned in protocol, but no outcomes are reported |
Ialongo 1994.
| Study characteristics | ||
| Methods | 14‐week, parallel trial with 3 arms:
|
|
| Participants | Number of participants screened: not stated Number of participants included: 48 (35 boys, 13 girls). A sample of 21 non‐clinical controls (13 boys and 8 girls) was also included in the trial Number of withdrawals: 7 families dropped out from pre‐test to post‐test: HD‐MPH 3, placebo 4 Numper of participants followed up: LD‐MPH 16, HD‐MPH 13, placebo 12 Diagnosis of ADHD: DSM‐III‐R (subtype not stated) Age: mean 7.97 years (SD 1.4, range 7‐11) IQ: ≤ 70 MPH‐naive: 98% Ethnicity: white (78.0%), African American (12.2%), Hispanic (9.8%) Country: USA Setting: outpatient clinic Comorbidity: CD 5, ODD 12 Comedication: no Other sociodemographics: middle‐income families. No significant differences between treatment groups or between treatment groups and dropouts in any participant and/or demographic characteristics at pre‐test, with the exception of ethnicity. A significantly greater proportion of African American children were included in the placebo condition than in the high‐dose condition Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 3 treatment conditions: medication placebo alone (n = 16), LD‐MPH (0.4 mg/kg) stimulant therapy (16) or HD‐MPH (0.8 mg/kg) stimulant therapy 16 Duration of intervention: 14 weeks Titration period: no Treatment compliance: 1 check on medication compliance consisted of periodic dispensing of medication over the course of the trial during follow‐up visits to staff paediatricians. In addition, a medical compliance questionnaire was completed anonymously by parents 1 month after post‐test assessments. 91.9% of parents in the medication conditions indicated that their child took the stimulant medication almost every day throughout the trial, whereas nearly all remaining families reported average usage of 3‐4 days a week |
|
| Outcomes |
ADHD symptoms
General behaviour
|
|
| Notes | Sample calculation: no information Ethics approval: no information Key conclusion of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: yes, 7 families dropped out due to intolerable side effects or lack of treatment efficacy Funding source: not declared Email correspondence with trial authors: August 2013 and January 2014. We emailed trial authors to request a copy of the protocol and additional information on, for example, the randomisation procedure and funding (Ramstad 2013a [pers comm]). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All medication was dispensed in a double‐blinded fashion |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All medication was dispensed in a double‐blinded fashion |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No description of imputation method Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified |
Jacobi‐Polishook 2009.
| Study characteristics | ||
| Methods | Single‐day, randomised, controlled, parallel, double‐blind trial with 2 arms investigating postural stability in 24 children with ADHD:
|
|
| Participants | Number of participants screened: 80 Number of participants included: 24 (22 boys, 2 girls) Number of participants followed up: 24 Number of withdrawals: 0 Diagnosis of ADHD: DSM‐IV (subtype not stated) Age: MPH mean 10.06 years (range 7‐16), placebo mean 10.88 years (range 7‐16) MPH‐naive: 0% Ethnicity: not stated Country: Israel Setting: outpatient clinic Comorbidity: not stated Comedication: no IQ: > 70 Other sociodemographics: none No significant difference in baseline demographics were noted between the 2 groups Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 5 mg of SA‐MPH or 5 mg placebo Number randomised to each group: MPH 12, placebo 12 Mean medication dosage: not stated Administration schedule: 5 mg x 1 (single dose) Duration of intervention: 1 day Washout before trial intervention: 24 h Titration period: none Treatment compliance: 100% |
|
| Outcomes |
Serious AEs
Non‐serious AEs
|
|
| Notes | Sample calculation: yes Ethics approval: no information Key conclusions of trial authors
Inclusion of MPH responders only/exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: yes; see 'Inclusion criteria' Any withdrawals due to AEs: no Funding source: not declared Any withdrawals due to AEs: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation using a table of random numbers |
| Allocation concealment (selection bias) | Low risk | Placebo pill was identical in appearance to the MPH pill |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and tester administering the examination were blinded to group assignments |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants and tester administering the examination were blinded to group assignments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts in either group Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Low risk | Outcomes reported according to protocol |
Jensen 1999 (MTA).
| Study characteristics | ||
| Methods | 14‐month multi‐centre, randomised, parallel clinical trial with 4 arms:
Phases: for the 2 groups receiving medication, an initial 28‐day titration period was provided. This titration phase was carried out as a randomised, double‐blind, placebo‐controlled, cross‐over trial with daily switching of MPH doses (placebo, low, middle and high). Once delivery of randomly assigned treatments by trial staff stopped at 14 months, the trial became an observational trial in which participants and families were free to choose their own treatment, but in the context of availability and barriers to care existing in their communities. The following follow‐up assessments took place after completion of the RCT at 10 months' follow‐up (24 months after randomisation), 3‐year follow‐up, 8‐year follow‐up and 10‐year follow‐up In our review, we will compare combined behavioural treatment (RCT) according to our protocol and will look at the medication treatment group as a cohort (observational) |
|
| Participants | Number of participants screened: 4541 Number of participants included: 579 Number of participants randomly assigned to MPH + behavioural treatment (combined treatment): 145, MPH 144, behavioural treatment 144 Number of participants followed up: combined treatment 142; medication 136; behavioural treatment 141 Number of withdrawals/dropouts: combined treatment 3; medication 8; behavioural treatment 3 Demographic data for combined treatment, behavioural treatment and medication management
Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to medication management, behavioural treatment or combined treatment Mean MPH dosage during main trial: combined treatment 31.2 mg/d, medication management: 37.8 mg/d Administration schedule: 3 times/d ‐ breakfast, lunch and in the afternoon Duration of intervention: 14 months Titration period: 4 weeks. After randomisation. Participants randomised to receive medication or combined treatment underwent a 4‐week, double‐blind titration phase. Treatment compliance: monthly pill counts, intermittent saliva measurements to monitor intake of MPH and encouragement for families to make up missed visits. "The study achieved a high degree of adherence to protocol." NB! For participants not attaining an adequate response to MPH during titration, alternate medications were titrated openly in the following order until a satisfactory choice was found: dextroamphetamine, pemoline, imipramine and others, if necessary approved by a cross‐site panel. Thus, 256 participants successfully completed titration; of these, 198 of 256 participants were assigned to an individually titrated best dose of MPH, and 26 were titrated to dextroamphetamine. 32 were given no medication because of a robust placebo response |
|
| Outcomes |
ADHD symptoms
Serious AEs
Non‐serious AEs Participants were provided up to 8 additional sessions when needed to address clinical emergencies or instances of possible trial attrition
For analysis of stimulant treatment duration in relation to substance use at 8‐year follow‐up, the primary outcome was the number of substances used in the past 6 months, to ensure that most stimulant treatment received would have preceded substance use. Component variables included the following: "drunk" once or more or drank alcohol ≥ 3‐4 times; ≥ 1 cigarettes/d in the past month (time frame exception specific to tobacco); marijuana ≥ 2 times; and any other illicit drug use or prescription medication misuse. Secondary analyses explored each class of substances separately. For analysis of stimulant treatment exposure over time in relation to substance use at 8‐year follow‐up, the primary outcome variable was substance use disorder in the past year for any substance (excluding tobacco). Secondary analyses explored alcohol and marijuana/other drug use disorders separately |
|
| Notes | Sample calculation: yes; power analysis, with 576 participants required Ethics approval: yes; approved by both local institutional review boards and NIH Office for Protection From Research Risk Comments from trial authors
Key conclusions of trial authors
Comments from review authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: yes, see exclusion criteria Any withdrawals due to AEs: 4 participants were removed during the lead‐in (titration period) because of prohibitive side effects: 1 child with buccal movements; another with skin picking; a third with depression, crying, sleep delay and appetite loss; and a 4th who was anorexic, listless and emotionally constricted Funding source: this trial was supported by several grants from the NIMH, Bethesda, Maryland Email correspondence with trial authors: January 2014‐June 2014. We sent several emails to the MTA group to request additional information. However, we were not able to obtain additional data. We did receive an email from Dr. Hinshaw confirming that the data on ADHD symptoms, parent‐rated, were wrong ‐ instead of a mean of 1.85, the correct mean was 0.85 for combined treatment after 14 months. We also received an email from Dr. Swanson in June 2014 stating that he would help with data collection. We wanted to conduct a reanalysis of data excluding those few participants not receiving MPH. Dr. Swanson provided several helpful comments on this and enclosed published articles, but we did not receive additional data, in part because of the time frame of this review. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done centrally by the NIMH Data Center, stratified by site in blocks of 16 (4 to each group). Stratified by 6 sites. Sealed, ordered envelopes were sent to sites for successive entries |
| Allocation concealment (selection bias) | Low risk | Sealed, ordered envelopes were sent to sites for successive entries. Treatment assignment was concealed until the family confirmed agreement to accept randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Treatment assignment was concealed until the family confirmed agreement to accept randomisation. After agreement on best dose, the blind was broken, and the agreed‐on dose (if not placebo) became the participant’s initial maintenance dose |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | After agreement on best dose, the blind was broken, and the agreed‐on dose (if not placebo) became the participant's initial maintenance dose. However, for some outcome measures, 3 strategies were devised to enlist blinded raters and objective observations |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT analyses. Despite high compliance, we checked whether compliance with assessments (i.e. missing data) could have changed our findings. We completed random‐effects regression analyses 2 ways: once with inclusion of all participants, and then including only participants who provided data over multiple time points during the trial. No differences emerged from these 2 sets of analyses. Of 289 participants randomly assigned to medication treatments, 33 (11%) did not finish titration. 17 refused medication and 1 moved away. 4 participants were removed during lead‐in because of prohibitive side effects: 1 child with buccal movements; another with skin picking; a 3rd with depression, crying, sleep delay, and appetite loss, and a 4th who was anorexic, listless, and emotionally constricted. Even though they did well in lead‐in, 7 additional participants stopped in the middle of titration because they could not follow titration procedures and 4 had excessive missing data and were not included. The remaining 256 participants (88.6%) successfully completed titration. Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): yes, for participants not attaining an adequate response to MPH during titration, alternate medications were titrated openly in the following order until a satisfactory choice was found: dextroamphetamine, pemoline, imipramine and others, if necessary approved by a cross‐site panel. Thus, 256 participants successfully completed titration; of these, 198 of 289 participants were assigned to an individually titrated best dose of MPH, and 26 were titrated to dextroamphetamine. 32 were given no medication because of a robust placebo response |
| Selective reporting (reporting bias) | Low risk | No indication of selective reporting |
Johnston 1988.
| Study characteristics | ||
| Methods | 2‐week cross‐over trial with 3 interventions:
Phases: to define rebound effects in 21 boys 4‐10 years of age, with a DSM‐III diagnosis of ADD and treated with MPH some days, and placebo other days |
|
| Participants | Number of participants screened: not stated Number of participants included: 21 Number of participants followed up: 21 (21 boys, 0 girls) Number of withdrawals: 0 Diagnosis of ADD: DSM‐III‐R (subtype not stated) Age: mean 7 years 7 months (range 4‐10 years) IQ: mean 101 (range 79‐120) MPH‐naive: not stated Ethnicity: not stated Country: USA Setting: outpatient clinic (summer treatment programme) Comorbidity: CD (n = 2), ODD (n = 17), learning disability (n = 9) Comedication: not stated Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to different drug condition orders of: 0.3 mg/kg MPH, 0.6 mg/kg MPH, 20 mg ER‐MPH, or placebo. Number randomised to each group: 0.3 mg/kg MPH twice daily: 21, 0.6 mg/kg MPH twice daily: 16 of the same 21 participants who received 0.3 mg/kg MPH, 20 mg ER‐MPH: 8, placebo, not stated. Within‐participant random sequence, condition varied daily Mean MPH dosage: not stated Administration schedule: twice daily, at breakfast and just before lunch Duration of intervention: 2 weeks. Note: it is not clear for how many days each boy received either of the MPH doses or placebo Washout before trial initiation: not stated Medication‐free period between interventions: not stated Titration period: not stated Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
Non‐serious AEs
|
|
| Notes | Sample calculation: none Ethics approval: no information Comment from review authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: not stated Any withdrawals due to AEs: no Funding source: not declared Email correspondence with trial authors: emailed trial authors to request additional information. Also asked whether this trial includes the Pelham 1989 reference. No answer from trial author, so we extracted data as from 2 different studies |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Order of drug condition for each child was randomly assigned over days |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Child, parent, teacher and programme counsellors were blinded to the condition. Active medication and placebo were disguised in gelatin capsules and pre‐packaged in individually dated envelopes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
Kaplan 1990.
| Study characteristics | ||
| Methods | 3 open trials, then a placebo‐controlled, double‐blind, cross‐over trial with 2 interventions:
|
|
| Participants | Number of participants screened: 6. Number of participants followed up: 6 (all boys) Number of withdrawals: 0. 3 outpatients were studied in an open‐label design Diagnosis of ADHD: DSM‐III (subtype not stated) Age: mean 14.4 years (range 13‐16) IQ: mean 86 (range 76‐97) MPH‐naive: 5 (55%) Ethnicity: not stated Country: USA Setting: outpatient clinic and inpatient ward Comorbidity: aggressive CD (100%) Comedication: yes; diphenhydramine 50 mg Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 2 possible drug condition orders of 0.6 mg/kg, with 30 mg as a ceiling for MPH and placebo Administration schedule: twice/d, 8:00 am and noon Mean MPH dosage: 0.47 mg/kg Duration of each medication condition: 3 weeks Washout before trial initiation: 1 week Medication‐free period between interventions: 20 h Titration period: 1 week after randomisation Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
|
|
| Notes | Sample calculation: no Ethics approval: no information Comment from trial authors
Key conclusion of trial authors
Inclusion of MPH responders only/exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: no Funding source: not declared Email correspondence with trial authors: March 2014. Emailed trial author twice to request additional information but have not received a reply. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | As the result of an oversight, assignment to MPH or placebo as the first condition was made in the absence of a specific randomisation formula. 5 of the 6 participants received placebo for the first condition and MPH for the second condition |
| Allocation concealment (selection bias) | Low risk | Assignment to order condition was determined with no knowledge of the particular participants involved; thus participants did not receive 1 condition or the other based on symptoms or any other systematic bias |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | In 2 cases, school vacation at the inpatient setting prevented teacher ratings from being obtained during 1 condition of the trial. In those 2 instances, the decision was made to use Conners' ratings obtained from the unit nurse for both the condition for which no teacher ratings were provided and the condition for which teacher ratings were given; thus comparisons were consistent in terms of rater. Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified |
Kelly 1989.
| Study characteristics | ||
| Methods | Double‐blind, cross‐over trial with 2 interventions:
Followed by long‐term follow‐up of 12 children out of 21 who continued to receive MPH. Follow‐up for an average of 16 months Phases
|
|
| Participants |
Cross‐over trial Number of participants screened: not stated Number of participants included: 21 (18 boys, 3 girls). 26 were initially included, but 5 children dropped out, 3 were removed by parents and 2 were disqualified because of a death in the family in 1 and a protocol procedural error in the other Number of participants followed up: 21, plus 2 participants who had been withdrawn but returned later for follow‐up Number of withdrawals to follow‐up: 0, but data for a few variables were not obtained for all participants Diagnosis of ADHD: DSM‐III Age: mean 9.3 years (range 8‐12) IQ: mean 100.7 MPH‐naive: 100% Ethnicity: white (62%), Hispanic/oriental [Asian]/black (14%), mixed race (24%) Country: USA Setting: outpatient clinic Comorbidity: oppositional disorder (14%), enuresis (38%) Comedication: not stated Other sociodemographics: 2‐parent household (95%) Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to MPH or placebo MPH dosage: between 0.3 and 0.6 mg/kg/d in the short‐term phase Administration schedule: 2 time points/d Duration of each medication condition: not stated Washout before trial initiation: from noon to the following morning (i.e. the next day) Titration period: no |
|
| Outcomes |
ADHD symptoms
|
|
| Notes | Sample calculation: not stated Ethics approval: yes Key conclusion of trial authors
Comment from review authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: no Funding source: CIBA Geigy Pharmaceuticals provided placebos Email correspondence with trial authors: January 2014. Trial authors not able to provide us with further information |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Child assigned in a double‐blind, cross‐over format to MPH followed by placebo or placebo followed by MPH |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Because data for a few variables were not obtained for all participants, a procedure for unbalanced analysis of variance was required and was accomplished by using the general linear model (GLM) procedure in the Statistical Analysis System (SAS) Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Unclear risk | Not protocol identified |
Kent 1995.
| Study characteristics | ||
| Methods | Double‐blind, cross‐over trial with 3 interventions:
Phases: the first 2 doses (7:00 am and noon) were unchanged from the open trial, whereas the 4:00 pm dose was 10 mg of MPH, 15 mg of MPH or placebo. Each of these three 4:00 pm medication conditions was administered in random order during the 12‐day period. Each medication condition was administered for a total of 4 days |
|
| Participants | Number of participants screened: not stated Number of participants included: 12 (11 boys, 1 girl) Number of participants followed up: not stated (but all 12 seem to appear in the results) Number of withdrawals: not stated Diagnosis of ADHD: DSM‐III‐R (subtype not stated) Age: mean 9.0 years (SD 2, range 5.5‐11.25) IQ: not stated MPH‐naive: 60% Ethnicity: not stated Country: USA Setting: inpatient ward Comorbidity: ODD (25%), learning disability and ODD (50%), CD (8%) Comedication: not stated Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to different possible drug condition orders of 10 mg or 15 mg MPH and placebo Mean MPH dosage: not stated Administration schedule: 7:00 am, noon and 4:00 pm Duration of each medication condition: 4 days Washout before trial initiation: none stated Medication‐free period between interventions: none Titration period: open titration of MPH was accomplished within 14 days of the child’s admission Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
Non‐serious AEs
|
|
| Notes | Ethics approval: informed consent for trial participation was obtained from each child's parent or guardian. All procedures were approved by the trial site's Human Subjects Research Review Committee Comment from trial authors
Key conclusions of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: yes; patients were excluded from consideration for trial participation if they had known hypersensitivity to MPH Any withdrawals due to AEs: none Funding source: this work was supported by the John and Maxine Bendheim Fellowship and by the Leon Lowenstein Foundation Email correspondence with trial authors: March 2014. Sent an email to trial authors to request additional information but have not received a response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Each of the three 4:00 pm medication conditions was administered in random order during the 12‐day double‐blind cross‐over period |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The identity of each day’s 4:00 pm dose was known only to the hospital pharmacist, until the child completed the 12‐day protocol |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Results are provided for all 12 children Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified |
Kent 1999.
| Study characteristics | ||
| Methods | Cross‐over trial with 3 interventions:
Phases: 3‐week, double‐blind, 2‐way cross‐over, long‐term (≥ 12 months) follow‐up |
|
| Participants | Number of participants screened: not stated Number of participants included: 50 (38 boys, 12 girls) Number of participants followed up: 43 Number of withdrawals: 13 Diagnosis of ADHD: DSM‐IV (subtype not stated) Age: mean not reported (range 4‐14 years) IQ: "overall normal intelligence" MPH‐naive: not stated Ethnicity: not stated Country: Canada Setting: outpatient clinic Comorbidity: depression (37%), anxiety (37%), learning disability (51%), CD (5%), "psychiatric disorder" (2%), Tourette’s disorder (12%), "other" (23%) Comedication: not stated Other sociodemographics: "Fifteen (30%) live in households that have a family income below the Canadian poverty line ($20,000/y)", 26 "rural", 9 "suburban", 14 "urban". 21 live with 1 biological parent, 24 live with both parents, 4 live with adoptive parents or "guardians" Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to different possible drug condition orders of 0.3 mg/kg MPH and 0.6 mg/kg MPH and placebo Mean MPH dosage: not stated Administration schedule: each new condition started on a Saturday morning (to allow parents’ observation/evaluation on weekend) Capsules given at 8:00 am and 12:00 pm. Conners' administered at baseline and on the last day of each week. A 30‐min semi‐structured follow‐up interview was conducted ≥ 12 months after completion of the trial Duration of each medication condition: 1 week Washout before trial initiation: not stated Medication‐free period between interventions: 12:00 pm to 8:00 am the following day Titration period: none Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
Non‐serious AEs
|
|
| Notes | Sample calculation: not stated Ethics approval: yes; the Research Ethics Board of the IWK Grace Health Centre approved the protocol Comments from trial authors
Key conclusion of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: yes, "No family found the MPT difficult, but 6 trials were incomplete, usually because of side effects” Funding source: Ms Kent was a summer medical student supported in part by the IWK Grace Research Foundation, Halifax, NovaScotia, and by the Pharmaceutical Manufacturers Association of Canada Studentship, Ottawa, Ontario Email correspondence with trial authors: August 2013. We received additional information from trial authors, but they were not able to provide the additional data that we requested |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Once enrolled in the MPT, the non‐blinded hospital pharmacist randomly assigned each child to a particular dosing schedule". "The capsules contained, in random order: placebo of the prescribed dose of MPH (Ritalin) hydrochloride (0.3 mg/kg or 0.6 mg/kg)" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Families (n = 50) with a child eligible for MPT were given 3 bottles of identical capsules". "The family, teacher, and physician were blinded for the order of medication" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "At the end of each trial the code was broken. The physician evaluated this information and made a clinical inference about the degree of response each week" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified |
Klorman 1990.
| Study characteristics | ||
| Methods | Cross‐over trial with 2 interventions:
Phases: 2 |
|
| Participants | Number of participants screened: not clear Number of participants included: 48. (42 boys, 6 girls) Number of participants followed up: appears to have been 48 Number of withdrawals: not clear Diagnosis of ADHD: DSM‐III Age: mean not stated (range 12‐18 years) IQ: mean 108.62 MPH‐naive: 46/48; 2 had brief trials in childhood Ethnicity: white (46) Country: USA Setting: outpatient clinic Comorbidity: oppositional and CD (24), oppositional not CD (12), anxiety (5), drug or alcohol abuse (2), depression (1) Comedication: no Other sociodemographics: double‐ or single‐parent family, predominantly middle class (mean Hollingshead socioeconomic status score of 48.8 (i.e. social class II)) Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 3 weeks of MPH and placebo Mean MPH dosage: 35.21 mg (± 5.94 (SD)). Range 15 mg (2 daily doses) to 40 mg (3 daily doses) Administration schedule: doses were gradually increased at the end of the 1st and 2nd weeks Washout before trial initiation: not described Titration period: after randomisation Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
Mean parent‐ and teacher‐reported Conners' hyperactivity and inattention scores were graphed but SD values were not. Also, the paper did not refer to measures of impulsivity or total scores. We could not use these data in our meta‐analyses because values were missing and trial authors were not able to provide supplemental data on this outcome Non‐serious AEs
|
|
| Notes | Sample calculation: not described Ethics approval: not described Key conclusion of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to averse events: Unclear, none mentioned Funding source: National Institute of Mental Health (NIMH) grant MH38118 Email correspondence with trial authors: April 2014. We obtained supplemental information/data from trial authors (Magnusson 2014a [pers comm]) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Received information from trial author (Magnusson 2014a [pers comm]) |
| Allocation concealment (selection bias) | Low risk | Received information from trial author (Magnusson 2014a [pers comm]) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Substances were dispensed in capsules of identical appearance" (p 703) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Parent and teacher ratings were used and participants were blinded to which capsule they were receiving |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified |
Kolko 1999.
| Study characteristics | ||
| Methods | Randomised, placebo‐controlled, cross‐over trial with 2 possible drug interventions and placebo, as well as 2 possible psychological interventions:
and
|
|
| Participants | Number of participants screened: 70 Number of participants included: 22 (all boys) Number of participants followed up: 16 Number of withdrawals: 6 Diagnosis of ADHD: DSM‐III‐R (subtypes not stated) Age: mean 9.6 years (range 6.9‐12.9) IQ: not stated MPH‐naive: not stated Ethnicity: African American (75%) Country: USA Setting: "partial hospitalisation" summer treatment programme Comorbidity: CD (44%), ODD (56%), anxiety disorder (18.8%), major depressive disorder (11.5%), dysthymia (6%), intermittent explosive disorder (6%), developmental articulation disorder (6%), asthma (12.5%) Comedication: not stated Other sociodemographics: 3 lived with 1 or both parents, 6 lived with grandparents, 1 lived with an aunt, 4 lived with non‐relatives, 2 lived with foster mother. 44% of families received welfare Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to different possible drug condition orders of 0.3 mg/kg and 0.6 mg/kg MPH and placebo Mean MPH dosage: not stated Administration schedule: 8:00 am and 11:30 am to 12.00 pm Duration of each medication condition: 1 day. Each medication condition was administered once per week for a total of 6 days during the trial Washout before trial initiation: 2 weeks Medication‐free period between interventions: no Titration period: none Treatment compliance: not stated Behavioural intervention: behaviour modification and no behaviour modification were alternated on a weekly basis for a total of 3 weeks per condition |
|
| Outcomes |
ADHD symptoms
Non‐serious AEs
|
|
| Notes | Sample calculation: not stated Ethics approval: not stated Key conclusions of trial authors
Comments from review authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: yes, 2 Funding source: not declared Email correspondence with trial author: January 2014. We were unable to obtain additional data (Nilausen 2014 [pers comm]) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Each MPH condition was administered once per week for a total of 6 days during the trial, and behaviour modification and no behaviour modification were alternated on a weekly basis, for a total of 3 weeks per condition. Thus, daily MPH conditions were crossed with 2, weekly behavioural intervention conditions |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is stated: "MPH or placebo was placed in identical opaque capsules" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Not stated Selection bias: exclusion of 2 participants with challenging behaviour |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
Kollins 2006 (PATS).
| Study characteristics | ||
| Methods | 8‐phase, 70‐week, multi‐centre trial (phase III trial) including:
If parents requested and clinicians agreed that participants were severely symptomatic, children could be moved directly into the medication phase that was concurrent with parent training. If participants did not tolerate the dosing in phase 4, they could enter the open‐label maintenance phase if they tolerated lower doses (e.g. 1.25 mg, 2.5 mg). If they tolerated all doses except 7.5 mg, they were eligible to enter phase 5, the cross‐over titration, with the planned week on a 7.5‐mg dose replaced by an additional 5‐mg week. After phase 5, cross‐over:
|
|
| Participants | Number of participants screened: 1915 Number of participants included in the trial: 303 (229 boys, 74 girls) Number of participants who completed the last phase of the trial: 8 Number of withdrawals during the trial: 295 Diagnosis of ADHD: DSM‐IV (combined (75%), hyperactive‐impulsive (25%), inattentive (0%)) Age: mean 4.41 years (range not reported) IQ: > 70 (mean 99.06) MPH‐naive: 100% Ethnicity: white (63%), African American (19%), Asian (2%), Hispanic or Latino (16%), American Indian or Alaskan Native (0.7%) Country: USA Setting: outpatient clinic Comorbidity: ODD (52%), communication disorder (22%), elimination disorder (i.e. encopresis, enuresis; 8%), specific phobia (8%), anxiety disorder (8%), developmental co‐ordination disorder (3%), CD (2%), pica (2%), adjustment disorder (1%), reactive attachment disorder (1%), OCD (0.7%), sleepwalking disorder (0.3%) Comedication: no Other sociodemographics: double‐parent family (76%), single‐parent family (18%), mean Hollingshead socioeconomic status 47.20 (SD 9.56) Phase 4, open‐label, safety lead‐in Number of participants included: 183 Number of participants followed up: 169 Number of withdrawals: 12 Number of participants leaving the trial phase and entering maintenance (phase 7): 2 Phase 5, cross‐over Number of participants included: 165 (122 boys, 43 girls) Number of participants followed up: 147 Number of withdrawals: 14 Number of participants leaving the trial phase and entering maintenance (phase 7): 4 Diagnosis of ADHD: DSM‐IV (combined (76%), hyperactive‐impulsive (24%), inattentive (0%)) Age: mean 4.74 years (range 3‐5.5) IQ: > 70 (mean 97.93) MPH‐naive: 100% Ethnicity: white (63%), African American (18%), Asian (1%), Hispanic or Latino (18%), American Indian or Alaskan Native (0.6%) Country: USA Comorbidity: ODD (55%), communication disorder (20%), elimination disorder (i.e. encopresis, enuresis; 8%), specific phobia (7%), anxiety disorder (10%), developmental co‐ordination disorder (4%), CD (3%), pica (2%), adjustment disorder (0.6%), reactive attachment disorder (2%), OCD (0.6%), sleepwalking disorder (0.6%) Comedication: no Sociodemographics: double‐parent family (79%), single‐parent family (21%), mean Hollingshead socioeconomic status 47.01 (SD 9.58) Phase 6, parallel Number of participants included: 114 (85 boys, 29 girls) Number of participants followed up: 77 Number of withdrawals: 1 Number of participants leaving the trial phase and entering maintenance (phase 7): 36 Diagnosis of ADHD: DSM‐IV (combined (75%), hyperactive‐impulsive (25%), inattentive (0%)) Age: mean 4.76 years (range not reported) IQ: > 70 (mean 97.45) MPH‐naive: 100% Ethnicity: white (65%), African American (17%), Asian (0.9%), Hispanic or Latino (17%), American Indian or Alaskan Native (0.9%) Country: USA Comorbidity: ODD (53%), communication disorder (22%), elimination disorder (i.e. encopresis, enuresis; 7%), specific phobia (7%), anxiety disorder (11%), developmental co‐ordination disorder (5%), CD (3%), pica (0.9%), adjustment disorder (0.9%), reactive attachment disorder (2%), OCD (0.9%), sleepwalking disorder (0.9%) Comedication: no Sociodemographics: double‐parent family (80%), single‐parent family (19%), mean Hollingshead socioeconomic status 47.61 (SD 9.45) Phase 7, open‐label maintenance Number of participants included: 140 (104 boys, 36 girls) Number of participants followed up: 95 Number of withdrawals: 45 Diagnosis of ADHD: DSM‐IV (combined (76.4%), hyperactive‐impulsive (23.6%), inattentive (0%)) Age: mean 4.4 years IQ: > 70 MPH‐naive: 100% Ethnicity: white (65.0%), African American (17.1%), Asian (1.4%), Hispanic (15.7%), American Indian (0.7%) Country: USA Comorbidity: ODD (52.9%), communication disorder (19.3%), anxiety disorder (11.4%) Comedication: no Sociodemographics: double‐parent family (81.4%), single‐parent family (18.6%), mean Hollingshead socioeconomic status 47.2 (SD 9.5) Inclusion criteria
Additional inclusion criteria for phase 4, open‐label lead‐in
Additional inclusion criteria for phase 5, cross‐over
Exclusion criteria
All cases were presented to a cross‐site panel of clinicians, and only patients for whom consensus indicated that all inclusion (and no exclusion) criteria were met could be enrolled |
|
| Interventions |
Phases 1‐3 Enrolment, parent training; baseline: no medical intervention Phases 4 to 8 Medical intervention (SA‐IR‐MPH) Phase 4, open‐label, safety lead‐in Titration: starting dose of 1.25 mg twice daily; increased to 7.5 mg 3 times daily Duration: 1 week Treatment compliance: not stated Phase 5, cross‐over Randomly assigned sequence of doses of 1.25 mg, 2.5 mg, 5.0 mg or 7.5 mg IR‐MPH and placebo administered 3 times daily for a week Medication‐free period between interventions: none Mean MPH dose: not stated Duration: 5 weeks Phase 6, parallel After a 24‐h medication washout, 4 weeks of randomly assigned treatment with a participant's optimal MPH dose as determined in phase 5, or placebo No of participants randomised to each group: MPH 61, placebo 53 Mean MPH dose: 14.22 mg/d, 0.7 mg/kg/d Duration: 4 weeks Treatment compliance: not stated Phase 7, open‐label, maintenance Maintenance starting doses were based on the best dose decision from cross‐over titration. Phase 5 placebo responders were maintained without medication for ≥ 4 weeks, unless their condition deteriorated, in which case open‐label treatment could be initiated. For any participant whose condition deteriorated, the MPH dose was gradually titrated for optimal response. The dosing regimen was adjusted to minimise some AEs to 3 times/d (at breakfast, around noon after lunch and at 3:30 pm), 7 days a week Mean MPH dose: increased from 14.04 mg/d (0.71 mg/kg/d) at month 1 to 19.94 mg/d (0.92 mg/kg/d) at month 10 Duration: 10 months Treatment compliance: not stated Phase 8, discontinuation Randomised, double‐blind, placebo discontinuation trial, in which an abrupt medication replacement consisted of placebo for half of the children, while others continued on their best MPH dose from the end of phase 7. Children returned to active medication if they met relapse criteria, in other words, CPRS or CTRS scores on the Hyperactivity/Impulsivity Index > 1.5 SD above age‐ and sex‐adjusted norms, or a clinician rating of 4 on the CGI‐S Duration: 6 weeks Treatment compliance: not stated. Those who opted out of the double‐blind phases would be allowed to continue on open‐label maintenance therapy. This greatly reduced incentive for families to remain in the double‐blind phases, especially if there was reason to suspect that a child had been randomly assigned to placebo |
|
| Outcomes |
ADHD symptoms
Serious AEs
General behaviour
Non‐serious AEs
|
|
| Notes | Sample calculation: yes. Target sample size stated in the online protocol 165. Sample to be randomly assigned 120 Ethics approval: yes; by institutional review boards at each trial site. The trial was monitored by the Data and Safety Monitoring Board of the NIMH Comments from trial authors
Key conclusions of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: yes, participants were selected for specific phases of the trial/interventions depending on response in earlier phases, as described in methods Withdrawals due to AEs: number of withdrawals due to AEs during medication phases: 21 (i.e. 11%) Number of participants leaving trial phase and entering maintenance (phase 7): 4 Funding source:
Email correspondence with trial authors: June 2014. We obtained supplemental information/data from the trial authors. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
|
| Allocation concealment (selection bias) | Low risk |
|
| Blinding of participants and personnel (performance bias) All outcomes | Low risk |
|
| Blinding of outcome assessment (detection bias) All outcomes | Low risk |
|
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Low risk |
|
Kollins 2021.
| Study characteristics | ||
| Methods | A 1‐week parallel‐trial with 2 arms:
|
|
| Participants | Number of participants screened: 178 (155 participants included in open‐label phase) Number of participants included: 150 randomised (92 male, 58 female) Number of participants followed up: 149 Number of withdrawals: 1 lost to follow‐up Diagnosis of ADHD: DSM‐5 (percentage of combined (88%), hyperactive‐impulsive (0%) and inattentive (12%)) Age: mean 9.6 (range 6‐12) IQ: not stated MPH‐naive: 89 (59.3%) Ethnicity: 76 white, 56 black/African American, 7 Asian, 10 multiracial, 1 other Country: USA Setting: outpatient clinic, laboratory classroom Comorbidity: some were specified as exclusion criteria Comedication: not stated Additional sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to receive either their optimised dose of SDX/d‐MPH (ER) or placebo for 7 days Number randomised to each group: MPH 74, placebo 76 Mean medication dosage: not stated Administration schedule: the appropriate blinded treatment was taken at home, once daily in the morning, on days 22–27 Duration (of (each) medication): 7 days Washout before trial initiation: 2 days Titration period: 3 weeks before randomisation Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
Serious AEs
General behavioral
Non‐serious AEs
|
|
| Notes | Sample calculation: yes (126) Ethics approval: the trial was approved by an Institutional Review Board. Comments from trial authors
Key conclusion of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH: yes Any withdrawals due to AEs: no Funding source: clinical research was funded by KemPharm, Inc. Funding for editorial and writing assistance in the form of proofreading, copyediting, and fact‐checking was provided by Corium, Inc Supplemental information regarding the risk of bias assessment was requested through personal email correspondence with the authors in July 2022 but no answer was received. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information about sequence generation available |
| Allocation concealment (selection bias) | Unclear risk | No information about method of allocation concealment available |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Randomisation was stratified by trial site. Neither the participant, the investigator, nor the sponsor knew a given participant’s treatment assignment. The appropriate blinded treatment was taken at home, once daily in the morning, on days 22–27 |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Randomisation was stratified by trial site. Neither the participant, the investigator, nor the sponsor knew a given participant’s treatment assignment. The appropriate blinded treatment was taken at home, once daily in the morning, on days 22–27 |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Efficacy analyses were conducted in the ITT population Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Low risk | No indication of selective reporting, all outcomes are reported |
Konrad 2004.
| Study characteristics | ||
| Methods | Double‐blind, placebo‐controlled, within‐participant trial of cross‐over design, lasting 6 days with 2 possible drug interventions and placebo:
The order of drug conditions was randomly assigned, with the restriction that higher doses should never be administered after placebo |
|
| Participants | Number of participants screened: not stated Number of participants included: 60 (44 boys, 16 girls) Number of participants followed up: 60 Number of withdrawals: 0 Diagnosis of ADHD: DSM‐IV (combined 47 (78%), inattentive 13 (22%)) Age: mean 10.8 years (SD 1.6, range 8‐12) IQ: mean 97.4 (SD 10.7, range not stated) MPH‐naive: 100% Ethnicity: not stated Country: Germany Setting: outpatient clinic and inpatient ward Comorbidity: ODD (n = 6; 10%), CD (n = 18; 30%), anxiety (n = 12; 18%), dyslexia (n = 19; 32%) Comedication: no Other sociodemographics: no significant differences in baseline demographics were noted between the 2 groups Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to "low‐dose" MPH (0.25 mg/kg), "high‐dose" MPH (0.5 mg/kg) and placebo Number randomised to each group: LD‐MPH 60; HD‐MPH 60; placebo 60 Mean MPH dosage: 9.2 mg (SD 2.2) for LD group (0.25 mg/kg), 18.4 mg (SD 5.4) for HD group (0.5 mg/kg) Administration schedule: medication was given between 7:00 am and 8:00 am for 6 days. "Cognitive testing began 60 minutes after medication ingestion and lasted 80 minutes". The order of drug conditions was randomly assigned, with the restriction that higher doses should never be administered after placebo. Thus, 11 orders were possible for the 6‐day procedure as a whole, and 6 orders were possible for the sequence of the 3 neuropsychological assessments. Children were assigned in equal numbers to the 6 orders Duration of intervention: 6‐day intervention, with each child receiving each intervention for 2 random days Titration period: before randomisation, 1 week of 0.3 mg/kg MPH for each participant "to ascertain tolerance" Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
|
|
| Notes | Sample calculation: not stated Ethics approval: yes; "the study was approved by the Medical Ethical Committee of the University Hospital of Aachen" Comments from trial authors
Key conclusions of trial authors
Comment from review authors
Inclusion of MPH responders only/exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: yes, before testing, all children were given 0.3 mg/kg MPH each day for ≥ 1 week to ascertain tolerance Any withdrawals due to AEs: not stated Funding source: German Society for the Advancement of Scientific Research (DFG grant KFO112) Email correspondence with trial authors: January 2014. We sent an email to the trial author to request additional information but have not received a reply |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Within the 6‐day protocol, the order of drug conditions was randomly assigned, with the restriction that higher doses should never be administered after placebo |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The active medication and placebo were prepared by a study protocol physician who was not involved in the assessment. All capsules were identical opaque gelatin capsules and were administered in a double‐blinded manner. Capsules containing placebo (lactose) or 0.25 mg/kg or 0.5 mg/kg doses of methylphenidate were prepared for each participant" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | As above |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "Due to computer problems, data for four children in one task in one condition were missing. As recommended by Tabachnik and Fidell (1996), these data were replaced by the average of the group per condition" Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified |
Konrad 2005.
| Study characteristics | ||
| Methods | Cross‐over trial with 2 interventions:
|
|
| Participants | Number of participants screened: not stated Number of participants included: 44 (37 boys (84%), 7 girls (16%)) Number of participants followed up: 44 Number of withdrawals: not stated Diagnosis of ADHD: DSM‐IV (combined type 100%) Age: mean 10.3 years (SD 1.9, range 8‐12) IQ: mean 98.1 MPH‐naive: not stated Ethnicity: not stated Country: Germany Setting: inpatient ward Comorbidity: not stated Comedication: no Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 6 possible drug condition orders of LD‐MPH 0.25 mg/kg or HD‐MPH 0.5 mg/kg and placebo Mean MPH dosage: 9.4 mg (SD 2.3) for the 0.25‐mg/kg dose; 18.6 mg (SD 5.3) for the 0.50‐mg/kg dose Administration schedule: not stated Time points: not stated Duration of each medication condition: 2 days Washout before trial initiation: not stated Medication‐free period between interventions: no Titration period: before testing, all children were given 0.30 mg/kg MPH each day for ≥ 1 week to ascertain tolerance Treatment compliance: not stated. Within the 6‐day protocol, the order of drug conditions was randomly assigned, with the restriction that the high dose never occurred right after placebo |
|
| Outcomes |
ADHD symptoms
|
|
| Notes | Sample calculation: not stated Ethics approval: yes; informed parental consent was obtained for all participants, and the trial was approved by the Medical Ethical Committee of the University Key conclusions of trial authors
Inclusion of MPH responders only/exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: yes; before testing, all children were given 0.30 mg/kg MPH each day for ≥ 1 week to ascertain tolerance Any withdrawals due to AEs: not stated Funding source: provided through a grant from the German Research Foundation (DFG grant: KFO112–TP5) Email correspondence with trial author: July 2015. Email sent to trial author to ask for additional information, but we have received no reply |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Within the 6‐day protocol, the order of drug conditions was randomly assigned, with the restriction that the high dose never was given immediately after placebo |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Active medication and placebo were prepared by a trial protocol physician who was not involved in the assessment. All capsules were identical opaque gelatin capsules and were administered in a double‐blind manner |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified |
Kortekaas‐Rijlaarsdam 2017.
| Study characteristics | ||
| Methods | A 2 ‐week cross‐over trial with 2 arms:
Phases: 1 |
|
| Participants | Number of participants screened: 78 Number of participants included: 65 Number of participants followed‐up: 63/61 (43 boys, 20 girls) Number of withdrawals: 4 Diagnosis of ADHD: DSM‐IV (subtype not stated) Age: mean 10.49 years (SD 1.24, range 8‐13) IQ: 97.68 (SD 13.82) MPH‐naive: if participants were MPH‐naive, they had to go through a medication titration at their treating physician and be at a stable dose for at least 3 weeks. Ethnicity: not stated Country: the Netherlands Setting: outpatient Comorbidity: not stated Comedication: not stated Additional sociodemographics: socioeconomic status: mean 5.24 (SD 0.86) Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 2 different medication orders of MPH and placebo Number randomised to each group: 33 received MPH‐placebo, 32 received placebo‐MPH Mean medication dosage: daily doses varied between 10 and 40 mg, with 27% of the children receiving 10 mg, 44% receiving 20 mg, 24% receiving 30 mg, and 5% receiving 40 mg Administration schedule: not stated Duration of each medication: 1 week Washout before trial initiation: 48 h Medication‐free period between interventions: 48 h Titration period: mean treatment before trial initiation: 30.7 months (SD 19.1) Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
|
|
| Notes | Sample calculation: yes, 63 Ethics approval: the current trial has been carried out in accordance with the Declaration of Helsinki and was approved by the local ethics committee. Key conclusion of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH: yes Any withdrawals due to AEs: 3 (2 not related to intervention) Funding source: unclear, but Shire was a collaborator Email correspondence with the trial authors: August and November 2021. We contacted the trial authors for information regarding risk of bias and first period data through personal email in August and November 2021, but no answer was received. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Our academic pharmacist, who was not in contact with any participants, was responsible for randomisation using predefined randomisation blocks to determine medication or placebo sequence" |
| Allocation concealment (selection bias) | Unclear risk | Nothing stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both active MPH and placebo capsules were inserted in other capsules to ensure visual equality |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Researchers, children, parents, and teachers were blinded to the intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT on 63 of 65 participants Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Low risk | All outcomes from protocol reported |
Kritchman 2019.
| Study characteristics | ||
| Methods | A single‐dose cross‐over‐trial with 2 arms:
Phases: 1 |
|
| Participants | Number of participants screened: no information Number of participants included: 20 (11 male, 9 female) Number of participants followed up: 20 Number of withdrawals: none Diagnosis of ADHD: not stated Age: 10.5 SD 1.99 (8‐18) IQ: not measured (based on clinical assessment there was no intellectual disability) MPH‐naive: not stated Ethnicity: not stated Country: Israel Setting: outpatient clinic Comorbidity: several comorbidities were exclusion criteria Comedication: not stated Additional sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 2 possible orders of 0.3 mg/kg IR‐MPH and placebo during 2 medication sessions. Assessments were made 45 min after drug administration. Number randomised to each group: not stated Mean medication dosage: not stated Administration schedule: single‐dose Duration (of (each) medication): single‐dose Washout before trial initiation: not stated Medication‐free period between interventions: no Titration period: NA Treatment compliance: not stated |
|
| Outcomes |
Non‐serious AEs
|
|
| Notes | Sample calculation: no Ethics approval: “Study protocol and consent form were approved by both the institutional review board (0009‐12‐SHA) and the national review board (20120239)” Comments from trial authors
Key conclusion of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH: no Any withdrawals due to AEs: no Funding source: Shalvata Mental Health Center Trial authors were contacted for information regarding risk of bias and first‐period data through personal email in August and Oktober 2021, but no answer was received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Nothing stated |
| Allocation concealment (selection bias) | Unclear risk | Nothing stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Mention of quadruple blinding, but no mention of method |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Mention of quadruple blinding, but no mention of method |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Unclear risk | Trial registration mention: “Patient's perspective will be measured by questionnaires assessing treatment adherence issues and patient's view regarding the use of placebo." This is not mentioned in the publication. |
Leddy 2009.
| Study characteristics | ||
| Methods | 9‐week RCT, cross‐over design, with 2 interventions:
Phases: 1 |
|
| Participants | Number of participants screened: 154 Number of participants included: 58 (both boys and girls) Number of participants followed up: not stated Number of withdrawals: not stated Diagnosis of ADHD: DSM‐IV (combined (95%), hyperactive‐impulsive (2%), inattentive (3%)) Age: mean not stated (range 6‐12 years) IQ: > 80 MPH‐naive: 19% Ethnicity: not stated Country: USA Setting: outpatient clinic (summer treatment programme) Comorbidity: ODD (52%), CD (10.5%) Comedication: no Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to different possible drug condition orders of 0.15 mg/kg MPH, 0.3 mg/kg MPH, 0.6 mg/kg MPH and placebo Mean MPH dosage: not stated Administration schedule: 3 time points Duration of each medication condition: 12 days for placebo, MPH 0.15 mg and MPH 0.3 mg; 9 days for MPH 0.6 mg Washout before trial initiation: not stated Medication‐free period between interventions: no Titration period: not stated Treatment compliance: not stated |
|
| Outcomes |
General behaviour
Non‐serious AEs
|
|
| Notes | Sample calculation: not stated Ethics approval: yes Key conclusion of trial authors
Inclusion of MPH responders only/exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: not stated Funding source: not declared Email correspondence with trial author: June 2014. Emailed the trial author to ask for raw data regarding side effects, data from the Disruptive Behavior Disorders Rating Scale and other data. Trial author responded to say he did not have them (Holmskov 2014 [pers comm]) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Drug dose varied daily on a randomized basis and included four conditions (...)" |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Double‐blind" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "Double‐blind" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information Selection bias (e.g. titration after randomisation → exclusion of methylphenidate non‐responders or placebo responders): unclear |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified |
Lehmkuhl 2002.
| Study characteristics | ||
| Methods | 4‐week, randomised, double‐blind, parallel trial with 2 arms:
|
|
| Participants | Number of participants screened: 102 Number of participants included: 85 (75 boys, 10 girls) Number of participants randomly assigned: MPH 43, placebo 42 Number of participants followed up: MPH 40, placebo 38 Number of withdrawals: MPH 3, placebo 4 Diagnosis of ADHD: DSM‐IV (combined (74.1%), hyperactive‐impulsive (0%), inattentive (24.7%), unspecified (1.2%)) Age: mean 9.8 years (range 6‐15) Mean IQ: MPH 104.8, placebo 102.7 (range 85‐146) MPH‐naive: 25 (29.4%) Ethnicity: German (82), other (3) Country: Germany Setting: outpatient clinic Comorbidity: ODD (51.8%), CD (9.4%), unspecified CD (3.5%), dysthymia (1.2%) Comedication: no, some of the participants were excluded from the ITT analysis due to comedication Other sociodemographics: the 2 groups were homogeneous in terms of sex distribution, school type, school class and nationality (at baseline, Barkley Side Effect Rating Scale‐D ratings for disturbed sleep, nightmares, sadness, weepiness, anxiety, drowsiness and nervous twitching were far more pronounced in group 1 than in group 2. These initial differences – except for sadness – levelled out during the 4‐week trial period) Additional CDs are less frequent in the group treated with medication (25 vs 30 participants), but this slight difference is not significant (Fisher's exact test, P value = 0.26) Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to ER‐MPH or placebo Mean MPH dosage: not stated Administration schedule: once daily after breakfast Duration of intervention: 4 weeks Titration period: weekly dose titration initiated after randomisation. Initially, 2 5 mg MPH/placebo tablets/d for 2 days, then 20 mg (1 ER‐MPH capsule/placebo) dosage increased to 40 mg and 60 mg, depending on weight and course of symptoms. Titration up to 40 mg and 60 mg MR‐MPH was possible in the 2nd and 3rd weeks of treatment, respectively (20 kg‐30 kg, maximum 20 mg MR‐MPH; 31 kg‐50 kg, maximum 40 mg MR‐MPH; < 50 kg, maximum 60 mg MR‐MPH) Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
Serious AEs
Non‐serious AEs
|
|
| Notes | Sample calculation: yes Ethics approval: approved by local university ethics committees Comments from trial authors
Key conclusions of trial authors
Comments from review authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: yes; 2 (appendicitis and aggression) Funding source: Medice Arzneimittel Pütter GmbH & Co. KG, Kuhloweg 37, D‐58638 Iserlohn Email correspondence with trial authors: April‐May 2014. We emailed trial authors twice but received no further information |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned to MPH or placebo by the following 4 strata: age (6‐8 years; 9‐11 years; 12‐16 years), sex, severity of the problem according to the teachers’ evaluation (Fremdbeurteilungsbogen für Hyper kinetische Störungen; sum‐score > 40 and < 40) and trial centre attended. Central randomisation was performed |
| Allocation concealment (selection bias) | Low risk | Central randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Trial drug (or placebo) was dispensed in packages containing a weekly supply that was blinded from medical personnel and parents |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Trial drug (or placebo) was dispensed in packages containing a weekly supply that was blinded from medical personnel and parents |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Low risk | No indication of selective reporting |
Lijffijt 2006.
| Study characteristics | ||
| Methods | 3‐week, randomised, double‐blind, cross‐over, within‐participant trial conducted to test MPH/placebo to assess correlations between measures of attention and inhibition with dopamine and norepinephrine blood levels:
|
|
| Participants | Number of participants screened: not stated Number of participants included: 15 (13 boys, 2 girls) Number of participants followed up: 15 Number of withdrawals: 0 Diagnosis of ADHD: DSM‐IV (combined (11), hyperactive‐impulsive (2), inattentive (2)) Age: mean 10.74 years (range 7‐13) IQ: mean 97.60 MPH‐naive: 0% Ethnicity: not stated Country: the Netherlands Setting: outpatient clinic Comorbidity: anxiety (n = 6), ODD (n = 5) Comedication: not stated Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to different possible drug condition orders of 0.5 mg/kg or 1.0 mg/kg MPH and placebo Mean MPH dosage: 22.67 mg Administration schedule: not stated Duration of each medication condition: 1 day Washout before trial initiation: 24 h before testing Medication‐free period between interventions: no Titration period: none/duration Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
General behaviour
Non‐serious AEs
|
|
| Notes | Sample calculation: no Ethics approval: yes; "The study was approved by the national medical ethical committee (CCMO)" Key conclusion of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: yes, see inclusion criteria Any withdrawals due to AEs: yes, "four participants were too fatigued after placebo or the 1.0 mg/kg dose to continue with the change task after they first completed the stop task" Funding source: not declared Email correspondence with trial authors: March 2014: we sent an email to the trial author to request additional information but have received no reply. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts. "All participants were familiar with the intake of MPH for at least 1 year" Selection bias (e.g. titration after randomisation → exclusion of methylphenidate non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
Lin 2014.
| Study characteristics | ||
| Methods | 8‐week, multi‐centre (31 sites in 5 countries), double‐blind, placebo‐controlled, comparator (OROS‐MPH), parallel trial with 3 interventions:
Phases
|
|
| Participants | Number of participants screened: 448 Number of participants included: 340 (70.6% boys, 29.4% girls) Number of participants followed up: 210 Number of withdrawals: 60 Diagnosis of ADHD: DSM‐IV‐TR (combined (70.9%), hyperactive‐impulsive (4.1%), inattentive (25%)) Age: mean 11.6 years (range 6‐17) IQ: not stated MPH‐naive: all participants treated with MPH were medication‐naive. 44% of placebo‐treated participants, 47% of edivoxetine‐treated participants in the 0.1 mg/kg/d arm and 49% of edivoxetine‐treated participants in each of the 0.2 mg/kg/d and 0.3 mg/kg/d arms had used stimulants previously Ethnicity: white (72.6%), African American (not stated), Asian (not stated), Hispanic (not stated), other (not stated) Country: USA, Canada, Taiwan, Mexico and Puerto Rico Setting: outpatient clinic Comorbidity: ODD ("less than 20%") Comedication: not stated Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 4 possible drug condition orders of 18 mg, 36 mg or 54 mg OROS‐MPH and placebo Mean MPH dosage: not stated Administration schedule: 1/d Duration of each medication condition: 8 weeks Washout before trial initiation: all MPH‐naive Medication‐free period between interventions: no Titration period: none, initiated after randomisation Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
Non‐serious AEs
|
|
| Notes | Sample calculation: not stated Ethics approval: yes Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: yes (3 participants) Funding source: Ely Lilly Email correspondence with trial authors: April 2015. We emailed trial authors to ask for supplemental information/data but have received no response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Interactive voice response system was used for randomisation and to determine which trial drug should be dispensed |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): yes, exclusion of MPH non‐responders (after randomisation) |
| Selective reporting (reporting bias) | High risk | Clear indication of selective reporting |
Lopez 2003.
| Study characteristics | ||
| Methods | Cross‐over trial with 3 interventions:
Phases: 4
Evaluated on day 0, randomly assigned to drug condition on days 7, 14, 21 and 28. 1 practice visit for a trial duration of 5 weeks in total |
|
| Participants | Number of participants screened: not stated Number of participants included: 36 (29 boys, 7 girls) Number of participants followed up: 36 Number of withdrawals: 0 Diagnosis of ADHD: DSM‐IV (subtype not stated) Age: mean 9 years (range 6‐12) IQ: not stated MPH‐naive: no Ethnicity: white (36%), African American (27%), Hispanic or other (36%) Country: USA Setting: outpatient clinic, laboratory classroom setting Co‐morbidity: not stated Co‐medication: not stated Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 24 (4 × 3 × 2 × 1) possible drug condition orders of IR‐MPH (Ritalin) 20 mg, ER‐MPH 18 mg (Concerta), ER‐MPH 36 mg (Concerta) and placebo Mean MPH dosage: not stated Administration schedule: not stated Duration of each medication condition: 1 day Washout before trial initiation: not stated Medication‐free period between interventions: on the morning following each trial period, participants resumed their regularly prescribed medication up to Thursday evening before the next trial period day on Saturday Titration period: all participants had been stabilised on an equivalent dose of 10 mg twice daily of MPH before trial entry Treatment compliance: no participants discontinued the trial prematurely |
|
| Outcomes |
ADHD symptoms
Non‐serious AEs
|
|
| Notes | Sample calculation: yes Ethics approval: yes Comment from trial authors
Key conclusion of trial authors
Comment from review authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: yes, all participants were stabilised previously on MPH Any withdrawals due to AEs: no Funding source: Novartis Email correspondence with trial authors: April 2014. We received supplemental information from trial authors. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Order of medication assignment was determined by random assignment by a computer programme |
| Allocation concealment (selection bias) | Unclear risk | Participants were randomly assigned to 4 treatment periods. For purposes of this trial, with the exception of the medicating nurse, all trial personnel were blinded to the medication administered to the child |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Single‐blind. For purposes of this trial, with the exception of the medicating nurse, all trial personnel were blinded to the medication administered to the child |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None reported. No participants discontinued the trial prematurely. Selection bias: no |
| Selective reporting (reporting bias) | Unclear risk | Protocol not identified |
Lufi 1997.
| Study characteristics | ||
| Methods | Cross‐over trial with 2 interventions:
Phases: participants randomly assigned to 3 weeks of MPH, 3 weeks of placebo. No washout. Assessment at baseline and by the end of each phase |
|
| Participants | Number of participants screened: not stated Number of participants included: 20 (18 boys, 2 girls) Number of participants followed up: 20 Number of withdrawals: 0 Diagnosis of ADHD: DSM‐IV (subtype not stated) Age: mean 9.23 years (± 1.62, range 7.17‐12.42) IQ: > 70 MPH‐naive: 100% Ethnicity: not stated Country: Israel Setting: outpatient clinic Comorbidity: none Comedication: no Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 2 possible drug condition orders of 10 mg MPH and placebo Mean MPH dosage: 10 mg/d Administration schedule: mornings Duration of each medication condition: 3 weeks Washout before trial initiation: all participants were treatment‐naive Medication‐free period between interventions: none Titration period: none Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
General behaviour
|
|
| Notes | Sample calculation: not stated Ethics approval: not stated Comment from trial authors
Key conclusions of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: no Funding source: not declared Email correspondence with trial authors: September 2013. We obtained supplemental information/data from trial authors |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Everyone involved in the assessment phase ...was blind to the type of medication..." All medications (both placebo and MPH) were given in identical capsules to prevent recognition of the true medication |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "...the parents, the teacher, the child and the psychologist who tested the child did not know what kind of medication the child was taking..." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified |
Lufi 2007.
| Study characteristics | ||
| Methods | 6‐week, randomised, double‐blind, placebo‐controlled, cross‐over trial conducted to detect the effects of MPH on co‐ordination and handwriting:
|
|
| Participants | Number of participants screened: 19 Number of participants included: 19 (12 boys, 7 girls) Number of participants followed up: 19 Number of withdrawals: 0 Diagnosis of ADHD: DSM‐IV (subtype not stated) Age: mean 9.51 years (range 7.08‐13.83) IQ: > 70 MPH‐naive: 0% Ethnicity: not stated Country: Israel Setting: outpatient clinic Comorbidity: not stated Comedication: no Other sociodemographics: "Participants were from a medium level social‐economic status" Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 2 possible drug condition orders of MPH and placebo Mean MPH dosage: 0.4 mg/kg Administration schedule: not stated Duration of each medication condition: 3 weeks Washout before trial initiation: no Medication‐free period between interventions: yes Titration period: not stated Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
General behaviour
|
|
| Notes | Sample calculation: not stated Ethics approval: not stated Comments from trial authors (limitations)
Key conclusions of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: unclear Any withdrawals due to AEs: no Funding source: not declared Email correspondence with trial authors: October 2013. We obtained additional information on IQ from the first trial author. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomized" |
| Allocation concealment (selection bias) | Unclear risk | "The study was designed as a double‐blind, randomized, crossover, placebo‐control procedure" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Each participant was given exactly 0.4 mg/kg of MPH in special capsules of MPH and a placebo to avoid recognition of the medication" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
Manos 1999.
| Study characteristics | ||
| Methods | 4‐week, double‐blind titration, placebo‐controlled protocol. Cross‐over trial with 2 interventions in 4 doses:
Phases
Manos 1999: "7 youths given MPH and 4 given Adderall [MAS] did not receive the 15 mg dose of medication. The decision to forego the 15 mg condition was based on the paediatrician’s assessment that the child was too young or underweight for this high dose and our assessment that the best dose had already been achieved at a lower dose" |
|
| Participants | Number of participants screened: 195 Number of participants included: 177. (33 boys, 9 girls) Number of participants followed up: 134 Number of withdrawals: 43 Diagnosis of ADHD: DSM‐IV (combined (55%), inattentive (45%)) Age: mean 10.1 years (SD not stated, range 5‐17) IQ: > 70 MPH‐naive: not stated Ethnicity: white (93%), African American (7%), Asian (0%), Hispanic (0%), others (0%) Country: USA Setting: outpatient clinic Comorbidity: no significant comorbid disorders. Although no formal comorbidity data are available, it appears that psychiatric comorbidity was modest in this cohort Comedication: not stated Sociodemographics: predominantly well educated Manos 1999 and Faraone 2002 (secondary reference under Manos 1999): 42 were participants matched to the MAS (Adderall) group in order of diagnostic category, age and sex. Only these 42 of 117 receiving MPH were compared with the MAS (Adderall) group Findling 2001a (secondary reference under Manos 1999): 195 youths entered the trial. Data for a best dose were provided for 177 participants: 111 in the MPH group, 66 in the MAS group Diagnosis of ADHD: inattentive (47%), combined (53%). Inattentive subtype is over‐represented in the older age group Age group: 4‐8 years (mean 6.35) 69 (57 boys); 8‐11 years (mean 9.47) 56 (45 boys); 11‐17.59 years (mean 13.64) 52 (41 boys) Other sociodemographics: no significant differences in baseline demographics were noted between groups Findling 2001b (secondary reference under Manos 1999): Number of participants included: 195 Number of participants completed: 137: MPH 82, MAS (Adderall) 55 Diagnosis of ADHD: DSM‐IV (combined (57%), inattentive (43%)) Age: mean 10 years (SD not stated, range 4‐17) IQ: > 70 Sex: 66 boys, 16 girls Ethnicity: white (84%), African American (6%), other (10%) Country: USA Comorbidity: without significant comorbid disorders Comedication: possible, but not recorded Sociodemographics: predominantly well educated. No differences in sex, ethnicity or ADHD subtype were found between MPH and MAS (Adderall) groups. No participants had a history of hypertension, hypotension or clinically significant cardiovascular disease Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to different orders of 5 mg, 10 mg or 15 mg MPH and placebo Mean MPH dosage: best dose 9.1 mg‐10.4 mg Administration schedule: morning (at 8.00 am) and noon Duration of each medication condition: 1 week Washout before trial initiation: none Titration period: none Treatment compliance: 11 terminated because of AEs |
|
| Outcomes |
ADHD symptoms Findling 2001a (secondary reference under Manos 1999): compared treatment for children and adolescents and weight‐adjusted dosing of MPH. Measurement instruments include the following
Best dose based only on Abbreviated Symptoms Questionnaire ‐ Teacher. Does not separate MPH and MAS in tables Non‐serious AEs
Findling 2001b (secondary reference under Manos 1999)
|
|
| Notes | Sample calculation: not stated Ethics approval: not stated Key conclusions of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: yes; 15 youths in the MAS sample had been tried on MPH before enrolment in this medication trial. Because of lack of response or serious side effects, these children discontinued use of MPH. A total of 37% of the MAS sample subsequently was composed of children who had unsuccessfully used MPH but successfully responded to MAS Any withdrawals due to AEs: yes; Findling 2001a (11/195) (secondary reference under Manos 1999), due to multiple AEs Funding source: in part by from Shire Pharmaceutical Development Incorporated to Dr. Faraone Email correspondence with trial authors: April 2014. Emailed trial authors twice to request additional data but have received no answer |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | First phase not blinded. MPH is a selected group |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Clinician, teacher and parent were blinded only to dose, not to medication |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Clinician, teacher and parent were blinded only to dose, not to medication |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Only best dose compared with placebo. Findling (Abbreviated Symptoms Questionnaire) + 30 participants. Of 43 participants with < 4 weeks of data, 30 had only 3 weeks of data because physicians considered them too young or too small to receive 15 mg before initiating protocol Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): yes |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified |
Martins 2004.
| Study characteristics | ||
| Methods | 4‐week, double‐blind, randomised, parallel trial with 2 interventions:
|
|
| Participants | Number of participants screened: not stated Number of participants included: 40 (all boys) Number of participants followed up: 38 Number of withdrawals: 2 (receiving MPH) Diagnosis of ADHD: DSM‐IV (combined (92.5%)) Age: mean MPH 9 years (SD 2.2, range 6‐14), placebo 9.6 years (SD 2.8, range 6‐14) IQ: mean, MPH 97.3, placebo 93.5 MPH‐naive: not stated Ethnicity: European‐Brazilian: MPH 16 (76.2%), placebo 15 (78.9%) Country: Brazil Setting: outpatient clinic Comorbidity: yes; conduct or ODD (MPH 57.2%, placebo 57.9%) Comedication: not stated, no psychiatric medication Other sociodemographics: monthly family income was calculated according to the following formula: total monthly income received by all members of the family (expressed in number of minimum wages) divided by the number of people in the family. A value lower than 0.7 (approximately USD 68 per family member per month) is usually an indicator of poverty in Brazil. MPH 3.3, placebo 2.4. No significant differences in baseline demographics were noted between the 2 groups Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to MPH 7 d/week or to MPH on weekdays and placebo on weekends Number randomised to each group: MPH 21, placebo 19 Mean MPH dosage: initial dose of MPH was 0.3 mg/kg/d the 1st week. Dose was raised to 0.5 mg/kg/d the 2nd week and to 0.70 mg/kg/d the 3rd and 4th weeks Administration schedule: twice/d: breakfast and lunch Duration of intervention: 4 weeks Titration period: none Treatment compliance: only 7 of 160 blister packs were returned with unused pills |
|
| Outcomes |
ADHD symptoms
Serious AEs
(Completed only by parents for assessment of side effects on weekends) |
|
| Notes | Sample calculation: no Ethics approval: approved by the Ethical Committee of the Hospital de Clínicas de Porto Alegre (HCPA) (approved as an International Review Board (IRB) by the Office for Human Research Protections, USA) Comments from trial authors
Key conclusion of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: no Funding source: MPH and placebo pills were supplied by Novartis Pharmaceuticals (São Paulo, Brazil) at no cost and without restrictions. No additional funding was requested or received from Novartis or any other commercial entity Email correspondence with trial authors: we have contacted trial authors several times to ask for additional information about data, but we have received no data from this trial that we can use |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised, double‐blind, parallel‐group design was used. Participants were randomly assigned to 1 of 2 groups, according to a computer‐derived algorithm (EPIINFO.06) |
| Allocation concealment (selection bias) | Low risk | Computer‐derived algorithm |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | MPH and placebo pills were of the same shape and colour |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double‐blind. No other information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Measured adherence to protocol by assessing returned, unopened blister packs. None of the findings in the analyses was significantly affected by the 2 participants (they did not follow the protocol as stated by researchers) from the MPH group who did not receive a few doses appropriately on weekends or on Monday. Some teacher ratings (8.5%) were missed because of the child’s absence from school on a specific day of evaluation or because of a school holiday Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Low risk | No indication of reporting bias |
Matthijssen 2019.
| Study characteristics | ||
| Methods | A 7‐week parallel discontinuation trial with 2 arms:
Phases: 1 |
|
| Participants | Number of participants screened: 530 Number of participants included: 104 Number of participants followed‐up: 94 (73 (77.7%) boys, 21 (22.3%) girls) Number of withdrawals: 25, 10 of whom did not receive allocated intervention Diagnosis of ADHD: no diagnosis by specified diagnostic tool required Age: MPH (continuation): mean 13.8 years (SD 2.2), placebo (discontinuation): mean 13.6 years (SD 2.2, range 8 years‐17 years) IQ: IQ < 70 was an exclusion criterion MPH‐naive: 0% (discontinuation trial) Ethnicity: European white ethnicity (MPH: n = 47 (100%), placebo: n = 46 (97.9%)) Country: the Netherlands Setting: outpatient Comorbidity: not stated Comedication: both medication and interventions used before trial initiation was allowed during the trial, as long as it did not fall within the exclusion criteria. 35 received comedication; of these: 32 received melatonin, 4 received hay fever medication, 2 received asthma medication, 1 received antipsychotic medication Additional sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 2 different groups, 1 continued either 36 or 54 mg of ER‐MPH for 7 weeks, the other discontinued medication by withdrawing gradually over 3 weeks period (week 1: 36 mg/d, week 2: 27 mg/d, week 3: 18 mg/d) followed by 4 weeks of complete placebo Number randomised to each group: discontinuation group (placebo) = 53, continuation group (ER‐MPH group) = 51 Mean medication dosage: continuation group at baseline: 0.93 mg/kg/d (SD 0.29) Administration schedule: participants used the same schedule for taking their medication as they did before the trial (e.g. daily or with weekend stops). Duration (of (each) medication): placebo group: 36 mg/d during week 1, 27 mg/d during week 2, 18 mg/d during week 3, and placebo for weeks 4 through 7. MPH group: 7 weeks of MPH Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
Serious AEs
General behaviour
Quality of life
Non‐serious AEs
|
|
| Notes | Sample calculation: yes, 120 (60 in each arm); “With this sample size, it is possible to detect an effect size (Cohen’sd) of 0.251 with a power of 0.80 and alpha of 0.05, as calculated with the program G*Power, version 3.1.” Sample size calculation was not met Ethics approval: the trial was approved by national and local institutional review board committees. Comments from trial authors
Key conclusion of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH: only children and adolescents who had used MPH for ≥ 2 years were included Any withdrawals due to AEs: yes, 1 Funding source: The Netherlands Organization for Health Research and development (ZonMw, grant 836011014) Email correspondence with trial authors: August and October 2021. We received information regarding risk of bias through personal email correspondence with the trial authors in August and October 2021 (Storm 2021d [pers comm]) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The trial pharmacy dispensed trial medication for either continued active medication or discontinuation according to 2 separate computer‐generated randomisation lists, for each dosage. A block‐randomisation of 6 was used to ensure even groups. |
| Allocation concealment (selection bias) | Low risk | Placebo capsules matched to medication. Dispensed by trial pharmacy |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The trial medication consisted of an over‐encapsulation of MPH in an osmotic‐controlled release oral delivery system (18 mg, 27 mg, 36 mg, and 54 mg). The over‐encapsulation was backfilled with lactose monohydrate for blinding purposes. Matching placebo capsules contained only the filler. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were not told the randomly determined treatment allocation of the trial participants. To ensure blinding the outcome assessors did not rate AEs and families were instructed not to discuss AEs with the outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "Analyses were conducted on the full data set, which included all participants who received at least 1 dose of the trial drug. In those who had withdrawn from the trial, we used ratings that were obtained at the time of trial termination." "A chi‐square test was used to analyse whether there was a difference between the two groups in the number of participants prematurely withdrawing from the trial." Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Low risk | No indication of selective reporting. The following outcomes will be part of separate reports: Barkley Side Effect Rating scale (BSERS), Family atmosphere questions, Parental Frustrations Questionnaire (PFQ), Child Depression Inventory (CDI) |
McBride 1988a.
| Study characteristics | ||
| Methods | Individual, double‐blind, cross‐over trial for 4 weeks with 2 interventions:
|
|
| Participants | Number of participants screened: not stated Number of participants included: 73 Number of participants followed up: 70 (53 boys, 17 girls) Number of withdrawals: 3 Diagnosis of ADHD: DSM‐III (77% met the criteria for ADD‐H) Mean age: MPH responders 8.5 (SD not stated), MPH non‐responders 9.5 (SD not stated). Range 6‐17 years Mean IQ: MPH responders 102 (SD 21), MPH non‐responders 89 (SD 23) MPH‐naive: 71 (97%) Ethnicity: not stated Country: USA Setting: outpatient clinic Comorbidity: not stated Comedication: carbamazepine or phenytoin or valproic acid or mephobarbital (6.4 receiving a combination of these drugs). Clonidine (n = 1) Sociodemographics: no information Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 2 possible drug condition orders of 0.3 mg/kg MPH and placebo. MPH was rounded to the nearest 1.25 mg Mean MPH dosage: no information, but mean dose during follow‐up was 0.36 mg/kg/dose Administration schedule: morning and 4 h later Duration of each medication condition: 2 weeks Washout before trial initiation: none Medication‐free period between interventions: none Titration period: none Treatment compliance: no information |
|
| Outcomes |
ADHD symptoms
Serious AEs
Non‐serious AEs
None of the parents of responders who had experienced side effects during the trial thought the effects were significant enough that they should not treat their child with MPH, and no side effects other than appetite suppression continued during regular therapy after the trial Follow‐up 6 months after: n = 33. 15 had no change in weight curves. 1 gained 7 kg beyond his original percentile |
|
| Notes | Sample calculation: no information Ethics approval: no information Comments from trial authors
Key conclusions of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: no Funding source: not declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | The pharmacist labelled the 2 sets of capsules as “Medicine A” and “Medicine B” in either order by coin flip for the first 22 trials, and then by using a random numbers table |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The manner of labelling was sealed by the pharmacist in an envelope that was not opened until the trial had ended and findings had been discussed with the parents |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Objectivity was lessened for a few parents because the decreased appetite associated with MPH led them to suspect which capsules contained the drug |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | In the few trials for which 1‐3 of 10 items on Conners' questionnaire had not been scores, the score was prorated based on 30 points maximum Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Low risk | No indication of selective reporting |
McCracken 2016.
| Study characteristics | ||
| Methods | An 8‐week parallel‐trial with 3 arms:
Phases: 2 |
|
| Participants | Number of participants screened: 323 Number of participants included: 212; 5 withdrew, before receiving trial drug, leaving 207 participants (142 (68%) boys, 65, (32%) girls) Number of participants followed‐up: 182 (ER‐d‐MPH = 61, IR‐guanfacine = 60, IR‐guanfacine+ER‐d‐MPH = 61) Number of withdrawals: 30 (including 5 before receiving trial drug) (ER‐d‐MPH = 9, IR‐guanfacine = 11, IR‐guanfacine+ER‐d‐MPH = 10) Diagnosis of ADHD: DSM‐IV (information available for 202 participants: 105 (51% ) combined, 5 (2%) hyperactive/impulsive, 92 (44%) inattentive) Age: mean 10.0 years (SD2.1, range 7‐14) IQ: mean 102.4 (SD 13.5) MPH‐naive: not stated Ethnicity: Hispanic (n = 44, 21.3%). Race reported: white (n = 143, 69%), African American (n = 36, 17%), Asian, Pacific Islander (n = 16, 8%), other (n = 12, 6%) Country: USA Setting: not stated Comorbidity: ODD 68 (33%) Comedication: CNS medication not allowed Additional Sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 3 different treatment sequences covering the periods baseline, weeks 1‐4, and weeks 5‐8 as follows:
ER‐d‐MPH (sequence 1 and 3) was given to participants < 25 kg at 5 mg on week 5, 10 mg to participants > 25 kg with an increase of 5 mg/week until week 7 IR‐guanfacine (sequence 2 and 3) was initiated at 0.5 mg then increased to 1 mg and increased by 0.5 mg each week until week 3. If Clinical‐Global Impression—Improvement ratings were either 1 or 2, no dose increases were made Placebo was mirrored Number randomised to each group: 70 ER‐d‐MPH, 71 IR‐guanfacine, 71 IR‐guanfacine + ER‐d‐MPH Mean medication dosage: mean final (week 8) daily doses of:
Mean mg/kg daily doses of guanfacine were 0.06 (+ 0.03) mg/kg/d for both guanfacine groups Administration schedule: twice daily
Duration (of (each) medication): IR‐guanfacine 8 weeks, ER‐d‐MPH 4 weeks, placebo (sequence 2) 4 weeks, placebo (sequence 1) 8 weeks Washout before trial initiation: not stated Medication‐free period between interventions: no Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
Serious AEs
Non‐serious AEs
|
|
| Notes | Sample calculation: not stated Ethics approval: yes; “All study procedures were approved by the University of California, Los Angeles (UCLA) institutional review board and were overseen by a data safety and monitoring board." Comments from trial authors
Key conclusion of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH: no Any withdrawals due to AEs: yes, IR‐guanfacine =1, Er‐d‐MPH 1, IR‐guanfacine + ER‐d‐MPH = 2 Funding source: NIMH Research Center grant P50MH077248, “Translational Research to Enhance Cognitive Control” (JTM) Email correspondence with trial authors: September 2021. We received supplemental information regarding risk of bias assessment through personal email correspondence with the trial authors in September 2021 (Storm 2021e [pers comm]) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised based on a computer‐generated program to yield a 1:1:1 allocation to the 3 different treatment sequences |
| Allocation concealment (selection bias) | Low risk | Dosing clinicians were blinded to group assignment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | At the time of submission of a participant found to be eligible into the centralised database, the program generated the treatment sequence assignment, which was then sent electronically to the Research Pharmacist only and was not shared with any trial staff. All staff involved in clinical assignments remained blinded, as were participants. Participants were blinded by use of uniform over‐encapsulation of drug or placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | A blinded clinician without knowledge of AEs completed the CGI‐S and ADHD‐RS‐IV at baseline and at the end of each within‐participant condition or last visit based on parent, participant and other available data. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): yes, exclusion of 1 non‐responder |
| Selective reporting (reporting bias) | Low risk | No indication of selective reporting. The secondary outcome Tests of Academic Performance is set in the second phase of the trial. Analysis has not yet been completed or published. |
McGough 2006.
| Study characteristics | ||
| Methods | Phase II, randomised, double‐blind, placebo‐controlled, laboratory‐classroom, cross‐over trial with a lead‐in open‐label dose‐optimisation phase:
|
|
| Participants | Number of participants screened: 93 entered the open‐label dose‐optimisation phase Number of participants included: 80 (from ITT: 57 boys (72%), 22 girls (28%)) Number of participants followed up: 79 Number of withdrawals: 1 participant discontinued because of "protocol violation" Diagnosis of ADHD: DSM‐IV‐R (combined 62 (79%), hyperactive‐impulsive 4 (5%), inattentive 13 (17%)) Age: mean 9.1 years (SD 1.7, range 6‐12) IQ: > 70 MPH‐naive: 37% Ethnicity: white 55 (70%), African American 8 (10%), Asian 2 (3%), other 14 (18%) Country: USA Setting: outpatient clinic Comorbidity: not stated Comedication: no Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 2 possible drug condition orders of MPH and placebo Number randomised to each group: cross‐over, 42 MPH/placebo, 38 placebo/MPH Mean MPH dosage: "At the end of the dose optimization phase of the study, the majority of patients (78%) were optimized to either the 16 mg or 20 mg dosage strengths" Administration schedule: once daily Duration of each medication condition: 1 week Washout before trial initiation: "up to 28 days" Medication‐free period between interventions: 4:00 pm to 7:00 am the next day (15 h) Titration period: 5‐week dose‐optimisation phase before randomisation Treatment compliance: "During the laboratory classroom period, 97% and 96% of participants were compliant with [methylphenidate transdermal system] MTS and placebo treatments respectively" |
|
| Outcomes |
ADHD symptoms
"The mean values of the CPRS‐R over the 11:00 am and 3:00 pm time points were used in the analysis" Non‐serious adverse outcomes
|
|
| Notes | Sample calculation: yes Ethics approval: yes Key conclusions of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: yes; "Participants were either known to be responsive to stimulants or‐naive to stimulant treatment". Participants were only randomised after the open‐label phase if they reached acceptable efficacy without unacceptable AEs. Any withdrawals due to AEs: no Funding source: Shire US Inc Email correspondence with trial authors: June 2014. We obtained supplemental information regarding risk of bias. Additional data were not available (Ramstad 2013a [pers comm]) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participants were randomized into either 1 week of [methylphenidate transdermal system] MTS or 1 week of [placebo transdermal system] PTS (in their individually optimized dose) and were crossed over to the opposite treatment the following week" From correspondence: "Participants were randomized centrally for each of the study conditions. Randomization codes were not available to site study staffs, but were provided to research pharmacies at each site which corresponded to a particular dose pack" (Ramstad 2014 [pers comm]) |
| Allocation concealment (selection bias) | Low risk | See above |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Phase‐II randomized, double‐blind, placebo‐controlled laboratory classroom, crossover study with a lead‐in open‐label dose optimization phase" From correspondence: "Active and inactive patches were identical in appearance" (Ramstad 2014 [pers comm]) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | See above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 1 participant excluded due to protocol violation Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Low risk | Trial protocol identified (NCT00466791), all outcomes reported |
McInnes 2007.
| Study characteristics | ||
| Methods | Cross‐over trial with 2 interventions:
Phases: 4 |
|
| Participants | Number of participants screened: 17 Number of participants included: 16 (12 boys, 4 girls) Number of participants followed up: 16 Number of withdrawals: 0 Diagnosis of ADHD: DSM‐IV‐TR (combined (63%), hyperactive‐impulsive (6%), inattentive (31%)) Age: mean 9.2 years (range 7‐12) IQ: mean 107.7 (range not reported) MPH‐naive: ~ 80% Ethnicity: not stated Country: Canada Setting: outpatient clinic Comorbidity: ODD (19%), CD (25%), generalised anxiety disorder (31%) Comedication: not stated Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 12 possible drug condition orders of low, medium and high MPH and placebo Mean MPH dosage: LD (mean 0.21 mg/kg to 0.33 mg/kg, SD 0.07 to 0.02); MD (mean 0.31 mg/kg to 0.43 mg/kg, SD 0.09 to 0.03); and HD (mean 0.42 mg/kg to 0.65 mg/kg, SD 0.13 to 0.15) MPH Administration schedule: 1/d at 9:00 am Duration of each medication condition: 1 day Washout before trial initiation: 48 h before trial Titration period: none Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
|
|
| Notes | Sample calculation: no Ethics approval: yes Comments from trial authors
Key conclusions of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: no Funding source: The Psychiatric Endowment Fund |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Multiple blind procedures", capsules identically packaged by pharmacists |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Examiner, who was kept blind to child’s medication status" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No LTFU Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | High risk | Symptom data not reported |
Merrill 2021.
| Study characteristics | ||
| Methods | A 6‐week cross‐over trial with 3 arms:
|
|
| Participants | Number of participants screened: not stated Number of participants included: 21 (all boys) Number of participants followed up: 21 Number of withdrawals: 0 Diagnosis of ADHD: not stated Age: mean 9.32 years, no range stated IQ: mean 105.52 MPH‐naive: not stated Ethnicity: not stated Country: USA Setting: summer treatment programme Comorbidity: not stated Comedication: not stated Additional sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to different drug condition orders of 0.3 mg/kg MPH, 0.6 mg/kg MPH and placebo twice daily Number randomised to each group: 21 Mean medication dosage: fixed‐dose trial with a period of 0.3 mg/kg twice/d (LD) and 0.6 mg/kg twice/d (HD) Administration schedule: not stated Duration (of (each) medication): not stated, but full duration of the trial was 6 weeks Washout before trial initiation: not stated Medication‐free period between interventions: not stated Titration period: none Treatment compliance: not stated |
|
| Outcomes |
General Behavior
|
|
| Notes | Sample calculation: no Ethics approval: not stated Comments from trial authors
Key conclusion of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH: not stated Any withdrawals due to AEs: no Funding source: not stated Supplemental information regarding trial design was requested through personal email correspondence with the authors in July 2022 but no answer was received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The trial is described as double‐blind but there is no information about the method |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Nothing stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the analysis Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Unclear risk | No trial protocol identified |
Moshe 2012.
| Study characteristics | ||
| Methods | 2‐week, randomised, double‐blind, cross‐over trial with 2 interventions:
|
|
| Participants | Number of participants screened: 78 Number of participants included: 57 (all boys) Number of participants followed up: 57 Number of withdrawals: 0 Diagnosis of ADHD: DSM‐IV (combined and hyperactive‐impulsive (53%), inattentive (47%)) Age: mean 9.5 years (range 7‐12) IQ: normal MPH‐naive: 100% Ethnicity: not stated Country: Israel Setting: outpatient clinic Comorbidity: no Comedication: no Other sociodemographics: representing all socioeconomic strata Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 2 possible drug condition orders of 0.3 mg/kg IR‐MPH and placebo MPH dose range: 6 mg‐12 mg Administration schedule: once daily Duration of each medication condition: 1 week Washout before trial initiation: no (drug‐naive) Titration period: no Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
|
|
| Notes | Sample calculation: yes Ethics approval: yes Comments from trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: no Funding source: none Email correspondence with trial authors: April‐October 2013. We received supplemental data from trial authors |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned with a table of random numbers |
| Allocation concealment (selection bias) | Low risk | Placebo (prepared as look‐alike capsules by the hospital pharmacy) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Low risk | No indication of selective reporting. According to trial authors, all planned outcomes were assessed and analysed |
Muniz 2008.
| Study characteristics | ||
| Methods | Randomised, multi‐centre, double‐blind, 5‐period, cross‐over trial in a laboratory classroom setting with 3 interventions:
Phases: ER‐d‐MPH 20 mg/d, ER‐d‐MPH 30 mg/d, ER‐d,l‐MPH 36 mg/d, ER‐d,l‐MPH 54 mg/d and placebo |
|
| Participants | Number of participants screened: 84 Number of participants included: 84 (55 boys, 29 girls) Number of participants followed up: 81 Number of withdrawals: 3 Diagnosis of ADHD: DSM‐IV (combined (89.3%), inattentive (10.7%)) Age: mean 9.5 years (SD 1.7, range 6‐12) IQ: not stated MPH‐naive: 0% Ethnicity: white (42.9%), African American (27.4%), Hispanic (28.6%), other (1.2%) Country: USA Setting: outpatient clinic Comorbidity: no Comedication: no Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 5 possible drug condition orders of ER‐d‐MPH 20 mg/d, 30 mg/d, 36 mg/d, 54 mg/d and placebo Mean MPH dosage: not stated Administration schedule: once daily. Morning dosing as 2 capsules Sunday to Saturday. 6 doses were administered at home (Sunday to Friday), and the Saturday dose was administered by research staff. This was repeated until all 5 treatments had been administered. Mean duration of exposure to trial medication was 7 days for all 5 treatments Washout before trial initiation: 6 days medication‐free Titration period: "On stabilized total daily dose or the nearest equivalent dose of 40 to 60 mg of d,l‐MPH or 20 to 30 mg d‐MPH for at least 2 weeks prior to the screening visit" initiated before randomisation Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
Serious AEs
General behaviour
Non‐serious AEs
|
|
| Notes | Sample calculation: yes; "It was determined that approximately 90 patients were required to detect a 0.05‐level treatment difference at 84% power assuming a difference and standard deviation of 3.5 and 11 for SKAMP‐Combined score, at 2 h post‐dose using a paired t‐test"
Ethics approval: no information Key conclusions of trial authors
Comment from review authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: yes; "Children recruited for this study had been stabilized on a total daily dose or the nearest equivalent dose of 40 to 60 mg of d,l‐MPH or 20 to 30 mg d‐MPH for at least 2 weeks prior to the screening visit" ‐ so presumably non‐responders to MPH and those experiencing intolerable AEs while taking MPH were not included Any withdrawals due to AEs: no Funding source: "This study was funded by Novartis Pharmaceuticals Corporation and reports the following involvement: design and conduct of the study; collection, management, analysis, and interpretation of data; preparation, review, and approval of the manuscript" Email correspondence with trial authors: July 2014. Emailed trial authors to ask for additional information but have not received a reply |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "A total of 84 subjects were randomized to receive treatment and were included in the efficacy and safety analyses" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "After the Practice Day, the first assigned treatment was dispensed to the parents as blinded capsules according to their child’s randomized sequence. To maintain blinding, all treatments were over‐encapsulated and the same number of capsules were given once daily for each sequence" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The ratings were based on the frequency and quality of behaviours as observed by three independent, blinded raters in each class. To maintain consistency throughout the study, the blinded observers were responsible for observing and rating the same 6 children at specified intervals throughout the 12‐hour testing period at each center" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "The intent‐to‐treat population included all randomized patients who took at least 1 dose of study medication and had at least 1 post‐dose efficacy measurement" Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Low risk | No selective outcome reporting |
Murray 2011.
| Study characteristics | ||
| Methods | Double‐blind, randomised, cross‐over, analogue classroom trial with 2 interventions:
Phases
|
|
| Participants | Number of participants screened: 89 were included in the open‐label phase Number of participants included: 68 (45 boys, 23 girls) Number of withdrawals: 2 Diagnosis of ADHD: DSM‐IV‐TR (combined (59%), hyperactive‐impulsive (4%), inattentive (37%)) Age: mean 10.75 years (range 5‐15) IQ: > 80 MPH‐naive: 65% Ethnicity: white (62%), African American (28%), Asian (not stated), Hispanic (not stated), other (10%) Country: USA Setting: outpatient clinic Comorbidity: anxiety (9%), depressive disorders (1%), learning disability (38%) Comedication: not stated Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 2 possible drug condition orders of OROS‐MPH and placebo Mean OROS‐MPH daily dosage: 47.6 mg Administration schedule: once daily, morning Average duration of OROS‐MPH treatment: not stated, but the medication was given under double‐blind condition on 1 day Duration of placebo intervention: 1 day Washout before trial initiation: up to 28 days Medication‐free period between interventions: no Titration period: before randomisation, up to 6 weeks Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
Serious AEs
Non‐serious AEs
|
|
| Notes | Sample calculation: yes Ethics approval: yes Key conclusion of trial authors
Inclusion of MPH responders only/exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: yes; a history of failed response to MPH was an exclusion criterion. During the open‐label phase before randomisation, 21 participants withdrew. Any withdrawals due to AEs: 2 Funding source: supported by Ortho‐McNeil Janssen Scientific Affairs, LLC Email correspondence with trial authors: June 2013‐June 2014. We have attempted to obtain supplemental efficacy data (SKAMP) and safety data from trial authors. We are awaiting data from the Yale Open Data Access Project |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind. Placebo and OROS‐MPH were matched in appearance |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 3 participants received OROS‐MPH on both laboratory school days in error; therefore, only data for their 1st laboratory school assessment were included in the analyses Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Low risk | Outcomes reported according to protocol |
Musten 1997.
| Study characteristics | ||
| Methods | Double‐blind, randomised, placebo‐controlled, cross‐over trial with 3 interventions:
|
|
| Participants | Number of participants screened: 109 Number of participants included: 54 met inclusion criteria; of these, the parents of 13 children refused MPH treatment. In the final sample, 41 children (26 boys, 5 girls) were included. Number of participants followed up: 31 Number of withdrawals: 10 Diagnosis of ADHD: DSM‐III‐R (subtype not stated) Regarding participants completing the trial Age: mean 58.07 months (approximately 4.8 years) (range 48‐70) IQ: mean 99.26 MPH‐naive: 93.5% Ethnicity: not stated Country: Canada Setting: outpatient clinic Comorbidity: ODD (84%), CD (19%), mood disorder (0%), OCD (0%), overanxious disorder (0%), somatisation disorder (0%), psychotic symptoms (0%) Comedication: not stated Other sociodemographics: combined parental income CAD 42,000: 2‐parent home (74%), single‐parent home (26%). Significant differences were observed between the treatment‐refused group (n = 13) and the treatment‐completed group (n = 31) on baseline symptoms assessed by Diagnostic Interview for Children and Adults‐Parents; SNAP; and Conners' hyperactivity ratings Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 6 possible drug condition orders of LD (0.3 mg/kg) and HD (0.5 mg/kg) MPH and placebo Administration schedule: twice/d, morning and lunch Duration of each medication condition: 7 to 10 days Washout before trial initiation: 48 h before screening assessment Titration period: none Treatment compliance: treatment compliance was determined by counting the number of pills returned to the researcher at the end of each assessment week Data on compliance: not stated |
|
| Outcomes |
ADHD symptoms
Non‐serious AEs
|
|
| Notes | Sample calculation: no Ethics approval: no information Comment from trial authors
Key conclusions of trial authors
Inclusion of MPH responders only/exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: unclear, reasons for withdrawals not stated Funding source: Health Canada grant Email correspondence with trial authors: June 2013. Personal email correspondence with trial author did not provide supplemental data and information as requested because of author's retirement, and because he has no access to the data because the trial took place 15 years ago. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Treatment was presented in a fully randomised order as prepared by the hospital's pharmacy department |
| Allocation concealment (selection bias) | Low risk | MPH and placebo were placed in orange gelatin capsules to disguise taste differences |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All participants, research personnel and medical personnel were unaware of the order of medication conditions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All participants, research personnel and medical personnel were unaware of the order of medication conditions |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only data for children completing the entire trial were analysed. Resons for withdrawals not stated Selection bias (e.g. titration after randomisation → exclusion): not stated |
| Selective reporting (reporting bias) | Unclear risk | Not possible to get a copy of the protocol |
NCT00409708.
| Study characteristics | ||
| Methods | A 12‐week parallel trial with 2 arms:
Phases: 1 or 2 (there is mention of a washout, but it appears to happen after the trial) |
|
| Participants | Number of participants screened: 142 Number of participants included: 109 randomised, baseline characteristics from 104 (66 boys, 38 girls) Number of participants followed‐up: 77 Number of withdrawals: 32 Diagnosis of ADHD: DSM‐IV (subtype not stated) Age: mean 8.4 years (SD 1.83, range 6‐12) IQ: not stated MPH‐naive: not stated Ethnicity: not stated Country: USA Setting: outpatient Comorbidity: some were exclusion criteria Comedication: not stated Additional sociodemographics: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to: receive either behavior therapy alone or with MPH. Number randomised to each group: 56 randomised to behavior therapy, 53 randomised to behavior therapy and MPH Mean medication dosage: not stated. Participants received between 10‐60 mg/d Administration schedule: not stated Duration of each medication: 12 weeks Washout before trial initiation: there is mention of a washout, but it appears to happen after the trial. Titration period: not stated Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
Serious AEs
Non‐serious AEs
|
|
| Notes | Sample calculation: no Ethics approval: not stated Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH: not stated Any withdrawals due to AEs: no Funding source: Novartis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Almost a third of participants withdrew. Reasons for withdrawals are not stated. Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): unclear |
| Selective reporting (reporting bias) | Low risk | Since no cytogenetic effects were observed, blood samples were not analysed for pharmacokinetics/pharmacodynamics, otherwise all outcomes reported |
NCT02039908.
| Study characteristics | ||
| Methods | A 4‐week cross‐over trial with 2 arms:
Phases: 3 phases (titration phase, double‐blind cross‐over phase, and 10‐month medication‐holiday‐trial) |
|
| Participants | Number of participants screened: not stated Number of participants included: 267 (213 boys, 54 girls) Number of participants followed‐up: 248 Number of withdrawals: 19 Diagnosis of ADHD: DSM‐IV (subtype not stated) Age: mean not reported (range 6‐12 years) IQ: > 80 MPH‐naive: not stated Ethnicity: Hispanic or Latino (n = 224, 83.9%), not Hispanic or Latino (n = 43, 16.1%). Race: Asian (n = 2, 0.7%), black or African American (n = 22, 8.2%), white (n = 237, 88.8), mixed race (n = 6, 2.2%) Country: USA Setting: outpatient, summer treatment programme Comorbidity: some were exclusion criteria; ODD, CD or a mood or anxiety disorder not requiring psychotropic medication were allowed Comedication: no psychotropic comedication allowed Additional sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to receive either 13 days of optimal‐dose MPH then a 2‐day medication/placebo probe followed by 13 days of placebo, or 13 days of placebo then a 2‐day medication/placebo probe followed by 13 days of optimal‐dose MPH Number randomised to each group: 129 medication first, 138 placebo first Mean medication dosage: not stated Administration schedule: not stated Duration of each medication: 13 days plus probe Washout before trial initiation: not stated Medication‐free period between interventions: yes, 2 days Titration period: 9 days during the first 2 weeks of the summer treatment camp, placebo‐controlled assessments of up to 4 different OROS‐MPH doses (18 mg, 27 mg, 36 mg, 54 mg; max dose not to exceed 2 mg/kg/d) will be conducted to establish each child’s optimal dose Treatment compliance: not stated |
|
| Outcomes |
Serious AEs
Non‐serious AEs
|
|
| Notes | Sample calculation: not stated Ethics approval: not stated No comments or conclusions Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH: yes, titration phase before randomisation Any withdrawals due to AEs: not stated Funding source: Florida International University Email correspondence with trial authors: August and October 2021. We contacted the trial authors for information regarding risk of bias and data through personal email in August and October 2021, but no answer was received. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Nothing stated |
| Allocation concealment (selection bias) | Unclear risk | Nothing stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Mention of blinding, but no mention of method |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Mention of blinding, but no mention of method |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 19 withdrawals, nothing further stated Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Low risk | All protocol outcomes reported |
NCT02293655.
| Study characteristics | ||
| Methods | A 4‐week parallel discontinuation trial with 2 arms:
|
|
| Participants | Number of participants screened: 204 Number of participants included: 109 (72 boys, 37 girls) Number of participants followed up: 100 Number of withdrawals: 9 (no reason given) Diagnosis of ADHD: DSM‐5 (type not stated) Age: range 7‐11 IQ: < 80 MPH‐naive: not stated Ethnicity: Hispanic or Latino (n = 9), not Hispanic or Latino (n = 100). Race: white (n = 88), more than one race (n = 10), black or African American (n = 8), Asian (n = 3) Country: USA Setting: outpatient Comorbidity: not stated Comedication: all psychiatric medications were exclusion criteria Additional sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to either discontinue MPH (and receive placebo) or remain on optimal dose of MPH (OROS‐MPH) for 4 weeks. Number randomised to each group: MPH = 17, placebo = 92 Mean medication dosage: not stated Administration schedule: once daily Duration (of (each) medication): 4 weeks Washout before trial initiation: none Medication‐free period between interventions: none Titration period: 4‐week placebo controlled MPH titration trial before randomisation (followed by a 4 weeks of maintenance phase at optimal dosage) Treatment compliance: not stated, but according to the protocol compliance will be elaborated at each visit |
|
| Outcomes |
ADHD symptoms
Serious AEs
General behavior
Non‐serious AEs
All outcomes assessed at baseline, during the maintenance period and 3 times during the discontinuation trial on/around day 1, 14 and 28 Only the Parent Vanderbilt ADHD‐RS Total Symptom Score and prevalence of side effect data were available for the review |
|
| Notes | Sample calculation: not stated Ethics approval: not stated Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH: yes; participants were only randomised if they completed the dose titration and maintenance phases Any withdrawals due to AEs: not stated Funding source: Children's Hospital Medical Center, Cincinnati |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information on method of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No information on method of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "With blinded randomisation during MPH titration Trial and Discontinuation Phase (through use of over‐encapsulated medication and identical placebos), the entire trial will be triple‐blind (i.e., participant families, trial staff, and teachers will be blinded to medication and dose)" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "With blinded randomisation during MPH Titration Trial and Discontinuation Phase (through use of over‐encapsulated medication and identical placebos), the entire trial will be triple‐blind (i.e., participant families, trial staff, and teachers will be blinded to medication and dose)" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information regarding reason for withdrawals and method of analysis Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): not stated |
| Selective reporting (reporting bias) | High risk | All outcomes have not been published yet |
NCT02536105.
| Study characteristics | ||
| Methods | A 4‐week cross‐over trial with 4 arms:
Phases: 3 (screening and wash‐out, open‐label titration phase, and double‐blind trial) |
|
| Participants | Number of participants screened: 88, of which 80 were included in the open‐label phase Number of participants included: 76 randomised in double‐blind trial (59 boys, 21 girls) Number of participants followed‐up: 67 in MPH tablet 1 group, 72 in placebo group, 66 in MPH tablet 2 group, 68 in oral suspension group Number of withdrawals: unclear Diagnosis of ADHD: DSM‐5 (subtype not stated) Age: mean 9.45 (SD 1.86, range 6‐12) IQ: not stated MPH‐naive: not stated Ethnicity: Asian (n = 2), black or African American (n = 14), white (n = 45), mixed race (n = 16), race unknown or not reported (n = 3) Country: USA Setting: outpatient Comorbidity: specific phobia, motor skills disorders, ODD, sleep disorders, elimination disorders, adjustment disorders, learning disorders, or communication disorders are allowed Comedication: no Additional sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 24 different medication orders of 1 week of 3 different types of MPH at optimised dosage (between 18‐72 mg) or placebo Number randomised to each group: not stated Mean medication dosage: not stated Administration schedule: not stated Duration of each medication: 1 week Washout before trial initiation: 3‐7 days (with the exception of atomoxetine, clonidine, guanfacine or a MAOI, which require a 28‐day washout) Medication‐free period between interventions: none Titration period: appears to be 8 weeks (until visit 8 in trial protocol) Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
Serious AEs
Non‐serious AEs
|
|
| Notes | Sample calculation: yes, 150 Ethics approval: yes Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH: yes, during the dose‐optimisation phase Any withdrawals due to AEs: not stated Funding source: Massachusetts General Hospital Email correspondence with trial authors: August 2021. We contacted the trial authors for information regarding risk of bias and first‐period data through personal email in August 2021; however, as the contact investigator has retired, we were unable to receive any answers. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The order of assignment will be generated by a statistician who is not involved in any of the trial procedures" |
| Allocation concealment (selection bias) | Low risk | Blinding ensured at external facility |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The external facility will ensure the packaging for the placebo and active are identical" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Only one staff member unblinded at all times for emergencies |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "The missing data will not be imputed and the incomplete individual observations will be included in the analyses as the non‐linear mixed effect modeling approach used to conduct the PK and the PK/PD [Pharmacokinetic‐Pharmacodynamic] analyses does not require complete data sets" Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | High risk | ADHD‐RS‐IV and CGI‐S not reported |
Newcorn 2008.
| Study characteristics | ||
| Methods | 20‐site, randomised, placebo‐controlled, double‐blind, 6‐week, parallel trial with 3 arms:
|
|
| Participants | Number of participants screened: 635 Number of participants included: 294 (211 boys, 83 girls) randomised to the arms used in this review (516 including the atomoxetine arm, not used in this review) Number of participants followed‐up: 237 (MPH 180, placebo 57) Number of withdrawals: 57 (MPH 40, placebo 17) DSM‐IV diagnosis of ADHD (combined (67%), hyperactive‐impulsive (1%), inattentive (32%)) Age: MPH mean 10.2, placebo mean 10.1 (range 6‐16) IQ: not stated MPH‐naive: 41%/121 Ethnicity: not stated Country: USA Setting: outpatient clinic Comorbidity: ODD (36%) Comedication: not stated Other sociodemographics: no significant differences in baseline demographics were noted between the 2 groups Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to OROS‐MPH or placebo Number of participants randomised: MPH 220, placebo 74 Mean MPH dosage: 39.9 mg/d (SD 14.6) or 1.26 mg/kg/d (SD 0.55) Administration schedule: single morning dose Duration of intervention: 6 weeks Titration period: none Treatment compliance: not stated. Participants were required to discontinue any psychoactive medication for ≥ 5 days before entering the trial |
|
| Outcomes |
ADHD symptoms
Quality of life
Non‐serious AEs
|
|
| Notes | Sample calculation: no Ethics approval: yes; approved by each site’s ethical review board Comments from trial authors
Key conclusion of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: yes Any withdrawals due to AEs: yes. 12 in assessment 1, and 3 in assessment 2 Funding source: Eli Lilly and Company Email correspondence with trial authors: November 2013 and January 2014. We requested additional data from trial authors but never received them |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Trial drugs were administered according to a double‐dummy design. Identically appearing capsules were used |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Trial drugs were administered according to a double‐dummy design. Identically appearing capsules were used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | NNTB was calculated for each treatment in relation to placebo and for atomoxetine in relation to MPH. The number of participants was chosen to have 90% power to declare non‐inferiority on the basis of a comparison of response rates, with a non‐inferiority margin of 15% Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Low risk | No indication of selective reporting |
Newcorn 2017a (flexible dose).
| Study characteristics | ||
| Methods | A 8‐week parallel trial with 3 arms:
Phases: 4 (up to 4 weeks screening and washout, 5‐week dose optimisation, 3 weeks maintenance dosage, 1 week follow‐up) |
|
| Participants | Number of participants screened: 628 Number of participants included: 464 randomised (278 included to relevant interventions MPH and placebo) 459 included in analysis (305 (66.4%) boys, 154 (33.6%) girls) Number of participants followed‐up: 380 Number of withdrawals: 84 (5 of them, after randomisation, were not included in the analysis; reason for withdrawal not stated) Diagnosis of ADHD: DSM‐IV‐TR (260 combined, 5 hyperactive‐impulsive and 194 inattentive) Age: 14.7 years (SD 1.37, range 13‐17 years) IQ: not stated MPH‐naive: not stated Ethnicity: Hispanic/Latino (n = 93), not Hispanic/Latino (n = 366). Race: white (n = 345), black/African American (n = 73), Native Hawaiian/Pacific Islander (n = 2), Asian (n = 7), American Indian or Alaska native (n = 2), other (n = 1), multiple (n = 29) Country: USA Setting: outpatient clinic Comorbidity: only ODD and mild stable asthma was not an exclusionary criterion Comedication: stable use of bronchodilator inhalers is not exclusionary Additional sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 3 different groups: LDX, MPH or placebo. Number randomised to each group: placebo 93, LDX 186, OROS‐MPH 185 Mean medication dosage: LDX 50.15 ± 12.501 mg/d, OROS‐MPH 44.47 ± 12.754 mg/d Administration schedule: not stated Duration (of (each) medication): 8 weeks Washout before trial initiation: 4‐week screening/washout period, specific length of washout not stated Titration period: during the first 5 weeks dosage was increased or decreased according to clinical assessment. LDX started at 30 mg/d and OROS‐MPH started at 18 mg/d Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
Serious AEs
Non‐serious AEs
|
|
| Notes | Sample calculation: no Ethics approval: yes. Trial protocols and related information were approved by either a central review board or institution‐specific review boards and appropriate regulatory agencies (US FDA, Therapeutic Product Directorate of Canada, Medical Products Agency of Sweden, Medical Research Council of Hungary, The Federal Institute for Drugs and Medical Devices [Bundesinstitut für Arzneimittel und Medizinprodukte] of Germany) before trial initiation. Key conclusion of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH: yes Any withdrawals due to AEs: yes, 20 Funding source: Shire Email correspondence with trial authors: September and October 2021. We contacted the trial authors for information regarding risk of bias through personal email in September and October 2021, but no answer was received. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised (2:2:1) using an interactive web‐response system (IWRS) to once‐daily LDX, OROS‐MPH, or placebo, respectively. |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | To maintain blinding, treatments were identical in appearance; participants were also instructed to take 1 capsule from 2 separate bottles |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No imputation for ADHD‐RS‐IV, LOCF for CGI‐S Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): yes, participants excluded due to lack of efficacy |
| Selective reporting (reporting bias) | Low risk | All outcomes reported according to protocol |
Newcorn 2017b (forced dose).
| Study characteristics | ||
| Methods | A 6‐week parallel trial with 3 arms:
Phases: 4 (4‐week screening/washout, 4 weeks forced titration, 2‐week maintenance dosage, 1‐week follow‐up phone‐call) |
|
| Participants | Number of participants screened: 778 Number of participants included: 549 (330 to relevant interventions). 547 started treatment (361 boys, 186 girls) Number of participants followed‐up: 464 Number of withdrawals: 85 (of which 2 were before treatment start) Diagnosis of ADHD: DSM‐IV‐TR (358 combined, 8 hyperactive‐impulsive and 181 inattentive) Age: 14.7 (SD 1.4, range 13‐17) IQ: not stated MPH‐naive: not stated Ethnicity: Hispanic/Latino (n = 101), not Hispanic/Latino (n = 446). Race: white (n = 401), black/African American (n = 110), native Hawaiian/Pacific Islander (n = 2), Asian (n = 8), American Indian or Alaska native (n = 3), other (n = 3), multiple (n = 20) Country: USA Setting: outpatient clinic Comorbidity: only ODD and mild stable asthma were not exclusionary criteria Comedication: stable use of bronchodilator inhalers is not exclusionary Additional sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 3 different groups of LDX, MPH or placebo Number randomised to each group: placebo 110, LDX 219, OROS‐MPH 220 Mean medication dosage: forced dosage: LDX week 1, 30 mg/d; week 2, 40 mg/d; week 3, 50 mg/d) and then in a 20‐mg increment (week 4) to a maximum of 70 mg/d; initial OROS‐MPH dose (18 mg/d) was increased weekly in 18‐mg increments to a maximum of 72 mg/d during weeks 2 through 4 Dosage could only be increased or be kept as it was Administration schedule: not stated Duration (of (each) medication): 6 weeks Washout before trial initiation: 4 weeks of screening/washout Titration period: 4 weeks Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
Serious AEs
Non‐serious AEs
|
|
| Notes | Sample calculation: no Ethics approval: yes. Trial protocols and related information were approved by either a central review board or institution‐specific review boards and appropriate regulatory agencies (US FDA, Therapeutic Product Directorate of Canada, Medical Products Agency of Sweden, Medical Research Council of Hungary, The Federal Institute for Drugs and Medical Devices [Bundesinstitut für Arzneimittel und Medizinprodukte] of Germany) before trial initiation. Key conclusion of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH: yes Any withdrawals due to AEs: yes, 30 (15 in LDX group) Funding source: Shire Email correspondence with trial authors: September and October 2021. We contacted the trial authors for information regarding risk of bias through personal email in September and October 2021, but no answer was received. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised (2:2:1) using an interactive web‐response system to once‐daily LDX, OROS‐MPH, or placebo, respectively |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | To maintain blinding, treatments were identical in appearance; participants were also instructed to take one capsule from 2 separate bottles |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No imputation for ADHD‐RS‐IV, LOCF for CGI‐S. Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): yes, participants excluded due to lack of efficacy |
| Selective reporting (reporting bias) | Low risk | Reported according to protocol |
Nikles 2006.
| Study characteristics | ||
| Methods | Cross‐over trial with 2 interventions
Phases: each trial included 3 pairs of treatment periods (n‐of‐1). Each pair contained the stimulant and the comparator stimulant or placebo. The child’s doctor individualised the dosing, and the order of drugs was randomly assigned within each pair. 2 treatment periods were included in a school week, and 2 days per treatment period (not including Wednesdays and weekends) |
|
| Participants | Number of participants screened: 108 (85 boys, 21 girls). 2 boys repeated n‐of‐1 trials; 22 (20%) trials were not completed Number of participants included: 86. (66 boys, 18 girls) Number of participants followed up: 86 Number of withdrawals: 10 (12%) trials were not completed Diagnosis of ADHD: DSM‐IV (subgroups not stated) Age: median 10 years (range 5‐16) IQ: not stated MPH‐naive: 36 were taking MPH, 47 dexamphetamine, 3 unknown pre‐trial medication Ethnicity: all were white (Nikles 2007 in Nikles 2006) Country: Australia Setting: outpatient clinic Comorbidity: not stated Comedication: not stated Other sociodemographics: caregiver's occupation: full‐time work 21%, part‐time or casual work 33%, unemployed or retired 5%, unpaid homemakers 25%, other 10%, unknown 5% Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 3 possible drug condition orders of individual doses of MPH, dexamphetamine and placebo Mean MPH dosage: not stated Administration schedule and time points: different for different individuals, as this is a series of n‐of‐1 trials Duration of each medication condition: 2 days each of MPH and placebo/week Washout before trial initiation: 40 h (from 4:00 pm Tuesday to 8:00 am Thursday) and 64 h (4:00 pm Friday to 8:00 am Monday) Titration period: not stated, but all were stabilised on an "optimal" dose of stimulant, initiated before randomisation Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms First 41 participants used at the end of each treatment period
From 42nd participant onwards used at the end of each treatment period
Non‐serious AEs
|
|
| Notes | Sample calculation: irrelevant Ethics approval: yes Key conclusion of trial authors
Comments from review authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: yes (n = 1, insomnia and depression) Funding source: The General Practice Evaluation Program, the Department of Health and Aged Care, Queensland Medical Laboratory, and the Royal Australian College of General Practitioners |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The order of drugs was randomly assigned within each pair"; "There were three pairs of treatment periods, with the order of drugs randomly assigned by a computer‐generated randomisation schedule within each" |
| Allocation concealment (selection bias) | Low risk | See above |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Patients, parents, doctors, and the research assistant were all blinded to medication order" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "A hospital pharmacy encapsulated the medication (crushing of tablets and production of identical capsules containing either medication or placebo)"; "Active medication was encapsulated and identical placebo capsules were produced by a hospital pharmacy" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Low risk | All outcomes are reported |
Oesterheld 1998.
| Study characteristics | ||
| Methods | Cross‐over trial with 3 interventions:
Phases: 5‐day trial lasting for 3 consecutive weeks, with 2 medication‐free days between phases |
|
| Participants | Number of participants screened: 30 Number of participants included: 4 Number followed up: 4 (2 boys, 2 girls) Number of withdrawals: 0 Diagnosis of ADHD: DSM‐IV (combined (66.7%), hyperactive‐impulsive (0%), inattentive (33.3%)) Age: mean 8.25 years (range 5‐11) IQ: mean 72.5 (range 63‐79) MPH‐naive: 100% Ethnicity: Native American (100%) Country: USA Setting: outpatient clinic (residential school, laboratory classroom) Comorbidity: not stated Comedication: no Other sociodemographics: not living with family Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to different possible drug condition orders of MPH (0.6 mg/kg), lactose placebo and vitamin C placebo Mean MPH dosage: not stated (range 10 mg/d to 17.5 mg/d) Administration schedule: 7:30 am, 11:00 am and 2:00 pm Duration of each medication condition: 5 days for 3 consecutive weeks Washout before trial initiation: not stated Medication‐free period between interventions: 2 days Titration period: none. Fixed dose Treatment compliance: medication given by nurse (directly observed treatment) |
|
| Outcomes |
ADHD symptoms
Non‐serious AEs
|
|
| Notes | Sample calculation: no Ethics approval: yes; Human Subjects Committee of the University of South Dakota's School of Medicine and the Research Committee of the Black Hills Children's Home Society Key conclusion of trial authors
Comment from review authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: no Funding source: a University of South Dakota/USF‐Mini Grant Email correspondence with trial authors: not able to find email addresses for trial authors because information is missing from the paper. Not possible to find on the Internet, etc. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The order of the trials was randomly determined |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | School carers, nurses, teachers and researchers were blinded as to whether treatment consisted of placebo or active agent. All agents were placed in identical capsules |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | On each day of each trial, a teacher, blinded to evaluation data, rated the child's behaviour in school using CTRS (39 items). The caregiver, blinded to evaluation data, completed CPRS (48 items) on a daily basis. School carers, nurses, teachers and researchers were blinded as to whether treatment consisted of placebo or active agent. All agents were placed in identical capsules |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified |
Overtoom 2003.
| Study characteristics | ||
| Methods | Double‐blind, randomised, cross‐over trial with 4 interventions:
|
|
| Participants | Number of participants screened: not stated Number of participants included: 16 Number of participants followed up: 16 (all boys) Number of withdrawals: 0 Diagnosis of ADHD: DSM‐III‐R (combined (100%)) Age: mean 10.4 years (range 7‐12) IQ: 95.4 MPH‐naive: 0 (0%) Ethnicity: not stated Country: the Netherlands Setting: outpatient clinic Comorbidity: ODD (n = 6), comorbid anxiety disorder (n = 1), specific developmental disorders (n = 3) Comedication: not stated Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 2 of the possible drug condition orders of MPH, desipramine, L‐dopa and placebo Mean MPH dosage: 15 mg Administration schedule: once, in the afternoon Duration of each medication condition: 1 day Washout before trial initiation: 3 days before for MPH users Medication‐free period between interventions: not stated Titration period: none Treatment compliance: 100% |
|
| Outcomes |
Non‐serious AEs
|
|
| Notes | Sample calculation: no
Ethics approval: yes Key conclusions of trial authors
Inclusion of MPH responders only/exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: yes; all participants were already stable on MPH Any withdrawals due to AEs: no Funding source: Netherlands Organisation for Scientific Research (NWO) Grant 575‐63‐082 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "A double‐blind randomised design was used" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
Palumbo 2008.
| Study characteristics | ||
| Methods | Multi‐centre, randomised, double‐blind, 16‐week, parallel trial with 2 × 2 factorial design:
2 successive 4‐week titration periods followed by an 8‐week maintenance period |
|
| Participants | Number of participants screened: 205 Number of participants included: 122 (98 boys, 24 girls) Number followed up: 93 (MPH 18, placebo 10, MPH + clonidine 24, clonidine 31) Number of withdrawals: 44 (MPH 11, placebo 20, MPH + clonidine 8, clonidine 5) DSM‐IV diagnosis of ADHD (combined (76%), hyperactive‐impulsive (4%), inattentive (20%)) Age: mean 9.5 (range not reported) IQ: > 70 MPH‐naive: 57 (46.7%) Ethnicity: white (77.9%), African American (10.7%), Hispanic (6.6%), other (4.9%) Country: USA Setting: outpatient clinic Co‐morbidity: ODD (47%), CD (9%) Co‐medication: not allowed, but all participants received protocol‐based behavioural interventions Other sociodemographics: participant groups were similar except for a higher percentage of white children in the clonidine group and some minor differences with regard to family history of ADHD and tics Inclusion criteria
Exclusion criteria
Previous use of MPH or clonidine was permitted |
|
| Interventions | Participants were randomly assigned to IR‐MPH or placebo Number randomised to each group: MPH 29, placebo 30, MPH + clonidine 32, clonidine 31 Mean MPH dosage: 0.76 ± 0.54 mg/kg/d (30.2 ± 18.9 mg/d (MPH‐only group) and 25.4 ± 18.2 mg/d (MPH + clonidine)) Administration schedule: 1‐3 times daily (morning, noon and afternoon) Duration of intervention: 12‐16 weeks Washout before trial initiation: 2 weeks Titration period: 8‐week flexible‐dose titration period (4 weeks for clonidine, then 4 weeks for MPH) initiated after randomisation Maintenance period of optimal dose: 8 weeks Treatment compliance: monitored using pill counts (results of monitoring not stated) |
|
| Outcomes |
ADHD symptoms
Quality of life
Non‐serious AEs
|
|
| Notes | Sample calculation: yes; sample size of 140 participants (35 per treatment group) was determined to provide between 80% and 90% power to detect a group difference (effects of clonidine)
Ethics approval: yes; approved by the institutional review board at each site Comments from trial authors
Key conclusions of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: moderate to severe AEs were cited as a reason for withdrawal in 8 participants: MPH + clonidine 5, clonidine 2, MPH 1
Funding source: NIH and National Institute of Neurological Disorders and Stroke Email correspondence with trial authors: March 2014 to June 2014. We attempted to obtain supplemental efficacy data from the trial authors |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation plan included stratification by centre (investigator) and by sexual maturity status (Prepubertal: Tanner I‐II, Pubertal: Tanner III‐V) |
| Allocation concealment (selection bias) | Low risk | Only the programmer in the Biostatistics Centre and the pharmacist knew the allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | MPH (or matching placebo) powder packaged in gelatin capsules |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All investigators, trial co‐ordinators, teachers, parents and children were blinded to treatment assignments |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Statistical analyses were performed according to the ITT principle, and last available observations were carried forward and imputed when needed for both efficacy and safety measures. However, only data from the ADHD‐RS for children who completed the titration period were analysed Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Low risk | No indication of selective reporting |
Pearson 2013.
| Study characteristics | ||
| Methods | 4‐week, cross‐over trial with 2 interventions:
Phases
|
|
| Participants | Number of participants screened: 94 Number of participants included: 24 (19 boys, 5 girls) Number of participants followed up: 24 Number of withdrawals: 0 Diagnosis of ADHD: DSM‐IV (combined 19, inattentive 5) Age: mean 8.8 years (range 7.1‐12.7) IQ: mean 85 (range 46‐112) MPH‐naive: 13 Ethnicity: white (n = 13), African American (n = 4), Asian (n = 1), Hispanic (n = 5), other (n = 1) Country: USA Setting: outpatient clinic Comorbidity: ODD (n = 5), OCD (n = 2), separation anxiety disorder (n = 1) Comedication: yes Other sociodemographics: Hollingshead 4 Factor Social Class 1.7 (0.9); Hollingshead 4 Factor socioeconomic status score 52.3 (10.8) Mean parental education level: mothers 15.8 years (SD = 2.3), fathers 17.2 years (SD = 3.1) Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 8 possible drug condition orders of 3 doses of MPH (low, moderate and high) and placebo Mean MPH dosage: low = 0.21 mg/kg; moderate = 0.35 mg/kg; high = 0.48 mg/kg Administration schedule: twice daily Duration of each medication condition: 1 week Washout before trial initiation: yes Medication‐free period between interventions: not stated Titration period: 1 week before randomisation Treatment compliance: "Parents completed a medication administration form, were asked about missing or late doses at weekly interviews and teachers were asked as well. Forms were verified by number of pills in returned vials. Families were asked again in case of discrepancy. Parents and teachers were also asked about unanswered items on questionnaires" 5 children discontinued the afternoon IR‐MPH dose due to behaviour concerns |
|
| Outcomes |
ADHD symptoms
Non‐serious AEs
|
|
| Notes | Sample calculation: no Ethics approval: yes Key conclusions of trial authors
Inclusion of MPH responders only/exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: yes, past treatment failure on a MPH trial was an exclusion criteria Any withdrawals due to AEs: no Funding source: grant number MH072263 from NIMH |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "We used the Digram balanced randomisation method that Dr. David Lane (a professor of both psychology and statistics at nearby Rice University) used to create the 8 balanced drug dose orders to which our 24 participants were assigned" |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | One‐week, single‐blind, "lead‐in‐dosing"; all trial personnel with participant contact were blind with respect to dosages given during the drug trial |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The study medication was prepared by the University of Texas Psychiatry Research Pharmacy: the Ritalin LA beads were mixed with (inert) placebo beads and placed in two opaque gelatin capsules, and the white generic IR‐MPH was crushed and mixed with cornstarch and placed in two size 1 gelatin capsules" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Blank was left for missing data Selection bias: yes; exclusion of placebo responder during single‐blind placebo washout week |
| Selective reporting (reporting bias) | Low risk | All outcomes are reported |
Pelham 1989.
| Study characteristics | ||
| Methods | Cross‐over trial with 2 interventions:
Phases
|
|
| Participants | Number of participants screened: not stated Number of participants included: 24 (12 boys, 12 girls) Number followed up: not stated Number of withdrawals: not stated Diagnosis of ADHD: DSM‐III (combined (not stated), hyperactive‐impulsive (19/24), inattentive (2/24)) Age: mean not reported (range: boys 5 years 6 months‐11 years; girls 5 years 8 months‐11 years 3 months) IQ: boys 100.8 (SD 14.23), girls 104.0 (SD 16.52) MPH‐naive: not stated Ethnicity: not stated Country: USA Setting: outpatient clinic (summer treatment programme) Comorbidity: attention deficit disorder (1/24), CD (5/24), ODD (15/24), learning disability (6/25) Comedication: yes; other doses of stimulants reported but not on trial days Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 2 possible drug condition orders of 0.3 mg/kg methylphenidate and placebo. "Each child received, in random order with condition varied daily, placebo twice a day and 0.3 mg MPH/kg twice a day." Mean MPH dosage: boys 9.8 mg (range 5.3‐16.9), girls 9.5 mg (range 5.2‐13.1) Administration schedule: twice daily at breakfast and at lunchtime Duration of each medication condition: not stated Washout before trial initiation: not stated Medication‐free period between interventions: no Titration period: none Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
General behaviour
|
|
| Notes | Sample calculation: no
Ethics approval: not stated Comment from trial authors
Key conclusions of trial authors
Comments from review authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: not stated Any withdrawals due to AEs: not stated Funding source: not declared Email correspondence with trial authors: October 2014. We received no supplemental information/data from trial authors. We asked authors whether this article was part of the Johnston 1988 trial but received no response, so we extracted the data as from 2 separate studies |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Each child received, in random order with condition varied daily, placebo and MPH |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Active medication and placebo were disguised in gelatin capsules and were pre‐packaged in individual, dated envelopes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated Selection bias (e.g. titration after randomisation → exclusion): unclear |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
Pelham 1990a.
| Study characteristics | ||
| Methods | Double‐blind, placebo‐controlled, cross‐over trial:
|
|
| Participants | Number of participants screened: not stated Number of participants included: 22 (all boys) Number of participants followed up: 22 Number of withdrawals: 0 Diagnosis of ADHD: DSM‐III‐R (subtype not stated) Age: mean 10.39 years (range 8.08‐13.17) IQ: mean 105.68 Methlyphenidate‐naive: not stated Ethnicity: not stated Country: USA Setting: outpatient clinic (summer treatment programme) Comorbidity: oppositional/defiant disorder (n = 9), CD (n = 4), "suggesting the presence of a learning disability", but IQ > 80 (n = 13) Comedication: not stated Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned different possible drug condition orders of MPH (10 mg twice/d, ER‐MPH 20 mg every morning) and placebo Mean MPH dosage: IR‐MPH 10 mg = 0.29 mg/kg Administration schedule: twice daily: IR‐MPH twice daily: morning and lunchtime; ER‐MPH once daily: morning Duration of each medication condition: 1 day, but in total 3‐6 days Washout before trial initiation: none Medication‐free period between interventions: not stated Titration period: no Treatment compliance: none All completed trial: not stated |
|
| Outcomes |
ADHD symptoms
Non‐serious AEs
|
|
| Notes | Sample calculation: no Ethics approval: not stated Comments from trial authors
Key conclusions of trial authors
Comments from review authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: no Funding source: not declared Email correspondence with trial authors: January 2014. No contact made through author correspondence |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Active medication and placebo were disguised in gelatin capsules and were pre‐packaged in individual daily pill reminders |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
Pelham 1993a.
| Study characteristics | ||
| Methods | Cross‐over trial with 2 interventions:
Phases: 3 |
|
| Participants | Number of participants screened: not stated Number of participants included: 31. Number of participants followed up: 31 (all boys) Number of withdrawals: 0 Diagnosis of ADHD: DSM‐III‐R (therefore no subtype) Age: mean 98.8 months (approximately 8.2 years; range 5.42‐9.92) IQ: mean 110.7 (range not stated) MPH‐naive: not stated Ethnicity: white (93.5%), African American (6.5%) Country: USA Setting: hospital (Summer Treatment Program) Comorbidity: ODD (32%), CD (48%) Comedication: not stated Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 3 possible drug condition orders of 0.3 mg/kg and 0.6 mg/kg MPH and placebo Mean MPH dosage (SD): low 8.1 mg (range 5‐15); high 16.0 mg (range 10‐22.5) Administration schedule: twice/d morning and midday; conditions were changed daily over 6 weeks Duration of each medication condition: 2 weeks overall (individual treatment condition 1 day) Washout before trial initiation: not described Titration period: none Treatment compliance: not reported |
|
| Outcomes |
ADHD symptoms
|
|
| Notes | Sample calculation: no Ethics approval: not stated Key conclusion of trial authors
Inclusion of MPH responders only/exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: no Funding source: not declared Email correspondence with trial author: June 2014. Emailed trial author to ask for additional information but have received no response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Referred to as a randomised trial but sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Capsules were identically packaged |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Referred to as double‐blind but not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Appears to be 100% follow‐up Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Unclear risk | Protocol not identified |
Pelham 1999.
| Study characteristics | ||
| Methods | 6‐week, within‐participant, double‐blind, placebo‐controlled, cross‐over design with 5 arms:
|
|
| Participants | Number of participants screened: 26 Number of participants included: 25 (21 boys, 4 girls) Number of participants followed up: 23 Number of withdrawals: 2 Diagnosis of ADHD: DSM‐IV (subtype not stated) Age: mean 9.6 years (range 5.8‐12.7) IQ: average intelligence MPH‐naive: not stated Ethnicity: white (88%) Country: USA Setting: outpatient clinic Comorbidity: ODD (n = 13), CD (n = 8) Comedication: not stated Other sociodemographics: "Median family income was US $40 000, with incomes ranging widely (from US $10 000 per year to US $100 000 per year)" Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to receive 5 different interventions changing on a daily basis. The 5 interventions were 10 mg and 17.5 mg of IR‐MPH (Ritalin), 7.5 and 12.5 mg of MAS (Adderall) and placebo Administration schedule: 2 time points Duration of each medication condition: 5‐day period Washout before trial initiation: not stated Medication‐free period between interventions: no Titration period: none initiated before/after randomisation Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
Non‐serious AEs
|
|
| Notes | Sample calculation: not stated Ethics approval: not stated Key conclusions of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: yes; 1 (exacerbation of tic disorder) Funding source: grants from the Shire Richwood Pharmaceutical Company and NIMH (Grants MH53554, MH45576 and MH50467) Email correspondence with trial authors: April 2014. We requested additional information from trial authors but have not received a reply |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomized" |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "The clinical team was not blind to medication condition when making their recommendations. Therefore, as a reliability check of the clinical team’s recommendations, one of the authors (J.W.) who was not involved in the clinical team meetings made independent recommendations based on the same data given to the clinical team. This rater was blind to drug condition except placebo" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "The clinical team was not blind to medication condition when making their recommendations. Therefore, as a reliability check of the clinical team’s recommendations, one of the authors (J.W.) who was not involved in the clinical team meetings made independent recommendations based on the same data given to the clinical team. This rater was blind to drug condition except placebo" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information Selection bias (e.g. titration after randomisation → exclusion): Unclear, not stated |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified |
Pelham 2001a.
| Study characteristics | ||
| Methods | Cross‐over trial with 3 interventions:
Phases: 3 |
|
| Participants | Number of participants screened: 70 Number of participants included: 68. (89% boys, 11% girls) Number of participants followed up: 68 (66 for ADHD symptoms) Number of withdrawals: 2 Diagnosis of ADHD: DSM‐IV (subtype not stated) Age: mean 9.1 years (range 6‐12) IQ: mean 104.8 MPH‐naive: 0% Ethnicity: white (94%), other (6%) Country: USA Setting: outpatient clinic (Summer Treatment Program) Comorbidity: ODD (43%), CD (37%) Comedication: no Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to different possible drug condition orders of IR‐MPH 3 times/d, Concerta MPH once/d, and placebo Mean MPH dosage: 0.75 mg/kg (SD 0.34) (3 dosing levels were used:
The dose level used for each child was based on that child’s MPH dosing before the start of the trial Administration schedule: IR‐MPH 3 times/d or ER‐MPH (Concerta) once daily Duration of each medication condition: 7 days Washout before trial initiation: not described Titration period: none Treatment compliance: "virtually 100%" |
|
| Outcomes |
ADHD symptoms
Non‐serious AEs
|
|
| Notes | Sample calculation: no Ethics approval: yes Comment from trial authors
Key conclusion of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: yes; all participants were required to be medicated with MPH and were receiving a stable dose for ≥ 4 weeks before the start of the trial Any withdrawals due to AEs: no Funding source: ALZA Corporation, the manufacturers of Concerta Email correspondence with trial author: June 2014. Emailed trial author to ask for additional information but have not received a reply |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Referred to as double‐blind; capsules were identical |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Referred to as double‐blind; capsules were identical |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Data reported for 66/70 for ADHD and behaviour Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
Pelham 2002.
| Study characteristics | ||
| Methods | Cross‐over trial with 2 interventions:
|
|
| Participants | Number of participants screened: not stated Number of participants included: 136 (all boys) Number of participants followed up: 136 Number of withdrawals: 0 Diagnosis of ADHD: DSM‐III‐R (therefore no subtype) Age: mean 9.7 years (range 7.6‐12.7) IQ: mean 104.5 MPH‐naive: not stated Ethnicity: white (81%), African American (15%) Country: USA Setting: Summer Treatment Program Comorbidity: ODD (53%), CD (24%) Comedication: not stated Other sociodemographics: median family income: USD 25,000, (range USD 10,000 to > USD 100,000) Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 2 possible drug condition orders of 0.3 mg/kg MPH and placebo Mean MPH dosage: 10 mg (SD 2.7) Administration schedule: twice daily at 7:45 am and 11:45 am Duration of each medication condition programme: 12 days (order randomly assigned daily over 6 weeks, doses administered over week days, except on Fridays); follow‐up: 30 days Washout before trial initiation: 2 weeks medication‐free baseline in programme, unclear for follow‐up Titration period: none Treatment compliance: not reported |
|
| Outcomes |
ADHD symptoms
General behaviour
|
|
| Notes | Sample calculation: no Ethics approval: yes Comment from trial authors
Key conclusion of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: no Funding source: NIMH (Grant MH48157) Email correspondence with trial authors: June 2014. Emailed trial authors to ask for additional information but have not received a reply |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Capsules were identical |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Raters were blinded to medication condition |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All of the outcomes used for this review are reported for 133 of the 136 boys. The reason for the missing data is not stated. Selection bias (e.g. titration after randomisation → exclusion): Unclear |
| Selective reporting (reporting bias) | Unclear risk | Although measures were rated daily, how data were aggregated/reported as a single result per phase is not clear. |
Pelham 2005.
| Study characteristics | ||
| Methods | 8‐day, multi‐centre, double‐blind, randomised, dose‐ranging, cross‐over trial with 2 interventions:
From a Summer Treatment Program |
|
| Participants | Number of participants screened: 36 Number of participants included: 36 (33 boys, 3 girls) Number of participants followed up: 36 Number of withdrawals: 0 Diagnosis of ADHD: DSM‐IV (subtype not stated) Age: mean 9.6 years (range 6‐13) IQ: mean 105.3 (SD 18) MPH‐naive: 15 (42%) Ethnicity: white (75%), African American (19%), Hispanic (3%), mixed African American/white (3%) Country: USA Setting: outpatient clinic (summer treatment programme) Comorbidity: not stated Comedication: no; antidepressants were withdrawn from 2 participants before trial enrolment Other sociodemographics: parents were manual workers (6%), clerical/sales workers (32%), technicians/semi‐professionals (23%) and executives/major professionals (32%) Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 8 possible drug conditions of MPH transdermal system and placebo MPH transdermal system dosage: 6.25 cm² (0.45 mg/h), 12.5 cm² (0.9 mg/h), 25 cm² (1.8 mg/h) Administration schedule: once/d (at 6:00 am and 7:00 am). Application sites were alternated each day between left and right hips Duration of each medication condition (crossed with time of application): 1‐day; patch worn for ≥ 12 h/d Duration of trial: 8 days Washout before trial initiation: no Medication‐free period between interventions: no Titration period: none Treatment compliance: 100%. Parents returned patches and dosing records to the trial site |
|
| Outcomes |
ADHD symptoms
Non‐serious AEs
|
|
| Notes | Sample calculation: yes (36‐48) Ethics approval: yes; institutional review board at each site approved the trial Comments from trial authors
Key conclusions of trial authors
Comment from review authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: no Funding source: Noven Pharmaceuticals. Furthermore, Dr. Pelham was supported by grants from National Institute on Alcohol Abuse and Alcoholism, National Institute on Drug Abuse; NIMH and National institute of Neurological Disorders and Stroke Email correspondence with trial authors: February‐March 2014. We attempted to obtain supplemental information regarding randomisation, allocation concealment, washout period and efficacy and safety data from trial authors but without success |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Trial sponsor produced random orders and prepared medication kits in numbered containers for each participant |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Treatment sequences were concealed until completion of the trial |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Treatment sequences were concealed until completion of the trial |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Evaluable participant data were analysed. As the result of record‐keeping difficulties at 1 site, efficacy data were excluded for 5 participants Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
Pelham 2011.
| Study characteristics | ||
| Methods | 3‐week, randomised, double‐blind, double‐dummy, cross‐over trial with 3 interventions:
Parents and children spent Friday evening to Sunday morning in a laboratory setting, where data collection took place |
|
| Participants | Number of participants screened: not stated Number of participants included: 10 (all boys) Number of participants followed up: 9 Number of withdrawals: 1 Diagnosis of ADHD: DSM‐IV (combined (80%), hyperactive‐impulsive (0%), inattentive (20%)) Age: mean 8.6 years (range 6.4‐9.7) IQ: mean 95.3 (range 83‐109) MPH‐naive: none; all receiving a stable dose of IR‐MPH before enrolment Ethnicity: white (50%), African American (20%), Asian (0%), Hispanic (0%), Native American (10%), other (20%) Country: USA Setting: outpatient clinic Comorbidity: ODD/CD (80%) Comedication: no other psychotropics Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to different drug orders of IR‐MPH, MPH transdermal system, and placebo IR‐MPH
MPH transdermal system
Duration of each medication condition: 1 week Washout before trial initiation: 48 h before trial (no washout between treatment periods) Titration period: none Treatment compliance: dosing and adhesion records were collected each week, but no data were available in the article |
|
| Outcomes |
ADHD symptoms
Non‐serious AEs
|
|
| Notes | Sample calculation: no Ethics approval: yes Key conclusion of trial authors
Comments from trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: yes; however, all participants had been receiving MPH previously, and this may explain the small number of side effects Any withdrawals due to AEs: yes; 1 receiving placebo Funding source: grant from Noven Pharmaceuticals (manufacturer of the MPH transdermal system) Email correspondence with trial authors: July 2014. Emailed trial authors to request additional information but have not received a reply |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random assignment; no information about how |
| Allocation concealment (selection bias) | Low risk | Double‐dummy procedure: all children received patches and capsules each day, with applicable placebos |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One participant discontinued the trial during the first week because of worsening behaviour while taking placebo and was not included in the analyses: all other participants completed the trial AEs: all participants receiving ≥ 1 dose of medication were included in the safety analysis Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Unclear risk | Not possible to find the protocol |
Pelham 2014.
| Study characteristics | ||
| Methods | 3‐week, randomised, controlled, cross‐over trial with 2 factors ‐ medication and behavioural intervention ‐ with daily medication changes:
|
|
| Participants | Number of participants screened: not stated Number of participants included: 48 (44 boys, 4 girls) Number of participants followed up: 47 Number of withdrawals: 1 Diagnosis of ADHD: DSM‐IV (subtypes not stated) Age: mean 9.35 years (range 5‐12) IQ: mean 106.33 (SD 14.61; range not stated) MPH‐naive: not stated Ethnicity: white (79%), African American (12.5%) Country: USA Setting: outpatient, Summer Treatment Program Comorbidity: not stated Comedication: not stated Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 3 possible drug condition orders of 0.15 mg/kg/dose MPH 3 times/d; 0.3 mg/kg/dose MPH 3 times/d; 0.6 mg/kg/dose MPH 3 times/d and placebo Mean MPH dosage: not clearly stated: "average doses were 5.4 mg (range 2.5‐10), 11 mg (range 6.25‐20), and 21 mg (range 11.25‐30), respectively" Administration schedule: 3 time points Duration of each medication condition: 0.15 mg dose for 4 days, 0.30 mg dose for 4 days and 0.6 mg dose for 3 days, switched daily Washout before trial initiation: not stated Medication‐free period between interventions: not stated Titration period: not stated Treatment compliance: "One child’s parents withdrew from the study after 2 days because of their concerns about possible side effects of the medication" |
|
| Outcomes |
ADHD symptoms
General behaviour
Non‐serious AEs
|
|
| Notes | Sample calculation: no Ethics approval: yes Comments from trial authors
Key conclusion of trial authors
Comment from review authors
Inclusion of MPH responders only/exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: yes; only participants not having any documented adverse response to MPH were included Any withdrawals due to AEs: yes (n = 1) Funding source: grant from the NIMH (MH62946). Dr. Pelham was funded by grants from the NIH (MH62946, MH69614, MH53554, MH69434, MH65899, MH78051, MH062946, NS39087, AA11873, DA12414, HD42080) and the Institute of Education Sciences (L03000665A). Dr. Fabiano was supported in part by a Ruth S. Kirschstein National Research Service Award Predoctoral Fellowship (1F31MH064243‐01A1) and by the Department of Education, Institute of Education Sciences (R324J06024, R324B06045) Email correspondence with trial authors: April 2015. We emailed trial authors to request supplemental information but have not received a response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "The children, their parents, and clinical staff members were uninformed of medication condition and only the research coordinator, pharmacist and medical director had access to the medication order. The medical director could reveal medication conditions in cases of severe side‐effect reports" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Observers were independent staff members who were not involved in the children’s treatment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only one participant withdrew from the trial. Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Unclear risk | No access to protocol. No description in clinicaltrials.gov |
Perez‐Alvarez 2009.
| Study characteristics | ||
| Methods | 12‐month, randomised, controlled, parallel trial with 2 different treatment strategies.
|
|
| Participants | Number of participants screened: not stated Number of participants included: 118 to the arms used for this review 150 in all 3 arms (boy:girl 4:1 for those with combined type; 1:3 for those with inattentive type) Number followed up: OROS‐MPH (Concerta) + psychology 59, humanistic psychology 59 Number of withdrawals: OROS‐MPH (Concerta) + psychology 0, humanistic psychology 0 Diagnosis of ADHD: DSM‐IV‐TR (combined (67%), hyperactive‐impulsive (0%), inattentive 33%)) Age: mean 10 years (range 7‐14) MPH‐naive: 100% Ethnicity: not stated Country: Spain Setting: outpatient clinic Comorbidity: no Comedication: no IQ: > 70 Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to OROS‐MPH (Concerta) + psychology or humanistic psychology Number assigned to each group: 59 to humanistic psychology, 59 to OROS‐MPH (Concerta) + psychology OROS‐MPH dose: not stated Administration schedule: not stated Duration of intervention: 12 months Titration period: not stated Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
|
|
| Notes | Sample calculation: no Ethics approval: yes Key conclusion of trial authors
Comment from review authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: no Funding: none. Research was part of the work day, participants were voluntary and no funding was needed to implement the trial Email correspondence with trial authors: June 2014. We received supplemental information regarding funding, ethics approval and protocol from the trial authors, although they were not able to send data from the SNAP, 4th Edition |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly assigned, but no information was given to explain how assignment was done |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Low risk | No indication of selective reporting, all planned analyses are described in the paper |
Pliszka 1990.
| Study characteristics | ||
| Methods | 4‐week, double‐blind, cross‐over trial, in which children were randomly assigned to the following:
|
|
| Participants | Number of participants screened: 79 (74 boys, 5 girls) Number of participants included: 46 Number of participants followed up: 43 (30 without anxiety, 13 with anxiety) Number of withdrawals: 3 Diagnosis of ADHD: DSM‐III (subtype not stated) Age: mean 9 years (range not reported) IQ: > 70 MPH‐naive: not stated Ethnicity: not stated Country: USA Setting: outpatient clinic Comorbidity: anxiety (30%), ODD (56.0%), CD (23.3%) (Participants who expressed transient anxiety or depression about consequences of punishment for misbehaviour were considered to not meet the criteria for an overanxious disorder) Comedication: no Other sociodemographics: none Inclusion criteria
Exclusion criteria
No patients met the criteria for any psychotic or depressive disorder. Participants were free of medication, as well as any other medical disorder. |
|
| Interventions | A total of 4 weeks of medication. First week always placebo; remaining 3 weeks, participants were randomly assigned to different orders of placebo and low‐ (0.25 mg/kg to 0.40 mg/kg) and high‐dose (0.45 mg/kg to 0.70 mg/kg) MPH. Doses were as follows: 5 mg and 10 mg for weight < 25 kg; for those overweight, the 2 dose conditions were 10 mg and 20 mg Administration schedule: twice/d, 7:00 am and 12 noon. Saturday, the dose was adjusted so that it was given 90 min before measurements were taken Duration of each medication condition: 1 week Washout before trial initiation: not relevant; did not take MPH before Medication‐free period between interventions: 17 h Titration period: none Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
|
|
| Notes | Sample calculation: no information Ethics approval: no information Comments from trial authors
Key conclusions of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: not stated Funding source: NIMH Email correspondence with trial authors: November 2013. We received from the trial authors supplemental information regarding participants' intellectual function and funding and additional data. The raw data no longer exist; therefore we cannot analyse data from different periods in the cross‐over trial, only endpoint data |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned to placebo and LD‐ and HD‐MPH |
| Allocation concealment (selection bias) | Unclear risk | Randomly assigned to placebo and LD‐ and HD‐MPH. Not stated how investigators allocated participants to the intervention |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The child, his or her parents, teachers and research assistants were all blinded to drug status |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The child, his or her parents, teachers and research assistants were all blinded to drug status |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Only 3 dropouts. All other participants were included in the final analysis Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): unclear, no reason for dropouts stated |
| Selective reporting (reporting bias) | Low risk | No indication of selective reporting |
Pliszka 2000.
| Study characteristics | ||
| Methods | 3‐week, randomised, double‐blind, placebo‐controlled, parallel trial with 3 arms:
|
|
| Participants | Number of participants screened: 73 Number of participants included: 58 Number randomly assigned: MPH 20, placebo 18 Number followed up: MPH 19, placebo 16 Number of withdrawals: MPH 1, placebo 2 Diagnosis of ADHD: DSM‐IV. DISC diagnosis of ADHD (subtype not stated) Age: mean 9 years (range not reported) IQ: > 75 MPH‐naive: MPH 5 (25%), placebo 1 (6%) Ethnicity: not stated Country: USA Setting: outpatient clinic Comorbidity: ODD (MPH 14%, placebo 10%), CD (MPH 1%, placebo 2%), anxiety disorder (MPH 20%, placebo 5%) Comedication: not stated Other sociodemographics: no significant difference in baseline demographics were noted between the 2 groups Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to IR‐MPH or placebo Mean MPH dosage: total dose 25.2 mg/d Administration schedule: 17 (85%) received ≥ 2 doses Time points: 1‐3 times/d: morning, after school and additional noon if needed Duration of intervention: 3 weeks Titration period: none Treatment compliance: not stated. Dosage was adjusted at the end of weeks 1 and 2 via an algorithm based on teacher and parent ratings |
|
| Outcomes |
ADHD symptoms
General behaviour
Non‐serious AEs
|
|
| Notes | Sample calculation: no Ethics approval: no Comment from trial authors
Key conclusion of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: yes, 3 Funding source: Shire Richwood Incorporated Email correspondence with trial authors: January 2014. Supplemental information received from trial authors |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned: "We used a random number generator to determine which of the 3 groups the child was assigned to" |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Parents, children, teachers and treating physicians were blind to medication status. Medication was crushed, mixed with a blue food powder and placed in opaque capsules |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Placebo participants were randomly assigned to follow MPH or MAS treatment algorithm. The blinded psychiatrist could not determine the child’s medication status simply by knowing which algorithm was being followed. Principal Investigator knew the medication status of participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Morning and afternoon IOWA scores were averaged at the end of the week Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Low risk | All planned outcomes were reported |
Pliszka 2007.
| Study characteristics | ||
| Methods | 5‐week double‐blind, cross‐over, placebo‐controlled trial, conducted to examine electrophysiological effects of MPH on inhibitory control in children with ADHD
|
|
| Participants | Number of participants screened: 12 Number of participants included: 12 (8 boys, 4 girls) Number of participants followed up: 12 Number of withdrawals: 0 Diagnosis of ADHD: DSM‐IV (combined (100%)) Age: mean 12.3 years (range 9‐15) IQ: not stated MPH‐naive: 10 Ethnicity: white (67%), African American (8%), Hispanic (25%) Country: USA Setting: not stated Comorbidity: ODD (45%) Comedication: not stated Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to different possible drug condition orders of 5 mg, 10 mg, 15 mg, 20 mg MPH and placebo Mean MPH dosage: not stated Administration schedule: 3 time points Duration of each medication condition: 7 days Washout before trial initiation: not stated Medication‐free period between interventions: 0 Titration period: none initiated before/after randomisation |
|
| Outcomes |
General behaviour
|
|
| Notes | Sample calculation: no Ethics approval: yes; trial approved by the Institutional Review Board of the University Comments from trial authors
Key conclusions of trial authors
Comment from review authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: no Funding source: NIMH Grant R01 MH63986 Email correspondence with trial authors: May 2014. Trial authors could not give us the necessary data (e.g. separate data for each intervention period); therefore we could not use CGI data |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: insufficient information regarding generation of random sequence allocation to permit a judgement of low or high risk of bias. |
| Allocation concealment (selection bias) | Unclear risk | From email: "one investigator was assigned on the basis of chance; the other remained blinded" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind. One investigator assigned on the basis of chance; the other remained blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | One investigator assigned on the basis of chance; the other remained blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified |
Pliszka 2017.
| Study characteristics | ||
| Methods | A 3 ‐week parallel trial with 2 arms:
Phases: 2 |
|
| Participants | Number of participants screened: 163 Number of participants included: 163 (161 were included in the ITT analysis; 113 (70.2%) boys, 48 (29.8% girls) Number of participants followed up: 138 Number of withdrawals: 25 Diagnosis of ADHD: DSM‐5. 145 (90.1%) combined, 0 (0%) predominantly hyperactive‐impulsive, 16 (9.9%) predominantly inattentive Age: mean 9.3 years (SD 1.79, range 6‐12 years) (of the ITT sample) IQ: not stated MPH‐naive: 0% Ethnicity: Hispanic/Latino (n = 34, 21.1%), non‐Hispanic/Latino (n = 126, 78.3%), missing (n = 1, 0.6%) (of the ITT sample). Race was reported from the ITT sample: white (n =105, 65.2%), black/African American (n = 45, 28.0%), Asian (n = 1, 0.6%), American Indian/Alaska native (n = 1, 0.6%), other (n = 9, 5.6%) Country: USA Setting: day clinic/research clinic or hospital Comorbidity: many disorders and somatic comorbidities were exclusion criteria Comedication: psychotropics not allowed. No use of prescription medication except those specified in exclusion criterion 9 and 10 Sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to DR/ER‐MPH oral capsules containing beads, titrated to 40, 60 or 80 mg (week 1, 2 and 3 respectively), or placebo matched to DR/ER‐MPH oral capsules but containing microcrystalline cellulose beads in place of MPH for 3 weeks Number randomised to each group: DR/ER‐MPH 82, placebo 81 Mean medication dosage: DR/ER‐MPH 68.1 mg (at endpoint) Administration schedule: once daily in the evening at 8:00 pm ± 30 min; “Participants were also permitted to adjust the evening dose time between 6:30 and 9:30 pm in 30‐ or 60‐minute increments per week to achieve optimal morning control of observed ADHD symptoms” Duration (of (each) medication): DR/ER‐MPH titrated to 40 mg 1st week, 60 mg 2nd week, 80 mg 3rd week (if increases were not tolerated 1‐down titration was allowed to a minimum of 40 mg), or placebo 3 weeks Washout before trial initiation: up to 2 weeks (phase 1). Stimulants, clonidine, and guanfacine required a >72‐h washout, and any other medication used to treat ADHD required a >7‐day washout before randomisation Treatment compliance: nothing stated besides percentage that completed the trial |
|
| Outcomes |
ADHD symptom severity
Serious AEs
General behaviour
Non‐serious AEs
|
|
| Notes | Sample calculation: yes; 180 with an estimated dropout rate of 20%‐25% = 70 participants per treatment arm after dropout Ethics approval: yes; “All participants and parents/legal guardians provided informed assent and consent, respectively, under procedures approved by each site’s institutional review board” Comments from trial authors
Key conclusion of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH: yes Any withdrawals due to AEs: yes, 5 Funding source: Ironshore Pharmaceuticals Email correspondence with trial authors: September 2021. We received supplemental information regarding risk of bias assessment through personal email correspondence with the trial authors in September 2021 (Storm 2021f [pers comm]) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Medication randomisation will be performed using an interactive web response system (IWRS). The IWRS will assign subjects to a treatment group based on the pre‐defined randomisation list." |
| Allocation concealment (selection bias) | Low risk | "A kit identification number corresponding to the assigned treatment will be assigned by IWRS." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Treatment assignments will be masked to the investigator, the sponsor, trial statistician, and the trial subjects." "If an investigator, site personnel performing assessments, or subject/parent/legal guardian is unblinded, the subject must be withdrawn from the trial, and procedures accompanying withdrawal are to be performed." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Treatment assignments will be masked to the investigator, the sponsor, trial statistician, and the trial subjects." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "The ADHD‐RS‐IV was analysed by using a mixed‐model repeated measures (MMRM) analysis that included the participant’s intercept as a random effect; treatment, trial center, and visit‐by‐treatment interaction as fixed effects; and baseline ADHD‐RS‐IV total score at Visit 2 as a covariate. The BSFQ and ADHD‐AM‐RS were also analysed using the MMRM, as described for the primary efficacy analysis." Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Quinn 2004.
| Study characteristics | ||
| Methods | Cross‐over trial with 3 interventions:
|
|
| Participants | Number of participants screened: not stated Number of participants included: 32 Number of participants followed up: 31 (all boys) Number of withdrawals: 1 Diagnosis of ADHD: DSM‐IV (combined (87.1%), hyperactive‐impulsive (9.7%), inattentive (3.2%)) Age: not stated (range 9‐12 years) IQ: > 80 MPH‐naive: 0% Ethnicity: not stated Country: USA and Canada Setting: outpatient clinic Comorbidity: (0%) Comedication: 0% for other medications for ADHD Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of the possible drug condition orders of d‐MPH, d,I‐MPH and placebo. Both the order of drugs and the dose sequence were randomly assigned Mean MPH dosage: d‐MPH 2.5 mg, 5 mg or 10 mg; d,l‐MPH 5 mg, 10 mg or 20 mg Administration schedule: once at 8.30 am Duration of each medication condition: 1 day. Interventions were separated by ≥ 6 days Washout before trial initiation: 24 h Medication‐free period between interventions: ≥ 24 h Titration period: none Treatment compliance: administered at the laboratory |
|
| Outcomes |
ADHD symptoms
Non‐serious AEs
|
|
| Notes | Sample calculation: no Ethics approval: approved by each centre’s institutional review board Comments from trial authors (limitations)
Key conclusions of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: yes; included only children stable on MPH Any withdrawals due to AEs: no Funding source: Celgene Email correspondence with trial authors: July ‐August 2014. We contacted trial authors to obtain safety data and supplemental information regarding randomisation, allocation concealment, blinding, handling of incomplete outcome data and outcomes, but we were not successful |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised. Both the order of drugs and the dose sequence were randomly assigned |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Except for first practice day, on which only participants were blinded, administration of doses was double‐blinded throughout the trial |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double‐blind. Blinded observers rated behaviour |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Trial authors report only 1 respondent LTFU, not related to side effects Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported. No protocol available |
Ramtvedt 2013.
| Study characteristics | ||
| Methods | 6‐week, cross‐over trial with 3 interventions:
Phases: 3 |
|
| Participants | Number of participants screened: not stated Number of participants included: 36. Number of participants followed up: 36 (29 boys, 7 girls) (n = 34 for AE data) Number of withdrawals: 0 Diagnosis of ADHD: DSM‐IV‐TR (combined (69%), hyperactive‐impulsive (3%), inattentive (28%)) Age: mean 11.4 years (range 9‐14) IQ: mean 90.9 MPH‐naive: 100% Ethnicity: not stated Country: Norway Setting: outpatient clinic Comorbidity: anxiety/depressive disorder (n = 9, 25%), ODD (n = 20, 55%), learning disability (n = 22, 61%) and Asperger syndrome (n = 1, 3%) Comedication: no Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 6 possible drug condition orders of MPH (30 mg/d‐40 mg/d), dextroamphetamine (10 mg/d‐20 mg/d) and placebo Mean MPH dosage: not stated Administration schedule: MPH, 3 time points (morning, lunch and afternoon); dextroamphetamine, 2 time points (morning and afternoon) Duration of each medication condition: 2 weeks (1st week: low dose (30 mg/d), 2nd week: high dose (40 mg/d)) Washout before trial initiation: no; all were stimulant‐naive Medication‐free period between interventions: yes; Saturday and Sunday (48 h) Titration period: 2 weeks initiated after randomisation Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
General behaviour
Non‐serious AEs
|
|
| Notes | Sample calculation: not stated Ethics approval: yes Comments from trial authors
Key conclusions of trial authors
Comments from review authors
Inclusion of MPH responders only/exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: no Funding source: first phase was conducted as part of ordinary clinical practice at Neuropsychiatric Unit, Østfold Hospital Trust. Second and third phases, data analysis and preparation of manuscript were sponsored by South‐Eastern Norway Regional Health Authority, and also by Østfold Hospital Trust and National Resource Centre for ADHD, both under the umbrella of South‐Eastern Norway Regional Health Authority Email correspondence with trial authors: April 2015. We obtained supplemental information or data regarding comedication, randomisation and blinding |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly and evenly assigned to each of 6 possible drug orders. "The children were assigned to the 6 possible drug condition orders by drawing numbers" |
| Allocation concealment (selection bias) | Unclear risk | Not clear |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants, parents, teachers and test administrators were blinded to drug order. 1 parent identified the drug order. Tablets of similar colours, shapes and textures were administered, but the drugs were not camouflaged in identical capsules |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Unclear risk | No access to protocol |
Rapport 1985.
| Study characteristics | ||
| Methods | Triple‐blind, randomised, cross‐over trial with 4 interventions:
Phases
|
|
| Participants | Number of participants screened: 22 Number of participants included: 14 (12 boys, 2 girls) Number of participants followed up: 11 Number of withdrawals: 1 Number of participants excluded during the trial: 2 Diagnosis of ADHD: DSM‐III (subtype not stated) Age: 8.3/7.75 years (range 6‐10) IQ: > 100 MPH‐naive: not stated Ethnicity: white (86%), not stated (14%) Country: USA Setting: outpatient clinic Comorbidity: not stated Comedication: no Other sociodemographics: low to middle socioeconomic status Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 24 possible drug condition orders of fixed doses of MPH (5 mg, 10 mg, 15 mg) and placebo. As a result of the small sample size, not all drug orders were used. Post hoc comparison showed that doses were distributed approximately equally across different positions in the sequence Administration schedule: once daily, in the morning Duration of each medication condition: 7 days Washout before trial initiation: none, but all weekly dosage changes occurred on Saturdays to control for potential rebound effects Titration period: none Treatment compliance: both used and unused envelopes with capsules were returned to control for medication compliance. No results regarding compliance were reported |
|
| Outcomes |
ADHD symptoms
No useable data |
|
| Notes | Sample calculation: not stated Ethics approval: not stated Key conclusion of trial authors
Comment from review authors
Inclusion of MPH responders only/exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: no Funding source: not declared Email correspondence with trial authors: July 2013. Trial authors informed us that original records were shredded after 20 years, and that they could not provide the requested data (Ramstad 2013c [pers comm]) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Order of drug administration was determined by randomly assigning each child to 1 of 24 possible drug disorders |
| Allocation concealment (selection bias) | Low risk | MPH was packaged by the pharmacist in coloured gelatin capsules to avoid detection of dose and taste. Capsules were placed in individually dated envelopes to ensure accurate dose administration |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Triple‐blind design (children, teachers and observers were blinded) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Teachers and observers were blind to when medication was administered and what specific doses were given |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): yes, data from 2 of 12 children in the present investigation were not included in the statistical analyses because they were classified as non‐responders according to results of the Paired Associates Learning test |
| Selective reporting (reporting bias) | Unclear risk | Not possible to receive a copy of the protocol |
Rapport 1987.
| Study characteristics | ||
| Methods | Double‐blind, placebo‐controlled, cross‐over trial with 2 interventions:
Phases: 5 MPH doses were given in a randomly assigned, counterbalanced sequence. Children received each dose for 6 consecutive days |
|
| Participants | Number of participants screened: 134. Children were screened for inclusion after referrals from paediatricians, psychiatrists and school personnel over a 5‐year period. Different publications reported different numbers of included children, ranging from 22‐76 children. Trial authors stated: "this is a sample of children who were recruited across several years with publications presenting findings over that time period. Thus, there were slight differences in sample composition as more participants were added. But essentially these studies included the same sample". Different publications reported different figures for missing data, number of withdrawals and other important information. This was included in our risk of bias assessment and is reported in the risk of bias table. 1‐day washout (Saturdays) was described in each publication Age: 10‐12 years. Reported ages varied across publications but, in most publications, ranged from 6‐11 years Diagnosis of ADHD: DSM‐III diagnosis of ADHD (subtype not stated) IQ: mean 101. DuPaul 1993 (in Rapport 1987), stated that children were of average or above average intelligence Sex: 37 boys and 5 girls (Rapport 1987) MPH‐naive: 100% (in both Rapport 1987 + other paper, DuPaul 1993). In Rapport 1987, 8 had experienced brief trials of stimulants within the previous 4 years Ethnicity: not stated Country: USA Setting: outpatient clinic Comorbidity: not stated Comedication: no children were taking medication before participating in the trial (DuPaul 1993 in Rapport 1987) Other sociodemographics: low to middle socioeconomic status (Hollingshead) (Rapport 1987 and DuPaul 1993 in Rapport 1987) Inclusion criteria
Exclusion criteria
We believe that this trial consists of 13 articles. When trial authors were asked about that, Dr. DuPaul answered that many of the publications based on the Rhode Island Construction Leadership Council (CLC) Clinic "were fairly the same study". Even though some publications describe 6 inclusion criteria and others describe 5, we believe that the publications are based on the same trial (Ramstad 2013b [pers comm]) |
|
| Interventions | Participants were randomly assigned to 1 of 5 possible drug condition orders of MPH and placebo. MPH was described by each child's paediatrician in the following doses: placebo, 5 mg, 10 mg, 15 mg and 20 mg. Fixed doses were prescribed (rather than mg/kg) Administration schedule: children were seen once a week at the Rhode Island Construction Leadership Council (CLC) Clinic. Baseline measures were obtained during the child's second clinic visit to allow familiarisation with clinic personnel and testing procedures. On subsequent testing days, children were administered a capsule of the active agent (MPH) or placebo. This procedure continued until each child received each dose for 6 consecutive days. All weekly dosage changes occurred on Sundays, and no medication was administrated on Saturdays, to allow for needed washout due to inter‐individual variation in serum and blood plasma levels following acute administration of MPH. All children described in the present trial were classified as favourable responders, whereas those whose performance did not improve to this extent were classified as non‐responders. These criteria were established a priori. All children described in the present trial were favourable responders based on the given criteria. (Children showing drug‐induced facilitation of performance ≥ 25% (i.e. a 25% drop in error rate) compared with baseline or placebo were classified as favourable responders, whereas those whose performance did not improve to this extent were classified as non‐responders) Treatment compliance: Rapport 1987 and DuPaul 1993 (in Rapport 1987): both used and unused envelopes were returned to the Rhode Island Construction Leadership Council (CLC) on a weekly basis to assess medication compliance. DuPaul 1993 and Denney 1999 (in Rapport 1987): Medication was properly administered nearly 100% of the time, with "make‐up" observation days scheduled after rare occasions when compliance was not obtained |
|
| Outcomes |
ADHD symptoms
Non‐serious AEs
|
|
| Notes | Sample calculation: no Ethics approval: not stated. From Kelly 1988 in Rapport 1987: believe the trial was approved by the Institutional Review Board IRB at the University of Rhode Island (the site of the trial), but Dr. Rapport could confirm this (Ramstad 2013c [pers comm]) Key conclusions of trial authors
Inclusion of MPH responders only/exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: not stated Funding source: none, either external or internal. This project was supported in part by a Biomedical Research Support Grant (Number S07 RR05712), which was awarded to the first trial author by the Biomedical Research Support Grant Program, Division of Research Resources, NIH Email correspondence with trial author: we contacted Dr. Rapport by email (Ramstad 2013c [pers comm]). He has stated that he does not have any supplementary data except those provided in these articles. We also contacted Dr. DuPaul (Ramstad 2013b [pers comm]) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Rapport 1987: "all children received each of the 5 MPH doses in a randomly assigned counterbalanced sequence" |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | All MPH and placebo doses were packaged in coloured gelatin capsules by the clinic's pharmacist. Capsules were sealed in individual daily‐dated envelopes. All teachers were blinded as to time of administration and specific dose |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided on blinding of outcome assessor |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): yes (4 participants) All children described in the present trial were classified as favourable responders, whereas those whose performance did not improve to this extent were classified as non‐responders. These criteria were established a priori. All children described in the present trial were favourable responders based on the given criteria. (Children showing drug‐induced facilitation of performance ≥ 25% (i.e. a 25% drop in error rate) compared with baseline or placebo were classified as favourable responders, whereas those whose performance did not improve to this extent were classified as non‐responders. |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
Rapport 2008.
| Study characteristics | ||
| Methods | Double‐blind, placebo‐controlled, 5‐week, cross‐over trial with 2 interventions:
Phases: baseline (1 week) and 5 cross‐over phases (1 week placebo and 1 week MPH each) |
|
| Participants | Number of participants screened: not stated Number of participants included: 65 Number of participants followed up: 65 (58 boys, 7 girls) Number of withdrawals: none Diagnosis of ADHD: DSM‐IV (all were of the combined subtype) Age: mean 8.56 years (SD 1.25; range 6‐11) IQ: mean 102.8 (SD 10.0; range not stated) MPH‐naive: 8 had experienced brief trials of stimulant therapy within the previous 4 years. None were prescribed psychostimulants immediately before the start of the current trial Ethnicity: white (100%) Country: USA Setting: outpatient clinic Comorbidity: not stated Comedication: not stated Other sociodemographics: low to middle Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to different possible drug condition orders of IR‐MPH (5 mg/d, 10 mg/d, 15 mg/d, 20 mg/d) and placebo Mean MPH dosage: not stated Administration schedule: once daily, in the morning Duration of each medication condition: 1 week Washout before trial initiation: none were prescribed psychostimulants immediately before the start of the current trial Medication‐free period between interventions: 1 day Titration period: none Treatment compliance: "nearly 100%; envelopes were returned on a weekly basis" |
|
| Outcomes |
Non‐serious AEs
|
|
| Notes | Sample calculation: not stated Ethics approval: yes Comments from trial authors
Key conclusion of trial authors
Comment from review authors
Inclusion of MPH responders only/exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: no Funding source: none Email correspondence with trial authors: April 2014. Obtained supplemental information regarding data (Ramstad 2013c [pers comm]) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | By email: "we used a computer‐generated random assignment (without replacement) procedure with an additional stipulation that a nearly equal number of children had to be assigned to each of the possible drug condition orders. Children were entered onto the list as they entered the trial and followed that particular order (of which coders, parents, teachers and evaluators were unaware throughout the trial)" (Magnusson 2014b [pers comm]) |
| Allocation concealment (selection bias) | Low risk | Same as above |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | MPH and placebo dosages were packaged in coloured gelatin capsules by the clinic's pharmacist to avoid detection of dose and taste |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Rater was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Information received by email: no dropouts (Magnusson 2014b [pers comm]) Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Low risk | No indication of selective reporting. All outcomes were reported |
Reitman 2001.
| Study characteristics | ||
| Methods | A 20‐day cross‐over trial with treatment shifting daily between 2 arms:
|
|
| Participants | Number of participants screened: not stated Number of participants included: 3 (1 boy, 2 girls) Number of participants followed‐up: 3 Number of withdrawals: none Diagnosis of ADHD: not stated Age: 6.3 years (range 6‐7) IQ: all participants were of normal intelligence. MPH‐naive: no Ethnicity: not stated Country: USA Setting: outpatient, summer treatment camp Comorbidity: not stated Comedication: not stated Additional sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to receive either MPH at individual dosage or placebo in a day‐by‐day cross‐over design. Placebo and MPH were combined with behavioral treatment and no treatment Number randomised to each group: not stated Mean medication dosage: 10 mg, 10 mg and 15 mg Administration schedule: not stated Duration (of (each) medication): not clearly stated, but from the text we assume that they received MPH and placebo for 10 days each Washout before trial initiation: not stated Medication‐free period between interventions: no Titration period: dosages were the ones that the children had responded to in a trial with classroom behaviour Treatment compliance: children received either medication or placebo each morning at home, and medication administration integrity was checked via phone or face‐to‐face confirmation each afternoon |
|
| Outcomes |
ADHD symptoms
|
|
| Notes | Sample calculation: no Ethics approval: not stated Comments from trial authors
Key conclusion of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH: yes, all children had shown previous response to their individual dosage Any withdrawals due to AEs: no Funding source: not declared Email correspondence with trial authors: none. We did not request supplemental information from the trial authors as no contact information was available. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Pill boxes were used to facilitate medication administration |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both staff and children were blind to medication status |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The observers were blind to medication status and completed the Conners ADHD index following the game each day |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No data imputed Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified |
Riggs 2011.
| Study characteristics | ||
| Methods | 16‐week, randomised, 11‐centre, parallel‐group trial with 2 arms:
Participants in both medication groups received manual‐standardised, individual CBT through motivational enhancement approaches throughout the 16‐week medication trial |
|
| Participants | Number of participants screened: 1334 prescreened, 450 were consented and screened. An unknown number was included in the pre‐randomisation titration phase. Number of participants included: 303 (239 boys, 64 girls) Number of participants followed up: 303 (MPH 151, placebo 152) Number of withdrawals 76 (MPH 33, placebo 43) Diagnosis of ADHD: DSM‐IV (combined (68.6%), hyperactive‐impulsive (2.6%), inattentive (28.1%)) Age: mean 16.5 years (SD 1.3; range 13‐18) IQ: all participants were cognitively normal MPH‐naive: not stated Ethnicity: Hispanic (15.2%) Race: white (61.7%), African American (23.2%), Asian (1.3%), Native American/Alaskan (1.0%), other (12.7%) Country: USA Setting: outpatient clinic Comorbidity: major depressive disorder (12.5%), CD (32.3%), non‐nicotine substance use disorder (100%) Comedication: drug/alcohol use Other sociodemographics: none Differences between groups : No statistically significant differences in baseline demographics were noted between the 2 groups Inclusion criteria
Exclusion criteria
Opiate dependence
Exploratory analyses of treatment response (ADHD‐RS)
|
|
| Interventions | Participants were randomly assigned to OROS‐MPH or placebo Number randomised to each group: MPH 151, placebo 152 Mean MPH dosage at the end of treatment: 68 mg Administration schedule: single dose in the morning Duration of intervention: 16 weeks Titration period: 2 weeks post‐randomisation Treatment compliance: 79% (pill counts), 82.3% (self‐reports) |
|
| Outcomes |
ADHD symptoms
Serious AEs
Non‐serious AEs
|
|
| Notes | Sample calculation: yes; sample size of 300 participants was calculated for ADHD‐RS Ethics approval: yes; Institutional Review Boards approved the protocol before participant enrolment Comments from trial authors
Key conclusions of trial authors
Inclusion of MPH responders only/exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: unclear. Participants underwent a 2‐week titration period prior to randomisation. It is not reported if any participants withdrew during the titration period due to AEs or lack of efficacy. Any withdrawals due to AEs: reasons for withdrawals not stated Funding source: OROS‐MPH and matching placebo were supplied to the Clinical Trials Network contract pharmacy (EMINENT Services Corporation) by McNeil Consumer and Specialty Pharmaceuticals (distributor for Concerta), at no cost. Comment from review authors
Email correspondence with trial authors: December 2013. We obtained supplemental information (IQ, allocation concealment and detailed description of 1 of the serious AEs) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned to OROS‐MPH or matching placebo in a 1:1 ratio, stratified by site and completed by computer at a centralised location |
| Allocation concealment (selection bias) | Low risk | Product was supplied in pre‐randomly assigned kits containing individual bottles. Kits and bottles were labelled with the protocol number and treatment/randomisation number. Labeling protected the trial, ensuring that it remained blinded, and indicated that the medication was investigational |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Sites were provided blinded medication bottles for each randomly assigned participant Masking: double‐blinded (participant, caregiver, investigator, outcomes assessor) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Masking: double‐blinded (participant, caregiver, investigator, outcomes assessor) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Primary analyses were ITT, including all randomly assigned trial participants Completers (N = 227) were not different from non‐completers (N = 76) in baseline demographic or clinical characteristics, and the proportion of completers did not differ by treatment assignment Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Low risk | No indication of selective reporting. |
Rubinsten 2008.
| Study characteristics | ||
| Methods | 5‐day, randomised, double‐blind, placebo‐controlled, cross‐over trial with 4 interventions and 11 possible orders:
|
|
| Participants | Number of participants screened: 170 Number of participants included: 18 Number of participants followed up: 18 (15 boys, 3 girls) Number of withdrawals: 0 Diagnosis of ADHD: DSM‐IV (combined (28%), hyperactive‐impulsive (0%), inattentive (72%)) Age: mean 9.73 years (range not reported) IQ: mean 104.44 MPH‐naive: 0% Ethnicity: not stated Country: Canada Setting: outpatient clinic Comorbidity: not stated Comedication: no Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 11 possible drug condition orders of MPH (10 mg, 15 mg and 20 mg) and placebo Mean MPH dosage: 0.27 mg/kg, 0.42 mg/kg and 0.58 mg/kg, respectively Administration schedule: not stated Duration of each medication condition: 1 day Washout before trial initiation: 24 h Medication‐free period between interventions: not stated Titration period: none Treatment compliance: not stated |
|
| Outcomes |
Non‐serious AEs
|
|
| Notes | Sample calculation: no Ethics approval: not stated Comment from trial authors
Key conclusion of trial authors
Comment from review authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: no Funding source: research was completed while Dr. Rubinsten was a post‐doctoral fellow at the Hospital for Sick Children (HSC), in Toronto, Canada, and was supported by the Rothschild Fellowship from Israel. It was undertaken, in part, through funding received from the Canadian Institutes of Health (CIHR: grant #MOP 64312), a CIHR post‐doctoral fellowship (A‐CB), and the Canada Research Chairs Program (RT) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Master randomisation tables were prepared by the research support pharmacist at the hospital using simple randomisation with restrictions. A balanced block 22 design was used |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information on allocation concealment provided to permit a judgement of low or high risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Examiner, psychiatrist, child and child’s family were not informed about the child’s randomisation order or daily medication status until trial completion. Placebo and active medication were prepared by the hospital pharmacist as powdered and packaged in an opaque gelatin capsule to prevent identification of content by colour, taste or volume. Each child’s medication was placed in an individually named and dated envelope to ensure accurate administration |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Examiner, psychiatrist, child and child’s family were not informed about the child’s randomisation order or daily medication status until trial completion |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified |
Samuels 2006.
| Study characteristics | ||
| Methods | Double‐blind, randomised, cross‐over trial with 2 interventions:
Phases: 2 |
|
| Participants | Number of participants screened: not stated Number of participants included: 6 Number of participants followed up: not stated Number of withdrawals: not stated Diagnosis of ADHD: DSM‐IV (subtype not stated) Age: mean (not stated) IQ: mean (not stated) MPH‐naive: not stated Ethnicity: white (100%) Country: USA Setting: outpatient clinic Comorbidity: not stated Comedication: not stated Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 2 possible drug condition orders of MPH and placebo Mean MPH dosage: not stated Administration schedule: each child was kept on his or her previous regimen of treatment and a corresponding placebo Duration of each medication condition: 24 h Washout before trial initiation: 3‐day run‐in Titration period: children were kept on stable medication doses and schedules Treatment compliance: not stated |
|
| Outcomes |
Non‐serious AEs
No separate data were available for MPH |
|
| Notes | Sample calculation: no Ethics approval: not stated Key conclusion of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: yes, all participants were already on a stable dose of MPH Any withdrawals due to AEs: unclear Funding source: not declared Email correspondence with trial authors: July 2014. Emailed trial authors to ask for additional information but have not received a reply |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described; "randomized in double‐blind fashion" |
| Allocation concealment (selection bias) | Unclear risk | "Randomized in double‐blind fashion" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Described as "double‐blind". All medications and placebo were identically packaged by the research pharmacy |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only participants who successfully underwent an ambulatory BP monitoring trial at the end of each phase were included in the primary analyses. The first trial included 17 participants, 13 of whom underwent the second trial. Of these, data from 11 participants (of whom 6 received MPH) were analysed. Unclear why the rest were not included. Selection bias (e.g. titration after randomisation → exclusion): unclear |
| Selective reporting (reporting bias) | Unclear risk | Protocol not found |
Schachar 1997a.
| Study characteristics | ||
| Methods | 12‐month, parallel RCT with 4 arms
Follow‐up at baseline (before treatment), at end of titration (3‐4 weeks) and after 4, 8 and 12 months of treatment Follow‐up trial of cohort completing the 12‐month RCT: participants were evaluated annually for 5 years |
|
| Participants | Number of participants screened: 302 Number of participants included: 91 included in the RCT (74 boys, 17 girls) Number of participants followed up: 63 (MPH 45, placebo 18) Number of withdrawals: 28 (MPH 1, placebo 27) Diagnosis of ADHD: DSM‐III‐R diagnosis of ADHD (subtype not stated) Mean age: MPH 8.4 years, placebo 8.3 years (range 6‐12) IQ: mean (MPH 110.3; placebo 107.6) MPH‐naive: 100% Ethnicity: not stated Country: Canada Setting: research unit at hospital Comorbidity: ODD (MPH 56.5%, placebo 44.4%), CD (MPH 6.5%, placebo 20.0%), anxiety (MPH 21.7%, placebo 24.4%) Comedication: no Other sociodemographics: placebo group had a higher score at baseline for psychosocial adversity than those assigned to the MPH group Psychosocial risk index: MPH 1.5%, placebo 2.7% Inclusion criteria Screening
Diagnostic evaluation
Final inclusion criteria
Exclusion criteria
|
|
| Interventions | Randomly assigned to 1 of the 4 treatment groups after stratification based on comorbid, conduct or oppositional disorder: MPH + parent training/parent support, placebo + parent training/parent support Number of participants randomised: MPH 46, placebo 45 Titration period: 3‐4 weeks (depending on child's weight) after randomisation Mean MPH dosage: target dose 0.7 mg/kg body weight, twice daily Administration schedule: breakfast and lunch Duration of intervention: 12 months Treatment compliance: MPH 80%, placebo 64% |
|
| Outcomes |
ADHD symptoms
General behaviour
Non‐serious adverse effects
|
|
| Notes | Sample calculation: yes Ethics approval: yes Comments from trial authors
Key conclusions of trial authors RCT
Follow‐up trial
Comments from review authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: yes. “Side effects were the stated reason for discontinuing MPH in five cases” Funding source: Medical Research Council of Canada, National Health Research Development Program of Canada and the Department of Psychiatry, The Hospital for Sick Children, Toronto. Placebo pills were provided by Ciba Geigy, Canada, Ltd Email correspondence with trial authors: September‐October 2013. Unfortunately, they were not able to provide supplemental data |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned after stratification based on the presence of comorbid conduct or oppositional disorder and assigned to 1 of the 4 treatment groups. Randomly assigned based on a random number table |
| Allocation concealment (selection bias) | Low risk | Concealed from the physician and participants before randomisation and maintained throughout the trial |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All participants (child, research staff, parents and teachers) were blinded to the medication assignment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All participants (child, research staff, parents and teachers) were blinded to the medication assignment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No imputation method: the effect of treatment was analysed among participants who adhered to their original medication assignment. At the 4‐month point, children not taking any medication were grouped with those taking placebo. When a participant switched from placebo to MPH treatment, they were counted within the MPH group Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Unclear risk | Not possible to find protocol |
Schachar 2008.
| Study characteristics | ||
| Methods | Single‐centre, randomised, double‐blind, 3‐way cross‐over trial with 2 interventions:
Phases: IR‐and multi‐layer (ER)‐release |
|
| Participants | Number of participants screened: not stated Number of participants included: 18 Number of participants followed up: 17 (15 boys, 2 girls) Number of withdrawals: 1 Diagnosis of ADHD: DSM‐IV diagnosis of ADHD (combined (100%)) Age: mean 11.3 years (range 6.8‐15.3) IQ: ≥ 85 MPH‐naive: 5 (29.4) Ethnicity: not stated Country: USA Setting: outpatient clinic, laboratory classroom setting Comorbidity: no Comedication: not stated Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 2 possible drug condition orders of IR‐MPH and ER‐MPH and placebo Mean MPH dosage: 31.2 mg/d (SD 11.7 mg/d; range 20‐60 mg/d), 1.2 mg/kg Administration schedule: twice daily, in the morning and at noon (4 h apart) Duration of each medication condition: 6 days Washout before trial initiation: 1 day Titration period: none. Participants who were receiving MPH at the time of trial entry received the dose of MPH that they were taking before entry into the trial Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
Non‐serious AEs
|
|
| Notes | Sample calculation: no Ethics approval: yes Key conclusion of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: yes Any withdrawals due to AEs: no Funding source: Purdue Pharma (Canada) Email correspondence with trial authors: May 2014. Emailed trial authors to ask for supplementary information but have not received a reply |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned to a treatment sequence according to a Latin square |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind; not further described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double‐blind; not further described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 1 withdrawal due to inability to swallow capsules, all other participants were included in efficacy analyses. All participants were included in safety analyses. Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol was published |
Schrantee 2016.
| Study characteristics | ||
| Methods | A 16‐week parallel‐trial with 2 arms:
Phases: 2:4 (1‐week actigraphy baseline, 16‐week parallel trial followed by 1‐week washout, 1‐week actigraphy follow‐up) |
|
| Participants | Number of participants screened: 75 boys Number of participants included: 50 (100% boys) Number of participants followed‐up: 48 (46 for sleep measurements) Number of withdrawals: 2 from the placebo group. Sleep outcomes were excluded for another 2 participants due to analytic errors Diagnosis of ADHD: DSM‐IV (21 combined, 1 hyperactive‐impulsive and 28 inattentive type) Age: placebo 11.3 MPH 11.4 (range 10‐12) IQ: MPH 103.22 (SD 21.01), placebo 103.35 (15.05) MPH‐naive: 100 % Ethnicity: not stated Country: the Netherlands Setting: outpatient Comorbidity: many were an exclusion criterion Comedication: many were an exclusion criterion Additional sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 2 different interventions, either flexible dose MPH (starting with 0.3 mg/kg day in 1‐2 doses) or placebo Number randomised to each group: 25 Mean medication dosage: MPH‐group 31.3043 mg/d (information from 23 participants) Administration schedule: individually fixed times during the day. 1‐2 times Duration (of (each) medication): 16 weeks Washout before trial initiation: NA Titration period: trial dosage can be increased weekly with 5‐10 mg/d to a maximum of 60 mg daily Treatment compliance: adherence to the trial medication was monitored at each of the 5 control visits |
|
| Outcomes |
Serious AEs
Non serious AEs
|
|
| Notes | Sample calculation: no Ethics approval: yes. The institutional review board of the Academic Medical Center approved the trial. Comments from trial authors
Key conclusion of trial authors
Comments from review authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH: no Any withdrawals due to AEs: yes, 1 in the placebo group Funding source: this trial was funded by faculty resources of the Academic Medical Center, University of Amsterdam, and by grant 11.32050.26 from the European Research Area Network Priority Medicines for Children (Sixth Framework Programme). Dr Rombouts was supported by Vici (Netherlands Organisation for Scientific Research), and Dr Andersen was supported by grant DA‐015403 from the National Institute on Drug Abuse. Email correspondence with trial authors: September 2021. We received supplemental information regarding mean medication dosage and vested interest through personal email correspondence with the trial authors in September 2021. (Storm 2021g [pers comm]). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned (1:1) using a permuted block randomisation scheme generated by the local Clinical Research Unit to either IR‐MPH or matching placebo treatment for 16 weeks |
| Allocation concealment (selection bias) | Low risk | The hospital pharmacy (Alkmaar) assigned participants to a specific allocation, using sequentially numbered containers. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The placebo tablet was identical to the MPH tablet with respect to appearance and was manufactured and labelled according to guidelines (2003/94/EG). The treating physician prescribed the medication under double‐blind conditions on clinical guidance (reduction in ADHD symptoms), in accordance with Dutch treatment guidelines. After trial end, blinding was checked with the participant and his psychiatrist as well as the trial investigators. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants as well as care providers and research personnel were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
“Missing values for the CBF (3.6% [14 of 392] due to dropout and 10.7% [42 of 392] in total) and clinical assessments (3.6% [14 of 392] due to dropout and 18.9% [74 of 392] in total) were replaced using nearest neighbor interpolation within age and medication group.” Comment: High‐risk method of analysis. Only 2 withdrawals from outcomes other than sleep, therefore low risk for general behaviour. Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Low risk | All outcomes from trial protocol reported |
Schulz 2010.
| Study characteristics | ||
| Methods | Double‐blind, randomised, multi‐centre, triple cross‐over design with 3 interventions:
|
|
| Participants | Number of patients screened: 147 Number of participants included: 147 (119 boys, 28 girls) Number of participants followed up: 139 Number of withdrawals: 1 Diagnosis of ADHD: DSM‐IV diagnosis (combined (55%), hyperactive‐impulsive (8%), inattentive (37%)) Age: mean 10.2 years (range 6‐14) IQ: not stated MPH‐naive: 0% Ethnicity: not stated Country: Germany Setting: outpatient clinic Comorbidity: 6.8% (disturbance in social behaviour (2.7%), initial insomnia (0.7%), ODD (1.36%), dysphaemia (0.68%), encopresis (0.68%)) Comedication: no Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Pre‐randomisation phase and 3 treatment periods of 7 days each. Participants were randomly assigned to 1 of 6 possible drug condition orders of 20 mg ER‐MPH (Ritalin), 20 mg ER‐MPH (Medikinet Retard) and placebo Number of participants: not stated Mean MPH dosage: 20 mg daily Administration schedule: once daily, morning Time points: 9:00 am Duration of each medication condition: 7 days Washout before trial initiation: none Medication‐free period between interventions: none Titration period: none Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
SeriousAEs
General behaviour
Non‐serious AEs
Clinically notable increased values for SBP were recorded under all 3 treatments: 15% on placebo, 17% on ER‐MPH (Ritalin) and 18% on ER‐MPH (Medikinet). Abnormal increases in DBP were noted in 5% of participants taking placebo and LA MPH (Ritalin) and in 3% of participants given ER‐MPH (Medikinet). Abnormal values in SBP among participants who had normal values at screening and at baseline were recorded in only 7 participants (3 given placebo, 3 taking ER‐MPH (Medikinet) and 2 taking ER‐MPH (Ritalin) ‐ as reported in the article). Changes in vital signs were attributed to sympathomimetic effects of MPH and were not considered clinically relevant |
|
| Notes | Sample calculation: yes
Ethics approval: yes Comments from trial authors
Key conclusions of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: yes; efficacy trial to prove non‐inferiority of ER‐MPH (Ritalin) vs ER‐MPH (Medikinet). Highly restrictive inclusion criterion ‐ responders only. Trial authors acknowledge "it might overestimate the treatment effect and the tolerability in the overall population, as no treatment naive patients have been included in this trial" Any withdrawals due to AEs: no Funding source: not declared. Trial aimed at showing efficacy of ER‐MPH (Ritalin) with purpose of obtaining marketing authorisation. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was centrally generated with a validated system that automated the random assignment of treatment sequences to randomization numbers in a specified ratio" |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "All treating physicians, other site staff, and patients, as well as data analysts and Novartis in‐house personnel, remained blinded from the time of randomization until database lock. The raters also did not have access to information about adverse events to maintain the blind" "To ensure blinding of the study, all capsules were overencapsulated in an identical optical design" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The scale was rated by three treatment‐blinded observers in the classroom who were specifically trained on the SKAMP scale" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomly assigned participants were included in the ITT population. Missing values for the primary endpoints were replaced by the worst value observed in another participant under the same treatment at the same assessment time. For non‐inferiority comparisons, the per‐protocol (PP) population consisting of participants without major protocol violations was considered primary Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified |
Schwartz 2004.
| Study characteristics | ||
| Methods | 2‐week, double‐blind, placebo‐controlled, cross‐over, randomised clinical trial with 2 interventions:
|
|
| Participants | Number of participants screened: not stated Number of participants included: 44 (37 boys, 7 girls) Number of participants followed up: 44 Number of withdrawals: none Diagnosis of ADHD: DSM‐IV (subtype not stated) Age: mean 9.2 years (range 6‐12) IQ: > 70 MPH‐naive: 16 Ethnicity: not stated Country: Canada Setting: outpatient clinic Comorbidity: CD (39%), ODD (32%), separation anxiety disorder (16%), major depression (7%) Comedication: no Other sociodemographics: income level category, mean 3.7 Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 2 possible drug condition orders of (0.5 mg/kg) MPH and placebo Mean MPH dosage: not stated Administration schedule: twice/day: morning/noon time points Duration of each medication condition: 7 days Washout before trial initiation: yes (2 weeks) Medication‐free period between interventions: not stated Titration period: none stated Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
Non‐serious AEs
|
|
| Notes | Sample calculation: post hoc power calculation Ethics approval: yes Comment from trial authors
Key conclusions of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: yes; exclusion of children with history of intolerance Any withdrawals due to AEs: no Funding source: grants from Le Fonds de la Recherche en Santé du Québec and the Canadian Institutes of Health Research. Email correspondence with trial authors: March‐April 2014. We emailed trial authors twice to ask for supplemental information regarding data on ADHD symptoms but have not received a reply |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "After baseline assessments, children randomly received either placebo or (...)" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified |
Sharp 1999.
| Study characteristics | ||
| Methods | Cross‐over trial with 3 interventions:
Phases
|
|
| Participants | Number of participants screened: 150 (girls) Number of participants included: 42 girls and 56 comparison boys Number of participants followed up: 42 Number of withdrawals: 1 girl given placebo at each phase Diagnosis of ADHD: DSM‐IV (subtype not stated) Mean age: 9.1 years (range 6.0‐12.7) Mean IQ: boys 109.3, girls 105.2 MPH‐naive: Some of the participants had no previous experience with stimulants. No information on exact number. Ethnicity: girls: white (67%), African American (19%), Hispanic (14%); boys: white (73%), African American (21%), Hispanic (4%), Asian (2%) Country: USA Setting: outpatient clinic, laboratory classroom setting Comorbidity: 71% boys, 69% girls; ODD (33% boys, 50% girls), CD (7% boys, 2% girls), major depression (0% boys, 7% girls), separation anxiety (0% boys, 2% girls), specific phobias (0% boys, 7% girls), trichotillomania (2% boys, 0% girls), tic disorders not otherwise specified (13% boys, 2% girls), enuresis (18% boys, 12% girls), reading disorder (5% boys, 8% girls) Comedication: not stated Other sociodemographics: socioeconomic status mean score 48.0 ± 25.8 (girls), 52.4 ± 26.9 (boys) Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 6 possible drug condition orders of MPH, dextroamphetamine and placebo Sharp 1999 Mean MPH dosage: week 1 mean dose 0.45 mg/kg, week 2 mean dose 0.85 mg/kg and week 3 mean dose 1.28 mg/kg Administration schedule: breakfast and lunch ‐ 5 days per week administered by nurse; administered by parent on weekends Elia 1991 (secondary reference under Sharp 1999): Mean MPH dosage: week one 0.9 mg/kg, week two 1.5 mg/kg and week three 2.5 mg/kg Administration schedule: 9:00 am and 1:00 pm throughout the entire week (7 days) Duration of each medication condition: 3 weeks Washout before trial initiation: 3‐week medication‐free period before trial Medication‐free period between interventions: none Titration period: none Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
Serious AEs Mean beneficial and adverse effects of dextroamphetamine and MPH were nearly identical for all ratings, including ratings of appetite problems. However, objectively verified significant decreases in body weight (drug main effect, F 10.27; P = 0.0002) were significantly greater for dextroamphetamine (mean change −1.1 ± 1.0 kg from baseline; P = 0.02) than for MPH (−0.4 ± 1.1 kg; not significant) Stimulant‐related AEs and body weight General behaviour
Non‐serious AEs
|
|
| Notes | Sample calculation: no Ethics approval: no information available Key conclusion of trial authors
Comment from review authors
Inclusion of MPH responders only/exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: no Funding source: not declared Email correspondence with trial authors: June 2014. We received supplemental information from Dr. Castellanos |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Order of medication (MPH, placebo or dextroamphetamine) was randomly assigned on the basis of a table administered by the research pharmacy. Dose escalation was fixed, with 2 ranges, depending on body weight (> or < 30 kg). 3 weeks (1 dose per week) in each phase. For most children, this was followed by random assignment to pemoline vs placebo after another washout period |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | MPH, dextroamphetamine and placebo were packaged in identical capsules by the NIH pharmacy and were administered by NH nurses in double‐blinded randomly assigned order |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All ratings were performed blind to treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Did not describe what they did with the missing data Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol published. |
Shiels 2009.
| Study characteristics | ||
| Methods | 3‐day, double‐blind, placebo‐controlled cross‐over trial with 3 arms:
|
|
| Participants | Number of participants screened: not stated Number of participants included: 49 (80% boys, 20% girls) Number of participants followed up: not stated Number of withdrawals: 0 Diagnosis of ADHD: DSM‐IV (combined 35 (71%), hyperactive‐impulsive 3 (6%), inattentive 11 (23%)) Age: mean 10.5 years (range 9‐12) IQ: mean 104 (range not stated) MPH‐naive: not stated Ethnicity: white (76%), African American (14%), other (10%) Country: USA Setting: outpatient clinic (summer research programme) Comorbidity: ODD (43%), CD (22%) Comedication: not stated Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were assigned to extended‐release MPH in "high" or "low" dose (range 18‐90 mg/d) No of participants randomised: not stated Mean MPH: LD 39 mg (Concerta), HD 73 mg (Concerta) Administration schedule: once daily Time points: in the morning, 90 min before cognitive task Duration of intervention: 7:30 am‐5:00 pm, Monday through Thursday Titration period: not stated Treatment compliance: not stated Washout before trial initiation: MPH 1 week if taking atomoxetine, 24 h if taking MPH |
|
| Outcomes |
General behaviour
Non‐serious AEs
|
|
| Notes | Sample calculation: not stated Ethics approval: not stated Key conclusions of trial authors
Comment from review authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: none Funding source: National Institute of Mental Health Email correspondence with trial authors: July 2014. Emailed trial authors to request additional information but have not received a response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "To maintain blinding, participants were given the same number of opaque capsules per day regardless of actual MPH dose" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified |
Silva 2005a.
| Study characteristics | ||
| Methods | 6‐week, randomised, single‐blind, placebo‐controlled, cross‐over trial with 5 interventions:
|
|
| Participants | Number of participants screened: not stated Number of participants included: 54 (34 boys, 20 girls) Number of participants followed up: 53 Number of withdrawals: 1 Diagnosis of ADHD: DSM‐IV (combined (70.4%), hyperactive‐impulsive (1.9%), inattentive (27.8%)) Age: mean 9.4 years (range 6‐12) IQ: > 80 MPH‐naive: 0% Ethnicity: white (63.0%), African American (14.8%), Asian (0%), other (22.2%) Country: USA Setting: laboratory classroom Comorbidity: not stated Comedication: not stated Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 10 possible drug condition orders of 20 mg ER‐MPH, 40 mg ER‐MPH, 18 mg OROS‐MPH, 36 mg OROS‐MPH and placebo Mean MPH dosage: not stated Administration schedule: once Duration of each medication condition: 1 day Washout before trial initiation: 1 day Medication‐free period between interventions: participants were instructed to continue to take their regularly prescribed medication Sundays through Thursdays between trial days; no medication was administered on Fridays to avoid possible carry‐over effects during the Saturday treatment period Titration period: none Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
Non‐serious AEs
Only 1 AE was considered to be drug‐related |
|
| Notes | Sample calculation: yes; 46 Ethics approval: approved by an independent investigational review board Comments from trial authors
Key conclusions of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: yes Any withdrawals due to AEs: no Funding source: Novartis Pharmaceuticals Corporation Email correspondence with trial authors: April 2014. Sent an email to trial authors to ask for additional data but have not receive a reply |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All trial personnel, with the exception of the nurse, pharmacist or physician dispensing medication, were blinded Trial medications were administered in an opaque container with a small aperture, so participants could not see them during administration |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The SKAMP was completed by blinded raters |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 1 participant received ER‐MPH 20 and 40 mg before discontinuing the trial. Reason not stated Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
Silva 2006.
| Study characteristics | ||
| Methods | Cross‐over trial with 2 interventions:
Phases: 2 cross‐over phases |
|
| Participants | Number of participants screened: not stated Number of participants included: 54 (38 boys, 16 girls) Number of participants followed up: 53 Number of withdrawals: 1 Diagnosis of ADHD: DSM‐IV (combined (90.7%), hyperactive‐impulsive (0%), inattentive (9.3%)) Age: mean 9.4 years (range 6‐12) IQ: not stated MPH‐naive: none Ethnicity: predominantly white Country: USA Setting: laboratory classroom Comorbidity: not stated Comedication: not stated Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 2 possible drug condition orders of 20 mg/d d‐MPH‐ER and placebo Mean MPH dosage: 20 mg/d Administration schedule: once daily; morning Duration of each medication condition: 1 week Washout before trial initiation: 1 day Medication‐free period between interventions: 1 day Titration period: none Treatment compliance: 100% in laboratory classroom sessions |
|
| Outcomes |
ADHD symptoms
Non‐serious AEs
|
|
| Notes | Sample calculation: yes Ethics approval: not stated Comments from trial authors
Key conclusion of trial authors
Inclusion of MPH responders only/exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: yes Any withdrawals due to AEs: yes; 1 (during placebo) Funding source: Novartis Email correspondence with trial authors: June 2014. Sent twice to trial author to ask for additional information but have not received a reply |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by computerised random number assignment |
| Allocation concealment (selection bias) | Low risk | Trial medication comprised 1 bottle of 5 capsules of blinded trial medication or 1 bottle of 5 matching placebo capsules |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind. Trial medication comprised 1 bottle of 5 capsules of blinded trial medication or 1 bottle of 5 matching placebo capsules |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | 3 independent blinded raters |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Safety population consisted of all participants who received ≥ 1 dose of trial medication. Efficacy population comprised all randomly assigned participants who provided valid efficacy measurements for both treatment periods Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified |
Silva 2008.
| Study characteristics | ||
| Methods | Randomised, multi‐centre, double‐blind, placebo‐controlled, cross‐over design with 2 interventions:
|
|
| Participants | Number of participants screened: 68 (45 boys, 23 girls) Number of participants included: 68 Number of participants followed up: 67 Number of withdrawals: 1 (from the placebo group) Diagnosis of ADHD: DSM‐IV (combined (82.4%), hyperactive‐impulsive (0%), inattentive (17.6 %)) Age: mean 9.5 years (range 6‐12) IQ: > 70 MPH‐naive: none Ethnicity: white (50%), African American (22.1%), Asian (0%), Hispanic (19.1%), other (8.8%) Country: USA Setting: outpatient clinic Comorbidity: not stated Comedication: not stated Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to ER‐d‐MPH or placebo Mean MPH dosage: 20 mg/d Administration schedule: once daily, in the morning Duration of each medication condition: 7 days Washout before trial initiation: 2 days Titration period: none Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
Non‐serious AEs
|
|
| Notes | Sample calculation: no Ethics approval: yes Comment from trial authors
Limitations
Key conclusions of trial authors
Inclusion of MPH responders only/exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: yes Any withdrawals due to AEs; yes Funding source: Novartis Email correspondence with trial authors: October 2013. We received supplemental information from trial authors regarding ethics approval and data. We were not able to obtain first period data |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random assignment |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All trial medications were identical in appearance for blinding purposes; double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Safety population consisted of all participants who took ≥ 1 dose of trial medication. Efficacy population included all randomly assigned participants who provided valid efficacy measurements for both treatment periods. Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Low risk | Outcomes according to protocol |
Smith 1998.
| Study characteristics | ||
| Methods |
Main trial: 8‐week intensive Summer Treatment Program, including a 6‐week, double‐blind, cross‐over trial with 4 interventions:
Follow‐up trial: retrospective follow‐up trial of 16 individuals who completed double‐blind, placebo‐controlled, cross‐over studies during 2 separate Summer Treatment Programs |
|
| Participants |
Main trial Number of participants screened: not stated Number of participants included: 49 Number of participants followed up: 46 (41 boys, 5 girls) Number of withdrawals: 3 Diagnosis of ADHD: DSM‐III‐R (subtype not stated) Age: mean 13.8 years (range 12‐17) IQ: mean 101 (range 65‐129) MPH‐naive: 28% Ethnicity: white (85%), African American (15%) Country: USA Setting: outpatient clinic (summer treatment program) Comorbidity: ODD (50%), CD (15%) Comedication: no Other sociodemographics: median family income USD 38,500 Inclusion criteria
Exclusion criteria
Follow‐up trial (Not used in this review) Number of participants included: 16 (all boys) Number of participants followed up: 16 Number of withdrawals: none Diagnosis of ADHD: DSM‐III‐R Age: mean (children) 10.2 years (range 8‐11); mean (adolescents) 12.7 years (range 12‐14.5) IQ: mean (children) 109; mean (adolescents) 107 MPH‐naive: 4 (25%) Ethnicity: white (100%) Country: USA Comorbidity: not stated Comedication: not stated Sociodemographics: family income (children USD 37,943; adolescents USD 49,650) |
|
| Interventions |
Main trial Participants were randomly assigned to 1 of the possible drug condition orders of 25 mg/d, 50 mg/d or 75 mg/d MPH and placebo Mean MPH dosage: 0.17 mg/kg Administration schedule: 3 times/d: 7:45 am, 11:45 am, 3:45 pm Duration of each medication condition: 6 days Washout before trial initiation: 2 weeks Medication‐free period between interventions: 16 h Titration period: none Treatment compliance: not stated Follow‐up trial (Not used in this review) Participants were randomly assigned to 1 of the possible drug condition orders of 0.3 mg/kg MPH and placebo Mean MPH dosage: 0.3 mg/kg Administration schedule: twice/d: 8:00 am and 12:00 pm Duration of each medication condition: 1 day Washout before trial initiation: 2 weeks Medication‐free period between interventions: 20 h Titration period: none Treatment compliance: not stated. Adolescents were evaluated on a protocol that included placebo and 3 doses of MPH. To facilitate comparison, doses for adolescents were converted to milligrams per kilogram, and the dose closest to 0.3 mg/kg was used in this trial |
|
| Outcomes |
ADHD symptoms
General behaviour
Non‐serious AEs
|
|
| Notes | Sample calculation: no
Ethics approval: not stated Comments from trial authors Main trial
Follow‐up trial
Key conclusions of trial authors Main trial
Follow‐up trial
Inclusion of MPH responders only/exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: no Funding source: grants from the National Institute on Drug Abuse, the NIMH, the National Institute on Alcohol Abuse and Alcoholism and the National Institute of Child Health and Human Development Email correspondence with trial authors: September 2014. We contacted trial authors twice to ask for supplemental information/data but have received no response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Medication conditions were randomly assigned daily, with each condition occurring once a week. Thus, adolescents received a mode of 6 replications of each medication condition |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind; no further information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double‐blind; no further information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "Some missing data was caused by holidays or absences from the program" Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
Smith 2004.
| Study characteristics | ||
| Methods | n‐of‐1 randomised, double‐blind, cross‐over trial investigating the effects of MPH (Ritalin) on the disruptive behaviour of a child diagnosed with ADHD. 1‐day antecedent analysis in the clinic followed by an extended school‐based trial, during which participants received MPH or placebo before evaluation Duration: 15 days |
|
| Participants | Number of participants screened: not stated Number of participants included: 1 boy Number of participants followed up: 1 Number of withdrawals: none Diagnosis of ADHD: DSM‐IV (subtype: not stated) Age: 11 years IQ: 124 MPH‐naive: no Ethnicity: not stated Country: USA Setting: outpatient clinic Comorbidity: not stated Comedication: not stated Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participant was randomly assigned to 1 of 2 possible drug condition orders of (20 mg/d ) IR‐MPH and placebo Outpatient clinic setting, duration 1 day: ingestion of 20 mg MPH or placebo 45 min before evaluation (randomised) Cross‐over 4 h later followed by 2nd evaluation School setting: 15 days (3 school weeks) Administration schedule: once daily, mornings, 45 min before 1st evaluation Medication‐free periods: on weekends; a total of 9 days with medication and 6 days without, randomly assigned throughout the trial period Titration period: no Washout period before trial initiation: none other than weekends Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
|
|
| Notes | Sample calculation: no Ethics approval: not stated Comments from trial authors
Key conclusion of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: no Funding source: not declared Email correspondence with trial authors: October 2013. We received from trial authors supplemental information regarding diagnostic criteria |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A third party, who was not involved in the clinical procedure, determined the selection of actual medication packs |
| Allocation concealment (selection bias) | Low risk | A third party, who was not involved in the clinical procedure, determined the selection of actual medication packs |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | A third party, not involved in the clinical procedures, determined the selection of actual medication packs. Thus, people conducting the direct evaluation were blinded to all medication manipulations (i.e. the child, parents, therapists and data collectors). However, no description was provided about whether medication and placebo pills were identical. "Prior to clinical assessment, two packets of medication were provided to the participant's parent. One package contained 20 mg of Ritalin and the other package contained a placebo" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | A third party, who was not involved in the clinical procedure, determined the selection of actual medication packs. Thus, people conducting the direct evaluation were blinded to all medication manipulations (i.e. the child, parents, therapists and data collectors) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified and no email from trial author |
Smithee 1998.
| Study characteristics | ||
| Methods | Cross‐over trial with 2 interventions:
|
|
| Participants | Number of participants screened: not stated Number of participants included: 26 (20 boys, 6 girls) Number of participants followed up: not stated Number of withdrawals: not stated Diagnosis of ADHD: DSM‐IV (combined (77% ), inattentive (23%)) Age: mean 9.63 years (range 6.5‐12) IQ: mean 99.71 MPH‐naive: 24 Ethnicity: not stated Country: USA Setting: outpatient clinic Comorbidity: ODD/CD (n = 13), anxiety disorders (n = 13), nocturnal enuresis (n = 4), vocal tics (n = 4), motor tics (n = 3) Comedication: not stated Other sociodemographics: middle class status; mean social class of 2.12 Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 2 possible drug condition orders of MPH and placebo Mean MPH dosage: 0.78 mg/kg/d Administration schedule: 3 times daily for 14 days per medication conditions Time points: morning, noon and 4:00 pm Duration of each medication condition: 14 days Washout before trial initiation: not stated Medication‐free period between interventions: no Titration period: on days 1‐3, participants received a dose 2.5 mg below their target dose of 0.3 mg/kg twice/d. On days 4‐7, dose was raised to 0.3 mg/kg twice/d, and on day 8, the 4:00 pm dose was added Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
Non‐serious AEs
Relatively few side effects were reported, probably in part because of adjustment in dosages in response to emergent symptoms |
|
| Notes | Sample calculation: no Ethics approval: not stated Comment from trial authors
Key conclusion of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: unclear, not stated Funding source: NIMH Grant MH 38228; Rafael Klorman Email correspondence with trial authors: March 2014. We received supplemental data from trial authors |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Medications were administered in random order |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Medications were administered in random order, under double‐blind conditions; capsules of identical appearance and taste |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | At the end of each phase, an investigator blind to pharmacological conditions administered a structured interview to the child’s parent concerning emergent side effects in the preceding period |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Reports of side effects led to bind reduction of dosages or omission of planned increments for 3 participants under MPH Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Unclear risk | Table with numbers of reported somatic effects not given |
Solanto 2009.
| Study characteristics | ||
| Methods | Cross‐over trial with 2 interventions:
Phases: week‐long exposure to placebo and to each of 3 different dosage regimens of IR‐MPH |
|
| Participants | Number of participants screened: not stated Number of participants included: 30 Number of participants followed up: 25 Number of withdrawals: 5 Diagnosis of ADHD: DSM‐IV (combined (60%), inattentive (40%)) Age: mean 8.53 (combined) and 9.20 (inattentive) IQ: not stated MPH‐naive: all but 1 were stimulant‐naive Ethnicity: not stated Country: USA Setting: hospital Comorbidity: ODD (combined 13%, predominantly inattentive 20%); learning disability (combined 40%, predominantly inattentive 20%); anxiety (combined 0%, predominantly inattentive 10%) Comedication: no Other sociodemographics: minority representation (combined 53%, predominantly inattentive 20%) Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 24 possible drug condition orders of LD‐, MD‐ and HD‐MPH and placebo Mean MPH dosage (Combined vs predominantly Inattentive): LD 0.50 ± 0.12 vs 0.44 ± 0.13; MD 0.83 ± 0.20 vs 0.73 ±‐0.21; HD 1.54 ± 0.31 vs 1.40 ± 0.38 Administration schedule: 3 times daily: morning, midday and 3:00 pm Duration of each medication condition: 1 week Washout before trial initiation: not required Medication‐free period between interventions: no Titration period: open‐label lead‐in week, not specified whether before or after randomisation of treatment Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
Non‐serious AEs
|
|
| Notes | Sample calculation: no Ethics approval: not stated Key conclusion of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: unclear if there was an open‐label titration phase before randomisation Any withdrawals due to AEs: yes; 2 Funding source: not declared Email correspondence with trial authors: April 2014. We received supplemental information from trial authors |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Low risk | Double‐blind, cross‐over with week‐long exposure to placebo and each of 3 different dosage regimens of IR‐MPH in randomly assigned order |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, cross‐over with week‐long exposure to placebo and each of 3 different dosage regimens of IR‐MPH in randomly assigned order. Trial medications were prepared and coded by the hospital pharmacy using identical gelatin capsules for active medication and placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Across the 4 placebo and drug conditions, data points were missing as follows: CPRS 1%, CTRS 6%, Parent SKAMP 1%, Teacher SKAMP 4%, Side Effects 0%. Missing scores were replaced by the mean of scores for that group in that condition Selection bias: titration conducted but unclear whether before or after randomisation. No participants were excluded after the 1‐week titration period |
| Selective reporting (reporting bias) | Low risk | Protocol identified; all outcomes reported |
Soleimani 2017.
| Study characteristics | ||
| Methods | A 4‐week cross‐over‐trial with 4 arms:
Phases: 3 phases (4‐week cross‐over with focus on ADHD symptoms followed by 1‐week washout period and 2‐week cross‐over with focus on DCD symptoms) |
|
| Participants | Number of participants screened: 28 Number of participants included: 17 (12 boys, 5 girls) Number of participants followed‐up: 16 Number of withdrawals: 1 Diagnosis of ADHD: DSM‐IV TR (100% combined type) Age: 7 years 6 months (± 18 months) (range 6‐11 years or 6‐12 years) IQ: 96.6 (± 12.5, range 80‐118) MPH‐naive: history of MPH usage, mean 22.9 days (range 0‑90 days) Ethnicity: not stated Country: Iran Setting: outpatient Comorbidity: all participants had DCD. No other comorbidities allowed Comedication: not stated Additional sociodemographics: number of siblings: 1.3 (SD 0.5, range 1‐2) Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 4 possible orders of 3 different doses (0.25, 0.5 or 1 mg/kg/d) of MPH (Ritalin) and placebo for 1 week each Number randomised to each group: not stated Mean medication dosage: not applicable (mean minimal effective dose per day was 17.3 mg (mean 0.85 mg/kg, ± 0.16)) Administration schedule: twice/d, 8:00 and 15:00 Duration (of (each) medication): 3 weeks of MPH with 3 different doses, 1 week of placebo Washout before trial initiation: no Medication‐free period between interventions: no Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
Results were only reported for the MPH intervention and no data have been included in our analysis |
|
| Notes | Sample calculation: yes. “The sample size was estimated from the previous double‐blind study. We estimated that the recruitment of 13 cases would provide 90% power to detect a significant difference between the effects of treatment on motor performance at a significance of P<0.05.” Ethics approval: yes Comments from trial authors
Key conclusion of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH: no Any withdrawals due to AEs: no Funding source: Guilan University of Medical Sciences Email correspondence with trial authors: August, October and November 2021. We contacted the trial authors for information regarding age of participants and first‐period data through personal email in August, October and November 2021, but no answer was received. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The patients were randomly allocated into one of the 4 groups using a random number generator. |
| Allocation concealment (selection bias) | Low risk |
Quote: "The key to the randomization was kept by the pharmacy and randomization was broken only in case of potential adverse events." Comment: no adverse events stated, and no statement of code breaking. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | These drugs were prepared by the Shafa Hospital Pharmacy and placed into similar gelatinous capsules. Drugs were labelled by trial participant number according to the randomisation schedule. Parents and the researchers were blinded to child’s drug condition and dosage. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Parents and the researchers were blinded to child’s drug condition and dosage. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 1 withdrawal Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Low risk | All outcomes from protocol reported |
Stein 1996.
| Study characteristics | ||
| Methods | 5‐week, triple‐blind, placebo‐controlled, cross‐over trial with 4 interventions:
|
|
| Participants | Number of participants screened: not stated Number of participants included: 25 (all boys) Number of participants followed up: 24 Number of withdrawals: 1 Diagnosis of ADHD: DSM‐III‐R (combined (88%), inattentive (12 %)) Age: mean 8.0 years (range 6‐12) IQ: not stated MPH‐naive: 44% Ethnicity: white (96%), Hispanic (4%) Country: USA Setting: outpatient clinic Comorbidity: ODD (28%), CD (12%) Comedication: no Other sociodemographics: Hollingshead socioeconomic status category I: 8 (33%), II: 8 (33%), III: 3 (12.5%), IV: 5 (20.8%) Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 4 possible drug condition orders of MPH and placebo Mean MPH dosage: 8.8 mg, 0.3 mg/kg/dose Administration schedule: twice/3 times daily: 8:00 am, 12:00 pm (and 2:00 pm) Duration of each medication condition: 1 week Washout before trial initiation: ≥ 5 days Medication‐free period between interventions: none Titration period: initiated after randomisation Treatment compliance: "there was generally good compliance, with a mean of 1.5 missed doses per child over the 5‐week course of the study" |
|
| Outcomes |
ADHD symptoms
Serious AEs
Non‐serious AEs
|
|
| Notes | Sample calculation: no Ethics approval: approved by the Institutional Review Board of the University of Chicago Comments from trial authors
Key conclusions of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: yes; 1 Funding source: the work was supported by the Smart Family Foundation Email correspondence with trial authors: July 2014. Supplemental information regarding randomisation and whether all planned outcomes were measured and reported was received from trial authors. Unfortunately, trial authors no longer had access to the dataset; therefore it has not been possible to receive supplemental data |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned to 1 of 4 different orders of drug administration. The research pharmacist used a random numbers table to assign participants to different orders of administration |
| Allocation concealment (selection bias) | Low risk | "All medication and placebos were prepared by the study pharmacist and placed in opaque gelatin capsules" In addition to the pharmacist, 1 investigator had access to the dosage code in the event of a medical emergency that necessitated breaking the blind |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Triple‐blinded: participants always took 3 capsules daily during the trial. "Active drug phase (b.i.d. or three t.i.d. [two or three times/day]) was always preceded by a titration phase. The purpose of the titration phase was to introduce a typical dose of MPH gradually, so that any observation of side effects during the b.i.d. and t.i.d. dosing phases could not be attributed to rapid introduction of MPH"; "All medication and placebos were prepared by the study pharmacist and placed in opaque gelatin capsules"; "In addition to the pharmacist, one investigator had access to the dosage code in the event of a medical emergency that necessitated breaking the blind" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Triple‐blinded: in addition to the pharmacist, one investigator had access to the dosage code in the event of a medical emergency that necessitated breaking the blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One dropout. Data collected on this participant were used in the descriptive information for baseline, 3 times/d and titration phases Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Low risk | All planned outcomes were measured and reported |
Stein 2003.
| Study characteristics | ||
| Methods | 4‐week, double‐blind, placebo‐controlled, cross‐over trial in which participants were randomly assigned to:
|
|
| Participants | Number of participants screened: not stated Number of participants included: 47 (33 boys, 14 girls) Number of participants followed up: 39 Number of withdrawals: 8 Diagnosis of ADHD: DSM‐IV (combined (68%), hyperactive‐impulsive (0%), inattentive (32%)) Age: mean 9.02 years (range 5 years 11 months‐16 years) IQ: mean 106.8 Stimulant‐naive: 70% Ethnicity: white (89.4%), African American (4.3%), Hispanic (2.1%), other (4.3%) Country: USA Setting: outpatient clinic Comorbidity: ODD (17%), encopresis/enuresis (10.6%), tic disorder (2.1%) Comedication: no Other sociodemographics: predominantly mid to upper socioeconomic status referral base of the clinic Inclusion criteria
Exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to different orders of OROS‐MPH (18 mg, 36 mg, 54 mg) and placebo. No child could start with the 54‐mg dose, and 1 child who weighed < 40 kg did not receive the 54‐mg dose to minimise potential side effects in smaller children Administration schedule: once daily Duration of each medication condition: 7 days Washout before trial initiation: 2‐week washout period for children who took stimulant medication before trial start Medication‐free period between interventions: no Titration period: none Treatment compliance: 92% of all trial medications were given |
|
| Outcomes |
ADHD symptoms
Non‐serious AEs
|
|
| Notes | Sample calculation: no Ethics approval: approved by the Institutional Review Boards of The University of Chicago, Children’s National Medical Center and the General Clinical Research Center Advisory Council Comments from trial authors
In interpreting the results, several limitations should be kept in mind
Key conclusions of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: yes; 8 Funding source: the NIMH, the General Clinical Research Center Program of the National Center for Research Resources, and the NIH, Department of Health and Human Services Correspondence with trial authors: August 2014. We contacted trial authors to request additional data. Trial authors no longer have access to the data |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Dosing schedules were assigned from a randomly ordered list of all dosing schedules |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Each medication differed in appearance, size and colour and, in the case of the 54‐mg condition, number of capsules. The placebo capsule was slightly larger than the MPH preparations. Trial authors conducted several analyses to investigate the impact of these differences in appearance on the findings of the trial (almost no impact on the results) but still considered risk of bias to be high. Parents and clinicians who were blinded to genotype and medication status rated ADHD symptoms, impairment and stimulant side effects each week |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Parents and clinicians who were blinded to genotype and medication status rated ADHD symptoms, impairment and stimulant side effects each week |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Any child who could not complete a treatment phase was classified as a premature discontinuation. Their data were included for all phases that were completed. No description on how many participants the analyses were based on. Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | High risk | Data were included for all phases that were completed. There is no description that reveals how many people the analyses were based on. In the abstract belonging to the trial (Stein et al. 2004), researchers write that they will "prospectively evaluate the effects of different doses of stimulant medication on these and other sleep problems". This is not provided in any of the articles included in the Stein 2003 trial. |
Stein 2011.
| Study characteristics | ||
| Methods | 8‐week, double‐blind, cross‐over trial comparing the following:
with a week of randomly assigned placebo within each drug period |
|
| Participants | Number of participants screened: 77 Number of participants included: 65 Number of participants followed up: 56 (41 boys, 15 girls) Number of withdrawals: 9 Demographic data on the 56 participants Diagnosis of ADHD: DSM‐IV (combined (67%), hyperactive‐impulsive (0%), inattentive (33%)) Age: mean 11.78 years (range 9‐17) IQ: > 70 MPH‐naive: 35.7% Ethnicity: white (41.0%), African American (41.0%), Asian (2%), Hispanic (7%), bi‐racial/mixed (9%) Country: USA Setting: outpatient clinic Comorbidity: ODD (n = 11, 29.7 %), enuresis (n = 3, 8.1 %), generalised anxiety disorder (n = 1, 2.7 %) and separation anxiety (n = 1, 2.7 %) Comedication: not stated Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 3 dose conditions of ER‐d‐MPH and ER‐MAS administered sequentially from lowest to highest dose with a randomly assigned week of placebo during each period MPH dosage: 10 mg, 20 mg and 25‐30 mg. Maximum dose was 25 mg in smaller children (i.e. < 35 kg) to minimise potential side effects Administration schedule: once daily Duration of each medication condition: 1 week Washout before trial initiation: 2‐day washout period before beginning of the trial. No washout period between treatment periods Titration period: none Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
Non‐serious AEs
|
|
| Notes | Sample calculation: no Ethics approval: approved by the Institutional Review Board of University of Illinois at Chicago Comment from trial authors
Key conclusions of trial authors
Comment from review authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: yes; see comments above Any withdrawals due to AEs: yes; 6 Funding source: investigator‐initiated trial sponsored by Novartis Pharmaceuticals, with additional support provided by the University of Illinois at Chicago, Center for Clinical and Translational Science. Email correspondence with trial authors: November and December 2013. We received supplemental data from trial authors. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Order of drug was randomly assigned so that 50% started with ER‐d‐MPH and 50% started with ER‐MAS; also a randomly assigned week of placebo during each period |
| Allocation concealment (selection bias) | Low risk | Weekly blister packs containing capsules of trial drug, which were indistinguishable from each other |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participant, caregivers, outcome assessors |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participant, caregivers, outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All trial participants who received ≥ 2 weeks of trial drug to ensure that all participants had been exposed to ≥ 1 week of active drug were analysed Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Low risk | Outcomes reported according to protocol |
Stoner 1994.
| Study characteristics | ||
| Methods | 2 n‐of‐1, double‐blind, placebo‐controlled, cross‐over, randomised controlled trial. Both cases received 4 levels of the following:
After the double‐blind trial, the code was broken, and the best dose was administered to the participant. Follow‐up data on the best dose were also measured. Follow‐up data were recorded anecdotally for the first case (Dan) and systematically for the second case (Bill) |
|
| Participants | Number of participants screened: 2 boys Number of participants included: 2 Number of participants followed up: 2 Number of withdrawals: none Diagnosis of ADHD: DSM‐III‐R (subtype not stated) Age: Dan 9 years, Bill 13 years IQ: not stated MPH‐naive: 100% Ethnicity: not stated Country: USA Setting: not stated Comorbidity: not stated Comedication: not stated Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to different orders of 3 doses of MPH and placebo Mean MPH dosage: not relevant Administration schedule: once daily at breakfast Duration of each medication condition: Dan received placebo for 3 days, and 5 days of treatment with 5 mg, 10 mg or 15 mg MPH consecutively. Bill received placebo for 3 days, and 3 days of treatment with 5 mg, 10 mg or 15 mg MPH consecutively Washout before trial initiation: treatment‐naive Medication‐free period between interventions: 24 h between doses Titration period: none Treatment compliance: parents were instructed to initial a monitoring form each time they gave a dose to their child after breakfast. No further description of this was provided Regarding the follow‐up trial: Dan 15 mg; Bill 10 mg (5 mg twice daily) |
|
| Outcomes |
General behaviour
Non‐serious AEs
|
|
| Notes | Sample calculation: not relevant Ethics approval: not stated Comments from trial authors
Key conclusion of trial authors
Comments from review authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: no Funding source: National Association of School Psychologists Email correspondence with trial author: January 2014. Wrote to trial author to request additional information regarding ethics approval, intellectual disability, etc., but have not received a response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The order of medication levels was determined randomly. In packing the envelopes, 1 of the trial authors arranged the coded envelopes according to the randomly determined order of trials |
| Allocation concealment (selection bias) | Low risk | Each medication level was prepared and packed in separate envelopes by a pharmacist |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Each dose of MPH and placebo was ground into a powder, mixed with an inert compound, and was sealed in a small coloured drug capsule, so that doses were identical in appearance and taste. Thus, the pharmacist, the data collectors, the participants and their families did not know the order of drug administration |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Each dose of MPH and placebo was ground into a powder, mixed with an inert compound, and was sealed in a small coloured drug capsule, so that doses were identical in appearance and taste. Thus, the pharmacist, the data collectors, the participants and their families did not know the order of drug administration |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data were provided Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol was identified, and no answer was received from the trial author |
Sumner 2010.
| Study characteristics | ||
| Methods | 3‐week, triple‐blind, randomised, cross‐over trial with 2 interventions:
|
|
| Participants | Number of participants screened: not stated Number of participants included: 31 Number of participants followed up: not stated Number of withdrawals: 1 Diagnosis of ADHD: DSM‐IV (subtype not stated) Age: mean not stated (range 6‐14 years) IQ: not stated MPH‐naive: not stated Ethnicity: not stated Country: USA Setting: outpatient clinic Comorbidity: none Comedication: none Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 2 possible drug condition orders: placebo, low, medium doses of OROS‐MPH; or low, medium doses of OROS‐MPH, placebo Doses: low (18 mg), medium (participants < 50 kg: 27 mg; participants > 50 kg: 36 mg) Administration schedule: not stated Duration of each medication condition: 1 week Washout before trial initiation: 1 week Titration period: no Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
|
|
| Notes | Sample calculation: no
Ethics approval: yes Comments from trial authors
Key conclusions of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: yes; exclusion of participants who have exhibited significant treatment‐limiting AEs while treated with MPH Any withdrawals due to AEs: not stated Funding source: it was not clear who sponsored the trial, but someone did (see authors' affiliations) Email correspondence with trial authors: August‐September 2013. We attempted to obtain supplemental information regarding characteristics of participants, withdrawals, funding and efficacy data from trial authors but without success |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned to 1 of 2 treatment sequence groups |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Triple‐blinded: trial participants, their parents/guardians and clinicians were blinded to trial assignment in terms of medications and dosing sequences. An unblinded prescribing physician and an unblinded trial co‐ordinator did not participate in clinical ratings. Placebo was matched to blinded medication, so that the 2 were indistinguishable |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | See above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One participant withdrew before the placebo visit and was not included in these analyses Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol was available |
Sunohara 1999.
| Study characteristics | ||
| Methods | Cross‐over trial with 3 arms:
Phases: 3 |
|
| Participants | Number of participants screened: not stated Number of participants included: 20 (16 boys, 4 girls) Number of participants followed up: 20 Number of withdrawals: 0 Diagnosis of ADHD: DSM‐III‐R (subtype not stated) Age: mean 10.5 years (range 10‐12) IQ: < 80 MPH‐naive: none Ethnicity: not stated Country: Canada Setting: outpatient clinic Comorbidity: ODD (n = 4), learning disability (n = 8) Comedication: not stated Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to LD‐MPH (mean 0.28 mg/kg) and HD‐MPH (mean 0.56 mg/kg) and placebo in the morning and afternoon Duration of each medication condition: 2 days Washout before trial initiation: no Titration period: no Treatment compliance: not stated |
|
| Outcomes |
Non‐serious AEs
|
|
| Notes | Sample calculation: not stated Ethics approval: no information Key conclusion of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: unclear Any withdrawals due to AEs: no Funding source: RESTRACOM graduate studentship for The Hospital for Sick Children Research Institute and Novartis Pharmaceuticals Email correspondence with trial authors: April 2014. Wrote to trial authors to ask for additional data but have not received a reply |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were followed up Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified |
Swanson 1998.
| Study characteristics | ||
| Methods | Randomised, double‐blind, cross‐over trial with 6 interventions:
|
|
| Participants | Number of participants screened: 36 Number of participants included: 33 (26 boys, 7 girls) Number of participants followed up: 29 Number of withdrawals: 4 Diagnosis of ADHD: DSM‐IV (subtype not stated) Age: mean 10.58 years (range 7‐14) IQ: > 80 MPH‐naive: 0% Ethnicity: not stated Country: USA Setting: outpatient clinic (laboratory classroom) Comorbidity: none Comedication: none Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 36 possible drug condition orders of unknown dose of MPH, placebo and 4 doses of MAS (Adderall) Mean MPH dosage: not stated Administration schedule: once, morning Duration of each medication condition: 7 days Washout before trial initiation: not stated Medication‐free period between interventions: none Titration period: none Treatment compliance: 30 of 33 participants completed the 7‐week trial |
|
| Outcomes |
ADHD symptoms
Serious AEs
Non‐serious AEs
|
|
| Notes | Sample calculation: no Ethics approval: not stated Comments from trial authors
Key conclusions of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: yes; only known MPH responders were included Any withdrawals due to AEs: no, not in the MPH or placebo group (2 in different MAS groups) Funding source: grant from Richwood Pharmaceutical Company Email correspondence with trial authors: January 2014. No reply has been received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Latin‐square design (with provisions for 36 participants) was used to determine the within‐participant order of administration of the 6 medication conditions, so that for each of the first 6 weeks, approximately one sixth of participants would be assigned to each of the 6 conditions. Separate randomisation was used to determine assignment of conditions across participants for week 7, to provide an opportunity to make up missed weeks |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | For each condition, a pharmacist prepared a set of 7 identical capsules, all containing placebo (lactose), 1 of the 4 doses of MAS or MPH |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 30 of 33 participants completed the 7‐week trial; 98% of data were collected as planned. For each individual, missing data (1.1% for SKAMP, 0% for Stimulant Side Effects) were replaced by the average of values from adjacent time points (or adjacent values for session 1 or 6). Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
Swanson 1999.
| Study characteristics | ||
| Methods | 4 week, double‐blind, randomised, cross‐over trial with 4 interventions of each 1 day duration
|
|
| Participants | Number of participants screened: not stated Number of participants included: 38 (33 boys, 5 girls) 4 withdrew before randomisation and 34 were included in the cross‐over trial Number of participants followed up: 31 Number of withdrawals: 3 Diagnosis of ADHD: DSM‐IV (subtype not stated) Age: mean 9.2 years (range 7‐12) IQ: not stated MPH‐naive: 0% Ethnicity: not stated Country: USA Setting: outpatient clinic (laboratory classroom) Comorbidity: not stated Comedication: not stated Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to different drug condition orders of 4 different interventions: MPH twice/d, flat and ascending, and placebo Mean MPH dosage: not stated. Nominal dose: 20 mg/d Administration schedule: 30‐min intervals from 7:30 am to 3:00 pm Duration of each medication condition: 1 day Washout before trial initiation: none Titration period: none (participants were kept on current standard treatment between trial days) Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
|
|
| Notes | Sample calculation: no Ethics approval: yes Comment from trial authors
Key conclusion of trial authors
Comment from review authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: yes, only participants on a stable dose of MPH were included Any withdrawals due to AEs: unclear. No information about withdrawals given Funding: ALZA Corporation, Palo Alto, California Email correspondence with trial authors: May 2014. Emailed trial authors to ask for additional information. No reply has been received. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "On subsequent Saturdays, each child received (in random order) 1 of 3 possible drug condition orders of MPH and placebo" |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All treatments were administered in identical capsules |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only completers were followed up Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): unclear, no information about withdrawals |
| Selective reporting (reporting bias) | Unclear risk | No protocol was published. |
Swanson 2002a.
| Study characteristics | ||
| Methods | Randomised, double‐blind, cross‐over design:
|
|
| Participants | Number of participants screened: not stated Number of participants included: 32 (28 boys, 4 girls) Number of participants followed up: 30 Number of withdrawals: 2, due to personal commitments Diagnosis of ADHD: DSM‐IV (combined (93.8%), hyperactive‐impulsive (6.2%), inattentive (0%)) Age: mean 9.9 years (range 7‐13) IQ: not stated MPH‐naive: 0%, all children in the trial were undergoing treatment with MPH when they were enrolled Ethnicity: not stated Country: USA Setting: outpatient clinic (laboratory classroom) Comorbidity: 0% Comedication: not stated Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 3 possible drug condition orders of 5 mg, 10 mg or 15 mg, 3 times/d or 18 mg/d, 36 mg/d or 54 mg/d administered in bolus at 7:30 am and once every 30 min for 8 h of MPH and placebo Mean MPH dosage: not stated Administration schedule: 3 time points Duration of each medication condition: 1 day Washout before trial initiation: not stated Medication‐free period between interventions: none Titration period: none Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
General behaviour
Non‐serious AEs
|
|
| Notes | Sample calculation: not stated Ethics approval: yes Comments from trial authors (limitations)
Key conclusions of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: yes, all children in the trial were undergoing treatment with MPH when they were enrolled Any withdrawals due to AEs: no Funding source: funding: ALZA Corporation Email correspondence with trial authors: trial authors were contacted twice by email and were asked for much supplemental information regarding data, but we have received no data; therefore, most of the data from this trial cannot be used in this review. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomized, 3‐way, crossover trial in which a double‐blind, double‐dummy procedure was used" |
| Allocation concealment (selection bias) | Unclear risk | Not enough information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not enough information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not enough information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 2 withdrawals, due to personal commitments Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Low risk | "Two additional items developed (from SKAMP) for the NIMH Collaborative Multisite Multimodal Treatment Study of Children With ADHD (MTA) (Greenhill et al., 2001) were included in the classroom ratings but were not included in the analyses of this study" |
Swanson 2002b.
| Study characteristics | ||
| Methods | Randomised, 3‐way, cross‐over trial with 2 interventions:
Phases: 3 |
|
| Participants | Number of participants screened: not stated Number of participants included: 64 (81.3% boys) Number of participants followed up: 59 Number of withdrawals: 3 Diagnosis of ADHD: DSM‐IV (combined (82.8%), hyperactive‐impulsive (not stated), inattentive (not stated)) Age: mean 9.2 years (range 6‐12) IQ: not stated MPH‐naive: none Ethnicity: white (82.8%), African American (not stated), Asian (not stated), Hispanic (not stated) Country: USA Setting: outpatient clinic (laboratory classroom) Comorbidity: not stated Comedication: not stated Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 6 possible drug condition orders of 5 mg, 10 mg or 15 mg, 3 times/d, or 18 mg, 36 mg or 54 mg OROS‐MPH and placebo Mean MPH dosage: not stated Administration schedule: 3 times/d 7:30 am, 11:30 am and 3:30 pm; OROS‐MPH 7:30 am Duration of each medication condition: 1 week Washout before trial initiation: not stated Medication‐free period between interventions: not stated Titration period: none Treatment compliance: no measure |
|
| Outcomes |
ADHD symptoms
Non‐serious AEs
|
|
| Notes | Sample calculation: not stated Ethics approval: not stated Key conclusion of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: yes; "The children with ADHD in the proof‐of‐concept and proof‐of‐product studies were selected based on a history of clinical response to stimulant medication, and this limits the extrapolation of the findings to the drug‐naive population" Any withdrawals due to AEs: no Funding: ALZA Corporation Email correspondence with trial authors: March 2014. We contacted trial authors twice by email and asked for supplemental information regarding data, but we have received no data; therefore most of the data from this trial cannot be used in this review. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Double‐blind procedures were implemented by administration of MPH or placebo in capsules |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, double‐dummy procedure was used to disguise the 3 treatments |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 5 participants not followed up |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
Swanson 2004b.
| Study characteristics | ||
| Methods | Multi‐centre, double‐blind, double‐dummy, placebo‐controlled, cross‐over trial in an analogue classroom setting comparing 3 treatment conditions
Each treatment intervention lasted a week |
|
| Participants | Number of participants screened: 214 Number of participants included: 184 (131 boys, 48 girls) Number of participants followed up: 157/184 ITT Number of withdrawals: 27 Diagnosis of ADHD: DSM‐IV (combined (82.2%), hyperactive‐impulsive (4.8%), inattentive (13.0 %)) Age: mean 9.6 years (range 6‐12) IQ: > 80 MPH‐naive: none Ethnicity: white (70%), African American (11.5%), Asian (1.7%), Hispanic (12.5%), other (5.3%) Country: USA Setting: outpatient clinic Comorbidity: approximately 25% had a comorbid condition; anxiety and ODD were most frequent. Girls had a greater rate of comorbid anxiety disorder (from Sonuga‐Barke 2007). Anxiety: 20.8 girls, 5.9 boys. ODD: 12.5 girls, 8.8 boys. Insomnia: 2.1 girls, 5.1 boys Comedication: not stated Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 6 possible drug condition orders of ER‐MPH (Metadate CD), ER‐MPH (Concerta), and placebo Dose: children treated with low doses (20 mg/d ) of MPH were randomly assigned to receive ER‐MPH (Metadate CD) 20 mg, ER‐MPH (Concerta) 18 mg, or placebo; those treated with medium doses (20 to 40 mg/d ) were randomly assigned to receive ER‐MPH (Metadate CD) 40 mg, ER‐MPH (Concerta) 36 mg, or placebo; children treated with high doses (40 mg/d ) were randomly assigned to receive ER‐MPH (Metadate CD) 60 mg, ER‐MPH (Concerta) 54 mg, or placebo Administration schedule: once daily in the morning Duration of each medication condition: 7 days Washout before trial initiation: no Medication‐free period between interventions: not stated Titration period: none Treatment compliance: regarding the medicine, not stated. 157 received all 3 levels of treatment and participated in all 7 classroom sessions |
|
| Outcomes |
ADHD symptoms
Non‐serious AEs
|
|
| Notes | Sample calculation: yes Ethics approval: yes Comments from trial authors
Limitations
Key conclusions of trial authors
Sex differences
Comment from review authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: yes Any withdrawals due to AEs: 3 trial participants discontinued because of AEs that were judged to be unrelated to medications (gastroenteritis: Concerta (n = 1); fever: placebo (n = 1); sunburn: placebo (n = 1)) Funding: Celltech Pharmaceuticals Incorporated Email correspondence with trial authors: June 2014. We emailed trial authors to obtain supplemental data. Unfortunately, data in the correct format are no longer accessible. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised |
| Allocation concealment (selection bias) | Unclear risk | Randomised |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, double‐dummy. Treatments were packaged according to a double‐dummy design. Each treatment pack contained a 1‐week supply of trial treatment, with each day’s supply consisting of 1 large capsule to accommodate the size of any dose level of Concerta (containing Concerta or placebo) and, depending on dose level, between 1 and 3 smaller Metadate CD‐sized capsules (containing Metadate CD or placebo) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data from participants finishing the whole trial (n = 157) and from the ITT population (n = 184) Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified |
Symons 2007.
| Study characteristics | ||
| Methods | Prospective, randomised, double‐blind, placebo‐controlled, cross‐over, single‐case designs were used to evaluate MPH administration for 3 school‐aged children with cerebral palsy and comorbid ADHD symptoms
Phases: 4 (including baseline) |
|
| Participants | Number of participants screened: not stated Number of participants included: 2 (1 boy, 1 girl) Number of participants followed up: 2 Number of withdrawals: none Diagnosis of ADHD: DSM‐IV (combined (100%), hyperactive‐impulsive (0%), inattentive (0%)) Age: mean 10.4 years (range 9‐11) IQ: > 70. Participant 2 = 92 non‐verbal and 96 performance non‐verbal (range not stated) MPH‐naive: not stated Ethnicity: not stated Country: USA Setting: outpatient clinic Comorbidity: cerebral palsy (100%), mild cognitive impairment (100%), autism spectrum disorder (50%), physical impairment (50%), learning disability (50%), other health impairment (50%) Comedication: not stated Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 3 possible drug condition orders of MPH (low or high dose) and placebo Mean MPH dosage: 2 doses of MPH administered: LD‐MPH (0.3 mg/kg) and HD‐MPH (0.5 mg/kg) Administration schedule: once daily, mornings, before the child’s school arrival Duration of each medication condition: order of drug/placebo administration was randomly assigned, and administration was provided for 5 consecutive school days in each condition. Drug treatment was not administered on weekends For participant 2, the order of administration was as follows: baseline, LD, placebo, HD For participant 3, the order of administration was as follows: baseline, placebo, LD, HD Washout before trial initiation: not stated Medication‐free period between interventions: weekends Titration period: not stated Treatment compliance: not stated |
|
| Outcomes |
General behaviour
Non‐serious AEs
|
|
| Notes | Sample calculation: no Ethics approval: not stated Key conclusions of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: not stated Any withdrawals due to AEs: no Funding source: McKnight LandGrant Professorship to the first author Email correspondence with trial authors: May 2014. Emailed trial author to request additional information but have not received a reply |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Order of drug/placebo administration was randomly assigned |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Parents, school personnel (i.e. teachers, teacher assistants) and research assistants responsible for observational data collection and coding were kept blind to drug/placebo conditions |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
Szobot 2004.
| Study characteristics | ||
| Methods | 4‐day, double‐blind, placebo‐controlled, randomised parallel‐group trial:
|
|
| Participants | Number of participants screened: not stated Number of participants included: 36 (all boys) Number of participants followed up: 36 (MPH 19, placebo 17) Number of withdrawals: 0 Diagnosis of ADHD: DSM‐IV, combined (MPH 84.6%, placebo 76.5%) Age: mean 11.6 years IQ: mean 94.7 MPH‐naive: not stated. But no psychiatric medication for the past 6 months Ethnicity: European‐Brazilian (89%) Country: Brazil Setting: outpatient clinic Comorbidity: CD and ODD (MPH 58.8%, placebo 68.4%), depressive disorder (MPH 5.2%, placebo 5.9%), multiple anxiety disorder (MPH 5.2%, placebo 0%), tic disorder (MPH 0%, placebo 5.9%) Comedication: not stated Other sociodemographics: low‐ to middle‐income families Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to MPH or placebo Number of participants randomised to each group: MPH 19, placebo 17 Mean MPH dosage: 0.72 mg/kg/d Administration schedule: twice/d: morning and lunchtime Duration of intervention: 4 days Titration period: first day 0.35 mg/kg Treatment compliance: good |
|
| Outcomes |
ADHD symptoms
Quality of life
Non‐serious AEs
|
|
| Notes | Sample calculation: no Ethics approval: approved by the Ethical Committee of the HCPA (approved as an IRB by the Office for Human Research Protections, USA) Comments from trial authors
Key conclusion of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: no Funding source: research funds from Hospital de Clínicas de Porto Alegre, FAPERGS and NOVARTIS Email correspondence with trial authors: October 2013. We received from the trial authors supplemental information regarding blinding and allocation concealment. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned on the basis of a computer‐derived algorithm (EPIINFO6) |
| Allocation concealment (selection bias) | Low risk | Only the doctor performing the randomisation knew the allocation, and he had nothing to do with data collection |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind: participants, parents and all research team members who had contact with participants. Both MPH and placebo pills were manufactured by a single pharmaceutical company; they had the same format and colour and were given to participants in 4 different blisters |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All research team members who had contact with participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo‐responders): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified |
Szobot 2008.
| Study characteristics | ||
| Methods | 6‐week cross‐over trial with 2 interventions
Group A: receiving SODAS MPH dosage: 0.3, 0.7 and 1.2 mg/kg/d for weeks 1, 2 and 3 and placebo for weeks 3, 4, and 6 Group B: receiving placebo for weeks 1, 2, and 3, and SODAS MPH dosage: 0.3, 0.7 and 1.2 mg/kg/d for weeks 4, 5, and 6 |
|
| Participants | Number of participants screened: 25 from a previous ADHD/substance misuse trial and 15 through advertising Number of participants included: 16 (100% boys) Number of participants followed up: 16 Number of withdrawals: 2 (both from group A; withdrawal rate 12.5%) Diagnosis of ADHD: DSM‐IV (combined 12 (75%), hyperactive/impulsive (n = 1), inattentive 3 (18.75%)) Mean age: group A 17.5 years (SD 2.33), group B 17.38 (SD 2.2) IQ: group A 79.43 (SD 16.66), group B 84.75 (SD 21.16) MPH‐naive: 100% Ethnicity: European‐Brazilian (group A 3 (37.5%), group B 7 (87.5%)) Country: Brazil Setting: outpatient clinic Comorbidity: CD (group A 100%, group B 75%); ODD (group A 25%, group B 37.5%); depression (group A 12.5%, group B 25%) Comedication: yes (marijuana and cocaine); group A: marijuana (100%) and cocaine (50%); group B: marijuana (87.5%) and cocaine (37.5%) Other sociodemographics: divorced parents (group A 37.5%, group B 50%); socioeconomic group A + B + C (group A 50%, group B 87.5%); group D + E (group A 50%, group B 12.5%) Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 2 possible drug condition orders of MPH and placebo Mean SODAS MPH dosage: 0.3, 0.7 and 1.2 mg/kg/d for weeks 1, 2 and 3 for group A, and for weeks 4, 5 and 6 for group B Administration schedule: morning dose time points Duration of each medication condition: 3 weeks Washout before trial initiation: no Medication‐free period between interventions: 24 h Titration period: none Treatment compliance: "Study compliance was assessed by self‐report, mother’s report and pill counting" |
|
| Outcomes |
ADHD symptoms
Serious AEs
Non‐serious AEs
|
|
| Notes | Sample calculation: not stated Ethics approval: "The project was approved by the Institutional Review Board (IRB) of Hospital de Clinicas de Porto Alegre (approved as an IRB by the Office for Human Research Protections, United States of America, IRB 00000921" Comments from trial authors
Key conclusions of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: 1 withdrawal resulted from a participant feeling "worse", "more restless" Funding source: "The ADHD outpatient program receives research support from Bristol‐Myers Squibb, Eli‐Lilly, Janssen‐Cilag and Novartis" Email correspondence with trial authors: May 2014. We received supplemental information from trial authors |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "One of the investigators (LAR) randomized the 16 subjects into groups A or B and prepared weekly blisters of medications for each participant" |
| Allocation concealment (selection bias) | High risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "A pharmacist packaged mPH‐SODAS and matching placebo in capsules so that the MPH‐SODAS and placebo could not be visually differentiated" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | From author correspondence: "all persons who evaluated outcome measures were blinded" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): unclear |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified |
Tannock 1989.
| Study characteristics | ||
| Methods | Within‐participant, cross‐over trial with 3 interventions:
2 different drug conditions each day |
|
| Participants | Number of participants screened: 16 Number of participants included: 12 Number of participants followed up: 12 (10 boys, 2 girls) Number of withdrawals: none Diagnosis of ADHD: DSM‐III ADD‐H (equivalent to 'combined' in later classifications) Age: mean 8.4 years (range 6‐11) IQ: mean 105 MPH‐naive: 5 received MPH previously. 3 were taking MPH at the time of referral and had a 48‐h pre‐trial washout Ethnicity: not stated Country: Canada Setting: outpatient clinic Comorbidity: ODD (n = 4), learning difficulties (n = 8; according to school record and defined as < 25th percentile on ≥ 1 subtests within the Wide Range Achievement Test (WRAT‐R)) Comedication: not stated Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 3 possible drug condition orders of 0.3 mg/kg or 1.0 mg/kg MPH and placebo Mean MPH dosage: not stated. Administration schedule: interval of 4 h separated morning and afternoon doses Duration of each medication condition: not stated Washout before trial initiation: 4 h Titration period: none Treatment compliance: not stated |
|
| Outcomes |
Non‐serious AEs
|
|
| Notes | Sample calculation: not stated Ethics approval: not stated Key conclusions of trial authors
Comment from review authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: no Funding source: Jointly funded by Ontario Mental Health Foundation (Grant Number 963‐86/88) and Health and Welfare Canada (Grant Number 6606‐3166‐42) Email correspondence with trial authors: sent email to trial authors to ask for additional information but have not received a reply. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The order of medication condition was randomized with the restrictions that each child receive two different medication conditions each day (e.g. high, low) occur with equal frequency in the morning and afternoon" "The order of these six combinations was randomized for each child" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The methylphenidate and placebo were packaged in coloured gelatin capsules by the hospital pharmacist to avoid detection of dose and taste, packaged in individual envelopes and dispensed by project staff 1 hour before testing" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified |
Tannock 1992.
| Study characteristics | ||
| Methods | Cross‐over trial with 3 interventions:
Phases: 3 separate drug conditions: placebo, LD (0.3 mg/kg) and HD (1.0 mg/kg) medication, with 2 test sessions each. Drug conditions changed on a daily basis: 6 test sessions plus baseline practice session |
|
| Participants | Number of participants included: 26 (24 boys, 2 girls) Number of participants followed up: 23 Number of withdrawals: 3 Diagnosis of ADHD: DSM‐III (subtype not stated) Age: mean 9.2 years (range not stated) IQ: mean 105.9 (SD 10.5) MPH‐naive (not clear; "9 children receiving stimulant medication prior to the present study") Ethnicity: not stated Country: Canada Setting: hospital/outpatient department Comorbidity: n = 12, oppositional disorder: 5, oppositional and CD: 3, oppositional and separation anxiety disorder: 1, oppositional and CD and major depression: 2, oppositional and CD and avoidant disorder: 1. Comedication: not clear Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to different drug condition orders of 3 possible interventions: LD‐MPH, or HD‐MPH, and placebo Mean MPH dosage: N/A (modal dose at 0.3 mg/kg = 7.5 mg (range 5.0 mg‐15 mg), modal dose at 1.0 mg/kg = 27.5 mg (range 17.5 mg‐47.5 mg)) Administration schedule: daily single dose Duration of each medication condition: 1 day Washout before trial initiation: 48 h before trial initiation Medication‐free periods between interventions: no Titration period: none Treatment compliance: capsules administered by project staff |
|
| Outcomes |
General behaviour
Non‐serious AEs
3 children exhibited unusual side effects leading to trial termination. One exhibited hypersensitivity (skin rash, urticaria and throat clearing), and 2 became somewhat disoriented and confused and complained of odd sensations in their limbs |
|
| Notes | Sample calculation: no Ethics approval: not stated Key conclusion of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: yes, child had to be scheduled to receive a trial with MPH independent of the present investigation Any withdrawals due to AEs: yes; 3 Funding source: grant from the Canadian Psychiatric Research Foundation and a Post‐Doctoral Fellowship by the Ontario Mental Health Foundation. Email correspondence with trial authors: April 2014. We emailed trial authors twice to ask for supplemental information/data but have not received a response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not clear |
| Allocation concealment (selection bias) | Low risk | Double‐blind, placebo‐controlled design. Drug order for the first 3 test sessions was randomly assigned, and each child retained the same order for the remaining 3 sessions |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, placebo‐controlled design. Medication packaged in coloured gelatin capsules to avoid detection of dose and taste. Each child followed the same routine for every test session |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not clear |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Incomplete outcome data for 3 respondents with unusual side effects Selection bias (e.g. titration after randomisation → exclusion): exclusion due to AEs |
| Selective reporting (reporting bias) | Unclear risk | No information presented on numbers of participants with side effects or outcomes for data on nausea, BP, etc. |
Tannock 1993.
| Study characteristics | ||
| Methods | Cross‐over trial with 3 interventions:
Phases
|
|
| Participants | Number of participants screened: not stated Number of participants included: 22 (and 16 healthy controls) Number of participants followed up: 22 (21 boys, 1 girl) Number of withdrawals: 0 Diagnosis of ADHD: DSM‐III (subtype not stated) Age: mean 9.4 years (range not stated) IQ: mean 105.5 (SD 11.3) MPH‐naive: not clear: "Thus all these children would have received a trial with psychostimulants independently of this study"; "8 children had been receiving stimulant medication prior to this study") Ethnicity: not stated Country: Canada Setting: outpatient clinic Comorbidity: 55%; oppositional disorder (n = 5), CD (n = 3), emotional disorder as well as oppositional or CD (n = 4), major depression (n = 2), avoidant disorder (n = 1), separation anxiety disorder (n = 1) and learning disorder (n = 6) Comedication: not clear Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 6 possible drug condition orders of (dose not stated) MPH and placebo Mean MPH dosage: not stated Administration schedule: 6 separate medication administrations: 2 placebo, 2 LD (0.3 mg/kg) and 2 HD (1.0 mg/kg). First 3 test sessions were ordered randomly, then were ordered repeatedly for last 3 sessions: daily for 6 days Duration of each medication condition: 3 h Washout before trial initiation: 48 h Medication‐free period between interventions: not clear Titration period: none Treatment compliance: "Medication was administered by project staff" |
|
| Outcomes |
Non‐serious AEs
|
|
| Notes | Sample calculation: no Ethics approval: not stated Key conclusions of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: unclear Withdrawals due to AEs: no Funding source: Canadian Psychiatric Research Foundation and the Medical Research Council of Canada. Email correspondence with trial authors: April 2014. Emailed trial authors twice for supplemental information/data but have received no response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Low risk | Double‐blind, placebo‐controlled, within‐participant design was used ...randomly assigned, counterbalanced sequence. Medications were prepared individually for each child and were packaged in coloured gelatin capsules to avoid detection of dose and taste |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, placebo‐controlled, within‐participant design was used ...randomly assigned, counterbalanced sequence. Medications were prepared individually for each child and were packaged in coloured gelatin capsules to avoid detection of dose and taste |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals were noted Selection bias (e.g. titration before randomisation → exclusion): no |
| Selective reporting (reporting bias) | Low risk | No indication of selective reporting, all outcomes reported |
Tannock 1995a.
| Study characteristics | ||
| Methods | 4‐day, randomised, double‐blind, placebo‐controlled, cross‐over trial with:
With initial 1‐day open‐label trial |
|
| Participants | Number of participants screened: 28 Number of participants included: 28 (25 boys, 3 girls) Number of participants followed up: 28 Number of withdrawals: 0 Diagnosis of ADHD: DSM‐III‐R (subtype not stated) Age: mean 8.9 years (range 7‐11) IQ: mean 106.5 (15.6) MPH‐naive: 80% Ethnicity: white (90%), other (10%) Country: Canada Setting: outpatient clinic Comorbidity: ODD or CD (35%), learning disabilities (39%) Comedication: not stated Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 3 doses of MPH (0.3 mg/kg, 0.6 mg/kg, 0.9 mg/kg) and placebo Mean MPH dosage: not stated Administration schedule: once daily Duration of each medication condition: 1 day Washout before trial initiation: for children receiving MPH before trial, washout period ≥ 48 h before trial, with no washout between periods Titration period: no, but all children participated in an initial 1‐day open trial with a 0.3 mg/kg dose of MPH to ascertain tolerance, before proceeding with the double‐blind, placebo‐controlled trial Treatment compliance: not stated |
|
| Outcomes |
Non‐serious AEs
|
|
| Notes | Sample calculation: yes Ethics approval: yes; Institutional Research Ethics Board Key conclusions of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: yes; 1‐day open‐label trial to ascertain tolerance towards medication, but all participants were analysed Any withdrawals due to AEs: no Funding source: Medical Research Council of Canada and Health and Welfare Canada Email correspondence with trial authors: August 2013. We received from the first trial authors supplemental information regarding dropouts, screening and ethics approval |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised |
| Allocation concealment (selection bias) | Low risk | All medication was prepared by the hospital pharmacy and was packaged in opaque gelatin capsules to avoid detection of dose and taste |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Children, parents, teachers and research staff were all blinded to medication conditions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Children, parents, teachers and research staff were all blinded to medication conditions |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data from all 28 participants were analysed Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Low risk | No indication of selective reporting |
Tannock 1995b.
| Study characteristics | ||
| Methods | Cross‐over trial with 4 interventions:
Phases: washout, baseline, trial 2 groups, ADHD and ADHD + anxiety ‐ were subjected to the same trial |
|
| Participants | Number of participants screened: 50 Number of participants included: 40 (34 boys, 6 girls) Number of participants followed up: 40 Number of withdrawals: none Diagnosis of ADHD: DSM‐III‐R (no subtype) Age: ADHD mean 9.4; ADHD + anxiety mean 9.1 (range 7‐11 years); IQ: > 80 MPH‐naive: not stated Ethnicity: white (90%), African American or Asian (10%) Country: Canada Setting: outpatient clinic Comorbidity (type: overanxious 27.5%, separation anxiety 5%, over anxiety and separation anxiety 10%, avoidant disorder with overanxious traits 2.5%, ODD 55%, CD 10%) Comedication: not stated Other sociodemographics: predominantly from middle‐class families Inclusion criteria
Exclusion criteria
Inclusion criteria for the ADHD + anxiety group ≥ 1 of the following
Children in the ADHD group did not meet any of these criteria for anxiety |
|
| Interventions | Participants were randomly assigned to 1 of 12 possible drug condition orders of 0.3 mg/kg, 0.6 mg/kg or 0.9 mg/kg MPH and placebo Mean MPH dosage: 0.3 mg/kg = 8.75 (± 2.2); 0.6 mg/kg = 17.75 (± 4.2); 0.9 mg/kg = 27.25 (± 5.6) Administration schedule: once/d Duration of each medication condition: 1 day Washout before trial initiation: 48 h when applicable Medication‐free period between interventions: not stated Titration period: no, but there was a 1‐day open trial on 0.3 mg/kg before proceeding to ascertain tolerance Treatment compliance: medication was administered at the laboratory |
|
| Outcomes |
Non‐serious AEs
|
|
| Notes | Sample calculation: not stated Ethics approval: not stated Key conclusion of trial authors
Comment from review authors
Inclusion of MPH responders only/exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: yes; see above Any withdrawals due to AEs: no Funding: in part by the Ontario Mental Health Foundation and the National Health Research and Development Program, Health Canada Email correspondence with trial authors: June 2014. Emailed trial authors twice to request supplemental information/data but have received no response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Drug order was counterbalanced and determined by random assignment, such that an approximately equal number of children were assigned to each of 12 possible orders |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Active medication and placebo were prepared by the hospital pharmacy, packaged in identical opaque gelatin capsules and administered in a double‐blind manner |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): 1‐day open trial to assess tolerance before main trial, but no children were excluded |
| Selective reporting (reporting bias) | Low risk | All outcomes are reported |
Tannock 2018.
| Study characteristics | ||
| Methods | A 10‐week to 4 months parallel‐trial with 2 arms, which were further parted into 3 trial arms of different reading therapy:
Phases: 2 phases (open‐label titration and double‐blind intervention) |
|
| Participants | Number of participants screened: 221 Number of participants included: 65 (49 boys, 16 girls) Number of participants followed‐up: 49 (15 teacher ratings and 1 parent rating not completed) Number of withdrawals: 16 (15 teacher ratings and 1 parent rating not completed) 13 participants changed their allocated intervention (8 from MPH group, 5 from placebo group) Diagnosis of ADHD: DSM‐IV (subtype not stated) Age: mean 8.5 years (SD 1.4, range 7‐11) IQ: 91.5 (SD 10.6) MPH‐naive: not stated Ethnicity: white (90%), African‐American (4%), Hispanic (1%), and Asian (5%) Country: Canada Setting: classroom laboratory in a clinical setting Comorbidity: all participants had a reading disorder. 29.3% had ODD 8.6% had CD Comedication: not stated Additional sociodemographics: socioeconomic status: 6.4 (SD 1.7) Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to receive either MPH at optimal dosage or placebo twice/d, while also receiving one of 3 reading interventions. Number randomised to each group: 39 to MPH, 26 to placebo. 13 participants changed their allocated intervention (8 from MPH group, 5 from placebo group) Mean medication dosage: not stated. Maximum oral dose of 0.7 mg/kg twice/d or 20 mg twice/d Administration schedule: twice/d, in the morning and before lunch Duration of each medication: 12‐13 weeks or 4‐5 months of MPH or placebo Washout before trial initiation: no information Titration period: 2‐3 week titration phase in which the dose of MPH or placebo was increased in 5 mg steps. Identical, scored, 10 mg pills of MPH and placebo were used Treatment compliance: research staff recorded the number of pills returned at trial completion (average of about 90%). |
|
| Outcomes |
ADHD symptoms
Serious AEs
General behaviour
Non‐serious AEs
|
|
| Notes | Sample calculation: no Ethics approval: no Comments from trial authors
Key conclusion of trial authors
Comments from review authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH: yes Any withdrawals due to AEs: not stated Funding source: an operating grant from the Canadian Institutes of Health Research (Grant #MT 13366), and by the donation of placebo medication from Novartis Pharmaceuticals. Email correspondence with the trial authors: September 2021. We sought supplemental information regarding details of the medical intervention through personal email correspondence with the authors in September 2021. Unfortunately the data are no longer available. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | 20% of parents (n = 13) requested a change in their child’s assigned medication condition (8 from placebo to MPH; 5 from MPH to placebo) |
| Allocation concealment (selection bias) | Low risk | Parents were then provided with a 4‐month supply of pills, each month packaged separately. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The MPH and placebo pills were identical in color, shape and size and were administered in a double‐blind manner twice daily (once in the morning before school and once at lunch)" Comment: 1 participant was unblinded during requested change of medication assignment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "We analysed behavioral data when both parent and teacher reports were present to ensure that results from these 2 sources could be compared" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "Within the initial 2‐week dose‐adjustment phase and prior to starting the academic programs, 20% of parents (n= 13) requested a change in their child’s assigned medication condition (8 from placebo to MPH; 5 from MPH to placebo)." Comment: an ITT analysis as well as an as‐treated analysis was made Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): there was a titration phase after randomisation |
| Selective reporting (reporting bias) | Unclear risk | No trial protocol identified |
Taylor 1987.
| Study characteristics | ||
| Methods | Double‐blind, randomised, placebo‐controlled, cross‐over trial with 2 arms
Each treatment period lasted for 3 weeks with a washout period of 1 week planned between treatments and up‐titration to optimum dosage during the 3 weeks |
|
| Participants | Number of participants screened: 64 Number of participants included: 39 (all boys). Of these, 26 had an ADHD diagnosis according to DSM‐III Number of participants followed up: 38 Number of withdrawals: 1 Diagnosis of ADHD: DSM‐III (n = 26; type not stated) The following data reflect the whole trial (n = 38) Age: mean 8.6 years (range 6‐10) IQ: all > 65; mean 93.4 MPH‐naive: 100% Ethnicity: not stated Country: UK Setting: outpatient clinic Comorbidity: no attentive or restless behaviour, but antisocial, disruptive or aggressive in conduct (n = 6), hyperactive but not antisocial or aggressive (n = 9), with both hyperactive and disruptive behaviour (n = 23), CD (n = 7), relationship problems (n = 2) and disturbance of emotions specific to childhood (n = 3) Comedication: not stated Other sociodemographics: 40% from broken homes Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 2 possible drug condition orders of MPH and placebo Mean MPH dosage: 9 received doses from 0.2 mg/kg to 0.5 mg/kg, 21 from 0.5 mg/kg to 0.9 mg/kg and 8 from 0.9 mg/kg to 1.4 mg/kg Administration schedule: time points not stated Duration of each medication condition: 3 weeks Washout before trial initiation: all were stimulant‐naive Medication‐free period between interventions: 1 week Titration period: a flexible dosage regimen was used after randomisation. Each child began with 5 mg daily, with dosage adjustments made at 2‐ to 3‐day intervals. The optimum dosage was assessed for each child, in the light of clinical response and the occurrence of side effects, to a maximum of 30 mg daily Treatment compliance: compliance with medication was rated as good or very good in 89% |
|
| Outcomes |
ADHD symptoms
Serious AEs
Non‐serious AEs
|
|
| Notes | Sample calculation: no Ethics approval: not stated Comment from trial authors
Key conclusion of trial authors
Comments from review authors
Inclusion of MPH responders only/exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no; all MPH treatment‐naive Any withdrawals due to AEs: yes; 1 in the placebo group Funding source: partially funded by grant from CIBA Ltd., which provided medicine and placebo Email correspondence with trial authors: October 2013. We received from trial authors no supplemental information regarding data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Children were randomly allocated to receive drug first or placebo first. This was carried out by pharmacy staff, who knew only the name and identifying number of each case |
| Allocation concealment (selection bias) | Low risk | Allocation was carried out by pharmacy staff, who knew only the name and identifying number of each case |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Tablets were dispensed to a trial member, who did not know what they contained and handed them to parents |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Psychiatrist supervising treatment also assessed and recorded side effects, physical findings and parents’ general impressions at the end of each treatment; other assessors of outcomes were blinded to the treatment given but also to possible clues arising from physical effects of the drug. Behavioural measures were carried out independently of one another by investigators blind to results of the other tests and to teacher ratings |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were missing for 3 children: 1 child had missing data on the Parental Account of Childhood Symptoms because he had been taken into care, and 2 children had missing data on the CTRS hyperactivity factor because they had been excluded from school. Method of imputation not described Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified |
Taylor 1993.
| Study characteristics | ||
| Methods | 4‐week, double‐blind, cross‐over trial with 3 interventions:
Each medication dose/placebo was given for 1 week |
|
| Participants | Number of participants screened: 57 Number of participants included: 57 (27 boys, 5 girls) Number of participants followed up: all 57 completed the trial but only 32 were included in the final analysis Number of withdrawals: 0, but 25 were not reported (due to non‐response and not enough data) Diagnosis of ADHD: DSM‐III‐R Age: mean 10.26 years (range 7.0‐12.9) IQ: mean 107 (SD 16.2; range 78‐139) MPH‐naive: 12 (37.5%) Ethnicity: not stated Country: Canada Setting: outpatient clinic Comorbidity: not stated Comedication: not stated Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to possible drug condition orders of, respectively, mean 6.72 mg (5 mg‐10 mg) and mean 11.88 mg (10 mg‐15 mg) MPH and placebo Administration schedule: morning and noon Duration of each medication condition: 1 week Washout before trial initiation: none Titration period: none Treatment compliance: no information |
|
| Outcomes |
Non‐serious AEs
|
|
| Notes | Sample calculation: no information Ethics approval: no information Key conclusion of trial authors
Inclusion of MPH responders only/exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: no Funding source: not declared Email correspondence with trial authors: January 2014. No supplemental information was received. We therefore have no more information on numbers of participants with non‐serious AEs. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised. All medication and placebo testing was conducted under double‐blind conditions with randomly assigned testing order |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Active medication and placebo substances were placed in identical red and white capsules in powder form. Matched on both taste and appearance |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | All medication and placebo testing was conducted under double‐blind conditions with randomly assigned testing order, but how blinding was done was not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Selection bias (e.g. titration after randomisation → exclusion): this series of 32 children with ADHD does not include an additional group of 12 children with ADHD, who after drug trials were deemed "non‐responders" as well as 13 participants on whom adequate Event‐Related Potentials data were not obtained |
| Selective reporting (reporting bias) | High risk | Did not report AE data |
Tervo 2002.
| Study characteristics | ||
| Methods | Triple‐blind, 3‐way, cross‐over trial with 2 interventions:
Phases
|
|
| Participants | Number of participants screened: not stated Number of participants included: 63 (49 boys, 14 girls) Number of participants followed up: 49 Number of withdrawals: 14 Diagnosis of ADHD: DSM‐IV (combined (70%), hyperactive‐impulsive (16%), inattentive (5%), other (9%)) Age: mean 9 years 10 months (SD 2 years 10 months; range not stated) IQ: not stated MPH‐naive: not stated Ethnicity: not stated Country: USA Setting: outpatient clinic Comorbidity: motor dysfunction (35%) Comedication: not stated Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 2 possible dose levels of IR‐MPH and placebo Mean MPH dosage: not stated Administration schedule: twice daily; time points not stated Duration of each medication condition: 6 days Washout before trial initiation: 1 day Titration period: none Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
General behaviour
Non‐serious AEs
|
|
| Notes | Sample calculation: no Ethics approval: no Comments from trial authors
Key conclusions of trial authors
Comments from review authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: yes Funding source: not declared Email correspondence with trial authors: April 2013. We received from trial authors the full dataset in Statistical Package for the Social Sciences(SPSS) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned |
| Allocation concealment (selection bias) | Low risk | Only the clinical pharmacist knew the sequence of phases |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Capsules of medication and placebo were made and dispensed as described by Barkley (1988). Tablets were crushed and placed within orange opaque gelatin capsules. Capsules disguised the taste of MPH and lactose placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Triple‐blind |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 14 of the 63 trials were excluded from the analysis because of inadequately completed outcome measures. 4 of the 14 children did not complete the trial because of adverse reactions to medication (e.g. irritability, headache, stomachache). These children received HD medication in the first trial interval Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol was published |
Tirosh 1993a.
| Study characteristics | ||
| Methods | 16‐day, double‐blind, cross‐over, counterbalanced trial with 2 interventions:
|
|
| Participants | Number of participants screened: not stated Number of participants included: 20 (16 boys, 4 girls) Number of participants followed up: 20 Number of withdrawals: 0 Diagnosis of ADHD: DSM‐III (ADD 30%, ADHD 70%) Age: mean 9.3 years (range 7‐12) IQ: mean 102 (SD 11) MPH‐naive: 100% Ethnicity: not stated Country: Israel Setting: outpatient clinic Comorbidity: no Comedication: no Other sociodemographics: middle (12), upper‐middle (5) and low (3) socioeconomic status of parents Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 2 possible drug condition orders of MPH and placebo MPH dose range: 0.3 mg/kg ‐0.5 mg/kg Administration schedule: twice daily Duration of each medication condition: 8 days Washout before trial initiation: no (participants were MPH‐naive) Titration period: no Treatment compliance: parents were asked to bring their packages of tablets back for pill count; no data |
|
| Outcomes |
ADHD symptoms
|
|
| Notes | Sample calculation: no Ethics approval: yes Key conclusions of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: no Funding source: none Email correspondence with trial authors: August 2013. Trial author stated that data were discarded (Ramstad 2013d [pers comm]). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation with a table of random numbers |
| Allocation concealment (selection bias) | Low risk | Look‐alike placebo tablets were supplied by the hospital pharmacy and were similarly administered |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
Tirosh 1993b.
| Study characteristics | ||
| Methods | Double‐blind, controlled, cross‐over trial with 2 interventions:
|
|
| Participants | Number of participants screened: unknown Number of participants included: 11 (8 boys, 3 girls) Number of participants followed up: 10 Number of withdrawals: 1 Diagnosis of ADHD: DSM‐III (subtype not stated) Age: median 9 years 8 months (range 6.9‐12.3 years) IQ: median 106 (range 92‐118) MPH‐naive: 100% Ethnicity: not stated Country: Israel Setting: outpatient clinic Comorbidity: none Comedication: none Other sociodemographics: low middle class (n = 2), middle class (n = 8) Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 2 possible drug condition orders of 0.3 mg/kg to 0.4 mg/kg MPH (total dose 10 mg‐15 mg) and placebo Mean MPH dosage: 0.3 mg/kg‐0.4 mg/kg Administration schedule: once daily at 7:30 am on 6 of the 8 days. During the other 2 days, a second dose was administered at 2:00 pm Duration of each medication condition: 8 days Washout before trial initiation: not stated Medication‐free period between interventions: 3 days Titration period: none Treatment compliance: as measured by returned package pill counts, this was rated as full compliance for the 10 children remaining in the trial |
|
| Outcomes |
ADHD symptoms
Non‐serious AEs
|
|
| Notes | Sample calculation: no Ethics approval: not stated Key conclusions of trial authors
Comment from review authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: yes; 1 Funding source: not declared Email correspondence with trial authors: December 2013. We were unable to receive supplemental data from trial authors because the trial is 20 years old (Ramstad 2013d [pers comm]). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | A drug‐placebo sequence was used for children assigned odd numbers in the trial and vice versa |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Look‐alike placebo tablets were supplied |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigator who analysed the data was unaware of the drug‐placebo sequence |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
Tourette's Syndrome Study Group 2002.
| Study characteristics | ||
| Methods | 16‐week, multi‐centre, randomised, double‐blind, placebo‐controlled, parallel trial with 4 arms:
Phases: 3
|
|
| Participants | Number of participants screened: not stated Number of participants included: 136 Number of participants followed up: 121 (MPH 33, placebo 25, clonidine 30, MPH + clonidine 33) Number of withdrawals: 19 (MPH 4, placebo 7, clonidine 4, clonidine + MPH 4) Diagnosis of ADHD: DSM‐IV: MPH: combined 32%, hyperactive‐impulsive 3%, inattentive 65%; placebo: combined 19%, hyperactive‐impulsive 0%, inattentive 81%; clonidine: combined 21%, hyperactive‐impulsive 3%, inattentive 76%; clonidine + MPH: combined 33%, hyperactive‐impulsive 3%, inattentive 64% Mean age: MPH 10.7 years (SD 2.0); placebo 9.7 years (SD 1.8); clonidine 9.7 years (SD 1.8); clonidine + MPH 10.6 years (SD 1.9). Total age range 7‐14 Sex: MPH 34 boys, 3 girls; placebo 29 boys, 3 girls; clonidine 29 boys, 5 girls; clonidine + MPH 24 boys, 9 girls Stimulant‐naive: 42% Ethnicity: MPH 81% white; placebo 94% white; clonidine 94% white; clonidine + MPH 85% white Country: USA Setting: outpatient clinic Comorbidity: tic disorder diagnosis 100%; primarily OCD and ODD. Furthermore, CD, generalised anxiety disorder and major depressive disorder. MPH: OCD (11%), ODD (33%). Placebo: OCD (22%), ODD (41%). Clonidine: OCD (15%), ODD (48%). Clonidine + MPH: OCD (16%), ODD (31%) Comedication: not stated IQ: > 70 Other sociodemographics: participant groups were similar, except that participants assigned to MPH (alone or in combination with clonidine) are approximately 1 year older, present a higher proportion of pubertal cases, show underrepresentation of the inattentive subtype of ADHD (and overrepresentation of the combined subtype) and have lower baseline Conners' Abbreviated Symptom Questionnaire (ASQ)‐Teacher scores. A higher proportion of girls was found in the combined treatment group Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to MPH (Ritalin; Novartis) alone, clonidine alone, clonidine + MPH or placebo Number randomised to each group: MPH 37, placebo 32, clonidine 34, MPH + clonidine 33 MPH dosage: MPH alone 25.7 mg/d, MPH + clonidine 26.1 mg/d Administration schedule: 2‐3 times daily Duration of intervention: 12 weeks (4‐week titration of MPH, 8‐week maintenance phase) Titration period: 4‐week initial dose titration period for clonidine, after which came 4‐week dose titration for MPH. Both titration periods took place after randomisation Treatment compliance: pill count monitored compliance |
|
| Outcomes |
ADHD symptoms
General behaviour
Quality of life
Non‐serious AEs
|
|
| Notes | Sample calculation: yes; 120 participants Ethics approval: yes; the protocol was approved by the institutional review board at each site Comment from trial authors
Key conclusions of trial authors
Comments from review authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: not stated Funding: the National Institute of Neurological Disorders and Stroke, the General Clinical Research Center, the National Center for Research Resources, the Tourette Syndrome Association Boeringer Ingelheim Inc. (particularly Dr. Virgil Dias), for supplying clonidine and matching placebo; Bausch and Lomb, Inc., for supplying small gifts for our study participants |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation. Stratification by centre (investigator) and sexual maturity status (prepubertal: Tanner stage I to II; pubertal: Tanner stage III to V). Blocking was used to ensure approximate balance among treatment groups within each stratum |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes that contained participants' treatment assignments |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinded: participants, clinicians, data collectors, outcome assessors, data analysts, data safety and monitoring committee, manuscript writers |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Only the programmer in the Biostatistics Center who generated the plan and the pharmacist in the Pharmacy Center who packaged and labelled the drug were aware of treatment assignments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Primary statistical analyses were performed according to the ITT principle and were based on all randomly assigned participants, as randomised. For analysis of outcome variables for efficacy, if a participant was missing a response at a particular visit, the last available observation for that participant was carried forward and imputed for that visit Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Low risk | No indication of selective reporting |
Tucker 2009.
| Study characteristics | ||
| Methods | Multi‐centre, open‐label RCT including behaviour treatment as co‐intervention with 2 arms
|
|
| Participants | Number of participants screened: 142 Number of participants included: 109 Number of participants followed up: 104 (66 boys, 38 girls) Number of withdrawals: 5 Diagnosis of ADHD: DSM‐IV (subtypes not described) Age: mean 8.4 years (range 6‐12) IQ: not stated; all age‐appropriate cognitive functioning MPH‐naive: 100% Ethnicity: white (73.1%), African American (24.0%), other (2.9%) Country: USA Setting: outpatient clinic Comorbidity: none Comedication: no Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to ER‐MPH plus behavioural treatment or to behavioural treatment alone. We considered this as ER‐MPH versus no intervention. Mean MPH dosage: not stated Administration schedule: once daily Duration of intervention: 3 months Titration period: initiated after randomisation. MPH was started at 10 mg/d and could be increased weekly in intervals of 10 mg/d to a maximum of 60 mg/d. MPH was administered to achieve the desired clinical effect with minimum or no side effects Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
Non‐serious AEs
|
|
| Notes | Sample calculation: yes Ethics approval: yes; approved by the ethics committees for all 17 trial centres Key conclusion of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no; all participants were MPH‐naive Any withdrawals due to AEs: no Funding source: Novartis Pharmaceuticals Corporation Email correspondence with trial authors: December 2013 and January 2014. Not able to contact trial authors |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised; no further description |
| Allocation concealment (selection bias) | Unclear risk | Randomisation was stratified by age group (6‐8 years and 9‐12 years) and by centre |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Evaluation of cytogenetic damage by blinded slide readers |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Outcome data reported for 68 of 109 randomly assigned participants Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified |
Ullmann 1985.
| Study characteristics | ||
| Methods | 8‐week, cross‐over trial:
Phases: 2
The trial was conducted over 3 years |
|
| Participants | Number of participants screened: not stated Number of participants included: 86 Number of participants followed up: 86 (67 boys, 19 girls) Number of withdrawals: 0 Diagnosis of ADHD: DSM‐III (ADD (15.1%), ADD‐H (70.9%), other (14.0%)) Age: mean 8.6 years (SD 1.8; range not stated) IQ: > 70 MPH‐naive: not stated Ethnicity: African American (17.4%), other (82.6%) Country: USA Setting: not stated Comorbidity: not stated Comedication: not stated Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 3 different doses of MPH (0.3 mg/kg, 0.5 mg/kg and 0.8 mg/kg) and placebo Administration schedule: once daily, before school Duration of each medication condition: 1 week Washout before trial initiation: dose taken in the morning to the next day's dose Titration period: 4 weeks Treatment compliance: "compliance were probably high" |
|
| Outcomes |
ADHD symptoms
|
|
| Notes | Sample calculation: no information Ethics approval: no information Comment from trial authors
Key conclusion of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: no Funding source: NIMH. Ciba‐Geigy provided medication and placebo Email correspondence with trial authors: not able to find first or second trial author's contact information; therefore not able to request supplemental data necessary for meta‐analyses |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random assignment |
| Allocation concealment (selection bias) | High risk | No description; only "methylphenidate in opaque capsules" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No description |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Teacher rating under blinded conditions |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were pooled for a total of 86 participants Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Unclear risk | Unable to obtain protocol |
Ullmann 1986.
| Study characteristics | ||
| Methods | Double‐blind, cross‐over trial in which participants were randomly assigned to the following conditions:
|
|
| Participants | Number of participants screened: not stated Number of participants included: 118. Number of participants followed up: 118 (92 boys, 26 girls) Number of withdrawals: 0 Diagnosis of ADHD: DSM‐III (subtype not stated) Age: mean 8.6 years (range 6‐14) IQ: > 70 MPH‐naive: not stated Ethnicity: white (81.4%), African American (18.6%) Country: USA Setting: school setting and outpatient clinic Comorbidity: not stated Comedication: not stated Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 3 different doses of MPH (0.3 mg/kg, 0.5 mg/kg and 0.8 mg/kg) and placebo Mean MPH dosage: not stated Administration schedule: not stated Duration of each medication condition: 1 week Washout before trial initiation: no information Titration period: none Treatment compliance: excellent compliance in all but a few children |
|
| Outcomes |
ADHD symptoms
Non‐serious AEs
Unable to obtain data on AEs, as could not locate contact details of the first or second trial author (see notes) |
|
| Notes | Sample calculation: no Ethics approval: no information Key conclusion of trial authors
Comment from trial authors
Comment from review authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: no Funding source: in part by a NIMH grant. Ciba‐Geigy provided medication and placebo. Email correspondence with trial authors: not able to find first or second trial author's contact information; therefore not able to obtain additional data (e.g. data (table) on adverse effects) from trial authors. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Low risk | Daily doses of MPH or placebo were placed in gelatin capsules to disguise taste and dose differences; medication for each week was packaged in a dated envelope |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Children, parents and teachers were blinded to dose order, as was the assistant who recorded data sent in by the teachers |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Children, parents and teachers were blinded to dose order, as was the assistant who recorded data sent in by the teachers |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
Urman 1995.
| Study characteristics | ||
| Methods | Double‐blind, placebo‐controlled, within‐participant (cross‐over) trial looking at cardiovascular effects of MPH (0.3 mg/kg, 0.6 mg/kg and 0.9 mg/kg) doses at baseline and after 60 and 120 min post‐MPH challenge in 2 groups of participants with ADHD
|
|
| Participants | Number of participants screened: not stated Number of participants included: 63 (58 boys, 5 girls) Number of participants followed up: 63 (ADHD without anxiety 34, ADHD with anxiety 29) Number of withdrawals: not stated Diagnosis of ADHD: DSM‐III‐R (but "82% of the ADHD and 93% of the ADHD/ANX [ADHD + anxiety] group" would meet the DSM‐IV criteria for a diagnosis of ADHD combined type) Age: ADHD without anxiety, mean 9.1 years (SD 1.3; range 6‐12); ADHD with anxiety, mean 8.7 years (SD 1.4) (overall range 6‐12) IQ: not stated MPH‐naive: 63 (100%) Ethnicity: white (90%), "African or Asian descent" (10%) Country: Canada Setting: not stated Comorbidity: ADHD group: ODD (14; 42%), CD (5; 15%); ADHD with anxiety group: ODD (11; 38%), CD (8; 28%). Also, in the ADHD with anxiety group, 3 met the DSM‐III criteria for overanxious disorder, 2 met criteria for separation anxiety disorder and 5 met criteria for both overanxious and separation anxiety disorders) Other sociodemographics: "The children tended to come from middle‐class families" Inclusion criteria ADHD with anxiety group
ADHD without anxiety group
Exclusion criteria ADHD without anxiety group
|
|
| Interventions | Participants were randomly assigned to 1 of 4 possible drug condition orders of placebo and MPH (0.3 mg/kg, 0.6 mg/kg or 0.9 mg/kg), given once daily over a 4‐day period. Dosage was based on a child’s body weight to the nearest 2.5 mg. Children followed the same routine for every test session with pre‐MPH measurements and with measurements at 60 min and again at 120 min after taking MPH Mean MPH dosage: not stated Duration of each medication condition: once daily, administered over a 4‐day period ‐ each medication condition given once to each child Washout before trial initiation: all were MPH‐naive Medication‐free period between interventions: not stated Treatment compliance: "Any child with missing data for a particular measure was excluded from the analysis of that measure", but trial authors did not indicate the overall quantity of missing data. "Medication was administered by a research nurse" |
|
| Outcomes |
Non‐serious AEs
|
|
| Notes | Sample calculation: no information Ethics approval: no information Comment from trial authors
Key conclusions of trial authors
Comments from review authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: no Funding source: in part by funds from the Medical Research Council of Canada and the Research Institute of the Hospital for Sick Children |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The order of medications was counterbalanced and determined by random assignment such that an approximately equal number of children received each dose on a given day in the trial" |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "All medication was prepared by the hospital pharmacy and packaged in opaque gelatin capsules, to avoid detection of dose and taste"; "Children, parents and research staff were blind to the medication conditions" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Medication was administered by a research nurse who was blind to children’s medication condition and classification of anxiety" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified |
Van der Meere 1999a.
| Study characteristics | ||
| Methods | 7‐week, randomised, double‐blind, placebo‐controlled, parallel‐group trial with 3 arms
|
|
| Participants | Number of participants screened: not stated Number of participants included: 48 (+24 in the clonidine group not used in this review) (62 boys, 10 girls) Number of participants followed up: 47 (MPH 23, placebo 24) Number of withdrawals: 1 (from the MPH group) Diagnosis of ADHD: DSM‐III‐R (subtype not stated) Age: mean 8.8 years (range 7‐12) IQ: > 70 MPH‐naive: 100% Ethnicity: mainly white Country: the Netherlands Setting: outpatient clinic Comorbidity: CD (11%), ODD (33%), depressive disorder (3%), overanxious disorder (1%), dysthymia (3%), generalised tonic‐clonic seizures (1%), ventricular septal defect (1%), congenital hypothyroidism (1%), precocious puberty (1%), deaf in right ear (1%), atresia in 1 ear (1%) Comedication: yes; for participants who had diseases that required it, for example, hypothyroidism Other sociodemographics: 44% from lower socioeconomic families Differences between groups No significant differences in age and IQ were noted between the 2 groups Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to MPH, placebo or clonidine Number of participants randomised to each group: MPH 24, placebo 24, clonidine 24 Total oral daily MPH dosage: 0.06 mg/kg Mean of the absolute MPH dose: 9.85 mg (2.26) Administration schedule: breakfast and lunchtime Duration of intervention: 7 weeks Titration: no, but dosage adjustments were made/allowed during first weeks Titration period: none Treatment compliance: maintained and checked by instructions (both oral and written) to both parents and child, and by counting tablets remaining at the end of treatment. Only 1 participant in the MPH group showed poor compliance |
|
| Outcomes |
ADHD symptoms
General behaviour
Non‐serious AEs
|
|
| Notes | Sample calculation: yes (≥ 15 participants in each group) Ethics approval: yes Key conclusion of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: no Funding source: grants from the Sophia Foundation for Medical Research and Boehringer Ingelheim BV, the Netherlands Email correspondence with trial authors: September 2013. We obtained supplemental information regarding a publication with information about randomisation and supplemental data |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The statistician sent the randomisation list to the pharmacist. Participants were then randomly assigned by a research pharmacist. To ensure blinding, pharmacists applied randomisation blocks at random at a length of 2 or 4 participants. MPH and matching placebos were used |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Teacher, parent, clinician, child and experimenter were blind to treatment conditions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Teacher, parent, clinician, child and experimenter were blind to treatment conditions |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT Selection bias: no, but dosage adjustments were made for several participants because of annoying AEs |
| Selective reporting (reporting bias) | Low risk | No indication of selective reporting |
Wallace 1994.
| Study characteristics | ||
| Methods | Double‐blind, single‐participant, randomised, cross‐over trial:
Conducted in 11 hospitalised children with ADHD |
|
| Participants | Number of participants screened: not stated Number of participants included: 11 Number followed up: 11 Number of withdrawals: 0 Diagnosis of ADHD: DSM‐III‐R (subtype not described) Age: mean 9 years 5 months (range 4‐13 years) IQ: not stated MPH‐naive: not stated Ethnicity: not stated Country: USA Setting: inpatient Comorbidity type: not stated Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 2 conditions: MPH and placebo on a daily basis Mean MPH dosage: not stated Administration schedule: daily Duration of each medication condition: MPH 2‐7 days, placebo 3‐6 days Washout before trial initiation: not described Medication‐free period between interventions: none Titration period: "Prior to trial commencement, all participants were treated with MPH for a period long enough to determine the dose believed to have optimal effectiveness and minimal side effects" Treatment compliance: not described |
|
| Outcomes |
ADHD symptoms
Serious AEs
Non‐serious AEs
|
|
| Notes | Sample calculation: not stated Ethics approval: not stated Comment from trial authors
Key conclusions of trial authors
Comment from review authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: yes, see titration before randomisation Any withdrawals due to AEs: no Funding source: The Veterans Administration Medical Center, Vermont Email correspondence with trial authors: we could find no contact information; therefore, we could not get additional data through personal email correspondence with trial authors |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence was determined by "coin toss method" |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "After completion of each trial, the treatment code was broken" Method of blinding not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data appear to have been reported. Selection bias (e.g. titration after randomisation → exclusion): no, but trial length was changed according to MPH response |
| Selective reporting (reporting bias) | High risk | Protocol not identified "Also trial length increased as effect size decreased, implying that if clinical effects were unconvincing, the trial was extended. The trials examined here did have trial length designated at commencement by the treating psychiatrist (generally 10 days). However, trials were extended, if after the predetermined number of days, the graphed data appeared equivocal." |
Wallander 1987.
| Study characteristics | ||
| Methods | Double‐blind, placebo‐controlled, cross‐over trial with 3 interventions:
|
|
| Participants | Number of participants screened: 28 (20 boys, 8 girls) Number of participants included: unclear Number of participants followed up: unclear Number of withdrawals: unclear Diagnosis of ADHD: DSM‐III (subtype not stated) Age: mean 8.4 years (range 6‐12) IQ: mean 79.64 MPH‐naive: 28 (100%) Ethnicity: not stated Country: USA Setting: outpatient clinic and inpatient ward Comorbidity: not stated Comedication: not stated Other sociodemographics: 3.52 (Hollingshead‐Redlich Index) Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of the possible drug condition orders of MPH (0.3 mg/kg and 0.6 mg/kg) and placebo Mean MPH dosage: no information Administration schedule: twice/d, 8:30 am and noon Duration of each medication condition: 12 days for inpatients and 19 days for outpatients (mean 15.25 days) Washout before trial initiation: none Medication‐free period between interventions: 68 h Titration period: none Treatment compliance: no information |
|
| Outcomes |
General behaviour
|
|
| Notes | Sample calculation: none Ethics approval: no information Key conclusions of trial authors
Comment from review authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: no Funding source: in part by Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) grants and the University of Southern California Faculty Research and Innovation Fund. Email correspondence with trial authors: December 2013. No supplemental information available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Receiving interventions in counterbalanced orders |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Agreement of 90% across all categories had to be reached with this validity observer Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Unclear risk | Protocol not identified |
Waxmonsky 2008.
| Study characteristics | ||
| Methods | 9‐week therapeutic summer camp consisting of a cross‐over trial with 4 interventions:
Varied daily within a cross‐over design of 3 intensities of behaviour modification therapy
Each lasted 3 weeks |
|
| Participants | Number of participants screened: 106 participants in the 2003 and 2004 Summer Treatment Program (University of Buffalo) Number of participants included: 101 (82 boys, 19 girls) (ADHD subgroup 33, ADHD + severe mood dysregulation (SMD) subgroup 68) Number of participants followed up: 99 Number of withdrawals: 2 Diagnosis of ADHD: DSM‐IV (combined (92%), hyperactive‐impulsive (not stated), inattentive (not stated)) Age: mean 8.5 years (range 5‐12) IQ: mean: 105 MPH‐naive: not stated Ethnicity: predominantly white Country: USA Setting: outpatient clinic (Summer Treatment Program) Comorbidity: ODD (54%), CD (12%) Comedication: not stated Other sociodemographics: predominantly middle class Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants attended a Summer Treatment Program each Monday through Friday for 9 weeks. Participants were randomly assigned possible drug condition orders of 0.15 mg/kg 3 times/d, 0.3 mg/kg 3 times/d and 0.6 mg/kg 3 times/d and placebo Average MPH dosages: 5 mg, 10 mg and 18 mg for 0.15 mg/kg, 0.3 mg/kg and 0.6 mg/kg doses, respectively Administration schedule: 7:45 am, 11:45 am and 3:45 pm Duration of each medication condition: each dose varied daily and was repeated 3 or 4 times within each behavioural treatment condition Trial duration: Monday through Friday for 9 weeks, totaling 45 days Washout before trial initiation: 1 week Medication‐free period between interventions: 0‐2 days Titration period: none Treatment compliance: not stated Cointervention: 3 behavioural conditions (no behaviour modification, low‐intensity behaviour modification and high‐intensity behaviour modification) are delivered in random order, with each condition lasting 3 weeks. Parents attended training sessions and implemented behaviour programmes at home |
|
| Outcomes |
Non‐serious AEs
|
|
| Notes | Sample calculation: not stated Ethics approval: yes; by the Health Sciences Institutional Review Board (IRB) of the University of Buffalo Comment from trial authors
Key conclusion of trial authors
Inclusion of MPH responders only/exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: yes; excluded patients with prior serious reactions to MPH Any withdrawals due to AEs: 1 withdrew because of tic‐like movements Funding source: NIMH Grant MH62946 and a Klingenstein Third Generation Foundation Fellowship in Child and Adolescent Depression Research Email correspondence with trial authors: August 2014. Obtained supplemental information regarding randomisation, allocation concealment and handling of missing data. Not possible to retrieve safety data |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random orders were generated by computer with the restrictions that each condition occurred at least once in each week. Children were assigned to previously generated codes at enrolment |
| Allocation concealment (selection bias) | Low risk | Because this was a cross‐over trial in which all children received all conditions multiple times, each child's entire 9‐week schedule was assigned at once. Treatment orders were concealed in an opaque envelope and were stored in a locked cabinet in the medication lab. Only authorised staff members had access to this cabinet. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind. Children, parents and staff were blinded to medication conditions. Placebo and MPH were packaged in identical opaque capsules to maintain blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind. Parents and staff were blinded to medication conditions. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Low risk | Outcomes reported according to protocol |
Weiss 2021.
| Study characteristics | ||
| Methods | A 4‐week parallel trial with 5 arms:
Phases: 4 (1 week washout, 2 weeks forced titration, 2 weeks stable dose, 6‐month open‐label, follow‐up trial) |
|
| Participants | Number of participants screened: 450 Number of participants included: 367; after exclusion of 1 trial site, 354 remained randomised (239 boys, 115 girls) Number of participants followed‐up: 323 Number of withdrawals: 31 (+ 13 from excluded trial site) Diagnosis of ADHD: DSM‐5 (255 combined, 5 hyperactive/impulsive, 94 inattentive) Age: placebo 14.1 mean years, MPH 14.2 mean years (range 12 to ≤ 17) IQ: > 80 MPH‐naive: 66% Ethnicity: not stated Country: Canada and USA Setting: outpatient Comorbidity: no allowed comorbidity specified. Many somatic and psychiatric disorders were exclusion criteria Comedication: a stable dose of melatonin was permitted (5.1% of participants); 1 participant in a MPH group received a hypnotic/sedative medicament Additional sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to either MPH PRC‐063 (25, 45, 70 or 85 mg) or placebo once/d. Number randomised to each group: first 367 were randomised (293 to MPH PRC‐063 groups and 74 to placebo). Due to major protocol violations, 13 participants from 1 site were excluded from the analysis, leaving 71 randomised to placebo and 283 randomised to MPH PRC‐063 groups (25 mg/d: n = 71, 45 mg/d: n = 69, 70 mg/d: n = 73, 85 mg/d: n = 70) Mean medication dosage: participants were evenly distributed to a forced dose of 25, 45, 70 or 85 mg/d. Administration schedule: once daily Duration (of (each) medication): 4 weeks Washout before trial initiation: 1 week Titration period: 2 weeks (blinded forced‐titration) Treatment compliance: compliance was evaluated by counting and recording the number of dispensed capsules and the number of returned capsules. Noncompliance was defined as missing ‡2 doses from a single bottle of trial medication. 83.7% compliance |
|
| Outcomes |
ADHD symptom severity
Serious AEs
General behaviour
Non‐serious AEs
As no SD was reported in the trial articles, we have not been able to use the ADHD Symptom or General Behavior data for the analysis. |
|
| Notes | Sample calculation: yes, 360 participants Ethics approval: yes. “For each study site, the study protocols were approved by an independent ethics committee or institutional review board, as appropriate. The studies were conducted in accordance with the Declaration of Helsinki, International Council for Harmonisation Good Clinical Practice (GCP), and all applicable local, state, and federal regulations.” (Weiss 2021a, p 2) Comments from trial authors
Key conclusion of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH: yes Any withdrawals due to AEs: yes, 10 participants all from the MPH PRC‐063 groups Funding source: Rhodes Pharmaceuticals, LP Email correspondence with trial authors: no correspondence |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Randomization schedules were generated by Y‐Prime, Inc., using an integrated web response system” |
| Allocation concealment (selection bias) | Low risk | Central allocation through Y‐Prime, Inc. and identically appearing drug containers and capsules |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “PRC‐063 and placebo were supplied in bottles, each containing 10 capsules. To maintain blinding, placebo and PRC‐063 at each dose were identical in appearance. No emergency unblinding occurred during the study.” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | “No emergency unblinding occurred during the study.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Due to multiple protocol violations and Good Clinical Practices (GCP) issues, it was necessary to exclude efficacy data for the 13 participants enrolled at one trial site from the primary efficacy analysis. A sensitivity analysis including data from this trial site was performed. As a further sensitivity analysis to assess the impact of missing data, the primary efficacy analysis for the full analysis population was repeated using Markov Chain Monte Carlo (MCMC) multiple imputation. Missing data were imputed 20 times. Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Low risk | All protocol outcomes reported |
Whalen 1990.
| Study characteristics | ||
| Methods | 2‐day cross‐over trial with 2 interventions
In a connected trial 25 children were randomised to the same interventions. The outcome of this trial included the social judgement processes during the different drug conditions. Outcomes from the trial included in this review were reported in this connected trial. |
|
| Participants | Number of participants screened: unknown Number of participants included: 24 Number of participants followed up: 24 (22 boys, 2 girls) Number of withdrawals: 0 ADHD diagnosis: trial 1 DSM‐III‐R Age: trial one mean 9 years 8 months (range 6.4‐13.2 years) IQ: no mental disability MPH‐naive: 0% Ethnicity: white (92%), African American (4%), Hispanic (4%) Country: USA Setting: outpatient clinic (Summer Treatment Program) Comorbidity: not stated Comedication: not stated Other sociodemographics: all were from middle‐ or low middle‐income backgrounds Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of the possible drug condition orders of 0.3 mg/kg MPH or placebo. Mean MPH dosage: 8.75 mg Administration schedule: twice/d, morning and lunchtime Duration of each medication condition: 1 day Washout before trial initiation: unknown Medication‐free period between interventions: medication and placebo were given on 2 consecutive days Titration period: none Treatment compliance: good (2 staff dispensed the medication for ingestion) |
|
| Outcomes |
General behaviour
|
|
| Notes | Sample calculation: no Ethics approval: no information Key conclusions of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: yes; only participants taking maintenance dosage of MPH or well titrated Any withdrawals due to AEs: no Funding source: not stated Email correspondence with trial authors: January 2014. No supplemental information has been received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both active medication and placebo were placed in opaque gelatin capsules. 2 staff, blinded to medication status, dispensed medication |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessments were made by independent healthy controls through video tapes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Low risk | No indication of selective reporting |
Wigal 2003.
| Study characteristics | ||
| Methods | Double‐blind, 2‐stage, cross‐over, pharmacokinetic and pharmacodynamic trial with 4 interventions
Phases: initial screening week, stage 1 cross‐over of IR‐MPH (Ritalin) vs placebo, stage 2 cross‐over of treatment C and treatment D. 4 weeks, with each trial period lasting 1 week |
|
| Participants | Number of participants screened: not stated Number of participants included: 27 Number of participants followed up: 25 (21 boys, 4 girls) Number of withdrawals: 4 Diagnosis of ADHD: DSM‐IV (subtype not stated) Age: mean 10 ± 1.4 years (range 7‐12) IQ: not stated MPH‐naive: not stated Ethnicity: white (88%), African American (8%), Asian (4%), Hispanic (0%), other (0%) Country: USA Setting: outpatient clinic (laboratory classroom and community) Comorbidity: not stated Comedication: not stated Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 2 possible drug condition orders of MPH and placebo in each of the 2 stages, 1 and 2, with each treatment period lasting 1 week. Stage 1
Stage 2 Half of the participants in each treatment group were randomly assigned to 20 mg/d dosage of MPH, the other half to 40 mg/d dosage
Mean MPH dosage: 20 mg/d or 40 mg/d Administration schedule: once daily or twice daily Time points: mornings and after lunch Duration of each medication condition: 1 week Washout before trial initiation: not stated Medication‐free period between interventions: 1 day (Sundays) Titration period: none Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
General behaviour
Non‐serious AEs
|
|
| Notes | Sample calculation: not stated Ethics approval: yes; trial protocol and participant's informed consent form were approved by the Institutional Review Board (Office of the Vice Chancellor for Research, University of California at Irvine) Key conclusion of trial authors
Comments from review authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: yes; positive MPH response was an inclusion criterion. Serious AEs were an exclusion criterion. Any withdrawals due to AEs: no Funding source: Celltech Americas Incorporated Email correspondence with trial authors: July 2014. Emailed trial authors twice for supplemental information but have received no response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Qualified participants were randomly assigned to a 2‐stage, double‐blind trial sequence consisting of 4 trial treatments, each lasting for a period of 1 week |
| Allocation concealment (selection bias) | Low risk | MPH (Ritalin) (10 mg) tablets were placed into capsule shells by Eurand Americas Incorporated. Resulting capsules were tested according to US Pharmacopeial Convention (USP) dissolution conditions for MPH tablets and showed a dissolution profile comparable with intact Ritalin tablets. All medications were supplied in white, opaque, size 3, hard gelatin capsule shells, and were packaged in blister cards to enhance compliance |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Ritalin (10 mg) tablets were placed into capsule shells by Eurand Americas Incorporated. Resulting capsules were tested according to US Pharmacopeial Convention (USP) dissolution conditions for MPH tablets and showed a dissolution profile comparable with intact Ritalin tablets. All medications were supplied in white, opaque, size 3, hard gelatin capsule shells, and were packaged in blister cards to enhance compliance |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): yes |
| Selective reporting (reporting bias) | Unclear risk | "Because the administration of the Side Effects Rating Form involved queries about specific adverse events, the frequency of adverse events reported in the parents’ and teachers’ Side Effect Rating Form was higher than that obtained from the reports elicited by general inquire (data not shown)" |
Wigal 2004.
| Study characteristics | ||
| Methods | 4‐week, randomised, double‐blind, placebo‐controlled, ITT, parallel multicentre trial with 3 arms:
|
|
| Participants | Number of participants screened: 174 Number of participants included: 132 (116 boys, 16 girls) Number of participants followed up: 119 (d‐MPH 42, dex,l‐MPH 40, placebo 37) Number of withdrawals: 13 (d‐MPH 2, dex,l‐MPH 6, placebo 5) Diagnosis of ADHD: DSM‐IV (combined (64%), hyperactive‐impulsive (1%), inattentive (35%)) Age: mean 9.8 years (range 6‐17) IQ: not stated. No mental disability MPH‐naive: 95 (72%) Ethnicity: white (78%), African American (14%), other (8%) Country: USA Setting: outpatient clinic Comorbidity: no Comedication: no Other sociodemographics: no significant differences in baseline demographics were noted between the 2 groups Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to IR‐MPH (dex‐MPH, dex,l‐ MPH) or placebo No of participants randomised to each group: d‐MPH 44, dex,l‐MPH 46, placebo 42 Mean MPH dosage: d‐MPH 18.25 mg/d, dex, l‐MPH 32.14 mg/d Administration schedule: twice daily in the morning (7:00 am‐8:00 am) and at noon (11:30 am‐2:30 pm) Duration of intervention: 4 weeks Titration period: maximum 3 weeks initiated after randomisation, included in the 4 weeks of intervention Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
Non‐serious AEs
|
|
| Notes | Sample calculation: yes Ethics approval: yes; approved by the institutional review board at each centre Comments from trial authors
Key conclusions of trial authors
Comment from review authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: yes, see exclusion criteria Any withdrawals due to AEs: yes. “parental consent was withdrawn for one patient due to deterioration of behavior on placebo”, and “AEs (two patients each in the d,l‐MPH and placebo groups)” Funding source: Celgene Corporation Email correspondence with trial authors: January 2014. Not possible to obtain data from trial authors |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised" |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Drug (d‐MPH and dex,l‐MPH) and placebo were identical in appearance |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Sample size calculations were based on a clinically meaningful effect size of 0.75 in the change in score over 4 weeks on the teacher‐rated SNAP between d‐MPH and placebo groups. Efficacy parameters were performed on the ITT sample, which included participants who received medication, had a baseline efficacy evaluation and had ≥ 1 post‐baseline efficacy evaluation Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol published |
Wigal 2011.
| Study characteristics | ||
| Methods | Double‐blind, randomised, cross‐over, analogue classroom trial with 2 interventions:
Phases
|
|
| Participants | Number of participants screened: not stated Number of participants included: 78 (55 boys, 23 girls) Number of participants followed up: 71 Number of withdrawals after randomisation: 0 Diagnosis of ADHD: DSM‐IV‐TR (81% combined, 0% hyperactive‐impulsive, 19% inattentive) Age: mean 10.1 years (range 9‐12 years) IQ: > 80 MPH‐naive: not stated Ethnicity: white (58%), African American (28%), other (14%) Country: USA Setting: outpatient clinic, laboratory classroom Comorbidity: anxiety (0%), depressive disorders (0%), learning disability (32%) Comedication: not stated Additional sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 2 possible drug condition orders of OROS‐MPH and placebo Mean OROS‐MPH daily dosage: 40.5 mg Administration schedule: once daily (morning) Average duration of OROS‐MPH treatment: 40 days; only a single blinded day Duration of placebo intervention: 1 day Washout before trial initiation: up to 28 days Medication‐free period between interventions: no Titration period: before randomisation, up to 6 weeks Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
Serious AEs
Non‐serious AEs
|
|
| Notes | Sample calculation: yes Ethics approval: yes Key conclusions of trial authors
Inclusion of MPH responders only/exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: yes; a history of failed response to MPH was an exclusion criterion. Only children demonstrating the required decrease in ADHD symptoms with OROS‐MPH within the labelled dosing range were included in the randomised phase of the study. Children who may have required a dose > 54 mg to achieve full therapeutic effect may also have been excluded. Any withdrawals due to AEs: no Funding source: supported by Ortho‐McNeil‐Janssen Scientific Affairs, LLC. Phase IV trial Email correspondence with trial authors: June 2013‐June 2014. We obtained supplemental efficacy data (SKAMP) and safety data. Awaiting data through the Yale Open Data Access Project |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Wigal 2011: "computer‐generated randomisation schedule" |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Wigal 2011: "blinding of investigators and participants maintained throughout the trial" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Wigal 2011: "blinding of investigators and participants maintained throughout the trial" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information on incomplete outcome data Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Low risk | All outcomes reported according to protocol |
Wigal 2013.
| Study characteristics | ||
| Methods | 4‐ to 6‐week, open‐label treatment (dose optimisation), cross‐over, 2‐week double‐blind trial with 2 interventions:
Phases: 2 |
|
| Participants | Number of participants screened: 45 (32 boys, 12 girls) entered the open‐label phase and were randomised at baseline visit Number of participants included: 39 entered the double‐blind cross‐over trial Number of participants followed up: 39 Number of withdrawals: 0 Diagnosis of ADHD: DSM‐IV (combined (70.5%), hyperactive‐impulsive (2.3%), inattentive (27.3%)) Age: mean 8.8 years (range 6‐12) IQ: not stated MPH‐naive: 0 (0%) Ethnicity: white 35 (79.5%), black/African American 4 (9.1%), Asian 3 (6.8%), other 2 (4.5%) Country: USA Setting: outpatient clinic Comorbidity: elimination disorder (4; 9.1%), ODD (8; 18.2%), specific phobias (2; 4.5%) Comedication: no Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to different sequences of MPH and placebo Mean MPH dosage: 32.8 mg/d Administration schedule: 4 times/d Duration of each medication condition: 1 week Washout before trial initiation: yes (1 day for stimulants) Medication‐free period between interventions: no Titration period: 3 weeks before randomisation Treatment compliance: 2 withdrawals of assent/consent, 2 AEs, 1 lack of efficacy, 1 LTFU, all during the open‐label phase |
|
| Outcomes |
ADHD symptoms
Non‐serious AEs 42 participants (93.3%) experienced a TEAE. 3 (6.7%) participants experienced severe adverse effects (affect lability, aggression and initial insomnia), and 2 (4.4%) participants had to discontinue medication (affect lability and aggression)
|
|
| Notes | Sample calculation: no Ethics approval: yes Comments from trial authors
Key conclusion of trial authors
Comments from review authors
Inclusion of MPH responders only/exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: yes, there was an open‐label titration phase before randomisation, during which 2 participants discontinued due to AEs and 1 due to lack of efficacy. Also participants had to be in treatment with suboptimal efficacy (no inclusion of placebo responders). Any withdrawals due to AEs: no Funding source: trial received funds from NextWave Pharmaceutics (Belden and Berry are with NextWave) Email correspondence with trial authors: emailed trial authors to request additional information but have not received a response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Results from open‐label phase |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Results from open‐label phase |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants in the double blind phase followed up Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Low risk | ADHD‐RS not reported (used only in the open phase) |
Wigal 2014.
| Study characteristics | ||
| Methods | Cross‐over trial with 2 interventions:
Phases: 4
|
|
| Participants | Number of participants screened: 32 Number of participants included: 26 (in open‐label phase; 12 boys, 10 girls) Number of participants followed up: 20 Number of withdrawals: 6 Diagnosis of ADHD: DSM‐IV‐TR (combined (n = 11), hyperactive‐impulsive (n = 3), inattentive (n = 12)) Age: mean 8.7 years (range 6‐12) IQ: mean not stated (range 86‐133) MPH‐naive: none Ethnicity: white (82%), black (9%), Asian (5%), Hispanic or Latino (23%), other (5%) Country: USA Setting: outpatient clinic, laboratory classroom setting Comorbidity: 11; generalised anxiety disorder, enuresis, ODD, chronic motor or vocal tic disorder, transient tic disorder Comedication: no Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 2 possible drug condition orders of MPH (15 mg, 20 mg, 30 mg or 40 mg) and placebo Mean MPH dosage: 32 mg Administration schedule: once/d Time points: in the morning Duration of each medication condition: 1 week Washout before trial initiation: 2 days Medication‐free period between interventions: not stated Titration period: yes; 2‐4 weeks before randomisation Treatment compliance: yes; verified at scheduled trial visits by trial personnel who examined documentation of drug dispensed, drug consumed and remaining drug, and recorded the information on the drug reconciliation form Compliance was calculated to be > 82% throughout the trial |
|
| Outcomes |
ADHD symptoms
Non‐serious AEs
There is not enough data on the CSHQ available for us to include it in the analysis. |
|
| Notes | Sample calculation: yes Ethics approval: yes Comment from trial authors
Key conclusions of trial authors
Inclusion of MPH responders only/exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: yes; 2 withdrew during the open‐label phase as the result of lack of efficacy Any withdrawals due to AEs: yes (n = 1) Funding source: Rhodes Pharmaceuticals L.P. Email correspondence with trial authors: April 2015. Obtained supplemental information from trial authors. Trial authors were contacted regarding CSHQ data in November 2021, but no answer was received. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomisation was determined by using a table of random numbers. Randomisation tables were provided to the randomisation monitor, who created unblinding envelopes and packaged the drug with blinded labels. |
| Allocation concealment (selection bias) | High risk | One participant received placebo at 2 periods ‐ the method was not efficacious |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All sponsor representatives, investigators, participants and independent raters remained blinded until after data were locked |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Yes; this has been done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT were used Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo‐responders): no; no exclusion of MPH non‐responders after randomisation |
| Selective reporting (reporting bias) | Low risk | No indication of selective reporting |
Wigal 2015.
| Study characteristics | ||
| Methods | A 1‐week parallel trial with 5 arms:
Phases: 4 (screening, double‐blind, open‐label, and safety follow‐up) |
|
| Participants | Number of participants screened: 280 Number of participants included: 230 (154 (67%) boys, 76 (33%) girls) Number of participants followed up: 221 Number of withdrawals: 9 Diagnosis of ADHD: DSM‐IV‐TR (140 combined, 6 hyperactive‐impulsive, 75 inattentive, 9 not reported) Age: mean 10.8 years (SD 3.0, range 6‐18) IQ: IQ < 80 was an exclusion criterion MPH‐naive: 146 of 221 (66%); "Two‐thirds of the sample were stimulant naïve, and one‐third of the sample (75/221) had a medication washout" Ethnicity: race was measured: white (n = 158, 68.7%), black (n = 53, 23%), Asian (n = 3, 1.3%), other (n = 16, 7%) Country: USA Setting: outpatient Comorbidity: many comorbidities were exclusion criteria (see exclusion criteria). Most common comorbidities were ODD (n = 22) and enuresis (n = 14) Comedication: CNS medication was an exclusion criterion, many illnesses were exclusion criteria, no further information Sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | 4 phases
Participants were randomly assigned to: 5 different groups, 4 receiving MPH‐MLR (biphentin MPH extended release capsules) of either 10 mg, 15 mg, 20 mg or 40 mg, or placebo capsules Number randomised to each group: MPH‐MLR 10 mg: 49, MPH‐MLR 15 mg: 44, MPH‐MLR 20 mg: 45, MPH‐MLR 40 mg: 45, placebo: 47 Patients weighing < 25 kg were not assigned to receive the 40 mg dose Mean medication dosage: forced‐dose: 10 mg, 15 mg, 20 mg or 40 mg Administration schedule: once daily at 10.00 am Duration (of (each) medication): 1 week Washout before trial initiation: minimum 48 h Treatment compliance: no information |
|
| Outcomes |
ADHD symptoms
Serious AEs
Non‐serious AEs
|
|
| Notes | Sample calculation: yes; “It was estimated that a total sample size of 225 would have 80 % power to detect a mean difference […]” Ethics approval: yes; “The study protocol, amendments, and informed consent form were reviewed and approved by an Institutional Review Board for each study site.” Comments from trial authors
Key conclusion of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH: yes, known hypersensitivity to MPH was an exclusion criteria Any withdrawals due to AEs: yes, 3 Funding source: Rhodes Pharmaceuticals […]. Medical writing assistance was provided by Linda Wagner, PharmD, from Excel Scientific Solutions and funded by Rhodes Pharmaceuticals LP Email correspondence with trial authors: September and November 2021. We contacted the trial authors for information regarding risk of bias and CSHQ data through personal email in September and November 2021, but no answer was received. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomised (1:1:1:1:1) to receive MPH‐MLR 10 mg, 15, 20, or 40 mg or placebo following a computer‐generated randomisation schedule with patients assigned the next random number arranged in an ABCDE block design with each letter representing one of the 5 treatment groups. There was no site stratification in randomization. Patients weighing <25 kg were not assigned to receive the 40 mg dose” |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "All trial treatments (MPH‐MLR 10, 15, 20, 30, 40, 50, 60 mg, placebo) were given orally once daily in the morning, no later than 10 a.m. and were packaged in bottles of ten capsules for a 1‐week dispensing interval and bottles of 30 for 4‐ and 8‐week dispensing intervals. Lot numbers used during the double‐blind phase were A07983‐2. 002L01 (10 mg), A07983‐002L02 (15 mg), A07983‐3. 002L03 (20 mg), A07983‐002L04 (40 mg), and A07983‐001L02 (placebo)" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "The model had class terms for treatment (5 levels), site (sites with less than ten patients were combined into a pseudo site), and a covariate term for baseline ADHD‐RS‐IV total score. The same model was applied to the ITT population as a sensitivity analysis. Subjects not completing the double‐blind phase had the decrease in total score set to zero for the sensitivity analysis." Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | High risk |
“Incidence of adverse findings using various measures of safety, tolerability, and quality of life assessments following administration of once daily Biphentin” (NCT01239030) Quality of life is mentioned as an outcome in the protocol but not in the full report. It is described that the C‐SSRS is assessed at screening and at the end of the double‐blind phase, but the results are not included. |
Wigal 2017.
| Study characteristics | ||
| Methods | A 1‐week parallel‐trial with 2 arms:
Phases: 3 (open‐label phase (6 weeks), double blind‐phase (1 week), follow‐up 1‐2 weeks after) |
|
| Participants | Number of participants screened: 90 entered the open‐label phase Number of participants included: 86 randomised in the double‐blind trial Number of participants followed up: 85 (ITT population, 53 (62.4%) boys, 32 (37.6%) girls) Number of withdrawals: 1 during double‐blind (lost to follow‐up) Diagnosis of ADHD: confirmed at screening (23 (27.1%) inattentive, 0 hyperactive/impulsive, 62 (72.9%) combined) Age: 9.6 years (SD 1.69, range 6‐12) IQ: not measured MPH‐naive: not stated Ethnicity: Hispanic/Latino (n = 13, 15.3%), non‐Hispanic/‐Latino (n = 72, 84.7%) Country: USA Setting: multi‐site, outpatient Comorbidity: not allowed Comedication: not allowed Additional sociodemographics: white (n = 49, 57.6%), black/African American (n = 30, 35.3%), Asian (n = 1, 1.2%), other (n = 5, 5.9%) Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 2 different groups, either placebo or ER‐MPH chewable tablet at optimised dose (between 20‐60 mg/d) Number randomised to each group: 44 placebo, 42 ER‐MPH chewable tablet Mean medication dosage: not stated Administration schedule: daily Duration (of (each) medication): 7 weeks of MPH for MPH group, 6 weeks MPH + 1 week placebo for placebo group Washout before trial initiation: a minimum of 24‐h before open‐label phase. No information on wash‐out period before double‐blind trial initiation Titration period: 6 weeks before double‐blind week. Starting at a dose of 20 mg, participants could either be increased or decreased by 10‐20 mg/d once a week to a maximum of 60 mg/d and a minimum of 20 mg/d, depending on a clinical judgement by the investigators Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
Serious AEs
Non‐serious AEs
|
|
| Notes | Sample calculation: no Ethics approval: yes; “The protocol, consent and assent forms, and the investigator’s brochure received institutional review board approval before initiation of the study” Comments from trial authors
Key conclusion of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH: yes Any withdrawals due to AEs: not stated for double‐blind period (1 during open‐label phase) Funding: this research was sponsored by NextWave Pharmaceuticals, a wholly owned subsidiary of Pfizer, Inc Email correspondence with trial authors: September and November 2021. We contacted the trial authors for information regarding risk of bias through personal email in September and November 2021, but no answer was received. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was conducted according to a fixed schedule using a permuted block design stratified by clinical site. |
| Allocation concealment (selection bias) | Low risk | The randomisation code was maintained centrally by the clinical supply group, and the trial team and investigator site personnel were blinded throughout the trial. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Mention of double‐blind, method not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The investigators who recorded the AEs did not conduct the efficacy evaluations, and the classroom raters collecting efficacy data had no duties outside the classroom and were not aware of AEs collected by the investigators. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Efficacy analyses were based on the ITT population, which included all participants who received at least 1 dose of the trial drug and had at least 1 post‐baseline assessment of the primary efficacy variable. Safety assessments were based on the enrolled safety population Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Low risk | All protocol outcomes are reported |
Wilens 2006b.
| Study characteristics | ||
| Methods | 2‐week, randomised, double‐blind, 15‐centre, parallel trial with 2 arms:
Phases: preceded by a 4‐week, open‐label, dose‐titration phase, and followed by an 8‐week, open‐label, follow‐up phase |
|
| Participants | Number of participants screened: 220 in the 4‐week dose‐titration phase Number of participants included: 177 (142 boys, 35 girls) Number of withdrawals: 44. MPH 16, placebo 28 Number of participants followed up: 171; number completing follow‐up: 135 Diagnosis of ADHD: DSM‐IV (subtype not stated) Age: mean 14.6 years (range 13‐18) IQ: not stated ADHD treatment‐naive: 24 Ethnicity: white (75.1%), African American (13.6%), other (11.3%) Country: USA Setting: outpatient clinic Comorbidity: not stated Comedication: not stated Other sociodemographics: the 2 groups were similar demographically, but the placebo group had a greater ratio of boys (P value < 0.04) Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to OROS‐MPH or placebo Number of participants randomised to each group: MPH 87, placebo 90 Mean MPH dosage: 0.84 mg/kg Administration schedule: once daily Duration of intervention: 2 weeks Titration period: 4 weeks initiated before randomisation. All participants initiated therapy at 18 mg/d, and clinical response was measured after 1 week. If response to treatment was inadequate, as per the a priori trial definition, the dose was titrated upward (in 18‐mg increments) at 1‐week intervals for up to 4 weeks, with maximum dose of 72 mg/d Treatment compliance: not stated; 8‐week, open‐label follow‐up on individualised dosage |
|
| Outcomes |
ADHD symptoms
Serious AEs
Non‐serious AEs
No participants experienced clinically important effects on ECG indexes, heart rate or BP during the trial |
|
| Notes | Sample calculation: yes Ethics approval: yes; trial was approved by the institutional review boards for all participating centres before the start of the trial Comments from trial authors
Key conclusions of trial authors
Inclusion of MPH responders only/exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: yes; criteria of response, defined as ≥ 30% improvement from baseline on the investigator‐scored ADHD‐RS. Participants who successfully completed the open‐label, dose‐titration phase were assigned a randomisation number Any withdrawals due to AEs: yes; 1 in the placebo group Funding source: McNeil Consumer and Specialty Pharmaceuticals Email correspondence with trial authors: December 2013 and Janurary 2014. Not able to make contact with trial authors |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, but method was not described |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Investigators were supplied with packages containing medication for each participant, as identified by randomisation number. Therefore, investigators and participants were blinded to whether a participant was receiving active medication or placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators were supplied with packages containing medication for each participant, as identified by randomisation number. Therefore, investigators and participants were blinded to whether a participant was receiving active medication or placebo |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Non‐responders not included. LOCF technique was used for all assessments in the double‐blind phase. Of 182 (83%) participants who successfully achieved the criteria for improvement at the dose‐titration phase, only 177 were randomly assigned to the double‐blind phase, because 5 reached the criteria after the double‐blind phase was closed. Of 177 randomly assigned participants, 1 did not enter the double‐blind phase, and efficacy data were not collected for another participant. Therefore, 175 participants were included in the efficacy analysis of the double‐blind phase, but 177 were included in the dosage and safety analysis. Selection bias: yes, participants were excluded due to lack of efficacy during the double blind phase |
| Selective reporting (reporting bias) | Low risk | No indication of selective reporting, outcomes according to protocol |
Wilens 2008.
| Study characteristics | ||
| Methods | Open‐label, 5‐week, dose‐optimisation period. This dose was maintained during the subsequent 3 weeks of the trial, except for 1 d/week of 3‐way cross‐over assessment. Randomised, double‐blind, 8‐centre, 3‐way, 3‐week, cross‐over trial with 3 interventions:
|
|
| Participants | Number of participants screened: 148 Number of participants included: 128 in open‐label, dose‐titration; 117 entered the blinded, randomised trial Number followed up: 127 for safety and 117 for efficacy Number of withdrawals: 2 (both withdrew from the analogue classroom phase but were included in the ITT efficacy analysis) Characteristics of the 127 followed up for safety Sex: 84 boys, 42 girls Diagnosis of ADHD: DSM‐IV (combined or hyperactive‐impulsive (92.1%) inattentive (not stated)) Age: mean 8.8 years (SD 1.84; range 6‐12) IQ: > 80 MPH‐naive: not stated Ethnicity: white (63.2%), African American (15.4%), Asian (not stated) Country: USA Comorbidity: not allowed Comedication: not stated Other sociodemographics: none Characteristics of the 117 followed up for efficacy Sex: 75 boys, 42 girls Diagnosis of ADHD: DSM‐IV (type not stated) Age: mean 8.8 years (range 6‐ 12) IQ: > 80 MPH‐naive: not stated Ethnicity: white (63.2%), African American (15.4%), Asian (0%), other (21.4%) Country: USA Setting: outpatient clinic (analogue class room) Comorbidity: not allowed Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
11 participants discontinued before the double‐blind randomisation phase, resulting in an ITT population of 117 participants 7 participants discontinued before the double‐blind randomisation phase, 3 were randomly assigned but did not undergo MPH transdermal system treatment 2 participants did not complete the analogue classroom phase of the trial: 1 because of an application site reaction, and 1 because of an AE (conjunctivitis), resulting in a total of 115 trial completers |
|
| Interventions | Participants were randomly assigned to 1 of 3 possible drug orders of MPH (4 and 6 h) and placebo Mean MPH dosage: 10 mg patch (n = 15), 15 mg patch (n = 34), 20 mg patch (n = 32) and 30 mg patch (n = 36) Administration schedule: once daily in the morning; patch worn for 9 h daily, and for 4 or 6 h for cross‐over assessments Duration of each medication condition: 1 day Participants were kept on their optimised dose between classroom sessions Washout before trial initiation: none; in‐between treatment with optimal dose of MPH Titration period: 5 weeks before randomisation Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
Quality of life
Non‐serious AEs
No clinically meaningful changes from baseline were observed in vital signs, ECG, urinalysis and haematological results or physical examinations. AEs were recorded from the time informed consent was signed until 30 days (week 12) after the last drug treatment |
|
| Notes | Sample calculation: yes; assuming a mean difference in SKAMP deportment score of 2.0 between active treatment and placebo, an SD of 5.0, a between correlation of 0.2, 90% power and a probability level of.05 (2‐sided), it was estimated that approximately 102 participants were needed to complete the double‐blind, cross‐over phase of the trial Ethics approval: yes; institutional review board at each site approved the trial Comments from trial authors
Key conclusion of trial authors
Comments from review authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: yes; participants with a history of failing to respond to psychostimulant treatment were also excluded Any withdrawals due to AEs: yes; 1 (conjunctivitis) Funding: Shire Development Incorporated Email correspondence with trial authors in April‐June 2014. Emailed trial authors twice to ask for additional data but never received a response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Single randomisation schedule prepared by an independent statistician using computer software that generated random numbers |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Each participant wore 2 patches prepared by an unblinded pharmacist to maintain treatment blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were only 2 withdrawals during the double blind phase Selection bias (e.g. titration after randomisation → exclusion of MPH non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Low risk | No indication of selective reporting, outcomes according to protocol |
Wilens 2010.
| Study characteristics | ||
| Methods | Blind, randomised, 4‐week, cross‐over trial with 2 interventions:
|
|
| Participants | Number of participants screened: unknown Number of participants included: 36 Number of participants followed up: 30 (25 boys, 5 girls) Number of withdrawals: 4 Diagnosis of ADHD: DSM‐IV (combined (53%), hyperactive‐impulsive (3%), inattentive (43%)) Age: mean 9.17 years (SD 1.84; range 6‐12) IQ: > 70 MPH‐naive: 14 (47%) Ethnicity: white (90%), African American (not stated), Asian (3%), Hispanic (not stated), other (7%) Country: USA Setting: outpatient clinic Comorbidity: oppositional disorder (70%), CD (7%), major depressive disorder (3%) Comedication: not stated Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 2 possible drug condition orders of MPH (20 mg) and placebo Mean MPH dosage: not stated Administration schedule: patch applied in morning and worn for 9 h Duration of each medication condition: 2 weeks Washout before trial initiation: none Titration period: 1 week after randomisation Treatment compliance: > 80% for all participants |
|
| Outcomes |
ADHD symptoms
Non‐serious AEs
|
|
| Notes | Sample calculation: no Ethics approval: not stated Comment from trial authors
Key conclusion of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: yes; participants with a history of no response or intolerability to MPH were excluded for ethical reasons Any withdrawals due to AEs: yes; 2 Funding source: trial and medication/placebo were funded by a grant through Shire Pharmaceuticals. Shire had no role in design, collection, analysis, interpretation, writing or decision to submit Email correspondence with trial authors: March/April 2014. We contacted the first trial author twice to ask for supplemental data but have not received a response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned |
| Allocation concealment (selection bias) | Low risk | Prescriptions filled by hospital pharmacy |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No additional information from trial author |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double‐blind (participant, caregiver, investigator) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT: participants who completed 1 week of treatment; LOCF Selection bias: yes. Excluded participants not following 1 week of treatment |
| Selective reporting (reporting bias) | Low risk | No indication of selective reporting, outcomes according to protocol |
Wilkison 1995.
| Study characteristics | ||
| Methods | Double‐blind, placebo‐controlled, cross‐over trial with 2 interventions:
Phases: 2 |
|
| Participants | Number of participants screened: not stated Number of participants included: 16 Number of participants followed up: 16 implied (16 boys, 0 girls) Number of withdrawals: not stated Diagnosis of ADHD: DSM‐III‐R (subtype not stated) Age: mean 10.2 years (range 8‐13) IQ: mean 112.6 MPH‐naive: 0% Ethnicity: not stated Country: USA Setting: outpatient clinic Comorbidity: no Comedication: not stated Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 2 possible drug condition orders of MPH (normal dose) and placebo Mean MPH dosage: 0.030 mg/kg (range 0.08 to 1.10 mg/kg/d) Administration schedule: not stated Duration of each medication condition: 36 h Washout before trial initiation: not stated Titration period: not stated Treatment compliance: not stated |
|
| Outcomes |
General behaviour
|
|
| Notes | Sample calculation: not described Ethics approval: not described Key conclusion of trial authors
Comment from review authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: yes, only participants who responded positively to MPH included Any withdrawals due to AEs: unclear Funding source: a University of Utah Biomedical Sciences Research Grant and a grant from the University Research Committee Email correspondence with trial authors: May 2014. We obtained supplemental information from trial authors (Magnusson 2014c [pers comm]) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | From correspondence: "generated by pharmacist" (Magnusson 2014c [pers comm]) |
| Allocation concealment (selection bias) | Low risk | From correspondence: "recruiter had no involvement in and was blinded to allocation sequence" (Magnusson 2014c [pers comm]) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | From correspondence: "pharmacist instructed parents (via instructions on pill bottle) regarding which pills should be administered and at what time" (Magnusson 2014c [pers comm]) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | See above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | From correspondence: "approximately 2% of skin conductance and heart rate data were affected by participant movement and were replaced with interpolated values Physiological data were lost for 2 participants with ADHD and for 2 participants without ADHD. For 3 of the remaining 28 participants, 1 of the 4 repeated measurements of interbeat intervals was replaced with the mean of the participant's diagnostic group because his ECG recordings were inadequate" (Magnusson 2014c [pers comm]) Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Low risk | No indication of reporting bias |
Wodrich 1998.
| Study characteristics | ||
| Methods | 3‐week cross‐over trial with 3 interventions:
Phases
|
|
| Participants | Number of participants screened: 123 ("treated in the clinic") Number of participants included: 57 (47 boys, 10 girls) Number of participants followed up: 57 Number of withdrawals: 66. Away on summer vacation (n = 30), teacher failed to complete all rating forms (n = 29), teacher marked 2 values indicated on a single dimension (n = 3), adverse medication side effect (n = 2), dropped out before child’s medication trial was completed (n = 2) Diagnosis of ADHD: DSM‐III‐R Age: mean 8.5 years (SD 2.1; range 6‐14) IQ: not stated MPH‐naive: not stated Ethnicity: white "Anglo" 54 (95%), Hispanic 3 (5%) Country: USA Setting: outpatient clinic Comorbidity: not stated Comedication: not stated Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 2 possible drug condition orders (5 mg MPH or 15 mg MPH) and placebo Mean MPH dosage: 10 mg/d, 30 mg/d and placebo Administration schedule: twice/d at 8:00 am and 12:00 pm Duration of each medication condition: 1 week Washout before trial initiation: approximately 20 h (lunchtime until "immediately before school" the next day) Titration period: not stated Treatment compliance: not stated Number of withdrawals: 2. Parents dropped out of the trial before their child’s medication trial was completed |
|
| Outcomes |
ADHD symptoms
Serious AEs
General behaviour
Non‐serious AEs
|
|
| Notes | Sample calculation: not stated Ethics approval: not stated Key conclusions of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: yes Funding source: not declared Email correspondence with trial authors: June 2014. We obtained supplemental information from trial authors. We contacted trial author to ask for missing data, but s/he was not able to supply the data |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Subjects then underwent a three‐week, triple‐blinded, cross‐over medication trial consisting of placebo, 5 mg, and 15 mg of MPH dose twice a day (immediately before school and at lunchtime)" (p 83); "Order of administration was counterbalanced so that equivalent numbers of children received each sequence" (p 83) |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Subjects then underwent a three‐week, triple‐blinded, cross‐over medication trial consisting of placebo, 5 mg, and 15 mg of MPH dose twice a day (immediately before school and at lunchtime). Each drug condition lasted 7 days (Thursday through Wednesday)" (p 83); "Doses were prepared and packaged for the three week trial by a licensed pharmacist who split MPH tablets and placed them with lactose into re‐sealable capsules. The placebo dose consisted of lactose‐filled capsules only. Equivalent numbers of capsules (three) were dispensed at each dosing time to maintain uniformity and disguise presence/amount of medication, as three capsules were required to encompass the largest dose (15 mg)" (p 83) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "This procedure was followed for three weeks, with the medication code being broken the final week and determination about medication efficacy and decision to continue addressed at that time" (p 84) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified |
Wolraich 2001.
| Study characteristics | ||
| Methods | 28‐day, randomised, parallel, double‐blind clinical trial with 3 arms:
|
|
| Participants | Number of participants screened: 405 Number of participants included: 312 (233 boys, 49 girls) Number of participants followed up: 206 (OROS‐MPH 79, IR‐MPH 81, placebo 46) Number of withdrawals: 71 (OROS‐MPH 15, IR‐MPH 13, placebo 43) Diagnosis of ADHD: DSM‐IV (combined (73.4%), hyperactive‐impulsive (7.1%), inattentive (19.5%)) Age: mean 9.0 years (range 6‐12) IQ: > 70 MPH‐naive: 20.2% Ethnicity: white (84.4%), African American (7.4%), Asian (0.4%), Hispanic (3.5%), other (4.3%) Country: USA Setting: outpatient clinic Comorbidity: ODD (41.8%), CD (11.3%), tic disorder (5.3%), anxiety disorder (1.4%), depression (0.7%) Comedication: not stated Other sociodemographics: none Inclusion criteria
Exclusion criteria
During the course of the trial, participants were allowed to receive behavioural interventions as long as the interventions had been initiated before the start of the trial and did not change during the trial. New behavioural therapy was not allowed during the course of the trial |
|
| Interventions | Average total daily dose: IR‐MPH 29.5 mg/d (0.90.4 mg/kg/d ), OROS‐MPH 34.3 mg/d (1.1 to 0.5 mg/kg/d ) No. of participants randomised to each group: OROS‐MPH 94, IR‐MPH 94, placebo 89 Administration schedule: 3 times/d Duration of intervention: 4 weeks Titration period: 4‐week titration period before randomisation (open‐label) for trial participants who had not received MPH for ADHD from their own practitioner in the 4 weeks before trial entry. Participants who had taken MPH during the 4 weeks before trial entry were assigned to a dose level based on their pre‐trial therapeutic dose and regimen Treatment compliance: not stated |
|
| Outcomes |
ADHD symptoms
Quality of life
|
|
| Notes | Sample size calculation: yes Ethics approval: yes Key conclusion of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: yes; 111 who had not received MPH before the study initially were enrolled into a dose‐titration study. One of these did not enrol in the randomised study because the 54‐mg dose was found to be ineffective Any withdrawals due to AEs: yes; 3 Funding source: ALZA Corporation Comments from review authors in July 2013: corresponded with first trial author, Wolraich, who answered all of our questions (Krogh 2013c [pers comm]). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Within each dose level, participants were randomly assigned equally to OROS‐MPH once/d, IR‐MPH (over‐encapsulated Ritalin) 3 times/d or placebo in a 3‐group parallel design. Stratified randomisation was conducted centrally at ALZA Corporation |
| Allocation concealment (selection bias) | Low risk | Double‐dummy |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind; double‐dummy |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind; double‐dummy |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | LOCF Selection bias (e.g. titration after randomisation → exclusion): no, but 59 discontinued due to lack of efficacy. |
| Selective reporting (reporting bias) | Unclear risk | Not able to get trial protocol |
Zeiner 1999.
| Study characteristics | ||
| Methods | Cross‐over trial with 2 interventions:
Phases: 7 weeks (3 weeks of medication, 1 week washout, 3 weeks of placebo) Zeiner 1995 (in Zeiner 1999): "extended treatment (mean duration 634 ± 130 days)" |
|
| Participants | Number of participants screened: 46 Number of participants included: 38 Number of participants followed up: 36 (36 boys, 0 girls) Number of withdrawals: 2 Diagnosis of ADHD: DSM‐III‐R or DSM‐IV (combined type (> 75%), hyperactive‐impulsive and inattentive types not stated) Age: mean 8.7 years (range 7‐11) IQ: mean 102 (range 79‐139) MPH‐naive: 100% Ethnicity: not stated Country: Norway Setting: outpatient clinic Comorbidity: ODD (23; 64%) Comedication: not stated Other sociodemographics: none Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to 1 of 2 possible drug condition orders of MPH (0.5 mg/kg) and placebo. 31 children received a morning dose of 10 mg or 15 mg (and 5 young boys received 7.5 mg) Mean MPH dosage: not stated; "0.55 mg/kg to 0.56 mg/kg daily across the entire duration of the extended treatment period" (Zeiner 1995 in Zeiner 1999) Administration schedule: capsules were given in 2 doses (at 8 am and 11.30 am) Duration of each medication condition: 3 weeks Washout before trial initiation: not relevant MPH‐naive: all Medication‐free period between interventions: 1 week Titration period: none Treatment compliance: no information |
|
| Outcomes |
ADHD symptoms
Assessments of the child were made during the last week of each trial period Non‐serious AEs
|
|
| Notes | Sample calculation: no
Ethics approval: no information Comment from trial authors
Key conclusions of trial authors
Comments from review authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no Any withdrawals due to AEs: no Funding: the Norwegian Medical Research Council, the Norwegian Public Health Association and the Legacy of Haldis and Josef Andresen Email correspondence with trial authors: May‐June 2014. We emailed Dr. Zeiner on 05 May 2014 and 02 June 2014 but never received a reply |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Children were randomly allocated to receive MPH first or placebo first |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "A randomized crossover, double‐blind design with methylphenidate and placebo". Placebo and MPH capsules that were used were identical, to ensure blindness to the drug condition |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "All raters were blind to the drug condition" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "The report from a teacher during one of the periods was missing for one child. Owing to technical problems or unwillingness to participate data were missing on some of the test measures [which measured side effects] for a few children" (Zeiner 1995 in Zeiner 1999) Selection bias: yes; only responders from the 7‐week trial were included in the extended‐treatment MPH group |
| Selective reporting (reporting bias) | Low risk | No selective outcome reporting |
Zeni 2009.
| Study characteristics | ||
| Methods | Randomised, cross‐over trial with 2 interventions:
Phases: 2; randomisation, cross‐over |
|
| Participants | Number of participants screened: 30 Number of participants included: 16 (9 boys, 7 girls) Number of participants followed up: 14 Number of withdrawals: 1 Diagnosis of ADHD: DSM‐IV (combined (78.6%), hyperactive (14.3%), inattentive (7.1%) out of n = 14) Age: mean 10.71 years (SD 1.86; range 8‐17) IQ: > 70 MPH‐naive: not stated Ethnicity: European‐Brazilian (71.4%), other (28.6%) Country: Brazil Setting: outpatient clinic Co‐morbidity: bipolar disorder (71.4%), borderline personality disorder (28.6%), anxiety disorder (57.1%), CD (57.1%), ODD (78.6%), psychosis (50%), out of n = 14 Co‐medication: aripiprazole (100%) Other sociodemographics: divorced parents (57.1%); socioeconomic level A + B + C: 92.9%; D + E: 7.1% Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to different possible drug condition orders of MPH (0.3 mg/kg for the 1st week, 0.7 mg/kg for the 2nd week) and placebo, both alongside aripiprazole Mean MPH dosage: 15 mg in the 1st week (10 mg in the morning and 5 mg in the afternoon), 35 mg in the 2nd week (20 mg in the morning and 15 mg in the afternoon) Administration schedule: twice/d (morning and afternoon) Duration of each medication condition: 2 weeks Washout before trial initiation: none (but recruited from 6‐week aripiprazole trial) Medication‐free period between interventions: not stated Titration period: none Treatment compliance: self‐report, mother’s report, pill counting in returned blister packs |
|
| Outcomes |
ADHD symptoms
General behaviour
Non‐serious AEs
|
|
| Notes | Sample calculation: no Ethics approval: yes Comments from trial authors
Key conclusions of trial authors
Exclusion of MPH non‐responders/children who have previously experienced AEs while taking MPH before randomisation: no. But only participants who reported improved borderline personality disorder in the previous aripiprazole trial were included. However, participants who reported that ADHD improved too much (as indicated by a score < 1.5 on the SNAP, 4th Edition) in the previous aripiprazole trial were excluded (see 'Exclusion criteria') Any withdrawals due to AEs: yes. 1; exacerbation of borderline personality disorder and ADHD requiring hospitalisation Funding source: research grants from Conselho Nacional de Desenvolvimento Cientifico e Tecnologico (CNPq, Brazil) (Grant 471761=03‐6) and Hospital de Clinicas de Porto Alegre (GPPG 03‐325). Aripiprazole was provided by Bristol‐Myers Squibb without restriction. Email correspondence with trial authors: May 2014. We obtained supplemental information regarding data. Trial authors provided us with data from the first period |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An independent third party randomly assigned participants to groups A and B |
| Allocation concealment (selection bias) | Low risk | A pharmacist packaged MPH and matching placebo in capsules, so they could not be differentiated by shape, colour, smell, weight or taste |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A pharmacist packaged MPH and matching placebo in capsules, so they could not be differentiated by shape, colour, smell, weight or taste |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | After all 4 assessments were completed, trial blinding was broken |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Analyses of primary and secondary outcome measures were performed using a mixed‐effects model (MEM) approach, which provides a flexible framework for analysis of repeated measures, while accounting for missing data (i.e. lost to follow‐up) Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Low risk | Outcomes reported according to protocol |
ABRS/ACRS/CARS: Conners' Abbreviated Rating Scale/Abbreviated Conners' Rating Scale/Conners' Abbreviated Rating Scale; ACTerRS: ADD/H: attention deficit disorder with hyperactivity Comprehensive Teacher Rating Scale; ADD: attention deficit disorder; ADD‐H: attention deficit disorder with hyperactivity; ADHD: attention deficit hyperactivity disorder; ADHD‐RS(‐IV/V): Attention Deficit Hyperactivity Disorder Rating Scale( 4th Edition/5th edition); ADI‐R: Autism Diagnostic Interview‐Revised; ADOS: Autism Diagnostic Observation Schedule; AEs: adverse events; ALT: alanine aminotransferase; AMP: amplitude of the sleep‐wake rhythm; ANOVA: analysis of variance; APRS: Abbreviated Parent Rating Scale; ASQ‐Teacher: attention deficit hyperactivity disorder Conners' Abbreviated Symptom Questionnaire for Teachers; ASQ‐parent: attention deficit hyperactivity disorder Conners' Abbreviated Symptom Questionnaire for parents; AST: aspartate aminotransferase
BMI: body mass index; BP: blood pressure; BRIEF: Behavior Rating Inventory of Executive Function;
CASS:S: Conners‐Wells Adolescent Self‐Report; CBCL: Child Behavior Checklist; CBT: cognitive behavioural therapy; CD: conduct disorder; CDC: Centers for Disease Control and Prevention; CDRS‐R: Children's Depression Rating Scale ‐ Revised; CGAS: Children’s Global Assessment Scale; CGI: Conners' Global Index; CGI(‐S): Clinical Global Impressions(‐Severity); CLAM: Conners, Loney and Milich Scale; CNS: central nervous system; CPRS(R‐S): Conners’ Parent Rating Scale (Revised, Short form); CRS: Conners' Rating Scale; C‐SSRS: Columbia Suicide Severity Rating Scale; CSHQ: Children’s or Adolescent Sleep Habits Questionnaire; CTRS(R‐S): Conners’ Teacher Rating Scale (Revised, Short form)
DBP: diastolic blood pressure; DCD: developmental co‐ordination disorder; DISC(‐4): Diagnostic Interview Schedule for Chldren (4th Edition); d‐MPH: dexmethylphenidate; d,l‐MPH: dex,levo‐threo‐methylphenidate; DR: delayed‐release; DR‐MPH: delayed‐release methylphenidate;DSM‐III:Diagnostic and Statistical Manual of Mental Disorders, Third Edition;DSM‐III‐R:Diagnostic and Statistical Manual of Mental Disorders, Third Edition, Revised; DSM‐IV:Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; DSM‐IV‐TR:Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision; DSM‐5:Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition;
ECG: electrocardiogram; EEG: electroencephalogram; ER: extended‐release; ER‐d‐MPH: extended‐release dexmethylphenidate; ER‐MAS: extended‐release mixed amphetamine sales; ER‐MPH: extended‐release methylphenidate; EqXL:Equasym;
FDA: Food and Drug Administration
HCG: human chorionic gonadotropin; HD: high‐dose; HD‐MPH: high‐dose methylphenidate;
ICD‐10:International Classification of Diseases, Tenth Revision; IR: immediate‐release; IR‐MPH: immediate‐release methylphenidate; ITT: intention‐to‐treat; IS: interdaily stability; IV: intradaily variability; IQ: Intelligence quotient
KBIT‐2: Kaufman Brief Intelligence Test; K‐SADS‐E/PL: Kiddie Schedule for Affective Disorders and Schizophrenia for School Age Children Epidemiological/Present and Lifetime version; ;
LA: long‐acting; LA‐MPH: long‐acting methylphenidate; LD: low‐dose; LD‐MPH: low‐dose methylphenidate; LDX: lisdexamphetamine; LLC: limited liability company; LOCF: last observation carried forward; L‐DOPA: levedopa; LTFU: loss to follow‐up; L5: the amount of activity during the 5 h with the lowest activity;
MAS: mixed amphetamine salts; MAOI: monoamine oxidase inhibitor; MD: moderate‐dose; MD‐MPH: moderate dose methylphenidate; MedDRA: Medical Dictionary for Regulatory Activities; MINI‐KID: Mini International Neuropsychiatric Interview for Children and Adolescents; MPH: methylphenidate; MPT: methylphenidate trial; MPH‐IR: Methylphenidate immediate release; MPH‐MLR: multilayer extended‐release bead methylphenidate capsule; MR: modified release; MR‐MPH: modified‐release methylphenidate; MTA: Multimodal Treatment Study of Children With ADHD;
NIH: National Institutes of Health; NIMH: National Institute of Mental Health; NNTB: number needed to treat for an additional beneficial outcome;
OCD: obsessive compulsive disorder; ODD: oppositional defiant disorder; ODT: orally disintegrating tablet; OROS‐MPH: osmotic‐controlled release oral delivery system methylphenidate; ORADUR‐MPH: long‐acting methylphenidate formulation; OTMP: Organisational, Time Management and Planning treatment;
PDD: pervasive developmental disorder; PICS: Parental Interview for Child Symptoms; PERMP: Permanent Product Measure of Performance; PREMB‐R AM: Parent Rating of Evening and Morning Behavior‐Revised, Morning; PREMB‐R PM: Parent Rating of Evening and Morning Behavior‐Revised, Evening; PSQI: Pittsburg Sleep Quality Inventory; PTS: placebo transdermal system; PTSD: post‐traumatic stress disorder
RCT: randomised clinical trial;
SA: short‐acting; SA‐MPH: short‐acting methylphenidate; SAERS: Serious Adverse Event Rating Scale; SCT: sluggish cognitive temp; SBP: systolic blood pressure; SD: standard deviation; SDX: serdexmethylphenidate; SDX/d‐MPH: serdexmethylphenidate/dexamphetamine‐methylphenidate; SE: standard error; SIB‐R: Scales of Independent Behavior Revised; SKAMP: Swanson, Kotkin, Agler, M‐Flynn, and Pelham Rating Scale; SNAP(‐IV): Swanson, Nolan and Pelham scale( 4th Edition); SODAS: spheroidal oral drug absorption system; SSRIs: selective serotonin reuptake inhibitors; STAI: Spielberger State‐Trait Anxiety Inventory; SWAN: Strength and Weakness of Attention‐Deficit/Hyperactivity Disorder‐symptoms and Normal Behavior Rating Scale;
TEAE: treatment emergent adverse event; TIB: total in‐bed time; TOTS: Loney’s Time on Task Scale; TRS: Teacher Rating Scale; TSH: thyroid stimulating hormone; TSSR: Tic Symptom Self‐Report Scale; TTI: Teacher Telephone Interview;
VADPRS: Vanderbilt ADHD Diagnostic Parent Rating Scale; VADTRS: Vanderbilt ADHD Diagnostic Teacher Rating Scale;
WASO: wake after sleep onset; WASI(‐II): Wechsler Abbreviated Scale of Intelligence( ‐ Second Edition); WBnumber: number of wake bouts; WBmean: mean wake bout time; WISC(‐II, III, IV, R): Wechsler Intelligence Scale for Children(‐2nd, 3rd, 4th, Revised edition); WRAT‐R: Wide Range Achievement Test;
XR: extended release;
YGTSS: Yale Global Tic Severity Scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ACTRN12608000059369 | Likely no relevant outcomes for our review as outcomes were structural imaging results: volume and activation of different brain regions |
| An 2013 | Likely no relevant outcome for our review as this was resting state brain function only |
| Anderson 2002 | Likely no relevant outcome for our review as this was functional magnetic resonance relaxometry only |
| Barkley 1988a | Likely no relevant outcomes for our review as outcomes were Home Situations Questionnaire, Parenting Stress Index, and Beck Depression Inventory |
| Barkley 1997 | Likely no relevant outcomes for our review as these were "2 questionnaires", and "Electronic apparatus" |
| Bart 2013 | Likely no relevant outcomes for our review as these were Movement Assessment Battery for Children – Second edition, and Online Continuous Performance Test |
| Bedard 2002 | Likely no relevant outcomes for our review as these were response interference, and Stroop Naming Speed |
| Bedard 2003 | Likely no relevant outcomes for our review as these were Parent Interview for Child Symptoms, Teacher Telephone Interview–IV, and Selective Stop‐Signal Task |
| Bedard 2004 | Likely no relevant outcomes for our review as these were Parent Interview for Child Symptoms; Teacher Telephone Interview–IV, Reading subtest/Wide Range Achievement Test ‐ 3, Word Attack and Word Identification subtests of the Woodcock Reading Mastery, and Test‐Revised Cambridge Neuropsychological Testing Automated Battery |
| Bedard 2007 | Likely no relevant outcomes for our review as these were focusing on working memory; Test of Word and Language Efficiency, Wide Range Achievement Test ‐ 3, Woodcock‐Johnson Tests of Achievement, and Cambridge Neuropsychological Test Automated Battery (CANTAB) Spatial Span |
| Beery 1994 | Likely no relevant outcomes for our review as these were focusing on behavioural management and behavioural disinhibition |
| Beery 2017 | Likely no relevant outcomes for our review as these was focusing on social behavior |
| Ben‐Pazi 2006 | Likely no relevant outcome for our review as this was focusing on hastening phenomena only |
| Bental 2008 | Likely no relevant outcomes for our review as these was focusing on reading measures |
| Beyer 2014 | Likely no relevant outcomes for our review as these were The Frankfurt Test and training of social affect |
| Bouziane 2019 | Likely no relevant outcome for our review as this was MRI measuring only |
| Brown 1984b | Likely no relevant outcome for our review as this was Children's Checking Task only |
| Buhrmester 1992 | Likely no relevant outcome for our review as this was focusing on prosocial behavior only |
| Campbell 1996 | Likely no relevant outcome for our review as this was reaction times on Tachistoscopic Task only |
| Carlson 1991 | Likely no relevant outcomes for our review as these were focusing on reaction times and other cognitive tasks |
| Carlson 1992 | Likely no relevant outcomes for our review as these were focusing on task and disruptive behaviour; academic work completion and accuracy |
| Cohen 2020 | Likely no relevant outcome for our review as this was focusing on Go/No‐Go Task only |
| Cox 2004b | Likely no relevant outcome for our review as this was focusing on driving performance ‐ measured by computer only |
| Cubillo 2014 | Likely no relevant outcome for our review as this were focusing on Stop Task combined with fMRI only |
| Cubillo 2020 | Likely no relevant outcome for our review as this was focusing on fMRI during Sustained Attention Task only |
| Dawson 1998 | Likely no relevant outcomes for our review as these were focusing on Mirsky's proposed factors of attention: sustained attention, focus/execute, encode, and stability of attention |
| De Sonneville 1991 | Likely no relevant outcomes for our review as these were focusing on specific attention function: sustained attention, information processing, response organization |
| DeVito 2008 | Likely no relevant outcomes for our review as these were focusing on Cambridge Gamble Task; measures of response inhibition and reflection‐impulsivity on the information Sampling Task |
| Dougherty 2016 | Likely no relevant outcomes for our review as these were focusing on Immediate Memory Task, GoStop Impulsivity Paradigm, Two Choice Impulsivity Paradigm |
| Evans 1986 | Likely no relevant outcomes for our review as these were focusing on verbal memory and learning |
| Fosco 2017 | Likely no relevant outcomes for our review as these were focusing on cognitive domains, including working memory, inhibitory control and attention |
| Fosco 2021 | Likely no relevant outcomes for our review as these were focusing on inhibitory control, visuospatial working memory, reaction time variability, and delay discounting |
| Fox 2014 | Likely no relevant outcomes for our review as these were focusing on memory tasks |
| Francis 2001 | Likely no relevant outcomes for our review as these were focusing story telling and story grammar analysis |
| Gan 1982 | Likely no relevant outcome for our review as this was focusing on performance on Paired Associate Learning task only |
| Golubchik 2018 | Likely no relevant outcome for our review as this was focusing on changes in school report cards only |
| González‐Carpio Hernández 2016 | Likely no relevant outcome for our review as this was focusing on creativity measurements only |
| Granger 1996 | Likely no relevant outcome for our review as this was focusing on social behaviour only |
| Grizenko 2010 | Likely no relevant outcomes for our review as these were focusing on academic behaviour; sustained attention; impulse inhibition control |
| Günther 2010 | Likely no relevant outcomes for our review as these were focusing on sustained attention measured by computer attention tests |
| Hadar 2020 | Likely no relevant outcomes for our review as these were focusing on Visuo‐Motor Attention Test and multiple cognitive tasks measuring auditory and visual executive functions |
| Halliday 1983 | Likely no relevant outcomes for our review as these were focusing on event‐related potentials |
| Hanisch 2004 | Likely no relevant outcomes for our review as these were focusing on computerised attention tasks |
| Hazel‐Fernandez 2006 | Likely no relevant outcome for our review as this was focusing on Paired Associates Learning task; Tower of Hanoi only |
| Helseth 2015 | Likely no relevant outcomes for our review as these were focusing on children’s rates of reinforcement for deviant peer behavior |
| Hinshaw 1989 | Likely no relevant outcome for our review as this was focusing on prosocial behaviour only |
| Hinshaw 1993 | Likely no relevant outcome for our review as this was focusing on antisocial behaviour only |
| Horowitz 2020 | Likely no relevant outcomes for our review as these were focusing on Sustained Attention to Response Task, N‐Back Task, Stroop Color and Word Task |
| Humphries 1979 | Likely no relevant outcome for our review as this was focusing on maze‐tracking performance only |
| Ishii‐Takahashi 2015 | Likely no relevant outcomes for our review as these were focusing on prefrontal haemodynamics measured by fNIRS |
| ISRCTN52376787 | Likely no relevant outcomes for our review as these were focusing on information about effects of MPH on cognitive function (including the possibility of cognitive toxicity) in children with ADHD, greater understanding of the underlying cognitive processes in ADHD, identification of potential cognitive deficits in ADHD. |
| JPRN‐UMIN000008831 | Likely no relevant outcomes for our review as these were focusing on fNIRS analysis and behavioral performance |
| JPRN‐UMIN000027533 | Likely no relevant outcome for our review as this was focusing on rsfMRI only |
| King 2009a | Likely no relevant outcomes for our review as these were focusing on social information processing |
| King 2009b | Likely no relevant outcomes for our review as these were focusing on laboratory provocation task, measuring hostile, instrumental, reactive, and proactive aggression |
| Kobayashi 2020 | Likely no relevant outcomes for our review as these were focusing on haemodynamic changes measured by fNIRS during observation of happy and angry facial expressions |
| Kowalczyk 2019 | Likely no relevant outcome for our review as these were focusing on sustained attention/vigilance task in a 3T MRI scanner only |
| Lange 2007 | Likely no relevant outcome for our review as these were focusing on reaction time, alertness, vigilance, and divided attention |
| Leitner 2007b | Likely no relevant outcomes for our review as these were focusing on gait; stride to stride variability, memory, visual‐spatial, verbal, and attention domains |
| Levi‐Shachar 2020 | Likely no relevant outcomes for our review as these were focusing on Dimensional Change Card Sort Test (DCCS) and the Flanker Inhibitory Control and Attention Test, Theory of Mind tests, oxytocin levels |
| Luman 2015 | Likely no relevant outcomes for our review as these were focusing on time production task, accuracy and response latency in an instrumental learning task |
| Malone 1988 | Likely no relevant outcomes for our review as these were focusing on word processing, reaction time, and cognitive decision task |
| Malone 1993 | Likely no relevant outcome for our review as this was focusing on impulsive responding only |
| Malone 1994 | Likely no relevant outcome for our review as this was focusing on right hemisphere dysfunction only |
| Martin 2007 | Likely no relevant outcomes for our review as these were focusing on depression; addiction rate; Continuous Performance Task; heart rate and blood pressure |
| Mazzetti 2022 | Likely no relevant outcome for our review as this was focusing on spatial attention task only |
| Mehta 2004 | Likely no relevant outcome for our review as this was focusing on working memory only |
| Merrill 2022 | Likely no relevant outcome for our review as these were focusing on novel mindwandering sustained attention to response task only |
| Milich 1989 | Likely no relevant outcome for our review as this was focusing on Continuous Performance Task only |
| Milich 1991 | Likely no relevant outcome for our review as this was focusing on task persistence only |
| Mizuno 2021 | Likely no relevant outcomes for our review as these were focusing on Sustained attention examined using a continuous performance test. MRI scans |
| Morris 2022 | Likely no relevant outcomes for our review as these were focusing on electrocardiographic and electrodermal outcomes |
| Nagashima 2015 | Likely no relevant outcomes for our review as these were focusing on oxy‐hb changes measured with fNIRS during Go/No‐Go Task |
| NCT00485797 | Likely no relevant outcome for our review as this was focusing on postural sway measures |
| NCT00778310 | Likely no relevant outcomes for our review as these were focusing on brain oxygenation level‐dependent signal in the fusiform gyrus and the amygdala on Concerta vs placebo |
| NCT02318017 | Likely no relevant outcomes for our review as these were focusing on electroencephalography data during neurocognitive tasks |
| NCT03788902 | Likely no relevant outcomes for our review as these were focusing on Theory of Mind, Faux‐Pas Recognition Test, and salivary oxytocin levels |
| NCT04349917 | Likely no relevant outcomes for our review as these were focusing on Go/No Go Task and fMRI |
| Novak 1995 | Likely no relevant outcomes for our review as these were focusing on reaction time and visuospatial attention |
| O'Toole 1997 | Likely no relevant outcome for our review as this was focusing on non‐verbal learning only |
| Pakdaman 2018 | Likely no relevant outcome for our review as this was focusing on neuropsychological tests only |
| Peeke 1984 | Likely no relevant outcome for our review as this was focusing on verbal information processing only |
| Pelham 1985 | Likely no relevant outcomes for our review as these were focusing on classroom academic and social behaviour |
| Pelham 1990b | Likely no relevant outcomes for our review as these were focusing on attention during baseball game; on task behaviour; and ability to answer question about the status of the game |
| Pelham 1992 | Likely no relevant outcomes for our review as these were self‐reported attribution and evaluation of behaviour |
| Pelham 1997 | Likely no relevant outcomes for our review as these were focusing on performance, self‐evaluation, persistence, and attributions on cognitive task |
| Pelham 2001b | Likely no relevant outcomes for our review as these were focusing on a large number of different measures of behaviour |
| Pelham 2017a | Likely no relevant outcomes for our review as these were focusing on daily contact reports, Good Day/Bad Day Questionnaire, and self‐perceived medication status |
| Pelham 2017b | Likely no relevant outcomes for our review as these were focusing mother and child relationship |
| Rapport 1995 | Likely no relevant outcome for our review as this was focusing on Paired Associates Learning task only |
| Ratzon 2017 | Likely no relevant outcomes for our review as these were focusing on driving skills tested by the STISIM Drive simulator |
| Richardson 1988 | Likely no relevant outcome for our review as this was focusing on reading achievement only |
| Rubia 2003 | Likely no relevant outcome for our review as this was focusing on motor timing only |
| Rubinson 2019 | Likely no relevant outcomes for our review as these were focusing on Go/No‐Go Task and electroencephalography |
| Sangal 2006 | Likely no relevant outcome for our review as this was focusing on auditory amplitude only |
| Silk 2012 | Likely no relevant outcome for our review as this was focusing on neural substrates only |
| Silk 2014 | Likely no relevant outcome for our review as this was focusing on a visual attention task only |
| Silk 2017 | Likely no relevant outcome for our review as this was focusing on rsfMRI only |
| Slama 2015 | Likely no relevant outcomes for our review as these were focusing on Continuous Performance Test and Counting Stroop Task |
| Smith 2013 | Likely no relevant outcome for our review as this was focusing on fMRI only |
| Solanto 1986 | Likely no relevant outcomes for our review as these were focusing on attention during play measured by locomotor activity; Children's Checking Test; and fine motor control |
| Solanto 1997 | Likely no relevant outcome for our review as this was focusing on Continuous Performance Test only |
| Srinivas 1992 | Likely no relevant outcomes for our review as these were focusing on sustained attention measured by computer attention tests |
| Strand 2012 | Likely no relevant outcome for our review as this was focusing on working memory only |
| Stray 2009 | Likely no relevant outcome for our review as this was focusing on motor functions only |
| Sunohara 1997 | Likely no relevant outcome for our review as this was focusing on event‐related potentials only |
| Sutoko 2019 | Likely no relevant outcome for our review as this was focusing on fNIRS during Go/No‐Go Task only |
| Swanson 1993 | Likely no relevant outcome for our review as this was focusing on impulsive responding |
| Szobot 2003 | Likely no relevant outcome for our review as this was focusing on cerebral blood flow only |
| Tannock 2000 | Likely no relevant outcomes for our review as these were focusing on naming speed and academic measures |
| Teicher 2003 | Likely no relevant outcomes for our review as these were focusing on computerised Contiuous Performance Test and fMRI |
| Teicher 2006 | Likely no relevant outcome for our review as this was focusing on rate‐dependent behavioural effects only |
| Teicher 2007 | Likely no relevant outcome for our review as this was focusing on McLean Motion Attention Test only |
| Tillery 2000 | Likely no relevant outcome for our review as this was focusing on auditory performance only |
| Trommer 1991 | Likely no relevant outcome for our review as this was focusing on Go‐No‐Go performance only |
| Tsang 2012 | Likely no relevant outcomes for our review as these were focusing on cognitive tasks, and emotional functions |
| Tucha 2006 | Likely no relevant outcome for our review as this was focusing on reaction time tasks only |
| Van den Driessche 2017 | Likely no relevant outcome for our review as this was focusing on mind blanking during Go/No‐Go Task only |
| Van Lith 2018 | Likely no relevant outcome for our review as this was focusing on region‐of‐interest analysis of fMRI data during fear learning only |
| Verbaten 1994 | Likely no relevant outcome for our review as this was focusing on Continuous Performance Test only |
| Waschbusch 2007 | Likely no relevant outcome for our review as this was focusing on academic‐oriented tasks only |
| Whalen 1987 | Likely no relevant outcome for our review as this was focusing on social behaviour only |
| Wong 2012 | Likely no relevant outcome for our review as this was focusing on Sternberg Working Memory fMRI Task only |
| Wu 2017 | Likely no relevant outcome for our review as this was focusing on brain activation during verbal working memory task only |
| Yarmolovsky 2017 | Likely no relevant outcome for our review as this was focusing on executive control test ‐ Stroop‐like task only |
ADHD: attention deficit hyperactivity disorder; fMRI: functional magnetic resonance imaging; fNIRS: functional near‐infrared spectroscopy; MPH: methylphenidate; MRI: magnetic resonance imaging; n: number; oxy‐hb: oxygen‐haemoglobin; rsfMRI: resting state functional magnetic resonance imaging; STISIM Drive: Systems Technology, Inc. Simulation Drive; 3T: 3 Tesla
Characteristics of studies awaiting classification [ordered by study ID]
Drtílková 1997.
| Methods | 2‐week RCT |
| Participants | 118 children |
| Interventions |
|
| Outcomes | |
| Notes | Article is written in Czech. We have not yet been able to have the article translated. |
Wang 2020.
| Methods | No information currently available |
| Participants | No information currently available |
| Interventions | No information currently available |
| Outcomes | No information currently available |
| Notes | No access with institutional ID. Authors contacted but have yet to reply |
ID: identifier; MPH: methylphenidate;RCT: randomised clinical trial
Characteristics of ongoing studies [ordered by study ID]
ChiCTR1800014945.
| Study name | Public title: Effect of the intervention combining medicine and education on school‐age children with attention deficit hyperactivity disorder Scientific title: as above |
| Methods | Randomised controlled parallel‐group trial Study duration: 6 months |
| Participants | Sample size (target): 120 Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes |
ADHD symptoms
Non‐serious AEs
Timing of outcome assessment not reported |
| Starting date | 22 January 2018‐1 January 2019 |
| Contact information | Name: Yu Guangjun Telephone: + 86 18917762998 Email: gjyu@shchildren.com.cn |
| Notes | Sponsor: School of Public Health, Shanghai Jiao Tong University Declaration of interests: not reported |
EUCTR2007‐004664‐46‐NL.
| Study name | Public title: not reported Scientific title: Influence of methylphenidate on sleep and circadian rhythm in children with attention‐deficit/hyperactivity disorder (ADHD) |
| Methods | Double‐blind, randomised controlled, parallel‐group trial Study duration: 14 months |
| Participants | Sample size (target): 140 Inclusion criteria
Exclusion criteria:
|
| Interventions |
|
| Outcomes |
Non‐serious AEs
Timing of outcome assessment not reported |
| Starting date | 29 July 2008 (date of registration). Starting date not reported |
| Contact information | Not reported |
| Notes | Sponsor: no sponsor Declaration of interests: not reported |
EUCTR2008‐001291‐71‐DE.
| Study name | Public title: not reported Scientific title: Electrophysiological correlates of putative endophenotypes of attention‐deficit/hyperactivity disorder (ADHD) |
| Methods | Single‐blind, randomised controlled, parallel‐group trial Study duration: 3 years |
| Participants | Sample size (target): 120 Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes |
Non‐serious AEs
Timing of outcome assessment not reported |
| Starting date | 23 July 2008 (date of registration). Starting date not reported |
| Contact information | Not available |
| Notes | Sponsor: University of Wuerzburg Declaration of interest: not reported |
EUCTR2008‐004425‐42‐NL.
| Study name | Public title: not reported Scientific title: Neurocognitive testing in children with ADHD |
| Methods | Randomised controlled, double blind cross‐over trial Study duration: 3 months |
| Participants | Sample size (target): 20 Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes |
ADHD symptoms
Non‐serious AEs
Timing of outcome assessments not reported |
| Starting date | 15 September 2008 (date of registration). Start date not reported |
| Contact information | Not reported |
| Notes | Sponsor: Centre for Human Drug Research Decleration of interests: not reported |
EUCTR2020‐003660‐11‐NL.
| Study name | Public title: Methylphenidate long‐term study Scientific title: Do effects of methylphenidate decline after long‐term use? A double‐blind, placebo‐controlled cross‐over study of effects of methylphenidate on cognitive functioning and real world behavior in treatment naive children compared to effects after 9 months of treatment in clinical practice |
| Methods | Randomized, double‐blind, placebo‐controlled cross‐over trial Study duration: 3 years |
| Participants | Sample size (target): 60 Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes |
General behaviour
Assessed at baseline, week 2 and week 40 |
| Starting date | 21 December 2020 (entered into trial registry). Start date not reported |
| Contact information | Name: Andrea Dietrich Affiliation: Accare, Lübeckweg 2, Groningen, 9723 HE, Netherlands Telephone: + 310613807225 |
| Notes | Sponsor: Accare Declaration of interest: not reported |
IRCT138804132000N2.
| Study name | Public title: Neurofeedback in the treatment of attention deficit hyperactivity disorder Scientific title: Effectiveness of neurofeedback versus methylphenidate in the treatment of children with attention deficit hyperactivity disorder |
| Methods | Parallel RCT Study duration: 3 months |
| Participants | Sample size (target): 90 Inclusion criteria:
Exclusion criteria:
|
| Interventions |
MPH dose will be adjusted based on optimal dose by a child and adolescent psychiatrist who visits all the participants. They will be assessed 1 week before the start and each month through the study. The last assessment will be on a week after the completion of the research. The rater will be blind to the groups of participants. |
| Outcomes |
ADHD symptoms
|
| Starting date | 22 December 2014 |
| Contact information | Name: Dr Javad Alaghband Rad, Associate Professor, Child & Adolescent Psychiatrist Affiliation: Tehran University of Medical Sciences, Roozbeh Hospital, South Kargar Street, Tehran Telephone: + 98 21 5541 9151 Email: alaghbandrad@gmail.com |
| Notes | Sponsor: Behavioral Sciences Research Center, Shahid Beheshti University of Medical Sciences Declaration of interests: not reported |
IRCT201701131556N94.
| Study name | Public title: Saffron versus methylphenidate in the treatment of attention deficit/hyperactivity disorder Scientific title: Saffron versus methylphenidate in the treatment of attention deficit/hyperactivity disorder: a double‐blind and randomized trial |
| Methods | Parallel RCT Study duration: 6‐weeks |
| Participants | Sample size (target): 40 Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes |
ADHD symptoms
|
| Starting date | 20 January 2017 |
| Contact information | Name: Shahin Akhondzadeh Telephone: + 98 21 5541 2222 Email: s.akhond@sina.tums.ac.ir |
| Notes | Sponsor: Tehran University of Medical Sciences Declaration of interests: not reported |
IRCT20190317043079N.
| Study name | Public title: The effect of neurofeedback/biofeedback on brain’s waves amplitude and attention deficit in children with ADHD Scientific title: The effectiveness of neurofeedback and heart rate variability training on QEEG baseline and sustained attention in children with ADHD |
| Methods | A randomised controlled parallel single‐blinded study without placebo Study duration: 12 weeks + 6 months follow‐up |
| Participants | Sample size (target): 160 Inclusion criteria
Exclusion criteria
|
| Interventions | There are 5 interventions in this study, the 2 that can be used for this review are:
|
| Outcomes |
ADHD symptoms
Timing of outcome assessments not reported |
| Starting date | 9 April 2021 |
| Contact information | Name: Soheila Hosseinzadeh Afilliation: Babol University of Medical Sciences 4717647745 Babol Iran (Islamic Republic of) Telephone: +98 11 3219 9592 Email: hoseinzadeh_soheila@yahoo.com |
| Notes | Sponsor: Babol University of Medical Sciences Declaration of interests: not reported |
Müller 2021.
| Study name | Public title: Methylphenidate for attention‐deficit/hyperactivity disorder in patients with Smith‐Magenis syndrome: protocol for a series of N‐of‐1 trials Scientific title: as above |
| Methods | Each trial will be randomised placebo‐controlled and double‐blind with multiple cross‐overs within a single patient. The trial will consist of a baseline period, a dose titration phase and 3 cycles each consisting of 1 period of placebo treatment and 1 period of MPH treatment both followed by a 1‐week washout period. Study duration: each trial will last for 14 weeks with a 3‐month follow‐up. |
| Participants | Sample size (target): 6 Inclusion criteria
Exclusion criteria
|
| Interventions |
Administered twice/d in a cross‐over design with a 1‐week washout period. |
| Outcomes |
ADHD symptoms
Numbers of serious AEs
Non‐serious AEs
|
| Starting date | Starting date not reported |
| Contact information | Email: a.m.vaneeghen@amsterdamumc.nl |
| Notes | Sponsor: the trial is financially sponsored by the Amsterdam UMC and healthcare institution’s Heeren Loo. Declaration of interests: no competing interest declared This is a published protocol for a series of N‐of‐1 trials. |
NCT00141050.
| Study name | Public title: Safety and efficacy study of dexmethylphenidate in children with ADHD Scientific title: as above |
| Methods | Randomised controlled double‐blind, cross‐over trial Study duration: not reported |
| Participants | Sample size (actual): 90 Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes |
ADHD symptoms
Total number of serious AEs
General behaviour
Non‐serious AEs
|
| Starting date | May 2005 (registration date). Starting date not reported |
| Contact information | Name: Matthew Brams, MD (Principal Investigator) Affiliation: Bayou City Research |
| Notes | Sponsor: Novartis Pharmaceuticals Declaration of interest: not reported |
NCT00254878.
| Study name | Public title: Placebo‐controlled comparison of two different brands of modified‐release oral dosage forms regarding safety and efficacy in children with attention deficit hyperactivity disorder (ADHD) aged 6‐14 Scientific title: A 7‐week multicenter, double‐blind, randomized, placebo‐controlled cross‐over evaluation of the efficacy and safety of two different brands of modified‐release oral dosage forms of methylphenidate‐hcl (20 mg, qd) in children with attention deficit hyperactivity disorder (ADHD) aged 6‐14 |
| Methods | Randomised controlled double‐blind, cross‐over trial Study duration: 7 weeks |
| Participants | Sample size: 130 (actual) Inclusion criteria:
Exclusion criteria:
|
| Interventions |
|
| Outcomes |
ADHD symptoms
Total numbers of serious AEs
General behaviour
Non‐serious AEs
|
| Starting date | October 2005 |
| Contact information | Not reported |
| Notes | Sponsor: Novartis Pharmaceuticals Declaration of interests: not reported |
NCT00414921.
| Study name | Public title: Preschool supplement to clonidine in ADHD (Kiddie‐CAT) Scientific title: as above |
| Methods | Randomised controlled parallel‐group trial Study duration: 16 weeks |
| Participants | Sample size (actual): 30 Inclusion criteria:
Exclusion criteria
|
| Interventions |
|
| Outcomes |
ADHD symptoms
Quality of life
Non‐serious AEs
|
| Starting date | September 2003 |
| Contact information | Only names of primary investigators available: Floyd Randy Sallee, MD/PhD; Oscar Bukstein, MD; Donna Palumbo, PhD; William Pelham, PhD |
| Notes | Sponsor: University of Cincinnati and National Institute of Neurological Disorders and Stroke (NINDS) Declaration of interests: not reported |
NCT00446537.
| Study name | Public title: Procedural learning in participants with ADHD Scientific title: not reported |
| Methods | Randomised controlled cross‐over trial Study duration: not reported |
| Participants | Sample size: not reported Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes | No outcomes reported |
| Starting date | Not reported |
| Contact information | Name: Esther Adi‐Japha, PhD Telephone: 972‐3‐5318704 Email: japhae@mail.biu.ac.il |
| Notes | Sponsor: Shaare Zedek Medical Center Declaration of interests: not reported |
NCT00485550.
| Study name | Public title: Comparison of atomoxetine plus either comparator or placebo in children with ADHD who haven't responded to stimulant therapy Scientific title: A randomized, double‐blind comparison of atomoxetine hydrochloride augmented with either extended‐release methylphenidate Hydrochloride (Concerta‐TM) or placebo in children with attention‐deficit/hyperactivity disorder (ADHD) who have not responded to stimulant mono therapy |
| Methods | Double‐blind, parallel‐group trial Study duration: not reported |
| Participants | Sample size (actual): 14 Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes |
ADHD symptoms
Numbers of serious AEs
Non‐serious AEs
|
| Starting date | January 2004 |
| Contact information | Telephone: 1‐877‐CTLILLY (1‐877‐285‐4559) or 1‐317‐615‐4559 Monday to Friday 9 AM to 5 PM Eastern time (UTC/GMT ‐ 5 h, EST) |
| Notes | Sponsor: Eli Lilly and Company Declaration of interests: not reported |
NCT02807870.
| Study name | Public title: Early interventions in children with attention deficit/hyperactivity disorder Scientific title: Early interventions in children with attention deficit/hyperactivity disorder: randomized controlled trial comparing methylphenidate parental training in treating preschool children with attention deficit /hyperactivity disorder |
| Methods | Randomised, double‐blind, parallel controlled trial Study duration: 8 weeks |
| Participants | Sample size (actual): 153 Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes |
ADHD symptom severity
General behaviour
Quality of life
|
| Starting date | June 2016 |
| Contact information | Name: Guilherme V Polanczyk (PI) Affiliation: University of Sao Paulo Medical School |
| Notes | Sponsor: University of Sao Paulo, Conselho Nacional de Desenvolvimento Científico e Tecnológico Declaration of interest: not reported |
Verlaet 2017.
| Study name | Public title: Effect of Pycnogenoll® on ADHD Scientific title: Effect of Pycnogenoll® on attention‐deficit hyperactivity disorder (ADHD): study protocol for a randomised controlled trial |
| Methods | Randomised, double‐blind, placebo‐ and active treatment‐controlled multicentre trial, with 3 parallel treatment arms Study duration: 10 weeks |
| Participants | Sample size (actual): 144 Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes |
ADHD symptoms
General behaviour
Non‐serious AEs
|
| Starting date | 1 September 2017 |
| Contact information | Name: Nina Hermans, PhD Affiliation: Department of Pharmaceutical Sciences, Laboratory of Nutrition and Functional Food Science, University of Antwerp, Belgium Email: Annelies.verlaet@uantwerpen.be |
| Notes | Sponsor: Fund for Scientific Research Flanders (Fonds Wetenschappelijk Onderzoek (FWO); FWO MAND 2013 ‐ 11U8316N 5 W) Declaration of interests: no competing interests declared |
ADD: attention deficit disorder; ADHD: attention deficit hyperactivity disorder; AE: adverse event; ARI: Affective Reactivity Index scale; ASA: acetylsalicylic acid; ASQ(‐T)/(‐P): Conners' Abbreviated Symptom Questionnaire (for teachers/parents); CD: conduct disorder; CGAS: Child Global Assessment Scales; CGI: Clinical Global Impressions; CPRS: Conners' Parent Rating Scale; CPT: Continuous Performance Test; d‐MPH: dexmethylphenidate; DSM(‐IV/‐5):Diagnostic and Statistical Manual of Mental Disorders (‐Fourth Edition/Fifth Edition);ECG: electroencephalogram; ER‐MPH: extended‐release methylphenidate; EST: Eastern Standard Time; GMT: Greenwich Mean Time; IDS‐2: Intelligence and Development 2nd Version; IR‐MPH: immediate‐release methylphenidate; IQ: intelligence quotient; K‐SADS‐PL: Kiddie Schedule for Affective Disorders and Schizophrenia for School Age Children Present and Lifetime version; MAOI: monoamine oxidase inhibitor; MAP‐DB: Multidimensional Assessment Profile of Disruptive Behavior; MPH: methylphenidate; ODD: oppositional defiant disorder; PI: principal investigator PICS: Parent Interview for Child Symptoms; QEEG: quantitative electroencephalography; RCT: randomised controlled trial; SBP: systolic blood pressure; SEQ: Social‐Emotional Questionnaire; SMS: Smith ‐ Magenis Syndrome; SNAP(‐IV): Swanson, Nolan and Pelham scale (Fourth Edition); UMC: University Medical Centre; UTC: Universal Time Coordinated; WISC‐III‐R: Wechsler Intelligence Scale for Children; 8‐OHdG: 8‐hydroxy‐2′‐deoxyguanosine
Differences between protocol and review
Methods
Criteria for considering studies for this review
Types of studies
We split the review into two systematic reviews to make the review more manageable. The Storebø 2015a review deals with benefits and harms of methylphenidate as reported by RCTs. Storebø 2018b assessed the risk of harms based on the findings of non‐randomised studies.
Types of participants
We decided to include trials in which at least 75% of participants were 18 years of age or younger, and the mean age of the trial population was 18 years of age or younger. We included two trials with such participants. These two trials included a few participants at 19 and 20 years of age and we thought it was better to include these trials than to exclude them. The effects of methylphenidate intervention on any outcome did not change when these two trials were removed from the analysis.
We decided to include trials in which at least 75% of participants had a normal intellectual quotient (IQ > 70). We included four trials with such participants. These four trials included a few participants with IQ below 70 and we thought it was better to include these trials than to exclude them. The effects of methylphenidate intervention on any outcome did not change when these four trials were removed from the analysis.
Types of outcome measures. Duration of studies
We changed the subdivision of duration from short term (≤ 6 months), medium term (6 to 12 months) and long term (> 12 months) to short term (≤ 6 months) and long term (> 6 months) because no trials had a duration of between 6 and 12 months. Only one trial (Jensen 1999 (MTA)), provided data on a duration longer than six months (14 months). We included the change in duration classification in analyses of ADHD symptoms and general behaviour.
Search methods for identification of studies Electronic searches
We did not search OpenGrey as this database closed down in 2020. We did not contact the medical authorities in the European Union for information about beneficial and adverse events. We did not ask for access to security updates and risk management plans of pharmaceutical companies due to lack of time and resources.
Data collection and analysis
Selection of studies and data extraction and management
More review authors than stated in the protocol screened titles and abstracts, extracted data, entered data into Review Manager 5 and conducted statistical analyses in Review Manager 5 (Review Manager 2020). More authors were added to the review as it became a bigger review than we expected at the protocol stage.
Measures of treatment effect. Continuous data
For the primary outcome of teacher‐rated attention deficit hyperactivity disorder (ADHD) symptoms, we recalculated the standardised mean difference (SMD) as mean difference (MD) on the ADHD‐Rating Scale (DuPaul 1991a), to check whether our result exceeded the minimum clinically important difference (MCID; Zhang 2005), for this specific rating scale. This was not stated in the protocol (Storebø 2012).
For the secondary outcome of quality of life, we recalculated the SMD as MD on the Child Health Questionnaire (Landgraf 1998), to check whether our results exceeded the MCID (Rentz 2005) for this specific rating scale. This was not stated in the protocol (Storebø 2012).
Dealing with missing data
We tried to obtain missing data by contacting the authors of the trials that we included in this review. When we were not able to obtain missing data, we conducted the analyses using the available (incomplete) data. We had intended to assess the impact of missing data by applying intention‐to‐treat as well as 'best‐case scenario' and 'worst‐case scenario' analyses. We could not use 'best‐case scenario' and 'worst‐case scenario' analyses in our assessment of benefits as there were no dichotomous outcomes. We decided not to use 'best‐case scenario' and 'worst‐case scenario' analyses in our assessment of adverse events, because we evaluated these analyses to be imprecise due to the high number of trials not reporting adverse events, and due to the high number of dropouts in the trials reporting adverse events. Moreover, we were unable to conduct intention‐to‐treat analyses for continuous outcomes due to lack of data for imputing means.
Data synthesis. Heterogeneity‐adjusted required information size and Trial Sequential Analysis
We performed a Trial Sequential Analysis on the total number of serious adverse events and on the total number of non‐serious adverse events only, as they were the only outcomes with dichotomous data with a substantial number of outcomes. Trial Sequential Analysis can be conducted on individual types of adverse events, but for this, the accrued information would represent a minute fraction of the required information size (RIS). We were not able to conduct a Trial Sequential Analysis for teacher‐, independent assessor‐ or parent‐rated outcomes, as the programme can be used only for MDs, not for SMDs.
Subgroup analysis and investigation of heterogeneity
We planned few subgroup analyses in our protocol. Due to large methodological and clinical heterogeneity in the included trials, we decided to conduct several post‐hoc subgroup analyses.
Types of scales (e.g. Conners' Teacher Rating Scale (CTRS; Conners 1998a) compared to Strengths and Weaknesses of ADHD Symptoms and Normal Behavior (SWAN) Scale (Swanson 2006). We did this subgroup analysis to test the potential differences in effect estimates between the numerous scales measuring comparable content.
Dose of methylphenidate (low dose (≤ 20 mg/d or ≤ 0.6 mg/kg/day) compared to moderate/high dose (> 20 mg/day or > 0.6 mg/kg/day)). We did this subgroup analysis to test the potential differences in effect estimates between doses, as this is very relevant for clinicians and patients.
Duration of treatment (short‐term trials (≤ 6 months) compared to long‐term trials (> 6 months)). We did this subgroup analysis to test the potential differences in effect estimates between short‐ compared to long‐term trials.
Trial design (parallel‐group trials compared to cross‐over trials (first period data and endpoint data)). We conducted this subgroup analysis as we pooled first‐period data with parallel‐group data and we used end‐of‐period data from cross‐over trials without adjusting for unit of analysis error.
Medication status before randomisation (medication‐naive (> 80% of included participants were medication‐naive) compared to not medication‐naive (< 20% of included participants were medication‐naive)). We did this subgroup analysis to test the potential differences in effect estimates between medication‐naïve and not medication‐naïve participants, as this is very relevant for clinicians and patients.
Risk of bias (trials at low risk of bias compared to trials at high risk of bias). We did this subgroup analysis to test the potential differences in effect estimates between trials at low risk of bias compared to those at high risk of bias.
Cohort selection bias (trials with enrichment design compared to trials without enrichment design). We did this subgroup analysis to test the potential differences in effect estimates between the trials that had used the enrichment design to those that did not use this design.
Vested interest (trials at high or unclear compared to trials at low risk of vested interests). There is empirical evidence showing that trials funded by industry might overestimate the benefits compared to trials not funded by industry and therefore we wanted to do this subgroup analysis.
Type of control group (trials with placebo control group compared to trials with no‐intervention control group). We wanted to test the impact of choice of control group on estimated intervention effects.
Differences between this update and the original review
Methods
Search methods for identification of studies. Electronic searches
We updated the search strategy to include additional brand names for methylphenidate. Instead of conducting two separate searches for efficacy and adverse effects, we combined these into a single search. We also added a new source of systematic reviews (Epistemonikos) for reference list checking.
Data collection and analysis. Assessment of risk of bias in included studies. Vested interests
We did not evaluate vested interest as a risk of bias domain in this update as this is not recommended by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Results
Description of studies
During this update, we found that one of the included trials was actually a subpublication of another trial (Bhat 2020). Therefore, the number of trials from the 2015 version is only 184 trials. Furthermore, we excluded one of the studies awaiting classification due to ineligible study design. Two studies that were included in the 2015 review based on reports of preliminary data had their study ID changed to match the primary reference included in the present version (Bhat 2020; Hawk 2018). One additional trial had its primary reference and study ID changed for naming consistency (Wigal 2011).
Included studies
In the 2015 version of this review (Storebø 2015b), we did not describe the trials that did not contribute to the analyses because they had no usable data. We have now added this information to the Included studies section and also made reference to these trials in the 'Main results' section of the abstract and additional Table 3.
Risk of bias in included studies
We did not assess all trials included in the 2015 review for enrichment (evaluated under the 'Notes' heading in all inclusion tables) and 'Selection bias' (evaluated under incomplete outcome data bias in all bias assessment tables). However, in this update, we reassessed all trials to include both of these assessments consistently throughout the review.
Contributions of authors
OJS has overall responsibility for the review and is a guarantor for the review.
OJS developed the design of the review and was responsible for the co‐ordination of the review.
CG, ES and MZ updated the background sections of the review.
OJS, HEC, JPS, JPR, MROS, PDR, and CMLH selected the studies.
MROS, CMLH, JPR, JPS, MS, and OJS extracted data and evaluated risk of bias.
OJS and HEC assessed the certainty of the body of evidence.
OJS, CGl and HEC interpreted the data.
OJS and CGl developed the analytical strategy.
MS, HEC, JPR, JPS, MROS, and OJS entered data into Review Manager 5.
OJS, MS, RK, HEC, JPR, and MROS conducted the statistical analysis.
All review authors participated in discussion and writing of the final review.
Sources of support
Internal sources
-
Psychiatric Research Unit, Region Zealand Psychiatry, Roskilde, Denmark
Ole Jakob Storebø, Johanne Pereira Ribeiro, Julie Schaug, Maja Rosenberg Overby Storm, and Erik Simonsen worked on this review during office hours
-
Copenhagen Trial Unit, Centre for Clinical Intervention Research, Copenhagen University Hospital, Denmark
Christian Gluud worked on this review during office hours
External sources
-
None, Other
N/A
Declarations of interest
Ole Jakob Storebø: works at Psychiatric Research Unit, Region Zealand Psychiatry, Denmark. He is an Editor for Cochrane Developmental, Psychosocial and Learning Problems (CDPLP) but was not involved in the editorial process for this review. He is also an Editor‐in‐Chief for the Scandinavian Journal of Child and Adolescent Psychiatry and Psychology. He has declared that he has no conflicts of interest.
Maja Rosenberg Overby Storm: has declared that she has no conflicts of interest.
Johanne Perieira Ribeiro: has declared that she has no conflicts of interest.
Christel‐Mie‐Lykke Huus: has declared that she has no conflicts of interest.
Pernille Darling Rasmussen: has declared that she has no conflicts of interest.
Julie Perrine Schaug: has declared that she has no conflicts of interest.
Henriette Edeman Callesen: has declared that she has no conflicts of interest.
Maria Skoog: has declared that she has no conflicts of interest.
Camilla Groth works at the Children's Department at Hillerød Hospital, Denmark, where she conducts paediatric clinical research. She has declared that she has no conflicts of interest.
Morris Zwi (MZ) is a Child and Adolescent Psychiatrist working part time in private practice; previously, he worked exclusively for the NHS for 32 years, before retiring. He is a former Editor for CDPLP and occasionally is consulted by the group for his expertise; however, he was not involved in the editorial process for this review and it has been over one year since he did any editorial work. MZ declares a payment from the Paediatric Medicines Expert Advisory Board of the Medicines and Healthcare Products Regulatory Agency for his attendance at their monthly/bimonthly (i.e. every two months) meetings.
Richard Kirubakaran: has declared that he has no conflicts of interest.
Erik Simonsen: has declared that he has no conflicts of interest.
Christian Gluud: is the Co‐ordinating Editor of the Cochrane Hepato‐Biliary Group. He has declared that he has no conflicts of interest.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Abikoff 2009 {published data only}
- Abikoff H, Nissley-Tsiopinis J, Gallagher R, Zambenedetti M, Seyffert M, Boorady R, et al. Effects of MPH-OROS on the organizational, time management, and planning behaviors of children with ADHD. Journal of the American Academy of Child and Adolescent Psychiatry 2009;48(2):166-75. [DOI: 10.1097/CHI.0b013e3181930626] [DOI] [PubMed] [Google Scholar]
- Concerta for organizational deficits in ADHD. Brown University Child and Adolescent Behavior Letter 2009;25(3):2. [DOI: 10.1002/cbl.20087] [DOI]
Ahmann 1993 {published data only}
- Ahmann PA, Waltonen SJ, Olson KA, Theye FW, Van Erem AJ, LaPlant RJ. Placebo-controlled evaluation of Ritalin side effects. Pediatrics 1993;91(6):1101-6. [DOI: 10.1542/peds.91.6.1101] [DOI] [PubMed] [Google Scholar]
Arnold 2004 {published data only}
- Arnold LE, Lindsay RL, Conners CK, Wigal SB, Levine AJ, Johnson DE, et al. A double-blind, placebo-controlled withdrawal trial of dexmethylphenidate hydrochloride in children with attention deficit hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 2004;14(4):542-54. [DOI: 10.1089/cap.2004.14.542] [DOI] [PubMed] [Google Scholar]
Barkley 1989b {published data only}
- Barkley RA, McMurray MB, Edelbrock CS, Robbins K. Side effects of methylphenidate in children with attention deficit hyperactivity disorder: a systemic, placebo-controlled evaluation. Pediatrics 1990;86(2):184-92. [DOI: 10.1542/peds.86.2.184] [DOI] [PubMed] [Google Scholar]
- Barkley RA, McMurray MB, Edelbrock CS, Robbins K. The response of aggressive and nonaggressive ADHD children to two doses of methylphenidate. Journal of the American Academy of Child and Adolescent Psychiatry 1989;28(6):873-81. [DOI: ] [DOI] [PubMed] [Google Scholar]
Barkley 1991 {published data only}
- Barkley RA, DuPaul GJ, McMurray MB. Attention deficit disorder with and without hyperactivity: clinical response to three dose levels of methylphenidate. Pediatrics 1991;87(4):519-31. [DOI: 10.1542/peds.87.4.519] [DOI] [PubMed] [Google Scholar]
- DuPaul GJ, Barkley RA, McMurray MB. Response of children with ADHD to methylphenidate: interaction with internalizing symptoms. Journal of the American Academy of Child and Adolescent Psychiatry 1994;33(6):894-903. [DOI: 10.1097/00004583-199407000-00016] [DOI] [PubMed] [Google Scholar]
Barkley 2000 {published data only}
- Barkley RA, Connor DF, Kwasnik D. Challenges to determining adolescent medication response in an outpatient clinical setting: comparing Adderall and methylphenidate for ADHD. Journal of Attention Disorders 2000;4(2):102-13. [DOI: 10.1177/108705470000400204] [DOI] [Google Scholar]
Barragán 2017 {published data only}
- Barragán E, Breuer D, Döpfner M. Efficacy and safety of omega-3/6 fatty acids, methylphenidate, and a combined treatment in children with ADHD. Journal of Attention Disorders 2017;21(5):433-41. [DOI: 10.1177/1087054713518239] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bedard 2008 {published data only}
- Bedard A-C, Tannock R. Anxiety, methylphenidate response, and working memory in children with ADHD. Journal of Attention Disorders 2008;11(5):546-57. [DOI: 10.1177/1087054707311213] [DOI] [PubMed] [Google Scholar]
Bhat 2020 {published data only}
- Ben AL, Grizenko N, Mbekou V, Lageix P, Baron C, Schwartz G, et al. Dopamine transporter gene and behavioral response to methylphenidate (MPH) in children with attention deficit hyperactivity disorder (ADHD). American Journal of Medical Genetics. Part A 2002;114(7):724-5. [DOI: 10.1002/ajmg.10971] [DOI] [Google Scholar]
- Bhat V, Sengupta SM, Grizenko N, Joober R. Therapeutic response in children with ADHD: role of observers and settings. World Journal of Pediatrics 2020;16(3):314-21. [DOI: 10.1007/s12519-019-00332-5] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Bhat V, Sengupta SM, Grizenko N, Joober R. Therapeutic response to methylphenidate in ADHD: role of child and observer gender. Journal of the Canadian Academy of Child and Adolescent Psychiatry 2020;29(1):44-52. [PMID: ] [PMID: ] [PMC free article] [PubMed] [Google Scholar]
- Fageera W, Chaumette B, Fortier MÈ, Grizenko N, Labbe A, Sengupta SM, et al. Association between COMT methylation and response to treatment in children with ADHD. Journal of Psychiatric Research 2021;135:86-93. [DOI: 10.1016/j.jpsychires.2021.01.008] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Fageera W, Grizenko N, Sengupta S M, Joober R. Using behavioral dynamic approaches to test for gene-by-gene interaction in modulating ADHD behaviors. Genetic Epidemiology 2016;40(7):634-5. [DOI: 10.1002/gepi.22001] [DOI] [Google Scholar]
- Fageera W, Grizenko N, Sengupta SM, Joober R. SNAP-25 (RS1051312) gene might be associated with placebo response in children with ADHD. Genetic Epidemiology 2016;40(7):634. [DOI: 10.1002/gepi.22001] [DOI] [Google Scholar]
- Fageera W, Grizenko N, Sengupta SM, Joober R. The association between COMT (VAL158MET) and DRD3 (SER-9-GLY) genotypes and methylphenidate side effects. Genetic Epidemiology 2016;40(7):634. [DOI: 10.1002/gepi.22001] [DOI] [Google Scholar]
- Fageera W, Grizenko N, Sengupta SM, Schmitz N, Joober R. COMT by DRD3 epistatic interaction in modulating behaviors in children with ADHD: a pharmaco-dynamic behavioral approach. Journal of Attention Disorders 2021;25(12):1720-30. [DOI: 10.1177/1087054720934191] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Fageera W, Sengupta S, Grizenko N, Joober R. SU6 - Synergistic effect of COMT and KIAA0319 genes in modulating ADHD behaviors. European Neuropsychopharmacology 2019;29(Suppl 3):S889-90. [DOI: 10.1016/j.euroneuro.2017.08.195] [DOI] [Google Scholar]
- Fageera W, Sengupta SM, Fortier M-È, Grizenko N, Babienco S, Labbe A, et al. Sex-dependent complex association of TPH2 with multiple dimensions of ADHD. Progress in Neuro-Psychopharmacology & Biological Psychiatry 2021;110:110296. [DOI: 10.1016/j.pnpbp.2021.110296] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Fageera W, Sengupta SM, Grizenko N, Joober R. M3 - Gene-gene interaction between COMT and NET in modulating ADHD behaviors. European Neuropsychopharmacology 2019;29(Suppl 3):S956. [DOI: 10.1016/j.euroneuro.2017.08.310] [DOI] [Google Scholar]
- Fageera W, Sengupta SM, Labbe A, Grizenko N, Joober R. DRD3 gene and ADHD: a pharmaco-behavioural genetic study. Neuromolecular Medicine 2018;20(4):515-24. [DOI: 10.1007/s12017-018-8504-z] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Fageera W, Traicu A, Sengupta SM, Fortier M-E, Choudhry Z, Labbe A, et al. Placebo response and its determinants in children with ADHD across multiple observers and settings: a randomized clinical trial. International Journal of Methods in Psychiatric Research 2017;27(1):e1572. [DOI: 10.1002/mpr.1572] [PMCID: PMC6877247] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortier ME, Sengupta SM, Grizenko N, Choudhry Z, Thakur G, Joober R. Genetic evidence for the association of the hypothalamic-pituitary-adrenal (HPA) axis with ADHD and methylphenidate treatment response. NeuroMolecular Medicine 2013;15(1):122-32. [DOI: 10.1007/s12017-012-8202-1] [DOI] [PubMed] [Google Scholar]
- Grizenko N, Bhat M, Schwartz G, Ter-Stepanian M, Joober R. Efficacy of methylphenidate in children with attention-deficit hyperactivity disorder and learning disabilities: a randomized crossover trial. Journal of Psychiatry & Neuroscience 2006;31(1):46-51. [PMCID: PMC1325066] [PMID: ] [PMC free article] [PubMed] [Google Scholar]
- Grizenko N, Cai E, Jolicoeur C, Ter-Stepanian M, Joober R. Effects of methylphenidate on acute math performance in children with attention-deficit hyperactivity disorder. Canadian Journal of Psychiatry 2013;58(11):632-9. [DOI: 10.1177/070674371305801109] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Grizenko N, Kovacina B, Amor LB, Schwartz G, Ter-Stepanian M, Joober R. Relationship between response to methylphenidate treatment in children with ADHD and psychopathology in their families. Journal of the American Academy of Child and Adolescent Psychiatry 2006;45(1):47-53. [DOI: 10.1097/01.chi.0000184932.64294.d9] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Grizenko N, Lachance M, Collard V, Lageix P, Baron C, Ben Amor L, et al. Sensitivity of tests to assess improvement in ADHD symptomatology. Canadian Child and Adolescent Psychiatry Review 2004;13(2):36-9. [PMCID: PMC2538631] [PMID: ] [PMC free article] [PubMed] [Google Scholar]
- Grizenko N, Qi Zhang DD, Polotskaia A, Joober R. Efficacy of methylphenidate in ADHD children across the normal and the gifted intellectual spectrum. Journal of the Canadian Academy of Child and Adolescent Psychiatry 2012;21(4):282-8. [PMCID: PMC3490529] [PMID: ] [PMC free article] [PubMed] [Google Scholar]
- Grizenko N, Rodrigues Pereira RM, Joober R. Sensitivity of scales to evaluate change in symptomatology with psychostimulants in different ADHD subtypes. Journal of the Canadian Academy of Child and Adolescent Psychiatry 2013;22(2):153-8. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Grizenko N, Bhat V, Sengupta S, Polotskaia A, Joober R. Relation between therapeutic response and side effects induced by methylphenidate as observed by parents and teachers of children with ADHD. BMC Psychiatry 2011;11:70. [DOI: 10.1186/1471-244X-11-70] [PMCID: PMC3095543] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00483106. Clinical and pharmacological study of attention deficit with hyperactivity disorder (ADHD). clinicaltrials.gov (first received 6 June 2007).
- Naumova D, Grizenko N, Sengupta SM, Joober R. DRD4 exon 3 genotype and ADHD: randomised pharmacodynamic investigation of treatment response to methylphenidate. World Journal of Biological Psychiatry 2019;20(6):486-95. [DOI: 10.1080/15622975.2017.1410221] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Sengupta S, Grizenko N, Schmitz N, Schwartz G, Bellingham J, Polotskaia A, et al. COMT Val108/158Met polymorphism and the modulation of task-oriented behavior in children with ADHD. Neuropsychopharmacology 2008;33(13):3069-77. [DOI: 10.1038/npp.2008.85] [PMCID: PMC2885152] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ter-Stepanian M, Grizenko N, Zappitelli M, Joober R. Clinical response to methylphenidate in children diagnosed with attention-deficit hyperactivity disorder and comorbid psychiatric disorders. Canadian Journal of Psychiatry 2010;55(5):305-12. [DOI: 10.1177/070674371005500506] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Thakur GA, Grizenko N, Sengupta SM, Schmitz N, Joober R. The 5-HTTLPR polymorphism of the serotonin transporter gene and short term behavioral response to methylphenidate in children with ADHD. BMC Psychiatry 2010;10:50. [DOI: 10.1186/1471-244X-10-50] [PMCID: PMC2905344] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur GA, Sengupta SM, Grizenko N, Choudhry Z, Joober R. Comprehensive phenotype/genotype analyses of the norepinephrine transporter gene (SLC6A2) in ADHD: relation to maternal smoking during pregnancy. PloS One 2012;7(11):e49616. [DOI: 10.1371/journal.pone.0049616] [PMCID: PMC3502190] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Biederman 2003b {published data only}
- Biederman J, Quinn D, Weiss M, Markabi S, Weidenman M, Edson K, et al. Efficacy and safety of Ritalin LA, a new, once daily, extended-release dosage form of methylphenidate, in children with attention deficit hyperactivity disorder. Pediatric Drugs 2003;5(12):833-41. [DOI: 10.2165/00148581-200305120-00006] [DOI] [PubMed] [Google Scholar]
Bliznakova 2007 {published data only}
- Bliznakova L, Gerstner S, Schmidt MH, Becker K. Methylphenidate double-blind trial: indication and performing [Der methylphenidat-doppelblindversuch - indikation und durchfuhrung]. Klinische Pädiatrie 2007;219(1):9-16. [DOI: 10.1055/s-2005-836823] [DOI] [PubMed] [Google Scholar]
Blum 2011 {published data only}
- Blum NJ, Jawad AF, Clarke AT, Power TJ. Effect of osmotic-release oral system methylphenidate on different domains of attention and executive functioning in children with attention-deficit-hyperactivity disorder. Developmental Medicine & Child Neurology 2011;53(9):843-9. [DOI: 10.1111/j.1469-8749.2011.03944.x] [DOI] [PubMed] [Google Scholar]
- NCT00530257. Study of the effects of osmotic-release oral system (OROS) methylphenidate (Concerta) on attention and memory (CHOP) [Effect of OROS-methylphenidate (Concerta) on different domains of attention and working memory in children with attention-deficit/hyperactivity disorder]. clinicaltrials.gov/ct2/show/NCT00530257 (first received 17 September 2007).
Borcherding 1990 {published data only}
- Borcherding BG, Keysor CS, Rapoport JL, Elia J, Amass J. Motor/vocal tics and compulsive behaviors on stimulant drugs: is there a common vulnerability? Psychiatry Research 1990;33(1):83-94. [DOI: 10.1016/0165-1781(90)90151-T] [DOI] [PubMed] [Google Scholar]
Brams 2008 {published data only}
- Brams M, Muniz R, Childress A, Giblin J, Mao A, Turnbow J, et al. A randomized, double-blind, crossover study of once-daily dexmethylphenidate in children with attention-deficit hyperactivity disorder: rapid onset of effect. CNS Drugs 2008;22(8):693-704. [DOI: 10.2165/00023210-200822080-00006] [DOI] [PubMed] [Google Scholar]
Brams 2012 {published data only}
- Brams M, Turnbow J, Pestreich L, Giblin J, Childress A, McCague K, et al. A randomized, double-blind study of 30 versus 20 mg dexmethylphenidate extended-release in children with attention-deficit/hyperactivity disorder: late-day symptom control. Journal of Clinical Psychopharmacology 2012;32(5):637-44. [DOI: 10.1097/JCP.0b013e3182677825] [DOI] [PubMed] [Google Scholar]
- Brams M, Turnbow J, Pestreich L. Erratum: a randomized, double-blind study of 30 versus 20 mg dexmethylphenidate extended-release in children with attention-deficit/ hyperactivity disorder: late-day symptom control. Journal of Clinical Psychopharmacology 2012;32(6):766. [DOI: 10.1097/JCP.0b013e3182761e12] [DOI] [PubMed] [Google Scholar]
- Dexmethylphenidate may be effective later in the day. Brown University Child & Adolescent Psychopharmacology Update 2012;14(11):8. [DOI: 10.1002/cpu.20175] [DOI]
- Muniz R, Pestreich L, McCague K, Padilla A, Brams M, Childress A. Extended-release dexmethylphenidate 30 mg improves late-day attention deficit hyperactivity disorder (ADHD) symptom control in children with ADHD: a randomized, double-blind crossover study. Journal of Child and Adolescent Psychopharmacology. Abstracts of the 50th Annual National Institute of Mental Health (NIMH) New Clinical Drug Evaluation Unit (NCDEU) Meeting; 2010 June 14–17; Boca Raton, Florida 2010;20(6):534-5. [Google Scholar]
- NCT00776009. Efficacy and safety of dex-methylphenidate extended release 30 mg versus 20 mg in children (6-12 years) with attention-deficit/hyperactivity disorder (ADHD) in a laboratory classroom setting [A randomized, multi-center, double-blind, placebo-controlled, cross-over study evaluating the safety and efficacy of dex-methylphenidate extended release 30 mg vs 20 mg as measured by Skamp-combined scores in children with attention-deficit/hyperactivity disorder (ADHD) in a laboratory classroom setting]. clinicaltrials.gov/ct2/show/NCT00776009 (first received 20 October 2008).
- Padilla A, Pestreich L, McCague K, Muniz R. Late-day attention deficit hyperactivity disorder (ADHD) symptom control improvement with extended-release dexmethylphenidate in children with ADHD of all ethnicities: a sub-analysis. Journal of Child and Adolescent Psychopharmacology. Abstracts of the 50th Annual National Institute of Mental Health (NIMH) New Clinical Drug Evaluation Unit (NCDEU) Meeting; 2010 June 14–17; Boca Raton, Florida 2010;20(6):534. [Google Scholar]
- Silva RR, Brams M, McCague K, Pestreich L, Muniz R. Extended-release dexmethylphenidate 30 mg/d versus 20 mg/d: duration of attention, behavior, and performance benefits in children with attention-deficit/hyperactivity disorder. Clinical Neuropharmacology 2013;36(4):117-21. [DOI: 10.1097/WNF.0b013e31829aa92c] [DOI] [PubMed] [Google Scholar]
Brown 1984a {published data only}
- Brown RT, Wynne ME, Slimmer LW. Attention deficit disorder and the effect of methylphenidate on attention, behavioral, and cardiovascular functioning. Journal of Clinical Psychiatry 1984;45(11):473-6. [PMID: ] [PubMed] [Google Scholar]
Brown 1985 {published data only}
- Brown RT, Wynne ME, Medenis R. Methylphenidate and cognitive therapy: a comparison of treatment approaches with hyperactive boys. Journal of Abnormal Child Psychology 1985;13(1):69-87. [DOI: 10.1007/BF00918373] [DOI] [PubMed] [Google Scholar]
Brown 1988 {published data only}
- Brown RT, Sexson SB. A controlled trial of methylphenidate in black adolescents. Attentional, behavioral, and physiological effects. Clinical Pediatrics 1988;27(2):74-81. [DOI: 10.1177/000992288802700204] [DOI] [PubMed] [Google Scholar]
- Brown RT, Sexson SB. Effects of methylphenidate on cardiovascular responses in attention deficit hyperactivity disordered adolescents. Journal of Adolescent Health Care 1989;10(3):179-83. [DOI: 10.1016/0197-0070(89)90229-5] [DOI] [PubMed] [Google Scholar]
Brown 1991 {published data only}
- Brown RT, Jaffe SL, Silverstein J, Magee H. Methylphenidate and hospitalized adolescents with conduct disorder: dose effects on classroom behavior, academic performance, and impulsivity. Journal of Youth and Adolescence 1991;20(5):501-18. [DOI: 10.1007/BF01540634] [DOI] [PubMed] [Google Scholar]
Buitelaar 1995 {published data only}
- Buitelaar JK, Swaab-Barneveld H, Van der Gaag RJ. Prediction of clinical response to methylphenidate in children with ADHD. In: X World Congress of Psychiatry; 1996 August 23-26; Madrid, Spain. Madrid: World Psychiatric Association, 1996.
- Buitelaar JK, Van der Gaag RJ, Swaab-Barneveld H, Kuiper M. Pindolol and methylphenidate in children with attention-deficit hyperactivity disorder. Clinical efficacy and side-effects. Journal of Child Psychology and Psychiatry 1996;37(5):587-95. [DOI: 10.1111/j.1469-7610.1996.tb01445.x] [DOI] [PubMed] [Google Scholar]
- Buitelaar JK, Van der Gaag RJ, Swaab-Barneveld H, Kuiper M. Prediction of clinical response to methylphenidate in children with attention-deficit hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry 1995;34(8):1025-32. [DOI: 10.1097/00004583-199508000-00012] [DOI] [PubMed] [Google Scholar]
Bukstein 1998 {published data only}
- Bukstein OG, Kolko DJ. Effects of methylphenidate on aggressive urban children with attention deficit hyperactivity disorder. Journal of Clinical Child Psychology 1998;27(3):340-51. [DOI: 10.1207/s15374424jccp2703_10] [DOI] [PubMed] [Google Scholar]
Butter 1983 {published data only}
- Butter HJ, Lapierre Y, Firestone P, Blank A. A comparative study of the efficacy of ACTH4-9 analog, methylphenidate, and placebo on attention deficit disorder with hyperkinesis. Journal of Clinical Psychopharmacology 1983;3(4):226-30. [PMID: ] [PubMed] [Google Scholar]
- Butter HJ, Lapierre Y, Firestone P, Blank A. Efficacy of ACTH 4-9 analog, methylphenidate, and placebo on attention deficit disorder with hyperkinesis. Progress in Neuro-Psychopharmacology & Biological Psychiatry 1984;8(4-6):661-4. [DOI: 10.1016/0278-5846(84)90032-0] [DOI] [PubMed] [Google Scholar]
Carlson 1995 {published data only}
- Carlson GA, Rapport MD, Kelly KL, Pataki CS. Methylphenidate and desipramine in hospitalized children with comorbid behavior and mood disorders: separate and combined effects on behavior and mood. Journal of Child and Adolescent Psychopharmacology 1995;5(3):191-204. [DOI: 10.1089/cap.1995.5.191] [DOI] [Google Scholar]
- Pataki CS, Carlson GA, Kelly KL, Rapport MD, Biancaniello TM. Side effects of methylphenidate and desipramine alone and in combination in children. Journal of the American Academy of Child and Adolescent Psychiatry 1993;32(5):1065-72. [DOI: 10.1097/00004583-199309000-00028] [DOI] [PubMed] [Google Scholar]
Carlson 2007 {published data only}
- Carlson GA, Dunn D, Kelsey D, Ruff D, Ball S, Ahrbecker L, et al. A pilot study for augmenting atomoxetine with methylphenidate: safety of concomitant therapy in children with attention-deficit/hyperactivity disorder. Child and Adolescent Psychiatry and Mental Health 2007;1:10. [DOI: 10.1186/1753-2000-1-10] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00485550. Comparison of atomoxetine plus either comparator or placebo in children with ADHD who haven't responded to stimulant therapy [A randomized, double-blind comparison of atomoxetine hydrochloride augmented with either extended-release methylphenidate hydrochloride (Concerta-tm) or placebo in children with attention-deficit/hyperactivity disorder (ADHD) who have not responded to stimulant mono therapy]. clinicaltrials.gov/ct2/show/NCT00485550 (first received 13 June 2007).
Castellanos 1997 {published data only}
- Castellanos FX, Giedd JN, Elia J, Marsh WL, Ritchie GF, Hamburger SD, et al. Controlled stimulant treatment of ADHD and comorbid Tourette's syndrome: effects of stimulant and dose. Journal of the American Academy of Child and Adolescent Psychiatry 1997;36(5):589-96. [DOI: 10.1097/00004583-199705000-00008] [DOI] [PubMed] [Google Scholar]
- Castellanos FX. Stimulants and tic disorders: from dogma to data. Archives of General Psychiatry 1999;56(4):337-8. [DOI: 10.1001/archpsyc.56.4.337] [DOI] [PubMed] [Google Scholar]
Chacko 2005 {published data only}
- Chacko A, Pelham WE, Gnagy EM, Greiner A, Vallano G, Bukstein O, et al. Stimulant medication effects in a summer treatment program among young children with attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry 2005;44(3):249-57. [DOI: 10.1097/00004583-200503000-00009] [DOI] [PubMed] [Google Scholar]
Childress 2009 {published data only}
- Childress A, Muniz R, Miller J, Arnold V, Harper L, Gerstner O, Borrello M, Thulasiraman A, Post A. Fixed-dose titration study of dexmethylphenidate extended release in children with ADHD: effects on teacher-rated scales. Annals of Neurology 2007;62(Suppl 11):S125. [DOI: 10.1002/ana.11635] [DOI] [Google Scholar]
- Childress AC, Spencer T, Lopez F, Gerstner O, Thulasiraman A, Muniz R, et al. Efficacy and safety of dexmethylphenidate extended-release capsules administered once daily to children with attention-deficit/hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 2009;19(4):351-61. [DOI: 10.1089/cap.2009.0007] [DOI] [PubMed] [Google Scholar]
- Childress AC, Spencer T, Lopez F, Gerstner O, Thulasiraman A, Muniz R, et al. Dose related safety and efficacy of dexmethylphenidate ER. Brown University Child & Adolescent Psychopharmacology Update 2009;11(11):7-8. [DOI: 10.1002/cpu.20103] [DOI] [PubMed] [Google Scholar]
- Greenbaum M, Muniz R, Brams M, Boellner S, Gerstner O, Borello M, et al. Cardiovascular safety of dexmethylphenidate extended release in children with ADHD. Annals of Neurology 2007;62(Suppl 11):S146. [DOI: 10.1002/ana.11638] [DOI] [Google Scholar]
- Lopez F, Muniz R, Joyce JM, Franklin ER, Gerstner O, Borello M, et al. Fixed-dose titration study of dexmethylphenidate extended release in children with ADHD: effects on parent-rated scales. Annals of Neurology 2007;62(Suppl 11):S122. [DOI: 10.1002/ana.11635] [DOI] [Google Scholar]
- NCT00763971. Efficacy, tolerability and safety of dexmethylphenidate HCL extended-release capsules in children with attention-deficit/hyperactivity disorder [A 5-week treatment, multi-center, double-blind, randomized, placebo-controlled, parallel-group, fixed-dose study of the efficacy, tolerability and safety of dexmethylphenidate HCL extended-release capsules administered once daily in pediatric children with attention-deficit/hyperactivity disorder]. clinicaltrials.gov/ct2/show/NCT00301236 (first received 10 March 2006).
Childress 2017 {published data only}
- Childress AC, Kollins SH, Cutler AJ, Marraffino A, Sikes CR. Efficacy, safety, and tolerability of an extended-release orally disintegrating methylphenidate tablet in children 6-12 years of age with attention-deficit/hyperactivity disorder in the laboratory classroom setting. Journal of Child and Adolescent Psychopharmacology 2017;27(1):66-74. [DOI: 10.1089/cap.2016.0002] [PMCID: PMC5326982] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress AC, Kollins SH, Cutler AJ, Marraffino A, Sikes CR. Open-label dose optimization of methylphenidate extended-release orally disintegrating tablet in a laboratory classroom study of children with attention-deficit/hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 2021;31(5):342-9. [DOI: 10.1089/cap.2020.0142] [PMID: ] [DOI] [PubMed] [Google Scholar]
- NCT01835548. NT0102 in the treatment of children with attention deficit hyperactivity disorder (ADHD) [A randomized, multicenter, double-blind, placebo controlled, parallel group study of nt0102 in children (ages 6 12 years) with attention-deficit hyperactivity disorder]. clinicaltrials.gov/ct2/show/NCT01835548 (first received 19 April 2013).
- Teuscher N, Sikes C, McMahen R, Engelking D. Population pharmacokinetic-pharmacodynamic modeling of a novel methylphenidate extended-release orally disintegrating tablet in pediatric patients with attention-deficit/hyperactivity disorder. Journal of the American Academy of Child & Adolescent Psychiatry 2017;56(Suppl 10):S288-9. [DOI: 10.1016/j.jaac.2017.09.382] [DOI] [Google Scholar]
- Teuscher NS, Sikes CR, McMahen R, Engelking D. Population pharmacokinetic-pharmacodynamic modeling of a novel methylphenidate extended-release orally disintegrating tablet in pediatric patients with attention-deficit/hyperactivity disorder. Journal of Clinical Psychopharmacology 2018;38(5):467-74. [DOI: 10.1097/JCP.0000000000000944] [PMID: ] [DOI] [PubMed] [Google Scholar]
Childress 2020a {published data only}
- Childress AC, Brams MN, Cutler AJ, Donnelly GA, Bhaskar S. Efficacy and safety of multilayer, extended-release methylphenidate (PRC-063) in children 6-12 years of age with attention-deficit/hyperactivity disorder: a laboratory classroom study. Journal of Child and Adolescent Psychopharmacology 2020;30(10):580-9. [DOI: 10.1089/cap.2020.0109] [PMCID: PMC7757528] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT03172481. PRC-063 classroom study in children (6-12 years of age) with ADHD [A phase 3, randomized, double-blind, placebo-controlled, parallel group, laboratory classroom study to evaluate the safety and efficacy of prc-063 compared to placebo in children (6-12 years of age) with ADHD]. clinicaltrials.gov/ct2/show/NCT03172481 (first received 11 June 2017).
Childress 2020b {published data only}
- Arnold V, Lopez F, Childress A, Po M, Gregg R, Sallee F, et al. Improvements in emotional lability with delayed-release and extended-release methylphenidate treatment in children with ADHD. Journal of the American Academy of Child & Adolescent Psychiatry 2021;60(Suppl 10):S257. [DOI: 10.1016/j.jaac.2021.09.412] [DOI] [Google Scholar]
- Childress AC, Cutler AJ, Marraffino A, DeSousa NJ, Incledon B, Sallee FR. Efficacy and safety of delayed-release and extended-release methylphenidate (DRr/ER-MPH) in children with ADHD: results from a pivotal phase 3 classroom trial. Journal of the American Academy of Child & Adolescent Psychiatry 2018;57(Suppl 10):S166. [DOI: 10.1016/j.jaac.2018.09.111] [DOI] [Google Scholar]
- Childress AC, Cutler AJ, Marraffino A, McDonnell MA, Turnbow JM, Brams M, et al. A randomized, double-blind, placebo-controlled study of HLD200, a delayed-release and extended-release methylphenidate, in children with attention-deficit/hyperactivity disorder: an evaluation of safety and efficacy throughout the day and across settings. Journal of Child and Adolescent Psychopharmacology 2020;30(1):2-14. [DOI: 10.1089/cap.2019.0070] [PMCID: PMC7041320] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress AC, Pliszka SR, Cutler AJ, Marraffino A, DeSousa NJ, Sallee FR, et al. Electronically monitored adherence rates with evening-dosed delayed-release and extended-release methylphenidate in children with ADHD. Journal of the American Academy of Child & Adolescent Psychiatry 2020;59(Suppl 10):S152-3. [DOI: 10.1016/j.jaac.2020.08.073] [DOI] [Google Scholar]
- Childress AC, Uchida CL, Po MD, DeSousa NJ, Incledon B. A post hoc comparison of prior ADHD medication dose and optimized delayed-release and extended-release methylphenidate dose in a pivotal phase iii trial. Clinical Therapeutics 2020;42(12):2332-40. [DOI: 10.1016/j.clinthera.2020.10.004] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Childress AC, Wigal SB, Marraffino A, DeSousa NJ, Incledon B, Sallee FR, et al. Clinical responses and symptomatic remissions achieved with delayed-release and extended-release methylphenidate in children with ADHD. Journal of the American Academy of Child & Adolescent Psychiatry 2019;58(Suppl 10):S286-7. [DOI: 10.1016/j.jaac.2019.08.440] [DOI] [Google Scholar]
- DeSousa N, Pliszka S, Wilens T, Childress A, Sallee R, Incledon B. Improvement in functional impairment during the early morning and late afternoon/evening bookends of the day: data from two phase 3 trials of evening-dosed delayed-release and extended-release methylphenidate in children with ADHD. Pediatrics 2020;146(1):590. [DOI: 10.1542/peds.146.1MA7.590a] [DOI] [Google Scholar]
- Faraone SV, Wilens T, Childress AC, Cutler AJ, Marraffino A, DeSousa NJ, et al. Achievement and maintenance of normalized levels of early morning and late afternoon/evening functional impairment with delayed-release and extended-release methylphenidate (DR/ER-MPH) in children with ADHD: post hoc analyses of before-school functioning. Journal of the American Academy of Child & Adolescent Psychiatry 2019;58(Suppl 10):S287-8. [DOI: 10.1016/j.jaac.2019.08.443] [DOI] [Google Scholar]
- McDonnell MA, Wigal S, Childress A, Kollins S, DeSousa N, Komolova M, et al. A phase III study of HLD200 in children with attention- deficit/hyperactivity disorder: safety results. Pediatrics 2018;141(1):253. [DOI: 10.1542/peds.141.1MA3.253] [DOI] [Google Scholar]
- McDonnell MA, Wigal S, Childress A, Kollins S, DeSousa N, Komolova M, et al. A treatment optimization study of HLD200 in children with attention-deficit/hyperactivity disorder. Pediatrics 2018;141(1):257. [DOI: 10.1542/peds.141.1MA3.257] [DOI] [Google Scholar]
- NCT02493777. A pivotal efficacy trial to evaluate HLD200 in children with ADHD in a classroom setting [Ph 3 multicenter OL treatment-optimized RDBPC forced-withdrawal, parallel GRP study to evaluate safety & efficacy of evening dosed HLD200, a novel DR/ER formulation (DELEXIS) of MPH HCL in children aged 6-12 with ADHD in classroom setting]. clinicaltrials.gov/ct2/show/NCT02493777 (first received 10 July 2015).
- Pliszka SR, Childress AC, Cutler AJ, Maraffino A, DeSousa NJ, Sallee FR, et al. Post hoc meta-analysis of morning and evening behaviors in children with ADHD treated with delayed-release and extended-release methylphenidate from two phase 3 studies. Journal of the American Academy of Child & Adolescent Psychiatry 2020;59(Suppl 10):155-6. [DOI: 10.1016/j.jaac.2020.08.082] [DOI] [Google Scholar]
Childress 2020c {published data only}
- Childress AC, Kollins SH, Adjei AL, Haddock P, Foehl HC, Mattingly G, et al. The efficacy and safety of methylphenidate hydrochloride (HCL) extended-release in preschool aged children with ADHD. Journal of the American Academy of Child & Adolescent Psychiatry 2018;57(Suppl 10):S209. [DOI: 10.1016/j.jaac.2018.09.241] [DOI] [Google Scholar]
- Childress AC, Kollins SH, Foehl HC, Newcorn JH, Mattingly G, Kupper RJ, et al. Randomized, double-blind, placebo-controlled, flexible-dose titration study of methylphenidate hydrochloride extended-release capsules (Aptensio XR) in preschool children with attention-deficit/hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 2020;30(2):58-68. [DOI: 10.1089/cap.2019.0085] [PMCID: PMC7047252] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02683265. A flexible-dose titration study of Aptensio XR in children ages 4 to under 6 years diagnosed with ADHD (EF003) [A randomized, double-blind, placebo-controlled, flexible-dose titration study of Aptensio XR® in children ages 4 to under 6 years diagnosed with attention deficit-hyperactivity disorder (ADHD)]. clinicaltrials.gov/ct2/show/NCT02683265 (first received 17 February 2016).
Chronis 2003 {published data only}
- Chronis A, Pelham WE Jr, Gnagy E, Roberts J, Aronoff H. The impact of late-afternoon stimulant dosing for children with ADHD on parent and parent-child domains. Journal of Clinical Child and Adolescent Psychology 2003;32(1):118-26. [DOI: 10.1207/S15374424JCCP3201_11] [DOI] [PubMed] [Google Scholar]
- Pelham WE, Gnagy EM, Chronis AM, Burrows-MacLean L, Fabiano GA, Onyango AN, et al. A comparison of morning-only and morning/late afternoon Adderall to morning-only, twice-daily, and three times-daily methylphenidate in children with attention-deficit/hyperactivity disorder. Pediatrics 1999;104(6):1300-11. [DOI: 10.1542/peds.104.6.1300] [DOI] [PubMed] [Google Scholar]
Coghill 2007 {published data only}
- Coghill DR, Rhodes SM, Matthews K. Chronic effects of the psychostimulant drug methylphenidate on neuropsychological functioning in drug-naive boys with hyperkinetic disorder (ADHD). In: Journal of Psychopharmacology. Summer Meeting of the British Association for Psychopharmacology; 2003 July 20-23; Cambridge, England. Vol. 17 (3). London: Sage Publications Ltd, 2003:A74. [WOS: 000185623500293]
- Coghill DR, Rhodes SM, Matthews K. The neuropsychological effects of chronic methylphenidate on drug-naive boys with attention-deficit/hyperactivity disorder. Biological Psychiatry 2007;62(9):954-62. [DOI: 10.1016/j.biopsych.2006.12.030] [DOI] [PubMed] [Google Scholar]
- Idema IM, Payne JM, Coghill D. Effects of methylphenidate on cognitive functions in boys with attention deficit hyperactivity disorder: does baseline performance matter? Journal of Consulting and Clinical Psychology 2021;89(7):615-25. [DOI: 10.1037/ccp0000662] [PMID: ] [DOI] [PubMed] [Google Scholar]
Coghill 2013 {published data only}
- Banaschewski T, Soutullo C, Lecendreux M, Johnson M, Zuddas A, Hodgkins P, et al. Health-related quality of life and functional outcomes from a randomized, controlled study of lisdexamfetamine dimesylate in children and adolescents with attention deficit hyperactivity disorder. CNS Drugs 2013;27(10):829-40. [DOI: 10.1007/s40263-013-0095-5] [PMCID: PMC3784063] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banaschewski T, Soutullo C, Lecendreux M, Johnson M, Zuddas A, Hodgkins P, et al. The child health and illness profile as a measure of health-related quality of life in stimulant-treated children and adolescents with ADHD. European Child & Adolescent Psychiatry 2013;22(Suppl 2):S125-6. [DOI: 10.1007/s00787-013-0423-9] [DOI] [Google Scholar]
- Coghill D, Banaschewski T, Lecendreux M, Soutollu C, Johnson M, Zuddas A, et al. The first European studies of lisdexamfetamine dimesylate in children and adolescents with attention-deficit/hyperactivity disorder. European Child & Adolescent Psychiatry. Abstracts of the 15th International Congress of ESCAP - European Society for Child and Adolescent Psychiatry; 2013 July 6-10, Dublin, Ireland 2013;22(2 Suppl):S125. [DOI: 10.1007/s00787-013-0423-9] [DOI] [Google Scholar]
- Coghill D, Banaschewski T, Lecendreux M, Soutullo C, Johnson M, Zuddas A, et al. European, randomized, phase 3 study of lisdexamfetamine dimesylate in children and adolescents with attention-deficit/hyperactivity disorder. European Neuropsychopharmacology 2013;23(10):1208-18. [DOI: 10.1016/j.euroneuro.2012.11.012] [DOI] [PubMed] [Google Scholar]
- Coghill D, Banaschewski T, Lecendreux M, Soutullo C, Johnson M, Zuddas A, et al. Post hoc comparison of the efficacy of lisdexamfetamine dimesylate and osmotic-release oral system methylphenidate in children and adolescents with ADHD. European Psychiatry 2013;28(Suppl 1):1. [DOI: 10.1016/S0924-9338(13)76103-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coghill DR, Banaschewski T, Lecendreux M, Soutullo C, Zuddas A, Adeyi B, et al. Post hoc analyses of the impact of previous medication on the efficacy of lisdexamfetamine dimesylate in the treatment of attention-deficit/hyperactivity disorder in a randomized, controlled trial. Neuropsychiatric Disease and Treatment 2014;10:2039-47. [DOI: 10.2147/NDT.S68273] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coghill DR, Banaschewski T, Lecendreux M, Zuddas A, Dittmann RW, Otero IH, et al. Efficacy of lisdexamfetamine dimesylate throughout the day in children and adolescents with attention-deficit/hyperactivity disorder: results from a randomized, controlled trial. European Child & Adolescent Psychiatry 2014;23(2):61-8. [DOI: 10.1007/s00787-013-0421-y] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doddamani L, Hodgkins P, Adeyi B, Squires LA, Civil R, Coghill DR. Functional impairment in children and adolescents with attention deficit hyperactivity disorder: results from short- and long-term studies of lisdexamfetamine dimesylate. In: Australian and New Zealand Journal of Psychiatry. Proceedings of the Royal Australian and New Zealand College of Psychiatrists, RANZCP Annual Congress; 2014 May 11-15; Perth, Australia. Vol. 48. Informa Healthcare, 2014:115. [EMBASE: 71565453]
- Gasior M, Coghill D, Soutullo C, Lyne A, Johnson M. Poster 7: efficacy and safety of lisdexamfetamine dimesylate in children and adolescents with ADHD: a phase 3, randomized, double-blind, multicenter, parallel-group, placebo- and active-controlled, dose-optimized study in Europe. Acta Neuropsychiatrica. Abstracts of the Scandinavian College of Neuropsychopharmacology 53rd Annual Meeting; 2012 April 25-27; Copenhagen, Denmark 2012;24(Suppl s1):24. [DOI: 10.1111/j.1601-5215.2012.00665.x] [DOI] [Google Scholar]
- Hodgkins P, Coghill DR, Soutullo CA, Bloomfield R, Gasior M, Johnson M. Poster 14: effect of lisdexamfetamine dimesylate on functional impairment in children and adolescents with attention-deficit/hyperactivity disorder. Acta Neuropsychiatrica. Abstracts of the Scandinavian College of Neuropsychopharmacology (SCNP) 53rd Annual Meeting; 2012 April 25-27; Copenhagen, Denmark 2012;24(Suppl s1):27-8. [DOI: 10.1111/j.1601-5215.2012.00665.x] [DOI] [Google Scholar]
- Hodgkins P, Setyawan J, Banaschewski T, Soutullo C, Lecendreux M, Johnson M, et al. Health utility scores in children and adolescents with attention-deficit/hyperactivity disorder: response to stimulant treatment. European Child & Adolescent Psychiatry. Abstracts of the 15th International Congress of ESCAP - European Society for Child and Adolescent Psychiatry; 2013 July 6-10; Dublin, Ireland 2013;22(Suppl 2):S127. [Google Scholar]
- NCT00763971. Randomized, double-blind safety and efficacy study of lisdexamfetamine dimesylate (LDX) in children and adolescents aged 6-17 [A phase III, randomised, double-blind, multicentre, parallel-group, placebo- and active-controlled, dose-optimisation safety and efficacy study of lisdexamfetamine dimesylate (LDX) in children and adolescents aged 6-17 with attention-deficit/hyperactivity disorder (ADHD)]. clinicaltrials.gov/ct2/show/NCT00763971 (first received 1 October 2008).
- Zuddas A, Banaschewski T, Lecendreux M, Soutullo C, Johnson M, Anderson C, et al. Clinical efficacy of lisdexamfetamine dimesylate in children and adolescents with ADHD: a post-hoc analysis. European Neuropsychopharmacology. Papers of the 25th European College of Neuropsychopharmacology Congress; 2012 October 13-17; Vienna, Austria 2012;22(Suppl 2):S431. [Google Scholar]
Connor 2000 {published data only}
- Connor DF, Barkley RA, Davis HT. A pilot study of methylphenidate, clonidine, or the combination in ADHD comorbid with aggressive oppositional defiant or conduct disorder. Clinical Pediatrics 2000;39(1):15-25. [DOI: 10.1177/000992280003900102] [PMID: ] [DOI] [PubMed] [Google Scholar]
Cook 1993 {published data only}
- Cook JR, Mausbach T, Burd L, Gascon GG, Slotnick HB, Patterson B, et al. A preliminary study of the relationship between central auditory processing disorder and attention deficit disorder. Journal of Psychiatry & Neuroscience 1993;18(3):130-7. [PMCID: PMC1188509] [PMID: ] [PMC free article] [PubMed] [Google Scholar]
- Cook JR. The Effects of Methylphenidate on Resource Allocation in the Mental Processing of ADD Children [PhD thesis]. University of North Dakota, 1989. [Google Scholar]
Corkum 2008 {published data only}
- Corkum P, Panton R, Ironside S, Macpherson M, Williams T. Acute impact of immediate release methylphenidate administered three times a day on sleep in children with attention-deficit/hyperactivity disorder. Journal of Pediatric Psychology 2008;33(4):368-79. [DOI: 10.1093/jpepsy/jsm106] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Ironside S, Davidson F, Corkum P. Circadian motor activity affected by stimulant medication in children with attention-deficit/hyperactivity disorder. Journal of Sleep Research 2010;19(4):546-51. [DOI: 10.1111/j.1365-2869.2010.00845.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Corkum 2020 {published data only}
- Corkum P, Begum EA, Rusak B, Rajda M, Shea S, MacPherson M, et al. The effects of extended-release stimulant medication on sleep in children with ADHD. Journal of the Canadian Academy of Child and Adolescent Psychiatry 2020;29(1):33-43. [PMCID: PMC7065567] [PMID: ] [PMC free article] [PubMed] [Google Scholar]
- Gendron M, Rusak B, Rajda M, Corkum PV. Assessing the impact of methylphenidate on sleep in children with ADHD using polysomnography and actigraphy. Sleep 2012;35(Abstract supplement):A374. [URL: static.primary.prod.gcms.the-infra.com/static/site/sleep/document/2012abstractsupplement.pdf?node=615167184db643e0186e&version=439372:72d2d41f9a60a7eb1c70 (accessed 21/09/22)] [Google Scholar]
- Morash-Conway J, Gendron M, Corkum P. The role of sleep quality and quantity in moderating the effectiveness of medication in the treatment of children with ADHD. Attention Deficit Hyperactivity Disorder 2017;9(1):31-8. [DOI: 10.1007/s12402-016-0204-7] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Waldon J, Begum E, Gendron M, Rusak B, Andreou P, Rajda M, et al. Concordance of actigraphy with polysomnography in children with and without attention-deficit/hyperactivity disorder. Journal of Sleep Research 2016;25(5):524-33. [DOI: 10.1111/jsr.12402] [PMID: ] [DOI] [PubMed] [Google Scholar]
Cox 2006 {published data only}
- Cox DJ, Merkel RL, Moore M, Thorndike F, Muller C, Kovatchev B. Relative benefits of stimulant therapy with OROS methylphenidate versus mixed amphetamine salts extended release in improving the driving performance of adolescent drivers with attention-deficit/hyperactivity disorder. Pediatrics 2006;118(3):e704-10. [DOI: 10.1542/peds.2005-2947] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Cox DJ, Moore M, Burket R, Merkel RL, Mikami AY, Kovatchev B. Rebound effects with long-acting amphetamine or methylphenidate stimulant medication preparations among adolescent male drivers with attention-deficit/hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 2008;18(1):1-10. [DOI: 10.1089/cap.2006.0141] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Mikami AY, Cox DJ, Davis MT, Wilson HK, Merkel RL, Burket R. Sex differences in effectiveness of extended-release stimulant medication among adolescents with attention-deficit/hyperactivity disorder. Journal of Clinical Psychology in Medical Settings 2009;16(3):233-42. [DOI: 10.1007/s10880-009-9165-8] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Wilson HK, Cox DJ, Merkel RL, Moore M, Coghill D. Effect of extended release stimulant-based medications on neuropsychological functioning among adolescents with attention-deficit/hyperactivity disorder. Archives of Clinical Neuropsychology 2006;21(8):797-807. [DOI: 10.1016/j.acn.2006.06.016] [PMID: ] [DOI] [PubMed] [Google Scholar]
CRIT124US02 {published data only}
- CRIT124US02. A multicenter, double-blind, randomized, placebo-controlled, crossover study evaluation the efficacy and safety of methylphenidate-hydrochloride-long-acting in female adolescents diagnosed with attention deficit/hyperactivity disorder (ADHD); updated 9 September 2005. novctrd.com/ctrdweb/trialresult/trialresults/pdf?trialResultId=1624.
Döpfner 2004 {published data only}
- Döpfner M, Gerber WD, Banaschewski T, Breuer D, Freisleder FJ, Gerber-von Müller G, et al. Comparative efficacy of once-a-day extended-release methylphenidate, two-times-daily immediate-release methylphenidate, and placebo in a laboratory school setting. European Child & Adolescent Psychiatry 2004;13(1 Suppl):i93-101. [DOI: 10.1007/s00787-004-1009-3] [DOI] [PubMed] [Google Scholar]
- Döpfner M, Schröder S, Schmidt J, Lehmkuhl G. Duration of action of a single dose of methylphenidate Retard compared to twice immediate-release methylphenidate in children and adolescents with ADHD [Wirkdauer einer einmaligen Gabe von Methylphenidat-Retard im Vergleich zu zweimaliger Gabe von schnell freisetzendem Methylphenidat bei Kindern und Jugendlichen mit ADHS]. Klinikum der Universität zu Köln, Klinik und Poliklinik für Psychiatrie und Psychotherapie des Kindes und Jugendalters 2003.
- Gerber WD, Gerber-von Müller G, Andrasik F, Niederberger U, Siniatchkin M, Kowalski JT, et al. The impact of a multimodal summer camp training on neuropsychological functioning in children and adolescents with ADHD: an exploratory study. Child Neuropsychology 2012;18(3):242-55. [DOI: 10.1080/09297049.2011.599115] [DOI] [PubMed] [Google Scholar]
- Gerber-von Müller G, Petermann U, Petermann F, Niederberger U, Stephani U, Siniatchkin M, et al. ADHD summer camp: development and evaluation of a multimodal intervention program [Das ADHD-summer-camp-entwicklung und evaluation eines multimodalen programms]. Kindheit und Entwicklung 2009;18(3):162-72. [DOI: 10.1026/0942-5403.18.3.162] [DOI] [Google Scholar]
- Lehmkuhl G. Double-blind, non-inferiority trial investigating the duration of action of Medikinet-retard vs immediate-release methylphenidate vs placebo across the day in children with attention deficit hyperactivity disorder (ADHD). Integrated final report. Phase III. Universitätsklinikum Essen. Project number Medikinet-retard (R) Trial 6520-0073-01 2005.
- Uebel H, Albrecht B, Kirov R, Heise A, Döpfner M, Freisleder FJ, et al. What can actigraphy add to the concept of labschool design in clinical trials? Current Pharmaceutical Design 2010;16(22):2434-42. [DOI: 10.2174/138161210791959845] [DOI] [PubMed] [Google Scholar]
Douglas 1986 {published data only}
- Douglas VI, Barr RG, O'Neill ME, Britton BG. Short term effects of methylphenidate on the cognitive, learning and academic performance of children with attention deficit disorder in the laboratory and the classroom. Journal of Child Psychology and Psychiatry, and Allied Disciplines 1986;27(2):191-211. [DOI: 10.1111/j.1469-7610.1986.tb02330.x] [DOI] [PubMed] [Google Scholar]
Douglas 1995 {published data only}
- Douglas VI, Barr RG, Desilets J, Sherman E. Do high doses of stimulants impair flexible thinking in attention-deficit hyperactivity disorder? Journal of the American Academy of Child and Adolescent Psychiatry 1995;34(7):877-85. [DOI: 10.1097/00004583-199507000-00011] [DOI] [PubMed] [Google Scholar]
DuPaul 1996 {published data only}
- DuPaul GJ, Anastopoulos AD, Kwasnik D, Barkley RA, McMurray MB, DuPaul GJ. Methylphenidate effects on children with attention deficit hyperactivity disorder: self-report of symptoms, side-effects, and self-esteem. Journal of Attention Disorders 1996;1(1):3-15. [DOI: 10.1177/108705479600100101] [DOI] [Google Scholar]
Duric 2012 {published data only}
- Duric NS, Assmus J, Gundersen D, Duric Golos A, Elgen IB. Multimodal treatment in children and adolescents with attention-deficit/hyperactivity disorder: a 6-month follow-up. Nordic Journal of Psychiatry 2017;71(5):386-94. [DOI: 10.1080/08039488.2017.1305446] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Duric NS, Assmus J, Gundersen D, Elgen IB. Neurofeedback for the treatment of children and adolescents with ADHD: a randomized and controlled clinical trial using parental reports. BMC Psychiatry 2012;12(1):1-8. [DOI: 10.1186/1471-244X-12-107] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01252446. Children with ADHD symptoms: comorbid conditions, cognitive and social performance (NF ADHD) [Children with ADHD symptoms: comorbid conditions, cognitive and social performance]. clinicaltrials.gov/ct2/show/NCT01252446 (first received 2 December 2010).
Epstein 2011 {published data only}
- Epstein JN, Brinkman WB, Froehlich T, Langberg JM, Narad ME, Antonini TN, et al. Effects of stimulant medication, incentives, and event rate on reaction time variability in children with ADHD. Neuropsychopharmacology 2011;36(5):1060-72. [DOI: 10.1038/npp.2010.243] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fabiano 2007 {published data only}
- Fabiano GA, Pelham WE Jr, Gnagy EM, Burrows-MacLean L, Coles EK, Chacko A, et al. The single and combined effects of multiple intensities of behavior modification and methylphenidate for children with attention deficit hyperactivity disorder in a classroom setting. School Psychology Review 2007;36(2):195-216. [DOI: 10.1080/02796015.2007.12087940] [DOI]
- Pelham WE, Burrows-Maclean L, Gnagy EM, Fabiano GA, Coles EK, Wymbs BT, et al. A dose-ranging study of behavioral and pharmacological treatment in social settings for children with ADHD. Journal of Abnormal Child Psychology 2014;42(6):1019-31. [DOI: 10.1007/s10802-013-9843-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
Findling 2006 {published data only}
- Findling RL, Quinn D, Hatch SJ, Cameron SJ, DeCory HH, McDowell M. Comparison of the clinical efficacy of twice-daily Ritalin and once-daily Equasym XL with placebo in children with attention deficit/hyperactivity disorder. European Child & Adolescent Psychiatry 2006;15(8):450-9. [DOI: 10.1007/s00787-006-0565-0] [DOI] [PubMed] [Google Scholar]
Findling 2007 {published data only}
- Findling RL, Short EJ, McNamara NK, Demeter CA, Stansbrey RJ, Gracious BL, et al. Methylphenidate in the treatment of children and adolescents with bipolar disorder and attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry 2007;46(11):1445-53. [DOI: 10.1097/chi.0b013e31814b8d3b] [DOI] [PubMed] [Google Scholar]
Findling 2008 {published data only}
- Faraone SV, Glatt SJ, Bukstein OG, Lopez FA, Arnold LE, Findling RL. Effects of once-daily oral and transdermal methylphenidate on sleep behavior of children with ADHD. Journal of Attention Disorders 2009;12(4):308-15. [DOI: 10.1177/1087054708314844] [DOI] [PubMed] [Google Scholar]
- Findling RL, Bukstein OG, Melmed RD, Lopez FA, Sallee FR, Arnold LE, et al. "A randomized, double-blind, placebo-controlled, parallel-group study of methylphenidate transdermal system in pediatric patients with attention-deficit/hyperactivity disorder": correction. Journal of Clinical Psychiatry 2008;69(2):329. [DOI: 10.4088/jcp.v69n0221e] [DOI] [PubMed] [Google Scholar]
- Findling RL, Bukstein OG, Melmed RD, López FA, Sallee FR, Arnold LE, et al. A randomized, double-blind, placebo-controlled, parallel-group study of methylphenidate transdermal system in pediatric patients with attention-deficit/hyperactivity disorder. Journal of Clinical Psychiatry 2008;69(1):149-59. Erratum in: Journal of Clinical Psychiatry. 2008;69(2):329. [DOI: 10.4088/jcp.v69n0120] [PMID: ] [DOI] [PubMed] [Google Scholar]
- NCT00444574. Safety and efficacy of MTS versus Concerta in pediatric patients (aged 6-12 years) with ADHD [A phase III, randomized, double-blind, multi-center, parallel-group, placebo-controlled, dose optimization study, designed to evaluate the safety and efficacy of methylphenidate transdermal system (MTS) vs Concerta® in pediatric patients aged 6-12 with attention-deficit/hyperactivity disorder (ADHD)]. clinicaltrials.gov/ct2/show/NCT00444574 (first received 8 March 2007).
- Swanson JM. Transdermal methylphenidate more effective than placebo for treating ADHD. Evidence-Based Mental Health 2008;11(4):118. [DOI: 10.1136/ebmh.11.4.118] [PMID: ] [DOI] [PubMed] [Google Scholar]
Findling 2010 {published data only}
- Findling RL, Katic A, Rubin R, Moon E, Civil R, Li Y. A 6-month, open-label, extension study of the tolerability and effectiveness of the methylphenidate transdermal system in adolescents diagnosed with attention-deficit/hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 2010;20(5):365-75. [DOI: 10.1089/cap.2009.0122] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Findling RL, Turnbow J, Burnside J, Melmed R, Civil R, Li Y. A randomized, double-blind, multicenter, parallel-group, placebo-controlled, dose-optimization study of the methylphenidate transdermal system for the treatment of ADHD in adolescents. CNS Spectrums 2010;15(7):419-30. [DOI: 10.1017/S1092852900000353] [DOI] [PubMed] [Google Scholar]
- Keating GM. Methylphenidate transdermal system in attention-deficit hyperactivity disorder in adolescentsy. Drugs in R&D 2012;12(3):171-3. [DOI: 10.2165/11207490-000000000-00000] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00501293. Evaluate the safety and efficacy of methylphenidate transdermal system (MTS) in adolescents aged 13-17 years with ADHD. https://clinicaltrials.gov/ct2/show/NCT00501293 2007.
Fine 1993 {published data only}
- Fine S, Johnston C. Drug and placebo side effects in methylphenidate-placebo trial for attention deficit hyperactivity disorder. Child Psychiatry and Human Development 1993;24(1):25-30. [DOI: 10.1007/BF02353715] [DOI] [PubMed] [Google Scholar]
- Johnston C, Fine S. Methods of evaluating methylphenidate in children with attention deficit hyperactivity disorder: acceptability, satisfaction and compliance. Journal of Pediatric Psychology 1993;18(6):717-30. [DOI: 10.1093/jpepsy/18.6.717] [DOI] [PubMed] [Google Scholar]
Firestone 1981 {published data only}
- Firestone P, Kelly MJ, Goodman JT, Davey J. Differential effects of parent training and stimulant medication with hyperactives: a progress report. Journal of the American Academy of Child and Adolescent Psychiatry 1981;20(1):135-47. [DOI: 10.1016/S0002-7138(09)60723-8] [DOI] [PubMed] [Google Scholar]
Fitzpatrick 1992a {published data only}
- Fitzpatrick PA, Klorman R, Brumaghim JT, Borgstedt AD. Effects of sustained-release and standard preparations of methylphenidate on attention deficit disorder. Journal of the American Academy of Child and Adolescent Psychiatry 1992;31(2):226-34. [DOI: 10.1097/00004583-199203000-00008] [DOI] [PubMed] [Google Scholar]
- Fitzpatrick PS. Effects of Sustained-Release and Standard Preparations of Methylphenidate on Attention Deficit Hyperactivity Disorder: Clinical Outcome, Performance, and Cognitive Event-Related Potentials [PhD thesis]. New York, USA: University of Rochester, 1990. [PROQUEST: 303856642] [Google Scholar]
Flapper 2008 {published data only}
- Flapper BCT, Houwen S, Schoemaker MM. Fine motor skills and effects of methylphenidate in children with attention-deficit-hyperactivity disorder and developmental coordination disorder. Developmental Medicine & Child Neurology 2006;48(3):165-9. [DOI: 10.1017/S0012162206000375] [DOI] [PubMed] [Google Scholar]
- Flapper BCT, Schoemaker MM. Effects of methylphenidate on quality of life in children with both developmental coordination disorder and ADHD. Developmental Medicine & Child Neurology 2008;50(4):294-9. [DOI: 10.1111/j.1469-8749.2008.02039.x] [DOI] [PubMed] [Google Scholar]
Forness 1992 {published data only}
- Forness SR, Cantwell DP, Swanson JM, Hanna GL, Youpa D. Differential effects of stimulant medication on reading performance of boys with hyperactivity with and without conduct disorder. Journal of Learning Disabilities 1991;24(5):304-10. [DOI: 10.1177/002221949102400507] [DOI] [PubMed] [Google Scholar]
- Forness SR, Swanson JM, Cantwell D, Guthrie D, Sena R. Response to stimulant medication across six measures of school-related performance in children with ADHD and disruptive behavior. Behavioral Disorders 1992;18(1):42-53. [DOI: 10.1177/019874299201800105] [DOI] [Google Scholar]
Froehlich 2011 {published data only}
- Froehlich TE, Epstein JN, Nick TG, Melguizo-Castro MS, Stein MA, Brinkman WB, et al. Pharmacogenetic predictors of methylphenidate dose-response in attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry 2011;50(11):1129-39.e2. [DOI: 10.1016/j.jaac.2011.08.002] [PMCID: PMC3225067] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Froehlich 2018 {published data only}
- Becker SP, Froehlich TE, Epstein JN. Effects of methylphenidate on sleep functioning in children with attention-deficit/hyperactivity disorder. Journal of Developmental and Behavioral Pediatrics 2016;37(5):395-404. [DOI: 10.1097/DBP.0000000000000285] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehlich TE, Antonini TN, Brinkman WB, Langberg JM, Simon JO, Adams R, et al. Mediators of methylphenidate effects on math performance in children with attention-deficit hyperactivity disorder. Journal of Developmental and Behavioral Pediatrics 2014;35(2):100-7. [DOI: 10.1097/DBP.0000000000000025] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehlich TE, Becker SP, Nick TG, Brinkman WB, Stein Mark A, Peugh J, et al. Sluggish cognitive tempo as a possible predictor of methylphenidate response in children with ADHD: A randomized controlled trial. Journal of Clinical Psychiatry 2018;79(2):1-18. [DOI: 10.4088/JCP.17m11553] [PMCID: PMC6558969] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehlich TE, Brinkman WiB, Peugh JL, Piedra AN, Vitucci DJ, Epstein JN. Pre-Existing Comorbid Emotional Symptoms Moderate Short-Term Methylphenidate Adverse Effects in a Randomized Trial of Children with Attention-Deficit/Hyperactivity Disorder. Journal of Child and Adolescent Psychopharmacology 2020;30(3):137-47. [DOI: 10.1089/cap.2019.0125] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu S, Piedra AN, Froehlich TE, Brinkman WB, Epstein J. Caregiver-reported side effects of methylphenidate and comorbid oppositional defiant disorder diagnosis in children with attention-deficit/hyperactivity disorder (ADHD). Journal of the American Academy of Child and Adolescent Psychiatry 2017 October;56(10 (Suppl)):282. [DOI: 10.1016/j.jaac.2017.09.363] [DOI] [Google Scholar]
- NCT01727414, Children's Hospital Medical Center Cincinnati. Attention Deficit Disorder Medication Response Study. https://clinicaltrials.gov/ct2/show/NCT01727414 2012.
- Orban SA, Karamchandani TA, Tamm L, Sidol CA, Peugh J, Froehlich TE, et al. Attention-deficit/hyperactivity disorder-related deficits and psychostimulant medication effects on comprehension of audiovisually presented educational material in children. Journal of Child and Adolescent Psychopharmacology 2018;28(10):727-38. [CENTRAL: PMC6306678] [DOI: 10.1089/cap.2018.0006] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner-Schuman ML, Froehlich TE, Kottyan LC, Brinkman WB, Ramsey LB, Epstein J. Pharmacogenomics of methylphenidate side effects in children with attention-deficit/hyperactivity disorder (ADHD). Journal of the American Academy of Child and Adolescent Psychiatry 2017;56(10 (Suppl)):289. [DOI: 10.1016/j.jaac.2017.09.383] [DOI] [Google Scholar]
Gadow 1990 {published data only}
- Gadow KD, Nolan EE, Paolicelli LM, Sprafkin J. A procedure for assessing the effects of methylphenidate on hyperactive children in public school settings. Journal of Clinical Child Psychology 1991;20(3):268-76. [DOI: 10.1207/s15374424jccp2003_5] [DOI] [Google Scholar]
- Gadow KD, Nolan EE, Sverd J, Sprafkin J, Paolicelli L. Methylphenidate in aggressive-hyperactive boys: I. Effects on peer aggression in public school settings. Journal of the American Academy of Child and Adolescent Psychiatry 1990;29(5):710-8. [DOI: 10.1097/00004583-199009000-00006] [DOI] [PubMed] [Google Scholar]
- Gadow KD, Paolicelli LM, Nolan EE, Schwartz J, Sprafkin J, Sverd J. Methylphenidate in aggressive hyperactive boys: II. Indirect effects of medication treatment on peer behavior. Journal of Child and Adolescent Psychopharmacology 1992;2(1):49-61. [DOI: 10.1089/cap.1992.2.49] [DOI] [PubMed] [Google Scholar]
Gadow 1995 {published data only}
- Gadow D, Nolan E, Sprafkin J, Sverd J. School observations of children with attention-deficit hyperactivity disorder and comorbid tic disorder: effects of methylphenidate treatment. Journal of Developmental and Behavioral Pediatrics 1995;16(3):167–76. [DOI: 10.1097/00004703-199506000-00004] [DOI] [PubMed] [Google Scholar]
- Gadow D, Sverd J, Sprafkin J, Nolan E, Ezor N. Efficacy of methylphenidate for attention-deficit hyperactivity disorder in children with tic disorder. In: Annual Progress in Child Psychiatry and Child Development. New York: Brunner/Mazel Publishers, 1996:494-522. [Google Scholar]
- Gadow KD, Nolan EE, Sverd J, Sprafkin J, Schwartz J. Anxiety and depression symptoms and response to methylphenidate in children with attention-deficit hyperactivity disorder and tic disorder. Journal of Clinical Psychopharmacology 2002;22(3):267-74. [DOI: 10.1097/00004714-200206000-00007] [DOI] [PubMed] [Google Scholar]
- Gadow KD, Nolan EE, Sverd J. Methylphenidate in hyperactive boys with comorbid tic disorder: II. Short-term behavioral effects in school settings. Journal of the American Academy of Child and Adolescent Psychiatry 1992;31(3):462-71. [DOI: 10.1097/00004583-199205000-00012] [DOI] [PubMed] [Google Scholar]
- Gadow KD, Sverd J, Sprafkin J, Nolan EE, Grossman S. Long-term methylphenidate therapy in children with comorbid attention-deficit hyperactivity disorder and chronic multiple tic disorder. Archives of General Psychiatry 1999;56(4):330-6. [DOI: 10.1001/archpsyc.56.4.330] [DOI] [PubMed] [Google Scholar]
- Nolan EE, Gadow KD. Children with ADHD and tic disorder and their classmates: behavioral normalization with methylphenidate. Journal of the American Academy of Child and Adolescent Psychiatry 1997;36(5):597-604. [DOI: 10.1097/00004583-199705000-00009] [DOI] [PubMed] [Google Scholar]
- Nolan EE, Gadow KD. Relation between ratings and observations of stimulant drug response in hyperactive children. Journal of Clinical Child Psychology 1994;23(1):78-90. [DOI: 10.1207/s15374424jccp2301_10] [DOI] [Google Scholar]
- Sprafkin J, Gadow KD. Double-blind versus open evaluations of stimulant drug response in children with attention-deficit hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 1996;6(4):215-28. [DOI: 10.1089/cap.1996.6.215] [DOI] [PubMed] [Google Scholar]
- Sverd J, Gadow KD, Nolan EE, Sprafkin J, Ezor SN. Methylphenidate in hyperactive boys with comorbid tic disorder: I. Clinic evaluations. Advances in Neurology 1992;58:271-81. [INIST-CNRS: 5607952] [URL: http://pascal-francis.inist.fr/vibad/index.php?action=getRecordDetail&idt=5607952] [PubMed] [Google Scholar]
Gadow 2007 {published data only}
- Gadow KD, Nolan E, Sprafkin J, Sverd J. School observations of children with attention-deficit hyperactivity disorder and comorbid tic disorder: effects of methylphenidate treatment. Journal of Developmental and Behavioral Pediatrics 1995;16(3):167-76. [DOI: 10.1097/00004703-199506000-00004] [DOI] [PubMed] [Google Scholar]
- Gadow KD, Nolan EE, Sverd J, Sprafkin J, Schneider J. Methylphenidate in children with oppositional defiant disorder and both comorbid chronic multiple tic disorder and ADHD. Journal of Child Neurology 2008;23(9):981-90. [DOI: 10.1177/0883073808315412] [DOI] [PubMed] [Google Scholar]
- Gadow KD, Sverd J, Nolan EE, Sprafkin J, Schneider J. Immediate-release methylphenidate for ADHD in children with comorbid chronic multiple tic disorder. Journal of the American Academy of Child and Adolescent Psychiatry 2007;46(7):840-8. [DOI: 10.1097/chi.0b013e31805c0860] [DOI] [PubMed] [Google Scholar]
- Gadow KD, Sverd J, Sprafkin J, Nolan EE, Ezor SN. Efficacy of methylphenidate for attention-deficit hyperactivity disorder in children with tic disorder. Archives of General Psychiatry 1995;52(6):444-55. [DOI: 10.1001/archpsyc.1995.03950180030005] [DOI] [PubMed] [Google Scholar]
- NCT00441649. Methylphenidate for treating children with ADHD and Tourette Syndrome [Methylphenidate treatment of ADHD in children with Tourette Syndrome]. clinicaltrials.gov/ct2/show/NCT00441649 (first received 1 March 2007).
Gadow 2011 {published data only}
- Gadow KD, Nolan EE. Methylphenidate and comorbid anxiety disorder in children with both chronic multiple tic disorder and ADHD. Journal of Attention Disorders 2011;15(3):246-56. [DOI: 10.1177/1087054709356405] [DOI] [PubMed] [Google Scholar]
Garfinkel 1983 {published data only}
- Garfinkel BD, Wender PH, Sloman L, O'Neill I. Tricyclic antidepressant and methylphenidate treatment of attention deficit disorder in children. Journal of the American Academy of Child and Adolescent Psychiatry 1983;22(4):343-8. [DOI: 10.1016/S0002-7138(09)60669-5] [DOI] [PubMed] [Google Scholar]
Gonzalez‐Heydrich 2010 {published data only}
- Gonzalez-Heydrich J, Whitney J, Hsin O, Mrakotsky C, MacMillan C, Torres A, et al. Tolerability of OROS-MPH 18 and 36 mg in paediatric epilepsy plus attention deficit/hyperactivity disorder (ADHD). Epilepsia. Proceedings of the 26th International Epilepsy Congress; 2005 August 28th - September 1st; Paris, France 2005;46(Suppl s6):179. [DOI: 10.1111/j.1528-1167.2005.460602.x] [DOI] [Google Scholar]
- Gonzalez-Heydrich J, Whitney J, Waber D, Forbes P, Hsin O, Faraone SV, et al. Adaptive phase I study of OROS methylphenidate treatment of attention deficit hyperactivity disorder with epilepsy. Epilepsy & Behavior 2010;18(3):229-37. [DOI: 10.1016/j.yebeh.2010.02.022] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Heydrich J. OROS methylphenidate for attention-deficit/hyperactivity disorder plus epilepsy. Pharmacy and Therapeutics 2006;31(12):725-6. [EMBASE: 2007065321] [Google Scholar]
- NCT00323947. Methylphenidate for treating attention deficit hyperactivity disorder in children with both ADHD and epilepsy. www.clinicaltrials.gov/ct2/show/NCT00323947 (accessed 1 May 2013).
Gorman 2006 {published data only}
- Chang HT. Effects of methylphenidate on performance and private speech of children with attention-deficit/hyperactivity disorder during the Tower of Hanoi task. Dissertation Abstracts International: Section B: The Sciences and Engineering 2001;62(1-B):540. [PROQUEST: 304727139] [Google Scholar]
- Gorman EB, Klorman R, Thatcher JE, Borgstedt AD. Effects of methylphenidate on subtypes of attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry 2006;45(7):808-16. [DOI: 10.1097/01.chi.0000214191.57993.dd] [DOI] [PubMed] [Google Scholar]
- Kopecky H, Chang HT, Klorman R, Thatcher JE, Borgstedt AD. Performance and private speech of children with attention-deficit/hyperactivity disorder while taking the Tower of Hanoi test: effects of depth of search, diagnostic subtype, and methylphenidate. Journal of Abnormal Child Psychology 2005;33(5):625-38. [DOI: 10.1007/s10802-005-6742-7] [DOI] [PubMed] [Google Scholar]
Green 2011 {published data only}
- Green T, Weinberger R, Diamond A, Berant M, Hirschfeld L, Frisch A, et al. The effect of methylphenidate on prefrontal cognitive functioning, inattention, and hyperactivity in velocardiofacial syndrome. Journal of Child and Adolescent Psychopharmacology 2011;21(6):589-95. [DOI: 10.1089/cap.2011.0042] [DOI] [PubMed] [Google Scholar]
- Green T, Weinberger R, Weizman A, Kotler M, Gothelf D. Effect of methylphenidate on neurocognitive functioning in velocardiofacial syndrome: a randomized placebo-controlled trial. Biological Psychiatry 2009;65(Suppl 8):147. [DOI: 10.1016/j.biopsych.2009.03.002] [DOI] [Google Scholar]
- NCT00768820. The psychiatric and cognitive phenotypes in velocardiofacial syndrome (VCFS) [The psychiatric and cognitive phenotypes in velocardiofacial syndrome (VCFS), Williams Syndrome (WS)and Fragile X Syndrome characterization, treatment and examining the connection to developmental and molecular factors]. clinicaltrials.gov/ct2/show/NCT00768820 (first received 8 October 2008).
Greenhill 2002 {published data only}
- Greenhill LL, Findling RL, Swanson JM, ADHD Study Group. A double-blind, placebo-controlled study of modified-release methylphenidate in children with attention-deficit/hyperactivity disorder. Pediatrics 2002;109(3):e39. [DOI: 10.1542/peds.109.3.e39] [DOI] [PubMed] [Google Scholar]
Greenhill 2006 {published data only}
- Greenhill LL, Muniz R, Ball RR, Levine A, Pestreich L, Jiang H. Efficacy and safety of dexmethylphenidate extended-release capsules in children with attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry 2006;45(7):817-23. [DOI: 10.1097/01.chi.0000220847.41027.5d] [DOI] [PubMed] [Google Scholar]
Gruber 2007 {published data only}
- Gruber R, Grizenko N, Schwartz G, Bellingham J, Guzman R, Joober R. Performance on the continuous performance test in children with ADHD is associated with sleep efficiency. Sleep 2007;30(8):1003-9. [DOI: 10.1093/sleep/30.8.1003] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber R, Grizenko N, Schwartz G, Benamor L, Ter-Stepanian M, Joober R. The associations between sleep and attention in children with attention deficit hyperactivity disorder while on placebo and while on methylphenidate. Sleep. Proceedings of the 19th Annual Meeting of the Associated Professional Sleep Societies; 2005 June 18-23; Denver, CO 2005;28:A93-4. [WOS: 000228906100277] [Google Scholar]
Hale 2011 {published data only}
- Hale JB, Reddy LA, Semrud-Clikeman M, Hain LA, Whitaker J, Morley J, et al. Executive impairment determines ADHD medication response: implications for academic achievement. Journal of Learning Disabilities 2011;44(2):196-212. [DOI: 10.1177/0022219410391191] [DOI] [PubMed] [Google Scholar]
- Kubas HA, Backenson EM, Wilcox G, Piercy JC, Hale JB. The effects of methylphenidate on cognitive function in children with attention-deficit/hyperactivity disorder. Postgraduate Medicine 2012;124(5):33-48. [DOI: 10.3810/pgm.2012.09.2592] [DOI] [PubMed] [Google Scholar]
Hawk 2018 {published data only}
- Ashare RL, Hawk LW Jr, Shiels K, Rhodes JD, Pelham WE Jr, Waxmonsky JG. Methylphenidate enhances prepulse inhibition during processing of task-relevant stimuli in attention-deficit/hyperactivity disorder. Psychophysiology 2010;47(5):838-45. [DOI: 10.1111/j.1469-8986.2010.01001.x] [PMCID: PMC2906628] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawk LW Jr, Fosco WD, Colder CR, Waxmonsky JG, Pelham WE Jr, Rosch KS. How do stimulant treatments for ADHD work? Evidence for mediation by improved cognition. Journal of Child Psychology and Psychiatry 2018;59(12):1271-81. [DOI: 10.1111/jcpp.12917] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Heriot 2008 {published data only}
- Heriot SA, Evans IM, Foster TM. Critical influences affecting response to various treatments in young children with ADHD: a case series. Child: Care, Health and Development 2008;34(1):121-33. [DOI: 10.1111/j.1365-2214.2007.00745.x] [DOI] [PubMed] [Google Scholar]
Hicks 1985 {published data only}
- Gualtieri CT, Hicks RE, Mayo JP, Schroeder SR. The persistence of stimulant effects in chronically treated children: further evidence of an inverse relationship between drug effects and placebo levels of response. Psychopharmacology 1984;83(1):44-7. [DOI: 10.1007/BF00427420] [DOI] [PubMed] [Google Scholar]
- Hicks RE, Gualtieri CT, Mayo JP, Schroeder SR, Lipton MA. Methylphenidate and homeostasis: drug effects on the cognitive performance of hyperactive children. In: Bloomingdale Lewis M, editors(s). Attention Deficit Disorder: Identification, Course and Treatment Rationale. New York, USA: SP Medical & Scientific Books, 1985:131-41. [Google Scholar]
Hoeppner 1997 {published data only}
- Hoeppner JA, Hale JB, Bradley AM, Byrnes M, Coury DL, Lennie L, et al. A clinical protocol for determining methylphenidate dosage levels in ADHD. Journal of Attention Disorders 1997;2(1):19-30. [DOI: 10.1177/108705479700200102] [DOI] [Google Scholar]
Horn 1991 {published data only}
- Horn WF, Ialongo NS, Pascoe JM, Greenberg G, Packard T, Lopez M, et al. Additive effects of psychostimulants, parent training, and self-control therapy with ADHD children. Journal of the American Academy of Child and Adolescent Psychiatry 1991;30(2):233-40. [DOI: 10.1097/00004583-199103000-00011] [DOI] [PubMed] [Google Scholar]
- Ialongo NS, Horn WF, Pascoe JM, Greenberg G, Packard T, Lopez M, et al. The effects of a multimodal intervention with attention-deficit hyperactivity disorder children: a 9-month follow-up. Journal of the American Academy of Child and Adolescent Psychiatry 1993;32(1):182-9. [DOI: 10.1097/00004583-199301000-00026] [DOI] [PubMed] [Google Scholar]
Huang 2021 {published data only}
- Huang Y-S, Yeh C-B, Chen C-H, Shang C-Y, Gau SS-F. A randomized, double-blind, placebo-controlled, two-way crossover clinical trial of Oradur-methylphenidate for treating children and adolescents with attention-deficit/hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 2021;31(3):164-78. [DOI: 10.1089/cap.2020.0104] [PMCID: PMC8066345] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02450890. Evaluate safety and efficacy of ORADUR®-methylphenidate in children and adolescents with ADHD [A phase III, multi-center, randomized, double-blind, placebo controlled, two-way cross-over clinical study to evaluate safety and efficacy of Oradur®-methylphenidate in children and adolescents with ADHD]. clinicaltrials.gov/ct2/show/NCT02450890 (first received 21 May 2015).
Ialongo 1994 {published data only}
- Ialongo NS, Lopez M, Horn WF, Pascoe JM, Greenberg G. Effects of psychostimulant medication on self-perceptions of competence, control, and mood in children with attention deficit hyperactivity disorder. Journal of Clinical Child Psychology 1994;23(2):161-73. [DOI: 10.1207/s15374424jccp2302_6] [DOI] [Google Scholar]
Jacobi‐Polishook 2009 {published data only}
- Jacobi-Polishook T, Shorer Z, Melzer I. The effect of methylphenidate on postural stability under single and dual task conditions in children with attention deficit hyperactivity disorder - a double blind randomized control trial. Journal of the Neurological Sciences 2009;280(1-2):15-21. [DOI: 10.1016/j.jns.2009.01.007] [DOI] [PubMed] [Google Scholar]
- NCT00485797. Effects of Ritalin on postural stability of hyper active children [Effects of methylphenidate on postural stability of children suffer from attention deficit hyperactivity disorder (ADHD) under single and dual task conditions]. clinicaltrials.gov/ct2/show/NCT00485797 (first received 13 June 2007).
Jensen 1999 (MTA) {published data only}
- Abikoff H. Tailored psychosocial treatments for ADHD: the search for a good fit. Journal of Clinical Child Psychology 2001;30(1):122-5. [DOI: 10.1207/S15374424JCCP3001_14] [DOI] [PubMed] [Google Scholar]
- Arnold LE, Abikoff HB, Cantwell DP, Conners CK, Elliott G, Greenhill LL, et al. National Institute of Mental Health Collaborative Multimodal Treatment Study of Children with ADHD (the MTA). Design challenges and choices. Archives of General Psychiatry 1997;54(9):865-70. [DOI: 10.1001/archpsyc.1997.01830210113015] [DOI] [PubMed] [Google Scholar]
- Arnold LE, Abikoff HB, Cantwell DP, Conners CK, Elliott GR, Greenhill LL, et al. NIMH Collaborative Multimodal Treatment Study of Children with ADHD (MTA): design, methodology, and protocol evolution. Journal of Attention Disorders 1997;2(3):141-58. [DOI: 10.1177/108705479700200301] [DOI] [Google Scholar]
- Arnold LE, Chuang S, Davies M, Abikoff HB, Conners CK, Elliott GR, et al. Nine months of multicomponent behavioral treatment for ADHD and effectiveness of MTA fading procedures. Journal of Abnormal Child Psychology 2004;32(1):39-51. [DOI: 10.1023/B:JACP.0000007579.61289.31] [DOI] [PubMed] [Google Scholar]
- Arnold LE, Elliott M, Sachs L, Bird H, Kraemer HC, Wells KC, et al. Effects of ethnicity on treatment attendance, stimulant response/dose, and 14-month outcome in ADHD. Journal of Consulting and Clinical Psychology 2003;71(4):713-27. [DOI: 10.1037/0022-006X.71.4.713] [DOI] [PubMed] [Google Scholar]
- Carey WB. What the multimodal treatment study of children with attention deficit/hyperactivity disorder did and did not say about the use of methylphenidate for attention deficits. Pediatrics 2000;105(4):863-4. [DOI: 10.1542/peds.105.4.863] [DOI] [PubMed] [Google Scholar]
- Conners CK, Epstein JN, March JS, Angold A, Wells KC, Klaric J, et al. Multimodal treatment of ADHD in the MTA: an alternative outcome analysis. Journal of the American Academy of Child and Adolescent Psychiatry 2001;40(2):159-67. [DOI: 10.1097/00004583-200102000-00010] [DOI] [PubMed] [Google Scholar]
- Galanter CA, Carlson GA, Jensen PS, Greenhill LL, Davies M, Li W, et al. Response to methylphenidate in children with attention deficit hyperactivity disorder and manic symptoms in the multimodal treatment study of children with attention deficit hyperactivity disorder titration trial. Journal of Child and Adolescent Psychopharmacology 2003;13(2):123-36. [DOI: 10.1089/104454603322163844] [DOI] [PubMed] [Google Scholar]
- Greene RW, Ablon JS. What does the MTA study tell us about effective psychosocial treatment for ADHD? Journal of Clinical Child Psychology 2001;30(1):114-21. [DOI: 10.1207/S15374424JCCP3001_13] [DOI] [PubMed] [Google Scholar]
- Greenhill LL, Abikoff HB, Arnold LE, Cantwell DP, Conners CK, Elliott G, et al. Medication treatment strategies in the MTA Study: relevance to clinicians and researchers. Journal of the American Academy of Child and Adolescent Psychiatry 1996;35(10):1304-13. [DOI: 10.1097/00004583-199610000-00017] [DOI] [PubMed] [Google Scholar]
- Greenhill LL, Swanson JM, Vitiello B, Davies M, Clevenger W, Wu M, et al. Impairment and deportment responses to different methylphenidate doses in children with ADHD: the MTA titration trial. Journal of the American Academy of Child and Adolescent Psychiatry 2001;40(2):180-7. [DOI: 10.1097/00004583-200102000-00012] [DOI] [PubMed] [Google Scholar]
- Hansen L, Thomsen PH. How to treat ADHD/DAMP? Is there a conclusive answer? A critical survey of the MTA trial. Ugeskrift for Laeger 2005;167(48):4555-9. [PMID: ] [PubMed] [Google Scholar]
- Hinshaw SP, March JS, Abikoff H, Arnold LE, Cantwell DP, Conners CK, et al. Comprehensive assessment of childhood attention-deficit hyperactivity disorder in the context of a multisite, multimodal clinical trial. Journal of Attention Disorders 1997;1(4):217-34. [DOI: 10.1177/108705479700100403] [DOI] [Google Scholar]
- Jensen PS, Arnold LE, Richters JE, Severe JB, Vereen D, Vitiello B, et al. A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. Archives of General Psychiatry 1999;56(12):1073-86. [DOI: 10.1001/archpsyc.56.12.1073] [DOI] [PubMed] [Google Scholar]
- Jensen PS, Arnold LE, Severe JB, Vitiello B, Hoagwood K. National Institute of Mental Health multimodal treatment study of ADHD follow-up: changes in effectiveness and growth after the end of treatment. Pediatrics 2004;113(4):762-9. [DOI: 10.1542/peds.113.4.762] [DOI] [PubMed] [Google Scholar]
- Jensen PS, Arnold LE, Swanson JM, Vitiello B, Abikoff HB, Greenhill LL, et al. 3-Year follow-up of the NIMH MTA study. Journal of the American Academy of Child and Adolescent Psychiatry 2007;46(8):989-1002. [DOI: 10.1097/CHI.0b013e3180686d48] [DOI] [PubMed] [Google Scholar]
- Jensen PS, Hinshaw SP, Kraemer HC, Lenora N, Newcorn JH, Abikoff HB, et al. ADHD comorbidity findings from the MTA study: comparing comorbid subgroups. Journal of the American Academy of Child and Adolescent Psychiatry 2001;40(2):147-58. [DOI: 10.1097/00004583-200102000-00009] [DOI] [PubMed] [Google Scholar]
- Jensen PS, Hinshaw SP, Swanson JM, Greenhill LL, Conners CK, Arnold LE, et al. Findings from the NIMH Multimodal Treatment Study of ADHD (MTA): implications and applications for primary care providers. Journal of Developmental and Behavioral Pediatrics 2001;22(1):60-73. [DOI: 10.1097/00004703-200102000-00008] [DOI] [PubMed] [Google Scholar]
- MTA Cooperative Group. National Institute of Mental Health Multimodal Treatment Study of ADHD follow-up: 24-month outcomes of treatment strategies for attention-deficit/hyperactivity disorder. Pediatrics 2004;113(4):754-61. [DOI: 10.1542/peds.113.4.754] [DOI] [PubMed] [Google Scholar]
- March JS, Swanson JM, Arnold LE, Hoza B, Conners CK, Hinshaw SP, et al. Anxiety as a predictor and outcome variable in the multimodal treatment study of children with ADHD (MTA). Journal of Abnormal Child Psychology 2000;28(6):527-41. [DOI: 10.1023/A:1005179014321] [DOI] [PubMed] [Google Scholar]
- Molina BSG, Flory K, Hinshaw SP, Greiner AR, Arnold LE, Swanson JM, et al. Delinquent behavior and emerging substance use in the MTA at 36 months: prevalence, course, and treatment effects. Journal of the American Academy of Child and Adolescent Psychiatry 2007;46(8):1028-40. [DOI: 10.1097/chi.0b013e3180686d96] [DOI] [PubMed] [Google Scholar]
- Molina BSG, Hinshaw SP, Arnold LE, Swanson JM, Pelham WE, Hechtman L, et al. Adolescent substance use in the multimodal treatment study of attention-deficit/hyperactivity disorder (ADHD) (MTA) as a function of childhood ADHD, random assignment to childhood treatments, and subsequent medication. Journal of the American Academy of Child and Adolescent Psychiatry 2013;52(3):250-63. [DOI: 10.1016/j.jaac.2012.12.014] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina BSG, Hinshaw SP, Swanson JM, Arnold LE, Vitiello B, Jensen PS, et al. The MTA at 8 years: prospective follow-up of children treated for combined-type ADHD in a multisite study. Journal of the American Academy of Child and Adolescent Psychiatry 2009;48(5):484-500. [DOI: 10.1097/CHI.0b013e31819c23d0] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00000388. Multimodal Treatment Study of children with attention deficit and hyperactivity disorder (MTA) [Multimodal Treatment Study of children with ADHD]. clinicaltrials.gov/ct2/show/NCT00000388 (first received 3 November 1999).
- Nieweg EH. Does ADHD medication stop working after 2-3 years? On the surprising, but little known follow-up of the MTA study [Is ADHD-medicatie na 2-3 jaar uitgewerkt? Over de verrassende, maar weinig bekende follow-up van het MTA-onderzoek]. Tijdschrift voor Psychiatrie 2010;52(4):245-54. [PMID: ] [PubMed] [Google Scholar]
- Owens EB, Hinshaw SP, Kraemer HC, Arnold LE, Abikoff HB, Cantwell DP, et al. Which treatment for whom for ADHD? Moderators of treatment response in the MTA. Journal of Consulting and Clinical Psychology 2003;71(3):540-52. [DOI: 10.1037/0022-006X.71.3.540] [DOI] [PubMed] [Google Scholar]
- Pelham WE Jr. The NIMH multimodal treatment study for attention-deficit hyperactivity disorder: just say yes to drugs alone? Canadian Journal of Psychiatry 1999;44(10):981-90. [DOI: 10.1177/070674379904401004] [DOI] [PubMed] [Google Scholar]
- Pelham WE, Gnagy EM, Greiner AR, Hoza B, Hinshaw SP, Swanson JM, et al. Behavioral versus behavioral and pharmacological treatment in ADHD children attending a summer treatment program. Journal of Abnormal Child Psychology 2000;28(6):507-25. [DOI: 10.1023/A:1005127030251] [DOI] [PubMed] [Google Scholar]
- Richters JE, Arnold LE, Jensen PS, Abikoff H, Conners CK, Greenhill LL, et al. NIMH collaborative multisite multimodal treatment study of children with ADHD: I. Background and rationale. Journal of the American Academy of Child and Adolescent Psychiatry 1995;34(8):987-1000. [DOI: 10.1097/00004583-199508000-00008] [DOI] [PubMed] [Google Scholar]
- Swanson J, Arnold LE, Kraemer H, Hechtman L, Molina B, Hinshaw S, et al. Evidence, interpretation, and qualification from multiple reports of long-term outcomes in the Multimodal Treatment Study of Children with ADHD (MTA): part I: executive summary. Journal of Attention Disorders 2008;12(1):4-14. [DOI: 10.1177/1087054708319345] [DOI] [PubMed] [Google Scholar]
- Swanson J, Arnold LE, Kraemer H, Hechtman L, Molina B, Hinshaw S, et al. Evidence, interpretation, and qualification from multiple reports of long-term outcomes in the Multimodal Treatment Study of Children with ADHD (MTA): part II: supporting details. Journal of Attention Disorders 2008;12(1):15-43. [DOI: 10.1177/1087054708319525] [DOI] [PubMed] [Google Scholar]
- Swanson JM, Elliott GR, Greenhill LL, Wigal T, Arnold LE, Vitiello B, et al. Effects of stimulant medication on growth rates across 3 years in the MTA follow-up. Journal of the American Academy of Child and Adolescent Psychiatry 2007;46(8):1015-27. [DOI: 10.1097/chi.0b013e3180686d7e] [DOI] [PubMed] [Google Scholar]
- Swanson JM, Hinshaw SP, Arnold LE, Gibbons RD, Marcus S, Hur K, et al. Secondary evaluations of MTA 36-month outcomes: propensity score and growth mixture model analyses. Journal of the American Academy of Child and Adolescent Psychiatry 2007;46(8):1003-14. [DOI: 10.1097/CHI.0b013e3180686d63] [DOI] [PubMed] [Google Scholar]
- Swanson JM, Kraemer HC, Hinshaw SP, Arnold LE, Conners CK, Abikoff HB, et al. Clinical relevance of the primary findings of the MTA: success rates based on severity of ADHD and ODD symptoms at the end of treatment. Journal of the American Academy of Child and Adolescent Psychiatry 2001;40(2):168-79. [DOI: 10.1097/00004583-200102000-00011] [DOI] [PubMed] [Google Scholar]
- The MTA Cooperative Group. Moderators and mediators of treatment response for children with attention-deficit/hyperactivity disorder: the multimodal treatment study of children with attention-deficit/hyperactivity disorder. Archives of General Psychiatry 1999;56(12):1088-96. [DOI: 10.1001/archpsyc.56.12.1088] [DOI] [PubMed] [Google Scholar]
- Vitiello B, Elliott GR, Swanson JM, Arnold LE, Hechtman L, Abikoff H, et al. Blood pressure and heart rate over 10 years in the Multimodal Treatment Study of Children with ADHD. American Journal of Psychiatry 2012;169(2):167-77. [DOI: 10.1176/appi.ajp.2011.10111705] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitiello B, Severe JB, Greenhill LL, Arnold LE, Abikoff HB, Bukstein OG, et al. Methylphenidate dosage for children with ADHD over time under controlled conditions: lessons from the MTA. Journal of the American Academy of Child and Adolescent Psychiatry 2001;40(2):188-96. [DOI: 10.1097/00004583-200102000-00013] [DOI] [PubMed] [Google Scholar]
Johnston 1988 {published data only}
- Johnston C, Pelham WE, Hoza J, Sturges J. Psychostimulant rebound in attention deficit disordered boys. Journal of the American Academy of Child and Adolescent Psychiatry 1988;27(6):806-10. [DOI: 10.1097/00004583-198811000-00026] [DOI] [PubMed] [Google Scholar]
- Pelham WE Jr, Sturges J, Hoza J, Schmidt C, Bijlsma JJ, Milich R, et al. Sustained release and standard methylphenidate effects on cognitive and social behavior in children with attention deficit disorder. Pediatrics 1987;80(4):491-501. [DOI: 10.1542/peds.80.4.491] [DOI] [PubMed] [Google Scholar]
- Pelham WE, Hoza J. Behavioral assessment of psychostimulant effects on ADD children in a summer day treatment program. In: Prinz R, editors(s). Advances in Behavioral Assessment of Children and Families. Vol. 3. Greenwich, CT: JAI Press, 1987:3-34. [Google Scholar]
- Pelham WE, Milich R, Walker JL. Effects of continuous and partial reinforcement and methylphenidate on learning in children with attention deficit disorder. Journal of Abnormal Psychology 1986;95(4):319-25. [DOI: 10.1037/0021-843X.95.4.319] [DOI] [PubMed] [Google Scholar]
Kaplan 1990 {published data only}
- Kaplan SL, Busner J, Kupietz S, Wassermann E, Segal B. Effects of methylphenidate on adolescents with aggressive conduct disorder and ADDH: a preliminary report. Journal of the American Academy of Child and Adolescent Psychiatry 1990;29(5):719-23. [DOI: 10.1097/00004583-199009000-00007] [DOI] [PubMed] [Google Scholar]
Kelly 1989 {published data only}
- Kelly PC, Cohen ML, Walker WO, Caskey OL, Atkinson AW. Self-esteem in children medically managed for attention deficit disorder. Pediatrics 1989;83(2):211-7. [DOI: 10.1542/peds.83.2.211] [DOI] [PubMed] [Google Scholar]
Kent 1995 {published data only}
- Kent JD, Blader JC, Koplewicz HS, Abikoff H, Foley CA. Effects of late-afternoon methylphenidate administration on behavior and sleep in attention-deficit hyperactivity disorder. Pediatrics 1995;96(2):320-5. [DOI: 10.1542/peds.96.2.320] [DOI] [PubMed] [Google Scholar]
Kent 1999 {published data only}
- Kent MA, Camfield CS, Camfield PR. Double-blind methylphenidate trials: practical, useful, and highly endorsed by families. Archives of Pediatrics & Adolescent Medicine 1999;153(12):1292-6. [DOI: 10.1001/archpedi.153.12.1292] [DOI] [PubMed] [Google Scholar]
Klorman 1990 {published data only}
- Klorman R, Brumaghim JT, Fitzpatrick PA, Borgstedt AD. Clinical effects of a controlled trial of methylphenidate on adolescents with attention deficit disorder. Journal of the American Academy of Child and Adolescent Psychiatry 1990;29(5):702-9. [DOI: 10.1097/00004583-199009000-00005] [DOI] [PubMed] [Google Scholar]
- Klorman R, Brumaghim JT, Fitzpatrick PA, Borgstedt AD. Methylphenidate reduces abnormalities of stimulus classification in adolescents with attention deficit disorder. Journal of Abnormal Psychology 1992;101(1):130-8. [DOI: 10.1037/0021-843X.101.1.130] [DOI] [PubMed] [Google Scholar]
- Klorman R, Brumaghim JT, Fitzpatrick PA, Borgstedt AD. Methylphenidate speeds evaluation processes of attention deficit disorder adolescents during a continuous performance test. Journal of Abnormal Child Psychology 1991;19(3):263-83. [DOI: 10.1007/BF00911231] [DOI] [PubMed] [Google Scholar]
Kolko 1999 {published data only}
- Kolko DJ, Bukstein OG, Barron J. Methylphenidate and behavior modification in children with ADHD and comorbid ODD or CD: main and incremental effects across settings. Journal of the American Academy of Child and Adolescent Psychiatry 1999;38(5):578-86. [DOI: 10.1097/00004583-199905000-00020] [DOI] [PubMed] [Google Scholar]
Kollins 2006 (PATS) {published data only}
- Abikoff HB, Vitiello B, Riddle MA, Cunningham C, Greenhill LL, Swanson JM, et al. Methylphenidate effects on functional outcomes in the Preschoolers with Attention-Deficit/Hyperactivity Disorder Treatment Study (PATS). Journal of Child and Adolescent Psychopharmacology 2007;17(5):581-92. [DOI: 10.1089/cap.2007.0068] [DOI] [PubMed] [Google Scholar]
- Anonymous. Young ADHD patients may improve with low-dose meds. Psychiatric Annals 2006;36(12):826. [URL: Retrieved from https://proxy3-bib.sdu.dk/login?url=https://www.proquest.com/scholarly-journals/young-adhd-patients-may-improve-with-low-dose/docview/217053192/se-2 (accessed 21/09/22)]
- At a glance... study design sought to balance rigor, subject safety. Brown University Child & Adolescent Psychopharmacology Update 2006;8(12):4-5. [DOI: 10.1002/cpu.20033] [DOI]
- Ghuman JK, Riddle MA, Vitiello B, Greenhill LL, Chuang SZ, Wigal SB, et al. Comorbidity moderates response to methylphenidate in the Preschoolers with Attention-Deficit/Hyperactivity Disorder Treatment Study (PATS). Journal of Child and Adolescent Psychopharmacology 2007;17(5):563-80. [DOI: 10.1089/cap.2007.0071] [DOI] [PubMed] [Google Scholar]
- Greenhill LL, Kollins S, Abikoff H, McCracken J, Riddle M, Swanson J, et al. Efficacy and safety of immediate-release methylphenidate treatment for preschoolers with ADHD. Journal of the American Academy of Child and Adolescent Psychiatry 2006;45(11):1284-93. [DOI: 10.1097/01.chi.0000235077.32661.61] [DOI] [PubMed] [Google Scholar]
- Greenhill LL, Kollins S, Abikoff H, McCracken J, Riddle M, Swanson J, et al. Erratum: "Efficacy and safety of immediate-release MPH treatment for preschoolers with ADHD". Journal of the American Academy of Child and Adolescent Psychiatry 2007;46(1):141. [DOI: 10.1097/01.chi.0000254217.97311.53] [DOI] [PubMed] [Google Scholar]
- Greenhill LL. Preschool ADHD treatment study (PATS): science and controversy. Economics of Neuroscience 2001;3(5):49-53. [EMBASE: 2001251865] [Google Scholar]
- Kollins S, Greenhill L, Swanson J, Wigal S, Abikoff H, McCracken J, et al. Rationale, design, and methods of the Preschool ADHD Treatment Study (PATS). Journal of the American Academy of Child and Adolescent Psychiatry 2006;45(11):1275-83. [DOI: 10.1097/01.chi.0000235074.86919.dc] [DOI] [PubMed] [Google Scholar]
- Kollins SH, Greenhill L. Evidence base for the use of stimulant medication in preschool children with ADHD. Infants and Young Children 2006;19(2):132-41. [DOI: 10.1097/00001163-200604000-00006] [DOI] [Google Scholar]
- MPH-related reductions in growth rates. Brown University Child & Adolescent Psychopharmacology Update 2006;8(12):6. [DOI: 10.1002/cpu.20033] [DOI]
- March JS. The preschool ADHD Treatment Study (PATS) as the culmination of twenty years of clinical trials in pediatric psychopharmacology. Journal of the American Academy of Child and Adolescent Psychiatry 2011;50(5):427-30. [DOI: 10.1016/j.jaac.2010.09.018] [DOI] [PubMed] [Google Scholar]
- McGough J, McCracken J, Swanson J, Riddle M, Kollins S, Greenhill L, et al. Pharmacogenetics of methylphenidate response in preschoolers with ADHD. Journal of the American Academy of Child and Adolescent Psychiatry 2006;45(11):1314-22. [DOI: 10.1097/01.chi.0000235083.40285.08] [DOI] [PubMed] [Google Scholar]
- NCT00018863. Treatment of attention deficit hyperactivity disorder in preschool-age children (PATS) [Methylphenidate efficacy and safety in ADHD preschoolers]. clinicaltrials.gov/ct2/show/NCT00018863 (first received 9 July 2001).
- PATS shows mixed effect of medication on functional outcomes. Brown University Child & Adolescent Psychopharmacology Update 2008;10(2):6. [DOI: 10.1002/cpu.20061] [DOI]
- PATS: efficacy of MPH in ADHD preschoolers. Brown University Child & Adolescent Psychopharmacology Update 2006;8(12):5-6. [DOI: 10.1002/cpu.20033] [DOI]
- PATS: safety and tolerability of MPH in ADHD preschoolers. Brown University Child & Adolescent Psychopharmacology Update 2006;8(12):2-4. [DOI: 10.1002/cpu.20033] [DOI]
- Reiff MI. Journal article reviews: attention-deficit/hyperactivity disorder. Journal of Developmental and Behavioral Pediatrics 2007;28(1):71-2. [URL: https://journals.lww.com/jrnldbp/Fulltext/2007/02000/Child_and_Adolescent_Psychiatry__The_Essentials.21.aspx (Accessed 21/09/22)]
- Riddle MA, Yershova K, Lazzaretto D, Paykina N, Yenokyan G, Greenhill L, et al. The Preschool Attention-Deficit/Hyperactivity Disorder Treatment Study (PATS) 6-year follow-up. Journal of the American Academy of Child and Adolescent Psychiatry 2013;52(3):264-78.e2. [DOI: 10.1016/j.jaac.2012.12.007] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle MA. New findings from the Preschoolers with Attention-Deficit/Hyperactivity Disorder Treatment Study (PATS). Journal of Child and Adolescent Psychopharmacology 2007;17(5):543-6. [DOI: 10.1089/cap.2007.17501] [DOI] [PubMed] [Google Scholar]
- Swanson J, Greenhill L, Wigal T, Kollins S, Stehli A, Davies M, et al. Stimulant-related reductions of growth rates in the PATS. Journal of the American Academy of Child and Adolescent Psychiatry 2006;45(11):1304-13. [DOI: 10.1097/01.chi.0000235075.25038.5a] [DOI] [PubMed] [Google Scholar]
- Vitiello B, Abikoff HB, Chuang SZ, Kollins SH, McCracken JT, Riddle MA, et al. Effectiveness of methylphenidate in the 10-month continuation phase of the Preschoolers with ADHD Treatment Study (PATS). Journal of Child and Adolescent Psychopharmacology 2007;17(5):593-603. [DOI: 10.1089/cap.2007.0058] [DOI] [PubMed] [Google Scholar]
- Wagner KD. Methylphenidate treatment of ADHD in preschoolers. Psychiatric Times 2007;24(3):47. [URL: link.gale.com/apps/doc/A160025094/AONE?u=anon~df44471e&sid=googleScholar&xid=67219b9e (accessed 21/09/22)] [Google Scholar]
- What are the effects of methylphenidate treatment in preschoolers with ADHD? Results from the Preschool ADHD Treatment Study (PATS). Brown University Child & Adolescent Psychopharmacology Update 2006;8(12):1-2. [DOI: 10.1002/cpu.20033] [DOI]
- Wigal T, Greenhill L, Chuang S, McGough J, Vitiello B, Skrobala A, et al. Safety and tolerability of methylphenidate in preschool children with ADHD. Journal of the American Academy of Child and Adolescent Psychiatry 2006;45(11):1294-303. [DOI: 10.1097/01.chi.0000235082.63156.27] [DOI] [PubMed] [Google Scholar]
Kollins 2021 {published data only}
- Kollins SH, Braeckman R, Guenther S, Barrett AC, Mickle TC, Oh C, et al. A randomized, controlled laboratory classroom study of serdexmethylphenidate and d-methyphenidate capsules in children with attention-deficit/hyperactivity disorder. Journal of child and adolescent psychopharmacology 2021;31(9):597-609. [DOI: 10.1089/cap.2021.0077] [DOI] [PubMed] [Google Scholar]
- NCT03292952. KP415 Classroom Study in Children (6-12 Years of Age) With ADHD. https://clinicaltrials.gov/ct2/show/NCT03292952.
Konrad 2004 {published data only}
- Konrad K, Günther T, Hanisch C, Herpertz-Dahlmann B. Differential effects of methylphenidate on attentional functions in children with attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry 2004;43(2):191-8. [DOI: 10.1097/00004583-200402000-00015] [DOI] [PubMed] [Google Scholar]
Konrad 2005 {published data only}
- Konrad K, Günther T, Heinzel-Gutenbrunner M, Herpertz-Dahlmann B. Clinical evaluation of subjective and objective changes in motor activity and attention in children with attention-deficit/hyperactivity disorder in a double-blind methylphenidate trial. Journal of Child and Adolescent Psychopharmacology 2005;15(2):180-90. [DOI: 10.1089/cap.2005.15.180] [DOI] [PubMed] [Google Scholar]
Kortekaas‐Rijlaarsdam 2017 {published data only}
- EUCTR2012-000492-17-NL. The effects of medical treatment with methylphenidate on academic activity and related skills in children with ADHD [The effects of long-acting methylphenidate on academic activity and related constructs in Children with ADHD: a randomised placebo controlled trial - ADHD and medication in the classroom]. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2012-000492-17-NL (first received 12 March 2012).
- Kortekaas-Rijlaarsdam AF, Luman M, Sonuga-Barke E, Bet P, Oosterlaan J. Methylphenidate-related improvements in math performance cannot be explained by better cognitive functioning or higher academic motivation: evidence from a randomized controlled trial. Journal of Attention Disorders 2020;24(13):1824-35. [DOI: 10.1177/1087054717713640] [PMCID: PMC7543012] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortekaas-Rijlaarsdam AF, Luman M, Sonuga-Barke E, Bet PM, Oosterlaan J. Short-term effects of methylphenidate on math productivity in children with attention-deficit/hyperactivity disorder are mediated by symptom improvements: evidence from a placebo-controlled trial. Journal of Clinical Psychopharmacology 2017;37(2):210-9. [DOI: 10.1097/JCP.0000000000000671] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kritchman 2019 {published data only}
- Kritchman M, Koubi M, Bloch AM, Bloch Y. Effect of methylphenidate on state anxiety in children with ADHD-a single dose, placebo controlled, crossover study. Frontiers in Behavioral Neuroscience 2019;13:106. [DOI: 10.3389/fnbeh.2019.00106] [PMCID: PMC6530494] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01798459. The effect of methylphenidate versus placebo on state anxiety in children with attention deficit hyperactivity disorder [A double blind randomized crossover study of the effect of methylphenidate versus placebo on state anxiety in children with attention deficit hyperactivity disorder]. clinicaltrials.gov/ct2/show/NCT01798459 (first received 25 Feburary 2013).
Leddy 2009 {published data only}
- Leddy JJ, Waxmonsky JG, Salis RJ, Paluch RA, Gnagy EM, Mahaney P, et al. Dopamine-related genotypes and the dose-response effect of methylphenidate on eating in attention-deficit/hyperactivity disorder youths. Journal of Child and Adolescent Psychopharmacology 2009;19(2):127-36. [DOI: 10.1089/cap.2008.046] [DOI] [PubMed] [Google Scholar]
Lehmkuhl 2002 {published data only}
- Döpfner M, Banaschewski T, Schmidt J, Uebel H, Schmeck K, Gerber WD, et al. Long-acting methylphenidate preparation in children with ADHD - a multicenter study [Langzeitwirksames methylphenidat bei kindern mit aufmerksamkeitsdefizit-hyperaktivitätsstörungen: eine multizentrische studie]. Nervenheilkunde: Zeitschrift fur interdisziplinaere Fortbildung 2003;22(2):85-92. [DOI: 10.1055/s-0038-1624375] [DOI] [Google Scholar]
- Lehmkuhl G. Placebo-controlled, double-blind multicenter trial on the efficacy of sustained-release methylphenidate in children suffering from attention deficit hyperactivity disorder (ADHD), Phase III. Integrated Final Report. Institut für medizinische Informatik, Biometrie und Epidemiologie, Universitätsklinikum Essen, Trial no 6520-9979-02, 1-9 2002.
- Sinzig J, Döpfner M, Lehmkuhl G, Uebel H, Schmeck K, Poustka F, et al. Long-acting methylphenidate has an effect on aggressive behavior in children with attention-deficit/hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 2007;17(4):421-32. [DOI: 10.1089/cap.2007.0011] [DOI] [PubMed] [Google Scholar]
- Sinzig JK, Döpfner M, Plück J, Banaschewski T, Stephani U, Lehmkuhl G, et al. Does a morning dose of methylphenidate retard reduce hyperkinetic symptoms in the afternoon? [Lassen sich hyperkinetische auffälligkeiten am nachmittag durch eine morgengabe von methylphenidat retard vermindern?]. Zeitschrift für Kinder- und Jugendpsychiatrie und Psychotherapie 2004;32(4):225-33. [DOI: 10.1024/1422-4917.32.4.225] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lijffijt 2006 {published data only}
- Lijffijt M, Kenemans JL, Ter Wal A, Quik EH, Kemner C, Westenberg H, et al. Dose-related effect of methylphenidate on stopping and changing in children with attention-deficit/hyperactivity disorder. European Psychiatry 2006;21(8):544-7. [DOI: 10.1016/j.eurpsy.2005.04.003] [DOI] [PubMed] [Google Scholar]
Lin 2014 {published data only}
- Lin DY, Kratochvil CJ, Xu W, Jin L, D'Souza DN, Kielbasa W, et al. A randomized trial of edivoxetine in pediatric patients with attention-deficit/hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 2014;24(4):190-200. [DOI: 10.1089/cap.2013.0043] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00922636. A study of pediatric patients with attention deficit/hyperactivity disorder [A fixed-dose, randomized, double-blind, placebo-controlled study of LY2216684 in pediatric patients with attention deficit/hyperactivity disorder]. clinicaltrials.gov/ct2/show/NCT00922636 (first received 17 June 2009).
Lopez 2003 {published data only}
- Lopez F, Silva R, Pestreich L, Muniz R. Comparative efficacy of two once daily methylphenidate formulations (Ritalin® LA™ 1 and Concerta ®) and placebo in children with attention deficit hyperactivity disorder across the school day. Paediatric Drugs 2003;5(8):545-55. [DOI: 10.2165/00148581-200305080-00005] [DOI] [PubMed] [Google Scholar]
- Lopez F, Silva R, Pestreich L, Muniz R. Erratum for: Comparative efficacy of two once daily methylphenidate formulations (Ritalin LA1 and Concerta) and placebo in children with attention deficit hyperactivity disorder across the school day. Pediatric Drugs 2003;5(12):832. Erratum for Pediatric Drugs 2003;5:545. [EMBASE: 2004010793] [DOI] [PubMed] [Google Scholar]
- Lopez FA, Silva RR, Pestreich L, Lee J, Muniz R. Comparative school-day efficacy of Ritalin LA, Concerta, and placebo in children with attention deficit hyperactivity disorder. Annals of Neurology. 32nd Annual Meeting of the Child Neurology Society; 2003 October 1-4; Miami Beach, Florida 2003;54 (Suppl 7):S143. [WOS: 000185260300393] [Google Scholar]
Lufi 1997 {published data only}
- Lufi D, Parish-Plass J, Gai E. The effect of methylphenidate on the cognitive and personality functioning of ADHD children. Israel Journal of Psychiatry and Related Sciences 1997;34(3):200-9. [PMID: ] [PubMed] [Google Scholar]
- Lufi D, Parish-Plass J. Randomised or not? Evidence Based Mental Health 2017;20:32. [DOI: 10.1136/eb-2016-102573] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lufi 2007 {published data only}
- Lufi D, Gai E. The effect of methylphenidate and placebo on eye-hand coordination functioning and handwriting of children with attention deficit hyperactivity disorder. Neurocase 2007;13(5):334-41. [DOI: 10.1080/13554790701851486] [PMID: ] [DOI] [PubMed] [Google Scholar]
Manos 1999 {published data only}
- Faraone SV, Short EJ, Biederman J, Findling RL, Roe C, Manos MJ. Efficacy of Adderall and methylphenidate in attention deficit hyperactivity disorder: a drug-placebo and drug-drug response curve analysis of a naturalistic study. International Journal of Neuropsychopharmacology 2002;5(2):121-9. [DOI: 10.1017/S1461145702002845] [DOI] [PubMed] [Google Scholar]
- Findling RL, Short EJ, Manos MJ. Developmental aspects of psychostimulant treatment in children and adolescents with attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry 2001;40(12):1441-7. [DOI: 10.1097/00004583-200112000-00015] [DOI] [PubMed] [Google Scholar]
- Findling RL, Short EJ, Manos MJ. Short-term cardiovascular effects of methylphenidate and Adderall. Journal of the American Academy of Child and Adolescent Psychiatry 2001;40(5):525-9. [DOI: 10.1097/00004583-200105000-00011] [DOI] [PubMed] [Google Scholar]
- Manos MJ, Short EJ, Findling RL. Differential effectiveness of methylphenidate and Adderall in school-age youths with attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry 1999;38(7):813-9. [DOI: 10.1097/00004583-199907000-00010] [DOI] [PubMed] [Google Scholar]
Martins 2004 {published data only}
- Martins S, Tramontina S, Polanczyk G, Eizirik M, Swanson JM, Rohde LA. Weekend holidays during methylphenidate use in ADHD children: a randomized clinical trial. Journal of Child and Adolescent Psychopharmacology 2004;14(2):195-206. [DOI: 10.1089/1044546041649066] [DOI] [PubMed] [Google Scholar]
Matthijssen 2019 {published data only}
- Matthijssen A-FM, Dietrich A, Bierens M, Deters RK, Van De Loo-Neus GH, Van Den Hoofdakker BJ, et al. Continued benefits of methylphenidate in ADHD after 2 years in clinical practice: a randomized placebo-controlled discontinuation study. American Journal of Psychiatry 2019;176(9):754-62. [DOI: 10.1176/appi.ajp.2019.18111296] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Matthijssen AM, Dietrich A, Bierens M, Deters RK, Van de Loo-Neus GH, Van den Hoofdakker BJ, et al. Effects of discontinuing methylphenidate on strengths and difficulties, quality of life and parenting stress. Journal of Child and Adolescent Psychopharmacology 2020;30(3):159-65. [DOI: 10.1089/cap.2019.0147] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Rosenau PT, Openneer TJ, Matthijssen A-FM, Van de Loo-Neus GH, Buitelaar JK, Van den Hoofdakker BJ, et al. Effects of methylphenidate on executive functioning in children and adolescents with ADHD after long-term use: a randomized, placebo-controlled discontinuation study. Journal of Child Psychology and Psychiatry 2021;62(12):1444-52. [DOI: 10.1111/jcpp.13419] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
McBride 1988a {published data only}
- McBride MC. An individual double-blind crossover trial for assessing methylphenidate response in children with attention deficit disorder. Journal of Pediatrics 1988;113(1 Pt 1):137-45. [DOI: 10.1016/S0022-3476(88)80548-1] [DOI] [PubMed] [Google Scholar]
McCracken 2016 {published data only}
- Bilder RM, Loo SK, McGough JJ, Whelan F, Helleman G, Sugar C, et al. Cognitive effects of stimulant, guanfacine, and combined treatment in child and adolescent attention-deficit/hyperactivity disorder. Biological Psychiatry 2016;79(9 Suppl 1):158S. [DOI: 10.1016/j.biopsych.2016.03.1054] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilder RM, Loo SK, McGough JJ, Whelan F, Hellemann G, Sugar C, et al. Cognitive effects of stimulant, guanfacine, and combined treatment in child and adolescent attention-deficit/hyperactivity disorder. Journal of the American Academy of Child & Adolescent Psychiatry 2016;55(8):667-73. [DOI: 10.1016/j.jaac.2016.05.016] [PMCID: PMC4964604] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo SK, Bilder RM, Cho AL, Sturm A, Cowen J, Walshaw P, et al. Effects of d-methylphenidate, guanfacine, and their combination on electroencephalogram resting state spectral power in attention-deficit/hyperactivity disorder. Journal of the American Academy of Child & Adolescent Psychiatry 2016;55(8):674-82.E1. [DOI: 10.1016/j.jaac.2016.04.020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken JT, McGough JJ, Loo SK, Levitt J, Del'Homme M, Cowen J, et al. Combined stimulant and guanfacine administration in attention-deficit/hyperactivity disorder: a controlled, comparative study. Journal of the American Academy of Child & Adolescent Psychiatry 2016;55(8):657-66.E1. [DOI: 10.1016/j.jaac.2016.05.015] [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken JT. AACAP Elaine Schlosser Lewis award for research on attention-deficit disorder: can we improve treatments for attention-deficit/hyperactivity disorder? Journal of the American Academy of Child & Adolescent Psychiatry 2017;56(Suppl 10):S130. [DOI: 10.1016/j.jaac.2017.09.005] [DOI] [Google Scholar]
- Michelini G. EEG predictors and correlates of methylphenidate, guanfacine, and combined treatment response in youth with ADHD. Journal of the American Academy of Child & Adolescent Psychiatry 2020;59(Suppl 10):S311. [DOI: 10.1016/j.jaac.2020.07.720] [DOI] [Google Scholar]
- NCT00429273. Single versus combination medication treatment for children with attention deficit hyperactivity disorder (project 1) [An eight-week, randomized, double-blind comparison of guanfacine, focalin XR, and the combination, with a twelve month open-label extension for the treatment of ADHD in pediatric subjects aged 7 to 14 years]. clinicaltrials.gov/ct2/show/NCT00429273 (first received 31 January 2007).
- Nurmi EL, Mallya A, Mallya KS, Hellemann GS, McGough J, Loo SK, et al. Pharmacogenetics of growth effects complicating ADHD treatment. Neuropsychopharmacology 2013;38(Suppl 2):S492-3. [DOI: 10.1038/npp.2013.281] [DOI] [Google Scholar]
- Nurmi EL, Mallya KS, McGough J, Loo SK, Bilder RM, Whelan F, et al. Pharmacogenetic moderators of methylphenidate and guanfacine response in children and adolescents with ADHD. Neuropsychopharmacology 2012;38(Suppl 1):S219. [DOI: 10.1038/npp.2012.220] [DOI] [Google Scholar]
- Sayer GR, McGough JJ, Levitt J, Cowen J, Sturm A, Castelo E, et al. Acute and long-term cardiovascular effects of stimulant, guanfacine, and combination therapy for attention-deficit/hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 2016;26(10):882-8. [DOI: 10.1089/cap.2015.0264] [PMCID: PMC5178010] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
McGough 2006 {published data only}
- McGough JJ, Wigal SB, Abikoff H, Turnbow JM, Posner K, Moon E. A randomized, double-blind, placebo-controlled, laboratory classroom assessment of methylphenidate transdermal system in children with ADHD. Journal of Attention Disorders 2006;9(3):476-85. [DOI: 10.1177/1087054705284089] [DOI] [PubMed] [Google Scholar]
- NCT00466791. Classroom study to assess efficacy and safety of MTS in pediatric patients aged 6-12 with ADHD [A phase II, randomized, double-blind, multi-center, placebo-controlled, dose optimization, analog classroom, crossover study designed to assess the time course of treatment effect, tolerability and safety of methylphenidate transdermal system (MTS) in pediatric patients aged 6-12 with attention-deficit/hyperactivity disorder (ADHD)]. clinicaltrials.gov/ct2/show/NCT00466791 (first received 27 April 2007).
- Wigal S, Turnbow J, Abikoff H, McGough J, Cohen J. Parent rated effects of transdermal methylphenidate in children with ADHD. In: International Journal of Neuropsychopharmacology. Abstracts from the XXVI Collegium Internationale Neuro-Psychopharmacologicum; 2008 July 13-17; Munich. Vol. 11 Suppl 1. 2008:232. [DOI: 10.1017/S1461145708009462] [DOI]
McInnes 2007 {published data only}
- McInnes A, Bedard A-C, Hogg-Johnson S, Tannock R. Preliminary evidence of beneficial effects of methylphenidate on listening comprehension in children with attention-deficit/hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 2007;17(1):35-49. [DOI: 10.1089/cap.2006.0051] [DOI] [PubMed] [Google Scholar]
Merrill 2021 {published data only}
- Merrill BM, Raiker JS, Evans SW, Gnagy EM, Pelham Jr WE. Cognitive mechanisms of methylphenidate in ADHD: Do improvements in sustained attention mediate behavioral improvements in the natural environment? Child Neuropsychology 2021;27(4):425-46. [DOI: 10.1080/09297049.2020.1862074] [DOI] [PubMed] [Google Scholar]
Moshe 2012 {published data only}
- Moshe K, Karni A, Tirosh E. Anxiety and methylphenidate in attention deficit hyperactivity disorder: a double-blind placebo-drug trial. Attention Deficit and Hyperactivity Disorders 2012;4(3):153-8. [DOI: 10.1007/s12402-012-0078-2] [DOI] [PubMed] [Google Scholar]
Muniz 2008 {published data only}
- Muniz R, Brams M, Mao A, McCague K, Pestreich L, Silva R. Efficacy and safety of extended-release dexmethylphenidate compared with d,l-methylphenidate and placebo in the treatment of children with attention-deficit/hyperactivity disorder: a 12-hour laboratory classroom study. Journal of Child and Adolescent Psychopharmacology 2008;18(3):248-56. [DOI: 10.1089/cap.2007.0015] [DOI] [PubMed] [Google Scholar]
- Silva R, Muniz R, McCague K, Childress A, Brams M, Mao A. Treatment of children with attention-deficit/hyperactivity disorder: results of a randomized, multicenter, double-blind, crossover study of extended-release dexmethylphenidate and D,L-methylphenidate and placebo in a laboratory classroom setting. Psychopharmacology Bulletin 2008;41(1):19-33. [PMID: ] [PubMed] [Google Scholar]
Murray 2011 {published data only}
- Armstrong RB, Damaraju CV, Ascher S, Schwarzman L, O'Neill J, Starr HL. Time course of treatment effect of OROS ® methylphenidate in children with ADHD. Journal of Attention Disorders 2012;16(8):697-705. [DOI: 10.1177/1087054711425772] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Murray DW, Childress A, Giblin J, Williamson D, Armstrong R, Starr HL. Effects of OROS methylphenidate on academic, behavioral, and cognitive tasks in children 9 to 12 years of age with attention-deficit/hyperactivity disorder. Clinical Pediatrics 2011;50(4):308-20. [DOI: 10.1177/0009922810394832] [DOI] [PubMed] [Google Scholar]
- NCT00799487. CONCERTA lab school study [Double-blind, randomized, placebo-controlled, crossover study evaluating the academic, behavioral and cognitive effects of Concerta on older children with ADHD (The ABC Study)]. clinicaltrials.gov/ct2/show/NCT00799487 (first received 1 December 2008).
Musten 1997 {published data only}
- Firestone P, Musten LM, Pisterman S, Mercer J, Bennett S. Short-term side effects of stimulant medication are increased in preschool children with attention-deficit/hyperactivity disorder: a double-blind placebo-controlled study. Journal of Child and Adolescent Psychopharmacology 1998;8(1):13-25. [DOI: 10.1089/cap.1998.8.13] [DOI] [PubMed] [Google Scholar]
- Musten LM, Firestone P, Pisterman S, Bennett S, Mercer J. Effects of methylphenidate on preschool children with ADHD: cognitive and behavioral functions. Journal of the American Academy of Child and Adolescent Psychiatry 1997;36(10):1407-15. [DOI: 10.1097/00004583-199710000-00023] [DOI] [PubMed] [Google Scholar]
- Musten LM. Efficacy of stimulant medication treatment of attention deficit hyperactivity disorder in preschool-aged children. Dissertation Abstracts International: Section B: The Sciences and Engineering 1998;59(3-B):1374. [PROQUEST: 304330121]
NCT00409708 {published data only}
- NCT00409708. Effects of methylphenidate on cellular abnormalities in children with attention deficit hyperactivity disorder (ADHD) [An open-label, behavioral-treatment-controlled evaluation of the effects of extended release methylphenidate on the frequency of cytogenetic abnormalities in children 6-12 years of age with attention deficit hyperactivity disorder (ADHD)]. clinicaltrials.gov/ct2/show/NCT00409708 (first received 11 December 2006).
NCT02039908 {published data only}
- NCT02039908. Examining tolerance to CNS stimulants in ADHD. clinicaltrials.gov/ct2/show/NCT02039908 (first received 20 January 2014).
- NCT02039908. Examining tolerance to CNS stimulants in ADHD: study protocol and statistical analysis plan; 22 January 2013. clinicaltrials.gov/ProvidedDocs/08/NCT02039908/Prot_SAP_000.pdf.
NCT02293655 {published data only}
- NCT02293655. The effects of ADHD medication (TEAM) study (TEAM) [The effects of ADHD medication (team) study: neurobehavioral effects of abrupt methylphenidate discontinuation]. clinicaltrials.gov/ct2/show/NCT02293655 February 2022.
NCT02536105 {published data only}
- NCT02536105. PK/PD pediactric ADHD classroom study [Pharmacokinetic pharmacodynamic studies of methylphenidate extended release products in pediatric attention deficit hyperactivity disorder]. clinicaltrials.gov/ct2/show/NCT02536105 (first received 31 August 2015).
Newcorn 2008 {published data only}
- Newcorn JH, Kratochvil CJ, Allen AJ, Casat CD, Ruff DD, Moore RJ, et al. Atomoxetine and osmotically released methylphenidate for the treatment of attention deficit hyperactivity disorder: acute comparison and differential response. American Journal of Psychiatry 2008;165(6):721-30. [DOI: 10.1176/appi.ajp.2007.05091676] [DOI] [PubMed] [Google Scholar]
- Newcorn JH, Kratochvil CJ, Allen AJ. Osmotically released methylphenidate compared to atomoxetine for ADHD. Brown University Child & Adolescent Psychopharmacology Update 2008;10(8):1. [DOI: 10.1002/cpu.20073] [DOI]
- Toplak ME. Osmotically released methylphenidate is more effective than atomoxetine in children and adolescents with ADHD. Evidence-Based Mental Health 2009;12(1):19. [DOI: 10.1136/ebmh.12.1.19] [PMID: ] [DOI] [PubMed] [Google Scholar]
Newcorn 2017a (flexible dose) {published data only}
- NCT01552915. Effectiveness of Vyvanse compared to Concerta in adolescents with attention-deficit/hyperactivity disorder [A phase 4, randomized, double-blind, multicenter, parallel-group, active-controlled, dose-optimization safety and efficacy study of SPD489 (Vyvanse®) compared with OROS-MPH (Concerta®) with a placebo reference arm, in adolescents aged 13-17 years with attention-deficit/hyperactivity disorder (ADHD)]. clinicaltrials.gov/ct2/show/NCT01552915 (first received 13 March 2012).
- Newcorn JH, Nagy P, Childress AC, Frick G, Yan B, Pliszka S. Randomized, double-blind, placebo-controlled acute comparator trials of lisdexamfetamine and extended-release methylphenidate in adolescents with attention-deficit/hyperactivity disorder. CNS Drugs 2017;31(11):999-1014. [DOI: 10.1007/s40263-017-0468-2] [PMCID: PMC5730627] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Newcorn 2017b (forced dose) {published data only}
- NCT01552902. Effectiveness of Vyvanse compared to Concerta in adolescents with attention-deficit/hyperactivity disorder [A phase 4, randomized, double-blind, multicenter, parallel-group, active-controlled, forced-dose titration, safety and efficacy study of SPD489 (Vyvanse®) compared with OROS-MPH (Concerta®) with a placebo reference arm, in adolescents aged 13-17 years with attention-deficit/hyperactivity disorder (ADHD)]. clinicaltrials.gov/ct2/show/NCT01552902 (first received 13 March 2012).
- Newcorn JH, Nagy P, Childress AC, Frick G, Yan B, Pliszka S. Randomized, double-blind, placebo-controlled acute comparator trials of lisdexamfetamine and extended-release methylphenidate in adolescents with attention-deficit/hyperactivity disorder. CNS drugs 2017;31(11):999-1014. [DOI: 10.1007/s40263-017-0468-2] [PMCID: PMC5730627] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nikles 2006 {published data only}
- Nikles CJ, Mitchell GK, Del Mar CB, Clavarino A, McNairn N. An n-of-1 trial service in clinical practice: testing the effectiveness of stimulants for attention-deficit/hyperactivity disorder. Pediatrics 2006;117(6):2040-6. [DOI: 10.1542/peds.2005-1328] [DOI] [PubMed] [Google Scholar]
- Nikles CJ, Mitchell GK, Del Mar CB, McNairn N, Clavarino A. Long-term changes in management following n-of-1 trials of stimulants in attention-deficit/hyperactivity disorder. European Journal of Clinical Pharmacology 2007;63(11):985-9. [DOI: 10.1007/s00228-007-0361-x] [DOI] [PubMed] [Google Scholar]
Oesterheld 1998 {published data only}
- Oesterheld JR, Kofoed L, Tervo R, Fogas B, Wilson A, Fiechtner H. Effectiveness of methylphenidate in Native American children with fetal alcohol syndrome and attention deficit/hyperactivity disorder: a controlled pilot study. Journal of Child and Adolescent Psychopharmacology 1998;8(1):39-48. [DOI: 10.1089/cap.1998.8.39] [DOI] [PubMed] [Google Scholar]
Overtoom 2003 {published data only}
- Overtoom CC, Verbaten MN, Kemner C, Kenemans JL, Van Engeland H, Buitelaar JK, et al. Effects of methylphenidate, desipramine, and L-dopa on attention and inhibition in children with attention deficit hyperactivity disorder. Behavioural Brain Research 2003;145(1-2):7-15. [DOI: 10.1016/S0166-4328(03)00097-4] [DOI] [PubMed] [Google Scholar]
Palumbo 2008 {published data only}
- Cannon M, Pelham WH, Sallee FR, Palumbo DR, Bukstein O, Daviss WB. Effects of clonidine and methylphenidate on family quality of life in attention-deficit/hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 2009;19(5):511-7. [DOI: 10.1089/cap.2009.0008] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daviss WB, Patel NC, Robb AS, McDermott MP, Bukstein OG, Pelham WE, et al. Clonidine for attention-deficit/hyperactivity disorder: II. ECG changes and adverse events analysis. Journal of the American Academy of Child and Adolescent Psychiatry 2008;47(2):189-98. [DOI: 10.1097/chi.0b013e31815d9ae4] [DOI] [PubMed] [Google Scholar]
- NCT00031395. Clonidine in attention deficit hyperactivity disorder (ADHD) in children [Clonidine in Attention deficit hyperactivity disorder (ADHD) Treatment (CAT)]. clinicaltrials.gov/ct2/show/NCT00031395 (first received 5 March 2002).
- Palumbo DR, Sallee FR, Pelham WE, Bukstein OG, Daviss WB, McDermott MP. Clonidine for attention-deficit/hyperactivity disorder: I. Efficacy and tolerability outcomes. Journal of the American Academy of Child and Adolescent Psychiatry 2008;47(2):180-8. [DOI: 10.1097/chi.0b013e31815d9af7] [DOI] [PubMed] [Google Scholar]
Pearson 2013 {published data only}
- Methylphenidate dosing improved behavior in children with ASD. Brown University Child & Adolescent Psychopharmacology Update 2013;15(8):4-5. [DOI: 10.1002/cpu.20193] [DOI]
- NCT00178503. Methylphenidate for attention deficit hyperactivity disorder and autism in children [ADHD symptoms in autism: cognition, behavior, treatment]. clinicaltrials.gov/ct2/show/NCT00178503 (first received 15 September 2005).
- Pearson DA, Santos CW, Aman MG, Arnold LE, Casat CD, Mansour R, et al. Effects of extended release methylphenidate treatment on ratings of attention-deficit/hyperactivity disorder (ADHD) and associated behavior in children with autism spectrum disorders and ADHD symptoms. Journal of Child and Adolescent Psychopharmacology 2013;23(5):337-51. [DOI: 10.1089/cap.2012.0096] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson DA, Santos CW, Aman MG, Arnold LE, Lane DM, Loveland KA, et al. Effects of extended-release methylphenidate treatment on cognitive task performance in children with autism spectrum disorder and attention-deficit/hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 2020;30(7):414-26. [DOI: 10.1089/cap.2020.0004] [PMCID: PMC7475091] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pelham 1989 {published data only}
- Pelham WE, Walker JL, Sturges J, Hoza J. Comparative effects of methylphenidate on ADD girls and ADD boys. Journal of the American Academy of Child and Adolescent Psychiatry 1989;28(5):773-6. [DOI: 10.1097/00004583-198909000-00021] [DOI] [PubMed] [Google Scholar]
Pelham 1990a {published data only}
- Pelham Jr WE, Greenslade KE, Vodde-Hamilton M, Murphy DA, Greenstein JJ, Gnagy EM, et al. Relative efficacy of long-acting stimulants on children with attention deficit-hyperactivity disorder: a comparison of standard methylphenidate, sustained-release methylphenidate, sustained-release dextroamphetamine, and pemoline. Pediatrics 1990;86(2):226-37. [DOI: 10.1542/peds.86.2.226] [DOI] [PubMed] [Google Scholar]
Pelham 1993a {published data only}
- Pelham J, Carlson C, Sams SE, Vallano G, Dixon MJ, Hoza B, et al. Separate and combined effects of methylphenidate and behavior modification on boys with attention deficit-hyperactivity disorder in the classroom. Journal of Consulting and Clinical Psychology 1993;61(3):506-15. [DOI: 10.1037/0022-006X.61.3.506] [DOI] [PubMed] [Google Scholar]
Pelham 1999 {published data only}
- Pelham WE, Aronoff HR, Midlam JK, Shapiro CJ, Gnagy EM, Chronis AM, et al. A comparison of Ritalin and Adderall: efficacy and time-course in children with attention-deficit/hyperactivity disorder. Pediatrics 1999;103(4):e43. [DOI: 10.1542/peds.103.4.e43] [DOI] [PubMed] [Google Scholar]
Pelham 2001a {published data only}
- Pelham WE, Gnagy EM, Burrows-Maclean L, Williams A, Fabiano GA, Morrisey SM, et al. Once-a-day Concerta methylphenidate versus three-times-daily methylphenidate in laboratory and natural settings. Pediatrics 2001;107(6):E105. [DOI: 10.1542/peds.107.6.e105] [DOI] [PubMed] [Google Scholar]
Pelham 2002 {published data only}
- Pelham WE, Hoza B, Pillow DR, Gnagy EM, Kipp HL, Greiner AR, et al. Effects of methylphenidate and expectancy on children with ADHD: behavior, academic performance, and attributions in a summer treatment program and regular classroom settings. Journal of Consulting and Clinical Psychology 2002;70(2):320-35. [DOI: 10.1037/0022-006X.70.2.320] [DOI] [PubMed] [Google Scholar]
Pelham 2005 {published data only}
- Chacko A, Pelham WE, Gnagy EM, Greiner A, Vallano G, Bukstein O, et al. Stimulant medication effects in a summer treatment program among young children with attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry 2005;44(3):249-57. [DOI: 10.1097/00004583-200503000-00009] [DOI] [PubMed] [Google Scholar]
- Pelham WE, Burrows-MacLean L, Gnagy EM, Fabiano GA, Coles EK, Tresco KE, et al. Transdermal methylphenidate, behavioral, and combined treatment for children with ADHD. Experimental and Clinical Psychopharmacology 2005;13(2):111-26. [DOI: 10.1037/1064-1297.13.2.111] [DOI] [PubMed] [Google Scholar]
- Pelham WE, Manos MJ, Ezzell CE, Tresco KE, Gnagy EM, Hoffman MT, et al. A dose-ranging study of a methylphenidate transdermal system in children with ADHD. Journal of the American Academy of Child and Adolescent Psychiatry 2005;44(6):522-9. [DOI: 10.1097/01.chi.0000157548.48960.95] [DOI] [PubMed] [Google Scholar]
Pelham 2011 {published data only}
- Pelham WE, Waxmonsky JG, Schentag J, Ballow CH, Panahon CJ, Gnagy EM, et al. Efficacy of a methylphenidate transdermal system versus t.i.d. methylphenidate in a laboratory setting. Journal of Attention Disorders 2011;15(1):28-35. [DOI: 10.1177/1087054709359163] [DOI] [PubMed] [Google Scholar]
Pelham 2014 {published data only}
- NCT00050622. Behavioral treatment, drug treatment, and combined treatment for attention deficit hyperactivity disorder (ADHD) [ADHD treatment: comparative and combined dosage effects]. clinicaltrials.gov/ct2/show/NCT00050622 (first received 17 December 2002).
- Pelham WE, Burrows-MacLean L, Gnagy EM, Fabiano GA, Coles EK, Wymbs BT, et al. A dose-ranging study of behavioral and pharmacological treatment in social settings for children with ADHD. Journal of Abnormal and Child Psychology 2014;42(6):1019-31. [DOI: 10.1007/s10802-013-9843-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
Perez‐Alvarez 2009 {published data only}
- Perez-Alvarez F, Serra-Amaya C, Timoneda-Gallart CA. Cognitive versus behavioral ADHD phenotype: what is it all about? Neuropediatrics 2009;40(1):32-8. [DOI: 10.1055/s-0029-1231055] [DOI] [PubMed] [Google Scholar]
Pliszka 1990 {published data only}
- Pliszka SR. Effect of anxiety on cognition, behavior, and stimulant response in ADHD. In: Chess S, Hertzig ME, editors(s). Annual Progress in Child Psychiatry and Child Development. London: Routledge, 1990:454-66. [DOI] [PubMed] [Google Scholar]
- Pliszka SR. Effect of anxiety on cognition, behavior, and stimulant response in ADHD. Journal of the American Academy of Child and Adolescent Psychiatry 1989;28(6):882-7. [DOI: 10.1097/00004583-198911000-00012] [DOI] [PubMed] [Google Scholar]
Pliszka 2000 {published data only}
- Pliszka SR, Browne RG, Olvera RL, Wynne SK. A double-blind, placebo-controlled study of Adderall and methylphenidate in the treatment of attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry 2000;39(5):619-26. [DOI: 10.1097/00004583-200005000-00016] [DOI] [PubMed] [Google Scholar]
Pliszka 2007 {published data only}
- NCT01069523. Electrophysiological effects of guanfacine extended release in attention deficit hyperactivity disorder (ADHD) [Electrophysiological effects of guanfacine extended-release (GXR) on inhibitory control in children with attention deficit/hyperactivity disorder (ADHD)]. clinicaltrials.gov/ct2/show/NCT01069523 (first received 17 February 2010).
- Pliszka SR, Liotti M, Bailey BY, Perez R, Glahn D, Semrud-Clikeman M. Electrophysiological effects of stimulant treatment on inhibitory control in children with attention-deficit/hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 2007;17(3):356-66. [DOI: 10.1089/cap.2006.0081] [DOI] [PubMed] [Google Scholar]
Pliszka 2017 {published data only}
- Arnold V, Pliszka S, Marraffino A, DeSousa N J, Incledon B, Sallee F R, et al. Efficacy and safety of HLD200 in children with attention-deficit/hyperactivity disorder: results from a pivotal phase 3 trial. CNS Spectrums 2017;22(1):103. [DOI: 10.1017/S1092852916000900] [DOI] [Google Scholar]
- Arnold VK, DeSousa NJ, Incledon B, Sallee FR, Wilens TE, Newcorn JH, et al. Pivotal phase 3 trial evaluating the efficacy and safety of HLD200, a novel delayed-release and extended-release formulation of methylphenidate, in children with attention-deficit/ hyperactivity disorder. Journal of the American Academy of Child & Adolescent Psychiatry 2016;55(Suppl 10):S170. [DOI: 10.1016/j.jaac.2016.09.218] [DOI] [Google Scholar]
- DeSousa N, Pliszka S, Wilens T, Childress A, Sallee R, Incledon B. Improvement in functional impairment during the early morning and late afternoon/evening bookends of the day: data from two phase 3 trials of evening-dosed delayed-release and extended-release methylphenidate in children with ADHD. Pediatrics 2020;146(1):590. [DOI: 10.1542/peds.146.1MA7.590a] [DOI] [Google Scholar]
- López FA, Faraone SV, Newcorn JH, Doll HA, Rhoten S, Lewis HB, et al. Effect of delayed-release and extended-release methylphenidate on caregiver strain and validation of psychometric properties of the Caregiver Strain Questionnaire: results from a phase 3 trial in children with attention-deficit/hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 2021;31(3):179-86. [DOI: 10.1089/cap.2020.0159] [PMCID: PMC8066344] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02520388. A pivotal efficacy trial to evaluate HLD200 in children with ADHD in a naturalistic setting [Ph 3 multicenter DBRCT parallel group study to evaluate safety & efficacy of evening-dosed HLD200, a novel delayed & extended release formulation (DELEXIS) of MPH HCL, on post-waking, early morning function in children aged 6-12 with ADHD]. clinicaltrials.gov/ct2/show/NCT02520388 (first received 11 August 2015).
- Pliszka S, Wilens T, Bostrom S, Arnold V, Marraffino A, Cutler A, et al. Effect of HLD200 on caregiver-reported ADHD symptom improvement in children with ADHD and caregiver strain: results from a phase 3 trial. Pediatrics 2018;142(1):810. [DOI: 10.1542/peds.142.1MA9.810] [DOI] [Google Scholar]
- Pliszka S, Wilens T, Bostrom S, Arnold V, Marraffino A, Cutler A, et al. Efficacy and safety of HLD200, a novel delayed-release and extended-release methylphenidate formulation, in children with attention-deficit/hyperactivity disorder: results from a pivotal phase 3 trial. ADHD Attention Deficit and Hyperactivity Disorders 2017;9:S39. [DOI: 10.1007/s12402-017-0224-y] [DOI]
- Pliszka S, Wilens T, Bostrom S, Arnold V, Marraffino A, Cutler A, et al. Efficacy and safety of HLD200, a novel delayed-release and extended-release methylphenidate formulation, in children with attention-deficit/hyperactivity disorder: results from a pivotal phase 3 trial. Pediatrics 2018;142(1 Meeting Abstract):794. [DOI: 10.1542/peds.142.1MA8.794] [ONLINE ISSN: 1098-4275] [PRINT ISSN: 0031-4005] [DOI] [Google Scholar]
- Pliszka SR, Arnold VK, Marraffino A, DeSousa NJ, Incledon B, Sallee FR, et al. 129 Effect of DR/ER-MPH on caregiver-reported ADHD symptom improvement in children with ADHD and caregiver strain: results from a phase 3 trial. CNS Spectrums 2018;23(1):81. [DOI: 10.1017/S1092852918000263] [DOI] [Google Scholar]
- Pliszka SR, Arnold VK, Marraffino A, DeSousa NJ, Incledon B, Sallee FR, et al. Effect of DR/ER-MPH on early morning and late afternoon/evening functioning in children with ADHD: analysis of PREMB-R items from a phase 3 trial. CNS Spectrums 2018;23(1):80-1. [DOI: 10.1017/S1092852918000251] [DOI] [Google Scholar]
- Pliszka SR, Arnold VK, Marraffino A, DeSousa NJ, Incledon B, Sallee FR, et al. Effect of delayed-release and extended-release methylphenidate (DR/ER-MPH) on all-day and early morning ADHD-related symptoms: analysis of ADHD-Rating Scale-IV and ADHD-AM-Rating Scale items from a phase 3 trial. Journal of the American Academy of Child & Adolescent Psychiatry 2018;57(Suppl 10):S169. [DOI: 10.1016/j.jaac.2018.09.119] [DOI] [Google Scholar]
- Pliszka SR, Childress AC, Cutler AJ, Maraffino A, DeSousa NJ, Sallee FR, et al. Post hoc meta-analysis of morning and evening behaviors in children with ADHD treated with delayed-release and extended-release methylphenidate from two phase 3 studies. Journal of the American Academy of Child & Adolescent Psychiatry 2020;59(Suppl 10):155-6. [DOI: 10.1016/j.jaac.2020.08.082] [DOI] [Google Scholar]
- Pliszka SR, Wilens TE, Bostrom S, Arnold VK, Marraffino A, Cutler AJ, et al. Efficacy and safety of HLD200, delayed-release and extended-release methylphenidate, in children with attention-deficit/hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 2017;27(6):474-82. [DOI: 10.1016/j.jaac.2016.07.331] [PMCID: PMC5567875] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pliszka SR. A phase 3 registration trial of delayed-release and extended-release methylphenidate (HLD200) in the treatment of early morning functioning impairments in children with attentiondeficit/hyperactivity disorder. Journal of the American Academy of Child & Adolescent Psychiatry 2016;55(Suppl 10):S315. [DOI: 10.1016/j.jaac.2016.07.331] [DOI] [Google Scholar]
- Wilens TE, Faraone SV, Hammerness P, Pliszka SR, DeSousa NJ, Sallee FR, et al. Clinically meaningful improvements with DR/ER-MPH in at-home functional impairment during the early morning, late afternoon, and evening in children with attention-deficit/hyperactivity disorder (ADHD). Journal of the American Academy of Child & Adolescent Psychiatry 2017;56(Suppl 10):S170. [DOI: 10.1016/j.jaac.2017.09.067] [DOI] [Google Scholar]
- Wilens TE, Pliszka SR, Arnold VK, Marraffino A, DeSousa NJ, Incledon B, et al. Consistent efficacy of DR/ER-MPH on early morning functioning in children with ADHD: analysis of BSFQ item ratings from a pivotal phase 3 trial. CNS Spectrums 2018;23(1):S82. [DOI: 10.1017/S1092852918000287] [DOI] [Google Scholar]
Quinn 2004 {published data only}
- Quinn D, Wigal S, Swanson J, Hirsch S, Ottolini Y, Dariani M, et al. Comparative pharmacodynamics and plasma concentrations of d-threo-methylphenidate hydrochloride after single doses of d-threo-methylphenidate hydrochloride and d,l-threo-methylphenidate hydrochloride in a double-blind, placebo-controlled, crossover laboratory school study in children with attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry 2004;43(11):1422-9. [DOI: 10.1097/01.chi.0000140455.96946.2b] [DOI] [PubMed] [Google Scholar]
Ramtvedt 2013 {published data only}
- NCT01220440. Comparing the efficacy of methylphenidate, dextroamphetamine and placebo in children diagnosed with ADHD. clinicaltrials.gov/ct2/show/NCT01220440 (first received 14 October 2010).
- Ramtvedt BE, Aabech HS, Sundet K. Minimizing adverse events while maintaining clinical improvement in a pediatric attention-deficit/hyperactivity disorder crossover trial with dextroamphetamine and methylphenidate. Journal of Child and Adolescent Psychopharmacology 2014;24(3):130-9. [DOI: 10.1089/cap.2013.0114] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramtvedt BE, Røinås E, Aabech HS, Sundet KS. Clinical gains from including both dextroamphetamine and methylphenidate in stimulant trials. Journal of Child and Adolescent Psychopharmacology 2013;23(9):597-604. [DOI: 10.1089/cap.2012.0085] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramtvedt BE, Sandvik L, Sundet K. Correspondence between children's and adults' ratings of stimulant-induced changes in ADHD behaviours in a crossover trial with medication-naive children. European Journal of Developmental Psychology 2014;11(6):687-700. [DOI: 10.1080/17405629.2014.927760] [DOI] [Google Scholar]
- Ramtvedt BE, Sundet K. Relationships between computer-based testing and behavioral ratings in the assessment of attention and activity in a pediatric ADHD stimulant crossover trial. Clinical Neuropsychologist 2014;28(7):1146-61. [DOI: 10.1080/13854046.2014.960453] [DOI] [PubMed] [Google Scholar]
Rapport 1985 {published data only}
- Rapport MD, DuPaul GJ, Stoner G, Birmingham BK, Masse G. Attention deficit disorder with hyperactivity: differential effects of methylphenidate on impulsivity. Pediatrics 1985;76(6):938-43. [DOI: 10.1542/peds.76.6.938] [DOI] [PubMed] [Google Scholar]
- Rapport MD, DuPaul GJ, Stoner G, Jones TJ. Comparing classroom and clinic measures of attention deficit disorder: differential, idiosyncratic, and dose-response effects of methylphenidate. Journal of Consulting and Clinical Psychology 1986;54(3):334-41. [DOI: 10.1037/0022-006X.54.3.334] [DOI] [PubMed] [Google Scholar]
- Rapport MD, DuPaul GJ. Methylphenidate: rate-dependent effects on hyperactivity. Psychopharmacology Bulletin 1986;22(1):223-8. [URL: https://books.google.dk/books?id=-YQVAQAAMAAJ&hl=da&pg=PA223#v=onepage&q&f=false (accessed 21/09/22)] [PubMed] [Google Scholar]
- Rapport MD, Stoner G, DuPaul GJ, Birmingham BK, Tucker S. Methylphenidate in hyperactive children: differential effects of dose on academic, learning, and social behavior. Journal of Abnormal Child Psychology 1985;13(2):227-43. [DOI: 10.1007/BF00910644] [DOI] [PubMed] [Google Scholar]
Rapport 1987 {published data only}
- Denney CB, Rapport MD. Predicting methylphenidate response in children with ADHD: theoretical, empirical, and conceptual models. Journal of the American Academy of Child and Adolescent Psychiatry 1999;38(4):393-401. [DOI: 10.1097/00004583-199904000-00011] [DOI] [PubMed] [Google Scholar]
- DuPaul GJ, Rapport MD, Vyse SA. ADDH and methylphenidate responders: effects on behavior controlled by complex reinforcement schedules. International Clinical Psychopharmacology 1988;3(4):349-61. [DOI: 10.1097/00004850-198810000-00006] [DOI] [PubMed] [Google Scholar]
- DuPaul GJ, Rapport MD. Does methylphenidate normalize the classroom performance of children with attention deficit disorder? Journal of the American Academy of Child and Adolescent Psychiatry 1993;32(1):190-8. [DOI: 10.1097/00004583-199301000-00027] [DOI] [PubMed] [Google Scholar]
- Kelly KL, Rapport MD, DuPaul GJ. Attention deficit disorder and methylphenidate: a multi-step analysis of dose-response effects on children's cardiovascular functioning. International Clinical Psychopharmacology 1988;3(2):167-81. [DOI: 10.1097/00004850-198804000-00007] [DOI] [PubMed] [Google Scholar]
- Rapport MD, Denney C, DuPaul GJ, Gardner MJ. Attention deficit disorder and methylphenidate: normalization rates, clinical effectiveness, and response prediction in 76 children. Journal of the American Academy of Child and Adolescent Psychiatry 1994;33(6):882-93. [DOI: 10.1097/00004583-199407000-00015] [DOI] [PubMed] [Google Scholar]
- Rapport MD, Denney C. Titrating methylphenidate in children with attention-deficit/hyperactivity disorder: is body mass predictive of clinical response? Journal of the American Academy of Child and Adolescent Psychiatry 1997;36(4):523-30. [DOI: 10.1097/00004583-199704000-00015] [DOI] [PubMed] [Google Scholar]
- Rapport MD, DuPaul GJ, Kelly KL. Attention deficit hyperactivity disorder and methylphenidate: the relationship between gross body weight and drug response in children. Psychopharmacology Bulletin 1989;25(2):285-90. [PMID: ] [PubMed] [Google Scholar]
- Rapport MD, Jones JT, DuPaul GJ, Kelly KL, Gardner MJ, Tucker SB, et al. Attention deficit disorder and methylphenidate: group and single-subject analyses of dose effects on attention in clinic and classroom settings. Journal of Clinical Child Psychology 1987;16(4):329-38. [DOI: 10.1207/s15374424jccp1604_6] [DOI] [Google Scholar]
- Rapport MD, Quinn SO, DuPaul GJ, Quinn EP, Kelly KL. Attention deficit disorder with hyperactivity and methylphenidate: the effects of dose and mastery level on children's learning performance. Journal of Abnormal Child Psychology 1989;17(6):669-89. [DOI: 10.1007/BF00917730] [DOI] [PubMed] [Google Scholar]
- Rapport MD, Randall R, Moffitt C. Attention-deficit/hyperactivity disorder and methylphenidate: a dose-response analysis and parent-child comparison of somatic complaints. Journal of Attention Disorders 2002;6(1):15-24. [DOI: 10.1177/108705470200600103] [DOI] [PubMed] [Google Scholar]
- Rapport MD, Stoner G, DuPaul GJ, Kelly KL, Tucker SB, Schoeler T. Attention deficit disorder and methylphenidate: a multilevel analysis of dose-response effects on children's impulsivity across settings. Journal of the American Academy of Child and Adolescent Psychiatry 1988;27(1):60-9. [DOI: 10.1097/00004583-198801000-00010] [DOI] [PubMed] [Google Scholar]
- Vyse SA, Rapport MD. The effects of methylphenidate on learning in children with ADDH: the stimulus equivalence paradigm. Journal of Consulting and Clinical Psychology 1989;57(3):425-35. [DOI: 10.1037/0022-006X.57.3.425] [DOI] [PubMed] [Google Scholar]
Rapport 2008 {published data only}
- Rapport MD, Kofler MJ, Coiro MM, Raiker JS, Sarver DE, Alderson RM. Unexpected effects of methylphenidate in attention-deficit/hyperactivity disorder reflect decreases in core/secondary symptoms and physical complaints common to all children. Journal of Child and Adolescent Psychopharmacology 2008;18(3):237-47. [DOI: 10.1089/cap.2007.0140] [DOI] [PubMed] [Google Scholar]
Reitman 2001 {published data only}
- Reitman D, Hupp SD, O'Callaghan PM, Gulley V, Northup J. The influence of a token economy and methylphenidate on attentive and disruptive behavior during sports with ADHD-diagnosed children. Behavior Modification 2001;25(2):305-23. [DOI: 10.1177/0145445501252007] [PMID: ] [DOI] [PubMed] [Google Scholar]
Riggs 2011 {published data only}
- NCT00264797. Attention deficit hyperactivity disorder (ADHD) in adolescents with substance use disorders (SUD) [Randomized controlled trial of osmotic-release methylphenidate (OROS-MPH) for attention deficit hyperactivity disorder (ADHD) in adolescents with substance use disorders (SUD)]. clinicaltrials.gov/ct2/show/NCT00264797 (first received 13 December 2005).
- NIDA-CTN-0028. Osmotic-release methylphenidate (OROS-MPH) for ADHD in adolescents with substance use disorders. datashare.nida.nih.gov/study/nida-ctn-0028 (accessed 28 February 2022).
- Riggs PD, Winhusen T, Davies RD, Leimberger JD, Mikulich-Gilbertson S, Klein C, et al. Randomized controlled trial of osmotic-release methylphenidate with cognitive-behavioral therapy in adolescents with attention-deficit/hyperactivity disorder and substance use disorders. Journal of the American Academy of Child and Adolescent Psychiatry 2011;50(9):903-14. [DOI: 10.1016/j.jaac.2011.06.010] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamm L, Adinoff B, Nakonezny PA, Winhusen T, Riggs P. Attention-deficit/hyperactivity disorder subtypes in adolescents with comorbid substance-use disorder. American Journal of Drug and Alcohol Abuse 2012;38(1):93-100. [DOI: 10.3109/00952990.2011.600395] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamm L, Trello-Rishel K, Riggs P, Nakonezny PA, Acosta M, Bailey G, et al. Predictors of treatment response in adolescents with comorbid substance use disorder and attention-deficit/hyperactivity disorder. Journal of Substance Abuse Treatment 2013;44(2):224-30. [DOI: 10.1016/j.jsat.2012.07.001] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warden D, Riggs PD, Min SJ, Mikulich-Gilbertson SK, Tamm L, Trello-Rishel K, et al. Major depression and treatment response in adolescents with ADHD and substance use disorder. Drug and Alcohol Dependence 2012;120(1-3):214-9. [DOI: 10.1016/j.drugalcdep.2011.08.001] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winhusen TM, Lewis DF, Riggs PD, Davies RD, Adler LA, Sonne S, et al. Subjective effects, misuse, and adverse effects of osmotic-release methylphenidate treatment in adolescent substance abusers with attention-deficit/hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 2011;21(5):455-63. [DOI: 10.1089/cap.2011.0014] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rubinsten 2008 {published data only}
- Rubinsten O, Bedard A-C, Tannock R. Methylphenidate has differential effects on numerical abilities in ADHD children with and without co-morbid mathematical difficulties. Open Psychology Journal 2008;1:11-7. [DOI: 10.2174/1874350100801010011] [DOI] [Google Scholar]
Samuels 2006 {published data only}
- Samuels JA, Franco K, Wan F, Sorof JM. Effect of stimulants on 24-h ambulatory blood pressure in children with ADHD: a double-blind, randomized, cross-over trial. Pediatric Nephrology 2006;21(1):92-5. [DOI: 10.1007/s00467-005-2051-1] [DOI] [PubMed] [Google Scholar]
Schachar 1997a {published data only}
- Charach A, Figueroa M, Chen S, Ickowicz A, Schachar R. Stimulant treatment over 5 years: effects on growth. Journal of the American Academy of Child and Adolescent Psychiatry 2006;45(4):415-21. [DOI: 10.1097/01.chi.0000199026.91699.20] [DOI] [PubMed] [Google Scholar]
- Charach A, Ickowicz A, Schachar R. Stimulant treatment over five years: adherence, effectiveness, and adverse effects. Journal of the American Academy of Child and Adolescent Psychiatry 2004;43(5):559-67. [DOI: 10.1097/00004583-200405000-00009] [DOI] [PubMed] [Google Scholar]
- Diamond IR, Tannock R, Schachar RJ. Response to methylphenidate in children with ADHD and comorbid anxiety. Journal of the American Academy of Child and Adolescent Psychiatry 1999;38(4):402-9. [DOI: 10.1097/00004583-199904000-00012] [DOI] [PubMed] [Google Scholar]
- Law SF, Schachar RJ. Do typical clinical doses of methylphenidate cause tics in children treated for attention-deficit hyperactivity disorder? Journal of the American Academy of Child and Adolescent Psychiatry 1999;38(8):944-51. [DOI: 10.1097/00004583-199908000-00009] [DOI] [PubMed] [Google Scholar]
- Schachar RJ, Tannock R, Cunningham C, Corkum PV. Behavioral, situational, and temporal effects of treatment of ADHD with methylphenidate. Journal of the American Academy of Child and Adolescent Psychiatry 1997;36(6):754-63. [DOI: 10.1097/00004583-199706000-00011] [DOI] [PubMed] [Google Scholar]
Schachar 2008 {published data only}
- Schachar R, Ickowicz A, Crosbie J, Donnelly GAE, Reiz JL, Miceli PC, et al. Cognitive and behavioral effects of multilayer-release methylphenidate in the treatment of children with attention-deficit/hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 2008;18(1):11-24. [DOI: 10.1089/cap.2007.0039] [DOI] [PubMed] [Google Scholar]
Schrantee 2016 {published data only}
- Bottelier MA, Schouw ML, Klomp A, Tamminga HG, Schrantee AG, Bouziane C, et al. The effects of psychotropic drugs on developing brain (ePOD) study: methods and design. BMC Psychiatry 2014;14:48. [DOI: 10.1186/1471-244X-14-48] [PMCID: PMC3930821] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NL2955 (NTR3103). Effects of methylphenidate on the developing brain [Effect modification by age of methylphenidate on the development of the dopaminergic system in the brain]. www.trialregister.nl/trial/2955 (first received 13 October 2011).
- Schrantee A, Tamminga HG, Bouziane C, Bottelier MA, Bron EE, Klein S, et al. A randomized clinical trial on the age-dependent effects of methylphenidate on the human dopaminergic system. Biological Psychiatry 2016;79(Suppl 9):S157. [DOI: 10.1016/j.biopsych.2016.03.1054] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrantee A, Tamminga HG, Bouziane C, Bottelier MA, Bron EE, Mutsaerts HJ, et al. Age-dependent effects of methylphenidate on the human dopaminergic system in young vs adult patients with attention-deficit/hyperactivity disorder: a randomized clinical trial. JAMA Psychiatry 2016;73(9):955-62. [DOI: 10.1001/jamapsychiatry.2016.1572] [PMCID: PMC5267166] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solleveld MM, Schrantee A, Baek HK, Bottelier MA, Lucassen PJ, Van Someren EJ, et al. Enduring effects of methylphenidate on sleep in children with attention-deficit/hyperactivity disorder: a double-blind randomized controlled trial. European Neuropsychopharmacology 2017;27(Suppl 4):S1111-2. [DOI: 10.1016/S0924-977X(17)31927-2] [DOI] [Google Scholar]
- Solleveld MM, Schrantee A, Baek HK, Bottelier MA, Tamminga HG, Bouziane C, et al. Effects of 16 weeks of methylphenidate treatment on Actigraph-assessed sleep measures in medication-naive children with ADHD. Frontiers in Psychiatry 2020;11:82. [DOI: 10.3389/fpsyt.2020.00082] [PMCID: PMC7058799] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamminga HG, Reneman L, Schrantee A, Bottelier MA, Bouziane C, Geurts HM, et al. Do effects of methylphenidate on cognitive performance last beyond treatment? A randomized placebo-controlled trial in boys and men with ADHD. European Neuropsychopharmacology 2021;46:1-13. [DOI: 10.1016/j.euroneuro.2021.02.002] [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Amlien I, Schrantee A, Rohani DA, Groote I, Bjørnerud A, et al. Methylphenidate effects on cortical thickness in children and adults with attention-deficit/hyperactivity disorder: a randomized clinical trial. American Journal of Neuroradiology 2020;41(5):758-65. [DOI: 10.3174/ajnr.A6560] [PMCID: PMC7228175] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schulz 2010 {published data only}
- EUCTR2006-006441-14-DE. A randomized, rater-blinded cross-over multicenter study comparing the clinical efficacy of Ritalin® LA (methylphenidate) treatment (20 or 40 mg orally o.d.) in children with ADHD under different breakfast conditions over two weeks. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2006-006441-14-DE (first received 9 March 2007).
- Schulz E, Fleischhaker C, Hennighausen K, Heiser P, Oehler K-U, Linder M, et al. A double-blind, randomized, placebo/active controlled crossover evaluation of the efficacy and safety of Ritalin®LA in children with attention-deficit/hyperactivity disorder in a laboratory classroom setting. Journal of Child and Adolescent Psychopharmacology 2010;20(5):377-85. [DOI: 10.1089/cap.2009.0106] [DOI] [PubMed] [Google Scholar]
Schwartz 2004 {published data only}
- Gruber R, Grizenko N, Schwartz G, Ben Amor L, Gauthier J, De Guzman R, et al. Sleep and COMT polymorphism in ADHD children: preliminary actigraphic data. Journal of the American Academy of Child and Adolescent Psychiatry 2006;45(8):982-9. [DOI: 10.1097/01.chi.0000220848.48650.10] [DOI] [PubMed] [Google Scholar]
- Schwartz G, Amor LB, Grizenko N, Lageix P, Baron C, Boivin DB, et al. Actigraphic monitoring during sleep of children with ADHD on methylphenidate and placebo. Journal of the American Academy of Child and Adolescent Psychiatry 2004;43(10):1276-82. [DOI: 10.1097/01.chi.0000135802.94090.93] [DOI] [PubMed] [Google Scholar]
Sharp 1999 {published data only}
- Borcherding BG, Keysor CS, Cooper TB, Rapoport JL. Differential effects of methylphenidate and dextroamphetamine on the motor activity level of hyperactive children. Neuropsychopharmacology 1989;2(4):255-63. [DOI: 10.1016/0893-133X(89)90029-8] [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Elia J, Kruesi MJ, Marsh WL, Gulotta CS, Potter WZ, et al. Cerebrospinal fluid homovanillic acid predicts behavioral response to stimulants in 45 boys with attention deficit/hyperactivity disorder. Neuropsychopharmacology 1996;14(2):125-37. [DOI: 10.1016/0893-133X(95)00077-Q] [DOI] [PubMed] [Google Scholar]
- Elia J, Borcherding BG, Rapoport JL, Keysor CS. Methylphenidate and dextroamphetamine treatments of hyperactivity: are there true nonresponders? Psychiatry Research 1991;36(2):141-55. [DOI: 10.1016/0165-1781(91)90126-A] [DOI] [PubMed] [Google Scholar]
- Elia J, Welsh PA, Gullotta CS, Rapoport JL. Classroom academic performance: improvement with both methylphenidate and dextroamphetamine in ADHD boys. Journal of Child Psychology and Psychiatry, and Allied Disciplines 1993;34(5):785-804. [DOI: 10.1111/j.1469-7610.1993.tb01071.x] [DOI] [PubMed] [Google Scholar]
- Schmidt ME, Kruesi MJ, Elia J, Borcherding BG, Elin RJ, Hosseini JM, et al. Effect of dextroamphetamine and methylphenidate on calcium and magnesium concentration in hyperactive boys. Psychiatry Research 1994;54(2):199-210. [DOI: 10.1016/0165-1781(94)90007-8] [DOI] [PubMed] [Google Scholar]
- Sharp WS, Walter JM, Marsh WL, Ritchie GF, Hamburger SD, Castellanos FX. ADHD in girls: clinical comparability of a research sample. Journal of the American Academy of Child and Adolescent Psychiatry 1999;38(1):40-7. [DOI: 10.1097/00004583-199901000-00018] [DOI] [PubMed] [Google Scholar]
Shiels 2009 {published data only}
- Shiels K, Hawk LW, Reynolds B, Mazzullo RJ, Rhodes JD, Pelham WE, et al. Effects of methylphenidate on discounting of delayed rewards in attention deficit/hyperactivity disorder. Experimental and Clinical Psychopharmacology 2009;17(5):291-301. [DOI: 10.1037/a0017259] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer SV, Hawk LW, Richards JB, Shiels K, Pelham WE, Waxmonsky JG. Stimulant treatment reduces lapses in attention among children with ADHD: the effects of methylphenidate on intra-individual response time distributions. Journal of Abnormal Child Psychology 2009;37(6):805-16. [DOI: 10.1007/s10802-009-9316-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Silva 2005a {published data only}
- Silva R, Muniz R, Pestreich LK, Brams M, Childress A, Lopez FA. Efficacy of two long-acting methylphenidate formulations in children with attention-deficit/hyperactivity disorder in a laboratory classroom setting. Journal of Child and Adolescent Psychopharmacology 2005;15(4):637-54. [DOI: 10.1089/cap.2005.15.637] [DOI] [PubMed] [Google Scholar]
Silva 2006 {published data only}
- Silva RR, Muniz R, Pestreich L, Childress A, Brams M, Lopez FA, et al. Efficacy and duration of effect of extended-release dexmethylphenidate versus placebo in schoolchildren with attention-deficit/hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 2006;16(3):239-51. [DOI: 10.1089/cap.2006.16.239] [DOI] [PubMed] [Google Scholar]
Silva 2008 {published data only}
- CRIT124EUS09. A randomized, multi-center, double-blind, cross-over study comparing the efficacy and safety of dexmethylphenidate (Novartis) 20 mg versus placebo in children (6-12 years) with attention-deficit/ hyperactivity disorder (ADHD) in a laboratory classroom setting. www.novctrd.com (assessed 28 February 2022). [URL: https://www.novctrd.com/#/clinicaltrialresults?category=StudyNumber&searchId=2139&searchName=CRIT124EUS09 (accessed 21/09/22)]
- Silva RR, Muniz R, Pestreich L, Brams M, Mao AR, Childress A, et al. Dexmethylphenidate extended-release capsules in children with attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry 2008;47(2):199-208. [DOI: 10.1097/chi.0b013e31815cd9a4] [DOI] [PubMed] [Google Scholar]
- Silva RR, Muniz R, Pestreich L, Lopez FA, Childress A, Wang J. Once-daily dexmethylphenidate: a placebo-controlled crossover study in children with attention-deficit/hyperactivity disorder. Annals of Neurology 2005;58(Suppl S9, Poster Session C):S109. [DOI: 10.1002/ana.11308] [DOI] [Google Scholar]
Smith 1998 {published data only}
- Evans SW, Pelham WE, Smith BH, Bukstein O, Gnagy EM, Greiner AR, et al. Dose-response effects of methylphenidate on ecologically valid measures of academic performance and classroom behavior in adolescents with ADHD. Experimental and Clinical Psychopharmacology 2001;9(2):163-75. [DOI: 10.1037/1064-1297.9.2.163] [DOI] [PubMed] [Google Scholar]
- Smith BH, Pelham WE, Evans S, Gnagy E, Molina B, Bukstein O, et al. Dosage effects of methylphenidate on the social behavior of adolescents diagnosed with attention-deficit hyperactivity disorder. Experimental and Clinical Psychopharmacology 1998;6(2):187-204. [DOI: 10.1037/1064-1297.6.2.187] [DOI] [PubMed] [Google Scholar]
- Smith BH, Pelham WE, Gnagy E, Yudell RS. Equivalent effects of stimulant treatment for attention-deficit hyperactivity disorder during childhood and adolescence. Journal of the American Academy of Child and Adolescent Psychiatry 1998;37(3):314-21. [DOI: 10.1097/00004583-199803000-00017] [DOI] [PubMed] [Google Scholar]
- Smith BH. Reliability, validity and unique contributions of self-reports by adolescents being treated for attention-deficit hyperactivity disorder. Dissertation Abstracts International: Section B: The Sciences and Engineering 1997;58(6-B):3328. [PROQUEST: 304343660]
Smith 2004 {published data only}
- Smith R, Larsen D, Derby KM, McLaughlin TF, Weber KP, Brown K, et al. A comparison of teacher checklists used over 15 days and a one-day antecedent analysis to conduct a medication trial. Psychology in the Schools 2004;41(2):235-40. [DOI: 10.1002/pits.10151] [DOI] [Google Scholar]
Smithee 1998 {published data only}
- Smithee JA, Klorman R, Brumaghim JT, Borgstedt AD. Methylphenidate does not modify the impact of response frequency or stimulus sequence on performance and event-related potentials of children with attention deficit hyperactivity disorder. Journal of Abnormal Child Psychology 1998;26(4):233-45. [DOI: 10.1023/A:1022698232481] [DOI] [PubMed] [Google Scholar]
Solanto 2009 {published data only}
- NCT00824317. Stimulant drug treatment of attention-deficit hyperactivity disorder (AD/HD), inattentive type [Stimulant drug treatment of AD/HD, inattentive type]. clinicaltrials.gov/ct2/show/NCT00824317 (first received 16 January 2009).
- Solanto M, Newcorn J, Vail L, Gilbert S, Ivanov I, Lara R. Stimulant drug response in the predominantly inattentive and combined subtypes of attention-deficit/hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 2009;19(6):663-71. [DOI: 10.1089/cap.2009.0033] [DOI] [PMC free article] [PubMed] [Google Scholar]
Soleimani 2017 {published data only}
- IRCT201107071483N2. Methylphenidate effect on fine motor skills of children with attention deficit/hyperactivity disorder [Evaluation of methylphenidate’s effect on motor skills disorder in children with ADHD and developmental coordination disorder referred to Shafa hospital, Rasht 1390]. en.irct.ir/trial/763 (first received 13 July 2011).
- Soleimani R, Kousha M, Zarrabi H, Tavafzadeh-Haghi SM, Jalali MM. The impact of methylphenidate on motor performance in children with both attention deficit hyperactivity disorder and developmental coordination disorder: a randomized double-blind crossover clinical trial. Iranian Journal of Medical Sciences 2017;42(4):354-61. [PMCID: PMC5523042] [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Stein 1996 {published data only}
- Stein MA, Blondis TA, Schnitzler ER, O'Brien T, Fishkin J, Blackwell B, et al. Methylphenidate dosing: twice daily versus three times daily. Pediatrics 1996;98(4 Pt 1):748-56. [DOI: 10.1542/peds.98.4.748] [DOI] [PubMed] [Google Scholar]
Stein 2003 {published data only}
- Stein MA, Sarampote C, Seymour K. Insomnia and tiredness in ADHD youth: relationship with methylphenidate dose, age, and weight. In: Pediatric Research. Annual Meeting of the Pediatric Academic Societies; 2004 May 4; San Francisco, CA. Vol. 55 (4). Baltimore: International Pediatric Research Foundation, 2004:74A. [WOS: 000220591100437]
- Stein MA, Sarampote CS, Waldman ID, Robb AS, Conlon C, Pearl PL, et al. A dose-response study of OROS methylphenidate in children with attention-deficit/hyperactivity disorder. Pediatrics 2003;112(5):e404. [DOI: 10.1542/peds.112.5.e404] [DOI] [PubMed] [Google Scholar]
- Stein MA, Seymour KE, Black DO, Sarampote CS, Robb A, Conlon C, et al. Effects and side effects of Concerta methylphenidate (MPH) in children with ADHD and comorbid internalizing symptoms. In: Pediatric Research. Annual Meeting of the Pediatric Academic Society; 2003 May 3-6; Seattle, Washington. Vol. 53 (4). Baltimore: International Pediatric Research Foundation, 2003:555A. [WOS: 000181897903139]
- Stein MA, Waldman ID, Sarampote CS, Seymour KE, Robb AS, Conlon C, et al. Dopamine transporter genotype and methylphenidate dose response in children with ADHD. Neuropsychopharmacology 2005;30(7):1374-82. [DOI: 10.1038/sj.npp.1300718] [DOI] [PubMed] [Google Scholar]
Stein 2011 {published data only}
- Lopez JA. Dose response of extended release dexmethylphenidate and mixed amphetamine salts on sleep of youth with attention deficit/hyperactivity disorder. McGill University 2013.
- NCT00393042. Sleep and tolerability study: comparing the effects of Adderall XR and Focalin XR [Sleep and tolerability of extended release dexmethylphenidate vs mixed amphetamine salts: a double blind, placebo controlled study (SAT Study)]. clinicaltrials.gov/ct2/show/NCT00393042 (first received 26 October 2006).
- Newcorn J, Stein M. HT-13-003 Dopamine transporter genotype and dose-response following treatment with psychostimulants: implications for optimization of acute and longer-term treatment. ADHD Attention Deficit and Hyperactivity Disorders 2015;7(Suppl 1):67. [DOI: 10.1007/s12402-015-0169-y] [DOI] [Google Scholar]
- Santisteban JA, Stein MA, Bergmame L, Gruber R. Effect of extended-release dexmethylphenidate and mixed amphetamine salts on sleep: a double-blind, randomized, crossover study in youth with attention-deficit hyperactivity disorder. CNS Drugs 2014;28(9):825-33. [DOI: 10.1007/s40263-014-0181-3] [PMCID: PMC4362706] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MA, Waldman I, Newcorn J, Bishop J, Kittles R, Cook EH Jr. Dopamine transporter genotype and stimulant dose-response in youth with attention-deficit/hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 2014;24(5):238-44. [DOI: 10.1089/cap.2013.0102] [PMCID: PMC4064733] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MA, Waldman ID, Charney E, Aryal S, Sable C, Gruber R, et al. Dose effects and comparative effectiveness of extended release dexmethylphenidate and mixed amphetamine salts. Journal of Child and Adolescent Psychopharmacology 2011;21(6):581-8. [DOI: 10.1089/cap.2011.0018] [DOI] [PMC free article] [PubMed]
- Wiebe S, Gruber R, Charney E, Aryal S, Waldman I, Newcorn J, et al. Sleep and emotional reactivity to extended release dexmethylphenidate versus mixed amphetamine salts: a double-blind, placebo controlled study. European Child & Adolescent Psychiatry. Proceedings of the Eunethydis 1st International ADHD Conference: From Data to Best Clinical Practice; 2010 May 26-28; Amsterdam, Netherlands 2010;19(Suppl 1):S82. [DOI: 10.1007/s00787-010-0117-5] [DOI] [Google Scholar]
Stoner 1994 {published data only}
- Stoner G, Carey SP, Ikeda MJ, Shinn MR. The utility of curriculum-based measurement for evaluating the effects of methylphenidate on academic performance. Journal of Applied Behavior Analysis 1994;27(1):101-13. [DOI: 10.1901/jaba.1994.27-101] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sumner 2010 {published data only}
- Sumner CR, Haynes VS, Teicher MH, Newcorn JH. Does placebo response differ between objective and subjective measures in children with attention-deficit/hyperactivity disorder? Postgraduate Medicine 2010;122(5):52-61. [DOI: 10.3810/pgm.2010.09.2201] [DOI] [PubMed] [Google Scholar]
Sunohara 1999 {published data only}
- Sunohara GA, Malone MA, Rovet J, Humphries T, Roberts W, Taylor MJ. Effect of methylphenidate on attention in children with attention deficit hyperactivity disorder (ADHD): ERP evidence. Neuropsychopharmacology 1999;21(2):218-28. [DOI: 10.1016/S0893-133X(99)00023-8] [DOI] [PubMed] [Google Scholar]
- Sunohara GA. Methylphenidate effects on focused and selective attention processing in children with ADHD. Dissertation Abstracts International: Section B: The Sciences and Engineering 1998;59(6-B):3113. [PROQUEST: 304395256]
Swanson 1998 {published data only}
- Swanson J, Wigal S, Greenhill L, Browne R, Waslick B, Lerner M, et al. Objective and subjective measures of the pharmacodynamic effects of Adderall in the treatment of children with ADHD in a controlled laboratory classroom setting. Psychopharmacology Bulletin 1998;34(1):55-60. [URL: https://www.proquest.com/openview/dca2df0222d3fd1edaa632ed4a9265e6/1?pq-origsite=gscholar&cbl=35891 (accessed 21/09/22)] [PubMed] [Google Scholar]
- Swanson JM, Wigal S, Greenhill LL, Browne R, Waslik B, Lerner M, et al. Analog classroom assessment of Adderall® in children with ADHD. Journal of the American Academy of Child and Adolescent Psychiatry 1998;37(5):519-26. [DOI: 10.1097/00004583-199805000-00014] [DOI] [PubMed] [Google Scholar]
Swanson 1999 {published data only}
- Swanson J, Gupta S, Guinta D, Flynn D, Agler D, Lerner M, et al. Acute tolerance to methylphenidate in the treatment of attention deficit hyperactivity disorder in children. Clinical Pharmacology and Therapeutics 1999;66(3):295-305. [DOI: 10.1016/S0009-9236(99)70038-X] [DOI] [PubMed] [Google Scholar]
- Wigal SB, Gupta S, Guinta D, Swanson JM. Reliability and validity of the SKAMP rating scale in a laboratory school setting. Psychopharmacology Bulletin 1998;34(1):47-53. [URL: https://books.google.dk/books?hl=no&lr=&id=8KorpzSlQggC&oi=fnd&pg=PA47&dq=Reliability+and+validity+of+the+SKAMP+rating+scale+in+a+laboratory+school+setting&ots=4kt8nR2cJM&sig=JHwtxhUicg-6IkMwVcBLw_9n4bg&redir_esc=y#v=onepage&q&f=false (accessed 21/09/22)] [PubMed] [Google Scholar]
Swanson 2002a {published data only}
- Swanson J, Gupta S, Lam A, Shoulson I, Lerner M, Modi N, et al. Development of a new once-a-day formulation of methylphenidate for the treatment of attention-deficit/hyperactivity disorder: proof-of-concept and proof-of-product studies. Archives of General Psychiatry 2003;60(2):204-11. [DOI: 10.1001/archpsyc.60.2.204] [DOI] [PubMed] [Google Scholar]
- Swanson J, Sadeh A, Lerner MA, Wigal SB. Comparison of the impact of OROS methylphenidate HCI with methylphenidate tid and placebo on the sleep of children with ADHD. Journal of Developmental and Behavioral Pediatrics 2000;21(5):387-8. [DOI: 10.1097/00004703-200010000-00028] [DOI] [Google Scholar]
- Swanson JM, Gupta S, Williams L, Agler D, Lerner M, Wigal S. Efficacy of a new pattern of delivery of methylphenidate for the treatment of ADHD: effects on activity level in the classroom and on the playground. Journal of the American Academy of Child and Adolescent Psychiatry 2002;41(11):1306-14. [DOI: 10.1097/00004583-200211000-00011] [DOI] [PubMed] [Google Scholar]
- Swanson JM, Wigal SB, Lemer MA. Comparison of the efficacy and safety of OROS methylphenidate HCI with methylphenidate tid and placebo in children with ADHD. In: Pediatric Academic Societies and the American Academy of Pediatrics Joint Meeting; 2000 May 12-16; Boston (MA), USA. 2000.
- Wigal S, Lerner M, Swanson J. Once-daily methylphenidate formulation: impact on academic productivity and activity levels of children with attention-deficit/hyperactivity disorder. European Neuropsychopharmacology 2002;12 (Suppl 3):416. [DOI: 10.1016/s0924-977x(02)80718-0] [DOI] [Google Scholar]
- Wigal S, Swanson JM, Lerner M. Comparison of duration of effect of OROS MPH with MPH tid in ADHD children. In: Annual Meeting of the American Psychiatric Association; 2001 May 5-10. New Orleans: American Psychiatric Association, 2001:34. [URL: https://psychiatry.org/getattachment/dd6290b1-3922-44c1-8203-0e996ea7ca46/am_syllabus_2001.pdf (accessed 21/09/22)]
Swanson 2002b {published data only}
- Swanson J, Gupta S, Lam A, Shoulson I, Lerner M, Modi N, et al. Development of a new once-a-day formulation of methylphenidate for the treatment of attention-deficit/hyperactivity disorder: proof-of-concept and proof-of-product studies. Archives of General Psychiatry 2003;60(2):204-11. [DOI: 10.1001/archpsyc.60.2.204] [DOI] [PubMed] [Google Scholar]
- Swanson J, Sadeh A, Lerner MA, Wigal SB. Comparison of the impact of OROS methylphenidate HCI with methylphenidate tid and placebo on the sleep of children with ADHD. Journal of Developmental and Behavioral Pediatrics 2000;21(5):387-8. [DOI: 10.1097/00004703-200010000-00028] [WOS: 000089857900028] [DOI] [Google Scholar]
- Swanson J, Wigal S, Lerner M. Treatment with a controlled-release formulation of methylphenidate for attention-deficit/hyperactivity disorder: onset and duration of effect. European Neuropsychopharmacology 2002;12 (Suppl 3):414. [DOI: 10.1016/s0924-977x(02)80714-3] [DOI] [Google Scholar]
- Swanson JM, Gupta S, Williams L, Agler D, Lerner M, Wigal S. Efficacy of a new pattern of delivery of methylphenidate for the treatment of ADHD: effects on activity level in the classroom and on the playground. Journal of the American Academy of Child and Adolescent Psychiatry 2002;41(11):1306-14. [DOI: 10.1097/00004583-200211000-00011] [DOI] [PubMed] [Google Scholar]
- Swanson JM, Wigal SB, Lemer MA. Comparison of the efficacy and safety of OROS methylphenidate HCI with methylphenidate tid and placebo in children with ADHD. In: Pediatric Academic Societies and the American Academy of Pediatrics Joint Meeting; 2000 May 12-16; Boston (MA), USA. 2000.
- Wigal S, Lerner M, Swanson J. Once-daily methylphenidate formulation: impact on academic productivity and activity levels of children with attention-deficit/hyperactivity disorder. European Neuropsychopharmacology 2002;12 (Suppl 3):416. [DOI: 10.1016/s0924-977x(02)80718-0] [DOI] [Google Scholar]
Swanson 2004b {published data only}
- NCT00381758. The COMACS Study: a comparison of methylphenidates in an analog classroom setting [A randomized, double-blind comparison of the time course of response to two extended-release oral delivery systems for methylphenidate in pediatric patients with attention deficit hyperactivity disorder in an analog classroom setting: The Comacs Study]. clinicaltrials.gov/ct2/show/NCT00381758 (first received 28 September 2006).
- Sonuga-Barke EJ, Coghill D, Markowitz JS, Swanson JM, Vandenberghe M, Hatch SJ. Sex differences in the response of children with ADHD to once-daily formulations of methylphenidate. Journal of the American Academy of Child and Adolescent Psychiatry 2007;46(6):701-10. [DOI: 10.1097/chi.0b013e31804659f1] [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ, Van Lier P, Swanson JM, Coghill D, Wigal S, Vandenberghe M, et al. Heterogeneity in the pharmacodynamics of two long-acting methylphenidate formulations for children with attention deficit/hyperactivity disorder. A growth mixture modelling analysis. European Child & Adolescent Psychiatry 2008;17(4):245-54. [DOI: 10.1007/s00787-007-0667-3] [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJS, Coghill D, Wigal T, DeBacker M, Swanson J. Adverse reactions to methylphenidate treatment for attention-deficit/hyperactivity disorder: structure and associations with clinical characteristics and symptom control. Journal of Child and Adolescent Psychopharmacology 2009;19(6):683-90. [DOI: 10.1089/cap.2009.0024] [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJS, Swanson JM, Coghill D, DeCory HH, Hatch SJ. Efficacy of two once-daily methylphenidate formulations compared across dose levels at different times of the day: preliminary indications from a secondary analysis of the COMACS study data. BMC Psychiatry 2004;4:28. [DOI: 10.1186/1471-244X-4-28] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonuge-Barke EJS, Swanson J, Hatch S, Van Lier P, Vandenberghe M. Heterogeneity in ADHD children's response to two long-acting methylphenidate formulations. Journal of Neural Transmission. Abstracts of the 39th International Danube Symposium for Neurological Sciences and Continuing Education and 1st International Congress on ADHD, from Childhood to Adult Disease 2007;114(7):LXXXIX. [DOI: 10.1007/s00702-007-0754-0] [DOI] [Google Scholar]
- Swanson JM, Wigal SB, Wigal T, Sonuga-Barke E, Greenhill LL, Biederman J, et al. A comparison of once-daily extended-release methylphenidate formulations in children with attention-deficit/hyperactivity disorder in the laboratory school (the Comacs Study). Pediatrics 2004;113(3 Pt 1):e206-16. [DOI: 10.1542/peds.113.3.e206] [DOI] [PubMed] [Google Scholar]
Symons 2007 {published data only}
- Symons FJ, Tervo RC, Kim O, Hoch J. The effects of methylphenidate on the classroom behavior of elementary school-age children with cerebral palsy: a preliminary observational analysis. Journal of Child Neurology 2007;22(1):89-94. [DOI: 10.1177/0883073807299965] [DOI] [PubMed] [Google Scholar]
Szobot 2004 {published data only}
- Szobot CM, Ketzer C, Parente MA, Biederman J, Rohde LA. The acute effect of methylphenidate in Brazilian male children and adolescents with ADHD: a randomized clinical trial. Journal of Attention Disorders 2004;8(2):37-43. [DOI: 10.1177/108705470400800201] [DOI] [PubMed] [Google Scholar]
Szobot 2008 {published data only}
- Szobot CM, Rohde LA, Katz B, Ruaro P, Schaefer T, Walcher M, et al. A randomized crossover clinical study showing that methylphenidate-SODAS improves attention-deficit/hyperactivity disorder symptoms in adolescents with substance use disorder. Brazilian Journal of Medical and Biological Research 2008;41(3):250-7. [DOI: 10.1590/s0100-879x2008005000011] [DOI] [PubMed] [Google Scholar]
Tannock 1989 {published data only}
- Tannock R, Schachar RJ, Carr RP, Chajczyk D, Logan GD. Effects of methylphenidate on inhibitory control in hyperactive children. Journal of Abnormal Child Psychology 1989;17(5):473-91. Erratum in: Journal of Abnormal Child Psychology 1990;18(1):119. [DOI: 10.1007/bf00916508] [DOI] [PubMed] [Google Scholar]
- Tannock R, Schachar RJ, Carr RP, Logan GD. Dose-response effects of methylphenidate on academic performance and overt behavior in hyperactive children. Pediatrics 1989;84(4):648-57. [DOI: 10.1542/peds.84.4.648] [DOI] [PubMed] [Google Scholar]
Tannock 1992 {published data only}
- Tannock R, Schachar R. Methylphenidate and cognitive perseveration in hyperactive children. Journal of Child Psychology and Psychiatry and Allied Disciplines 1992;33(7):1217-28. [DOI: 10.1111/j.1469-7610.1992.tb00940.x] [DOI] [PubMed] [Google Scholar]
Tannock 1993 {published data only}
- Tannock R, Schachar RJ, Logan GD. Does methylphenidate induce overfocusing in hyperactive children? Journal of Clinical Child Psychology 1993;22(1):28-41. [DOI: 10.1207/s15374424jccp2201_3] [DOI] [Google Scholar]
Tannock 1995a {published data only}
- Tannock R, Schachar R, Logan G. Methylphenidate and cognitive flexibility: dissociated dose effects in hyperactive children. Journal of Abnormal Child Psychology 1995;23(2):235-66. [DOI: 10.1007/bf01447091] [DOI] [PubMed] [Google Scholar]
Tannock 1995b {published data only}
- Tannock R, Fine J, Heintz T, Schachar RJ. A linguistic approach detects stimulant effects in two children with attention-deficit hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 1995;5(3):177-89. [DOI: 10.1089/cap.1995.5.177] [DOI] [Google Scholar]
- Tannock R, Ickowicz A, Schachar R. Differential effects of methylphenidate on working memory in ADHD children with and without comorbid anxiety. Journal of the American Academy of Child and Adolescent Psychiatry 1995;34(7):886-96. [DOI: 10.1097/00004583-199507000-00012] [DOI] [PubMed] [Google Scholar]
Tannock 2018 {published data only}
- Tannock R, Frijters JC, Martinussen R, White EJ, Ickowicz A, Benson NJ, et al. Combined modality intervention for ADHD with comorbid reading disorders: a proof of concept study. Journal of Learning Disabilities 2018;51(1):55-72. [DOI: 10.1177/0022219416678409] [PMID: ] [DOI] [PubMed] [Google Scholar]
Taylor 1987 {published data only}
- Taylor E, Schachar R, Thorley G, Wieselberg HM, Everitt B, Rutter M. Which boys respond to stimulant medication? A controlled trial of methylphenidate in boys with disruptive behaviour. Psychological Medicine 1987;17(1):121-43. [DOI: 10.1017/s0033291700013039] [DOI] [PubMed] [Google Scholar]
Taylor 1993 {published data only}
- Taylor MJ, Voros JG, Logan WJ, Malone MA. Changes in event-related potentials with stimulant medication in children with attention deficit hyperactivity disorder. Biological Psychology 1993;36(3):139-56. [DOI: 10.1016/0301-0511(93)90015-z] [DOI] [PubMed] [Google Scholar]
Tervo 2002 {published data only}
- Tervo RC, Azuma S, Fogas B, Fiechtner H. Children with ADHD and motor dysfunction compared with children with ADHD only. Developmental Medicine & Child Neurology 2002;44(6):383-90. [DOI: 10.1111/j.1469-8749.2002.tb00832.x] [DOI] [PubMed] [Google Scholar]
Tirosh 1993a {published data only}
- Tirosh E, Elhasid R, Kamah SC, Cohen A. Predictive value of placebo methylphenidate. Pediatric Neurology 1993;9(2):131-3. [DOI: 10.1016/0887-8994(93)90049-i] [DOI] [PubMed] [Google Scholar]
Tirosh 1993b {published data only}
- Tirosh E, Sadeh A, Munvez R, Lavie P. Effects of methylphenidate on sleep in children with attention-deficient hyperactivity disorder. An activity monitor study. American Journal of Diseases of Children 1993;147(12):1313-5. [DOI: 10.1001/archpedi.1993.02160360055018] [DOI] [PubMed] [Google Scholar]
Tourette's Syndrome Study Group 2002 {published data only}
- Deputy SR. Treatment of ADHD in children with tics: a randomized controlled trial. Clinical Pediatrics 2002;41(9):736. [DOI: 10.1212/wnl.58.4.527] [DOI] [PubMed] [Google Scholar]
- Kurlan R, Goldberg J. Clonidine and methylphenidate were effective for attention deficit hyperactivity disorder in children with comorbid tics. Evidence-Based Medicine 2002;7(5):157. [DOI: 10.1136/ebmh.5.4.122] [DOI] [PubMed] [Google Scholar]
- Kurlan R, Tourette Syndrome Study Group. Treatment of attention-deficit-hyperactivity disorder in children with Tourette's syndrome (TACT Trial). Annals of Neurology. Abstracts from the American Neurological Association's 125th Annual Meeting; 2000 October 15-18; Boston, Massachusetts 2000;48(6):953. [DOI: ] [DOI] [Google Scholar]
- Tourette's Syndrome Study Group. Treatment of ADHD in children with tics: a randomized controlled trial. Neurology 2002;58(4):527-36. [DOI: 10.1212/wnl.58.4.527] [DOI] [PubMed] [Google Scholar]
Tucker 2009 {published data only}
- Tucker JD, Suter W, Petibone DM, Thomas RA, Bailey NL, Zhou Y, et al. Cytogenetic assessment of methylphenidate treatment in pediatric patients treated for attention deficit hyperactivity disorder. Mutation Research 2009;677(1-2):53-8. [DOI: 10.1016/j.mrgentox.2009.05.005] [DOI] [PubMed] [Google Scholar]
- Zhou Y, Muni R, Tucke JD, Kumar V. Extended-release methylphenidate exposure and the frequency of cytogenetic abnormalities in children with attention-deficit-hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology. Proceedings of the 49th Annual National Institute of Mental Health (NIMH) New Clinical Drug Evaluation Unit (NCDEU) Meeting; 2009 June 29- July 2; Hollywood, Florida 2009;19(6):785. [DOI: 10.1089/cap.2009.1963] [DOI] [Google Scholar]
Ullmann 1985 {published data only}
- Ullmann RK, Sleator EK. Attention deficit disorder children with or without hyperactivity: which behaviors are helped by stimulants? Clinical Pediatrics 1985;24(10):547-51. [DOI: 10.1177/000992288502401001] [DOI] [PubMed] [Google Scholar]
Ullmann 1986 {published data only}
- Ullmann RK, Sleator EK. Responders, nonresponders, and placebo responders among children with attention deficit disorder: importance of a blinded placebo evaluation. Clinical Pediatrics 1986;25(12):594-9. [DOI: 10.1177/000992288602501201] [DOI] [PubMed] [Google Scholar]
Urman 1995 {published data only}
- Urman R, Ickowicz A, Fulford P, Tannock R. An exaggerated cardiovascular response to methylphenidate in ADHD children with anxiety. Journal of Child and Adolescent Psychopharmacology 1995;5(1):29-37. [DOI: 10.1089/cap.1995.5.29] [DOI] [Google Scholar]
Van der Meere 1999a {published data only}
- Gunning WB. A Controlled Trial of Clonidine in Hyperkinetic Children. Rotterdam: Erasmus Universiteit Rotterdam, 1992. [Google Scholar]
- Van der Meere J, Gunning B, Stemerdink N. The effect of methylphenidate and clonidine on response inhibition and state regulation in children with ADHD. Journal of Child Psychology and Psychiatry and Allied Disciplines 1999;40(2):291-8. [DOI: 10.1017/s0021963098003424] [DOI] [PubMed] [Google Scholar]
Wallace 1994 {published data only}
- Wallace AE, Kofoed LL. Statistical analysis of single case studies in the clinical setting: the example of methylphenidate trials in children with attention-deficit hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 1994;4(3):141-50. [DOI: 10.1089/cap.1994.4.141] [DOI] [Google Scholar]
Wallander 1987 {published data only}
- Wallander JL, Schroeder SR, Michelli JA, Gualtieri CT. Classroom social interactions of attention deficit disorder with hyperactivity children as a function of stimulant medication. Journal of Pediatric Psychology 1987;12(1):61-76. [DOI: 10.1093/jpepsy/12.1.61] [DOI] [PubMed] [Google Scholar]
Waxmonsky 2008 {published data only}
- NCT00050622. Behavioral treatment, drug treatment, and combined treatment for attention deficit hyperactivity disorder (ADHD) [ADHD treatment: comparative and combined dosage effects]. clinicaltrials.gov/ct2/show/NCT00050622 (first received 17 December 2002).
- Waxmonsky J, Pelham WE, Gnagy E, Cummings MR, O'Connor B, Majumdar A, et al. The efficacy and tolerability of methylphenidate and behavior modification in children with attention-deficit/hyperactivity disorder and severe mood dysregulation. Journal of Child and Adolescent Psychopharmacology 2008;18(6):573-88. [DOI: 10.1089/cap.2008.065] [DOI] [PMC free article] [PubMed] [Google Scholar]
Weiss 2021 {published data only}
- Kollins SH, Cutler AJ, Khattak S, Weiss MD, Donnelly G, Reiz JL. A six-month, open-label, multicenter study of the safety and efficacy of PRC-063 in adolescents with ADHD. Journal of the American Academy of Child & Adolescent Psychiatry 2016;55(Suppl 10):S217. [DOI: 10.1016/j.jaac.2016.09.361] [DOI]
- Kollins SH, Cutler AJ, Khattak S, Weiss MD, Donnelly G, Reiz SJ. A randomized double-blind placebo-controlled multicenter study measuring the efficacy and safety of a novel, extended-release formulation of methylphenidate (PRC-063) in adolescents with attention-deficit/hyperactivity disorder. Journal of the American Academy of Child & Adolescent Psychiatry 2016;55(Suppl 10):S217-8. [DOI: 10.1016/j.jaac.2016.09.362] [DOI] [Google Scholar]
- NCT02139111. PRC-063 in adolescent ADHD [A phase III, randomized, double-blind, placebo-controlled, parallel-arm, multi-center study measuring the efficacy and safety of PRC-063 in adolescent ADHD patients]. clinicaltrials.gov/ct2/show/NCT02139111 (first received 15 May 2014).
- Quinn D, Donnelly GA, Reiz J. Effect of PRC-063 on executive functioning in adolescents with ADHD in a randomized, double-blind, placebo-controlled, multi-center study with a six-month open label extension. ADHD Learning 2016.
- Weiss MD, Cutler AJ, Kollins SH, Donnely GA. Efficacy and safety of a long-acting multilayer-release methylphenidate formulation (PRC-063) in the treatment of adolescent attention-deficit/hyperactivity disorder: a randomized, double-blind clinical trial with a 6-month open-label extension. Journal of Child and Adolescent Psychopharmacology 2021;31(9):610-22. [DOI: 10.1089/cap.2021.0034] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Weiss MD, Surman C, Khullar A, Owens J, He E, Cataldo M, et al. Effect of a multilayer, extended-release methylphenidate formulation (PRC-063) on sleep in adolescents with attention-deficit/hyperactivity disorder: a randomized, double-blind, fixed-dose, placebo-controlled trial followed by a 6-month open-label follow-up. Journal of Child and Adolescent Psychopharmacology 2021;31(9):623-30. [DOI: 10.1089/cap.2021.0087] [PMID: ] [DOI] [PubMed] [Google Scholar]
Whalen 1990 {published data only}
- Whalen CK, Henker B, Granger DA. Ratings of medication effects in hyperactive children: viable or vulnerable? Behavioral Assessment 1989;11(2):179-99. [URL: https://psycnet.apa.org/record/1989-40814-001 (accessed 21/09/22)] [Google Scholar]
- Whalen CK, Henker B, Granger DA. Social judgment processes in hyperactive boys: effects of methylphenidate and comparisons with normal peers. Journal of Abnormal Child Psychology 1990;18(3):297-316. [DOI: 10.1007/bf00916567] [DOI] [PubMed] [Google Scholar]
- Whalen CK, Henker B, Hinshaw SP, Granger DA. Externalizing behavior disorders, situational generality, and the type A behavior pattern. Child Development 1989;60(6):1453-62. [DOI: 10.1111/j.1467-8624.1989.tb04016.x] [DOI] [PubMed] [Google Scholar]
Wigal 2003 {published data only}
- Wigal SB, Sanchez DY, DeCory HH, D'Imperio JM, Swanson JM. Selection of the optimal dose ratio for a controlled-delivery formulation of methylphenidate. Journal of Applied Research 2003;3(1):46-63. [URL: http://www.jarcet.com/articles/Vol3Iss1/DECORY.htm] [Google Scholar]
Wigal 2004 {published data only}
- Wigal S, Swanson JM, Feifel D, Sangal RB, Elia J, Casat CD, et al. A double-blind, placebo-controlled trial of dexmethylphenidate hydrochloride and d,l-threo-methylphenidate hydrochloride in children with attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry 2004;43(11):1406-14. [DOI: 10.1097/01.chi.0000138351.98604.92] [DOI] [PubMed] [Google Scholar]
Wigal 2011 {published data only}
- Armstrong RB, Damaraju CV, Ascher S, Schwarzman L, O'Neill J, Starr HL. Time course of treatment effect of OROS® methylphenidate in children with ADHD. Journal of Attention Disorders 2012;16(8):697-705. [DOI: 10.1177/1087054711425772] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Significant effect of OROS MPH ER on attention. Brown University Child & Adolescent Psychopharmacology Update 2010;12(1):7-8. [DOI: 10.1002/cpu.20107] [DOI]
- Wigal SB, Wigal T, Schuck S, Brams M, Williamson D, Armstrong RB, et al. Academic, behavioral, and cognitive effects of OROS ® methylphenidate on older children with attention-deficit/hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 2011;21(2):121-31. [DOI: 10.1089/cap.2010.0047] [PMCID: PMC3080768] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson D, Murray DW, Damaraju CV, Ascher S, Starr HL. Methylphenidate in children with ADHD with or without learning disability. Journal of Attention Disorders 2014;18(2):95-104. [DOI: 10.1177/1087054712443411] [PMID: ] [DOI] [PubMed] [Google Scholar]
Wigal 2013 {published data only}
- Liquid version of methylphenidate shows efficacy in school trial. Brown University Child & Adolescent Psychopharmacology Update 2013;15(3):1; 2-3. [DOI: 10.1002/cpu.20183] [DOI]
- NCT00904670. Quillivant oral suspension (Quillivant XR) in the treatment of attention deficit hyperactivity disorder (ADHD) [NWP06 in the treatment of children with attention deficit hyperactivity disorder (ADHD)- a laboratory classroom study]. clinicaltrials.gov/ct2/show/NCT00904670 (first received 20 May 2009).
- Palumbo DR, Belden HW, Berry SA. Methylphenidate extended-release oral suspension (MEROS) improves ADHD-rating scale and permanent product measure of performance scores in children with ADHD. Annals of Neurology 2015;78(Suppl 19):166. [DOI: 10.1002/ana.24477] [DOI] [Google Scholar]
- Robb AS, Findling RL, Childress AC, Berry SA, Belden HW, Wigal SB. Efficacy, safety, and tolerability of a novel methylphenidate extended-release oral suspension (MEROS) in ADHD. Journal of Attention Disorders 2017;21(14):1180-91. [DOI: 10.1177/1087054714533191] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Wigal SB, Childress AC, Belden HW, Berry SA. NWP06, an extended-release oral suspension of methylphenidate, improved attention-deficit/hyperactivity disorder symptoms compared with placebo in a laboratory classroom study. Journal of Child and Adolescent Psychopharmacology 2013;23(1):3-10. [DOI: 10.1089/cap.2012.0073] [PMCID: PMC3696913] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wigal 2014 {published data only}
- NCT01269463. Time course of response to methylphenidate HCL ER capsules in children 6 to 12 years with ADHD in classroom setting [A randomized, double-blind study of the time course of response to biphentin methylphenidate hydrochloride ER capsules compared to placebo in children 6 to 12 years with attention deficit hyperactivity disorder in analog classroom setting]. clinicaltrials.gov/ct2/show/NCT01269463 (first received 4 January 2011).
- Owens J, Weiss M, Nordbrock E, Mattingly G, Wigal S, Greenhill LL, et al. Effect of aptensio XR (methylphenidate HCL extended-release) capsules on sleep in children with attention-deficit/hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 2016;26(10):873-81. [DOI: 10.1089/cap.2016.0083] [PMCID: PMC5178023] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigal SB, Greenhill LL, Nordbrock E, Connor DF, Kollins SH, Adjei A, et al. A randomized placebo-controlled double-blind study evaluating the time course of response to methylphenidate hydrochloride extended-release capsules in children with attention-deficit/hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 2014;24(10):562-9. [DOI: 10.1089/cap.2014.0100] [PMCID: PMC4268556] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wigal 2015 {published data only}
- NCT01239030. Efficacy and safety of methylphenidate HCl ER capsules in children and adolescents with ADHD [A randomized, parallel, double-blind efficacy and safety study of biphentin methylphenidate HCL extended release capsules compared to placebo in children and adolescents 6 to 18 years with attention deficit hyperactivity disorder (ADHD)]. clinicaltrials.gov/ct2/show/NCT01239030 (first received 11 November 2010).
- Owens J, Weiss M, Nordbrock E, Mattingly G, Wigal S, Greenhill LL, et al. Effect of Aptensio XR (methylphenidate HCl extended-release) capsules on sleep in children with attention-deficit/hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 2016;26(10):873-81. [DOI: 10.1089/cap.2016.0083] [PMCID: PMC5178023] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss M, Childress A, Nordbrock E, Adjei AL, Kupper RJ, Mattingly G. Characteristics of ADHD symptom response/remission in a clinical trial of methylphenidate extended release. Journal of Clinical Medicine 2019;8(4):461. [DOI: 10.3390/jcm8040461] [PMCID: PMC6517933] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigal SB, Adjei AL, Childress A, Chang WW, Kupper RJ. A study of methylphenidate extended-release capsules in a randomized, double-blind, placebo-controlled protocol in children and adolescents with ADHD. CNS Spectrums 2015;20(1):67-8. [DOI: 10.1017/S1092852914000765] [DOI] [Google Scholar]
- Wigal SB, Nordbrock E, Adjei AL, Childress A, Kupper RJ, Greenhill L. Efficacy of methylphenidate hydrochloride extended-release capsules (Aptensio XRTM) in children and adolescents with attention-deficit/hyperactivity disorder: a phase III, randomized, double-blind study. CNS drugs 2015;29(4):331-40. [DOI: 10.1007/s40263-015-0241-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wigal 2017 {published data only}
- NCT01654250. NWP09 in children with attention deficit hyperactivity disorder (ADHD) [A multicenter, dose-optimized, double-blind, randomized, placebo-controlled study to evaluate the efficacy of NWP09 in pediatric patients with attention deficit hyperactivity disorder (ADHD) in a laboratory classroom]. clinicaltrials.gov/ct2/show/NCT01654250 (first received 31 July 2012).
- Wigal SB, Childress A, Berry SA, Belden H, Walters F, Chappell P, et al. Efficacy and safety of a chewable methylphenidate extended-release tablet in children with attention-deficit/hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 2017;27(8):690-9. [DOI: 10.1089/cap.2016.0177] [PMCID: PMC5651935] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigal SB, Childress AC, Berry S, Belden H, Chappell PB, Wajsbrot D, et al. Efficacy of methylphenidate extendedrelease chewable tablets in children with attention-deficit/hyperactivity disorder: open-label, dose-optimization period outcomes. Journal of American Academy of Child and Adolescent Psychopharmacology 2016;55(Suppl 10):164-5. [DOI: 10.1016/j.jaac.2016.09.202] [DOI] [Google Scholar]
Wilens 2006b {published data only}
- Biederman J. Effectiveness and safety of the once-daily OROS formulation of methylphenidate in adolescents with attention-deficit/hyperactivity disorder. European Neuropsychopharmacology 2003;13 (Suppl 4):S448. [DOI: 10.1016/s0924-977x(03)92351-0] [DOI] [Google Scholar]
- Greenhill LL. Safety and efficacy of OROS MPH in adolescents with ADHD. In: 156th Annual Meeting of the American Psychiatric Association; 2003 May 17-22; San Francisco, CA. San Francisco, 2003:16. [URL: https://www.psychiatry.org/getattachment/b3a019bd-6525-4640-a69e-ed330bf178ec/am_syllabus_2003.pdf (accessed 21/09/22)]
- McGough JJ, McBurnett K, Bukstein O, Wilens TE, Greenhill L, Lerner M, et al. Once-daily OROS methylphenidate is safe and well tolerated in adolescents with attention-deficit/hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 2006;16(3):351-6. [DOI: 10.1089/cap.2006.16.351] [DOI] [PubMed] [Google Scholar]
- NCT00249353. An effectiveness and safety study of Concerta® (methylphenidate hydrochloride) in the treatment of adolescents with attention deficit hyperactivity disorder [An evaluation of the safety and effectiveness of Concerta® (methylphenidate hydrochloride), up to 72 mg daily, in adolescents with attention deficit hyperactivity disorder]. Clinicaltrials.gov/ct2/show/NCT00249353 (first received 7 November 2005).
- Newcorn JH, Stein MA, Cooper KM. Dose-response characteristics in adolescents with attention-deficit/hyperactivity disorder treated with OROS methylphenidate in a 4-week, open-label, dose-titration study. Journal of Child and Adolescent Psychopharmacology 2010;20(3):187-96. [DOI: 10.1089/cap.2009.0102] [DOI] [PubMed] [Google Scholar]
- Oral system methylphenidate for teen ADHD. Brown University Child & Adolescent Psychopharmacology Update 2006;8(3):4-5. [DOI: 10.1002/cpu.20015] [DOI]
- Wilens TE, McBurnett K, Bukstein O, McGough J, Greenhill L, Lerner M, et al. Multisite controlled study of OROS methylphenidate in the treatment of adolescents with attention-deficit/hyperactivity disorder. Archives of Pediatrics & Adolescent Medicine 2006;160(1):82-90. [DOI: 10.1001/archpedi.160.1.82] [DOI] [PubMed] [Google Scholar]
Wilens 2008 {published data only}
- López FA, Landgraf JM, Wilens TE. Quality of life and parent satisfaction with the methylphenidate transdermal system. European Neuropsychopharmacology 2008;18(Suppl 4):S562. [DOI: 10.1016/S0924-977X(08)70856-3] [DOI] [Google Scholar]
- López FA, Wilens TE, Wigal SB, Turnbow JM. Effects of variable wear times on transdermal methylphenidate in attention-deficit/hyperactivity disorder. European Neuropsychopharmacology 2008;18(Suppl 4):S561-2. [DOI: 10.1016/S0924-977X(08)70855-1] [DOI] [Google Scholar]
- Manos M, Frazier TW, Landgraf JM, Weiss M, Hodgkins P. HRQL and medication satisfaction in children with ADHD treated with the methylphenidate transdermal system. Current Medical Research and Opinion 2009;25(12):3001-10. [DOI: 10.1185/03007990903388797] [PMID: ] [DOI] [PubMed] [Google Scholar]
- NCT00151970. Efficacy, safety and tolerability of SPD485 in children aged 6-12 diagnosed with ADHD [A phase IIb, randomized, double-blind, multi-center, placebo-controlled, dose-optimization, 3-way cross-over, analog classroom study to assess the efficacy, duration of effect, tolerability and safety of 4- and 6-hour wear times of methylphenidate transdermal system (MYS) in pediatric subjects aged 6-12 with attention-deficit/hyperactivity disorder (ADHD)]. clinicaltrials.gov/ct2/show/NCT00151970 (first received 9 September 2005).
- Wilens TE, Boellner SW, López FA, Turnbow JM, Wigal SB, Childress AC, et al. Varying the wear time of the methylphenidate transdermal system in children with attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry 2008;47(6):700-8. [DOI: 10.1097/CHI.0b013e31816bffdf] [PMID: ] [DOI] [PubMed] [Google Scholar]
Wilens 2010 {published data only}
- NCT00586157. Study of medication patch to treat children ages 6-12 with ADHD [Efficacy and safety/tolerability of methylphenidate transdermal system (MTS) for before-school dysfunction in children with attention deficit hyperactivity disorder (ADHD)]. clinicaltrials.gov/ct2/show/NCT00586157 (first received 4 January 2008).
- Wilens T, Hammerness P, Utzinger L, Georgiopoulos A, Doyle R, Brodziak K, et al. Before-school ADHD symptoms and functioning in youth treated with the methylphenidate transdermal patch (MTS). Journal of Child and Adolescent Psychopharmacology 2009;19(6):785-6. [DOI: 10.1089/cap.2009.1963] [DOI] [Google Scholar]
- Wilens TE, Hammerness P, Martelon MK, Brodziak K, Utzinger L, Wong P. A controlled trial of the methylphenidate transdermal system on before-school functioning in children with attention-deficit/hyperactivity disorder. Journal of Clinical Psychiatry 2010;71(5):548-56. [DOI: 10.4088/JCP.09m05779pur] [PMID: ] [DOI] [PubMed] [Google Scholar]
Wilkison 1995 {published data only}
- Wilkison PC, Kircher JC, McMahon WM, Sloane HN. Effects of methylphenidate on reward strength in boys with attention-deficit hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry 1995;34(7):897-901. [DOI: 10.1097/00004583-199507000-00013] [PMID: ] [DOI] [PubMed] [Google Scholar]
Wodrich 1998 {published data only}
- Wodrich DL, Kush JC. The effect of methylphenidate on teachers' behavioral ratings in specific school situations. Psychology in the Schools 1998;35(1):81-8. [DOI: ] [DOI] [Google Scholar]
Wolraich 2001 {published data only}
- Greenhill LL. Efficacy and safety of once-daily methylphenidate HCl, standard methylphenidate and placebo in children with ADHD. In: 153rd Annual Meeting of the American Psychiatric Association; 2000 May 13-18; Chicago, Illinois. Chicago, 2000:238. [URL: https://psychiatry.org/getattachment/e5c6e55e-e5cb-426a-af0b-396aee0f102b/am_newresearch_2000.pdf (accessed 21/09/22)]
- Greenhill LL. Evaluation of the efficacy and safety of Concerta (Methylphenidate HCI) extended-release tablets, Ritalin, and placebo in children with ADHD. Neurology 2000;54(7):A420-1. [WOS: 000086557801120] [Google Scholar]
- Swanson J, Greenhill L, Pelham W, Wilens T, Wolraich M, Abikoff H, et al. Initiating Concerta (TM) (OROS methylphenidate HCl) qd in children with attention-deficit hyperactivity disorder. Journal of Clinical Research 2000;3:59-76. [URL: https://experts.umn.edu/en/publications/initiating-concertatm-oros-methylphenidate-hcl-qd-in-children-wit (accessed 21/09/22)] [Google Scholar]
- Wolraich ML, Greenhill LL, Pelham W, Swanson J, Wilens T, Palumbo D, et al. Randomized, controlled trial of OROS methylphenidate once a day in children with attention-deficit/hyperactivity disorder. Pediatrics 2001;108(4):883-92. [DOI: 10.1542/peds.108.4.883] [DOI] [PubMed] [Google Scholar]
- Wolraich ML. Efficacy and safety of OROS(R) methylphenidate HCl (MPH) extended-release tablets (CONCERTA (TM)), conventional MPH, and placebo in children with ADHD. International Journal of Neuropsychopharmacology. Proceedings of the XXIIst CINP Congress; 2000 July 9-13; Brussels, Belgium 2000;3 Suppl 1:S329. [DOI: 10.1017/S1461145700009998] [DOI] [Google Scholar]
- Wolraich ML. Evaluation of efficacy and safety of OROS methylphenidate HCI (MPH) extended-release tablets, methylphenidate tid, and placebo in children with ADHD. Pediatric Research 2000;47(4):36A. [WOS: 000086155300208] [Google Scholar]
Zeiner 1999 {published data only}
- Zeiner P, Bryhn G, Bjercke C, Truyen K, Strand G. Response to methylphenidate in boys with attention-deficit hyperactivity disorder. Acta Paediatrica 1999;88(3):298-303. [DOI: 10.1111/j.1651-2227.1999.tb01100.x] [DOI] [PubMed] [Google Scholar]
- Zeiner P. Body growth and cardiovascular function after extended treatment (1.75 years) with methylphenidate in boys with attention-deficit hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 1995;5(2):129-38. [DOI: 10.1089/cap.1995.5.129] [DOI] [Google Scholar]
- Zeiner P. Do the beneficial effects of extended methylphenidate treatment in boys with attention-deficit hyperactivity disorder dissipate rapidly during placebo treatment? Nordic Journal of Psychiatry 1999;53(1):55-60. [DOI: 10.1080/080394899426738] [DOI] [Google Scholar]
Zeni 2009 {published data only}
- NCT00305370. Aripiprazole associated with methylphenidate in children and adolescents with bipolar disorder and ADHD [Aripiprazole associated with methylphenidate in children and adolescents with bipolar disorder and ADHD: a randomized cross-over placebo controlled trial]. clinicaltrials.gov/ct2/show/NCT00305370 (first received 21 March 2006).
- Zeni CP, Tramontina S, Ketzer CR, Pheula GF, Rohde LA. Methylphenidate combined with aripiprazole in children and adolescents with bipolar disorder and attention-deficit/hyperactivity disorder: a randomized crossover trial. Journal of Child and Adolescent Psychopharmacology 2009;19(5):553-61. [DOI: 10.1089/cap.2009.0037] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
ACTRN12608000059369 {published data only}
- ACTRN12608000059369. A structural and functional imaging research of children with attention deficit hyperactivity disorder (ADHD) [Structural and functional imaging maps of children with attention deficit hyperactivity disorder (ADHD) using magnetic resonance Imaging (MRI) scan of the brain and methylphenidate (MPH)]. anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12608000059369 (first received 30 January 2008).
An 2013 {published data only}
- An L, Cao X-H, Cao Q-J, Sun L, Yang L, Zou Q-H, et al. Methylphenidate normalizes resting-state brain dysfunction in boys with attention deficit hyperactivity disorder. Neuropsychopharmacology 2013;38(7):1287-95. [DOI: 10.1038/npp.2013.27] [DOI] [PMC free article] [PubMed] [Google Scholar]
Anderson 2002 {published data only}
- Anderson CM, Polcari A, Lowen SB, Renshaw PF, Teicher MH. Effects of methylphenidate on functional magnetic resonance relaxometry of the cerebellar vermis in boys with ADHD. American Journal of Psychiatry 2002;159(8):1322-8. [DOI: 10.1176/appi.ajp.159.8.1322] [DOI] [PubMed] [Google Scholar]
Barkley 1988a {published data only}
- Barkley RA, Fischer M, Newby RF, Breen MJ. Development of a multimethod clinical protocol for assessing stimulant drug response in children with attention deficit disorder. Journal of Clinical Child Psychology 1988;17(1):14-24. [DOI: 10.1207/s15374424jccp1701_3] [DOI] [Google Scholar]
Barkley 1997 {published data only}
- Barkley RA, Koplowitz S, Anderson T, McMurray MB. Sense of time in children with ADHD: effects of duration, distraction, and stimulant medication. Journal of the International Neuropsychological Society 1997;3(4):359-69. [DOI: 10.1017/S1355617797003597] [DOI] [PubMed] [Google Scholar]
Bart 2013 {published data only}
- Bart O, Daniel L, Dan O, Bar-Haim Y. Influence of methylphenidate on motor performance and attention in children with developmental coordination disorder and attention deficit hyperactive disorder. Research in Developmental Disabilities 2013;34(6):1922-7. [DOI: 10.1016/j.ridd.2013.03.015] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Bart O, Podoly T, Bar-Haim Y. A preliminary study on the effect of methylphenidate on motor performance in children with comorbid DCD and ADHD. Research in Developmental Disabilities 2010;31(6):1443-7. [DOI: 10.1016/j.ridd.2010.06.014] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bedard 2002 {published data only}
- Bedard A-C, Ickowicz A, Tannock R. Methylphenidate improves Stroop naming speed, but not response interference, in children with attention deficit hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 2002;12(4):301-9. [DOI: 10.1089/104454602762599844] [DOI] [PubMed] [Google Scholar]
Bedard 2003 {published data only}
- Bedard A-C, Ickowicz A, Logan GD, Hogg-Johnson S, Schachar R, Tannock R. Selective inhibition in children with attention-deficit hyperactivity disorder off and on stimulant medication. Journal of Abnormal Child Psychology 2003;31(3):315-27. [DOI: 10.1023/A:1023285614844] [DOI] [PubMed] [Google Scholar]
Bedard 2004 {published data only}
- Bedard A-C, Martinussen R, Ickowicz A, Tannock R. Methylphenidate improves visual-spatial memory in children with attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry 2004;43(3):260-8. [DOI: 10.1097/00004583-200403000-00006] [DOI] [PubMed] [Google Scholar]
Bedard 2007 {published data only}
- Bedard A-C, Jain U, Hogg Johnson S, Tannock R. Effects of methylphenidate on working memory components: influence of measurement. Journal of Child Psychology and Psychiatry 2007;48(9):872-80. [DOI: 10.1111/j.1469-7610.2007.01760.x] [DOI] [PubMed] [Google Scholar]
Beery 1994 {published data only}
- Beery SH. Behavioral disinhibition, anxiety, and response to methylphenidate and behavior management in children with attention deficit hyperactivity disorder. Dissertation Abstracts International: Section B: The Sciences and Engineering 1994;56(2-B):1096. [PROQUEST: 9519723] [Google Scholar]
Beery 2017 {published data only}
- Beery SH, Quay HC, Pelham WE Jr. Differential response to methylphenidate in inattentive and combined subtype ADHD. Journal of Attention Disorders 2017;21(1):62-70. [DOI: 10.1177/1087054712469256] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ben‐Pazi 2006 {published data only}
- Ben-Pazi H, Shalev RS, Gross-Tsur V, Bergman H. Age and medication effects on rhythmic responses in ADHD: possible oscillatory mechanisms? Neuropsychologia 2006;44(3):412-6. [DOI: 10.1016/j.neuropsychologia.2005.05.022] [DOI] [PubMed] [Google Scholar]
Bental 2008 {published data only}
- Bental B, Tirosh E. The effects of methylphenidate on word decoding accuracy in boys with attention-deficit/hyperactivity disorder. Journal of Clinical Psychopharmacology 2008;28(1):89-92. [DOI: 10.1097/jcp.0b013e3181603f0e] [DOI] [PubMed] [Google Scholar]
Beyer 2014 {published data only}
- Beyer von Morgenstern S, Becker I, Sinzig J. Improvement of facial affect recognition in children and adolescents with attention-deficit/hyperactivity disorder under methylphenidate. Acta Neuropsychiatrica 2014;26(4):202-8. [DOI: 10.1017/neu.2013.55] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bouziane 2019 {published data only}
- Bouziane C, Filatova OG, Schrantee A, Caan MW, Vos FM, Reneman L. White matter by diffusion MRI following methylphenidate treatment: a randomized control trial in males with attention-deficit/hyperactivity disorder. Radiology 2019;293(1):186-92. [DOI: 10.1148/radiol.2019182528] [PMID: ] [DOI] [PubMed] [Google Scholar]
Brown 1984b {published data only}
- Brown RT, Wynne ME. Sustained attention in boys with attention deficit disorder and the effect of methylphenidate. Pediatric Nursing 1984;10(1):35-9. [PMID: ] [PubMed] [Google Scholar]
Buhrmester 1992 {published data only}
- Buhrmester D, Whalen CK, Henker B, MacDonald V, Hinshaw SP. Prosocial behavior in hyperactive boys: effects of stimulant medication and comparison with normal boys. Journal of Abnormal Child Psychology 1992;20(1):103-21. [DOI: 10.1007/BF00927119] [DOI] [PubMed] [Google Scholar]
Campbell 1996 {published data only}
- Campbell L, Malone MA, Kershner JR, Roberts W, Humphries T, Logan WJ. Methylphenidate slows right hemisphere processing in children with attention-deficit/hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 1996;6(4):229-39. [DOI: 10.1089/cap.1996.6.229] [DOI] [PubMed] [Google Scholar]
Carlson 1991 {published data only}
- Carlson CL, Pelham WE Jr, Swanson JM, Wagner JL. A divided attention analysis of the effects of methylphenidate on the arithmetic performance of children with attention-deficit hyperactivity disorder. Journal of Child Psychology and Psychiatry and Allied Disciplines 1991;32(3):463-71. [DOI: 10.1111/j.1469-7610.1991.tb00324.x] [DOI] [PubMed] [Google Scholar]
Carlson 1992 {published data only}
- Carlson CL, Pelham WE Jr, Milich R, Dixon J. Single and combined effects of methylphenidate and behavior therapy on the classroom performance of children with attention-deficit hyperactivity disorder. Journal of Abnormal Child Psychology 1992;20(2):213-32. [DOI: 10.1007/BF00916549] [DOI] [PubMed] [Google Scholar]
Cohen 2020 {published data only}
- Cohen J, Eom K, Henry T, Bricken C, Cejas D, Politte L, et al. Dynamic reconfiguration of functional brain networks in ADHD after methylphenidate administration relates to improvements in response control. Biological Psychiatry 2020;87(Suppl 9):S19. [DOI: 10.1016/j.biopsych.2020.02.074] [DOI] [Google Scholar]
Cox 2004b {published data only}
- Cox DJ, Humphrey JW, Merkel RL, Penberthy JK, Kovatchev B. Controlled-release methylphenidate improves attention during on-road driving by adolescents with attention-deficit/hyperactivity disorder. Journal of the American Board of Family Medicine 2004;17(4):235-9. [DOI: 10.3122/jabfm.17.4.235] [DOI] [PubMed] [Google Scholar]
- Cox DJ, Merkel RL, Penberthy JK, Kovatchev B, Hankin CS. Impact of methylphenidate delivery profiles on driving performance of adolescents with attention-deficit/hyperactivity disorder: a pilot study. Journal of the American Academy of Child and Adolescent Psychiatry 2004;43(3):269-75. [DOI: 10.1097/00004583-200403000-00007] [DOI] [PubMed] [Google Scholar]
Cubillo 2014 {published data only}
- Cubillo A, Smith AB, Barrett N, Giampietro V, Brammer MJ, Simmons A, et al. Shared and drug-specific effects of atomoxetine and methylphenidate on inhibitory brain dysfunction in medication-naive ADHD boys. Cerebral Cortex 2014;24(1):174-85. [DOI: 10.1093/cercor/bhs296] [PMCID: PMC3862268] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cubillo 2020 {published data only}
- Cubillo A, Kowalczyk O, Smith AB, Barrett N, Giampietro V, Brammer M, et al. Shared normalisation of sustained attention-related brain dysfunctions of stimulants and non-stimulant medications in ADHD. Biological Psychiatry 2020;87(Suppl 9):S18. [DOI: 10.1016/j.biopsych.2020.02.072] [DOI] [Google Scholar]
Dawson 1998 {published data only}
- Dawson NL. Effects of methylphenidate on neuropsychological functions myelomeningocele. Dissertation Abstracts International: Section B: The Sciences and Engineering 1998;(12-B):6805. [PROQUEST: 304371447]
De Sonneville 1991 {published data only}
- De Sonneville LM, Njiokiktjien C, Hilhorst RC. Methylphenidate-induced changes in ADDH information processors. Journal of Child Psychology and Psychiatry and Allied Disciplines 1991;32(2):285-95. [DOI: 10.1111/j.1469-7610.1991.tb00307.x] [DOI] [PubMed] [Google Scholar]
DeVito 2008 {published data only}
- DeVito EE, Blackwell AD, Clark L, Kent L, Dezsery AM, Turner DC, et al. Methylphenidate improves response inhibition but not reflection-impulsivity in children with attention deficit hyperactivity disorder (ADHD). Psychopharmacology 2009;202(1-3):531-9. [DOI: 10.1007/s00213-008-1337-y] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVito EE, Blackwell AD, Kent L, Ersche KD, Clark L, Salmond CH, et al. The effects of methylphenidate on decision making in attention-deficit/hyperactivity disorder. Biological Psychiatry 2008;64(7):636-9. [DOI: 10.1016/j.biopsych.2008.04.017] [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVito EE, Sahakian BJ. Response to comments on 'Methylphenidate improves response inhibition but not reflection impulsivity in children with attention deficit hyperactivity disorder (ADHD)'. Psychopharmacology 2009;203(1):187. [DOI: 10.1007/s00213-008-1383-5] [DOI] [PubMed] [Google Scholar]
Dougherty 2016 {published data only}
- Dougherty DM, Olvera RL, Acheson A, Hill-Kapturczak N, Ryan SR, Mathias CW. Acute effects of methylphenidate on impulsivity and attentional behavior among adolescents comorbid for ADHD and conduct disorder. Journal of Adolescence 2016;53:222-30. [DOI: 10.1016/j.adolescence.2016.10.013] [PMCID: PMC5116269] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Evans 1986 {published data only}
- Evans RW, Gualtieri CT, Amara I. Methylphenidate and memory: dissociated effects in hyperactive children. Psychopharmacology 1986;90(2):211-6. [DOI: 10.1007/BF00181244] [DOI] [PubMed] [Google Scholar]
Fosco 2017 {published data only}
- Fosco WD, White CN, Hawk LW Jr. Acute stimulant treatment and reinforcement increase the speed of information accumulation in children with ADHD. Journal of Abnormal Child Psychology 2017;45(5):911-20. [DOI: 10.1007/s10802-016-0222-0] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fosco 2021 {published data only}
- Fosco WD, Rosch KS, Waxmonsky JG, Pelham WE, Hawk LW. Baseline performance moderates stimulant effects on cognition in youth with ADHD. Experimental and Clinical Psychopharmacology 2021;29(4):302-7. [DOI: 10.1037/pha0000374] [PMCID: PMC8388131] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fox 2014 {published data only}
- Fox O, Adi-Japha E, Karni A. The effect of a skipped dose (placebo) of methylphenidate on the learning and retention of a motor skill in adolescents with attention deficit hyperactivity disorder. European Neuropsychopharmacology 2014;24(3):391-6. [DOI: 10.1016/j.euroneuro.2013.11.005] [DOI] [PubMed] [Google Scholar]
Francis 2001 {published data only}
- Francis S, Fine J, Tannock R. Methylphenidate selectively improves story retelling in children with attention deficit hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 2001;11(3):217-28. [DOI: 10.1089/10445460152595540] [DOI] [PubMed] [Google Scholar]
Gan 1982 {published data only}
- Gan J, Cantwell DP. Dosage effects of methylphenidate on paired associate learning: positive/negative placebo responders. Journal of the American Academy of Child Psychiatry 1982;21(3):237-42. [DOI: 10.1016/S0002-7138(09)60876-1] [DOI] [PubMed] [Google Scholar]
Golubchik 2018 {published data only}
- Golubchik P, Hamerman H, Manor I, Peskin M, Weizman A. Effectiveness of parental training, methylphenidate treatment, and their combination on academic achievements and behavior at school of children with attention-deficit hyperactivity disorder. International Clinical Psychopharmacology 2018;33(4):229-32. [DOI: 10.1097/YIC.0000000000000218] [PMID: ] [DOI] [PubMed] [Google Scholar]
González‐Carpio Hernández 2016 {published data only}
- González-Carpio Hernández G, Serrano Selva JP. Medication and creativity in attention deficit hyperactivity disorder (ADHD). Psicothema 2016;28(1):20-5. [DOI: 10.7334/psicothema2015.126] [PMID: ] [DOI] [PubMed] [Google Scholar]
Granger 1996 {published data only}
- Granger DA, Whalen CK, Henker B, Cantwell C. ADHD boys' behavior during structured classroom social activities: effects of social demands, teacher proximity, and methylphenidate. Journal of Attention Disorders 1996;1(1):16-30. [DOI: 10.1177/108705479600100102] [DOI] [Google Scholar]
Grizenko 2010 {published data only}
- Grizenko N, Paci M, Joober R. Is the inattentive subtype of ADHD different from the combined/hyperactive subtype? Journal of Attention Disorders 2010;13(6):649-57. [DOI: 10.1177/1087054709347200] [DOI] [PubMed] [Google Scholar]
Günther 2010 {published data only}
- Günther T, Herpertz-Dahlmann B, Konrad K. Sex differences in attentional performance and their modulation by methylphenidate in children with attention-deficit/hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 2010;20(3):179-86. [DOI: 10.1089/cap.2009.0060] [DOI] [PubMed] [Google Scholar]
Hadar 2020 {published data only}
- Hadar Y, Hocherman S, Lamm O, Tirosh E. Auditory and visual executive functions in children and response to methylphenidate: a randomized controlled trial. Journal of Attention Disorders 2020;24(2):235-45. [DOI: 10.1177/1087054717700978] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Hadar Y, Hocherman S, Lamm O, Tirosh E. The visuo-motor attention test in boys with attention deficit hyperactivity disorder (ADHD): methylphenidate-placebo randomized controlled trial. Child Psychiatry and Human Development 2021;52(1):96–103. [DOI: 10.1007/s10578-020-00993-8] [PMID: ] [DOI] [PubMed] [Google Scholar]
Halliday 1983 {published data only}
- Halliday R, Callaway E, Naylor H. Visual evoked potential changes induced by methylphenidate in hyperactive children: dose/response effects. Electroencephalography and Clinical Neurophysiology 1983;55(3):258-67. [DOI: 10.1016/0013-4694(83)90203-1] [DOI] [PubMed] [Google Scholar]
Hanisch 2004 {published data only}
- Hanisch C, Konrad K, Günther T, Herpertz-Dahlmann B. Age-dependent neuropsychological deficits and effects of methylphenidate in children with attention-deficit/hyperactivity disorder: a comparison of pre- and grade-school children. Journal of Neural Transmission 2004;111(7):865-81. [DOI: 10.1007/s00702-003-0056-0] [DOI] [PubMed] [Google Scholar]
Hazel‐Fernandez 2006 {published data only}
- Hazel-Fernandez LA, Klorman R, Wallace JM, Cook S. Methylphenidate improves aspects of executive function in African American children with ADHD. Journal of Attention Disorders 2006;9(4):582-9. [DOI: 10.1177/1087054705284243] [DOI] [PubMed] [Google Scholar]
Helseth 2015 {published data only}
- Helseth SA, Waschbusch DA, Gnagy EM, Onyango AN, Burrows-MacLean L, Fabiano GA, et al. Effects of behavioral and pharmacological therapies on peer reinforcement of deviancy in children with ADHD-only, ADHD and conduct problems, and controls. Journal of Consulting and Clinical Psychology 2015;83(2):280-92. [DOI: 10.1037/a0038505] [PMCID: PMC4380669] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hinshaw 1989 {published data only}
- Hinshaw SP, Buhrmester D, Heller T. Anger control in response to verbal provocation: effects of stimulant medication for boys with ADHD. Journal of Abnormal Child Psychology 1989;17(4):393-407. [DOI: 10.1007/BF00915034] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Hinshaw SP, Henker B, Whalen CK, Erhardt D, Dunnington RE. Aggressive, prosocial, and nonsocial behavior in hyperactive boys: dose effects of methylphenidate in naturalistic settings. Journal of Consulting and Clinical Psychology 1989;57(5):636-43. [DOI: 10.1037/0022-006X.57.5.636] [DOI] [PubMed] [Google Scholar]
Hinshaw 1993 {published data only}
- Hinshaw SP, Heller T, McHale JP. Covert antisocial behavior in boys with attention-deficit hyperactivity disorder: external validation and effects of methylphenidate. Journal of Consulting and Clinical Psychology 1992;60(2):274-81. [DOI: 10.1037/0022-006X.60.2.274] [DOI] [PubMed] [Google Scholar]
Horowitz 2020 {published data only}
- Horowitz I, Avirame K, Naim-Feil J, Rubinson M, Moses E, Gothelf D, et al. The interactive effects of test-retest and methylphenidate administration on cognitive performance in youth with ADHD: a double-blind placebo-controlled crossover study. Psychiatry Research 2020;291:113056. [DOI: 10.1016/j.psychres.2020.113056] [PMID: ] [DOI] [PubMed] [Google Scholar]
Humphries 1979 {published data only}
- Humphries T, Swanson J, Kinsbourne M, Yiu L. Stimulant effects on persistence of motor performance of hyperactive children. Journal of Pediatric Psychology 1979;4(1):55-66. [DOI: 10.1093/jpepsy/4.1.55] [DOI] [Google Scholar]
Ishii‐Takahashi 2015 {published data only}
- Ishii-Takahashi A, Takizawa R, Nishimura Y, Kawakubo Y, Hamada K, Okuhata S, et al. "Neuroimaging-aided prediction of the effect of methylphenidate in children with attention-deficit hyperactivity disorder: a randomized controlled trial": corrigendum. Neuropsychopharmacology 2015;40(12):2852. [DOI: 10.1038/npp.2015.154] [PMCID: PMC4864663] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii-Takahashi A, Takizawa R, Nishimura Y, Kawakubo Y, Hamada K, Okuhata S, et al. Neuroimaging-aided prediction of the effect of methylphenidate in children with attention-deficit hyperactivity disorder: a randomized controlled trial. Neuropsychopharmacology 2015;40(12):2676-85. Erratum in: Neuropsychopharmacology 2015; 40(12):2852. [DOI: 10.1038/npp.2015.128] [PMCID: PMC4864654] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
ISRCTN52376787 {published data only}
- ISRCTN52376787. Working memory in children with attention-deficit/hyperactivity disorder (ADHD): the impact of methylphenidate (MPH). www.isrctn.com/ISRCTN52376787 (first received 23 January 2004). [DOI: 10.1186/ISRCTN52376787] [DOI]
JPRN‐UMIN000008831 {published data only}
- JPRN-UMIN000008831. Neurophysiological analysis of ADHD: an exploratory neuroimaging study using functional near-infrared spectroscopy (fNIRS). upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000010374 (first received 10 September 2012).
JPRN‐UMIN000027533 {published data only}
- JPRN-UMIN000027533. A research for the effect of the treatment in children with attention-deficit/hyperactivity disorder using resting state functional magnetic resonance imaging. upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000031550 (first received 1 June 2017).
King 2009a {published data only}
- King S, Waschbusch DA, Pelham WE, Frankland BW, Corkum PV, Jacques S. Subtypes of aggression in children with attention deficit hyperactivity disorder: medication effects and comparison with typical children. Journal of Clinical Child and Adolescent Psychology 2009;38(5):619-29. [DOI: 10.1080/15374410903103619] [DOI] [PubMed] [Google Scholar]
King 2009b {published data only}
- King S, Waschbusch DA, Pelham WE Jr, Frankland BW, Andrade BF, Jacques S, et al. Social information processing in elementary-school aged children with ADHD: medication effects and comparisons with typical children. Journal of Abnormal Child Psychology 2009;37(4):579-89. [DOI: 10.1007/s10802-008-9294-9] [DOI] [PubMed] [Google Scholar]
Kobayashi 2020 {published data only}
- Kobayashi M, Ikeda T, Tokuda T, Monden Y, Nagashima M, Mizushima SG, et al. Acute administration of methylphenidate differentially affects cortical processing of emotional facial expressions in attention-deficit hyperactivity disorder children as studied by functional near-infrared spectroscopy. Neurophotonics 2020;7(2):025003. [DOI: 10.1117/1.NPh.7.2.025003] [PMCID: PMC7201297] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kowalczyk 2019 {published data only}
- Kowalczyk OS, Cubillo AI, Smith A, Barrett N, Giampietro V, Brammer M, et al. Methylphenidate and atomoxetine normalise frontoparietal activation in ADHD during sustained attention. ADHD Attention Deficit and Hyperactivity Disorders 2019;11(1):S38-9. [DOI: 10.1007/s12402-019-00295-7] [DOI] [Google Scholar]
Lange 2007 {published data only}
- Lange KW, Tucha LT, Walitza S, Sontag TA, Stasik D, Linder M, et al. Effects of methylphenidate on multiple components of attention in children with attention deficit hyperactivity disorder. Journal of Neural Transmission. Abstracts of the 39th International Danube Symposium for Neurological Sciences and Continuing Education and 1st International Congress on ADHD, from Childhood to Adult Disease; 2007 June 2-5; Wurzburg, Germany 2007;114 (7):LXXV. [DOI: 10.1007/s00702-007-0754-0] [DOI] [Google Scholar]
Leitner 2007b {published data only}
- Leitner Y, Barak R, Giladi N, Peretz C, Eshel R, Gruendlinger L, et al. Gait in attention deficit hyperactivity disorder: effects of methylphenidate and dual tasking. Journal of Neurology 2007;254(10):1330-8. [DOI: 10.1007/s00415-006-0522-3] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Leitner Y, Doniger GM, Barak R, Simon ES, Hausdorff JM. A novel multidomain computerized cognitive assessment for attention-deficit hyperactivity disorder: evidence for widespread and circumscribed cognitive deficits. Journal of Child Neurology 2007;22(3):264-76. [DOI: 10.1177/0883073807299859] [PMID: ] [DOI] [PubMed] [Google Scholar]
Levi‐Shachar 2020 {published data only}
- Levi-Shachar O, Gvirts HZ, Goldwin Y, Bloch Y, Shamay-Tsoory S, Zagoory-Sharon O, et al. Correction: the effect of methylphenidate on social cognition and oxytocin in children with attention deficit hyperactivity disorder. Neuropsychopharmacology 2020;45(2):438. [DOI: 10.1038/s41386-019-0533-2] [PMCID: PMC6901490] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi-Shachar O, Gvirts HZ, Goldwin Y, Bloch Y, Shamay-Tsoory S, Zagoory-Sharon O, et al. The effect of methylphenidate on social cognition and oxytocin in children with attention deficit hyperactivity disorder. Neuropsychopharmacology 2020;45(2):367-73. Erratum in: Neuropsychopharmacology 2020;45(2):438. [DOI: 10.1038/s41386-019-0522-5] [PMCID: PMC6901562] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Luman 2015 {published data only}
- Luman M, Goos V, Oosterlaan J. Instrumental learning in ADHD in a context of reward: intact learning curves and performance improvement with methylphenidate. Journal of Abnormal Child Psychology 2015;43(4):681-91. [DOI: 10.1007/s10802-014-9934-1] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Luman M, Papanikolau A, Oosterlaan J. The unique and combined effects of reinforcement and methylphenidate on temporal information processing in attention-deficit/hyperactivity disorder. Journal of Clinical Psychopharmacology 2015;35(4):414-21. [DOI: 10.1097/JCP.0000000000000349] [PMID: ] [DOI] [PubMed] [Google Scholar]
Malone 1988 {published data only}
- Malone MA, Kershner JR, Siegel L. The effects of methylphenidate on levels of processing and laterality in children with attention deficit disorder. Journal of Abnormal Child Psychology 1988;16(4):379-95. [DOI: 10.1007/BF00914170] [DOI] [PubMed] [Google Scholar]
Malone 1993 {published data only}
- Malone MA, Swanson JM. Effects of methylphenidate on impulsive responding in children with attention-deficit hyperactivity disorder. Journal of Child Neurology 1993;8(2):157-63. [DOI: 10.1177/088307389300800209] [DOI] [PubMed] [Google Scholar]
Malone 1994 {published data only}
- Malone MA, Couitis J, Kershner JR, Logan WJ. Right hemisphere dysfunction and methylphenidate effects in children with attention-deficit/hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 1994;4(4):245-53. [DOI: 10.1089/cap.1994.4.245] [DOI] [Google Scholar]
Martin 2007 {published data only}
- Martin CA, Guenthner G, Bingcang C, Rayens MK, Kelly TH. Measurement of the subjective effects of methylphenidate in 11- to 15-year-old children with attention-deficit/hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 2007;17(1):63-73. [DOI: 10.1089/cap.2006.0020] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mazzetti 2022 {published data only}
- Mazzetti C, Damatac CG, Sprooten E, ter Huurne N, Buitelaar JK, Jensen O. Dorsal-to-ventral imbalance in the superior longitudinal fasciculus mediates methylphenidate's effect on beta oscillations in ADHD. Psychophysiology 2022;59:1-17. [DOI: 10.1111/psyp.14008] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mehta 2004 {published data only}
- Mehta MA, Goodyer IM, Sahakian BJ. Methylphenidate improves working memory and set-shifting in AD/HD: relationships to baseline memory capacity. Journal of Child Psychology and Psychiatry and Alllied Disciplines 2004;45(2):293-305. [DOI: 10.1111/j.1469-7610.2004.00221.x] [DOI] [PubMed] [Google Scholar]
Merrill 2022 {published data only}
- Merrill BM, Raiker JS, Mattfeld AT, Macphee FL, Ramos MC, Zhao X, et al. Mind-wandering and childhood ADHD: experimental manipulations across laboratory and naturalistic settings. Research on Child and Adolescent Psychopathology 2022;5:1-11. [DOI: 10.1007/s10802-022-00912-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Milich 1989 {published data only}
- Milich R, Licht BG, Murphy DA, Pelham WE. Attention-deficit hyperactivity disordered boys' evaluations of and attributions for task performance on medication versus placebo. Journal of Abnormal Psychology 1989;98(3):280-4. [DOI: 10.1037/0021-843X.98.3.280] [DOI] [PubMed] [Google Scholar]
Milich 1991 {published data only}
- Milich R, Carlson CL, Pelham WE, Licht BG. Effects of methylphenidate on the persistence of ADHD boys following failure experiences. Journal of Abnormal Child Psychology 1991;19(5):519-36. [DOI: 10.1007/BF00925818] [DOI] [PubMed] [Google Scholar]
Mizuno 2021 {published data only}
- Mizuno Y, Cai W, Spekar K, Makita K, Takiguchi S, Tomoda A, et al. Effects of methylphenidate on aberrant brain network dynamics in children with attention-deficit/hyperactivity disorder: a randomized controlled clinical trial. Biological Psychiatry 2021;89:108. [DOI: 10.1016/j.biopsych.2021.02.278] [DOI] [Google Scholar]
Morris 2022 {published data only}
- Morris SSj, Musser ED, Tenenbaum RB, Ward AR, Raiker JS, Coles EK. Methylphenidate improves autonomic functioning among youth with attention-deficit/hyperactivity disorder. Research on Child and Adolescent Psychopathology 2022;50:591-603. [DOI: 10.1007/s10802-021-00870-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nagashima 2015 {published data only}
- Nagashima M, Monden Y, Dan I, Mizutani T, Ikeda T, Shimoizumi H, et al. E-019 fNIRS-based assessment of MPH effect in drug-naive ADHD: a double-blind, placebo-controlled study. Japanese Society of Child Neurology 2015;47(Suppl 1):S211. [DOI: 10.11251/ojjscn.47.S110] [DOI] [Google Scholar]
NCT00485797 {published data only}
- NCT00485797. Effects of Ritalin on postural stability of hyper active children [Effects of methylphenidate on postural stability of children suffer From attention deficit hyperactivity disorder (ADHD) under single and dual task conditions]. clinicaltrials.gov/ct2/show/NCT00485797 (first received 13 June 2007).
NCT00778310 {published data only}
- NCT00778310. Neuroimaging of the effects of Concerta in the treatment of ADHD [Functional neuroimaging of acute Concerta treatment of attention-deficit/hyperactivity disorder (ADHD): differences across development]. clinicaltrials.gov/ct2/show/NCT00778310 (first received 23 October 2008).
NCT02318017 {published data only}
- NCT02318017. Psychostimulants effects on brain functional connectivity in youth with attention deficit hyperactivity disorder [An electroencephalography study of the effects of psychostimulants on dynamic patterns of cognitive task-based functional connectivity in youths with attention deficit hyperactivity disorder]. clinicaltrials.gov/ct2/show/NCT02318017 (first received 17 December 2014).
NCT03788902 {published data only}
- NCT03788902. OT and social cognition in children with ADHD: impact of MPH [Oxytocin and social cognition in children with attention deficit hyperactivity disorder: impact of methylphenidate]. clinicaltrials.gov/ct2/show/NCT03788902 (first received 28 December 2018).
NCT04349917 {published data only}
- NCT04349917. Large-scale brain organization during cognitive control in ADHD. clinicaltrials.gov/ct2/show/NCT04349917 (first received 16 April 2020).
Novak 1995 {published data only}
- Novak GP, Solanto M, Abikoff H. Spatial orienting and focused attention in attention deficit hyperactivity disorder. Psychophysiology 1995;32(6):546-59. [DOI: 10.1111/j.1469-8986.1995.tb01231.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
O'Toole 1997 {published data only}
- O'Toole K, Abramowitz A, Morris R, Dulcan M. Effects of methylphenidate on attention and nonverbal learning in children with attention-deficit hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry 1997;36(4):531-8. [DOI: 10.1097/00004583-199704000-00016] [DOI] [PubMed] [Google Scholar]
Pakdaman 2018 {published data only}
- Pakdaman F, Irani F, Tajikzadeh F, Jabalkandi SA. The efficacy of Ritalin in ADHD children under neurofeedback training. Neurological Sciences 2018;39(12):2071-8. [DOI: 10.1007/s10072-018-3539-3] [PMID: ] [DOI] [PubMed] [Google Scholar]
Peeke 1984 {published data only}
- Peeke S, Halliday R, Callaway E, Prael R, Reus V. Effects of two doses of methylphenidate on verbal information processing in hyperactive children. Journal of Clinical Psychopharmacology 1984;4(2):82-8. [DOI: 10.1097/00004714-198404020-00004] [DOI] [PubMed] [Google Scholar]
Pelham 1985 {published data only}
- Pelham WE, Bender ME, Caddell J, Booth S, Moorer SH. Methylphenidate and children with attention deficit disorder. Dose effects on classroom academic and social behavior. Archives of General Psychiatry 1985;42(10):948-52. [DOI: 10.1001/archpsyc.1985.01790330028003] [PMID: ] [DOI] [PubMed] [Google Scholar]
Pelham 1990b {published data only}
- Pelham WE, McBurnett K, Harper GW, Milich R, Murphy DA, Clinton J, et al. Methylphenidate and baseball playing in ADHD children: who's on first? Journal of Consulting and Clinical Psychology 1990;58(1):130-3. [DOI: 10.1037/0022-006X.58.1.130] [PMID: ] [DOI] [PubMed] [Google Scholar]
Pelham 1992 {published data only}
- Pelham WE, Murphy DA, Vannatta K, Milich R, Licht BG, Gnagy EM, et al. Methylphenidate and attributions in boys with attention-deficit hyperactivity disorder. Journal of Consulting and Clinical Psychology 1992;60(2):282-92. [DOI: 10.1037/0022-006X.60.2.282] [PSYCINFO: 1994-34504-001] [DOI] [PubMed] [Google Scholar]
- Pelham WE, Murphy DA, Vannatta K, Milich R. Methylphenidate and attributions in boys with attention-deficit hyperactivity disorder. In: Hertzig ME, Farber EA, editors(s). Annual Progress in Child Psychiatry and Child Development. American Psychological Association, 1993:242-65. [ISBN: 9780876307298] [Google Scholar]
Pelham 1997 {published data only}
- Pelham WE, Hoza B, Kipp HL, Gnagy EM, Trane ST. Effects of methylphenidate and expectancy on ADHD children's performance, self-evaluations, persistence, and attributions on a cognitive task. Experimental and Clinical Psychopharmacology 1997;5(1):3-13. [DOI: 10.1037/1064-1297.5.1.3] [DOI] [PubMed] [Google Scholar]
Pelham 2001b {published data only}
- Pelham WE Jr, Waschbusch DA, Hoza B, Pillow DR, Gnagy EM. Effects of methylphenidate and expectancy on performance, self-evaluations, persistence, and attributions on a social task in boys with ADHD. Experimental and Clinical Psychopharmacology 2001;9(4):425-37. [DOI: 10.1037/1064-1297.9.4.425] [DOI] [PubMed] [Google Scholar]
Pelham 2017a {published data only}
- Pelham WE Jr, Gnagy EM, Sibley MH, Kipp HL, Smith BH, Evans SW, et al. Attributions and perception of methylphenidate effects in adolescents with ADHD. Journal of Attention Disorders 2017;21(2):129-36. [DOI: 10.1177/1087054713493320] [PMID: ] [DOI] [PubMed] [Google Scholar]
Pelham 2017b {published data only}
- Pelham WE Jr, Meichenbaum DL, Smith BH, Sibley MH, Gnagy EM, Bukstein O. Acute effects of MPH on the parent-teen interactions of adolescents with ADHD. Journal of Attention Disorders 2017;21(2):158-67. [DOI: 10.1177/1087054713480833] [PMID: ] [DOI] [PubMed] [Google Scholar]
Rapport 1995 {published data only}
- Rapport MD, Loo S, Denney C. The paired associate learning task: is it an externally valid instrument for assessing methylphenidate response in children with attention deficit disorder? Journal of Psychopathology and Behavioral Assessment 1995;17(2):125-44. [DOI: 10.1007/BF02229014] [DOI] [Google Scholar]
Ratzon 2017 {published data only}
- Ratzon NZ, Lunievsky EK, Ashkenasi A, Laks J, Cohen HA. Simulated driving skills evaluation of teenagers with attention deficit hyperactivity disorder before driving lessons. American Journal of Occupational Therapy 2017;71(3):7103220010p1-8. [DOI: 10.5014/ajot.2017.020164] [PMID: ] [DOI] [PubMed] [Google Scholar]
Richardson 1988 {published data only}
- Kupietz SS, Winsberg BG, Sverd J. Learning ability and methylphenidate (Ritalin(®)) plasma concentration in hyperkinetic children: a preliminary investigation. Journal of the American Academy of Child and Adolescent Psychiatry 1982;21(1):27-30. [DOI: 10.1097/00004583-198201000-00006] [DOI] [PubMed] [Google Scholar]
- Richardson E, Kupietz SS, Winsberg BG, Maitinsky S, Mendell N. Effects of methylphenidate dosage in hyperactive reading-disabled children: II. Reading achievement. Journal of the American Academy of Child and Adolescent Psychiatry 1988;27(1):78-87. [DOI: 10.1097/00004583-198801000-00012] [DOI] [PubMed] [Google Scholar]
Rubia 2003 {published data only}
- Rubia K, Noorloos J, Smith A, Gunning B, Sergeant J. Motor timing deficits in community and clinical boys with hyperactive behavior: the effect of methylphenidate on motor timing. Journal of Abnormal Child Psychology 2003;31(3):301-13. [DOI: 10.1023/A:1023233630774] [DOI] [PubMed] [Google Scholar]
Rubinson 2019 {published data only}
- Rubinson M, Horowitz I, Naim-Feil J, Gothelf D, Levit-Binnun N, Moses E. Effects of methylphenidate on the ERP amplitude in youth with ADHD: a double-blind placebo-controlled cross-over EEG study. PloS One 2019;14(5):e0217383. [DOI: 10.1371/journal.pone.0217383] [PMCID: PMC6544236] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinson M, Horowitz I, Naim-Feil J, Gothelf D, Moses E, Levit-Binnun N. Electroencephalography functional networks reveal global effects of methylphenidate in youth with attention deficit/hyperactivity disorder. Brain Connectivity 2019;9(5):437-50. [DOI: 10.1089/brain.2018.0630] [PMID: ] [DOI] [PubMed] [Google Scholar]
Sangal 2006 {published data only}
- Sangal RB, Sangal JM. Attention-deficit/hyperactivity disorder: use of cognitive evoked potential (P300) to predict treatment response. Clinical Neurophysiology 2006;117(9):1996-2006. [DOI: 10.1016/j.clinph.2006.06.004] [DOI] [PubMed] [Google Scholar]
Silk 2012 {published data only}
- Silk T. Resting-state functional connectivity anomalies in ADHD and responses to methylphenidate medication. Brain Connectivity 2012;2(4):A128. [DOI: 10.1089/brain.2012.1500] [DOI]
Silk 2014 {published data only}
- Silk TJ, Newman DP, Eramudugolla R, Vance A, Bellgrove MA. Influence of methylphenidate on spatial attention asymmetry in adolescents with attention deficit hyperactivity disorder (ADHD): preliminary findings. Neuropsychologia 2014;56:178-83. [DOI: 10.1016/j.neuropsychologia.2014.01.015] [PMID: ] [DOI] [PubMed] [Google Scholar]
Silk 2017 {published data only}
- Silk TJ, Malpas C, Vance A, Bellgrove MA. The effect of single-dose methylphenidate on resting-state network functional connectivity in ADHD. Brain Imaging and Behavior 2017;11(5):1422-31. [DOI: 10.1007/s11682-016-9620-8] [PMID: ] [DOI] [PubMed] [Google Scholar]
Slama 2015 {published data only}
- Slama H, Fery P, Verheulpen D, Vanzeveren N, Van Bogaert P. Cognitive improvement of attention and inhibition in the late afternoon in children with attention-deficit hyperactivity disorder (ADHD) treated with osmotic-release oral system methylphenidate. Journal of Child Neurology 2015;30(8):1000-9. [DOI: 10.1177/0883073814550498] [PMID: ] [DOI] [PubMed] [Google Scholar]
Smith 2013 {published data only}
- Smith A, Cubillo A, Barrett N, Giampietro V, Simmons A, Brammer M, et al. Neurofunctional effects of methylphenidate and atomoxetine in boys with attention-deficit/hyperactivity disorder during time discrimination. Biological Psychiatry 2013;74(8):615-22. [DOI: 10.1016/j.biopsych.2013.03.030] [DOI] [PubMed] [Google Scholar]
Solanto 1986 {published data only}
- Solanto MV. Behavioral effects of low-dose methylphenidate in childhood attention deficit disorder: implications for a mechanism of stimulant drug action. Journal of the American Academy of Child and Adolescent Psychiatry 1986;25(1):96-101. [DOI: 10.1016/S0002-7138(09)60604-X] [DOI] [PubMed] [Google Scholar]
Solanto 1997 {published data only}
- Solanto MV, Wender EH, Bartell SS. Effects of methylphenidate and behavioral contingencies on sustained attention in attention-deficit hyperactivity disorder: a test of the reward dysfunction hypothesis. Journal of Child and Adolescent Psychopharmacology 1997;7(2):123-36. [DOI: 10.1089/cap.1997.7.123] [DOI] [PubMed] [Google Scholar]
Srinivas 1992 {published data only}
- Srinivas NR, Hubbard JW, Quinn D, Midha KK. Enantioselective pharmacokinetics and pharmacodynamics of dl-threo-methylphenidate in children with attention deficit hyperactivity disorder. Clinical Pharmacology and Therapeutics 1992;52(5):561-8. [DOI: 10.1038/clpt.1992.185] [DOI] [PubMed] [Google Scholar]
Strand 2012 {published data only}
- Strand MT, Hawk LW Jr, Bubnik M, Shiels K, Pelham WE Jr, Waxmonsky JG. Improving working memory in children with attention-deficit/hyperactivity disorder: the separate and combined effects of incentives and stimulant medication. Journal of Abnormal Child Psychology 2012;40(7):1193-207. [DOI: 10.1007/s10802-012-9627-6] [DOI] [PMC free article] [PubMed]
Stray 2009 {published data only}
- Stray LL, Stray T, Iversen S, Ruud A, Ellertsen B. Methylphenidate improves motor functions in children diagnosed with hyperkinetic disorder. Behavioral and Brain Functions 2009;5(21):1-12. [DOI: 10.1186/1744-9081-5-21] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sunohara 1997 {published data only}
- Sunohara GA, Voros JG, Malone MA, Taylor MJ. Effects of methylphenidate in children with attention deficit hyperactivity disorder: a comparison of event-related potentials between medication responders and non-responders. International Journal of Psychophysiology 1997;27(1):9-14. [DOI: 10.1016/s0167-8760(97)00746-0] [PMID: ] [DOI] [PubMed] [Google Scholar]
Sutoko 2019 {published data only}
- Sutoko S, Monden Y, Tokuda T, Ikeda T, Nagashima M, Kiguchi M, et al. Distinct methylphenidate-evoked response measured using functional near-infrared spectroscopy during go/no-go task as a supporting differential diagnostic tool between attention-deficit/hyperactivity disorder and autism spectrum disorder comorbid children. Frontiers in Human Neuroscience 2019;13:7. [DOI: 10.3389/fnhum.2019.00007] [PMCID: PMC6375904] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Swanson 1993 {published data only}
- Swanson J, McBurnett K, Wigal T, Pfiffner LJ. Effect of stimulant medication on children with attention deficit disorder: a "review of reviews". Exceptional Children 1993;60(2):154-61. [DOI: 10.1177/001440299306000209] [DOI] [Google Scholar]
Szobot 2003 {published data only}
- Szobot CM, Ketzer C, Cunha RD, Parente MA, Langleben DD, Acton PD, et al. The acute effect of methylphenidate on cerebral blood flow in boys with attention-deficit/hyperactivity disorder. European Journal of Nuclear Medicine and Molecular Imaging 2003;30(3):423-6. [DOI: 10.1007/s00259-002-1082-0] [DOI] [PubMed] [Google Scholar]
Tannock 2000 {published data only}
- Tannock R, Martinussen R, Frijters J. Naming speed performance and stimulant effects indicate effortful, semantic processing deficits in attention-deficit/hyperactivity disorder. Journal of Abnormal Child Psychology 2000;28(3):237-52. [DOI: 10.1023/A:1005192220001] [DOI] [PubMed] [Google Scholar]
Teicher 2003 {published data only}
- Teicher MH, Polcari A, Anderson CM, Andersen SL, Lowen SB, Navalta CP. Rate dependency revisited: understanding the effects of methylphenidate in children with attention deficit hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 2003;13(1):41-51. [DOI: 10.1089/104454603321666180] [DOI] [PubMed] [Google Scholar]
Teicher 2006 {published data only}
- Teicher MH, Polcari A, Foley M, Valente E, McGreenery CE, Chang WW, et al. Methylphenidate blood levels and therapeutic response in children with attention-deficit hyperactivity disorder: I. Effects of different dosing regimens. Journal of Child and Adolescent Psychopharmacology 2006;16(4):416-31. [DOI: 10.1089/cap.2006.16.416] [DOI] [PubMed] [Google Scholar]
Teicher 2007 {published data only}
- Teicher MH, Polcari A, McGreenery CE. Objective measures of activity and attention accurately identify methylphenidate doses associated with optimal clinical response in children with ADHD. Biological Psychiatry 2007;61(8 Suppl):66S. [DOI: 10.1016/j.biopsych.2007.03.008] [DOI] [Google Scholar]
- Teicher MH, Polcari A, McGreenery CE. Utility of objective measures of activity and attention in the assessment of therapeutic response to stimulants in children with attention-deficit/hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology 2008;18(3):265-70. [DOI: 10.1089/cap.2007.0090] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tillery 2000 {published data only}
- Tillery KL, Katz J, Keller WD. Effects of methylphenidate (Ritalin) on auditory performance in children with attention and auditory processing disorders. Journal of Speech, Language, and Hearing Research 2000;43(4):893-901. [DOI: 10.1044/jslhr.4304.893] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Tillery KL. A double-blind study of the Central Auditory Processing and Auditory Continuous Test performances of children with attention deficit hyperactivity disorder and central auditory processing disorder under Ritalin and placebo conditions. Dissertation Abstracts International: Section A: Humanities and Social Sciences 1998;58(7-A):2536. [PROQUEST: 9801350]
Trommer 1991 {published data only}
- Trommer BL, Hoeppner JA, Zecker SG. The go-no go test in attention deficit disorder is sensitive to methylphenidate. Journal of Child Neurology 1991;6(1 Suppl):S128-31. [DOI: 10.1177/0883073891006001S13] [DOI] [PubMed] [Google Scholar]
Tsang 2012 {published data only}
- Tsang TW, Kohn MR, Clarke SD, Williams LM. Cognition and emotion in child and adolescent ADHD. Biological Psychiatry 2012;71(Suppl 8):74S. [DOI: 10.1016/j.biopsych.2012.02.012] [DOI] [Google Scholar]
Tucha 2006 {published data only}
- Tucha O, Prell S, Mecklinger L, Bormann-Kischkel C, Kübber S, Linder M, et al. Effects of methylphenidate on multiple components of attention in children with attention deficit hyperactivity disorder. Psychopharmacology 2006;185(3):315-26. [DOI: 10.1007/s00213-006-0318-2] [DOI] [PubMed] [Google Scholar]
Van den Driessche 2017 {published data only}
- Van den Driessche C, Bastian M, Peyre H, Stordeur C, Acquaviva E, Bahadori S, et al. Attentional lapses in attention-deficit/hyperactivity disorder: blank rather than wandering thoughts. Psychological Science 2017;28(10):1375-86. [DOI: 10.1177/0956797617708234] [PMID: ] [DOI] [PubMed] [Google Scholar]
Van Lith 2018 {published data only}
- Van Lith K, Veltman DJ, Cohn MD, Pape LE, Van den Akker-Nijdam ME, Van Loon AW, et al. Effects of methylphenidate during fear learning in antisocial adolescents: a randomized controlled fMRI trial. Journal of the American Academy of Child and Adolescent Psychiatry 2018;57(12):934-43. [DOI: 10.1016/j.jaac.2018.06.026] [PMID: ] [DOI] [PubMed] [Google Scholar]
Verbaten 1994 {published data only}
- Verbaten MN, Overtoom CC, Koelega HS, Swaab-Barneveld H, Van der Gaag RJ, Buitelaar J, et al. Methylphenidate influences on both early and late ERP waves of ADHD children in a continuous performance test. Journal of Abnormal Child Psychology 1994;22(5):561-78. [DOI: 10.1007/BF02168938] [DOI] [PubMed] [Google Scholar]
Waschbusch 2007 {published data only}
- Waschbusch DA, Craig R, Pelham WE Jr, King S. Self-handicapping prior to academic-oriented tasks in children with attention deficit/hyperactivity disorder (ADHD): medication effects and comparisons with controls. Journal of Abnormal Child Psychology 2007;35(2):275-86. [DOI: 10.1007/s10802-006-9085-0] [DOI] [PubMed] [Google Scholar]
Whalen 1987 {published data only}
- Whalen CK, Henker B, Swanson JM, Granger D, Kliewer W, Spencer J. Natural social behaviors in hyperactive children: dose effects of methylphenidate. Journal of Consulting and Clinical Psychology 1987;55(2):187-93. [DOI: 10.1037/0022-006X.55.2.187] [DOI] [PubMed] [Google Scholar]
Wong 2012 {published data only}
- Wong CG, Stevens MC. The effects of stimulant medication on working memory functional connectivity in attention-deficit/hyperactivity disorder. Biological Psychiatry 2012;71(5):458-66. [DOI: 10.1016/j.biopsych.2011.11.011] [PMCID: PMC4120250] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wu 2017 {published data only}
- Wu Z, Hoogman M, Cao Q, An L, Yang L, Bralten J, et al. Laterality of activation patterns in boys with attention-deficit/hyperactivity disorder and effects of methylphenidate during verbal working memory task. ADHD Attention Deficit and Hyperactivity Disorders 2015;7(Suppl 1):S36. [DOI: 10.1007/s12402-015-0169-y] [DOI] [Google Scholar]
- Wu Z-M, Bralten J, An L, Cao Q-J, Cao X-H, Sun L, et al. Verbal working memory-related functional connectivity alterations in boys with attention-deficit/hyperactivity disorder and the effects of methylphenidate. Journal of Psychopharmacology 2017;31(8):1061-9. [DOI: 10.1177/0269881117715607] [PMID: ] [DOI] [PubMed] [Google Scholar]
Yarmolovsky 2017 {published data only}
- Yarmolovsky J, Szwarc T, Schwartz M, Tirosh E, Geva R. Hot executive control and response to a stimulant in a double-blind randomized trial in children with ADHD. European Archives of Psychiatry and Clinical Neuroscience 2017;267(1):73-82. [DOI: 10.1007/s00406-016-0683-8] [PMCID: PMC5253147] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Drtílková 1997 {published data only}
- Drtílková I, Mišurec J, Balaštíková B. Methylphenidate, amphetaminil and mesocarb in children with hyperkinetic disorder: predicting value changes the pharmo-EEG profile of psychostimulatory substances [Metylfenidat, amfetaminil a mesocarb u deti s hyperkinetickou poruchou: prediktivni hodnota zmen farmakologickeho EEG profilu psychostimulacnich latek]. Ceska a Slovenska Psychiatrie 1997;93(Suppl 3):44-53. [ISSN: 0069-2336] [Google Scholar]
Wang 2020 {published data only}
References to ongoing studies
ChiCTR1800014945 {published data only}
- ChiCTR1800014945. Effect of the intervention combining medicine and education on school-age children with attention deficit hyperactivity disorder. www.chictr.org.cn/showprojen.aspx?proj=24003 (first received 24 February 2018).
EUCTR2007‐004664‐46‐NL {published data only}
- EUCTR2007-004664-46-NL. Influence of methylphenidate on sleep and circadian rhythm in children with attention-deficit/hyperactivity disorder (ADHD). www.clinicaltrialsregister.eu/ctr-search/trial/2007-004664-46/NL (first received 15 November 2007).
EUCTR2008‐001291‐71‐DE {published data only}
- EUCTR2008-001291-71-DE. Electrophysiological correlates of putative endophenotypes of attention-deficit/hyperactivity disorder (ADHD). www.clinicaltrialsregister.eu/ctr-search/trial/2008-001291-71/DE (first received 9 June 2008).
EUCTR2008‐004425‐42‐NL {published data only}
- EUCTR2008-004425-42-NL. Neurocognitive testing in children with ADHD. www.clinicaltrialsregister.eu/ctr-search/trial/2008-004425-42/NL (first received 27 June 2008).
EUCTR2020‐003660‐11‐NL {published data only}
- EUCTR2020-003660-11-NL. Do effects of methylphenidate decline after long-term use? A double-blind, placebo-controlled cross-over study of effects of methylphenidate on cognitive functioning and real world behavior in treatment naïve children compared to effects after 9 months of treatment in clinical practice [Verminderen de effecten van methylfenidaat na langdurig gebruik? Een dubbelblind, placebo-gecontroleerde cross-over onderzoek naar de effecten van methylfenidaat op cognitief functioneren en gedrag in de klas en thuis bij kinderen vooraf aan de start van de behandeling met methylfenidaat en na 9 maanden behandeling in de klinische praktijk]. www.clinicaltrialsregister.eu/ctr-search/trial/2020-003660-11/NL (first received 21 December 2020).
IRCT138804132000N2 {published data only}
- IRCT138804132000N2. Neurofeedback in the treatment of attention deficit hyperactivity disorder [Effectiveness of neurofeedback versus methylphenidate in the treatment of children with attention deficit hyperactivity disorder]. en.irct.ir/trial/1599 (first received 15 February 2013).
IRCT201701131556N94 {published data only}
- IRCT201701131556N94. Saffron versus methylphenidate in the treatment of attention deficit hyperactivity disorder [Saffron versus methylphenidate in the treatment of attention deficit/hyperactivity disorder: a double-blind and randomized trialSaffron versus methylphenidate in the treatment of attention deficit hyperactivity disorder]. en.irct.ir/trial/943 (first received 13 January 2017).
IRCT20190317043079N {published data only}
- The effect of neurofeedback/biofeedback on brain’s waves amplitude and attention deficit in children with ADHD. https://en.irct.ir/trial/55030 2021.
Müller 2021 {published data only}
- Müller AR, Zinkstok JR, Rommelse NN, Van den Ven PM, Roes KC, Wijburg FA, et al. Methylphenidate for attention-deficit/hyperactivity disorder in patients with Smith-Magenis syndrome: protocol for a series of N-of-1 trials. Orphanet Journal of Rare Diseases 2021;16(380):1-9. [DOI: 10.1186/s13023-021-02003-z] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT00141050 {published data only}
- NCT00141050. Safety and efficacy study of dexmethylphenidate in children with ADHD. clinicaltrials.gov/ct2/show/NCT00141050 (first received 1 September 2005).
NCT00254878 {published data only}
- NCT00254878. Placebo-controlled comparison of two different brands of modified-release oral dosage forms regarding safety and efficacy in children with attention deficit hyperactivity disorder (ADHD) aged 6-14 [A 7 week multicenter, double-blind, randomized, placebo-controlled cross-over evaluation of the efficacy and safety of two different brands of modified-release oral dosage forms of methylphenidate-hcl (20 mg, q.d.) in children with attention deficit hyperactivity disorder (ADHD) aged 6 - 14]. clinicaltrials.gov/ct2/show/NCT00254878 (first received 17 November 2005).
NCT00414921 {published data only}
- NCT00414921. Preschool supplement to clonidine in ADHD (Kiddie-CAT). clinicaltrials.gov/ct2/show/NCT00414921 (first received 22 December 2006).
NCT00446537 {published data only}
- NCT00446537. Procedural learning in participants with ADHD. clinicaltrials.gov/ct2/show/NCT00446537 (first received 13 March 2007).
NCT00485550 {published data only}
- NCT00485550. Comparison of atomoxetine plus either comparator or placebo in children with ADHD who haven't responded to stimulant therapy [A randomized, double-blind comparison of atomoxetine hydrochloride augmented with either extended-release methylphenidate hydrochloride (Concerta™) or placebo in children with attention-deficit/hyperactivity disorder (ADHD) who have not responded to stimulant mono therapy]. clinicaltrials.gov/ct2/show/NCT00485550 (first received 13 June 2007).
NCT02807870 {published data only}
- NCT02807870. Early interventions in children with attention deficit/hyperactivity disorder [Early interventions in children with attention deficit/hyperactivity disorder: randomized controlled trial comparing methylphenidate parental training in treating preschool children with attention deficit/hyperactivity disorder]. clinicaltrials.gov/ct2/show/NCT02807870 (first received 21 June 2016).
Verlaet 2017 {published data only}
- NCT02700685. Effect of Pycnogenol® on ADHD [Effect of a polyphenol-rich plant extract on attention-deficit hyperactivity disorder (ADHD): a randomized, double blind, placebo and active product controlled multicenter trial]. clinicaltrials.gov/ct2/show/NCT02700685 (first received 7 March 2016).
- Verlaet AA, Ceulemans B, Verhelst H, Van West D, De Bruyne T, Pieters L, et al. Effect of Pycnogenol® on attention-deficit hyperactivity disorder (ADHD): study protocol for a randomised controlled trial. Trials 2017;18(1):145. [DOI: 10.1186/s13063-017-1879-67-1879-6] [PMCID: PMC5370458] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Aasberg 1978
- Aasberg M, Montgomery SA, Perris C, Schalling D, Sedvall G. A comprehensive psychopathological rating scale. Acta Psychiatrica Scandinavica. Supplementum 1978;57(S271):5-27. [DOI: 10.1111/j.1600-0447.1978.tb02357.x] [DOI] [PubMed] [Google Scholar]
Abikoff 1980
- Abikoff H, Gittelman R, Klein DF. Classroom observation code for hyperactive children: a replication of validity. Journal of Consulting and Clinical Psychology 1980;48(5):555-65. [DOI: 10.1037/0022-006X.48.5.555] [DOI] [PubMed] [Google Scholar]
Achenbach 1986
- Achenbach TM, Edelbrock CS. Manual for the Teacher's Report Form and Teacher Version of the Child Behavior Profile. Burlington, VT: University of Vermont, Department of Psychiatry, 1986. [Google Scholar]
Achenbach 1991
- Achenbach TM. Integrative Guide to the 1991 CBCL/4-18, YSR, and TRF Profiles. Burlington, VT: University of Vermont, Department of Psychiatry, 1991. [Google Scholar]
Achenbach 1991a
- Achenbach TM. Manual for the Child Behavior Checklist/4-18 and 1991 Profile. Burlington, VT: University of Vermont, Department of Pyshciatry, 1991. [Google Scholar]
Achenbach 1991b
- Achenbach TM. Manual for the Teacher Report Form. Burlington, VT: University of Vermont, Department of Psychiatry, 1991. [Google Scholar]
Achenbach 2001
- Achenbach TM, Rescorla LA. Manual for the ASEBA School-Age Forms and Profiles. Burlington, VT: ASEBA, 2001. [Google Scholar]
AIM‐C 2013
- AIM-C 2015. ADHD Impact module - child. bit.ly/1fgWlKo (accessed 6 July 2015).
Akinbami 2011
- Akinbami LJ, Liu X, Pastor PN, Reuben CA. Attention deficit hyperactivity disorder among children aged 5-17 years in the United States, 1998-2009. NCHS Data Brief 2011;70:1-8. [PMID: ] [PubMed] [Google Scholar]
Aman 1996
- Aman MG, Tassé MJ, Rojahn J, Hammer D. The Nisonger CBRF: a child behavior rating form for children with developmental disabilities. Research in Developmental Disabilities 1996;17(1):41-57. [DOI: 10.1016/0891-4222(95)00039-9] [DOI] [PubMed] [Google Scholar]
American Academy of Pediatrics 2011
- Subcommittee on Attention-Deficit/Hyperactivity Activity Disorder, Steering Committee on Quality Improvement and Management. ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics 2011;128(5):1007-22. [DOI: 10.1542/peds.2011-2654] [DOI] [PMC free article] [PubMed] [Google Scholar]
Angold 1995
- Angold A, Prendergast M, Cox A, Harrington R, Simonoff E, Rutter M. The Child and Adolescent Psychiatric Assessment (CAPA). Psychological Medicine 1995;25(4):739-53. [DOI: 10.1017/S003329170003498X] [DOI] [PubMed] [Google Scholar]
APA 1968
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorder-DSM-II. Washington, DC: APA, 1968. [Google Scholar]
APA 1980
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-III). 3rd edition. Washington, DC: American Psychiatric Association, 1980. [Google Scholar]
APA 1987
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM III-R). 3rd revised edition. Washington, DC: American Psychiatric Association, 1987. [Google Scholar]
APA 1994
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV). 4th edition. Washington, DC: American Psychiatric Association, 1994. [Google Scholar]
APA 2000
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) Text revision. 4th edition. Washington, DC: American Psychiatric Publishing, 2000. [Google Scholar]
APA 2013
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5). 5th edition. Washington, DC: American Psyciatric Association, 2013. [Google Scholar]
Austic 2015
- Austic EA. Peak ages of risk for starting nonmedical use of prescription stimulants. Drug and Alcohol Dependence 2015;152(1):224-9. [DOI: 10.1016/j.drugalcdep.2015.03.034] [PMCID: PMC4458195] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bachmann 2017
- Bachmann CJ, Wijlaars LP, Kalverdijk LJ, Burcu M, Glaeske G, Schuiling-Veninga CC, et al. Trends in ADHD medication use in children and adolescents in five western countries, 2005-2012. European Neuropsychopharmacology 2017;27(5):484-93. [DOI: 10.1016/j.euroneuro.2017.03.002] [PMID: ] [DOI] [PubMed] [Google Scholar]
Banaschewski 2016a
- Banaschewski T, Gerlach M, Becker K, Holtmann M, Döpfner M, Romanos M. Trust, but verify. The errors and misinterpretations in the Cochrane analysis by OJ Storebo and colleagues on the efficacy and safety of methylphenidate for the treatment of children and adolescents with ADHD. Zeitschrift für Kinder-und Jugendpsychiatrie und Psychotherapie 2016;44(4):307-14. [DOI: 10.1024/1422-4917/a000433] [PMID: ] [DOI] [PubMed] [Google Scholar]
Banaschewski 2016b
- Banaschewski T, Buitelaar J, Chui CS, Coghill D, Cortese S, Simonoff E, et al. Methylphenidate for ADHD in children and adolescents: throwing the baby out with the bathwater. Evidence-Based Mental Health 2016;19(4):97-9. [DOI: 10.1136/eb-2016-102461] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Barkley 1977a
- Barkley RA. A review of stimulant drug research with hyperactive children. Journal of Child Psychology and Psychiatry 1977;18(2):137-65. [DOI: 10.1111/j.1469-7610.1977.tb00425.x] [DOI] [PubMed] [Google Scholar]
Barkley 1981
- Barkley RA. The use of psychopharmacology to study reciprocal influences in parent-child interaction. Journal of Abnormal Child Psychology 1981;9(3):303-10. [DOI: 10.1007/BF00916834] [DOI] [PubMed] [Google Scholar]
Barkley 1987
- Barkley RA, Edelbrock CS. Assessing Situational Variation in Children's Behavior Problems: The Home and School Situations Questionnaires. Greenwich, CT: JAI Press, 1987. [Google Scholar]
Barkley 1988b
- Barkley RA. Child behavior rating scales and checklists. In: Rutter M, Tuma AH, Lann I, editors(s). Assessment and Diagnosis in Child Psychopathology. New York: Guilford Press, 1988:113-55. [Google Scholar]
Barkley 1989a
- Barkley RA. Hyperactive girls and boys: stimulant drug effects on mother-child interactions. Journal of Child Psychology and Psychiatry 1989;30(3):379-90. [DOI: 10.1111/j.1469-7610.1989.tb00253.x] [DOI] [PubMed] [Google Scholar]
Barkley 1990
- Barkley RA, McMurray MB, Edelbrock CS, Robbins K. Side effects of methylphenidate in children with attention deficit hyperactivity disorder: a systemic, placebo-controlled evaluation. Pediatrics 1990;86(2):184-92. [DOI: 10.1542/peds.86.2.184] [DOI] [PubMed] [Google Scholar]
Barkley 1991a
- Barkley RA. Attention-Deficit Hyperactivity Disorder: A Clinical Workbook. New York: Guilford Press, 1991. [Google Scholar]
Barkley 1991b
- Barkley RA, DuPaul GJ, McMurray MB. Attention deficit disorder with and without hyperactivity: clinical response to three dose levels of methylphenidate. Pediatrics 1991;87(4):519-31. [DOI: 10.1542/peds.87.4.519] [DOI] [PubMed] [Google Scholar]
Barkley 1998
- Barkley RA, Murphy KR, Bauermeister JJ. Attention-Deficit Hyperactivity Disorder: A Clinical Workbook. 2nd edition. New York: Guilford Press, 1998. [Google Scholar]
Becker 1993
- Becker MP, Balagtas CC. Marginal modeling of binary cross-over data. Biometrics 1993;49(4):997-1009. [DOI: 10.2307/2532242] [DOI] [PubMed] [Google Scholar]
Berkey 1996
- Berkey CS, Mosteller F, Lau J, Antman E. Uncertainty of the time of first significance in random effects cumulative meta-analysis. Controlled Clinical Trials 1996;17(5):357-71. [DOI: 10.1016/S0197-2456(96)00014-1] [DOI] [PubMed] [Google Scholar]
Biederman 2003a
- Biederman J. Pharmacotherapy for attention-deficit/hyperactivity disorder (ADHD) decreases the risk for substance abuse: findings from a longitudinal follow-up of youths with and without ADHD. Journal of Clinical Psychiatry 2003;64(Suppl 11):3-8. [PMID: ] [PubMed] [Google Scholar]
Bladder 2009
- Blader JC, Schooler NR, Jensen PS, Pliszka SR, Kafantaris V. Adjunctive divalproex versus placebo for children with ADHD and aggression refractory to stimulant monotherapy. American Journal of Psychiatry 2009;166(12):1392-401. [DOI: 10.1176/appi.ajp.2009.09020233] [PMCID: PMC2940237] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bloch 2009
- Bloch MH, Panza KE, Landeros-Weisenberger A, Leckman JF. Meta-analysis: treatment of attention-deficit/hyperactivity disorder in children with comorbid tic disorders. Journal of the American Academy of Child and Adolescent Psychiatry 2009;48(9):884-93. [DOI: 10.1097/CHI.0b013e3181b26e9f] [DOI] [PMC free article] [PubMed] [Google Scholar]
Block 1998
- Block SL. Attention-deficit disorder: a paradigm for psychotropic medication intervention in pediatrics. Pediatric Clinics of North America 1998;45(5):1053-83. [DOI: 10.1016/S0031-3955(05)70062-6] [DOI] [PubMed] [Google Scholar]
BNF 2020
- British National Formulary. Methylphenidate hydrochloride: indications and dose. www.medicinescomplete.com/mc/bnfc/2011/PHP2479‐methylphenidate‐hydrochloride.htm (accessed 15 January 2020). [DOI: 10.18578/BNF.995526496] [DOI]
Boesen 2017
- Boesen K, Saiz LC, Erviti J, Storebø OJ, Gluud C, Gøtzsche PC, et al. The Cochrane Collaboration withdraws a review on methylphenidate for adults with attention deficit hyperactivity disorder. BMJ Evidence-Based Medicine 2017;22(4):143-7. [DOI: 10.1136/ebmed-2017-110716] [PMCID: PMC5537554] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Boesen 2022
- Boesen K, Paludan-Müller AS, Gøtzsche PC, Jørgensen KJ. Extended-release methylphenidate for attention deficit hyperactivity disorder (ADHD) in adults. Cochrane Database of Systematic Reviews 2022, Issue 2. Art. No: CD012857. [DOI: 10.1002/14651858.CD012857.pub2] [PMCID: PMC8869321 (available on 2023-02-24)] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bradley 1937
- Bradley Charles. The behavior of children receiving benzedrine. American Journal of Psychiatry 1937;94(3):577-85. [DOI: ] [Google Scholar]
Brok 2008
- Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta-analysis. Journal of Clinical Epidemiology 2008;61(8):763-9. [DOI: 10.1016/j.jclinepi.2007.10.007] [DOI] [PubMed] [Google Scholar]
Brok 2009
- Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta-analysis may be inconclusive - trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta-analysis. International Journal of Epidemiology 2009;38(1):287-98. [DOI: 10.1093/ije/dyn188] [DOI] [PubMed] [Google Scholar]
Bruni 1996
- Bruni O, Ottaviano S, Guidetti V, Romoli M, Innocenzi M, Cortesi F, et al. The Sleep Disturbance Scale for Children (SDSC). Construction and validation of an instrument to evaluate sleep disturbances in childhood and adolescence. Journal of Sleep Research 1996;5(4):251-61. [DOI: 10.1111/j.1365-2869.1996.00251.x] [DOI] [PubMed] [Google Scholar]
Buitelaar 2022
- Buitelaar J, Bölte S, Brandeis D, Caye A, Christmann N, Cortese S, et al. Toward precision medicine in ADHD. Frontiers in Behavioral Neuroscience 2022;16:900981. [DOI: 10.3389/fnbeh.2022.900981] [PMCID: PMC9299434] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Burnett 2021
- Burnett T, Jennison C. Adaptive enrichment trials: what are the benefits? Statistics in Medicine 2021;40(3):690-711. [DOI: 10.1002/sim.8797] [PMCID: PMC7839594] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bussing 2008
- Bussing R, Fernandez M, Harwood M, Hou W, Garvan CW, Eyberg SM, et al. Parent and teacher SNAP-IV ratings of attention deficit hyperactivity disorder symptoms: psychometric properties and normative ratings from a school district sample. Assessment 2008;15(3):317-28. [DOI: 10.1177/1073191107313888] [DOI] [PMC free article] [PubMed] [Google Scholar]
CADDRA 2011
- Canadian Attention Deficit Hyperactivity Disorder Resource Alliance. Canadian ADHD Practice Guidelines. 3rd edition. Toronto: CADDRA, 2011. [Google Scholar]
Candido 2021
- Candido RC, Menezes de Padua CA, Golder S, Junqueira DR. Immediate release methylphenidate for attention deficit hyperactivity disorder (ADHD) in adults. Cochrane Database of Systematic Reviews 2021, Issue 1. Art. No: CD013011. [DOI: 10.1002/14651858.CD013011.pub2] [PMCID: PMC8092481] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Castellanos 2006
- Castellanos FX, Sonuga-Barke EJ, Milham MP, Tannock R. Characterizing cognition in ADHD: beyond executive dysfunction. Trends in Cognitive Sciences 2006;10(3):117-23. [DOI: 10.1016/j.tics.2006.01.011] [DOI] [PubMed] [Google Scholar]
Catala‐Lopez 2017
- Catalá-López F, Hutton B, Núñez-Beltrán A, Page MJ, Ridao M, Saint-Gerons DM, et al. The pharmacological and non-pharmacological treatment of attention deficit hyperactivity disorder in children and adolescents: a systematic review with network meta-analyses of randomised trials. PloS One 2017;12(7):e0180355. [DOI: 10.1371/journal.pone.0180355] [PMCID: PMC5507500] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Caye 2017
- Caye A, Machado JD, Rohde LA. Evaluating parental disagreement in ADHD diagnosis: can we rely on a single report from home? Journal of Attention Disorders 2017;21(7):561-566. [DOI: 10.1177/1087054713504134] [DOI] [PubMed] [Google Scholar]
Cerrillo‐Urbina 2018
- Cerrillo-Urbina AJ, García-Hermoso A, Pardo-Guijarro MJ, Sánchez-López M, Santos-Gómez JL, Martínez-Vizcaíno V. The effects of long-acting stimulant and nonstimulant medications in children and adolescents with attention-deficit/hyperactivity disorder: a meta-analysis of randomized controlled trials. Journal of Child and Adolescent Psychopharmacology 2018;28(8):494-507. [DOI: 10.1089/cap.2017.0151] [PMID: ] [DOI] [PubMed] [Google Scholar]
Chambers 1985
- Chambers WJ, Puig-Antich J, Hirsch M, Paez P, Ambrosini PJ, Tabrizi MA, et al. The assessment of affective disorders in children and adolescents by semistructured interview. Test-retest reliability of the schedule for affective disorders and schizophrenia for school-age children, present episode version. Archives of General Psychiatry 1985;42(7):696-702. [DOI: 10.1001/archpsyc.1985.01790300064008] [DOI] [PubMed] [Google Scholar]
Chan 2013
- Chan A-W, Tetzlaff JM, Altman DG, Laupacis A, Gøtzsche PC, Krleža-Jerić K, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Annals of Internal Medicine 2013;158(3):200-7. [DOI: 10.7326/0003-4819-158-3-201302050-00583] [DOI] [PMC free article] [PubMed]
Chang 2014
- Chang Z, Lichtenstein P, Halldner L, D'Onofrio B, Serlachius E, Fazel S, et al. Stimulant ADHD medication and risk for substance abuse. Journal of Child Psychology and Psychiatry 2014;55(8):878-85. [DOI: 10.1111/jcpp.12164] [PMCID: PMC4147667] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chang 2017
- Chang Z, Quinn PD, Hur K, Gibbons RD, Sjölander A, Larsson H, et al. Association between medication use for attention-deficit/hyperactivity disorder and risk of motor vehicle crashes. JAMA Psychiatry 2017;74(6):597-603. [DOI: 10.1001/jamapsychiatry.2017.0659] [PMCID: PMC5539840] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Charach 2011
- Charach A, Yeung E, Climans T, Lillie E. Childhood attention-deficit/hyperactivity disorder and future substance use disorders: comparative meta-analyses. Journal of the American Academy of Child and Adolescent Psychiatry 2011;50(1):9-21. [DOI: 10.1016/j.jaac.2010.09.019] [DOI] [PubMed] [Google Scholar]
Charach 2013
- Charach A, Carson P, Fox S, Ali MU, Beckett J, Lim CG. Interventions for preschool children at high risk for ADHD: a comparative effectiveness review. Pediatrics 2013;131(5):e1584-604. [DOI: 10.1542/peds.2012-0974] [DOI] [PubMed] [Google Scholar]
Chen 2006
- Chen W, Taylor E. Parental account of children's symptoms (PACS), ADHD phenotypes and its applications to molecular genetic studies. In: Oades RD, editors(s). Attention-Deficit/Hyperactivity Disorders and the Hyperkinetic Syndrome: Current Ideas and Ways Forward. Hauppage, NY: Nova Science Publishing, 2006:3-20. [Google Scholar]
Cherland 1999
- Cherland E, Fritzpark R. Psychotic side effects of psychostimulants: a 5-year review. Canadian Journal of Psychiatry 1999;44(8):811-3. [DOI: 10.1177/070674379904400810] [DOI] [PubMed] [Google Scholar]
Chervin 2000
- Chervin RD, Hedger K, Dillon JE, Pituch KJ. Pediatric Sleep Questionnaire (PSQ): validity and reliability of scales for sleep-disordered breathing, snoring, sleepiness, and behavioral problems. Sleep Medicine 2000;1(1):21-32. [DOI: 10.1016/S1389-9457(99)00009-X] [DOI] [PubMed] [Google Scholar]
Childress 2019
- Childress AC, Komolova M, Sallee FR. An update on the pharmacokinetic considerations in the treatment of ADHD with long-acting methylphenidate and amphetamine formulations. Expert Opinion on Drug Metabolism & Toxicology 2019;15(11):937-74. [DOI: 10.1080/17425255.2019.1675636] [DOI] [PubMed] [Google Scholar]
Cipriani 2018
- Cipriani A, Adamo N, Del Giovane C, Coghill D, Banaschewski T, Hollis C, et al. Unbalanced risk-benefit analysis of ADHD drugs–Authors' reply. Lancet Psychiatry 2018;5(11):871-3. [DOI: 10.1016/S2215-0366(18)30396-1] [PMID: ] [DOI] [PubMed] [Google Scholar]
Clemow 2014
- Clemow DB, Walker DJ. The potential for misuse and abuse of medications in ADHD: a review. Postgraduate Medicine 2014;126(5):64-81. [DOI: 10.3810/pgm.2014.09.2801] [PMID: ] [DOI] [PubMed] [Google Scholar]
Coghill 2021
- Coghill D, Banaschewski T, Cortese S, Asherson P, Brandeis D, Buitelaar J, et al. The management of ADHD in children and adolescents: bringing evidence to the clinic: perspective from the European ADHD Guidelines Group (EAGG). European Child & Adolescent Psychiatry 2021 Oct 22 [Epub ahead of print]. [DOI: 10.1007/s00787-021-01871-x] [PMCID: PMC8532460] [PMID: ] [DOI] [PMC free article] [PubMed]
Conners 1985
- Conners CK, Barkley RA. Rating scales and checklists for child psychopharmacology. Psychopharmacology Bulletin 1985;21(4):809-43. [PMID: ] [PubMed] [Google Scholar]
Conners 1995
- Conners CK. The Conners' Parent Rating Scales. Toronto (ON): Multi-Health Systems, 1995. [Google Scholar]
Conners 1997a
- Conners CK. Conners' Rating Scales. Revised edition. North Tonowanda: Multi-Health Systems, 1997. [Google Scholar]
Conners 1997b
- Conners CK, Wells KC, Parker JD, Sitarenios G, Diamond JM, Powell JW. A new self-report scale for assessment of adolescent psychopathology: factor structure, reliability, validity, and diagnostic sensitivity. Journal of Abnormal Child Psychology 1997;25(6):487-97. [DOI: 10.1023/A:1022637815797] [DOI] [PubMed] [Google Scholar]
Conners 1998a
- Conners CK, Sitarenios G, Parker JDA, Epstein JN. Revision and restandardization of the Conners Teacher Rating Scale (CTRS-R): factor structure, reliability, and criterion validity. Journal of Abnormal Child Psychology 1998;26(4):279-91. [DOI: 10.1023/A:1022606501530] [DOI] [PubMed] [Google Scholar]
Conners 1998b
- Conners CK, Sitarenios G, Parker JD, Epstein JN. The revised Conners' Parent Rating Scale (CPRS-R): factor structure, reliability, and criterion validity. Journal of Abnormal Child Psychology 1998;26(4):257-68. [DOI: 10.1023/A:1022602400621] [DOI] [PubMed] [Google Scholar]
Conners 2001
- Conners CK. Conners Rating Scales, Revised. North Tonawanda: Multi-Health Systems, 2001. [Google Scholar]
Conners 2008
- Conners CK. Conners 3rd Edition. Toronto: Multi-Health Systems Inc, 2008. [Google Scholar]
Conners 2009
- Conners C. Conners Early Childhood. North Tonawanda, NY: MultiHealth Systems, Inc, 2009. [Google Scholar]
Connor 2002
- Connor DF. Preschool attention deficit hyperactivity disorder: a review of prevalence, diagnosis, neurobiology, and stimulant treatment. Journal of Developmental and Behavioral Pediatrics 2002;23(Suppl 1):S1-S9. [DOI: 10.1097/00004703-200202001-00002] [DOI] [PubMed] [Google Scholar]
Cooper 2011
- Cooper WO, Habel LA, Sox CM, Chan KA, Arbogast PG, Cheetham TC, et al. ADHD drugs and serious cardiovascular events in children and young adults. New England Journal of Medicine 2011;365(20):1896-904. [DOI: 10.1056/NEJMoa1110212] [PMCID: PMC4943074] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Corkum 2007
- Corkum P, Andreou P, Schachar R, Tannock R, Cunningham C. The Telephone Interview Probe: a novel measure of treatment response in children with attention deficit hyperactivity disorder. Educational and Psychological Measurement 2007;67(1):169-85. [DOI: 10.1177/0013164406292038] [DOI] [Google Scholar]
Cortese 2018
- Cortese S, Adamo N, Del Giovane C, Mohr-Jensen C, Hayes AJ, Carucci S, et al. Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: a systematic review and network meta-analysis. Lancet Psychiatry 2018;5(9):727-38. [DOI: 10.1016/S2215-0366(18)30269-4] [PMCID: PMC6109107] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cox 2004a
- Cox DJ, Merkel RL, Penberthy JK, Kovatchev B, Hankin CS. Impact of methylphenidate delivery profiles on driving performance of adolescents with attention-deficit/hyperactivity disorder: a pilot study. Journal of the American Acadademy of Child and Adolescent Psychiatry 2004;43(3):269-75. [DOI: 10.1097/00004583-200403000-00007] [DOI] [PubMed] [Google Scholar]
CTU 2022
- Copenhagen Trial Unit. TSA - trial sequential analysis. https://ctu.dk/tsa/ (accessed May 2022).
Curtin 2002
- Curtin F, Elbourne D, Altman DG. Meta-analysis combining parallel and cross-over clinical trials. III: The issue of carry-over. Statistics in Medicine 2002;21(15):2161-73. [DOI: 10.1002/sim.1207] [DOI] [PubMed] [Google Scholar]
da Costa 2013
- da Costa BR, Nüesch E, Rutjes AW, Johnston BC, Reichenbach S, Trelle S, et al. Combining follow-up and change data is valid in meta-analyses of continuous outcomes: a meta-epidemiological study. Journal of Clinical Epidemiology 2013;66(8):847-55. [DOI: 10.1016/j.jclinepi.2013.03.009] [DOI] [PubMed] [Google Scholar]
Dalsgaard 2015
- Dalsgaard S, Leckman JF, Mortensen PB, Nielsen HS, Simonsen M. Effect of drugs on the risk of injuries in children with attention deficit hyperactivity disorder: a prospective cohort study. Lancet Psychiatry 2015;2(8):702-9. [DOI: 10.1016/S2215-0366(15)00271-0] [PMID: ] [DOI] [PubMed] [Google Scholar]
Deeks 2022
- Deeks JJ, Higgins JP, Altman DG (editors). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Di Lorenzo 2021
- Di Lorenzo R, Balducci J, Poppi C, Arcolin E, Cutino A, Ferri P, et al. Children and adolescents with ADHD followed up to adulthood: a systematic review of long-term outcomes. Acta Neuropsychiatrica 2021;33(6):283-98. [DOI: 10.1017/neu.2021.23] [DOI] [PubMed] [Google Scholar]
Drug Usage Statistics 2013‐2019
- Methylphenidate: Drug Usage Statistics, United States, 2013-2019. clincalc.com/DrugStats/Drugs/Methylphenidate (accessed 5 March 2022).
DuPaul 1991a
- DuPaul GJ. Parent and teacher ratings of ADHD symptoms: psychometric properties in a community-based sample. Journal of Clinical Child Psychology 1991;20(3):245-53. [DOI: 10.1207/s15374424jccp2003_3] [DOI] [Google Scholar]
DuPaul 1992
- DuPaul GJ, Barkley RA. Situational variability of attention problems: psychometric properties of the Revised Home and School Situations Questionnaires. Journal of Clinical Child Psychology 1992;21(2):178-88. [DOI: 10.1207/s15374424jccp2102_10] [DOI] [Google Scholar]
Döpfner 2000
- Döpfner M, Lehmkuhl G. Diagnostik-System für Psychische Störungen im Kindes- und Jugendalter nach ICD-10 und DSM-IV (DISYPS-KJ). Bern: Huber, 2000. [Google Scholar]
Döpfner 2008
- Döpfner M, Görtz-Dorten A, Lehmkuhl G. Diagnostik-System für Psychische Störungen im Kindes- und Jugendalter nach ICD-10 und DSM-IV. DISYPS-II. Bern: Huber, 2008. [Google Scholar]
Efstratopoulou 2013
- Efstratopoulou M, Simons J, Janssen R. Concordance among physical educators’, teachers’, and parents’ perceptions of attention problems in children. Journal of Attention Disorders 2013;17(5):437-43. [DOI: 10.1177/1087054711431698] [DOI] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta-analyses involving cross-over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140-9. [DOI: 10.1093/ije/31.1.140] [PMID: ] [DOI] [PubMed] [Google Scholar]
Engert 2008
- Engert V, Pruessner JC. Dopaminergic and noradrenergic contributions to functionality in ADHD: the role of methylphenidate. Current Neuropharmacology 2008;6(4):322-8. [DOI: 10.2174/157015908787386069] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Epstein 2014
- Epstein T, Patsopoulos NA, Weiser M. Immediate-release methylphenidate for attention deficit hyperactivity disorder (ADHD) in adults. Cochrane Database of Systematic Reviews 2014, Issue 9. Art. No: CD005041. [DOI: 10.1002/14651858.CD005041.pub2] [DOI] [PubMed] [Google Scholar]
Epstein 2016
- Epstein T, Patsopoulos NA, Weiser M. Immediate‐release methylphenidate for attention deficit hyperactivity disorder (ADHD) in adults. Cochrane Database of Systematic Reviews 2016, Issue 5. Art. No: CD005041. [DOI: 10.1002/14651858.CD005041.pub3] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ercan 2001
- Ercan ES, Amado S, Somer O, Çıkoğlu S. Development of a test battery for attention deficit hyperactivity and disruptive behavior disorders [Dikkat eksikligi hiperaktivite bozuklugu ve yikici davranis bozukluklari icin bir test bataryasi gelistirme cabasi]. Turkish Journal of Child and Adolescent Mental Health 2001;8(3):132-44. [Google Scholar]
Fabiano 2006
- Fabiano GA, Pelham WE Jr, Waschbusch DA, Gnagy EM, Lahey BB, Chronis AM, et al. A practical measure of impairment: psychometric properties of the impairment rating scale in samples of children with attention deficit hyperactivity disorder and two school-based samples. Journal of Clinical Child and Adolescent Psychology 2006;35(3):369-85. [DOI: 10.1207/s15374424jccp3503_3] [DOI] [PubMed] [Google Scholar]
Faltinsen 2018a
- Faltinsen EG, Gluud C, Simonsen E, Zwi M, Storebø OJ. Unbalanced risk-benefit analysis of ADHD drugs. Lancet. Psychiatry 2018;5(11):870. [DOI: 10.1016/S2215-0366(18)30334-1] [PMID: ] [DOI] [PubMed] [Google Scholar]
Faltinsen 2018b
- Faltinsen EG, Storebø OJ, Jakobsen JC, Boesen K, Lange T, Gluud C. Network meta-analysis: the highest level of medical evidence? BMJ Evidence-Based Medicine 2018;23(2):56-9. [DOI: 10.1136/bmjebm-2017-110887] [PMID: ] [DOI] [PubMed] [Google Scholar]
Faltinsen 2019
- Faltinsen EG, Storebø OJ, Gluud C. Methodological concerns with network meta-analysis on drugs for attention deficit hyperactivity disorder. European Child & Adolescent Psychiatry 2019;28(1):145-6. [DOI: 10.1007/s00787-018-1164-6] [PMID: ] [DOI] [PubMed] [Google Scholar]
Faraone 2002
- Faraone SV, Biederman J, Roe C. Comparative efficacy of Adderall and methylphenidate in attention-deficit/hyperactivity disorder: a meta-analysis. Journal of Clinical Psychopharmacology 2002;22(5):468-73. [DOI: 10.1097/01.jcp.0000033401.43191.26] [DOI] [PubMed] [Google Scholar]
Faraone 2006
- Faraone SV, Biederman J, Spencer TJ, Aleardi M. Comparing the efficacy of medications for ADHD using meta-analysis. Medscape General Medicine 2006;8(4):4. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Faraone 2009
- Faraone SV. Using meta-analysis to compare the efficacy of medications for attention-deficit/hyperactivity disorder in youths. Pharmacy and Therapeutics 2009;34(12):678-94. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Faraone 2010
- Faraone SV, Glatt SJ. A comparison of the efficacy of medications for adult attention-deficit/hyperactivity disorder using meta-analysis of effect sizes. Journal of Clinical Psychiatry 2010;71(6):754-63. [DOI: 10.4088/JCP.08m04902pur] [DOI] [PubMed] [Google Scholar]
Faraone 2018
- Faraone SV, Hammerness PG, Wilens TE. Reliability and validity of the Before-School Functioning Scale in children with ADHD. Journal of Attention Disorders 2018;22(11):1040-8. [DOI: 10.1177/1087054714564623] [PMID: ] [DOI] [PubMed] [Google Scholar]
Fazel 2015
- Fazel M. Methylphenidate for ADHD. BMJ 2015;351:h5875. [DOI: 10.1136/bmj.h5875] [PMID: ] [DOI] [PubMed] [Google Scholar]
Fischer 1998
- Fischer M, Newby RF. Use of the restricted academic task in ADHD dose-response relationships. Journal of Learning Disabilities 1998;31(6):608-12. [DOI: 10.1177/002221949803100611] [DOI] [PubMed] [Google Scholar]
Fish 1985
- Fish B. Children's Psychiatric Rating Scale (scoring). Psychopharmacology Bulletin 1985;21(4):1764-985. [Google Scholar]
Fitzpatrick 1992b
- Fitzpatrick PA, Klorman R, Brumaghim JT, Borgstedt AD. Effects of sustained-release and standard preparations of methylphenidate on attention deficit disorder. Journal of the American Academy of Child and Adolescent Psychiatry 1992;31(2):226-34. [DOI: 10.1097/00004583-199203000-00008] [DOI] [PubMed] [Google Scholar]
Frodl 2012
- Frodl T, Skokauskas N. Meta-analysis of structural MRI studies in children and adults with attention deficit hyperactivity disorder indicates treatment effects. Acta Psychiatrica Scandinavica 2012;125(2):114-26. [DOI: 10.1111/j.1600-0447.2011.01786.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Gadow 1994
- Gadow KD, Sprafkin J. Child Symptom Inventories Manual. Stony Brook, NY: Checkmate Plus, 1994. [Google Scholar]
Gadow 1996
- Gadow KD, Sprafkin J, Nolan EE. ADHD School Observation Code. New York: Checkmate Plus, 1996. [Google Scholar]
Gale 1986
- Gale J, Pfefferbaum B, Suhr MA, Overall JE. The Brief Psychiatric Rating Scale for Children: a reliability study. Journal of Clinical Child Psychology 1986;15(4):341-5. [DOI: 10.1207/s15374424jccp1504_9] [DOI] [Google Scholar]
Gerlach 2017
- Gerlach M, Banaschewski T, Coghill D, Rohde LA, Romanos M. What are the benefits of methylphenidate as a treatment for children and adolescents with attention-deficit/hyperactivity disorder? ADHD Attention Deficit and Hyperactivity Disorders 2017;9(1):1-3. [DOI: 10.1007/s12402-017-0220-2] [PMID: ] [DOI] [PubMed] [Google Scholar]
Gilmore 2001
- Gilmore A, Milne R. Methylphenidate in children with hyperactivity: review and cost-utility analysis. Pharmacoepidemology and Drug Safety 2001;10(2):85-94. [DOI: 10.1002/pds.564] [DOI] [PubMed] [Google Scholar]
Gioia 2000
- Gioia GA, Isquith PK, Guy SC, Kenworthy L. Test review Behavior Rating Inventory of Executive Function. Child Neuropsychology 2000;6(3):235-8. [DOI: 10.1076/chin.6.3.235.3152] [DOI] [PubMed] [Google Scholar]
Gittleman‐Klein 1976
- Gittleman-Klein R, Klein DF. Methylphenidate effects in learning disabilities: psychometric changes. Archives of General Psychiatry 1976;33(6):655-64. [DOI: 10.1001/archpsyc.1976.01770060003001] [DOI] [PubMed] [Google Scholar]
Gluud 2015
- The Nordic Trial Alliance Working Group 6 on Transparency and Registration. Report on transparency and registration in clinical research in the Nordic countries. bit.ly/1S2H1Np (accessed 30 April 2015).
Goyette 1978
- Goyette CH, Conners CK, Ulrich RF. Normative data on revised Conners Parent and Teacher Rating Scales. Journal of Abnormal Child Psychology 1978;6(2):221-36. [DOI: 10.1007/BF00919127] [DOI] [PubMed] [Google Scholar]
Guy 1976
- Guy W. ECDEU Assessment Manual for Psychopharmacology Revised, 1976. Rockville, MD, USA: Department of Health and Human Services, 1976. [DHEW PUBLICATION NUMBER: ADM 91-338] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction - GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383-94. [DOI] [PubMed] [Google Scholar]
Habel 2011
- Habel LA, Cooper WO, Sox CM, Chan KA, Fireman BH, Arbogast PG, et al. ADHD medications and risk of serious cardiovascular events in young and middle-aged adults. JAMA 2011;306(24):2673-83. [DOI: 10.1001/jama.2011.1830] [PMCID: PMC3350308] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hanwella 2011
- Hanwella R, Senanayake Mi, De Silva V. Comparative efficacy and acceptability of methylphenidate and atomoxetine in treatment of attention deficit hyperactivity disorder in children and adolescents: a meta-analysis. BMC Psychiatry 2011;11:176. [DOI: 10.1186/1471-244X-11-176] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hartman 2007
- Hartman CA, Rhee SH, Willcutt EG, Pennington, BF. Modeling rater disagreement for ADHD: are parents or teachers biased? Journal of Abnormal Child Psychology 2007;35(4):536-42. [DOI: 10.1007/s10802-007-9110-y] [PMID: ] [DOI] [PubMed] [Google Scholar]
Heal 2006
- Heal DJ, Pierce DM. Methylphenidate and its isomers. CNS Drugs 2006;20(9):713-38. [DOI: 10.2165/00023210-200620090-00002] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. https://training.cochrane.org/handbook/archive/v5.
Higgins 2022a
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Hodgkins 2013
- Hodgkins P, Setyawan J, Mitra D, Davis K, Quintero J, Fridman M, et al. Management of ADHD in children across Europe: patient demographics, physician characteristics and treatment patterns. European Journal of Pediatrics 2013;172(7):895-906. [DOI: 10.1007/s00431-013-1969-8] [PMCID: PMC3701791] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hoekstra 2016
- Hoekstra PJ, Buitelaar JK. Is the evidence base of methylphenidate for children and adolescents with attention-deficit/hyperactivity disorder flawed? European Child & Adolescent Psychiatry 2016;25(4):339-40. [DOI: 10.1007/s00787-016-0845-2] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hoffmann 2017
- Hoffmann TC, Del Mar C. Clinicians' expectations of the benefits and harms of treatments, screening, and tests: a systematic review. JAMA Internal Medicine 2017;177(3):407-19. [DOI: 10.1001/jamainternmed.2016.8254] [PMID: ] [DOI] [PubMed] [Google Scholar]
Holden 2013
- Holden SE, Jenkins-Jones S, Poole CD, Morgan CL, Coghill D, Currie CJ. The prevalence and incidence, resource use and financial costs of treating people with attention deficit/hyperactivity disorder (ADHD) in the United Kingdom (1998 to 2010). Child and Adolescent Psychiatry and Mental Health 2013;11(7):34. [DOI: 10.1186/1753-2000-7-34] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hollis 1999
- Hollis S, Campbell F. What is meant by intention to treat analysis? Survey of published randomised controlled trials. BMJ 1999;319(7211):670-4. [DOI: 10.1136/bmj.319.7211.670] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hollis 2016
- Hollis C. Methylphenidate for ADHD: have Cochrane got it wrong this time?; 10 March-11April 2016. Available at www.nationalelfservice.net/mental-health/adhd/methylphenidate-for-adhd-have-cochrane-got-it-wrong-this-time/.
Holmskov 2014 [pers comm]
- Holmskov M. Data requested [personal communication]. Email to: Dr Leddy 11 June 2014.
Humphrey 1982
- Humphrey LL. Children's and teachers' perspectives on children's self-control: the development of two rating scales. Journal of Consulting and Clinical Psychology 1982;50(5):624-33. [DOI: 10.1037/0022-006X.50.5.624] [DOI] [PubMed] [Google Scholar]
ICH 1996
- ICH Expert Working Group. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. ICH harmonised tripartite guideline. Guideline for good clinical practice E6(R1). http://bit.ly/1B0jeJg (accessed 4 March 2014).
Iversen 2006
- Iversen L. Neurotransmitter transporters and their impact on the development of psychopharmacology. British Journal of Pharmacology 2006;147(S1):S82-8. [DOI: 10.1038/sj.bjp.0706428] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jakobsen 2013
- Jakobsen JC, Gluud C. The necessity of randomised clinical trials. British Journal of Medicine and Medical Research 2013;3(4):1453-68. [DOI: 10.9734/BJMMR/2013/3208] [DOI] [Google Scholar]
Jakobsen 2014
- Jakobsen JC, Wetterslev J, Winkel P, Lange T, Gluud C. Thresholds for statistical and clinical significance in systematic reviews with meta-analytic methods. BMC Medical Research Methodology 2014;14(1):120. [DOI: 10.1186/1471-2288-14-120] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jensen 2001
- Jensen PS, Hinshaw SP, Kraemer HC, Lenora N, Newcorn JH, Abikoff HB, et al. ADHD comorbidity findings from the MTA study: comparing comorbid subgroups. Journal of the American Academy of Child and Adolescent Psychiatry 2001;40(2):147-58. [DOI: 10.1097/00004583-200102000-00009] [DOI] [PubMed] [Google Scholar]
Kadesjö 2001
- Kadesjö B, Gillberg C. The comorbidity of ADHD in the general population of Swedish school-age children. Journal of Child Psychology and Psychiatry and Allied Disciplines 2001;42(4):487-92. [DOI: 10.1017/S0021963001007090] [DOI] [PubMed] [Google Scholar]
Kadesjö 2002
- Kadesjö B. ADHD in Children and Adults [ADHD hos barn och vuxna]. Stockholm: Socialstyrelsen, 2002. [Google Scholar]
Kambeitz 2014
- Kambeitz J, Romanos M, Ettinger U. Meta-analysis of the association between dopamine transporter genotype and response to methylphenidate treatment in ADHD. Pharmacogenomics Journal 2014;14(1):77-84. [DOI: 10.1038/tpj.2013.9] [DOI] [PubMed] [Google Scholar]
Kanjwal 2012
- Kanjwal K, Saeed B, Karabin B, Kanjwal Y, Grubb BP. Use of methylphenidate in the treatment of patients suffering from refractory postural tachycardia syndrome. American Journal of Therapeutics 2012;19(1):2-6. [DOI: 10.1097/MJT.0b013e3181dd21d2] [DOI] [PubMed] [Google Scholar]
Kelsey 2004
- Kelsey DK, Sumner CR, Casat CD, Coury DL, Quintana H, Saylor KE, et al. Once-daily atomoxetine treatment for children with attention-deficit/hyperactivity disorder, including an assessment of evening and morning behavior: a double-blind, placebo-controlled trial. Pediatrics 2004;114(1):e1-8. [DOI: 10.1542/peds.114.1.e1] [DOI] [PubMed] [Google Scholar]
Kendall 1979
- Kendall PC, Wilcox LE. Self-control in children: development of a rating scale. Journal of Consulting and Clinical Psychology 1979;47(6):1020-9. [DOI: 10.1037/0022-006X.47.6.1020] [DOI] [PubMed] [Google Scholar]
Kidd 2000
- Kidd PM. Attention deficit/hyperactivity disorder (ADHD) in children: rationale for its integrative management. Alternative Medicine Review 2000;5(5):402-28. [PMID: ] [PubMed] [Google Scholar]
Kimko 1999
- Kimko HC, Cross JT, Abernethy DR. Pharmacokinetics and clinical effectiveness of methylphenidate. Clinical Pharmacokinetics 1999;37(6):457–70. [DOI: 10.2165/00003088-199937060-00002] [DOI] [PubMed] [Google Scholar]
King 2006
- King S, Griffin S, Hodges Z, Weatherly H, Asseburg C, Richardson G, et al. A systematic review and economic model of the effectiveness and cost-effectiveness of methylphenidate, dexamfetamine and atomoxetine for the treatment of attention deficit hyperactivity disorder in children and adolescents. Health Technology Assessment 2006;10(23):iii-iv, xiii-146. [DOI: 10.3310/hta10230] [DOI] [PubMed]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta-analyses. Annals of Internal Medicine 2001;135(11):982-9. [DOI: 10.7326/0003-4819-135-11-200112040-00010] [DOI] [PubMed] [Google Scholar]
Korang 2020
- Korang SK, Juul S, Nielsen EE, Feinberg J, Siddiqui F, Ong G, et al. Vaccines to prevent COVID-19: a protocol for a living systematic review with network meta-analysis including individual patient data (The LIVING VACCINE Project). Systematic Reviews 2020;9(1):262. [DOI: 10.1186/s13643-020-01516-1] [PMCID: PMC7678579] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Krogh 2013a [pers comm]
- Krogh HB. Requested data from the trial [personal communication]. Email to: Dr Brams 23 September 2013.
Krogh 2013b [pers comm]
- Krogh HB. Requestion data on inclusion criteria [personal communication]. Email to: Dr Cox 8 October 2013.
Krogh 2013c [pers comm]
- Krogh HB. Data received from author [personal communication]. Email to: Dr Wolraich 2 July 2013.
Krogh 2014a [pers comm]
- Krogh HB. Randomisation procedure and analyses used in our trial [personal communication]. Email to: Russel Barkley 15 February 2014.
Krogh 2014b [pers comm]
- Krogh HB. Data requested [personal communication]. Email to: Dr Johnson June 2014.
Krogh 2014c [pers comm]
- Krogh HB. Data regarding randomisation procedure. Email to: Dr Klorman 21 January 2014.
Krogh 2019
- Krogh HB, Storebø OJ, Faltinsen E, Todorovac A, Ydedahl-Jensen E, Magnusson FL, et al. Methodological advantages and disadvantages of parallel and crossover randomised clinical trials on methylphenidate for attention deficit hyperactivity disorder: a systematic review and meta-analyses. BMJ Open 2019;9(3):e026478. [DOI: 10.1136/bmjopen-2018-026478] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lachar 1986
- Lachar D, Klein R, Boersma D. Personality Inventory for Children: approaches to actuarial interpretation in clinic and school settings. In: Knoff H, editors(s). The Assessment of Child and Adolescent Personality. New York: Guilford Press, 1986:273-308. [Google Scholar]
Ladd 1996
- Ladd GW, Profilet SM. The Child Behavior Scale: a teacher-report measure of young children's aggressive, withdrawn, and prosocial behaviors. Developmental Psychology 1996;32(6):1008-24. [DOI: 10.1037/0012-1649.32.6.1008] [DOI] [Google Scholar]
Landgraf 1998
- Landgraf JM, Maunsell E, Speechley KN, Bullinger M, Campbell S, Abetz L, et al. Canadian-French, German and UK versions of the Child Health Questionnaire: methodology and preliminary item scaling results. Quality of Life Research 1998;7(5):433-45. [DOI: 10.1023/A:1008810004694] [PMID: ] [DOI] [PubMed] [Google Scholar]
Landgraf 2002
- Landgraf JM, Rich M, Rappaport L. Measuring quality of life in children with attention-deficit/hyperactivity disorder and their families: development and evaluation of a new tool. Archives of Pediatrics and Adolescent Medicine 2002;156(4):384-91. [DOI: 10.1001/archpedi.156.4.384] [DOI] [PubMed] [Google Scholar]
Lange 2010
- Lange KW, Reichl S, Lange KM, Tucha L, Tucha O. The history of attention deficit hyperactivity disorder. Attention Deficit and Hyperactivity Disorder 2010;2(4):241-55. [DOI: 10.1007/s12402-010-0045-8.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lau 1995
- Lau J, Schmid CH, Chalmers TC. Cumulative meta-analysis of clinical trials builds evidence for exemplary medical care. Journal of Clinical Epidemiology 1995;48(1):45-57. [DOI: 10.1016/0895-4356(94)00106-Z] [DOI] [PubMed] [Google Scholar]
Laursen 2020
- Laursen DR, Hansen C, Paludan-Müller AS, Hróbjartsson A. Active placebo versus standard placebo control interventions in pharmacological randomised trials (Protocol). Cochrane Database of Systematic Reviews 2020, Issue 7. Art. No: MR000055. [DOI: 10.1002/14651858.MR000055] [DOI] [Google Scholar]
Laursen 2022
- Laursen DR, Nejstgaard CH, Bjørkedal E, Frost AD, Hansen MR, Paludan-Müller AS, et al. Impact of active placebo controls on estimated drug effects in randomised trials: a systematic review of trials with both active placebo and standard placebo. Cochrane Database of Systematic Reviews In press. [CENTRAL: Art. No.: MR000055] [DOI] [PMC free article] [PubMed]
Lavigne 2012
- Lavigne JV, Dulcan MK, LeBailly SA, Binns HJ. Can parent reports serve as a proxy for teacher ratings in medication management of attention-deficit hyperactivity disorder? Journal of Developmental & Behavioral Pediatrics 2012;33(4):336-42. [DOI: 10.1097/DBP.0b013e31824afea1] [DOI] [PubMed] [Google Scholar]
Leckman 1988
- Leckman JF, Towbin K, Ort SI. Clinical assessment of tic disorder severity. In: Cohen DJ, Bruun RD, Leckman JF, editors(s). Tourette's Syndrome and Tic Disorders: Clinical Understanding and Treatment. Oxford: John Wiley & Sons, 1988:55-78. [Google Scholar]
Leckman 1989
- Leckman JF, Riddle MA, Hardin MT, Ort SI, Swartz KL, Stevenson J, et al. The Yale Global Tic Severity Scale: initial testing of a clinician-rated scale of tic severity. Journal of the American Academy of Child and Adolescent Psychiatry 1989;28(4):566-73. [DOI: 10.1097/00004583-198907000-00015] [DOI] [PubMed] [Google Scholar]
Li 2017
- Li Y, Gao J, He S, Zhang Y, Wang Q. An evaluation on the efficacy and safety of treatments for attention deficit hyperactivity disorder in children and adolescents: a comparison of multiple treatments. Molecular Neurobiology 2017;54(9):6655-69. [DOI: 10.1007/s12035-016-0179-6] [PMID: ] [DOI] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. Journal of Clinical Epidemiology 2009;62(10):e1-e34. [DOI: 10.1016/j.jclinepi.2009.06.006] [DOI] [PubMed] [Google Scholar]
Lichtenstein 2012
- Lichtenstein P, Halldner L, Zetterqvist J, Sjölander A, Serlachius E, Fazel S, et al. Medication for attention deficit-hyperactivity disorder and criminality. New England Journal of Medicine 2012;367(21):2006-14. [DOI: 10.1056/NEJMoa1203241] [PMCID: PMC3664186] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Light 2015
Loney 1982
- Loney J, Milich R. Hyperactivity, inattention, and aggression in clinical practice. In: Wolraich M, Routh DK, editors(s). Advances in Developmental and Behavioral Pediatrics. Vol. 3. Greenwich: JAI Press, 1982:113-47. [Google Scholar]
Loney 1984
- Loney J. A short parent scale for subgrouping childhood hyperactivity, aggression. In: Annual Meeting of the American Psychological Association; 1984 August 24-28; Toronto. Toronto, 1984. [DOI: 10.1037/0003-066X.39.6.709] [DOI]
Lundh 2012
- Lundh A, Sismondo S, Lexchin J, Busuioc OA, Bero L. Industry sponsorship and research outcome. Cochrane Database of Systematic Reviews 2012, Issue 12. Art. No: MR000033. [DOI: 10.1002/14651858.MR000033.pub2] [DOI] [PubMed] [Google Scholar]
Lundh 2018
- Lundh A, Lexchin J, Mintzes B, Schroll JB, Bero L. Industry sponsorship and research outcome: systematic review with meta-analysis. Intensive Care Medicine 2018;44(10):1603-12. [DOI: 10.1007/s00134-018-5293-7] [PMID: ] [DOI] [PubMed] [Google Scholar]
Magnusson 2014a [pers comm]
- Magnusson FL. Received information on randomisation and allocation concealment procedure [personal communication]. Email to: Dr Klorman 17 April 2014.
Magnusson 2014b [pers comm]
- Magnusson FL. Data received from author [personal communication]. Email to: Dr Rapport 8 May 2014.
Magnusson 2014c [pers comm]
- Magnusson FL. Data received from author [personal communication]. Email to: Dr Kircher May 2014.
Maia 2014
- Maia CR, Cortese S, Caye A, Deakin TK, Polanczyk GV, Polanczyk CA, et al. Long-term efficacy of methylphenidate immediate-release for the treatment of childhood ADHD: a systematic review and meta-analysis. Journal of Attention Disorders 2014;21(1):3-13. [DOI: 10.1177/1087054714559643] [DOI] [PubMed] [Google Scholar]
Marsee 2007
- Marsee MA, Frick PJ. Exploring the cognitive and emotional correlates to proactive and reactive aggression in a sample of detained girls. Journal of Abnormal Child Psychology 2007;35(6):969-81. [DOI: 10.1007/s10802-007-9147-y] [DOI] [PubMed] [Google Scholar]
McCarthy 2018
- McCarthy S, Neubert A, Man KK, Banaschewski T, Buitelaar J, Carucci S, et al. Effects of long-term methylphenidate use on growth and blood pressure: results of the German Health Interview and Examination Survey for Children and Adolescents (KiGGS). BMC Psychiatry 2018;18(1):327. [DOI: 10.1186/s12888-018-1884-7] [PMCID: PMC6180569] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
McGough 2006a
- McGough JJ, Wigal SB, Abikoff H, Turnbow JM, Posner K, Moon E. A randomized, double-blind, placebo-controlled, laboratory classroom assessment of methylphenidate transdermal system in children with ADHD. Journal of Attention Disorders 2006;9(3):476-85. [DOI: 10.1177/1087054705284089] [DOI] [PubMed] [Google Scholar]
Mendelsohn 1978
- Mendelsohn M, Erdwins C. The Disruptive Behavior Scale: an objective assessment of unmanageable social behavior in adolescents. Journal of Clinical Psychology 1978;34(2):426-8. [DOI: ] [DOI] [PubMed] [Google Scholar]
Milich 1980
- Milich R, Roberts MA, Loney J, Caputo J. Differentiating practice effects and statistical regression on the Conners Hyperkinesis Index. Journal of Abnormal Child Psychology 1980;8(4):549-52. [DOI: 10.1007/BF00916506] [DOI] [PubMed] [Google Scholar]
Miller 1997
- Miller LS, Koplewicz HS, Klein RG. Teacher ratings of hyperactivity, inattention, and conduct problems in preschoolers. Journal of Abnormal Child Psychology 1997;25(2):113-9. [DOI: 10.1023/A:1025727428097] [DOI] [PubMed] [Google Scholar]
Moffit 2007
- Moffit TE, Melchior M. Why does the worldwide prevalence of childhood attention deficit hyperactivity disorder matter? American Journal of Psychiatry 2007;146(6):856-8. [DOI: 10.1176/ajp.2007.164.6.856] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad A, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analysis? Lancet 1998;352(9128):609-13. [DOI: 10.1016/S0140-6736(98)01085-X] [DOI] [PubMed] [Google Scholar]
Moher 2010
- Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux J, et al. Research methods and reporting: CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ Clinical Research Edition 2010;340:c869. [DOI: 10.1136/bmj.c869] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2015
- Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Systematic Reviews 2015;4:1. [DOI: 10.1186/2046-4053-4-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Molina 2009
- Molina BS, Hinshaw SP, Swanson JM, Arnold LE, Vitiello B, Jensen PS, et al. The MTA at 8 years: prospective follow-up of children treated for combined-type ADHD in a multisite study. Journal of the American Academy of Child and Adolescent Psychiatry 2009;48(5):484-500. [DOI: 10.1097/CHI.0b013e31819c23d0] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moncrieff 2004
- Moncrieff J, Wessely S, Hardy R. Active placebos versus antidepressants for depression. Cochrane Database of Systematic Reviews 2004, Issue 1. Art. No: CD003012. [DOI: 10.1002/14651858.CD003012.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
MTA 1999a
- MTA Cooperation Group. A 14 month randomised clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. Archives of General Psychiatry 1999;56(12):1073-86. [DOI: 10.1001/archpsyc.56.12.1073] [DOI] [PubMed] [Google Scholar]
MTA 1999b
- MTA Cooperative Group. A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. Archives of General Psychiatry 1999;56(12):1073-1086. [DOI: 10.1001/archpsyc.56.12.1073] [DOI] [PubMed] [Google Scholar]
Murray 2007
- Murray DW, Kollins SH, Hardy KK, Abikoff HB, Swanson JM, Cunningham C, et al. Parent versus teacher ratings of attention-deficit/hyperactivity disorder symptoms in the Preschoolers with Attention-Deficit/Hyperactivity Disorder Treatment Study (PATS). Journal of Child and Adolescent Psychopharmacology 2007;17(5):605-19. [DOI: 10.1089/cap.2007.0060] [DOI] [PubMed] [Google Scholar]
Murray 2009
- Murray DW, Bussing R, Fernandez M, Hou W, Garvan CW, Swanson JM, et al. Psychometric properties of teacher SKAMP ratings from a community sample. Assessment 2009;16(2):193-208. [DOI: 10.1177/1073191108326924] [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2018
- National Institute for Health and Care Excellence. Attention Deficit Hyperactivity Disorder: Diagnosis and Management. London, UK: National Institute for Health and Care Excellence, 2018. [BOOKSHELF ID: NBK493361] [PMID: ] [PubMed] [Google Scholar]
Nigg 2005
- Nigg JT, Casey BJ. An integrative theory of attention-deficit/hyperactivity disorder based on the cognitive and affective neurosciences. Development and Psychopathology 2005;17(3):785-806. [DOI: 10.1017/S0954579405050376] [DOI] [PubMed] [Google Scholar]
Nilausen 2014 [pers comm]
- Nilausen TD. Requested data [personal communication]. Email to: Dr Kolko 13 January 2014.
Owens 2000
- Owens JA, Spirito A, McGuinn M. The Children's Sleep Habits Questionnaire (CSHQ): psychometric properties of a survey instrument for school-aged children. Sleep 2000;23(8):1043-51. [DOI: 10.1093/sleep/23.8.1d] [DOI] [PubMed] [Google Scholar]
Padilha 2018
- Padilha SC, Virtuoso S, Tonin FS, Borba HH, Pontarolo R. Efficacy and safety of drugs for attention deficit hyperactivity disorder in children and adolescents: a network meta-analysis. European Child & Adolescent Psychiatry 2018;27(10):1335-45. [DOI: 10.1007/s00787-018-1125-0] [PMID: ] [DOI] [PubMed] [Google Scholar]
Page 2022
- Page MJ, Higgins JP, Sterne JA. Chapter 13: Assessing risk of bias due to missing results in a synthesis. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Pasini 2007
- Pasini A, Paloscia C, Alessandrelli R, Porfirio MC, Curatolo P. Attention and executive functions profile in drug naive ADHD subtypes. Brain & Development 2007;29(7):400-8. [DOI: 10.1016/j.braindev.2006.11.010] [DOI] [PubMed] [Google Scholar]
Pelham 1993b
- Pelham WE Jr. Pharmacotherapy for children with attention-deficit hyperactivity disorder. School Psychology Review 1993;22(2):199-227. [DOI: 10.1080/02796015.1993.12085647] [DOI] [Google Scholar]
Pelham 1996
- Pelham WE, Hoza B. Intensive treatment: a summer treatment pro-gram for children with ADHD. In: Hibbs E, Jensen P, editors(s). Psychosocial Treatments for Child andAdolescent Disorders: Empirically Based Strategies for Clinical Practice. New York: APA Press, 1996:311-340. [DOI: 10.1037/10196-013] [DOI] [Google Scholar]
Pelham 2005a
- Pelham WE, Burrows-MacLean L, Gnagy EM, Fabiano GA, Coles EK, Tresco KE, et al. Transdermal methylphenidate, behavioral, and combined treatment for children with ADHD. Experimental and Clinical Psychopharmacology 2005;13(2):111-26. [DOI: 10.1037/1064-1297.13.2.111] [DOI] [PubMed] [Google Scholar]
Pereira Ribeiro 2022
Pfefferbaum‐Levine 1983
- Pfefferbaum-Levine B, Overall JE. Diagnostic factor structure of the Children's Psychiatric Rating Scale (CPRS). Journal of Clinical Child Psychology 1983;12(2):167-73. [DOI: 10.1080/15374418309533126] [DOI] [Google Scholar]
Pliszka 2007a
- Pliszka S, AACAP Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry 2007;46(7):894-921. [DOI: 10.1097/chi.0b013e318054e724] [DOI] [PubMed] [Google Scholar]
Polanczyk 2007
- Polanczyk G, Rohde LA. Epidemiology of attention-deficit/hyperactivity disorder across the lifespan. Current Opinion in Psychiatry 2007;20(4):386-92. [DOI: 10.1097/YCO.0b013e3281568d7a] [DOI] [PubMed] [Google Scholar]
Polanczyk 2014
- Polanczyk GV, Willcutt EG, Salum GA, Kieling C, Rohde LA. ADHD prevalence estimates across three decades: an updated systematic review and meta-regression analysis. International Journal of Epidemiology 2014;43(2):434-42. [DOI: 10.1093/ije/dyt261] [DOI] [PMC free article] [PubMed] [Google Scholar]
Polderman 2007
- Polderman TJ, Derks EM, Hudziak JJ, Verhulst FC, Posthuma D, Boomsma DI. Across the continuum of attention skills: a twin study of the SWAN ADHD rating scale. Journal of Child Psychology and Psychiatry 2007;48(11):1080-7. [DOI: 10.1111/j.1469-7610.2007.01783.x] [DOI] [PubMed] [Google Scholar]
Posner 2011
- Posner K, Brown GK, Stanley B, Brent DA, Yershova KV, Oquendo MA, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. American Journal of Psychiatry 2011;168(12):1266-77. [DOI: 10.1176/appi.ajp.2011.10111704] [PMCID: PMC3893686] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Poznanski 1983
- Poznanski EO, Cook SC, Carroll BJ, Corzo H. Use of the Children's Depression Rating Scale in an inpatient psychiatric population. Journal of Clinical Psychiatry 1983;44(6):200-3. [PMID: ] [PubMed] [Google Scholar]
Punja 2013
- Punja S, Zorzela L, Hartling L, Urichuk L, Vohra S. Long-acting versus short-acting methylphenidate for paediatric ADHD: a systematic review and meta-analysis of comparative efficacy. BMJ Open 2013;3(3):e002312. [DOI: 10.1136/bmjopen-2012-002312] [DOI] [PMC free article] [PubMed] [Google Scholar]
Raman 2015
- Raman SR, Marshall SW, Gaynes BN, Haynes K, Naftel AJ, Stürmer T. An observational study of pharmacological treatment in primary care of children with ADHD in the United Kingdom. Psychiatric Services 2015;66(6):617-24. [DOI: 10.1176/appi.ps.201300148] [DOI] [PubMed] [Google Scholar]
Ramstad 2013a [pers comm]
- Ramstad E. Data requested from author [personal communication]. Email to: Dr Ialongo 1 August 2013.
Ramstad 2013b [pers comm]
- Ramstad E. Request on data from the Rhode Island CLC study [personal communication]. Email to: Dr DuPaul 5 September 2013.
Ramstad 2013c [pers comm]
- Ramstad E. Data requestioned [personal communication]. Email to: Dr Rapport 22 July 2013.
Ramstad 2013d [pers comm]
- Ramstad E. Data requested from author [personal communication]. Email to: Dr Tirosh 1 August 2013.
Ramstad 2014 [pers comm]
- Ramstad E. Data received from authors [personal communication]. Email to: Dr Vitiello July 2014.
Ravens‐Sieberer 1998
- Ravens-Sieberer AU, Bullinger M. Assessing health-related quality of life in chronically ill children with the German KINDL: first psychometric and content analytical results. Quality of Life Research 1998;7(5):399-407. [DOI: 10.1023/a:1008853819715] [PMID: ] [DOI] [PubMed] [Google Scholar]
Reichow 2013
- Reichow B, Volkmar FR, Bloch MH. Systematic review and meta-analysis of pharmacological treatment of the symptoms of attention-deficit/hyperactivity disorder in children with pervasive developmental disorders. Journal of Autism and Developmental Disorders 2013;43(10):2435-41. [DOI: 10.1007/s10803-013-1793-z] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rentz 2005
- Rentz AM, Matza LS, Secnik K, Swensen A, Revicki DA. Psychometric validation of the Child Health Questionnaire (CHQ) in a sample of children and adolescents with attention-deficit/hyperactivity disorder. Quality of Life Research 2005;14(3):719-34. [DOI: 10.1007/s11136-004-0832-9] [DOI] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: The Cochrane Collaboration, 2020.
Ribeiro 2021
- Ribeiro JP, Arthur EJ, Gluud C, Simonsen E, Storebø OJ. Does methylphenidate work in children and adolescents with attention deficit hyperactivity disorder? Pediatric Reports 2021;13(3):434-43. [DOI: 10.3390/pediatric13030050] [PMCID: PMC8396049] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Riley 2004
- Riley AW, Forrest CB, Starfield B, Rebok GW, Robertson JA, Green BF. The Parent Report Form of the CHIP-Child Edition: reliability and validity. Medical Care 2004;42(3):210-20. [DOI: 10.1097/01.mlr.0000114909.33878.ca] [DOI] [PubMed] [Google Scholar]
Romanos 2016
- Romanos M, Reif A, Banaschewski T. Methylphenidate for attention-deficit/hyperactivity disorder. JAMA 2016;316(9):994-5. [DOI: 10.1001/jama.2016.10279] [PMID: ] [DOI] [PubMed] [Google Scholar]
Rossi 2010
- Rossi S. Australian Medicines Handbook (online); 2010. Available at amhonline.amh.net.au.
Routh 1978
- Routh D. Hyperactivity. In: Magrab P, editors(s). Psychological Management of Paediatric Problems. Baltimore: University Park Press, 1978. [Google Scholar]
Rowe 1997
- Rowe KS, Rowe KJ. Norms for parental ratings on Conners' Abbreviated Parent-Teacher Questionnaire: implications for the design of behavioral rating inventories and analyses of data derived from them. Journal of Abnormal Child Psychology 1997;25(6):425-51. [DOI: 10.1023/A:1022678013979] [DOI] [PubMed] [Google Scholar]
Savovic 2018
- Savovic J, Turner RM, Mawdsley D, Jones HE, Beynon R, Higgins JP, et al. Association between risk-of-bias assessments and results of randomized trials in Cochrane reviews: the ROBES meta-epidemiologic study. American Journal of Clinical Epidemiology 2018;187(5):1113-22. [DOI: 10.1093/aje/kwx344] [DOI] [PMC free article] [PubMed] [Google Scholar]
Savović 2012a
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomised controlled trials: combined analysis of meta-epidemiological studies. Health Technology Assessment 2012;16(35):1-82. [DOI: 10.3310/hta16350] [DOI] [PubMed] [Google Scholar]
Savović 2012b
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Annals of Internal Medicine 2012;157(6):429–38. [DOI: 10.7326/0003-4819-157-6-201209180-00537] [DOI] [PubMed] [Google Scholar]
Scahill 2000
- Scahill L, Schwab-Stone M. Epidemiology of ADHD in school-age children. Child and Adolescent Psychiatric Clinics of North America 2000;9(3):541-55. [DOI: 10.1016/S1056-4993(18)30106-8] [DOI] [PubMed] [Google Scholar]
Schachar 1997b
- Schachar RJ, Tannock R, Cunningham C, Corkum PV. Behavioral, situational, and temporal effects of treatment of ADHD with methylphenidate. Journal of the American Academy of Child and Adolescent Psychiatry 1997;36(6):754-63. [DOI: 10.1097/00004583-199706000-00011] [DOI] [PubMed] [Google Scholar]
Schachter 2001
- Schachter HM, Pham B, King J, Langford S, Moher D. How efficacious and safe is short-acting methylphenidate for the treatment of attention-deficit disorder in children and adolescents? A meta-analysis. Canadian Medical Association Journal 2001;165(11):1475-88. [URL: http://www.cmaj.ca/content/165/11/1475.short] [PMC free article] [PubMed] [Google Scholar]
Schmidt 2009
- Schmidt S, Petermann F. Developmental psychopathology: attention deficit hyperactivity disorder (ADHD). BMC Psychiatry 2009;9(1):58. [DOI: 10.1186/1471-244X-9-58] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schubert 2010
- Schubert I, Köster I, Lehmkuhl G. The changing prevalence of attention-deficit/hyperactivity disorder and methylphenidate prescriptions: a study of data from a random sample of insurees of the AOK Health Insurance Company in the German State of Hesse, 2000-2007. Deutsches Ärzteblatt International 2010;107(36):615-21. [DOI: 10.3238/arztebl.2010.0615] [PMCID: PMC2947846] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408-12. [DOI: 10.1001/jama.1995.03520290060030] [DOI] [PubMed] [Google Scholar]
Schulz 2012
- Schulz KP, Fan J, Bédard AC, Clerkin SM, Ivanov I, Tang CY, et al. Common and unique therapeutic mechanisms of stimulant and nonstimulant treatments for attention-deficit/hyperactivity disorder. Archives of General Psychiatry 2012;69(9):952-61. [DOI: 10.1001/archgenpsychiatry.2011.2053] [DOI] [PubMed] [Google Scholar]
Schünemann 2022
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al., editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook.
Sergeant 2003
- Sergeant JA, Geurts H, Huijbregts S, Scheres A, Oosterlaan J. The top and the bottom of ADHD: a neuropsychological perspective. Neuroscience and Biobehavioural Reviews 2003;27(7):583-92. [DOI: 10.1016/j.neubiorev.2003.08.004] [DOI] [PubMed] [Google Scholar]
Shaffer 1983
- Shaffer D, Gould MS, Brasic J, Ambrosini P, Fisher P, Bird H, et al. A Children's Global Assessment Scale (CGAS). Archives of General Psychiatry 1983;40(11):1228-31. [DOI: 10.1001/archpsyc.1983.01790100074010] [DOI] [PubMed] [Google Scholar]
Shaw 2012
- Shaw M, Hodgkins P, Caci H, Young S, Kahle J, Woods AG, et al. A systematic review and analysis of long-term outcomes in attention deficit hyperactivity disorder: effects of treatment and non-treatment. BMC Medicine Sep 2012;10:99. [DOI: 10.1186/1741-7015-10-99] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shaw 2016
- Shaw P. Quantifying the benefits and risks of methylphenidate as treatment for childhood attention-deficit/hyperactivity disorder. JAMA 2016;315(18):1953-5. [DOI: 10.1001/jama.2016.3427] [PMID: ] [DOI] [PubMed] [Google Scholar]
SIGN 2009
- Scottish Intercollegiate Guidelines Network. Management of attention deficit and hyperkinetic disorders in children and young people. http://www.sign.ac.uk/guidelines/fulltext/112/ (accessed 30 April 2015).
Silva 2005b
- Silva RR, Alpert M, Pouget E, Silva V, Trosper S, Reyes K, et al. A rating scale for disruptive behavior disorders, based on the DSM-IV item pool. Psychiatric Quarterly 2005;76(4):327-39. [DOI: 10.1007/s11126-005-4966-x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Solanto 1998
- Solanto MV. Neuropsychopharmacological mechanisms of stimulant drug action in attention-deficit hyperactivity disorder: a review and integration. Behavioural Brain Research 1998;94(1):127-52. [DOI: 10.1016/S0166-4328(97)00175-7] [DOI] [PubMed] [Google Scholar]
Sonuga‐Barke 2007
- Sonuga-Barke EJS, Coghill D, Markowitz JS, Swanson JM, Vandenberghe M, Hatch SJ. Sex differences in response of children with ADHD to once-daily formulations of methylphenidate. Journal of American Academy of Child & Adolescent Psychiatry 2007;46(6):701-10. [DOI: 10.1097/chi.0b013e31804659f1] [DOI] [PubMed] [Google Scholar]
Spencer 2013
- Spencer TJ, Brown A, Seidman LJ, Valera EM, Makris N, Lomedico A, et al. Effect of psychostimulants on brain structure and function in ADHD: a qualitative literature review of MRI-based neuroimaging studies. Journal of Clinical Psychiatry 2013;74(9):902-17. [DOI: 10.4088/JCP.12r08287] [PMCID: PMC3801446] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sprafkin 1986
- Sprafkin J, Grayson P, Gadow K, Nolan EE, Paolicelli LM. Code for Observing Social Activity (COSA). Stony Brook: State University of New York, Department of Psychiatry and Behavioral Science, 1986. [Google Scholar]
Steinhausen 1993
- Steinhausen H-C. LF - Teacher Questionnaire after Rutter, Tizard and Whitmore - German edited version [Lehrerfragebogen nach Rutter, Tizard und Whitmore - Deutsche bearbeitete Fassung]. In: Psychische Störungen bei Kindern und Jugendlichen. Lehrbuch der Kinder- und Jugendpsychiatrie. München: Urban Und Schwarzenberg, 1993. [Google Scholar]
Stevenson 1989
- Stevenson RD, Wolraich ML. Stimulant medication therapy in the treatment of children with attention deficit hyperactivity disorder. Pediatric Clinics of North America 1989;36(5):1183-97. [DOI: 10.1016/S0031-3955(16)36764-5] [DOI] [PubMed] [Google Scholar]
Storebø 2015b [pers comm]
- Storebø OJ, Gluud C. Criticism to "Immediate-release methylphenidate for attention deficit hyperactivity disorder (ADHD) in adults" [personal communication]. Email to: T Epstein via Wiley Online Feedback form 10 May 2015.
Storebø 2016a
- Storebø OJ, Gluud C. Methylphenidate for ADHD: have Cochrane got it wrong this time? Responses to Chris Hollis; 11 March-10 June 2016. Available at www.nationalelfservice.net/mental-health/adhd/methylphenidate-for-adhd-have-cochrane-got-it-wrong-this-time.
Storebø 2016b
- Storebø OJ, Simonsen E, Gluud C. Methylphenidate for attention-deficit/hyperactivity disorder in children and adolescents. JAMA 2016;315(18):2009-10. [DOI: 10.1001/jama.2016.3611] [PMID: ] [DOI] [PubMed] [Google Scholar]
Storebø 2016c
- Storebø OJ, Zwi M, Moreira-Maia CR, Skoog M, Camilla G, Gillies D, et al. Response to "Trust, but verify" by Banaschewski et al. Zeitschrift für Kinder- und Jugendpsychiatrie und Psychotherapie 2016;44(5):334-5. [DOI: 10.1024/1422-4917/a000472] [PMID: ] [DOI] [PubMed] [Google Scholar]
Storebø 2016d
- Storebø OJ, Zwi M, Krogh HB, Moreira-Maia CR, Holmskov M, Gillies D, et al. Evidence on methylphenidate in children and adolescents with ADHD is in fact of 'very low quality'. BMJ Evidence-Based Mental Health 2016;19(4):100-2. [DOI: 10.1136/eb-2016-102499] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Storebø 2016e
- Storebø OJ, Simonsen E, Gluud C. Methylphenidate for attention-deficit/hyperactivity disorder-reply. JAMA 2016;316(9):995. [DOI: 10.1001/jama.2016.10300] [PMID: ] [DOI] [PubMed] [Google Scholar]
Storebø 2016f
- Storebø OJ, Simonsen E, Gluud C. The evidence base of methylphenidate for children and adolescents with attention-deficit hyperactivity disorder is in fact flawed. European Child & Adolescent Psychiatry 2016;25(9):1037-8. [DOI: 10.1007/s00787-016-0855-0] [PMID: ] [DOI] [PubMed] [Google Scholar]
Storebø 2018a
- Storebø OJ, Faltinsen E, Zwi M, Simonsen E, Gluud C. The jury is still out on the benefits and harms of methylphenidate for children and adolescents with attention-deficit/hyperactivity disorder. Clinical Pharmacology & Therapeutics 2018;104(4):606-9. [DOI: 10.1002/cpt.1149] [PMID: ] [DOI] [PubMed] [Google Scholar]
Storebø 2018b
- Storebø OJ, Pedersen N, Ramstad E, Kielsholm ML, Nielsen SS, Krogh HB, et al. Methylphenidate for attention deficit hyperactivity disorder (ADHD) in children and adolescents – assessment of adverse events in non‐randomised studies. Cochrane Database of Systematic Reviews 2018, Issue 5. Art. No: CD012069. [DOI: 10.1002/14651858.CD012069.pub2] [PMCID: PMC6494554] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Storebø 2021
- Storebø OJ, Gluud C. Methylphenidate for ADHD rejected from the WHO Essential Medicines List due to uncertainties in benefit-harm profile. BMJ Evidence-Based Medicine 2021;26(4):172-5. [DOI: 10.1136/bmjebm-2019-111328] [PMID: ] [DOI] [PubMed] [Google Scholar]
Storm 2021b [pers comm]
- Storm M. Data Requested [personal communication]. Email from Dr Froehlich 30 august 2021.
Storm 2021 [pers comm]
- Storm M. Data requested [personal communication]. Email from Dr Childress 30 august 2021.
Storm 2021c [pers comm]
- Overby M. Data requested [personal communication]. Email from Dr Hawk 8 October 2021.
Storm 2021d [pers comm]
- Storm M. Data requested [personal communication]. Email from Dr Mathijsen 20 October 2021.
Storm 2021e [pers comm]
- Storm M. Data requested [personal communication]. Email from Dr McCracken 7 september 2021.
Storm 2021f [pers comm]
- Storm M. Data requested [personal communication]. Email from Dr Pliszka 8 September 2021.
Storm 2021g [pers comm]
- Storm M. Data requested [personal communication]. Email from Dr Reneman 6 September 2021.
Sutton 2003
- Sutton V, Sumner C, Allen AJ, Feng W, Schuh K, Michelson D. Validity, reliability, and responsiveness of the DPREMB-R scale for ADHD. In: Scientific Proceedings of the 50th Anniversary Meeting of the American Academy of Child and Adolescent Psychiatry (AACAP); 2003 Oct 14-19; Miami (FL). 2003:169-70.
Swanson 1992
- Swanson JM. School-based Assessments and Interventions for ADD Students. Irvine, CA: KC Publishing, 1992. [Google Scholar]
Swanson 2004a
- Swanson JM, Wigal SB, Wigal T, Sonuga-Barke E, Greenhill LL, Biederman J, et al. A comparison of once-daily extended-release methylphenidate formulations in children with attention-deficit/hyperactivity disorder in the laboratory school (the COMACS Study). Pediatrics 2004;113(3):e206-16. [DOI: 10.1542/peds.113.3.e206] [DOI] [PubMed] [Google Scholar]
Swanson 2006
- Swanson JM, Schuck S, Mann M, Carlson C, Hartman K, Sergeant JA, et al. Categorical and dimensional definitions and evaluations of symptoms of ADHD: the SNAP and SWAN Rating Scales. http://bit.ly/1Kj9akF (accessed 4 March 2015). [PMC free article] [PubMed]
Swanson 2009
- Swanson J. The MTA follow-up into adolescence: implications for personalized treatment. In: Oral presentation at The International Society for Research in Child and Adolescent Psychopathology (ISRCAP). 2009.
Taylor 2004
Thorlund 2009
- Thorlund K, Devereaux PJ, Wetterslev J, Guyatt G, Ioannidis JP, Thabane L, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta-analysis? International Journal of Epidemiology 2009;38(1):276-86. [DOI: 10.1093/ije/dyn179] [DOI] [PubMed] [Google Scholar]
Timimi 2004
- Timimi S, Taylor E. ADHD is best understood as a cultural construct. British Journal of Psychiatry 2004;184(1):8-9. [DOI: 10.1192/bjp.184.1.8] [DOI] [PubMed] [Google Scholar]
Torrance 1982
- Torrance GW, Boyle MH, Horwood SP. Application of multi-attribute utility theory to measure social preferences for health states. Operations Research 1982;30(6):1043-69. [DOI: 10.1287/opre.30.6.1043] [DOI] [PubMed] [Google Scholar]
Trecenõ 2012
- Trecenõ C, Martín Arias LH, Sáinz M, Salado I, García Ortega P, Velasco V, et al. Trends in the consumption of attention deficit hyperactivity disorder medications in Castilla y León (Spain): changes in the consumption pattern following the introduction of extended release methylphenidate. Pharmacoepidemiology and Drug Safety 2012;21(4):435-41. [DOI: 10.1002/pds.2348] [PMID: ] [DOI] [PubMed] [Google Scholar]
Turgay 1994
- Turgay A. Disruptive Behavior Disorders: Child and Adolescent Screening and Rating Scales for Children, Adolescents, Parents and Teachers. West Blomfield, Michigan: Integrative Therapy Institute Publication, 1994. [Google Scholar]
Ullmann 1984
- Ullmann RK, Sleator EK, Sprague RL. A new rating scale for diagnosis and monitoring of ADD children. Psychopharmacology Bulletin 1984;20(1):160-4. [PMID: PMID: 6718641] [PubMed] [Google Scholar]
US FDA 2011
- US Food and Drug Administration. Communication about an ongoing safety review of stimulant medications used in children with attention-deficit/hyperactivity disorder (ADHD). http://1.usa.gov/1M5AYoq (accessed 6 May 2011).
Van der Meere 1999b
- Van der Meere J, Gunning B, Stemerdink N. The effect of methylphenidate and clonidine on response inhibition and state regulation in children with ADHD. Journal of Child Psychology and Psychiatry and Allied Disciplines 1999;40(2):291-8. [DOI: 10.1111/1469-7610.00443] [DOI] [PubMed] [Google Scholar]
Van der Oord 2008
- Van der Oord S, Prins PJ, Oosterlaan J, Emmelkamp PM. Efficacy of methylphenidate, psychosocial treatments and their combination in school-aged children with ADHD: a meta-analysis. Clinical Psychology Review 2008;28(5):783-800. [DOI: 10.1016/j.cpr.2007.10.007] [DOI] [PubMed] [Google Scholar]
Visser 2014
- Visser SN, Danielson ML, Bitsko RH, Holbrook JR, Kogan MD, Ghandour RM, et al. Trends in the parent-report of health care provider-diagnosed and medicated attention-deficit/hyperactivity disorder: United States, 2003-2011. Journal of the American Academy of Child and Adolescent Psychiatry 2014;53(1):34-46.e2. [DOI: 10.1016/j.jaac.2013.09.001] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wahler 1976
- Wahler RG, House AE, Stambaugh EE. Ecological Assessment of Child Problem Behavior: A Clinical Package for Home, School, and Institutional Settings. Oxford: Pergamon, 1976. [Google Scholar]
Ward 1993
- Ward MF, Wender PH, Reimherr FW. The Wender Utah Rating Scale: an aid in the retrospective diagnosis of childhood attention deficit hyperactivity disorder. American Journal of Psychiatry 1993;150(6):885-90. [DOI: ] [DOI] [PubMed] [Google Scholar]
Wechsler 1945
- Wechsler D. A standardized memory scale for clinical use. Journal of Psychology: Interdisciplinary and Applied 1945;19(1):87-95. [DOI: 10.1080/00223980.1945.9917223] [DOI] [Google Scholar]
Wenthur 2016
- Wenthur CJ. Classics in chemical neuroscience: methylphenidate. ACS Chemical Neuroscience 2016;7(8):1030-40. [DOI: 10.1021/acschemneuro.6b00199] [PMID: ] [DOI] [PubMed] [Google Scholar]
Wetterslev 2008
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta-analysis. Journal of Clinical Epidemiology 2008;61(1):64-75. [DOI: 10.1016/j.jclinepi.2007.03.013] [DOI] [PubMed] [Google Scholar]
Wetterslev 2009
- Wetterslev J, Thorlund K, Brok J, Gluud C. Estimating required information size by quantifying diversity in random-effects model meta-analyses. BMC Medical Research Methodology 2009;9:86. [DOI: 10.1186/1471-2288-9-86] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wetterslev 2017
- Wetterslev J, Jakobsen JC, Gluud C. Trial sequential analysis in systematic reviews with meta-analysis. BMC Medical Research Methodology volume 2017;17(1):1-18. [DOI: 10.1186/s12874-017-0315-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 1988
- World Health Organization. International classification of diseases— ninth revision (ICD-9). Weekly Epidemiological Record 1988;63(45):343-4. [URL: https://apps.who.int/iris/handle/10665/226922] [Google Scholar]
WHO 1992
- World Health Organization. The ICD-10 Classification of Mental and Behavioural Disorders. Clinical Descriptions and Diagnostic Guidelines. Geneva: WHO, 1992. [Google Scholar]
WHO 2019
- World Health Organization. ICD-11 World Health Organisation. https://icd.who.int/en/ 2019.
WHO 2021
- World Health Organization. The Selection and Use of Essential Medicines: Report of the WHO Expert Committee on Selection and Use of Essential Medicines, 2021 (including the 22nd WHO Model List of Essential Medicines and the 8th WHO Model List of Essential Medicines for Children). WHO Technical Report Series, No 1035. Geneva: World Health Organization, 2021. [ISBN: 9789240041134] [URL: Available at www.who.int/publications/i/item/9789240041134] [Google Scholar]
Wigal 1998
- Wigal SB, Gupta S, Guinta D, Swanson JM. Reliability and validity of the SKAMP rating scale in a laboratory school setting. Psychopharmacology Bulletin 1998;34(1):47-53. [URL: Available at: tinyurl.com/yutxvn5h] [PubMed] [Google Scholar]
Wilens 2006a
- Wilens TE, Gignac M, Swezey A, Monuteaux MC, Biederman J. Characteristics of adolescents and young adults with ADHD who divert or misuse their prescribed medications. Journal of the American Academy of Child and Adolescent Psychiatry 2006;45(4):408-14. [DOI: 10.1097/01.chi.0000199027.68828.b3] [DOI] [PubMed] [Google Scholar]
Wilkinson 2006
- Wilkinson GS, Robertson GJ. Wide Range Achievement Test 4. Lutz, FL: Psychological Assessment Resources, 2006. [Google Scholar]
Wolraich 2003
- Wolraich ML, Lambert W, Doffing MA, Bickman L, Simmons T, Worley K. Psychometric properties of the Vanderbilt ADHD Diagnostic Parent Rating Scale in a referred population. Journal of Pediatric Psychology 2003;28(8):559-67. [DOI: 10.1093/jpepsy/jsg046] [DOI] [PubMed] [Google Scholar]
Wolraich 2019
- Wolraich ML, Hagan JF, Allan C, Chan E, Davison D, Earls M, et al. Clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics 2019;144(4):e20192528. [DOI: 10.1542/peds.2019-2528] [PMCID: PMC7067282] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta-epidemiological study. BMJ 2008;336(7644):601–5. [DOI: 10.1136/bmj.39465.451748.AD] [DOI] [PMC free article] [PubMed] [Google Scholar]
Woodcock 2001
- Woodcock RW, Mcgrew KS, Mather N. Woodcock-Johnson III Tests of Achievement. Itasca, IL: Riverside Publishers, 2001. [Google Scholar]
Young 1978
- Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. British Journal of Psychiatry 1978;133(11):429-35. [DOI: 10.1192/bjp.133.5.429] [DOI] [PubMed] [Google Scholar]
Zhang 2005
- Zhang S, Faries DE, Vowles M, Michelson D. ADHD Rating Scale IV: psychometric properties from a multinational study as clinician-administered instrument. International Journal of Methods in Psychiatric Research 2005;14(4):186-201. [DOI: 10.1002/mpr.7] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zito 2000
- Zito J, Safer DJ, DosReis S, Gardner JF, Boles M, Lynch FT. Trends in the prescribing of psychotropic medications to preschoolers. JAMA 2000;283(8):1025-30. [DOI: 10.1001/jama.283.8.1025] [DOI] [PubMed] [Google Scholar]
Zoëga 2011
- Zoëga H, Furu K, Halldórsson M, Thomsen PH, Sourander A, Martikainen JE. Use of ADHD drugs in the Nordic countries: a population-based comparison study. Acta Psychiatrica Scandinavica 2011;123(5):360-7. [DOI: 10.1111/j.1600-0447.2010.01607.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Storebø 2012
- Storebø OJ, Rosendal S, Skoog M, Groth C, Bille T, Buch Rasmussen K, et al. Methylphenidate for attention deficit hyperactivity disorder (ADHD) in children and adolescents. Cochrane Database of Systematic Reviews 2012, Issue 5. Art. No: CD009885. [DOI: 10.1002/14651858.CD009885] [DOI] [Google Scholar]
Storebø 2015a
- Storebø OJ, Ramstad E, Krogh HB, Nilausen TD, Skoog M, Holmskov M, et al. Methylphenidate for children and adolescents with attention deficit hyperactivity disorder (ADHD). Cochrane Database of Systematic Reviews 2015, Issue 11. Art. No: CD009885. [DOI: 10.1002/14651858.CD009885.pub2] [PMCID: PMC8763351] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Storebø 2015b
- Storebø OJ, Krogh HB, Ramstad E, Moreira-Maia CR, Holmskov M, Skoog M, et al. Methylphenidate for attention-deficit/hyperactivity disorder in children and adolescents: Cochrane systematic review with meta-analyses and trial sequential analyses of randomised clinical trials. BMJ 2015;351:h5203. [DOI: 10.1136/bmj.h5203] [PMCID: PMC4659414] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Storebø 2016
- Storebø OJ, Simonsen E, Gluud C. Methylphenidate for attention-deficit/hyperactivity disorder in children and adolescents. JAMA 2016;315(18):2009-10. [DOI: 10.1001/jama.2016.3611] [PMID: ] [DOI] [PubMed] [Google Scholar]


