Abstract
Nicosulfuron, one of the most widely used selective herbicides in corn field, can effectively control annual and perennial grass weeds, sedges, and some broadleaf weeds. The residual phytotoxicity of nicosulfuron in soil and water has become increasingly prominent. Therefore, an efficient method for detection of nicosulfuron was critical to ensure the sustainable and healthy development of agriculture and the ecological environment. In this paper, five nicosulfuron haptens which contained carboxyl group or aldehyde groups were designed and synthesized, and an indirect competitive immunoassay was developed for the first time. The assay showed an IC50 of 8.42 ng/mL and had negligible cross reactivities toward other sulfonylurea herbicides. In the spike and recovery studies, the recovery rate from soil samples was 95%–104%, and that of wheat roots was 92%–98%, which showed a good correlation with LC-MS analysis for nicosulfuron. The immunoassay was then used to quantify nicosulfuron concentration which could cause the obvious phytotoxic symptoms to wheat. Obvious symptoms of nicosulfuron phytotoxicity in wheat root was observed at the concentration of 0.068±0.006 mg/kg (ELISA result) which was consistent with 0.072±0.007 mg/kg obtained by LC-MS. The developed immunoassay method is an effective tool for environment contamination monitoring.
Keywords: nicosulfuron, hapten synthesis, polyclonal antibody, environment contamination monitoring
1. Introduction
Sulfonylurea herbicides are a class of highly efficient herbicides developed and used since 1970s. They stop the growth of sensitive plants by inhibiting the activity of acetolactate synthase in plants (Brown, 2010; Chaleff and Mauvais, 1984). Now, sulfonylurea herbicides are widely used in the world to control weeds in corn, wheat, rice, and other agricultural fields. Of course, the exceptional high potency of this compound class cautions that care must be taken to avoid contamination of the environment and other crops. Nicosulfuron (1-(4,6-dimethoxy-2-pyrimidinyl)-3-[3-(dimethylcarbamoyl)-2-pyridylsulfonyl]urea), a representative compound among the sulfonylurea herbicide, is mainly used for the control of annual and perennial grass weeds, sedges and some broadleaf weeds in corn fields. The herbicide is not safe to some corn varieties. For example, sweet corn and popcorn were sensitive to nicosulfuron (Wang et al., 2021). In addition, corn is sensitive to nicosulfuron before the 2-leaf stage and after the 10-leaf stage. Therefore, it is generally used at the 3–5 leaf stage of maize seedlings. With the continuous use of nicosulfuron, its residues could be found in soils and that could affect soil enzyme activities and microbial communities (Pap et al., 2022). Additionally, residues of nicosulfuron in the soil might cause residual toxicity to rotational crops, such as wheat. In addition, nicosulfuron was considered relatively mobile in soil, and could be transferred to various environmental water bodies by leaching and runoff, causing damage to some aquatic organisms (Seguin et al., 2001). Thus, as mentioned above it is important to develop an efficient and sensitive analytical method for environmental monitoring which can aid in proper use of this effective and highly potent herbicide.
Currently, the detection and analysis of sulfonylurea herbicides or nicosulfuron (Table 1) are mainly done by using high-performance liquid chromatography/electrospray ionization-mass spectrometry (Furlong et al., 2000), nano-liquid chromatography (Serra-Mora et al., 2020), high-performance liquid chromatography with UV detection (Tian et al., 2022), magnetic solid-phase extraction technique coupled with ultra-high-performance liquid chromatography (Wang et al., 2019) and other instrumental techniques. The aforementioned methods bear drawbacks such as need for expensive equipment and professionally trained operators.
Table 1.
Some reported assays for the dertermination of nicosulfuron.
| number | detection methods | IC50 | LOD or LOQ | samples | ref |
|---|---|---|---|---|---|
|
| |||||
| 1 | HPLC-UV | – | 14.5 μg/L | water | (Polati et al., 2006) |
| 2 | HPLC-MSn | – | 6.9 μg/L | water | (Polati et al., 2006) |
| 3 | Molecular imprinting | – | 0.45 μg/L | water | (Ren and Chen, 2015) |
| 4 | Fluorescence sensor | – | 2.99 μg/L | water | (Liu et al., 2021) |
| 5 | Luminescent sensor | – | 1.52 mg/L | water | (Ma et al., 2022} |
In recent years, the enzyme-linked immunosorbent assays (ELISA) played an important role in detection of pesticides and other environmental pollutants (Li et al., 2020; Qiao and Cai, 2021; Xu et al., 2022). In addition, the immunosensor based on the reaction of antigen-antibody showed many advantages in rapid detection of small molecular weight pollutants such as mycotoxins(Myndrul et al., 2017a; Myndrul et al., 2017b; Roman et al., 2018). The advantage of ELISA is that it often does not require complicated sample pretreatment, and it can rapidly and efficiently generate analytical data. An ELISA method has been developed for the detection of several sulfonylurea herbicides such as bensulfuron-methyl(Jae et al., 2002), chlorimuron-methyl(Sheedy and Hall, 2001), chlorsulfuron(Eremin et al., 2002) and metsulfuron-methyl(Knopp et al., 1999). However, there is no immunoassay method for nicosulfuron at present. Antibody quality is a key factor affecting the sensitivity and specificity of ELISA, however choice of hapten is critical for obtaining high-quality antibodies(Chen et al., 2021; Huang et al., 2022; Li et al., 2017). Similarity to the structure of the target analyte, as well as heterogeneity of the immunizing and the coating antigens are key factors to be taken into consideration during hapten design. Generally, the structure of the immunizing hapten should be more similar to the target analyte. So, the position and nature of the spacer arm are two important factors that influence hapten performance(Cevallos-Cedeño et al., 2018). Also, many studies have shown that use of the heterologous hapten for immunizing and coating can significantly improve the sensitivity and specificity of an immunoassay (Ding et al., 2017; Natalia et al., 2015).
In this study, design and synthesis of five nicosulfuron haptens are reported. One polyclonal antibody was produced and a quantitative indirect competitive immunoassay selective to nicosulfuron was developed for the first time. The performance of the optimized ELISA was evaluated on spiked soil and wheat root samples, and validated by LC-MS in a blind fashion. The ELISA developed here provides a sensitive and convenient method for environmental monitoring of nicosulfuron and can also aid in proper use of this herbicide.
2. Materials and methods
2.1. Synthesis of haptens and preparation of immunogens and coating antigens
All chemicals and reagents used for the synthesis of haptens were of analytical grade and used as received without further purification. The progress of the reactions was detected by analytical thin layer chromatography (TLC) performing on Merck TLC silica gel 60 F254 plates. Flash colum chromatography was performed on silica gel (100–200 mesh) from Qingdao Haiyang. NMR spectra were recorded on Bruker 400 MHz spectrometer (Brüker, Switzerland) in chloroform-d (CDCl3) or dimethyl sulfoxide-d6. HRMS spectra were recorded on Thermo Q Exactive Focus in ESI. The synthetic route toward haptens is shown in Scheme 1 and experimental details are listed in the supporting information.
Scheme 1.
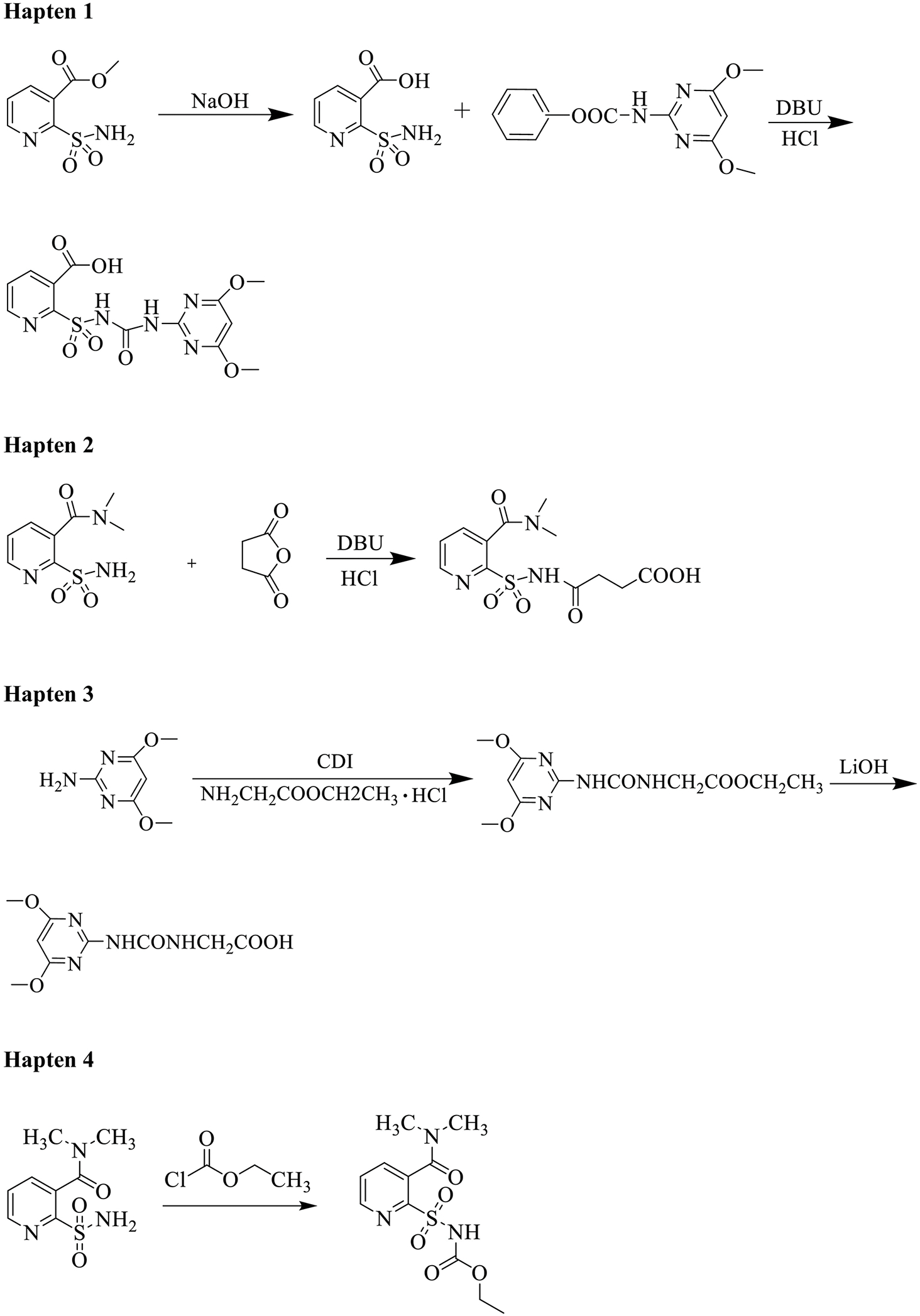
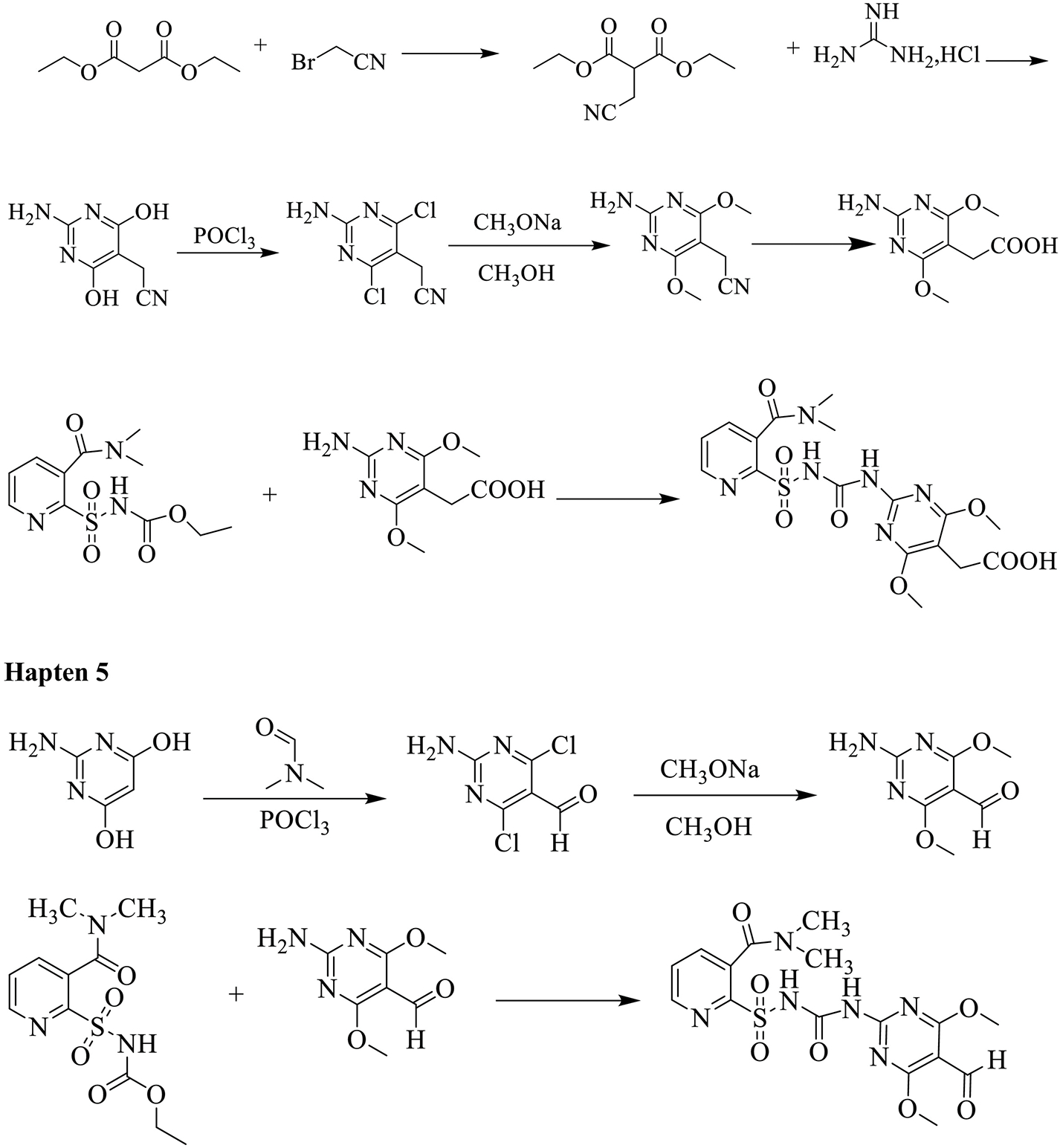
Synthetic route for nicosulfuron haptens.
Haptens (haptens 1–4) with a reactive carboxylic acid group (-COOH) were conjugated to proteins by a sulfo-N-hydroxysuccinimide (NHS) method and hapten 5 with an aldehyde group (-CHO) was conjugated by the schiff base formation method (Scheme 2). Hapten 4 and Hapten 5 were conjugated to thyroglobulin (Thy) for immunogen preparation. Haptens 1–5 were conjugated to bovine serum albumin (BSA) for coating antigen screening.
Scheme 2.
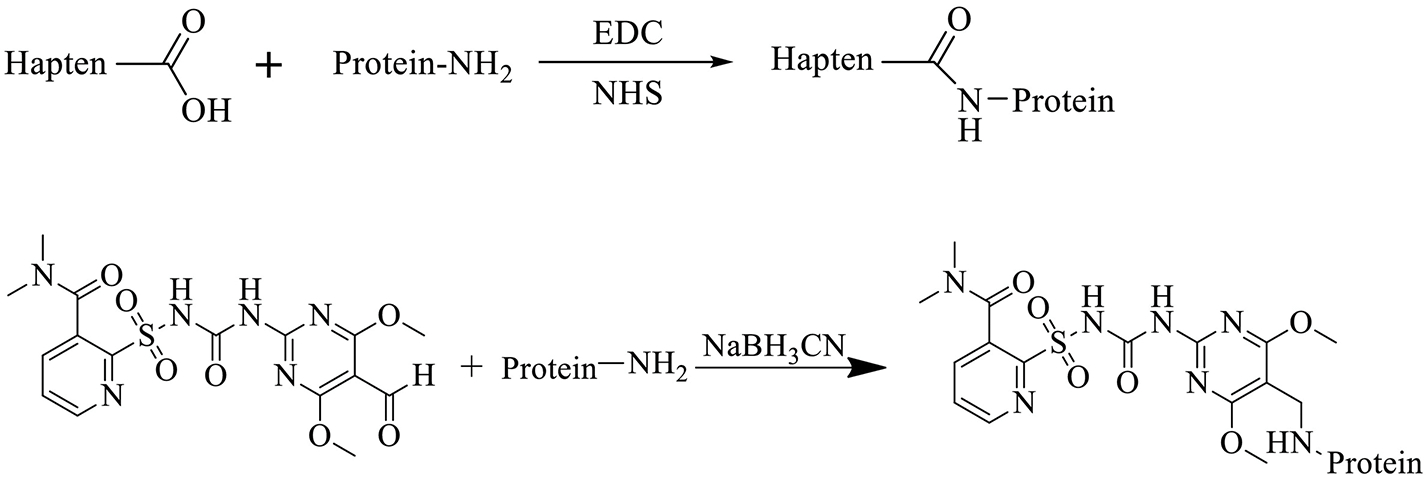
Preparation of hapten-protein conjugates based on the sulfo-N-hydroxysuccinimide method and schiff base formation method. Protein = BSA and Thy.
Sulfo-N-hydroxysuccinimide method
Haptens 1–4 were coupled covalently with available amines of the carrier protein (BSA or Thy) using a sulfo-N-hydroxysuccinimide method(Natalia et al., 2015). Briefly, each hapten (0.06 mmol) was dissolved in 3 mL of dry dimethylformamide (DMF) with NHS (0.072 mmol) and 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC, 0.07 mmol) and the mixture was stirred overnight at room temperature. The active ester was added dropwise to the solution of protein (25 mg) in 6 mL borate buffer (0.05 M, pH=8.0) with vigorous stirring over 30 min at room temperature. The reaction was continued at 4°C for 3 h by slow stirring to complete the conjugation, and then the resulting conjugates were dialyzed in PBS over 72 h at 4 °C, and stored at −20 °C until further use.
Schiff base formation method
Hapten 5 was coupled to carrier proteins (BSA or Thy) using Schiff base formation as previously described(Huo et al., 2019). Briefly, hapten 5 (0.05 mmol) dissolved in 100 μL DMSO was added slowly to the solution of carrier protein (BSA or Thy; 50 mg) dissolved in 10 mL of carbonate buffer (pH=9) with gentle stirring. The mixture was stirred for 1 h at room temperature followed by addition of 100 μL of 5 M cyanoborohydride in 1 N NaOH. The reaction mixture was allowed to react for 3 h at room temperature. The resulting conjugates were dialyzed in PBS over 72 h at 4 °C, and stored at −20 °C until further use.
2.2. Immunization and antiserum preparation for nicosulfuron
Hapten 4-Thy and hapten 5-Thy were used to produce the polyclonal antibodies. The antisera #101 and #102 were obtained from the rabbits immunized by hapten 5-Thy, while antisera #103 and #104 were obtained from the rabbits immunized by hapten 4-Thy. In brief, two New Zealand white rabbits were immunized with each of the immunogens emulsified with complete Freund’s adjuvant. The rabbits received booster injections with 2 weeks intervals, and were bled 7 days after the forth booster injection. The serum was collected by centrifugation, stored at −20°C, and used without purification for development of the indirect competitive ELISA.
2.3. Indirect competitive inhibition ELISA
The hapten-protein conjugates using the BSA as carrier protein were screened as potential coating antigens for each serum. In further assay development, a checkerboard procedure was used to determine the optimal dilution of the selected coating antigen and antibody.
The procedure for the ELISAs was as follows: A microtiter plate was coated with the optimal concentration of coating antigen diluted in coating buffer (100 μL/well) overnight at 4°C and then the solution was replaced with blocking buffer (3% BSA in 1×PBS, 200 μL/well) for 1 h at room temperature. The plate was washed three times with PBST (0.05% Tween-20 in 1×PBS), and then 50 μL of nicosulfuron standard (or sample) solutions and an equal volume of anti-nicosulfuron antiserum diluted in 1×PBS were added. The plate was incubated for 1 h at room temperature and then washed five times with PBST. Goat anti-rabbit IgG-horseradish peroxidase was added (1/10000 dilution; 100 μL/well) and the plate was incubated for 1 h at room temperature. After washing five times with PBST, 3,3’, 5,5’-tetramethylbenzidine (TMB) substrate solution was added (100 μL/well) and was left to develop color for about 15 min at room temperature. Finally, the reaction was stopped by addition of 2 M H2SO4 (50 μL/well), and the absorbance at 450 nm was measured. OriginPro 8.1 (OriginLab, Northampton, MA) was used for processing of the analytical data.
2.4. Cross-reactivity (CR)
The selectivity of antiserum obtained from rabbits #103 was evaluated by analyzing standard solutions of nicosulfuron 1, and its structurally similar compounds, tribenuron-methyl, 2; bensulfuron-menthyl, 3; sulfometuron-methyl, 4; mesosulfuron-methyl, 5; pyrazosulfuron-ethyl, 6; cinosulfuron, 7; chlorsulfuron, 8; rimsulfuron, 9; chlorimuron-ethyl, 10; thifensulfuron-methyl, 11 and metsulfuron-methyl, 12 (Figure 1). The CR was calculated as (IC50 of nicosulfuron /IC50 of tested compound) ×100.
Figure 1.
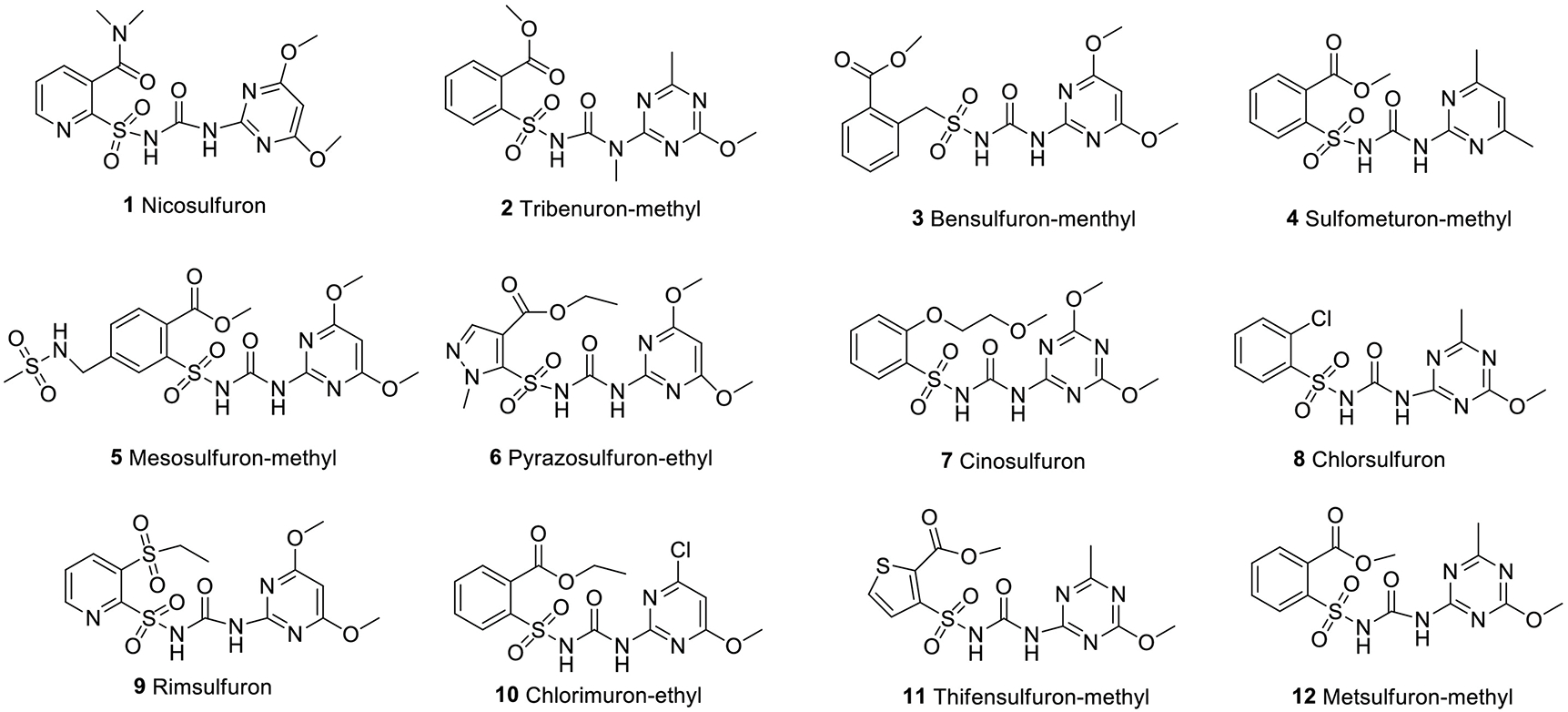
Structures of nicosulfuron 1, and structurally related compounds 2–12 tested for cross-reactivity.
2.5. Analysis of spiked samples
To evaluate the performance of the developed immunoassays, spike and recovery studies were performed using wheat root and soil. Blank samples were proved to be free of nicosulfuron by LC-MS.
Root samples of the wheat grown under greenhouse conditions for 1 week were collected, washed with clean water, dried with absorbent paper, and then the roots were frozen with liquid nitrogen, grinded and fortified with nicosulfuron (1 mg/mL in acetonitrile) to final concentrations of 0.16, 0.76, 3.6 mg/kg of root sample. Soil samples were fortified with nicosulfuron (1 mg/mL in acetonitrile) to final concentrations of 0.16, 0.76, 3.6 mg/kg. The wheat roots and soil fortified samples (1.0 g) were extracted using 2.5 mL of 25 mM PBS containing 25% acetonitrile. After vortexing, the mixtures were centrifuged at 4000 rpm for 15 min, the supernatants were collected and diluted appropriately with 25 mM PBS. All the spiked samples were filtered through a 0.22 μm filter and before analysis by immunoassay and LC-MS.
For the LC-MS procedure, samples (2 μL) were injected on an AB5500 liquid chromatography system and the separation was carried out on a Bonshell C18 column (50 mm×2.1 mm, 2.7 μm) at 35°C. Water containing 0.1% (v:v) formic acid (solution A) and acetonitrile (solution B) were used as the mobile phase with a flow rate of 0.3 mL/min and the gradient was given in Table S-1. The LC system was connected to a 4000 Qtrap mass spectrometeroperated in a negative ESI mode and multiple reaction monitoring mode. The optimized ion source parameters and MRM method are shown in Table S-2 and S-3, respectively.
2.6. Evaluation of the nicosulfuron phytotoxicity to wheat and determination of its concentration
The soil samples were sprayed with different amounts of nicosulfuron and after thorough mixing, the final concentrations of nicosulfuron in the soil were 0.0125 mg/kg, 0.025 mg/kg, 0.05 mg/kg, 0.1 mg/kg, 0.2 mg/kg, 0.4 mg/kg, 0.8 mg/kg, respectively. The above-treated soil samples were used to cultivate wheat under greenhouse conditions. The soil sample without nicosulfuron was used as blank control. Each treatment was repeated three times. After one week of cultivation, when the wheat in the treatment group showed obvious symptoms of phytotoxicity compared to the control group, the soil and wheat root samples were collected to quantify residual nicosulfuron by the ELISA and LC-MS at the same time. The extraction and analysis followed the same procedures as the spiked samples.
3. Results and discussion
3.1. Design and synthesis of haptens
Nicosulfuron is a small molecule herbicide with molecular weight of less than 1000 Da, and as such it must be conjugated to a large carrier protein to elicit an immune response. It is necessary to modify or redesign the structure of the pesticide molecule to synthesize the corresponding hapten which is critical to the sensitivity and selectivity of the ELISA method. Since sulfonylurea herbicides are relatively similar in structure, if we want to obtain a highly selective antibody against nicosulfuron, it is important to preserve the unique dimethylcarbamoyl functional group which can distinguish nicosulfuron from other sulfonylurea herbicides. Based on this consideration, hapten 4 and hapten 5 to be used for immunizing haptens were designed and synthesized. Additionally, hapten 1, hapten 2 and hapten 3 used for coating antigen were also designed and synthesized.
The design principle for hapten 4 and hapten 5 was to retain the overall structural characteristics of nicosulfuron, but with an attachment of an aldehyde or carboxyl group as distal as possible from the key dimethylcarbamoyl functional group. Hapten 1, hapten 2 and hapten 3 were mainly used for preparation of coating antigens and all had carboxyl group for coupling to the protein through an amide bond. In this study, we applied two methods, EDC/sulfo-N-hydroxysuccinimide and Schiff base formation, for the conjugation of hapten with carrier protein. EDC/Sulfo-N-hydroxysuccinimide is a common method to combine the carboxyl group of hapten with carrier protein. 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC)/Sulfo-N-hydroxysuccinimide reacts with carboxyl group to form an intermediate activated ester, which then reacts with the amino group on protein to form the conjugate. It is important to not use the same method of amide bond formation with the immunizing hapten without separation of the active intermediate. One can in fact raise antibodies to a urea formed by reaction of the EDC with a carrier protein. This is easily avoided but not using the same coupling method for coating and immunizing antigens or by using a two step coupling process. Schiff base formation can be easily performed by reacting aldehyde group of hapten with the amino group of the protein, then the labile Schiff base intermediate is reduced into a stable secondary amine in the presence of sodium cyanoborohydride.
3.2. Screening of the sera and coating antigen combinations
The antisera #101 and #102 were obtained from the rabbits immunized by hapten 5-Thy, while antisera #103 and #104 were obtained from the rabbits immunized by hapten 4-Thy. All four sera were screened against five coating antigens using checkerboard titration. The results showed, as expected that (Table 2)the four sera had a better response in terms of sensitivity against their corresponding homologous coating antigen (coating antigen 4 and coating antigen 5) compared the heterologous coating antigens (coating antigen 1–3). Sera #102, #103 and #104 also had high to moderate binding to heterologous antigens (coating antigen 2 and coating antigen 3). There is well known that use of heterologous coating antigens could improve the ELISA sensitivity. Therefore, we focused on determining the sensitivity of several heterologous pairs, and expectedly, the sensitivity of the heterologous pairs was significantly higher than those of the homologous pairs. For example, in the homologous competition assay for serum #103, the IC50 value was 326 ng/mL (coating antigen 4), while in the heterologous assay (coating antigen 2) the IC50 was 12.3 ng/mL.
Table 2.
Antiserum titer response against various coating antigens.
| Coat antigen (Dilution 1000-fold) | # 101 |
# 102 |
# 103 |
# 104 |
||||
|---|---|---|---|---|---|---|---|---|
| Dilution fold |
Dilution fold |
Dilution fold |
Dilution fold |
|||||
| 1000 | 10,000 | 1000 | 10,000 | 1000 | 10,000 | 1000 | 10,000 | |
|
| ||||||||
| Coating antigen-1 | − | − | − | − | − | − | − | − |
| Coating antigen-2 | − | − | +++ | ++ | +++ | + | +++ | − |
| Coating antigen-3 | − | − | ++ | + | ++ | + | ++ | + |
| Coating antigen-4 | +++ | − | ++ | − | +++ | +++ | +++ | + |
| Coating antigen-5 | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ |
Note: −, absorbance <0.3; +, absorbance 0.3–0.6: ++, absorbance 0.6–0.9; and +++, absorbance >0.9.
After screening, serum #103 from the rabbit immunized by hapten 4-Thy was selected to develop a competitive assay in heterologous format with the hapten 2-BSA as coating antigen.
3.3. Parameter optimization to improve sensitivity of nicosulfuron detection
Coating antigen and antibody concentrations as well as other assay parameters such as ionic strength and pH are often optimized to improve immunoreactions between coating antigen and antibody and increase immunoassay sensitivity. The combination of serum #103 (diluted 2000-fold) and coating antigen 2 (diluted 2000-fold) showed the lowest IC50. The ELISA showed the highest Amax/IC50 and lower IC50 at pH 7.4 and 25 mM PBS buffer (Fig. S2 and Fig. S3). Organic solvents play an important role in the sample pretreatment and preparation of standard solutions of analyte, but high concentrations of organic solvents generally alter the activity of antibodies. Therefore, it is necessary to evaluate the effect of organic solvents on immunoreactions. Acetonitrile and DMSO were selected due to solubility considerations. The results showed that DMSO had a more pronounced effect on immunoassay performance compared to acetonitrile. Negligible effect on ELISA was observed at the acetonitrile concentration of 2.5% (Fig. S4 and Fig. S5). Using optimized parameters, the ELISA standard curve was obtained by plotting the mean values of OD450 versus the concentration of nicosulfuron using OriginPro 8.1 (OriginLab, Northampton, MA) (Fig. 2). The IC50 value, LOD and linear range (IC10 to IC90) were 8.42, 0.11 and 0.11 to 432.89 ng/mL respectively.
Figure 2.
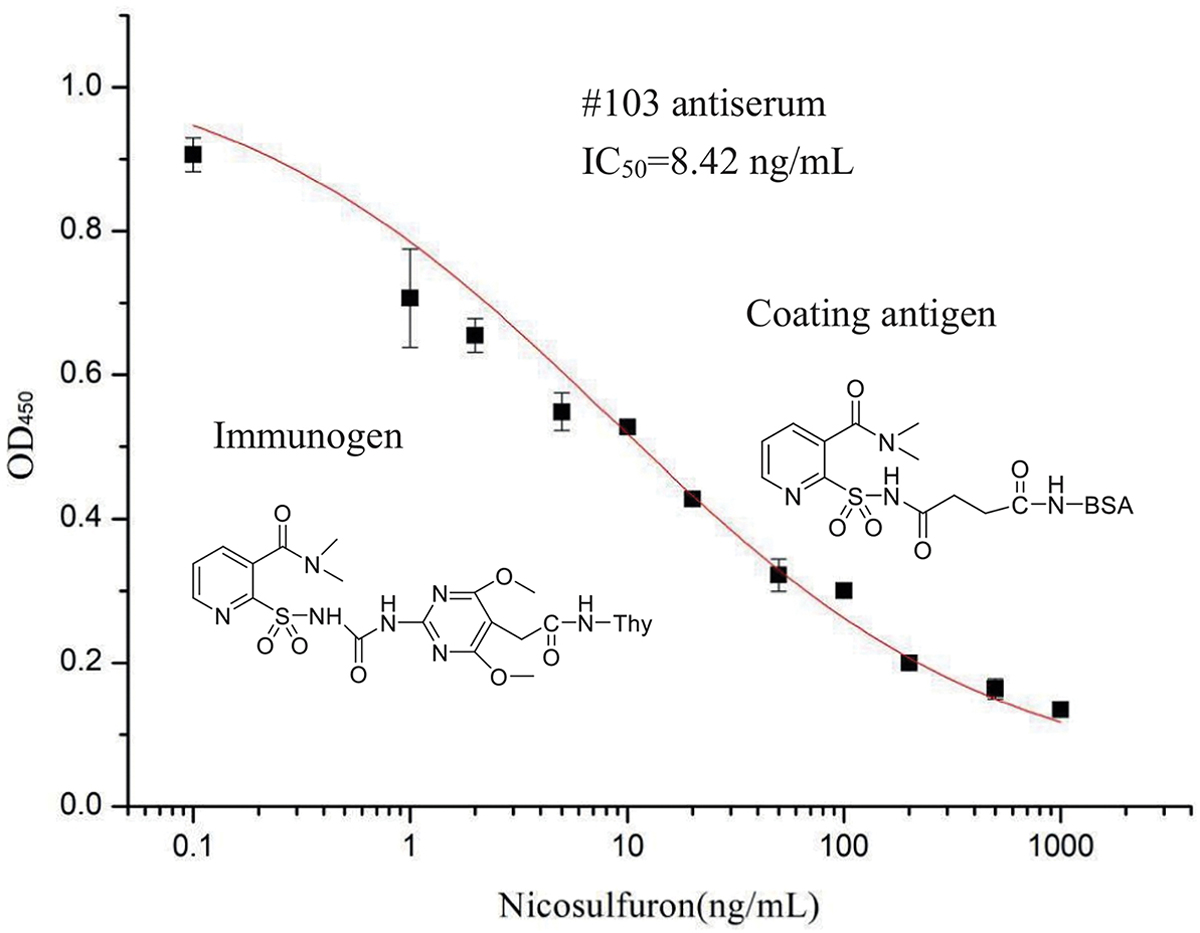
Standard curves for nicosulfuron by ELISA. Serial dilutions of nicosulfuron standard were mixed with antiserum #103 in optimized buffer. Then 100 μL of the mixtures were added to the coating antigen 2-coated wells. Each point represents the mean value of three replicates.
3.4. Selectivity of the developed immunoassay for nicosulfuron
In actual detection, the immunoassays often have cross reactions with analogs of the target compound. Therefore, hapten 4 and hapten 5, mimicking the structure of nicosulfuron to the greatest extent and using of heterologous coating antigens were performed with the ultimate goal to obtain an antibody with excellent selectivity and sensitivity. The antibody #103/coating antigen 2 combination was further tested for cross-reactivity with 11 nicosulfuron analogs. As shown in Table 3, the antibody had excellent selectivity toward nicosulfuron, since negligible cross reactivities were observed to all the other 11 analogs. Surprisingly, negligible cross reactivity in the developed ELISA was observed for nicosulfuron analogs like bensulfuron-methyl, rimsulfuron and chlorimuron-ethyl, even though they all have similar structure to nicosulfuron. The structure of the hapten 5 used for immunization was essentially the same as that of nicosulfuron, except an added aldehyde group used for coupling to the carrier protein. It is speculated that the obtained resulting antibody could recognize unique structural features of nicosulfuron such as dimethylcarbamoyl, key function distinguishing nicosulfuron from its analogs.
Table 3.
Cross-reactivity of the antiserum against nicosulfuron structural analogs. Antiserum #103/coating antigen 2 was used to evaluate the cross-reactivity.
| Compound | Cross reactivity (%) | Compound | Cross reactivity (%) |
|---|---|---|---|
|
| |||
| Nicosulfuron | 100 | Cinosulfuron | <0.1 |
| Tribenuron-methyl | <0.1 | Chlorsulfuron | <0.1 |
| Bensulfuron-menthl | <0.1 | Rimsulfuron | <0.1 |
| Sulfometuron-methl | <0.1 | Chlorimuron-ethyl | <0.1 |
| Mesosulfuron-methl | <0.1 | Thifensulfuron-methl | <0.1 |
| Pyrazosulfuron-ethyl | <0.1 | Metesulfuron-methyl | <0.1 |
3.5. Analysis of spiked samples and validation with LC-MS
Matrix effect can significantly interfere with performance of the immunoassay, and affect the accuracy of the analytical results. Therefore, researchers try to reduce matrix effect in immunoassay through various methods. Dilution of the sample is the easiest and common method to minimize or eliminate matrix interferences. In this study, the effects of residual nicosulfuron in soil on the growth of sensitive crop wheat were determined. Therefore wheat root and soil were selected for the matrix effect evaluation. Samples for evaluation of matrix effects were confirmed to be free of nicosulfuron by LC-MS (LOQ=0.2 ng/mL, LOD=0.005 ng/mL) analysis. The matrix interferences were tested at 5-fold, 10-fold, 20-fold, 40-fold, and 80-fold dilutions (the total dilution was 10-, 20-, 40-, 80-, and 160-fold, after mixing with antibody). The results (Figure 3) show that assays performed with 80-fold diluted soil and wheat root matrixes, had lower IC50 and a higher Amax/IC50 compared to the assay buffer. Also, the standard inhibition curve obtained for 80-fold diluted sample matrix was similar to that obtained with the assay buffer. Thus, an 80-fold dilution of soil and wheat root samples minimized matrix interferences in the ELISA, and was chosen for the developed assay.
Figure 3.
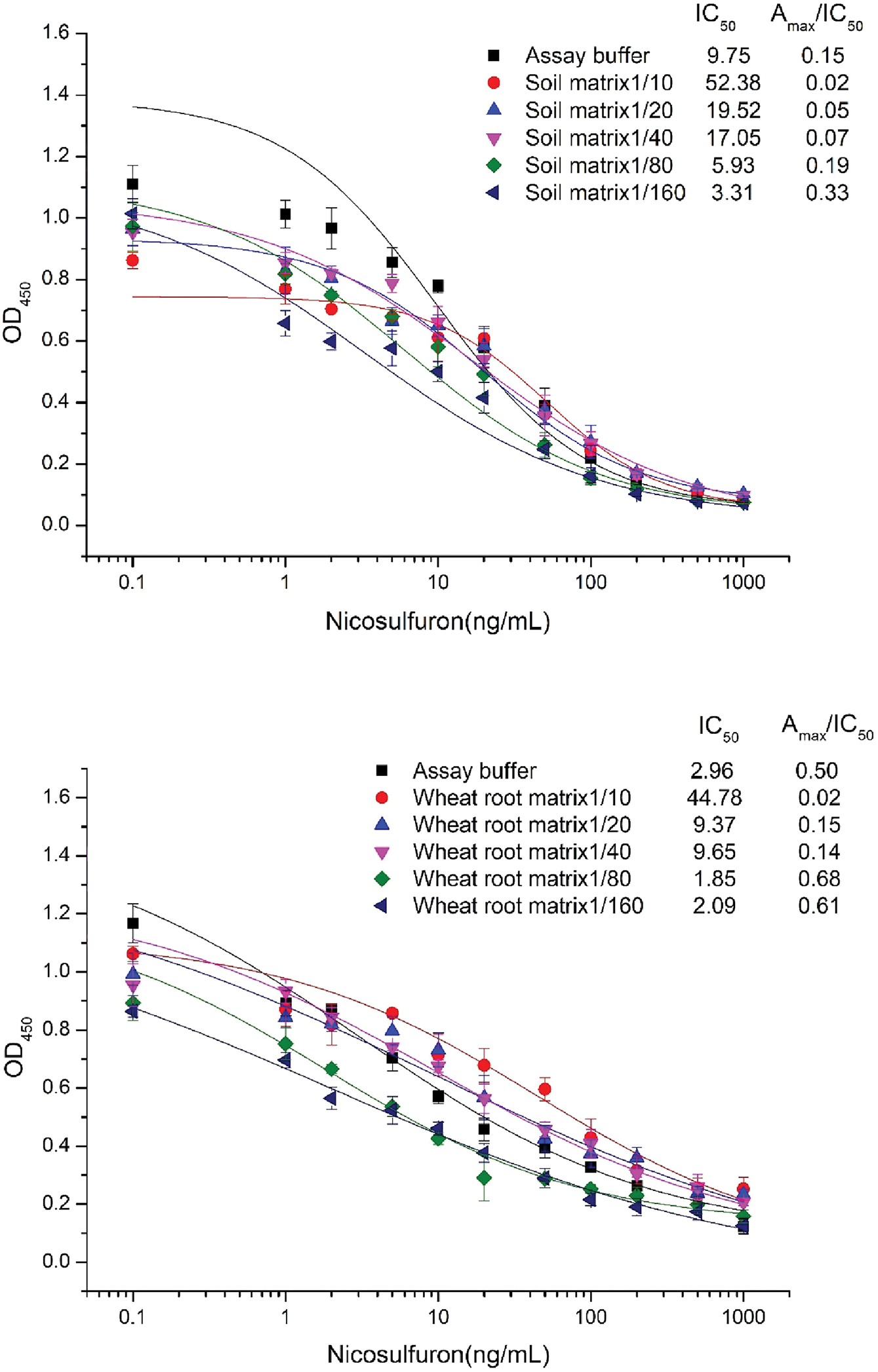
The effect of wheat root and soil matrix on the performance of antiserum #103 based ELISA.
To validate the reliability of the ELISA developed here, the method was applied to determine nicosulfuron in the spiked wheat root and soil samples which were confirmed to be nicosulfuron-free by LC-MS. The wheat root and soil spiked with three different concentrations of nicosulfuron (0.16, 0.76, 3.6 mg/kg) were analyzed by ELISA. Average recoveries for the wheat root ranged from 92 to 98%, while, for the soil, the average recovery ranged from 95 to 104%. All the spiked samples of wheat root and soil were analyzed by LC-MS to evaluate the accuracy and precision of the developed ELISA method. Recovery results for nicosulfuron in spiked samples were consistent between ELISA and LC-MS (Table 4).
Table 4.
Spike-recovery results for wheat root and soil samples determined by ELISA and LC-MS.
| Sample | Spiked concentration, dilution 80-fold (ng/mL) | ELISA (ng/mL) | Average recovery (%) | CV (%) | LC-MS (ng/mL) | Average recovery (*) | CV (%) |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Wheat root | 2 | 1.99 ± 0.27 | 92 | 13.56 | 1.79 ± 0.076 | 79 | 4.25 |
| 9.5 | 9.72 ± 0.63 | 102 | 6.48 | 9.15 ± 0.24 | 86 | 2.62 | |
| 45 | 43.98 ± 3.46 | 98 | 7.86 | 47.45 ± 7.22 | 103 | 15.21 | |
| Soil | 2 | 1.89 ± 0.105 | 95 | 5.55 | 1.87 ± 0.10 | 94 | 5.34 |
| 9.5 | 9.54 ± 0.337 | 101 | 3.53 | 9.43 ± 0.34 | 99 | 3.60 | |
| 45 | 46.81 ± 0.086 | 104 | 1.83 | 42.85 ± 1.41 | 97 | 3.29 | |
3.6. Nicosulfuron phytotoxicity on wheat and determination of its concentration
It had been reported previously that 0.5 mg/kg nicosulfuron in soil could cause phytotoxicity to wheat (Zhang et al., 2020). In this study, the growth status of wheat planted in the soil with different concentrations of nicosulfuron was determined. The final concentrations of nicosulfuron in the soil were 0.0125 mg/kg, 0.025 mg/kg, 0.05 mg/kg, 0.1 mg/kg, 0.2 mg/kg, 0.4 mg/kg and 0.8 mg/kg, respectively. One week after sowing the wheat, its visual symptoms of phytotoxicity were observed, and the fresh weight of the aboveground and underground parts were measured. At nicosulfuron concentration of 0.1 mg/kg, wheat showed obvious symptoms of phytotoxicity, and the root length and shoot growth were significantly inhibited compared to control (Figure 4). The results of fresh weight determination were consistent with visual symptoms. At nicosulfuron concentration of 0.1 mg/kg or above, the fresh weight of the aboveground and underground parts of wheat was significantly different from the control (Figure 4).
Figure 4.
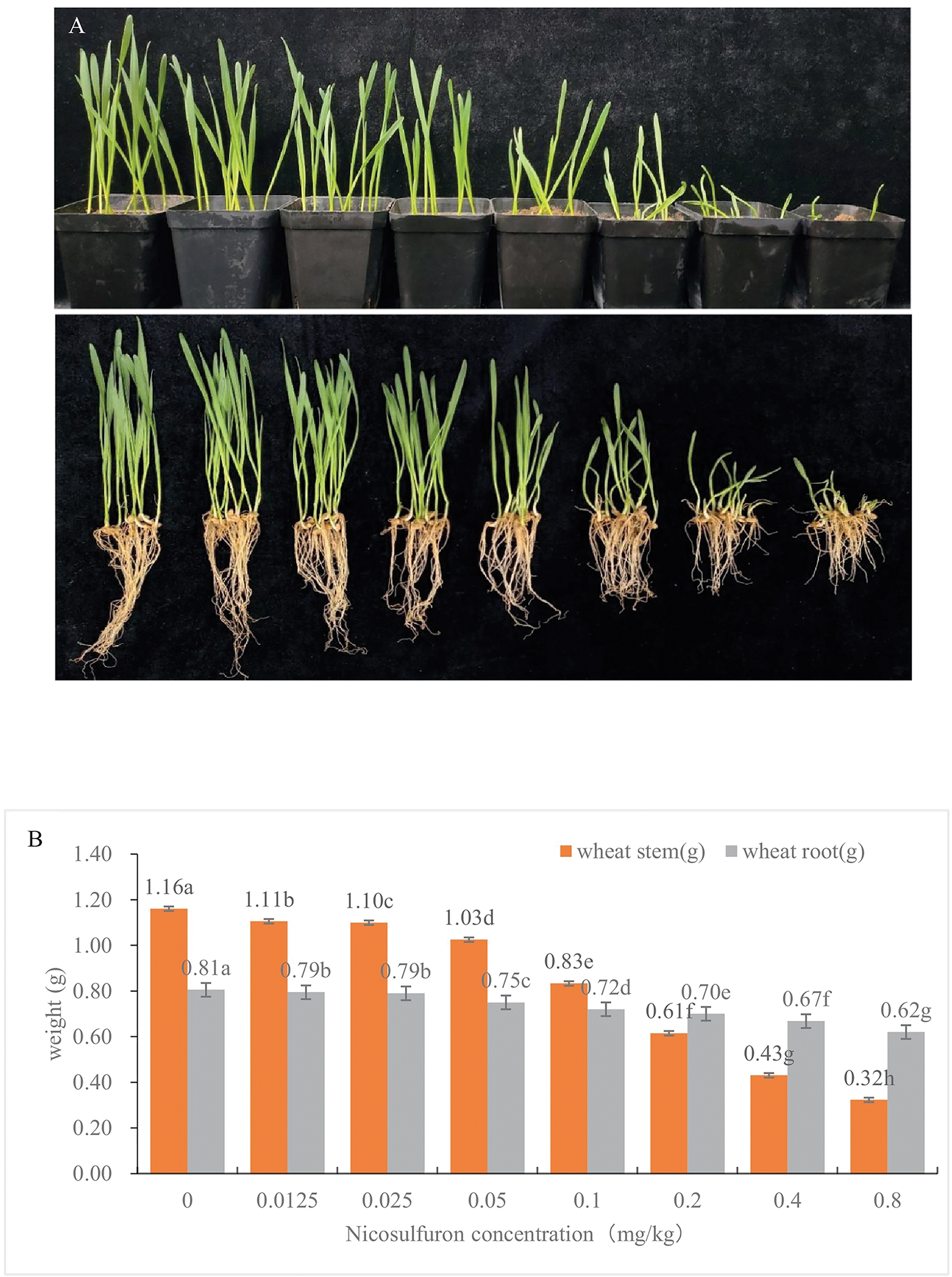
The visual symptoms (A) and fresh weight of stem and root (B) of wheat planted in the soil with different concertation of nicosulfuron. The concentrations of nicosulfuron in the soil from left to right are 0 mg/kg, 0.0125 mg/kg, 0.025 mg/kg, 0.05 mg/kg, 0.1 mg/kg, 0.2 mg/kg, 0.4 mg/kg and 0.8 mg/kg, respectively.
Next, nicosulfuron residues in seven soil samples and wheat root samples were quantified by ELISA and LC-MS, and the results (Table 5) from both methods showed a good correlation. For example, when the final concentration of nicosulfuron applied in the soil was 0.1 mg/kg, the actual concentrations of nicosulfuron in soil samples and wheat root samples detected by ELISA were 0.101±0.011 mg/kg and 0.068±0.006 mg/kg, respectively, while LC-MS detected 0.087±0.009 mg/kg and 0.072±0.007 mg/kg nicosulfuron respectively. The above results further verified the sensitivity and accuracy of the ELISA method developed in this study.
Table 5.
Quantification of nicosulfuron in the wheat root and soil samples determined by ELI5A and LC-MS.
| Sample | # | Nicosulfuron concentration (mg/kg) | Collection time, days after treatment | ELISA (mg/kg) | CV (%) | LC-MS (mg/kg) | CV (%) |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Soil | 1 | 0.0125 | 7 | 0.012 ± 0.001 | 8.33 | 0.011 ± 0.001 | 7.69 |
| 2 | 0.025 | 7 | 0.020 ± 0.002 | 10.00 | 0.019 ± 0.003 | 15.78 | |
| 3 | 0.05 | 7 | 0.041 ± 0.004 | 9.76 | 0.044 ± 0.002 | 4.54 | |
| 4 | 0.1 | 7 | 0.101 ± 0.011 | 10.89 | 0.087 ± 0.009 | 10.34 | |
| 5 | 0.2 | 7 | 0.196 ± 0.012 | 6.12 | 0.188 ± 0.014 | 7.45 | |
| 6 | 0.4 | 7 | 0.407 ± 0.025 | 6.14 | 0.366 ± 0.012 | 3.27 | |
| 7 | 0.8 | 7 | 0.652 ± 0.058 | 8.90 | 0.690 ± 0.043 | 6.23 | |
| Wheat root | 1 | 0.0125 | 7 | 0.012 ± 0.001 | 8.33 | 0.013 ± 0.001 | 7.69 |
| 2 | 0.025 | 7 | 0.020 ± 0.002 | 10.00 | 0.020 ± 0.001 | 5.00 | |
| 3 | 0.05 | 7 | 0.041 ± 0.002 | 4.88 | 0.039 ± 0.004 | 10.26 | |
| 4 | 0.1 | 7 | 0.068 ± 0.006 | 8.82 | 0.072 ± 0.007 | 9.72 | |
| 5 | 0.2 | 7 | 0.183 ± 0.015 | 8.20 | 0.175 ± 0.019 | 10.86 | |
| 6 | 0.4 | 7 | 0.377 ± 0.011 | 2.91 | 0.348 ± 0.012 | 3.45 | |
| 7 | 0.8 | 7 | 0.511 ± 0.044 | 8.61 | 0.512 ± 0.036 | 7.03 | |
4. Conclusion
The use of ultra-efficient herbicides plays an important role in reducing the number of pesticides used and protecting the environment. Nicosulfuron remains the main herbicide used in corn fields in the world. The development of efficient and rapid detection methods is of great significance to guide the proper use of nicosulfuron. In the study, five nicosulfuron haptens were designed and synthesized based on the principles of hapten design. After immunization of rabbits, obtained serum #103 exhibited excellent selectivity for nicosulfuron, with no cross-reactivity to nicosulfuron analogs. Finally, a sensitive and selective ELISA based on the antiserum #103 and coating antigen 2 was successfully developed. Developed immunoassay had excellent sensitivity with IC50 of 8.42 ng/mL and accuracy for the detection of nicosulfuron contamination in the environmental soil samples. Also, the detection method and the phenotype of sensitive wheat were combined to verify that low concentrations of nicosulfuron could still cause phytotoxicity to wheat. Overall, we obtain a high selective polyclonal antibody, which is an efficient way to develop the immunoassay for monitoring the proper use of nicosulfuron and its presence in the environment.
Supplementary Material
Acknowledgment
This work was supported by the National Natural Science Foundation of China (32102246, 32001927 and 31871981), the Natural Science Foundation of Hebei Province (C2020204116) and the Starting Scientific Research Foundation for the Introduced Talents of Hebei Agricultural University (YJ201963). Partial support was orivuded by NIH-NIEHS (RIVER Award) R35 ES030443-01 and NIH-NIEHS (Superfund Award) P42 ES004699. We thank animal experiment center of Jiangsu University for the rabbit immunizations services.
Footnotes
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
CRediT authorship contribution statement
Qing-Qing Gao, Jing-Qian Huo: Methodology, writing-original draft preparation. Lai Chen, Xiao-Tong Xu, Wei-Hong Zhang: Sample preparation and data analysis. Qing-Qing Gao, Dong-Chen Yang, Xiao-Tong Xu, Bin Jia: Assay development and Validation. Bogdan Barnych, Bruce D. Hammock: Reviewing and Editing. Jin-Lin Zhang, Jing-Qian Huo: Conceptualization and Supervision.
Supporting Information
Synthetic procedures for haptens; MS spectra for hapten 4 and hapten 5; Effects of ionic strength, pH, DMSO and acetonitrile on the performance of ELISA for nicosulfuron; LC gradient; Mass spectrometric source parameters; MRM method.
References
- Brown HM. Mode of action, crop selectivity, and soil relations of the sulfonylurea herbicides. Pest Management Science 2010; 29: 263–281. [Google Scholar]
- Cevallos-Cedeño R, Agulló C, Abad-Somovilla A, Abad-Fuentes A, Mercader JV. Hapten design and antibody generation for immunoanalysis of spirotetramat and spirotetramat-enol. ACS Omega 2018; 3: 11950–11957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaleff RS, Mauvais CJ. Acetolactate synthase is the site of action of two sulfonylurea herbicides in higher plants. Science 1984; 224: 1443–1445. [DOI] [PubMed] [Google Scholar]
- Chen ZJ, Wu HL, Xiao ZL, Fu HJ, Xu ZL. Rational hapten design to produce high-quality antibodies against carbamate pesticides and development of immunochromatographic assays for simultaneous pesticide screening. Journal of Hazardous Materials 2021; 412: 125241. [DOI] [PubMed] [Google Scholar]
- Ding Y, Hua X, Sun N, Yang J, Deng J, Shi H, et al. Development of a phage chemiluminescent enzyme immunoassay with high sensitivity for the determination of imidaclothiz in agricultural and environmental samples. Science of The Total Environment 2017; 609: 854–860. [DOI] [PubMed] [Google Scholar]
- Eremin SA, Ryabova IA, Yakovleva JN, Yazynina EV, Zherdev AV, Dzantiev BB. Development of a rapid, specific fluorescence polarization immunoassay for the herbicide chlorsulfuron. Analytica chimica acta 2002, 468: 229–236. [Google Scholar]
- Furlong ET, Burkhardt MR, Gates PM, Werner SL, Battaglin WA. Routine determination of sulfonylurea, imidazolinone, and sulfonamide herbicides at nanogram-per-liter concentrations by solid-phase extraction and liquid chromatography/mass spectrometry. Science of the Total Environment 2000; 248: 135–146. [DOI] [PubMed] [Google Scholar]
- Huang L, Chen H, Cui P, Ding Y, Wang M, Hua X. Development of immunoassay based on rational hapten design for sensitive detection of pendimethalin in environment. Science of The Total Environment 2022; 830: 154690–154697. [DOI] [PubMed] [Google Scholar]
- Huo J, Barnych B, Li Z, Wan D, Li D, Vasylieva N, et al. Hapten synthesis, antibody development, and a highly sensitive indirect competitive chemiluminescent enzyme immunoassay for detection of dicamba. Journal of Agricultural and Food Chemistry 2019; 67: 5711–5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jae Koo, Lee Ki, Chang Ahn, et al. Development of an immunoassay for the residues of the herbicide bensulfuron-methyl. Journal of Agricultural and Food Chemistry 2002; 50: 1791–1803. [DOI] [PubMed] [Google Scholar]
- Knopp A, Knopp D, Niessner R. ELISA determination of the sulfonylurea herbicide metsulfuron-methyl in different water types. Environmental Science & Technology 1999; 33: 358–361. [Google Scholar]
- Li P, Zhang W, Zhang Z, Zhang Q, Chen ZY. Fundamentals of hapten-protein conjugate synthesis to obtain high-quality antibodies for analysis of food and environmental contaminants. Current Organic Chemistry 2017; 21: 2606–2611. [Google Scholar]
- Li ZF, Dong JX, Vasylieva N, Cui YL, Hammock BD. Highly specific nanobody against herbicide 2,4-dichlorophenoxyacetic acid for monitoring of its contamination in environmental water. Science of The Total Environment 2020; 753: 141950–141959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myndrul V, Viter R, Savchuk M, Koval M, Starodub N, Silamielis V, et al. Gold coated porous silicon nanocomposite as a substrate for photoluminescence-based immunosensor suitable for the determination of Aflatoxin B1. Talanta 2017a; 175: 297–304. [DOI] [PubMed] [Google Scholar]
- Myndrul V, Viter R, Savchuk M, Shpyrka N, Erts D, Jevdokimovs D, et al. Porous silicon based photoluminescence immunosensor for rapid and highly-sensitive detection of Ochratoxin A. Biosensors & Bioelectronics 2017b; 102: 661–667. [DOI] [PubMed] [Google Scholar]
- Natalia Vasylieva, Ki Chang, Ahn Bogdan, et al. Development of an immunoassay for the detection of the phenylpyrazole insecticide fipronil. Environmental Science & Technology 2015; 49: 10038–10047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pap SM, Popovi B, Stoji N, Danojevi D, Pucarevi M, Ervenski J, et al. The environmental issue of pesticide residues in agricultural soils in Serbia. International Journal of Environmental Science and Technology 2022; 19: 1–14. [Google Scholar]
- Qiao Y, Cai Q. Gold nanoparticles-amplified indirect competitive enzyme-linked immunosorbent assay of BDE-121 using a monoclonal antibody. Journal of nanoscience and nanotechnology 2021; 21: 5036–5043. [DOI] [PubMed] [Google Scholar]
- Roman Viter, Maryna Savchuk, Igor Iatsunskyi, et al. Analytical, thermodynamical and kinetic characteristics of photoluminescence immunosensor for the determination of Ochratoxin A. Biosensors & Bioelectronics 2018; 99: 237–243. [DOI] [PubMed] [Google Scholar]
- Seguin F, Druart JC, Cohu RL. Effects of atrazine and nicosulfuron on periphytic diatom communities in freshwater outdoor lentic mesocosms. Annales de Limnologie - International Journal of Limnology 2001; 37: 3–8. [Google Scholar]
- Serra-Mora P, Herráez-Hernández R, Campíns-Falcó P. Minimizing the impact of sample preparation on analytical results: In-tube solid-phase microextraction coupled on-line to nano-liquid chromatography for the monitoring of tribenuron methyl in environmental waters. Science of The Total Environment 2020; 721: 137732–137740. [DOI] [PubMed] [Google Scholar]
- Sheedy C, Hall JC. Immunoaffinity purification of chlorimuron-ethyl from soil extracts prior to quantitation by enzyme-linked immunosorbent assay. Journal of Agricultural & Food Chemistry 2001; 49: 1151–7. [DOI] [PubMed] [Google Scholar]
- Tian C, Wu Z, He M, Chen B, Hu B. Amino functionalized magnetic covalent organic framework for magnetic solid-phase extraction of sulfonylurea herbicides in environmental samples from tobacco land. Journal of separation science. 2022; 45: 1746–1756. [DOI] [PubMed] [Google Scholar]
- Wang DD, Zhao Y, Yang M, Guo HM, Yang ZH. Magnetic polydopamine modified with deep eutectic solvent for the magnetic solid-phase extraction of sulfonylurea herbicides in water samples. Journal of Chromatography A 2019; 1601: 53–59. [DOI] [PubMed] [Google Scholar]
- Wang J, Gao H, Guo Z, Meng Y, Yang Q. Adaptation responses in C4 photosynthesis of sweet maize (Zea mays L.) exposed to nicosulfuron. Ecotoxicology and Environmental Safety 2021; 214: 112096–112107. [DOI] [PubMed] [Google Scholar]
- Xu L, Yang F, Dias A, Zhang X. Development of quantum dot-linked immunosorbent assay (QLISA) and ELISA for the detection of sunset yellow in foods and beverages. Food Chemistry 2022; 385: 132648–132654. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Yang D, Si H, Wang J, Zhang J. Biotransformation of the herbicide nicosulfuron residues in soil and seven sulfonylurea herbicides by Bacillus subtilis YB1: A climate chamber study. Environmental Pollution 2020; 263: 114492–114500. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


