Abstract
While almost half of all mammal species are rodents, records of albinism in free‐ranging rodents are very rare. Australia has a large and diverse assemblage of native rodent species, but there are no records of free‐ranging albino rodents in the published literature. In this study, we aim to improve our understanding of the occurrence of albinism in Australian rodent species by collating contemporary and historic records of this condition and providing an estimate of its frequency. We found 23 records of albinism (i.e., a complete loss of pigmentation), representing eight species, in free‐ranging rodents native to Australia, with the frequency of albinism being generally <0.1%. Our findings bring the total number of rodent species in which albinism has been recorded globally to 76. While native Australian species represent only 7.8% of the world's murid rodent diversity, they now account for 42.1% of murid rodent species known to exhibit albinism. We also identified multiple concurrent albino records from a small island population of rakali (Hydromys chrysogaster) and discuss the factors that may contribute to the relatively high frequency (2%) of the condition on this island. We suggest that the small number of native albino rodents recorded in mainland Australia over the last 100 years means that traits associated with the condition are likely deleterious within populations and are thus selected against.
Keywords: albino, mammal, mouse, Muridae, pelage, rat
While almost half of all mammal species are rodents, records of albinism in free‐ranging rodents are very rare, and there there are no records of albinism in native Australian rodents in the published literature. In this study, we aim to improve our understanding of the occurrence of albinism in Australian rodent species by collating contemporary and historic records of this condition and providing an estimate of its frequency. We found 23 records of albinism (representing eight species) in free‐ranging rodents native to Australia. The addition of these records represents a considerable (11.8%) increase in the number of rodent species, globally, with albinism recorded, and nearly doubles the number of murid rodent species from 11 to 19 (1.3%–2.2%).
1. INTRODUCTION
Mammals exhibit diverse fur and skin colouration patterns that have evolved for purposes such as camouflage, intra‐ and interspecific signaling, and thermoregulation (Caro & Mallarino, 2020). Hypopigmentary congenital disorders, such as albinism and leucism, cause atypical colouration in mammals due to a deficiency of melanin—the main pigment that determines the color of skin, fur, and eyes (Hiller, 1983). While there are several forms of albinism (McCardle, 2012), true or complete albinism is characterized by the total absence of melanin, and consequently, albino mammals typically exhibit white or off‐white/straw‐colored pelage, with pale skin and pink eyes (Guillery et al., 1999; Searle, 1968). Leucism is a partial or total melanin reduction in fur or skin pigmentation (Phillips & Wilson, 1965), but unlike true albinism, it rarely affects skin and does not affect the eyes (Miller, 2005; van Grouw, 2006). Albinism is rare and typically affects one in several thousand individuals (Fernández et al., 2021). It is generally (though not always, e.g., Sage, 1962) passed on via an autosomal recessive inheritance pattern (i.e., offspring must inherit one copy of the recessive gene from both parents), and an elevated frequency of albinism typically reflects low‐genetic diversity within a population (Bensch et al., 2000; Holyoak, 1978).
Records of true albinism in free‐ranging mammals are particularly uncommon (Acevedo et al., 2009; Dunlop et al., 2019; Romero et al., 2018; Zortéa & Silva, 2018), likely due to the decreased fitness associated with the condition. For example, albinism can contribute to reduced visual ability (Guillery et al., 1999), decreased sense of smell (Keeler, 1942), increased UV sensitivity (Polanowski et al., 2012), reduced heat absorption in cold waters for marine mammals (Würsig et al., 2009), increased conspicuousness to predators (Prather, 1961), and potentially an increased likelihood of infanticide (Leroux et al., 2021). As such, free‐ranging albino mammals may be less likely to reach reproductive maturity, although there are several known exceptions (e.g. Dunlop et al., 2019; Polanowski et al., 2012; Esteve & Jefferyt, 1998).
Despite the rarity of the condition, albinism is often associated with rodents. Some Muridae species, particularly the brown rat (also known as the Norway or laboratory rat; Rattus norvegicus), have been selectively bred for albinism for over a century for domestication (e.g., as pets) and research purposes (Beermann et al., 2004; Castle, 1947; Castle & Allen, 1903; Hou & Protopopova, 2022; Modlinska & Pisula, 2020; Noirot, 1966; Novikova & Grigoryan, 2020). However, albinism in free‐ranging rodents appears to be uncommon and has been recorded in fewer than 2% of rodent species globally (Nations et al., 2021; Romero et al., 2018).
Australia has a diverse and ubiquitous assemblage of murid rodents that comprised at least 67 species before European invasion (Menkhorst & Knight, 2004; Roycroft et al., 2021; van Dyck & Strahan, 2008). Since that time, however, at least 13 native rodent species have become extinct, and an additional 25 species have declined enough in distribution and abundance to warrant listing as threatened at State or Federal levels (DAWE, 2021; DELWP, 2021; Northern Territory Government, 2021; OEH, 2021; South Australian Government, 2021). Four invasive rodent species also occur in the country: the brown rat, black rat (R. rattus), pacific rat (R. exulans), and house mouse (Mus musculus; Menkhorst & Knight, 2004; van Dyck & Strahan, 2008). Despite the ubiquity of rodents in Australia, to our knowledge, there are no records of native albino rodents in the published literature (Romero et al., 2018). In the gray literature, however, there is a recent mention of albinism in a population of rakali (Hydromys chrysogaster) on Barrow Island, Western Australia, with multiple albino rakali sightings on the island in recent years (Bettink, 2016). Barrow Island (202 km2) is located 90 km northeast of Onslow and represents a taxonomically distinct population that is typically smaller with lighter pelage compared to populations on the Australian mainland (Bettink, 2016).
In this study, we aim to improve our understanding of the occurrence of complete or true albinism (i.e., a total loss of pigmentation) in Australian rodent species. To achieve this, we attempt to collate all records of the condition in Australia, both contemporary and historic, using three distinct methods:
An examination of historic records by searching a national, digitized newspaper database, and auditing museum collections.
A survey of contemporary ecologists and naturalists, requesting albino rodent records and a summary of their rodent trapping data to better understand the frequency of the condition.
A camera trap survey of a population that is known to produce albino individuals on occasion (rakali on Barrow Island) to better understand the frequency of the condition at that location.
Notably, the patterns of albinism inheritance were not investigated as part of this study.
2. MATERIALS AND METHODS
2.1. Historic records survey
To collate historic records of albino rodents in Australia, we first completed a search of historical (gray literature) records on the Trove digitized newspaper database (https://trove.nla.gov.au/); a freely available, comprehensive digital collection of Australian newspaper articles, gazettes, and other resources dating back to 1762. We searched Trove on the May 24, 2021, using the search terms ‘albin rodent’, ‘albin rat’, and ‘albin mouse’. These search terms returned 224 articles, of which 200 (89.29%) were removed after screening as they were either duplicates or fell outside the scope of our search (e.g., records of captive rodents, records from outside Australia; Figure A1).
We then audited seven Australian State and Territory museum collections (Australian Museum, Australian National Wildlife Collection, Museum and Art Gallery of the Northern Territory, Museums Victoria, South Australian Museum, Tasmanian Museum and Art Gallery, and Western Australian Museum) for albino specimens with the assistance of mammal curators or collection managers from each institution. They searched their respective institution's collections database for any mention of the word ‘albino’ attached to native murid specimen records and visually inspected collections for any abnormally pale rodent specimens. Notably, the collection at the Queensland Museum was inaccessible for the duration of our survey; therefore, this collection was not audited.
There are several large collections of Australian rodent specimens housed in international museums. We searched international museum online databases (American Museum of Natural History [AMNH], British Natural History Museum, Field Museum of Natural History, Museum of Vertebrate Zoology [MVZ], and Smithsonian National Museum of Natural History) for records of Australian rodents with albinism but did not identify any. Some online databases lacked a notes field (AMNH, MVZ), so while Australian rodent specimens were found, there was no scope for identifying albinism if it were present. We also contacted mammalogy curators, collection managers, or research associates at several of these museums – no respondents could recall any relevant specimens within their collections, and visual inspection of the MVZ collection returned no albinos.
2.2. Contemporary ecologist and naturalist survey
We contacted 69 ecologists and naturalists with extensive experience conducting small mammal trapping surveys in Australia and asked whether they had any contemporary records of albino rodents. If they had recorded an albino, we asked for the year, month, and location of the detection, as well as the species, sex, and weight of the animal. To better understand the frequency with which albino individuals are detected, we also requested a summary of their rodent trapping data. Specifically, we asked for location (region and state), species, sample period (years), and sample size (i.e., the number of individuals captured during the sample period). We received responses regarding albino records from 57 ecologists and naturalists and trapping summaries from 23 ecologists and naturalists. In total, 77 species‐specific trapping summaries were provided (some ecologists provided summaries for more than one species) representing 30 species (28 native, 2 non‐native). Sample sizes for each species were combined, and we estimated the frequency of albinism only for those species with adequate sample sizes (≥1000 individuals) by dividing the number of albino individuals by the sample size. Notably, some of the larger sample sizes provided were estimated to the nearest hundred (Table A1). The complete trapping summary is available in the Appendix (Table A1). Importantly, we acknowledge that this survey of contemporary ecologists and naturalists, while extensive, was neither systematic nor exhaustive; by virtue of the scale of the task, there remain ecologists that we have not consulted and datasets we have not accessed. As such, there may be additional recent albino rodent records that are not included here.
2.3. Barrow Island camera survey
The current population size of rakali on Barrow Island is not well‐known, but previous estimates have indicated that there may be approximately 150 individual rakali on the island (Bettink, 2016). To better understand the frequency of albinism in this population, we conducted a camera trap survey across 36 sites on the island (Figure 1). Barrow Island is a flat, dry island with hummock grassland (Triodia spp.) as the dominant vegetation, dotted with large termite mounds. The coastline includes rugged limestone cliffs, caves, and beaches. There is no fresh water, except during the summer monsoonal season when heavy rainfall and freshwater runoff are associated with cyclonic activity. Camera traps (Reconyx PC900, Reconyx, Wisconsin) were placed in limestone cliff habitat within 50 m of the shore along the islands' coast and were separated by a minimum of 750 m (mean 1377 m, 750–4700 m). Cameras were deployed in October 2019 and collected 476 days later in January 2021. During this time, cameras were operational for approximately 400 days, having SD cards and batteries changed, when possible, as Covid‐19 restricted visits to the island. Cameras sometimes failed due to heat, salt spray, cyclones, as well as “evisceration” of batteries by white‐bellied sea eagles (Haliaeetus leucogaster). At each site, cameras were attached to a 1.2 m picket on a 40 cm right angle bracket, facing the ground. As fishes comprise a large part of the diet of rakali, we used a perforated tin of sardines as a scent lure, secured at the base of each stake. Cameras were set to record five images per trigger event at moderate sensitivity, with no delay between possible trigger events. Each photo sequence was treated as a single point in time, and a detection event was defined as a set of images separated by at least 15 min. Images were processed and rakali were identified by E. Sanders.
FIGURE 1.
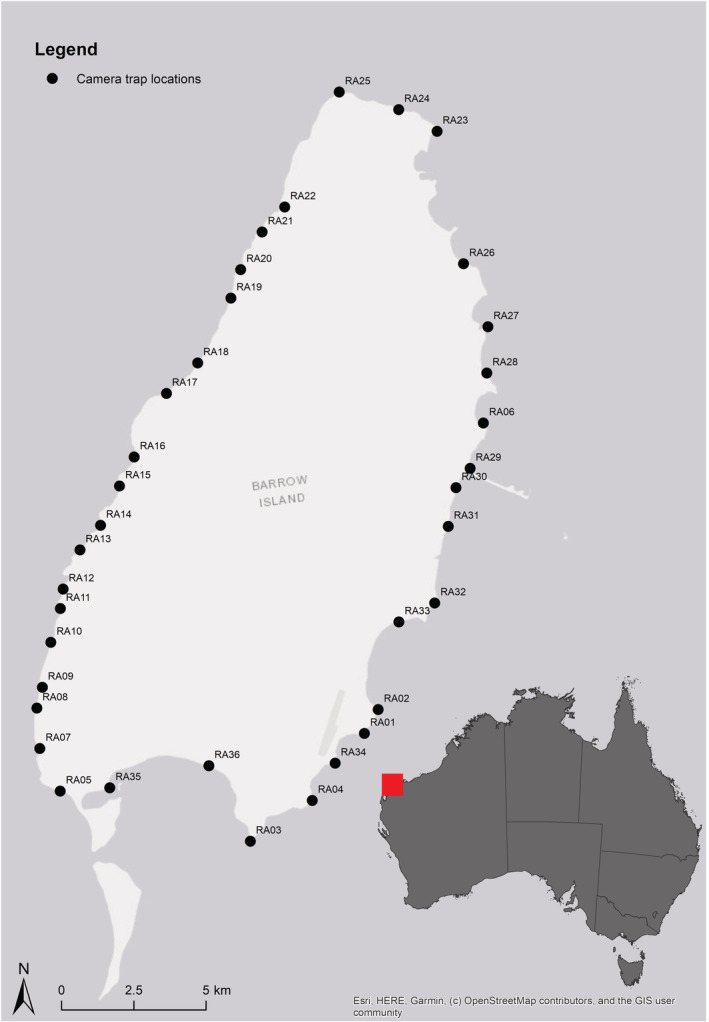
Map showing the locations of the camera traps deployed to survey rakali on Barrow Island, Western Australia.
3. RESULTS
3.1. Historic records survey
Our search of the Trove digitized newspaper database revealed 24 records of free‐ranging albino rodents in Australia. Of these, 16 were records of unidentified species (Table A2), five were records of invasive species, and three were records of native species (Table 1). The records of native species comprised one rakali from Victoria (recorded in 1937) and two long‐haired rats (R. villosissimus) from Queensland (recorded in 1940 and 1951; Table 1). Of the non‐native species, there were three records of the brown rat and two of the house mouse (Table 1).
TABLE 1.
Records of albinism in Australian rodents sourced from the historic (gray literature and museum) survey, a contemporary ecologist/naturalist survey, and a camera trap survey for rakali on Barrow Island.
| Genus | Species | Year (month) | State (region) | Sex (weight) | Survey method | Source |
|---|---|---|---|---|---|---|
|
Hydromys |
Rakali (H. chrysogaster) | 1937 | Vic (Melbourne) | Historic (gray literature) | M. E. Freame | |
| 2019 (Oct) | WA (Barrow Island) | Barrow Island camera trap | Judy Dunlop, Emmalie Sanders | |||
| 2019 (Oct) | WA (Barrow Island) | Barrow Island camera trap | ||||
| 2019 (Nov) | WA (Barrow Island) | Barrow Island camera trap | ||||
| 2007 | WA (Barrow Island) | F (455 g) | Contemporary |
Keith Morris |
||
| 2008 | WA (Barrow Island) | F (430 g) | Contemporary | |||
| 2011 | WA (Barrow Island) | M (605 g) | Contemporary | |||
| 2011 | WA (Barrow Island) | Contemporary |
Pendoley Environmental |
|||
| 2015 | WA (Barrow Island) | Contemporary | ||||
| 2015 | WA (Barrow Island) | Contemporary | ||||
| Pseudomys | Ash‐gray mouse (P. albocinereus) | 1988 (Nov) | WA (Goldfields) | M (23 g) | Contemporary | Chris Dickman |
| Desert mouse (P. desertor) | 2001 (March) | QLD (Simpson Desert) | Contemporary | |||
| Heath mouse (P. shortridgei) | 2012 (June) | Vic (Grampians National Park) | M | Contemporary | Susannah Hale | |
| Rattus | Bush rat (R. fuscipes) | 2021 (April) | Vic (Otway Ranges) | Contemporary | Michael Loughnan | |
| 1980 (July) | ACT (Brindabella Ranges) | F (75 g) | Contemporary | Chris Dickman | ||
| 1991 (July) | NSW (Chichester State Forest) | M (82 g) | Contemporary | |||
| 2021 (Jan) | Vic (Otway Ranges) | F (125 g) | Contemporary | Darcy Watchorn | ||
| Brown rat (R. norvegicus)* | 1891 | Unknown | Historic (gray literature) | The Sydney Morning Herald | ||
| 1931 | NSW (Surry Hills) | Historic (gray literature) | Daily Pictorial (Sydney) | |||
| 1945 | NSW (Tallong) | Historic (gray literature) | Goulburn Evening Post | |||
| Canefield rat (R. sordidus) | 1939 | Qld (Macknade) | M | Historic (museum) | Australian Museum | |
| Long‐haired rat (R. villosissimus) | 1940 | QLD (Unknown) | Historic (gray literature) | T. A. J. Carnegie & W. Wells | ||
| 1951 | QLD (Unknown) | Historic (gray literature) | J. Brooke | |||
| 1991 (July) | QLD (Simpson Desert) | M (69 g) | Contemporary | Chris Dickman | ||
| 2011 (Aug) | QLD (Simpson Desert) | F (80 g) | Contemporary | |||
| Unknown | Unknown founder location (captive born) | Historic (museum) |
South Australian Museum |
|||
| Unknown | Unknown founder location (captive born) | Historic (museum) | ||||
| Uromys | Giant white‐tailed rat (U. caudimaculatus) | pre‐1900 | QLD (Unknown) | Historic (museum) | Australian Museum | |
| Mus | House mouse (Mus musculus)* | 1914 | WA (North Perth) | Historic (gray literature) | The Daily News (Perth) | |
| 1916 | WA (Donnybrook) | Historic (gray literature) | The West Australian | |||
| 1999 | QLD (Emerald) | F (11 g) | Contemporary | Chris Dickman |
Note: There were 31 records of albino rodents identified from our surveys, including eight records from the gray literature survey (three native, five non‐native), four records from the museum survey (two wild‐born, two captive‐born), 16 records from the contemporary survey (15 native, one non‐native), and three records from the camera trap survey on Barrow Island. Non‐native species are denoted by an asterisk next to the species name. Month, region, and weight of specimen record are provided in parentheses where data were available. The complete contemporary survey list, which includes references and rodent species for which no albinos were recorded, can be found in the Appendix (Table A1).
Abbreviations: State abbreviations: ACT, Australian Capital Territory; NSW, New South Wales; QLD, Queensland; Tas, Tasmania; Vic, Victoria; WA, Western Australia. Sex abbreviations: M, male; F, female.
Auditing of museum collections returned two records of wild‐caught native albino or ‘part albino’ rodent specimens (Table 1): a giant white‐tailed rat (Uromys caudimaculatus) and a canefield rat (Rattus sordidus) (Table 1) from the Australian Museum (New South Wales). The giant white‐tailed rat specimen has been lost; remaining notes label the specimen as ‘albino’ with an estimated collection date pre‐1900. The canefield rat was a skin specimen, collected from a sugar mill in 1939; it was labeled as ‘semi‐albino’, but appears to have no pigmentation in the skin or fur except a faint pale gray at the base of the dorsal hairs (pers. comm. Sandy Ingleby; Figure 2). The faint colouration and the absence of eyes (by nature of being a dry specimen) mean leucism cannot be ruled out. In addition, there were two records of captive‐born long‐haired rats from the South Australian Museum (Table 1). Of these, only one (spirit) specimen remains; it is a confirmed true albino with red eyes (Figure 2). There are, however, no data available on the founder source location or generation of the captive‐born animals.
FIGURE 2.
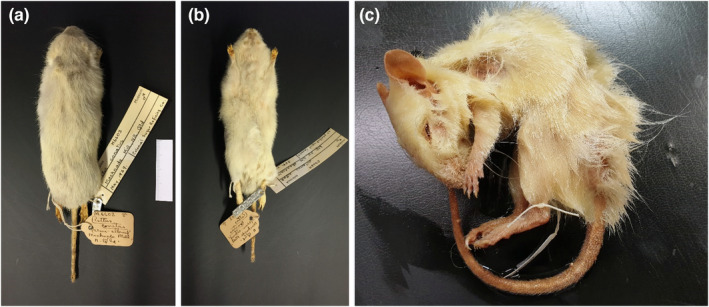
Images of two preserved albino rodent bodies identified from our survey of Australian museums: (a) dorsal image of a canefield rat (Rattus sordidus), which was labeled as ‘semi‐albino’ from the Australian Museum (New South Wales) but appears to have no pigmentation in the skin or fur except a faint pale gray at the base of the dorsal hairs (pers. comm. Sandy Ingleby; image credit Sandy Ingleby); (b) ventral image of the same canefield rat (image credit Sandy Ingleby); (c) image of a captive‐born long‐haired rat (R. villosissimus) from the South Australian Museum. No data were available on the founder source location or generation of the captive‐born animals (image credit David Stemmer).
3.2. Contemporary ecologist/naturalist survey
We collated approximately 53,159 contemporary records of live‐trapped, individual free‐ranging rodents in Australia; representing 30 species from 10 genera (Table A2). There were 16 records of free‐ranging albino rodents (dating from 1980–2021) representing six species native to Australia and one non‐native species (Table 1; Figure 3). The most common species in which albinism was recorded was the rakali with six detections (all from Barrow Island) and the bush rat (Rattus fuscipes) with four detections, followed by the long‐haired rat with two detections, and the heath mouse (Pseudomys shortridgei), ash‐gray mouse (P. albocinereus), desert mouse (P. desertor), and the non‐native house mouse each with one detection (Table 1). Most animals were live‐trapped, except for one bush rat that was recorded on a white‐flash camera trap, and three rakali that were opportunistically observed during nocturnal fauna surveys (Table 1; Figure 3). The frequency of albinism was calculated for the bush rat (0.03%), long‐haired rat (0.06%), desert mouse (0.08%), heath mouse (0.09%), and the non‐native house mouse (0.01%). The frequency of albinism for the rakali and ash‐gray mouse were not calculated, as these species had sample sizes <1000 (Table A2).
FIGURE 3.
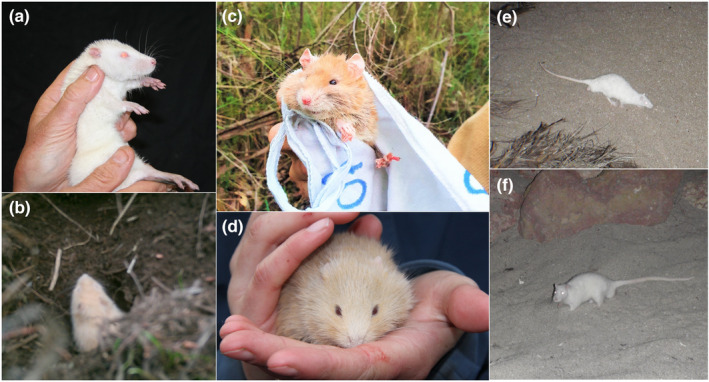
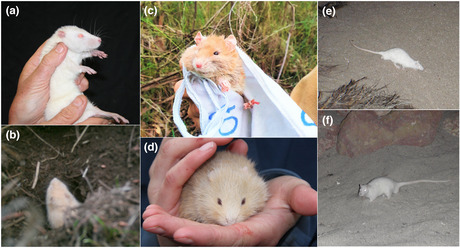
Photographs of free‐ranging albino rodents native to Australia; (a) a female albino rakali (Hydromys chrysogaster) on Barrow Island, Western Australia (image credit: Keith Morris); (b) an albino bush rat (Rattus fuscipes) captured on a camera trap in the Otway Ranges, Victoria (image credit: Michael Loughnan); (c) a female albino bush rat from the Otway Ranges, Victoria, exhibiting the off‐white/straw colored pelage not uncommon with albino mammals; this rat also had red, as opposed to pink, eyes (image credit: Darcy Watchorn); (d) a male albino heath mouse (Pseudomys shortridgei) from the Grampians National Park, Victoria (image credit: Susie Hale); (e) an albino rakali observed on Barrow Island (image credit: Pendoley Environmental); (f) an albino rakali observed on Barrow Island (image credit: Pendoley Environmental).
The sex ratio of albino rodents (where data were available) was equal, with six females and six males. Body weight information was available for 10 records, and the majority of individuals were relatively lightweight (Menkhorst & Knight, 2001), although no obvious signs of poor body condition or high parasite load were reported for these animals. No other physical abnormalities were reported.
3.3. Barrow Island camera survey
We recorded a total of 285 rakali detections on Barrow Island, with rakali recorded at 34 of the 36 camera sites. There were seven detections of albino rakali (Figure 4) from a total of three sites (RA01, RA02, and RA36). Sites RA01 are RA02 were approximately 1 km apart, and while it is possible for a rakali to traverse this distance and be detected at both sites, at least two individuals could be differentiated in the camera trap images (one individual had a distinctive tail kink). Site RA36 is over 5 km from the RA01 and RA02 sites (Figure 1), and this distance is longer than overland traverses previously recorded in the species (Gardner & Serena, 1995; Harris, 1978; Vernes, 1998). Therefore, it is likely that a minimum of three albino rakali were detected on Barrow Island (Table 1), and that the frequency of albinism in this population was at least 2%, assuming a population size of 150 individuals (Bettink, 2016).
FIGURE 4.

Photographs of free‐ranging rakali from the camera trap survey on Barrow Island, Western Australia, undertaken from October 2019–January 2021: (a) an image of a non‐albino rakali from site RA36, (b) an image of an albino rakali from site RA36, (c) an image of a non‐albino rakali from site RA02, and (d) an image of an albino rakali from site RA02.
4. DISCUSSION
Our surveys uncovered 23 native, free‐ranging albino murid rodent records from across Australia. These records represent eight species, of which five are old endemics (rakali, giant white‐tailed rat, ash‐gray mouse, desert mouse, and heath mouse) and three are new endemics (the canefield rat, long‐haired rat, and bush rat). To our knowledge, this represents the first occasion that records of albinism in free‐ranging rodents native to Australia have been recorded in the published literature.
Records of albinism in free‐ranging rodents are rare; a review by Romero et al. (2018) found that albino specimens have been reported in just 64 of 2683 species in the order Rodentia (sensu Upham et al., 2022), with no records from Australian species. Since 2018, four more rodent species have been added to that list (Dalapicolla et al., 2020; Nations et al., 2021; Stumpp et al., 2019; van der Geer, 2019). The addition of the eight Australian species reported in this study represents a considerable (11.8%) increase in the number of rodent species, globally, with albinism recorded, and nearly doubles the number of murid rodent species from 11 to 19 (1.3% to 2.2%). We also identified seven records of albinism in two of Australia's four invasive rodent species, the house mouse and brown rat; however, albinism has been previously reported in these species (see Romero et al., 2018).
In line with Romero et al. (2018), the results from our contemporary survey also emphasize the rarity of the condition in rodents, with our 16 records (representing six native species and one non‐native species) arising from a sample of over 53,000 individuals (representing 30 species). We are assuming, however, that the encounter with an albino animal would be both obvious enough for most ecologists and naturalists to recognize and of enough interest to record. Albinism was infrequently recorded in our contemporary survey, with approximately 1 in 3300 bush rats, 1 in 1700 long‐haired rats, 1 in 1250 desert mice, 1 in 1100 heath mice, and 1 in 10,000 house mice recorded with the condition. In comparison to other estimates, however, these frequencies are relatively high. Some estimates of albinism frequency in mammals suggest 1 in every 10,000 individuals, however, species‐ and population‐specific estimates remain uncommon; most published literature on free‐ranging albino mammals reports isolated and opportunistic observations of individuals with the condition and do not estimate frequency (e.g., Acevedo et al., 2009; Dunlop et al., 2019; Ferron & Laplante, 2013; Funasaka et al., 2015; Ribeiro & de Siqueira‐Silva, 2020; Zortéa & Silva, 2018).
The frequency of albinism can, however, increase under certain conditions, such as inbreeding among small isolated populations or between closely related individuals (Caro, 2009). The rakali population on Barrow Island has been isolated for at least 8000 years and, as a result, likely has little genetic diversity and exhibits the effects of genetic drift and inbreeding depression (Bettink, 2016). Our survey of contemporary ecologists and naturalists identified six albino rakali records from Barrow Island, and our camera trap survey suggests that there were at least three albino individuals persisting on the island probably simultaneously (three records within 1 month). Our estimate that at least 1 in 50 individuals (2%) of the Barrow Island rakali population are albino is considerably higher than our other estimates from the contemporary survey, which ranged from 1 in ~1100 to 1 in 10,000 (0.01%–0.1%). In other small populations of wild mammals, low‐genetic diversity can lead to naturally occurring albino phenotypes, such as with the Japanese mole (Mogera imaizumii; Tsuchihashi et al., 2011), western lowland gorilla (Gorilla gorilla gorilla; Prado‐Martinez et al., 2013), and Asinara donkey (Equus asinus; Utzeri et al., 2016); however, the genetic diversity and population structure of rakali on Barrow Island are still poorly known. In contrast to the presence of dark morphs of rakali in Australia's south, albino colouration may have fewer deleterious effects for animals on Barrow Island (Bettink, 2016), which has no feral predators and is characterized by bright white sandy beaches and pale limestone coast. We note, however, that the three albino rakali detected in our camera survey were only detected in the first 4 months of the survey period (early Oct 2019 – late Jan 2020), even though the cameras remained in situ for a further 12 months (until January 2021). It is possible that these individuals suffered from predation, as the number of rakali detections was higher in the second half of the survey. As previously mentioned, Barrow Island has no introduced predators, so predation on the island would be limited to nocturnal or crepuscular birds of prey, large varanids (Varanus giganteus), or conspecific predation. We, along with Bettink (2016), recommend further investigation into the genetic diversity and population demographics of the rakali on Barrow Island.
Excluding the Barrow Island Hydromys, eight of the 14 records of albinism in Australian rodents were from arid and semi‐arid species (ash gray mouse, desert mouse, long‐haired rat) which exhibit an irruptive (boom‐bust) population cycle (Dickman et al., 1999; Greenville et al., 2012; Madsen & Shine, 1999). These dramatic fluctuations typically occur in arid and semi‐arid environments in response to pulses in primary productivity catalyzed by rainfall events, which occur unreliably in these environments (Dickman et al., 1999; Greenville et al., 2012; Madsen & Shine, 1999). Significant rainfall events can facilitate a pulse of productivity (Whitford & Duval, 2019) after which rodent populations often erupt—a period known as the ‘boom’ phase (Dickman et al., 1999, 2010; Madsen & Shine, 1999; Plomley, 1972). This intense response to rainfall is driven by high‐reproductive rates, increased survival, and the potential for females to birth multiple litters in a year. In these instances, greater resource availability and recruitment may increase both the likelihood of albino offspring being produced (higher number of births rather than an increase in proportion of albino births) and the likelihood of their survival to adulthood by improving access to food and shelter resources, hence reducing competition and predation pressures. Our contemporary records of albinism in the desert mouse and long‐haired rat were made after prolonged wet periods when populations of these species were high (Dickman et al., 2014; Greenville et al., 2013, 2016); however, an in‐depth investigation into the influence of rainfall on the frequency of albinism detections in Australian rodents was considered beyond the scope of this research.
The sex ratio reported in this study was equal; rakali, bush rats, and long‐haired rats—which account for the majority of those records—often exhibit even sex ratios (Predavec & Dickman, 1994; Press, 1987; Smart et al., 2011; Speldewinde et al., 2013; Valentine et al., 2009), although populations of long‐haired rats can skew towards either males or females (Carstairs, 1976; Predavec & Dickman, 1994). Furthermore, a survey of rakali by Bettink (2016) identified a male‐skewed sex bias on Barrow Island, albeit from a relatively small sample (n = 11). Most individuals with bodyweight data were relatively lightweight (Menkhorst & Knight, 2001); however, due to the small sample size of albino individuals, we were unable to infer any potential influence of albinism on sex ratio or body condition in this study, although instances of poor body condition were not reported, and we are not aware of albinism influencing the sex ratio of free‐ranging mammal populations.
5. CONCLUSION
Our investigation has shed light on the occurrence of albinism in Australian rodents; indeed, our addition of eight Australian species represents a considerable increase in the number of rodent species in which albinism has been recorded globally. We do note, however, that due to the rarity of albinism occurrence, we were unable to rigorously test how factors such as population dynamics, rainfall, climate, habitat type, or other possible environmental factors influence the occurrence of albinism in Australian rodents, or the fate of albino individuals. Nonetheless, we have demonstrated that albinism is an extremely rare condition in Australia and suggest that the traits associated with the condition are deleterious and thus selected against. The rakali population on Barrow Island represents a unique opportunity to further investigate whether (and if so, how) albinism confers a disadvantage on fitness and survival. The continued compilation of albinism accounts within Rodentia, both in Australia and around the world, will improve our understanding of trait‐based selection and the possible effects that this rare phenotypic condition may have on survivorship.
AUTHOR CONTRIBUTIONS
Darcy Watchorn: Conceptualization (lead); data curation (lead); formal analysis (supporting); investigation (lead); methodology (lead); visualization (lead); writing – original draft (lead); writing – review and editing (lead). Chris Dickman: Investigation (supporting); methodology (supporting); project administration (supporting); visualization (supporting); writing – original draft (supporting); writing – review and editing (supporting). Judy Dunlop: Methodology (supporting); visualization (supporting); writing – original draft (supporting); writing – review and editing (supporting). Emmalie Sanders: Investigation (supporting); methodology (supporting); visualization (supporting); writing – original draft (supporting); writing – review and editing (supporting). Molly Watchorn: Investigation (supporting); methodology (supporting); visualization (supporting); writing – original draft (supporting); writing – review and editing (supporting). Phoebe A Burns: Investigation (supporting); methodology (supporting); project administration (supporting); visualization (supporting); writing – original draft (supporting); writing – review and editing (supporting).
FUNDING INFORMATION
DJW was supported by a Deakin University Postgraduate Research Scholarship. No additional external funding was provided for this research.
ACKNOWLEDGMENTS
We acknowledge the Wurundjeri People as the Traditional Custodians of the land on which this research was conducted. We would also like to acknowledge the Traditional Owners of the lands on which all the contemporary surveys referenced in this paper were undertaken. We thank Neal Birch, Harry Moore, and Bas van der Ploeg, all of whom provided superb assistance to JD with the Barrow Island surveys. We are grateful to Keith Morris and the DBCA Barrow Island Reserves Officers for their support of Barrow work, and to Karen Bettink for her excellent research on the Barrow Island rakali population. We thank the museum curators, collection managers and research associates who provided information or access to specimens: Cath Kemper, Chris Conroy, Chris Wilson, David Stemmer, Gavin Dally, Jon Nations, Kenny Travouillon, Kevin Rowe, Kris Helgen, Leo Joseph, Neil Duncan, Nicole Zehntner, and Sandy Ingleby. We also wish to thank the ecologists and naturalists who contributed to our contemporary survey, and we especially thank those who provided summaries of their rodent capture data, including: Andrew Cockburn, Angela Sanders, Barbara Wilson, Billie Lazenby, Chris MacGregor, David Lindenmayer, Greg Hollis, Ian Radford, John White, Julian Di Stefano, Keith Bellchambers, Luke Leung, Michael Driessen, Michael Loughnan, Nick Clemann, Rosie Hohnen, Sarah Legge, Susannah Hale, Thomas Madson, Tim Doherty, and Vivianna Miritis.
TABLE A1.
The total number of individual rodents captured (including albinos and non‐albinos), as identified by our contemporary ecologist and naturalist survey.
| Genus | Species | Number of individuals | Ecologist/Naturalist | Reference |
|---|---|---|---|---|
| Conilurus |
Brush‐tailed rabbit rat (Conilurus penicillatus) |
95 | Ian Radford | |
| Hydromys |
Rakali (Hydromys chrysogaster) |
1 | Ian Radford | |
| Leggadina |
Lakeland Downs mouse (Leggadina lakedownensis) |
71 |
Ian Radford, Sarah Legge |
(Legge et al., 2019) |
| Mesembriomys |
Black‐footed tree‐rat (Mesembriomys gouldii) |
1 | Ian Radford | |
|
Golden‐backed tree‐rat (Mesembriomys macrurus) |
102 | Ian Radford | ||
| Mus |
House mouse (Mus musculus)** |
~9230 |
Billie Lazenby, Chris Dickman, Greg Hollis, Ian Radford, John Wight, Michael Driessen, Phoebe Burns, Susie Hale, Vivianna Miritis |
(Lazenby et al., 2018) |
| Notomys |
Mitchell's hopping mouse (Notomys mitchellii) |
~ 125 |
Andrew Cockburn, Nick Clemann, Tim Doherty |
(Cockburn, 1981) |
|
Spinifex hopping mouse (Notomys alexis) |
~ 6000 | Chris Dickman | ||
| Pseudomys |
Ash‐gray mouse (Pseudomys albocinereus) |
109 | Chris Dickman | |
|
Central pebble‐mound Pseudomys (Pseudomys johnsoni) |
12 | Ian Radford | ||
|
Delicate mouse (Pseodomys delicatulus) |
397 |
Ian Radford, Sarah Legge |
(Legge et al., 2019) | |
|
Desert mouse (Pseudomys desertor) |
1331 |
Chris Dickman, Ian Radford |
||
|
Heath mouse (Pseudomys shortridgei) |
2346 |
Andrew Cockburn, Angela Sanders, John Wight, Julian Di Stefano, Phoebe Burns, Susie Hale |
(Cockburn et al., 1981; di Stefano et al., 2011) | |
|
New Holland mouse (Pseudomys novaehollandiae) |
779 |
Billie Lazenby, Greg Hollis, Michael Driessen, Phoebe Burns |
(Lazenby et al., 2018) | |
|
Sandy inland mouse (Pseudomys hermannsburgensis) |
~ 8121 |
Chris Dickman, Tim Doherty |
||
|
Silky Mouse (Pseudomys apodemoides) |
~1248 |
Andrew Cockburn, Nick Clemann |
(Cockburn, 1981; di Stefano et al., 2011) | |
|
Smoky mouse (Pseudomys fumeus) |
399 |
Phoebe Burns |
(Ford et al., 2003) | |
|
Western chestnut mouse (Pseodomys nanus) |
1045 |
Ian Radford, Sarah Legge |
(Legge et al., 2019) | |
|
Western mouse (Pseudomys occidentalis) |
137 | Angela Sanders | ||
| Rattus |
**Black rat (Rattus rattus) |
171 |
John Wight, Phoebe Burns, Susie Hale |
|
|
Bush rat (Rattus fuscipes) |
~12,201 |
Angela Sanders, Barbara Wilson, Chris Dickman, Darcy Watchorn, David Lindenmayer, Chis MacGregor, Michael Loughnan, Phoebe Burns, Rosie Hohnen, Vivianna Miritis |
||
|
Cape York rat (Rattus leucopus) |
296 | Luke Leung | (Leung, 1999b) | |
|
Dusky rat (Rattus colletti) |
1372 | Thomas Madsen | (Madsen & Shine, 1999; Madsen, Ujvari, Shine, Buttemer, & Olsson, 2006; Madsen, Ujvari, Shine, & Olsson, 2006; Ujvari et al., 2016) | |
|
Long‐haired rat (Rattus villosissimus) |
3207 |
Chris Dickman, Keith Bellchambers (AWC) |
||
|
Pale field rat (Rattus tunneyi) |
1573 |
Ian Radford, Sarah Legge |
(Legge et al., 2019) | |
|
Swamp rat (Rattus lutreolus) |
635 |
Billie Lazenby, Michael Driessen, John Wight, Phoebe Burns, Susie Hale |
(Lazenby et al., 2018) | |
| Zyzomys |
Common rock rat (Zyzomys argurus) |
1181 |
Ian Radford, Sarah Legge |
(Legge et al., 2019) |
|
Kimberley rock rat (Zyzomys woodwardi) |
386 | Ian Radford | ||
| Melomys |
Cape York melomys (Melomys capensis) |
343 | Luke Leung | (Leung, 1999a) |
|
Grassland melomys (Melomys burtoni) |
206 | Ian Radford |
Note: The non‐native species are denoted by two asterisks. References are provided where applicable.
TABLE A2.
The 24 historic records of albinism in rodents in Australia collated from the Trove digitized newspaper database that were identified to species.
| Genus | Species | Year | State (region) |
|---|---|---|---|
| Hydromys | Rakali (Hydromys chrysogaster) | 1937 | Vic (Melbourne) |
| Rattus | Long‐haired rat (Rattus villosissimus) | 1940 | QLD (Boulia) |
| 1951 | QLD (Connemarra) | ||
| **Brown rat (Rattus norvegicus) | 1891 | Unknown | |
| 1931 | NSW (Surry Hills) | ||
| 1945 | NSW (Tallong) | ||
| Mus | **House mouse (Mus musculus) | 1914 | WA (North Perth) |
| 1916 | WA (Donnybrook) | ||
| Unknown | Unknown | 1859 | TAS (Bryn Estyn) |
| 1904 | VIC (Kyneton) | ||
| 1917 | NSW (Forbes) | ||
| 1917 | NSW (Forbes) | ||
| 1923 | VIC (Melbourne) | ||
| 1927 | TAS (Myalla) | ||
| 1928 | WA (Dowerin) | ||
| 1932 | NSW (Unknown) | ||
| 1933 | NSW (Grafton) | ||
| 1934 | VIC (Lubeck) | ||
| 1936 | QLD (Ipswich) | ||
| 1940 | NSW (Bowral) | ||
| 1940 | QLD (Mount Garnet) | ||
| 1950 | NSW (Manning River) | ||
| 1951 | SA (Osborne) | ||
| 1955 | SA (Parkside) |
Note: The non‐native species are denoted by two asterisks.
Abbreviations: NSW, New South Wales; QLD, Queensland; TAS, Tasmania; Vic, Victoria; WA, Western Australia.
FIGURE A1.
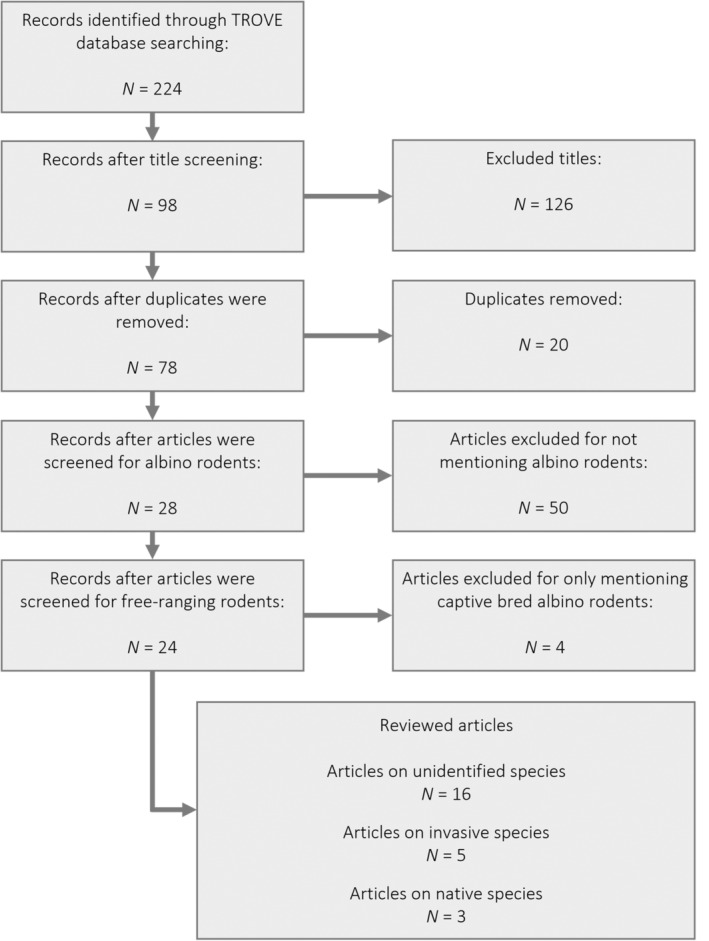
Flow diagram outlining the screening of the albino rodent literature from the Trove digitized newspaper database, resulting in the final list of 24 articles/free‐ranging albino records.
Watchorn, D. , Dickman, C. , Dunlop, J. , Sanders, E. , Watchorn, M. , & Burns, P. (2023). Ghost rodents: Albinism in Australian rodent species. Ecology and Evolution, 13, e9942. 10.1002/ece3.9942
DATA AVAILABILITY STATEMENT
Data pertaining to historic records, contemporary ecologist/naturalist surveys, and Barrow Island camera surveys are summarized in the manuscript and are available in full in the Appendix.
REFERENCES
- Acevedo, J. , Aguayo‐Lobo, A. , & Torres, D. (2009). Albino Weddell seal at cape Shirreff, Livingston Island, Antarctica. Polar Biology, 32, 1239–1243. 10.1007/s00300-009-0680-8 [DOI] [Google Scholar]
- Beermann, F. , Orlow, S. J. , & Lamoreux, M. L. (2004). The Tyr (albino) locus of the laboratory mouse. Mammalian Genome, 15, 749–758. 10.1007/s00335-004-4002-8 [DOI] [PubMed] [Google Scholar]
- Bensch, S. , Hansson, B. , Hasselquist, D. , & Nielsen, B. O. (2000). Partial albinism in a semi‐isolated population of great reed warblers. Hereditas, 133, 167–170. [DOI] [PubMed] [Google Scholar]
- Bettink, K. A. (2016). Shedding light on rakali: Genetic and morphological differentiation in the Australo‐Papuan golden‐bellied water rat (Hydromys chrysogaster). In With notes on the Barrow Island population shedding light on rakali. The University of Western Australia. [Google Scholar]
- Caro, T. (2009). Contrasting coloration in terrestrial mammals. Philosophical Transactions of the Royal Society B: Biological Sciences, 364, 537–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caro, T. , & Mallarino, R. (2020). Coloration in mammals. Trends in Ecology and Evolution, 35, 357–366. 10.1016/j.tree.2019.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstairs, J. L. (1976). Population dynamics and movements of Rattus villosissimus (Waite) during the 1966‐69 plague at Brunette downs, NT. Wildlife Research, 3, 1–9. [Google Scholar]
- Castle, W. E. (1947). The domestication of the rat. Proceedings of the National Academy of Sciences of the United States of America, 33, 109–117. 10.1073/pnas.33.5.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle, W. E. , & Allen, G. M. (1903). The heredity of albinism. Proceedings of the American Academy of Arts and Sciences, 38, 603. 10.2307/20021812 [DOI] [Google Scholar]
- Cockburn, A. (1981). Diet and habitat preference of the silky desert mouse, Pseudomys apodemoides (Rodentia). Wildlife Research, 8, 475–497. [Google Scholar]
- Cockburn, A. , Braithwaite, R. W. , & Lee, A. K. (1981). The response of the heath rat, Pseudomys shortridgei, to pyric succession: A temporally dynamic life‐history strategy. The Journal of Animal Ecology, 50, 649–666. [Google Scholar]
- Dalapicolla, J. , de Oliveira Roth, P. R. , & Percequillo, A. R. (2020). First record of albinism in spiny rats of genus Proechimys (Rodentia: Echimyidae) from Western Amazon. Mammalia, 84, 605–609. [Google Scholar]
- DAWE . (2021). EPBC act list of threatened Fauna. Australian Government. https://www.environment.gov.au/cgi‐bin/sprat/public/publicthreatenedlist.pl?wanted=fauna. Accessed 11 October 2021. [Google Scholar]
- DELWP . (2021). Flora and Fauna guarantee act 1988 – threatened list august 2021. Victorian State Government. https://www.environment.vic.gov.au/__data/assets/pdf_file/0031/536089/FFG‐Threatened‐List‐August‐2021‐v2.pdf. Accessed 11 October 2021. [Google Scholar]
- di Stefano, J. , Owen, L. , Morris, R. , Duff, T. O. M. , & York, A. (2011). Fire, landscape change and models of small mammal habitat suitability at multiple spatial scales. Austral Ecology, 36, 638–649. [Google Scholar]
- Dickman, C. , Wardle, G. , Foulkes, J. , & de Preu, N. (2014). Desert complex environments. In Biodiversity and environmental change: Monitoring, challenges and direction (pp. 379–424). CSIRO Publishing. [Google Scholar]
- Dickman, C. R. , Greenville, A. C. , Beh, C. L. , Tamayo, B. , & Wardle, G. M. (2010). Social organization and movements of desert rodents during population ‘booms’ and ‘busts’ in Central Australia. Journal of Mammalogy, 91, 798–810. 10.1644/09-MAMM-S-205.1 [DOI] [Google Scholar]
- Dickman, C. R. , Mahon, P. S. , Masters, P. , & Gibson, D. F. (1999). Long‐term dynamics of rodent populations in arid Australia: The influence of rainfall. Wildlife Research, 26, 389–403. 10.1071/WR97057 [DOI] [Google Scholar]
- Dunlop, J. , Peacock, D. , Moore, H. , & Cowan, M. (2019). Albinism in Dasyurus species – a collation of historical and modern records. Australian Mammalogy, 42, 114–118. 10.1071/AM19014 [DOI] [Google Scholar]
- Esteve, J. V. , & Jeffery, G. (1998). Reduced retinal deficits in an albino mammal with a cone rich retina: A study of the ganglion cell layer at the area centralis of pigmented and albino grey squirrels. Vision research, 38(6), 937–940. [DOI] [PubMed] [Google Scholar]
- Fernández, A. , Hayashi, M. , Garrido, G. , Montero, A. , Guardia, A. , Suzuki, T. , & Montoliu, L. (2021). Genetics of non‐syndromic and syndromic oculocutaneous albinism in human and mouse. Pigment Cell and Melanoma Research, 34, 786–799. [DOI] [PubMed] [Google Scholar]
- Ferron, J. , & Laplante, P. (2013). Report of an albino red squirrel from Sainte‐Luce. Northeastern Naturalist 20. [Google Scholar]
- Ford, F. , Cockburn, A. , & Broome, L. (2003). Habitat preference, diet and demography of the smoky mouse, Pseudomys fumeus (Rodentia: Muridae), in South‐Eastern New South Wales. Wildlife Research, 30, 89–101. [Google Scholar]
- Funasaka, N. , Kirihata, T. , Kato, H. , & Ohsumi, S. (2015). The first record of a true albino common bottlenose dolphin Tursiops truncatus from Japan. Mammal Study, 40, 19–22. [Google Scholar]
- Gardner, J. L. , & Serena, M. (1995). Observations on activity patterns, population and den characteristics of the water rat Hydromys chrysogaster along Badger Creek, Victoria. Australian Mammalogy, 18, 71–75. [Google Scholar]
- Greenville, A. C. , Wardle, G. M. , & Dickman, C. R. (2012). Extreme climatic events drive mammal irruptions: Regression analysis of 100‐year trends in desert rainfall and temperature. Ecology and Evolution, 2, 2645–2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenville, A. C. , Wardle, G. M. , & Dickman, C. R. (2013). Extreme rainfall events predict irruptions of rat plagues in Central Australia. Austral Ecology, 38, 754–764. 10.1111/aec.12033 [DOI] [Google Scholar]
- Greenville, A. C. , Wardle, G. M. , Nguyen, V. , & Dickman, C. R. (2016). Population dynamics of desert mammals: Similarities and contrasts within a multispecies assemblage. Ecosphere, 7, e01343. 10.1002/ECS2.1343 [DOI] [Google Scholar]
- Guillery, R. W. , Jeffery, G. , & Saunders, N. (1999). Visual abnormalities in albino wallabies: A brief note. Journal of Comparative Neurology, 403(1), 33–38. [PubMed] [Google Scholar]
- Harris, W. F. (1978). An ecological study of the Australian water‐rat (Hydromys chrysogaster: Geoffroy) in Southeast Queensland. University of Queensland. [Google Scholar]
- Hiller, I. (1983). The Young Naturlist. In ‘The Louise Lindsey Merrick Texas Environmental Series’ . pp. 28–31. (The Louise Lindsey Merrick Texas Environmental Series.).
- Holyoak, D. T. (1978). Variable albinism of the flight feathers as an adaptation for recognition of individual birds in some Polynesian populations of Acrocephalus warblers. Ardea, 66, 112–117. [Google Scholar]
- Hou, C. Y. , & Protopopova, A. (2022). Rats as pets: Predictors of adoption and surrender of pet rats (Rattus norvegicus domestica) in British Columbia, Canada. PLoS One, 17, 1–21. 10.1371/journal.pone.0264262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeler, C. E. (1942). The association of the black (non‐agouti) gene with behavior: In the Norway rat. Journal of Heredity, 33, 371–384. [Google Scholar]
- Lazenby, B. T. , Bell, P. , Driessen, M. M. , Pemberton, D. , & Dickman, C. R. (2018). Evidence for a recent decline in the distribution and abundance of the new Holland mouse (Pseudomys novaehollandiae) in Tasmania, Australia. Australian Mammalogy, 41, 179–185. [Google Scholar]
- Legge, S. , Smith, J. G. , James, A. , Tuft, K. D. , Webb, T. , & Woinarski, J. C. Z. (2019). Interactions among threats affect conservation management outcomes: Livestock grazing removes the benefits of fire management for small mammals in Australian tropical savannas. Conservation Science and Practice, 1, 1–13. 10.1111/csp2.52 [DOI] [Google Scholar]
- Leroux, M. , Monday, G. , Chandia, B. , Akankwasa, J. W. , Zuberbühler, K. , Hobaiter, C. , Crockford, C. , Townsend, S. W. , Asiimwe, C. , & Fedurek, P. (2021). First observation of a chimpanzee with albinism in the wild: Social interactions and subsequent infanticide. American Journal of Primatology, e23305, e23305. 10.1002/AJP.23305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung, L. K. P. (1999a). Ecology of Australian tropical rainforest mammals. II. The Cape York melomys, Melomys capensis (Muridae: Rodentia). Wildlife Research, 26, 307–316. 10.1071/WR96043 [DOI] [Google Scholar]
- Leung, L. K. P. (1999b). Ecology of Australian tropical rainforest mammals. III. The Cape York rat, Rattus leucopus (Muridae: Rodentia). Wildlife Research, 26, 317–328. 10.1071/WR96044 [DOI] [Google Scholar]
- Madsen, T. , & Shine, R. (1999). Rainfall and rats: Climatically‐driven dynamics of a tropical rodent population. Austral Ecology, 24, 80–89. 10.1046/j.1442-9993.1999.00948.x [DOI] [Google Scholar]
- Madsen, T. , Ujvari, B. , Shine, R. , Buttemer, W. , & Olsson, M. (2006). Size matters: Extraordinary rodent abundance on an Australian tropical flood plain. Austral Ecology, 31, 361–365. 10.1111/j.1442-9993.2006.01564.x [DOI] [Google Scholar]
- Madsen, T. , Ujvari, B. , Shine, R. , & Olsson, M. (2006). Rain, rats and pythons: Climate‐driven population dynamics of predators and prey in tropical Australia. Austral Ecology, 31, 30–37. 10.1111/j.1442-9993.2006.01540.x [DOI] [Google Scholar]
- McCardle, H. (2012). Albinism in wild vertebrates. Texas State University‐San Marcos. [Google Scholar]
- Menkhorst, P. , & Knight, F. (2001). Field guide to the mammals of Australia. Oxford University Press. [Google Scholar]
- Menkhorst, P. , & Knight, F. (2004). A field guide to the mammals of Australia. Oxford University Press Melbourne. [Google Scholar]
- Miller, J. (2005). All About Albinism. Missouri Conservationist, 66, 5–7. [Google Scholar]
- Modlinska, K. , & Pisula, W. (2020). The natural history of model organisms the Norway rat, from an obnoxious pest to a laboratory pet. ELife, 9, 899–908. 10.7554/eLife.50651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nations, J. A. , Mursyid, A. , Busta, R. D. , Adrian, S. P. , Handika, H. , Achmadi, A. S. , & Esselstyn, J. A. (2021). The first report of albinism in a Sundaland endemic rodent. Mammalia, 85, 168–172. 10.1515/mammalia-2020-0047 [DOI] [Google Scholar]
- Noirot, E. (1966). Ultra‐sounds in young rodents. I. Changes with age in albino mice. Animal Behaviour, 14, 459–462. [DOI] [PubMed] [Google Scholar]
- Northern Territory Government . (2021). Threatened animals listed under the Territory Parks and Wildlife Conservation Act 1976 . https://nt.gov.au/environment/animals/threatened‐animals. Accessed 11 October 2021.
- Novikova, Y. P. , & Grigoryan, E. N. (2020). Early appearance of aging signs in the retinal pigment epithelium in young albino rats. Russian Journal of Developmental Biology, 51, 377–386. 10.1134/s1062360420060065 [DOI] [Google Scholar]
- OEH . (2021). Threatened biodiversity profile search . https://www.environment.nsw.gov.au/threatenedSpeciesApp/. Accessed 11 October 2021.
- Phillips, C. J. , & Wilson, N. (1965). A partially albino bandicoot from New Guinea. Journal of Mammalogy, 46, 698–699. [PubMed] [Google Scholar]
- Plomley, N. J. B. (1972). Some notes on plagues of small mammals in Australia. Journal of Natural History, 6, 363–384. [Google Scholar]
- Polanowski, A. M. , Robinson‐Laverick, S. M. , Paton, D. , & Jarman, S. N. (2012). Variation in the tyrosinase gene associated with a white humpback whale (Megaptera novaeangliae). Journal of Heredity, 103, 130–133. 10.1093/jhered/esr108 [DOI] [PubMed] [Google Scholar]
- Prado‐Martinez, J. , Hernando‐Herraez, I. , Lorente‐Galdos, B. , Dabad, M. , Ramirez, O. , Baeza‐Delgado, C. , Morcillo‐Suarez, C. , Alkan, C. , Hormozdiari, F. , Raineri, E. , Estellé, J. , Fernandez‐Callejo, M. , Valles, M. , Ritscher, L. , Schöneberg, T. , de la Calle‐Mustienes, E. , Casillas, S. , Rubio‐Acero, R. , Melé, M. , … Marques‐Bonet, T. (2013). The genome sequencing of an albino Western lowland gorilla reveals inbreeding in the wild. BMC Genomics, 14, 1–8. 10.1186/1471-2164-14-363/FIGURES/3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prather, E. E. (1961). A comparison of production of albino and normal channel catfish . In Proceedings of the annual conference of the southeastern Association of Game and Fish Commissioners. pp. 302–303.
- Predavec, M. , & Dickman, C. R. (1994). Population dynamics and habitat use of the long‐haired rat (Rattus villosissimus) in South‐Western Queensland. Wildlife Research, 21, 57–65. 10.1071/WR9940001 [DOI] [Google Scholar]
- Press, A. J. (1987). Comparison of the demography of populations of Rattus fuscipes living in cool temperate rain‐forests and dry Sclerophyll forests. Wildlife Research, 14, 45–63. 10.1071/WR9870045 [DOI] [Google Scholar]
- Ribeiro, R. , & de Siqueira‐Silva, D. H. (2020). First report of complete albinism in Mazama americana (Erxleben, 1777) in the biological Reserve of Tapirapé, oriental Amazon, Brazil. Acta Scientiarum. Biological Sciences, 42, 1–7. [Google Scholar]
- Romero, V. , Racines‐Márquez, C. E. , & Brito, J. (2018). A short review and worldwide list of wild albino rodents with the first report of albinism in Coendou rufescens (Rodentia: Erethizontidae). Mammalia, 82, 509–515. 10.1515/mammalia-2017-0111 [DOI] [Google Scholar]
- Roycroft, E. , MacDonald, A. J. , Moritz, C. , Moussalli, A. , Portela Miguez, R. , & Rowe, K. C. (2021). Museum genomics reveals the rapid decline and extinction of Australian rodents since European settlement. Proceedings of the National Academy of Sciences, 118, e2021390118. 10.1073/pnas.2021390118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage, B. L. (1962). Albinism and melanism in birds. British Birds, 55, 201–225. [Google Scholar]
- Searle, A. G. (1968). Comparative genetics of coat colour in mammals. Comparative genetics of coat colour in mammals.
- Smart, C. , Speldewinde, P. C. , & Mills, H. R. (2011). Influence of habitat characteristics on the distribution of the water‐rat (Hydromys chrysogaster) in the greater Perth region, Western Australia. Journal of the Royal Society of Western Australia, 94, 533–539. [Google Scholar]
- South Australian Government . (2021). South Australia National Parks and Wildlife Act 1972 . version: 19.3.2021. https://www.legislation.sa.gov.au/LZ/C/A/NATIONAL PARKS AND WILDLIFE ACT 1972/CURRENT/1972.56.AUTH.PDF
- Speldewinde, P. C. , Close, P. , Weybury, M. , & Comer, S. (2013). Habitat preference of the Australian water rat (Hydromys chrysogaster) in a coastal wetland and stream, two peoples bay, South‐Western Australia. Australian Mammalogy, 35, 188–194. 10.1071/AM12001 [DOI] [Google Scholar]
- Stumpp, R. , Casali, D. , Cunha, H. , & Paglia, A. (2019). Complete albinism in Oxymycterus dasytrichus (Schinz 1821)(Rodentia: Cricetidae). Mammalia, 83, 281–286. [Google Scholar]
- Tsuchihashi, A. , Tamate, H. , & Yokohata, Y. (2011). Frequent occurrence of partial albinism in lesser Japanese moles (Mogera imaizumii) on Kinkazan Island, Miyagi prefecture, Northeastern Japan. Mammal Study, 36, 141–146. 10.3106/041.036.0304 [DOI] [Google Scholar]
- Ujvari, B. , Brown, G. , Shine, R. , & Madsen, T. (2016). Floods and famine: Climate‐induced collapse of a tropical predator‐prey community. Functional Ecology, 30, 453–458. 10.1111/1365-2435.12505 [DOI] [Google Scholar]
- Upham, N. , Burgin, C. , Widness, J. , Liphardt, S. , Parker, C. , Becker, M. , Rochon, I. , & Huckaby, D. (2022). Mammal diversity database. Version 1.9. https://zenodo.org/record/6407053#.YvG5DnZByUk
- Utzeri, V. J. , Bertolini, F. , Ribani, A. , Schiavo, G. , Dall'Olio, S. , & Fontanesi, L. (2016). The albinism of the feral Asinara white donkeys (Equus asinus) is determined by a missense mutation in a highly conserved position of the tyrosinase (TYR) gene deduced protein. Animal Genetics, 47, 120–124. 10.1111/age.12386 [DOI] [PubMed] [Google Scholar]
- Valentine, L. E. , Wilson, B. A. , Reaveley, A. , Huang, N. , Johnson, B. , & Brown, P. (2009). Patterns of ground‐dwelling vertebrate biodiversity in the Gnangara sustainability strategy study area. Report, Department of Environment and Conservation. [Google Scholar]
- van der Geer, A. A. E. (2019). Effect of isolation on coat colour polymorphism of Polynesian rats in Island Southeast Asia and the Pacific. PeerJ, 7, e6894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dyck, S. , & Strahan, R. (2008). The mammals of Australia. New Holland Pub Pty Limited. [Google Scholar]
- van Grouw, H. (2006). Not every white bird is an albino: Sense and nonsense about colour aberrations in birds. Dutch Birding, 28, 79–89. [Google Scholar]
- Vernes, K. (1998). Observation of a long‐range overland movement event by an adult common water rat, Hydromys chrysogaster . Australian Mammalogy, 20, 409–410. [Google Scholar]
- Whitford, W. G. , & Duval, B. D. (2019). Ecology of desert systems. Academic Press. [Google Scholar]
- Würsig, B. , Perrin, W. F. , & Thewissen, J. G. M. (2009). Encyclopedia of marine mammals. Academic Press. [Google Scholar]
- Zortéa, M. , & Silva, M. C. (2018). Albinism in the striped spear‐nosed bat Gardnerycteris crenulatum (Chiroptera: Phyllostomidae) with an updated list of albino bats in the world. Mammalia, 82, 78–84. 10.1515/mammalia-2016-0080 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data pertaining to historic records, contemporary ecologist/naturalist surveys, and Barrow Island camera surveys are summarized in the manuscript and are available in full in the Appendix.


