Abstract
Background
Both behavioural support (including brief advice and counselling) and pharmacotherapies (including nicotine replacement therapy (NRT), varenicline and bupropion) are effective in helping people to stop smoking. Combining both treatment approaches is recommended where possible, but the size of the treatment effect with different combinations and in different settings and populations is unclear.
Objectives
To assess the effect of combining behavioural support and medication to aid smoking cessation, compared to a minimal intervention or usual care, and to identify whether there are different effects depending on characteristics of the treatment setting, intervention, population treated, or take‐up of treatment.
Search methods
We searched the Cochrane Tobacco Addiction Group Specialised Register in July 2015 for records with any mention of pharmacotherapy, including any type of NRT, bupropion, nortriptyline or varenicline.
Selection criteria
Randomized or quasi‐randomized controlled trials evaluating combinations of pharmacotherapy and behavioural support for smoking cessation, compared to a control receiving usual care or brief advice or less intensive behavioural support. We excluded trials recruiting only pregnant women, trials recruiting only adolescents, and trials with less than six months follow‐up.
Data collection and analysis
Search results were prescreened by one author and inclusion or exclusion of potentially relevant trials was agreed by two authors. Data was extracted by one author and checked by another.
The main outcome measure was abstinence from smoking after at least six months of follow‐up. We used the most rigorous definition of abstinence for each trial, and biochemically validated rates if available. We calculated the risk ratio (RR) and 95% confidence interval (CI) for each study. Where appropriate, we performed meta‐analysis using a Mantel‐Haenszel fixed‐effect model.
Main results
Fifty‐three studies with a total of more than 25,000 participants met the inclusion criteria. A large proportion of studies recruited people in healthcare settings or with specific health needs. Most studies provided NRT. Behavioural support was typically provided by specialists in cessation counselling, who offered between four and eight contact sessions. The planned maximum duration of contact was typically more than 30 minutes but less than 300 minutes. Overall, studies were at low or unclear risk of bias, and findings were not sensitive to the exclusion of any of the six studies rated at high risk of bias in one domain. One large study (the Lung Health Study) contributed heterogeneity due to a substantially larger treatment effect than seen in other studies (RR 3.88, 95% CI 3.35 to 4.50). Since this study used a particularly intensive intervention which included extended availability of nicotine gum, multiple group sessions and long term maintenance and recycling contacts, the results may not be comparable with the interventions used in other studies, and hence it was not pooled in other analyses. Based on the remaining 52 studies (19,488 participants) there was high quality evidence (using GRADE) for a benefit of combined pharmacotherapy and behavioural treatment compared to usual care, brief advice or less intensive behavioural support (RR 1.83, 95% CI 1.68 to 1.98) with moderate statistical heterogeneity (I² = 36%).
The pooled estimate for 43 trials that recruited participants in healthcare settings (RR 1.97, 95% CI 1.79 to 2.18) was higher than for eight trials with community‐based recruitment (RR 1.53, 95% CI 1.33 to 1.76). Compared to the first version of the review, previous weak evidence of differences in other subgroup analyses has disappeared. We did not detect differences between subgroups defined by motivation to quit, treatment provider, number or duration of support sessions, or take‐up of treatment.
Authors' conclusions
Interventions that combine pharmacotherapy and behavioural support increase smoking cessation success compared to a minimal intervention or usual care. Updating this review with an additional 12 studies (5,000 participants) did not materially change the effect estimate. Although trials differed in the details of their populations and interventions, we did not detect any factors that modified treatment effects apart from the recruitment setting. We did not find evidence from indirect comparisons that offering more intensive behavioural support was associated with larger treatment effects.
Keywords: Adult, Female, Humans, Male, Behavior Therapy, Behavior Therapy/methods, Combined Modality Therapy, Combined Modality Therapy/methods, Counseling, Counseling/methods, Randomized Controlled Trials as Topic, Smoking, Smoking/therapy, Smoking Cessation, Smoking Cessation/methods, Tobacco Use Cessation Devices
Plain language summary
Does a combination of stop smoking medication and behavioural support help smokers to stop?
Background
Behavioural support (such as brief advice and counselling) and medications (including varenicline, bupropion, and nicotine replacement therapies like patches or gum) help people quit smoking. Many guidelines recommend combining medication and behavioural support to help people stop smoking, but it is unclear if some combinations are more effective than others, or if the combination of medication and behavioural support works better in some settings or groups than in others.
Study Characteristics
In July 2015 we searched for studies which tested combinations of behavioural support and medication to help smokers to stop compared to usual care or brief behavioural support. People who smoked were recruited mainly in health care settings. Some trials only enrolled people who said they wanted to try to quit at that time, but some included people who weren't planning to quit. Studies had to report how many people had stopped smoking after at least six months.
Results
We found 53 studies with a total of over 25,000 participants. One very large study found a large benefit. It gave intensive support including nicotine gum, multiple group sessions, and long term contact to help people stay quit or encourage additional quit attempts. Because it was not typical of most treatment programmes, it was not included when we estimated the likely benefit, although it shows that such intensive support can be very effective. Based on the remaining 52 studies, we found high quality evidence that using a combination of behavioural support and medication increases the chances of successfully quitting after at least six months. Combining the results suggests that the chance of success is increased by 70 to 100 percent compared to just brief advice or support. There was some evidence that the effect tended to be larger when participants were recruited in healthcare settings. There was no clear evidence that providing more contact increased the number of people who quit smoking at six months or longer. .
Summary of findings
Summary of findings for the main comparison. Combined pharmacotherapy and behavioural interventions for smoking cessation.
| Combined pharmacotherapy and behavioural interventions for smoking cessation | ||||||
| Patient or population: People who smoke Settings: Community and healthcare settings Intervention: Combined pharmacotherapy and behavioural interventions, compared to brief advice or usual care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Combined pharmacotherapy and behavioural interventions | |||||
|
Cessation at longest follow‐up (all but Lung Health Study) Follow‐up: 6 months+ |
86 per 10001 | 157 per 1000 (144 to 170) | RR 1.83 (1.68 to 1.98) | 19488 (52 studies) | ⊕⊕⊕⊕ high2 | |
| Cessation at longest follow‐up (Lung Health Study only) Follow‐up: mean 12 months | 90 per 1000 | 350 per 1000 (302 to 406) | RR 3.88 (3.35 to 4.5) | 5887 (1 study) | ⊕⊕⊕⊝ moderate3 | Substantially larger treatment effect than seen in other studies. Particularly intensive intervention, hence not included in main analysis. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Baseline risk calculated as mean control group risk for both comparisons 2 Some evidence of asymmetry in a funnel plot; excess of small trials detecting larger effects. However, in a sensitivity analysis, removing smaller studies did not markedly decrease the pooled estimate. 3 Downgraded due to indirectness. As this study had a particularly intensive intervention, the results may not be generalisable to real world treatment programmes.
Background
Giving up smoking is the most effective way for people who smoke to reduce their risk of smoking related disease and premature death. Behavioural support and pharmacotherapies help people to stop smoking. Behavioural support interventions include written materials containing advice on quitting, multisession group therapy programmes or individual counselling sessions in person or by telephone. Providing standard self‐help materials alone seems to have a small effect on success, but there is evidence of a benefit of individually tailored self‐help materials or more intensive advice or counselling (Lancaster 2005; Hartmann‐Boyce 2014). Nicotine replacement therapy (NRT), varenicline, bupropion, cytisine and nortriptyline all increase the long‐term success of quit attempts (Cahill 2013). Many clinical practice guidelines recommend that healthcare providers offer people who are prepared to make a quit attempt both classes of intervention on the basis that they may have an additive or even multiplicative effect. This approach assumes that the two types of treatment have complementary modes of action, and may independently improve the chances of maintaining long‐term abstinence. However, it is recognised that many people who use pharmacotherapy will not take up the offer of intensive behavioural support. NRT products are available over the counter (OTC) without a prescription in many countries, and people who purchase them may not access any specific behavioural support. People who obtain prescriptions for pharmacotherapies are more likely to receive some support, but this may be focused on explaining proper use of the drug rather than on behavioural counselling. Surveys suggest that the proportion of people who use both types of treatment when attempting to stop smoking is low (Shiffman 2008; Kotz 2009b). The aim of this review is to assess the the effect of the combined intervention of pharmacotherapy and behavioural support, compared to using neither type of treatment, or receiving only brief advice or behavioural support.
Other Cochrane Tobacco Addiction reviews have evaluated the separate effects of behavioural and pharmaceutical interventions. In order to quantify these individual effects, these reviews restrict inclusion to trials where the intervention under investigation is unconfounded. By unconfounded, we mean that trials of pharmacotherapies had to provide the same amount of behavioural support (materials, advice, counselling contacts) to all participants whether they receive active treatment or a placebo or no medication. Likewise, when behavioural interventions are evaluated there must be no systematic difference in the offer of medications between the active and control arms of the trial.
The findings from reviews of unconfounded trials support the use of combined pharmacological and behavioural therapy, but do not provide a direct estimate of the size of the benefit to be expected from combining the two types of treatment. The aim of this review is to synthesize the evidence from trials that directly evaluate the use of an intervention combining pharmacotherapy and behavioural support, where the control condition includes neither pharmacotherapy nor the same intensity of behavioural support. The control will involve either usual care, or brief advice. If the pharmacotherapy and the behavioural support components exert independent effects on successful cessation, these trials might be expected to give considerably larger treatment effects than would be achieved from either the behavioural or the medication component alone. However, other factors may affect the size of this effect. In particular, pragmatic trials of interventions in healthcare settings may find smaller effects than placebo‐controlled pharmacotherapy studies in research settings, as delivery of the intervention components may be lower. To address this, we set out to identify moderators that might lead to heterogeneity in effects of combined treatment, including the motivation of participants, the nature of the treatment setting, and the type of therapist. We also aimed to categorize by the intensity of behavioural support, based on number and duration of contacts, in order to evaluate whether the intensity of the behavioural support affected treatment success. Previous meta‐analyses have suggested that there is a dose response, with more contacts improving outcomes amongst people receiving pharmacotherapy (Fiore 2008 table 6.23). We also considered the degree to which participants used both medication and behavioural components as a possible explanation for any heterogeneity.
For this review we identified trials of interventions that combined pharmacotherapies (including NRT, varenicline, bupropion, cytisine, or nortriptyline) with behavioural support (tailored materials, brief advice, in person or telephone counselling) and that compared outcomes against a control group that received either usual care or a brief cessation component (i.e. advice to quit but no other behavioural support or medication common to the intervention). A companion review will evaluate whether more intensive behavioural support improves cessation outcomes for people using pharmacotherapy, using the direct evidence from trials that compare different levels of behavioural support for people receiving any type of pharmacotherapy for smoking cessation (Stead 2015).
Objectives
To assess the effect of combining behavioural support and medication to aid smoking cessation, compared to using neither, and to identify whether there are different effects depending on characteristics of the treatment setting, intervention, population treated, or take‐up of treatment.
Methods
Criteria for considering studies for this review
Types of studies
Randomized or quasi‐randomized controlled trials. We did not exclude studies on the basis of publication status or language of publication.
Types of participants
We included trials that recruited people who smoke in any setting, with the exception of trials which only recruit pregnant women or adolescents. These populations are considered in specific reviews. Trial participants did not need to be selected according to their interest in quitting or their suitability for pharmacotherapy.
Types of interventions
We included interventions for increasing smoking cessation that included behavioural support and the availability of pharmacotherapy, regardless of type of pharmacotherapy. We excluded trials where fewer than 20% of participants were eligible for or used pharmacotherapy. The provision of written information or brief instructions on correct use of the pharmacotherapy was not regarded as behavioural support. The control group should not have been systematically offered pharmacotherapy but we did not exclude studies where some control group participants obtained medication from other sources. Control group participants could be offered usual care, self‐help materials or brief advice on quitting, but support had to have been of a lower intensity than that given to intervention participants.
Types of outcome measures
Following the standard methodology of the Cochrane Tobacco Addiction Group, the primary outcome is smoking cessation at the longest follow‐up using the strictest definition of abstinence, that is, preferring sustained over point prevalence abstinence and using biochemically validated rates where available. We also noted any other abstinence outcomes reported and conducted sensitivity analyses to test if the choice of outcome affected the results of meta‐analysis. We excluded trials reporting less than six months follow‐up from the start of intervention.
Search methods for identification of studies
We identified trials from the Cochrane Tobacco Addiction Specialised Register (the Register). This is generated from regular searches of the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, and PsycINFO for trials of smoking cessation or prevention interventions. The most recent search of the Register was in July 2015. At the time of the search the Register included the results of searches of the CENTRAL to issue 4, 2015; MEDLINE (via OVID) from 1946 to update 20150501; EMBASE (via OVID) from 1974 to week 201519 and PsycINFO (via OVID) from 1967 to update 20150506. See the Tobacco Addiction Group Module in the Cochrane Library for full search strategies and a list of other resources searched.
We searched the Register for records with any mention of pharmacotherapy in title, abstract or indexing terms (see Appendix 1 for the final search strategy). We checked titles and abstracts to identify trials of interventions for smoking cessation that combined pharmacotherapy with behavioural support. We also checked the excluded study lists of reviews of behavioural therapies and pharmacotherapy for trials excluded because pharmacotherapy was confounded with additional behavioural support compared to the control group. Trials with a factorial design that varied both pharmacotherapy and behavioural conditions were also considered for inclusion. We also tested an additional MEDLINE search using the smoking related terms and design limits used in the standard register search and the MeSH terms ‘combined modality therapy’ or (Drug Therapy and (exp Behavior therapy or exp Counseling)). This search retrieved a subset of records already screened for the inclusion in the Register and was used to assess whether it might retrieve studies where there was no mention of a specific cessation pharmacotherapy in the title, abstract or indexing. We did not find any additional studies from this.
Data collection and analysis
Selection of studies
LS identified potentially relevant trial reports according to the criteria above. Areas of uncertainty were discussed with PK and TL. LS and either TL or PK extracted data.
Data extraction and management
We extracted the following information from trial reports:
Country and setting of trial
Method of recruitment, including any selection by motivation to quit
Method of sequence generation
Method of allocation concealment
Characteristics of participants including gender, age, smoking rate
Characteristics of intervention deliverer
Intervention components: type, dose and duration of pharmacotherapy, type and duration of behavioural support
Control group components
Outcomes: primary outcome length of follow‐up and definition of abstinence; other follow‐ups and abstinence definitions; use of biochemical validation; loss to follow‐up.
Assessment of risk of bias in included studies
Studies were evaluated on the basis of the randomization procedure and allocation concealment, incomplete outcome data assessment and any other bias (Schulz 2002a; Schulz 2002b; Higgins 2011). Publication bias was assessed using a funnel plot.
Measures of treatment effect
Trial effects were expressed as a relative risk (RR): (quitters in treatment group/total randomized to treatment group)/ (quitters in control group/total randomized to control group).
Dealing with missing data
Numbers lost to follow‐up were reported by group where available. Following standard Cochrane Tobacco Addiction Group methods, people lost to follow‐up were assumed to be smoking and included in the denominators for calculating the RR. Any deaths during follow‐up were reported separately and excluded from denominators.
Assessment of heterogeneity
We considered pooling all trials comparing combined therapy to usual care/minimal intervention control if heterogeneity as assessed by the I² statistic (Higgins 2003) was less than 50%.
Data synthesis
For groups of trials where meta‐analysis was judged appropriate, relative risks were pooled using a Mantel‐Haenszel fixed‐effect model, and a pooled estimate with 95% confidence intervals reported.
If trials had more than one intervention condition we compared the most intensive combination of behavioural support and pharmacotherapy to the control in the main analysis.
Subgroup analysis and investigation of heterogeneity
We undertook planned subgroup analyses by setting, participant selection, intervention provider, number of sessions, total duration of contact, and take‐up of treatment. The subgroups are listed below.
Setting
Recruited in healthcare settings
Recruited as community volunteers
Participant selection
Selected for willingness to make a quit attempt/ high take‐up of pharmacotherapy
Not selected for interest in quitting/ low take‐up of pharmacotherapy
Not explicitly selected but study procedures and participant characteristics suggested that most participants were willing to make a quit attempt
Provider
Usual care provider
Specialist in smoking cessation
Peer group counsellor (ex‐smoker)
Intensity
We conducted alternative analyses of intensity adapted from two of the categories used in the US Guidelines (Fiore 2008). We used planned contact time and number of sessions where possible. If this was variable or unclear we used any report of actual delivery.
We categorised total amount of contact time as zero (if the only support was sent by mail), 1 to 30 minutes (collapsing one to three and 4 to 30 US guideline categories), 31 to 90, 91 to 300, and over 300 minutes.
Number of person‐to‐person sessions was categorised as zero, one to three (instead of zero to one and two to three as used in US guidelines), four to eight and over eight sessions.
Treatment take‐up (compliance with medication and behavioural support)
We expected this group of trials to include some pragmatic studies where participants are offered treatment but did not use all the components offered. After pilot testing, we categorised studies into three groups:
High – over 70% starting pharmacotherapy and receiving at least one session of support (where applicable)
Moderate – Over 30% starting pharmacotherapy and over 50% receiving at least one session of support
Low – less than 30% starting pharmacotherapy or less than 50% receive at least one session of support
We did not separate trials using different pharmacotherapies in initial analyses but we considered the type of pharmacotherapy as an explanation for remaining heterogeneity.
Where there was evidence from subgroup analyses of clinically relevant differences between categories in any of the subgroups above, we used meta regression (Stata) to explore whether any of the characteristics were effect modifiers, including each of the characteristics as categorical (dummy) variables. To assess possible effect modification according to the intensity of intervention received, we also included number of sessions, amount of contact time and take‐up together in a single meta‐regression analysis. Since these analyses were not pre‐specified they are hypothesis generating only.
Sensitivity analysis
We considered the sensitivity of the results to changes in the cut‐off range for categories outlined above. If data for multiple outcomes were provided, we conducted sensitivity analyses to test if the choice of outcome affected the results. We also conducted sensitivity analyses removing smaller studies to assess possible impact of publication bias.
Results
Description of studies
Results of the search
The original register search retrieved approximately 2200 records, approximately 400 additional records were screened for this update. Most of the records that did report trials of interventions for smoking cessation were not relevant because they were placebo‐controlled trials of pharmacotherapies, in which the behavioural support was the same for intervention and control conditions. We identified 41 studies for inclusion in the original review and a further 12 for the update. Many included studies were identified via more than one study report. All reports related to a study are listed in the reference section with the primary report used for data extraction identified. Trials are identified by the first author and year of publication of the main study report. A further 90 studies are listed as excluded.
Included studies
We identified 53 studies that met all inclusion criteria with a total of more than 25,000 participants. Most have been published since 2000, with the earliest published in 1988. One trial had almost 6000 participants (Lung Health Study) and one over 2000 (Hollis 2007). Thirty‐one trials had more than 100 participants in the intervention arm and most of the remainder had more than 50 in the intervention arm. Twelve trials are new for this update (Murray 2013; Prochaska 2014; Rigotti 2014; Stockings 2014; Winhusen 2014; Bernstein 2015; Haas 2015; Hickman 2015; Lee 2015; Peckham 2015; Perez‐Tortosa 2015)
About half the studies were conducted in the USA. Of the others there were five from Canada (Wilson 1988; Reid 2003; Ratner 2004; Chouinard 2005; Lee 2015), four from Australia (Vial 2002; Wakefield 2004; Baker 2006; Stockings 2014), three from Denmark (Tonnesen 2006; Villebro 2008; Thomsen 2010), three from Spain (Juarranz Sanz 1998; Rodriguez 2003; Perez‐Tortosa 2015), four from the UK (Molyneux 2003; Binnie 2007; Murray 2013; Peckham 2015) and one each from Brazil (Otero 2006), Italy (Segnan 1991), the Netherlands (Kotz 2009), Sweden (Sadr Azodi 2009), Japan (Hanioka 2010) and Hong Kong (Chan 2010).
Details of can be found in the Characteristics of included studies table. Appendix 2 tabulates the following study characteristics: setting and provider; selection by motivation; number and total duration of contact categories; and level of take‐up of treatment.
Trial setting and recruitment
A high proportion of trials were conducted in healthcare settings and/or recruited people with specific health needs. These included ten trials in general (non psychiatric) hospital inpatients (Simon 1997; Lewis 1998; Vial 2002; Molyneux 2003; Reid 2003; Chouinard 2005; Mohiuddin 2007; Brandstein 2011; Murray 2013; Rigotti 2014), one in emergency room patients (Bernstein 2015), five in patients awaiting admission for surgery (Ratner 2004; Villebro 2008; Sadr Azodi 2009; Thomsen 2010; Lee 2015), three in psychiatric hospital inpatients (Stockings 2014; Prochaska 2014; Hickman 2015), three for other mental health patients (Baker 2006; Hall 2006; Peckham 2015), four in outpatient substance abuse treatment programmes (Cooney 2007; Reid 2008; Carmody 2012; Winhusen 2014), one in an AIDS clinic (Wewers 2000), two for people with cancer (Wakefield 2004; Duffy 2006), and one for cancer survivors (Emmons 2005). Eight trials recruited patients of primary care clinics (Wilson 1988; Ockene 1991; Segnan 1991; Juarranz Sanz 1998; Katz 2004; Perez‐Tortosa 2015 (diabetic patients); Haas 2015) or primary care and women's health clinics (Wewers 2009), and two recruited dental clinic patients (Binnie 2007; Hanioka 2010). One recruited Chinese men with erectile dysfunction (Chan 2010). Three recruited people identified as having mild airway obstruction (Lung Health Study; Kotz 2009) or COPD (Tonnesen 2006). One recruited employees at annual occupational health checks (Rodriguez 2003). Okuyemi 2007 recruited residents of low‐income public housing departments. Schauffler 2001 recruited members of health maintenance organisations and Velicer 2006 recruited Veterans Administration medical centre patients, in both cases using proactive telephone contact. An 2006 recruited Veterans Administration medical centre patients by mail. Hall 2002, Otero 2006 and McCarthy 2008 recruited community volunteers motivated to quit and Hollis 2007 recruited callers to a quitline seeking cessation assistance.
Selection by motivation to quit
We tried to classify trials according to whether or not willingness to make a quit attempt was required for study eligibility. In some studies motivation was an explicit requirement, including four trials that enrolled motivated volunteers for pharmacotherapy trials involving placebos (Hall 2002; Tonnesen 2006; McCarthy 2008; Kotz 2009). In some study reports there was no mention of motivation as a requirement for inclusion. Some of these trials did recruit some people with no plans to quit smoking. In others, the method of recruitment or the requirement to adhere to a protocol made it seem likely that only people interested in attempting to quit would enrol. We grouped the studies as follows:
Motivation required (22 trials, 42%): Simon 1997; Lewis 1998; Wewers 2000; Hall 2002; Vial 2002; Reid 2003; Rodriguez 2003; An 2006; Baker 2006; Otero 2006; Tonnesen 2006; Cooney 2007; Hollis 2007; McCarthy 2008; Reid 2008; Kotz 2009: Chan 2010; Hanioka 2010; Carmody 2012; Rigotti 2014; Winhusen 2014; Peckham 2015.
Motivation not required but participants likely to have been interested in quitting (10 trials, 19%): Juarranz Sanz 1998; Molyneux 2003; Wakefield 2004; Mohiuddin 2007; Okuyemi 2007; Villebro 2008; Sadr Azodi 2009, Wewers 2009; Brandstein 2011; Haas 2015.
Not selected by motivation (21 trials, 40%): Wilson 1988; Ockene 1991; Segnan 1991; Lung Health Study; Schauffler 2001; Katz 2004; Ratner 2004; Chouinard 2005; Emmons 2005; Duffy 2006; Hall 2006; Velicer 2006; Binnie 2007; Thomsen 2010; Hickman 2015; Lee 2015; Murray 2013; Bernstein 2015; Perez‐Tortosa 2015, Prochaska 2014; Stockings 2014.
Participant characteristics
Trials typically had between 35 to 65% female participants. Two trials recruited only women (Wewers 2009; Thomsen 2010) and one only men (Chan 2010). Five trials in the US Veterans Administration medical system had higher proportions of men (Simon 1997; An 2006; Velicer 2006; Cooney 2007; Carmody 2012) as did a Spanish workplace trial (Rodriguez 2003). The average age typically ranged from low 40's to mid 50's. The age was younger in a trial amongst survivors of childhood cancer (Emmons 2005).
Provider characteristics
Most counselling and support was provided by specialist cessation counsellors or trained trial personnel. In a small subgroup the intervention was largely given by usual care providers including general practitioners/family physicians (Ockene 1991; Segnan 1991; Juarranz Sanz 1998; Perez‐Tortosa 2015), dental hygienists (Binnie 2007), dentists and dental hygienist (Hanioka 2010) or occupational physicians (Rodriguez 2003). Two studies used peer group counsellors (Emmons 2005;Wewers 2000) and one used trained lay advisers (Wewers 2009).
Intervention characteristics
The typical intervention involved multiple contacts with a specialist cessation adviser or counsellor, with most participants using some pharmacotherapy and receiving multiple contacts. However, there was a great deal of variation, including some interventions which involved making pharmacotherapy and behavioural components available to a large population in which take‐up of treatment was low (Schauffler 2001), or providing a brief intervention to all participants and offering stepped care for those willing to set a quit date (Reid 2003; Katz 2004). One intervention was delivered entirely by mail or prerecorded phone messages, using an expert system for tailoring contact (Velicer 2006) and two by telephone counselling alone (Hollis 2007; Haas 2015). All others included some face‐to‐face contact but additional sessions was sometimes provided by telephone. More than half the trials (n = 28, 53%) offered between four and eight sessions and a quarter (n = 13) over eight sessions. The modal category for contact time was 91 to 300 minutes (n = 22, 42%), with 17 (32%) offering between 31 and 90 minutes and eight (15%) over 300 minutes. We categorised interventions according to the maximum planned contact unless session duration was not described, so the typical time per participant would have been smaller, even in studies where the take‐up of treatment was high.
The treatment offered to the control group typically involved brief advice and self‐help materials.
Excluded studies
We list 90 studies as excluded. In most of these there was no difference between treatment conditions in the use of pharmacotherapy, and the trial tested different types or amounts of adjunct behavioural support. Trials of this type contribute to a separate review (Stead 2015). A small number of studies did not report six month or longer follow‐up. Reasons for exclusion can be found in the Characteristics of excluded studies table.
Risk of bias in included studies
Figure 1 presents review authors' judgements about each risk of bias item as percentages across all included studies. We did not judge any studies to be at risk of 'other' bias.
1.
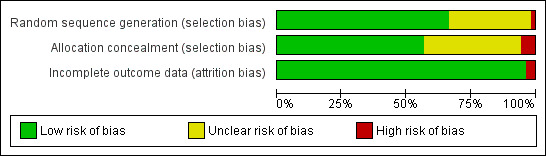
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
All studies reported that treatment allocation was random, with more than half explicitly describing an adequate method of generating the randomization schedule. One cluster randomized study was judged at high risk of bias because the unit of assignment was the ward, and an imbalance in the type of ward assigned to each condition lead to an imbalance in participant characteristics (Murray 2013). Thirty (57%) reported a procedure for allocation concealment that we judged to be at a low risk of bias. Three studies were judged at high risk of selection bias (Wilson 1988; Duffy 2006; Perez‐Tortosa 2015), two of which were cluster randomized. The participants in Wilson 1988 were recruited by receptionists who could not be blind to practice condition, and there were baseline differences in consent rate and in motivation to quit between conditions. Some participants in Perez‐Tortosa 2015 were recruited in health centres after allocation. Duffy 2006 recruited cancer patients with either smoking, alcohol or depression problems, and had more smokers in the intervention group suggesting possible recruitment bias. The other 20 (38%) trial reports gave too little information about allocation procedures to be certain that the risk of bias was low, and were hence judged to be at unclear risk of bias in this domain.
Blinding
We did not formally evaluate blinding of participants, providers or other personnel. It was almost always unclear whether or not participants would have known that they were in a control condition, but most controls did include advice and support for smoking cessation. Providers could not have been blind to treatment condition. Self‐reported smoking status was biochemically validated at longest follow‐up in 35 studies, with twelve of these measuring cotinine and the remainder carbon monoxide (CO). Seven studies either did not collect samples at final follow‐up (Sadr Azodi 2009; Bernstein 2015; Lee 2015) or did not obtain samples from enough participants and did not report validated quit rates (Reid 2003; Katz 2004; Villebro 2008; Hickman 2015). Eleven studies did not attempt any type of validation (Ockene 1991; Schauffler 2001; Emmons 2005; An 2006; Duffy 2006; Otero 2006; Velicer 2006; Hollis 2007; Thomsen 2010; Brandstein 2011; Haas 2015).
Incomplete outcome data
We classified two studies at high risk of attrition bias. In Hanioka 2010 a number of control participants declined consent once told their treatment group. In Perez‐Tortosa 2015 a number of participants were excluded from analyses because of a lack of baseline data, and losses to follow‐up were also excluded from reported analyses. In other studies, later dropouts were counted as continuing smokers, and all other trials were classified as low risk of bias due to loss to follow‐up. Most trials lost less than 20% of each condition. There were a small number of trials in which the proportion lost to follow‐up was over 20% and also differed between groups. Of the trials at potential risk of bias, Binnie 2007 and Villebro 2008 had high and differential losses, but since both were small trials any effect on the meta‐analysis of different assumptions would be small. Hall 2006 and Hollis 2007 both had relatively high losses but both reported that different assumptions about the smoking status of those lost to follow‐up would not be likely to alter their relative effects.
Effects of interventions
See: Table 1
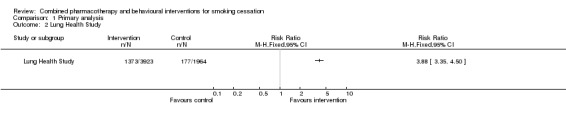
A pooled estimate combining all 53 included studies using a Mantel‐Haenszel fixed‐effect model had a very high level of heterogeneity (I² = 70%, data not shown). This heterogeneity was attributable to the Lung Health Study which showed a very strong intervention effect (relative risk [RR] 3.88, 95% confidence interval [CI] 3.35 to 4.50). This study had a particularly intensive intervention; the behavioural component was a group‐based 12 session course, and nicotine gum was available without charge for six months. Removing this study from the meta‐analysis reduced heterogeneity (I² = 36%), and a benefit of intervention was still detected (RR 1.83, 95% CI 1.68 to 1.98, 19319 participants, Figure 2, Analysis 1.1, Table 1). Only three trials (Wakefield 2004; Ratner 2004; Okuyemi 2007) had lower quit rates in the intervention than the control group and all had wide confidence intervals.
2.
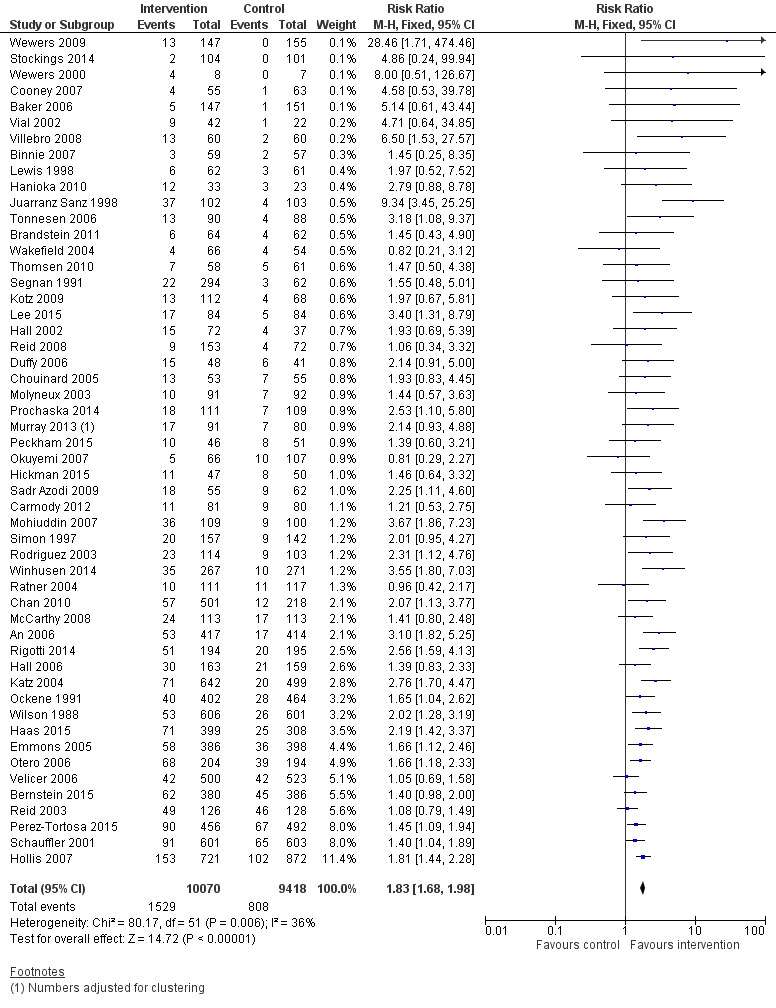
Combined intervention versus control. Cessation at longest follow‐up.
1.1. Analysis.
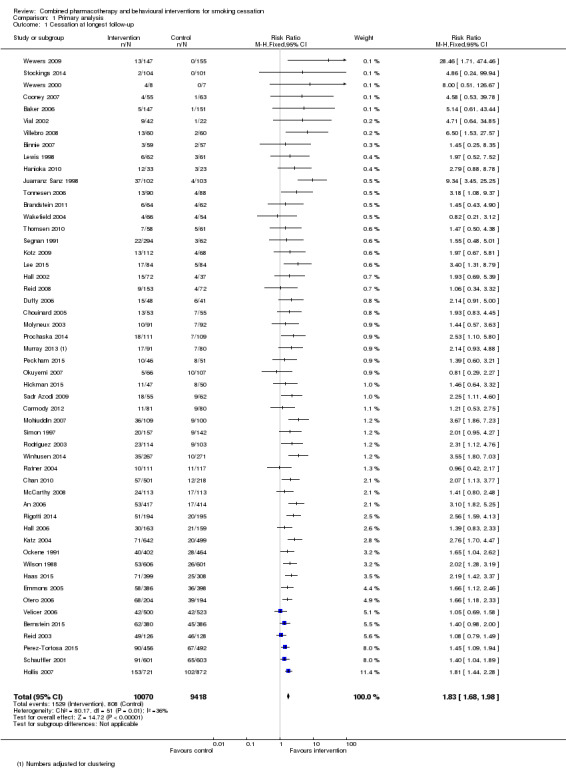
Comparison 1 Primary analysis, Outcome 1 Cessation at longest follow‐up.
There was some evidence of asymmetry in a funnel plot; there was an excess of small trials detecting larger effects, suggesting the possibility of publication or other bias. In a sensitivity analysis, removing smaller studies did not markedly decrease the pooled estimate.
Subgroup analyses
Appendix 2 lists study characteristics used for subgroup analyses for each included study. All the subgroup analyses reported below exclude the Lung Health Study.
Effect of setting
The pooled estimate for trials that recruited participants in healthcare settings (RR 1.97, 95% CI 1.79 to 2.18, 43 trials, 13863 participants) was significantly higher than that for trials that recruited volunteers in other settings (RR 1.53, 95% CI 1.33 to 1.76, 8 trials, 4906 participants) (Analysis 2.1). There were more small trials with large and significant effects in the healthcare subgroup, and a number of these had notably low quit rates in the control arms.
2.1. Analysis.
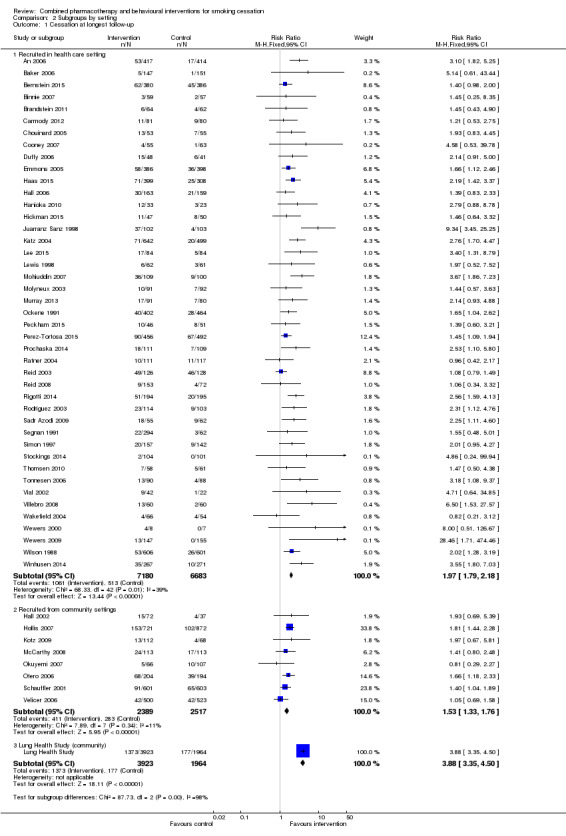
Comparison 2 Subgroups by setting, Outcome 1 Cessation at longest follow‐up.
Effect of selection by motivation to quit
We did not detect evidence that the relative effect of the intervention differed according to whether participants were prepared to make a quit attempt or not (Analysis 3.1). The subgroup of participants selected for motivation had a slightly larger estimated effect (RR 1.90, 95% CI 1.68 to 2.15, 22 trials, 7088 participants) than the 'Not selected' subgroup (RR 1.60, 95% CI 1.42 to 1.80, 20 trials, 10138 participants); the largest effect estimate was in the subgroup of 10 trials that did not explicitly select for motivation, but that seemed unlikely to recruit unmotivated participants (RR 2.71, 95% CI 2.11 to 3.49, 2262 participants). There was also considerable heterogeneity in this subgroup (I² = 61%), for which there was no obvious explanation. In a meta‐regression, motivation to quit was not found to be an effect modifier (p = 0.09). In this update there was no longer any sign that average control group quit rates were higher in the more motivated populations.
3.1. Analysis.
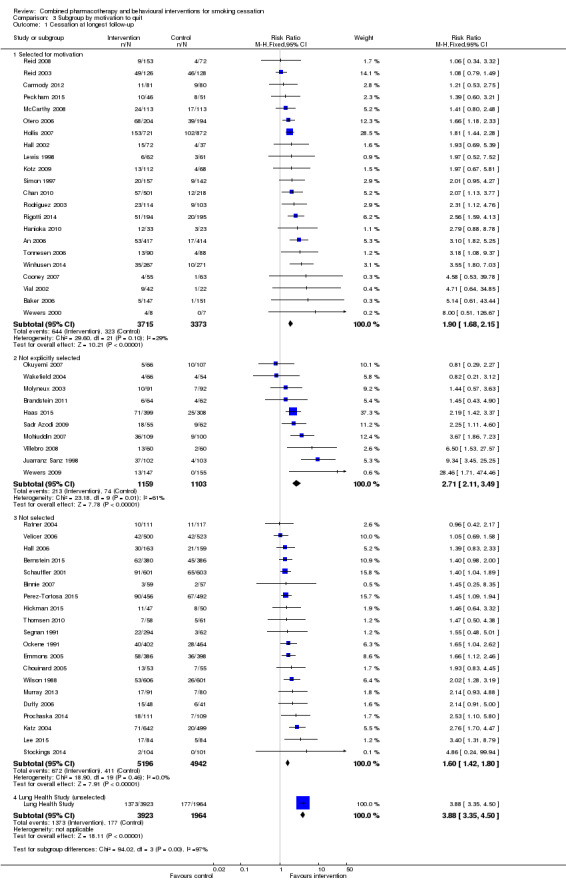
Comparison 3 Subgroup by motivation to quit, Outcome 1 Cessation at longest follow‐up.
Effect of provider
The behavioural intervention was provided by specialists in cessation counselling in 39 of the trials. In nine trials, the support/counselling was given by a non specialist healthcare professional involved in usual care. In a further two trials behavioural support was provided by a peer counsellor, one of which was a very small pilot (Wewers 2000). One trial used trained lay advisers (Wewers 2009). One trial (Velicer 2006) had no person‐to‐person contact and behavioural support was provided by an 'expert system' generating individualised written materials and prerecorded telephone messages. There was no important difference between the specialist care subgroup (RR 1.81, 95% CI 1.64 to 1.99, I² = 25%, 39 trials, 12252 participants) and the subgroup where counselling was linked to usual care (RR 2.03, 95% CI 1.70 to 2.43, I² = 54%, 9 trials, 5112 participants) (Analysis 4.1). In the previous version of the review there was a difference by provider that might have been attributable to confounding with take‐up of treatment and duration of contact. For this update, we did three separate exploratory meta‐regressions controlling for type of provider, take‐up of treatment and duration of contact; none was an effect modifier (p‐values 0.37, 0.08 and 0.46 respectively).
4.1. Analysis.
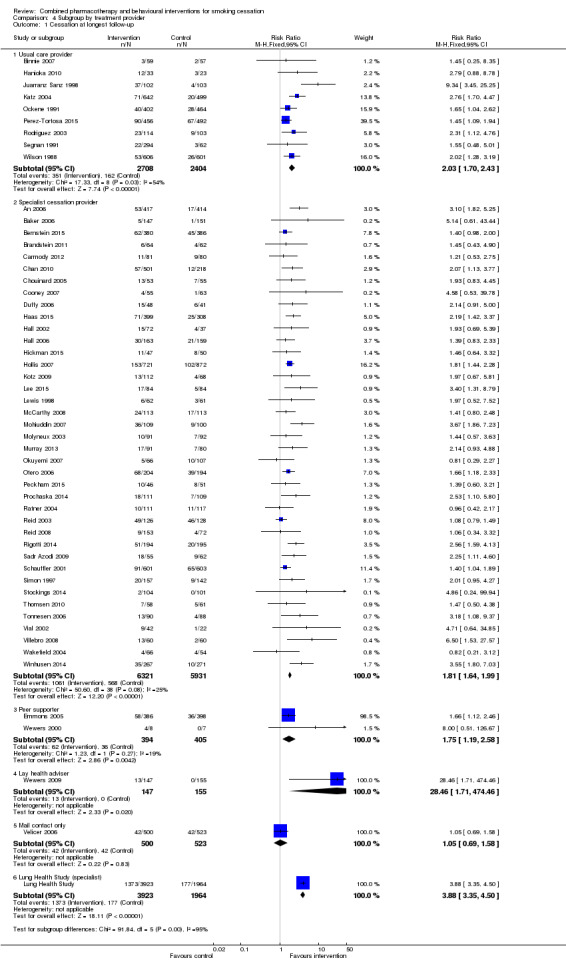
Comparison 4 Subgroup by treatment provider, Outcome 1 Cessation at longest follow‐up.
Effect of intensity
We categorised trials by intended number of sessions and planned total duration of contact. Not all interventions prescribed a fixed number or standardised length of sessions, and not all participants received all planned contacts. Unsurprisingly there was some correlation between number of sessions and duration of contact; for example, all interventions that intended to provide at least 300 minutes of contact had at least four sessions scheduled. Where there was personal contact, there was only weak evidence that studies offering more sessions had larger effects (Analysis 5.1); the subgroup of trials offering eight or more sessions had the largest estimate (RR 2.10, 95% CI 1.65 to 2.68, 13 trials, 2270 participants) but CIs overlapped. One to three and four to eight session categories had almost the same effects. There was no clear evidence that increasing the duration of personal contact increased the effect either (Analysis 6.1). Estimates for each subgroup overlapped. There was more heterogeneity in the 31 to 90 minute category (I² = 55%), partially attributable to Juarranz Sanz 1998, (RR 9.34). In an exploratory meta‐regression neither number (p = 0.85) nor duration (p = 0.46) alone or in combination (p = 0.73) were effect modifiers, nor was take‐up in combination with these (p = 0.36).
5.1. Analysis.
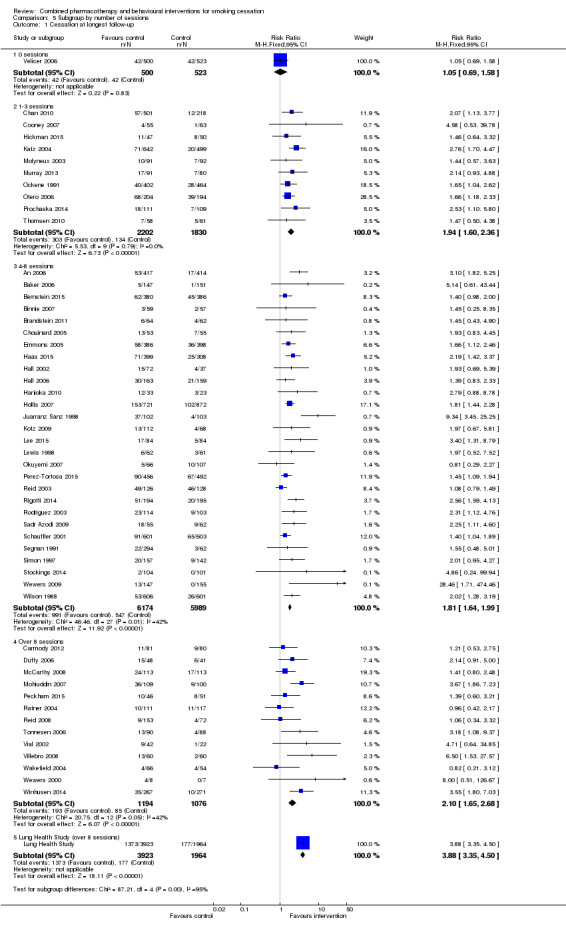
Comparison 5 Subgroup by number of sessions, Outcome 1 Cessation at longest follow‐up.
6.1. Analysis.
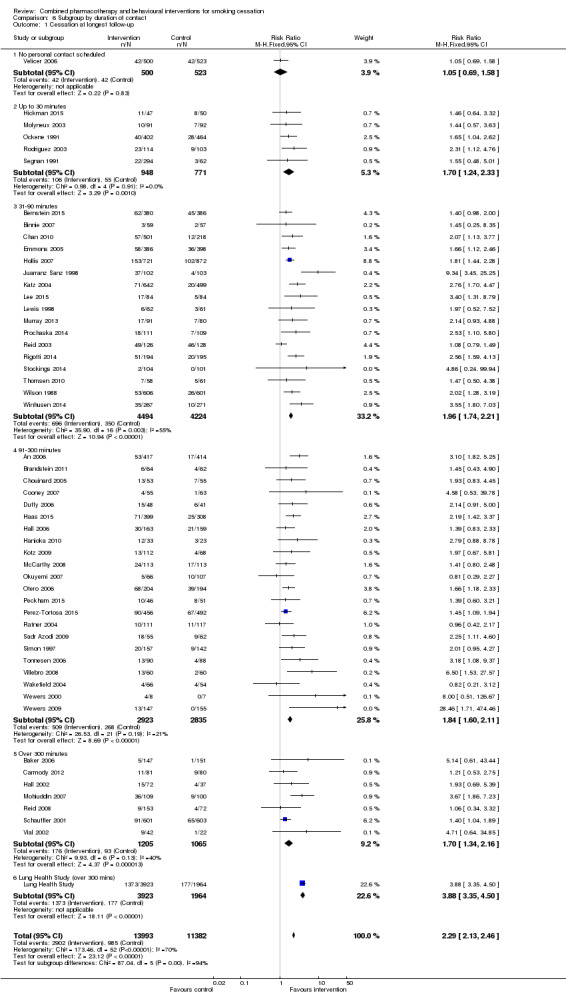
Comparison 6 Subgroup by duration of contact, Outcome 1 Cessation at longest follow‐up.
Effect of differences in treatment take‐up
Only three trials were classified as 'low take‐up of treatment' (Katz 2004; Reid 2003; Schauffler 2001), and in these the estimated effect was smaller, whilst there was little difference between the 18 that were moderate and 29 that were high (Analysis 7.1). As noted above in the analyses of intensity, there was no longer any evidence from meta‐regression of an effect of intensity even in the subgroup of 'high take‐up' trials.
7.1. Analysis.
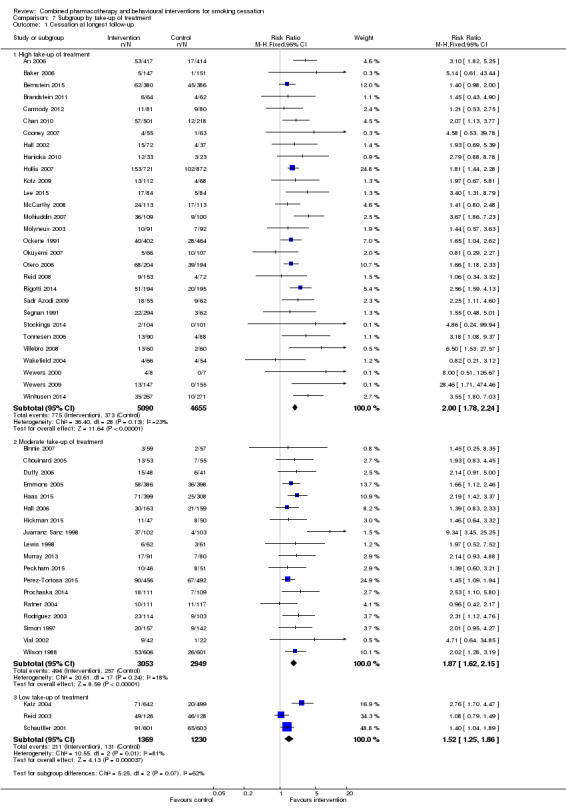
Comparison 7 Subgroup by take‐up of treatment, Outcome 1 Cessation at longest follow‐up.
Direct tests of intensity of support
Two trials compared multiple intensities of support. In both cases the more intensive condition was compared to the control in the primary analysis. Hollis 2007 offered up to four additional telephone calls in the intensive counselling condition compared to a 30 to 40 minute motivational interview and a single follow‐up call in the moderate condition but this did not significantly increase quit rates. Higher intensity participants had on average only about 14 minutes more contact. Otero 2006 randomized to one, two, three or four weekly hour‐long sessions but pooled reported outcomes for one to two and three to four sessions. Direct comparisons of different intensities of behavioural therapy as adjuncts to pharmacotherapy are covered in a separate review (Stead 2015).
Discussion
Behavioural support and certain pharmacotherapies increase the chance of successful cessation for people trying to quit. These effects have been confirmed by reviews of trials that synthesise the results of trials of both modalities of treatment. Nicotine replacement therapy (Stead 2012), bupropion and nortriptyline (Hughes 2014) and varenicline and cytisine (Cahill 2012) increase quit rates. Group therapy (Stead 2005), individual counselling (Lancaster 2005) and telephone counselling (Stead 2013) have all been shown to be successful ways of delivering behavioural support for cessation with some support for individually tailored written self‐help materials (Hartmann‐Boyce 2014). The effects of the two treatment modalities are largely assumed to be independent, although behavioural support may influence correct use of medication. Although guidelines support combining approaches where possible, the size of effect that might be expected when both therapies are combined has not been clear.
Summary of main results
In this review of 53 trials evaluating interventions that combine pharmacotherapy with behavioural support, we found, unsurprisingly, that the combination improves quit rates compared to no treatment or a minimum intervention (see Table 1). This finding is in accord with the evidence that each type of intervention is effective when evaluated independently. A strength of the evidence from these trials is that the results are largely consistent, with little evidence of clinically important heterogeneity even though they use a wide range of approaches in many different populations and settings.
One trial demonstrated a large benefit of a multimodal therapy. The Lung Health Study, conducted in the early 1990s, achieved a cessation rate of 35% at one year in the intervention group, compared with 9% for the control. The investigators also reported sustained benefits after five years, and demonstrated reduced mortality in the intervention group. As noted above, this was a particularly intensive intervention. Participants were offered maintenance sessions, and repeat treatment was available for those failing to quit. In addition, all participants had mild airway impairment, and intervention group participants were further randomized to use a bronchodilator or placebo inhaler. This component might have increased motivation for quitting, and the relatively high rates of cessation in the control group would support this.
The pooled estimate for the remaining 52 trials (RR 1.83, 95% CI 1. 68 to 1.98) suggests that a combined intervention might typically increase cessation success by 70 to 100%. Most of the trials in this review offered one or more types of NRT, or bupropion. Based on estimates from Cochrane reviews of the effects for NRT alone (pooled estimate from 117 trials RR 1.60, 95% CI 1.53 to 1.68, Stead 2012) and bupropion alone (pooled estimate from 44 trials, RR 1.62, 95% CI 1.49 to 1.76, Hughes 2014), the additional benefit from the behavioural component might seem small. However it may be misleading to directly compare these estimates, and we did not attempt any formal statistical comparison.
There are important differences between the trials included in this review and typical pharmacotherapy studies that should be noted. Pharmacotherapy trials included in meta‐analyses typically have a placebo control, but the control group also receives identical behavioural support to the active therapy group. The intensity may vary from brief advice on correct use of pharmacotherapy and provision of self‐help materials, to multiple counselling sessions. The trial protocol may call for frequent contact with a clinical research centre, even if counselling contact is limited. Participants may have high expectations for the effect of treatment, but also the knowledge that they could be receiving placebo. In contrast, in the studies included in this review the control groups had limited support, but this typically involved advice that could be classified as a cessation intervention in other contexts. Additionally, it was generally unclear whether controls would have known the components of the active intervention and we did not attempt to assess the risk of bias from lack of blinding. In almost all the trials, intervention group participants would have known they were receiving active medication, but without the connotations of receiving a 'new' drug. Apart from the small number of included trials that were placebo‐controlled factorial studies of medication and behavioural components (Hall 2006; Tonnesen 2006; McCarthy 2008), trials in this review had pragmatic designs and the intervention typically involved an offer of treatment. Actual use of medication and take‐up of a full programme of behavioural support was not uniform across trials.
We had previously detected weak evidence for possible effect modifiers using subgroup analyses and meta‐regression. For most of the subgroups, any differential effects have become smaller and were no longer evident in meta‐regressions. We did find evidence of larger effects when trials recruited participants in health care settings. We did not find evidence that the type of provider, the motivation of the participants, the intensity of behavioural support or the amount of support taken up by participants affected the estimates of relative effects. Almost all of the interventions in this review involved multiple sessions, with some studies providing or offering more than eight sessions and as much as eight hours of contact, although the typical intensity was much lower. We no longer detected evidence from indirect comparisons that increasing contact increased quit success in the trials with the highest take‐up of treatment. Stronger evidence for a dose‐response trend might have been obscured by the multiple differences between trials. We used meta‐regression for exploratory analyses of some potential effect modifiers but the relatively small number of trials and large number of variables reduces the power of this approach. We might not have been able to identify or quantify possible moderators. For example, more intensive support might have been tested in 'hard to treat' populations but it is not clear how this might be characterised. It seems unlikely that the number of supportive contacts and their length would have absolutely no effect on outcome, but our findings suggest that the added benefit from offering more intensive support may be small. One possible explanation is that the use of pharmacotherapy attenuates the importance of the behavioural support. Healthcare providers have an important role in convincing smokers of the importance of attempting to quit and making pharmacotherapy and behavioural support available. We did not find evidence from indirect comparison that counselling by trained specialists was critical for success; in fact the estimated treatment effect was higher in the smaller group of trials where the behavioural support was provided by non specialists, although we do not think great importance should be attached to this finding.
Overall completeness and applicability of evidence
These trials have been undertaken in a very wide range of settings using different providers of care and amongst different populations. The populations include people with mental illness and smoking related diseases. The relative homogeneity of their results therefore supports the general applicability of the evidence. Although most of the trials provided one or more types of NRT, and a small number offered bupropion, there is no reason to suppose that the results would not apply to interventions that offered varenicline.
Using a pharmacotherapy and accessing behavioural support will increase the chance of giving up smoking, but people who smoke are unlikely to use this combination when making a quit attempt. A US survey in 2003 found that only 5.9% of those making a quit attempt in the previous year had used combined behavioural and pharmacologic treatment (Shiffman 2008). An English survey of smokers between 2006 and 2012 found that 4.8% of people attempting to quit smoking had used both a prescription pharmacotherapy and specialist behavioural support (Kotz 2014).
Quality of the evidence
The majority of studies were judged to be at low or unclear risk of bias, and only five of the included studies were judged to be at high risk of bias in one or more domains. The results of the meta‐analysis were not sensitive to the exclusion of any single trial. Excluding studies that did not use biochemical validation did not reduce the effect size. The largest study, Hollis 2007, was atypical in that all contact was telephone‐based, via a quitline (most other studies included some face‐to‐face contact); it also had a potential methodological weakness due to losses to follow‐up and lack of biochemical validation, although we did not judge these to put it at a high risk of bias. Excluding this study did not alter the effect estimate.
Potential biases in the review process
We used the Cochrane Tobacco Addiction Specialised Register to identify studies. The Register includes reports of trials identified from the major bibliographic databases. There is no straightforward term for the type of intervention we were interested in, but we screened any trial report that mentioned a pharmacotherapy. It is possible that the Register does not include all relevant trial reports or that we failed to identify some. Our methods for data extraction and analysis are those used for other Cochrane reviews. The practice of imputing missing data as smoking is standard practice for primary and secondary research in smoking cessation and has the advantage that absolute cessation rates are not inflated by ignoring loss to follow‐up. Bias in the relative effect will only be introduced if misclassification differs for people who are lost from the intervention condition compared to the control. If proportionately more of those who are lost in the control group are assumed to be smokers but have in fact quit then the treatment effect would be overestimated.
Agreements and disagreements with other studies or reviews
The results of this review are broadly in agreement with other reviews and guidelines (Hughes 1995; Reus 2008). US Guidelines (Fiore 2008) endorse a dose‐response relationship for total amount of contact time (up to 300 minutes) and number of sessions, as well as session length. Their meta‐analyses suggest clear trends although there were not necessarily significant differences between adjacent categories. There were however clear differences between for example 4 to 30 minutes of contact time (odds ratio (OR) 1.9, 95% CI 1.5 to 2.3) and 91 to 300 minutes (OR 3.2, 95% CI 2.3 to 4.6) (Fiore 2008 table 6.9) and between two to three treatment sessions (OR 1.4, 95% CI 1.1 to 1.7) and over eight sessions (OR 2.3, 95% CI 2.1 to 3.0) (Fiore 2008 table 6.10). Our estimates comparing subgroups of trials classified by within trial differences in intensity show less clear evidence of a dose‐response effect, although they do not exclude there being one.
Authors' conclusions
Implications for practice.
Interventions that combine pharmacotherapy and behavioural support increase smoking cessation success in a wide range of settings and populations, compared to a minimal intervention or usual care. This suggests that clinicians should encourage smokers to use both types of aid. Offering more intensive behavioural support was not shown to be associated with larger treatment effects; this may be because intensive interventions are more difficult to deliver consistently to participants.
Implications for research.
It is unlikely that further trials will alter the main findings of this review, although they may contribute to further understanding about the effects of treatment in particular settings or in populations of smokers.
What's new
| Date | Event | Description |
|---|---|---|
| 16 February 2016 | New search has been performed | Searches updated; twelve new studies included. |
| 16 February 2016 | New citation required but conclusions have not changed | Two additional authors. No material change to pooled estimates. |
History
Protocol first published: Issue 1, 2010 Review first published: Issue 10, 2012
| Date | Event | Description |
|---|---|---|
| 14 November 2012 | Amended | Contact details updated. Reference to companion review 'Behavioural interventions as adjuncts to pharmacotherapy for smoking cessation' updated to reflect publication in issue 12, 2012. |
Appendices
Appendix 1. Register Search
Version of the search used in the Cochrane Register of Studies:
1 NRT:TI,AB,KW 679
2 (nicotine NEAR (replacement OR patch* OR transdermal OR gum OR lozenge* OR sublingual OR inhaler* OR inhalator* OR oral OR nasal OR spray)):TI,AB,KW 1796
3 (Bupropion OR zyban OR wellbutrin):TI,AB,KW,MH,EMT 546
4 (varenicline OR champix OR chantix):TI,AB,KW,MH,EMT 278
5 combined modality therapy:MH,KW 213
6 ((behavio?r therapy) AND (drug therapy)):KW,MH,EMT,TI,AB 62
7 ((counsel*) AND (*drug therapy)):KW,MH,EMT,TI,AB 185
8 #1 OR #2 OR #3 OR #4 OR #5 2454
9 #6 OR #7 OR #8 2509
10 #9 AND INREGISTER 2200
Appendix 2. Summary of included study characteristics
| Study | Recruitment setting/ Provider |
Selected for motivation to quit |
Sessions/ Duration |
Take‐up |
| An 2006 | Veterans Administration medical centres/ Cessation specialist (telephone counsellor) |
Selected | 4‐8 / 91‐300 mins | High |
| Baker 2006 | Community Health Agencies (mental health patients)/ Cessation specialist |
Selected | 4‐8 / >300 mins | High |
| Bernstein 2015 | Emergency department/ Specialist (trained research assistant) |
Not selected | 4‐8 / 31‐90 mins | High |
| Binnie 2007 | Periodontology clinic/ Dental Hygienist (UC) |
Not selected | 4‐8 / 31‐90 mins | Moderate |
| Brandstein 2011 | Hospital inpatients/ Cessation specialist (telephone counsellor) |
Not explicit | 4‐8 / 91‐300 mins | High |
| Carmody 2012 | Alcohol treatment patients/ Cessation specialists |
Selected | >8 / >300 mins | High |
| Chan 2010 | Clinic patients and volunteers/ Cessation specialist |
Selected | 1‐3 / 31‐90 mins | High |
| Chouinard 2005 | Hospital inpatients/ Cessation specialist (reseach nurse) |
Not selected | 4‐8 / 91‐300 mins | Moderate |
| Cooney 2007 | Substance abuse programmes/ Cessation specialist |
Selected | 1‐3 / 91‐300 mins | High |
| Duffy 2006 | ENT clinic cancer patients/ Cessation specialist |
Not selected | >8 / 91‐300 mins | Moderate |
| Emmons 2005 | Childhood cancer survivors study/ Peer counsellor |
Not selected | 4‐8 / 31‐90 mins | Moderate |
| Haas 2015 | Primary care patients/ Cessation specialist |
Not explicit | 4‐8 / 91‐300 mins | Moderate |
| Hall 2002 | Community/ Cessation specialist |
Selected | 4‐8 />300 mins | High |
| Hall 2006 | Mental health clinics/ Cessation specialist |
Not selected | 4‐8 / 91‐300 mins | Moderate |
| Hanioka 2010 | Dental clinics/ Dentists and dental hygienists (UC) |
Selected | 4‐8 / 91‐300 mins | High |
| Hickman 2015 | Psychiatric inpatients/ Cessation specialist (study staff) |
Not selected | 1‐3 / 4‐30 mins | Moderate |
| Hollis 2007 | Community/ Cessation specialist (telephone counsellor) |
Selected | 4‐8 / 31‐90 mins | High |
| Juarranz Sanz 1998 | Primary Care Clinic/ General practitioner (UC) |
Not explicit | 4‐8 / 31‐90 mins | Moderate |
| Katz 2004 | Primary Care Clinic/ UC (low take‐up of specialist referral) |
Not selected | 1‐3 / 31‐90 mins | Low |
| Kotz 2009 | Community/ Cessation specialist (respiratory nurse) |
Selected | 4‐8 / 91‐300 mins | High |
| Lee 2015 | Presurgical clinic/ Nurse & specialist (telephone counsellor) |
Not selected | 4‐8 / 31‐90 mins | Moderate |
| Lewis 1998 | Hospital inpatients/ Cessation specialist (research nurse) |
Selected | 4‐8 / 31‐90 mins | Moderate |
| Lung Health Study | Community/ Cessation specialist |
Not selected | >8 / >300 mins | High |
| McCarthy 2008 | Community/ Cessation specialist |
Selected | >8 / 91‐300 mins | High |
| Mohiuddin 2007 | Hospital inpatients/ Cessation specialist |
Not explicit | >8 / >300 mins | High |
| Molyneux 2003 | Hospital inpatients/ Cessation specialist |
Not explicit | 1‐3 / up to 30 mins | High |
| Murray 2013 | Hospital inpatients/ Cessation specialists |
Not selected | 1‐3 31‐90 minutes | Moderate |
| Ockene 1991 | Primary Care Clinic/ Physician resident (UC) |
Not selected | 1‐3 / up to 30 mins | High |
| Okuyemi 2007 | Community/ Cessation specialist |
Not explicit | 4‐8 / 91‐300 mins | High |
| Otero 2006 | Community/ Cessation specialist |
Selected | 1‐3 / 91‐300 mins | High |
| Peckham 2015 | Mental Health Services/ Trained mental health professional |
Selected | >8 / 91‐300 mins | Moderate |
| Perez‐Tortosa 2015 | Primary care (diabetic patients)/ Primary care teams |
Not selected | 4‐8/ 91‐300 mins | Moderate |
| Prochaska 2014 | Psychiatric inpatients/ Cessation specialist |
Not selected | 1‐3/ 31‐90 mins | Moderate |
| Ratner 2004 | Preadmission clinic/ Specialist (Trained nurse) |
Not selected | >8 / 91‐300 mins | Moderate |
| Reid 2003 | Hospital inpatients/ Specialist (nurse counsellor) |
Selected | 4‐8 / 31‐90 mins | Low |
| Reid 2008 | Drug & alcohol dependence treatment/ Cessation specialist |
Selected | >8 / >300 mins | High |
| Rigotti 2014 | Hospital inpatients/ Cessation specialist |
Selected | 4‐8/ 31‐90 mins | High |
| Rodriguez 2003 | Worksite occupation health/ Occupational physician (UC) |
Selected | 4‐8 / up to 30 mins | Moderate |
| Sadr Azodi 2009 | Presurgical clinics/ Cessation specialist |
Not explicit | 4‐8 / 91‐300 mins | High |
| Schauffler 2001 | Community/ Cessation specialist |
Not selected | 4‐8 / >300 mins | Low |
| Segnan 1991 | Primary Care Clinics/ GP (UC) |
Not selected | 4‐8 / up to 30 mins | High |
| Simon 1997 | Hospital inpatients/ Cessation specialist |
Selected | 4‐8 / 91‐300 mins | Moderate |
| Stockings 2014 | Psychiatric inpatients/ Cessation specialist |
Not selected | 4‐8 / 31‐90 mins | High |
| Thomsen 2010 | Surgical clinics/ Cessation specialist |
Not selected | 1‐3 / 31‐90 mins | Unclear |
| Tonnesen 2006 | Outpatient chest clinic/ Specialist (trained nurse) |
Selected | >8 / 91‐300 mins | High |
| Velicer 2006 | Community/ Expert system, no provider |
Not selected | No personal contact | N/A |
| Vial 2002 | Hospital inpatients/ Specialist (Pharmacist) |
Selected | >8 / >300 mins | Moderate |
| Villebro 2008 | Presurgical clinic/ Specialist (trial nurse) |
Not explicit | >8 / 91‐300 mins | High |
| Wakefield 2004 | Cancer treatment units/ Specialist (trial co‐ordinator) |
Not explicit | >8 / 91‐300 mins | High |
| Wewers 2000 | AIDS clinical trial unit/ Peer counsellor |
Selected | >8 / 91‐300 mins | High |
| Wewers 2009 | Primary Care Clinics/ Lay health adviser |
Not explicit | 4‐8 / 91‐300 mins | High |
| Wilson 1988 | Primary Care Clinics/ Family physician (UC) |
Not selected | 4‐8 / 31‐90 mins | Moderate |
| Winhusen 2014 | Substance use disorder clinics Cessation specialist |
Selected | >8 / 31‐90 mins | High |
Data and analyses
Comparison 1. Primary analysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cessation at longest follow‐up | 52 | 19488 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.83 [1.68, 1.98] |
| 2 Lung Health Study | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
1.2. Analysis.
Comparison 1 Primary analysis, Outcome 2 Lung Health Study.
Comparison 2. Subgroups by setting.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cessation at longest follow‐up | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Recruited in health care setting | 43 | 13863 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.97 [1.79, 2.18] |
| 1.2 Recruited from community settings | 8 | 4906 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [1.33, 1.76] |
| 1.3 Lung Health Study (community) | 1 | 5887 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.88 [3.35, 4.50] |
Comparison 3. Subgroup by motivation to quit.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cessation at longest follow‐up | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Selected for motivation | 22 | 7088 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.90 [1.68, 2.15] |
| 1.2 Not explicitly selected | 10 | 2262 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.71 [2.11, 3.49] |
| 1.3 Not selected | 20 | 10138 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.60 [1.42, 1.80] |
| 1.4 Lung Health Study (unselected) | 1 | 5887 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.88 [3.35, 4.50] |
Comparison 4. Subgroup by treatment provider.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cessation at longest follow‐up | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Usual care provider | 9 | 5112 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.03 [1.70, 2.43] |
| 1.2 Specialist cessation provider | 39 | 12252 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.81 [1.64, 1.99] |
| 1.3 Peer supporter | 2 | 799 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [1.19, 2.58] |
| 1.4 Lay health adviser | 1 | 302 | Risk Ratio (M‐H, Fixed, 95% CI) | 28.46 [1.71, 474.46] |
| 1.5 Mail contact only | 1 | 1023 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.69, 1.58] |
| 1.6 Lung Health Study (specialist) | 1 | 5887 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.88 [3.35, 4.50] |
Comparison 5. Subgroup by number of sessions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cessation at longest follow‐up | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 0 sessions | 1 | 1023 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.69, 1.58] |
| 1.2 1‐3 sessions | 10 | 4032 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.94 [1.60, 2.36] |
| 1.3 4‐8 sessions | 28 | 12163 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.81 [1.64, 1.99] |
| 1.4 Over 8 sessions | 13 | 2270 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.10 [1.65, 2.68] |
| 1.5 Lung Health Study (over 8 sessions) | 1 | 5887 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.88 [3.35, 4.50] |
Comparison 6. Subgroup by duration of contact.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cessation at longest follow‐up | 53 | 25375 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.29 [2.13, 2.46] |
| 1.1 No personal contact scheduled | 1 | 1023 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.69, 1.58] |
| 1.2 Up to 30 minutes | 5 | 1719 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.70 [1.24, 2.33] |
| 1.3 31‐90 minutes | 17 | 8718 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.96 [1.74, 2.21] |
| 1.4 91‐300 minutes | 22 | 5758 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.84 [1.60, 2.11] |
| 1.5 Over 300 minutes | 7 | 2270 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.70 [1.34, 2.16] |
| 1.6 Lung Health Study (over 300 mins) | 1 | 5887 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.88 [3.35, 4.50] |
Comparison 7. Subgroup by take‐up of treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cessation at longest follow‐up | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 High take‐up of treatment | 29 | 9745 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.00 [1.78, 2.24] |
| 1.2 Moderate take‐up of treatment | 18 | 6002 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.87 [1.62, 2.15] |
| 1.3 Low take‐up of treatment | 3 | 2599 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [1.25, 1.86] |
Comparison 8. Subgroup by treatment take‐up, specialist support only.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cessation at longest follow‐up | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 High take‐up of treatment | 25 | 8452 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.01 [1.78, 2.27] |
| 1.2 Moderate take‐up of treatment | 12 | 2525 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.84 [1.49, 2.28] |
| 1.3 Low take‐up of treatment | 2 | 1458 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [1.02, 1.58] |
8.1. Analysis.
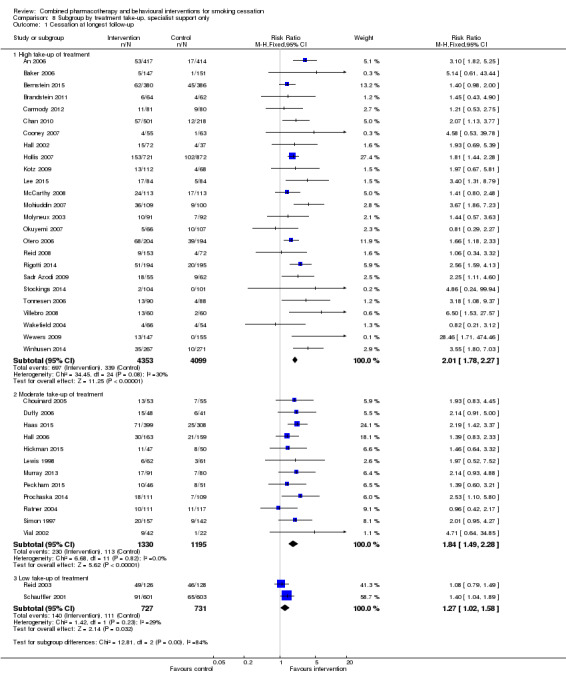
Comparison 8 Subgroup by treatment take‐up, specialist support only, Outcome 1 Cessation at longest follow‐up.
Comparison 9. Subgroup by number of sessions, high take‐up only.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cessation at longest follow‐up | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 1‐3 sessions | 5 | 2284 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.74 [1.37, 2.22] |
| 1.2 4‐8 sessions | 15 | 5669 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.01 [1.72, 2.33] |
| 1.3 Over 8 sessions | 9 | 1792 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.33 [1.76, 3.09] |
9.1. Analysis.
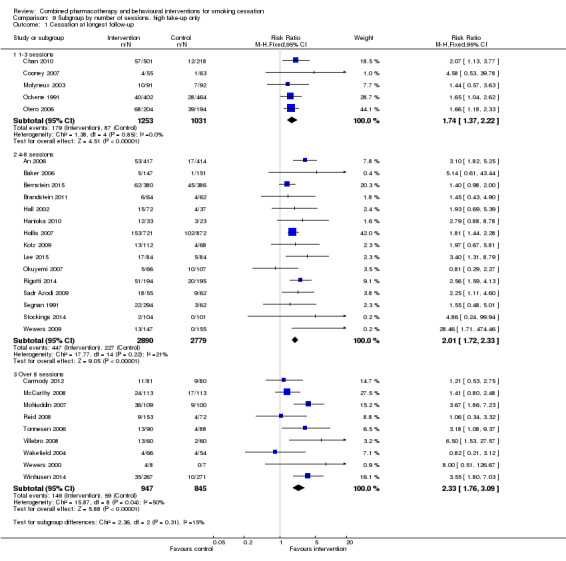
Comparison 9 Subgroup by number of sessions, high take‐up only, Outcome 1 Cessation at longest follow‐up.
Comparison 10. Subgroup by duration of contact, high take‐up only.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cessation at longest follow‐up | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 up to 30 minutes | 3 | 1405 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.60 [1.08, 2.36] |
| 1.2 31‐90 minutes | 7 | 4378 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.96 [1.66, 2.31] |
| 1.3 91‐300 minutes | 14 | 2960 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.14 [1.74, 2.62] |
| 1.4 Over 300 minutes | 5 | 1002 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.20 [1.46, 3.32] |
10.1. Analysis.
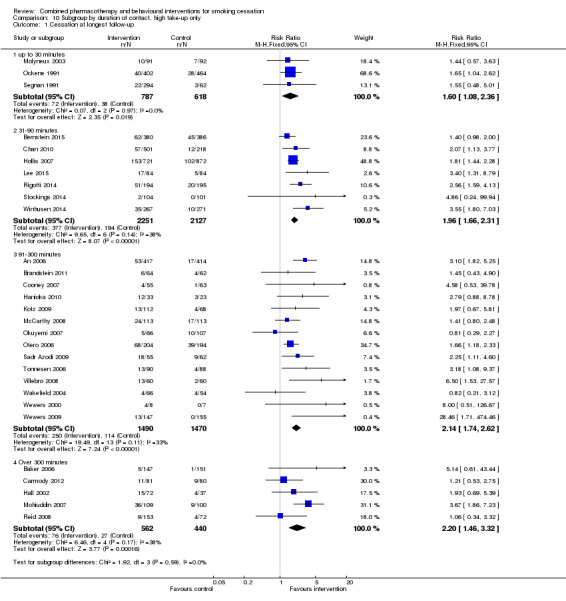
Comparison 10 Subgroup by duration of contact, high take‐up only, Outcome 1 Cessation at longest follow‐up.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
An 2006.
| Methods | Setting: 5 Veterans Administration medical centres, USA Recruitment: by mail, prepared to quit in next 30 days | |
| Participants | 821 smokers interested in quitting (excludes 16 deaths, 1 withdrawal); 91% M, av. age 57, av. cpd 26. 26% had > 7d abstinence in previous year, 44% ever use of bupropion, 82% ever use NRT Provider: Specialist, telephone counsellors |
|
| Interventions | 1. Mailed S‐H and standard care; opportunity for intervention during routine care and referral to individual or group cessation programmes. NRT & bupropion avail on formulary 2. As 1, plus proactive TC, modified California helpline protocol, 7 calls over 2m, relapse sensitive schedule additional calls possible, multiple quit attempts. NRT & bupropion available, could be mailed directly after screening & primary provider approval for bupropion | |
| Outcomes | Abstinence at 12m (sustained from 6m, 7‐day PP also reported) Validation: none | |
| Notes | Pharmacotherapy was available to control group, but intervention substantially increased use; 86% vs 30% reported use at 3m. Treatment effect greater for sustained quitting than PP. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 16 deaths (10 I; 6 C) & 1 withdrawal excluded from denominators. Other losses assumed smoking |
Baker 2006.
| Methods | Setting: research clinics, Sydney & Newcastle, Australia Recruitment: referrals, mainly from community health agencies, interested in quitting | |
| Participants | 298 smokers with non acute psychotic disorder; 48% F, av. age 37, av. cpd 30, 57% schizophrenia or schizo‐affective disorder Provider: Trained cessation therapist | |
| Interventions | 1. Treatment as usual: Assessment interview & S‐H books for patient & supporter 2. As 1 plus 8 x 1‐hour sessions (weekly x 6, 8 & 10wks), motivational interviewing & CBT & nicotine patch (21 mg for 8wks incl tapering) | |
| Outcomes | Continuous abstinence at 12m (PP also reported) Validation: CO < 10 ppm | |
| Notes | One participant claiming abstinence at 12m had CO >10 ppm attributable to continued cannabis use and was classified as abstinent. Unclear if this person in the continuously abstinent or PP category | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Participants were informed that they would be randomly assigned to one of two conditions at the end of the initial assessment interview, which was achieved simply by asking them to draw a sealed envelope from a set of envelopes in which there was initially an equal distribution of treatment/control allocations at each site.' |
| Allocation concealment (selection bias) | Unclear risk | Unclear if envelopes were opaque and if participants kept to allocated condition |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 17% lost to follow‐up at 12m, no significant difference between groups |
Bernstein 2015.
| Methods | Setting: emergency department (ED), USA Recruitment: ED patients (both admitted and released), not selected for motivation |
|
| Participants | 778 smokers (averaging ≥ 5 cpd); 48% M, av. age 40, av. cpd 11 Provider: research assistant trained in motivational techniques & specialist counsellor |
|
| Interventions | 1. Control: self‐help brochure with quitline contact details 2. As 1 plus 10‐15 min motivational interview delivered by a research assistant trained in motivational techniques, 6 week supply of nicotine patches and gum, faxed referral to state quitline for proactive counselling, call from nurse 3 days after ED visit |
|
| Outcomes | Abstinence: 7 day PP at 12 months Validation: CO only at 3 months |
|
| Notes | New for 2015 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | random plan generator |
| Allocation concealment (selection bias) | Low risk | blinded staff member prepared opaque consecutively numbered envelopes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up 21.5% (82) I , 22% (82) C. Denominators exclude 6 deaths in I, 2 deaths & 2 duplicate enrolments in C |
Binnie 2007.
| Methods | Setting: Periodontology clinic in dental hospital, Scotland Recruitment: Patients attending for treatment invited to enrol, not selected for motivation | |
| Participants | 116 smokers (excludes 1 death, 1 withdrawal), 13% pre‐contemplators, 45% contemplators at baseline; 71% F, av. age ˜42, median 20 cpd Provider: Trained dental hygienist | |
| Interventions | 1. Usual care 2. 5As based intervention from hygienist at visits for periodontal treatment. Median visits 6‐7. Duration not specified. Free NRT (patch or gum) available, number using not specified | |
| Outcomes | Sustained abstinence at 12 months (abstinent at 3, 6, 12m) Validation: Saliva cotinine < 20 ng/ml, CO at 3m & 6m. | |
| Notes | Intervention did not define number and duration of sessions; classified as 4‐8 sessions, 31‐90 minutes. Number of people who received NRT not specified. Classifed as Moderate for treatment take‐up; subgroup results not sensitive to recoding as High. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized using minimisation method |
| Allocation concealment (selection bias) | Low risk | 'The randomisation process was set up by the project statistician and was implemented independently from the recruitment process.' |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 death, 1 withdrawal before treatment excluded from C denominator. Lost to follow‐up: 26/59 (44%) I, 34/57 (60%) C. Losses included as smokers; exclusion would reduce point estimate, but CIs wide. |
Brandstein 2011.
| Methods | Setting: Single hospital, California, USA Recruitment: Inpatients who had quit smoking during hospitalisation (not explicitly selected for motivation to remain abstinent) | |
| Participants | 126 smokers of >10 cpd prior to hospitalization, 65% M, av. age 47 Provider: Specialist, telephone counsellors (Bedside counselling from Respiratory Therapist for all participants) |
|
| Interventions | 1. Enhanced Intervention: brief bedside counselling, 21 mg nicotine patch for 8 weeks (including tapering period) provided at discharge. Proactive telephone counselling from California Smokers' Helpline; initial call 30 min, up to x5 10‐15 min contacts. Final contact ˜ 2m post discharge 2. Usual care, same bedside counselling as 1 |
|
| Outcomes | Self reported prolonged (180 day) abstinence at 6m Validation: None; all participants asked to provide a saliva sample 'as a way of enhancing self‐report accuracy'. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'The PI used computer generated randomization lists so that randomization was stratified by the RT [respiratory therapist] and subjects were allocated to treatment condition using blocks of four.' |
| Allocation concealment (selection bias) | Low risk | 'Randomization took place after the RT collected baseline data, provided bedside counselling, and obtained consent; thus RTs were blind to group assignment during those procedures.' |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 37.5% I, 43.6% C lost at 6m, similar between groups. Counted as smokers in MA |
Carmody 2012.
| Methods | Setting: Two Veterans Affairs Drug & Alcohol Treatment (DAT) programmes, USA Recruitment: DAT patients reporting alcohol as primary drug, with at least 7 days abstinence, interested in quitting |
|
| Participants | 162 smokers (≥5 cpd); 97% M, av.age 50, av.cpd 17 Provider: Specialist counsellor |
|
| Interventions | 1. Usual care; referral to smoking cessation programme that provided brief smoking cessation counselling & guideline‐concordant medications 2. Individual CBT, 16 sessions over 26 weeks, combination NRT; patch for 16 weeks, lozenge for 26 weeks |
|
| Outcomes | Abstinence at 12m, 7 day PP (Sustained and prolonged abstinence measured but not reported in paper) Validation: CO < 10 ppm |
|
| Notes | New for 2015. Previously listed as excluded because control group could potentially receive pharmacotherapy. Number of quitters estimated from graphs, assuming that denominators were numbers followed up. Intervention participants attended an average of 8 sessions and 16 participants (27%) attended all 16 sessions 1 Intervention group death between 12‐26 weeks |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer randomisation |
| Allocation concealment (selection bias) | Unclear risk | no information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 33% I, 29% C lost at 12m |
Chan 2010.
| Methods | Setting: Clinics, Hong Kong Recruitment: Community volunteers & clinic patients, motivated to quit | |
| Participants | 719 male smokers with erectile dysfunction, av. age 49, av. cpd 20 Provider: Specialist counsellor |
|
| Interventions | 1, Counselling at 0, 1, 4wks, ˜15 min, NRT (patch or gum) for 2 wks. +/‐ 5 min adherence intervention (pooled for cessation outcomes) 2. Brief advice, 10 min, S‐H materials |
|
| Outcomes | Abstinence at 6m (PP) Validation: cotinine 115 ng/mL, CO > 8ppm |
|
| Notes | Study stopped early before reaching target, when abstinence differences significant. Not included in subgroup by setting as recruitment included both community volunteers and clinic patients |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned in 2 stages, no details reported |
| Allocation concealment (selection bias) | Unclear risk | No details given, 'single blind' |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 24‐30% lost to follow‐up |
Chouinard 2005.
| Methods | Setting: Canada Recruitment: Inpatients with cardiovascular disease (Myocardial Infarction, angina, Congestive Heart Failure) or Peripheral Vascular Disease, unselected by motivation | |
| Participants | 168 past‐month smokers; 27% M, av.age 56, 60% in preparation or action SoC Provider: Research nurse (specialist) |
|
| Interventions | 1. Counselling: 1 face to face session, 10‐60 mins, av. 40 mins, based on Transtheoretical Model, included component to enhance social support from a significant family member, 6 telephone calls over 2m post‐discharge. Advised to use pharmacotherapy, mainly NRT 2. In hospital counselling only (not used in analysis) 3. Usual care cessation advice (6% used pharmacotherapy) | |
| Outcomes | Abstinence at 6m (sustained at 2m & 6m) Validation: Urine cotinine or CO | |
| Notes | 2 compared to 3 in main analysis. Sustained abstinence rate identical for 2 and 3. Classified as Moderate take‐up; 39% used pharmacotherapy but 75% received 6 phone calls; subgroup results not sensitive to recoding as High. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Cluster randomized in groups of 3‐6 'to prevent contamination between groups', method not described |
| Allocation concealment (selection bias) | Low risk | 'Individuals not familiar with the study were in charge of the randomization procedure which included inserting the information into envelopes that were sealed and would be opened by the investigator only at the time of recruitment.' |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 death in intervention group and 3 ineligible for follow‐up excluded from denominators in analysis |
Cooney 2007.
| Methods | Setting: 2 Veterans Administration outpatient substance abuse programmes, USA Recruitment: Patients in treatment programme, interested in smoking cessation & alcohol treatment | |
| Participants | 118 smokers, ≥ 10 cpd, (excludes 15 early dropouts); 89% M, av. age 47, av. cpd 25 Provider: cessation specialist |
|
| Interventions | 1. Brief advice; 5As model, 15 min session & 5 min follow‐up, no offer of NRT 2. Intensive intervention; 3x 60 min individual sessions, free NRT (21 mg patch for up to 8 wks including tapering) Both delivered concurrently with 3 week intensive substance abuse programme (15 meetings) | |
| Outcomes | Abstinence at 6 months (PP) Validation: CO < 10 ppm | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized, method not stated |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 9 I & 6 C pre‐therapy dropouts excluded. Lost to follow‐up: 9/55 (16%) I, 14/63 (22%) C. Losses included as smokers; exclusion would reduce point estimate, but CIs wide. |
Duffy 2006.
| Methods | Setting: ENT clinics at 4 hospitals, USA Recruitment: Patients with head & neck cancer who screened positive for smoking, alcohol problem or depression, not selected for motivation | |
| Participants | 89 current smokers used in MA, out of 184 trial participants who also included 26 quit within last month and 21 within last 6m. Demographics are for all participants; 16% F, av.age 57 Provider: Trained nurse specialist |
|
| Interventions | 1. Telephone counselling and offer of NRT or bupropion or combination; 9‐11 CBT based calls, linked to use of CBT workbook. Smokers with problem drinking or depression received counselling for these too. 2. Enhanced usual care with assessment and referral | |
| Outcomes | Abstinence at 6m (self‐reported sustained) Validation: none | |
| Notes | Total contact time not stated but estimated as 91‐300 mins based on sessions lasting 10 to 30 mins. Number of current smokers who were prescribed medication unclear, but likely to have been at least 30%. Classified as Moderate take‐up; subgroup results not sensitive to recoding. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized, method not described |
| Allocation concealment (selection bias) | High risk | No details given. Smokers were a higher proportion of the intervention than control groups, and a higher proportion of those randomized than those who refused, raising possibility of selection bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 22 in total (including non smokers) lost to follow‐up, evenly distributed. Losses included as smokers. |
Emmons 2005.
| Methods | Setting: Childhood Cancer Survivors Study cohort, USA Recruitment: Smokers contacted via telephone to assess eligibility and enrol, not selected for motivation | |
| Participants | 794 smokers (excludes 2 deaths in control); 47% F, av. age 31, av. cpd 12, 18% pre‐contemplators, 39% contemplators Provider: Peer, trained cancer survivor | |
| Interventions | 1. S‐H control. Mailed manual (Clearing the Air) & letter from study physician 2. Peer counselling. Up to 6 calls in 7m period, by trained cancer survivor. Motivational, tailored to SoC. Free NRT available. Individually tailored materials before 1st call & other materials during intervention. | |
| Outcomes | Abstinence at 12m (7‐day PP) Validation: none (warning that samples might be requested) | |
| Notes | No data on average number of calls. Longer term follow‐up, assessed at 2‐4 years, reported in Emmons 2009. Not used in MA ‐ sustained rates not reported. PP rates increased from 12m and remained higher in counselling group (20.6% vs 17.6%, P<.0003). 29% of intervention group requested and used NRT as part of intervention. At 8m 33% I and 8% C reported use of NRT in period |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up at 12m; 24% I, 19% C. All included as smokers in MA. |
Haas 2015.
| Methods | Setting: 13 primary care practices, USA Recruitment: Electronic health records used to identify low SES smokers who had visited a clinic in previous month, recruited via IVR system, not explicitly selected for motivation but 77% planning to quit in 30 days |
|
| Participants | 707 smokers (any smoking in previous week); 68% F, av. age 50, av. cpd 15 Provider: specialists |
|
| Interventions | 1. Usual care control, no offer of treatment after recruitment 2. Intervention: telephone‐based motivational counselling (up to 4 calls, total 75‐100 minutes over 8‐10 weeks), access to free nicotine patches 6 weeks, referrals to community resources to address socio‐contextual mediators of tobacco use, coordination with primary care clinician |
|
| Outcomes | Abstinence: 7 day PP at 9 months Validation: none |
|
| Notes | New for 2015 update 64% I used NRT, vs 44% C. 69% spoke with tobacco treatment specialist at least once. Classified as moderate treatment take up. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Randomization was performed in batches based on the date of the clinic visit “ ‐ Randomization appears to have been based on alternation, but this should have resulted in balanced groups |
| Allocation concealment (selection bias) | Low risk | Randomization occurred before recruitment which was automated using IVR technology. No opportunity for bias to be introduced |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 36% I, 32% C lost to follow up |
Hall 2002.
| Methods | Setting: USA Recruitment: Community volunteers motivated to quit. Exclusion criteria included current MDD | |
| Participants | 220 smokers of ≥ 10 cpd (109 in relevant arms); 40‐47% female, av. age 37‐43, av. cpd 20‐23; 33% had history of MDD Provider: masters level counsellors | |
| Interventions | 3 x 2 factorial design Pharmacotherapies: bupropion (300 mg/d for 12 wks), nortriptyline (up to 100 mg/d titrated to serum 50‐150 ng/mL for 12 wks), or placebo 1. Medical Management (MM) control: physician advice, S‐H, 10‐20 min 1st visit, 5 min at 2, 6, 11wks) 2. Psychological Intervention (PI) as MM plus 5x 90 min group sessions at 4, 5, 5, 7, 11wks). Group size 3‐11 | |
| Outcomes | PP at 1 yr (47wks post‐quit date). Prolonged abstinence not reported by cell. Validation: CO ≤ 10 ppm, urine cotinine ≤ 60 ng/mL | |
| Notes | Bupropion or nortriptyline with PI vs placebo with MM in main comparison | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized, method not specified, |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 19% lost to follow‐up at 1y, no difference by group, included in ITT analysis |
Hall 2006.
| Methods | Setting: Four mental health O/P clinics, CA, USA Recruitment: Provider referral, invitation letters, fliers. Motivation to quit not required | |
| Participants | 322 psychiatric outpatients, daily smokers, treated for depression (unipolar); 70% F, av. age 42, av. cpd 15. Provider: cessation specialist | |
| Interventions | 1. Control: Referrals to SC programmes + SC guide 2. Intervention: (i) Counsellor‐led 15‐min computerized assessments and feedback at 3, 6, and 12m, using SoC framework (ii) For those in contemplation/preparation, offered SC programme of counselling (6 x 30 mins over 8 wks), + NRT, or bupropion (2nd line). SC programme made available to any Int pt requesting it, regardless of stage. | |
| Outcomes | 7‐day PP at 18m (Also reported at 3, 6, 12m) Validation: Expired CO ≤ 10 ppm | |
| Notes | 34% (53) entered cessation & had pharmacotherapy. Classified as Moderate take‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized; "allocation list was computer‐generated by statistical staff" |
| Allocation concealment (selection bias) | Low risk | "after completing the baseline assessment, interviewers randomly assigned participants to conditions from within stratified blocks, according to the number of cigarettes smoked per day and the participants' stage of change". Possibly not concealed, but risk of bias assessed as low. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses reported for all time points: 6m: I 23%, C 25%; 12m: I 31%, C 30%; 18m: I 25%, C 31%. Authors calculated propensity scores to estimate the effects of missing data on outcomes and there was no evidence that missing data caused bias. Main analyses in report used completers, losses treated as smoking in this MA |
Hanioka 2010.
| Methods | Setting: 19 dental clinics, Japan Recruitment: Dental patients willing to stop smoking within 1 month |
|
| Participants | 56 adult smokers attending dental clinics in Japan, 29% F, av, age ˜48, av. cpd ˜25 (excludes 14 I & 21 C who declined participation after randomization but before consent) Providers: Dentists & dental hygienists |
|
| Interventions | 1. Free nicotine patches for 6 weeks, information about nicotine gum. 5 counselling visits at baseline, 2, 4, 8, & 12wks 2. No intervention |
|
| Outcomes | Abstinence at 12 m (3, 6, 12m continuous abstinence) Validation: Saliva cotinine < 20 ng/mL |
|
| Notes | Total duration of contact averaged 116 mins (87‐146) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details of method of sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Assignment cards in envelopes provided a priori to clinics; allocated as subjects agreed to participate but before consent. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Participants were given details of their treatment after allocation, and more control than intervention declined after allocation and before consent. 15 later dropouts included as smokers |
Hickman 2015.
| Methods | Setting: psychiatric units, urban public hospital, USA Recruitment: inpatients, not selected for motivation |
|
| Participants | 100 smokers (≥5 cpd prior to hospitalisation), 35% F, av. age 40, av. cpd 19 Provider: specialists (study staff) |
|
| Interventions | 1. Usual care, NRT available on ward to manage withdrawal 2. Transtheoretical model (TTM)‐tailored, computer‐assisted intervention with printed report at baseline, 3 & 6 m, stage matched manual, individual counselling during hospitalisation (1 x 15‐30 min session), NRT available for 10 w post discharge |
|
| Outcomes | Abstinence at 12 m (7‐day PP) Validation: CO < 10 ppm (collateral reports only for 31.6% of people reporting abstinence at 12m) |
|
| Notes | New for 2015 update. Found via author search for reports of ongoing studies. Described as a replication and extension of Prochaska 2014, with same NCT number. Participants recruited in 2009/2010 2 deaths in intervention, 1 in control, all after end of intervention |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'computer‐generated random assignment program stratified by baseline cigarettes per day (>15) and stage of change' |
| Allocation concealment (selection bias) | Low risk | 'Research staff blinded to the randomization schedule.' |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up 10% (5) I, 4% (2) C. 3 deaths (2 I, 1 C) excluded from randomized denominators |
Hollis 2007.
| Methods | Setting: Community‐based telephone quitline programme, Oregon USA Recruitment: Callers invited to participate; assumed to be fully or partly motivated to quit. | |
| Participants | 4614 smokers; 40% M, av. age 41, av. cpd 21. Provider: cessation specialist, telephone based |
|
| Interventions | Factorial design; 3 levels of counselling, +/‐ offer of up to 8 weeks of free nicotine patches. No face‐to‐face contact. 1. Control (Brief): 15 min call + referral material + tailored S‐H materials. [Mean 1 session, 20 min contact time] 2. Moderate: 40 min call + brief call to encourage use of community services, tailored S‐H materials. [Mean 2.0 sessions, 47 min contact] 3. Intensive: As 2, plus offer of ≤ 4 additional calls. [Mean 2.9 sessions, 60 min contact] Each call incorporated MI techniques, stage assessment, RP as needed. | |
| Outcomes | Abstinence at 12m (30 day PP). Also assessed at 6m Validation: none | |
| Notes | Of those offered NRT, 80% accepted the first 5‐week regimen and 25%/28% requested a second 3‐week refill and there were no differences across the three levels of behavioural intensity. 3 with NRT vs 1 without NRT used in main analysis, but little difference between moderate and intensive outcomes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "a computer algorithm randomly assigned participants" |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 35% control, 30% intervention lost at 12 months, included in analyses as smokers. Authors report sensitivity analyses using imputation for missing data. Some evidence that effect of NRT, but not behavioural support, might be over estimated using missing = smoking assumption for all losses, but less evident when only 'active refusers' assumed to be smoking |
Juarranz Sanz 1998.
| Methods | Setting: Primary care clinic, Spain Recruitment: Patients of clinic proactively recruited by phone, unclear whether motivation to quit required | |
| Participants | 205 smokers; 48% M, av. age 38, av. cpd 23 Provider: primary care provider | |
| Interventions | 1. Initial counselling 35 min, phone call 2d post quit date, visits at 2wks,1m, 3m, 6m. Nicotine patch for 8‐12 wks, dose and duration tailored. 2. No intervention | |
| Outcomes | Abstinence at 6m, self‐reported prolonged Validation: CO ≤ 8 ppm | |
| Notes | No further details could be obtained about the intervention provider | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random, but possibly alternated |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 10 lost to follow‐up, included as smokers |
Katz 2004.
| Methods | Setting: 8 primary care clinics, USA Recruitment: smokers attending for non‐emergency visits | |
| Participants | 1141 smokers (>1 cpd) 56% F, age 43/40, median cpd 20/15 Providers: Usual care clinicians & trained nurses (classified as usual care provider) | |
| Interventions | 1. Intervention based on AHRQ guidelines. Training in brief advice for intake clinicians, vital signs stamp. Patients willing to set TQD offered proactive telephone counselling (2 calls, pre & post TQD) by trained nurse, smokers of over 10 cpd offered NRT 2. Control. Information about guidelines, no specific advice on counselling. | |
| Outcomes | Sustained abstinence at 2m & 6m Validation: saliva cotinine. Poor response, similar return & misreport rates. Validated sustained rates not reported. | |
| Notes | Study also included a baseline assessment. Only data from smokers recruited during implementation period used here. Compliance: 183 intervention patients were willing to set a quit date so eligible for counselling and NRT; 148/642 (23%) had some counselling, 164/642 (25.5%) had NRT; 144 received both components. 29% of all intervention participant reported NRT use during follow‐up versus 11% in control. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Cluster randomized by clinic, method not described |
| Allocation concealment (selection bias) | Low risk | Participants enrolled by completing an exit interview with researcher, not determined by clinic |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4‐8% lost to follow‐up |
Kotz 2009.
| Methods | Setting: University research unit, Netherlands Recruitment: Volunteers from community & health centres, interested in quitting & reporting respiratory symptoms | |
| Participants | 296 people with at least 10 pack‐yrs of smoking, and evidence of mild to moderate airflow limitation at spirometry; 39% F, av. age 54, av. cpd 23 Provider: respiratory nurse |
|
| Interventions | 1. High intensity counselling (4 weekly 40 min individual sessions) & nortriptyline for 7 weeks. No information given about spirometry results. 2. As 1 including confrontational counselling about spirometry findings (not used in this review) 3. Referred to GP for low intensity smoking cessation treatment, no information about spirometry results | |
| Outcomes | Prolonged abstinence at 52 weeks (weeks 5‐52) Validation: urine cotinine < 50 ng/mL at 5, 26 & 52 weeks | |
| Notes | 1 vs 3 compared in analysis. Test of confrontational counselling not covered in this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using computerised system, initially 1:1:1 ratio, then altered to 3:3:1 with block size 7 |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 17% lost in 1 vs 22% in 3, included as smokers |
Lee 2015.
| Methods | Setting: Hospital preadmission clinic, Canada Recruitment: elective surgery patients screened at pre‐admission clinic appointment then contacted via written letter inviting them to participate |
|
| Participants | 168 smokers (≥2 cpd) awaiting elective surgery. 54.5% F, av. age: 48, av. cpd 16 Provider: preadmission nurse brief counselling + specialist helpline counsellors |
|
| Interventions | 1. Usual care 'inconsistent perioperative smoking cessation advice from nurses, surgeons, or anesthesiologists, but no further study‐specific smoking cessation intervention.' 2. Brief counseling (<5 mins) by the preadmission nurse, 6 weeks nicotine patch, S‐H materials, referral to quitline, at least 4 quitline calls offered (est duration 31‐90) |
|
| Outcomes | Abstinence: 7 day PP at 1 year Validation: 30 days post‐op but not at 1 year |
|
| Notes | New for 2015 update 48% of intervention group did not get telephone counselling. All given NRT so included in high take‐up subgroup although no explicit report of use |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | randomization was computer generated; randomly permutated blocks |
| Allocation concealment (selection bias) | Low risk | "Allocation was concealed by consecutively numbered sealed opaque envelopes" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up 30% (24) I, 20% (17) C. A proportion of these due to change or cancellation of surgery. |
Lewis 1998.
| Methods | Setting: hospital, USA Recruitment: inpatients (excluding some cardiac conditions) interested in quitting | |
| Participants | 185 hospitalised adults; self‐reported ‘regular use’ for at least one year. 44% F, av. age 43, av. cpd 24 Provider: research nurse | |
| Interventions | 1. Minimal care (MC): motivational message from physician to quit plus pamphlet 2. Counselling and nicotine patch (CAP). 3. Counselling and placebo patch (CPP). (not included in this review) In addition groups 2 & 3 received a motivational message & instructions on patch use from physician, 4 sessions of telephone counselling by nurse based on cognitive behavioural therapy and motivational interviewing. | |
| Outcomes | Abstinence at 6m (7 day PP) Validation: CO ≤ 10 ppm | |
| Notes | 2 compared to 1 in this review. No information about compliance with treatment except 'Patch compliance was not related to outcome among patients'. Classified as Moderate take‐up but subgroup results not sensitive to recoding as High. Also contributes to reviews of NRT, nursing and hospital interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized: predetermined computer‐generated code |
| Allocation concealment (selection bias) | Low risk | Study staff blind to active/placebo patch condition so assignment code likely to have been blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up reported. 10 self‐reported quitters refused CO validation and counted as smokers |
Lung Health Study.
| Methods | Setting: 10 study centres, USA Recruitment: Healthy smokers with mild airway obstruction, not required to be interested in quitting | |
| Participants | 5887 smokers; 37% F, av. age 48, av. cpd 31 Providers: specialist counsellors |
|
| Interventions | 1. Advice from study physician with stress on high risk of COPD, 12 group sessions over 10 weeks, beginning on quit day, initially 4 sessions/week, 2 mg nicotine gum available for 6 months, maintenance and recycling sessions offered long term. Cessation intervention participants also randomized to bronchodilator or placebo arms, pooled here. 2. Usual Care, no intervention | |
| Outcomes | Abstinence at 1 year (PP) (also assessed annually for 5 years, data not used here) Validation: CO at each visit, cotinine at 1y | |
| Notes | Numbers quit at 1y estimated from graph. At 5y sustained abstinence rates reported to be approximately 22% vs 5% | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized, computer generated separately for each centre, blocks of random permutations of varied length (Reported in Connett 1993) |
| Allocation concealment (selection bias) | Low risk | Centralised verification of eligibility & allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ˜95% follow‐up at 1y. Non attenders counted as smokers |
McCarthy 2008.
| Methods | Setting: clinic, USA Recruitment: community volunteers motivated to enrol in trial of cessation medication | |
| Participants | 463 smokers (226 in relevant arms); 50% female, av. age 36‐41 across arms, av.cpd 22 Providers: trained college‐aged or bachelor's level staff, supervised by experienced counsellor | |
| Interventions | Factorial trial of bupropion or placebo pharmacotherapy and counselling versus support 1. Bupropion & counselling; 8 x 10 min sessions, 2 prequit, TQD, 5 over 4 wks, additional office visits without counselling 2. Bupropion & psychoeducation (not used in this review) 3. Placebo & counselling (not used in this review) 4. Placebo & psychoeducation about medication, support & encouragement. Same no. of office visits, 80 mins less contact time than 1. | |
| Outcomes | 7 day PP abstinence at 12m Validation: CO ≤10 ppm | |
| Notes | 1 vs 4 used as a test of combined intervention. Others arms do not contribute to this review. Classified as 91‐300 mins because of additional contact time during office visits. Also contributes to Cochrane reviews of antidepressants (Hughes 2014) (collapsing behavioural conditions) and individual behavioural counselling (Lancaster 2005) (collapsing pharmacotherapy) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Random number table' |
| Allocation concealment (selection bias) | Low risk | 'Staff who screened and enrolled participants were unaware of the experimental condition to be assigned' |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | In relevant arms 24 (11%) failed to attend quit date visit and 62 (27%) lost to follow‐up at 12m, no difference by condition, all included as smokers in ITT analysis |
Mohiuddin 2007.
| Methods | Setting: Hospital, USA Recruitment: Inpatients with diagnosis of acute coronary syndrome (including MI) or decompensated CHF, admitted to critical care unit, invited to participate, not selected for motivation to quit, but high rate of refusal amongst eligible patients | |
| Participants | 209 smokers; 37% F, av. age 55, av. cpd I 26, C 22 (p=.03); no information about baseline motivation Providers: Physician and trained tobacco counsellor or nurse. | |
| Interventions | All participants received standardised 30 min in‐hospital counselling & S‐H materials 1. Intervention: Inpatient counselling. Individualised pharmacotherapy (NRT and/or bupropion). Weekly group meetings (60 min session for up to 3m) with trained tobacco counsellor (content: behavioural counselling, social support, relaxation training, risk factor management). 2. Control: inpatient counselling & S‐H only. | |
| Outcomes | Sustained abstinence at 24m (PP abstinence and 12m outcomes also reported) Validation: CO | |
| Notes | Pharmacotherapy used by 75% in intervention (bupropion 7%; NRT 28%; combination 40%) compared to 17% control (bupropion 1%; NRT 5%; combination 11%) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "using simple randomization, without block assignment" |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | <5% lost to follow‐up, included as smokers. 3 deaths in I and 12 in C also retained in denominators |
Molyneux 2003.
| Methods | Setting: hospital, UK Recruitment: hospital inpatients, invited to participate, not selected for motivation to quit, but 'expected to comply with protocol' and high rate of refusal amongst eligible patients | |
| Participants | 274 smokers (183 in relevant arms) admitted to medical and surgical wards, smoked in last 28 days; 60% M, av. age 60, median cpd 17, 81% had previous quit attempt; no information about baseline motivation Providers: research doctor or nurse trained in cessation counselling | |
| Interventions | 1. Usual Care, no smoking advice 2. Brief (20 min) bedside counselling + advice leaflet + advice on NRT 3. As 2 plus 6 week course of patient's choice of NRT product (patch, gum, inhalator, sublingual tablet nasal spray) | |
| Outcomes | Continuous abstinence at 12m Validation: CO < 10 ppm at 3 & 12m | |
| Notes | 3 vs 1 as test of combined brief counselling and offer of pharmacotherapy. 96% in condition 3 accepted NRT, few other participants obtained NRT. Also contributes to Cochrane reviews of individual counselling (Lancaster 2005) (2 vs 1) and interventions in hospitalised patients (Rigotti 2012). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'List generated for each centre allocating equally in random permuted blocks of nine.' |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 68 (37%) lost to follow‐up included as smokers in analysis |
Murray 2013.
| Methods | Setting: 18 acute medical wards in 1 hospital, UK Recruitment: Inpatients, unselected for motivation |
|
| Participants | 493 smokers (within 4 weeks of admission); 40% F, av. age 56, av. cpd not reported Provider: Specialists (research team) |
|
| Interventions | Pharmacotherapy: Dual nicotine replacement therapy was most common (patch, inhalator) but varenicline was also used. 1. Usual care: possible advice to quit & cessation support 2. Intervention: brief advice & offer of support. Those accepting support received tailored support, pharmacotherapy (usually patch, inhalator or both). |
|
| Outcomes | Abstinence: continuous at 6 months Validation: CO < 10 ppm |
|
| Notes | Intracluster correlation coefficient (ICC) was 0.07, higher than expected. Numbers in meta‐analysis adjusted to 17/91 vs 7/80 to allow for clustering (as described in Cochrane Handbook 16.3.4/5). Paper reports OR adjusted for clustering and stratification of 1.53 (0.60 to 3.91) compared to 2.40 (0.94, 6.12) for adjusted data so we also tested sensitivity of overall results to this study Median number of behavioural support sessions 1, IQ range 0‐2 NRT used by 50% I, 29% C |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Cluster randomized: admission ward was unit of randomization, small number of clusters led to imbalance in patient characteristics |
| Allocation concealment (selection bias) | Low risk | Clinical and research staff and patients were aware of group assignment, and Intervention group participants were recruited soon after admission whilst control group participants recruited prior to discharge. However 'Although the study design precluded blinding, ward staff were unaware of the exact details of the study, and patients were specifically not informed of the components of the intervention being tested. Consent ... was only sought for the follow‐up measures, for which the procedure was identical for both groups. It is therefore unlikely that knowledge of missing out on treatment influenced the results gained in the study,' so judged low risk. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 26% I, 32% C lost to follow up. 14 deaths in I, 10 in UC |
Ockene 1991.
| Methods | Setting: US primary care residency programme (physicians in training) Recruitment: unselected patients in 5 primary care clinics | |
| Participants | 1286 smoking patients not selected for motivation to quit Providers: 196 primary care physicians in training | |
| Interventions | 1. Advice only 2. Patient‐centred counselling, written materials, asked to schedule follow‐up visit, follow‐up letter (not used in this review) 3. Patient‐centred counselling and offer of prescription for nicotine gum (Each group was further randomised to minimal (no calls) or intensive follow‐up by telephone (3 calls over 6m) from a health educator (HE) but no main effects or interactions were noted and no results were presented at 12 months so this factor is not analysed here) | |
| Outcomes | Sustained abstinence at 12m (reported in Ockene 1994) (6 & 12m). PP also reported Validation: none | |
| Notes | Adjusted rates used in analysis. All physicians received training in minimal vs intensive interventions and delivered them according to random allocation of patient. 12m PP abstinence showed no effect of intervention. 69% of group 3 accepted prescription and received at least 1 box of gum. Also contributes to Cochrane reviews of physician advice and NRT | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized, no further information provided |
| Allocation concealment (selection bias) | Unclear risk | Each physician delivered 1 of the 3 interventions according to instructions in a packet for each patient. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 17% of total sample unreachable and treated as smokers in analyses. 25 others not included |
Okuyemi 2007.
| Methods | Setting: 20 low‐income public housing developments, USA Recruitment: residents attending community health fairs, no contraindications to NRT, not selected for motivation | |
| Participants | 173 smokers; ˜70% F, av. age 43, av. cpd ˜17. ‘Although we did not screen for motivation as part of our study inclusion criteria, motivation to quit was moderately high in both groups at baseline’ Providers: specialist counsellors |
|
| Interventions | Intervention: MI counselling in‐person at weeks 0 & 3, phone on day 10, wk 5 & 20. 8 week supply of 4 mg nicotine gum Control: Same schedule of MI for increasing fruit & veg consumption, free supplies, cookbook | |
| Outcomes | Abstinence at 6m (7‐day PP) Validation: CO ≤10 ppm | |
| Notes | No correction for clustering. Length of sessions not reported, estimated as 91‐300 mins. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Cluster randomized by housing unit, stratified by elderly vs family development |
| Allocation concealment (selection bias) | Low risk | Treatment assignment was revealed to the research staff only after each health fair was completed. A timed e‐mail was sent to the study coordinator at 6:00 p.m. after each health fair was complete along with a sealed envelope containing the randomization code. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 19 (28.8%) I, 23 (21.5%) C lost to follow‐up, included as smokers. |
Otero 2006.
| Methods | Setting: community, Brazil Recruitment: volunteers, wanting to quit | |
| Participants | 1199 smokers (includes 254 non‐attenders); 63% female, av.age 42, 46% smoked >20 cpd Providers: trained doctors, nurses or psychologists | |
| Interventions | Factorial design; NRT or no NRT, and 5 levels of behavioural support collapsed into 3 for reported analyses. Nicotine patch 21mg or 14mg based on dependence, for 8wks including tapering 1. Single 20 min session ‐ classified as brief intervention control in meta‐analysis 2. Cognitive behavioural, 1 or 2 weekly x1 hr group sessions 3. As 2, with 3 or 4 weekly sessions. Maintenance or recycling sessions provided to all groups at 3, 6, 12m. | |
| Outcomes | Abstinence at 12m (7 day PP) Validation: none | |
| Notes | Condition 3 with either dose of patch compared to condition 1 without patch in primary meta‐analysis. Similar outcomes for condition 2 as for 3. Classified in 1‐3 session, 91‐300 minute subgroups 29% of no‐patch group participants asked for nicotine patch after the 3m follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized, stratified by age & sex, by independent specialist |
| Allocation concealment (selection bias) | Low risk | Trial administrators blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Non‐participants and losses to follow‐up included as smokers |
Peckham 2015.
| Methods | Setting: Mental health services, UK Recruitment: referral from mental health & primary care. Selected for motivation to quit or cut down |
|
| Participants | 97 smokers with severe mental illness (SMI), 40% F, av. age 47, av. cpd 24 Provider: Mental health professional (MHSCP) trained to deliver smoking cessation behavioral support, (GP for pharmacotherapy) |
|
| Interventions | 1. Usual NHS quit smoking service with no specific adaptation, could include pharmacotherapy 2. 'Bespoke' smoking cessation intervention tailored to SMI: patients encouraged to reduce smoking, set own quit dates, and make multiple attempts. Pharmacotherapy prescribed by GP, type not stipulated by most often NRT. Time estimated at 91‐300 mins |
|
| Outcomes | Abstinence: 7 day PP at 12 months Validation: CO < 10 ppm |
|
| Notes | New for 2015. 41/46 participants attended at least one session and the mean number of sessions was 10. Limited information about use of pharmacotherapy, but had to be obtained separately from GP; coded as moderate take up. Two intervention participants did not have CO verification, classified as smoking in this analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated centralised randomization |
| Allocation concealment (selection bias) | Low risk | "The researcher contacted a secure randomisation line run by the York Trials Unit and, once given the details of the patient’s allocation, immediately informed the patient of his or her allocation and set up the first appointment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up 28% (13) I, 31% (16) C |
Perez‐Tortosa 2015.
| Methods | Setting: 43 primary care teams, Spain Recruitment: diabetic patients during visits, or were selected by simple random sampling from a list of diabetic smokers. Not selected for motivation |
|
| Participants | 948 (after exclusion of 129 without recorded baseline stage of change, not included in analyses) diabetic smokers (any smoking in past 7 days at recruitment) (Aged over 14 but predominantly adults). 24% F, av. age 60, av. cpd 15 Provider: primary care teams |
|
| Interventions | 1. Usual care 2. Intensive, individualized intervention using motivational interview, therapies & medications based on stage of change. Up to 8 visits over 12 months for those in preparation/action stages. Median visits 4 (2‐6), contact time 100 mins |
|
| Outcomes | Abstinence: continued abstinence at 1 year Validation: CO level of less than 6 ppm |
|
| Notes | New for 2015 update No details of how many people used medications or what type, coded as moderate treatment take‐up |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Cluster randomized by primary care centre |
| Allocation concealment (selection bias) | High risk | ‘Patients were recruited as they visited the primary care team or alternatively were selected by simple random sampling from a list of diabetic smokers’. Patients recruited after centres allocated, and baseline differences, so judged at high risk for selection bias |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 129 without baseline stage of change data were excluded from analyses. Loss to follow‐up 24% (111) I, 23.4% (115) C, not included in reported analyses, reincluded in denominators for this MA. |
Prochaska 2014.
| Methods | Setting: Inpatient psychiatric unit, USA Recruitment/ motivation: Research staff identified patients based on medical records, not selected for motivation to quit |
|
| Participants | 224 psychiatric inpatients; 37.5% F, av.age 40, av cigs/day 19 Provider: study counsellor/ computers |
|
| Interventions | 1. Intervention; access to nicotine patch for 10 weeks; completion of a computer‐delivered, Transtheoretical Model‐‐‐tailored intervention program with printed individualized report tailored to stage of change, temptations, decisional balance, and the processes of change; a stage‐tailored print manual; a 15‐ to 30‐minute cessation counselling session 2. Usual care |
|
| Outcomes | Abstinence: 7 day PP at 18 months Validation: CO less than 10 ppm |
|
| Notes | Numbers quit calculated from percentages | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'computer‐generated random assignment stratified by cpd prior to hospitalization' |
| Allocation concealment (selection bias) | Low risk | 'Research staff were blinded to the randomization schedule' |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up at 18m 20% intervention, 18% control. No evidence that missing data biased effects. |
Ratner 2004.
| Methods | Setting: Preadmission clinic, teaching hospital, Canada Recruitment: Smokers awaiting surgery, not selected for motivation | |
| Participants | 237 smokers; 52% F, av. age 49, av. cpd 12; 16.5% precontemplators, 35% contemplators Provider: research nurse |
|
| Interventions | Intervention: Initiated 1‐3 weeks before surgery: 15 min face‐to‐face counselling, materials, nicotine gum, quit kit, hotline number. Post‐operative visit, 9 telephone calls, weekly for 1 m, biweekly for 2 m Control: usual care | |
| Outcomes | Abstinence at 12m (7 day PP) Validation: cotinine <100 ng/mL based on mailed 'Nicotest' strips,. Self reported non smokers failing to return strips are classified as smokers in this analysis. 'it was not possible to verify whether participants had tested their own urine' | |
| Notes | No information on % using NRT or other medication, classified as moderate take‐up of treatment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes opened after baseline data collection |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 30 I & 29 C lost to follow‐up treated as smokers. 9 deaths excluded from denominators |
Reid 2003.
| Methods | Setting: Cardiac hospital, Canada Recruitment: Inpatients with myocardial infarction, coronary artery bypass graft, coronary angioplasty, coronary angiography, motivated to quit | |
| Participants | 254 current smokers (smoked in month before admission); av. age 54 yrs Providers: specialist nurse counsellors |
|
| Interventions | Intervention: Brief nurse counselling at bedside (5‐10 mins) + booklet . Nurse call at 4 wks; if smoking, offered 3 x 20 min in‐person counselling sessions (wks 4,8,12) and nicotine patch recommended for 8 wks. Nonsmokers reinforced and reminded about relapse prevention Control: Brief nurse counselling (5‐10 mins) + self‐help booklet (same in hospital as intervention group) | |
| Outcomes | Abstinence at 12m (7‐day PP) Validation: Random sample of 25 self‐reported non‐smokers asked for CO validation; 91% validated, similar in both arms. Results not adjusted for this. | |
| Notes | Classified as 4‐8 sessions, 31‐90 mins. Classified as low take‐up because only 26% scheduled to receive 4 week intervention due to continued smoking | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization stratified by diagnosis on admission, degree of nicotine dependence using random numbers table |
| Allocation concealment (selection bias) | Low risk | Concealed until after assessment and initial counselling |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 19.5% I 9.5% C lost to follow‐up treated as smokers. 2 deaths included using last smoking status. |
Reid 2008.
| Methods | Setting: drug & alcohol dependence treatment centres, USA Recruitment: outpatients with current drug or alcoholic dependence or methadone maintained, interested in quitting | |
| Participants | 225 smokers (≥10 cpd), 49% F, av. age 41, av. cpd 21 Providers: Specialist counsellors |
|
| Interventions | 1. Intervention: Nicotine patch for 8wks, Intensive behavioral therapy; 9 group sessions over 7wks, mood management & CBT components. 2. Treatment as usual (offered cessation treatment after end of trial) | |
| Outcomes | Abstinence at 26wks (PP) Validation: CO ≤ 10 ppm | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization in 2:1 ratio 'computer generated, using permuted blocks of six, stratified by site and sex' |
| Allocation concealment (selection bias) | Low risk | ‘study statistician, who had no other contact with site study staff, performed the randomization, and staff were blind as to stratification and block size strategies’ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 11 intervention and 4 control participants did not complete treatment. Denominator used for abstinence rates unclear, but results not sensitive to different assumptions |
Rigotti 2014.
| Methods | Setting: hospital, USA Recruitment: inpatients planning to quit smoking after discharge and willing to use medication Providers: Specialist counsellors |
|
| Participants | 397 smokers (≥1 cp in month before admission), 52% F, av.age 53, av. cpd 17 | |
| Interventions | 1. Intervention: Choice of free medication for up to 90 days. 30 day supply at discharge. Interactive voice response calls at 2, 14, 30, 60, & 90 days, encouraged to request counsellor call back 2. Control: Advice on post discharge medication & recommendation to call quitline. Physicians advised to prescribe medication |
|
| Outcomes | Abstinence at 6 months (PP) (Continuous abstinence also reported, but not validated) Validation: saliva cotinine < 10 ng/ml or CO < 9 ppm |
|
| Notes | 4 deaths in each group by 6 month, not included in MA denominators. Medication (79% vs 59%) and counselling (37% vs 23%) was higher in intervention than control during 1st month after discharge. Relative effect not sensitive to choice of outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'randomly assigned (1:1) to sustained care or standard care in permuted blocks of 8, stratified by daily cigarette consumption (<10 vs ≥10) and admitting service (cardiac vs other)' |
| Allocation concealment (selection bias) | Low risk | 'Treatment assignment was concealed in sequentially numbered sealed envelopes within each stratum. Research staff opened the next envelope corresponding to the participant’s randomization stratum.' |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up not significantly different; 22% C, 17% I. Sensitivity analyses did not alter findings |
Rodriguez 2003.
| Methods | Setting: 3 worksites, Spain. Recruitment: at annual medical check‐up, motivated to quit smoking | |
| Participants | 218 smokers; 86% M, av. age 43 Provider: occupational physician |
|
| Interventions | 1. Intervention: 5‐8 mins structured counselling + further contacts at 2 days, 15 days & 3m. NRT based on Fagerstrom score; < 5 counselling only; 5‐7 8 wks x14 mg nicotine patch; > 7 4 wks x 21 mg, 4wks x 14mg, 4wks x 7mg. Could be increased if necessary. 2. Control: minimal (30‐60 secs) sporadic unstructured advice, usually at annual medical check up | |
| Outcomes | Continuous abstinence (7 day PP at each assessment) at 12m. Validation: expired CO <= 10 ppm at each assessment | |
| Notes | Control group participants also contacted at 2 & 15 days and most appear to have also made quit attempts. Classified as moderate treatment take‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Numbered sealed opaque envelopes, opened after enrolment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 death in intervention group excluded from denominator, no other reported losses to follow‐up |
Sadr Azodi 2009.
| Methods | Setting: Pre‐surgical clinics, 3 hospitals, Sweden Recruitment: Smokers due to undergo elective surgery for primary hernia repair, laparoscopic cholecystectomy or hip or knee prosthesis. Not selected for motivation to quit, but unwillingness to quit smoking a major reason for refusal to enrol | |
| Participants | 117 smokers; 47% F, av. age 55, av. cpd 15; no information about baseline motivation Provider: trained nurse counsellor |
|
| Interventions | Intervention: weekly counselling 4 wks pre‐ to 4 wks post‐op, face‐to‐face or by telephone, free choice of NRT product, Control: Standard care | |
| Outcomes | Abstinence at 12m. Post operative complications were primary trial outcomes Validation: CO ≤10 ppm at 2‐3 weeks post op, no validation at 12m | |
| Notes | Smoking cessation was validated by CO in exhaled air. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified block randomization |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7/55 (13%) I 10/62 (16%) C lost to 12‐month follow‐up, treated as smokers |
Schauffler 2001.
| Methods | Setting: 2 health maintenance organisations (HMOs), USA Recruitment: Members of HMOs recruited by phone, no obligation to quit | |
| Participants | 1204 smokers (excludes 342 who did not return consent form). No demographic information provided Providers: specialist counsellors |
|
| Interventions | 1. Notification of access to smoking cessation treatment covered by HMO: free NRT (patch or gum ordered by phone, dose tailored to smoking history) and free American Lung Association programmes (4‐7 sessions over 2‐4 weeks) 2. Self‐help kit including video & pamphlet | |
| Outcomes | Abstinence at 12m from introduction of benefit (PP). No quit date set so period since quit day not known Validation: none | |
| Notes | Low use of treatment: 25% I vs 14% C study completers reported use of NRT during study period. 1.2% I vs 1.1% C reported participation in a behavioural programme. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | randomized, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 12% discontinued & 15% lost to follow‐up, similar across groups. Paper calculates % quit excluding discontinuations; all losses counted as smokers in this MA, giving marginally more conservative RR |
Segnan 1991.
| Methods | Setting: 44 general practices, Italy Recruitment: Consecutive eligible patients attending on study days (unselected) | |
| Participants | 923 smoking general practice attenders aged 20‐60; ˜35% F, av. age ˜45, av. cpd ˜15 Providers: GPs who had undergone a 3‐hr training session | |
| Interventions | 1. Advice and leaflet 2. Repeated counselling (follow‐up at 1, 3, 6, 9m) (not used in this review) 3. Repeated counselling plus nicotine gum 4. Repeated counselling plus spirometry (not used in this review) | |
| Outcomes | Sustained abstinence at 12m (sustained for 3m by self‐report) Validation: Urinary cotinine < 100 ng/mg | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Blocked treatment allocation based on a sequence of random numbers' |
| Allocation concealment (selection bias) | Low risk | 'closed, numbered envelopes ... provided to each GP at the beginning of the study ... envelopes were indistinguishable from the outside ... research staff checked physicians' compliance with the procedure for assignment by comparing envelope numbers and dates of recruitment' |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6% refused, 7% untraced at 12m, included as smokers |
Simon 1997.
| Methods | Setting: Veterans Administration hospital, USA Recruitment: smokers undergoing non‐cardiac surgery, prepared to make quit attempt | |
| Participants | 324 smokers (smoked within 2 wks of admission); 98% M, av. age 54, av. cpd 20 Providers: public health educator | |
| Interventions | Intervention: single counselling session (30‐60 min) prior to discharge, 5 follow‐up counselling calls over 3m (based on social learning theory and stages of change). Video, prescription for NRT (gum or patch, dose not stated, for 3m) if no contraindications. Control: Brief pre‐discharge counselling (10 min) and S‐H materials. | |
| Outcomes | Abstinence at 12m (7 day PP) Validation: serum or saliva cotinine < 15 ng/ml. 6 self reports confirmed only by 'significant other' classified as smokers in the meta‐analysis. 2 NRT users classed as quit. | |
| Notes | Approx 65% intervention and 17% control used NRT. Not associated with quitting in either group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Random list of assignments' |
| Allocation concealment (selection bias) | Low risk | 'Sealed opaque envelopes opened on formal enrolment' |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 25 (8%) lost to follow‐up included as smokers, 11 (7%) intervention & 14 (9%) control group deaths excluded from denominators in report and in this meta‐analysis |
Stockings 2014.
| Methods | Setting: Inpatient psychiatric facility, Australia Recruitment: Newly admitted current smoker, not selected for motivation |
|
| Participants | 2015 inpatient smokers, 46% F, av. age 50, av. cpd 23 Providers: project officer (provided motivational interview) & counsellor |
|
| Interventions | 1. Treatment as usual: brief advice, NRT provided during admission and for 3 days at discharge, post discharge smoking care plan 2. As 1 plus 10‐15 min motivational interview at enrolment, self‐help materials, 2 week NRT at discharge, telephone counselling every 2 weeks for 4 months, 12 weeks tailored NRT (typically combination therapy), referral to quitline & community support groups |
|
| Outcomes | Continuous abstinence at 6 months (7 day PP also reported) Validation: CO < 10 ppm |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated by a statistician independent of research team. |
| Allocation concealment (selection bias) | Low risk | ' ... each consenting participant drawing a sequentially numbered envelope from a series of envelopes containing an equal distribution of control and intervention...All project and clinical staff working in the hospital setting and follow‐up interviewers were blinded to the randomization sequence.' |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 64.4% followed up at 6 months 'and did not differ between treatment conditions at any time point' 'There were no significant differences in characteristics for those who completed the follow‐up assessments compared to those who did not, at any time point. |
Thomsen 2010.
| Methods | Setting: 3 surgical units, Denmark Recruitment: Women scheduled for breast cancer surgery in near future, not selected for motivation |
|
| Participants | 130 women with breast cancer; av. age 57, av. cpd NS Providers: specialist counsellor |
|
| Interventions | 1. Counselling session 45‐90 mins, using MI. NRT offered free of charge perioperatively. 2. Usual care control, no systematic advice |
|
| Outcomes | Sustained abstinence at 12m (from 2 days pre‐op, blinded telephone interviews) Validation: none |
|
| Notes | No information on number of participants who used NRT, so not included in subgroup for take‐up of treatment. All intervention group received counselling | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized, stratified by dept and procedure, method not stated |
| Allocation concealment (selection bias) | Unclear risk | No details reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7 I and 3 C withdrew before receiving any intervention, excluded from denominators. 1 death in control group excluded, other dropouts treated as smokers in this analysis. |
Tonnesen 2006.
| Methods | Setting: 7 chest clinics, Denmark Recruitment: outpatient attender motivated to enrol in trial of cessation medication | |
| Participants | 370 smokers of >1 cpd with COPD (178 in arms of interest); 52% F, av. age 61, av. cpd 20 Providers: 20 nurses with cessation experience, trained to support medication use and provide standardised counselling | |
| Interventions | Factorial trial. Nicotine sublingual tablet versus placebo and high versus low support 1. High support: 7 x 20‐30min clinic visits (0, 2, 4, 8, 12 wks, 6m, 12m) & 5 x 10min phone calls (1, 6, 10 wks, 4½m, 9m), total contact time 4½ hrs. 2. Low support: 4 clinic visits (0, 2 wks, 6m, 12m) & 6 phone calls (1, 4, 6, 9, 12 wks, 9m), total time 2½ hrs | |
| Outcomes | Sustained abstinence at 12m (validated at all visits from wk 2, PP also reported) Validation: CO < 10 ppm | |
| Notes | NRT and high support versus placebo and low support used for meta‐analysis.Therapists were not full time specialist counsellors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomization list at each centre |
| Allocation concealment (selection bias) | Unclear risk | Allocation process not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 82 (22%) of total in trial lost to follow‐up, 14 deaths, all included as smokers (support condition not specified for dropouts or deaths) |
Velicer 2006.
| Methods | Setting: Community, USA Recruitment: Proactive approach by telephone to smokers at Veterans Administration medical center, not selected for motivation | |
| Participants | 2054 smokers (1023 in relevant arms); 23% F, av age 51, 40% precontemplators, 40% contemplators, 20% preparers Provider: expert system only |
|
| Interventions | 1. Stage‐based S‐H manuals; participants sent manual for current stage and next stage on 2. As 1 plus 6wks nicotine patch if in appropriate stage, reassessed for NRT eligibility at 6 & 10m 3. As 2 plus one expert system feedback report 4. As 3 plus regular automated telephone counselling using prerecorded messages | |
| Outcomes | Abstinence at 30m, sustained for 6m Validation: none, telephone assessors blind to condition | |
| Notes | None of the interventions involved any personal contact. 4 vs 1 used as test of combined intervention. In condition 4 79% received NRT, 60% used the telephone system at least once | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐based random number generator |
| Allocation concealment (selection bias) | Low risk | Allocation done after completion of survey. Randomized participants who did not return consent form are excluded from further analyses |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 39% lost incl 8% refused by 30m, no significant differences between groups. Different treatments of missing data reported not to have altered pattern of results. |
Vial 2002.
| Methods | Setting: Single hospital, Australia Recruitment: inpatients, motivated to quit | |
| Participants | 102 (only 64 followed to 6m), 44% F, av. age 52 Providers: pharmacists |
|
| Interventions | 1. Initial counselling session from hospital pharmacist, up to 16 further weekly meetings after discharge, nicotine patch dispensed weekly at visits. 2. Initial counselling from hospital pharmacist, further sessions with a community pharmacist, same availability of patch 3. Minimal intervention, brief advice only | |
| Outcomes | Continuous abstinence at 12 months Validation: CO ≤ 8 ppm | |
| Notes | 1 & 2 versus 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer randomized, block size 10 |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Due to time constraints only 64 of the randomized participants had been enrolled early enough to be followed at 12m, of whom 19 could not be reached |
Villebro 2008.
| Methods | Setting: Pre‐surgical assessment clinic, Denmark Recruitment: Smokers due to undergo elective hip or knee replacement surgery, not selected for motivation | |
| Participants | 120 daily smokers; 57% F, av. age 65, av. cpd 15; no information about baseline motivation Provider: trial nurse specialist |
|
| Interventions | Intervention: weekly counselling from 6‐8 weeks prior to surgery to 10 days post‐op. Individualised NRT. Strong encouragement to quit but option to reduce consumption by ≥50%. Control: Standard care | |
| Outcomes | Abstinence 1 year after surgery Validation: CO only for those who participated in focus group interviews | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified block randomisation |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4/60 I & 15/60 C not reached at 1y, included as smokers |
Wakefield 2004.
| Methods | Setting: Radiation therapy, medical oncology & haematology departments of a single hospital, Australia Recruitment: cancer patients, not selected for interest in quitting, but number refused enrolment due to lack of interest | |
| Participants | 137 smokers; 38% F, av. age 52, av. cpd 21 Provider: Counsellor |
|
| Interventions | 1. Motivational interviewing by single counsellor, unspecified number and duration of sessions, advice to use NRT if motivated and smoking >15 cpd. 2. Brief advice, SH materials | |
| Outcomes | Abstinence at 6m, prolonged for 3m Validation: cotinine < 400 nmol/mmol or CO < 8 ppm if using NRT | |
| Notes | Average of 11 contacts, 18 min duration, 209 mins total contact/patient. 81% of intervention and 42% of controls reported use of NRT by 6m | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'randomized' |
| Allocation concealment (selection bias) | Unclear risk | no details given. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 32 (23%) lost to follow‐up, included as smokers. 17 deaths excluded from denominators |
Wewers 2000.
| Methods | Setting: AIDS clinical trial unit, Ohio, USA Recruitment: HIV seropositive volunteers, interested in quitting | |
| Participants | 15 male smokers, av. age 40, av. cpd 27 Provider: peer educator |
|
| Interventions | Intervention: Initial session, TQD set, SH materials, wk3 visit, nicotine patch 24 + 4wk), weekly telephone contact from peer educator for 8wk Control: mailed SH, written quit advice from nurse | |
| Outcomes | Continuous abstinence 8 months post intervention (no smoking since quit date) Validation: CO <8 ppm | |
| Notes | Pilot study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'randomly assigned' |
| Allocation concealment (selection bias) | Unclear risk | no details given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 1 control reached at 8 months but 7 known to be smoking at 8 weeks so no impact on continuous abstinence outcomes. |
Wewers 2009.
| Methods | Setting: 14 primary care and women’s health clinics, Ohio, USA Recruitment: Women who had attended clinics in past 2y invited to enrol. Not explicitly selected for motivation |
|
| Participants | 302 women. Av. age not stated, majority under 50, av. cpd 17.2 for intervention, 19.4 for control, p = 0.05 Provider: trained lay health adviser |
|
| Interventions | 1. Intervention from health adviser. 8x 30‐40 min sessions over 12wks. Nicotine patch 21 mg for 8wks. 2. Letter from physician advising cessation, S‐H guide |
|
| Outcomes | Abstinence at 12m (prolonged) (PP was primary trial outcome) Validation: Saliva cotinine < 14 ng/mL |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Randomly assigned' but mentions a biostatistics core staff member suggesting central randomisation |
| Allocation concealment (selection bias) | Low risk | Baseline data collection and eligibility assessed using computer assisted interview before random assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses higher in intervention (22.4%) than control (14.8%) but all missing assumed smoking |
Wilson 1988.
| Methods | Setting: 70 family practices, Canada Recruitment: smokers attending regular appointments, not selected for motivation | |
| Participants | 1207 patients in 46 practices (in relevant arms); ˜64% F, majority aged 25‐44, approx 1/3 smoked >20 cpd Providers: Physician (usual care) |
|
| Interventions | 1. Trained physician asked for a decision to set quit date. At quit date appointment explained correct use of 2 mg gum, x4 supportive follow‐ups over 2m. All visits ˜10 min. Prescription for nicotine gum 2. Untrained physician offered gum prescription, no other advice or follow‐up (not used in this review) 3. Usual care | |
| Outcomes | Abstinence at 12m (sustained for 3m) Validation: saliva cotinine ≤ 0.057 µmol/L, or saliva thiocyanate ≤ µ1724 mol/L if the patient was still using nicotine gum. | |
| Notes | Intervention patients more motivated to quit, so adjusted mean cessation rates used. For meta‐analysis we estimated number of quitters without a correction for clustering within practices; the number of clusters was large, cluster size small (average 28 patients/cluster) and cessation rates did not vary significantly among practices within treatment groups. Less than 65% of intervention used any gum, 27% used only a few pieces. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Cluster randomized by practice |
| Allocation concealment (selection bias) | High risk | Receptionists recruited the first one or two eligible smokers each day. Usual care group physicians were not alerted but other physicians prompted to offer intervention. Recruitment consent rates lower for intervention (76%) than control (91%), and baseline differences in motivation to quit. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4.5% classified as smokers, who might have quit |
Winhusen 2014.
| Methods | Setting: 12 outpatient substance use disorder (SUD) programmes, USA Recruitment: adults enrolled in outpatient SUD, interested in quitting |
|
| Participants | 538 smokers (≥7 cpd), 46% F, av. age 36, av. cpd 16 Provider: Interventionists |
|
| Interventions | 1. Intervention: treatment as usual + extended‐release (XL) bupropion (300mg for 10w + 3d taper) and nicotine inhaler (6–16 nicotine cartridges per day for 10w + 3w taper), counselling, 10 x 10 min weekly sessions, prize‐based contingency management for smoking abstinence. 2. Treatment as usual; SUD treatment as usually provided at the participating site. |
|
| Outcomes | Abstinence at 6 months, 7 day PP (Primary outcome for study was stimulant abstinence) Validation: CO <8 ppm. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'eligible participants are stratified within sites, according to whether a stimulant‐positive (amphetamines or cocaine) UDS result is obtained during screening, and randomized 1:1 at a centralized site' (from Winhusen 2012) |
| Allocation concealment (selection bias) | Low risk | Central randomisation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up 21% (57) I, 20% (53) C, 'no group differences on completion rate or reasons for non‐completion' |
AHRQ ‐ Agency for Healthcare Research; av ‐ average; C ‐ control; CO ‐ carbon monoxide; COPD ‐ chronic obstructive pulmonary disease; CBT ‐ cognitive behavioural therapy; cpd ‐ cigarettes per day; d ‐ day; F ‐ female; GP ‐ general practitioner; I ‐ intervention; m ‐ month(s); M ‐ male; MA ‐ meta‐analysis; MDD ‐ major depressive disorder; MI ‐ motivational interviewing; NRT ‐ nicotine replacement therapy; NS ‐ not specified; O/P ‐ outpatient; PI ‐ principal investigator; PP ‐ point prevalence abstinence; RP ‐ relapse prevention; RR ‐ risk ratio; SC ‐ smoking cessation; S‐H ‐ self‐help; SoC ‐ Stage of Change; TC ‐ telephone counselling; TQD ‐ target quit date; wk(s) ‐ week(s)
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ahluwalia 2006 | Factorial trial crossing nicotine gum/ placebo and two counselling approaches. Matched intensity so no minimal intervention control relevant to this review. (See Lancaster 2005, Individual behavioural counselling for smoking cessation) |
| Alterman 2001 | Comparison of three levels of behaviour support as adjuncts to NRT. Included in Stead 2015. |
| Andrews 2007 | Cluster randomized with only two clusters so not possible to estimate the intraclass correlation. |
| Aveyard 2007 | Comparison of two levels of behaviour support as adjuncts to NRT. Included in Stead 2015. |
| Berndt 2014 | The no intervention control group was a historical control before wards randomised to the two interventions. Other two arms contribute to Stead 2015. |
| Bernstein 2011 | Short follow‐up (three months). |
| Bock 2008 | Main intervention was motivational interviewing. Both intervention and control participants interested in quitting were offered NRT. |
| Bock 2014 | All participants received NRT. Included in Stead 2015. |
| Boyle 2007 | Comparison of two levels of behaviour support as adjuncts to pharmacotherapy. Included in Stead 2015. |
| Brown 2007 | Factorial trial of bupropion/placebo and mood management CBT or standard cessation CBT. Both behavioural interventions were intensive. |
| Brown 2013 | Comparison of two types of behavioural support as adjuncts to NRT. Included in Stead 2015. |
| BTS 1983 | Difference between levels of behavioural support was provision of self‐help materials. |
| Buchanan 2004 | Only three months follow‐up. |
| Bushnell 1997 | All participants offered free NRT. Comparison between two types of support programme. Included in Stead 2015. |
| Calabro 2012 | Comparison of two levels of behavioural support as adjuncts to NRT. Included in Stead 2015. |
| Campbell 1995 | Short‐follow‐up (end of treatment at 16 weeks). Delayed intervention control. |
| Chan 2011 | Planned as a trial of smoking reduction using NRT and counselling; participants selected for lack of motivation to quit. (Did report cessation outcomes, inclusion would not affect conclusions.) |
| Christenhusz 2007 | All participants offered bupropion, test of different intensities of counselling. Also excluded from Stead 2015 because provision and cost of pharmacotherapy differed between arms. |
| Costello 2011 | All participants received NRT and a behavioural intervention. Compared two intensities of counselling. Also excluded from Stead 2015 because only 5 weeks follow‐up. |
| Cropsey 2008 | Waiting list control with delayed intervention. Outcomes reported for early and delayed intervention participants together. |
| Cropsey 2013 | Did not assess number of quitters. |
| Ellerbeck 2009 | All participants received offers of free pharmacotherapy, test of different levels of telephone counselling. Included in Stead 2015. |
| Ferguson 2012 | Some level of pharmacotherapy available to both intervention and control arms. |
| Fiore 2004 | All participants offered NRT, test of different types of counselling. Included in Stead 2015. |
| Fraser 2014 | NRT only 2 weeks and complex control and interventions (5 arms with 32 combinations) |
| Gariti 2009 | Factorial trial comparing two levels of counselling intensity in combination with one of two pharmacotherapies. |
| Gifford 2011 | All participants offered bupropion, test of different levels of behavioural support. Included in Stead 2015. |
| Ginsberg 1992 | All participants offered NRT, test of different levels of behavioural support. Included in Stead 2015. |
| Gordon 2010 | Very little difference in use of pharmacotherapy between interventions (based on 3As or 5As) and control; 30% prescribed pharmacotherapy in intervention vs 20% in control. |
| Hall 1985 | No low intensity control. Two arms included in Stead 2015. |
| Hall 1987 | No low intensity control. Factorial trial of nicotine gum or placebo and high or low intensity behavioural support. The low intensity treatment involved five 60 minute meetings. Included in Stead 2015. |
| Hall 1994 | No low intensity control. Trial compared two high intensity behavioural interventions as adjuncts to NRT in a selected population. Included in Stead 2015. |
| Hall 1996 | No low intensity control. Factorial trial comparing two high intensity interventions, and nicotine gum versus placebo. Also excluded from Stead 2015. |
| Hall 1998 | No low intensity control. Factorial trial comparing two high intensity interventions (as used in Hall 1994), and nortriptyline versus placebo. Included in Stead 2015. |
| Hall 2004 | No low intensity control. Factorial trial comparing extended behavioural intervention and nortriptyline as adjuncts to nicotine patch. Also excluded from Stead 2015. |
| Hall 2009 | No low intensity control. Factorial trial comparing brief and extended behavioural intervention and nortriptyline or placebo as adjuncts to nicotine patch. Included in Stead 2015. |
| Hall 2011 | All participants received bupropion and behavioural support, trial of extended support. Also excluded from Stead 2015. |
| Hegaard 2003 | Study population pregnant smokers, not eligible |
| Hokanson 2006 | Intervention consisted of motivational interviewing and offer of pharmacotherapy. Control group received advice to quit smoking as part of diabetes education programme. Similar numbers of subjects in both groups sought medication (bupropion or NRT) so does not test a combined approach. |
| Huber 1988 | No minimal intervention control; test of nicotine gum versus behavioural support versus combination. |
| Huber 2003 | Both nicotine gum groups had high intensity behavioural support. Control was a waiting list. Included in Stead 2015. |
| Humfleet 2013 | All participants offered NRT, test of different types of counselling. Included in Stead 2015. |
| Ingersoll 2009 | Only 3 months follow‐up. Test of motivational interviewing as adjunct to nicotine patch therapy. Also excluded from Stead 2015. |
| Japuntich 2006 | All participants received bupropion, trial of adjunct internet support. Also excluded from Stead 2015. |
| Jennings 2014 | Only 16 week follow up. |
| Jorenby 1995 | All participants received nicotine patch. Factorial trial of dosage and level of behavioural support. Included in Stead 2015. |
| Joseph 2004 | Intervention and control did not differ on use of pharmacotherapy or intensity of behavioural support. Test of timing in relation to alcohol dependence treatment. |
| Joyce 2008 | Test of reimbursement for pharmacotherapy and counselling. |
| Katz 2002 | Non randomized pilot study for Katz 2004. Before & after study in one clinic with four clinics as controls. |
| Killen 2008 | Test of extended support as adjunct to combined pharmacotherapy. Included in Stead 2015. |
| Kinnunen 2008 | All participants received nicotine gum and brief counselling. Tested efficacy of additional exercise intervention or a matched contact condition that did not involve further counselling. |
| Lacasse 2008 | Only 18% of intervention participants received NRT. Trial in hospital inpatients, detected no effect of 5As behavioural intervention including brief counselling and postdischarge phone calls. |
| Lando 1997 | Test of telephone counselling as adjunct to NRT. Included in Stead 2015. |
| Levine 2010 | No minimal intervention control; behavioural interventions were matched for intensity, specifically tested a weight related intervention. |
| Lifrak 1997 | Comparison of two levels of behavioural support as adjuncts to NRT. Included in Stead 2015. |
| Lloyd‐Richardson 2009 | Comparison of two levels of behavioural support as adjuncts to NRT. Included in Stead 2015. |
| MacLeod 2003 | Test of telephone counselling as adjunct to NRT. Included in Stead 2015. |
| Marshall 1985 | All participants received nicotine gum. Trial of additional follow‐up. Also excluded from Stead 2015. |
| Martin 1997 | No minimal support condition; behavioural conditions differed in theoretical basis but not intensity. |
| Mochizuki 2004 | Only three months follow‐up. Small study of pharmacist advice as adjunct to NRT. |
| Nilsson 1996 | Only four months follow‐up. Intervention was offer of group support and free NRT. |
| Okuyemi 2006 | All participants received same intensity of motivational interviewing, group sessions and offer of NRT. Tested different targets for motivational interviewing. Also excluded from Stead 2015. |
| Okuyemi 2013 | Comparison of two levels of behavioural support as adjuncts to NRT. Included in Stead 2015. |
| Pakhale 2015 | Pharmacotherapy use very similar in each condition although provided via different process. |
| Park 2011 | Non randomized, historical control design. |
| Prochaska 2015 | A large proportion of participants were adolescents and the use of NRT was very low |
| Reid 1999 | Compared two levels of behavioural support as adjuncts to NRT. Included in Stead 2015. |
| Rohsenow 2014 | Comparison of two levels of behavioural support as adjuncts to NRT. Included in Stead 2015. |
| Rovina 2009 | All participants received either a behavioural intervention, pharmacotherapy, or combination. Included in Stead 2015. |
| Schmitz 2007 | All participants received same intensity of group based therapy, compared cognitive behavioural to supportive approaches. See Cochrane review of group based behavioural therapies (Stead 2005). |
| Schnoll 2005 | Behavioural interventions similar in intensity as adjuncts to nicotine patch, and only three months follow‐up |
| Shiffman 2000 | Short follow‐up (12 weeks from start of treatment). Study of computer tailored materials as adjunct to nicotine gum. |
| Shiffman 2001 | Short follow‐up (12 weeks from start of treatment). Study of computer tailored materials as adjunct to nicotine patch. |
| Simon 2003 | Comparison of two levels of behavioural support as adjuncts to NRT. Included in Stead 2015. |
| Smith 2001 | Comparison of three levels of behavioural support as adjuncts to NRT. Included in Stead 2015. |
| Solomon 2000 | Comparison of two levels of behavioural support as adjuncts to NRT. Included in Stead 2015. |
| Solomon 2005 | Comparison of two levels of behavioural support as adjuncts to NRT. Included in Stead 2015. |
| Sorensen 2003 | Short follow‐up (pre‐operative period) |
| Stein 2006 | Comparison of two levels of behavioural support as adjuncts to NRT. Included in Stead 2015. |
| Strecher 2005 | Short follow‐up (12 weeks from start of treatment). Study of web‐based tailored materials as adjunct to nicotine patch. |
| Swan 2003 | All participants received bupropion, factorial trial of dose and intensity of behavioural support. Included in Stead 2015. |
| Swan 2010 | Comparison of three levels of behavioural support as adjuncts to varenicline. Included in Stead 2015. |
| Ward 2001 | Compared two group‐based behavioural interventions similar in intensity as adjuncts to nicotine patch, see Stead 2005 'Group behaviour therapy programmes for smoking cessation' |
| Wiggers 2006 | All participants received NRT, test of additional behavioural support. Included in Stead 2015. |
| Williams 2010 | Comparison of two levels of behavioural support as adjuncts to NRT. Included in Stead 2015. |
| Wolfenden 2005 | Only three month follow‐up. Test of multifaceted intervention including offer of NRT at preoperative clinics. |
| Wolfenden 2008 | Not fully randomized; pilot study in which part of the control group was a historical control, from follow‐up of a previous trial |
| Wu 2009 | Included in Stead 2015. |
| Yalcin 2014 | Compared two types of behavioural support as adjuncts to pharmacotherapy. Included in Stead 2015. |
| Yu 2006 | Short follow‐up (12 weeks from start of treatment). Test of behavioural support adjunct to NRT. Also excluded from Stead 2015. |
CBT ‐ cognitive behavioural therapy; NRT ‐ nicotine replacement therapy
Characteristics of ongoing studies [ordered by study ID]
Aung 2013.
| Trial name or title | Effective Smoking Cessation Augmented PackagE (ESCAPE) Evidence‐based new service package vs. routine package to stop smoking |
| Methods | Setting: non‐ communicable disease clinics in primary care units, Thailand Recruitment/ motivation: Patients at high risk of CVD recruited during routine visit to primary care units; had to be willing to attempt quitting |
| Participants | 328 |
| Interventions | Intervention; Support from primary care nurse at 3 monthly visits, CO measurement and feedback, family member trained to offer support and monitoring, nicotine gum if needed for withdrawal symptoms Control: 5As approach at first visit |
| Outcomes | Abstinence: continuous 6 months at 1 year Verification: CO |
| Starting date | |
| Contact information | myo@juntendo.ac.jp |
| Notes | ISRCTN89315117 |
Cummins 2012.
| Trial name or title | Smoking cessation in Hospitalized Smokers |
| Methods | Randomized 2 x 2 factorial study |
| Participants | Hospitalised patients from 2 San Diego County healthcare systems |
| Interventions | Provision of nicotine patch at discharge/ no NRT. Proactive telephone counselling from California Quitline/ no counselling |
| Outcomes | Abstinence at 2 and 6 months |
| Starting date | August 2011 |
| Contact information | Shu‐Hong Zhu: szhu@ucsd.edu |
| Notes | NCT01289275. Brandstein 2011 was a pilot for this study |
Duffy 2012.
| Trial name or title | Dissemination of the nurse‐administered Tobacco Tactics intervention versus usual care in six Trinity community hospitals: study protocol for a comparative effectiveness trial |
| Methods | Setting: hospitalized smokers, USA Recruitment/ motivation: nurses will identify smokers, then enrolled by research assistant |
| Participants | 1528 hospitalized smokers, 18 and older |
| Interventions | Intervention: Pharmacotherapy: nicotine replacement (patch, gum, and/or lozenge), bupropion, combination, varenicline (prescribed based on a smoking cessation management tool) and nurse‐administered Tobacco Tactics intervention Control: usual care |
| Outcomes | Abstinence: 7 day PP at 6 months Validation: NicAlert urinary test |
| Starting date | July 2011 |
| Contact information | Sonia A Duffy, bump@umich.edu |
| Notes | NCT01309217 |
Fu 2014.
| Trial name or title | Improved Effectiveness of Smoking Cessation Programs for Minnesota Priority Populations |
| Methods | Setting: adult smokers enrolled in the Minnesota Health Care Programs (MHCP), a state‐funded health insurance plan for low‐income persons, USA Recruitment/ motivation: baseline participant screening survey conducted prior to randomization |
| Participants | 2500 adult smokers enrolled in the Minnesota Health Care Programs |
| Interventions | 1. Proactive outreach combined with free NRT and telephone counseling (PRO+NRT+TC) 2. Usual care (Smoking cessation products: patch, gum, lozenge, inhaler and nasal spray |
| Outcomes | Abstinence: 6 month prolonged abstinence at 1 year Validation: not specified |
| Starting date | February 2011 |
| Contact information | Steven Fu, Steven.Fu@va.gov |
| Notes | NCT01123967 |
Metse 2014.
| Trial name or title | Evaluating the efficacy of an integrated smoking cessation intervention for mental health patients: study protocol for a randomised controlled trial |
| Methods | Setting: acute mental illness inpatients (psychiatry), Australia Recruitment/Setting: Research staff recruited participants; unselected |
| Participants | 800 |
| Interventions | 1. Inpatient brief motivational interview, package of self‐help material, post discharge: up to 12 weeks NRT, 16 weeks telephone support, proactive Quitline referral 2. Standard hospital and discharge smoking cessation care |
| Outcomes | Abstincence: prolonged cessation and 7 day PP at 12 months Validation: CO |
| Starting date | November 16 2012 |
| Contact information | alexandra.metse@uon.edu.au |
| Notes | ACTRN12612001042831 |
NCT01320462.
| Trial name or title | Smoking Cessation Program in the Preadmission Clinic: The Combination of Counseling, Pharmacotherapy and Quit Line |
| Methods | Setting: Canada Recruitment/motivation: unselected |
| Participants | 296 |
| Interventions | 1. counselling, pharmacotherapy, and quit line ‐ structured preoperative counselling, pharmacotherapy with varenicline for three months, and referral to the quit line (Smokers' Helpline) for proactive telephone counseling and follow up. 2. control ‐ brief advice regarding smoking cessation and provision of the quit line's information. |
| Outcomes | Abstinence: continuous abstinence, 1 year after surgery |
| Starting date | December 2010 |
| Contact information | Dr. Frances Chung, Staff Anesthesiologist, University Health Network, Toronto |
| Notes |
Prochaska 2014b.
| Trial name or title | Tobacco treatment in inpatient psychiatry settings |
| Methods | RCT recruiting in 5 acute inpatient psychiatry units, San Francisco |
| Participants | 693 adult smokers, psychiatric inpatients |
| Interventions | Transtheoretical model (TTM)‐tailored computer‐delivered intervention, motivational enhancement and cognitive–behavioral counselling, and nicotine‐replacement therapy |
| Outcomes | Smoking cessation |
| Starting date | 2009 |
| Contact information | Judith Prochaska |
| Notes | Publication reports baseline data. No results identified |
Thomas 2013.
| Trial name or title | Give up for Good, A pharmacist‐led system‐change smoking cessation intervention for smokers admitted to Australian public hospitals |
| Methods | RCT in 3 public hospitals, Australia |
| Participants | 600 inpatient smokers |
| Interventions | Intervention: Pharmacist will help prepare a quit plan, discuss/provide pharmacotherapy, link to primary care support, & follow‐up 4 weeks post discharge Control: usual care |
| Outcomes | Biochemically verified 7‐day PP abstinence at 6 & 12 months |
| Starting date | 2012 |
| Contact information | Johnson George |
| Notes |
Urdapilleta‐Herrera 2013.
| Trial name or title | Bupropion together with cognitive‐conductual therapy (CBT) |
| Methods | RCT |
| Participants | 45 smokers |
| Interventions | 1. Bupropion & cognitive behavioural therapy 2. Placebo |
| Outcomes | Abstinence at 6 months |
| Starting date | |
| Contact information | |
| Notes |
Weaver 2015.
| Trial name or title | Feasibility of delivering a quitline based smoking cessation intervention in lung cancer patients receiving outpatient treatment: a pilot study |
| Methods | RCT in 13 National Cancer Institute Community Clinical Oncology Program (CCOP) sites, USA |
| Participants | 146 cancer patients who are scheduled to receive or currently receiving surgery, radiation or chemotherapy OR have received one or more of the following within the last 6 months; surgery, last radiation treatment, or last chemotherapy treatment in a community outpatient setting. |
| Interventions | Intervention: brief in‐person counselling, quitline telephone counselling, and 6 weeks of nicotine replacement Control: usual care, advice to quit & self‐help materials |
| Outcomes | Abstinence at 24 weeks |
| Starting date | October 2011 |
| Contact information | Kathryn Weaver |
| Notes | Preliminary results presented in conference abstract. |
Wong 2008.
| Trial name or title | The Chinese Community Smoking Cessation Project |
| Methods | Prospective, randomized clinical trial, set in the Chinese community in San Francisco, CA, USA |
| Participants | 464 Chinese Americans with medical conditions, av. 9 cpd |
| Interventions | Intervention: physician advice, in‐person counselling with nicotine replacement therapy, 5 telephone calls Control: physician advice and self‐help manual only Recruitment and intervention and control treatments were culturally tailored |
| Outcomes | Biochemically validated self reported abstinence at 6, 12 and 24 months |
| Starting date | Ran from 2001 to 2007 |
| Contact information | Candice C. Wong, Candice.Wong@ucsf.edu |
| Notes | Study completed but full trial report not available |
Differences between protocol and review
A second planned objective of the review was to evaluate the effect of increasing behavioural support for people using pharmacotherapy. This is now addressed in a companion review (Stead 2015).
The categories used for some subgroup analyses were altered: we distinguished between no person‐to‐person contact and between one and three sessions rather than using the US Guideline categories of zero to one and two to three, and collapsed one to three minutes and 4 to 30 minutes for contact time. We added categories of peer group counsellor and lay counsellor to provider type, and we added a category of studies that did not explicitly select for motivation but where study procedures or participant characteristics suggested that participants were typically motivated to quit.
We did not use a brief/moderate/intensive subgroup analysis because it did not help discriminate between studies.
Contributions of authors
LS & TL jointly conceived the review and wrote the protocol.
LS designed the search strategy and prescreened results. LS and TL or PK agreed on study inclusion and data extraction. TF conducted meta‐regressions for the update.
All authors contributed to the text and are responsible for the analyses and conclusions.
Sources of support
Internal sources
Nuffield Department of Primary Care Health Sciences, University of Oxford, UK.
External sources
NHS, National Institute for Health Research, UK.
National School for Health Research, School for Primary Care Research, UK.
Declarations of interest
No authors have any conflicts of interest to report.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
An 2006 {published data only}
- An LC, Partin M, Zhu SH, Arikian NJ, Nelson DB, Nugent SM, et al. Delivery of bupropion SR as part of a telephone counseling intervention for veteran smokers (POS1‐040). Society for Research on Nicotine and Tobacco 10th Annual Meeting February 18‐21, Phoenix, Arizona. 2004.
- An LC, Zhu S‐H, Nelson DB, Arikian NJ, Nugent S, Partin MR, et al. Benefits of telephone care over primary care for smoking cessation. Archives of Internal Medicine 2006;166:536‐42. [DOI] [PubMed] [Google Scholar]
- An LC, Zhu SH, Arikian NJ, Nelson DB, Nugent SM, Partin M, et al. Telestop: a randomized trial of increased access to behavioral and pharmacological therapy for smoking cessation (abstract). Nicotine & Tobacco Research 2005;7(4):689. [Google Scholar]
- Smith M, Fu S, Nugent S, Nelson D, Joseph A, An L. A cost‐effective telehealth intervention for smoking cessation. Journal of General Internal Medicine 2010;25(Suppl 3):208. [Google Scholar]
Baker 2006 {published data only}
- Baker A, Richmond R, Haile M, Lewin TJ, Carr VJ, Taylor RL, et al. A randomized controlled trial of a smoking cessation intervention among people with a psychotic disorder. American Journal of Psychiatry 2006;163(11):1934‐42. [DOI] [PubMed] [Google Scholar]
- Baker A, Richmond R, Haile M, Lewin TJ, Carr VJ, Taylor RL, et al. Characteristics of smokers with a psychotic disorder and implications for smoking interventions. Psychiatry Research 2007;150(2):141‐52. [DOI] [PubMed] [Google Scholar]
- Baker A, Richmond R, Kay‐Lambkin F, Lewin TJ, Carr VJ. Randomized controlled trial of a smoking cessation intervention among people with a psychotic disorder: 3‐year follow‐up (PA2‐2). Society for Research on Nicotine and Tobacco 13th Annual Meeting February 21‐24, Austin, Texas. 2007.
- Baker A, Richmond R, Lewin TJ, Kay‐Lambkin F. Cigarette smoking and psychosis: naturalistic follow up 4 years after an intervention trial. Australian and New Zealand Journal of Psychiatry 2010;44(4):342‐50. [CENTRAL: 750490; CRS: 9400123000005634; PUBMED: 20307166] [DOI] [PubMed] [Google Scholar]
- Richmond R, Baker A, Haile MJ, Carr V, Lewin T, Wilhelm K, et al. Randomized control trial of nicotine patch and cognitive behavioral therapy among smokers with psychotic illness (PA5‐4). Society for Research on Nicotine and Tobacco 10th Annual Meeting February 18‐21, Phoenix, Arizona. 2004.
- Richmond RL, Baker A, Haile M, Carr V, Lewin T, Wilhelm K, et al. Intervention for tobacco dependence among people with a psychotic illness: RCT with one year outcome. Nicotine & Tobacco Research 2005;7(4):681. [Google Scholar]
Bernstein 2015 {published data only}
- Bernstein SL, D'Onofrio G, Rosner J, O'Malley S, Makuch R, Busch S, et al. Successful tobacco dependence treatment in low‐income emergency department patients: a randomized trial. Academic Emergency Medicine 2015;66(2):140‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Binnie 2007 {published data only}
- Binnie VI, McHugh S, Jenkins W, Borland W, MacPherson LM. A randomised controlled trial of a smoking cessation intervention delivered by dental hygienists: A feasibility study. BMC Oral Health 2007;7:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brandstein 2011 {published and unpublished data}
- Brandstein K. A proactive smoking cessation intervention with hospitalized smokers: A randomized controlled trial. Dissertation Abstracts International 2012;72(8‐B):4600. [Google Scholar]
- Brandstein K. A proactive smoking cessation intervention with hospitalized smokers: A randomized controlled trial. PhD Dissertation, University of California San Diego & San Diego State University 2011.
Carmody 2012 {published data only}
- Carmody TP, Delucchi K, Duncan CL, Banys P, Simon JA, Solkowitz SN, et al. Intensive intervention for alcohol‐dependent smokers in early recovery: a randomized trial. Drug and Alcohol Dependence 2012;122(3):186‐94. [CENTRAL: 832176; CRS: 9400123000012551; EMBASE: 2012211083; PUBMED: 22014532] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmody TP, Delucchi K, Simon JA, Duncan CL, Solkowitz SN, Huggins J, et al. Expectancies regarding the interaction between smoking and substance use in alcohol‐dependent smokers in early recovery. Psychology of Addictive Behaviors 2012;26(2):358‐63. [CENTRAL: 836797; CRS: 9400123000011228; EMBASE: 2013459892; PUBMED: 21707127] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chan 2010 {published data only}
- Chan SS, Leung DY, Abdullah AS, Lo SS, Yip AW, Kok WM, et al. Smoking‐cessation and adherence intervention among Chinese patients with erectile dysfunction. American Journal of Preventive Medicine 2010;39(3):251‐8. [DOI] [PubMed] [Google Scholar]
Chouinard 2005 {published data only}
- Chouinard MC, Robichaud‐Ekstrand S. The effectiveness of a nursing inpatient smoking cessation program in individuals with cardiovascular disease. Nursing Research 2005;54(4):243‐54. [DOI] [PubMed] [Google Scholar]
Cooney 2007 {published data only}
- Cooney N, Pilkey D, Steinberg H, Cooney J, Litt M, Oncken C. Ecological momentary assessment of alcohol‐tobacco interactions after concurrent alcohol‐tobacco treatment. Society for Research on Nicotine and Tobacco 11th Annual Meeting, 20‐23 March 2005; Prague, Czech Republic. 2005.
- Cooney N, Pilkey D, Steinberg H, Cooney J, Litt M, Oncken C. Efficacy of intensive versus brief smoking cessation treatment concurrent with intensive outpatient alcohol treatment (POS4‐62). Society for Research on Nicotine and Tobacco 9th Annual Meeting February 19‐22 New Orleans, Louisiana. 2003.
- Cooney NL, Litt MD, Cooney JL, Pilkey DT, Steinberg HR, Oncken CA. Concurrent brief versus intensive smoking intervention during alcohol dependence treatment. Psychology of Addictive Behaviors 2007;21:570‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney NL, Litt MD, Cooney JL, Pilkey DT, Steinburg HR, Oncken CA. Alcohol and tobacco cessation in alcohol‐dependent smokers: analysis of real‐time reports. Psychology of Addictive Behaviors 2007;21:277‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MM, Grant C, Cooper S, Cooney JL. Anxiety and smoking cessation outcomes in alcohol‐dependent smokers. Nicotine & Tobacco Research 2013;15(2):364‐75. [CENTRAL: 865747; CRS: 9400107000000696; PUBMED: 22955245] [DOI] [PubMed] [Google Scholar]
Duffy 2006 {published data only}
- Duffy SA, Ronis DL, Valenstein M, Lambert MT, Fowler KE, Gregory L, et al. A tailored smoking, alcohol, and depression intervention for head and neck cancer patients. Cancer Epidemiology, Biomarkers & Prevention 2006;15(11):2203‐8. [DOI] [PubMed] [Google Scholar]
- Duffy SA, Scheumann AL, Fowler KE, Darling‐Fisher C, Terrell JE. Perceived difficulty quitting predicts enrollment in a smoking‐cessation program for patients with head and neck cancer. Oncology Nursing Forum 2010;37(3):349‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Emmons 2005 {published data only}
- Emmons KM, Puleo E, Mertens A, Gritz ER, Diller L, Li FP. Long‐term smoking cessation outcomes among childhood cancer survivors in the Partnership for Health Study. Journal of Clinical Oncology 2009;27:52‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmons KM, Puleo E, Park E, Gritz ER, Butterfield RM, Weeks JC, et al. Peer‐delivered smoking counseling for childhood cancer survivors increases rate of cessation: the Partnership for Health Study. Journal of Clinical Oncology 2005;23:6516‐23. [DOI] [PubMed] [Google Scholar]
- Park ER, Puleo E, Butterfield RM, Zorn M, Mertens AC, Gritz ER, et al. A process evaluation of a telephone‐based peer‐delivered smoking cessation intervention for adult survivors of childhood cancer: the Partnership for Health Study. Preventive Medicine 2006;42:435‐42. [DOI] [PubMed] [Google Scholar]
Haas 2015 {published data only}
- Haas JS, Linder JA, Park ER, Gonzalez I, Rigotti NA, Klinger EV, et al. Proactive tobacco cessation outreach to smokers of low socioeconomic status: a randomized clinical trial. JAMA Internal Medicine 2015;175(2):218‐26. [CENTRAL: 1047636; CRS: 9400050000000150; EMBASE: 2015718788; PUBMED: 25506771] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hall 2002 {published data only}
- Hall SM, Humfleet GL, Reus VI, Munoz RF, Hartz DT, Maude‐Griffin R. Psychological intervention and antidepressant treatment in smoking cessation. Archives of General Psychiatry 2002;59:930‐6. [DOI] [PubMed] [Google Scholar]
- Hall SM, Lightwood JM, Humfleet GL, Bostrom A, Reus VI, Munoz R. Cost‐effectiveness of bupropion, nortriptyline, and psychological intervention in smoking cessation. Journal of Behavioral Health Services & Research 2005;32:381‐92. [DOI] [PubMed] [Google Scholar]
Hall 2006 {published data only}
- Barnett PG, Wong W, Hall S. The cost‐effectiveness of a smoking cessation program for out‐patients in treatment for depression. Addiction 2008;103(5):834‐40. [DOI] [PubMed] [Google Scholar]
- Hall SM, Tsoh JY, Prochaska JJ, Eisendrath S, Rossi JS, Redding CA, et al. Treatment for cigarette smoking among depressed mental health outpatients: A randomized clinical trial. American Journal of Public Health 2006;96(10):1808‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haug NA, Hall SM, Prochaska JJ, Rosen AB, Tsoh JY, Humfleet G, et al. Acceptance of nicotine dependence treatment among currently depressed smokers. Nicotine & Tobacco Research 2005;7:217‐224. [DOI] [PubMed] [Google Scholar]
- Prochaska JJ, Hall SM, Tsoh JY, Eisendrath S, Rossi JS, Redding CA, et al. Treating tobacco dependence in clinically depressed smokers: effect of smoking cessation on mental health functioning. American Journal of Public Health 2008;98(3):446‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochaska JJ, Rossi JS, Redding CA, Rosen AB, Tsoh JY, Humfleet GL, et al. Depressed smokers and stage of change: implications for treatment interventions. Drug and Alcohol Dependence 2004;76:143‐51. [DOI] [PubMed] [Google Scholar]
Hanioka 2010 {published data only}
- Hanioka T, Ojima M, Tanaka H, Naito M, Hamajima N, Matsuse R. Intensive smoking‐cessation intervention in the dental setting. Journal of Dental Research 2010;89(1):66‐70. [DOI] [PubMed] [Google Scholar]
Hickman 2015 {published data only}
- Hickman NJ 3rd, Delucchi KL, Prochaska JJ. Treating tobacco dependence at the intersection of diversity, poverty, and mental illness: A randomized feasibility and replication trial. Nicotine & Tobacco Research 2015;17(8):101‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hollis 2007 {published data only}
- Hollis JF, McAfee TA, Fellows JL, Zbikowski SM, Stark M, Riedlinger K. The effectiveness and cost effectiveness of telephone counselling and the nicotine patch in a state tobacco quitline. Tobacco Control 2007;16(Suppl 1):i53‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Juarranz Sanz 1998 {published data only}
- Juarranz Sanz M, Soriano Llora T, Reyes Martin G, Sanchez Sachez MI. Efficacy of the treatment with substitute of nicotine in the smoking cessation from the Primary Health Care. Revista de Medicina Familiar y Comunitaria 1998;8(6):376‐83. [Google Scholar]
Katz 2004 {published data only}
- Katz DA, Muehlenbruch DR, Brown RL, Fiore MC, Baker TB. Effectiveness of implementing the Agency for Healthcare Research and Quality smoking cessation clinical practice guideline: a randomized, controlled trial. Journal of the National Cancer Institute 2004;96(8):594‐603. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kotz 2009 {published data only}
- Kotz D, Wesseling G, Huibers MJ, Schayck OC. Efficacy of confrontational counselling for smoking cessation in smokers with previously undiagnosed mild to moderate airflow limitation: study protocol of a randomized controlled trial. BMC Public Health 2007;7:332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotz D, Wesseling G, Huibers MJH, Schayck OCP. Efficacy of confronting smokers with airflow limitation for smoking cessation. European Respiratory Journal 2009;33:754‐62. [DOI] [PubMed] [Google Scholar]
Lee 2015 {published data only}
- Lee SM, Landry J, Jones PM, Buhrmann O, Morley‐Forster P. Long‐term quit rates after a perioperative smoking cessation randomized controlled trial. Anesthesia and Analgesia 2015;120(3):582‐7. [CENTRAL: 1066736; CRS: 9400131000001133; PUBMED: 25695576] [DOI] [PubMed] [Google Scholar]
- Lee SM, Landry J, Jones PM, Buhrmann O, Morley‐Forster P. The effectiveness of a perioperative smoking cessation program: A randomized clinical trial. Anesthesia and Analgesia 2013;117(3):605‐13. [CENTRAL: 876186; CRS: 9400126000000174; EMBASE: 2013565919; PUBMED: 23868890] [DOI] [PubMed] [Google Scholar]
Lewis 1998 {published data only}
- Lewis SF, Piasecki TM, Fiore MC, Anderson JE, Baker TB. Transdermal nicotine replacement for hospitalized patients: A randomized clinical trial. Preventive Medicine 1998;27:296‐303. [DOI] [PubMed] [Google Scholar]
Lung Health Study {published data only}
- Anthonisen NR, Skeans MA, Wise RA, Manfreda J, Kanner RE, Connett JE, et al. The effects of a smoking cessation intervention on 14.5‐year mortality: a randomized clinical trial. Annals of Internal Medicine 2005;142:233‐39. [DOI] [PubMed] [Google Scholar]
- Bjornson W, Rand C, Connett JE, Lindgren P, Nides M, Pope F, et al. Gender differences in smoking cessation after 3 years in the Lung Health Study. American Journal of Public Health 1995;85:223‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connett JE, Kusek JW, Bailey WC, O'Hara P, Wu M. Design of the Lung Health Study: a randomized clinical trial of early intervention for chronic obstructive pulmonary disease. Controlled Clinical Trials 1993;14(2 Suppl):3S‐19S. [DOI] [PubMed] [Google Scholar]
- Nides MA, Rakos RF, Gonzales D, Murray RP, Tashkin DP, Bjornson‐Benson WM, et al. Predictors of initial smoking cessation and relapse through the first 2 years of the Lung Health Study. Journal of Consulting & Clinical Psychology 1995;63:60‐9. [DOI] [PubMed] [Google Scholar]
- O'Hara P, Grill J, Rigdon MA, Connett JE, Lauger GA Johnston JJ. Design and results of the initial intervention program for the Lung Health Study. Preventive Medicine 1993;22:304‐15. [DOI] [PubMed] [Google Scholar]
- Wise RA, Kanner RE, Lindgren P, Connett JE, Altose MD, Enright PL, et al. The effect of smoking intervention and an inhaled bronchodilator on airways reactivity in COPD: the Lung Health Study. Chest 2003;124(2):449‐58. [DOI] [PubMed] [Google Scholar]
McCarthy 2008 {published data only}
- McCarthy DE. Mechanisms of tobacco cessation treatment: Self‐report mediators of counseling and bupropion sustained release treatment. Dissertation Abstracts International: Section B: The Sciences and Engineering 2007;67(9‐B):5414. [Google Scholar]
- McCarthy DE, Piasecki TM, Jorenby DE, Lawrence DL, Shiffman S, Baker TB. A multi‐level analysis of non‐significant counseling effects in a randomized smoking cessation trial. Addiction (Abingdon, England) 2010;105(12):2195‐208. [CENTRAL: 780680; CRS: 9400123000005902; EMBASE: 20840173; PUBMED: 20840173] [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy DE, Piasecki TM, Lawrence DL, Jorenby DE, Shiffman S, Baker TB. Psychological mediators of bupropion sustained‐release treatment for smoking cessation. Addiction 2008;103:1521‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy DE, Piasecki TM, Lawrence DL, Jorenby DE, Shiffman S, Fiore MC, et al. A randomized controlled clinical trial of bupropion SR and individual smoking cessation counseling. Nicotine & Tobacco Research 2008;10:717‐29. [DOI] [PubMed] [Google Scholar]
- Minami H, McCarthy DE, Jorenby DE, Baker TB. An Ecological Momentary Assessment analysis of relations among coping, affect and smoking during a quit attempt. Addiction 2011;106:641‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mohiuddin 2007 {published data only}
- Mohiuddin SM, Mooss AN, Hunter CB, Grollmes TL, Cloutier DA, Hilleman DE. Intensive smoking cessation intervention reduces mortality in high‐risk smokers with cardiovascular disease. Chest 2007;131:446‐52. [DOI] [PubMed] [Google Scholar]
Molyneux 2003 {published data only}
- Molyneux A, Lewis S, Leivers U, Anderton A, Antoniak M, Brackenridge A, et al. Clinical trial comparing nicotine replacement therapy (NRT) plus brief counselling, brief counselling alone, and minimal intervention on smoking cessation in hospital inpatients. Thorax 2003;58:484‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Murray 2013 {published data only}
- Murray RL, Leonardi‐Bee J, Marsh J, Jayes L, Li J, Parrott S, et al. Systematic identification and treatment of smokers by hospital based cessation practitioners in a secondary care setting: cluster randomised controlled trial. BMJ (Clinical Research Ed.) 2013;347:f4004. [CENTRAL: 873086; CRS: 9400107000001505; EMBASE: 2013464666; PEER: Reviewed Journal: 2013‐26055‐001; PUBMED: 23836616] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ockene 1991 {published data only}
- Hebert JR, Kristeller J, Ockene JK, Landon J, Luippold R, Goldberg RJ, et al. Patient characteristics and the effect of three physician‐ delivered smoking interventions. Preventive Medicine 1992;21:557‐73. [DOI] [PubMed] [Google Scholar]
- Ockene JK, Kristeller J, Goldberg R, Amick TL, Pekow PS, Hosmer D, et al. Increasing the efficacy of physician‐delivered smoking interventions: a randomized clinical trial. Journal of General Internal Medicine 1991;6:1‐8. [DOI] [PubMed] [Google Scholar]
- Ockene JK, Kristeller J, Pbert L, Hebert JR, Luippold R, Goldberg RJ, et al. The physician‐delivered smoking intervention project: can short‐term interventions produce long‐term effects for a general outpatient population?. Health Psychology 1994;13:278‐81. [DOI] [PubMed] [Google Scholar]
- Rosal MC, Ockene JK, Hurley TG, Kalan K, Hebert JR. Effectiveness of nicotine‐containing gum in the physician‐ delivered smoking intervention study. Preventive Medicine 1998;27(2):262‐7. [DOI] [PubMed] [Google Scholar]
Okuyemi 2007 {published data only}
- Ahluwalia JS, Nollen N, Kaur H, James AS, Mayo MS, Resnicow K. Pathway to health: cluster‐randomized trial to increase fruit and vegetable consumption among smokers in public housing. Health Psychology 2007;26(2):214‐21. [DOI] [PubMed] [Google Scholar]
- Boardman T, Catley D, Grobe JE, Little TD, Ahluwalia JS. Using motivational interviewing with smokers: do therapist behaviors relate to engagement and therapeutic alliance?. Journal of Substance Abuse Treatment 2006;31(4):329‐39. [DOI] [PubMed] [Google Scholar]
- Okuyemi KS, James AS, Mayo MS, Nollen N, Catley D, Choi WS, et al. Pathways to health: a cluster randomized trial of nicotine gum and motivational interviewing for smoking cessation in low‐income housing. Health Education & Behavior 2007;34(1):43‐54. [DOI] [PubMed] [Google Scholar]
Otero 2006 {published data only}
- Otero UB, Perez CA, Szklo M, Esteves GA, dePinho MM, Szklo AS, et al. Randomized clinical trial: effectiveness of the cognitive‐behavioral approach and the use of nicotine replacement transdermal patches for smoking cessation among adults in Rio de Janeiro, Brazil [Portuguese] [Ensaio clinico randomizado: efetividade da abordagem cognitivo‐comportamental e uso de adesivos transdermicos de reposicao de nicotina, na cessacao de fumar, em adultos residentes no Municipio do Rio de Janeiro, Brasil]. Cadernos de Saude Publica 2006;22:439‐49. [DOI] [PubMed] [Google Scholar]
Peckham 2015 {published data only}
- Gilbody S, Peckham E, Man M‐S, Mitchell N, Li J, Becque T, et al. Bespoke smoking cessation for people with severe mental ill health (SCIMITAR): A pilot randomised controlled trial. The Lancet Psychiatry 2015;2(5):395‐402. [DOI] [PubMed] [Google Scholar]
- Peckham E, Man MS, Mitchell N, Li J, Becque T, Knowles S, et al. Smoking Cessation Intervention for severe Mental Ill Health Trial (SCIMITAR): a pilot randomised control trial of the clinical effectiveness and cost‐effectiveness of a bespoke smoking cessation service. Health Technology Assessment (Winchester, England) 2015;19(25):1‐148. [CRS: 9400131000001435; EMBASE: 2015892977; PUBMED: 25827850] [DOI] [PMC free article] [PubMed] [Google Scholar]
Perez‐Tortosa 2015 {published data only}
- Perez‐Tortosa S, Roig L, Manresa JM, Martin‐Cantera C, Puigdomenech E, Roura P, et al. Continued smoking abstinence in diabetic patients in primary care: a cluster randomized controlled multicenter study. Diabetes Research and Clinical Practice 2015;107(1):94‐103. [CENTRAL: 1051143; CRS: 9400131000000622; EMBASE: 2015750171; PUBMED: 25444354] [DOI] [PubMed] [Google Scholar]
- Roig L, Perez S, Prieto G, Martin C, Advani M, Armengol A, et al. Cluster randomized trial in smoking cessation with intensive advice in diabetic patients in primary care. ITADI Study. BMC Public Health 2010;10:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Prochaska 2014 {published data only}
- Prochaska JJ, Hall SE, Delucchi K, Hall SM. Efficacy of initiating tobacco dependence treatment in inpatient psychiatry: a randomized controlled trial. American Journal of Public Health 2014;104(8):1557‐65. [CENTRAL: 1014299; CRS: 9400129000003152] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochaska JJ, Hall SE, Hall SM. Stage‐tailored tobacco cessation treatment in inpatient psychiatry. Psychiatric Services (Washington, D.C.) 2009;60(6):848. [CRS: 9400131000001867; PUBMED: 19487360] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ratner 2004 {published data only}
- Bottorff JL, Johnson JL, Moffat B, Fofonoff D, Budz B, Groening M. Synchronizing clinician engagement and client motivation in telephone counseling. Qualitative Health Research 2004;14:462‐77. [DOI] [PubMed] [Google Scholar]
- Ratner PA, Johnson JL, Richardson CG, Bottorff JL, Moffat B, Mackay M, et al. Efficacy of a Smoking‐Cessation Intervention for Elective‐Surgical Patients. Research in Nursing and Health 2004;27:148‐61. [DOI] [PubMed] [Google Scholar]
Reid 2003 {published data only}
- Reid R, Pipe A, Higginson L, Johnson K, D'Angelo MS, Cooke D, et al. Stepped care approach to smoking cessation in patients hospitalized for coronary artery disease. Journal of Cardiopulmonary Rehabilitation 2003;23(3):176‐82. [DOI] [PubMed] [Google Scholar]
Reid 2008 {published data only}
- Reid MS, Fallon B, Sonne S, Flammino F, Nunes EV, Jiang H, et al. Smoking cessation treatment in community‐based substance abuse rehabilitation programs. Journal of Substance Abuse Treatment 2008;35(1):68‐77. [DOI] [PubMed] [Google Scholar]
- Reid MS, Jiang H, Fallon B, Sonne S, Rinaldi P, Turrigiano E, et al. Smoking cessation treatment among patients in community‐based substance abuse rehabilitation programs: Exploring predictors of outcome as clues toward treatment improvement. American Journal of Drug and Alcohol Abuse 2011;37(5):472‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid MS, Jiang H, Fallon B, Sonne S, Rinaldi P, Turrigiano E, et al. Smoking cessation treatment among patients in community‐based substance abuse rehabilitation programs: Exploring predictors of outcome as clues toward treatment improvement. American Journal of Drug and Alcohol Abuse 2011;37(5):472‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonne SC, Nunes EV, Jiang H, Tyson C, Rotrosen J, Reid MS. The relationship between depression and smoking cessation outcomes in treatment‐seeking substance abusers. American Journal on Addictions 2010;19:111‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rigotti 2014 {published data only}
- Japuntich SJ, Regan S, Viana J, Tymoszczuk J, Reyen M, Levy DE, et al. Comparative effectiveness of post‐discharge interventions for hospitalized smokers: study protocol for a randomized controlled trial. Trials 2012;13:124. [CENTRAL: 863876; CRS: 9400107000000040; PUBMED: 22852832] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigotti NA, Japuntich S, Regan S, Kelley JH, Chang Y, Reyen M, et al. Promoting smoking cessation after hospital discharge: The Helping Hand randomized controlled comparative effectiveness trial. Journal of General Internal Medicine 2013;28(1 Suppl):S160. [CENTRAL: 993940; CRS: 9400130000000359; EMBASE: 71292966] [Google Scholar]
- Rigotti NA, Regan S, Levy DE, Japuntich S, Chang Y, Park ER, et al. Sustained care intervention and postdischarge smoking cessation among hospitalized adults a randomized clinical trial. JAMA 2014;312(7):719‐28. [CENTRAL: 1000144; CRS: 9400129000002791; EMBASE: 2014554016; PUBMED: 25138333] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rodriguez 2003 {published data only (unpublished sought but not used)}
- Guallar‐Castillon P, Lafuente Urdinguio P, Garteizaurrekoa Dublang P, Sainz Martinez O, Diez Azcarate JI, Foj Aleman M, et al. Probability of success in tobacco quitting during the course of two simple medical interventions [Probabilidad de exito en el abandono del tabaco en el curso de dos intervenciones sencillas para dejar de fumar]. Revista Espanol de Salud Publica 2003;77(1):117‐24. [PubMed] [Google Scholar]
- Rodriguez‐Artalejo F, Lafuente Urdinguio P, Guallar‐Castillon P, Garteizaurrekoa Dublang P, Sainz Martinez O, Diez Azcarate JI, et al. One year effectiveness of an individualised smoking cessation intervention at the workplace: a randomised controlled trial. Occupational Environmental Medicine 2003;60(3):358‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sadr Azodi 2009 {published data only}
- Sadr Axodi O, Lindstrom D, Adami J, Tonnesen H, Nasell H, Gilljam H, et al. The efficacy of a smoking cessation programme in patients undergoing elective surgery: a randomised clinical trial. Anaesthesia 2009;64(3):259‐65. [DOI] [PubMed] [Google Scholar]
Schauffler 2001 {published data only}
- Schauffler HH, McMenamin S, Olson K, Boyce‐Smith G, Rideout JA, Kamil J. Variations in treatment benefits influence smoking cessation: results of a randomised controlled trial. Tobacco Control 2001;10(2):175‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Segnan 1991 {published data only}
- Segnan N, Ponti A, Battista RN, Senore C, Rosso S, Shapiro SH, et al. A randomized trial of smoking cessation interventions in general practice in Italy. Cancer Causes and Control 1991;2:239‐46. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Senore C, Battista RN, Ponti A, Segnan N, Shapiro SH, Rosso S, et al. Comparing participants and nonparticipants in a smoking cessation trial: Selection factors associated with general practitioner recruitment activity. Journal of Clinical Epidemiology 1999;52:83‐9. [DOI] [PubMed] [Google Scholar]
- Senore C, Battista RN, Shapiro SH, Segnan N, Ponti A, Rosso S, et al. Predictors of smoking cessation following physicians' counseling. Preventive Medicine 1998;27(3):412‐21. [DOI] [PubMed] [Google Scholar]
Simon 1997 {published data only}
- Simon JA, Solkowitz SN, Carmody TP, Browner WS. Smoking cessation after surgery ‐ A randomized trial. Archives of Internal Medicine 1997;157:1371‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Stockings 2014 {published data only}
- The effect of an integrated smoking care program on the reduction and abstinence of smoking in mental health inpatients following discharge from hospital. Http://www.anzctr.org.au/trial_view.aspx?ID=83938 2012. [CRS: 9400123000016492]
- Stockings EA, Bowman JA, Baker AL, Terry M, Clancy R, Wye PM, et al. Impact of a postdischarge smoking cessation intervention for smokers admitted to an inpatient psychiatric facility: a randomized controlled trial. Nicotine & Tobacco Research 2014;16(11):1417‐28. [CENTRAL: 1036731; CRS: 9400129000003421; EMBASE: 2014920180; PUBMED: 24939916] [DOI] [PubMed] [Google Scholar]
- Stockings EA, Bowman JA, Wiggers J, Baker AL, Terry M, Clancy R, et al. A randomised controlled trial linking mental health inpatients to community smoking cessation supports: a study protocol. BMC Public Health 2011;11:570. [CENTRAL: 810681; CRS: 9400123000011878; EMBASE: 21762532; 6272; PUBMED: 21762532] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thomsen 2010 {published data only}
- Thomsen T, Tonnesen H, Okholm M, Kroman N, Maibom A, Sauerberg ML, et al. Brief smoking cessation intervention in relation to breast cancer surgery: a randomized controlled trial. Nicotine & Tobacco Research 2010;12(11):1118‐24. [DOI] [PubMed] [Google Scholar]
Tonnesen 2006 {published data only}
- Tonnesen P, Mikkelsen K, Bremann L. Nurse‐conducted smoking cessation in patients with COPD using nicotine sublingual tablets and behavioral support. Chest 2006;130(2):334‐42. [DOI] [PubMed] [Google Scholar]
Velicer 2006 {published data only}
- Velicer WF, Friedman RH, Fava JL, Gulliver SB, Keller S, Sun X, et al. Evaluating nicotine replacement therapy and stage‐based therapies in a population‐based effectiveness trial. Journal of Consulting and Clinical Psychology 2006;74(6):1162‐72. [DOI] [PubMed] [Google Scholar]
Vial 2002 {published data only}
- Vial RJ, Jones TE, Ruffin RE, Gilbert AL. Smoking cessation program using nicotine patches linking hospital to the community. Journal of Pharmacy Practice and Research 2002;32(1):57‐62. [Google Scholar]
Villebro 2008 {published data only}
- Moller AM, Pedersen T, Villebro N, Norgaard P. Impact of lifestyle on perioperative smoking cessation and postoperative complication rate. Preventive Medicine 2003;36:704‐9. [DOI] [PubMed] [Google Scholar]
- Moller AM, Villebro N, Pedersen T, Tonnesen H. Effect of preoperative smoking intervention on postoperative complications: A randomised clinical trial. Lancet 2002;359:114‐7. [DOI] [PubMed] [Google Scholar]
- Villebro NM, Pedersen T, Moller AM, Tonnesen H. Long‐term effects of a preoperative smoking cessation programme. The Clinical Respiratory Journal 2008;2:175‐82. [DOI] [PubMed] [Google Scholar]
Wakefield 2004 {published data only}
- Wakefield M, Olver I, Whitford H, Rosenfeld E. Motivational interviewing as a smoking cessation intervention for patients with cancer: randomized controlled trial. Nursing Research 2004;53(6):396‐405. [DOI] [PubMed] [Google Scholar]
Wewers 2000 {published data only}
- Wewers ME, Neidig JL, Kihm KE. The feasibility of a nurse‐managed, peer‐led tobacco cessation intervention among HIV‐positive smokers. Journal of the Association of Nurses In Aids Care 2000;11(6):37‐44. [DOI] [PubMed] [Google Scholar]
Wewers 2009 {published data only}
- Hood NE, Ferketich AK, Paskett ED, Wewers ME. Treatment adherence in a lay health adviser intervention to treat tobacco dependence. Health Education Research 2013;28(1):72‐82. [CENTRAL: 921000; CRS: 9400107000000711; PEER: Reviewed Journal: 2013‐02978‐007; PUBMED: 22843347] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troyer MB, Ferketich AK, Murray DM, Paskett ED, Wewers ME. Characteristics of rural Appalachian women who enroll in a tobacco dependence treatment clinical trial. Nicotine & Tobacco Research 2011;13(9):880‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wewers ME, Ferketich AK, Harness J, Paskett ED. Effectiveness of a nurse‐managed, lay‐led tobacco cessation intervention among Ohio Appalachian women. Cancer Epidemiology, Biomarkers & Prevention 2009;18:3451‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilson 1988 {published data only}
- Wilson DM, Taylor DW, Gilbert JR, Best JA, Lindsay EA, Willms DG, et al. A randomized trial of a family physician intervention for smoking cessation. Journal of the American Medical Association 1988;260(11):1570‐4. [PubMed] [Google Scholar]
Winhusen 2014 {published data only}
- Winhusen T, Stitzer M, Woody G, Brigham G, Kropp F, Ghitza U, et al. Design considerations for a study to evaluate the impact of smoking cessation treatment on stimulant use outcomes in stimulant‐dependent individuals. Contemporary Clinical Trials 2012;33(1):197‐205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winhusen TM, Brigham GS, Kropp F, Lindblad R, Gardin JG2, Penn P, et al. A randomized trial of concurrent smoking‐cessation and substance use disorder treatment in stimulant‐dependent smokers. Journal of Clinical Psychiatry 2014;75(4):336‐43. [CENTRAL: 883292; CRS: 9400129000001165; EMBASE: 2014305114; PUBMED: 24345356] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Ahluwalia 2006 {published data only}
- Ahluwalia JS, Okuyemi K, Nollen N, Choi WS, Kaur H, Pulvers K, et al. The effects of nicotine gum and counseling among African American light smokers: A 2 x 2 factorial design. Addiction 2006;101:883‐91. [DOI] [PubMed] [Google Scholar]
- Berg CJ, Thomas JL, An LC, Guo H, Collins T, Okuyemi Kolawole S, et al. Change in smoking, diet, and walking for exercise in Blacks. Health Education & Behavior 2012;39(2):191‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nollen NL, Mayo MS, Sanderson CL, Okuyemi KS, Choi WS, Kaur H, et al. Predictors of quitting among African American light smokers enrolled in a randomized, placebo‐controlled trial. Journal of General Internal Medicine 2006;21:590‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuyemi KS, Cox LS, Nollen NL, Snow TM, Kaur H, Choi W, et al. Baseline characteristics and recruitment strategies in a randomized clinical trial of African‐American light smokers. American Journal of Health Promotion 2007;21:183‐91. [DOI] [PubMed] [Google Scholar]
- Okuyemi KS, Faseru B, Sanderson CL, Bronars CA, Ahluwalia JS. Relationship between menthol cigarettes and smoking cessation among African American light smokers. Addiction 2007;102:1979‐86. [DOI] [PubMed] [Google Scholar]
- Okuyemi KS, Pulvers KM, Cox LS, Thomas JL, Kaur H, Mayo MS, et al. Nicotine dependence among African American light smokers: a comparison of three scales. Addictive Behaviors 2007;32:1989‐2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Alterman 2001 {published data only}
- Alterman AI, Gariti P, Cook TG, Cnaan A. Nicodermal patch adherence and its correlates. Drug & Alcohol Dependence 1999;53:159‐65. [DOI] [PubMed] [Google Scholar]
- Alterman AI, Gariti P, Mulvaney F. Short‐ and long‐term smoking cessation for three levels of intensity of behavioral treatment. Psychology of Addictive Behaviors 2001;15:261‐4. [PubMed] [Google Scholar]
Andrews 2007 {published data only}
- Andrews JO, Felton G, Wewers ME, Waller J, Tingen M. The effect of a multi‐component smoking cessation intervention in African American women residing in public housing. Research in Nursing & Health 2007;30(1):45‐60. [DOI] [PubMed] [Google Scholar]
Aveyard 2007 {published data only}
- Aveyard P, Brown K, Saunders C, Alexander A, Johnstone E, Munafo MR, et al. Weekly versus basic smoking cessation support in primary care: a randomised controlled trial. Thorax 2007;62:898‐903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves C. Smoking cessation trial may be missing the point [comment]. Thorax 2008;63:291‐2. [PubMed] [Google Scholar]
Berndt 2014 {published data only}
- Berndt N, Bolman C, Froelicher ES, Mudde A, Candel M, Vries H, et al. Effectiveness of a telephone delivered and a face‐to‐face delivered counseling intervention for smoking cessation in patients with coronary heart disease: a 6‐month follow‐up. Journal of Behavioral Medicine 2014;37(4):709‐24. [CENTRAL: 1000936; CRS: 9400107000001638; PUBMED: 23760610] [DOI] [PubMed] [Google Scholar]
- Berndt N, Bolman C, Lechner L, Mudde A, Verheugt FW, Vries H. Effectiveness of two intensive treatment methods for smoking cessation and relapse prevention in patients with coronary heart disease: study protocol and baseline description. BMC Cardiovascular Disorders 2012;12:33. [CENTRAL: 840357; CRS: 9400123000016156; EMBASE: 2012574240; PUBMED: 22587684] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bernstein 2011 {published data only}
- Bernstein SL, Bijur P, Cooperman N, Jearld S, Arnsten JH, Moadel A, et al. A randomized trial of a multicomponent cessation strategy for emergency department smokers. Academic Emergency Medicine 2011;18:575‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein SL, Bijur P, Cooperman N, Jearld S, Arnsten JH, Moadel A, et al. Efficacy of an emergency department‐based multicomponent intervention for smokers with substance use disorders. Journal of Substance Abuse Treatment 2013;44(1):139‐42. [CENTRAL: 867792; CRS: 9400107000000027; PUBMED: 22763199] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bock 2008 {published data only}
- Bock BC, Becker BM, Niaura RS, Partridge R, Fava JL, Trask P. Smoking cessation among patients in an emergency chest pain observation unit: outcomes of the Chest Pain Smoking Study (CPSS). Nicotine & Tobacco Research 2008;10(10):1523‐31. [DOI] [PubMed] [Google Scholar]
Bock 2014 {published data only}
- Bock BC, Papandonatos GD, Dios MA, Abrams DB, Azam MM, Fagan M, et al. Tobacco cessation among low‐income smokers: motivational enhancement and nicotine patch treatment. Nicotine & Tobacco Research 2014;16(4):413‐22. [CENTRAL: 986341; CRS: 9400050000000050; EMBASE: 2014185069; PUBMED: 24174612] [DOI] [PMC free article] [PubMed] [Google Scholar]
Boyle 2007 {published data only}
- Boyle RG, Solberg LI, Asche SE, Boucher JL, Pronk NP, Jensen CJ. Offering telephone counseling to smokers using pharmacotherapy. Nicotine & Tobacco Research 2005;7(Suppl 1):S19‐27. [DOI] [PubMed] [Google Scholar]
- Boyle RG, Solberg LI, Asche SE, Maciosek MV, Boucher JL, Pronk NP. Proactive recruitment of health plan smokers into telephone counseling. Nicotine & Tobacco Research 2007;9:581‐9. [DOI] [PubMed] [Google Scholar]
Brown 2007 {published data only}
- Brown RA, Niaura R, Lloyd‐Richardson EE, Strong DR, Kahler CW, Abrantes AM, et al. Bupropion and cognitive‐behavioral treatment for depression in smoking cessation. Nicotine & Tobacco Research 2007;9(7):721‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong DR, Kahler CW, Leventhal AM, Abrantes AM, Lloyd‐Richardson E, Niaura R, et al. Impact of bupropion and cognitive‐behavioral treatment for depression on positive affect, negative affect, and urges to smoke during cessation treatment. Nicotine & Tobacco Research 2009;11:1142‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brown 2013 {published data only}
- Brown RA, Reed KM, Bloom EL, Minami H, Strong DR, Lejuez CW, et al. Development and preliminary randomized controlled trial of a distress tolerance treatment for smokers with a history of early lapse. Nicotine & Tobacco Research 2013;15(12):2005‐15. [CENTRAL: 915430; CRS: 9400129000000262; EMBASE: 2013716990; PUBMED: 23884317] [DOI] [PMC free article] [PubMed] [Google Scholar]
BTS 1983 {published data only}
- British Thoracic Society. Comparison of four methods of smoking withdrawal in patients with smoking related diseases. Report by a subcommittee of the Research Committee of the British Thoracic Society. BMJ 1983;286:595‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Buchanan 2004 {published data only}
- Buchanan LM, Banna M, White A, Moses S, Siedlik C, Wood M. An exploratory study of multicomponent treatment intervention for tobacco dependency. Journal of Nursing Scholarship 2004;36:324‐30. [DOI] [PubMed] [Google Scholar]
Bushnell 1997 {published data only}
- Bushnell FK, Forbes B, Goffaux J, Dietrich M, Wells N. Smoking cessation in military personnel. Military Medicine 1997;162(11):715‐9. [PubMed] [Google Scholar]
Calabro 2012 {published data only}
- Calabro KS, Marani S, Yost T, Segura J, Mullin Jones M, Nelson S, et al. Project SUCCESS: results from a randomized controlled trial. ISRN Public Health 2012:Article ID 913713. [CENTRAL: 836719; CRS: 9400123000016091] [Google Scholar]
- Prokhorov AV, Marani S, Vost T, Luca M, Mullen M. Project SUCCESS: students using computerized coaching to end smoking successfully [PA2‐3]. Society for Research on Nicotine & Tobacco 17th Annual Meeting, February 16‐19, Toronto. 2011:18. [CENTRAL: 795274; CRS: 9400123000006112; 31113]
Campbell 1995 {published data only}
- Campbell BK, Wander N, Stark MJ, Holbert T. Treating cigarette‐smoking in drug‐abusing clients. Journal of Substance Abuse Treatment 1995;12:89‐94. [DOI] [PubMed] [Google Scholar]
Chan 2011 {published data only}
- Chan SS, Leung DY, Abdullah AS, Wong VT, Hedley AJ, Lam TH. A randomized controlled trial of a smoking reduction plus nicotine replacement therapy intervention for smokers not willing to quit smoking. Addiction 2011;106(6):1155‐63. [DOI] [PubMed] [Google Scholar]
Christenhusz 2007 {published data only}
- Christenhusz L, Pieterse M, Seydel E, PJ. Prospective determinants of smoking cessation in COPD patients within a high intensity or a brief counseling intervention. Patient Education & Counseling 2007;66(2):162‐6. [DOI] [PubMed] [Google Scholar]
- Christenhusz LC, Prenger R, Pieterse ME, Seydel ER, Palen J. Cost‐effectiveness of an intensive smoking cessation intervention for COPD outpatients. Nicotine & Tobacco Research 2012;14(6):657‐63. [CENTRAL: 834174; CRS: 9400123000014488; EMBASE: 2012309134; PUBMED: 22180589] [DOI] [PubMed] [Google Scholar]
Costello 2011 {published data only}
- Costello MJ, Sproule B, Victor JC, Leatherdale ST, Zawertailo L, Selby P. Effectiveness of pharmacist counseling combined with nicotine replacement therapy: a pragmatic randomized trial with 6,987 smokers. Cancer Causes & Control 2011;22(2):167‐80. [DOI] [PubMed] [Google Scholar]
Cropsey 2008 {published data only}
- Berg CJ, Ahluwalia JS, Cropsey K. Predictors of adherence to behavioral counseling and medication among female prisoners enrolled in a smoking cessation trial. Journal of Correctional Health Care 2013;19(4):236‐47. [CENTRAL: 1000792; CRS: 9400129000001078; PUBMED: 23955055] [DOI] [PubMed] [Google Scholar]
- Cropsey K, Eldridge G, Weaver M, Villalobos G, Stitzer M, Best A. Smoking cessation intervention for female prisoners: addressing an urgent public health need. American Journal of Public Health 2008;98(10):1894‐901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cropsey KL, Jackson DO, Hale GJ, Carpenter MJ, Stitzer ML. Impact of self‐initiated pre‐quit smoking reduction on cessation rates: results of a clinical trial of smoking cessation among female prisoners. Addictive Behaviors 2011;36(1‐2):73‐8. [CENTRAL: 794056; CRS: 9400123000006091] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cropsey KL, Weaver MF, Eldridge GD, Villalobos GC, Best AM, Stitzer ML. Differential success rates in racial groups: results of a clinical trial of smoking cessation among female prisoners. Nicotine & Tobacco Research 2009;11(6):690‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cropsey 2013 {published data only}
- Cropsey KL, Hendricks PS, Jardin B, Clark CB, Katiyar N, Willig J, et al. A pilot study of screening, brief intervention, and referral for treatment (SBIRT) in non‐treatment seeking smokers with HIV. Addictive Behaviors 2013;38(10):2541‐6. [CENTRAL: 875522; CRS: 9400107000001206; EMBASE: 2013410228; PEER: Reviewed Journal: 2013‐27387‐013; PUBMED: 23787030] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ellerbeck 2009 {published data only}
- Cox LS, Cupertino AP, Mussulman LM, Nazir N, Greiner KA, Mahnken JD, et al. Design and baseline characteristics from the KAN‐QUIT disease management intervention for rural smokers in primary care. Preventive Medicine 2008;47(2):200‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellerbeck EF, Mahnken JD, Cupertino AP, Cox LS, Greiner KA, Mussulman LM, et al. Effect of varying levels of disease management on smoking cessation: a randomized trial. Annals of Internal Medicine 2009;150(7):437‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ferguson 2012 {published data only}
- Coleman T, McEwen A, Bauld L, Ferguson J, Lorgelly P, Lewis S. Protocol for the Proactive Or Reactive Telephone Smoking CeSsation Support (PORTSSS) trial. Trials [Electronic Resource] 2009;10:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson J, Docherty G, Bauld L, Lewis S, Lorgelly P, Boyd KA, et al. Effect of offering different levels of support and free nicotine replacement therapy via an English national telephone quitline: randomised controlled trial. BMJ March 2012;344:e1696. [DOI: 10.1136/bmj.e1696] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fiore 2004 {published data only}
- Fiore MC, McCarthy DE, Jackson TC, Zehner ME, Jorenby DE, Mielke M, et al. Integrating smoking cessation treatment into primary care: An effectiveness study. Preventive Medicine 2004;38:412‐20. [DOI] [PubMed] [Google Scholar]
Fraser 2014 {published data only}
- Fraser D, Kobinsky K, Smith SS, Kramer J, Theobald WE, Baker TB. Five population‐based interventions for smoking cessation: a MOST trial. Translational Behavioral Medicine 2014;4(4):382‐90. [CENTRAL: 1040081; CRS: 9400129000003838; EMBASE: 2015657527; PUBMED: 25584087] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gariti 2009 {published data only}
- Gariti P, Levin S, Whittingham T, Barou D, Kampman KM, Lynch K, et al. Why do those who request smoking treatment fail to attend the first appointment?. Journal of Substance Abuse Treatment 2008;35:62‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gariti P, Lynch K, Alterman A, Kampman K, Xie H, Varillo K. Comparing smoking treatment programs for lighter smokers with and without a history of heavier smoking. Journal of Substance Abuse Treatment 2009;37(3):247‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gifford 2011 {published data only}
- Gifford EV, Kohlenberg BS, Hayes SC, Pierson HM, Piasecki MP, Antonuccio DO, et al. Does acceptance and relationship focused behavior therapy contribute to bupropion outcomes? A randomized controlled trial of functional analytic psychotherapy and acceptance and commitment therapy for smoking cessation. Behavior Therapy 2011;42(4):700‐15. [CENTRAL: 814642; CRS: 9400123000012398; 6939; PUBMED: 22035998] [DOI] [PubMed] [Google Scholar]
Ginsberg 1992 {published data only}
- Ginsberg D, Hall SM, Rosinski M. Partner support, psychological treatment, and nicotine gum in smoking treatment: an incremental study. International Journal of the Addictions 1992;27:503‐14. [DOI] [PubMed] [Google Scholar]
Gordon 2010 {published data only}
- Gordon JS, Andrews JA, Albert DA, Crews KM, Payne TJ, Severson HH. Tobacco cessation via public dental clinics: results of a randomized trial. American Journal of Public Health 2010;100(7):1307‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hall 1985 {published data only}
- Hall SM, Killen JD. Psychological and pharmacological approaches to smoking relapse prevention. NIDA Research Monograph 1985;53:131‐43. [PubMed] [Google Scholar]
- Hall SM, Tunstall C, Rugg D, Jones R, Benowitz N. Nicotine gum and behavioral treatment in smoking cessation. Journal of Consulting and Clinical Psychology 1985;53(2):256‐8. [DOI] [PubMed] [Google Scholar]
Hall 1987 {published data only}
- Hall SM, Tunstall CD, Ginsberg D, Benowitz NL, Jones RT. Nicotine gum and behavioral treatment: a placebo controlled trial. Journal of Consulting and Clinical Psychology 1987;55:603‐5. [DOI] [PubMed] [Google Scholar]
Hall 1994 {published data only}
- Hall SM, Munoz RF, Reus VI. Cognitive‐behavioral intervention increases abstinence rates for depressive‐history smokers. Journal of Consulting and Clinical Psychology 1994;62:141‐6. [DOI] [PubMed] [Google Scholar]
Hall 1996 {published data only}
- Hall SM, Munoz RF, Reus VI, Sees KL, Duncan C, Humfleet GL, et al. Mood management and nicotine gum in smoking treatment ‐ a therapeutic contact and placebo‐controlled study. Journal of Consulting and Clinical Psychology 1996;64:1003‐09. [DOI] [PubMed] [Google Scholar]
Hall 1998 {published data only}
- Hall SM, Reus VI, Munoz RF, Sees KL, Humfleet G, Hartz DT, et al. Nortriptyline and cognitive‐behavioral therapy in the treatment of cigarette smoking. Archives of General Psychiatry 1998;55:683‐90. [DOI] [PubMed] [Google Scholar]
- Hall SM, Reus VI, Munoz RF, Sees KL, Humfleet GL, Frederick S. Nortriptyline and cognitive‐behavioral treatment of cigarette smoking. CPDD Annual Meeting. San Juan, Puerto Rico, 1996:52.
Hall 2004 {published data only}
- Hall SM, Humfleet GL, Reus VI, Munoz RF, Cullen J. Extended nortriptyline and psychological treatment for cigarette smoking. American Journal of Psychiatry 2004;161(11):2100‐7. [DOI] [PubMed] [Google Scholar]
Hall 2009 {published data only}
- Hall SM, Humfleet GL, Munoz RF, Reus VI, Robbins JA, Prochaska JJ. Extended treatment of older cigarette smokers. Addiction 2009;104:1043‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks PS, Delucchi KL, Hall SM. Mechanisms of change in extended cognitive behavioral treatment for tobacco dependence. Drug & Alcohol Dependence 2010;109:114‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hall 2011 {published data only}
- Hall SM, Humfleet GL, Muñoz RF, Reus VI, Prochaska JJ, Robbins JA. Using extended cognitive behavioral treatment and medication to treat dependent smokers. American Journal of Public Health 2011;101(12):2349‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hegaard 2003 {published data only}
- Hegaard HK, Kjaergaard H, Moller LF, Wachmann H, Ottesen B. Multimodal intervention raises smoking cessation rate during pregnancy. Acta Obstetricia et Gynecologica Scandinavica 2003;82(9):813‐9. [DOI] [PubMed] [Google Scholar]
Hokanson 2006 {published data only}
- Hokanson JM, Anderson RL, Hennrikus DJ, Lando HA, Kendall DM. Integrated tobacco cessation counseling in a diabetes self‐management training program: a randomized trial of diabetes and reduction of tobacco. The Diabetes Educator 2006;32(4):562‐70. [DOI] [PubMed] [Google Scholar]
Huber 1988 {published data only}
- Huber D. Combined and separate treatment effects of nicotine chewing gum and self‐control method. Pharmacopsychiatry 1988;21:461‐2. [DOI] [PubMed] [Google Scholar]
Huber 2003 {published data only}
- Huber D, Gastner J. Smoking cessation: A comparison of behavior therapy, nicotine replacement therapy and their combination. Verhaltenstherapie und Verhaltenshmedizin 2003;24:167‐85. [Google Scholar]
Humfleet 2013 {published data only}
- Humfleet GL, Hall SM, Delucchi KL, Dilley JW. A randomized clinical trial of smoking cessation treatments provided in HIV clinical care settings. Nicotine & Tobacco Research 2013;15(8):1436‐45. [CENTRAL: 861196; CRS: 9400123000017501; EMBASE: 2013472501; OI:SOURCE:: NLM. PMC3715392 [Available on 08/01/14]; PUBMED: 23430708] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ingersoll 2009 {published data only}
- Ingersoll KS, Cropsey KL, Heckman CJ. A test of motivational plus nicotine replacement interventions for HIV positive smokers. AIDS & Behavior 2009;13(3):545‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Japuntich 2006 {published data only}
- Japuntich SJ, Zehner ME, Smith SS, Jorenby DE, Valdez JA, Fiore MC, et al. Smoking cessation via the internet: a randomized clinical trial of an internet intervention as adjuvant treatment in a smoking cessation intervention. Nicotine & Tobacco Research 2006;8(Suppl 1):S59‐67. [DOI] [PubMed] [Google Scholar]
Jennings 2014 {published data only}
- Jennings C, Kotseva K, Bacquer D, Hoes A, Velasco J, Brusaferro S, et al. Effectiveness of a preventive cardiology programme for high CVD risk persistent smokers: the EUROACTION PLUS varenicline trial. European Heart Journal 2014;35(21):1411‐20. [CENTRAL: 996426; CRS: 9400129000002224; EMBASE: 2014386940; PUBMED: 24616337] [DOI] [PubMed] [Google Scholar]
Jorenby 1995 {published data only}
- Jorenby DE, Smith SS, Fiore MC, Hurt RD, Offord KP, Croghan IT, et al. Varying nicotine patch dose and type of smoking cessation counseling. JAMA 1995;274:1347‐52. [PubMed] [Google Scholar]
Joseph 2004 {published data only}
- Joseph AM, Nelson DB, Nugent SM, Willenbring ML. Timing of alcohol and smoking cessation (TASC): smoking among substance use patients screened and enrolled in a clinical trial. Journal of Addictive Disease 2003;22:87‐107. [DOI] [PubMed] [Google Scholar]
- Joseph AM, Willenbring ML, Nugent SM, Nelson DB. A randomized trial of concurrent versus delayed smoking intervention for patients in alcohol dependence treatment. Journal of Studies on Alcohol 2004;65(6):681‐91. [DOI] [PubMed] [Google Scholar]
Joyce 2008 {published data only}
- Joyce GF, Niaura R, Maglione M, Mongoven J, Larson‐Rotter C, Coan J, et al. The effectiveness of covering smoking cessation services for medicare beneficiaries. Health Services Research 2008;43(6):2106‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Katz 2002 {published data only}
- Katz DA, Muehlenbruch DR, Brown RB, Fiore MC, Baker TB. Effectiveness of a clinic‐based strategy for implementing the AHRQ Smoking Cessation Guideline in primary care. Preventive Medicine 2002;35:293‐301. [DOI] [PubMed] [Google Scholar]
Killen 2008 {published data only}
- Killen JD, Fortmann SP, Schatzberg AF, Arredondo C, Murphy G, Hayward C, et al. Extended cognitive behavior therapy for cigarette smoking cessation. Addiction 2008;103(8):1381‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kinnunen 2008 {published data only}
- Kinnunen T, Leeman RF, Korhonen T, Quiles ZN, Terwal DM, Garvey AJ, et al. Exercise as an adjunct to nicotine gum in treating tobacco dependence among women. Nicotine & Tobacco Research 2008;10(4):689‐703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen T, Goodwin A, Miesmaa P, Dupuis EA, Kinnunen T. Smoking cessation program with exercise improves cardiovascular disease biomarkers in sedentary women. Journal of Women's Health 2011;20(7):1051‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lacasse 2008 {published data only}
- Lacasse Y, Lamontagne R, Martin S, Simard S, Arsenault M. Randomized trial of a smoking cessation intervention in hospitalized patients. Nicotine & Tobacco Research 2008;10(7):1215‐21. [DOI] [PubMed] [Google Scholar]
Lando 1997 {published data only}
- Lando HA, Rolnick S, Klevan D, Roski J, Cherney L, Lauger G. Telephone support as an adjunct to transdermal nicotine in smoking cessation. American Journal of Public Health 1997;87:1670‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolnick SJ, Klevan D, Cherney L, Lando HA. Nicotine replacement therapy in a group model HMO. HMO Practice 1997;11:34‐7. [PubMed] [Google Scholar]
Levine 2010 {published data only}
- Levine MD, Perkins KA, Kalarchian MA, Cheng Y, Houck PR, Slane JD, et al. Bupropion and cognitive behavioral therapy for weight‐concerned women smokers. Archives of Internal Medicine 2010;170:543‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lifrak 1997 {published data only}
- Lifrak P, Gariti P, Alterman AI, McKay J, Volpicelli J, Sparkman T, et al. Results of two levels of adjunctive treatment used with the nicotine patch. American Journal on Addictions 1997;6:93‐8. [PubMed] [Google Scholar]
Lloyd‐Richardson 2009 {published data only}
- Lloyd‐Richardson EE, Stanton CA, Papandonatos GD, Shadel WG, Stein M, Tashima K, et al. Motivation and patch treatment for HIV+ smokers: a randomized controlled trial. Addiction 2009;104(11):1891‐900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton CA, Lloyd‐Richardson EE, Papandonatos GD, Dios MA, Niaura R. Mediators of the relationship between nicotine replacement therapy and smoking abstinence among people living with HIV/AIDS. AIDS Education & Prevention 2009;21(3 Suppl):65‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
MacLeod 2003 {published data only}
- Macleod ZR, Charles MA, Arnaldi VC, Adams IM. Telephone counselling as an adjunct to nicotine patches in smoking cessation: a randomised controlled trial. Medical Journal of Australia 2003;179:349‐52. [DOI] [PubMed] [Google Scholar]
Marshall 1985 {published data only}
- Marshall A, Raw M. Nicotine chewing gum in general practice: effect of follow up appointments. British Medical Journal 1985;290:1397‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Martin 1997 {published data only}
- Martin JE, Calfas KJ, Patten CA, Polarek M, Hofstetter CR, Noto J, et al. Prospective evaluation of three smoking interventions in 205 recovering alcoholics: one‐year results of project SCRAP‐tobacco. Journal of Consulting and Clinical Psychology 1997;65:190‐4. [DOI] [PubMed] [Google Scholar]
- Patten CA, Martin JE, Calfas KJ, Brown SA, Schroeder DR. Effect of three smoking cessation treatments on nicotine withdrawal in 141 abstinent alcoholic smokers. Addictive Behaviors 2000;25:301‐6. [DOI] [PubMed] [Google Scholar]
Mochizuki 2004 {published data only}
- Mochizuki M, Hatsugaya M, Rokujoh E, Arita E, Hashiguchi M, Shimizu N, et al. [Randomized controlled study on the effectiveness of community pharmacists' advice for smoking cessation by Nicorette‐‐evaluation at three months after initiation]. Yakugaku Zasshi 2004;124(12):989‐95. [DOI] [PubMed] [Google Scholar]
Nilsson 1996 {published data only}
- Nilsson P, Lundgren H, Soderstrom M, Fagerstrom KO, Nilsson Ehle P. Effects of smoking cessation on insulin and cardiovascular risk factors‐‐a controlled study of 4 months' duration. Journal of Internal Medicine 1996;240(4):189‐94. [DOI] [PubMed] [Google Scholar]
Okuyemi 2006 {published data only}
- Okuyemi KS, Thomas JL, Hall S, Nollen NL, Richter KP, Jeffries SK, et al. Smoking cessation in homeless populations: A pilot clinical trial. Nicotine & Tobacco Research 2006;8(5):689‐99. [DOI] [PubMed] [Google Scholar]
Okuyemi 2013 {published data only}
- Goldade K, Jarlais D, Everson‐Rose SA, Guo H, Thomas J, Gelberg L, et al. Knowing quitters predicts smoking cessation in a homeless population. American Journal of Health Behavior 2013;37(4):517‐24. [CENTRAL: 993944; CRS: 9400107000000703; PEER: Reviewed Journal: 2013‐08953‐009; PUBMED: 23985232] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldade K, Whembolua GL, Thomas J, Eischen S, Guo H, Connett J, et al. Designing a smoking cessation intervention for the unique needs of homeless persons: A community‐based randomized clinical trial. Clinical Trials 2011;8(6):744‐54. [CENTRAL: 814647; CRS: 9400123000012422; 6866; PUBMED: 22167112] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuyemi KS, Goldade K, Whembolua G‐L, Thomas JL, Eischen S, Sewali B, et al. Motivational interviewing to enhance nicotine patch treatment for smoking cessation among homeless smokers: a randomized controlled trial. Addiction 2013;108(6):1136‐44. [CENTRAL: 921008; CRS: 9400107000001568; PEER: Reviewed Journal: 2013‐16822‐021; PUBMED: 23510102] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuyemi KS, Goldade K, Whembolua GL, Thomas JL, Eischen S, Guo H, et al. Smoking characteristics and comorbidities in the power to quit randomized clinical trial for homeless smokers. Nicotine & Tobacco Research 2013;15(1):22‐8. [CENTRAL: 814589; CRS: 9400103000000015; EMBASE: 2012756123; PUBMED: 22589422] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner C, Sewali B, Olayinka A, Eischen S, Wang Q, Guo H, et al. Smoking cessation in homeless populations: who participates and who does not. Nicotine & Tobacco Research 2014;16(3):369‐72. [CENTRAL: 977672; CRS: 9400129000000793] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pakhale 2015 {published data only}
- Pakhale S, Baron J, Armstrong MA, Garde A, Reid RD, Alvarez G, et al. A pilot randomized controlled trial of smoking cessation in an outpatient respirology clinic. Canadian Respiratory Journal [Revue Canadienne de Pneumologie] 2015;22(2):91‐6. [CRS: 9400131000001433; EMBASE: 2015888546; PUBMED: 25647168] [DOI] [PMC free article] [PubMed] [Google Scholar]
Park 2011 {published data only}
- Park ER, Japuntich S, Temel J, Lanuti M, Pandiscio J, Hilgenberg J, et al. A smoking cessation intervention for thoracic surgery and oncology clinics: A pilot trial. Journal of Thoracic Oncology 2011;6(6):1059‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Prochaska 2015 {published data only}
- Grana RA, Ramo DE, Fromont SC, Hall SM, Prochaska JJ. Correlates of tobacco dependence and motivation to quit among young people receiving mental health treatment. Drug and Alcohol Dependence 2012;125(1‐2):127‐31. [CENTRAL: 840375; CRS: 9400123000016714; PUBMED: 22560677] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochaska JJ, Fromont SC, Ramo DE, Young‐Wolff KC, Delucchi K, Brown RA, et al. Gender differences in a randomized controlled trial treating tobacco use among adolescents and young adults with mental health concerns. Nicotine & Tobacco Research 2015;17(4):479‐85. [CRS: 9400131000001562; PUBMED: 25762759] [DOI] [PMC free article] [PubMed] [Google Scholar]
Reid 1999 {published data only}
- D'Angelo ME, Reid RD, Brown KS, Pipe AL. Gender differences in predictors for long‐term smoking cessation following physician advice and nicotine replacement therapy. Canadian Journal of Public Health 2001;92(6):418‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid RD, Pipe A, Dafoe WA. Is telephone counselling a useful addition to physician advice and nicotine replacement therapy in helping patients to stop smoking? A randomized controlled trial. Canadian Medical Association Journal 1999;160(11):1577‐81. [PMC free article] [PubMed] [Google Scholar]
Rohsenow 2014 {published data only}
- Rohsenow DJ, Martin RA, Monti PM, Colby SM, Day AM, Abrams DB, et al. Motivational interviewing versus brief advice for cigarette smokers in residential alcohol treatment. Journal of Substance Abuse Treatment 2014;46(3):346‐55. [CENTRAL: 959232; CRS: 9400130000000480; EMBASE: 2014038589; PUBMED: 24210533] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohsenow DJ, Monti PM, Colby SM, Martin RA. Brief interventions for smoking cessation in alcoholic smokers. Alcoholism, Clinical and Experimental Research 2002;26(12):1950‐1. [CENTRAL: 434257; CRS: 9400123000003065; PUBMED: 12500132] [DOI] [PubMed] [Google Scholar]
Rovina 2009 {published data only}
- Rovina N, Nikoloutsou I, Katsani G, Dima E, Fransis K, Roussos C, et al. Effectiveness of pharmacotherapy and behavioral interventions for smoking cessation in actual clinical practice. Therapeutic Advances in Respiratory Disease 2009;3:279‐87. [DOI] [PubMed] [Google Scholar]
Schmitz 2007 {published data only}
- Schmitz JM, Stotts AL, Mooney ME, Delaune KA, Moeller GF. Bupropion and cognitive‐behavioral therapy for smoking cessation in women. Nicotine & Tobacco Research 2007;9(6):699‐709. [DOI] [PubMed] [Google Scholar]
Schnoll 2005 {published data only}
- Schnoll RA, Rothman RL, Wielt DB, Lerman C, Pedri H, Wang H, et al. A randomized pilot study of cognitive‐behavioral therapy versus basic health education for smoking cessation among cancer patients. Annals of Behavioral Medicine 2005;30(1):1‐11. [DOI] [PubMed] [Google Scholar]
Shiffman 2000 {published data only}
- Hollis JF, McAfee TA, Fellows JL, Zbikowski SM, Stark M, Riedlinger K. The effectiveness and cost effectiveness of telephone counselling and the nicotine patch in a state tobacco quitline. Tobacco Control 2007;16(Suppl 1):i53‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shiffman 2001 {published data only}
- Shiffman S, Paty JA, Rohay JM, Marino ME, Gitchell JG. The efficacy of computer‐tailored smoking cessation material as a supplement to nicotine patch therapy. Drug & Alcohol Dependence 2001;64(1):35‐46. [DOI] [PubMed] [Google Scholar]
Simon 2003 {published data only}
- Simon JA, Carmody TP, Hudes ES, Snyder E, Murray J. Intensive smoking cessation counseling versus minimal counseling among hospitalized smokers treated with transdermal nicotine replacement: a randomized trial. American Journal of Medicine 2003;114(7):555‐62. [DOI] [PubMed] [Google Scholar]
Smith 2001 {published data only}
- Smith SS, Jorenby DE, Fiore MC, Anderson JE, Mielke MM, Beach KE, et al. Strike while the iron is hot: can stepped‐care treatments resurrect relapsing smokers?. Journal of Consulting and Clinical Psychology 2001;69:429‐39. [DOI] [PubMed] [Google Scholar]
Solomon 2000 {published data only}
- Solomon LJ, Scharoun GM, Flynn BS, Secker‐Walker RH, Sepinwall D. Free nicotine patches plus proactive telephone peer support to help low‐income women stop smoking. Preventive Medicine 2000;31(1):68‐74. [DOI] [PubMed] [Google Scholar]
Solomon 2005 {published data only}
- Solomon LJ, Marcy TW, Howe KD, Skelly JM, Reinier K, Flynn BS. Does extended proactive telephone support increase smoking cessation among low‐income women using nicotine patches?. Preventive Medicine 2005;40(3):306‐13. [DOI] [PubMed] [Google Scholar]
Sorensen 2003 {published data only}
- Sorensen LT, Jorgensen T. Short‐term preoperative smoking cessation intervention does not affect postoperative complications in colorectal surgery: A randomised clinical trial. Colorectal Disease 2003;5:347‐52. [DOI] [PubMed] [Google Scholar]
Stein 2006 {published data only}
- Stein MD, Anderson BJ, Niaura R. Smoking cessation patterns in methadone‐maintained smokers. Nicotine & Tobacco Research 2007;9(3):421‐8. [DOI] [PubMed] [Google Scholar]
- Stein MD, Weinstock MC, Herman DS, Anderson BJ. Respiratory symptom relief related to reduction in cigarette use. Journal of General Internal Medicine 2005;20(10):889‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MD, Weinstock MC, Herman DS, Anderson BJ, Anthony JL, Niaura R. A smoking cessation intervention for the methadone‐maintained. Addiction 2006;101(4):599‐607. [DOI] [PubMed] [Google Scholar]
Strecher 2005 {published data only}
- Strecher VJ, Shiffman S, West R. Moderators and mediators of a web‐based computer‐tailored smoking cessation program among nicotine patch users. Nicotine & Tobacco Research 2006;8(Suppl 1):S95‐101. [DOI] [PubMed] [Google Scholar]
- Strecher VJ, Shiffman S, West R. Randomized controlled trial of a web‐based computer‐tailored smoking cessation program as a supplement to nicotine patch therapy. Addiction 2005;100(5):682‐8. [DOI] [PubMed] [Google Scholar]
Swan 2003 {published data only}
- Jack LM, Swan GE, Thompson E, Curry SJ, McAfee T, Dacey S, et al. Bupropion SR and smoking cessation in actual practice: methods for recruitment, screening, and exclusion for a field trial in a managed‐care setting. Preventive Medicine 2003;36:585‐93. [DOI] [PubMed] [Google Scholar]
- Javitz HS, Swan GE, Zbikowski SM, Curry SJ, McAfee TA, Decker DL, et al. Cost‐effectiveness of different combinations of bupropion SR dose and behavioral treatment for smoking cessation: a societal perspective. American Journal of Managed Care 2004;10:217‐26. [PubMed] [Google Scholar]
- Swan GE, Jack LM, Curry S, Chorost M, Javitz H, McAfee T, et al. Bupropion SR and counseling for smoking cessation in actual practice: Predictors of outcome. Nicotine & Tobacco Research 2003;5:911‐21. [DOI] [PubMed] [Google Scholar]
- Swan GE, Jack LM, Javitz HS, McAfee T, McClure JB. Predictors of 12‐month outcome in smokers who received bupropion sustained‐release for smoking cessation. Central Nervous System Drugs 2008;22(3):239‐56. [DOI] [PubMed] [Google Scholar]
- Swan GE, Javitz HS, Jack LM, Curry SJ, McAfee T. Heterogeneity in 12‐month outcome among female and male smokers. Addiction 2004;99:237‐50. [DOI] [PubMed] [Google Scholar]
- Swan GE, McAfee T, Curry SJ, Jack LM, Javitz H, Dacey S, et al. Effectiveness of bupropion sustained release for smoking cessation in a health care setting: a randomized trial. Archives of Internal Medicine 2003;163:2337‐44. [DOI] [PubMed] [Google Scholar]
- Swan GE, Valdes AM, Ring HZ, Khroyan TV, Jack LM, Ton CC, et al. Dopamine receptor DRD2 genotype and smoking cessation outcome following treatment with bupropion SR. Pharmacogenomics Journal 2005;5:21‐9. [DOI] [PubMed] [Google Scholar]
Swan 2010 {published data only}
- Catz SL, Jack LM, McClure JB, Javitz HS, Deprey M, Zbikowski SM, et al. Adherence to varenicline in the COMPASS smoking cessation intervention trial. Nicotine & Tobacco Research 2011;13(5):361‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halperin AC, McAfee TA, Jack LM, Catz SL, McClure JB, Deprey TM, et al. Impact of symptoms experienced by varenicline users on tobacco treatment in a real world setting. Journal of Substance Abuse Treatment 2009;36(4):428‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure JB, Jack L, Deprey M, Catz S, McAfee T, Zbikowski S, et al. Canary in a coal mine? Interest in bupropion SR use among smokers in the COMPASS trial. Nicotine & Tobacco Research 2008;10(12):1815‐16. [DOI] [PubMed] [Google Scholar]
- McClure JB, Swan GE, Jack L, Catz SL, Zbikowski SM, McAfee TA, et al. Mood, side‐effects and smoking outcomes among persons with and without probable lifetime depression taking varenicline. Journal of General Internal Medicine 2009;24(5):563‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan GE, McClure JB, Jack LM, Zbikowski SM, Javitz HS, Catz SL, et al. Behavioral counseling and varenicline treatment for smoking cessation. American Journal of Preventive Medicine 2010;38(5):482‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zbikowski SM, Jack LM, McClure JB, Deprey M, Javitz HS, McAfee TA, et al. Utilization of services in a randomized trial testing phone‐ and web‐based interventions for smoking cessation. Nicotine & Tobacco Research 2011;13(5):319‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ward 2001 {published data only}
- Ward T. Using psychological insights to help people quit smoking. Journal of Advanced Nursing 2001;34(6):754‐9. [DOI] [PubMed] [Google Scholar]
Wiggers 2006 {published data only}
- Wiggers LC, Oort FJ, Dijkstra A, Haes JC, Legemate DA, Smets EM. Cognitive changes in cardiovascular patients following a tailored behavioral smoking cessation intervention. Preventive Medicine 2005;40:812‐21. [DOI] [PubMed] [Google Scholar]
- Wiggers LC, Smets EM, Oort FJ, Peters RJ, Storm‐Versloot MN, Vermeulen H, et al. The effect of a minimal intervention strategy in addition to nicotine replacement therapy to support smoking cessation in cardiovascular outpatients: a randomized clinical trial. European Journal of Cardiovascular Prevention & Rehabilitation 2006;13:931‐7. [DOI] [PubMed] [Google Scholar]
- Wiggers LC, Smets EM, Oort FJ, Storm‐Versloot MN, Vermeulen H, Loenen LB, et al. Adherence to nicotine replacement patch therapy in cardiovascular patients. International Journal of Behavioral Medicine 2006;13:79‐88. [DOI] [PubMed] [Google Scholar]
- Wiggers LCW, Oort FJ, Peters RJG, Legemate DA, Haes HCJM, Smets EMA. Smoking cessation may not improve quality of life in atherosclerotic patients. Nicotine & Tobacco Research 2006;8(4):581‐9. [DOI] [PubMed] [Google Scholar]
Williams 2010 {published data only}
- Williams JM, Steinberg ML, Zimmermann MH, Gandhi KK, Stipelman B, Budsock PD, et al. Comparison of two intensities of tobacco dependence counseling in schizophrenia and schizoaffective disorder. Journal of Substance Abuse Treatment 2010;38(4):384‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wolfenden 2005 {published data only}
- Wolfenden L, Wiggers J, Knight J, Campbell E, Rissel C, Kerridge R, et al. A programme for reducing smoking in pre‐operative surgical patients: randomised controlled trial. Anaesthesia 2005;60:172‐9. [DOI] [PubMed] [Google Scholar]
Wolfenden 2008 {published data only}
- Wolfenden L, Wiggers J, Campbell E, Knight J. Pilot of a preoperative smoking cessation intervention incorporating post‐discharge support from a Quitline. Health Promotion Journal of Australia 2008;19(2):158‐60. [DOI] [PubMed] [Google Scholar]
Wu 2009 {published data only}
- Wu D, Ma GX, Zhou K, Zhou D, Liu A, Poon AN. The effect of a culturally tailored smoking cessation for Chinese American smokers. Nicotine & Tobacco Research 2009;11:1448‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yalcin 2014 {published data only}
- Yalcin BM, Unal M, Pirdal H, Karahan TF. Effects of an anger management and stress control program on smoking cessation: a randomized controlled trial. Journal of the American Board of Family Medicine 2014;27(5):645‐60. [CENTRAL: 1014527; CRS: 9400129000003402] [DOI] [PubMed] [Google Scholar]
Yu 2006 {published data only}
- Yu H, Zang Y, Lin J. The effect of the abstinence from smoking with nicotine replacement therapy combining with psychological and behavior intervention. Zhongguo Kangfu Yixue Zazhi 2006;21(12):1104‐6. [Google Scholar]
References to ongoing studies
Aung 2013 {published data only}
- Aung MN, Yuasa M, Lorga T, Moolphate S, Fukuda H, Kitajima T, et al. Evidence‐based new service package vs. routine service package for smoking cessation to prevent high risk patients from cardiovascular diseases (CVD): study protocol for randomized controlled trial. Trials 2013;14(1):419. [CENTRAL: 910729; CRS: 9400129000000199] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cummins 2012 {published data only}
- Cummins S, Zhu SH, Gamst A, Kirby C, Brandstein K, Klonhoff‐Cohen H, et al. Nicotine patches and quitline counseling to help hospitalized smokers stay quit: Study protocol for a randomized controlled trial. Trials 2012;13(1):128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Duffy 2012 {published data only}
- Duffy SA, Ronis DL, Titler MG, Blow FC, Jordan N, Thomas PL, et al. Dissemination of the nurse‐administered Tobacco Tactics intervention versus usual care in six Trinity community hospitals: study protocol for a comparative effectiveness trial. Trials 2012;13:125. [CENTRAL: 863868; CRS: 9400123000018182; EMBASE: 2013011304; PUBMED: 22852834] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fu 2014 {published data only}
- Fu SS, Ryn M, Burgess DJ, Nelson D, Clothier B, Thomas JL, et al. Proactive tobacco treatment for low income smokers: study protocol of a randomized controlled trial. BMC Public Health 2014;14:337. [CRS: 9400129000003458] [DOI] [PMC free article] [PubMed] [Google Scholar]
Metse 2014 {published data only}
- Metse AP, Bowman JA, Wye P, Stockings E, Adams M, Clancy R, et al. Evaluating the efficacy of an integrated smoking cessation intervention for mental health patients: study protocol for a randomised controlled trial. Trials 2014;15:266. [CENTRAL: 994729; CRS: 9400129000003086; EMBASE: 2014463699; PUBMED: 24996596] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT01320462 {published data only}
- NCT01320462. Smoking cessation program in the preadmission clinic. clinicaltrials.gov/show/NCT01320462 (accessed 24 July 2015). [CRS: 9400123000011466]
Prochaska 2014b {published data only}
- Prochaska JJ, Fromont SC, Delucchi K, Young‐Wolff KC, Benowitz NL, Hall S, et al. Multiple risk‐behavior profiles of smokers with serious mental illness and motivation for change. Health Psychology 2014;33(12):1518‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thomas 2013 {published data only}
- Thomas D, Abramson MJ, Bonevski B, Taylor S, Poole S, Weeks GR, et al. A pharmacist‐led system‐change smoking cessation intervention for smokers admitted to Australian public hospitals (GIVE UP FOR GOOD): study protocol for a randomised controlled trial. Trials 2013;14:148. [CENTRAL: 863879; CRS: 9400107000000317; EMBASE: 2013336506; PUBMED: 23693155] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D, Abramson MJ, Bonevski B, Taylor S, Poole SG, Weeks GR, et al. Quitting experiences and preferences of smokers admitted to Australian public hospitals participating in a randomised controlled trial. Asia‐Pacific Journal of Clinical Oncology 2014;10:202. [CENTRAL: 1049898; CRS: 9400050000000157; EMBASE: 71741036] [Google Scholar]
Urdapilleta‐Herrera 2013 {published data only}
- Urdapilleta‐Herrera E, Piña‐Rosales MF, Vargas‐Rojas MI, Ramírez‐Venegas A, Sansores‐Martínez R. Bupropion together with cognitive‐conductual therapy (CBT) is an alternative for a long‐term abstinence of smoking [Abstract]. European Respiratory Society Annual Congress, 2013 Sept 7‐11, Barcelona, Spain 2013;42(Suppl 57):682s [3347]. [CENTRAL: 989603; CRS: 9400050000000070] [Google Scholar]
Weaver 2015 {published data only}
- Weaver K, Kaplan S, Urbanic J, Case D, Zbikowski S, Dakhil CSR, et al. Incorporating evidence‐based smoking cessation into community oncology practices: Feasibility and preliminary efficacy of an enhanced quitline‐based smoking cessation intervention for cancer survivors. Psycho‐oncology 2015;24(Suppl 2):32. [Google Scholar]
- Weaver KE, Kaplan S, Griffin L, Urbanic J, Zbikowski S, Danhauer SC. Satisfaction with a Quitline‐based Smoking Cessation Intervention among Cancer Survivors. Cancer Epidemiology, Biomarkers & Prevention 2015;24(4):759. [CRS: 9400131000001709; PUBMED: 25834151] [Google Scholar]
Wong 2008 {published data only}
- Wong CC, Tsoh JY, Tong EK, Hom FB, Cooper B, Chow EA. The Chinese community smoking cessation project: A community sensitive intervention trial. Journal of Community Health 2008;33(6):363‐73. [DOI] [PubMed] [Google Scholar]
Additional references
Cahill 2012
- Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database of Systematic Reviews 2012, Issue 4. [DOI: 10.1002/14651858.CD006103.pub6] [DOI] [PubMed] [Google Scholar]
Cahill 2013
- Cahill K, Stevens S, Perera R, Lancaster T. Pharmacological interventions for smoking cessation: an overview and network meta‐analysis. Cochrane Database of Systematic Reviews 2013, Issue 5. [DOI: 10.1002/14651858.CD009329.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fiore 2008
- Fiore MC, Jaén CR, Baker TB, et al. Treating Tobacco Use and Dependence: 2008 Update. Clinical Practice Guideline. AHRQ publication No. 00‐0032. Rockville, MD: US Dept of Health and Human Services. Public Health Services, May 2008. [Google Scholar]
Hartmann‐Boyce 2014
- Hartmann‐Boyce J, Lancaster T, Stead LF. Print‐based self‐help interventions for smoking cessation. Cochrane Database of Systematic Reviews 2014, Issue 6. [DOI: 10.1002/14651858.CD001118.pub3] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (eds). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Hughes 1995
- Hughes JR. Combining behavioral therapy and pharmacotherapy for smoking cessation: an update. NIDA Research Monograph 1995;150:92‐109. [PubMed] [Google Scholar]
Hughes 2014
- Hughes JR, Stead LF, Hartmann‐Boyce J, Cahill K, Lancaster T. Antidepressants for smoking cessation. Cochrane Database of Systematic Reviews 2014, Issue 1. [DOI: 10.1002/14651858.CD000031.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kotz 2014
- Kotz D, Brown J, West R. Prospective cohort study of the effectiveness of smoking cessation treatments used in the "real world". Mayo Clinic Proceedings 2014;89(10):1360‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lancaster 2005
- Lancaster T, Stead LF. Individual behavioural counselling for smoking cessation. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD001292.pub2] [DOI] [PubMed] [Google Scholar]
Reus 2008
- Reus VI, Smith BJ. Multimodal techniques for smoking cessation: a review of their efficacy and utilisation and clinical practice guidelines. International Journal of Clinical Practice 2008;62(11):1753‐68. [DOI] [PubMed] [Google Scholar]
Rigotti 2012
- Rigotti NA, Clair C, Munafò MR, Stead LF. Interventions for smoking cessation in hospitalised patients. Cochrane Database of Systematic Reviews 2012, Issue 5. [DOI: 10.1002/14651858.CD001837.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schulz 2002a
- Schulz KF, Grimes DA. Generation of allocation sequences in randomised trials: chance, not choice. The Lancet 2002;359(9305):515‐9. [DOI] [PubMed] [Google Scholar]
Schulz 2002b
- Schulz KF, Grimes DA. Allocation concealment in randomised trials: defending against deciphering. The Lancet 2002;359(9306):614‐8. [DOI] [PubMed] [Google Scholar]
Shiffman 2008
- Shiffman S, Brockwell SE, Pillitteri JL, Gitchell JG. Use of smoking‐cessation treatments in the United States. American Journal of Preventive Medicine 2008;34(2):102‐11. [DOI] [PubMed] [Google Scholar]
Stead 2005
- Stead LF, Lancaster T. Group behaviour therapy programmes for smoking cessation. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD001007.pub2] [DOI] [PubMed] [Google Scholar]
Stead 2012
- Stead LF, Perera R, Bullen C, Mant D, Hartmann‐Boyce J, Cahill K, Lancaster T. Nicotine replacement therapy for smoking cessation. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD000146.pub4] [DOI] [PubMed] [Google Scholar]
Stead 2013
- Stead LF, Hartmann‐Boyce J, Perera R, Lancaster T. Telephone counselling for smoking cessation. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD002850.pub3] [DOI] [PubMed] [Google Scholar]
Stead 2015
- Stead LF, Koilpillai P, Lancaster T. Additional behavioural support as an adjunct to pharmacotherapy for smoking cessation. Cochrane Database of Systematic Reviews 2015, Issue 10. [DOI: 10.1002/14651858.CD009670.pub3] [DOI] [PubMed] [Google Scholar]


