Keywords: fibrosis, inflammation, macrophages, strength
Abstract
Physical inactivity has many detrimental effects on health, yet the impact of physical inactivity in early life on muscle health in adulthood remains unknown. Early postnatal malnutrition has prolonged effects into adulthood and we propose that early postnatal (P) physical inactivity would have similar negative effects. To test this hypothesis, we exposed postnatal mice (∼P28, C57BL/6J) to 14 days of physical inactivity (shortly after weaning, from ∼P28 to P42 days of age) in the form of muscle disuse with hindlimb unloading (HU). After this early-life physical inactivity, they were allowed to normally ambulate until 5 mo of age (P140, adulthood) when they underwent 14 days of HU with and without 7-day recovery. They were then tested for physical function (grip strength) and muscles were extracted and weighed. Immunofluorescence was carried out on these muscle cross sections for analysis of myofiber cross-sectional area (fCSA), macrophage density (CD68+ cells), and extracellular matrix (ECM) area. Muscle weights and fCSA and myofiber diameter were used to quantify changes in muscle and fiber size. Compared with age-matched controls, no notable effects of early-life physical inactivity (HU) on skeletal muscle and myofiber size were observed. However, a significant reduction in adult grip strength was observed in those exposed to HU early in life. This was associated with reduced muscle macrophages and increased ECM area. Exposure to a short period of early life disuse has negative enduring effects into adulthood impacting grip strength, muscle macrophages, and muscle composition as low muscle quality.
NEW & NOTEWORTHY We demonstrate that early life disuse resulted in less grip strength in adulthood. Analysis of muscle composition demonstrated no loss of whole muscle or myofiber size indicating lower muscle quality akin to premature aging. This poor muscle quality was characterized by altered muscle macrophages and extracellular matrix area. We demonstrate intriguing correlations between this loss of grip strength and muscle macrophages and also area of noncontractile tissue in the muscle.
INTRODUCTION
Physical activity comprehensively improves overall health in a manner that cannot be duplicated pharmaceutically (1), whereas physical inactivity weakens the function of multiple organ systems and accelerates chronic disease risk, functional decline, and premature mortality (1). This negative impact of physical inactivity has primarily been evaluated in middle-aged and older adults who we know are more susceptible to the effects of physical inactivity than young adults (1). Less is known regarding the negative impact of physical inactivity on youth.
Adolescents (2), children (3–5), and infants (5, 6) in the United States have a high prevalence of physical inactivity. The long-term effects of hospitalization conditions for early childhood are of concern and they are often accompanied by months to years of physical inactivity (7, 8). Thus, little is known regarding the impact of physical inactivity on children, specifically during key stages of skeletal muscle development (9), and its long-term consequences. Maintenance of muscle mass and strength reflects functional capacity with direct implications on longevity and quality of life (8). Dodds et al. (10) suggested that muscle mass and function may be influenced by a variety of factors such as genetics, lifestyle factors (e.g., diet and physical activity), chronic illness, endocrine, and early life exposures.
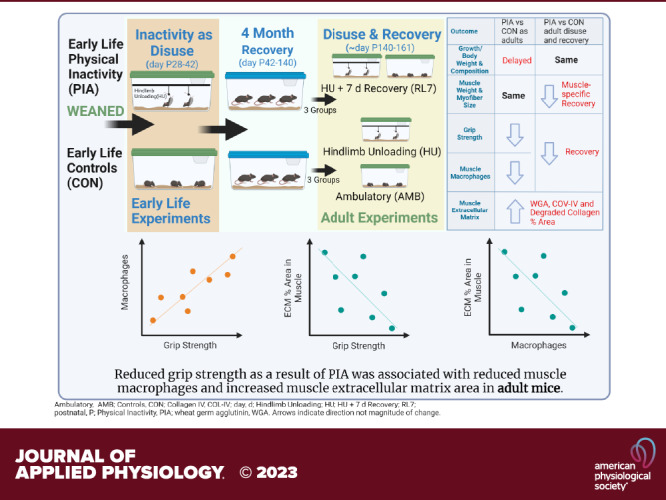
We propose that the effects of physical inactivity are substantial during the key periods of skeletal muscle development and may have detrimental, long-lasting impacts on health later in life. Several classic studies clearly indicate physical inactivity rapidly precipitates severe skeletal muscle loss and impaired regrowth in postnatal muscle (11–18). Additional detrimental effects have been reported on bone (19, 20) and motor control (19–21). A common assumption is that the “resilience of youth” may overcome the negative effects of physical inactivity. Yet, compelling evidence indicates that pre- and postnatal malnutrition during key periods of muscle development causes long-lasting and permanent effects (22–25) on muscle and overall health. We propose that early postnatal physical inactivity has similar effects into adulthood on muscle mirroring those of malnutrition and that this pilot study of physical inactivity in early life in the form of muscle disuse will attenuate growth and cause muscle atrophy and weakness in adulthood. Secondarily, we sought to determine if early life physical inactivity altered the response to disuse and a recovery period in adulthood.
To test this hypothesis, we exposed postnatal mice (∼P28, C57BL/6J) to 14 days of physical inactivity (shortly after weaning, from ∼28 to 42 days of age) in the form of muscle disuse with hindlimb unloading (HU). After this early life of physical inactivity, they were allowed to normally ambulate until 5 mo of age (P140, adulthood).
METHODS
Animals and Experiments
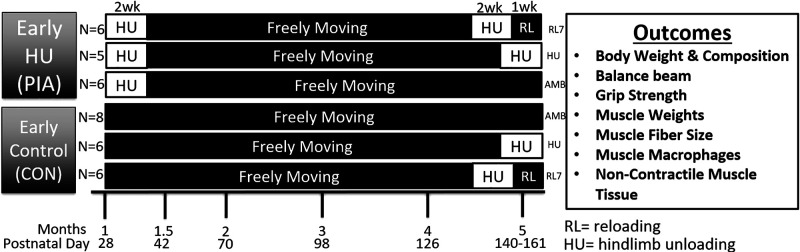
Male C57BL/6 mice bred in-house (Jackson Laboratories) were used in this study. Mice were initially divided into two groups: early life HU [physical inactivity (PIA); n = 17] and early life control (CON; n = 20; Fig. 1). In the PIA group, we exposed postnatal (P) mice, shortly after weaning (∼P28), to 14 days of physical inactivity (from ∼28 to 42 days of age) in the form of muscle disuse with HU (2/cage) conducted as we have previously done (26) with modifications to allow for the rapid growth of the mouse. As these postnatal mice are rapidly growing more frequent visits (twice per day) were needed to adjust the mouse to maintain disuse. As the limbs were growing the unloading high had to be manually adjusted to ensure the hindlimbs did not contact the floor of the cage. PIA (unloading) was conducted as soon as early in independent life as possible following animal transfer to our facility. This period of independent life demonstrates the fastest growth rate and as such may be an important time for programming optimal growth. Meanwhile, the CON animals were able to freely ambulate in their cage (2–3 animals/cage). Both groups had ad libitum access to food (standard chow) and water during the experimental periods. Following day P42 all animals were placed into standard cages and allowed to freely ambulate in their cage (2–3 animals/cage) until 140 days old (early adulthood). During this (recovery) time the food consumption and weight were assessed weekly and body composition was assessed monthly for all of the animals. At early adulthood between P126 and P140, the second phase of experiments was started. In the second phase, both the PIA and CON groups were split into three subgroups each: adult ambulatory controls [PIA-AMB (n = 6) and CON-AMB (n = 8)], 14 days of hindlimb unloading [PIA-HU (n = 5) and CON-HU (n = 6)], and HU followed by 7 days of reloading [PIA-RL7 (n = 6) and CON-RL7 (n = 6)] as performed previously (26). Animals were housed with ad libitum access to food and water and maintained on a 12:12-h light-dark cycle. All experimental procedures were approved by The University of Utah Institutional Animal Care and Use Committee. Whole body tissue composition (lean, water, and fat mass) was assayed with a Minispec MQ20 NMR analyzer (Bruker, Rheinstetten, Germany) after the conclusion of early life PIA and then every month until the day before tissue collection to assess longitudinal changes in lean and fat measurement over time. Grip strength was conducted in the second phase of experiments longitudinally on the groups undergoing HU and reloading. The balance beam test was only conducted on the Adult Control and Adult PIA-RL7 groups. On day 14 of HU, after 7 days of reloading or for ambulatory control, mice were fasted for 5 h and then euthanized under isoflurane followed by cervical dislocation. Triceps, tibialis anterior (TA)/extensor digitorum longus (EDL) combined, plantaris, soleus, and gastrocnemius muscles were rapidly dissected, weighed, frozen in liquid nitrogen or OCT (Fisher) in isopentane, and stored at −80°C for later analysis. Heart weights were also taken.
Figure 1.
Experimental design and outcomes for the assessment of physical inactivity as hindlimb unloading in early postnatal mice and effects in early adulthood. CON, control; PIA, physical inactivity.
Immunofluorescence and histology.
Frozen OCT-embedded hindlimb muscle samples (soleus, plantaris, gastrocnemius, and tibialis anterior/extensor digitorum longus) were sectioned on a cryostat (HM525 NX Cryostat; Thermo Fisher, Waltham, MA). Immunofluorescent stained slides were observed with a Leica DMi8 fully automated wide-field fluorescence light microscope (Leica; Wetzlar, Germany) with the ×10 objective lens. Images were acquired using a high-sensitivity Hamamatsu ORCA-ER cooled CCD camera (Hamamatsu Photonics; Hamamatsu, Japan). Example images of the gastrocnemius representing the average value of each group for the triceps surae for the below assays are shown in Supplemental Figs. S1–S3.
Myofiber type was assessed with overnight incubation of sections with BA.D5 (1:75; Developmental Studies Hybridoma Bank, Univ. of Iowa) in 1:2 rations of PBS:supernatant of SC.71 and BF.F3 (Developmental Studies Hybridoma Bank, Univ. of Iowa) to visualize myosin heavy chain I (MHC I), myosin heavy chain IIa (MHC IIa), and myosin heavy chain IIa (MHC IIb) when incubated in Alexa Fluor 647 (Cat. No. A21242, 1:250; Invitrogen, Carlsbad, CA), Alexa Fluor 555 (Cat. No. A21426, 1:500; Invitrogen, Carlsbad, CA) and Alexa Fluor 488 (Cat. No. A21121, 1:500; Invitrogen), respectively. In addition, the primary laminin antibody (1:200, Sigma, Cat. No. L9393) was included with the DSHB antibodies overnight at 4°C, followed by secondary antibody AMCA (Vector, Cat. No. Cl-1000) designated myofiber borders for assessment of myofiber-type-specific cross-sectional area (CSA). Negative stained fibers were considered to be myosin heavy chain IIx (MHC IIx). Myofiber CSA was measured using semiautomatic muscle analysis with the segmentation of histology, a MATLAB application (SMASH) alongside ImageJ software as conducted previously (26). Manual macrophage identification sections of the triceps surae were prepared and analyzed as previously conducted (26). Briefly, manual macrophage identification sections of the triceps surae were blocked in M.O.M. (Vector, Burlingame, CA) and 2.5% NHS (Vector), counterstained with anti-mouse dystrophin antibody (1:100; Santa Cruz Biotechnology, Dallas, TX) and then with anti-rat CD68 antibody (1:100; Bio-Rad, Hercules, CA). Anti-rat secondary antibody (1:250, AF555, Invitrogen) and anti-mouse secondary antibody (1:500, A488, Invitrogen) were applied and then mounted in a DAPI-containing mounting medium (Vector), imaged, and then were analyzed using ImageJ.
Assessment of collagen IV and biotinylated-collagen hybridizing peptide (B-CHP; degraded collagen) cross sections of the triceps surae were as previously conducted (27). Briefly, to assess collagen IV and biotinylated-collagen hybridizing peptide (B-CHP; degraded collagen) cross sections of the triceps surae were fixed in −20°C acetone, blocked with streptavidin and biotin (Vector, Cat. No. SP-2001), and incubated in 15 μM B-CHP (3-Helix) following manufacturer instructions. Primary collagen IV antibody (Abcam, Cat. No. ab6586) was added with B-CHP and incubated for 1 h at room temperature, followed by overnight incubation at 4°C. DyLight 594 (1:200, Thermo Fisher, Cat. No. SA-5549) and secondary antibody Alexa Fluor 647 (Invitrogen, Cat. No. 21245) were utilized for B-CHP and collagen IV, respectively. B-CHP and collagen IV were analyzed using ImageJ.
Grip strength.
Whole body grip strength was assessed utilizing a grip strength meter with a mesh wire attachment (Columbus Instruments, Columbus, OH) as previously conducted (27, 28). The protocol is explained in detail by Castro and Kuang (29), but in brief, mice would be encouraged to ambulate across to the front of the mesh wire briefly before being pulled by the base of their tail parallel to the mesh wire until they released their grip. Three trials were conducted, and peak force was recorded. The average of the two highest peak force measurements was assessed as whole body grip strength. The grip strength measurements were conducted by one investigator throughout the study. The tests for all cohorts were conducted on the same day and the investigator was blinded as to the early life group.
Physical function via the balance beam.
The balance beam test of physical function (30) was conducted on adult ambulatory control mice and adult 7-day reload mice only since the condition of HU prohibited familiarization sessions and loading of the mice limbs. After 1 day of familiarization, the tests were performed 2 days in a row with trial one on the first day and trial two on the second day. The time for each mouse to cross a 1-m long wide (12 mm) and then narrow (6 mm) beam was measured (i.e., beam latency).
Statistical Analysis
All data are shown as means ± SE and were checked for major violations of normality. Tissue weight was also examined as tissue weight relative to body weight and then lean mass. To examine changes in body weight, tissue composition, muscle weight, food intake, and myofiber outcomes at one-time point, we used a two-way ANOVA with the factors of Early Experiment (CON vs. PIA) and Adult Experiment (CON, HU, and RL). Specific changes within each factor were compared with Šídák’s multiple comparisons test. To examine changes in body weight and tissue composition across the experimental periods over time, we used mixed-effects linear regression, with one between-groups factor (early PIA) and one repeated-measures factor (time). Significance was set as P ≤ 0.05, and trends were noted as 0.05 < P < 0.10. Analyses were conducted and graphs were created in GraphPad Prism 9.3.1.
RESULTS
Body Weight, Tissue Composition, and Food Intake
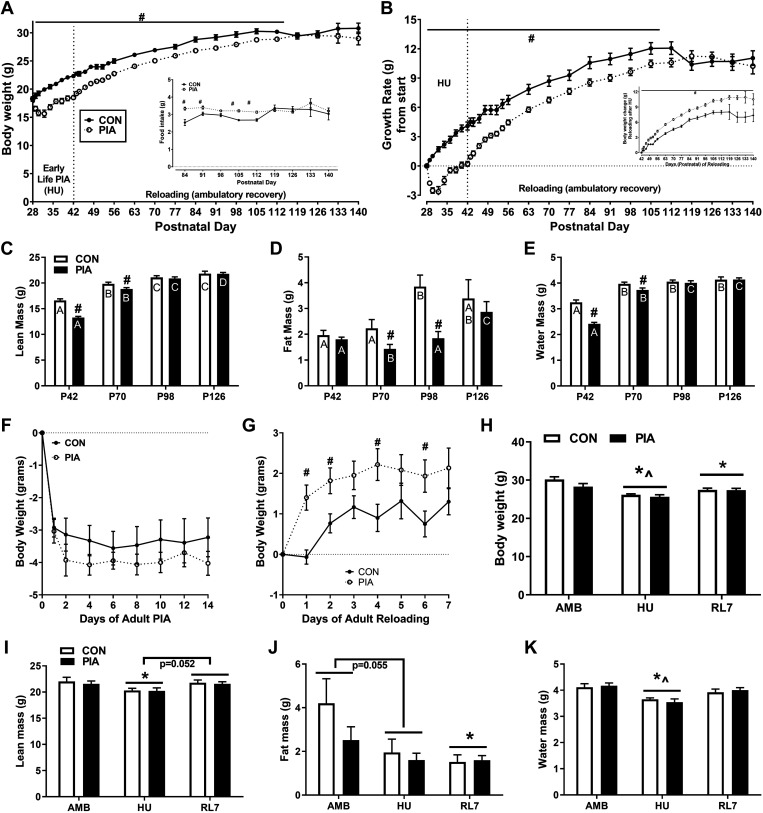
Body weight was similar in CON and PIA at time 0, but different between CON and PIA on days 1–84 of the experiment (P < 0.05; Fig. 2A). Growth rate (change from day 0) was significantly greater in CON versus PIA from day 1 to day 77 (P < 0.05; Fig. 2B). Change in body weight during the reloading period after HU was significantly greater in PIA versus CON from day 16 to the end of the experiment (P < 0.05; Fig. 2B, inset).
Figure 2.
Body weight (A), growth rate (B), lean mass (C), fat mass (D), water mass (E) of male C57BL/6 mice in early life ambulatory controls (CON) and those exposed to early life PIA (PIA) as hindlimb unloading (HU) in the early postnatal period (days P28–42) and during 4-mo recovery (days P42–140) and effects in early adulthood when exposed to HU and HU + 7 days of reloading (RL7). Body weight changes during PIA (F) growth rate during 7 days of reloading following HU (G) in early adulthood mice in ambulatory controls (CON) and those exposed to early life PIA (PIA). Early life controls (CON) and PIA (PIA) mice body weight (H) and tissue composition (I–K) at sack in early adulthood (5 mo) in ambulatory controls (AMB), exposed to HU or HU + 7 days of reloading. PIA, physical inactivity.
Daily food intake (data not shown) during the P28- to P42-day period of early life CON or PIA was greater during HU and periods of the recovery period (Fig. 2A, inset) than control for both absolute (grams daily) and relative (grams/body weight per day) intakes (P < 0.05)
Tissue composition during the ambulatory recovery period is shown in Fig. 2, C–E. Following PIA in the form of HU, lean mass and water mass were depressed in PIA versus CON at P42 and P70 (P < 0.05). The CON group plateaued their lean mass at about P98 whereas the PIA group demonstrated a delay in lean mass development (P < 0.05). Fat mass development was attenuated in the PIA group compared with CON at P70 and P98 with a pattern of a late rebound at P126 (P < 0.05).
For the adult experiments, there was no difference in the loss of body weight during HU (Fig. 2F). However, the PIA group experienced a more rapid increase in body weight during reloading following adult experiments (P < 0.05; Fig. 2G). Body weight decreased from AMB to HU (and AMB to RL7 in both groups (P < 0.05), but increased from HU to RL7 (P < 0.05; Fig. 2H). Lean mass at sack was decreased from AMB to HU (P < 0.05) and strongly tended to increase from HU to RL7 (P = 0.052) in both groups (Fig. 2I). Fat mass strongly tended to decrease from AMB to HU (P = 0.055) and was decreased from AMB to RL7 (P < 0.05; Fig. 2J). Water mass was decreased from AMB to HU (P < 0.05) and increased from HU to RL7 (P < 0.05) in both groups (Fig. 2K).
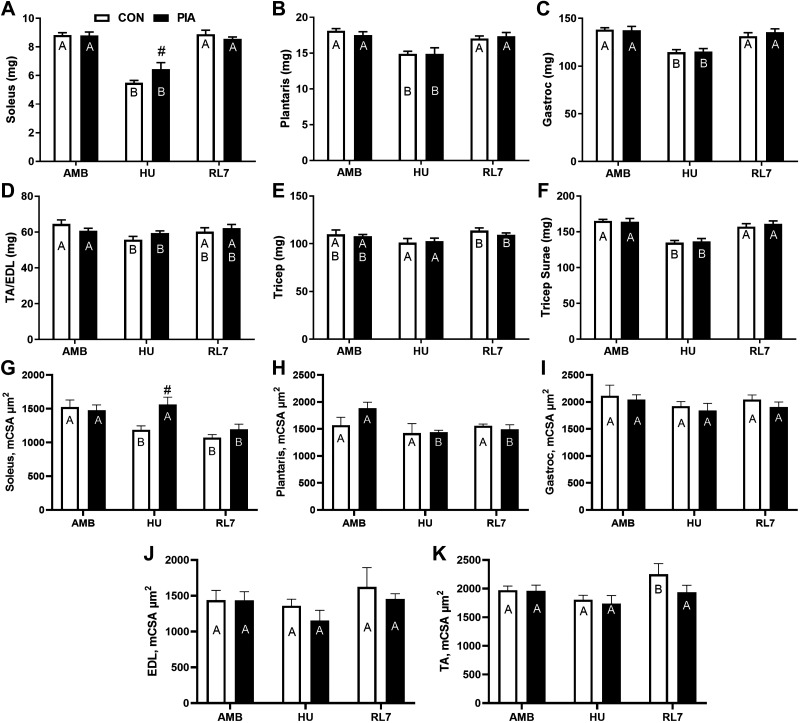
Muscle and Heart Weights
Absolute soleus, plantaris, gastrocnemius, and triceps surae (soleus, plantaris, and gastrocnemius combined) mass decreased from AMB to HU (P < 0.05) and then recovered from HU to RL7 (P < 0.05). For muscle and heart tissue only, the soleus demonstrated a group difference between CON and PIA and this was at the HU time point (P < 0.05). The TA/EDL demonstrated a decrease from AMB to HU (P < 0.05). Triceps weight increased from HU to RL7 (P < 0.05) for absolute and relative to BW data. There was no change in absolute heart size for absolute or relative (to body weight or lean mass) mass.
Relative to body weight (data not shown) the soleus also increased from AMB to RL7 (P < 0.05) and a group difference between CON and PIA and this was at the HU time point (P < 0.05). Relative to body weight (data not shown), there were no differences in the plantaris. Relative to body weight (data not shown), there was a decrease from AMB to HU (P < 0.05) and then evidence for recovery from HU to RL7 (P < 0.05) in the gastrocnemius and triceps surae with no changes in the TA/EDL.
Myofiber MHC Cross-Sectional Area and Composition
Mean myofiber cross-sectional area (mCSA) shown in Fig. 3, G–K displayed no differences at AMB or RL7 between PIA and CON in any of the investigated muscles. Other than a higher mCSA in the soleus in PIA (P < 0.05), there were no differences between PIA and CON in any of the other muscles at HU. Gastroc and EDL mCSA were unchanged across the adult experiments. And there were minor differences in the soleus, plantaris, and TA across the adult experiments. Soleus mCSA was reduced (P < 0.05) from AMB at HU and RL7 for CON and at RL7 for PIA. Plantaris mCSA was reduced (P < 0.05) from AMB at HU and RL7 for PIA. TA mCSA was increased (P < 0.05) from AMB at RL7 for CON.
Figure 3.
Muscle wet weight (A–F) and mean myofiber cross-sectional area (G–K) of male C57BL/6 mice exposed to physical inactivity (PIA) as hindlimb unloading (HU) or as early life ambulatory controls (CON) in the early postnatal period (days P28–42) examined in early adulthood (∼5 mo/P150) as ambulatory controls (AMB) or mice exposed to HU or HU + 7 days of reloading (RL7). Data are means ± SE. Different letters within each experimental group (CON, PIA) indicate differences (P < 0.05) across adult experimental conditions (AMB, HU, RL7). #P < 0.05 vs. CON in that condition.
Fiber-type-specific CSA is shown in Supplemental Table S1 with example images of the gastrocnemius in Supplemental Fig. S1, A and B. Soleus MHC I CSA was lower in HU and RL7 versus AMB in CON (P < 0.05) and lower in PIA at RL7 versus AMB (P < 0.05), yet PIA was greater than CON at RL7. Soleus MHC IIa CSA was lower in HU and RL7 versus Ambl in CON (P < 0.05) and in RL7 (P < 0.1) versus AMB in PIA. Soleus MHC IIx CSA was less in RL7 versus AMB in CON (P < 0.1) and less in CON at HU compared with PIA (P < 0.05).
Plantaris fiber-type CSA was mostly unchanged. Only plantaris MHC IIb CSA was as less (P < 0.1) at HU versus AMB in PIA. Gastrocnemius fiber-type CSA was not significantly different by group or across adult experiments.
TA/EDL fiber-type CSA showed some differences across group and adult experiments. TA MHC IIa CSA was lower in HU and RL7 versus AMB in PIA (P < 0.05) and compared with CON at HU (P < 0.05) and RL7 (P < 0.1). TA MHC IIb CSA was lower in HU versus AMB in CON (P < 0.05) and lower in HU and RL7 versus AMB in PIA. TA MHC IIb CSA was lower in PIA at HU and RL7 compared with CON (P < 0.05). TA MHC IIx CSA was lower in HU and RL7 versus AMB in PIA and compared with CON at those conditions (P < 0.05).
EDL MHC IIa CSA was lower in HU versus AMB in PIA (P < 0.05) and compared with CON at HU (P < 0.05). EDL MHC IIb CSA was lower in HU versus AMB in PIA (P < 0.05. EDL MHC IIx CSA was lower in PIA and compared with CON at HU and RL7 (P < 0.1).
Fiber-Type Composition
Fiber-type composition (Supplemental Table S2) displayed no differences across adult experiments or between PIA and CON in any of the investigated muscles with a few exceptions. Plantaris MHC IIa percent tended to be greater (P < 0.1) in HU versus AMB in PIA. EDL MHC IIx percent tended to be greater (P < 0.1) in HU versus AMB in CON and greater (P < 0.1) at AMB in CON versus PIA.
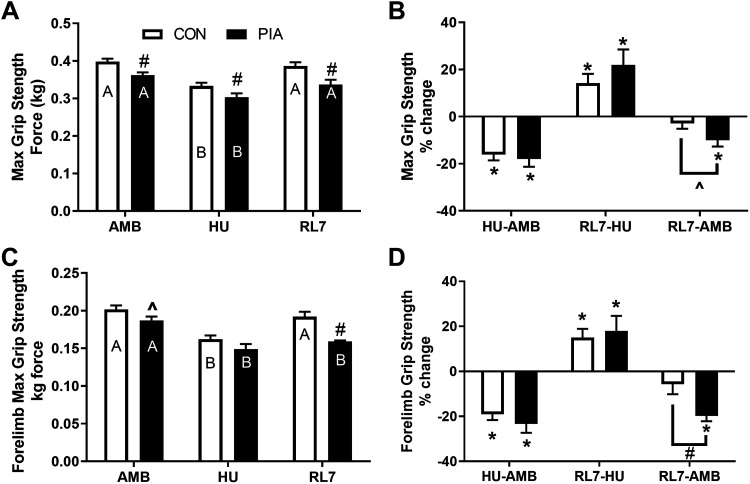
Maximum Grip Strength
Figure 4 shows the grip strength results taken during the adult experiments. Maximum grip strength of all the limbs was reduced in PIA versus CON at all time points, was decreased following HU and restored at RL7 (P < 0.05). Examination of the percent change differences revealed that the change from AMB to RL7 was significant (P < 0.05) and different from CON in the PIA group indicating impaired recovery (P < 0.1). Maximum grip strength of the forelimbs only was reduced in PIA versus CON at AMB (P < 0.1) and RL7 (P < 0.05). Both groups demonstrated loss of strength following HU (P < 0.05), but the PIA group did not recover at RL7, unlike CON (P < 0.05). Examination of the percent change differences revealed that the change from AMB to RL7 was significant (P < 0.05) and different from CON in the PIA group indicating impaired recovery (P < 0.05).
Figure 4.
Maximum grip strength of all limbs (A and B) or forelimbs only (C and D) with absolute (A and C) and change data (B and D) of male C57BL/6 mice exposed to physical inactivity (PIA) as hindlimb unloading (HU) or as early life ambulatory controls (CON) in the early postnatal period (days P28–42) examined in early adulthood (∼5 mo/P150) as ambulatory controls (AMB) or mice exposed to HU or HU + 7 days of reloading (RL7). Data are means ± SE. Different letters within each experimental group (CON, PIA) indicate differences (P < 0.05) across adult experimental conditions (AMB, HU, RL7). #P < 0.05 vs. CON in that condition. For % change data *P < 0.05 is a significant change.
Balance Beam
There were no statistical differences between the groups for time to traverse the 12- or 6-mm balance beams (Supplemental Fig. S2). There was a nonstatistical trend for the 7-day RL mice in the PIA group to have a greater beam latency than CON mice.
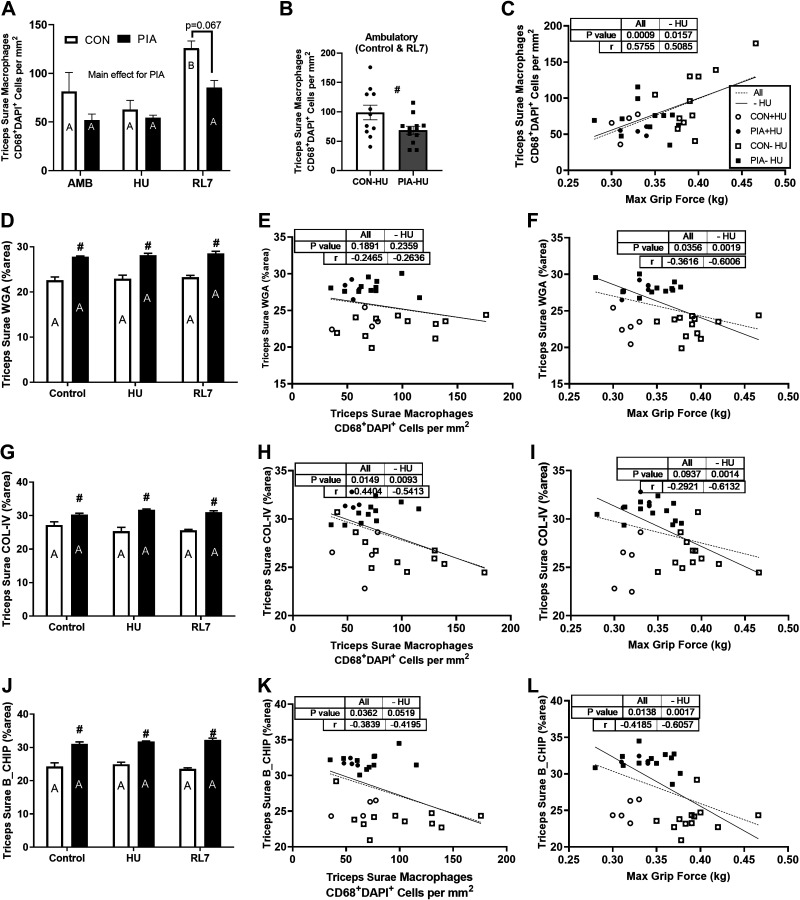
Triceps Surae Muscle Macrophages via Immunofluorescence
CD68+ DAPI+ cells were defined as macrophages in skeletal muscle (Fig. 5, A and B) with example images of the gastrocnemius in Supplemental Fig. S3. When pooling the ambulatory adult experimental groups (controls and reloads) together there was a main effect for a reduced (P < 0.05) muscle macrophage content for PIA versus CON. There was an increase in muscle macrophages at RL7 compared with HU (P < 0.05) for CON. This increase in muscle macrophages at RL7 showed a trend to be reduced (P = 0.067) in PIA versus CON.
Figure 5.
Triceps surae muscle macrophages (A and B) and extracellular matrix (D, G, and J) via immunofluorescence of male C57BL/6 mice exposed to physical inactivity (PIA) as hindlimb unloading (HU) or as early life ambulatory controls (CON) in the early postnatal period (days P28–42) examined in early adulthood (∼5 mo/P150) as ambulatory controls (AMB) or mice exposed to HU or HU + 7 days of reloading (RL7). The triceps surae muscle macrophage content for ambulatory controls and reload experiments (B). The relationship between triceps surae muscle macrophages and maximum grip force with all samples or ambulatory only (HU excluded; C). Wheat germ agglutinin (WGA) % area as a marker for the extracellular matrix (D) with correlations with all samples or ambulatory only (HU excluded) against macrophages (E) and maximum grip force (F). Collagen IV (COL-IV) % area (G) with correlations with all samples or ambulatory only (HU excluded) against macrophages (H) and maximum grip force (I). Degraded collagen (B-CHIP; J) with correlations with all samples or ambulatory only (HU excluded) against macrophages (K) and maximum grip force (L) Data are means ± SE. Different letters within each experimental group (CON, PIA) indicate differences (P < 0.05) across adult experimental conditions (AMB, HU, RL7). #P < 0.05 vs. CON in that condition.
Triceps Surae % Area Extracellular Matrix via Immunofluorescence
The extracellular matrix was impacted by PIA (Fig. 5, G and J) with example images of the gastrocnemius in Supplemental Fig. S4. The % area WGA, COL-IV, and B-CHIP was greater (P < 0.05) in PIA versus CON at all time points with no effect on the adult experiments.
Correlations
A greater density of triceps surae muscle macrophages (CD68+ DAPI+ cells) was positively correlated with maximum grip strength (P < 0.001; r = 0.576) for all experimental groups and for the ambulatory (control and reload) groups only (P < 0.05; r = 0.509; Fig. 5C).
A greater % area of WGA was not associated with muscle macrophages (Fig. 5E) for all experimental groups (P > 0.1; r = −0.247) and for the ambulatory (control and reload) groups only (P > 0.1; r = −0.264). However, a greater % area of WGA was negatively associated with maximum grip strength (Fig. 5C) for all experimental groups (P < 0.05; r = −0.362) and for the ambulatory (control and reload) groups only (P < 0.01; r = −0.601).
A greater % area of COL-IV was negatively associated with muscle macrophages (Fig. 5H) for all experimental groups (P < 0.05; r = −0.440) and for the ambulatory (control and reload) groups (P < 0.01; r = −0.541). A greater % area of COL-IV displayed a trend to be negatively associated with maximum grip strength (Fig. 5I) for all experimental groups (P < 0.1; r = −0.292) and a significant association for the ambulatory (control and reload) groups only (P < 0.001; r = −0.613).
A greater % area of degraded collagen (B-CHIP) was negatively associated with muscle macrophages (Fig. 5K) for all experimental groups (P < 0.05; r = −0.384) and a trend for the ambulatory (control and reload) groups (P = 0.052; r = −0.420). A greater % area of COL-IV was negatively associated with maximum grip strength (Fig. 5L) for all experimental groups (P < 0.05; r = −0.417) and a significant association for the ambulatory (control and reload) groups only (P < 0.001; r = −0.606).
DISCUSSION
Our main finding is that although early life PIA did not induce significant muscle atrophy, it did result in muscle weakness (via grip strength) in adulthood, even after 4 mo of recovery. This muscle weakness was associated with reduced muscle macrophages and increased % area of extracellular matrix in muscle. This represents low muscle quality as dynapenia and potential for premature aging, which should be explored in future investigations.
Previous research has indicated the effects of muscle disuse and immediate recovery early in life are more severe compared with older rodents on outcomes of muscle and myofiber size (11, 12, 14, 16–18), fiber-type composition (11, 12, 14), myogenesis (31, 32). Additional detrimental effects have been reported on bone (19, 20) and motor control (19–21) thus theoretically negatively impacting general postnatal muscle development. Yet the effects of early life physical inactivity in the form of disuse atrophy on longer-term muscle health into early adulthood, are less understood. Previous studies have shown that early life enrichment of cage conditions to enhance physical activity by providing wheels (33), climbing opportunities (34), or treadmill training (35) promotes some beneficial postnatal muscle adaptations into adulthood. Other studies have looked at early postnatal continuous PIA as HU in rats for 3 mo (36) or continuous PIA in a moderately small cage with no effect of recovery (34, 37). We sought to examine the effect of a more intense period of PIA as modeled by HU for a shorter period of time during postnatal development, yet with a long recovery of normal ambulation. Thus, shortly after weaning, we exposed postnatal mice 4–5 wk of age (∼P28) to 14 days of PIA (muscle disuse) and then allowed normal ambulation until early adulthood (∼5 mo/P140). For humans, this is equivalent to about half a year of PIA during the first few years of life and 25–30 yr of age as early adulthood.
This pilot study investigation determined that PIA as disuse in the early life postnatal period caused an attenuated growth rate as indicated by reduced body weights and lower lean and water tissue composition. This is in line with previous studies showing attenuated postnatal growth during HU (12, 14, 36) and during some other forms of physical inactivity (37). For the next 4 mo, we followed their recovery until early adulthood (5 mo)—when long bone growth is typically complete in C57BL/6J mice (38). During the initial recovery, the PIA mice demonstrated a rebound of growth rate to match the early-life ambulatory controls at around P98 (14 wk of recovery). This was demonstrated by restored lean and water mass, however, fat mass had a more delayed recovery until about P126 (20 wk). This pattern reflects a similar pattern to “thrifty catch-up” metabolic programming (39). These are periods where a deficit in growth or a loss of weight is followed by a weight supercompensation or weight regain and are termed “thrifty catch-up.” These “thrifty catch-up” events are considered to increase the risk of metabolic morbidity and early mortality throughout the lifecycle to program weight gain and accelerate disease progression (39). An in-depth investigation of this potential metabolic “programming” as a result of early life PIA should be an area of future investigation.
In line with the similar lean mass findings in adulthood between CON and PIA mice, we discovered no differences in whole muscle size or myofiber-type cross-sectional area in several muscles in adulthood as a result of early-life PIA. A classic investigation in chicks suggested that the earlier the initiation and the longer the duration of disuse during the postnatal growth period the more severe the atrophy and impaired the regrowth (15). The lack of major differences in muscle and myofiber size is not that all surprising because although our initiation of disuse at 4–5 wk (∼P28) was early, it could have been as early as 3 wk of age (P21) when the mice are weaned. Also, the duration of disuse we utilized is comparatively minimal at 14 days. Although 14 days is long for a mouse (∼6 human months) this length of time may not have been sufficient to maintain a reduced muscle and myofiber size in our mice as adults. One previous study exposed 4d postnatal rats to 3 mo of HU and then demonstrated smaller solei compared with controls even after a subsequent 3 mo of recovery at 6 mo of age (36). This maintained effect from the disuse was likely a factor of the prolonged period of disuse (3 mo) compared with ours (2 wk) and the different species used (rats vs. mice). Although muscle and myofiber size differences were not detectable in early adulthood, we anticipate that early-life physical inactivity could cause premature sarcopenia at the whole muscle and myofiber size level in middle age (12+ mo) and that increased severity and/or duration of early life physical inactivity could result in more pronounced deficits in muscle health later in adult life.
Using grip strength assessment, we demonstrate weakness in adulthood as a result of early life PIA, even after 4 mo of recovery. To our knowledge, this is the first such report regarding grip strength after a long recovery from early life physical inactivity. A recent study took 15-wk-old mice and exposed them to 19 wk without climbing opportunities to demonstrate reduced grip strength (34). These mice were not early postnatal, being 15–33 wk old, and these effects were not after a recovery period but during the physical inactivity intervention (34) as a distinction from our experiments. The grip strength weakness we demonstrated after this 4-mo recovery was not concomitant with reduced physical function assessed via the balance beam tests. This may have been a factor of the small sample size in this pilot study or the lack of sensitivity of the test to delineate these types of functional changes after a 4-mo recovery. However, the reduced grip strength was associated with increased extracellular matrix area, collagen IV area, and degraded collagen area in addition to reduced macrophage density in the plantar flexor muscles of the hindlimb, the triceps surae. This association was surprising because the grip strength measurement involves both the forelimbs and the hindlimbs and the % area of ECM and macrophage density assessment was limited to the plantar flexors (triceps surae) of the hindlimb, which are muscles doubtlessly recruited during the test, but in addition to other muscles. To our knowledge, this is the first report to demonstrate that a potent early life disuse stimulus can cause longer lasting (4 mo later) reductions in muscle macrophage content and increased % area ECM and degraded collagen in muscle.
This association between the % area of ECM, COL-IV and degraded collagen, and grip strength could indicate impaired force transmission (40, 41) as a result of increased collagen IV in the ECM is a potential sign of fibrosis (42) in those muscles. Although exercise training suggests increased collagen and collagen turnover, this is normally associated with the optimal reorganization and cross-linking of the matrix (43), whereas the increased ECM in these cases and other cases of disuse and aging (27, 44) are typical of reduced force capacity suggesting suboptimal organization of the matrix. It would be intriguing to determine in future studies the nature of the dysfunction of the ECM of mice exposed to this early-life PIA. As we demonstrated increased levels of degraded collagen (as B-CHIP), it is possible that the collagen turnover is insufficiently increased in failed attempts to repair the ECM. This increased collagen IV in muscle is akin to that found in weaker and older (23 mo) rodents (45) and combined with the grip strength reductions may be an indication of the first steps toward premature aging in our early adulthood mice similar to the pattern of low muscle quality demonstrated by others (46). It is known that muscle macrophages have important roles in collagen turnover that are dysregulated in aging (47–49). The timely balance of pro- and inflammatory macrophages specifically regulates this turnover. Thus, increased ECM area and collagen IV content in those muscles should be investigated in the future as it may be related to an insufficient muscle macrophage content to promote turnover and removal of the degraded and suboptimally cross-linked collagen.
In a second round of experiments at early adulthood (5 mo), the mice that encountered early-life PIA were again exposed to disuse and a 7-day recovery period. With the exception of the soleus, early-life disuse did little to alter the whole muscle or myofiber size response to disuse and recovery from disuse in adulthood. The early-life PIA mice exposed to HU in adulthood demonstrated less reduction in soleus muscle size and mean myofiber CSA compared with CON mice. Nakamura et al. (35) exposed rats to 8 wk of treadmill running starting at 4 wk of age or had sedentary controls and both groups of animals were exposed to HU for 7 days at 20 wk of age. They found that previous early postnatal treadmill running attenuated the atrophy of the plantaris following HU in adulthood. This is in contrast to a study where 3-wk old rats were treated as cage controls or exposed to 8 wk of small cage reduction followed by a week of HU as juveniles (37). They showed that prior continuous moderate PIA worsened soleus atrophy following a week of HU. Our work demonstrated very few effects of the prior early life PIA on the adult muscle size response to HU and 7 days of recovery as ∼P150 adults with the exception of the soleus. The soleus paradoxically demonstrated attenuated atrophy as demonstrated by whole muscle and myofiber in the PIA mice. Although the soleus is an important muscle for ambulation, its size is minuscule compared with the other muscles so this finding may be functionally irrelevant.
The typical increase in macrophages during recovery from disuse (26) in the triceps surae appeared to be attenuated with early-life PIA. More specifically, the early-life PIA mice under ambulatory conditions of both control and recovery had less macrophages than CON mice which may have contributed to the greater area of ECM in PIA mice as discussed earlier. ECM (WGA % area), degraded collagen, and collagen IV area were unchanged in response to these adulthood experiments of disuse and recovery in early-life ambulatory control mice or early-life PIA mice even after 4 mo of recovery suggesting a degree of permanence to this PIA event that occurred in early postnatal life. In adulthood, early life PIA mice also had an impaired ability to recover grip strength losses during reloading following disuse. This may be linked, in part, to the early life PIA mice specifically demonstrating a reduced size of fast twitch-specific myofibers in the anterior hindlimb muscles, in particular the tibialis anterior, during their adulthood disuse and reloading. However, this may also be linked to the trend for a reduced macrophage content during the adult reloading period akin to that demonstrated elsewhere (27, 44). Regardless of the mechanism, the inability to recover grip strength in response to disuse in early adulthood is a concerning phenomenon.
Limitations
As this was a pilot study, it is possible that our smaller sample size was insufficient to detect differences in some of our outcomes. However, even with smaller sample sizes, we were able to detect notable differences in other outcomes such as grip strength and triceps surae noncontractile tissue and macrophages. Also, it is likely that other mechanisms than macrophages and noncontractile tissue accumulation in muscle may have contributed to the grip strength differences and changes, Indeed, neuromuscular changes in response to hindlimb unloading and reloading are a major contributing factor to the loss of force following disuse (50–52). To address this, we examined the relationship between grip strength and the noncontractile tissue area on the ambulatory condition and with HU animals excluded.
Significance and Conclusions
The negative impact of PIA is substantial during key periods of skeletal muscle development and may have detrimental, long-lasting effects on adult health. We demonstrated that early-life PIA in the form of disuse did not result in sustained muscle atrophy, but it did cause muscle weakness in early adulthood. This muscle weakness was associated with reduced muscle macrophage content and increased % area of noncontractile tissue in muscle. This may represent low muscle quality and the potential for premature aging, which needs future exploration. It is relevant to elucidate the effect of early-life physical inactivity on the physiology and progression of aging so interventional strategies can be designed to prevent this premature shortening of health span.
DATA AVAILABILITY
Data will be made available upon reasonable request.
SUPPLEMENTAL DATA
Supplemental Figs. S1–S4 and Supplemental Tables S1 and S2: https://doi.org/10.6084/m9.figshare.21534936.
GRANTS
This work was supported by the Miami University College of Education Health and Society Seed Grants, Miami University Committee for Faculty Research (to P.T. Reidy) and the Miami University Undergraduate Summer Scholars Program (to B. Jevnikar). Support was also provided by the National Institute on Aging at the National Institute of Health Grants R01 AG050781 (to M. J. Drummond) and by the National Institute of Arthritis and Musculoskeletal and Skin Diseases at the National Institutes of Health F32 AR072481 (to P. T. Reidy).
DISCLAIMERS
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
P.T.R. and M.J.D. conceived and designed research; P.T.R., R.W.W., D.K.F., J.J.P., Z.S.M., A.I.M., and N.M.M.P.d.H. performed experiments; P.T.R., A.D.S., B.E.J., A.K.D., R.W.W., A.A.K., J.M.M., and N.M.M.P.d.H. analyzed data; P.T.R., A.D.S., B.E.J., A.K.D., A.A.K., J.M.M., Z.S.M., and M.J.D. interpreted results of experiments; P.T.R. prepared figures; P.T.R., Z.S.M., and M.J.D. drafted manuscript; P.T.R., A.D.S., B.E.J., A.K.D., R.W.W., A.A.K., J.M.M., D.K.F., J.J.P., A.I.M., N.M.M.P.d.H., and M.J.D. edited and revised manuscript; P.T.R., A.D.S., B.E.J., A.K.D., R.W.W., A.A.K., J.M.M., D.K.F., J.J.P., Z.S.M., A.I.M., N.M.M.P.d.H., and M.J.D. approved final version of manuscript.
ACKNOWLEDGMENTS
Microscopy work was performed at the Live Microscopy Core in the Department of Pharmacology & Systems Physiology at the University of Cincinnati. Graphical abstract created with BioRender and published with permission.
REFERENCES
- 1. Booth FW, Roberts CK, Thyfault JP, Ruegsegger GN, Toedebusch RG. Role of inactivity in chronic diseases: evolutionary insight and pathophysiological mechanisms. Physiol Rev 97: 1351–1402, 2017. doi: 10.1152/physrev.00019.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.CDC. Physical Activity Facts (Online). https://www.cdc.gov/healthyschools/physicalactivity/facts.htm [2021 May 24].
- 3. Tucker P. The physical activity levels of preschool-aged children: a systematic review. Early Child Res Q 23: 547–558, 2008. doi: 10.1016/j.ecresq.2008.08.005. [DOI] [Google Scholar]
- 4. Pereira JR, Cliff DP, Sousa-Sá E, Zhang Z, Santos R. Prevalence of objectively measured sedentary behavior in early years: systematic review and meta-analysis. Scand J Med Sci Sports 29: 308–328, 2019. doi: 10.1111/sms.13339. [DOI] [PubMed] [Google Scholar]
- 5. Janssen X, Martin A, Hughes AR, Hill CM, Kotronoulas G, Hesketh KR. Associations of screen time, sedentary time and physical activity with sleep in under 5s: a systematic review and meta-analysis. Sleep Med Rev 49: 101226, 2020. doi: 10.1016/j.smrv.2019.101226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hallam RA, Bargreen K, Fouts HN, Lessard L, Skrobot C. The use of infant confinement equipment in community-based child care centers: an analysis of centers participating in a statewide quality rating and improvement system. Matern Child Health J 22: 694–701, 2018. doi: 10.1007/s10995-018-2438-9. [DOI] [PubMed] [Google Scholar]
- 7. Ness KK, Krull KR, Jones KE, Mulrooney DA, Armstrong GT, Green DM, Chemaitilly W, Smith WA, Wilson CL, Sklar CA, Shelton K, Srivastava DK, Ali S, Robison LL, Hudson MM. Physiologic frailty as a sign of accelerated aging among adult survivors of childhood cancer: a report from the St Jude Lifetime cohort study. J Clin Oncol 31: 4496–4503, 2013. doi: 10.1200/JCO.2013.52.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McGregor RA, Cameron-Smith D, Poppitt SD. It is not just muscle mass: a review of muscle quality, composition and metabolism during ageing as determinants of muscle function and mobility in later life. Longev Healthspan 3: 9, 2014. doi: 10.1186/2046-2395-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Roberts MD, Ruegsegger GN, Brown JD, Booth FW. Mechanisms associated with physical activity behavior: insights from rodent experiments. Exerc Sport Sci Rev 45: 217–222, 2017. doi: 10.1249/JES.0000000000000124. [DOI] [PubMed] [Google Scholar]
- 10. Dodds RM, Roberts HC, Cooper C, Sayer AA. The epidemiology of sarcopenia. J Clin Densitom 18: 461–466, 2015. doi: 10.1016/j.jocd.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Asmussen G, Miersch H, Soukup T. The influence of suspension hypokinesia on contractile properties of slow and fast twitch muscles of young growing and adult rats. Biomed Biochim Acta 48: S426–S431, 1989. [PubMed] [Google Scholar]
- 12. Elder GC, McComas AJ. Development of rat muscle during short- and long-term hindlimb suspension. J Appl Physiol (1985) 62: 1917–1923, 1987. doi: 10.1152/jappl.1987.62.5.1917. [DOI] [PubMed] [Google Scholar]
- 13. Haida N, Fowler WM Jr, Abresch RT, Larson DB, Sharman RB, Taylor RG, Entrikin RK. Effect of hind-limb suspension on young and adult skeletal muscle. I. Normal mice. Exp Neurol 103: 68–76, 1989. doi: 10.1016/0014-4886(89)90187-8. [DOI] [PubMed] [Google Scholar]
- 14. Saitoh A, Okumoto T, Nakano H, Wada M, Katsuta S. Age effect on expression of myosin heavy and light chain isoforms in suspended rat soleus muscle. J Appl Physiol (1985) ) 86: 1483–1489, 1999. doi: 10.1152/jappl.1999.86.5.1483. [DOI] [PubMed] [Google Scholar]
- 15. Shear CR. Effects of disuse on growing and adult chick skeletal muscle. J Cell Sci 48: 35–54, 1981. doi: 10.1242/jcs.48.1.35. [DOI] [PubMed] [Google Scholar]
- 16. Simard C, Lacaille M, Vallieres J. Effects of hypokinesia/hypodynamia on contractile and histochemical properties of young and old rat soleus muscle. Exp Neurol 97: 106–114, 1987. doi: 10.1016/0014-4886(87)90285-8. [DOI] [PubMed] [Google Scholar]
- 17. Steffen JM, Fell RD, Geoghegan TE, Ringel LC, Musacchia XJ. Age effects on rat hindlimb muscle atrophy during suspension unloading. J Appl Physiol (1985) 68: 927–931, 1990. doi: 10.1152/jappl.1990.68.3.927. [DOI] [PubMed] [Google Scholar]
- 18. Shear CR. Cross-sectional myofibre and myofibril growth in immobilized developing skeletal muscle. J Cell Sci 29: 297–312, 1978. doi: 10.1242/jcs.29.1.297. [DOI] [PubMed] [Google Scholar]
- 19. Delcour M, Massicotte VS, Russier M, Bras H, Peyronnet J, Canu MH, Cayetanot F, Barbe MF, Coq JO. Early movement restriction leads to enduring disorders in muscle and locomotion. Brain Pathol 28: 889–901, 2018. doi: 10.1111/bpa.12594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ohira Y, Kawano F, Wang XD, Sudoh M, Iwashita Y, Majima HJ, Nonaka I. Irreversible morphological changes in leg bone following chronic gravitational unloading of growing rats. Life Sci 79: 686–694, 2006. doi: 10.1016/j.lfs.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 21. Walton KD, Lieberman D, Llinas A, Begin M, Llinás RR. Identification of a critical period for motor development in neonatal rats. Neuroscience 51: 763–767, 1992. doi: 10.1016/0306-4522(92)90517-6. [DOI] [PubMed] [Google Scholar]
- 22. Winick M. The role of early nutrition in subsequent development and optimal future health. Bull N Y Acad Med 65: 1020–1025, 1989. [PMC free article] [PubMed] [Google Scholar]
- 23. Martins VJB, Toledo Florêncio TMM, Grillo LP, do Carmo P Franco M, Martins PA, Clemente APG, Santos CDL, de Fatima A Vieira M, Sawaya AL. Long-lasting effects of undernutrition. Int J Environ Res Public Health 8: 1817–1846, 2011. doi: 10.3390/ijerph8061817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Leszczynski EC, Visker JR, Ferguson DP. The effect of growth restriction on voluntary physical activity engagement in mice. Med Sci Sports Exerc 51: 2201–2209, 2019. doi: 10.1249/MSS.0000000000002040. [DOI] [PubMed] [Google Scholar]
- 25. Pendergrast LA, Leszczynski EC, Visker JR, Triplett AN, Ferguson DP. Early life undernutrition reduces maximum treadmill running capacity in adulthood in mice. Appl Physiol Nutr Metab 45: 240–250, 2020. doi: 10.1139/apnm-2019-0023. [DOI] [PubMed] [Google Scholar]
- 26. Reidy PT, McKenzie AI, Mahmassani ZS, Petrocelli JJ, Nelson DB, Lindsay CC, Gardner JE, Morrow VR, Keefe AC, Huffaker TB, Stoddard GJ, Kardon G, O'Connell RM, Drummond MJ. Aging impairs mouse skeletal muscle macrophage polarization and muscle-specific abundance during recovery from disuse. Am J Physiol Endocrinol Physiol 317: E85–E98, 2019. doi: 10.1152/ajpendo.00422.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Petrocelli JJ, Mahmassani ZS, Fix DK, Montgomery JA, Reidy PT, McKenzie AI, de Hart NM, Ferrara PJ, Kelley JJ, Eshima H, Funai K, Drummond MJ. Metformin and leucine increase satellite cells and collagen remodeling during disuse and recovery in aged muscle. FASEB J 35: e21862, 2021. doi: 10.1096/fj.202100883R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McKenzie AI, Reidy PT, Nelson DS, Mulvey JL, Yonemura NM, Petrocelli JJ, Mahmassani ZS, Tippetts TS, Summers SA, Funai K, Drummond MJ. Pharmacological inhibition of TLR4 ameliorates muscle and liver ceramide content after disuse in previously physically active mice. Am J Physiol Regul Integr Comp Physiol 318: R503–R511, 2020. doi: 10.1152/ajpregu.00330.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Castro B, Kuang S. Evaluation of muscle performance in mice by treadmill exhaustion test and whole-limb grip strength assay. Bio Protoc 7: e2237, 2017. doi: 10.21769/BioProtoc.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Luong TN, Carlisle HJ, Southwell A, Patterson PH. Assessment of motor balance and coordination in mice using the balance beam. J Vis Exp: 2376, 2011. doi: 10.3791/2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Darr KC, Schultz E. Hindlimb suspension suppresses muscle growth and satellite cell proliferation. J Appl Physiol (1985) 67: 1827–1834, 1989. doi: 10.1152/jappl.1989.67.5.1827. [DOI] [PubMed] [Google Scholar]
- 32. Schultz E, Darr KC, Macius A. Acute effects of hindlimb unweighting on satellite cells of growing skeletal muscle. J Appl Physiol (1985) 76: 266–270, 1994. doi: 10.1152/jappl.1994.76.1.266. [DOI] [PubMed] [Google Scholar]
- 33. Smith HK, Merry TL. Voluntary resistance wheel exercise during post-natal growth in rats enhances skeletal muscle satellite cell and myonuclear content at adulthood. Acta Physiol (Oxf) 204: 393–402, 2012. doi: 10.1111/j.1748-1716.2011.02350.x. [DOI] [PubMed] [Google Scholar]
- 34. Roemers P, Hulst Y, van Heijningen S, van Dijk G, van Heuvelen MJG, De Deyn PP, van der Zee EA. Inducing physical inactivity in mice: preventing climbing and reducing cage size negatively affect physical fitness and body composition. Front Behav Neurosci 13: 221, 2019. doi: 10.3389/fnbeh.2019.00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nakamura K, Ohsawa I, Masuzawa R, Konno R, Watanabe A, Kawano F. Running training experience attenuates disuse atrophy in fast-twitch skeletal muscles of rats. J Appl Physiol (1985) 123: 902–913, 2017. doi: 10.1152/japplphysiol.00289.2017. [DOI] [PubMed] [Google Scholar]
- 36. Kawano F, Takeno Y, Nakai N, Higo Y, Terada M, Ohira T, Nonaka I, Ohira Y. Essential role of satellite cells in the growth of rat soleus muscle fibers. Am J Physiol Cell Physiol 295: C458–C467, 2008. doi: 10.1152/ajpcell.00497.2007. [DOI] [PubMed] [Google Scholar]
- 37. Yoshihara T, Natsume T, Tsuzuki T, Chang S-W, Kakigi R, Machida S, Sugiura T, Naito H. Long-term physical inactivity exacerbates hindlimb unloading-induced muscle atrophy in young rat soleus muscle. J Appl Physiol (1985) 130: 1214–1225, 2021. doi: 10.1152/japplphysiol.00494.2020. [DOI] [PubMed] [Google Scholar]
- 38. Glatt V, Canalis E, Stadmeyer L, Bouxsein ML. Age-related changes in trabecular architecture differ in female and male C57BL/6J mice. J Bone Miner Res 22: 1197–1207, 2007. doi: 10.1359/jbmr.070507. [DOI] [PubMed] [Google Scholar]
- 39. Dulloo AG, Jacquet J, Seydoux J, Montani JP. The thrifty “catch-up fat” phenotype: its impact on insulin sensitivity during growth trajectories to obesity and metabolic syndrome. Int J Obes (Lond) 30, Suppl 4: S23–35, 2006. [Erratum in Int J Obes (Lond) 34: 1230, 2010]. doi: 10.1038/sj.ijo.0803516. [DOI] [PubMed] [Google Scholar]
- 40. Csapo R, Gumpenberger M, Wessner B. Skeletal muscle extracellular matrix - what do we know about its composition, regulation, and physiological roles? A narrative review. Front Physiol 11: 253, 2020. doi: 10.3389/fphys.2020.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gillies AR, Lieber RL. Structure and function of the skeletal muscle extracellular matrix. Muscle Nerve 44: 318–331, 2011. doi: 10.1002/mus.22094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zanotti S, Saredi S, Ruggieri A, Fabbri M, Blasevich F, Romaggi S, Morandi L, Mora M. Altered extracellular matrix transcript expression and protein modulation in primary Duchenne muscular dystrophy myotubes. Matrix Biol 26: 615–624, 2007. doi: 10.1016/j.matbio.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 43. Brightwell CR, Latham CM, Thomas NT, Keeble AR, Murach KA, Fry CS. A glitch in the matrix: the pivotal role for extracellular matrix remodeling during muscle hypertrophy. Am J Physiol Cell Physiol 323: C763–C771, 2022. doi: 10.1152/ajpcell.00200.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fix DK, Mahmassani ZS, Petrocelli JJ, de Hart NMMP, Ferrara PJ, Painter JS, Nistor G, Lane TE, Keirstead HS, Drummond MJ. Reversal of deficits in aged skeletal muscle during disuse and recovery in response to treatment with a secrotome product derived from partially differentiated human pluripotent stem cells. GeroScience 43: 2635–2652, 2021. doi: 10.1007/s11357-021-00423-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kovanen V, Suominen H, Peltonen L. Effects of aging and life-long physical training on collagen in slow and fast skeletal muscle in rats. A morphometric and immuno-histochemical study. Cell Tissue Res 248: 247–255, 1987. doi: 10.1007/BF00218191. [DOI] [PubMed] [Google Scholar]
- 46. Ge X, Cho A, Ciol MA, Pettan-Brewer C, Snyder J, Rabinovitch P, Ladiges W. Grip strength is potentially an early indicator of age-related decline in mice. Pathobiol Aging Age Relat Dis 6: 32981, 2016. [Erratum in Pathobiol Aging Age Relat Dis 6: 33718, 2016]. doi: 10.3402/pba.v6.32981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wang Y, Wehling-Henricks M, Samengo G, Tidball JG. Increases of M2a macrophages and fibrosis in aging muscle are influenced by bone marrow aging and negatively regulated by muscle-derived nitric oxide. Aging Cell 14: 678–688, 2015. doi: 10.1111/acel.12350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cui C-Y, Driscoll RK, Piao Y, Chia CW, Gorospe M, Ferrucci L. Skewed macrophage polarization in aging skeletal muscle. Aging Cell 18: e13032, 2019. doi: 10.1111/acel.13032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rahman FA, Angus SA, Stokes K, Karpowicz P, Krause MP. Impaired ECM remodeling and macrophage activity define necrosis and regeneration following damage in aged skeletal muscle. Int J Mol Sci 21: 4575, 2020. doi: 10.3390/ijms21134575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Stodieck LS, Greybeck BJ, Cannon CMA, Hanson AM, Young MH, Simske SJ, Ferguson VL. In vivo measurement of hindlimb neuromuscular function in mice. Muscle Nerve 45: 536–543, 2012. doi: 10.1002/mus.22294. [DOI] [PubMed] [Google Scholar]
- 51. Kawano F, Ishihara A, Stevens JL, Wang XD, Ohshima S, Horisaka M, Maeda Y, Nonaka I, Ohira Y. Tension- and afferent input-associated responses of neuromuscular system of rats to hindlimb unloading and/or tenotomy. Am J Physiol Regul Integr Comp Physiol 287: R76–R86, 2004. doi: 10.1152/ajpregu.00694.2003. [DOI] [PubMed] [Google Scholar]
- 52. Morey-Holton ER, Globus RK. Hindlimb unloading rodent model: technical aspects. J Appl Physiol (1985) 92: 1367–1377, 2002. doi: 10.1152/japplphysiol.00969.2001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figs. S1–S4 and Supplemental Tables S1 and S2: https://doi.org/10.6084/m9.figshare.21534936.
Data Availability Statement
Data will be made available upon reasonable request.