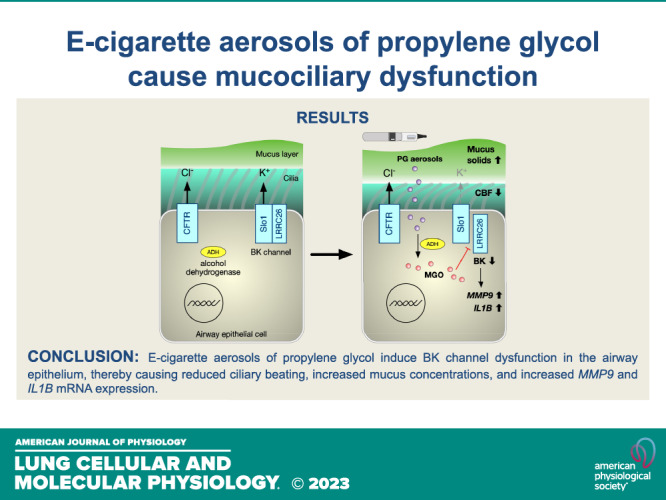
Keywords: airway epithelium, e-cigarette, propylene glycol, vaping
Abstract
Propylene glycol (PG) is a common delivery vehicle for nicotine and flavorings in e-cigarette (e-cig) liquids and is largely considered safe for ingestion. However, little is known about its effects as an e-cig aerosol on the airway. Here, we investigated whether pure PG e-cig aerosols in realistic daily amounts impact parameters of mucociliary function and airway inflammation in a large animal model (sheep) in vivo and primary human bronchial epithelial cells (HBECs) in vitro. Five-day exposure of sheep to e-cig aerosols of 100% PG increased mucus concentrations (% mucus solids) of tracheal secretions. PG e-cig aerosols further increased the activity of matrix metalloproteinase-9 (MMP-9) in tracheal secretions. In vitro exposure of HBECs to e-cig aerosols of 100% PG decreased ciliary beating and increased mucus concentrations. PG e-cig aerosols further reduced the activity of large conductance, Ca2+-activated, and voltage-dependent K+ (BK) channels. We show here for the first time that PG can be metabolized to methylglyoxal (MGO) in airway epithelia. PG e-cig aerosols increased levels of MGO and MGO alone reduced BK activity. Patch-clamp experiments suggest that MGO can disrupt the interaction between the major pore-forming BK subunit human Slo1 (hSlo1) and the gamma regulatory subunit LRRC26. PG exposures also caused a significant increase in mRNA expression levels of MMP9 and interleukin 1 beta (IL1B). Taken together, these data show that PG e-cig aerosols cause mucus hyperconcentration in sheep in vivo and HBECs in vitro, likely by disrupting the function of BK channels important for airway hydration.
INTRODUCTION
The widespread adoption of e-cigarette (e-cig) use has been aided by the belief that vaping e-liquids is less harmful than smoking combustible cigarettes. E-liquids typically comprise nicotine, flavorings, and the delivery vehicles propylene glycol (PG) and vegetable glycerin (VG). Although nicotine and certain flavorings can elicit adverse effects in the airway (1), PG and VG are generally regarded as innocuous. However, recent evidence suggests that exposure to aerosols of PG and/or VG can have damaging effects in the airway (2–5). Here, we set out to determine whether PG e-cig aerosols alone impact mucociliary clearance (MCC) and airway inflammation in a large animal model in vivo and in primary human bronchial epithelial cells (HBECs) in vitro under conditions mimicking normal e-cig use.
PG is commonly used as a food additive and is on the Generally Recognized as Safe (GRAS) list of the Food and Drug Administration (FDA). However, the GRAS designation of PG is based on oral ingestion studies without testing for safety after inhalation. Recent studies show that PG/VG exposure can decrease glucose uptake and alter the metabolism of HBECs in vitro (3). Aerosols of PG and VG can also induce inflammation in HBECs (4), though this is likely dependent on the e-cig device and exposure conditions. In mice, chronic inhalation of sole PG/VG aerosols did not cause inflammation but was shown to disrupt lipid homeostasis and lead to greater morbidity and mortality after virus challenge (2). Furthermore, it is known that aerosol generation from PG- and VG-containing e-liquids can create harmful byproducts, such as reactive oxygen species (ROS) (6), formaldehyde (7), and acrolein (8), in addition to leeching toxic heavy metals such as selenium (9, 10). However, the generation of these byproducts is typically associated with increased wattages of e-cig devices, at times exceeding levels reported by users.
In airway homeostasis, ion channels sustain adequate airway surface liquid (ASL) volume for proper ciliary movement and mucus hydration (11). Cystic fibrosis transmembrane conductance regulator (CFTR) serves an important role for this function since CFTR dysfunction results in thickening of the mucus layer, water absorption, and impaired ciliary beating (12). Mucus hyperconcentration is strongly correlated with decreased MCC (13) and protracted episodes of impaired MCC can cause chronic inflammation as observed in cystic fibrosis (14) and bronchiectasis patients (15). Beyond CFTR, other ion channels, including the large conductance, Ca2+-activated, and voltage-dependent K+ (BK) channel, play important roles in ASL hydration (16–18). Our previous study showed that both CFTR and BK channel activities are impaired by PG/VG aerosols containing nicotine (19). We and others have shown that aerosols of VG, but not PG, can reduce CFTR function in vitro (20, 21). However, the effects of sole PG aerosols on other ion channels in the airway epithelium have yet to be adequately explored.
Therefore, we were interested in the contribution of PG e-cig aerosols in realistic daily amounts to mucociliary dysfunction and airway inflammation both in vitro and in vivo. We focused on matrix metalloproteinase-9 (MMP-9) because of its known role as a mediator of inflammation in chronic obstructive pulmonary disease (COPD) (22). Moreover, the activities of proteases, including MMP-9, were found to be elevated in the lungs of e-cig users who vaped e-liquids containing nicotine and flavorings (23). Here, we show that 5-day exposure of sheep to e-cig aerosols of PG causes mucus hyperconcentration and a significant increase in MMP-9 activity in tracheal secretions. Primary HBECs exposed to aerosols of PG for 5 days further show increased mucus concentrations with associated decreases in ciliary beating. Aerosols of PG did not impact CFTR function, but significantly reduced BK channel activity. Importantly, we found that exposure of HBECs to PG increases levels of methylglyoxal (MGO), a PG metabolite, that itself can reduce BK channel conductance. Collectively, these data describe a new pathway by which PG e-cig aerosols can cause mucociliary dysfunction.
MATERIALS AND METHODS
E-Cig Device and e-Liquids
The eVic Supreme with a Delta 23 atomizer (Joyetech, Shenzen, China) was used for all exposures as previously described (24). The C3 atomizer head contains 3 coils with a resistance of 1.4 Ω. The e-liquid was 100% PG (American E-liquid Store, Wauwatosa, WI) and quality control was assured by Pace Engineering Concepts (Delafield, WI). The 100% PG e-liquid was custom-made for research purposes and contained no nicotine or flavoring additives.
Animal Study Design
Only adult female sheep (ewes) were used because male sheep are naturally more aggressive and thus not amenable for long-term experimentation without the use of general anesthesia that could affect measurements. All procedures were approved by the Mount Sinai Medical Center Animal Research Committee. Conscious ewes were nasally intubated, and their tracheal secretions and plasma were collected as previously described (24, 25).
E-Cig Aerosol Exposure for Sheep
Nasally intubated sheep were exposed to aerosols generated from an eVic Supreme containing 100% PG e-liquid. Aerosols were drawn into a 60-mL syringe and then delivered into the trachea only during inspiration, at a frequency of 20 breaths/min and a tidal volume of 500 mL, via the inspiration tubing of a piston respirator.
Concentration (% Solids) Measurements of Sheep Mucus
Percent solids of sheep mucus were measured and calculated as previously described (24, 26).
Measurement of MMP-9 Activity
MMP-9 activity was measured from sheep tracheal secretions using a Human Active MMP-9 Fluorokine E kit (Cat. No. F9M00; R&D Systems, Minneapolis, MN) that was first validated for use with sheep.
Protein Concentrations with ELISA
ELISA assays were performed following manufacturer’s instructions. For sheep plasma samples, Nori Sheep TGF-β1 ELISA kit (Cat. No. GR106127; Genorise, Berwyn, PA), Nori Sheep IL-6 ELISA kit ( Cat. No. GR106329; Genorise), and Nori Sheep IL-8 ELISA kit (Cat. No. GR106453; Genorise) were used.
Air-Liquid Interface Cultures
Lungs were provided by organ procurement agencies, including the Life Alliance Organ Recovery Agency at the University of Miami (Miami, FL), LifeCenter Northwest (Bellevue, WA), the Nevada Donor Network (Las Vegas, NV), and the Midwest Transplant Network (Westwood, KS) after obtaining IRB-approved consent for research. HBECs were isolated from de-identified donor lungs of never-smoking individuals rejected for transplant after informed consent. Donor lung demographics are listed in Table 1. Donors did not suffer from any documented airway diseases. HBECs were cultured at the air-liquid interface (ALI) according to published methods (27). HBECs were fully differentiated at the ALI for ≥4 wk before e-cig aerosol exposures and as previously described (19, 24).
Table 1.
Characteristics of never-smoker donor lungs
| Donor Lung Characteristics | Age, yr |
|---|---|
| Combined, n = 29 | |
| Median | 23 |
| Range | 14–65 |
| Female, n = 13 | |
| Median | 20 |
| Range | 14–62 |
| Male, n = 16 | |
| Median | 24 |
| Range | 14–65 |
E-Cig Aerosol Exposure for HBECs In Vitro
Using the VC-1 exposure robot (Vitrocell, Waldkirch, Germany) under slightly modified ISO parameters (19, 24), a puff of e-cig aerosol was generated from 100% PG e-liquid using the Delta 23 atomizer set to 3.2–3.6 V for a power setting of ∼7.0 W during 3 s of aerosol collection. Batteries were fully charged before each exposure. ALI cultures in Transwell inserts were exposed to either filtered air or 50 puffs of aerosol (55 mL per puff, applied once every 20 s for 16.7 min). Each puff of e-cig aerosol was diluted with humidified air (relative humidity >50%) at 0.25 L/min. A constant vacuum was applied at 5 mL/min to generate sufficient flow for the e-cig aerosol to gently apply onto the ALI culture surface. HBECs were exposed to 50 puffs per session in the morning and evening (100 total puffs/day), for five or seven consecutive days. Basolateral media was changed every other day and the apical surface was unwashed during the experiments.
Ciliary Beat Frequency Measurements
Ciliary beat frequency (CBF) recordings were performed as previously described (28) on ALI cultures within a 3-mm radius from the center (free of influence by liquid meniscus) using a Basler acA645 camera (Basler, Ahrensburg, Germany) mounted on a Zeiss Axiovert running SAVA software (29). CBF was recorded 24 h after last exposure.
Mucus Concentration (% Solids) Measurements of ALI Cultures
The percentage solids of mucin-containing fluid on top of ALI cultures was measured according to published methods of wet and dry weights using a UMX2 microbalance (Mettler-Toledo) (30, 31). Percent mucus solids was measured 24 h after last exposure.
Immunofluorescence Staining
Immunofluorescence staining of HBECs was performed as previously described (32). Fixed cultures were incubated with anti-MUC5AC antibody (MA1-38223; Thermo Fisher Scientific, Waltham, MA) at 0.4 µg/mL and anti-MUC5B antibody (Cat. No. 37–7400; Thermo Fisher Scientific) at 2 µg/mL overnight at 4°C. Cultures were then incubated with Alexa Fluor 555 donkey anti-mouse IgG (Cat. No. A31570; Thermo Fisher Scientific) at 1 µg/mL and Alexa Fluor 488 donkey anti-rabbit IgG (Cat. No. A21206; Thermo Fisher Scientific) at 1 µg/mL for 1 h at room temperature. Hoechst 33258 (Cat. No. H3569; Thermo Fisher Scientific) was used at 2 µg/mL for 10 min at room temperature.
Confocal Imaging and Image Processing
Z-stack images were obtained using a Nikon C2+ confocal microscope (Nikon Instruments, Tokyo, Japan) with a 20× objective from six different points on each culture. Quantification of surface area staining was measured from collapsed z-stack images using ImageJ software (Bethesda, MD). Briefly, images were converted to 8-bit grayscale and thresholded using Otsu’s method (33). The % surface area labeling was measured as the percentage of pixels in the image using the Nucleus Counter plugin for ImageJ.
LDH Cytotoxicity Assay
Cytotoxicity was assessed by measuring lactate dehydrogenase (LDH) in basolateral media from HBECs exposed to e-cig aerosols for 5 days using the CyQUANT LDH Cytotoxicity Assay kit (Thermo Fisher Scientific).
In Vitro Ion Channel Measurements
HBECs were mounted in Ussing chambers connected to a VCC MC6 or MC8 voltage clamp unit (Physiologic Instruments, San Diego, CA). CFTR function was measured as the change in short-circuit current (ISC) caused by CFTR inhibition with 10 µM CFTRinh-172 (Cat. No. C2992; MilliporeSigma, Burlington, MA) after CFTR stimulation with 10 µM forskolin (Cat. No. F3197; MilliporeSigma) in the presence of 10 µM amiloride (Cat. No. A7410; MilliporeSigma) under a basolateral-to-apical chloride gradient as previously described (24, 34). BK channel function was measured as the change in ISC caused by stimulation with 10 µM ATP (Cat. No. A9187; MilliporeSigma) in the presence of 10 µM amiloride under a basolateral-to-apical K+ gradient as previously described (16, 35).
Methylglyoxal and d-Lactate Measurements
Levels of methylglyoxal and d-lactate were measured in basolateral media of HBECs using a Methylglyoxal Assay Kit (Cat. No. ab273284; Abcam, Cambridge, MA) and a d-lactate Assay Kit (Cat. No. ab83429; Abcam) according to manufacturer’s instructions.
Patch-Clamp Measurements
BK channels were expressed in Xenopus oocytes by injecting complementary RNA for the human pore-forming subunit (hSlo1), alone or in combination with that for the human regulatory subunit LRRC26 in a 1:1 ratio by weight and studied 2–7 days after injection. Potassium currents were recorded from excised patches in 0 Ca2+ using the patch-clamp technique in the inside-out configuration. The external pipette solution contained (in mM) 140 K-methanesulfonic acid (K-MES), 2 MgCl2, 6 HCl, and 20 HEPES. The internal solution contained 140 K-MES, 10 HCl, 5 EGTA, and 20 HEPES. The pH of all solutions was adjusted to 7.2 with MES. Patch-clamp experiments were performed using an Axopatch 200B Amplifier (Axon Instruments), PATCHMASTER acquisition software (HEKA, Holliston, MA), and Igor Pro software (WaveMetrics Inc., Lake Oswego, OR) for graphing and data analysis as previously described (36). On patch excision into MGO-free internal solution, currents were evoked by a series of 50 ms depolarizations to various voltages (0 to 180 mV, in 45 mV steps) with p/−4 leak subtraction from a holding potential of −80 mV, repeated every 30 s. After allowing currents to stabilize for ∼5 min the patch was perfused with MGO-free solution for at least 5 min to record baseline activity (control), then perfused with 5 mM MGO for at least 10 min, followed in some cases by perfusion again with MGO-free solution (wash). Patches were perfused using a gravity-driven “sewer-pipe” system with separate capillary ports for control and MGO solution placed in close proximity to the pipette tip, requiring less than 1 s for solution change.
Assessing mRNA Expression with Quantitative Real-Time PCR
RNA was isolated from HBECs using the E.Z.N.A. Total RNA Kit (OMEGA Bio-tek, Norcross, GA) and cDNA was made using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). qPCR was performed for each sample using a TaqMan Universal Master Mix (Thermo Fisher Scientific) mixed with TaqMan primers for target genes which include IL1B (Hs01555410_m1), IL6 (Hs00985639_m1), IL8 (Hs00174103_m1), MMP9 (Hs00234579_m1), and TGFB1 (Hs01365601_m1). GAPDH (Hs99999905_m1) was used as endogenous control. Expression data was generated by ΔΔCT method using the threshold cycle (CT) value of target gene and GAPDH.
RESULTS
PG E-Cig Aerosols Increase Mucus Concentrations and MMP-9 Activity in Ovine Airways In Vivo
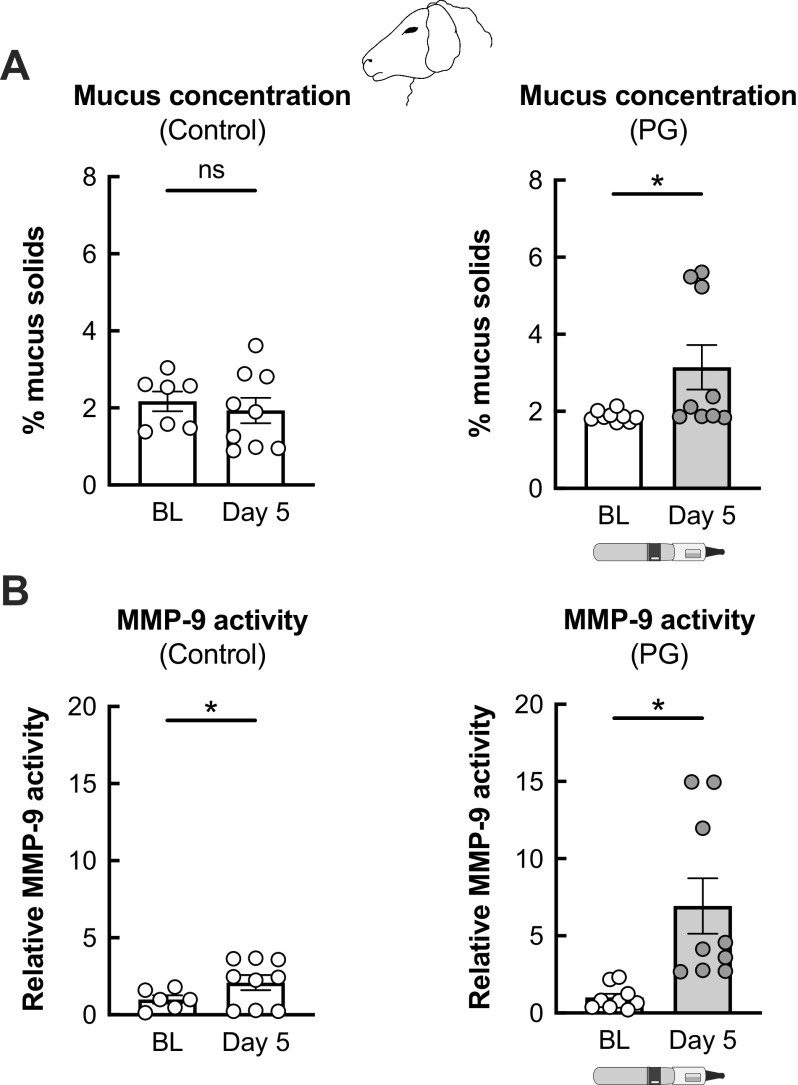
We used our established e-cig exposure model in sheep to determine the effects of PG aerosols in the airway (24). Adult ewes were exposed to 80 puffs (40 puffs per session twice daily) with ≥ 240 s daily puff time of 100% PG aerosols generated by the eVic Supreme for 5 days. Blood and tracheal secretions were collected at baseline and on day 5 after the last exposure. Mucus concentrations (as measured by percentage of mucus solids) in tracheal secretions from sham-exposed (control) sheep remained unchanged from baseline after 5 days (Fig. 1A). However, 5-day exposure of sheep to PG aerosols led to a significant increase in mucus concentrations in tracheal secretions (Fig. 1A). MMP-9 activity measured from tracheal secretions increased by twofold in nonexposed sheep after 5 days (Fig. 1B), although this was likely due to intubation which can cause some local inflammation. On the other hand, PG aerosols caused a sevenfold increase in MMP-9 activity in sheep after 5 days of exposure (Fig. 1B).
Figure 1.
Airway effects of PG e-cig aerosols in a large animal model (sheep). A: sham-treated, control sheep show no significant changes in mucus concentrations (measured as % mucus solids from tracheal secretions) after 5 days. Five-day exposure of sheep to 100% PG e-cig aerosols causes a significant increase in mucus concentrations. n ≥ 7 from 3 sheep for control. n = 9 from 3 sheep for PG. B: sham-treated, control sheep show an increase in MMP-9 activity measured from tracheal secretions after 5 days, likely due to nasal intubation. Five-day exposure of sheep to PG aerosols causes a significant increase in MMP-9 activity. n ≥ 6 from ≥ 2 sheep for control. n = 9 from 3 sheep for PG. Data are presented as means ± SE. *P < 0.05, ns = not significant. Data were analyzed using a mixed-effects model. PG, propylene glycol.
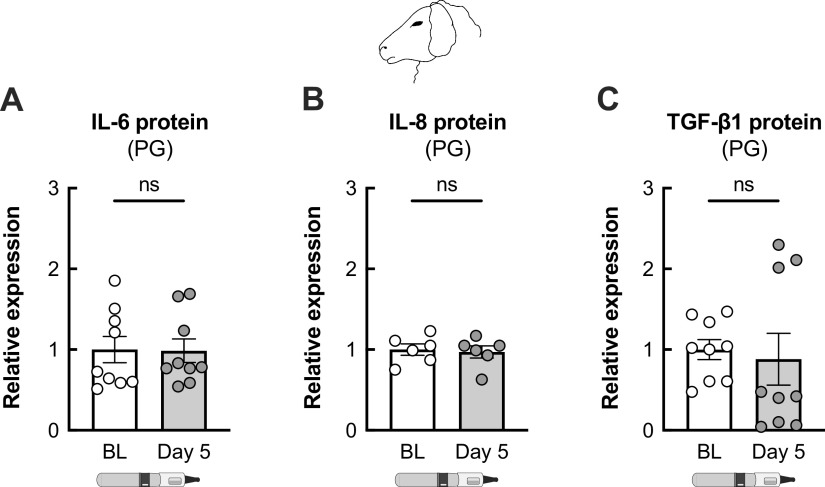
We recently showed that 5-day exposure of sheep to e-cig of aerosols of VG causes a significant increase in plasma levels of TGF-β1 (21). However, exposure of sheep to aerosols of PG did not induce an increase in plasma levels of inflammatory markers, including IL-6, IL-8, and TGF-β1, after 5 days (Fig. 2).
Figure 2.
Plasma levels of inflammatory markers are unchanged in sheep exposed to PG e-cig aerosols. Five-day exposure of sheep to e-cig aerosols of PG does not cause a significant change in plasma levels of IL-6 (A), IL-8 (B), or TGF-β1 (C) proteins. n ≥ 6 from ≥ 2 sheep. Data are presented as means ± SE. ns = not significant. Data were analyzed using a mixed-effects model. PG, propylene glycol.
PG E-Cig Aerosols Increase Mucus Concentrations and Decrease Ciliary Beating in HBECs In Vitro
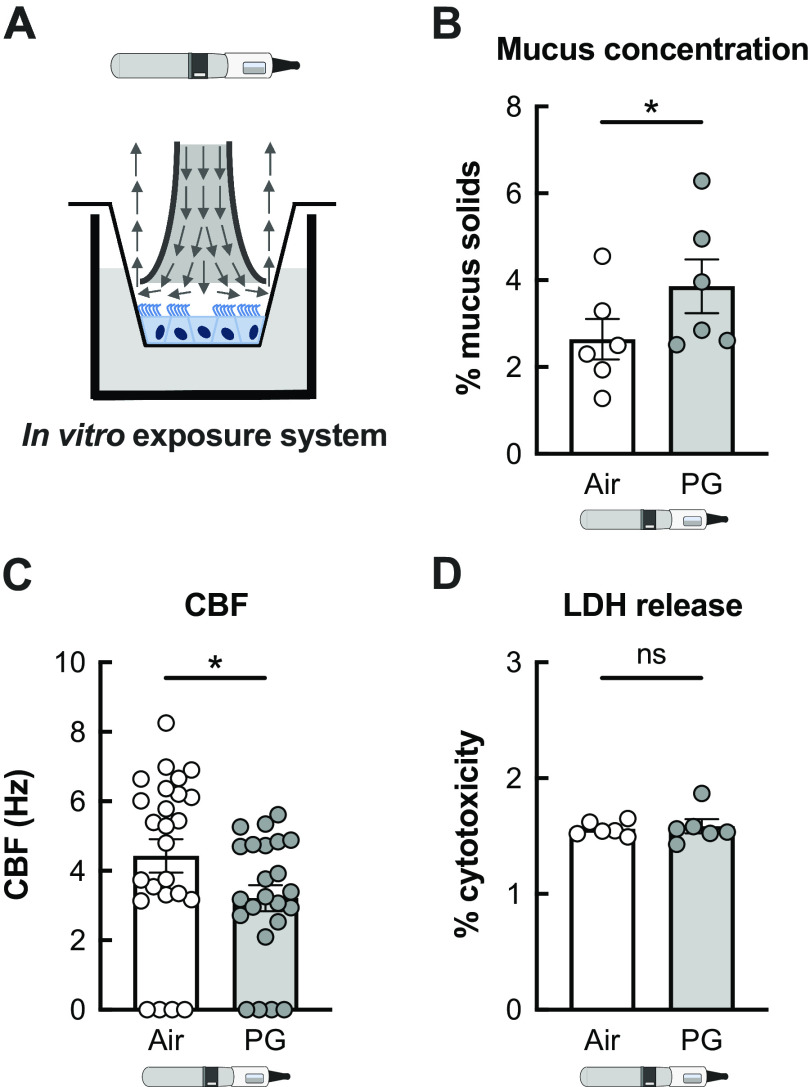
We next exposed primary HBECs to PG e-cig aerosols to investigate the mechanisms through which PG e-cig aerosols cause mucociliary dysfunction. HBECs from donors who never smoked or vaped were exposed to 100% PG e-cig aerosols for 5 days using the VC-1 exposure robot to mirror the 5-day exposure in our sheep model (Fig. 3A). Mucus concentrations and ciliary beat frequency (CBF) were measured in air-exposed (control) and aerosol-exposed cultures 24 h after the last exposure to 100 puffs per day (50 puffs per session twice daily) with a 3 s duration per puff (total 300 s daily puff time). PG aerosols significantly increased mucus concentrations and reduced CBF in HBECs after 5 days compared with air-exposed controls (Fig. 3, B and C), consistent with our findings in sheep. Importantly, 5-day exposure of HBECs to PG e-cig aerosols did not cause cytotoxicity (Fig. 3D).
Figure 3.
PG e-cig aerosols induce mucociliary dysfunction in primary HBECs in vitro. A: representative schema illustrating exposure of fully differentiated primary HBECs to e-cig aerosols. Arrows indicate direction of aerosol flow. B: mucus concentrations are significantly increased in HBECs exposed to PG e-cig aerosols after 5 days compared with air-exposed controls. n = 6 from 5 lungs. C: ciliary beat frequency (CBF) of HBECs exposed to PG e-cig aerosols is significantly reduced after 5 days compared with air-exposed controls. n ≥ 23 from 5 lungs. D: 5-day exposure of HBECs to PG e-cig aerosols does not affect cytotoxicity as assessed by lactate dehydrogenase (LDH) release into basolateral media. n = 6 lungs. Data are presented as means ± SE. *P < 0.05, ns = not significant. Data were analyzed by two-tailed t test (A and C) or mixed-effects model (B) after assessing normality by Shapiro–Wilk. HBECs, human bronchial epithelial cells; PG, propylene glycol.
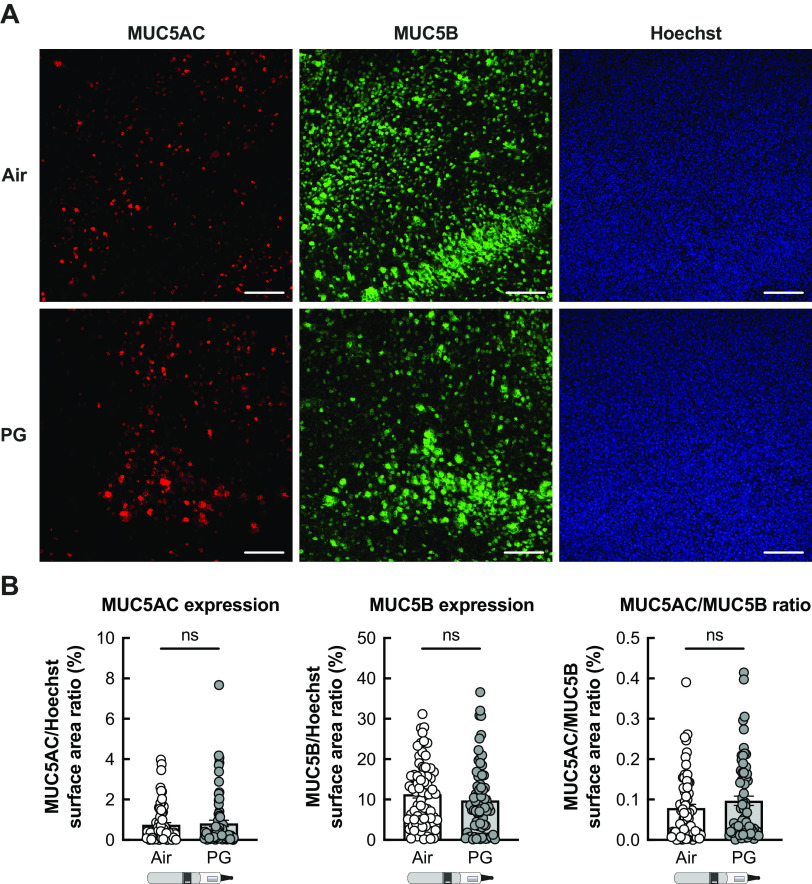
Given the increase in mucus concentrations, we tested whether PG e-cig aerosols affected staining for mucin 5AC (MUC5AC) or mucin 5B (MUC5B). However, no significant change in MUC5AC or MUC5B expression was observed in PG aerosol-exposed HBECs (Fig. 4, A and B). Higher MUC5AC/MUC5B ratios are associated with severity of muco-obstructive diseases (37, 38). Although MUC5AC/MUC5B ratios trended to increase in PG-exposed HBECs after 5 days, the change was not significant when compared with air controls (Fig. 4B).
Figure 4.
PG e-cig aerosols do not affect MUC5AC and MUC5B production in HBECs in vitro. A: representative confocal image stacks of MUC5AC and MUC5B expression and Hoechst (nuclei) stain in HBECs exposed to air or PG e-cig aerosols for 7 days. Scale bar, 100 µm. B: quantification of MUC5AC and MUC5B expression expressed as a ratio of surface area labeling of MUC5AC/Hoechst and MUC5B/Hoechst, respectively. MUC5AC/MUC5B ratio represents the ratio of MUC5AC surface area labeling to MUC5B surface area labeling for each individual image. n = 72 images from 12 lungs. Data are presented as means ± SE. ns = not significant. Data were analyzed using a mixed-effects model. HBECs, human bronchial epithelial cells; PG, propylene glycol.
PG E-Cig Aerosols Increase Levels of MGO That Cause BK Dysfunction in HBECs In Vitro
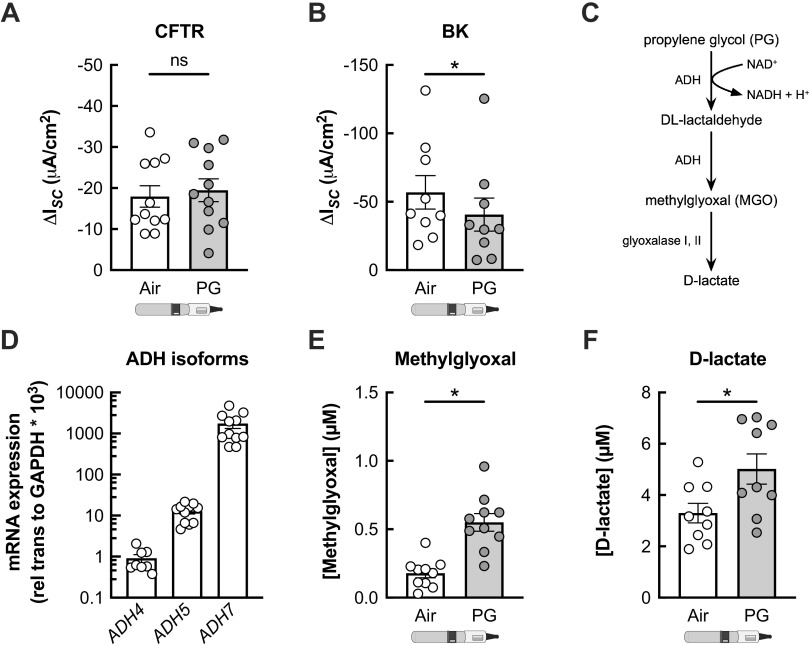
We next investigated whether PG e-cig aerosols affect the conductance of apical ion channels important for MCC. HBECs were exposed to PG aerosols for 7 days for these experiments. Seven-day exposure of HBECs to PG e-cig aerosols did not significantly impact CFTR function (Fig. 5A). However, PG aerosols caused a significant reduction in BK channel activity compared with air controls (Fig. 5B).
Figure 5.
PG e-cig aerosols increase levels of methylglyoxal (MGO) to inhibit BK channel function in HBECs in vitro. A: 7-day exposure of HBECs to propylene glycol (PG) e-cig aerosols does not significantly change CFTR conductance compared with air-exposed controls. n = 11 lungs. B: PG e-cig aerosols cause a significant reduction in BK activity after 7 days compared with air-exposed controls. n = 9 lungs. C: schematic depicting metabolism of propylene glycol (PG) into methylglyoxal (MGO), which is then converted into d-lactate. ADH = alcohol dehydrogenase. D: mRNA expressions of ADH isoforms ADH4, ADH5, and ADH7 in fully differentiated HBECs from nonsmoker donors. n ≥ 8 lungs. E and F: levels of methylglyoxal (E) and d-lactate (F) are significantly increased in the basolateral media of HBECs after 7 days of PG aerosol exposure compared with air-exposed controls. n ≥ 9 lungs. Data are presented as means ± SE. *P < 0.05. Data were analyzed by two-tailed t test (A, E, and F) or two-tailed Wilcoxon test (B) after assessing normality by Shapiro-Wilk. BK, large conductance, Ca2+-activated, and voltage-dependent K+; HBECs, human bronchial epithelial cells; ns, not significant.
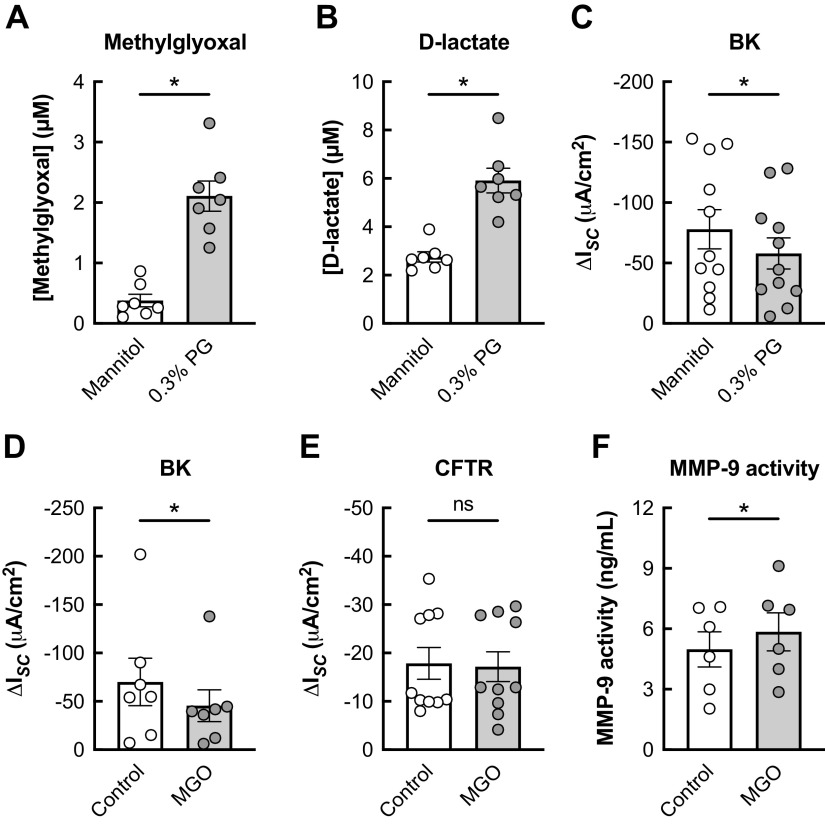
PG is metabolized by alcohol dehydrogenase (ADH) into DL-lactaldehyde, which can be converted to MGO. MGO can be further turned into d-lactate through the glyoxalase pathway (Fig. 5C). Humans express seven different ADH isoforms (39). In fully differentiated HBECs, ADH5 and ADH7 are the predominant isoforms (Fig. 5D). Seven-day exposure of HBECs to PG e-cig aerosols significantly increased MGO and d-lactate levels in the basolateral media (Fig. 5, E and F). Since thermal decomposition of PG by the atomizer coil could catalyze MGO formation (40–43), we tested whether MGO levels are increased in HBECs directly exposed to PG (0.3% v/v) in the basolateral media. PG was used at this concentration because higher concentrations of PG cause a significant increase in the osmolality of the solution (3). Exposure of HBECs to 0.3% PG for 24 h caused a significant increase in MGO and d-lactate levels in the basolateral media compared with an isosmotic control of 0.74% mannitol (Fig. 6, A and B). BK channel function was also significantly decreased in HBECs exposed to 0.3% PG compared with the isosmotic control (Fig. 6C). Basolateral MGO (1 µM) caused a significant reduction in BK channel activity but did not impact CFTR function (Fig. 6, D and E), similar to PG aerosols. Finally, basolateral MGO significantly increased MMP-9 activity levels measured in the basolateral media after 24 h (Fig. 6F).
Figure 6.
Basolateral PG inhibits BK channel function in HBECs in vitro. A and B: basolateral levels of methylglyoxal (A) and d-lactate (B) are significantly increased in HBECs 24 h after exposure to basolateral 0.3% PG compared with 0.74% mannitol (isosmotic) control. n = 7 lungs. C: 24-h exposure of HBECs to 0.3% PG causes a significant reduction in BK activity compared with mannitol control. n = 11 from 7 lungs. D: 24-h exposure of HBECs to basolateral MGO (1 µM) causes a significant decrease in BK function. n = 7 lungs. E: basolateral MGO does not significantly change CFTR conductance compared with controls after 24 h. n = 10 from 6 lungs. F: 24-h exposure of HBECs to basolateral MGO causes a significant increase in MMP-9 activity measured in the basolateral media. n = 6 from 3 lungs. Data are presented as means ± SE. *P < 0.05, ns = not significant. Data were analyzed by two-tailed t test (A–D and F) or two-tailed Wilcoxon test (E) after assessing normality by Shapiro-Wilk. BK, large conductance, Ca2+-activated, and voltage-dependent K+; HBECs, human bronchial epithelial cells; MGO, methylglyoxal; PG, propylene glycol.
Basolateral levels of MGO and d-lactate are also significantly increased, whereas BK channel activity is significantly decreased, in HBECs 5 days after PG aerosol exposure (Supplemental Fig. S1). The increase in mucus concentrations and reduced CBF observed in PG-exposed HBECs is consistent with BK channel dysfunction (Supplemental Fig. S2) (16).
MGO Modifies the Interaction between BK Channel Subunits Slo1 and LRRC26
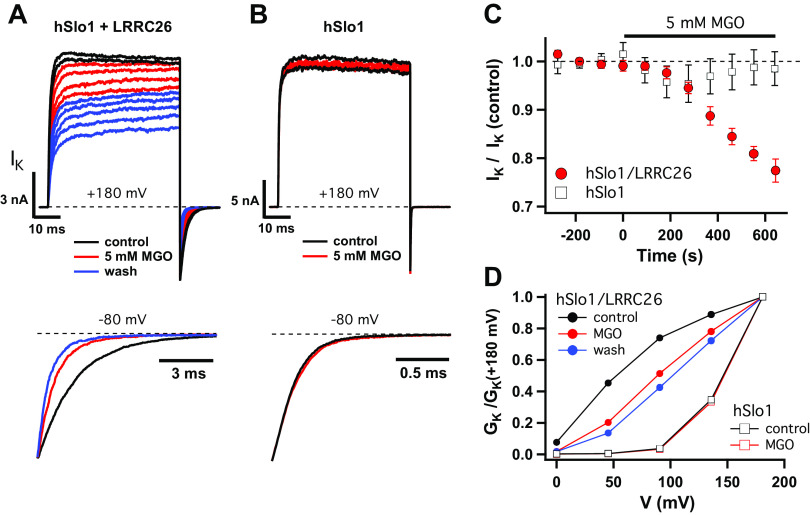
MGO can modify arginine residues to form irreversible adducts (44), and therefore might act directly to modify BK channels and inhibit their function. Slo1, encoded by the KCNMA1 gene, is the main α subunit of the BK channel, and forms channels on its own. However, co-assembly of Slo1 with the γ1 regulatory subunit LRRC26 enhances voltage-dependent activation and is required in nonexcitable cells, including airway epithelial cells, for BK channels to activate at physiological voltages (17, 18, 45). Moreover, a cluster of arginine residues in the intracellular c-terminal “tail” of LRRC26 is known to be crucial for the efficient association of LRRC26 with Slo1, and hence their functional interaction (46). We therefore tested the possibility that MGO disrupts the interaction between LRRC26 and Slo1 in patch clamp experiments using human (hSlo1) channels expressed in Xenopus oocytes. Membrane patches were excised from oocytes expressing hSlo1 and LRRC26, or hSlo1 alone, and K+ current was recorded in response to voltage pulses in the absence of intracellular Ca2+ (5 mM EGTA) before, during, and after perfusing the intracellular side of the patch with 5 mM MGO for at least 10 min. Higher concentrations of MGO were used for these experiments to test for acute, direct effects on BK channel function under conditions where intracellular pathways are essentially eliminated. Outward current evoked at +180 mV as well as the inward tail current following the voltage pulse decreased gradually during MGO treatment for channels containing LRRC26 (Fig. 7, A and C) but not for hSlo1 alone (Fig. 7, B and C), suggesting that LRRC26 or its interaction with hSlo1 is modified. This effect was not reversed by MGO washout. In fact, current continued to decrease following MGO treatment, although patches were continuously perfused with MGO-free solution (Fig. 7A, blue traces).
Figure 7.
MGO modifies the interaction between hSlo1 and LRRC26. A and B: representative BK channel currents evoked from Xenopus oocytes expressing hSlo1 and LRRC26 (A) or hSlo1 alone (B) at +180 mV. Each trace is the average of 6 sweeps (acquired over 3 min). Outward current during the pulse as well as the inward tail current following the pulse decrease during MGO (5 mM) treatment (red) for channels containing LRRC26 (A), but not for Slo1 alone (B). Lower panels show kinetic changes in normalized tail currents. C: time course of MGO action. Data shown as means ± SE for 3 experiments each in the presence and absence of LRRC26 with each point representing the average of 3 sweeps (acquired over 90 s) at +180 mV. D: conductance-voltage (GK-V) relations, normalized to +180 mV from the experiments in A and B, show MGO produces a positive shift in the voltage dependence of activation. BK, large conductance, Ca2+-activated, and voltage-dependent K+; MGO, methylglyoxal.
That hSlo1/LRRC26 channels are gradually and irreversibly inhibited by MGO is consistent with chemical modification, most likely involving arginine residues in LRRC26 known to be important for Slo1/LRRC26 interaction. A delay of ∼2 min was observed between MGO application and the onset of inhibition (Fig. 7C), consistent with a requirement for modifying multiple residues and/or a delay in the dissociation of modified subunits. The latter could also potentially account for increased inhibition following MGO washout. Although the effects of MGO on the kinetics and voltage dependence of BK channel activity did not reach a steady state and were not characterized in detail, they appear consistent with a partial inhibition of LRRC26 action. The decrease in current was accompanied by a threefold speeding of deactivation, evident from normalized tail currents in Fig. 7A (lower panel), partially recapitulating to the major kinetic difference between control traces in the presence and absence of LRRC26 (Fig. 7, A and B). Likewise, normalized conductance-voltage (GK-V) relations show that MGO alters the voltage dependence of hSlo1/LRRC26, shifting the threshold for activation to more positive voltages, again qualitatively consistent with the impact of LRRC26 removal (Fig. 7D). Thus, MGO likely inhibits BK channel function by disrupting interaction between hSlo1 and LRRC26.
PG E-Cig Aerosols Increase MMP-9 Expression in HBECs In Vitro
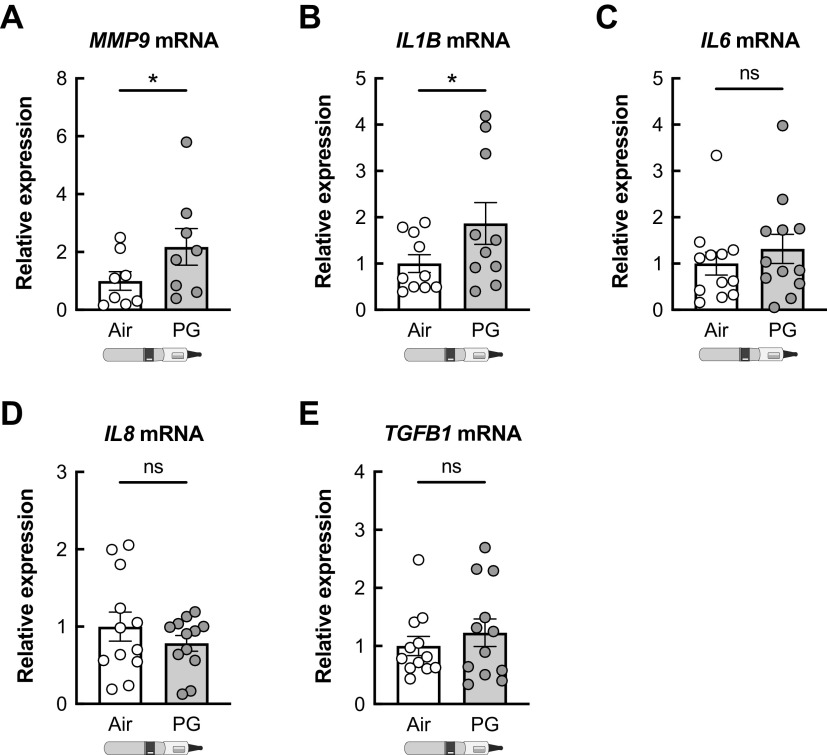
HBECs exposed to PG aerosols for 7 days showed no significant changes in IL6, IL8, and TGFB1 mRNAs compared with air-exposed controls (Fig. 8). However, there was a significant increase in IL1B and MMP9 mRNA expression levels (Fig. 8).
Figure 8.
Inflammatory markers in HBECs exposed to PG e-cig aerosols. Exposure of HBECs to e-cig aerosols of PG significantly increased the expression of MMP9 (A) and IL1B (B) mRNAs after 7 days. IL6 (C), IL8 (D), and TGFB1 (E) mRNA expressions are statistically unchanged in HBECs after 7 days of PG exposure compared with air controls. n ≥ 8 lungs. Data are presented as means ± SE. *P < 0.05. Data were analyzed by two-tailed t test (A) or two-tailed Wilcoxon test (B–E) after assessing normality by Shapiro–Wilk. HBECs, human bronchial epithelial cells; ns, not significant; PG, propylene glycol.
DISCUSSION
This study demonstrates that PG e-cig aerosols, in the absence of nicotine and flavorings, cause mucus hyperconcentration both in primary HBECs in vitro and sheep in vivo. PG aerosols further induced significant increases in MMP9 expression in HBECs and MMP-9 activity in the sheep airway. Mechanistically, PG aerosols impair BK channel function via production of the cytotoxic molecule MGO. Together, these data show that metabolism of a common e-liquid constituent by the airway epithelium can further contribute to the harmful effects of e-cig aerosols.
Mucus hyperconcentration is correlated with decreased MCC (30) and lung function (15), and is considered an emerging predictive property of chronic bronchitis (31). Growing evidence suggests that e-cig use is associated with increased risk of chronic bronchitis. Adolescent e-cig users were found to have increased rates of chronic bronchitis symptoms, including chronic cough, congestion or phlegm, compared with those who never used e-cigs (47). A recent report using data from the Population Assessment of Tobacco and Health (PATH) study further showed that former or current e-cig use is associated with increased odds of developing wheezing-related respiratory symptoms in young adults (age 18–24) with no previous history of respiratory disease (48). Although nicotine and other toxicants are likely to mediate some of the damaging effects of e-cig aerosols in the airway (1), our data provide further evidence that the common solvent PG could contribute as well.
Our experiments reveal for the first time that PG e-cig aerosols can reduce BK channel conductance in HBECs in vitro. BK channels are apically expressed potassium channels that are part of an apical loop current that favors chloride secretion and is critically important for proper ASL hydration (16). Inhibiting BK channels, either pharmacologically with paxilline or by shRNA-mediated knockdown of KCNMA1 (hSlo1), the pore-forming α subunit, causes a significant reduction in CBF and a significant increase in mucus concentrations in HBECs in vitro (16). Reduction of BK channel function by inflammatory mediators also results in associated reductions in ASL volumes, CBF, and increased mucus concentrations, further highlighting the importance of BK channels for proper MCC (17, 18, 26, 32, 35).
The ability of PG aerosols to cause BK channel dysfunction is likely to be at least partly mediated by MGO, an α-dicarbonyl compound produced from PG that is detected in e-cig aerosols (40–43). MGO is known to be toxic and cause necrosis of the nasal airway epithelium in mice at concentrations lower than diacetyl (49). When heated, PG can be oxidized to form MGO. However, PG can also be metabolized into MGO by ADH. ADH isoforms are expressed in the airway epithelium and contribute to the conversion of PG into MGO as evidenced by our experiments with basolaterally administered PG. Interestingly, MGO alone reduced BK activity but had no effect on CFTR function. This is consistent with a previous study that reported that aerosols of PG had no effect on CFTR activity (20), as well as our studies here demonstrating a lack of change in CFTR conductance in response to sole PG aerosols in vitro.
It is conceivable that reduction of BK channel activity by MGO could reflect reduced channel expression or pharmacological inhibition, as MGO has been shown to alter mRNA instability of ATP-sensitive K+ channels and also reversibly modulate their function by direct interaction (50, 51). However, our results suggest that the ability of MGO to chemically modify arginine residues (44) together with the essential role of regulatory LRRC26 subunits for BK channel function in the airway epithelium can account for the specific effect of MGO on this channel. Although the main pore-forming subunit of BK channels (Slo1) contains several arginine residues that are important for voltage- or Ca2+-sensing, and therefore contribute to BK channel activity (52, 53), we observed no effect of acute application of a high (5 mM) concentration of MGO on hSlo1 channel function. By contrast, channels containing LRRC26 were strongly and irreversibly inhibited by the same treatment, consistent with a partial reduction in LRRC26 action, suggesting that chemical modification by MGO can disrupt the interaction of LRRC26 with hSlo1. BK channel activity in nonexcitable airway epithelial cells under physiological conditions requires LRRC26 so any disruption between LRRC26 and hSlo1 will inhibit BK conductance (17, 18, 26, 35). LRRC26 contains an unusual cluster of six arginine residues (291RARRRRLR298) within its c-terminal tail, and progressive deletion or mutation of these residues results in both inhibition of channel activity and reduced physical association of LRRC26 with the channel, consistent with a progressive decrease in the affinity of LRRC26 for hSlo1 (46). Outstanding questions include the identity of critical arginine residues in LRRC26 that are modified by MGO to reduce channel activity, whether additional arginine residues in LRRC26 and/or hSlo1 also contribute, and if prolonged MGO treatment can completely eliminate the contribution of LRRC26 to BK channel activity.
We further found that PG aerosols increased MMP-9 activity in sheep tracheal secretions and MMP9 and IL1B mRNA expressions in exposed HBECs. IL1B encodes IL-1β, a proinflammatory cytokine that can also induce MMP-9 expression (54). The increase in MMP-9 activity is particularly noteworthy given that MMP-9 levels are associated with inflammation and airway remodeling in chronic respiratory diseases (22). Increased MMP-9 activity has also been demonstrated to contribute to mucus production by modulating the expression of MUC5AC (55, 56). Although PG aerosols increased MMP-9 activity in HBECs, we did not observe a corresponding increase in MUC5AC expression. Thus, the increase in mucus concentrations resulting from PG exposure can likely be attributed to BK channel dysfunction.
Importantly, our in vivo data in sheep are consistent with a recent human study showing increased expression of neutrophil elastase and increased expression and activity of MMP-2 and MMP-9 in bronchoalveolar lavage (BAL) fluid of chronic e-cig users (23). However, the authors concluded that increased protease activity was nicotine-dependent, and all enrolled users in the study used their preferred flavorings. As our study focused on nicotine- and flavoring-free e-cig aerosols, we speculate that PG aerosols alone can elicit a protease and inflammatory response that could be further exacerbated by nicotine and/or flavorings. Although PG only aerosols did not increase plasma levels of some inflammatory markers in sheep, it is important to note that systemic markers of inflammation have only been reported thus far after chronic exposure of nicotine-containing e-cig aerosols in mice (57) and in e-cig users who vaped nicotine-containing products (58).
In conclusion, this study demonstrates that aerosols from PG-containing e-cig liquid vehicles negatively impact airway ion transport, mucus concentrations, and protease expression and activity in naïve airway epithelial cells and in sheep trachea. These findings underscore how PG aerosols, even in the absence of nicotine or flavors, can possibly have harmful effects on the airway.
DATA AVAILABILITY
Data will be made available on reasonable request.
SUPPLEMENTAL DATA
Supplemental Figs. S1 and S2: https://doi.org/10.6084/m9.figshare.21950684.v1.
GRANTS
This work was supported by the Flight Attendant Medical Research Institute (CIA No. 130033 to M.S.); the James and Esther King Florida Biomedical Research Program (Grant No. 5JK02 to M.S.); and the National Institutes of Health (F32 HL140729 to S.C.; R01 HL157942 to M.D.K. and M.S.; and R01 HL133240 and R01 HL139365 to M.S.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.D.K., S.C., N.B., L.S., J.S., F.T.H., and M.S. conceived and designed research; M.D.K., S.C., N.B., L.S., N.S., J.S.D., M.Y., J.S., F.T.H., and M.S. performed experiments; M.D.K., S.C., N.B., L.S., N.S., J.S.D., M.Y., J.S., F.T.H., and M.S. analyzed data; M.D.K., S.C., N.B., L.S., N.S., J.S., F.T.H., and M.S. interpreted results of experiments; M.D.K., S.C., L.S., F.T.H., and M.S. prepared figures; M.D.K., M.S., and S.C. drafted manuscript; M.D.K., S.C., N.B., N.S., J.S., F.T.H., and M.S. edited and revised manuscript; M.D.K., S.C., N.B., L.S., N.S., J.S.D., M.Y., J.S., F.T.H.; and M.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the Life Alliance Organ Recovery Agency at the University of Miami, LifeCenter Northwest in Seattle, WA, Nevada Donor Network in Las Vegas, NV, and Midwest Transplant Network in Kansas City, KS for providing the lungs. We thank Dr. Brian Button (University of North Carolina) for providing the mucus solids measurement technique, and Tony Pace (PEC, LLC) for producing the 100% PG e-liquid used in this study.
REFERENCES
- 1. Gotts JE, Jordt SE, McConnell R, Tarran R. What are the respiratory effects of e-cigarettes? BMJ 366: l5275, 2019. [Erratum in BMJ 367: l5980, 2019]. doi: 10.1136/bmj.l5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Madison MC, Landers CT, Gu BH, Chang CY, Tung HY, You R, Hong MJ, Baghaei N, Song LZ, Porter P, Putluri N, Salas R, Gilbert BE, Levental I, Campen MJ, Corry DB, Kheradmand F. Electronic cigarettes disrupt lung lipid homeostasis and innate immunity independent of nicotine. J Clin Invest 129: 4290–4304, 2019. doi: 10.1172/JCI128531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Woodall M, Jacob J, Kalsi KK, Schroeder V, Davis E, Kenyon B, Khan I, Garnett JP, Tarran R, Baines DL. E-cigarette constituents propylene glycol and vegetable glycerin decrease glucose uptake and its metabolism in airway epithelial cells in vitro. Am J Physiol Lung Cell Mol Physiol 319: L957–L967, 2020. doi: 10.1152/ajplung.00123.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Escobar YH, Nipp G, Cui T, Petters SS, Surratt JD, Jaspers I. In vitro toxicity and chemical characterization of aerosol derived from electronic cigarette humectants using a newly developed exposure system. Chem Res Toxicol 33: 1677–1688, 2020. doi: 10.1021/acs.chemrestox.9b00490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Scheffler S, Dieken H, Krischenowski O, Förster C, Branscheid D, Aufderheide M. Evaluation of E-cigarette liquid vapor and mainstream cigarette smoke after direct exposure of primary human bronchial epithelial cells. Int J Environ Res Public Health 12: 3915–3925, 2015. doi: 10.3390/ijerph120403915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lerner CA, Sundar IK, Yao H, Gerloff J, Ossip DJ, McIntosh S, Robinson R, Rahman I. Vapors produced by electronic cigarettes and e-juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung. PLoS One 10: e0116732, 2015. doi: 10.1371/journal.pone.0116732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jensen RP, Luo W, Pankow JF, Strongin RM, Peyton DH. Hidden formaldehyde in e-cigarette aerosols. N Engl J Med 372: 392–394, 2015. doi: 10.1056/NEJMc1413069. [DOI] [PubMed] [Google Scholar]
- 8. Bekki K, Uchiyama S, Ohta K, Inaba Y, Nakagome H, Kunugita N. Carbonyl compounds generated from electronic cigarettes. Int J Environ Res Public Health 11: 11192–11200, 2014. doi: 10.3390/ijerph111111192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Williams M, Ventura J, Loza A, Wang Y, Talbot P. Chemical elements in electronic cigarette solvents and aerosols inhibit mitochondrial reductases and induce oxidative stress. Nicotine Tob Res 22: S14–S24, 2020. doi: 10.1093/ntr/ntaa193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Olmedo P, Goessler W, Tanda S, Grau-Perez M, Jarmul S, Aherrera A, Chen R, Hilpert M, Cohen JE, Navas-Acien A, Rule AM. Metal concentrations in e-cigarette liquid and aerosol samples: the contribution of metallic coils. Environ Health Perspect 126: 027010, 2018. doi: 10.1289/EHP2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bustamante-Marin XM, Ostrowski LE. Cilia and mucociliary clearance. Cold Spring Harb Perspect Biol 9: a028241, 2017. doi: 10.1101/cshperspect.a028241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ghosh A, Boucher RC, Tarran R. Airway hydration and COPD. Cell Mol Life Sci 72: 3637–3652, 2015. doi: 10.1007/s00018-015-1946-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mall MA, Danahay H, Boucher RC. Emerging concepts and therapies for mucoobstructive lung disease. Ann Am Thorac Soc 15: S216–S226, 2018. doi: 10.1513/AnnalsATS.201806-368AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hill DB, Long RF, Kissner WJ, Atieh E, Garbarine IC, Markovetz MR, Fontana NC, Christy M, Habibpour M, Tarran R, Forest MG, Boucher RC, Button B. Pathological mucus and impaired mucus clearance in cystic fibrosis patients result from increased concentration, not altered pH. Eur Respir J 52: 1801297, 2018. doi: 10.1183/13993003.01297-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ramsey KA, Chen ACH, Radicioni G, Lourie R, Martin M, Broomfield A, Sheng YH, Hasnain SZ, Radford-Smith G, Simms LA, Burr L, Thornton DJ, Bowler SD, Livengood S, Ceppe A, Knowles MR, Noone PG Sr, Donaldson SH, Hill DB, Ehre C, Button B, Alexis NE, Kesimer M, Boucher RC, McGuckin MA. Airway mucus hyperconcentration in non-cystic fibrosis bronchiectasis. Am J Respir Crit Care Med 201: 661–670, 2020. doi: 10.1164/rccm.201906-1219OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Manzanares D, Gonzalez C, Ivonnet P, Chen RS, Valencia-Gattas M, Conner GE, Larsson HP, Salathe M. Functional apical large conductance, Ca2+-activated, and voltage-dependent K+ channels are required for maintenance of airway surface liquid volume. J Biol Chem 286: 19830–19839, 2011. doi: 10.1074/jbc.M110.185074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Manzanares D, Krick S, Baumlin N, Dennis JS, Tyrrell J, Tarran R, Salathe M. Airway surface dehydration by transforming growth factor β (TGF-β) in cystic fibrosis is due to decreased function of a voltage-dependent potassium channel and can be rescued by the drug pirfenidone. J Biol Chem 290: 25710–25716, 2015. doi: 10.1074/jbc.M115.670885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Manzanares D, Srinivasan M, Salathe ST, Ivonnet P, Baumlin N, Dennis JS, Conner GE, Salathe M. IFN-γ-mediated reduction of large-conductance, Ca2+-activated, voltage-dependent K+ (BK) channel activity in airway epithelial cells leads to mucociliary dysfunction. Am J Physiol Lung Cell Mol Physiol 306: L453–L462, 2014. doi: 10.1152/ajplung.00247.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Garcia-Arcos I, Geraghty P, Baumlin N, Campos M, Dabo AJ, Jundi B, Cummins N, Eden E, Grosche A, Salathe M, Foronjy R. Chronic electronic cigarette exposure in mice induces features of COPD in a nicotine-dependent manner. Thorax 71: 1119–1129, 2016. doi: 10.1136/thoraxjnl-2015-208039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lin VY, Fain MD, Jackson PL, Berryhill TF, Wilson LS, Mazur M, Barnes SJ, Blalock JE, Raju SV, Rowe SM. Vaporized E-cigarette liquids induce ion transport dysfunction in airway epithelia. Am J Respir Cell Mol Biol 61: 162–173, 2019. doi: 10.1165/rcmb.2017-0432OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kim MD, Chung S, Dennis JS, Yoshida M, Aguiar C, Aller SP, Mendes ES, Schmid A, Sabater J, Baumlin N, Salathe M. Vegetable glycerin e-cigarette aerosols cause airway inflammation and ion channel dysfunction. Front Pharmacol 13: 1012723, 2022. doi: 10.3389/fphar.2022.1012723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Churg A, Zhou S, Wright JL. Series “matrix metalloproteinases in lung health and disease”: Matrix metalloproteinases in COPD. Eur Respir J 39: 197–209, 2012. doi: 10.1183/09031936.00121611. [DOI] [PubMed] [Google Scholar]
- 23. Ghosh A, Coakley RD, Ghio AJ, Muhlebach MS, Esther CR Jr, Alexis NE, Tarran R. Chronic e-cigarette use increases neutrophil elastase and matrix metalloprotease levels in the lung. Am J Respir Crit Care Med 200: 1392–1401, 2019. doi: 10.1164/rccm.201903-0615OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chung S, Baumlin N, Dennis JS, Moore R, Salathe SF, Whitney PL, Sabater J, Abraham WM, Kim MD, Salathe M. Electronic cigarette vapor with nicotine causes airway mucociliary dysfunction preferentially via TRPA1 receptors. Am J Respir Crit Care Med 200: 1134–1145, 2019. doi: 10.1164/rccm.201811-2087OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Terryah ST, Fellner RC, Ahmad S, Moore PJ, Reidel B, Sesma JI, Kim CS, Garland AL, Scott DW, Sabater JR, Carpenter J, Randell SH, Kesimer M, Abraham WM, Arendshorst WJ, Tarran R. Evaluation of a SPLUNC1-derived peptide for the treatment of cystic fibrosis lung disease. Am J Physiol Lung Cell Mol Physiol 314: L192–L205, 2018. doi: 10.1152/ajplung.00546.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim MD, Baumlin N, Yoshida M, Polineni D, Salathe SF, David JK, Peloquin CA, Wanner A, Dennis JS, Sailland J, Whitney P, Horrigan FT, Sabater JR, Abraham WM, Salathe M. Losartan rescues inflammation-related mucociliary dysfunction in relevant models of cystic fibrosis. Am J Respir Crit Care Med 201: 313–324, 2020. doi: 10.1164/rccm.201905-0990OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fulcher ML, Gabriel S, Burns KA, Yankaskas JR, Randell SH. Well-differentiated human airway epithelial cell cultures. Methods Mol Med 107: 183–206, 2005. doi: 10.1385/1-59259-861-7:183. [DOI] [PubMed] [Google Scholar]
- 28. Salathe M, Bookman RJ. Mode of Ca2+ action on ciliary beat frequency in single ovine airway epithelial cells. J Physiol 520: 851–865, 1999. doi: 10.1111/j.1469-7793.1999.00851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sisson JH, Stoner JA, Ammons BA, Wyatt TA. All-digital image capture and whole-field analysis of ciliary beat frequency. J Microsc 211: 103–111, 2003. doi: 10.1046/j.1365-2818.2003.01209.x. [DOI] [PubMed] [Google Scholar]
- 30. Anderson WH, Coakley RD, Button B, Henderson AG, Zeman KL, Alexis NE, Peden DB, Lazarowski ER, Davis CW, Bailey S, Fuller F, Almond M, Qaqish B, Bordonali E, Rubinstein M, Bennett WD, Kesimer M, Boucher RC. The relationship of mucus concentration (hydration) to mucus osmotic pressure and transport in chronic bronchitis. Am J Respir Crit Care Med 192: 182–190, 2015. doi: 10.1164/rccm.201412-2230OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Button B, Anderson WH, Boucher RC. Mucus hyperconcentration as a unifying aspect of the chronic bronchitic phenotype. Ann Am Thorac Soc 13 Suppl 2: S156–S162, 2016. doi: 10.1513/AnnalsATS.201507-455KV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim MD, Baumlin N, Dennis JS, Yoshida M, Kis A, Aguiar C, Schmid A, Mendes E, Salathe M. Losartan reduces cigarette smoke-induced airway inflammation and mucus hypersecretion. ERJ Open Res 7: 00394–02020, 2021. doi: 10.1183/23120541.00394-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Otsu N. A threshold selection method from gray-level histograms. IEEE Transactions on Systems, Man, and Cybernetics 9: 62–66, 1979. doi: 10.1109/TSMC.1979.4310076. [DOI] [Google Scholar]
- 34. Sailland J, Grosche A, Baumlin N, Dennis JS, Schmid A, Krick S, Salathe M. Role of Smad3 and p38 signalling in cigarette smoke-induced CFTR and BK dysfunction in primary human bronchial airway epithelial cells. Sci Rep 7: 10506, 2017. doi: 10.1038/s41598-017-11038-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bengtson CD, Kim MD, Anabtawi A, He J, Dennis JS, Miller S, Yoshida M, Baumlin N, Salathe M. Hyperglycaemia in CF adversely affects BK channel function critical for mucus clearance. Eur Respir J 57: 2000509, 2020. doi: 10.1183/13993003.00509-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sun L, Horrigan FT. A gating lever and molecular logic gate that couple voltage and calcium sensor activation to opening in BK potassium channels. Sci Adv 8: eabq5772, 2022. doi: 10.1126/sciadv.abq5772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kesimer M, Ford AA, Ceppe A, Radicioni G, Cao R, Davis CW, Doerschuk CM, Alexis NE, Anderson WH, Henderson AG, Barr RG, Bleecker ER, Christenson SA, Cooper CB, Han MK, Hansel NN, Hastie AT, Hoffman EA, Kanner RE, Martinez F, Paine R 3rd, Woodruff PG, O'Neal WK, Boucher RC. Airway mucin concentration as a marker of chronic bronchitis. N Engl J Med 377: 911–922, 2017. doi: 10.1056/NEJMoa1701632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Radicioni G, Ceppe A, Ford AA, Alexis NE, Barr RG, Bleecker ER, Christenson SA, Cooper CB, Han MK, Hansel NN, Hastie AT, Hoffman EA, Kanner RE, Martinez FJ, Ozkan E, Paine R 3rd, Woodruff PG, O'Neal WK, Boucher RC, Kesimer M. Airway mucin MUC5AC and MUC5B concentrations and the initiation and progression of chronic obstructive pulmonary disease: an analysis of the SPIROMICS cohort. Lancet Respir Med 9: 1241–1254, 2021. doi: 10.1016/S2213-2600(21)00079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Edenberg HJ. The genetics of alcohol metabolism: role of alcohol dehydrogenase and aldehyde dehydrogenase variants. Alcohol Res Health 30: 5–13, 2007. [PMC free article] [PubMed] [Google Scholar]
- 40. Uchiyama S, Ohta K, Inaba Y, Kunugita N. Determination of carbonyl compounds generated from the E-cigarette using coupled silica cartridges impregnated with hydroquinone and 2,4-dinitrophenylhydrazine, followed by high-performance liquid chromatography. Anal Sci 29: 1219–1222, 2013. doi: 10.2116/analsci.29.1219. [DOI] [PubMed] [Google Scholar]
- 41. Uchiyama S, Senoo Y, Hayashida H, Inaba Y, Nakagome H, Kunugita N. Determination of chemical compounds generated from second-generation e-cigarettes using a sorbent cartridge followed by a two-step elution method. Anal Sci 32: 549–555, 2016. doi: 10.2116/analsci.32.549. [DOI] [PubMed] [Google Scholar]
- 42. Saliba NA, El Hellani A, Honein E, Salman R, Talih S, Zeaiter J, Shihadeh A. Surface chemistry of electronic cigarette electrical heating coils: effects of metal type on propylene glycol thermal decomposition. J Anal Appl Pyrolysis 134: 520–525, 2018. doi: 10.1016/j.jaap.2018.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Azimi P, Keshavarz Z, Lahaie Luna M, Cedeno Laurent JG, Vallarino J, Christiani DC, Allen JG. An unrecognized hazard in e-cigarette vapor: preliminary quantification of methylglyoxal formation from propylene glycol in e-cigarettes. Int J Environ Res Public Health 18: 385, 2021. doi: 10.3390/ijerph18020385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Oya T, Hattori N, Mizuno Y, Miyata S, Maeda S, Osawa T, Uchida K. Methylglyoxal modification of protein. Chemical and immunochemical characterization of methylglyoxal-arginine adducts. J Biol Chem 274: 18492–18502, 1999. doi: 10.1074/jbc.274.26.18492. [DOI] [PubMed] [Google Scholar]
- 45. Yan J, Aldrich RW. LRRC26 auxiliary protein allows BK channel activation at resting voltage without calcium. Nature 466: 513–516, 2010. doi: 10.1038/nature09162. [DOI] [PubMed] [Google Scholar]
- 46. Li Q, Guan X, Yen K, Zhang J, Yan J. The single transmembrane segment determines the modulatory function of the BK channel auxiliary gamma subunit. J Gen Physiol 147: 337–351, 2016. doi: 10.1085/jgp.201511551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. McConnell R, Barrington-Trimis JL, Wang K, Urman R, Hong H, Unger J, Samet J, Leventhal A, Berhane K. Electronic cigarette use and respiratory symptoms in adolescents. Am J Respir Crit Care Med 195: 1043–1049, 2017. doi: 10.1164/rccm.201604-0804OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Xie W, Tackett AP, Berlowitz JB, Harlow AF, Kathuria H, Galiatsatos P, Fetterman JL, Cho J, Blaha MJ, Hamburg NM, Robertson RM, DeFilippis AP, Hall ME, Bhatnagar A, Benjamin EJ, Stokes AC. Association of electronic cigarette use with respiratory symptom development among US young adults. Am J Respir Crit Care Med 205: 1320–1329, 2022. doi: 10.1164/rccm.202107-1718OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hubbs AF, Kreiss K, Cummings KJ, Fluharty KL, O'Connell R, Cole A, Dodd TM, Clingerman SM, Flesher JR, Lee R, Pagel S, Battelli LA, Cumpston A, Jackson M, Kashon M, Orandle MS, Fedan JS, Sriram K. Flavorings-related lung disease: a brief review and new mechanistic data. Toxicol Pathol 47: 1012–1026, 2019. doi: 10.1177/0192623319879906. [DOI] [PubMed] [Google Scholar]
- 50. Yang Y, Konduru AS, Cui N, Yu L, Trower TC, Shi W, Shi Y, Jiang C. Acute exposure of methylglyoxal leads to activation of KATP channels expressed in HEK293 cells. Acta Pharmacol Sin 35: 58–64, 2014. doi: 10.1038/aps.2013.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yang Y, Li S, Konduru AS, Zhang S, Trower TC, Shi W, Cui N, Yu L, Wang Y, Zhu D, Jiang C. Prolonged exposure to methylglyoxal causes disruption of vascular KATP channel by mRNA instability. Am J Physiol Cell Physiol 303: C1045–C1054, 2012. doi: 10.1152/ajpcell.00020.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Latorre R, Castillo K, Carrasquel-Ursulaez W, Sepulveda RV, Gonzalez-Nilo F, Gonzalez C, Alvarez O. Molecular determinants of BK channel functional diversity and functioning. Physiol Rev 97: 39–87, 2017. doi: 10.1152/physrev.00001.2016. [DOI] [PubMed] [Google Scholar]
- 53. Yang H, Zhang G, Cui J. BK channels: multiple sensors, one activation gate. Front Physiol 6: 29, 2015. doi: 10.3389/fphys.2015.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Liang KC, Lee CW, Lin WN, Lin CC, Wu CB, Luo SF, Yang CM. Interleukin-1beta induces MMP-9 expression via p42/p44 MAPK, p38 MAPK, JNK, and nuclear factor-kappaB signaling pathways in human tracheal smooth muscle cells. J Cell Physiol 211: 759–770, 2007. doi: 10.1002/jcp.20992. [DOI] [PubMed] [Google Scholar]
- 55. Deshmukh HS, Case LM, Wesselkamper SC, Borchers MT, Martin LD, Shertzer HG, Nadel JA, Leikauf GD. Metalloproteinases mediate mucin 5AC expression by epidermal growth factor receptor activation. Am J Respir Crit Care Med 171: 305–314, 2005. doi: 10.1164/rccm.200408-1003OC. [DOI] [PubMed] [Google Scholar]
- 56. Deshmukh HS, Shaver C, Case LM, Dietsch M, Wesselkamper SC, Hardie WD, Korfhagen TR, Corradi M, Nadel JA, Borchers MT, Leikauf GD. Acrolein-activated matrix metalloproteinase 9 contributes to persistent mucin production. Am J Respir Cell Mol Biol 38: 446–454, 2008. doi: 10.1165/rcmb.2006-0339OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Crotty Alexander LE, Drummond CA, Hepokoski M, Mathew D, Moshensky A, Willeford A, Das S, Singh P, Yong Z, Lee JH, Vega K, Du A, Shin J, Javier C, Tian J, Brown JH, Breen EC. Chronic inhalation of e-cigarette vapor containing nicotine disrupts airway barrier function and induces systemic inflammation and multiorgan fibrosis in mice. Am J Physiol Regul Integr Comp Physiol 314: R834–R847, 2018. [Erratum in Am J Physiol Regul Integr Comp Physiol 323: R483, 2022]. doi: 10.1152/ajpregu.00270.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Singh KP, Lawyer G, Muthumalage T, Maremanda KP, Khan NA, McDonough SR, Ye D, McIntosh S, Rahman I. Systemic biomarkers in electronic cigarette users: implications for noninvasive assessment of vaping-associated pulmonary injuries. ERJ Open Res 5: 00182–02019, 2019. doi: 10.1183/23120541.00182-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figs. S1 and S2: https://doi.org/10.6084/m9.figshare.21950684.v1.
Data Availability Statement
Data will be made available on reasonable request.