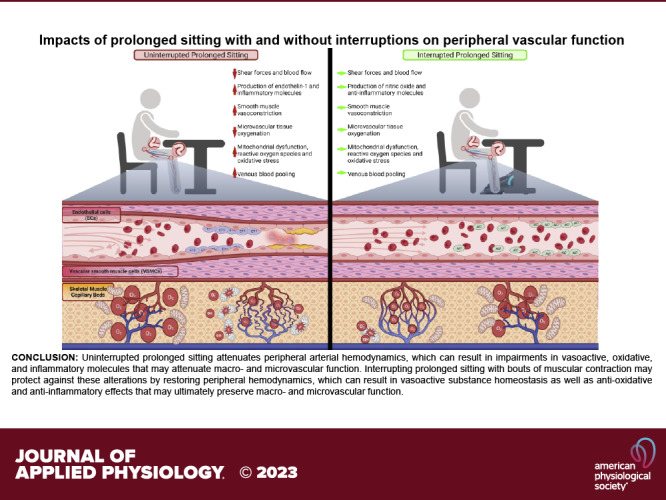
Keywords: macrovascular function, microcirculation, peripheral arterial disease, prolonged sitting, shear stress
Abstract
Sitting time is associated with increased risks for subclinical atherosclerosis and cardiovascular disease development, and this is thought to be partially due to sitting-induced disturbances in macro- and microvascular function as well as molecular imbalances. Despite surmounting evidence supporting these claims, contributing mechanisms to these phenomena remain largely unknown. In this review, we discuss evidence for potential mechanisms of sitting-induced perturbations in peripheral hemodynamics and vascular function and how these potential mechanisms may be targeted using active and passive muscular contraction methods. Furthermore, we also highlight concerns regarding the experimental environment and population considerations for future studies. Optimizing prolonged sitting investigations may allow us to not only better understand the hypothesized sitting-induced transient proatherogenic environment but to also enhance methods and devise mechanistic targets to salvage sitting-induced attenuations in vascular function, which may ultimately play a role in averting atherosclerosis and cardiovascular disease development.
INTRODUCTION
Sedentary behaviors, particularly time spent in the seated position, have been on the rise in the United States. Adults and children alike currently spend a substantial amount of their daily lives seated, with adults sitting for 6–8 h per day and youths totaling nearly 7 h per day (1–4). With our nation being heavily reliant on technology for work and leisure purposes, it is plausible to expect that time in the seated position may persist or potentially escalate. This is concerning as sitting time has been identified as an independent risk factor for the manifestation of metabolic impairments, cardiovascular diseases, and all-cause mortality (5, 6).
Prolonged sitting, identified as sitting uninterrupted for 1 h or more at a time (7, 8), places unique challenges on the cardiovascular system, with a recent meta-analysis and a review both indicating that substantial vascular impairments may occur in the lower extremities following a prolonged sitting bout (9, 10). It has been suggested that these cardiovascular perturbations may be partially due to “arterial bending” at the hip and knee joints and elevations in hydrostatic pressure that alter local hemodynamics, collectively contributing to sitting-induced disturbances in macro- and microvascular function as well as circulating molecules (7, 8, 10–15). Importantly, impairments in the macro- and microcirculation are classified as contributing factors in the development of lower-extremity atherosclerotic diseases (16–18), and sitting time alone was recently identified to be linked to subclinical atherosclerosis and lower-extremity vascular disease (19, 20). Therefore, preventing sitting-induced disruptions in vascular function may be an area of interest to reduce risks for atherosclerosis.
Prophylactic exercise strategies and breaking-up prolonged sitting with skeletal muscle contractions with activities such as walking (21), fidgeting (22), cycling/pedaling (8, 23), simple resistance exercises (24, 25), and passive leg movements (8) have been shown to have potential protective effects against sitting-induced disturbances in vascular function in healthy and disease populations. These findings are reasonably promising, as averting sitting-induced alterations in vascular function may yield a considerable amount of protection against developing lower-extremity atherosclerosis. However, the mechanism(s) underlying this vascular protection during prolonged sitting is not fully understood. Therefore, this review will focus on 1) the potential mechanisms of sitting-induced alterations in peripheral hemodynamics including macrovascular function, microvascular function, and circulating molecular imbalances, 2) potential protective mechanisms by muscular contractions against prolonged sitting-induced disturbances in vascular function and circulating molecules, and 3) suggestions regarding the experimental environment and study populations for future work.
IMPACTS OF PROLONGED SITTING ON PERIPHERAL HEMODYNAMICS
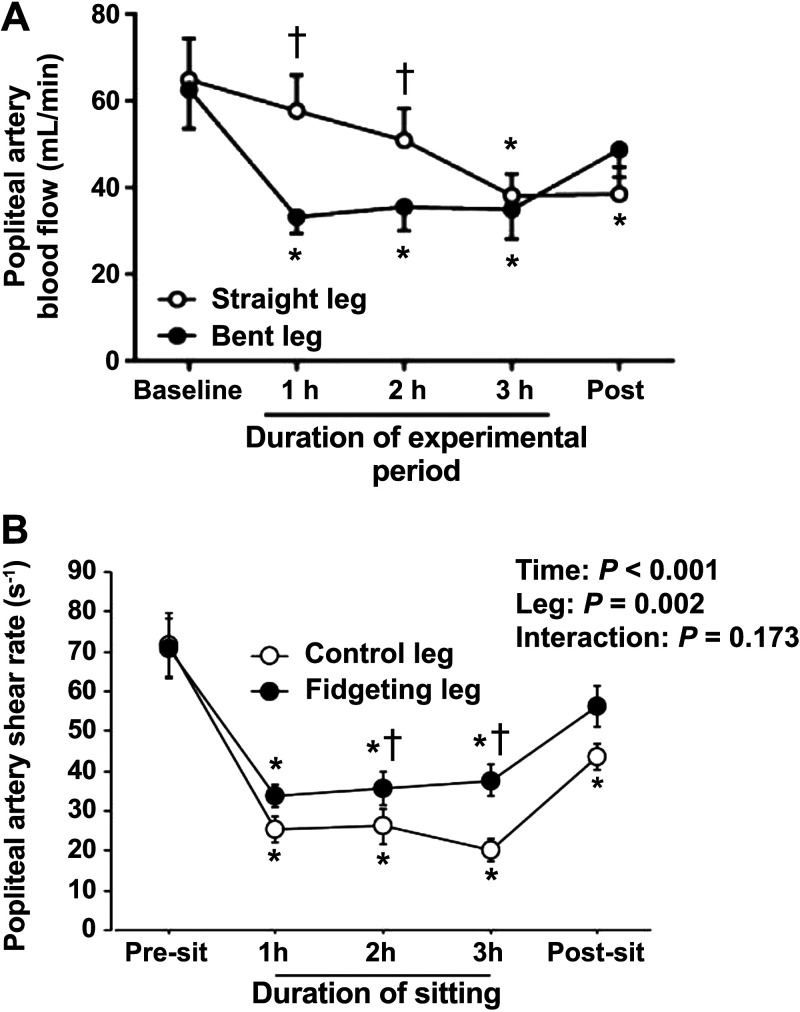
Hemodynamics refers to the distribution and forces of blood flow throughout the circulatory system. The vascular endothelium is continuously subjected to the frictional forces produced by arterial blood flow, otherwise known as shear stress (26, 27). The endothelium reacts to these forces and conveys information via biochemical signals to regulate the phenotypic changes required for maintaining vascular homeostasis (26, 27). These endothelial-mediated responses are contingent on the nature of the shear stress patterns. Unidirectional laminar shear stress stimulates the synthesis of antiatherogenic vasodilatory molecules such as nitric oxide (NO) and prostacyclin (28). On the contrary, regions of disturbed laminar shear stress stimulate production of potent proatherogenic substances and molecules such as endothelin-1 (ET-1) and vascular adhesion molecules (28). Furthermore, low arterial shear stress can upregulate endothelial-derived reactive oxygen species (ROS) and can blunt endogenous antioxidant mechanisms, which may generate a pro-oxidative shift in the redox balance (29, 30). Atherosclerotic plaques have been reported to commonly manifest near arterial bifurcations, and this may be partly due to the bifurcation’s structural complexity that fosters an unfavorable local hemodynamic environment that is associated with vasoactive metabolite imbalances, enhanced inflammatory signaling, and greater ROS (29–34). It may be possible that repetitive and/or prolonged exposure to local hemodynamic impairments could contribute to lower-extremity atherosclerotic diseases (10). In particular, the seated position may mimic a similar environment to arterial bifurcations due to the “bent artery” morphology created by 90-degree angles at the hip and knee joints. Walsh et al. (11) revealed that 3 h of leg bending, similar to the angulation of the seated position, produces hemodynamic disturbances in the popliteal artery when compared with a straight limb (Fig. 1A). Therefore, the “bent artery” position associated with the seated posture may be a key contributor to sitting-induced changes in hemodynamics.
Figure 1.
The effects of the bent leg position and intermittent active muscular contractions (fidgeting, 1 min on/4 min off) during prolonged sitting on popliteal artery hemodynamics. A: the bent leg position associated with prolonged sitting produces reductions in popliteal artery blood flow when compared with an otherwise straight limb (mL/min), n = 12, *P < 0.05 vs. Baseline, †P < 0.05 vs. straight leg. B: mean popliteal artery shear rate (s−1) was greater in the fidgeting leg compared with the nonfidgeting control leg when measured 2 min after the 1-min fidgeting bout. n = 11, *P < 0.05 vs. baseline †P < 0.05 vs. control leg. [Adapted and reused with permission from Walsh et al. (11), under a Creative Commons Attribution 4.0 International License, and from Morishima et al. (22).]
Hemodynamic impairments due to prolonged sitting have been well reported. Thosar et al. (21, 35) performed two studies that investigated the impacts of prolonged sitting (3 h) in healthy young males on superficial femoral artery hemodynamics using Doppler ultrasound (Table 1). The authors showed reductions in the superficial femoral artery mean and antegrade shear rates after just 1 h of sitting (21, 35). In line with these investigations, we found reduced common femoral artery mean shear rate and blood flow after prolonged sitting for 2.5 h (8). Furthermore, sitting-induced hemodynamic alterations are consistently present in other downstream arteries, including reduced shear rate and blood flow in the popliteal and posterior tibial arteries (14, 36, 38, 39) (Table 1). Although these findings are largely in agreement, it is important to note that shear rate provides an estimation of shear stress, which is a limitation for Doppler ultrasound. A recent study by Hou et al. (13) used computational modeling techniques to understand how the arterial deformation due to the seated position (i.e., arterial bending) can impact external iliac artery shear stress in vivo (Table 1). After 30 min in a seated position, a greater area of the external iliac artery was exposed to higher oscillatory shear index, low shear stress, and alterations in transverse wall shear stress (indicates the average wall shear stress component over the cardiac cycle that is perpendicular to the mean wall shear stress vector) when compared with a supine position (13). Based on these studies, we can infer that the prolonged leg arterial bending that is associated with the seated position can impair hemodynamics, which may be a factor in producing a potential transient proatherogenic environment (10). Therefore, devising methods that salvage arterial hemodynamics may prevent the formation of this proatherogenic phenotype during prolonged sitting.
Table 1.
Impacts of uninterrupted prolonged sitting on peripheral hemodynamics, macrovascular function, microvascular function, and vasoactive molecules
| References | Sex and Age of Subjects, yr | Sitting Duration, h | Location | Outcomes |
|---|---|---|---|---|
|
Peripheral hemodynamics
| ||||
| Credeur et al. (36) | M and F, 26 ± 7 (n = 20) | 3 | Posterior tibial artery | ↓ Mean shear rate ↓ Oscillatory shear index |
| Horiuchi et al. (37) | M and F, 22 ± 4 (n = 20) | 3 | Popliteal artery | ↓ Blood flow |
| Hou et al. (13) | M, 23 ± 2 (n = 5) | 0.5 | External iliac artery | ↑ Oscillatory shear index ↓ Retrograde flow ↓ Shear stress |
| Park et al. (8) | M and F, 24 ± 2 (n = 14) | 2.5 | Common femoral artery | ↓ Mean shear rate ↓ Blood flow |
| Restaino et al. (38) | M, 27 ± 1 (n = 11) | 6 | Popliteal artery | ↓ Mean blood flow ↓ Mean blood velocity ↓ Mean shear rate |
| Restaino et al. (39) | M, 26 ± 1 (n = 10) | 3 | Popliteal artery | ↓ Mean shear rate ↓ Blood flow |
| Shvartz et al. (40) | M, 25 (n = 8) | 5 | Thigh and calf | ↔ Thigh blood flow ↓ Calf blood flow |
| Thosar et al. (35) | M, 24 ± 4 (n = 12) | 3 | Superficial femoral artery | ↓ Antegrade shear rate ↓ Mean shear rate ↔ Retrograde shear rate |
| Thosar et al. (21) | M, 24 ± 4 (n = 12) | 3 | Superficial femoral artery | ↓ Antegrade shear rate ↓ Mean shear rate ↔ Retrograde shear rate |
| Walsh et al. (11) | M and F, 26 ± 1 (n = 12) | 3 | Popliteal artery | ↓ Mean shear rate ↓ Antegrade shear rate ↑ Retrograde shear rate |
| Macrovascular function | ||||
| Barone Gibbs et al. (41) | M and F, 42 ± 12 (n = 25) | 10 | Peripheral and central PWV | ↔ Carotid-femoral PWV ↔ Carotid-radial PWV ↑ Carotid-ankle PWV |
| Climie et al. (25) | M and F, 57 ± 12 (n = 19) | 5 | Superficial femoral artery FMD | ↓ Superficial femoral artery FMD |
| Credeur et al. (36) | M and F, 26 ± 7 (n = 20) | 3 | Posterior tibial artery FMD and central PWV | ↑ Carotid-femoral PWV ↓ Posterior tibial FMD |
| Evans et al. (42) | M and F, 22 ± 3 (n = 20) | 3 | Central PWV | ↔ Carotid-femoral PWV |
| Headid et al. (7) | M and F, 22 ± 2 (n = 12) | 2.5 | Popliteal artery FMD, peripheral and central PWV | ↓ Popliteal artery FMD ↔ Carotid-femoral PWV ↔ Carotid-radial PWV ↔ Carotid-distal PWV |
| Morishima et al. (22) | M and F, 26 ± 1 (n = 11) | 3 | Popliteal artery FMD | ↓ Popliteal artery FMD |
| Morishima et al. (15) | M and F, 27 ± 1 (n = 15) | 3 | Popliteal artery FMD | ↓ Popliteal artery FMD |
| O'Brien et al. (43) | M and F, 24 ± 3 (n = 20) | 3 | Popliteal artery FMD | ↓ Popliteal artery FMD |
| Park et al. (8) | M and F, 24 ± 2 (n = 14) | 2.5 | Popliteal artery FMD, peripheral and central PWV | ↓ Popliteal artery FMD ↔ Carotid-femoral PWV ↔ Carotid-radial PWV ↔ Carotid-ankle PWV ↔ Femoral-ankle PWV |
| Restaino et al. (38) | M, 27 ± 1 (n = 11) | 6 | Popliteal artery FMD | ↓ Popliteal artery FMD |
| Restaino et al. (39) | M, 26 ± 1 (n = 10) | 3 | Popliteal artery FMD | ↓ Popliteal artery FMD |
| Taylor et al. (24) | M and F, 62 ± 8 (n = 24) | 7 | Femoral artery FMD | ↔ Femoral artery FMD |
| Thosar et al. (35) | M, 24 ± 4 (n = 12) | 3 | Superficial femoral artery FMD | ↓ Superficial femoral artery FMD |
| Thosar et al. (21) | M, 24 ± 4 (n = 12) | 3 | Superficial femoral artery FMD | ↓ Superficial femoral artery FMD |
| Microvascular function | ||||
| Clime et al. (25) | M and F, 57 ± 12 (n = 19) | 5 | Femoral artery reactive hyperemia | ↔ Shear rate AUC |
| Credeur et al. (36) | M and F, 26 ± 7 (n = 20) | 3 | Posterior tibial artery reactive hyperemia | ↓ Posterior tibial artery AUC |
| Decker et al. (44) | M and F, 24 ± 4 (n = 26) | 1.5 | Passive leg movement | ↓ Common femoral artery peak blood flow ↔ Common femoral artery blood flow AUC |
| Garten et al. (23) | M and F, 57 ± 1 (n = 10) | 3 | Passive leg movement | ↓ Common femoral artery blood flow AUC |
| Headid et al. (7) | M and F, 22 ± 2 (n = 12) | 2.5 | Medial gastrocnemius reactive hyperemia | ↓ Tissue oxygenation index recovery rate |
| Horiuchi et al. (45) | M and F, 21 ± 2 (n = 20) | 3 | Medial gastrocnemius reactive hyperemia | ↓ Tissue oxygenation index AUC ↓ Tissue oxygenation index recovery rate |
| Kurosawa et al. (46) | M, 23 ± 1 (n = 9) | 8 | Lateral gastrocnemius tissue oxygenation | ↓ Oxygenated hemoglobin ↓ Total hemoglobin |
| O'Brien et al. (43) | M and F, 24 ± 3 (n = 20) | 3 | Popliteal artery reactive hyperemia | ↓ Shear rate AUC |
| Park et al. (8) | M and F, 24 ± 2 (n = 14) | 2.5 | Medial gastrocnemius reactive hyperemia | ↓ Tissue oxygenation index recovery rate |
| Restaino et al. (38) | M, 27 ± 1 (n = 11) | 6 | Popliteal artery reactive hyperemia | ↓ Popliteal mean blood flow AUC ↓ Popliteal artery shear AUC |
| Restaino et al. (39) | M, 26 ± 1 (n = 10) | 3 | Popliteal artery reactive hyperemia | ↓ Blood flow AUC ↓ Shear rate AUC |
| Vranish et al. (47) | M and F, 23 ± 4 (n = 18) | 3 | Popliteal artery reactive hyperemia | ↓ Popliteal artery blood velocity AUC |
| Vasoactive molecules | ||||
| Ballard et al. (48) | M, 21 ± 2 (n = 11) | 3 | Plasma oxidant status and endothelin-1 | ↔ Malondialdehyde ↔ Endothelin-1 |
| Climie et al. (25) | M and F, 57 ± 12 (n = 19) | 5 | NO bioavailability, endothelin-1, and inflammatory makers | ↑ Endothelin-1 |
| ↔ Plasma nitrate/nitrite ↔ Intracellular adhesion molecule-1 ↔ Vascular cell adhesion molecule-1 | ||||
| Decker et al. (44) | M and F, 24 ± 4 (n = 26) | 1.5 | Whole blood superoxide | ↔ Superoxide concentration |
| Evans et al. (49) | M and F, 22 ± 3 (n = 20) | 3 | Endothelial microparticles, circulating angiogenic cells, endothelin-1 | ↓ Endothelial microparticles ↔ Circulating angiogenic cells ↔ Endothelin-1 |
| Garten et al. (23) | M and F, 57 ± 1 (n = 10) | 3 | Plasma oxidant status and inflammatory markers | ↔ Malondialdehyde ↔ Superoxide dismutase ↔ Interleukin-6 |
| Park et al. (8) | M and F, 24 ± 2 (n = 14) | 2.5 | NO bioavailability and endothelin-1 | ↓ Total plasma nitrate/nitrite ↔ Endothelin-1 ↓ Total plasma nitrate/nitrite to endothelin-1 ratio |
| Taylor et al. (24) | M and F, 62 ± 8 (n = 24) | 7 | Endothelin-1 | ↔ Endothelin-1 |
AUC, area under the curve; F, female; FMD, flow-mediated dilation; M, male; PWV, pulse-wave velocity. ↑, increase; ↓, decrease; ↔, no change.
Our group and others suggested that prolonged sitting-mediated increases in venous pooling may be a potential target for preventing these hemodynamic disturbances (7, 8, 37–40), but recent work has revealed that addressing venous pooling alone is not sufficient for hemodynamic preservation (37). Horiuchi and Stoner (37) performed a study that prevented sitting-induced venous pooling with compression stockings, but these stockings were not capable of averting sitting-induced reductions in leg arterial blood flow. This indicates that methods should be directly targeted at improving arterial hemodynamics rather than reducing venous pooling alone. Of note, our group and others have used intermittent active skeletal muscle contractions to prevent sitting-induced alterations in hemodynamics during prolonged sitting (8, 21, 22, 24, 25). Morishima et al. (22) showed that intermittent active skeletal muscle contractions (i.e., “fidgeting” 1 min on/4 min off) could produce higher levels of mean popliteal artery shear rate when compared with the nonfidgeting control limb during 3 h of prolonged sitting (Fig. 1B). We hypothesize that a key contributing mechanism underlying these protective effects is muscle afferent activation. Group III (mechanosensitive) and group IV (metabosensitive) afferents are the major mechanisms that stimulate central and peripheral cardiovascular responses during skeletal muscle contraction (50, 51). Previous human studies have used passive leg movements to activate group III afferents and active leg movements to activate both group III and IV afferents (52, 53). We recently applied both passive and active leg movement stimuli to break up prolonged sitting in healthy young adults, and we found that active leg movements could preserve arterial hemodynamics while passive leg movements only could induce modest protective effects (8). We concluded that group IV afferent activation, prompted by the metabolites produced during active muscular contractions, is required to preserve arterial hemodynamics during prolonged sitting. However, investigations regarding the contribution of local skeletal muscle metabolites (e.g., potassium, adenosine, hydrogen ions, etc.), by methods such as intramuscular metabolite injection with postexercise circulatory occlusion (PECO), are warranted to fully confirm group IV afferent activation.
Although most of the sitting literature primarily attributes hemodynamic alterations to the hypothesized “bent artery” morphology, we and others have also proposed that activation of muscle sympathetic nerve activity (MSNA) may be an interesting contributing mechanism (7, 8, 10, 21). Postural changes from the supine to the seated position have been reported to increase MSNA in adults (54), and Padilla et al. (55) showed that increases in leg MSNA are associated with perturbations in shear rate patterns, such as greater retrograde shear rate and oscillatory shear index. Considering the relationship between MSNA with the seated posture and hemodynamic alterations, we and others have speculated that increased MSNA may play a role in hemodynamic disturbances, reduced leg blood flow, and increased peripheral vascular resistance during prolonged sitting (7, 8, 10, 38). Interestingly, low-intensity skeletal muscle contractions by cycling have been shown to reduce MSNA (56). This may imply that utilizing intermittent low-intensity skeletal muscle contractions during prolonged sitting may increase laminar shear stress and/or reduce turbulent flow, retrograde flow, and the contribution of MSNA during sitting, which may concomitantly protect against sitting-induced hemodynamic disturbances (56). Future investigations should incorporate use of MSNA and lower extremity hemodynamics to understand the complex interplay of these factors during prolonged sitting both with and without intermittent skeletal muscle contractions, and the intensity of the intermittent skeletal muscle contractions, similar to the fidgeting, calf raises, and cycling from previous work, should be considered (8, 22, 42). In addition to MSNA, future studies should consider investigating cardiovagal baroreflex sensitivity, which may give additional insight into autonomic nervous system function. O’Brien et al. (57) demonstrated that breaking up sedentary time is a stronger predictor of cardiovagal baroreflex sensitivity than total sedentary time alone, which may make cardiovagal baroreflex sensitivity an important addition to understanding prolonged sitting with and without activity breaks on autonomic regulation.
IMPACTS OF PROLONGED SITTING ON MACROVASCULAR FUNCTION
The macrocirculation includes large arteries and veins, which transport and distribute blood throughout the body. The major functional components of these vessels include the endothelium and the smooth muscle. Under normal physiological conditions, the endothelium responds to hemodynamic alterations and produces vasoactive signaling molecules, such as NO and ET-1 among others, which act on both the endothelium and the smooth muscle to regulate vascular tone (i.e., vasodilation and vasoconstriction) (26, 27, 58). Endothelial and smooth muscle structure and function are known to be altered in disease conditions, such as atherosclerosis, which can severely compromise macrovascular homeostasis.
Although the mechanisms underlying macrovascular dysfunction are not well established, endothelial dysfunction is often characterized by vasoactive molecule dysregulation, excessive ROS, and upregulated inflammatory signaling cascades (59). Furthermore, these factors may also induce structural and functional changes at the level of the vascular smooth muscle such as calcification, changes in extracellular matrix proteins (elastin and collagen), and reduced arterial compliance (60). Even though these changes in the smooth muscle are oftentimes chronic and manifest over several years, alterations in endothelial function can be detected before evidence of atherosclerotic lesions, which suggests that endothelial dysfunction may be a key contributor to the early development of atherosclerotic diseases (61–63). Interestingly, acute bouts of prolonged sitting just a few hours in duration have been found to impair macrovascular endothelial function, most particularly in the lower extremities (7, 8, 21, 22, 35, 36, 38, 39, 43) (Table 1). Considering that endothelial dysfunction has been classically referred to as a “hallmark” of cardiovascular diseases and atherosclerosis, we hypothesize that persistent exposure to sitting-induced attenuations in macrovascular endothelial function may be a factor driving the relationship between sitting time and cardiovascular disease development (5, 6).
Flow-mediated dilation (FMD) is considered the “gold standard” noninvasive assessment for endothelial function, which has been the primary assessment used by the prolonged sitting literature to evaluate macrovascular endothelial function in the upper and lower extremities. Briefly, FMD is performed using a Doppler ultrasound system to assess the change in arterial diameter (% change from initial baseline diameter) in response to an increase in arterial shear stress following a short period of arterial occlusion (i.e., reactive hyperemia) (64, 65). We and others have consistently shown that an acute bout of prolonged sitting attenuates FMD in the superficial femoral artery, popliteal artery, and posterior tibial artery (7, 8, 15, 22, 36, 38, 39, 43). It is hypothesized that these sitting-induced impairments in endothelial function may render the lower extremities more susceptible to atherosclerosis, especially when considering that individuals spend a substantial amount of their waking hours in the seated position (1–4, 10).
Impaired local hemodynamic-induced changes in circulating molecules and cellular signaling factors have been commonly proposed as key contributors to sitting-induced attenuations in lower extremity endothelial function (7, 8, 22, 35, 38, 39). As discussed, the seated posture is associated with greater amounts of low and oscillatory shear rates in the lower extremity conduit arteries (14, 21, 35, 36, 38, 39, 66). Previous work has shown that oscillatory and low shear stress can increase endothelial cell-derived ROS and downregulate endothelial nitric oxide synthase (eNOS) expression and NO production (29, 30, 67). On the contrary, laminar shear stress can theoretically oppose these effects by stimulating upregulation of endothelial antioxidant mechanisms, eNOS-derived NO production, and reductions in vasoconstrictor and pro-inflammatory molecules (67–71). Human in vivo work has been in support of these factors, as experimentally induced increases in brachial artery oscillatory and retrograde shear rates (by forearm cuff compression of 25–75 mmHg) have been shown to attenuate conduit artery endothelial function, likely due to imbalances in endothelial-derived vasoactive substances and redox-related mechanisms (72, 73). Therefore, determining methods that produce favorable flow and shear stress profiles during prolonged sitting may serve to protect against sitting-induced decrements in macrovascular endothelial function, which may provide additional insight into shear-mediated mechanisms, such as changes in molecular and signaling factors, underlying endothelial dysfunction.
Investigations have primarily used local heating and sitting interruptions with intermittent active muscular contractions to increase local muscular metabolism and upregulate favorable shear stress profiles that ultimately preserve macrovascular endothelial function during prolonged sitting (8, 21, 22, 24, 25, 39). For instance, we revealed that intermittent active muscular contraction is required to protect macrovascular endothelial function during prolonged sitting, which may be partially due to upregulated local muscular metabolism and arterial shear stress (Fig. 2A), with reduced MSNA or improvements in cardiovagal baroreflex sensitivity also being potential contributing factors (8). However, the mechanisms underlying the protective effects of increased mean and antegrade shear rates on endothelial function have remained poorly investigated. Padilla and Fadel (10) suggested that increases in ET-1 concentrations may be a key contributor to sitting-induced reductions in endothelial function. They hypothesized that increased ET-1 concentrations may upregulate ROS and reduce total plasma nitrate and nitrite, markers of NO bioavailability, and they proposed that these ET-1 concentrations can be reduced when the endothelium is exposed to laminar shear stress (10, 74). In support of this notion, Climie et al. (25) found that resistance exercises performed for 3 min every 30 min during prolonged sitting can attenuate sitting-induced increases in ET-1 concentrations, but no changes in plasma NO bioavailability were noted. In agreement with their findings, we found that active pedaling on an under-desk foot elliptical for 2 min every 30 min during prolonged sitting can reduce ET-1 concentrations but does not change NO bioavailability (8). Furthermore, we reported that these reductions in ET-1 produced a favorable shift in the vasodilator-to-vasoconstrictor balance when compared with control prolonged sitting (no muscular contractions) (8). We hypothesized that intermittent increases in mean arterial shear rate allowed for the preservation of an antiatherogenic phenotype (i.e., preserved vasodilator-to-vasoconstrictor balance). Collectively, and in support of the hypothesis by Padilla and Fadel (10), these findings suggest that increased ET-1 concentrations and vasoactive substance imbalances may be key mechanisms underlying sitting-induced attenuations in macrovascular endothelial function during prolonged sitting (8, 10, 25).
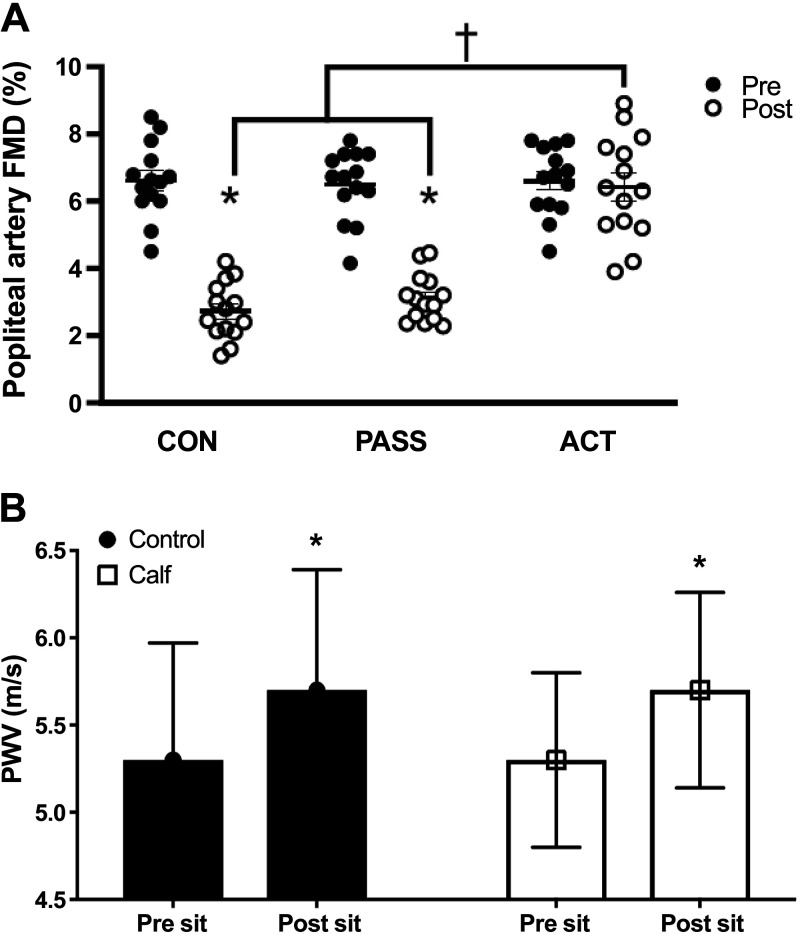
Figure 2.
Measurements of popliteal artery flow-mediated dilation (FMD) pre- and postprolonged sitting in a control group (CON, no movement), passive leg movement group (PASS, passive leg movements every 30 min for 2 min at 1 Hz), and active leg movement group (ACT, active leg movements every 30 min for 2 min at 1 Hz at a 13-W workload) and central arterial stiffness [assessed by carotid-to-femoral pulse-wave velocity (PWV)] in response to 3 h of prolonged sitting both with and without intermittent active skeletal muscle contractions (i.e., 10 calf raises at 0.33 Hz every 10 min). A: FMD decreased after sitting in CON and PASS but was preserved in ACT. n = 14, *P < 0.05 vs. Pre, †P < 0.05 vs. ACT. B: carotid-to-femoral PWV (m/s) increased after sitting in both conditions. n = 20, *P < 0.05 vs. Pre sit. [Adapted and reused with permission from Park et al. (8) and Evans et al. (42).]
In addition to sitting-induced changes in macrovascular endothelial function, it is important to consider acute changes in arterial compliance. Arterial stiffness, although a chronic manifestation that is attributed to a large array of pathological sequela (60), has been assessed by pulse-wave velocity (PWV) in response to acute prolonged sitting. Carotid-to-femoral PWV is considered the noninvasive “gold standard” assessment of central arterial stiffness in the field of cardiovascular physiology (75). Carotid-to-femoral PWV has been reported to acutely increase (∼ Δ 0.3–0.4 m/s) after 3 h of prolonged sitting in young adults (Fig. 2B) (36, 42), and carotid-to-ankle PWV, an indicator of peripheral arterial stiffness, has been shown to increase during prolonged sitting in overweight/obese adults with elevated blood pressure (41) (Table 1). These studies suggested that these increases in PWV may be rather trivial (36, 41, 42), as changes in PWV ≥ 1.0 m/s may be required to be considered a clinically impactful change (76). However, it has been hypothesized that repetitive exposure to these modest increases in PWV may pose potential cardiovascular consequences (36). Even though we tend to agree with this hypothesis, it is unlikely that structural changes occur due to an acute bout of prolonged sitting, which makes investigation of moment-to-moment changes in potential mechanisms that may have an immediate impact on PWV necessary to understand how prolonged sitting may acutely affect PWV. Future investigations assessing arterial compliance should consider that central and peripheral PWV assessment may be sensitive to these potential changes in MSNA, hemodynamics, blood pressure, and peripheral vascular resistance, which may be of interest to include in forthcoming work.
IMPACTS OF PROLONGED SITTING ON MICROVASCULAR FUNCTION
The primary role of the skeletal muscle microcirculation is to regulate blood flow and oxygen delivery to meet the local metabolic demand (77–80). These processes rely on the complex interplay of the skeletal muscle resistance vessels (i.e., feed arteries and arterioles) and capillary networks. The resistance vessel endothelium responds to biochemical signals to regulate vascular tone and resultant blood flow to the tissue, and capillary networks make appropriate adjustments via recruitment and distension according to local metabolism. Aging and disease negatively impact these regulatory functions of the skeletal muscle microcirculation, and the potential mechanisms by which this dysregulation occurs are multifaceted and poorly understood, with some proposed factors including low-grade inflammation upregulated pro-oxidative signaling cascades (16, 81, 82). We and others hypothesize that repetitive exposure to transient proatherogenic environments, such as prolonged sitting, may foster these unfavorable environments and play a role in impairments in microvascular function and the pathobiology of lower-extremity atherosclerosis (7, 8, 83).
The majority of the prolonged sitting literature has focused on alterations in macrovascular endothelial function, but investigations have shown recent interest in the microcirculation. The microcirculatory response to prolonged sitting has been assessed both indirectly and directly with Doppler ultrasound and near-infrared spectroscopy (NIRS), respectively. Doppler ultrasound has been used to quantify the area under the curve (AUC) for mean blood flow and blood velocity in response to reactive hyperemia in conduit arteries, and this work has demonstrated that microvascular reactivity is blunted after 3–6 h of prolonged sitting in the upper and lower extremities (36, 38, 39, 43, 47, 83) (Table 1). Consistent with these findings, investigations assessing microvascular reactivity with Doppler ultrasound during passive leg movement have revealed that microvascular impairments are present after 1.5–3 h of prolonged sitting (23, 44) (Table 1). Continuous-wave NIRS, which uses near-infrared light to quantify tissue oxygenation status, has been used by our group and others to directly assess the leg microcirculation in response to prolonged sitting (7, 8, 45, 46) (Table 1). These studies revealed impairments in tissue oxygenation index (TOI) recovery rate and TOI AUC during reactive hyperemia after 2.5–3 h of sitting (7, 8, 45) and reductions in oxygen delivery to the periphery throughout 8 h of sitting (46).
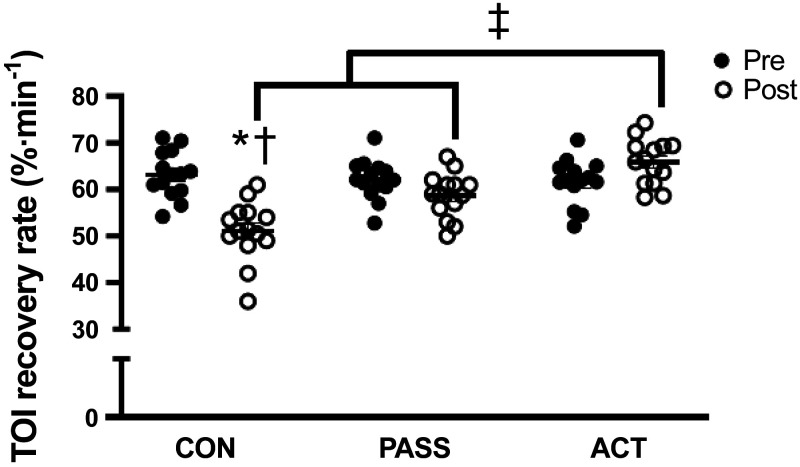
Our group and others have used active and passive skeletal muscle contractions before, during, and after prolonged sitting in the attempt to prevent or reverse sitting-induced microvascular impairments. Methods such as using a high-intensity exercise session before prolonged sitting (3 h) (23) and a 10-min walk (∼1,000 steps) after prolonged sitting (6 h) (38) have been shown to prevent and reverse sitting-induced reductions in microvascular function, respectively. In addition, interrupting prolonged sitting (3 h) with modified squats can protect microvascular function as assessed by the TOI recovery slope and AUC (45). Similarly, we found that interrupting prolonged sitting (2.5 h) with active pedaling on an under-desk foot elliptical can protect the TOI recovery slope (Fig. 3) (8). Furthermore, and unique to our work, we found that intermittent passive leg movements using the same under-desk foot elliptical during prolonged sitting (2.5 h) can also salvage the TOI recovery slope (Fig. 3) (8).
Figure 3.
Measurements of microvascular function [tissue oxygenation index (TOI) recovery rate, % min−1] pre- and postprolonged sitting in a control group (CON, no movement), passive leg movement group (PASS, passive leg movements every 30 min for 2 min at 1 Hz), and active leg movement group (ACT, active leg movements every 30 min for 2 min at 1 Hz at a 13 W workload). TOI recovery rate was lower after sitting in CON, and post-CON was less than post-PASS and post-ACT. Post-ACT was greater than post-CON and post-PASS. n = 14, *P < 0.05 vs. Pre, †P < 0.05 vs. PASS, ‡P < 0.05 vs. ACT. [Adapted and reused with permission from Park et al. (8).]
We and others have hypothesized that the protective effects of passive and active muscular contractions on the microcirculation during prolonged sitting are likely due to the complex interplay of several mechanisms, such as favorable shifts in hemodynamic profiles that preserve circulating vasoactive metabolite and redox homeostasis (8, 23, 38, 45). Interestingly, the roles of hemodynamic profiles and vasoactive metabolites show some differences between passive and active muscular contractions. In our previous work, there was a detectable increase in mean arterial shear rate during passive movement bouts, which likely played a role in protecting microvascular function (8). Despite the level of microvascular protection supplied by passive leg movements, active leg movements provided a greater amount (8). This is speculated to be due to the contribution of skeletal muscle metabolism and central hemodynamics (increased blood pressure, heart rate, contractility, etc.) that provoked a more robust increase in mean arterial shear rate compared with passive leg movement, and this greater increase in shear rate is the likely mechanism that preserved circulating NO bioavailability and the vasodilator-to-vasoconstrictor substance balance (8). In line with these findings, Climie et al. (25) found that breaking up prolonged sitting with active muscular contraction can counteract sitting-induced increases in ET-1 concentration, which is a potent endothelial cell-produced vasoconstrictor, and Garten et al. (23) showed that a prophylactic aerobic exercise session preserved passive leg movement-induced hyperemia, which is indicative of maintained NO bioavailability. Interestingly, Garten et al. also noted that both conditions in their study (control and prior exercise bout) were not accompanied by changes in plasma oxidant status, suggesting that oxidative stress may not play an immediate role in sitting-induced attenuations in microvascular function (23). Collectively, these studies suggest that increasing local skeletal muscle metabolism that prompts the release of vasodilators plays an important role in supporting microvascular function and promoting an otherwise antiatherogenic environment (8, 84, 85).
Unfortunately, noninvasive assessments of the microcirculation pose several experimental considerations that must be acknowledged. The conduit artery AUC response (blood velocity and/or flow) to postocclusive reactive hyperemia has been commonly used as a surrogate marker of microvascular function (36, 38, 39, 43, 47, 83), but the analysis process can be extensive with a large margin for error depending on access to edge-detection software and sonography experience (86). Furthermore, the lack of consensus for the most reliable methodological approach for conduit artery AUC (i.e., peak or mean values, normalized values, etc.) poses additional experimental considerations (86). The passive leg movement technique has been used frequently to quantify microvascular function in both healthy and clinical populations (23, 44, 87). The assessment is also considered less technically challenging, thus making it a more attractive method for investigators with less sonography experience and limited software access (87). However, it is important to note that assessments of conduit artery AUC and passive leg movement-induced hyperemia indirectly quantify microvascular reactivity. As such, direct assessments of the microcirculation should be considered for future work. We and others have used NIRS to assess the microvascular response to postocclusive reactive hyperemia and have reported the reperfusion slope (i.e., TOI recovery slope) and TOI AUC (7, 8, 45). Although these have been well-accepted metrics for NIRS, the signals can be altered by adipose tissue thickness, which should be used as a covariate when performing data analyses in future investigations (86). Furthermore, other techniques such as microdialysis, laser Doppler flowmetry, and/or optical coherence tomography should be used in forthcoming studies to provide a more comprehensive and direct assessment of the microcirculation.
IMPACT OF PROLONGED SITTING ON VASOACTIVE MOLECULES
To date, the sitting literature has primarily focused on functional assessments of systemic vascular function. Despite the insight provided by these studies, the contribution of molecular mechanisms to these functional deficits remains relatively underexplored. These aspects may be important to consider, as atherosclerosis is associated with alterations in vasoactive metabolites as well as upregulation of proinflammatory and pro-oxidative signaling cascades. Information regarding how prolonged sitting may impact these molecular factors may provide a better understanding of how the hypothesized transient proatherogenic environment is generated by prolonged sitting (7, 8, 83).
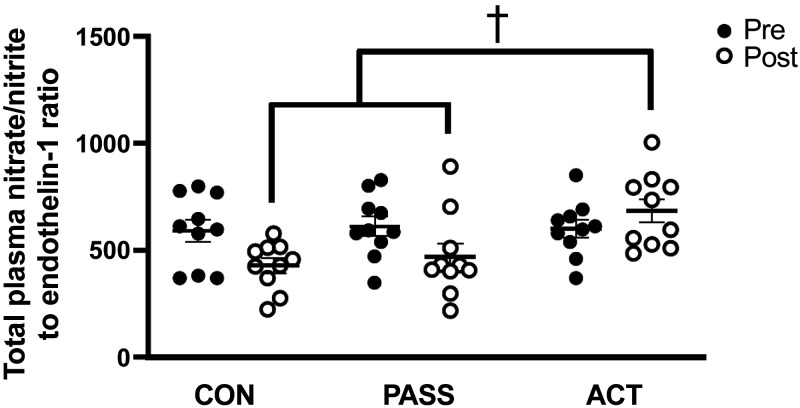
We and others have investigated circulating biomarkers from whole blood and plasma samples in response to prolonged sitting, which have included assessments of oxidant status, inflammatory molecules, vasoactive metabolites, endothelial microparticles, and adhesion molecules (Table 1). Thosar et al. (88) and Decker et al. (44) used antioxidant intake (vitamin C, 1,000–1,500 mg) to investigate the potential role(s) of acute oxidative stress during prolonged sitting for 1.5–3 h. They noted that vitamin C intake can ablate sitting-induced alterations in lower extremity microvascular function in males but not in females (44, 88). However, no changes in whole blood superoxide concentrations were noted in response to sitting with or without vitamin C intake (44). Furthermore, studies investigating prolonged sitting (3 h) with and without prior exercise bouts have shown no alterations in markers of plasma redox status (malondialdehyde and superoxide dismutase) or inflammation (interleukin-6) in healthy young males (23, 48). On the contrary, studies investigating circulating vasoactive metabolites during prolonged sitting have shown some inconsistent results in healthy and clinical populations. We and Climie et al. (25) reported that ET-1 concentrations rise during prolonged sitting (5 h) and total plasma nitrate/nitrite levels are lower after prolonged sitting (2.5 h) (8, 25), which resulted in an unfavorable shift in vasoactive metabolite balance (Fig. 4) (8), but Evans et al. (49) and Taylor et al. (24) showed no changes in ET-1 concentrations during prolonged sitting (3–7 h) regardless of intermittent skeletal muscle contractions. In addition, other work has revealed that prolonged sitting (3 h) reduces levels of circulating endothelial microparticles independent of interruptions with active skeletal muscle contractions (49), whereas vascular and cellular adhesion molecules remain unaltered during prolonged sitting (5 h) both with and without sitting interruptions (25).
Figure 4.
Measurements of total plasma nitrate/nitrite to endothelin-1 ratio pre- and postprolonged sitting in a control group (CON, no movement), passive leg movement group (PASS, passive leg movements every 30 min for 2 min at 1 Hz), and active leg movement group (ACT, active leg movements every 30 min for 2 min at 1 Hz at a 13-W workload). Total plasma nitrate/nitrite to endothelin-1 ratio was lower in post-CON and post-PASS when compared with post-ACT. n = 14, †P < 0.05 vs. PASS. [Adapted and reused with permission from Park et al. (8).]
With the limited amount of literature on circulating biomarkers in response to prolonged sitting, there remains a substantial amount of uncertainty regarding their complex interplay. For instance, investigations have focused on markers of inflammation, plasma oxidant status, and vasoactive metabolites, but little work has been performed regarding upstream factors to these molecules. Future work should incorporate greater investigation of endothelial microparticles and vascular adhesion molecules (25, 89), which are known to be associated with elevated cardiovascular disease risk (90). In addition, investigation of other molecular factors may be of-interest, such as microRNAs and extracellular vesicles. Investigations of these factors may provide additional insight into alterations in cellular signaling that may ultimately affect downstream factors pertaining to inflammation, oxidative stress, and/or vasoactive metabolites.
Furthermore, production of these circulating factors in response to prolonged sitting may be altered due to several modifiable and nonmodifiable variables such as age, health status, dietary habits, and physical activity level. For example, healthy young populations often have intact homeostatic regulation even when exposed to low levels of acute physiological stress (e.g., prolonged sitting), which may make it difficult to detect any true changes in these circulating biomarkers. Age and health or disease status may inadvertently affect the pro- and antiatherosclerotic circulating factors that are often assessed in response to prolonged sitting. Although these concerns and concepts have been proposed in brief by our group and others (8, 23, 44, 48, 91), proper execution of these considerations in future studies is paramount to understanding sitting-induced changes in circulating biomarkers of inflammation, oxidative stress, and vascular function.
In conjunction with circulating biomarkers, to our knowledge, only one study has investigated the effects of breaking up prolonged sitting on skeletal muscle gene expression (vastus lateralis) in overweight/obese adults (92). They noted that interrupting prolonged sitting (5 h) with light- and moderate-intensity treadmill walking every 30 min supports increased expression of triglyceride and carbohydrate metabolism-related genes as well as genes that modulate anti-inflammatory and antioxidative cascades (92). Further use of skeletal muscle biopsies, such as biopsies downstream from the vastus lateralis, such as the gastrocnemius, should be included to further investigate the impacts of prolonged sitting on the expression of redox- and inflammatory-relevant genes and proteins, mitochondrial respiration and function, and antioxidant enzyme activity to better characterize prolonged sitting-induced molecular changes at the level of the skeletal muscle.
SEX DIFFERENCES AND OTHER CONSIDERATIONS
The role of sex as a biological variable in the prolonged sitting literature has remained a relatively underinvestigated area. Several of the earlier investigations that have provided a large amount of the groundwork in prolonged sitting research only included male participants (21, 35, 39, 88). Recent studies have sought to investigate sex differences, but they have shown some mixed results regarding peripheral macro- and microvascular function. After 3 h of prolonged sitting, Vranish et al. (47) reported that males demonstrated attenuated popliteal artery FMD compared with females, but O’Brien et al. (43) reported no differences between sexes. On the contrary, studies focusing on sex differences in the microcirculation have shown more consistencies in findings. Three studies reported that males and females exhibit similar reductions in microvascular function following 1.5–3 h of prolonged sitting (43, 44, 47). The authors of these studies as well as other reviews on prolonged sitting and cardiovasomobility have discussed some of these sex differences in detail pertaining to sex hormones, which may be an area of interest to explore further (10, 44, 47, 93). Regardless, future studies should power adequately to investigate potential sex differences, especially considering the recent urgency and priority placed on sex as a biological variable in cardiovascular research (94). As such, the age of menarche, menstrual cycle phases, hormonal contraceptive use, as well as other factors specific to female physiology need to be appropriately considered in the design of these studies (95).
Other potential confounding factors to consider in future investigations include habitual physical activity and overall physical fitness level. Short-term reductions in daily physical activity, such as reduced average daily step count for 5–7 days, can profoundly impact popliteal artery FMD, circulating endothelial microparticles, and even skeletal muscle citrate synthase activity (96, 97). Participants should maintain their normal physical activity levels throughout study enrollment, including average daily step count and planned exercise activities, with intense exercise avoided during the 24-h window before each experimental visit. Future work should consider reporting average daily step count (from accelerometers or commercially available fitness trackers) and physical fitness levels (such as true or estimated V̇o2max) and/or minutes of moderate-to-vigorous physical activity per week as a component of participant characteristics as we and others have done previously (7, 8, 44).
More adequately resembling day-to-day living conditions should be considered in future work. Most protocols have typically been performed following an overnight fast (7, 8, 21, 35, 42) or after a standardized breakfast (24, 25), whereas other studies have focused on the impacts of prolonged sitting in the postprandial state (prolonged sitting after a standardized meal or meal replacement for participants) on vascular function and metabolic hormone regulation (41, 98, 99). Future work should consider including a standardized meal before and/or snacks during sitting protocols to better reflect day-to-day living habits, especially when experiments last several hours.
Another aspect that should be considered to reflect day-to-day living conditions is the conditions of the investigational environment. Prolonged sitting generally occurs during work and leisure activities in spaces such as shared offices, conference rooms, lecture classrooms, and auditoriums. Oftentimes, these spaces are densely populated and not well ventilated, which causes carbon dioxide (CO2) produced by natural respiration to accumulate (100). In fact, these levels often climb to nearly four to five times that of normal atmospheric concentrations in these spaces (∼1,500 ppm CO2) in as little as 1–2 h, thus classifying them as “mild hypercapnic environments” (7, 8, 100). Acute exposure to mild hypercapnic conditions alone can place a substantial amount of stress on the cardiovascular and autonomic nervous systems, resulting in alterations in ventilation, heart rate, blood pressure, and hemodynamics. Although these cardiovascular and autonomic responses to a mild hypercapnic environment are otherwise “normal” and warranted to maintain homeostasis, they may generate an additional level of physiological stress that could intensify the negative effects of prolonged sitting on vascular function. We recently reported that a mild hypercapnic environment (∼1,500 ppm CO2) can intensify the negative effects of prolonged sitting on the peripheral circulation in healthy young adults, suggesting that individuals sitting in these conditions may endure greater cardiovascular consequences than the sitting literature has reported thus far (7, 8).
It is important to note that our previous prolonged sitting studies in mild hypercapnic environments were performed in a healthy young adult population (age: ∼ 20–26 yr) (7, 8). Our participants averaged ∼12,000 steps per day and were “recreationally active” (7, 8). This poses issues with the external validity of our findings, as other populations may be more susceptible to prolonged sitting in these environments. For example, according to the US Bureau of Labor Statistics in 2020, occupations in sectors such as office and administrative support, legal firms, and business and financial operations sit for >75% of their workdays and are on average ∼42–46 yr old (middle aged). Considering that sitting time has been identified as an independent risk factor for cardiovascular and metabolic diseases (5, 6), it is only logical to shift the investigational focus to populations who are frequently predisposed to prolonged sitting in these mild hypercapnic environments. Finally, future work should dive deeper into potential contributing mechanisms to sitting-induced alterations in vascular function in these populations to develop novel and efficacious strategies for vascular protection, which may reduce the likelihood of vascular dysfunction and the development of cardiovascular diseases in these at-risk populations.
CONCLUSIONS
Sitting time is an often-overlooked independent behavioral risk factor for the development of cardiovascular diseases and all-cause mortality (5, 6), and we hypothesize that the “bent artery” morphology associated with the seated position is a major initiator of sitting-induced changes in vascular function. With adults and children spending nearly 6–8 h per day in the seated position (1–4), it is important that our field focuses on interventions that are effective at targeting these functional changes. Within the past decade, the sitting literature has revealed that using intermittent skeletal muscle contractions can be effective in negating some of these sitting-induced impairments on the vasculature. However, several gaps in the literature persist. Future prolonged sitting research should 1) investigate other potential contributing mechanisms to sitting-induced vascular dysfunction and the protective mechanisms of muscular contraction (e.g., MSNA, skeletal muscle metabolites and activation of muscle afferents, smooth muscle function, skeletal muscle gene/protein expression and mitochondrial function, etc.), 2) carefully consider the investigational environment, as many prolonged sitting bouts occur in mild hypercapnic environments that further exacerbate prolonged sitting’s negative effects (7), and 3) focus on populations who may be more at risk for regular bouts of prolonged sitting (e.g., office and administrative support workers, legal professionals, sedentary middle-aged and older-aged individuals, etc.). These future investigations will allow for researchers to optimize therapeutic strategies that prevent sitting-induced vascular dysfunction in populations who may be more susceptible to prolonged sitting, which may play a role in averting metabolic and cardiovascular disease development.
GRANTS
This work was supported by the National Institutes of Health (NIH) Grants R01 HD106911-01A1 and R01 AG077803; NIH National Institute of General Medical Sciences, which funds the Great Plains IdeA-CTR Network (U54 GM115458); the University of Nebraska Collaboration Initiative Grant (32105); the NASA Nebraska Space Grant Fellowship; and the NASA Nebraska Space Grant (NNX15AI09H and 80NSSC20M0112).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
E.J.P., M.F.A., and S.-Y.P. prepared figures; E.J.P. and S.-Y.P. drafted manuscript; E.J.P., M.F.A., and S.-Y.P. edited and revised manuscript; E.J.P., M.F.A., and S.-Y.P. approved final version of manuscript.
ACKNOWLEDGMENTS
Graphical abstract image created with BioRender.com and published with permission.
REFERENCES
- 1. Gremaud AL, Carr LJ, Simmering JE, Evans NJ, Cremer JF, Segre AM, Polgreen LA, Polgreen PM. Gamifying accelerometer use increases physical activity levels of sedentary office workers. J Am Heart Assoc 7: e007735, 2018. doi: 10.1161/JAHA.117.007735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Healy GN, Winkler EA, Owen N, Anuradha S, Dunstan DW. Replacing sitting time with standing or stepping: associations with cardio-metabolic risk biomarkers. Eur Heart J 36: 2643–2649, 2015. doi: 10.1093/eurheartj/ehv308. [DOI] [PubMed] [Google Scholar]
- 3. Pate RR, Mitchell JA, Byun W, Dowda M. Sedentary behaviour in youth. Br J Sports Med 45: 906–913, 2011. doi: 10.1136/bjsports-2011-090192. [DOI] [PubMed] [Google Scholar]
- 4. Matthews CE, Chen KY, Freedson PS, Buchowski MS, Beech BM, Pate RR, Troiano RP. Amount of time spent in sedentary behaviors in the United States, 2003-2004. Am J Epidemiol 167: 875–881, 2008. doi: 10.1093/aje/kwm390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Henschel B, Gorczyca AM, Chomistek AK. Time spent sitting as an independent risk factor for cardiovascular disease. Am J Lifestyle Med 14: 204–215, 2020. doi: 10.1177/1559827617728482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Patel AV, Bernstein L, Deka A, Feigelson HS, Campbell PT, Gapstur SM, Colditz GA, Thun MJ. Leisure time spent sitting in relation to total mortality in a prospective cohort of US adults. Am J Epidemiol 172: 419–429, 2010. doi: 10.1093/aje/kwq155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Headid RJ, Pekas EJ, Wooden TK, Son W-M, Layec G, Shin J, Park S-Y. Impacts of prolonged sitting with mild hypercapnia on vascular and autonomic function in healthy recreationally active adults. Am J Physiol Heart Circ Physiol 319: H468–H480, 2020. doi: 10.1152/ajpheart.00354.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Park SY, Wooden TK, Pekas EJ, Anderson CP, Yadav SK, Slivka DR, Layec G. Effects of passive and active leg movements to interrupt sitting in mild hypercapnia on cardiovascular function in healthy adults. J Appl Physiol (1985) 132: 874–887, 2022. doi: 10.1152/japplphysiol.00799.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Paterson C, Fryer S, Zieff G, Stone K, Credeur DP, Barone Gibbs B, Padilla J, Parker JK, Stoner L. The effects of acute exposure to prolonged sitting, with and without interruption, on vascular function among adults: a meta-analysis. Sports Med 50: 1929–1942, 2020. doi: 10.1007/s40279-020-01325-5. [DOI] [PubMed] [Google Scholar]
- 10. Padilla J, Fadel PJ. Prolonged sitting leg vasculopathy: contributing factors and clinical implications. Am J Physiol Heart Circ Physiol 313: H722–H728, 2017. doi: 10.1152/ajpheart.00326.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Walsh LK, Restaino RM, Martinez-Lemus LA, Padilla J. Prolonged leg bending impairs endothelial function in the popliteal artery. Physiol Rep 5: e13478, 2017. doi: 10.14814/phy2.13478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thosar SS, Johnson BD, Johnston JD, Wallace JP. Sitting and endothelial dysfunction: the role of shear stress. Med Sci Monit 18: RA173–RA180, 2012. doi: 10.12659/msm.883589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hou J, Li X, Li Z, Yin L, Chen X, Liang F. An in vivo data-based computational study on sitting-induced hemodynamic changes in the external iliac artery. J Biomech Eng 144: 021007, 2022. doi: 10.1115/1.4052292. [DOI] [PubMed] [Google Scholar]
- 14. Padilla J, Sheldon RD, Sitar DM, Newcomer SC. Impact of acute exposure to increased hydrostatic pressure and reduced shear rate on conduit artery endothelial function: a limb-specific response. Am J Physiol Heart Circ Physiol 297: H1103–H1108, 2009. doi: 10.1152/ajpheart.00167.2009. [DOI] [PubMed] [Google Scholar]
- 15. Morishima T, Restaino RM, Walsh LK, Kanaley JA, Padilla J. Prior exercise and standing as strategies to circumvent sitting-induced leg endothelial dysfunction. Clin Sci (Lond) 131: 1045–1053, 2017. doi: 10.1042/CS20170031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Park SY, Pekas EJ, Anderson CP, Kambis TN, Mishra PK, Schieber MN, Wooden TK, Thompson JR, Kim KS, Pipinos II. Impaired microcirculatory function, mitochondrial respiration, and oxygen utilization in skeletal muscle of claudicating patients with peripheral artery disease. Am J Physiol Heart Circ Physiol 322: H867–H879, 2022. doi: 10.1152/ajpheart.00690.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Beckman JA, Duncan MS, Damrauer SM, Wells QS, Barnett JV, Wasserman DH, Bedimo RJ, Butt AA, Marconi VC, Sico JJ, Tindle HA, Bonaca MP, Aday AW, Freiberg MS. Microvascular disease, peripheral artery disease, and amputation. Circulation 140: 449–458, 2019. doi: 10.1161/CIRCULATIONAHA.119.040672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Heinen Y, Stegemann E, Sansone R, Benedens K, Wagstaff R, Balzer J, Rassaf T, Lauer T, Kelm M, Heiss C. Local association between endothelial dysfunction and intimal hyperplasia: relevance in peripheral artery disease. J Am Heart Assoc 4: e001472, 2015. doi: 10.1161/JAHA.114.001472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lim SS, Huang CC, Hsu PF, Lin CC, Wang YJ, Ding YZ, Liou TL, Wang YW, Huang SS, Lu TM, Chen JW, Chan WL, Lin SJ, Leu HB. Prolonged sitting time links to subclinical atherosclerosis. J Chin Med Assoc 85: 51–58, 2022. doi: 10.1097/JCMA.0000000000000672. [DOI] [PubMed] [Google Scholar]
- 20. Asfour M, Baskovski E, Esenboğa K, Kumbasar D. Association between lower extremity arterial disease and various sitting positions. Anatol J Cardiol 26: 180–188, 2022. doi: 10.5152/AnatolJCardiol.2021.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Thosar SS, Bielko SL, Mather KJ, Johnston JD, Wallace JP. Effect of prolonged sitting and breaks in sitting time on endothelial function. Med Sci Sports Exerc 47: 843–849, 2015. doi: 10.1249/MSS.0000000000000479. [DOI] [PubMed] [Google Scholar]
- 22. Morishima T, Restaino RM, Walsh LK, Kanaley JA, Fadel PJ, Padilla J. Prolonged sitting-induced leg endothelial dysfunction is prevented by fidgeting. Am J Physiol Heart Circ Physiol 311: H177–H182, 2016. doi: 10.1152/ajpheart.00297.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Garten RS, Scott MC, Zúñiga TM, Hogwood AC, Fralin RC, Weggen J. A prior high-intensity exercise bout attenuates the vascular dysfunction resulting from a prolonged sedentary bout. J Phys Act Health 16: 916–924, 2019. doi: 10.1123/jpah.2018-0568. [DOI] [PubMed] [Google Scholar]
- 24. Taylor FC, Dunstan DW, Homer AR, Dempsey PC, Kingwell BA, Climie RE, Owen N, Cohen ND, Larsen RN, Grace M, Eikelis N, Wheeler MJ, Townsend MK, Maniar N, Green DJ. Acute effects of interrupting prolonged sitting on vascular function in type 2 diabetes. Am J Physiol-Heart Circ Physiol 320: H393–H403, 2020. doi: 10.1152/ajpheart.00422.2020. [DOI] [PubMed] [Google Scholar]
- 25. Climie RE, Wheeler MJ, Grace M, Lambert EA, Cohen N, Owen N, Kingwell BA, Dunstan DW, Green DJ. Simple intermittent resistance activity mitigates the detrimental effect of prolonged unbroken sitting on arterial function in overweight and obese adults. J Appl Physiol (1985) 125: 1787–1794, 2018. [Erratum in J Appl Physiol (1985) 128: 1685, 2020]. doi: 10.1152/japplphysiol.00544.2018. [DOI] [PubMed] [Google Scholar]
- 26. Davies PF. Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat Clin Pract Cardiovasc Med 6: 16–26, 2009. doi: 10.1038/ncpcardio1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhou J, Li YS, Chien S. Shear stress-initiated signaling and its regulation of endothelial function. Arterioscler Thromb Vasc Biol 34: 2191–2198, 2014. doi: 10.1161/ATVBAHA.114.303422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 399: 601–605, 1999. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 29. Chao Y, Ye P, Zhu L, Kong X, Qu X, Zhang J, Luo J, Yang H, Chen S. Low shear stress induces endothelial reactive oxygen species via the AT1R/eNOS/NO pathway. J Cell Physiol 233: 1384–1395, 2018. doi: 10.1002/jcp.26016. [DOI] [PubMed] [Google Scholar]
- 30. Hosoya T, Maruyama A, Kang MI, Kawatani Y, Shibata T, Uchida K, Warabi E, Noguchi N, Itoh K, Yamamoto M. Differential responses of the Nrf2-Keap1 system to laminar and oscillatory shear stresses in endothelial cells. J Biol Chem 280: 27244–27250, 2005. [Erratum in J Biol Chem 280: 29988, 2005. Warabi, Eiji [added]; Noguchi, Noriko [added], and in J Biol Chem 282: 15312, 2007]. doi: 10.1074/jbc.M502551200. [DOI] [PubMed] [Google Scholar]
- 31. Kröger K, Kucharczik A, Hirche H, Rudofsky G. Atherosclerotic lesions are more frequent in femoral arteries than in carotid arteries independent of increasing number of risk factors. Angiology 50: 649–654, 1999. doi: 10.1177/000331979905000805. [DOI] [PubMed] [Google Scholar]
- 32. Morbiducci U, Kok AM, Kwak BR, Stone PH, Steinman DA, Wentzel JJ. Atherosclerosis at arterial bifurcations: evidence for the role of haemodynamics and geometry. Thromb Haemost 115: 484–492, 2016. doi: 10.1160/TH15-07-0597. [DOI] [PubMed] [Google Scholar]
- 33. Chatterjee S, Fisher AB. Mechanotransduction in the endothelium: role of membrane proteins and reactive oxygen species in sensing, transduction, and transmission of the signal with altered blood flow. Antioxid Redox Signal 20: 899–913, 2014. doi: 10.1089/ars.2013.5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Traub O, Berk BC. Laminar shear stress: mechanisms by which endothelial cells transduce an atheroprotective force. Arterioscler Thromb Vasc Biol 18: 677–685, 1998. doi: 10.1161/01.atv.18.5.677. [DOI] [PubMed] [Google Scholar]
- 35. Thosar SS, Bielko SL, Wiggins CC, Wallace JP. Differences in brachial and femoral artery responses to prolonged sitting. Cardiovasc Ultrasound 12: 50, 2014. doi: 10.1186/1476-7120-12-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Credeur DP, Miller SM, Jones R, Stoner L, Dolbow DR, Fryer SM, Stone K, McCoy SM. Impact of prolonged sitting on peripheral and central vascular health. Am J Cardiol 123: 260–266, 2019. doi: 10.1016/j.amjcard.2018.10.014. [DOI] [PubMed] [Google Scholar]
- 37. Horiuchi M, Stoner L. Effects of compression stockings on lower-limb venous and arterial system responses to prolonged sitting: a randomized cross-over trial. Vasc Med 26: 386–393, 2021. doi: 10.1177/1358863X20988899. [DOI] [PubMed] [Google Scholar]
- 38. Restaino RM, Holwerda SW, Credeur DP, Fadel PJ, Padilla J. Impact of prolonged sitting on lower and upper limb micro- and macrovascular dilator function. Exp Physiol 100: 829–838, 2015. doi: 10.1113/EP085238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Restaino RM, Walsh LK, Morishima T, Vranish JR, Martinez-Lemus LA, Fadel PJ, Padilla J. Endothelial dysfunction following prolonged sitting is mediated by a reduction in shear stress. Am J Physiol Heart Circ Physiol 310: H648–H653, 2016. doi: 10.1152/ajpheart.00943.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shvartz E, Gaume JG, White RT, Reibold RC. Hemodynamic-responses during prolonged sitting. J Appl Physiol Respir Environ Exerc Physiol 54: 1673–1680, 1983. doi: 10.1152/jappl.1983.54.6.1673. [DOI] [PubMed] [Google Scholar]
- 41. Barone Gibbs B, Kowalsky RJ, Perdomo SJ, Taormina JM, Balzer JR, Jakicic JM. Effect of alternating standing and sitting on blood pressure and pulse wave velocity during a simulated workday in adults with overweight/obesity. J Hypertens 35: 2411–2418, 2017. doi: 10.1097/HJH.0000000000001463. [DOI] [PubMed] [Google Scholar]
- 42. Evans WS, Stoner L, Willey Q, Kelsch E, Credeur DP, Hanson ED. Local exercise does not prevent the aortic stiffening response to acute prolonged sitting: a randomized crossover trial. J Appl Physiol (1985) 127: 781–787, 2019. doi: 10.1152/japplphysiol.00318.2019. [DOI] [PubMed] [Google Scholar]
- 43. O'Brien MW, Johns JA, Williams TD, Kimmerly DS. Sex does not influence impairments in popliteal endothelial-dependent vasodilator or vasoconstrictor responses following prolonged sitting. J Appl Physiol (1985) 127: 679–687, 2019. doi: 10.1152/japplphysiol.00887.2018. [DOI] [PubMed] [Google Scholar]
- 44. Decker KP, Feliciano PG, Kimmel MT, Hogwood AC, Weggen JB, Darling AM, Richardson JW, Garten RS. Examining sex differences in sitting-induced microvascular dysfunction: insight from acute vitamin C supplementation. Microvasc Res 135: 104147, 2021. doi: 10.1016/j.mvr.2021.104147. [DOI] [PubMed] [Google Scholar]
- 45. Horiuchi M, Stoner L. Macrovascular and microvascular responses to prolonged sitting with and without bodyweight exercise interruptions: a randomized cross-over trial. Vasc Med 27: 127–135, 2022. doi: 10.1177/1358863X211053381. [DOI] [PubMed] [Google Scholar]
- 46. Kurosawa Y, Nirengi S, Tabata I, Isaka T, Clark JF, Hamaoka T. Effects of prolonged sitting with or without elastic garments on limb volume, arterial blood flow, and muscle oxygenation. Med Sci Sports Exerc 54: 399–407, 2022. doi: 10.1249/MSS.0000000000002822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Vranish JR, Young BE, Kaur J, Patik JC, Padilla J, Fadel PJ. Influence of sex on microvascular and macrovascular responses to prolonged sitting. Am J Physiol Heart Circ Physiol 312: H800–H805, 2017. doi: 10.1152/ajpheart.00823.2016. [DOI] [PubMed] [Google Scholar]
- 48. Ballard KD, Duguid RM, Berry CW, Dey P, Bruno RS, Ward RM, Timmerman KL. Effects of prior aerobic exercise on sitting-induced vascular dysfunction in healthy men. Eur J Appl Physiol 117: 2509–2518, 2017. doi: 10.1007/s00421-017-3738-2. [DOI] [PubMed] [Google Scholar]
- 49. Evans WS, Hanson ED, Shill DD, Landers-Ramos RQ, Stoner L, Willey Q, Credeur DP, Prior SJ. Sitting decreases endothelial microparticles but not circulating angiogenic cells irrespective of lower leg exercises: a randomized cross-over trial. Exp Physiol 105: 1408–1419, 2020. [DOI] [PubMed] [Google Scholar]
- 50. McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol 224: 173–186, 1972. doi: 10.1113/jphysiol.1972.sp009887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Coote JH, Hilton SM, Perez-Gonzalez JF. The reflex nature of the pressor response to muscular exercise. J Physiol 215: 789–804, 1971. doi: 10.1113/jphysiol.1971.sp009498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Trinity JD, Amann M, McDaniel J, Fjeldstad AS, Barrett-O'Keefe Z, Runnels S, Morgan DE, Wray DW, Richardson RS. Limb movement-induced hyperemia has a central hemodynamic component: evidence from a neural blockade study. Am J Physiol Heart Circ Physiol 299: H1693–H1700, 2010. doi: 10.1152/ajpheart.00482.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Group III and IV muscle afferents contribute to ventilatory and cardiovascular response to rhythmic exercise in humans. J Appl Physiol (1985) 109: 966–976, 2010. doi: 10.1152/japplphysiol.00462.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ng AV, Johnson DG, Callister R, Seals DR. Muscle sympathetic nerve activity during postural change in healthy young and older adults. Clin Auton Res 5: 57–60, 1995. doi: 10.1007/BF01845500. [DOI] [PubMed] [Google Scholar]
- 55. Padilla J, Young CN, Simmons GH, Deo SH, Newcomer SC, Sullivan JP, Laughlin MH, Fadel PJ. Increased muscle sympathetic nerve activity acutely alters conduit artery shear rate patterns. Am J Physiol Heart Circ Physiol 298: H1128–H1135, 2010. doi: 10.1152/ajpheart.01133.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Saito M, Tsukanaka A, Yanagihara D, Mano T. Muscle sympathetic nerve responses to graded leg cycling. J Appl Physiol (1985) 75: 663–667, 1993. doi: 10.1152/jappl.1993.75.2.663. [DOI] [PubMed] [Google Scholar]
- 57. O'Brien MW, Al-Hinnawi A, Wu Y, Petterson JL, Shivgulam ME, Johns JA, Frayne RJ, Kimmerly DS. The influence of habitual breaks in sedentary time on cardiovagal baroreflex function. Appl Physiol Nutr Metab 46: 1143–1146, 2021. doi: 10.1139/apnm-2021-0246. [DOI] [PubMed] [Google Scholar]
- 58. Michiels C. Endothelial cell functions. J Cell Physiol 196: 430–443, 2003. doi: 10.1002/jcp.10333. [DOI] [PubMed] [Google Scholar]
- 59. Hadi HA, Carr CS, Al Suwaidi J. Endothelial dysfunction: cardiovascular risk factors, therapy, and outcome. Vasc Health Risk Manag 1: 183–198, 2005. [PMC free article] [PubMed] [Google Scholar]
- 60. Lacolley P, Regnault V, Segers P, Laurent S. Vascular smooth muscle cells and arterial stiffening: relevance in development, aging, and disease. Physiol Rev 97: 1555–1617, 2017. doi: 10.1152/physrev.00003.2017. [DOI] [PubMed] [Google Scholar]
- 61. Lusis AJ. Atherosclerosis. Nature 407: 233–241, 2000. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation 109, Suppl 1: III27–III32, 2004. doi: 10.1161/01.CIR.0000131515.03336.f8. [DOI] [PubMed] [Google Scholar]
- 63. McLenachan JM, Williams JK, Fish RD, Ganz P, Selwyn AP. Loss of flow-mediated endothelium-dependent dilation occurs early in the development of atherosclerosis. Circulation 84: 1273–1278, 1991. doi: 10.1161/01.cir.84.3.1273. [DOI] [PubMed] [Google Scholar]
- 64. Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, Lloyd JK, Deanfield JE. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet 340: 1111–1115, 1992. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- 65. Harris RA, Nishiyama SK, Wray DW, Richardson RS. Ultrasound assessment of flow-mediated dilation. Hypertension 55: 1075–1085, 2010. doi: 10.1161/HYPERTENSIONAHA.110.150821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Malek AM, Alper SL, Izumo S. Hemodynamic shear stress and its role in atherosclerosis. JAMA 282: 2035–2042, 1999. doi: 10.1001/jama.282.21.2035. [DOI] [PubMed] [Google Scholar]
- 67. De Keulenaer GW, Chappell DC, Ishizaka N, Nerem RM, Alexander RW, Griendling KK. Oscillatory and steady laminar shear stress differentially affect human endothelial redox state: role of a superoxide-producing NADH oxidase. Circ Res 82: 1094–1101, 1998. doi: 10.1161/01.res.82.10.1094. [DOI] [PubMed] [Google Scholar]
- 68. Nadaud S, Philippe M, Arnal JF, Michel JB, Soubrier F. Sustained increase in aortic endothelial nitric oxide synthase expression in vivo in a model of chronic high blood flow. Circ Res 79: 857–863, 1996. doi: 10.1161/01.res.79.4.857. [DOI] [PubMed] [Google Scholar]
- 69. Inoue N, Ramasamy S, Fukai T, Nerem RM, Harrison DG. Shear stress modulates expression of Cu/Zn superoxide dismutase in human aortic endothelial cells. Circ Res 79: 32–37, 1996. doi: 10.1161/01.res.79.1.32. [DOI] [PubMed] [Google Scholar]
- 70. Masatsugu K, Itoh H, Chun TH, Ogawa Y, Tamura N, Yamashita J, Doi K, Inoue M, Fukunaga Y, Sawada N, Saito T, Korenaga R, Ando J, Nakao K. Physiologic shear stress suppresses endothelin-converting enzyme-1 expression in vascular endothelial cells. J Cardiovasc Pharmacol 31, Suppl 1: S42–S45, 1998. doi: 10.1097/00005344-199800001-00014. [DOI] [PubMed] [Google Scholar]
- 71. Shyy YJ, Hsieh HJ, Usami S, Chien S. Fluid shear stress induces a biphasic response of human monocyte chemotactic protein 1 gene expression in vascular endothelium. Proc Natl Acad Sci USA 91: 4678–4682, 1994. doi: 10.1073/pnas.91.11.4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Thijssen DH, Dawson EA, Tinken TM, Cable NT, Green DJ. Retrograde flow and shear rate acutely impair endothelial function in humans. Hypertension 53: 986–992, 2009. doi: 10.1161/HYPERTENSIONAHA.109.131508. [DOI] [PubMed] [Google Scholar]
- 73. Johnson BD, Mather KJ, Newcomer SC, Mickleborough TD, Wallace JP. Vitamin C prevents the acute decline of flow-mediated dilation after altered shear rate patterns. Appl Physiol Nutr Metab 38: 268–274, 2013. doi: 10.1139/apnm-2012-0169. [DOI] [PubMed] [Google Scholar]
- 74. Duerrschmidt N, Wippich N, Goettsch W, Broemme HJ, Morawietz H. Endothelin-1 induces NAD(P)H oxidase in human endothelial cells. Biochem Biophys Res Commun 269: 713–717, 2000. doi: 10.1006/bbrc.2000.2354. [DOI] [PubMed] [Google Scholar]
- 75. Townsend RR, Wilkinson IB, Schiffrin EL, Avolio AP, Chirinos JA, Cockcroft JR, Heffernan KS, Lakatta EG, McEniery CM, Mitchell GF, Najjar SS, Nichols WW, Urbina EM, Weber T; American Heart Association Council on Hypertension. Recommendations for improving and standardizing vascular research on arterial stiffness: a scientific statement from the American Heart Association. Hypertension 66: 698–722, 2015. doi: 10.1161/HYP.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol 55: 1318–1327, 2010. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 77. Rahman M, Siddik AB. Anatomy, arterioles. In: StatPearls. Treasure Island, FL: StatPearls Publishing, 2022. [PubMed] [Google Scholar]
- 78. Fronek K, Zweifach BW. Microvascular pressure distribution in skeletal muscle and the effect of vasodilation. Am J Physiol 228: 791–796, 1975. doi: 10.1152/ajplegacy.1975.228.3.791. [DOI] [PubMed] [Google Scholar]
- 79. Sweeney TE, Sarelius IH. Arteriolar control of capillary cell flow in striated muscle. Circ Res 64: 112–120, 1989. doi: 10.1161/01.res.64.1.112. [DOI] [PubMed] [Google Scholar]
- 80. Eriksson E, Lisander B. Changes in precapillary resistance in skeletal muscle vessels studied by intravital microscopy. Acta Physiol Scand 84: 295–305, 1972. doi: 10.1111/j.1748-1716.1972.tb05181.x. [DOI] [PubMed] [Google Scholar]
- 81. Park SY, Kwon OS, Andtbacka RHI, Hyngstrom JR, Reese V, Murphy MP, Richardson RS. Age-related endothelial dysfunction in human skeletal muscle feed arteries: the role of free radicals derived from mitochondria in the vasculature. Acta Physiol (Oxf) 222: e12947, 2018. doi: 10.1111/apha.12893. [DOI] [PubMed] [Google Scholar]
- 82. Park SH, Kwon OS, Park SY, Weavil JC, Hydren JR, Reese V, Andtbacka RHI, Hyngstrom JR, Richardson RS. Vasodilatory and vascular mitochondrial respiratory function with advancing age: evidence of a free radically mediated link in the human vasculature. Am J Physiol Regul Integr Comp Physiol 318: R701–R711, 2020. doi: 10.1152/ajpregu.00268.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Vranish JR, Young BE, Stephens BY, Kaur J, Padilla J, Fadel PJ. Brief periods of inactivity reduce leg microvascular, but not macrovascular, function in healthy young men. Exp Physiol 103: 1425–1434, 2018. doi: 10.1113/EP086918. [DOI] [PubMed] [Google Scholar]
- 84. Delp M, Laughlin M. Regulation of skeletal muscle perfusion during exercise. Acta Physiol Scand 162: 411–419, 1998. doi: 10.1046/j.1365-201X.1998.0324e.x. [DOI] [PubMed] [Google Scholar]
- 85. Thomas GD, Segal SS. Neural control of muscle blood flow during exercise. J Appl Physiol (1985) 97: 731–738, 2004. doi: 10.1152/japplphysiol.00076.2004. [DOI] [PubMed] [Google Scholar]
- 86. Rosenberry R, Nelson MD. Reactive hyperemia: a review of methods, mechanisms, and considerations. Am J Physiol Regul Integr Comp Physiol 318: R605–R618, 2020. doi: 10.1152/ajpregu.00339.2019. [DOI] [PubMed] [Google Scholar]
- 87. Gifford JR, Richardson RS. CORP: Ultrasound assessment of vascular function with the passive leg movement technique. J Appl Physiol (1985) 123: 1708–1720, 2017. doi: 10.1152/japplphysiol.00557.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Thosar SS, Bielko SL, Wiggins CC, Klaunig JE, Mather KJ, Wallace JP. Antioxidant vitamin C prevents decline in endothelial function during sitting. Med Sci Monit 21: 1015–1021, 2015. doi: 10.12659/MSM.893192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Nosova EV, Yen P, Chong KC, Alley HF, Stock EO, Quinn A, Hellmann J, Conte MS, Owens CD, Spite M, Grenon SM. Short-term physical inactivity impairs vascular function. J Surg Res 190: 672–682, 2014. doi: 10.1016/j.jss.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Galkina E, Ley K. Vascular adhesion molecules in atherosclerosis. Arterioscler Thromb Vasc Biol 27: 2292–2301, 2007. doi: 10.1161/ATVBAHA.107.149179. [DOI] [PubMed] [Google Scholar]
- 91. Block G, Jensen CD, Morrow JD, Holland N, Norkus EP, Milne GL, Hudes M, Dalvi TB, Crawford PB, Fung EB, Schumacher L, Harmatz P. The effect of vitamins C and E on biomarkers of oxidative stress depends on baseline level. Free Radic Biol Med 45: 377–384, 2008. doi: 10.1016/j.freeradbiomed.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Latouche C, Jowett JB, Carey AL, Bertovic DA, Owen N, Dunstan DW, Kingwell BA. Effects of breaking up prolonged sitting on skeletal muscle gene expression. J Appl Physiol (1985) 114: 453–460, 2013. doi: 10.1152/japplphysiol.00978.2012. [DOI] [PubMed] [Google Scholar]
- 93. Trinity JD, Drummond MJ, Fermoyle CC, McKenzie AI, Supiano MA, Richardson RS. Cardiovasomobility: an integrative understanding of how disuse impacts cardiovascular and skeletal muscle health. J Appl Physiol (1985) 132: 835–861, 2022. doi: 10.1152/japplphysiol.00607.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Lindsey ML, LeBlanc AJ, Ripplinger CM, Carter JR, Kirk JA, Hansell Keehan K, Brunt KR, Kleinbongard P, Kassiri Z. Reinforcing rigor and reproducibility expectations for use of sex and gender in cardiovascular research. Am J Physiol Heart Circ Physiol 321: H819–H824, 2021. doi: 10.1152/ajpheart.00418.2021. [DOI] [PubMed] [Google Scholar]
- 95. Chang DH, Dumanski SM, Ahmed SB. Female sex-specific considerations to improve rigor and reproducibility in cardiovascular research. Am J Physiol Heart Circ Physiol 324: H279–H287, 2022. doi: 10.1152/ajpheart.00462.2022. [DOI] [PubMed] [Google Scholar]
- 96. Boyle LJ, Credeur DP, Jenkins NT, Padilla J, Leidy HJ, Thyfault JP, Fadel PJ. Impact of reduced daily physical activity on conduit artery flow-mediated dilation and circulating endothelial microparticles. J Appl Physiol (1985) 115: 1519–1525, 2013. doi: 10.1152/japplphysiol.00837.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Edwards SJ, Shad BJ, Marshall RN, Morgan PT, Wallis GA, Breen L. Short-term step reduction reduces citrate synthase activity without altering skeletal muscle markers of oxidative metabolism or insulin-mediated signaling in young males. J Appl Physiol (1985) 131: 1653–1662, 2021. doi: 10.1152/japplphysiol.00650.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Bailey DP, Maylor BD, Orton CJ, Zakrzewski-Fruer JK. Effects of breaking up prolonged sitting following low and high glycaemic index breakfast consumption on glucose and insulin concentrations. Eur J Appl Physiol 117: 1299–1307, 2017. doi: 10.1007/s00421-017-3610-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Peddie MC, Kessell C, Bergen T, Gibbons TD, Campbell HA, Cotter JD, Rehrer NJ, Thomas KN. The effects of prolonged sitting, prolonged standing, and activity breaks on vascular function, and postprandial glucose and insulin responses: a randomised crossover trial. PLoS One 16: e0244841, 2021. doi: 10.1371/journal.pone.0244841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Vehvilainen T, Lindholm H, Rintamaki H, Paakkonen R, Hirvonen A, Niemi O, Vinha J. High indoor CO2 concentrations in an office environment increases the transcutaneous CO2 level and sleepiness during cognitive work. J Occup Environ Hyg 13: 19–29, 2016. doi: 10.1080/15459624.2015.1076160. [DOI] [PubMed] [Google Scholar]