Abstract
Introduction
In 2004, the U.S. President’s Emergency Plan for AIDS Relief (PEPFAR), with CDC as a major U.S. government implementing agency, began providing HIV antiretroviral therapy (ART) worldwide. Through suppression of HIV viral load, effective ART reduces morbidity and mortality among persons with HIV infection and prevents vertical and sexual transmission.
Methods
To describe program impact, data were analyzed from all PEPFAR programs and from six countries that have conducted nationally representative Population-based HIV Impact Assessment (PHIA) surveys, including PEPFAR programmatic data on the number of persons with HIV infection receiving PEPFAR-supported ART (2004–2022), rates of viral load coverage (the proportion of eligible persons with HIV infection who received a viral load test) and viral load suppression (proportion of persons who received a viral load test with <1,000 HIV copies per mL of blood) (2015–2022), and population viral load suppression rates in six countries that had two PHIA surveys conducted during 2015–2021. To assess health system strengthening, data on workforce and laboratory systems were analyzed.
Results
By September 2022, approximately 20 million persons with HIV infection in 54 countries were receiving PEPFAR-supported ART (62% CDC-supported); this number increased 300-fold from the 66,550 reported in September 2004. During 2015–2022, viral load coverage more than tripled, from 24% to 80%, and viral load suppression increased from 80% to 95%. Despite increases in viral load suppression rates and health system strengthening investments, variability exists in viral load coverage among some subpopulations (children aged <10 years, males, pregnant women, men who have sex with men [MSM], persons in prisons and other closed settings [persons in prisons], and transgender persons) and in viral load suppression among other subpopulations (pregnant and breastfeeding women, persons in prisons, and persons aged <20 years).
Conclusions and implications for public health practice
Since 2004, PEPFAR has scaled up effective ART to approximately 20 million persons with HIV infection in 54 countries. To eliminate HIV as a global public health threat, achievements must be sustained and expanded to reach all subpopulations. CDC and PEPFAR remain committed to tackling HIV while strengthening public health systems and global health security.
Introduction
The U.S. President’s Emergency Plan for AIDS Relief (PEPFAR) was announced in January 2003 and remains the largest commitment by any nation to address a single disease. PEPFAR’s core aim is to address health inequities in access to HIV services. The initial goal was to prevent 7 million infections, treat 2 million persons, and provide humane care for persons suffering from AIDS and for children orphaned by AIDS.† At the time, approximately 30 million persons with HIV infection were estimated to live on the African continent, including 3 million children and adolescents aged <15 years; however, only 50,000 were receiving antiretroviral therapy (ART).§ Since 2004, PEPFAR has supported partner governments’ expansion of ART delivery while strengthening health systems. Through viral load suppression, effective ART reduces morbidity and mortality among persons with HIV infection (1); it also prevents vertical transmission from mothers with HIV infection to their infants if the mother is on ART and the HIV-exposed infant receives prophylaxis; and prevents sexual transmission when viral load is undetectable (<200 copies per mL of blood) (2–5).
PEPFAR, led and coordinated by the U.S. Department of State, uses a whole-of-government approach for global HIV/AIDS response, implemented by seven U.S. government departments and agencies, including CDC.¶ As the U.S. agency responsible for protecting public health, CDC couples its core area investments in public health workforce development, surveillance, and laboratory capacity with scientific and technical expertise and data-driven approaches to fight the global HIV epidemic and other threats to global health security.**
PEPFAR supports the Sustainable Development Goals and the Joint United Nations Programme on HIV/AIDS’ (UNAIDS) fast-track strategy to end the AIDS epidemic as a global threat by 2030: that 95% of persons with HIV infection know their status, that 95% of those with known status receive ART, and that 95% of those receiving ART achieve viral load suppression.†† Worldwide in 2021, an estimated 38.4 million persons had HIV infection; 650,000 AIDS-related deaths and 1.5 million new infections occurred.§§ An estimated 28.7 million persons with HIV infection were receiving ART, and among those receiving ART, an estimated 92% had suppressed viral loads. To assess PEPFAR-supported program impact and health system–strengthening investments, programmatic data from all PEPFAR programs and survey data for six countries with more than one Population-based HIV Impact Assessment (PHIA) survey were analyzed.¶¶
Methods
To describe program impact, PEPFAR Monitoring, Evaluation, and Reporting*** programmatic data were analyzed by age, sex, and subpopulation (pregnant or breastfeeding women and key populations, including female sex workers, men who have sex with men (MSM), transgender persons, persons who inject drugs, and persons in prisons), and proportion of CDC contribution; analyses were stratified by fiscal year (October–September).††† Before October 2018, persons with HIV infection receiving PEPFAR-supported ART were defined as persons currently receiving ART and for whom ≤90 days had elapsed after missing a scheduled ART pickup; in October 2018, this definition changed to persons currently receiving ART for whom ≤28 days had elapsed after missing a scheduled ART pickup. A proxy rate for viral load coverage was calculated as the percentage of persons with HIV infection receiving ART for ≥6 months with documented receipt of a viral load test within the previous 12 months. As an indicator of ART effectiveness, viral load suppression was defined as <1,000 HIV copies per mL of blood, and the viral load suppression rate was calculated as the number of persons with HIV infection with viral load suppression among those who received a viral load test. Using data from the PEPFAR-supported, CDC-led PHIA surveys, population viral load suppression rates by sex and age group (15–24, 25–34, 35–49, and ≥50 years) were analyzed for six countries (Eswatini, Lesotho, Malawi, Uganda, Zambia, and Zimbabwe) that completed two surveys during 2015–2021.§§§
PEPFAR Monitoring, Evaluation, and Reporting data were analyzed to describe health system strengthening investments. The workforce includes the number of health care workers (including lay, clinical, pharmacy, and laboratory workers) who provide HIV- or tuberculosis (TB)-related prevention, treatment, or other HIV-related services in community, clinic, or other settings. Molecular testing capacity was defined as the existence of a facility with dedicated infrastructure and staff members trained to conduct HIV early infant diagnosis, viral load, or TB molecular diagnostic testing. Laboratory continuous quality improvement enrollment was defined as participation in activities aimed at ensuring diagnostic accuracy and reliability supported by a recognized laboratory continuous quality improvement program. Accreditation was defined as achieving the highest standard of clinical laboratory quality as assessed by a nationally, regionally, or internationally recognized accrediting body. This activity was reviewed by CDC and conducted consistent with applicable federal law and CDC policy.¶¶¶
Results
During 2004–2022, the number of persons with HIV infection receiving PEPFAR-supported ART increased 300-fold, from 66,550 to 20,166,110, in 54 countries (Figure 1). During 2015–2022, the annual number of persons with HIV infection who received a viral load test increased 605%, from 2,109,749 to 14,875,130, and the overall viral load coverage rate increased 233%, from 24% (2,109,749 of 8,806,300 eligible persons who received a viral load test) to 80% (14,875,130 of 18,573,406) (Figure 2) (Table 1). During 2017–2022, viral load coverage rates increased to approximately 75% among women, men, and persons aged <10, 10–19, and ≥20 years; among pregnant women, viral load coverage increased 72%, from 18% to 31%. During 2020–2022, viral load coverage increased from 70% to 85% among female sex workers, from 62% to 83% among persons who inject drugs, and from 64% to 78% among MSM. Among transgender persons, viral load coverage decreased 6%, from 71% to 67%, and among persons in prisons, coverage decreased 24%, from 75% to 57%.
FIGURE 1.
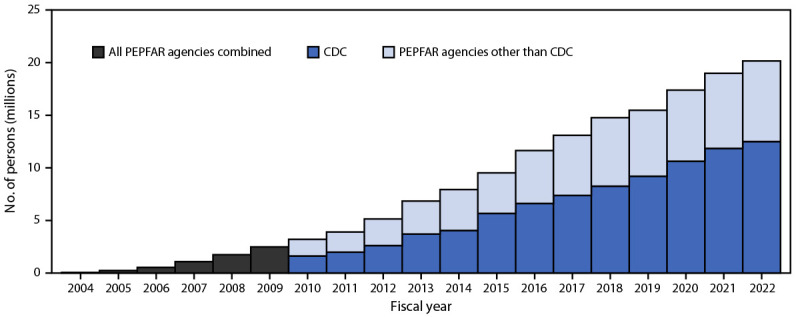
Cumulative number of persons with HIV infection receiving antiretroviral therapy supported by the U.S. President’s Emergency Plan for AIDS Relief,* by CDC and other agencies† — worldwide,§ fiscal years 2004–2022¶
Abbreviations: ART = antiretroviral therapy; PEPFAR = U.S. President’s Emergency Plan for AIDS Relief.
* Data on support provided by CDC and other PEPFAR agencies available for fiscal years 2010–2022.
† PEPFAR agencies include the U.S. Agency for International Development, the U.S. Department of Health and Human Services and its agencies (CDC, Health Resources and Service Administration, and National Institutes of Health), the U.S. Department of Defense, the Peace Corps, the U.S. Department of Labor, the U.S. Department of Commerce, and the U.S. Department of the Treasury.
§ As of September 30, 2022: Angola, Benin, Botswana, Brazil, Burkina Faso, Burma, Burundi, Cameroon, Colombia, Cote d'Ivoire, Democratic Republic of the Congo, Dominican Republic, El Salvador, Eswatini, Ethiopia, Ghana, Guatemala, Haiti, Honduras, India, Indonesia, Jamaica, Kazakhstan, Kenya, Kyrgyzstan, Laos, Lesotho, Liberia, Malawi, Mali, Mozambique, Namibia, Nepal, Nicaragua, Nigeria, Panama, Papua New Guinea, Peru, Philippines, Rwanda, Senegal, Sierra Leone, South Africa, South Sudan, Tajikistan, Tanzania, Thailand, Togo, Trinidad and Tobago, Uganda, Ukraine, Vietnam, Zambia, and Zimbabwe.
¶ Fiscal years are October–September.
FIGURE 2.
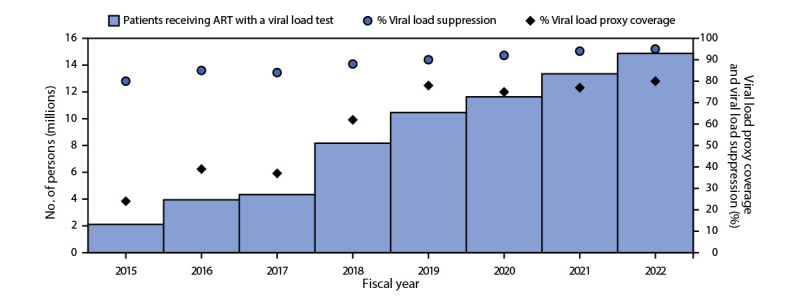
Number of persons with HIV infection receiving antiretroviral therapy supported by the U.S. President’s Emergency Plan for AIDS Relief with a viral load test,* viral load proxy coverage rate,† and viral load suppression rate§ — worldwide,¶ fiscal years 2015–2022**
Abbreviations: ART = antiretroviral therapy; PEPFAR = U.S. President’s Emergency Plan for AIDS Relief.
* Viral load test result documented in the patient record or laboratory information system.
† Proxy viral load coverage rate was calculated as the percentage of persons with HIV infection receiving ART for ≥6 months with documented receipt of a viral load test within the preceding 12 months.
§ Viral load suppression was defined as <1,000 HIV copies per mL of blood; suppression rate was calculated as the number of persons with HIV infection with viral load suppression among those who received a viral load test.
¶ As of September 30, 2022: Angola, Benin, Botswana, Brazil, Burkina Faso, Burma, Burundi, Cameroon, Colombia, Cote d'Ivoire, Democratic Republic of the Congo, Dominican Republic, El Salvador, Eswatini, Ethiopia, Ghana, Guatemala, Haiti, Honduras, India, Indonesia, Jamaica, Kazakhstan, Kenya, Kyrgyzstan, Laos, Lesotho, Liberia, Malawi, Mali, Mozambique, Namibia, Nepal, Nicaragua, Nigeria, Panama, Papua New Guinea, Peru, Philippines, Rwanda, Senegal, Sierra Leone, South Africa, South Sudan, Tajikistan, Tanzania, Thailand, Togo, Trinidad and Tobago, Uganda, Ukraine, Vietnam, Zambia, and Zimbabwe.
** Fiscal years are October–September.
TABLE 1. Summary of programmatic data on viral load testing,* proxy viral load coverage,† and viral load suppression,§ by age group, sex, and subpopulation — worldwide, fiscal years 2015–2022¶.
| Characteristic (program fiscal years, 2015–2022)¶ | Baseline programmatic data** |
Programmatic data from September 30, 2022 |
Change§§ in VL proxy coverage, baseline–2022, % | Change¶¶ in VL suppression, baseline–2022, % | ||||
|---|---|---|---|---|---|---|---|---|
| No. of eligible†† persons receiving VL test/total eligible persons | VL proxy coverage rate, % | VL suppression rate, % | No. of eligible†† persons receiving VL test/total eligible persons | VL proxy coverage rate, % | VL suppression rate, % | |||
|
Overall
|
2,109,749/8,806,300
|
24
|
80
|
14,875,130/18,573,406
|
80
|
95
|
233
|
19
|
|
Age group, yrs (2017–2022)
| ||||||||
| <10 |
147,143/313,426 |
47 |
67 |
249,530/314,058 |
79 |
84 |
68 |
25 |
| 10–19 |
240,705/496,053 |
49 |
68 |
620,686/729,305 |
85 |
88 |
73 |
29 |
| ≥20 |
3,649,299/8,403,258 |
43 |
86 |
13,941,086/17,202,330 |
81 |
96 |
88 |
12 |
| Unknown*** |
249,150/2,832,066 |
9 |
88 |
63,828/327,713 |
19 |
94 |
111 |
7 |
|
Sex††† (2017–2022)
| ||||||||
| Female |
2,760,201/7,695,526 |
36 |
86 |
9,768,760/12,034,211 |
81 |
95 |
125 |
10 |
| Male |
1,339,638/4,029,411 |
33 |
84 |
5,106,370/6,539,195 |
78 |
95 |
136 |
13 |
|
Pregnant and breastfeeding women with HIV infection§§§ (2017–2022)
|
|
|||||||
| Pregnant women |
80,652/438,315 |
18 |
95 |
150,818/487,608 |
31 |
92 |
72 |
−3 |
| Breastfeeding women |
82,255 |
NA |
85 |
399,082 |
NA |
94 |
— |
11 |
|
Key populations (2020–2022)
| ||||||||
| Female sex workers |
58,378/83,095 |
70 |
93 |
199,435/233,652 |
85 |
97 |
21 |
4 |
| Men who have sex with men |
51,317/79,983 |
64 |
94 |
165,352/210,926 |
78 |
97 |
22 |
3 |
| Persons in prisons and other enclosed settings |
18,605/24,821 |
75 |
93 |
22,836/39,805 |
57 |
93 |
–24 |
0 |
| Persons who inject drugs |
33,716/54,394 |
62 |
93 |
79,822/96,228 |
83 |
96 |
34 |
3 |
| Transgender persons | 2,352/3,328 | 71 | 89 | 7,120/10,700 | 67 | 96 | −6 | 8 |
Abbreviations: ART = antiretroviral therapy; NA = not applicable; PEPFAR = U.S. President’s Emergency Plan for AIDS Relief; VL = viral load.
* VL test result documented in the patient record or laboratory information system.
† Proxy VL coverage rate was calculated as the percentage of persons with HIV infection who received ART for ≥6 months with documented receipt of a VL test within the preceding 12 months.
§ VL suppression was defined as <1,000 HIV copies per mL of blood; suppression rate was calculated as the number of persons with HIV infection with VL suppression among those who received a VL test.
¶ Each characteristic was calculated using data from the end of the fiscal year from which they were available to the end of fiscal year 2022.
** The year of comparison for each group varied based on the quality and availability of data for analyses and is indicated in the row parentheses.
†† Eligible persons are those who have received ART for ≥6 months, derived from the number of persons receiving PEPFAR-supported ART during the two preceding quarters.
§§ Calculated as ([VL coverage 2022 − VL coverage baseline]/VL coverage baseline) * 100.
¶¶ Calculated as ([VL suppression 2022 − VL suppression baseline]/VL suppression baseline) * 100.
*** The value for age unknown includes 327,713 persons who received ART and were eligible for a VL test, reported in aggregate age groups only (i.e., <15 years and ≥15 years). Because of the proxy nature of the indicator, data reporting discrepancies for age group (<15 years and ≥15 years versus age disaggregates in ≤5-year age bands) might be observed.
††† PEPFAR indicators are disaggregated by biologic sex (male or female), where applicable.
§§§ The number of breastfeeding women receiving ART is not reported in PEPFAR monitoring, evaluation, and reporting.
During 2015–2022, the viral load suppression rate among those receiving testing increased from 80% (1,691,232 persons with viral load suppression of 2,109,749 who received a viral load test) to 95% (14,146,647 of 14,875,130) (Figure 2). During 2017–2022, the viral load suppression rate increased among women, men, persons aged <10, 10–19, and ≥20 years, pregnant women, and breastfeeding women. Males, females, and those aged ≥20 years reached viral load suppression rates of ≥95% in 2022 (Table 1). By 2022, the viral load suppression rate among female sex workers, MSM, transgender persons, and persons who inject drugs reached ≥95%, but among persons in prisons, remained unchanged, at 93%.
PHIA survey results demonstrated increased population viral load suppression rates in all six assessed countries, with overall viral load suppression rates in the first and second surveys ranging from 59.2% (Zambia) to 73.1% (Eswatini) and 75.4% (Uganda) to 88.6% (Eswatini), respectively (Table 2). Across all surveys, with few exceptions, population viral load suppression rates were higher in older than in younger persons, and higher in women than in men.
TABLE 2. Population viral load suppression prevalence* results from Population-based HIV Impact Assessment surveys in countries supported by the U.S. President’s Emergency Plan for AIDS Relief† — six African countries, 2015–2021.
| Country, age group, yrs | Population viral load suppression rate,% |
% Change from survey 1 to survey 2 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Survey 1 |
Survey 2 |
||||||||
| Male | Female | Total | Male | Female | Total | Male | Female | Total | |
|
Eswatini
| |||||||||
|
All ages
|
67.6
|
76.0
|
73.1
|
86.1
|
90.1
|
88.6
|
18.5
|
14.1
|
15.5
|
| 15–24 |
32.9 |
55.5 |
50.6
|
80.5 |
76.1 |
77.1
|
47.6 |
20.6 |
26.5
|
| 25–34 |
54.8 |
73.5 |
68.4
|
62.9 |
85.7 |
80.4
|
8.1 |
12.2 |
12.0
|
| 35–49 |
71.5 |
82.7 |
78.5
|
88.9 |
93.8 |
91.9
|
17.4 |
11.1 |
13.4
|
| ≥50 |
86.4 |
85.3 |
85.8
|
94.3 |
96.3 |
95.3
|
7.9 |
11.0 |
9.5
|
|
Lesotho
| |||||||||
|
All ages
|
63.4
|
70.5
|
67.6
|
77.1
|
83.4
|
81.0
|
13.7
|
12.9
|
13.4
|
| 15–24 |
51.3 |
50.9 |
51.0
|
61.7 |
65.6 |
64.7
|
10.4 |
14.7 |
13.7
|
| 25–34 |
46.1 |
64.6 |
57.9
|
58.7 |
77.6 |
72.3
|
12.6 |
13.0 |
14.4
|
| 35–49 |
67.5 |
78.3 |
73.3
|
78.3 |
87.8 |
83.5
|
10.8 |
9.5 |
10.2
|
| ≥50 |
84.3 |
80.6 |
82.3
|
90.7 |
90.7 |
90.7
|
6.4 |
10.1 |
8.4
|
|
Malawi
| |||||||||
|
All ages
|
60.9
|
73.1
|
68.3
|
85.5
|
88.4
|
87.3
|
24.6
|
15.3
|
19.0
|
| 15–24 |
37.2 |
49.7 |
46.0
|
75.0 |
73.2 |
73.8
|
37.8 |
23.5 |
27.8
|
| 25–34 |
48.2 |
70.1 |
62.9
|
74.0 |
82.6 |
80.1
|
25.8 |
12.5 |
17.2
|
| 35–49 |
66.0 |
78.5 |
73.2
|
87.6 |
92.7 |
90.8
|
21.6 |
14.2 |
17.6
|
| ≥50 |
73.7 |
81.6 |
78.0
|
90.9 |
93.0 |
92.0
|
17.2 |
11.4 |
14.0
|
|
Uganda
| |||||||||
|
All ages
|
53.6
|
62.9
|
59.6
|
69.8
|
78.3
|
75.4
|
16.2
|
15.4
|
15.8
|
| 15–24 |
32.5 |
44.9 |
42.5
|
43.5 |
57.8 |
54.7
|
11.0 |
12.9 |
12.2
|
| 25–34 |
38.7 |
57.9 |
52.6
|
51.9 |
75.0 |
68.8
|
13.2 |
17.1 |
16.2
|
| 35–49 |
60.1 |
71.4 |
66.3
|
75.1 |
84.9 |
80.9
|
15.0 |
13.5 |
14.6
|
| ≥50 |
65.0 |
79.4 |
73.0
|
85.4 |
90.2 |
88.0
|
20.4 |
10.8 |
15.0
|
|
Zambia
| |||||||||
|
All ages
|
57.2
|
60.4
|
59.2
|
85.5
|
86.6
|
86.2
|
28.3
|
26.2
|
27.0
|
| 15–24 |
36.7 |
33.6 |
34.3
|
70.1 |
71.2 |
70.9
|
33.4 |
37.6 |
36.6
|
| 25–34 |
36.7 |
56.1 |
50.4
|
72.6 |
83.4 |
81.0
|
35.9 |
27.3 |
30.6
|
| 35–49 |
61.8 |
70.8 |
66.9
|
87.7 |
89.9 |
89.1
|
25.9 |
19.1 |
22.2
|
| ≥50 |
79.7 |
73.5 |
76.6
|
93.0 |
91.7 |
92.3
|
13.3 |
18.2 |
15.7
|
|
Zimbabwe
| |||||||||
|
All ages
|
54.1
|
63.8
|
59.8
|
73.0
|
79.8
|
77.3
|
18.9
|
16.0
|
17.5
|
| 15–24 |
40.1 |
47.9 |
45.3
|
49.2 |
66.2 |
60.6
|
9.1 |
18.3 |
15.3
|
| 25–34 |
36.2 |
54.2 |
48.7
|
52.4 |
70.7 |
65.7
|
16.2 |
16.5 |
17.0
|
| 35–49 |
55.8 |
70.5 |
63.9
|
76.6 |
82.4 |
80.2
|
20.8 |
11.9 |
16.3
|
| ≥50 | 71.6 | 78.8 | 75.1 | 84.5 | 91.0 | 88.1 | 12.9 | 12.2 | 13.0 |
* Viral load suppression was defined as <1,000 copies per mL of blood; suppression rate was calculated as the number of persons with HIV infection with viral load suppression among those who received a viral load test.
† Eswatini: survey 1 was conducted during 2016–2017, and survey 2 during 2021; Lesotho: survey 1 was conducted during 2016–2017, and survey 2 during 2020; Malawi: survey 1 was conducted during 2015–2016, and survey 2 during 2020–2021; Uganda: survey 1 was conducted during 2016–2017, and survey 2 during 2020–2021; Zambia: survey 1 was conducted during 2016, and survey 2 during 2021; and Zimbabwe: survey 1 was conducted during 2015–2016, and survey 2 during 2021.
In 2022, the PEPFAR-supported workforce included 371,760 health care workers in approximately 70,000 community, clinic, or other settings. During 2017–2022, the number of PEPFAR-supported facilities with a molecular laboratory increased by 115%, from 926 to 1,995; the number of PEPFAR-supported facilities with one or more laboratory enrolled in a continuous quality improvement program increased by 112%, from 795 to 1,687; and those that were accredited increased by 194%, from 103 to 303.
In 2010, approximately one half of persons with HIV infection receiving PEPFAR-supported ART received services through CDC implementing partners (Figure 1). By September 2022, CDC implementing partners supported 62% (12,566,736 of 20,166,110 persons with HIV infection receiving PEPFAR-supported ART) of the PEPFAR total. Among the total PEPFAR-supported workforce in 2022, 42% were supported through CDC implementing partners.
Discussion
The cumulative program impact of PEPFAR among 54 countries reached approximately 20.2 million persons with HIV infection with lifesaving ART by September 2022, a 300-fold increase from 2004. PEPFAR-supported ART is effective, as demonstrated by program data indicating that the UNAIDS target for viral load suppression was achieved in 2022, and by PHIA survey data indicating increased viral load suppression rates at the population level (i.e., not restricted to persons with HIV infection receiving PEPFAR-supported ART). By providing effective ART, PEPFAR’s investments have helped avert new HIV infections (6) and have led to sustained declines in all-cause mortality.**** For example, in Uganda, the first PEPFAR-supported country, ART scale-up since 2004 has helped to avert an estimated 500,000 infections, including approximately 230,000 infections among HIV-exposed infants, and 600,000 HIV-related deaths (7). In Eswatini, national HIV incidence decreased by nearly one half and viral load suppression doubled during 2011–2016 (8).
PEPFAR program impact is founded on strengthened health systems. Improvements in laboratory capacity, including molecular testing and continuous quality improvement activities described in this report, have supported the full HIV cascade of care (9), including accurate HIV diagnosis, treatment, and viral load monitoring of ART effectiveness. Investments reflect PEPFAR’s commitment to local public health system strengthening for broader pandemic preparedness and response. Under PEPFAR’s current 5-year strategy,†††† the United States aims to eliminate the HIV/AIDS pandemic as a public health threat by 2030, while sustainably strengthening public health systems.
Through PEPFAR, CDC is at the forefront of global ART scale-up efforts. CDC receives approximately 50% of PEPFAR funding for HIV treatment and supports approximately 60% of all persons receiving ART through PEPFAR. The PEPFAR-supported CDC-led PHIA surveys have provided rigorous estimates of critical HIV indicators by age group, sex, and subnational geographic units. Other PEPFAR investments achieved through CDC have strengthened surveillance systems, such as health and laboratory information systems for patient and program monitoring, as well as HIV case reporting. The PEPFAR laboratory continuous quality improvement program (Strengthening Laboratory Management Toward Accreditation§§§§) has provided practical tools for resource-limited settings to improve quality management systems and prepare laboratories for accreditation¶¶¶¶ (10). CDC provides leadership in the use of multiple data sources to continually identify gaps in HIV service delivery for policy and program action (11,12).
Beyond HIV, PEPFAR investments in public health system strengthening have had additional benefits, including improving global health security. For example, during the COVID-19 pandemic, PEPFAR-supported countries demonstrated the resilience of PEPFAR investments by protecting and advancing HIV response gains (13,14), while also responding to COVID-19. In Nigeria, an ART surge in nine states supported by CDC through PEPFAR rapidly increased the total number of persons with HIV infection receiving ART by 26% (110,815) during April–September 2020 alone (15,16). PEPFAR investments have been leveraged for public health emergency response. Workforce investments have trained and deployed large numbers of health care workers not only to prevent, diagnose, and treat HIV and provide quality care for persons with HIV infection, but also to identify, track, and contain other health threats such as cholera, Ebola virus disease, and COVID-19.***** During April 2020–March 2021, a total of 109 PEPFAR-supported centralized HIV viral load and early infant diagnosis laboratories and 138 decentralized HIV and TB sites reported conducting approximately 3.4 million SARS-CoV-2 tests in 16 countries (17).
Despite these achievements, 10 million persons with HIV infection worldwide (in countries with and without PEPFAR support) were not receiving ART in 2021, and gaps exist among certain subpopulations. Global HIV control cannot be achieved without prioritizing health equity. For example, although overall viral load coverage rates have increased over time, rates were lower among children aged <10 years, males, pregnant women, MSM, persons in prisons, and transgender persons. Similarly, whereas overall viral load suppression rates reached the UNAIDS target of 95% of persons with HIV infection receiving ART, rates were lower among pregnant and breastfeeding women and persons in prisons, and much lower for persons aged <20 years, including children and adolescents with HIV infection. Results from PHIA surveys further highlight lower viral load suppression rates among younger age groups and among men compared with women. Stigma and discrimination remain important barriers to health equity. In sub-Saharan Africa, for example, HIV prevalence among MSM and transgender women is significantly higher than it is in the general population (18). Understanding the root causes including structural determinants of health for the observed differences and addressing potential factors leading to health disparities is essential to eliminate HIV as a global public health threat.
The findings in this report are subject to at least six limitations. First, indicator definitions and the systems to collect and report data have evolved over time, which might have affected data quality and results observed. Second, the countries, number of sites reporting, changes in national HIV guidelines (i.e., prevention, treatment, ART initiation criteria, recommended ART regimens, and monitoring), and the ability for persons with HIV infection to access services at any site have also evolved, which might have affected results observed. Third, misclassification of patients in certain subpopulations might have occurred if this information was not disclosed and captured by medical records. Fourth, viral load coverage analyses used aggregate program data, and as such, reported viral load proxy coverage rates could differ from actual viral load coverage rates. Fifth, because some facilities might have more than one laboratory, the number of laboratories might have been underreported. Finally, programmatic data cannot be directly compared with PHIA results, which are derived through representative sampling methods.
Since 2004, PEPFAR has scaled up ART to approximately 20 million persons with HIV infection worldwide, managing a chronic disease at an unprecedented level while strengthening public health systems through workforce, surveillance, and laboratory capacity investments. To eliminate HIV as a global public health threat, achievements in HIV services must be sustained and expanded to reach all subpopulations. PEPFAR remains committed to supporting partner governments to eliminate HIV as a global public health threat while strengthening public health systems and global health security.
Summary.
What is already known about this topic?
The U.S. President’s Emergency Plan for AIDS Relief (PEPFAR) began providing HIV antiretroviral therapy (ART) worldwide in 2004. Through viral load suppression, effective ART improves health outcomes and prevents transmission.
What is added by this report?
By 2022, approximately 20 million persons with HIV infection in 54 countries received PEPFAR-supported ART (62% CDC-supported); this number represents an increase of 300-fold from 66,550 in 2004. During 2015–2022, viral load suppression rates increased from 80% to 95% among those who received testing.
What are the implications for public health practice?
To eliminate HIV as a global public health threat, achievements must be sustained and expanded to reach all subpopulations. PEPFAR remains committed to tackling HIV while strengthening public health systems and global health security.
Acknowledgments
The U.S. President’s Emergency Plan for AIDS Relief (PEPFAR) partner governments; civil society organizations; site staff members; implementing partners; PEPFAR interagency viral load and early infant diagnosis community of practice; Population-based HIV Impact Assessment support teams (Eswatini, Lesotho, Malawi, Uganda, Zambia, and Zimbabwe); Division of Global HIV and TB, Center for Global Health, CDC.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflicts of interest were disclosed.
Footnotes
These authors contributed equally to this report.
https://www.unaids.org/en/resources/documents/2022/in-danger-global-aids-update; https://www.unaids.org/en/resources/fact-sheet
For persons with HIV infection receiving PEPFAR-supported ART, overall data were available for 2004–2022; data by agency were analyzed for 2010–2022; and data by age, sex, and key population were analyzed for 2022. For viral load, overall data were available for 2015–2022; data by age, sex, and among pregnant or breastfeeding women were available for 2017–2022; data on key populations were available for 2020–2022; and data were not available to calculate viral load proxy coverage among breastfeeding women. Data on human resources to support HIV and TB services were available for 2022. Data on laboratory capacity were available for 2017–2022. PEPFAR indicators are disaggregated by biologic sex (male or female), where applicable.
Population viral load suppression rate was calculated as the number of persons with HIV infection with viral load suppression (<1,000 HIV copies per mL of blood) among all persons identified with HIV infection. PHIA survey data sets are available for public download from the PHIA Project team portal (https://phia-data.icap.columbia.edu/datasets). Eswatini: survey 1 was conducted during 2016–2017, and survey 2 during 2021; Lesotho: survey 1 was conducted during 2016–2017, and survey 2 during 2020; Malawi: survey 1 was conducted during 2015–2016, and survey 2 during 2020–2021; Uganda: survey 1 was conducted during 2016–2017, and survey 2 during 2020–2021; Zambia: survey 1 was conducted during 2016, and survey 2 during 2021; and Zimbabwe: survey 1 was conducted during 2015–2016, and survey 2 during 2021.
45 C.F.R. part 46.102(l)(2), 21 C.F.R. part 56; 42 U.S.C. Sect. 241(d); 5 U.S.C. Sect. 552a; 44 U.S.C. Sect. 3501 et seq.
Contributor Information
Simon Agolory, CDC.
Josef Amann, CDC.
Brittney Baack, CDC.
Stephanie Behel, CDC.
Anand Date, CDC.
Jeff Hanson, CDC.
William P. Killam, CDC
Hetal Patel, CDC.
Sadhna Patel, CDC.
Rituparna Pati, CDC.
Laura Porter, CDC.
Alicia Warner, CDC.
Tadesse Wuhib, CDC.
Clement Zeh, CDC.
Ana Carolina Faria E Silva Santelli, CDC Brazil Country Office.
Giselle Guevara, CDC Caribbean Regional Office.
Rosa Elena Morales, CDC Central America Regional Office.
Alexandre Kunumboa Ekra, CDC Côte d'Ivoire Country Office.
Francois Kitenge, CDC Democratic Republic of the Congo Country Office.
Luis Bonilla, CDC Dominican Republic Country Office.
Sikhathele Mazibuko, CDC Eswatini Country Office.
Tekeste Damena, CDC Ethiopia Country Office.
Patrice Joseph, CDC Haiti Country Office.
Sunita Upadhyaya, CDC India Country Office.
Indira Aitmagambetova, CDC Kazakhstan Country Office.
Jane Mwangi, CDC Kenya Country Office.
Nazira Usmanova, CDC Kyrgzstan Country Office.
Douangchanh Xaymounvong, CDC Laos Country Office.
Mugyenyi Asiimwe, CDC Lesotho Country Office.
Maida Alice, CDC Malawi Country Office.
Gillian Jessina Masamha, CDC Mozambique Country Office.
Gram Mutandi, CDC Namibia Country Office.
Solomon Odafe, CDC Nigeria Country Office.
Lacson Romel, CDC Philippines Country Office.
Canisious Musoni, CDC Rwanda Country Office.
Mary Mogashoa, CDC South Africa Country Office.
Alex Bolo, CDC South Sudan Country Office.
Aziz Nabidzhonov, CDC Tajikistan Country Office.
George Mgomella, CDC Tanzania Country Office.
Rangsima Lolekha, CDC Thailand Country Office.
Stella Alamo-Talisuna, CDC Uganda Country Office.
Nataliya Podolchak, CDC Ukraine Country Office.
Chi K Nguyen, CDC Vietnam Country Office.
Silas Quaye, CDC West Africa Regional Program Country Office.
Annie Mwila, CDC Zambia Country Office.
Ponesai Nyika, CDC Zimbabwe Country Office.
References
- 1.Braitstein P, Brinkhof MW, Dabis F, et al. ; ART Cohort Collaboration (ART-CC) groups. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet 2006;367:817–24. 10.1016/S0140-6736(06)68337-2 [DOI] [PubMed] [Google Scholar]
- 2.Siegfried N, van der Merwe L, Brocklehurst P, Sint TT. Antiretrovirals for reducing the risk of mother-to-child transmission of HIV infection. Cochrane Database Syst Rev 2011;(7):CD003510. 10.1002/14651858.CD003510.pub3 [DOI] [PubMed] [Google Scholar]
- 3.Cohen MS, Chen YQ, McCauley M, et al. ; HPTN 052 Study Team. Antiretroviral therapy for the prevention of HIV-1 transmission. N Engl J Med 2016;375:830–9. 10.1056/NEJMoa1600693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodger AJ, Cambiano V, Bruun T, et al. ; PARTNER Study Group. Risk of HIV transmission through condomless sex in serodifferent gay couples with the HIV-positive partner taking suppressive antiretroviral therapy (PARTNER): final results of a multicentre, prospective, observational study. Lancet 2019;393:2428–38. 10.1016/S0140-6736(19)30418-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bavinton BR, Pinto AN, Phanuphak N, et al. ; Opposites Attract Study Group. Viral suppression and HIV transmission in serodiscordant male couples: an international, prospective, observational, cohort study. Lancet HIV 2018;5:e438–47. 10.1016/S2352-3018(18)30132-2 [DOI] [PubMed] [Google Scholar]
- 6.Heaton LM, Bouey PD, Fu J, et al. Estimating the impact of the US President’s Emergency Plan for AIDS Relief on HIV treatment and prevention programmes in Africa. Sex Transm Infect 2015;91:615–20. 10.1136/sextrans-2014-051991 [DOI] [PubMed] [Google Scholar]
- 7.Dirlikov E, Kamoga J, Talisuna SA, et al. ; PEPFAR Uganda. Scale-up of HIV antiretroviral therapy and estimation of averted infections and HIV-related deaths—Uganda, 2004–2022. MMWR Morb Mortal Wkly Rep 2023;72:90–4. 10.15585/mmwr.mm7204a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nkambule R, Philip NM, Reid G, et al. HIV incidence, viremia, and the national response in Eswatini: two sequential population-based surveys. PLoS One 2021;16:e0260892. 10.1371/journal.pone.0260892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Medland NA, McMahon JH, Chow EP, Elliott JH, Hoy JF, Fairley CK. The HIV care cascade: a systematic review of data sources, methodology and comparability. J Int AIDS Soc 2015;18:20634. 10.7448/IAS.18.1.20634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yao K, Maruta T, Luman ET, Nkengasong JN. The SLMTA programme: transforming the laboratory landscape in developing countries. Afr J Lab Med 2014;3:194. 10.4102/ajlm.v3i2.194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Justman JE, Mugurungi O, El-Sadr WM. HIV population surveys—bringing precision to the global response. N Engl J Med 2018;378:1859–61. 10.1056/NEJMp1801934 [DOI] [PubMed] [Google Scholar]
- 12.Birx D, Zaidi I. Forward: measuring progress toward epidemic control. J Acquir Immune Defic Syndr 2021;87(Suppl 1):S1. 10.1097/QAI.0000000000002700 [DOI] [PubMed] [Google Scholar]
- 13.Fisher KA, Patel SV, Mehta N, et al. ; PEPFAR Strategic Information Study Group. Lessons learned from programmatic gains in HIV service delivery during the COVID-19 pandemic—41 PEPFAR-supported countries, 2020. MMWR Morb Mortal Wkly Rep 2022;71:447–52. 10.15585/mmwr.mm7112a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bachanas PJ, Chun HM, Mehta N, et al. Protecting the gains: analysis of HIV treatment and service delivery programme data and interventions implemented in 19 African countries during COVID-19. J Int AIDS Soc 2022;25:e26033. 10.1002/jia2.26033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boyd AT, Jahun I, Dirlikov E, et al. Expanding access to HIV services during the COVID-19 pandemic—Nigeria, 2020. AIDS Res Ther 2021;18:62. 10.1186/s12981-021-00385-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dirlikov E, Jahun I, Odafe SF, et al. ; CDC Nigeria ART Surge Team. Rapid scale-up of an antiretroviral therapy program before and during the COVID-19 pandemic—nine states, Nigeria, March 31, 2019–September 30, 2020. MMWR Morb Mortal Wkly Rep 2021;70:421–6. 10.15585/mmwr.mm7012a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Romano ER, Sleeman K, Hall-Eidson P, et al. Contribution of PEPFAR-supported HIV and TB molecular diagnostic networks to COVID-19 testing preparedness in 16 countries. Emerg Infect Dis 2022;28:S59–68. 10.3201/eid2813.220789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kloek M, Bulstra CA, van Noord L, Al-Hassany L, Cowan FM, Hontelez JAC. HIV prevalence among men who have sex with men, transgender women and cisgender male sex workers in sub-Saharan Africa: a systematic review and meta-analysis. J Int AIDS Soc 2022;25:e26022. 10.1002/jia2.26022 [DOI] [PMC free article] [PubMed] [Google Scholar]


