Abstract
Purpose
Accelerated atherosclerosis is a major complication in patients with end-stage renal disease and it plays an important role in the pathogenesis of erectile dysfunction (ED). However, the association between aortic calcification burden and the severity of ED remains unclear. The aim of the present study was to investigate this association in men undergoing dialysis.
Materials and Methods
This cross-sectional study included 71 men undergoing peritoneal dialysis and/or hemodialysis between July 2016 and May 2018 at Mutsu General Hospital. ED was assessed with the Sexual Health Inventory for Men (SHIM). Patients were divided into the mild/moderate (SHIM score ≥8) and severe ED groups (SHIM score ≤7). Aortic calcification index (ACI) was examined as a clinical indicator of abdominal aortic calcification. Multivariable logistic regression analysis was performed to identify the significant factors associated with severe ED.
Results
The median age of the study participants was 64 years; all had ED, with 64.8% having severe ED. In the multivariable analyses, a slight association was observed between ankle-brachial index and severe ED (odds ratio [OR], 0.058; p=0.072), whereas ACI was significantly associated with severe ED (OR, 1.022; p=0.022).
Conclusions
Aortic calcification burden was independently associated with severe ED.
Keywords: Atherosclerosis, Dialysis, End-stage renal disease, Erectile dysfunction, Vascular calcification
INTRODUCTION
Erectile dysfunction (ED) is a frequent complication in men with end-stage renal disease (ESRD), and previous studies have reported that its prevalence reaches 80% [1,2].
ED has a negative impact on the quality of life (QOL) of men with ESRD as well as in healthy men [3]. More severe ED is also associated with a lower QOL in men undergoing hemodialysis (HD) [4]. However, only 1% of men consult with their primary physician about erectile problems [5], despite ED being a potentially treatable complication even in men undergoing dialysis [6]. Therefore, physicians are encouraged to proactively ask about patients’ sexual problems to improve their QOL. Because there are currently no robust indicators for severe ED, identifying these is crucial to provide appropriate treatment and eventually improve QOL in such men. Moreover, ED is a well-known predictor of future cardiovascular events [7] and symptoms of ED precede clinically overt cardiovascular disease (CVD) by 2 to 3 years [8,9]. Thus, investigating the pathogenesis of ED is also important.
Aortic calcification is one of the markers for atherosclerosis and can be simply and quantitatively measured by computed tomography (CT) images [10,11]. Because accelerated atherosclerosis is a major complication in patients with ESRD [12], it plays an important role in the pathogenesis of ED [13,14]. Therefore, we hypothesized that aortic calcification burden might be a useful indicator for severe ED in men undergoing dialysis.
The present study aimed to investigate the association between aortic calcification burden and the severity of ED in men undergoing peritoneal dialysis (PD) and/or HD.
MATERIALS AND METHODS
1. Ethics statement
This study was conducted in accordance with the ethical standards of the Declaration of Helsinki and was approved by the ethics review board of Mutsu General Hospital (authorization number: H28-2). All participants provided written informed consent.
2. Patient selection
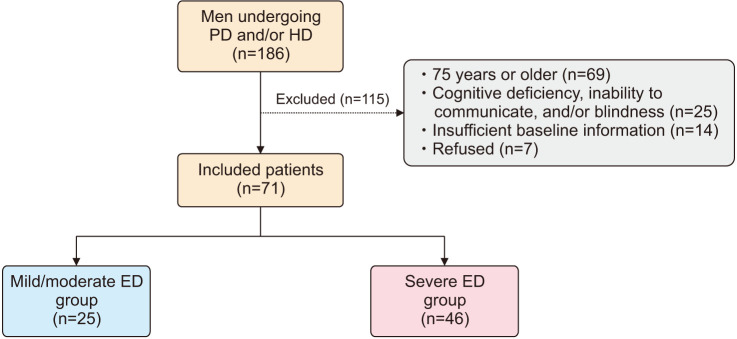
This cross-sectional study assessed 186 men undergoing PD and/or HD between July 2016 and May 2018 at Mutsu General Hospital. Among the 186 men identified, 115 were excluded based on the following exclusion criteria: (1) aged 75 years or older; (2) cognitive deficiency, inability to communicate, and/or blindness; (3) insufficient baseline information; and (4) refusal to participate in this study. Finally, 71 men undergoing PD and/or HD were included (Fig. 1).
Fig. 1. Patient selection. The number of included and excluded patients is shown. PD: peritoneal dialysis, HD: hemodialysis, ED: erectile dysfunction.
3. Evaluation of variables
The following variables were analyzed: age, body mass index (BMI), hypertension (HTN), dyslipidemia, diabetes mellitus (DM), CVD, depression, laboratory values, cardio-ankle vascular index (CAVI), ankle-brachial index (ABI), education level, marital status, smoking status, current habitual drinking, and medications (i.e., β-blockers, calcium [Ca]-blockers, thiazide diuretics, spironolactone, methyldopa, clonidine, and antidepressants). BMI was calculated as dry weight in kilograms divided by the square of height in meters. HTN was defined as taking antihypertensive medications and/or having a systolic blood pressure of ≥140 mmHg and/or a diastolic blood pressure of ≥90 mmHg. Dyslipidemia was defined as taking lipid lowering medications and/or having a low-density lipoprotein (LDL) cholesterol of ≥140 mg/dL, a triglyceride concentration of ≥150 mg/dL, and/or high-density lipoprotein cholesterol of <40 mg/dL. DM was defined as taking glycemic control medications and/or having a history of type 2 DM. The following variables were measured as part of routine clinical examination: CAVI, ABI, and laboratory blood test, including serum hemoglobin, creatinine, albumin, Ca, inorganic phosphorus, intact parathyroid hormone (iPTH), total cholesterol, LDL cholesterol, and triglycerides. Serum total testosterone was measured for research purpose. Blood sampling was performed in the morning. Blood was drawn after 12 hours of fasting and serum was separated by centrifugation with 3,000 rpm for 5 minutes. Laboratory values except for total testosterone were measured using an enzymatic assay with an automatic analyzer (BioMajesty 6070 G; Nihon denshi, Tokyo, Japan). Serum total testosterone was measured using chemiluminescent immunoassay. Adjusted Ca levels were calculated using Payne’s formula [15]. Self-reported depression was assessed using the vitality questionnaire of the Short Form 36 Health Survey. Patients who had responses to the questionnaire such as “all of the time” or “most of the time” and who were taking antidepressants were considered as having depression [16]. CAVI and ABI were measured using the VaSera VS-1500A device (Fukuda Denshi Co., Tokyo, Japan). The averages of right and left CAVI and ABI values were used for analyses.
4. Evaluation of erectile dysfunction
ED was assessed using the Sexual Health Inventory for Men (SHIM): a validated abbreviated version of the International Index of Erectile Function [17]. The SHIM scores are interpreted as follows: no ED (≥22), mild ED (17–21), moderate ED (8–16), and severe ED (≤7). Accordingly, men were divided into the mild/moderate (SHIM score ≥8) and severe ED groups (SHIM score ≤7) (Fig. 1).
5. Measurement of aortic calcification index
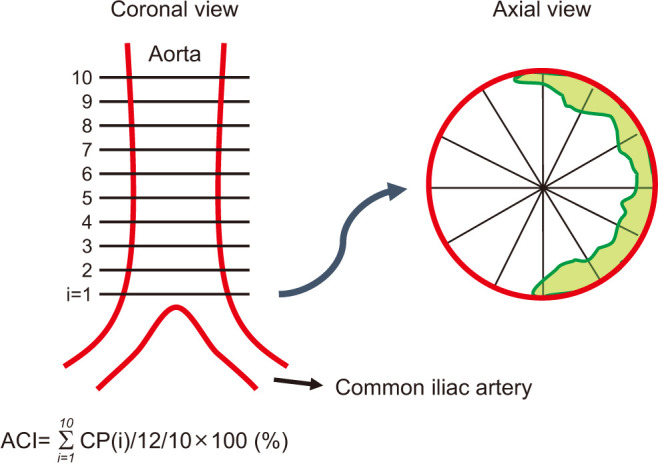
Aortic calcification index (ACI) was examined as a clinical indicator of abdominal aortic calcification. ACI was quantitatively measured using abdominal CT images (Revolution EVO; GE Healthcare Japan, Tokyo, Japan) by evaluating 10 slices of the aorta scanned at 10-mm intervals above the bifurcation of the common iliac arteries as previously described [18]. Each slice was divided into 12 sectors, and the numbers of sectors with calcification were counted. For example, if 8 out of 12 sectors were calcified in slice 1, this was scored as 8/12=66.7% (Fig. 2). The ACI (%) was calculated by averaging the percentage of calcification-positive sectors in slices 1–10. ACI was measured by a single investigator in a blinded manner. All abdominal CT examinations were performed as an annual routine screening for renal cell carcinoma within 6 months before or after evaluating the SHIM score.
Fig. 2. Measurement of aortic calcification index (ACI). ACI was quantitatively measured using abdominal computed tomography images by evaluating 10 slices of the aorta scanned at 10-mm intervals above the bifurcation of the common iliac arteries. Each slice was divided into 12 sectors, and the numbers of sectors with calcification were counted. The ACI (%) was calculated by averaging the percentage of calcification-positive sectors in slices 1–10. The green area in the axial view indicates calcification. CP: calcification profile.
6. Statistical analysis
Statistical analyses were performed using SPSS version 24.0 (IBM Corp., Armonk, NY, USA), GraphPad Prism 5.03 (GraphPad Software, San Diego, CA, USA), and R 3.5.1 (The R Foundation for Statistical Computing, Vienna, Austria). Quantitative variables were expressed as median with interquartile range. Categorical variables were compared using the Fisher exact test or chi-squared test. Quantitative variables were compared using the Mann–Whitney U-test. Correlation was analyzed using Spearman’s rank correlation coefficient. The optimal cutoff value of ACI for severe ED was calculated with the receiver operating characteristic curve. Uni- and multivariable logistic regression analyses were performed to identify the significant factors associated with severe ED. Since ABI and ACI are similar indices for evaluating atherosclerosis, we included these in multivariable regression models separately. Thus, two types of multivariable regression analyses were performed. Statistical significance was set at p<0.05.
RESULTS
1. Patient background
The median age and dialysis duration of the study participants were 64 years and 44 months, respectively. Among the 71 patients, 8 (11.3%), 60 (84.5%), and 3 (4.2%) were undergoing PD, HD, and a combination of once-weekly HD with PD, respectively (Table 1).
Table 1. Patient background.
| Variable | All (n=71) | Mild/moderate ED group (n=25) | Severe ED group (n=46) | p-value | |
|---|---|---|---|---|---|
| Age, y | 64 (55–66) | 58 (46–65) | 65 (58–67) | 0.009 | |
| Body mass index, kg/m2 | 23 (20–28) | 25 (20–29) | 23 (20–25) | 0.284 | |
| Hypertension | 65 (91.5) | 24 (96.0) | 41 (89.1) | 0.414 | |
| Dyslipidemia | 46 (64.8) | 15 (60.0) | 31 (67.4) | 0.533 | |
| Diabetes mellitus | 42 (59.2) | 16 (64.0) | 26 (56.5) | 0.540 | |
| Cardiovascular disease | 24 (33.8) | 7 (28.0) | 17 (37.0) | 0.446 | |
| Depression | 4 (5.6) | 1 (4.0) | 3 (6.5) | >0.999 | |
| Type of dialysis | 0.746 | ||||
| PD | 8 (11.3) | 4 (16.0) | 4 (8.7) | ||
| HD | 60 (84.5) | 20 (80.0) | 40 (87.0) | ||
| Combination of PD and HD | 3 (4.2) | 1 (4.0) | 2 (4.3) | ||
| Duration of dialysis, mo | 44 (11–86) | 26 (4–74) | 53 (14–102) | 0.079 | |
| Medications | |||||
| β-blockers | 30 (42.3) | 11 (44.0) | 19 (41.3) | 0.826 | |
| Calcium-blockers | 28 (39.4) | 12 (48.0) | 16 (34.8) | 0.276 | |
| Thiazide diuretics | 1 (1.4) | 0 (0.0) | 1 (2.2) | >0.999 | |
| Laboratory blood test | |||||
| Hemoglobin, g/dL | 12 (11–13) | 12 (11–13) | 12 (10–12) | 0.427 | |
| Creatinine, mg/dL | 11 (9.2–13.0) | 11 (9.5–13.0) | 11 (8.8–13.0) | 0.923 | |
| Phosphorus, mg/dL | 4.6 (3.9–5.8) | 5.1 (4.3–6.9) | 4.2 (3.7–5.6) | 0.056 | |
| Adjusted calcium, mg/dL | 8.8 (8.3–9.2) | 8.7 (8.2–9.1) | 8.8 (8.4–9.4) | 0.235 | |
| iPTH, pg/mL | 125 (78–214) | 147 (90–244) | 106 (63–189) | 0.043 | |
| Total cholesterol, mg/dL | 148 (123–196) | 152 (133–189) | 148 (111–207) | 0.805 | |
| LDL-cholesterol, mg/dL | 68 (50–95) | 65 (50–92) | 68 (55–96) | 0.466 | |
| Triglyceride, mg/dL | 137 (103–173) | 171 (110–174) | 118 (92–141) | 0.211 | |
| Testosterone, ng/dL | 420 (221–561) | 281 (229–523) | 467 (220–581) | 0.190 | |
| Smoking status | >0.999 | ||||
| Never | 15 (21.1) | 5 (20.0) | 10 (21.7) | ||
| Former | 44 (62.0) | 16 (64.0) | 28 (60.9) | ||
| Current | 12 (16.9) | 4 (16.0) | 8 (17.4) | ||
| Current habitual drinking | 18 (25.4) | 5 (20.0) | 13 (28.3) | 0.572 | |
| Post-high school education | 47 (66.2) | 17 (68.0) | 30 (65.2) | 0.813 | |
| Having current sexual partner | 40 (56.3) | 14 (56.0) | 26 (56.5) | 0.966 | |
| Cardio-ankle vascular index | 9.6 (8.3–11.0) | 8.7 (8.1–11.0) | 9.8 (8.9–11.0) | 0.088 | |
| Ankle-brachial index | 1.1 (0.9–1.1) | 1.1 (1.0–1.2) | 1.0 (0.9–1.1) | 0.087 | |
| Aortic calcification index, % | 61 (28–85) | 28 (10–75) | 70 (37–90) | <0.001 | |
| SHIM score | 4 (1–11) | 14 (11–19) | 2 (1–4) | <0.001 | |
Values are presented as median (interquartile range) or number (%).
ED: erectile dysfunction, PD: peritoneal dialysis, HD: hemodialysis, iPTH: intact parathyroid hormone, LDL: low-density lipoprotein, SHIM: sexual health inventory for men.
Median SHIM score was 4.0. The prevalence of any level of ED was 100%. Of those, 25 (35.2%) and 46 (64.8%) patients reported mild/moderate (mild/moderate ED group) and severe ED (severe ED group), respectively (Fig. 1). No patient received any type of treatment for ED at the time of investigation.
No significant differences in patient background were observed between both groups except for age, iPTH, and ACI (Table 1). No patients were taking spironolactone, methyldopa, or clonidine in both groups.
2. Correlation between aortic calcification index and SHIM score
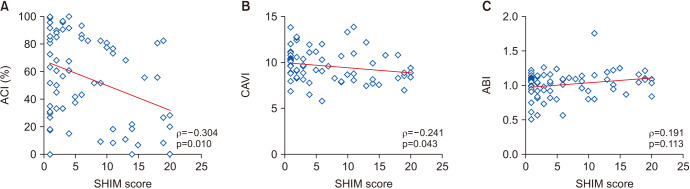
Spearman’s rank correlation test demonstrated a significant negative correlation between SHIM scores and ACI (ρ=−0.304, p=0.010; Fig. 3A) and between SHIM scores and CAVI (ρ=−0.241, p=0.043; Fig. 3B). On the other hand, no significant correlation was observed between SHIM scores and ABI (ρ=0.191, p=0.113; Fig. 3C).
Fig. 3. Correlations between the Sexual Health Inventory for Men (SHIM) scores and aortic calcification index (ACI) (A), cardio-ankle vascular index (CAVI) (B), and ankle-brachial index (ABI) (C) were analyzed using Spearman’s rank correlation coefficient.
3. Association between aortic calcification index and the severity of erectile dysfunction
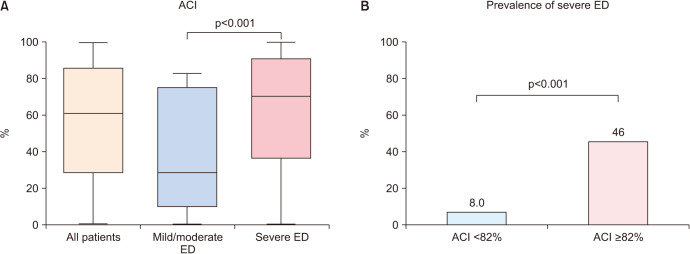
The median ACI in all patients was 61% (Table 1, Fig. 4A). The severe ED group had significantly higher ACI than the mild/moderate ED group (Table 1, Fig. 4A; 70% vs. 28%, respectively, p<0.001). When we focused on only patients undergoing HD (n=57), similar result was observed (62% vs. 52%, respectively, p=0.022; Supplement Fig. 1).
Fig. 4. Association between aortic calcification index (ACI) and the Sexual Health Inventory for Men (SHIM) score. ACI was compared between the mild/moderate and severe erectile dysfunction (ED) groups using the Mann–Whitney U-test (A). The prevalence of severe ED was compared between patients with ACI <82% and ACI ≥82% using the Fisher exact test (B).
The optimal cutoff value of ACI for severe ED was 82% (Supplement Fig. 2); patients with ACI ≥82% had significantly higher prevalence of severe ED than those with ACI <82% (46% vs. 8.0%, respectively, p<0.001; Fig. 4B).
4. Uni- and multivariable analyses for severe erectile dysfunction
In the univariable analyses, age, ABI, and ACI were significantly associated with severe ED (Table 2). In the multivariable analysis (Model 1; including ABI), a slight association was observed between ABI and severe ED (odds ratio [OR], 0.058; 95% confidence interval [CI], 0.003–1.295; p=0.072; Table 3) and only age was significantly and independently associated with severe ED (OR, 1.072; 95% CI, 1.013–1.135; p=0.016; Table 3). In the multivariable analysis (Model 2; including ACI), only ACI was significantly and independently associated with severe ED (OR, 1.022; 95% CI, 1.003–1.041; p=0.022; Table 3). When we focused on only patients undergoing HD, only ACI was associated with severe ED (OR, 1.019; 95% CI, 1.000–1.039; p=0.050; Supplement Table 1).
Table 2. Univariable analyses for severe erectile dysfunction.
| Variable | Factor | p-value | Odds ratio | 95% confidence interval |
|---|---|---|---|---|
| Age | Continuous | 0.013 | 1.072 | 1.015–1.132 |
| Body mass index | Continuous | 0.491 | 0.971 | 0.893–1.056 |
| Hypertension | Positive | 0.340 | 0.342 | 0.038–3.100 |
| Dyslipidemia | Positive | 0.534 | 1.378 | 0.502–3.783 |
| Diabetes mellitus | Positive | 0.541 | 0.731 | 0.268–1.994 |
| Type of dialysis | PD | 0.359 | 0.500 | 0.114–2.200 |
| Duration of dialysis | Continuous | 0.051 | 1.010 | 1.000–1.020 |
| β-blockers | Taking | 0.826 | 0.896 | 0.335–2.396 |
| Calcium-blockers | Taking | 0.278 | 0.578 | 0.214–1.558 |
| Serum iPTH level | Continuous | 0.161 | 0.997 | 0.992–1.001 |
| Serum testosterone level | Continuous | 0.176 | 1.002 | 0.999–1.005 |
| Smoking status | Current | 0.786 | 1.000 | 0.999–1.001 |
| Depression | Positive | 0.663 | 1.674 | 0.165–17.00 |
| Cardio-ankle vascular index | Continuous | 0.257 | 1.183 | 0.885–1.581 |
| Ankle-brachial index | Continuous | 0.049 | 0.045 | 0.002–0.987 |
| Aortic calcification index | Continuous | 0.003 | 1.027 | 1.009–1.045 |
PD: peritoneal dialysis, iPTH: intact parathyroid hormone.
Table 3. Multivariable analyses for severe erectile dysfunction.
| Model and variable | Factor | p-value | Odds ratio | 95% confidence interval | |
|---|---|---|---|---|---|
| Model 1 | |||||
| Age | Continuous | 0.016 | 1.072 | 1.013–1.135 | |
| Ankle-brachial index | Continuous | 0.072 | 0.058 | 0.003–1.295 | |
| Model 2 | |||||
| Age | Continuous | 0.141 | 1.046 | 0.985–1.111 | |
| Aortic calcification index | Continuous | 0.022 | 1.022 | 1.003–1.041 | |
DISCUSSION
Although accelerated atherosclerosis is a major complication and plays an important role in the pathogenesis of ED in men undergoing dialysis [12,13,14,19], to the best of our knowledge, no study has investigated the impact of aortic calcification burden on the severity of ED, and this is the first study to investigate this association. We found that ACI was significantly associated with severe ED. Patients undergoing dialysis might be reticent to discuss their sexual problems with a physician and some patients perceive ED as part of the normal aging process rather than complication [14,20]. Thus, these results might be helpful for identifying and eventually treating men suffering from severe ED.
In the present study, we evaluated aortic calcification burden as a surrogate marker of atherosclerosis. Ideally, the condition of the internal pudendal artery should be evaluated because this artery is responsible for the arteriogenic ED [21]. However, it is difficult to evaluate atherosclerosis of the internal pudendal artery in clinical practice because of its size [22]. Another alternative site is the coronary artery. Several studies reported a negative correlation between coronary artery calcification burden and the International Index of Erectile Function 5 (IIEF-5) score without adjustment for other confounding variables in men undergoing HD [23,24]. However, it is also difficult to measure its calcification burden due to the facility restriction [22]. On the other hand, aortic calcification burden can be easily and quantitatively measured by general CT images [18]. It takes only 1 to 2 minutes to analyze a single subject. Because atherosclerosis theoretically affects all arteries at the same time to the same extent [25], it is reasonable to measure aortic calcification burden as a surrogate marker of atherosclerosis of penile arteries. Other non-invasive surrogate markers of arteriosclerosis include the CAVI, ABI, and intima-media thickness (IMT) of carotid arteries. Stolic and Bukumiric [26] reported a negative correlation between the IMT of carotid arteries and IIEF-5 score in men undergoing HD. However, because they could not adjust for other confounding variables, the conclusion was not definitive. Regarding CAVI and ABI, these indices were not selected as independent factors associated with severe ED in the present study (Table 2, 3). Moreover, it was reported that accurate ABI values might not be obtained in cases with high arterial calcification burden [27]. Therefore, aortic calcification burden might be a better surrogate marker of atherosclerosis of the penile arteries, considering its correlation with the severity of ED, ease of measurement, and non-invasiveness. However, to our knowledge, no study has demonstrated the correlation between aortic calcification burden and penile artery calcification burden. Further studies are needed to address this issue.
Although it is well-known that the pathogenesis of ED in men undergoing dialysis is multifactorial [2,5,28,29,30], it remains unclear which factor has the greatest contribution to the severity of ED. Collaborative Depression and Sexual Dysfunction in Hemodialysis Working Group [1] conducted a multinational cross-sectional study with relatively larger sample size to identify correlates for severe ED in men undergoing HD. Age, HTN, DM, depression, endocrine abnormalities, and unmarried status were found to be significantly associated with severe ED in a multivariable model. However, they did not evaluate atherosclerosis, which may play a crucial role in the pathogenesis of ED in men undergoing dialysis [13,14]. Although many possible factors (i.e., age, HTN, dyslipidemia, DM, anemia, mineral metabolism disorders, depression, serum testosterone level, smoking status, habitual drinking, marital status, and medications) were analyzed in the present study, only age and aortic calcification burden were significantly associated with severe ED. Those results suggest that arteriogenic factors might contribute more to severe ED than other factors in men undergoing dialysis.
Vascular calcification has been historically classified as (1) tunica intima calcification associated with atherosclerosis and (2) tunica media calcification mediated by a phenotypic change of vascular smooth muscle cells into bone forming cells and associated with disturbances in the metabolism of Ca, phosphorus, and vitamin D [31]. Tunica media calcification is particularly common in patients with ESRD [32,33]. Although we evaluated aortic calcification burden using CT images in the present study, CT is not sensitive enough to identify the exact location of calcification. Thus, we could not evaluate the association between the location of calcification and the severity of ED. Further study is needed to address this issue.
The present study has several limitations. First, the cross-sectional study design does not allow the determination of cause-and-effect associations. Second, a relatively small number of men were enrolled, and the number of men undergoing PD was also small. Third, we did not evaluate the imbalance in the hypothalamic–pituitary–gonadal axis, hyperprolactinemia, and zinc deficiency, which might increase the prevalence of ED in men undergoing dialysis [13,34]. Fourth, only a single examiner was tasked to measure all ACIs and the inter-observer agreement was not evaluated. Fifth, there were time lags between CT scan and ED evaluation using the SHIM. Therefore, the actual aortic calcification burden might be changed at the time of investigation. Finally, we could not compare the aortic calcification burden between patients with and without ED because all patients had ED. Despite these limitations, the present study demonstrated a negative impact of aortic calcification burden on the severity of ED in men undergoing dialysis.
CONCLUSIONS
Aortic calcification burden was independently associated with severe ED. This result might be helpful for clinicians to identify and eventually treat men suffering from severe ED.
Footnotes
Conflict of Interest: The authors have nothing to disclose.
Funding: This work was supported by a Grant-in-Aid for Scientific Research (No. 17K11119, 18K09157, and 19H05556) from the Japan Society for the Promotion of Science.
- Conceptualization: NF.
- Data curation: NF, MM.
- Validation: SH, YT, TO, HY, Takahiro Yoneyama, YH, KY, CO.
- Formal analysis: NF.
- Funding acquisition: SH, Tohru Yoneyama, CO.
- Investigation: NF.
- Methodology: NF.
- Project administration: NF.
- Supervision: SH, CO.
- Writing – original draft: NF.
- Writing – review & editing: all authors.
Data Sharing Statement
The data required to reproduce these findings cannot be shared at this time due to legal and ethical reasons.
Supplementary Materials
Supplementary materials can be found via https://doi.org/10.5534/wjmh.210230.
Association between aortic calcification index (ACI) and the Sexual Health Inventory for Men (SHIM) score in patients undergoing hemodialysis (HD). ACI was compared between the mild/moderate and severe erectile dysfunction (ED) groups using the Mann–Whitney U-test in patients undergoing HD.
Optimal cutoff values of aortic calcification index (ACI) for severe erectile dysfunction (ED). The optimal cutoff value of ACI was calculated using the receiver operating characteristic curve.
Univariable analyses for severe erectile dysfunction in patients undergoing hemodialysis
References
- 1.Collaborative Depression and Sexual Dysfunction in Hemodialysis Working Group. Vecchio M, Palmer S, De Berardis G, Craig J, Johnson D, et al. Prevalence and correlates of erectile dysfunction in men on chronic haemodialysis: a multinational cross-sectional study. Nephrol Dial Transplant. 2012;27:2479–2488. doi: 10.1093/ndt/gfr635. [DOI] [PubMed] [Google Scholar]
- 2.Rosas SE, Joffe M, Franklin E, Strom BL, Kotzker W, Brensinger C, et al. Prevalence and determinants of erectile dysfunction in hemodialysis patients. Kidney Int. 2001;59:2259–2266. doi: 10.1046/j.1523-1755.2001.00742.x. [DOI] [PubMed] [Google Scholar]
- 3.Lew-Starowicz M, Gellert R. The sexuality and quality of life of hemodialyzed patients--ASED multicenter study. J Sex Med. 2009;6:1062–1071. doi: 10.1111/j.1743-6109.2008.01040.x. [DOI] [PubMed] [Google Scholar]
- 4.Fernandes GV, dos Santos RR, Soares W, de Lima LG, de Macêdo BS, da Fonte JE, et al. The impact of erectile dysfunction on the quality of life of men undergoing hemodialysis and its association with depression. J Sex Med. 2010;7:4003–4010. doi: 10.1111/j.1743-6109.2010.01993.x. [DOI] [PubMed] [Google Scholar]
- 5.Arslan D, Aslan G, Sifil A, Cavdar C, Celebi I, Gamsari T, et al. Sexual dysfunction in male patients on hemodialysis: assessment with the International Index of Erectile Function (IIEF) Int J Impot Res. 2002;14:539–542. doi: 10.1038/sj.ijir.3900937. [DOI] [PubMed] [Google Scholar]
- 6.Türk S, Karalezli G, Tonbul HZ, Yildiz M, Altintepe L, Yildiz A, et al. Erectile dysfunction and the effects of sildenafil treatment in patients on haemodialysis and continuous ambulatory peritoneal dialysis. Nephrol Dial Transplant. 2001;16:1818–1822. doi: 10.1093/ndt/16.9.1818. [DOI] [PubMed] [Google Scholar]
- 7.Uddin SMI, Mirbolouk M, Dardari Z, Feldman DI, Cainzos-Achirica M, DeFilippis AP, et al. Erectile dysfunction as an independent predictor of future cardiovascular events: the multi-ethnic study of atherosclerosis. Circulation. 2018;138:540–542. doi: 10.1161/CIRCULATIONAHA.118.033990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanaka Y, Bundy JD, Allen NB, Uddin SMI, Feldman DI, Michos ED, et al. Association of erectile dysfunction with incident atrial fibrillation: the multi-ethnic study of atherosclerosis (MESA) Am J Med. 2020;133:613–620.e1. doi: 10.1016/j.amjmed.2019.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gowani Z, Uddin SMI, Mirbolouk M, Ayyaz D, Billups KL, Miner M, et al. Vascular erectile dysfunction and subclinical cardiovascular disease. Curr Sex Health Rep. 2017;9:305–312. doi: 10.1007/s11930-017-0137-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Albanese I, Khan K, Barratt B, Al-Kindi H, Schwertani A. Atherosclerotic calcification: Wnt is the hint. J Am Heart Assoc. 2018;7:e007356. doi: 10.1161/JAHA.117.007356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kälsch H, Lehmann N, Moebus S, Hoffmann B, Stang A, Jöckel KH, et al. Aortic calcification onset and progression: association with the development of coronary atherosclerosis. J Am Heart Assoc. 2017;6:e005093. doi: 10.1161/JAHA.116.005093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindner A, Charra B, Sherrard DJ, Scribner BH. Accelerated atherosclerosis in prolonged maintenance hemodialysis. N Engl J Med. 1974;290:697–701. doi: 10.1056/NEJM197403282901301. [DOI] [PubMed] [Google Scholar]
- 13.Kaufman JM, Hatzichristou DG, Mulhall JP, Fitch WP, Goldstein I. Impotence and chronic renal failure: a study of the hemodynamic pathophysiology. J Urol. 1994;151:612–618. doi: 10.1016/s0022-5347(17)35030-9. [DOI] [PubMed] [Google Scholar]
- 14.Bellinghieri G, Savica V, Santoro D. Vascular erectile dysfunction in chronic renal failure. Semin Nephrol. 2006;26:42–45. doi: 10.1016/j.semnephrol.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 15.Payne RB, Little AJ, Williams RB, Milner JR. Interpretation of serum calcium in patients with abnormal serum proteins. Br Med J. 1973;4:643–646. doi: 10.1136/bmj.4.5893.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soma O, Hatakeyama S, Imai A, Matsumoto T, Hamano I, Fujita N, et al. Relationship between frailty and lower urinary tract symptoms among community-dwelling adults. Low Urin Tract Symptoms. 2020;12:128–136. doi: 10.1111/luts.12292. [DOI] [PubMed] [Google Scholar]
- 17.Ramanathan R, Mulhall J, Rao S, Leung R, Martinez Salamanca JI, Mandhani A, et al. Predictive correlation between the International Index of Erectile Function (IIEF) and Sexual Health Inventory for Men (SHIM): implications for calculating a derived SHIM for clinical use. J Sex Med. 2007;4:1336–1344. doi: 10.1111/j.1743-6109.2007.00576.x. [DOI] [PubMed] [Google Scholar]
- 18.Tsushima M, Terayama Y, Momose A, Funyu T, Ohyama C, Hada R. Carotid intima media thickness and aortic calcification index closely relate to cerebro- and cardiovascular disorders in hemodialysis patients. Int J Urol. 2008;15:48–51. doi: 10.1111/j.1442-2042.2007.01925.x. discussion 51-2. [DOI] [PubMed] [Google Scholar]
- 19.Lue TF. Erectile dysfunction. N Engl J Med. 2000;342:1802–1813. doi: 10.1056/NEJM200006153422407. [DOI] [PubMed] [Google Scholar]
- 20.Nassir A. Sexual function in male patients undergoing treatment for renal failure: a prospective view. J Sex Med. 2009;6:3407–3414. doi: 10.1111/j.1743-6109.2009.01411.x. [DOI] [PubMed] [Google Scholar]
- 21.Pereira JA, Bilhim T, Rio Tinto H, Fernandes L, Martins Pisco J, Goyri-O'Neill J. Radiologic anatomy of arteriogenic erectile dysfunction: a systematized approach. Acta Med Port. 2013;26:219–225. [PubMed] [Google Scholar]
- 22.Matsuura T, Abe T, Onoda M, Ikarashi D, Sugimura J, Komaki T, et al. Pelvic artery calcification score is a marker of vascular calcification in male hemodialysis patients. Ther Apher Dial. 2018;22:509–513. doi: 10.1111/1744-9987.12668. [DOI] [PubMed] [Google Scholar]
- 23.Roy N, Rosas SE. Erectile dysfunction and coronary artery calcification in incident dialysis patients. J Nephrol. 2021;34:1521–1529. doi: 10.1007/s40620-021-00994-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inci K, Hazirolan T, Aki FT, Oruc O, Tombul T, Tasar C, et al. Coronary artery calcifications in hemodialysis patients and their correlation with the prevalence of erectile dysfunction. Transplant Proc. 2008;40:77–80. doi: 10.1016/j.transproceed.2007.11.046. [DOI] [PubMed] [Google Scholar]
- 25.Montorsi P, Ravagnani PM, Galli S, Rotatori F, Briganti A, Salonia A, et al. Common grounds for erectile dysfunction and coronary artery disease. Curr Opin Urol. 2004;14:361–365. doi: 10.1097/00042307-200411000-00012. [DOI] [PubMed] [Google Scholar]
- 26.Stolic RV, Bukumiric ZM. Intima-media thickness of carotid arteries and erectile dysfunction in hemodialysis patients. Hemodial Int. 2010;14:510–514. doi: 10.1111/j.1542-4758.2010.00493.x. [DOI] [PubMed] [Google Scholar]
- 27.Ohtake T, Oka M, Ikee R, Mochida Y, Ishioka K, Moriya H, et al. Impact of lower limbs' arterial calcification on the prevalence and severity of PAD in patients on hemodialysis. J Vasc Surg. 2011;53:676–683. doi: 10.1016/j.jvs.2010.09.070. [DOI] [PubMed] [Google Scholar]
- 28.Bommer J, Ritz E, del Pozo E, Bommer G. Improved sexual function in male haemodialysis patients on bromocriptine. Lancet. 1979;2:496–497. doi: 10.1016/s0140-6736(79)91553-8. [DOI] [PubMed] [Google Scholar]
- 29.Chopp RT, Mendez R. Sexual function and hormonal abnormalities in uremic men on chronic dialysis and after renal transplantation. Fertil Steril. 1978;29:661–666. doi: 10.1016/s0015-0282(16)43341-8. [DOI] [PubMed] [Google Scholar]
- 30.Massry SG, Goldstein DA, Procci WR, Kletzky OA. Impotence in patients with uremia: a possible role for parathyroid hormone. Nephron. 1977;19:305–310. doi: 10.1159/000180907. [DOI] [PubMed] [Google Scholar]
- 31.Coll B, Betriu A, Martínez-Alonso M, Amoedo ML, Arcidiacono MV, Borras M, et al. Large artery calcification on dialysis patients is located in the intima and related to atherosclerosis. Clin J Am Soc Nephrol. 2011;6:303–310. doi: 10.2215/CJN.04290510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moe SM, O'Neill KD, Duan D, Ahmed S, Chen NX, Leapman SB, et al. Medial artery calcification in ESRD patients is associated with deposition of bone matrix proteins. Kidney Int. 2002;61:638–647. doi: 10.1046/j.1523-1755.2002.00170.x. [DOI] [PubMed] [Google Scholar]
- 33.Ballanti P, Silvestrini G, Pisanò S, De Paolis P, Di Giulio S, Mantella D, et al. Medial artery calcification of uremic patients: a histological, histochemical and ultrastructural study. Histol Histopathol. 2011;26:191–200. doi: 10.14670/HH-26.191. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki E, Nishimatsu H, Oba S, Takahashi M, Homma Y. Chronic kidney disease and erectile dysfunction. World J Nephrol. 2014;3:220–229. doi: 10.5527/wjn.v3.i4.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Association between aortic calcification index (ACI) and the Sexual Health Inventory for Men (SHIM) score in patients undergoing hemodialysis (HD). ACI was compared between the mild/moderate and severe erectile dysfunction (ED) groups using the Mann–Whitney U-test in patients undergoing HD.
Optimal cutoff values of aortic calcification index (ACI) for severe erectile dysfunction (ED). The optimal cutoff value of ACI was calculated using the receiver operating characteristic curve.
Univariable analyses for severe erectile dysfunction in patients undergoing hemodialysis
Data Availability Statement
The data required to reproduce these findings cannot be shared at this time due to legal and ethical reasons.