Abstract
Purpose
Despite the significant role of varicocele in the pathogenesis of male infertility, the impact of varicocele repair (VR) on conventional semen parameters remains controversial. Only a few systematic reviews and meta-analyses (SRMAs) have evaluated the impact of VR on sperm concentration, total motility, and progressive motility, mostly using a before-after analytic approach. No SRMA to date has evaluated the change in conventional semen parameters after VR compared to untreated controls. This study aimed to evaluate the effect of VR on conventional semen parameters in infertile patients with clinical varicocele compared to untreated controls.
Materials and Methods
A literature search was performed using Scopus, PubMed, Embase, and Cochrane databases following the Population Intervention Comparison Outcome (PICOS) model (Population: infertile patients with clinical varicocele; Intervention: VR [any technique]; Comparison: infertile patients with clinical varicocele that were untreated; Outcome: sperm concentration, sperm total count, progressive sperm motility, total sperm motility, sperm morphology, and semen volume; Study type: randomized controlled trials and observational studies).
Results
A total of 1,632 abstracts were initially assessed for eligibility. Sixteen studies were finally included with a total of 2,420 infertile men with clinical varicocele (1,424 patients treated with VR vs. 996 untreated controls). The analysis showed significantly improved post-operative semen parameters in patients compared to controls with regards to sperm concentration (standardized mean difference [SMD] 1.739; 95% CI 1.129 to 2.349; p<0.001; I2=97.6%), total sperm count (SMD 1.894; 95% CI 0.566 to 3.222; p<0.05; I2=97.8%), progressive sperm motility (SMD 3.301; 95% CI 2.164 to 4.437; p<0.01; I2=98.5%), total sperm motility (SMD 0.887; 95% CI 0.036 to 1.738; p=0.04; I2=97.3%) and normal sperm morphology (SMD 1.673; 95% CI 0.876 to 2.470; p<0.05; I2=98.5%). All the outcomes showed a high inter-study heterogeneity, but the sensitivity analysis showed that no study was sensitive enough to change these results. Publication bias was present only in the analysis of the sperm concentration and progressive motility. No significant difference was found for the semen volume (SMD 0.313; 95% CI -0.242 to 0.868; I2=89.7%).
Conclusions
This study provides a high level of evidence in favor of a positive effect of VR to improve conventional semen parameters in infertile men with clinical varicocele. To the best of our knowledge, this is the first SRMA to compare changes in conventional semen parameters after VR with changes in parameters of a control group over the same period. This is in contrast to other SRMAs which have compared semen parameters before and after VR, without reference to a control group. Our findings strengthen the available evidence and have a potential to upgrade professional societies’ practice recommendations favoring VR to improve conventional semen parameters in infertile men.
Keywords: Male infertility, Semen, Varicocele
INTRODUCTION
Infertility is estimated to impact 15% of couples attempting to conceive worldwide [1]. The male partner is solely responsible in 17.1% of cases [2]. Varicoceles have been identified as the most common surgically correctable cause of male infertility [3]. They are common in the general male population being present in 15% of healthy men [4]. Additionally, 35% to 44% of men with primary infertility and 45% to 81% of men with secondary infertility have a varicocele [5,6]. In a study conducted by the World Health Organization (WHO), it was shown that men with varicocele had lower sperm concentration and motility than men without varicocele [7]. Another study carried out in healthy men in Europe identified deterioration in sperm quality even in men with a grade 1 varicocele [4].
The American Urological Association (AUA) and the American Society for Reproductive Medicine (ASRM) recommend surgical varicocele repair (VR) of clinical varicoceles in non-azoospermic infertile men with abnormal semen parameters (moderate recommendation; moderate evidence level) [8]. Unfortunately, the term “abnormal semen parameters” is not defined. These recommendations are based primarily on the study by Wang et al [9], in which the effect of different VR techniques on sperm concentration and sperm motility were meta-analyzed. In the latter study, surgical techniques with an inguinal and subinguinal approach had the greatest improvement in sperm concentration and sperm motility parameters (compared with the retroperitoneal technique) [8]. The European Association of Urology (EAU) also recommends VR in infertile men with a clinical varicocele, abnormal semen parameters, and without other male causes of infertility (strong recommendation; high evidence level) [10]. Again, the term “abnormal semen parameters” is not defined. These recommendations are essentially based on two meta-analyses (MAs) that reported an improvement of sperm concentration (mean difference [MD] 9.71 and 12.32×106/mL) [11,12], total sperm motility (MD 9.92% and 10.86%), progressive sperm motility (MD 9.69%), and sperm morphology (MD 3.16%) after microsurgical VR or surgical VR [11,12]. The European Academy of Andrology (EAA) asked the question differently, assessing the management of oligo-astheno-teratozoospermia (OAT) [13]. Based on the WHO study, which found a varicocele in a quarter of the 9,304 men with OAT [7], the EAA advises discussing VR in infertile couples whose male partner has a palpable varicocele associated with OAT, highlighting the improvement of semen parameters [11,12] and/or fertility rates after VR [14,15].
Hence, although surgical VR is recommended in infertile men with a clinical varicocele and abnormal semen parameters, the sperm parameter(s) used to determine the indication for VR and to assess its efficacy are not detailed. According to a recent worldwide survey, it is also unclear whether VR is indicated or not in cases of isolated oligozoospermia, isolated asthenozoospermia, or isolated teratozoospermia [16].
Despite the well-established role of varicocele in the pathogenesis of male infertility, current literature does not provide enough data to draw firm conclusions as to the impact of VR on semen quality and male fertility potential. Several MAs have shown that it has a positive effect of VR on semen parameters [11,12,17,18]. However, the studies published to date addressing the effect of VR on semen characteristics have been criticized for many reasons such as the inclusion of men with subclinical varicoceles or normal semen parameters, poor study designs, significant drop-outs after randomization, selective reporting or inadequate sample size. Therefore, the aim of this systematic review and meta-analysis (SRMA) was to investigate the impact of VR on conventional semen parameters (semen volume and sperm concentration, total sperm count, total motility, progressive motility, and morphology) in infertile men with clinical varicocele when compared to a control group of infertile men with clinical varicocele that did not undergo VR (i.e., no treatment). The results of the current report will provide an update of the impact of VR on semen parameters in infertile men by adding newer studies and analysing outcomes in comparision to a control group.
MATERIALS AND METHODS
1. Search strategy
A systematic search was conducted from May 2021 to August 2021 using Scopus, PubMed, Embase, and Cochrane databases. In the initial query string on Scopus, the entry term “varicoc*” was searched in combination (AND) with the terms “management” OR “embolization” OR “microsurg*” OR “micro-surg*” OR “repair” OR “correction” OR “treatment” OR “ligation” OR “surg*” OR “operation” OR “radiolog*”. Alternative entry term was “varicocelectomy”. To identify studies evaluating the effect of VR on semen parameters and natural pregnancy, additional keywords, were “sperm*” OR “semen” OR “ejaculate” OR “azoosperm*” OR “oligozoosperm*” OR “asthenozoosperm*” OR “teratozoosperm*” OR “necrozoosperm*” OR “fragmentation” OR “dna” OR “chromatin” OR “sdf” OR “azoo*” or “oxida*” OR “ros” OR “orp” OR “seminal” OR “astheno*” OR “terato*” OR “oligo*” OR “oxygen” OR “pregnan*” OR “conception” OR “child” OR “conceive*” OR “scsa” OR “tunel” OR “apoptiosis” OR “time (AND) to (AND) pregnanc*” OR “fertil*” OR “offspring*”. The query “LIMIT-TO ( DOCTYPE , “ar” )” was used to limit retrieve only original studies. The same combination of terms was used for PubMed, Embase, and Cochrane databases.
The search strategy was performed in compliance with the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) guidelines [19] and the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) [20]. Abstracts of the retrieved articles were independently screened by researchers in duplicate and assessed to confirm their eligibility by 48 researchers, who worked in pairs. Disagreements were resolved by a third person.
The protocol of this SRMA has been registered in the PROSPERO database (ID 329842).
2. Selection criteria
This SRMA included all published studies on infertile men with clinical varicocele that measured conventional semen parameters in varicocele-treated patients vs. untreated controls. There was no language restriction as translation to English was performed by native language researchers. All eligible studies were selected following the PICOS (Population, Intervention, Comparison, Outcome, Study type) model (Supplement Table 1) [21]. Studies on azoospermia, subclinical varicocele, animal studies, in vitro studies, case reports, and case series were excluded.
3. Data extraction
Data extraction was performed by 37 well-trained researchers (24 active researchers and 13 assistant team leaders) divided in four teams, under the supervision of twelve team leaders and co-team leaders. The following data were collected: study design, characteristics of varicocele (laterality, grade, classification used for grading) and VR (method, laterality), time from VR to last follow-up evaluation, number of patients and controls, semen analysis data - semen volume (mL), sperm concentration (×106/mL), total sperm count (×106), sperm vitality (%), total sperm motility (%), progressive motility (%), normal morphology (%), in the infertile men that underwent VR versus a control group that received no treatment. Data were extracted by one researcher and verified by a second researcher. Any disagreements were resolved by a third senior researcher.
4. Quality assessment
The quality of evidence of the included studies was assessed using the following checklists: the Cambridge Quality Checklist [22], which was applied to observational studies (case-control and cohort), the Cochrane Risk of Bias [23], the Jadad score [24], and the CONSORT (consolidated standards of reporting trials) guidelines [25] which were applied to randomized controlled trials (RCTs). Each study was independently evaluated by two researchers, and disagreements were resolved by a third researcher.
5. Statistical analysis
1) Quantitative data analysis
We used Comprehensive Meta-Analysis Software (Version 2; Biostat Inc., Englewood, NJ, USA) for performing MA on quantitative data. We chose the standardized mean difference (SMD) as the effect size for statistical comparison between cases and controls, which allows the comparison even between those studies which did not measure the outcome using the same instruments. Cochran’s Q test and heterogeneity index (I2) were used to assess heterogeneity across pooled studies, with p<0.10 considered as statistically significant [26]. I2 is an indicator of heterogeneity and lies between 0% and 100%, where <25% suggests low heterogeneity, 50% suggests moderate heterogeneity, and 75% suggests high heterogeneity [27]. The pooled effect size was calculated using either the fixed effect or random effects models, depending upon the level of heterogeneity. In case of low heterogeneity, the fixed effect model was adopted while in case of significant heterogeneity, random effects model was adopted. High resolution forest plots were generated for the SMD and confidence interval (CI) for all studies and the pooled data [28].
2) Cumulative analysis
Cumulative analysis was performed to assess the chronological trend of statistical significance over a period of time. For this, the effect size and the corresponding CI were calculated after the addition of each new study in a temporal manner. The trend of p-value and statistical inference was used to draw inferences regarding the strength of the association, its vulnerability to variations and the history of variations.
3) Sensitivity analysis
The pooled effect size and the corresponding CI were calculated after exclusion of one study at a time. A study resulting in the change of inference upon its exclusion was labelled as a ‘sensitive study’.
4) Publication bias analysis
Publication bias was qualitatively analysed by the asymmetry of the funnel plot, which suggested some missing studies on one side of the graph. For quantitative analysis of publication bias, we employed Egger’s intercept test, which evaluated statistical significance of publication bias. In case of the presence of publication bias, unbiased estimates were calculated using the‘trim and fill’ method [29].
The results were independently verified by four experienced biostatisticians (Rajender Singh, Ahmed Harraz, Ralf Henkel, Nicolas Garrido), and their independent analysis showed full agreement. The results reported in this article were analyzed by Rajender Singh.
RESULTS
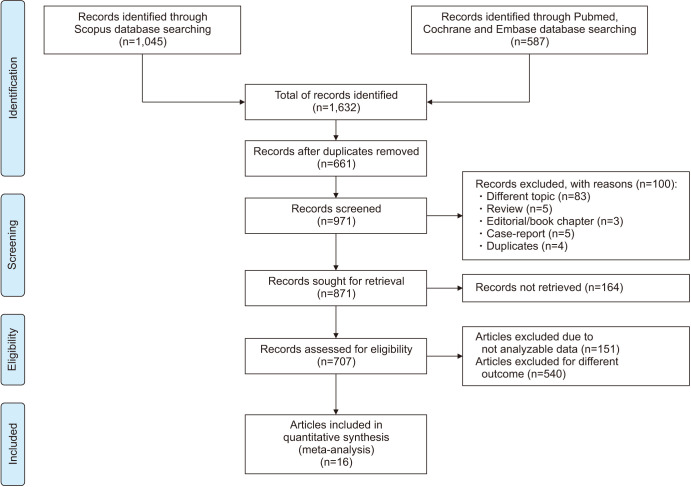
Using the above-mentioned search strategy, 1,632 abstracts were extracted. After the removal of 661 duplicates, 971 abstracts were assessed. Of these, 100 articles were identified by title and abstract as review articles, case reports, book chapters, papers on different topics or duplicates and were judged ineligible. Of the remaining 871 articles, 164 full-texts were not found, whereas 151 were definitively excluded after reading the full-text because they did not contain analyzable data. Finally, 537 articles included data of VR in the context of male infertility treatment. Of these, 19 studies assessing the impact of VR versus no treatment on basic semen parameters were included in the present analysis (Fig. 1). A full list of excluded studies, with reason, is provided in Supplement Table 2.
Fig. 1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow-chart.
Among the 19 included studies, 5 reported data on semen volume [30,31,32,33,34], 13 reported sperm concentration [30,31,34,35,36,37,38,39,40,41,42,43,44], 5 reported total sperm count [30,34,36,37,45], 7 reported total sperm motility [30,31,32,33,34,35,36], 9 reported progressive sperm motility [30,36,37,38,39,41,42,43,44], and 11 reported data on sperm morphology [30,32,33,35,36,37,38,39,41,42,43]. The main characteristics of the included studies are shown in Table 1.
Table 1. Main characteristics of the included studies.
| First author | Year | Study design | Patients (n) | Controls (n) | Type of VR | Timing of the evaluation (mo) | Outcome | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SV | SC | TSC | TM | PM | SM | |||||||
| Turgut [35] | 2020 | Observational | 52 | 36 | Microsurgical | 3 | x | x | x | |||
| Gomaa [30] | 2018 | Observational | 45 | 45 | Microsurgical | 6 | x | x | x | x | x | x |
| Ketabchi [45] | 2018 | RCT | 24 | 28 | Microsurgical | 6 | x | |||||
| McGarry [31] | 2015 | Observational | 290 | 155 | Microsurgical | 6 | x | x | x | |||
| Gokce [36] | 2013 | Observational | 168 | 138 | Microsurgical | 3 | x | x | x | x | x | |
| Mansour Ghanaie [37] | 2012 | RCT | 68 | 68 | NMI | 12 | x | x | x | x | ||
| Ghanem [38] | 2011 | Observational | 64 | 27 | Embolization | 12 | x | x | x | |||
| Abdel-Meguid [39] | 2011 | RCT | 73 | 72 | Microsurgical | x | x | x | ||||
| Sathya Srini [40] | 2011 | Observational | 100 | 100 | Microsurgical | 12 | x | |||||
| Seo [32] | 2010 | Observational | 25 | 25 | Microsurgical | 6 | x | x | x | |||
| Çakan [33] | 2008 | Observational | 28 | 23 | Unspecified | 12 | x | x | x | |||
| Di Bisceglie [41] | 2007 | Observational | 38 | 40 | Embolization | 6 | x | x | x | |||
| Di Bisceglie [42] | 2003 | Observational | 223 | 77 | Embolization | 6 | x | x | x | |||
| Grasso [43] | 2000 | RCT | 34 | 34 | Microsurgical | 12 | x | x | x | |||
| Okuyama [44] | 1988 | Observational | 141 | 83 | HR | 84 | x | x | ||||
| Nilsson [34] | 1979 | RCT | 51 | 45 | NMI | 74 | x | x | x | x | ||
HR: high retroperitoneal, NMI: non-microsurgical, PM: progressive motility, RCT: randomized controlled trial, SC: sperm concentration, SM: sperm morphology, SV: semen volume, TM: total motility, TSC: total sperm count, VR: varicocele repair.
1. Quality of evidence of included studies
Among the 19 included studies, 11 had an observational design and were evaluated using the Cambridge quality checklist; 5 were RCTs and were evaluated using the Cochrane risk bias for RCTs, the CONSORT, and the Jadad’s tools (Table 2).
Table 2. Quality of evidence assessment (results of the Cambridge Quality Checklist [22], Cochrane Risk of Bias for Randomized controlled trials [23], CONSORT guidelines [25] and Jadad score [24]).
| First author | Year | Cambridge Quality Checklist | Cochrane risk of bias for RCTs | CONSORT guidelines (0–25) | Jadad (1–5) | |||
|---|---|---|---|---|---|---|---|---|
| Checklist for correlates (0–5) | Checklist for risk factors (1–3) | Checklist for causal risk factor (1–7) | Total score (2–15) | |||||
| Turgut [35] | 2020 | 1 | 2 | 4 | 7 | |||
| Gomaa [30] | 2018 | 2 | 3 | 6 | 11 | |||
| Ketabchi [45] | 2018 | High | 15 | 1 | ||||
| McGarry [31] | 2015 | 3 | 2 | 4 | 9 | |||
| Gokce [36] | 2013 | 1 | 2 | 6 | 9 | |||
| Mansour Ghanaie [37] | 2012 | High | 14 | 1 | ||||
| Ghanem [38] | 2011 | 2 | 3 | 6 | 11 | |||
| Abdel-Meguid [39] | 2011 | High | 23 | 5 | ||||
| Sathya Srini [40] | 2011 | 1 | 3 | 6 | 10 | |||
| Seo [32] | 2010 | 0 | 2 | 6 | 8 | |||
| Çakan [33] | 2008 | 3 | 2 | 6 | 11 | |||
| Di Bisceglie [41] | 2007 | 1 | 3 | 6 | 10 | |||
| Di Bisceglie [42] | 2003 | 3 | 2 | 4 | 9 | |||
| Grasso [43] | 2000 | High | 16 | 2 | ||||
| Okuyama [44] | 1988 | 1 | 3 | 6 | 10 | |||
| Nilsson [34] | 1979 | Low | 10 | 2 | ||||
CONSORT: consolidated standards of reporting trials, High: high quality evidence, Low: low quality evidence, RCT: randomized controlled trial.
Although the Cambridge scale does not enstablish a precise threshold to differentiate between high quality and low quality studies, out of a total score of 15, 6 studies scored ≥10 [30,33,38,40,41,44], while 5 studies scored 7 to 9 [31,32,35,36,42].
Among the RCTs, the quality of 4 out 5 studies was ranked as high using the Cochrane’s tool [37,39,43,45]. The CONSORT and the JADAD’s scales both identified the best quality study among the 4 [39]. Only one RCTs was judged as of low quality [34].
2. Semen volume
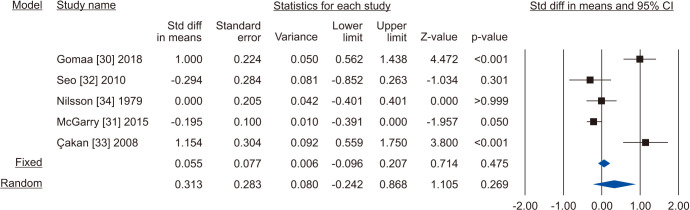
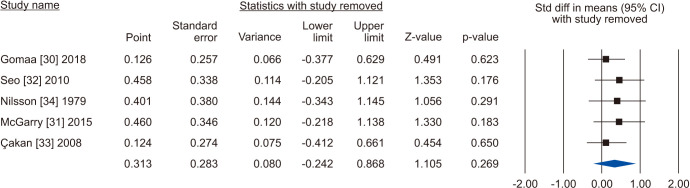
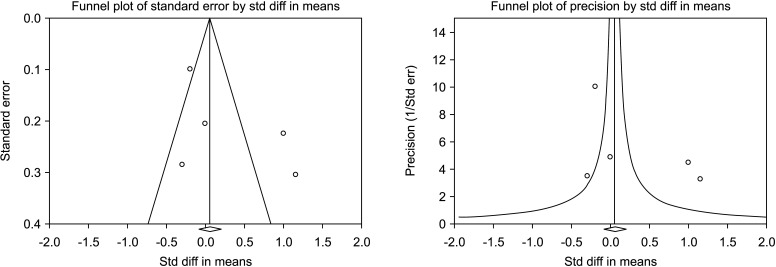
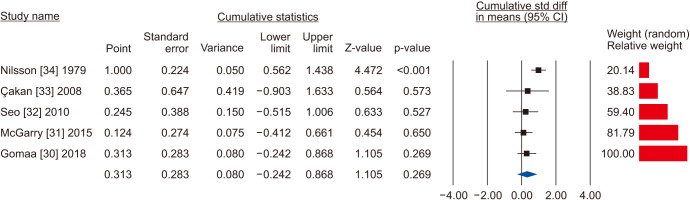
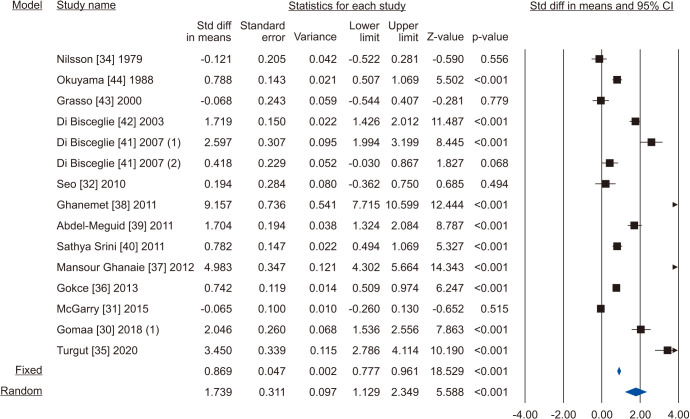
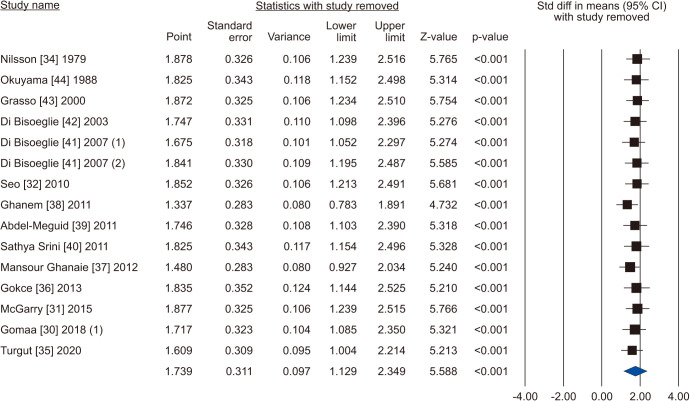
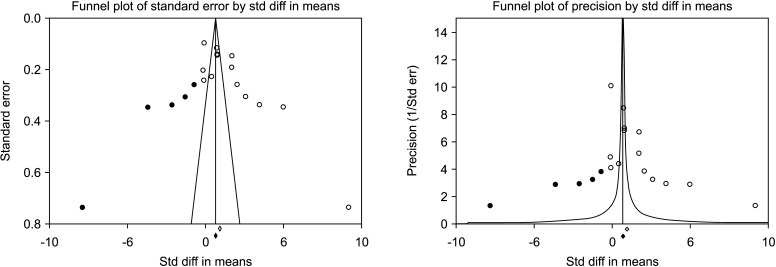
Five [30,31,32,33,34] including 732 patients, 439 cases and 293 controls, reported changes of semen volume. At random effect, SMD was 0.313 (95% CI -0.242 to 0.868; I2=89.7%; p>0.05). Hence, VR non-significantly increased the semen volume (Fig. 2). At the sensitivity analysis no study was sensitive enough to change this conclusion (Fig. 3). Also, Egger’s regression model and funnel plots reported no risk of bias (intercept=4.23; 95% CI -4.94 to 13.40; p=0.23) (Fig. 4). Cumulative analysis showed consistent non-significant change in semen volume, except the first study [34] (Fig. 5).
Fig. 2. Forest plot of the semen volume in infertile men with varicocele repair compared to no treatment.
Fig. 3. Forest plot of the semen volume in infertile men with varicocele repair compared to no treatment: sensitivity analysis.
Fig. 4. Funnel plot of the semen volume in infertile men with varicocele repair compared to no treatment.
Fig. 5. Forest plot of the semen volume in infertile men with varicocele repair compared to no treatment: cumulative analysis.
3. Sperm concentration
Fourteen studies including 2,267 subjects (1,347 cases vs. 920 controls) evaluated the sperm concentration parameter [30,31,32,34,35,36,37,38,39,40,41,42,43,44]. Due to the presence of significant heterogeneity, as demonstrated by the Q-test (Q-value=579.085; p<0.001) and the I2=97.6%, the random effect model was used. There was a significant increase in sperm concentration in favour of VR (SMD 1.739; 95% CI 1.129 to 2.349; p<0.001) (Fig. 6). No study was significantly sensitive enough to alter the above results (Fig. 7). There was a significant publication bias as demonstrated by the Egger’s test (p<0.001) and the asymmetrical funnel plots (Fig. 8). Cumulative analysis showed that sperm concentration achieved significant association with the addition of Di Bisceglie et al [41] in the pool and has stayed significant after that (Fig. 9).
Fig. 6. Forest plot of the sperm concentration in infertile men with varicocele repair compared to no treatment.
Fig. 7. Forest plot of the sperm concentration in infertile men with varicocele repair compared to no treatment: sensitivity analysis.
Fig. 8. Funnel plot of the sperm concentration in infertile men with varicocele repair compared to no treatment.
Fig. 9. Forest plot of the sperm concentration in infertile men with varicocele repair compared to no treatment: cumulative analysis.
4. Total sperm count
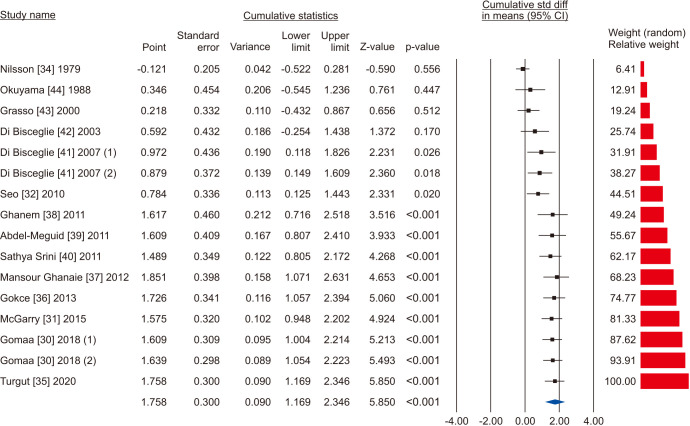
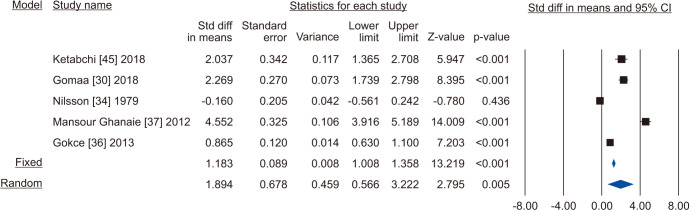
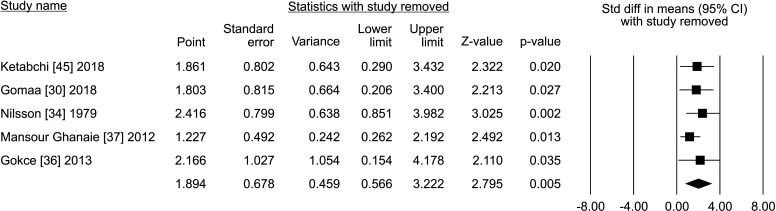
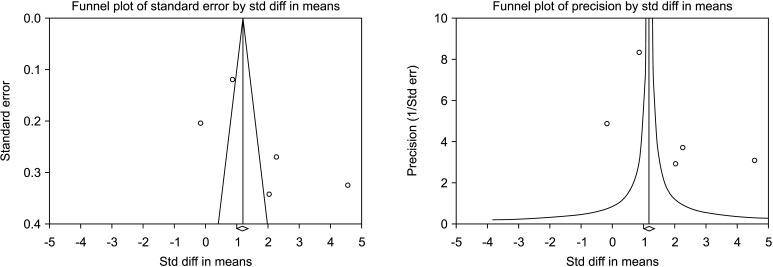
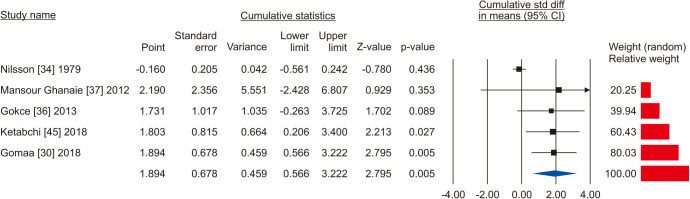
Five studies reported the total sperm count in patients treated with VR against no treatment, for a total of 1,073 patients, including 622 cases and 451 controls [30,34,36,37,45]. The analysis showed a significant improvement in the total sperm count (SMD 1.894; 95% CI 0.566 to 3.222; p<0.05; I2=97.8%) (Fig. 10), and no study was sensitive enough to change this conclusion (Fig. 11). There wasn’t significant publication bias as demonstrated by the Egger’s test (p>0.05) and the symmetrical funnel plots (Fig. 12). Cumulative analysis showed that total sperm count achieved significance with the addition of Ketabchi et al [45] and stayed significant after that (Fig. 13).
Fig. 10. Forest plot of the total sperm count in infertile men with varicocele repair compared to no treatment.
Fig. 11. Forest plot of the total sperm count in infertile men with varicocele repair compared to no treatment: sensitivity analysis.
Fig. 12. Funnel plot of the total sperm count in infertile men with varicocele repair compared to no treatment.
Fig. 13. Forest plot of the total sperm count in infertile men with varicocele repair compared to no treatment: cumulative analysis.
5. Progressive sperm motility
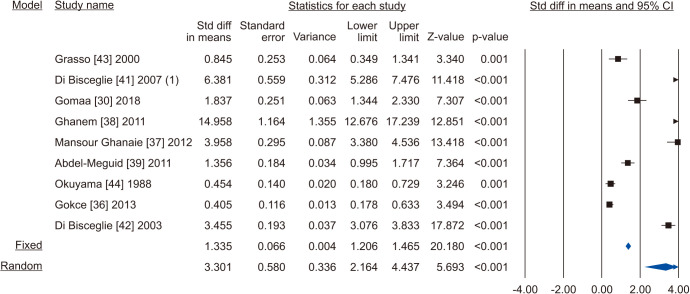
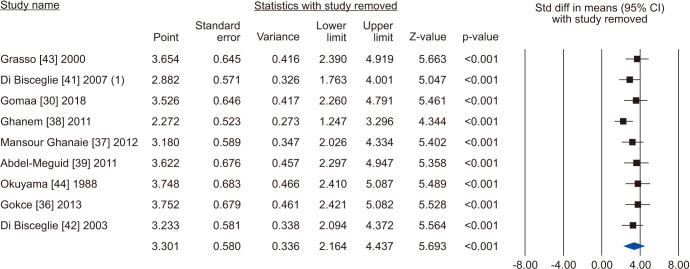
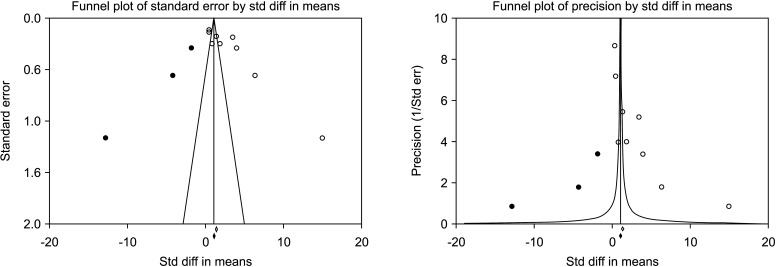
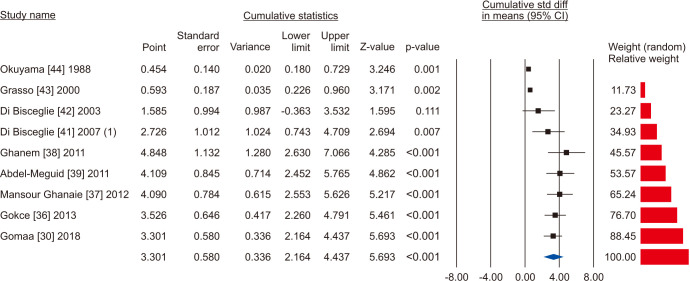
Nine studies assessing the effect of VR on sperm progressive motility were eligible for the analysis [30,36,37,38,39,41,42,43,44]. The statistical analysis of 1,438 cases (854 cases vs. 584 controls) on progressive sperm motility indicated considerable heterogenity (I2=98.5%); however, VR demonstrated improvement in all nine studies. The effect of VR were more distinct in the series of Mansour Ghanaie et al [37] and Di Bisceglie et al [42]. The pooled analysis indicates that VR enhanced progressive sperm motility significantly (SMD 3.301; 95% CI 2.164 to 4.437; p<0.01) (Fig. 14). At the sensitivity analysis, no study was sensitive enought to change this conclusion (Fig. 15). Furthermore, publication bias was present, as demonstrated by the Egger’s test (p<0.001) and the asymmetrical funnel plots (Fig. 16). The adjusted values were SMD 3.239 (95% CI 0.030 to 2.448). Cumulative analysis showed that progressive sperm motility became significant after the addition of Di Bisceglie et al [41] and has stayed significant after that (Fig. 17).
Fig. 14. Forest plot of the progressive sperm motility in infertile men with varicocele repair compared to no treatment.
Fig. 15. Forest plot of the progressive sperm motility in infertile men with varicocele repair compared to no treatment: sensitivity analysis.
Fig. 16. Funnel plot of the progressive sperm motility in infertile men with varicocele repair compared to no treatment.
Fig. 17. Forest plot of the progressive sperm motility in infertile men with varicocele repair compared to no treatment: cumulative analysis.
6. Total sperm motility
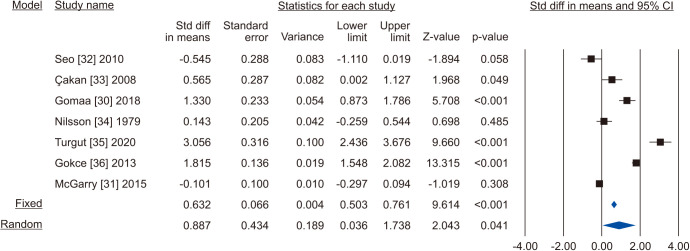
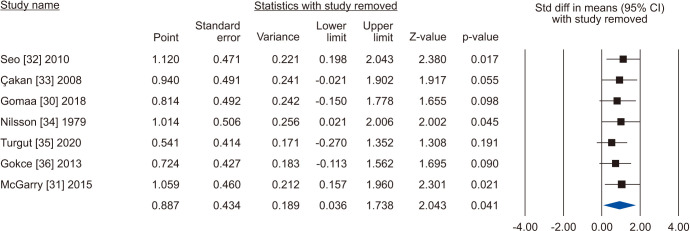
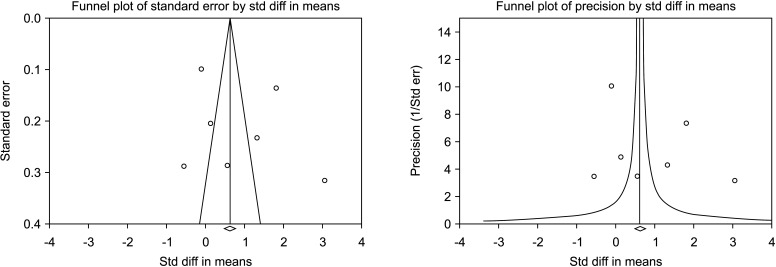
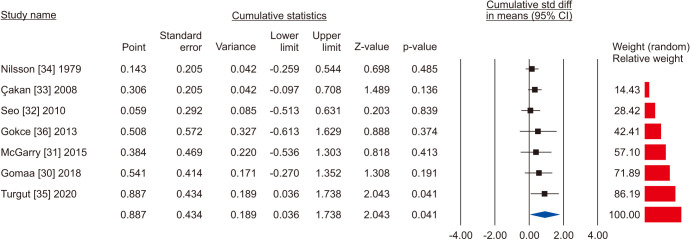
The random-effects model of 7 studies involving 1,126 participants, 659 who underwent VR and 467 controls [30,31,32,33,34,35,36] showed that there was a significant improvement in total sperm motility in the VR group (SMD 0.887; 95% CI 0.036 to 1.738; p=0.041) (Fig. 18). The interstudy heterogeneity was high (I2=97.3%). No study was sensitive enough to change the conclusion that VR improves total sperm motility (Fig. 19). No publication bias was found, as demonstrated by the Egger test results (p>0.05) and the symmetry of the funnel plots (Fig. 20). Cumulative analysis showed that total sperm motility is slightly significant and is highly vulnerable to changes with the addition of further studies (Fig. 21).
Fig. 18. Forest plot of the total sperm motility in infertile men with varicocele repair compared to no treatment.
Fig. 19. Forest plot of the total sperm motility in infertile men with varicocele repair compared to no treatment: sensitivity analysis.
Fig. 20. Funnel plot of the total sperm motility in infertile men with varicocele repair compared to no treatment.
Fig. 21. Forest plot of the total sperm motility in infertile men with varicocele repair compared to no treatment: cumulative analysis.
7. Sperm morphology
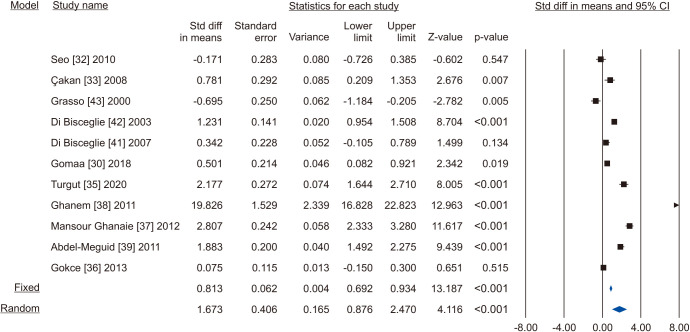
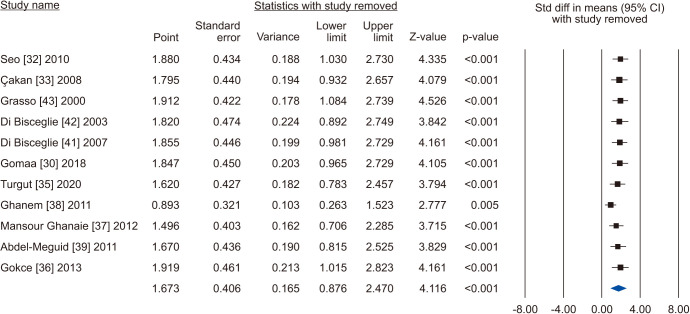
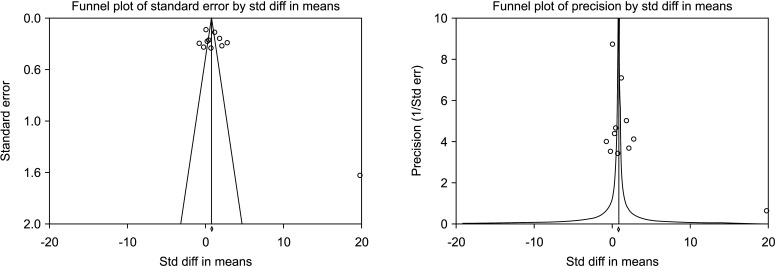
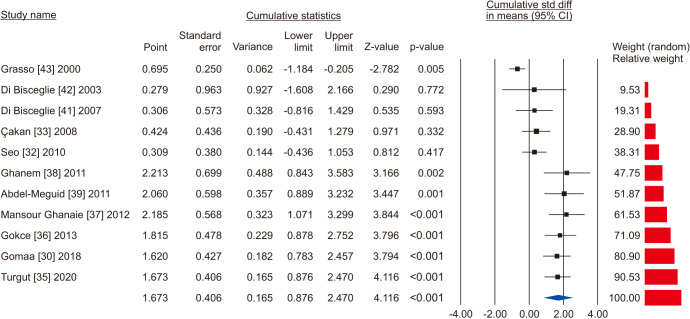
A total of 11 studies included normal sperm morphology in their outcome, overall including 818 cases and 585 controls [30,32,33,35,36,37,38,39,41,42,43]. There was a significant improvement in normal sperm morphology (SMD 1.673; 95% CI 0.876 to 2.470; p<0.05) (Fig. 22). Furthermore, significant inter-study heterogety was found (I2=98.5%). Also, sensitivity analysis was performed and no study was sensitive enough to change this conclusion (Fig. 23). Furthermore, publication bias was not present, as as demonstrated by the Egger’s test (p>0.05) and the symmetrical funnel plots (Fig. 24). Cumulative analysis showed that sperm morphology achieved significance with the addition of Ghanem et al [38] and has stayed significant after that (Fig. 25).
Fig. 22. Forest plot of the sperm morphology in infertile men with varicocele repair compared to no treatment.
Fig. 23. Forest plot of the sperm morphology in infertile men with varicocele repair compared to no treatment: sensitivity analysis.
Fig. 24. Funnel plot of the sperm morphology in infertile men with varicocele repair compared to no treatment.
Fig. 25. Forest plot of the sperm morphology in infertile men with varicocele repair compared to no treatment: cumulative analysis.
DISCUSSION
Varicocele remains one of the most controversial topics in male infertility. Although most international fertility societies agree on the indication for VR in cases of male infertility with clinically palpable varicocele and abnormal semen parameters [13,14,46], there are still some clinicians who doubt the beneficial effect of VR on semen parameters and fertility status [47,48,49].
Results of the current MA indicate significant improvements in sperm concentration (SMD 1.864; p<0.001), total sperm count (SMD 1.894; p<0.05), total sperm motility (SMD 0.887; p=0.04), progressive sperm motility (SMD 3.301; p<0.01), and normal sperm morphology (SMD 1.673; p<0.05) in infertile men with clinically palpable varicocele that underwent VR when compared to men who did not undergo VR. VR was also associated with an increase in semen volume but this was not statistically significant. No publication bias could be found in the analyzed studies semen volume, total sperm count, total sperm motility, and normal morphology. For sperm concentration and progressive motility, no study was sensitive enough to change the conclusion of our findings. However, the results of the current MA are limited by the high heterogeneity noted among the included studies.
Over the last 30 years, nearly 2,000 original articles have been published on varicocele and about half of them have evaluated the impact of varicocele and VR on semen parameters [50]. Agarwal et al [12] described the positive effect of VR on semen parameters in a MA of 17 studies. The authors evaluated infertile men with bilateral or unilateral clinically palpable varicoceles and at least one abnormality in semen parameters. The study results reported that sperm concentration, morphology, and sperm motility were significantly increased after microsurgical or high-ligation VR. Baazeem et al [11] performed a similar MA evaluating 17 RCTs before and after varicocelectomy. The results of the latter MA demonstrated that VR resulted in a significant increase in total and progressive sperm motility as well as in sperm concentration. Schauer et al [17] examined the effects of three surgical approaches of VR: subinguinal, inguinal, and high ligation. VR resulted in significant improvement in sperm count and motility independently of the surgical approach. Asafu-Adjei et al [18] performed a MA evaluating the outcome of VR classified by varicocele grade. They concluded that VR showed improvement in sperm concentration and motility across all varicocele grades, but the greatest improvement was observed with higher grade varicoceles. Results emerging from these MAs have led to guidelines from international societies [8,10,13] regarding the indications for VR in the management of clinical varicocele.
Previous MAs explored the effect of VR on semen parameters [11,12,17,51]. All these studies adopted a statistical approach that compared preoperative and postoperative semen analyses. They found statistically significant improvement of different semen parameters post-operatively (Table 3). The authors of these studies suggested that the post-operative improvement of semen parameters is not a mere finding due to repetition of testing but is a result of VR. However, the lack of proper control group in these studies left room for doubt on the efficacy of VR.
Table 3. Evidence coming from previous meta-analyses on varicocele repair by comparing preoperative and postoperative semen analyses.
| Study | Number of articles | Number of cases | Findings |
|---|---|---|---|
| Agarwal et al, 2007 [12] | 17 | 1,231 for count 1,015 for motility 528 for morphology |
Significant increase of sperm concentration by 9.71 millions/mL (95% CI, 7.34–12.08; p=0.00001). Significant increase of average sperm motility by 9.92% (95% CI, 4.90–14.95; p=0.0001). Significant improvement of sperm morphology with an estimated change of 3.16% (95% CI, 0.72–5.60; p=0.01). |
| Baazeem et al, 2011 [11] | 22 for concentration 17 for motility 5 for progressive motility |
Significant improvement of sperm concentration after varicocelectomy (ranging from 4.0 to 60.0 million sperm) (95% CI, 9.45–15.19; p<0.0001). Significant improvement of total sperm motility after varicocelectomy (ranging from 2.7% to 21.4%) (95% CI, 7.07–14.65; p<0.0001). Significant improvement of progressive sperm motility after varicocelectomy (ranging from 5.0% to 15.7%) (95% CI, 4.86–14.52; p=0.003). |
|
| Birowo et al, 2020 [51] | 7 | 289 | Significant increase of sperm concentration by 9.59 million per mL (mean difference: 9.59; 95% CI, 7.80–11.38; p<0.00001). Significant increase of progressive sperm motility by 8.66% after varicocelectomy (mean difference: 8.66; 95% CI, 6.96–10.36; p<0.00001). Significant increase of normal sperm morphology by 2.73% (mean difference: 2.73; 95% CI, 0.65–4.80; p=0.01). |
CI: confidence interval.
For researchers opposing VR as a treatment for male infertility, the main concern is that spontaneous improvement of semen parameters can occur even without intervention and this was not accounted for in various studies due to defective analysis resulting from lack of a proper control group [48,52]. During the last decade, several MA studies, including Cochrane Database of systematic reviews [13,53,54], have primarily focused on the differences in pregnancy and/or life birth rates reported with “treated versus non-treated” varicoceles. Surprisingly, no previously published MA has addressed the comparison of the “after treatment versus after no treatment” variations in conventional semen parameters among patients with varicocele.
In the current MA, we have sought to avoid the shortcomings of previous studies (which compared pre- and post-operative data), and provide more reliable conclusions by only including studies that compared postoperative semen parameters of the treatment group (infertile men where varicocele is repaired) versus the parameters after a similar period of time in the control group (infertile patients with varicocele that were not treated). A proper control group is the key to establish a cause-and-effect relationship of a certain variable [55].
To the best of our knowledge, the present study is the first MA primarily assessing the impact of VR on conventional semen parameters in a population of infertile men with clinical varicocele, as compared to no treatment (Table 4). The findings of our MA are in favour of a positive effect of VR on conventional semen parameters among infertile patients with clinical varicoceles. Our “after treatment versus after no treatment” study comparison should provide a more robust, and higher level of evidence than the other previously published MAs that used the usual “before and after treatment” approach.
Table 4. Summary of the findings of the present study.
| Outcome | (No. of studies) No. of participants |
Results | Heterogeneity | Sensitivity analysis | Risk of bias | Conclusion |
|---|---|---|---|---|---|---|
| SV | (5) 732 total participants 439 treated 293 controls |
SMD 0.313; 95% CI -0.242 to 0.868; p>0.05 | Significant heterogeneity, I2=89.7% | No study was sensitive enough to alter the results. | No significant publication bias, Egger’s test (p=0.23) and symmetrical funnel plots. | Non-significant increase in SV. |
| SC | (13) 2,267 total participants 1,347 treated 920 controls |
SMD 1.864; 95% CI 1.249 to 2.479; p<0.001 | Significant heterogeneity, I2=97.6% | No study was sensitive enough to alter the results. | Significant publication bias, Egger’s test (p<0.001) and asymmetrical funnel plots. | Significant increase in SC, favoring treatment. |
| TSC | (5) 1,073 total participants 622 treated 451 controls |
SMD 1.894; 95% CI 0.566 to 3.222; p<0.05 | Significant heterogeneity, I2=97.8% | No study was sensitive enough to alter the results. | No significant publication bias, Egger’s test (p>0.05) and symmetrical funnel plots. | Significant increase in TSC, favoring treatment. |
| SPM | (9) 1,438 total participants 854 treated 584 controls |
SMD 3.301; 95% CI 2.164 to 4.437; p<0.01 | Significant heterogeneity, I2=98.5% | No study was sensitive enough to alter the results. | Significant publication bias, Egger’s test (p<0.001) and asymmetrical funnel plots. | Significant increase in SPM, favoring treatment. |
| TM | (7) 1,126 total participants 659 treated 467 controls |
SMD 0.887; 95% CI 0.036 to 1.738; p=0.041 | Significant heterogeneity, I2=97.3% | No study was sensitive enough to alter the results. | No significant publication bias, Egger’s test (p>0.05) and symmetrical funnel plots. | Significant increase in TM, favoring treatment. |
| SM | (11) 1,403 total participants 818 treated 585 controls |
SMD 1.673; 95% CI 0.876 to 2.470; p<0.05 | Significant heterogeneity, I2=98.5% | No study was sensitive enough to alter the results. | No significant publication bias, Egger’s test (p>0.05) and symmetrical funnel plots. | Significant increase in SM, favoring treatment. |
CI: confidence interval, SC: sperm concentration, SM: sperm morphology, SMD: standardized mean difference, SPM: sperm progressive motility, SV: semen volume, TM: total motility, TSC: total sperm count.
The primary outcome of the present MA is semen parameters. These represent additional important end point to pregnancy or live birth which are emphasized by other MA on VR [53]. In a recently published global survey, with 574 respondents, exploring the general attitude of clinicians toward the management of varicocele, an increase in the pregnancy rate or in the live birth rate was considered as the primary outcome measure of the success of VR by only 16.3% and the 9.8% of respondents, respectively. Importantly, the 58.7% agreed that a significant improvement in semen parameters could be considered a successful outcome of VR, even if normal values are not reached [16]. This may reflect the understanding that pregnancy and live birth depend upon both male and female factors. VR could improve semen conventional parameters, but the improvement itself alone may not lead to a proportionate increase in pregnancy rates since it depends on several factors in both the male and the female. Therefore, conventional semen parameters represent a valid outcome measure of VR.
Despite the interesting findings of the current MA, there are a few limitations. First, only a few RCTs fulfilled the criteria for inclusion in the analysis. Second, significant heterogeneity was found for all tested outcomes. Third, risk of publication bias was remarkable for studies addressing the impact of VR on sperm concentration and progressive sperm motility. Fourth, there is a concern regarding the quality of the included studies (Table 4).
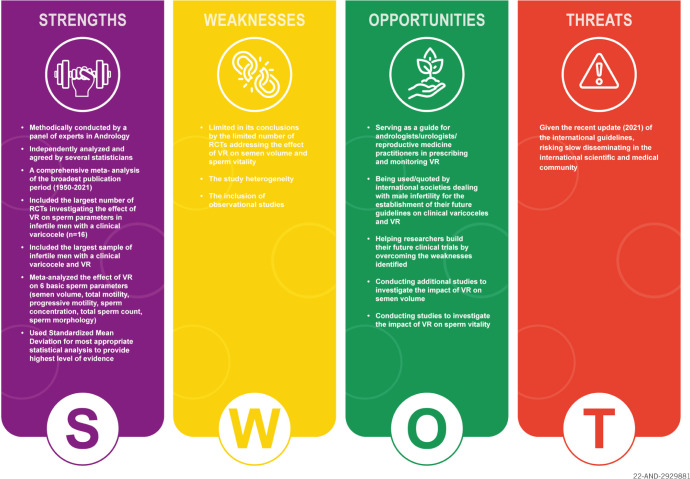
1. SWOT analysis
The highlights of our MA are summarized in a SWOT analysis (SWOT stands for strengths [S], weaknesses [W], opportunities [O], and threats [T]) (Fig. 26). The “strengths” (S) and “weaknesses” (W) are related to the internal environment while the “opportunities” (O), and “threats” (T) are all external factors that can either enhance or hinder the development of a subject, respectively [56]. Using the SWOT method, we summarize the main advantages and limitations of our MA on the topic of VR in infertile men with clinical varicocele. We also summarize the factors that could limit the use of the results of this MA and address the opportunities that could ensure the wide diffusion of this MA in the medical and scientific community.
Fig. 26. Strengths, Weaknesses, Opportunities, and Threats (SWOT) analysis. RCT: randomized controlled trial, VR: varicocele repair.
CONCLUSIONS
This SRMA of controlled studies, provides further evidence that VR significantly improves conventional semen parameters. To the best of our knowledge, this is the first SRMA to compare changes in conventional semen parameters after VR with changes in parameters of a control group over the same period. This is in contrast to other SRMAs which have compared semen parameters before and after VR, without reference to a control group. Our findings strengthen the available evidence and have a potential to upgrade professional societies’ practice recommendations favoring VR to improve conventional semen parameters in infertile men.
Acknowledgements
Authors wish to thank the members of the Global Andrology Forum for their support.
Footnotes
Conflict of Interest: The authors have nothing to disclose.
Funding: None.
- Conceptualization: RC, AA, Rupin S.
- Data curation: AH.
- Formal analysis: Rajender S.
- Investigation: FB, Ramadan S.
- Methodology: TAAAMH.
- Project administration: AA, Rupin S.
- Software: Rajender S.
- Visualization: all authors.
- Writing – original draft: all authors.
- Writing – review & editing: all authors.
Supplementary Materials
Supplementary materials can be found via https://doi.org/10.5534/wjmh.220142.
The PICOS (Population, Intervention, Comparison, Outcome, Study type) model [21] of the current systematic review and meta-analysis
Excluded studies with reasons
References
- 1.Thonneau P, Marchand S, Tallec A, Ferial ML, Ducot B, Lansac J, et al. Incidence and main causes of infertility in a resident population (1,850,000) of three French regions (1988-1989) Hum Reprod. 1991;6:811–816. doi: 10.1093/oxfordjournals.humrep.a137433. [DOI] [PubMed] [Google Scholar]
- 2.Odisho AY, Nangia AK, Katz PP, Smith JF. Temporal and geospatial trends in male factor infertility with assisted reproductive technology in the United States from 1999-2010. Fertil Steril. 2014;102:469–475. doi: 10.1016/j.fertnstert.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 3.Freeman S, Bertolotto M, Richenberg J, Belfield J, Dogra V, Huang DY, et al. members of the ESUR-SPIWG WG. Ultrasound evaluation of varicoceles: guidelines and recommendations of the European Society of Urogenital Radiology Scrotal and Penile Imaging Working Group (ESUR-SPIWG) for detection, classification, and grading. Eur Radiol. 2020;30:11–25. doi: 10.1007/s00330-019-06280-y. [DOI] [PubMed] [Google Scholar]
- 4.Damsgaard J, Joensen UN, Carlsen E, Erenpreiss J, Blomberg Jensen M, Matulevicius V, et al. Varicocele is associated with impaired semen quality and reproductive hormone levels: a study of 7035 healthy young men from six European countries. Eur Urol. 2016;70:1019–1029. doi: 10.1016/j.eururo.2016.06.044. [DOI] [PubMed] [Google Scholar]
- 5.Gorelick JI, Goldstein M. Loss of fertility in men with varicocele. Fertil Steril. 1993;59:613–616. [PubMed] [Google Scholar]
- 6.Jarow JP, Coburn M, Sigman M. Incidence of varicoceles in men with primary and secondary infertility. Urology. 1996;47:73–76. doi: 10.1016/s0090-4295(99)80385-9. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. The influence of varicocele on parameters of fertility in a large group of men presenting to infertility clinics. Fertil Steril. 1992;57:1289–1293. [PubMed] [Google Scholar]
- 8.Schlegel PN, Sigman M, Collura B, De Jonge CJ, Eisenberg ML, Lamb DJ, et al. Diagnosis and treatment of infertility in men: AUA/ASRM guideline part I. Fertil Steril. 2021;115:54–61. doi: 10.1016/j.fertnstert.2020.11.015. [DOI] [PubMed] [Google Scholar]
- 9.Wang J, Xia SJ, Liu ZH, Tao L, Ge JF, Xu CM, et al. Inguinal and subinguinal micro-varicocelectomy, the optimal surgical management of varicocele: a meta-analysis. Asian J Androl. 2015;17:74–80. doi: 10.4103/1008-682X.136443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Minhas S, Bettocchi C, Boeri L, Capogrosso P, Carvalho J, Cilesiz NC, et al. EAU Working Group on Male Sexual and Reproductive Health. European Association of Urology guidelines on male sexual and reproductive health: 2021 update on male infertility. Eur Urol. 2021;80:603–620. doi: 10.1016/j.eururo.2021.08.014. [DOI] [PubMed] [Google Scholar]
- 11.Baazeem A, Belzile E, Ciampi A, Dohle G, Jarvi K, Salonia A, et al. Varicocele and male factor infertility treatment: a new meta-analysis and review of the role of varicocele repair. Eur Urol. 2011;60:796–808. doi: 10.1016/j.eururo.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 12.Agarwal A, Deepinder F, Cocuzza M, Agarwal R, Short RA, Sabanegh E, et al. Efficacy of varicocelectomy in improving semen parameters: new meta-analytical approach. Urology. 2007;70:532–538. doi: 10.1016/j.urology.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 13.Colpi GM, Francavilla S, Haidl G, Link K, Behre HM, Goulis DG, et al. European Academy of Andrology guideline management of oligo-astheno-teratozoospermia. Andrology. 2018;6:513–524. doi: 10.1111/andr.12502. [DOI] [PubMed] [Google Scholar]
- 14.Marmar JL, Agarwal A, Prabakaran S, Agarwal R, Short RA, Benoff S, et al. Reassessing the value of varicocelectomy as a treatment for male subfertility with a new meta-analysis. Fertil Steril. 2007;88:639–648. doi: 10.1016/j.fertnstert.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 15.Kroese AC, de Lange NM, Collins J, Evers JL. Surgery or embolization for varicoceles in subfertile men. Cochrane Database Syst Rev. 2012;10:CD000479. doi: 10.1002/14651858.CD000479.pub5. [DOI] [PubMed] [Google Scholar]
- 16.Shah R, Agarwal A, Kavoussi P, Rambhatla A, Saleh R, Cannarella R, et al. Consensus and diversity in the management of varicocele for male infertility: results of a global practice survey and comparison with guidelines and recommendations. World J Mens Health. 2022 doi: 10.5534/wjmh.220048. [Epub] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schauer I, Madersbacher S, Jost R, Hübner WA, Imhof M. The impact of varicocelectomy on sperm parameters: a meta-analysis. J Urol. 2012;187:1540–1547. doi: 10.1016/j.juro.2011.12.084. [DOI] [PubMed] [Google Scholar]
- 18.Asafu-Adjei D, Judge C, Deibert CM, Li G, Stember D, Stahl PJ. Systematic review of the impact of varicocele grade on response to surgical management. J Urol. 2020;203:48–56. doi: 10.1097/JU.0000000000000311. [DOI] [PubMed] [Google Scholar]
- 19.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 20.Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647. doi: 10.1136/bmj.g7647. Erratum in: BMJ 2016;354:i4086. [DOI] [PubMed] [Google Scholar]
- 21.Methley AM, Campbell S, Chew-Graham C, McNally R, Cheraghi-Sohi S. PICO, PICOS and SPIDER: a comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv Res. 2014;14:579. doi: 10.1186/s12913-014-0579-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murray J, Farrington DP, Eisner MP. Drawing conclusions about causes from systematic reviews of risk factors: the Cambridge Quality Checklists. J Exp Criminol. 2009;5:1–23. [Google Scholar]
- 23.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 24.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 25.Schulz KF, Altman DG, Moher D CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332. doi: 10.1136/bmj.c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods. 2006;11:193–206. doi: 10.1037/1082-989X.11.2.193. [DOI] [PubMed] [Google Scholar]
- 27.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andrade C. Mean difference, standardized mean difference (SMD), and their use in meta-analysis: as simple as it gets. J Clin Psychiatry. 2020;81:20f13681. doi: 10.4088/JCP.20f13681. [DOI] [PubMed] [Google Scholar]
- 29.Duval S, Tweedie R. A nonparametric “trim and fill” method of accounting for publication bias in meta-analysis. J Am Stat Assoc. 2000;95:89–98. [Google Scholar]
- 30.Gomaa MD, Motawaa MA, Al-Nashar AM, El-Sakka AI. Impact of subinguinal varicocelectomy on serum testosterone to estradiol ratio in male patients with infertility. Urology. 2018;117:70–77. doi: 10.1016/j.urology.2018.03.039. [DOI] [PubMed] [Google Scholar]
- 31.McGarry P, Alrabeeah K, Jarvi K, Zini A. Is varicocelectomy beneficial in men previously deemed subfertile but with normal semen parameters based on the new guidelines? A retrospective study. Urology. 2015;85:357–362. doi: 10.1016/j.urology.2014.10.031. [DOI] [PubMed] [Google Scholar]
- 32.Seo JT, Kim KT, Moon MH, Kim WT. The significance of microsurgical varicocelectomy in the treatment of subclinical varicocele. Fertil Steril. 2010;93:1907–1910. doi: 10.1016/j.fertnstert.2008.12.118. [DOI] [PubMed] [Google Scholar]
- 33.Çakan M, Bakirtas H, Aldemir M, Demirel F, Altug U. Results of varicocelectomy in patients with isolated teratozoospermia. Urol Int. 2008;80:172–176. doi: 10.1159/000112609. [DOI] [PubMed] [Google Scholar]
- 34.Nilsson S, Edvinsson A, Nilsson B. Improvement of semen and pregnancy rate after ligation and division of the internal spermatic vein: fact or fiction? Br J Urol. 1979;51:591–596. doi: 10.1111/j.1464-410x.1979.tb03609.x. [DOI] [PubMed] [Google Scholar]
- 35.Turgut H. The effect of varicocelectomy on the pregnancy rate in patients with severe oligospermia. Niger J Clin Pract. 2020;23:1744–1747. doi: 10.4103/njcp.njcp_173_20. [DOI] [PubMed] [Google Scholar]
- 36.Gokce MI, Gülpınar O, Süer E, Mermerkaya M, Aydos K, Yaman O. Effect of performing varicocelectomy before intracytoplasmic sperm injection on clinical outcomes in non-azoospermic males. Int Urol Nephrol. 2013;45:367–372. doi: 10.1007/s11255-013-0394-2. [DOI] [PubMed] [Google Scholar]
- 37.Mansour Ghanaie M, Asgari SA, Dadrass N, Allahkhah A, Iran-Pour E, Safarinejad MR. Effects of varicocele repair on spontaneous first trimester miscarriage: a randomized clinical trial. Urol J. 2012;9:505–513. [PubMed] [Google Scholar]
- 38.Ghanem MA, Safan MA, Ghanem AA, Dohle GR. The role of varicocele sclerotherapy in men with severe oligo-astheno-teratozoospermia. Asian J Androl. 2011;13:867–871. doi: 10.1038/aja.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abdel-Meguid TA, Al-Sayyad A, Tayib A, Farsi HM. Does varicocele repair improve male infertility? An evidence-based perspective from a randomized, controlled trial. Eur Urol. 2011;59:455–461. doi: 10.1016/j.eururo.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 40.Sathya Srini V, Belur Veerachari S. Does varicocelectomy improve gonadal function in men with hypogonadism and infertility? Analysis of a prospective study. Int J Endocrinol. 2011;2011:916380. doi: 10.1155/2011/916380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Di Bisceglie C, Bertagna A, Baldi M, Lanfranco F, Tagliabue M, Gazzera C, et al. Varicocele sclerotherapy improves serum inhibin B levels and seminal parameters. Int J Androl. 2007;30:531–536. doi: 10.1111/j.1365-2605.2007.00747.x. [DOI] [PubMed] [Google Scholar]
- 42.Di Bisceglie C, Fornengo R, Grosso M, Gazzera C, Mancini A, Andriani B, et al. Follow-up of varicocele treated with percutaneous retrograde sclerotherapy: technical, clinical and seminal aspects. J Endocrinol Invest. 2003;26:1059–1064. doi: 10.1007/BF03345250. [DOI] [PubMed] [Google Scholar]
- 43.Grasso M, Lania C, Castelli M, Galli L, Franzoso F, Rigatti P. Low-grade left varicocele in patients over 30 years old: the effect of spermatic vein ligation on fertility. BJU Int. 2000;85:305–307. doi: 10.1046/j.1464-410x.2000.00437.x. [DOI] [PubMed] [Google Scholar]
- 44.Okuyama A, Fujisue H, Matsui T, Doi Y, Takeyama M, Nakamura N, et al. Surgical repair of varicocele: effective treatment for subfertile men in a controlled study. Eur Urol. 1988;14:298–300. doi: 10.1159/000472964. [DOI] [PubMed] [Google Scholar]
- 45.Ketabchi AA, Salajegheh S. The effects of acupuncture treatment in infertile patients with clinical varicocele. Nephrourol Mon. 2018;10:e65451 [Google Scholar]
- 46.Schlegel PN, Sigman M, Collura B, De Jonge CJ, Eisenberg ML, Lamb DJ, et al. Diagnosis and treatment of infertility in men: AUA/ASRM guideline part II. J Urol. 2021;205:44–51. doi: 10.1097/JU.0000000000001520. [DOI] [PubMed] [Google Scholar]
- 47.Silber SJ. The varicocele dilemma. Hum Reprod Update. 2001;7:70–77. doi: 10.1093/humupd/7.1.70. [DOI] [PubMed] [Google Scholar]
- 48.Kantartzi PD, Goulis ChD, Goulis GD, Papadimas I. Male infertility and varicocele: myths and reality. Hippokratia. 2007;11:99–104. [PMC free article] [PubMed] [Google Scholar]
- 49.Silber S. The varicocele argument resurfaces. J Assist Reprod Genet. 2018;35:1079–1082. doi: 10.1007/s10815-018-1160-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Agarwal A, Finelli R, Durairajanayagam D, Leisegang K, Henkel R, Salvio G, et al. Comprehensive analysis of global research on human varicocele: a scientometric approach. World J Mens Health. 2022 doi: 10.5534/wjmh.210202. [Epub] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Birowo P, Rahendra Wijaya J, Atmoko W, Rasyid N. The effects of varicocelectomy on the DNA fragmentation index and other sperm parameters: a meta-analysis. Basic Clin Androl. 2020;30:15. doi: 10.1186/s12610-020-00112-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baker HW, Kovacs GT. Spontaneous improvement in semen quality: regression towards the mean. Int J Androl. 1985;8:421–426. doi: 10.1111/j.1365-2605.1985.tb00855.x. [DOI] [PubMed] [Google Scholar]
- 53.Persad E, O'Loughlin CA, Kaur S, Wagner G, Matyas N, Hassler-Di Fratta MR, et al. Surgical or radiological treatment for varicoceles in subfertile men. Cochrane Database Syst Rev. 2021;4:CD000479. doi: 10.1002/14651858.CD000479.pub6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kroese ACJ, de Lange NM, Collins J, Evers JLH, Marjoribanks J. Surgery or embolization for varicoceles in subfertile men: summary of a Cochrane review. Fertil Steril. 2014;102:1553–1555. [Google Scholar]
- 55.Hinkelmann K, Kempthorne O. Design and analysis of experiments. 2nd ed. Vol. 1, Introduction to experimental design. Hoboken (NJ): Wiley; 2008. [Google Scholar]
- 56.Teoli D, Sanvictores T, An J. In: StatPearls. Abai B, Abu-Ghosh A, Acharya AB, Acharya U, Adhia SG, Sedeh PA, editors. Treasure Island (FL): StatPearls Publishing; 2022. SWOT analysis. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The PICOS (Population, Intervention, Comparison, Outcome, Study type) model [21] of the current systematic review and meta-analysis
Excluded studies with reasons