Abstract
Background
Combined treatment with BRAFi and/or MEK inhibitors (MEKi) improves outcomes in advanced melanoma patients in comparison with monotherapy.
Objective
We aim to report real-world treatment efficacy and safety of vemurafenib (V) and vemurafenib + cobimetinib (V + C) from 10 years of practice.
Patients and Methods
A total of 275 consecutive patients with unresectable or metastatic BRAF mutated melanoma started first-line V or V + C treatment between 1 October 2013 and 31 December 2020. Survival analyses were performed using the Kaplan–Meier method, and Log-rank and Chi-square tests were used for comparison between groups.
Results
The estimated median overall survival (mOS) was 10.3 months in the V group, and 12.3 months in the V + C group (p = 0.0005; HR = 1.58, 95% CI 1.2–2.1), although the latter group of patients had lactate dehydrogenase elevated numerically more often. Estimated median progression-free survival (mPFS) was 5.5 months in the V group, and 8.3 months in the V + C group (p = 0.0002; HR = 1.62, 95% CI 1.3–2.1). Complete response, partial response, stable disease, and progressive disease as best responses were recorded in the V/V + C groups in 7%/10%, 52%/46%, 26%/28%, and 15%/16% of patients, respectively. The numbers of patients with any grade of adverse effects were similar in both groups.
Conclusions
We confirmed significant improvement in the mOS and mPFS of unresectable and/or metastatic BRAF mutated-melanoma patients treated outside clinical trials with V + C as compared with V, with no major increase in toxicity for the combination.
Key Points
| This is the first study with such a long follow-up, over 10 years, comparing the efficacy and safety of vemurafenib monotherapy with the combination of vemurafenib and cobimetinib. |
| It is also the first study to compare the efficacy and safety of vemurafenib monotherapy with the combination of vemurafenib and cobimetinib carried out on such a large group of patients treated in real clinical practice. |
Introduction
Constitutive activation of the RAS/RAF/MEK/ERK signaling pathway caused by mutations in the BRAF gene is a key mechanism of proliferation of melanoma cells. These genetic alterations are found in approximately 50% of patients with melanoma. The development and introduction into routine clinical use of selective BRAF inhibitors (BRAFi) significantly improved the outcomes of patients with unresectable or metastatic BRAF-mutated melanoma treatment [1].
The first approved BRAFi was vemurafenib. The BRIM 3 randomized phase III study demonstrated that vemurafenib (V) improved progression-free survival (PFS), overall survival (OS), and overall response rate (ORR) compared with dacarbazine in patients with previously untreated advanced/unresectable melanoma, although the majority of patients progressed after 6–7 months [2, 3]. At the cellular level, the most common mechanism of acquired resistance to BRAFi monotherapy is the reactivation of downstream MAP signaling [4]. The discovery of this phenomenon initiated translational research and subsequent trials with agents targeting MEK kinase. In the next step, a combination strategy of MEK kinase inhibitor (MEKi) with BRAFi was investigated. The co-BRIM study provided data on the efficacy of the combination of BRAFi (vemurafenib) with MEKi (cobimetinib) [5]. In the coBRIM study, progression was observed significantly later with median PFS (mPFS) of 12.6 months in the combination arm (vemurafenib + cobimetinib, V + C) compared with 7.2 months in the vemurafenib + placebo (V + P) arm. Similarly, patients in the V + C combination arm achieved longer median OS (mOS) than those in the V arm (22.5 months vs 17.4 months, respectively) [6]. Moreover, the COMBI-d trial with another combination of BRAF and MEK inhibitors (dabrafenib + trametinib) confirmed that this strategy delays drug resistance development and improved mOS to 25.1 versus 18.7 months for dabrafenib monotherapy [7].
Combined treatment with vemurafenib and cobimetinib is one of the three combinations of BRAF and MEK inhibitors available. Dabrafenib + trametinib (D+T) and encorafenib + binimetinib (E+B) are also used in patients with BRAF-mutated melanoma according to the European Society for Medical Oncology (ESMO) and American Society of Clinical Oncology (ASCO) guidelines, with level 1 evidence. The combination of these kinase inhibitors is the preferred treatment option (over immunotherapy) in the group of symptomatic patients with rapid disease dynamics due to the high response rate and the rapid onset of disease control [8]. The advantages of combined therapy with BRAF and MEK inhibitors over BRAFi monotherapy were confirmed in phase III clinical trials, so the use of BRAFi alone is limited only to patients with baseline MEKi contraindications or with limiting adverse events (AEs) occurring during this therapy. There are practically no data that compare the efficacy and safety of V and V + C in everyday clinical practice, or long-term analyses beyond 5 years. Therefore, we present an analysis of the efficacy and safety of real-world treatment in patients treated with V and V + C over 10 years of routine practice.
Materials and Methods
Patients
In this observational study, we collected data from adult patients who started first-line therapy for advanced/metastatic BRAF-mutated melanoma between 1 February 2013 and 31 December 2020. The observation data cut-off was 31 December 2021. We included all consecutive patients treated in recent major referral oncology trials in Poland. The enrollees were treated in the first line with vemurafenib monotherapy or vemurafenib and cobimetinib combination. All eligible patients had the diagnosis confirmed by pathologists experienced in skin cancer pathology and confirmed BRAF mutation status, as well as computed tomography (CT)-based disease staging at the beginning of treatment no later than 28 days. All patients were treated outside of clinical trials.
All patients were treated until disease progression, unacceptable toxicity of therapy, death, or withdrawal of consent to treatment, whichever came first. For all patients, the first radiological evaluation was performed between weeks 8 and 10 after the beginning of therapy, and then every 3 months as required by national treatment reimbursement guidelines (https://www.gov.pl/web/zdrowie/choroby-onkologiczne; drug program B.59). Response to treatment was assessed according to the RECIST 1.1 criteria [9]. The date of death was confirmed by the Polish National Cancer Registry System through the personal identification number of all patients at the data cutoff.
Data Collection
Clinical data of patients collected at the time of the beginning of treatment included age, sex, location of the primary lesion, stage of the disease according to TNM (tumor, node, metastasis; staging system) (AJCC 8th edition), lactate dehydrogenase activity (LDH), and ECOG (East Cooperative Oncology Group) status. Data on response to treatment and information on the type of therapy used in the second line were also collected.
OS was defined as the time between the date of start of treatment and the date of death. PFS was defined as the time between the date of the start of treatment and the first date of documented radiological or clinical progression determined by the investigator, or death from any cause, whichever occurred first. Participants who died without a reported progression were considered to have progressed on the date of their death. The survival of participants who did not progress or who died was censored on the date of their last visit/follow-up. The total response rate (ORR) was defined as CR (complete response) + PR (partial response), and the disease control rate (DCR) was defined as CR + PR + SD (stable disease) [9, 10]. Treatment response was evaluated according to RECIST 1.1 [10].
Data on the occurrence of AEs during treatment were collected from health record data. Only cases with available AE data were included in the analysis. Treatment toxicity data was evaluated according to Common Terminology Criteria for Adverse Events (CTCAE) v4.0 [11].
Data Analysis
The characteristics of the patients were analyzed with descriptive statistics. Progression-free survival (PFS) and overall survival (OS) were calculated with the Kaplan–Meier method and a logarithmic rank test was used to assess differences between survival curves. The Cox proportional hazard model was used for multivariate analysis. All variables with a p-value < 0.1 in the univariate analysis were included in the multivariate model. Also, 95% confidence intervals (CI) were reported. Differences were considered statistically significant if the p-values were < 0.05 [12]. Analysis was performed with Statistica software version 13.3.
Results
Characteristics of the Study Groups
A total of 275 patients were analyzed in the study, including 119 (43%) patients treated with vemurafenib and 156 (57%) with vemurafenib + cobimetinib. The median age in the study group was 58 years (range 25–90 years). Most of the patients performed well with ECOG performance status (PS) 0 or 1 (89%). Ninety-nine (36%) patients had brain metastases at the beginning of treatment (stage IVd TNM8). There were no differences between the groups in baseline factors (age, sex, primary location of melanoma, ECOG PS, and location of metastases). There were more patients in the V + C group with an elevated baseline LDH level. The baseline characteristics of the patient groups are summarized in Table 1.
Table 1.
Baseline patient characteristics
| Characteristic | Vemurafenib n = 119 (43%) |
Vemurafenib + cobimetinib n = 156 (57%) |
p-value |
|---|---|---|---|
| Age at the start of therapy | |||
| Median (range) | 59 (25–83) | 57 (25–90) | 0.2470 |
| ≥ 65 years | 34 (29%) | 42 (27%) | 0.7622 |
| ≥ 75 years | 7 (6%) | 16 (10%) | 0.2364 |
| Gender | |||
| Males | 72 (60%) | 95 (61%) | 0.9473 |
| Females | 47 (40%) | 61 (39%) | |
| Weight | |||
| Median (range) | 76 (46–143) | 78 (46–165) | 0.2692 |
| > 70 kg | 63 (62%) | 82 (69%) | 0.2674 |
| ≤ 70 kg | 38 (38%) | 36 (31%) | |
| Location of the primary tumor | |||
| Skin | 114 (96%) | 149 (96%) | 0.9085 |
| MUP | 5 (4%) | 7 (4%) | |
| ECOG | |||
| 0 | 34 (31%) | 50 (32%) | 0.4669 |
| 1 | 70 (63%) | 92 (59%) | |
| 2 | 7 (6%) | 12 (8%) | |
| 3 | 0 | 2 (1%) | |
| No data | 8 (7%) | 0 | |
| LDH level | |||
| Normal | 54 (50%) | 54 (37%) | 0.0289 |
| > Normal | 53 (50%) | 93 (63%) | |
| > 2 × normal | 28 (%) | 41 (%) | |
| No data | 12 (10%) | 9 (6%) | |
| Brain metastasis | |||
| No | 83 (70%) | 93 (60%) | 0.0816 |
| Yes | 36 (30%) | 63 (40%) | |
| Liver metastasis | |||
| No | 88 (74%) | 101 (65%) | 0.1010 |
| Yes | 31 (26%) | 55 (35%) | |
| Number of localization of metastasis | |||
| ≤ 2 | 49 (41%) | 75 (48%) | 0.2540 |
| > 2 | 70 (59%) | 81 (52%) | |
| TNM stage (AJCC 8th Edition) | |||
| III | 2 (2%) | 6 (4%) | |
| M1a | 25 (21%) | 24 (15%) | 0.2822 |
| M1b | 14 (12%) | 17 (11%) | |
| M1c | 42 (35%) | 46 (30%) | |
| M1d | 36 (30%) | 63 (40%) | |
AJCC American Joint Committee on Cancer, ECOG Eastern Cooperative Oncology Group (performance status), LDH lactate dehydrogenase, MUP melanoma of unknown primary location, TNM tumor, node, metastasis (staging system)
Efficacy of Vemurafenib and Vemurafenib + Cobimetinib Therapy
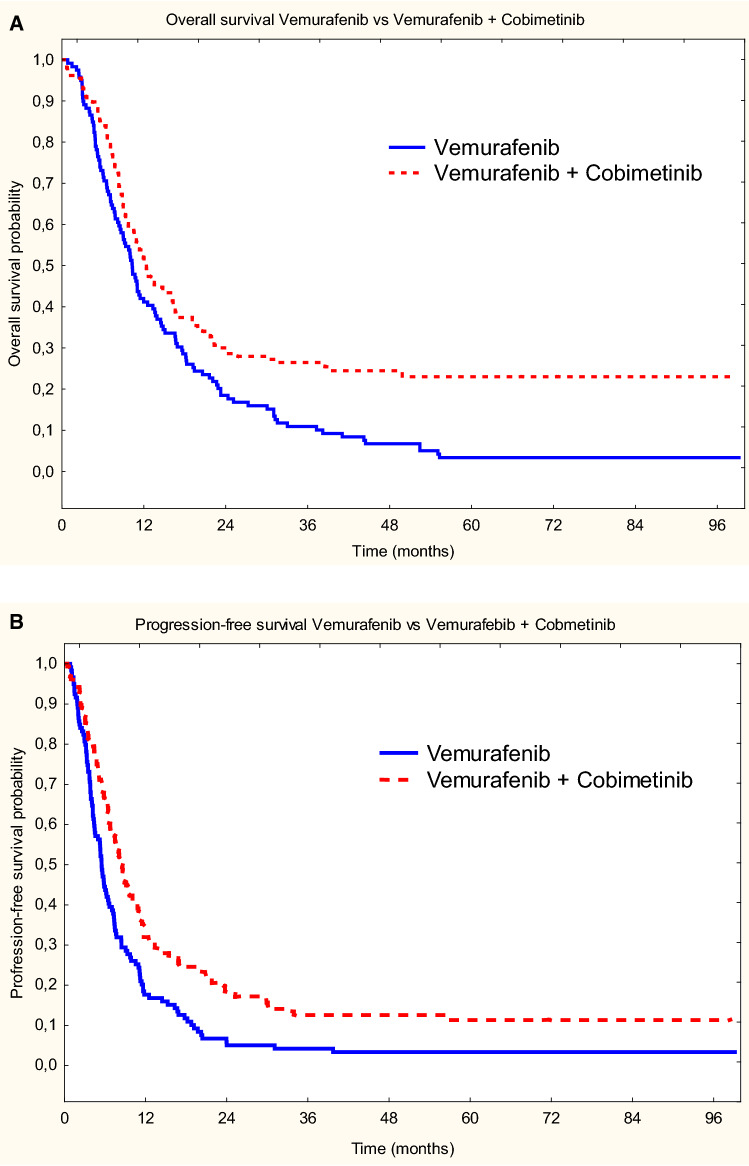
The estimated median OS (mOS) in the V group was 10.3 months while in V + C it was 12.2 months (p = 0.0005; HR = 1.58, 95% CI 1.2–2.1) (Fig. 1A). The estimated OS rates at 3, 5, and 7 years were 11%, 3%, 3% in the V-group patients and 26%, 23%, 23% in patients in the V + C group, respectively. The estimated median progression-free survival (mPFS) of the V group was 5.5 months, while in the V + C cohort it was 8.3 months (p = 0.0002; HR = 1.62, 95% CI 1.3–2.1) (Fig. 1B). Furthermore, in the V group, the estimated PFS rates at 3, 5, and 7 years were 4%, 3%, and 3% in the V group, and 13%, 12%, 12% in the V + C group, respectively. At the time of analysis in group V, therapy was continued by one (1%) patient and in the V + C cohort by 16 (10%) patients. It should be noted that in group V, only one patient (1%) achieved the 5-year duration of treatment (7 years and 9 months), while in group V + C, 5-year duration of treatment was reached in 15 (10%) patients (including eight over 5 years). CR, PR, SD, and PD were recorded in the V/V + C group in 7 (10%), 52 (46%), 26 (28%), and 15 (16%) patients, respectively (Table 2). The median follow-up was 11.2 months (range 0.4–99.2).
Fig. 1.
A Overall survival for vemurafenib vs vemurafenib + cobimetinib. B Progression-free survival for vemurafenib vs vemurafenib + cobimetinib
Table 2.
Characteristic of the outcomes of first-line treatment with vemurafenib (V) versus vemurafenib + cobimetinib (V + C) in the study groups
| Factors | V | V + C | p-value |
|---|---|---|---|
| Duration of treatment | |||
| Median (range) months | 5.3 (0.7–93.1)* | 8.4 (0.4–98.5)* | 0.0001 |
| Best overall tumor response | |||
| CR | 8 (7%) | 15 (10%) | 0.7226 |
| PR | 62 (52%) | 72 (46%) | |
| SD | 31 (26%) | 44 (28%) | |
| PD | 18 (15%) | 25 (16%) | |
| ORR (CR+PR) | 70 (59%) | 87 (56%) | 0.6120 |
| DCR (CR+PR+SD) | 101 (85%) | 131 (84%) | 0.8386 |
| Reason for end of therapy | |||
| Continuing first line | 1 (1%) | 16 (10%) | |
| Progression | 107 (90%) | 109 (70%) | |
| Death | 5 (4%) | 14 (9%) | |
| Complications | 5 (4%) | 15 (10%) | |
| Consent withdrawal | 1 (1%) | 2 (1%) | 0.0003 |
| Dose changes | |||
| Dose reduction |
Once: 17 (14%) Twice: 1 (15) |
Once: 23 (15%) Twice: 4 (3%) Only V: 2 (1%) |
|
| Interruption in treatment | 27 (23%) |
V + C: 40 (26%) Only C: 4 (3%) |
|
| Treatment discontinuation | 5 (4%) | 15 (10%) | |
| Second-line treatment | |||
| None | 67 (56%) | 88 (56%) | 0.0962 |
| Immunotherapy | 40 (34%) | 62 (40%) | |
| Anti-PD-1/anti-CTLA-4 | 13 (11%)/27 (23%) | 56 (36%)/6 (4%) | |
| Chemotherapy | 12 (10%) | 6 (4%) | |
CR complete response, DCR disease control rate, ORR overall response rate, OS overall survival, PD progressive disease, PFS progression-free survival, PR partial response, SD stable disease
*Data based on the last follow-up
Prognostic Factors for OS and PFS
In a univariate analysis in the V and V + C groups, a significant positive effect on the duration of OS and PFS had an absence of brain metastases (p = 0.0014, p < 0.0001 and p = 0.0074, p < 0.0001, respectively), normal activity of LDH (p = 0.0004, p = 0.0005 and p = 0.0019, p < 0.0001, respectively), fewer than three sites of metastasis (p = 0.0156, p = 0.0006 and p = 0.0312, p = 0.0006, respectively), ECOG 0 (p = 0.0026, p = 0.0002 and p = 0.0011, p < 0.0001, respectively). Age < 65 years (p = 0.1213, p = 0.3662 and p = 0.2744, p = 0.6951, respectively), gender (p = 0.7527, p = 0.3556 and p = 0.5966, p = 0.4714, respectively), location of the primary lesion (p = 0.3889, p = 0.9445 and p = 0.9458, p = 0.4959, respectively), weight (p = 0.0881, p = 0.3888 and p = 0.3921, p = 0.0628, respectively), liver metastasis (p = 0.6886, p = 0.3348 and p = 0.2226, p = 0.5321, respectively) was correlated with neither OS nor duration of PFS.
According to multivariate analysis, the unfavorable prognostic factors for OS in both groups were higher ECOG, brain metastasis, and elevated LDH level. Furthermore, the unfavorable prognostic factors for PFS in the V group were higher ECOG, presence of brain metastasis, and elevated LDH activity, as well as higher ECOG in the V + C group (Table 3).
Table 3.
Results of the multivariate Cox proportional hazards models for OS and PFS
| Parameter | Category | Vemurafenib | Vemurafenib + cobimetinib | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OS | PFS | OS | PFS | ||||||||||
| p-value | HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | ||
| Number of metastasis location | < 3 | 0.5425 | 0.87 | 0.5–1.4 | 0.6183 | 0.89 | 0.6–1.4 | 0.2413 | 0.78 | 0.5–1.2 | 0.9049 | 0.97 | 0.6–1.6 |
| Brain metastasis | No | 0.0043 | 0.45 | 0.3–0.8 | 0.0074 | 0.51 | 0.3–0.8 | 0.0096 | 0.588 | 0.4–0.9 | 0.0716 | 0.65 | 0.4–1.1 |
| LDH level | Normal | 0.0002 | 042 | 0.3–0.7 | 0.0182 | 0.60 | 0.4–0.9 | 0.0245 | 0.608 | 0.4–0.9 | 0.2218 | 0.75 | 0.5–1.2 |
| ECOG | 0 | 0.0190 | 0.07 | 0.01–1.1 | 0.0070 | 0.05 | 0.01–0.6 | 0.0037 | 0.23 | 0.1–0.8 | < 0.0001 | < 0.0001 | 0.0–0.0 |
| ECOG | 1 | 0.4567 | 0.16 | 0.01–2.3 | 0.2284 | 0.10 | 0.01–1.2 | 0.8607 | 0.33 | 0.1–1.4 | < 0.0001 | < 0.0001 | 0.0–0.0 |
| Weight | ≤ 70 kg | 0.0504 | 1.62 | 1.0–2.6 | 0.2914 | 0.77 | 0.5–1.2 | ||||||
95% CI 95% confidence interval, HR hazard ratio, OS overall survival, PFS progression-free survival, LDH lactate dehydrogenase, ECOG Eastern Cooperative Oncology Group (performance status)
Safety of Vemurafenib and Vemurafenib + Cobimetinib Therapy
The numbers of patients with any grade of AE or G3/G4 were similar in both groups (p = 0.3556 and p = 0.8345, respectively): in the V group there were 57 (48%)/15 (13%) and in the V + C group there were 66 (42%)/21 (13%) patients. In the V group there were 17 (14%), and in V + C there were 22 (14%) patients with more than one AE (Table 4). The only notable difference in the toxicity of both types of treatment was related to skin complications, which were more common in patients who received vemurafenib alone (37% vs 20.5%).
Table 4.
Adverse events during vemurafenib or vemurafenib with cobimetinib treatment
| irAEs | Vemurafenib n = 119 |
Vemurafenib + cobimetinib n = 156 |
p-value (for all AEs) | ||
|---|---|---|---|---|---|
| All grades n (%) |
G3 or G4 n (%) |
All grades n (%) |
G3 or G4 n (%) |
||
| All AEs | 96 (81) | 19 (16) | 103 (66) | 26 (17) | |
| Dermatological—rash | 27 (23) | 6 (5) | 25 (16) | 8 (5) | 0.1223 |
| Dermatological—hyperkeratosis | 8 (7) | 6 (5) | 1 (0.5) | 1 (0.5) | |
| Dermatological—photosensitivity | 2 (2) | 0 | 3 (2) | 2 (1) | |
|
Dermatological—other [including skin cancer] |
6 (5) [1 (1)] |
1 (1) |
3 (2) [1 (1)] |
0 | |
| Fatigue | 2 (2) | 0 | 3 (2) | 1 (0.5) | 0.8818 |
| AST/ALT/bilirubin elevation | 19 (16) | 2 (2) | 26 (17) | 1 (0.5) | 0.9626 |
| Diarrhea/gastrointestinal inflammation | 2 (2) | 0 | 3 (2) | 2 (1) | 0.0552 |
| Cardiovascular/QTc prolongation | 1 (1)/2 (2) | 1 (1)/0 | 7 (4.5)/1 (0.5) | 3 (2)/0 | 0.1788 |
| Creatinine elevation | 7 (6) | 0 | 7 (4.5) | 0 | 0.7855 |
| Hematological (neutropenia, leucopenia, anemia) | 8 (7) | 3 (3) | 5 (3) | 3 (2) | 0.1508 |
| Fever | 6 (5) | 0 | 6 (4) | 4 (2.5) | 0.6320 |
| Ocular | 2 (2) | 0 | 5 (3) | 0 | 0.4165 |
| Rheumatological | 2 (2) | 0 | 7 (4.5) | 2 (1) | 0.1441 |
| Electrolyte dysregulation | 2 (2) | 0 | 1 (0.5) | 1 (0.5) | 0.4121 |
AEs adverse events, ALT alanine aminotransferase, AST aspartate aminotransferase, irAEs immune-related adverse events
Discussion
This retrospective analysis, conducted on a large Polish cohort, confirmed the advantage in the effectiveness of combined therapy V + C over V alone, without a significant increase in the toxicity of treatment, in patients with metastatic melanoma in first-line therapy. Statistically significant differences were observed between monotherapy with V + C and V in mPFS (8.3 vs 5.5 months) and mOS (12.3 vs 10.3 months). However, there was no benefit from combination therapy in relation to ORR (56% vs 59%), which was reported in the coBRIM study [5]. No differences between the groups in relation to the achieved objective response was probably influenced by a higher percentage of patients with a high tumor burden, as indicated by the number of patients with elevated LDH levels in the V + C group (63% vs 50%). Our ORR result is consistent with the pooled analysis of the BRIM 2,3,7 and co-BRIM studies, which showed a correlation between the depth of response and prognostic factors such as LDH level or the presence of liver metastases [13]. The survival outcomes obtained in our analysis are very similar to those presented by Mesti et al. in the only available study that compares the efficacy of V with that of V + C in a real-world population of patients with metastatic melanoma. mPFS was 5 versus 7.9 months and mOS was 6.7 versus 9.7 months in monotherapy V and V + C, respectively. In the above-mentioned study by Mesti et al., mPFS was very similar to the mPFS observed in our population (5.5 vs 8.3 months, V vs V + C). On the contrary, the mOS in the Slovenian cohort was shorter than in the Polish (10.3 vs 12.3 months, V vs V + C). We believe that differences in mOS between the Polish and Slovenian populations could have been influenced by the treatment applied in subsequent lines. The baseline population characteristics were similar in both studies [14].
Compared with the coBRIM results, our patients achieved shorter mPFS and mOS [6]. These discrepancies are consequences of the selection and rigorous qualification criteria for treatment in clinical trials compared with those used in routine practice. In our analysis, the percentage of patients with unfavorable prognostic factors was higher than in the coBRIM population. Patients with an elevated baseline level of LDH and ECOG/PS 1 or worse comprised 53% and 69% of our population, while only 47% and 30% of the coBRIM population, respectively. Moreover, there were no patients with brain metastases in the coBRIM population compared with 36% in our population [5].
In the MELANIS study carried out on a French population, which included patients treated with V + C, the survival outcomes of the patients were assessed in the entire group and divided into patients who met the criteria for inclusion in the coBRIM study (coBRIM- and non-coBRIM-like population). The mOS of non-co-BRIM-like patients was shorter than that of co-BRIM-like patients (14.7 months vs 25.4 months, respectively), but there were practically no differences in mPFS (7.2 months vs 7.7 months). However, the small size of the co-BRIM-like group (47 patients) and the heterogeneity of the population (51.4% received previous systemic treatment) make it impossible to clearly interpret the results of this study [12]. Likewise, Ismail et al., in a large analysis (435 patients) conducted on a real-world Dutch population treated with first-line BRAFi and MEKi (D+T 86% and V + C 14%), divided patients according to the inclusion criteria for the COMBI-d study into trial eligible and ineligible. Out of 435 patients, more than half (52%) were defined as ineligible, mainly due to the presence of metastasis to the CNS (76%) and poor performance status (39% ECOG ≥ 2). Trial eligible patients lived almost twice as long as ineligible (mOS 17.3 vs 8.9 months) [15].
We also conclude that there is a group of patients in whom treatment with BRAFi and MEKi can provide long-term responses (PFS). In BRIM3, 11% of patients treated with vemurafenib remained progression free after 4 years; in BRIM7, 20.3% of patients treated with V + C in the first line remained progression free after 5 years; and in the coBRIM study, 14% and 10% of patients in the V + C and V + P group were progression free after 5 years [6, 16, 17]. These patients had favorable baseline characteristics (PS0, normal LDH level, M1c) and a good response to the applied therapy (CR or PR) [6, 17]. Similar data were obtained in a large (435 patients) real-world Dutch population treated with first-line BRAFi and MEKi (D+T 86% and V + C 14%). A plateau in the curves for PFS and OS was also observed after 3 years [15].
Our data confirmed previous observations from the pooled analysis of BRIM-2, BRIM-3, BRIM-7, and coBRIM clinical trials on the prognostic significance of LDH level and performance status (ECOG) for OS and data from the real-world MELANIS study, where in addition to the above-mentioned, OS was influenced by the presence of brain metastases [12, 18]. Performing multivariate Cox analysis, we identified clinical baseline parameters which predict therapy outcomes. In the V group, the independent predictors of PFS and OS were LDH, ECOG PS, and brain metastases; similarly, in the V + C group these factors were found to be predictors of OS. However, in the V + C group analysis that identified only ECOG PS as an independent predictor of PFS, LDH and brain metastases had no significant influence on PFS. These data, with regard to the prognostic value of LDH and ECOG, are partly consistent with the results obtained from clinical trial analyses.
Based on data from four clinical trials with V ± C (BRIM2,3,7 and coBRIM), including 310 patients treated with V + C and 717 patients treated with V, Hauschild et al. first identified prognostic significance factors and then created a prognostic tree taking into account the strength of the influence of a given factor on therapy results [18]. LDH, ECOG PS, and baseline sum of the longest diameters of target lesions (SLD) were prognostic for OS. The established correlation was significant for the entire population, as well as for the V and V + C subgroups. On the other hand, contrary to the results obtained by us, in Hauschild's analysis, the prognostic factors of PFS for V and V + C, apart from ECOG PS, were the level of LDH, the presence or absence of liver metastases, and the SLD [18]. The factor that most determined PFS was the baseline level of LDH, which had no significant impact on PFS in our group of patients treated with V + C. However, Hauschild showed that among patients with elevated LDH, there is a subgroup of patients with a prognosis similar to that of patients with normal LDH; these are patients with LDH ≤ 2 × the upper limit of normal (ULN), ECOG PS0 and SLD 44 mm [18].
Sorich et al. analyzed the prognostic model for dabrafenib + trametinib (D+T), dividing patients according to the level of LDH and the number of organs containing metastases into four prognostic subgroups [(1) LDH < ULN and fewer than three metastatic organ sites; (2) LDH < ULN and three or more metastatic organ sites; (3) LDH 1–2 × ULN; and (4) LDH ≥ 2 × ULN] and found application in the group of patients treated with V + C. Using data from the BRIM-3 and co-BRIM studies, Sorich et al. showed that patients treated with V + C, divided into subgroups according to the criteria presented, differ significantly in terms of PFS and OS achieved [19]. In contrast, our analysis did not show a significant impact of the number of metastasis locations on the treatment outcomes achieved by patients in both the V + C and V monotherapy groups. Whereas in the MELANIS early access program group of patients treated with V + C, differences in achieved PFS and OS were observed depending on the presence or absence of brain metastases, normal versus elevated LDH, and ECOG PS < 2 versus ECOG PS 2. However, the independent negative prognostic factors for OS were the presence of brain metastases, ECOG PS 2 status, and liver impairment, while for PFS only the ECOG PS ≥ 2 was relevant [12]. At the same time, the Dermatologic Cooperative Oncology Group conducted a multicenter retrospective study to identify patient and pretreatment characteristics associated with the results of vemurafenib therapy. For both PFS and OS, five predictors have been detected: ECOG PS, pretreatment with immunotherapy, LDH levels, age > 55 years, and pretreatment with chemotherapy. For OS, in addition to the factors listed above, pretreatment with kinase inhibitors and male sex were predictors. Of all predictors, elevated LDH and ECOG PS < 1 had the strongest negative impact on survival in V-treated patients, while for shorter PFS, the strongest predictor was ECOG PS < 1 (HR for LDH > ULN = 1.7 (1.28–2.24); p = 0.00023; HR for ECOG < 1 = 1.99 (1.49–2.64); p < 0.00001) [20]. These data taken together indicated that to better predict the response of V + C therapy, a further multivariate analysis should be performed. That analysis must include all factors discussed above, in particular, LDH level, presence of meta in the CNS, and overall performance status distinguishing between ECOG PS0, PS1 and PS2.
The discrepancy between the results presented in our analysis and the results of the analysis from clinical trials is due to the fact that different clinical parameters were investigated in each of the analyses. For example, in our analysis, the presence of brain metastases was taken into account, which was one of the exclusion criteria for participation in the clinical trials analyzed. The importance of performance status and the presence of brain metastases as a prognostic factor was confirmed in the real world.
In our study, both the proportion of patients experiencing side effects, G3 and G4 side effects, and the total number of side effects were similar in both groups. The only notable difference in the toxicity of both types of treatments was related to skin complications, which were more common in patients who received vemurafenib alone (40% vs 24%). The increased number of skin AEs in patients treated with vemurafenib is mainly due to the increased proliferation of keratinocytes caused by paradoxical activation of the MAPK pathway by inhibition of BRAF kinase. Clinically, this leads to the development of hyperkeratosis and secondary neoplasms such as keratoacanthoma and squamous cell carcinoma (cuSCC) [21].
This was reflected in the results of the coBRIM study, where the following percentages of the above AEs were recorded in the V + P and V + C arms: cuSCC 12.6% versus 4%, keratoacanthoma 9.3% versus 1.6%, and hyperkeratosis 27.2% versus 10.1% [22]. The safety profile of both drugs in real practice is much more favorable than the results of clinical trials demonstrate. The percentages of patients experiencing adverse effects, severe AEs, and the consequences of their occurrence, such as dose modification or discontinuation of treatment, are much less frequently observed. Despite a similar percentage of AEs, especially AE G3 and G4, the number of patients who discontinued treatment in our study due to unacceptable toxicity was higher in the V + C group than in the V + P group (10% vs 4%). This is consistent with the results of the coBRIM study, where twice as many patients in the V + C arm discontinued treatment due to AEs (17% vs 9%) [6].
Our results in terms of the toxicity of both therapies are very similar to the results obtained in a similar population in Slovenia. In the Mesti report presenting Slovenian data, more AEs were reported, 83% for V and 63% for V + C, respectively, and while the percentages of AE G3 and above were similar to those observed in our population, they constituted only 17% of AEs in group V and only 6% in the V + C group (in Poland, 13% vs 13.5%, respectively) [14]. In a study focusing on the safety of vemurafenib in clinical practice, the number of registered AEs was also lower than in clinical trials of this drug as monotherapy (BRIM-3). Particularly large differences were found in AE G3–G4 (37% vs 74% BRIM-3), but a similar percentage of patients discontinued treatment due to AEs (7% vs 7% BRIM-3) [17, 23, 24]. Significant differences in the number of registered AEs in our study and other real-world studies compared with the coBRIM study may result largely from different monitoring guidelines of patients undergoing treatment in routine clinical practice compared with the trial. In the coBRIM study, each patient underwent a thorough cardiological, ophthalmological, and dermatological evaluation on the day of the initiation of treatment, on the first day of the second cycle, and then every three cycles. Echocardiography and specialist ophthalmological examination with optical coherence tomography allowed the detection of a decrease in LVEF and ophthalmic disorders that were clinically silent. In our patients, echocardiography and ophthalmological examination were performed prior to treatment, and then only in case of clinical symptoms. Thus, no clinically silent cardiological and ophthalmic AEs were detected.
A limitation of our work is the retrospective nature, which can cause recall bias and consequently under-reporting of AEs, but an undoubted advantage of our work is the large number of patients included in the analysis and the patients' profile reflecting the real-world population of patients with metastatic melanoma. Long-term follow-up is also a great advantage, as it provides information about the usually small but very important group of patients who obtain long-term benefits from treatment. What is especially important is that the addition of a MEK kinase inhibitor allowed for the improvement of survival rates without increasing the toxicity of the treatment.
Conclusions
These analyses confirmed significant improvements in the results of treatment with V + C therapy over V monotherapy in patients with unresectable and/or metastatic BRAF-mutated melanoma. In the V + C group, more CRs were achieved. Furthermore, in routine practice, no major increase in the toxicity of combination therapy is demonstrated compared with monotherapy.
Declarations
Funding
The article was not financed and was based on the authors' own work. This research did not receive any specific grants from funding agencies in the public, commercial or nonprofit sectors.
Conflict of Interest
PR has received honoraria for lectures and advisory boards outside of the scope of the study from MSD, BMS, Novartis, Pierre Fabre, Sanofi, and Merck. JM - grants and consultancies - BMS, MSD. Fees and honoraria: AMC, BMS, Novartis, Roche, Merck; BCS, RD, GKW, WB, TK, MDS, BMS, Novartis, Roche, Pierre Fabre, MSD; RS, BMS, MSD, Astellas Pharma; JM, BMS, GlaxoSmithKline, Roche, MSD, Novartis, Pierre Fabre; NKK, BMS, Roche; MZ, BMS, MSD, Novartis, for lectures and advisory boards outside of the scope of the study. KP, BZ, KO declare that they have no conflicts of interest that might be relevant to the contents of this manuscript.
Ethics Approval
The study was approved by the bioethics committee, ethical board approval, number KB/430-74 /20. This is a retrospective, noninterventional study, and all data analyzed were collected as a part of routine clinical practice for diagnosis and treatment. All patients signed an informed consent form for treatment according to standard operating procedures in our hospitals. In addition, patients were diagnosed and treated following national guidelines and policies. Treatment was covered according to the reimbursement regulations of the National Health Fund (NFZ, Poland), based on the recommendations of the Polish Agency for Health Technology Assessment and Tariff System (AOTMiT).
Consent to Participate and to Publish
All patients signed an informed consent form for treatment according to the standard operating procedures in our hospitals. All data have been anonymized.
Data Availability
All data generated or analyzed during this study are available upon reasonable request.
Code Availability
Not applicable.
Author Contributions
KP: investigation, methodology, original draft writing, AMC: formal analysis, review and editing, PR: review and editing, BCS: conceptualization, data curation, supervision, writing - review and editing. Resources: all authors All authors read and approved the final manuscript.
Footnotes
Karolina Piejko and Bożena Cybulska-Stopa contributed equally to this study.
Anna M. Czarnecka and Piotr Rutkowski contributed equally to this study.
References
- 1.Patel H, Yacoub N, Mishra R, et al. Current advances in the treatment of BRAF-mutant melanoma. Cancers (Basel) 2020;12(2):482. doi: 10.3390/cancers12020482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364(26):2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McArthur GA, Chapman PB, Robert C, et al. Safety and efficacy of vemurafenib in BRAF(V600E) and BRAF(V600K) mutation-positive melanoma (BRIM-3): extended follow-up of a phase 3, randomised, open-label study. Lancet Oncol. 2014;15(3):323–332. doi: 10.1016/S1470-2045(14)70012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rizos H, Menzies AM, Pupo GM, et al. BRAF inhibitor resistance mechanisms in metastatic melanoma: spectrum and clinical impact. Clin Cancer Res. 2014;20(7):1965–1977. doi: 10.1158/1078-0432.CCR-13-3122. [DOI] [PubMed] [Google Scholar]
- 5.Larkin J, Ascierto PA, Dréno B, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med. 2014;371(20):1867–1876. doi: 10.1056/NEJMoa1408868. [DOI] [PubMed] [Google Scholar]
- 6.Ascierto PA, Dréno B, Larkin J, et al. 5-Year outcomes with cobimetinib plus vemurafenib in BRAFV600 mutation-positive advanced melanoma: extended follow-up of the coBRIM study. Clin Cancer Res. 2021;27(19):5225–5235. doi: 10.1158/1078-0432.CCR-21-0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Long GV, Stroyakovskiy D, Gogas H, et al. Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: a multicentre, double-blind, phase 3 randomised controlled trial. Lancet. 2015;386(9992):444–451. doi: 10.1016/S0140-6736(15)60898-4. [DOI] [PubMed] [Google Scholar]
- 8.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) NCCN.org NCCN Guidelines for Patients®; 2022. http://www.nccn.org/patients(online).
- 9.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1,1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 10.Płużański A. Evaluation of response to treatment—criteria RECIST 11. NOWOTWORY J Oncol. 2014;64(4):331–335. doi: 10.5603/NJO.2014.0055. [DOI] [Google Scholar]
- 11.U S Department of Health and Human Services. Common terminology criteria for adverse events (CTCAE) version 4.0, vol. 4, no. 03 (2009).
- 12.Meyer N, Pérol D, Duval-Modeste AB, et al. Survival in adult patients with BRAFV600 mutation-positive advanced melanoma: a noninterventional ambispective study of patients with cobimetinib combined with vemurafenib during the French early access program: MELANIS study. Melanoma Res. 2022;32(4):269–277. doi: 10.1097/CMR.0000000000000833. [DOI] [PubMed] [Google Scholar]
- 13.Lewis KD, Larkin J, Ribas A, et al. Impact of depth of response on survival in patients treated with cobimetinib ± vemurafenib: pooled analysis of BRIM-2, BRIM-3, BRIM-7 and coBRIM. Br J Cancer. 2019;121(7):522–528. doi: 10.1038/s41416-019-0546-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ocvirk J, Rebersek M, Boc M, Mesti T. Patients with metastatic melanoma treated with vemurafenib as monotherapy or in combination with cobimetinib. J Clin Oncol. 2017;35(15 suppl):e21016. doi: 10.1200/JCO.2017.35.15_suppl.e21016. [DOI] [Google Scholar]
- 15.Ismail RK, Suijkerbuijk KPM, de Boer A, et al. Long-term survival of patients with advanced melanoma treated with BRAF-MEK inhibitors. Melanoma Res. 2022;32(6):460–468. doi: 10.1097/CMR.0000000000000832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ribas A, Daud A, Pavlick AC, et al. Extended 5-year follow-up results of a phase Ib study (BRIM7) of vemurafenib and cobimetinib in BRAF-mutant melanoma. Clin Cancer Res. 2020;26(1):46–53. doi: 10.1158/1078-0432.CCR-18-4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chapman PB, Robert C, Larkin J, et al. Vemurafenib in patients with BRAFV600 mutation-positive metastatic melanoma: final overall survival results of the randomized BRIM-3 study. Ann Oncol. 2017;28(10):2581–2587. doi: 10.1093/annonc/mdx339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hauschild A, Larkin J, Ribas A, et al. Modeled prognostic subgroups for survival and treatment outcomes in BRAF V600-mutated metastatic melanoma: pooled analysis of 4 randomized clinical trials. JAMA Oncol. 2018;4(10):1382–1388. doi: 10.1001/jamaoncol.2018.2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sorich MJ, Rowland A, Hopkins AM. Validation of dabrafenib-trametinib prognostic groups in patients treated with vemurafenib and cobimetinib for advanced BRAF-mutated melanoma. Melanoma Res. 2020;30(3):268–271. doi: 10.1097/CMR.0000000000000634. [DOI] [PubMed] [Google Scholar]
- 20.Ugurel S, Loquai C, Kähler K, et al. A multicenter DeCOG study on predictors of vemurafenib therapy outcome in melanoma: pretreatment impacts survival. Ann Oncol. 2015;26(3):573–582. doi: 10.1093/annonc/mdu573. [DOI] [PubMed] [Google Scholar]
- 21.Su F, Viros A, Milagre C, et al. RAS mutations in cutaneous squamous-cell carcinomas in patients treated with BRAF inhibitors. N Engl J Med. 2012;366(3):207–215. doi: 10.1056/NEJMoa1105358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dréno B, Ribas A, Larkin J, et al. Incidence, course, and management of toxicities associated with cobimetinib in combination with vemurafenib in the coBRIM study. Ann Oncol. 2017;28(5):1137–1144. doi: 10.1093/annonc/mdx040. [DOI] [PubMed] [Google Scholar]
- 23.Larkin J, Del Vecchio M, Ascierto PA, et al. Vemurafenib in patients with BRAF (V600) mutated metastatic melanoma: an open-label, multicentre, safety study. Lancet Oncol. 2014;15(4):436–444. doi: 10.1016/S1470-2045(14)70051-8. [DOI] [PubMed] [Google Scholar]
- 24.Heinzerling L, Eigentler TK, Fluck M, et al. Tolerability of BRAF/MEK inhibitor combinations: adverse event evaluation and management. ESMO Open. 2019;4(3):e000491. doi: 10.1136/esmoopen-2019-000491. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are available upon reasonable request.