Abstract
Necroptosis is a form of regulated necrosis involved in various pathological diseases. The process of necroptosis is controlled by receptor-interacting kinase 1 (RIPK1), RIPK3, and pseudokinase mixed lineage kinase domain-like protein (MLKL), and pharmacological inhibition of these kinases has been shown to have therapeutic potentials in a variety of diseases. In this study, using drug repurposing strategy combined with high-throughput screening (HTS), we discovered that AZD4547, a previously reported FGFR inhibitor, is able to interfere with necroptosis through direct targeting of RIPK1 kinase. In both human and mouse cell models, AZD4547 blocked RIPK1-dependent necroptosis. In addition, AZD4547 rescued animals from TNF-induced lethal shock and inflammatory responses. Together, our study demonstrates that AZD4547 is a potent and selective inhibitor of RIPK1 with therapeutic potential for the treatment of inflammatory disorders that involve necroptosis.
Keywords: AZD4547, RIPK1, inhibitors, SIRS model, anti-necroptosis
Introduction
Necroptosis is a form of programmed necrotic cell death characterized by cell swelling and cell membrane permeabilization, followed by release of immunostimulatory intracellular components [1, 2], thereby ensuing inflammatory responses in vivo [3, 4]. Recent studies suggested that necroptosis is involved in a variety of pathological processes, including inflammatory, infectious and degenerative disorders [5–7]. Upon stimulation with tumor necrosis factor α (TNFα), TNF receptor 1 (TNFR1) induces the ubiquitylation of receptor-interacting protein kinase 1 (RIPK1) and the transient formation of TNF-signaling complex (TNF-RSC or Complex I) along with other components, which promotes the activation of NF-κB signaling pathway and the expression of pro-survival genes [6, 8, 9]. Generally, blocking of the activation of RIPK1 repressors, such as inhibitor κB kinases (IKKs), TGFβ activated kinase-1 (TAK1) or inhibitors of apoptosis (IAPs), leads to the caspase 8 dependent-apoptosis following RIPK1 activation [10, 11]. When caspase-8 is absent or inhibited by caspase inhibitors such as z-VAD, RIPK1 binds and phosphorylates with RIPK3 to initiate the formation of the necrosome complex [12, 13]. The activated RIPK3 in turn mediates the phosphorylation of mixed-lineage kinase domain-like pseudokinase (MLKL) to trigger plasma membrane permeabilization and promote necroptosis [14]. Thus, together with MLKL, the two serine/threonine kinases, RIPK1 and RIPK3 have been considered as the core components of the necroptotic programs and the potential drug targets for therapeutic intervention in necroptosis‐associated diseases [11].
Necrostatin‐1 (Nec-1), the first reported necroptosis inhibitor by selective targeting RIPK1, was discovered in 2005 [15]. Although Nec-1 and its improved analog Nec-1s exhibit remarkable kinome selectivity and display significant therapeutic effects in multiple experimental disease models, insufficient potency and poor pharmacokinetic profiles limit them for further drug development [8, 11]. Recently, the first-in-class selective RIPK1 inhibitor (GSK2982772) has successfully completed Phase I clinical trials in psoriasis and rheumatoid arthritis (RA) [16, 17], but the RIPK1 activity variation in different species limits the explorations in animal disease models for in vivo therapeutic potentials [18]. In addition, although several compounds targeting RIPK3 [19] and MLKL [20], the downstream of RIPK1, were developed, few of them have been reported to enter human clinical trials. Therefore, it is important to discover novel necroptosis inhibitors for diseases associated with this pathway.
In this study, using a drug repurposing approach, we identified the selective FGFR inhibitor AZD4547, which recently completed Phase II clinical trial [21], as a novel inhibitor of necroptosis through cellular anti-necroptosis screening. Further studies confirmed that AZD4547 blocks necroptosis in both human and murine cells at similar drug concentrations. Biochemical assay and molecular docking demonstrated that AZD4547 selectively inhibits RIPK1 as a non-classical ATP-competitive type II kinase inhibitor. Moreover, pre-treatment of AZD4547 blocks TNF-induced lethal shock and inflammatory responses in mouse models. Taken together, these findings suggested that as a potent inhibitor of necroptosis, AZD4547 has potential therapeutic benefit in necroptosis-related indications.
Materials and methods
Chemical reagents
Z-VAD-FMK, SMAC mimetic (BV6), AZD4547, erdafitinib, infigratinib, cycloheximide (CHX), GSK583, GSK872, propidium iodide (PI), Hoechst 33342, lipopolysaccharide (LPS) and polyinosinic-polycytidylic acid sodium (Poly(I:C)) were purchased from MCE (Shanghai, China). Nec-1 was purchased from Targetmol. Recombinant TNFα (10602-HNAE) and IFNβ (50708-MCCH) were purchased from Sino Bio.
Cell lines and cell culture
The cell lines HT29, TOV-21G, L929, U937, RAW264.7 and HEK-293T were purchased from Cobioer Biosciences Co., Ltd. (Nanjing, China). TOV-21G, U937 were cultured in PRMI 1640 (Corning, USA) with 10% fetal bovine serum (VivaCell, Shanghai, China). HT29, L929, RAW264.7 and HEK-293T were cultured in Dulbecco’s Modified Eagle Medium (DMEM) (Corning, USA) with 10% FBS. All cell lines were cultured in culture media with 1% penicillin/streptomycin at 37 °C with 5% CO2. All cell lines were authenticated by STR. Mouse bone marrow derived macrophages (BMDM) were isolated from 6–8 weeks-old C57BL/6 mice bone marrow and differentiated for 5 to 7 days in DMEM supplemented with 10% FBS and 30% supernatant from L929 cells [22].
Small chemical library high-throughput screen (HTS)
TOV-21G cells, grown in 384-well plates, were induced to undergo necroptosis with 20 ng/mL TNFα, 2 μM BV6 and 20 μM zVAD (TSZ) for 24 h. Compounds from a clinical inhibitor library were delivered into each well at a final concentration of 0.5 μM. Cell viability was determined by Cell Titer-Glo assay after 16 h culturing. The Nec-1 was added at a final concentration of 10 μM as positive control.
Immunoblotting and immunoprecipitation
Immunoblotting analysis was performed following the standard protocol. Antibodies against the following proteins were used for immunoblot analysis: p-RIP (S166) (65746, Cell Signaling Technology), RIP (3493, Cell Signaling Technology), p-RIP (S166) (31122, Cell Signaling Technology), p-RIP3 (93654, Cell Signaling Technology), RIP3 (S227) (13526, Cell Signaling Technology), RIP3 (17563–1-AP, Proteintech), p-RIP3 (T231 + S232) (ab222320, Abcam), p-MLKL (S358) (91689, Cell Signaling Technology), MLKL (14993, Cell Signaling Technology), MLKL (66675-1-Ig, Proteintech), p-MLKL (S345) (ab196436, Abcam), IκBα (10268-1-AP, Proteintech), GAPDH (HC301-02, TransGen Biotech), rabbit IgG HRP-linked antibody (7074, Cell Signaling Technology) and mouse IgG HRP-linked antibody (7076, Cell Signaling Technology).
For endogenous immunoprecipitation (IP), cells were placed on ice after treatment and cell lysates were prepared in IP buffer (P0013, Beyotime). Cell lysates were then incubated with the corresponding IP antibodies and samples were incubated overnight with gentle rotation at 4 °C. Protein A/G Magnetic Beads (HY-K0202, MCE) were incubated with the antigen-antibody complex for 4 h the following day. Beads were washed four times in IP buffer and immunoprecipitates were eluted by boiling in 2 × Laemmli buffer and analyzed by immunoblotting blot. VeriBlot (ab131366, Abcam) was used as secondary antibodies for immunoblotting.
Enzymatic assays
The ADP-Glo® kinase assay (Promega) was used to test compounds for its RIPK1/2/3 kinase inhibition effects. The proteins of RIPK1 (VA7591) and RIPK2 (v4084) were purchased from Promega and the protein of RIPK3 (R09-10G) was purchased from SignalChem. The kinase reaction was performed according to manufacturer’s instructions.
ATP competitive assay
AZD4547 was generally diluted into 4 concentrations (30 nM, 20 nM, 10 nM, and 5 nM). Eight concentrations were used (150 μM to 5 μM) for ATP competition experiments. 2.5 µL RIPK1 was incubated with AZD4547 for 60 min in reaction buffer followed by addition of 2.5 µL ATP/substrate mixture. The assay was conducted for 1 h at room temperature. Then, 5 μL of ADP-Glo reagent was added into each well to stop the reaction and consumed the remaining ADP within 40 min. At the end, 10 µL kinase detection reagent was added and incubated for 30 min at room temperature. The luminescence signal was readout with an envision Perkin Elmer plate reader (Envision, PE, USA).
Plasmid construction and transfection
The full length RIPK1, RIPK1 (S161A) and RIPK3 were obtained by PCR and site-mutagenesis from cDNA and cloned into pcDNA3.1 plasmid. 293 T cells were transfected with RIPK1 or RIPK3 plasmids for 12 h using LipoFiter™ (Hanbio, HB-LF-1000) according to the manufacturer’s instructions, then the cell lysates were analyzed by immunoblotting.
PI uptake assay
The cells of optimized density (0.5 × 106–1 × 106/well) were seeded in 6-well plates. Cells were pretreated with indicated compounds for 1 h, then stimulated with TSZ for 12 h. PI (1 μg/mL) and Hoechst 33342 (1 μg/mL) were added into culture medium for 30 min before detection. Representative images were taken by fluorescence microscope (Thermo Fisher, EVOS M5000) and analyzed by ImageJ software.
TNF-induced SIRS model
C57 BL/6 J female mice were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd (Beijing, China), housed in a specific pathogen-free facility and used according to the animal care regulations of Hefei Institutes of Physical Science Chinese Academy of Sciences (Approval no. DWLL-2020–26). The efficacy of AZD4547 was tested in mice using a TNF-driven systemic inflammatory response syndrome model (SIRS). A total of 5 mice per dose group were orally administered HKI solution (0.5% methylcellulose/0.4% Tween 80 in ddH2O) or AZD4547 at doses of 5 and 10 mg/kg 1 h before i.v. administration of recombinant TNF-α (50 μg/mouse, 10602-HNAE, Sino Biological), whereas zVAD-FMK (CAS No: 161401-82-7, MedChemExpress, Shanghai, China) was injected intraperitoneally (i.p.). zVAD-FMK was given 15 min before (250 mg) and 1 h after (100 mg) TNF. Temperature loss in the mice was measured by a rectal probe. Plasma samples and tissue samples of ileum and liver were collected at designated times after injection. Mouse IL-1α (70-EK201A/2-96) and IL-6 (70-EK206/3-96) ELISA on blood serum was performed using ELISA kits from MultiScience in accordance with the manufacturer’s instructions.
Statistical analysis
Statistical analyses were performed by using Prism version 8 (GraphPad Software). The statistical significance of differences between groups was evaluated using the Student’s t test or two-way ANOVA for multiple comparisons. (*P < 0.05, **P < 0.01, ***P < 0.001).
Results
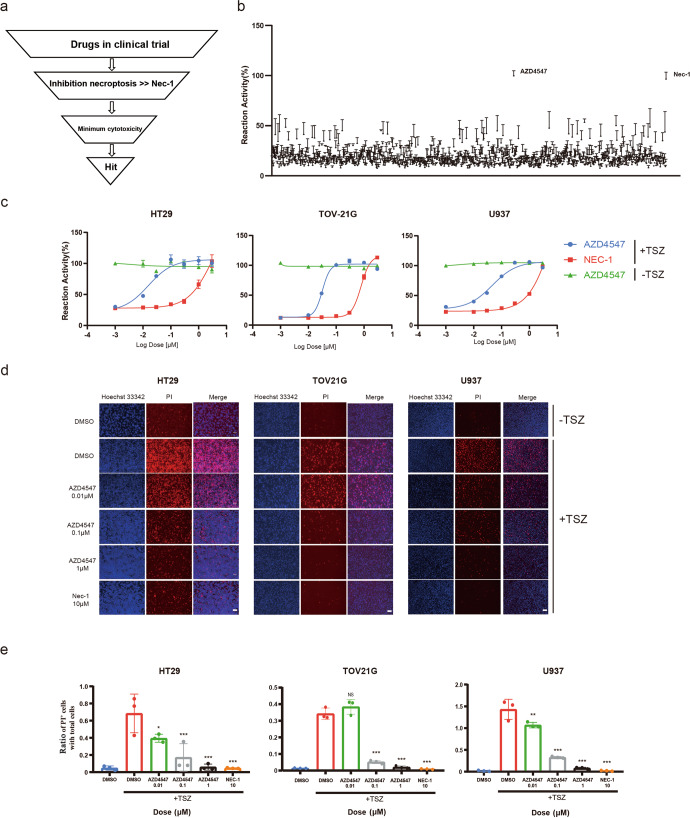
Identification of AZD4547 as a potent necroptosis inhibitor by high-throughput screening
To discover new necroptosis inhibitors, we established the classical TNF-mediated cell necroptosis system and performed high-throughput screening (HTS) of a chemical library consisting over 1000 compounds in clinal trials for their ability to block TNFα-induced necroptosis. Necroptosis was induced in TOV21G cells by treatment with TNF‐α, Smac mimetic (BV6), and caspase inhibitor z‐VAD‐FMK (TSZ), and the cell viability was measured by Cell Titer-Glo assay. To exclude the possible general cytotoxic effects, the compounds were all tested at a concentration of 0.5 μM for cytotoxicity evaluation in the meantime (Fig. 1a). In addition, we chose the well characterized RIPK1 inhibitor Necrostatin-1 (Nec-1) as positive control (Fig. 1a). Interestingly, the selective FGFR inhibitor AZD4547 came up as the most potent molecule in rescuing cells from necroptotic cell death among all the drugs tested (Fig. 1b). Next, we chose another three cell lines to further confirm and evaluate the results from HTS. The results showed that, compared to the Nec-1, AZD4547 efficiently rescued TSZ-induced cell death dose‐dependently at concentrations under 0.1 μM (HT29 EC50 = 0.019 μM; TOV21G EC50 = 0.031 μM; U937 EC50 = 0.052 μM; Fig. 1c). Moreover, we did not observe significant cell cytotoxicity of AZD4547 in the three cell lines up to 10 μM, suggesting desirable therapeutic window (Fig. 1c). We then used propidium iodide (PI) uptake assay to monitor the TNF-induced necroptosis in three cell lines directly. The result showed that PI-positive cells, which is a marker for membrane permeability during necroptosis, decreased in the presence of AZD4547 in response to TSZ induction as measured by fluorescence microscope (Fig. 1d). Quantitative analysis indicated that, consistent with above cell viability results, AZD4547 significantly reduced the relative PI-positive area in a dose-dependent manner (Fig. 1e).
Fig. 1. Identification of AZD4547 as a potent necroptosis inhibitor by HTS.
a Schematic overview of drug screen workflow. b Identification of necroptosis inhibitor by cellular screening with clinical trials inhibitor library. TOV21G cells were pretreated with each compound (0.5 μM) for 30 min and then stimulated with TSZ for 16 h to induce necroptosis. Cell survival was determined by CellTiter-Glo assay and normalized to untreated control cells. c HT29, TOV21G or U937 cells were pretreated with DMSO or AZD4547 at the indicated concentrations and then stimulated with TSZ for 24 h. Cell viability was analyzed by CellTiter‐Glo assay. d Fluorescent images of HT29, TOV-21G or U937 cells cultured in the presence of indicated compounds and stimulated with TSZ. The cells were stained as indicated. Bar = 100 μm. e Quantitative results of relative PI-positive area in Fig. 1d.
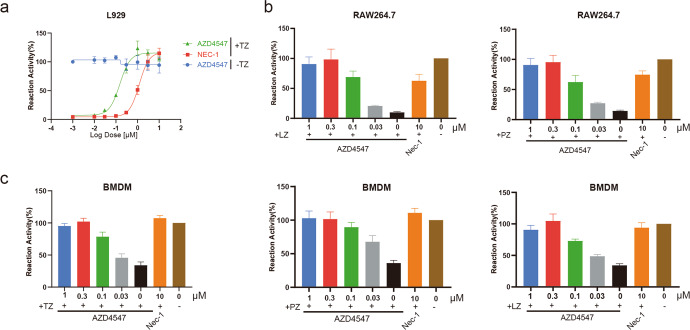
To recapitulate the inhibitory effect of AZD4547 on murine cell models, we first tested AZD4547 in L929 cells. As we expected, AZD4547 potently protected the cells from necroptosis induced by TNF-α and z-VAD-FMK (TZ) dose-dependently (Fig. 2a). Other than necroptosis induced by TNF-α, it is also known that activated Toll-like receptors can also trigger necroptosis [9, 23–25]. Therefore, we next examined the effects of AZD4547 in immortalized macrophage cell line RAW264.7 treated with poly(I:C) (PZ) or LPS (LZ) plus z-VAD-FMK. The results showed that AZD4547 robustly blocked necroptosis mediated by both TLR3 and TLR4 at doses under 0.1 μM (Fig. 2b). Consistently, in mouse bone marrow derived macrophages (BMDM), AZD4547 efficiently inhibited necroptosis induced by TZ, PZ or LZ at the same doses (Fig. 2c). Taken together, these results demonstrated that AZD4547 is a potent inhibitor of necroptosis in both human and mouse cells.
Fig. 2. AZD4547 efficiently blocks necroptosis triggered by various death receptor ligands in murine cells.
a L929 cells were pretreated with AZD4547 at the indicated concentrations followed by stimulation with TZ for 24 h. b RAW264.7 cells were pretreated with AZD4547 at the indicated concentration followed by stimulation with LZ or PZ for 24 h. c BMDM cells were pretreated with AZD4547 at the indicated concentration followed by stimulation with TZ, PZ or LZ for 24 h. All cell viability was analyzed by CellTiter‐Glo assay. Results shown are means ± SD.
AZD4547 blocks necroptosis independent of FGFR or TNF-induced NF-κB activation
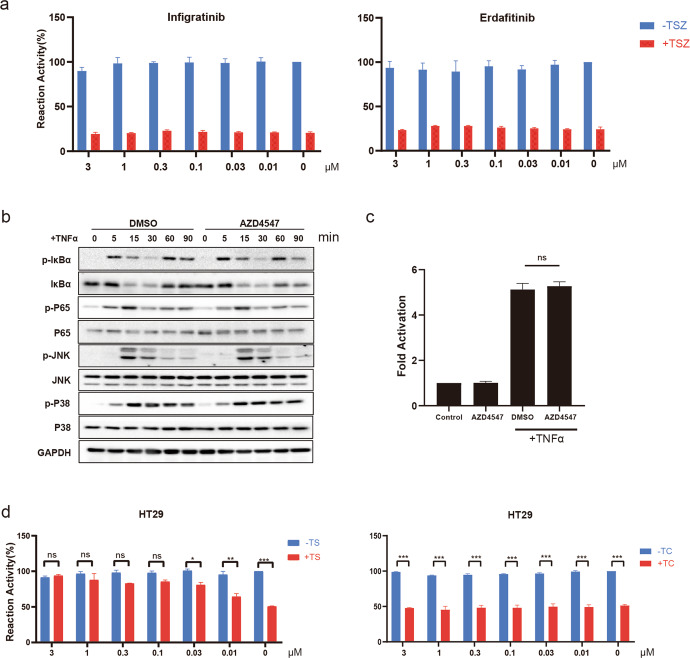
Given that AZD4547 was reported as a selective FGFR inhibitor, to rule out the possibility that AZD4547 blocks necroptosis through interfering FGFR function, we tested two FDA approved FGFR inhibitors, erdafitinib and infigratinib. As expected, both of them were unable to rescue TSZ-induced necroptosis in the dose ranges we tested (Fig. 3a). Based on TNFR1 induces the transient formation of plasma membrane-associated signaling complex (TNF-RSC or Complex I) [8] upon stimulation of TNFα, in order to determine the effect of AZD4547 on the formation of the RIPK1 involved-Complex I, we next tested whether TNF-induced activity of NF-κB was affected by AZD4547. Immunoblot analysis revealed that pretreatment with AZD4547 did not affect the downstream signaling of NF-κB and MAPK in response to TNFα stimulation (Fig. 3b). In addition, AZD4547 had no effect on TNFα-induced NF-κB luciferase reporter activity (Fig. 3c). These results suggested that AZD4547 has no effect on NF‐κB signaling pathway activated through TNF-α and the formation of Complex I.
Fig. 3. AZD4547 blocks necroptosis independent of FGFR or TNF-induced NF-κB activation.
a TOV21G cells were pretreated with infigratinib (left panel) or erdafitinib (right panel) at the indicated concentrations followed by stimulation with TSZ for 24 h. Cell viability was analyzed by CellTiter‐Glo assay. b HT29 cells were pretreated with AZD4547 (1 μM) for 30 min followed by stimulation with TNFα (200 ng/mL) at the indicated time points. c 293 T cells were transfected with a firefly luciferase reporter to construct a promoter sequence containing NF-κB binding sites. After 24 h, cells were treated indicated for 4 h. d HT29 cells were pretreated with DMSO or AZD4547 and then stimulated with TNFα (20 ng/mL) plus Smac mimetic BV6 (2 μM; TS) for 24 h (left). HT29 cells were pretreated with DMSO or AZD4547 and then stimulated with TNFα (20 ng/mL) plus cycloheximide (5 μg/mL; TC) for 24 h (right). Cell viability was analyzed by CellTiter‐Glo assay. *P < 0.05, **P < 0.01, ***P < 0.001.
Considering AZD4547 was developed as a kinase inhibitor, we next studied the published KINOMEscan data to find potential kinase targets that could affect necroptotic signaling. Of note, at a concentration of 10 μM, AZD4547 was found to show high affinity with RIPK1 kinase (0% of control) [26], which is known for its role in necroptosis, suggesting that RIPK1 is a target of AZD4547. Therefore, we set out to verify the effect of AZD4547 on RIPK1 activity. Previous studies had shown that TNFα cotreatment together with Smac mimetic (TS) or cycloheximide (TC) could induce apoptosis through RIPK1-dependent or independent pathways [27, 28]. As we expected, our results showed that AZD4547 protected cells from TS-induced, but not TC-induced, apoptosis in HT29 (Fig. 3d), suggesting that the anti-necroptosis properties of AZD4547 may be due to its RIPK1 kinase activity.
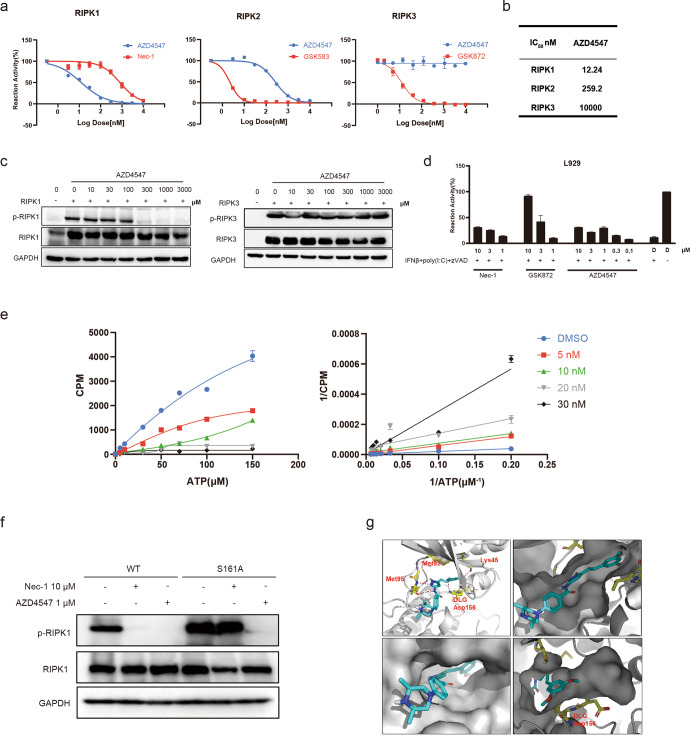
AZD4547 selectively targets RIPK1 among RIPK kinases
To investigate whether AZD4547 directly targets RIPK1 and explore the selectivity of AZD4547 in RIPK family, we performed in vitro kinase assay with three RIPK kinases. The results showed that AZD4547 potently impaired the kinase activity of RIPK1, with IC50 value of 12 nM, which is consistent with its cellular activity in necroptosis assay (Fig. 4a). In addition, AZD4547 was at least 20-fold more selective for RIPK1 over RIPK2 and exhibited no inhibition on RIPK3 up to 10 μM (Fig. 4b). For further verification in cellular context, we tested AZD4547 in cells overexpressing RIPK1 and RIPK3. As we expected, AZD4547 dose-dependently inhibited the auto-phosphorylation of RIPK1, but not RIPK3 (Fig. 4c). Here, we also chose a selective RIPK3 inhibitor GSK872 [29] as positive control to validate our assay system (Fig. S1a). Next, we proceeded with another reported RIPK1-independent necroptosis assay to verify that the anti-necroptotic effect of AZD4547 was solely dependent on RIPK1. In agreement with previous studies [25, 30], selective RIPK3 inhibitor GSK872 significantly rescued the necroptosis dose-dependently, while the RIPK1 inhibitor Nec-1 and AZD4547 did not display protective effect of cells even to 10 μM (Fig. 4d).
Fig. 4. AZD4547 is a highly selective RIPK1 inhibitor.
a In vitro kinase activity was performed with recombinant RIPK1, RIPK2 or RIPK3. b The IC50 values of AZD4547 in three RIPKs family members. c 293 T cells were transfected with RIPK1 (left panel) or RIPK3 (right panel). After 12 h, the cells were treated with AZD4547 at the indicated concentrations for 4 h. Cell lysates were then analyzed by immunoblotting. d L929 cells were primed with IFN-β (50 units/mL) for 24 h, then treated with 10 μg/mL poly(I:C) plus 20 μM zVAD and different concentrations of compounds indicated for 24 h. Cell viability was analyzed by CellTiter‐Glo assay with DMSO(D)as control. e Kinetic study of AZD4547 against RIPK1 with varied concentrations (5 nM, 10 nM, 20 nM and 30 nM respectively) of ATP. Michaelis–Menten plot (left panel) and Lineweaver-Burk plot (right panel) for RIPK1 with AZD4547. f 293 T cells were transfected with RIPK1-WT or S161A mutant for 24 h. AZD4547 or Nec-1 was added post 6 h transfection. Cell lysates were harvested and subjected to Western blot analysis. g Molecular modeling of the binding mode of AZD4547 with kinase domain of human RIPK1 (PDB ID: 6NYH).
In an attempt to study the role of AZD4547 with respect to RIPK1 inhibition, enzyme kinetics analysis was performed. With the increasing concentrations of ATP, Lineweaver-Burk plot showed that all the curves intersected the Y-intercept at zero, indicating that AZD4547 is an ATP-competitive inhibitor (Fig. 4e). To further analyze the binding mode between AZD4547 and RIPK1 kinase, we constructed RIPK1 S161A mutation, which lost the binding activity with Nec-1 as reporter previously, to see whether AZD4547 can occupy the allosteric hydrophobic pocket adjacent to activation loop [31]. The results showed that AZD4547, not Nec-1, effectively inhibited the activation of both WT-RIPK1 and S161A mutant (Fig. 4f), which indicated that AZD4547 might not fit type-III binding model. Using computer-aided structural analysis, we then docked AZD4547 to both the active and inactive confirmation of RIPK1 (PDB ID: 6NYH) (Fig. 4g) and found that AZD4547 fits well into the ATP pocket of the inactive conformation and mainly binds to the residues K45, M95, and D156 of RIPK1. One O atom of the methoxy group forms a hydrogen bond with the K45 and the other O atom forms a hydrogen bond of the DLG-moiety (D156). The O atom of the carbonyl and the N atom of pyrazole form bidentate hydrogen bonds with the hinge residue M95. Dimethylpiperazine extended to the solvent front. It is interesting to note that AZD4547 adapted a non-classical type II binding mode with RIPK1 with no fragment occupying the allosteric pocket.
Since our detection system is based on the enzymatic activity of RIPK1, in order to prove the binding mode, we generated a RIPK1 K45A mutant and tested the effect of AZD4547 on the phosphorylation levels of RIPK1 K45A. The results showed that, K45A mutation significantly affected the phosphorylation level of RIPK1, which is consistent with recent studies [32]. And the effects of AZD4547 on RIPK1 K45A mutants were investigated, the results showed that AZD4547 could hardly block RIPK1 K45A phosphorylation activity compared to WT-RIPK1 (Fig. S2). Considering the M95 is a hinge residue of RIPK1, and the hydrogen bonds were formed with the N and O of the amide on the backbone, the mutagenesis cannot remove the amide on the backbone. Since D156 site is the key catalytic site of DLG, and a single mutation here results in complete loss of enzymatic activity of RIPK1 as reported. Therefore, we ended up performing the mutagenesis and validation analysis only for K45 residue. Taken together, all these findings are consistent with our molecular docking results, suggesting that AZD4547 binds and selectively inhibits RIPK1 as a non-classical ATP-competitive type II kinase inhibitor.
AZD4547 inhibits necroptosis signaling pathway and blocks necrosome formation
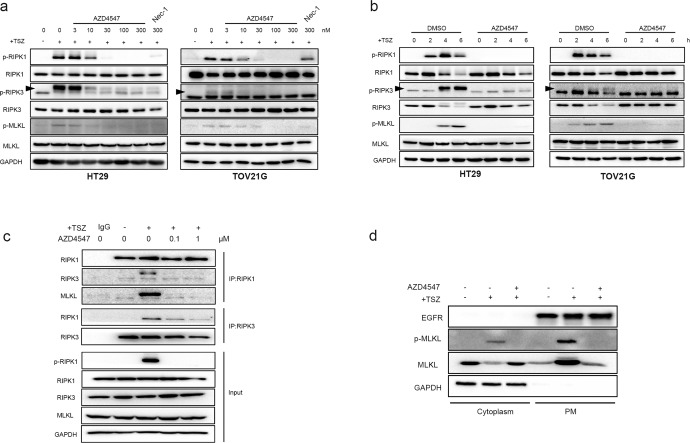
Given that AZD4547 acts as an ATP-competitive RIPK1 inhibitors in vitro, we then determined to know whether the drug would abolish the downstream necroptosis signaling in cellular context. Our immunoblotting results demonstrated that AZD4547 inhibited the phosphorylation of RIPK1 and its downstream RIPK3 and MLKL in a dose-dependent manner when necroptosis was induced by TSZ in HT29 and TOV21G cell lines (Fig. 5a). Similar results were also found in murine L929 cells (Fig. S1b). Moreover, we also investigated the time course of necroptosis signal pathway activation after TSZ stimulation. Accompany with the increase in phosphorylation of RIPK1 2 h post stimulation, the total RIPK1 and RIPK3 decreased gradually [33]. Consistently, the AZD4547 robustly blocked necroptosis-related signaling and reduce the degradation of RIPK1 and RIPK3 (Fig. 5b). Since RIPK1 activation is essential for the necrosome formation, we next examined the effect of AZD4547 on the interaction between RIPK1, RIPK3 and MLKL. We found that the interactions of RIPK1 with RIPK3 and MLKL were abolished by AZD4547 (Fig. 5c), suggesting that AZD4547 blocked TNFα-induced necrosome complex formation by inhibiting the activation of RIPK1 kinase. Finally, considering that oligomerized MLKL translocated from cytoplasm to cell membrane is a hallmark event for necroptosis [34], we evaluated the cellular distribution of MLKL in TSZ-induced HT29 cells. The results demonstrated that AZD4547 blocked the phosphorylation of MLKL and the relocation of MLKL to plasma membrane (Fig. 5d).
Fig. 5. AZD4547 inhibits necroptosis signaling pathway and blocks necrosome formation.
a HT29 and TOV21G cells were treated with the indicated compound for 1 h prior to the treatment of TSZ for additional 4 h. Cell lysates were harvested and subjected to Western blot analysis for phosphorylation levels of RIPK1, RIPK3, and MLKL. The arrows indicated the target bands. b The effects of AZD4547 on phosphorylation of RIPK1, RIPK3 and MLKL in TSZ treated HT29 and TOV21G cells. HT29 and TOV21G cells were pretreated with DMSO or 0.5 μM AZD4547 for 1 h, then treated with TSZ for 0, 2, 4 and 6 h. Indicated proteins were detected by immunoblotting. c HT29 cells were treated with the same conditions as Fig. 5a. Cell lysates were co-immunoprecipitated with anti‐RIPK1 or RIPK3 antibody (IP: RIPK1 or RIPK3) and analyzed by immunoblotting with indicated antibodies. d HT29 cells were treated with AZD4547 for 1 h prior to the treatment of TSZ for additional 6 h. The cells were collected and then separated into cytoplasmic and membrane proteins. The levels of MLKL and p-MLKL were analyzed by Western blotting. GAPDH was used as cytoplasm protein control. EGFR was used as membrane protein control.
AZD4547 protects animal from TNF‐induced SIRS in vivo
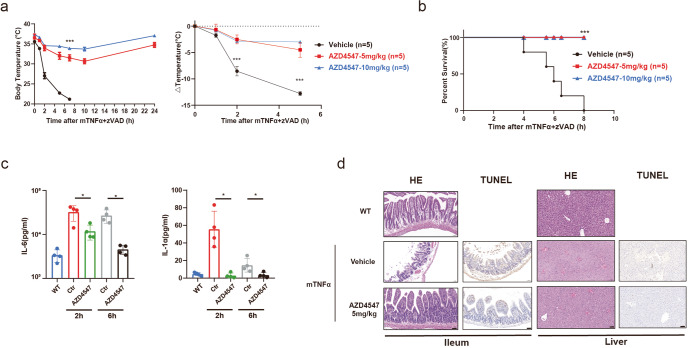
To explore whether AZD4547 has protective effects against RIPK-dependent inflammation in vivo, we established systemic inflammatory response syndrome (SIRS) mouse model, characterized by with hypothermia, increased serum levels of cytokines and chemokines, by injection of TNF and zVAD-FMK in mice [35]. Using this model, we evaluated the in vivo therapeutic effect of AZD4547. While TNF injection induced rapid body temperature drop and resulted in animal death (Fig. 6a, b), pretreatment with AZD4547 protected animals from hypothermia and death in a dose-dependent manner (Fig. 6a, b). AZD4547 showed 66% and 77% protection from temperature reduction over 5 h at doses of 5 and 10 mg/kg, respectively (Fig. 6a). Furthermore, the serum levels of proinflammatory cytokines IL‐6 and IL-1α were significantly ameliorated by AZD4547 treatment at 2 and 6 h in TNF-induced SIRS (Fig. 6c). Meantime, H&E and TUNEL staining results post TNF-α injection 4 h demonstrated that the damage of ileum and liver was also attenuated by AZD4547 treatment (Fig. 6d). Moreover, we also evaluated the effect of AZD4547 on the level of RIPK1 phosphorylation in the ileum and liver of animal models. Results showed that the p-RIPK1 was robustly decreased post-AZD4547 treatment compared to vehicle and normal groups (Fig. S3), suggesting that AZD4547 blocks necroptosis through targeting RIPK1 in animal models. Taken together, these results demonstrate that AZD4547 protects against TNF‐induced SIRS in vivo.
Fig. 6. AZD4547 protects mice from TNF‐induced SIRS.
a C57BL/6 J mice were pretreated with or without AZD4547 (5 or 10 mg/kg) and then induced SIRS with TNFα and z‐VAD‐FMK. The body temperature (left panel) and reduction in body temperature over time (right panel) were measured at indicated time. b Survival curve of the vehicle control and AZD4547 treated mice after TNFα treatment are shown (n = 5). c After SIRS induction for 2 or 6 h, serum levels of IL‐6 and IL-1α were determined by ELISA. d Representative section of H&E and TUNEL staining of the ileum and liver with or without AZD4547 treatment after TNF-α injection 4 h later. *P < 0.05, ***P < 0.001. TUNEL assay was not performed for WT group.
Discussion
Recently, more and more studies reported disease amelioration by pharmacological inhibition or genetic deficiency of the key signaling molecules in the necrotic signaling pathway in various preclinical models, including inflammatory bowel disease (IBD), psoriatic inflammation, rheumatoid arthritis (RA), and chronic neurodegenerative diseases [6, 11, 36–38]. Although there have been many drugs developed against RIPK3 and MLKL, few of them are limited in clinical use to date due to potential toxicity of target [29] or poor pharmacokinetic profiles [11, 20]. Up to now, more RIPK1 inhibitors have been developed than the other necroptosis‐associated targets and made fast progress. The first RIPK1 inhibitor Nec-1 [15] and its analogs have been limited from further drug development due to unsatisfactory potency and poor pharmacokinetic profiles. Recently, GSK2982772 became the first RIPK1 inhibitor entering phase I/IIa clinical trials for the treatment of inflammatory disorders, such as ulcerative colitis, psoriasis, and rheumatoid arthritis [39, 40]. However, the effect of GSK2982772 on RIPK1 activity varies in different species [39], which might limit the explorations of their in vivo therapeutic potential in animal disease models. In addition, the brain-penetrant RIPK1 inhibitor DNL747 also initiated phase I clinical trials to treat neurodegeneration diseases, such as Alzheimer disease (AD) and amyotrophic lateral sclerosis [6, 41]. Interestingly, some studies reported the application of RIPK1 inhibitors in regulating tumor-related process, including inhibiting tumor metastasis by blocking tumor-cell-induced endothelial cell necroptosis [42] or enhancing tumor immunotherapy by regulating the tolerogenicity of tumor-associated macrophages in pancreatic cancer [43]. Unfortunately, no clinical trials have been approved so far. Beside the structure-based drug design, drug repurposing has been considered as a more efficient drug development strategy towards faster and safer clinical use [44]. Earlier studies have found the kinase domains of both RIPK1 and RIPK3 share similar sequence with the kinase domain of B-Raf, thus multiple B-Raf inhibitors were tested and only dabrafenib could rescue cells from necroptosis by direct targeting of RIPK3 [45]. Recently, using drug repurposing strategy, pan-Raf inhibitors LY3009120 [37] and TAK-632 [33] were identified as potent inhibitors of necroptosis by targeting RIPK1 and RIPK3. Moreover, a cellular screening identified multitargeting kinase inhibitors sorafenib, pazopanib, and ponatinib as necroptosis inhibitors that displayed anti-necrosis function at submicromolar concentrations [46, 47]. Taken together, characterization and development of novel, selective, and nontoxic inhibitors of necroptosis have the significance for both basic biological research and treatment of clinical disorders associated with necroptotic cell death.
In this study, we performed phenotypic HTS of compound library containing drugs that are currently in clinical trials in order to identify novel inhibitors of necroptosis. Out of the screening, we found selective FGFR inhibitor AZD4547 that recently has been completed the phase II clinical trial. Compared to the other reported TKIs that block necroptosis, AZD4547 exhibited lower cytotoxicity throughout the dose ranges effective for necroptosis blockade and achieved nanomolar level activity in cells. Meantime, AZD4547 displayed similar activity in both human and mouse cells. Interestingly, two FDA-approved selective FGFR inhibitor, erdafitinib and infigratinib showed no anti-necrosis function in our dose range detection. Further studies confirmed that AZD4547 selectively inhibited RIPK1, but not RIPK3, thereby blocking necrotic signaling pathways and the formation of necrosomes, and ultimately inhibited the occurrence of cell necrosis. Finally, oral administration of AZD4547 protected mice against TNF‐induced SIRS in vivo, suggesting its potential for therapeutic application in necroptosis‐associated diseases.
As an FGFR inhibitor, AZD4547 exerts a therapeutic effect in some inflammation-related models, such as acute kidney injury, the cecal ligation and puncture (CLP)-induced sepsis and hepatic fibrosis [48–50]. Studies suggest that inhibition of FGFR attenuates the LPS-induced TLR4 activation, and affects the activation of NF-κB signaling, thereby restricting the secretion of inflammation-related factors [50, 51]. In addition, studies also suggested that FGF plays a role in regulating the innate immune response [52]. Here, we found there is little to no effect of AZD4547 on TNFα-induced NF-κB signaling. Considering the differences between cell types and animal models, for example, the CLP and LPS induce bacterial inflammatory response, while TNFα induces a sterile inflammatory response, and the differences between TLR4 and TNF receptors, further research is needed to clarify how the FGFR functions during inflammation.
Compared to TNF-induced sterile SIRS that mimics the acute hyperinflammatory phase of SIRS, the septic CLP model closely resembles the clinical SIRS that develops after peritonitis and is more relevant for clinical conditions [53]. Current studies suggest that RIPK-dependent necrotic processes are involved in the development of the CLP model [35, 54]. Recently, Huang et al. reported the protective effect of AZD4547 against excessive inflammatory damage in CLP septic mice model [49], suggesting that AZD4547 may play potential roles in anti-inflammatory through RIPK1 in vivo. In this study, we report that AZD4547, a previously reported FGFR inhibitor, is able to interfere with necroptosis through direct targeting of RIPK1 kinase. In both human and mouse cell models, AZD4547 blocked RIPK1-dependent necroptosis. Importantly, we found that AZD4547 acts by affecting RIPK1, rather than FGFR, in rescuing animals from TNF-induced lethal shock and inflammatory responses, which are consistent with recent studies showing that RIPK1 plays a role in inflammation, including sepsis [6]. We believe that our findings could be complementary to previous studies that AZD4547 exerts anti-inflammatory effects through FGFR signaling, in which a possible role for RIPK1 was not ruled out. Taken together, AZD4547 acts mainly by inhibiting RIPK1 under TNF-induced non-bacterial inflammatory response, thus expanding the clinical indications for AZD4547.
Although AZD4547 exhibited potent RIPK1 activity, several important issues concerning the in vivo use of AZD4547 as a necroptosis inhibitor should be taken into account. Firstly, from the clinical studies that have been disclosed so far, about 90% patients experienced at least one adverse event (AE) following AZD4547 treatment [55, 56]. Therefore, limits in the relationship between risks and therapeutic benefits for AZD4547 in long-term treatment of patients with chronic diseases associated with inflammation. Secondly, the potential off‐target effects of AZD4547 may affect its protective activity in necroinflammation in vivo. For example, from the published KINOMEscan data [26], we found AZD4547 has high affinity with the c-KIT, thus associates with the risk for myelosuppression [57, 58]. Based on the above existing issues, further administration on target-based structural modifications of AZD4547 to optimize high potent and selective drugs for RIPK1 related inflammatory diseases will be put in more effort.
Supplementary information
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant Nos, 32171479, 82104198, 82103976), the Natural Science Foundation of Anhui Province (Grant No, 2108085QH377), the China Postdoctoral Science Foundation (Grant No. 2020M671916), the Collaborative Innovation Program of Hefei Science Center, CAS (Grant No. 2021HSC-CIP014), the Frontier Science Key Research Program of CAS (Grant QYZDB-SSW-SLH037), and the CASHIPS Director’s Fund (Grant Nos. YZJJZX202011, YZJJ2022QN41, YZJJ2021QN38). We are also grateful for the support of the Youth Innovation Promotion Association of CAS support (No. 2022453) for JY. A portion of this work was supported by the High Magnetic Field Laboratory of Anhui Province.
Author contributions
WCW, JL, and QSL conceived and joint-supervised the study; ZWW and ALW designed experiments and analyzed data; ZWW, FMZ, RJ, JL, SQ, and LJS performed experiments; BLW performed docking analysis; ZWW and JY drafted the manuscript; WCW, JL, and QSL revised the manuscript. All authors discussed the results and commented on the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Zuo-wei Wang, Feng-ming Zou, Ao-li Wang
Contributor Information
Jing Liu, Email: jingliu@hmfl.ac.cn.
Wen-chao Wang, Email: wwcbox@hmfl.ac.cn.
Qing-song Liu, Email: qsliu97@hmfl.ac.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41401-022-00993-5.
References
- 1.Sarhan M, Land WG, Tonnus W, Hugo CP, Linkermann A. Origin and consequences of necroinflammation. Physiol Rev. 2018;98:727–80. doi: 10.1152/physrev.00041.2016. [DOI] [PubMed] [Google Scholar]
- 2.Wallach D, Kang TB, Dillon CP, Green DR. Programmed necrosis in inflammation: Toward identification of the effector molecules. Science. 2016;352:aaf2154. doi: 10.1126/science.aaf2154. [DOI] [PubMed] [Google Scholar]
- 3.Kaczmarek A, Vandenabeele P, Krysko DV. Necroptosis: the release of damage-associated molecular patterns and its physiological relevance. Immunity. 2013;38:209–23. doi: 10.1016/j.immuni.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Pasparakis M, Vandenabeele P. Necroptosis and its role in inflammation. Nature. 2015;517:311–20. doi: 10.1038/nature14191. [DOI] [PubMed] [Google Scholar]
- 5.Degterev A, Ofengeim D, Yuan J. Targeting RIPK1 for the treatment of human diseases. Proc Natl Acad Sci USA. 2019;116:9714–22. doi: 10.1073/pnas.1901179116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mifflin L, Ofengeim D, Yuan J. Receptor-interacting protein kinase 1 (RIPK1) as a therapeutic target. Nat Rev Drug Discov. 2020;19:553–71. doi: 10.1038/s41573-020-0071-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ito Y, Ofengeim D, Najafov A, Das S, Saberi S, Li Y, et al. RIPK1 mediates axonal degeneration by promoting inflammation and necroptosis in ALS. Science. 2016;353:603–8. doi: 10.1126/science.aaf6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dondelinger Y, Darding M, Bertrand MJ, Walczak H. Poly-ubiquitination in TNFR1-mediated necroptosis. Cell Mol Life Sci. 2016;73:2165–76. doi: 10.1007/s00018-016-2191-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Newton K. RIPK1 and RIPK3: critical regulators of inflammation and cell death. Trends Cell Biol. 2015;25:347–53. doi: 10.1016/j.tcb.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Dondelinger Y, Jouan-Lanhouet S, Divert T, Theatre E, Bertin J, Gough PJ, et al. NF-kappaB-independent role of IKKalpha/IKKbeta in preventing RIPK1 kinase-dependent apoptotic and necroptotic cell death during TNF signaling. Mol Cell. 2015;60:63–76. doi: 10.1016/j.molcel.2015.07.032. [DOI] [PubMed] [Google Scholar]
- 11.Martens S, Hofmans S, Declercq W, Augustyns K, Vandenabeele P. Inhibitors targeting RIPK1/RIPK3: old and new drugs. Trends Pharmacol Sci. 2020;41:209–24. doi: 10.1016/j.tips.2020.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildford M, et al. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137:1112–23. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaiser WJ, Upton JW, Long AB, Livingston-Rosanoff D, Daley-Bauer LP, Hakem R, et al. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature. 2011;471:368–72. doi: 10.1038/nature09857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun L, Wang H, Wang Z, He S, Chen S, Liao D, et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell. 2012;148:213–27. doi: 10.1016/j.cell.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 15.Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1:112–9. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- 16.Tompson DJ, Davies C, Scott NE, Cannons EP, Kostapanos M, Gross AS, et al. Comparison of the pharmacokinetics of RIPK1 Inhibitor GSK2982772 in healthy Western and Japanese subjects. Eur J Drug Metab Pharmacokinet. 2021;46:71–83. doi: 10.1007/s13318-020-00652-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weisel K, Berger S, Thorn K, Taylor PC, Peterfy C, Siddall H, et al. A randomized, placebo-controlled experimental medicine study of RIPK1 inhibitor GSK2982772 in patients with moderate to severe rheumatoid arthritis. Arthritis Res Ther. 2021;23:85. doi: 10.1186/s13075-021-02468-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris PA, King BW, Bandyopadhyay D, Berger SB, Campobasso N, Capriotti CA, et al. DNA-Encoded library screening identifies benzo[b][1,4]oxazepin-4-ones as highly potent and monoselective receptor interacting protein 1 kinase inhibitors. J Med Chem. 2016;59:2163–78. doi: 10.1021/acs.jmedchem.5b01898. [DOI] [PubMed] [Google Scholar]
- 19.Park HH, Park SY, Mah S, Park JH, Hong SS, Hong S, et al. HS-1371, a novel kinase inhibitor of RIP3-mediated necroptosis. Exp Mol Med. 2018;50:1–15. doi: 10.1038/s12276-018-0152-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rubbelke M, Fiegen D, Bauer M, Binder F, Hamilton J, King J, et al. Locking mixed-lineage kinase domain-like protein in its auto-inhibited state prevents necroptosis. Proc Natl Acad Sci USA. 2020;117:33272–81. doi: 10.1073/pnas.2017406117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chae YK, Hong F, Vaklavas C, Cheng HH, Hammerman P, Mitchell EP, et al. Phase II study of AZD4547 in patients with tumors harboring aberrations in the FGFR pathway: results from the NCI-MATCH Trial (EAY131) Subprotocol W. J Clin Oncol. 2020;38:2407–17. doi: 10.1200/JCO.19.02630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Didierlaurent A, Brissoni B, Velin D, Aebi N, Tardivel A, Kaslin E, et al. Tollip regulates proinflammatory responses to interleukin-1 and lipopolysaccharide. Mol Cell Biol. 2006;26:735–42. doi: 10.1128/MCB.26.3.735-742.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He S, Liang Y, Shao F, Wang X. Toll-like receptors activate programmed necrosis in macrophages through a receptor-interacting kinase-3-mediated pathway. Proc Natl Acad Sci USA. 2011;108:20054–9. doi: 10.1073/pnas.1116302108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Najjar M, Saleh D, Zelic M, Nogusa S, Shah S, Tai A, et al. RIPK1 and RIPK3 kinases promote cell-death-independent inflammation by toll-like receptor 4. Immunity. 2016;45:46–59. doi: 10.1016/j.immuni.2016.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaiser WJ, Sridharan H, Huang C, Mandal P, Upton JW, Gough PJ, et al. Toll-like receptor 3-mediated necrosis via TRIF, RIP3, and MLKL. J Biol Chem. 2013;288:31268–79. doi: 10.1074/jbc.M113.462341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gray N. AZD4547 KINOMEscan. 2013.
- 27.Wang L, Du F, Wang X. TNF-alpha induces two distinct caspase-8 activation pathways. Cell. 2008;133:693–703. doi: 10.1016/j.cell.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 28.He S, Wang L, Miao L, Wang T, Du F, Zhao L, et al. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell. 2009;137:1100–11. doi: 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 29.Mandal P, Berger SB, Pillay S, Moriwaki K, Huang C, Guo H, et al. RIP3 induces apoptosis independent of pronecrotic kinase activity. Mol Cell. 2014;56:481–95. doi: 10.1016/j.molcel.2014.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou T, Wang Q, Phan N, Ren J, Yang H, Feldman CC, et al. Identification of a novel class of RIP1/RIP3 dual inhibitors that impede cell death and inflammation in mouse abdominal aortic aneurysm models. Cell Death Dis. 2019;10:226. doi: 10.1038/s41419-019-1468-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xie T, Peng W, Liu Y, Yan C, Maki J, Degterev A, et al. Structural basis of RIP1 inhibition by necrostatins. Structure. 2013;21:493–9. doi: 10.1016/j.str.2013.01.016. [DOI] [PubMed] [Google Scholar]
- 32.Shutinoski B, Alturki NA, Rijal D, Bertin J, Gough PJ, Schlossmacher MG, et al. K45A mutation of RIPK1 results in poor necroptosis and cytokine signaling in macrophages, which impacts inflammatory responses in vivo. Cell Death Differ. 2016;23:1628–37. doi: 10.1038/cdd.2016.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen X, Zhuang C, Ren Y, Zhang H, Qin X, Hu L, et al. Identification of the Raf kinase inhibitor TAK-632 and its analogues as potent inhibitors of necroptosis by targeting RIPK1 and RIPK3. Br J Pharmacol. 2019;176:2095–108. doi: 10.1111/bph.14653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen X, Li W, Ren J, Huang D, He WT, Song Y, et al. Translocation of mixed lineage kinase domain-like protein to plasma membrane leads to necrotic cell death. Cell Res. 2014;24:105–21. doi: 10.1038/cr.2013.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duprez L, Takahashi N, Van Hauwermeiren F, Vandendriessche B, Goossens V, Vanden Berghe T, et al. RIP kinase-dependent necrosis drives lethal systemic inflammatory response syndrome. Immunity. 2011;35:908–18. doi: 10.1016/j.immuni.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 36.Duan X, Liu X, Liu N, Huang Y, Jin Z, Zhang S, et al. Inhibition of keratinocyte necroptosis mediated by RIPK1/RIPK3/MLKL provides a protective effect against psoriatic inflammation. Cell Death Dis. 2020;11:134. doi: 10.1038/s41419-020-2328-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang C, Luo Y, He Q, Liu S, He A, Yan J. A pan-RAF inhibitor LY3009120 inhibits necroptosis by preventing phosphorylation of RIPK1 and alleviates dextran sulfate sodium-induced colitis. Clin Sci (Lond) 2019;133:919–32. doi: 10.1042/CS20181081. [DOI] [PubMed] [Google Scholar]
- 38.Yu Z, Jiang N, Su W, Zhuo Y. Necroptosis: a novel pathway in neuroinflammation. Front Pharmacol. 2021;12:701564. doi: 10.3389/fphar.2021.701564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harris PA, Berger SB, Jeong JU, Nagilla R, Bandyopadhyay D, Campobasso N, et al. Discovery of a first-in-class receptor interacting protein 1 (RIP1) kinase specific clinical candidate (GSK2982772) for the treatment of inflammatory diseases. J Med Chem. 2017;60:1247–61. doi: 10.1021/acs.jmedchem.6b01751. [DOI] [PubMed] [Google Scholar]
- 40.Weisel K, Scott N, Berger S, Wang S, Brown K, Powell M, et al. A randomised, placebo-controlled study of RIPK1 inhibitor GSK2982772 in patients with active ulcerative colitis. BMJ Open Gastroenterol. 2021;8:e000680. doi: 10.1136/bmjgast-2021-000680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perova T, Grandal I, Nutter LMJ, Papp E, Matei IR, Beyene J, et al. Therapeutic potential of spleen tyrosine kinase inhibition for treating high-risk precursor B cell acute lymphoblastic leukemia. Sci Transl Med. 2014;6:236ra62. doi: 10.1126/scitranslmed.3008661. [DOI] [PubMed] [Google Scholar]
- 42.Strilic B, Yang L, Albarran-Juarez J, Wachsmuth L, Han K, Muller UC, et al. Tumour-cell-induced endothelial cell necroptosis via death receptor 6 promotes metastasis. Nature. 2016;536:215–8. doi: 10.1038/nature19076. [DOI] [PubMed] [Google Scholar]
- 43.Wang W, Marinis JM, Beal AM, Savadkar S, Wu Y, Khan M, et al. RIP1 kinase drives macrophage-mediated adaptive immune tolerance in pancreatic cancer. Cancer Cell. 2018;34:757–74 e7. doi: 10.1016/j.ccell.2018.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fulda S. Repurposing anticancer drugs for targeting necroptosis. Cell Cycle. 2018;17:829–32. doi: 10.1080/15384101.2018.1442626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li JX, Feng JM, Wang Y, Li XH, Chen XX, Su Y, et al. The B-Raf(V600E) inhibitor dabrafenib selectively inhibits RIP3 and alleviates acetaminophen-induced liver injury. Cell Death Dis. 2014;5:e1278. doi: 10.1038/cddis.2014.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martens S, Jeong M, Tonnus W, Feldmann F, Hofmans S, Goossens V, et al. Sorafenib tosylate inhibits directly necrosome complex formation and protects in mouse models of inflammation and tissue injury. Cell Death Dis. 2017;8:e2904. doi: 10.1038/cddis.2017.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fauster A, Rebsamen M, Huber KV, Bigenzahn JW, Stukalov A, Lardeau CH, et al. A cellular screen identifies ponatinib and pazopanib as inhibitors of necroptosis. Cell Death Dis. 2015;6:e1767. doi: 10.1038/cddis.2015.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen X, Zhang X, Xu J, Zhao Y, Bao J, Zheng Z, et al. AZD4547 attenuates lipopolysaccharide-induced acute kidney injury by inhibiting inflammation: the role of FGFR1 in renal tubular epithelial cells. Drug Des Devel Ther. 2020;14:833–44. doi: 10.2147/DDDT.S224343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang Y, Wang F, Li H, Xu S, Xu W, Pan X, et al. Inhibition of fibroblast growth factor receptor by AZD4547 protects against inflammation in septic mice. Inflammation. 2019;42:1957–67. doi: 10.1007/s10753-019-01056-4. [DOI] [PubMed] [Google Scholar]
- 50.Lou D, Han J, Zhou L, Ma H, Xv J, Shou J, et al. Fibroblast growth factor receptor 1 antagonism attenuates lipopolysaccharide-induced activation of hepatic stellate cells via suppressing inflammation. Exp Ther Med. 2018;16:2909–16. doi: 10.3892/etm.2018.6586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhu L, Weng Z, Shen P, Zhou J, Zeng J, Weng F, et al. S100B regulates inflammatory response during osteoarthritis via fibroblast growth factor receptor 1 signaling. Mol Med Rep. 2018;18:4855–64. doi: 10.3892/mmr.2018.9523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fitzpatrick EA, Han X, Xiao Z, Quarles LD. Role of fibroblast growth factor-23 in innate immune responses. Front Endocrinol (Lausanne) 2018;9:320. doi: 10.3389/fendo.2018.00320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rittirsch D, Huber-Lang MS, Flierl MA, Ward PA. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc. 2009;4:31–6. doi: 10.1038/nprot.2008.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen H, Li Y, Wu J, Li G, Tao X, Lai K, et al. RIPK3 collaborates with GSDMD to drive tissue injury in lethal polymicrobial sepsis. Cell Death Differ. 2020;27:2568–85. doi: 10.1038/s41418-020-0524-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Paik PK, Shen R, Berger MF, Ferry D, Soria JC, Mathewson A, et al. A phase Ib open-label multicenter study of AZD4547 in patients with advanced squamous cell lung cancers. Clin Cancer Res. 2017;23:5366–73. doi: 10.1158/1078-0432.CCR-17-0645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saka H, Kitagawa C, Kogure Y, Takahashi Y, Fujikawa K, Sagawa T, et al. Safety, tolerability and pharmacokinetics of the fibroblast growth factor receptor inhibitor AZD4547 in Japanese patients with advanced solid tumours: a Phase I study. Invest New Drugs. 2017;35:451–62. doi: 10.1007/s10637-016-0416-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Galanis A, Levis M. Inhibition of c-Kit by tyrosine kinase inhibitors. Haematologica. 2015;100:e77–9. doi: 10.3324/haematol.2014.117028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kwak Y, Cho H, Hur W, Sim T. Antitumor effects and mechanisms of AZD4547 on FGFR2-deregulated endometrial cancer cells. Mol Cancer Ther. 2015;14:2292–302. doi: 10.1158/1535-7163.MCT-15-0032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.