Abstract
Background
Many clinical trials have reported that low-dose aspirin decreases the risk of pre-eclampsia in women with prior pre-eclampsia. However, its impact in a real-world population has not been fully assessed.
Objectives
To assess the rates of low-dose aspirin initiation during pregnancy in women with a history of pre-eclampsia, and to evaluate the impact of low-dose aspirin in prevention of pre-eclampsia recurrence in a real-world population.
Study Design
CONCEPTION is a French nationwide cohort study which uses data from the country’s National Health Data System database. We included all women in France who gave birth at least twice between 2010–2018, and who had pre-eclampsia during their first pregnancy. Every dispensing of low-dose aspirin (75–300 mg) between the beginning of their second pregnancy and 36 weeks of gestation (WG) was identified. We used Poisson regression models to estimate the adjusted incidence rate ratios (aIRRs) of receiving aspirin at least once during their second pregnancy. In women who had early and/or severe pre-eclampsia during their first pregnancy, we estimated the IRRs of pre-eclampsia recurrence during their second pregnancy according to the aspirin therapy.
Results
In 28,467 women who were included in the study, the aspirin initiation rate during the second pregnancy ranged from 27.8% for women in whose first pregnancy the pre-eclampsia was mild and late, to 79.9% for those women whose pre-eclampsia was severe and early. Just over half (54.3%) of those treated with aspirin-initiated treatment before 16 WG and adhered to treatment. Compared with women with mild and late pre-eclampsia, the aIRRs (95% CI) for receiving aspirin at least once during the second pregnancy were 1.94 (1.86–2.03) for women with severe and late pre-eclampsia, 2.34 (2.17–2.52) for those with early and mild pre-eclampsia, and 2.87 [2.74–3.01] for those with early and severe pre-eclampsia E. Social deprivation was associated with a lower initiation of aspirin (IRR = 0.74 [0.70–0.78]). Aspirin was not associated with a lower risk of mild and late pre-eclampsia, severe and late pre-eclampsia, or mild and early pre-eclampsia during the second pregnancy. The aIRRs for severe and early pre-eclampsia during the second pregnancy were 0.77 (0.62–0.95) for women who received prescribed aspirin at least once, 0.71 (0.5–0.89) for those who initiated aspirin therapy before 16 WG, and 0.60 (0.47–0.77) for those who adhered to aspirin treatment throughout their second pregnancy. The risk of severe and early pre-eclampsia was lower only when the prescribed mean daily dose was ≥ 100 mg/day.
Conclusion
In women with a history of pre-eclampsia, aspirin initiation during a second pregnancy and adherence to the prescribed dosage were largely insufficient, especially for women experiencing social deprivation. Aspirin initiated before 16 WG at a dose ≥ 100 mg/day was associated with a lower risk of severe and early pre-eclampsia.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40265-023-01842-3.
Key Points
| In women with a history of pre-eclampsia: |
| Aspirin initiated before 16 WG at a dose ≥ 100 mg/day was associated with a lower risk of early and severe pre-eclampsia. |
| Only 43.6% of the women included received prescribed aspirin at least once. |
| Social deprivation was associated with a significantly higher risk of pre-eclampsia recurrence. |
Introduction
Pre-eclampsia (PE) is a complex hypertensive disorder of pregnancy combining hypertension and proteinuria, which can lead to severe complications such as eclampsia, HELLP syndrome, and placental abruption [1, 2]. It chiefly occurs during the first pregnancy and is responsible for more than 500,000 fetal and neonatal deaths, and 70,000 maternal deaths worldwide annually [3]. In France, its estimated age-standardised prevalence is 3.1% in nulliparous women and 1.2% in parous women [4].
Since 1985, many clinical studies have reported that low-dose aspirin decreases the risk of pre-eclampsia in high-risk women [5–12]. The most recent Cochrane review found that low-dose aspirin was associated with a statistically significant (18%) lower risk of pre-eclampsia [13]. On the strength of this finding, most national and international guidelines now recommend that high-risk women receive low-dose aspirin to prevent pre-eclampsia [3, 14–18]. Unfortunately, there is a large degree of heterogeneity in these guidelines, especially regarding the definition of high-risk women and the initiation dosage [14]. Although some of them suggest a wide indication for aspirin therapy, including all women with diabetes, hypertension or obesity [15], the most consensual indication is prior pre-eclampsia. In France, guidelines in place since 2008 recommend low-dose aspirin only for women with a history of severe or early pre-eclampsia [17].
While the benefits of low-dose aspirin to prevent pre-eclampsia have been quite well established in clinical trials, its impact on a real-world population has not been fully assessed. Discrepancies are often observed between the therapeutic effect of a treatment in clinical trials and in real-world studies, mainly due to differences in study populations, therapeutic indications, treatment protocols, and adherence to therapy [19]. The latter dimension is particularly important since the level of the impact of low-dose aspirin on pre-eclampsia prevention has been associated with the level of adherence [20]. Neither the extent to which medical guidelines for aspirin-based pre-eclampsia prevention are followed, nor the determinants of aspirin therapy initiation and adherence have been studied in any great depth.
The present study aimed to assess the rates of low-dose aspirin initiation during pregnancy in women with a history of pre-eclampsia, to identify the factors associated with aspirin therapy initiation and adherence, and to evaluate the impact of low-dose aspirin in prevention of pre-eclampsia recurrence in a real-world population.
Material and Methods
Data Source
CONCEPTION (Cohort of Cardiovascular risk in Pregnancy) is a prospective cohort study aimed at exploring the epidemiology of hypertensive disorders of pregnancy and cardiovascular diseases during pregnancy, as well as their repercussions on future cardiovascular health in mothers. It includes all women who gave birth in France between 1 January 2010 and 31 December 2018. The study’s methodology and characteristics are described elsewhere [4, 21]. CONCEPTION is based on data from the French National Health Insurance Information (Système National des Données de Santé, or SNDS) database, which contains comprehensive information on all healthcare expenditures reimbursed by France’s national health insurance system for almost the entire population (~67,000,000 people) [22, 23]. The SNDS mainly comprises two information sources: the National Hospital Discharge Database (PMSI), which records information on public and private hospital stays (whether inpatient or outpatient)—including diagnoses—under ICD-10 codes, and the Interscheme Consumption Data Mart (DCIR), which records information on outpatient care. In particular, the DCIR records reimbursements for drug purchases, medical consultations, and other healthcare expenditures.
Study Population
In the present analysis, we included all women who gave birth for the first time in France after 22 weeks of gestation (WG) between 1 January 2010 and 31 December 2018, who then had a second pregnancy during the same period. Women aged < 15 or > 49 years, those with a medical history of stroke, acute coronary syndrome or heart injury, were excluded as it would not be possible to determine whether they were treated with aspirin during their second pregnancy for secondary prevention of cardiovascular disease, or for prevention of pre-eclampsia. From this initial population, we then identified women diagnosed with pre-eclampsia during their first pregnancy, using the ICD-10 codes O14 (pre-eclampsia) and O15 (eclampsia) in their hospital stay for delivery. Of these, only women with a second delivery after 22 WG between 2010 and 2018 were included.
Definition of Pre-Eclampsia
For the first and second pregnancies, we identified pre-eclampsia phenotypes using the following definitions. We considered pre-eclampsia to be severe when it was recorded as such (ICD-10 code 014.1), or when eclampsia (O15) or HELLP syndrome (O14.2) was diagnosed. It was considered mild for all other situations. Pre-eclampsia was defined as ‘early’ when occurring before 34 WG, and ‘late’ after that time.
Aspirin Use
Every dispensing of low-dose aspirin (75300 mg) between the beginning of the second pregnancy and 36 WG was identified for each woman included. We computed the proportion of days covered with aspirin therapy by dividing the total number of aspirin units delivered (pills or powder) by the number of days during the treatment period, which ran from the first dispensing of aspirin to 36 WG, maternal death, or a diagnosis of pre-eclampsia, whichever came first. We assumed that aspirin was taken on the basis of one unit a day, irrespective of the dose. A proportion of days covered (PDC) ≥ 80% was used as a proxy for aspirin adherence [24]. Aspirin intake was defined in three different ways: at least one dispensing during the pregnancy, at least one dispensing before 16 WG, and at least one dispensing before 16 WG with a PDC ≥ 80%.
We also computed the mean daily dose of aspirin, by dividing the total amount of aspirin dispensed by the treatment period. This mean daily dose was divided into three categories: 0–75 mg/day, 75–100 mg/day and ≥ 100 mg/day.
Women’s Characteristics and Covariables
For each pregnancy, we collected the following data: the delivery mode (vaginal or Caesarean section), whether it was a multiple or singleton pregnancy, intrauterine foetal death (IUFD), obesity- and pregnancy-related haemorrhaging from ICD-10 codes provided in hospital records. Preterm birth was defined as delivery before 37 WG. Tobacco use was identified using an algorithm combining specific hospital coding and delivery of prescribed nicotine replacement therapy before or during pregnancy. Pre-existing diabetes was identified using an algorithm based on delivery of antidiabetic drugs on three different dates in the year preceding pregnancy (or on two dates if at least one large package of 90 pills of antidiabetic drugs was delivered). Gestational diabetes was identified using an algorithm combining the delivery of insulin and glucose strips, or a diagnosis of diabetes during pregnancy in women with no pre-existing diabetes. Persons who benefitted from Universal Medical Coverage (CMUc)—a social benefit in France for those whose income is below a certain ceiling—were defined as living in social deprivation.
Statistical Analysis
The characteristics of the women and their first and second pregnancies were described with numbers and percentages for categorical variables, and means and standard deviations for continuous variables. These characteristics were stratified according to whether prescribed aspirin was dispensed in the second pregnancy, and compared using the Chi-squared test for categorical variables, and Student’s t-test for continuous variables. We then described the numbers and percentages for aspirin dispensing during the second pregnancy according to the severity and the timing (early or late) of pre-eclampsia during the first pregnancy. We also described the numbers and percentages of women who consulted a general practitioner, a cardiologist, an obstetrician-gynaecologist or a midwife at least once in the year before the beginning of their second pregnancy (or in the time since the first delivery if the inter-pregnancy interval was < 1 year) and 20 WG of the second pregnancy, stratified by social deprivation or no social deprivation.
We used Poisson regression models to estimate the crude and fully-adjusted incidence rate ratios (IRRs) and 95% confidence interval (CI) of receiving aspirin at least once during the second pregnancy according to the severity and timing of the first pre-eclampsia, the characteristics of women at the beginning of the second pregnancy (age, social deprivation, obesity, diabetes, tobacco use, chronic hypertension), and the characteristics of the first pregnancy (multiple pregnancy, gestational diabetes, gestational hypertension).
Finally, in women who had early or severe pre-eclampsia during the first pregnancy, we used Poisson regression models to estimate the IRRs and 95% CI of late pre-eclampsia, severe pre-eclampsia, early pre-eclampsia and ‘early or late pre-eclampsia’ during the second pregnancy according to aspirin intake and mean daily aspirin dose, adjusted for the same covariates.
Results
After excluding 3632 women with a history of cardiovascular disease and/or aged <15 years or >49 years, a total of 2,829,744 women with first deliveries in France between 2010 and 2018 were identified (Fig. 1). Among them, 82,820 (2.9%) had pre-eclampsia during their first pregnancy. Of these, 28,467 women had a second pregnancy in the same period and constituted our study population. In terms of aspirin therapy, 43.6% received prescribed low-dose aspirin at least once during their second pregnancy. Of these, 54.3% initiated their treatment before 16 WG and adhered to it throughout their pregnancy (≥ 80% days covered by aspirin therapy).
Fig. 1.
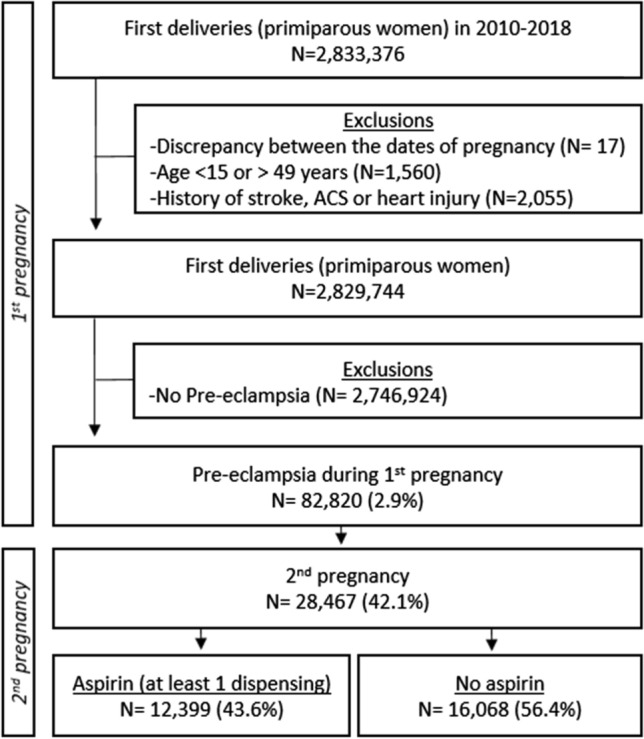
Flow chart. ACS acute coronary syndrome
The characteristics of the study population according to the dispensing of aspirin or no aspirin are displayed in Table 1. Women who received aspirin were older at the beginning of their second pregnancy than untreated women (31.0 vs 30.0 years). They were also significantly more likely to have chronic hypertension and to have had a multiple first pregnancy, a Caesarean section, a preterm first birth, IUFD, and gestational diabetes during their first pregnancy. However, they were significantly less likely to live in social deprivation (13.7% vs 20.8%).
Table 1.
Characteristics according to prescribed dispensing of aspirin
| At least one dispensing of aspirin | Total | ||||||
|---|---|---|---|---|---|---|---|
| No | Yes | p-value | |||||
| (N=16,068) | (N=12,399) | (N=28,467) | |||||
| N | % | N | % | N | % | ||
| Characteristics at the beginning of the 2nd pregnancy | |||||||
| Mean age (SD) (years) | 30.0 | 5.0 | 31.0 | 4.7 | <0.0001 | 30.5 | 4.9 |
| Social deprivation | 3338 | 20.8 | 1693 | 13.7 | <0.0001 | 5031 | 17.7 |
| Obesity | 1935 | 12.0 | 1392 | 11.2 | 0.0336 | 3327 | 11.7 |
| Diabetes | 265 | 1.7 | 231 | 1.9 | 0.1716 | 496 | 1.7 |
| Tobacco use | 1328 | 8.3 | 1067 | 8.6 | 0.3046 | 2395 | 8.4 |
| Chronic hypertension | 2396 | 14.9 | 2938 | 23.7 | <0.0001 | 5334 | 18.7 |
| Characteristics of the 1st pregnancy | |||||||
| Multiple pregnancy | 621 | 3.9 | 295 | 2.4 | <0.0001 | 916 | 3.2 |
| Caesarean section | 7594 | 47.3 | 8821 | 71.1 | <0.0001 | 16415 | 57.7 |
| Preterm birth (< 37 WG) | 3619 | 22.5 | 7711 | 62.2 | <0.0001 | 11330 | 39.8 |
| IUFD | 14 | 0.1 | 34 | 0.3 | 0.0001 | 48 | 0.2 |
| Gestational diabetes | 1725 | 10.7 | 1101 | 8.9 | <0.0001 | 2826 | 9.9 |
| Obstetrical haemorrhage | 1407 | 8.8 | 1052 | 8.5 | 0.4180 | 2459 | 8.6 |
| PE subtypes (1st pregnancy) | <0.0001 | ||||||
| Mild and late PE | 11295 | 70.3 | 4341 | 35.0 | 15636 | 54.9 | |
| Severe and late PE | 3522 | 21.9 | 4161 | 33.6 | 7683 | 27.0 | |
| Mild and early | 484 | 3.0 | 857 | 6.9 | 1341 | 4.7 | |
| Severe and early PE | 767 | 4.8 | 3040 | 24.5 | 3807 | 13.4 | |
IUFD intra-uterine foetal death, PE pre-eclampsia, SD standard deviation, WG weeks of gestation
Aspirin initiation rates depended greatly on the severity (mild/severe) and the timing (early/late) of pre-eclampsia during their first pregnancy (Table 2). Specifically, only 27.8% of women who had mild and late pre-eclampsia had received prescribed aspirin at least once during their second pregnancy, 24.3% received a first aspirin before 16 WG (87% of those who received aspirin), while 11.1% received aspirin before 16 WG and adhered to therapy throughout the pregnancy (39.9% of those who received aspirin). On the contrary, 79.9% of women who had early and severe pre-eclampsia during the first pregnancy had received prescribed aspirin at least once during their second pregnancy, 73.7% received a first aspirin before 16 WG (92.2% of those who received aspirin), and 58.6% received it before 16 WG and adhered to therapy throughout the pregnancy (73.3% of those who received aspirin).
Table 2.
Prescribed dispensing of aspirin during the second pregnancy according to pre-eclampsia subtype during the first pregnancy
| Dispensing of aspirin during the second pregnancy | ||||||
|---|---|---|---|---|---|---|
| At least 1 delivery | At least 1 delivery ≤ 16 WG | At least 1 delivery ≤ 16 WG and PDC ≥ 80% | ||||
| N | % | N | % | N | % | |
| PE subtype during 1st pregnancy | ||||||
| Mild and late PE | 4341 | 27.8 | 3792 | 24.3 | 1738 | 11.1 |
| Severe and late PE | 4161 | 54.2 | 3708 | 48.3 | 2195 | 28.6 |
| Mild and early | 857 | 63.9 | 780 | 58.2 | 573 | 42.7 |
| Severe and early PE | 3040 | 79.9 | 2806 | 73.7 | 2232 | 58.6 |
| Total | 12,399 | 43.6 | 11,086 | 38.9 | 6738 | 23.7 |
PDC proportion of days covered, PE pre-eclampsia, WG weeks of gestation
The dispensing of aspirin also depended on social deprivation (Supplemental Table 1). Only 33.7% of women living in social deprivation received prescribed aspirin at least once during their second pregnancy, 27.4% began the treatment before 16 WG (81.4% of those who received aspirin), and 15.0% received their first aspirin before 16 WG and adhered to therapy throughout the pregnancy (44.6% of those who received aspirin).
Comparing women with mild and late pre-eclampsia (Table 3), the adjusted IRRs (95% CI) of being treated with aspirin (at least one dispensing) during the second pregnancy were 1.94 (1.86–2.03) for women with severe and late pre-eclampsia, 2.34 (2.17–2.52) for early and mild pre-eclampsia, and 2.87 (2.74–3.01) for early and severe pre-eclampsia. Older maternal age and chronic hypertension were also associated with aspirin dispensing during the second pregnancy. On the contrary, living in social deprivation (aIRR = 0.74 [0.70–0.78]) and having a multiple first pregnancy (aIRR = 0.67 [0.59–0.75]) were negatively associated with aspirin therapy.
Table 3.
Incidence rate ratios of being treated with aspirin during the second pregnancy, in women with pre-eclampsia during their first pregnancy
| At least one aspirin dispensing | IRR (95% CI) of receiving aspirin during the second pregnancy | |||
|---|---|---|---|---|
| N | % | Crude | Fully-adjusted | |
| According to the characteristics at the beginning of the 2nd pregnancy | ||||
| Maternal age | ||||
| < 20 years | 76 | 19.7 | 0.49 (0.39–0.61) | 0.60 (0.48–0.75) |
| [20–30] | 5273 | 40.2 | Ref | Ref |
| [30–40] | 6660 | 47.2 | 1.18 (1.13–1.22) | 1.13 (1.09–1.17) |
| ≥ 40 years | 390 | 46.6 | 1.16 (1.05–1.29) | 1.08 (0.97–1.19) |
| Social deprivation | 1693 | 33.7 | 0.74 (0.70–0.78) | 0.74 (0.70–0.78) |
| Obesity | 1392 | 41.8 | 0.96 (0.90–1.01) | 0.96 (0.91–1.02) |
| Diabetes | 231 | 46.6 | 1.07 (0.94–1.22) | 1.01 (0.89–1.15) |
| Tobacco use | 1067 | 44.6 | 1.03 (0.96–1.09) | 1.03 (0.96–1.09) |
| Chronic HT | 2938 | 55.1 | 1.35 (1.29–1.40) | 1.16 (1.11–1.21) |
| Multiple pregnancy | 295 | 32.2 | 0.73 (0.65–0.82) | 0.67 (0.59–0.75) |
| Gestational diabetes | 1101 | 39.0 | 0.88 (0.83–0.94) | 0.92 (0.86–0.98) |
| Gestational hypertension | 1551 | 46.8 | 1.08 (1.03–1.14) | 0.93 (0.88–0.98) |
| Mild and late PE | 4341 | 27.8 | Ref | Ref |
| Severe and late PE | 4161 | 54.2 | 1.95 (1.87–2.04) | 1.94 (1.86–2.03) |
| Mild and early PE | 857 | 63.9 | 2.30 (2.14–2.48) | 2.34 (2.17–2.52) |
| Severe and early PE | 3040 | 79.9 | 2.88 (2.75–3.01) | 2.87 (2.74–3.01) |
CI confidence interval, HT hypertension, IRR incidence risk ratio, PE pre-eclampsia
*Adjusted for all characteristics of the first pregnancy and the characteristics at the beginning of the second pregnancy
In women who had early and/or severe pre-eclampsia during their first pregnancy (N= 12,831), we estimated the crude and adjusted IRRs of having pre-eclampsia during the second pregnancy according to whether they received aspirin therapy (Table 4, crude results in supplemental Table 2). Aspirin therapy was not associated with a lower risk of mild and late pre-eclampsia, severe and late pre-eclampsia, or mild and early pre-eclampsia during the second pregnancy, irrespective of treatment adherence and the mean daily dose. On the contrary, receiving prescribed aspirin at least once was associated with a statistically significant lower risk of severe and early pre-eclampsia during the second pregnancy (aIRR = 0.77 [0.62–0.95]). The risk of early and severe pre-eclampsia was even lower when aspirin was initiated before 16 WG (aIRR = 0.71 [0.57–0.89]), and in women who adhered to treatment throughout their pregnancy (aIRR = 0.60 [0.47–0.77]). Aspirin therapy was not associated with a lower risk of any pre-eclampsia subtype when the mean daily dose was < 100 mg. In contrast, a mean daily dose ≥ 100 mg was associated with a lower risk of severe and early pre-eclampsia (aIRR= 0.67 [0.53–0.85]). Social deprivation was associated with a significantly higher risk of pre-eclampsia recurrence (IRR = 1.24 [1.12–1.36]).
Table 4.
Adjusted incidence risk ratios (aIRR) of developing pre-eclampsia during the 2nd pregnancy according to aspirin use, in women with early and/or severe pre-eclampsia during the 1st pregnancy
| Mild and late PE during the 2nd pregnancy (N = 1143) | Severe and late PE during the 2nd pregnancy (N = 632) | Mild and early PE during the 2nd pregnancy (N = 209) | Severe and early PE during the 2nd pregnancy (N = 463) | Total PE during the 2nd pregnancy (N = 2,447) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | aIRR | N | aIRR | N | aIRR | N | aIRR | N | adjusted IRR | |
| Aspirin purchases | ||||||||||
| No aspirin | 378 | Ref | 213 | Ref | 64 | Ref | 155 | Ref | 810 | Ref |
| At least one | 765 | 1.06 (0.92–1.22) | 419 | 0.98 (0.82–1.18) | 145 | 0.92 (0.67–1.27) | 308 | 0.77 (0.62–0.95) | 1637 | 0.98 (0.90–1.08) |
| ≤ 16 WG | 691 | 1.04 (0.90–1.20) | 365 | 0.94 (0.78–1.14) | 131 | 0.92 (0.66–1.27) | 267 | 0.71 (0.57–0.89) | 1454 | 0.93 (0.85–1.01) |
| ≤ 16 WG and PDC ≥ 80% | 500 | 1.08 (0.92–1.27) | 266 | 0.96 (0.78–1.19) | 91 | 0.84 (0.59–1.21) | 175 | 0.60 (0.47–0.77) | 1032 | 0.93 (0.86–1.01) |
| Mean daily dose | ||||||||||
| No aspirin | 378 | Ref | 213 | Ref | 64 | Ref | 155 | Ref | 810 | Ref |
| 0–75 mg/j | 213 | 1.02 (0.86–1.21) | 113 | 0.95 (0.75–1.20) | 41 | 1.04 (0.70–1.55) | 108 | 1.10 (0.85–1.41) | 475 | 1.03 (0.92–1.16) |
| 75–100 mg/j | 176 | 1.05 (0.87–1.26) | 98 | 1.01 (0.79–1.29) | 31 | 0.93 (0.60–1.45) | 59 | 0.77 (0.56–1.04) | 364 | 0.99 (0.87–1.12) |
| ≥ 100 mg/j | 376 | 1.03 (0.88–1.21) | 208 | 0.99 (0.81–1.22) | 73 | 0.86 (0.60–1.23) | 141 | 0.67 (0.53–0.85) | 798 | 0.95 (0.85–1.05) |
The IRRs were estimated using Poisson regression models adjusted for the characteristics of the first and second pregnancies. This analysis was performed in women with early and/or severe pre-eclampsia during the 1st pregnancy, as this was the only indication for aspirin treatment in French guidelines
IRR incidence risk ratio, PDC proportion of days covered (by aspirin), PE pre-eclampsia, WG weeks of gestation
Discussion
In this nationwide real-world study, it was found that the rates of initiation of aspirin therapy and adherence during a second pregnancy in women with a history of pre-eclampsia, were largely insufficient, especially in those living with social deprivation. Moreover, the latter sub-group was less likely to initiate treatment before 16 WG (the recommended timeframe). In women with severe or early pre-eclampsia during their first pregnancy, aspirin treatment during the second pregnancy was associated with a lower risk of severe and early pre-eclampsia. This effect was statistically significant only when the mean daily dose was ≥ 100 mg and therapy was initiated before 16 WG.
According to 2008 French guidelines [17], women with early or severe pre-eclampsia during their first pregnancy should receive low-dose aspirin during subsequent pregnancies to prevent the risk of pre-eclampsia recurrence. Other national and international guidelines recommend this prevention action irrespective of the timing and severity of the first pre-eclampsia [14]. In our study, only 54% of women with severe pre-eclampsia during their first pregnancy received prescribed aspirin at least once during their second pregnancy, and less than one-third adhered to therapy throughout the pregnancy. These rates were higher in women with early pre-eclampsia. Unsurprisingly, women whose first pre-eclampsia was mild and late were unlikely to initiate aspirin (27.8%) during their second pregnancy, since this indication was not recognised by French guidelines; then again, these figures may be higher in countries where different guidelines apply. Better diffusion of guidelines among physicians and better medical surveillance of at-risk women, especially those living in social deprivation, could improve these rates. In a randomised controlled trial, Wright et al found that 86% of women had good adherence to aspirin therapy during their pregnancy (≥ 80% days covered) [20]. Our results suggest that real-world adherence could be well below this figure. Poor initiation and adherence rates represent a loss of opportunity for both women and children, resulting in an excess number of avoidable maternofoetal complications. This is particularly unfortunate, given the extensive evidence of the safety of low-dose aspirin during pregnancy, with no significant impact on the foetus and only a 6% excess risk of post-partum haemorrhage [13].
Besides the severity and the timing of the first pre-eclampsia, our results showed that older maternal age and chronic hypertension were also associated with aspirin therapy during the second pregnancy. Although not recognised as a therapeutic indication by French guidelines, these two conditions are known to be risk factors of pre-eclampsia. In 2016, Bartsch et al reported that chronic hypertension was the second most important risk factor for pre-eclampsia after previous pre-eclampsia [25]. Consequently, many international and national guidelines recommend that women with chronic hypertension receive aspirin to prevent pre-eclampsia [14].
Women living in social deprivation were 26% less likely to receive aspirin than those not living in social deprivation. Moreover, a complementary analysis found that deprived women who received aspirin were less adherent and initiated treatment later in the pregnancy. The latter result is an issue of concern which must be tackled, since socioeconomic status is also a risk factor of pre-eclampsia recognised by experts [15]. It may reflect the lack of medical follow-up of these women during pregnancy, less access to healthcare, and a lower health literacy level [26, 27]. It most probably does not reflect the costs involved, as aspirin is a low-cost drug and is fully reimbursed in France. Efforts should therefore be made to improve the medical follow-up of socially deprived women during their pregnancy, in order to reduce social inequalities in health (Supplemental Table 1). Moreover, women who experience pre-eclampsia should receive adequate information about their higher risk of recurrence and of later cardiovascular disease, during their postpartum stay [26, 28, 29].
In our study, women with pre-eclampsia during a multiple first pregnancy were also less likely to be treated with aspirin during their second pregnancy. Clinicians may be aware that pre-eclampsia in multiple pregnancies is a particular subtype of pre-eclampsia, mainly the result of a placental ischaemia linked to feto-placental inadequacy, and has a lower risk of recurrence [30].
Our result showed that the risk of early and/or severe pre-eclampsia recurrence was lower in women treated with aspirin, which is consistent with findings from previous studies and especially clinical trials. In a meta-analysis of randomised controlled trials, Roberge et al found that when aspirin was initiated at ≤ 16 WG, the risk of pre-eclampsia and severe pre-eclampsia was significantly lower, and that there was a dose-response effect [10]. In another meta-analysis, the same authors reported that aspirin was associated with a lower risk of preterm pre-eclampsia, but not term pre-eclampsia [9]. This effect was found only when aspirin was initiated at ≤ 16 WG and at a mean daily dose ≥ 100 mg. The trials cited above were performed in different populations and with different protocols (in terms of treatment indication, aspirin dose, definition of outcomes, etc.), hence the discrepancies between the meta-analyses. The validation of these results in real-world population-based studies is therefore valuable to assess the relevance and the effectiveness of treatment guidelines for the prevention of pre-eclampsia.
Our study focused on the prevention of pre-eclampsia recurrence using low-dose aspirin therapy in women who had early or severe pre-eclampsia during their first pregnancy, since this has been the only indication for aspirin therapy recognised by the French college of obstetrics and gynaecology in 2008 [17]. In these women, aspirin was associated with a lower risk of severe or early pre-eclampsia—but not of late and mild pre-eclampsia—only when initiated before 16 WG. This finding reflects the pathophysiology of pre-eclampsia and the pharmacological action of aspirin. Two phenotypes of pre-eclampsia are commonly described: an early-onset form, which results from poor placentation, and a late-onset form, which is more related to maternal factors [31, 32]. Given that aspirin was found elsewhere to improve trophoblast function by modulating the production of cytokines and inhibiting antiangiogenic factors, one can understand why it mainly reduces the risk of early pre-eclampsia [33].
We found a lower risk of early and severe pre-eclampsia only when the mean daily dose was ≥ 100 mg. This is an important finding since the recommended dose of aspirin varies greatly between national and international guidelines and it still a cause for debate. For instance, the World Health Organization and the American College of Obstetricians and Gynaecologists recommend a daily dose 75 mg and 81 mg, respectively, while the European Societies of Cardiology (ESC) and Hypertension (ESH) recommend a daily dose of 100 or 160 mg [18].
Our study has several strengths. Its population-based design, made possible by the use of France’s national medico-administrative database SNDS, enabled us to exhaustively identify all women who had pre-eclampsia during their first pregnancy and who had a second pregnancy in France between 2010 and 2018. A previous report highlighted that the identification of pre-eclampsia in the SNDS is accurate, with a sensitivity of 83% [34]. Thanks to this research approach, our study included a much larger population than clinical trials and meta-analyses on this topic to date. Accordingly, our study has excellent statistical power in terms of exploring the impact of aspirin on pre-eclampsia recurrence according to aspirin dosage, the moment of initiation, and adherence to therapy. We were also able to assess the rate of aspirin dispensing, and the factors associated receiving prescribed aspirin in women with pre-eclampsia during their first pregnancy.
The study also has limitations. First, because of its observational nature, associations found between aspirin therapy and pre-eclampsia may be subject to an indication bias. Nonetheless, the fact that our results were consistent with those of clinical trials to date leads us to believe that the adjustment performed on the models was sufficient to avoid such bias. Second, we assumed that all dispensed aspirin was taken by women; however, some medication boxes may have been lost or patients may have simply stopped taking medication before the end of their pregnancy. Nevertheless, aspirin dispensing is a better indicator of treatment intake than prescription or questionnaires; the PDC method is regarded as a gold standard to assess medication adherence in cohort studies, for it easily summarises adherence over a study period [24]. Third, as aspirin is an inexpensive drug in France, some women may have bought it without prescription. Such purchases would therefore not have been reimbursed and consequently not identified in our analysis. Moreover, we did not know the indication aspirin; it may have been prescribed for other indications than prior pre-eclampsia such as antiphospholipid antibodies syndrome. Finally, our results on the association between low-dose aspirin therapy and a lower risk of pre-eclampsia recurrence apply only to the second pregnancy since we did not study subsequent pregnancies.
Conclusion
Aspirin therapy initiation and adherence levels to prevent recurrent pre-eclampsia were largely insufficient in France, especially in women living in social deprivation. This represents a loss of opportunity for these women and their children. In women with severe or early pre-eclampsia during their first pregnancy, initiating aspirin before 16 WG during their second pregnancy was associated with a 40% lower risk of severe and early pre-eclampsia recurrence. This observational result needs to be confirmed by further randomised controlled trials. Progress still needs to be made to limit the immediate and long-term burden of pre-eclampsia, especially given the growing evidence that pre-eclampsia is a major risk factor of long-term cardiovascular diseases.
Supplementary Information
Below is the link to the electronic supplementary material.
Declarations
Conflict of interest
S.K. reports, outside the submitted work, nonfinancial support from Lilly France, Novo Nordisk, Novartis Pharma, Roche diabetes care, Lifescan, Abbott France, Sanofi, ViiV Healthcare, Servier, Becton Dickinson, and personal fees from Icomed, Pascaleo, BT3SI, M3global research. J.B. reports, outside the submitted work, personal fees from Abbott, Bayer, Bottu, Ferring, Steripharma, Kantar, Teriak, personal fees and non-financial support from Pfizer, Quantum Genomics, personal fees from Sanofi and Servier. All other authors (GL, CG, AG, NR, CDT, VT, GPB, JB, and VO) declare no conflict of interest that might be relevant to this work.
Funding
This work was supported by the French Cardiology Federation (Thematic grant 2019: cardiovascular diseases in women), the French Hypertension Society (SFHTA) and the French Hypertension Research Foundation (FRHTA). The funders had no role in the study design, data collection, data analysis, decision to publish, or drafting of the manuscript.
Authors’ contributions
VO, JB, CDT, SK, VT, NR and GPB conceived and designed the analysis. GL, AG and CG collected the data and performed the analysis. GL wrote the paper under the supervision of VO and JB.
Ethical approval
In line with French national regulations and ethics committee, Institutional Review Board (IRB) approval was not required for this study.
Data availability
Santé Publique France—the French public health agency—has full and chronic access to the SNDS (governmental deliberation no. 2016–316, 13 October 2016). We cannot share National Health Data System data as they are only available on a secure portal. Authorization to access this portal needs registration and clearance.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
References
- 1.Chappell LC, Cluver CA, Kingdom J, Tong S. Pre-eclampsia. Lancet. 2021;398(10297):341–354. doi: 10.1016/S0140-6736(20)32335-7. [DOI] [PubMed] [Google Scholar]
- 2.Hutcheon JA, Lisonkova S, Joseph KS. Epidemiology of pre-eclampsia and the other hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol. 2011;25(4):391–403. doi: 10.1016/j.bpobgyn.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 3.Brown MA, Magee LA, Kenny LC, Karumanchi SA, McCarthy FP, Saito S, Hall DR, Warren CE, Adoyi G, Ishaku S. International Society for the Study of Hypertension in P Hypertensive Disorders of Pregnancy: ISSHP classification, diagnosis, and management recommendations for international practice. Hypertension. 2018;72(1):24–43. doi: 10.1161/HYPERTENSIONAHA.117.10803. [DOI] [PubMed] [Google Scholar]
- 4.Olie V, Moutengou E, Grave C, Deneux-Tharaux C, Regnault N, Kretz S, Gabet A, Mounier-Vehier C, Tsatsaris V, Plu-Bureau G, Blacher J. Prevalence of hypertensive disorders during pregnancy in France (2010–2018): the nationwide CONCEPTION study. J Clin Hypertens (Greenwich) 2021;23(7):1344–1353. doi: 10.1111/jch.14254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beaufils M, Uzan S, Donsimoni R, Colau JC. Prevention of pre-eclampsia by early antiplatelet therapy. Lancet. 1985;1(8433):840–842. doi: 10.1016/S0140-6736(85)92207-X. [DOI] [PubMed] [Google Scholar]
- 6.Askie LM, Duley L, Henderson-Smart DJ, Stewart LA, Group PC. Antiplatelet agents for prevention of pre-eclampsia: a meta-analysis of individual patient data. Lancet. 2007;369(9575):1791–1798. doi: 10.1016/S0140-6736(07)60712-0. [DOI] [PubMed] [Google Scholar]
- 7.Bujold E, Roberge S, Lacasse Y, Bureau M, Audibert F, Marcoux S, Forest JC, Giguere Y. Prevention of preeclampsia and intrauterine growth restriction with aspirin started in early pregnancy: a meta-analysis. Obstet Gynecol. 2010;116(2 Pt 1):402–414. doi: 10.1097/AOG.0b013e3181e9322a. [DOI] [PubMed] [Google Scholar]
- 8.Henderson JT, Whitlock EP, O'Connor E, Senger CA, Thompson JH, Rowland MG. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia: a systematic evidence review for the US preventive services task force. Ann Intern Med. 2014;160(10):695–703. doi: 10.7326/M13-2844. [DOI] [PubMed] [Google Scholar]
- 9.Roberge S, Bujold E, Nicolaides KH. Aspirin for the prevention of preterm and term preeclampsia: systematic review and metaanalysis. Am J Obstet Gynecol. 2018;218(3):287–293. doi: 10.1016/j.ajog.2017.11.561. [DOI] [PubMed] [Google Scholar]
- 10.Roberge S, Nicolaides K, Demers S, Hyett J, Chaillet N, Bujold E. The role of aspirin dose on the prevention of preeclampsia and fetal growth restriction: systematic review and meta-analysis. Am J Obstet Gynecol. 2017;216(2):110–120. doi: 10.1016/j.ajog.2016.09.076. [DOI] [PubMed] [Google Scholar]
- 11.Rolnik DL, Wright D, Poon LC, O'Gorman N, Syngelaki A, de Paco MC, Akolekar R, Cicero S, Janga D, Singh M, Molina FS, Persico N, Jani JC, Plasencia W, Papaioannou G, Tenenbaum-Gavish K, Meiri H, Gizurarson S, Maclagan K, Nicolaides KH. Aspirin versus placebo in pregnancies at high risk for preterm preeclampsia. N Engl J Med. 2017;377(7):613–622. doi: 10.1056/NEJMoa1704559. [DOI] [PubMed] [Google Scholar]
- 12.Meher S, Duley L, Hunter K, Askie L. Antiplatelet therapy before or after 16 weeks' gestation for preventing preeclampsia: an individual participant data meta-analysis. Am J Obstet Gynecol. 2017;216(2):121–128. doi: 10.1016/j.ajog.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 13.Duley L, Meher S, Hunter KE, Seidler AL, Askie LM. Antiplatelet agents for preventing pre-eclampsia and its complications. Cochrane Database Syst Rev. 2019;2019:10. doi: 10.1002/14651858.CD004659.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scott G, Gillon TE, Pels A, von Dadelszen P, Magee LA. Guidelines-similarities and dissimilarities: a systematic review of international clinical practice guidelines for pregnancy hypertension. Am J Obstet Gynecol. 2022;226(2S):S1222–S1236. doi: 10.1016/j.ajog.2020.08.018. [DOI] [PubMed] [Google Scholar]
- 15.ACOG Committee Opinion No 743: low-dose aspirin use during pregnancy. Obstet Gynecol. 2018;132(1):e44–e52. doi: 10.1097/AOG.0000000000002708. [DOI] [PubMed] [Google Scholar]
- 16.WHO. WHO recommendations for prevention and treatment of pre-eclampsia and eclampsia. 2011. [PubMed]
- 17.Pottecher T, Luton D, Zupan V, Collet M. Multidisciplinary management of severe pre-eclampsia (PE). Experts’ guidelines 2008. Annales Françaises d’Anesthésie et de Réanimation. 2008;28:275–281. doi: 10.1016/j.annfar.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 18.Regitz-Zagrosek V, Roos-Hesselink JW, Bauersachs J, Blomstrom-Lundqvist C, Cifkova R, De Bonis M, Iung B, Johnson MR, Kintscher U, Kranke P, Lang IM, Morais J, Pieper PG, Presbitero P, Price S, Rosano GMC, Seeland U, Simoncini T, Swan L, Warnes CA, Group ESCSD. 2018 ESC Guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J. 2018;39(34):3165–241. [DOI] [PubMed]
- 19.Kim HS, Lee S, Kim JH. Real-world evidence versus randomized controlled trial: clinical research based on electronic medical records. J Korean Med Sci. 2018;33(34):e213. doi: 10.3346/jkms.2018.33.e213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wright D, Poon LC, Rolnik DL, Syngelaki A, Delgado JL, Vojtassakova D, de Alvarado M, Kapeti E, Rehal A, Pazos A, Carbone IF, Dutemeyer V, Plasencia W, Papantoniou N, Nicolaides KH. Aspirin for evidence-based preeclampsia prevention trial: influence of compliance on beneficial effect of aspirin in prevention of preterm preeclampsia. Am J Obstet Gynecol. 2017;217(6):685. doi: 10.1016/j.ajog.2017.08.110. [DOI] [PubMed] [Google Scholar]
- 21.Boucheron P, Lailler G, Moutengou E, Regnault N, Gabet A, Deneux-Tharaux C, Kretz S, Grave C, Mounier-Vehier C, Tsatsaris V, Plu-Bureau G, Blacher J, Olie V. Hypertensive disorders of pregnancy and onset of chronic hypertension in France: the nationwide CONCEPTION study. Eur Heart J. 2021;2:2. doi: 10.1093/eurheartj/ehab686. [DOI] [PubMed] [Google Scholar]
- 22.Tuppin P, de Roquefeuil L, Weill A, Ricordeau P, Merliere Y. French national health insurance information system and the permanent beneficiaries sample. Rev Epidemiol Sante Publique. 2010;58(4):286–290. doi: 10.1016/j.respe.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 23.Quantin C, Cottenet J, Vuagnat A, Prunet C, Mouquet MC, Fresson J, Blondel B. Quality of perinatal statistics from hospital discharge data: comparison with civil registration and the 2010 National Perinatal Survey. J Gynecol Obstet Biol Reprod (Paris) 2014;43(9):680–690. doi: 10.1016/j.jgyn.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 24.Forbes CA, Deshpande S, Sorio-Vilela F, Kutikova L, Duffy S, Gouni-Berthold I, Hagstrom E. A systematic literature review comparing methods for the measurement of patient persistence and adherence. Curr Med Res Opin. 2018;34(9):1613–1625. doi: 10.1080/03007995.2018.1477747. [DOI] [PubMed] [Google Scholar]
- 25.Bartsch E, Medcalf KE, Park AL, Ray JG. High Risk of Pre-eclampsia Identification G. Clinical risk factors for pre-eclampsia determined in early pregnancy: systematic review and meta-analysis of large cohort studies. BMJ. 2016;353:1753. doi: 10.1136/bmj.i1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tavananezhad N, Bolbanabad AM, Ghelichkhani F, Effati-Daryani F, Mirghafourvand M. The relationship between health literacy and empowerment in pregnant women: a cross-sectional study. BMC Pregnan Childbirth. 2022;22(1):351. doi: 10.1186/s12884-022-04686-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thomson K, Moffat M, Arisa O, Jesurasa A, Richmond C, Odeniyi A, Bambra C, Rankin J, Brown H, Bishop J, Wing S, McNaughton A, Heslehurst N. Socioeconomic inequalities and adverse pregnancy outcomes in the UK and Republic of Ireland: a systematic review and meta-analysis. BMJ Open. 2021;11(3):e042753. doi: 10.1136/bmjopen-2020-042753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rossiter C, Henry A, Roberts L, Brown MA, Gow M, Arnott C, Salisbury J, Ruhotas A, Hehir A, Denney-Wilson E. Optimising mothers' health behaviour after hypertensive disorders of pregnancy: a qualitative study of a postnatal intervention. BMC Public Health. 2022;22(1):1259. doi: 10.1186/s12889-022-13590-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lui NA, Jeyaram G, Henry A. Postpartum interventions to reduce long-term cardiovascular disease risk in women after hypertensive disorders of pregnancy: a systematic review. Front Cardiovasc Med. 2019;6:160. doi: 10.3389/fcvm.2019.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hernandez-Diaz S, Toh S, Cnattingius S. Risk of pre-eclampsia in first and subsequent pregnancies: prospective cohort study. BMJ. 2009;338:b2255. doi: 10.1136/bmj.b2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.von Dadelszen P, Magee LA, Roberts JM. Subclassification of preeclampsia. Hypertens Pregnan. 2003;22(2):143–148. doi: 10.1081/PRG-120021060. [DOI] [PubMed] [Google Scholar]
- 32.Staff AC. The two-stage placental model of preeclampsia: An update. J Reprod Immunol. 2019;134–135:1–10. doi: 10.1016/j.jri.2019.07.004. [DOI] [PubMed] [Google Scholar]
- 33.Panagodage S, Yong HE, Da Silva CF, Borg AJ, Kalionis B, Brennecke SP, Murthi P. Low-dose acetylsalicylic acid treatment modulates the production of cytokines and improves trophoblast function in an in vitro model of early-onset preeclampsia. Am J Pathol. 2016;186(12):3217–3224. doi: 10.1016/j.ajpath.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 34.Pierron A, Revert M, Goueslard K, Vuagnat A, Cottenet J, Benzenine E, Fresson J, Quantin C. Evaluation of the metrological quality of the medico-administrative data for perinatal indicators: A pilot study in 3 university hospitals. Rev Epidemiol Sante Publique. 2015;63(4):237–246. doi: 10.1016/j.respe.2015.05.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Santé Publique France—the French public health agency—has full and chronic access to the SNDS (governmental deliberation no. 2016–316, 13 October 2016). We cannot share National Health Data System data as they are only available on a secure portal. Authorization to access this portal needs registration and clearance.


