Abstract
Background and Objective
Quantifying exposure to drugs for personalized dose adjustment is of critical importance in patients with tuberculosis who may be at risk of treatment failure or toxicity due to individual variability in pharmacokinetics. Traditionally, serum or plasma samples have been used for drug monitoring, which only poses collection and logistical challenges in high-tuberculosis burden/low-resourced areas. Less invasive and lower cost tests using alternative biomatrices other than serum or plasma may improve the feasibility of therapeutic drug monitoring.
Methods
A systematic review was conducted to include studies reporting anti-tuberculosis drug concentration measurements in dried blood spots, urine, saliva, and hair. Reports were screened to include study design, population, analytical methods, relevant pharmacokinetic parameters, and risk of bias.
Results
A total of 75 reports encompassing all four biomatrices were included. Dried blood spots reduced the sample volume requirement and cut shipping costs whereas simpler laboratory methods to test the presence of drug in urine can allow point-of-care testing in high-burden settings. Minimal pre-processing requirements with saliva samples may further increase acceptability for laboratory staff. Multi-analyte panels have been tested in hair with the capacity to test a wide range of drugs and some of their metabolites.
Conclusions
Reported data were mostly from small-scale studies and alternative biomatrices need to be qualified in large and diverse populations for the demonstration of feasibility in operational settings. High-quality interventional studies will improve the uptake of alternative biomatrices in guidelines and accelerate implementation in programmatic tuberculosis treatment.
Key Points
| Dried blood spots with a reduced sample volume requirement, high sample stability, and low shipping costs facilitate therapeutic drug monitoring in remote settings using a centralized laboratory service. |
| Simple semi-quantitative methods using urine or saliva can serve as point-of-care testing in high-burden settings. |
| Hair samples can provide information on drug exposure over a longer period of time. |
Introduction
Anti-tuberculosis (TB) drugs act in a concentration-dependent manner and suboptimal circulating drug concentrations have been associated with poor outcomes, including acquired drug resistance [1–4]. Individual pharmacokinetic variability is difficult to predict without direct measurement, and early detection of suboptimal drug concentrations enables clinicians to optimize the dose to prevent treatment failures and avoid adverse effects due to toxic drug concentrations [5].
Measuring drug concentrations via serum or plasma has been considered the gold standard for therapeutic drug monitoring (TDM). However, TDM poses many challenges such as uncomfortable sampling methods, requirement for highly trained personnel from the sample collection to analysis, and dry-ice shipping, which all lead to high costs or a lack of availability in TB-endemic settings where poor treatment outcomes are more common and TDM may be of the most benefit [6]. Performing TDM with dried blood spots (DBS), urine, saliva, and hair, in lieu of regular serum or plasma sampling is gaining popularity owing to the relatively simple sample collection and specimens that do not require cold-chain transport [7, 8].
For DBS, a single drop of blood obtained via an automatic lancet can be collected by healthcare workers or patients themselves with minimal discomfort and without the need for trained phlebotomists [9]. The small sample volume makes this method more suitable in pediatric patients as well as patients unable to undergo large-volume venous sampling during intensive pharmacokinetic studies [10]. Dried blood spot cards can be shipped at an ambient temperature reducing the need for shipments on dry ice, thereby reducing shipping costs [9, 10].
Urine collection offers an inexpensive point-of-care testing option with minimal processing for quantifying the excretion of drugs with known and relatively fixed proportions of renal elimination [11–13]. For example, colorimetric methods to qualitatively detect isoniazid in urine by the Arkansas method have been commercialized (IsoScreen; GFC Diagnostics Ltd, Oxforshire, UK) and used extensively to estimate adherence in patients with active TB or latent TB infection, or in patients receiving isoniazid preventative therapy [14]. Colorimetric analytical procedures have also been developed to quantitatively measure rifampin, pyrazinamide, and levofloxacin in the urine of patients with TB [11–13, 15].
Similarly, saliva offers another biomatrix with simple sampling methods that may be more cost effective, with the ability to be implemented across a wide variety of patient populations [16, 17]. Saliva is a low-protein matrix and the drug concentrations quantified in this matrix may more accurately reflect the proportion of medication that is non-protein bound [16]. The ability of many anti-TB drugs to be distributed into oral fluid makes saliva a promising alternative matrix for performing drug monitoring in the field with simple equipment and very little extra processing [17–20].
DBS, urine, and saliva metrics provide snapshots of drug concentrations either at one timepoint or over one dosing interval that can be used to estimate important pharmacokinetic parameters such as peak concentration (Cmax) and the total area under the concentration–time curve (AUC) for a dosing interval. However, cumulative exposure throughout the treatment period is not captured by these metrics. Measuring drug concentrations in hair, especially of drugs with short half-lives such as isoniazid [21] and linezolid [22], may be more representative of long-term pharmacokinetic exposure that is dependent upon the four parameters of absorption, distribution, metabolism, and elimination, but also patterns of adherence to prescribed medications, a potentially important feature for anti-TB care where treatment courses are long [23].
The aim of this systematic review was to assess the current state of knowledge of studies comparing TB medication concentrations in DBS, urine, saliva, and hair with plasma or serum concentrations, define the product development stage of these methods based on the published literature, and explore if TDM using these alternative matrices would be feasible for anti-TB care in programmatic settings.
Methods
First-line and second-line anti-TB drugs were included in this systematic search [24]. PubMed and Web of Science were searched in May 2022 for the keywords (isoniazid OR rifampin OR pyrazinamide OR ethambutol OR rifapentine OR levofloxacin OR moxifloxacin OR gatifloxacin OR amikacin OR capreomycin OR kanamycin OR streptomycin OR ethionamide OR prothionamide OR cycloserine OR terizidone OR linezolid OR clofazimine OR bedaquiline OR delamanid OR pretomanid OR paraaminosalicylic acid OR imipenem/cilastatin OR imipenem OR cilastatin OR meropenem OR amoxicillin/clavulanate OR amoxicillin OR clavulanate OR thiacetazone) AND (saliva OR urine OR hair OR dried * spot OR volumetric absorptive microsample*) AND (tuberculosis OR TB). There was no limit on publication dates. Reproducibility of results was checked by a second reviewer by conducting a search using the same keywords. Two independent reviewers screened titles and abstracts for eligibility after duplicates were removed. A full-text review was performed on the remaining reports and articles. Non-human studies, commentaries, and studies that did not collect DBS, urine, saliva, or hair samples were excluded. References were screened to include relevant articles. The Preferred Reporting Items for Systematic reviews and Meta-analyses (PRISMA) was used for this review [25].
Data extraction was performed to determine study population, sample size, sampling, analytical methods used to determine concentrations, comparative serum and/or plasma concentrations, and if the obtained results were used to perform drug monitoring. Ratios of the concentrations of individual drugs within the alternate biomatrix to serum and/or plasma concentrations were calculated if Cmax or AUC values were available.
Risk of bias was assessed for all included studies using the Risk Of Bias In Nonrandomized Studies-of Interventions (ROBINS-I) tool, which evaluates the risk of bias in estimates of effectiveness or safety of an intervention from studies that did not use randomization to allocate interventions [26]. As no validated tool for risk bias assessment was available for pharmacokinetic studies, ROBINS-I was adopted by making changes to the classification of interventions and deviations from intended interventions sections, as they were not applicable to pharmacokinetic studies. For each drug under every biomatrix, the technology readiness level (TRL) was assessed from a scale of 1 (basic research) to 9 (launch operations) [27], and details on the level assessment are described in Table 1.
Table 1.
TRL to test readiness of implementing alternative biomatrix in programmatic settings
| TRL score | Description | Interpretation in context |
|---|---|---|
| 9 | Actual system proven in operational environment | Alternative matrix proven to be used in lieu of plasma/serum for drug monitoring |
| 8 | System complete and qualified | Alternative matrix assays validated with gold-standard comparisons (i.e., with reported pharmacokinetic parameters and alternative matrix-gold standard ratios) |
| 7 | System model or prototype demonstration in operational environment | Alternative matrix assays tested in patients with tuberculosis |
| 6 | Technology demonstrated in relevant environment | Alternative matrix assays tested in healthy human volunteers ingesting study medications |
| 5 | Technology validated in relevant environment | Alternative matrix assays tested in spiked healthy human samples |
| 4 | Technology validated in laboratory | Alternative matrix assays validated in laboratory |
| 3 | Experimental proof of concept | Non-human sample proof-of-concept studies of alternative matrix |
| 2 | Technology concept formulated | Assays to quantify drug concentrations in alternative matrix developed |
| 1 | Basic principles observed | Principles of using alternative matrix observed |
Figure adapted from https://www.twi-global.com/technical-knowledge/faqs/technology-readiness-levels
TRL technology readiness level
Results
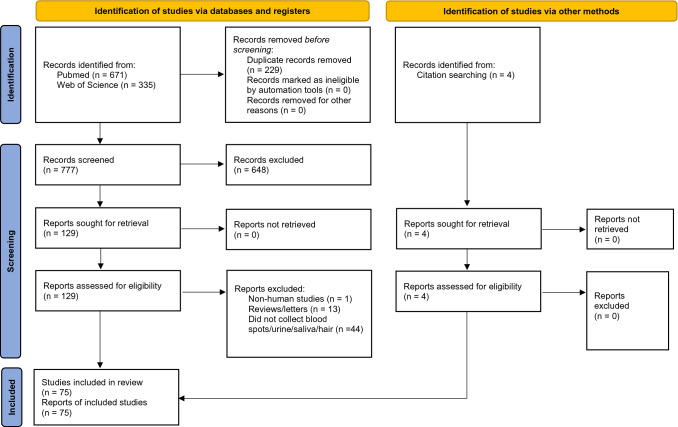
A total of 671 articles were found in PubMed and 335 in Web of Science for the search terms resulting in 777 articles after 229 duplicate reports were removed. Of the remaining articles, 648 records were excluded as they were not relevant based on title and abstract screening. A full-text assessment was performed for 129 articles and 58 articles were excluded for reasons stated in Fig. 1. Four articles were included from searching references, leading to a final total of 75 articles included in the systematic review.
Fig. 1.
Flowchart of the search of reports included in this systematic review. Chart from Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. doi: 10. 1136 / bmj. n71 (http://www.prisma-statement.org)
DBS
Table 2 summarizes the information of studies focusing on the development and validation of a bioanalytical method to quantify anti-TB drugs in the DBS matrix (n = 8). The majority of studies (87.5%) were performed on patients with TB while one study was conducted in healthy volunteers [28], and one in pediatric patients [29]. Most of the studies were small in size and ranged from 6 to 26 subjects. Plasma and DBS samples were collected 1 week to 10 days after treatment initiation. Dried blood spots were generated through a finger prick [29, 30] or pipetting venous dried blood spot (VDBS) onto paper, [28, 31] or both [32, 33]. For comparative plasma samples, both intensive and sparse sampling methods were applied, while finger-prick DBS specimens were mostly collected by a sparse sampling strategy. Considering the quantification method, liquid chromatography-tandem mass spectrometry was the most common apparatus applied for both DBS and plasma matrices (seven of eight studies). The methods were also validated with criteria according to the guidelines for bioanalytical method validation with accuracy and precision ≤ 20% relative error and coefficient of variation respectively for quality-control samples at the lower limit of quantification and ≤ 15% for other quality-control samples. As clinical validation is highly recommended [9], the agreement between DBS and plasma analysis data was assessed in all studies including two or more methods such as simple linear regression, Passing–Bablok regression, Deming regression, Bland–Altman plots, and predictive performance of plasma concentrations from DBS. Pharmacokinetic parameters, including Cmax and/or AUC, were calculated for plasma and/or DBS in two studies [29, 33]. Information on sample size [34], the duration between treatment initiation and sample collection [30, 32, 34, 35], DBS sampling times [34, 35], and drug concentrations [28–32, 34, 35] was not provided in some studies. All included studies were estimated to have a low overall risk of bias under various categories (Table 2). As studies comparing DBS and plasma presented DBS-plasma ratios, the TRL score was 8 for rifampin, ethambutol, and linezolid. Albeit the small sample size, measuring DBS was performed in mostly patients with TB, and the TRL scores for isoniazid, pyrazinamide, moxifloxacin, and clarithromycin were 7, indicating the technology of using DBS was demonstrated in an operational environment (Table 6). The TRL score for rifapentine was 6 as the study was performed in healthy volunteers rather than patients with TB [28].
Table 2.
List of studies reporting DBS sampling, serum/plasma-DBS comparative methods, and risk of bias
| Drug | Study | Population type | Sample size | Sampling | Sampling times | Analytical method | DBS-serum/plasma ratio | DBS-serum/plasma comparison methods | Risk of bias (overall) |
|---|---|---|---|---|---|---|---|---|---|
| Rifampin | Martial et al. [29] | TB (pediatric) | 15 | Days 7–10 | 0, 2, 4, and 8 h post-dose | LC-MS/MS | 1.33 | Ratios, Passing–Bablok regression, Bland–Altman plots, predictive performance of plasma from DBS | Low |
| Vu et al. [30] | TB | 12 | ND | 1, 2, and 4 h post-dose | LC-MS/MS | ND | Linear regression, Deming regression | Low | |
| Rifapentine | Parsons et al. [28] | HV | 26 | 1st and 14th dose | 0, 0.5, 1, 2, 4, 5, 8, 12, 24, 34, 48, and 72 h post-dose | LC-MS/MS | ND | Bland–Altman plots | Low |
| Isoniazid | Lee et al. [35] | TB | 10 | ND | ND | UPLC-MS/MS | ND | Passing–Bablok regression, Bland–Altman plot | Low |
| Pyrazinamide | Martial et al. [29] | TB (pediatric) | 15 | Days 7–10 | 0, 2, 4, and 8 h post-dose | LC-MS/MS | 1.23 | Ratios, Passing–Bablok regression, Bland–Altman plots, predictive performance of plasma from DBS | Low |
| Ethambutol | Martial et al. [29] | TB (pediatric) | 15 | Days 7–10 | 0, 2, 4, and 8 h post-dose | LC-MS/MS | 1.96 | Ratios, Passing–Bablok regression, Bland–Altman plots, predictive performance of plasma from DBS | Low |
| Moxifloxacin | Bradmadhi et al. [31] | TB | 15 | After > 3 doses | 2 h post-dose | UPLC-MS/MS | ND | Deming regression, Bland–Altman plots | Low |
| Vu et al. [32] | TB | 6 | Not provided | 0, 2, and 8 h post-dose | LC-MS/MS | ND | Linear regression, Passing–Bablok regression | Low | |
| Linezolid | Baietto et al. [34] | ND | ND | ND | Not provided | UPLC-PDA | ND | Passing–Bablok regressions, Bland–Altman analysis | Low |
| Vu et al. [33] | TB | 8 | After > 7 days | 0, 2, and 8 h post-dose | LC-MS/MS | DBS: 1.20 | Ratios, Passing–Bablok regressions, Bland–Altman analysis | Low | |
| Clarithromycin | Vu et al. [30] | TB | 12 | ND | 0, 2, and 8 h post-dose | LC-MS/MS | ND | Linear regression, Deming regression | Low |
DBS dried blood spots, h hours, HV healthy volunteers, LC-MS/MS liquid chromatography-mass spectrometry/mass spectrometry, ND not described, TB tuberculosis, UPLC-PDA ultra-performance liquid chromatography-photo diode array
Table 6.
Data indicating TRL of each alternative matrix for drugs found in this systematic review
| Drug | DBS | Urine | Saliva | Hair | ||||
|---|---|---|---|---|---|---|---|---|
| TRL score | Summary | TRL score | Summary | TRL score | Summary | TRL score | Summary | |
| Rifampin | 8 | Results from one of the two studies reported [29, 30], additional studies needed | 7 | Ratios not provided in quantitative studies [11, 15, 42, 44, 47]. Other studies qualitative | 8 | Reported ratios low. Poor diffusion in saliva noted [74, 77] | No data | |
| Rifapentine | 6 | Studies in patients with TB needed [28] | 7 | Urine Cmax, AUC not reported [47] | No data | No data | ||
| Isoniazid | 7 | DBS and plasma concentrations were measured in the included study, but Cmax , AUC, and DBS-plasma ratio not reported [35] | 7 | Ratios not provided in quantitative studies [36, 38, 49, 50, 59, 67, 69]. Other studies qualitative | 8 | Wide range of reported ratios [50, 74, 77] | 7 | Wide range of reported hair concentrations at different timepoints [84] in one study. Ratios for other studies needed |
| Pyrazinamide | 7 | Study performed in pediatric patients [29], studies in adults needed | 7 | Urine-serum ratio not reported [12] | No data | 7 | Studies reporting hair-plasma ratios needed | |
| Ethambutol | 8 | Reported DBS-plasma ratio low [29] | No data | No data | 7 | Studies reporting hair-plasma ratios needed | ||
| Levofloxacin | No data | 7 | Urine-serum ratio not reported [13] | 8 | Wide range of reported ratios [20, 79] | 7 | Studies reporting hair-plasma ratios needed | |
| Moxifloxacin | 7 | DBS and plasma concentrations were measured in included studies, but Cmax , AUC, and DBS-plasma ratio not reported [31, 32] | No data | 8 | Wide range of reported ratios [75, 78] | 7 | Studies reporting hair-plasma ratios needed | |
| Amikacin | No data | No data | 8 | Undetectable levels in saliva [81] | No data | |||
| Ethionamide | No data | 6 | Both included studies qualitative [72, 73] | No data | 7 | Studies reporting hair-plasma ratios needed | ||
| Cycloserine | No data | 7 | Urine-plasma ratio not reported. Wide urine concentrations in different tested methods in same study [71] | No data | No data | |||
| Linezolid | 8 | Ratio promising in one study, but conversion factor may be needed [33] | No data | TRL 8 | Wide range of reported ratios [78, 80] | 7 | Studies reporting hair-plasma ratios needed | |
| Clofazimine | No data | No data | No data | 7 | Studies reporting hair-plasma ratios needed | |||
| Bedaquiline | No data | No data | No data | 7 | Studies reporting hair-plasma ratios needed | |||
| Delamanid | No data | No data | No data | 7 | Studies reporting hair-plasma ratios needed | |||
| Pretomanid | No data | No data | No data | 7 | Studies reporting hair-plasma ratios needed | |||
| Clarithromycin | 7 | DBS and plasma concentrations measured, but Cmax , AUC, and DBS-plasma ratio not reported [30] | No data | 8 | Correction factor to be applied [80] | No data | ||
AUC area under the concentration–time curve, Cmax peak concentration, DBS dried blood spot, TRL technology readiness level
Urine
A total of 43 articles were found to determine rifampin, rifapentine, isoniazid, pyrazinamide, ethionamide, levofloxacin, and cycloserine in urine. Study populations comprised healthy volunteers and adult and pediatric patients with drug-susceptible drug-resistant TB or patients with latent TB infection. The sample size ranged between one and 650 participants. Dosage, sample collection, and drug analytical methods are listed in Table 3. Visual detection using the Arkansas method was the most common method of testing adherence among patients taking isoniazid. Seventeen studies quantitatively measured drugs in urine, and seven of the 17 studies compared urine concentration with serum. Only one study [36] described a procedure for reporting the absence of isoniazid in urine to the treating physician to monitor adherence.
Table 3.
List of studies reporting urine sampling, serum/plasma-urine comparative methods, and risk of bias
| Drug | Study | Population type | Sample size | Sampling | Urine sampling times | Analytical method | Urine-serum/plasma ratio | Urine-serum/plasma comparison methods | Risk of bias (overall) |
|---|---|---|---|---|---|---|---|---|---|
| Rifampin | Burkhardt et al. [37] | TB | 319 | ND | 2, 4, 6, 8, and 24 h post-dose | Chemical reaction and visual detection | N/A | N/A | Moderate |
| Chatterjee et al. [40] | HV | 1 | N/A | N/A | Fluorescence quenching | N/A | N/A | Low | |
| Eidus et al. [41] | Volunteers | 9 | Day of dose administration | 0, 1, 2, 4, 6, 8, 12, and 24 h post-dose | Chemical reaction and visual detection | N/A | N/A | Low | |
| Espinosa-Mansilla et al. [42] | TB | 1 | ND | ND | Chromatography with photometric detection | N/A | N/A | Low | |
| Meissner et al. [43] | TB | 174 | ND | ND | Visual detection with color reference | N/A | N/A | Low | |
| Mitchison et al. [44] | TB | 19 | ND | 0, 2, 4, 8, 12, 24, 28, 32, 36, and 48 h post-dose | Plate diffusion assay | N/A | N/A | Low | |
| Mqoqi et al. [45] | TB | 270 | ND | ND | Chemical reaction and visual detection | N/A | N/A | Low | |
| Palanduz et al. [46] | TB (pediatric) | 45 | 0.5, 1, 2, 3, 4, 5, and 6 months after treatment initiation | Second urine after medication ingestion | Chemical reaction and visual detection | N/A | N/A | Low | |
| Sirgel et al. [47] | TB | Study 1: 57; Study 2: 46 | Study 1: 2 days before to 5 days after; Study 2: day of visit | Study 1: baseline, 24, 48, 72, 96, and 120 h post-dose; Study 2: 2-h intervals for 8 h post-dose | Study 1: microbiologic assay, visual detection after addition of chemicals; Study 2: HPLC | N/A | N/A | Low | |
| Szipsky et al. [15] | TB (pediatric) | 12 | Two weeks after treatment initiation | 2 h post-dose | Colorimetry, mobile phone/light box | ND | Correlations, receiver operating characteristic curve for target Cmax , and AUC0–24 | Moderate | |
| Wardman et al. [48] | TB | 113 | ND | ND | Visual detection, chemical reaction, chromatographic methods (unspecified) | N/A | N/A | Low | |
| Zentner et al. [11] | TB + HV | 45 | HV: On dose administration day. TB: ND | HV: 4 h, 8 h post-dose. TB: 8 h post-dose | Colorimetry | ND | Correlation, receiver operator characteristic curve | Moderate | |
| Rifapentine | Sirgel et al. [47] | TB | Study 1: 57; Study 2: 46 | Study 1: 2 days before to 5 days after; study 2: day of visit | Study 1: baseline, 24, 48, 72, 96, and 120 h post-dose; study 2: 2-h intervals for 8 h post-dose | Study 1: microbiologic assay, visual detection after addition of chemicals; Study 2: HPLC | N/A | N/A | Low |
| Isoniazid | Amlabu et al. [49] | IPT (pediatric) | 41 | Visit day | 4 and 24 h after dose in daily therapy. 4, 48, and 72 h after dose in intermittent therapy. | Arkansas method + HPLC-MS/MS | N/A | N/A | Low |
| Anusiem et al. [50] | HV | 5 | After first-dose administration | 0, 1, 2, 3, 4, 5, 6, 7, 8, 12, 24, and 48 h post-dose | Spectrophotometry | ND | ND | Low | |
| Burkhardt et al. [37] | TB | 319 | ND | 2, 4, 6, 8, and 24 h post-dose | Chemical reaction (visual detection) | N/A | N/A | Moderate | |
| Eidlitz-Markus et al. [51] | LTBI (adults, pediatric, adolescents) | 105 | During routine follow-up | Once | Arkansas method | N/A | N/A | Low | |
| Elizaga et al. [52] | TB + HV | 51 | ND | ND | Arkansas method | N/A | N/A | Low | |
| Ellard et al. [39] | HV | 39 | On the day of study | Sub-study 1: 0, 0–1, 1–2, 2–3, 3–4, 4–6, 6–8, 8–10, 10–12, 12–21, 21–23, 23–25, 25–27 or, 0, 0–10, 10–12, 12–14, 14–16, 16–18, 18–21, 21–23, 23–25 h post-dose | Fluorimetry and visual detection | N/A | N/A | Moderate | |
| Sub-studies 2 and 3: 0, 0–1, 1–2, 2–3, 23.5, 23.5–24.5. 0, 0–1, 17.5, 17.5–18.5 | |||||||||
| Sub-study 4: 0, 0–2, 2–4, 4–6, 23.5–24.5, 47.5–48.5 | |||||||||
| Sub-study 5: 0, 0–1, 1–2, 2–3, 3–4, 4–6, 6–8, 8–10, 10–12, 12–24 | |||||||||
| Guerra et al. [53] | TB + IPT | 94 | ND | 24 h after observed ingestion | Arkansas method | N/A | N/A | Low | |
| Hamilton et al. [54] | TB + HV | 1673 samples (unknown participant number) | ND | ND | Chemical reaction (visual detection) | N/A | N/A | Low | |
| Hanifa et al. [55] | TB + HV | 213 | At least 3 days after therapy initiation | 6, 12, and 24 h post-dose | Arkansas method | N/A | N/A | Low | |
| Hashiguchi et al. [38] | HV | 4 | On the day of study | 0–4 h, 4–8 h, 8–12 h, and 12–24 h post-dose | Thin-layer chromatography and HPLC validation | N/A | N/A | Moderate | |
| Kendall et al. [56] | IPT | 296 | On enrollment day | One sample on enrollment day | Arkansas method | N/A | N/A | Low | |
| LaCourse et al. [57] | IPT (pediatric) | 150 | ND | ND | Visual detection using dipstick (Arkansas method) | N/A | N/A | Low | |
| Macfadyen et al. [58] | TB | 440 | ND in parent study. Day of first isoniazid ingestion in validation study | Once during random home visit or follow-up (0, 2, 4, 6, 9, and 24 h post-dose in validation study) | Paper test: visual detection | N/A | N/A | Low | |
| Macintyre et al. [36] | TB | 173 | ND | ND | HPLC | N/A | N/A | Low | |
| Meissner et al. [43] | TB | 234 | ND | ND | Dipstick (in-house Arkansas method compared to Taxo INH strips) | N/A | N/A | Low | |
| Mishra et al. [59] | TB + HV | 15 | ND | ND | Micellar liquid chromatography | N/A | N/A | Low | |
| Mqoqi et al. [45] | TB | 270 | ND | ND | Chemical reaction (visual detection) | N/A | N/A | Low | |
| Narain et al. [60] | ND | 4044 samples (unknown participant number) | During study visits | 24, 48, and 72 h post-dose | Visual detection (Belles-Littleman filter paper spot test) | N/A | N/A | Low | |
| Nicolau et al. [14] | TB , LTBI, non-TB | 195 | ND | ND | IsoScreen method (visual detection) | N/A | N/A | Low | |
| Palanduz et al. [46] | TB (pediatric) | 45 | 0.5, 1, 2, 3, 4, 5, and 6 months after treatment initiation | Second urine after medication ingestion | Chemical reaction (visual detection) | N/A | N/A | Low | |
| Perry et al. [61] | LTBI (adolescents) | 194 | Once a month for 9 months | Once per visit | Arkansas method | N/A | N/A | Low | |
| Schmitz et al. [62] | LTBI (adults and adolescents) | 26 | Day of visit | ND | Arkansas method | N/A | N/A | Low | |
| Schraufnagel et al. [63] | TB | 94 | ND | ND | Arkansas method | N/A | N/A | Low | |
| Sirgel et al. [47] | TB | Study 1: 52; Study 2: 46 | Study 1: ND; Study 2: between 4 and 6 weeks after therapy initiation | Study 1: ND; Study 2: 2-h intervals for 8 h | Mycodyn Uritec test strips, chemical reaction (visual detection), HPLC | N/A | N/A | Low | |
| Soobratty et al. [64] | TB , LTBI | 105 | Day of visit | 12, 24, 48, and 72 h post-dose | IsoScreen method (visual detection) | N/A | N/A | Low | |
| Subbaraman et al. [65] | TB | 650 | Random | Once during random home visit | Arkansas method | N/A | N/A | Low | |
| Szakacs et al. [66] | IPT + HV | 306 | Day of visit | 0, 24, 36, and 72 h post-dose in healthy volunteers | Visual detection, chromatography | N/A | N/A | Low | |
| Venho et al. [67] | TB | 26 | ND | 24 h post-dose | Spectrophotometry | ND | N/A | Low | |
| Whitfield et al. [68] | TB , LTBI | 191 | ND | ND | Arkansas method | N/A | N/A | Low | |
| Zhao et al. [69] | ND | 6 | ND | ND | Fluorimetry with silver nanocluster sheets | ND | ND | Low | |
| Pyrazinamide | Burkhardt et al. [37] | TB | 319 | ND | 2, 4, 6, 8, and 24 h post-dose | Chemical reaction (visual detection) | N/A | N/A | Moderate |
| Palanduz et al. [46] | TB (pediatric) | 45 | 0.5, 1, 2, 3, 4, 5, and 6 months after treatment initiation | Second urine after medication ingestion | Chemical reaction (visual detection) | N/A | N/A | Low | |
| Pines et al. [70] | TB | ND | ND | ND | Visual detection | N/A | N/A | Low | |
| Zentner et al. [12] | HV, TB | 45 | HV: Day of drug intake; TB: within 2 months of therapy initiation | HV: 4 h, 8 h post-dose; TB: 4 h post-dose | Colorimetry | ND | Receiver operating characteristic curve | Moderate | |
| Levofloxacin | Rao et al. [13] | TB + HV | 16 | HV: day of dose administration; TB: > 2 weeks after therapy initiation | 0–4, 4–8, and 8–24 h intervals post-dose | Colorimetry | ND | Correlation, receiver operating characteristic curve | Moderate |
| Cycloserine | Mattila et al. [71] | TB + HV | 11 | ND | 8 h after last dose | Chemical assay, bioassay | ND | ND | Low |
| Ethionamide | Eidus et al. [72] | HV | 8 | After first-dose administration | 2–8 h post-dose | Chemical reaction (visual detection) | N/A | N/A | Low |
| Eidus et al. [73] | HV | 14 | After first-dose administration | 1, 2, 3, 6, 7, 8, 10, 12, 14, 18, 24, 26, 28, 30, and 32 h post-dose | Chemical reaction (visual detection) | N/A | N/A | Low |
h hours, HPLC high performance liquid chromatography, HPLC-MS/MS high performance liquid chromatography mass spectrometry/mass spectrometry, HV healthy volunteers, IPT isoniazid preventative therapy, LTBI latent TB infection, N/A not applicable, ND not described, TB tuberculosis
Studies were assessed for the risk of bias. All participants included in one study [37] were male, causing a moderate risk of bias in the selection of participants into the study. Of the four participants enrolled into one study [38], results were reported for three participants, leading to a moderate risk of bias due to missing data. Another study [39] reported only cumulative apparent excretion for a metabolite of isoniazid instead of the parent compound, causing a moderate risk of bias in selection of the reported results. High-performance liquid chromatography was used to measure serum concentrations for rifampin [11, 15], pyrazinamide [12], and levofloxacin [13] whereas, colorimetry with a spectrophotometer was used to measure urine concentrations, leading to a moderate risk of bias in the measurement of outcomes due to the different analytical instruments used. All other studies [14, 36, 40–73] had a low overall risk of bias (Table 3). Urine had a TRL score of 7 (Table 6) for all drugs except ethionamide found in this systematic search, as most studies were performed in patients with TB in different settings, but the absence of urine-serum/plasma ratios prevents urine from being used prospectively to perform TDM. Both studies testing the presence of ethionamide were performed in healthy volunteers, resulting in a TRL score of 6.
Saliva
Studies comparing saliva and serum were found for two first-line drugs, rifampin and isoniazid, and five second-line anti-TB medications, levofloxacin, moxifloxacin, linezolid, amikacin, and clarithromycin. Patients with TB and healthy volunteers comprised the study population and sample sizes ranged from 6 to 45 participants. Liquid chromatography-tandem mass spectrometry was the most common instrument for drug quantification, followed by spectrophotometry (Table 4). A novel mobile ultraviolet-visible spectrophotometry was repurposed to detect levofloxacin [18, 20] and linezolid [19] in saliva. The duration between treatment initiation and sample collection [74] and saliva sampling times [75] were not provided for two studies. The risk of bias was assessed, and one study [76] had a moderate risk of bias because of the selection of participants in the study as all participants were female. Remaining studies [18–20, 50, 74, 77–81] had a low overall risk of bias (Table 4). The TRL score for all saliva studies was 8 (ultraviolet-visible as they were performed mostly in patients with TB on drug regimens similar to those found in programmatic settings and most studies performed saliva-plasma/serum comparisons.
Table 4.
List of studies reporting salivary sampling, serum/plasma-saliva comparative methods, and risk of bias
| Drug | Study | Population type | Sample size | Sampling | Saliva sampling times | Analytical method | Saliva-serum/plasma ratio | Saliva-serum/plasma comparison methods | Risk of bias (overall) |
|---|---|---|---|---|---|---|---|---|---|
| Rifampin | Gurumurthy et al. [74] | TB | 30 | ND | 1, 2, 3, 6, and 8 h post-dose | Plate diffusion assay/microbiological methods | 0.07–0.13 | Ratios | Low |
| van den Elsen et al. [77] | TB | 11 | > 2 weeks | 0, 0.5, 1, 2, 3, 4, and 6 h post-dose | LC-MS/MS | Paired conc: 0.126 (0.109–0.154) AUC0–24: 0.154 (0.127–0.162) | Ratios, Passing–Bablok regression, Bland–Altman plots | Low | |
| Isoniazid | Anusiem et al. [50] | HV | 5 | Day of visit | 0, 0.5, 1, 1.5, 2, 2.5, 3, 4, 5, 6, 7, 8, 12, and 24 h post-dose | Spectrophotometry | AUC0–24: 0.14 | Ratios | Low |
| Gurumurthy et al. [74] | TB | 30 | ND | 1, 2, 3, 6, and 8 h post-dose | Chemical reaction and ultraviolet detection | Calculated Cmax ratio for slow acetylators: 0.95. Calculated Cmax ratio for rapid acetylators: 0.94 | Calculated ratios | Low | |
| Ofoefule et al. [76] | HV | 6 | Day of visit | 1, 2, 3, 4, 5, 6, 7, 8, and 24 h post-dose | ND | N/A | N/A | Moderate | |
| van den Elsen et al. [77] | TB | 8 | > 2 weeks | Pre-dose, 0.5, 1, 2, 3, 4, and 6 h post-dose | LC-MS/MS | Paired conc.: 0.763 (0.413–1.158) AUC0–24: 0.824 (0.492–1.2) | Ratios, Passing–Bablok regression, Bland–Altman plots | Low | |
| Levofloxacin | Alffenaar et al. [18] | HV | 6 | ND | ND | Spectrophotometry (mobile nanophotometer) | N/A | N/A | Low |
| Ghimire et al. [79] | TB | 23 | First visit: 15–30 days. Second visit: 45–60 days | 0, 1, 2, 4, and 8 h post-dose | LC-MS/MS | First visit, Cmax : 0.68 (0.53–0.97) AUC0–24: 0.69 (0.53–0.99). Second visit, Cmax : 0.73 (0.66–1.18) AUC0-24: 0.74 (0.59–0.93) | Ratios, Passing–Bablok regression, Bland–Altman plots | Low | |
| Mohamed et al. [20] | TB | 45 | > 2 weeks | 1 and 4 h post-dose | Spectrophotometry (mobile nanophotometer) | Cmax : 0.76 AUC0–24: 0.7 | Calculated ratios, Passing–Bablok regression | Low | |
| Moxifloxacin | Kumar et al. [75] | HV | 24 | Day of visit | ND | HPLC | 0.54 | Ratio | Low |
| van den Elsen et al. [78] | TB | 15 | > 2 weeks | 0, 1, 2, 3, 4, and 8 h post-dose | LC-MS/MS | Paired con.c: 1 (0.68–1.35) AUC0–24: 0.89 (0.61–1.14) | Ratios, Passing–Bablok regression, Bland–Altman plots | Low | |
| Linezolid | Bolhuis et al. [80] | TB | 7 | > 2 weeks | 0, 1, 2, 3, 4, 8, and 12 h post-dose | HPLC-MS/MS | AUC0–12: 0.97 | Ratios, Passing–Bablok regression, Bland–Altman plots | Low |
| Kim et al. [19] | HV | 6 | ND | ND | Spectrophotometer (mobile nanophotometer) | N/A | N/A | Low | |
| van den Elsen et al. [78] | TB | 7 | > 2 weeks | 0, 1, 2, 3, 4, and 8 h post-dose | LC-MS/MS | Paired conc.: 0.76 (0.64–0.85) AUC0–24: 0.81 (0.74–0.88) | Ratios, Passing–Bablok regression, Bland–Altman plots | Low | |
| Amikacin | van den Elsen et al. [81] | TB | 6 | > 2 weeks | 0, 1, 2, 3, 4, and 8 h post-dose | Particle-enhanced turbidimetric inhibition immunoassay | Up to 0.18 | Ratios | Low |
| Clarithromycin | Bolhuis et al. [80] | TB | 7 | > 2 weeks | 0, 1, 2, 3, 4, 8, and 12 h post-dose | HPLC-MS/MS | Reported = 3.07 | Ratios, Passing–Bablok regression, Bland–Altman plots | Low |
AUC area under the concentration–time curve, Cmax maximum concentration, conc. concentration, h hours, HPLC high-performance liquid chromatography, HPLC-MS/MS high-performance liquid chromatography-mass spectrometry/mass spectrometry, HV healthy volunteers, LC-MS/MS liquid chromatography-mass spectrometry/mass spectrometry, N/A not applicable, ND not described, TB tuberculosis
Hair
A total of 13 articles reported on measured hair concentrations of three first-line TB drugs (isoniazid, pyrazinamide, and ethambutol) and eight second-line drugs (levofloxacin, moxifloxacin, linezolid, clofazimine, bedaquiline, pretomanid, ethionamide, and delamanid). Apart from parent compounds, three articles also measured TB drug metabolites in hair (acetyl-INH [21, 82] and DM-6705 [83], a metabolite of delamanid). Study populations comprised both adults and pediatric patients, and sample sizes ranged from two to 264 participants. Liquid chromatography-tandem mass spectrometry was used in all studies to quantify the various anti-TB drugs from hair (Table 5). Only two [22, 84] of the 13 studies performed comparative pharmacokinetic studies in plasma as well as hair samples, and simple scatter plots were used to demonstrate correlations. All studies were assessed for the risk of bias and two studies had a moderate risk of bias because of the selection of participants as one study [85] had 98% female participants and the other [22] enrolled all male participants. Other studies [21, 82–84, 86–92] had a low overall risk of bias. Similar to urine and saliva, the TRL score for hair was 7 (Table 5) as all studies were performed in patients with TB in operational settings.
Table 5.
List of studies reporting hair sampling, serum/plasma-hair comparative methods, and risk of bias
| Drug | Study | population type | Sample size | Sampling | Hair sampling times | Analytical method | Hair-serum/plasma ratio | Hair-serum/plasma comparison methods | Risk of bias (overall) |
|---|---|---|---|---|---|---|---|---|---|
| Isoniazid | Eisenhut et al. [82] | TB + LTBI | 40 | ND | Once during study | HPLC/MS | N/A | N/A | Low |
| Gerona et al. [88] | TB | 30 | > 14 days | Once during visit | LC-MS/MS | N/A | N/A | Low | |
| Gerona et al. [86] | TB + LTBI | 18 | Variable | Once during visit | LC-MS/MS | N/A | N/A | Low | |
| Mave et al. [21] | TB | 264 | 1, 5, and 6 months | Once during each visit | LC-MS/MS | N/A | N/A | Low | |
| Mave et al. [84] | TB (pediatric) | 16 | 2, 4, and 6 months | Once during each visit | LC-MS/MS | Calculated ratio between median hair conc. and serum 2 month AUC0–6 : 0.05 at 2 months, 0.09 at 4 months, 0.04 at 6 months | Calculated ratios, Correlation | Low | |
| Mave et al. [89] | TB (pediatric) | 38 | 1, 2, 4, and 6 months | Once during each visit | LC-MS/MS | N/A | N/A | Low | |
| Metcalfe et al. [85] | LTBI | 28 | 3 and 6 months | Once during each visit | LC-MS/MS | N/A | N/A | Moderate | |
| Metcalfe et al. [90] | TB | 46 | Median 87 days | Once during visit | LC-MS/MS | N/A | N/A | Low | |
| Reckers et al. [92] | TB | 96 | ND | ND | LC-MS/MS | N/A | N/A | Low | |
| Pyrazinamide | Gerona et al. [88] | TB | 30 | > 14 days | Once during visit | LC-MS/MS | N/A | N/A | Low |
| Gerona et al. [87] | TB | 2 | ND | ND | LC-MS/MS | N/A | N/A | Low | |
| Mave et al. [21] | TB | 264 | 1, 5, and 6 months after therapy initiation | Once during each visit | LC-MS/MS | N/A | N/A | Low | |
| Metcalfe et al. [85] | TB | 57 | Median 144 days | Once during study | LC-MS/MS | N/A | N/A | Moderate | |
| Metcalfe et al. [90] | TB | 47 | Median 87 days | Once during visit | LC-MS/MS | N/A | N/A | Low | |
| Reckers et al. [92] | TB | 96 | ND | ND | LC-MS/MS | N/A | N/A | Low | |
| Ethambutol | Gerona et al. [88] | TB | 30 | > 14 days | Once during visit | LC-MS/MS | N/A | N/A | Low |
| Metcalfe et al. [85] | TB | 57 | Median 144 days | Once during study | LC-MS/MS | N/A | N/A | Moderate | |
| Metcalfe et al. [90] | TB | 47 | Median 87 days | Once during visit | LC-MS/MS | N/A | N/A | Low | |
| Reckers et al. [92] | TB | 96 | ND | ND | LC-MS/MS | N/A | N/A | Low | |
| Levofloxacin | Gerona et al. [88] | TB | 30 | > 14 days | Once during visit | LC-MS/MS | N/A | N/A | Low |
| Gerona et al. [87] | TB | 2 | ND | ND | LC-MS/MS | N/A | N/A | Low | |
| Metcalfe et al. [85] | TB | 57 | Median 144 days | Once during study | LC-MS/MS | N/A | N/A | Moderate | |
| Metcalfe et al. [90] | TB | 47 | Median 87 days | Once during visit | LC-MS/MS | N/A | N/A | Low | |
| Reckers et al. [92] | TB | 96 | ND | ND | LC-MS/MS | N/A | N/A | Low | |
| Moxifloxacin | Gerona et al. [88] | TB | 30 | > 14 days | Once during visit | LC-MS/MS | N/A | N/A | Low |
| Gerona et al. [87] | TB | 2 | ND | ND | LC-MS/MS | N/A | N/A | Low | |
| Metcalfe et al. [85] | TB | 57 | Median 144 days | Once during study | LC-MS/MS | N/A | N/A | Moderate | |
| Metcalfe et al. [90] | TB | 47 | Median 87 days | Once during visit | LC-MS/MS | N/A | N/A | Low | |
| Reckers et al. [92] | TB | 96 | ND | ND | LC-MS/MS | N/A | N/A | Low | |
| Linezolid | Gerona et al. [88] | TB | 30 | > 14 days | Once during visit | LC-MS/MS | N/A | N/A | Low |
| Gerona et al. [87] | TB | 2 | ND | ND | LC-MS/MS | N/A | N/A | Low | |
| Metcalfe et al. [85] | TB | 57 | Median 144 days | Once during study | LC-MS/MS | N/A | N/A | Moderate | |
| Metcalfe et al. [90] | TB | 47 | Median 87 days | Once during visit | LC-MS/MS | N/A | N/A | Low | |
| Reckers et al. [92] | TB | 96 | ND | ND | LC-MS/MS | N/A | N/A | Low | |
| Wasserman et al. [22] | TB | 6 | < 3 months | Once during visit | LC-MS/MS | ND | Correlation coefficient 0.84 (scatterplot) | Moderate | |
| Clofazimine | Gerona et al. [88] | TB | 30 | > 14 days | Once during visit | LC-MS/MS | N/A | N/A | Low |
| Metcalfe et al. [85] | TB | 57 | Median 144 days | Once during study | LC-MS/MS | N/A | N/A | Moderate | |
| Metcalfe et al. [90] | TB | 47 | Median 87 days | Once during visit | LC-MS/MS | N/A | N/A | Low | |
| Reckers et al. [92] | TB | 96 | ND | ND | LC-MS/MS | N/A | N/A | Low | |
| Bedaquiline | Gerona et al. [88] | TB | 30 | > 14 days | Once during visit | LC-MS/MS | N/A | N/A | Low |
| Metcalfe et al. [85] | TB | 57 | Median 144 days | Once during study | LC-MS/MS | N/A | N/A | Moderate | |
| Metcalfe et al. [90] | TB | 25 | Median 87 days | Once during visit | LC-MS/MS | N/A | N/A | Low | |
| Metcalfe et al. [91] | TB | 4 | ND | ND | LC-MS/MS | N/A | N/A | Low | |
| Reckers et al. [92] | TB | 96 | ND | ND | LC-MS/MS | N/A | N/A | Low | |
| Pretomanid | Gerona et al. [88] | TB | 30 | > 14 days | Once during visit | LC-MS/MS | N/A | N/A | Low |
| Metcalfe et al. [85] | TB | 57 | Median 144 days | Once during study | LC-MS/MS | N/A | N/A | Moderate | |
| Metcalfe et al. [90] | TB | 47 | Median 87 days | Once during visit | LC-MS/MS | N/A | N/A | Low | |
| Reckers et al. [92] | TB | 96 | ND | ND | LC-MS/MS | N/A | N/A | Low | |
| Ethionamide | Gerona et al. [88] | TB | 30 | > 14 days | Once during visit | LC-MS/MS | N/A | N/A | Low |
| Metcalfe et al. [85] | TB | 57 | Median 144 days | Once during study | LC-MS/MS | N/A | N/A | Moderate | |
| Metcalfe et al. [90] | TB | 47 | Median 87 days | Once during visit | LC-MS/MS | N/A | N/A | Low | |
| Delamanid | Reckers et al. [83] | TB | 12 | ND | ND | LC-MS/MS | N/A | N/A | Low |
AUC area under the concentration–time curve, conc. concentration, HPLC/MS high-performance liquid chromatography/mass spectrometry, LC-MS/MS liquid chromatography-mass spectrometry/mass spectrometry, LTBI latent TB infection, N/A not applicable, ND not described, TB tuberculosis
Discussion
This systematic review sought to explore opportunities for performing TDM for anti-TB drugs in alternative biological matrices to serum or plasma, specifically DBS, urine, saliva, and hair. We found that numerous classes of anti-TB drugs have been studied in quantitative or semi-quantitative assays in the alternative matrices, but few have been carried forward beyond diagnostic accuracy work to translate into dose adjustment. Studies within certain matrices such as DBS and saliva have been more comprehensive in reporting diagnostic accuracy, comparing levels to relevant pharmacokinetic parameters in serum or plasma, while studies in urine and hair have focused primarily on predicting medication adherence (Table 6).
Performance characteristics for each alternative biomatrix described in this systematic review are important to consider. For instance, from our search results for DBS, comparisons between plasma and DBS were performed for rifampin, pyrazinamide, ethambutol, moxifloxacin, and linezolid. The study by Martial et al. [29], conducted in children, had DBS to plasma ratios of 0.75 for rifampin, 0.81 for pyrazinamide, and 0.51 for ethambutol. While the ratios were acceptable for rifampin and pyrazinamide, ethambutol concentrations in DBS may be unsuitable to predict plasma concentrations because of low precision. The authors attribute the lower ratio of rifampin and pyrazinamide to peripheral distribution variability in children [29]. Linezolid showed good agreement between DBS and plasma with a ratio of 1.2 and a narrow range [33]. Linezolid concentrates more in erythrocytes than plasma and the differences in binding capacity cause linezolid concentrations to be higher in blood, hence, the authors proposed conversion factors to determine corresponding plasma values [33, 93]. High sample stability was also observed, making monitoring with DBS feasible for linezolid, which can reduce under-exposures or over-exposures in as many as 40% of patients [93]. These features may have broad applicability given the widespread roll out of linezolid in rifampin-resistant TB regimens for both improved efficacy and mitigating common exposure-related toxicities of linezolid [94]. For drugs such as rifapentine, isoniazid, moxifloxacin, and clarithromycin, studies with fewer than 30 participants were found, and the absence of reported DBS-plasma/serum ratios precluded prediction of clinical applicability. Although DBS can be a more convenient alternative to collecting whole blood for drug quantifications, especially in very young children and other participants unable to undergo multiple large-volume blood draws, there is a need for validated sample collection and measurement techniques [95] before blood spots can be used in lieu of plasma/serum for drug monitoring.
In contrast to the other biomatrices, urine has been utilized to monitor adherence to anti-TB treatment for over five decades. This earlier usage was borne from the misguided assumption that treatment failure arose from a patient’s inability or unwillingness to take medications as prescribed. Currently, variable adherence is understood as an expected response to TB treatment, but prescribed dose and individual pharmacokinetic variability also largely influence drug exposure and treatment outcome [96, 97]. Thus, there have been advances to use urine colorimetric methods for quantification within a medication dosing interval in an attempt to make a more precise dose adjustment in response to an individual’s pharmacokinetic variability. For example, the earlier visual detection of color change upon adding chemicals to the patented IsoScreen kit to detect isoniazid semi-qualitatively has been adapted to measure concentrations of various drugs [12, 13]. To reduce the use of laboratory demands further, a mobile phone color reader with a standardized light box has been used to quantify rifampin concentrations in urine [15]. However, only a few of the identified studies in this review quantitatively measured concentrations of drugs in urine, distinct from the semi-quantitative methods used for the measurement of adherence [11–13, 15, 50]. Although testing for adherence has been well validated for rifampin and isoniazid, a lack of reported urine-plasma/serum ratios in quantitative studies makes it difficult to identify urine threshold concentrations that may be predictive of optimum plasma exposure. Furthermore, while urine assays may be relatively simple to implement owing to an easier sample collection for all ages, including the presence of special urine collection bags for pediatric patients, and simple quantification methods, the identified studies did not consistently report on factors such as patient hydration, urine pH [98], and the presence of other co-morbid conditions affecting renal clearance.
Most studies of the saliva matrix reported concentrations in ratio to serum or plasma values allowing interpretation as to whether some drugs were more or less fitting for this platform. For example, rifampin, arguably the most important anti-TB drug, had the lowest ratio of 0.07 of saliva:plasma concentrations observed in one study [74], making the use of saliva to predict plasma concentrations challenging. Rifampin saliva concentrations were low despite assured adequate dosing [74, 77], likely due to strong binding of rifampin to plasma proteins and poor diffusion into the salivary glands [99]. A wide range of saliva-plasma ratios was reported for isoniazid, levofloxacin, and linezolid that could be due to varying dosing and sampling methods across studies. The highest ratio was observed for clarithromycin of 3.07 in Bolhuis et al. [80]. Higher ratios may allow for easy detection in saliva. This may be promising for other infectious diseases, as clarithromycin or other macro/azalides are more indicated for treating non-tuberculous mycobacteria. More important than the actual ratio is the inter-patient and intra-patient variability in the ratio as it would allow the incorporation of an appropriate correction factor where the ratio is reproducible. To illustrate, isoniazid is not bound to plasma proteins and can easily diffuse into saliva [100], yet the inter-study variability of saliva-plasma ratios among Anusiem et al. [50], Gurumurthy et al. [74], and van den Elsen et al. [77] suggests that salivary flow and pH might influence concentrations and well-designed pharmacokinetic studies would be needed before a reliable correction factor can be applied. However, saliva TDM appears possible in the treatment of rifampin-resistant/multi-drug-resistant TB for the key drugs of the fluoroquinolone class (levofloxacin and moxifloxacin) and linezolid. These drugs have also been measured using a novel, mobile, micro-volume, ultraviolet-visible spectrophotometer [18, 19], which can quantify salivary drug concentrations as demonstrated at the bedside in at least one study among patients with drug-resistant TB in Tanzania [20].
The systematic review did identify a relatively recent increase in the number of studies attempting to quantify drug exposure from hair samples in a range of cohorts with both drug-susceptible and drug-resistant TB. As a representative example, in a study by Mave et al. [21], hair samples were collected at 2, 4, and 6 months after isoniazid therapy initiation where isoniazid and acetyl-isoniazid concentrations were decreasing over time, which the authors suggested might indicate important changes in adherence patterns. Additionally, for a drug such as isoniazid that is unstable in plasma, DBS, and urine over long periods and requires cold-chain transport from serum or plasma, hair may offer an advantage for the measurement of cumulative drug exposure over time due to the relative stability of isoniazid in this biomatrix [21]. Overall, however, comparative studies of hair concentrations with gold standard plasma or serum concentrations were few as plasma and serum measurements cover different durations of exposure compared with hair. Concentrations in hair are an indicator of the average level of drug over a period of weeks or months, and contemporaneous plasma or serum measurement would only reflect a more recent drug intake, usually during a single dosing interval. In future studies, a different type of comparison between plasma or serum and hair could involve comparing a steady-state drug concentration in serum over a clinically relevant period (utilizing peak and trough concentrations) with hair concentrations in the same span of time.
This systematic review was not without limitations. A validated tool for assessment of the risk of bias of bioanalytical-pharmacokinetic types of studies was not available, but we instead modified the ROBINS-I for this purpose. Hence, a validated tool would be needed to properly assess the risk of bias in pharmacokinetic studies to avoid inappropriate risk classification. Some studies were performed in healthy volunteers or spiked samples, which could limit the extrapolation of findings to patients with TB, particularly those treated with multi-drug regimens.
Despite these limitations of early-stage studies, TDM using DBS, urine, saliva, or hair would be of immense benefit in TB-endemic regions and therefore randomized controlled trials enrolling diverse populations including adults, adolescents, and children with drug-susceptible drug-resistant TP from various ethnicities are needed. Dosage regimens in these studies must be most indicative of dosages administered in clinical and programmatic settings, and paired plasma/serum-alternative matrix sampling should be obtained for full pharmacokinetic curves and for additional population-pharmacokinetic studies that inform dose adjustment strategies. Population pharmacokinetic modeling and pharmacokinetic-pharmacodynamic studies could help predict the most appropriate individual dose, and model-informed precision dosing could also be utilized in predicting sampling schedules and exposures in alternative matrices [101]. Variable factors need to be taken into consideration to provide high-level evidence for TDM and these include volume and hematocrit effects for DBS [9]; pH, fraction of drug eliminated renally, hydration, renal function for urine [98]; salivary flow and pH [17]; and understanding relevant serum exposures from hair concentrations [23]. Having validated analytical methods for plasma and or serum and the alternative matrix, and the ability to calculate plasma-matrix ratios from AUC values form important components of a rigorous pharmacokinetic study design [17]. Last, with TDM more commonly performed among both inpatients and outpatients [7], there is also a need to determine the cost effectiveness and financial implications that TDM might pose to individuals and service providers in TB-endemic settings [102, 103].
Conclusions
Despite the readiness of alternative matrix assays to be performed in operational settings and considerable promise for the use of alternative matrices for personalized dose adjustment, assays from DBS, urine, saliva, and hair must be subjected to well-designed studies with diverse study populations on TB treatment, using consistent sample collection methods and validated analytical techniques for both serum or plasma and the alternative biomatrix to increase the uptake in guidelines and accelerate implementation in programmatic TB treatment.
Declarations
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. Prakruti S. Rao, Nisha Modi, Yingda L. Xie, and Scott K. Heysell were supported by the National Institutes of Health grant R01 AI137080.
Conflict of interest
Prakruti S. Rao, Nisha Modi, Nam-Tien Tran Nguyen, Dinh Hoa Vu, Yingda L. Xie, Monica Gandhi, Roy Gerona, John Metcalfe, Scott K. Heysell, and Jan-Willem C. Alffenaar have no conflicts of interest that are directly relevant to the content of this article.
Ethics approval
Data used in this study were collected according to the principles of Declaration of Helsinki. Approval was granted by institutional review boards or independent ethics committees for each study from which data were used in this manuscript.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Authors’ contributions
Conceptualization: JWA, SKH. Data extraction and assembling first draft: PSR. All authors contributed to the preparation and critical revision of the manuscript.
Footnotes
Nisha Modi, Nam-Tien Tran Nguyen, Dinh Hoa Vu and Yingda L. Xie contributed equally, authors listed in alphabetical order.
References
- 1.Pasipanodya JG, McIlleron H, Burger A, Wash PA, Smith P, Gumbo T. Serum drug concentrations predictive of pulmonary tuberculosis outcomes. J Infect Dis. 2013;208:1464–1473. doi: 10.1093/infdis/jit352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Srivastava S, Pasipanodya JG, Meek C, Leff R, Gumbo T. Multidrug-resistant tuberculosis not due to noncompliance but to between-patient pharmacokinetic variability. J Infect Dis. 2011;204:1951–1959. doi: 10.1093/infdis/jir658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zheng X, Davies Forsman L, Bao Z, Xie Y, Ning Z, Schön T, et al. Drug exposure and susceptibility of second-line drugs correlate with treatment response in patients with multidrug-resistant tuberculosis: a multicentre prospective cohort study in China. Eur Respir J. 2022;59:2101925. doi: 10.1183/13993003.01925-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zheng X, Bao Z, Forsman LD, Hu Y, Ren W, Gao Y, et al. Drug exposure and minimum inhibitory concentration predict pulmonary tuberculosis treatment response. Clin Infect Dis. 2021;73:e3520–e3528. doi: 10.1093/cid/ciaa1569. [DOI] [PubMed] [Google Scholar]
- 5.Alffenaar JWC, Stocker SL, Forsman LD, Garcia-Prats A, Heysell SK, Aarnoutse RE, et al. Clinical standards for the dosing and management of TB drugs. Int J Tuberc Lung Dis. 2022;26:483–499. doi: 10.5588/ijtld.22.0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reynolds J, Heysell SK. Understanding pharmacokinetics to improve tuberculosis treatment outcome. Expert Opin Drug Metab Toxicol. 2014;10:813–823. doi: 10.1517/17425255.2014.895813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim HY, Byashalira KC, Heysell SK, Märtson A-G, Mpagama SG, Rao P, et al. Therapeutic drug monitoring of anti-infective drugs: implementation strategies for 3 different scenarios. Ther Drug Monit. 2022;44:3–10. doi: 10.1097/FTD.0000000000000936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alffenaar J-WC, Gumbo T, Dooley KE, Peloquin CA, Mcilleron H, Zagorski A, et al. Integrating pharmacokinetics and pharmacodynamics in operational research to end tuberculosis. Clin Infect Dis. 2020;70:1774–80. [DOI] [PMC free article] [PubMed]
- 9.Capiau S, Veenhof H, Koster RA, Bergqvist Y, Boettcher M, Halmingh O, et al. Official International Association for Therapeutic Drug Monitoring and Clinical Toxicology guideline: development and validation of dried blood spot-based methods for therapeutic drug monitoring. Ther Drug Monit. 2019;41:409–430. doi: 10.1097/FTD.0000000000000643. [DOI] [PubMed] [Google Scholar]
- 10.Vu D, Alffenaar J, Edelbroek P, Brouwers J, Uges D. Dried blood spots: a new tool for tuberculosis treatment optimization. Curr Pharm Des. 2011;17:2931–2939. doi: 10.2174/138161211797470174. [DOI] [PubMed] [Google Scholar]
- 11.Zentner I, Schlecht HP, Khensouvann L, Tamuhla N, Kutzler M, Ivaturi V, et al. Urine colorimetry to detect low rifampin exposure during tuberculosis therapy: a proof-of-concept study. BMC Infect Dis. 2016;16:242. doi: 10.1186/s12879-016-1576-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zentner I, Modongo C, Zetola NM, Pasipanodya JG, Srivastava S, Heysell SK, et al. Urine colorimetry for therapeutic drug monitoring of pyrazinamide during tuberculosis treatment. Int J Infect Dis. 2018;68:18–23. doi: 10.1016/j.ijid.2017.12.017. [DOI] [PubMed] [Google Scholar]
- 13.Rao P, Zhdanova S, Ogarkov O, Orlova E, Ebers A, Stroup S, et al. Urine colorimetry for levofloxacin pharmacokinetics and personalized dosing in people with drug-resistant tuberculosis. Int J Mycobacteriol. 2020;9:411–416. doi: 10.4103/ijmy.ijmy_186_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nicolau I, Tian L, Menzies D, Ostiguy G, Pai M. Point-of-care urine tests for smoking status and isoniazid treatment monitoring in adult patients. PLoS ONE. 2012;7:e45913. doi: 10.1371/journal.pone.0045913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Szipszky C, Van Aartsen D, Criddle S, Rao P, Zentner I, Justine M, et al. Determination of rifampin concentrations by urine colorimetry and mobile phone readout for personalized dosing in tuberculosis treatment. J Pediatr Infect Dis Soc. 2021;10:104–111. doi: 10.1093/jpids/piaa024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raju KSR, Taneja I, Singh SP, Wahajuddin. Utility of noninvasive biomatrices in pharmacokinetic studies: noninvasive biomatrices in pharmacokinetics. Biomed Chromatogr. 2013;27:1354–66. [DOI] [PubMed]
- 17.van den Elsen SHJ, Oostenbrink LM, Heysell SK, Hira D, Touw DJ, Akkerman OW, et al. Systematic review of salivary versus blood concentrations of antituberculosis drugs and their potential for salivary therapeutic drug monitoring. Ther Drug Monit. 2018;40:17–37. doi: 10.1097/FTD.0000000000000462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alffenaar J-WC, Jongedijk EM, van Winkel CAJ, Sariko M, Heysell SK, Mpagama S, et al. A mobile microvolume UV/visible light spectrophotometer for the measurement of levofloxacin in saliva. J Antimicrob Chemother. 2021;76:423–9. [DOI] [PMC free article] [PubMed]
- 19.Kim HY, Ruiter E, Jongedijk EM, Ak HK, Marais BJ, Pk B, et al. Saliva-based linezolid monitoring on a mobile UV spectrophotometer. J Antimicrob Chemother. 2021;76:1786–1792. doi: 10.1093/jac/dkab075. [DOI] [PubMed] [Google Scholar]
- 20.Mohamed S, Mvungi HC, Sariko M, Rao P, Mbelele P, Jongedijk EM, et al. Levofloxacin pharmacokinetics in saliva as measured by a mobile microvolume UV spectrophotometer among people treated for rifampicin-resistant TB in Tanzania. J Antimicrob Chemother. 2021;76:1547–1552. doi: 10.1093/jac/dkab057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mave V, Kadam D, Gaikwad S, Kinikar A, Aguilar D, Chavan A, et al. Measuring TB drug levels in the hair in adults and children to monitor drug exposure and outcomes. Int J Tuberc Lung Dis. 2021;25:52–60. doi: 10.5588/ijtld.20.0574. [DOI] [PubMed] [Google Scholar]
- 22.Wasserman S, Huo S, Ky K, Malig YN, Esmail A, Dheda K, et al. Correlation of linezolid hair concentrations with plasma exposure in patients with drug-resistant tuberculosis. Antimicrob Agents Chemother. 2020;64:e02145–e2219. doi: 10.1128/AAC.02145-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alffenaar JWC, Marais BJ, Heysell SK. Measuring anti-TB drug concentrations in hair: unlocking the door to cumulative drug exposure and treatment outcome. Int J Tuberc Lung Dis. 2021;25:3–5. doi: 10.5588/ijtld.20.0797. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization. Meeting report of the WHO expert consultation on drug-resistant tuberculosis treatment outcome definitions, 17–19 November 2020. Geneva: World Health Organization; 2021. https://apps.who.int/iris/handle/10665/340284. Accessed 13 July 2022.
- 25.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [PMC free article] [PubMed]
- 26.Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Héder M. From NASA to EU: the evolution of the TRL scale in Public Sector Innovation. Innov J Public Sect Innov J. 2017;22(2):2–22.
- 28.Parsons TL, Marzinke MA, Hoang T, Bliven-Sizemore E, Weiner M, Mac Kenzie WR, et al. Quantification of rifapentine, a potent antituberculosis drug, from dried blood spot samples using liquid chromatographic-tandem mass spectrometric analysis. Antimicrob Agents Chemother. 2014;58:6747–6757. doi: 10.1128/AAC.03607-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martial LC, Kerkhoff J, Martinez N, Rodríguez M, Coronel R, Molinas G, et al. Evaluation of dried blood spot sampling for pharmacokinetic research and therapeutic drug monitoring of anti-tuberculosis drugs in children. Int J Antimicrob Agents. 2018;52:109–113. doi: 10.1016/j.ijantimicag.2018.04.020. [DOI] [PubMed] [Google Scholar]
- 30.Vu DH, Koster RA, Bolhuis MS, Greijdanus B, Altena RV, Nguyen DH, et al. Simultaneous determination of rifampicin, clarithromycin and their metabolites in dried blood spots using LC-MS/MS. Talanta. 2014;121:9–17. doi: 10.1016/j.talanta.2013.12.043. [DOI] [PubMed] [Google Scholar]
- 31.Brahmadhi A, Chen MX, Wang S-Y, Cho Y-Y, Yu M-C, Lee C-H, et al. Determination of fluoroquinolones in dried plasma spots by using microwave-assisted extraction coupled to ultra-high performance liquid chromatography-tandem mass spectrometry for therapeutic drug monitoring. J Pharm Biomed Anal. 2021;195:113821. doi: 10.1016/j.jpba.2020.113821. [DOI] [PubMed] [Google Scholar]
- 32.Vu DH, Koster RA, Alffenaar JWC, Brouwers JRBJ, Uges DRA. Determination of moxifloxacin in dried blood spots using LC-MS/MS and the impact of the hematocrit and blood volume. J Chromatogr B Anal Technol Biomed Life Sci. 2011;879:1063–1070. doi: 10.1016/j.jchromb.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 33.Vu DH, Bolhuis MS, Koster RA, Greijdanus B, de Lange WCM, van Altena R, et al. Dried blood spot analysis for therapeutic drug monitoring of linezolid in patients with multidrug-resistant tuberculosis. Antimicrob Agents Chemother. 2012;56:5758–5763. doi: 10.1128/AAC.01054-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baietto L, D’Avolio A, Ariaudo A, Corcione S, Simiele M, Cusato J, et al. Development and validation of a new UPLC-PDA method to quantify linezolid in plasma and in dried plasma spots. J Chromatogr B Anal Technol Biomed Life Sci. 2013;936:42–47. doi: 10.1016/j.jchromb.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 35.Lee K, Jun S-H, Choi M-S, Song SH, Park JS, Lee JH, et al. Application of the isoniazid assay in dried blood spots using the ultra-performance liquid chromatography-tandem mass spectrometry. Clin Biochem. 2017;50:882–885. doi: 10.1016/j.clinbiochem.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 36.Macintyre CR, Goebel K, Brown GV. Patient knows best: blinded assessment of nonadherence with antituberculous therapy by physicians, nurses, and patients compared with urine drug levels. Prev Med. 2005;40:41–45. doi: 10.1016/j.ypmed.2004.04.045. [DOI] [PubMed] [Google Scholar]
- 37.Burkhardt KR, Nel EE. Monitoring regularity of drug intake in tuberculous patients by means of simple urine tests. S Afr Med J. 1980;57:981–985. [PubMed] [Google Scholar]
- 38.Hashiguchi M, Ohno K, Sakuma A, Hino F, Tanaka T, Ohtsuji M, et al. A simplified method for detecting isoniazid compliance in patients receiving antituberculosis chemotherapy. J Clin Pharmacol. 2002;42:151–156. doi: 10.1177/00912700222011184. [DOI] [PubMed] [Google Scholar]
- 39.Ellard GA, Jenner PJ, Downs PA. An evaluation of the potential use of isoniazid, acetylisoniazid and isonicotinic acid for monitoring the self-administration of drugs. Br J Clin Pharmacol. 1980;10:369–381. doi: 10.1111/j.1365-2125.1980.tb01773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chatterjee K, Kuo CW, Chen A, Chen P. Detection of residual rifampicin in urine via fluorescence quenching of gold nanoclusters on paper. J Nanobiotechnology. 2015;13:46. doi: 10.1186/s12951-015-0105-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eidus L, Harnanansingh AM. Simple procedures for checking rifampin in urine. Am Rev Respir Dis. 1969;100:738–739. doi: 10.1164/arrd.1969.100.5.738. [DOI] [PubMed] [Google Scholar]
- 42.Espinosa-Mansilla A, Acedo-Valenzuela MI, Muñoz de la Peña A, Cañada Cañada F, Salinas López F. Determination of antitubercular drugs in urine and pharmaceuticals by LC using a gradient flow combined with programmed diode array photometric detection. Talanta. 2002;58:273–80. [DOI] [PubMed]
- 43.Meissner PE, Musoke P, Okwera A, Bunn JEG, Coulter JBS. The value of urine testing for verifying adherence to anti-tuberculosis chemotherapy in children and adults in Uganda. Int J Tuberc Lung Dis. 2002;6:903–908. [PubMed] [Google Scholar]
- 44.Mitchison DA, Allen BW, Miller AB. Detection of rifampicin in urine by a simple microbiological assay. Tubercle. 1970;51:300–304. doi: 10.1016/0041-3879(70)90023-1. [DOI] [PubMed] [Google Scholar]
- 45.Mqoqi NP, Churchyard GA, Kleinschmidt I, Williams B. Attendance versus compliance with tuberculosis treatment in an occupational setting: a pilot study. S Afr Med J. 1997;87:1517–1521. [PubMed] [Google Scholar]
- 46.Palanduz A, Gultekin D, Kayaalp N. Follow-up of compliance with tuberculosis treatment in children: monitoring by urine tests. Pediatr Pulmonol. 2003;36:55–57. doi: 10.1002/ppul.10314. [DOI] [PubMed] [Google Scholar]
- 47.Sirgel FA, Maritz JS, Venter A, Langdon G, Smith PJ, Donald PR. Monitoring the ingestion of anti-tuberculosis drugs by simple non-invasive methods. Int J Pharm. 2006;307:182–187. doi: 10.1016/j.ijpharm.2005.09.033. [DOI] [PubMed] [Google Scholar]
- 48.Wardman AG, Knox AJ, Muers MF, Page RL. Profiles of non-compliance with antituberculous therapy. Br J Dis Chest. 1988;82:285–289. doi: 10.1016/0007-0971(88)90070-8. [DOI] [PubMed] [Google Scholar]
- 49.Amlabu V, Mulligan C, Jele N, Evans A, Gray D, Zar HJ, et al. Isoniazid/acetylisoniazid urine concentrations: markers of adherence to isoniazid preventive therapy in children. Int J Tuberc Lung Dis. 2014;18:528–530. doi: 10.5588/ijtld.13.0730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anusiem CA, Brown SA, Ezejiofor NA, Barikpoar E, Orisakwe OE. Isoniazid pharmacokinetics in the presence of ofloxacin and norfloxacin antibiotics. Am J Ther. 2018;25:e397–404. doi: 10.1097/MJT.0000000000000032. [DOI] [PubMed] [Google Scholar]
- 51.Eidlitz-Markus T, Zeharia A, Baum G, Mimouni M, Amir J. Use of the urine color test to monitor compliance with isoniazid treatment of latent tuberculosis infection. Chest. 2003;123:736–739. doi: 10.1378/chest.123.3.736. [DOI] [PubMed] [Google Scholar]
- 52.Elizaga J, Friedland JS. Monitoring compliance with antituberculous treatment by detection of isoniazid in urine. Lancet. 1997;350:1225–1226. doi: 10.1016/S0140-6736(05)63457-5. [DOI] [PubMed] [Google Scholar]
- 53.Guerra RL, Conde MB, Efron A, Loredo C, Bastos G, Chaisson RE, et al. Point-of-care Arkansas method for measuring adherence to treatment with isoniazid. Respir Med. 2010;104:754–757. doi: 10.1016/j.rmed.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hamilton EJ, Jessamine AG, Eidus L. Specificity of the isoniazid drop test for control of domiciliary treatment of tuberculosis. Can Med Assoc J. 1964;90:695–697. [PMC free article] [PubMed] [Google Scholar]
- 55.Hanifa Y, Mngadi K, Lewis J, Fielding K, Churchyard G, Grant AD. Evaluation of the Arkansas method of urine testing for isoniazid in South Africa. Int J Tuberc Lung Dis. 2007;11:1232–1236. [PubMed] [Google Scholar]
- 56.Kendall EA, Durovni B, Martinson NA, Cavalacante S, Masonoke K, Saraceni V, et al. Adherence to tuberculosis preventive therapy measured by urine metabolite testing among people with HIV. AIDS. 2020;34:63–71. doi: 10.1097/QAD.0000000000002380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.LaCourse SM, Leon D, Panpradist N, Richardson BA, Maleche-Obimbo E, Mecha J, et al. Urine biomarker assessment of infant adherence to isoniazid prophylaxis. Pediatr Infect Dis J. 2021;40:e43–e45. doi: 10.1097/INF.0000000000002936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Macfadyen DM, Heffernan JF. Urine testing for isoniazid in the supervision of out-patient oral chemotherapy for pulmonary tuberculosis: the failure of a routine service. Bull World Health Organ. 1967;36:847–852. [PMC free article] [PubMed] [Google Scholar]
- 59.Mishra P, Albiol-Chiva J, Bose D, Durgbanshi A, Peris-Vicente J, Carda-Broch S, et al. Optimization and validation of a chromatographic method for the quantification of isoniazid in urine of tuberculosis patients according to the European Medicines Agency guideline. Antibiotics (Basel). 2018;7:107. doi: 10.3390/antibiotics7040107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Narain R, Rao MS, Naganna K, Bagga AS. Estimating the population consuming isoniazid by urine test. Am Rev Respir Dis. 1971;104:122–125. doi: 10.1164/arrd.1971.104.1.122. [DOI] [PubMed] [Google Scholar]
- 61.Perry S, Hovell MF, Blumberg E, Berg J, Vera A, Sipan C, et al. Urine testing to monitor adherence to TB preventive therapy. J Clin Epidemiol. 2002;55:235–238. doi: 10.1016/S0895-4356(01)00470-X. [DOI] [PubMed] [Google Scholar]
- 62.Schmitz KE, Hovell MF, Wong CA, Kelley NJ, Nilsen D, Blumberg EJ, et al. The reliability and practicality of the Arkansas method assay of isoniazid adherence. Clin Nurs Res. 2010;19:131–143. doi: 10.1177/1054773810363473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schraufnagel DE, Stoner R, Whiting E, Snukst-Torbeck G, Werhane MJ. Testing for isoniazid: an evaluation of the Arkansas method. Chest. 1990;98:314–316. doi: 10.1378/chest.98.2.314. [DOI] [PubMed] [Google Scholar]
- 64.Soobratty MR, Whitfield R, Subramaniam K, Grove G, Carver A, O’Donovan GV, et al. Point-of-care urine test for assessing adherence to isoniazid treatment for tuberculosis. Eur Respir J. 2014;43:1519–1522. doi: 10.1183/09031936.00132613. [DOI] [PubMed] [Google Scholar]
- 65.Subbaraman R, Thomas BE, Kumar JV, Thiruvengadam K, Khandewale A, Kokila S, et al. Understanding nonadherence to tuberculosis medications in India using urine drug metabolite testing: a cohort study. Open Forum Infect Dis. 2021;8:ofab190. [DOI] [PMC free article] [PubMed]
- 66.Szakacs TA, Wilson D, Cameron DW, Clark M, Kocheleff P, Muller FJ, et al. Adherence with isoniazid for prevention of tuberculosis among HIV-infected adults in South Africa. BMC Infect Dis. 2006;6:97. doi: 10.1186/1471-2334-6-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Venho VM, Koskinen R. The effect of pyrazinamide, rifampicin and cycloserine on the blood levels and urinary excretion of isoniazid. Ann Clin Res. 1971;3:277–280. [PubMed] [Google Scholar]
- 68.Whitfield R, Cope GF. Point-of-care test to monitor adherence to anti-tuberculous treatment. Ann Clin Biochem. 2004;41:411–413. doi: 10.1258/0004563041731637. [DOI] [PubMed] [Google Scholar]
- 69.Zhao Y, Zhang X, Jia C, Wu J, Tang H, Shang J, et al. A simple signal-on strategy for fluorescent detection of tuberculostatic drug isoniazid based on Ag clusters-MnO(2) sheets nanoplatform. Colloids Surf B Biointerfaces. 2021;201:111627. doi: 10.1016/j.colsurfb.2021.111627. [DOI] [PubMed] [Google Scholar]
- 70.Pines A, Richardson RJ. A simple table test for the detection of pyrazinamide in the urine. Tubercle. 1964;45:166–168. doi: 10.1016/S0041-3879(64)80075-1. [DOI] [PubMed] [Google Scholar]
- 71.Mattila MJ, Nieminen E, Tiitinen H. Serum levels, urinary excretion, and side-effects of cycloserine in the presence of isoniazid and p-aminosalicylic acid. Scand J Respir Dis. 1969;50:291–300. [PubMed] [Google Scholar]
- 72.Eidus L, Harnanansingh AM. A urine test for control of ingestion of ethionamide. Am Rev Respir Dis. 1968;98:315–316. doi: 10.1164/arrd.1968.98.2.315. [DOI] [PubMed] [Google Scholar]
- 73.Eidus L, Jessamine AG, Harnanansingh AM. Evaluation of a urinary colour test in the surveillance of ethionamide medication. Can Med Assoc J. 1968;99:413–415. [PMC free article] [PubMed] [Google Scholar]
- 74.Gurumurthy P, Rahman F, Narayana AS, Sarma GR. Salivary levels of isoniazid and rifampicin in tuberculous patients. Tubercle. 1990;71:29–33. doi: 10.1016/0041-3879(90)90057-F. [DOI] [PubMed] [Google Scholar]
- 75.Kumar AKH, Sudha V, Srinivasan R, Ramachandran G. Simple and rapid liquid chromatography method for determination of moxifloxacin in saliva. J Chromatogr B Anal Technol Biomed Life Sci. 2011;879:3663–3667. doi: 10.1016/j.jchromb.2011.09.047. [DOI] [PubMed] [Google Scholar]
- 76.Ofoefule SI, Onyeagba OE, Orisakwe OE. Effects of pefloxacin on urinary and salivary concentrations of isoniazid in six healthy female volunteers. Am J Ther. 2000;7:313–316. doi: 10.1097/00045391-200007050-00008. [DOI] [PubMed] [Google Scholar]
- 77.van den Elsen SHJ, Akkerman OW, Wessels M, Jongedijk EM, Ghimire S, van der Werf TS, et al. Dose optimisation of first-line tuberculosis drugs using therapeutic drug monitoring in saliva: feasible for rifampicin, not for isoniazid. Eur Respir J. 2020;56:2000803. doi: 10.1183/13993003.00803-2020. [DOI] [PubMed] [Google Scholar]
- 78.van den Elsen SHJ, Akkerman OW, Jongedijk EM, Wessels M, Ghimire S, van der Werf TS, et al. Therapeutic drug monitoring using saliva as matrix: an opportunity for linezolid, but challenge for moxifloxacin. Eur Respir J. 2020;55:1901903. doi: 10.1183/13993003.01903-2019. [DOI] [PubMed] [Google Scholar]
- 79.Ghimire S, Maharjan B, Jongedijk EM, Kosterink JGW, Ghimire GR, Touw DJ, et al. Evaluation of saliva as a potential alternative sampling matrix for therapeutic drug monitoring of levofloxacin in patients with multidrug-resistant tuberculosis. Antimicrob Agents Chemother. 2019;63:e02379–e2418. doi: 10.1128/AAC.02379-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bolhuis MS, van Altena R, van Hateren K, de Lange WCM, Greijdanus B, Uges DRA, et al. Clinical validation of the analysis of linezolid and clarithromycin in oral fluid of patients with multidrug-resistant tuberculosis. Antimicrob Agents Chemother. 2013;57:3676–3680. doi: 10.1128/AAC.00558-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.van den Elsen SHJ, Akkerman OW, Huisman JR, Touw DJ, van der Werf TS, Bolhuis MS, et al. Lack of penetration of amikacin into saliva of tuberculosis patients. Eur Respir J. 2018;51:1702024. doi: 10.1183/13993003.02024-2017. [DOI] [PubMed] [Google Scholar]
- 82.Eisenhut M, Thieme D, Schmid D, Fieseler S, Sachs H. Hair Analysis for determination of isoniazid concentrations and acetylator phenotype during antituberculous treatment. Tuberc Res Treat. 2012;2012:327027. doi: 10.1155/2012/327027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Reckers A, Huo S, Esmail A, Dheda K, Bacchetti P, Gandhi M, et al. Development and validation of a liquid chromatography-tandem mass spectrometry method for quantifying delamanid and its metabolite in small hair samples. J Chromatogr B Analyt Technol Biomed Life Sci. 2021;1169:122467. doi: 10.1016/j.jchromb.2020.122467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mave V, Kinikar A, Kagal A, Nimkar S, Koli H, Khwaja S, et al. Isoniazid concentrations in hair and plasma area-under-the-curve exposure among children with tuberculosis. PLoS ONE. 2017;12:e0189101. doi: 10.1371/journal.pone.0189101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Metcalfe J, Bacchetti P, Esmail A, Reckers A, Aguilar D, Wen A, et al. Diagnostic accuracy of a liquid chromatography-tandem mass spectrometry assay in small hair samples for rifampin-resistant tuberculosis drug concentrations in a routine care setting. BMC Infect Dis. 2021;21:99. doi: 10.1186/s12879-020-05738-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gerona R, Wen A, Chin AT, Koss CA, Bacchetti P, Metcalfe J, et al. Quantifying isoniazid levels in small hair samples: a novel method for assessing adherence during the treatment of latent and active tuberculosis. PLoS ONE. 2016;11:e0155887. doi: 10.1371/journal.pone.0155887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gerona R, Wen A, Koss C, Bacchetti P, Gandhi M, Metcalfe J. A multi-analyte panel for non-invasive pharmacokinetic monitoring of second-line anti-tuberculosis drugs. Int J Tuberc Lung Dis. 2016;20:991–992. doi: 10.5588/ijtld.16.0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gerona R, Wen A, Aguilar D, Shum J, Reckers A, Bacchetti P, et al. Simultaneous analysis of 11 medications for drug resistant TB in small hair samples to quantify adherence and exposure using a validated LC-MS/MS panel. J Chromatogr B Anal Technol Biomed Life Sci. 2019;1125:121729. doi: 10.1016/j.jchromb.2019.121729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mave V, Chandanwale A, Kinikar A, Khadse S, Kagal A, Gupte N, et al. Isoniazid hair concentrations in children with tuberculosis: a proof of concept study. Int J Tuberc Lung Dis. 2016;20:844–847. doi: 10.5588/ijtld.15.0882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Metcalfe J, Bacchetti P, Gerona R, Esmail A, Dheda K, Gandhi M. Association of anti-tuberculosis drug concentrations in hair and treatment outcomes in MDR- and XDR-TB. ERJ Open Res. 2019;5:00046–2019. doi: 10.1183/23120541.00046-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Metcalfe J, Gerona R, Wen A, Bacchetti P, Gandhi M. An LC-MS/MS-based method to analyze the anti-tuberculosis drug bedaquiline in hair. Int J Tuberc Lung Dis. 2017;21:1069–1070. doi: 10.5588/ijtld.17.0408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Reckers A, Wen A, Aguilar D, Bacchetti P, Gandhi M, Metcalfe J, et al. Validated LC-MS/MS panel for quantifying 11 drug-resistant TB medications in small hair samples. J Vis Exp. 2020. 10.3791/60861. [DOI] [PubMed]
- 93.Pea F, Furlanut M, Cojutti P, Cristini F, Zamparini E, Franceschi L, et al. Therapeutic drug monitoring of linezolid: a retrospective monocentric analysis. Antimicrob Agents Chemother. 2010;54:4605–4610. doi: 10.1128/AAC.00177-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.World Health Organization . Rapid communication: key changes to the treatment of drug-resistant tuberculosis. Geneva: World Health Organization; 2022. [Google Scholar]
- 95.Zuur MA, Veenhof H, Aleksa A, VanʼtBoveneind-Vrubleuskaya N, Darmawan E, Hasnain MG, et al. Quality assessment of dried blood spots from patients with tuberculosis from 4 countries. Ther Drug Monit. 2019;41:714–718. doi: 10.1097/FTD.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 96.Pasipanodya JG, Srivastava S, Gumbo T. Meta-analysis of clinical studies supports the pharmacokinetic variability hypothesis for acquired drug resistance and failure of antituberculosis therapy. Clin Infect Dis. 2012;55:169–177. doi: 10.1093/cid/cis353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.O’Donnell MR, Daftary A, Frick M, Hirsch-Moverman Y, Amico KR, Senthilingam M, et al. Re-inventing adherence: toward a patient-centered model of care for drug-resistant tuberculosis and HIV. Int J Tuberc Lung Dis. 2016;20:430–434. doi: 10.5588/ijtld.15.0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sand TE, Jacobsen S. Effect of urine pH and flow on renal clearance of methotrexate. Eur J Clin Pharmacol. 1981;19:453–456. doi: 10.1007/BF00548590. [DOI] [PubMed] [Google Scholar]
- 99.Boman G, Ringberger VA. Binding of rifampicin by human plasma proteins. Eur J Clin Pharmacol. 1974;7:369–373. doi: 10.1007/BF00558209. [DOI] [PubMed] [Google Scholar]
- 100.Boxenbaum HG, Berkersky I, Mattaliano V, Kaplan SA. Plasma and salivary concentrations of isoniazid in man: preliminary findings in two slow acetylator subjects. J Pharmacokinet Biopharm. 1975;3:443–456. doi: 10.1007/BF01059476. [DOI] [PubMed] [Google Scholar]
- 101.Alffenaar J-WC, de Steenwinkel JEM, Diacon AH, Simonsson USH, Srivastava S, Wicha SG. Pharmacokinetics and pharmacodynamics of anti-tuberculosis drugs: an evaluation of in vitro, in vivo methodologies and human studies. Front Pharmacol. 2022;13:1063453. [DOI] [PMC free article] [PubMed]
- 102.Touw DJ, Neef C, Thomson AH, Vinks AA. Cost-effectiveness of Therapeutic Drug Monitoring Committee of the International Association for Therapeutic Drug Monitoring and Clinical Toxicology. Cost-effectiveness of therapeutic drug monitoring: a systematic review. Ther Drug Monit. 2005;27:10–7. [DOI] [PubMed]
- 103.Margineanu I, Akkerman O, Cattaneo D, Goletti D, Marriott DJE, Migliori GB, et al. Practices of therapeutic drug monitoring in tuberculosis: an international survey. Eur Respir J. 2022;59:2102787. doi: 10.1183/13993003.02787-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]