Abstract
Background
Candidemia is a pervasive problem associated with significant morbidity and mortality in health care settings. This study aimed to determine the changing distribution of Candida species and the emergence of uncommon species.
Methods
This was a cross-sectional study performed in two Cairo University hospitals between 2019 and 2020. All Candida species isolates recovered from blood cultures of adults and pediatrics patients admitted to the hospitals were included. Candida isolates were identified by chromogenic Candida agar and Vitek2 YST identification card. Candida kefyr was confirmed by chip array.
Results
Candida species were responsible for 1.6% of bloodstream infections in adults and 10.8% in pediatric patients. C. albicans was the most prevalent species representing 27.8% in adults and 48.3% in pediatrics. Non-albicans species (NAC) represented the most isolated Candida species among adults and pediatrics (72.2% and 51.6%, respectively) with the predominance of C. tropicalis (27.8% and 22.5%, respectively) followed by C. parapsilosis (16.7% and 10.8%, respectively). The uncommon Candida, which is Candida species other than C. albicans, C. parapsilosis, C. tropicalis, C. glabrata, and C. krusei, represents 16.6% and 14% of all candidemia in adults and pediatrics, respectively. Only one of each of C. lusitaniae, C. utilis, and C. kefyr were detected in adults. C. lusitaniae was the most frequently recovered uncommon Candida among pediatrics resulting in 6.4% of candidemia followed by C. famata (4.3%), C. utilis (2.2%), and C. kefyr (1.1%).
Conclusions
C. albicans is still the primary species isolated from pediatrics and adults with candidemia despite the considerable shift to the non-albicans species. C. tropicalis and C. parapsilosis are the most prevalent NAC. The increased prevalence of uncommon Candida species is alarming and necessitates a prompt stewardship program.
Keywords: Candida albicans, Non-albicans Candida, Candidaparapsilosis, Candidemia, Uncommon Candida, Candida kefyr
Introduction
Candida species are opportunistic fungal pathogens capable of causing a variety of infections in humans including mucosal and invasive candidiasis [1]. Candidemia, the most notable among invasive candidiasis, is a growing problem in tertiary care hospitals all over the world [2]. It is one of the most common causes of bloodstream infections(BSIs) (22%) in the United States, nevertheless bacterial BSI is much more common [3]. The global incidence of candidemia has significantly increased in the last years, which may be attributed to the widespread use of immunosuppressive therapies and broad-spectrum antibiotics. The wide use of antifungal prophylaxis also leads to infection with less susceptible Candida spp. [4–6]. Candidemia is associated with higher rates of morbidity and mortality in healthcare settings, especially among critically ill or immunocompromised patients or those with complicated medical conditions [7]. Although Candida albicans (C. albicans) is still considered a major pathogen associated with candidemia, a progressive shift from a dominance of C. albicans to non-albicans Candida (NAC) species has been notified by several countries [8, 9]. More than 90% of candidemia cases are attributed to one of the following five species (C. albicans, C. parapsilosis, C. tropicalis, C. glabrata, and C. krusei) [10, 11]. Recently, the emergence of Candidemia caused by other uncommon species such as C. guilliermondii, C. lusitaniae, and C. kefyr represents a new health threat to hospitalized patients [12–14]. The epidemiology and outcome of candidemia differ between pediatric and adult patients. Candidemia appears to be more frequent in neonates and young infants than in adults and is associated with a better outcome [15]. As the prevalence of Candida species differs between countries, regions, institutions, and different species that have variable antifungal resistance profiles it is necessary to determine the local epidemiology of candidemia for optimization of prevention and treatment [16].
This study aimed to determine the prevalence of Candida bloodstream infections in children and adults, the species distribution, and the emergence of rare Candida species from patients in two hospitals in Egypt.
Materials and methods
Study design
This retrospective cross-sectional study was performed in the clinical microbiology laboratories in two of Cairo University Hospitals (Kasr El-Ainy and CUSPH Cairo University Specialized Pediatric Hospital) from January 2019 to January 2020. All Candida species isolated from blood culture specimens from patients with septicemia were included in the study.
Isolation of Candida and species identification
Nearly, 3 ml of blood collected from pediatric patients suspected of septicemia was inoculated into a pediatric blood culture bottle (BACTEC Peds Plus/F) and introduced into an automated Blood culture system BACTEC 9050 (Becton Dickinson, USA). For an adult patient, 5–10 ml of blood was collected in the specialized tubes for the BacT/ALERT 3D (bioMérieux, France) automated blood culture system. After the system was alarmed for a positive culture, Gram staining was done to confirm the presence of yeast cells. Subculture on blood agar was performed. Candida isolates were identified up to species level using chromogenic Candida agar (Oxoid, England) and Vitek 2 YST identification card (bioMérieux, France). Candida kefyr was first isolated in our hospital so it was confirmed by chip array.
DNA extraction of Candida kefyr
DNA extraction and purification were performed using NucleoSpin Tissue mini kit (Macherey–Nagel Gmbh &Co., Germany), according to the manufacturer’s protocol. The DNA was stored at − 20 °C until used in PCR and chip hybridization.
PCR amplification and hybridization
After DNA extraction, the DNA was amplified by PCR with primers supplied with the kit and hybridized onto the LCD chip. The DNA was amplified in a total volume of 25 µl using Taq polymerase (Platinum Taq®, Invitrogen GmbH, Karlsruhe, Germany), according to the instructions of the manufacturer of the LCD chip (Fungi 2.1; Chipron GmbH, Berlin, Germany). The cycling conditions were set to 3 min at 95 °C (initial denaturation), 30 s at 94 °C, 45 s at 56 °C, 45 s at 72 °C (35–45 repetitions for amplification), 3 min at 72 °C for final extension, and cooling at 4 °C, in a Bio-Rad Thermal Cycler PTC-200. An aliquot of 7 µL of the PCR products was run on a 2% agarose gel. In a second step, PCR product was spotted manually on LCD chips and hybridization was performed according to the instruction manual. According to the manufacturer, the array can discriminate between 25 different fungal species or species clusters, such as C. albicans, C. glabrata, C. tropicalis, C. parapsilosis, C. krusei, C. dubliniensis, C. guilliermondii, C. pelliculosa, C. lusitaniae, C. lambica, C. kefyr, Aspergillus niger complex, Aspergillus fumigatus, Aspergillus flavus, Aspergillus terreus, Aspergillus nidulans, Mucor spp., Rhizomucor pusillus, Rhizomucor oryzae/Rhizomucor arrhizus, Rhizomucor azygosporus/Rhizomucor microspores, Rhizomucor stolonifer, Cryptococcus neoformans, Paecilomyces variotii, Scedosporium prolificans, and Lichtheimia (Absidia) corymbifera. For scanning and final analysis, a combination of a transmission light scanner and image analysis software supplied by the manufacturer was used.
Statistical analysis
Descriptive statistics were used to summarize the study findings. Qualitative and quantitative data values were expressed as frequency along with percentage.
Results
During the year 2019, 3321 blood cultures from adult patients were analyzed. 34.1% (1134/3321) of blood cultures were positive for at least one organism, though only 1.6% (18/1134) of positive ones were positive for Candida. Among pediatric patients, 28.9% of blood cultures were positive with 10.8% (93/860) being positive for Candida species.
Among adult patients with candidemia, C. albicans represented only 27.8% (5/18) while non-albicans species represented the most isolated Candida species 72.2% (13/18) (Fig. 1). C. tropicalis was the most common NAC causing candidemia in adults. C. tropicalis represented 27.8% of all candidemia cases followed by C. parapsilosis and C. glabrata (16.7, 11.1% respectively). Only one C. lusitaniae, C. utilis, and C. kefyr (5.5%) were detected in adults. The uncommon Candida, which was Candida species other than C. albicans, C. parapsilosis, C. tropicalis, C. glabrata, and C. krusei, represented 16.6% of all candidemia (Fig. 2).
Fig. 1.
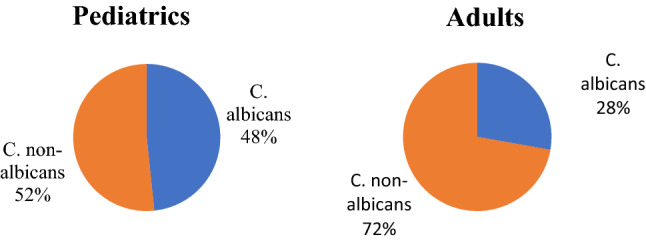
Prevalence of C. albicans and non-albicans species in pediatrics and adults
Fig. 2.
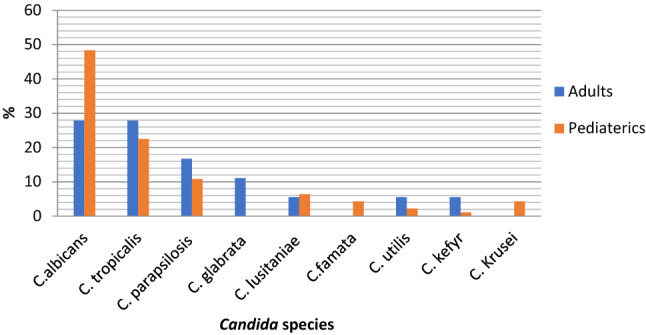
Candida species distribution among children and adults
While in pediatrics, there was a higher prevalence of C. albicans than in adults but still a predominance of non-albicans species. C. albicans represented 48.3% (45/93) while non-albicans 51.6% (48/93) (Fig. 1). The most common non-albicans species-causing candidemia was C. tropicalis (22.5%) followed by C. parapsilosis (10.8%), C. lusitaniae (6.4%), C. krusei (4.3%), C. famata (4.3%), and C. utilis (2.2%). One C. kefyr (1.1%) was also isolated from pediatric patients. The uncommon Candida species represented 14% of all candidemia (Fig. 2).
Discussion
Candidemia is an alarming problem in the health care setting associated with significant mortality rates, prolonged hospital stay, and high health-related costs. The incidence of candidemia is raising globally which necessitates careful monitoring and management [17, 18]. C. albicans, C. glabrata, C. parapsilosis, C. tropicalis, and C. krusei are held responsible for the majority of candidemia episodes [10, 11]. In our study, we observed that C. albicans is still the principal species responsible for candidemia in pediatrics and adults (48.3% and 27.8%). This was in agreement with several studies showing that C. albicans is still the primary cause and most frequently isolated species in different countries including the United States (67%), China (36.1%), Italy (61.2%) Kuwait (32%), Saudi Arabia (38.3%) and Egypt (36%) [19–24].
Despite the high prevalence of C. albicans in this study, there was a predominance of the non-albicans species in both pediatric patients and adults (51.6% and 72.2%, respectively). Recently, the shift from C. albicans dominance to the non-albicans has been witnessed by several studies on the national and international level [24–29]. The worldwide reports of increased resistance to antifungal agents such as fluconazole and echinocandins especially with NAC together with the considerable shift in species distribution are alarming and call for prompt antifungal stewardship programs and crucial identification of Candida isolates to species level to allow targeted management [30].
In our study, C. tropicalis was the most isolated NAC from adults followed by C. parapsilosis and C. glabrata. This was consistent with a study in a tertiary care university hospital in Turkey in which C. tropicalis caused 14% of all candidemia cases in the general surgery department followed by C. glabrata (12%) and C. parapsilosis (8%) [31]. In parallel with our results, C. tropicalis was the most common species isolated from patients with solid and hematological malignancies in Taiwan (41.9%) and exhibited the highest resistance rate to azoles [32]. C. glabrata, in contrast to our study, was the primary NAC causing candidemia in adult patients from Israel (40%) despite the dominance of C. tropicalis in hematology–oncology patients [26]. Also in Denmark, Finland, Norway, and Sweden, C. glabrata was the foremost isolated NAC in disagreement with our results [33]. In an Egyptian study among ICU patients, C. krusei was the most common NAC isolated (18.5%) followed by C. parapsilosis (20%), C. tropicalis (16%), and C. glabrata (10%) [34].
Among pediatric patients, we observed that the most common NAC causing candidemia in this study was C. tropicalis (22.5%) followed by C. parapsilosis (10.8%). This was in agreement with a study done in India in which 84% of candidemia episodes in children were attributed to NAC with C. tropicalis being the foremost recovered species followed by C. parapsilosis [29]. In concordance with our results, some studies in Middles East regions reported the dominance of C. tropicalis among NAC Candidemia [35]. In Saudi Arabia at a children’s hospital at King Fahad Medical City, between January 2010 and January 2011, the most NAC included were C. tropicalis (23%) and C. parapsilosis (13.1%). Candida famata (5.7%), C. lusitaniae (4.1%) and C. glabrata (2.5%) [36]. Similar to our results, C. tropicalis was the 2nd most common cause of candidemia after C. albicans in Egyptian study during 2017 [24]. In contrast to our study, C parapsilosis was reported as the most notable NAC species among infants and children in several studies and this has been attributed to its association with catheters, which results from its capability to stick on plastic and the power to thrive in parenteral nutrition [23, 37]. In addition, in a study in the PICU of Mansoura University Children’s Hospital, Egypt, over 1 year, C. parapsilosis accounted for 25% of NAC candidemia followed by C. tropicalis, and C. glabrata (17% and 8%, respectively) [38]. Similar to our results, C. glabrata and C. krusei were reported infrequent causes of fungemia in pediatrics [23, 39].
The variation in species distribution could be attributed to geographical variation and certain patient-related factors as age, associated co-morbidities such as malignancies, surgery, central venous catheters insertion [40]. In addition, the types of antifungals empirically used in the region may influence the distribution of species. It was documented that the use of fluconazole promotes C. glabrata and C. krusei infections while caspofungin usage to a lesser extent promotes infection by C. parapsilosis, C. glabrata, and C. krusei [41].
Seldom seen Candida species isolated from blood are increasingly reported in the literature in the last years [12, 42–44]. This is presumably due to the advances in mycological identification methods allowing identification of isolates to species level. However, the possibility of increased emergence of previously “nonpathogenic” species as true opportunistic pathogens should be considered especially with rising numbers of immunocompromised individuals.
In our study, the uncommon Candida species resulted in 14% and 16.6% of candidemia cases in pediatrics and adults, respectively. We reported a higher incidence of uncommon candidemia than in several studies. In general, the documented rates of uncommon Candida resulting in BSIs is less than 10% [13, 45–47]. Higher frequencies are observed also among cancer patients in MD Anderson Cancer Center hospital and a pediatric medical center in Taiwan (12% and 14%, respectively) [48].
In pediatrics, C. lusitaniae was the most frequently recovered uncommon Candida species followed by C. famata and C. utilis. In contrast to our results, C. guilliermondii has been the most frequently isolated uncommon Candida species among pediatric patients in several studies [12, 43, 49].
C. lusitaniae is an opportunistic yeast pathogen that is often mentioned by the capability to develop resistance to amphotericin B during treatment and may manifest as breakthrough infection in immunocompromised patients on amphotericin B therapy [50]. In line with our results, C. lusitaniae was recovered from 4% of the pediatric patients in a multicenter observational study in the United States from 2007 to 2011 [51]. A slightly lower incidence of C. lusitaniae candidemia was demonstrated in Kuwait. C. lusitaniae was detected at a higher frequency among infants less than 1 year resulting in 14.6% of invasive infections in Prospective Antifungal Therapy registry (PATH) data [44]. Also, C. lusitaniae was the second most common cause and accounted for 18.8% of candidemia caused by uncommon Candida species in a medical center in Taiwan [12].
Among adults, C. lusitaniae, C. utilis, and C. kefyr were recovered at a low frequency and each of them constituted 5.5% of candidemia cases. In contrast to our study, C. guilliermondii (41%) was the most commonly recovered from cancer patients in a retrospective study over 16 years period in Texas followed by C. lusitaniae (28%), C. kefyr (19%), C. famata (10%), and C. dublinensis (1%) [13]. On the other hand in a study at a university hospital in China analyzing candidemia among cancer patients found that C. lusitaniae and C. famata were held responsible for 3.8% of candidemia [45]. Limited data is available in the literature about the prevalence and species distribution of uncommon Candida in Egypt.
C. kefyr is a rare emerging Candida species that can cause candidemia especially in patients with hematologic malignancies [52]. It inhabits the gastrointestinal tract and is associated with the consumption of dairy products harboring this species [53]. In our study, C. kefyr was reported for the first time in our hospitals with a very rare frequency. Only one isolate was detected in each of the adult and pediatric groups (5.5% and 1.1%, respectively). The rate of isolation of C. kefyr ranges from 0.4% to 22.2% in different countries. The highest incidence (22.2%) was demonstrated in a surveillance study in France over 12-year periods [54]. On the other hand, the frequency of C. kefyr isolation from blood specimens was only as low as 0.4% in a 10-year study in Canada [55]. In a children's hospital in India, four C. kefyr (9%) were recovered from the blood of patients with candidemia [29]. Although C. kefyr is thought to have a high susceptibility to all antifungal drugs, recent studies reported acquiring resistance to echinocandins during treatment [56, 57].
Our study has several limitations that to be considered. First, the limited number of candidemia caused by individual uncommon Candida species. Second, we lack the information about the clinical history, underlying risk factors, and prior use of antibiotics or antifungals that could help to clarify the causes of shifting to non-albicans candidemia and the emergence of rare species. Third, we did not perform an antifungal susceptibility profile that is very important to assess the resistance pattern of NAC and infrequent Candida species and establish a policy for institutional empirical antifungal usage.
In conclusion, Candida species is an alarming pathogen causing BSIs in pediatrics and adults. Although C. albicans is still the primary species isolated from pediatrics and adults with candidemia, a notable shift to NAC candidemia especially by C. tropicalis and C. parapsilosis was observed in our study. We noted a higher incidence of candidemia attributable to uncommon Candida which necessitate analyzing the risk factors associated with this emergence and studying the antifungal susceptibility pattern of these species. C. kefyr was isolated for the first time in our hospital. The increased resistance associated with NAC and the correlation between the empirical uses of antifungals and the emergence of uncommon Candida species reported in the literature prioritizes the antifungal stewardship program and targeted management.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest. The authors have no relevant financial or non-financial interests to disclose.
Funding interest
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). No funds, grants, or other support was received.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
References
- 1.Gudlaugsson O, Gillespie S, Lee K, Berg JV, Hu J, Messer S, et al. Attributable mortality of nosocomial candidemia, revisited. Clin Infect Dis. 2003;37:1172–1177. doi: 10.1086/378745. [DOI] [PubMed] [Google Scholar]
- 2.Delaloye J, Calandra T. Invasive candidiasis as a cause of sepsis in the critically ill patient. Virulence. 2014;5:161–169. doi: 10.4161/viru.26187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Magill SS, Edwards JR, Bamberg W, Beldavs ZG, Dumyati G, Kainer MA, et al. Multistate point-prevalence survey of health care–associated infections. N Engl J Med. 2014;370:1198–1208. doi: 10.1056/NEJMoa1306801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gamaletsou MN, Walsh TJ, Zaoutis T, Pagoni M, Kotsopoulou M, Voulgarelis M, Panayiotidis P, Vassilakopoulos T, Angelopoulou MK, Marangos M, Spyridonidis A, Kofteridis D, Pouli A, Sotiropoulos D, Matsouka P, Argyropoulou A, Perloretzou S, Leckerman K, Manaka A, Oikonomopoulos P, Daikos G, Petrikkos G, Sipsas NV. A prospective, cohort, multicentre study of candidaemia in hospitalized adult patients with haematological malignancies. Clin Microbiol Infect. 2014;20:O50–O57. doi: 10.1111/1469-0691.12312. [DOI] [PubMed] [Google Scholar]
- 5.Goemaere B, Becker P, Van Wijngaerden E, Maertens J, Spriet I, Hendrickx M, et al. Increasing candidaemia incidence from 2004 to 2015 with a shift in epidemiology in patients preexposed to antifungals. Mycoses. 2018;61:127–133. doi: 10.1111/myc.12714. [DOI] [PubMed] [Google Scholar]
- 6.Wille MP, Guimarães T, Furtado GH, Colombo AL. Historical trends in the epidemiology of candidaemia: analysis of an 11-year period in a tertiary care hospital in Brazil. Mem Inst Oswaldo Cruz. 2013;108:288–292. doi: 10.1590/S0074-02762013000300005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Russo A, Falcone M, Fantoni M, Murri R, Masucci L, Carfagna P, Ghezzi MC, Posteraro B, Sanguinetti M, Venditti M. Risk factors and clinical outcomes of candidaemia in patients treated for Clostridium difficile infection. Clin Microbiol Infect. 2015;21:493.e1–493.e4. doi: 10.1016/j.cmi.2014.12.024. [DOI] [PubMed] [Google Scholar]
- 8.Juyal D, Sharma M, Pal S, Rathaur VK, Sharma N. Emergence of non-albicans Candida species in neonatal candidemia. N Am J Med Sci. 2013;5:541. doi: 10.4103/1947-2714.118919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Papadimitriou-Olivgeris M, Spiliopoulou A, Kolonitsiou F, Bartzavali C, Lambropoulou A, Xaplanteri P, et al. Increasing incidence of candidaemia and shifting epidemiology in favor of Candida non-albicans in a 9-year period (2009–2017) in a university Greek hospital. Infection. 2019;47:209–216. doi: 10.1007/s15010-018-1217-2. [DOI] [PubMed] [Google Scholar]
- 10.Klingspor L, Tortorano AM, Peman J, Willinger B, Hamal P, Sendid B, Velegraki A, Kibbler C, Meis JF, Sabino R, Ruhnke M, Arikan-Akdagli S, Salonen J, Dóczi I. Invasive Candida infections in surgical patients in intensive care units: a prospective, multicentre survey initiated by the European Confederation of Medical Mycology (ECMM) (2006–2008) Clin Microbiol Infect. 2015;21:87.e1–87.e10. doi: 10.1016/j.cmi.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 11.Tan B, Chakrabarti A, Li R, Patel A, Watcharananan S, Liu Z, et al. Incidence and species distribution of candidaemia in Asia: a laboratory-based surveillance study. Clin Microbiol Infect. 2015;21:946–953. doi: 10.1016/j.cmi.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 12.Tsai MH, Hsu JF, Yang LY, Pan YB, Lai MY, Chu SM, Huang HR, Chiang MC, Fu RH, Lu JJ. Candidemia due to uncommon Candida species in children: new threat and impacts on outcomes. Sci Rep. 2018;8:15239. doi: 10.1038/s41598-018-33662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jung DS, Farmakiotis D, Jiang Y, Tarrand JJ, Kontoyiannis DP. Uncommon Candida species fungemia among cancer patients, Houston, Texas, USA. Emerg Infect Dis. 2015;21:1942. doi: 10.3201/eid2111.150404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Charsizadeh A, Mirhendi H, Nikmanesh B, Eshaghi H, Rahmani M, et al. Candidemia in children caused by uncommon species of Candida. Arch Pediatr Infect Dis. 2018;6:e11895. doi: 10.5812/pedinfect.11895. [DOI] [Google Scholar]
- 15.Mantadakis E, Pana ZD, Zaoutis T. Candidemia in children: epidemiology, prevention and management. Mycoses. 2018;61:614–622. doi: 10.1111/myc.12792. [DOI] [PubMed] [Google Scholar]
- 16.Arendrup MC, Perlin DS. Echinocandin resistance: an emerging clinical problem? Curr Opin Infect Dis. 2014;27:484. doi: 10.1097/QCO.0000000000000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koehler P, Stecher M, Cornely OA, Koehler D, Vehreschild MJ, Bohlius J, et al. Morbidity and mortality of candidaemia in Europe: an epidemiologic meta-analysis. Clin Microbiol Infect. 2019;25:1200–1212. doi: 10.1016/j.cmi.2019.04.024. [DOI] [PubMed] [Google Scholar]
- 18.Brunetti G, Navazio AS, Giuliani A, Giordano A, Proli EM, Antonelli G, et al. Candida blood stream infections observed between 2011 and 2016 in a large Italian University Hospital: a time-based retrospective analysis on epidemiology, biofilm production, antifungal agents consumption and drug-susceptibility. PLoS ONE. 2019;14:e0224678. doi: 10.1371/journal.pone.0224678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alobaid K, Khan Z. Epidemiologic characteristics of adult candidemic patients in a secondary hospital in Kuwait: a retrospective study. J Mycol Med. 2019;29:35–38. doi: 10.1016/j.mycmed.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Al-Dorzi HM, Sakkijha H, Khan R, Aldabbagh T, Toledo A, Ntinika P, Al Johani SM, Arabi YM. Invasive candidiasis in critically ill patients: a prospective cohort study in two tertiary care centers. J Intensive Care Med. 2020;35:542–553. doi: 10.1177/0885066618767835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mencarini J, Mantengoli E, Tofani L, Riccobono E, Fornaini R, Bartalesi F, et al. Evaluation of candidemia and antifungal consumption in a large tertiary care Italian hospital over a 12-year period. Infection. 2018;46:469–476. doi: 10.1007/s15010-018-1139-z. [DOI] [PubMed] [Google Scholar]
- 22.Lin S, Chen R, Zhu S, Wang H, Wang L, Zou J, et al. Candidemia in adults at a tertiary hospital in China: clinical characteristics, species distribution, resistance, and outcomes. Mycopathologia. 2018;183:679–689. doi: 10.1007/s11046-018-0258-5. [DOI] [PubMed] [Google Scholar]
- 23.Benedict K, Roy M, Kabbani S, Anderson EJ, Farley MM, Harb S, et al. Neonatal and pediatric candidemia: results from population-based active laboratory surveillance in four US locations, 2009–2015. J Pediatr Infect Dis Soc. 2018;7:e78–e85. doi: 10.1093/jpids/piy009. [DOI] [PubMed] [Google Scholar]
- 24.Khairat SM, Sayed AM, Nabih M, Soliman NS, Hassan YM. Prevalence of Candida blood stream infections among children in tertiary care hospital: detection of species and antifungal susceptibility. Infect Drug Resist. 2019;12:2409. doi: 10.2147/IDR.S196972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Castanheira M, Messer SA, Rhomberg PR, Pfaller MA. Antifungal susceptibility patterns of a global collection of fungal isolates: results of the SENTRY Antifungal Surveillance Program (2013) Diagn Microbiol Infect Dis. 2016;85:200–204. doi: 10.1016/j.diagmicrobio.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 26.Israel S, Amit S, Israel A, Livneh A, Nir-Paz R, Korem M. The epidemiology and susceptibility of candidemia in Jerusalem, Israel. Front Cell Infect Microbiol. 2019;9:352. doi: 10.3389/fcimb.2019.00352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hassan DM, Yousef RHA, Abu Elhamed WA, Ali AA, Madkour LA. Candidemia in the neonatal intensive care unit: insights on epidemiology and antifungal drug susceptibility patterns. Arch Pediatr Infect Dis. 2019;7:e81090. [Google Scholar]
- 28.Fu J, Ding Y, Wei B, Wang L, Xu S, Qin P, et al. Epidemiology of Candida albicans and non-C. albicans of neonatal candidemia at a tertiary care hospital in western China. BMC Infect Dis. 2017;17:329. doi: 10.1186/s12879-017-2423-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaur R, Jaggi S, Dhakad M, Rawat D. An etiological and antifungal profile of candidemia in children. Int J Community Med Public Health. 2019;6:3899–3904. doi: 10.18203/2394-6040.ijcmph20193990. [DOI] [Google Scholar]
- 30.Whaley SG, Berkow EL, Rybak JM, Nishimoto AT, Barker KS, Rogers PD. Azole antifungal resistance in Candida albicans and emerging non-albicans Candida species. Front Microbiol. 2017;7:2173. doi: 10.3389/fmicb.2016.02173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Önal U, Metin DY, Karaca C, Polat SH, Ersin S, Taşbakan MI. Retrospective evaluation of candidemic patients among general surgery department in a tertiary care university hospital. Turk J Surg. 2019;35:210. doi: 10.5578/turkjsurg.4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu P-F, Liu W-L, Hsieh M-H, Hii I-M, Lee Y-L, Lin Y-T, et al. Epidemiology and antifungal susceptibility of candidemia isolates of non-albicans Candida species from cancer patients: non-albicans candidemia in cancer patients. Emerg Microbes Infect. 2017;6:1–7. doi: 10.1038/emi.2017.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hesstvedt L, Arendrup MC, Poikonen E, Klingpor L, Friman V, Nordøy I, et al. Differences in epidemiology of candidaemia in the Nordic countries—what is to blame? Mycoses. 2017;60:11–19. doi: 10.1111/myc.12535. [DOI] [PubMed] [Google Scholar]
- 34.Abass E, Mohamed S, El-Kholy I, Zaki S. Incidence of ICU-acquired candidemia in a tertiary care hospital in Cairo, Egypt. Egypt J Microbiol. 2019;54:55–61. doi: 10.21608/ejm.2019.14099.1104. [DOI] [Google Scholar]
- 35.Omrani AS, Makkawy EA, Baig K, Baredhwan AA, Almuthree SA, Elkhizzi NA, et al. Ten-year review of invasive Candida infections in a tertiary care center in Saudi Arabia. Saudi Med J. 2014;35:821–826. [PubMed] [Google Scholar]
- 36.Almoosa Z, Ahmed GY, Omran A, AlSarheed A, Alturki A, Alaqeel A, et al. Invasive candidiasis in pediatric patients at King Fahad Medical City in Central Saudi Arabia: a 5-year retrospective study. Saudi Med J. 2017;38:1118. doi: 10.15537/smj.2017.11.21116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palazzi DL, Arrieta A, Castagnola E, Halasa N, Hubbard S, Brozovich AA, et al. Candida speciation, antifungal treatment and adverse events in pediatric invasive candidiasis: results from 441 infections in a prospective, multi-national study. Pediatr Infect Dis J. 2014;33:1294–1296. doi: 10.1097/INF.0000000000000431. [DOI] [PubMed] [Google Scholar]
- 38.Hegazi M, Abdelkader A, Zaki M, El-Deek B. Characteristics and risk factors of candidemia in pediatric intensive care unit of a tertiary care children’s hospital in Egypt. J Infect Dev Ctries. 2014;8:624–634. doi: 10.3855/jidc.4186. [DOI] [PubMed] [Google Scholar]
- 39.Dornbusch HJ, Manzoni P, Roilides E, Walsh TJ, Groll AH. Invasive fungal infections in children. Pediatr Infect Dis J. 2009;28:734–737. doi: 10.1097/INF.0b013e3181b076b1. [DOI] [PubMed] [Google Scholar]
- 40.Guinea J. Global trends in the distribution of Candida species causing candidemia. Clin Microbiol Infect. 2014;20:5–10. doi: 10.1111/1469-0691.12539. [DOI] [PubMed] [Google Scholar]
- 41.Lortholary O, Desnos-Ollivier M, Sitbon K, Fontanet A, Bretagne S, Dromer F, et al. Recent exposure to caspofungin or fluconazole influences the epidemiology of candidemia: a prospective multicenter study involving 2,441 patients. Antimicrob Agents Chemother. 2011;55:532–538. doi: 10.1128/AAC.01128-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pfaller MA, Moet GJ, Messer SA, Jones RN, Castanheira M. Candida bloodstream infections: comparison of species distributions and antifungal resistance patterns in community-onset and nosocomial isolates in the SENTRY Antimicrobial Surveillance Program, 2008–2009. Antimicrob Agents Chemother. 2011;55:561–566. doi: 10.1128/AAC.01079-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu W-L, Lai C-C, Li M-C, Wu C-J, Ko W-C, Hung Y-L, et al. Clinical manifestations of candidemia caused by uncommon Candida species and antifungal susceptibility of the isolates in a regional hospital in Taiwan, 2007–2014. J Microbiol Immunol Infect. 2019;52:612–619. doi: 10.1016/j.jmii.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 44.Pfaller MA, Andes DR, Diekema DJ, Horn DL, Reboli AC, Rotstein C, et al. Epidemiology and outcomes of invasive candidiasis due to non-albicans species of Candida in 2,496 patients: data from the Prospective Antifungal Therapy (PATH) registry 2004–2008. PLoS ONE. 2014;9:e101510. doi: 10.1371/journal.pone.0101510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li D, Xia R, Zhang Q, Bai C, Li Z, Zhang P. Evaluation of candidemia in epidemiology and risk factors among cancer patients in a cancer center of China: an 8-year case-control study. BMC Infect Dis. 2017;17:536. doi: 10.1186/s12879-017-2636-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xiao M, Sun Z-Y, Kang M, Guo D-W, Liao K, Chen SC-A, et al. Five-year National Surveillance of invasive candidiasis: species distribution and azole susceptibility from the China hospital invasive fungal surveillance net (CHIF-NET) study. J Clin Microbiol. 2018;56:e00577–e618. doi: 10.1128/JCM.00577-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen SC, Marriott D, Playford E, Nguyen Q, Ellis D, Meyer W, et al. Candidaemia with uncommon Candida species: predisposing factors, outcome, antifungal susceptibility, and implications for management. Clin Microbiol Infect. 2009;15:662–669. doi: 10.1111/j.1469-0691.2009.02821.x. [DOI] [PubMed] [Google Scholar]
- 48.Tang HJ, Liu WL, Lin HL, Lai CC. Epidemiology and prognostic factors of candidemia in cancer patients. PLoS ONE. 2014;9:e99103. doi: 10.1371/journal.pone.0099103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Santolaya ME, Alvarado T, Queiroz-Telles F, Colombo AL, Zurita J, Tiraboschi IN, et al. Active surveillance of candidemia in children from Latin America: a key requirement for improving disease outcome. Pediatr Infect Dis J. 2014;33:e40–e44. doi: 10.1097/INF.0000000000000039. [DOI] [PubMed] [Google Scholar]
- 50.Atkinson BJ, Lewis RE, Kontoyiannis DP. Candida lusitaniae fungemia in cancer patients: risk factors for amphotericin B failure and outcome. Sabouraudia. 2008;46:541–546. doi: 10.1080/13693780801968571. [DOI] [PubMed] [Google Scholar]
- 51.Steinbach WJ, Roilides E, Berman D, Hoffman JA, Groll AH, Bin-Hussain I, et al. Results from a prospective, international, epidemiologic study of invasive candidiasis in children and neonates. Pediatr Infect Dis J. 2012;31:1252–1257. doi: 10.1097/INF.0b013e3182737427. [DOI] [PubMed] [Google Scholar]
- 52.Dufresne SF, Marr KA, Sydnor E, Staab JF, Karp JE, Lu K, et al. Epidemiology of Candida kefyr in patients with hematologic malignancies. J Clin Microbiol. 2014;52:1830–1837. doi: 10.1128/JCM.00131-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Khan Z, Ahmad S, Al-Obaid K, Joseph L, Chandy R. Candida kefyr as a cause of bloodstream infection and adjunctive role of biomarkers in its diagnosis. J Mycol Med. 2015;25:71–75. doi: 10.1016/j.mycmed.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 54.Bretagne S, Renaudat C, Desnos-Ollivier M, Sitbon K, Lortholary O, Dromer F. Predisposing factors and outcome of uncommon yeast species-related fungaemia based on an exhaustive surveillance programme (2002–14) J Antimicrob Chemother. 2017;72:1784–1793. doi: 10.1093/jac/dkx045. [DOI] [PubMed] [Google Scholar]
- 55.Al-Rawahi GN, Roscoe DL. Ten-year review of candidemia in a Canadian tertiary care centre: predominance of non-albicans Candida species. Can J Infect Dis Med Microbiol. 2013;24:e65–e68. doi: 10.1155/2013/929717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fekkar A, Meyer I, Brossas J, Dannaoui E, Palous M, Uzunov M, et al. Rapid emergence of echinocandin resistance during Candida kefyr fungemia treatment with caspofungin. Antimicrob Agents Chemother. 2013;57:2380–2382. doi: 10.1128/AAC.02037-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pfaller M, Boyken L, Hollis R, Kroeger J, Messer S, Tendolkar S, et al. In vitro susceptibility of invasive isolates of Candida spp. to anidulafungin, caspofungin, and micafungin: six years of global surveillance. J Clin Microbiol. 2008;46:150–156. doi: 10.1128/JCM.01901-07. [DOI] [PMC free article] [PubMed] [Google Scholar]


