Abstract
The use of electronic cigarettes (e-cigarettes) has increased rapidly in the United States, especially among high school students. e-Cigarettes contain some recognized carcinogens and may induce DNA damage in oral cells. The aim of this review is to summarize studies reporting DNA adducts or other types of DNA damage in oral cells in vitro or in vivo upon exposure to e-cigarette vapor and to evaluate the possible connections between e-cigarette exposure and oral cancer. Three databases including PubMed, Scopus, and EMBASE and gray literature were searched for articles published up to April 24, 2022. After screening 321 articles, we extracted 27 for further investigation. Based on the inclusion criteria, 22 articles were eligible for this review. The in vitro studies demonstrate that e-cigarette liquid or vapor can induce DNA damage, oxidative stress, DNA double-stranded breaks, apoptosis, cytotoxicity, and genotoxicity in different types of oral cells. The clinical studies showed that e-cigarette users have significantly higher levels of Nʹ-nitrosonornicotine, acrolein DNA adducts, metanuclear anomalies, gene regulation, and lactate dehydrogenase enzyme expression and significantly lower levels of apurinic/apyrimidinic sites than non-users. Comparison of micronuclei levels between e-cigarette users and non-users gave inconsistent results. e-Cigarettes are implicated in DNA damage to oral cells, but publications to date present limited evidence. Future studies with larger sample sizes are required to investigate the long-term consequences of e-cigarette use.
Keywords: DNA damage, e-cigarettes, oral cells
Graphical Abstract
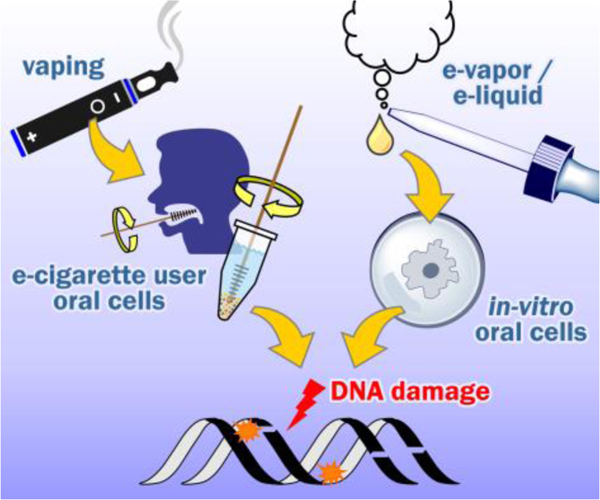
According to published studies, electronic cigarettes may induce DNA adducts or other types of DNA damage in oral cells in vitro or in vivo upon exposure to e-cigarette vapor. However, controversy exists. Further studies are required to investigate the long-term consequences of e-cigarette use.
1 |. INTRODUCTION
Electronic cigarettes (e-cigarettes) are generally regarded as safer alternatives to conventional cigarette smoking, but their potentially harmful effects have not been fully evaluated. e-Cigarettes are particularly popular among young adults and teens, and their use has increased rapidly.1 The percentage of high school students who have used e-cigarettes increased from 1.5% in 2011 to 20.8% in 2018.2
A variety of compounds in addition to nicotine have been detected in the refill solutions and aerosols of e-cigarettes. These include other tobacco alkaloids, tobacco-specific nitrosamines, formaldehyde, acetaldehyde, acrolein, metals, polycyclic aromatic hydrocarbons (PAHs), and propylene glycol or glycerin.3 Most of these chemicals were also found in conventional cigarette smoke and some cause DNA damage that may be related to cancer onset.4
DNA damage is central to the mechanism of carcinogenesis by cigarette smoke (Figure 1).5 The chemicals in cigarette smoke can react directly with DNA or, after metabolism, form addition products with the DNA bases and phosphates.4 These addition products, termed DNA adducts, can cause miscoding during DNA replication, leading to permanent changes in coding regions of DNA. When these mutations occur in critical regions of critical genes such as RAS and TP53, the result can be loss of normal cellular growth control mechanisms and the initiation of cancer.7 e-Cigarettes have far fewer carcinogens and substantially lower amounts of carcinogens than cigarette smoke, but the same basic mechanism of DNA damage may apply.8
FIGURE 1.
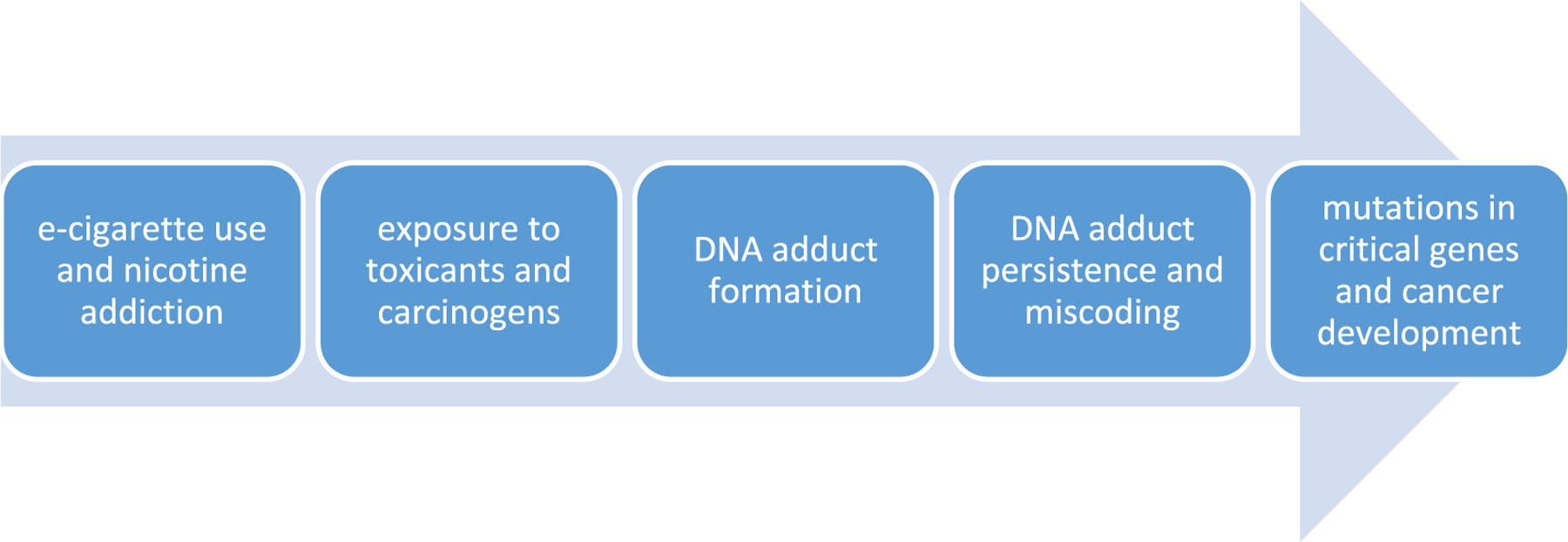
Overview of the pathway potentially leading from electronic cigarette (e-cigarette) use to DNA adduct formation, mutations, and cancer as established for cigarette smoking5,6 [Colour figure can be viewed at wileyonlinelibrary.com]
The first contact of the various chemicals inhaled with the e-aerosol takes place in the oral cavity, and information on the effects of this interaction comes mainly from animal or in vitro studies.1,9 Several relevant reviews have recently been published. e-Cigarette exposure can induce several oral health effects, including dysbiosis, inflammation, periodontal diseases, deterioration of dental and gingival health, and changes to the oral microbiome.1,10 e-Cigarette exposure was also implicated in adverse effects on head and neck, and oral cells in terms of aberrant morphology, cytotoxicity, oxidative stress, reduced viability, delayed fibroblast migration, and genotoxicity.1,11 e-Cigarette users have elevated levels of biomarkers of certain volatile organic compounds (VOCs; e.g., acrylamide, acrolein, and acrylonitrile), metals (e.g., cadmium and selenium), and propylene glycol compared with non-users and may also be exposed to other carcinogenic chemicals (e.g., benzene and chromium).12 There are also studies to investigate the association between e-cigarette exposure and head and neck cancer.9,13,14 They did not find enough evidence to support the proposal that e-cigarettes are carcinogenic to the head, neck, and oral cavity, but further studies are required.14 Longitudinal studies are also warranted for future research.11
According to the above reviews, e-cigarettes are toxic to oral cells, contain carcinogenic agents, and may have the potential to induce oral cancer. The aim of this review is to present an updated summary of studies reporting DNA adducts or other DNA damage in oral cells in vitro or in vivo exposed to e-cigarette vapor, primarily focusing on clinical studies of e-cigarette users. We are investigating the possible connections among e-cigarette exposure, DNA damage in oral cells, and oral cancer based on published studies. This review will update current knowledge of e-cigarette effects on DNA adducts and related damage in oral cavity cells.
2 |. SEARCH STRATEGY AND SELECTION CRITERIA
We conducted a systematic review of the literature within three main electronic databases (Medline/PubMed, Scopus, and EMBASE) to identify all articles examining oral cell DNA damage induced by e-cigarettes. The literature search was conducted using the following electronic search strategy: (“electronic cigarette” OR e-cigarette OR e-vapor OR e-liquid) AND (oral OR gingival OR tongue OR saliva OR buccal OR mouth) AND (“DNA damage” OR “DNA adduct” OR “DNA strand break” OR cytotoxicity OR genotoxicity) from inception until April 24, 2022, and it was restricted to peer-reviewed articles published in English. We also searched cross-references to complement the evidence given in this review. Publications were excluded if they were not peer reviewed. The search flow chart is shown in Figure 2.
FIGURE 2.
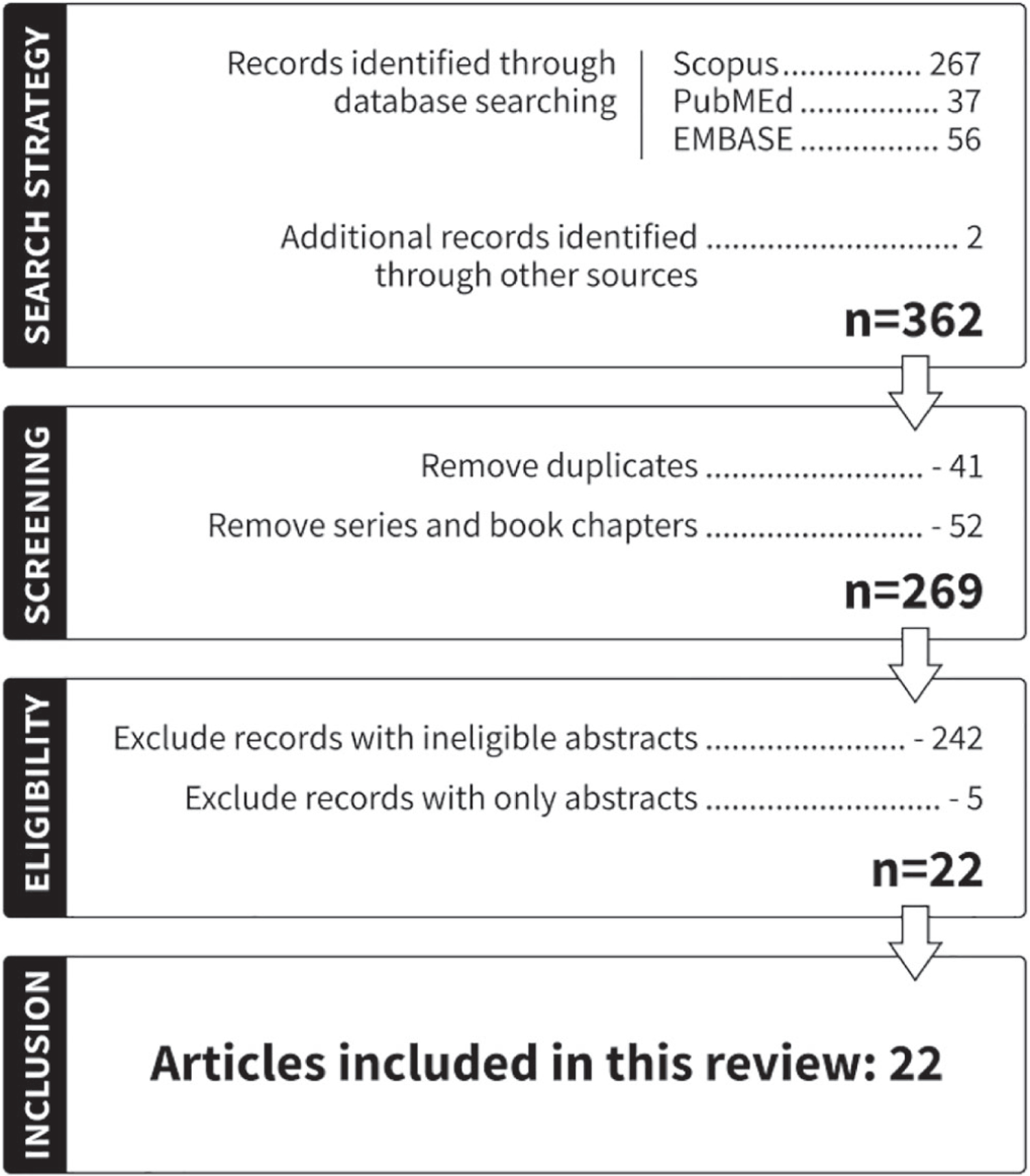
Flowchart of publications included in the systematic review
3 |. RESULTS
3.1 |. DNA damage in oral cells: In vitro experiments
The DNA damage, cytotoxicity, and genotoxicity of e-cigarette vapor or liquid exposure to oral cells in vitro have been addressed in several studies, as summarized in Table 1.
TABLE 1.
Studies investigating DNA damage and related effects in electronic cigarette (e-cigarette)-exposed oral cells
| Cell types | e-Cigarette types | DNA damage | Reference |
|---|---|---|---|
| Normal human oral keratinocytes | e-Cigarette aerosol | Oxidative stress and cytotoxicity | Ji et al. 201615 |
| Human gingival epithelial cells | e-Cigarette vapor | Altered cellular morphology, cytotoxicity, increased apoptosis, and lactate dehydrogenase activity | Rouabhia et al. 201716 |
| Human gingival fibroblasts | Nicotine-containing and nicotine-free fluids of e-cigarettes | Increased reactive oxidation species production and cytotoxicity, followed by apoptosis | Sancilio et al. 201617 |
| Human periodontal ligament fibroblasts, human gingival epithelium progenitors pooled, and epigingival 3D epithelium | e-Cigarettes with flavorings | Increased oxidative stress, carbonyl stress, inflammatory response, and DNA damage along with histone deacetylase 2 reduction via receptor for advanced glycation end products-dependent mechanisms | Sundar et al. 201618 |
| Healthy oropharyngeal mucosa | Flavored e-cigarette liquids | Cytotoxicity, DNA fragmentation, and mutation | Welz et al. 201619 |
| Normal epithelial and head and neck squamous cell carcinoma cell lines | Nicotine-containing and nicotine-free vapor extract from two popular e-cigarette brands | Increased DNA strand breaks, reduced cell viability, and clonogenic survival, along with increased rates of apoptosis and necrosis | Yu et al. 201620 |
| Human epithelial normal bronchial cells and human premalignant dysplastic oral mucosal keratinocyte cells | e-Cigarette aerosol | Oxidative DNA damage | Ganapathy et al. 201721 |
| Human oral keratinocyte cell line | Condensate of e-cigarette aerosol | Induced CYP1A1/1B1 and enhanced benzo[a]pyrene metabolism by activating the aryl hydrocarbon receptor | Sun et al. 201922 |
| Human oral squamous cell carcinoma cells and normal human gingival fibroblast cells | e-Cigarette aerosol | Induced cytotoxicity and intracellular oxidative stress | Ureña et al. 202023 |
| Human gingival fibroblasts | e-Cigarette vapor | Higher metabolic activity, no caspase 3/7 activation, and no significant differences in the amount of apoptosis/necrosis | Vermehren et al. 202024 |
| Gingival fibroblast/gingival mesenchymal stem cells (GF/G-MSCs) | e-Cigarette aerosol | No significant effects on DNA damage, cellular dedifferentiation, cellular proliferation, or viability of GF/G-MSCs | El-Mouelhy et al. 202225 |
| 3 immortalized oral epithelial cell lines | e-Cigarette-generated aerosols from 10 flavored e-liquid products with and without nicotine | Cell toxicity, oxidative stress, lipid peroxidation, and genotoxicity in terms of micronuclei formation | Tellez et al. 202126 |
| Human epithelial oral cells | e-Cigarette aerosol | Enhanced cytotoxicity and cell death in a dose-dependent manner and expressed upregulation of inflammatory cytokines up to 3.0-fold | Ramenzoni et al. 202227 |
Studies have found that e-cigarette exposure induced single or unspecific DNA adducts or relevant DNA damage in oral cells. Sun et al.22 found that a condensate of e-cigarette aerosol enhanced the rate of benzo[a]pyrene (BaP) tetraol formation several folds in a human oral keratinocyte cell line. BaP tetraol comprises the isomers of the hydrolysis products of a carcinogenic metabolite of BaP, anti-7,8-dihydroxy-9,10-epoxy-7,8,9,10-tetrahydro BaP (BPDE). e-Cigarette aerosol likely induces CYP1A1/1B1 and enhances BaP metabolism by activating the aryl hydrocarbon receptor. Using a primer-anchored DNA damage detection assay (q-PADDA) and an 8-oxo-deoxyguanosine ELISA assay to measure DNA damage, Ganapathy et al.21 found that e-cigarette extracts induced “bulky” DNA damage and oxidative DNA damage (in the form of 8-oxo-deoxyguanosine) in human oral and lung epithelial cells. Yu et al.20 exposed cell lines (both normal oral cells and head and neck squamous cell carcinoma [SCC] cells) to e-cigarette vapor with and without nicotine. They found that e-cigarette vapors induced cell apoptosis and necrosis and caused DNA strand breaks regardless of the presence or absence of nicotine. Welz et al.19 incubated human oropharyngeal mucosal cells with three e-liquids separately. They found that e-liquids are cytotoxic to human oropharyngeal mucosal cells and can induce significant DNA damage. These studies obviously demonstrate the diverse composition of e-cigarette vapor and liquid, and their capability to cause different types of DNA damage in vitro in oral cells.
DNA damage also accompanied the formation of reactive oxygen species or increased oxidative stress, followed by genotoxicity and cytotoxicity to oral cells. Ureña et al.23 tested third-generation e-cigarettes induction of cytotoxicity and oxidative stress in normal and cancerous human oral cell lines. They found that e-cigarette aerosols generated from only one of the eight liquids tested using a new atomizer induced cytotoxicity against two human oral cells in vitro. The e-cigarette aerosol also induced an increase of intracellular oxidative stress. Ji et al.15 exposed normal human oral keratinocytes to e-cigarette aerosol and found that toxic substances (e.g., nanoparticles and chemicals) in e-cigarettes may partially induce oxidative stress, resulting in cytotoxicity to oral epithelial cells. Ramenzoni et al.27 compared the toxicities of e-cigarette liquid aerosols with traditional cigarette smoke in human epithelial oral cells. Similar to traditional cigarette smoke, e-cigarette aerosols had adverse effects by enhancing cytotoxicity and cell death in a dose-dependent manner and caused upregulation of inflammatory cytokines up to 3.0-fold. Rouabhia et al.16 exposed human gingival epithelial cells to e-cigarette vapor, which resulted in altered cellular morphology, cytotoxicity, increased apoptosis, and lactate dehydrogenase (LDH) activity. These associated adverse effects of e-cigarette vapor exposure imply possible complications resulting from DNA damage.
Nicotine and flavors have been involved in the evaluation of e-cigarette DNA damage in some studies. Both nicotine-containing and nicotine-free e-cigarette fluids induced increased reactive oxygen species production in human gingival fibroblasts (HGFs) after 24 h, followed by apoptosis after 48 h of exposure. This implies that chemicals other than nicotine in e-cigarette liquids induced cytotoxicity in HGFs.17 Sundar et al.18 showed that e-cigarettes with flavorings caused several effects on human periodontal ligament fibroblasts, human gingival epithelium progenitors pooled, or epigingival 3D epithelium, including increased oxidative stress, carbonyl stress, inflammatory responses, and/or DNA damage. Similar damage was induced in human umbilical vein endothelial cells by e-cigarette aerosol extract.28 Tellez et al.26 evaluated the dose response for cytotoxicity and genotoxicity of e-cigarette-generated aerosols from 10 flavored e-liquid products with and without nicotine compared with unflavored ones in 3 immortalized oral epithelial cell lines. Three e-liquids caused >20% cell toxicity. Nine products induced significant levels of oxidative stress up to 2.4-fold. Dose–response increases up to 12-fold were seen for individual cell lines in terms of lipid peroxidation. Micronuclei formation indicative of genotoxicity was increased up to fivefold by some products.26 Based on these studies, nicotine and flavor additives in e-cigarettes may need to be taken into account when considering the DNA-damaging effects of e-cigarette fluids.
There are also studies demonstrating no significant effects related to DNA damage. El-Mouelhy et al.25 evaluated the effect of e-cigarette aerosol, cannabis, and conventional cigarette smoke on gingival fibroblast/gingival mesenchymal stem cells (GF/G-MSCs) of never smokers. e-Cigarettes showed no significant effects on DNA damage, cellular dedifferentiation, cellular proliferation, or viability of GF/G-MSCs. Vermehren et al.24 investigated cell-damaging effects on HGFs. Compared with controls, e-cigarettes-stimulated HGFs showed higher metabolic activity but no caspase 3/7 activation, nor significant differences in the amount of apoptosis/necrosis. Inconsistent findings related to DNA damage may result from the variety of experimental conditions including exposure doses and duration, types of in vitro cells, and e-cigarette brands. More details will be discussed in Section 4.
3.2 |. DNA damage in oral cells: Clinical studies
Pulmonary effects of e-cigarettes in both in vitro and in vivo models as well as investigation of mechanisms of the inflammatory response and oxidative stress are generally the main focuses of current research. There are limited studies comparing DNA damage in oral cells between e-cigarette users and non-users (Table 2).
TABLE 2.
Clinical studies of DNA damage or relevant factors measurement due to electronic cigarette (e-cigarette) exposure
| Oral cell type | DNA damage measurement | Nonsmoker |
e-Cigarette users |
Significance | Reference | ||
|---|---|---|---|---|---|---|---|
| N | Mean/median | N | Mean/median | ||||
| Oral mucosa | Micronuclei | 20 | 0.012 ± 0.0056 (/1000 cells) | 22 | 0.028 ± 0.024 (/1000 cells) | NS | Franco et al. 201629 |
| Saliva | Nʹ-nitrosonornicotine | 19 | 0.25 ± 0.28 pg/ml | 20 | 14.6 ± 23.1 pg/ml | S | Bustamante et al. 201830 |
| Oral epithelium | Aberrantly expressed transcripts and molecular pathway | 27 | 42 | S | Tommasi et al. 201931 | ||
| Oral buccal cells | AP sites | 35 | 6.0 per 107 nt | 30 | 3.3 per 107 nt | S | Guo et al. 202132 |
| Buccal samples | Tumor suppressor TP53 upregulation | 0 | 3 | N/A | Hamad et al. 202133 | ||
| Cytobrush of tongue and mouth | Metanuclear anomalies | 27 |
aKaryolysis: 5 ± 2 aBroken eggs: 0 ± 1 aNuclear bud: 0 ± 1 |
20 |
aKaryolysis: 30 ± 40 aBroken eggs: 2 ±4 aNuclear bud: 4 ±1 |
S | Schwarzmeier et al. 202134 |
| Cytobrush of tongue and mouth | Micronuclei | 27 |
aMicronuclei: 0 ± 2.2 aMicronucleated cells: 0± 2 |
20 |
aMicronuclei: 2.4 ± 0.2 aMicronucleated cells: 2 ±1 |
NS | Schwarzmeier et al. 202134 |
| Buccal mucosa | Micronuclei | 20 | Micronuclei: 1.95 ± 1.05 (/1000 cells) Micronucleated cells: 1.40 ± 0.68 (/1000 cells) |
23 | Micronuclei: 3.21 ± 1.12 (/1000 cells) Micronucleated cells: 2.39 ± 1.07 (/1000 cells) |
S | Pop et al. 202135 |
| Saliva | Lactate dehydrogenase | 30 | 21.45 ± 15.30 mU/ml | 29 | 35.15 ± 24.34 mU/ml | S | Pandarathodiyil et al. 202136 |
| Oral buccal cells | ɣ-OH-Acr-dGuo | 20 | 21 fmol/μmol dGuo | 20 | 179 fmol/μmol dGuo | S | Cheng et al. 202237 |
Abbreviations: AP, apurinic/apyrimidinic; dGuo, deoxyguanosine; N/A, not available; NS, not statistically significant; S, statistically significant; ɣ-OH-Acr-dGuo, (8R/S)-3-(2ʹ-deoxyribos-1ʹ-yl)-5,6,7,8-tetrahydro-8-hydroxypyrimido[1,2-a]purine-10(3H)one.
Estimated from graphs.
3.2.1 |. Acrolein DNA adducts
Acrolein is considered probably carcinogenic to humans (Group 2A) by the International Agency for Research on Cancer (IARC).38 Acrolein reacts with deoxyguanosine (dGuo) to form 1,N2-propano-deoxyguanosine adducts in DNA: (6R/S)-3-(2ʹ-deoxyribos-1ʹ-yl)-5,−6,7,8-tetrahydro-6-hydroxypyrimido[1,2-a]purine-10(3H)one (α-OH-Acr-dGuo) and (8R/S)-3-(2ʹ-deoxyribos-1ʹ-yl)-5,6,7,8-tetrahydro-8-hydroxypyrimido[1,2-a]purine-10(3H)one (ɣ-OH-Acr-dGuo). In a recent study by our group, we used a validated liquid chromatography-nanoelectrospray ionization-high-resolution tandem mass spectrometry method to quantify ɣ-OH-Acr-dGuo in oral buccal cells of e-cigarette users and non-users of any nicotine containing product. e-Cigarette usage was confirmed by analysis of urinary biomarkers (total nicotine equivalents and cyanoethyl mercapturic acid). The level of ɣ-OH-Acr-dGuo was 179 fmol/μmol dGuo (range 5.0–793 fmol/μmol dGuo) in e-cigarette users whereas that in non-users was 21.0 fmol/μmol dGuo (range 5.0–539 fmol/μmol dGuo; p = 0.001).37 Levels of ɣ-OH-Acr-dGuo in oral cells of cigarette smokers were 446 fmol/μmol dGuo (range 158–5830 fmol/μmol dGuo), similar to an earlier study of this adduct in oral cells of smokers, and significantly higher than that in e-cigarette users.39 Although the potential consequences of ɣ-OH-Acr-dGuo with respect to oral pathologies in e-cigarette users remain to be determined, our results present a warning signal. Increased DNA adduct formation from acrolein in the oral cavity could suggest possible elevated cancer risk.
3.2.2 |. Apurinic/apyrimidinic sites in DNA
Carcinogens and toxicants in e-cigarettes and tobacco products may also result in the formation of apurinic/apyrimidinic (AP) sites and initiation of the carcinogenic process. Our group recently optimized a liquid chromatography-nanoelectrospray ionization-high-resolution tandem mass spectrometry method to analyze AP sites in buccal cell DNA of 35 nonsmokers, 30 smokers, and 30 e-cigarette users. AP sites in e-cigarette users (median 3.3 per 107 nt) were significantly lower than in smokers (median 5.7 per 107 nt) and nonsmokers (median 6.0 per 107 nt).32 We hypothesized that propylene glycol in the e-cigarettes may inhibit bacterial growth in oral cells, resulting in reduced inflammation and related effects, and reduced AP site levels in e-cigarette user DNA. AP sites correlated with Nʹ-nitrosonornicotine (NNN) and the related compound 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) exposure in rats.40
3.2.3 |. Other relevant studies
Carcinogens
NNN was detected in the saliva of e-cigarette users (n = 20) by Bustamante et al. with a mean concentration of 14.6 ± 23.1 pg/ml compared with 0.28 ± 0.25 pg/ml in non-users (n = 19).30 NNN and NNK have been classified as Group I human carcinogens by the IARC.41 NNN is a potent oral cavity and esophageal carcinogen in rats treated with 14 ppm of this compound in the drinking water.42 Metabolic activation of NNN and NNK results in formation of reactive electrophiles that modify DNA to produce a variety of products including methyl, 4-(3-pyridyl)-4-oxobutyl (POB), and 4-(3-pyridyl)-4-hydroxybutyl (PHB) adducts that have been previously characterized.42–44 The detection of NNN in saliva may imply the potential formation of DNA adducts and induced DNA damage in e-cigarette users.
Multiple studies of urinary or blood toxicant and carcinogen biomarkers have examined potential carcinogen exposure in e-cigarette users versus non-users of any tobacco or nicotine product, as summarized in three recent reviews.12,45,46 These biomarkers include tobacco-specific nitrosamines, nicotine, PAHs, VOCs, flame retardants, metals, propylene glycol, and their metabolites. These studies suggest potential increased exposure of e-cigarette users to genotoxic carcinogens such as acrolein and acrylonitrile, but the results of biomarker studies published to date are not conclusive.
Micronuclei
Micronuclei were measured as a cancer risk factor.35 Franco et al.29 recruited 65 subjects from three groups—cigarette smokers (n = 23), e-cigarette users (n = 22), and nonsmokers (n = 20)—to examine micronuclei, indicators of genomic instability, in their oral mucosa. Micronuclei were significantly lower comparing e-cigarette users with smokers. No significant difference was found between e-cigarette users and nonsmokers. Pop et al.35 evaluated micronuclei in the oral mucosa of smokers (n = 25) and e-cigarette users (n = 23) compared with nonsmokers (n = 20). The mean micronuclei values and micronucleated cells in smokers and e-cigarette users were both significantly higher than in nonsmokers (p < 0.05). There were no significant differences between smokers and e-cigarette users. These results implied that e-cigarettes have similar cytotoxicity as cigarette smoke.
Schwarzmeier et al.34 investigated cytogenetic and cytotoxic damage through the evaluation of micronuclei and metanuclear anomalies in the oral mucosa of e-cigarette users. They collected oral samples from four groups of people: e-cigarette users (n = 20), smokers (n = 22), former smokers (n = 22), and non-users (n = 27). Micronuclei were higher in smokers than in the other three groups; there was no difference in micronuclei between e-cigarette users and non-users. Metanuclear anomalies in terms of karyolysis, binucleation, broken eggs, and nuclear buds were significantly higher in e-cigarette users compared with non-users. Metanuclear anomalies may relate to chromosomal instability, DNA damage, and cell death.34 These results implied some potential genotoxicity of e-cigarette vapor on oral cells.
Gene regulation
The deregulation of genes involved in crucial cellular functions in humans may result in the onset of cancer. Studies have investigated the regulation of cancer-related genes in oral cells of e-cigarette users to provide insights into the potential biological consequences of exposure to carcinogenic compounds.31,33
Tommasi et al.31 examined the genome-wide regulation of genes and associated molecular pathways in oral cells of e-cigarette users (n = 42), smokers (n = 24), and nonsmokers (n = 27) through RNA-sequencing analysis. Similar to smokers, vapers had deregulation of critically important genes and molecular pathways in the oral epithelium associated with cancer.
Gene regulation after e-cigarette exposure was also explored by Hamad et al.33 They collected buccal and blood samples from three subjects (two males and one female) from three visits. They found that the tumor suppressor gene TP53 was significantly upregulated in buccal samples. TP53 expression was dependent on puff volume and flow rate in both buccal and blood cells.
Enzyme expression
LDH, a cytoplasmic enzyme, catalyzes the conversion of glucose into pyruvic acid during aerobic glycolysis. LDH levels increase as oxidative stress or oxidative damage occurs in the body and thus acts as an indicator of cell damage or cell death.47 Pandarathodiyil et al.36 examined the levels of LDH in the saliva of e-cigarette users (n = 29) and compared the data with cigarette smokers (n = 29) and non-users (n = 30). The mean values for salivary LDH activity levels for e-cigarette users and smokers were significantly higher than the control groups (p < 0.05). There was no significant difference in salivary LDH activity level in e-cigarette users when compared with smokers. This study confirmed the cytotoxic and harmful effects of e-cigarettes on the oral mucosa.
4 |. SUMMARY, DISCUSSION, AND FUTURE RESEARCH
We present both in vitro and clinical studies designed to understand DNA damage and associated adverse effects of e-cigarettes. On one hand, most of the in vitro studies reviewed here demonstrated that, under various conditions, e-cigarette liquid or vapor can induce DNA damage, oxidative stress, and cytotoxicity in different types of oral cells or established oral cell lines. However, two studies focused on gingival-related cells and found that e-cigarettes did not induce significant cytotoxicity when compared with control cells.24,25 On the other hand, the clinical studies showed inconsistent results. The cancer-causing chemical NNN, the acrolein DNA adduct ɣ-OH-Acr-dGuo, metanuclear anomalies, gene regulation, and LDH enzyme expression were significantly higher in e-cigarette users compared with non-users. AP sites were lower in e-cigarette users than non-users. Two studies on micronuclei showed opposite results when comparing e-cigarette users with non-users.29,35
The above review focused on comparing exposures in e-cigarette users and controls. What about the DNA damage and adverse effects of e-cigarettes relative to cigarette smoke? The emissions of some cigarette smoke toxicants from e-cigarettes have been reported either undetectable or only a few percent of those found in cigarette smoke.48 Therefore, the adverse effects of e-cigarette use were generally found less than those of smokers. Salivary levels of NNN in e-cigarette users were dramatically lower than in smokers.30 Acrolein DNA adducts in e-cigarette users were less than 50% of those in the smokers.37 Smokers had ~50% more differentially aberrantly expressed transcripts than e-cigarette users.31 The number of affected targets in the “Rho family GTPases signaling pathway” was three times higher in smokers than in e-cigarette users.31 Micronuclei were also significantly higher in smokers than in e-cigarette users in two studies in which micronuclei were not significantly different between e-cigarette users and non-users.29,34 However, the presence of toxicants in e-cigarette aerosols, even at comparatively low levels, suggests that e-cigarette use is not risk-free.48 Levels of the carbonyls formaldehyde, acetaldehyde, and acrolein can approach those from traditional tobacco cigarettes.8 Eshraghian and Al-Delaimy49 reviewed articles identifying chemicals in e-liquid and aerosols, and 22 of them were found in both e-liquids and aerosols. Some of these such as benzene, chromium, formaldehyde, NNK, and NNN are toxic and carcinogenic. Benzene from e-cigarettes at levels of 100 μg/m3 or higher, as reported in one study, will not be of negligible risk.50
Nicotine and flavors are major components of e-cigarettes. Flavors may play some role in the toxicity of e-cigarettes. Fruit-flavored e-liquids (FLs) showed higher levels of toxicity than tobacco-flavored e-liquids (TL) in a study by Welz et al.19 FLs also induced more serious DNA damage than TL. Other studies also found flavored e-liquids caused more harmful effects than unflavored ones.13,18,26 To reduce the popularity of e-cigarettes among youth, the U.S. Food and Drug Administration, in January 2020, issued a policy prioritizing enforcement prohibiting the sale of any flavored, cartridge-based e-cigarette product other than a tobacco-or menthol-flavored product.51 There is controversy about the role which nicotine may play in e-cigarettes with respect to DNA damage. The nicotine content of e-cigarettes and traditional cigarettes is similar, and we have detected similar concentrations of urinary cotinine, the main nicotine metabolite, in e-cigarette users and smokers.32 In the in vitro oral cell exposure studies reviewed here, Welz et al. found that in epithelial cells, short-term treatment induces up to a 5-fold increase in cell death without nicotine and up to a 10-fold increase with nicotine as compared with untreated controls (p < 0.001).19 However, other human oral cell studies found similar toxic effects of nicotine-containing and nicotine-free e-cigarettes.17,20,26
There are limitations to the studies cited in this review. Only a small body of literature was identified that was relevant to e-cigarettes and DNA damage in oral cells. The studies in this review used different methods, and different brands and different concentrations of e-cigarette liquid or vapor. Toxic effects are expected to be dose dependent, and the relationship of doses used in the in vitro studies to normal human exposure amounts is uncertain. Exposure times also varied among the studies. The techniques used to generate e-cigarette aerosols may also bias the results. Ureña et al. found that the age of the atomizer influenced the tested toxicities of e-cigarette aerosols.23 The adverse effects of e-cigarettes may also vary from brand to brand, and some brands seem have higher toxic effects than others.23 e-Cigarette power will also affect emissions. The reaction of oral cavity cells in specific locations relative to the e-cigarette exposure may also differ. Sample preparation and sample analysis methods also varied among the studies. In addition, most of the studies examined the overall effects of mixed components of e-cigarettes. It is hard to conclude which toxicants or carcinogens may contribute to the observed DNA damage. The relatively small sample size of each study group in the clinical studies is also a limitation (mostly n < 30; Table 2).
Tobacco smoking is a well-established risk factor for the development of head and neck cancer.13 The health effects of e-cigarette research are still lagging relative to the popularity of these products with consumers. There is not enough evidence to support the carcinogenicity of e-cigarettes.13 One study reported that a young adult was diagnosed with HPV-negative SCC of the oral cavity that was rapidly progressive and fatal. This person had an extensive history of vaping using nicotine-delivery systems.52 Nguyen et al. have reported two cases of oral cancer in chronic exclusive e-cigarette users with no other apparent risk factors.53 There is also evidence suggesting that e-cigarettes may play active roles in the pathogenesis of other malignancies such as lung and bladder cancers.13 However, animal models regarding carcinogenesis of e-cigarettes to the oral cavity are still lacking. Additional biomarkers indicating the early onset of cancer are also required to better understand the effects of e-cigarettes.
Future studies should move on from in vitro to in vivo studies and directly compare effects of tobacco smoke with e-cigarette aerosol. In addition, more thoroughly designed prospective cohort studies with larger sample sizes and longer periods of observation are necessary to establish the safety of e-cigarettes. Future research must also examine a wide variety of e-cigarettes. The relation between single toxicants in e-cigarettes with specific DNA damage also requires further investigation. There is an urgent need to educate health professionals about the potential detrimental effects of e-cigarettes on the oral cavity and to encourage them to recommend safer smoking cessation aids until more research is conducted.13
ACKNOWLEDGEMENTS
This study was supported by grant numbers CA-203851 and CA-259652 from the U.S. National Cancer Institute (NIH) and the Food and Drug Administration Center for Tobacco Products. The content is solely the responsibility of the authors and does not necessarily represent the views of the NIH or the Food and Drug Administration. We thank Bob Carlson for editorial support.
Funding information
U.S. National Cancer Institute (NIH), Grant/Award Numbers: CA-259652, CA-203851; Food and Drug Administration Center for Tobacco Products
REFERENCES
- 1.Szumilas P, Wilk A, Szumilas K, Karakiewicz B. The effects of e-cigarette aerosol on oral cavity cells and tissues: a narrative review. Toxics 2022;10(2):74. doi: 10.3390/toxics10020074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Brien D, Long J, Quigley J, Lee C, McCarthy A, Kavanagh P. Association between electronic cigarette use and tobacco cigarette smoking initiation in adolescents: a systematic review and meta-analysis. BMC Public Health 2021;21(1):954. doi: 10.1186/s12889-021-10935-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hecht SS, Carmella SG, Kotandeniya D, et al. Evaluation of toxicant and carcinogen metabolites in the urine of e-cigarette users versus cigarette smokers. Nicotine Tob Res 2015;17(6):704–709. doi: 10.1093/ntr/ntu218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma B, Stepanov I, Hecht SS. Recent studies on DNA adducts resulting from human exposure to tobacco smoke. Toxics 2019;7(1):16. doi: 10.3390/toxics7010016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hecht SS, Hatsukami DK. Smokeless tobacco and cigarette smoking: chemical mechanisms and cancer prevention. Nat Rev Cancer 2022; 22(3):143–155. doi: 10.1038/s41568-021-00423-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hecht SS. Tobacco smoke carcinogens and lung cancer. J Natl Cancer Inst 1999;91(14):1194–1210. doi: 10.1093/jnci/91.14.1194 [DOI] [PubMed] [Google Scholar]
- 7.Hecht SS. Oral cell DNA adducts as potential biomarkers for lung cancer susceptibility in cigarette smokers. Chem Res Toxicol 2017;30(1): 367–375. doi: 10.1021/acs.chemrestox.6b00372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Margham J, McAdam K, Cunningham A, et al. The chemical complexity of e-cigarette aerosols compared with the smoke from a tobacco burning cigarette. Front Chem 2021;9:743060. doi: 10.3389/fchem.2021.743060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Szukalska M, Szyfter K, Florek E, et al. Electronic cigarettes and head and neck cancer risk—current state of art. Cancers (Basel) 2020; 12(11):3274. doi: 10.3390/cancers12113274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang I, Sandeep S, Rodriguez J. The oral health impact of electronic cigarette use: a systematic review. Crit Rev Toxicol 2020;50(2):97–127. doi: 10.1080/10408444.2020.1713726 [DOI] [PubMed] [Google Scholar]
- 11.Wilson C, Tellez Freitas CM, Awan KH, Ajdaharian J, Geiler J, Thirucenthilvelan P. Adverse effects of e-cigarettes on head, neck, and oral cells: a systematic review. J Oral Pathol Med 2022;51(2): 113–125. doi: 10.1111/jop.13273 [DOI] [PubMed] [Google Scholar]
- 12.Hiler M, Weidner A-S, Hull LC, Kurti AN, Mishina EV. Systemic biomarkers of exposure associated with ends use: a scoping review. Tob Control 2021;tobaccocontrol-2021-056896. doi: 10.1136/tobaccocontrol-2021-056896 [DOI] [PubMed] [Google Scholar]
- 13.Flach S, Maniam P, Manickavasagam J. E-cigarettes and head and neck cancers: a systematic review of the current literature. Clin Otolaryngol 2019;44(5):749–756. doi: 10.1111/coa.13384 [DOI] [PubMed] [Google Scholar]
- 14.Raj AT, Sujatha G, Muruganandhan J, et al. Reviewing the oral carcinogenic potential of e-cigarettes using the Bradford Hill criteria of causation. Transl Cancer Res 2020;9(4):3142–3152. doi: 10.21037/tcr.2020.01.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ji EH, Sun B, Zhao T, et al. Characterization of electronic cigarette aerosol and its induction of oxidative stress response in oral keratinocytes. PLoS ONE 2016;11(5):e0154447. doi: 10.1371/journal.pone.0154447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rouabhia M, Park HJ, Semlali A, Zakrzewski A, Chmielewski W, Chakir J. E-cigarette vapor induces an apoptotic response in human gingival epithelial cells through the caspase-3 pathway. J Cell Physiol 2017;232(6):1539–1547. doi: 10.1002/jcp.25677 [DOI] [PubMed] [Google Scholar]
- 17.Sancilio S, Gallorini M, Cataldi A, di Giacomo V. Cytotoxicity and apoptosis induction by e-cigarette fluids in human gingival fibroblasts. Clin Oral Investig 2016;20(3):477–483. doi: 10.1007/s00784-015-1537-x [DOI] [PubMed] [Google Scholar]
- 18.Sundar IK, Javed F, Romanos GE, Rahman I. E-cigarettes and flavorings induce inflammatory and pro-senescence responses in oral epithelial cells and periodontal fibroblasts. Oncotarget 2016;7(47): 77196–77204. doi: 10.18632/oncotarget.12857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Welz C, Canis M, Schwenk-Zieger S, et al. Cytotoxic and genotoxic effects of electronic cigarette liquids on human mucosal tissue cultures of the oropharynx. J Environ Pathol Toxicol Oncol 2016;35(4): 343–354. doi: 10.1615/JEnvironPatholToxicolOncol.2016016652 [DOI] [PubMed] [Google Scholar]
- 20.Yu V, Rahimy M, Korrapati A, et al. Electronic cigarettes induce DNA strand breaks and cell death independently of nicotine in cell lines. Oral Oncol 2016;52:58–65. doi: 10.1016/j.oraloncology.2015.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ganapathy V, Manyanga J, Brame L, et al. Electronic cigarette aerosols suppress cellular antioxidant defenses and induce significant oxidative DNA damage. PLoS ONE 2017;12(5):e0177780. doi: 10.1371/journal.pone.0177780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun Y-W, Kosinska W, Guttenplan JB. E-cigarette aerosol condensate enhances metabolism of benzo(a)pyrene to genotoxic products, and induces CYP1A1 and CYP1B1, likely by activation of the aryl hydrocarbon receptor. Int J Environ Res Public Health 2019;16(14):2468. doi: 10.3390/ijerph16142468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ureña JF, Ebersol LA, Silakov A, Elias RJ, Lambert JD. Impact of atomizer age and flavor on in vitro toxicity of aerosols from a third-generation electronic cigarette against human oral cells. Chem Res Toxicol 2020;33(10):2527–2537. doi: 10.1021/acs.chemrestox.0c00028 [DOI] [PubMed] [Google Scholar]
- 24.Vermehren MF, Wiesmann N, Deschner J, Brieger J, Al-Nawas B, Kämmerer PW. Comparative analysis of the impact of e-cigarette vapor and cigarette smoke on human gingival fibroblasts. Toxicol in Vitro 2020;69:105005. doi: 10.1016/j.tiv.2020.105005 [DOI] [PubMed] [Google Scholar]
- 25.El-Mouelhy ATM, Nasry SA, Abou El-Dahab O, Sabry D, Fawzy El-Sayed K. In vitro evaluation of the effect of the electronic cigarette aerosol, cannabis smoke, and conventional cigarette smoke on the properties of gingival fibroblasts/gingival mesenchymal stem cells. J Periodontal Res 2022;57(1):104–114. doi: 10.1111/jre.12943 [DOI] [PubMed] [Google Scholar]
- 26.Tellez CS, Juri DE, Phillips LM, et al. Cytotoxicity and genotoxicity of e-cigarette generated aerosols containing diverse flavoring products and nicotine in oral epithelial cell lines. Toxicol Sci 2021;179(2):220–228. doi: 10.1093/toxsci/kfaa174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramenzoni LL, Schneider A, Fox SC, et al. Cytotoxic and inflammatory effects of electronic and traditional cigarettes on oral gingival cells using a novel automated smoking instrument: an in vitro study. Toxics 2022;10(4):179. doi: 10.3390/toxics10040179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anderson C, Majeste A, Hanus J, Wang S. E-cigarette aerosol exposure induces reactive oxygen species, DNA damage, and cell death in vascular endothelial cells. Toxicol Sci 2016;154(2):332–340. doi: 10.1093/toxsci/kfw166 [DOI] [PubMed] [Google Scholar]
- 29.Franco T, Trapasso S, Puzzo L, Allegra E. Electronic cigarette: role in the primary prevention of oral cavity cancer. Clin Med Insight 2016;9: 7–12. PMID: CMENT.S40364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bustamante G, Ma B, Yakovlev G, et al. Presence of the carcinogen Nʹ-nitrosonornicotine in saliva of e-cigarette users. Chem Res Toxicol 2018;31(8):731–738. doi: 10.1021/acs.chemrestox.8b00089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tommasi S, Caliri AW, Caceres A, et al. Deregulation of biologically significant genes and associated molecular pathways in the oral epithelium of electronic cigarette users. Int J Mol Sci 2019;20(3):738. doi: 10.3390/ijms20030738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo J, Ikuemonisan J, Hatsukami DK, Hecht SS. Liquid chromatography-nanoelectrospray ionization-high-resolution tandem mass spectrometry analysis of apurinic/apyrimidinic sites in oral cell DNA of cigarette smokers, e-cigarette users, and nonsmokers. Chem Res Toxicol 2021;34(12):2540–2548. doi: 10.1021/acs.chemrestox.1c00308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hamad SH, Brinkman MC, Tsai Y-H, et al. Pilot study to detect genes involved in DNA damage and cancer in humans: potential biomarkers of exposure to e-cigarette aerosols. Gene 2021;12(3):448. doi: 10.3390/genes12030448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwarzmeier LÂT, da Cruz BS, Ferreira CCP, et al. E-cig might cause cell damage of oral mucosa. Oral Surg Oral Med Oral Pathol Oral Radiol 2021;131(4):435–443. doi: 10.1016/j.oooo.2020.11.009 [DOI] [PubMed] [Google Scholar]
- 35.Pop AM, Coros R, Stoica AM, Monea M. Early diagnosis of oral mucosal alterations in smokers and e-cigarette users based on micronuclei count: a cross-sectional study among dental students. Int J Environ Res Public Health 2021;18(24):13246. doi: 10.3390/ijerph182413246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pandarathodiyil AK, Ramanathan A, Garg R, et al. Lactate dehydrogenase levels in the saliva of cigarette and e-cigarette smokers (vapers): a comparative analysis. Asian Pac J Cancer Prev 2021;22(10):3227–3235. doi: 10.31557/APJCP.2021.22.10.3227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng G, Guo J, Carmella SG, et al. Increased acrolein-DNA adducts in buccal brushings of e-cigarette users. Carcinogenesis 2022;43(5): 437–444. doi: 10.1093/carcin/bgac026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marques MM, Beland FA, Lachenmeier DW, et al. Carcinogenicity of acrolein, crotonaldehyde, and arecoline. Lancet Oncol 2021;22(1):19–20. doi: 10.1016/S1470-2045(20)30727-0 [DOI] [PubMed] [Google Scholar]
- 39.Paiano V, Maertens L, Guidolin V, Yang J, Balbo S, Hecht SS. Quantitative liquid chromatography-nanoelectrospray ionization-high-resolution tandem mass spectrometry analysis of acrolein-DNA adducts and etheno-DNA adducts in oral cells from cigarette smokers and nonsmokers. Chem Res Toxicol 2020;33(8):2197–2207. doi: 10.1021/acs.chemrestox.0c00223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guo J, Chen H, Upadhyaya P, Zhao Y, Turesky RJ, Hecht SS. Mass spectrometric quantitation of apurinic/apyrimidinic sites in tissue DNA of rats exposed to tobacco-specific nitrosamines and in lung and leukocyte DNA of cigarette smokers and nonsmokers. Chem Res Toxicol 2020;33(9):2475–2486. doi: 10.1021/acs.chemrestox.0c00265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.International Agency for Research on Cancer (IARC). Agents classifed by the IARC monographs, volumes 1–132 2012; https://monographs.iarc.who.int/list-of-classifications. Accessed August 24, 2022.
- 42.Zhao L, Balbo S, Wang M, et al. Quantitation of pyridyloxobutyl-DNA adducts in tissues of rats treated chronically with (R)- or (S)-Nʹ-nitrosonornicotine (NNN) in a carcinogenicity study. Chem Res Toxicol 2013;26(10):1526–1535. doi: 10.1021/tx400235x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lao Y, Villalta PW, Sturla SJ, Wang M, Hecht SS. Quantitation of pyridyloxobutyl DNA adducts of tobacco-specific nitrosamines in rat tissue DNA by high-performance liquid chromatography-electrospray ionization-tandem mass spectrometry. Chem Res Toxicol 2006;19(5): 674–682. doi: 10.1021/tx050351x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang S, Wang M, Villalta PW, Lindgren BR, Lao Y, Hecht SS. Quantitation of pyridyloxobutyl DNA adducts in nasal and oral mucosa of rats treated chronically with enantiomers of Nʹ-nitrosonor-nicotine. Chem Res Toxicol 2009;22(5):949–956. doi: 10.1021/tx900040j [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Akiyama Y, Sherwood N. Systematic review of biomarker findings from clinical studies of electronic cigarettes and heated tobacco products. Toxicol Rep 2021;8:282–294. doi: 10.1016/j.toxrep.2021.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bjurlin MA, Matulewicz RS, Roberts TR, et al. Carcinogen biomarkers in the urine of electronic cigarette users and implications for the development of bladder cancer: a systematic review. Eur Urol Oncol 2021;4(5):766–783. doi: 10.1016/j.euo.2020.02.004 [DOI] [PubMed] [Google Scholar]
- 47.Narang AP, Greval RS, Chopra H, Kalra CS. The role of two enzymes (LDH and PHI) and a tumour marker (CEA) in the prognostic evaluation of head and neck malignancy. Indian J Otolaryngol Head Neck Surg 2001;53(1):76–80. doi: 10.1007/BF02910990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Margham J, McAdam K, Forster M, et al. Chemical composition of aerosol from an e-cigarette: a quantitative comparison with cigarette smoke. Chem Res Toxicol 2016;29(10):1662–1678. doi: 10.1021/acs.chemrestox.6b00188 [DOI] [PubMed] [Google Scholar]
- 49.Eshraghian EA, Al-Delaimy WK. A review of constituents identified in e-cigarette liquids and aerosols. Tob Prev Cessat 2021;7(February): 10–10, 15. doi: 10.18332/tpc/131111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pankow JF, Kim K, McWhirter KJ, et al. Benzene formation in electronic cigarettes. PLoS ONE 2017;12(3):e0173055. doi: 10.1371/journal.pone.0173055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.United States Food and Drug Administration. FDA finalizes enforcement policy on unauthorized flavored cartridge-based e-cigarettes that appeal to children, including fruit and mint 2020. https://www.fda.gov/news-events/press-announcements/fda-finalizes-enforcement-policy-unauthorized-flavored-cartridge-based-e-cigarettes-appeal-children. Accessed August 22, 2022.
- 52.Klawinski D, Hanna I, Breslin NK, Katzenstein HM, Indelicato DJ. Vaping the venom: oral cavity cancer in a young adult with extensive electronic cigarette use. Pediatrics 2021;147(5):e2020022301. doi: 10.1542/peds.2020-022301 [DOI] [PubMed] [Google Scholar]
- 53.Nguyen H, Kitzmiller JP, Nguyen KT, Nguyen CD, Bui TC. Oral carcinoma associated with chronic use of electronic cigarettes. Otolaryngology 2017;7(2):3. doi: 10.4172/2161-119X.1000304 [DOI] [Google Scholar]


