Abstract
Purpose of Review
Discoid lateral meniscus (DLM) is a well-known meniscus variant, and comprises excess and thickened meniscal tissue, altered collagen ultrastructure, and peripheral instability. This article presents a comprehensive review on current knowledge of DLM, focusing on pathology in parallel with surgical techniques and outcomes.
Recent Findings
A paradigm shift in surgical management of DLM is taking place as knee surgeons are seeing more patients with long-term sequelae of partial lateral meniscectomy, the standard treatment for DLM for many years. Surgical treatment has evolved alongside the understanding of DLM pathology. A new classification system has been proposed and optimal surgical techniques described in recent years. This article highlights up-to-date evidence and techniques in management of both acute DLM tears and joint restoration following subtotal meniscectomy for DLM.
Summary
Surgical management of DLM must be tailored to individual pathology, which is variable within the diagnosis of DLM. We present an algorithm for management of DLM and discuss future directions for the understanding and treatment of this debilitating condition.
Keywords: Discoid, Meniscal repair, Meniscal transplantation, Knee preservation
Introduction
Discoid meniscus is a common meniscus variant that can become symptomatic at any age. The true prevalence is unknown as discoid menisci are often asymptomatic [1•, 2]. Estimated as present in 4–5% of the population [3], discoid lateral meniscus (DLM) is not yet fully understood in both etiology and comprehensive pathology. Epidemiologic data has shown incidence of symptomatic DLM as 3.2 per 100,000 person-years [1•]. As a result, management strategies are widely variable and outcomes unreliable. First described in 1889 [4], “discoid meniscus” refers to thickened meniscal tissue with abnormal mobility/peripheral stability [5–8]. The pathology can be simplified into three “S’s” of the discoid meniscus: Size, Stability and microscopic Structure. Discoid menisci are hyperthickened (Size). They often have compromised peripheral Stability. Lastly, discoid menisci have abnormal collagen (Structure). These all play a role in the clinical challenges posed by the DLM.
Classification
The Watanabe classification system is the most frequently utilized in the literature [9]. The Watanabe classification describes three types of DLM: complete being full disk morphology covering the entire tibial plateau, incomplete being increased width but still in semilunar shape, and Wrisberg ligament variant being normal or thickened lateral meniscus that is lacking peripheral attachments of the posterior horn (coronary ligaments), stabilized posteriorly only by the Wrisberg meniscofemoral ligament. A more recent classification system has been described in light of possible overlap between complete/incomplete and Wrisberg ligament type menisci: specifically that the meniscus can be thickened while also having peripheral instability. This classification system was established by the Pediatric Research in Sports Medicine (PRiSM) organization and subclassifies based on four main elements of DLM: meniscal width, meniscal height, peripheral stability, and meniscal tear. Width is classified as W0 to W2 (normal to near complete > 90% of plateau coverage respectively). Height is H0 or H1 (normal height or thicker than normal height, respectively). Stability is classified as S0 describing no instability, SA anterior half instability, SP posterior half instability, and SAP with both. Regarding tear classification, T0 denotes no tear. THA/THP describe horizontal tears in anterior or posterior meniscus. THAP describes a horizontal tear throughout anterior and posterior tissue. TDA/TDP/TDAP is the same anatomic locations with degenerative/complex/radial tearing [10]. While descriptive, no classification system to date has been able to prognosticate natural history or surgical outcomes.
Natural History
Incidence of contralateral DLM has been reported from 72% [11, 12] to 97% [13]. It has been reported that 17% of patients after surgery for DLM will ultimately undergo surgery on the contralateral knee [11]. Additionally, DLM does seem to does seem to have a variable association with osteochondritis dissecans of the lateral femoral condyle [14–18].
Pathophysiology
The presence of thickened meniscal tissue is congenital [4], however the underlying reason for peripheral instability associated with the DLM is not yet clear. Theories include congenital absence of coronary ligaments, or peripheral ligament failure from repetitive loading and excessive shear stress incurred by the thickened meniscus. It is possible that both conditions exist.
In addition to the morphological abnormalities of thickening and peripheral instability, the structural integrity of the DLM differs from the normal meniscus. The collagen has been found to be both quantitatively and qualitatively inferior to non-discoid lateral menisci [19••, 20••, 21]. In a histological study, circumferential fibers in DLM were distorted with significant myxoid degeneration, voids, and metaplasia, as well as heterogeneic organization, most markedly affecting the posterior horn compared to the homogeneous collagen organization in non-discoid menisci [20••]. It is not yet clear whether the thickened yet brittle meniscus leads to peripheral instability from increased shear stress at the meniscocapsular junction, or if the peripheral meniscocapsular insufficiency leads to hypermobility and increased tearing rates of the DLM. This abnormal microstructure and collagen may portend inferior outcomes when standard meniscal surgical treatments are applied to this non-standard tissue.
Clinical Presentation
Presentation of DLM varies and may not present until adulthood. Clinical awareness should be present in the setting of a child with a flexion contracture, atraumatic lateral pain, or meniscus tears resulting from relatively low energy injuries. Patients typically present with lateral knee pain with or without mechanical symptoms. Physical exam comprises standard evaluation of the entire lower extremity with meniscal testing. In the case of an unstable DLM, an audible and or visible clunk may result during range of motion testing. If the DLM is fixed in a displaced position or very large, it may cause a motion limiting block. The presence of swelling is common but not always present. Lateral joint line tenderness is the most typical physical exam finding.
Imaging
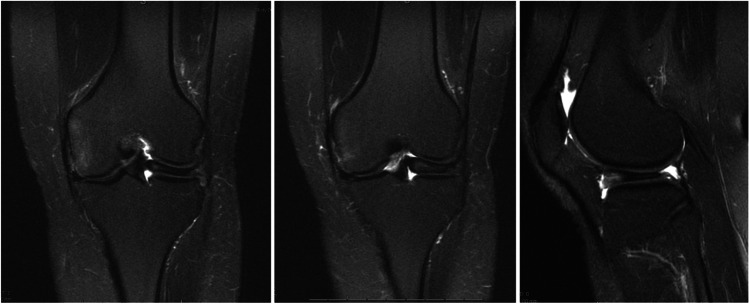
Plain radiographs may demonstrate squaring of the lateral femoral condyle, concavity of the lateral tibial plateau, increased lateral joint space, and/or hypoplasia of the lateral tibial spine. The presence of all four findings carries a 76% positive predictive value for DLM in children aged 10–16 years of age [22]. However, a normal x-ray is often the case and should not rule out the presence of a DLM (Fig. 1). Magnetic resonance imaging (MRI) will show a universal, yet variable, amount of excess meniscus tissue. As described earlier, the DLM may also show thickening in the form of increased height either centrally or throughout. There is often increased intrasubstance signal within the meniscus. This is often mistaken for a horizontal cleavage tear as it appears as a horizontal increased T2 signal within the meniscus, but more likely represents the increased water content and myxoid degeneration characterized in DLM (Fig. 2). The presence of tears and/or peripheral instability is also variable and requires thorough evaluation of sagittal and coronal series).
Fig. 1.
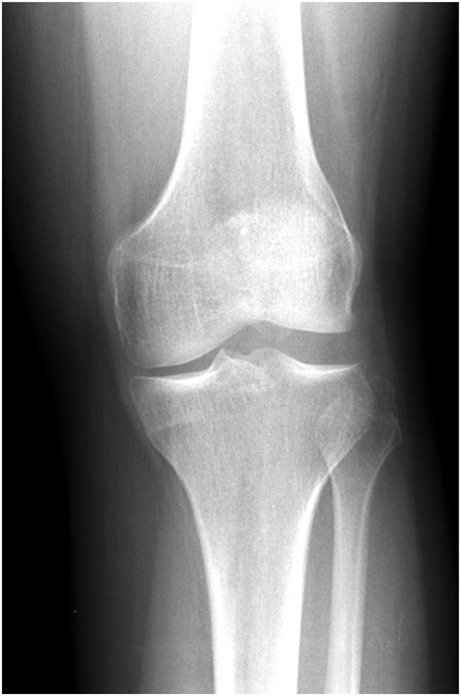
Anteroposterior radiograph of the left knee of a skeletally mature patient demonstrating all four radiographic findings of DLM: squaring of the lateral femoral condyle, concavity of the lateral tibial plateau, increased lateral joint space, and hypoplasia of the lateral tibial spine
Fig. 2.
MRI T2 coronal × 2 and sagittal images demonstrating a skeletally mature patient with DLM. Increased T2 signal seen within the meniscal tissue as a horizontal strip represents intrasubstance degeneration with myxoid tissue. This is seen most clearly on T2 images due to increased water content due to structural dysregulation within the meniscal tissue. Hypoplasia of the lateral femoral condyle is also appreciated
When to Treat
Surgical intervention for DLM has the following indications: symptomatic tears, symptomatic instability and persistent pain with or without mechanical symptoms (i.e. flexion contracture in a child). Pain without tearing or instability should be elucidated from more common causes of knee pain such as patellofemoral pain or distal iliotibial band syndrome. These patients may require a course of physical therapy, steroid injections, etc. prior to surgery to rule out other causes of pain before surgical intervention is warranted. The presence of DLM alone without symptoms does not warrant surgical intervention, however the patient should be counseled regarding risk of tearing if incidentally observed on imaging and/ or for the contralateral knee.
Surgical Techniques
Surgical techniques are varied and outcomes unreliable due to altered tissue quality and meniscal hypermobility. Surgical techniques have advanced alongside our understanding of discoid pathology. Complete excision with total meniscectomy was reported from the 1960s to the 1980s [23], with poor long-term outcomes [24, 25] and is something that should be avoided if at all possible. Repair was first described in 1990 [26] and this has become an important element of management of DLM. Arthroscopic management is universal at this point with general principles of creating a normal, or more normal, volume of meniscus tissue, repairing tears, and restoring stability with the goal of maximizing durability and joint preservation.
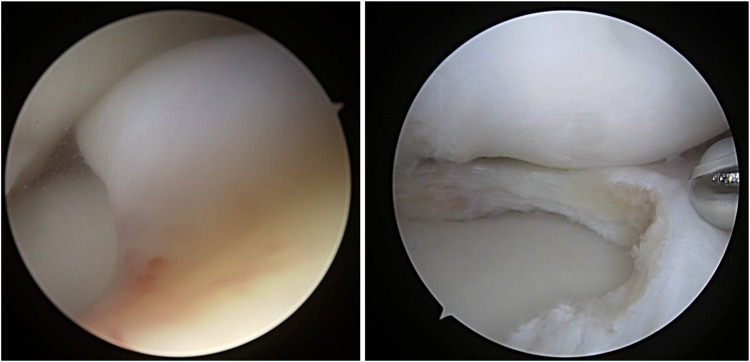
Intrasubstance degeneration (ID) is an important factor for the operating surgeon to consider in management of DLM. ID will typically be revealed once saucerization is begun and the inner substance of the meniscus is exposed. This abnormal tissue is well demonstrated in arthroscopic images in Fig. 3. ID is often misinterpreted as a horizontal cleavage tear on both MRI and intraoperatively, however it is important to recognize this as treatment is different than standard degenerative cleavage tears. Techniques are described in the next section followed by outcomes.
Fig. 3.
Arthroscopic images of a left knee demonstrating near complete coverage of the lateral plateau followed by saucerization revealing myxoid ID tissue (same knee as MRI images in Fig. 2)
Saucerization
The goal of saucerization to preserve more meniscal width than previously thought: retaining 10 to 15 mm of tissue [27, 28••] as opposed to the classic guidelines of 6–8 mm [29]. Yamasaki et al. assessed 45 knees with DLM undergoing saucerization with repair as indicated and identified an inverse correlation between residual meniscal width and functional outcomes and joint preservation, with 5 mm of residual meniscal width being the threshold for radiographic degenerative changes at mean 40 months postoperatively [30••]. Additionally, several studies have shown that meniscal width decreases significantly in the first months to years postoperative from DLM surgery [31, 32]. Smaller residual meniscal width postoperatively has also been associated with extrusion of the lateral meniscus at 2 years [33]. A separate study reports meniscal width in non-discoid menisci to be 12–15 mm [28••]. These findings suggest that a larger meniscal width than previously thought should be maintained when saucerizing DLM.
Repair
The operating surgeon should be prepared to repair when operating on DLM. As discussed, ID will be unveiled as saucerization is performed and the DLM will often appear as a superior and inferior leaflet. Surgical options are to resect one or both leaflets, or to repair. This will depend on the tissue quality and volume as determined by the operating surgeon. Techniques for repair have been described that take into account the altered ultrastructure and biomechanics of the DLM [34, 35••].
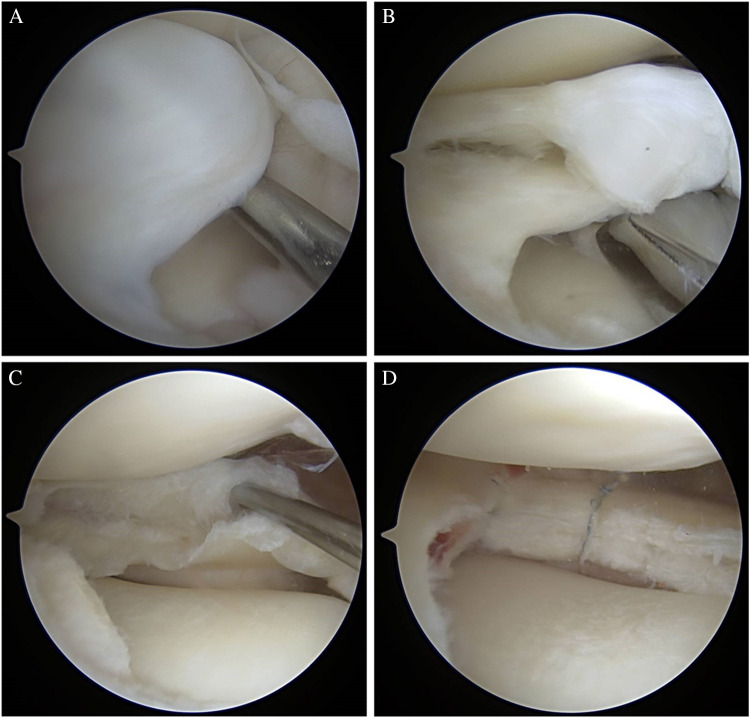
Optimal technique comprises first arthroscopic assessment of the meniscus for tissue quality, thickness, and stability. Next, limited saucerization is performed leaving the aforementioned 10–15 mm of meniscal width. At this point, examination of the residual meniscal tissue will allow the surgeon to identify ID and intrasubstance tearing as well as any peripheral instability. Once the presence and extent of ID has been established, any loose or myxoid tissue is debrided and the leaflets are probed for repair potential. If a cleavage plane is present from either horizontal tear or compromised ID tissue, repair should be performed to bring the two leaflets together. A far medial accessory portal can be useful in accessing the anterior half of the meniscus as described by a senior author of this article [35••]. Following debridement of any myxoid tissue, trephination within the seam of the tear to promote capsular bleeding, and freshening of the tear edges with a rasp is performed ahead of repair. Nonabsorbable suture (0, 2–0, or small tape) is placed circumferentially around the meniscus in haybale fashion using an all-inside meniscal based suture passing device (Scorpion — Arthrex, Naples, FL; Novostitch — Smith & Nephew, Andover, MA), compressing the superior and inferior leaflets together. Repair sutures are placed 4–5 mm apart to maximize compressive forces. Figure 4 demonstrates an example of this repair technique.
Fig. 4.
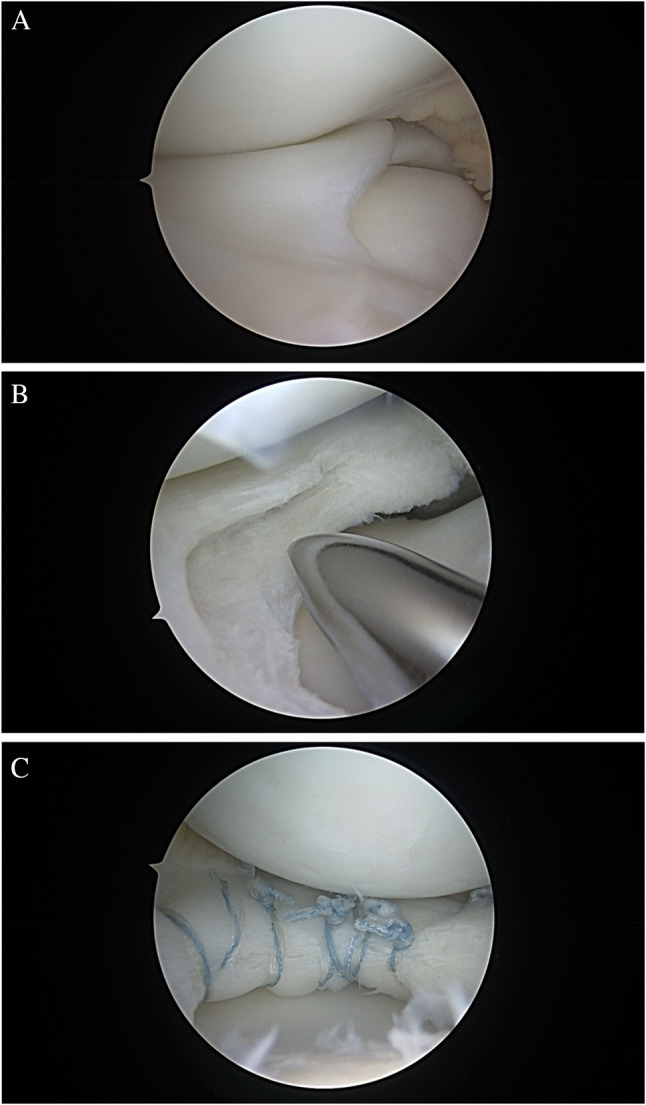
A, B, C: Arthroscopic images of a right knee DLM saucerization and repair. Image A: discoid tissue without obvious external tearing. Image B: following saucerization with ID revealed, with similar appearance to horizontal cleavage tear. Image C: following all-inside haybale suture placement
All inside capsular based repair devices (ex. Fiberstitch — Arthrex, Naples, FL; Fast-Fix Smith & Nephew, Andover, MA) can also be used. If peripheral instability is present following intrasubstance repair as above, then meniscocapsular repair may be required as well, or alternatively capsular-based repair techniques can be utilized for intrasubstance repair (Fig. 5). This can be performed with all-inside meniscocapsular anchor implants, all inside meniscal based suture passing devices or inside-out/outside-in per surgeon preference. Lastly, biological augmentation commonly performed via marrow venting is recommended following meniscal repair to improve healing. This can be performed in the intercondylar notch or using the accessory far medial portal into the medial non-articular cortex of the medial femoral condyle. The authors’ preferred technique involves marrow venting using a microfracture awl or motorized awl on the medial aspect of the medial femoral condyle using the far medial accessory portal created for repair. Utilizing these DLM-specific techniques will maximize residual tissue volume, quality, and stability.
Fig. 5.
A–D: arthroscopic images of a right knee. (A) DLM with full coverage of the lateral tibial plateau. (B–C) ID revealed as sauceration is performed. (D) all-inside capsular-based anchors being placed with similar compression of leaflets to Fig. 4 images. The difference in this case is the repair technique with capsular-based repair stitches in this figure and all-meniscal suture without capsular inclusion in Fig. 4
Outcomes
Degenerative changes have been widely described following surgical treatment of DLM [23, 25, 36••, 37] with progression to symptomatic lateral compartment osteoarthritis in 50% of knees 8 years following surgical treatment [36••]. Further, 59% of patients experienced retear by the same time point [36••]. Partial lateral meniscectomy or saucerization alters the limb axis, with slight progression in valgus alignment occurring after saucerization for DLM in patients with normal mechanical axes [38•]. In treating individuals with baseline valgus alignment, these changes must be taken into account when treating DLM. A large series of 419 knees undergoing surgery (saucerization + / − repair) for DLM revealed a 17% reoperation rate at median 20 months postoperative, with 94% of reoperations performed for retear [39]. Reviewing outcomes of repair, it is difficult to elucidate the durability of meniscal repair for DLM compared to saucerization alone, as repair is performed at the surgeon’s discretion and is not always indicated. Studies comparing partial meniscectomy/saucerization with or without repair therefore yields similar outcomes between the groups [39–42].
Managing the long-term complications of chondral disease, meniscal deficiency, and possible valgus deformity requires thorough evaluation and discussion with the patient and their family. Multiple procedures are frequently required. Goals are to restore the knee joint as much as possible, which may require meniscal transplantation, cartilage preservation procedures, and/ or osteotomy to correct alignment and offload the lateral compartment. Meniscal allograft transplantation (MAT) has been described in the setting of subtotal lateral meniscectomy for DLM [43–46].
While controversial, MAT as soon as meniscal deficiency is identified, even prior to symptoms, has been recommended [43] and is often the practice of a senior author on this paper (JLP). Early MAT is associated with superior outcomes [23] and is cost-effective to delay arthroplasty [47]. Improved pain and function have been widely described for MAT in the setting of failed arthroscopic treatment of DLM [48–52]. However, even with salvage procedures such as MAT, while improved compared to preoperative function, outcomes are still inferior when performed for DLM as compared to non-discoid lateral meniscus [49, 50]. It is theorized that the bony anatomy formed alongside DLM (condylar prominence and squaring) may contribute to these differences.
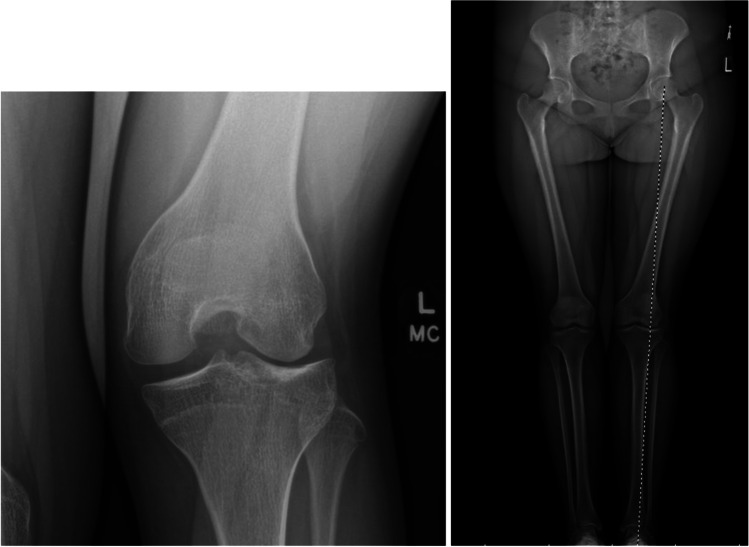
Osteotomy, typically at the distal femur, may also be required if progressive valgus deformity has developed and/or to offload the lateral compartment for any cartilage procedures. Limited long-term data has been described for both MAT and osteotomy, but studies assessing these procedures specifically following DLM meniscectomy show good clinical outcomes [53, 54]. The case in Fig. 6 demonstrates a 15-year-old patient status post subtotal lateral meniscectomy for DLM, now with 6 degrees valgus deformity and meniscal deficiency.
Fig. 6.
AP and standing alignment radiographs of an adolescent patient s/p subtotal lateral meniscectomy for DLM, now with 6 degrees valgus deformity
Treatment Algorithm
In brief, surgical treatment is recommended for symptomatic DLM as follows:
Intraoperatively, the surgeon should first perform limited saucerization with 10–15 mm residual width.
Next, one should assess ID and repair with haybale technique (can be all-meniscal based with suture passing device and knots, or capsular based with anchor-based suture implants, depending on peripheral stability).
Following intrasubstance repair, assess periphery and augment repair to capsule if needed.
Separately, in a patient presenting status post partial meniscectomy with ongoing symptoms, standard radiographs including standing alignment radiographs and MRI should be performed. All pathologies should be addressed which may include MAT, osteotomy, and cartilage transplantation. Diagnostic arthroscopy may be required first in order to assess meniscus and cartilage before definitive cartilage or meniscal transplantation.
Future Directions
Our understanding of DLM continues to advance and therefore so does our ability to optimize treatment. The order of pathology is still not fully understood in a “chicken or the egg” scenario: if the peripheral mobility is a result of the thickened tissue and increased shear stress on peripheral attachments, or if the peripheral deficiency is also congenital and increases tear risk in the DLM. Identifying stepwise progression of pathology may help elucidate early treatment options or potentially prophylactic interventions to avoid ongoing pain and frequent need for multiple surgeries. Future directions for studying DLM also include refining repair techniques to account for abnormal tissue. Further biologic augmentation of repairs may offer some improved healing potential but has not been thoroughly studied.
Declarations
Conflict of Interest
Dr Pace is a consultant for Arthrex and JRF Ortho. He serves as a committee member for AOSSM and PRiSM. Dr Mandelbaum and Dr Campbell have no relevant conflicts of interest regarding the subject of this review article.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Sabbag OD, et al. Incidence and treatment trends of symptomatic discoid lateral menisci: an 18-year population-based study. Orthop J Sports Med. 2018;6:2325967118797886. doi: 10.1177/2325967118797886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grimm NL, et al. Demographics and epidemiology of discoid menisci of the knee: analysis of a large regional insurance database. Orthop J Sports Med. 2020;8:2325967120950669. doi: 10.1177/2325967120950669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rohren EM, Kosarek FJ, Helms CA. Discoid lateral meniscus and the frequency of meniscal tears. Skeletal Radiol. 2001;30:316–320. doi: 10.1007/s002560100351. [DOI] [PubMed] [Google Scholar]
- 4.Young R. The external semilunar cartilage as a complete disc. London: Williams and Norgate; 1889. [Google Scholar]
- 5.Bellier G, Dupont JY, Larrain M, Caudron C, Carlioz H. Lateral discoid menisci in children. Arthroscopy. 1989;5:52–56. doi: 10.1016/0749-8063(89)90092-3. [DOI] [PubMed] [Google Scholar]
- 6.Dickhaut SC, DeLee JC. The discoid lateral-meniscus syndrome. J Bone Joint Surg Am Vol. 1982;64:1068–1073. doi: 10.2106/00004623-198264070-00018. [DOI] [PubMed] [Google Scholar]
- 7.Ahn JH, Shim JS, Hwang CH, Oh WH. Discoid lateral meniscus in children: clinical manifestations and morphology. J Pediatr Orthop. 2001;21:812–816. doi: 10.1097/01241398-200111000-00022. [DOI] [PubMed] [Google Scholar]
- 8.Aichroth PM, Patel DV, Marx CL. Congenital discoid lateral meniscus in children A follow-up study and evolution of management. J Bone Joint Surg Br Vol. 1991;73:932–936. doi: 10.1302/0301-620X.73B6.1955439. [DOI] [PubMed] [Google Scholar]
- 9.Watanabe M, Takeda S, Ikeuchi H. Atlas of arthroscopy. Berlin: Springer-Verlag; 1969. [Google Scholar]
- 10.Lee RJ, et al. Reliability of a new arthroscopic discoid lateral meniscus classification system: a multicenter video analysis. Am J Sports Med. 2022;50:1245–1253. doi: 10.1177/03635465221076857. [DOI] [PubMed] [Google Scholar]
- 11.Bae JH, et al. Incidence of bilateral discoid lateral meniscus in an Asian population: an arthroscopic assessment of contralateral knees. Arthroscopy. 2012;28:936–941. doi: 10.1016/j.arthro.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Liu WX, Zhao JZ, Huangfu XQ, He YH, Yang XG. Prevalence of bilateral Discoid Lateral Menisci (DLM) in patients operated for symptomatic DLM with a follow-up study on their asymptomatic contralateral knees: a Magnetic Resonance Imaging (MRI) assessment. BMC Musculoskelet Disord. 2015;16:172. doi: 10.1186/s12891-015-0626-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahn JH, Lee SH, Yoo JC, Lee HJ, Lee JS. Bilateral discoid lateral meniscus in knees: evaluation of the contralateral knee in patients with symptomatic discoid lateral meniscus. Arthroscopy. 2010;26:1348–1356. doi: 10.1016/j.arthro.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 14.Kamei G, et al. Characteristic shape of the lateral femoral condyle in patients with osteochondritis dissecans accompanied by a discoid lateral meniscus. J Orthop Sci. 2012;17:124–128. doi: 10.1007/s00776-011-0190-8. [DOI] [PubMed] [Google Scholar]
- 15.Bulgheroni E, Mattioli L, Bulgheroni P. Evolution of osteochondritis dissecans of the lateral femoral condyle combined with discoid meniscus. Joints. 2017;5:114–117. doi: 10.1055/s-0037-1603673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishikawa M, Adachi N, Nakamae A, Deie M, Ochi M. Progression of stable juvenile osteochondritis dissecans after 10 years of meniscectomy of the discoid lateral meniscus. J Pediatr Orthop B. 2017;26:487–490. doi: 10.1097/BPB.0000000000000316. [DOI] [PubMed] [Google Scholar]
- 17.Takigami J, et al. Predictive factors for osteochondritis dissecans of the lateral femoral condyle concurrent with a discoid lateral meniscus. Knee Surg Sports Traumatol Arthrosc. 2018;26:799–805. doi: 10.1007/s00167-017-4451-8. [DOI] [PubMed] [Google Scholar]
- 18.Mochizuki T, Tanifuji O, Sato T, Watanabe S, Endo N. Predictive factors for developing osteochondritis dissecans after surgery for discoid lateral meniscus are younger age and shorter meniscal width. Knee Surg Sports Traumatol Arthrosc. 2021;29:100–108. doi: 10.1007/s00167-019-05750-6. [DOI] [PubMed] [Google Scholar]
- 19.Atay OA, et al. Discoid meniscus: an ultrastructural study with transmission electron microscopy. Am J Sports Med. 2007;35:475–478. doi: 10.1177/0363546506294678. [DOI] [PubMed] [Google Scholar]
- 20.Papadopoulos A, Kirkos JM, Kapetanos GA. Histomorphologic study of discoid meniscus. Arthroscopy. 2009;25:262–268. doi: 10.1016/j.arthro.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 21.Choi YH, et al. Collagenous ultrastructure of the discoid meniscus: a transmission electron microscopy study. Am J Sports Med. 2017;45:598–603. doi: 10.1177/0363546516674181. [DOI] [PubMed] [Google Scholar]
- 22.Ha CW, et al. The utility of the radiographic condylar cut-off sign in children and adolescents with complete discoid lateral meniscus. Knee Surg Sports Traumatol Arthrosc. 2017;25:3862–3868. doi: 10.1007/s00167-016-4361-1. [DOI] [PubMed] [Google Scholar]
- 23.Ikeuchi H. Arthroscopic treatment of the discoid lateral meniscus. Technique and long-term results. Clin Orthop Relat Res. 1982;19–28. [PubMed]
- 24.Manzione M, Pizzutillo PD, Peoples AB, Schweizer PA. Meniscectomy in children: a long-term follow-up study. Am J Sports Med. 1983;11:111–115. doi: 10.1177/036354658301100301. [DOI] [PubMed] [Google Scholar]
- 25.Raber DA, Friederich NF, Hefti F. Discoid lateral meniscus in children. Long-term follow-up after total meniscectomy. J Bone Joint Surg Am Vol. 1998;80:1579–1586. doi: 10.2106/00004623-199811000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Woods GW, Whelan JM. Discoid meniscus. Clin Sports Med. 1990;9:695–706. doi: 10.1016/S0278-5919(20)30717-1. [DOI] [PubMed] [Google Scholar]
- 27.Hashimoto Y, et al. Arthroscopic saucerization with inside-out repair and anterocentral shift of a discoid lateral meniscus with retention of adequate volume of residual meniscus. Arthrosc Tech. 2021;10:e2553–e2557. doi: 10.1016/j.eats.2021.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gamble JG, et al. Radial width of the lateral meniscus at the popliteal hiatus: relevance to saucerization of discoid lateral menisci. Am J Sports Med. 2022;50:138–141. doi: 10.1177/03635465211056661. [DOI] [PubMed] [Google Scholar]
- 29.Lee CR, Bin SI, Kim JM, Lee BS, Kim NK. Arthroscopic partial meniscectomy in young patients with symptomatic discoid lateral meniscus: an average 10-year follow-up study. Arch Orthop Trauma Surg. 2018;138:369–376. doi: 10.1007/s00402-017-2853-1. [DOI] [PubMed] [Google Scholar]
- 30.Yamasaki S, et al. Risk factors associated with knee joint degeneration after arthroscopic reshaping for juvenile discoid lateral meniscus. Am J Sports Med. 2017;45:570–577. doi: 10.1177/0363546516668623. [DOI] [PubMed] [Google Scholar]
- 31.Nishino K, Hashimoto Y, Tsumoto S, Yamasaki S, Nakamura H. Morphological changes in the residual meniscus after reshaping surgery for a discoid lateral meniscus. Am J Sports Med. 2021;49:3270–3278. doi: 10.1177/03635465211033586. [DOI] [PubMed] [Google Scholar]
- 32.Matsuo T, et al. Post-operative deformation and extrusion of the discoid lateral meniscus following a partial meniscectomy with repair. Knee Surg Sports Traumatol Arthrosc. 2017;25:390–396. doi: 10.1007/s00167-016-4393-6. [DOI] [PubMed] [Google Scholar]
- 33.Mochizuki T, Tanifuji O, Watanabe S, Sato T, Endo N. The postoperative shorter meniscal width was the risk factor of lateral meniscal extrusion in the middle portion for juvenile and adolescent knees with discoid lateral meniscus. Knee Surg Sports Traumatol Arthrosc. 2021;29:2857–2866. doi: 10.1007/s00167-020-06188-x. [DOI] [PubMed] [Google Scholar]
- 34.Zuke WA, Cvetanovich GL, Go B, Forsythe B. Arthroscopic saucerization and all-inside repair of a delaminated discoid lateral meniscus. Arthrosc Tech. 2017;6:e1387–e1391. doi: 10.1016/j.eats.2017.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pace JL, Luczak SB, Kanski G, Fitzsimmons KP, Kakazu R. Discoid lateral meniscus saucerization and treatment of intrasubstance degeneration through an accessory medial portal using a small arthroscope. Arthrosc Tech. 2021;10:e2165–e2171. doi: 10.1016/j.eats.2021.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sabbag OD, et al. High rate of recurrent meniscal tear and lateral compartment osteoarthritis in patients treated for symptomatic lateral discoid meniscus: a population-based study. Orthop J Sports Med. 2019;7:2325967119856284. doi: 10.1177/2325967119856284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Habata T, et al. Long-term clinical and radiographic follow-up of total resection for discoid lateral meniscus. Arthroscopy. 2006;22:1339–1343. doi: 10.1016/j.arthro.2006.07.039. [DOI] [PubMed] [Google Scholar]
- 38.He Y, Chen H, Fan Y, Zhou Y, Bao W. Partial resection of lateral discoid meniscus changes lower limb axial alignment - a retrospective cohort study. Knee. 2022;37:171–179. doi: 10.1016/j.knee.2022.06.010. [DOI] [PubMed] [Google Scholar]
- 39.Logan CA, et al. Symptomatic discoid meniscus in children and adolescents: a review of 470 cases. J Pediatr Orthop. 2021;41:496–501. doi: 10.1097/BPO.0000000000001907. [DOI] [PubMed] [Google Scholar]
- 40.Yoo WJ, et al. Arthroscopic treatment for symptomatic discoid meniscus in children: midterm outcomes and prognostic factors. Arthroscopy. 2015;31:2327–2334. doi: 10.1016/j.arthro.2015.06.032. [DOI] [PubMed] [Google Scholar]
- 41.Ahn JH, et al. Long-term results of arthroscopic reshaping for symptomatic discoid lateral meniscus in children. Arthroscopy. 2015;31:867–873. doi: 10.1016/j.arthro.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 42.Ng YH, Tan SHS, Lim AKS, Hui JH. Meniscoplasty leads to good mid-term to long-term outcomes for children and adolescents with discoid lateral meniscus. Knee Surg Sports Traumatol Arthrosc. 2021;29:352–357. doi: 10.1007/s00167-020-05929-2. [DOI] [PubMed] [Google Scholar]
- 43.Jiang D, et al. Comparative study on immediate versus delayed meniscus allograft transplantation: 4- to 6-year follow-up. Am J Sports Med. 2014;42:2329–2337. doi: 10.1177/0363546514541653. [DOI] [PubMed] [Google Scholar]
- 44.Riboh JC, Tilton AK, Cvetanovich GL, Campbell KA, Cole BJ. Meniscal allograft transplantation in the adolescent population. Arthroscopy. 2016;32:1133–1140 e1131. doi: 10.1016/j.arthro.2015.11.041. [DOI] [PubMed] [Google Scholar]
- 45.Lee BS, Bin SI, Kim JM. Articular cartilage degenerates after subtotal/total lateral meniscectomy but radiographic arthrosis progression is reduced after meniscal transplantation. Am J Sports Med. 2016;44:159–165. doi: 10.1177/0363546515612076. [DOI] [PubMed] [Google Scholar]
- 46.Kocher MS, Tepolt FA, Vavken P. Meniscus transplantation in skeletally immature patients. J Pediatr Orthop B. 2016;25:343–348. doi: 10.1097/BPB.0000000000000291. [DOI] [PubMed] [Google Scholar]
- 47.Ramme AJ, Strauss EJ, Jazrawi L, Gold HT. Cost effectiveness of meniscal allograft for torn discoid lateral meniscus in young women. Phys Sportsmed. 2016;44:278–282. doi: 10.1080/00913847.2016.1197762. [DOI] [PubMed] [Google Scholar]
- 48.Kim JM, Bin SI. Meniscal allograft transplantation after total meniscectomy of torn discoid lateral meniscus. Arthroscopy. 2006;22:1344–1350 e1341. doi: 10.1016/j.arthro.2006.07.048. [DOI] [PubMed] [Google Scholar]
- 49.Yoon KH, Lee SH, Park SY, Jung GY, Chung KY. Meniscus allograft transplantation for discoid lateral meniscus: clinical comparison between discoid lateral meniscus and nondiscoid lateral meniscus. Arthroscopy. 2014;30:724–730. doi: 10.1016/j.arthro.2014.02.017. [DOI] [PubMed] [Google Scholar]
- 50.Ren S, et al. Anatomical knee variables result in worse outcomes of lateral meniscal allograft transplantation with discoid lateral menisci than with nondiscoid lateral menisci. Knee Surg Sports Traumatol Arthrosc. 2021;29:4146–4153. doi: 10.1007/s00167-021-06509-8. [DOI] [PubMed] [Google Scholar]
- 51.Lee DW, Kim JG, Ha JK, Kim WJ. Simultaneous osteoperiosteal autologous iliac crest graft and lateral meniscus allograft transplantation for osteochondral lesion with bony defect and lateral discoid meniscus tear. Knee Surg Relat Res. 2016;28:165–171. doi: 10.5792/ksrr.2016.28.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang SI. Meniscal allograft transplantation for symptomatic knee after meniscectomy of torn discoid medial meniscus: report of three cases. Acta Orthop Traumatol Turc. 2018;52:70–74. doi: 10.1016/j.aott.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zaffagnini S, Espinosa M, Neri MP, Marcacci M, Grassi A. Treatment of meniscal deficiency with meniscal allograft transplantation and femoral osteotomy in a patient with history of lateral discoid meniscus: 15-year follow-up case report. JBJS Case Connect. 2020;10:e0079. doi: 10.2106/JBJS.CC.19.00079. [DOI] [PubMed] [Google Scholar]
- 54.Smith RA, Vandenberg CD, Pace JL. Management of long-term complications in the setting of lateral meniscal deficiency after saucerization of a discoid lateral meniscus in an adolescent patient: a case report and review of the literature. JBJS Case Connect. 2018;8:e102. doi: 10.2106/JBJS.CC.18.00054. [DOI] [PubMed] [Google Scholar]