Abstract
Thermostable enzymes are enzymes that can withstand elevated temperatures as high as 50 °C without altering their structure or distinctive features. The potential of thermostable enzymes to increase the conversion rate at high temperature has been identified as a key factor in enhancing the efficiency of industrial operations. Performing procedures at higher temperatures with thermostable enzymes minimises the risk of microbial contamination, which is one of the most significant benefits. In addition, it helps reduce substrate viscosity, improve transfer speeds, and increase solubility during reaction operations. Thermostable enzymes offer enormous industrial potential as biocatalysts, especially cellulase and xylanase, which have garnered considerable amount of interest for biodegradation and biofuel applications. As the usage of enzymes becomes more common, a range of performance-enhancing applications are being explored. This article offers a bibliometric evaluation of thermostable enzymes. Scopus databases were searched for scientific articles. The findings indicated that thermostable enzymes are widely employed in biodegradation as well as in biofuel and biomass production. Japan, the United States, China, and India, as along with the institutions affiliated with these nations, stand out as the academically most productive in the field of thermostable enzymes. This study’s analysis exposed a vast number of published papers that demonstrate the industrial potential of thermostable enzymes. These results highlight the significance of thermostable enzyme research for a variety of applications.
Keywords: Industrial enzymes, Bioprocess, Biodegradation, Biofuel, Research trends, Bibliometric, VOSviewer
Background
Recent developments in biological based materials for a variety of applications have begun to penetrate the industrial sector [1, 2]. As a result, several industries are now taking steps to transition from chemical-based manufacturing to clean biological manufacturing. [3–5]. Enzymes are proteins that operate as biological catalysts in biological systems, speeding up reactions and catalyzing chemical reaction functions [6–8]. Enzymes are increasingly being used in a wide range of industrial processes due to their great specificity of action. Their benefits include their efficiency in speeding chemical reactions and their selectivity in distinguishing between potential substrates [6, 9, 10]. Enzymes may aid in the development of environmentally friendly processes by displacing toxic chemicals used in industrial manufacturing [11–13].
Enzymes can be utilized in many industrial production processes, enabling the creation of environmentally friendly technology processes without creation of waste and production of hazardous chemicals such as detergent formulations, cheese production, the leather industry and pharmaceuticals industry [8, 14–17]. However, the most major difficulty to the widespread commercial deployment of enzymes is their intrinsic fragility under rigorous industrial operations conditions, one of which is that they cannot sustain the process's high temperature. The majority of enzymes lose activity at higher temperatures, which are typically between 25 °C and 37 °C [18]. Enzymes derived from thermophiles have affected the interest of numerous businesses, including the pharmaceutical, detergent, textile, food, feed industries, leather, and paper, as well as biorefineries [19, 20]. The term for these enzymes is “thermostable enzymes”.
Thermostable enzymes are enzymes that can resist high temperatures, typically between 45 °C and 120 °C [2]. These enzymes not only survive at high temperatures, but they also work in severe environments where humans cannot exist. Thermostable enzymes provide numerous benefits to the industrial sector, including a rapid growth rate, a reduced risk of contamination, a reduction in liquid viscosity, and improved solubility in polymeric substrates and oil [21]. The resistance of thermostable enzymes to proteolysis and chemical denaturation is greater. With these benefits, it is possible to slow down the process of denaturation, which is essential for commercial preparations, and to store them at room temperature for a longer half-life. Many different enzymes have been identified from thermophiles, including cellulases, amylases, xylanases, pectinases, proteases, and lipases [22–25].
Numerous thermophilic microbial taxa, such as Bacillus, Clostridium, Pyrococcus, Thermus, Thermotoga, and Aquifex, produce unique enzymes including α-amylase, lipase, cellulase, xylanase, alkaline phosphatase, polymerase and ligase [18]. Taq polymerase was the first thermostable enzyme to be reported in 1976 [26]. It was discovered from thermal springs of Yellowstone National Park in 1969 and was isolated from Thermus Aquaticus. The ideal temperature for activity was determined to be 75–80 °C, with a half-life of 2 h at 92.5 °C, 40 min at 95 °C, and 9 min at 97.5 °C [27]. Formerly, DNA polymerases obtained from Escherichia coli were used in polymerase chain reaction (PCR) methods [28]. Nevertheless, they lost their enzymatic activity at high temperatures, necessitating the addition of a new polymerase enzyme after each cycle of denaturation and primer hybridization, which was time-consuming and costly. As a result, the availability of thermostable Taq DNA polymerase has an impact on the PCR development process since it can survive the 95 °C required for DNA strand separation without denaturing. Thermophilic microorganisms are occupying several biological niches, including hot springs, deep marine, volcanic sites, compost and deep organic landfills [29]. However, they have been widely investigated in hot springs around the world and are abundant in nature [30]. Hot springs have been identified as natural habitats that are ideal for thermophile colonization.
Since 1970, numerous studies have been undertaken on thermostable enzymes. As this protein differs from mesophilic enzymes in its structural properties and adaptations to the harsh environment, the majority of the studies have been oriented to studying these characteristics [25, 31]. Thermophiles have rigid cell walls, a high G + C concentration of DNA contents that ultimately alters these protein structure [32]. This component causes these enzymes to have distinct hydrogen bonds, electrostatic interactions, hydrophilic contacts, metal binding and loop deletion or shortening, which eventually results in a superior conformational shape. Enzyme thermostability is typically an intrinsic feature determined by the primary protein structure. The thermostable enzymes were more stable than the mesophilic enzymes because they had larger levels of non-polar amino acids [33]. These amino acids increase the hydrophobicity of proteins, which is directed towards the catalytic pocket and increases the rigidity of proteins [34]. Furthermore, the higher charged of amino acids also enhance the electrostatic interactions in the outer part of protein leading greater ion pair interaction [35]. Studies also revealed that thermostable enzymes contain higher disulphide bonds and hydrophobic bonds [36]. These criteria make the structure of the enzymes more rigid and lead to better folding of the conformational.
Technologies for data extraction and synthesis are currently essential due to the abundance of data available. Bibliometric analysis is a statistical methodology that use [37, 38] to analyze and evaluate a significant number of scientific research articles in various fields of knowledge. Bibliometric is very important for uncovering developing trends in a specific topic or field by identifying the relationship of core research or authors across all publications, journal performance, collaboration patterns, and research constituents [39]. The findings of academic publishing analysis have proven to be an excellent method of measuring the impact of research trends [40]. As a result, the researcher can find knowledge gaps concerning the issue and develop new original ideas for investigation and contribution to the specific research topic. Through bibliometric analysis, it is possible to gain a comprehensive understanding of a certain topic and its relationship to specific databases. Utilizing interaction charts, this popular method provides a simple and easy assessment of selected works. The publication year, the most-cited articles, journals, authors, and fields of study were all examined in this study's bibliometric analysis of thermostable enzymes. Current trends in thermostable enzymes are examined in light of relevant research.
Scientific literature research
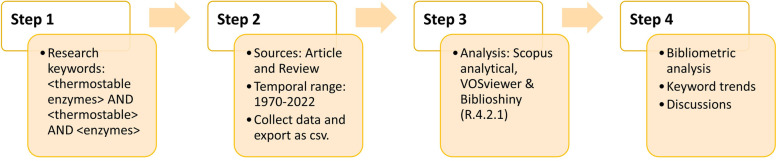
The Scopus database (www.scopus.com) was recovered in September 2022 using the search terms "thermostable enzyme" AND "thermostable" AND "enzymes" (Fig. 1), with only research publications and review studies included. There were no time restrictions placed on the search, and all publications up to the search year (1970–2022). This allows us to estimate the global research output on thermostable enzymes from a large number of high-quality journal papers. The "analyze results" feature of Scopus's search tools displays the publication year and topic matter of the chosen work. The articles we gathered were examined using VOSviewer version 1.6.15, which was used to import all data into a Microsoft Excel spreadsheet (csv) (www.vosviewer.com). VOSviewer is a software tool used in bibliometric analysis by constructing and visualising the networks of publications, documents, sources, authors, organizations, and countries. These networks can be built via citation, bibliographic coupling, co-citation, or co-authorship relationships. It also provides network mapping of the relationships between bibliometric networks and the topic of interest. In this study, the data were collected to determine the most productive countries, the most highly referenced journals, authors, publications and research trends based on keyword analysis. Several different types and units of analysis were used to construct the results.
Fig. 1.
Flowchart of bibliometric search strategy
Analysis of publications
General analysis
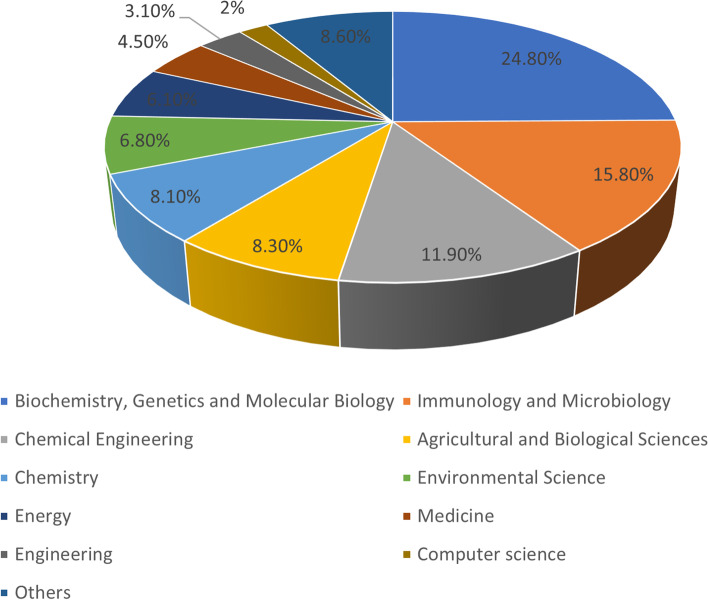
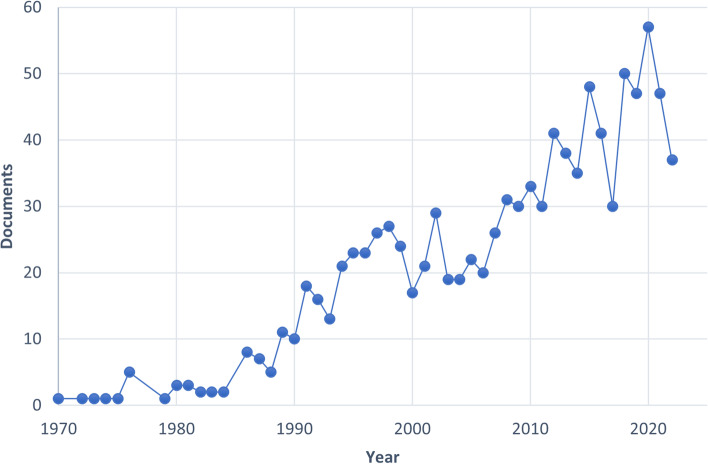
The aforementioned search method accumulates 1090 articles, which were divided into 11 categories of research topics (Fig. 2). The search for articles and reviews was narrowed down to 1010 publications. 97% (n = 3925) of them were written in English, 1.03% (n = 42) in Chinese and 1.97% in other languages. The first study on thermostable enzymes published was the purification of thermostable isoleucyl-tRNA synthetase from Bacillus stearothermophilus in 1972 [41]. After 1995, the number of articles increased each year, finally surpassing 20 and going over 40. The distribution of articles by study area from 1970 to 2022 is shown in Fig. 3. Qualitative trend analysis for each study area with more than 100 publications between 2017 and 2022 was conducted to examine the most common use of thermostable enzymes during the preceding five years (biochemistry, genetic and molecular biology, immunology and microbiology, chemical engineering, chemistry and agricultural and biological sciences).
Fig. 2.
The main research topic related to thermostable enzymes (Scopus)
Fig. 3.
Annual scientific production of articles on thermostable enzymes
Biochemistry, genetics and molecular biology
Study on the isolation and characterization [42–45], expression and purification [46–50], gene cloning, structural function analysis, improving catalytic efficiency, site-directed mutagenesis, protein crystallization, computational simulations, rational engineering to improve enzymes activity [51–56] preservation of enzymes, indigenous thermophilic exploration, cell-free enzymatic, polymerase synthesis, marine thermophiles, enzymatic purification and biodegradation [57–59]. The most important enzymes: Cellulose, xylanase, lipase and amylase.
Immunology and microbiology
Study on immunogenicity of subunit vaccine [60], biosensor, detection of organic pollutants [61], enzymatic bioreceptor [62], applications to in vitro biosynthesis, substrate specificity, improvement catalytic performance, biomedical, preparation of pharmacologically active icaritin, in vitro antioxidant activity, cancer prodrug-mediated therapies or gene therapy applications [63–65]. The most important enzymes: Esterase-2, endoglucanase, aldehyde dehydrogenase, cellulose, xylanase and laccase.
Chemical engineering
Study on dye-linked L-lactate dehydrogenase [66], lignification [67], dishwashing machine [68], degradation of lignocellulose [69], biodegradable polymer, biotransformation, fine chemical industry, bio-bleaching and dye decolorizing agent [70], renewable bioethanol [71] and enzyme immobilization for the hydrolysis reaction [72]. The most important enzymes: Lipase, cellulase, xylanase, glucosidase and amylase.
Agricultural and biological sciences
Study on class III peroxidases (POX) plants [73], Calotropis procera root peroxidase (CPrP) [74], oxidoreductive enzymes, microalgal and cyanobacterial [75] and bioremediate phenol from petroleum effluent [57]. The most important enzymes: Amylase, peroxidase, esterase.
Chemistry
Enzymes-based sensor [76], Flavoenzyme dye-linked L-lactate dehydrogenase (Dye-LDH) [66], degradation of poly (lactic acid), PLA, biodegradation of xenobiotics [77], aromatic compounds and lactic acid, enzyme immobilization on carboxymethyl cellulose (CMC)-hydrogel, organic chemistry, synthetic catalyst and bioremediation—dimethylformamidase (DMFase) [78]. The most important enzymes: Dehydrogenase, peroxidases, phosphatase and pectate lyse.
Research trends
According to the results of a general analysis, most research on thermostable enzymes has been carried out in the fields of biochemistry, genetics and molecular biology [79, 80]. All studies on this topic, were focuses more on strategies used to enhance thermostable enzymes, such as molecular, characterization, genetic alteration to improve catalytic activity and purification. Although the quest for thermophiles began 40 years ago, the discovery of important and novel thermostable enzymes continues to rise, making the search for and isolation of thermophiles an essential topic of research. However, based on the industrial potential of thermostable enzymes, the majority of research focuses on lignocellulosic biodegradation for biofuel production. The biodegradable process requires severe conditions for the hydrolysis of lignocellulosic biomass [67, 79]. Enzymatic degradation is the most effective and environmentally safe method for converting complex lignocellulose polymers into fermentable monosaccharides, compared to chemical and physical procedures. Cellulase and xylanase are the thermostable enzymes that are involved in this industrial sector [4, 5, 12]. The usefulness of thermostable cellulases and xylanases is primarily determined by their productivity, thermostability, specific activity, broad pH range and broad substrate specificity. Using genetic engineering, expression control and enzyme immobilization, the thermostability of thermophile cellulase and xylanase has been increased in order to expand their industrial applications. Most approaches involve site-directed mutagenesis, whereas cloning was used to increase the enzymes' stability.
Top research institutions and countries
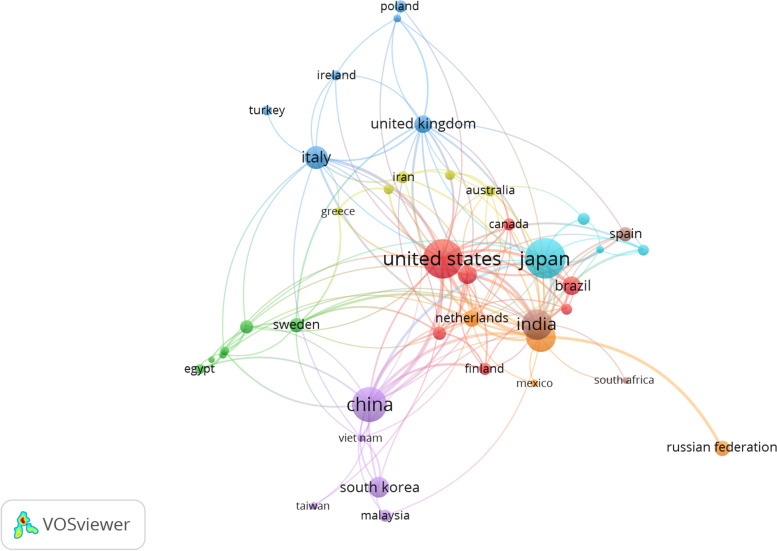
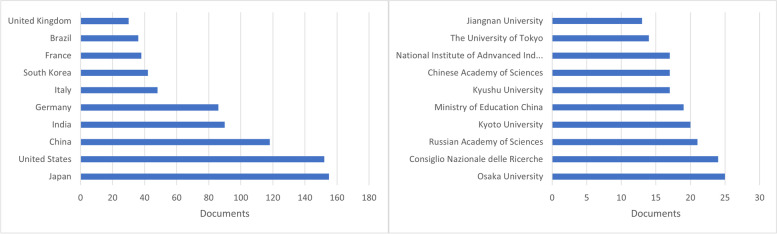
A review of publications by nation revealed that the top 10 countries represented 79.90% (n = 807) of all articles (Fig. 4). Japan came in first with 15.6% of all papers produced, followed by the United States (15%), China (11.88%) and India (9.10%). The top 10 institutions with the most publications included three Chinese universities and five Japanese universities. Other countries, such as India, Germany, South Korea and the United States (US), did not have any institutions in the top 10, while the US had just one. From search results analysis based on document affiliations, Osaka University Japan was the top research institution for the study of thermostable enzymes with 25 documents followed by the Consiglio Nazionale delle Ricerche Italy, the Russian Academy of Sciences, Kyoto University and the Ministry of Education, China (Fig. 5).
Fig. 4.
Collaborative networks between the 20 most productive countries in the research of thermostable enzymes according to a bibliometric analysis of the Scopus database
Fig. 5.
Number of publications per country and top research institutions on thermostable enzyme research based on the Scopus database
Most global cited documents
We examined the number of citations of articles published between 1976 and 2022 to identify the most cited articles of recent times and determined that highly referred publications are often older. As indicated in Table 1, Holland (1991) [81] had the most referenced articles throughout this time span, with 2,147 citations. The 5'-3' exonuclease activity of Taq DNA polymerase from Thermus aquaticus was used by the authors to create a simple and effective approach for identifying PCR products. This enzyme is frequently employed for PCR amplification because of its exceptional heat resistance. At 72 °C, nucleotides are integrated at a rate of 2 and 4 kb/min, while their half-life at 95 °C is 40 min.
Table 1.
Top 10 most cited publications on thermostable lipase in the Scopus database (1970–2022)
| Author | Year | Journal | Title | Total Citation | Citation |
|---|---|---|---|---|---|
| Holland PM, Abramson RD, Watson R, and David Gelfand H | 1991 | Proc Natl Acad Sci U S A | Detection of specific polymerase chain reaction product by utilizing the 5' -* 3' exonuclease activity of Thermus aquaticus DNA polymerase | 2147 | [81] |
| Haki GD and Rakshit SK | 2003 | Bioresour Technol | Developments in industrially important thermostable enzymes: a review | 885 | [82] |
| Barany, F | 1991 | Proc Natl Acad Sci U S A | Genetic disease detection and DNA amplification using cloned thermostable ligase | 647 | [83] |
| Fernandez-Lafuente, R | 2010 | J Mol Catal B Enzym | Lipase from Thermomyces lanuginosus: Uses and prospects as an industrial biocatalyst | 449 | [84] |
| Klibanov AM | 1983 | Adv Appl Microbiol | Stabilization of Enzymes against Thermal Inactivation | 406 | [85] |
| Turner P, Mamo G and Karlsson EN | 2007 | Microb Cell Fact | Potential and utilization of thermophiles and thermostable enzymes in biorefining | 386 | [86] |
| Sterner R and Liebl W | 2001 | Crit Rev Biochem Mol Biol | Thermophilic Adaptation of Proteins | 318 | [87] |
| Zhulidov PA, Bogdanova EA, Shcheglov AS, Vagner LL, Khaspekov GL, Kozhemyako VB, Matz MV, Meleshkevitch E, Moroz LL, Lukyanov SA, Shagin DA | 2004 | Nucleic Acids Res | Simple cDNA normalization using kamchatka crab duplex-speci®c nuclease | 316 | [88] |
| Berka RM, Grigoriev et al | 2011 | Nat Biotechnol | Comparative genomic analysis of the thermophilic biomass-degrading fungi Myceliophthora thermophila and Thielavia terrestris | 309 | [89] |
| Eom S, Wang J, Steitz T | 1996 | Nature | Structure of Taq polymerase with DNA polymerase active site | 305 | [90] |
The second most cited review article, ‘Developments in Industrially Significant Thermostable Enzymes: A Review ‘ by Haki and Rakshit [82], with 883 citations. The authors are connected to the Thai Asian Institute of Technology's Bioprocess Technology Program (AIT). The number of applications for enzymes has increased as a result of the creation of thermostable enzymes, as this review article explains. Due to their inherent stability, thermophilic organisms have discovered a variety of economic applications as a result of the numerous studies that have been conducted to identify them. The food industry (which synthesizes amino acids), the petroleum, chemical and paper sectors are the next largest users of thermostable enzymes in the starch sector [91–93].
Barany is the author of third most cited article which published in the same journal as Holland 1991, Journal of Proceeding Natl Acad Sci USA. Barany is a researcher from the Cornell University Medical College, New York has conducted a study on genetic disease detection and DNA amplification using cloned thermostable ligase from Thermus aquaticus [83].
Fernandez-Lafuente is the fourth most cited review article with total citation about 449. Fernandez-Lafuente is a researcher from the Instituto de Catálisis-CSIC, Spain. The most cited article in 2010 is specific for thermostable lipase from Thermomyces laguginosus which available in both soluble and immobilized form [84].
Most relevant authors
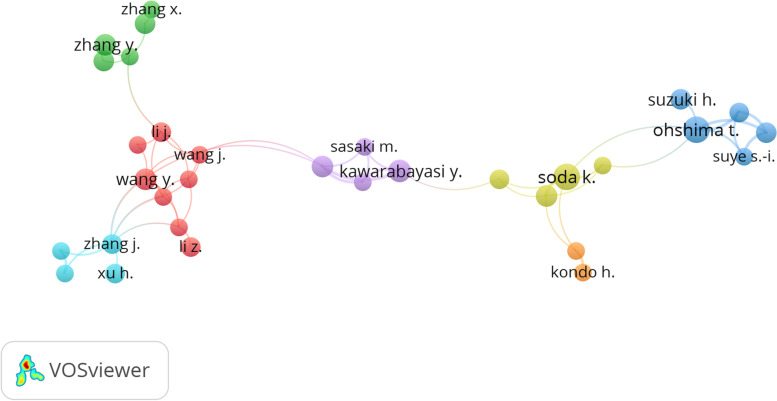
Rossi Mosè E, a researcher at the Consiglio Nazionale delle Ricerche in Rome, Italy, is the most cited and notable author. His 445 papers were cited 11,991 times in 6737 different documents. The author has written 13 articles on the study of thermostable enzymes, and the paper titled "Crystal structure of the most catalytically effective carbonic anhydrase enzyme, SazCA from the thermophilic bacterium Sulfurihydrogenibium azorense" has received the most citations, with 62 citations [52]. Oh Deokkun, a scholar at Konkuk University in Seoul, South Korea, is the second most cited author (8286 citations). The most recent publication, which has 74 citations, was released in 2011 where the research was conducted on cloning and expression of thermostable cellobiose 2-epimerase, a from Caldicellulosiruptor saccharolyticus into Escherichia coli as expression host [44].
The collaborative network among authors has been analyzed. From the VOSviewer bibliometric analysis in Fig. 6, there are seven clusters of authors that connected with each other on thermostable enzyme research, but Rossi Mosè and Oh Deokkun were not included in the collaborative network. However, Ohshima, T (Cluster 3) is the third most cited author (3315 citations) and a researcher from Osaka Institute of Technology, Japan. He has connections with the fourth most cited author, Soda. K (Cluster 4) who is from the Institute of Chemical Research, Kyoto University. Both of them published a review paper together in 1989 with the title’Thermostable amino acid dehydrogenases: applications and gene cloning ‘ which was cited by 33 authors [94]. Cluster 1 consists of eight connected authors, which are Gao, R., Wang, Y., Wang, Xiaojuan, Wang, Zhongyu (Jilin University, China). They are members of the same research group at Key Laboratory for Molecular Enzymology and Engineering.
Fig. 6.
The top 30 most productive authors collaborate in thermostable lipase research according to a bibliometric analysis of the Scopus database
Recent research
Recent research on thermostable lipase was analyzed in the Scopus database from 2018–2022 (5 years). From the results, Febbraio Ferdinando from Consiglio Nazionale delle Ricerche, Rome, Italy (same affiliations with the most relevant authors, Rossi Mosè) have much more research on enzyme-based biosensor by using thermostable lipase. The most recent research paper on biosensor fluorescent detection of organophosphate pesticides using the thermostable enzyme esterase-2 from Alicyclobacillus acidocaldarius (EST2) with a lipase-like Ser-His-Asp catalytic triad was published in 2022, and is a promising candidate as a bioreceptor for the development of biosensor [62].
Huiying Luo, a researcher at the Chinese Academy of Agricultural Sciences in Beijing, China, studies thermostable xylanase and cellulase, which are commonly used to decompose lignocellulosic biomass and have potential applications in the feed and fuel industries [95, 96]. This study sheds light on the underlying mechanism and methods of modifying xylanase for commercial use.
Most relevant journals
The Journal of Applied Microbiology and Biotechnology, published by Springer Nature, ranked first, with 43 papers and 1066 citations. Table 2 shows that 20% of all publications on the subject may be attributed to the top 10 journals. From 43 documents, the highest cited paper is written by Bragger (1989) with research on extremely thermophilic archaebacteria and eubacteria with 101 citations [97]. This article demonstrated the isolation of 36 thermophilic eubacteria for extracellular amylase, hemicellulase (xylanase), cellulase, protease, pectinase and lipase activities. As shown in Table 3, the journal had an impact factor of 3.3 and a CiteScore of 8.8 in 2019; thus, it received an average of 8.8 citations per article published. This publication is of tremendous relevance in the field since, as its title suggests, it focuses primarily on the application of microorganism-derived enzymes in biotechnology.
Table 2.
Top 10 of the most relevant sources for thermostable enzymes research
| Journal | Country | Articles No | Publisher | Impact factor | Cite score | H-index |
|---|---|---|---|---|---|---|
| Applied Microbiology and Biotechnology | Germany | 43 | Springer Verlag | 3.3 | 8.8 | 236 |
| Enzyme And Microbial Technology | United States | 36 | Elsevier | 3.705 | 6.0 | 153 |
| Bioscience, Biotechnology and Biochemistry | United Kingdom | 18 | Oxford University Press | 2.337 | 3.3 | 123 |
| Biotechnology Letters | Netherlands | 17 | Springer Nature | 2.716 | 4.0 | 114 |
| Applied Biochemistry and Biotechnology | United States | 16 | American Society for Microbiology | 7.8 | 339 | |
| Journal Of Biochemistry | United Kingdom | 16 | Oxford University Press | 3.241 | 4.5 | 120 |
| Applied And Environmental Microbiology | United states | 15 | American Society for Microbiology | 2.926 | 7.8 | 339 |
| Biotechnology for Biofuels | United Kingdom | 15 | Biomed Central Ltd | 7.670 | 11.5 | 108 |
| International Journal of Biological Macromolecules | Netherlands | 15 | Elsevier | 8.025 | 11.6 | 144 |
| Bioresource Technology | United Kingdom | 13 | Elsevier | 11.889 | 17.4 | 317 |
Table 3.
Keyword clusters analysis of scientific publication of thermostable enzymes in the Scopus collection database (1976–2022)
| Cluster | Items |
|---|---|
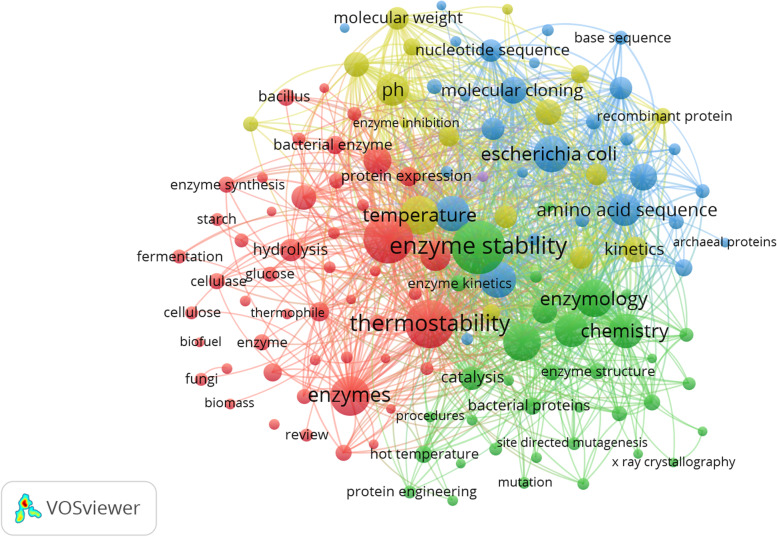
| 1 | Amylase, bacillus, bacteria, bacterial enzymes, bacterial strains, bacterium, beta glucosidase, biocatalyst, biofuel, biomass, biosynthesis, biotechnology, cellulase, cellulose, enzyme assay, enzyme immobilization, enzyme synthesis, fermentation, fungi, genetic engineering, geobacillus, geobacillus stearotherm, glucose, hydrolysis, microbiology, phylogeny, starch, thermodynamic stability, thermophile, thermophilic bacteria, thermostability, thermostable enzyme, triacylglycerol lipase, xylan endo 1,3 beta xyloses |
| 2 | Bacterial protein, biocatalysis, catalysis, catalytic domain, chemistry, crystal structure, x-ray crystallography, enzyme active site, enzyme structure, enzymology, glycosidase, glycoside hydrolases, mutagenesis, protein confirmation, protein denaturation, protein engineering, protein stability |
| 3 | Amino acid, archeal proteins, cloning, molecular DNA, Escherichia coli, gene expression, gene sequence, hydrogen-ion concentration, isolation and purification, molecular cloning, molecular genetics, nucleotide sequences, polymerase chain reaction, protein expression, protein purification, recombinant protein, sequence alignment, sequence homology, thermotoga maritima, thermus, thermus thermophilus |
| 4 | Bacterial enzymes, enzyme analysis, enzymes inhibition, enzymes kinetics, enzyme purification, enzyme specificity, enzyme substrate, substrate specificity, substrates |
| 5 | Thermotoga maritama |
The Journal of Enzyme and Microbial Technology, with 36 articles, is the second most relevant publication in the field. Its publications had to do with technological advancements. Bioresource Technology is the most prominent journal on the subject, with an impact factor of 11.889 and 13 articles. This journal published Haki & Rakshit (2003), which is one of the top 10 most referenced papers with 885 citations. Their principal fields of publication were Bioscience, Biotechnology, and Biochemistry ranked third among the most cited journals with twenty citations for 18 published publications. 33% of the top ten journals were published in the United States, 30% in the United Kingdom, 21% in Germany, and 16% in the Netherlands. Thus, 67% of the journals were European and 33% were North American.
Keyword trends analysis
The purpose of keyword co-occurrence analysis is to identify emerging trends and hot subjects, and it is an important method for tracking scientific progress. The findings of the trend analysis for the time periods utilising keywords with at least 30 occurrences are displayed in Fig. 7 and Table 3. The result revealed that there were 7996 keywords in the 1,010 articles, and 135 keywords appeared 30 times or more. For better interpretation of results, the terms used for the literature search were omitted from Fig. 7. From the results, 5 clusters were obtained from total keywords. Analysis of the keyword trend revealed that studies were associated with amylase, beta glucosidase, biofuel, cellulase, and cellulose are included in cluster 1 (Table 3). We highlight the fact that research on thermostable enzymes and biofuel production began to emerge strongly in this time range.
Fig. 7.
Keyword trend analysis of scientific publications on thermostable enzymes in the Scopus collections database (1976-2022)
Conclusions
This study has detailed the current research status on thermostable enzymes that has increased throughout the years. We detected a trend toward the application of thermostable enzymes in several industrial research domains, notably for ecologically friendly approaches to address pollution and bioremediation. Enzymatic production of biodiesel is expected to be a trend in the coming years, encouraged by the increasing interest in natural components and green technologies. The usage of thermostable enzymes in industrial applications is expected to increase especially in biodegradable of lignocellulosic biomass for biofuel production. However, we noticed weak collaboration links between researchers from different nations, and organizations which have to be developed to increase knowledge diffusion. There has been an increasing amount of study and Japan remains ahead in both the sum of publications and total citation frequency in this sector. Thus, it is not difficult to forecast that this area of research is expected to continue to rapidly increase and that more papers will be published in the coming years. For future research, it will be necessary to create strategies for developing thermostable enzymes that can be employed extensively in the biofuel, biodegradation, food, pharmaceutical, textile, bio-based, and animal feed industries. Enzymes are frequently denatured by high temperatures, strong acids and bases, organic solvents, and other harsh conditions, compromising their catalytic capabilities and limiting their applicability in industrial processes. Discovering new thermostable enzymes in extreme environments or performing molecular modification of existing enzymes with poor thermostability using emerging protein engineering technology are now effective methods for getting new thermostable enzymes.
Acknowledgements
Not applicable.
Abbreviations
- CSV
Comma Separated Value (CSV)
- RNA
Ribonucleic acid
- DNA
Deoxyribonucleic acid
- PCR
Polymerase chain reaction
- POX
Peroxidases
- CPrP
Calotropis procera root peroxidase
- LDH
L-lactate dehydrogenase
- PLA
Poly lactic acid
- CMC
Carboxymethyl cellulose
- CA
Carnonic anhydrase
- DMFase
Dimethylformamidase
Authors’ contributions
CHAC analyzed and interpreted the data regarding the data collections, analysis and was a major contributor in writing the manuscript. All authors read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
All research manuscripts and review data utilised in this study were retrieved in CSV format from the Scopus database (attached in supplementary materials). This link yielded all of the evaluated search results.https://www.scopus.com/term/analyzer.uri?sid=4e9621c056d6ac82d181afb073c5e641&origin=resultslist&src=s&s=TITLE-ABS-KEY%28thermostable-enzymes%2c+thermostable%2c+enzymes%29&sort=plf-f&sdt=cl&sot=b&sl=58&count=1022&analyzeResults=Analyze+results&cluster=scosubtype%2c%22ar%22%2ct%2c%22re%22%2ct%2bscopubstage%2c%22aip%22%2cf%2bscosrctype%2c%22d%22%2cf&txGid=12bdad479e4e1e91daabb8fc725d2e54
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kumar B, Verma P. Enzyme mediated multi-product process: a concept of bio-based refinery. Ind Crops Prod. 2020 doi: 10.1016/j.indcrop.2020.112607. [DOI] [Google Scholar]
- 2.Rigoldi F, Donini S, Redaelli A, Parisini E, Gautieri A. Review: engineering of thermostable enzymes for industrial applications. APL Bioeng. 2018 doi: 10.1063/1.4997367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradu P, Biswas A, Nair C. et al. (2022) Recent advances in green technology and Industrial Revolution 4.0 for a sustainable future. Environ Sci Pollut Res. 10.1007/s11356-022-20024-4 [DOI] [PMC free article] [PubMed] [Retracted]
- 4.Nargotra P, Vaid S, Bajaj BK (2016) Cellulase production from Bacillus subtilis SV1 and its application potential for saccharification of ionic liquid pretreated pine needle biomass under one pot consolidated bioprocess. Fermentation.10.3390/fermentation2040019
- 5.Sharma S, SharmaV, Nargotra, P. et al. (2020) Bioprocess development for production of a process-apt xylanase with multifaceted application potential for a range of industrial processes. SN Appl Sci. 10.1007/s42452-020-2541-6
- 6.Robinson PK (2015) Enzymes: principles and biotechnological applications. Essays Biochem.10.1042/bse0590001 [DOI] [PMC free article] [PubMed]
- 7.Bommarius AS, Paye MF (2013) Stabilizing biocatalysts. Chem Soc Rev. 10.1039/C3CS60137D [DOI] [PubMed]
- 8.Choi JM, Han SS, Kim HS. Industrial applications of enzyme biocatalysis: current status and future aspects. Biotechnol Adv. 2015;33(7):1443–54. doi: 10.1016/j.biotechadv.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 9.Agarwal PK (2006) Enzymes: An integrated view of structure, dynamics and function. Microb Cell Fact 5. doi:10.1186/1475-2859-5-2 [DOI] [PMC free article] [PubMed]
- 10.Neet KE (1998) Enzyme catalytic power minireview series. J Biol Chem. 10.1074/jbc.273.40.25527 [DOI] [PubMed]
- 11.Singh S, Bajaj BK (2017) Potential application spectrum of microbial proteases for clean and green industrial production. Energy Ecol Environ. 10.1007/s40974-017-0076-5
- 12.Vaid S, Bajaj BK (2017) Production of ionic liquid tolerant cellulase from Bacillus subtilis G2 using agroindustrial residues with application potential for saccharification of biomass under one pot consolidated bioprocess. Waste Biomass Valoriz.10.1007/s12649-016-9626-x
- 13.Jegannathan KR, Nielsen PH (2013) Environmental assessment of enzyme use in industrial production–a literature review. J. Clean. Prod. 10.1016/j.jclepro.2012.11.005
- 14.Madhavan A, Sindhu R, Binod P, Sukumaran RK, Pandey A (2017) Strategies for design of improved biocatalysts for industrial applications. Bioresour Technol. 10.1016/j.biortech.2017.05.031 [DOI] [PubMed]
- 15.Cherif S, Mnif S, Hadrich F, Abdelkafi S, Sayadi S (2011) A newly high alkaline lipase: an ideal choice for application in detergent formulations. Lipids Health Dis. 10.1186/1476-511X-10-221 [DOI] [PMC free article] [PubMed]
- 16.Madhavan A, Arun KB, Binod P, Sirohi R, Tarafdar A, Reshmy R, Awasthi MK, Sindhu R (2021) Design of novel enzyme biocatalysts for industrial bioprocess: harnessing the power of protein engineering, high throughput screening and synthetic biology. Bioresource Technology. 10.1016/j.biortech.2020.124617 [DOI] [PubMed]
- 17.Alfa MJ, Jackson M (2001) A new hydrogen peroxide-based medical-device detergent with germicidal properties: Comparison with enzymatic cleaners. AJIC. [DOI] [PubMed]
- 18.Sharma S, Vaid S, Bhat B, Singh S, Bajaj BK (2019) Chapter 17 -Thermostable Enzymes for Industrial Biotechnology. Adv Enzyme chnology. 10.1016/B978-0-444-64114-4.00017-0
- 19.Desai AA (2011) Sitagliptin manufacture: a compelling tale of green chemistry, process intensification, and industrial asymmetric catalysis. Angew Chem. 10.1002/anie.201007051 [DOI] [PubMed]
- 20.Maervoet VET, De Mey M, Beauprez J, De Maeseneire S, Soetaert WK (2011) Enhancing the microbial conversion of glycerol to 1,3-propanediol using metabolic engineering. Org Process Res Dev. 10.1021/op1001929
- 21.[22] Arbab S, Ullah H, Khan MIU, Khattak MNK, Zhang J, Li K, Hassan IU (2022) Diversity and distribution of thermophilic microorganisms and their applications in biotechnology. J Basic Microbiol.10.1002/jobm.202100529 [DOI] [PubMed]
- 22.Yachmenev VG, Bertoniere NR, Blanchard EJ (2002) Intensification of the bio-processing of cotton textiles by combined enzyme/ultrasound treatment. J Chem Technol Biot. 10.1002/jctb.579
- 23.Wang ZX, Shi XX, Chen GR, Ren ZH, Luo L, Yan J (2006) A new synthesis of alpha-arbutin via Lewis acid catalyzed selective glycosylation of tetra-O-benzyl-alpha-D-glucopyranosyl trichloroacetimidate with hydroquinone. Carbohydr Re. 10.1016/j.carres.2006.04.022 [DOI] [PubMed]
- 24.Maijala P, Kleen M, Westin C, Poppius-Levlin K, Herranen K, Lehto JH, et al (2008) Biomechanical pulping of softwood with enzymes and white-rot fungus Physisporinus rivulosus. Enzym Microb Technol. 10.1016/j.enzmictec.2007.11.017
- 25.Reetz MT, Carballeira JD, Vogel A (2006) Iterative saturation mutagenesis on the basis of B factors as a strategy for increasing protein thermostability. Angew Chem. 10.1002/anie.200602795 [DOI] [PubMed]
- 26.Chien A, Edgar DB, Trela JM (1976) Deoxyribonucleic acid polymerase from the extreme thermophile Thermus aquaticus. J Bacteriol.10.1128/jb.127.3.1550-1557.1976 [DOI] [PMC free article] [PubMed]
- 27.Lawyer FC, Stoffel S, Saiki RK, Chang SY, Landre PA, Abramson RD, Gelfand DH (1993) High-level expression, purification, and enzymatic characterization of full-length Thermus aquaticus DNA polymerase and a truncated form deficient in 5' to 3' exonuclease activity".PCR Methods and Applications. 10.1101/gr.2.4.275 [DOI] [PubMed]
- 28.Mullis K, Faloona F, Scharf S, Saiki R, Horn G, Erlich H (1986) Specific enzymatic amplification of DNA in vitro: the polymerase chain reaction. Cold Spring Harb Symp Quant Biol. 10.1101/sqb.1986.051.01.032 [DOI] [PubMed]
- 29.Mehta, Deepika, Satyanarayana T (2013). Diversity of Hot Environments and Thermophilic Microbes. Thermophilic Microbes in Environmental and Industrial Biotechnology. 10.1007/978-94-007-5899-5_1
- 30.Luo Z, Miao J, Li G, Du Y, Yu X (2017) A recombinant highly thermostable β-mannanase (ReTMan26) from thermophilic Bacillus subtilis (TBS2) expressed in Pichia pastoris and its pH and temperature stability. Appl. Biochem. Biotechnol. 10.1007/s12010-017-2397-4 [DOI] [PubMed]
- 31.Siegel JB, Zanghellini A, Lovick HM, Kiss G, Lambert AR, St.Clair JL, et al. (2010) Computational design of an enzyme catalyst for a stereoselective bimolecular Diels–Alder reaction. 10.1126/science.1190239 [DOI] [PMC free article] [PubMed]
- 32.Hickey DA, Singer GAC (2004) Genomic and proteomic adaptations to growth at high temperature. Genome Biol. 10.1186/gb-2004-5-10-117 [DOI] [PMC free article] [PubMed]
- 33.Das R, Gerstein M (2000) The stability of thermophilic proteins: a study based on comprehensive genome comparison, Funct. Integr. Genomics. doi: 10.1007/s101420000003 [DOI] [PubMed]
- 34.Goodenough PW, Jenkins JA (1991) Protein engineering to change thermal stability for food enzymes. Biochem Soc Trans. 10.1042/bst0190655 [DOI] [PubMed]
- 35.Sadeghi M, Naderi-Manesh H, Zarrabi M, Ranjbar B (2006) Effective factors in thermostability of thermophilic proteins. Biophys Chem. 10.1016/j.bpc.2005.09.018 [DOI] [PubMed]
- 36.Li WF, Zhou XX, Lu P (2005) Structural features of thermozymes. Biotechnol Adv. 10.1016/j.biotechadv.2005.01.002 [DOI] [PubMed]
- 37.Kahar UM, Latif NA, Amran SI, Liew KJ, Goh KM (2022) A bibliometric analysis and review of pullulan-degrading enzymes-past and current trends. Catalysts.10.3390/catal12020143
- 38.Almeida FLC, Castro MPJ (2021) Travália BM, Forte MBS. Trends in lipase immobilization: Bibliometric review and patent analysis. Process Biochemistry. doi: 10.1016/j.procbio.2021.07.005.
- 39.Donthu N, Kumar S, Mukherjee D, Pandey N, Lim WM (2021) How to conduct a bibliometric analysis: An overview and guidelines. J Bus Res. 10.1016/j.jbusres.2021.04.070
- 40.Chen X, Chen J, Wu D, Xie Y, Li J (2016) Mapping the Research Trends by Co-word Analysis Based on Keywords from Funded Project. Procedia Computer Science. 10.1016/j.procs.2016.07.140
- 41.Charlier J, Grosjean H (1972) Isoleucyl‐Transfer Ribonucleic Acid Synthetase from Bacillus stearothermophilus 1. Properties of the Enzyme. European Journal of Biochemistry. 10.1111/j.1432-1033. 1972.tb01681.x [DOI] [PubMed]
- 42.Rastogi G, Muppidi GL, Gurram RN, Adhikari A, Bischoff KM, Hughes SR, Apel WA, Bang SS, Dixon DJ, Sani RK (2009) Isolation and characterization of cellulose-degrading bacteria from the deep subsurface of the Homestake gold mine, Lead, South Dakota, USA. Journal of Industrial Microbiology and Biotechnology. 10.1007/s10295-009-0528-9 [DOI] [PubMed]
- 43.Bibel M, Brettl C, Gosslar U, Kriegshäuser G, Liebl W (1998) Isolation and analysis of genes for amylolytic enzymes of the hyperthermophilic bacterium Thermotoga maritima. FEMS Microbiol Lett. 10.1016/S0378-1097(97)00420-5 [DOI] [PubMed]
- 44.Park CS, Kim JE, Choi JG, Oh DK (2011) Characterization of a recombinant cellobiose 2-epimerase from Caldicellulosiruptor saccharolyticus and its application in the production of mannose from glucose. Appl Microbiol Biotechnol. 10.1007/s00253-011-3403-3 [DOI] [PubMed]
- 45.Li H, Zhang X (2005) Characterization of thermostable lipase from thermophilic Geobacillus sp. TW1. Protein Expression and Purification. 10.1016/j.pep.2005.03.011 [DOI] [PubMed]
- 46.Pritsa AA, Kyriakidis DA (2011) L-asparaginase of Thermus thermophilus: Purification, properties and identification of essential amino acids for its catalytic activity. Molecular and Cellular Biochemistry. 10.1023/A:1011066129771 [DOI] [PubMed]
- 47.Breccia JD, Siñeriz F, Baigorí MD, Castro GR, Hatti-Kaul R (1998) Purification and characterization of a thermostable xylanase from Bacillus amyloliquefaciens. Enzyme Microbial Technol. 10.1016/S0141-0229(97)00102-6
- 48.Nawani N, Kaur J (2000) Purification, characterization and thermostability of lipase from a thermophilic Bacillus sp. J33. Molecular and Cellular Biochemistry. doi:10.1023/a:1007047328301 [DOI] [PubMed]
- 49.Yamada T, Akutsu N, Miyazaki K, Kakinuma K, Yoshida M, Oshima T (1990) Purification, catalytic properties, and thermal stability of Threo-DS-3-isopropylmalate dehydrogenase coded by leuB gene from an extreme thermophile, Thermus thermophilus strain HB8. J Biochem.10.1093/oxfordjournals.jbchem.a123220 [DOI] [PubMed]
- 50.Chen W, Chen H, Xia Y, Zhao J, Tian F, Zhang H (2008) Production, purification, and characterization of a potential thermostable galactosidase for milk lactose hydrolysis from Bacillus stearothermophilus. J Dairy Sci. 10.3168/jds.2007-617 [DOI] [PubMed]
- 51.Dion M, Fourage L, Hallet JN, Colas B (1999) Cloning and expression of a β-glycosidase gene from Thermus thermophilus. Sequence and biochemical characterization of the encoded enzyme. Glycoconjugate J. 10.1023/A:1006997602727 [DOI] [PubMed]
- 52.De Simone G, Monti SM, Alterio V, Buonanno M, De Luca V, Rossi M, Carginale V, Supuran CT, Capasso C, Di Fiore A (2015) Crystal structure of the most catalytically effective carbonic anhydrase enzyme known, SazCA from the thermophilic bacterium Sulfurihydrogenibium azorense. Bioorganic Med Chem Lett. 10.1016/j.bmcl.2015.02.068 [DOI] [PubMed]
- 53.Knapp S, De Vos WM, Rice D, Ladenstein R (1997) Crystal structure of glutamate dehydrogenase from the hyperthermophilic eubacterium Thermotoga maritima at 3.0 Å resolution. J Mol Biol. 10.1006/jmbi.1996.0900 [DOI] [PubMed]
- 54.Del Arco J, Sánchez-Murcia PA, Mancheño JM, Gago F, Fernández-Lucas J (2018) Characterization of an atypical, thermostable, organic solvent- and acid-tolerant 2'-deoxyribosyltransferase from Chroococcidiopsis thermalis. Appl Microbiol Biotechnol. 10.1007/s00253-018-9134-y [DOI] [PubMed]
- 55.Cruz G, Acosta J, Mancheño JM, Del Arco J, Fernández-Lucas J (2022) Rational design of a Thermostable 2′-Deoxyribosyltransferase for Nelarabine Production by Prediction of Disulfide Bond Engineering Sites. Int J Mol Sci. 10.3390/ijms231911806 [DOI] [PMC free article] [PubMed]
- 56.Kumar S, Bhardwaj VK, Guleria S, Purohit R, Kumar S (2022) Improving the catalytic efficiency and dimeric stability of Cu, Zn superoxide dismutase by combining structure-guided consensus approach with site-directed mutagenesis. Biochimica et Biophysica Acta – Bioenergetics. 10.1016/j.bbabio.2021.148505 [DOI] [PubMed]
- 57.Maurya A, Bhattacharya A, Khare SK (2020) Enzymatic Remediation of Polyethylene Terephthalate (PET)–based polymers for effective management of plastic wastes: an overview. Front Bioengineering Biotechnol. 10.3389/fbioe.2020.602325 [DOI] [PMC free article] [PubMed]
- 58.Petroll K, Care A, Bergquist PL, Sunna A (2020) A novel framework for the cell-free enzymatic production of glucaric acid. Metabolic Engineering. 10.1016/j.ymben.2019.11.003 [DOI] [PubMed]
- 59.Coelho GD, Ballaminut N, Thomaz DV, Gomes Machado KM. Characterization of a thermostable Deconica castanella Laccase and application toward pentachlorophenol degradation. Prep Biochem Biotechnol. 2019;49(9):908–915. doi: 10.1080/10826068.2019.1636280. [DOI] [PubMed] [Google Scholar]
- 60.Chichester JA, Musiychuk K, de la Rosa P, Horsey A, Stevenson N, Ugulava N, Rabindran S, Palmer GA, Mett V, Yusibov V (2007) Immunogenicity of a subunit vaccine against Bacillus anthracis. Vaccine. 10.1016/j.vaccine.2007.01.068 [DOI] [PubMed]
- 61.Barbieri MV, Rodrigues ACM, Febbraio F (2022) Monitoring of pesticide amount in water and drinkable food by a fluorescence-based biosensor. EFSA Journal. 10.2903/j.efsa.2022.e200403 [DOI] [PMC free article] [PubMed]
- 62.Rodrigues ACM, Barbieri MV, Febbraio F (2022) Monitoring of pesticide amount in fruit and vegetables by a fluorescence-based sensor. EFSA Journal. 10.2903/j.efsa.2022.e200419 [DOI] [PMC free article] [PubMed]
- 63.Rykov SV, Selimzyanova AI, Nikolaeva AY, Lazarenko VA, Tsurin NV, Akentyev PI, Zverlov VV, Liebl W, Schwarz WH, Berezina OV (2022) Unusual substrate specificity in GH family 12: structure–function analysis of glucanases Bgh12A and Xgh12B from Aspergillus cervinus, and Egh12 from Thielavia terrestris. Appl Microbiol Biotechnol. 10.1007/s00253-022-11811-7 [DOI] [PubMed]
- 64.Vachher M, Sen A, Kapila R, Nigam A (2021) Microbial therapeutic enzymes: A promising area of biopharmaceuticals. Current Research in Biotechnology. Volume. 10.1016/j.crbiot.2021.05.006.
- 65.[66] Panigrahi P, Chand D, Mukherji R, Ramasamy S, Suresh CG (2015) Sequence and structure-based comparative analysis to assess, identify and improve the thermostability of penicillin G acylases. J Industrial Microbiol Biotechnol.10.1007/s10295-015-1690-x [DOI] [PubMed]
- 66.Satomura T, Uno K, Kurosawa N, Sakuraba H, Ohshima T, Suye SI (2021) Characterization of a novel thermostable dye-linked L-lactate dehydrogenase complex and its application in electrochemical detection. Int J Mol Sci. 10.3390/ijms222413570 [DOI] [PMC free article] [PubMed]
- 67.Long L, Tian D, Zhai R, Li X, Zhang Y, Hu J, Wang F, Saddler J (2018) Thermostable xylanase-aided two-stage hydrolysis approach enhances sugar release of pretreated lignocellulosic biomass. Bioresource Technol. 10.1016/j.biortech.2018.02.104 [DOI] [PubMed]
- 68.Naganthran A, Masomian M, Rahman RNZRA, Ali MSM, Nooh HM (2017) Improving the efficiency of new automatic dishwashing detergent formulation by addition of thermostable lipase, protease and amylase. Molecules.10.3390/molecules22091577 [DOI] [PMC free article] [PubMed]
- 69.Ajeje SB, Hu Y, Song G, Peter SB, Afful RG, Sun F, Asadollahi MA, Amiri H, Abdulkhani A, Sun H (2021) Thermostable Cellulases / Xylanases from Thermophilic and Hyperthermophilic Microorganisms: Current Perspective. Front Bioengineering Biotechnol. 10.3389/fbioe.2021.794304 [DOI] [PMC free article] [PubMed]
- 70.Panwar V, Sheikh JN, Dutta T (2020) Sustainable Denim Bleaching by a Novel Thermostable Bacterial Laccase. Appl Biochem Biotechnol. 10.1007/s12010-020-03390-y [DOI] [PubMed]
- 71.Shokrkar H, Ebrahimi S, Zamani M (2017) Bioethanol production from acidic and enzymatic hydrolysates of mixed microalgae culture. Fuel.10.1016/j.fuel.2017.03.090
- 72.Sun G, Huang Z, Zhang Z, Liu Y, Li J, Du G, Lv X, Liu L (2022) A Two-Step Cross-Linked Hydrogel Immobilization Strategy for Diacetylchitobiose Deacetylase. Catalysts.10.3390/catal12090932
- 73.Silva FA, Albuquerque LM, Martins TF, de Freitas JA, Vasconcelos IM, Queiroz de Freitas D, Moreno FBMB, Monteiro-Moreira ACO, Oliveira JTA (2022) A peroxidase purified from cowpea roots possesses high thermal stability and displays antifungal activity against Colletotrichum gloeosporioides and Fusarium oxysporum. Biocatalysis and Agricultural Biotechnology.10.1016/j.bcab.2022.102322
- 74.Oliveira FKD, Santos LO, Buffon JG (2021) Mechanism of action, sources, and application of peroxidases. Food Res Int. 10.1016/j.foodres.2021.110266 [DOI] [PubMed]
- 75.Ikram SF, Kumar D, Singh V, Tripathi BN, Kim BH (2021) Microalgal and cyanobacterial diversity of two selected hot springs of garhwal Himalaya, Uttarakhand, India. Fundamental and Applied Limnology. 10.1127/fal/2021/1366
- 76.Tani Y, Tanaka K, Yabutani T, Mishima Y, Sakuraba H, Ohshima T, Motonaka J (2008) Development of a d-amino acids electrochemical sensor based on immobilization of thermostable d-Proline dehydrogenase within agar gel membrane. Analytica Chimica Acta. 10.1016/j.aca.2008.04.063 [DOI] [PubMed]
- 77.Coleman NV, Rich DJ, Tang FHM, Vervoort RW, Maggi F (2020) Biodegradation and Abiotic Degradation of Trifluralin: A Commonly Used Herbicide with a Poorly Understood Environmental Fate. Environ Sci Technol. 10.1021/acs.est.0c02070 [DOI] [PubMed]
- 78.Arya CK, Yadav S, Fine J, Casanal A, Chopra G, Ramanathan G, Vinothkumar KR, Subramanian R (2020) A 2-Tyr-1-carboxylate Mononuclear Iron Center Forms the Active Site of a Paracoccus Dimethylformamidase. Angewandte Chemie - International Edition. 10.1002/anie.202005332 [DOI] [PMC free article] [PubMed]
- 79.You S, Zha Z, Li J, Zhang W, Bai Z, Hu Y, Wang X, Chen Y, Chen Z, Wang J, Luo H (2021) Improvement of XYL10C_∆N catalytic performance through loop engineering for lignocellulosic biomass utilization in feed and fuel industries. Biotechnol Biofuels. 10.1186/s13068-021-02044-3 [DOI] [PMC free article] [PubMed]
- 80.Krska D, Larsbrink J (2020) Investigation of a thermostable multi-domain xylanase-glucuronoyl esterase enzyme from Caldicellulosiruptor kristjanssonii incorporating multiple carbohydrate-binding modules. Biotechnol Biofuels. 10.1186/s13068-020-01709-9 [DOI] [PMC free article] [PubMed]
- 81.Holland PM, Abramson RD, Watson R, Gelfand DH (1991) Detection of specific polymerase chain reaction product by utilizing the 5′ → 3′ exonuclease activity of Thermus aquaticus DNA polymerase. Proceedings of the National Academy of Sciences of the United States of America. 10.1073/pnas.88.16.7276 [DOI] [PMC free article] [PubMed]
- 82.Haki GD, Rakshit SK (2003) Developments in industrially important thermostable enzymes: a review. Bioresource Technol. 10.1016/S0960-8524(03)00033-6 [DOI] [PubMed]
- 83.Barany F (1991) Genetic disease detection and DNA amplification using cloned thermostable ligase. Proceedings of the National Academy of Sciences of the United States of America. 10.1073/pnas.88.1.189 [DOI] [PMC free article] [PubMed]
- 84.Fernandez-Lafuente R (2010) Lipase from Thermomyces lanuginosus: Uses and prospects as an industrial biocatalyst. Journal of Molecular Catalysis B: Enzymatic. 10.1016/j.molcatb.2009.11.010
- 85.Klibanov AM (1983) Stabilization of Enzymes against Thermal Inactivation. Adv Appl Microbiol. 10.1016/S0065-2164(08)70352-6 [DOI] [PubMed]
- 86.Turner P, Mamo G, Karlsson EN (2007) Potential and utilization of thermophiles and thermostable enzymes in biorefining. Microb Cell Fact.10.1186/1475-2859-6-9 [DOI] [PMC free article] [PubMed]
- 87.Sterner R, Liebl W (2001) Thermophilic adaptation of proteins. Crit Rev Biochem Mol Biol. 10.1080/20014091074174 [DOI] [PubMed]
- 88.Zhulidov PA, Bogdanova EA, Shcheglov AS, Vagner LL, Khaspekov GL, Kozhemyako VB, Matz MV, Meleshkevitch E, Moroz LL, Lukyanov SA, Shagin DA (2004) Simple cDNA normalization using kamchatka crab duplex-specific nuclease. Nucleic Acids Res. 10.1093/nar/gnh031 [DOI] [PMC free article] [PubMed]
- 89.Berka R, Grigoriev I, Otillar R. et al. (2011) Comparative genomic analysis of the thermophilic biomass-degrading fungi Myceliophthora thermophila and Thielavia terrestris. Nat Biotechnol. 10.1038/nbt.1976 [DOI] [PubMed]
- 90.Eom S, Wang J, Steitz T (1996) Structure of Taq polymerase with DNA at the polymerase active site. Nature. 10.1038/382278a0 [DOI] [PubMed]
- 91.De Mot R, Van Oudendijck E, Verachtert H (1984) Production of extracellular debranching activity by amylolytic yeasts. Biotechnol Lett. 10.1007/BF00135686
- 92.De Rosa M, Morana A, Riccio A, Gambacorta A, Trincone A, Incani O (1994) Lipids of the Archaea: A new tool for bioelectronics. Biosensors Bioelectronics.10.1016/0956-5663(94)80064-2
- 93.Demirjian DC, Morís-Varas F, Cassidy, CS (2001) Enzymes from extremophiles. Curr Opinion Chem Biol. 10.1016/S1367-5931(00)00183-6 [DOI] [PubMed]
- 94.Toshihisa Ohshima, Kenji Soda (1989) Thermostable amino acid dehydrogenases: applications and gene cloning. Trends Biotechnol.10.1016/0167-7799(89)90106-6
- 95.Ding S, Liu X, Hakulinen N, Taherzadeh MJ, Wang Y, Wang Y, Qin X, Wang X, Yao B, Luo H, Tu T (2022) Boosting enzymatic degradation of cellulose using a fungal expansin: Structural insight into the pretreatment mechanism. Bioresource Technol. 10.1016/j.biortech.2022.127434 [DOI] [PubMed]
- 96.Wang J, You S, Zha Z et al. (2021) Loop engineering of a thermostable GH10 xylanase to improve low-temperature catalytic performance for better synergistic biomass-degrading abilities. Res Square.10.21203/rs.3.rs-466274/v1 [DOI] [PubMed]
- 97.Bragger JM, Daniel RM, Coolbear T. et al. (1989) Very stable enzymes from extremely thermophilic archaebacteria and eubacteria. Appl Microbiol Biotechnol. 10.1007/BF00270794
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All research manuscripts and review data utilised in this study were retrieved in CSV format from the Scopus database (attached in supplementary materials). This link yielded all of the evaluated search results.https://www.scopus.com/term/analyzer.uri?sid=4e9621c056d6ac82d181afb073c5e641&origin=resultslist&src=s&s=TITLE-ABS-KEY%28thermostable-enzymes%2c+thermostable%2c+enzymes%29&sort=plf-f&sdt=cl&sot=b&sl=58&count=1022&analyzeResults=Analyze+results&cluster=scosubtype%2c%22ar%22%2ct%2c%22re%22%2ct%2bscopubstage%2c%22aip%22%2cf%2bscosrctype%2c%22d%22%2cf&txGid=12bdad479e4e1e91daabb8fc725d2e54