Abstract
Objective
To evaluate the economic value of nivolumab versus docetaxel for advanced non-small cell lung cancer (aNSCLC) treatment after platinum-based chemotherapy in adults without epidermal growth factor receptor/anaplastic lymphoma kinase aberrations in China.
Methods
Partitioned survival models evaluated lifetime costs and benefits of nivolumab versus docetaxel by squamous and non-squamous histologies from a Chinese healthcare payer perspective. Progression-free disease, progressed disease, and death health states were considered over a 20-year time horizon. Clinical data were derived from the CheckMate pivotal Phase III trials (ClinicalTrials.gov identifiers: NCT01642004, NCT01673867, NCT02613507); patient-level survival data were extrapolated using parametric functions. China-specific health state utilities, healthcare resource utilisation, and unit costs were applied. Sensitivity analyses explored uncertainty.
Results
Nivolumab resulted in extended survival (1.489 and 1.228 life-years [1.226 and 0.995 discounted]) and quality-adjusted survival benefits (1.034 and 0.833 quality-adjusted life-years) at additional costs of ¥214,353 (US$31,829) and ¥158,993 (US$23,608) versus docetaxel in squamous and non-squamous aNSCLC, respectively. Nivolumab was associated with higher acquisition costs, lower subsequent treatment costs, and lower adverse event management costs than docetaxel in both histologies. Drug acquisition costs, discount rate for outcomes, and average body weight were key model drivers. Stochastic results aligned with the deterministic results.
Conclusions
Nivolumab yielded survival and quality-adjusted survival benefits at incremental cost versus docetaxel in aNSCLC. As a traditional healthcare payer perspective was applied, the true economic benefit of nivolumab may be underestimated as not all treatment benefits and costs of relevance to society were considered.
Supplementary Information
The online version contains supplementary material available at 10.1007/s41669-022-00383-x.
Key Points for Decision Makers
| This is the first economic evaluation of nivolumab for patients with advanced non-small cell lung cancer (aNSCLC) in China based on individual patient-level data. |
| Nivolumab was associated with survival and quality-adjusted survival benefits versus docetaxel for treatment of aNSCLC. |
| Treatment with nivolumab is anticipated to provide value to the Chinese healthcare system and direct healthcare benefits to Chinese patients with aNSCLC. |
Introduction
Lung cancer is the most common type of cancer and the leading cause of cancer deaths in China, with an estimated 774,323 new cases and 690,567 deaths in 2018 [1]. The age-standardised incidence of lung cancer in China was 35.1 per 100,000 in 2018, and the age-standardised mortality rate was 30.9 per 100,000 [1]. About 80–85% of lung cancer cases are categorised as non-small cell lung cancer (NSCLC), which includes the histology subtypes squamous and non-squamous [2]. Approximately 25–30% of all lung cancers are squamous-cell carcinomas [2].
The majority of NSCLC cases are diagnosed at an advanced stage (IIIB or IV), when the cancer has already spread to distant sites [3]. The prognosis for advanced NSCLC (aNSCLC) is poor [3]. Data from the US National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) Program report that 57% of lung and bronchus cancers had already spread distally at time of diagnosis, and the estimated 5-year survival rate (all races, both sexes, based on the SEER 18 database, 2009–2015) for cancer diagnosed at this stage was 5.2% [3]. In addition to the mortality burden, aNSCLC substantially impairs quality of life (QOL) for patients [4, 5] and their caregivers [6, 7].
Some patients with NSCLC have genetic factors that contribute to tumour progression, such as epidermal growth factor receptor (EGFR) mutations or anaplastic lymphoma kinase (ALK) alterations [8]. In Western countries, EGFR mutations were found in approximately 16–20% of cases of NSCLC (or aNSCLC) [9, 10]; the frequency of EGFR mutations is much higher in China (around 40–50%) [11–13]. ALK alterations have been found to occur in about ≤ 10% of patients with NSCLC (or aNSCLC) in China (8–10%) [12, 13] and Western countries (4%) [10]. Although targeted therapies are available for patients with NSCLC who have genomic alterations [10], treatment options that can prolong survival for those who test negative for EGFR/ALK aberrations have been limited.
In June 2018, nivolumab, a programmed death-1 immune checkpoint-inhibitor, was approved in China for the treatment of locally advanced or metastatic NSCLC after prior platinum-based chemotherapy in adult patients without EGFR or ALK aberrations by the National Medical Products Administration (NMPA; formerly China National Drug Administration) [14], based on positive Phase III clinical trial results [15–17]. In global trials (CheckMate 017 [15] and CheckMate 057 [16]), nivolumab significantly improved the primary endpoint of overall survival (OS) in a broad population of previously treated patients, and was associated with reduced frequency of grade 3/4 adverse events (AEs) and improved QOL versus docetaxel in patients with squamous or non-squamous aNSCLC, respectively [15, 17–19]. Outcomes of nivolumab in CheckMate 078, which was conducted in Asian patients (90.7% Chinese) with squamous or non-squamous aNSCLC without ALK or EGFR aberrations [17], were consistent with those in the global trials [20], implying that outcomes in this context were not impacted by Asian ethnicity.
The Chinese clinical guidelines (2020) second-line therapy class I recommendation for patients with stage IV non-squamous NSCLC with performance status 0–2 is nivolumab, docetaxel or pemetrexed and the class II recommendation is pembrolizumab (for patients with PD-L1 ≥ 1) or atezolizumab [21]. The class I recommendation for patients with stage IV squamous with performance status of 0–2 is nivolumab or docetaxel; the class II recommendation for second-line treatment is pembrolizumab (for patients with PD-L1 ≥1), atezolizumab, gemcitabine or vinorelbine monotherapy, or afatinib (if not eligible for chemotherapy or immuno-oncology treatment) [21]. Patients with NSCLC and a performance status of 3–4 are to receive best supportive care [21].
Our objective was to evaluate the economic value of nivolumab versus docetaxel in the treatment of squamous and non-squamous aNSCLC after prior chemotherapy in adults without EGFR/ALK aberrations in China, applying the most relevant available data to the specific context of China. At the time of analysis nivolumab was not reimbursed in China.
Methods
Model Design
This analysis evaluated lifetime costs and health outcomes of nivolumab versus docetaxel by squamous and non-squamous histologies. For the cost-effectiveness analysis, we developed a cohort-based partitioned survival model with three mutually exclusive health states: progression-free (PF), progressed disease (PD), and death (ESM Online Resource Fig. 1). Use of a partitioned survival model has been extensively validated and applied in numerous previous technology appraisals [22, 23]. Health state occupancy was calculated using progression-free survival (PFS) and OS extrapolations, as described below. The model used a 1-week cycle length and a 20-year lifetime horizon, the latter based on the interval between the average age of the patient group and average life expectancy at birth in China. The median age of patients was 63 years in CheckMate 017 [15], 62 years in CheckMate 057 [16], and 60 years in CheckMate 078 [17]. The perspective was that of a healthcare payer in China; however, patient co-payments were included.
Model outcomes included absolute and incremental life-years (LYs), quality-adjusted life-years (QALYs), aggregated and disaggregated (total and incremental) costs associated with treatments, and incremental costs per LY and per QALY gained, expressed as the incremental cost-effectiveness ratio (ICER), for nivolumab versus docetaxel treatment. These outcomes were selected as they are typical of cost-effectiveness analyses of therapy interventions.
Costs and quality-adjusted life-years were discounted at 3% per year, and LYs are reported as both not discounted and discounted at 3% per year based on expert recommendation.
Input Data
Clinical data (individual patient-level data) were derived from the pivotal CheckMate Phase III trials (CheckMate 017 [NCT01642004], CheckMate 057 [NCT01673867], and CheckMate 078 [NCT02613507]). As oncology models for advanced disease can be sensitive to long-term survival estimates, survival data (PFS and OS) were derived from CheckMate 017 (squamous aNSCLC) and CheckMate 057 (non-squamous aNSCLC), because these global Phase III trials provided substantially longer survival follow-up (minimum follow-up, CheckMate 017 [squamous aNSCLC]: 64.2 months, CheckMate 057 [non-squamous aNSCLC]: 64.5 months) than CheckMate 078 (minimum follow-up, 37.3 months) [24, 25]. More mature data from both CheckMate 017 and CheckMate 057 reduce the uncertainty around long-term OS and PFS extrapolations in cost-effectiveness models, and therefore yield more robust ICERs [26]. In addition, median duration of treatment was similar across all three studies for each treatment arm. Given the consistency of outcomes across CheckMate 017, CheckMate 057, and CheckMate 078, irrespective of ethnicity, this approach was considered appropriate for a Chinese population.
As the patients in CheckMate 078 were predominantly Chinese, inputs related to patient characteristics, frequency of grade 3/4 AEs, subsequent treatment patterns, and QOL were taken from CheckMate 078, where possible, to further increase the specificity of the analysis to the target population.
Survival Data
The proportion of patients in each health state was estimated using parametric curves fitted to OS and PFS data from CheckMate 017 and CheckMate 057 (5-year, across both histologies). In terms of data comparability with CheckMate 078, the 3-year pooled hazard ratio (HR) for OS with nivolumab versus docetaxel in squamous/non-squamous aNSCLC was comparable for CheckMate 017 and 057 (3-year pooled, HR 0.70; 95% confidence interval [CI] 0.61–0.81) [20] and CheckMate 078 (3-year aNSCLC, HR 0.75; 95% CI 0.61–0.93) [25]. The HR for PFS was comparable for CheckMate 017 and 057 (3-year pooled, HR 0.80; 95% CI 0.69–0.92) [20] and CheckMate 078 (3-year aNSCLC, HR 0.78; 95% CI 0.64–0.96) [25].
For robust and accurate cost-effectiveness estimates of nivolumab in aNSCLC, the extrapolations from fitted parametric distributions to the survival data were evaluated using established methodology [18]. The choice of distribution for each survival dataset was informed by statistical goodness-of-fit assessed using Akaike information criterion/Bayesian information criterion statistics, visual inspection, and comprehensive testing of the proportional hazard assumption for nivolumab and docetaxel. In addition, extrapolations were assessed by both clinical plausibility and coherence with external data sources, by comparing extrapolated survival outcomes against long-term CheckMate 003 data [27] (for nivolumab) and conditional survival data from real-world US SEER data [3], as recommended by the National Institute for Health and Care Excellence (NICE) for England and Wales [28], and were tested with health economic and clinical experts. The ability of the functional forms to match predictions from validation datasets based on study populations with longer follow-up is of particular importance in evaluating immuno-oncology (I-O) treatments versus prior standards of care. Notably, in CheckMate 017 and 057, a substantial number of patients treated with the I-O agent nivolumab were still alive in the early database locks and responses and survival for these patients have shown to be durable [15, 16, 20, 29, 30].
In squamous aNSCLC, a dependent survival model was fitted for the docetaxel arm of CheckMate 017 based on a minimum follow-up of 60 months using the spline 2-knot hazards curve, with the HR for OS reported in CheckMate 017 (HR 0.61) applied to the fitted curve to estimate the nivolumab arm (ESM Online Resource Fig. 2). Nivolumab PFS was estimated from CheckMate 017 data using a 1-knot spline odds model, and docetaxel PFS was estimated from CheckMate 017 data using a 1-knot spline hazard model (ESM Online Resource Fig. 3).
In non-squamous aNSCLC, nivolumab OS was estimated using the log-normal model based on a minimum follow-up of 60 months from CheckMate 057 (ESM Online Resource Fig. 4). Docetaxel OS was estimated using a log-normal model based on minimum follow-up of 18 months from CheckMate 057 (ESM Online Resource Fig. 5) to minimise any effects of crossover, as patients who discontinued docetaxel treatment could cross over to receive nivolumab. A curve fit to data with a minimum follow-up of 18 months was chosen because only 0.7% of the docetaxel patients had crossed over to nivolumab at this time point; thus, the OS was less likely to be confounded by crossover than docetaxel survival with 60 months of follow-up. Following the OS landmark of docetaxel patients at 18 months, the parametric curve selection validation was based on conditional survival estimates from real-world databases including SEER. Nivolumab PFS was estimated using a 2-knot spline odds model (ESM Online Resource Fig. 6) and docetaxel PFS was estimated using a 2-knot spline odds model (ESM Online Resource Fig. 7), both using a minimum follow-up of 60 months from CheckMate 057. Progression-free survival was not affected by crossover. The different methods used for the survival data are based on the goodness-of-fit for the survival curves in the relevant CheckMate trials.
Adverse Event Data
Treatment-related grade 3/4 AEs with ≥1% incidence in either treatment arm were included in the model, derived from CheckMate 078 (ESM Online Resource Table 1) because this trial was specific to a predominantly Chinese population and thus reflects the safety profile in Chinese patients.
Utility Data
Health state utilities for patients in the PF or PD health states were derived using EuroQoL five dimensions three-level questionnaire (EQ-5D-3L) data collected in CheckMate 078, converted to utility values using Chinese tariffs (ESM Online Resource Table 2). The ‘death’ state utility was assumed to be zero. Treatment-specific utility values across histologies were used in the base case, while overall utility values (independent of treatment received) and aggregated values by health state for squamous and non-squamous separately were used in a series of scenario analyses.
Cost Inputs
The model included costs of disease management in the PF and PD states, drug acquisition, drug administration, management of grade 3/4 AEs, treatments received in subsequent lines of therapy, and end-of-life care. Costs in Chinese Yuan Renminbi are 2019 values or were inflated to 2019 values from older sources and were converted to US$ using the May 3, 2019, currency conversion rate at www.xe.com. Healthcare resource use and unit costs for administration, disease management, and terminal (end-of-life) care were derived using real-world evidence (RWE; Bristol Myers Squibb data on file). RWE inputs were obtained from a retrospective study undertaken to quantify healthcare resource utilisation and direct medical costs associated with aNSCLC across 24 consecutive months (1 January 2015 to 31 December 2016) in 10 Chinese hospitals. Costs and resource use for 3425 patients were estimated by histology and line of treatment; disease management costs per 4 weeks were ¥564 (US$84) in the PF health state and ¥500 (US$74) in the PD health state (ESM Online Resource Table 3).
At a licensed dose of 3 mg/kg every 2 weeks, the cost per dose of nivolumab was ¥18,520 (US$2750) [31], assuming that patients received two 10 mL vials, based on the average patient body weight in CheckMate 078. At a dose of 75 mg/m2 every 3 weeks, the cost per dose of docetaxel was ¥9100 (US$1351) (ESM Online Resource Table 4). CheckMate 017/057/078 dosing protocols indicated treatment until progression or unacceptable toxicity; patients who received nivolumab could continue beyond initial disease progression if the investigator determined that the patient was receiving clinical benefit [15–17]. However, in the model, patients in the PF health state were assumed to have received treatment with nivolumab biweekly for up to 2 years (2-year stopping rule). The maximum duration of treatment of immune-therapy is uncertain at present given the lack of long-term data; however, this base-case assumption of 2 years’ maximum treatment duration was based on the CheckMate 003 trial, which included a 96-week stopping rule (approximately 2 years) accompanied by a subsequent survival plateau out to 6 years, and patterns of data from a randomised study of pembrolizumab in second-line treatment of advanced NSCLC that included a 2-year stopping rule [32, 33]. The clinical acceptability of terminating nivolumab treatment at 2 years for patients who are still on therapy at this point has been further supported by the Swedish Dental and Pharmaceutical Benefits Agency (TLV committee) and NICE [19, 34–36]. Full reimbursement for the entire extrapolated treatment duration was used for docetaxel. Drug administration costs were estimated at ¥180 (US$27) per intravenous administration, derived from the RWE study (Bristol Myers Squibb data on file).
China-specific grade 3/4 AE disease management costs were identified by a systematic literature review. Where possible, the cost per episode for each grade 3/4 AE was estimated using average costs reported in the published literature. This included anaemia, bone marrow failure, fatigue, febrile neutropenia, neutropenia, and decreased white blood cell count. For other grade 3/4 AEs, where no costs were found in published sources, costs were estimated through key opinion leader interviews (ESM Online Resource Table 5). The total per-episode treatment cost of each AE was multiplied by the AE incidence from CheckMate 078 (ESM Online Resource Table 1) and included in the first week of treatment in the model, per standard accepted practice for health technology assessment agencies.
Subsequent treatment costs were estimated based on CheckMate 078 data. Upon progression on or after treatment with either nivolumab or docetaxel, it was assumed that 43–48% of patients would go on to receive best supportive care and palliative care, in line with the CheckMate 078 study. It was further assumed that the remaining proportion of patients would receive subsequent systemic anti-cancer therapy with a duration of 2.6 months after nivolumab or 4.4 months after docetaxel. Subsequent treatment distribution was based on the distribution of the top four treatments reported in CheckMate 078 in each arm, used to represent Chinese clinical practice (ESM Online Resource Table 6). Based on these assumptions and input data, the cost per person receiving subsequent therapy after disease progression was estimated at ¥47,197 (US$7008) for nivolumab and ¥97,782 (US$14,519) for docetaxel.
Terminal-care costs account for costs in the last month (30 days) of a patient’s life and were applied as a one-off cost to all patients newly entering the death state over the time horizon. The cost of terminal care was estimated from the RWE study (Bristol Myers Squibb data on file) at ¥149,019 (US$22,127) per patient.
Sensitivity Analyses
One-way (deterministic) sensitivity analyses were conducted by varying key parameters (body weight/surface area, costs, duration of subsequent treatment, utilities) by their 95% CIs or ±20% of the base-case values based on data availability. The discount rate for costs and QALYs varied from 0–6%.
Probabilistic sensitivity analyses were conducted by sampling the value of key parameters in the model from probabilistic distributions, using 1000 iterations (ESM Online Resource Table 7).
Scenario Analyses
Scenario analyses included applying histology-specific or treatment-specific utilities or both:
Base case: CheckMate 078 derived utilities using a treatment-specific approach across both histologies
Scenario 1: CheckMate 078 derived utilities using a histology-specific but not treatment-specific approach
Scenario 2: Use of CheckMate 017 or CheckMate 057 overall histology-specific utilities
Additional scenarios include alternative PFS and OS parametric curves; reimbursement of nivolumab for the entire extrapolated treatment duration; 2-year maximum treatment duration for docetaxel; disease management costs ± 50%; and AE management costs ± 50%.
Results
Base Case
Compared with docetaxel, in both squamous and non-squamous aNSCLC, nivolumab was associated with survival and quality-adjusted survival benefits, at additional cost (Table 1). With nivolumab treatment, patients with squamous aNSCLC were projected to gain an additional 1.489 LYs (undiscounted; 1.226 discounted LYs) and 1.034 QALYs versus those receiving docetaxel; in non-squamous aNSCLC, the additional gain was projected to be 1.228 LYs (undiscounted; 0.995 discounted LYs) and 0.833 QALYs. The incremental cost anticipated for nivolumab versus docetaxel was ¥214,353 (US$31,829) in squamous aNSCLC and ¥158,993 (US$23,608) in non-squamous aNSCLC. The incremental cost per LY gained for nivolumab versus docetaxel in squamous and non-squamous aNSCLC was ¥143,961 (US$21,376; ¥174,821 discounted [US$25,959]) and ¥129,477 (US$19,227; ¥159,834 discounted [US$23,733]), respectively. The ICER for nivolumab versus docetaxel was ¥207,388 (US$31,537) per QALY gained in squamous aNSCLC and ¥190,919 (US$29,033) per QALY gained in non-squamous aNSCLC.
Table 1.
Base case incremental results for nivolumab compared with docetaxel
| aNSCLC histology | Incremental costs (¥ [US$a]) | Incremental LYs, undiscounted | Incremental LYs, discounted | Incremental QALYs | Incremental cost per LYG, undiscounted (¥ [US$a]) | Incremental cost per LYG, discounted (¥ [US$a]) | Incremental cost per QALY (¥ [US$a]) |
|---|---|---|---|---|---|---|---|
| Deterministic | |||||||
| Squamous | 214,353 (31,829) | 1.489 | 1.226 | 1.034 | 143,961 (21,376) | 174,821 (25,959) | 207,388 (30,794) |
| Non-squamous | 158,993 (23,608) | 1.228 | 0.995 | 0.833 | 129,477 (19,226) | 159,834 (23,733) | 190,919 (28,349) |
| Stochastic | |||||||
| Squamous | 245,149 (36,401) | – | – | 1.143 | – | – | 214,536 (31,856) |
| Non-squamous | 187,809 (27,887) | – | – | 0.854 | – | – | 219,971 (32,663) |
LYs were not included within the probabilistic sensitivity analyses
aNSCLC advanced non-small cell lung cancer, LY life-year, LYG life-year gained, QALY quality-adjusted life-year
aConversion using exchange rate ¥1 = US$0.148487; May 3, 2019; www.xe.com
In squamous aNSCLC, patients receiving nivolumab incurred higher treatment acquisition costs (¥303,143 [US$45,013]) than docetaxel (¥58,827 [US$8735]) (Table 2). Patients receiving nivolumab incurred lower subsequent treatment costs (¥19,152 [US$2567] versus ¥46,448 [US$6897], respectively) and grade 3/4 AE management costs (¥91 [US$16] versus ¥4229 [US$628], respectively) versus docetaxel. A similar pattern was observed in non-squamous aNSCLC, with higher treatment acquisition costs incurred for patients receiving nivolumab (¥268,098 [US$39,809]) versus docetaxel (¥78,872 [US$11,712]) and lower subsequent treatment costs (¥19,355 [US$2874] versus ¥46,383 [US$6887]) and AE management costs (¥91 [US$16] versus ¥4229 [US$628]) reported for patients receiving nivolumab versus docetaxel, respectively.
Table 2.
Base case disaggregated per-patient cost breakdown for nivolumab and docetaxel
| Total cost (¥ [US$a]) | Cost breakdown (¥ [US$a]) | |||||
|---|---|---|---|---|---|---|
| Disease managementb | Treatment acquisition | Treatment administration | Subsequent treatment | Grade 3/4 AEs | ||
| Squamous aNSCLC | ||||||
| Nivolumab | 478,830 (71,100) | 153,499 (22,793) | 303,143 (45,013) | 2945 (437) | 19,152 (2844) | 91 (14) |
| Docetaxel | 264,477 (39,271) | 152,790 (22,687) | 59,827 (8735) | 1183 (176) | 46,448 (6897) | 4229 (628) |
| Non-squamous aNSCLC | ||||||
| Nivolumab | 443,980 (65,925) | 153,831 (22,842) | 268,098 (39,809) | 2605 (387) | 19,355 (2874) | 91 (15) |
| Docetaxel | 284,986 (42,317) | 153,944 (22,859) | 78,872 (11,712) | 1560 (232) | 46,383 (6887) | 4229 (628) |
AE adverse event, aNSCLC, advanced non-small cell lung cancer
aConversion using exchange rate ¥1 = US$0.148487; May 3, 2019; www.xe.com
bDisease management costs include computed tomography, positron emission tomography, magnetic resonance, x-rays, ultrasound, radiology, radiation therapy, laboratory tests, and other organ systems (gastrointestinal, genitourinary, cardiac, nervous, reproductive)
Scenario Analysis
Alternative utilities or extrapolation curve choices had minimal impact on the ICERs in squamous and non-squamous aNSCLC (Table 3). Reimbursement of nivolumab treatment beyond 2 years increased the ICER of nivolumab versus docetaxel in both squamous and non-squamous aNSCLC whereas limiting the duration of treatment of docetaxel for 2 years increased the ICER in non-squamous aNSCLC. Increasing or decreasing disease management costs by 50% had a modest impact on the ICERs, while increasing or decreasing grade 3/4 AE costs by 50% had a negligible impact on the ICERs (Table 3).
Table 3.
Results of scenario analyses
| Scenario | ICER cost/QALY (¥ [US$a]) | ||
|---|---|---|---|
| NSQ aNSCLC | SQ aNSCLC | ||
| Base-case ICER | 190,919 (28,349) | 207,388 (30,794) | |
| 1 | CheckMate 078 derived utilities using an overall (not treatment-specific) approach that is histology-specific | 199,193 (29,578) | 215,589 (32,012) |
| 2 | Use of CheckMate 017 or CheckMate 057 overall histology-specific utilities | 208,822 (31,007) | 228,029 (33,859) |
| 3 | Alternative PFS and OS parametric curves: | ||
| OS nivolumab: generalised gamma (for NSQ), loglogistic for SQ | 181,806 (26,996) | 224,006 (33,262) | |
| OS docetaxel: 1-knot spline normal (for NSQ), loglogistic for SQ | 182,775 (27,140) | 198,957 (29,543) | |
| PFS nivolumab: 1-knot spline hazard (for NSQ), 2-knot hazard spline (for SQ) | 185,714 (27,576) | 211,432 (31,395) | |
| PFS docetaxel: Log normal (for NSQ), 2-knot hazard spline (for SQ) | 189,451 (28,131) | 207,480 (30,808) | |
| 4 | Reimbursement of nivolumab for the entire extrapolated treatment duration | 666,699 (98,996) | 682,151 (101,291) |
| 5 | 2-year maximum treatment duration for docetaxel | 193,006 (28,659) | 248,950 (36,966) |
| 6 | Disease management costs: | ||
| +50% | 195,240 (28,991) | 211,754 (31,443) | |
| −50% | 186,599 (27,708) | 203,022 (30,146) | |
| 7 | AE management costs: | ||
| +50% | 188,435 (27,980) | 205,386 (30,497) | |
| − 50% | 193,404 (28,718) | 209,390 (31,092) | |
AE adverse event, aNSCLC advanced non-small cell lung cancer, NSQ non-squamous cell carcinoma, OS overall survival, PFS progression-free survival, QALY quality-adjusted life-year, SQ squamous cell carcinoma
aConversion using exchange rate ¥1 = US$0.148487; May 3, 2019; www.xe.com
Sensitivity Analysis
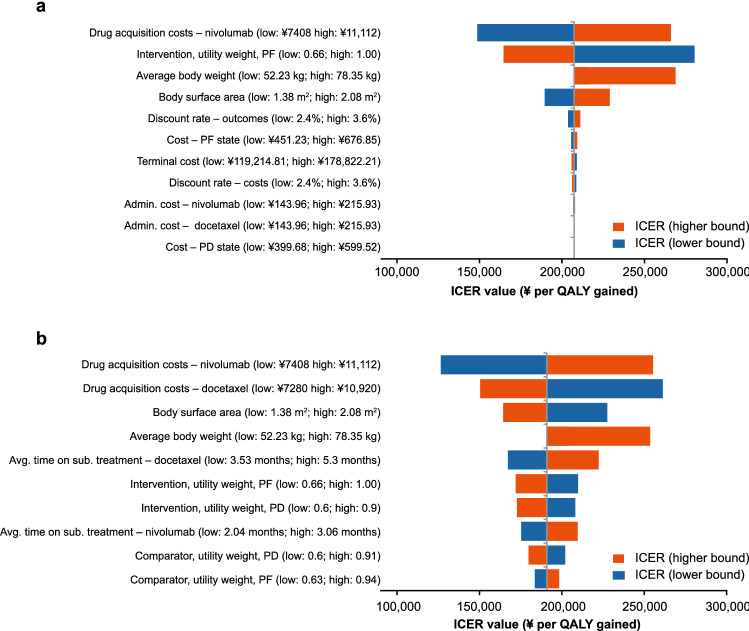
One-way sensitivity analyses demonstrated that drug acquisition costs, intervention utility weight, average body weight, and body surface area were key model drivers for both squamous and non-squamous aNSCLC (Fig. 1). Probabilistic sensitivity analysis indicated that the stochastic results aligned with the deterministic results (Table 1, ESM Online Resource Fig. 8). The probability of nivolumab of being a cost-effective treatment option compared with docetaxel at willingness-to-pay thresholds of ¥180,000 (US$26,727), ¥200,000 (US$29,697) and ¥250,000 (US$37,122) per QALY gained, was 30.6%, 43.2%, and 66.5%, respectively for squamous aNSCLC and 27.0%, 38.5%, and 66.5%, respectively, for non-squamous aNSCLC (ESM Online Resource Fig. 9).
Fig. 1.
Deterministic sensitivity analysis in (a) squamous aNSCLC and (b) non-squamous aNSCLC. The results for body weight reflect the effect of vial sharing. Only an increase or decrease in weight sufficient to need a new vial of nivolumab or docetaxel (calculated via body surface area) would have an impact on the results. For average patient body weight, there is no bar for lower parameter value because the reduction in patient weight used in the sensitivity analysis did not lead to a change in vial calculations for nivolumab and therefore the ICER was unaffected. admin. administration, aNSCLC advanced non-small cell lung cancer, avg. average, ICER incremental cost-effectiveness ratio, PD progressed disease, PF progression-free, sub. subsequent
Discussion
Clinical trial results demonstrated that nivolumab is associated with substantial increased survival benefits versus docetaxel in both squamous and non-squamous aNSCLC [15–17]. To our knowledge, this analysis is the first economic evaluation of nivolumab in patients with aNSCLC in China that utilises the totality of the available clinical evidence for nivolumab in pre-treated NSCLC. The results indicate that nivolumab would be associated with extended survival and quality-adjusted survival benefits in squamous aNSCLC (an additional 1.489 LYs [undiscounted; 1.226 discounted LYs]) and 1.034 QALYs) and in non-squamous aNSCLC (an additional 1.228 LYs [undiscounted; 0.995 discounted LYs] and 0.833 QALYs), and incremental costs of ¥214,353 (US$31,829 [37]) in squamous aNSCLC and ¥158,993 (US$23,608) in non-squamous aNSCLC versus docetaxel. The ICER for nivolumab versus docetaxel was ¥207,388 (US$30,794) per QALY gained in squamous aNSCLC and ¥190,919 (US$28,349) per QALY gained in non-squamous aNSCLC.
The ICER value is considered as one of the decision-making criteria for payers; however, no officially recognised willingness-to-pay threshold has been set in China to date. In early 2018, the International Society for Pharmacoeconomics and Outcomes Research issued a report to define elements of value in healthcare, which indicated that several potential elements could be incorporated to produce further scenarios for different value-assessment settings [38]. Although there is no current consensus on the dimensions of value in China, a consideration of these can strategically support payers in their decision making to: (1) improve the efficiency and performance of health insurance funding; (2) lead the value orientation of the drug quality system; and (3) build a high-quality, value-based service delivery system [39].
Although there is no officially recognised willingness-to-pay threshold in China, a threshold of 3× per-capita gross domestic product (GDP) per QALY has been used frequently in Chinese cost-effectiveness analyses [40–42]. In China, the per-capita national GDP in 2019 (based on end-of-year population) was ¥70,078 (US$10,406) [43], with considerable regional disparity reflecting China’s unbalanced economic development. These regional variations present a key challenge in determining a national willingness-to-pay threshold. For squamous aNSCLC, the ICER for nivolumab versus docetaxel in our analysis was ¥207,388 (US$30,794) per QALY, approximately 3.0× the 2019 national per-capita GDP and about 0.8× the 2019 per-capita GDP in Beijing or Shanghai. For non-squamous aNSCLC, the ICER for nivolumab versus docetaxel in our analysis was below the threshold of 2.7× the 2019 national per-capita GDP (¥190,919 [US$28,349] per QALY).
The base-case cost-effectiveness results for nivolumab versus docetaxel in aNSCLC in China are comparable with published estimates for other lung cancer therapies in aNSCLC in China (Table 4) [40, 44, 45]. Icotinib or gefitinib as first-line treatments for aNSCLC had ICERs of ¥135,890 (US$19,809) to ¥195,407 (US$28,485) per QALY versus pemetrexed plus cisplatin, and osimertinib had an ICER of ¥329,836 (US$48,081) per QALY versus chemotherapy in aNSCLC after progression following first-line EGFR tyrosine kinase inhibitor therapy.
Table 4.
Cost-effectiveness of other cancer therapies in NSCLC in China
| Drug | Control arm | Study perspective | ICER, US$ per QALY | Indication |
|---|---|---|---|---|
| Osimertinib [44] | Chemotherapy | Payer and the healthcare system | 48,081 | NSCLC after progression following first-line EGFR TKI therapy |
| Gefitinib [40] |
Pemetrexed plus cisplatin alone |
Chinese healthcare system | 28,485 | First-line treatments for aNSCLC |
| Gefitinib strategy with PAP [40] | Pemetrexed plus cisplatin alone | Chinese healthcare system | 22,577 | First-line treatments for aNSCLC |
| Icotinib [40] | Pemetrexed plus cisplatin alone | Chinese healthcare system | 19,809 | First-line treatments for aNSCLC |
| Bevacizumab [45] | Pemetrexed plus cisplatin | Social perspective | 299,155 | Maintenance therapy for metastatic NSCLC |
| Nivolumab versus docetaxel in the present analysis for comparison | ||||
| Nivolumab | Docetaxel | Healthcare payer in China | 33,802a | Squamous |
| Nivolumab | Docetaxel | Healthcare payer in China | 25,204a | Non-squamous |
aConversion using exchange rate ¥1 = US$0.148487; May 3, 2019; www.xe.com
aNSCLC advanced non-small cell lung cancer, EGFR epidermal growth factor receptor, ICER incremental cost-effectiveness ratio, NSCLC non-small cell lung cancer, PAP patient assistance programme, QALY quality-adjusted life-year, TKI tyrosine kinase inhibitor
The results of the cost-effectiveness analyses of nivolumab versus docetaxel for patients with squamous/non-squamous histology in the present study were also similar to previously published studies of nivolumab in other countries that used comparable time horizons and approach. The reported gains were 1.28/1.03 LY and 0.85/0.67 LY for Sweden and Canada, respectively [46] and 1.49/1.23 LY for the UK [26]. Nivolumab is approved for reimbursement in most countries, and such approvals and widespread use suggest acceptable value for money [14, 47, 48]. But, because of differences in healthcare systems, costs, and economic development, caution should be applied when comparing results across countries.
Two recent cost-effectiveness studies in China for nivolumab versus docetaxel in NSCLC have been reported. In Liu et al, nivolumab resulted in a LY gain of 0.30 (~3.6 months) versus docetaxel over a lifetime horizon, compared with incremental LYs of 1.235 and 1.330 for patients with squamous and non-squamous histology, respectively, in the present analysis [49]. Thus, the Liu et al study reports a substantially higher ICER value for nivolumab versus docetaxel (US$93,307 per QALY) [49] compared to the present histology-specific results. These differences may be explained by differing methods used to select survival extrapolation and for the validation process for long-term estimates. Liu et al. fitted Weibull parametric models to digitised docetaxel survival curves from CheckMate 078 [49]. However, the extrapolations appear to underpredict the Kaplan–Meier data from the trial and no validation was undertaken to increase confidence in their clinical plausibility and coherence with other data sources [17, 49]. Liu et al have further assumed proportional hazards, which may not be appropriate given that unlike chemotherapies, I-O therapies can be associated with delayed but durable responses, which can translate into improved long-term survival [50]. Thus, I-O treatments are often associated with complex hazard functions, which limit the reliability of standard parametric models, especially with short duration of follow-up [50, 51]. To address this, the analyses in the present study utilised independent parametric curves for nivolumab and docetaxel that not only provided a better fit to the respective underlying trial but also underwent extensive validation (including clinical validation) to increase the reliability of the outcomes. However, it is important to note that survival extrapolation was validated with external data from the USA, as long-term data—such as those from SEER—are not available in a Chinese population.
A second study by Zhang et al in 2020 reported the cost effectiveness of second-line nivolumab versus docetaxel for platinum-treated aNSCLC from a Chinese health service system perspective [52]. This study considerably underestimated the survival benefit for nivolumab and docetaxel, as well as the incremental benefit of nivolumab in terms of QALYs, resulting in a comparably high ICER value (US$ 72,128 per QALY) [52]. The authors estimated that OS at the end of 2 years was close to zero; however, in the CheckMate 078 study in predominately Chinese patients, 28% and 18% of patients were alive at 2 years and 19% and 12% were alive at 3 years in nivolumab and docetaxel groups, respectively [25, 52].
Although the first published economic assessment of nivolumab in second-line+ NSCLC was based on treat-to-progression dosing [53], subsequent health technology assessment opinions indicate that a 2-year treatment-stopping rule may be appropriate and reflective of clinical practice [27, 32, 33, 35, 36, 54]. Moreover, the long-term survival benefits of I-O therapies in second-line+ NSCLC trials with stopping rules (CheckMate 003) have shown to be similar to those without stopping rules (CheckMate 017 and 057) [55] and in CheckMate 078, very few (< 9%) patients were treated for longer than 2 years [56]. Additional data of the effects of stopping treatment at 2 years will emerge with the 2-year stopping rule now widely adopted in NSCLC trials [57–61] and future trials.
In our model, several factors may potentially overestimate costs with nivolumab and therefore our reported ICERs should be considered conservative. First, disease management costs were assumed to be equal for nivolumab and docetaxel. However, it is likely that treatment-specific monitoring costs (a disease management component cost that could not be estimated specifically due to lack of data) would be lower for nivolumab owing to its improved safety profile versus docetaxel. Second, improved survival and QOL with nivolumab may reduce the use of traditional Chinese medicine and other pseudo-adjuvants, which are widely procured by hospitals for cancer patients receiving chemotherapy in China. Pseudo-adjuvants were reported to account for 28.1% of total procurement costs for all anti-tumour drugs in Shanghai in 2015 [62]. Our model did not consider the concurrent use of these non-cancer-directive treatment agents in the chemotherapy arm and did not account for any increase in the use of traditional Chinese medicine with docetaxel. Only docetaxel was considered as a relevant comparator, reflecting the standard of care in China at the time of our study, which is a limitation of this analysis. Third, the model did not consider the possibility of administering nivolumab in the outpatient setting due to its favourable safety profile and convenience, while patients receiving chemotherapy in China will usually be hospitalized [63]. In a study of 1002 patients, only one was treated in the outpatient setting (Bristol Myers Squibb data on file). Administration of nivolumab in an outpatient setting could have the potential to save resources associated with inpatient treatment and reduce the pressure on hospitals.
In addition to the cost elements described, nivolumab may provide a good illustration of the broader societal value that can be achieved through improved health outcomes. A study in Canada explored these issues via an analysis of nivolumab in squamous aNSCLC from a broad societal perspective, including costs and benefits related to caregiver burden, value to current non-patients based on the probability that they may become sick in the future (value of insurance), the option value of treatment and the value placed by patients with cancer on durable survival gains (value of hope) [53]. The study found that about half of the incremental benefit of nivolumab was omitted when using a conventional payer perspective [53]. Our analysis included only direct costs to the healthcare payer in China without considering wider societal benefits; thus, it would likely produce a higher estimate of the ICER of nivolumab. Including other dimensions of value, such as those explored in the Canadian study, could better describe the value offered by nivolumab in this setting to patients in China, their families and to society, and therefore better inform decision making. Even if the scientific spill-over of these broader value dimensions cannot be incorporated into the current economic model, this information is useful to enrich the perspectives of value assessment in China in the future.
Since its launch in China, nivolumab has presented an additional treatment option with important clinical value and long-term survival prospects for previously treated patients. Our model has demonstrated the economic value of nivolumab, which would be further improved when considering the patient assistance programme or its potential inclusion in the national reimbursement drug list, as a price reduction/discount would likely be required.
Conclusion
This is the first economic evaluation undertaken for nivolumab in a Chinese context using patient-level data. Nivolumab was associated with survival and quality-adjusted survival benefits at incremental cost versus docetaxel in aNSCLC. Nivolumab is therefore expected to deliver direct healthcare benefits to Chinese patients and value to the Chinese healthcare system. The true clinical and economic value of nivolumab is expected to be greater than estimated herein, as this analysis adopted a traditional healthcare payer perspective, where not all treatment benefits and costs of relevance to society have been captured.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We acknowledge the RWE database analysis by Happy Life Technology Ltd, local market insights by Liya Fan, and Kate Young and Ritika Jain of Parexel International for health economic insights. Medical writing and editorial assistance were funded by Bristol Myers Squibb and provided by Breanne Landry, PhD, from Parexel International.
Declarations
Funding
This analysis was funded by Bristol Myers Squibb. Development of the health economic model, data collection, and data interpretation were funded by Bristol Myers Squibb. Data analysis for this study was partly funded by Bristol Myers Squibb.
Conflicts of interest
S. Hu is a Health Economics Professor employed by the School of Public Health, Fudan University. Z. Tang was an employee of Sino-American Shanghai Squibb Pharmaceuticals Ltd at the time the work was undertaken. J.P. Harrison, N. Hertel, J.R. Penrod, J.R. May, and A. Juarez-Garcia are employees of Bristol Myers Squibb and report stock ownership in Bristol Myers Squibb. O. Holdgate was an employee of Parexel International at the time the work was undertaken.
Ethics approval
Not applicable
Consent to participate
Not applicable
Consent for publication
Not applicable
Availability of data and material
The economic model used in this study is not publicly available to protect commercial-in-confidence information. Details of inputs used to generate the analysis are presented within the Supplemental Materials.
Authors’ contributions
ZT, JPH, NH, JRP, JRM, and OH contributed to the study conception and design. SH, ZT, JPH, NH, JRP, JRM, AJ-G, and OH performed material preparation, data collection, and analysis. All authors reviewed previous versions of the manuscript and have read and approved the final manuscript.
Footnotes
Zhiliu Tang was working at Affiliation 2 at the time of the study, and clarify that GSK was not involved in the study.
References
- 1.International Agency for Research on Cancer. Cancer today. Data visualization tools for exploring the global cancer burden in 2018. http://gco.iarc.fr/today/home. Accessed 7 Dec 2018.
- 2.American Cancer Society. What is non-small cell lung cancer? https://www.cancer.org/cancer/non-small-cell-lung-cancer/about/what-is-non-small-cell-lung-cancer.html. Accessed 7 Dec 2018.
- 3.National Cancer Institute Surveillance Epidemiology and End Results. Cancer stat facts: lung and bronchus cancer. https://seer.cancer.gov/statfacts/html/lungb.html. Accessed 31 May 2019.
- 4.Chouaid C, Agulnik J, Goker E, et al. Health-related quality of life and utility in patients with advanced non-small-cell lung cancer: a prospective cross-sectional patient survey in a real-world setting. J Thorac Oncol. 2013;8(8):997–1003. doi: 10.1097/JTO.0b013e318299243b. [DOI] [PubMed] [Google Scholar]
- 5.Hechtner M, Eichler M, Wehler B, et al. Quality of life in NSCLC survivors—a multicenter cross-sectional study. J Thorac Oncol. 2019;14(3):420–435. doi: 10.1016/j.jtho.2018.11.019. [DOI] [PubMed] [Google Scholar]
- 6.Jassem J, Penrod JR, Goren A, et al. Caring for relatives with lung cancer in Europe: an evaluation of caregivers' experience. Qual Life Res. 2015;24(12):2843–2852. doi: 10.1007/s11136-015-1028-1. [DOI] [PubMed] [Google Scholar]
- 7.Wood R, Taylor-Stokes G, Lees M. The humanistic burden associated with caring for patients with advanced non-small cell lung cancer (NSCLC) in three European countries—a real-world survey of caregivers. Support Care Cancer. 2019;27(5):1709–1719. doi: 10.1007/s00520-018-4419-3. [DOI] [PubMed] [Google Scholar]
- 8.Kris MG, Johnson BE, Berry LD, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA. 2014;311(19):1998–2006. doi: 10.1001/jama.2014.3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosell R, Moran T, Queralt C, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361(10):958–967. doi: 10.1056/NEJMoa0904554. [DOI] [PubMed] [Google Scholar]
- 10.Suh JH, Johnson A, Albacker L, et al. Comprehensive genomic profiling facilitates implementation of the National Comprehensive Cancer Network guidelines for lung cancer biomarker testing and identifies patients who may benefit from enrollment in mechanism-driven clinical trials. Oncologist. 2016;21(6):684–691. doi: 10.1634/theoncologist.2016-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi Y, Li J, Zhang S, et al. Molecular epidemiology of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology—mainland China subset analysis of the PIONEER study. PLoS ONE. 2015;10(11):e0143515. doi: 10.1371/journal.pone.0143515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wen S, Dai L, Wang L, et al. Genomic signature of driver genes identified by target next-generation sequencing in Chinese non-small cell lung cancer. Oncologist. 2019;24(11):e1070–e1081. doi: 10.1634/theoncologist.2018-0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liang H, Song X, Zhang Y, et al. Real-world data on EGFR/ALK gene status and first-line targeted therapy rate in newly diagnosed advanced non-small cell lung cancer patients in Northern China: a prospective observational study. Thorac Cancer. 2019;10(7):1521–1532. doi: 10.1111/1759-7714.13090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bristol Myers Squibb. Press release: China National Drug Administration approves country’s first immuno-oncology agent, Opdivo (nivolumab injection), for previously treated non-small cell lung cancer (NSCLC). https://news.bms.com/press-release/corporatefinancial-news/china-national-drug-administration-approves-countrys-first-imm. Accessed 19 Mar 2019.
- 15.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu YL, Lu S, Cheng Y, et al. Nivolumab versus docetaxel in a predominantly Chinese patient population with previously treated advanced NSCLC: CheckMate 078 randomized phase III clinical trial. J Thorac Oncol. 2019;14(5):867–875. doi: 10.1016/j.jtho.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 18.Latimer N. NICE DSU Technical Support Document 14: Survival analysis for economic evaluations alongside clinical trials—extrapoloation with patient-level data. http://nicedsu.org.uk/wp-content/uploads/2016/03/NICE-DSU-TSD-Survival-analysis.updated-March-2013.v2.pdf. Accessed 5 May 2019. [PubMed]
- 19.Tandvårds- och läkemedelsförmånsverket (TLV). Prissökningar i databsen. http://www.tlv.se/beslut/sok/lakemedel. Accessed 8 Apr 2019.
- 20.Vokes EE, Ready N, Felip E, et al. Nivolumab versus docetaxel in previously treated advanced non-small-cell lung cancer (CheckMate 017 and CheckMate 057): 3-year update and outcomes in patients with liver metastases. Ann Oncol. 2018;29(4):959–965. doi: 10.1093/annonc/mdy041. [DOI] [PubMed] [Google Scholar]
- 21.Guo Z, Chuang L, Liu Z, et al. Chinese Society of Clinical Oncology (CSCO) diagnosis and treatment guidelines for lung cancer (version 2020). People's Medical Publishing House. 2020.
- 22.National Institute of Health and Care Excellence. Nivolumab for previously treated squamous non-small-cell lung cancer: Technology appraisal guidance [TA483]. www.nice.org.uk/guidance/ta483. Accessed 16 Mar 2020.
- 23.National Institute of Health and Care Excellence. Nivolumab for previously treated non-squamous non-small-cell lung cancer: Technology appraisal guidance [TA484]. www.nice.org.uk/guidance/ta484. Accessed 16 Mar 2020.
- 24.Borghaei H, Gettinger S, Vokes EE, et al. Five-year outcomes from the randomized, phase III trials CheckMate 017 and 057: nivolumab versus docetaxel in previously treated non-small-cell lung cancer. J Clin Oncol. 2021; JCO2001605. [DOI] [PMC free article] [PubMed]
- 25.Cheng Y, Wu Y-L, Lu S, et al. Three-year follow-up from CheckMate 078: nivolumab versus docetaxel in a predominantly Chinese patient population with previously treated advanced non-small cell lung cancer. September 16–20, 2020; Xiamen, China. [DOI] [PubMed]
- 26.Rothwell B, Kiff C, Ling C, et al. Cost effectiveness of nivolumab in patients with advanced, previously treated squamous and non-squamous non-small-cell lung cancer in England. Pharmacoecon Open. 2021;5(2):251–60. Epub 2020 Dec 17. [DOI] [PMC free article] [PubMed]
- 27.Gettinger S, Horn L, Jackman D, et al. Five-year follow-up of nivolumab in previously treated advanced non-small-cell lung cancer: results from the CA209-003 study. J Clin Oncol. 2018;36(17):1675–1684. doi: 10.1200/JCO.2017.77.0412. [DOI] [PubMed] [Google Scholar]
- 28.National Institute of Health and Care Excellence. Guide to the methods of technology appraisal 2013: Process and methods [PMG9]. www.nice.org.uk/process/pmg9/chapter/foreword. Accessed 7 Aug 2020. [PubMed]
- 29.Horn L, Spigel DR, Vokes EE, et al. Nivolumab versus docetaxel in previously treated patients with advanced non-small-cell lung cancer: two-year outcomes from two randomized, open-label, phase III trials (CheckMate 017 and CheckMate 057) J Clin Oncol. 2017;35(35):3924–3933. doi: 10.1200/JCO.2017.74.3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gettinger S, Borghaei H, Brahmer J, et al. Five-year outcomes from the randomized, phase 3 trials CheckMate 017/057: nivolumab vs docetaxel in previously treated NSCLC. J Thorac Oncol. 2019;14(10, Supplement):OA14.04. doi: 10.1016/j.jtho.2019.08.486. [DOI] [Google Scholar]
- 31.Bristol Myers Squibb. Provincial bidding price from Yaozhi. https://db.yaozh.com/yaopinzhongbiao. Accessed 19 Nov 2019.
- 32.Gettinger SN, Horn L, Gandhi L, et al. Overall survival and long-term safety of nivolumab (anti-programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol. 2015;33(18):2004–2012. doi: 10.1200/JCO.2014.58.3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herbst RS, Garon EB, Kim D-W, et al. LBA4 Long-term follow-up in the KEYNOTE-010 study of pembrolizumab (pembro) for advanced NSCLC, including in patients (pts) who completed 2 years of pembro and pts who received a second course of pembro. Ann Oncol. 2018 doi: 10.1093/annonc/mdy511.003. [DOI] [Google Scholar]
- 34.National Institute of Health and Care Excellence. Technology appraisal guidance: Nivolumab for advanced squamous non-small-cell lung cancer after chemotherapy (TA655). www.nice.org.uk/guidance/ta655. Accessed 27 Nov 2020.
- 35.National Institute of Health and Care Excellence. Final appraisal document: Nivolumab for advanced squamous non-small-cell lung cancer after chemotherapy [TA655]. https://www.nice.org.uk/guidance/ta655/documents/final-appraisal-determination-document. Accessed 18 May 2021.
- 36.National Institute of Health and Care Excellence. Final appraisal document: Nivolumab for previously treated non-squamous non-small-cell lung cancer [TA484]. https://www.nice.org.uk/guidance/ta484/documents/final-appraisal-determination-document. Accessed 18 May 2021.
- 37.XE Currency Converter. www.xe.com Accessed 3 May 2019.
- 38.Lakdawalla DN, Doshi JA, Garrison LP, Jr, et al. Defining elements of value in health care—a health economics approach: an ISPOR Special Task Force report [3] Value Health. 2018;21(2):131–139. doi: 10.1016/j.jval.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 39.China Joint Study Partnership, World Bank Group, World Health Organization, et al. Healthy China: deepening health reform in China building high-quality and value-based service delivery. Policy Summary. 2016. https://openknowledge.worldbank.org/bitstream/handle/10986/31458/9781464812637.pdf. Accessed 5 Jun 2019.
- 40.Lu S, Ye M, Ding L, et al. Cost-effectiveness of gefitinib, icotinib, and pemetrexed-based chemotherapy as first-line treatments for advanced non-small cell lung cancer in China. Oncotarget. 2017;8(6):9996–10006. doi: 10.18632/oncotarget.14310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shi Q, Hu S, Furnback WE, et al. The cost-effectiveness of a NSCLC patient assistance program for pemetrexed maintenance therapy in People's Republic of China. Clinicoecon Outcomes Res. 2017;9:99–106. doi: 10.2147/CEOR.S119818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wan XM, Peng LB, Ma JA, et al. Economic evaluation of nivolumab as a second-line treatment for advanced renal cell carcinoma from US and Chinese perspectives. Cancer. 2017;123(14):2634–2641. doi: 10.1002/cncr.30666. [DOI] [PubMed] [Google Scholar]
- 43.National Bureau of Statistics of China. Annual by province. http://data.stats.gov.cn/english/easyquery.htm?cn=C01. Accessed 9 May 2022.
- 44.Wu B, Gu X, Zhang Q. Cost-effectiveness of osimertinib for EGFR mutation-positive non-small cell lung cancer after progression following first-line EGFR TKI therapy. J Thorac Oncol. 2018;13(2):184–193. doi: 10.1016/j.jtho.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 45.Zheng H, Xie L, Zhan M, et al. Cost-effectiveness analysis of the addition of bevacizumab to chemotherapy as induction and maintenance therapy for metastatic non-squamous non-small-cell lung cancer. Clin Transl Oncol. 2018;20(3):286–293. doi: 10.1007/s12094-017-1715-1. [DOI] [PubMed] [Google Scholar]
- 46.Chaudhary MA, Holmberg C, Lakhdari K, et al. Cost-effectiveness of nivolumab in squamous and non-squamous non-small cell lung cancer in Canada and Sweden: an update with 5-year data. J Med Econ. 2021;24(1):607–619. doi: 10.1080/13696998.2021.1917139. [DOI] [PubMed] [Google Scholar]
- 47.Bristol Myers Squibb. Press release: Bristol-Myers Squibb’s Opdivo (nivolumab) receives expanded FDA approval in previously-treated metastatic non-small cell lung cancer (NSCLC), offering improved survival to more patients https://news.bms.com/press-release/bristol-myers-squibbs-opdivo-nivolumab-receives-expanded-fda-approval-previously-treat. Accessed 15 Dec 2019.
- 48.Bristol Myers Squibb. Press release: European Commission approves expanded use of Opdivo® (nivolumab) to include previously treated metastatic non-squamous non-small cell lung cancer https://news.bms.com/press-release/european-commission-approves-expanded-use-opdivo-nivolumab-include-previously-treated. Accessed 15 Dec 2019.
- 49.Liu Q, Luo X, Peng L, et al. Nivolumab versus docetaxel for previously treated advanced non-small cell lung cancer in China: a cost-effectiveness analysis. Clin Drug Investig. 2020;40(2):129–137. doi: 10.1007/s40261-019-00869-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen TT. Statistical issues and challenges in immuno-oncology. J Immunother Cancer. 2013;1:18. doi: 10.1186/2051-1426-1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Santi I, Johal S, Yuan Y, et al. The impact of response at a landmark on overall survival: Implication for the economic evaluation of the value of immuno-oncology treatment in non-small cell lung cancer. Presented at: International Society for Pharmacoeconomics and Outcomes Research (ISPOR) 23rd Annual International Meeting, 19–23 May 2018; Baltimore, MD, USA. Poster number: PRM7.
- 52.Zhang L, Zeng X, Cai H, et al. Cost-effectiveness of second-line nivolumab for platinum-treated advanced non-small-cell lung cancer. J Comp Eff Res. 2020;9(18):1301–1309. doi: 10.2217/cer-2020-0053. [DOI] [PubMed] [Google Scholar]
- 53.Shafrin J, Skornicki M, Brauer M, et al. An exploratory case study of the impact of expanding cost-effectiveness analysis for second-line nivolumab for patients with squamous non-small cell lung cancer in Canada: Does it make a difference? Health Policy. 2018;122(6):607–613. doi: 10.1016/j.healthpol.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 54.Smare C, Venkatachalam M, Medin E, et al. An economic evaluation of nivolumab for the treatment of squamous and non-squamous NSCLC in the Swedish setting. Nord J Health Econ. 2019;7(1):47–64.
- 55.Antonia SJ, Borghaei H, Ramalingam SS, et al. Four-year survival with nivolumab in patients with previously treated advanced non-small-cell lung cancer: a pooled analysis. Lancet Oncol. 2019;20(10):1395–1408. doi: 10.1016/S1470-2045(19)30407-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang J, Lu S, Zhou C, et al. 2-year follow-up from CheckMate 078: nivolumab versus docetaxel in a predominantly Chinese patient population with previously treated advanced non-small cell lung cancer (NSCLC). Presented at: 22nd Annual Meeting of the Chinese Society of Clinical Oncology; 18-22 Sep 2019; Xiamen, China. Abstract number: 5225.
- 57.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 58.Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N Engl J Med. 2019;381(21):2020–2031. doi: 10.1056/NEJMoa1910231. [DOI] [PubMed] [Google Scholar]
- 59.Gandhi L, Rodriguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 60.Mok TSK, Wu YL, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393(10183):1819–1830. doi: 10.1016/S0140-6736(18)32409-7. [DOI] [PubMed] [Google Scholar]
- 61.Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 62.He J, Yang Y, Wu W, et al. Analysis on procurement and clinical use of antitumor drugs in Shanghai. Chin Health Res. 2017;20(3).
- 63.Garfield DH, Brenner H, Lu L. Practicing western oncology in Shanghai, China: one group's experience. J Oncol Pract. 2013;9(4):e141–e144. doi: 10.1200/JOP.2012.000811. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.