Abstract
Introduction
Natalizumab, a therapy for relapsing–remitting multiple sclerosis (RRMS), is associated with a risk of progressive multifocal leukoencephalopathy (PML). Over the last several years, practitioners have used off-label extended interval dosing (EID) of natalizumab to reduce PML risk, despite the absence of a large-scale efficacy evaluation.
Methods
We conducted a retrospective, multicenter cohort study among adults with RRMS receiving stable standard interval dosing (SID), defined as a ≥ 12-month consecutive period of ≥ 11 natalizumab infusions/year in France. We compared the 12-month risk difference of remaining relapse-free (primary endpoint) between patients who switched to EID (≤ 9 natalizumab infusions) and those who remained on SID, with a noninferiority margin of − 11%. We used propensity score methods such as inverse probability treatment weighting (IPTW) and 1:1 propensity score matching (PSM). Secondary endpoints were annualized relapse rate, disease progression, and safety.
Results
Baseline characteristics were similar between patients receiving EID (n = 147) and SID (n = 156). The proportion of relapse-free patients 12 months postbaseline was 142/147 in the EID (96.6%) and 144/156 in the SID group (92.3%); risk difference (95% CI) 4.3% (− 1.3 to 9.8%); p < 0.001 for non-inferiority. There were no significant differences between relapse rates (0.043 vs. 0.083 per year, respectively; p = 0.14) or Expanded Disability Status Scale mean scores (2.43 vs. 2.72, respectively; p = 0.18); anti-JC virus index values were similar (p = 0.23); and no instances of PML were reported. The comparisons using IPTW (n = 306) and PSM (n = 204) were consistent.
Conclusion
These results support the pertinence of using an EID strategy for RRMS patients treated with natalizumab.
Clinical Trials
gov identifier (NCT04580381).
Supplementary Information
The online version contains supplementary material available at 10.1007/s40120-023-00440-5.
Keywords: Extended interval dosing, Multiple sclerosis, Natalizumab, Real-world evidence
Key Summary Points
| Natalizumab, a therapy for relapsing–remitting multiple sclerosis (RRMS), is associated with a risk of progressive multifocal leukoencephalopathy (PML), a severe but rare opportunistic infection of the central nervous system. |
| Over the last several years, practitioners have used off-label extended interval dosing (EID) of natalizumab to reduce PML risk, despite the absence of a large-scale efficacy evaluation. |
| We conducted a real-world, retrospective, multicenter, noninferiority study using lack of relapse as the primary clinical endpoint and evaluating the efficacy in French patients with RRMS who switched to natalizumab EID (> 5-week interval) after at least 12 months of SID in comparison with those who continued on SID. |
| The proportion of relapse-free patients at 12 months postbaseline was similar for the EID and SID groups; there were no significant differences between relapse rates or Expanded Disability Status Scale mean scores, and no instances of PML were reported. |
| These results indicate that patients established on the approved dosing of 300 mg natalizumab Q4W can switch to Q6W EID with no loss in clinical efficacy. |
Introduction
Many innovative drugs have become available to treat active relapsing–remitting multiple sclerosis (RRMS). Natalizumab, an anti-α4β1 integrin receptor monoclonal antibody, is highly efficacious in reducing relapse rates, radiological activity, and disability progression in large-scale randomized trials [1–3] and real-world studies [4–6]. However, natalizumab treatment is associated with progressive multifocal leukoencephalopathy (PML), a severe but rare opportunistic infection of the central nervous system [7, 8]. Therefore, the therapeutic advantages of natalizumab need to be weighed against the risk of PML. Risk mitigation strategies have been developed, mainly based on monitoring three risk factors: the presence of anti-JC virus (JCV) antibodies, prior immunosuppressant use, and duration of natalizumab treatment beyond 2 years [9].
Extended interval dosing (EID) has emerged as another potential strategy for natalizumab-associated PML risk mitigation. Natalizumab was approved with 300-mg infusions at the standard interval dosing (SID) every 4 weeks (Q4W) [10]. A combination of pharmacological studies and observations of clinical disease activity after natalizumab cessation have suggested that dosing intervals > 4 weeks could potentially maintain a high level of efficacy while reducing the risk of PML by allowing a base level of immunological surveillance [2, 11–18].
Over the last several years, practitioners worldwide have been using off-label EID of natalizumab at variable dosing intervals to reduce the risk of PML, despite the absence of a large-scale efficacy evaluation. In a retrospective analysis of the TOUCH (Tysabri Outreach: Unified Commitment to Health) Prescribing Program, an average EID period of every 6 weeks (Q6W) was associated with a significant reduction of PML risk in comparison with SID in anti-JCV antibody-positive patients with RRMS [19]; however, this database does not capture effectiveness outcomes. Other retrospective studies have suggested that natalizumab's effectiveness in terms of clinical or radiologic outcomes is not diminished after switching to an EID regimen of approximately 6 weeks after at least 6 months of stable SID treatment, though these studies are generally limited by the lack of well-matched treatment cohorts and variable definitions of EID [20–24]. However, in a study of patients who were treated for at least 1 year with SID and then switched to an every-12-week schedule, the 12-week EID schedule did not adequately control radiologic or clinical disease activity [25], and recent models of natalizumab efficacy showed that disease activity is more likely to return with a switch to dosing intervals greater than Q6W [26].
Recently published results of the randomized, controlled phase 3b trial NOVA (NCT03689972) have demonstrated the efficacy of natalizumab in patients with RRMS who were stable on Q4W dosing for at least 1 year and were randomized to Q6W dosing in comparison with patients who remained on Q4W dosing. The primary endpoint was the number of new or newly enlarging T2 lesions at 72 weeks. Analysis of the NOVA primary endpoint and secondary magnetic resonance imaging (MRI) and clinical endpoints suggest that patients who are stable on natalizumab Q4W dosing can switch to Q6W dosing without a clinically meaningful loss of efficacy [27].
In this context, we conducted the first French real-world, retrospective, multicenter, noninferiority study using lack of relapse as the primary clinical endpoint and evaluating the efficacy in patients with RRMS who switched to natalizumab EID (> 5-week interval) after at least 12 months of SID in comparison with those who continued on SID.
Methods
Design
This was a retrospective, multicenter, noninferiority study in patients with RRMS in real-world clinical practice in France. The study was conducted in accordance with the Declaration of Helsinki and was approved by the local ethics committee in Caen (file number 1454), and it is registered in the ClinicalTrials.gov database (NCT04580381). The patients’ informed consent to the study was collected when they were included in the EDMUS (European Database for Multiple Sclerosis) follow-up programs or through an informative document validated by the local ethics committee and provided by each center. According to a standardized procedure, our study collected demographic, clinical, and radiological characteristics of eligible natalizumab-treated patients in each participating center through January 5, 2020. Patients were selected from the EDMUS database and medical files from a secured local database (Percy Hospital, Clamart, France). Patients were assigned to the SID or EID group according to inclusion criteria. The statistical analysis was performed at the main investigative center in Caen, France.
Objectives
Our main objective was the evaluation of the EID strategy’s efficacy in RRMS in terms of relapse rate. Secondary objectives comprised the effectiveness of the EID strategy with regard to disability progression and clinical disease activity and a safety evaluation in terms of PML.
Inclusion and Data Collection
Patients were included if they were at least 18 years old and had received at least 11 infusions of natalizumab over 12 months as disease-modifying monotherapy for RRMS using the approved dosing and before the period of interest. Patients who were pregnant during the period of interest or the previous 12 months were excluded. Additionally, patients were excluded if their natalizumab infusion history, clinical history, or both were not available; if they had a dosing gap, defined as ≥ 12 weeks between any two doses; or if they had an overdose, defined as < 3 weeks between any two doses.
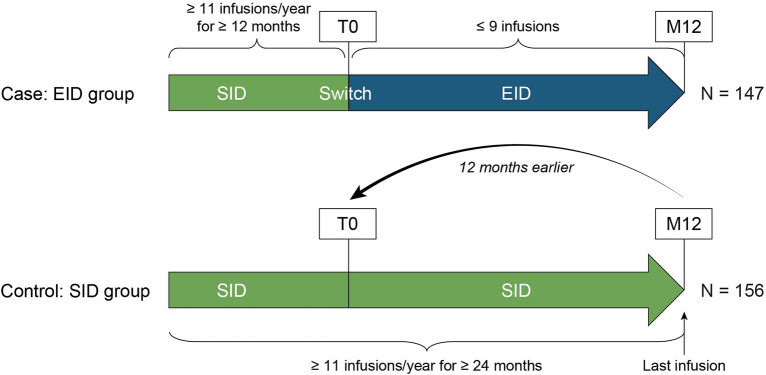
According to the following strategy, patients were assigned to one of two groups. Patients were assigned to the EID group if the 12 months of SID were followed by ≤ 9 natalizumab infusions in the next 12 months. Patients were assigned to the SID group if they had a continuous 24-month period of SID, defined as ≥ 11 infusions per year, without any period of EID in their therapeutic history (Fig. 1).
Fig. 1.
Study design
Baseline demographic and clinical characteristics included age at the start of the period of interest (hereafter referred to as baseline); body mass index (BMI); RRMS disease duration at baseline; previous disease-modifying therapies; disability at baseline evaluated by the Expanded Disability Status Scale (EDSS); natalizumab therapy duration, defined as the period in months between the first infusion and the date specified as baseline; and anti-JCV serology status. T2 FLAIR MR image files were collected at baseline and 12 months when available.
Outcomes
Our primary endpoint was the proportion of patients presenting with no relapse 12 months after baseline. Our secondary efficacy endpoints comprised disability progression evaluated by the EDSS score; the annualized relapse rate (ARR), measured at 12 months and characterized by the number of clinical relapses diagnosed by the attending neurologist according to clinical criteria recorded during follow-up; the overall efficacy in relation to clinical disease activity (defined as the absence of a clinical relapse and no worsening of EDSS at 12 months, which is defined as the absence of EDSS progression or < 1 point as previously published [28]); and change in lesion volume detected by T2 fluid-attenuated inversion recovery.
Centralized MRI examinations were performed at baseline and 12 months to evaluate the total lesion volume and its evolution (new lesions or enlargement of preexisting T2 lesions). Automated analysis software was used with an algorithm based on a convolutional neural network allowing the integration of multiple levels of volumetric data interpretation with autocorrections and operating according to a learning method (Pixyl La Tronche, France) [29].
Safety endpoints included PML cases reported in the patients’ medical files and JCV index evolution during follow-up when available.
Statistical Analyses
Power and Planned Sample Size
In the retrospective database, we had anticipated that the number of patients who switched to EID would be 109. With three hypothetical SID controls per EID case, we computed a priori that by assessing a frequency of 80% in the primary outcome, 5% alpha risk, and 80% power, this sample would provide a noninferiority margin of 11%.
The ratio of controls per case was not reached, and we decided to analyze all available data with the planned empiric noninferiority margin.
Statistical Analysis Plan
Characteristics of patients were described by treatment group (SID vs. EID) as count (percentage) and mean (standard deviation) or median (interquartile range) for qualitative and quantitative variables, respectively.
The percentage of success, defined as no relapse, was compared between the SID and EID groups by the noninferiority test for the risk difference with a noninferiority margin of −11% using the Farrington–Manning test.
Baseline differences between treatment-defined groups may differ because the two groups were not randomized. Therefore, we analyzed our data using propensity score methods. We used two different strategies to address the potential channeling bias for the endpoints, namely, inverse probability treatment weighting (IPTW) and propensity score matching (PSM). We modeled the probability of being in an EID group with a non-parsimonious logistic regression that included the following variables: age, sex, BMI, smoking, ARR at inclusion, number of relapses at inclusion, and the duration of natalizumab exposure at inclusion. First, we used the inverse probability of treatment weighting to weight the noninferiority risk difference test (stabilized weights) for the primary endpoint, the generalized linear model with a Poisson distribution for the ARR, the Mann–Whitney U test for quantitative secondary endpoints, and the chi-square test for qualitative secondary endpoints. Second, we performed a one-to-one procedure (macro ONETOMANY) to match treatment groups based on their propensity scores. Then, we analyzed this paired population using the noninferiority risk difference test for the primary endpoint, a generalized linear model with a Poisson distribution taking into account matched data for the ARR, the Wilcoxon signed-rank test for quantitative secondary endpoints, and the McNemar test for qualitative secondary endpoints.
The shrinkage method was used as a sensitivity analysis to assess the robustness of IPTW by excluding observations of the most extreme weights computed from the propensity score. For the propensity-score-matching model, we performed a sensitivity analysis using a multivariate generalized linear model with a binomial and Poisson distribution, taking into account the matched data for at least one relapse and ARR at 12 months to adjust for therapy groups and ARR at baseline.
Raw baseline characteristics were compared between groups using the chi-square or Fisher exact test for categorical variables and the Student t or Mann–Whitney U test for continuous variables according to their distribution. After adjusting with the IPTW method, we compared baseline characteristics using the chi-square or Fisher exact test for categorical variables and the Student t or Mann–Whitney U test for continuous variables according to their distribution by weighting on stabilized weights. Baseline characteristics were compared between groups before and after the matching procedure using the standardized mean differences.
A p value of less than 0.05 was considered significant; all p values were two-tailed. No adjustment was performed. Statistical analyses were performed using SAS statistical software, version 9.4 (SAS Institute Inc., Cary, NC, USA), and R software, version 3.6.2 (R Foundation for Statistical Computing).
Results
Patients
In this multicenter cohort study, 400 patients were eligible for inclusion across the five recruiting centers. Among this cohort, 15 patients (3.7%) presented at least 1 of the exclusion criteria, 61 patients (15%) did not meet the inclusion criteria, and 21 patients (5.2%) were excluded because of missing data (Fig. S1 in the Supplementary Information). Thus, 303 patients (156 patients in the SID group and 147 in the EID group) were analyzed. Clinical characteristics at baseline are shown in Table 1. The dosing interval in the EID group was 5 or 6 weeks. The features of both the unadjusted and IPTW populations are summarized in Table 1. After adjustment by stabilized IPTW, no significant differences were observed between the groups, except that the duration of natalizumab exposure at inclusion was significantly longer in the EID group. In the sensitivity analysis by the shrinkage IPTW approach, no significant differences were observed between the groups (Table S1 in the Supplementary Information). A total of 102 patients in the EID group were 1:1 matched to 102 patients in the SID group. After matching, no significant differences were observed between the groups (Fig. S2 in the Supplementary Information).
Table 1.
Baseline characteristics in the raw and IPTW populations
| Baseline characteristics | Raw data | IPTW | ||||
|---|---|---|---|---|---|---|
| SID group (n = 156) | EID group (n = 147) | p valuea | SID group (wn = 161.0) | EID group (wn = 145.1) | p valueb | |
| Age (years), mean (SD) | 40.2 (10.6) | 39.3 (9.7) | 0.467 | 40.9 (10.9) | 39.8 (9.5) | 0.378 |
| Female, n (%) | 123 (79) | 110 (75) | 0.408 | 123.0 (76) | 112.5 (78) | 0.801 |
| BMI(kg/m2), median (IQR)c | 24.6 (23.0–25.0) | 24.6 (21.7–25.9) | 0.192 | 24.6 (22.9–25.0) | 24.6 (21.6–26.2) | 0.317 |
| Weight (kg), mean (SD)d | 70.2 (15.7) | 68.3 (15.5) | 0.374 | 69.4 (15.1) | 68.3 (15.9) | 0.600 |
| ≤ 80 kg, n (%)d | 132 (85) | 117 (80) | 0.254 | 86.3 (79) | 107.7 (78) | 0.735 |
| Any disease-modifying therapy before natalizumab, n (%) | 132 (85) | 121 (82) | 0.590 | 137.4 (85) | 119.1 (82) | 0.444 |
| RRMS disease duration at baseline (months), median (IQR) | 132.5 (72.0–205.0) | 139.0 (83.0–199.0) | 0.915 | 125.0 (67.0–195.0) | 142 (84.0–200.0) | 0.233 |
| ARR at baseline, median (IQR) | 0.50 (0.23–0.70) | 0.40 (0.21–0.67) | 0.214 | 0.40 (0.00–0.70) | 0.46 (0.22–0.67) | 0.350 |
| Duration of natalizumab exposure at baseline, median (IQR), months | 28.5 (12.0–73.5) | 27.0 (21.0–54.0) | 0.259 | 21.0 (12.0–63.0) | 26.0 (21.0–54.0) | 0.018 |
| Number of relapses at baseline, median (IQR) | 5.0 (3.0–9.0) | 4.0 (3.0–7.0) | 0.127 | 4.0 (2.0–8.0) | 5.0 (3.0–8.0) | 0.785 |
| EDSS score at baseline, median (IQR)d | 2.0 (1.0–4.0) | 2.0 (1.0–4.0) | 0.617 | 2.5 (1.0–4.0) | 2.5 (1.0–4.0) | 0.788 |
| Positive JCV serology at baseline, n (%)b | 39 (26) | 37 (29) | 0.588 | 38.7 (25) | 35.2 (28) | 0.587 |
Variables included in the propensity score model are shown in boldface type
ARR annualized relapse rate, BMI body mass index, EDSS Expanded Disability Status Scale, EID extended interval dosing, IPTW inverse probability treatment weighting, IQR interquartile range, JCV JC virus, RRMS relapsing–remitting multiple sclerosis, SD standard deviation, SID standard interval dosing, wn weighted number
ap value of the Student t test or Mann–Whitney U test for continuous variables and the chi-square test or Fisher exact test for qualitative variables
bp value of the Student t test or Mann–Whitney U test for continuous variables and the chi-square test or Fisher exact test for qualitative variables, weighted on stabilized weights
cImputation by the mean
dBody weight was missing for 70 (23%), EDSS score was missing for 10 (3%), and anti-JCV serostatus was missing for 25 (8%) patients, respectively
Primary Endpoint
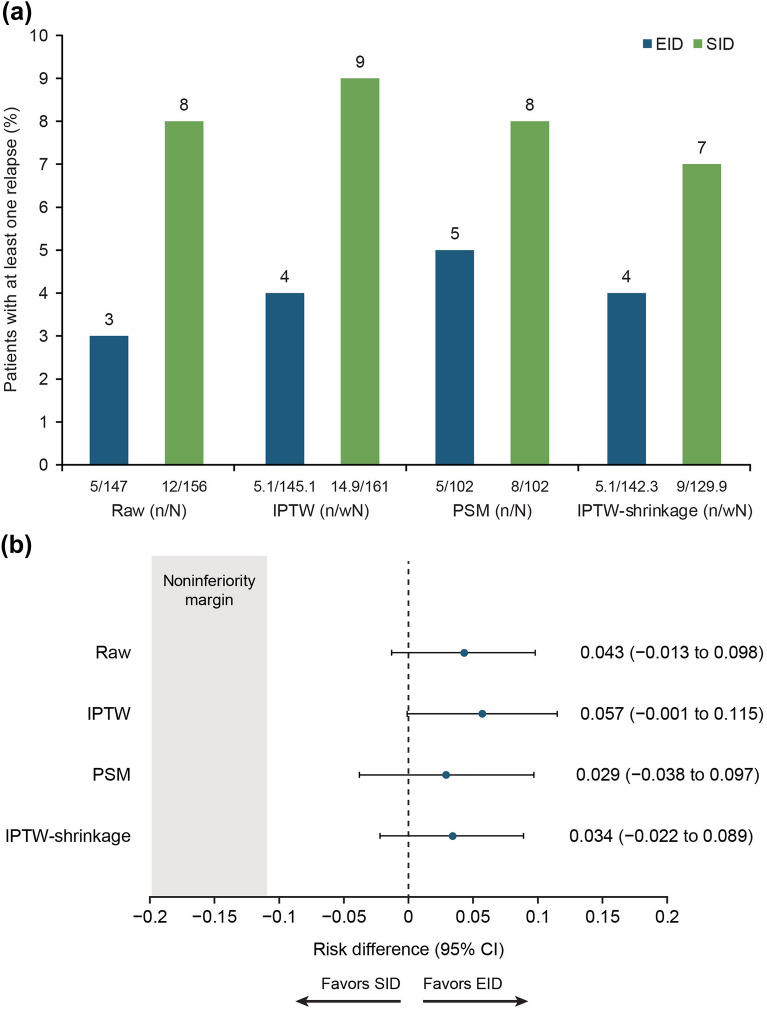
The noninferiority analysis comparing patients free from relapse during the period of interest (ARR = 0) in the unadjusted, IPTW, and PSM models is presented in Fig. 2. In the unadjusted model, the event’s occurrence in the EID group (97%) was significantly noninferior in comparison with the SID group (92%) (risk difference [RD] = 0.043; 95% confidence interval [CI] −0.013 to 0.09; p < 0.001). In the stabilized IPTW and PSM models, similar results were observed (RD = 0.057; 95% CI − 0.001 to 0.115; p < 0.001; RD = 0.029; 95% CI − 0.038 to 0.097; p < 0.001, respectively). These results were confirmed in the sensitivity analysis by the shrinkage approach (RD = 0.034; 95% CI − 0.022 to 0.089; p < 0.001) (Fig. 2). In the sensitivity multivariate analysis of the absence of relapse at 12 months, no significant difference was observed between the two groups adjusted for treatment and ARR at baseline (p = 0.410 and p = 0.333, respectively) (Table S2 in the Supplementary Information).
Fig. 2.
a Percentage of patients with at least one relapse during the follow-up according to therapy group and b risk differences between therapy groups
Secondary Endpoints
Unadjusted Model
In the SID group, 12 (8%) patients had at least one relapse during the study period, and in the EID group, 5 (3%) patients had at least one relapse. The comparison of 12-month outcomes between both groups is shown in Table 2. At 12 months after inclusion, the ARR was not significantly different between the two groups (0.083 ± 0.300 in the SID group vs. 0.041 ± 0.231 in the EID group; p = 0.149), nor was the difference in EDSS score (2.72 ± 1.85 in the SID group vs. 2.43 ± 1.74 in the EID group; p = 0.180). No significant difference was observed between the SID and EID groups for the overall clinical disease activity at 12 months (126 [81%] patients in the SID group vs. 111 [86%] in the EID group; p = 0.236) and the worsening of EDSS score from baseline to 12 months (20 [13%] patients in the SID group and 14 [11%] in the EID group; p = 0.610).
Table 2.
Secondary outcomes in the SID and EID groups in the unadjusted, IPTW, and PSM models and sensitivity analysis by the shrinkage IPTW approach
| Outcomes | Raw data | IPTW | Matched | IPTW (shrinkage) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SID group (n = 156) | EID group (n = 147) | p valuea | SID group (wn = 161.0) | EID group (wn = 145.1) | p valueb | SID group (n = 102) | EID group (n = 102) | p valuec | SID group (wn = 129.9) | EID group (wn = 142.3) | p valueb | |
| ARR at 12 months, mean (SD) | 0.083 (0.300) | 0.041 (0.231) | 0.149 | 0.098 (0.320) | 0.043 (0.238) | 0.084 | 0.088 (0.318) | 0.059 (0.275) | 0.475 | 0.076 (0.270) | 0.044 (0.239) | 0.282 |
| EDSS score at 12 months, mean (SD) | 2.72 (1.85) | 2.43 (1.74) | 0.180 | 2.75 (1.79) | 2.56 (1.80) | 0.368 | 2.65 (1.87) | 2.54 (1.76) | 0.642 | 2.71 (1.75) | 2.51 (1.77) | 0.366 |
| Worsening of EDSS score from baseline to 12 months, n (%) | 20 (13) | 14 (11) | 0.610 | 26.5 (16) | 14.8 (11) | 0.172 | 13 (13) | 12 (12) | > 0.999 | 17.2 (13) | 14.8 (11) | 0.607 |
| Overall clinical disease activity at 12 months, n (%) | 126 (81) | 111 (86) | 0.236 | 121.2 (75) | 116.1 (86) | 0.022 | 82 (80) | 82 (84) | 0.690 | 105.3 (81) | 113.4 (86) | 0.314 |
Overall clinical disease activity: nondisease activity (no worsening of EDSS at 12 months and no relapse in the last 12 months)
ARR annualized relapse rate, EDSS Expanded Disability Status Scale, EID extended interval dosing, IPTW inverse probability treatment weighting, PSM propensity score matching, SD standard deviation, SID standard interval dosing, wn weighted number
ap value obtained by a univariate generalized linear model with a Poisson distribution for the ARR, by the Student t test for quantitative variables, and by the chi-square test for qualitative variables
bp value obtained by a univariate generalized linear model with a Poisson distribution for the ARR, by the Student t test for quantitative variables, and by the chi-square test for qualitative variables, weighted on stabilized weights
cp value obtained by a univariate generalized linear model with a Poisson distribution for the ARR taking into account matched data, the paired Student t test for quantitative variables, and the McNemar test for qualitative variables
IPTW Model
At 12 months after inclusion, the mean (standard deviation [SD]) ARR was not significantly different between both groups (SID, 0.098 [0.320] vs. EID, 0.043 [0.238]; p = 0.084), nor was there a significant difference in the EDSS score and the worsening of EDSS score (Table 2). A significant difference was observed in the overall clinical disease activity (75% in the SID group vs. 86% in the EID group; p = 0.022). Similar results were observed in the sensitivity analyses by the shrinkage approach, except for overall clinical disease activity (Table 2). Excluding the most extreme weights in the baseline characteristics, the overall clinical disease activity at 12 months was not significantly different between the two groups (81% in the SID group vs. 86% in the EID group; p = 0.314).
PSM Model
At 12 months after inclusion, the mean (SD) ARR was not significantly different between the SID (0.088 [0.318]) and the EID (0.059 [0.275]) groups (p = 0.475), nor were the EDSS score results (Table 2). No significant difference was observed between the SID and EID groups for overall clinical disease activity at 12 months (82 [80%] in the SID group vs. 82 [84%] in the EID group; p = 0.690) or the worsening of EDSS score from baseline to 12 months (13 [13%] patients in the SID group vs. 12 [12%] in the EID group; p > 0.999). In the sensitivity multivariate analysis of ARR at 12 months, no significant difference was observed between both groups adjusted for the therapy group and ARR at baseline (p = 0.543 and p = 0.152, respectively) (Table S2 in the Supplementary Information).
MRI
Baseline and 12-month MRI data were available for 92 patients. At 12 months after inclusion, the median change in lesion volume from baseline did not differ significantly (p = 0.852) between the SID group (n = 44, change = −0.28) and the EID group (n = 48, change = −0.38) (see Table S3 in the Supplementary Information).
Safety Endpoints
The anti-JCV index values are described for all patients who had the value at baseline and/or at 12 months in Table S4 in the Supplementary Information. In patients who had an anti-JCV index value at 12 months (n = 258), no significant difference was observed between the two groups in terms of raw index (SID median 0.00, interquartile range [IQR] [0.00; 0.52]; EID median 0.00, IQR [0.00; 0.47]; p = 0.627). The anti-JCV index was only available in 133 patients (63 SID, 70 EID) at both times (at baseline and 12 months). No significant difference was observed in the variation in JCV index between baseline and 12-month follow-up (SID median variation 0.00, IQR [− 0.05; 0.06]; EID median difference 0.00, IQR [− 0.19; 0.00]; p = 0.230). No events of PML were reported throughout the 12-month study period.
Discussion
Real-world data based on more diverse and representative patient populations can complement randomized clinical trial data by providing important information to help assess and monitor responses to disease-modifying therapies in individual patients. These data represent the first report of the effectiveness of natalizumab EID in a French cohort of patients with RRMS.
In this retrospective study of French patients with RRMS treated with natalizumab SID for at least 12 months who then switched to EID or remained on SID, the proportion of EID patients in the overall patient cohort who were free of relapse from baseline to month 12 was significantly noninferior in comparison with SID patients (primary endpoint). Similar results were obtained for EID and SID patients matched by IPTW and 1:1 PSM, supporting the finding in the overall patient group.
Analyses of the study’s secondary endpoints were consistent with the primary endpoint. There were no significant differences in EDSS scores for both dosing groups at 12 months. From baseline to month 12, ARR was not significantly different for EID and SID patients, and the proportions of patients in each group with EDSS worsening were similar. An analysis of the overall clinical disease activity endpoint, a composite clinical endpoint comprising ARR and EDSS score over 12 months, also demonstrated no significant difference for natalizumab EID and SID patients. The available MRI data for this French cohort of patients also indicated no significant differences between treatment groups for change in lesion volume.
Previous retrospective studies of natalizumab EID have used variable definitions of EID and variable requirements for the length of the lead-in period of SID before switching to EID. Despite the lack of a strictly defined EID protocol, our results from patients using approximately a Q6W EID are generally consistent with those in previous retrospective cohort studies of the clinical efficacy of natalizumab EID vs. SID. Notably, our findings of noninferiority of EID compared to SID are consistent with a prior study in 360 Italian patients of the noninferiority of natalizumab EID (median dosing interval of 6 weeks) assessed by ARR for EID versus SID over approximately 2 years [22]. Additionally, another Italian cohort of 2092 patients found that EID (median interval of 43 days) did not lead to a significant difference in relapse rate, EDSS worsening, or overall clinical disease activity in comparison with SID [21].
The safety outcomes of this study were limited to the incidence of PML and related PML risk factors, including the anti-JCV index value. No PML was reported in either group over 12 months in this study. The incidence of PML in the setting of SID is rare enough that small studies with a short follow-up such as this would not be expected to detect a significant reduction in PML incidence between EID and SID. Additionally, no difference was observed in the anti-JCV index at baseline and after 12 months. Although the small sample size limits these findings, they are concordant with the previous retrospective analysis of EID safety [19].
Conclusions from this study are generally limited by the observational, retrospective design, including potential missing data points. Even though differences in treatment groups were adjusted using IPTW and PSM, residual confounding from unmeasured covariates may remain. In addition, there is a potential for treatment decision bias based on factors such as MS severity and PML risk, which may have affected the decision to initiate EID or stay with SID in some individuals. Additionally, possible center effects from a lack of a standardized evaluation protocol could lead to differences in EDSS scoring and MRI evaluations, knowing that the low percentage of MRI evaluations performed at baseline and during follow-up severely limits information regarding subclinical inflammation in this cohort.
Specific study limitations include the small sample size, and the planned ratio of SID to EID patients was not reached. The smaller cohort size was accounted for by adjusting the statistical methods (1:1 matching for SID and EID rather than the planned 3:1 matching). Also, the number of relapses was lower than expected, increasing the statistical power needed to demonstrate noninferiority.
This study provides a real-world counterpart to the prospective randomized phase 3 trial comparing the efficacy of Q4W and Q6W dosing (NOVA). The similarities between this study and NOVA—specifically the inclusion requirements for patients to have at least 12 months of natalizumab SID before the start of the study and a targeted EID period of Q6W—are consistent with those of the ongoing randomized clinical trial of natalizumab efficacy in patients who switched from stable treatment with Q4W–Q6W dosing in comparison with those who remained on Q4W [27]. The MRI outcome data, although limited by missing data, may also enable comparisons with the radiological results from NOVA.
Conclusion
These results provide additional real-world evidence of the clinical noninferiority of natalizumab EID compared to SID. The efficacy observed here and in other retrospective analyses, combined with the extensive retrospective safety results of natalizumab EID in US patients with MS [19], provides valuable information to healthcare providers who make treatment decisions for their patients with MS. The combined retrospective efficacy and safety work indicates that patients established on the approved dosing of 300 mg natalizumab Q4W can switch to Q6W EID with no loss in clinical efficacy.
As work to define a generalizable EID protocol continues, the prospect of personalized dosing schedules based on individual pharmacokinetic responses to natalizumab infusion may lead to even more effective dosing strategies, as demonstrated by van Kempen and coworkers, who monitored natalizumab serum concentrations to determine the optimal EID period to maintain efficacy [30].
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank all study participants. The authors thank Chevanne Damien, Abrous Mouloud, Noellie Freitas, and Christina Faroul Celine Callier (CRA) for their technical assistance.
Funding
This study was sponsored by Caen University Hospital, with additional funding (including the journal’s fee) provided by Biogen. All operational aspects of the study, including monitoring, data collection, and statistical analyses, were managed by Caen University Hospital. The funder had no role in the study design, data collection, analysis, interpretation, or manuscript writing.
Medical Writing and/or Editorial Assistance
The authors were assisted in preparing the manuscript by Luke Ward, Ph.D., and John Watson, Ph.D., of Ashfield MedComms (Middletown, CT, USA). Celia Nelson of Ashfield MedComms copyedited and styled the manuscript per journal requirements. Biogen provided funding for writing support.
Authorship
All named authors meet the International Committee of Medical Journal Editors criteria for authorship for this manuscript and take responsibility for the integrity of the work. The authors provided final approval of all content and were responsible for the decision to submit it for publication.
Author Contributions
Juliette Pelle and Gilles Defer helped with drafting and revising this manuscript, played a major role in data acquisition, aided in designing the study, and were responsible for the analysis and interpretation of data. Anais R. Briant and Jean-Jacques Parienti helped with drafting and revising this manuscript, aided in designing the study, and were responsible for the analysis and interpretation of data. Pierre Branger, Nathalie Derache, Charlotte Arnaud, Christine Lebrun-Frenay, Mikael Cohen, Lydiane Mondot, Jerome De Seze, Kevin Bigaut, Nicolas Collongues, Laurent Kremer, Damien Ricard, Flavie Bompaire, Charlotte Ohlmann, Magali Sallansonnet-Froment, Jonathan Ciron, Damien Biotti, and Beatrice Pignolet helped with drafting and revising this manuscript and played a major role in data acquisition.
Prior Presentation
Portions of this study were presented at the 8th European Academy of Neurology Congress, June 25–28, 2022 (Vienna, Austria).
Disclosures
Juliette Pelle reports no disclosures relevant to the manuscript. Anais R. Briant reports no disclosures relevant to the manuscript. Pierre Branger reported receiving personal compensation for consulting, serving on a scientific advisory board, speaking, or other activities with Alexion, Biogen, Bristol Myers Squibb, Merck, Novartis, and Sanofi Genzyme outside the submitted work. Nathalie Derache reported receiving personal fees from Biogen, Novartis, Merck, and Roche outside the submitted work. Charlotte Arnaud reports no disclosures relevant to the manuscript. Christine Lebrun-Frenay reported serving as principal investigator of the Teriflunomide in Radiologically Isolated Syndrome (TERIS) study, on the steering committee for the Arise study, and as co-president of the Observatoire Français de la Sclérose En Plaques (OFSEP) scientific committee. Mikael Cohen reported receiving personal fees from Biogen, Merck, Novartis, Roche, Alexion, Ad Scientiam, Teva, and Sanofi outside the submitted work. Lydiane Mondot reports no disclosures relevant to the manuscript. Jerome De Seze reported receiving personal fees from Biogen, Roche, Sanofi, Alexion, Bristol Myers Squibb, Actelion, and Teva outside the submitted work. Kevin Bigaut reported fees as a consultant or for presentations in partnership with Biogen, Roche, Novartis, and BMS/Celgene. Nicolas Collongues reports no disclosures relevant to the manuscript. Laurent Kremer has received personal compensation for consulting, serving on a scientific advisory board, speaking, or other activities with Biogen, Novartis, Merck, Sanofi Genzyme, Roche, and Alexion outside the submitted work. Damien Ricard reports no disclosures relevant to the manuscript. Flavie Bompaire reports no disclosures relevant to the manuscript. Charlotte Ohlmann reports no disclosures relevant to the manuscript. Magali Sallansonnet-Froment reports no disclosures relevant to the manuscript. Jonathan Ciron reported receiving personal fees from Biogen, Novartis, Merck, Sanofi Genzyme, Roche, Bristol Myers Squibb, and Alexion outside the submitted work. Damien Biotti reported receiving personal fees from Biogen, Novartis, Merck, Sanofi Genzyme, Roche, Teva, Bristol Myers Squibb, and Alexion outside the submitted work. Beatrice Pignolet reports no disclosures relevant to the manuscript. Jean-Jacques Parienti received honoraria and grants from ViiV, Gilead, and Merck outside the submitted work. Gilles Defer reported receiving personal fees from Biogen, Bristol Myers Squibb, Merck Serono, Novartis, Sanofi Genzyme, and Teva Pharmaceuticals outside the submitted work.
Compliance with Ethics Guidelines
The study was conducted in accordance with the Declaration of Helsinki and approved by the local Ethics Committee in Caen (file number 1454) and is registered in the ClinicalTrials.gov database (NCT04580381). The patients’ informed consent to the study was collected when they were included in the EDMUS (European Database for Multiple Sclerosis) follow-up programs or through an informative document validated by the local ethics committee and provided by each center.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Havrdova E, Galetta S, Hutchinson M, et al. Effect of natalizumab on clinical and radiological disease activity in multiple sclerosis: a retrospective analysis of the Natalizumab Safety and Efficacy in Relapsing–Remitting Multiple Sclerosis (AFFIRM) study. Lancet Neurol. 2009;8:254–260. doi: 10.1016/S1474-4422(09)70021-3. [DOI] [PubMed] [Google Scholar]
- 2.Miller DH, Khan OA, Sheremata WA, et al. A controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2003;348:15–23. doi: 10.1056/NEJMoa020696. [DOI] [PubMed] [Google Scholar]
- 3.Polman CH, O'Connor PW, Havrdova E, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354:899–910. doi: 10.1056/NEJMoa044397. [DOI] [PubMed] [Google Scholar]
- 4.Butzkueven H, Kappos L, Wiendl H, et al. Long-term safety and effectiveness of natalizumab treatment in clinical practice: 10 years of real-world data from the Tysabri Observational Program (TOP). J Neurol Neurosurg Psychiatry. 2020;91:660–8. [DOI] [PMC free article] [PubMed]
- 5.Horakova D, Uher T, Krasensky J, et al. Long-term effectiveness of natalizumab on MRI outcomes and no evidence of disease activity in relapsing-remitting multiple sclerosis patients treated in a Czech Republic real-world setting: a longitudinal, retrospective study. Mult Scler Relat Disord. 2020;46:102543. [DOI] [PubMed]
- 6.Perumal J, Fox RJ, Balabanov R, et al. Outcomes of natalizumab treatment within 3 years of relapsing-remitting multiple sclerosis diagnosis: a prespecified 2-year interim analysis of STRIVE. BMC Neurol. 2019;19:116–127. doi: 10.1186/s12883-019-1337-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bloomgren G, Richman S, Hotermans C, et al. Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N Engl J Med. 2012;366:1870–1880. doi: 10.1056/NEJMoa1107829. [DOI] [PubMed] [Google Scholar]
- 8.Ho P-R, Koendgen H, Campbell N, Haddock B, Richman S, Chang I. Risk of natalizumab-associated progressive multifocal leukoencephalopathy in patients with multiple sclerosis: a retrospective analysis of data from four clinical studies. Lancet Neurol. 2017;16:925–933. doi: 10.1016/S1474-4422(17)30282-X. [DOI] [PubMed] [Google Scholar]
- 9.McGuigan C, Craner M, Guadagno J, et al. Stratification and monitoring of natalizumab-associated progressive multifocal leukoencephalopathy risk: recommendations from an expert group. J Neurol Neurosurg Psychiatry. 2016;87:117–125. doi: 10.1136/jnnp-2015-311100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Biogen. Tysabri® (natalizumab): prescribing information. Cambridge: Biogen, Inc; 2021.
- 11.Sheremata WA, Vollmer TL, Stone LA, Willmer-Hulme AJ, Koller M. A safety and pharmacokinetic study of intravenous natalizumab in patients with MS. Neurology. 1999;52:1072. doi: 10.1212/WNL.52.5.1072. [DOI] [PubMed] [Google Scholar]
- 12.Defer G, Mariotte D, Derache N, et al. Increase of infusion interval do not impair natalizumab efficacy in RR-MS patients: a pilot study based on monthly monitoring of CD49d. Neurology. 2012;78:P06.166. doi: 10.1212/WNL.78.1_MeetingAbstracts.P06.166. [DOI] [Google Scholar]
- 13.Khoy K, Mariotte D, Defer G, Petit G, Toutirais O, Le Mauff B. Natalizumab in multiple sclerosis treatment: from biological effects to immune monitoring. Front Immunol. 2020;11:549842. doi: 10.3389/fimmu.2020.549842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Defer G, Mariotte D, Derache N, et al. CD49d expression as a promising biomarker to monitor natalizumab efficacy. J Neurol Sci. 2012;314:138–142. doi: 10.1016/j.jns.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 15.van Kempen ZL, Leurs CE, Witte BI, et al. The majority of natalizumab-treated MS patients have high natalizumab concentrations at time of re-dosing. Mult Scler. 2018;24:805–810. doi: 10.1177/1352458517708464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhovtis Ryerson L, Li X, Goldberg JD, et al. Pharmacodynamics of natalizumab extended interval dosing in MS. Neurol Neuroimmunol Neuroinflamm. 2020;7:e672. doi: 10.1212/NXI.0000000000000672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Killestein J, Vennegoor A, Strijbis EM, et al. Natalizumab drug holiday in multiple sclerosis: poorly tolerated. Ann Neurol. 2010;68:392–395. doi: 10.1002/ana.22074. [DOI] [PubMed] [Google Scholar]
- 18.Borriello G, Prosperini L, Marinelli F, Fubelli F, Pozzilli C. Observations during an elective interruption of natalizumab treatment: a post-marketing study. Mult Scler. 2011;17:372–375. doi: 10.1177/1352458510392098. [DOI] [PubMed] [Google Scholar]
- 19.Zhovtis Ryerson L, Foley J, Chang I, et al. Risk of natalizumab-associated PML in patients with MS is reduced with extended interval dosing. Neurology. 2019;93:e1452–e1462. doi: 10.1212/WNL.0000000000008243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bomprezzi R, Pawate S. Extended interval dosing of natalizumab: a two-center, 7-year experience. Ther Adv Neurol Disord. 2014;7:227–231. doi: 10.1177/1756285614540224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chisari CG, Grimaldi LM, Salemi G, et al. Clinical effectiveness of different natalizumab interval dosing schedules in a large Italian population of patients with multiple sclerosis. J Neurol Neurosurg Psychiatry. 2020;91:1297–1303. doi: 10.1136/jnnp-2020-323472. [DOI] [PubMed] [Google Scholar]
- 22.Clerico M, De Mercanti SF, Signori A, et al. Extending the interval of natalizumab dosing: is efficacy preserved? Neurotherapeutics. 2020;17:200–207. doi: 10.1007/s13311-019-00776-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaufman M, Cree BAC, De Sèze J, et al. Radiologic MS disease activity during natalizumab treatment interruption: findings from RESTORE. J Neurol. 2015;262:326–336. doi: 10.1007/s00415-014-7558-6. [DOI] [PubMed] [Google Scholar]
- 24.Zhovtis Ryerson L, Frohman TC, Foley J, et al. Extended interval dosing of natalizumab in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2016;87:885–889. doi: 10.1136/jnnp-2015-312940. [DOI] [PubMed] [Google Scholar]
- 25.Trojano M, Ramió-Torrentà L, Grimaldi LME, et al. A randomized study of natalizumab dosing regimens for relapsing–remitting multiple sclerosis. Mult Scler. 2021;27:2240–2253. doi: 10.1177/13524585211003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang I, Muralidharan KK, Campbell N, Ho PR. Modeling the efficacy of natalizumab in multiple sclerosis patients who switch from every-4-week dosing to extended-interval dosing. J Clin Pharmacol. 2021;61:339–348. doi: 10.1002/jcph.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Foley J, Defer G, Zhovtis Ryerson L, et al. Comparison of switching to 6-week dosing of natalizumab versus continuing with 4-week dosing in patients with relapsing-remitting multiple sclerosis (NOVA): a randomised, controlled, open-label, phase 3b trial. Lancet Neurol. 2022;21:608–19. [DOI] [PubMed]
- 28.Kalincik T, Horakova D, Spelman T, et al. Switch to natalizumab versus fingolimod in active relapsing-remitting multiple sclerosis. Ann Neurol. 2015;77:425–435. doi: 10.1002/ana.24339. [DOI] [PubMed] [Google Scholar]
- 29.Commowick O, Istace A, Kain M, et al. Objective evaluation of multiple sclerosis lesion segmentation using a data management and processing infrastructure. Sci Rep. 2018;8:13650. doi: 10.1038/s41598-018-31911-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Kempen ZLE, Hoogervorst ELJ, Wattjes MP, et al. Personalized extended interval dosing of natalizumab in MS: a prospective multicenter trial. Neurology. 2020;95:e745–e754. doi: 10.1212/WNL.0000000000009995. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.