Abstract
Introduction
Angiographic vasospasm (VSP), the narrowing of intracranial arteries, is a complication of aneurysmal subarachnoid hemorrhage (aSAH) and often results in delayed cerebral ischemia (DCI) and cerebral infarction. The objective of this systematic review was to summarize the clinical burden of angiographic VSP and its related complications (DCI and cerebral infarction) after aSAH.
Methods
Systematic searches of MEDLINE, Embase, and the Cochrane Library were conducted (in January 2021) in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines to identify studies reporting clinical outcomes of angiographic VSP and its related complications after aSAH. Study outcomes included measures of functional status (modified Rankin Scale [mRS], Glasgow Outcome Scale [GOS], extended Glasgow Outcome Scale [GOS-E], modified Barthel Index, or the modified National Institutes of Health Stroke Scale), cognitive status (Montreal Cognitive Assessment or the Mini Mental State Exam), clinical events (rebleeding), and mortality. Study selection, data extraction, and qualitative analyses were conducted.
Results
Of 5704 abstracts reviewed, 110 studies were selected: 20 comparative and 39 regression-based studies were included in the qualitative synthesis, 51 descriptive studies were excluded. Most studies (51) were observational and conducted in a single country (53). The occurrence of angiographic VSP and its related complications after aSAH resulted in significantly poorer functional outcomes in three of nine comparative and 11 of 13 regression-based studies, measured by the mRS, and in five of six comparative and eight of nine regression-based studies, measured by the GOS and GOS-E. Angiographic VSP and its related complications were significantly associated with poor cognitive status in all five regression-based studies. Numerically or significantly higher mortality rates in patients with versus those without angiographic VSP and its related complications were reported in five of ten comparative studies and in eight of nine regression-based studies. Six studies looked at specific VSP populations (e.g., by severity or timing of VSP).
Conclusion
Patients with angiographic VSP and its related complications often had poor functional, neurological, and cognitive outcomes and reduced odds of survival both in hospital and at follow-up. We estimate that angiographic VSP and its related complications, DCI and cerebral infarction, lead to an approximately threefold higher odds of poor functional and cognitive outcomes, and about a twofold increase in the odds of death.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40120-022-00436-7.
Keywords: Aneurysmal subarachnoid hemorrhage, Angiographic vasospasm, Cerebral infarction, Clinical burden, Delayed cerebral ischemia, Systematic review
Plain Language Summary
Aneurysmal subarachnoid hemorrhage is a medical emergency in which an aneurysm, a weakened outpouching of a cerebral blood vessel, ruptures causing bleeding in the subarachnoid space. Components from the bleeding can trigger a process leading to the constriction of cerebral arteries, called angiographic vasospasm. Angiographic vasospasm is a frequent occurrence after aneurysmal subarachnoid hemorrhage and can also result in delayed cerebral ischemia and cerebral infarction, which can severely impact patients’ health. This study summarizes the published literature to describe the clinical burden that patients may experience due to angiographic vasospasm, delayed cerebral ischemia, and cerebral infarction after aneurysmal subarachnoid hemorrhage. The evidence from these studies emphasizes numerous clinical consequences that patients may experience. These patients may suffer from diminished neurological and intellectual activity, leading to disability and a loss of functional independence in everyday activities. Angiographic vasospasm and its related complications also reduce the chances of survival, both in the hospital and at follow-up. The considerable clinical burden associated with angiographic vasospasm, delayed cerebral ischemia, and cerebral infarction highlights the importance of their prevention.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40120-022-00436-7.
Key Summary Points
| Why carry out this study? |
| This may be the first study to systematically review the clinical burden caused by angiographic vasospasm (VSP) and its related complications (delayed cerebral ischemia [DCI] and cerebral infarction) after aneurysmal subarachnoid hemorrhage (aSAH). |
| What was learned from this study? |
| Patients with aSAH experience considerable clinical burden associated with angiographic VSP, DCI, and cerebral infarction. |
| Angiographic VSP, DCI, and cerebral infarction after aSAH were associated with poor functional outcomes in terms of disability or dependence in daily activities. |
| Angiographic VSP, DCI, and cerebral infarction after aSAH were predictive of later impaired cognitive abilities. |
| Patients with angiographic VSP, DCI, and cerebral infarction after aSAH also experienced reduced odds of survival both in hospital and at follow-up. |
Introduction
Aneurysmal subarachnoid hemorrhage (aSAH) is caused by rupture of an aneurysm and leads to life-threatening bleeding in the subarachnoid space [1, 2]. Up to 70% of patients with aSAH experience angiographic vasospasm (VSP) [3], defined as the angiographic finding of narrowed cerebral arteries [4]. Angiographic VSP usually begins around day 3, peaks in intensity between days 8 and 11, and resolves within 21 days [5]. It is a serious complication of aSAH that can lead to VSP-related complications such as delayed cerebral ischemia (DCI, also known as DIND [delayed ischemic neurological deficit] and defined as a decline in consciousness of ≥ 2 points on the Glasgow Coma Scale [GCS] or the occurrence of a new focal neurological deficit, lasting for > 1 h, after exclusion of other potential causes of clinical deterioration [4]) and cerebral infarction (defined radiologically as the presence of a new hypodensity on computed tomography [CT] scan located in a vascular distribution [6]). It has been postulated that in addition to angiographic VSP, other factors, including cortical spreading depression, microthrombosis, microcirculatory constriction, and capillary transit time heterogeneity may contribute to DCI and delayed cerebral infarction [7, 8].
Clinical deterioration due to DCI occurs in 20–50% of patients with aSAH and begins 4–14 days after securing the aneurysm [5]. Angiographic VSP after aSAH is also a strong predictor of cerebral infarction, with the incidence of cerebral infarction increasing with increasing severity of angiographic VSP as measured by angiography, rising to > 50% in patients with severe angiographic VSP following aSAH [9]. In addition, approximately 80–90% of patients with cerebral infarction have evidence of angiographic VSP [6, 10, 11]. Both DCI and cerebral infarction are predictors of poor long-term clinical outcomes [3, 4].
This study completes our endeavors to elucidate the burden of angiographic VSP and its related complications after aSAH. Previously [12], we identified a substantial direct and indirect economic burden and poorer health-related quality of life that was long-lasting in aSAH patients who developed angiographic VSP and its related complications, compared with those who did not. Here we report an up-to-date understanding of the clinical burden of angiographic VSP and its related complications after aSAH based on a systematic review of relevant literature that reported comparative or regression-based analyses of aSAH patients.
Methods
Search Strategy
Systematic searches were conducted in indexed literature databases to identify pertinent peer-reviewed studies that were relevant to the research question: What is the clinical burden of patients who had experienced VSP and its related complications after aSAH?
The search strategies, study selection, and data extraction used here are similar to those published in our systematic review of the economic and humanistic burden of angiographic VSP and its related complications after aSAH [12]. A systematic search of MEDLINE, Embase, and the Cochrane Library was conducted on 8 January 2021, in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) 2020 guidelines [13]. Searches were developed on separate search terms for the disease (“subarachnoid hemorrhage” or “vasospasm”) and outcomes of interest. Initially, no restrictions on time or language were applied. Conference abstracts, editorials, and narrative reviews were excluded.
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors. For full details of the search strategy, see Electronic Supplementary Material (ESM) Tables S1 and S2.
Study Selection
Publications identified through the searches were screened against the Population, Intervention, Comparators, Outcomes, and Study (PICOS) design eligibility criteria (Table 1). Following the removal of duplicate references, two reviewers independently reviewed the final list of abstracts against these criteria, with consensus reached by mutual agreement or by a third reviewer when discrepancies arose. Due to the high volume of literature identified, further inclusion criteria were systematically applied after abstract screening was completed to increase the sensitivity of the search. Only studies published after 2010, with a sample size > 30, that mentioned VSP, DCI, or cerebral infarction in the title or abstract were included. Additionally, studies that examined only the diagnosis or epidemiology of VSP were excluded. Cross-checking of eligible data from our previous systematic review [12] was also undertaken.
Table 1.
The Population, Intervention, Comparators, Outcomes, and Study (PICOS) design eligibility criteria
| PICOS design criteria | Description |
|---|---|
| Population | Patients (any age) with VSP or its related complications following aSAH after clipping surgery or coiling |
| Interventions/Comparators | Any treatment received in the ICU and post-ICU setting |
| Outcomes | Including—but not limited to—measures of: mortality, clinical events, cognitive status, functional status |
| Study design |
Clinical trials (RCTs, NRS) Observational studies (any study design): real-world studies, hospital databases, or chart reviews Excluded: commentaries, expert reviews, case reports |
| Other considerations | Minimum sample size: ≥ 2 individuals |
| Exclusion criteria | Studies not meeting the inclusion criteria outlined above |
aSAH aneurysmal subarachnoid hemorrhage, ICU intensive care unit, NRS non-randomized study, RCT randomized controlled trial, VSP vasospasm
After full-text screening, using the double review process described above, the included clinical studies were systematically categorized into three groups: comparative studies (i.e., analyzed clinical outcomes for patients with VSP/DCI vs. those without), regression-based studies (i.e., reported findings based on a regression-type of analysis that estimated the probability of a clinical outcome using VSP/DCI severity as a predictor), and descriptive studies (i.e., reported clinical findings for patients with VSP/DCI without a reference or control group).
Data Extraction
For the comparative and regression-based studies, data on the objective, study characteristics, patient characteristics, medication and outcomes of each study were extracted by a single reviewer using a predetermined extraction sheet. Study outcomes included measures of functional status, cognitive status, clinical events, and mortality (ESM Table S3). Full validation of the extracted data was conducted by a second reviewer against clean copies of the references.
Clinical burden related to functional outcomes was measured using the modified Rankin Scale (mRS), which is the preferred primary clinical outcome measure in acute stroke research [14], the Glasgow Outcome Scale (GOS) [15] or the extended GOS (GOS-E) [16].
Other measures of functional status included the modified Barthel Index [17] and the modified National Institutes of Health Stroke Scale (mNIHSS) [18].
Clinical burden related to cognitive outcomes was assessed using the Montreal Cognitive Assessment (MoCA) [19] or the Mini-Mental State Exam (MMSE) [20].
Clinical burden related to clinical events (rebleeding) and mortality were also assessed.
Synthesis of Results
The high degree of heterogeneity observed in the included studies prevented meta-analyses of the study outcomes. Therefore, the results are presented qualitatively by identifying trends in the outcomes across the studies considering differences in study and patient characteristics, and grouped according to the methodological approach used in the study (i.e., comparative or regression-based).
The definition of angiographic VSP and multivariate adjustments used in the studies were considered in the data synthesis.
VSP can be diagnosed by angiography (angiographic VSP), transcranial Doppler (TCD) (defined as mean flow velocity in any vessel > 120 cm/s) or as DCI (i.e., a decline in consciousness of ≥ 2 points on the GSC or the occurrence of a new focal neurological deficit, lasting for > 1 h, after exclusion of other potential causes of clinical deterioration) [3, 4].
Diagnosis of VSP in each study was coded to reflect ≥ 1 of the following four categories: angiographic VSP (diagnosed by digital subtraction angiography [DSA], computed tomography angiography [CTA], or magnetic resonance angiography [MRA]), DCI (clinical VSP), and others, such as TCD VSP or combined diagnosis (VSP with DCI or VSP with cerebral infarction). The term “cerebral VSP” refers to VSP determined using a combination of angiographic, TCD, and/or clinical diagnosis, or when the method used to diagnose VSP was not reported.
For regression-based studies reporting multivariate analyses, four variables were extracted: age, premorbid history of hypertension, neurological status at admission measured by the World Federation of Neurosurgical Societies (WFNS) grade, and Fisher grade at admission.
Results
Included Studies
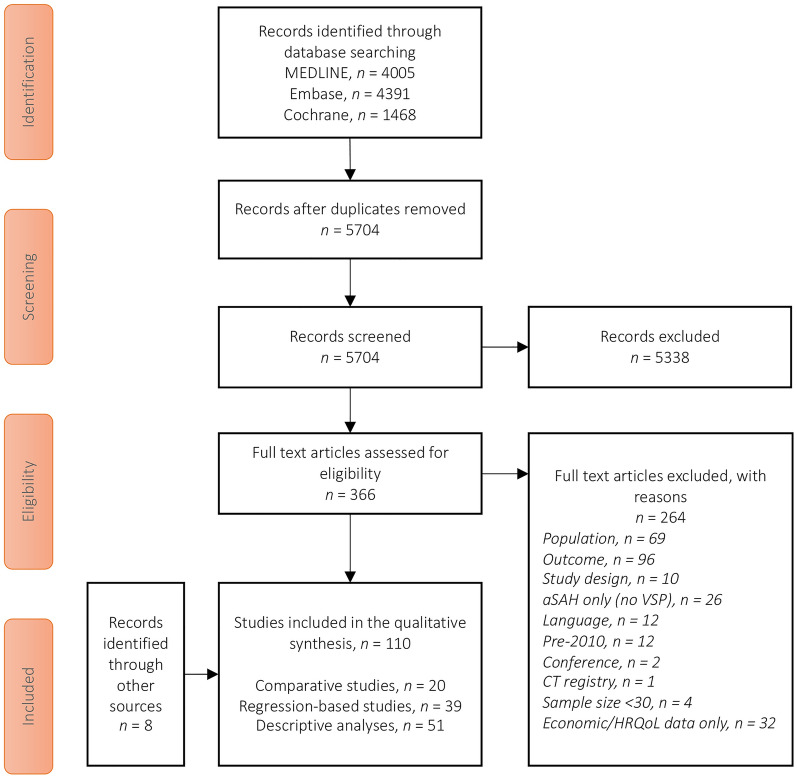
Searches in MEDLINE (4005 hits), Embase (4391 hits), and Cochrane (1468 hits) identified 5704 abstracts for review after duplicates were removed. From these, 366 records were selected for full-text review and 264 articles were excluded, leaving 102 studies meeting the inclusion criteria. An additional eight eligible studies reporting clinical outcomes were identified from our previous systematic review [12]. Therefore, the final number of studies analyzed was 110 (Fig. 1).
Fig. 1.
The Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) diagram showing the study selection process. aSAH Aneurysmal subarachnoid hemorrhage, CT clinical trial, HRQoL health-related quality of life,
Data on the clinical burden of angiographic VSP and its related complications after aSAH were identified from 20 comparative studies [21–40] and 39 regression-based studies [41–79] (study characteristics are presented in ESM Table S4). The remaining 51 studies were purely descriptive and are not included (study listing available on request).
Most of the studies were observational (19 of 20 comparative studies, 32 of 39 regression-based studies), the majority of which were conducted retrospectively (15 of 19 comparative studies, 27 of 32 regression-based studies). Only four comparative studies and five regression-based studies were based on prospective designs. Eight studies were based on clinical trials, including seven post hoc analyses of data (5 from the CONSCIOUS-1 trial) [35, 41, 45, 56, 60, 61, 67] and a small (n = 66) randomized controlled trial (RCT) study from India [68] (ESM Table S4).
Most studies (20 of 20 comparative studies, 33 of 39 regression-based studies) were conducted in a single country (ESM Table S4). Two countries together comprised half of the included studies: Germany (13 studies) [22, 27, 30, 33, 36, 43, 48, 49, 53, 54, 63, 65, 78] and the USA (14 studies) [23, 24, 32, 37, 38, 44, 47, 50, 51, 55, 58, 64, 74, 76]. Six studies were based on data from multiple countries: Aldakkan et al. 2017 [41] and five post hoc analyses of the CONSCIOUS-1 trial [45, 56, 60, 61, 67].
Among the studies, 17 of 20 comparative studies and 28 of 39 regression-based studies were conducted in single centers (ESM Table S4). Seven regression-based studies collected data from a large number (i.e., > 30) of centers, including five post hoc analyses of the CONSCIOUS-1 trial [45, 56, 60, 61, 67] and two studies based in Japan [62, 73].
Patient numbers varied widely across the studies, ranging from 34 patients [22] to 1647 patients [29] for the comparative studies and from 40 patients [46] to 17,343 patients [62] for the regression-based studies (ESM Table S4).
Studies also varied considerably in the period of data coverage and length of data collection. For the comparative studies, data were collected from as early as 2002 [34, 37] to 2017 [26], with data collection periods ranging from < 1 year [26] to 9 years [29, 33, 34, 39]. Regression-based studies tended to examine data from earlier time periods and have a wider data collection period. The earliest regression-based studies collected data as early as August 1996 to June 2013 [44] and from April 1996 to April 2014 [70] while the most recent study reported data from January 2013 to July 2019 [58]. Data collection periods for regression-based studies ranged from 1 year [54] to 21 years [59].
VSP diagnosis varied across the studies, with most studies relying on angiographic criteria (15 of 20 comparative studies, 32 of 39 regression-based studies) and the use of multiple (i.e., at least two) diagnostic criteria more common than a single-criteria approach (ESM Table S5).
Of the 39 regression-based studies, 32 reported adjusting for a number of different factors to predict clinical outcomes for angiographic VSP patients, with age the most commonly employed adjustment factor, followed by WFNS grade (ESM Table S6).
Patient Characteristics
Patient populations were broadly comparable between the studies. However, a few studies included a highly selective patient population, such as those who were in need of an external ventricular drain [22], had received specific anesthetics [23], or had undergone specific treatments, such as anticonvulsants [32]. Patient characteristics of age, hypertension, Fisher grade, and WFNS grade were selected to describe the patient population at baseline (ESM Tables S7–S11).
Age was a commonly reported patient characteristic, available in 19 of 20 comparative studies and 36 of 39 regression-based studies (ESM Table S7). Among the comparative studies, most reported mean or median patient ages in the age range of 50–59 years, with the average age of patients ranging from a mean of 43 ± 11 years in the new symptomatic subgroup of Tekle et al. [38] to a mean of 65 (range 46–87) years in Uozumi et al. [39]. Likewise, among the regression-based studies, reported mean or median patient ages were predominantly in the age range 50–59 years with some exceptions due to specific subpopulations investigated. The youngest patients (mean 44.2 ± 13 years) were reported in the control arm of a small RCT of propofol in Indian aSAH patients [68]. The oldest (mean 81 ± 5 years) were from a study that examined aSAH in advanced age (i.e., ≥ 75 years) [62].
Pre-existing hypertension was a less commonly reported patient characteristic, only available in nine of 20 comparative studies and 15 of 39 regression-based studies (ESM Table S8). There was heterogeneity in terms of the proportion of the study population with pre-existing hypertension, ranging from 29% (in the severe VSP subgroup of Mortimer et al. [35]) to 64% (in the new symptomatic VSP subgroup of Tekle et al. [38]) in comparative studies and from 18% [66] to 77% [76] in regression-based studies.
Most studies included a patient population with relatively high disease severity at baseline using the Fisher scale or the modified Fisher scale (ESM Tables S9, S10). Among the comparative studies, most patients had a Fisher grade of ≥ 3. Likewise, in regression-based studies most patients had a Fisher grade of ≥ 3, with the exception of two studies with 60% aSAH patients with Fisher grade 2 [42, 77] and a small RCT of propofol in Indian aSAH patients, which enrolled approximately 60% of patients with Fisher grade 1–2 [68].
In contrast, studies varied widely in terms of the WFNS grades of patients recruited (ESM Table S11). Some studies recruited patients with low-severity aSAH (i.e., WFNS 1–2) mostly [76, 79] or exclusively [41, 46], while other studies investigated patients from the entire range of WFNS grades (i.e., WFNS 1–5) [25, 26, 30, 33, 34, 36, 52, 70, 71, 76, 77].
Clinical Burden
Angiographic VSP-Related Complications: DCI and Cerebral Infarction
As this systematic review aimed to describe and quantify the clinical burden of angiographic VSP and its related complications (i.e., DCI and new cerebral infarction) after aSAH, it was considered necessary to assess the correlations between angiographic VSP, DCI and new cerebral infarction. While causality in the classical sense cannot be derived from such correlations, they at least are complications whose burden is directly related to angiographic VSP.
A statistically significant relationship between angiographic VSP and DCI was reported in two comparative studies [30, 40] and three regression-based studies [41, 44, 53], and between TCD-defined VSP and DCI in one comparative study [25] (ESM Table S12).
Angiographic VSP and cerebral infarction were investigated directly as separate outcomes in five studies. Two comparative studies [31, 37] suggested that cerebral infarction was higher in patients with angiographic VSP. Three regression-based studies [44, 59, 71] found an association between angiographic VSP and cerebral infarction, but suggested that other factors also contribute to whether angiographic VSP leads to cerebral infarction (ESM Table S13).
Functional Status: Modified Rankin Scale
Three [26, 29, 39] of nine comparative studies and 11 [48, 49, 52–54, 60, 63, 65, 71, 73, 74] of 13 regression-based studies found that patients with angiographic VSP or VSP-related complications have significantly poorer functional outcomes, as measured by the mRS, both at discharge and follow-up, than patients without angiographic VSP or VSP-related complications. In six studies [33, 38, 44, 59, 66, 76] that looked at specific VSP populations (e.g., by severity or timing of angiographic VSP, TCD-defined VSP or cerebral VSP), the outcomes were mixed.
In comparative studies, patients with angiographic VSP or DCI-related infarction had significantly poorer mRS functioning at 3 [29, 39] or 6 months [26] post aSAH than patients without angiographic VSP or DCI-related infarction. In contrast, patients with versus those without angiographic VSP or DCI had similar mRS functional outcomes at discharge in three studies [23, 25, 31], and at 3 months [27] or 18 months [21] in one study each. Similar functional outcomes at 3 months were also reported in one study between patients with severe angiographic VSP and those with mild or no angiographic VSP [35] (ESM Table S14).
Two comparative studies assessed the mRS of specific VSP populations (e.g., by severity of cerebral VSP or TCD-defined VSP). One of these identified significantly better functional outcomes (mRS score 0–2) at 6 months in patients with cerebral VSP lasting > 14 days compared with matched-pair patients with cerebral VSP lasting ≤ 14 days [33]. The second study, in which all patients had TCD-defined VSP, found no significant difference in the proportion of patients with poor functional outcome (mRS 3–6) at discharge who developed a new episode of TCD-defined VSP in a previously asymptomatic artery or not [38] (ESM Table S14).
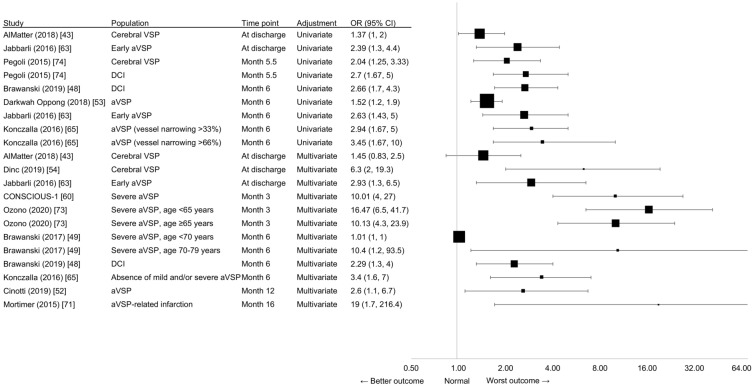
In studies that employed a regression-based approach, five [48, 53, 63, 65, 74] of six studies found an association between angiographic VSP or DCI and unfavorable functional outcome on the mRS using univariate analysis (Fig. 2). Patients who had angiographic VSP or DCI were significantly more likely to exhibit neurological dysfunction after 6 months [48, 53]. Early angiographic VSP (i.e., angiographic VSP within 72 h post aSAH) independently predicted unfavorable outcome at discharge and at the 6 months’ follow-up [63]. Increasing severity of angiographic VSP (i.e., angiographic VSP vessel narrowing > 33% and > 66%) was significantly associated with an increasing likelihood of unfavorable outcome (mRS > 2) at 6 months post aSAH [65]. Patients without cerebral VSP or DCI after aSAH had significantly better odds of good functional outcome (mRS 0 or 1) after a mean follow-up of 5.5 months [74].
Fig. 2.
Clinical burden, as measured by functional outcome (mRS scores, OR), from regression-based studies. The size of the square corresponds to the number of patients assessed. The OR of AlMatter et al. [43], Jabbarli et al. [63], Konczalla et al. [65], and Pegoli et al. [74] have been transformed so that the results point in the same direction as the other studies, meaning that OR < 1 indicates the “better outcome”, OR = 1 indicates the “same outcome”, and OR > 1 indicates the “worst outcome”. The transformation equation is as follows: if OR = (a/b)/(c/d), ORt = 1/OR = 1/((a/b)/(c/d)) = (c/d)/(a/b), with ORt = transformed ORs. aVSP Angiographic vasospasm, CI confidence interval, DCI delayed cerebral ischemia, mRS modified Rankin scale, OR odds ratio, VSP vasospasm
One single-center, retrospective cohort study of 693 aSAH patients (only n = 177 [25.5%]) identified with cerebral VSP found that cerebral VSP was not significantly associated with worse mRS outcome at discharge using univariate analysis [43].
Eleven studies adjusted for the effects of confounding variables that affect outcome potentially independent of angiographic VSP and DCI (Fig. 2). Of these, nine studies [48, 49, 52, 54, 60, 63, 65, 71, 73] found angiographic VSP, severe angiographic VSP (defined as permanent deficit and/or appearance of low-density area on CT [73] or vessel diameter > 66% [49]), cerebral VSP, or DCI was a significant predictor of poor outcome at discharge [54, 63] or 3 months [60, 73], 6 months [48, 49, 65], or 1 year or later [52, 71] using multivariate regression analysis.
Two studies found no significant association between cerebral VSP or DCI and poor outcome using multivariate regression analysis. A single-center, retrospective cohort study of 693 aSAH patients (only n = 177 [25.5%] identified with cerebral VSP) found no association between cerebral VSP and worse mRS outcome at discharge [43]. One retrospective observational cohort study of 237 aSAH patients found DCI was not significantly predictive of poor outcome at 3 months [75].
Four regression-based studies looked at specific VSP populations. A prospective, longitudinal cohort study of 1286 aSAH patients found that ultra-early angiographic VSP (defined as angiographic VSP within the first 48 h of aSAH) was not significantly associated with poor functional outcome at 3 months using multivariate regression analysis [44]. A single-center retrospective study of 80 patients with angiographic VSP reported a significant (p = 0.01) association between cerebral infarction and poor outcome (mRS 3–6) at 3 months [59]. Another retrospective case series, which assessed functional mRS outcomes at 1 month and ≥ 12 months post aSAH in 88 aSAH patients with DCI, found no significant association between patients with severe versus mild-to-moderate cerebral VSP using univariate analysis [66]. Finally, a retrospective cohort study of 159 aSAH patients who underwent endovascular treatment for angiographic VSP (including intra-arterial vasodilator infusion and/or balloon angioplasty) found no significant association between angiographic VSP and mRS at discharge after adjusting for confounding factors [76].
Functional Status: Glasgow Outcome Scale Score and Extended Glasgow Outcome Scale Score
Five [22, 28, 30, 36, 40] of six comparative studies and eight [45–47, 57, 61, 69, 77, 78] of nine regression-based studies found that patients with angiographic VSP, TCD-defined VSP, cerebral VSP, or VSP-related DCI post aSAH had poorer neurological outcomes, measured by the GOS and GOS-E, both at discharge and at follow-up, compared with those without angiographic VSP, TCD-defined VSP, cerebral VSP, or VSP-related DCI.
Among the comparative studies (ESM Tables S15, S16), one small (< 55 patients) prospective study found that patients with VSP-related DCI, compared to those without, had significantly higher odds of poor neurological status (GOS < 4) at discharge (OR 5.4, 95% CI 1.2–24; p = 0.03), which persisted 3-months after aSAH (odds ratio [OR] 10, 95% confidence interval [CI] 2.0–49; p < 0.01) [22], and another reported that aSAH patients with angiographic VSP had significantly worse neurological outcomes on the GOS-E scale at 6 months (p = 0.01 vs. those without angiographic VSP) [40]. Retrospective studies found that aSAH patients with cerebral VSP had significantly poorer neurological outcomes at discharge, as assessed by the GOS (p < 0.001 vs. no cerebral VSP) (n = 224) [28], and that severe TCD-defined VSP predicted poorer neurological outcomes (GOS 1–3) at 3, 6, and 12-months post aSAH compared with mild or moderate TCD-defined VSP (all p < 0.01) (n = 142) [36]. Similarly, aSAH patients with angiographic VSP had poorer GOS-E scores at discharge (p = 0.01 vs. no angiographic VSP) but not at 3–6 months follow-up, whereas patients with DCI had poorer GOS-E scores at both discharge and at 3–6 months of follow-up (p < 0.001 and p = 0.002 vs, no DCI, respectively) (n = 138) [30].
Conversely, a post hoc analysis of prospectively acquired clinical trial data found no significant differences in discharge GOS or 90-day GOS (favorable outcome dichotomized as GOS 4–5) between patients with mild or no cerebral angiographic VSP (n = 63) and patients with severe angiographic VSP (n = 17) [35] (ESM Table S15).
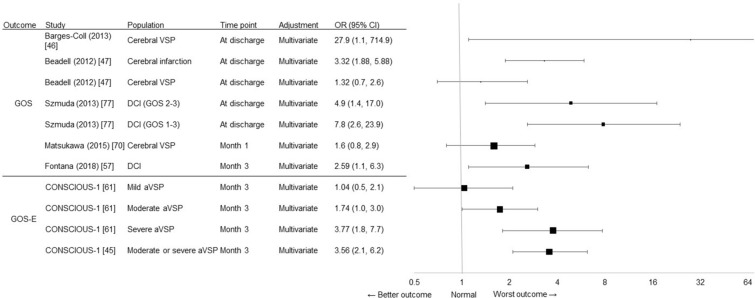
Multivariate regression-based studies (Fig. 3) found that cerebral VSP predicted poor neurological outcome at discharge (GOS 1–2) in a small prospective study of 40 patients with aSAH [46], while the presence of DCI increased the odds by > 2-fold for an unfavorable neurological outcome (GOS 1–3) at 3 months post aSAH in a retrospective analysis of 270 patients with aSAH [57]. Likewise, a retrospective analysis of short-term outcomes after aSAH found that the presence of DCI increased the odds by > 4-fold for an unfavorable outcome (GOS 2, 3) at discharge and by > 7-fold when the evaluation of morbidity included death (GOS 1–3), even after controlling for confounding factors [77]. In a retrospective review of 374 patients with aSAH, cerebral infarction, but not cerebral VSP, was independently predictive of poor GOS at discharge [47]. Post hoc analyses of data from the CONSCIOUS-1 (Clazosentan to Overcome Neurological iSChemia and Infarction OccUrring after Subarachnoid hemorrhage) trial found that both moderate and severe, but not mild angiographic VSP, predicted poor GOS-E at 3 months [61], with a > 3-fold increase in the odds of poor neurological outcomes identified for moderate or severe angiographic VSP compared with mild or no angiographic VSP [45]. Similarly, a single-center retrospective study of 176 patients identified DCI as a significant predictor of unfavorable outcomes (GOS 1–3) at 1 year after aSAH (p = 0.01) using proportional odds logistic regression analysis [78]. A retrospective analysis found that aSAH patients (n = 112) with cerebral infarction due to angiographic VSP were significantly (p < 0.0001) more likely to experience a poor outcome (GOS 1–3) at discharge than a good outcome using univariate analysis [69].
Fig. 3.
Clinical burden, as measured by functional outcome (OR) reported in regression-based studies based on GOS scores and GOS-E scores. The size of the square corresponds to the number of patients assessed. GOS Glasgow Outcome Scale, GOS-E extended Glasgow Outcome Scale
Finally, in a univariate analysis of a retrospectively collected series of 460 aSAH patients, cerebral VSP was significantly related to poor outcome at 30 days (OR not reported; p = 0.024), but this association was not significant by multivariate logistic regression analysis (Fig. 3) [70].
Functional Status: Other Measures
Two studies reported less commonly used measures of neurological functioning. In a retrospective analysis of 299 aSAH patients with stress-induced cardiomyopathy, cerebral VSP was significantly associated with poorer functional outcomes measured using the modified Barthel Index at both 3 and 12 months (p value not stated) [64]. In contrast, a retrospective analysis of 74 aSAH patients found similar neurological functioning measured using the mNIHSS at 3 months regardless of the occurrence or not of TCD-defined VSP (median mNIHSS 7.5 [range 1–25.5] vs. 6 [range 2–10], respectively, for patients with vs. those without TCD-defined VSP; p value not reported) [27].
Cognitive Impairment
All five regression-based studies [42, 58, 67, 68, 79] found that angiographic VSP, TCD-defined VSP, and DCI were significantly associated with poor cognitive status measured using the MoCA or the MMSE (ESM Table S17).
DCI (p = 0.022), but not cerebral VSP, was significantly predictive of cognitive impairment (MoCA < 22) at discharge in a retrospective review of aSAH patient records from 2013 to 2019 [58]. A prospective observational study found that cerebral infarction due to DCI (MoCA < 26) accounted for 38% of the variance in cognitive outcomes at 3 months [79]. A prospective observational study of 82 aSAH patients reported that the number of days with DCI was a significant risk factor for cognitive dysfunction (MoCA < 21) at 1 year, even after accounting for confounding factors [42].
In a post hoc analysis of CONSCIOUS-1, significantly lower mean MMSE scores were identified at 12 weeks after aSAH in patients with severe angiographic VSP versus those with no angiographic VSP (18 vs. 28, respectively; Kruskal–Wallis, p < 0.0001), with no apparent differences between patients with moderate, mild, or no angiographic VSP [67]. Cerebral infarction due to DCI accounted for 31% of the variance in cognitive outcomes (MMSE < 27) at 3 months by a multiple regression analysis [79]. Finally, the occurrence of TCD-defined VSP predicted cognitive impairment at discharge by multivariate analysis in a small (n = 66) RCT study from India that employed a Hindi-language modification of the MMSE [68].
Mortality
Five [25–27, 34, 37] of ten comparative studies and eight [50, 55, 57, 63, 64, 72, 73, 78] of nine regression-based studies found numerically or significantly higher mortality rates in patients with than in patients without angiographic VSP or its related complications. In two studies [33, 59] that looked at specific VSP populations (e.g., by severity or timing of angiographic or cerebral VSP), the outcomes were mixed.
In comparisons of patients with versus those without angiographic VSP or its related complications (ESM Table S18), only one small retrospective study reported significantly higher in-hospital mortality rates in patients with DCI than in those without DCI (p < 0.0001; n = 137) [37]. In addition, a retrospective review of patient health records identified a marginally higher in-hospital mortality rate in aSAH patients who developed DCI than in those who didn’t (16.5 vs. 13.7%, respectively, p-value not reported; n = 463) [34].
Four studies (including 2 retrospective studies [24, 31], 1 prospective study [22], and 1 post hoc analysis of a RCT [35]) found in-hospital mortality rates were not significantly different between patients with versus those without angiographic VSP [31, 35], DCI [22], or delayed infarction [24]. One outlying retrospective study identified lower in-hospital mortality in patients with versus those without DCI, albeit without statistical significance (p = 0.357) [21].
The evidence reporting mortality by VSP or DCI status at later time points was more consistent across three studies (ESM Table S18). In small prospective studies, mortality rates were twofold higher in patients with DCI than in patients without DCI at day 21 (p value not reported) [25] and significantly higher at 6 months in patients with angiographic VSP compared with those without (p < 0.001) [26]. In a small retrospective study, the rate of mortality at 3 months in patients with TCD-defined VSP was more than twice that of patients without TCD-defined VSP (p value not reported) [27].
One comparative study that assessed the mRS of a specific VSP population found that significantly more patients with cerebral VSP lasting ≤ 14 days died in hospital (p < 0.0001) or within 6 months (p < 0.001) than patients with cerebral VSP lasting > 14 days [33].
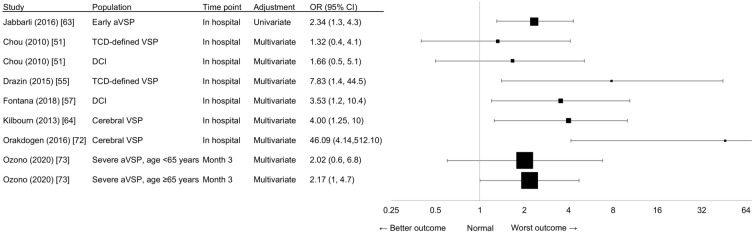
Evidence from regression-based studies was generally consistent with the proposition that angiographic VSP and its related complications were associated with mortality (Fig. 4). A retrospective study of 531 patients found that in-hospital mortality increased twofold if angiographic VSP occurred within 72 h after aSAH using univariate analysis [63]. Three retrospective studies that investigated in-hospital mortality using multivariate analysis found that cerebral VSP reduced the odds of in-hospital survival by 75% [64], TCD-defined VSP increased the odds of in-hospital mortality by > 7-fold [55], and patients with DCI were 3.5-fold more likely to die in hospital [57]. A large retrospective study of 1124 patients identified severe angiographic VSP as a significant risk factor for mortality at 3 months in elderly patients aged ≥ 65 years, but not in patients aged < 65 years using multivariate analysis [73]. A smaller retrospective study of 114 aSAH patients identified cerebral VSP as an independent risk factor for mortality using multivariate analysis, although the degree of uncertainty (measured by the width of the 95% CI) was large (OR 46.093, 95% CI 4.149–512.105; p = 0.002) [72].
Fig. 4.
Clinical outcome, with death (OR) as indicator, reported in regression-based studies. The size of the square corresponds to the number of patients assessed. The ORs of Kilbourn et al. [64] have been transformed so that the results point in the same direction as the other studies, meaning that OR < 1 indicates “best outcome”, OR = 1 indicates “same outcome”, and OR > 1 indicates “worst outcome”. The transformation equation is as follows: if OR = (a/b)/(c/d), ORt = 1/OR = 1/((a/b)/(c/d)) = (c/d)/ (a/b), with ORt = transformed ORs. TCD Transcranial Doppler
Conversely, one retrospective study of 198 aSAH patients [51] found no increased odds of in-hospital mortality in patients with TCD-defined VSP or DCI using multivariate analysis.
Two studies did not report effect sizes but only significant values. A large retrospective study (n > 5000) of the U.S. National Inpatient Sample database found that cerebral VSP significantly increased in-hospital mortality (p < 0.0012) using multivariable regression modeling [50]. A single-center retrospective study of 176 aSAH patients found that DCI was a significant predictor of mortality by day 30 (p = 0.019), but not at 1 year (p = 0.177), by proportional odds logistic regression analysis [78].
One single-center retrospective study in 80 aSAH patients with angiographic VSP, which investigated a specific VSP population, found that cerebral infarction and death at 3 months were significantly associated (p = 0.003) [59].
Discussion
In this study we summarize the impact of angiographic VSP, DCI, and cerebral infarction post aSAH on functional outcomes (measured using the mRS, GOS, GOS-E, modified Barthel Index, and NIHSS), cognitive impairment (measured using the MoCA and MMSE) and mortality. We were unable to find other similar, comprehensive studies. Our analyses of 20 comparative and 39 regression-based studies found that the vast majority report a significant detrimental effect of angiographic VSP and its related complications, DCI and cerebral infarction, on outcomes of patients with aSAH (Fig. 5). Together with our previous publication [12], this study completes our synthesis of evidence across the overall (i.e., clinical, economic, and humanistic) burden of disease of angiographic VSP and its related complications after aSAH.
Fig. 5.
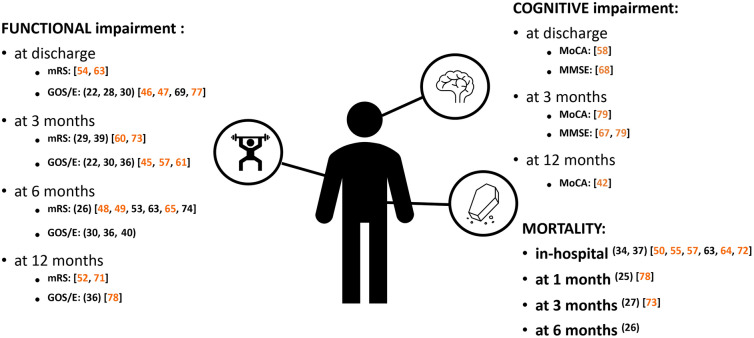
Evidence showing the clinical impact of cerebral vasospasm and its related complications, delayed cerebral ischemia and cerebral infarction, on patients after aneurysmal subarachnoid hemorrhage, by outcome and time point. The figure only summarizes evidence reporting an association between cerebral vasospasm and its related complications with negative clinical outcomes. Functional evidence based on the Barthel Index and mNIHSS tools are not shown. References in parentheses indicate comparative studies, while references in square brackets indicate regression-based studies. Reference numbers highlighted in orange indicate outcomes based on multivariate evidence alone or combined with univariate evidence; reference numbers in black indicate outcomes based on univariate evidence alone. GOS/E Glasgow Outcome Scale/extended Glasgow Outcome Scale, MMSE Mini-Mental State Examination, MoCA Montreal Cognitive Assessment
We were unable to conduct a meta-analysis due to the high degree of heterogeneity between studies. Nevertheless, eight of 15 comparative and 19 of 22 regression-based studies identified significantly poorer functional outcomes, as measured by the mRS, GOS, and GOS-E, in aSAH patients who experience angiographic VSP and its related complications than in those who do not. Most studies showed that these complications are associated with some functional impairment early on in the initial hospital stay, but that effects of angiographic VSP and its related complications become more apparent after 3 or 6 months [31, 35, 43]. One explanation may be that the clinical burden of the initial aSAH event overshadows the effect of angiographic VSP and its complications.
This analysis found that it was difficult to accurately estimate the effect size of angiographic VSP and its related complications on outcomes after aSAH. From the regression-based studies analyzed here, the median OR (interquartile range [IQR]) is 2.7 (2.2, 7.2) for functional outcome measured by the mRS, with the broad range arising from the often small population size of studies. However, if this median is used as a reference case, patients who experience angiographic VSP, DCI and cerebral infarction post aSAH have an approximately threefold higher odds of a poor functional outcome than those who do not. The regression-based evidence when the outcome measure is the GOS/GOS-E was virtually the same, with a reference case median OR (IQR) of 2.6 (1.5, 4.3).
Regarding the cognitive status of aSAH patients, despite the lower corpus of evidence, all five regression-based studies identified a significant association between angiographic VSP and its related complications and poor cognitive outcome measured using the MoCA or the MMSE. Notably, cognitive impairment was present at discharge, 3 months, and even at 1 year post aSAH in a prospective observational study of 82 aSAH patients [42]. This result is consistent with the findings of a prospective study that was published after our search was conducted [80], which reported significantly worse neuropsychological functioning in aSAH patients with DCI for up to 3 months after the hemorrhage compared with non-DCI patients. Similar to functional impairment, cognitive impairment appears to be irreversible. The effect size of cerebral infarction due to DCI on cognitive impairment is substantial, and, in one prospective observational study, accounted for 38% of the variance in cognitive outcomes (MoCA < 26) and 31% of the variance in cognitive outcomes (MMSE < 27) at 3 months [79]. The median OR (minimum, maximum) of 3 (1.3, 3.9) for cognitive abilities as per the MoCA score indicates a worst outcome in patients with angiographic VSP or VSP-related complications versus those without.
Most comparative and regression-based analyses found that patients with angiographic VSP or DCI had increased odds of in-hospital mortality [33, 37, 55, 57, 63] or reduced odds of in-hospital survival [64] that is present for up to 6 months [25–27, 33, 73]. This is consistent with the findings of a large retrospective study of the U.S. National Inpatient Sample database that reported a significant association between cerebral VSP and in-hospital mortality [50]. In terms of the effect size, the median OR (IQR) for mortality in aSAH patients with these complications was 2.3 (1.8, 5.9), meaning that these patients have an approximately twofold higher odds of dying compared with aSAH patients without such complications. We found one regression-based study that reported no significant differences in in-hospital mortality between patients with versus those without TCD-defined VSP [51]. However, the authors opined that the study was underpowered to detect such a difference. Similarly, four comparative studies [22, 24, 31, 35] did not identify increased mortality in patients with angiographic VSP and its related complications compared to those without such complications. However, in-hospital mortality in patients with angiographic VSP versus non-VSP patients was limited by the low number of deaths in these studies. For example, a post hoc analysis of an RCT reported that no patients with severe angiographic VSP (n = 17) and only two of 63 patients with none/mild angiographic VSP died in hospital [35].
Increasing severity of angiographic VSP, the presence of DCI or cerebral infarction, and older age may also adversely affect functional and cognitive outcomes and increase the likelihood of death. For example, the severity of VSP, but not the occurrence of VSP, per se, appears to be a significant factor driving cognitive dysfunction [67] and poorer functional outcomes [36, 45, 61, 65]. The sometimes not reported relationship of the occurrence of angiographic VSP to functional outcome and other outcomes may be because mild and moderate angiographic VSP are included and could lead to less DCI and cerebral infarction events than severe VSP [9]. Likely for similar reasons, DCI or cerebral infarction, but not angiographic VSP or cerebral VSP, were significantly predictive of cognitive impairment [58] and poor functional outcome [30, 47]. Finally, there may be an interaction between angiographic VSP and age. We found increased odds for mortality in elderly aSAH patients aged ≥ 65 years with severe angiographic VSP versus those with non-severe angiographic VSP, but not in patients aged < 65 years of age [73], while the occurrence of severe angiographic VSP was a significant predictor of an unfavorable functional outcome in aSAH patients aged 70–79 years, but not in those aged < 70 years [49].
Despite the majority of studies showing the clinical burden associated with angiographic VSP, DCI, and cerebral infarction, some studies report contradictory outcomes. A limitation of some studies may be their relatively small sample size, which may underpower a study to evaluate clinically important associations. A limitation specific to Abulhasan et al. [21] is that the mRS score used to assess the functional outcome was retrospectively calculated from medical records, which is less accurate than in-person assessment. The time point of measurement may also explain non-significant results. Indeed, for measurements taken in-hospital or at discharge, it is harder to disassociate clinical impairment due to the initial aSAH event versus the presence of complications, such as angiographic VSP, DCI, and cerebral infarction.
Strengths and Limitations
The strengths of this study are the comprehensive screening of the literature, resulting in 110 studies including 42,631 patients for analysis. The likelihood of having more heterogeneous results due to differences in the standard of care was limited by excluding studies published before 2010.
There are also a number of limitations. Most studies collected data in single centers, which may restrict the generalizability of the findings. Most studies were retrospective, and the data obtained from these are more likely to be inaccurate and biased by missing data and incomplete and unaudited variables and outcomes. Further limitations include the heterogeneity of the studies, and the different definitions of angiographic VSP, DCI and cerebral infarction. Indeed, clinical heterogeneity in the prevention and treatment of angiographic VSP across the studies could influence the mechanisms that lead to poor neurological outcomes in aSAH patients and skew the results. Almost all patients with aSAH develop some angiographic VSP, so heterogeneity in the severity and breadth of angiographic VSP between studies also could affect the results.
We also did not include the 51 descriptive studies because the association between VSP and a particular outcome was not reported.
Conclusion
This systematic review found that angiographic VSP and its related complications, DCI and cerebral infarction, were associated with worse clinical outcome, higher mortality, and poorer cognitive function in the majority of studies of patients with aSAH. We estimate that angiographic VSP and its related complications, DCI and cerebral infarction, lead to an approximately threefold higher odds of poor functional and cognitive outcomes, and about a twofold increase in the odds of death.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
The Systematic Literature Review, the journal’s Rapid Service Fee, and medical writing services were funded by Idorsia Pharmaceuticals Ltd, Switzerland.
Medical writing assistance
Medical writing assistance was provided by Melanie Gatt (PhD), an independent medical writer. Support for this assistance was funded by Idorsia Pharmaceuticals Ltd.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author contributions
François-Xavier Chalet, Orestis Briasoulis, Eric J. Manalastas, Darren A. Talbot, Juliette C. Thompson, and R. Loch Macdonald contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Juliette C. Thompson and Eric J. Manalastas. All authors commented on all versions of the manuscript, and read and approved the final manuscript.
Disclosures
Juliette C. Thompson and Eric J. Manalastas from Visible Analytics Ltd received fees from Idorsia Pharmaceuticals Ltd for the conduct of the systematic literature review. François-Xavier Chalet is an employee and shareholder of Idorsia Pharmaceuticals Ltd. Darren A. Talbot is an employee and shareholder of Idorsia Pharmaceuticals Ltd. Orestis Briasoulis is an employee and shareholder of Idorsia Pharmaceuticals Ltd. R. Loch Macdonald is a consultant for Acasti Pharma, BioProducts Laboratory, CSL Behring, and Idorsia Pharmaceuticals Ltd.
Compliance with ethics guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
References
- 1.D'Souza S. Aneurysmal subarachnoid hemorrhage. J Neurosurg Anesthesiol. 2015;27:222–240. doi: 10.1097/ana.0000000000000130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Macdonald RL, Schweizer TA. Spontaneous subarachnoid haemorrhage. Lancet. 2017;389:655–666. doi: 10.1016/s0140-6736(16)30668-7. [DOI] [PubMed] [Google Scholar]
- 3.Frontera JA, Fernandez A, Schmidt JM, et al. Defining vasospasm after subarachnoid hemorrhage: what is the most clinically relevant definition? Stroke. 2009;40:1963–1968. doi: 10.1161/strokeaha.108.544700. [DOI] [PubMed] [Google Scholar]
- 4.Vergouwen MD, Vermeulen M, van Gijn J, et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary research group. Stroke. 2010;41:2391–2395. doi: 10.1161/strokeaha.110.589275. [DOI] [PubMed] [Google Scholar]
- 5.Dorsch NW, King MT. A review of cerebral vasospasm in aneurysmal subarachnoid haemorrhage part I: incidence and effects. J Clin Neurosci. 1994;1:19–26. doi: 10.1016/0967-5868(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 6.Rabinstein AA, Weigand S, Atkinson JL, Wijdicks EF. Patterns of cerebral infarction in aneurysmal subarachnoid hemorrhage. Stroke. 2005;36:992–997. doi: 10.1161/01.STR.0000163090.59350.5a. [DOI] [PubMed] [Google Scholar]
- 7.Geraghty JR, Testai FD. Delayed cerebral ischemia after subarachnoid hemorrhage: beyond vasospasm and towards a multifactorial pathophysiology. Curr Atheroscler Rep. 2017;19:50. doi: 10.1007/s11883-017-0690-x. [DOI] [PubMed] [Google Scholar]
- 8.Østergaard L, Aamand R, Karabegovic S, et al. The role of the microcirculation in delayed cerebral ischemia and chronic degenerative changes after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2013;33:1825–1837. doi: 10.1038/jcbfm.2013.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crowley RW, Medel R, Dumont AS, et al. Angiographic vasospasm is strongly correlated with cerebral infarction after subarachnoid hemorrhage. Stroke. 2011;42:919–923. doi: 10.1161/STROKEAHA.110.597005. [DOI] [PubMed] [Google Scholar]
- 10.Weidauer S, Lanfermann H, Raabe A, Zanella F, Seifert V, Beck J. Impairment of cerebral perfusion and infarct patterns attributable to vasospasm after aneurysmal subarachnoid hemorrhage: a prospective MRI and DSA study. Stroke. 2007;38:1831–1836. doi: 10.1161/strokeaha.106.477976. [DOI] [PubMed] [Google Scholar]
- 11.Brami J, Chousterman B, Boulouis G, et al. Delayed cerebral infarction is systematically associated with a cerebral vasospasm of large intracranial arteries. Neurosurgery. 2020;86:E175–E183. doi: 10.1093/neuros/nyz340. [DOI] [PubMed] [Google Scholar]
- 12.Thompson JC, Chalet FX, Manalastas EJ, Hawkins N, Sarri G, Talbot DA. Economic and humanistic burden of cerebral vasospasm and its related complications after aneurysmal subarachnoid hemorrhage: a systematic literature review. Neurol Ther. 2022;11:597–620. doi: 10.1007/s40120-022-00348-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. J Clin Epidemiol. 2021;134:178–189. doi: 10.1016/j.jclinepi.2021.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Lees KR, Bath PM, Schellinger PD, et al. Contemporary outcome measures in acute stroke research: choice of primary outcome measure. Stroke. 2012;43:1163–1170. doi: 10.1161/STROKEAHA.111.641423. [DOI] [PubMed] [Google Scholar]
- 15.Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975;1:480–484. doi: 10.1016/s0140-6736(75)92830-5. [DOI] [PubMed] [Google Scholar]
- 16.Jennett B, Snoek J, Bond MR, Brooks N. Disability after severe head injury: observations on the use of the Glasgow Outcome Scale. J Neurol Neurosurg Psychiatry. 1981;44:285–293. doi: 10.1136/jnnp.44.4.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shah S, Vanclay F, Cooper B. Improving the sensitivity of the Barthel Index for stroke rehabilitation. J Clin Epidemiol. 1989;42:703–709. doi: 10.1016/0895-4356(89)90065-6. [DOI] [PubMed] [Google Scholar]
- 18.Lyden PD, Lu M, Levine SR, Brott TG, Broderick J. A modified national institutes of health stroke scale for use in stroke clinical trials. Stroke. 2001;32:1310–1317. doi: 10.1161/01.STR.32.6.1310. [DOI] [PubMed] [Google Scholar]
- 19.Nasreddine ZS, Phillips NA, Bédirian V, et al. The montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 20.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 21.Abulhasan YB, Ortiz Jimenez J, Teitelbaum J, Simoneau G, Angle MR. Milrinone for refractory cerebral vasospasm with delayed cerebral ischemia. J Neurosurg. 2020;134:971–982. doi: 10.3171/2020.1.JNS193107. [DOI] [PubMed] [Google Scholar]
- 22.Appel D, Seeberger M, Schwedhelm E, et al. Asymmetric and symmetric dimethylarginines are markers of delayed cerebral ischemia and neurological outcome in patients with subarachnoid hemorrhage. Neurocrit Care. 2018;29:84–93. doi: 10.1007/s12028-018-0520-1. [DOI] [PubMed] [Google Scholar]
- 23.Athiraman U, Aum D, Vellimana AK, et al. Evidence for a conditioning effect of inhalational anesthetics on angiographic vasospasm after aneurysmal subarachnoid hemorrhage. J Neurosurg. 2020;133:152–158. doi: 10.3171/2019.3.JNS183512. [DOI] [PubMed] [Google Scholar]
- 24.Brown RJ, Kumar A, Dhar R, Sampson TR, Diringer MN. The relationship between delayed infarcts and angiographic vasospasm after aneurysmal subarachnoid hemorrhage. Neurosurgery. 2013;72:702–707. doi: 10.1227/NEU.0b013e318285c3db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Budohoski KP, Czosnyka M, Smielewski P, et al. Impairment of cerebral autoregulation predicts delayed cerebral ischemia after subarachnoid hemorrhage: a prospective observational study. Stroke. 2012;43:3230–3237. doi: 10.1161/STROKEAHA.112.669788. [DOI] [PubMed] [Google Scholar]
- 26.Ding CY, Cai HP, Ge HL, Yu LH, Lin YX, Kang DZ. Is admission lipoprotein-associated phospholipase A2 a novel predictor of vasospasm and outcome in patients with aneurysmal subarachnoid hemorrhage? Neurosurgery. 2020;86:122–131. doi: 10.1093/neuros/nyz041. [DOI] [PubMed] [Google Scholar]
- 27.Ehlert A, Schmidt C, Wolfer J, et al. Molsidomine for the prevention of vasospasm-related delayed ischemic neurological deficits and delayed brain infarction and the improvement of clinical outcome after subarachnoid hemorrhage: a single-center clinical observational study. J Neurosurg. 2016;124:51–58. doi: 10.3171/2014.12.JNS13846. [DOI] [PubMed] [Google Scholar]
- 28.Filipce V, Caparoski A. The effects of vasospasm and re-bleeding on the outcome of patients with subarachnoid hemorrhage from ruptured intracranial aneurysm. Pril (Makedon Akad Nauk Umet Odd Med Nauki) 2015;36:77–82. doi: 10.1515/prilozi-2015-0081. [DOI] [PubMed] [Google Scholar]
- 29.Haegens NM, Gathier CS, Horn J, Coert BA, Verbaan D, van den Bergh WM. Induced hypertension in preventing cerebral infarction in delayed cerebral ischemia after subarachnoid hemorrhage. Stroke. 2018;49:2630–2636. doi: 10.1161/STROKEAHA.118.022310. [DOI] [PubMed] [Google Scholar]
- 30.Hurth H, Birkenhauer U, Steiner J, Schlak D, Hennersdorf F, Ebner FH. Delayed cerebral ischemia in patients with aneurysmal subarachnoid hemorrhage—serum D-dimer and C-reactive protein as early markers. J Stroke Cerebrovasc Dis. 2020;29:104558. doi: 10.1016/j.jstrokecerebrovasdis.2019.104558. [DOI] [PubMed] [Google Scholar]
- 31.Jeon YT, Lee JH, Lee H, et al. The postoperative C-reactive protein level can be a useful prognostic factor for poor outcome and symptomatic vasospasm in patients with aneurysmal subarachnoid hemorrhage. J Neurosurg Anesthesiol. 2012;24:317–324. doi: 10.1097/ANA.0b013e31826047a2. [DOI] [PubMed] [Google Scholar]
- 32.Karamchandani RR, Fletcher JJ, Pandey AS, Rajajee V. Incidence of delayed seizures, delayed cerebral ischemia and poor outcome with the use of levetiracetam versus phenytoin after aneurysmal subarachnoid hemorrhage. J Clin Neurosci. 2014;21:1507–1513. doi: 10.1016/j.jocn.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 33.Konczalla J, Brawanski N, Bruder M, Senft C, Platz J, Seifert V. Outcome of patients with long-lasting cerebral vasospasm after subarachnoid hemorrhage: is prolonged treatment for cerebral vasospasm worthwhile? A matched-pair analysis. World Neurosurg. 2016;88:488–496. doi: 10.1016/j.wneu.2015.10.033. [DOI] [PubMed] [Google Scholar]
- 34.Lee H, Perry JJ, English SW, et al. Clinical prediction of delayed cerebral ischemia in aneurysmal subarachnoid hemorrhage. J Neurosurg. 2019;130:1914–1921. doi: 10.3171/2018.1.JNS172715. [DOI] [PubMed] [Google Scholar]
- 35.Mortimer AM, Steinfort B, Faulder K, et al. The detrimental clinical impact of severe angiographic vasospasm may be diminished by maximal medical therapy and intensive endovascular treatment. J Neurointerv Surg. 2015;7:881–887. doi: 10.1136/neurintsurg-2014-011403. [DOI] [PubMed] [Google Scholar]
- 36.Sakr Y, Dunisch P, Santos C, et al. Poor outcome is associated with less negative fluid balance in patients with aneurysmal subarachnoid hemorrhage treated with prophylactic vasopressor-induced hypertension. Ann Intensive Care. 2016;6:25. doi: 10.1186/s13613-016-0128-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanelli PC, Anumula N, Gold R, et al. Outcomes-based assessment of a new reference standard for delayed cerebral ischemia related to vasospasm in aneurysmal subarachnoid hemorrhage. Acad Radiol. 2012;19:1066–1074. doi: 10.1016/j.acra.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tekle WG, Chaudry SA, Hassan AE, et al. High risk of new episode of symptomatic vasospasm in unaffected arteries in subarachnoid hemorrhage patients receiving targeted endovascular treatment for symptomatic focal vasospasm. Neurocrit Care. 2014;20:399–405. doi: 10.1007/s12028-013-9825-2. [DOI] [PubMed] [Google Scholar]
- 39.Uozumi Y, Mizobe T, Miyamoto H, et al. Decreased serum sodium levels predict symptomatic vasospasm in patients with subarachnoid hemorrhage. J Clin Neurosci. 2017;46:118–123. doi: 10.1016/j.jocn.2017.08.037. [DOI] [PubMed] [Google Scholar]
- 40.Vrsajkov V, Jevđić J, Mihajlovic D, Pajtić V, Lazukić A, Pantić-Vrsajkov J. Ischemic lesion on computed tomography after subarachnoid hemorrhage: good correlation with angiographic vasospasm and worse outcome. Neurosurg Q. 2016;26:225–229. doi: 10.1097/WNQ.0000000000000159. [DOI] [Google Scholar]
- 41.Aldakkan A, Mansouri A, Jaja BN, Alotaibi NM, Macdonald RL, Subarachnoid Hemorrhage International Trialists Collaborators Predictors of delayed cerebral ischemia in patients with aneurysmal subarachnoid hemorrhage with asymptomatic angiographic vasospasm on admission. World Neurosurg. 2017;97:199–204. doi: 10.1016/j.wneu.2016.09.096. [DOI] [PubMed] [Google Scholar]
- 42.Ali A, Tanirgan G, Sabanci PA, et al. Relation of gray-white matter ratio with long-term cognitive functions and quality of life in patients with mild to moderate aneurysmal subarachnoid hemorrhage: a prospective observational study. Acta Neurochir (Wien) 2018;160:181–189. doi: 10.1007/s00701-017-3374-y. [DOI] [PubMed] [Google Scholar]
- 43.AlMatter M, Aguilar Pereza M, Bhogal P, Hellstern V, Ganslandt O, Henkes H. Results of interdisciplinary management of 693 patients with aneurysmal subarachnoid hemorrhage: clinical outcome and relevant prognostic factors. Clin Neurol Neurosurg. 2018;167:106–111. doi: 10.1016/j.clineuro.2018.02.022. [DOI] [PubMed] [Google Scholar]
- 44.Al-Mufti F, Roh D, Lahiri S, et al. Ultra-early angiographic vasospasm associated with delayed cerebral ischemia and infarction following aneurysmal subarachnoid hemorrhage. J Neurosurg. 2017;126:1545–1551. doi: 10.3171/2016.2.JNS151939. [DOI] [PubMed] [Google Scholar]
- 45.Ayling OG, Ibrahim GM, Alotaibi NM, Gooderham PA, Macdonald RL. Dissociation of early and delayed cerebral infarction after aneurysmal subarachnoid hemorrhage. Stroke. 2016;47:2945–2951. doi: 10.1161/STROKEAHA.116.014794. [DOI] [PubMed] [Google Scholar]
- 46.Barges-Coll J, Perez-Neri I, Avendano J, Mendez-Rosito D, Gomez-Amador JL, Rios C. Plasma taurine as a predictor of poor outcome in patients with mild neurological deficits after aneurysmal subarachnoid hemorrhage. J Neurosurg. 2013;119:1021–1027. doi: 10.3171/2013.4.JNS121558. [DOI] [PubMed] [Google Scholar]
- 47.Beadell NC, Thompson EM, Delashaw JB, Cetas JS. The deleterious effects of methamphetamine use on initial presentation and clinical outcomes in aneurysmal subarachnoid hemorrhage. J Neurosurg. 2012;117:781–786. doi: 10.3171/2012.7.JNS12396. [DOI] [PubMed] [Google Scholar]
- 48.Brawanski N, Kashefiolasl S, Won SY, et al. Does aneurysm side influence the infarction side and patients outcome after subarachnoid hemorrhage? PLoS ONE. 2019;14:e0224013. doi: 10.1371/journal.pone.0224013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brawanski N, Kunze F, Bruder M, et al. Subarachnoid hemorrhage in advanced age: comparison of patients aged 70–79 years and 80 years and older. World Neurosurg. 2017;106:139–144. doi: 10.1016/j.wneu.2017.06.056. [DOI] [PubMed] [Google Scholar]
- 50.Chotai S, Patel PD, Liles C, et al. Impact of neurovascular comorbidities and complications on outcomes after procedural management of intracranial aneurysm: part 2, ruptured intracranial aneurysm. World Neurosurg. 2021;146:e270–e312. doi: 10.1016/j.wneu.2020.10.091. [DOI] [PubMed] [Google Scholar]
- 51.Chou CH, Reed SD, Allsbrook JS, Steele JL, Schulman KA, Alexander MJ. Costs of vasospasm in patients with aneurysmal subarachnoid hemorrhage. Neurosurgery. 2010;67:345–351. doi: 10.1227/01.NEU.0000371980.08391.71. [DOI] [PubMed] [Google Scholar]
- 52.Cinotti R, Putegnat JB, Lakhal K, et al. Evolution of neurological recovery during the first year after subarachnoid haemorrhage in a French university centre. Anaesth Crit Care Pain Med. 2019;38:251–257. doi: 10.1016/j.accpm.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 53.Darkwah Oppong M, Iannaccone A, Gembruch O, et al. Vasospasm-related complications after subarachnoid hemorrhage: the role of patients' age and sex. Acta Neurochir (Wien) 2018;160:1393–1400. doi: 10.1007/s00701-018-3549-1. [DOI] [PubMed] [Google Scholar]
- 54.Dinc N, Quick-Weller J, Tritt S, et al. Vasospasm of the basilar artery following spontaneous SAH-clinical observations and implications for vascular research. Neurosurg Rev. 2019;42:983–989. doi: 10.1007/s10143-018-1015-4. [DOI] [PubMed] [Google Scholar]
- 55.Drazin D, Rosner J, Nuno M, et al. Type of admission is associated with outcome of spontaneous subarachnoid hemorrhage. Int J Stroke. 2015;10:529–533. doi: 10.1111/ijs.12005. [DOI] [PubMed] [Google Scholar]
- 56.Dumont AS, Crowley RW, Monteith SJ, et al. Endovascular treatment or neurosurgical clipping of ruptured intracranial aneurysms: effect on angiographic vasospasm, delayed ischemic neurological deficit, cerebral infarction, and clinical outcome. Stroke. 2010;41:2519–2524. doi: 10.1161/STROKEAHA.110.579383. [DOI] [PubMed] [Google Scholar]
- 57.Fontana V, Bond O, Spadaro S, et al. Red cell distribution width after subarachnoid hemorrhage. J Neurosurg Anesthesiol. 2018;30:319–327. doi: 10.1097/ANA.0000000000000459. [DOI] [PubMed] [Google Scholar]
- 58.Geraghty JR, Lara-Angulo MN, Spegar M, Reeh J, Testai FD. Severe cognitive impairment in aneurysmal subarachnoid hemorrhage: predictors and relationship to functional outcome. J Stroke Cerebrovasc Dis. 2020;29:105027. doi: 10.1016/j.jstrokecerebrovasdis.2020.105027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hosmann A, Rauscher S, Wang WT, et al. Intra-arterial papaverine-hydrochloride and transluminal balloon angioplasty for neurointerventional management of delayed-onset post-aneurysmal subarachnoid hemorrhage vasospasm. World Neurosurg. 2018;119:e301–e312. doi: 10.1016/j.wneu.2018.07.138. [DOI] [PubMed] [Google Scholar]
- 60.Ibrahim GM, Macdonald RL. The effects of fluid balance and colloid administration on outcomes in patients with aneurysmal subarachnoid hemorrhage: a propensity score-matched analysis. Neurocrit Care. 2013;19:140–149. doi: 10.1007/s12028-013-9860-z. [DOI] [PubMed] [Google Scholar]
- 61.Ibrahim GM, Vachhrajani S, Ilodigwe D, et al. Method of aneurysm treatment does not affect clot clearance after aneurysmal subarachnoid hemorrhage. Neurosurgery. 2012;70:102–109. doi: 10.1227/NEU.0b013e31822e5a8e. [DOI] [PubMed] [Google Scholar]
- 62.Ido K, Kurogi R, Kurogi A, et al. Effect of treatment modality and cerebral vasospasm agent on patient outcomes after aneurysmal subarachnoid hemorrhage in the elderly aged 75 years and older. PLoS ONE. 2020;15:e0230953. doi: 10.1371/journal.pone.0230953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jabbarli R, Reinhard M, Shah M, et al. Early vasospasm after aneurysmal subarachnoid hemorrhage predicts the occurrence and severity of symptomatic vasospasm and delayed cerebral ischemia. Cerebrovasc Dis. 2016;41:265–272. doi: 10.1159/000443744. [DOI] [PubMed] [Google Scholar]
- 64.Kilbourn KJ, Levy S, Staff I, Kureshi I, McCullough L. Clinical characteristics and outcomes of neurogenic stress cadiomyopathy in aneurysmal subarachnoid hemorrhage. Clin Neurol Neurosurg. 2013;115:909–914. doi: 10.1016/j.clineuro.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 65.Konczalla J, Brawanski N, Platz J, Senft C, Kashefiolasl S, Seifert V. Aneurysm location as a prognostic outcome factor after subarachnoid hemorrhage from internal carotid artery aneurysms and potential impact for further experimental subarachnoid hemorrhage models. World Neurosurg. 2016;92:273–278. doi: 10.1016/j.wneu.2016.04.086. [DOI] [PubMed] [Google Scholar]
- 66.Lannes M, Teitelbaum J, del Pilar CM, Cardoso M, Angle M. Milrinone and homeostasis to treat cerebral vasospasm associated with subarachnoid hemorrhage: the Montreal Neurological Hospital protocol. Neurocrit Care. 2012;16:354–362. doi: 10.1007/s12028-012-9701-5. [DOI] [PubMed] [Google Scholar]
- 67.Macdonald RL, Hunsche E, Schuler R, Wlodarczyk J, Mayer SA. Quality of life and healthcare resource use associated with angiographic vasospasm after aneurysmal subarachnoid hemorrhage. Stroke. 2012;43:1082–1088. doi: 10.1161/STROKEAHA.111.634071. [DOI] [PubMed] [Google Scholar]
- 68.Mahajan C, Chouhan RS, Rath GP, et al. Effect of intraoperative brain protection with propofol on postoperative cognition in patients undergoing temporary clipping during intracranial aneurysm surgery. Neurol India. 2014;62:262–268. doi: 10.4103/0028-3886.136908. [DOI] [PubMed] [Google Scholar]
- 69.Matano F, Fujiki Y, Mizunari T, et al. Serum glucose and potassium ratio as risk factors for cerebral vasospasm after aneurysmal subarachnoid hemorrhage. J Stroke Cerebrovasc Dis. 2019;28:1951–1957. doi: 10.1016/j.jstrokecerebrovasdis.2019.03.041. [DOI] [PubMed] [Google Scholar]
- 70.Matsukawa H, Tanikawa R, Kamiyama H, et al. Effects of clot removal by meticulous irrigation and continuous low-dose intravenous nicardipine on symptomatic cerebral vasospasm in patients with aneurysmal subarachnoid hemorrhage treated by clipping. World Neurosurg. 2015;84:1798–1803. doi: 10.1016/j.wneu.2015.07.069. [DOI] [PubMed] [Google Scholar]
- 71.Mortimer AM, Steinfort B, Faulder K, et al. Institution of sustained endovascular treatment prior to clinical deterioration in patients with severe angiographic vasospasm: a retrospective observational study of clinico-radiological outcomes. J Neuroradiol. 2015;42:176–183. doi: 10.1016/j.neurad.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 72.Orakdogen M, Emon ST, Somay H, Engin T, Ates O, Berkman MZ. Prognostic factors in patients who underwent aneurysmal clipping due to spontaneous subarachnoid hemorrhage. Turk Neurosurg. 2016;26:840–848. doi: 10.5137/1019-5149.JTN.13654-14.1. [DOI] [PubMed] [Google Scholar]
- 73.Ozono I, Ikawa F, Hidaka T, et al. Risk factor for poor outcome in elderly patients with aneurysmal subarachnoid hemorrhage based on post hoc analysis of the modified WFNS scale study. World Neurosurg. 2020;141:e466–e473. doi: 10.1016/j.wneu.2020.05.196. [DOI] [PubMed] [Google Scholar]
- 74.Pegoli M, Mandrekar J, Rabinstein AA, Lanzino G. Predictors of excellent functional outcome in aneurysmal subarachnoid hemorrhage. J Neurosurg. 2015;122:414–418. doi: 10.3171/2014.10.JNS14290. [DOI] [PubMed] [Google Scholar]
- 75.Rass V, Gaasch M, Kofler M, et al. Fluid intake but not fluid balance is associated with poor outcome in nontraumatic subarachnoid hemorrhage patients. Crit Care Med. 2019;47:e555–e562. doi: 10.1097/CCM.0000000000003775. [DOI] [PubMed] [Google Scholar]
- 76.Sokolowski JD, Chen CJ, Ding D, et al. Endovascular treatment for cerebral vasospasm following aneurysmal subarachnoid hemorrhage: predictors of outcome and retreatment. J Neurointerv Surg. 2018;10:367–374. doi: 10.1136/neurintsurg-2017-013363. [DOI] [PubMed] [Google Scholar]
- 77.Szmuda T, Słoniewski P, Waszak PM, Kindrachuk M, Olijewski W. Short- and long-term outcome of surgically treated ruptured internal carotid artery aneurysms. Acta Neuropsychologica. 2013;11:403–417. doi: 10.5604/17307503.1090469. [DOI] [Google Scholar]
- 78.Voellger B, Rupa R, Arndt C, Carl B, Nimsky C. Outcome after interdisciplinary treatment for aneurysmal subarachnoid hemorrhage—a single center experience. Medicina (Kaunas) 2019 doi: 10.3390/medicina55110724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wong GK, Lam S, Ngai K, et al. Evaluation of cognitive impairment by the Montreal cognitive assessment in patients with aneurysmal subarachnoid haemorrhage: prevalence, risk factors and correlations with 3 month outcomes. J Neurol Neurosurg Psychiatry. 2012;83:1112–1117. doi: 10.1136/jnnp-2012-302217. [DOI] [PubMed] [Google Scholar]
- 80.Stienen MN, Germans MR, Zindel-Geisseler O, et al. Longitudinal neuropsychological assessment after aneurysmal subarachnoid hemorrhage and its relationship with delayed cerebral ischemia: a prospective Swiss multicenter study. J Neurosurg. 2022;137(6):1742-50. 10.3171/2022.2.jns212595. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.