Background:
The aim of this study was to evaluate whether the Nerbridge, an artificial polyglycolic acid conduit with collagen matrix, is comparable to direct nerve suture in a rat sciatic nerve injury model in a short-gap interposition (SGI) setting.
Methods:
Sixty-six female Lewis rats were randomly divided into the sham group (n = 13); no reconstruction (no-recon) group (n = 13; rat model with 10 mm sciatic nerve defect); direct group (n = 20; rat sciatic nerve injury directly connected by 10-0 Nylon); and SGI group (n = 20; sciatic nerve injury repaired using 5-mm Nerbridge). Motor function and histological recovery were evaluated. The sciatic nerve and gastrocnemius muscle were harvested for quantification of the degree of nerve regeneration and muscle atrophy.
Results:
The SGI and direct groups achieved equal recovery in both functional and histological outcomes. At weeks 3 and 8 postsurgery, there was a significant improvement in the sciatic functional index of the SGI group when compared with that of the no-recon group (P < 0.05). Furthermore, the direct and SGI groups had less muscle atrophy at 4 and 8 weeks postsurgery compared with the no-recon group (P < 0.05). The axon density and diameter at the distal site in the SGI group were significantly higher than that in the no-recon group and comparable to that in the direct and sham groups.
Conclusion:
An artificial nerve conduit has equal potential as direct suture in motor nerve reconstruction when used in the SGI setting.
Takeaways
Question: Is the Nerbridge, an artificial PGA/collagen-based bilayer conduit, comparable to direct nerve suture in a short-gap interposition setting?
Findings: The short-gap interposition group showed comparable results to the direct suture group and superior results to negative control in terms of both functional and histological assessment up to 8 weeks postoperatively in a rat sciatic nerve repair model.
Meaning: Artificial polyglycolic acid/collagen-based bilayer conduit in a short-gap interposition setting provides regenerative potential comparable to that of direct suture.
INTRODUCTION
Peripheral nerve injury (PNI) is a common pathological condition that often results from traumatic or sports injuries, traffic accidents, and natural disasters.1 After PNI, a series of cellular and molecular events called Wallerian degeneration occurs, and axon regeneration is enhanced.2,3 However, it is difficult to achieve complete function and structure recovery after nerve injury due to the lack of an appropriate microenvironment to support the adhesion and migration of Schwan cells.4 In the clinical setting, nerve defects longer than 4 mm are not suitable for direct suturing because of detrimental tension along the nerve and retardation of healing.5 Currently, autologous nerve transplantation is regarded as the gold standard to repair such nerve injuries for bridging the injured site; however, donor-site morbidity is inevitable. Under these circumstances, an artificial nerve conduit (ANC) is an alternative option to achieve better outcomes.
Many natural and synthetic ANCs have been developed to facilitate axonal guidance and provide a similar microenvironment to enhance nerve regeneration.6,7 With an ever-growing understanding of the complex interaction between cells and their microenvironment in tissues, the focus is being shifted on preserving the microenvironment capability of scaffolds. The key role in this is that of the extracellular matrix (ECM), which controls cell migration, proliferation, and adhesion. Collagen is a vital component of the endoneurium environment. Several clinical and preclinical studies confirmed that collagen scaffolds not only promote axon regeneration but also prevent fibrotic tissue infiltration.8–10 On the other hand, polyglycolic acid (PGA) has played an important role in the surgical field as a suture material because of its biodegradability and high biocompatibility. Nerbridge, an artificial PGA conduit filled with a collagen matrix, which was approved by the U.S. Food and Drug Administration in 2016, could have the potential to be a helpful tool for nerve reconstruction.
Several reports have stated that the interposition of ANC is comparable to direct suturing. Boeckstyns et al11 conducted a multicenter randomized control study (RCT) using a collagen tube and showed comparable data between the ANC and direct suturing in a 2-cm gap, in terms of sensory recovery and muscle function over 2 years. Weber et al12 also conducted a multicenter RCT and showed favorable outcomes for a PGA-based ANC in a 4-mm gap and comparable outcomes in a 5- to 7-mm gap, compared with direct suturing in terms of sensory recovery. These clinical studies were conducted using single-layer ANC, and the clinical or experimental results of bi-layer ANC, such as Nerbridge, are still unclear. Moreover, it is still controversial whether the interposition of the ANC, instead of direct suturing in nerve repair, is feasible in terms of nerve regeneration in the motor nerve. It is generally considered that when interposing the ANC between the nerve gap, nerve axon growth through the conduit may take time and cause problems with regeneration. To answer these questions, our study aimed to evaluate whether an ANC is comparable to direct nerve suture in a short-gap (<5 mm) setting in motor nerve repair.
MATERIAL AND METHODS
Animals
Female Lewis rats (n = 66; age: 8 weeks; weight: 250 g) were purchased from Japan SLC. The animal experiments were performed following the Guide for Animal Experimentation of Nagoya University School of Medicine and Aichi Cancer Center, and were approved by the Institutional Animal Care and Use Committee of both institutions. The animals had free access to standard food and water and were housed in an animal care facility maintained on a 12-hour light/dark cycle at 24°C.
Surgical Procedure
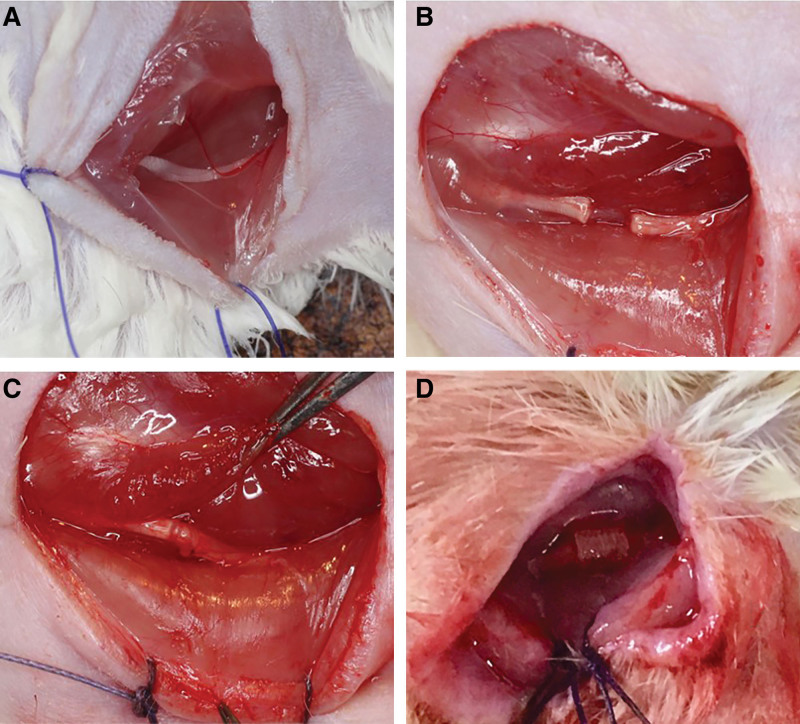
The animals were anesthetized with isoflurane gas (3% induction; 2% maintenance), and the hair on the right femur was removed before surgery. A 2-cm incision was made in the right thigh. After separating the dorsolateral gluteal muscles, the sciatic nerve was carefully isolated and exposed. The rats were divided randomly into the following four groups (Fig. 1): (1) The sham group served as a positive control, in which the sciatic nerve was exposed without interference (n = 13); (2) the no reconstruction (no-recon) group served as the negative control in which a 10-mm nerve gap was made (n = 13); (3) the direct group (n = 20) and (4) the SGI group (n = 20) served as the experimental groups in which the sciatic nerve injury was repaired with 10-0 Nylon sutures directly (direct group) or with 5-mm Nerbridge (SGI group) under a microscope. Following the operation, the skin was closed using 4-0 Nylon sutures. In the SGI group, proximal and distal nerve stumps were pulled into the conduit for 1 mm, resulting in a 3-mm nerve gap between the stumps. In each group, the animals were divided randomly to evaluate functional recovery (sham: n = 4; no-recon: n = 4; direct: n = 10; SGI: n = 10), histology, and muscle atrophy (sham: n = 9; no-recon: n = 9; direct: n = 10; SGI: n = 10).
Fig. 1.
Description of the surgery. A, Sham group: the sciatic nerve was exposed without any interference. B, No-recon group: a 10-mm nerve segment was made by micro scissors without reconstruction. C, Direct group: the sciatic nerve was transected and repaired with 10-0 nylon directly. D, SGI group: the sciatic nerve was transected and bridged using 0.5 mm Nerbridge. Arrows indicate the reconstruction site in the experimental group (C and D). No-recon group: No reconstruction group; SGI group: short-gap interposition group.
Functional Recovery Assessment
The functional gait of the rats was assessed using CatWalk XT (Noldus Information Technology, Wageningen, the Netherlands), which quantifies diverse static and dynamic rat gait parameters. The rat gait was recorded at 1, 2, 3, 4, and 8 weeks postsurgery. Three completed runs per trial were recorded for each rat. The sciatic functional index (SFI) was calculated according to a method reported by Brown et al.13
Nerve Histological Studies
The sciatic nerves of the four groups were harvested at 8 weeks postsurgery and fixed in 10% formalin. The longitudinal specimens were cut into 5-μm paraffin sections and stained using hematoxylin and eosin (H&E); immunohistochemical staining for the S100 protein was also performed. Next, we grossly observed the degree of nerve regeneration, vascularization, and neuroma formation using H&E and IHC staining. For transection, especially in the experimental group, the proximal site (PS) and the distal site (DS) were stained with H&E, 0.1% toluidine blue, and Masson trichrome. The axon density and diameter of the four groups were measured for all the specimens stained with toluidine blue using an image analysis software (Image J, Bethesda, Md.). We randomly selected 20 areas per specimen and counted the number of axons under high magnification (40×). The mean diameter of the axon was calculated using the formula √ (a × b), where “a” is the longest diameter of the axon and “b” is the shortest diameter passing perpendicularly. Approximately 100 axons were measured per sample. In addition, the percentage area of collagen deposition in the direct and SGI groups was calculated for all the specimens stained with Masson trichrome using the same software.
Gastrocnemius Muscle Atrophy Evaluation
Rats were euthanized by inhalation using isoflurane overdose (>4%) at 2, 4, and 8 weeks postsurgery. The healthy and injured gastrocnemius muscles (GMs) were harvested and weighed. Next, the muscles were fixed in 10% formalin, and the specimens were embedded in paraffin and sectioned into 4-μm slices, which were subjected to Masson trichrome staining. The average area (S) of the muscle fibers was measured using ImageJ software, and the mean diameter of the muscle fibers was calculated using the formula: 2√(S/π).14 The result was expressed as the ratio of the injured side to the healthy side of the GM both in mass (GM mass ratio) and diameter (GM diameter ratio).
Statistical Analysis
All data are expressed as mean ± SD. The t test, one-way analysis of variance, and posthoc Tukey test were used for analysis. GraphPad Prism 6 (San Diego, Ca.) software was used for the statistical analyses and plot preparation. Statistical significance was set at a P value less than 0.05.
RESULTS
Functional Recovery Assessment
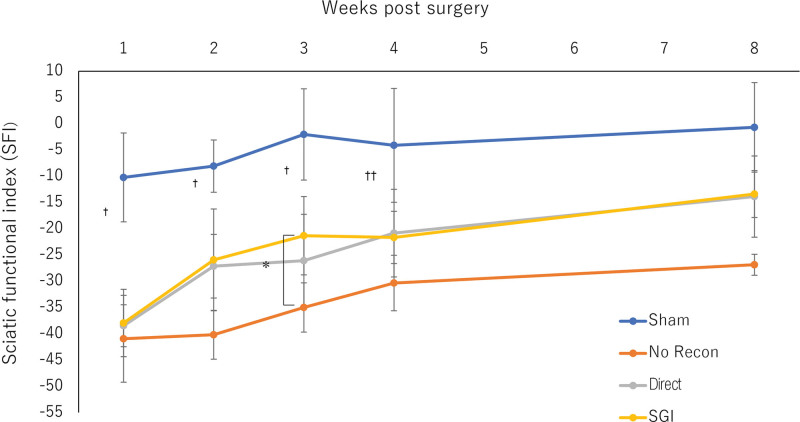
Generally, none of the rats showed infections or signs of autotomy throughout the experiment. All animals showed progressive improvement in mobility after surgery, indicating good recovery (Fig. 2). Motor recovery indicates regeneration of the nerve structure, which was assessed through rat gait analysis. However, at weeks 1, 2, and 3 postsurgery, there was a statistically significant difference in the SFI between the sham and other groups (P < 0.05). At week 4, the SGI and no-recon groups still showed significant differences in the SFI compared with the sham group (P < 0.05). However, at week 3 postsurgery, the SGI group showed enhanced recovery of nerve function compared with that in the no-recon group (P < 0.05). Furthermore, the no-recon group still differed significantly from all other groups at week 8 (P < 0.05), but there was no difference among the sham, direct, and SGI groups.
Fig. 2.
Functional recovery assessment. SFI of the four groups over an 8-week observation period. During postoperative weeks 1, 2, and 3, there is a statistical difference in the SFI between the sham and all other groups (†P < 0.05: Sham vs all other groups). At week 4, a significant difference in the SFI was still observed between the SGI, no-recon group, and sham groups (††P < 0.05: Sham vs SGI vs no-recon groups). At weeks 3 and 8, a significant accelerated improvement of the SFI was observed in the SGI group when compared with the no-recon group (*P < 0.05: SGI vs no-recon groups; #P < 0.05: All others vs no-recon group). No-recon group: No reconstruction group; SGI group: short-gap interposition group.
Nerve Histology and Histomorphometry
Gross Observation
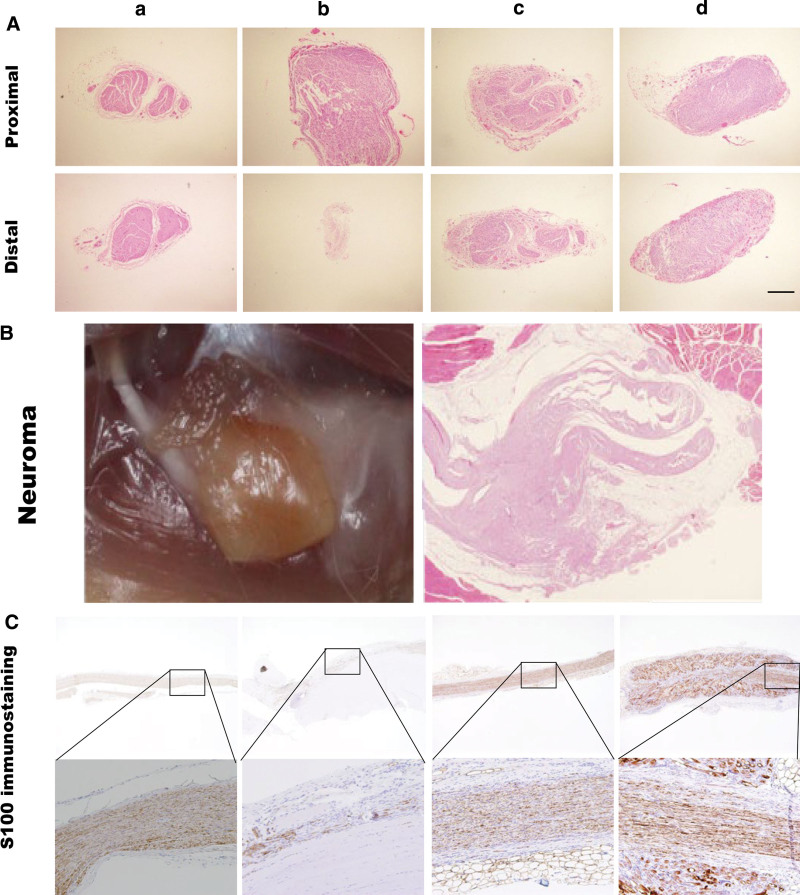
H&E and S100 immunohistochemical staining were used to assess the histology of the regenerated nerve. The degree of nerve regeneration in all four groups was observed in the transverse sections of the PS and DS through H&E staining. At the DS, the sham, direct, and SGI groups showed a more organized nerve structure and regular axon arrangement compared with that in the no-recon group. An ordered structure, including the perineurium and endoneurium, was observed in these three groups (Fig. 3A). Although there was no obvious difference between the SGI and direct groups in nerve regeneration, one of the specimens in the latter group showed neuroma formation (Fig. 3B). Longitudinal histology of nerve regeneration for S100 immunohistochemical analysis showed that nerve fibers in the SGI and direct groups were nearly completely regenerated. Compared with the no-recon group, nerve fibers in the these groups were arranged in an organized pattern along the long axis (Fig. 3C).
Fig. 3.
A, Histology of the regenerated nerve by H&E staining and S100 immunohistochemical staining at week 8 postsurgery. a, Sham group. b, No-recon group. c, Direct group. d, SGI group. H&E staining of transverse section of the proximal and DSs. Bar = 500 μm. B, Neuroma: image and H&E staining of the neuroma formed at the DS in the direct group. Bar = 1000 μm. C, S100 immunohistochemical staining: longitudinal section of the nerve. Images at higher magnification (10×) are the areas highlighted by squares. Bar = 1000 μm. No-recon group: No reconstruction group; SGI group: short-gap interposition group; H&E: hematoxylin and eosin.
Morphometric Analysis
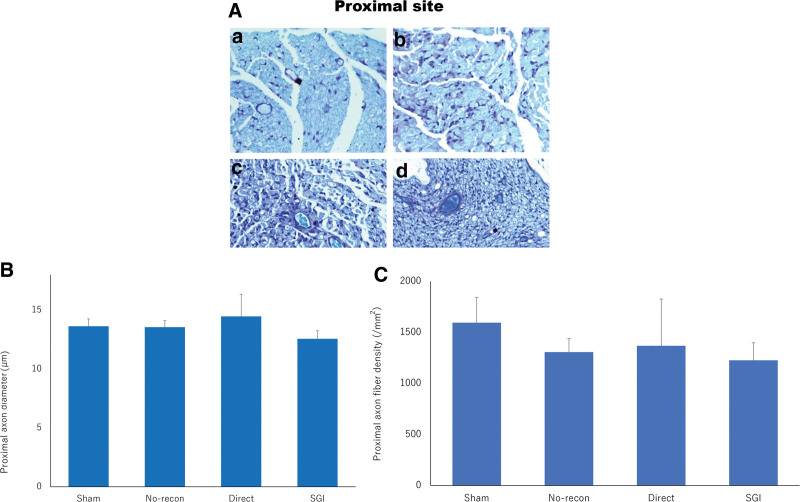
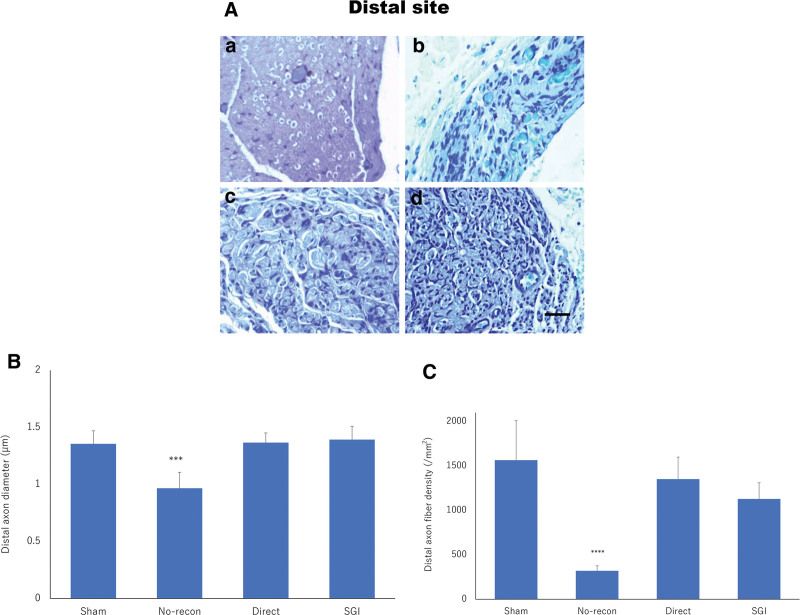
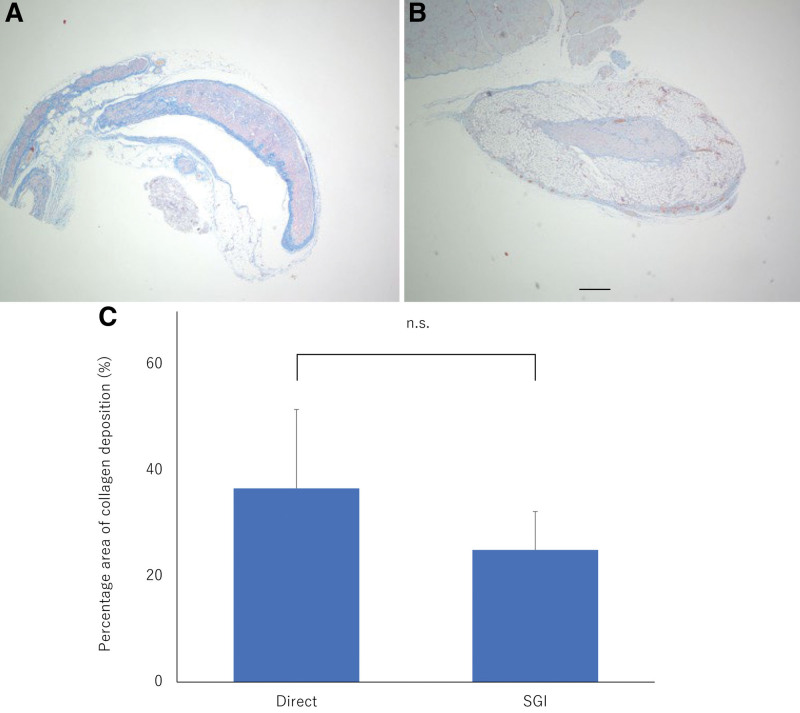
Nerve histomorphometry demonstrated that myelinated axons were arranged in clusters surrounded by thin connective tissue. In the PS, no obvious morphological difference was observed between the toluidine blue-stained specimens of the sham, direct, SGI, and no-recon groups supported by qualitative analysis of the axon diameter and density (Fig. 4). At the DS, animals treated with direct suture and Nerbridge showed that regenerated nerves were well-arranged and had a high degree of myelination, similar to what was seen in the sham group. A significant number of regenerated axons could be identified in the direct and SGI groups (Fig. 5A). Meanwhile, the results of axon diameter (P < 0.001; Fig. 5B) and axon density (P < 0.0001; Fig. 5C) were significantly different between the SGI, direct, sham, and no-recon groups, but no difference was observed among the SGI, direct, and sham groups. In addition, at week 8 postsurgery, Masson trichrome-stained nerve transected sections demonstrated a larger area of collagen deposition in the direct group than that in the SGI group; however, no significant difference was detected between the SGI and direct groups (Fig. 6).
Fig. 4.
Toluidine blue staining and quantitative analysis of the PS. a, Sham group. b, No-recon group. c, Direct group. d, SGI group. A, Toluidine blue staining of the PS for all four groups at 8 weeks postsurgery. Bar = 50 μm. B-C, The quantification shows similar axon diameter (B) and axon density (C) among all four groups. Graphs represent the measurement in the sham (n = 2), no-recon (n = 3), direct (n = 6), and SGI (n = 7) groups. No-recon group: No reconstruction group; SGI group: short-gap interposition group.
Fig. 5.
Toluidine blue staining and quantitative analysis of the DS. a, Sham group. b, No-recon group. c, Direct group. d, SGI group. A, Toluidine blue staining of the DS for all four groups at 8 weeks postsurgery. Bar = 50 μm. The quantification shows a significant difference between the no-recon and SGI groups both in axon diameter B, and axon density C, at the DS. Graphs represent the measurement in the sham (n = 2), no-recon (n = 3), direct (n = 6), and SGI (n = 7) groups. ***P < 0.001, ****P < 0.0001. No-recon group: No reconstruction group; SGI group: short-gap interposition group.
Fig. 6.
Masson trichrome-stained nerve transected sections and percentage area of collagen deposition. A-B, Masson trichrome staining at the DS for the direct (A) and SGI group (B) at 8 weeks postsurgery. C, Difference in the percentage area of collagen deposition between the two groups. The quantification shows no significant difference between the direct group and SGI group in the percentage area of collagen deposition. The graph represents the measurement in the direct (n = 7) and SGI (n = 7) groups. SGI group, short-gap interposition group; ns, no statistical difference.
GM Atrophy Analysis
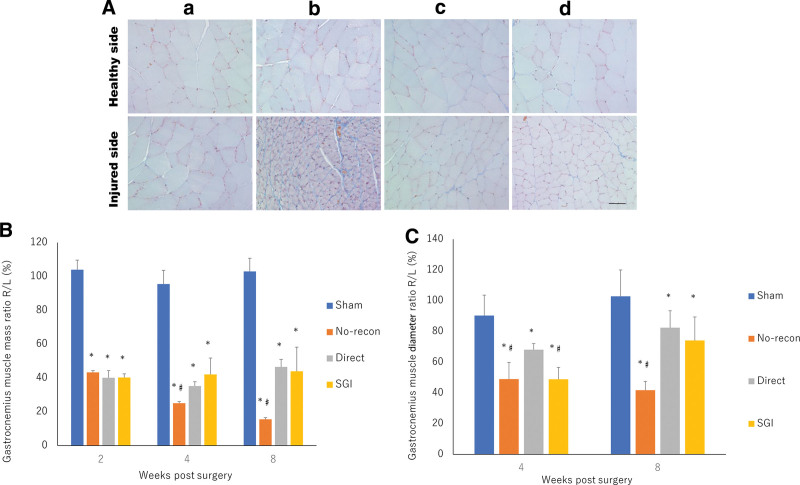
Muscle atrophy was assessed using the GM mass ratio and GM diameter ratio. Microscopic images of both the healthy and tested sides of the GM muscle at week 8 postsurgery are shown in Fig. 7A. GM mass ratio assessment revealed the occurrence of denervation-related muscle atrophy in the no-recon, direct, and SGI groups from week 2 postsurgery (P < 0.05). However, weight loss was prevented in the SGI and direct groups compared with that in the no-recon group at weeks 4 and 8 postsurgery (P < 0.05), and the SGI group did not differ from the direct group in the GM mass ratio (Fig. 7B). The GM diameter ratio indicated that the direct group did significantly differ from the SGI and no-recon groups at week 4 postsurgery (P < 0.05), which is in conflict with the GM mass ratio analysis. However, this contradiction demonstrated the prevention of muscle fiber loss during atrophy progression in the SGI group. Moreover, at week 8 postsurgery, there was no significant difference between the SGI and direct groups, which was confirmed with GM mass ratio analysis, and the GM diameter ratio in the no-recon group was significantly lower than that in all other groups at week 8 postsurgery. (Fig. 7C).
Fig. 7.
Masson trichrome-stained GM and quantitative analysis of GM atrophy degree. a, Sham group. b, No-recon group. c, Direct group. d, SGI group. A, Masson trichrome staining of the GM of the healthy and injured sides at 8 weeks postsurgery. Bar = 50 μm. B, Assessment of the GM mass ratio at 2, 4, and 8 weeks postsurgery. *P < 0.05, vs sham group; #P < 0.05, vs direct group. C, Evaluation of the GM diameter ratio at 4 and 8 weeks postsurgery. *P < 0.05, vs sham group; #P < 0.05, vs direct group. No-recon group: No reconstruction group; SGI group: short-gap interposition group.
DISCUSSION
In the present study, we demonstrated for the first time that a PGA/collagen-based bilayer nerve conduit (Nerbridge) promoted nerve regeneration in a rat sciatic nerve injury model. Our results indicated that the Nerbridge implantation and direct suture are equally effective in enhancing motor functional recovery and leading to nerve regeneration and can significantly prevent GM atrophy. Furthermore, under gross observation, the group treated with a nerve conduit showed a lower degree of fibrosis compared with that in the direct group. Several mechanisms may explain why nerve regeneration facilitated by PGA/collagen-based bilayer nerve conduit is equal to that induced by direct suture. The advantages of this bilayer nerve conduit are mentioned hereinafter.
In recent decades, natural materials have been frequently used for tissue engineering of nerves because they are biocompatible, favor the migration of SCs, and prevent the occurrence of toxic effects.15 The outer layer of the bilayer design, the PGA, is a member of the linear aliphatic polyester family that has been widely used as a synthetic polymer for nerve implantation because of its biodegradation and biocompatibility characteristics in the past decades.12,16 Neither the polymer nor its degradation products are toxic in vivo.17 On the other hand, PGA is likely to provide better suture and mechanical integrity.
Collagen is a major class of insoluble fibers in the ECM and is responsible for framework modeling of connective tissues. In nerve repair, collagen can initiate several cellular processes, including induction of transcription of various genes in nerve cells.18 Besides, some studies have suggested the strong chemotropism of growing neurites and axons in the presence of collagen.19 Thus, collagen-based nerve conduits have been used in many preclinical studies for peripheral nerve regeneration.10,20–22 According to those reports, limited histological recovery and signs of inflammation and neuroma formation occurred, which is consistent with our results. As for clinical work, recently, Atsuhiko Iwao et al reported three cases of obturator nerve injury repaired by collagen-based ANC.23 In addition, two cases using a 20 mm or 25 mm length of the PGA-collagen tube for the repair of facial nerve defect reported the enhancement of frontal muscle movement.24 Those reports suggest the potential benefits of a collagen-based ANC in the treatment of PNIs patients. In contrast to Nerbridge, direct suture or autologous nerve grafts are secured with stitches, resulting in the formation of a connective tissue capsule on the graft surface, isolating the graft from the normal tissue. Furthermore, the collagen scaffold, as a biomaterial, prevents fibrous tissue infiltration and provides a suitable microenvironment for the growth of severed axons and supporting SCs. Additionally, our S100 immunohistochemical staining showed that almost all regenerated axons crossed the DS to reenter the distal nerve stump. Nerve quantification results confirmed similar functional recovery of collagen scaffold with direct suture. Moreover, collagen scaffolds have been confirmed to enhance angiogenesis by interacting with endothelial cells and by promoting the secretion of vascular endothelial growth factor in PNI.25,26
It is known that denervation of the target muscle causes damage to motor nerves, followed by alterations in various structures including the neuromuscular junction and cell ultrastructure. Thus, a decrease in the muscle fiber size and number leads to muscle atrophy. Once the muscle is re-innervated, its function is restored and atrophy is stopped. As the sciatic nerve majorly innervates the GM, the GM was taken as representative of the target muscle of the sciatic nerve in this study at week 8 postsurgery, and the GM mass and diameter ratios showed no significant difference between the SGI and direct groups. These findings are consistent with the functional recovery assessment noted. Interestingly, at week 4 postsurgery, the GM mass ratio and GM diameter ratio showed conflicting tendencies, suggesting a decrease in the muscle fiber size in the no-recon group compared with that in the SGI group. Therefore, although the muscle diameter of the SGI group did not show an obvious recovery compared with that in the direct group at week 8 postsurgery, the contradiction indicates the prevention of muscle fiber loss, which also confirms the effects of the PGA/collagen bilayer nerve conduit on nerve regeneration and re-innervation of the target muscle.
In the current study, we successfully demonstrated in the experimental level that the use of ANC is comparable to direct suture for gaps less than 5 mm. We believe that this finding can be used to expand the clinical indications of ANC and that its use can be effective in the following clinical situations: (1) direct suture for the short gap of the nerve is possible but results in tension to some degree; (2) diameters between the proximal stump and distal stump are different, which causes difficulty in performing direct suture; and (3) the adventitia of the nerves are injured when implementing direct suture. Besides, tension caused by direct suture would promote fibrous infiltration and neuroma formation27; accordingly, extra surgery might be necessary for excising neuromas, which reduces the efficiency of direct suture. Given the limitations of direct suture in the above conditions, despite the cost of ANC being high, it would be a beneficial alternative due to aiding nerve suturing. However, with respect to any future wide clinical implementation, it would be ideal to conduct cost analysis including different types of artificial nerves. On the other hand, using ANC in our study has another limitation when it comes to clinical cases of long-distance gap (>30 mm), in which autologous nerve grafting should be performed.28 Clinical studies, including RCTs,11,12,29 have reported that ANC is generally equivalent to autologous nerve transplantation for gaps of 3 cm or less in sensory and motor nerves. However, there have been only a few reports on gaps larger than 3 cm, possibly due to poor results.30 This is thought to be due to the lack of Schwann cells and nerve growth factors in ANC that promote the growth of the nerve axon when the gap is long. Hence, attempts to introduce Schwann cells and growth factors into ANC have been studied.31,32 However, it would be better to investigate larger nerve gaps in a rat model in the future.
As regards the timing of evaluation, some researchers use 12-weeks postsurgery as the time point for the end of the repair in the rat sciatic nerve injury model.9 Although we found functional and histological differences between the non-recon group and the experimental group (direct group and SGI group) at the 8-week time point, a longer study period may provide new insights into nerve recovery as well as muscle recovery. Furthermore, in this study, we compared nerve repair between the ANC and direct suturing. Future studies can be performed by comparing the nerve repair achieved through the ANC with that of autologous nerve grafting.
CONCLUSIONS
Taken together, the results of the present study demonstrated that, in the repair of peripheral nerve defects, the collagen-based bilayer conduit induces functional axon regeneration to a degree similar to that obtained by the direct suture when used in a short-gap setting. Additionally, Nerbridge could prevent fibrosis infiltration in normal tissue and promote vascularization. These findings may provide an efficient treatment approach for nerve injuries.
ACKNOWLEDGMENTS
The authors would like to thank Prof. Hitoshi Hirata for allowing us to use the CatWalk XT. The authors would also like to thank the Biopathology Institute Co., Ltd. for histological and immunohistochemical staining. Y. Li would like to thank the Otsuka Toshimi Scholarship Foundation for financial support (21-78). The ANC used in this study (Nerbridge) was provided by Toyobo Co., Ltd, Japan.
Footnotes
Published online 27 March 2023.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
REFERENCES
- 1.Li R, Liu Z, Pan Y, et al. Peripheral nerve injuries treatment: a systematic review. Cell Biochem Biophys. 2014;68:449–454. [DOI] [PubMed] [Google Scholar]
- 2.Jiang L, Jones S, Jia X. Stem cell transplantation for peripheral nerve regeneration: current options and opportunities. Int J Mol Sci. 2017;18:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coleman MP, Conforti L, Buckmaster EA, et al. An 85-kb tandem triplication in the slow Wallerian degeneration (Wlds) mouse. Proc Natl Acad Sci U S A. 1998;95:9985–9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pinho AC, Fonseca AC, Serra AC, et al. Peripheral nerve regeneration: current status and new strategies using polymeric materials. Adv Healthc Mater. 2016;5:2732–2744. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt CE, Leach JB. Neural tissue engineering: strategies for repair and regeneration. Annu Rev Biomed Eng. 2003;5:293–347. [DOI] [PubMed] [Google Scholar]
- 6.Wieringa PA, Gonçalves de Pinho AR, Micera S, et al. Biomimetic architectures for peripheral nerve repair: a review of biofabrication strategies. Adv Healthc Mater. 2018;7:e1701164. [DOI] [PubMed] [Google Scholar]
- 7.Daly WT, Knight AM, Wang H, et al. Comparison and characterization of multiple biomaterial conduits for peripheral nerve repair. Biomaterials. 2013;34:8630–8639. [DOI] [PubMed] [Google Scholar]
- 8.Yu W, Zhao W, Zhu C, et al. Sciatic nerve regeneration in rats by a promising electrospun collagen/poly(ε-caprolactone) nerve conduit with tailored degradation rate. BMC Neurosci. 2011;12:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bozkurt A, Boecker A, Tank J, et al. Efficient bridging of 20 mm rat sciatic nerve lesions with a longitudinally micro-structured collagen scaffold. Biomaterials. 2016;75:112–122. [DOI] [PubMed] [Google Scholar]
- 10.Klein S, Vykoukal J, Felthaus O, et al. Collagen type I conduits for the regeneration of nerve defects. Materials (Basel). 2016;9:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boeckstyns ME, Sørensen AI, Viñeta JF, et al. Collagen conduit versus microsurgical neurorrhaphy: 2-year follow-up of a prospective, blinded clinical and electrophysiological multicenter randomized, controlled trial. J Hand Surg Am. 2013;38:2405–2411. [DOI] [PubMed] [Google Scholar]
- 12.Weber RA, Breidenbach WC, Brown RE, et al. A randomized prospective study of polyglycolic acid conduits for digital nerve reconstruction in humans. Plast Reconstr Surg. 2000;106:1036–45; discussion 1046. . [DOI] [PubMed] [Google Scholar]
- 13.Brown CJ, Mackinnon SE, Evans PJ, et al. Self-evaluation of walking-track measurement using a sciatic function index. Microsurgery. 1989;10:226–235. [DOI] [PubMed] [Google Scholar]
- 14.Mula J, Lee JD, Liu F, et al. Automated image analysis of skeletal muscle fiber cross-sectional area. J Appl Physiol (1985). 2013;114:148–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fornasari BE, Carta G, Gambarotta G, et al. Natural-Based biomaterials for peripheral nerve injury repair. Front Bioeng Biotechnol. 2020;8:554257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsumoto K, Ohnishi K, Kiyotani T, et al. Peripheral nerve regeneration across an 80-mm gap bridged by a polyglycolic acid (PGA)-collagen tube filled with laminin-coated collagen fibers: a histological and electrophysiological evaluation of regenerated nerves. Brain Res. 2000;868:315–328. [DOI] [PubMed] [Google Scholar]
- 17.Wang X, Hu W, Cao Y, et al. Dog sciatic nerve regeneration across a 30-mm defect bridged by a chitosan/PGA artificial nerve graft. Brain. 2005;128:1897–1910. [DOI] [PubMed] [Google Scholar]
- 18.Jones JM, Cohen RL, Chambers DA. Collagen modulates gene activation of plasminogen activator system molecules. Exp Cell Res. 2002;280:244–254. [DOI] [PubMed] [Google Scholar]
- 19.Guthrie S, Lumsden A. Neuroprotocoles: A Companion Method in Neurosciences. San Diego: Academic Press; 1994. [Google Scholar]
- 20.Bozkurt A, Lassner F, O’Dey D, et al. The role of microstructured and interconnected pore channels in a collagen-based nerve guide on axonal regeneration in peripheral nerves. Biomaterials. 2012;33:1363–1375. [DOI] [PubMed] [Google Scholar]
- 21.Zhuang H, Bu S, Hua L, et al. Gelatin-methacrylamide gel loaded with microspheres to deliver GDNF in bilayer collagen conduit promoting sciatic nerve growth. Int J Nanomedicine. 2016;11:1383–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suzuki H, Araki K, Matsui T, et al. Value of a novel PGA-collagen tube on recurrent laryngeal nerve regeneration in a rat model. Laryngoscope. 2016;126:E233–E239. [DOI] [PubMed] [Google Scholar]
- 23.Iwao A, Yagi M, Imamura Y, et al. Intraoperative obturator nerve injury reconstructed using a PGA-collagen tube: three case reports. Gynecologic Oncology Reports. 2022;41:100977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakamura Y, Takanari K, Ebisawa K, et al. Repair of temporal branch of the facial nerve with novel polyglycolic acid-collagen tube: a case report of two cases. Nagoya J Med Sci. 2020;82:123–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perng CK, Wang YJ, Tsi CH, et al. In vivo angiogenesis effect of porous collagen scaffold with hyaluronic acid oligosaccharides. J Surg Res. 2011;168:9–15. [DOI] [PubMed] [Google Scholar]
- 26.Li G, Han Q, Lu P, et al. Construction of dual-biofunctionalized chitosan/collagen scaffolds for simultaneous neovascularization and nerve regeneration. Research (Wash D C). 2020;2020:2603048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Terzis J, Faibisoff B, Williams B. The nerve gap: suture under tension vs. graft. Plast Reconstr Surg. 1975;56:166–170. [PubMed] [Google Scholar]
- 28.Lundborg G. Alternatives to autologous nerve grafts. Handchir Mikrochir Plast Chir. 2004;36:1–7. [DOI] [PubMed] [Google Scholar]
- 29.Dienstknecht T, Klein S, Vykoukal J, et al. Type I collagen nerve conduits for median nerve repairs in the forearm. J Hand Surg Am. 2013;38:1119–1124. [DOI] [PubMed] [Google Scholar]
- 30.Ichihara S, Inada Y, Nakamura T. Artificial nerve tubes and their application for repair of peripheral nerve injury: an update of current concepts. Injury. 2008;39:29–39. [DOI] [PubMed] [Google Scholar]
- 31.Pabari A, Yang SY, Mosahebi A, et al. Recent advances in artificial nerve conduit design: strategies for the delivery of luminal fillers. J Control Release. 2011;156:2–10. [DOI] [PubMed] [Google Scholar]
- 32.Daly W, Yao L, Zeugolis D, et al. A biomaterials approach to peripheral nerve regeneration: bridging the peripheral nerve gap and enhancing functional recovery. J R Soc Interface. 2012;9:202–221. [DOI] [PMC free article] [PubMed] [Google Scholar]