PURPOSE
Neoadjuvant chemotherapy (NAC) has proven survival benefits for patients with invasive urothelial carcinoma of the bladder, yet its role for upper tract urothelial carcinoma (UTUC) remains undefined. We conducted a multicenter, single-arm, phase II trial of NAC with gemcitabine and split-dose cisplatin (GC) for patients with high-risk UTUC before extirpative surgery to evaluate response, survival, and tolerability.
METHODS
Eligible patients with defined criteria for high-risk localized UTUC received four cycles of split-dose GC before surgical resection and lymph node dissection. The primary study end point was rate of pathologic response (defined as < ypT2N0). Secondary end points included progression-free survival (PFS), overall survival (OS), and safety and tolerability.
RESULTS
Among 57 patients evaluated, 36 (63%) demonstrated pathologic response (95% CI, 49 to 76). A complete pathologic response (ypT0N0) was noted in 11 patients (19%). Fifty-one patients (89%) tolerated at least three complete cycles of split-dose GC, 27 patients (47%) tolerated four complete cycles, and all patients proceeded to surgery. With a median follow up of 3.1 years, 2- and 5-year PFS rates were 89% (95% CI, 81 to 98) and 72% (95% CI, 59 to 87), while 2- and 5-year OS rates were 93% (95% CI, 86 to 100) and 79% (95% CI, 67 to 94), respectively. Pathologic complete and partial responses were associated with improved PFS and OS compared with nonresponders (≥ ypT2N any; 2-year PFS 100% and 95% v 76%, P < .001; 2-year OS 100% and 100% v 80%, P < .001).
CONCLUSION
NAC with split-dose GC for high-risk UTUC is a well-tolerated, effective therapy demonstrating evidence of pathologic response that is associated with favorable survival outcomes. Given that these survival outcomes are superior to historical series, these data support the use of NAC as a standard of care for high-risk UTUC, and split-dose GC is a viable option for NAC.
BACKGROUND
Upper tract urothelial carcinoma (UTUC) is a rare and highly lethal form of urothelial cancer, constituting 5%-10% of urothelial carcinomas.1 Disease prognosis is predominately driven by advanced stage and high-grade disease at presentation. Muscle-invasive disease (≥ pT2) is present in 60% of radical nephroureterectomy (RNU) cases, and the 5-year cancer-specific mortality rates for patients with pT2, pT3, and pT4 disease are 21%, 35%, and 59%, respectively. Additionally, high-grade disease, irrespective of stage, defines a high-risk population with poor prognosis following surgery alone.2
CONTEXT
Key Objective
High-risk upper tract urothelial carcinoma (UTUC) has a poor prognosis despite radical nephroureterectomy. While there is now strong evidence to support the usage of adjuvant chemotherapy to improve survival outcomes, only a minority of patients will be eligible for this treatment. There is a paucity of prospective data in the neoadjuvant setting, during which more patients may be able to tolerate cisplatin-based therapy. This phase II trial prospectively evaluated neoadjuvant gemcitabine and split-dose cisplatin (GC) followed by radical surgery for patients with high-risk UTUC.
Knowledge Generated
Neoadjuvant split-dose GC for high-risk UTUC is well tolerated, does not significantly delay surgery, and has substantial activity. Progression-free survival and overall survival with this therapy are superior to historical series without it.
Relevance
This study provides an alternative dose schedule of gemcitabine and split-dose cisplatin in the neoadjuvant setting for high-risk UTUC while providing favorable outcomes, potentially increasing the number of patients eligible for perioperative chemotherapy.
Because of the rarity of high-risk UTUC, its optimal management is frequently extrapolated from urothelial carcinoma of the bladder (UCB) literature, especially the role of perioperative chemotherapy. For patients with UCB, neoadjuvant cisplatin-based chemotherapy (NAC) before radical cystectomy and pelvic lymph node dissection (LND) improves long-term survival compared with surgery alone.3,4 Outcomes are comparable for patients with complete response and those with pathologic downstaging following systemic therapy, characterized by eradication of the muscle-invasive component. Therefore, pathologic downstaging (≤ ypT1N0) represents a frequent choice of efficacy end point in modern neoadjuvant trials in UCB.5
However, accurate staging of UTUC is particularly difficult, as ureteroscopic biopsy specimens are unable to reliably determine depth of invasion before extirpative surgery.6 For patients with locally advanced disease after RNU, there is now level 1 evidence to support adjuvant chemotherapy usage on the basis of the phase III POUT (Peri-Operative chemotherapy versus sUrveillance in upper Tract urothelial cancer) trial.7 Unfortunately, up to 85% of patients will have stage three or greater chronic kidney disease (CKD) after RNU, severely limiting the proportion of patients eligible for cisplatin-based therapy in this setting.8 Despite the strong rationale for NAC, there has been a paucity of prospective data to formally support its use in high-risk UTUC. The only reported trial to date is the Eastern Cooperative Oncology Group and American College of Radiology Imaging Network (ECOG-ACRIN) 8141 trial, which demonstrated pathologic complete response and downstaging rates of 14% and 62%, respectively, in 29 patients eligible for NAC with accelerated methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC), although the study was closed prematurely because of poor accrual in the gemcitabine and carboplatin arm for cisplatin-ineligible patients.9
This study prospectively evaluated the use of neoadjuvant gemcitabine and split-dose cisplatin (GC) for patients with high-risk UTUC. The chemotherapy regimen was derived from effective dosing parameters, with increased tolerability, previously established in patients with UCB with comparable oncologic risk.5,10 The primary outcome was pathologic response rate; secondary outcomes were treatment safety, cancer progression, and survival.
METHODS
Eligibility
This is a multicenter, single-arm, phase II trial of neoadjuvant split-dose GC in patients with localized high-grade UTUC (ClinicalTrials.gov identifier: NCT01261728). The four trial sites included Memorial Sloan Kettering (New York, NY), Hartford Hospital (Hartford, CT), Lehigh Valley Health Network (Allentown, PA), and Mayo Clinic—Arizona (Scottsdale, AZ). Primary inclusion criteria were high-risk patients defined as histologically confirmed diagnosis of high-grade UTUC (ie, by endoscopic or percutaneous biopsy) and/or radiographically visible invasive disease (cT2-T4a N0/X M0) with positive selective urinary cytology (high-grade cytology collected from ipsilateral kidney and/or ureter). Variant histology on biopsy was not excluded. Hydronephrosis associated with tumor on imaging was classified as invasive as conventionally defined. Carcinoma in situ was excluded except when accompanied by high-grade papillary disease. Patients had to be cisplatin-eligible (estimated glomerular filtration rate [eGFR] ≥ 55 mL/min per 1.73 m2, Karnofsky performance status [KPS] ≥ 70%, no pre-existing grade three peripheral neuropathy or hearing impairment, New York Heart Association class III or IV heart failure, or recent cardiovascular event) and medically appropriate surgical candidates per the treating surgeon. Concomitant non–muscle-invasive UCB was acceptable if treatable by transurethral resection.
This study was approved by the institutional review boards of participating sites and performed in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. All patients provided written informed consent before study entry.
Procedures
Patients were prescribed four cycles of split-dose GC: gemcitabine 1,000 mg/m2 and cisplatin 35 mg/m2 once daily on days 1 and 8 of a 21-day cycle. This modification of conventional GC,11 with removal of day 15 gemcitabine and incorporation of split-dose cisplatin, has been shown to improve tolerability and the toxicity profile,5,10 an important consideration in patients with high possibility of hydronephrosis and/or renal parenchymal involvement at presentation. Although the original trial of split-dose cisplatin was performed in a locally advanced and metastatic UCB population,10 there has since been further evidence demonstrating excellent pathologic outcomes with a favorable toxicity profile in the neoadjuvant setting.5
Surgical intervention, by open or minimally invasive approach per surgeon discretion, was recommended within 12 weeks of chemotherapy completion and included RNU for tumors involving the renal pelvis and/or proximal ureter, or distal ureterectomy in selected patients assessed to have only distal ureteral involvement. Per Protocol (online only), all patients underwent ipsilateral templated LND, which was performed as previously described.12
Patients underwent a scheduled examination and toxicity assessment using National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0) on a standardized protocol (Data Supplement, online only). Imaging was performed before initiation and after completion of chemotherapy to evaluate imaging response. Correlative assessments of radiographic features and genomic analyses were also performed (Data Supplement).
End Points and Statistical Analyses
The primary end point was presence or absence of pathologic response following chemotherapy—defined as < ypT2N0 at the surgical pathological specimen per the American Joint Committee on Cancer seventh edition criteria.13 This end point was determined on the basis of NAC trials in UCB,3,4,14 as no similar evidence in UTUC was available at the time of study design. Several studies have since been published that have demonstrated the utility of pathologic response as an oncologic end point in UTUC.15-18 Hereafter, we refer to patients with pathologic response following NAC as responders, and those with residual muscle-invasive and/or nodal disease (≥ ypT2N any) as nonresponders, as per convention in NAC trials in UCB. Patients with progressive disease before surgery, and any patients unable to undergo surgery or refusing surgery were considered nonresponders for the primary end point analysis.
In a retrospective review of the Memorial Sloan Kettering surgical database that included 250 patients with high-grade UTUC undergoing RNU without NAC, 106 (42%) had < pT2 disease on final pathology. These high-grade patients reflect the target population of this trial, and thus, the threshold for treatment efficacy has been set at 40% for < pT2 disease. Although this internal review was performed at the time of study conception, there have since been several other modern series published supporting the continued validity of this threshold.15,19-21 The study was a single-arm trial with a Simon's minimax two-stage design. The pathologic response proportion would be considered promising if > 60% (alternative hypothesis) and unacceptable if < 40% (null hypothesis), on the basis of post-NAC response rates in UCB3,22,23 and on observed rates of < pT2N0 disease among patients receiving upfront surgical management of high-risk UTUC as above. With type I and type II error rates at 0.05 and 0.10, respectively, the study had a 90% probability of rejecting the null hypothesis if the true percentage of responders was ≥ 60%, and a 5% probability of rejecting the null hypothesis if the true percentage of responders was < 40%. The first stage required > 12 responders out of 29 enrolled patients. At the end of the study, treatment would be deemed effective if 28 or more pathologic responses were identified among 54 treated patients.
The secondary end points included pathologic complete response rate, progression-free survival (PFS) and overall survival (OS), and safety and tolerability. Our original protocol included time to progression as a secondary end point, but to increase comparability of our results with the literature, PFS was also evaluated and reported hereafter. PFS and OS were estimated using the Kaplan-Meier method. PFS was measured from the date of surgery until the first date that systemic recurrence was objectively documented or death. Systemic recurrence was defined as either metastatic or local nonurothelial recurrence (ie, lymph node or soft tissue within the retroperitoneum or pelvis). Because of the multifocal nature of urothelial tumors, this definition excluded new or recurrent tumors of the urethra, bladder, ureters, or renal pelvis, which were defined as urothelial recurrence. Patients without documented recurrence were censored at last follow-up. The surgical complication rate was defined as the total number of complications divided by the number of patients.
RESULTS
Patient Characteristics
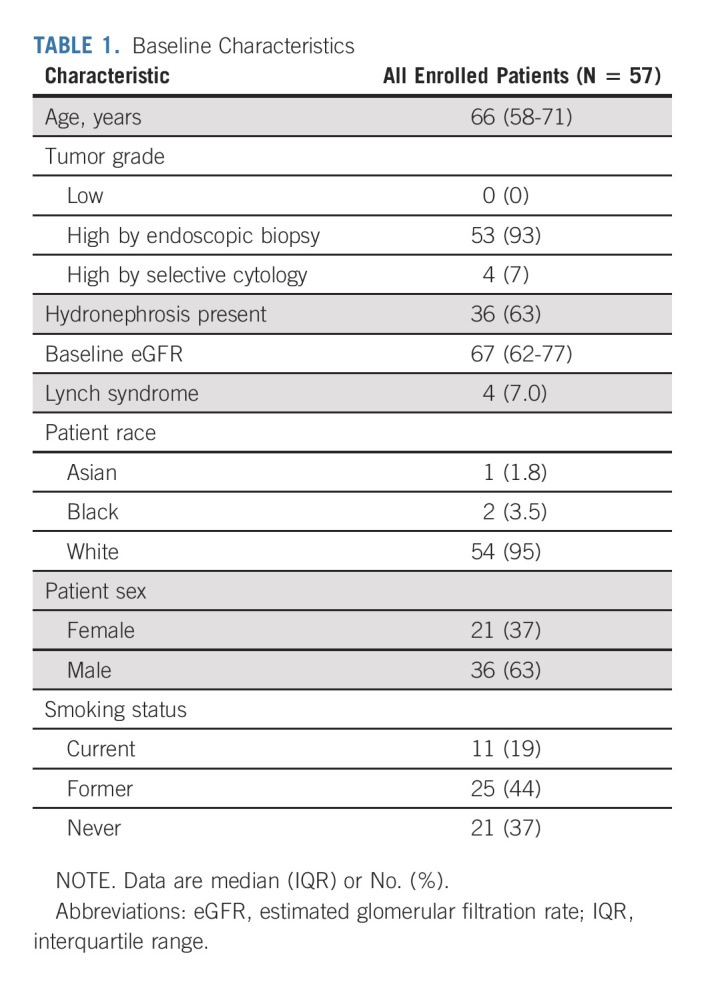
Between December 2010 and April 2019, 57 patients were enrolled and included for analysis (Table 1). All patients had radiographic cT2-4 disease on baseline imaging, 53 (93%) had high-grade histology on biopsy, and the four (7%) without a biopsy had high-grade cytology.
TABLE 1.
Baseline Characteristics
Fifty-one patients (89%) received at least three complete cycles of split-dose GC. Six (11%) received three cycles, 18 (31%) received three and a half cycles, and 27 (47%) received all four cycles. Only six patients (11%) received fewer than three complete cycles (two cycles: n = 1 [1.8%]; one cycle: n = 2 [3.5%]; and 0 cycles: n = 3 [5.3%]). Overall, 33 patients (58%) required toxicity-related dose modifications: dose delays in five (9%), dose reductions in 12 (21%), and early discontinuation in eight (14%). Toxicities that resulted in early discontinuation included thromboembolic events (n = 3 [5%]), renal dysfunction (n = 2 [4%]), and cardiovascular events (n = 1 [2%]).
Treatment-related toxicities (Data Supplement) were observed in 53 patients (93%). Grade ≥ 3 toxicities (Table 2) occurred in 42 patients (74%). Four patients (7%) had febrile neutropenia. There were no treatment-related deaths. All patients were able to proceed to surgery.
TABLE 2.
Grade 3 or Higher Chemotherapy-Related Toxicities
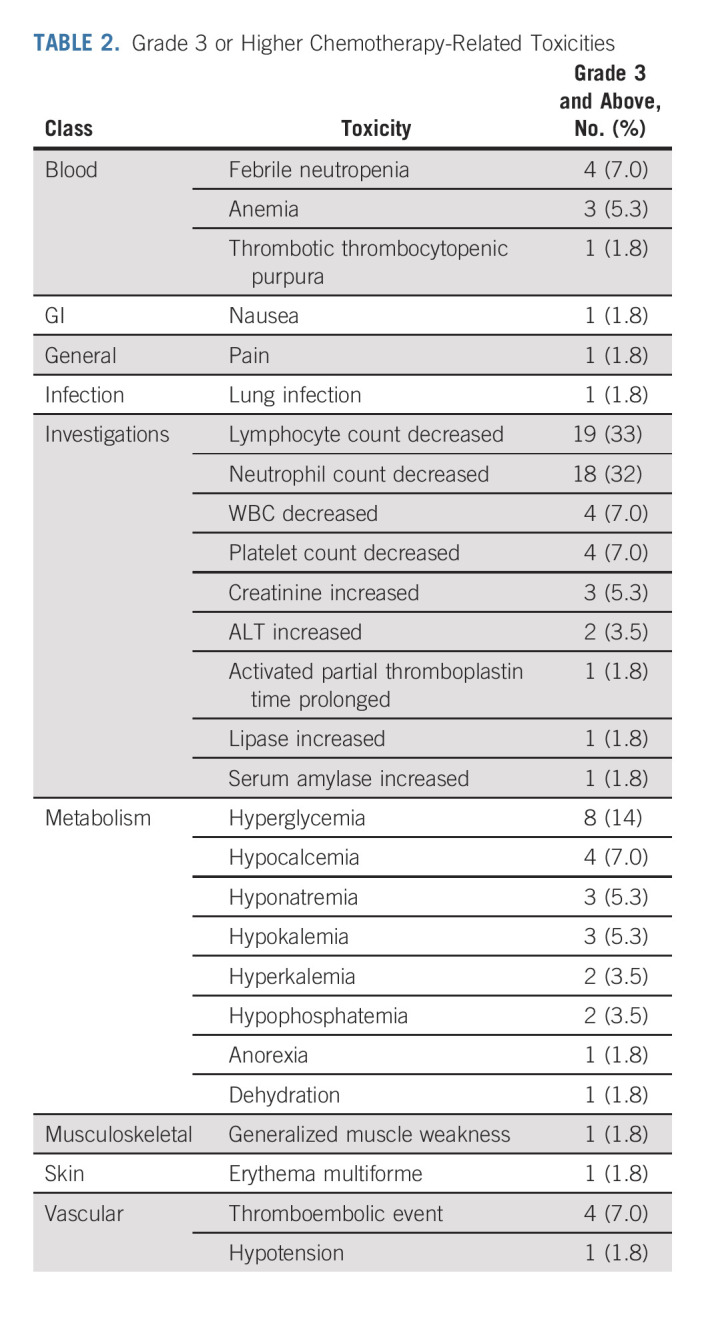
The median duration from chemotherapy completion to surgery was 7 weeks (interquartile range [IQR], 5.0-8.1). No patients demonstrated clinical or radiographic disease progression before surgery. Forty-seven patients (82%) underwent minimally invasive surgery. Fifty-four patients (95%) underwent RNU and three (5%) underwent distal ureterectomy. Fifty-three patients (93%) underwent a templated LND with a median yield of 18 lymph nodes (IQR, 11-23). The 30-day rate of grade ≥ 3 surgical complications was 11%.
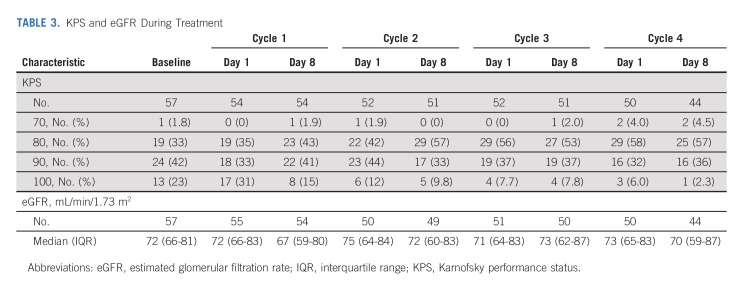
Renal function was minimally affected during chemotherapy; median eGFR was 72 (IQR, 66-81) at baseline and 70 (IQR, 59-87) after chemotherapy (Table 3). Renal function was severely affected by surgery; median 7-day, 12-month, and 24-month postsurgery eGFR values were 41 (IQR, 33-51), 42 (IQR, 35-59), and 40 (IQR, 34-52), respectively. Within 90 days of surgery, stage 4 CKD (eGFR < 30) occurred in 25% of patients, although no patients required dialysis. KPS did decline during chemotherapy, although the proportion of patients with a KPS ≥ 80 (equivalent to ECOG 1 or 0) was minimally affected.
TABLE 3.
KPS and eGFR During Treatment
Oncologic Outcomes
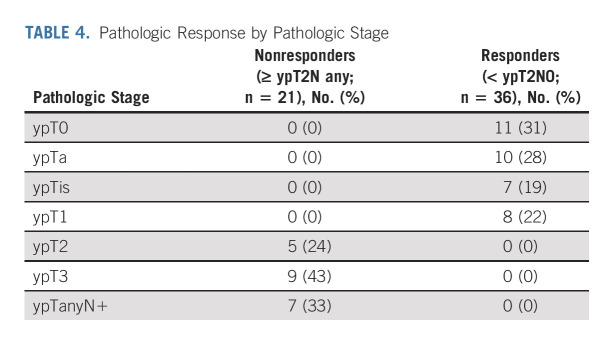
Thirty-sixty of 57 patients (63%) met the primary end point of pathologic response (< ypT2N0; 95% CI, 49 to 76); 11 (19%) had pathologic complete response (ypT0N0; Table 4). Six patients with residual muscle-invasive disease had variant histologies: micropapillary (n = 1), squamous (n = 4), or nested (n = 1). Of the four patients with Lynch syndrome, pathology included ypT2N0 (n = 1), ypT2Nx (n = 1), and ypTaN0 (n = 2). At a median follow-up of 3.1 years (IQR, 2.0-6.1 years) among survivors, there were 19 PFS events and 11 patients who died.
TABLE 4.
Pathologic Response by Pathologic Stage
Of note, when the first stage ended, 16 of 29 patients demonstrated a pathologic response, meeting the threshold to continue into the second stage. The original plan was to include 54 evaluable patients, and to reject the null hypothesis if 28 of 54 patients demonstrated a pathologic response: this threshold was met in the first 54 patients, with 33 responding to treatment.
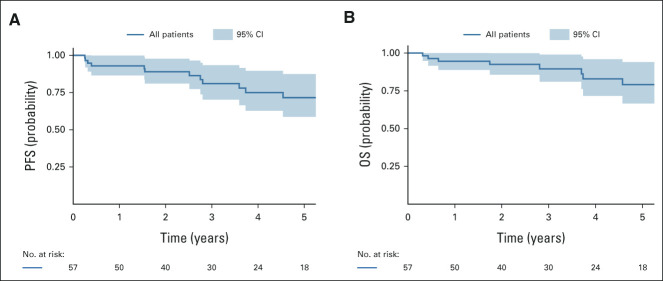
Kaplan-Meier curves for PFS and OS are presented in Figure 1 and stratified by pathologic response in Figure 2. Two-year PFS rate was 89% (95% CI, 81 to 98), and 5-year PFS rate was 72% (95% CI, 59 to 87). PFS was significantly superior among complete and partial responders compared with nonresponders (P < .001); 2-year PFS rates were, respectively, 100% and 95% vs 76%, and 5-year PFS rates were, respectively, 100% and 86% versus 47%. Two-year OS rate was 93% (95% CI, 86 to 100), and 5-year OS rate was 79% (95% CI, 67 to 94). Similarly, OS was significantly superior among complete and partial responders compared with nonresponders (P < .001); 2-year OS rates were, respectively, 100% and 100% versus 80%, and 5-year OS rates were, respectively, 100% and 90% versus 58%.
FIG 1.
Kaplan-Meier curves for PFS and OS. OS, overall survival; PFS, progression-free survival.
FIG 2.
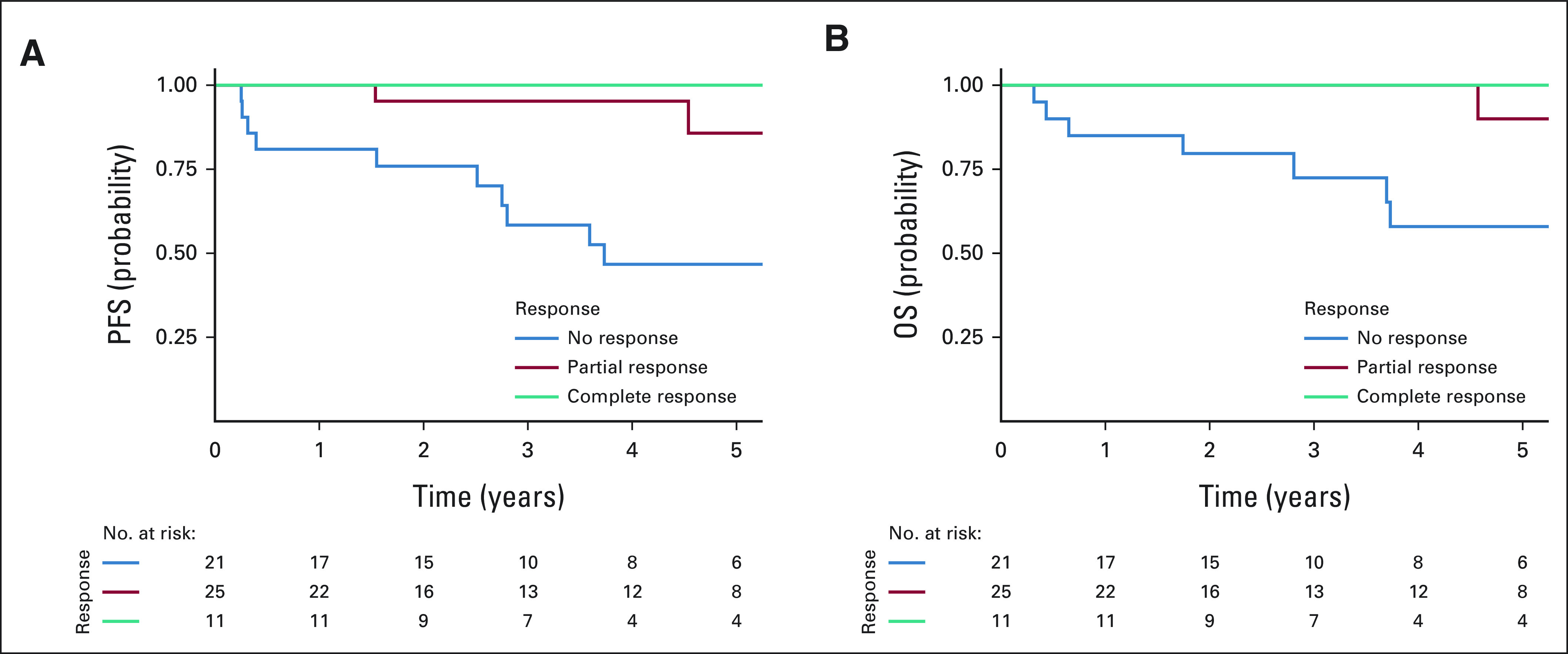
Kaplan-Meier curves for PFS and OS stratified by pathologic response. OS, overall survival; PFS, progression-free survival.
DISCUSSION
To our knowledge, this multicenter phase II trial is the first and only fully accrued prospective study evaluating the role of NAC followed by effective surgery for high-risk UTUC. The primary outcome, a 63% pathologic response rate, met the prespecified criteria to be considered promising. Coupled with the 19% complete response rate and excellent 2- and 5-year PFS and OS rates, our results offer support that neoadjuvant split-dose GC has substantial activity in UTUC. Patients who had a pathologic response at the time of surgery had clearly superior long-term oncologic outcomes than patients who did not have a pathologic response, echoing studies of NAC for UCB.3,5,14 Our results can be compared with prior historical series of high-risk UTUC treated with RNU and without NAC, in which 2- and 5-year OS rates have ranged from 68% to 84% and 29% to 62%, respectively.15,24-26 Several other historical series have also reported recurrence-free survival or relapse-free survival with definitions equivalent to our PFS outcome (ie, metastasis, local nonurothelial recurrence, or death). These 2- and 5-year survival outcomes have ranged from 50% to 76% and 40% to 68%, respectively.21,24,27 Because of the rarity of the disease, this single-arm trial required over 10 years to complete. Since there are currently no completed randomized controlled phase III neoadjuvant trials, this is the strongest evidence available at this time. Furthermore, this multimodal therapy was safe and well tolerated, suggesting that similar to UCB, patients with high-risk UTUC should be offered NAC followed by extirpative surgery as an optimal treatment option for their disease.
Our results contribute to the scarce prospective literature on the role of multimodality management and perioperative chemotherapy for patients with UTUC. To date, only two prospective trials on perioperative chemotherapy for UTUC have been reported. The ECOG-ACRIN 8141 trial9 planned to enroll 30 cisplatin-eligible and 30 cisplatin-ineligible patients and treat them with either accelerated MVAC, or gemcitabine and carboplatin, respectively. The study was closed prematurely because of poor accrual, with only six patients enrolled in the gemcitabine and carboplatin arm. Of the 29 patients eligible for MVAC, the pathologic complete response and downstaging rates were 14% and 62%, respectively, which our findings are consistent with. With a median follow-up of 21.1 months, the median recurrence-free survival and OS was not reached. The phase III POUT trial randomly assigned 260 patients with pT2-4 pN0-3 UTUC to platinum-based adjuvant chemotherapy or surveillance.7 In this trial, adjuvant platinum-based chemotherapy significantly improved disease-free survival compared with surveillance at a median follow-up of 30.3 months, while OS data remain immature.
However, the use of cisplatin-based chemotherapy for UTUC is frequently limited by renal dysfunction: stage three or greater CKD is present in roughly 40% of patients with newly diagnosed UTUC and up to 85% of patients following RNU.8,28 In the POUT trial, 44% of patients treated in the adjuvant setting initially received carboplatin, and 11% who initially received cisplatin were subsequently switched to carboplatin because of progressive renal impairment. The use of carboplatin for UC remains contentious—subgroup analysis in the POUT trial indicated that the effect size with carboplatin may be smaller than that of cisplatin, although the trial was not powered to test the difference. A series of prior neoadjuvant trials with carboplatin-based chemotherapy in UCB have demonstrated low levels of clinical activity.29 Given this limitation, it could be argued that in the neoadjuvant setting, more patients are eligible, and treatment may be better tolerated. The use of NAC may be most critical for patients who are predicted to have poor renal function post-RNU and who become cisplatin-ineligible. Although all patients in our study were required to be cisplatin-eligible, which may potentially confer a prognostic advantage, this decision was made to mitigate the risk of treatment-related toxicities in patients with pre-existing medical conditions, as the disease-specific inclusion criteria were intended to select for patients with high risk of recurrence or metastatic dissemination following surgery alone.
Our study demonstrates that split-dose cisplatin-based NAC is safe, feasible, and effective in UTUC. Using split-dose cisplatin, we were able to maximize treatment delivery; the majority of patients were able to receive both adequate chemotherapy (≥ three cycles) and timely surgery. Similar to patients with UCB treated with split-dose GC,4 to date, no patients have required dialysis. Overall complication rates, length of stay, and perioperative data such as blood loss and duration of surgery were comparable with those in similar published series.19
The primary limitation to our study is the long accrual period. This is partly because of the rarity of the disease and higher proportion of cisplatin-ineligibility inherent in UTUC; our experience is not unique or singular in this case, as seen in the inability of the ECOG-ACRIN trial to reach their enrollment target. However, the long accrual time did allow for the ability to provide longer-term data on survival end points. Moreover, neoadjuvant studies for UTUC are more challenging because accurate preoperative staging is difficult, given the more limited access to the upper tracts and the absence of standardized imaging criteria. Endoscopic resection to the degree performed in the bladder is not feasible or safe in the upper tract, and so, complete resection for staging or as an adjunct to treatment is not possible. As previously noted, biopsy specimens from the upper tract are unable to reliably determine depth of invasion. So, this must be considered in the interpretation of our pathologic response rate. However, our pathologic complete response rate (ypT0N0) of 19% demonstrates that there is oncologic activity with the regimen used, as the primary tumors are not completely resected during the diagnostic evaluation. Although selection for cisplatin-based NAC is challenging and may lead to overtreatment, all patients met defined high-risk criteria in this study. Furthermore, the multicenter effort ensured our ability to complete our enrollment, paralleling the multicenter nature of the POUT trial, which reaffirms that a collaborative and cooperative approach is essential in clinical trials for rare diseases to improve outcomes.
In conclusion, this is a fully accrued phase II multicenter trial, which successfully demonstrates the feasibility, safety, and clinical activity of neoadjuvant split-dose GC followed by radical surgery for high-risk UTUC, with superior clinical outcomes compared with historical series. This single-arm trial required over 10 years to complete because of the rarity of the disease, and there are no completed randomized controlled phase III neoadjuvant trials at this time. These results lend significant support to the consideration of NAC as a standard of care to allow for expansion of perioperative cisplatin-based chemotherapy to more patients with UTUC, and split-dose GC is a viable option for NAC.
ACKNOWLEDGMENT
The authors thank Janet Novak, PhD, ELS(D), of Memorial Sloan Kettering Cancer Center for editing the manuscript.
Jonathan A. Coleman
Travel, Accommodations, Expenses: Digital Angiography Reading Center
Uncompensated Relationships: Steba Biotech, AngioDynamics
Wesley Yip
Consulting or Advisory Role: Gilead Sciences, SAI MedPartners
Daniel D. Sjoberg
Consulting or Advisory Role: OPKO Diagnostics
Bernard H. Bochner
Consulting or Advisory Role: Olympus
Eugene J. Pietzak
Honoraria: UpToDate
Consulting or Advisory Role: Merck, Chugai Pharma, QED Therapeutics, Janssen, Urogen pharma
Research Funding: Janssen
A. Ari Hakimi
Consulting or Advisory Role: Merck
Kwanghee Kim
Stock and Other Ownership Interests: Arvinas Inc
Hikmat A. Al-Ahmadie
Consulting or Advisory Role: AstraZeneca/MedImmune, Janssen Biotech, PAIGE.AI
Suresh G. Nair
Stock and Other Ownership Interests: Moderna Therapeutics, Novavax, Biontech, Gilead Sciences
Research Funding: Bristol Myers Squibb (Inst), Merck (Inst), Nektar (Inst)
Angelo A. Baccala Jr
Honoraria: Myriad Genetics
Consulting or Advisory Role: Myriad Genetics
Speakers' Bureau: Myriad Genetics, Pfizer
Gopa Iyer
Consulting or Advisory Role: Bayer, Janssen, Mirati Therapeutics, Basilea, Flare Therapeutics, Loxo/Lilly
Speakers' Bureau: Gilead Sciences, Lynx Group
Research Funding: Mirati Therapeutics (Inst), Novartis (Inst), Debiopharm Group (Inst), Bayer (Inst), Janssen (Inst), Seattle Genetics (Inst)
Jonathan E. Rosenberg
Honoraria: UpToDate, Medscape, Peerview, Research To Practice, Clinical Care Options, Physicans' Education Resource, MJH Life Sciences, EMD Serono, Pfizer
Consulting or Advisory Role: Lilly, Merck, Roche/Genentech, AstraZeneca/MedImmune, Bristol Myers Squibb, Seattle Genetics, Bayer, BioClin Therapeutics, QED Therapeutics, Pharmacyclics, GlaxoSmithKline, Janssen Oncology, Astellas Pharma, Boehringer Ingelheim, Pfizer/EMD Serono, Mirati Therapeutics, Immunomedics, Tyra Biosciences, Infinity Pharmaceuticals, Gilead Sciences, Hengrui Pharmaceutical, Alligator Bioscience, Imvax
Research Funding: Genentech/Roche (Inst), Seattle Genetics (Inst), Bayer (Inst), AstraZeneca (Inst), QED Therapeutics (Inst), Astellas Pharma (Inst)
Patents, Royalties, Other Intellectual Property: Predictor of platinum sensitivity (Inst)
Min Y. Teo
Consulting or Advisory Role: Janssen Oncology
Research Funding: Bristol Myers Squibb (Inst), Clovis Oncology (Inst), Pharmacyclics (Inst)
Dean F. Bajorin
Honoraria: Bristol Myers Squibb/Medarex
Consulting or Advisory Role: Merck, Bristol Myers Squibb Foundation
Research Funding: Novartis (Inst), Merck (Inst), Bristol Myers Squibb (Inst), AstraZeneca (Inst), Astellas Pharma (Inst), Seattle Genetics/Astellas (Inst)
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in part at the American Urological Association Annual Meeting, Chicago, IL, May 5, 2019; and in full at the American Society of Clinical Oncology Genitourinary Cancers Symposium, San Francisco, CA, February 18, 2022.
SUPPORT
Funding was received from the Thompson Family Foundation (J.A.C. and K.K.), the Sidney Kimmel Center for Prostate and Urologic Cancers, NIH/NCI Cancer Center Support Grant P30 CA008748, and Ruth L. Kirschstein National Research Service Award T32CA082088 (W.Y.).
CLINICAL TRIAL INFORMATION
AUTHOR CONTRIBUTIONS
Conception and design: Jonathan A. Coleman, Wesley Yip, Daniel D. Sjoberg, Bernard H. Bochner, Guido Dalbagni, Harry W. Herr, Angelo A. Baccala Jr, Andrea B. Apolo, Gopa Iyer, Dean F. Bajorin
Financial support: Jonathan A. Coleman
Administrative support: Jonathan A. Coleman, Nicole E. Benfante
Provision of study materials or patients: Jonathan A. Coleman, S. Machele Donat, Harry W. Herr, Timothy F. Donahue, Eugene J. Pietzak, Ricardo G. Alvim, Anoop M. Meraney, Steven J. Shichman, Jeffrey M. Kamradt, Suresh G. Nair, Angelo A. Baccala Jr, Paul Palyca, Muhammad A. Rizvi, Antonio F. Muina, Jonathan E. Rosenberg, Min Y. Teo, Dean F. Bajorin
Collection and assembly of data: Jonathan A. Coleman, Wesley Yip, Nathan C. Wong, Harry W. Herr, Eugene K. Cha, Eugene J. Pietzak, Hikmat A. Al-Ahmadie, Ricardo G. Alvim, Nicole E. Benfante, Anoop M. Meraney, Jeffrey M. Kamradt, Paul Palyca, Bradley W. Lash, Muhammad A. Rizvi, Antonio F. Muina, Andrea B. Apolo, Gopa Iyer, Jonathan E. Rosenberg
Data analysis and interpretation: Jonathan A. Coleman, Wesley Yip, Nathan C. Wong, Daniel D. Sjoberg, S. Machele Donat, Harry W. Herr, A. Ari Hakimi, Kwanghee Kim, Hikmat A. Al-Ahmadie, H. Alberto Vargas, Ricardo G. Alvim, Soleen Ghafoor, Andrea B. Apolo, Gopa Iyer, Jonathan E. Rosenberg, Min Y. Teo, Dean F. Bajorin
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Multicenter Phase II Clinical Trial of Gemcitabine and Cisplatin as Neoadjuvant Chemotherapy for Patients With High-Grade Upper Tract Urothelial Carcinoma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Jonathan A. Coleman
Travel, Accommodations, Expenses: Digital Angiography Reading Center
Uncompensated Relationships: Steba Biotech, AngioDynamics
Wesley Yip
Consulting or Advisory Role: Gilead Sciences, SAI MedPartners
Daniel D. Sjoberg
Consulting or Advisory Role: OPKO Diagnostics
Bernard H. Bochner
Consulting or Advisory Role: Olympus
Eugene J. Pietzak
Honoraria: UpToDate
Consulting or Advisory Role: Merck, Chugai Pharma, QED Therapeutics, Janssen, Urogen pharma
Research Funding: Janssen
A. Ari Hakimi
Consulting or Advisory Role: Merck
Kwanghee Kim
Stock and Other Ownership Interests: Arvinas Inc
Hikmat A. Al-Ahmadie
Consulting or Advisory Role: AstraZeneca/MedImmune, Janssen Biotech, PAIGE.AI
Suresh G. Nair
Stock and Other Ownership Interests: Moderna Therapeutics, Novavax, Biontech, Gilead Sciences
Research Funding: Bristol Myers Squibb (Inst), Merck (Inst), Nektar (Inst)
Angelo A. Baccala Jr
Honoraria: Myriad Genetics
Consulting or Advisory Role: Myriad Genetics
Speakers' Bureau: Myriad Genetics, Pfizer
Gopa Iyer
Consulting or Advisory Role: Bayer, Janssen, Mirati Therapeutics, Basilea, Flare Therapeutics, Loxo/Lilly
Speakers' Bureau: Gilead Sciences, Lynx Group
Research Funding: Mirati Therapeutics (Inst), Novartis (Inst), Debiopharm Group (Inst), Bayer (Inst), Janssen (Inst), Seattle Genetics (Inst)
Jonathan E. Rosenberg
Honoraria: UpToDate, Medscape, Peerview, Research To Practice, Clinical Care Options, Physicans' Education Resource, MJH Life Sciences, EMD Serono, Pfizer
Consulting or Advisory Role: Lilly, Merck, Roche/Genentech, AstraZeneca/MedImmune, Bristol Myers Squibb, Seattle Genetics, Bayer, BioClin Therapeutics, QED Therapeutics, Pharmacyclics, GlaxoSmithKline, Janssen Oncology, Astellas Pharma, Boehringer Ingelheim, Pfizer/EMD Serono, Mirati Therapeutics, Immunomedics, Tyra Biosciences, Infinity Pharmaceuticals, Gilead Sciences, Hengrui Pharmaceutical, Alligator Bioscience, Imvax
Research Funding: Genentech/Roche (Inst), Seattle Genetics (Inst), Bayer (Inst), AstraZeneca (Inst), QED Therapeutics (Inst), Astellas Pharma (Inst)
Patents, Royalties, Other Intellectual Property: Predictor of platinum sensitivity (Inst)
Min Y. Teo
Consulting or Advisory Role: Janssen Oncology
Research Funding: Bristol Myers Squibb (Inst), Clovis Oncology (Inst), Pharmacyclics (Inst)
Dean F. Bajorin
Honoraria: Bristol Myers Squibb/Medarex
Consulting or Advisory Role: Merck, Bristol Myers Squibb Foundation
Research Funding: Novartis (Inst), Merck (Inst), Bristol Myers Squibb (Inst), AstraZeneca (Inst), Astellas Pharma (Inst), Seattle Genetics/Astellas (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1. Munoz JJ, Ellison LM. Upper tract urothelial neoplasms: Incidence and survival during the last 2 decades. J Urol. 2000;164:1523–1525. [PubMed] [Google Scholar]
- 2. Collà Ruvolo C, Nocera L, Stolzenbach LF, et al. Incidence and survival rates of contemporary patients with invasive upper tract urothelial carcinoma. Eur Urol Oncol. 2021;4:792–801. doi: 10.1016/j.euo.2020.11.005. [DOI] [PubMed] [Google Scholar]
- 3. Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349:859–866. doi: 10.1056/NEJMoa022148. [DOI] [PubMed] [Google Scholar]
- 4. Collaboration ABCAM-a. Neoadjuvant chemotherapy in invasive bladder cancer: Update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. Eur Urol. 2005;48:202–205. doi: 10.1016/j.eururo.2005.04.006. discussion 5-6. [DOI] [PubMed] [Google Scholar]
- 5. Iyer G, Tully CM, Zabor EC, et al. Neoadjuvant gemcitabine-cisplatin plus radical cystectomy-pelvic lymph node dissection for muscle-invasive bladder cancer: A 12-year experience. Clin Genitourin Cancer. 2020;18:387–394. doi: 10.1016/j.clgc.2020.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Clements T, Messer JC, Terrell JD, et al. High-grade ureteroscopic biopsy is associated with advanced pathology of upper-tract urothelial carcinoma tumors at definitive surgical resection. J Endourol. 2012;26:398–402. doi: 10.1089/end.2011.0426. [DOI] [PubMed] [Google Scholar]
- 7. Birtle A, Johnson M, Chester J, et al. Adjuvant chemotherapy in upper tract urothelial carcinoma (the POUT trial): A phase 3, open-label, randomised controlled trial. Lancet. 2020;395:1268–1277. doi: 10.1016/S0140-6736(20)30415-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shao IH, Lin YH, Hou CP, et al. Risk factors associated with ineligibility of adjuvant cisplatin-based chemotherapy after nephroureterectomy. Drug Des Devel Ther. 2014;8:1985–1990. doi: 10.2147/DDDT.S72197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Margulis V, Puligandla M, Trabulsi EJ, et al. Phase II trial of neoadjuvant systemic chemotherapy followed by extirpative surgery in patients with high grade upper tract urothelial carcinoma. J Urol. 2020;203:690–698. doi: 10.1097/JU.0000000000000644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hussain SA, Stocken DD, Riley P, et al. A phase I/II study of gemcitabine and fractionated cisplatin in an outpatient setting using a 21-day schedule in patients with advanced and metastatic bladder cancer. Br J Cancer. 2004;91:844–849. doi: 10.1038/sj.bjc.6602112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. von der Maase H, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: Results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. 2000;18:3068–3077. doi: 10.1200/JCO.2000.18.17.3068. [DOI] [PubMed] [Google Scholar]
- 12. Matin SF, Sfakianos JP, Espiritu PN, et al. Patterns of lymphatic metastases in upper tract urothelial carcinoma and proposed dissection templates. J Urol. 2015;194:1567–1574. doi: 10.1016/j.juro.2015.06.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edge SB, Byrd DR, Compton CC, et al. AJCC Cancer Staging Manual. ed 7. New York, NY: Springer; 2010. [Google Scholar]
- 14. Splinter TA, Scher HI, Denis L, et al. The prognostic value of the pathological response to combination chemotherapy before cystectomy in patients with invasive bladder cancer. European Organization for Research on Treatment of Cancer—Genitourinary Group. J Urol. 1992;147:606–608. doi: 10.1016/s0022-5347(17)37318-4. [DOI] [PubMed] [Google Scholar]
- 15. Porten S, Siefker-Radtke AO, Xiao L, et al. Neoadjuvant chemotherapy improves survival of patients with upper tract urothelial carcinoma. Cancer. 2014;120:1794–1799. doi: 10.1002/cncr.28655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Martini A, Daza J, Poltiyelova E, et al. Pathological downstaging as a novel endpoint for the development of neoadjuvant chemotherapy for upper tract urothelial carcinoma. BJU Int. 2019;124:665–671. doi: 10.1111/bju.14719. [DOI] [PubMed] [Google Scholar]
- 17. Singla N, Christie A, Freifeld Y, et al. Pathologic stage as a surrogate for oncologic outcomes after receipt of neoadjuvant chemotherapy for high-grade upper tract urothelial carcinoma. Urol Oncol. 2020;38:933.e7–933.e12. doi: 10.1016/j.urolonc.2020.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Foerster B, Abufaraj M, Petros F, et al. Efficacy of preoperative chemotherapy for high risk upper tract urothelial carcinoma. J Urol. 2020;203:1101–1108. doi: 10.1097/JU.0000000000000737. [DOI] [PubMed] [Google Scholar]
- 19. Liao RS, Gupta M, Schwen ZR, et al. Comparison of pathological stage in patients treated with and without neoadjuvant chemotherapy for high risk upper tract urothelial carcinoma. J Urol. 2018;200:68–73. doi: 10.1016/j.juro.2017.12.054. [DOI] [PubMed] [Google Scholar]
- 20. Matin SF, Margulis V, Kamat A, et al. Incidence of downstaging and complete remission after neoadjuvant chemotherapy for high-risk upper tract transitional cell carcinoma. Cancer. 2010;116:3127–3134. doi: 10.1002/cncr.25050. [DOI] [PubMed] [Google Scholar]
- 21. Krabbe LM, Eminaga O, Shariat SF, et al. Postoperative nomogram for relapse-free survival in patients with high grade upper tract urothelial carcinoma. J Urol. 2017;197:580–589. doi: 10.1016/j.juro.2016.09.078. [DOI] [PubMed] [Google Scholar]
- 22. Dash A, Pettus JA, Herr HW, et al. A role for neoadjuvant gemcitabine plus cisplatin in muscle-invasive urothelial carcinoma of the bladder: A retrospective experience. Cancer. 2008;113:2471–2477. doi: 10.1002/cncr.23848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: A randomised controlled trial. International collaboration of trialists. Lancet. 1999;354:533–540. [PubMed] [Google Scholar]
- 24. Martinez-Salamanca JI, Shariat SF, Rodriguez JC, et al. Prognostic role of ECOG performance status in patients with urothelial carcinoma of the upper urinary tract: An international study. BJU Int. 2012;109:1155–1161. doi: 10.1111/j.1464-410X.2011.10479.x. [DOI] [PubMed] [Google Scholar]
- 25. Manabe D, Saika T, Ebara S, et al. Comparative study of oncologic outcome of laparoscopic nephroureterectomy and standard nephroureterectomy for upper urinary tract transitional cell carcinoma. Urology. 2007;69:457–461. doi: 10.1016/j.urology.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 26. Zeuschner P, Vollmer SG, Linxweiler J, et al. Robot-assisted versus open radical nephroureterectomy for urothelial carcinoma of the upper urinary tract: A retrospective cohort study across ten years. Surg Oncol. 2021;38:101607. doi: 10.1016/j.suronc.2021.101607. [DOI] [PubMed] [Google Scholar]
- 27. Zennami K, Sumitomo M, Takahara K, et al. Two cycles of neoadjuvant chemotherapy improves survival in patients with high-risk upper tract urothelial carcinoma. BJU Int. 2021;127:332–339. doi: 10.1111/bju.15230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Raman JD, Lin YK, Kaag M, et al. High rates of advanced disease, complications, and decline of renal function after radical nephroureterectomy. Urol Oncol. 2014;32:47.e9–47.e14. doi: 10.1016/j.urolonc.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 29. Einstein DJ, Sonpavde G. Treatment approaches for cisplatin-ineligible patients with invasive bladder cancer. Curr Treat Options Oncol. 2019;20:12. doi: 10.1007/s11864-019-0609-6. [DOI] [PubMed] [Google Scholar]
- 30. Winer AG, Vertosick EA, Ghanaat M, et al. Prognostic value of lymph node yield during nephroureterectomy for upper tract urothelial carcinoma. Urol Oncol. 2017;35:151.e9–151.e15. doi: 10.1016/j.urolonc.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]