Background:
During the opioid epidemic, misuse of acetaminophen-opioid products resulted in supratherapeutic acetaminophen ingestions and cases of hepatotoxicity. In 2014, the US Food and Drug Administration (FDA) limited the amount of acetaminophen in combination products to 325 mg, and the US Drug Enforcement Administration (DEA) changed hydrocodone/acetaminophen from schedule III to schedule II. This study assessed whether these federal mandates were associated with changes in acetaminophen-opioid supratherapeutic ingestions.
Methods:
We identified emergency department encounters at our institution of patients with a detectable acetaminophen concentration and manually reviewed these charts.
Results:
We found a decline in acetaminophen-opioid supratherapeutic ingestions after 2014. A downtrend in hydrocodone/acetaminophen ingestions accompanied a relative increase in codeine/acetaminophen ingestions from 2015 onwards.
Conclusion:
This experience at one large safety net hospital suggests a beneficial impact of the FDA ruling in reducing likely unintentional acetaminophen supratherapeutic ingestions, carrying a risk of hepatotoxicity, in the setting of intentional opioid ingestions.
INTRODUCTION
During the opioid epidemic, people misusing acetaminophen-opioid products resulted in supratherapeutic acetaminophen ingestions and cases of hepatotoxicity.1 In 2014, the US Food and Drug Administration (FDA) limited the amount of acetaminophen in combination products to 325 mg, and the US Drug Enforcement Administration (DEA) changed hydrocodone/acetaminophen from schedule III to schedule II. This study highlights the association of federal mandates with changes in acetaminophen-opioid supratherapeutic ingestions in a large Texas county hospital from 2011 to 2020.
METHODS
From our electronic health record, we identified emergency department encounters between January 1, 2011 and December 31, 2020 of patients ≥18 years old with a detectable acetaminophen concentration (>10 mcg/mL). Using a standardized abstraction form, trained abstractors manually reviewed these charts to identify encounters involving acetaminophen-opioid supratherapeutic ingestions and extracted product(s) ingested, intent, amount, and disposition. We defined supratherapeutic ingestions as >10 mcg/mL serum acetaminophen concentration with report of taking more than the therapeutic dose.2 We report descriptive statistics and performed chi-square analysis for change in the rate of encounters per year before and after 2014.
RESULTS
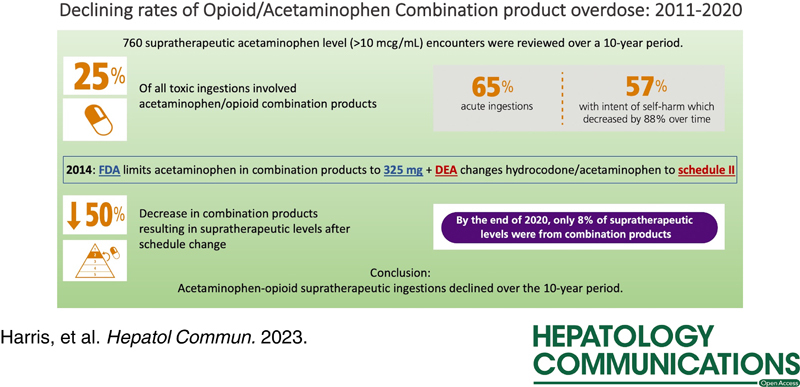
Of 760 encounters with supratherapeutic ingestions, 186 (25%) involved acetaminophen-opioid products. Most patients were Whites (83%), non-Hispanic (76%), and female (54%). The percentage of supratherapeutic acetaminophen-opioid ingestions decreased from 34% before 2014 to 17% from 2014 onward (p<0.01). (Figure 1A) The percentage of acetaminophen-opioid supratherapeutic ingestion encounters out of the total number of encounters with acetaminophen concentration >10 mcg/mL also declined from 34% in 2011 to 8% by 2020 (Figure 1D). Most ingestions were acute (65%), with intent at self-harm (57%). The rate of intentional acetaminophen-opioid supratherapeutic encounters decreased over the study period (Figure 1C). Most patients were not treated with N-acetylcysteine and only 3% developed acute liver failure with no mortality. Dispositions from the emergency department were discharged home (37%), admitted to a noncritical care unit (27%), admitted to a psychiatric care facility (18%), admitted to a critical care unit (15%), and left against medical advice (3%). A downtrend in hydrocodone/acetaminophen ingestions accompanied a relative increase in codeine/acetaminophen ingestions from 2015 onward (Figure 1B).
FIGURE 1.
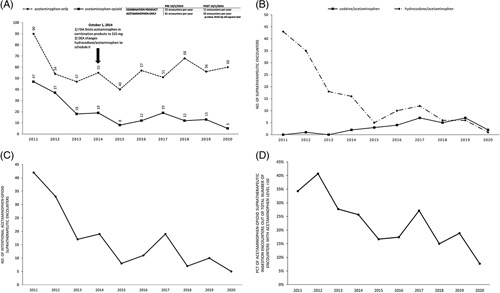
(A) Number of encounters involving supratherapeutic ingestion of acetaminophen-only or acetaminophenopioid products per year. (B) Number of encounters involving supratherapeutic ingestion of codeine/acetaminophen versus hydrocodone/acetaminophen per year. Of note, encounters involving ingestion of oxycodone/acetaminophen, tramadol/acetaminophen, or propoxyphene/acetaminophen were not included in this figure due to very low prevalence. (C) Number of intentional supratherapeutic ingestions of acetaminophen-opioid products per year. (D) Percentage by year of acetaminophen-opioid supratherapeutic ingestion encounters out of the total number of encounters with acetaminophen level >10.
DISCUSSION
Our study shows a decline in acetaminophen-opioid supratherapeutic ingestions at our institution after 2014, where the rate of hospitalized APAP-associated acute liver injury or acute liver failure per 100,000 adult admissions is 73.4/100,000.3 As hydrocodone/acetaminophen supratherapeutic ingestions declined, codeine-acetaminophen, which remained schedule III, may have replaced some hydrocodone-acetaminophen prescriptions. This trend is consistent with findings from a study comparing opioid analgesic exposures reported to Texas Poison Centers during the 6 months before and after the hydrocodone schedule change.4 In Texas, schedule II drugs (those with high abuse risk but accepted medical uses) require either triplicate prescription or official forms issued by the Texas Department of Public Safety to prescribers. Schedule III drugs do not require this additional level of documentation. Although most acetaminophen-opioid ingestions were described as intentional, we suspect that people who intentionally overdose for self-harm may be intending opioid overdose rather than acetaminophen overdose. Studies from early in the opioid epidemic (2000–2010) showed an increase in hepatotoxicity from acetaminophen-opioid medications compared with prior years. This experience at one large safety net hospital suggests a beneficial impact of the FDA ruling in reducing likely unintentional acetaminophen supratherapeutic ingestions, carrying a risk of hepatotoxicity, in the setting of intentional opioid ingestions.
Acknowledgments
CONFLICT OF INTEREST
William M. Lee consults for Seagen, Veristat, and GlaxoSmithKline. The remaining authors have no conflicts to report.
Footnotes
Abbreviations: FDA, US Food and Drug Administration; DEA, Drug Enforcement Administration.
Contributor Information
Elizabeth Harris, Email: lizzy4910@gmail.com.
Michael Harms, Email: Michael.Harms@phhs.org.
Dazhe Cao, Email: dazhe.cao@utsouthwestern.edu.
Courtney Prestwood, Email: courtneyprestwood@gmail.com.
Lister DeBinya, Email: lister.debinya@utsouthwestern.edu.
Kurt Kleinschmidt, Email: kurt.kleinschmidt@utsouthwestern.edu.
Amy Young, Email: Amy.Young@utsouthwestern.edu.
Srishti Saha, Email: Srishti.Saha@utsouthwestern.edu.
Jody Rule, Email: jody.rule@utsouthwestern.edu.
Kristin Alvarez, Email: Kristin.Alvarez@phhs.org.
Sandeep R. Das, Email: Sandeep.Das@utsouthwestern.edu.
William M. Lee, Email: william.lee@utsouthwestern.edu.
REFERENCES
- 1. Bond GR, Ho M, Woodward RW. Trends in hepatic injury associated with unintentional overdose of paracetamol (acetaminophen) in products with and without opioid. Drug Safety. 2012;35:149–57. [DOI] [PubMed] [Google Scholar]
- 2. Daly FFS, O'Malley GF, Heard K, Bogdan GM, Dart RC. Prospective evaluation of repeated supratherapeutic acetaminophen (paracetamol) ingestion. Ann Emerg Med. 2004;44:393–8. [DOI] [PubMed] [Google Scholar]
- 3. Long A, Magrath M, Mihalopoulos M, Rule JA, Agrawal D, Haley R, et al. Changes in epidemiology of acetaminophen overdoses in an urban county hospital after 20 years. Am J Gastroenterol. 2022;117:1324–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Haynes A, Kleinschmidt K, Forrester MB, Young A. Trends in analgesic exposures reported to Texas Poison Centers following increased regulation of hydrocodone. Clin Toxicol. 2016;54:434–440. [DOI] [PubMed] [Google Scholar]


