Abstract
In the past decade, liver organoids have evolved rapidly as valuable research tools, providing novel insights into almost all types of liver diseases, including monogenic liver diseases, alcohol-associated liver disease, metabolic-associated fatty liver disease, various types of (viral) hepatitis, and liver cancers. Liver organoids in part mimic the microphysiology of the human liver and fill a gap in high-fidelity liver disease models to a certain extent. They hold great promise to elucidate the pathogenic mechanism of a diversity of liver diseases and play a crucial role in drug development. Moreover, it is challenging but opportunistic to apply liver organoids for tailored therapies of various liver diseases. The establishment, applications, and challenges of different types of liver organoids, for example, derived from embryonic, adult, or induced pluripotent stem cells, to model different liver diseases, are presented in this review.
INTRODUCTION
Approximately 2 million people worldwide die each year from liver diseases, many of which are tricky to treat. Liver transplantation is the only option in the advanced stage, leading to an increased medical and economic burden.1 Therefore, there is an urgent need for innovative tools to better understand the pathology of liver diseases to develop better treatments. Immortalized cell lines are the most common models in a lab but are limited by numerous genetic and functional alterations that compromise the authenticity of modeling liver disease.2 Primary human hepatocytes, regarded as the gold standard for evaluating hepatic metabolism, are limited by their loss of proliferative capacity in vitro.3 Liver organoids, defined as organ-specific 3-dimensional (3D) structures grown from pluripotent stem cells (PSCs), liver-resident adult stem cells (ASCs), and/or hepatocytes, are capable of overcoming the proliferation limitation and confer advantages for the maintenance of metabolic function. In addition, organoids, which allow the generation of cell-cell or cell-ECM interactions in all 3 dimensions, could more closely resemble the architectural and functional properties of in vivo tissues and show the potential to serve as personalized hepatic model systems toward disease modeling, drug screening, and drug toxicity testing, whereas in 2-dimensional monolayer cultures, interactions are limited to the horizontal plane.4,5
Stem cell–derived organoids can be differentiated into multiple liver cell types and can self-organize by cell sorting and lineage commitment to form organ-like tissues that recapitulate the key features of the liver.6–8 To mimic the cell niche, the hepatic progenitors or differentiated hepatic cells are placed in an artificial extracellular matrix (ECM), such as Matrigel or noncanonical bioengineered supporting matrix, which allows the development of 3D organoids.7 PSCs, including induced PSCs (iPSCs) and embryonic stem cells, differentiate into liver organoids through 3 major stages3,9,10 (Figure 1). Compared with liver tissue–derived organoids, organoids generated with PSCs are more accessible and achieve comparable maturity in functional metabolism.3 Furthermore, PSCs-derived liver organoids present fetal characteristics before differentiation, opening avenues for the exploration of liver development. Nonetheless, attention should also be paid to the ethical issue in the use of embryonic stem cells as primary materials for liver organoid establishment.11
FIGURE 1.
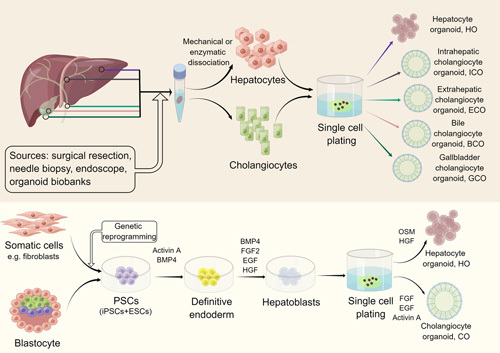
Scheme of liver organoid formation. Adult stem cells (ASCs), primary hepatocytes, or cholangiocytes could be obtained from primary liver tissue, intrahepatic/extrahepatic bile duct, gallbladder, or human bile by means of surgical resection, needle biopsy, and endoscope brushing. After mechanical or enzymatic dissociation, single hepatocytes or cholangiocytes are plated on ECM that contains exclusive growth factors and chemical molecules. In addition, liver organoids can be derived from PSCs, including iPSCs and ESCs. PSCs are firstly induced into definitive endoderm and then into hepatoblasts, which can differentiate into hepatocyte or cholangiocyte organoids with careful modulation of specific signaling pathway modeling liver development (relative growth factors in the medium are listed above). Abbreviations: BMP4, bone morphogenetic protein 4; ESCs, embryonic stem cells; iPSCs, induced pluripotent stem cells; PSCs, pluripotent stem cells.
ASCs include leucine-rich repeat-containing G protein–coupled receptor 5–positive (Lgr5+) hepatic progenitors12 and epithelial cell adhesion molecule–positive (EpCAM+) biliary epithelial cells.13 Hepatic epithelial cells, including primary cholangiocytes14,15 or primary hepatocytes,16,17 can be isolated from the human liver, intrahepatic14 or extrahepatic bile duct,15 gallbladder,15,18 or bile19–21 samples by surgical resections, needle biopsies, or endoscopic retrograde cholangiopancreatography brushing. Organoids derived from ASCs, primary hepatocytes, or cholangiocytes can be expanded in vitro before differentiating into liver organoids when cued with selected growth factors and small molecules22 (Figure 1). Liver tissue–derived epithelial organoids exhibit high-grade genetic stability and represent the tissue of origin, making them an attractive research tool for disease modeling,5 whereas tissue-derived liver organoids are limited in accessibility to human samples and have a relatively narrow differentiation capacity, although these organoids are expandable and could maintain mature functionality.3 Although transgenic reprogramming is needed for iPSCs, they are also promising alternatives for disease modeling, as they can be derived from nonhepatic cells, which circumvents the need for primary liver tissue that can only be obtained through invasive procedures.23
Liver organoids have rapidly advanced our knowledge of hepatic biology24 and serve as preclinical tools for drug screening and toxicity assessment,25 personalized treatment,26 regenerative medicine,27 and disease mechanism investigation28 in modeling of liver diseases (Figure 2). With substantial reviews dealing with the generation and application of liver organoids,5,25,27,29–32 we concentrate here on the advancements of liver organoids in modeling the variety of liver diseases. At the end of the review, the frontier advancements of liver organoids are addressed and outstanding questions are discussed.
FIGURE 2.
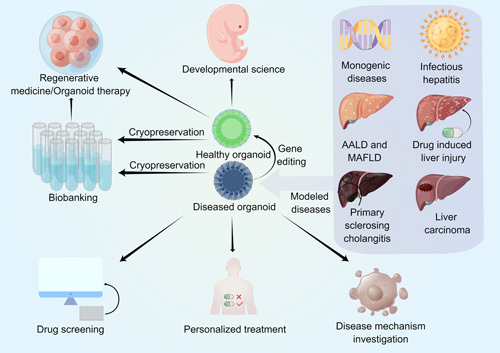
Biomedical applications of liver organoids. Healthy liver organoids derived from healthy donors can mimic organogenesis, and thus hold great promise for developmental science. Healthy organoid transplantation into injured individuals showed terrific therapeutic effects, indicating that healthy organoids are prospective in regenerative medicine. Both healthy organoids and diseased organoids derived from patients with different types of liver diseases, including monogenic liver diseases, infectious hepatitis, AALD, MAFLD, and liver carcinoma, could be cryopreserved as biobanks for further experiments such as disease mechanism investigation. Diseased organoids are also subjected to drug screening and toxicity testing, indicating great candidates for personalized treatment. Abbreviations: AALD, alcohol-associated liver disease; MAFLD, metabolic-associated fatty liver disease.
LIVER ORGANOIDS IN MODELING MONOGENIC AND/OR NEONATAL LIVER DISEASES
Alagille syndrome
Alagille syndrome (AGS) is a multisystem disorder caused by heterozygous mutations in the neurogenic locus notch homolog protein (Notch) signaling pathway, including Jagged1 (JAG1) and/or NOTCH2, which is manifested by bile duct paucity and chronic cholestasis.33 Utilizing biopsy from an AGS patient, Huch and colleagues constructed human 3D AGS organoids, displaying pronounced apoptosis of biliary cells in the organoid lumens.13 Andersson et al34 cultured handpicked bile duct fragments into liver organoids from JAG1 Ndr/Ndr mice and human controls and found that AGS organoids derived from JAG1 Ndr/Ndr mice showed delayed and uncontrolled differentiation and collapsed after a few days in culture, indicating structural rather than developmental defects (Table 1). To validate the role of Notch signaling in the bile duct–specific differentiation of liver organoids, Vyas et al68 added a Notch inhibitor to the culture medium and observed an attenuated maturation of bile duct structures and a significant reduction in the expression of transcription factors regulating bile duct development, implying a similar phenotype of AGS.
TABLE 1.
Summary of liver organoids modeling noncancer liver diseases
| Modeled disease | Species source(s) | Cell sources | Culture system | Modeled phenotypes | References |
|---|---|---|---|---|---|
| Alagille syndrome | Human | Liver biopsies from 3 AGS patients | Matrigel | Failure to upregulate biliary makers (CK19 and CK7); Inability to integrate into epithelium; Increased apoptosis in the organoid lumen |
13 |
| Alagille syndrome | Mouse | Bile duct fragment from JAG1 Ndr/Ndr mice | Matrigel | Disrupted bile duct morphogenesis and delayed differentiation | 34 |
| Alpha-1 antitrypsin deficiency | Human | Liver biopsies from AATD patients | Matrigel | A1AT protein aggregation, reduced A1AT protein secretion, and increased apoptosis within the differentiated organoids; Reduced ability of supernatants from differentiated organoids to block elastase activity |
13 |
| Alpha-1 antitrypsin deficiency | Human | Liver biopsies from ZZ, MZ AATD patients | Matrigel | Intracellular aggregation and lower secretion of A1AT protein; Lower expression of albumin and apolipoprotein B |
35 |
| Wilson disease | Canine | iPSCs from COMMD1-deficient dog models | Matrigel | Higher intracellular copper accumulation | 36,37 |
| Wilson disease | Human | Intrahepatic cholangiocytes from patients with Wilson disease | Matrigel | Increased sensitivity to copper treatment | 38 |
| Ornithine transcarbamylase deficiency | Human | iPSCs from a OTCD patient | Matrigel | Reduced urea cycle activity | 39 |
| Cystic fibrosis–associated liver disease | Human | iPSCs from CF patients | Matrigel | Formation of branched ductal structures and impaired FIS | 40 |
| Cystic fibrosis–associated liver disease | Human | iPSCs from a CF patient | Matrigel | Loss of function to regulate intracellular chloride concentration | 41 |
| Cystic fibrosis–associated liver disease | Human | Intrahepatic cholangiocytes from CF patients | Matrigel | Impaired FIS | 38 |
| Wolman disease | Human | iPSCs from Wolman disease patients | Matrigel | Prominent steatosis and fibrosis | 42 |
| Polycystic liver disease | Human | iPSCs from a PLD patient | Matrigel | Increased organoid size responsive to secretin and decreased organoid size responsive to octreotide and somatostatin | 41 |
| Biliary atresia | Human | Liver biopsies from BA patients | Matrigel | Lack of basal positioning nucleus, misorientation of cilia, and lower expression of ZO-1; Lower expression of developmental and functional markers; Increased permeability; Aberrant expression of F-actin, β-catenin, and Ezrin |
43 |
| Biliary atresia | Human | EPCAM+ cells from liver biopsies of BA patients | Matrigel | Aberrant morphology and apical-basal organization; Beta-amyloid deposition around bile duct |
44 |
| Biliary atresia | Mouse | Murine neonatal extrahepatic cholangiocytes | Collagen-Matrigel mixture | Disrupted cellular polarity and increased permeability of biliary epithelium | 45 |
| Biliary atresia | Human | Fetal liver, adult liver, and bile duct | Matrigel | Morphological changes consistent with BA | 46 |
| Methylmalonic acidemia | Human | Intrahepatic cholangiocytes from MMA patients | Matrigel | Increased propionylcarnitine concentration compared with controls | 38 |
| Alcohol-associated liver disease | Human | EtOH-treated hFLMC/hEHO | Matrigel | Enhanced oxidative stress; Responsive injury and fibrogenesis to EtOH treatment; Upregulation of genes encoding the lipogenic-associated enzymes and transcription factors |
47 |
| NAFLD | Feline | FFA-treated liver organoids derived from ASCs | Matrigel | Increased intracellular lipid accumulation; Upregulation of PLIN2, CPT1A, and PPARG |
48,49 |
| NAFLD | Human | FFA-treated liver organoids derived from PSCs | Matrigel | Increased intracellular lipid accumulation; Increased metabolites regarding lipid metabolism; Enriched gene sets for fatty acid metabolism |
3 |
| NAFLD | Human | Oleic acid-treated liver organoids derived from healthy and diseased PSCs | Matrigel | Increased intracellular lipid accumulation; Enlarged cells size and hepatocyte ballooning; Overexpression of inflammatory cytokines |
42 |
| NAFLD | Human | Lactate, pyruvate, and octanoic acid-treated liver organoids derived from intrahepatic cholangiocytes | Matrigel | Increased intracellular lipid accumulation, triglyceride, diacylglycerol, and glucose level; Distinct mitochondrial impairment |
2 |
| NASH | Mouse | Liver organoids derived from MCD diet-induced NASH mice models | Matrigel | Upregulation of collagen I and α-SMA; Occurrence of EMT |
50 |
| NASH | Human | Liver organoids derived from liver tissue of NASH patients | Matrigel | Increased intracellular lipid accumulation | 51 |
| Hepatitis B | Human | iPSCs | Microwell culture system | Upregulation of HBV infection-promoting factors, including NTCP, glypican 5, PPARA, and CEBPA; Permissiveness of HBV infection and progeny HBV propagation; Hepatic dysfunction after HBV infection |
52 |
| Hepatitis B | Human | Differentiated liver progenitor-like cells | Matrigel | Expression of host factors essential to HBV entry and replication, including NTCP, RXRA, and HNF4A; Permissive of HBV infection |
53 |
| Hepatitis B | Human | ASCs from HBV-infected patients | Matrigel | Expression of NTCP; Permissiveness for HBV infection and replication, and progeny HBV propagation |
54 |
| Hepatitis C | Human | iPSC-derived hepatic progenitors | Inverted colloid crystal scaffold | Expression of proteins responsible for HCVcc entry and HCV packaging; Permissiveness for genotype 2a HCV reporter virus infection |
55 |
| Hepatitis E | Human | ASCs from human adult and fetal liver | Matrigel | Permissiveness for HEV infection and replication; Activation of innate defense; Positive response to recombinant IFN-α |
56 |
| COVID-19 | Human | Human PSC-derived adult hepatocyte and cholangiocyte. | Matrigel | Expression of ACE2; Permissiveness for SARS-CoV-2 infection; Upregulation of chemokines and relative inflammatory pathways similar as primary pulmonary autopsy samples from patients with COVID-19 |
57 |
| COVID-19 | Human | Bile duct–derived progenitor cells | Matrigel | Expression of ACE2 and TMPPSS2; Significant cholangiocyte tropism of SARS-CoV-2 infection; Disrupted cholangiocyte barrier and bile acid transportation |
58 |
| Hepatic Plasmodium infection | Human and simian | Primary hepatocytes | Macroporous cellusponge | Expression of CD81; Maturation of schizonts and hypnozoites of Plasmodium cynomolgi and Plasmodium vivax into blood-invasive merosomes |
59 |
| Drug-induced liver injury | Human | iPSCs and ESCs | Matrigel | Higher expression and induction rate of CYP450 enzymes (CYP3A4, 1A2, 2C9); Hepatic injury caused by APAP |
60 |
| Drug-induced liver injury | human | iPSCs | Perfusable chip system | Higher expression and induction rate of CYP450 enzymes (CYP3A4, 2C9, 2B6); Hepatic injury caused by APAP |
61 |
| Drug-induced liver injury | Human | iPSCs and ESCs | Matrigel | Higher expression of CYP450 enzymes (CYP3A4, 1A2, 2A6, 2E1); Hepatic injury caused by APAP, trovafloxacin, and troglitazone |
3 |
| Drug-induced liver injury | Human | iPSCs and ESCs | 384-well based high-speed live imaging platform | Expression of CYP450 enzymes (CYP3A4, 1A2, 2C9, 7A1); Hepatic response to 283 drugs |
62 |
| Drug-induced liver fibrosis | Human | HepaRG cell line and iPSC-derived HSCs | Matrigel | Expression of CYP450 enzyme (CYP3A4); APAP-induced HSC activation, collagen secretion, and deposition |
63 |
| Drug-induced phospholipidosis | Human | ASCs | Matrigel | Expression of CYP450 enzyme (CYP3A4); Upregulation of LAMP2 and presentation of characteristic lamellar bodies with exposure to amiodarone, sertraline, and amikacin |
64 |
| Primary sclerosing cholangitis | Human | Cholangiocytes from patients with primary sclerosing cholangitis | Matrigel | Cellular senescence and macrophage accumulation; Smaller size and slower growth rate compared with normal controls; Lack of central lumens |
65 |
| Primary sclerosing cholangitis | Human | Cholangiocytes from bile, common bile duct, and liver explant of patients with primary sclerosing cholangitis | Matrigel | Primary sclerosing cholangitis-related autoimmune dysregulation | 20 |
| Primary sclerosing cholangitis | Human | Cholangiocytes from bile of patients with primary sclerosing cholangitis | Matrigel | Reactive immune phenotypes under inflammatory stimuli | 21 |
| Hyperuricemia | Human | Primary human hepatocyte from paracarcinomatous liver tissue | Matrigel | Higher uric acid level in the supernatant of HUA organoids; Sensitive to allopurinol |
66,67 |
Abbreviations: A1AT, alpha-1 antitrypsin; AATD, alpha-1 antitrypsin deficiency; ACE2, angiotensin I converting enzyme 2; AGS, Alagille syndrome; APAP, acetaminophen; ASC, adult stem cell; BA, Biliary atresia; CEBPA, CCAAT/enhancer-binding protein alpha; CF, cystic fibrosis; CFLD, cystic fibrosis–associated liver disease; CK, cytokeratin; COMMD1, copper metabolism domain containing 1; CPT1A, carnitine palmitoyl transferase 1A; CYP, cytochrome; EMT, epithelial-mesenchymal transition; EPCAM, epithelial cell adhesion molecule; ESC, embryonic stem cell; EtOH, ethanol; FFA, free fatty acid; FIS, forskolin-induced swelling; HCVcc, cell cultured HCV; hFLMC/hEHO, human fetal mesenchymal cells/human ESC-derived hepatic organoids; HNF4A, hepatocyte nuclear factor 4 alpha; HUA, hyperuricemia; IFN-α, interferon-alpha; iPSC, induced pluripotent stem cells; JAG1, jagged1; LAMP2, lysosome-associated membrane protein 2; MCD, methionine deficient and choline deficient; MDS, mitochondrial DNA depletion syndrome; MMA, methylmalonic acidemia; NTCP, sodium-taurocholate cotransporting polypeptide; OTCD, ornithine transcarbamylase deficiency; PLD, polycystic liver disease; PLIN2, perilipin 2; PPARA, peroxisome proliferator-activated receptor alpha; PPARG, peroxisome proliferator-activated receptor gamma; RXRA, retinoid X receptor A; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TMPPSS2, transmembrane serine protease 2; ZO-1, zona occludens-1; α-SMA, alpha-smooth muscle actin.
Alpha-1 antitrypsin deficiency
Alpha-1 antitrypsin (A1AT) deficiency (AATD) is a genetic disorder resulting from mutations in the A1AT gene (SERPINA1) encoding the serine protease inhibitor family A member 1. The most common, nondisease-causing mutation is named the “M” allele, and the most frequent disease-associated mutations are referred to as the “S” and “Z” alleles.69 A1AT is produced primarily by hepatocytes and acts as a serine protease inhibitor, protecting against tissue damage and destruction caused by neutrophil elastase. Patients with AATD have low serum A1AT levels and manifest with hepatic dysfunction and chronic obstructive lung disease.70 Biopsies from 3 patients with AATD were used to generate organoids, in which A1AT protein aggregation, reduced protein secretion, and decreased elastase inhibition seemed similar to matched donor tissue.13 Furthermore, Gómez-Mariano and colleagues established and compared organoids with normal, MM, and deficient MZ and ZZ genotypes. They observed the reduced expression of hepatocyte markers, such as albumin, apolipoprotein B, and SERPINA1, and higher intracellular Z-A1AT polymers in ZZ and MZ organoids35 (Table 1), suggesting that liver organoids are bona fide models of AATD phenotype.
Wilson disease
Wilson disease, caused by mutations in the ATP7B gene, is a rare autosomal recessive disorder characterized by excessive copper accumulation in the liver and brain, leading to severe hepatic and subsequent neurological dysfunction.71 ATP7B interacts with the copper metabolism domain containing 1 (COMMD1), a protein that is mutated in Bedlington terriers with hereditary copper toxicosis similar to human Wilson disease.72 Nantasanti et al36 generated organoids from livers of these dogs with COMMD1 deficiency, which exhibited higher copper accumulation. With Dsred-expressing lentiviral vectors, the COMMD1 gene was transferred to COMMD1-deficient organoids, which normalized the intracellular copper concentrations.36 Furthermore, 24 hours after the exposure to copper, COMMD1-deficient organoids exhibited reduced cell viability, whereas gene-corrected and wild-type organoids were not affected.36 COMMD1-deficient dogs undergoing autologous intraportal transplantation of single cells derived from gene-corrected organoids survived up to 2 years.37 Notably, intrahepatic cholangiocyte organoids (ICOs) have been developed with biopsy sample of 1 patient with ATP7B mutation, and ICOs displayed reduced cell viability after copper treatment38 (Table 1).
Urea cycle disorders
The liver converts excess ammonia to urea through the urea cycle, and congenital defects in urea cycle enzymes or transporters can lead to urea cycle disorders. Defects in 2 enzymes of the urea cycle, ornithine transcarbamylase (OTC) and argininosuccinate synthetase 1 (ASS1), result in OTC deficiency (OTCD) and citrullinemia type 1 (CT1), respectively.73 Matsumoto and colleagues generated iPSC-derived organoids from a severe OTCD patient and subsequently corrected the mutation through CRISPR/Cas939 (Table 1). The gene-edited organoids showed significant improvement in urea production.39 The expression level of 26 key genes of hepatocytes was evaluated at different stages. However, except for OTC and HNF4α, no significant differences in gene expression between gene-edited iPSC-derived organoids and their unedited counterparts were observed, which might be because of the instability of the mutant transcript in unedited iPSC organoids.39 To assess the role of an ASS1 mutation in liver organoids, Akbari and colleagues transfected CT1 patient-derived iPSCs with vectors containing full-length ASS1 cDNA or empty control vectors and thereby generated corresponding transgenic organoids. Higher ammonia concentrations and lower ureagenesis levels were detected in conditioned media of CT1 organoids, and these CT1 phenotypes were rescued to normal levels by the re-expression of wild-type ASS1 in the CT1 organoids.74
Cystic fibrosis–associated liver disease
Cystic fibrosis (CF) is caused by mutations in the CF transmembrane conductance regulator (CFTR), which helps to maintain the balance of salt, particularly chloride, and water on many surfaces in the body.75 Defective chloride channels lead to various epithelial dysfunctions, including the biliary epithelium.75 Patients with CF-associated liver disease (CFLD) are often present with hepatic dysfunction due to biliary cirrhosis.76 hPSC-derived cholangiocyte organoids could form epithelialized cystic structures, which express markers found in mature bile ducts, including the CFTR.40 Those cholangiocyte organoids displayed epithelial functions, such as CFTR-mediated fluid secretion, validated by the regulation of cyst swelling. Forskolin, an activator of the adenosine 3′, 5′- cAMP pathway, is widely used to induce swelling in the cyst, which could functionally indicate CFTR function and provide insights for the CF organoid studies. CF organoids were formed by iPSC-derived cholangiocytes from patients homozygous for the most common CFTR F508del mutation and liver samples from a patient compound heterozygous for CFTR F508del and R1162X40,41 (Table 1). Because of CFTR mutations, impaired forskolin-induced swelling occurred in these CF organoids, which indicated the malfunction of CFTR to transport chloride and corresponded to bile viscosity seen in CF patients.38,40,41 CFTR correctors (VX-809 and VX-661) stabilize CFTR by filling into a hydrophobic pocket in the first transmembrane domain and linking together 4 helices that are thermodynamically unstable.77 CFTR potentiator (VX-770) binds at the site involved in channel gating and stabilizes the open conformation of CFTR.78 A relevant clinical trial has been successfully completed.79,80 As expected, impaired forskolin-induced swelling in human CF organoids was salvageable by a combination of CFTR correctors (VX-809 and VX-661) and CFTR potentiator (VX-770).38,40,41
Wolman disease
Wolman disease is caused by mutations in the enzyme lysosomal acid lipase (LAL) that hydrolyzes cholesteryl esters and triglycerides. Hepatocytes from patients with Wolman disease have a massive accumulation of cholesteryl esters and triglycerides, accompanied by lethal steatohepatitis and fibrosis.71 Wolman disease–specific iPSC-derived multicellular organoids composed of hepatocyte-like, stellate-like, and Kupffer-like cells showed prominent steatosis, aggressive fibrosis, and increased stiffness, consistent with the clinical presentation of Wolman disease42 (Table 1). Increased lipid accumulation in the hepatocyte could be rescued by exposure to recombinant LAL protein.42 Importantly, FGF19 could ameliorate steatosis and fibrosis in the Wolman organoids by alleviating oxidative stress.42
Polycystic liver disease
iPSC-derived organoids were established from polycystic liver disease (PLD) patients. In PLD, increased cholangiocyte proliferation and fluid secretion are key features, and cholangiocyte cAMP is an important regulator of these processes. Octreotide is a synthetic analog of somatostatin known to inhibit cAMP and clinically used to suppress and/or revere PLD progression41 (Table 1). Importantly, octreotide treatment significantly reduced the size of PLD organoids and reproduced the effects of the drug in vitro.41
Biliary atresia
Biliary atresia (BA) is an obstructive cholangiopathy often occurring in neonates and involving both extrahepatic and intrahepatic bile ducts.81 Cholangiocyte organoids derived from liver biopsies of infants with BA exhibited halted development, reduced the expression of developmental and functional markers (Cytokeratin 7, CK7; EpCAM; transporters Aquaporin 1, AQP1; CFTR; Somatostatin receptor 2, SSTR2), reduced the population of ciliated cells, abnormal cell polarity, and increased epithelial permeability.43,44 Furthermore, it has been reported that the FGF2 and EGF promote the maturation of BA organoids and repair the cellular defects when added to the organoid culture medium.43 Biliatresone, a plant toxin, resulted in monolayer disruption and lumen obstruction of mouse extrahepatic cholangiocyte organoids, phenotyping the pathology of BA.45,82 Beta-amyloid, mainly recognized in Alzheimer disease, has been shown to accumulate around BA organoids and beta-amyloid treatment–induced aberrant morphology and transcriptional changes reminiscent of BA organoids over normal organoids44 (Table 1). Moreover, cholangiocyte organoids were sensitive to rotavirus and supported the entire life cycle of rotavirus. Rotavirus-infected organoids were shriveled and disorganized, partially recapitulating the development of BA. In addition, mycophenolic acid, interferon-alpha, and monoclonal neutralizing antibody targeting rotavirus VP7 protein could prevent cytopathogenesis of rotavirus-infected cholangiocyte organoids.46 Biliatresone-induced, beta-amyloid-induced, and rotavirus-induced organoids that recapitulated BA, to some extent, might contribute to the identification of key cellular and molecular targets involved in epithelial injury and bile duct obstruction. In contrast, these organoids are in a dilemma to fully feature BA as their phenotypes might come from different pathological mechanisms.
Methylmalonic acidemia
ICOs have been generated from liver tissues from methylmalonic acidemia patients.38 Propionylcarnitine was an intermediate product of branched-chain amino acid metabolism and was used as a clinical biomarker. Compared with controls, expanded ICO cells and culture media exhibited a significant increase in propionylcarnitine38 (Table 1).
LIVER ORGANOIDS IN MODELING ALCOHOL-ASSOCIATED LIVER DISEASE
Alcohol-associated liver disease is a complex process that includes a wide spectrum of hepatic lesions from steatosis to cirrhosis, even severe liver failure. It is characterized by oxidative stress, disturbed metabolism, inflammation, modifications in the regeneration process, and bacterial translocation.83 By incorporating hepatic stellate cells (HSCs substitute and human fetal mesenchymal cells (hFLMCs) into human embryonic stem cells–derived hepatic organoids (hEHOs), Wang et al47 created a coculture hepatic multitissue organoid, referred to as hFLMC/hEHO, that carries both alcohol dehydrogenase and cytochrome P450 (CYP450) protein 2E1 (CYP2E1), which metabolizes ethanol (EtOH) to acetaldehyde accompanied by ROS production, further inducing oxidative stress and steatosis in AALD (Table 1). After EtOH treatment, enhanced activity of CYP2E1, responsive damage, such as upregulated secretion of alanine aminotransferase, aspartate aminotransferase, and lactate dehydrogenase, and reduced organoid viability were observed, and hFLMC/hEHO displayed enhanced fibrogenesis and enhanced oxidative stress.47 Moreover, excessive fat accumulation, upregulation of genes encoding lipogenic-associated enzymes and transcription factors, as well as activated proinflammatory IL-1 and IL-17 signaling were observed in hFLMC/hEHO compared with controls without EtOH treatment.47
LIVER ORGANOIDS IN MODELING METABOLIC ASSOCIATED FATTY LIVER DISEASE
There has been a significant rise in the prevalence of metabolic associated fatty liver disease (MAFLD) with complex pathogenesis. The need for effective and safe therapy has spurred the development of both in vivo and in vitro models to elucidate the disease pathogenesis and provides insights for the identification of potential treatment targets and preclinical tests of the therapeutic efficacy of drugs.84 Liver organoids have extended the map of the MAFLD models. Liver organoids have been generated from different species, including cats,48,49 dogs,48 mice,2,50 and humans,2,3,42 and used to model MAFLD (Table 1). Kruitwagen et al48 established the long-term feline liver organoids, which, compared with their mouse, human, and dog counterparts, exhibited more pronounced intracellular lipid accumulation when treated with excess free fatty acids. Moreover, free fatty acid–treated organoids exhibited typical MAFLD features, including a distinct increase in the metabolites from lipid metabolism, inflammatory cytokines, intracellular lipid droplets3,42,48 and hepatocyte ballooning, and a fibrogenic response.42 They found that supplementation with T863 (a diacylglycerol O-acyltransferase 1 inhibitor) and 5-aminoimidazole-4-carboxamide 1-β-D-ribofuranoside (an AMP kinase activator) significantly alleviated lipid (triacylglycerol) accumulation and enhanced the viability of lipid-loaded hepatic organoids.48,49 Although liver organoids aided in the approaches to explore potential drug targets and detect the toxicity of potential therapeutic compounds for MAFLD, the application of this system was limited because of the failure to replicate the multicellular milieu of the intact liver.
A subset of patients with MAFLD will progress to NASH, characterized by inflammation and fibrosis that usually progresses to cirrhosis and predisposes to HCC.85 In the search for a diagnostic marker for NASH, Elbadawy et al50 generated NASH organoids from mice fed a methionine-deficient and choline-deficient diet and found that Areg (amphiregulin) and Igf2bp2 (insulin-like growth factor 2 mRNA-binding protein 2) were the promising diagnostic biomarkers for NASH, as they were specifically elevated in all NASH organoids compared with those from control mice. Nonetheless, these NASH findings in animal organoid models might not correspond to the findings in humans. Thus, future studies are needed to clarify the efficacy of these genes in the diagnosis of NASH, because mouse models cannot fully recapitulate the pathological mechanism underlying human NASH disease. Notably, McCarron et al51 demonstrated that human organoids generated from irreversibly damaged NASH livers exhibited distinct upregulation of inflammation, liver fibrosis, and tumor biomarkers. Intriguingly, NASH organoids exhibited significant permissiveness to the severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2) vesicular stomatitis pseudovirus, which might play a role in NASH-related COVID-19.51
LIVER ORGANOIDS IN MODELING INFECTIOUS HEPATITIS
Hepatitis B/C/E
Liver organoids derived from hiPSCs have been shown to be highly susceptible to HBV infection,52 as are liver organoids generated from primary hepatocytes.53,54 Liver organoids displayed high expression of the HBV entry receptor, the sodium-taurocholate cotransporting polypeptide52–54 (Table 1). Replication and production of the infectious progeny HBV by organoids have also been observed when challenged by the sera of HBV-infected patients.52,54 Moreover, HBV-induced hepatic dysfunction of liver organoids was decreased by treatment with myrcludex (an HBV entry inhibitor) and entecavir (an anti-HBV nucleotide).52 In addition to in vitro infection of originally HBV-free liver organoids, also HBV-containing organoids were generated from HBV-infected patients.53,54 Fu et al53 validated the potency of entecavir and Cas9/HBV-single guide RNAs (targeting HBV covalent closed circle DNA) either independently or in combination to prevent the rebound of HBV replication in hepatitis B patient–derived organoids. De Crignis et al54, by transcriptome analysis of patient-derived liver organoids, identified a novel early gene signature for liver cancers, providing a potential biomarker to surveil and prevent the progression from HBV infection to HCC. In addition, human liver organoids might serve as a promising preclinical platform to monitor the hepatotoxicity caused by drugs used to treat hepatitis B.54.
Liver organoids, demonstrating a high expression of HCV entry-related proteins (scavenger receptor, class B, type 1; cluster of differentiation 81, CD81), were highly permissive to HCV infection, while 2D cells were less susceptible to HCV infection55 (Table 1).
Human liver–derived hepatocyte and cholangiocyte organoids have been shown to support HEV replication throughout the life cycle56 (Table 1). HEV infection impacted liver organoids mildly on morphological characteristics but robustly on metabolic response, especially in the IFN signaling pathway, which mattered in the early innate immune defense against a broad range of viruses.56 After screening HEV-infected liver organoids with anti-HEV drugs, brequinar and homoharringtonine were identified as potent inhibitors of HEV infection, which were also effective against the ribavirin-resistant variant harboring the G1634R mutation.56
COVID-19–induced hepatitis
SARS-CoV-2, which caused the COVID-19 pandemic, typically leads to severe respiratory failure, but sometimes also affects other organs like liver, heart, and kidney.86 Yang et al57 showed that human PSC (hPSC)–derived hepatocyte and cholangiocyte organoids expressing angiotensin 1 converting enzyme 2 (ACE2, a putative receptor of SARS-CoV-2) were highly permissive to SARS-CoV-2 infection. SARS-CoV-2–infected cholangiocyte organoids induced a robust increase in secreted chemokines and upregulation of inflammatory pathways, as also seen in the primary human COVID-19 pulmonary biopsy specimens.57 In addition, Zhao et al58 reported the existence of a proportion of ACE2+/transmembrane serine protease 2+ (TMPPSS2+) cholangiocytes in bile duct organoids, to which the SARS-CoV-2 displayed notable tropism (Table 1). Notably, SARS-CoV-2 deteriorated the bile duct cell barrier and bile acid transport by regulating the expression of tight junctions and bile acid transport–related genes and stimulating the expression of several key apoptotic factors. This indicated that hepatic dysfunction in COVID-19 patients was partly due to the direct cholangiocyte injury caused by SARS-CoV-2.58
Protozoan parasite–induced hepatitis
Liver organoids were capable of demonstrating the intact liver stage of Plasmodium cynomolgi and Plasmodium vivax and have been validated as predictive platforms for antiplasmodial and antirelapse drug screening59 (Table 1).
LIVER ORGANOIDS IN MODELING DRUG-INDUCED LIVER INJURY
DILI is a hepatic adverse reaction to drugs or other chemical agents.62 hPSC-derived liver organoids expressed a phase II detoxification enzyme at the liver tissue level3 and modulated the DILI of acetaminophen (APAP),60,61 trovafloxacin, and troglitazone.3 Importantly, Shinozawa et al62 designed a liver organoid–based toxicity screening (LoT) assay to analyze the hepatoxicity of 238 marketed drugs (32 negative controls and 206 reported DILI agents) and showed high predictive values of the LoT system with 88.7% sensitivity and 88.9% specificity. The results of the LoT system were comparable to or higher than those of primary hepatocyte-based models.62 Furthermore, liver organoids emulated DILI susceptibility in individuals in different disease contexts. For example, liver organoids exposed to troglitazone after unsaturated fatty acid treatment exhibited substantial fragmentation due to cell death, recapitulating potential DILI in the context of MAFLD.62 In addition, Howell et al87 generated murine hepatocyte organoids that expressed a variety of drug metabolism and transport proteins required for drug metabolisms, such as CYP3A, glutathione-S-transferase alpha, and multidrug resistance protein 1A. Despite there being continual increased CTP3A4 activity in iPSC-derived hepatic organoids after a month of culture, fetal markers such as AFP and CYP3A7 remained highly expressed in the organoids, which might lead to the deviation of drug assessment results.9
Aside from acute DILI, Leite et al88 developed liver organoids as a readout to model chronic drug-induced liver fibrosis by analyzing the gene expression associated with HSC activation, as well as collagen accumulation in the liver organoids after repeated exposure to fibrotic compounds, such as methotrexate and allyl alcohol. Intriguingly, APAP, which causes acute liver injury, could also result in chronic liver fibrosis through the activation of HSCs.63,88 In addition, ASC-derived liver organoids treated with amiodarone, sertraline, or amikacin showed significant upregulation of lysosome-associated membrane protein 2, lamellar bodies, and reduction of cell viability, as manifested in drug-induced phospholipidosis, a phospholipid storage disease caused by the formation of phospholipid-drug complexes in lysosomes64 (Table 1).
LIVER ORGANOIDS IN MODELING PRIMARY SCLEROSING CHOLANGITIS
Primary sclerosing cholangitis (PSC) is a progressive fibroinflammatory biliary disorder characterized by multifocal bile duct strictures and dilations, and distressfully, it lacks definitive etiology and efficient pharmacotherapy.89 Cholangiocyte senescence and immune responsiveness have been reported to be predominant phenotypes of PSC.89,90 Loarca and colleagues established senescent cholangiocyte organoids with senescent cholangiocytes, or cholangiocytes from patients with PSC, and secretory/normal controls with healthy human cholangiocytes. Senescent cholangiocyte organoids responded negatively to secretin stimulation, whereas secretory controls enlarged in the luminal area after secretin stimulation.65 Compared with secretory organoids, senescent organoids showed higher expression of cellular senescence markers, smaller size, slower growth rate, and more significant macrophage accumulation. This recapitulated the cellular senescence and immune responsiveness in the primary sclerosing cholangitis.65 Notably, half of the senescent organoids and all of the PSC-derived organoids failed to form central lumens while secretory controls developed integrated lumens.65 Soroka et al20,21 extracted human cholangiocyte organoids from bile specimens of PSC patients who underwent diagnostic or therapeutic endoscopic retrograde cholangiopancreatography (Table 1). On the basis of the methodology of Soroka and colleagues, Reich et al91 constructed human bile-derived organoids and found that IL8 stimulation declined the expression of biliary epithelium–protective Takeda G protein–coupled receptor-5 (TGR5) mRNA, indicating the pathogenesis of PSC.
LIVER ORGANOIDS IN MODELING HYPERURICEMIA
The liver is the major organ for purine anabolism and uric acid production, and hyperuricemia (HUA) is a metabolic disorder resulting from abnormal purine metabolism.66 Hou et al66,67 established liver organoids from human paracarcinomatous tissue and found a high expression of key enzymes of purine metabolism, including adenosine deaminase and xanthine oxidase. Then, they induced liver organoids to HUA organoids by the xanthine oxidase substrate xanthine. A higher uric acid level in the supernatant of HUA organoids was detected, and allopurinol, the most common drug for gout, significantly lowered uric acid production by HUA organoids66,67 (Table 1). Puerarin was reported to have obvious therapeutic effects on HUA.92 In a range of concentrations, the puerarin66 and 2 histidine dipeptides, carnosine and anserine,67 had uric acid–lowering effects with HUA organoids.
LIVER ORGANOIDS IN MODELING LIVER CANCERS
Primary liver cancer (PLC), mainly comprising HCC and cholangiocarcinoma, accounts for the second highest cancer-related mortality worldwide.93 Liver cancer organoids, also termed “tumoroids,” provide an amenable system to study the occurrence and progression of PLC, as well as a drug-testing platform (Table 2). From surgically resected PLC tissues, Broutier et al,94 Li et al,95 and Wang et al96 have successfully established organoids that in part preserve the histological characteristics, tumorogenic and metastatic properties, and genomic and transcriptomic landscapes of the parental tumor tissues even after long-term expansion in vitro, thereby facilitating patient-specific drug testing. PLC organoids generated by diagnostic needle biopsies of PLC patients and diethylnitrosamine-induced murine liver tumors have also been reported.97 However, establishing PLC organoids from very well-differentiated liver tumor tissues (with <5% of proliferating cells) remained infeasible so far.5,94 In addition, organoids of hepatoblastoma, the most common childhood liver cancer, have been successfully developed in the dish with freshly resected patient tumors for drug screening.98 Coculture systems with other hepatic cell types, such as endothelial cells and cancer-associated fibroblasts (CAFs), allowed the investigation of the tumor microenvironment as well.99,100 Importantly, Neal and colleagues reported PDOs from tumor biopsies consisting of bile duct ampullary adenocarcinoma. The PDOs preserved diverse immune cells, including T, B, natural killer, natural killer T cells, and macrophages, as well as fibroblast stroma. These PDOs faithfully recapitulated the TCR repertoire of parental tumor tissue and immune checkpoint blockade in vitro.101
TABLE 2.
Summary of liver organoids modeling primary liver cancer
| Species source(s) | Cell sources | Culture system | Applications | Limitations | References |
|---|---|---|---|---|---|
| Human | Surgically resected PLC tissues | Matrigel | Identification of potential prognostic biomarkers and therapeutic targets; Patient-specific drug sensitivity testing |
Paucity of immune system and stromal components | 94–96 |
| Human | Needle biopsies of PLC | Matrigel | Potential attribution to establish patient-specific liver cancer organoid biobank and develop tailored medicine | Poor success rate to establish HCC organoids | 97 |
| Human | Surgically resected hepatoblastoma tissues | Matrigel | Medium-throughput drug screening; Individualized drug sensitivity testing |
Relatively low yield rate | 98 |
| Human | HCC cells, primary fibroblasts, microvascular endothelial cells | Matrigel | Demonstration of the role of nonparenchymal cells to support liver tumor organoids | No further investigation of possible therapeutic target in the signaling pathway to do with nonparenchymal cells | 99 |
| Human and mouse | CAFs and PLC cells | Matrigel | Anticancer drug sensitivity testing | Paucity of immune cells in the cocultures | 100 |
Abbreviations: CAF, cancer-associated fibroblast; CC, cholangiocarcinoma; PDX, patient-derived xenograft; PLC, primary liver cancer; TIC, tumor initiating cells.
CONCLUSIONS
Liver organoids are now robustly established models for diverse liver diseases, including monogenic liver diseases, alcohol-associated liver disease, MAFLD, various types of hepatitis, and liver cancers, to study the pathogenesis, immune interaction, drug toxicity, and drug efficacy in a personalized manner. In addition, PDOs competent for long-term deposition in liquid-nitrogen or −80 °C environment21,65 are eligible to constitute a valuable biobank for preclinical and/or clinical research. This was extremely vital to the research on rare liver diseases such as monogenic inherited liver diseases due to the rarity of the primary tissue materials. Owning stable genomic recapitulation of in vivo tissues, liver organoids manipulated by gene-editing technology, including but not limited to CRISPR/Cas9, also holds great promise for investigating selected gene function in various liver diseases.17,102,103 Recently, large-scale preclinical and even clinical trials were designed to validate the efficacy and safety of drugs62,104 based on the high-throughput liver organoids, which would boost the prospect of liver organoids serving as an exciting platform for the pharmaceutical industry to minimize the potential for DILI. The successful generation of liver organoids that combine hepatocytes and cholangiocytes with hepatobiliary connections would further the drug development.105 Despite the visible progress of liver organoids, certain issues still remain.
hPSC-derived organoids have been reported to be less mature.106 Although hPSC-derived liver organoids showed the key features of mature hepatocytes such as significant albumin secretion, potent mitochondrial respiration, enriched gene sets involved in the drug metabolism, bile acid, and lipid metabolism,3 PSC-derived organoids retain a fetal-like state even in prolonged culture, which might be explained by the culture conditions that support both hepatic and biliary cells, hindering the further maturation of these cells.9 Similarly, adult liver organoids were also positive for progenitor markers and early hepatocyte specification markers, indicative of cell immaturity.48 In some cell clusters within single organoids, mature hepatocyte markers such as albumin were negative, indicating that there were different maturation levels within an organoid.48 It has been found that the gut microbiome or microbiome-derived metabolites enhanced the maturity of PSC-derived liver organoids, which could partially promote the terminal differentiation of hepatocytes.107
As is known, a pathological microenvironment plays a crucial role in disease development. However, most of the hitherto reported liver organoids comprise only hepatic parenchymal cells (hepatocytes and/or cholangiocytes), limiting those liver organoids to modeling disease components such as the interaction of other nonparenchymal cells (such as hepatic stellate cells, kupffer cells, and immune cells) in the pathogenesis of specific liver diseases. Indeed, coculture systems containing HSCs63,88,108 and Kupffer cells,42,109 but still without adaptive immune components, have been reported. Hopefully, Neal et al101 reported that PDOs might direct further incorporation of immune cells and nonimmune stromal elements into organoids to advance the pathological interpretation of the liver disease microenvironment.
Despite robust development in the last decade, the liver organoid technique has several unsolved questions, including the following: (1) experimental liver organoids particularly used for drug screening are constrained by commercially expensive growth factors and significant methodology variations, including various tissue sources or cell lines, different organoid protocols among different labs, interbatch or intrabatch effect; (2) there is a lack of advanced liver organoid differentiation protocols for obtaining more complex integrity of liver organoids en bloc with multiple cell types and de novo vasculature; (3) given the immunological disadvantage of Matrigel, which is extracted from the ECM of the Engelbreth-Holm-Swarm mouse sarcoma and negatively impacts the therapeutic application of organoids, alternative xenofree bioengineered materials such as polyethylene glycol hydrogel enzymatically cross-linked by the activated transglutaminase factor XIIIa, polyisocyanopeptides, and their derivatives have been developed for the safe application of liver organoids in regenerative medicine.110 Also, a nanofibrillar hydrogel with controllable stiffness for organoid culture was developed to overcome the complex and heterogeneous ingredients of Matrigel.111 However, the current knowledge of xenofree hydrogels is still limited, and biocompatible and immuotolerant biomaterials are in need to be developed to support liver organoid formation for clinical approaches.
Collectively, liver organoids hold vital promise to model various liver diseases and recapitulate their pathological characteristics at different stages. Revising the methodologies, reinforcing the supporting materials, and incorporating multiple cell types will make liver organoids a leading platform from bench to bedside (Table 3).
TABLE 3.
Summary of liver organoids modeling secondary liver cancers
| Original tumor sources | Species source | Cell sources | Culture system | Applications | Limitations | References |
|---|---|---|---|---|---|---|
| CRC | Human | CRLM samples | Matrigel | Patient-specific anticancer drug screening | Incomplete recapitulation of CRLM phenotypes | 97 |
| CRC and GOC | Human | Liver metastases from CRC and GOC | Matrigel | Anticancer drug sensitivity testing | Restrained experimental scale | 98 |
| CRC | Human | CRLM samples | Matrigel | Drug screening; Individualized treatment |
Lack of coclinical trails | 99 |
| Rectal cancer | Human | Surgically resected initial and recurrent liver metastases from rectal cancer | Matrigel | Spatio-temporal pharmacogenomic analysis; Drug screening |
Inconsistent drug response of PDOs and liver metastases | 100 |
Abbreviations: CRC, colorectal carcinoma; CRLM, CRC liver metastases; GOC, gastroesophageal carcinoma; PDO, patient-derived organoid.
AUTHOR CONTRIBUTIONS
Xi-cheng Sun collected and analyzed the literature and wrote the manuscript. De-fu Kong selected the subject material and participated in the writing. Klaas Nico Faber developed the concept and revised the manuscript. Kang He designed and developed the concept and polished the manuscript. Qiang Xia developed the concept and revised the manuscript.
FUNDING INFORMATION
This study was supported by the Project of the Shanghai Municipal Health Commission (20204Y0012), the Innovative Research Team of High-Level Local Universities in Shanghai (SSMU-ZDCX20180802), the National Natural Science Foundation of China (81972205), the Project of Shanghai Key Clinical Specialties (shslczdzk05801), the Seed Fund of Renji Hospital (RJZZ18-010), and the Shenkang 3-year action plan (SHDC2020CR2003A, SHDC2020CR5012).
CONFLICT OF INTEREST
The authors have no conflicts to report.
Footnotes
Abbreviations: α-SMA, alpha-smooth muscle; A1AT, alpha-1 antitrypsin; AALD, alcohol-associated liver disease; AATD, alpha-1 antitrypsin deficiency; ACE2, angiotensin 1 converting enzyme 2; ADA, adenosine deaminase; AGS, Alagille syndrome; AALD, alcohol-associated liver disease; APAP, acetaminophen; AQP1, Aquaporin 1; Areg, amphiregulin; ASCs, adult stem cells; ASS1, argininosuccinate synthetase 1; BA, biliary atresia; BMP4, bone morphogenetic protein 4; CAFs, cancer-associated fibroblasts; CC, cholangiocarcinoma; CEBPA, CCAAT/enhancer-binding protein alpha; CF, cystic fibrosis; CFLD, cystic fibrosis–associated liver disease; CFTR, cystic fibrosis transmembrane conductance regulator; CK7, Cytokeratin 7; COMMD1, copper metabolism domain containing 1; CRC, colorectal carcinoma; CRISPR, clustered regularly interspaced short palindromic repeat; CRLM, CRC liver metastases; CT1, citrullinemia type 1; CYP2E1, CYP450 protein 2E1; CYP450, cytochrome P450; ECM, extracellular matrix; EpCAM, epithelial cell adhesion molecule; ESCs, embryonic stem cells; EtOH, ethanol; FFAs, free fatty acids; FIS, forskolin-induced swelling; GOC, gastroesophageal carcinoma; HCVcc, cell cultured HCV; hEHOs, human ESC–derived hepatic organoids; hFLMCs, human fetal mesenchymal cells; HNF4A, hepatocyte nuclear factor 4 alpha; HSCs, hepatic stellate cells; HUA, hyperuricemia; ICOs, intrahepatic cholangiocyte organoids; IFN-α, interferon-alpha; Igf2bp2, insulin-like growth factor 2 mRNA-binding protein 2; iPSCs, induced PSCs; JAG1, Jagged1; KCs, Kupffer cells; LAL, lysosomal acid lipase; Lgr5, leucine-rich-repeat-containing G protein–coupled receptor 5; LoT, liver organoid–based toxicity screening; MAFLD, metabolic-associated fatty liver disease; MCD, methionine- and choline deficient; MMA, methylmalonic acidemia; MDS, mitochondrial DNA depletion syndrome; NK, natural killer; Notch, neurogenic locus notch homolog protein; NTCP, sodium-taurocholate co-transporting polypeptide; OSM, oncostatin M; OTC, ornithine transcarbamylase; OTCD, OTC deficiency; PDOs, patient-derived organoids; PLC, primary liver cancer; PLD, polycystic liver disease; PLIN2, perilipin 2; PPARG, peroxisome proliferator–activated receptor gamma; PSC, primary sclerosing cholangitis; PSCs, pluripotent stem cells; RXRA, retinoid X receptor A; SARS-CoV-2, severe acute respiratory syndrome-coronavirus 2; SERPINA1, serine protease inhibitor family A member 1; SSTR2, somatostatin receptor 2; TGR5, Takeda G protein–coupled receptor-5; TMPPSS2, transmembrane serine protease 2; UCD, urea cycle disorder; XO, xanthine oxidase.
Xi-Cheng Sun and De-fu Kong contributed equally to this article.
Search strategy and selection criteria: Data for this review were collected from databases, including MEDLINE, Web of Science, EMBASE, and Google scholar, using the following search terms: ‘liver organoids’ OR ‘hepatic organoids’, various liver disease names AND ‘organoids’, ‘liver cancer organoids’ OR ‘liver carcinoma organoids’ OR ‘hepatocarcinoma organoids’ OR ‘cholangiocarcinoma organoids’ OR ‘hepatic tumoroids’. Articles with well-known concepts and published in English between 2010 and 2022 were included.
Contributor Information
Xi-Cheng Sun, Email: sunxckjwn@163.com.
De-fu Kong, Email: d.kong@umcg.nl.
Jie Zhao, Email: zhaojie@renji.com.
Klaas Nico Faber, Email: k.n.faber@umcg.nl.
Qiang Xia, Email: xiaqiang@shsmu.edu.cn.
Kang He, Email: hekang929@163.com.
REFERENCES
- 1. Asrani SK, Devarbhavi H, Eaton J, Kamath PS. Burden of liver diseases in the world. J Hepatol. 2019;70:151–71. [DOI] [PubMed] [Google Scholar]
- 2. Wang L, Li M, Yu B, Shi S, Liu J, Zhang R, et al. Recapitulating lipid accumulation and related metabolic dysregulation in human liver-derived organoids. J Mol Med (Berl). 2022;100:471–84. [DOI] [PubMed] [Google Scholar]
- 3. Mun SJ, Ryu J-S, Lee M-O, Son YS, Oh SJ, Cho H-S, et al. Generation of expandable human pluripotent stem cell-derived hepatocyte-like liver organoids. J Hepatol. 2019;71:970–85. [DOI] [PubMed] [Google Scholar]
- 4. Garnier D, Li R, Delbos F, Fourrier A, Collet C, Guguen-Guillouzo C, et al. Expansion of human primary hepatocytes in vitro through their amplification as liver progenitors in a 3D organoid system. Sci Rep. 2018;8:8222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Prior N, Inacio P, Huch M. Liver organoids: from basic research to therapeutic applications. Gut. 2019;68:2228–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lancaster MA, Knoblich JA. Organogenesis in a dish: modeling development and disease using organoid technologies. Science. 2014;345:1247125. [DOI] [PubMed] [Google Scholar]
- 7. Kretzschmar K, Clevers H. Organoids: modeling development and the stem cell Niche in a dish. Dev Cell. 2016;38:590–600. [DOI] [PubMed] [Google Scholar]
- 8. Li Y, Yang X, Plummer R, Hayashi Y, Deng XS, Nie YZ, et al. Human pluripotent stem cell-derived hepatocyte-like cells and organoids for liver disease and therapy. Int J Mol Sci. 2021;22:10471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ramli MNB, Lim YS, Koe CT, Demircioglu D, Tng W, Gonzales KAU, et al. Human pluripotent stem cell-derived organoids as models of liver disease. Gastroenterology. 2020;159:1471–1486 e1412. [DOI] [PubMed] [Google Scholar]
- 10. Wu F, Wu D, Ren Y, Huang Y, Feng B, Zhao N, et al. Generation of hepatobiliary organoids from human induced pluripotent stem cells. J Hepatol. 2019;70:1145–58. [DOI] [PubMed] [Google Scholar]
- 11. King NM, Perrin J. Ethical issues in stem cell research and therapy. Stem Cell Res Ther. 2014;5:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Prior N, Hindley CJ, Rost F, Meléndez E, Lau WWY, Göttgens B, et al. Lgr5(+) stem and progenitor cells reside at the apex of a heterogeneous embryonic hepatoblast pool. Development. 2019;146:103689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huch M, Gehart H, Ruben, Hamer K, Blokzijl F, Monique, et al. Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell. 2015;160:299–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Broutier L, Andersson-Rolf A, Hindley CJ, Boj SF, Clevers H, Koo BK, et al. Culture and establishment of self-renewing human and mouse adult liver and pancreas 3D organoids and their genetic manipulation. Nat Protoc. 2016;11:1724–43. [DOI] [PubMed] [Google Scholar]
- 15. Sampaziotis F, Justin AW, Tysoe OC, Sawiak S, Godfrey EM, Upponi SS, et al. Reconstruction of the mouse extrahepatic biliary tree using primary human extrahepatic cholangiocyte organoids. Nature Medicine. 2017;23:954–63. [DOI] [PubMed] [Google Scholar]
- 16. Hu H, Gehart H, Artegiani B, LÖpez-Iglesias C, Dekkers F, Basak O et al. Long-Term Expansion of Functional Mouse and Human Hepatocytes as 3D Organoids. Cell. 2018;175:1591-1606.e19. [DOI] [PubMed]
- 17. Hendriks D, Artegiani B, Hu H, Chuva De Sousa Lopes S, Clevers H. Establishment of human fetal hepatocyte organoids and CRISPR–Cas9-based gene knockin and knockout in organoid cultures from human liver. Nature Protocols. 2021;16:182–217. [DOI] [PubMed] [Google Scholar]
- 18. Lugli N, Kamileri I, Keogh A, Malinka T, Sarris ME, Talianidis I, et al. R‐spondin 1 and noggin facilitate expansion of resident stem cells from non‐damaged gallbladders. EMBO Rep. 2016;17:769–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Roos FJM, Verstegen MMA, Muñ7oz Albarinos L, Roest HP, Poley JW, Tetteroo GWM, et al. Human bile contains cholangiocyte organoid-initiating cells which wxpand as functional cholangiocytes in non-canonical Wnt stimulating conditions. Front Cell Dev Biol. 2020;8:630492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Soroka CJ, Assis DN, Alrabadi LS, Roberts S, Cusack L, Jaffe AB, et al. Bile‐derived organoids from patients with primary sclerosing cholangitis recapitulate their inflammatory immune profile. Hepatology. 2019;70:871–82. [DOI] [PubMed] [Google Scholar]
- 21. Soroka CJ, Assis DN, Boyer JL. Patient-Derived Organoids from Human Bile: An In Vitro Method to Study Cholangiopathies. Springer; 2019:363–372. [DOI] [PubMed] [Google Scholar]
- 22. Marsee A, Roos FJM, Verstegen MMA, Gehart H, de Koning E, Lemaigre F, et al. Building consensus on definition and nomenclature of hepatic, pancreatic, and biliary organoids. Cell Stem Cell. 2021;28:816–32. [DOI] [PubMed] [Google Scholar]
- 23. Olgasi C, Cucci A, Follenzi A. iPSC-derived liver organoids: a journey from drug screening, to disease modeling, arriving to regenerative medicine. Int J Mol Sci. 2020;21:6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Blutt SE, Crawford SE, Bomidi C, Zeng XL, Broughman JR, Robertson M, et al. Use of human tissue stem cell-derived organoid cultures to model enterohepatic circulation. Am J Physiol Gastrointest Liver Physiol. 2021;321:G270–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brooks A, Liang X, Zhang Y, Zhao CX, Roberts MS, Wang H, et al. Liver organoid as a 3D in vitro model for drug validation and toxicity assessment. Pharmacol Res. 2021;169:105608. [DOI] [PubMed] [Google Scholar]
- 26. Aberle MR, Burkhart RA, Tiriac H, Olde Damink SWM, Dejong CHC, Tuveson DA, et al. Patient-derived organoid models help define personalized management of gastrointestinal cancer. Br J Surg. 2018;105:e48–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Peng WC, Kraaier LJ, Kluiver TA. Hepatocyte organoids and cell transplantation: what the future holds. Exp Mol Med. 2021;53:1512–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Clevers H. Modeling development and disease with organoids. Cell. 2016;165:1586–97. [DOI] [PubMed] [Google Scholar]
- 29. Caiazza C, Parisi S, Caiazzo M. Liver organoids: updates on disease modeling and biomedical applications. Biology (Basel). 2021;10:835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gough A, Soto-Gutierrez A, Vernetti L, Ebrahimkhani MR, Stern AM, Taylor DL. Human biomimetic liver microphysiology systems in drug development and precision medicine. Nat Rev Gastroenterol Hepatol. 2021;18:252–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhu X, Zhang B, He Y, Bao J. Liver organoids: formation strategies and biomedical applications. Tissue Eng Regen Med. 2021;18:573–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schneeberger K, Spee B, Costa P, Sachs N, Clevers H, Malda J. Converging biofabrication and organoid technologies: the next frontier in hepatic and intestinal tissue engineering? Biofabrication. 2017;9:013001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mitchell E, Gilbert M, Loomes KM. Alagille syndrome. Clin Liver Dis. 2018;22:625–41. [DOI] [PubMed] [Google Scholar]
- 34. Andersson ER, Chivukula, Hankeova S, Sjöqvist M, Tsoi YL, Ramsköld D, Masek J, et al . Mouse model of Alagille syndrome and mechanisms of Jagged1 missense mutations. Gastroenterology. 2018;154:1080–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gómez-Mariano G, Matamala N, Martínez S, Justo I, Marcacuzco A, Jimenez C, et al. Liver organoids reproduce alpha-1 antitrypsin deficiency-related liver disease. Hepatol Int. 2020;14:127–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nantasanti S, Spee B, Kruitwagen HS, Chen C, Geijsen N, Oosterhoff LA, et al. Disease modeling and gene therapy of copper storage disease in canine hepatic organoids. Stem Cell Reports. 2015;5:895–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kruitwagen HS, Oosterhoff LA, van Wolferen ME, Chen C, Nantasanti Assawarachan S, Schneeberger K, et al. Long-term survival of transplanted autologous canine liver organoids in a COMMD1-deficient dog model of metabolic liver disease. Cells. 2020;9:410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lehmann V, Schene IF, Ardisasmita AI, Liv N, Veenendaal T, Klumperman J, et al. The potential and limitations of intrahepatic cholangiocyte organoids to study inborn errors of metabolism. J Inherit Metab Dis. 2022;45:353–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zabulica M, Jakobsson T, Ravaioli F, Vosough M, Gramignoli R, Ellis E, et al. Gene editing correction of a urea cycle defect in organoid stem cell derived hepatocyte-like cells. Int J Mol Sci. 2021;22:1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ogawa M, Ogawa S, Bear CE, Ahmadi S, Chin S, Li B, et al. Directed differentiation of cholangiocytes from human pluripotent stem cells. Nature Biotechnology. 2015;33:853–61. [DOI] [PubMed] [Google Scholar]
- 41. Sampaziotis F, de Brito MC, Madrigal P, Bertero A, Saeb-Parsy K, Soares FAC, et al. Cholangiocytes derived from human induced pluripotent stem cells for disease modeling and drug validation. Nat Biotechnol. 2015;33:845–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ouchi R, Togo S, Kimura M, Shinozawa T, Koido M, Koike H, et al. Modeling steatohepatitis in humans with pluripotent stem cell-derived organoids. Cell Metabolism. 2019;30:374–384.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Amarachintha SP, Mourya R, Ayabe H, Yang L, Luo Z, Li X, et al. Biliary organoids uncover delayed epithelial development and barrier function in biliary atresia. Hepatology. 2021;75:89-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Babu RO, Lui VCH, Chen Y, Yiu RSW, Ye Y, Niu B, et al. Beta-amyloid deposition around hepatic bile ducts is a novel pathobiological and diagnostic feature of biliary atresia. J Hepatol. 2020;73:1391–403. [DOI] [PubMed] [Google Scholar]
- 45. Waisbourd-Zinman O, Koh H, Tsai S, Lavrut PM, Dang C, Zhao X, et al. The toxin biliatresone causes mouse extrahepatic cholangiocyte damage and fibrosis through decreased glutathione and SOX17. Hepatology. 2016;64:880–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chen S, Li P, Wang Y, Yin Y, de Ruiter PE, Verstegen MMA, et al. Rotavirus infection and cytopathogenesis in human biliary organoids potentially recapitulate biliary atresia development. mBio. 2020;11:e01968–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wang S, Wang X, Tan Z, Su Y, Liu J, Chang M, et al. Human ESC-derived expandable hepatic organoids enable therapeutic liver repopulation and pathophysiological modeling of alcoholic liver injury. Cell Res. 2019;29:1009–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kruitwagen HS, Oosterhoff LA, Vernooij I, Schrall IM, van Wolferen ME, Bannink F, et al. Long-term adult feline liver organoid cultures for disease modeling of hepatic steatosis. Stem Cell Rep. 2017;8:822–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Haaker MW, Kruitwagen HS, Vaandrager AB, Houweling M, Penning LC, Molenaar MR, et al. Identification of potential drugs for treatment of hepatic lipidosis in cats using an in vitro feline liver organoid system. J Vet Intern Med. 2020;34:132–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Elbadawy M, Yamanaka M, Goto Y, Hayashi K, Tsunedomi R, Hazama S, et al. Efficacy of primary liver organoid culture from different stages of non-alcoholic steatohepatitis (NASH) mouse model. Biomaterials. 2020;237:119823. [DOI] [PubMed] [Google Scholar]
- 51. McCarron S, Bathon B, Conlon DM, Abbey D, Rader DJ, Gawronski K, et al. Functional characterization of organoids derived from irreversibly damaged NASH patient liver. Hepatology. 2021;74:1825–44. [DOI] [PubMed] [Google Scholar]
- 52. Nie Y-Z, Zheng Y-W, Miyakawa K, Murata S, Zhang R-R, Sekine K, et al. Recapitulation of hepatitis B virus–host interactions in liver organoids from human induced pluripotent stem cells. EBioMedicine. 2018;35:114–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fu GB, Huang WJ, Zeng M, Zhou X, Wu HP, Liu CC, et al. Expansion and differentiation of human hepatocyte-derived liver progenitor-like cells and their use for the study of hepatotropic pathogens. Cell Res. 2019;29:8–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. De Crignis E, Hossain T, Romal S, Carofiglio F, Moulos P, Khalid MM, et al. Application of human liver organoids as a patient-derived primary model for HBV infection and related hepatocellular carcinoma. eLife. 2021;10:e60747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ng SS, Saeb-Parsy K, Blackford SJI, Segal JM, Serra MP, Horcas-Lopez M, et al. Human iPS derived progenitors bioengineered into liver organoids using an inverted colloidal crystal poly (ethylene glycol) scaffold. Biomaterials. 2018;182:299–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Li P, Li Y, Wang Y, Liu J, Lavrijsen M, Li Y, et al. Recapitulating hepatitis E virus-host interactions and facilitating antiviral drug discovery in human liver-derived organoids. Sci Adv. 2022;8:eabj5908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yang L, Han Y, Nilsson-Payant BE, Gupta V, Wang P, Duan X, et al. A hHuman pluripotent stem cell-based platform to study SARS-CoV-2 tropism and model virus infection in human cells and organoids. Cell Stem Cell. 2020;27:125–36 e127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zhao B, Ni C, Gao R, Wang Y, Yang L, Wei J, et al. Recapitulation of SARS-CoV-2 infection and cholangiocyte damage with human liver ductal organoids. Protein & Cell. 2020;11:771–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chua ACY, Ananthanarayanan A, Ong JJY, Wong JY, Yip A, Singh NH, et al. Hepatic spheroids used as an in vitro model to study malaria relapse. Biomaterials. 2019;216:119221. [DOI] [PubMed] [Google Scholar]
- 60. Sgodda M, Dai Z, Zweigerdt R, Sharma AD, Ott M, Cantz T. A scalable approach for the generation of human pluripotent stem cell-derived hepatic organoids with sensitive hepatotoxicity features. Stem Cells Dev. 2017;26:1490–504. [DOI] [PubMed] [Google Scholar]
- 61. Wang Y, Wang H, Deng P, Chen W, Guo Y, Tao T, et al. In situ differentiation and generation of functional liver organoids from human iPSCs in a 3D perfusable chip system. Lab Chip. 2018;18:3606–16. [DOI] [PubMed] [Google Scholar]
- 62. Shinozawa T, Kimura M, Cai Y, Saiki N, Yoneyama Y, Ouchi R, et al. High-fidelity drug-induced liver injury screen using human pluripotent stem cell–derived organoids. Gastroenterology. 2021;160:831–46.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Coll M, Perea L, Boon R, Leite SB, Vallverdú J, Mannaerts I, et al. Generation of hepatic stellate cells from human pluripotent stem cells enables in vitro modeling of liver fibrosis. Cell Stem Cell. 2018;23:101–13.e107. [DOI] [PubMed] [Google Scholar]
- 64. Lee J-Y, Han H-J, Lee S-J, Cho E-H, Lee H-B, Seok J-H, et al. Use of 3D human liver organoids to predict drug-iInduced phospholipidosis. Int J Mol Sci. 2020;21:2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Loarca L, De Assuncao TM, Jalan-Sakrikar N, Bronk S, Krishnan A, Huang B, et al. Development and characterization of cholangioids from normal and diseased human cholangiocytes as an in vitro model to study primary sclerosing cholangitis. Lab Invest. 2017;97:1385–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hou C, Hu Y, Jiang H, Xu Z, Sha W, Liu J, et al. Establishment of a 3D hyperuricemia model based on cultured human liver organoids. Free Radic Biol Med. 2022;178:7–17. [DOI] [PubMed] [Google Scholar]
- 67. Hou C, Sha W, Xu Z, Hu Y, Amakye WK, Yao M, et al. Culture and establishment of self-renewing human liver 3D organoids with high uric acid for screening antihyperuricemic functional compounds. Food Chem. 2022;374:131634. [DOI] [PubMed] [Google Scholar]
- 68. Vyas D, Baptista PM, Brovold M, Moran E, Gaston B, Booth C, et al. Self‐assembled liver organoids recapitulate hepatobiliary organogenesis in vitro. Hepatology. 2018;67:750–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Greene CM, Marciniak SJ, Teckman J, Ferrarotti I, Brantly ML, Lomas DA, et al. α1-antitrypsin deficiency. Nat Rev Dis Primers. 2016;2:16051. [DOI] [PubMed] [Google Scholar]
- 70. Santangelo S, Scarlata S, Poeta ML, Bialas AJ, Paone G, Incalzi RA. Alpha-1 antitrypsin deficiency: current perspective from genetics to diagnosis and therapeutic approaches. Curr Med Chem. 2017;24:65–90. [DOI] [PubMed] [Google Scholar]
- 71. Haugabook SJ, Ferrer M, Ottinger EA. In vitro and in vivo translational models for rare liver diseases. Biochim Biophys Acta Mol Basis Dis. 2019;1865:1003–018. [DOI] [PubMed] [Google Scholar]
- 72. Favier RP, Spee B, Schotanus BA, van den Ingh TS, Fieten H, Brinkhof B, et al. COMMD1-deficient dogs accumulate copper in hepatocytes and provide a good model for chronic hepatitis and fibrosis. PLoS One. 2012;7:e42158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Matsumoto S, Häberle J, Kido J, Mitsubuchi H, Endo F, Nakamura K. Urea cycle disorders-update. J Hum Genet. 2019;64:833–47. [DOI] [PubMed] [Google Scholar]
- 74. Akbari S, Sevinç GG, Ersoy N, Basak O, Kaplan K, Sevinç K, et al. Robust, long-term culture of endoderm-derived hepatic organoids for disease modeling. Stem Cell Reports. 2019;13:627–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Cutting GR. Cystic fibrosis genetics: from molecular understanding to clinical application. Nat Rev Genet. 2015;16:45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Leeuwen L, Fitzgerald DA, Gaskin KJ. Liver disease in cystic fibrosis. Paediatr Respir Rev. 2014;15:69–74. [DOI] [PubMed] [Google Scholar]
- 77. Fiedorczuk K, Chen J. Mechanism of CFTR correction by type I folding correctors. Cell. 2022;185:158–68.e11. [DOI] [PubMed] [Google Scholar]
- 78. Liu F, Zhang Z, Levit A, Levring J, Touhara KK, Shoichet BK, et al. Structural identification of a hotspot on CFTR for potentiation. Science. 2019;364:1184–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Dekkers JF, Wiegerinck CL, De Jonge HR, Bronsveld I, Janssens HM, De Winter-De Groot KM, et al. A functional CFTR assay using primary cystic fibrosis intestinal organoids. Nature Med. 2013;19:939–45. [DOI] [PubMed] [Google Scholar]
- 80. Ramsey BW, Davies J, McElvaney NG, Tullis E, Bell SC, Dřevínek P, et al. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med. 2011;365:1663–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Bezerra JA, Wells RG, Mack CL, Karpen SJ, Hoofnagle JH, Doo E, et al. Biliary atresia: clinical and research challenges for the twenty-first century. Hepatology. 2018;68:1163–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Fried S, Gilboa D, Har-Zahav A, Lavrut PM, Du Y, Karjoo S, et al. Extrahepatic cholangiocyte obstruction is mediated by decreased glutathione, Wnt and Notch signaling pathways in a toxic model of biliary atresia. Sci Rep. 2020;10:7599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Lam D, Dan YY, Chan YS, Ng HH. Emerging liver organoid platforms and technologies. Cell Regen. 2021;10:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018;24:908–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Pierantonelli I, Svegliati-Baroni G. Nonalcoholic fatty liver disease: basic pathogenetic mechanisms in the progression from NAFLD to NASH. Transplantation. 2019;103:e1–13. [DOI] [PubMed] [Google Scholar]
- 86. Cho J, Lee J, Sia CH, Koo CS, Tan BY, Hong W, et al. Extrapulmonary manifestations and complications of severe acute respiratory syndrome coronavirus 2 infection: a systematic review. Singapore Med J. 2021. doi: 10.11622/smedj.2021100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Howell L, Jenkins RE, Lynch S, Duckworth C, Kevin Park B, Goldring C. Proteomic profiling of murine biliary-derived hepatic organoids and their capacity for drug disposition, bioactivation and detoxification. Arch Toxicol. 2021;95:2413–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Leite SB, Roosens T, El Taghdouini A, Mannaerts I, Smout AJ, Najimi M, et al. Novel human hepatic organoid model enables testing of drug-induced liver fibrosis in vitro. Biomaterials. 2016;78:1–10. [DOI] [PubMed] [Google Scholar]
- 89. Karlsen TH, Folseraas T, Thorburn D, Vesterhus M. Primary sclerosing cholangitis—a comprehensive review. J Hepatol. 2017;67:1298–323. [DOI] [PubMed] [Google Scholar]
- 90. Tabibian JH, O’Hara SP, Splinter PL, Trussoni CE, LaRusso NF. Cholangiocyte senescence by way of N-ras activation is a characteristic of primary sclerosing cholangitis. Hepatology. 2014;59:2263–2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Reich M, Spomer L, Klindt C, Fuchs K, Stindt J, Deutschmann K, et al. Downregulation of TGR5 (GPBAR1) in biliary epithelial cells contributes to the pathogenesis of sclerosing cholangitis. J Hepatol. 2021;75:634–46. [DOI] [PubMed] [Google Scholar]
- 92. Xing ZH, Ma YC, Li XP, Zhang B, Zhang MD. Research progress of puerarin and its derivatives on anti-inflammatory and anti-gout activities. Zhongguo Zhong Yao Za Zhi. 2017;42:3703–08. [DOI] [PubMed] [Google Scholar]
- 93. Qiu Z, Li H, Zhang Z, Zhu Z, He S, Wang X, et al. A pharmacogenomic landscape in human liver cancers. Cancer Cell. 2019;36:179–93.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Broutier L, Mastrogiovanni G, Verstegen MM, Francies HE, Gavarró LM, Bradshaw CR, et al. Human primary liver cancer–derived organoid cultures for disease modeling and drug screening. Nature Medicine. 2017;23:1424–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Li L, Knutsdottir H, Hui K, Weiss MJ, He J, Philosophe B, et al. Human primary liver cancer organoids reveal intratumor and interpatient drug response heterogeneity. JCI Insight. 2019;4:e121490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Wang S, Wang Y, Xun X, Zhang C, Xiang X, Cheng Q, et al. Hedgehog signaling promotes sorafenib resistance in hepatocellular carcinoma patient-derived organoids. J Exp Clin Cancer Res. 2020;39:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Nuciforo S, Fofana I, Matter MS, Blumer T, Calabrese D, Boldanova T, et al. Organoid models of human liver cancers derived from tumor needle biopsies. Cell Reports. 2018;24:1363–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Saltsman JA, Hammond WJ, Narayan NJC, Requena D, Gehart H, Lalazar G, et al. A human organoid model of aggressive hepatoblastoma for disease modeling and drug testing. Cancers (Basel). 2020;12:2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Wang Y, Takeishi K, Li Z, Cervantes-Alvarez E, Collin de l’Hortet A, Guzman-Lepe J, et al. Microenvironment of a tumor-organoid system enhances hepatocellular carcinoma malignancy-related hallmarks. Organogenesis. 2017;13:83–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Liu J, Li P, Wang L, Li M, Ge Z, Noordam L, et al. Cancer-associated fibroblasts provide a stromal niche for liver cancer organoids that confers trophic effects and therapy resistance. Cell Mol Gastroenterol Hepatol. 2021;11:407–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Neal JT, Li X, Zhu J, Giangarra V, Grzeskowiak CL, Ju J, et al. Organoid modeling of the tumor immune microenvironment. Cell. 2018;175:1972–88 e1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Artegiani B, Van Voorthuijsen L, Lindeboom RGH, Seinstra D, Heo I, Tapia P, et al. Probing the tumor suppressor function of BAP1 in CRISPR-engineered human liver organoids. Cell Stem Cell. 2019;24:927–43.e26. [DOI] [PubMed] [Google Scholar]
- 103. Fumagalli A, Drost J, Suijkerbuijk SJE, Van Boxtel R, De Ligt J, Offerhaus GJ, et al. Genetic dissection of colorectal cancer progression by orthotopic transplantation of engineered cancer organoids. Proc Natl Acad Sci U S A. 2017;114:E2357–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Vlachogiannis G, Hedayat S, Vatsiou A, Jamin Y, Fernández-Mateos J, Khan K, et al. Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science. 2018;359:920–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Tanimizu N, Ichinohe N, Sasaki Y, Itoh T, Sudo R, Yamaguchi T, et al. Generation of functional liver organoids on combining hepatocytes and cholangiocytes with hepatobiliary connections ex vivo. Nat Commun. 2021;12:3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Dedhia PH, Bertaux-Skeirik N, Zavros Y, Spence JR. Organoid models of human gastrointestinal development and disease. Gastroenterology. 2016;150:1098–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Mun SJ, Lee J, Chung KS, Son MY, Son MJ. Effect of microbial short-chain fatty acids on CYP3A4-mediated metabolic activation of human pluripotent stem cell-derived liver organoids. Cells. 2021;10:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Nguyen DG, Funk J, Robbins JB, Crogan-Grundy C, Presnell SC, Singer T, et al. Bioprinted 3D primary liver tissues allow assessment of organ-level response to clinical drug induced toxicity in vitro. PLoS One. 2016;11:e0158674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Sendi H, Mead I, Wan M, Mehrab-Mohseni M, Koch K, Atala A, et al. miR-122 inhibition in a human liver organoid model leads to liver inflammation, necrosis, steatofibrosis and dysregulated insulin signaling. PLoS One. 2018;13:e0200847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Kaur S, Kaur I, Rawal P, Tripathi DM, Vasudevan A. Non-matrigel scaffolds for organoid cultures. Cancer Lett. 2021;504:58–66. [DOI] [PubMed] [Google Scholar]
- 111. Prince E, Cruickshank J, Ba-Alawi W, Hodgson K, Haight J, Tobin C, et al. Biomimetic hydrogel supports initiation and growth of patient-derived breast tumor organoids. Nat Commun. 2022;13:1466. [DOI] [PMC free article] [PubMed] [Google Scholar]


