ABSTRACT
Acute pancreatitis is an infrequent but clinically significant complication of immune checkpoint inhibitor (ICI) therapy. Guidelines recommend high-dose steroids and withdrawal of ICI in patients with severe ICI-induced pancreatitis. Management of steroid-refractory ICI pancreatitis is unclear. Infliximab is used to treat select extrapancreatic immune-related adverse events, but its role in ICI pancreatitis remains undefined. To our knowledge, we describe the first case of ICI pancreatitis successfully treated with infliximab after inadequate steroid response (recurrent pancreatitis on multiple attempted steroid tapers). Infliximab may be a viable treatment of steroid-refractory ICI pancreatitis. Further study of its potential effectiveness may improve guideline-directed care.
KEYWORDS: immune checkpoint inhibitor, immune-related adverse event, pancreatitis, infliximab
INTRODUCTION
Immune checkpoint inhibitors (ICIs) are increasingly used in the treatment of advanced malignancies and can result in a range of immune-related adverse events (irAEs) of varying severity. Gastrointestinal irAEs can affect the colon, small intestine, stomach, liver, and/or pancreas1; gastroenterologists are frequently called upon to diagnose and treat these irAEs. ICI-induced pancreatic injury occurs in approximately 1%–4% of patients treated with ICI(s).2–5 ICI-induced pancreatic injury presents variably from asymptomatic hyperlipasemia and/or hyperamylasemia to symptomatic acute pancreatitis.2,3 Current guidelines recommend high-dose steroids and withdrawal or cessation of ICIs in patients with ICI-induced pancreatitis.6,7 However, management of patients with ICI-induced pancreatitis refractory to steroids or recurrent during a steroid taper is unclear. In the absence of a viable second-line treatment option, patients with steroid-refractory ICI-induced pancreatitis may have to continue using high-dose steroids, with their associated risks. Fatality from steroid-refractory ICI pancreatitis has also been reported.8
The tumor necrosis factor-alpha (TNF-α) inhibitor, infliximab, is a well-established treatment option in steroid-refractory irAEs, including colitis, myocarditis, inflammatory arthritis, and pneumonitis.6,7 To our knowledge, we present the first case to describe steroid-refractory ICI pancreatitis treated with infliximab.
CASE REPORT
A 73-year-old White man with recurrent metastatic melanoma to the lung was treated with pembrolizumab (a programmed cell death-1 inhibitor). His medical history was notable for rheumatoid arthritis, which was stable, asymptomatic, and last treated 11 months earlier with rituximab. He had not experienced any prior ICI-related irAEs. After his third cycle of pembrolizumab, the patient developed new epigastric and left upper quadrant pain with radiation to his back. He had a serum lipase of 289 U/L, which rose to 467 U/L the next day, over 7 times the laboratory upper limit of normal of 60 U/L. Positron computed tomography imaging demonstrated pancreatic edema with fat stranding consistent with acute pancreatitis (Figure 1). Serum calcium and lipids were within normal limits, and there was no personal or family history of pancreatitis or cholelithiasis, nor personal alcohol or tobacco use. He was diagnosed with pembrolizumab (ICI-)induced acute pancreatitis (grade 3/moderate severity pancreatitis by Common Terminology Criteria for Adverse Events and mild-acute interstitial edematous pancreatitis by the revised Atlanta classification).9
Figure 1.
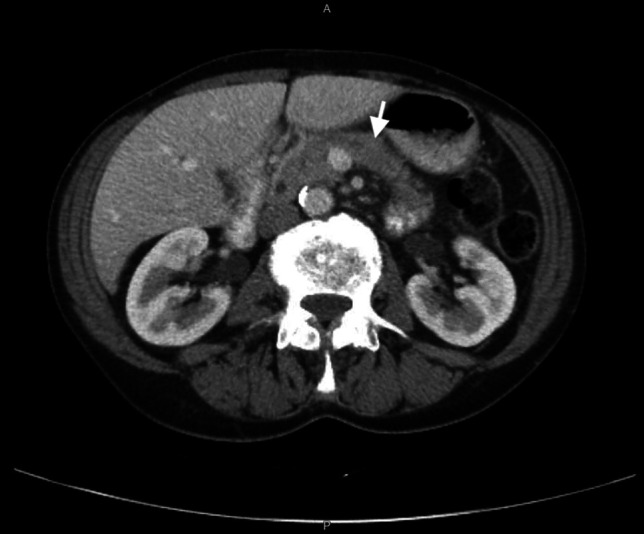
Computed tomography evidence of acute pancreatitis attributed to pembrolizumab use. New diffuse pancreatic enlargement (white arrow) and adjacent fat stranding are visualized.
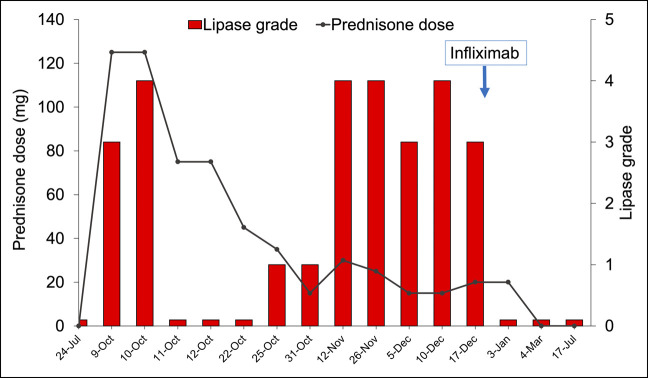
The patient was hospitalized for intravenous fluids and intravenous methylprednisolone (1 mg/kg), followed by an oral prednisone taper. A time line of his steroid dosing and grade of hyperlipasemia is presented in Figure 2. His pain temporarily resolved with steroids, but when his prednisone dose was tapered from 20 to 15 mg daily, he developed recurrent epigastric pain and elevated serum lipase (482 U/L). His prednisone dose was increased back to 30 mg daily, and his lipase downtrended (280 U/L) and abdominal pain transiently improved. On a repeat attempt to taper to 15 mg daily, he developed recurrent epigastric pain and hyperlipasemia (474 U/L). Upper and lower endoscopies were performed to evaluate for concomitant luminal ICI-related inflammation (gastritis, enteritis, or colitis). Endoscopy demonstrated mild, nonspecific pathologic changes inconsistent with ICI gastritis or colitis or another identifiable etiology.
Figure 2.
Treatment course with steroids and then with infliximab for immune checkpoint inhibitor-induced acute pancreatitis. Serum lipase was graded according to the Common Terminology Criteria for Adverse Events, version 5.0; higher grades correspond to higher severity. Grade 1: serum lipase >ULN–1.5 times the ULN. Grade 2: >1.5–2.0 times ULN, or >2.0–5.0 times ULN and asymptomatic. Grade 3: >2.0–5.0 times ULN with signs or symptoms, or >5.0 times ULN and asymptomatic. Grade 4: >5.0 times ULN with signs or symptoms. Intravenous methylprednisolone doses were converted to equivalent oral prednisone doses using a conversion factor of 1.25. ULN, upper limit of normal.
Infliximab (5 mg/kg) was administered for symptomatic ICI pancreatitis given 2 failed attempts to taper oral steroids. After a single infusion, his abdominal pain and lipase improved without recurrence. He was able to wean promptly from steroids. Repeat abdominal computed tomography imaging 5 and 8 months after infliximab showed a normal pancreatic size and contour without evidence of pancreatitis. The patient has not had recurrent pancreatitis in over 2 years of follow-up.
DISCUSSION
To our knowledge, this is the first case report to describe infliximab therapy for steroid-refractory ICI-induced acute pancreatitis. The improvement in symptomatic, serologic, and radiographic evidence of pancreatic inflammation in this patient suggests that infliximab may be a viable second-line treatment of ICI-induced pancreatitis that does not respond to steroids or rapidly recurs during steroid taper. Current guidelines detail the use of infliximab for numerous extrapancreatic irAEs, but do not define its role in immune-mediated pancreatitis. Pancreatic toxicity, although rare, can have fatal consequences.8 In addition, long-term complications of pancreatitis, including exocrine pancreatic insufficiency and diabetes mellitus, can significantly affect quality of life. Gastroenterologists are increasingly involved in the management of patients with ICI pancreatitis and must be aware of first-line and adjunctive treatment options.
Several mechanisms support the possible effectiveness of infliximab, a chimeric TNF-blocking monoclonal antibody, in ICI pancreatitis. In acute pancreatitis, TNF-α is a central proinflammatory mediator induced in pancreatic antigen-presenting cells, signaling toward acinar cell apoptosis or necrosis. In ICI-induced colitis, clinical data support a key role of TNF-α, and myeloid cells demonstrate transcriptional changes induced by this cytokine.10 Given the response of patients to TNF-α blockade and guidelines supporting infliximab in the treatment of numerous irAEs,6,7 the pathologic role of TNF-α in the inflammatory state of ICI-induced pancreatitis and a potentially beneficial role of TNF-α blockade in its treatment are both very plausible.
The use of infliximab has potential side effects, including hypersensitivity reactions, reactivation of tuberculosis or hepatitis B, and increased susceptibility to infections. However, steroid-sparing agents have the potential to decrease the risks of prolonged high-dose steroid use and/or chronic sequelae from ICI toxicities.
One relevant consideration for the use of infliximab is whether another ICI-induced complication for which infliximab is a second-line therapy is concurrently present. Given the frequent presentation with multiple simultaneous or sequential irAEs,11 this patient underwent upper and lower endoscopies to rule out concurrent luminal ICI toxicities. The effectiveness of infliximab for ICI-induced pancreatitis may be further evaluated in individuals who develop simultaneous ICI-induced pancreatitis and another irAE for which infliximab is an established treatment.
Further studies are warranted to validate the effectiveness of infliximab specifically in patients with steroid-refractory ICI-induced pancreatitis and explore the impact of TNF-α blockade on long-term complications, including pancreatic insufficiency secondary to pancreatitis and cancer outcomes.
DISCLOSURES
Author contributions: MJ Townsend and S. Grover were each involved in the conception, design, and composition of this work. FS Hodi made significant contributions in the revision of this work. MJ Townsend is the article guarantor.
Financial disclosure: MJ Townsend has no conflicts of interest or financial ties to disclose. FS Hodi reports grants and personal fees from Bristol-Myers Squibb, personal fees from Merck, personal fees from EMD Serono, grants and personal fees from Novartis, personal fees from Surface, personal fees from Compass Therapeutics, personal fees from Apricity, personal fees from Sanofi, personal fees from Pionyr, personal fees from Torque, personal fees from Bicara, personal fees from Checkpoint Therapeutics, personal fees from Genentech/Roche, personal fees from Bioentre, personal fees from Gossamer, personal fees from Iovance, personal fees from Trillium, personal fees from Catalym, personal fees from Immunocore, personal fees from Amgen, personal fees from Kairos, personal fees from Rheos, personal fees from Zumutor, and personal fees from Corner Therapeutics, outside the submitted work; in addition, FS Hodi has a patent Methods for Treating MICA-Related Disorders (#20100111973) with royalties paid; a patent tumor antigens and uses thereof (#7250291) issued; a patent Angiopoietin-2 Biomarkers Predictive of Anti-immune checkpoint response (#20170248603) pending; a patent Compositions and Methods for Identification, Assessment, Prevention, and Treatment of Melanoma using PD-L1 Isoforms (#20160340407) pending; a patent Therapeutic peptides (#20160046716) pending; a patent Therapeutic Peptides (#20140004112) pending; a patent Therapeutic Peptides (#20170022275) pending; a patent Therapeutic Peptides (#20170008962) pending; a patent Therapeutic Peptides (#9402905) issued; a patent Methods of Using Pembrolizumab and Trebananib pending; a patent Vaccine Compositions and Methods for Restoring NKG2D Pathway Function Against Cancers (#10279021) issued; a patent Antibodies that Bind to MHC Class I Polypeptide-related Sequence A (#10106611) issued; and a patent Anti-Galectin Antibody Biomarkers Predictive of Anti-Immune Checkpoint and Anti-Angiogenesis Responses (#20170343552) pending. S. Grover is a gastroenterology editor (employment) at UpToDate and Wolters Kluwer Inc.
Previous presentation: This case was presented previously as an abstract/poster at the American College of Gastroenterology Annual Scientific Meeting; October 26, 2021; Las Vegas, Nevada.
Informed consent was obtained for this case report.
Contributor Information
Matthew J. Townsend, Email: matthew.townsend@duke.edu.
F. Stephen Hodi, Email: stephen_hodi@dfci.harvard.edu.
REFERENCES
- 1.Xing P, Zhang F, Wang G, et al. Incidence rates of immune-related adverse events and their correlation with response in advanced solid tumours treated with NIVO or NIVO+IPI: A systematic review and meta-analysis. J Immunother Cancer. 2019;7(1):341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.George J, Bajaj D, Sankaramangalam K, et al. Incidence of pancreatitis with the use of immune checkpoint inhibitors (ICI) in advanced cancers: A systematic review and meta-analysis. Pancreatology. 2019;19(4):587–94. [DOI] [PubMed] [Google Scholar]
- 3.Abu-Sbeih H, Tang T, Lu Y, et al. Clinical characteristics and outcomes of immune checkpoint inhibitor-induced pancreatic injury. J Immunother Cancer. 2019;7(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Su Q, Zhang XC, Zhang CG, Hou YL, Yao YX, Cao BW. Risk of immune-related pancreatitis in patients with solid tumors treated with immune checkpoint inhibitors: Systematic assessment with meta-analysis. J Immunol Res. 2018;2018:1027323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grimmelmann I, Momma M, Zimmer L, et al. Lipase elevation and type 1 diabetes mellitus related to immune checkpoint inhibitor therapy: A multicentre study of 90 patients from the German Dermatooncology Group. Eur J Cancer. 2021;149:1–10. [DOI] [PubMed] [Google Scholar]
- 6.Thompson JA, Schneider BJ, Brahmer J, et al. Management of immunotherapy-related toxicities, version 1.2022: NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2022;20(4):387–405. [DOI] [PubMed] [Google Scholar]
- 7.Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2018;36(17):1714–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ueno M, Tsuji Y, Yokoyama T, et al. Fatal immune checkpoint inhibitor-related pancreatitis. Intern Med. 2021;60(24):3905–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Common Terminology Criteria for Adverse Events (CTCAE): Version 5.0. U.S. Department of Health and Human Services National Institutes of Health, National Cancer Institute: Cincinnati, OH, 2017. [Google Scholar]
- 10.Dougan M, Luoma AM, Dougan SK, Wucherpfennig KW. Understanding and treating the inflammatory adverse events of cancer immunotherapy. Cell. 2021;184(6):1575–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shimozaki K, Sukawa Y, Beppu N, et al. Multiple immune-related adverse events and anti-tumor efficacy: Real-world data on various solid tumors. Cancer Manag Res. 2020;12:4585–93. [DOI] [PMC free article] [PubMed] [Google Scholar]