Abstract
Objectives:
CT-scan hyperdensities (HD) are described in more than 60% of all paranasal sinus fungus ball (FB) cases. Two types can be distinguished according to their density: calcium and metal types. We aimed to establish the prevalence and density of the HD observed in sphenoid and maxillary sinus FB and their relation to dental factors.
Methods:
This retrospective study included 64 patients operated in a tertiary referral center for unilateral maxillary or sphenoid FB diagnosed by histology or mycology. Pre-operative CT scans were analyzed by three independent observers (two ENT and one radiologist).
Results:
There were 45 maxillary FB and 19 sphenoid FB. 63 FB showed HD. Metal-type HD were observed in 28 maxillary FB but not in sphenoid sinuses. Among maxillary FB, the prevalence of endodontic treatment was significantly more significant on the FB side than on the healthy side (p = 0.02). The prevalence of endodontic treatment on the pathological side was more significant in the metal-type group than in the group without metal-type HD (p = 0.01). Isolated calcium-type HD were evidenced in 17 maxillary FB and 18 sphenoid FB (p = 0.019).
Conclusion:
This study highlights the existence of two different types of HD in FBs of the paranasal sinuses with an association between metal-type HD and endodontic treatments.
Keywords: Fungi, Sinusitis, Maxillary Sinus, Sphenoid Sinus, Root Canal Obturation
Introduction
Fungus ball (FB) is the most frequent type of non-invasive fungal sinusitis in Western countries. The first diagnostic criterion described by DeShazo is: « radiological evidence of sinus opacification with or without associated flocculent calcifications ». 1 The prevalence of hyperdensities (HD) in FB ranges from 60 to 100%, using radiological and CT-scan data. 1–6 They have been described in several ways, including metal-dense spots, iron-like signals, flocculent calcifications, and microcalcifications. 7–10 Krennmair, Lugmayr, and Lenglinger classified maxillary HD into two types depending on their density in Hounsfield units (HUs). Inorganic HD have a density >1500 HU, while organic HD are considered physiological with a density <1500 HU. 11,12
Two theories have been propounded to explain the formation of FB. The aerogenic theory states that airborne fungus spores can penetrate the sinus and become pathogenic in anaerobic conditions. 13 The odontogenic theory posits that fungi colonize the sinus through the intrasinusal extrusion of endodontic treatment material. 14 To date, metallic HD in maxillary FB have been considered the consequence of the extrusion of root filling material after endodontic procedures. 3,11,14,15 Moreover, an association between endodontic material and maxillary sinus aspergillosis has been shown. 16
On the opposite, metallic HD have rarely been described in sphenoid sinus FB. 17,18 Their pattern has been described as calcifications due to the precipitation of calcium salts in the necrotic center of the FB. 8,19,20 The dental origin in sphenoid sinus FB seems less probable than in the maxillary sinuses owing to the absence of direct contact between the dental roots and the sinus mucosa.
Further data in the exploration of FB physiopathology may be gained by studying the different patterns of sinus HD. We hypothesized that the pattern of HD found in sinus FB might be related to their physiopathology and might differ depending on their location and the presence of dental factors. This study aimed to describe the CT-scan sphenoid and maxillary FB patterns and analyze the relationship between dental pathologies and these patterns.
Methods and materials
The institutional review board approved this single-center retrospective observational study of the University Hospital of Bordeaux. We analyzed patients' data for sinus FB in the Department of Otorhinolaryngology between August 2006 and June 2016. Only locations within the maxillary or sphenoid sinus were included. All FB had to be proven on pathological and/or mycological examination (direct examination after Grocott-Gomori stain and/or culture on Sabouraud dextrose agar). The final analysis included only the patients who met these preliminary conditions, with a sinus CT-scan exam available in DICOM format on CD or the local imaging network. Patients with mucosal fungal invasion on pathological examination and those with bilateral FB were also excluded, because the second aim of the study was to compare the dentogenic factors between both sides. Epidemiological data were recorded for the remaining patients: gender, age at surgery, pre-operative symptomatology, and mycological data.
Three observers analyzed all CT scans independently: two ENT (NR junior, PLB senior) and one neuroradiologist (SM, senior). CT scans were read with multiplanar reconstructions: axial views parallel to the hard palate line and coronal reconstructions perpendicular to the hard palate. No low-dose CTs were included, and the average DLP (dose–length product) was 80 mGy/cm. All CT scans were read with a window width of 3000 and a length of 500. The presence of HD in the sinus content affected by FB was evaluated in each patient. Intrasinusal HD were classified as metal-type HD and calcium-type HD from their radiological features as follows (Figure 1): metal-type HD appeared as rounded, well-defined HD with a density higher than that of the adjacent bone, calcium-type HD were defined as punctuated, poorly defined bone-dense signals and were distinguished from linear peripheral mucosa calcifications. Mean, minimal and maximal densities were measured for calcium-type HD and metal-type HD. The minimal region of interest (ROI) area required was 2 mm². In the event of metal-type HD, the density of the endodontic material was systematically measured if present. Osseous modifications of the sinus walls were recorded: sinus wall thickening/sclerosis, sinus wall erosion, and ostial dilation. CT scans were read with Dx-MM 6.0 DICOM software (Medasys S.A., Clamart, France).
Figure 1.
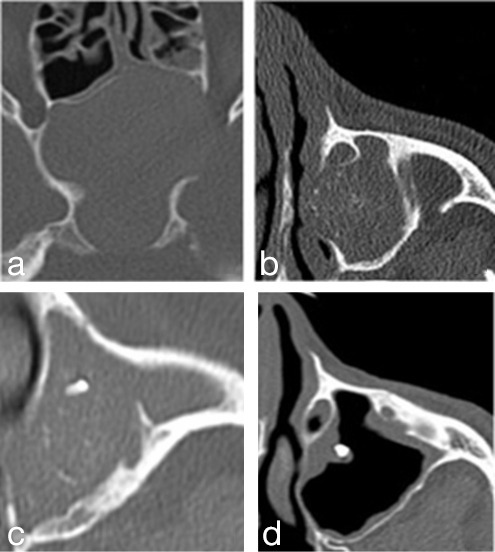
Radiological patterns observed in this study. (a) no hyperdensities – neutral opacification of sphenoid sinus (50 mA, 100 kV, WW350 WL2000); (b) flocculent isolated calcium-type hyperdensities (50 mA, 100 kV, WW350 WL2000); (c) association of metal-like hyperdensity with peripheric calcifications (70 mA, 100 kV, WW400 WL2000); (d) isolated metal-type hyperdensity without sinus opacification (70 mA, 100 kV, WW400 WL2000).
Endodontic treatment and extraction in the maxillary dental arches were assessed for the FB and the healthy sides. Canines, premolars, and molars were analyzed individually owing to their contact with the maxillary sinus floor. Every single tooth was classified as one of the following: non-analyzable (insufficient CT volume, bilateral third molar absence or extraction), no contact with the sinus, endodontic treatment (with or without overfilling), or dental extraction. This classification is illustrated in Figure 2. The prevalence of endodontic treatment or dental extraction was defined as the number of patients having one or more endodontic treatments or dental extractions in the whole group.
Figure 2.
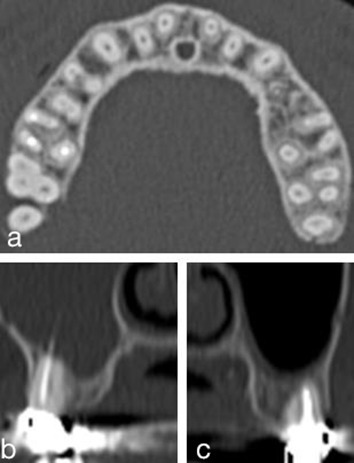
Dentogenic factors: dental extraction in axial view; root filling patterns evaluated in coronal views: extrusion into sinus lumen vs non-extrusion.
Quantitative variables were expressed as mean ± SD. Interobserver agreement for quantitative parameters was evaluated using the interclass correlation coefficient, and the mean values between the three observers were retained for further analysis. Mean densities were compared by using the Student’s t-test. Concerning qualitative parameters, interobserver agreement was evaluated with Fleiss' κ. Qualitative data were retained for analysis when at least two of the three observers agreed; otherwise, a second reading was performed. Associations between the type of HD and dental factors were obtained with χ2 or Fisher exact tests. A p-value < 0.05 was considered statistically significant. All analyses were performed with GraphPad Prism 6.01 for Windows (GraphPad Software Inc, San Diego, CA).
Results
Epidemiology
Over a period of 10 years and 5 months, 168 patients underwent surgery for presumed paranasal sinus FB in the Otorhinolaryngology Department at the University Hospital of Bordeaux. Six patients were excluded owing to bilateral FB. Pathological or mycological examination failed to prove the presence of fungus in 32 patients. 66 patients had no CT-scan DICOM data available. We included the 64 remaining patients. All patients and/or relatives gave oral or written informed consent to use their medical data. FB was located in the maxillary sinus in 45 patients and the sphenoid sinus in 19 patients. All patients underwent endonasal endoscopic surgery except one operated by a Caldwell-Luc approach. The mean age at surgery was 61.9 ± 14.9 years. The mean age at surgery did not differ significantly between maxillary or sphenoid FB, 60.2 ± 2.2 vs 66.0 ± 3.5 respectively, p = 0.16. Symptoms of chronic rhinosinusitis were found in 67 and 74% of maxillary and sphenoid FB, respectively.
Pathological examination was positive for clusters of hyphae in 60 patients (94%). The direct examination was positive in 50 cases (78%), whereas culture was positive only in 20 cases (31%). Aspergillus species were identified in 18 cultures (90%): Aspergillus fumigatus (n = 16), Aspergillus flavus (n = 2). The other fungi identified were Schizophillum com. and Fusarium spp.
Sinus hyperdensities evaluation
CT scan HD were observed in all FB patients except in one with a sphenoid sinus location. Four radiological patterns were observed: no HD, calcium-type HD, metal-type HD, and association of calcium-type and metal-type HD. These patterns are illustrated in Figure 1.
Maxillary sinus FB exhibited metal-type HD in 28 cases out of 45 (62%). Among these cases, metal-type HD were associated with calcium-type HD in 18 maxillary sinuses. Isolated calcium-type HD were observed in 17 cases of maxillary sinus FB (38%). Conversely, metal-type HD were never observed in sphenoid sinus FB, whereas isolated calcium-type HD were observed in 18 cases (95%). Metal-type HD were significantly associated with a maxillary location, whereas calcium-type HD were associated with a sphenoid sinus location (Table 1).
Table 1.
Main results illustrating different types of HD, density measurements in HUs, radiological features, and dental evaluations (NC)
| Maxillary sinus (N = 45) | Sphenoid sinus (N = 19) | Total (N = 64) | p-value | ||
|---|---|---|---|---|---|
| Presence of HD | Isolated calcium-like HD | 17 (38%) | 18 (95%) | 35 (55%) | 0.019 |
| Metal + Calcium like HD | 28 (62%) | 0 (0%) | 28 (44%) | 0.0003 | |
| Isolated metal-like HD | 10 (22%) | 0 (0%) | 10 (16%) | NC | |
| No HD | 0 | 1 (5%) | 1 (1.6%) | NC | |
| Densities (HU) Mean ± SD (range) | Calcium-like HD | 701 ± 394 (131–1578) | 679 ± 372 (143–1261) | 694 ± 371 (131–1578) | 0.25 |
| Metal-like HD | 4149 ± 343 (2516–13100) | NC | |||
| Endodontic treatment on FB side | Prevalence | 35 (78%) | 8 (42%) | 45 (70%) | 0.02 |
| Mean Nbr ± SD | 1.3 ± 1.5 | 0.7 ± 0.2 | 1.1 ± 1 | 0.04 | |
| Bone modifications | Sclerosis | 41 (91%) | 18 (95%) | 59 (92%) | 1 |
| Bone lysis | 28 (62%) | 8 (42%) | 36 (56%) | 0.14 | |
| Ostium dilation | 25 (56%) | 9 (47%) | 34 (53%) | 0.36 | |
| Dental extraction on FB side | Prevalence | 31 (69%) | 9 (47%) | 40 (63%) | 0.27 |
| Mean Nbr ± SD | 1.4 ± 0.2 | 1.2 ± 0.3 | 1.3 ± 1.3 | 0.25 | |
FB, fungus ball; HD, hyperdensities; HU, Hounsfield unit; NC, not calculated.
Fleiss' κ showed an almost perfect interobserver agreement for metal-type HD (κ = 0.94) and a substantial agreement for calcium-type HD evaluation (κ = 0.68). The mean density on CT scans was 4149 ± 343 HU for metal-type HD and 694 ± 50 HU for calcium-type HD (p < 0.0001) (Table 1). There was no difference between the mean densities of maxillary and sphenoid calcium-type HD (p = 0.25). Mean interclass correlation coefficients (ICC) showed excellent interobserver agreement for the densities of calcium-type HD (ICC = 0.73) and nearly perfect agreement for metal-type HD (ICC = 0.96). Concerning bone analysis, sclerosis was observed in 52 FB (81%), bone erosion in 21 cases (33%), and ostial dilation in 34 cases (53%) (Table 1).
Dental factors
Dental factors were analyzed in all the patients. The prevalence of endodontic treatment on the FB side was significantly higher in maxillary FB than in sphenoid FB (78% vs 42%; p = 0.02). Endodontic treatment overfilling was only observed on the FB side in 31% (14 patients) with maxillary sinus FB. Among the maxillary FB group, the prevalence of endodontic treatment was higher on the FB side than on the healthy side (78% vs 47%; p = 0.01). The difference in the prevalence of endodontic treatment between the FB side and the healthy side was not significant for sphenoid sinus FB (42% vs 42%; p = 1). The prevalence of dental extractions on the FB side was not significantly different between maxillary and sphenoid FB (69% vs 47%; p = 0.27). Among the maxillary FB group, the prevalence of dental extractions was not significantly different between the FB side and the healthy side (69% vs 67%; p = 0.82). The difference in the prevalence of dental extractions between the FB side and the healthy side was not significant in sphenoid FB (47% vs 68%; p = 0.19).
Association between hyperdensities and dental factors
To assess the association between metal-type HD and endodontic treatment in maxillary sinus FB, we performed a subgroup analysis between patients with metal-type HD and those without metal-type HD. On the FB side, the prevalence of endodontic treatment was higher in the group with metal-type HD than in the group without (89% vs 59%, respectively, p = 0.01). On the healthy side, the prevalence was not significantly different between both groups (46% vs 47%, p = 0.79). In the group of maxillary sinus, FB with metal-type HD, the prevalence of endodontic treatment on the FB side was statistically higher than on the healthy side (89% vs 46%, p = 0.028). The prevalence was not significantly different in the group without metal-type HD (59% for the FB side vs 47% for the healthy side, p = 0.7). These results are illustrated in Table 2. The mean density of endodontic treatment material in maxillary sinus FB with metal-type HD was 3816 ± 420 HU. The densities of metal-type HD and endodontic treatment exhibited a significant positive correlation (Spearman coefficient ρ = 0.91, p = 0.0001).
Table 2.
Relationship between endodontic treatment prevalence and radiological patterns within the maxillary fungus ball subgroup
| Endodontic treatment prevalence in maxillary FB (n = 45) | FB side | Healthy side | p-value |
|---|---|---|---|
| FB with metal-HD (n = 28) | 25 (89%) | 13 (46%) | 0.028 |
| FB without metal-HD (n = 17) | 10 (59%) | 8 (47%) | 0.7 |
| p -value | 0.01 | 0.79 |
FB, fungus ball; HD, hyperdensities.
Discussion
This study highlights two types of HD observed in maxillary or sphenoid FB. Metal-type HD seems specific to the maxillary sinus, and calcium-type HD can be found both in maxillary and sphenoid locations. A strong association was observed between maxillary FB, metal-type HD, and endodontic treatments.
The characteristics of FB HD according to their subjective and/or objective densities have been rarely studied to date. 9,11,12,21,22 We can now describe metal- and calcium-type HD with a significant interobserver agreement and a large difference in mean objective densities. Krennmair, Lenglinger et al described two types of HD in maxillary FB: organic type with a density <1500 HU and inorganic type with a density >1500 HU. 11,12 This 1500 HU cut-off is close to our findings with a maximal density of 1578 HU for calcium-type HD (range 131–1578) and a minimum density of 2516 HU for metal-type HD (range 2516–13,100), findings that are closer to the 2000 HU cut-off described later by Lenglinger et al. 12
The patterns of HD differed between maxillary and sphenoid sinus FB. Maxillary sinuses exhibited metal-type HD in 62% of cases, with or without an association with calcium-type HD. The association between metal-type HD and maxillary sinus FB has been widely described, and its prevalence ranges from 67.5 to 94%. 7,11,15,18,23 On the contrary, in our series, calcium-type HD occurred in both locations. Furthermore, sphenoid FB never exhibited metal-type HD but showed isolated calcium-type HD in 95% of the cases, which is close to the prevalence rates of previous studies. 21,24,25 These differences point to a specific fungal behavior that could be related to the physiopathology of the FB.
Stammberger et al analyzed the biochemical content of FB and found high concentrations of calcium phosphate, a little calcium sulfate, and traces of heavy metals. 20,26 The denser areas were correlated with the highest concentrations of calcium salts, which correspond to the central necrotic areas of FB. These necrotic areas with calcium salts could be due to the metabolism of the fungus. Consequently, the presence of HD in any sinus cavity may simply be fortuitous and have no link with the etiology of FB. While our results show a strong association between endodontic treatments and maxillary FB, they concern only the group with metal-type HD, as investigated by other authors. 7,14,15,17 In other words, while the dental care is statistically related to the presence of an FB, not all FB seem to be related to dental care.
Even though our study was limited by the retrospective gathering of data with a possible underestimation of previous endodontic treatments, it appears crucial to understand the pathogenesis of FB to make this distinction. The endodontic treatment and material can promote fungal growth in the maxillary sinus, and the association between endodontic treatments and FB has been reported. Mensi et al observed a prevalence of 89% for endodontic treatments in maxillary FB. 7 However, their analysis included all teeth except the incisors, but the canines and the first premolars are rarely close to the maxillary floor, leading to an overestimated prevalence. Nicolai et al published a study of 120 patients with maxillary FB in whom 104 had a maxillary treated tooth on the ipsilateral side-of the FB (86.7%). 17 On the other hand, Dufour et al found overfilling of a dental cavity in only 18 (10.4%) of 173 patients with maxillary sinus FB. 5 Therefore, the statistical relationship is not sufficient to establish a causal link. Moreover, the incidence of endodontic treatments with or without overfilling in the population without a FB should be established.
In the case of a metal-type HD, it is still impossible to know whether it is due to excessive endodontic treatment or whether the fungus arises from a small amount of extruded material in the sinus. Until now, metal-type HD has been suspected to be due to extruded root filling material, given the positive correlation between the density of such material and metal-type HD, as in our study. 11,12 However, this density correlation does not mean a causal relationship. Moreover, in our work, almost all density measurements were limited to 3071 HU due to the CT-scan devices' technical specifications. This technical limitation probably led to an underestimation of the densities of endodontic materials and metal-type HD without biasing the calcium-type HD evaluation. In the five CT scans in which densities >3071 HU were recorded, the mean maximal densities of metal-type HD and endodontic material tended to correlate (9569 ± 2883 HU and 8188 ± 4760 HU, respectively). The high densities of endodontic treatments are due to their high concentration in heavy metals such as zinc, copper, lead, silver bismuth, and iron. 27,28 This composition is entirely different from that found by Stammberger et al in FB. 20,26 Zinc oxide, and bismuth salts have been shown to promote fungal growth in vitro 29,30 and probably play a role in the sinus concretions of maxillary FB. 28,31 However, the authors did not analyze specifically the metal-type HD themselves compared to calcium-type HD and the remaining FB.
This odontogenic theory cannot explain the other locations of FB, so another mechanism leading to their formation may be involved. Milosev et al first described the aerogenic theory, which holds that spores inhaled into the sinus may become pathogenic in anaerobic conditions. 11 However, computational fluid dynamic studies have demonstrated a very low flow velocity or no flow into the paranasal sinuses, 32–34 supporting the theory that maxillary sinuses are not aerated through the ostio–meatal complex. In fact, the role of the maxillary sinus is to produce nitric oxide, which passes from the sinus to the main airflow toward the nasal cavity. 35–37 Moreover, these studies demonstrated sterile sinus cultures, but only bacteriological cultures were performed and not mycological cultures. Finally, Kostamo et al showed that chronic hyperplastic sinusitis and fungal sinusitis are not associated with exposure to moisture. 38 However, whatever the mechanism, fungal spores are thought to reach the sinus cavity and develop FB. The penetration of fungal spores through the ostio–meatal complex could be assisted by a wider ostio–meatal complex, yet no patient develops an FB after middle antrostomy. Complex fluid dynamic changes like sneezing could be a cause. Alternatively, endodontic sealers may become porous several years after endodontic treatment. 39 These complex physiopathological mechanisms are still unclear and need further studies to be fully elucidated. Finally, few authors have observed metal-type HD in sphenoid FB. 17,18 The odontogenic theory cannot account for these findings owing to the distance between the two sites. A more plausible is the high accumulation of calcium salts in the fungal mass.
Conclusions
This study highlights two types of HD observed in maxillary or sphenoid FB. Metal-type HD seems specific to the maxillary sinus, while calcium-type HD can be found both in maxillary and sphenoid locations. We found a strong association between endodontic treatments, maxillary FB, and metal-type HD. The findings fuel the debate about FB physiopathology: FB with metal-type HD could be linked to an “endodontic” etiology, while FB without metal-type HD could be the consequence of other causes allowing the penetration of fungal spores into the sinus cavity. Further studies with comparative spectrophotometric evaluations of metal-type HD and endodontic treatment could help unravel the physiopathology of FB and give substance to the odontogenic theory.
Footnotes
Acknowledgment: The authors would like to thank Dr Ray Cooke for his linguistic assistance.
Disclosure: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Ahmed Alharbi, Email: dralahmadi3@gmail.com.
Nicolas Reville, Email: nicolas.reville@chu-bordeaux.fr.
Sandrine Molinier, Email: sandrine.molinier@chu-bordeaux.fr.
Pierre-Louis Bastier, Email: plbastier@orange.fr.
Ludovic de Gabory, Email: ludovic.de-gabory@chu-bordeaux.fr.
REFERENCES
- 1. deShazo RD, O’Brien M, Chapin K, Soto-Aguilar M, Swain R, Lyons M, et al. Criteria for the diagnosis of sinus mycetoma. J Allergy Clin Immunol 1997; 99: 475–85. doi: 10.1016/s0091-6749(97)70073-3 [DOI] [PubMed] [Google Scholar]
- 2. Kopp W, Fotter R, Ebner F, Beaufort F, Stammberger H. Radiological aspects of aspergillosis in the paranasal sinuses. Eur J Radiol 1986; 6: 178–80. [PubMed] [Google Scholar]
- 3. Kopp W, Fotter R, Steiner H, Beaufort F, Stammberger H. Aspergillosis of the paranasal sinuses. Radiology 1985; 156: 715–16. doi: 10.1148/radiology.156.3.4023231 [DOI] [PubMed] [Google Scholar]
- 4. Robey AB, O’Brien EK, Richardson BE, Baker JJ, Poage DP, Leopold DA. The changing face of paranasal sinus fungus balls. Ann Otol Rhinol Laryngol 2009; 118: 500–505. doi: 10.1177/000348940911800708 [DOI] [PubMed] [Google Scholar]
- 5. Dufour X, Kauffmann-Lacroix C, Ferrie JC, Goujon JM, Rodier MH, Klossek JM. Paranasal sinus fungus ball: epidemiology, clinical features and diagnosis. A retrospective analysis of 173 cases from A single medical center in france, 1989-2002. Med Mycol 2006; 44: 61–67. doi: 10.1080/13693780500235728 [DOI] [PubMed] [Google Scholar]
- 6. Nomura K, Asaka D, Nakayama T, Okushi T, Matsuwaki Y, Yoshimura T, et al. Sinus fungus ball in the japanese population: clinical and imaging characteristics of 104 cases. Int J Otolaryngol 2013; 2013: 731640. doi: 10.1155/2013/731640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mensi M, Piccioni M, Marsili F, Nicolai P, Sapelli PL, Latronico N. Risk of maxillary fungus ball in patients with endodontic treatment on maxillary teeth: a case-control study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2007; 103: 433–36. doi: 10.1016/j.tripleo.2006.08.014 [DOI] [PubMed] [Google Scholar]
- 8. Eloy P, Grenier J, Pirlet A, Poirrier AL, Stephens JS, Rombaux P. Sphenoid sinus fungall ball: a retrospective study over a 10- year period. Rhinology 2013; 51: 181–88. doi: 10.4193/Rhino12.114 [DOI] [PubMed] [Google Scholar]
- 9. Klossek JM, Serrano E, Péloquin L, Percodani J, Fontanel JP, Pessey JJ. Functional endoscopic sinus surgery and 109 mycetomas of paranasal sinuses. Laryngoscope 1997; 107: 112–17. doi: 10.1097/00005537-199701000-00021 [DOI] [PubMed] [Google Scholar]
- 10. Yoon JH, Na DG, Byun HS, Koh YH, Chung SK, Dong HJ. Calcification in chronic maxillary sinusitis: comparison of CT findings with histopathologic results. AJNR Am J Neuroradiol 1999; 20: 571–74. [PMC free article] [PubMed] [Google Scholar]
- 11. Krennmair G, Lenglinger F. Maxillary sinus aspergillosis: diagnosis and differentiation of the pathogenesis based on computed tomography densitometry of sinus concretions. J Oral Maxillofac Surg 1995; 53: 657–63. doi: 10.1016/0278-2391(95)90164-7 [DOI] [PubMed] [Google Scholar]
- 12. Lenglinger FX, Krennmair G, Müller-Schelken H, Artmann W. Radiodense concretions in maxillary sinus aspergillosis: pathogenesis and the role of CT densitometry. Eur Radiol 1996; 6: 375–79. doi: 10.1007/BF00180617 [DOI] [PubMed] [Google Scholar]
- 13. Milosev B, el-Mahgoub S, Aal OA, el-Hassan AM. Primary aspergilloma of paranasal sinuses in the sudan. A review of seventeen cases. Br J Surg 1969; 56: 132–37. doi: 10.1002/bjs.1800560213 [DOI] [PubMed] [Google Scholar]
- 14. Beck-Mannagetta J, Necek D. Radiologic findings in aspergillosis of the maxillary sinus. Oral Surg Oral Med Oral Pathol 1986; 62: 345–49. doi: 10.1016/0030-4220(86)90019-8 [DOI] [PubMed] [Google Scholar]
- 15. Legent F, Billet J, Beauvillain C, Bonnet J, Miegeville M. The role of dental canal fillings in the development of aspergillus sinusitis. a report of 85 cases. Arch Otorhinolaryngol 1989; 246: 318–20. doi: 10.1007/BF00463584 [DOI] [PubMed] [Google Scholar]
- 16. Tomazic PV, Dostal E, Magyar M, Lang-Loidolt D, Wolf A, Koele W, et al. Potential correlations of dentogenic factors to the development of clinically verified fungus balls: A retrospective computed tomography-based analysis. Laryngoscope 2016; 126: 39–43. doi: 10.1002/lary.25416 [DOI] [PubMed] [Google Scholar]
- 17. Nicolai P, Lombardi D, Tomenzoli D, Villaret AB, Piccioni M, Mensi M, et al. Fungus ball of the paranasal sinuses: experience in 160 patients treated with endoscopic surgery. Laryngoscope 2009; 119: 2275–79. doi: 10.1002/lary.20578 [DOI] [PubMed] [Google Scholar]
- 18. Ledderose GJ, Braun T, Betz CS, Stelter K, Leunig A. Functional endoscopic surgery of paranasal fungus ball: clinical outcome, patient benefit and health-related quality of life. Eur Arch Otorhinolaryngol 2012; 269: 2203–8. doi: 10.1007/s00405-012-1925-7 [DOI] [PubMed] [Google Scholar]
- 19. Tanaka H, Sakae T, Mishima H, Yamamoto H. Calcium phosphate in aspergillosis of the maxillary sinus. Scanning Microsc 1993; 7: 1241–45. [PubMed] [Google Scholar]
- 20. Stammberger H, Jakse R, Beaufort F. Aspergillosis of the paranasal sinuses X-ray diagnosis, histopathology, and clinical aspects. Ann Otol Rhinol Laryngol 1984; 93: 251–56. doi: 10.1177/000348948409300313 [DOI] [PubMed] [Google Scholar]
- 21. Seo Y-J, Kim J, Kim K, Lee J-G, Kim CH, Yoon JH. Radiologic characteristics of sinonasal fungus ball: an analysis of 119 cases. Acta Radiol 2011; 52: 790–95. doi: 10.1258/ar.2011.110021 [DOI] [PubMed] [Google Scholar]
- 22. Krennmair G, Lenglinger F, Müller-Schelken H. Computed tomography (CT) in the diagnosis of sinus aspergillosis. J Craniomaxillofac Surg 1994; 22: 120–25. doi: 10.1016/s1010-5182(05)80022-8 [DOI] [PubMed] [Google Scholar]
- 23. Broglie MA, Tinguely M, Holzman D. How to diagnose sinus fungus balls in the paranasal sinus? an analysis of an institution’s cases from january 1999 to december 2006. Rhinology 2009; 47: 379–84. doi: 10.4193/Rhin09.026 [DOI] [PubMed] [Google Scholar]
- 24. Kim TH, Na KJ, Seok JH, Heo SJ, Park JH, Kim JS. A retrospective analysis of 29 isolated sphenoid fungus ball cases from A medical centre in korea (1999-2012). Rhinology 2013; 51: 280–86. doi: 10.4193/Rhino12.145 [DOI] [PubMed] [Google Scholar]
- 25. Bowman J, Panizza B, Gandhi M. Sphenoid sinus fungal balls. Ann Otol Rhinol Laryngol 2007; 116: 514–19. doi: 10.1177/000348940711600706 [DOI] [PubMed] [Google Scholar]
- 26. Stammberger H, Jakse R, Raber J. Aspergillus mycoses of the paranasal sinuses. detection and analysis of roentgen opaque structures in fungal concretions. HNO 1983; 31: 161–67. [PubMed] [Google Scholar]
- 27.. Ørstavik D. Endodontic filling materials: Endodontic filling materials. Endod Top 2014;31(1):53-67. [Google Scholar]
- 28. Jafari F, Jafari S. Composition and physicochemical properties of calcium silicate based sealers: a review article. J Clin Exp Dent 2017; 9: e1249–55. doi: 10.4317/jced.54103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Alharbi SA, Mashat BH, Al-Harbi NA, Wainwright M, Aloufi AS, Alnaimat S. Bismuth-inhibitory effects on bacteria and stimulation of fungal growth in vitro. Saudi J Biol Sci 2012; 19: 147–50. doi: 10.1016/j.sjbs.2012.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Willinger B, Beck-Mannagetta J, Hirschl AM, Makristathis A, Rotter ML. Influence of zinc oxide on aspergillus species: a possible cause of local, non-invasive aspergillosis of the maxillary sinus. Mycoses 1996; 39: 361–66. doi: 10.1111/j.1439-0507.1996.tb00154.x [DOI] [PubMed] [Google Scholar]
- 31. Nicolai P, Mensi M, Marsili F, Piccioni M, Salgarello S, Gilberti E, et al. Maxillary fungus ball: zinc-oxide endodontic materials as a risk factor. Acta Otorhinolaryngol Ital 2015; 35: 93–96. [PMC free article] [PubMed] [Google Scholar]
- 32. Xiong G, Zhan J-M, Jiang H-Y, Li J-F, Rong L-W, Xu G, et al. Computational fluid dynamics simulation of airflow in the normal nasal cavity and paranasal sinuses. Am J Rhinol 2008; 22: 477–82. doi: 10.2500/ajr.2008.22.3211 [DOI] [PubMed] [Google Scholar]
- 33. Xi J, Yuan JE, Si XA, Hasbany J. Numerical optimization of targeted delivery of charged nanoparticles to the ostiomeatal complex for treatment of rhinosinusitis. Int J Nanomedicine 2015; 10: 4847–61. doi: 10.2147/IJN.S87382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. de Gabory L, Reville N, Baux Y, Boisson N, Bordenave L. Numerical simulation of two consecutive nasal respiratory cycles: toward a better understanding of nasal physiology. Int Forum Allergy Rhinol 2018; 8: 676–85. doi: 10.1002/alr.22086 [DOI] [PubMed] [Google Scholar]
- 35. Lundberg JO, Rinder J, Weitzberg E, Lundberg JM, Alving K. Nasally exhaled nitric oxide in humans originates mainly in the paranasal sinuses. Acta Physiol Scand 1994; 152: 431–32. doi: 10.1111/j.1748-1716.1994.tb09826.x [DOI] [PubMed] [Google Scholar]
- 36. Lundberg JO, Farkas-Szallasi T, Weitzberg E, Rinder J, Lidholm J, Anggåard A, et al. High nitric oxide production in human paranasal sinuses. Nat Med 1995; 1: 370–73. doi: 10.1038/nm0495-370 [DOI] [PubMed] [Google Scholar]
- 37. Sobin J, Engquist S, Nord CE. Bacteriology of the maxillary sinus in healthy volunteers. Scand J Infect Dis 1992; 24: 633–35. doi: 10.3109/00365549209054650 [DOI] [PubMed] [Google Scholar]
- 38. Kostamo K, Richardson M, Malmberg H, Ylikoski J, Ranta H, Toskala E. Does the triad of fungi, bacteria and exposure to moisture have an impact on chronic hyperplastic sinusitis? Indoor Air 2005; 15: 112–19. doi: 10.1111/j.1600-0668.2004.00322.x [DOI] [PubMed] [Google Scholar]
- 39. Ortiz FG, Jimeno EB. Analysis of the porosity of endodontic sealers through micro-computed tomography: A systematic review. J Conserv Dent 2018; 21: 238–42. doi: 10.4103/JCD.JCD_346_17 [DOI] [PMC free article] [PubMed] [Google Scholar]


