Abstract
Background:
Demodex parasites are the most common ectoparasites in humans. One of the factors in the pathogenesis of an increase in parasite density is immunosuppression. In this prospective study, we aimed to evaluate the effect of phototherapy-induced immunosuppression on Demodex density.
Material and Methods:
Thirty-five patients receiving phototherapy were included in the study. The number of parasites in the samples taken from the right cheek, left cheek, forehead, nose and chin of the patients, by standardized skin surface biopsy method, were recorded before phototherapy and in the third month of treatment.
Results:
Of the 35 patients, the female-to-male ratio was found to be 2.1:1. There was no statistically significant difference between the ages of the male and female patients. The increase in the number of parasites in the right cheek, left cheek, nose and chin in the third month was statistically significant; whereas the increase in the forehead was not.
Conclusion:
The results of our study showed that phototherapy can cause an increase in Demodex density, and these findings are compatible with those of other studies in the literature. Since our study aims to evaluate density at the beginning and the end of the third month of phototherapy, it differs from other studies by indicating the effect of phototherapy more accurately.
Keywords: Demodex, immunosuppression, phototherapy
Introduction
Demodex parasites are part of the large microbiome that lives on human skin.[1] The most common places are on the face; cheeks, forehead, nasolabial area and eyelashes, also they can be found in the scalp, trunk and genital area. They are obligatory parasites and can be seen on the skin without any clinical symptoms.[2,3] The most widely used method in practice to evaluate the number of parasites is the “standard superficial skin biopsy (SSSB)” method. The SSSB method was described by Marks and Dawber. Since it is a semi-invasive method, it is suitable for multiple samples to be taken from a patient, as in our study.[4] An area of 1 cm2 is marked on the slide and a drop of cyanoacrylate adhesive is put on this area. The area to be sampled is cleaned with alcohol. The adhesive-dropped surface of the slide has adhered to the area. After about one minute, when the adhesive dries, the slide is pulled slowly to separate it from the skin surface. Thus, the follicular component is collected. Immersion oil is dripped onto the sample, covered with a coverslip and examined under a light microscope at x10 magnification and then x40 magnification. The sample taken should be examined within one hour.[4,5] In this examination, the detection of 5 or more parasites in an area of 1 cm2 is considered significant and the clinical picture is called “demodicidosis.”[6] Demodicidosis gives non-specific clinical signs such as rash, itching, follicular scale, papulopustular eruption, rough and sandpaper-like feel.[7]
The increase in density of demodex parasites and their penetration into the dermis shows an inflammation-initiating feature.[3] It is thought that parasites escape from the immune system by suppressing the innate immune response of the host.[8] With protease inhibitors released by parasites, the complement system is inhibited, the immune mediators of the host are broken down and inflammatory mediators increase.[9] A large number of parasites mechanically block the hair follicle and sebaceous glands, disrupting the skin barrier, and both this blockage and the chitin skeleton of the parasites and released enzymes stimulate the inflammatory response.[1] Although many factors such as age, gender, genetic factors, ultraviolet (UV) exposure, skin sebum and pH level, hygiene and body mass index are responsible for the occurrence of demodicidosis, immunosuppression is the most important risk factor.[2,3,10] Systemic and local immunosuppressive conditions increase the parasite density and T cell defect is considered the most important factor for parasite invasion.[2,10]
Phototherapy refers to the use of UV light in the treatment of skin diseases.[11] Although the mechanism of phototherapy is not well-known, it is thought to function through its antiproliferative effect, apoptosis and suppression of the immune system.[12,13]
Material and Methods
Patients and study design
In this prospective study, 35 patients who started phototherapy at İzmir Katip Çelebi University Atatürk Training and Research Hospital Dermatology Clinic between 29.03.2018 and 29.06.2018 were included. There was no gender or clinical diagnosis limitation for the patients. Patients who started local Psoralen UVA (PUVA) treatment in which the face did not receive UV, patients with lesions on their faces, using the topical immunosuppressive treatment on their faces and those younger than 18 years of age were excluded from the study. None of the patients were using any systemic immunosuppressive medication. The ages, genders and clinical diagnoses of the patients included in the study were recorded. Parasite examinations were performed before receiving their first treatment and in the third month of their treatment. Samples were taken from the right cheek, left cheek, forehead, nose and chin regions of all patients by standardized superficial skin biopsy method (SSSB) and parasite numbers were recorded for each zone. In other words, the patients included in our study were only those who received phototherapy and did not use any systemic immunosuppressive therapy, and there was no lesion in the facial areas we sampled.
Statistical analysis
Statistical analysis of the data was performed in IBM SPSS Statistics Version 22 program. All data were presented as n (%), mean ± standard deviation and median (minimum-maximum). Mann Whitney U analysis was used to compare the ages of male and female cases and Wilcoxon Signed Ranks analysis was used to compare baseline and 3-month measurements. A P value of less than 0.05 was considered statistically significant.
Results
Demographic features
Of the 35 patients included in the study, 24 (69%) were female and 11 (31%) were male. The age range of the patients varied between 18 and 87. The mean age of women was 45.17 ± 19.52 and the mean age of men were 38.73 ± 17.92. There was no statistically significant difference between the ages of the female and male patients (p > 0.05) [Table 1].
Table 1.
Average age distribution by gender of the patients
| Gender | n | % | Age | Z | P | |
|---|---|---|---|---|---|---|
|
| ||||||
| Ort.±SS | Median (Min.-Max.) | |||||
| Male | 11 | 31,4 | 38,73±17,92 | 36 (19-67) | −0,782 | 0,434 |
| Female | 24 | 68,6 | 45,17±19,52 | 47 (15-87) | ||
| Total | 35 | 100,0 | 43,14±19,01 | 45 (15-87) | ||
Clinical diagnoses
Distribution of diagnoses; nine patients (25.7%) had psoriasis vulgaris, nine patients (25.7%) had vitiligo, six patients (17.1%) had parapsoriasis, four patients (11.4%) had pruritus, three patients (8.6%) had mycosis fungoides, one patient (2.9%) had alopecia totalis, one patient (2.9%) had hypertrophic lichen planus, one patient (2.9%) had pityriasis rubra pilaris, and one patient (2.9%) had pityriasis lichenoides chronica [Table 2].
Table 2.
Clinical diagnosis
| Diagnosis | n | % |
|---|---|---|
| Alopecia Totalis | 1 | 2,9 |
| Hypertrophic lichen planus | 1 | 2,9 |
| Mycosis Fungoides | 3 | 8,6 |
| Parapsoriasis | 6 | 17,1 |
| Pityriasis lichenoides chronica | 1 | 2,9 |
| Pityriasis Rubra Pilaris | 1 | 2,9 |
| Pruritus | 4 | 11,4 |
| Psoriasis Vulgaris | 9 | 25,7 |
| Vitiligo | 9 | 25,7 |
| Total | 35 | 100,0 |
Phototherapy type
All of the 35 patients included in the study were receiving narrowband UVB (dbUVB) treatment three days a week and the patients did not interrupt the treatment during the three months.
Parasite numbers
Before the first session of phototherapy and in the third month, the number of Demodex parasites was evaluated and recorded using the SSSB method from the right cheek, left cheek, forehead, nose and chin regions. The increase in the number of parasites in the right cheek, left cheek, nose and chin in the third month was statistically significant (p 0.05); whereas the increase in the forehead was not statistically significant (p > 0.05) [Table 3]. The highest parasite numbers were observed on the cheeks of the patients in our study. The microscopic photo shows the increase in demodex density in samples taken from the left cheek area of a patient before and after treatment [Figures 1 and 2].
Table 3.
Parasite numbers before and 3rd month of phototheraphy
| Ort.±SS | Median (Min.-Max.) | Z | P | |
|---|---|---|---|---|
| Right cheek | ||||
| Before | 11,23±23,24 | 2 (0113) | −3,332 | 0,001 |
| 3. month | 19,57±38,86 | 4 (0-172) | ||
| Left cheek | ||||
| Before | 13,8±29,37 | 3 (0-152) | −3,278 | 0,001 |
| 3. month | 20,8±36,21 | 7 (0-160) | ||
| Forehead | ||||
| Before | 6,66±14,78 | 0 (0-71) | −1,770 | 0,077 |
| 3. month | 8,86±14,72 | 2 (0-50) | ||
| Nose | ||||
| Before | 0,6±1,12 | 0 (0-5) | −2,852 | 0,004 |
| 3. month | 2,11±4,12 | 0 (0-21) | ||
| Chin | ||||
| Before | 1,37±2,03 | 0 (0-7) | −3,477 | 0,001 |
| 3. month | 5,94±14,86 | 2 (0-83) |
Figure 1.
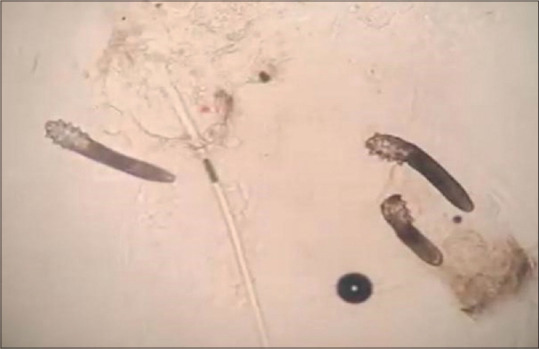
An image showing the density of demodex on a patient's left cheek before phototherapy
Figure 2.
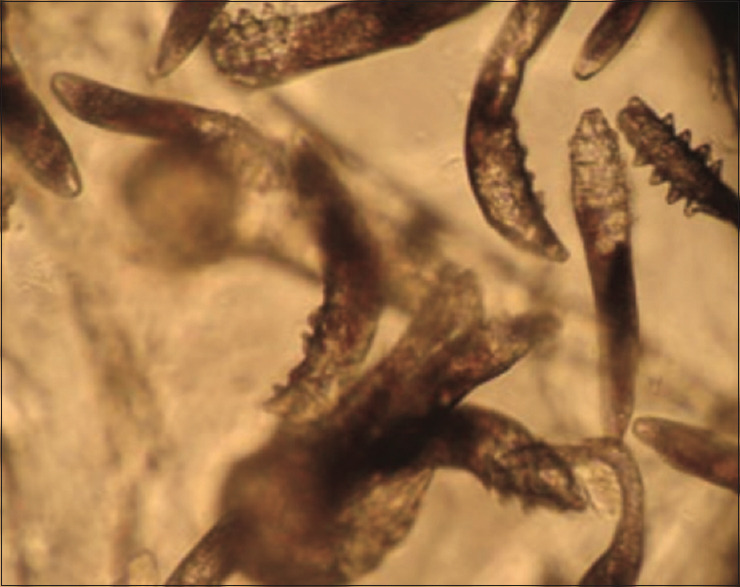
An image showing the density of demodex on a patient's left cheek in the third-month phototherapy
Discussion
Demodex parasites settle in hair follicles and sebaceous glands and they can live without any clinical symptoms. The increase in the number of Demodex parasites or their penetration into the dermis is considered to initiate inflammation.[2,3] Although the mechanisms of inflammation by parasites are not well-known, it is thought that parasites escape from the immune system by suppressing the innate immune response of the host.[1,8,14] The parasites which increase their number with local immunosuppression they create, interact with the cells in the pilosebaceous unit and stimulate the release of inflammatory mediators.[1,15]
Rosacea is the disease most associated with Demodex, and many studies have been published on this topic. In many studies, the density of parasites in rosacea was found to be higher than in the control groups.[16]
In a study conducted on patients with type-2 diabetes mellitus (DM), it was found that the number of Demodex and mean parasite sizes were higher than in the control group. It is thought that cellular and humoral immune system dysfunction and peripheral vascular insufficiency may be effective in the increase of the parasite.[17,18] Cellular immunity is suppressed during pregnancy and the secretion of sebaceous glands increases. Even though, a significant increase was not found in a study investigating whether there was an increase in parasite density.[19]
In another study, an increase in Demodex density was observed in patients treated with immunosuppressive drugs such as topical steroid, tacrolimus and pimecrolimus.[20] In a study conducted in a group of patients with haematological malignancies who received chemotherapy, the density of Demodex parasites was found to be higher than the control group.[21] Increasing parasite density was detected in patients with renal transplant in a case series of four patients,[22] also parasite increase has been shown in paediatric cases diagnosed with Acute Lymphocytic Leukemia.[23] Clinical findings related to demodicidosis have also been reported in patients with acquired immunodeficiency syndrome.[24] As a result, many studies are showing that immunosuppression increases parasite density.[1]
Phototherapy is used in the treatment of many diseases in dermatology. The basis of mechanism of action is based on the immunosuppression effect. In addition to immunosuppression, hyperplasia that develops in sebaceous glands due to the UV effect has been reported to be effective in the increase of Demodex density. There are two studies in the literature on the effect of immunosuppression caused by phototherapy and sebaceous hyperplasia on demodicidosis.[8] In a study, papulopustular lesions due to Demodex were described on the face and upper body of patients receiving UVB treatment at 311 nm wavelength.[25] The study of Kulaç et al.[10] was more comprehensive, and patients who received PUVA and dbUVB treatment were selected regardless of the number of sessions, and the control group consisted of healthy individuals. As a result of this study, a significant increase was found in the Demodex density of patients who received phototherapy compared to the control group and the increase in patients receiving PUVA was higher than in patients receiving dbUVB.
In our study, before the treatment of patients who received phototherapy and in the third month of their treatment; we compared the numbers of Demodex parasites we obtained from the right cheek, left cheek, forehead, nose and chin using SSSB. Two previous studies consisted of patients who received phototherapy regardless of the number and duration of sessions. We tried to put forward the effect of phototherapy on the formation of demodicidosis in a more realistic way with the evaluation made before and three months after phototherapy. All 35 patients included in our study were receiving dbUVB three days a week. Kulaç et al.[10] claimed in their study that PUVA treatment was a more effective cause of demodicidosis than dbUVB treatment. In our study, no comparison could be made since there was no patient receiving PUVA therapy. Longer-term studies are required in larger patient series to demonstrate the effect of phototherapy on parasite density.
Our conclusion and recommendation from our study are that demodicidosis should be kept in mind in the differential diagnosis of patients receiving phototherapy when an eruption develops on the face and trunk during treatment.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Moran EM, Foley R, Powell FC. Demodex and rosacea revisited. Clin Dermatol. 2017;35:195–200. doi: 10.1016/j.clindermatol.2016.10.014. [DOI] [PubMed] [Google Scholar]
- 2.Dokuyucu R, Kaya ÖA, Yula E, Üstün Ġ, Bayram F, Gökçe C. The presence of Demodex foliculorum in various obese groups according to BMI levels. Arch Iran Med. 2016;19:210–4. [PubMed] [Google Scholar]
- 3.Yun CH, Yun JH, Beak JO, Roh JY, Lee JR. Demodex mite density determinations by standardized skin surface biopsy and direct microscopic examination and their relations with clinical types and distribution patterns. Ann Dermatol. 2017;29:137–42. doi: 10.5021/ad.2017.29.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aşkın Ü, Seçkin D. Comparison of the two techniques for measurement of the density of Demodex folliculorum: Standardized skin surface biopsy and direct microscopic examination. Br J Dermatol. 2010;162:1124–6. doi: 10.1111/j.1365-2133.2010.09645.x. [DOI] [PubMed] [Google Scholar]
- 5.Aytekin S, Göktay F, Yaşar Ş, Gizlenti S. Tips and tricks on Demodex density examination by standardized skin surface biopsy. Eur Acad Dermatol Venerol. 2016;30:e126–8. doi: 10.1111/jdv.13402. [DOI] [PubMed] [Google Scholar]
- 6.Chang YS, Huang YC. Role of demodex mite infestation in rosacea: A systematic review and meta-analysis. J Am Acad Dermatol. 2017;77:441–7. doi: 10.1016/j.jaad.2017.03.040. [DOI] [PubMed] [Google Scholar]
- 7.Forton FMN, De Maertelaer V. Two consecutive standardized skin surface biopsies: An improved sampling method to evaluate Demodex density as a diagnostic tool for rosacea and demodicosis. Acta Derm Venerol. 2017;97:242–6. doi: 10.2340/00015555-2528. [DOI] [PubMed] [Google Scholar]
- 8.Forton FMN. Papulopustular rosacea, skin immunity and Demodex: Pityriasis folliculorum as a missing link. Eur Acad Dermatol Venerol. 2012;26:19–28. doi: 10.1111/j.1468-3083.2011.04310.x. [DOI] [PubMed] [Google Scholar]
- 9.Chen W, Plewig G. Human demodicosis: Revisit and a proposed classification. Br J Dermatol. 2014;170:1219–25. doi: 10.1111/bjd.12850. [DOI] [PubMed] [Google Scholar]
- 10.Kulaç M, Çiftçi GH, Karaca G, Çetinkaya Z. Clinical importance of Demodex folliculorum in patients receiving phototheraphy. Int J Dermatol. 2008;47:72–7. doi: 10.1111/j.1365-4632.2007.03336.x. [DOI] [PubMed] [Google Scholar]
- 11.Ferahbaş A. Fototerapi uygulama şekilleri ve protokolleri. Türkderm. 2010;44:67–72. [Google Scholar]
- 12.Şavk E. Foto (kemo) terapinin immünolojisi. Türkderm. 2010;44:62–6. [Google Scholar]
- 13.Akyol M. Fototerapi. Turkderm Arch Turk Dermatol Venerol. 2016;50:13–7. [Google Scholar]
- 14.Akilov OE, Mumcuoğlu KY. Association between human demodicosis and HLA class I. Clin Exp Dermatol. 2003;28:70–3. doi: 10.1046/j.1365-2230.2003.01173.x. [DOI] [PubMed] [Google Scholar]
- 15.Hay RJ. Demodex and skin disease- false creation or palpable form? Br J Dermatol. 2014;170:1214–8. doi: 10.1111/bjd.13015. [DOI] [PubMed] [Google Scholar]
- 16.Casas C, Paul C, Lahfa M, Livideanu B, Lejeune O, Alvarez-Georges S, et al. Quantification of Demodex folliculorum by PCR in rosacea and its relationship to skin immune activation. Exp Dermatol. 2012;21:906–10. doi: 10.1111/exd.12030. [DOI] [PubMed] [Google Scholar]
- 17.Akdeniz S, Bahçeci M, Tuzcu AK, Harman M, Alp S, Bahçeci S. Is Demodex folliculorum larger in diabetic patients? Eur Acad Dermatol Venerol JEADV. 2002;16:532–48. doi: 10.1046/j.1468-3083.2002.00545_7.x. [DOI] [PubMed] [Google Scholar]
- 18.Hom MM, Mastrota KM, Schachter SE. Demodex: Clinical cases and diagnostic protocol. Optometry Vision Sci. 2013;90:198–205. doi: 10.1097/OPX.0b013e3182968c77. [DOI] [PubMed] [Google Scholar]
- 19.Esen Aydıngöz Ġ, Dervent B, Güney O. Demodex folliculorum in pregnancy. Int J Dermatol. 2000;39:743–5. doi: 10.1046/j.1365-4362.2000.00013.x. [DOI] [PubMed] [Google Scholar]
- 20.Dolenc-Voljc M, Pohar M, Lunder T. Density of Demodex folliculorum in perioral dermatitis. Acta Derm Venerol. 2005;85:211–5. doi: 10.1080/00015550510030069. [DOI] [PubMed] [Google Scholar]
- 21.Seyhan ME, Karıncalıoğlu Y, Bayram N, Aycan Ö, Kuku I. Density of Demodex folliculorum in haematological malignancies. J Int Med Res. 2004;32:411–5. doi: 10.1177/147323000403200410. [DOI] [PubMed] [Google Scholar]
- 22.Chovatiya RJ, Colegio OR. Demodicosis in renal transplant recipients. Am J Transplant. 2016;16:712–6. doi: 10.1111/ajt.13462. [DOI] [PubMed] [Google Scholar]
- 23.Herron MD, O’Reilly MA, Vanderhooft SL. Refractory Demodex folliculitis in five children with acute lymphoblastic leukemia. Pediatr Dermatol. 2005;22:407–11. doi: 10.1111/j.1525-1470.2005.00103.x. [DOI] [PubMed] [Google Scholar]
- 24.Kosik-Bogacka DI, Lanocha N, Lanocha A, Czepita D, Grobelny A, Zdziarska B, et al. Demodex folliculorum and Demodex brevis in healthy and immunocompromised patients. Ophthalmic Epidemiyol. 2013;20:159–63. doi: 10.3109/09286586.2013.789532. [DOI] [PubMed] [Google Scholar]
- 25.Aytekin S. Outbreak of demodex folliculitis on the face and upper trunk during 311-nm UVB therapy for psoriasis. Eur Acad Dermatol Venerol JEADV. 2004;18:221–42. doi: 10.1111/j.1468-3083.2004.00898.x. [DOI] [PubMed] [Google Scholar]


