Abstract
Alopecia areata (AA) is type of autoimmune, T-cell-mediated disease with abnormal expression of MHC Class I, a common reason for non-scarring hair loss. Familial Mediterranean fever (FMF) is a hereditary autoinflammatory disease characterized by periodic fever and serositis. Various diseases and conditions that may be related to FMF have been reported. It has been known that patients with FMF are vulnerable to MHC Class I-related diseases. The co-existence of the two MHC Class I group-associated entities, FMF and AA, has not been reported in the literature. Here, we present three cases with AA and FMF and discuss the possible common pathway in their pathogenesis.
Keywords: Alopecia areata, Familial Mediterranean fever, MHC class 1
Introduction
Alopecia areata is characterized by non-scarring well-demarcated alopecic patches. Although its aetiopathogenesis has not been fully elucidated, the collapse in the immune privilege (IP) of the anagen hair follicle (HF) was proved. It has been suggested that MHC-I expression is more important than MHC-II expression and it may facilitate autoimmune attack of hair follicles by CD8+ cells.[1] Moreover, ectopic MHC-I expression in the HF epithelium and presentation of autoantigens to CD8+ T cells may play a role in the development of AA.[2] A correlation is found between the CD8+ T cell density and the severity of disease.[3]
Familial Mediterranean fever is a kind of autoinflammatory disease that occurs as a result of a mutated MEFV gene, which encodes the pyrin protein.[4] The prevalence of MHC-II-spectrum autoimmune diseases is similar between FMF patients and general population.[5] On the other hand, inflammatory diseases such as spondyloarthritis, inflammatory bowel diseases, vasculitis, demyelinating diseases and glomerulonephritis, most of which are classified as MHC class I group diseases (MHC-I-opathies), are more common in FMF patients. Co-existence of the two MHC-I group-associated entities, FMF and AA, has not been reported in the literature. Here, we presented three patients with AA and FMF, and discussed their possible common pathogenetic relationship.
Case-1
A 23-year-old man presented to our clinic with the complaint of hair loss on the scalp, beard, eyebrows, eyelashes and body sites which started 1 year ago. He was also diagnosed with FMF 5 years ago and was taking colchicine 1.5 mg/day. On his dermatological examination, he had a complete loss of eyebrows, eyelashes, body hairs and scalp hair, [Figure 1] in addition to multiple longitudinal ridges on the fingernails. On his trichoscopic examination, there were numerous yellow dots, empty follicles and a small number of vellus hairs. His laboratory tests were within normal limits. He was diagnosed with alopecia universalis and tried to be sensitized with topical diphencyprone but sensitization could not be achieved. Sulfasalazine treatment was initiated because he refused systemic corticosteroid treatment. At the end of the third month, as no clinical improvement could be achieved, tofacitinib treatment was started 10 mg/day. In the third of the tofacitinib treatment, partial hair regrowth occurred. He is still on tofacitinib therapy.
Figure 1.
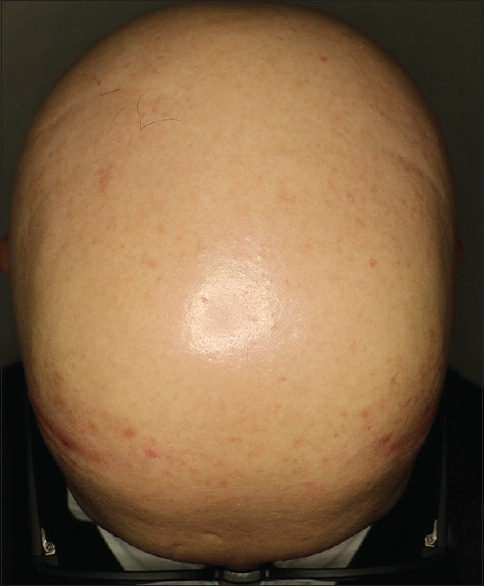
Complete hair loss on the scalp
Case-2
A 7-year-old male patient presented with 6-month history of hair loss on the scalp. In his medical history, he had FMF, Behçet's disease and hypothyroidism and he was on colchicine tablet 0.5 mg/day for Behçet's disease and levothyroxine sodium 25 mg/day treatment. There was a third-degree consanguineous marriage between his parents. In his family history; his mother had Behçet's disease, his brother had FMF and his father had a history of AA. On his dermatological examination, there were multiple alopecic patches on vertex, occipital and parietal areas [Figure 2a]. On trichoscopic evaluation; black dots, exclamation mark hairs and numerous vellus hairs were observed [Figure 2b]. Trachyonychia was observed on his all fingernails [Figure 3a and b] and toenails. In laboratory investigations; he has a low TSH level (0.0180 uIU/mL), anti-TPO and anti-Tg tests were positive (325.38 IU/mL and 406.78 IU/mL). Azelaic acid cream and prednicarbate lotion were started. Hair regrowth was observed within 2 months but hair loss has occurred on another side of the scalp.
Figure 2.
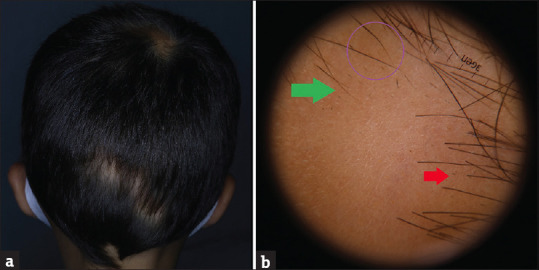
(a) Multiple alopecic patches on vertex, occipital and parietal areas. (b) Black dots (red arrow), exclamation mark hairs (purple circle) and numerous vellus hairs (green arrow)
Figure 3.
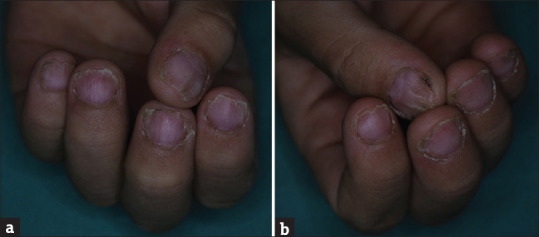
(a and b) Trachyonychia on his all fingernails
Case-3
A 24-year-old woman applied with widespread hair loss for 3 months. She was diagnosed with FMF 6 years ago and is taking colchicine tablet 1 mg/day. There was a second degree consanguineous marriage between her parents. Her brother and cousin were diagnosed with FMF. On her dermatological examination, multiple alopecic patches were observed on her scalp [Figure 4a]. On trichoscopic evaluation; there were numerous yellow dots, a small number of black dots, exclamation mark hairs and vellus hairs [Figure 4b]. In laboratory tests, no abnormalities were observed, except for a weakly positive anti-nuclear antibody. The patient was diagnosed as AA and a monthly triamcinolone acetonide 5 mg/mL intralesional injection was started. During the treatment period, the patient was diagnosed with ulcerative colitis (UC). Sulfasalazine was added because of both the insufficient clinical response to hair loss and also treatment for UC. It could not be continued due to nausea and topical diphencyprone treatment was initiated. The gastroenterologist decided to follow-up with her without treatment for UC.
Figure 4.
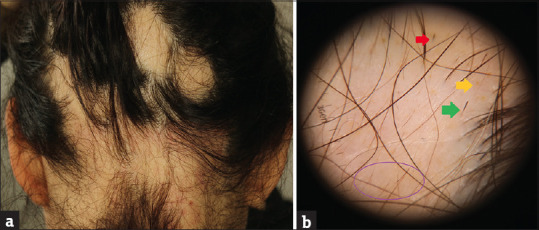
(a) Multiple alopecic patches. (b) Numerous yellow dots (yellow arrow), a small number of black dots (red arrow), exclamation mark hairs (green arrow) and vellus hairs (purple circle)
Discussion
Alopecia areata is a multifactorial autoimmune disease that is characterized by IP collapse in anagen HF.[6] Normalization of ectopic MHC-I expression by treatment is therapeutic in some MHC Class I-related diseases like AA, autoimmune uveitis, multiple sclerosis, fetal loss and allograft rejection.[7] It has been known that patients with FMF are vulnerable to MHC Class I-related diseases such as UC, Crohn's disease, Behçet's disease and ankylosing spondylitis. One of our patients (case 2) has Behçet's disease in addition to FMF and AA, also his mother has Behçet's disease and his brother has FMF. Another patient (case 3) was diagnosed with UC in addition to FMF and AA. It seems that the relationship between AA and FMF is not surprising. In fact, we think that in addition to AA and FMF, other MHC Class I-related diseases can be observed in some patients.
Despite autoinflammation and autoimmunity being classified in separate categories, it is noteworthy that there are some similarities in their pathogenesis. In both of them, changes in innate and adaptive immunity cause organ-specific damage, genetic predisposition and repeated systemic inflammatory attacks are common features. Although autoimmune diseases are considered adaptive immunity-mediated disorders, it is thought that innate immunity and inflammasome formation are involved in the pathogenesis of autoimmune diseases.[8]
In the pathogenesis of FMF, the activation of the inflammasome and the formation of autoinflammation as a result of the mutation of the MEFV gene encoding pyrin have been held responsible.[4] In addition, IL-1 β has been identified as a common cytokine combining adaptive and innate immunity.[8,9] IL-1 β may play a role in common pathogenesis of AA and FMF. Colchicine is frequently used in the treatment of FMF and it shows its effect by interrupting mitosis and cell transport systems. As a result of this, side effects of colchicine are observed on organs with a high rate of cell turnover such as gastrointestinal tracts, bone marrow and hair. Although anagen effluvium is observed in colchicum toxicity, however, hair loss may not always occur.[10]
In conclusion, both AA and FMF are MHC Class I-related diseases. There may be an association between FMF and AA like other MHC Class I-related diseases. Moreover, the existence of similarities between autoimmune and autoinflammatory disorders indicates varying degrees of interaction between them. The genetic predisposition and the role of immunity in both FMF and AA indicate that there may be common pathways in their pathogenesis. Further studies are needed to demonstrate their relationship.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil
Conflicts of interest
There are no conflicts of interest.
References
- 1.Sterkens A, Lambert J, Bervoets A. Alopecia areata: A review on diagnosis, immunological etiopathogenesis and treatment options. Clin Exp Med. 2021;21:215–30. doi: 10.1007/s10238-020-00673-w. [DOI] [PubMed] [Google Scholar]
- 2.Gilhar A, Schrum AG, Etzioni A, Waldmann H, Paus R. Alopecia areata: Animal models illuminate autoimmune pathogenesis and novel immunotherapeutic strategies. Autoimmun Rev. 2016;15:726–35. doi: 10.1016/j.autrev.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strazzulla LC, Wang EHC, Avila L, Lo Sicco K, Brinster N, Christiano AM, et al. Alopecia areata: Disease characteristics, clinical evaluation, and new perspectives on pathogenesis. J Am Acad Dermatol. 2018;78:1–12. doi: 10.1016/j.jaad.2017.04.1141. [DOI] [PubMed] [Google Scholar]
- 4.Özen S, Batu ED, Demir S. Familial mediterranean fever: Recent developments in pathogenesis and new recommendations for management. Front Immunol. 2017;8:253. doi: 10.3389/fimmu.2017.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tufan A, Lachmann HJ. Familial mediterranean fever, from pathogenesis to treatment: A contemporary review. Turkish J Med Sci. 2020;50:1591–610. doi: 10.3906/sag-2008-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gilhar A, Etzioni A, Paus R. Review: Alopecia areata. N Engl J Med. 2012;366:1515–25. doi: 10.1056/NEJMra1103442. [DOI] [PubMed] [Google Scholar]
- 7.Ito T, Ito N, Bettermann A, Tokura Y, Takigawa M, Paus R. Collapse and restoration of MHC Class-I-dependent immune privilege exploiting the human hair follicle as a model. Am J Pathol. 2004;164:623–34. doi: 10.1016/S0002-9440(10)63151-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El-Shebiny EM, Zahran ES, Shoeib SA, Habib ES. Bridging autoinflammatory and autoimmune diseases. Egypt J Intern Med. 2021;33:11. [Google Scholar]
- 9.Watad A, Bragazzi NL, Adawi M, Shoenfeld Y, Comaneshter D, Cohen AD, et al. FMF Is associated with a wide spectrum of MHC Class I- and allied SpA disorders but not with classical MHC Class II-associated autoimmune disease: Insights from a large cohort study. Front Immunol. 2019;10:2733. doi: 10.3389/fimmu.2019.02733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu V, Juhasz M, Chiang A, Mesinkovska NA. Alopecia and associated toxic agents: A systematic review. Skin Appendage Disord. 2018;4:245–60. doi: 10.1159/000485749. [DOI] [PMC free article] [PubMed] [Google Scholar]


