Abstract
Aim
This study was performed to evaluate the efficacy of a multidisciplinary approach incorporating neoadjuvant chemoradiotherapy with S1 (S1‐NACRT) for resectable pancreatic ductal adenocarcinoma.
Methods
The medical records of 132 patients who received S1‐NACRT for resectable pancreatic ductal adenocarcinoma from 2010 to 2019 were reviewed. The S1‐NACRT regimen consisted of S1 at a dose of 80‐120 mg/body/day together with 1.8 Gy of radiation in 28 fractions. The patients were re‐evaluated 4 weeks after S1‐NACRT completion, and a pancreatectomy was then considered.
Results
Adverse events of S1‐NACRT ≥grade 3 occurred in 22.7% of the patients, and 1.5% discontinued therapy. Of the 112 patients who underwent a pancreatectomy, 109 underwent R0 resection. Adjuvant chemotherapy with relative dose intensity ≥50% was administered to 74.1% of the patients who underwent resection. The median overall survival of all patients was 47 months, and the median overall survival and recurrence‐free survival of patients who underwent resection was 71 and 32 months, respectively. According to the multivariate analyses of prognostic factors for overall survival in patients who underwent resection, negative margin status (hazard ratio: 0.182; P = 0.006) and relative dose intensity of adjuvant chemotherapy ≥50% (hazard ratio 0.294; P < 0.001) were independent prognostic factors of overall survival.
Conclusions
A multidisciplinary approach incorporating S1‐NACRT for resectable pancreatic ductal adenocarcinoma demonstrated acceptable tolerability and good local control and resulted in comparable survival benefits.
Keywords: chemoradiotherapy, neoadjuvant therapy, pancreatic ductal carcinoma, S1 (combination), surgical oncology
This study was performed to evaluate the efficacy of a multidisciplinary approach incorporating neoadjuvant chemoradiotherapy with S1 (S1‐NACRT) for resectable pancreatic ductal adenocarcinoma. The median overall survival of all patients was 47 months, and the median overall survival and recurrence‐free survival of patients who underwent resection were 71 months and 32 months, respectively. A multidisciplinary approach incorporating S1‐NACRT for resectable pancreatic ductal adenocarcinoma demonstrated acceptable tolerability and good local control and resulted in comparable survival benefits.
1. INTRODUCTION
While invasive ductal adenocarcinoma of the pancreas (PDAC) has a poor prognosis, 1 resectable PDAC (R‐PDAC) is a form of the disease that may be cured. 2 The long‐term outcome of R‐PDAC has gradually improved due to the efficacy of adjuvant chemotherapy (ACT). 3 , 4 , 5 However, its prognosis remains far from satisfactory. As a type of ACT, neoadjuvant therapy (NAT), such as neoadjuvant chemotherapy (NACT), which aims to control micrometastases and preoperatively selects candidates for resection, improves the long‐term outcomes of PDAC. 6 , 7 , 8 , 9 , 10 , 11 Moreover, R0 resection is one of the factors associated with long‐term survival. 12 Therefore, another type of NAT, neoadjuvant chemoradiotherapy (NACRT), has been employed to provide intensive local control of cancer and deliver systemic control with anticancer drugs. 6 , 7 , 11 The roles of NACRT and NACT should be clarified.
At Komagome Hospital, surgical resection with intraoperative radiotherapy (IORT) followed by adjuvant chemoradiotherapy (ACRT) has been administered to patients with R‐PDAC for the past two decades before the current study was initiated. 13 However, ACRT was frequently not completed as scheduled due to the poor physical condition of most patients, and the long‐term outcomes have been unsatisfactory despite the regimen's ability to provide good local control. Thus, in 2008, our protocol was shifted to CRT before surgery when evidence of treatment that improved the prognosis of R‐PDAC was scarce. 14 Eventually, in 2009, the NACRT regimen was standardized to the current NACRT with S1 (S1‐NACRT) regimen. During that time, although S1 is an oral anticancer agent based on tegafur that was a concomitant drug with proven efficacy as an ACT for R‐PDAC and as part of NACRT for borderline resectable PDAC (BR‐PDAC), 6 , 11 its efficacy as part of NACRT for R‐PDAC has remained uncertain. However, because improved efficacy of S1‐NACRT has been observed, we have continued to administer S1‐NACRT to patients with R‐PDAC at Komagome Hospital until NACT with gemcitabine and S‐1 (GS‐NACT) became the standard of care for these patients in Japan in 2019.
The current study has not been conducted as prospective clinical research because we have performed a multidisciplinary approach incorporating S1‐NACRT for R‐PDAC as one of our tailor‐made therapies, which has been provided within the available realm of the National Health Insurance. However, because we have performed S1‐NACRT for R‐PDAC with a standardized protocol for more than a decade and obtained comparable results, we retrospectively investigated its safety and efficacy.
2. METHODS
The medical records of patients who received S1‐NACRT for R‐PDAC between October 2009 and December 2019 at Komagome Hospital were retrospectively reviewed. The study design was approved by the institutional review board of the hospital (Approved number: 2621).
2.1. Patients
During the study period, all patients diagnosed with R‐PDAC were considered candidates for S1‐NACRT. The following were the exclusion criteria: patients who declined NACRT; those with a gastrointestinal obstruction due to tumor invasion; those who were deemed unsuitable for NACRT because of a comorbidity, active infection, or other reasons; those with a concomitant malignancy; those who were not diagnosed with pancreatic cancer by histological examination using pretreatment biopsy or surgical specimen; those who were enrolled in a prospective multicenter trial; and those who were judged as unsuitable for surgery by the authors. A radiologist (M.S.) reviewed pretreatment imaging studies to confirm that the findings were consistent with R‐PDAC as defined in the National Comprehensive Cancer Network (NCCN) guidelines. 2 In the current study, borderline resectable tumors, such as BR‐PV defined in the NCCN guidelines, 2 were not included in the cohorts of T1‐T3 disease defined in the UICC TNM. 15 , 16
2.2. Diagnosis
Diagnosis was based on imaging studies primarily focused on enhanced computed tomography (CT). Since 2016, gadolinium‐ethoxybenzyl‐diethylene‐triaminepentaacetic acid‐magnetic resonance imaging (EOB‐MRI) has been concomitantly performed to more accurately detect occult liver metastases. Treatment was initiated after histological or cytological examination confirmed the presence of adenocarcinoma.
2.3. NACRT
S1 was administered concomitantly on the same day as radiotherapy. The S1 dose was adjusted by body surface at a dose of 80‐120 mg/body/day (the mean dose was calculated at 71.8 mg/m2/day) (Figure S1). Until 2018, external beam radiotherapy was administered with three‐dimensional conformal radiotherapy, typically with four ports; subsequently, intensity‐modulated radiotherapy (IMRT) was used for all cases. Figure S2 contains representative images of radiation planning. A total of 50.4 Gy was delivered in 28 fractions of 1.8 Gy each on weekdays. Gross tumor volume (GTV) was defined as the primary tumor and any involved nodes. The internal margin was added to the GTV based on four‐dimensional CT or radioscopy according to respiratory motion (defined as the total GTV). The clinical target volume (CTV) comprised the total GTV, its surrounding and uniform 5‐mm margin, and the area of soft tissue and lymph nodes around the celiac and superior mesenteric arteries (SMAs) where the most critical dissection margins are found. Furthermore, a 5‐mm margin was added to the CTV to establish the planning target volume.
Reassessment was performed 4 weeks after the last day of NACRT. All adverse events were recorded in accordance with the Common Terminology Criteria for Adverse Events 5.0 guidelines. 17
2.4. Pancreatectomy
Surgical resection was planned for 6‐8 weeks after NACRT completion for patients diagnosed with resectable disease and who were considered capable of tolerating a pancreatectomy based on a re‐evaluation.
Resection comprised a systematic pancreatectomy with regional lymph node and adjacent soft tissue dissection using the artery‐first approach. 18 , 19 The portal and/or superior mesenteric veins were resected and reconstructed if they were unable to be isolated smoothly, regardless of whether tumor invasion had occurred. The nerve plexus surrounding the SMA was essentially preserved. Postoperative complications were classified following the Clavien‐Dindo classification. 20 Pancreatic fistula, delayed gastric emptying, and postoperative hemorrhage were graded according to the International Study Group of Pancreatic Surgery classification. 21 , 22 , 23
The histological effect of NACRT and margin status were assessed according to the classification proposed by Evans et al 24 and the 0‐mm rule, 25 respectively.
2.5. ACT and postoperative follow‐up
Adjuvant chemotherapy was introduced after the recovery of oral dietary intake and absence of infection was confirmed. S1 monotherapy was the first‐choice regimen. 4 The ACT protocol with S1 specified a dose identical to that of NACRT for two consecutive weeks followed by a 1‐week interval or four consecutive weeks followed by a 2‐week interval for a total of 24 weeks. The mean S1 dose was 72.1 mg/m2/day. S1 administration every other day was considered for patients who could not tolerate the standard ACT protocols. 26 Some patients, such as those with an allergy to one or more of the S1 components, received gemcitabine‐based ACT, in which gemcitabine was administered in six cycles of 1000 mg/m2 on days 1, 8, and 15 every 28 days. 3
Postoperative follow‐up was performed by outpatient interview in addition to laboratory tests, including tumor marker tests, at least every 3 months and an imaging study every 3‐4 months. Patients with recurrence received the standard of care at that time.
2.6. Statistical analysis
Continuous data were presented as medians (ranges). Categorical variables were compared using the χ2 test. Overall survival (OS) and recurrence‐free survival (RFS) were defined as the duration between the date of treatment initiation and the date of death and the duration between the date of treatment initiation and diagnosis of first recurrence, respectively. Survival curves were generated using the Kaplan‐Meier method, and differences between curves were assessed using the log‐rank test. Factors with P < 0.25 on univariate analyses were included in a multivariate analysis using a Cox proportional hazards model with step‐wise regressions. Cutoff levels of 500 U/mL, 150 U/mL, and 30 mm for CA19‐9, DUPAN‐2, and tumor size, respectively, were used to dichotomize the variables. 10 , 27 , 28 The relative dose intensity (RDI) of ACT was calculated by dividing the administered dose by the planned dose. If recurrence was diagnosed before the ACT was completed, a dose until a recurrence was diagnosed was defined as the planned dose. P < 0.05 was considered to indicate statistical significance in all the analyses, which were performed using JMP PRO software, version 15.2.1 (SAS Institute Inc.) and SPSS version 28.0 (IBM).
3. RESULTS
3.1. Patient background
A flow diagram of 180 patients diagnosed with R‐PDAC is shown in Figure 1. Of the 180 patients, 45 were scheduled to undergo upfront surgery for the abovementioned reasons, whereas two patients received NACRT with a combination of S1 and gemcitabine. One patient was not diagnosed with pancreatic cancer by histological examination using pretreatment biopsy or surgical specimen. Finally, the remaining 132 patients who received S1‐NACRT were enrolled. For radiotherapy, 22 patients underwent IMRT. The patient characteristics are listed in Table 1. Of the 132 patients, 81 (61.4%) had a tumor in the pancreatic head, and 108 (81.8%) had T3 disease according to the UICC TNM seventh edition. 15 The CA19‐9 level was higher than normal before NACRT was initiated in 81 patients (61.4%).
FIGURE 1.
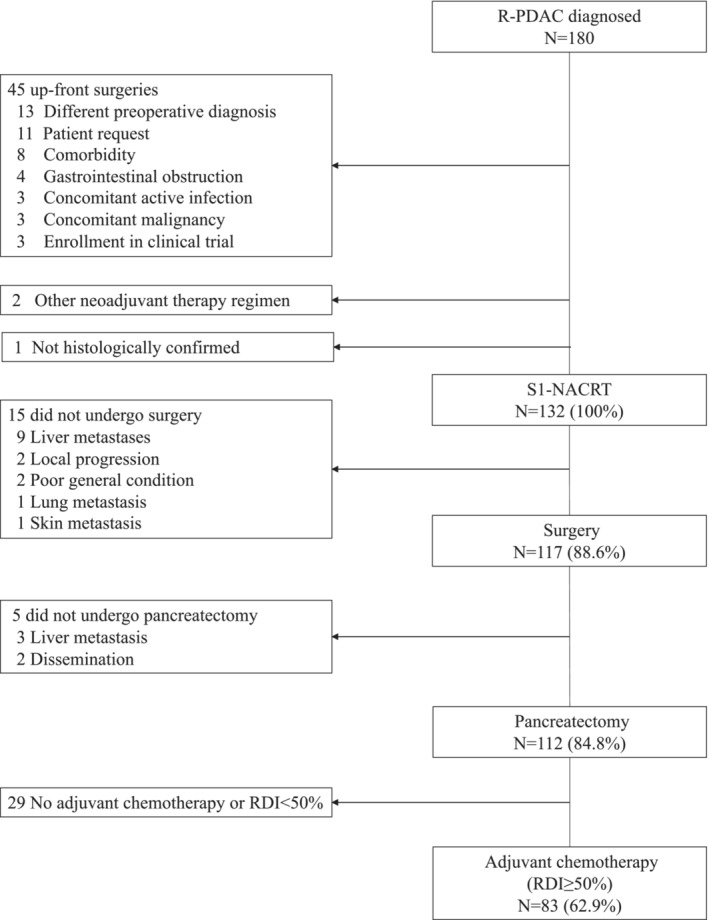
Patient flow (CONSORT diagram). RDI, relative dose intensity; R‐PDAC, resectable pancreatic ductal adenocarcinoma; S1‐NACRT, neoadjuvant chemoradiotherapy with S1.
TABLE 1.
Patient background
| n = 132 | |
|---|---|
| Age, year | 71 (36‐86) |
| Sex | |
| Male | 66 (50) |
| Female | 66 (50) |
| ASA | |
| 1 | 24 (18.2) |
| 2 | 107 (81.1) |
| 3 | 1 (0.8) |
| Performance status | |
| 0 | 105 (79.5) |
| 1 | 26 (19.7) |
| 2 | 1 (0.8) |
| Dominant location of tumor, n | |
| Head | 81 (61.4) |
| Body | 32 (24.2) |
| Tail | 19 (14.4) |
| Tumor size, mm | 23 (10‐63) |
| Clinical T stage (UICC 8th) | |
| T1b | 2 (1.5) |
| T1c | 44 (33.3) |
| T2 | 78 (59.1) |
| T3 | 8 (6.1) |
| Clinical T stage (UICC 7th) | |
| T1 | 15 (11.4) |
| T2 | 9 (6.8) |
| T3 | 108 (81.8) |
| Histological diagnosis | |
| Confirmed | 122 (92.4) |
| CA19‐9 levels, U/mL | 83.1 (<0.4‐11700) |
| DUPAN‐2 levels, U/mL a | 110 (<25‐>1600) |
| Type of radiation | |
| IMRT | 22 (16.7) |
Note: Expressed as median (range) or number (percentage).
Abbreviations: ASA, American Society of Anesthesiologists; IMRT, intensity‐modulated radiotherapy; UICC, Union for International Cancer Control.
Data of five patients are missing.
3.2. Adverse events and response rate to NACRT
The outcomes of NACRT are summarized in Table 2. Thirty patients (22.7%) experienced adverse events (≥grade 3). Decreased white blood cell count was the most common adverse event. Two patients (1.5%) terminated NACRT due to adverse events. The disease and local control rates were 88.6% and 96.2%, respectively. Most cases of disease progression were caused by distant metastases.
TABLE 2.
Adverse event and response of NACRT (n = 132)
| Adverse events | Grade 1‐2 | Grade 3 | Grade 4 | ≥Grade 3 a | ||
|---|---|---|---|---|---|---|
| White blood cell decrease | 98 (74.2) | 13 (9.8) | 0 (0) | 13 (9.8) | ||
| Neutrophil decrease | 78 (59.1) | 9 (6.8) | 0 (0) | 9 (6.8) | ||
| Anemia | 108 (81.8) | 0 (0) | 0 (0) | 0 (0) | ||
| Platelet count decrease | 101 (76.5) | 0 (0) | 1 (0.8) | 0 (0) | ||
| Febrile neutropenia | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| Blood bilirubin increased | 7 (5.3) | 1 (0.8) | 0 (0) | 1 (0.8) | ||
| AST increased | 8 (6) | 4 (3) | 1 (0.8) | 4 (3) | ||
| ALT increased | 9 (6.8) | 5 (3.8) | 0 (0) | 5 (3.8) | ||
| Creatinine increased | 2 (1.5) | 0 (0) | 0 (0) | 0 (0) | ||
| Anorexia | 19 (14.4) | 4 (3) | 0 (0) | 4 (3) | ||
| Diarrhea | 5 (3.8) | 1 (0.8) | 0 (0) | 1 (0.8) | ||
| Nausea | 22 (16.7) | 4 (3) | 0 (0) | 4 (3) | ||
| Taste alteration | 3 (2.3) | 0 (0) | 0 (0) | 0 (0) | ||
| Mucositis | 2 (1.5) | 0 (0) | 0 (0) | 0 (0) | ||
| Skin rash | 4 (3) | 1 (0.8) | 0 (0) | 1 (0.8) | ||
| Dermatitis | 5 (3.8) | 0 (0) | 0 (0) | 0 (0) | ||
| Stomatitis | 5 (3.8) | 0 (0) | 0 (0) | 0 (0) | ||
| Gastric ulcer | 1 (0.8) | 0 (0) | 0 (0) | 0 (0) | ||
| Fatigue | 7 (5.3) | 0 (0) | 0 (0) | 0 (0) | ||
| Fever/Sepsis | 12 (9.1) | 3 (2.3) | 0 (0) | 3 (2.3) | ||
| Any adverse events | 110 (83.3) | 30 (22.7) | 2 (1.5) | 30 (22.7) | ||
| Initial S1 dose, mg/m2/day | 71.9 (49‐87.9) | |||||
| Diagnosis to treatment, days | 16 (1‐60) | |||||
| Completion of treatment | 130 (98.5) | |||||
| Prolongation of treatment | 27 (20.5) | |||||
| Dose reduction | 20 (15.2) | |||||
| RDI of S1 | 100 (32‐100) | |||||
| Post NACRT tumor size, mm | 20 (0‐60) | |||||
| Tumor shrinkage rate, % | 89.1 (0‐129) | |||||
| Response (CR/PR/SD/PD) | 1//31/85/15 | |||||
| Reason of PD | ||||||
| Distant metastases | 10 (7.6) | |||||
| Local progression | 5 (3.8) | |||||
| Post NACRT CA19‐9, U/mL | 22.2 (<0.4‐7940) | |||||
| CA19‐9 decrease | 112 (84.8) | |||||
| Reduction rate of CA19‐9, % | 50.4 (2‐1009) | |||||
| Post NACRT DUPAN‐2, U/mL b | 47 (<25‐>1600) | |||||
| DUPAN‐2 decrease c | 107 (88.4) | |||||
| Reduction rate of DUPAN‐2, % c | 67.9 (4‐379) | |||||
Note: Expressed as median (range) or number (percentage).
Abbreviations: CR, complete response; NACRT, neoadjuvant chemoradiotherapy; PD, progression of disease; PR, partial response; RDI, relative dose intensity; SD, stable disease.
Counted as one if multiple in one patient.
Data of seven patients are missing.
Data of 11 patients are missing.
3.3. Surgical outcome
Of 132 patients, 11, two, and two did not receive surgery owing to distant metastases, local progression, and worsening general condition, respectively. Of the remaining 117 patients who were scheduled for surgery, 112 (95.7%) underwent pancreatectomy, and five did not undergo pancreatectomy because their tumors were found to be unresectable (liver metastases and peritoneal dissemination in three and two patients, respectively) prior to surgery. Pancreaticoduodenectomy, distal pancreatectomy, and total pancreatectomy were performed in 62, 45, and five patients (55.4%, 40.2%, and 4.5%), respectively. Concomitant portal vein resection was performed in 25 patients (22.3%).
The surgical outcomes are presented in Table 3. Grade B or C postoperative pancreatic fistula developed in 13 patients (11.6%). Seventeen patients (15.2%) experienced a major complication (≥grade 3a). One patient died on postoperative day 13 due to intra‐abdominal hemorrhage from a branch of the SMA, which was unrelated to the pancreatic fistula. The surgical mortality rate was 0.9%. All five patients who developed portal vein thrombosis did not undergo concomitant portal vein resection. Of the five patients, two had a pancreatic fistula. All patients recovered with medication. All bloodstream infections that developed in six patients were caused by central or peripheral venous catheter infection. An intra‐abdominal abscess developed in eight patients, and they were all recovered by percutaneous drainage or antibiotics.
TABLE 3.
Surgical outcome
| No of resected patients | 112 (84.8) | ||||
| Reason for aborted resection | |||||
| Distant metastases | 16 (12.1) | ||||
| Liver metastasis | 12 (9.1) | ||||
| Dissemination | 2 (1.5) | ||||
| Lung metastases | 1 (0.8) | ||||
| Skin metastases | 1 (0.8) | ||||
| Local progression | 2 (1.5) | ||||
| Poor general condition | 2 (1.5) | ||||
| Operative procedure | |||||
| PD | 62 (55.4) | ||||
| DP | 45 (40.2) | ||||
| TP | 5 (4.5) | ||||
| PD | DP | ||||
| Portal vein resection | 25 (22.3) | 21 (33.9) | 2 (4.4) | ||
| Laparoscopic approach | 0 (0) | 33 (73.3) | |||
| PpPD | 46 (74.2) | ||||
| SSPPD | 14 (22.6) | ||||
| Operative time, min | 388 (147‐869) | 425 (259‐869) | 326 (147‐537) | ||
| Blood loss, mL | 485 (0‐4450) | 690 (160‐4450) | 150 (0‐2250) | ||
| Postoperative complication | |||||
| Pancreatic fistula a | 13 (11.6) | 8 (12.9) | 5 (11.1) | ||
| Delayed gastric emptying a | 9 (8) | 3 (4.8) | 6 (13.3) | ||
| Portal vein thrombosis | 5 (4.5) | 3 (4.8) | 2 (4.4) | ||
| Hemorrhage a | 4 (3.6) | 3 (4.8) | 1 (2.2) | ||
| Intraabdominal abscess | 8 (7.1) | 3 (4.8) | 4 (8.9) | ||
| Cholangitis | 4 (3.6) | 4 (6.5) | |||
| Blood stream infection | 6 (5.4) | 4 (6.5) | 1 (2.2) | ||
| Intestinal perforation | 1 (0.9) | 1 (1.6) | |||
| Ischemic colitis | 1 (0.9) | 1 (1.6) | |||
| Deep venous thrombosis | 1 (0.9) | 1 (1.6) | |||
| CD ≥ grade 2, n | 43 (38.4) | 24 (38.7) | 16 (35.6) | ||
| CD ≥ grade 3, n | 17 (15.2) | 12 (19.4) | 5 (11.1) | ||
| Mortality | 1 (0.9) | 1 (1.6) | |||
| Postoperative stay, day | 19 (7‐116) | 21 (11‐116) | 15 (7‐108) | ||
Note: Expressed as median (range) or number (percentage).
Abbreviations: CD, Clavien‐Dindo classification; DP, distal pancreatectomy; PD, pancreaticoduodenectomy; PF, pancreatic fistula; PpPD, pylorus preserving pancreaticoduodenectomy; SSPPD, subtotal stomach preserving pancreaticoduodenectomy; TP, total pancreatectomy.
Grade B/C in ISGPS.
3.4. Pathological findings
Pathological evaluation revealed that seven and five patients (both, 10.7%) exhibited grades 3 and 4 histological responses, respectively. Five patients with grade 4 were histologically diagnosed with pancreatic cancer by preoperative examination. Twenty‐seven patients (24.1%) had lymph node involvement. Microscopic negative surgical margins were achieved in 109 patients, for a R0 resection rate of 97.3%.
3.5. ACT and recurrence
Adjuvant chemotherapy was administered to 89 patients (79.5%), most of whom (92.1%) received S1. Seventy patients (62.5%) started ACT within 10 weeks following their pancreatectomy. The number of patients whose RDI of ACT until a recurrence was diagnosed was <50% of the planned dose, including those who were never administered ACT, was 29 (25.9%). The reasons that the RDI was <50% or why ACT was not employed were gastrointestinal symptoms and treatment refusal in 13 (11.6%) and nine (8.0%) patients, respectively. Recurrences developed in 56 patients, including in 20, 12, and eight with liver, lung, and multiple organ metastases, respectively. As a treatment for recurrence, chemotherapy consisting of nanoparticulate albumin‐bound paclitaxel (nab‐PTX) or a combination chemotherapy regimen consisting of oxaliplatin, irinotecan, fluorouracil, and leucovorin (FOLFIRINOX) was administered to 24 patients (Table 4).
TABLE 4.
Pathological findings and postoperative course
| n = 112 | |
|---|---|
| Histological response grade 1/2a/2b/3/4 | 13/61/26/7/5 |
| Size of residual tumor, mm | 21 (0‐170) |
| Pathological T stage (UICC 8th) | |
| No residue tumor/Tis/T1a/T1b/T1c/T2/T3 | 5/3/6/9/35/45/9 |
| Pathological T stage (UICC 7th) | |
| No residue tumor/Tis/T1/T2/T3 | 5/3/33/16/55 |
| Pathological N stage (UICC 8th) | |
| N0/N1/N2 | 85/26/1 |
| Number of resected lymph nodes | 25 (3‐65) |
| Number of positive lymph nodes | 0 (0‐4) |
| TMN stage (UICC 8th) | |
| 0/IA/IB/IIA/IIB/III | 8/38/32/7/26/1 |
| TMN stage (UICC 7th) | |
| 0/IA/IB/IIA/IIB | 8/28/14/35/27 |
| Negative microscopic resection margin | 109 (97.3) |
| Postoperative adjuvant chemotherapy | |
| S1/Gemcitabine/No adjuvant chemotherapy | 82/7/23 |
| Initial dose of adjuvant chemotherapy, mg/m2/day a | 71.7 (56.9‐80.0) |
| Interval from surgery to adjuvant chemotherapy, day | 49 (10‐211) |
| Relative dose intensity of adjuvant chemotherapy, % | 100 (10‐100) |
| Reasons of aborted adjuvant chemotherapy b | 29 (25.9) |
| Gastrointestinal symptoms | 13 (11.6) |
| Anorexia | 7 (6.3) |
| Diarrhea | 7 (6.3) |
| Patient request | 9 (8.0) |
| Exacerbation of comorbidity | 7 (6.3) |
| Refractory ascites | 2 (1.8) |
| Portal vein thrombosis | 1 (0.9) |
| Postoperative hemorrhage | 1 (0.9) |
| Neutropenia | 1 (0.9) |
| Recurrences | 56 (50) |
| Liver | 20 (17.9) |
| Lung | 12 (10.7) |
| Dissemination | 7 (6.3) |
| Remnant pancreas | 4 (3.6) |
| Local recurrence | 3 (2.7) |
| Bone | 1 (0.9) |
| Lymph node | 1 (0.9) |
| Multiple organs | 8 (7.1) |
| Chemotherapy after recurrences | |
| nab‐PTX | 20 (17.9) |
| nab‐PTX and FOLFIRINOX | 2 (1.8) |
| nab‐PTX and liposomal irinotecan | 2 (1.8) |
Abbreviations: FOLFIRINOX, a combination chemotherapy regimen consisting of oxaliplatin, irinotecan, fluorouracil, and leucovorin; nab‐PTX, nanoparticulate albumin bound paclitaxel; UICC, Union for International Cancer Control.
Value of 82 patients who administered S1.
Relative dose intensity under 50% or no adjuvant chemotherapy. Duplications are included.
3.6. Long‐term outcome
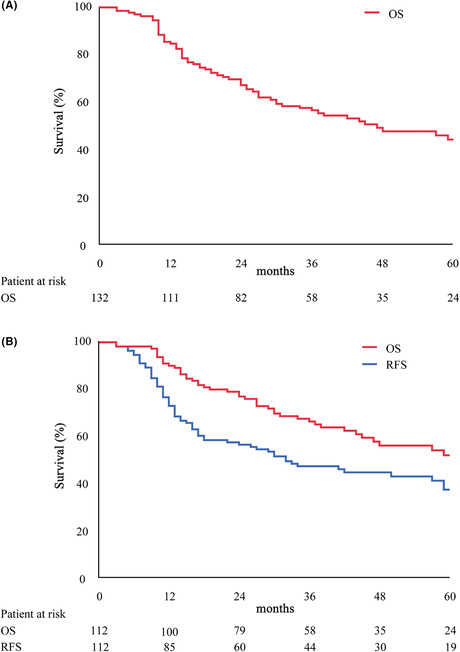
The 3‐year OS (3y OS) and 5‐year OS (5y OS) for the entire cohort were 56.7% and 44.5%, respectively, with a median OS of 47 months according to intention‐to‐treat‐analysis (Figure 2A). For 112 patients who underwent pancreatectomy (pancreatectomy cohort), the 3y OS, 5y OS, median OS, 3y RFS, 5y RFS, and median RFS were 66.5%, 52.2%, 71 months, 47.6%, 37.7%, and 32 months, respectively (Figure 2B). In the pancreatectomy cohort, no interruption of NACRT, negative margin status, and an RDI of ACT ≥50% were significant prognostic factors that affected the OS according to the univariate analysis, whereas negative margin status and an RDI of ACT ≥50% were independent prognostic factors of the OS according to the multivariate analysis (negative margin status: hazard ratio [HR], 0.182; confidence interval [CI], 0.054‐0.618; P = 0.006; RDI of ACT ≥50%: HR, 0.294; CI, 0.166‐0.521; P < 0.001) (Table 5).
FIGURE 2.
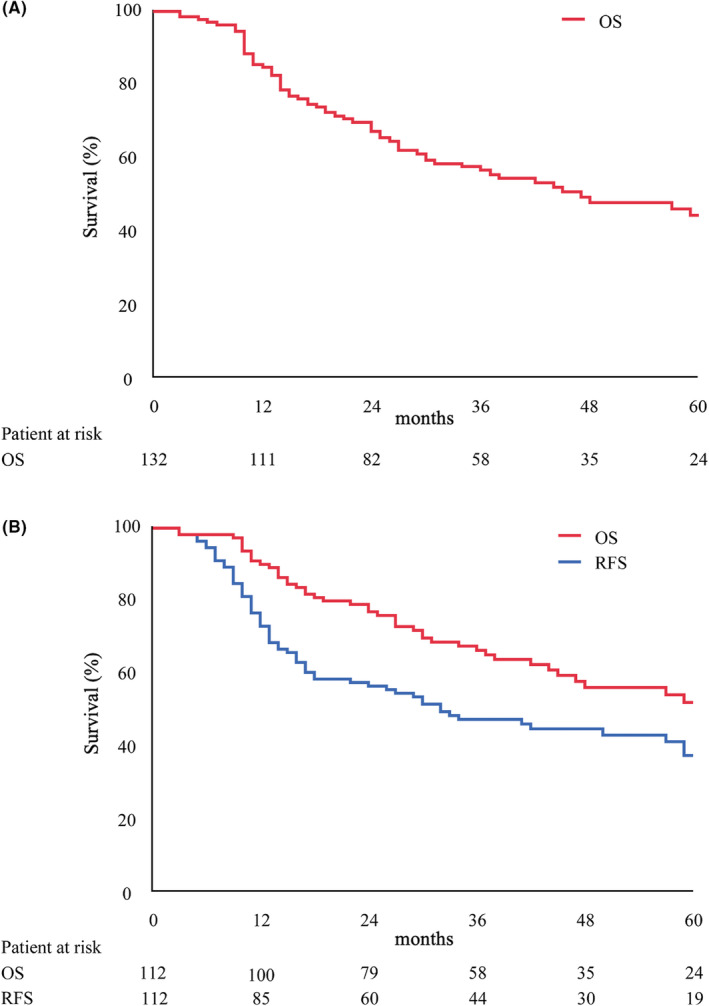
Survival curves. (A) Overall survival (OS) of whole study cohort. (B) OS and recurrence‐free survival (RFS) of resected cohort.
TABLE 5.
Univariate and multivariate analysis for overall survival in the resected cohort (n = 112)
| n | MST, m | Univariate | Multivariate | ||
|---|---|---|---|---|---|
| P | HR | P | |||
| Age (y) | |||||
| ≤70 | 54 | 79 | 0.122 | 0.318 | |
| >70 | 58 | 44 | |||
| Sex | |||||
| Male | 56 | 48 | 0.253 | ||
| Female | 56 | 79 | |||
| Pretreatment size | |||||
| ≤30 mm | 96 | 62 | 0.627 | ||
| >30 mm | 16 | 71 | |||
| Tumor location | |||||
| Head | 65 | 48 | 0.127 | 0.352 | |
| Body/Tail | 47 | 79 | |||
| Pretreatment CA 19‐9 | |||||
| ≤500 U/mL | 99 | 71 | 0.726 | ||
| >500 U/mL | 13 | 48 | |||
| Pretreatment DUPAN2 a | |||||
| ≤150 U/mL | 64 | 59 | 0.645 | ||
| >150 U/mL | 43 | 71 | |||
| Type of radiotherapy | |||||
| IMRT | 21 | N.R. | 0.229 | 0.242 | |
| Conventional | 91 | 59 | |||
| Interruption of NACRT | |||||
| No interruption | 89 | 79 | 0.016 | 0.161 | |
| With interruption | 23 | 45 | |||
| Dose reduction | |||||
| Full dose | 103 | 62 | 0.674 | ||
| Reduction | 9 | N.R. | |||
| Preoperative Size | |||||
| ≤30 mm | 100 | 71 | 0.860 | ||
| >30 mm | 12 | N.R. | |||
| Preoperative CA 19‐9 | |||||
| ≤37 U/mL | 75 | 45 | 0.564 | ||
| >37 U/mL | 37 | 71 | |||
| Preoperative DUPAN2 b | |||||
| ≤150 U/mL | 84 | 79 | 0.584 | ||
| >150 U/mL | 24 | 71 | |||
| Margin status | |||||
| R0 | 109 | 71 | <0.001 | 0.182 | 0.006 |
| R1 | 3 | 14 | |||
| Lymph node status | |||||
| Negative | 85 | 71 | 0.621 | ||
| Positive | 27 | 48 | |||
| Histological response c | |||||
| Grade 1, 2a, 2b | 100 | 59 | 0.109 | 0.129 | |
| Grade 3, 4 | 12 | N.R. | |||
| RDI of AC | |||||
| ≥50% | 83 | 82 | <0.001 | 0.294 | <0.001 |
| None or <50% | 29 | 27 | |||
Abbreviations: AC, adjuvant chemotherapy; HR, hazard ratio; MST, median survival time; N.R., not reached; NACRT, neoadjuvant chemoradiotherapy; RDI, relative dose intensity.
Data of five patients were missing.
Data of four patients were missing.
EVANS classification.
4. DISCUSSION
Together with ACT, NAT is expected to further improve the survival of patients with R‐PDAC. 2 In Japan, based on the high‐quality evidence from the randomized controlled trial conducted by Uesaka et al, 4 upfront surgery followed by S1‐ACT became the standard of care for patients with R‐PDAC in 2016. Subsequently, the results of a randomized controlled trial comparing GS‐NACT with upfront surgery in patients with potentially resectable PDAC (Prep‐02/JSAP05) were reported in 2019 and demonstrated the superiority of GS‐NACT over upfront surgery in terms of OS (median of OS: GS‐NACT 36.7 months vs upfront surgery 26.6 months, P = 0.015). 9 Consequently, the Japan Pancreas Society changed their recommendation regarding the standard of care for R‐PDAC from upfront surgery to GS‐NACT. 29 The current study was initiated earlier than these studies and was conducted until the current standard of care was established after comparable results were obtained, such as a median of OS 71 months in the pancreatectomy cohort.
Here, negative margin status and RDI of ACT were identified as independent prognostic factors for long‐term survival. Cancer therapies that aim to cure were divided based on the following two perspectives: local and systemic control. Compared with previous studies of NACT, although 81.8% of study patients had T3 disease, the results of the indicators of local control, such as the R0 resection rate of 97.3%, a regional lymph node metastasis rate of 24.1%, and grades 3 and 4 histological effects proportion of 10.7%, were superior. 8 , 30 , 31 This indicates that S1‐NACRT has a greater capacity for intensive local control. However, due to the appearance of distant metastases, pancreatectomy was not performed in 12.1% of patients (Table 3); consequently, the long‐term results of the entire cohort were not superior to those of previous studies of NACT. 8 , 30 , 31 Conversely, regarding systemic control, the majority of the patients was administered a full dose of S1 during NACRT with a similar length of treatment to that of the standard protocol of previous studies (1.5 months); however, it is a single‐drug treatment, making it insufficient as a systemic control therapy for pancreatic cancer. Radiotherapy may have complemented the OS with enhanced local control. Furthermore, in the current study, the induction rate of ACT was high (79.5%), which may have been caused by not using strong chemotherapy as NACT. Consequently, the long‐term results may be comparable to those of previous studies of NACT. These results imply that while S1‐NACRT can become one of the alternative treatments to NACTs, which employ a stronger regimen, systemic control has a greater impact on long‐term outcomes than local control, even if local control is a significant prognostic factor.
S1‐NACRT has the potential to result in better long‐term outcomes, such as by administering this regimen between NACT and pancreatectomy within the current standard multidisciplinary treatment strategy, which consists of NACT and pancreatectomy followed by an adequate dose of ACT. When considering such a long treatment duration of the current standard multidisciplinary treatment, maintaining a patient's digestive system is essential; this is accomplished by avoiding dissecting the tissue, such as the nerve plexus, surrounding the celiac axis (CA) and/or SMA. 32 , 33 , 34 In this regard, local control by S1‐NACRT can play a role in securing negative surgical margins and maintaining a patient's digestive condition by preserving the surrounding tissue around the CA and/or SMA. Appropriate indication criteria for S1‐NACRT, including for BR‐PDAC, should be considered.
This study had several limitations. First, because it was a single‐center study, the results should be verified by a multicenter study. Second, 47 (26.1%) of 180 patients who were diagnosed with R‐PDAC at the study center did not receive S1‐NACRT, although arbitrary patient selection based on tumor biology was not performed. Third, this was a retrospective study, and thus, several strategies included during the study, such as the radiotherapy equipment, preoperative imaging study protocol, ACT agent, and treatment after recurrences, varied throughout the study period.
In conclusion, the strategy incorporating S1‐NACRT for R‐PDAC demonstrated acceptable tolerability, good local control, and comparable survival benefits. S1‐NACRT has the potential to be part of a multidisciplinary treatment strategy for PDAC owing to its potent power in local control.
DISCLOSURE
Conflict of Interest: None of the authors have any conflict of interest or financial ties to disclose.
Author Contributions: The conception or design of the work: MM, GH, KT, SN, YO, S. Hayakawa, MS, S. Horiguchi, MK, and YS. Analysis or interpretation of data for the work: MM, GH, KT, SN, YO, and YS. Drafting the work: MM, S. Hayakawa. Revising the work: GH, KT, SN, YO, MS, S. Horiguchi, MK, YS. All the authors approved the final version of manuscript and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
ETHICAL APPAROVAL
The protocol for this research project has been approved by a suitably constituted Ethics Committee of the institution and it conforms to the provisions of the Declaration of Helsinki. Committee of the institutional review board of Komagome Hospital, Approval No. 2621. All informed consent was obtained from the subjects and/or guardians.
Supporting information
Figure S1
Figure S2
ACKNOWLEDGEMENT
The authors would like to thank Makoto Saito (Clinical Research Support Center, Tokyo Metropolitan Cancer and Infectious Diseases Center, Komagome Hospital) for his expert advice on the analyses, and Enago (www.enago.jp) for the English language review.
Matsumura M, Honda G, Tani K, Nemoto S, Ome Y, Hayakawa S, et al. The outcome of a multidisciplinary approach incorporating neoadjuvant chemoradiotherapy with S1 for resectable pancreatic ductal adenocarcinoma. Ann Gastroenterol Surg. 2023;7:306–317. 10.1002/ags3.12624
REFERENCES
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. [DOI] [PubMed] [Google Scholar]
- 2. National comprehensive cancer network guidelines . Pancreatic adenocarcinoma versoin 2. 2021. https://www.nccn.org/guidelines/. Accessed 9 Nov 2021.
- 3. Oettle H, Neuhaus P, Hochhaus A, Hartmann JT, Gellert K, Ridwelski K, et al. Adjuvant chemotherapy with gemcitabine and long‐term outcomes among patients with resected pancreatic cancer: the CONKO‐001 randomized trial. JAMA. 2013;310(14):1473–81. [DOI] [PubMed] [Google Scholar]
- 4. Uesaka K, Boku N, Fukutomi A, Okamura Y, Konishi M, Matsumoto I, et al. Adjuvant chemotherapy of S‐1 versus gemcitabine for resected pancreatic cancer: a phase 3, open‐label, randomised, non‐inferiority trial (JASPAC 01). Lancet. 2016;388(10041):248–57. [DOI] [PubMed] [Google Scholar]
- 5. Conroy T, Hammel P, Hebbar M, Ben Abdelghani M, Wei AC, Raoul JL, et al. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med. 2018;379(25):2395–406. [DOI] [PubMed] [Google Scholar]
- 6. Fujii T, Satoi S, Yamada S, Murotani K, Yanagimoto H, Takami H, et al. Clinical benefits of neoadjuvant chemoradiotherapy for adenocarcinoma of the pancreatic head: an observational study using inverse probability of treatment weighting. J Gastroenterol. 2017;52(1):81–93. [DOI] [PubMed] [Google Scholar]
- 7. Eguchi H, Takeda Y, Takahashi H, Nakahira S, Kashiwazaki M, Shimizu J, et al. A prospective, open‐label, multicenter phase 2 trial of neoadjuvant therapy using full‐dose gemcitabine and S‐1 concurrent with radiation for Resectable pancreatic ductal adenocarcinoma. Ann Surg Oncol. 2019;26(13):4498–505. [DOI] [PubMed] [Google Scholar]
- 8. Macedo FI, Ryon E, Maithel SK, Lee RM, Kooby DA, Fields RC, et al. Survival outcomes associated with clinical and pathological response following neoadjuvant FOLFIRINOX or gemcitabine/nab‐paclitaxel chemotherapy in resected pancreatic cancer. Ann Surg. 2019;270(3):400–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Unno M, Motoi F, Matsuyama Y, Satoi S, Matsumoto I, Aosasa S. Randomized phase II/III trial of neoadjuvant chemotherapy with gemcitabine and S‐1 versus upfront surgery for resectable pancreatic cancer (Prep‐02/JSAP‐05). J Clin Oncol. 2019;37(suppl 4):189. [DOI] [PubMed] [Google Scholar]
- 10. Inoue Y, Saiura A, Oba A, Ono Y, Mise Y, Ito H, et al. Neoadjuvant gemcitabine and nab‐paclitaxel for borderline resectable pancreatic cancers: intention‐to‐treat analysis compared with upfront surgery. J Hepatobiliary Pancreat Sci. 2020;15:143–55. [DOI] [PubMed] [Google Scholar]
- 11. Takahashi S, Ohno I, Ikeda M, Konishi M, Kobayashi T, Akimoto T, et al. Neoadjuvant S‐1 with concurrent radiotherapy followed by surgery for borderline Resectable pancreatic cancer: a phase II open‐label multicenter prospective trial (JASPAC05). Ann Surg. 2020. 10.1097/sla.0000000000004535. Publish Ahead of Print. [DOI] [PubMed] [Google Scholar]
- 12. Demir IE, Jäger C, Schlitter AM, Konukiewitz B, Stecher L, Schorn S, et al. R0 versus R1 resection matters after pancreaticoduodenectomy, and less after distal or Total pancreatectomy for pancreatic cancer. Ann Surg. 2018;268(6):1058–68. [DOI] [PubMed] [Google Scholar]
- 13. Okamoto A, Matsumoto G, Tsuruta K, Baba H, Karasawa K, Kamisawa T, et al. Intraoperative radiation therapy for pancreatic adenocarcinoma: the Komagome hospital experience. Pancreas. 2004;28(3):296–300. [DOI] [PubMed] [Google Scholar]
- 14. Kobayashi S, Honda G, Kurata M, Okuda Y, Sakamoto K, Karasawa K, et al. A phase I study of S‐1 and gemcitabine with concurrent radiotherapy in patients with non‐metastatic advanced pancreatic cancer. Hepato‐Gastroenterology. 2015;62(140):1031–6. [PubMed] [Google Scholar]
- 15. Sobin LH, Gospodarowicz MK, Wittekind CH. International Union against Cancer (UICC). TNM classification of malignant tumors. 7th ed. New York, NY: Wiley; 2009. [Google Scholar]
- 16. Brierley JD, Gospodarowicz MK, Wittekind C. International Union against Cancer (UICC). TNM classification of malignant tumors. 8th ed. New York, NY: Wiley; 2017. [Google Scholar]
- 17. U.S. Department of Health and Human Services . Common Terminology Criteria for Adverse Events (CTCAE) Version 50 2017. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_50/. Accessed 7 Aug 2022.
- 18. Ome Y, Seyama Y, Doi M, Muto J. Laparoscopic distal pancreatectomy for left‐sided pancreatic cancer using the "Caudo‐dorsal artery first approach". Ann Surg Oncol. 2019;26(13):4464–5. [DOI] [PubMed] [Google Scholar]
- 19. Nakao A. The mesenteric approach in pancreatoduodenectomy. Dig Surg. 2016;33(4):308–13. [DOI] [PubMed] [Google Scholar]
- 20. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, et al. The 2016 update of the international study group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery. 2017;161(3):584–91. [DOI] [PubMed] [Google Scholar]
- 22. Wente MN, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the international study Group of Pancreatic Surgery (ISGPS). Surgery. 2007;142(5):761–8. [DOI] [PubMed] [Google Scholar]
- 23. Wente MN, Veit JA, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, et al. Postpancreatectomy hemorrhage (PPH): an international study Group of Pancreatic Surgery (ISGPS) definition. Surgery. 2007;142(1):20–5. [DOI] [PubMed] [Google Scholar]
- 24. Evans DB, Varadhachary GR, Crane CH, Sun CC, Lee JE, Pisters PW, et al. Preoperative gemcitabine‐based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26(21):3496–502. [DOI] [PubMed] [Google Scholar]
- 25. Markov P, Satoi S, Kon M. Redefining the R1 resection in patients with pancreatic ductal adenocarcinoma. J Hepatobiliary Pancreat Sci. 2016;23(9):523–32. [DOI] [PubMed] [Google Scholar]
- 26. Masuda T, Watanabe M, Fujitaka K, Hamai K, Ishikawa N, Doi M, et al. Alternate‐day administration of S‐1 for elderly patients with advanced non‐small‐cell lung carcinoma: a prospective feasibility study. Mol Clin Oncol. 2018;9(5):539–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kondo N, Murakami Y, Uemura K, Nakagawa N, Takahashi S, Ohge H, et al. Comparison of the prognostic impact of pre‐ and post‐operative CA19‐9, SPan‐1, and DUPAN‐II levels in patients with pancreatic carcinoma. Pancreatology. 2017;17(1):95–102. [DOI] [PubMed] [Google Scholar]
- 28. Okada K, Kawai M, Tani M, Hirono S, Miyazawa M, Shimizu A, et al. Predicting factors for unresectability in patients with pancreatic ductal adenocarcinoma. J Hepatobiliary Pancreat Sci. 2014;21(9):648–53. [DOI] [PubMed] [Google Scholar]
- 29. Okusaka T, Nakamura M, Yoshida M, Kitano M, Uesaka K, Ito Y, et al. Clinical practice guidelines for pancreatic cancer 2019 from the Japan pancreas society: a synopsis. Pancreas. 2020;49(3):326–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mokdad AA, Minter RM, Zhu H, Augustine MM, Porembka MR, Wang SC, et al. Neoadjuvant therapy followed by resection versus upfront resection for resectable pancreatic cancer: a propensity score matched analysis. J Clin Oncol. 2017;35(5):515–22. [DOI] [PubMed] [Google Scholar]
- 31. EM OR, Perelshteyn A, Jarnagin WR, Schattner M, Gerdes H, Capanu M, et al. A single‐arm, nonrandomized phase II trial of neoadjuvant gemcitabine and oxaliplatin in patients with resectable pancreas adenocarcinoma. Ann Surg. 2014;260(1):142–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nimura Y, Nagino M, Takao S, Takada T, Miyazaki K, Kawarada Y, et al. Standard versus extended lymphadenectomy in radical pancreatoduodenectomy for ductal adenocarcinoma of the head of the pancreas: long‐term results of a Japanese multicenter randomized controlled trial. J Hepatobiliary Pancreat Sci. 2012;19(3):230–41. [DOI] [PubMed] [Google Scholar]
- 33. Jang JY, Kang MJ, Heo JS, Choi SH, Choi DW, Park SJ, et al. A prospective randomized controlled study comparing outcomes of standard resection and extended resection, including dissection of the nerve plexus and various lymph nodes, in patients with pancreatic head cancer. Ann Surg. 2014;259(4):656–64. [DOI] [PubMed] [Google Scholar]
- 34. Jang JY, Kang JS, Han Y, Heo JS, Choi SH, Choi DW, et al. Long‐term outcomes and recurrence patterns of standard versus extended pancreatectomy for pancreatic head cancer: a multicenter prospective randomized controlled study. J Hepatobiliary Pancreat Sci. 2017;24(7):426–33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Figure S2


