This cohort study compares the effectiveness of adding fludrocortisone to hydrocortisone vs hydrocortisone alone among patients with septic shock using target trial emulation.
Key Points
Question
What is the comparative effectiveness of fludrocortisone added to hydrocortisone vs hydrocortisone alone among patients with septic shock?
Findings
In this multicenter cohort study among 88 275 patients with septic shock receiving norepinephrine who initiated hydrocortisone treatment, the addition of fludrocortisone to hydrocortisone was associated with a 3.7% lower adjusted absolute risk difference in the primary composite outcome of mortality or discharge to hospice compared with initiation of hydrocortisone alone.
Meaning
Among patients with septic shock receiving norepinephrine who initiated hydrocortisone treatment, the addition of fludrocortisone was associated with lower rates of the composite of death or discharge to hospice compared with hydrocortisone alone.
Abstract
Importance
Patients with septic shock may benefit from the initiation of corticosteroids. However, the comparative effectiveness of the 2 most studied corticosteroid regimens (hydrocortisone with fludrocortisone vs hydrocortisone alone) is unclear.
Objective
To compare the effectiveness of adding fludrocortisone to hydrocortisone vs hydrocortisone alone among patients with septic shock using target trial emulation.
Design, Setting, and Participants
This retrospective cohort study from 2016 to 2020 used the enhanced claims-based Premier Healthcare Database, which included approximately 25% of US hospitalizations. Participants were adult patients hospitalized with septic shock and receiving norepinephrine who began hydrocortisone treatment. Data analysis was performed from May 2022 to December 2022.
Exposure
Addition of fludrocortisone on the same calendar day that hydrocortisone treatment was initiated vs use of hydrocortisone alone.
Main Outcome and Measures
Composite of hospital death or discharge to hospice. Adjusted risk differences were calculated using doubly robust targeted maximum likelihood estimation.
Results
Analyses included 88 275 patients, 2280 who began treatment with hydrocortisone-fludrocortisone (median [IQR] age, 64 [54-73] years; 1041 female; 1239 male) and 85 995 (median [IQR] age, 67 [57-76] years; 42 136 female; 43 859 male) who began treatment with hydrocortisone alone. The primary composite outcome of death in hospital or discharge to hospice occurred among 1076 (47.2%) patients treated with hydrocortisone-fludrocortisone vs 43 669 (50.8%) treated with hydrocortisone alone (adjusted absolute risk difference, −3.7%; 95% CI, −4.2% to −3.1%; P < .001).
Conclusions and Relevance
In this comparative effectiveness cohort study among adult patients with septic shock who began hydrocortisone treatment, the addition of fludrocortisone was superior to hydrocortisone alone.
Introduction
Sepsis occurs in approximately 1.7 million US hospitalizations1 and in more than a third of hospitalizations that result in death.2 Septic shock—the most severe form of sepsis in which vasoplegia and cardiovascular organ dysfunction necessitate the use of vasopressor medications to support blood pressure—is associated with fatality rates greater than 30%.3 In patients with septic shock who require ongoing support with vasopressors, guidelines4 suggest adding corticosteroid therapy (weak recommendation, moderate-quality evidence), with a recommendation for use of intravenous hydrocortisone at a dose of 200 mg/d. These guidelines are based on randomized clinical trials5,6,7,8,9 and subsequent meta-analyses10,11 that found shortened shock duration and potentially reduced mortality with corticosteroids. However, the individual clinical trials that demonstrated improved mortality were limited to interventions that paired hydrocortisone with the mineralocorticoid fludrocortisone,8,9 not trials comparing hydrocortisone alone with placebo. One randomized clinical trial (Combination of Corticotherapy and Intensive Insulin Therapy for Septic Shock [COIITSS])12 showed a statistically nonsignificant 2.9% lower absolute mortality among patients randomized to combination hydrocortisone-fludrocortisone as compared with hydrocortisone alone; however, the COIITSS trial was underpowered due to underestimated control group mortality and a sample size chosen to detect only a large effect size (12.5% risk difference) generally not present13,14 in critical care trials.
Given the potential for a clinically significant benefit of hydrocortisone-fludrocortisone combination therapy as compared with hydrocortisone alone in septic shock, we used target trial emulation to evaluate the effectiveness of the addition of fludrocortisone to hydrocortisone vs hydrocortisone alone in patients with septic shock admitted to US hospitals.
Methods
Target Trial
We used observational data to emulate a target trial15,16 (eTable 1 in Supplement 1) that would randomize hospitalized adults with septic shock who were within 3 days of hospital admission, and who had initiated hydrocortisone treatment, to receive fludrocortisone within the same calendar day, or usual care, in an unblinded fashion. The hypothetical trial would follow participants until hospital discharge for the primary composite outcome of hospital mortality or discharge to hospice care.
Study Population
We used the Premier Healthcare Database 2016-2020, an enhanced claims-based database designed for measuring quality and health care utilization that contains claims data (eg, demographics and International Classification of Diseases diagnosis and procedures codes) and hospital day–indexed billing information with minimal missing data (<0.01% of variable fields are missing).17 Preliminary missing or invalid data received by the database is returned to source hospitals for correction prior to final data release.18 Approximately 25% of all US inpatient hospitalizations are included in the database (because hospitals choose to participate, the included hospitals represent a nonrandom sample of US hospitals but have characteristics similar to those in the American Hospital Association Database).19 Included patients were those admitted to intensive care or intermediate care units with septic shock who received norepinephrine and began hydrocortisone treatment within 3 days of hospital admission. We excluded patients younger than 18 years and those with alternative indications for fludrocortisone (primary adrenal insufficiency, orthostatic hypotension, and congenital adrenal hyperplasia). Patients with septic shock were identified using the International Classification of Diseases, Tenth Revision (ICD-10) code for septic shock (ie, explicit septic shock) as the admitting diagnosis or with the present on admission classifier. We chose to include patients with an explicit septic shock diagnosis, rather than using other claims-based strategies to identify septic shock,20 due to the near 100% specificity and positive predictive value20,21 of the explicit septic shock definition and because limiting to patients with explicit septic shock diagnoses results in a population with high mortality20,22 (ie, patients likely to benefit from initiation of corticosteroid treatment). There is moderate correlation (Pearson coefficient, 0.64) between the explicit septic shock definition and Sepsis-3–based algorithms.20 Study day 0 was defined as the calendar day in which hydrocortisone treatment was first initiated (in the hydrocortisone monotherapy arm), or the day that hydrocortisone and fludrocortisone were co-initiated (in the combination therapy arm). Detailed eligibility criteria are in eTable 2 in Supplement 1.
Treatment Assignment
Treatment assignment was based on whether enteral fludrocortisone treatment was initiated on the same calendar day that hydrocortisone treatment was initiated (hereafter referred to as combination “hydrocortisone-fludrocortisone” for those receiving fludrocortisone and “hydrocortisone-alone” for those not receiving fludrocortisone). Patients initially started on hydrocortisone treatment who then received fludrocortisone on subsequent days were assigned to the hydrocortisone-only group consistent with intention-to-treat principles. Unlike prior clinical trials7,9 that randomized patients to 7 days of corticosteroids, the specification of treatment assignment in our study emulated a hypothetical trial that randomized patients to an initial corticosteroid strategy without specification of subsequent doses or duration. Treatment assignments were ascertained using hospital billing data (eTable 3 in Supplement 1). Because granularity was limited to the calendar day, the time from hydrocortisone to fludrocortisone initiation was not known and included possibilities that fludrocortisone preceded hydrocortisone (increasing the risk of misclassification) or that fludrocortisone was given up to 24 hours after hydrocortisone (increasing the risk of immortal time bias). Consistent with our assumption that fludrocortisone was given concurrent with or shortly after hydrocortisone, analysis of electronic health record data (n = 58) from the Medical Information Mart for Intensive Care Database23 showed a median (IQR) time from hydrocortisone treatment initiation to fludrocortisone treatment initiation of 120 (0-840) minutes among patients with septic shock (eMethods in Supplement 1).
Outcomes
Outcomes were ascertained from study day 0 (start of hydrocortisone or hydrocortisone-fludrocortisone) until hospital discharge. The primary outcome was the composite of hospital death or discharge to hospice. Secondary outcomes were hospital death, vasopressor-free days, and hospital-free days by day 28. “Free day” outcomes were calculated as 28 minus the number of days of therapy (vasopressor use or hospitalization during the index hospitalization), with patients who died in the hospital assigned 0 “free days.” We calculated the proportion of patients who developed hypernatremia and health care–associated infection in each treatment arm to assess for potential complications of corticosteroid treatment.
Covariates
We used directed acyclic graphs24 (eFigure 1 in Supplement 1) to identify covariates on or before study day 0 that were likely to confound the association between treatment assignment and outcomes. Included covariates were age; sex; health insurance type; hospital discharge quarter and year; validated measures of comorbidity burden25 and acute organ dysfunction26,27; major surgery28; history of congestive heart failure or connective tissue disease; pneumonia present on admission; resuscitative fluid volume; enteral administration of medications other than fludrocortisone (as the receipt of enteral medications may reflect lower severity of acute illness); time from hospital admission and norepinephrine initiation to treatment assignment; use of etomidate, kidney replacement therapy, vasopressors, and invasive mechanical ventilation; assessments of the hypothalamic-pituitary-adrenal axis; surgical care unit admission; admission hospital; and hospital teaching status, size, caseload, and US census region. We used the most recent value for covariates with more than 1 entry. Variable definitions are shown in eTable 3 in Supplement 1.
Statistical Analysis
Covariate balance was assessed using absolute standardized mean differences (SMDs) between treatment assignments. Unadjusted survival curves were constructed using the Kaplan-Meier estimator.29 Unadjusted proportions and risk differences were calculated by treatment assignment. We calculated adjusted absolute risk differences (adjusted mean differences for continuous outcomes) and 95% CIs using doubly robust targeted maximum likelihood estimation (TMLE)30 and an ensemble machine learner (Super Learner).31,32 Targeted maximum likelihood estimation provides semiparametric, locally efficient substitution estimators and yields valid estimates of the treatment effect when models estimating the probability of treatment assignment or the probability of the outcome are correctly specified (doubly robust). We conducted subgroup analyses stratified by age, sex, history of congestive heart failure, and days from hospital admission to initiation of corticosteroid treatment.
Analyses were performed with R software, version 4.0.5 (R Foundation for Statistical Computing). Alpha was 2-sided and set at .05 for the primary outcome TMLE analysis. We did not adjust for multiple comparisons; thus, all analyses other than the primary outcome should be viewed as hypothesis generating. The protocol for this study was previously deposited in an online repository.33 This study was designated as not human participants research by Boston University’s Institutional Review Board (#H-41795). The design of this study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.34 Additional analysis details are included in the eMethods in Supplement 1.
Sensitivity Analyses
We conducted multiple additional analyses to assess the robustness of the findings under alternative assumptions and to assess the risk of bias (see eMethods in Supplement 1 for additional details). Briefly, we calculated E-values to estimate the strength of association between unmeasured confounders, treatment assignment, and the primary outcome that would be needed to bring the association between treatment assignment and outcome to zero.35,36 We conducted a negative control analysis using an outcome of blood transfusion to assess the risk of residual confounding.37 We repeated analyses after excluding patients discharged in 2020 to minimize effects from the COVID-19 pandemic. To minimize the potential for immortal time bias,38 we repeated analyses among patients who met inclusion criteria only on hospital day 1. To assess the robustness of results to possible covariate misclassification, we repeated analyses among patients who met inclusion criteria on hospital day 2 or 3 and classified covariates using variables from the day prior to treatment assignment. Last, we explored the robustness of findings to potential residual confounding by indication, secular changes in sepsis treatment, and patient illness severity, using the difference-in-differences method39 that compared changes in outcomes before and after hospital-level adoption of fludrocortisone following the March 2018 publication of the Activated Protein C and Corticosteroids for Human Septic Shock (APROCCHSS) trial9—the largest clinical trial showing mortality benefit of combination hydrocortisone-fludrocortisone compared with placebo.
Results
Study Population and Baseline Characteristics
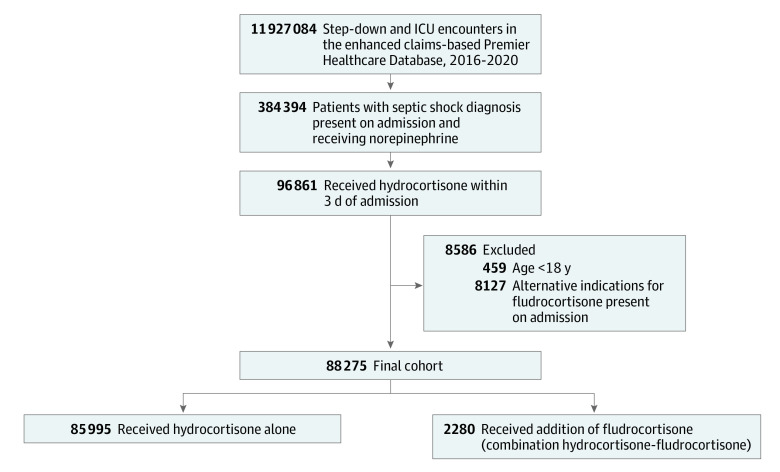
Among the 384 394 patients with septic shock who received norepinephrine, 88 275 received hydrocortisone within 3 days of hospitalization, met eligibility criteria, and were included in analyses (Figure 1). Among included patients, 85 995 (97.4%) were treated with hydrocortisone alone (median [IQR] age, 67 [57-76] years; 42 136 female; 43 859 male), and 2280 (2.6%) were treated with combination hydrocortisone-fludrocortisone (median [IQR] age, 64 [54-73] years; 1041 female; 1239 male). The median (IQR) time from norepinephrine initiation to hydrocortisone initiation was 0 (0-1) days in both treatment groups. Patients who received hydrocortisone-fludrocortisone were more likely to receive medications other than fludrocortisone via the enteral route (83.1%) compared with patients who received hydrocortisone alone (60.4%; SMD, 0.52). Baseline characteristics related to admission hospital also differed between treatment assignments (Table 1).
Figure 1. Study Flow Diagram.
Among the 287 533 patients with septic shock who received norepinephrine but who did not receive hydrocortisone, 101 611 (35.3%) died. ICU indicates intensive care unit.
Table 1. Baseline Covariates for Patients With Septic Shock Receiving Norepinephrine Who Received Hydrocortisone Treatment.
| Variable | Hydrocortisone alone (n = 85 995) | Hydrocortisone and fludrocortisone (n = 2280) | Absolute SMD | Absolute SMD after inverse probability of treatment weighting |
|---|---|---|---|---|
| Age, median (IQR), y | 67 (57-76) | 64 (54-73) | 0.20 | 0.06 |
| Sex, No. (%) | ||||
| Female | 42 136 (49.0) | 1041 (45.7) | 0.07 | 0.05 |
| Male | 43 859 (51.0) | 1239 (54.3) | ||
| Health insurance type, No. (%) | ||||
| Commercial | 12 479 (14.5) | 318 (13.9) | 0.15 | 0.08 |
| Medicaid | 12 000 (14.0) | 427 (18.7) | ||
| Medicare | 56 551 (65.8) | 1389 (60.9) | ||
| Self-pay | 2692 (3.1) | 94 (4.1) | ||
| Other | 2273 (2.6) | 52 (2.3) | ||
| Elixhauser comorbidity score POA, median (IQR) | 6 (4-7) | 6 (4-7) | 0.04 | 0.05 |
| CHF POA, No. (%) | 31 663 (36.8) | 848 (37.2) | 0.01 | 0.02 |
| Connective tissue disease POA, No. (%) | 6461 (7.5) | 117 (5.1) | 0.10 | 0.08 |
| Pneumonia POA, No. (%) | 32 306 (37.6) | 899 (39.4) | 0.04 | 0.02 |
| Major surgery per HCUP on or before day of hydrocortisone initiation, No. (%) | 8380 (9.7) | 148 (6.5) | 0.12 | 0.10 |
| Acute organ dysfunction, No. (%) | ||||
| Respiratory | 36 518 (42.5) | 1123 (49.3) | 0.14 | 0.02 |
| Hematologic | 26 125 (30.4) | 724 (31.8) | 0.03 | 0.06 |
| Hepatic | 10 906 (12.7) | 291 (12.8) | 0.00 | 0.07 |
| Renal | 60 416 (70.3) | 1621 (71.1) | 0.02 | 0.01 |
| Time from hospital admission to hydrocortisone initiation, No. (%) | ||||
| 0 d | 45 835 (53.3) | 1052 (46.1) | 0.15 | 0.02 |
| 1 d | 32 576 (37.9) | 1029 (45.1) | ||
| 2 d | 7584 (8.8) | 199 (8.7) | ||
| Time from norepinephrine initiation to hydrocortisone treatment, No. (%) | ||||
| 0 d | 60 262 (70.1) | 1434 (62.9) | 0.17 | 0.02 |
| 1 d | 22 431 (26.1) | 771 (33.8) | ||
| 2 d | 3302 (3.8) | 75 (3.3) | ||
| Volume of resuscitative fluids on day of hydrocortisone initiation, median (IQR), mL | 2000 (0-4500) | 2500 (500-5000) | 0.16 | 0.02 |
| Enteral medication administration other than fludrocortisone on day of hydrocortisone initiation, No. (%) | 51 974 (60.4) | 1895 (83.1) | 0.52 | 0.29 |
| Serum cortisol measured on day of hydrocortisone initiation, No. (%) | 14 130 (16.4) | 321 (14.1) | 0.07 | 0.02 |
| Cosyntropin administered on day of hydrocortisone initiation, No. (%) | 392 (0.5) | 5 (0.2) | 0.04 | 0.06 |
| Etomidate use on or before day of hydrocortisone initiation, No. (%) | 22 652 (26.3) | 710 (31.1) | 0.11 | 0.03 |
| Kidney replacement therapy on or before day of hydrocortisone initiation, No. (%) | 7301 (8.5) | 216 (9.5) | 0.04 | 0.02 |
| Vasopressor use on day of hydrocortisone initiation, No. (%) | ||||
| Dopamine | 5715 (6.6) | 74 (3.2) | 0.16 | 0.09 |
| Epinephrine | 17 987 (20.9) | 483 (21.2) | 0.01 | 0.03 |
| Phenylephrine | 21 445 (24.9) | 565 (24.8) | 0.00 | 0.02 |
| Vasopressin | 45 077 (52.4) | 1551 (68.0) | 0.32 | 0.09 |
| Vasopressor count on day of hydrocortisone initiation, median (IQR) | 2 (1-3) | 2 (1-3) | 0.13 | 0.01 |
| Invasive mechanical ventilation on day of hydrocortisone initiation, No. (%) | 50 981 (59.3) | 1491 (65.4) | 0.13 | 0.00 |
| US Census region, No. (%) | ||||
| Midwest | 18 548 (21.6) | 480 (21.1) | 0.17 | 0.13 |
| Northeast | 10 954 (12.7) | 388 (17.0) | ||
| South | 39 823 (46.3) | 897 (39.3) | ||
| West | 16 670 (19.4) | 515 (22.6) | ||
| Teaching hospital status, No. (%) | 44 347 (51.6) | 1640 (71.9) | 0.43 | 0.18 |
| Hospital bed number, No. (%) | ||||
| 0-99 | 2912 (3.4) | 62 (2.7) | 0.29 | 0.13 |
| 100-199 | 11 286 (13.1) | 177 (7.8) | ||
| 200-299 | 14 549 (16.9) | 311 (13.6) | ||
| 300-399 | 14 199 (16.5) | 280 (12.3) | ||
| 400-499 | 10 792 (12.5) | 342 (15.0) | ||
| ≥500 | 32 257 (37.5) | 1108 (48.6) | ||
| Hospital caseload, median (IQR) | 232 (119-394) | 370 (189-503) | 0.42 | 0.15 |
| Surgical care unit, No. (%) | 3221 (3.7) | 175 (7.7) | 0.17 | 0.03 |
| Discharge quarter/y, No. (%) | ||||
| 1/2016 | 3709 (4.3) | 10 (0.4) | 0.95 | 0.31 |
| 2/2016 | 3435 (4.0) | 8 (0.4) | ||
| 3/2016 | 3363 (3.9) | 13 (0.6) | ||
| 4/2016 | 3673 (4.3) | 16 (0.7) | ||
| 1/2017 | 4336 (5.0) | 13 (0.6) | ||
| 2/2017 | 4198 (4.9) | 9 (0.4) | ||
| 3/2017 | 3967 (4.6) | 10 (0.4) | ||
| 4/2017 | 4340 (5.0) | 15 (0.7) | ||
| 1/2018 | 4929 (5.7) | 35 (1.5) | ||
| 2/2018 | 4297 (5.0) | 233 (10.2) | ||
| 3/2018 | 4212 (4.9) | 191 (8.4) | ||
| 4/2018 | 4659 (5.4) | 189 (8.3) | ||
| 1/2019 | 5204 (6.1) | 237 (10.4) | ||
| 2/2019 | 4786 (5.6) | 194 (8.5) | ||
| 3/2019 | 4525 (5.3) | 182 (8.0) | ||
| 4/2019 | 4916 (5.7) | 164 (7.2) | ||
| 1/2020 | 5078 (5.9) | 224 (9.8) | ||
| 2/2020 | 4116 (4.8) | 201 (8.8) | ||
| 3/2020 | 4000 (4.7) | 180 (7.9) | ||
| 4/2020 | 4252 (4.9) | 156 (6.8) | ||
Abbreviations: CHF, congestive heart failure; HCUP, Healthcare Cost and Utilization Project; POA, present on admission; SMD, standardized mean difference.
Primary Outcome
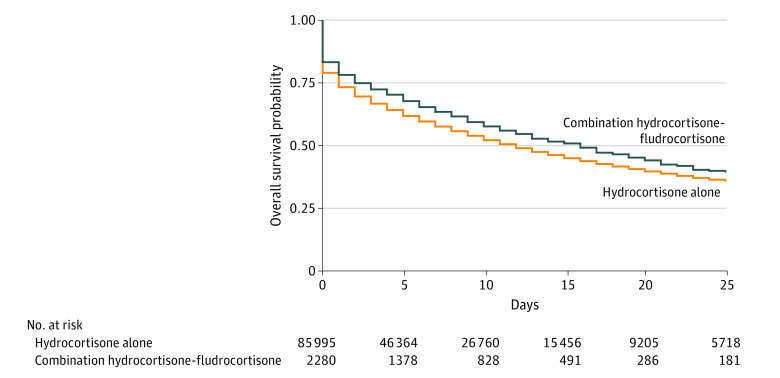
The median (IQR) number of days of follow-up was 6 (2-13) in patients treated with hydrocortisone-fludrocortisone and 5 (1-12) in those treated with hydrocortisone alone. The median (IQR) duration of treatment was 3 (1-4) days in the hydrocortisone-fludrocortisone group and 3 (2-6) in the hydrocortisone alone group. The median (IQR) total dose of hydrocortisone on study day 0 was 225 (200-300) mg among patients who received hydrocortisone-fludrocortisone and 200 (100-300) mg among patients who received hydrocortisone alone. The median (IQR) total dose of fludrocortisone was 0.1 (0.1-0.1) mg. Among patients who received hydrocortisone-fludrocortisone, 1076 (47.2%) died or were discharged to hospice vs 43 669 (50.8%) for those who received hydrocortisone alone. Figure 2 shows unadjusted survival curves by treatment assignment. In the adjusted TMLE analysis, receipt of hydrocortisone-fludrocortisone was associated with an adjusted absolute risk difference of −3.7% (95% CI, −4.2% to −3.1%; P < .001; E-value, 1.37) in hospital mortality or discharge to hospice compared with hydrocortisone alone (Table 2, eFigure 2 in Supplement 1). Results were similar in the sensitivity analyses (eTables 4-6 in Supplement 1, Table 2). There was no difference by treatment assignment for the negative control outcome of blood transfusion after study day 0 (hydrocortisone-fludrocortisone: 28.3%; hydrocortisone alone: 29.9%; adjusted risk difference, −0.3%; 95% CI, −0.8% to 0.1%). The direction of effect favored hydrocortisone-fludrocortisone in all prespecified subgroups (eTable 7 in Supplement 1).
Figure 2. 28-Day Survival Among Patients With Septic Shock Treated With Hydrocortisone-Fludrocortisone or Hydrocortisone Alone.
Shown are unadjusted Kaplan-Meier survival curves for the composite outcome of hospital death or discharge to hospice for patients in the 28 days after initiation of corticosteroid treatment among adult patients with septic shock.
Table 2. Primary and Sensitivity Targeted Maximum Likelihood Estimation Analyses for Hospital Death or Discharge to Hospice.
| Hospital death or discharge to hospice | Hydrocortisone alone | Hydrocortisone and fludrocortisone | Unadjusted risk difference (95% CI) | Adjusted risk difference (95% CI) | P value |
|---|---|---|---|---|---|
| No. patients with events/total No. of patients (%) | |||||
| Primary analysis (n = 88 275) | 43 669/85 995 (50.8) | 1076/2280 (47.2) | −3.6 (−5.7 to −1.5) | −3.7 (−4.2 to −3.1) | <.001 |
| Sensitivity analyses | |||||
| Met inclusion criteria on hospital day 1 (n = 46 887) | 22 303/45 835 (48.7) | 452/1052 (43.0) | −5.7 (−8.7 to −2.6) | −5.2 (−5.8 to −4.6) | <.001 |
| Met inclusion criteria on hospital day 2 or 3 with covariates ascertained on the day before treatment assignment (n = 41 388) | 21 366/40 160 (53.2) | 624/1228 (50.8) | −2.4 (−5.2 to 0.4) | −2.2 (−3.2 to −1.3) | <.001 |
| Excluding 2020 (n = 70 068) | 34 072/68 549 (49.7) | 696/1519 (45.8) | −3.9 (−6.4 to −1.3) | −4.0 (−4.6 to −3.5) | <.001 |
Secondary Outcomes
The rate of hospital death was 39.3% among patients who received hydrocortisone-fludrocortisone and 42.7% among patients who received hydrocortisone (adjusted risk difference, −3.7%; 95% CI, −4.2% to −3.3%). Vasopressor-free days and hospital-free days were higher among patients who received hydrocortisone-fludrocortisone (Table 3). The adjusted mean difference in vasopressor-free days and hospital-free days comparing patients treated with hydrocortisone-fludrocortisone vs hydrocortisone alone was 0.9 (95% CI, 0.8-1.1) days and 0.7 (95% CI, 0.6-0.8) days, respectively. The proportions of patients with incident hypernatremia (8872 of 78 484 [11.3%] for hydrocortisone alone; 236 of 2066 [11.4%] for hydrocortisone-fludrocortisone) and health care-associated infection (811 of 82 783 [1.0%] for hydrocortisone alone; 31 of 2175 [1.4%] for hydrocortisone-fludrocortisone) were similar between treatment arms.
Table 3. Secondary Outcomes Using Targeted Maximum Likelihood Estimation.
| Outcome (n = 88 275) | Hydrocortisone alone | Hydrocortisone and fludrocortisone | Unadjusted risk difference (95% CI) | Adjusted risk difference (95% CI) | P value |
|---|---|---|---|---|---|
| No. patients with events/total No. of patients (%) | |||||
| Hospital death | 36 713/85 995 (42.7) | 896/2280 (39.3) | −3.4 (−5.5 to −1.3) | −3.7 (−4.2 to −3.3) | <.001 |
| Blood transfusion negative control outcome | 25 716/85 995 (29.9) | 645/2280 (28.3) | −1.6 (−3.5 to 0.3) | −0.3 (−0.8 to 0.1) | .12 |
| Mean days (95% CI) | Unadjusted risk difference (95% CI) | Adjusted risk difference (95% CI) | P value | ||
| Vasopressor-free days | 12.9 (12.9 to 13.0) | 13.8 (13.3 to 14.4) | 0.9 (0.3 to 1.5) | 0.9 (0.8 to 1.1) | <.001 |
| Hospital-free days | 8.4 (8.3 to 8.4) | 8.7 (8.3 to 9.1) | 0.3 (−0.1 to 0.7) | 0.7 (0.6 to 0.8) | <.001 |
Difference-in-Differences
We identified 3521 patients admitted to future fludrocortisone “adopter” hospitals (ie, those in the top quartile of hospitals that increased their use of hydrocortisone-fludrocortisone after APROCCHSS) and 7510 admitted to control hospitals prior to publication of APROCCHSS,9 and 5464 admitted to adopter hospitals and 9784 admitted to control hospitals after publication of APROCCHSS. The percentage of patients who received fludrocortisone in addition to hydrocortisone increased from 0.4% pre-APROCCHSS to 12.6% post-APROCCHSS among patients admitted to adopter hospitals and remained stable (0.3% to 0.3%) among patients admitted to control hospitals. There was no evidence (interaction term β, 0.0006; 95% CI, −0.0010 to 0.0030) that outcome trends between adopter and control hospitals were different in the pre-APROCCHSS period (eFigure 3 in Supplement 1). Hospital death or discharge to hospice occurred in 51.6% of patients admitted to adopter hospitals and 49.0% of patients admitted to control hospitals pre-APROCCHSS and 52.6% (+1.0%) in adopter hospitals and 52.2% (+3.2%) of patients in controls post-APROCCHSS, resulting in an adjusted difference-in-difference estimator of −2.0% (−3.9% to −0.2%), a lower probability of hospital death or discharge to hospice for patients admitted to fludrocortisone adopter hospitals after publication of APROCCHSS9 compared with patients admitted to control hospitals. There was no evidence of bias from the blood transfusion falsification test (difference-in-difference estimator, 1.3%; 95% CI, −0.4% to 2.9%).
Discussion
In this cohort study, we used a large, multicenter, enhanced claims-based database to emulate a clinical trial that would compare the effectiveness of fludrocortisone added to hydrocortisone vs hydrocortisone alone among patients with septic shock. We found that fludrocortisone added to hydrocortisone was associated with increased hospital survival, shorter length of stay, and decreased shock duration compared with hydrocortisone alone. The primary outcome effect estimate was similar to the risk reduction of the COIITSS clinical trial (−2.9% absolute risk reduction),12 a previous and potentially underpowered randomized clinical trial that also compared hydrocortisone-fludrocortisone to hydrocortisone alone. Findings provide additional evidence that the addition of fludrocortisone to hydrocortisone may be superior to hydrocortisone alone among patients with septic shock.
Despite the findings of prior clinical trials8,9 that showed reduced mortality with combined hydrocortisone-fludrocortisone as compared with placebo, guidelines4 recommend hydrocortisone alone in patients with septic shock with persistent vasopressor requirements. Rationale for recommendations to use hydrocortisone alone during septic shock include the “negative” (ie, statistically nonsignificant) findings from the COIITSS clinical trial12 and potentially adequate mineralocorticoid effects of hydrocortisone. However, published mineralocorticoid equivalences are based on sodium-retaining potency40,41 and do not account for pleotropic mineralocorticoid effects, including activation of innate immunity and facilitation of clearance of increased alveolar fluid (a hallmark of acute respiratory distress syndrome, a common comorbidity in patients with septic shock) by alveolar epithelial cells.42,43,44 We speculate that differences in these pleotropic effects between fludrocortisone and hydrocortisone may explain the lower mortality associated with combination hydrocortisone-fludrocortisone vs hydrocortisone alone.
Our results inform the feasibility of future studies and clinical care. Based on our results and assuming 50% mortality among patients given hydrocortisone alone, a 1:1 randomized clinical trial comparing hydrocortisone alone to hydrocortisone-fludrocortisone would need to enroll 5724 participants to have 80% power (α = .05) to detect a difference at least as large as that identified in our study. In addition, future Bayesian network meta-analyses seeking to compare hydrocortisone to hydrocortisone-fludrocortisone using existing placebo-controlled randomized clinical trials should consider using our observational effect estimates to inform prior probabilities. Last, in absence of these future studies, our results suggest that clinicians seeking to optimize the use of corticosteroids in septic shock should consider adding fludrocortisone when initiating hydrocortisone treatment.
Limitations
Our study has limitations. Although results were robust to sensitivity analyses, were similar to those from a prior clinical trial,12 and would only be altered in the presence of an unmeasured confounder with a 37% or greater association with treatment assignment and outcome (E-value, 1.37), the observational nature of our study increases the risk of residual unmeasured confounding as compared with a randomized trial. Although we used validated scores to estimate severity of acute organ dysfunction with similar performance to the sequential organ failure score27 and adjusted for admission hospital to account for between-center practice pattern variation, the Premier Healthcare Database does not contain comprehensive electronic medical record physiological/vital sign data or vasopressor doses that, because not included in models, may increase the risk of unmeasured cofounding. However, a difference-in-differences design sensitivity analysis less subject to residual confounding by individual patient characteristics yielded complementary results showing that higher vs lower hospital-level adoption of fludrocortisone after publication of the APROCCHSS trial9 was associated with improved outcomes. Last, there are several limitations related to the Premier Healthcare Database having granularity only to the level of the calendar day. First, in our primary analysis we classified covariates that were present on study day 0 as preexposure (rather than postexposure), an assumption that is unverifiable based on the limited granularity of the data set. However, a sensitivity analysis limited to patients who met inclusion criteria and were assigned treatment status on hospital day 2 or 3, with covariates defined based on values on the day prior to treatment assignment, yielded similar results to the primary analysis, suggesting that primary results were not fully explained by covariate misclassification. Second, it is possible that initiation of fludrocortisone treatment did not occur concurrently with hydrocortisone administration but instead occurred after hydrocortisone initiation but still on the same calendar day, potentially increasing the risk of immortal time bias within the same day. The unadjusted survival curve showing separation between treatment assignments starting on study day 0 could suggest evidence of immortal time bias, although the treatment arms continue to separate through study day 4, a time period consistent with survival curve separation in the APROCCHSS trial.9 In addition, analysis of the separate MIMIC-IV database23 suggested that the median time from hydrocortisone to fludrocortisone initiation is only 2 hours.
Conclusions
In this multicenter observational effectiveness cohort study of patients with septic shock who were started on hydrocortisone treatment, the addition of fludrocortisone was superior to hydrocortisone alone across multiple patient outcomes, including mortality. These results, among more than 88 000 patients, showed similar absolute risk reduction estimates to a previous clinical trial and provide additional evidence for combination hydrocortisone-fludrocortisone therapy for patients with septic shock for whom clinicians choose to initiate corticosteroid therapy.
eMethods: Statistical analysis plan
eTable 1: Comparison between target trial and the observational study of the effectiveness of fludrocortisone added to hydrocortisone versus hydrocortisone alone in patients with septic shock
eTable 2: Study eligibility criteria
eTable 3: Study variable definitions
eTable 4: Baseline covariates in the sensitivity analysis cohort excluding patients discharged in 2020
eTable 5: Baseline covariates in the sensitivity analysis cohort limited to patients who met inclusion criteria on hospital day 1
eTable 6: Baseline covariates in the sensitivity analysis cohort limited to patients who met inclusion criteria on hospital days 2 or 3 and covariates were defined on the day before treatment assignment
eTable 7: Subgroup-analyses for the adjusted risk difference of hospital death or hospice discharge
eFigure 1: Directed acyclic graph
eFigure 2: Distribution of propensity scores
eFigure 3: Trends in hospital death or discharge hospice among patients with septic shock on norepinephrine and hydrocortisone from 2016-2017
eReferences
Data Sharing Statement
References
- 1.Rhee C, Dantes R, Epstein L, et al. ; CDC Prevention Epicenter Program . Incidence and trends of sepsis in US hospitals using clinical vs claims data, 2009-2014. JAMA. 2017;318(13):1241-1249. doi: 10.1001/jama.2017.13836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu V, Escobar GJ, Greene JD, et al. Hospital deaths in patients with sepsis from 2 independent cohorts. JAMA. 2014;312(1):90-92. doi: 10.1001/jama.2014.5804 [DOI] [PubMed] [Google Scholar]
- 3.Bauer M, Gerlach H, Vogelmann T, Preissing F, Stiefel J, Adam D. Mortality in sepsis and septic shock in Europe, North America and Australia between 2009 and 2019—results from a systematic review and meta-analysis. Crit Care. 2020;24(1):239. doi: 10.1186/s13054-020-02950-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evans L, Rhodes A, Alhazzani W, et al. Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock 2021. Crit Care Med. 2021;49(11):e1063-e1143. doi: 10.1097/CCM.0000000000005337 [DOI] [PubMed] [Google Scholar]
- 5.Gordon AC, Mason AJ, Thirunavukkarasu N, et al. ; VANISH Investigators . Effect of early vasopressin vs norepinephrine on kidney failure in patients with septic shock: the VANISH randomized clinical trial. JAMA. 2016;316(5):509-518. doi: 10.1001/jama.2016.10485 [DOI] [PubMed] [Google Scholar]
- 6.Sprung CL, Annane D, Keh D, et al. ; CORTICUS Study Group . Hydrocortisone therapy for patients with septic shock. N Engl J Med. 2008;358(2):111-124. doi: 10.1056/NEJMoa071366 [DOI] [PubMed] [Google Scholar]
- 7.Venkatesh B, Finfer S, Cohen J, et al. ; ADRENAL Trial Investigators and the Australian–New Zealand Intensive Care Society Clinical Trials Group . Adjunctive glucocorticoid therapy in patients with septic shock. N Engl J Med. 2018;378(9):797-808. doi: 10.1056/NEJMoa1705835 [DOI] [PubMed] [Google Scholar]
- 8.Annane D, Sébille V, Charpentier C, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA. 2002;288(7):862-871. doi: 10.1001/jama.288.7.862 [DOI] [PubMed] [Google Scholar]
- 9.Annane D, Renault A, Brun-Buisson C, et al. ; CRICS-TRIGGERSEP Network . Hydrocortisone plus fludrocortisone for adults with septic shock. N Engl J Med. 2018;378(9):809-818. doi: 10.1056/NEJMoa1705716 [DOI] [PubMed] [Google Scholar]
- 10.Rygård SL, Butler E, Granholm A, et al. Low-dose corticosteroids for adult patients with septic shock: a systematic review with meta-analysis and trial sequential analysis. Intensive Care Med. 2018;44(7):1003-1016. doi: 10.1007/s00134-018-5197-6 [DOI] [PubMed] [Google Scholar]
- 11.Rochwerg B, Oczkowski SJ, Siemieniuk RAC, et al. Corticosteroids in sepsis: an updated systematic review and meta-analysis. Crit Care Med. 2018;46(9):1411-1420. doi: 10.1097/CCM.0000000000003262 [DOI] [PubMed] [Google Scholar]
- 12.Annane D, Cariou A, Maxime V, et al. ; COIITSS Study Investigators . Corticosteroid treatment and intensive insulin therapy for septic shock in adults: a randomized controlled trial. JAMA. 2010;303(4):341-348. doi: 10.1001/jama.2010.2 [DOI] [PubMed] [Google Scholar]
- 13.Harhay MO, Wagner J, Ratcliffe SJ, et al. Outcomes and statistical power in adult critical care randomized trials. Am J Respir Crit Care Med. 2014;189(12):1469-1478. doi: 10.1164/rccm.201401-0056CP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abrams D, Montesi SB, Moore SKL, et al. Powering bias and clinically important treatment effects in randomized trials of critical illness. Crit Care Med. 2020;48(12):1710-1719. doi: 10.1097/CCM.0000000000004568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hernán MA, Robins JM. Using big data to emulate a target trial when a randomized trial is not available. Am J Epidemiol. 2016;183(8):758-764. doi: 10.1093/aje/kwv254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hernán MA. Methods of public health research—strengthening causal inference from observational data. N Engl J Med. 2021;385(15):1345-1348. doi: 10.1056/NEJMp2113319 [DOI] [PubMed] [Google Scholar]
- 17.Lindenauer PK, Pekow P, Wang K, Mamidi DK, Gutierrez B, Benjamin EM. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Engl J Med. 2005;353(4):349-361. doi: 10.1056/NEJMoa041895 [DOI] [PubMed] [Google Scholar]
- 18.Schneeweiss S, Seeger JD, Landon J, Walker AM. Aprotinin during coronary-artery bypass grafting and risk of death. N Engl J Med. 2008;358(8):771-783. doi: 10.1056/NEJMoa0707571 [DOI] [PubMed] [Google Scholar]
- 19.Premier Healthcare Database White Paper . Data that informs and performs. Published online March 2, 2020. Accessed November 9, 2021. https://products.premierinc.com/downloads/PremierHealthcareDatabaseWhitepaper.pdf
- 20.Rhee C, Jentzsch MS, Kadri SS, et al. ; Centers for Disease Control and Prevention (CDC) Prevention Epicenters Program . Variation in identifying sepsis and organ dysfunction using administrative versus electronic clinical data and impact on hospital outcome comparisons. Crit Care Med. 2019;47(4):493-500. doi: 10.1097/CCM.0000000000003554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iwashyna TJ, Odden A, Rohde J, et al. Identifying patients with severe sepsis using administrative claims: patient-level validation of the angus implementation of the international consensus conference definition of severe sepsis. Med Care. 2014;52(6):e39-e43. doi: 10.1097/MLR.0b013e318268ac86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whittaker SA, Mikkelsen ME, Gaieski DF, Koshy S, Kean C, Fuchs BD. Severe sepsis cohorts derived from claims-based strategies appear to be biased toward a more severely ill patient population. Crit Care Med. 2013;41(4):945-953. doi: 10.1097/CCM.0b013e31827466f1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson A, Bulgarelli L, Pollard T, Horng S, Celi LA, Mark R. MIMIC-IV (version 0.4). PhysioNet. doi: 10.13026/A3WN-HQ05 [DOI]
- 24.Shrier I, Platt RW. Reducing bias through directed acyclic graphs. BMC Med Res Methodol. 2008;8(1):70. doi: 10.1186/1471-2288-8-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elixhauser A, Friedman B, Stranges E. Septicemia in US hospitals, 2009: Statistical Brief #122. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Agency for Healthcare Research and Quality (US); 2006. Accessed February 23, 2021. https://www.ncbi.nlm.nih.gov/books/NBK65391/
- 26.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303-1310. doi: 10.1097/00003246-200107000-00002 [DOI] [PubMed] [Google Scholar]
- 27.Bosch NA, Law AC, Rucci JM, Peterson D, Walkey AJ. Predictive validity of the sequential organ failure assessment score versus claims-based scores among critically ill patients. Ann Am Thorac Soc. 2022;19(6):1072-1076. doi: 10.1513/AnnalsATS.202111-1251RL [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Healthcare Cost & Utilization Project . Procedure classes refined for ICD-10-PCS. Accessed June 8, 2022. https://hcup-us.ahrq.gov/toolssoftware/procedureicd10/procedure_icd10.jsp#elements
- 29.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457-481. doi: 10.1080/01621459.1958.10501452 [DOI] [Google Scholar]
- 30.van der Laan MJ, Rubin D. Targeted maximum likelihood learning. Int J Biostat. 2006;2(1). doi: 10.2202/1557-4679.1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van der Laan MJ, Polley EC, Hubbard AE. Super learner. Stat Appl Genet Mol Biol. 2007;6(1):e25. doi: 10.2202/1544-6115.1309 [DOI] [PubMed] [Google Scholar]
- 32.Gruber S, van der Laan M. tmle: An R package for targeted maximum likelihood estimation. J Stat Softw. 2012;51:1-35. doi: 10.18637/jss.v051.i1323504300 [DOI] [Google Scholar]
- 33.Bosch N. Comparative effectiveness of fludrocortisone and hydrocortisone to hydrocortisone in patients with septic shock: a target trial emulation. Published online June 9, 2022. Accessed June 28, 2022. https://osf.io/gkrqn/
- 34.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344-349. doi: 10.1016/j.jclinepi.2007.11.008 [DOI] [PubMed] [Google Scholar]
- 35.Mathur MB, Ding P, Riddell CA, VanderWeele TJ. Web site and R package for computing E-values. Epidemiology. 2018;29(5):e45-e47. doi: 10.1097/EDE.0000000000000864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med. 2017;167(4):268-274. doi: 10.7326/M16-2607 [DOI] [PubMed] [Google Scholar]
- 37.Lipsitch M, Tchetgen Tchetgen E, Cohen T. Negative controls: a tool for detecting confounding and bias in observational studies. Epidemiology. 2010;21(3):383-388. doi: 10.1097/EDE.0b013e3181d61eeb [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vail EA, Gershengorn HB, Wunsch H, Walkey AJ. Attention to immortal time bias in critical care research. Am J Respir Crit Care Med. 2021;203(10):1222-1229. doi: 10.1164/rccm.202008-3238CP [DOI] [PubMed] [Google Scholar]
- 39.Zhou H, Taber C, Arcona S, Li Y. Difference-in-differences method in comparative effectiveness research: utility with unbalanced groups. Appl Health Econ Health Policy. 2016;14(4):419-429. doi: 10.1007/s40258-016-0249-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brunton LL, Hilal-Dandan R, Knollmann BC. Goodman & Gilman’s: The Pharmacological Basis of Therapeutics. 13th ed. McGraw Hill Medical; 2018. Accessed June 27, 2022. https://accessmedicine.mhmedical.com/book.aspx?bookid=2189 [Google Scholar]
- 41.Kaufman DA. Glucocorticoid therapy in septic shock in adults. In: UpToDate. Wolters Kluwer; 2022. Accessed June 27, 2022. https://www.uptodate.com/contents/glucocorticoid-therapy-in-septic-shock-in-adults#H1330646853
- 42.Heming N, Sivanandamoorthy S, Meng P, Bounab R, Annane D. Immune effects of corticosteroids in sepsis. Front Immunol. 2018;9:1736. doi: 10.3389/fimmu.2018.01736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Annane D. Why my steroid trials in septic shock were “positive”. Crit Care Med. 2019;47(12):1789-1793. doi: 10.1097/CCM.0000000000003889 [DOI] [PubMed] [Google Scholar]
- 44.Suzuki S, Tsubochi H, Suzuki T, et al. Modulation of transalveolar fluid absorption by endogenous aldosterone in adult rats. Exp Lung Res. 2001;27(2):143-155. doi: 10.1080/019021401750069384 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods: Statistical analysis plan
eTable 1: Comparison between target trial and the observational study of the effectiveness of fludrocortisone added to hydrocortisone versus hydrocortisone alone in patients with septic shock
eTable 2: Study eligibility criteria
eTable 3: Study variable definitions
eTable 4: Baseline covariates in the sensitivity analysis cohort excluding patients discharged in 2020
eTable 5: Baseline covariates in the sensitivity analysis cohort limited to patients who met inclusion criteria on hospital day 1
eTable 6: Baseline covariates in the sensitivity analysis cohort limited to patients who met inclusion criteria on hospital days 2 or 3 and covariates were defined on the day before treatment assignment
eTable 7: Subgroup-analyses for the adjusted risk difference of hospital death or hospice discharge
eFigure 1: Directed acyclic graph
eFigure 2: Distribution of propensity scores
eFigure 3: Trends in hospital death or discharge hospice among patients with septic shock on norepinephrine and hydrocortisone from 2016-2017
eReferences
Data Sharing Statement