Abstract
Background:
Stimulant medications such as methylphenidate (MPH) are the frontline treatment for Attention-Deficit/Hyperactivity Disorder (ADHD). Despite their well-documented efficacy, the mechanisms by which stimulants improve clinical outcomes are not clear. The current study evaluated whether MPH effects on classroom behavior were mediated by improved cognitive functioning.
Methods:
Children with ADHD (n=82;9–12 years old) participated in a week-long summer research camp, consisting of cognitive testing, classroom periods, and recreational activities. After a baseline day, participants completed a three-day randomized, double-blind, placebo-controlled trial of MPH (at doses approximating 0.3 and 0.6 mg/kg of immediate-release MPH dosed TID). Cognitive domains included inhibitory control (Stop Signal Task and prepulse inhibition of startle), attention (Continuous Performance Task and reaction time variability), and working memory (forward and backward spatial span). Clinical outcomes included math seatwork productivity and teacher-rated classroom behavior. A within-subjects path-analytic approach was used to test mediation. MPH-placebo and dose-response contrasts were used to evaluate drug effects.
Results:
MPH improved seatwork productivity and teacher ratings (ds=1.4 and 1.1) and all domains of cognition (ds=.3–1.1). Inhibitory control (SST) and working memory backward uniquely mediated the effect of MPH (vs. placebo) on productivity. Only working memory backward mediated the impact of MPH on teacher-rated behavior. The dose-response (0.6 vs. 0.3 mg/kg) effects were more modest for clinical outcomes (ds=0.4 and 0.2) and cognition (ds=0–0.3); there was no evidence of cognitive mediation of the clinical dose-response effects.
Conclusions:
These findings are novel in demonstrating that specific cognitive processes mediate clinical improvement with stimulant treatment for ADHD. They converge with work on ADHD theory, neurobiology, and treatment development in suggesting that inhibitory control and working memory may be mechanisms of stimulant treatment response in ADHD. More work is necessary to evaluate the degree to which these findings generalize to chronic treatment, a broader array of clinical outcomes, and non-stimulant treatments.
Keywords: ADHD, methylphenidate, cognition, mediation
The identification of treatment mechanisms is critical for evaluating theory, advancing personalized medicine, and developing novel, targeted therapies (e.g., Kazdin, 2007; Kraemer, Wilson, Fairburn, & Agras, 2002). In Attention-Deficit/Hyperactivity Disorder (ADHD), as in many other disorders, demonstration of how even the most well-supported treatments work has proven challenging. Stimulant medications such as methylphenidate (MPH) are the frontline pharmacotherapy for school-age children with Attention-Deficit/Hyperactivity Disorder (ADHD; Pliszka 2007); decades of clinical studies document their efficacy in the short-term management of ADHD, improving target behavior and symptom ratings. In parallel, decades of basic laboratory research demonstrate that stimulants improve a range of cognitive processes that are implicated in ADHD (e.g., Coghill et al., 2014a; Cortese et al., 2015). Though these independent lines of research are consistent with hypotheses that stimulant effects on cognitive processes are causal in the clinical effects, they provide only indirect evidence that treatment-related improvement in cognitive function accounts for, or mediates, treatment-related clinical improvement. As highlighted in the framework of Kazdin (2007; see also Kraemer et al., 2002; Mackinnon et al., 2008), direct tests of mediation are necessary (but not sufficient) to evaluate candidate treatment mechanisms. Such work requires the assessment of potential mediators in the context of a strong treatment outcome design, such as a randomized controlled trial (RCT), in order to concurrently assess treatment effects on clinical outcomes (Figure 1, “c” path) and the hypothesized mediators (“a” path). Given the challenges of such designs, it is perhaps not surprising that they are rarely implemented in the ADHD literature (c.f., Froehlich et al., 2014; Kortekaas-Rijlaarsdam et al., 2017). The present study addresses this gap by evaluating the degree to which MPH effects on basic cognitive processes mediate the effects of MPH on classroom functioning, a key clinical outcome for youth with ADHD.
Figure 1.
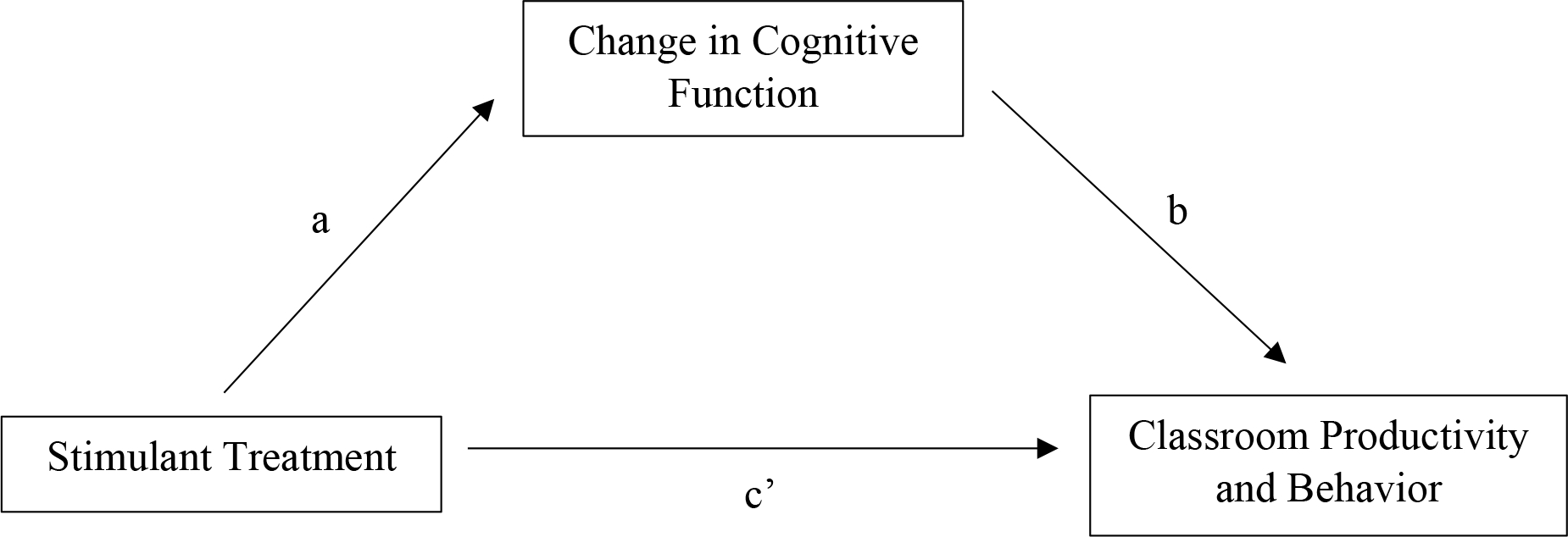
Sample mediation model.
Clinical Effects of Stimulants
There is strong evidence that stimulants reduce parent- and teacher-reported ADHD symptoms (Faraone & Buitelaar, 2010; MTA Cooperative Group, 1999) and improve classroom behavior (Pelham et al., 2014; Prasad et al., 2013), including increasing on-task behavior and the amount of school work completed (Baweja et al., 2016; Prasad et al., 2013). Moreover, clinical improvement can be realized with acute treatment in natural and laboratory settings (e.g., Pelham et al., 2001), allowing the initial evaluation of mediation in a more feasible short-term RCT. The setting for the present work, an analogue classroom, was chosen to balance feasibility and internal validity with clinical relevance. School-related impairment is a common referral concern, and laboratory school analogues have become the predominant paradigm for evaluating the efficacy of novel stimulant agents in children with ADHD (Wigal & Wigal, 2006).
Candidate Mediators of ADHD Treatment Response
Kazdin (2007) recommends using theory to guide the selection of multiple mediators that are evaluated concurrently. Etiologic theories of ADHD posit that deficits in core cognitive processes, such as behavioral inhibition (Barkley, 1997), attention (Castellanos & Tannock, 2002; Douglas, 1999), and working memory (Rapport et al., 2008) give rise to behavioral symptoms and related impairment. From these theories, it follows that amelioration of ADHD-related deficits in cognition should result in clinical improvement. Indeed, there is evidence that maturation of cognitive function predicts ADHD remission (e.g., Halperin et al., 2008; Karalunas et al., 2017; c.f., Coghill et., 2014b). In ADHD intervention work, cognitive functions have been hypothesized as treatment mechanisms for stimulant and non-stimulant medications (e.g., Arnsten, 2010; Gamo et al., 2010; Robbins & Arnsten, 2009) and are common targets for the development of non-pharmacologic interventions (see e.g., Cortese et al., 2015; Halperin et al., 2011; 2013).
Following additional recommendations for selecting candidate mediators (Kraemer et al., 2002), we targeted basic processes that robustly discriminate between children with and without ADHD at the group level and have demonstrated sensitivity to MPH. As briefly detailed below, these criteria led us to focus on three domains: inhibitory control, working memory, and attention. Meta-analytic evidence suggests that these domains demonstrate robust diagnostic group differences (Willcutt et al., 2005; Huang-Pollock, Karalunas, Tam, & Moore, 2012; Martinussen, Hayden, Hogg-Johnson, & Tannock, 2005) and beneficial effects of MPH (Coghill et al., 2014a; see also Pietrzak, Mollica, Maruff, & Snyder, 2006).
We assessed two indicators for each cognitive domain. Within inhibitory control, we focused on the most influential measure of response inhibition in the ADHD literature- stop signal reaction time (SSRT) from the stop task (Lipszyc & Schachar, 2010); we recently reported that MPH normalized SSRT in the present sample (Rosch et al., 2016). We also assessed a psychophysiological index of inhibitory function- prepulse inhibition (PPI) of the startle reflex. PPI during task-relevant stimuli is disrupted in ADHD (Conzelmann et al., 2010; Hawk et al., 2003), and MPH reverses this deficit (Ashare et al., 2010 focused on the first 50 participants in the present study; Hawk et al., 2003). On tasks of working memory, MPH generally improves the manipulation component of memory, with less clear evidence that MPH improves storage capacity (Bedard & Tannock, 2008; cf. Bedard et al., 2007). We assessed both sub-domains of visual-spatial working memory in the present study (see Shiels et al., 2008). Attention was assessed with a widely-used Continuous Performance Task (CPT; see Huang-Pollock et al., 2012); we also examined reaction time (RT) variability on a simple discrimination task (Leth-Steensen, Elbaz, & Douglas, 2000). MPH increases CPT hit rate (Bubnik et al., 2015; Solanto, Wender, & Bartell, 1997) and reduces reaction time variability, an effect observed among the first 50 participants in the current study (Spencer et al., 2009).
The Full Mediation Framework
A novel and critical question is the extent to which individual differences in the cognitive effects of stimulants actually account for individual differences in the clinical effects of stimulants. There are only two published studies on this topic (Froehlich et al., 2014; Kortekaas-Rijlaarsdam et al., 2017; see also Hinshaw, 2007). Both prior studies were medication titration trials of children with ADHD. Froehlich et al. examined cognitive and classroom performance at baseline and again after a 4-week, double-blind crossover MPH titration trial; at post-titration, children were randomized to their clinically-optimal dose of MPH (n=47) or placebo (n=46). Cognitive performance was averaged across 2 incentive × 3 event rate conditions for each outcome. In Kortekaas-Rijlaarsdam et al., 65 children completed a double-blind, randomized crossover evaluation of their typical MPH dose and placebo. Brief cognitive and math productivity assessments were completed individually at each child’s school.
Although these studies were groundbreaking in applying the full logic of mediation to stimulant mechanisms in ADHD, the results were disappointing. In particular, there was a surprising lack of stimulant effects on key cognitive measures (“a” path in Figure 1), precluding tests of mediation for most (Froehlich et al., 2014) or all (Kortekass-Rijlaarsdam et al., 2017) cognitive processes.
The present study improves upon these initial efforts in several ways. Titration studies are useful clinically but are not ideal for evaluating mediation. Mediation explicitly seeks to explain variability in treatment response (Kraemer et al., 2002). By contrast, titration studies vary the treatment across participants in a manner that is designed to restrict variability in clinical response – but that variability is of central interest in mediational analyses. The titration approach may be even more problematic in understanding stimulant treatment for ADHD, as the dose-response functions for clinical and cognitive measures may differ (see, e.g., Coghill, 2014a; Rosch et al., 2016; Pietrzak et al., 2006). We take an approach more aligned with mediation theory and methodology by evaluating clinical and cognitive performance under two standard therapeutic doses of MPH and placebo. This approach provides a broader assessment of stimulant treatment and allows the evaluation of dose-response relationships (see Kazdin, 2007).
We took steps to improve statistical power and the reliability and validity of our outcomes, including the use of a fully within-subjects design, a baseline practice day to minimize the impact of practice effects, two active medication assessments, and a large sample size (n=82). For clinical outcomes, we examined mediation for both academic productivity and teacher ratings; in contrast to prior mediation efforts, children completed three small-group classroom periods in each condition, facilitating reliability and ecological validity. For candidate mediators, we conducted a head-to-head comparison of leading theory-based cognitive processes with demonstrated sensitivity to stimulant treatment, administered under standard testing conditions (c.f. the complex manipulation of MPH × incentive × event rate in Froehlich et al. and the abbreviated cognitive battery administered in the school setting by Kortekaas-Rijlaarsdam et al.). Following initial evaluation of each putative mediator in separate models, we examined the degree to which they uniquely mediated treatment outcome when considered together.
Method
Participants
Participants were 82 children (9–12 years old) with a DSM-IV (American Psychiatric Association, 2000) diagnosis of ADHD (see Table 1 for participant characteristics). Diagnoses were made based on a structured computerized parent clinical interview (Diagnostic Interview Schedule for Children Version IV (DISC-IV); Shaffer et al., 2000), as well as parent and teacher rating scales of behavior (Disruptive Behavior Disorder (DBD) rating scale; Pelham et al., 1992) and impairment (Fabiano et al., 2006); ratings were completed based on behavior off of medication (see Rosch et al., 2016 for detailed diagnostic procedures and inclusion/exclusion criteria).
Table 1.
Participant characteristics.
| Age, mean (SD) | 10.8 (1.1) |
| Gender (%male:female) | 74:26 |
| % stimulant naïve | 21 |
| WISC Full-Scale IQ, mean (SD) | 103 (14) |
| Ethnicity (%white:black:other) | 81:12:7 |
| % Comorbid ODD | 44 |
| % Comorbid CD | 27 |
| Hyp/Imp symptoms, mean (SD) | |
| Parent | 15.2 (5.9) |
| Teacher | 11.6 (7.1) |
| Inattentive symptoms, mean (SD) | |
| Parent | 18.7 (5.6) |
| Teacher | 14.6 (6.3) |
Note. Symptoms represent the total score of items within each subtype domain on the DBD-rating scale (range of 0–27).
Setting and Procedures
Cohorts of 3–5 children participated in a week-long Summer Research Camp that ran 7:30am to 5pm Monday through Friday. Camp days consisted of computerized cognitive tasks (to assess candidate mediators) and three academic periods (to assess clinical outcomes), which were interspersed with recreational activities.
During both cognitive testing and the classroom periods, a low-intensity behavioral modification system was in place to ensure that markedly disruptive behavior did not invalidate data (see e.g., Fabiano et al., 2014). A basic set of rules (follow directions, stay in your assigned area, use materials appropriately, try your best) was reviewed at the beginning of each classroom or cognitive testing period, and children began with a set number of points that varied depending on the length of time of the activity. Note that points were not contingent on cognitive or math performance. The first two rule violations resulted in verbal warnings; subsequent rule violations led to response cost. Consequently, it was rare for a child to have more than two rule violations per period. Points were exchanged for small toys and/or gift cards at the end of each day.
Medication Assessment
Most participants (n=58) had prior experience with stimulant medication. Participants currently taking stimulants (n=31) discontinued use at least 24 hours prior to baseline testing. One participant had previously taken atomoxetine but had discontinued use several months prior to study participation. After a baseline, stimulant-free day (Monday) to familiarize participants with study activities and minimize to the impact of novelty or practice during the medication evaluation, children completed a 3-day, randomized, double-blind, placebo-controlled medication assessment. Medication and placebo were administered in identical opaque capsules by study staff upon arrival to camp (~7:45 am; 90 minutes prior to testing). Active doses were extended-release osmotic-release oral system (OROS) MPH at the nearest commercially-available equivalents to 0.3 and 0.6 mg/kg TID immediate-release MPH. Doses ranged from 27 to 90 mg (dose was capped at 90 mg for safety reasons). Mean low and high doses were 1.06 mg/kg (SD=0.12) and 2.02 mg/kg (SD=0.23), respectively.
To promote tolerability, the 24 children (29% of the sample) who were either stimulant-naïve or were previously taking MPH at doses <40% of the 0.6 mg/kg dose had their medication order restricted to ensure they received the 0.3 mg/kg dose prior to the 0.6 mg/kg dose. Otherwise, dosing order was counterbalanced across participants. Based on daily assessments of blood pressure, heart rate, and side effect ratings by staff and parents, this regimen was well-tolerated among the participants in the present report. However, data for two additional children are excluded because we withheld the 0.6 mg/kg dose after observing moderate motor tics on the 0.3 mg/kg dose.
Cognitive Assessments
Children completed tasks in individual testing rooms. Tasks were programmed in E-Prime 1.1 and presented on 43-cm (diagonal) monitors, unless otherwise noted. Task order was randomized between participants. All tasks either included alternate forms across days (math seatwork, WM, CPT, PPI) or had built-in randomization procedures (SST, choice RT task) to mitigate concerns about memorization of task stimuli. Given space limitations, cognitive methods are presented in brief; we refer readers interested in additional details to papers demonstrating MPH effects among initial participants in the current study.
Inhibitory control.
Stop Signal Task (SST).
The SST (see Rosch et al., 20161, for details) involves pressing a button to indicate which direction an arrow faces (equal p ←, →; the “go” stimulus; Logan, Cowan, & Davis, 1984). After a brief “go” practice to emphasize quick responding, a “stop” practice introduced the auditory stop signal (1000 Hz tone; p=.25). Children were instructed to withhold responding whenever the “go” stimulus was followed by the stop signal. Next, children completed 4 blocks of 64 trials. The initial stop delay (SD) occurred 350 ms post-stimulus onset and adjusted dynamically in 50 ms increments to maximize the probability that the child could inhibit on ~50% of stop trials. Stop signal reaction time (SSRT = mean RT – mean SD) was the primary outcome; smaller SSRTs reflect better inhibitory control.
Prepulse inhibition (PPI) of startle.
In brief (see Ashare et al., 20102, for details), children were asked to discriminate between short (p=.67; 5 s duration) and long (p=.33; 8 s duration) tones of one pitch (p=.5) and to ignore tones of another pitch (400-and 1200-Hz; 70-dB). Auditory startle probes (50-ms, 100-dB white noise) were presented binaurally during most tones (p=.25 for each of 120-, 180-, and 4500-ms stimulus onset asynchronies [SOAs]) and during the inter-trial interval (ITI) on the remaining trials. The eyeblink startle response was measured bilaterally from orbicularis oculi electromyography and scored following standard procedures. Percent PPI ([(Mprepulse_trials − MITI_trials)/(MITI_trials)] × −100) was computed for each Day/Medication × Pitch (attended vs. ignored) × short-lead SOA (120, 180 ms) × Eye condition, examined for outliers, and aggregated across eye and SOA. Outlying or missing data in one or more cells is common in this paradigm, resulting in exclusion of up to 50% of participants even in single-session studies; in the present study, complete data were available for 71% (n=58) of the sample. Greater attentional modification of PPI (%PPI attend − %PPI ignore) difference scores reflect better sensitivity of this early inhibitory process to contextual demands.
Attention.
A-X CPT.
The A-X CPT is a widely-used measure of attention (see Bubnik et al., 2015; current task adapted from Halperin et al., 1988). Participants attended to a long stream of characters and were instructed to press the space bar only when they observed an “X” that was immediately preceded by an “A.” A 20-trial practice preceded 400 task trials (target p=.1; stimulus duration=150 ms; ISI=1500 ms). Though number of hits is often examined, there was a ceiling effect in the present sample (see also Huang-Pollock et al., 2012), with 16 children with perfect hit rates on the placebo day. Therefore, we examined mean hit RT.
Intra-individual RT variability - XO Choice Discrimination Task.
Children completed a simple discrimination task (“X” vs. “O”) by pressing buttons as quickly as possible while maintaining accuracy (see Spencer et al., 20093, for details). The task included 10 practice trials and 100 test trials (2.8-s stimulus duration; 1-s ITI). RT variability was quantified with the ex-Gaussian parameter tau, which provides an estimate of the skewed tail of an RT distribution (Leth-Steensen et al., 2000). Tau was computed in MATLAB (Mathworks, Inc., Natick, MA) using the DISTRIB toolbox (Lacouture & Cousineau, 2008).
Visuo-spatial working memory.
The computerized visuo-spatial working memory task (see Shiels et al., 2008) was adapted from the Corsi’s Block Tapping task (Milner, 1971) and previous visuo-spatial paradigms (e.g., Bedard & Tannock, 2008). The task presented an array of 10 white boxes on a black background. On each trial, a sequence of smiley faces (☺) appeared within these squares at a rate of one square per second.
Forward span- storage.
To assess short-term storage of visuo-spatial information, children were asked to click on the squares in the same order in which the smiley faces appeared. After a practice trial, children completed trials that advanced in difficulty from a two-location sequence to a maximum of an eight-location sequence, with two trials in each difficulty level. The task terminated when the child missed both trials in the same level.
Backward span- manipulation.
To assess mental manipulation of visuo-spatial information, children were instructed to click on the squares in the reverse order that they were presented. The task structure paralleled that of the Forward Span. For each direction (Forward and Backward), the total number of correct trials served as the measure of working memory.
Classroom Outcome Measures
Children attended 30-min classroom periods at 10:15 a.m., noon, and 3:30 p.m. each day. After reviewing classroom rules, children completed 20 minutes of individualized math seatwork (based on the intake assessment of the maximum difficulty at which a child could complete 5 problems in one minute; if needed, difficulty level was adjusted on the baseline day). Seatwork packets contained more problems than could be completed in 20 minutes, and children were instructed to accurately complete as many problems as possible. Consistent with prior work (see Baweja et al., 2016; Prasad et al., 2013), the primary classroom outcome was productivity; the total number of math problems completed was averaged across the three classroom periods within medication condition (accuracy was uniformly high because difficulty was tailored to each child’s level.)
We examined teacher ratings of Inattention/Overactivity on the modified-IOWA Scale (Pelham et al., 1989) as a secondary clinical outcome; ratings were made blind to treatment condition and were aggregated across classes within condition.
Data Reduction and Analytic Plan
Premature responses (<150 ms) were excluded for RT-based tasks. The primary variables for each mediation analysis were difference scores representing the contrast between 1) placebo vs. the average of 0.3 and 0.6 mg/kg MPH, and 2) 0.3 vs. 0.6 mg/kg MPH. The difference scores were screened for outliers and conformation to distributional assumptions. Outliers >3 SDs were windsorized; no problematic skewness or kurtosis was observed.
Mediation was assessed using a within-subjects path-analytic approach with bootstrapped confidence intervals for evaluating statistical significance (Judd, Kenny, & McClelland, 2001; Montoya & Hayes, 2017). Separate models were first run with each potential mediator. Significant mediators in individual models were then included in a parallel multiple mediator model to assess whether each process contributed uniquely to mediation. Mediation was assessed first for the MPH-placebo contrast (difference score of the average of 0.3 and .0.6 mg/kg MPH vs. placebo) and then for the dose-response contrast (difference score of 0.3 vs. 0.6 mg/kg).
Results
MPH Effects
Clinical outcomes – the c path.
As shown in Figure 2a, MPH significantly increased math productivity, MPH vs. placebo F(1,81)=120.2, p<.001, in a dose-dependent manner, 0.3 vs. 0.6 mg/kg MPH F(1,81)= 11.2, p=.001. MPH also dose-dependently improved teacher ratings, MPH vs. placebo F(1,81)=88.7, p<.001; 0.3 vs. 0.6 mg/kg MPH F(1,81)=4.9, p=.03.
Figure 2.
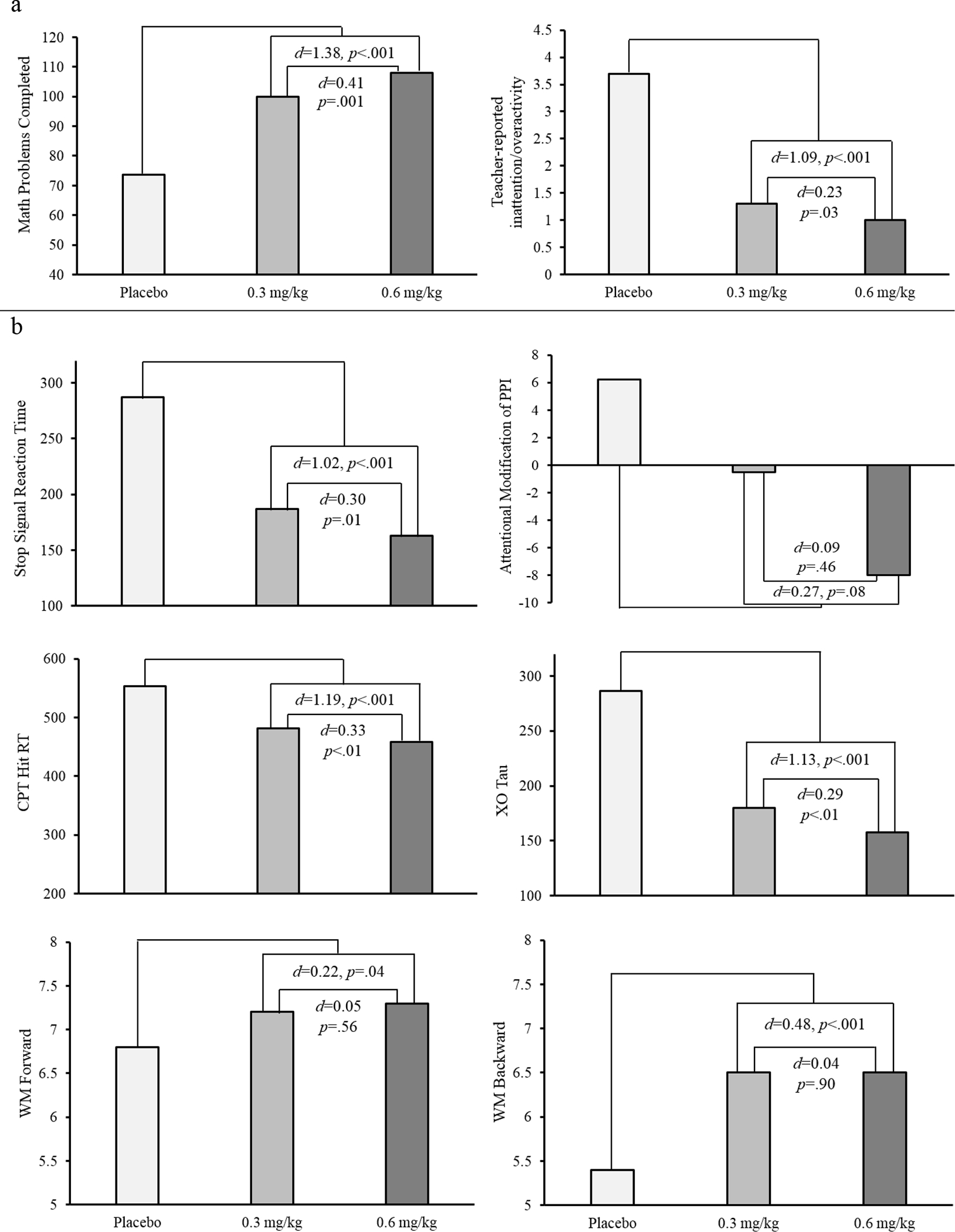
Methylphenidate effects on clinical outcomes (panel a) and candidate mediators (panel b).
Teacher ratings are from the Inattention/Overactivity subscale of the IOWA Conners rating scale. PPI= prepulse inhibition (represents the change in prepulse inhibition in attended vs. ignored trials); CPT= continuous performance task. Contrasts represent the medication effect (placebo vs. the average of the low and high doses) and the dose-response effect (low vs. high dose).
Cognitive outcomes – the a path.
All cognitive variables were enhanced by MPH compared to placebo, all Fs>4.3, all ps<.05 (see Figure 2b). Inhibitory control (SSRT) and attention (tau and hit RT on the CPT) also demonstrated dose-response effects, Fs>4.5, ps<.05.
Mediation
A significant indirect effect for the MPH vs. placebo effect on math productivity was observed for inhibitory control on the SST (b=8.2; 95% CI [2.5, 15.0]; see Table 2); medication effects on SSRT accounted for 9.8% of the variance in MPH effects on math productivity. Working memory backward also mediated the MPH vs. placebo effect on math productivity (b= 2.8; 95% CI [0.6, 5.4]), accounting for 10.1% of the variance in productivity. As shown in Table 2, the remaining cognitive measures did not significantly mediate the medication effect on math productivity. When included in a parallel multiple mediator model, both SST inhibitory control and working memory backward continued to uniquely mediate the MPH – math productivity relationship (inhibitory control b=7.4; 95% CI [1.7, 14.5]; working memory b=2.6; 95% CI [0.4, 5.0]; total indirect effect b=10.0; 95% CI [4.1, 17.3]). Together, MPH effects on both inhibitory control and working memory accounted for 18% of the variance in MPH effects on math productivity. None of the cognitive variables mediated the dose-response effect on math productivity.
Table 2.
Mediation results for classroom productivity
| MPH vs. Placebo | Low (0.3 mg/kg) vs. High (0.6 mg/kg) | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| a (b, SE) | b (b, SE) | Indirect Effect (b, SE) | 95% CI | a (b, SE) | b (b, SE) | Indirect Effect (b, SE) | 95% CI | |
|
| ||||||||
| SSRT | 104.3 (9.3)*** | 0.1 (0.03)** | 8.2 (3.2)** R2 =.10 |
2.5, 15.0 | 22.3 (7.9)** | 0.01 (0.03) | 0.3 (0.7) R2 =.002 |
−1.2, 1.7 |
| Percent PPI | −7.4 (4.2)+ | 0.2 (0.1)* | −1.4 (1.0) R2 =.08 |
−3.5, 0.3 | −2.9 (5.0) | −0.1 (0.06)+ | 0.3 (0.7) R2 =.07 |
−0.7, 2.0 |
| CPT Hit RT | 81.3 (7.0)*** | 0.03 (0.04) | 2.2 (3.4) R2 =.03 |
−4.2, 9.3 | 20.6 (6.7)** | −0.001 (0.03) | −0.02 (0.8) R2 =.01 |
−1.7, 1.4 |
| XO Tau | 115.8 (9.4)*** | 0.03 (0.02) | 3.1 (2.7) R2 =.02 |
−2.2, 8.6 | 18.6 (6.8)** | 0.07 (0.03)* | 1.2 (0.8)+ R2 =.08 |
−0.04, 2.9 |
| WM Forward | 0.4 (0.2)* | 2.5 (1.3)+ | 1.1 (0.8) R2 =.09 |
−0.1, 3.0 | 0.1 (0.2) | 1.2 (0.8) | 0.1 (0.3) R2 =.02 |
−0.4, 0.9 |
| WM Backward | 1.1 (0.2)*** | 2.7 (1.1)* | 2.8 (1.2)* R2 =.10 |
0.6, 5.4 | −0.1 (0.2) | 1.0 (1.2) | −0.08 (0.3) R2 =.02 |
−0.9, 0.5 |
Note. Classroom productivity is the number of math seatwork problems completed. SSRT= stop signal reaction time; PPI= prepulse inhibition (represents the change in prepulse inhibition in attended vs. ignored trials); CPT= continuous performance task; MPH is the average of the low (0.3 mg/kg) and high (0.6 mg/kg) doses.
p<.01,
p<.05,
p<.01,
p<.0001.
For teacher ratings, only working memory backward mediated the MPH vs. placebo effect (indirect effect b= 0.4; 95% CI [0.1, 0.7]; see Table 3 for mediation results for all tasks), accounting for 16% of the variance in clinical outcome. There was no evidence of mediation for the dose-response effect on teacher ratings4.
Table 3.
Mediation results for teacher ratings
| MPH vs. Placebo | Low (0.3 mg/kg) vs. High (0.6 mg/kg) | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| a (b, SE) | b (b, SE) | Indirect Effect (b, SE) | 95% CI | a (b, SE) | b (b, SE) | Indirect Effect (b, SE) | 95% CI | |
|
| ||||||||
| SSRT | 104.3 (9.3)*** | 0.00 (0.003) | −0.03 (0.3) R2=.10 |
−0.5, 0.5 | 22.3 (7.9)** | 0.004 (0.002) | 0.1 (0.1) R2=.06 |
−0.002, 0.2 |
| Percent PPI | −8.0 (4.5)+ | 0.01 (0.02) | −0.1 (0.2) R2=.13 |
−0.5, 0.1 | −2.3 (5.2) | 0.003 (0.004) | −0.01 (0.03) R2=.07 |
−0.1, 0.1 |
| CPT Hit RT | 81.3 (7.0)*** | 0.003 (0.004) | 0.3 (0.3) R2=.03 |
−0.3, 0.9 | 20.6 (6.7)** | 0.002 (0.003) | 0.04 (0.1) R2=.01 |
−0.1, 0.2 |
| XO Tau | 115.8 (9.4)*** | 0.003 (0.003) | 0.4 (0.3) R2=.09 |
−0.2, 1.0 | 18.6 (6.8)** | 0.002 (0.002) | 0.04 (0.1) R2=.01 |
−0.04, 0.2 |
| WM Forward | 0.4 (0.2)* | 0.2 (0.1) | 0.1 (0.1) R2=.06 |
−0.03, 0.2 | 0.1 (0.2) | 0.04 (0.1) | 0.003 (0.02) R2=.02 |
−0.03, 0.1 |
| WM Backward | 1.1 (0.2)*** | 0.3 (0.1)** | 0.4 (0.1)** R2=.16 |
0.1, 0.7 | −0.1 (0.2) | 0.1 (0.1) | −0.01 (0.03) R2=.02 |
−0.08, 0.04 |
Note. Teacher ratings are from the Inattention/Overactivity subscale of the IOWA Conners rating scale. SSRT= stop signal reaction time; PPI= prepulse inhibition (represents the change in prepulse inhibition in attended vs. ignored trials); CPT= continuous performance task; MPH is the average of the low (0.3 mg/kg) and high (0.6 mg/kg) doses.
p<.01,
p<.05,
p<.01,
p<.0001.
Discussion
The present study provided the first demonstration that the acute effects of methylphenidate on select cognitive processes partially accounted for, or mediated, individual differences in treatment effects in a classroom setting. To appreciate the significance of this work, and the limitations that remain to be addressed, we consider the findings in the context of general treatment mechanism frameworks (e.g., Kazdin, 2007; Kraemer et al., 2002) and specific aspects of ADHD theory, research, and practice.
Despite the importance of elucidating treatment mechanisms, their evaluation has largely been limited to indirect evidence (e.g., Kazdin, 2007; Kraemer et al., 2002). Certainly, that is the case in the ADHD literature. Decades of research show that stimulant medication, the frontline pharmacotherapy for pediatric ADHD, reduces symptoms and impairment (Path ‘c’ in Figure 1; MTA Group, 1999; Pelham et al., 2014). A separate longstanding literature demonstrates that stimulants ameliorate the cognitive deficits implicated in many theoretical models of ADHD (Path ‘a’ in Figure 1; e.g., Coghill et al., 2014a; Cortese et al., 2015).
The next critical step requires concurrent assessment of candidate mechanisms, preferably multiple theory-based mechanisms, and clinical outcomes under both treatment and control conditions, in order to directly evaluate mediation (e.g., Kazdin, 2007). In ADHD research, very few studies have collected such data (Froehlich et al., 2014; Kortekaas-Rijlaarsdam et al., 2017). A range of methodological factors may have contributed to their limited ability to test mediation (see introduction), including the lack of robust stimulant effects on cognition.
In the present study, we assessed two conceptually and methodologically strong indices in each of three key cognitive domains: inhibitory control, attention, and working memory. Compared to placebo, MPH improved one or both indices in each domain with effect sizes in the medium-to-large range (see Figure 2b). However, only MPH effects on inhibitory control on the SST and working memory manipulation on the spatial span task actually mediated the MPH effect on classroom productivity, together accounting for 18% of the variance. Working memory manipulation also mediated the MPH effect on teacher-reported classroom behavior (inattention/overactivity), explaining 16% of the variance.
The novel findings of significant treatment mediation are encouraging, but it is important to note that the majority of the variance in clinical outcomes remained unexplained. This does not appear to be due to insensitivity of the measures to MPH. Indeed, the attention measures (CPT hit RT and tau on the XO task) exhibited the largest effects of MPH in the present work (both ds > 1.1), but neither mediated stimulant effects on classroom productivity or teacher ratings. Of course, the absence of mediation by these two RT-based attention tasks does not allow us to rule out attention as a stimulant treatment mechanism. Indeed, a ceiling effect resulted in our inability to evaluate CPT hit rate, a more typical index of sustained attention, as a mediator. Future work should consider more difficult CPT variations as well as the examination of changes in attention over time (e.g., Fosco & Hawk, 2017; Huang-Pollock et al., 2012). Despite the possibility of a Type II error when it comes to attention, the present findings highlight an important principle of treatment mechanism evaluation: direct evaluation of mediation is critical for ruling out many of the wide range of basic processes a treatment might improve to a subset that can actually statistically account for individual differences in treatment outcome (e.g., Kazdin, 2007). The present work subjected a panel of cognitive indices central to ADHD theory to such a direct test, and only indices of response inhibition and working memory manipulation survived.
The present demonstration of cognitive mediation of clinical outcomes helps to open a bottleneck in understanding of ADHD treatment. As others have detailed (e.g., Kazdin, 2007; Kraemer et al., 2002), elucidation of treatment mechanisms facilitates and constrains theory, provides clearer targets for refining or repurposing current treatments and screening of new treatment approaches, and may foster tailoring of treatments (e.g., making use of the substantial cognitive heterogeneity that characterizes ADHD; Nigg et al., 2005). It also provides evidence that the clinical and cognitive effects of stimulants are linked, in contrast to the recent suggestion that the two domains are independent (e.g., Coghill et al., 2014b).
However, a full understanding of treatment mechanisms is never the result of a single study (Kazdin, 2007), and future work is needed to address several gaps not addressed by the present work. Statistical mediation is a necessary but not sufficient condition for demonstrating a treatment mechanism. Because a mediator may simply be a correlate of the true mechanism, one would ideally independently manipulate the putative mechanism and observe a later change in the clinical outcome (e.g., Kazdin, 2007). This would demonstrate that the mediator changes before the outcome, or temporal precedence. Temporal precedence can be most clearly evaluated for treatment effects that develop slowly, over days, weeks, or even months (see Judd et al., 2001; Kraemer et al., 2001). However, temporal precedence is nearly impossible to demonstrate for treatments like stimulants that produce acute cognitive and clinical effects – effects that occur within 60–90 minutes of dosing and dissipate within 12 hours of dosing (see, e.g., Pelham et al., 2001; Bubnik et al., 2015; Rosch et al., 2016). That is, one can choose to measure one process hours or days before the other, but in actuality, the basic and clinical effects likely occur so closely together in time that it would be futile to try to demonstrate that change in a hypothesized mediator preceded the change in clinical behavior.
Given this intractable problem for demonstrating temporal precedence for putative mediators of stimulants, we turn to the broader context of causal precedence (e.g., Baron & Kenny, 1986; Judd et al., 2001), which relies on theory (see also Kazdin, 2007). As noted in the introduction, decades of theory on ADHD posit that deficits in basic cognitive processes give rise to the symptoms, clinically-relevant behavior, and impairment associated with ADHD. Extending this logic, theory consistently posits that stimulants (and non-stimulants such as atomoxetine; Arnsten, 2010) improve clinical behavior via improvements in basic cognitive processes, not the reverse causal pathway. Finally, numerous cognitive training programs have also been developed explicitly on the premise that enhancing cognitive function will result in better clinical functioning for children with ADHD (unfortunately, this work generally has not directly evaluated mediation; for a review, see Cortese et al., 2015). Thus, although temporal precedence cannot be demonstrated empirically, logic and theory strongly suggest that changes in basic processes causally precede changes in clinical outcomes.
We hope that the current study serves as a catalyst for more systematic and integrative evaluation of ADHD treatment mechanisms. The present investigation is strong on internal validity, but weak on external validity. That is not an uncommon starting point when breaking new ground, but it is vital that future work address key limitations of the present work. First, the majority of children had a history of well-tolerated treatment with stimulant medication, and the study was not adequately powered to evaluate the possible moderating role of treatment history. Second, data were collected in a summer research camp with clinical outcomes focusing on an analogue classroom. This permitted strong experimental control but does not reflect the environments in which children’s behavior is most problematic. Third, we examined a narrow range of clinical outcomes, which limits generalizability. Our primary outcome was a self-paced math task. It is plausible that classroom activities with different demands (e.g., attending to a teacher’s lesson, during which the pace of information is outside of the child’s control), and clinical outcomes in other contexts (e.g., home, recreational and social activities) rely on different cognitive processes than those we studied. Fourth, it is critical to determine whether our findings for acute intervention (one day per treatment condition) generalize to more ecologically-valid treatment durations, as well as varying combinations of medication and behavioral intervention. It should also be noted that repeated assessments of cognition and clinical outcome over time may yield even stronger evidence of mediation, both because of improved reliability of measurement with aggregation and because of the improved ability to evaluate temporal precedence.
Conclusions
Building upon decades of largely independent literatures demonstrating the clinical and cognitive effects of stimulants among children with ADHD, the present work provides the first evidence that stimulant effects on specific cognitive processes (namely working memory and inhibitory control) actually account for, or partially mediate, individual differences in clinical response to stimulants. Rather than being the final word on the topic, this work provides important proof of principle: We can explicitly evaluate and understand how our ADHD treatments work. We call for greater integration of experimental psychopathology, behavioral pharmacology, and treatment outcome research in evaluating existing treatments and developing future mechanism-targeted therapies.
Key Points.
Mechanisms of stimulant treatment response are poorly understood. The present study evaluated a panel of candidate cognitive mediators and classroom outcomes under placebo and two active doses (0.3 and 0.6 mg/kg) of methylphenidate.
Methylphenidate effects on inhibitory control and working memory mediated medication effects on math seatwork productivity. Working memory also mediated medication effects on teacher-rated classroom behavior.
These findings converge with ADHD theory, neurobiology, and treatment development in suggesting that inhibitory control and working memory may be mechanisms of stimulant treatment response.
Acknowledgements
We thank Jerry Richards and Rosemary Tannock, for invaluable contributions to the overall design of the project and input on numerous clinical and methodological details; Beth Gnagy and Greg Fabiano for assistance with making a summer research camp work; Dominica Vito and Brian Gangloff for project coordination; Mark Kugotowski for e-prime programming (programs available from the authors); Louise Cooper and the Research Pharmacy at UB, the many trainees who worked long hours on the project; and the children and families who allowed us to work and play with them in the name of science. This work was sponsored by R01 MH069434 to LWH.
Footnotes
Rosch et al. (2016) reports SST data on MPH effects for the full sample in the current study.
Ashare et al. (2010) presents data from the first 50 participants of the current sample.
Spencer et al. (2009) reports data from the first 50 participants from the current sample.
When participants with a restricted medication order (n=24) are excluded from analyses, dose effects on SSRT mediate the dose-response effect of MPH on classroom teacher ratings (b=0.12; 95% CI [0.002, 0.27]; R2 = 0.11). The pattern of results for all other analyses were the same when participants with a restricted order were excluded.
References
- American Psychiatric Association. (2000). Diagnostic and statistical manual of mental disorders, 4th ed. Washington, D.C.: American Psychiatric Association. [Google Scholar]
- Arnsten AF (2010). The use of α−2A adrenergic agonists for the treatment of attention-deficit/hyperactivity disorder. Expert Review of Neurotherapeutics, 10, 1595–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashare RL, Hawk LW, Shiels K, Rhodes JD, Pelham WE, & Waxmonsky JG (2010). Methylphenidate enhances prepulse inhibition during processing of task-relevant stimuli in attention-deficit/hyperactivity disorder. Psychophysiology, 47, 838–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baweja R, Mattison RE, & Waxmonsky JG (2015). Impact of attention-deficit/hyperactivity disorder on school performance: What are the effects of medication? Pediatric Drugs, 17, 459–477. [DOI] [PubMed] [Google Scholar]
- Bedard A, Jain U, Hogg-Johnson S, & Tannock R (2007). Effects of methylphenidate on working memory components: Influence of measurement. Journal of Child Psychology and Psychiatry, 48, 872–880. [DOI] [PubMed] [Google Scholar]
- Bedard A, & Tannock R Anxiety, methylphenidate response, and working memory in children with ADHD. Journal of Attention Disorder, 11, 546–557. [DOI] [PubMed] [Google Scholar]
- Bubnik MG, Hawk LW, Pelham WE, Waxmonsky JG, & Rosch KS (2015). Reinforcement enhances vigilance among children with ADHD: Comparisons to typically-developing children and to the effects of Methylphenidate. Journal of Abnormal Child Psychology, 43, 149–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coghill D (2014). Editorial: Acknowledging complexity and heterogeneity in causality-implications of recent insights into neuropsychology of childhood disorders for clinical practice. Journal of Child Psychology and Psychiatry, 55, 737–740. [DOI] [PubMed] [Google Scholar]
- Coghill D, Seth S, Pedrosos S, Usala T, Currie J, & Gagliano A (2014). Effects of methylphenidate on cognitive functions in children and adolescents with attention-deficit/hyperactivity disorder: Evidence from a systematic review and a meta-analysis. Biological Psychiatry, 76, 603–615. [DOI] [PubMed] [Google Scholar]
- Coghill DR, Hayward D, Rhodes SM, Grimmer C, & Matthews K (2014). A longitudinal examination of neuropsychological and clinical functioning in boys with attention deficit hyperactivity disorder (ADHD): Improvements in executive functioning do not explain clinical improvement. Psychological Medicine, 44, 1087–1099. [DOI] [PubMed] [Google Scholar]
- Conzelmann A, Pauli P, Mucha RF, Jacob CP, Gerdes A, Ramonos J,…& Fallgatter AJ (2010). Early attentional deficits in an attention-to-prepulse paradigm in ADHD adults. Journal of Abnormal Psychology, 119, 594–603. [DOI] [PubMed] [Google Scholar]
- Cortese S, Ferrin M, Brandeis D, Buitelaar J, Daley D, Dittmann RW, ... & Zuddas A (2015). Cognitive training for attention-deficit/hyperactivity disorder: meta-analysis of clinical and neuropsychological outcomes from randomized controlled trials. Journal of the American Academy of Child & Adolescent Psychiatry, 54, 164–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraone SV, & Buitelaar J (2010). Comparing the efficacy of stimulants for ADHD in children and adolescents using meta-analysis. European Child and Adolescent Psychiatry, 19, 353–364. [DOI] [PubMed] [Google Scholar]
- Fosco WD, & Hawk LW Jr. (2017). Relating lab to life: Decrements in attention over time predict math productivity among children with ADHD. Child Neuropsychology, 23, 148–158. [DOI] [PubMed] [Google Scholar]
- Froehlich TE, Antonini TN, Brinkman WB, Langberg JM, Simon JO, Adams R, & Matheson H (2014). Mediators of methylphenidate effects on math performance in children with attention-deficit/hyperactivity disorder. Journal of Developmental and Behavioral Pediatrics, 35, 100–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamo NJ, Wang M, & Arnsten AF (2010). Methylphenidate and atomoxetine enhance prefrontal function through α 2-adrenergic and dopamine D 1 receptors. Journal of the American Academy of Child & Adolescent Psychiatry, 49, 1011–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halperin JM, & Healey DM (2011). The influences of environmental enrichment, cognitive enhancement, and physical exercise on brain development: Can we alter the developmental trajectory of ADHD? Neuroscience & Biobehavioral Reviews, 35, 621–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halperin JM, Marks DJ, Bedard ACV, Chacko A, Curchack JT, Yoon CA, & Healey DM (2013). Training executive, attention, and motor skills: a proof-of-concept study in preschool children with ADHD. Journal of Attention Disorders, 17, 711–721. [DOI] [PubMed] [Google Scholar]
- Halperin JM, Trampush JW, Miller CJ, Marks DJ, & Newcorn JH (2008). Neuropsychological outcome in adolescents/young adults with childhood ADHD: Profiles of persisters, remitters and controls. Journal of Child Psychology and Psychiatry, 49, 958–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halperin JM, Wolf LE, Pasculvaca DM, Newcorn JH, Healy JM, O’Brien JD, Morganstein A, & Young JG (1988). Differential assessment of attention and impulsivity in children. Journal of the American Academy of Child and Adolescent Psychiatry, 27, 326–329. [DOI] [PubMed] [Google Scholar]
- Hawk LW Jr., Yartz AR, Pelham WE Jr., & Lock TM (2003). The effects on methylphenidate on prepulse inhibition during attended and ignored prestimuli among boys with attention-deficit hyperactivity disorder. Psychopharmacology, 165, 118–127. [DOI] [PubMed] [Google Scholar]
- Hinshaw SP (2007). Moderators and mediators of treatment outcome for youth with ADHD: Understanding for whom and how interventions work. Ambulatory Pediatrics, 7, 91–100. [DOI] [PubMed] [Google Scholar]
- Huang-Pollock CL, Karalunas SL, Tam H, & Moore AN (2012). Evaluating vigilance deficits in ADHD: A meta-analysis of CPT performance. Journal of Abnormal Psychology, 121, 360–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judd CM, Kenny DA, McClelland GH (2001). Estimating and testing mediation and moderation in within-subject designs. Psychological Methods, 6, 115–134. [DOI] [PubMed] [Google Scholar]
- Karalunas SL, Gustafsson HC, Dieckmann NF, Tipsord J, Mitchell SH, & Nigg JT (2017). Heterogeneity in development of aspects of working memory predicts longitudinal attention deficit hyperactivity disorder symptom change. Journal of Abnormal Psychology, 126, 774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazdin AE (2007). Mediators and mechanisms of change in psychotherapy research. Annual Reviews of Clinical Psychology, 3, 1–27. [DOI] [PubMed] [Google Scholar]
- Kortekaas-Rijlaardsdam AF, Luman M, Sonuga-Barke E, Bet P, & Oosterlaan J (2017). Methylphenidate-related improvements in math performance cannot be explained by better cognitive functioning or higher academic motivation: Evidence from a randomized control trial. Journal of Attention Disorders, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer HC, Wilson GT, Fairburn CG, & Agras WS (2002). Mediators and moderators of treatment effects in randomized clinical trials. Archives of General Psychiatry, 59, 877–884. [DOI] [PubMed] [Google Scholar]
- Lacouture Y, & Cousineau D (2008). How to use MATLAB to fit the ex-Gaussian and other probability functions to a distribution of response times. Tutorials in Quantitative Methods for Psychology, 4, 35–45. [Google Scholar]
- Leth-Steensen C, Elbaz ZK, Douglas VI (2000). Mean response times, variability and skew in the responding of ADHD children: A response time distributional approach. Acta Psychologica, 104, 167–190. [DOI] [PubMed] [Google Scholar]
- Lipszyc J, & Schachar R (2010). Inhibitory control and psychopathology: A meta-analysis of the stop signal task. Journal of the International Neuropsychological Society, 16, 1064–1076. [DOI] [PubMed] [Google Scholar]
- Logan GD, Cowan WB, & Davis KA (1984). On the ability to inhibit simple and choice reaction time responses: A model and a method. Journal of Experimental Psychology: Human Perception and Performance, 10, 276–291. [DOI] [PubMed] [Google Scholar]
- Martinussen R, Hayden J, Hogg-Johnson S, & Tannock R (2005). A meta-analysis of working memory impairments in children with Attention-Deficit/Hyperactivity Disorder. Journal of the American Academy of Child and Adolescent Psychiatry, 44, 377–384. [DOI] [PubMed] [Google Scholar]
- Montoya AK, & Hayes AF (2017). Two-condition within-participant statistical mediation analysis: A path-analytic framework. Psychological Methods, 22, 6–27. [DOI] [PubMed] [Google Scholar]
- MTA Cooperative Group. (1999). 14-month randomized clinical trial of treatment strategies for attention deficit hyperactivity disorder. Archives of General Psychiatry, 56, 1073–1086. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Willcutt EG, Doyle AE, & Sonuga-Barke ES (2005). Causal heterogeneity in Attention-Deficit/ Hyperactivity Disorder: Do we need neuropsychologically impaired subtypes?. Biological Psychiatry, 57, 1224–1230. [DOI] [PubMed] [Google Scholar]
- Pelham WE, Burrows-McLean L, Gnagy EM, Fabiano GA, Coles EK, Wymbs BT, … Waschbusch DA (2014). A dose-ranging study of behavioral and pharmacological treatment in social settings for children with ADHD. Journal of Abnormal Child Psychology, 42, 1019–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham WE, Gnagy EM, Greenslade KE, & Milich R (1992). Teacher ratings of DSM-III-R symptoms for the disruptive behavior disorders. Journal of the American Academy of Child and Adolescent Psychiatry, 31, 210–218. [DOI] [PubMed] [Google Scholar]
- Pelham WE, Milich R, Murphy DA, & Murphy HA (1989). Normative data on the IOWA Conners teacher rating scale. Journal of Clinical Child Psychology, 18, 259–262. [Google Scholar]
- Pietrzak RH, Mollica CM, Maruff P, & Snyder PJ (2006). Cognitive effects of immediate-release methylphenidate in children with attention-deficit/hyperactivity disorder. Neuroscience & Biobehavioral Reviews, 30, 1225–1245. [DOI] [PubMed] [Google Scholar]
- Pliszka S (2007). Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. Journal of the American Academy of Child & Adolescent Psychiatry, 46, 894–921. [DOI] [PubMed] [Google Scholar]
- Prasad V, Brogan E, Mulvaney C, Grainge M, Stanton W, & Sayal K (2013). How effective are drug treatments for children with ADHD at improving on-task behaviour and academic achievement in the school classroom? A systematic review and meta-analysis. European Child and Adolescent Psychiatry, 22, 203–216. [DOI] [PubMed] [Google Scholar]
- Rapport MD, Alderson RM, Kofler MJ, Sarver DE, Bolden J, & Sims V (2008). Working memory deficits in boys with Attention-deficit/Hyperactivity Disorder: The contribution of central executive and subsystem processes. Journal of Abnormal Child Psychology, 36, 825–837. [DOI] [PubMed] [Google Scholar]
- Robbins TW, & Arnsten AF (2009). The neuropsychopharmacology of fronto-executive function: monoaminergic modulation. Annual Review of Neuroscience, 32, 267–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosch KS, Fosco WD, Pelham WE, Waxmonsky JG, Bubnik MG, & Hawk LW, (2016). Reinforcement and stimulant medication ameliorate deficient response inhibition in children with Attention-Deficit/Hyperactivity Disorder. Journal of Abnormal Child Psychology, 44, 309–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, & Schwab-Stone ME (2000). NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): Description, differences from previous versions, and reliability of some common diagnoses. Journal of the American Academy of Child and Adolescent Psychiatry, 39, 28–38. [DOI] [PubMed] [Google Scholar]
- Shiels K, Hawk LW, Lysczek CL, Tannock R, Pelham WE, Spencer SV, Gangloff BP, & Waschbusch DA (2008). The effects of incentives on visual-spatial working memory in children with Attention-Deficit/Hyperactivity Disorder. Journal of Abnormal Child Psycholgoy, 36, 903–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solanto MV, Wender EH, & Bartell SS (1997). Effects of methylphenidate and behavioral contingencies on sustained attention in attention-deficit hyperactivity disorder: a test of the reward dysfunction hypothesis. Journal of Child and Adolescent Psychopharmacology, 7, 123–136. [DOI] [PubMed] [Google Scholar]
- Spencer SV, Hawk LW, Richards JB, Shiels K, Pelham WE, & Waxmonsky JG (2009). Stimulant treatment reduces lapses in attention among children with ADHD: The effects of methylphenidate on intra-individual response time distributions. Journal of Abnormal Child Psychology, 37, 805–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigal SB, & Wigal TL (2006). The laboratory school protocol: Its origin, use, and new applications. Journal of Attention Disorders, 10, 92–111. [DOI] [PubMed] [Google Scholar]


