Abstract
Introduction
Posterior ring apophyseal fracture (PRAF) is characterized by the separation of bone fragments and sometimes coexists with lumbar disc herniation (LDH). However, how often these conditions coexist and the details of the clinical course remain unclear.
Methods
We analyzed 200 patients who underwent surgical treatment for LDH at our hospital from January 2016 to December 2020. Among these, we reviewed 21 patients who underwent microendoscopic surgery to treat PRAF. They consisted of 11 male and 10 female patients, ranging in age from 15 to 63 years. The average age was 32.8 months, and the average follow-up period was 39.8 years. We performed simple roentgenography and magnetic resonance imaging for all patients and computed tomography for about 80% of the patients. We evaluated the type of PRAF fragment (Takata classification), disease level, Japanese Orthopedic Association (JOA) score, Roland-Morris Disability Questionnaire (RDQ) score, operating time, intraoperative blood loss, and perioperative complications.
Results
A total of 10.5% of patients with LDH also had PRAF. The mean JOA score significantly improved from 10.6 ± 5.7 points before surgery to 21.4 ± 5.1 points at the final observation (p < 0.05). The mean RDQ score significantly improved from 17.1 ± 4.5 preoperatively to 5.5 ± 0.5 at the final observation (p < 0.05). The average operation time was 88.6 minutes. There were no complications requiring early surgery that were due to postoperative infection or epidural hematoma, but one patient required reoperation.
Conclusion
This study showed that PRAF coexisted with LDH in about 10% of cases, and the outcomes of surgical treatment were generally good. Computed tomography is recommended to improve the diagnostic rate and assist with surgical planning and intraoperative decision-making.
Keywords: ct scan, ldh, praf, co-existence, computed tomography (ct ), endoscopic surgery, surgical outcomes research, lumbar disc herniation surgery, posterior ring apophysis fracture
Introduction
Lumbar posterior ring apophyseal fracture (PRAF) is characterized by the separation of bone fragments at the posterior rim of the superior and inferior lumbar vertebral endplates, where the ring apophysis and the adjacent vertebral body are usually incompletely fused [1,2]. PRAF is uncommon and is complicated by lumbar disc herniation (LDH) in young patients [3]. PRAF is a fissure between the fragile cartilage and bony endplates in childhood caused by acute or chronic repetitive mechanical stimulation secondary to external forces such as those occurring in sports or trauma. However, there are some theories that PRAF is a fracture of the vertebral body endplate or that it is unrelated to trauma. There is no uniform consensus on its cause [4]. PRAF is sometimes missed and is often difficult to treat. The true frequency of PRAF is difficult to estimate and easily underestimated because simple roentgenography (X-ray) and magnetic resonance imaging (MRI) cannot clearly detect lesions. Many authors have argued for surgical treatment when conservative therapy is ineffective [3,5]. Because of its rarity and diversity of classification, there is no consensus on whether decompression requires herniorrhaphy only, simultaneous fragment removal, or extensive laminectomy with fusion. [6-9]. However, the treatment options for PRAF with LDH have not yet been fully explored. This study was performed to investigate how often PRAF and LDH coexist and assess the clinical course at our hospital.
Materials and methods
We retrospectively reviewed the data of 200 consecutive patients who underwent surgical treatment of LDH at the Kyushu Central Hospital, Fukuoka, Japan, from January 2016 to December 2020. Among them, we focused on 21 patients who underwent microendoscopic surgery to treat PRAF. The patients comprised 11 men and 10 women, ranging in age from 15 to 63 years (mean age, 39.8 years) at the time of surgery. The disease level was L4/5 in seven patients and L5/S1 in 14 patients. Takata types I, II, and III PRAF were present in three, two, and 16 patients, respectively [3]. The follow-up period ranged from 12 to 96 months (average, 32.5 months). The clinical records were analyzed for the following data: type of imaging test performed, Japanese Orthopedic Association (JOA) score, Roland-Morris Disability Questionnaire (RDQ) score, operating time, intraoperative blood loss, and perioperative complications. All statistical analyses were performed with Easy R (EZR) (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria) [10]. Numerical variables are expressed as the mean ± standard error of the mean. The Student’s t-test was performed; if the p-value was <0.05, the null hypothesis of no difference was rejected. This study was reviewed and approved by the Institutional Ethics Committee of Kyushu Central Hospital (approval application no. 310).
Results
PRAF was found in 21 of 200 consecutive patients (Table 1).
Table 1. Patient characteristics.
LDH: lumbar disc herniation; MED: microendoscopic discectomy; FESS: fully endoscopic spine surgery; F: female; M: male
| Case | Age (in years) | Sex | Levels of LDH | Takata Classification | Surgical treatment | Bone resection |
| 1 | 45 | F | L4/5 | 3 | MED | No |
| 2 | 44 | F | L5/S | 3 | MED | No |
| 3 | 40 | F | L5/S | 3 | MED | No |
| 4 | 45 | F | L4/5 | 3 | MED | No |
| 5 | 40 | M | L5/S | 2 | MED | No |
| 6 | 63 | F | L5/S | 3 | MED | No |
| 7 | 29 | M | L4/5 | 1 | MED | No |
| 8 | 29 | M | L4/5 | 3 | MED | No |
| 9 | 26 | M | L5/S | 3 | MED | No |
| 10 | 25 | F | L5/S | 1 | MED | No |
| 11 | 62 | M | L5/S | 3 | MED | No |
| 12 | 56 | M | L5/S | 3 | MED | No |
| 13 | 57 | F | L4/5 | 3 | MED | No |
| 14 | 44 | F | L5/S | 3 | MED | No |
| 15 | 38 | M | L5/S | 3 | MED | No |
| 16 | 27 | M | L5/S | 3 | MED | No |
| 17 | 30 | M | L5/S | 3 | MED | No |
| 18 | 35 | M | L5/S | 3 | MED | No |
| 19 | 44 | F | L5/S | 3 | MED | No |
| 20 | 15 | M | L4/5 | 1 | MED | No |
| 21 | 41 | F | L4/5 | 2 | FESS | Yes |
PRAF and LDH coexisted in 10.5% of patients. X-ray and MRI were performed in all cases, whereas computed tomography (CT) was performed in about 80% of cases. The JOA score was improved after surgery in all patients. The mean score was 10.6 ± 4.67 points before surgery and 21.4 ± 5.11 points at the final follow-up (p < 0.05). The RDQ score was also improved after surgery in all patients. The mean score was 17.1 ± 4.78 points before surgery and 5.55 ± 0.55 points at the final follow-up (p < 0.05). The mean operation time was 88.6 ± 33.3 minutes. The blood loss was minimal and difficult to measure in most patients, but a few patients had a blood loss of about 100 ml. Bone fragment removal was noted in the operative record of only one patient. There were no complications requiring early surgery due to postoperative infection or epidural hematoma, but one patient required reoperation because of a recurrent hernia (Table 2).
Table 2. Clinical outcomes in patients with PRAF.
PRAF: posterior ring apophyseal fracture; JOA score: Japanese Orthopedic Association score; RDQ: Roland–Morris Disability Questionnaire
| Characteristic | Outcomes |
| Operation time (in minutes) (Mean±SD) | 88.6±4.30 |
| Preoperative JOA score (Mean±SD) | 10.6 ± 4.67 |
| Postoperative JOA score (Mean±SD) | 21.4 ± 5.11 |
| Preoperative RDQ (Mean±SD) | 17.1 ± 4.78 |
| Postoperative RDQ (Mean±SD) | 5.55 ± 0.55 |
| Reoperation /recurrent hernia | 4.76%(1/21) |
Discussion
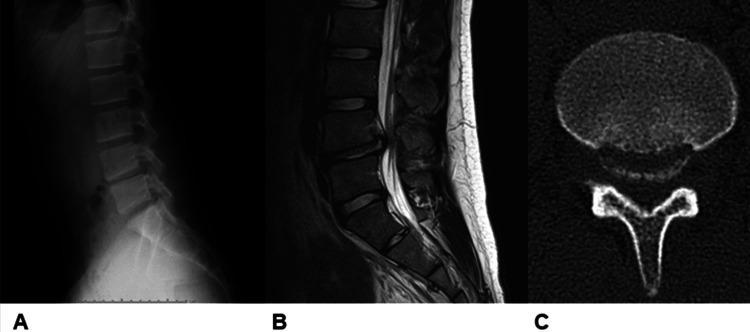
PRAF is rarely associated with disc herniation and is typically seen in 5% to 8% of patients with LDH [1-5]. It is more common in male patients and most common among patients in their late teens and 20s. PRAF is also seen in patients in their 30s and 40s, but rarely beyond the fifth decade of life [5,11-13]. In our study, the rate of coexistence of PRAF and LDH was slightly higher than in previous reports by various other authors. PRAF with LDH is probably more common in adults than is generally recognized. It is difficult to capture bone fragments with X-ray and MRI. Therefore, CT is essential for imaging of PRAF [3,14]. In the present case series, one patient was diagnosed with PRAF based on postoperative CT despite the fact that preoperative X-ray and MRI showed no obvious bone fragments, and the surgeon had confirmed intraoperatively that no free hernia was present on the cephalad and medial sides (Figure 1).
Figure 1. A case in which PRAF was difficult to diagnose by preoperative X-ray and MRI but could be diagnosed by a postoperative CT scan (case 20).
A: A preoperative X-ray showed no obvious bone fragments; B: A preoperative MRI showed no obvious bone fragments; C: A postoperative computed tomography led to the diagnosis of Takata type I posterior ring apophyseal fracture
Because of the rarity of PRAF with LDH, a consensus on the treatment strategy has not been reached. Surgery is the treatment of choice for lumbar buttock pain and leg pain refractory to conservative treatment. There is no consensus on whether decompression requires herniorrhaphy alone, simultaneous fragment removal, or extensive laminectomy with fusion. However, the treatments available for PRAF with LDH have not yet been fully investigated [5,15-20]. Shirado et al. [17] reported that good results can be obtained with herniotomy alone and that bone fragment resection is not mandatory. By contrast, Epstein and Epstein [2] and Epstein [4] reported that movable bone fragments are involved in neurological symptoms and that bone fragment resection is necessary because nerve root impingement is not adequately resolved. Additionally, Savini et al. [12] stated that treatment should be combined with fusion for iatrogenic instability due to extensive laminectomy and facetectomy for wide-based fragments.
A CT scan is desirable to improve the diagnosis rate, surgical planning, and intraoperative decision-making. Our current strategy is to first perform preoperative CT measurements to determine the position of the bone fragments and discs and measure the width of the lamina window, then confirm intraoperatively nerve decompression and bone fragment mobility, and finally determine the need for bony fragment removal based on the mobility of the nerve root after hernia removal and the instability of the bony fragment. Several options are available if bone resection is required. Bone fragment removal has been attempted under microscopy, the microendoscopic discectomy (MED) system, and, more recently, fully endoscopic spine surgery (FESS). Because the goal of surgery is to obtain adequate nerve decompression, it is essential to plan for bony resection sufficient to achieve that goal. If removal of the bony component is necessary, it is important to ensure an adequate visual field and working space. Some authors have suggested that bilateral or total discectomy is necessary for central lesions with large, wide basal bone fragments. We believe that in some cases, endoscopy can be utilized to remove the dissected bone fragments beyond the midline with unilateral entry. Matsumoto et al. [21] reported that MED is more feasible for patients with a lateral type of fragment. They treated PRAF with MED and reported good results, achieving a cure in 18 patients with PRAF. Patients with unilateral and central-type bone fragments were also compared in their study. The results indicated that the mean recovery rate of the JOA score was significantly higher in patients with unilateral than central fragments, with a mean follow-up of 21.1 months. The authors argued that the MED technique, which facilitates the removal of lateral bone fragments and nerve root retraction, is more effective in patients with lateral fragments. The MED technique is minimally invasive but requires appropriate patient selection and a skilled surgical technique [21]. Okada et al. [22] reported that it is possible to resect bone fragments using a unilateral or bilateral approach, even those with a wide base, using a curved Kerrison rongeur or chisel. FESS can be approached close to the midline of the symptomatic side, and simultaneous contralateral decompression is possible from one side. Therefore, it is a useful option for the removal of bone fragments. Few publications have focused on the application of FESS. Zuo et al. [23] reported good results in 9 of 57 cases of disc herniation associated with angle dissection that had ossified fragments, and they performed total removal of the fragments using FESS. We performed FESS and intraoperatively found strong tension after hernia removal in some cases, which allowed for good bone fragment processing.
Limitations
This study had several limitations. First, the surgical technique and treatment of bone lesions were chosen at the discretion of the attending physician. In reality, because PRAF is not common, what treatment modality is best remains controversial. The different physiological characteristics of younger and older patients may have influenced the surgical technique. Second, preoperative CT scans were not performed in all cases. The use of preoperative CT has recently decreased. The reasons for this trend include the decreased need for CT myelography because of improved MRI resolution and the risks of contrast agents and CT radiation exposure. Although younger patients are more conscious of avoiding CT exposure, it is desirable to perform CT at the level of the lower lumbar spine (the site of predilection) by narrowing the CT imaging range. CT-like MRI, which has been the focus of much attention in recent years, may help to solve this problem. Third, this was a single-hospital retrospective observational study, and the small number of patients may have influenced the clinical outcomes. Prospective studies involving multiple centers and large numbers of patients are desirable.
Conclusions
We evaluated the coexistence rate of PRAF with LDH and the surgical outcomes at our hospital. The coexistence rate was 10.5%; thus, it is not rare. The outcomes were generally good. Although there is still no consensus on the treatment strategy for PRAF, we believe that some patients require bone fragment treatment. When bone resection is necessary, it is preferable to have a bone fragment treatment option. A CT scan is recommended to improve the diagnostic rate of PRAF and to assist in surgical planning and intraoperative decision-making.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study
Animal Ethics
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
References
- 1.Fracture of the lumbar vertebral ring apophysis imitating disc herniation. Albeck MJ, Madsen FF, Wagner A, Gjerris F. Acta Neurochir (Wien) 1991;113:52–56. doi: 10.1007/BF01402115. [DOI] [PubMed] [Google Scholar]
- 2.Limbus lumbar vertebral fractures in 27 adolescents and adults. Epstein NE, Epstein JA. Spine (Phila Pa 1976) 1991;16:962–966. doi: 10.1097/00007632-199108000-00017. [DOI] [PubMed] [Google Scholar]
- 3.Fracture of the posterior margin of a lumbar vertebral body. Takata K, Inoue S, Takahashi K, Ohtsuka Y. https://pubmed.ncbi.nlm.nih.gov/3356726/ J Bone Joint Surg Am. 1988;70:589–594. [PubMed] [Google Scholar]
- 4.Lumbar surgery for 56 limbus fractures emphasizing noncalcified type III lesions. Epstein NE. Spine (Phila Pa 1976) 1992;17:1489–1496. doi: 10.1097/00007632-199212000-00008. [DOI] [PubMed] [Google Scholar]
- 5.A review of current treatment of lumbar posterior ring apophysis fracture with lumbar disc herniation. Wu X, Ma W, Du H, Gurung K. Eur Spine J. 2013;22:475–488. doi: 10.1007/s00586-012-2580-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Usefulness of the round endcap expandable cage placed on the vertebral ring apophysis in anterior spinal reconstruction. Okuwaki S, Tatsumura M, Eto F, Funayama T, Yamazaki M. Cureus. 2022;14:0. doi: 10.7759/cureus.23586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.A short-segment fusion strategy using a wide-foot-plate expandable cage for vertebral pseudarthrosis after an osteoporotic vertebral fracture. Taiji R, Takami M, Yukawa Y, et al. J Neurosurg Spine. 2020;33:1–8. doi: 10.3171/2020.5.SPINE2062. [DOI] [PubMed] [Google Scholar]
- 8.Corpectomy cage subsidence with rectangular versus round endcaps. Deukmedjian AR, Manwaring J, Le TV, Turner AW, Uribe JS. J Clin Neurosci. 2014;21:1632–1636. doi: 10.1016/j.jocn.2013.12.028. [DOI] [PubMed] [Google Scholar]
- 9.Posterior ring apophysis separation combined with lumbar disc herniation in adults: a 10-year experience in the surgical management of 87 cases. Akhaddar A, Belfquih H, Oukabli M, Boucetta M. J Neurosurg Spine. 2011;14:475–483. doi: 10.3171/2010.11.SPINE10392. [DOI] [PubMed] [Google Scholar]
- 10.Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Kanda Y. Bone Marrow Transplant. 2013;48:452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.The ring apophysis of the human vertebra; contribution to human osteogeny. II. BI EM, CO JW. https://pubmed.ncbi.nlm.nih.gov/14850517/ J Bone Joint Surg Am. 1951;33-A:783–787. [PubMed] [Google Scholar]
- 12.Posterior lumbar apophyseal fractures. Savini R, Di Silvestre M, Gargiulo G, Picci P. Spine (Phila Pa 1976) 1991;16:1118–1123. doi: 10.1097/00007632-199109000-00018. [DOI] [PubMed] [Google Scholar]
- 13.Posterior limbus vertebral lesions causing lumbosacral radiculopathy and the cauda equina syndrome. Baba H, Uchida K, Furusawa N, et al. Spinal Cord. 1996;34:427–432. doi: 10.1038/sc.1996.76. [DOI] [PubMed] [Google Scholar]
- 14.Limbus lumbar and sacral vertebral fractures. Mendez JS, Huete IL, Tagle PM. Neurol Res. 2002;24:139–144. doi: 10.1179/016164102101199675. [DOI] [PubMed] [Google Scholar]
- 15.Posterior retroextramarginal disc hernia (PREMDH): definition, diagnosis, and treatment. Scarfò GB, Muzii VF, Mariottini A, Bolognini A, Cartolari R. Surg Neurol. 1996;46:205–211. doi: 10.1016/0090-3019(96)00154-1. [DOI] [PubMed] [Google Scholar]
- 16.Clinical significance of ring apophysis fracture in adolescent lumbar disc herniation. Chang CH, Lee ZL, Chen WJ, Tan CF, Chen LH. Spine (Phila Pa 1976) 2008;33:1750–1754. doi: 10.1097/BRS.0b013e31817d1d12. [DOI] [PubMed] [Google Scholar]
- 17.Lumbar disc herniation associated with separation of the ring apophysis: is removal of the detached apophyses mandatory to achieve satisfactory results? Shirado O, Yamazaki Y, Takeda N, Minami A. Clin Orthop Relat Res. 2005;431:120–128. doi: 10.1097/01.blo.0000150457.47232.fd. [DOI] [PubMed] [Google Scholar]
- 18.Lumbar disc herniation associated with separation of the posterior ring apophysis: analysis of five surgical cases and review of the literature. Asazuma T, Nobuta M, Sato M, Yamagishi M, Fujikawa K. Acta Neurochir (Wien) 2003;145:461–466. doi: 10.1007/s00701-003-0044-z. [DOI] [PubMed] [Google Scholar]
- 19.Three-dimensional finite element analysis of the pediatric lumbar spine. Part I: pathomechanism of apophyseal bony ring fracture. Sairyo K, Goel VK, Masuda A, et al. Eur Spine J. 2006;15:923–929. doi: 10.1007/s00586-005-1026-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fracture of the posterior margin of the lumbar spine: case report after an acute, unique, and severe trauma. Molina V, Court C, Dagher G, Pourjamasb B, Nordin JY. Spine (Phila Pa 1976) 2004;29:0–7. doi: 10.1097/01.brs.0000148151.23560.c4. [DOI] [PubMed] [Google Scholar]
- 21.Microendoscopic discectomy for lumbar disc herniation with bony fragment due to apophyseal separation. Matsumoto M, Watanabe K, Tuji T, et al. Minim Invasive Neurosurg. 2007;50:335–339. doi: 10.1055/s-2007-993202. [DOI] [PubMed] [Google Scholar]
- 22.Microendoscope-assisted decompression surgery with resection of bony fragment for treating a separation of lumbar posterior ring apophysis in young athletes. Okada M, Yoshida M, Minamide A, Nomura K, Maio K, Yamada H. Global Spine J. 2021;11:889–895. doi: 10.1177/2192568220929290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Short-term effectiveness of percutaneous endoscopic spine surgery for treatment of lumbar disc herniation with posterior ring apophysis separation [Article in Chinese] Zuo Y, Kong Q, Li X, Xu L, Hu S, Zeng J, Song Y. https://pubmed.ncbi.nlm.nih.gov/25639049/ Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2014;28:1353–1357. [PubMed] [Google Scholar]