Abstract
Cirrhosis is an important cause of morbidity and mortality in people with chronic liver disease worldwide. In 2019, cirrhosis was associated with 2.4% of global deaths. Owing to the rising prevalence of obesity and increased alcohol consumption on the one hand, and improvements in the management of hepatitis B virus and hepatitis C virus infections on the other, the epidemiology and burden of cirrhosis are changing. In this Review, we highlight global trends in the epidemiology of cirrhosis, discuss the contributions of various aetiologies of liver disease, examine projections for the burden of cirrhosis, and suggest future directions to tackle this condition. Although viral hepatitis remains the leading cause of cirrhosis worldwide, the prevalence of non-alcoholic fatty liver disease (NAFLD) and alcohol-associated cirrhosis are rising in several regions of the world. The global number of deaths from cirrhosis increased between 2012 and 2017, but age-standardized death rates (ASDRs) declined. However, the ASDR for NAFLD-associated cirrhosis increased over this period, whereas ASDRs for other aetiologies of cirrhosis declined. The number of deaths from cirrhosis is projected to increase in the next decade. For these reasons, greater efforts are required to facilitate primary prevention, early detection and treatment of liver disease, and to improve access to care.
Subject terms: Liver cirrhosis, Epidemiology
In this Review, Huang et al. highlight global trends in the epidemiology of cirrhosis, including contributions of various aetiologies of liver disease, and consider what needs to be done to address projected increases in the burden of cirrhosis.
Key points
Hepatitis C virus (HCV) infection remains the leading cause of global deaths related to cirrhosis, followed by alcohol-associated liver disease.
The global burden of cirrhosis associated with non-alcoholic fatty liver disease (NAFLD) has increased substantially in the past decade.
In the Americas, the dominant cause of cirrhosis is shifting from viral hepatitis to NAFLD and alcohol-associated liver disease.
The COVID-19 pandemic has set back progress in the elimination of HCV and hepatitis B virus, and most countries are not on track to meet the WHO viral hepatitis elimination targets.
The focus of care should be shifted upstream towards primary prevention and early detection of liver disease to reduce the global burden of cirrhosis.
Introduction
Cirrhosis is an important cause of morbidity and mortality among patients with chronic liver disease1. Cirrhosis can lead to hepatocellular carcinoma (HCC) and hepatic decompensation, including ascites, hepatic encephalopathy and variceal bleeding1–7, and is a leading cause of death worldwide — it was associated with 2.4% of global deaths in 2019 (ref. 8). The major aetiologies of cirrhosis are hepatitis B virus (HBV) and hepatitis C virus (HCV) infection, alcohol-associated liver disease and non-alcoholic fatty liver disease (NAFLD)9,10. However, the past decade has seen major changes in the aetiology and burden of liver disease11–16.
Increasing HBV vaccination coverage and improved availability of effective antivirals against HBV have contributed to a reduction in the global age-standardized death rates (ASDRs) for HBV-associated cirrhosis12,17–20. Similarly, since 2015, safe and effective directly acting antivirals (DAAs) have revolutionized treatment of HCV infection, although the full impact of DAAs on the global burden of HCV-associated cirrhosis is unclear12,21–23. Despite the rising consumption of alcohol24,25, ASDRs for alcohol-associated cirrhosis have decreased, although under-reporting and under-diagnosis are a concern26. By contrast, the obesity and diabetes epidemic has led to a rapid rise in the prevalence of NAFLD, and ASDRs for NAFLD-associated cirrhosis have increased26.
Estimates of the global burden of cirrhosis and of the contributions made by various causes of liver disease are important for practitioners, researchers and health-care policymakers to inform clinical practice, provide directions for research and guide the use of resources, respectively. In this Review, we highlight global trends in the epidemiology of cirrhosis, discuss the contributions of various aetiologies of liver disease, examine projections for the burden of cirrhosis, and suggest future directions for reducing the burden of cirrhosis.
Prevalence of cirrhosis
The Global Burden of Disease (GBD) Study provides a comprehensive overview of the estimated global burden of cirrhosis and chronic liver diseases (which are collectively referred to as cirrhosis in the GBD Study)27,28. In the GBD Study 2017, the estimated number of people with compensated cirrhosis was 112 million worldwide, corresponding to an age-standardized global prevalence of compensated cirrhosis of 1,395 cases per 100,000 population12.
The data used in the GBD Study depended on the quality of each country’s registry27, and where data were not available, modelling was used to extrapolate from previous trends. Such extrapolation could have introduced bias, which would have reduced the accuracy of these prevalence estimates. In regions with lower standards of health care, cirrhosis is likely to be under-reported owing to a lack of disease awareness and access to care. In light of these limitations, interpretation of data from the GBD Study requires caution, but the study remains an important source of information about the burden of cirrhosis.
Several country-specific, population-based studies conducted in Europe have examined the prevalence of cirrhosis on the basis of non-invasive tests. Estimates of prevalence in these studies have ranged from 0.3% to 0.8%29. However, such data on the global prevalence of cirrhosis are limited.
Aetiologies of liver disease
The prevalence of HBV and HCV infection among individuals with cirrhosis was estimated in a large systematic review and meta-analysis of 520 studies (including 1,376,503 individuals from 86 countries or territories) published between 1993 and 2021 (ref. 30). Although the primary aim of this study was to evaluate the prevalence of HBV and HCV infection, data on heavy alcohol consumption and NAFLD were also reported where available. The analysis included only studies of unselected patients with cirrhosis, who were assumed to be representative of the population at each centre.
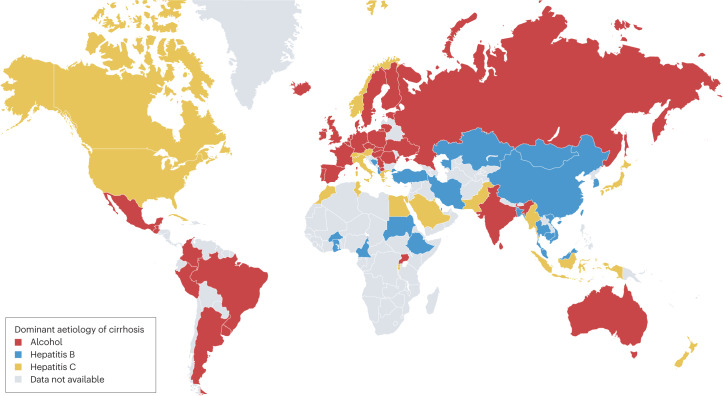
Globally, among individuals with cirrhosis, 42% had HBV infection and 21% had HCV infection. By WHO region, the prevalence of HBV infection among patients with cirrhosis was highest in the Western Pacific region (59%) and lowest in the Americas (5%), whereas the highest prevalence of HCV infection among patients with cirrhosis was in the Eastern Mediterranean region (70%) and the lowest was in Africa and the Western Pacific (both 13%). The proportion of patients with cirrhosis and heavy alcohol use was high in Europe (16–78%) and the Americas (17–52%) and was generally lower in Asia (0–41%). Data on the prevalence of NAFLD among patients with cirrhosis in this study were more limited, but estimates ranged from 2% in South Korea and Brazil to 18% in Canada (when considering estimates based on at least three studies). The dominant reported cause of cirrhosis varied by country (Fig. 1).
Fig. 1. Dominant reported aetiology of cirrhosis from 1993 to 2021.
Data were obtained from a systematic review of cirrhosis that included studies published during the period 1993–2021 (ref. 30).
Interpretation of the data from this large meta-analysis requires caution. The primary aim of the meta-analysis was to evaluate the prevalence of viral hepatitis, rather than heavy alcohol use or NAFLD, in cirrhosis, so studies relevant to alcohol use and NAFLD might have been omitted. In addition, many of the included studies did not account for multiple aetiologies of cirrhosis. We speculate that cirrhosis has more than one cause in a substantial proportion of patients, particularly considering the growing prevalence of obesity and increasing alcohol consumption.
In addition, the concept of metabolic-associated fatty liver disease (MAFLD) is likely to alter the apparent aetiology of cirrhosis. MAFLD is defined — on the basis of recently proposed criteria31,32 — as hepatic steatosis with obesity, type 2 diabetes mellitus or other factors associated with metabolic dysfunction without the need to exclude alternative causes of chronic liver disease, such as viral hepatitis or alcohol consumption32. Data on the global burden of MAFLD-associated cirrhosis are limited, but we speculate that the burden of MAFLD-associated cirrhosis will increase over time owing to an increasing proportion of individuals with more than one cause of liver disease, such as concomitant alcohol consumption and NAFLD, or concomitant NAFLD and viral hepatitis. Finally, as the studies included in this meta-analysis spanned nearly 30 years, the data might not reflect the trends in the past decade.
Trends in the aetiology of cirrhosis
Studies from across the world provide insights into trends in the aetiology of cirrhosis (Supplementary Table 1); these trends are discussed by WHO region in the sections that follow. Many of the larger studies conducted in North America, Europe and the Western Pacific were based on data from administrative disease registries and relied on International Classification of Diseases (ICD) codes (Box 1) to identify cases of cirrhosis; this approach is susceptible to bias related to incomplete records or incorrect coding. By contrast, studies in which clinical criteria and chart review were used to identify cases of cirrhosis are less susceptible to bias, but many such studies included modest sample sizes and, consequently, might not fully reflect trends in aetiology.
Similarly, the definitions of NAFLD-associated cirrhosis varied across studies, so interpretation of data based on these definitions requires caution. Criteria for NAFLD as a cause of cirrhosis have been proposed to enable consistent enrolment into clinical trials33. These criteria enable categorization of the likelihood that NAFLD is the cause of cirrhosis (definite, probable or possible) on the basis of histological evidence and metabolic risk factors33. However, these criteria have not been uniformly adopted and might require further validation.
Box 1 International Classification of Diseases codes for cirrhosis.
Multiple studies have been conducted in North America, Europe and Australia to examined the use of International Classification of Diseases 9 (ICD-9) and ICD-10 codes to identify cirrhosis36,113–121. These studies showed that ICD codes used to identify cirrhosis generally have a relatively high positive predictive value but, when used in isolation, they have only modest sensitivity for identifying cirrhosis36,113,115.
Algorithms that use a combination of ICD codes, or a combination of ICD codes and laboratory results, could improve diagnostic accuracy36,113,115,122. One systematic review has identified nine ICD-10 codes that have been used to identify cirrhosis most frequently in the literature118. When validated in Europe and North America, this set of nine ICD-10 codes had greater sensitivity than the code set most frequently used in the literature121, and maintained a high positive predictive value (83–89%). This consensus code set could, therefore, be a useful tool for identification of cirrhosis in large databases and health records, and should be further validated in regions outside North America and Europe118.
The Americas
Multiple studies conducted in the Americas indicate a shift in the dominant cause of cirrhosis over the past 10–20 years. In a population-based study conducted in Indiana, USA, analysis of the incident cases of cirrhosis from 2004 to 2014 (n = 9,261)16 identified increases in the proportion of patients with alcohol-associated cirrhosis (0.8% per year) and NAFLD-associated cirrhosis (0.6% per year), whereas the proportion with viral hepatitis decreased by 1.4% per year16. In a prospective study in 1,717 people with cirrhosis at five hospitals in Texas, USA, between 2016 and 2019, the major causes of cirrhosis were cured HCV infection (33%), alcohol consumption (31%) and NAFLD (23%), highlighting the shift away from active viral hepatitis as the dominant cause of cirrhosis34. Similarly, in an analysis of population-based administrative health-care data from 159,549 people with cirrhosis in Ontario, Canada, the most common causes of incident cirrhosis between 2000 and 2017 were NAFLD (53%) and alcohol consumption (24%)35. However, this study differed from other studies conducted in Canada, in which the leading cause of cirrhosis was HCV infection — some degree of misclassification bias might have contributed to the high estimated proportion of cirrhosis caused by NAFLD36,37.
Changes in the aetiology of cirrhosis over time in Mexico were assessed in a study including 4,584 people who were diagnosed with incident cirrhosis in six tertiary hospitals between 2000 and 2019 (ref. 38). In this study, diagnostic criteria for MAFLD31,32, rather than NAFLD, were used. MAFLD was identified as the third most common cause of incident cirrhosis in 2000 (14%) but had become the leading cause of incident cirrhosis (36%) in 2019. By contrast, HCV infection was the leading cause of cirrhosis in 2000 (45%) but had declined by 2019 (11%). The proportion of cirrhosis cases due to alcohol consumption increased from 28% to 33% during the study period. However, the way in which patients who fulfilled the criteria for MAFLD and had concomitant viral hepatitis or high alcohol consumption were classified was not clear38. Finally, a study of the United Network for Organ Sharing database from 2014 to 2019 determined that NAFLD and alcohol consumption have become the most common causes of liver disease among people without HCC waiting for a liver transplant39.
Taken together, these data suggest that the aetiology of cirrhosis in the Americas is shifting from active HBV and HCV infection towards resolved or treated viral hepatitis, alcohol consumption and NAFLD. These data are in line with increases in obesity and alcohol consumption in the Americas40–42.
Europe
As in the Americas, studies in Europe indicate a change in the dominant aetiology of cirrhosis. Analysis of data from all hospital admissions in Germany from 2005 to 2018 revealed that the prevalence of NAFLD-associated cirrhosis increased fourfold during the study period43. Nevertheless, alcohol consumption remained the dominant cause of cirrhosis in Germany in 2018, accounting for 52% of cirrhosis cases; NAFLD and NASH accounted for only 3% and 1%, respectively. In Sweden, in a cohort study in patients with cirrhosis who visited a tertiary hospital, the proportion of patients with cirrhosis due to viral hepatitis declined from 43% in the first 5 years (2004–2008) of the study to 31% in the final 4 years (2014–2017), whereas the proportion due to NAFLD increased from 6% to 15% in the same period44. Similarly, in Italy, in a multicentre study in patients with cirrhosis (n = 832) at 16 hospitals45, the proportions of people with cirrhosis due to alcohol consumption and HCV infection decreased, whereas the proportion of people with cirrhosis due to NAFLD increased when compared with a historical cohort (2001)46.
Collectively, these data indicate that the prevalence of NAFLD-associated cirrhosis is increasing in Europe, whereas the prevalence of alcohol-associated cirrhosis seems to be decreasing, possibly in response to public health measures such as the enforcement of a minimum price for alcohol and increased alcohol taxation41,47,48. The data also suggest that the prevalence of HCV-associated and HBV-associated cirrhosis is declining in Europe.
Africa, the Eastern Mediterranean and Southeast Asia
Limited data are available on trends in the aetiology of cirrhosis in Africa, the Eastern Mediterranean and Southeast Asia, as many studies conducted in these regions have included modest sample sizes30. More data are required from these regions to accurately determine trends in the aetiology of cirrhosis and to identify and address the gaps in linkage to care.
In Africa, research on liver diseases is a major unmet need. Estimates from the GBD Study determined that most cases of cirrhosis in Africa were related to HBV infection, alcohol consumption and HCV infection, but no other up-to-date population-based data exist for the aetiologies of cirrhosis12,49,50. Data from larger studies of HCC in Africa can provide some indication of the relative contributions that each aetiology of liver disease makes to the prevalence of cirrhosis, although some people with HCC do not have cirrhosis, so caution is required in interpreting the data. In one study, analysis of 1,251 people with HCC (all of whom had cirrhosis) from Egypt determined that the dominant aetiology was HCV infection (84%), followed by other or unknown causes (12%), HBV–HCV co-infection (2%) and HBV infection (1%)51. In the same study, analysis of 1,315 people with HCC (66% of whom had cirrhosis) from other African countries (Nigeria, Ghana, the Ivory Coast, Cameroon, Sudan, Ethiopia, Tanzania and Uganda) determined that the most common aetiology was HBV infection (55%), followed by other or unknown causes (22%), alcohol consumption (13%) and HCV infection (6%)51.
In the Eastern Mediterranean region, single-centre studies suggest that viral hepatitis remains the dominant cause of cirrhosis. For example, in one study of 953 Jewish and 95 Arab Bedouin individuals with cirrhosis at a tertiary hospital in Israel determined that the most common cause of cirrhosis was HCV infection (39%) among Jewish individuals and NAFLD (21%) among Bedouin individuals52. In Qatar, study of 109 individuals with cirrhosis who were admitted to an intensive care unit showed that the most common aetiology of cirrhosis was HCV infection (34%), followed by alcohol consumption (26%), cryptogenic liver disease (24%) and HBV infection (21%)53. However, population-based data from this region are lacking and are needed to get a more accurate understanding of aetiology.
Data from the Southeast Asia region are similarly limited. In one study conducted in India, the most common aetiology among 4,413 patients with cirrhosis from 11 hospitals was alcohol consumption (34%), followed by other causes (29%), HBV infection (18%), HCV infection (17%) and NAFLD (2%)54. However, among 192 people with cirrhosis who underwent endoscopic band ligation in a hospital in Pakistan, cirrhosis was attributed to HCV infection in 63% and to HBV infection in 19%55.
The Western Pacific
Studies in the Western Pacific region have shown that NAFLD-associated and alcohol-associated cirrhosis are increasing in this region, but viral hepatitis remains the dominant cause of cirrhosis30. These trends were demonstrated in a large study in 48,621 individuals with cirrhosis identified on the basis of clinical criteria at 79 hospitals in Japan56. Comparison of aetiology in 2007 with that in 2014–2016 demonstrated an increase in NAFLD-associated cirrhosis (2% to 9%) and alcohol-associated cirrhosis (14% to 25%). The proportion of cirrhosis cases due to HCV and HBV infection declined (from 59% to 40% and from 14% to 9%, respectively), although these remained the largest contributors. In another study in 15,716 patients with cirrhosis at five university hospitals in South Korea between 2000 and 2014, the proportion of cirrhosis cases due to NAFLD, alcohol consumption and HCV infection increased over the study period57. However, the study periods of both of these studies were before the widespread availability of DAA therapy for HCV infection, which is likely to reduce the proportion of cirrhosis cases that are due to HCV infection over time.
Also in the Western Pacific region, analysis of data from 1,582 individuals with a new diagnosis of cirrhosis at a hospital in China determined that the proportion of NAFLD-associated cirrhosis cases was higher (3%) in the last 2 years of the study (2012–2013) than the average (2%) over the entire study period (2003–2013)58. In Taiwan, analysis of data from 18,423 individuals with HCC who were diagnosed between 1981 and 2001 showed that the percentage of HBV-associated HCC decreased from 82% to 66% among men and from 67% to 41% among women over the study period owing to an increase in HCV-associated HCC59. Despite the continued dominance of viral hepatitis as a cause of cirrhosis in the Western Pacific region, tracking changes in the aetiology of cirrhosis over time remains important, as the rates of obesity and alcohol consumption continue to rise in parallel with increasing economic wealth24,41,60–63.
Decompensated cirrhosis
Hepatic decompensation is potentially preventable with antiviral therapy (in HBV-associated and HCV-associated cirrhosis) and lifestyle changes (in alcohol-associated and NAFLD-associated cirrhosis), but epidemiological data suggest that decompensated cirrhosis is increasing in prevalence. In prior studies in patients with compensated cirrhosis, transition from a compensated state to a decompensated state (including ascites, hepatic encephalopathy and variceal bleeding) occurred at a rate of 5–12% per year64–66. Data from the GBD Study 2017 indicate that the global number of prevalent cases of decompensated cirrhosis increased from 5.2 million in 1990 to 10.6 million in 2017, corresponding to an increase in the estimated age-standardized prevalence of decompensated cirrhosis from 110.6 per 100,000 population in 1990 to 132.5 per 100,000 population in 2017 (ref. 12). In 2017, the proportion of decompensated cirrhosis associated with HBV and HCV infection, alcohol consumption, NAFLD and other causes was 28%, 25%, 23%, 9% and 16%, respectively12.
Acute-on-chronic liver failure (ACLF) is characterized by acute decompensation of chronic liver disease that is associated with organ failure and carries a high risk of mortality67,68. Several definitions exist for ACLF, including criteria from the Asia Pacific Association for the Study of the Liver69, the European Association for the Study of the Liver–Chronic Liver Failure (EASL–CLIF)70, and the North American Consortium for the Study of End-Stage Liver71. ACLF has been reviewed in detail elsewhere67,72. A meta-analysis of 30 studies determined that the global prevalence of ACLF (defined by the EASL–CLIF criteria) among patients admitted with decompensated cirrhosis was 35%73. Alcohol consumption was the underlying cause of chronic liver disease in 45% of people with ACLF globally; regional pooled estimates ranged from 24% in North America to 55% in Europe73. The global 90-day mortality associated with ACLF was 58% and ranged from 41% in North America to 68% in Southeast Asia73.
In combination, these data highlight the need for increased efforts to identify liver disease at an earlier stage. Earlier identification would enable the use of preventive measures, thereby reducing the burden of decompensation74.
Deaths associated with cirrhosis
The GBD Study 2019 provides a comprehensive global overview of the estimated mortality associated with cirrhosis (and chronic liver diseases)27. Although deaths attributed to liver cancer were excluded from this analysis, the proportions of liver cancer due to the various aetiologies of liver disease were used as co-variates in determining the proportion of cirrhosis deaths due to the various aetiologies of liver disease. The analysis provided global and regional estimates for the number of deaths and ASDRs associated with cirrhosis in 2019 (Table 1).
Table 1.
Deaths associated with cirrhosis in 2019 by location and aetiology of liver disease
| Category | Number of deathsa (95% UI) | ASDR per 100,000 population (95% CI) |
|---|---|---|
| Location | ||
| Global | 1,472,000 (1,375,000–1,579,000) | 18.0 (16.8–19.3) |
| Africa | 188,000 (159,000–225,000) | 35.2 (30.3–41.2) |
| Eastern Mediterranean | 146,000 (118,000–176,000) | 36.2 (29.3–43.4) |
| Europe | 223,000 (208,000–237,000) | 16.0 (14.9–17.0) |
| Americas | 216,000 (202,000–231,000) | 17.4 (16.3–18.6) |
| Southeast Asia | 443,000 (397,000–502,000) | 25.0 (22.4–28.3) |
| Western Pacific | 253,000 (225,000–284,000) | 9.6 (8.6–10.8) |
| Aetiology | ||
| Hepatitis C | 395,000 (336,000–459,000) | 4.8 (4.1–5.6) |
| Hepatitis B | 331,000 (279,000–392,000) | 4.0 (3.4–4.8) |
| Alcohol | 372,000 (315,000–438,000) | 4.5 (3.8–5.3) |
| NASH | 134,000 (96,000–177,000) | 1.7 (1.2–2.2) |
| Other | 240,000 (188,000–303,000) | 3.0 (2.4–3.8) |
aData for the global and regional number of deaths estimated in the Global Burden of Disease Study 2019 were obtained from the GBD Results Tool27. In the Global Burden of Disease Study 2019, the number of deaths was estimated using a multistep algorithm based on International Classification of Disease diagnosis coding, a systematic literature review to develop inputs of disease epidemiology, and a Bayesian meta-regression modelling methodology. Where data for certain countries or regions were not available, the Global Burden of Disease Study 2019 results depended on modelling and past trends, potentially resulting in discrepancies in the accuracy of the data. ASDR, age-standardized death rate; CI, confidence interval; NASH, non-alcoholic steatohepatitis; UI, uncertainty interval.
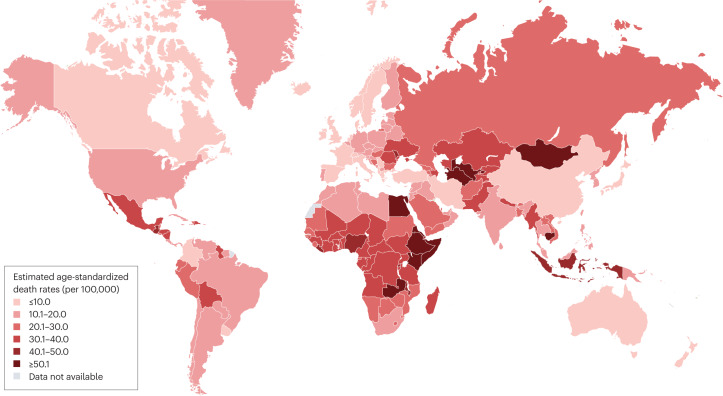
The estimated number of deaths associated with cirrhosis worldwide in 2019 was 1,472,000 (ref. 27). This number had increased by 10% from 2010 (ref. 27). The absolute number of deaths associated with cirrhosis was lowest in the Eastern Mediterranean region (146,000) and highest in Southeast Asia (443,000). The estimated global ASDR for cirrhosis in 2019 was 18 deaths per 100,000 population27 (Table 1). By region, the lowest estimated ASDR was in the Western Pacific (9.6 deaths per 100,000 population) and the highest was in the Eastern Mediterranean region (36.2 deaths per 100,000 population). The disconnect between the number of cirrhosis-related deaths and the ASDR in the Eastern Mediterranean region results from the relatively small and young population in that region75. The estimated ASDR in different countries (Fig. 2) ranged from 3.3 deaths per 100,000 population in Singapore to 126.7 deaths per 100,000 population in Egypt.
Fig. 2. Estimated age-standardized death rates due to cirrhosis in 2019 by country.
Data for the age-standardized death rate in 2019 were estimated in the Global Burden of Disease Study 2019 and these data were obtained from the GBD Results Tool27. Where data for countries or regions were unavailable, the Global Burden of Disease Study 2019 results depended on modelling and past trends, potentially resulting in discrepancies in the accuracy of the data.
The number of deaths associated with cirrhosis with different aetiologies (Table 1) ranged from 134,241 deaths for NAFLD-associated cirrhosis to 395,022 for HCV-associated cirrhosis. The corresponding estimated ASDRs ranged from 1.7 deaths per 100,000 population for NAFLD-associated cirrhosis to 4.8 deaths per 100,000 population for HCV-associated cirrhosis27. However, a substantial proportion of cases of cirrhosis that were categorized as having ‘other causes’ in the GBD Study might have been due to NAFLD or occult HBV infection, which would mean that the figures for NAFLD-associated and HBV-associated cirrhosis are underestimates.
Data from the GBD Study 2017 has provided insight into temporal trends in deaths associated with cirrhosis. Between 2012 and 2017, the number of deaths associated with cirrhosis increased by 9%, although the global ASDR for cirrhosis declined from 17.1 to 16.5 deaths per 100,000 population (ref. 26). The disconnect between the number of deaths and ASDR is due to population growth and ageing. The ASDRs for HBV-associated cirrhosis, HCV-associated cirrhosis and alcohol-associated cirrhosis also decreased by 1.4%, 0.5% and 0.4%, respectively, between 2012 and 2017. By contrast, the ASDR for NAFLD-associated cirrhosis increased by 0.3% over the same period26. However, cardiovascular diseases are the leading cause of death among patients with NAFLD, highlighting the need for a multidisciplinary approach to this condition76–78.
Predictions
Several modelling studies been done to project the burden of various aetiologies of cirrhosis over the next decade22,35,79–82. However, these studies should be interpreted with caution. The input data were largely derived from administrative databases, which are susceptible to bias, including under-reporting and misclassification bias. In addition, fibrosis can regress after antiviral treatment or lifestyle modification but few of these modelling studies accounted for the possibility of such regression83–86. Despite these limitations, the resulting data serve as an important reference to guide public health policymakers and researchers.
HCV-associated cirrhosis
Integration of a literature review, a Delphi process and Markov modelling to forecast the burden of incident decompensated HCV-associated cirrhosis in 2030 led to an estimated increase from 148,000 cases in 2020 to 174,000 in 2030 worldwide22. However, the rate of cases per 100,000 population was projected to remain relatively stable. The same modelling study also provided an estimate of the prevalence of viraemic HCV infection, which indicated that the global hepatitis elimination target for 2030 is unlikely to be attained should current trends persist87.
Caution must be exercised when interpreting the estimates from this study, as sufficient data for model generation were available for fewer than half of the countries. In addition, treatment rates were uncertain, especially for countries in which a substantial proportion of treatments were exported for overseas use. Nevertheless, the COVID-19 pandemic has set back the progress made in HCV elimination88,89 (Box 2), and these data serve as a call to action for an increased emphasis on HCV elimination.
Box 2 Impacts of the COVID-19 pandemic on cirrhosis.
Population-based analyses conducted in the USA determined that mortality due to cirrhosis increased markedly during the COVID-19 pandemic123. Evidence from multiple countries has demonstrated that alcohol consumption increased substantially during the pandemic97,124–128, and the observed increase in mortality was mainly related to alcohol-associated cirrhosis123.
Among patients with COVID-19 and chronic liver disease in the US National COVID Cohort Collaborative, the presence of cirrhosis was associated with an increased risk of mortality (adjusted HR 3.3)129.
In the early phase of the COVID-19 pandemic, cirrhosis referrals, hospitalizations and clinic visits declined substantially, which might have contributed to lower quality of care, delayed presentation and loss of follow-up95,96,130,131.
A global study conducted at 44 international centres determined that consultations, testing and treatment rates for hepatitis B virus and hepatitis C virus infection decreased substantially between January 2019 and December 2020, and this decrease was related to the COVID-19 pandemic89.
Modelling of a 1-year delay to the hepatitis C elimination programmes of 110 countries owing to the COVID-19 pandemic indicated that such a delay would result in 72,300 excess liver-related deaths (likely to be related to HCV-associated cirrhosis in the majority of these cases) over the next 10 years relative to the situation with no delay132.
HBV-associated cirrhosis
Data on the projected global burden of HBV-associated cirrhosis are limited. Projections in one study suggest that the incidence of HBV infection will fall by 2030 but that HBV-related deaths will increase by 39% between 2015 and 2030 (ref. 81). Data from the GBD Study 2019 showed that only four countries had attained the WHO Global Health Sector Strategy on Viral Hepatitis 2020 interim impact target of a 10% reduction in deaths between 2015 and 2019 (ref. 90). Furthermore, despite the availability of vaccines and life-saving antiviral therapy, HBV infection remains severely under-diagnosed, so a minority of treatment-eligible patients receive antiviral treatment91,92, and the COVID-19 pandemic has hindered HBV elimination efforts globally89 (Box 2). Together, these observations highlight that HBV is likely to remain a major threat to public health in the next decade, and increased political will and resources are required to eliminate HBV, which remains a major cause of cirrhosis and HCC worldwide.
Alcohol-associated cirrhosis
In a study in the USA, data from the National Vital Statistics System, the National Institute on Alcohol Abuse and Alcoholism, the National Death Index, the National Epidemiologic Survey on Alcohol and Related Conditions, and other published data were used to predict outcomes from alcohol-associated liver disease in the USA between 2019 and 2040 (ref. 82). The result was a predicted increase of 77% in the age-standardized incidence of decompensated alcohol-associated cirrhosis, from 9.9 cases per 100,000 patient-years in 2019 to 17.5 cases per 100,000 patient-years in 2040, should current trends be left unchecked82.
In a study conducted in Canada, cirrhosis incidence rates were calculated from the crude rates observed in Ontario between 2000 and 2017, and projections of incidence were calculated on the basis of the estimated population of Ontario from 2018 to 2040 (ref. 35). The incidence of cirrhosis (of all aetiologies) was projected to increase by 9% between 2018 and 2040, with a steady increase in the age-standardized incidence rate for alcohol-associated cirrhosis.
The projected burden of alcohol-associated cirrhosis in these studies might be underestimated owing to under-reporting. In addition, neither study accounted for comorbid diseases, such as concomitant HCV infection, which could increase the rate of fibrosis progression. Nevertheless, these data indicate that the burden of alcohol-associated cirrhosis in North America is likely to increase further unless alcohol-related policies are instituted or specific therapies become available for alcohol-associated liver disease. Data for the projected burden of alcohol-associated cirrhosis outside North America are limited.
One additional point to consider is that the COVID-19 pandemic resulted in an increase in alcohol consumption in many countries (Box 2). This increase in alcohol consumption could further increase the global burden of alcohol-associated cirrhosis in the coming years93–98.
NAFLD-associated cirrhosis
Several studies have generated predictions about the burden of NAFLD-associated cirrhosis by 2030. In one study, the prevalence of NAFLD in China, France, Germany, Italy, Japan, Spain, the UK and the USA was predicted on the basis of published data, expert consensus and country-level prevalence of obesity and type 2 diabetes mellitus80. Markov modelling was then used to project the burden of NAFLD-associated cirrhosis in 2030 (ref. 80). The smallest projected increase in the prevalence of compensated NAFLD-associated cirrhosis cases was 64% in Japan and the largest projected increase was 156% in France. The smallest projected increase in the prevalence of decompensated NAFLD-associated cirrhosis cases was 75% in Japan and the largest projected increase was 187% in France.
A similar methodology was used in another study to forecast the burden of NAFLD-associated cirrhosis in 2030 in Hong Kong, Singapore, South Korea and Taiwan79. From 2019 to 2030, incident decompensated NAFLD-associated cirrhosis was projected to increase by 65% in Hong Kong, 85% in South Korea and 100% in Singapore and Taiwan. In both studies, definitions of NAFLD varied between the input sources, contributing to variation in the projected prevalence of NAFLD.
In the Canadian study of cirrhosis discussed above36, projections indicated that NAFLD-associated cirrhosis would account for 75% of incident cases of cirrhosis in Ontario by 2040. These data require cautious interpretation, as the proportion of historical cirrhosis cases due to NAFLD was much higher than estimates in other Canadian studies36,37. Taken together, however, these data highlight the growing burden of NAFLD-associated cirrhosis and the need for urgent measures to control the underlying metabolic risk factors at a global and regional level.
Future directions
Liver disease is often under-diagnosed, and many individuals present late with decompensated cirrhosis99,100. Disadvantaged and under-served communities are often disproportionately affected by liver disease but often lack timely access to appropriate care91,101,102. Despite advances in the diagnosis, treatment and management of cirrhosis, medical interventions often have limited benefit on long-term survival, necessitating the use of resource-heavy treatments such as liver transplantation7,103–106. Therefore, the focus of care should be shifted upstream, from the management of complications to prevention and early treatment74.
As an example, consumption of alcohol per capita (alcohol consumption within one calendar year in litres of pure alcohol in people aged ≥15 years) in Europe has declined over time (12.3 l in 2005 to 9.8 l in 2016), and this decline is related to policies that enforce a minimum price for alcohol and increased alcohol taxation26,41,47,48. The strong political will required to implement these policies has contributed to a reduction in mortality from alcohol-associated cirrhosis in Europe over the past decade26,41,47,48. By contrast, in the Americas, Western Pacific and Southeast Asia, ASDRs due to alcohol-associated cirrhosis increased during the same time period26. A consensus statement published in 2021 highlighted the fact that most countries in the world lack a national strategy for NAFLD, reflecting the low priority of this disease in national health agendas107. Greater efforts are required at national and regional levels to implement public health policies that target the metabolic risk factors for liver diseases, such as high-sugar foods, lack of physical activity and heavy alcohol consumption108.
A case-finding approach could help to detect patients with early cirrhosis or advanced fibrosis, and thereby facilitate treatment. In a population-based, prospective cohort study conducted in Germany, a structured screening programme (a combination of routine serum tests including aspartate aminotransferase, alanine aminotransferase and platelet levels) of individuals participating in health check-ups was associated with 59% higher odds of detecting early cirrhosis compared with routine care, after excluding individuals with decompensated cirrhosis109. The American Gastroenterological Association has proposed a clinical care pathway to facilitate risk stratification and management of individuals with NAFLD in primary care, endocrinology and obesity medicine, which could strengthen linkage to tertiary care but requires prospective validation110.
Outreach efforts to improve screening, treatment and linkage to care for viral hepatitis are effective but are not widely adopted22,111. The Polaris Observatory estimated that only 1% of prevalent cases of HCV infection worldwide were treated in 2020, and <10% of treatment-eligible individuals with HBV infection received antiviral treatment22,81. These sobering figures highlight the uphill task faced by the global hepatology community, and underscore the need for stronger multidisciplinary collaboration between primary care physicians, preventive care specialists, nurse practitioners, infectious disease specialists, hepatologists, patient representatives and policymakers.
Conclusions
The aetiology of cirrhosis is changing, and the global burden of NAFLD-associated cirrhosis is steadily rising in parallel with the epidemic of obesity and type 2 diabetes mellitus. Global alcohol consumption continues to rise, and national policies are required to reduce the burden of alcohol-associated cirrhosis. Despite the availability of effective antiviral therapies for HCV and HBV infection, most countries are not on track to meet the WHO viral hepatitis elimination targets. The burden of cirrhosis remains substantial owing to under-diagnosis and under-treatment of chronic liver disease, and the number of deaths and cases of decompensated cirrhosis are projected to rise in the next decade. More resources should be directed towards primary prevention, early detection of liver disease and linkage to care to reduce the global burden of cirrhosis.
Supplementary information
Acknowledgements
R.L. receives funding support from NCATS (5UL1TR001442), NIDDK (U01DK061734, U01DK130190, R01DK106419, R01DK121378, R01DK124318, P30DK120515), NHLBI (P01HL147835) and NIAAA (U01AA029019). D.Q.H. receives funding support from the Singapore Ministry of Health’s NMRC Research Training Fellowship (MOH-000595-01). M.A. acknowledges partial support from the Chilean government through the Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT grant 1191145).
Author contributions
All authors contributed to all aspects of the manuscript.
Peer review
Peer review information
Nature Reviews Gastroenterology & Hepatology thanks M.-L. Yu, who co-reviewed with P.-Y. Hsu, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Competing interests
D.Q.H. has served as an advisory board member for Eisai and Gilead. N.A.T. receives institutional grant support from DURECT Corporation, Eiger Pharmaceuticals, Gilead Sciences, Glaxo-Smith-Kline, Helio Health and Roche-Genentech. F.T. serves as a consultant to Abbvie, Alnylam, Boehringer-Ingelheim, CSL Behring, Falk, Gilead, Intercept, Inventiva, Ionis, Novartis, Novo Nordisk and Pfizer. His institute has received research grants from Allergan, Bristol Myers Squibb, Gilead and Inventiva. L.L.G. serves as a consultant to Novo Nordisk and Pfizer, and her institutes have received research grants from Alexion, Gilead, Novo Nordisk, Sobi Int. and Vingmed. M.A. receives support from the Chilean government through the Fondo Nacional De Ciencia y Tecnología de Chile (FONDECYT no. 1191145) and the Comisión Nacional de Investigación, Ciencia y Tecnología (CONICYT, AFB170005, CARE, Chile, UC). E.B. serves as a consultant to AstraZeneca, Boehringer-Ingelheim, Eli Lilly, Gilead, Intercept, Inventiva, Novartis, Novo Nordisk, Merck, MSD and Pfizer. Her institute has received a research grant from Gilead. R.L. serves as a consultant to 89 Bio, Aardvark Therapeutics, Altimmune, Anylam/Regeneron, Amgen, Arrowhead Pharmaceuticals, AstraZeneca, Bristol Myers Squibb, CohBar, Eli Lilly, Galmed, Gilead, Glympse bio, Hightide, Inipharma, Intercept, Inventiva, Ionis, Janssen, Madrigal, Metacrine, NGM Biopharmaceuticals, Novartis, Novo Nordisk, Merck, Pfizer, Sagimet, Theratechnologies, Terns Pharmaceuticals and Viking Therapeutics. In addition his institutes have received research grants from Arrowhead Pharmaceuticals, Astrazeneca, Boehringer-Ingelheim, Bristol Myers Squibb, Eli Lilly, Galectin Therapeutics, Galmed Pharmaceuticals, Gilead, Intercept, Hanmi, Intercept, Inventiva, Ionis, Janssen, Madrigal Pharmaceuticals, Merck, NGM Biopharmaceuticals, Novo Nordisk, Merck, Pfizer, Sonic Incytes and Terns Pharmaceuticals. He is also a co-founder of LipoNexus.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Review criteria PubMed was searched from inception to June 2022 using the terms ‘cirrhosis’, ‘end-stage liver disease’ and ‘liver cirrhosis’ without language restrictions. Guidelines, original articles and reviews were evaluated. Data for the global and regional number and age-adjusted rate of deaths estimated in the Global Burden of Disease (GBD) Study 2019 were obtained from the GBD Results Tool27. The GBD Study was used to identify trends in the mortality rates of cirrhosis112, but not temporal trends in the prevalence of the aetiologies of cirrhosis, as limited data on these trends were available within the GBD framework. Individual country-specific and region-specific studies were, therefore, selected to provide data from diverse geographical locations on temporal trends in the prevalence of the aetiologies of cirrhosis. When multiple studies originating from the same country were available, studies that provided data for temporal trends in aetiologies of cirrhosis were preferentially selected.
Supplementary information
The online version contains supplementary material available at 10.1038/s41575-023-00759-2.
References
- 1.Ginès P, et al. Liver cirrhosis. Lancet. 2021;398:1359–1376. doi: 10.1016/S0140-6736(21)01374-X. [DOI] [PubMed] [Google Scholar]
- 2.Tapper EB, Ufere NN, Huang DQ, Loomba R. Review article: current and emerging therapies for the management of cirrhosis and its complications. Aliment. Pharmacol. Ther. 2022;55:1099–1115. doi: 10.1111/apt.16831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tapper EB, Parikh ND. Mortality due to cirrhosis and liver cancer in the United States, 1999-2016: observational study. BMJ. 2018;362:k2817. doi: 10.1136/bmj.k2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang DQ, et al. Hepatocellular carcinoma incidence in alcohol-associated cirrhosis: systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 2022 doi: 10.1016/j.cgh.2022.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tan DJH, et al. Clinical characteristics, surveillance, treatment allocation, and outcomes of non-alcoholic fatty liver disease-related hepatocellular carcinoma: a systematic review and meta-analysis. Lancet Oncol. 2022;23:521–530. doi: 10.1016/S1470-2045(22)00078-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tan DJH, et al. Global burden of liver cancer in males and females: changing etiological basis and the growing contribution of NASH. Hepatology. 2022 doi: 10.1002/hep.32758. [DOI] [PubMed] [Google Scholar]
- 7.Ajmera V, et al. Liver stiffness on magnetic resonance elastography and the MEFIB index and liver-related outcomes in nonalcoholic fatty liver disease: a systematic review and meta-analysis of individual participants. Gastroenterology. 2022;163:1079–1089.e5. doi: 10.1053/j.gastro.2022.06.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The Global Health Observatory. Global health estimates: Leading causes of death. WHOhttps://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-leading-causes-of-death. (2023).
- 9.Moon AM, Singal AG, Tapper EB. Contemporary epidemiology of chronic liver disease and cirrhosis. Clin. Gastroenterol. Hepatol. 2020;18:2650–2666. doi: 10.1016/j.cgh.2019.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loomba R, Friedman SL, Shulman GI. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell. 2021;184:2537–2564. doi: 10.1016/j.cell.2021.04.015. [DOI] [PubMed] [Google Scholar]
- 11.Asrani SK, Devarbhavi H, Eaton J, Kamath PS. Burden of liver diseases in the world. J. Hepatol. 2019;70:151–171. doi: 10.1016/j.jhep.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 12.GBD 2017 Cirrhosis Collaborators. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020;5:245–266. doi: 10.1016/S2468-1253(19)30349-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang DQ, et al. Changing global epidemiology of liver cancer from 2010 to 2019: NASH is the fastest growing cause of liver cancer. Cell Metab. 2022;34:969–977.e2. doi: 10.1016/j.cmet.2022.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang DQ, El-Serag HB, Loomba R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2021;18:223–238. doi: 10.1038/s41575-020-00381-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Golabi P, et al. Burden of non-alcoholic fatty liver disease in Asia, the Middle East and North Africa: Data from Global Burden of Disease 2009-2019. J. Hepatol. 2021;75:795–809. doi: 10.1016/j.jhep.2021.05.022. [DOI] [PubMed] [Google Scholar]
- 16.Orman ES, et al. Trends in characteristics, mortality, and other outcomes of patients with newly diagnosed cirrhosis. JAMA Netw. Open. 2019;2:e196412. doi: 10.1001/jamanetworkopen.2019.6412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen VL, et al. Anti-viral therapy is associated with improved survival but is underutilised in patients with hepatitis B virus-related hepatocellular carcinoma: real-world east and west experience. Aliment. Pharmacol. Ther. 2018;48:44–54. doi: 10.1111/apt.14801. [DOI] [PubMed] [Google Scholar]
- 18.Terrault NA, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560–1599. doi: 10.1002/hep.29800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.European Association for the Study of the Liver EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J. Hepatol. 2017;67:370–398. doi: 10.1016/j.jhep.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 20.Sarin SK, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol. Int. 2016;10:1–98. doi: 10.1007/s12072-015-9675-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghany MG, Morgan TR, AASLD-IDSA Hepatitis C Guidance Panel Hepatitis C guidance 2019 update: American Association for the Study of Liver Diseases–Infectious Diseases Society of America recommendations for testing, managing, and treating hepatitis C virus infection. Hepatology. 2020;71:686–721. doi: 10.1002/hep.31060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Polaris Observatory HCV Collaborators. Global change in hepatitis C virus prevalence and cascade of care between 2015 and 2020: a modelling study. Lancet Gastroenterol. Hepatol. 2022;7:396–415. doi: 10.1016/S2468-1253(21)00472-6. [DOI] [PubMed] [Google Scholar]
- 23.European Association for the Study of the Liver EASL recommendations on treatment of hepatitis C: final update of the series. J. Hepatol. 2020;73:1170–1218. doi: 10.1016/j.jhep.2020.08.018. [DOI] [PubMed] [Google Scholar]
- 24.Manthey J, et al. Global alcohol exposure between 1990 and 2017 and forecasts until 2030: a modelling study. Lancet. 2019;393:2493–2502. doi: 10.1016/S0140-6736(18)32744-2. [DOI] [PubMed] [Google Scholar]
- 25.Rehm J, Shield KD. Global burden of alcohol use disorders and alcohol liver disease. Biomedicines. 2019;7:99. doi: 10.3390/biomedicines7040099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paik J, et al. The global burden of liver cancer (LC) and chronic liver diseases (CLD) is driven by non-alcoholic steatohepatitis (NASH) and alcohol liver disease (ALD) [abstract GS008] J. Hepatol. 2022;77(Suppl. 1):S5–S7. doi: 10.1016/S0168-8278(22)00435-4. [DOI] [Google Scholar]
- 27.GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1204–1222. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ginès P, et al. Population screening for liver fibrosis: toward early diagnosis and intervention for chronic liver diseases. Hepatology. 2022;75:219–228. doi: 10.1002/hep.32163. [DOI] [PubMed] [Google Scholar]
- 30.Alberts CJ, et al. Worldwide prevalence of hepatitis B virus and hepatitis C virus among patients with cirrhosis at country, region, and global levels: a systematic review. Lancet Gastroenterol. Hepatol. 2022;7:724–735. doi: 10.1016/S2468-1253(22)00050-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eslam M, et al. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158:1999–2014.e1. doi: 10.1053/j.gastro.2019.11.312. [DOI] [PubMed] [Google Scholar]
- 32.Ng CH, Huang DQ, Nguyen MH. NAFLD versus MAFLD: prevalence, outcomes and implications of a change in name. Clin. Mol. Hepatol. 2022 doi: 10.3350/cmh.2022.0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noureddin M, et al. Attribution of nonalcoholic steatohepatitis as an etiology of cirrhosis for clinical trials eligibility: recommendations from the multi-stakeholder liver forum. Gastroenterology. 2020;159:422–427.e1. doi: 10.1053/j.gastro.2020.04.039. [DOI] [PubMed] [Google Scholar]
- 34.El-Serag HB, et al. Risk factors for cirrhosis in contemporary hepatology practices–findings from the Texas Hepatocellular Carcinoma Consortium Cohort. Gastroenterology. 2020;159:376–377. doi: 10.1053/j.gastro.2020.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Flemming JA, Djerboua M, Groome PA, Booth CM, Terrault NA. NAFLD and alcohol-associated liver disease will be responsible for almost all new diagnoses of cirrhosis in Canada by 2040. Hepatology. 2021;74:3330–3344. doi: 10.1002/hep.32032. [DOI] [PubMed] [Google Scholar]
- 36.Philip G, Djerboua M, Carlone D, Flemming JA. Validation of a hierarchical algorithm to define chronic liver disease and cirrhosis etiology in administrative healthcare data. PLoS ONE. 2020;15:e0229218. doi: 10.1371/journal.pone.0229218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sharma SA, et al. Toronto HCC risk index: a validated scoring system to predict 10-year risk of HCC in patients with cirrhosis. J. Hepatol. 2017 doi: 10.1016/j.jhep.2017.07.033. [DOI] [PubMed] [Google Scholar]
- 38.Gonzalez-Chagolla A, et al. Cirrhosis etiology trends in developing countries: transition from infectious to metabolic conditions. Report from a multicentric cohort in central Mexico. Lancet Reg. Health Am. 2022;7:100151. doi: 10.1016/j.lana.2021.100151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wong RJ, Singal AK. Trends in liver disease etiology among adults awaiting liver transplantation in the United States, 2014-2019. JAMA Netw. Open. 2020;3:e1920294. doi: 10.1001/jamanetworkopen.2019.20294. [DOI] [PubMed] [Google Scholar]
- 40.Ward ZJ, et al. Projected U.S. state-level prevalence of adult obesity and severe obesity. N. Engl. J. Med. 2019;381:2440–2450. doi: 10.1056/NEJMsa1909301. [DOI] [PubMed] [Google Scholar]
- 41.World Health Organization. Global status report on alcohol and health 2018 (WHO, 2018).
- 42.Díaz LA, et al. Liver diseases in Latin America: current status, unmet needs, and opportunities for improvement. Curr. Treat. Options Gastroenterol. 2022;20:261–278. doi: 10.1007/s11938-022-00382-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gu W, et al. Trends and the course of liver cirrhosis and its complications in Germany: Nationwide population-based study (2005 to 2018) Lancet Reg. Health Eur. 2022;12:100240. doi: 10.1016/j.lanepe.2021.100240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hagström H, et al. Etiologies and outcomes of cirrhosis in a large contemporary cohort. Scand. J. Gastroenterol. 2021;56:727–732. doi: 10.1080/00365521.2021.1912167. [DOI] [PubMed] [Google Scholar]
- 45.Stroffolini T, et al. Characteristics of liver cirrhosis in Italy: evidence for a decreasing role of HCV aetiology. Eur. J. Intern. Med. 2017;38:68–72. doi: 10.1016/j.ejim.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 46.Stroffolini T, et al. Characteristics of liver cirrhosis in Italy: results from a multicenter national study. Dig. Liver Dis. 2004;36:56–60. doi: 10.1016/j.dld.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 47.World Health Organization. Equity, social determinants and public health programmes (WHO, 2010).
- 48.World Health Organization. European action plan to reduce the harmful use of alcohol 2012–2020 (WHO, 2012).
- 49.Vento S, Dzudzor B, Cainelli F, Tachi K. Liver cirrhosis in sub-Saharan Africa: neglected, yet important. Lancet Glob. Health. 2018;6:e1060–e1061. doi: 10.1016/S2214-109X(18)30344-9. [DOI] [PubMed] [Google Scholar]
- 50.Mokdad AA, et al. Liver cirrhosis mortality in 187 countries between 1980 and 2010: a systematic analysis. BMC Med. 2014;12:145. doi: 10.1186/s12916-014-0145-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang JD, et al. Characteristics, management, and outcomes of patients with hepatocellular carcinoma in Africa: a multicountry observational study from the Africa Liver Cancer Consortium. Lancet Gastroenterol. Hepatol. 2017;2:103–111. doi: 10.1016/S2468-1253(16)30161-3. [DOI] [PubMed] [Google Scholar]
- 52.Tailakh MA, et al. Liver cirrhosis, etiology and clinical characteristics disparities among minority population. J. Immigr. Minor. Health. 2022;24:1122–1128. doi: 10.1007/s10903-021-01263-y. [DOI] [PubMed] [Google Scholar]
- 53.Elzouki AN, et al. Predicting mortality of patients with cirrhosis admitted to medical intensive care unit: an experience of a single tertiary center. Arab. J. Gastroenterol. 2016;17:159–163. doi: 10.1016/j.ajg.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 54.Mukherjee PS, et al. Etiology and mode of presentation of chronic liver diseases in India: a multi centric study. PLoS ONE. 2017;12:e0187033. doi: 10.1371/journal.pone.0187033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alvi H, Zuberi BF, Rasheed T, Ibrahim MA. Evaluation of endoscopic variceal band ligation sessions in obliteration of esophageal varices. Pak. J. Med. Sci. 2020;36:37–41. doi: 10.12669/pjms.36.2.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Enomoto H, et al. Transition in the etiology of liver cirrhosis in Japan: a nationwide survey. J. Gastroenterol. 2020;55:353–362. doi: 10.1007/s00535-019-01645-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jang WY, et al. Changes in characteristics of patients with liver cirrhosis visiting a tertiary hospital over 15 years: a retrospective multi-center study in Korea. J. Korean Med. Sci. 2020;35:e233. doi: 10.3346/jkms.2020.35.e233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xiong J, et al. Non-alcoholic steatohepatitis-related liver cirrhosis is increasing in China: a ten-year retrospective study. Clinics. 2015;70:563–568. doi: 10.6061/clinics/2015(08)06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lu S-N, et al. Secular trends and geographic variations of hepatitis B virus and hepatitis C virus-associated hepatocellular carcinoma in Taiwan. Int. J. Cancer. 2006;119:1946–1952. doi: 10.1002/ijc.22045. [DOI] [PubMed] [Google Scholar]
- 60.Schmidt LA, Room R. Alcohol and inequity in the process of development: contributions from ethnographic research. Int. J. Alcohol. Drug. Res. 2013;1:41–55. doi: 10.7895/ijadr.v1i1.38. [DOI] [Google Scholar]
- 61.Wang H, Ma L, Yin Q, Zhang X, Zhang C. Prevalence of alcoholic liver disease and its association with socioeconomic status in north-eastern China. Alcohol. Clin. Exp. Res. 2014;38:1035–1041. doi: 10.1111/acer.12321. [DOI] [PubMed] [Google Scholar]
- 62.Charatcharoenwitthaya P, Liangpunsakul S, Piratvisuth T. Alcohol-associated liver disease: East versus West. Clin. Liver Dis. 2020;16:231–235. doi: 10.1002/cld.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fan JG, Kim SU, Wong VW. New trends on obesity and NAFLD in Asia. J. Hepatol. 2017;67:862–873. doi: 10.1016/j.jhep.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 64.D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J. Hepatol. 2006;44:217–231. doi: 10.1016/j.jhep.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 65.Jepsen P, Ott P, Andersen PK, Sørensen HT, Vilstrup H. Clinical course of alcoholic liver cirrhosis: a Danish population-based cohort study. Hepatology. 2010;51:1675–1682. doi: 10.1002/hep.23500. [DOI] [PubMed] [Google Scholar]
- 66.Fleming KM, Aithal GP, Card TR, West J. The rate of decompensation and clinical progression of disease in people with cirrhosis: a cohort study. Aliment. Pharmacol. Ther. 2010;32:1343–1350. doi: 10.1111/j.1365-2036.2010.04473.x. [DOI] [PubMed] [Google Scholar]
- 67.Arroyo V, Moreau R, Jalan R. Acute-on-chronic liver failure. N. Engl. J. Med. 2020;382:2137–2145. doi: 10.1056/NEJMra1914900. [DOI] [PubMed] [Google Scholar]
- 68.Hernaez R, Solà E, Moreau R, Ginès P. Acute-on-chronic liver failure: an update. Gut. 2017;66:541–553. doi: 10.1136/gutjnl-2016-312670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sarin SK, et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific association for the study of the liver (APASL): an update. Hepatol. Int. 2019;13:353–390. doi: 10.1007/s12072-019-09946-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moreau R, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426–1437.e9. doi: 10.1053/j.gastro.2013.02.042. [DOI] [PubMed] [Google Scholar]
- 71.O’Leary JG, et al. NACSELD acute-on-chronic liver failure (NACSELD-ACLF) score predicts 30-day survival in hospitalized patients with cirrhosis. Hepatology. 2018;67:2367–2374. doi: 10.1002/hep.29773. [DOI] [PubMed] [Google Scholar]
- 72.Arroyo V, et al. Acute-on-chronic liver failure in cirrhosis. Nat. Rev. Dis. Prim. 2016;2:16041. doi: 10.1038/nrdp.2016.41. [DOI] [PubMed] [Google Scholar]
- 73.Mezzano G, et al. Global burden of disease: acute-on-chronic liver failure, a systematic review and meta-analysis. Gut. 2022;71:148–155. doi: 10.1136/gutjnl-2020-322161. [DOI] [PubMed] [Google Scholar]
- 74.Karlsen TH, et al. The EASL-Lancet Liver Commission: protecting the next generation of Europeans against liver disease complications and premature mortality. Lancet. 2022;399:61–116. doi: 10.1016/S0140-6736(21)01701-3. [DOI] [PubMed] [Google Scholar]
- 75.GBD 2019 Demographics Collaborators. Global age-sex-specific fertility, mortality, healthy life expectancy (HALE), and population estimates in 204 countries and territories, 1950–2019: a comprehensive demographic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1160–1203. doi: 10.1016/S0140-6736(20)30977-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Adams LA, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–121. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 77.Huang D, et al. Shared mechanisms between cardiovascular disease and NAFLD. Semin. Liver Dis. 2022 doi: 10.1055/a-1930-6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Younossi ZM, et al. Nonalcoholic steatofibrosis independently predicts mortality in nonalcoholic fatty liver disease. Hepatol. Commun. 2017;1:421–428. doi: 10.1002/hep4.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Estes C, et al. Modelling NAFLD disease burden in four Asian regions – 2019-2030. Aliment. Pharmacol. Ther. 2020;51:801–811. doi: 10.1111/apt.15673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Estes C, et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016-2030. J. Hepatol. 2018;69:896–904. doi: 10.1016/j.jhep.2018.05.036. [DOI] [PubMed] [Google Scholar]
- 81.Razavi-Shearer D, et al. The disease burden of hepatitis B and hepatitis C from 2015 to 2030: the long and winding road [abstract OS050] J. Hepatol. 2022;77(Suppl. 1):S43. doi: 10.1016/S0168-8278(22)00496-2. [DOI] [Google Scholar]
- 82.Julien J, Ayer T, Bethea ED, Tapper EB, Chhatwal J. Projected prevalence and mortality associated with alcohol-related liver disease in the USA, 2019-40: a modelling study. Lancet Public. Health. 2020;5:e316–e323. doi: 10.1016/S2468-2667(20)30062-1. [DOI] [PubMed] [Google Scholar]
- 83.Hsu WF, et al. Hepatitis C virus eradication decreases the risks of liver cirrhosis and cirrhosis-related complications (Taiwanese chronic hepatitis C cohort) J. Gastroenterol. Hepatol. 2021;36:2884–2892. doi: 10.1111/jgh.15538. [DOI] [PubMed] [Google Scholar]
- 84.Sanyal AJ, et al. Cirrhosis regression is associated with improved clinical outcomes in patients with nonalcoholic steatohepatitis. Hepatology. 2021 doi: 10.1002/hep.32204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xie YD, Feng B, Gao Y, Wei L. Effect of abstinence from alcohol on survival of patients with alcoholic cirrhosis: A systematic review and meta-analysis. Hepatol. Res. 2014;44:436–449. doi: 10.1111/hepr.12131. [DOI] [PubMed] [Google Scholar]
- 86.Chang TT, et al. Long-term entecavir therapy results in the reversal of fibrosis/cirrhosis and continued histological improvement in patients with chronic hepatitis B. Hepatology. 2010;52:886–893. doi: 10.1002/hep.23785. [DOI] [PubMed] [Google Scholar]
- 87.Cox AL, et al. Progress towards elimination goals for viral hepatitis. Nat. Rev. Gastroenterol. Hepatol. 2020;17:533–542. doi: 10.1038/s41575-020-0332-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tergast TL, et al. Updated epidemiology of hepatitis C virus infections and implications for hepatitis C virus elimination in Germany. J. Viral Hepat. 2022;29:536–542. doi: 10.1111/jvh.13680. [DOI] [PubMed] [Google Scholar]
- 89.Kondili LA, et al. Impact of the COVID-19 pandemic on hepatitis B and C elimination: an EASL survey. JHEP Rep. 2022;4:100531. doi: 10.1016/j.jhepr.2022.100531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.GBD 2019 Hepatiitis B Collaborators. Global, regional, and national burden of hepatitis B, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Gastroenterol. Hepatol. 2022;7:796–829. doi: 10.1016/S2468-1253(22)00124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ye Q, et al. Substantial gaps in evaluation and treatment of patients with hepatitis B in the US. J. Hepatol. 2022;76:63–74. doi: 10.1016/j.jhep.2021.08.019. [DOI] [PubMed] [Google Scholar]
- 92.Le MH, et al. Chronic hepatitis B prevalence among foreign-born and U.S.-born adults in the United States, 1999-2016. Hepatology. 2020;71:431–443. doi: 10.1002/hep.30831. [DOI] [PubMed] [Google Scholar]
- 93.Julien J, et al. Effect of increased alcohol consumption during COVID-19 pandemic on alcohol-associated liver disease: a modeling study. Hepatology. 2022;75:1480–1490. doi: 10.1002/hep.32272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bittermann T, Mahmud N, Abt P. Trends in liver transplantation for acute alcohol-associated hepatitis during the COVID-19 pandemic in the US. JAMA Netw. Open. 2021;4:e2118713. doi: 10.1001/jamanetworkopen.2021.18713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Toyoda H, Huang DQ, Le MH, Nguyen MH. Liver care and surveillance: the global impact of the COVID-19 pandemic. Hepatol. Commun. 2020;4:1751–1757. doi: 10.1002/hep4.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tan EX-X. Impact of COVID-19 on liver transplantation in Hong Kong and Singapore: a modelling study. Lancet Reg. Health West. Pac. 2021;16:100262. doi: 10.1016/j.lanwpc.2021.100262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lee BP, Dodge JL, Leventhal A, Terrault NA. Retail alcohol and tobacco sales during COVID-19. Ann. Intern. Med. 2021;174:1027–1029. doi: 10.7326/M20-7271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.White AM, Castle I-JP, Powell PA, Hingson RW, Koob GF. Alcohol-related deaths during the COVID-19 pandemic. JAMA. 2022 doi: 10.1001/jama.2022.4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hussain A, et al. Decompensated cirrhosis is the commonest presentation for NAFLD patients undergoing liver transplant assessment. Clin. Med. 2020;20:313–318. doi: 10.7861/clinmed.2019-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Trebicka J, et al. The PREDICT study uncovers three clinical courses of acutely decompensated cirrhosis that have distinct pathophysiology. J. Hepatol. 2020;73:842–854. doi: 10.1016/j.jhep.2020.06.013. [DOI] [PubMed] [Google Scholar]
- 101.Lee BP, Dodge JL, Terrault NA. Geographic density of gastroenterologists is associated with decreased mortality from alcohol-associated liver disease. Clin. Gastroenterol. Hepatol. 2022 doi: 10.1016/j.cgh.2022.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Oluyomi AO, El-Serag HB, Olayode A, Thrift AP. Neighborhood-level factors contribute to disparities in hepatocellular carcinoma incidence in Texas. Clin. Gastroenterol. Hepatol. 2022 doi: 10.1016/j.cgh.2022.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.de Franchis R, Bosch J, Garcia-Tsao G, Reiberger T, Ripoll C. Baveno VII – Renewing consensus in portal hypertension. J. Hepatol. 2022;76:959–974. doi: 10.1016/j.jhep.2021.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Liver transplantation. J. Hepatol. 2016;64:433–485. doi: 10.1016/j.jhep.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 105.Lucey MR, et al. Long-term management of the successful adult liver transplant: 2012 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Liver Transpl. 2013;19:3–26. doi: 10.1002/lt.23566. [DOI] [PubMed] [Google Scholar]
- 106.Sharpton SR, et al. Gut metagenome-derived signature predicts hepatic decompensation and mortality in NAFLD-related cirrhosis. Aliment. Pharmacol. Ther. 2022;56:1475–1485. doi: 10.1111/apt.17236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lazarus JV, et al. Advancing the global public health agenda for NAFLD: a consensus statement. Nat. Rev. Gastroenterol. Hepatol. 2021 doi: 10.1038/s41575-021-00523-4. [DOI] [PubMed] [Google Scholar]
- 108.Díaz LA, et al. The establishment of public health policies and the burden of non-alcoholic fatty liver disease in the Americas. Lancet Gastroenterol. Hepatol. 2022;7:552–559. doi: 10.1016/S2468-1253(22)00008-5. [DOI] [PubMed] [Google Scholar]
- 109.Labenz C, et al. Structured early detection of asymptomatic liver cirrhosis: results of the population-based liver screening program SEAL. J. Hepatol. 2022;77:695–701. doi: 10.1016/j.jhep.2022.04.009. [DOI] [PubMed] [Google Scholar]
- 110.Kanwal F, et al. Clinical care pathway for the risk stratification and management of patients with nonalcoholic fatty liver disease. Gastroenterology. 2021;161:1657–1669. doi: 10.1053/j.gastro.2021.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rozenberg-Ben-Dror K, et al. Improving quality of hepatitis B care in the Veteran’s Health Administration. Clin. liver Dis. 2022;19:213–218. doi: 10.1002/cld.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Paik JM, Golabi P, Younossi Y, Mishra A, Younossi ZM. Changes in the global burden of chronic liver diseases from 2012 to 2017: the growing impact of NAFLD. Hepatology. 2020;72:1605–1616. doi: 10.1002/hep.31173. [DOI] [PubMed] [Google Scholar]
- 113.Nehra MS, et al. Use of administrative claims data for identifying patients with cirrhosis. J. Clin. Gastroenterol. 2013;47:e50–e54. doi: 10.1097/MCG.0b013e3182688d2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hayward KL, et al. ICD-10-AM codes for cirrhosis and related complications: key performance considerations for population and healthcare studies. BMJ Open Gastroenterol. 2020;7:e000485. doi: 10.1136/bmjgast-2020-000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ramrakhiani NS, et al. Validity of international classification of diseases, tenth revision, codes for cirrhosis. Dig. Dis. 2021;39:243–246. doi: 10.1159/000510981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mapakshi S, Kramer JR, Richardson P, El-Serag HB, Kanwal F. Positive predictive value of international classification of diseases, 10th revision, codes for cirrhosis and its related complications. Clin. Gastroenterol. Hepatol. 2018;16:1677–1678. doi: 10.1016/j.cgh.2018.01.042. [DOI] [PubMed] [Google Scholar]
- 117.Bengtsson B, Askling J, Ludvigsson JF, Hagström H. Validity of administrative codes associated with cirrhosis in Sweden. Scand. J. Gastroenterol. 2020;55:1205–1210. doi: 10.1080/00365521.2020.1820566. [DOI] [PubMed] [Google Scholar]
- 118.Shearer JE, et al. Systematic review: development of a consensus code set to identify cirrhosis in electronic health records. Aliment. Pharmacol. Ther. 2022;55:645–657. doi: 10.1111/apt.16806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ratib S, West J, Crooks CJ, Fleming KM. Diagnosis of liver cirrhosis in England, a cohort study, 1998-2009: a comparison with cancer. Am. J. Gastroenterol. 2014;109:190–198. doi: 10.1038/ajg.2013.405. [DOI] [PubMed] [Google Scholar]
- 120.Thygesen SK, Christiansen CF, Christensen S, Lash TL, Sørensen HT. The predictive value of ICD-10 diagnostic coding used to assess Charlson comorbidity index conditions in the population-based Danish National Registry of Patients. BMC Med. Res. Methodol. 2011;11:83. doi: 10.1186/1471-2288-11-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kramer JR, et al. The validity of viral hepatitis and chronic liver disease diagnoses in Veterans Affairs Administrative databases. Aliment. Pharmacol. Ther. 2008;27:274–282. doi: 10.1111/j.1365-2036.2007.03572.x. [DOI] [PubMed] [Google Scholar]
- 122.Goldberg D, Lewis J, Halpern S, Weiner M, Lo Re V., 3rd Validation of three coding algorithms to identify patients with end-stage liver disease in an administrative database. Pharmacoepidemiol. Drug. Saf. 2012;21:765–769. doi: 10.1002/pds.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kim D, Alshuwaykh O, Dennis BB, Cholankeril G, Ahmed A. Trends in etiology-based mortality from chronic liver disease before and during COVID-19 pandemic in the United States. Clin. Gastroenterol. Hepatol. 2022;20:2307–2316.e3. doi: 10.1016/j.cgh.2022.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pollard MS, Tucker JS, Green HD., Jr. Changes in adult alcohol use and consequences during the COVID-19 pandemic in the US. JAMA Netw. Open. 2020;3:e2022942. doi: 10.1001/jamanetworkopen.2020.22942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.The Lancet Gastroenterology Hepatology. Drinking alone: COVID-19, lockdown, and alcohol-related harm. Lancet Gastroenterol. Hepatol. 2020;5:625. doi: 10.1016/S2468-1253(20)30159-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.NANOS Research. COVID-19 and increased alcohol consumption: NANOS poll summary report (Canadian Centre on Substance Use and Addiction, 2020).
- 127.Vanderbruggen N, et al. Self-reported alcohol, tobacco, and cannabis use during COVID-19 lockdown measures: results from a web-based survey. Eur. Addict. Res. 2020;26:309–315. doi: 10.1159/000510822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sidor A, Rzymski P. Dietary choices and habits during COVID-19 lockdown: experience from Poland. Nutrients. 2020;12:1657. doi: 10.3390/nu12061657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ge J, Pletcher MJ, Lai JC. Outcomes of SARS-CoV-2 infection in patients with chronic liver disease and cirrhosis: a national COVID cohort collaborative study. Gastroenterology. 2021;161:1487–1501.e5. doi: 10.1053/j.gastro.2021.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mahmud N, Hubbard RA, Kaplan DE, Serper M. Declining cirrhosis hospitalizations in the wake of the COVID-19 pandemic: a national cohort study. Gastroenterology. 2020;159:1134–1136.e3. doi: 10.1053/j.gastro.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tapper EB, Asrani SK. The COVID-19 pandemic will have a long-lasting impact on the quality of cirrhosis care. J. Hepatol. 2020;73:441–445. doi: 10.1016/j.jhep.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Blach S, et al. Impact of COVID-19 on global HCV elimination efforts. J. Hepatol. 2021;74:31–36. doi: 10.1016/j.jhep.2020.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.