Abstract
Introduction
Since the first COVID-19 messenger RNA vaccines became available globally for emergency or conditional use, post-marketing surveillance activities have been implemented for the monitoring of any adverse events that might arise in daily clinical practice and were not detected earlier during clinical trials.
Methods
Safety data concerning the BNT162b2 and the mRNA-1273 COVID-19 vaccines were collected from the Vaccine Adverse Event Reporting System (VAERS) for the period from December 2020 to October 15, 2021. In addition to a descriptive analysis of individuals who experienced an adverse event after vaccination, a case-non-case analysis was performed by using the Reporting Odds Ratio with 95 % confidence interval as statistical parameter for detecting differences in reporting rates between the two mRNA vaccines.
Results
At the cut-off date, a total of 758,040 reports were submitted to VAERS, of which 439,401 were related to the Pfizer-BioNTech (BNT162b2) vaccine and 318,639 to the Moderna vaccine (mRNA-1273). Most common adverse events following immunization for both mRNA vaccines were headache, fatigue, pyrexia, dizziness, nausea, pain, chills, and pain in extremity. A disproportionality was found for BNT162b2 as compared with mRNA-1273 for some events of special interest, such as myocarditis [ROR 2.00; 95 % confidence interval (CI), 1.93–2.06], Bell’s palsy (1.34; 1.29–1.39), and anaphylactic shock (3.23; 2.96–3.53).
Conclusion
Even if some rare adverse events were identified, our survey of post-marketing surveillance has provided further evidence of the favourable safety profile of mRNA vaccines.
Keywords: mRNA vaccine, COVID-19, Safety, Pharmacovigilance
1. Introduction
The devasting worldwide impact of the COVID-19 pandemic, declared by the World Health Organization on March 11, 2020 [1], was soon followed by the identifying of the genetic sequence of SARS-CoV-2, the coronavirus that causes COVID-19. Shortly after, many centres of the scientific community moved towards the development of an effective vaccine able to contrast the spread of this dangerous infection. A striking feature of the vaccine development landscape has been the broad range of technology platforms being evaluated, including novel approaches not previously used in licensed vaccines [2]. Among these, the BNT162b2 and the mRNA-1273 COVID-19 vaccines have led since the beginning to relevant results and are still the basis of the currently U.S. and E.U. vaccination campaigns. Both are lipid nanoparticle-formulated mRNA-based vaccines which encode the prefusion stabilised, membrane-anchored SARS-CoV-2 full-length spike protein [3], [4]. These candidates have moved from conception to large-scale implementation within a year, an unprecedented speed compared to the traditional vaccine development pathway which takes on average over 10 years, and even compared with the accelerated 5-year timescale for development of the first Ebola vaccine [2].
The Pfizer-BioNTech vaccine (BNT162b2) has been the first COVID-19 vaccine available in the United States under Emergency Use Authorization (EUA) since December 11, 2020, for the prevention of COVID-19 illness as a two-dose primary series in individuals 16 years of age and older [5], as well as the first one receiving from the Food and Drug Administration (FDA) the full approval on August 23, 2021, whit the brand name Comirnaty® [6], [7]. Similarly, the Moderna vaccine (mRNA-1273), developed by Moderna and the Vaccine Research Center at the National Institute of Allergy and Infectious Diseases (NIAID) within the National Institutes of Health (NIH), has been authorised for emergency use as a two-dose primary series for individuals 18 or older since December 18, 2020 [8]. On January 31, 2022, the FDA granted the second full approval for Moderna’s COVID-19 vaccine, now marketed as Spikevax® [9].
As with all medicinal products, regardless of whether they are made available for emergency use or under full approval, post-authorisation pharmacovigilance activities are required to ensure vaccine safety and effectiveness over time. Although no safety concerns have emerged during clinical trials [3], [4], unexpected adverse events could arise following global distribution of COVID-19 vaccines. Rare, long-term or serious outcomes associated with a vaccine may not be identified in phase 3 trials because of limited sample size, restrictive inclusion criteria, limited duration of follow-up, and characteristics of trial participants which may be different from those of the population ultimately receiving the vaccine. Furthermore, there is still limited knowledge with mRNA platforms [10]. With the aim of providing further evidence on possible vaccine-related adverse reactions in clinical practice, we captured from the Vaccine Adverse Event Reporting System (VAERS) all adverse events following immunization (AEFIs) reported with the BNT162b2 and the mRNA-1273 COVID-19 vaccines in the real clinical setting from December 2020 to October 15, 2021.
2. Methods
Post-vaccination data were collected from the Vaccine Adverse Event Reporting System (VAERS) [11], which is one of the largest databases freely and readily available to the public. VAERS is the national reporting system designed to detect early safety warnings for vaccines after they are authorised or licensed for use by the U.S. regulatory agency, and currently even for COVID-19 vaccines available after issuance of an EUA. It is co-managed by the Centers for Disease Control and Prevention (CDC) and the Food and Drug Administration (FDA), as part of the larger vaccine safety system in the United States that helps make sure vaccines are safe [12]. Adverse event information captured from VAERS includes the type of vaccine received, the date of immunization, the receipt date, patient’s characteristics such as age class and sex, and a description of the toxicity, including time of onset and seriousness according to the U.S. Code of Federal Regulations [13]. Of the three vaccines to date available in the United States to protect against COVID-19 disease, we focused on the analysis of the BNT162b2 and the mRNA-1273 vaccines. Considering that both mRNA vaccines received an Emergency Use Authorization on December 2020, we take into account a period between that month and October 15, 2021.
2.1. Descriptive analysis
In order to collect reports of adverse events concerning people who received an mRNA-based COVID-19 vaccine, we searched the VAERS database by selecting all AEFI reports, regardless of location, with BNT162b2 or mRNA-1273 vaccines reported as suspected medicinal products submitted up to the cut-off date of October 15, 2021. Since a VAERS report may include one or more adverse events, we counted separately all events reported after receipt of mRNA vaccines, regardless of the time of AEFI onset since vaccination. Based on the reports collected, we performed a descriptive analysis to define the distribution by sex and age class of individuals who experienced at least one adverse event after receiving a COVID-19 vaccine dose. For each vaccine under review, it was checked if the most occurring events were listed in the corresponding Summary of the Product Characteristics (SPCs) to ascertain the notoriety of the adverse reactions.
2.2. Statistical analysis
For adverse events with reporting frequency ≥ 2, a case-non-case analysis was performed by using the Reporting Odds Ratio (ROR) with 95 % confidence interval as statistical parameter. Case-non-case studies are among the most used methods to assess drug safety in daily clinical setting. The aim is to compare the incidence of a drug-related adverse event of interest with the incidence of the same adverse reaction in the remaining records of the database. However, since the medicinal products analysed are vaccines, we considered the use of the whole database to be inappropriate. Therefore, the statistical analysis was carried out by comparing the two mRNA vaccines. If ROR is > 1, a disproportion for the vaccine-event pair can be assumed and an early warning of a potential safety problem with a vaccine can be provided.
3. Results
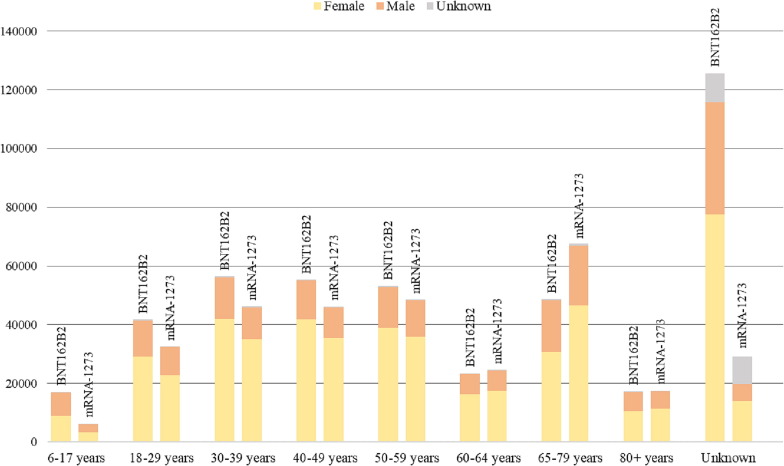
All reports collected from VAERS are first classified by patient age and sex (see Fig. 1 and Table S1 in the Supplementary Appendix). The most reported AEFIs that occurred after immunization with the BNT162b2 or the mRNA-1273 COVID-19 vaccines are listed in Table 1 , while the disproportionality analysis results are shown, with a focus on the most statistically significant events, in Table 2 . For both Table 1, Table 2, the data were processed through the exclusion of those events that were not true adverse reactions, such as “accidental underdose”, “product use issue”, “SARS-CoV-2 test”, “pulmonary physical examination” and several others. Lastly, Table 3 shows the Reporting Odds Ratios of adverse events of special interest determined for both mRNA vaccines.
Fig 1.
Age and sex of patients who experienced at least one adverse event following immunization with the BNT162b2 and the mRNA-1273 COVID-19 vaccines.
Table 1.
Most reported adverse events for the BNT162b2 and the mRNA-1273 COVID-19 vaccines in VAERS.
| Events | N | % | Events | N | % | ||
|---|---|---|---|---|---|---|---|
| BNT162b2 | Headache | 75,455 | 3.63 | mRNA-1273 | Headache | 57,089 | 4.19 |
| Fatigue | 62,762 | 3.02 | Pyrexia | 52,635 | 3.86 | ||
| Pyrexia | 56,432 | 2.71 | Fatigue | 48,706 | 3.57 | ||
| Dizziness | 45,777 | 2.20 | Chills | 44,465 | 3.26 | ||
| Nausea | 44,114 | 2.12 | Pain | 39,224 | 2.88 | ||
| Pain | 42,801 | 2.06 | Pain in extremity | 33,578 | 2.46 | ||
| Chills | 42,232 | 2.03 | Nausea | 32,558 | 2.39 | ||
| Pain in extremity | 35,572 | 1.71 | Dizziness | 28,105 | 2.06 | ||
| Dyspnoea | 29,064 | 1.40 | Myalgia | 23,411 | 1.72 | ||
| Arthralgia | 28,267 | 1.36 | Injection site pain | 23,274 | 1.71 | ||
| Myalgia | 27,889 | 1.34 | Injection site erythema | 22,693 | 1.67 | ||
| COVID-19 | 23,226 | 1.12 | Arthralgia | 19,572 | 1.44 | ||
| Body temperature | 22,649 | 1.09 | Rash | 18,660 | 1.37 | ||
| Malaise | 20,281 | 0.98 | Pruritus | 18,373 | 1.35 | ||
| Asthenia | 19,648 | 0.95 | Injection site swelling | 17,606 | 1.29 | ||
| Rash | 18,184 | 0.87 | Injection site pruritus | 16,556 | 1.21 | ||
| Vomiting | 17,778 | 0.86 | Dyspnoea | 15,490 | 1.14 | ||
| Paraesthesia | 17,119 | 0.82 | Erythema | 14,623 | 1.07 | ||
| Pruritus | 16,335 | 0.79 | Asthenia | 12,889 | 0.95 | ||
| Chest pain | 16,274 | 0.78 | Vomiting | 12,785 | 0.94 | ||
| Diarrhoea | 15,618 | 0.75 | Injection site warmth | 11,978 | 0.88 | ||
| Lymphadenopathy | 15,539 | 0.75 | Diarrhoea | 10,489 | 0.77 | ||
| Hypoaesthesia | 14,948 | 0.72 | Peripheral swelling | 10,016 | 0.73 | ||
| Cough | 13,843 | 0.67 | Urticaria | 9613 | 0.71 | ||
| Vaccination site pain | 13,092 | 0.63 | Injection site rush | 9401 | 0.69 | ||
| Syncope | 12,601 | 0.61 | Feeling abnormal | 9385 | 0.69 | ||
| SARS-CoV-2 test positive | 12,403 | 0.60 | Malaise | 9310 | 0.68 | ||
| Hyperhidrosis | 12,382 | 0.60 | Hyperhidrosis | 8871 | 0.65 | ||
| Feeling abnormal | 11,780 | 0.57 | Vaccination site pain | 8788 | 0.64 | ||
| Injection site pain | 11,240 | 0.54 | Paraesthesia | 8744 | 0.64 | ||
| Palpitations | 10,918 | 0.53 | Lymphadenopathy | 8688 | 0.64 | ||
| Urticaria | 10,891 | 0.52 | COVID-19 | 8333 | 0.61 | ||
| Chest discomfort | 10,025 | 0.48 | Hypoaesthesia | 8131 | 0.60 | ||
| Erythema | 9504 | 0.46 | Cough | 7453 | 0.55 | ||
| Back pain | 8310 | 0.40 | Chest pain | 7406 | 0.54 | ||
| Tinnitus | 8279 | 0.40 | Rash erythematous | 6700 | 0.49 | ||
| Heart rate increased | 8147 | 0.39 | Swelling | 6343 | 0.47 | ||
| Peripheral swelling | 7912 | 0.38 | SARS-CoV-2 test positive | 6104 | 0.45 | ||
| Oropharyngeal pain | 7905 | 0.38 | Skin warm | 5976 | 0.44 | ||
| Loss of consciousness | 7782 | 0.37 | Decreased appetite | 5741 | 0.42 | ||
| Tremor | 7738 | 0.37 | Vaccination site erythema | 5669 | 0.42 | ||
| Drug ineffective | 7645 | 0.37 | Tremor | 5588 | 0.41 | ||
| Condition aggravated | 7204 | 0.35 | Back pain | 5586 | 0.41 | ||
| Feeling hot | 6836 | 0.33 | Syncope | 5553 | 0.41 | ||
| Hypertension | 6644 | 0.32 | Palpitations | 5538 | 0.41 | ||
| Neck pain | 6617 | 0.32 | Chest discomfort | 5421 | 0.40 | ||
| Tachycardia | 6554 | 0.32 | Influenza like illness | 5235 | 0.38 | ||
| Decreased appetite | 6520 | 0.31 | Feeling hot | 5205 | 0.38 | ||
| Influenza like illness | 6330 | 0.30 | Rash pruritic | 5172 | 0.38 | ||
| Blood pressure increased | 6264 | 0.30 | Heart rate increased | 5074 | 0.37 |
Table 2.
Adverse events with higher Reporting Odds Ratio (ROR) reported with mRNA-1273 compared to BNT162b2 and reported with BNT162b2 compared to mRNA-1273.
| Events | N mRNA-1273 |
N BNT162b2 |
ROR | CI_low | CI_up | Events | N BNT162b2 |
N mRNA-1273 |
ROR | CI_low | CI_up | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mRNA-1273 as compared to BNT162b2 | Application site swelling | 15 | 1 | 22.88 | 1.94 | 269.45 | BNT162b2 as compared to mRNA-1273 | Disease recurrence | 1625 | 15 | 71.07 | 59.41 | 85.03 |
| Injection site papule | 46 | 4 | 17.54 | 8.05 | 38.23 | Gamma-glutamyltransferase increased | 106 | 2 | 34.75 | 10.78 | 112.00 | ||
| Injection site rash | 9401 | 1057 | 13.65 | 13.36 | 13.96 | Drug ineffective | 7645 | 186 | 27.04 | 26.16 | 27.95 | ||
| Injection site pruritus | 16,556 | 1917 | 13.32 | 13.11 | 13.54 | Acute stress disorder | 41 | 1 | 26.88 | 2.79 | 259.16 | ||
| Injection site induration | 4492 | 521 | 13.19 | 12.76 | 13.63 | Mutism | 38 | 1 | 24.91 | 2.55 | 243.06 | ||
| Injection site hypersensitivity | 144 | 17 | 12.92 | 9.78 | 17.07 | Multisystem inflammatory syndrome in children |
35 | 1 | 22.95 | 2.32 | 226.88 | ||
| Injection site warmth | 11,978 | 1509 | 12.21 | 11.97 | 12.44 | Sleep disorder due to a general medical condition | 34 | 1 | 22.29 | 2.24 | 221.46 | ||
| Injection site inflammation | 967 | 122 | 12.10 | 11.18 | 13.09 | Disseminated bacillus Calmette-Guerin infection | 30 | 1 | 19.67 | 1.94 | 199.70 | ||
| Vaccination site anaesthesia | 27 | 4 | 10.30 | 4.33 | 24.51 | Base excess | 133 | 5 | 17.44 | 9.94 | 30.59 | ||
| Injection site irritation | 242 | 36 | 10.26 | 8.56 | 12.28 | Sleep disorder due to general medical condition, inomnia type |
52 | 2 | 17.05 | 4.87 | 59.60 | ||
| Injection site erythema | 22,693 | 3456 | 10.17 | 10.03 | 10.31 | Anti-platelet antibody | 74 | 3 | 16.17 | 6.70 | 39.03 | ||
| Injection site cellulitis | 305 | 46 | 10.12 | 8.66 | 11.81 | Reaction to excipient | 49 | 2 | 16.06 | 4.56 | 56.62 | ||
| Administration site pain | 37 | 6 | 9.41 | 4.92 | 18.00 | Blood pressure diastolic decreased | 69 | 3 | 15.08 | 6.20 | 36.69 | ||
| Growing pains | 18 | 3 | 9.15 | 3.00 | 27.92 | Cerebellar haemorrhage | 46 | 2 | 15.08 | 4.24 | 53.64 | ||
| Injection site plaque | 12 | 2 | 9.15 | 1.95 | 42.94 | Ocular vascular disorder | 46 | 2 | 15.08 | 4.24 | 53.64 | ||
| Injection site reaction | 4002 | 670 | 9.14 | 8.83 | 9.45 | Complicated appendicitis | 21 | 1 | 13.77 | 1.26 | 149.90 | ||
| Injection site urticaria | 1175 | 209 | 8.58 | 8.03 | 9.17 | International normalised ratio abnoraml | 21 | 1 | 13.77 | 1.26 | 149.90 | ||
| Injection site swelling | 17,606 | 3426 | 7.93 | 7.81 | 8.05 | Dyslalia | 61 | 3 | 13.33 | 5.40 | 32.93 | ||
| Injection site pustule | 30 | 6 | 7.63 | 3.85 | 15.12 | Cerebellar syndrome | 20 | 1 | 13.11 | 1.19 | 144.28 | ||
| Endoscopy upper gastrointestinal tract abnormal | 10 | 2 | 7.63 | 1.54 | 37.77 | Viral pharyngitis | 20 | 1 | 13.11 | 1.19 | 144.28 | ||
| Left ventricular enlargement | 10 | 2 | 7.63 | 1.54 | 37.77 | Pharyngeal disorder | 38 | 2 | 12.46 | 3.40 | 45.61 | ||
| Nasal mucosal blistering | 10 | 2 | 7.63 | 1.54 | 37.77 | Pharyngeal inflammation | 38 | 2 | 12.46 | 3.40 | 45.61 | ||
| Vaccination complication | 2789 | 603 | 7.07 | 6.79 | 7.36 | Allergic reaction to excipient | 19 | 1 | 12.46 | 1.12 | 138.64 | ||
| Injection site oedema | 176 | 38 | 7.07 | 5.79 | 8.62 | Upper airway obstruction | 19 | 1 | 12.46 | 1.12 | 138.64 | ||
| Optical coherence tomography abnoraml | 9 | 2 | 6.86 | 1.34 | 35.15 | Macroglossia | 18 | 1 | 11.80 | 1.05 | 132.97 |
Table 3.
Reporting Odds Ratios of adverse events of special interest as measured by comparing BNT162b2 with mRNA-1273 (ROR1) and vice-versa (ROR2).
| Events | N BNT162b2 | N mRNA-1273 | ROR1 | ROR2 | CI_low1 | CI_up1 | CI_low2 | CI_up2 |
|---|---|---|---|---|---|---|---|---|
| Myocarditis | 3989 | 1310 | 2.00 | 0.50 | 1.93 | 2.06 | 0.47 | 0.53 |
| Pericarditis | 2785 | 743 | 2.46 | 0.41 | 2.36 | 2.56 | 0.38 | 0.44 |
| Bell's palsy | 2891 | 1417 | 1.34 | 0.75 | 1.29 | 1.39 | 0.71 | 0.79 |
| Guillain-Barré syndrome | 843 | 295 | 1.87 | 0.53 | 1.74 | 2.02 | 0.48 | 0.60 |
| Anaphylactic reaction | 5004 | 1233 | 2.66 | 0.38 | 2.59 | 2.74 | 0.35 | 0.40 |
| Anaphylactic shock | 700 | 142 | 3.23 | 0.31 | 2.96 | 3.53 | 0.26 | 0.37 |
| Thrombosis | 3291 | 1291 | 1.67 | 0.60 | 1.61 | 1.73 | 0.57 | 0.63 |
| Deep vein thrombosis | 3156 | 1086 | 1.91 | 0.52 | 1.84 | 1.98 | 0.49 | 0.56 |
| Thrombocytopenia | 1424 | 403 | 2.31 | 0.43 | 2.19 | 2.45 | 0.39 | 0.48 |
3.1. Descriptive analysis for the BNT162b2 vaccine
At the data cut-off date of October 15, 2021, and after 243,856,565 doses of the Pfizer-BioNTech candidate have been administered [12], a total of 439,401 individuals experienced at least one adverse event following immunization with the BNT162b2 vaccine (Table S1). Reports for male patients were 131,024 (29.82 %) and those for females were 296,124 (67.39 %). For 12,253 reports (2.79 %), sex of the patients was not stated. Over half of the patients (230,505; 52.46 %) belonged to the age class between 18 and 64 years old. Of the remaining reports, 17,263 (3.93 %) concerned patients aged less than 18 years and 66,035 (15.03 %) people aged 65 years or above. Based on the information retrieved from VAERS, it was not possible to trace the age range of as many as 125,598 individuals (28.58 %). The number of reports related to BNT162b2 submitted to VAERS increased until April (1.79 % reports received in December 2020, 7.24 % in January, 7.44 % in February, 10.02 % in March, 12.53 % in April 2021), and then remained stable in the following months of the study period (11.57 % in May, 11.33 % in June, 12.28 % in July, 12.44 % in August, 11.58 % in September 2021). Overall, 2,078,743 adverse events were reported for the Pfizer-BioNTech vaccine and were described as non-serious for 353,618 patients (80.48 %). As shown in Table 1, the top five most common reaction were headache (75,455 events; 3.63 %), fatigue (62,762; 3.02 %), pyrexia (56,432; 2.71 %), dizziness (45,777; 2.20 %), and nausea (44,114; 2.12 %). For the majority of BNT162b2 recipients (166,896; 37.98 %), the reported events were early onset and observed on the same day of immunization.
3.2. Descriptive analysis for the mRNA-1273 vaccine
Between December 18, 2020, and October 15, 2021, a total of 155,267,673 Moderna vaccine doses were administered [12] and 318,639 recipients reported a related adverse event to VAERS (Table S1). Most of the reports (221,940; 69.65 %) concerned female patients, while only 85,011 (26.68 %) were related to males. For 11,688 (3.67 %) sex was not reported. Regarding the belonging age class, 198,064 patients (62.16 %) were homogeneously distributed in the range 18–64 years, 85,075 (26.70 %) were aged 65 years and over, and 6,266 (1.97 %) were under 18 years of age. In the other 29,234 cases (9.17 %), the information about the patient's age class was missing. Most reports related to the mRNA-1273 vaccine were received from January to April (12.01 % in January, 10.89 % in February, 12.44 % in March, 12.92 % in April 2021) and in August 2021 (25.66 %). Vaccine-related adverse events that have been recovered overall from VAERS were 1,362,754 and occurred on the same day patients received the administration into 115,774 (36.33 %) of them. The most frequently reported events were headache (57,089 events; 4.19 %), pyrexia (52,635; 3.86 %), fatigue (48,706; 3.57 %), chills (44,465; 3.26 %), and pain (39,224; 2.88 %) (Table 1). Among all patients immunized with the mRNA-1273 vaccine, 30,573 (9.59 %) experienced serious reactions.
3.3. Statistical analysis
The disproportionality study was performed by comparing the incidence rate of AEFIs related to the mRNA-1273 vaccine with that reported for the BNT162b2 vaccine and vice-versa. Overall, there were 477 statistically significant events reported for the Moderna vaccine and 1,759 for the Pfizer-BioNTech one. As shown in Table 2, among the mRNA-1273 vaccine-event pairs with higher ROR, we found application site swelling [ROR 22.88; 95 % confidence interval (CI), 1.94 to 269.45], injection site papule (17.54; 8.05 to 38.23), injection site rash (13.65; 13.36 to 13.96), injection site pruritus (13.32; 13.11 to 13.54), and injection site induration (13.19; 12.76 to 13.63). The results of the analysis for the BNT162b2 vaccine revealed in the top places events such as disease recurrence (ROR 71.07; 95 % CI, 59.41 to 85.03), gamma-glutamyltransferase increased (34.75; 10.78 to 112.00), drug ineffective (27.04; 26.16 to 27.95), acute stress disorder (26.88; 2.79 to 259.16), and mutism (24.91; 2.55 to 243.06). Other AEFIs we have focused on, given the latest safety concerns from other post-approval pharmacovigilance activities, were myocarditis, pericarditis, Bell’s palsy, Guillain-Barré syndrome, anaphylactic reaction, anaphylactic shock, thrombosis, deep vein thrombosis, and thrombocytopenia. For such events a disproportionality was found for the BNT162b2 vaccine as compared to the mRNA-1273 vaccine (Table 3).
4. Discussion
From December 2020 through October 15, 2021, 399,124,238 total doses of mRNA COVID-19 vaccines were administered in the United States. Of these, 243,856,565 were BNT162b2 and 155,267,673 mRNA-1273 vaccines [14].
Giving the gravity of the public health emergency and the importance of making a vaccine available as soon as possible, the U.S. Food and Drug Administration (FDA) recommended in October 2020 that data from phase 3 studies included a median follow-up duration of at least 2 months after completion of the full vaccination regimen to support distribution of an investigational vaccine under EUA [15]. To counterbalance the extremely short time frame that led to the necessary safety data to COVID-19 vaccines authorisation, programs of passive pharmacovigilance for the monitoring of adverse events were implemented and are still warranted to ensure a favourable risk–benefit ratio.
The analysis of safety surveillance data from VAERS highlights that female population was more prone to express an adverse event after being immunized with either mRNA vaccines as compared to the male population. This sex difference could be the result of a greater predisposition of females to develop an AEFI following both BNT162b2 and mRNA-1273 vaccines, or a greater proneness of women to report any possible adverse events than men. Already during the first 6 months of the U.S. COVID-19 vaccination programme, a remarkably excess in the rates of adverse events emerged in female individuals for both mRNA vaccines [16]. Furthermore, the survey by Green et al based on Israeli data also confirmed a higher rate of adverse events after immunization with the Pfizer-BioNTech vaccine in females than in males [17].
Patients who experienced an AEFI with either product were mostly aged ≥ 18 years, with the highest proportion among Moderna recipients between 65 and 79 years. Since December 2020, the Pfizer-BioNTech and the Moderna vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) became available under EUA as a two-dose primary series for individuals over 16 and 18 years of age, respectively [5], [8], with older people first in line. Later, on May 10, 2021, FDA amended the Emergency Use Authorization for BNT162b2 to include those 12 through 15 years of age [18], and this accounts for the higher number of reports belonging to the age class 6–17 years (17,090; 3.89 %) compared with the mRNA-1273 vaccine (6,172; 1.94 %).
Most reported adverse events in VAERS were almost the same for each mRNA vaccine. Notably, the top eight AEFIs by frequency of reporting related to both Pfizer-BioNTech and Moderna vaccines were headache, fatigue, pyrexia, dizziness, nausea, pain, chills, and pain in extremity. Such pattern is largely consistent with systemic vaccine reactogenicity and with safety data that formed the basis for emergency or conditional authorisations globally. On the Summary of Product Characteristics (SPCs) events such headache, fatigue, pyrexia, and chills are listed as very common adverse reactions (≥1/10) for both medicinal products, while dizziness and pain in extremity are included among uncommon adverse reactions (≥1/1,000 to less than 1/100) for mRNA-1273 and BNT162b2, respectively [19], [20]. In comparison, data from VAERS, covering a period of about 10 months, show for these events lower incidence rates than those highlighted by the safety profile of both SPCs, which may be related to the voluntary nature of VAERS reports and to a minimal under-reporting expected for non-serious events.
From the case-non-case analysis, performed by using the Reporting Odds Ratio (ROR), a disproportionality has arisen for the mRNA-1273 vaccine compared to BNT162b2 for many vaccination site reactions like application site swelling, injection site papule, injection site rash, injection site pruritus, and injection site induration. Although the local reactogenicity profile of mRNA vaccines did not differ substantially, these data might suggest a higher association of injection site side effects with the Moderna product than with the Pfizer-BioNTech one. Consistent with our findings, local injection site reactions as well as systemic reactions were found more frequently after mRNA-1273 versus BNT162b2 during the initial 6 months of U.S. safety monitoring, especially among female participants and individuals younger than 65 years [16]. The safety profile of the mRNA-1273 vaccine for the median 2-months follow up showed that adverse events at the injection site were mainly grade 1 or 2 in severity and lasted a few days from the onset [4].
Following the widespread introduction of mRNA vaccines, some adverse events occurring after immunization emerged as possible rare side effects leading to active surveillance programs to further investigate whether the signal represented an actual risk. Some AEFIs reported after the receipt of messenger RNA vaccines that most of all have led to great concern were myocarditis, pericarditis, Bell’s palsy, Guillain-Barré syndrome, anaphylaxis, and thrombosis. On the basis of our statistical analysis, such events seem to be more associated with the BNT162b2 vaccine than with the mRNA-1273 vaccine. For myocarditis and pericarditis, Gallo et al also found a consistent largely increased risk for the Pfizer-BioNTech vaccine compared to the Moderna vaccine among men aged 18–24 years [21]. As both findings are from a retrospective analysis, additional studies are required to better asses the observed link.
After early reports of a small number of cases of cardiac inflammation after the Pfizer-BioNTech vaccine emerged in Israel as early as late April [22], a “likely association” between mRNA vaccines and myocarditis and pericarditis was declared by the CDC Advisory Committee on Immunization Practices on June 23, 2021 [23]. According to the World Health Organization/International Society and Federation of Cardiology Task Force [24], myocarditis is defined as an inflammatory disorder of the heart muscle and commonly manifest itself in chest pain, shortness of breath, and feelings of having a fast-beating, fluttering, or pounding heart [25]. Although individual reports of myocarditis in our study were not stratified according to sex and age class, several studies have already reported that the highest incidence of cases that occurred in temporal proximity to the vaccination are observed among male adolescents and young adults within the first week after the second vaccine dose [26], [27]. According to data also drawn from the Vaccine Adverse Event Reporting System, the CDC estimated in June 2021 the link between COVID-19 mRNA vaccines and myocarditis at an incidence of about 4.78 cases per million overall [28]. Although a disproportion of myocarditis has emerged for BNT162b2 compared to the mRNA-1273 vaccine (ROR 2.00; 95 % CI, 1.93 to 2.06), vaccine-related myocarditis remains a rare event compared with the millions who have been given the vaccine. Furthermore, as reported by the president of the British Cardiovascular Society to The BMJ, myocarditis is for the vast majority of people a benign, self-limiting condition that can be easily treated with NSAIDs [23].
After administration of 13.8 million doses of Pfizer-BioNTech and Moderna COVID-19 vaccines to the U.S. population during the first month of the vaccination program, cases of confirmed anaphylaxis have been observed with a CDC-estimated incidence of 4.5 per million doses administered [29]. Anaphylaxis is a life-threating multisystem reaction that occur rarely after vaccination, with onset typically within minutes to hours [30]. Overall, reports of anaphylactic reactions following mRNA vaccination submitted to VAERS until the cut-off date were 6,237. The incidence rate of anaphylaxis, which we estimated to be 15.63 per million mRNA vaccine doses, appears to be approximately 10 times as high as the incidence reported in the first month of COVID-19 vaccine safety monitoring. However, since we have considered all the cases of anaphylactic reaction retrieved from VAERS, our estimate rate might be higher than the actual one. In the analysis of 6-months safety surveillance data from the Vaccine Safety Datalink, Klein et al evaluated an incidence rate of confirmed anaphylaxis with Brighton Collaboration criteria at 4.8 per million BNT162b2 doses and 5.1 per million mRNA-1273 doses [10]. Our findings, in contrast, showed a significant higher rate of anaphylactic reactions reported after administration of the Pfizer-BioNTech vaccine compared to the Moderna vaccine (ROR 2.66; 95 % CI, 2.59 to 2.74). mRNA vaccines are built on the same lipid-based nanoparticle delivery system; however, they differ in the respective lipid mixtures, and it is possible that such component accounts for different incidence rates. Although the technology behind mRNA vaccines is not new, it was not previously used in licensed vaccines. There is therefore no prior experience that informs the likelihood or explains the mechanism of allergic reactions with mRNA vaccines [31]. According to worldwide reports [10], [32], [33], however, there is evidence that most cases of anaphylaxis after receipt of either mRNA vaccines occurred in female patients, which might suggest a potential involvement of hormonal regulation in the development of anaphylaxis. Despite this, anaphylaxis is a treatable condition and the adherence to the current CDC guidance on use of mRNA COVID-19 vaccines [34] and management of anaphylaxis [35] allows to halt the progression of life-threating symptoms in the great majority of cases.
The phase 3 trials of both COVID-19 mRNA vaccines noted an imbalance of cases of Bell’s palsy in the vaccine group compared with the placebo group [3], [4]. Nonetheless, a causal relationship could not be established and continued monitoring was strongly recommended for facial paralysis. On October 15, 2021, we identified 4,308 cases (0.13 %) of Bell’s palsy among the 3,441,497 AEFIs reported for both vaccines. In a previous disproportionality analysis performed through the Bayesian neural network method, facial paralysis-related events were estimated to be 0.63 % of the total adverse events reported for either mRNA vaccine in the World Health Organization pharmacovigilance database [36]. Although the study did not specify the actual number of cases of Bell’s palsy, which is an idiopathic form, this estimate is somewhat higher than that in our survey. These data suggest greater confidence against the initial concern regarding a higher risk of Bell’s palsy after mRNA vaccination.
Other serious adverse events that may occur after COVID-19 vaccination, though rarely, are thrombosis with thrombocytopenia syndrome (TTS) and Guillain-Barré syndrome (GBS), which were reported mostly in people who got the Johnson & Johnson’s Janssen vaccine [37]. As of October 15, 2021, a total of 4,582 cases of thrombosis following administration of either mRNA vaccine were recovered from VAERS. Nevertheless, the reporting rate found in the present safety surveillance analysis certainly exceeds the actual cases of vaccine-related TTS. The definition of thrombosis with thrombocytopenia syndrome (TTS), also known as vaccine-induced immune thrombotic thrombocytopenia (VITT), is based on the combined presence of a thrombosis and new onset thrombocytopenia. Its diagnosis requires the presence of anti-PF4/heparin antibodies detected by ELISA or other laboratory investigations, including D-dimer test and serotonin release assay. To support the definitive diagnosis, all potential cases of TTS usually undergo a case review by health professionals to confirm that patients have been vaccinated against COVID-19 within last 30 days and to rule out those events with alternative explanation for the condition, such as an heparin exposure within the previous 100 days [38]. Since cases of thrombosis submitted to VAERS were not confirmed as consistent with TTS according to such criteria, we were not able to compare the actual reporting rate of TTS for mRNA vaccines with that estimated by the CDC last December for the Janssen COVID-19 vaccine [39]. However, based on available data, there is not an increased risk for TTS after mRNA COVID-19 vaccination, which is actually recommended by CDC and FDA for some patients, especially women ages 30–49 years, who are at higher risk of developing this rare adverse event [37].
To conclude, several important limitations of the present post-marketing safety surveillance survey should be considered. The U.S. Vaccine Adverse Event Reporting System (VAERS) accepts voluntary reports of adverse events that occur following vaccination from healthcare professionals, vaccine manufacturers, and the public. Vaccine providers and recipients are encouraged to report any clinically significant health problem that occur after the administration of any vaccine, whether or not they believe the vaccine was the cause, and this leads to potential biases. In addition, the reports may include incomplete, inaccurate, coincidental, and unverified information, which could make difficult to obtain satisfactory data and also prevent duplicate detection [40]. A notable strength of the present survey is that under-reporting, at least for serious events, is expected to be very low as the data were collected during the most intense safety monitoring campaign ever. Although VAERS alone cannot be used to establish a causal relationship between a vaccine and an adverse event, it is able as an early warning system to provide preliminary information on possible safety issues with a vaccine.
5. Conclusion
Our post-marketing surveillance survey has provided further evidence on the safety profile of mRNA vaccines in real-world clinical settings. Even if no evident safety issues emerged, a disproportionality was found for BNT162b2 compared to mRNA-1273 for some events of interest, such as myocarditis, Bell’s palsy, and anaphylactic reaction. Nevertheless, the known health benefits of mRNA vaccination still far overweight the known risks of COVID-19 illness and its related possibly severe complications, such as long-term health problems, hospitalization, and even death.
6. Statements and declarations
Ethics approval and patient consent: The manuscript does not contain clinical studies or patient data. For this type of study, ethics committee approval and formal consent are not required.
Availability of data: The data that support the findings of this study are available from the corresponding author upon reasonable request.
Authors’ Contributions: Substantial contributions to conception or design of the work (D.M., G.S.L., NM, MB), or the acquisition (G.B.), analysis (G.S.L., G.B., D.M.) or interpretation of data for the work (G.S.L., D.M., N.M., G.B., M.B.). Drafting of the work (G.S.L., D.M.) or revising it critically for important intellectual content (N.M., M.B., G.B.). All authors approved the submitted final version to be published. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding information: no external funding source, and no editorial or manuscript support.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2023.03.054.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- 1.World Health Organization. WHO Director-General’s opening remarks at the media briefing on COVID-19 - 11 March 2020. February 8, 2022. (https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020).
- 2.Thanh Le T., Andreadakis Z., Kumar A., Gómez Román R., Tollefsen S., Saville M., et al. The COVID-19 vaccine development landscape. Nat Rev Drug Discov. 2020;19(5):305–306. doi: 10.1038/d41573-020-00073-5. [DOI] [PubMed] [Google Scholar]
- 3.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oliver S., Gargano J., Marin M., Wallace M., Curran K.G., Chamberland M., et al. The Advisory Committee on Immunization Practices' Interim Recommendation for Use of Pfizer-BioNTech COVID-19 Vaccine - United States, December 2020. MMWR Morb Mortal Wkly Rep. 2020;69(50):1922–1924. doi: 10.15585/mmwr.mm6950e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Food and Drug Administration. Comirnaty and Pfizer-BioNTech COVID-19 Vaccine. February 8, 2022. (https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/comirnaty-and-pfizer-biontech-covid-19-vaccine).
- 7.Krause P.R., Gruber M.F. Emergency Use Authorization of Covid Vaccines - Safety and Efficacy Follow-up Considerations. N Engl J Med. 2020;383(19):e107. doi: 10.1056/NEJMp2031373. [DOI] [PubMed] [Google Scholar]
- 8.Oliver S., Gargano J., Marin M., Wallace M., Curran K.G., Chamberland M., et al. The Advisory Committee on Immunization Practices' Interim Recommendation for Use of Moderna COVID-19 Vaccine - United States, December 2020. MMWR Morb Mortal Wkly Rep. 2021;69(5152):1653–1656. doi: 10.15585/mmwr.mm695152e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Food and Drug Administration. Spikevax and Moderna COVID-19 Vaccine. February 8, 2022. (https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/spikevax-and-moderna-covid-19-vaccine).
- 10.Klein N.P., Lewis N., Goddard K., Fireman B., Zerbo O., Hanson K.E., et al. Surveillance for Adverse Events After COVID-19 mRNA Vaccination. JAMA. 2021;326(14):1390. doi: 10.1001/jama.2021.15072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. About The Vaccine Adverse Event Reporting System (VAERS). February 10, 2022. (https://wonder.cdc.gov/controller/datarequest/D8;jsessionid=C2D605C8C4483B0B97F1BCDCAA8C).
- 12.Centers for Disease Control and Prevention. Vaccine Adverse Event Reporting System (VAERS). February 10, 2022. (https://www.cdc.gov/vaccinesafety/ensuringsafety/monitoring/vaers/index.html).
- 13.Food and Drug Administration. CFR - Code of Federal Regulations Title 21. February 10, 2022. (https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=312.32).
- 14.Centers for Disease Control and Prevention. COVID-19 Vaccinations in the United States. October 15, 2021. (https://covid.cdc.gov/covid-data-tracker/#vaccinations_vacc-total-admin-rate-total).
- 15.Food and Drug Administration. Emergency Use Authorization for Vaccines to Prevent COVID-19. February 28, 2022. (https://www.fda.gov/regulatory-information/search-fda-guidance-documents/emergency-use-authorization-vaccines-prevent-covid-19).
- 16.Rosenblum H.G., Gee J., Liu R., Marquez P.L., Zhang B., Strid P., et al. Safety of mRNA vaccines administered during the initial 6 months of the US COVID-19 vaccination programme: an observational study of reports to the Vaccine Adverse Event Reporting System and v-safe. Lancet Infect Dis. 2022;22(6):802–812. doi: 10.1016/S1473-3099(22)00054-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Green M.S., Peer V., Magid A., Hagani N., Anis E., Nitzan D. Gender Differences in Adverse Events Following the Pfizer-BioNTech COVID-19 Vaccine. Vaccines (Basel) 2022;10(2):233. doi: 10.3390/vaccines10020233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Food and Drug Administration. FDA Approves First COVID-19 Vaccine. February 25, 2022. (https://www.fda.gov/news-events/press-announcements/fda-approves-first-covid-19-vaccine).
- 19.European Medicine Agency. Comirnaty® Summary of Product Characteristics. March 3, 2022. (https://www.ema.europa.eu/en/documents/product-information/comirnaty-epar-product-information_en.pdf).
- 20.European Medicine Agency. Spikevax® Summary of Product Characteristics. March 3, 2022. (https://www.ema.europa.eu/en/documents/product-information/spikevax-previously-covid-19-vaccine-moderna-epar-product-information_en.pdf).
- 21.Gallo K., Goede A., Mura C., Abel R., Moahamed B., Preissner S., et al. A Comparative Analysis of COVID-19 Vaccines Based on over 580,000 Cases from the Vaccination Adverse Event Reporting System. Vaccines (Basel) 2022;10(3):408. doi: 10.3390/vaccines10030408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ministry of Health. Surveillance of Myocarditis (Inflammation of the Heart Muscle) Cases Between December 2020 and May 2021 (Including). March 5, 20(https://www.gov.il/en/departments/news/01062021-03).
- 23.Wise J. Covid-19: Should we be worried about reports of myocarditis and pericarditis after mRNA vaccines? BMJ. 2021;373 doi: 10.1136/bmj.n1635. [DOI] [PubMed] [Google Scholar]
- 24.Richardson P., McKenna W., Bristow M., et al. Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the Definition and Classification of cardiomyopathies. Circulation. 1996;93:841–842. doi: 10.1161/01.cir.93.5.841. [DOI] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention. Myocarditis and Pericarditis After mRNA COVID-19 Vaccination. March 8, 2022. (https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/myocarditis.html).
- 26.Witberg G., Barda N., Hoss S., Richter I., Wiessman M., Aviv Y., et al. Myocarditis after Covid-19 Vaccination in a Large Health Care Organization. N Engl J Med. 2021;385(23):2132–2139. doi: 10.1056/NEJMoa2110737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mevorach D., Anis E., Cedar N., Bromberg M., Haas E.J., Nadir E., et al. Myocarditis after BNT162b2 mRNA Vaccine against Covid-19 in Israel. N Engl J Med. 2021;385(23):2140–2149. doi: 10.1056/NEJMoa2109730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wallace M, Oliver S. COVID-19 mRNA vaccines in adolescents and young adults: benefit-risk discussion. March 8, 2022. (https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-06/05-COVID-Wallace-508.pdf).
- 29.Gee J., Marquez P., Su J., Calvert G.M., Liu R., Myers T., et al. First Month of COVID-19 Vaccine Safety Monitoring - United States, December 14, 2020-January 13, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(8):283–288. doi: 10.15585/mmwr.mm7008e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McNeil M.M., DeStefano F. Vaccine-associated hypersensitivity. J Allergy Clin Immunol. 2018;141(2):463–472. doi: 10.1016/j.jaci.2017.12.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Longo D.L., Castells M.C., Phillips E.J. Maintaining Safety with SARS-CoV-2 Vaccines. N Engl J Med. 2021;384(7):643–649. doi: 10.1056/NEJMra2035343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sobczak M., Rafał P.R. The risk of anaphylaxis behind authorized COVID-19 vaccines: a meta-analysis. Clin Mol Allergy. 2022;20(1):1. doi: 10.1186/s12948-022-00167-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Somiya M., Mine S., Yasukawa K., Ikeda S. Sex differences in the incidence of anaphylaxis to LNP-mRNA COVID-19 vaccines. Vaccine. 2021;39(25):3313–3314. doi: 10.1016/j.vaccine.2021.04.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Centers for Disease Control and Prevention. Interim Clinical Considerations for Use of COVID-19 Vaccines Currently Approved or Authorized in the United States. March 10, 2022. (https://www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html).
- 35.Centers for Disease Control and Prevention. Interim Considerations: Preparing for the Potential Management of Anaphylaxis after COVID-19 Vaccination. March 10, 2022. (https://www.cdc.gov/vaccines/covid-19/clinical-considerations/managing-anaphylaxis.html).
- 36.Renoud L., Khouri C., Revol B., Lepelley M., Perez J., Roustit M., et al. Association of Facial Paralysis With mRNA COVID-19 Vaccines: A Disproportionality Analysis Using the World Health Organization Pharmacovigilance Database. JAMA Intern Med. 2021;181(9):1243. doi: 10.1001/jamainternmed.2021.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Centers for Disease Control and Prevention. Selected Adverse Events Reported after COVID-19 Vaccination. March 14, 2022. (https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/adverse-events.html).
- 38.World Health Organization. Guidance for clinical case management of thrombosis with thrombocytopenia syndrome (TTS) following vaccination to prevent coronavirus disease (COVID-19). March 14, 2022. (https://www.who.int/publications/i/item/WHO-2019-nCoV-TTS-2021.1).
- 39.See I. CDC Awardee COVID-19 Vaccination Planning Meeting. March 14, 2022. (https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-12-16/02-COVID-See-508.pdf).
- 40.Vaccine Adverse Event Reporting System. VAERS Data. March 15, 2022. (https://vaers.hhs.gov/data.html).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.