Abstract
Background
Central Africa is one of the largest areas of high endemicity for human T-cell leukemia virus-1 (HTLV-1). However, no preventive measures are yet implemented to reduce its transmission, which can be sexual, from mother-to-child, or through contaminated blood products. Rare zoonotic transmissions from nonhuman primates (NHPs) have also been reported in this region. Here we investigated the HTLV-1 prevalence and associated risk factors in a rural population in Cameroon.
Methods
From 2019 to 2021, we performed a cross-sectional survey in the eastern region of Cameroon. HTLV-1 infection was first screened by ELISA, then tested by western blot and envelope gene targeted polymerase chain reaction. Risk factors associated with HTLV-1 infection were identified by logistic regression in univariable and multivariable analyses.
Results
Among 3400 participants, HTLV-1 prevalence was 1.1% (95% confidence interval [CI], .7–1.5). Factors independently associated with HTLV-1 infection were Pygmy ethnicity (adjusted odd ratio [aOR], 2.9; 95% CI, 1.3–6.2), history of surgery (aOR, 6.3; 95% CI, 2.2–17.8), and NHP bite (aOR, 6.6; 95% CI, 2.2–19.8).
Conclusions
These results suggest both iatrogenic and zoonotic transmission of HTLV-1 in Cameroon. Further studies are needed to assess the risk of nosocomial transmission of HTLV-1, to guide public health authorities in implementing preventive measures to control HTLV-1 transmission.
Keywords: Cameroon, HTLV-1, central Africa, cross-sectional survey, epidemiology, nosocomial, zoonoses
Among 3,400 rural adult participants from East Cameroon, HTLV-1 prevalence was 1.1%. Independent factors associated with the infection were Pygmy ethnicity, history of surgery and non-human primate bite, suggesting both nosocomial and zoonotic transmission of HTLV-1 in this region.
Human T-cell leukemia virus type 1 (HTLV-1) infects at least 5 to 10 million people worldwide, with specific foci of high prevalence, such as Japan, South and Central America, and sub-Saharan Africa [1]. HTLV-1 infection remains asymptomatic in the majority of cases but can lead to 2 severe and irreversible diseases: a lymphoproliferative malignancy (adult T-cell leukemia) and a chronic neuromyelopathy (tropical spastic paraparesis/HTLV-1–associated myelopathy). HTLV-1 infection is also associated to other inflammatory diseases (such as uveitis, infective dermatitis, or bronchiectasis) [2]. While the lifetime risk of developing associated diseases is estimated to be around 4%–7%, the infection has been associated with a higher, as yet unexplained, mortality rate [3].
HTLV-1 infection can be acquired through sexual intercourse, primarily from men to women; from mothers to infants during prolonged breastfeeding; or through parenteral transmission, such as through contaminated blood transfusions, organ transplants, or needle sharing among drug users. It is generally accepted that in the few endemic countries where blood screening for HTLV-1 is implemented for blood donors (such as Japan, Brazil, United States, and some Caribbean and European countries) and for pregnant women (in Japan and French overseas territories), transmission of HTLV-1 is mainly through sexual intercourse. However, in most of the other endemic countries, especially in the African continent, the role of each mode of transmission and the associated risk factors remain largely unknown. Furthermore, in central Africa, which represents the largest high HTLV-1 endemic area, zoonotic transmission from infected nonhuman primates (NHP) to humans is also possible. Indeed, simian T-cell leukemia virus type 1 (STLV-1), the HTLV-1 simian homologue, infects a large variety of NHPs in the Old World. In central Africa, HTLV-1 and STLV-1 strains are close and cannot be separated into distinct phylogenetic lineages according to species of origin. Indeed, some human and simian strains are identical [4]. By convention, the names HTLV and STLV refer to the species in which the virus was identified. However, zoonotic transmissions have been very rarely reported and only in central Africa, in populations frequently in contact with NHPs, particularly hunters, and have been associated with NHP bites [5].
Central Africa is a hotspot for HTLV-1 infection with prevalence as high as 5%–15% in the adult rural population of Gabon [6, 7]. In Cameroon, previous studies have shown a prevalence ranging from 1% to 2% in the adult population, with higher prevalence in the Pygmy ethnic group [8–11]. However, the risk factors associated with HTLV-1 infection in this country remain largely unknown. Furthermore, despite this relatively high prevalence, there are currently no preventive measure to control HTLV-1 transmission. Identification of risk factors is fundamental to better understand the mode of transmission of HTLV-1 in the population, which will help public health authorities implement preventive measure to control HTLV-1 transmission.
METHODS
Study Population
Villages and rural roadside settlements were invited to participate in the study, and all volunteers over the age of 15 years were included. Epidemiological data, including demographic information, medical history, and contacts with wild animals (especially monkeys and apes) during hunting and/or butchering activities, were collected on a standardized questionnaire. A whole-blood sample of 5–10 mL was collected in EDTA tubes from all participants. Samples were transported in coolers and centrally processed 48 to 72 hours after sampling. Plasma and peripheral blood buffy coat (PBBC) were obtained and stored frozen at −80°C.
Ethics
Ethical approval was obtained from the Institutional Review Board of Institut Pasteur and the Comité National d’Ethique du Cameroun (permit number 019/02/1136/CE/CNERSH/SP). Prior to field sampling, detailed information and explanation of the study were provided to local authorities, the village chief, and the community, and written informed consent was obtained from all participants. Written informed consent was obtained from the parents or legal guardians of minor children. Free medical consultation was offered to all participants.
HTLV-1 Serology and Molecular Testing
Plasma samples were subjected to an enzyme-linked immunosorbent assay (ELISA; HTLV-I/II ELISA 4.0; MP Biomedicals) for the detection of HTLV-1/2 antibodies. All reactive samples were then subjected to a confirmatory western blot assay (HTLV BLOT 2.4; MP Biomedicals). Profiles were defined according to the manufacturer’s instructions. For all samples with a nonnegative profile, high-molecular-weight DNA was extracted from the PBBC using the QIAamp blood minikit (Qiagen). Polymerase chain reaction (PCR) was performed to amplify an 885-bp fragment of the env gene with primers Env11 and Env22, as previously described [12]. Amplicons were visualized by electrophoresis in a 1.5% agarose gel, stained with ethidium bromide, and amplicons of expected size were sent to the Eurofins sequencing platform at Cochin Hospital (Paris, France) for sequencing. HTLV-1 genotypes were identified by BLAST searches of the obtained sequences, using the GenBank database (http://www.ncbi.nlm.nih.gov/BLAST) and a phylogenetic tree was constructed from the sequence alignment of the 522-bp env fragment obtained by the maximum likelihood method, with Seaview version 4 software.
A positive HTLV-1 infection was defined as an HTLV-1–like western blot profile (HTLV-1, HTLV-1/2, HTLV) or an indeterminate western blot profile associated with a positive PCR.
Statistical Analyses
HTLV-1 prevalence and 95% confidence intervals (CIs) were calculated and compared between groups using the χ2 test or Fisher exact test, accordingly. We determined odds ratios (OR) using logistic regression. All variables with a P value <.20 were included in the multivariable analyses. Variables retained in the final model were considered statistically significant if the P values were <.05. Multiple imputations were performed to replace missing values for the variable “hospitalization with or without surgery,” using the multivariable imputation by chained equations (MICE) method [13]. All analyses were performed using STATA 15.0 software (Stata Corporation).
RESULTS
Description of the Population
Between March 2019 and March 2021, the study was conducted in 71 villages (Supplementary Figure 1). A total of 3433 individuals participated in the survey, of which 33 were rejected because of missing or incomplete questionnaire (31) or missing blood samples (2). The analysis was therefore carried out on 3400 people, aged between 15 and 90 years (mean age, 39.9 years), of whom 1850 were women (54%) and 1550 men. Of these, 2630 (77%) were from the local Bantu ethnic group, 656 (19%) from the Baka Pygmy ethnic group, 108 (3%) were from another region of Cameroon, and for 6 information was not available (Table 1).
Table 1.
HTLV-1 Prevalence According to Demographic Characteristics
| Characteristics | No. (%) | nHTLV-1 | Prevalence, % | OR (95% CI) | P Value |
|---|---|---|---|---|---|
| Total | 3400 (100) | 36 | 1.1 | ||
| Sex | |||||
| ȃMale | 1550 (46) | 14 | 0.9 | 1 | .42 |
| ȃFemale | 1850 (54) | 22 | 1.2 | 1.3 (.7–2.6) | … |
| Age category, y | |||||
| ȃ15–30 | 1260 (37) | 9 | 0.7 | 1 | .03* |
| ȃ31–50 | 1206 (35) | 10 | 0.8 | 1.2 (.5–2.9) | … |
| ȃ51–90 | 934 (27) | 17 | 1.8 | 2.6 (1.1–5.8) | … |
| Ethnic group | |||||
| ȃBantu | 2630 (77) | 22 | 0.8 | 1 | .006** |
| ȃPygmy | 656 (19) | 14 | 2.1 | 2.6 (1.3–5.1) | … |
| ȃOthers | 108 (3) | 0 | 0 | … | … |
| ȃUnknown | 6 (<1) | 0 | 0 | … | … |
| Department | |||||
| ȃHaut-Nyong | 1669 (49) | 8 | 0.5 | 1 | .002** |
| ȃKadey | 252 (7) | 1 | 0.4 | 0.8 (.1–6.6) | … |
| ȃBoumba and Ngoko | 1479 (44) | 27 | 1.8 | 3.9 (1.8–8.5) | … |
Abbreviations: CI, confidence interval; HTLV-1, human T-cell leukemia virus; nHTLV-1, number of infected individuals; OR, odds ratio. *P < .05, **P < .01.
Frequency of Potential Risk Factors
Of the potential risk factors associated with HTLV-1, 62% of participants reported a history of hospitalization and 13% a history of surgery. The most common surgery was hernia repair, which accounted for 63% of all surgeries, followed by gyneco-obstetric surgeries (26%). Other surgical procedures included tumors, abscesses, or trauma. Blood transfusion was reported by 3% of participants, mostly women, and was primarily associated with anemia during pregnancy or blood loss during delivery. The other most common reasons for transfusion were malaria infection, surgery, and traumatic accident.
Cultural scarification was reported by 77% participants and was more common in the Pygmy ethnic group (96%) than in the Bantu ethnic group (73%) but was as equally common in men and women. Exposure to wildlife, particularly NHPs, was extremely frequent in the study population, whether through consumption, bushmeat cutting, or hunting practices. The frequency of hunting was 83% among Bantu men and 92% among Pygmy men, while 26 and 68% of Bantu and Pygmy women, respectively, had ever or regularly accompanied men on hunts. Almost all participants consumed NHP meat (92%) and 55% of men had ever hunted NHPs. NHP injuries were reported by 59 individuals for bites (41 monkey, 16 gorilla, and 2 chimpanzee) and 11 for scratches (7 monkey and 5 gorilla). Most injuries occurred during hunting or accidental encounters, while one-fifth of bites were inflicted by NHPs kept as pets. Overall, nearly 3% of the study population reported ever keeping NHPs as pets in households.
HTLV-1 Serology and Genotypes
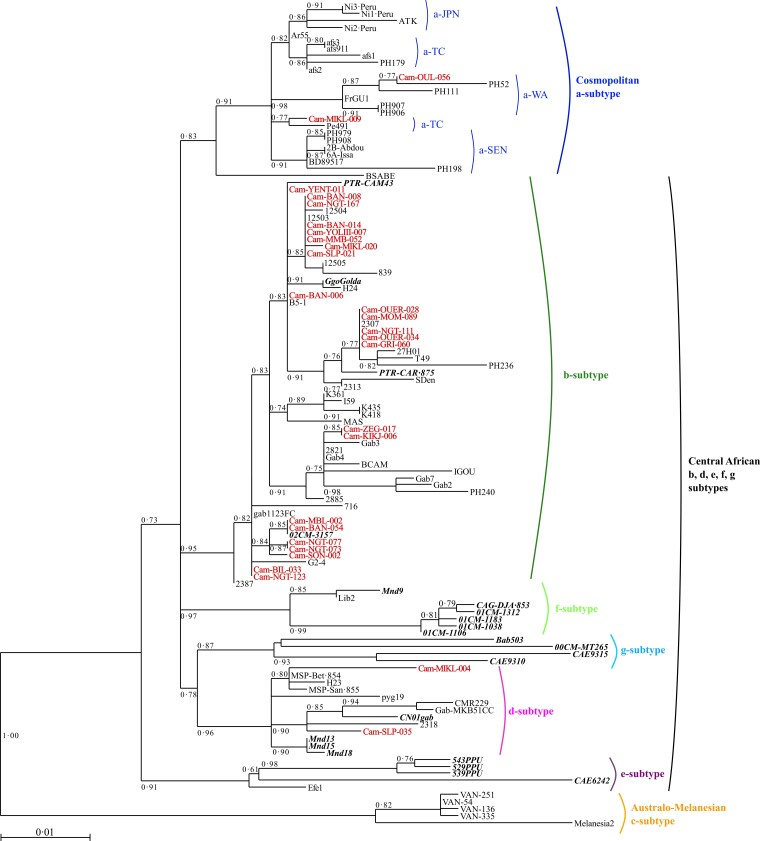
Antibodies against HTLV-1 were detected in 403 plasma samples (12%) with the ELISA test. Of these, HTLV-1 infection was confirmed by western blot for 35 (8.5%), having either an HTLV-1 (29), HTLV (2), or HTLV-1/2 (4) profile. Two had an HTLV-2 profile (0.7%), 199 had an indeterminate profile (49%), and 166 were negative (41%). Molecular amplification was performed on DNA extracted from the PBBC of all nonnegative western blots, for which a buffy coat was available (236/237). The HTLV-1 env fragment was amplified for 27 samples (24 HTLV-1, 2 HTLV-1/2, and 1 indeterminate) (Supplementary Table 1). Of these, 23 were of the central African HTLV-1b subtype, with 98.1%–100% nucleotide identity. Two belonged to the central African HTLV-1d subtype with 97.5% nucleotide identity, and the other 2 belonged to the cosmopolitan HTLV-1a subtype (1 from the West African group and 1 from the transcontinental group), with 98.2% nucleotide identity. HTLV-1b and d subgroups have been found in Bantu and Pygmy individuals, while the 2 HTLV-1a strains have been found only in Bantus. There is high variability in the b genotype, and the new strains are grouped into different clusters, supported by high bootstrap values, containing both human and simian strains (Figure 1).
Figure 1.
Phylogenetic tree generated by the maximum likelihood method with a 522-bp fragment of the env gene. Phylogenetic comparisons were performed with the 522-nucleotide env gp21 gene fragment obtained from 113 human T-cell leukemia virus 1 (HTLV-1) isolates, including the 27 sequences from this study (in red) and 86 previously published sequences. The GenBank accession numbers of the new sequences from the rural population of Cameroon are ON130946–ON130972. Simian T-cell leukemia virus 1 (STLV-1) strains are shown in bold italic typeface. The phylogeny was derived by the maximum likelihood method with the generalized time reversible (GTR) model. Horizontal branch lengths are drawn to scale, with the bar indicating 0.01 nucleotide replacements per site. Maximum posterior probabilities were calculated and reported on the maximum likelihood tree (threshold value ≥0.50). a-TC, a-JPN, a-SEN, a-WA, correspond to the transcontinental, Japanese, Senegalese, and West African clades of the a-genotype, respectively.
Considering all HTLV-1–like western blot profiles (HTLV-1, HTLV-1/2, and HTLV) and positive PCR for indeterminate profiles, HTLV-1 infection was detected in 36 individuals, giving a prevalence of 1.06% (95% CI, 0.7%–1.5%; Supplementary Table 2).
Factors Associated With HTLV-1 Infection
The prevalence of HTLV-1 increased with age (> 50 years) and was higher in the Pygmy ethnic group and in the Boumba and Ngoko department (Table 1). There was no difference in prevalence between men and women. In univariable analyses, HTLV-1 infection was associated with history of hospitalization (with or without history of surgery) and marginally with transfusion, but not with scarification. HTLV-1 infection was marginally associated with history of transfusion, only among those who received more than 1 blood poach (Table 2). Among surgical procedures, only hernia surgery was associated with a higher risk of HTLV-1 infection, while obstetric procedures (such as cesarean delivery, cystectomy) were not.
Table 2.
Univariable Analysis of Iatrogenic Risk Factors Associated With HTLV-1 Infection
| Risk Factor | No. (%) | nHTLV-1 | Prevalence, % | OR (95% CI) | P Value |
|---|---|---|---|---|---|
| Total | 3400 (100) | 36 | 1.1 | ||
| History of hospitalization and surgery | |||||
| ȃNever hospitalized | 1282 (38) | 6 | 0.5 | 1 | .009** |
| ȃHospitalization without surgery | 1463 (43) | 18 | 1.2 | 2.7 (1.1–6.7) | … |
| ȃHospitalization with history of surgery | 453 (13) | 11 | 2.4 | 5.3 (2.0–14.4) | … |
| ȃHospitalization, history of surgery unknown | 196 (6) | 1 | 0.5 | 1.1 (.1–9.1) | … |
| ȃHospitalization unknown | 6 (0.2) | 0 | 0 | … | … |
| Number of hospitalizations | |||||
| ȃ0 | 1284 (38) | 6 | 0.5 | 1 | .04* |
| ȃ1 | 1011 (30) | 18 | 1.8 | 3.9 (1.5–9.8) | … |
| ȃ2–4 | 681 (20) | 8 | 1.2 | 2.5 (.9–7.3) | … |
| ȃ≥5 | 321 (9) | 4 | 1.2 | 2.7 (.8–9.6) | … |
| ȃNA | 103 (3) | 0 | 0 | … | … |
| Number of surgeries | |||||
| ȃ0 | 2746 (81) | 24 | 0.9 | 1 | .04* |
| ȃ1 | 371 (11) | 9 | 2.4 | 2.8 (1.3–6.1) | … |
| ȃ2 | 84 (2) | 1 | 1.2 | 1.4 (.2–10.2) | … |
| ȃ3–4 | 27 (1) | 1 | 3.7 | 4.4 (.6–33.5) | … |
| ȃNA | 172 (5) | 1 | 1 | … | … |
| Surgery | |||||
| ȃHernia | 308 (9) | 8 | 2.6 | 2.9 (1.3–6.4) | .007** |
| ȃGyneco-obstetric | 128 (4) | 2 | 1.6 | 1.3 (.3–5.5) | .75 |
| ȃOthera | 44 (1) | 2 | 4.5 | 4.6 (1.1–19.6) | .02* |
| ȃNot specified | 10 (0.3) | 0 | 0 | … | … |
| Transfusion | |||||
| ȃNo | 3269 (96) | 33 | 1 | 1 | .09 |
| ȃYes | 112 (3) | 3 | 2.7 | 2.7 (.8–9.0) | … |
| ȃUnknown | 19 (1) | 0 | 0 | … | … |
| Number of blood pouches | |||||
| ȃ0 | 3269 (97) | 33 | 1 | 1 | .001*** |
| ȃ1 | 33 (1) | 0 | 0 | … | … |
| ȃ2–4 | 39 (1) | 3 | 7.7 | 8.2 (2.4–27.9) | … |
| ȃUnknown | 40 (1) | 0 | 0 | … | … |
| Scarification | |||||
| ȃNo | 759 (22) | 5 | 0.7 | 1 | .24 |
| ȃYes | 2608 (77) | 30 | 1.2 | 1.8 (.7–4.5) | … |
| ȃUnknown | 33 (1) | 1 | 3.0 | … | … |
Abbreviations: CI, confidence interval; HTLV-1, human T-cell leukemia virus; NA, not available; nHTLV-1, number of infected individuals; OR, odds ratio. *P < .05; **P < .01; ***P < .001.
Others surgery included road accident, abdominal, urological surgery (appendicitis), abscesses.
Among practices involving contact with wild animals, HTLV-1 infection was associated in univariable analyses with practices such as accompanying hunting, hunting apes, keeping an NHP as a pet (both great apes and monkeys), injuries during bushmeat butchering, and bites from an NHP. The association was stronger for bites inflicted by gorillas than by monkey species. The small number of individuals bitten by chimpanzees does not allow us to conclude any association with HTLV-1 infection. For some individuals, information on bite history was either missing or suspected but not confirmed. The high prevalence observed in this group could therefore be related to an undisclosed bite incident but cannot be confirmed. In addition, we found no association with bushmeat consumption or butchering, either for all wild animals or specifically for NHP (Table 3).
Table 3.
Univariable Analysis of Zoonotic Risk Factors Associated With HTLV-1 Infection
| Risk Factors | No. (%) |
nHTLV-1 | Prevalence, % | OR (95% CI) | P Value |
|---|---|---|---|---|---|
| Hunting | |||||
| ȃNo | 2075 (61) | 25 | 1.2 | 1 | .45 |
| ȃYes | 1314 (38.6) | 11 | 0.8 | .7 (.3–1.4) | … |
| ȃUnknown | 11 (0.3) | 0 | 0.0 | … | … |
| Accompanying to hunt | |||||
| ȃNo | 2393 (70.4) | 19 | 0.8 | 1 | .04* |
| ȃYes | 779 (22.9) | 14 | 1.8 | 2.3 (1.1–4.6) | … |
| ȃUnknown | 228 (6.7) | 3 | 1.3 | 1.7 (.5–5.7) | … |
| Hunting NHPa | |||||
| ȃNo | 2097 (61.7) | 17 | 0.8 | 1 | .16 |
| ȃYes | 1133 (33.3) | 15 | 1.3 | 1.6 (.8–3.3) | … |
| ȃUnknown | 170 (5.0) | 4 | 2.4 | … | … |
| Hunting monkeys | |||||
| ȃNo | 2111 (62) | 17 | 0.8 | 1 | .14 |
| ȃYes | 1119 (33) | 15 | 1.3 | 1.7 (.8–3.4) | … |
| ȃUnknown | 170 (5) | 4 | 2.4 | … | … |
| Hunting apes | |||||
| ȃNo | 3004 (88) | 23 | 0.8 | 1 | <.001*** |
| ȃYes | 319 (9) | 10 | 3.1 | 4.2 (2.0–8.9) | … |
| ȃUnknown | 77 (2) | 3 | 3.9 | … | … |
| Bushmeat cutting | |||||
| ȃNo | 518 (15.2) | 6 | 1.2 | 1 | .81 |
| ȃYes | 2880 (84.7) | 30 | 1.0 | .9 (.4–2.2) | … |
| ȃUnknown | 2 (0.1) | 0 | 0.0 | … | … |
| Injury while cutting | |||||
| ȃNo | 1603 (47.1) | 13 | 0.8 | 1 | .15 |
| ȃYes | 1727 (50.8) | 23 | 1.3 | 1.7 (.8–3.3) | … |
| ȃUnknown | 70 (2.1) | 0 | 0.0 | … | … |
| Cutting NHP meat | |||||
| ȃNo | 1062 (31.2) | 12 | 1.1 | 1 | .72 |
| ȃYes | 2316 (68.1) | 23 | 1.0 | .9 (.4–1.8) | … |
| ȃUnknown | 22 (0.6) | 1 | 4.5 | … | … |
| Consumption of NHP meat | |||||
| ȃNo | 255 (7.5) | 2 | 0.8 | 1 | .65 |
| ȃYes | 3138 (92.3) | 34 | 1.1 | 1.4 (.3–5.8) | … |
| ȃUnknown | 7 (0.2) | 0 | 0.0 | … | … |
| Keeping an NHP as pets | |||||
| ȃNo | 3301 (97.1) | 32 | 1.0 | 1 | .002** |
| ȃYes | 95 (2.8) | 4 | 4.2 | 4.5 (1.6–13.0) | … |
| ȃUnknown | 4 (0.1) | 0 | 0.0 | … | … |
| Bitten by an NHP | |||||
| ȃNo | 3300 (97.1) | 29 | 0.9 | 1 | <.001*** |
| ȃYes | 59 (1.7) | 4 | 6.8 | 8.2 (2.8–24.2) | … |
| ȃUnknown | 41 (1.2) | 3 | 7.3 | … | … |
| NHP bite category | |||||
| ȃNo bite | 3300 (97.1) | 33 | 0.9 | 1 | <.001*** |
| ȃMonkey bite | 41 (1.2) | 1 | 2.4 | 2.8 (.4–21.2) | … |
| ȃGorilla bite | 16 (0.5) | 3 | 18.8 | 26 (7–96) | … |
| ȃChimp bite | 2 (0.06) | 0 | 0 | … | … |
| ȃUnknown | 41 (1.2) | 3 | 7.3 | … | … |
| Scratched by an NHP | |||||
| ȃNo | 3347 (98.5) | 33 | 1.0 | 1 | NS |
| ȃYes | 11 (0.3) | 0 | 0 | … | … |
| ȃUnknown | 41 (1.2) | 3 | 7.3 | … | … |
Abbreviations: CI, confidence interval; HTLV-1, human T-cell leukemia virus; nHTLV-1, number of infected individuals; NS, not significant; OR, odds ratio.
Nonhuman primate including apes and monkeys. *P < .05; **P < .01; ***P < .001.
Variables included in the initial multivariable models were age category, ethnic group, history of hospitalization and/or surgery, history of transfusion, accompaniment to hunting, NHP hunting (apes only), keeping an NHP as pet, NHP bite, and injury while butchering bushmeat. Factors independently associated with HTLV-1 infection in the multivariable analysis were ethnic group (Pygmy aOR, 2.9), history of hospitalization with surgery (aOR, 6.3), and a history of NHP bite (aOR, 6.6) (Table 4).
Table 4.
Multivariable Analysis Factors Associated With HTLV-1 Infection
| Variables | aOR | 95% CI | P Value |
|---|---|---|---|
| Ethnic group | |||
| ȃBantu | 1 | … | … |
| ȃPygmy | 2.9 | 1.3–6.2 | .007** |
| History of hospitalization and surgery | |||
| ȃNever hospitalized | 1 | … | .002** |
| ȃHospitalization without surgery | 2.4 | .9–6.2 | … |
| ȃHospitalization with history of surgery | 6.3 | 2.2–17.8 | … |
| Bitten by an NHP | |||
| ȃNo | 1 | … | … |
| ȃYes | 6.6 | 2.2–19.8 | .001** |
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval. **P < .01.
DISCUSSION
Our large survey in eastern Cameroon showed a high prevalence of HTLV-1 in the adult rural population. HTLV-1 prevalence was higher in the Pygmy ethnic population and was associated with a history of surgery and NHP bites, suggesting both iatrogenic and zoonotic transmission of HTLV-1. A higher prevalence in the Pygmy ethnic group has been previously reported in studies conducted in central Africa and may indicate either a founder effect driving this difference or higher risk factors leading to a higher rate of exposure and transmission in this population [6, 8, 9]. Although in endemic areas HTLV-1 prevalence increases with age and is generally higher in women, we observed no sex difference in our study, similarly to previous studies in Cameroon [8, 10]. One possible explanation could be that, while sexual transmission results in higher prevalence in women, hunting activities are more frequent among men, who therefore are exposed to a higher risk of zoonotic transmission.
Central African STLV-1 and HTLV-1 strains (b and d–f genotypes), sequenced in NHPs and humans, respectively, are genetically close with a few indistinguishable strains. Infection with these strains could result from a zoonotic transmission, while on the contrary, the cosmopolitan a genotype, which has only been sequenced in humans, could only be acquired from interhuman transmission. Zoonotic transmission has already been demonstrated in individuals frequently in contact with NHPs, with a higher risk of infection in individuals bitten by NHPs in Cameroon [5]. This bite transmission may be enabled by the presence of infected cells, either in the saliva or in the oral blood of the NHPs, as has been demonstrated in humans [14, 15]. In our study, gorilla bites result in a significantly higher risk of HTLV-1 transmission compared to monkey bites. STLV-1 have been detected in several gorillas in Cameroon; however, the low number of tested individuals is not sufficient to conclude on a higher prevalence in wild gorilla populations [16]. This difference could also be the result of an increasing risk of transmission with the severity of the bite, as observed in a previous study in Cameroon [5]. However, other contacts, such as scratching, cutting up bushmeat, or eating NHP meat, do not appear to represent a risk of HTLV-1 transmission, consistent with other studies [6, 17, 18]. Despite the likely high prevalence of STLV-1 and frequent contact through hunting in various rural sub-Saharan African population, zoonotic transmission appears to be rather rare and has been reported in only a few studies, in Cameroon, Gabon, and Guinea-Bissau [5, 6, 19, 20]. Nevertheless, the extremely high frequency of contact with NHPs in this rural population, and the strong association of HTLV-1 prevalence with NHP bites in our study in Cameroon, highlight a significant risk of zoonotic transmission in this rural population, and may underline a higher exposure to other simian virus, particularly foamy virus, HTLV-3, and HTLV-4 [21–23].
Furthermore, in our study, a history of surgery, particularly hernia repair, emerged as an independent risk factor for HTLV-1. The association with HTLV-1 infection and history of hospitalization or surgery have been reported in very few studies in Africa. Only 2 studies, conducted in rural and urban adult populations of Gabon and Guinea-Bissau, respectively, showed that the number of hospitalizations was an independent risk factor for HTLV-1 infection [6, 20]. In Gabon, history of hospitalization and surgery were also suggested as potential risk factors in univariable analyses by another study conducted in a rural adult population, while in Peru, history of dental surgeries was associated with HTLV-1 prevalence in pregnant women [7, 24].
Parenteral transmission of HTLV-1 is primarily known to occur through untested blood transfusion via infected lymphocytes [25]. Blood donations are not currently tested for HTLV-1 in Cameroon, and to date there has been no study to assess the prevalence of HTLV-1 in blood donors and the resulting risk of parenteral transmission in the country. In our study, this risk appeared elevated as HTLV-1 prevalence was 7.7% among recipients of 2 or more blood pouches. However, in the multivariable analysis, history of blood transfusion was not associated with HTLV-1 infection whereas a history of surgery, particularly for hernia, was a risk factor, independent of blood transfusion. Hernia surgery is the most common surgery and may be performed in local health centers or during health campaigns. This result could therefore suggest past iatrogenic transmission of HTLV-1, through the use of infected syringes or nonsterile equipments. However, this result should be interpreted with caution. Indeed, while the cross-sectional approach of this survey does not allow us to determine the chronology between an identified risk factor and the acquisition of the infection, it seems unlikely that HTLV-1 infection could result in an increased risk of hernia. On the other hand, the pathology could also lead the patient to first consult a traditional healer with risky practices before hospitalization or surgery. The origin of such iatrogenic transmission of HTLV-1 should be thus studied more in detail by further specific work.
Viral transmission through shared needles has been demonstrated primarily for HTLV-2 infection among intravenous drug users, and the high prevalence of HTLV-1 among drug users tends to suggest parenteral transmission of HTLV-1 as well [26, 27]. This is also supported by 2 studies, both performed in older populations, showing an association between HTLV-1 infection and a history of intravenous injection or blood transfusion in Democratic Republic of Congo, and intramuscular injection for trypanosomiasis prophylactic treatment in Central African Republic [28, 29]. In addition, some practices performed in specific communities have also been suggested as a risk factor for HTLV-1 infection in different countries, such as ornamental scarification in Guinea-Bissau or self-inflicted flagellation in the United Kingdom [20, 30].
Our current results show evidence of both nosocomial and zoonotic transmission of HTLV-1 in the rural population of central Africa. In these populations, the major modes of HTLV-1 transmission are the sexual and mother-to-child routes, as there is little to no preventive measures against sexually transmitted infections and no alternative to breast feeding. Although the nosocomial and zoonotic transmissions are therefore minor compared to these modes, these are significant findings with a clear public health impact. Firstly, the high frequency of contact with NHPs in this population and the resulting zoonotic transmission of HTLV-1 suggest a significant risk of subsidiary transmission of other zoonotic pathogens (in particular other retroviruses, such as foamy virus, HTLV-3, and HTLV-4) and emphasize the risk of emergence of new pathogens. Secondly, while HTLV-1 infection has been previously associated with a history of hospitalization and/or surgery in sub-Saharan Africa, iatrogenic transmission of HTLV-1 remains poorly studied and could be relatively easily prevented. Our results confirm transmission of HTLV-1 in rural settings and highlight the substantial need for further assessment to identify procedures at risk for iatrogenic transmission and alert public health authorities to issue specific public health guidance to reduce HTLV-1 transmission in the population.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Jill-Léa Ramassamy, Unité d’Epidémiologie et Physiopathologie des Virus Oncogènes, Institut Pasteur, Université de Paris Cité, CNRS UMR 3569, Paris, France.
Chanceline Bilounga Ndongo, Direction de la Lutte Contre la Maladie, les Epidémies et les Pandémies. Ministère de la Santé Publique, Yaoundé, Cameroun; Faculté de Médecine et des Sciences Pharmaceutiques, l’Université de Douala, Douala, Cameroun.
Patrick Nnuka, Direction de la Lutte Contre la Maladie, les Epidémies et les Pandémies. Ministère de la Santé Publique, Yaoundé, Cameroun.
Maëlle Antunes, Unité d’Epidémiologie et Physiopathologie des Virus Oncogènes, Institut Pasteur, Université de Paris Cité, CNRS UMR 3569, Paris, France.
Margot Le Mener, Unité d’Epidémiologie et Physiopathologie des Virus Oncogènes, Institut Pasteur, Université de Paris Cité, CNRS UMR 3569, Paris, France.
Edouard Betsem a Betsem, Université de Yaoundé I, Yaoundé, Cameroun.
Richard Njouom, Unité de Virologie, Centre Pasteur du Cameroun, Yaoundé, Cameroun.
Olivier Cassar, Unité d’Epidémiologie et Physiopathologie des Virus Oncogènes, Institut Pasteur, Université de Paris Cité, CNRS UMR 3569, Paris, France.
Arnaud Fontanet, Unité de Recherche et d’Expertise Epidémiologie des Maladies Emergentes, Institut Pasteur, Paris, France; Conservatoire National des Arts et Métiers, Unité PACRI, Paris, France.
Antoine Gessain, Unité d’Epidémiologie et Physiopathologie des Virus Oncogènes, Institut Pasteur, Université de Paris Cité, CNRS UMR 3569, Paris, France.
Notes
Acknowledgments. We thank Dr Melo Forchu Ozeru and Dr Otseng Abbe Arthur for their clinical expertise during the different field surveys. We also thank the Institut de Recherche pour le Développement in Yaoundé and all the members of the Virology unit of Centre Pasteur du Cameroun for their logistical assistance.
Disclaimer . The funders of this study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Financial support. This work was supported by the European Union as part of the EboSursy project (grant number FOOD/2016/379-660 to J. L. R.); and the Institut Pasteur, France and the Centre National de la Recherche Scientifique, UMR 3569, through the Investissement d’Avenir as part of a Laboratoire d’Excellence French research program, Integrative Biology of Emerging Infectious Diseases (grant number ANR10-LBX- 62 IBEID to A. G.). Funding to pay the Open Access publication charges for this article was provided by the European Union.
References
- 1. Gessain A, Cassar O. Epidemiological aspects and World distribution of HTLV-1 infection. Front Microbiol 2012; 3:388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Proietti FA, Carneiro-Proietti ABF, Catalan-Soares BC, Murphy EL. Global epidemiology of HTLV-I infection and associated diseases. Oncogene 2005; 24:6058. [DOI] [PubMed] [Google Scholar]
- 3. Schierhout G, McGregor S, Gessain A, Einsiedel L, Martinello M, Kaldor J. Association between HTLV-1 infection and adverse health outcomes: a systematic review and meta-analysis of epidemiological studies. Lancet Infect Dis 2020; 20:133–43. [DOI] [PubMed] [Google Scholar]
- 4. Mahieux R, Chappey C, Georges-Courbot M-C, et al. . Simian T-cell lymphotropic virus type 1 from Mandrillus sphinx as a simian counterpart of human T-cell lymphotropic virus type 1 subtype D. J Virol 1998; 72:10316–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Filippone C, Betsem E, Tortevoye P, et al. . A severe bite from a nonhuman primate is a major risk factor for HTLV-1 infection in hunters from Central Africa. Clin Infect Dis 2015; 60:1667–76. [DOI] [PubMed] [Google Scholar]
- 6. Djuicy DD, Mouinga-Ondémé A, Cassar O, et al. . Risk factors for HTLV-1 infection in Central Africa: a rural population-based survey in Gabon. PLoS Negl Trop Dis 2018; 12:e0006832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Caron M, Besson G, Padilla C, et al. . Revisiting human T-cell lymphotropic virus types 1 and 2 infections among rural population in Gabon, Central Africa thirty years after the first analysis. PLoS Negl Trop Dis 2018; 12:e0006833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Filippone C, Bassot S, Betsem E, et al. . A new and frequent human T-cell leukemia virus indeterminate western blot pattern: epidemiological determinants and PCR results in Central African inhabitants. J Clin Microbiol 2012; 50:1663–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mauclère P, Le Hesran J-Y, Mahieux R, et al. . Demographic, ethnic, and geographic differences between human T cell lymphotropic virus (HTLV) type I-seropositive carriers and persons with HTLV-I gag-indeterminate western blots in Central Africa. J Infect Dis 1997; 176:505–9. [DOI] [PubMed] [Google Scholar]
- 10. Mauclère P, Afonso PV, Meertens L, et al. . HTLV-2B strains, similar to those found in several Amerindian tribes, are endemic in Central African Bakola pygmies. J Infect Dis 2011; 203:1316–23. [DOI] [PubMed] [Google Scholar]
- 11. Rodgers MA, Vallari AS, Harris B, et al. . Identification of rare HIV-1 group N, HBV AE, and HTLV-3 strains in rural south Cameroon. Virology 2017; 504:141–51. [DOI] [PubMed] [Google Scholar]
- 12. Vermeulen M, Sykes W, Coleman C, et al. . The prevalence of human T-lymphotropic virus type 1 & 2 (HTLV-1/2) in South African blood donors. Vox Sang 2019; 114:451–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van Buuren S, Boshuizen HC, Knook DL. Multiple imputation of missing blood pressure covariates in survival analysis. Stat Med 1999; 18:681–94. [DOI] [PubMed] [Google Scholar]
- 14. Lins L, de Carvalho VJU, Rego FFA, et al. . Oral health profile in patients infected with HTLV-1: clinical findings, proviral load, and molecular analysis from HTLV-1 in saliva. J Med Virol 2012; 84:1428–36. [DOI] [PubMed] [Google Scholar]
- 15. Offen D, Achiron A, Wasserman L, et al. . HTLV-1 in mouthwash cells from a TSP/HAM patient and asymptomatic carriers. Arch Virol 1998; 143:1029–34. [DOI] [PubMed] [Google Scholar]
- 16. Nerrienet E, Meertens L, Kfutwah A, Foupouapouognigni Y, Ayouba A, Gessain A. Simian T cell leukaemia virus type I subtype B in a wild-caught gorilla (Gorilla gorilla gorilla) and chimpanzee (Pan troglodytes vellerosus) from Cameroon. J Gen Virol 2004; 85:25–9. [DOI] [PubMed] [Google Scholar]
- 17. Halbrook M, Gadoth A, Shankar A, et al. . Human T-cell lymphotropic virus type 1 transmission dynamics in rural villages in the democratic republic of the Congo with high nonhuman primate exposure. PLoS Negl Trop Dis 2021; 15:e0008923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mossoun A, Calvignac-Spencer S, Anoh AE, et al. . Bushmeat hunting and zoonotic transmission of simian T-lymphotropic virus 1 in tropical West and Central Africa. J Virol 2017; 91:e02479-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kazanji M, Mouinga-Ondémé A, Lekana-Douki-Etenna S, et al. . Origin of HTLV-1 in hunters of nonhuman primates in Central Africa. J Infect Dis 2015; 211:361–5. [DOI] [PubMed] [Google Scholar]
- 20. Melbye M, Poulsen A-G, Gallo D, et al. . HTLV-1 infection in a population-based cohort of older persons in Guinea-Bissau, West Africa: risk factors and impact on survival. Int J Cancer 1998; 76:293–8. [DOI] [PubMed] [Google Scholar]
- 21. Betsem E, Rua R, Tortevoye P, Froment A, Gessain A. Frequent and recent human acquisition of simian foamy viruses through apes’ bites in Central Africa. PLoS Pathog 2011; 7:e1002306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Richard L, Mouinga-Ondémé A, Betsem E, et al. . Zoonotic transmission of two new strains of human T-lymphotropic virus type 4 in hunters bitten by a gorilla in Central Africa. Clin Infect 2016; 63:800–3. [DOI] [PubMed] [Google Scholar]
- 23. Wolfe ND, Heneine W, Carr JK, et al. . Emergence of unique primate T-lymphotropic viruses among Central African bushmeat hunters. Proc Natl Acad Sci U S A 2005; 102:7994–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zurita S, Costa C, Watts D, et al. . Prevalence of human retroviral infection in Quillabamba and Cuzco, Peru: a new endemic area for human T cell lymphotropic virus type 1. Am J Trop Med Hyg 1997; 56:561–5. [DOI] [PubMed] [Google Scholar]
- 25. Murphy EL. Infection with human T-lymphotropic virus types-1 and -2 (HTLV-1 and -2): implications for blood transfusion safety. Transfus Clin Biol 2016; 23:13–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Murphy EL, Watanabe K, Nass CC, Ownby H, Williams A, Nemo G. Evidence among blood donors for a 30-year-old epidemic of human T lymphotropic virus type II infection in the United States. J Infect Dis 1999; 180:1777–83. [DOI] [PubMed] [Google Scholar]
- 27. Etzel A, Shibata GY, Rozman M, Jorge ML, Damas CD, Segurado AA. HTLV-1 and HTLV-2 infections in HIV-infected individuals from santos, Brazil: seroprevalence and risk factors. J Acquir Immune Defic Syndr 2001; 26:185–90. [DOI] [PubMed] [Google Scholar]
- 28. Hogan CA, Iles J, Frost EH, et al. . Epidemic history and iatrogenic transmission of blood-borne viruses in mid-20th century Kinshasa. J Infect Dis 2016; 214:353–60. [DOI] [PubMed] [Google Scholar]
- 29. Pépin LA-C, Mamadou-Yaya F, et al. . Iatrogenic transmission of human T cell lymphotropic virus type 1 and hepatitis C virus through parenteral treatment and chemoprophylaxis of sleeping sickness in colonial equatorial Africa. Clin Infect Dis 2010; 51:777–84. [DOI] [PubMed] [Google Scholar]
- 30. Tang AR, Taylor GP, Dhasmana D. Self-flagellation as possible route of human T-cell lymphotropic virus type-1 transmission. Emerg Infect Dis 2019; 25:811–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.