Abstract
Background
Cross-neutralizing capacity of antibodies against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants is important in mitigating (re-)exposures. Role of antibody maturation, the process whereby selection of higher affinity antibodies augments host immunity, to determine SARS-CoV-2 neutralizing capacity was investigated.
Methods
Sera from SARS-CoV-2 convalescents at 2, 6, or 10 months postrecovery, and BNT162b2 vaccine recipients at 3 or 25 weeks postvaccination, were analyzed. Anti-spike IgG avidity was measured in urea-treated ELISAs. Neutralizing capacity was assessed by surrogate neutralization assays. Fold change between variant and wild-type neutralization inferred the breadth of neutralizing capacity.
Results
Compared with early-convalescent, avidity indices of late-convalescent sera were significantly higher (median, 37.7 [interquartile range 28.4–45.1] vs 64.9 [57.5–71.5], P < .0001). Urea-resistant, high-avidity IgG best predicted neutralizing capacity (Spearman r = 0.49 vs 0.67 [wild-type]; 0.18–0.52 vs 0.48–0.83 [variants]). Higher-avidity convalescent sera better cross-neutralized SARS-CoV-2 variants (P < .001 [Alpha]; P < .01 [Delta and Omicron]). Vaccinees only experienced meaningful avidity maturation following the booster dose, exhibiting rather limited cross-neutralizing capacity at week 25.
Conclusions
Avidity maturation was progressive beyond acute recovery from infection, or became apparent after the booster vaccine dose, granting broader anti-SARS-CoV-2 neutralizing capacity. Understanding the maturation kinetics of the 2 building blocks of anti-SARS-CoV-2 humoral immunity is crucial.
Keywords: SARS-CoV-2, antibody maturation, avidity, neutralization breadth, variants of concern
Avidity maturation augments host immunity following a natural infection and/or vaccination. For protection against SARS-CoV-2, avidity maturation was progressive beyond acute recovery from infection or became apparent after the booster vaccine dose, and granted broader neutralizing capacity against variant strains.
Given the reported coronavirus disease 2019 (COVID-19) reinfections and vaccine breakthrough infections, it is unlikely that convalescent or vaccinated individuals will attain lifelong immune protection [1, 2]. Therefore, the magnitude of partial immunity those individuals acquire is an important issue. In the convalescent phase and thereafter, circulating levels of neutralizing immunoglobulin G (IgG) that target the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) receptor binding domain (RBD) are highly correlated with residual protectivity [3, 4]. However, the durability of anti-spike IgGs and their potential cross-neutralizing capacity against rapidly evolving SARS-CoV-2 variants are primary concerns in mitigating future re-exposures [5].
Avidity maturation is the biological process whereby antigen-driven selection of higher-affinity antibodies augments the host's long-lasting protective immunity. Continuous antigen presentation at germinal centers promotes the fine-tuning of the antibody's complementarity toward the epitope. The kinetics and potential implications of maturing antibody avidity in the context of immune protection against SARS-CoV-2 and its emerging variants of concern (VOCs) remains to be fully elucidated [6–8]. In the present study, we correlated the kinetics of serum antibody avidity maturation, which followed SARS-CoV-2 natural infections, as well as vaccine administrations, to the magnitude and breadth of in vitro neutralizing capacity. The immune status in individuals who have experienced COVID-19 before or after their receipt of a SARS-CoV-2 vaccine is often referred to as “hybrid immunity” [9], and is known to confer enhanced protection against variable SARS-CoV-2 strains [10]. Our observations provide a logical perspective on the variable durability and resilience of the 2 building blocks, the natural infection immunity and the vaccine-elicited immune response, needed for the acquisition of hybrid immunity.
METHODS
Human Subjects and Sample Collection
From June 2020 to February 2021, a total 462 convalescent individuals, who had been affected by and had recovered from COVID-19 preceding the emergence of the SARS-CoV-2 VOCs (during March–November 2020) [11], were recruited and asked to provide a single serum sample at Osaka Metropolitan University and collaborating institutions. Although none of the samples were serial collections from the same patients, the mixed cohort from different regions shared the predominant SARS-CoV-2 genotypes, belonging to B.1 or B.1.1 lineages [12, 13]. Seventeen serum samples from 11 recipients of the BNT162b2 SARS-CoV-2 mRNA vaccine, recruited during March–April 2021, were collected at week 3 and week 25 after the first dose. Analyses were conducted in accordance with the 1964 Declaration of Helsinki and its later amendments. This research was approved by the Ethical Committee of Osaka Metropolitan University Graduate School of Medicine (No. 2020-003). All participants provided written informed consent prior to enrollment.
Anti-Spike IgG Measurement
Abbott SARS-CoV-2 IgG II Quant assays (6S6023) were run in accordance with the manufacturer's instructions.
Avidity Enzyme-Linked Immunosorbent Assay
Serum samples were tested for their avidity towards the SARS-CoV-2 spike antigen using an enzyme-linked immunosorbent assay (ELISA)-based assay (EI 2606–9601; Euroimmun) [14]. ELISA assays were first run in accordance with the manufacturer's instructions. Next, to detect solely the high-avidity antibodies, replicate samples were treated with urea (5.5 M for 10 minutes at 37°C) following incubation of the spike antigen-coated plate with serum samples. Optical density 450 (OD450) of a sample without urea treatment was indicative of the total anti-spike IgG titer, while that following urea treatment (urea resistant) was indicative of the high-avidity IgG fraction. The avidity index (AI) was calculated as: AI = {(OD450 of sample with 5.5 M urea) − (OD450 of negative control with 5.5 M urea)}/{(OD450 of sample without urea) – (OD450 of negative control without urea)} × 100 [%]. Serum samples were diluted 1:101 for analysis, except for the postvaccine sera, which required a higher dilution of 1:201 to bring the OD450 signals into linear range.
Neutralizing Capacity
Neutralizing capacity against wild-type (WT) and VOC SARS-CoV-2 was quantified using the GenScript SARS-CoV-2 sVNT (L00847-A; GenScript), a competition ELISA-based surrogate virus neutralization test (sVNT). Obtained signals have been shown to strongly correlate with the results of the conventional live-virus neutralization test [15, 16]. The assay protocol followed the manufacturer's instructions. Serum samples and controls were preincubated to neutralize WT RBD (WT; GenScript, Z03594), or B.1.1.7 (Alpha; GenScript, Z03595), B.1.617.2 (Delta; GenScript, Z03614), or B.1.1.529 (Omicron; GenScript, Z03730) RBD variants conjugated with horseradish peroxidase. The inhibition rate (%inhibition) was calculated as: %inhibition = {1 − (OD450 of sample)/(OD450 of negative control)} × 100 [%].
Breadth of Neutralizing Capacity
For quantitative assessment of the serum antibodies’ abilities to cross-neutralize SARS-CoV-2 and its variants, the sVNT % inhibition values were converted to the linearly scaled World Health Organization (WHO) International Standard units, IU/mL [17, 18]. We first obtained %inhibition data for 4-fold serially diluted WHO International Standard (20/136) samples by testing them in the sVNT platform using WT and VOC RBD antigens. Inhibitory dose-response standard curves were generated by performing a 4-parameter logistic equation curve fit against the log-transformed IU/mL concentration values and their corresponding %inhibition data (GraphPad Prism, version 9). A standard curve was plotted uniquely for each WT and VOC RBD antigen. Next, the sVNT %inhibition values obtained from patient sera, tested at optimized dilutions (up to 1:30, adjusted to bring the OD450 signals into quantitative range of the standard curves), were converted to neutralizing titer (IU/mL) by interpolation from the inhibitory dose-response standard curves. Neutralizing titer (IU/mL) against WT (NTWT) or VOC RBD antigens (NTAlpha, NTDelta, or NTOmicron) were computed from the standard curve specifically generated for each antigen, because they demonstrated unique inhibitory dose-response relationships. The fold changes in neutralizing titer between the VOCs and WT (NTAlpha/NTWT, NTDelta/NTWT, and NTOmicron/NTWT) were calculated to serve as the index for the “breadth of neutralizing capacity.” Similar indices have served as quantitative measures of relative neutralizing potency towards VOC strains [19]. For direct inspection purposes on the role of avidity maturation to determine the breadth of neutralizing capacity, the combined 55 sera from early (month 2) and late convalescence (month 10) were regrouped into low-avidity (below the 50th percentile) and high-avidity (50th percentile and above) sera based on rank, from bottom to top, in their AIs.
Statistical Analysis
Differences in titer, avidity, and the magnitude/breadth of neutralizing capacity of sera were tested by Mann-Whitney test. For the longitudinal anti-spike IgG titer plot, a linear fit was performed, and Pearson correlation coefficient was calculated. Spearman correlation coefficient was calculated to assess the strength of the correlation between antibody titer and in vitro neutralizing capacity. P values less than .05 were considered statistically significant.
RESUTS
Serum Anti–SARS-CoV-2 Antibodies Decay, While Maturing in Avidity, Over Time
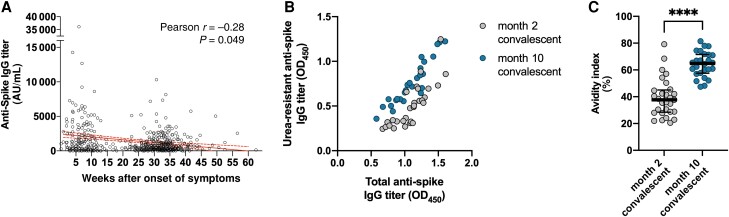
As repeatedly reported, serum levels of circulating anti-spike IgG showed longitudinal decline in titer in SARS-CoV-2 convalescent individuals (Pearson r = −0.28, P = .049; Figure 1A) [20]. To further investigate the qualitative evolution of serum antibodies, representative subsets of sera matched by their total anti-spike IgG titers (median OD450 1.12 [interquartile range, IQR, 0.93–1.25] vs 1.09 [IQR, 0.89–1.26], P = .98; Figure 1B) were selected from early- and late-convalescent phases (n = 29, median 8 weeks [IQR, 7–9 weeks] and n = 26, median 37 weeks [IQR, 36–40 weeks] after onset of symptoms, respectively), and compared of their serum antibody avidity characteristics. Polyclonal antibodies present in the late-convalescent sera were more avid against the SARS-CoV-2 spike antigen, and thus their OD450 were relatively resistant to urea treatment (Figure 1B). The difference in maturity of convalescent sera was reflected in the AI, with that of late-convalescent sera demonstrating higher AIs (median AI 37.7 [IQR, 28.4–45.1] vs 64.9 [IQR, 57.5–71.5], P < .0001; Figure 1C), indicating a progressive maturation process that extends months beyond the acute phase of COVID-19.
Figure 1.
Evolution of anti-SARS-CoV-2 IgG in convalescent individuals. A, Anti-spike IgG levels of individuals observed across the convalescent phase, and their linear fit (solid line) with 95% confidence intervals (dashed lines). B, Resistance of the OD450 signal against urea treatment in subsets of sera from early- (n = 29) and late-convalescent (n = 26) samples, matched by total anti-spike IgG titers. C, Avidity index of antibodies present in early- (n = 29) and late-convalescent (n = 26) sera. Horizontal lines and error bars represent medians and interquartile ranges. ****P < .0001. Abbreviations: IgG, immunoglobulin G; AU, arbitrary unit; OD450, optical density at 450 nm; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Higher-Avidity Antibodies Exert Broadened Neutralizing Capacity Against VOCs
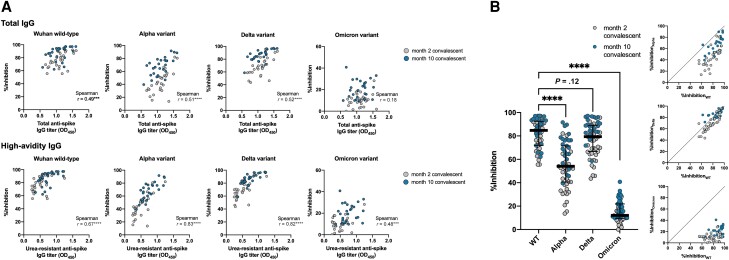
Next, we investigated the functional consequences of enhanced antibody avidity. Compared with the raw OD450 values (Figure 2A, upper), the urea-resistant OD450 signals from the avidity ELISA (Figure 2A, lower) better correlated with the inhibition rate (%inhibition) obtained from the sVNT. The results indicated a central role for high-affinity anti-spike IgG antibodies in determining in vitro neutralizing capacity against SARS-CoV-2 WT and variant RBDs.
Figure 2.
Central role of high-avidity IgG in neutralizing SARS-CoV-2. A, Neutralizing capacity of antibodies present in early- (n = 29) and late-convalescent sera (n = 26) against wild-type and variant SARS-CoV-2, as measured by surrogate virus neutralization, and plotted against total (upper) and urea-resistant, high-avidity anti-spike IgG titers (lower). B, Resistance of SARS-CoV-2 variants against neutralization by convalescent sera. Horizontal lines and error bars represent medians and interquartile ranges. ***P < .001, ****P < .0001. Abbreviations: IgG, immunoglobulin G; OD450, optical density at 450 nm; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; WT, wild type.
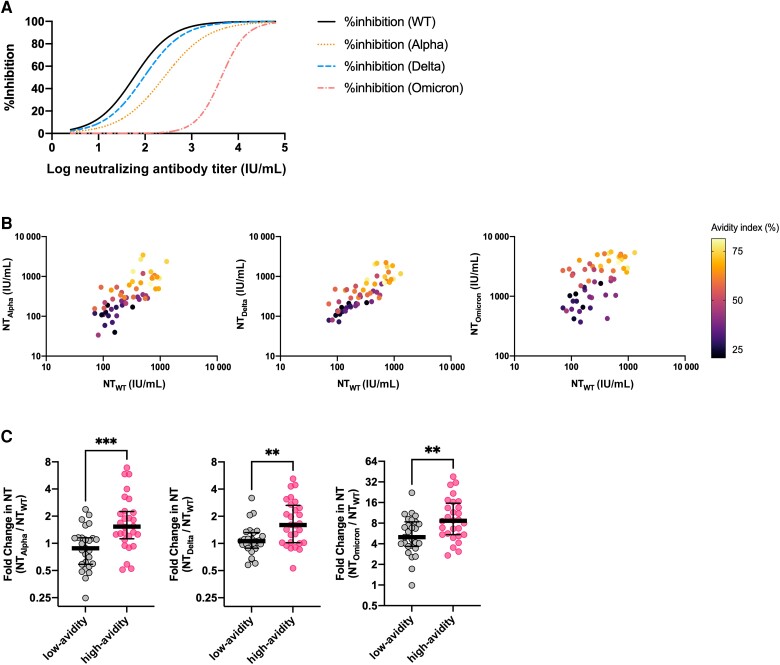
We next tested the hypothesis that serum antibody avidity determines the tolerability against RBD variations. As previously reported, the Alpha, Delta, and Omicron variants were more resistant to neutralization by convalescent sera, showing attenuated inhibition rates in the sVNT (Figure 2B). To further compare the cross-neutralizing capacity of individual sera against different VOCs, in other words the breadth of neutralizing capacity, we attempted a linear scale conversion of the neutralizing titer by modifying previously employed methods [17]. Briefly, the neutralizing titer (IU/mL) of each convalescent sample was computed from their %inhibition values through interpolation from standard curves that were generated by testing serially diluted WHO International Standard (20/136) sera (Figure 3A). The breadth of neutralizing capacity was expressed as the NT against the Alpha, Delta, and Omicron VOC RBDs (NTAlpha, NTDelta, and NTOmicron, respectively) relative to the NT against the WT RBD (NTWT) in fold changes. Interestingly, when NTs against SARS-CoV-2 VOCs were plotted against that of WT, with respect to the serum antibody's AI, sera of higher AIs tended to show higher NTAlpha, NTDelta, and NTOmicron values relative to NTWT (Figure 3B). Next, the fold change in NT against SARS-CoV-2 VOCs relative to the WT was compared between low-avidity (below the 50th percentile) and high-avidity (50th percentile and above) serum samples, regrouped from the combined 55 sera from early (month 2) and late convalescence (month 10) based on rank in their degree of antibody maturation. Higher-avidity sera showed greater fold change in NT across the different SARS-CoV-2 VOCs (Figure 3C). Together, the results indicated that sera of higher AIs exhibited enhanced breadth of neutralizing capacity and better tolerated the variations in SARS-CoV-2 RBD.
Figure 3.
Enhanced neutralization breadth of higher-avidity anti-SARS-CoV-2 IgG. A, Four-parameter logistic standard curves for conversion of inhibition rates to neutralizing antibody titers in WHO International Standard units. B, Cross-neutralizing capacity of convalescent sera against the Alpha (left), Delta (middle), and Omicron variants (right). The samples’ avidity indices are depicted according to the color scale shown. C, Relative inhibition potency towards SARS-CoV-2 variants indexed to the wild-type neutralizing titer, indicating the breadth of neutralizing capacity per serum. Sera from early- (month 2) and late-convalescence (month 10) were regrouped into low-avidity (below the 50th percentile) and high-avidity (50th percentile and above) sera based on rank, from bottom to top, in their degree of antibody maturation. Horizontal lines and error bars represent medians and interquartile ranges. **P < .01, ***P < .001. Abbreviations: IgG, immunoglobulin G; IU, international standard unit; NT, neutralizing titer; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; WT, wild type.
Vaccinees Experience Significant Avidity Maturation Following the Booster Dose
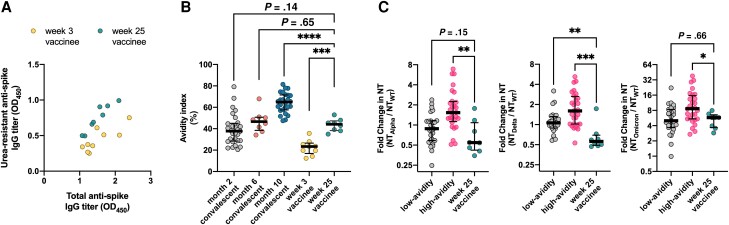
Serum antibody avidity maturation in the vaccine-elicited immune responses was next assessed through analysis of sera from recipients of the BNT162b2 SARS-CoV-2 mRNA vaccine (vaccinees). Sera collected from vaccinees at week 3 (n = 9) and week 25 (n = 8) after the first dose were matched for their total anti-spike IgG titers (median OD450 1.37 [IQR, 1.23–1.90] vs 1.48 [IQR, 1.20–1.75], P = 1.0; Figure 4A), and their kinetics of AI evolution were further compared with those of convalescent individuals. Here, while appreciating the limited number of available samples, 8 additional sera from mid-convalescence (6 months postrecovery), were included for the purpose of time-matched comparison between vaccinees and convalescents. Importantly, serum antibodies present in the sera of week 25 vaccinees demonstrated significantly higher AIs compared with those of week 3 vaccinees (median 44.2 [IQR, 38.3–47.4] vs 23.4 [IQR, 16.9–26.6], P = .0002), indicating that the second dose of the vaccine had boosted the hosts’ maturation of antibody avidity. The AIs of week 25 (approximate month 6) vaccinees (44.2 [IQR, 38.3–47.4]) were slightly higher than those of early convalescents (month 2, 37.7 [IQR, 28.4–45.1]), although not statistically significant (P = .14), and were similar to those of mid-convalescents (month 6, 46.6 [IQR, 38.4–50.7]) (Figure 4B).
Figure 4.
Functional evolution of anti-SARS-CoV-2 IgG in recipients of the BNT162b2 SARS-CoV-2 mRNA vaccine. A, Resistance of the OD450 signal against urea treatment in subsets of sera from vaccine recipients at week 3 (n = 9) and week 25 (n = 8) postvaccination, matched by total anti-spike IgG titers. B, Avidity index of antibodies present in week 3 (n = 9) and week 25 (n = 8) vaccinees in comparison with convalescent individuals. C, Relative inhibition potency of week 25 vaccinee sera (n = 8) towards SARS-CoV-2 variants plotted against that of convalescent individuals. Sera from early (month 2) and late convalescence (month 10) were regrouped into low-avidity (below the 50th percentile) and high-avidity (50th percentile and above) sera based on rank, from bottom to top, in their degree of antibody maturation. *P < .05, **P < .01, ***P < .001, ****P < .0001. Abbreviations: IgG, immunoglobulin G; OD450, optical density at 450 nm; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; WT, wild type.
Contribution of the Matured IgG Fraction to Neutralizing Capacity Among Vaccinees
Interestingly, in contrast to the convalescent sera and the week 25 vaccinee sera, the neutralizing capacity of the sera of week 3 vaccinees showed a relatively weak correlation (Spearman r = 0.52, P = .16) with their urea-resistant anti-spike IgG titer. The correlation was rather strong (Spearman r = 0.87, P < .01) when the levels of neutralizing capacity were plotted against total anti-spike IgG titers. This indicates that high-avidity antibodies contribute less to neutralizing capacity early in the vaccine response and become a stronger determinant only after the second dose (Spearman r = 0.71, P = .06). Reflecting their intermediacy in the process of avidity maturation, the breadth of neutralizing capacity of the week 25 vaccinees’ sera remained limited to an extent comparable to that of the low-avidity subgroup of convalescent sera (Figure 4C).
DISCUSSION
Antibody avidity maturation in COVID-19 convalescents was observed as an ongoing process that surprisingly extended months beyond the acute phase. This finding supports the notion that SARS-CoV-2 proteins persist in tissues to continuously fuel the germinal centers for antigen presentation, even during late convalescence [20]. More importantly, the high-avidity neutralizing IgGs, which are the result of continuous B-cell evolution, remain in the circulation of late convalescents, fortifying for viral re-encounters by exerting enhanced cross-neutralizing capacity against emerging SARS-COV-2 variants.
In theory, immature plasma cells produce antibodies that possess conformationally flexible antigen-binding surfaces, which at the cost of lowered specificity and affinity are capable of recognizing diverse antigens (polyreactivity hypothesis) [21]. In contrast, mature plasma cells produce antibodies of preorganized and rigid paratopes to take more energetically favorable configurations with improved complementarity against epitopes [22]. This general tendency towards paratope rigidification upon avidity maturation, while favorable in terms of enhancing specificity, is seemingly detrimental for broad cross-reactivity. In the case of SARS-CoV-2–neutralizing antibodies, however, our results show that high-avidity antibodies exert broader protection against evolving variants, granting long-lasting protective immunity. This paradoxical relationship between affinity maturation and the broadening in reactivity of SARS-CoV-2–neutralizing antibodies is worthy of note. The molecular basis of B-cell evolution is explained by the acquisition of somatic hypermutations [23]. The aforementioned rigidification of paratopes is only one possible consequence of such somatic hypermutations. Mutations may lead to increased buried surface area upon complex formation, strengthening antibody-antigen binding. B cells may alternatively acquire amino acid substitutions that result in better complementarity of the antigen binding site. Additive molecular contacts at the paratope-epitope interface, through the addition of novel polar or hydrophobic bonds, may also underlie the better complementarity [24]. The increase in variety of the structural determinants decreases the dependency of the paratope-epitope interaction on specific amino acid residues [25]. Thus, these diverse biophysical mechanisms of antibody affinity maturation all provide explanations for the efficacy of high-affinity antibodies in neutralizing SARS-CoV-2 variants.
Given that the majority of the world's population has relied largely on the SARS-CoV-2 vaccines for immunological protection, our secondary interest was how the vaccine-elicited immune responses were different from natural infection-acquired immunity. The kinetics of affinity maturation of SARS-CoV-2–specific antibodies have recently been shown to correlate with the longevity of immune protection following natural SARS-CoV-2 infection [26–28]. As observed in the present study, the higher potency of the high-avidity antibodies in neutralizing SARS-CoV-2 may counter the longitudinal decay in quantity and explain the durability of infection-acquired immune protection. Interestingly, the time course of avidity maturation seemed variable between infection- and vaccine-elicited antibodies. Indeed, evident avidity maturation had followed the booster dose of the mRNA vaccine. The cross-neutralizing capacity of vaccinees’ sera against SARS-CoV-2 variants, however, remained limited up to 6 months postvaccination, possibly reflecting their intermediacy in the process of antibody maturation. Large-scale clinical trials in real-world settings have shown greater protection associated with infection-acquired immunity than with vaccine-elicited immune responses [29, 30]. The present study further enriches the evidence for, and provides an immunological basis to, the greater protection associated with infection-acquired immunity. Additionally, the urea-resistant fraction of the antibody titer was predictive of neutralizing capacity only following the second dose of the vaccine. In contrast to the limited variety of epitopes recurrently targeted across convalescent individuals [31], the vaccine-elicited immune response is mapped to a broadened epitope landscape [32, 33]. During the initial response to the SARS-CoV-2 vaccine, the majority of urea-resistant signals obtained from our avidity ELISA were possibly from a widely dispersed, nonneutralizing antibody repertoire that still dominated the week 3 vaccinees’ sera. The second vaccine dose seemingly facilitated the positive selection of neutralizing over nonneutralizing epitopes. Collectively, the dynamics of epitope selection, as well as avidity maturation, seem unique to each mode of acquired humoral immunity, the natural infection immunity and the vaccine-elicited immune response [34].
Furthermore, circulating IgG may serve as sources of passive immunotherapeutics [35]. In such a context, their extent of maturity may affect cross-neutralizing capacity and immunotherapy outcome. Immunotherapy using convalescent sera has unfortunately remained investigational for COVID-19, although it is expected to be beneficial for those with deficient active immunity [36, 37]. To mitigate the emergence of immune-escaping variants in future pandemics, assessing avidity to stratify the potency of donated sera may be a reasonable strategy for effective immunotherapy.
Limitations of the present study include (1) the avidity indices and %inhibition values shown in Figure 1 and Figure 2, respectively, were obtained from a nonserial collection of convalescent serum samples, and thus may reflect effects or differences deriving from variances among individuals, independent of the chronological evolution of antibodies; (2) sVNT was used instead of the standard live-virus neutralization test to quantify the neutralizing capacity of sera; (3) the cellular compartment was not studied, which also plays decisive roles in immune protection; (4) the timing of sampling was different between convalescent sera and postvaccination sera; and (5) the observation period of vaccinees was limited, so the long-term consequences remain elusive.
In conclusion, the present study showed that the continuous process of avidity maturation, extending beyond late convalescence, grants broader neutralizing capacity and robust protection that stand relatively resilient against emerging SARS-CoV-2 variants. With immunopotentiation through repeat vaccinations becoming a pivotal strategy to accomplish herd immunity, understanding the longitudinal evolution of vaccine-induced immune responses and the incremental protective effects of booster vaccinations, which will also occur in convalescent individuals (hybrid immunity), remains critical.
Contributor Information
Yu Nakagama, Department of Virology and Parasitology, Graduate School of Medicine, Osaka Metropolitan University, Osaka, Japan; Research Center for Infectious Disease Sciences, Graduate School of Medicine, Osaka Metropolitan University, Osaka, Japan.
Katherine Candray, Department of Virology and Parasitology, Graduate School of Medicine, Osaka Metropolitan University, Osaka, Japan; Research Center for Infectious Disease Sciences, Graduate School of Medicine, Osaka Metropolitan University, Osaka, Japan; Centro Nacional de Investigaciones Científicas de El Salvador, San Salvador, El Salvador.
Natsuko Kaku, Department of Virology and Parasitology, Graduate School of Medicine, Osaka Metropolitan University, Osaka, Japan; Research Center for Infectious Disease Sciences, Graduate School of Medicine, Osaka Metropolitan University, Osaka, Japan.
Yuko Komase, Department of Respiratory Internal Medicine, St Marianna University, Yokohama Seibu Hospital, Yokohama, Japan.
Maria-Virginia Rodriguez-Funes, National Rosales Hospital, San Salvador, El Salvador.
Rhina Dominguez, El Salvador National Institute of Health, San Salvador, El Salvador.
Tomoya Tsuchida, Division of General Internal Medicine, St Marianna University School of Medicine, Kawasaki, Japan.
Hiroyuki Kunishima, Department of Infectious Diseases, St Marianna University School of Medicine, Kawasaki, Japan.
Etsuko Nagai, Department of Infectious Diseases and Applied Immunology, Institute of Medical Science, The University of Tokyo, Tokyo, Japan.
Eisuke Adachi, Department of Infectious Diseases and Applied Immunology, Institute of Medical Science, The University of Tokyo, Tokyo, Japan.
Dieudonné Mumba Ngoyi, Institut National de Recherche Biomedicale, Kinshasa, Democratic Republic of the Congo.
Mari Yamasue, Department of Respiratory Medicine and Infectious Diseases, Oita University Faculty of Medicine, Oita, Japan.
Kosaku Komiya, Department of Respiratory Medicine and Infectious Diseases, Oita University Faculty of Medicine, Oita, Japan.
Kazufumi Hiramatsu, Department of Respiratory Medicine and Infectious Diseases, Oita University Faculty of Medicine, Oita, Japan.
Naoto Uemura, Department of Respiratory Medicine and Infectious Diseases, Oita University Faculty of Medicine, Oita, Japan.
Yuki Sugiura, Center for Cancer Immunotherapy and Immunobiology, Kyoto University Graduate School of Medicine, Kyoto, Japan.
Mayo Yasugi, Graduate School of Veterinary Science, Osaka Metropolitan University, Osaka, Japan; Asian Health Science Research Institute, Osaka Metropolitan University, Osaka, Japan; Osaka International Research Center for Infectious Diseases, Osaka Metropolitan University, Osaka, Japan.
Yuka Yamagishi, Department of Clinical Infectious Diseases, Aichi Medical University, Aichi, Japan.
Hiroshige Mikamo, Department of Clinical Infectious Diseases, Aichi Medical University, Aichi, Japan.
Satoshi Shiraishi, Department of Respiratory Medicine, Osaka City Juso Hospital, Osaka, Japan.
Takehiro Izumo, Department of Respiratory Medicine, Japanese Red Cross Medical Center, Tokyo, Japan.
Sachie Nakagama, Department of Virology and Parasitology, Graduate School of Medicine, Osaka Metropolitan University, Osaka, Japan; Research Center for Infectious Disease Sciences, Graduate School of Medicine, Osaka Metropolitan University, Osaka, Japan.
Chihiro Watanabe, Department of Virology and Parasitology, Graduate School of Medicine, Osaka Metropolitan University, Osaka, Japan; Research Center for Infectious Disease Sciences, Graduate School of Medicine, Osaka Metropolitan University, Osaka, Japan.
Yuko Nitahara, Department of Virology and Parasitology, Graduate School of Medicine, Osaka Metropolitan University, Osaka, Japan; Research Center for Infectious Disease Sciences, Graduate School of Medicine, Osaka Metropolitan University, Osaka, Japan.
Evariste Tshibangu-Kabamba, Department of Virology and Parasitology, Graduate School of Medicine, Osaka Metropolitan University, Osaka, Japan; Research Center for Infectious Disease Sciences, Graduate School of Medicine, Osaka Metropolitan University, Osaka, Japan.
Hiroshi Kakeya, Department of Virology and Parasitology, Graduate School of Medicine, Osaka Metropolitan University, Osaka, Japan; Department of Infection Control Science, Graduate School of Medicine, Osaka Metropolitan University, Osaka, Japan.
Yasutoshi Kido, Department of Virology and Parasitology, Graduate School of Medicine, Osaka Metropolitan University, Osaka, Japan; Research Center for Infectious Disease Sciences, Graduate School of Medicine, Osaka Metropolitan University, Osaka, Japan.
Notes
Acknowledgments. The authors thank all participants of the study and, for technical assistance, Mrs Mika Oku from Osaka Metropolitan University. Data acquisition was partly performed at the Research Support Platform, Graduate School of Medicine, Osaka Metropolitan University.
Financial support. This work was supported by the Japan Agency for Medical Research and Development (grant numbers JP20jk0110021 to Y. N., and JP20wm0125003 and JP20he1122001 to Y. K.); the Japan Society for the Promotion of Science KAKENHI (grant numbers 21K09078 to N. K. and 22K15927 to Y. N.); the Osaka Metropolitan University Strategic Research Grant (grant number OCU-SRG2021_YR09 to Y. N.); the Osaka Metropolitan University Special Reserves Fund for COVID-19; and the Shinya Yamanaka Laboratory COVID-19 Private Fund. Funding to pay the Open Access publication charges for this article was provided by the Japan Society for the Promotion of Science.
References
- 1. Hacisuleyman E, Hale C, Saito Y, et al. . Vaccine breakthrough infections with SARS-CoV-2 variants. N Engl J Med 2021; 384:2212–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. To KK-W, Hung IF-N, Ip JD, et al. . Coronavirus disease 2019 (COVID-19) re-infection by a phylogenetically distinct severe acute respiratory syndrome coronavirus 2 strain confirmed by whole genome sequencing. Clin Infect Dis 2021; 73:e2946–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Khoury DS, Cromer D, Reynaldi A, et al. . Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med 2021; 27:1205–11. [DOI] [PubMed] [Google Scholar]
- 4. Bergwerk M, Gonen T, Lustig Y, et al. . COVID-19 breakthrough infections in vaccinated health care workers. N Engl J Med 2021; 385:1474–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cromer D, Juno JA, Khoury D, et al. . Prospects for durable immune control of SARS-CoV-2 and prevention of reinfection. Nat Rev Immunol 2021; 21:395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bauer G. The potential significance of high avidity immunoglobulin G (IgG) for protective immunity towards SARS-CoV-2. Int J Infect Dis 2021; 106:61–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bauer G, Struck F, Schreiner P, Staschik E, Soutschek E, Motz M. The challenge of avidity determination in SARS-CoV-2 serology. J Med Virol 2021; 93:3092–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nakagama Y, Nitahara Y, Kaku N, Tshibangu-Kabamba E, Kido Y. A dual-antigen SARS-CoV-2 serological assay reflects antibody avidity. J Clin Microbiol 2022; 60:e0226221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. World Health Organization . Interim statement on hybrid immunity and increasing population seroprevalence rates. https://www.who.int/news/item/01-06-2022-interim-statement-on-hybrid-immunity-and-increasing-population-seroprevalence-rates. Accessed 4 November 2022.
- 10. Nakagama S, Nakagama Y, Komase Y, et al. . The impact of prior COVID-19 on vaccine response and the resultant hybrid immunity are age-dependent. MedRxiv, https://doi.org/2022.09.19.22280079, 19September2022, preprint: not peer reviewed. [Google Scholar]
- 11. Adachi T, Ayusawa M, Ujiie M, et al. . Novel coronavirus infection COVID-19 medical practice guidelines, version 7.2 [in Japanese]. https://www.mhlw.go.jp/content/000936623.pdf. Accessed 1 July 2022.
- 12. Nakagama Y, Komase Y, Candray K, et al. . Serological testing reveals the hidden COVID-19 burden among health care workers experiencing a SARS-CoV-2 nosocomial outbreak. Microbiol Spectr 2021; 9:e0108221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nakagama Y, Rodriguez-Funes MV, Dominguez R, et al. . Cumulative seroprevalence among healthcare workers after the first wave of the COVID-19 pandemic in El Salvador, Central America. Clin Microbiol Infect 2022; 28:1508–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pichler D, Baumgartner M, Kimpel J, et al. . Marked increase in avidity of SARS-CoV-2 antibodies 7–8 months after infection is not diminished in old age. J Infect Dis 2021; 224:764–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tan CW, Chia WN, Qin X, et al. . A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2–spike protein–protein interaction. Nat Biotechnol 2020; 38:1073–8. [DOI] [PubMed] [Google Scholar]
- 16. Mariën J, Michiels J, Heyndrickx L, et al. . Evaluation of a surrogate virus neutralization test for high-throughput serosurveillance of SARS-CoV-2. J Virol Methods 2021; 297:114228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Taylor SC, Hurst B, Martiszus I, et al. . Semi-quantitative, high throughput analysis of SARS-CoV-2 neutralizing antibodies: measuring the level and duration of immune response antibodies post infection/vaccination. Vaccine 2021; 39:5688–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. National Institute for Biological Standards and Control. First WHO international standard for anti-SARS-CoV-2 immunoglobulin, human. (NIBSC code: 20/136). https://www.nibsc.org/documents/ifu/20-136.pdf. Accessed 1 July 2022.
- 19. Moriyama S, Adachi Y, Sato T, et al. . Temporal maturation of neutralizing antibodies in COVID-19 convalescent individuals improves potency and breadth to circulating SARS-CoV-2 variants. Immunity 2021; 54:1841–1852.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gaebler C, Wang Z, Lorenzi JCC, et al. . Evolution of antibody immunity to SARS-CoV-2. Nature 2021; 591:639–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Furukawa K, Akasako-Furukawa A, Shirai H, Nakamura H, Azuma T. Junctional amino acids determine the maturation pathway of an antibody. Immunity 1999; 11:329–38. [DOI] [PubMed] [Google Scholar]
- 22. Manivel V, Sahoo NC, Salunke DM, Rao KVS. Maturation of an antibody response is governed by modulations in flexibility of the antigen-combining site. Immunity 2000; 13:611–20. [DOI] [PubMed] [Google Scholar]
- 23. Victora GD, Nussenzweig MC. Germinal centers. Annu Rev Immunol 2012; 30:429–57. [DOI] [PubMed] [Google Scholar]
- 24. Mishra AK, Mariuzza RA. Insights into the structural basis of antibody affinity maturation from next-generation sequencing. Front Immunol 2018; 9:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Muecksch F, Weisblum Y, Barnes CO, et al. . Affinity maturation of SARS-CoV-2 neutralizing antibodies confers potency, breadth, and resilience to viral escape mutations. Immunity 2021; 54:1853–1868.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Luo YR, Chakraborty I, Yun C, Wu AHB, Lynch KL. Kinetics of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibody avidity maturation and association with disease severity. Clin Infect Dis 2021; 73:e3095–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Löfström E, Eringfält A, Kötz A, et al. . Dynamics of IgG-avidity and antibody levels after COVID-19. J Clin Virol 2021; 144:104986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chia WN, Zhu F, Ong SWX, et al. . Dynamics of SARS-CoV-2 neutralising antibody responses and duration of immunity: a longitudinal study. Lancet Microbe 2021; 2:e240–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gazit S, Shlezinger R, Perez G, et al. . Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) naturally acquired immunity versus vaccine-induced immunity, reinfections versus breakthrough infections: a retrospective cohort study. Clin Infect Dis 2022; 75:e545–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hall V, Foulkes S, Insalata F, et al. . Protection against SARS-CoV-2 after COVID-19 vaccination and previous infection. N Engl J Med 2022; 386:1207–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tong P, Gautam A, Windsor IW, et al. . Memory B cell repertoire for recognition of evolving SARS-CoV-2 spike. Cell 2021; 184:4969–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nitahara Y, Nakagama Y, Kaku N, et al. . High-resolution linear epitope mapping of the receptor binding domain of SARS-CoV-2 spike protein in COVID-19 mRNA vaccine recipients. Microbiol Spectr 2021; 9:e0096521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Greaney AJ, Loes AN, Gentles LE, et al. . Antibodies elicited by mRNA-1273 vaccination bind more broadly to the receptor binding domain than do those from SARS-CoV-2 infection. Sci Transl Med 2021; 13:eabi9915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang Z, Muecksch F, Schaefer-Babajew D, et al. . Naturally enhanced neutralizing breadth against SARS-CoV-2 one year after infection. Nature 2021; 595:426–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tang J, Lee Y, Ravichandran S, et al. . Epitope diversity of SARS-CoV-2 hyperimmune intravenous human immunoglobulins and neutralization of variants of concern. iScience 2021; 24:103006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Takakuwa T, Nakagama Y, Yasugi M, et al. . Discrepant antigen-specific antibody responses causing SARS-CoV-2 persistence in a patient receiving B-cell-targeted therapy with rituximab. Intern Med 2021; 60:3827–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Takita M, Yoshida T, Tsuchida T, et al. . Low SARS-CoV-2 antibody titers may be associated with poor clinical outcomes for patients with severe COVID-19. Sci Rep 2022; 12:9147. [DOI] [PMC free article] [PubMed] [Google Scholar]