Abstract
Background
The aim of this study was to investigate safety and immunogenicity of vaccine formulations against respiratory syncytial virus (RSV) containing the stabilized prefusion conformation of RSV fusion protein (RSVPreF3).
Methods
This phase 1/2, randomized controlled, observer-blind study enrolled 48 young adults (YAs; aged 18–40 years) and 1005 older adults (OAs; aged 60–80 years) between January and August 2019. Participants were randomized into equally sized groups to receive 2 doses of unadjuvanted (YAs and OAs) or AS01-adjuvanted (OAs) vaccine or placebo 2 months apart. Vaccine safety and immunogenicity were assessed until 1 month (YAs) or 12 months (OAs) after second vaccination.
Results
The RSVPreF3 vaccines boosted humoral (RSVPreF3-specific immunoglobulin G [IgG] and RSV-A neutralizing antibody) responses, which increased in an antigen concentration-dependent manner and were highest after dose 1. Compared to prevaccination, the geometric mean frequencies of polyfunctional CD4+ T cells increased after each dose and were significantly higher in adjuvanted than unadjuvanted vaccinees. Postvaccination immune responses persisted until end of follow-up. Solicited adverse events were mostly mild to moderate and transient. Despite a higher observed reactogenicity of AS01-containing vaccines, no safety concerns were identified for any assessed formulation.
Conclusions
Based on safety and immunogenicity profiles, the AS01E-adjuvanted vaccine containing 120 μg of RSVPreF3 was selected for further clinical development.
Clinical Trials Registration
Keywords: AS01 adjuvant, RSV neutralizing antibodies, cell-mediated immunity, F protein, respiratory syncytial virus
Vaccine formulations against respiratory syncytial virus (RSV) containing the stabilized prefusion conformation of RSV fusion protein (RSVPreF3) were well tolerated and immunogenic. Based on safety/immunogenicity profiles, the AS01E-adjuvanted vaccine containing 120 μg of RSVPreF3 was selected for further clinical development.
Respiratory syncytial virus (RSV) is a common pathogen causing typical respiratory tract infections (RTIs) with seasonal winter peaks in temperate climates [1]. Based on the clinical presentation alone, disease caused by RSV is usually indistinguishable from other respiratory viral diseases. Despite relative antigenic stability of RSV and its fusion (F) protein [2–4], natural infection induces incomplete short-lasting immunity and does not prevent subsequent infections [1], as shown by recurrent infections in children and adults [5].
Severe RSV-associated lower respiratory tract disease (RSV-LRTD) occurs in older adults (OAs; usually ≥60 years old), especially those with underlying medical conditions [6, 7]. In 2015, an estimated 1.5 million OAs suffered from RSV-related acute RTI in industrialized countries, of whom approximately 14.5% were hospitalized [8]. RSV-related morbidity and mortality are high in OAs and are anticipated to increase as the world population ages.
Although immunological correlates associated with susceptibility to severe RSV illness in OAs are not well understood, age-related decline in innate and adaptive immune response to RSV is well documented [9]. Diverse humoral and cell-mediated immune (CMI) responses are engaged to combat RSV at local and systemic levels [5]. Lower serum and mucosal RSV-specific antibody levels, shortages of naive lymphocytes, and shifts in adaptive CMI response increase the risk for RSV infection and severe illness in OAs [10].
Currently, no prophylactic treatment or vaccine against RSV-LRTD in OAs exists [11]. An effective RSV vaccine in OAs will likely need to boost/induce potent and durable RSV neutralizing antibody (nAb) responses, as well as restore/elicit RSV-specific T-cell responses [12]. This study is part of efforts to develop a vaccine against RSV-LRTD caused by the 2 RSV subtypes (RSV-A and/or RSV-B) in OAs. The present RSV investigational vaccine contains the highly conserved RSV F protein [3,4], stabilized in its trimeric prefusion conformation (RSVPreF3), with or without AS01-based adjuvant (AS01E and AS01B). The RSVPreF3 was chosen because it displays important antigenic sites [3,4], while the AS01 adjuvant system was selected for its ability to induce robust specific helper CD4+ T-cell responses, and rapid and durable humoral and cellular responses when combined with protein antigens [13–15], also in OAs [16–18].
The overall objectives of this phase 1/2 study were to evaluate safety, reactogenicity, and immunogenicity of RSVPreF3 vaccine formulations in young adults (YAs; 18–40 years of age) and OAs (60–80 years of age). The YAs received only the unadjuvanted formulation, whereas OAs received both unadjuvanted and AS01-adjuvanted formulations. The results of this study were used to support selection of a formulation for further vaccine development to prevent RSV-LRTD in OAs.
METHODS
Ethical Approvals
This phase 1/2, randomized, placebo-controlled, observer-blind study (NCT03814590) was conducted at multiple centers in the United States (18 centers) and Belgium (3 centers), according to the Declaration of Helsinki and Good Clinical Practice guidelines. Study documents were independently reviewed and approved by national, regional, or institutional review boards or independent ethics committees. Participants provided written informed consent.
Investigational Vaccine Formulations
The investigational vaccine formulations are based on the RSVPreF3 antigen derived from the F protein of the RSV-A2 strain [19]. RSV F protein is the main surface virus antigen, well-conserved across RSV-A and -B subtypes [3,4], and induces potent nAb responses [20].
Nine different RSVPreF3-based investigational vaccine formulations and placebo were assessed. RSVPreF3 formulations included 3 different RSVPreF3 antigen concentrations (30, 60, or 120 µg), and were unadjuvanted, or adjuvanted using AS01E or AS01B (see details in Supplementary Information). Placebo consisted of 150 mM sodium chloride solution.
Study Participants
The study was conducted in 2 parts: Part A enrolled healthy YAs aged 18–40 years, and part B enrolled OAs aged 60–80 years.
Eligible participants were individuals of appropriate age at the time of first vaccination, who were able to comply with the protocol (according to investigators’ opinion). OAs needed to reside in an environment allowing free mixing with the general population, and/or to bear primary responsibility for self-care and daily living activities. Exclusion criteria are listed in the Supplementary Information.
Factorial Study Design and Conduct
In parts A and B, 2 doses of investigational vaccine or placebo were administered intramuscularly into the deltoid region of the nondominant arm, 2 months apart (day 1 and day 61) (Figure 1 and details in Supplementary Information). Intercurrent independent data monitoring committee (IDMC) evaluations of unblinded safety data occurred after each vaccination at designated timepoints in parts A and B and were required for study continuation (details in Supplementary Information).
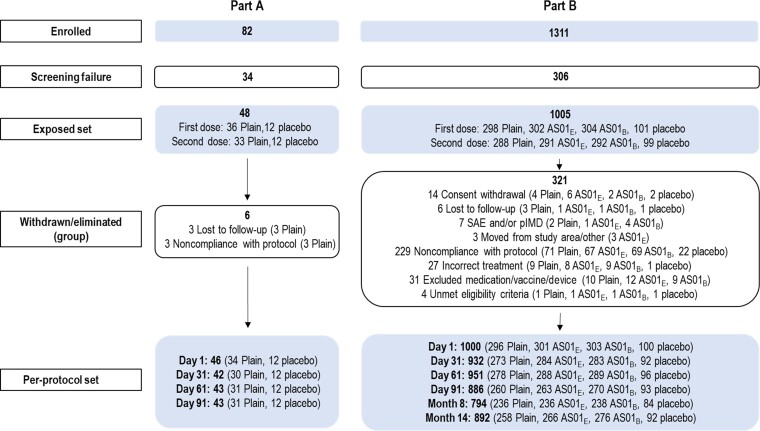
Figure 1.
Study design. Relevant points in the study timeline are designated as day (D) and month (M). N indicates number of participants in each study part/group. Dose 1 and 2 indicate vaccine and placebo administration timepoints. Blood sample S was the blood sample drawn for analysis of hematological (blood cell counts and hemoglobin levels) and biochemical (alanine and aspartate aminotransferases, creatinine, blood urea nitrogen, and uric acid) laboratory parameters. Blood sample H was the blood sample collected to measure concentrations of RSVPreF3-specific immunoglobulin and titers of respiratory syncytial virus-specific neutralizing antibodies. Blood sample C was the blood sample collected for analysis of polyfunctional T-cell responses. 30 μg, 60 μg, and 120 μg indicate RSVPreF3 antigen concentration. Abbreviations: AS01B and AS01E, adjuvanted vaccine formulations with the corresponding vaccine adjuvant systems; IDMC, independent data monitoring committee; Plain, unadjuvanted vaccine formulations; RSVPreF3, prefusion conformation of the respiratory syncytial virus F protein.
Young adults were followed up until day 91 (see details in Supplementary Information). Part B was split over 2 sequentially conducted steps, part B1 (100 participants) and part B2 (905 participants), in which OAs were randomized into 10 equally sized groups (1:1:1:1:1:1:1:1:1:1). One group received placebo, while the remaining 9 groups received unadjuvanted (30-Plain, 60-Plain, 120-Plain), AS01E-adjuvanted (30-AS01E, 60-AS01E, 120-AS01E), or AS01B-adjuvanted (30-AS01B, 60-AS01B, 120-AS01B) vaccine formulations. OAs were followed up until 12 months after the second vaccination (Figure 1).
Randomization and Blinding
Study participants were randomized using a centralized randomization system on the internet. To minimize selection bias, the randomization algorithm used a minimization procedure for center and sex in both parts, and additionally age in part B.
The study was observer-blinded in parts A and B until day 91, and single-blinded in part B between day 91 and study end. Investigators, site staff, and study staff were partially unblinded at a group level for the long-term evaluation subset (all participants from part B1 and those receiving a selected level of antigen or placebo in B2), from whom blood samples were drawn at last study visit.
Study Objectives and Endpoints
The primary objective was to evaluate the safety and reactogenicity of 2 doses of investigational vaccine administered at day 1 and day 61, until day 91. The secondary safety objective was to evaluate safety and reactogenicity of 2 doses of the investigational vaccine until month 14.
Main secondary immunogenicity objectives were to characterize the humoral and CMI responses, including dose dependence, related to the investigational RSV vaccine formulations administered at day 1 and day 61, until day 91. See Supplementary Table 1 and Supplementary Information for further details on study objectives and endpoints.
Safety and Reactogenicity Evaluation
Events leading to withdrawals, pregnancies (in YAs only), and intercurrent medical conditions were collected from day 1 until study end (day 91 in YAs and month 14 in OAs) (see further details in Supplementary Information, including a list of solicited adverse events [AEs] and their grading scale). Causality between AEs and vaccine administration was determined based on investigators’ clinical judgement.
Immunogenicity Evaluation
Humoral immune response was assessed by measuring RSV-A and RSV-B nAb titers by in-house neutralization assays, and RSVPreF3-specific immunoglobulin G (IgG) and RSVPreF3-epitope-specific (RSB1) antibody concentrations by in-house enzyme-linked immunosorbent assays (ELISAs; competition ELISA used for RSB1) (see details on assays in Supplementary Information).
Fc-mediated antibody functionalities were evaluated in a post hoc analysis as described in Supplementary Information [21,22].
To evaluate CMI responses, frequencies of RSVPreF3-specific CD4+ and CD8+ T cells were measured after in vitro stimulation and background subtraction, using intracellular cytokine staining on peripheral blood mononuclear cells. Results were computed as frequencies per 1 million CD4+ or CD8+ T cells, with the lower limit of quantification of 590 (see details in Supplementary Information).
Statistical Analyses
Collected data were analyzed using descriptive statistics. In parts A and B, the exposed set (ES) was used for safety and demographic analyses, while the per-protocol set (PPS) was used for primary immunogenicity analyses. Since >10% of participants in part B were excluded from the PPS due to protocol deviations, vaccine immunogenicity was also analyzed on the ES for day 91, month 8, and month 14. Missing or invalid data were not considered. See further details on study sample size determination and ES- and PPS-based analyses in the Supplementary Information.
Categorical demographic variables were computed using frequencies (number/percentage), while descriptive statistics (mean, median, standard deviation, range) were calculated for continuous variables. Frequency of AEs was reported with numbers, percentages, and 95% confidence interval, per vaccine dose and overall. Humoral immune responses were evaluated as geometric mean concentrations (GMCs; for RSVPreF3-specific IgG) and geometric mean titers (GMTs; for nAb) of RSV-specific antibodies.
CMI responses were evaluated by descriptive statistics (median with minimum and maximum [min, max]) at each designated timepoint: geometric mean frequency (GMF) of RSVPreF3-specific CD4+ and CD8+ T cells and geometric mean ratio (GMR) of frequency of RSVPreF3-specific CD4+ T cells, at each postvaccination timepoint over prevaccination.
Statistical comparisons between groups were conducted in part B (see details in Supplementary Information) in terms of RSV-A nAb and CD4+ T-cell responses. Vaccine formulations were individually compared to placebo and among each other per adjuvant group using an analysis of covariance model.
RESULTS
Study Duration and Participants
Participants were enrolled between 21 January and 9 August 2019. All participants received their last dose by 23 October 2019 and completed the day 91 timepoint before the coronavirus disease 2019 pandemic (March 2020). The study ended on 23 February 2021. In part B, 1005 participants received at least 1 vaccine dose or placebo (ES), while 970 received 2 doses (Figure 2). Up to 211 participants were excluded from PPS at different timepoints. The long-term evaluation subset included all participants from part B1 and participants from part B2 who received placebo and the formulations containing 120 μg of RSVPreF3 antigen, for follow-up at month 14 (see details in Supplementary Information).
Figure 2.
Consolidated Standards of Reporting Trials (CONSORT) flow diagram of participant cohorts in study parts A and B. Abbreviations: AS01B and AS01E, adjuvanted vaccine formulations with the corresponding vaccine adjuvant systems; pIMD, potential immune-mediated disease; Plain, unadjuvanted vaccine formulations; RSVPreF3, prefusion conformation of the respiratory syncytial virus F protein; SAE, serious adverse event.
The demographic characteristics of all groups were comparable and well balanced in OAs (Table 1). The mean age at first vaccination was 67.6 years. Study participants were more commonly female (57.0%), non-Hispanic/Latino (96.4%), and White (92.2%). See Supplementary Information for demographic results in YAs.
Table 1.
Demographic Characteristics of Study Participants (Exposed Set)
| Participants | Adjuvanta | RSVPreF3 Antigen Concentration | No. | Age in Years at First Vaccination, Mean (SD) | Sex, No. (%) | Ethnicity, No. (%) | Race, No. (%) | ||
|---|---|---|---|---|---|---|---|---|---|
| Female | Not Hispanic or Latino | White | Black/African American | Otherb | |||||
| Young adults (18─40 y) | Plain | 30 µg | 12 | 31.2 (7.0) | 8 (66.7) | 12 (100) | 10 (83.3) | 2 (16.7) | 0 (0.0) |
| 60 µg | 12 | 26.5 (4.0) | 7 (58.3) | 11 (91.7) | 10 (83.3) | 1 (8.3) | 1 (8.3) | ||
| 120 µg | 12 | 29.9 (6.1) | 7 (58.3) | 11 (91.7) | 10 (83.3) | 2 (16.7) | 0 (0.0) | ||
| Placebo | 12 | 31.6 (5.6) | 9 (75.0) | 12 (100) | 11 (91.7) | 1 (8.3) | 0 (0.0) | ||
| Older adults (60─80 y) | Plain | 30 µg | 101 | 67.3 (5.6) | 58 (57.4) | 99 (98.0) | 95 (94.1) | 5 (5.0) | 1 (1.0) |
| 60 µg | 97 | 67.8 (5.6) | 54 (55.7) | 91 (93.8) | 90 (92.8) | 7 (7.2) | 0 (0.0) | ||
| 120 µg | 100 | 67.9 (4.9) | 57 (57.0) | 94 (94.0) | 93 (93.0) | 4 (4.0) | 0 (0.0) | ||
| AS01E | 30 µg | 101 | 67.8 (5.1) | 58 (57.4) | 98 (97.0) | 88 (87.1) | 12 (11.9) | 1 (1.0) | |
| 60 µg | 101 | 67.1 (5.6) | 57 (56.4) | 98 (97.0) | 94 (93.1) | 7 (6.9) | 0 (0.0) | ||
| 120 µg | 100 | 67.6 (5.2) | 57 (57.0) | 97 (97.0) | 93 (93.0) | 5 (5.0) | 1 (1.0) | ||
| AS01B | 30 µg | 103 | 67.6 (4.9) | 59 (57.3) | 99 (96.1) | 92 (89.3) | 11 (10.7) | 0 (0.0) | |
| 60 µg | 100 | 67.5 (4.9) | 58 (58.0) | 98 (98.0) | 93 (93.0) | 7 (7.0) | 0 (0.0) | ||
| 120 µg | 101 | 67.5 (4.9) | 57 (56.4) | 97 (96.0) | 92 (91.1) | 7 (6.9) | 1 (1.0) | ||
| Plain | Placebo | 101 | 68.1 (5.7) | 58 (57.4) | 98 (97.0) | 97 (96.0) | 4 (4.0) | 0 (0.0) | |
Abbreviations: RSVPReF3, prefusion conformation of the respiratory syncytial virus F protein; SD, standard deviation.
AS01B and AS01E, adjuvanted vaccine formulations with the corresponding vaccine adjuvant systems; Plain, unadjuvanted vaccine formulations.
Includes American Indian/Alaska Native and Asian participants.
Safety and Reactogenicity
Summary of Safety Results
IDMC evaluations identified no safety concerns precluding use of any vaccine formulation throughout the study. Proportions of participants reporting at least 1 solicited AE within 7 days and at least 1 unsolicited AE within 30 days were similar after each vaccination (data not shown). See Supplementary Information and Supplementary Figures 1 and 2 for safety results in YAs.
Safety Results in OAs
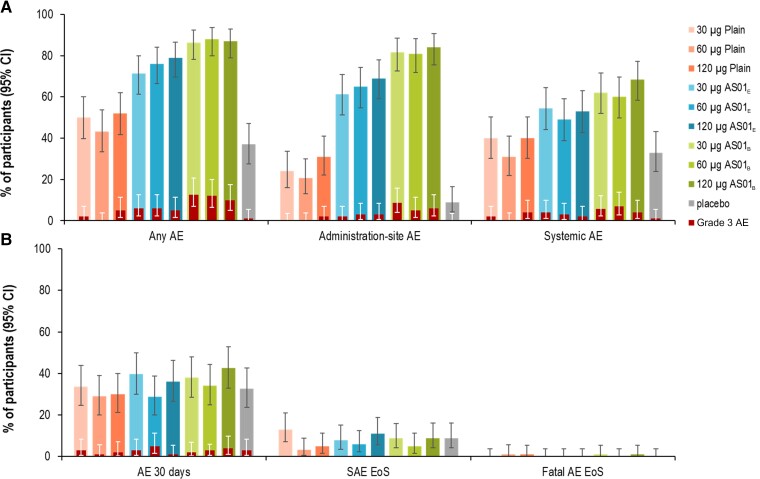
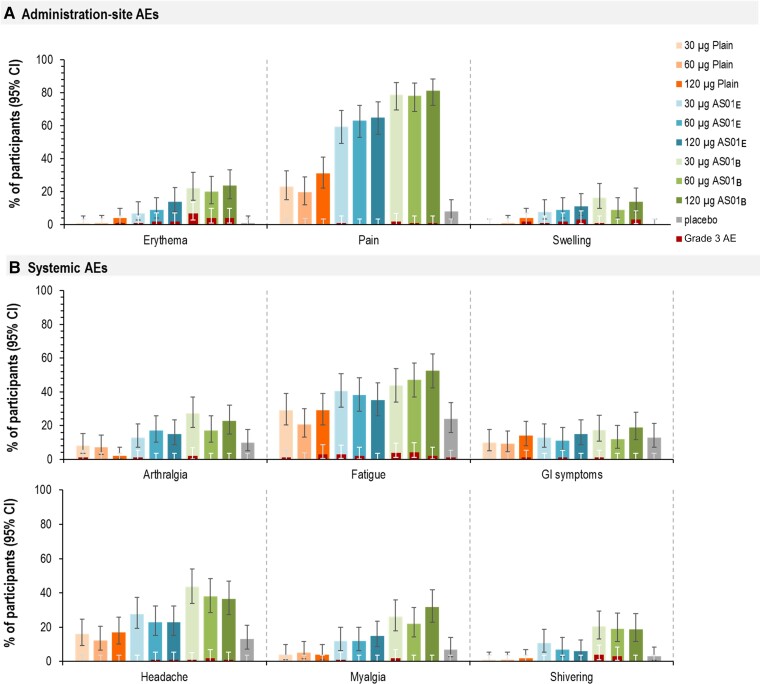
Within 7 days after any vaccination, 43.3%–52.0% of Plain, 71.3%–79.0% of AS01E, 86.4%–88.0% of AS01B, and 37.0% of placebo recipients reported at least 1 solicited AE (Figure 3). Solicited AEs were most frequent in AS01 groups (trend toward higher AE frequency in AS01B than AS01E) compared to Plain and placebo groups. The most frequently reported administration-site solicited AE was pain (8.0% in placebo, and 19.6% [60-Plain] to 81.2% [120-AS01B] in RSVPreF3 vaccine recipients) (Figure 4, Supplementary Table 2). Grade 3 solicited administration site events occurred in 0.0%–2.0% (Plain), 2.0%–3.0% (AS01E), and 5.0%–8.7% (AS01B) of RSVPreF3 vaccine recipients but not in the placebo group (Figure 3). The trend toward higher reactogenicity of the AS01-based formulations (highest for AS01B) was observed also for systemic solicited AEs (Figure 3). Fatigue (24.0% in placebo and 20.6% [60-Plain] to 52.5% [120-AS01B] in RSVPreF3 vaccine recipients) and headache (13.0% in placebo and 12.4% [60-Plain] to 43.7% [30-AS01B] in RSVPreF3 vaccine recipients) were the most frequent systemic solicited AEs (Figure 4, Supplementary Table 2). Most solicited systemic AEs were considered vaccine-related. Grade 3 solicited systemic AEs were reported by up to 1.0% of placebo, 3.0% (120-Plain), 3.0% (30-AS01E), and 4.0% (60-AS01B) of RSVPreF3 vaccine recipients (fatigue). All solicited AEs reported within 7 days of any administered dose were mostly mild and transient in nature (Supplementary Table 3).
Figure 3.
Summary of solicited adverse events (AEs) reported within 7 days after any dose (A) and unsolicited AEs (any within 30 days after any dose, serious AE [SAE], or fatality until study end) (B) in part B (older adults [60–80 years of age]) (exposed set). B, Percentages of participants experiencing at least 1 AE from the following categories and periods: any unsolicited AE within 30 days after any vaccination; an SAE until study end; fatal outcome until study end. 30 µg, 60 µg, and 120 µg indicate prefusion conformation of the respiratory syncytial virus F protein antigen concentration. Abbreviations: AE, adverse event; AS01B and AS01E, adjuvanted vaccine formulations with the corresponding vaccine adjuvant systems; CI, confidence interval; EoS, end of study; Plain, unadjuvanted vaccine formulation; SAE, serious adverse event.
Figure 4.
Solicited administration-site (A) and systemic adverse events (B) reported within 7 days after any vaccination in part B (older adults [60–80 years of age]) (exposed set). 30 µg, 60 µg, and 120 µg indicate prefusion conformation of the respiratory syncytial virus F protein antigen concentration. Abbreviations: AE, adverse event; AS01B and AS01E, adjuvanted vaccine formulations with the corresponding vaccine adjuvant systems; CI, confidence interval; GI, gastrointestinal; Plain, unadjuvanted vaccine formulation.
Within 30 days after vaccination, similar proportions of placebo (32.7%) and RSVPreF3 vaccine recipients (28.7% [60-AS01E] to 42.6% [120-AS01B]) reported unsolicited AEs (Figure 3, Supplementary Table 4). Between 1.0% and 5.0% of participants reported at least 1 grade 3 AE. Of all unsolicited AEs considered vaccine related (4.1% [60-Plain] to 19.8% [120-AS01B]), only 1 was grade 3 (constipation, in 30-AS01B). From first vaccination up to day 91 and month 14, 1.0% and 8.9% of placebo, up to 5.9% and 12.9% of Plain, and up to 5.0% and 11.0% of adjuvanted RSVPreF3 vaccine recipients reported at least 1 serious AE (SAE) (Supplementary Tables 4 and 5). No SAEs were considered vaccine-related (Supplementary Tables 4 and 5). Seven participants were withdrawn due to SAEs or potential immune-mediated diseases (pIMDs) until study end (1 each in 60-Plain, 120-Plain, 30-AS01E, and 30-AS01B, and 3 in 120-AS01B). Four participants died: 1 due to unknown reason (120-Plain), 1 to aortic aneurysm/cardiorespiratory arrest/hemorrhagic shock (60-Plain), 1 to cardiac arrest/respiratory distress (30-AS01B), and 1 to stage 4 lung carcinoma (120-AS01B). Four pIMDs were reported between day 91 and month 14: gout (placebo), autoimmune encephalitis (30-Plain), Bell’s palsy (60-AS01E), and rheumatoid arthritis (120-AS01E), but none were considered vaccine related.
Immunogenicity Results
Summary of Immunogenicity Results
All tested participants had detectable baseline RSV-specific antibodies (RSVPreF3-specific IgG, RSV-A- and RSV-B-specific nAb) due to previous RSV exposure(s). Baseline RSVPreF3-specific CD4+ T-cell frequencies were below the lower limit of quantification (Figures 5 and 6; Supplementary Figures 3, 5, and 6). RSVPreF3 vaccination robustly increased antibody levels and CD4+ T-cell frequencies compared to prevaccination (Figures 5 and 6; Supplementary Figures 3–5). Results from analyses on ES and PPS were comparable for all immunogenicity parameters (data not shown).
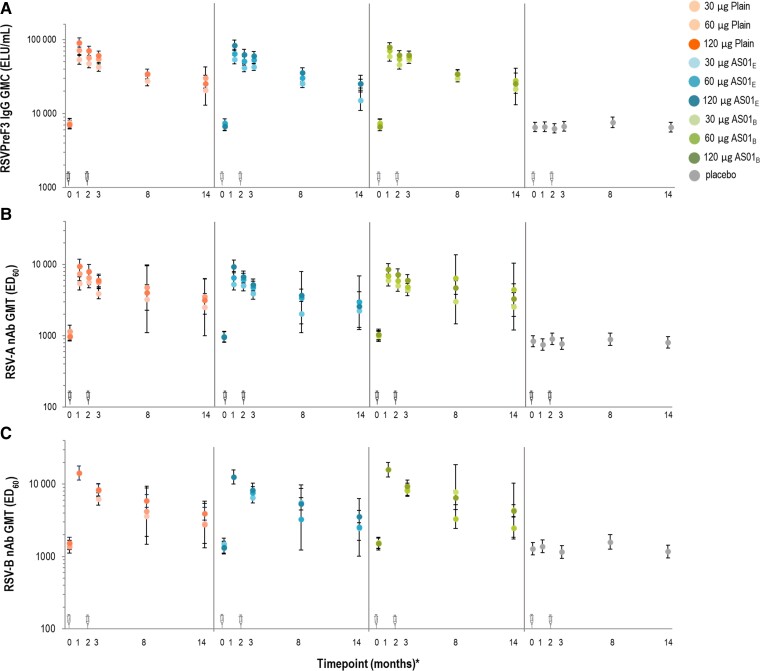
Figure 5.
Prefusion conformation of the respiratory syncytial virus F protein (RSVPreF3)-specific immunoglobulin G geometric mean concentration (A), respiratory syncytial virus (RSV) A-specific neutralizing antibody (nAb) geometric mean titer (GMT) (B), and RSV-B-specific nAb GMT values (C) in part B (older adults [60–80 years of age]) (per-protocol set). *The timepoints in months (0, 1, 2, and 3) reflect days 1 (vaccination 1), 31, 61 (vaccination 2), and 91, respectively. Month 14 data derive from the long-term evaluation subset. C, The data at timepoint month 1 (day 31) were only tested for formulations with the selected concentration of the RSVPreF3 antigen (120 μg) and placebo; the participants were also vaccinated twice and at same timepoints, but the blood samples were not analyzed on day 61. Error bars represent 95% confidence intervals. The syringe symbols above the x-axis designate vaccination timepoints. 30 µg, 60 µg, and 120 µg indicate prefusion conformation of the respiratory syncytial virus F protein antigen concentration. Abbreviations: AS01B and AS01E, adjuvanted vaccine formulations with the corresponding vaccine adjuvant systems; ED60, estimated dilution 60; ELU, enzyme-linked immunosorbent assay unit; GMC, geometric mean concentration; GMT, geometric mean titer; IgG, immunoglobulin G; nAb, neutralizing antibody; Plain, unadjuvanted vaccine formulations; RSV, respiratory syncytial virus; RSVPreF3, prefusion conformation of the respiratory syncytial virus F protein.
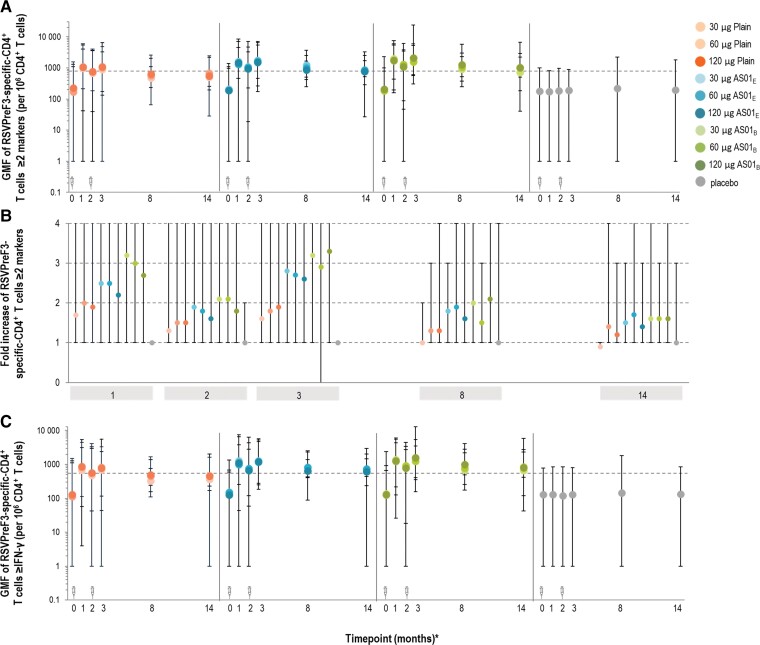
Figure 6.
Geometric mean frequency (GMF) (A) and fold increase (calculated as the ratio of T-cell concentration relative to day 1 values for the corresponding vaccine formulation) (B) in prefusion conformation of the respiratory syncytial virus F protein (RSVPreF3)-specific CD4+ T cells expressing at least 2 markersa and geometric mean frequency of RSVPreF3-specific CD4+ T cells expressing at least interferon gamma (IFN-γ)b (C) in part B (older adults [60–80 years of age]) (per-protocol set). aAt least 2 of the following in vitro markers: interleukin (IL) 2, CD40 ligand, tumor necrosis factor-α, IFN-γ. bAt least IFN-γ among IFN-γ, IL-13, and IL-17. *The timepoints in months (0, 1, 2, and 3) reflect days 1 (vaccination 1), 31, 61 (vaccination 2), and 91, respectively. Month 14 data derive from the long-term evaluation subset. A and C, The syringe symbols above the x-axis designate vaccination timepoints; dotted lines represent the assay cutoff of 590. GMF values are plotted as the median; error bars denote the range (min, max). 30 µg, 60 µg, and 120 µg indicate RSVPreF3 antigen concentration. Abbreviations: AS01B and AS01E, adjuvanted vaccine formulations with the corresponding vaccine adjuvant systems; GMF, geometric mean frequency; IFN-γ, interferon gamma; Plain, unadjuvanted vaccine formulations; RSVPreF3, prefusion conformation of the respiratory syncytial virus F protein.
Immunogenicity Results in OAs
All RSVPreF3 vaccine formulations induced higher RSV-A nAb response compared to placebo at day 31 and day 91 (P < .0001) (Figure 5). Mean fold increases (postvaccination over prevaccination) of RSV-A nAb GMTs in RSVPreF3 recipients ranged from 5.6 to 9.9 on day 31, 3.8 to 6.6 on day 91, and 2.7 to 4.4 at month 14 (Supplementary Table 6). A positive linear effect of the RSVPreF3 antigen concentration was demonstrated in terms of RSV-A nAb GMTs (all vaccine groups) (P < .0001; Figure 5 and Supplementary Information). The AS01-based adjuvant did not significantly affect GMTs of RSV-A nAb (P > .025). Similar to RSV-A, the investigational RSVPreF3 vaccine formulations induced robust RSV-B nAb, RSVPreF3-specific IgG and RSB1 responses in OAs (Figure 5, Supplementary Table 7). Fold increases (postvaccination over prevaccination) in RSVPreF3-specific IgG GMCs were 7.2–12.8 on day 31, 5.5–9.3 on day 91, and 2.6–4.5 at month 14 (Supplementary Table 6).
Fold increase ratios of RSVPreF3 IgG over RSV-A and RSV-B nAb were similar at each timepoint and for all vaccine formulations, for both RSV-A (0.6–1.6) and RSV-B (1.0–1.9) (Supplementary Figure 4).
A post hoc analysis of humoral functional features induced 1 month after 1 dose of the RSVPreF3 vaccine demonstrated a polyfunctional profile, with significant induction of immunoglobulin A (IgA) and activation of natural killer (NK) cells, granulocytes, and the complement pathway (details in Supplementary Information and Supplementary Figure 7).
Mean baseline GMFs of CD4+ T cells expressing at least 2 activation markers (among interleukin [IL] 2, CD40L, tumor necrosis factor–α, and interferon gamma [IFN-γ]) were lower in OAs (86.2 [min, max: 1, 1087] to 142.5 [min, max: 1, 1537]) than YAs (232.2 [min, max: 4, 1057] to 457.6 [min, max: 218, 1549]). The GMFs of these polyfunctional RSVPreF3-specific CD4+ T cells increased distinctly in OAs after the first vaccine dose for all vaccine formulations (Figure 6; Supplementary Table 6).
The second vaccine dose transiently increased the relative frequency of polyfunctional RSVPreF3-specific CD4+ T cells on day 91, but not above day 31 levels (P > .025) (Figure 6). Compared to prevaccination, the GMF of these CD4+ T cells increased by 1.7–3.2 on day 31, 1.3–2.1 on day 61, and 1.6–3.3 on day 91 (Supplementary Table 6).
The presence of any adjuvant significantly increased CMI responses compared to no adjuvant on day 31 (GMR, 1.33 [AS01E] and 1.65 [AS01B]; P < .0001). A statistically significant effect of AS01B over AS01E was demonstrated (GMR, 1.23; P = .0001).
GMFs of RSVPreF3-specific CD4+ T cells expressing at least IFN-γ (among IFN-γ, IL-13, and IL-17) followed similar trends as GMFs of RSVPreF3-specific CD4+ T cells expressing at least 2 markers (Figure 6).
Vaccination with RSVPreF3 formulations did not detectably increase CD8+ T-cell responses compared to placebo (Supplementary Figure 8).
DISCUSSION
Given the RSV seasonal spread and incomplete and limited duration of immunity after natural infection, vaccination to prevent RSV-induced LRTD could significantly reduce the disease burden in OAs. The present data demonstrate that the investigational RSVPreF3-based vaccine has an acceptable safety profile and elicits RSV-specific humoral and CMI responses persisting up to 12 months after a 2-dose vaccination. Given the immunological benefit of the formulations with the highest RSVPreF3 antigen level (120 µg) and the less reactogenic AS01E adjuvant, the 120-AS01E formulation has been selected for further clinical evaluation.
Vaccine formulations were well tolerated in YAs and OAs. In OAs, solicited administration-site AEs were most frequently reported in adjuvanted RSVPreF3 vaccine recipients, followed by unadjuvanted formulations and placebo. A tendency toward lower reactogenicity of AS01E- compared to AS01B-containing formulations was observed. Although adjuvanted formulations were more reactogenic, few participants reported grade 3 solicited AEs, which were transient. Frequencies of unsolicited AEs, SAEs, and pIMDs were similar among all groups, and no SAEs or pIMDs were considered vaccine-related, supporting the acceptable safety profile of the RSVPreF3 formulations.
Due to previous RSV exposure, YAs and OAs participants had measurable levels of prevaccination RSVPreF3-specific IgG and nAb. All RSVPreF3 vaccine formulations induced robust immune responses after the first vaccine dose in terms of RSVPreF3-specific IgG levels, RSV-specific nAb titers, and polyfunctional RSVPreF3-specific CD4+ T cells, in YAs and OAs. No effect on CD8+ T-cell levels was observed, regardless of the RSVPreF3 antigen dose or adjuvant presence.
Humoral immune responses (RSVPreF3-specific IgG concentrations, and RSV-A and RSV-B nAb titers) were highest on day 31, without added effect of the second vaccine dose. Adjuvant presence did not impact the observed IgG and nAb responses, consistent with recently published immunogenicity profiles of RSVPreF-based vaccines in adults aged 18–50 years [23]. Also, unadjuvanted and AlOH-adjuvanted RSVPreF-based vaccines were previously found to comparably boost RSV-specific nAb responses, which were highest after the first vaccine dose [23–26]. In OAs, a positive linear effect of increased RSVPreF3 antigen concentrations on RSV-A-specific nAb titers was observed, with 120 µg formulations being the most potent. This is in agreement with previous findings of higher immunogenicity observed with higher RSVPreF antigen concentrations administered per different schedules in nonpregnant women [19,27] or a 2-dose schedule in OAs [28].
Although RSV-specific antibody levels declined over time, IgG concentrations and nAb titers remained above prevaccination and placebo levels at 12 months postvaccination. In OAs, GMRs of fold increase in RSVPreF3-specific IgG and RSV-A and RSV-B nAb levels were 0.6–1.6 and 1.2–1.9, respectively. This balanced induction of RSVPreF3-specific IgG and RSV-A and RSV-B nAb indicates that the investigated RSVPreF3 vaccine formulations may protect OAs recipients against RSV-LRTD [29].
Beyond virus neutralization, a role of Fc-mediated humoral functionalities in protection against RSV has been proposed [30]. Antibody-dependent cellular cytotoxicity was suggested as part of the protective mechanism induced by an adenoviral vector encoding RSVPreF in small animal models [31]. Functional antibody profiling in nonhuman primate RSV challenge models demonstrated that the RSV-F vaccine–induced Fc-mediated ability to drive NK degranulation and complement deposition was linked to protection [32]. Interestingly, induction of RSVPreF-specific IgA was also associated with viral control in this model, in alignment with previous findings in humans [33,34]. The present system serology data showed significant increases in IgA titers and Fc-mediated functionalities, such as complement deposition and NK activation, after 1 dose of the selected formulation. These findings thus suggest potential clinical efficacy of the selected RSVPreF3 formulation in OAs, though no immunological correlate of protection has yet been established.
The apparent lower baseline frequencies of RSV-specific CD4+ T cells in OAs likely reflect the age-associated loss of RSV-specific CMI [9]. While antigen concentrations did not influence CD4+ T-cell responses within each formulation, the addition of adjuvant resulted in higher CD4+ T-cell frequencies. The stimulating effect of AS01-based adjuvants on cellular immunity in OAs is well documented [16,17]. This adjuvant-mediated boosting of CD4+ T-cell responses in OAs to similar levels as in YAs after each vaccination and the persistence of this response underscore the ability of adjuvanted RSVPreF3 vaccine formulations to boost CMI despite demonstrated immunosenescence in OAs [35]. A robust and durable RSV-specific CMI response is especially beneficial in OAs, given that waning cellular immunity may prevent efficient virus clearance and therefore increase susceptibility to severe RSV infections [9,10,29]. The increased polyfunctional CD4+ response indicates that CD4+ T cells may still be recruited in OAs.
A limitation of this study was that the OA participants, with minimal medical history, are likely not representative of the general OA population that may have more comorbidities. This selection may have led to fewer AEs and a better immune response to investigational vaccines. Also, >10.0% of OA participants were excluded from the PPS for immunogenicity analyses. However, analyses on ES and PPS yielded similar results, so this is unlikely to bias the presented data.
The main strengths of this study are its factorial staggered design, the stringent oversight by the IDMC to ensure maximal participant safety, the number of tested vaccine formulations, and the high numbers of enrolled participants for a phase 1/2 study.
In conclusion, the 120-AS01E formulation has been selected for further clinical development as a single-dose schedule vaccine, based on its ability to boost humoral and CMI responses in the target OA population and its clinically acceptable safety profile. Future studies, such as the ongoing phase 3 trials of the selected RSVPreF3 vaccine formulation (NCT04886596 and NCT04732871), will shed further light on the durability and protective capacity of the vaccine-induced RSV-specific immune responses in OAs.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Isabel Leroux-Roels, Center for Vaccinology, Ghent University Hospital, Ghent, Belgium.
Matthew G Davis, Rochester Clinical Research, Rochester, New York, USA.
Katie Steenackers, Vaccine and Infectious Disease Institute, University of Antwerp, Wilrijk, Belgium.
Brandon Essink, Meridian Clinical Research Omaha, Omaha, Nebraska, USA.
Corinne Vandermeulen, Department of Public Health and Primary Care, Leuven University Vaccinology Center, KU Leuven, Leuven, Belgium.
Charles Fogarty, Lung and Chest Medical Associates, Spartanburg Medical Research, Spartanburg, South Carolina, USA.
Charles P Andrews, IMA Clinical Research, San Antonio, Texas, USA.
Edward Kerwin, Crisor, LLC, c/o Clinical Research Institute of Southern Oregon, Medford, Oregon, USA.
Marie-Pierre David, GSK, Wavre, Belgium.
Laurence Fissette, GSK, Wavre, Belgium.
Carline Vanden Abeele, GSK, Wavre, Belgium.
Delphine Collete, GSK, Rixensart, Belgium.
Magali de Heusch, GSK, Wavre, Belgium.
Bruno Salaun, GSK, Rixensart, Belgium.
Nathalie De Schrevel, GSK, Rixensart, Belgium.
Juliane Koch, GSK, Wavre, Belgium.
Céline Verheust, GSK, Wavre, Belgium.
Nancy Dezutter, GSK, Wavre, Belgium.
Frank Struyf, GSK, Wavre, Belgium.
Narcisa Mesaros, GSK, Wavre, Belgium.
Jelena Tica, GSK, Wavre, Belgium.
Veronica Hulstrøm, GSK, Wavre, Belgium.
Notes
Author contributions . B. E., C. Va., J. K., C. Ve., M.-P. D., L. F., N. D. S., N. D., F. S., N. M., and J. T. contributed to the conceptualization of the study. I. L.-R., E. K., D. C., M. G. D., K. S., C. F., C. P. A., M. d. H., B. E., C. Va., N. M., B. S., J. K., C. Ve., M.-P. D., N. D. S., J. T., and V. H. contributed to data collection or generation. B. S., N. D. S., and J. T. contributed to methodology. M.-P. D., L. F., C. V. A., and J. T. contributed to formal analysis. I. L.-R., E. K., D. C., C. Va., N. M., B. S., J. K., C. Ve., M.-P. D., L. F., C. V. A., N. D. S., N. D., J. T., and V. H. were involved in data analysis/interpretation. J. T. drafted the original draft of the manuscript. All authors contributed to manuscript review and editing and approved the final version of the manuscript.
Acknowledgments. We thank all study participants and their families as well as the staff at the participating institutions. We also appreciate the time and contribution of the Independent Data Monitoring Committee. We thank the technical assistance of staff at GSK Clinical Laboratory Science Laboratories and GSK clinical, in particular Anne-Marie Camier, Frederique Bossiroy, Ouafae Bouchikhi, Stephanie Howard, Geetha Kamath, Stacey Lewis, Anne Meulemans, Amoolya Modi, Margaret Schultz, and Melanie Steffens. We also thank SeromYx Inc for the system serology work, and the Modis platform for editorial assistance and manuscript coordination, on behalf of GSK. Irena Zurnic Bönisch provided medical writing support, and Stéphanie Deroo coordinated manuscript development and provided editorial support.
Data availability. The study report including the protocol is available on the GSK Clinical Study Register (https://www.gsk-studyregister.com/). Anonymized individual participant data and study documents can be requested for further research from: www.clinicalstudydatarequest.com (study ID 208851).
Disclaimer. GlaxoSmithKline Biologicals SA was involved in all stages of the study conduct and analysis and took responsibility for all costs associated with the development and the publishing of the present manuscript.
Financial support. Funding to pay the Open Access publication charges for this article was provided by GlaxoSmithKline Biologicals SA.
References
- 1. Simoes EA. Respiratory syncytial virus infection. Lancet 1999; 354:847–52. [DOI] [PubMed] [Google Scholar]
- 2. Ascough S, Paterson S, Chiu C. Induction and subversion of human protective immunity: contrasting influenza and respiratory syncytial virus. Front Immunol 2018; 9:323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hause AM, Henke DM, Avadhanula V, Shaw CA, Tapia LI, Piedra PA. Sequence variability of the respiratory syncytial virus (RSV) fusion gene among contemporary and historical genotypes of RSV/A and RSV/B. PLoS One 2017; 12:e0175792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mas V, Nair H, Campbell H, Melero JA, Williams TC. Antigenic and sequence variability of the human respiratory syncytial virus F glycoprotein compared to related viruses in a comprehensive dataset. Vaccine 2018; 36:6660–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Russell CD, Unger SA, Walton M, Schwarze J. The human immune response to respiratory syncytial virus infection. Clin Microbiol Rev 2017; 30:481–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med 2005; 352:1749–59. [DOI] [PubMed] [Google Scholar]
- 7. Falsey AR, McElhaney JE, Beran J, et al. . Respiratory syncytial virus and other respiratory viral infections in older adults with moderate to severe influenza-like illness. J Infect Dis 2014; 209:1873–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shi T, Denouel A, Tietjen AK, et al. . Global disease burden estimates of respiratory syncytial virus–associated acute respiratory infection in older adults in 2015: a systematic review and meta-analysis. J Infect Dis 2020; 222:S577–83. [DOI] [PubMed] [Google Scholar]
- 9. Cherukuri A, Patton K, GasserRA, Jret al. . Adults 65 years old and older have reduced numbers of functional memory T cells to respiratory syncytial virus fusion protein. Clin Vaccine Immunol 2013; 20:239–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Malloy AM, Falsey AR, Ruckwardt TJ. Consequences of immature and senescent immune responses for infection with respiratory syncytial virus. Curr Top Microbiol Immunol 2013; 372:211–31. [DOI] [PubMed] [Google Scholar]
- 11. Boyoglu-Barnum S, Chirkova T, Anderson LJ. Biology of infection and disease pathogenesis to guide RSV vaccine development. Front Immunol 2019; 10:1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Graham BS, Modjarrad K, McLellan JS. Novel antigens for RSV vaccines. Curr Opin Immunol 2015; 35:30–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Didierlaurent AM, Laupeze B, Di Pasquale A, Hergli N, Collignon C, Garcon N. Adjuvant system AS01: helping to overcome the challenges of modern vaccines. Expert Rev Vaccines 2017; 16:55–63. [DOI] [PubMed] [Google Scholar]
- 14. Garcon N, Van Mechelen M. Recent clinical experience with vaccines using MPL- and QS-21-containing adjuvant systems. Expert Rev Vaccines 2011; 10:471–86. [DOI] [PubMed] [Google Scholar]
- 15. Leroux-Roels G, Marchant A, Levy J, et al. . Impact of adjuvants on CD4(+) T cell and B cell responses to a protein antigen vaccine: results from a phase II, randomized, multicenter trial. Clin Immunol 2016; 169:16–27. [DOI] [PubMed] [Google Scholar]
- 16. Chlibek R, Smetana J, Pauksens K, et al. . Safety and immunogenicity of three different formulations of an adjuvanted varicella-zoster virus subunit candidate vaccine in older adults: a phase II, randomized, controlled study. Vaccine 2014; 32:1745–53. [DOI] [PubMed] [Google Scholar]
- 17. Schwarz TF, Volpe S, Catteau G, et al. . Persistence of immune response to an adjuvanted varicella-zoster virus subunit vaccine for up to year nine in older adults. Hum Vaccin Immunother 2018; 14:1370–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Voic H, de Vries RD, Sidney J, et al. . Identification and characterization of CD4(+) T cell epitopes after Shingrix vaccination. J Virol 2020; 94:e01641–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schwarz TF, Johnson C, Grigat C, et al. . Three dose levels of a maternal respiratory syncytial virus vaccine candidate are well tolerated and immunogenic in a randomized trial in non-pregnant women. J Infect Dis 2022; 225:2067–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ngwuta JO, Chen M, Modjarrad K, et al. . Prefusion F-specific antibodies determine the magnitude of RSV neutralizing activity in human sera. Sci Transl Med 2015; 7:309ra162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Boudreau CM, Yu WH, Suscovich TJ, Talbot HK, Edwards KM, Alter G. Selective induction of antibody effector functional responses using MF59-adjuvanted vaccination. J Clin Invest 2020; 130:662–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Suscovich TJ, Fallon JK, Das J, et al. . Mapping functional humoral correlates of protection against malaria challenge following RTS, S/AS01 vaccination. Sci Transl Med 2020; 12:eabb4757. [DOI] [PubMed] [Google Scholar]
- 23. Ruckwardt TJ, Morabito KM, Phung E, et al. . Safety, tolerability, and immunogenicity of the respiratory syncytial virus prefusion F subunit vaccine DS-Cav1: a phase 1, randomised, open-label, dose-escalation clinical trial. Lancet Resp Med 2021; 9:1111–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Beran J, Lickliter JD, Schwarz TF, et al. . Safety and immunogenicity of 3 formulations of an investigational respiratory syncytial virus vaccine in nonpregnant women: results from 2 phase 2 trials. J Infect Dis 2018; 217:1616–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Langley JM, Aggarwal N, Toma A, et al. . A randomized, controlled, observer-blinded phase 1 study of the safety and immunogenicity of a respiratory syncytial virus vaccine with or without alum adjuvant. J Infect Dis 2017; 215:24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Walsh EE, Falsey AR, Scott DA, et al. . A randomized phase 1/2 study of a respiratory syncytial virus prefusion F vaccine. J Infect Dis 2022; 225:1357–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schwarz TF, McPhee RA, Launay O, et al. . Immunogenicity and safety of 3 formulations of a respiratory syncytial virus candidate vaccine in nonpregnant women: a phase 2, randomized trial. J Infect Dis 2019; 220:1816–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Williams K, Bastian AR, Feldman RA, et al. . Phase 1 safety and immunogenicity study of a respiratory syncytial virus vaccine with an adenovirus 26 vector encoding prefusion F (Ad26.RSV.preF) in adults aged ≥60 years. J Infect Dis 2020; 222:979–88. [DOI] [PubMed] [Google Scholar]
- 29. Stephens LM, Varga SM. Considerations for a respiratory syncytial virus vaccine targeting an elderly population. Vaccines 2021; 9:624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. van Erp EA, Luytjes W, Ferwerda G, van Kasteren PB. Fc-mediated antibody effector functions during respiratory syncytial virus infection and disease. Front Immunol 2019; 10:548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Saeland E, van der Fits L, Bolder R, et al. . Immunogenicity and protective efficacy of adenoviral and subunit RSV vaccines based on stabilized prefusion F protein in pre-clinical models. Vaccine 2022; 40:934–44. [DOI] [PubMed] [Google Scholar]
- 32. Zohar T, Hsiao JC, Mehta N, et al. . Upper and lower respiratory tract correlates of protection against respiratory syncytial virus following vaccination of nonhuman primates. Cell Host Microbe 2022; 30:41–52.e5. [DOI] [PubMed] [Google Scholar]
- 33. Walsh EE, Falsey AR. Humoral and mucosal immunity in protection from natural respiratory syncytial virus infection in adults. J Infect Dis 2004; 190:373–8. [DOI] [PubMed] [Google Scholar]
- 34. Habibi MS, Jozwik A, Makris S, et al. . Impaired antibody-mediated protection and defective IgA B-cell memory in experimental infection of adults with respiratory syncytial virus. Am J Respir Crit Care Med 2015; 191:1040–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lambert ND, Ovsyannikova IG, Pankratz VS, Jacobson RM, Poland GA. Understanding the immune response to seasonal influenza vaccination in older adults: a systems biology approach. Expert Rev Vaccines 2012; 11:985–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.