Abstract
Background
The impact of COVID-19 on the epidemiology, clinical characteristics, and infection spectrum of viral and bacterial respiratory infections in Western China is unknown.
Methods
We conducted an interrupted time series analysis based on surveillance of acute respiratory infections (ARI) in Western China to supplement the available data.
Results
The positive rates of influenza virus, Streptococcus pneumoniae, and viral and bacterial coinfections decreased, but parainfluenza virus, respiratory syncytial virus, human adenovirus, human rhinovirus, human bocavirus, non-typeable Haemophilus influenzae, Mycoplasma pneumoniae, and Chlamydia pneumoniae infections increased after the onset of the COVID-19 epidemic. The positive rate for viral infection in outpatients and children aged <5 years increased, but the positive rates of bacterial infection and viral and bacterial coinfections decreased, and the proportion patients with clinical symptoms of ARI decreased after the onset of the COVID-19 epidemic. Non-pharmacological interventions reduced the positive rates of viral and bacterial infections in the short term but did not have a long-term limiting effect. Moreover, the proportion of ARI patients with severe clinical symptoms (dyspnea and pleural effusion) increased in the short term after COVID-19, but in the long-term, it decreased.
Conclusions
The epidemiology, clinical characteristics, and infection spectrum of viral and bacterial infections in Western China have changed, and children will be a high-risk group for ARI after the COVID-19 epidemic. In addition, the reluctance of ARI patients with mild clinical symptoms to seek medical care after COVID-19 should be considered. In the post-COVID-19 era, we need to strengthen the surveillance of respiratory pathogens.
Introduction
After the 2019 novel coronavirus disease (COVID-19) outbreak, many countries adopted control measures to deal with this disease [12, 33, 36, 39]. In mainland China, all provinces launched the “first-level response” in January 2020 to control the COVID-19 epidemic [26]. During the COVID-19 pandemic, the control measures adopted by mainland China included blocking communities, traffic control, restricting entry and exit, and working at home [10, 20, 36]. These non-pharmaceutical interventions (NPIs) can help people maintain sufficient social distance to block the transmission of SARS-CoV-2 [31]. Studies have shown that NPIs applied during the COVID-19 pandemic had a major impact on other diseases as well. For example, although it has been reported that COVID-19 control measures had an impact on cancer, respiratory infections, digestive infections, zoonotic diseases, and sexually transmitted diseases [8, 26, 29, 30], these studies mainly examined the impact of NPIs on the incidence, mortality, or diagnosis rates of these diseases, but not on the epidemiology, clinical characteristics, and infection spectrum. Studies have been conducted in Europe, Brazil, North America, and Korea to explore the impact of COVID-19 on viral and bacterial respiratory infections [1, 15, 35, 37], but in mainland China, the only such studies were conducted in the eastern and southern regions [6, 23, 38], and there is a need to carry out such studies in Western China, in particular because the level of medical care, climate, and environment of Western China differ from those of other regions of China [25]. Here, we used an interrupted time series (ITS) design to explore the immediate and long-term impact of the COVID-19 outbreak on the epidemiology, infection spectrum, and clinical characteristics of viral and bacterial respiratory infections. Our findings can help in the surveillance and prevention of viral and bacterial respiratory infections in Western China in the post-COVID-19 era.
Materials and methods
Case sources
Active surveillance of acute respiratory infections (ARI) was conducted from January 2010 to December 2020 in Western China (Gansu, Qinghai, Xinjiang, and Inner Mongolia), and at least 5000 ARI cases were recorded over each five-year period. The case definition of ARI consisted of three components: (1) acute infection manifestations including at least one of the following: fever, elevated or decreased white blood cell counts or abnormal white blood cell profiles, chills, decreased body temperature (considering age); (2) respiratory clinical manifestations including at least one of the following: cough (new or worsening), sputum production, shortness of breath, abnormal breath sounds on auscultation, or chest pain; and (3) chest X-ray suggestive of inflammatory changes in the lungs. For patients meeting the inclusion criteria, physicians or nurses at the sentinel hospital used a standard questionnaire developed by the CDC (China Center for Disease Control and Prevention) to collect basic demographic information and clinical characteristics, and specimens were collected at the same time. Informed consent was obtained from patients for this surveillance program, and this study was approved by the Ethics Review Committee of the Gansu Provincial Center for Disease Control and Prevention (approval number 2018-007).
Specimen collection
Each ARI surveillance laboratory collected blood specimens, nasal/pharyngeal swabs, sputum, nasopharyngeal aspirates, bronchoalveolar lavage fluid, thoracentesis fluid specimens, and urine specimens from ARI patients. Blood specimens, respiratory specimens and urine specimens were sent for examination as soon as possible after collection. Blood culture bottles were sent for testing within 24 hours and were stored at room temperature (15-30°C). Other specimens were stored at 4-8°C before sending for testing.
Virological methods
Viral nucleic acid from influenza virus (Flu), parainfluenza virus (PIV), respiratory syncytial virus (RSV), human metapneumovirus (HMPV), human adenovirus (HAdV), human coronavirus (HCoV), human rhinovirus (HRV), and human bocavirus (HBoV) were extracted using commercial kits (Invitrogen/QIAGEN/Roche/Promega) and automated nucleic acid extraction equipment (QIAGEN/Roche/bioMerieux Applied Biosystems) for nucleic acid testing by polymerase chain reaction (PCR) or reverse transcription polymerase chain reaction (RT-PCR).
Bacteriological methods
In this study, bacteriological testing was performed by culturing Pseudomonas aeruginosa (PA), Klebsiella pneumoniae (KP), Staphylococcus aureus (SA), Streptococcus pneumoniae (SP), non-typeable Haemophilus influenzae (NTHi), Mycoplasma pneumoniae (MP), and Chlamydia pneumoniae (CP). During sampling, we placed whole-blood specimens in blood culture bottles for bacterial culture, while sputum, nasopharyngeal aspirate, bronchoalveolar lavage fluid, and thoracentesis fluid specimens were inoculated onto blood agar medium, MacConkey agar medium, and chocolate agar medium for bacterial culture after homogenization, pH neutralization, or bacterial enrichment, and the plates were incubated at 35 ℃ and 5-10% CO2 in a humid environment for 18-24 hours. After whole-blood specimens, sputum, nasopharyngeal aspirate, bronchoalveolar lavage fluid, and thoracentesis fluid specimens were cultured, we performed preliminary isolation and identification of PA, KP, SA, SP, and NTHi based on colony morphology, Gram staining, and biochemical methods (using the API biochemical identification system or the VITEK automatic bacterial identification instrument). In addition, serum specimens were used for CP antibody detection, and nasal/pharyngeal swab specimens were used for MP and CP detection. Urine specimens from ARI patients were not used for bacterial culture but were used for antigen detection of SP. For specimens with negative results for bacterial isolation, we used commercial kits (Invitrogen/QIAGEN/Roche/Promega) and automated nucleic acid extraction equipment (QIAGEN/Roche/bioMerieux/Applied Biosystems) to extract bacterial nucleic acids and then used PCR or real-time PCR to detect PA, KP, SA, SP, NTHi, MP, and CP.
Interrupted time series design
This study used an ITS design to analyze the immediate and long-term impact of the COVID-19 outbreak on the positive rates of viral and bacterial infections in ARI patients. We used January 2020 as the intervention time point and the positivity rate as the outcome variable Y. The pre-intervention time variable was X1 (taking values of 1, 2, 3, ..., n), the intervention variable was X2 (0 prior to January 2020, 1 from January 2020), the post-intervention time variable was X3 (0 prior to January 2020, with values of 0, 1, 2, …, n in order by month from January 2020), and ε was the random error term not explained by the outcome variable. The ITS model was:
where β0 is the rate at the beginning, β1 represents the trend in the rate before COVID-19, β2 is the level change in the rate in the short term (within January 2020), which reflects the immediate impact of COVID-19 on the rate, β3 is the slope change in the rate after the onset of the COVID-19 epidemic (from January 2020 to December 2020), which reflects the long-term impact of COVID-19 on the rate, and β1+β3 represents the trend in the rate after COVID-19 [2, 22, 32].
Statistical analysis
This study included the epidemiology, clinical signs and symptoms, and laboratory test results for respiratory pathogens for 12,805 ARI patients. Age was expressed as the median and interquartile range (IQR), rates were compared using Pearson's chi-square test, and rates for different groups were then subjected to multiple comparisons if statistically significant differences were found. Fitting of ITS data was done using a Poisson regression model. Because the number of ARI patients did not obey the Poisson distribution, we modeled directly using the number of monthly targeted cases and then included the number of ARI patients (log-transformed) as an offset variable in order to transform back to rates. In addition, we used Fourier terms (pairs of sine and cosine functions) in ITS to adjust the seasonality/periodicity of the rate. Pearson's chi-square test and ITS were performed using Stata 15.0 software, and the histograms were produced using Origin 2021. All testing was two-sided with significance determined at P < 0.05.
Results
Comparative analysis of the epidemiology of ARI in Western China before and after COVID-19
A total of 12,805 ARI patients were surveilled in sentinel hospitals in Western China from 2010 to 2020. These included 7774 (60.71%) males and 5031 (39.29%) females. The minimum age of ARI patients was 0 years, the maximum age was 108 years, and the median age was 13 years (IQR: 4-53 years). There were no statistically significant differences in the gender distribution and the positive rate of viral infection in ARI of the patients before and after the onset of the COVID-19 epidemic (P > 0.05) (Table 1). However, there were differences in the age distribution of the patients and the seasonal distribution of ARI before and after COVID-19 (P < 0.001). Multiple comparisons showed that the proportion of ARI patients aged <5 years increased but the proportion of ARI patients aged 15-59 years decreased after COVID-19. The proportion of ARI cases occurring in spring and autumn increased and those in summer and winter decreased after COVID-19 (P < 0.05). The proportion of outpatients decreased but the proportion of inpatients increased after COVID-19 (P < 0.05). The positive rate of bacterial infection in ARI patients decreased after COVID-19 (P < 0.001), and the positive rate of viral and bacterial coinfections in ARI patients decreased after COVID-19 (P < 0.001).
Table 1.
Comparative analysis of the epidemiology of ARI in Western China before and after COVID-19
| Variable | Number (%) of acute respiratory infections | χ2 | P-value | ||
|---|---|---|---|---|---|
| 2010-2019 | 2020 | ||||
| Gender | Male | 7319 (60.67) | 455 (61.40) | 0.158 | 0.691 |
| Female | 4745 (39.33) | 286 (38.60) | |||
| Age | <5 | 3613 (29.95) | 273 (36.84) | 29.06 | <0.001 |
| 5-14 | 2550 (21.14) | 161 (21.73) | |||
| 15-59 | 3462 (28.70) | 150 (20.24) | |||
| ≥60 | 2439 (20.22) | 157 (21.19) | |||
| Season | Spring | 2543 (21.08) | 208 (28.07) | 40.47 | <0.001 |
| Summer | 2465 (20.43) | 117 (15.79) | |||
| Autumn | 3454 (28.63) | 247 (33.33) | |||
| Winter | 3602 (29.86) | 169 (22.81) | |||
| Consultations | Outpatient | 5292 (43.87) | 295 (39.81) | 4.667 | 0.031 |
| Inpatient | 6772 (56.13) | 446 (60.19) | |||
| Viral infection | Positive | 2760 (22.88) | 165 (22.27) | 0.148 | 0.701 |
| Negative | 9304 (77.12) | 576 (77.73) | |||
| Bacterial infection | Positive | 1286 (10.66) | 19 (2.56) | 49.99 | <0.001 |
| Negative | 10778 (89.34) | 722 (97.44) | |||
| Viral and bacterial co-infections | Positive | 366 (3.03) | 3 (0.40) | 17.241 | <0.001 |
| Negative | 11698 (96.97) | 738 (99.60) | |||
Changes in the viral and bacterial infection spectrum of ARI cases in Western China before and after COVID-19
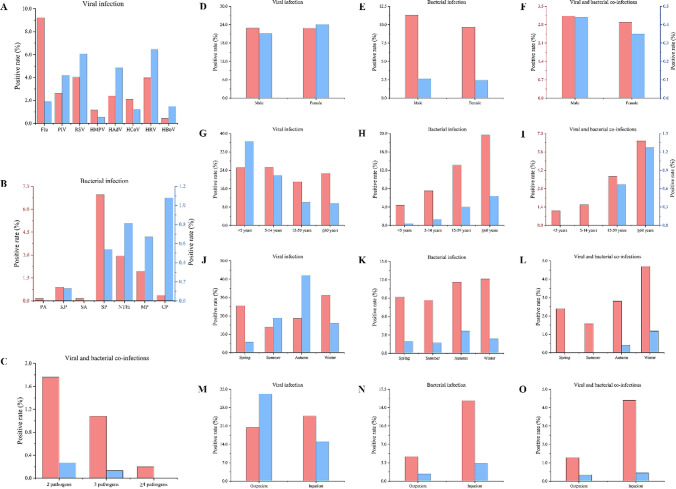
Before COVID-19, Flu had the highest positive rate (9.22%), and HBoV had the lowest (0.44%). After COVID-19, HRV had the highest positive rate (6.47%), and HMPV had the lowest (0.54%) (Fig. 1A). The greatest decrease in the positive rate of Flu (growth rate = -79.50%) was observed after COVID-19, and a decrease in the positive rates of HMPV (growth rate = -53.45%) and HCoV (growth rate = -42.11%) was also observed. However, the greatest increase in the positive rate of HBoV (growth rate = 236.36%) was observed after COVID-19, and an increase in the positive rate of PIV (growth rate = 58.94%), RSV (growth rate = 49.88%), HAdV (growth rate = 102.50%), and HRV (growth rate = 62.56%) was also observed. Before COVID-19, SP had the highest positive rate (6.95%), and PA (0.16%) and SA (0.16%) had the lowest (Fig. 1B). After COVID-19, CP had the highest positive rate (1.08%), and PA and SA were not detected. The greatest decrease in the positive rate of PA (growth rate = -100.00%) and SA (growth rate = -100.00%) was observed after COVID-19, and KP (growth rate = -85.56%), SP (growth rate = -92.23%), NTHi (growth rate = -72.45%), and MP (growth rate = -65.28%) also showed a decrease in the positive rate. Before COVID-19, the positive rate of viral and bacterial coinfections in ARI patients was 3.03%, with coinfections with two pathogens (1.76%) being the most frequent and coinfections with four or more pathogens (0.16%) being the least frequent (Table 1 and Fig. 1C). After COVID-19, the rates of coinfections with two (growth rate = -84.66%), three (growth rate = -87.96%), and four or more pathogens (growth rate = -100.00%) decreased.
Fig. 1.
Changes in ARI in Western China before and after COVID-19. Red bars indicate the positive rate before COVID-19, and blue bars indicate the positive rate after COVID-19.
Changes in the positive rates of viral and bacterial infections in ARI patients with different characteristics before and after COVID-19
After COVID-19, the positive rate of bacterial infections in male (growth rate = -76.66%) and female (growth rate = -74.61%) patients decreased (Fig. 1E), and the positive rate of viral and bacterial coinfections in male (growth rate = -85.94%) and female (growth rate = -87.89%) patients also decreased (Fig. 1F). The positive rate of viral infection in patients aged <5 years (growth rate = 45.76%) increased, but in patients aged 5-14 years (growth rate = -13.80%), 15-59 years (growth rate = -47.40%), and ≥60 years (growth rate = -57.72%), it decreased (Fig. 1G). The positive rate of bacterial infection in patients aged <5 years (growth rate = -91.59%), 5-14 years (growth rate = -83.53%), 15-59 years (growth rate = -69.56%), and ≥60 years (growth rate = -67.63%) decreased (Fig. 1H), and the positive rate of viral and bacterial coinfections in patients aged <5 years (growth rate = -100.00%), 5-14 years (growth rate = -100.00%), 15-59 years (growth rate = -82.04%), and ≥60 years (growth rate = -80.28%) also decreased (Fig. 1I). After COVID-19, the positive rate of viral infections in ARI patients decreased in spring (growth rate = -77.39%) and winter (growth rate = -48.65%) but increased in summer (growth rate = 34.29%) and autumn (growth rate = 125.55%) (Fig. 1J). The positive rate of bacterial infection in ARI patients in spring (growth rate = -79.13%), summer (growth rate = -80.21%), autumn (growth rate = -68.65%), and winter (growth rate = -80.51%) decreased (Fig. 1K), and the positive rate of viral and bacterial coinfections in ARI patients in spring (growth rate = -100.00%), summer (growth rate = -100.00%), autumn (growth rate = -85.77%), and winter (growth rate = -74.84%) also decreased (Fig. 1L). The positive rate of viral infection in outpatients (growth rate = 62.76%) increased, but in inpatients (growth rate = -39.46%) it decreased (Fig. 1M). The positive rate of bacterial infection in outpatients (growth rate = -70.88%) and inpatients (growth rate = -78.10%) decreased (Fig. 1N). The positive rate of viral and bacterial coinfections in outpatients (growth rate = -73.44%) and inpatients (growth rate = -89.77%) also decreased (Fig. 1O).
Comparative analysis of the clinical characteristics of ARI patients in Western China before and after COVID-19
After COVID-19, the proportion of ARI patients with fever and pleural effusion increased (P < 0.05) (Table 2). However, the proportion of ARI patients with cough, runny nose, expectoration, chest pain, dyspnea, headache, fatigue, and pulmonary rales decreased after COVID-19 (P < 0.05). There was no significant difference in the proportion of ARI patients with sore throat, tachypnoea, abdominal pain, or diarrhea before and after COVID-19 (P > 0.05).
Table 2.
Comparative analysis of the clinical characteristics of ARI patients in Western China before and after COVID-19
| Symptom | Number (%) of acute respiratory infections | χ2 | P-value | ||
|---|---|---|---|---|---|
| 2010-2019 | 2020 | ||||
| Fever | Yes | 10144 (84.08) | 668 (90.15) | 19.531 | <0.001 |
| No | 1920 (15.92) | 73 (9.85) | |||
| Cough | Yes | 9065 (75.14) | 516 (69.64) | 11.232 | 0.001 |
| No | 2999 (24.86) | 225 (30.36) | |||
| Runny nose | Yes | 2764 (22.91) | 96 (12.96) | 39.890 | <0.001 |
| No | 9300 (77.09) | 645 (87.04) | |||
| Sore throat | Yes | 3166 (26.24) | 185 (24.97) | 0.589 | 0.443 |
| No | 8898 (73.76) | 556 (75.03) | |||
| Expectoration | Yes | 5014 (41.56) | 244 (32.93) | 21.500 | <0.001 |
| No | 7050 (58.44) | 497 (67.07) | |||
| Chest pain | Yes | 1022 (8.47) | 44 (5.94) | 5.872 | 0.015 |
| No | 11042 (91.53) | 697 (94.06) | |||
| Tachypnoea | Yes | 1287 (10.67) | 80 (10.80) | 0.012 | 0.913 |
| No | 10777 (89.33) | 661 (89.20) | |||
| Dyspnea | Yes | 1331 (11.03) | 55 (7.42) | 9.428 | 0.002 |
| No | 10733 (88.97) | 686 (92.58) | |||
| Headache | Yes | 2017 (16.72) | 80 (10.80) | 17.884 | <0.001 |
| No | 10047 (83.28) | 661 (89.20) | |||
| Fatigue | Yes | 3018 (25.02) | 101 (13.63) | 49.125 | <0.001 |
| No | 9046 (74.98) | 640 (86.37) | |||
| Abdominal pain | Yes | 285 (2.36) | 13 (1.75) | 1.135 | 0.287 |
| No | 11779 (97.64) | 728 (98.25) | |||
| Diarrhea | Yes | 300 (2.49) | 17 (2.29) | 0.107 | 0.743 |
| No | 11764 (97.51) | 724 (97.71) | |||
| Pulmonary rales | Yes | 2574 (21.34) | 83 (11.20) | 43.609 | <0.001 |
| No | 9490 (78.66) | 658 (88.80) | |||
| Pleural effusion | Yes | 554 (4.59) | 47 (6.34) | 4.785 | 0.029 |
| No | 11511 (95.41) | 694 (93.66) | |||
Interrupted time series analysis in the impact of COVID-19 on the epidemiology of ARI in Western China
The proportion of ARI patients aged <5 years and 15-59 years decreased by 55.90% and increased by 109.70% immediately after COVID-19 (within January 2020), respectively (Table 3). The slope in the proportion of ARI patients aged <5 years and 15-59 years increased by 21.40% and decreased by 20.50%, respectively, after COVID-19 (from January 2020 to December 2020), and the proportion of ARI patients aged <5 years and 15-59 years increased by 19.20% and decreased by 20.50%, respectively, per month after COVID-19. However, we cannot determine whether NPIs after COVID-19 had an immediate or long-term impact on the hospitalization rate of ARI patients. The positive rates of viral infection and bacterial infection in ARI patients decreased by 78.10% and 23.50%, respectively, immediately after COVID-19. The slope in the positive rates of viral infection and bacterial infection in ARI patients increased by 20.10% and 4.00%, respectively, after COVID-19, and the positive rates of viral infection and bacterial infection in ARI patients increased by 21.70% and 5.30%, respectively, per month after COVID-19.
Table 3.
Immediate and long-term impact of COVID-19 on the epidemiology of ARI in Western China
| Group | Trend | RR | 95%CI | P-value |
|---|---|---|---|---|
| <5 years | Trend before COVID-19 (January 2018 to December 2019) | 0.982 | 0.973 to 0.991 | <0.001 |
| Level change after COVID-19 (within January 2020) | 0.441 | 0.314 to 0.619 | <0.001 | |
| Trend change after COVID-19 (January 2020 to December 2020) | 1.214 | 1.166 to 1.263 | <0.001 | |
| Trend after COVID-19 (January 2020 to December 2020) | 1.192 | 1.145 to 1.240 | <0.001 | |
| 15-59 years | Trend before COVID-19 (January 2018 to December 2019) | 1.000 | 0.989 to 1.011 | 0.983 |
| Level change after COVID-19 (within January 2020) | 2.097 | 1.581 to 2.782 | <0.001 | |
| Trend change after COVID-19 (January 2020 to December 2020) | 0.795 | 0.751 to 0.842 | <0.001 | |
| Trend after COVID-19 (January 2020 to December 2020) | 0.795 | 0.751 to 0.842 | <0.001 | |
| Inpatient | Trend before COVID-19 (January 2018 to December 2019) | 1.003 | 0.996 to 1.009 | 0.422 |
| Level change after COVID-19 (within January 2020) | 0.923 | 0.755 to 1.129 | 0.437 | |
| Trend change after COVID-19 (January 2020 to December 2020) | 0.997 | 0.969 to 1.026 | 0.842 | |
| Trend after COVID-19 (January 2020 to December 2020) | 1.000 | 0.972 to 1.029 | 0.991 | |
| Viral infection | Trend before COVID-19 (January 2018 to December 2019) | 1.013 | 1.003 to 1.024 | 0.014 |
| Level change after COVID-19 (within January 2020) | 0.219 | 0.147 to 0.328 | <0.001 | |
| Trend change after COVID-19 (January 2020 to December 2020) | 1.201 | 1.145 to 1.259 | <0.001 | |
| Trend after COVID-19 (January 2020 to December 2020) | 1.217 | 1.160 to 1.276 | <0.001 | |
| Bacterial infection | Trend before COVID-19 (January 2018 to December 2019) | 1.013 | 1.006 to 1.020 | <0.001 |
| Level change after COVID-19 (within January 2020) | 0.765 | 0.619 to 0.946 | 0.010 | |
| Trend change after COVID-19 (January 2020 to December 2020) | 1.040 | 1.012 to 1.068 | <0.001 | |
| Trend after COVID-19 (January 2020 to December 2020) | 1.053 | 1.025 to 1.081 | <0.001 |
RR, rate ratio; 95% CI, 95% confidence interval
Interrupted time series analysis of the impact of COVID-19 on the clinical characteristics of ARI patients in Western China
The proportion of ARI patients with cough, chest pain, and pulmonary rales decreased by 17.80%, 56.40%, and 39.20%, and the proportion of ARI patients with dyspnea increased by 87.10% immediately after COVID-19 (within January 2020) (Table 4). The slope in the proportion of ARI patients with runny nose, expectoration, dyspnea, headache, fatigue, and pleural effusion decreased by 12.80%, 7.80%, 8.30%, 7.60%, 9.90%, and 10.90%, respectively, after COVID-19 (from January 2020 to December 2020), and the slope in the proportion of ARI patients with chest pain increased by 16.40% after COVID-19. The proportion of ARI patients with runny nose, expectoration, dyspnea, headache, fatigue, and pleural effusion decreased by 11.70%, 6.60%, 9.50%, 7.80%, 10.70%, and 9.00%, respectively, per month after COVID-19, and the proportion of ARI patients with chest pain increased by 14.60% per month after COVID-19.
Table 4.
Immediate and long-term impact of COVID-19 on the clinical characteristics of ARI patients in Western China
| Symptom | Trend | RR | 95% CI | P-value |
|---|---|---|---|---|
| Fever | Trend before COVID-19 (January 2018 to December 2019) | 1.005 | 0.999 to 1.010 | 0.112 |
| Level change after COVID-19 (within January 2020) | 0.958 | 0.812 to 1.129 | 0.607 | |
| Trend change after COVID-19 (January 2020 to December 2020) | 0.993 | 0.971 to 1.016 | 0.548 | |
| Trend after COVID-19 (January 2020 to December 2020) | 0.998 | 0.975 to 1.020 | 0.830 | |
| Cough | Trend before COVID-19 (January 2018 to December 2019) | 1.004 | 0.998 to 1.010 | 0.189 |
| Level change after COVID-19 (within January 2020) | 0.822 | 0.686 to 0.985 | 0.034 | |
| Trend change after COVID-19 (January 2020 to December 2020) | 1.004 | 0.980 to 1.029 | 0.739 | |
| Trend after COVID-19 (January 2020 to December 2020) | 1.008 | 0.984 to 1.034 | 0.512 | |
| Runny nose | Trend before COVID-19 (January 2018 to December 2019) | 1.013 | 1.001 to 1.024 | 0.029 |
| Level change after COVID-19 (within January 2020) | 0.880 | 0.620 to 1.250 | 0.477 | |
| Trend change after COVID-19 (January 2020 to December 2020) | 0.872 | 0.820 to 0.928 | <0.001 | |
| Trend after COVID-19 (January 2020 to December 2020) | 0.883 | 0.831 to 0.939 | <0.001 | |
| Expectoration | Trend before COVID-19 (January 2018 to December 2019) | 1.012 | 1.004 to 1.021 | 0.003 |
| Level change after COVID-19 (within January 2020) | 0.924 | 0.731 to 1.169 | 0.511 | |
| Trend change after COVID-19 (January 2020 to December 2020) | 0.922 | 0.889 to 0.957 | <0.001 | |
| Trend after COVID-19 (January 2020 to December 2020) | 0.934 | 0.900 to 0.968 | <0.001 | |
| Chest pain | Trend before COVID-19 (January 2018 to December 2019) | 0.984 | 0.965 to 1.004 | 0.115 |
| Level change after COVID-19 (within January 2020) | 0.436 | 0.211 to 0.904 | 0.026 | |
| Trend change after COVID-19 (January 2020 to December 2020) | 1.164 | 1.068 to 1.268 | 0.001 | |
| Trend after COVID-19 (January 2020 to December 2020) | 1.146 | 1.052 to 1.248 | 0.002 | |
| Dyspnea | Trend before COVID-19 (January 2018 to December 2019) | 0.987 | 0.969 to 1.006 | 0.178 |
| Level change after COVID-19 (within January 2020) | 1.871 | 1.172 to 2.987 | 0.009 | |
| Trend change after COVID-19 (January 2020 to December 2020) | 0.917 | 0.849 to 0.991 | 0.028 | |
| Trend after COVID-19 (January 2020 to December 2020) | 0.905 | 0.840 to 0.976 | 0.009 | |
| Headache | Trend before COVID-19 (January 2018 to December 2019) | 0.998 | 0.984 to 1.011 | 0.743 |
| Level change after COVID-19 (within January 2020) | 1.120 | 0.733 to 1.710 | 0.601 | |
| Trend change after COVID-19 (January 2020 to December 2020) | 0.924 | 0.866 to 0.987 | 0.018 | |
| Trend after COVID-19 (January 2020 to December 2020) | 0.922 | 0.864 to 0.984 | 0.015 | |
| Fatigue | Trend before COVID-19 (January 2018 to December 2019) | 0.996 | 0.985 to 1.008 | 0.540 |
| Level change after COVID-19 (within January 2020) | 1.110 | 0.779 to 1.581 | 0.565 | |
| Trend change after COVID-19 (January 2020 to December 2020) | 0.901 | 0.846 to 0.959 | 0.001 | |
| Trend after COVID-19 (January 2020 to December 2020) | 0.897 | 0.843 to 0.955 | 0.001 | |
| Pulmonary rales | Trend before COVID-19 (January 2018 to December 2019) | 0.983 | 0.972 to 0.993 | 0.001 |
| Level change after COVID-19 (within January 2020) | 0.608 | 0.415 to 0.890 | 0.011 | |
| Trend change after COVID-19 (January 2020 to December 2020) | 0.990 | 0.931 to 1.053 | 0.750 | |
| Trend after COVID-19 (January 2020 to December 2020) | 0.973 | 0.915 to 1.034 | 0.378 | |
| Pleural effusion | Trend before COVID-19 (January 2018 to December 2019) | 1.021 | 0.997 to 1.045 | 0.091 |
| Level change after COVID-19 (within January 2020) | 1.556 | 0.871 to 2.782 | 0.136 | |
| Trend change after COVID-19 (January 2020 to December 2020) | 0.891 | 0.814 to 0.976 | 0.013 | |
| Trend after COVID-19 (January 2020 to December 2020) | 0.910 | 0.831 to 0.996 | 0.040 |
RR, rate ratio; 95% CI, 95% confidence interval
Discussion
NPIs have a significant limiting effect on the epidemic of COVID-19 and also have some limiting effects on other diseases [8, 11, 15, 16, 18]. In this study, we found that the epidemiology of ARI patients in Western China changed after COVID-19. One of these changes was that the proportion of children aged <5 years increased after COVID-19. This may be related to a significant decrease in the stringency of NPIs after China's COVID-19 prevention and control strategy shifted from “first-level response” to “normalized control” in May 2020 [26], and many kindergartens started to reopen during the “normalized-control” period [5]. However, children aged <5 years are a high-risk group for ARI, and therefore, decreasing the stringency of NPIs would be expected to increase the incidence of ARI in children [14]. Secondly, awareness of personal disease prevention measures in children is lower than in other age groups, and NPIs are less effective for children than for adults [28]. This may have contributed to the increased rate of ARI in children aged <5 years after COVID-19. Therefore, in the post-COVID-19 era, we should focus on the prevention of ARI in children. RSV is one of the most common pathogens causing ARI in children aged <5 years [7, 9], and this study found the positive rate of RSV after COVID-19 to be higher than that before COVID-19, which may also be one of the reasons for the increase in the proportion of ARI patients aged <5 years after COVID-19. We also found that the number of ARI patients was lower in winter 2020 than that in winter during the pre-COVID-19 period. January to February 2020 (winter) was the most severe period of the COVID-19 epidemic in China, and implementation of the highest level of public health control during that period inevitably reduced the incidence of ARI [21, 34]. After that, with the effective control of COVID-19, we observed an increase in the rate of hospitalization of ARI patients. It is known that a higher proportion of ARI patients with milder clinical symptoms are seen as outpatients and that a higher proportion of ARI patients with more-severe symptoms are hospitalized. During the COVID-19 pandemic, some ARI patients with milder symptoms might have refused to go to the hospital for fear of developing COVID-19 [10], while ARI patients with more-severe symptoms are admitted to the hospital for essential treatment, led to a higher relative proportion of hospitalization of ARI patients after COVID-19.
After COVID-19, the positive rate of Flu decreased significantly, while the positive rate of PIV, RSV, and HRV increased, which is consistent with the results of other similar studies [9, 24]. A study also found that RSV outbreaks occurred after downgrading the stringency of NPIs [9]. Therefore, surveillance of respiratory viruses such as RSV needs to be strengthened in the future. After COVID-19, the positive rate of bacterial infection in ARI patients decreased, but the positive rate of viral infection in ARI patients did not change. In particular, the positive rate of bacterial infection (especially SP, NTHi, and MP) decreased significantly in Western China after COVID-19, which is consistent with findings from many countries around the world [3, 4, 13]. A study has found that the dramatic decline in infection rates of many respiratory bacteria after COVID-19 corresponded to the timing of government responses to COVID-19 and changes in the movement of people [4]. Therefore, the decrease in the positive rate of bacterial infections after COVID-19 might have been due to NPIs [19], and even the low-stringency NPIs still had a significant limiting effect on bacterial infection. We also found that the positive rate of viral and bacterial coinfections in ARI patients decreased significantly after COVID-19, regardless of gender, age, level of treatment, or season. This is due to the overall decrease in bacterial respiratory infections due to NPIs. The positive rate of viral infection in ARI patients aged <5 years increased after COVID-19, as was also seen in South Africa, where NPIs during COVID-19 changed the infection spectrum, making children more likely to be infected with other respiratory viruses [27]. The proportion of ARI patients with clinical symptoms decreased after COVID-19, which may be related to the decreased positive rates of viral and bacterial infections after COVID-19. Previous studies have shown that HMPV infection or coinfection with HMPV and other respiratory viruses increases the risk of pneumonia and severe clinical symptoms [6]. In addition, coinfections with respiratory pathogens also increase the risk and severity of clinical symptoms in ARI patients [17]. Thus, the decrease in the positive rate of respiratory pathogens after COVID-19 has caused a decrease in the proportion of ARI patients with clinical symptoms [6].
ITS analysis showed a decrease in the proportion of ARI patients aged <5 years in the short term after COVID-19, which may be related to the closure of the kindergarten after COVID-19. We also found a decrease in the positive rates of both respiratory viral and bacterial infections in the short term after COVID-19, suggesting that NPIs had an equally significant limiting effect on the transmission of respiratory pathogens. However, in the long term, the proportion of ARI patients aged <5 years and the positive rates of respiratory viral and bacterial infections increased throughout the post-COVID-19 period, which may be related to the reopening of kindergartens and children's low awareness of protective measures. This finding suggests that we need to be vigilant for the resurgence of respiratory viruses and bacteria in the future. In addition, changes in the seasonality, positive rate, and infection spectrum of respiratory pathogens caused by NPIs during the COVID-19 pandemic may lead to a decline in the population's immunity to these pathogens [6, 7]. It has been shown that RSV antibody levels in women and infants decreased after COVID-19 [6]. This could lead to larger and more-serious outbreaks of some respiratory pathogens. Therefore, for Western China, where the level of medical resources is not highly developed [25], it is very important to strengthen the surveillance of respiratory pathogens to protect the health of the people in this region and also to reduce the impact on medical resources. ITS analysis also showed that the proportion of ARI patients with cough, chest pain, and pulmonary rales decreased in the short term after COVID-19. This phenomenon may be related to traffic control, community closures, and fear of developing COVID-19, which discouraged some ARI patients with less-severe clinical symptoms from actively seeking care. However, the proportion of ARI patients with severe clinical symptoms (dyspnea and pleural effusion) increased in the short term after COVID-19 due to the urgent need for treatment. Thus, there was an increase in the proportion of ARI patients with dyspnea and pleural effusion in the short term after COVID-19.
Our study fills a gap in our knowledge about changes in ARI in Western China and contributes to their future prevention and control, but it has some limitations. First, changes in the epidemiology, clinical characteristics, and pathogen spectrum of ARI might have been due to decreased ARI surveillance after COVID-19 rather than NPIs. Second, the COVID-19 pandemic is likely to have changed the behavior of people seeking medical care, resulting in a higher proportion of infections with respiratory pathogens remaining undetected. Third, our study did not consider other factors that may impact the clinical characteristics, such as pre-visit medications or improvements in treatment. Finally, the ARI surveillance program was discontinued at the end of 2020, and subsequent developments were therefore not investigated.
Acknowledgements
We are grateful to the School of Public Health of Lanzhou University and Gansu CDC for their support for this study.
Author contributions
TSS, XSZ, and XWR conceived and designed the study. LM, DHL, TRW, and RL collected and cleaned data. TSS, XZ, XFL, HMZ, and XPS analyzed the data. TSS, XZ, and XSZ wrote the drafts of the paper. TSS, ML, and JSL interpreted the findings. XZ, DSY, and XWR revised drafts of the paper. NJ, XSZ, XFL, and DSY checked the analysis codes. All authors read and approved the final manuscript.
Funding
This work was supported by the Natural Science Foundation of Gansu Province (grant numbers 20JR10RA598 and 18JR3RA040) and the National Science and Technology Major Special Projects (grant number 2017ZX10103006).
Data availability
The data that support this study are available from the corresponding author upon reasonable request.
Declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Ethics statement
Informed consent was obtained from patients for this surveillance program, and this study was approved by the Ethics Review Committee of the Gansu Provincial Center for Disease Control and Prevention (approval number 2018-007).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Tianshan Shi, Xin Zhao and Xiaoshu Zhang contributed equally to this article.
References
- 1.Baker RE, Park SW, Yang W, Vecchi GA, Metcalf CJE, Grenfell BT. The impact of COVID-19 nonpharmaceutical interventions on the future dynamics of endemic infections. Proc Natl Acad Sci USA. 2020;117:30547–30553. doi: 10.1073/pnas.2013182117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernal JL, Cummins S, Gasparrini A. Interrupted time series regression for the evaluation of public health interventions: a tutorial. Int J Epidemiol. 2017;46:348–355. doi: 10.1093/ije/dyw098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borchers A, Pieler T. Programming pluripotent precursor cells derived from Xenopus embryos to generate specific tissues and organs. Genes (Basel) 2010;1:413–426. doi: 10.3390/genes1030413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brueggemann AB, Jansen van Rensburg MJ, Shaw D, McCarthy ND, Jolley KA, Maiden MCJ, van der Linden MPG, Amin-Chowdhury Z, Bennett DE, Borrow R, Brandileone MC, Broughton K, Campbell R, Cao B, Casanova C, Choi EH, Chu YW, Clark SA, Claus H, Coelho J, Corcoran M, Cottrell S, Cunney RJ, Dalby T, Davies H, de Gouveia L, Deghmane AE, Demczuk W, Desmet S, Drew RJ, du Plessis M, Erlendsdottir H, Fry NK, Fuursted K, Gray SJ, Henriques-Normark B, Hale T, Hilty M, Hoffmann S, Humphreys H, Ip M, Jacobsson S, Johnston J, Kozakova J, Kristinsson KG, Krizova P, Kuch A, Ladhani SN, Lam TT, Lebedova V, Lindholm L, Litt DJ, Martin I, Martiny D, Mattheus W, McElligott M, Meehan M, Meiring S, Molling P, Morfeldt E, Morgan J, Mulhall RM, Munoz-Almagro C, Murdoch DR, Murphy J, Musilek M, Mzabi A, Perez-Arguello A, Perrin M, Perry M, Redin A, Roberts R, Roberts M, Rokney A, Ron M, Scott KJ, Sheppard CL, Siira L, Skoczynska A, Sloan M, Slotved HC, Smith AJ, Song JY, Taha MK, Toropainen M, Tsang D, Vainio A, van Sorge NM, Varon E, Vlach J, Vogel U, Vohrnova S, von Gottberg A, Zanella RC, Zhou F. Changes in the incidence of invasive disease due to Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis during the COVID-19 pandemic in 26 countries and territories in the Invasive Respiratory Infection Surveillance Initiative: a prospective analysis of surveillance data. Lancet Digit Health. 2021;3:e360–e370. doi: 10.1016/S2589-7500(21)00077-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiu SS, Cowling BJ, Peiris JSM, Chan ELY, Wong WHS, Lee KP. Effects of nonpharmaceutical COVID-19 interventions on pediatric hospitalizations for other respiratory virus infections, Hong Kong. Emerg Infect Dis. 2022;28:62–68. doi: 10.3201/eid2801.211099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cui A, Xie Z, Xu J, Hu K, Zhu R, Li Z, Li Y, Sun L, Xiang X, Xu B, Zhang R, Gao Z, Zhang Y, Xu W. Comparative analysis of the clinical and epidemiological characteristics of human influenza virus versus human respiratory syncytial virus versus human metapneumovirus infection in nine provinces of China during 2009–2021. J Med Virol. 2022;94:5894–5903. doi: 10.1002/jmv.28073. [DOI] [PubMed] [Google Scholar]
- 7.Di Mattia G, Nenna R, Mancino E, Rizzo V, Pierangeli A, Villani A, Midulla F. During the COVID-19 pandemic where has respiratory syncytial virus gone? Pediatr Pulmonol. 2021;56:3106–3109. doi: 10.1002/ppul.25582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dinmohamed AG, Visser O, Verhoeven RHA, Louwman MWJ, van Nederveen FH, Willems SM, Merkx MAW, Lemmens VEPP, Nagtegaal ID, Siesling S. Fewer cancer diagnoses during the COVID-19 epidemic in the Netherlands. Lancet Oncol. 2020;21:750–751. doi: 10.1016/S1470-2045(20)30265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eden JS, Sikazwe C, Xie R, Deng YM, Sullivan SG, Michie A, Levy A, Cutmore E, Blyth CC, Britton PN, Crawford N, Dong X, Dwyer DE, Edwards KM, Horsburgh BA, Foley D, Kennedy K, Minney-Smith C, Speers D, Tulloch RL, Holmes EC, Dhanasekaran V, Smith DW, Kok J, Barr IG, Australian RSVsg, Off-season RSV epidemics in Australia after easing of COVID-19 restrictions. Nat Commun. 2022;13:2884. doi: 10.1038/s41467-022-30485-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fei H, Yinyin X, Hui C, Ni W, Xin D, Wei C, Tao L, Shitong H, Miaomiao S, Mingting C, Keshavjee S, Yanlin Z, Chin DP, Jianjun L. The impact of the COVID-19 epidemic on tuberculosis control in China. Lancet Reg Health West Pac. 2020;3:100032. doi: 10.1016/j.lanwpc.2020.100032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geng MJ, Zhang HY, Yu LJ, Lv CL, Wang T, Che TL, Xu Q, Jiang BG, Chen JJ, Hay SI, Li ZJ, Gao GF, Wang LP, Yang Y, Fang LQ, Liu W. Changes in notifiable infectious disease incidence in China during the COVID-19 pandemic. Nat Commun. 2021;12:6923. doi: 10.1038/s41467-021-27292-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guan D, Wang D, Hallegatte S, Davis SJ, Huo J, Li S, Bai Y, Lei T, Xue Q, Coffman D, Cheng D, Chen P, Liang X, Xu B, Lu X, Wang S, Hubacek K, Gong P. Global supply-chain effects of COVID-19 control measures. Nat Hum Behav. 2020;4:577–587. doi: 10.1038/s41562-020-0896-8. [DOI] [PubMed] [Google Scholar]
- 13.Haapanen M, Renko M, Artama M, Kuitunen I. The impact of the lockdown and the re-opening of schools and day cares on the epidemiology of SARS-CoV-2 and other respiratory infections in children—a nationwide register study in Finland. EClinicalMedicine. 2021;34:100807. doi: 10.1016/j.eclinm.2021.100807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heikkinen T, Booy R, Campins M, Finn A, Olcen P, Peltola H, Rodrigo C, Schmitt HJ, Schumacher F, Teo S, Weil-Olivier C. Should healthy children be vaccinated against influenza? A consensus report of the Summits of Independent European Vaccination Experts. Eur J Pediatr. 2006;165:223–228. doi: 10.1007/s00431-005-0040-9. [DOI] [PubMed] [Google Scholar]
- 15.Huh K, Jung J, Hong J, Kim M, Ahn JG, Kim JH, Kang JM. Impact of nonpharmaceutical interventions on the incidence of respiratory infections during the coronavirus disease 2019 (COVID-19) outbreak in Korea: a nationwide surveillance study. Clin Infect Dis. 2021;72:e184–e191. doi: 10.1093/cid/ciaa1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones D, Neal RD, Duffy SRG, Scott SE, Whitaker KL, Brain K. Impact of the COVID-19 pandemic on the symptomatic diagnosis of cancer: the view from primary care. Lancet Oncol. 2020;21:748–750. doi: 10.1016/S1470-2045(20)30242-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jongbloed M, Leijte WT, Linssen CFM, van den Hoogen BG, van Gorp ECM, de Kruif MD. Clinical impact of human metapneumovirus infections before and during the COVID-19 pandemic. Infect Dis (Lond) 2021;53:488–497. doi: 10.1080/23744235.2021.1887510. [DOI] [PubMed] [Google Scholar]
- 18.Kennedy DM, Zambrano GJ, Wang Y, Neto OP. Modeling the effects of intervention strategies on COVID-19 transmission dynamics. J Clin Virol. 2020;128:104440. doi: 10.1016/j.jcv.2020.104440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim YK, Choi YY, Lee H, Song ES, Ahn JG, Park SE, Lee T, Cho HK, Lee J, Kim YJ, Jo DS, Kang HM, Lee JK, Kim CS, Kim DH, Kim HM, Choi JH, Eun BW, Kim NH, Cho EY, Kim YK, Oh CE, Kim KH, Choi EH. Differential impact of nonpharmaceutical interventions on the epidemiology of invasive bacterial infections in children during the coronavirus disease 2019 pandemic. Pediatr Infect Dis J. 2022;41:91–96. doi: 10.1097/INF.0000000000003414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lei H, Xu M, Wang X, Xie Y, Du X, Chen T, Yang L, Wang D, Shu Y. Nonpharmaceutical interventions used to control COVID-19 reduced seasonal influenza transmission in China. J Infect Dis. 2020;222:1780–1783. doi: 10.1093/infdis/jiaa570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li ZJ, Yu LJ, Zhang HY, Shan CX, Lu QB, Zhang XA, Ren X, Zhang CH, Wang YF, Lin SH, Xu Q, Jiang BG, Jiang T, Lv CL, Chen JJ, Gao GF, Yang WZ, Wang LP, Yang Y, Fang LQ, Liu W, Chinese Centers for Disease C, Prevention Etiology Surveillance Study Team of Acute Respiratory I Broad Impacts of Coronavirus Disease 2019 (COVID-19) Pandemic on Acute Respiratory Infections in China: An Observational Study. Clin Infect Dis. 2022;75:e1054–e1062. doi: 10.1093/cid/ciab942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Linden A. Conducting interrupted time-series analysis for single- and multiple-group comparisons. Stata J. 2015;15:480–500. doi: 10.1177/1536867X1501500208. [DOI] [Google Scholar]
- 23.Liu P, Xu M, Cao L, Su L, Lu L, Dong N, Jia R, Zhu X, Xu J. Impact of COVID-19 pandemic on the prevalence of respiratory viruses in children with lower respiratory tract infections in China. Virol J. 2021 doi: 10.1186/s12985-021-01627-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu P, Xu M, Lu L, Ma A, Cao L, Su L, Dong N, Jia R, Zhu X, Xu J. The changing pattern of common respiratory and enteric viruses among outpatient children in Shanghai, China: two years of the COVID-19 pandemic. J Med Virol. 2022;94:4696–4703. doi: 10.1002/jmv.27896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu X, Gao W, Yan H. Measuring and decomposing the inequality of maternal health services utilization in Western Rural China. Bmc Health Serv Res. 2014 doi: 10.1186/1472-6963-14-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma C, Guo X, Wang L, Li W, Liu S, Lin F, Xu W (2022) The impact of the COVID-19 pandemic on the incidence and mortality of zoonotic diseases in China. BMJ Glob Health. 10.1136/bmjgh-2021-007109 [DOI] [PMC free article] [PubMed]
- 27.Ogunbayo AE, Mogotsi MT, Sondlane H, Nkwadipo KR, Sabiu S, Nyaga MM (2022) Pathogen profile of children hospitalised with severe acute respiratory infections during COVID-19 pandemic in the free state Province, South Africa. Int J Environ Res Public Health. 10.3390/ijerph191610418 [DOI] [PMC free article] [PubMed]
- 28.Olsen SJ, Winn AK, Budd AP, Prill MM, Steel J, Midgley CM, Kniss K, Burns E, Rowe T, Foust A, Jasso G, Merced-Morales A, Davis CT, Jang Y, Jones J, Daly P, Gubareva L, Barnes J, Kondor R, Sessions W, Smith C, Wentworth DE, Garg S, Havers FP, Fry AM, Hall AJ, Brammer L, Silk BJ. Changes in influenza and other respiratory virus activity during the COVID-19 pandemic-United States, 2020–2021. Am J Transplant. 2021;21:3481–3486. doi: 10.1111/ajt.16049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richards M, Anderson M, Carter P, Ebert BL, Mossialos E. The impact of the COVID-19 pandemic on cancer care. Nat Cancer. 2020;1:565–567. doi: 10.1038/s43018-020-0074-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rybak A, Levy C, Angoulvant F, Auvrignon A, Gembara P, Danis K, Vaux S, Levy-Bruhl D, van der Werf S, Bechet S, Bonacorsi S, Assad Z, Lazzati A, Michel M, Kaguelidou F, Faye A, Cohen R, Varon E, Ouldali N. Association of nonpharmaceutical interventions during the COVID-19 pandemic with invasive pneumococcal disease, pneumococcal carriage, and respiratory viral infections among children in France. JAMA Netw Open. 2022;5:e2218959. doi: 10.1001/jamanetworkopen.2022.18959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scotta MC, Kern LB, Polese-Bonatto M, Azevedo TR, Varela FH, Zavaglia GO, Fernandes IR, de David CN, Fazolo T, da Costa MSC, de Carvalho FC, Sartor ITS, Zavascki AP, Stein RT. Impact of rhinovirus on hospitalization during the COVID-19 pandemic: a prospective cohort study. J Clin Virol. 2022;156:105197. doi: 10.1016/j.jcv.2022.105197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi T, Meng L, Li D, Jin N, Zhao X, Zhang X, Liu Y, Zheng H, Zhao X, Li J, Shen X, Ren X. Effect of different vaccine strategies for the control of Japanese encephalitis in mainland China from 1961 to 2020: a quantitative analysis. Vaccine. 2022;40:6243–6254. doi: 10.1016/j.vaccine.2022.09.030. [DOI] [PubMed] [Google Scholar]
- 33.Steffen R, Lautenschlager S, Fehr J. Travel restrictions and lockdown during the COVID-19 pandemic-impact on notified infectious diseases in Switzerland. J Travel Med. 2020 doi: 10.1093/jtm/taaa180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang JL, Abbasi K. What can the world learn from China's response to covid-19? BMJ. 2021;375:n2806. doi: 10.1136/bmj.n2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang JW, Bialasiewicz S, Dwyer DE, Dilcher M, Tellier R, Taylor J, Hua H, Jennings L, Kok J, Levy A, Smith D, Barr IG, Sullivan SG. Where have all the viruses gone? Disappearance of seasonal respiratory viruses during the COVID-19 pandemic. J Med Virol. 2021;93:4099–4101. doi: 10.1002/jmv.26964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tian H, Liu Y, Li Y, Wu C-H, Chen B, Kraemer MUG, Li B, Cai J, Xu B, Yang Q, Wang B, Yang P, Cui Y, Song Y, Zheng P, Wang Q, Bjornstad ON, Yang R, Grenfell BT, Pybus OG, Dye C. An investigation of transmission control measures during the first 50 days of the COVID-19 epidemic in China. Science. 2020;368:638–+. doi: 10.1126/science.abb6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Varela FH, Scotta MC, Polese-Bonatto M, Sartor ITS, Ferreira CF, Fernandes IR, Zavaglia GO, de Almeida WAF, Arakaki-Sanchez D, Pinto LA, Nader Bastos GA, Nasi LA, Falavigna M, Pitrez PM, Stein RT, Group COs Absence of detection of RSV and influenza during the COVID-19 pandemic in a Brazilian cohort: likely role of lower transmission in the community. J Glob Health. 2021;11:05007. doi: 10.7189/jogh.11.05007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang H, Zheng Y, de Jonge MI, Wang R, Verhagen LM, Chen Y, Li L, Xu Z, Wang W. Lockdown measures during the COVID-19 pandemic strongly impacted the circulation of respiratory pathogens in Southern China. Sci Rep. 2022;12:16926. doi: 10.1038/s41598-022-21430-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiao Y, Torok ME. Taking the right measures to control COVID-19. Lancet Infect Dis. 2020;20:523–524. doi: 10.1016/S1473-3099(20)30152-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support this study are available from the corresponding author upon reasonable request.