ABSTRACT
We performed a scoping review to identify the extent of the literature describing the use of iloprost in the treatment of frostbite. Iloprost is a stable synthetic analog of prostaglandin I2. As a potent inhibitor of platelet aggregation and vasodilator, it has been used to address the post-rewarming reperfusion injury in frostbite. The search using iloprost and frostbite as key words and MeSH terms yielded 200 articles. We included in our review the literature examining iloprost for the treatment of frostbite in humans in the form of primary research, conference proceedings and abstracts. Twenty studies published from 1994 to 2022 were selected for analysis. The majority were retrospective case series consisting of a homogeneous population of mountain sport enthusiasts. A total of 254 patients and over 1000 frostbitten digits were included among the 20 studies. The larger case series demonstrated a decrease in amputation rates relative to untreated patients. Primary gaps in the literature include a paucity of randomised trials and relatively limited study populations to date. While the case evidence is promising, a multi-centre collaboration would be crucial to adequately power prospective randomised studies to definitively determine if iloprost has a role in the treatment of frostbite.
KEYWORDS: Iloprost, prostacyclin, vasodilator, frostbite, cold injury, amputation, review
Introduction
Frostbite is a cold environment injury that can have significant consequences, including amputation. Frostbite is an occurrence of high-latitude locations and can affect the health of indigenous people living in northern communities and people active in polar regions. However, there are still knowledge gaps in frostbite management and resources can be limited in remote communities, increasing challenges in management.
Frostbite occurs when the tissues are exposed to temperatures below their freezing point. Ice crystal formation (intracellular and extracellular) causes cell dehydration and shrinkage, electrolyte disturbances, lipid and protein denaturation and resultant cell injury and death. [1] Following thawing, a cascade of events mediated by thromboxane A2, prostaglandin F2alpha (PGF2α), bradykinin and histamine causes inflammation, vasoconstriction of arterioles and venules, thrombus formation and emboli in the microvessels [2]. This leads to progressive ischaemia and further cell death, resulting in necrosis [1].
In humans, frostbite most commonly affects upper and lower limbs and digits but can also affect other parts of the body such as the nose and ears. Frostbite can be described as deep or superficial and “degrees” of severity have been used, like in burns. As per Cauchy et al. frostbite classification, frostbite severity varies from Grade 1 injury, which involves most distal parts of fingers and toes, without cyanosis, to Grade 4 frostbite, where the cyanotic injury or haemorrhagic blisters extend proximal to the metacarpal phalangeal or metatarsal phalangeal joints [3]. The risk of amputation rises quickly as the grade increases from a rate of 30% proximal to the distal phalanx to 100% proximal to the metacarpals/tarsals [3]. Other factors also affect outcomes: delayed presentation to healthcare facility, multiple freeze-thaw cycles and delay in pharmacologic treatment initiation [4,5].
Frostbite management has evolved slowly in the past century. William Mills, an Alaskan physician, pioneered research in frostbite patients in 1960s, including the rewarming method (rapid immersion of affected body parts in hot water, using a whirlpool with antiseptic solution) [6]. In 1980s, Robson, Heggers and McCauley developed early pharmacological management and human treatment protocols based on animal studies. They found an elevated amount of PGF2α and thromboxane B2 in the fluid aspirated from frostbite blisters. They hypothesised that those arachidonic acid metabolites and inflammatory mediators were responsible for the vasoconstriction and platelet aggregation leading to dermal ischaemia [7,8]. They incorporated anti-prostaglandin agents (acetylsalicylic acid and ibuprofen) as well as specific thromboxane inhibitors (topical Aloe vera) to frostbite treatment protocols in Chicago and Detroit [8–11] (Figure 1).
Figure 1.
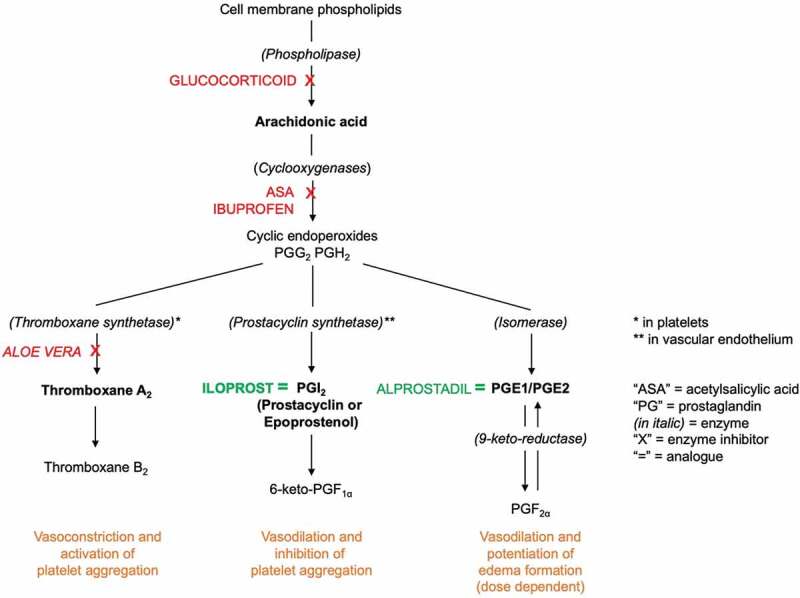
Conversion of arachidonic acid to thromboxane and prostaglandins and their known analogues, inhibitors and roles (adapted from references 8, 22, 23 and 24).
Thrombolytics
In the early 1990s, the Hennepin County Medical Center in Minneapolis piloted the use of thrombolytics in the treatment of frostbite [12]. Thrombolytics or recombinant tissue plasminogen activators (rtPA) work by binding to fibrin in a thrombus and activating plasminogen, which causes local fibrinolysis and prevents enlargement of blood clots in frostbitten extremities [13]. Several thrombolytics, mainly alteplase, have been used in the treatment of frostbite with over 15 published studies, totalling over 300 patients [4,14]. In two systematic reviews on the use of thrombolytics in the treatment of frostbite published in 2019, the limb salvage rates ranged from 0% to 100% [4,14]. However, the quality of the evidence was low and some authors suggest to reserve their use to research protocols [14]. Studies have demonstrated a rapid loss of efficacy with delay of administration of thrombolytics. Nygaard et al. showed that the time from rewarming to alteplase significantly impacted amputation rate, with each hour of delayed treatment leading to an additional 27% loss of tissue [15]. As per the aforementioned study, alteplase should ideally be initiated within 4 h of rewarming, with some benefits observed up to 12 h post-rewarming [15].
Thrombolytics can be administered intra-arterial or intravenous. However, the intra-arterial route of administration can be challenging in limited resource settings given the need for contrast administration and angiography to perform catheter-directed intra-arterial thrombolysis. In Drinane et al. 2019 systematic review, intra-arterial and intravenous thrombolysis had similar limb salvage rates of 76.4% and 77.3% [4]. Thrombolytics carry a risk of bleeding and haemorrhage. In a recent study detailing bleeding complications in severe frostbite patients treated with intravenous alteplase, 8.4% of patients had bleeding resulting in change of management or intervention [13].
Fibrinolysis can have a procoagulant effect (clot extension and reformation); therefore, anticoagulants, unfractionated heparin (UFH) and low-molecular weight heparins (LMWH), have been used as adjunctive therapy in thrombolytics protocols [2]. LMWH has a number of advantages over UFH: LMWH are administered subcutaneously as opposed to an intravenous infusion, they have a more predictable anticoagulant effect, and they are less likely to induce immune-mediated thrombocytopenia [16]. Heparin is not recommended as monotherapy in frostbite owing to insufficient data [2].
Iloprost
Iloprost was developed in Germany in 1980s and has been used in Europe intravenously for Raynaud’s disease, peripheral arterial disease, Buerger’s Disease, critical leg ischaemia and systemic sclerosis [17–20]. Iloprost use in the treatment of frostbite was first documented by Groechenig in Austria [21].
Iloprost is a stable synthetic analog of prostaglandin I2 (prostacyclin or epoprostenol) mimicking its endogenous properties: potent inhibition of platelet aggregation and vasodilation [22,23] (Figure 1). When platelets become activated after endothelial injury, prostacyclin prevents them from adhering to the surrounding endothelium [24]. It is known that the balance between prostacyclin and thromboxane governs vascular tone and iloprost inhibits the vasoconstriction induced by thromboxane A2 [22,23]. Iloprost may also have fibrinolytic properties by stimulating the release of endogenous tissue plasminogen activator or counteracting its inhibition [23]. Iloprost may reduce oxidative stress from free radicals, making it a favourable agent to mitigate the reperfusion injury [23]. Finally, it could also decrease neutrophil adhesion and chemotaxis but the evidence is tenuous [23].
Iloprost administered intravenously is distributed into tissues within 4 min and is eliminated within 30 min. Its effect on platelet aggregation is quickly reversible (within 2 h) but its prostacyclin mimetic effect may interrupt the vicious cycle of activation and amplification between activated platelets, leukocytes and damaged endothelium because long-lasting effects have been observed in patients with chronic ischaemia [23]. It is suggested that intermittent iloprost treatment augments endogenous prostanoid activity sufficiently to interrupt the cycle of activation and amplification, thereby restoring physiological balance [23].
Tolerance to iloprost is variable but adverse reactions are clearly dose related [23]. Headaches and facial flushing are the most common adverse reactions and can generally be avoided by titrating up the dosage progressively [23]. They also cease shortly after the infusion is discontinued. A recent study in patients with systemic sclerosis observed that overweight and obese patients were more susceptible to iloprost adverse effects [25].
Of interest, iloprost decreases peripheral vascular resistance and mean arterial blood pressure with only a mild increase in heart rate and cardiac index [23]. It increases renal blood flow but has a natriuretic effect, which is independent of the haemodynamic change [23]. Due to these characteristics, iloprost is currently investigated in septic shock patients with persistence of microperfusion disorders [26].
Other prostaglandin analogues
Iloprost is not available in the United States, and there are anecdotal reports of the use of epoprostenol in frostbite. The ephemeral nature of epoprostenol hinders its use clinically [23]. It has a shorter elimination half-life (3–6 min compared to 30 min for iloprost) and is more chemically unstable than iloprost [23]. In pulmonary arterial hypertension, due to its pharmacokinetics, epoprostenol has to be given by continuous intravenous administration via a permanent central venous catheter and a portable infusion pump [27]. Problems in the delivery system resulting in an abrupt interruption in the administration of the drug or inadvertent administration of a bolus have been associated with serious consequences in patients with pulmonary arterial hypertension, including death [28]. Where other prostanoids are not available, epoprostenol has also been used to treat patients with severe Raynaud’s Phenomenon refractory to conventional treatments and when acute therapy is needed, in intravenous infusions of 6–24 hours for 2 to 5 days [29]. We could find reports of its use for frostbite in three publications from the United States [30–32] as well as upon communication with American peers.
Alprostadil, also known as prostaglandin E1, is another prostaglandin used in frostbite for its vasodilating properties, but the experience is limited to Korea, the United States and China [33–35]. It has been used as an intravenous infusion for 24 h along with NSAIDs [33] or as an adjunct to thrombolysis, injected intra-arterial prior to thrombolytic administration to dilate the spasmodic microvessels [34,35].
There is a growing number of publications on the use of iloprost in frostbite but there are small patient numbers in each study, and the data is heterogeneous given the lack of standardised treatment protocols. The limited available evidence was not amenable to a systematic review, therefore we conducted a scoping review using methodological guidelines developed by international experts [36,37].
Objectives
The aim of this study is to scope the literature on the use of iloprost for the treatment of frostbite to describe this area of research. The study is particularly interested in all primary research conducted to date in order to characterise how this question has been examined and what gaps in knowledge exist. As a scoping review, rather than commenting on the effectiveness of iloprost to treat frostbite, the study instead aims to describe how this question has been investigated. Specifically, the study addresses the following questions:
What methodologies have been used to study the role of iloprost in the management of frostbite?
In what populations (e.g. geographic location of study, cause of frostbite, patients’ comorbidities, severity of frostbite, extent of injury) has iloprost been studied?
What iloprost treatment protocols have been studied (dose, route, duration)?
What treatments have been used along with iloprost (method of rewarming, NSAIDs, thrombolytics, anticoagulants, antiplatelets, wound care, Hyperbaric Oxygen Therapy, etc.)?
What outcomes are being used to measure the efficacy of iloprost for the treatment of frostbite and what is the reported efficacy of iloprost?
What is known about the safety of iloprost in the management of frostbite?
Methods
Protocol registration
As recommended by scoping review guidelines [37], the protocol was published on the free, open-access platform Open Science Framework to promote knowledge translation and methodological transparency.
Inclusion criteria
To be included in the review, the literature must:
Be primary research examining iloprost for the treatment of frostbite in humans, or
Be a conference or congress proceeding or abstract.
Primary research was included because the paper’s goal is to describe how, where and on whom iloprost has been studied to treat frostbite. In line with the overarching goals of a scoping review to include a breadth of knowledge in various forms, grey literature conference proceedings and abstracts were included. This ensured that any data not officially published in peer-reviewed journals were captured in the analysis.
The following literature types were excluded:
Any review methodology,
Treatment guidelines or protocols,
Grey literature (government reports, news articles, blog posts, etc.) apart from the above, or
Studies including any non-freezing cold injury (e.g. perniosis/chilblains, Trench foot disease or frostnip).
Reviews and treatment guidelines/protocols were excluded because the goal of the study is to describe the primary literature, rather than any larger analysis of or expert opinion on this literature. By excluding non-primary literature, the review will be able to focus more directly on delineating how iloprost for the treatment of frostbite has been studied thus far, and what gaps in research exist. Similarly, grey literature that did not pertain to primary research on iloprost for frostbite (i.e. Government reports, news articles, blog posts) were excluded because this form of knowledge will not contribute to a description of the nature of scientific knowledge regarding iloprost for frostbite, nor any gaps in this area of inquiry. Articles describing iloprost for non-freezing cold injuries were excluded as these injuries represent clinical entities distinct from frostbite that are not the subject of this review.
In order to capture the complete temporal and geographic study of iloprost for frostbite, no restriction on publication date, language, or location was used. Non-English articles were translated using a combination of Google Translate and input from native-language speakers.
Search strategy
The search strategy was constructed in consultation with a Doctor of Philosophy in Library Sciences. Medline, Embase, CINAHL, EBM Reviews were searched with a combination of iloprost and frostbite as key words as well as using MeSH terms in title, abstract and all fields. Web of Science, Scopus and Medline Conference proceedings were searched with the same key word and MeSH term strategy. Finally, a targeted search for grey literature was conducted using a list of pertinent conferences. See Figure 2 for a complete list. Articles were saved and organised in Zotero. The most recent search was executed on 15 December 2022.
Figure 2.
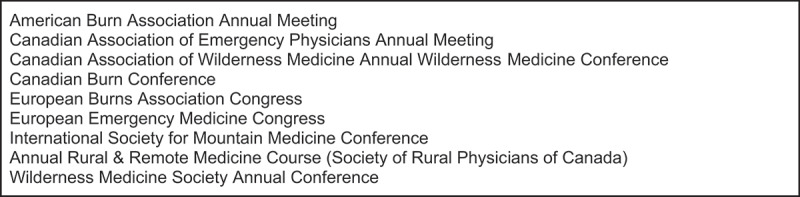
Grey literature sources.
Search
Ovid MEDLINE(R) and Epub Ahead of Print, In-Process, In-Data-Review & Other Non-Indexed Citations, Daily and Versions <1946 to 15 December 2022>
1 frostbite.sh. 2435
-
2 iloprost.sh. 2115
3 1 and 2 16
4 iloprost.ti. or iloprost.ab. 2637
5 2 or 4 3011
6 frostbite.ti. or frostbite.ab. 2817
7 1 or 6 2236
8 5 and 7 23
Selection of studies
Two reviewers (JG and AP) performed an initial title/abstract screen to exclude ineligible articles. A short list of articles was then generated which underwent a full-text review. A final list of articles to be included in the analysis was generated using the predetermined inclusion and exclusion criteria. The inclusion and exclusion of studies was documented on a Google Drive Sheet. Any disagreements on study eligibility were reconciled by discussion and consensus with the third reviewer (DMJ).
Data extraction
All data were extracted independently by the three authors from eligible studies using a Google Drive Sheet. The following variables were extracted when available: city and country where the patients were treated, study design, number of patients treated with iloprost, patient demographics (age, sex, comorbid conditions), event leading to frostbite, frostbite severity, extremities involved (lower/upper/both), number of digits affected, time from injury to rewarming and rewarming to iloprost initiation, rewarming method, iloprost dose and duration, other medications/therapies used (including Hyperbaric Oxygen Therapy), wound care, imaging used, digit amputation/salvage rate and adverse reactions.
Data analysis
A basic descriptive analysis was performed (number of studies, localisation, number of patients treated with iloprost, and number of digits involved).
Results
Selection of studies
Synthesis of results
Figure 3 presents a flow diagram of the process for selection of studies. Twenty studies were included in this review. Table 1 presents the characteristics of the studies included. The language of the included study is specified below the author name/year if different from English. There is a mention of “(Abstract)” below the author's name/year if the study was published as a conference or congress proceeding. The total number of patients treated with iloprost from all studies, percentage of male patient, patient age (mean) and the total number of digits affected (when available) is also reported.
Figure 3.
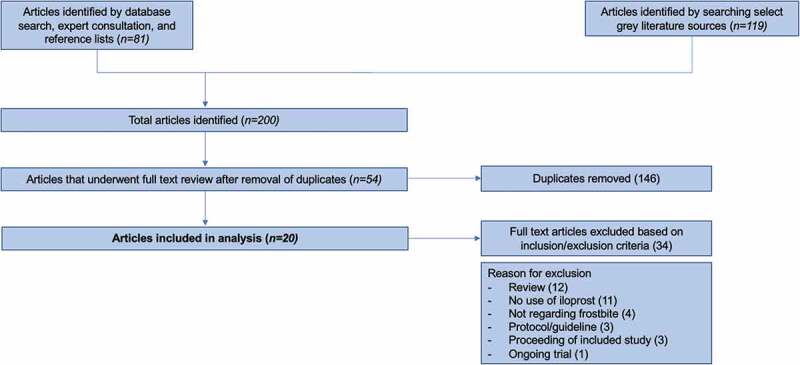
Selection of studies flow diagram.
Table 1.
Overall characteristics of included studies.
| Included Study | Country | Study Design | Number of patients treated with iloprost | Percentage of male patients | Patient age, mean | Predisposing event | Frostbite severity | Number of digits affected |
|---|---|---|---|---|---|---|---|---|
| Groechenig 1994 [21] | Austria | Case report | 5 | 80% | 35 | Sport, substance use, paralysis | 2nd and 3rd degree | - |
| Hödl 2005 [38] [German] |
Austria | Case report | 2 | 100% | 37 | Sport | 1st, 2nd and 3rd degree | 20 |
| Roche-Nagle et al. 2008 [39] | Ireland | Case report | 1 | 100% | 47 | Homelessness, substance use | Severe | 10 |
| Imray et al. 2009 [40] | England | Case report | 1 | - | n/a | Sport | - | 8 |
| Cauchy et al. 2011 [41] | France | Randomised controlled trial | 32 | 100% | 32 | Sport | Stage 2–4 | 301 |
| Gorjanc et al. 2012 [42] [Slovenian] |
Slovenia | Case series | 7 | - | n/a | Sport | Deep | - |
| Haik J et al. 2016 [43] | Nepal, Israel | Case series | 4 | 75% | 30 | Sport | Grade 1–4 | - |
| Cauchy et al. 2016 [44] (Abstract) |
France | Case series (iloprost versus other therapies) | 78 | - | - | - | Grade 2–4 | - |
| Poole & Gauthier 2016 [45] | Canada | Case report | 2 | 100% | 45 | Sport | Grade 3 | 10 |
| Lindford et al. 2017 [46] | Finland | Case series | 4 | - | - | n/a | Severe | n/a |
| Pandey et al. 2018 [47] | Nepal | Case series | 5 | 100% | 39 | Sport | Grade 2–4 | 34 |
| Gorjanc et al. 2018 [48] (Abstract) |
Slovenia | Case report | 2 | 100% | 25 | Sport | Deep | - |
| Irarrazaval et al. 2018 [49] | Nepal | Case report | 1 | 100% | 60 | Sport | Grade 2 and 3 | 5 |
| Jud et al. 2019 [50] | Austria | Case report | 1 | 100% | 45 | Sport | Grade 2 | - |
| Lorenzo-Villalba et al. 2021 [51] | France | Case report | 1 | 0% | 83 | Chronic disease (Cold Agglutinin Disease) | - | - |
| Poole et al. 2021 [52] | Canada | Case series | 22 | 77% | 39 | Sport, substance use, car trouble, work, psychiatric disorder | Grade 2–4 | 142 |
| MacLennan et al. 2021 [53] | Canada | Case report | 1 | 100% | 48 | Car trouble | Grade 2 | 1 |
| Magnan et al. 2021 [54] | Switzerland, France | Prospective single-arm study | 58 | 93% | 33 | Sport, work, homelessness | Grade 3 and 4 | 238 |
| Magnan et al. 2022 [55] | Switzerland | Case report | 1 | 100% | 36 | Sport | Grade 3 | 10 |
| Crooks et al. 2022 [56] | Canada | Case series (iloprost versus standard of care) | 26 | 89% | 36 | Substance use, car trouble, work, sport, unknown | Grade 2–4 | 242 |
| All studies | n/a | n/a | 254 | 88% | 42 | n/a | n/a | >1000 |
“-”= information not available, “n/a”= not applicable.
Frostbite treatment details are presented in Table 2, including iloprost dose and duration, other medications used, description of wound care when available and the use of Hyperbaric Oxygen Therapy (HBOT).
Table 2.
Relevant treatment data extracted.
| Included Study | Rewarming method | Time from injury or rewarming to iloprost | Iloprost dose | Iloprost duration | Other medications used | Wound care | HBOT |
|---|---|---|---|---|---|---|---|
| Groechenig 1994 [21] | - | - | 0.5 to 2 ng/kg/min continuous IV | 14–42 days | Cortisone LMWH |
- | No |
| Hödl 2005 [38] | - | 1 to 9 days from injury | 10 to 25 mcg IV daily | 3 to 8 weeks | ASA Analgesic Clindamycin Dextran Enoxaparin Flucloxacillin Neuroleptic |
Octenisept solution Aspiration of blisters Bacitracin/neomycin powder Strips between digits Cotton bandage Aquacel dressing |
No |
| Roche-Nagle et al. 2008 [39] | - | - | - | - | ASA Antibiotics Heparin NSAIDs |
- | No |
| Imray et al. 2009 [40] | - | 5 days from rewarming | - | 5 days | - | - | No |
| Cauchy et al. 2011 [41] | Rapid | < or >12 hours | 0.5 to 2 ng/kg/min for 6 hours IV daily | 8 days | ASA Alteplase |
- | No |
| Gorjanc et al. 2012 [42] | Rapid | <3 days from injury in majority | 1 to 2 ng/kg/min for 6 hours IV daily | 5 to 10 days | ASA Amox/clav Dalteparin/enoxaparin Pentoxifylline |
- | 10 to 30 days |
| Haik J et al. 2016 [43] | - | 1 day from rewarming | 2 ng/kg/min for 6 hours IV daily | 6 days | ASA Cephalexin Enoxaparin Ibuprofen |
Debridement Vaseline gauze Gauze soaked in mafenid acetate |
30 sessions |
| Cauchy et al. 2016 [44] | Rapid | - | - | - | ASA Alteplase (“tPA”; IV) |
- | No |
| Poole & Gauthier 2016 [45] | Passive and rapid | <24 to 48 hours from rewarming | 0.5 to 2 ng/kg/min for 6 hours IV daily | 5 days | Fentanyl Ibuprofen Morphine |
Hydrotherapy whirlpool daily Aspiration of clear blister Low-adherent dressings Topical Aloe vera |
No |
| Lindford et al. 2017 [46] | Rapid | 28 hours from injury in 1 case | 0.5 to 2 ng/kg/min for 6 hours IV daily | 2 to 3 days | ASA Alteplase (IA) Cephalosporin Enoxaparin Opioid analgesia Pantoprazole Papaverine Paracetamol Regional nerve block Simvastatin UFH |
Debridement of blisters Polyurethane finger or Silver-based foam dressings |
No |
| Pandey et al. 2018 [47] | Passive | 32 to 72 hours from injury | 2 to 10 mcg/hour for 6 hours IV daily (per patient weight; titrated up to 2 ng/kg/min) | 5 days | ASA Ceftriaxone Cephalexin |
Draining of large blisters Non adherent dressing |
No |
| Gorjanc et al. 2018 [48] | - | - | - | - | Amox/clav Enoxaparin Ibuprofen Pantoprazole Pentoxifylline |
- | Yes |
| Irarrazaval et al. 2018 [49] | Rapid | 75 hours from injury | 2 ng/kg/min for 6 hours IV daily | 4 days | ASA Cephalexin Ibuprofen Sildenafil |
Daily dressing change | 5 days |
| Jud et al. 2019 [50] | - | - | 20 mcg IV daily | 10 days | ASA Enoxaparin |
Draining of lesions Topical betamethasone |
No |
| Lorenzo-Villalba et al. 2021 [51] | Passive | 8 days from injury | n/a | 5 days | ASA Bendamustine and rituximab (for Cold Agglutinin Disease) |
Evacuation of hematomas | No |
| Poole et al. 2021 [52] | Passive and rapid | <1 to 55 hours from presentation | 2 to 10 mcg/hour for 6 hours IV daily (per patient weight; titrated up to 2 ng/kg/min) | 1 to 5 days | Alteplase (IV) Ibuprofen UFH |
Daily hydrotherapy Debridement and aspiration of clear blisters Topical aloe vera |
No |
| MacLennan et al. 2021 [53] | - | - | 2 to 10 mcg/hour for 6 hours IV daily | 5 days | - | - | No |
| Magnan et al. 2021 [54] | Rapid | <6 to 72 hours (majority 12 to 48 hours) from injury | 8 to 10 mcg/hour for 6 hours IV daily | 7 days | ASA Amox/clav |
Topical hyaluronic acid daily | 14 sessions |
| Magnan et al. 2022 [55] | Passive | 70 hours from injury | 10 mcg/hour for 6 hours IV daily | 7 days | ASA Amox/clav Heparin |
Daily warm bath/betadine Bandage with hyaluronic acid Early physiotherapy |
30 sessions |
| Crooks et al. 2022 [56] | - | <12 to >72 hours (majority<12 hours) from injury to presentation | 2 to 10 mcg/hour for 6 hours IV daily (maximum 50 mcg/day) | 5 days | ASA Alteplase IV/IA (Grade 4) Antibiotics Ibuprofen Nitroglycerin UFH (Grade 4) |
- | No |
“HBOT” = Hyperbaric Oxygen Therapy, “-” = information not available, “IV” = intravenous, “IA” = intra-arterial, “ASA” = Acetylsalicylic acid.
“Amox/clav” = Amoxicillin/clavulanic acid, “LMWH” = Low Molecular Weight Heparin”, “UFH” = Unfractionated heparin.
Finally, the main treatment outcomes measured, outcomes reported, and adverse drug events reported are summarised in Table 3. Only outcomes of patients treated with iloprost are reported to simplify and summarise pertinent outcomes to this review. Iloprost outcomes are presented as n (%). Results from statistical analysis (chi-square or Fisher’s exact tests, analysis of variance, Mann – Whitney test, logistic regressions, and subgroup analysis) are not included in the table for simplification purposes but are reported in the discussion when pertinent.
Table 3.
Relevant outcome data extracted.
| Included Study | Outcome measured | Outcome | Adverse drug event |
|---|---|---|---|
| Groechenig 1994 [21] | Patients requiring amputation | 0/5 (0%) | - |
| Hödl 2005 [38] | Affected digits requiring amputation | 5/20 (25%) | None |
| Roche-Nagle et al. 2008 [39] | Affected limbs requiring amputation | 2/2 (100%) | - |
| Imray et al. 2009 [40] | Affected digits appearance and function | Full function regained | - |
| Cauchy et al. 2011 [41] | Risk of patient requiring amputation based on bone scan at day 8 | 0/16 (0%) iloprost3/16 (19%) iloprost + alteplase | Hot flashes (55%), nausea (25%), palpitation (15%), vomiting (5%) |
| Affected digits requiring amputation according to treatment | 0/142 (0%) iloprost5/159 (3%) iloprost + alteplase | ||
| Affected digits requiring amputation according to severity of frostbite | Stage 2 Stage 3 Stage 40/64 (0%) 0/74 (0%) 0/3 (0%) iloprost2/60 (3%) 0/74 (0%) 3/25 (12%) iloprost + alteplase | ||
| Affected digits requiring amputation according to time to treatment | <12 hours >12 hours0/79 (0%) 0/63 (0%) iloprost2/144 (1%) 3/15 (20%) iloprost + alteplase | ||
| Gorjanc et al. 2012 [42] | Patients requiring amputation | 4/7 (57%) | Dizziness and hypotension requiring discontinuation of iloprost (29%) |
| Haik J et al. 2016 [43] | Patients requiring amputation | 2/4 (50%) | - |
| Cauchy et al. 2016 [44] | Patients requiring amputation according to severity of frostbite | Grade 3 Grade 42/41 (5%) 4/6 (67%) iloprost3/11 (27%) 4/9 (44%) iloprost + alteplase | - |
| Poole & Gauthier 2016 [45] | Affected digits requiring amputation | 0/10 (0%) | None |
| Lindford et al. 2017 [46] | Overall digital salvage rate* | 78% | None |
| Pandey et al. 2018 [47] | Affected digits requiring amputation | 13/34 (38%) | Headache (40%), nausea (40%), hypotension requiring dose reduction (20%) |
| Gorjanc et al. 2018 [48] | n/a | n/a | - |
| Irarrazaval et al. 2018 [49] | Affected digits requiring amputation | 5/5 (100%), partially | - |
| Jud et al. 2019 [50] | Patient requiring amputation | 0/1 (0%) | - |
| Lorenzo-Villalba et al. 2021 [51] | Affected limbs requiring amputation | 4/4 (100%) | Hypotension and headache limiting dose increase (100%) |
| Poole et al. 2021 [52] | Patients requiring amputation | 4/22 (18%) | Headache (50%), flushing (36%), tachycardia (36%), nausea (27%), vomiting (9%), dizziness (9%), hypotension (4%), bleeding (4%) |
| Affected digits requiring amputation according to severity | Grade 2 Grade 3 Grade 40/59 (0%) 0/25 (0%) 29/58 (50%) | ||
| MacLennan et al. 2021 [53] | Visual microvascular assessment and treatment response | Flow defect visible and improved 6 hours after iloprost infusion | None |
| Magnan et al. 2021 [54] | Preserved segments** per patient (mean) compared to historical group | 13 iloprost + HBOT 6 iloprost alone |
- |
| Amputated rays*** compared to historical group | 0 iloprost + HBOT 2 iloprost alone |
||
| Magnan et al. 2022 [55] | Affected digits requiring amputation | 0/10 (0%) | - |
| Crooks et al. 2022 [56] | Affected digits requiring amputation according to frostbite severity compared to standard care | Grade 2 Grade 3 Grade 40/44 (0%) 18/102 (18%) 44/96 (46%) | Tachycardia requiring dose reduction (12%), headache (8%), headache requiring iloprost discontinuation (4%) |
“-” = information not available
* Calculated using the total number of digits at risk minus number of digits amputated divided by total number of digits at risk multiplied by 100
** Each phalanx and each metacarpal or metatarsal is defined as a segment. Preserved segments was defined as the difference between the number of segments with frostbite and lost segments
*** 4 segments comprise a ray (3 segments for the thumb or the hallux)
Discussion
Summary of evidence
Iloprost has garnered interest in the treatment of frostbite, a condition where in advanced cases the risk of amputation is high without treatment. We analysed 20 studies describing the use of iloprost for frostbite, published from 1994 to 2022. The vast majority of studies examining this question are retrospective case series consisting of the fairly homogenous population of extreme mountain sport enthusiasts. Nevertheless, there exists a base of scientific knowledge, with 254 treated patients identified in our review, including over 1000 frostbitten digits, from which further inquiry can be pursued. The primary gaps in the literature include the paucity of high-quality randomised trials and relatively limited study populations to date. Below we will discuss our results in detail as framed through the guiding questions of this study.
Where and in what populations has iloprost for frostbite been studied?
According to the publications available, treatment of frostbite with iloprost was first described in Austria, then Ireland, England, France, Slovenia, Nepal and Israel (Table 1). Publications from Canada arose in 2016. No studies from the United States could be found. The geography of studies can likely be explained by the increased prevalence of frostbite in these countries and the limited availability of iloprost, as it is not commercially available in the United States and only available in Canada through Health Canada Special Access Program since 2015.
The population in which iloprost has been studied is fairly homogeneous. Most patients identified by this review were males in their 40s. There were no children or pregnant patients identified. Sports was the leading cause of frostbite, present as a predisposing event in 15 studies [21,38,40–43,45,47–50,52,54–56]. Most patients developed frostbite while engaging in sports such as alpinism, hiking, climbing, mountaineering, trekking, running and paragliding. Many injuries occurred at high altitude where the risk of poor outcome may increase due to hypoxaemia, impaired vasodilation and dehydration [57].
Substance use only contributed to frostbite in four published studies [21,39,52,56]. Crooks et al.’s retrospective case comparison studied a predominantly inner-city population [56]. Of the 26 cases treated with iloprost, 81% were smokers, 73% used alcohol regularly, 12% used opioids, 50% used stimulants and 35% were unhoused. lntoxication was the circumstance resulting in frostbite injury in 19 patients (73%). More data is required to generalise the treatment effect of iloprost in frostbite. Focusing on adventure athletes may result in describing the best-case scenario of treating healthy individuals without comorbidities.
The terminology used to describe frostbite severity varied across studies. Cauchy’s classification [3] was the most commonly used, but several studies did not specify the scale they used to grade frostbite severity. The most published data described patients with frostbite severity of Grade 2 to 4. Some studies graded the injuries clinically (visual grading), while others used imaging (angiography or bone scan) to grade severity. MacLennan et al. used intravenous indocyanine green fluorescence to visualise microvasculature and assess iloprost treatment response [53]. This raises the issue of grading clinically versus using imaging studies such as angiography, nuclear medicine or fluorescence, especially in resource-limited settings. A gap in the literature exists that validates clinical grading relative to advanced imaging. Future studies should explicitly state how frostbite is graded in order for potential treatment effects to be identified for specific frostbite grades and to enable comparison between study populations.
In all studies, frostbite occurred on the hands, feet, or digits. The number of digits affected was not consistently detailed but totalled over 1000. The distribution of affected digits, whether upper of lower extremities, was not consistently described. Some authors suggest that the long-term outcome may be affected by the location of the lesions [5]. No studies included other areas that can be affected by frostbite, such as the torso, face, ears or nose.
How has iloprost for frostbite been studied?
The majority of publications were case reports (11), followed by case series (7), one prospective single-arm study and one randomised controlled trial (Table 1). There is a paucity of prospective, randomised clinical trials comparing iloprost’s efficacy and/or safety to other treatments for frostbite. Only one randomised controlled trial was identified, which enrolled 47 patients and compared iloprost to iloprost and alteplase as well as to buflomedil (all combined with acetylsalicylic acid) [41]. A subsequent publication from Cauchy et al. with the same treatment arms (as well as an additional group who did not receive any treatment) described a larger number of participants (N = 131) [44]. However, it was only published in the form of an abstract and the details are limited.
The number of patients treated with iloprost in each study ranged from 1 to 78 patients, totalling 254 patients. The largest studies published were Cauchy et al. 2016 (N = 78), Magnan et al. 2022 (N = 58), Cauchy et al. 2011 (N = 32), Crooks et al. 2022 (N = 26) and Poole et al. 2021 (N = 22). There exists the possibility of cases overlap between the different publications owing to the fact that four groups produced two cases series each (Cauchy et al., Gorjanc et al., Poole et al. and Magnan et al.) [41,42,44,45,48,52,54,55]. At most, the overlap would produce redundancy in 37 patients.
While case reports and case series generally cannot produce widely generalisable conclusions, in this case they have helped to lay a foundation of clinical experience and knowledge regarding the use of iloprost to treat frostbite. Given the fact that frostbite is a relatively rare disease, there will likely need to be longstanding, multicenter collaboration in order to adequately power randomised controlled trials.
What iloprost protocols have been studied to treat frostbite and what other therapies have been included?
We identified a diverse range of treatment protocols in which iloprost was used to treat frostbite (Table 2). Several studies began treatment with rewarming of the affected tissues. However, the rewarming method was not consistently described. Eight studies reported rapid rewarming with immersion in hot water [41,42,44–46,49,52,54]. Others simply stated that rewarming had occurred.
The time from frostbite injury or rewarming to iloprost treatment ranged from less than one hour to nine days. Some reported time from the injury or first onset of symptoms to presentation to hospital, while others reported time from rewarming to treatment initiation (warm ischaemia time). The most common iloprost dose used for frostbite was 0.5 to 2 ng/kg/min or 2 to 10 mcg/hour infused for 6 h daily, which is consistent with dosing used in Raynaud’s [17]. However, the duration of daily iloprost infusions varied from 1 to 10 days, with some extending the infusions to 56 days. The optimal iloprost duration in frostbite is yet to be determined.
Almost all studies reported numerous concomitant therapies with iloprost. The most commonly used were non-steroidal anti-inflammatories (acetylsalicylic acid, ibuprofen) in 16 studies [38,39,41–44,46–52,54–56]. There is uncertainty as to whether acetylsalicylic acid or ibuprofen is effective in frostbite. Since their use was popularised in 1980s, this hypothesis has not been verified [8–11].
Alteplase was used in five studies [41,44,46,52,56] and mostly in combination with iloprost. In the Cauchy et al. randomised controlled trial, the risk of amputation was lower in the iloprost group compared to the iloprost and alteplase group, however there were more severely frostbitten digits in the iloprost and alteplase group [41]. The benefit of the combination has yet to be confirmed. A randomised control trial is underway in Finland to compare iloprost to alteplase efficacy [58].
Unfractionated heparin (UFH) and low molecular weight heparins (LMWH) were also frequently used in the studies included in this review. As described previously, evidence supports the use of heparin as adjunctive therapy in thrombolytic protocols, but heparin or enoxaparin/dalteparin have been used in conjunction with iloprost alone in eight of the studies included in this review [21,38,39,42,43,48,50,55]. Whether UFH or LMWH offer additional benefit when combined with iloprost requires further investigation.
Wound care was not consistently described and varied. Debridement and daily hydrotherapy were common.
Hyperbaric oxygen therapy (HBOT) was used in six studies [42,43,48,49,54,55]. Many studies did not describe their HBOT protocol beyond the number of days they used the treatment for. Magnan et al. (2021) provided a detailed description of how HBOT was used in their study [54]. They initiated the first HBOT session as close to the time of injury as possible. The duration was 150 min at 2.5 atmosphere absolutes (ATA), which is equivalent to 2.5 times the atmospheric pressure at sea level. Patients received daily HBOT sessions for 150 min at 2.5 ATA for seven days while in hospital and another seven days after discharge, for a total of 14 days. This protocol for HBOT was the most detailed of all the studies in this review.
What outcomes measures are being used in the study of iloprost for the treatment of frostbite?
Most studies reported the rate of amputation of the area affected by frostbite (Table 3). Some used the number of digits amputated over the total patient digits affected to calculate a rate of amputation, some reported the amputation rate per frostbite severity, while others used patients requiring one or more amputations over patients affected by frostbite. Magnan et al. described their amputation rate by segment (each phalanx or each metacarpal or metatarsal) and by ray (four segments or three segments for the thumb or the hallux) [54]. The use of a standard quantification tool such as the Hennepin score would be useful to compare future studies results [59].
Some studies presented pictures of wound appearance or described digit and limb function on follow-up [38–40,42,45–47,49–51,55]. There was often limited information on potential chronic issues in patients who avoided amputation such as vasomotor disturbances, neuropathic and nociceptive pain or damage to skeletal structures [60]. The duration of follow-up was often short.
With regards to safety and adverse reactions, 10 of 20 studies described iloprost tolerance [38,41,42,45–47,51–53,56].
What were the studies’ findings?
Within the aforementioned methodological limitations, the literature suggests that treatment of frostbite with iloprost is associated with reduced amputation rates (Table 3). Some of the strongest evidence comes from Cauchy et al. (2011) [41]. Percentage of amputation of affected digits across treatment arms and for all stages of frostbite were 39.6% in the buflomedil group, 3.1% in the iloprost and alteplase group, and 0% in the iloprost alone group.
In their later publication, Cauchy et al. (2016) also demonstrated an association between iloprost and reduced amputation rates in the treatment of frostbite [44]. For grade 4 frostbite, the amputation rate in patients receiving no pharmacologic intervention or aspirin and buflomedil was 100% but the amputation rate was 67% with iloprost and 44% when combining iloprost and alteplase.
Poole and Gauthier, across their 2016 and 2021 papers, showed a combined digit amputation rate of 0% for Grade 2 and Grade 3, and 50% for Grade 4 frostbite [45,52].
Crooks et al. also showed reduced amputations among patients treated with iloprost [56]. They included 90 patients, where 26 were treated with iloprost and 64 received standard care. For Grade 2 frostbite severity, there were no amputations in either group. However, significantly lower digital amputation rates were seen among patients treated with iloprost compared to controls for Grade 3 (18% vs 44%, p < 0.001) and Grade 4 (46% vs 95%, p < 0.001) severities.
Interestingly, Magnan et al. (2021) showed that patients treated with iloprost in combination with HBOT required less amputation than patients treated with iloprost alone [54]. The authors compared a prospective group of patients with Grades 3 and 4 frostbite treated with iloprost and HBOT to a historical control group who had received iloprost alone. Patients who received iloprost and HBOT had a significantly higher number of preserved segments per patient (mean 13) compared to a historical group treated with iloprost alone (mean 6) and the odds ratio was significantly higher by 45-fold (p < 0.001).
The association between iloprost treatment and reduced amputation rates was not seen in all studies. For example, a case series by Haik et al. showed that two of four patients with varying degrees of frostbite severity required amputation [43]. One patient with Grade 3 and 4 injuries to both hands required 10 digital amputations. Another patient with Grade 4 and Grade 2 to 3 injuries to their right hand and left foot, respectively, required a right palm and four left-toe amputations. Another case report by Irarrazaval et al. described a patient with Grades 2 and 3 frostbite who required some degree of amputation for each digit despite iloprost treatment [49]. One publication described the case of an unfortunate elderly patient with Cold Agglutinin Disease and Raynaud’s who presented seven days after cold exposure with extensive cyanosis to feet and hands [51]. The patient was treated unsuccessfully with iloprost and eventually passed. Some authors suggest that even if amputation was unavoidable in certain subjects, amputations were performed on a smaller scale with iloprost [42].
While there is still no direct comparison of iloprost versus alteplase, in the study from Lindford et al., iloprost was used if there was a contraindication to thrombolysis, angiographic findings suggesting vasospasm or nonresponse to thrombolysis [46]. One patient had a minimal response to alteplase but had complete reperfusion after iloprost infusion, suggesting iloprost may have benefits when thrombolytics failed [46].
Iloprost is believed to maintain its efficacy even if initiated later post-rewarming compared to thrombolytics. In Cauchy et al.’s studies, treatment benefit with iloprost was evident even if treatment was started more than 12 h after injury [41] and up to 48 h [44]. Pandey et al. attempted to evaluate if delayed iloprost use up to 72 h would help reduce tissue loss in Grades 3 and 4 frostbite [47]. In their case, a series of patients evacuated from Himalayan peaks, four received treatment between 48 and 72 h from injury. Two of the patients treated with iloprost had excellent results with minimal tissue loss and two had good results with tissue loss less than expected. One patient with a poor outcome likely experienced a freeze-thaw-refreeze injury. Other authors observed iloprost benefit despite delayed initiation of iloprost 70-h post injury [55] and five-day post-rewarming [40].
Relatively minor side effects (headache, nausea, flushing) were common, which is consistent with the non-frostbite literature [17–20]. Occasionally more rare but serious side effects (symptomatic hypotension, bleeding) resulted in iloprost discontinuation [42,56]. There was limited information on the safety and tolerability of iloprost and alteplase/heparin combination.
As illustrated in Table 3, most studies show some association between iloprost and reduced amputation rates. Adverse effects from iloprost were generally infrequent and mild, though some more clinically significant adverse events were reported. Given the significant limitations of study design and population across the vast majority of studies found in this review, adequately powered randomised controlled trials are needed to demonstrate the efficacy and safety of iloprost to treat frostbite.
Limitations
A scoping review methodology is limited in general insofar as it cannot determine a treatment’s efficacy. In our case specifically, this scoping review does not and cannot comment on whether iloprost is effective for the treatment of frostbite. Rather, this scoping review maps how iloprost has been studied to date, summarises the studies’ findings, and identifies gaps in research.
We opted to include various forms of publications, including grey literature. Two studies included in this review were published in scientific journals’ “Correspondence” section [21,41] and two were international conferences’ abstracts [44,48]. They were short publications with limited details.
Conclusions
This scoping review highlights how the vast majority of data on iloprost for frostbite has come from retrospective case analysis of young, healthy males, who developed frostbite engaging in high altitude or cold weather activities. There is a paucity of high quality, prospective, randomised data derived from heterogeneous study populations. In addition, this study serves as a road map for the ongoing inquiry of iloprost to treat frostbite. Key issues to be addressed by future research are utilising a consensus frostbite severity grading scale, carefully selecting an iloprost dosing and adjuvant regime and employing reproducible and clinically meaningful outcome measurements. Given frostbite’s low prevalence, multi-centre collaboration will be crucial to adequately power any prospective randomised studies in order to definitively determine if iloprost has a role in the treatment of frostbite.
Acknowledgments
The authors would like to thank Dr. Trina Fyfe, Health Sciences Librarian at the University of Northern British Columbia, for her help with the literature search. The authors would also like to thank Aleksandra Neumann, Library Technician at the College of Physicians and Surgeons of British Columbia Library Services for retrieving articles unavailable to the authors.
Funding Statement
The authors received no funding to perform this scoping review.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Handford C, Thomas O, Imray CHE.. Frostbite. Emerg Med Clin North Am. 2017;35(2):281–15. [DOI] [PubMed] [Google Scholar]
- [2].McIntosh SE, Freer L, Grissom CK, et al. Wilderness medical society clinical practice guidelines for the prevention and treatment of frostbite: 2019 update. Wilderness Environ Med. 2019;30(4S):S19–32. [DOI] [PubMed] [Google Scholar]
- [3].Cauchy E, Chenille E, Marchand V, et al. Retrospective study of 70 cases of severe frostbite lesions: a proposed new classification scheme. Wilderness Environ Med. 2001;12(4):248–255. [DOI] [PubMed] [Google Scholar]
- [4].Drinane J, Kotamarti VS, O’connor C, et al. Thrombolytic salvage of threatened frostbitten extremities and digits: a systematic review. J Burn Care Res. 2019;40(5):541–549. [DOI] [PubMed] [Google Scholar]
- [5].Drinane J, Heiman AJ, Ricci JA, et al. Thrombolytic salvage of the frostbitten upper extremity: a systematic review. Hand (N Y). 2022;17(3):397–404. doi: 10.1177/1558944720940065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Mills WJ, Whaley R, Fish W. Frostbite: experience with rapid rewarming and ultrasonic therapy. Part III. 1961. Alaska Med. 1993;35(1): 19–27. Part III. 1961. [PubMed] [Google Scholar]
- [7].Robson MC, Heggers JP. Evaluation of hand frostbite blister fluid as a clue to pathogenesis. J Hand Surg Am. 1981;6(1):43–47. [DOI] [PubMed] [Google Scholar]
- [8].Heggers JP, Robson MC, Manavalen K, et al. Experimental and clinical observations on frostbite. Ann Emerg Med. 1987;16(9):1056–1062. [DOI] [PubMed] [Google Scholar]
- [9].McCauley RL, Hing DN, Robson MC, et al. Frostbite injuries: a rational approach based on the pathophysiology. J Trauma. 1983;23(2):143–147. doi: 10.1097/00005373-198302000-00013 [DOI] [PubMed] [Google Scholar]
- [10].Heggers JP, Phillips LG, McCauley RL, et al. Frostbite: experimental and clinical evaluations of treatment. J Wilderness Med. 1990;1(1):27–32. doi: 10.1580/0953-9859-1.1.27 [DOI] [Google Scholar]
- [11].McCauley RL, Heggers JP, Robson MC. Frostbite. Postgrad Med. 1990;88(8):67–77. [DOI] [PubMed] [Google Scholar]
- [12].Skolnick AA. Early data suggest clot-dissolving drug may help save frostbitten limbs from amputation. JAMA. 1992;267(15):2008–2010. [PubMed] [Google Scholar]
- [13].Murphy J, Endorf FW, Winters MK, et al. Bleeding complications in patients with severe frostbite injury. J Burn Care Res. 2022;irac180. doi: 10.1093/jbcr/irac180. [DOI] [PubMed] [Google Scholar]
- [14].Hutchison RL, Miller HM, Michalke SK. The use of tPA in the treatment of frostbite: a systematic review. Hand (N Y). 2019;14(1):13–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Nygaard RM, Lacey AM, Lemere A, et al. Time matters in severe frostbite: assessment of Limb/digit salvage on the individual patient level. J Burn Care Res. 2017;38(1):53–59. [DOI] [PubMed] [Google Scholar]
- [16].Cutlip D, Lincoff AM. Anticoagulant therapy in non-ST elevation acute coronary syndromes. In: Verheugt F, editor. UpToDate. Waltham (MA): Wolters Kluwer N.V.; 2022. [Google Scholar]
- [17].Levien TL. Advances in the treatment of Raynaud’s phenomenon. Vasc Health Risk Manag. 2010;6:167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Robertson L, Andras A. Prostanoids for intermittent claudication. Cochrane Database Syst Rev. 2013;(4). CD000986. doi: 10.1002/14651858.CD000986.pub3 [DOI] [PubMed] [Google Scholar]
- [19].Vietto V, Franco JV, Saenz V, et al. Prostanoids for critical limb ischaemia. Cochrane Database Syst Rev. 2018;1(1). CD006544. doi: 10.1002/14651858.CD006544.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Cacione DG, Macedo CR, Do Carmo Novaes F, et al. Pharmacological treatment for Buerger’s disease. Cochrane Database Syst Rev. 2020;5(5):CD011033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Groechenig E. Treatment of frostbite with iloprost. Lancet. 1994;344(8930):1152–1153. [DOI] [PubMed] [Google Scholar]
- [22].Weissman G. Prostaglandins in acute inflammation current concepts. Kalamazoo, Michigan: Scope Publication. The Upjohn Company; 1980. [Google Scholar]
- [23].Grant SM, Goa KI. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in peripheral vascular disease, myocardial ischaemia and extracorporeal circulation procedures. Drugs. 1992;43(6):889–924. [DOI] [PubMed] [Google Scholar]
- [24].Cotran RS, Kumar V, Collins T. Robbins pathologic basis of disease. 6th ed ed. Philadelphia, PA: Elsevier Science; 1999. [Google Scholar]
- [25].Bixio R, Adami G, Bertoldo E, et al. Higher body mass index is associated with a lower iloprost infusion rate tolerance and higher iloprost-related adverse events in patients with systemic sclerosis. Ther Adv Musculoskelet Dis. 2022;14:1759720X221137125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Legrand M, Oufella HA, De Backer D, et al. I-MICRO trial investigators. The I-MICRO trial, Ilomedin for treatment of septic shock with persistent microperfusion defects: a double-blind, randomized controlled trial-study protocol for a randomized controlled trial. Trials. 2020;21(1):601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Cristo Ropero MJ, Cruz-Utrilla A, Escribano-Subias MP. Epoprostenol for the treatment of pulmonary arterial hypertension. Expert Rev Clin Pharmacol. 2021;14(8):1005–1013. [DOI] [PubMed] [Google Scholar]
- [28].The Institute for Safe Medication Practices Canada (ISMP Canada) . Alert: interruption of epoprostenol (flolan) infusion leads to patient’s death. ISMP Canada Safety Bulletins. 2011;11(2): [Accessed December 22, 2022]; Available online at. https://ismpcanada.ca/bulletin/alert-interruption-of-epoprostenol-flolan-infusion-leads-to-patients-death/ [Google Scholar]
- [29].Cruz JE, Ward A, Anthony S, et al. Evidence for the use of epoprostenol to treat raynaud’s phenomenon with or without digital ulcers. Ann Pharmacother. 2016;50(12):1060–1067. [DOI] [PubMed] [Google Scholar]
- [30].Khan SL, Parikh R, Mooncai T, et al. Barriers to frostbite treatment at an academic medical center. Am J Emerg Med. 2019;37(8):.e1601.3–1601.5. [DOI] [PubMed] [Google Scholar]
- [31].Eslami Do A, Lauver B. Utilization of epoprostenol in the management of severe frostbite. CHEST Annual Meeting, Medical Student/resident Case Report Posters. Chicago, IL, USA; October 23 2019;(Abstract). doi: 10.1016/j.chest.2019.08.2054 [DOI] [Google Scholar]
- [32].Bello JW, Rickrode GA, Harlan NP, et al. Systemic prostacyclin analogues for frostbite require careful monitoring. J Burn Care Res. 2022;44(2):irac178. doi: 10.1093/jbcr/irac178 [DOI] [PubMed] [Google Scholar]
- [33].Woo EK, Lee JW, Hur GY, et al. Proposed treatment protocol for frostbite: a retrospective analysis of 17 cases based on a 3-year single-institution experience. Arch Plast Surg. 2013;40(5):510–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Patel N, Srinivasa DR, Srinivasa RN, et al. Intra-arterial thrombolysis for extremity frostbite decreases digital amputation rates and hospital length of stay. Cardiovasc Intervent Radiol. 2017;40(12):1824–1831. [DOI] [PubMed] [Google Scholar]
- [35].Zhang N, Yu X, Zhao J, et al. Management and outcome of feet deep frostbite injury (III and IV degrees): a series report of 36 cases. Int J Low Extrem Wounds. 2022;21(3):325–331. [DOI] [PubMed] [Google Scholar]
- [36].Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-Scr): checklist and explanation. Ann Intern Med. 2018;169(7):467–473. [DOI] [PubMed] [Google Scholar]
- [37].Peters MDJ, Marnie C, Tricco AC, et al. Updated methodological guidance for the conduct of scoping reviews. JBI Evid Synth. 2020;18(10):2119–2126. [DOI] [PubMed] [Google Scholar]
- [38].Hödl S. Zur Therapie der Erfrierungen [Treatment of freezing injury]. Wien Med Wochenschr. 2005;1551:199–203. [German]. doi: 10.1007/s10354-005-0165-5 [DOI] [PubMed] [Google Scholar]
- [39].Roche-Nagle G, Murphy D, Collins A, et al. Frostbite: management options. Eur J Emerg Med. 2008;15(3):173–175. [DOI] [PubMed] [Google Scholar]
- [40].Imray C, Grieve A, Dhillon S, et al. Cold damage to the extremities: frostbite and non-freezing cold injuries. Postgrad Med J. 2009;85(1007):481–488. [DOI] [PubMed] [Google Scholar]
- [41].Cauchy E, Cheguillaume B, Chetaille E. A controlled trial of a prostacyclin and rt-PA in the treatment of severe frostbite. N Engl J Med. 2011;364(2):189–190. [DOI] [PubMed] [Google Scholar]
- [42].Gorjanc J, Ahčan UG, Veselko M, et al. Modern management of patients with frostbite. ZdravVestn. 2012;81(10). Accessed on December 30, 2022. [Slovenian] Available from. https://vestnik.szd.si/index.php/ZdravVest/article/view/599 [Google Scholar]
- [43].Haik J, Brown S, Liran A, et al. Deep Frostbite: the question of adjuvant treatment. Isr Med Assoc J. 2016;18(1):56–57. [PubMed] [Google Scholar]
- [44].Cauchy E, Chetaille E, Pham E, et al. Iloprost with and without rt-PA: treatment of 131 cases of severe frostbite. 7th World Congress of Mountain and Wilderness Med. Telluride, Colorado. July 30 - August 4. 2016;273: 432. (Abstract). doi: 10.1016/j.wem.2016.06.029 [DOI] [Google Scholar]
- [45].Poole A, Gauthier J. Treatment of severe frostbite with iloprost in northern Canada. CMAJ. 2016;188(17–18):1255–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Lindford A, Valtonen J, Hult M, et al. The evolution of the Helsinki frostbite management protocol. Burns. 2017;43(7):1455–1463. doi: 10.1016/j.burns.2017.04.016 [DOI] [PubMed] [Google Scholar]
- [47].Pandey P, Vadlamudi R, Pradhan R, et al. Case report: severe frostbite in extreme altitude climbers-the Kathmandu iloprost experience. Wilderness Environ Med. 2018;29(3):366–374. [DOI] [PubMed] [Google Scholar]
- [48].Gorjanc J, Mekjavic I, Havlicek T, et al. The planica protocol for frostbite management - 2 case reports. XII international society for mountain medicine world congress on mountain medicine. Kathmandu, Nepal; November 21–24 2018. Abstract 33861. doi: 10.1089/ham.2018.29015.abstracts [DOI] [Google Scholar]
- [49].Irarrazaval S, Besa P, Cauchy E, et al. Case report of frostbite with delay in evacuation: field use of iloprost might have improved the outcome. High Alt Med Biol. 2018;19(4):382–387. [DOI] [PubMed] [Google Scholar]
- [50].Jud P, Hafner F, Brodmann M. Frostbite of the hands after paragliding: a chilling experience. Lancet. 2019;394(10216):2282. [DOI] [PubMed] [Google Scholar]
- [51].Lorenzo-Villalba N, Andres E, Guerrero-Niño J, et al. Frostbite and cold agglutinin disease: coexistence of two entities leading to poor clinical outcomes. Medicina (Kaunas). 2021;57(6):592.doi: 10.3390/medicina57060592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Poole A, Gauthier J, MacLennan M. Management of severe frostbite with iloprost, alteplase and heparin: a Yukon case series. CMAJ Open. 2021;9(2):E585–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].MacLennan M, Poole A, Gauthier J. Use of fluorescence to visualize response to iloprost treatment for frostbite. CMAJ. 2021;193(31):E1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Magnan M-A, Gavet-Ageron A, Louge P, et al. Hyperbaric oxygen therapy with iloprost improves digit salvage in severe frostbite compared to iloprost alone. Medicina (Kaunas). 2021;57(11):1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Magnan DM, Gelsomino M, Louge P, et al. Successful delayed hyperbaric oxygen therapy and iloprost treatment on severe Frostbite at high altitude. High Alt Med Biol. 2022;23(3):294–297. [DOI] [PubMed] [Google Scholar]
- [56].Crooks S, Shaw BH, Andruchow JE, et al. Effectiveness of intravenous prostaglandin to reduce digital amputations from frostbite: an observational study. CJEM. 2022;24(6):622–629. [DOI] [PubMed] [Google Scholar]
- [57].Cauchy E, Davis CB, Pasquier M, et al. A new proposal for management of severe frostbite in the austere environment. Wilderness Environ Med. 2016;27(1):92–99. [DOI] [PubMed] [Google Scholar]
- [58].European Union Clinical Trials Register . Prospective randomised non-inferiority Nordic frostbite treatment study comparing tPA and iloprost therapy. (Ongoing). EudraCT Number: 2018-000712-15. Available online at: https://www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2018-000712-15 (Accessed December 27, 2022).
- [59].Nygaard RM, Whitley AB, Fey RM, et al. The Hennepin score: quantification of frostbite management efficacy. J Burn Care Res. 2016;37(4):e317–22. [DOI] [PubMed] [Google Scholar]
- [60].Regli IB, Strapazzon G, Falla M, et al. Long-term sequelae of frostbite-a scoping review. Int J Environ Res Public Health. 2021;18(18):9655. [DOI] [PMC free article] [PubMed] [Google Scholar]


