Abstract
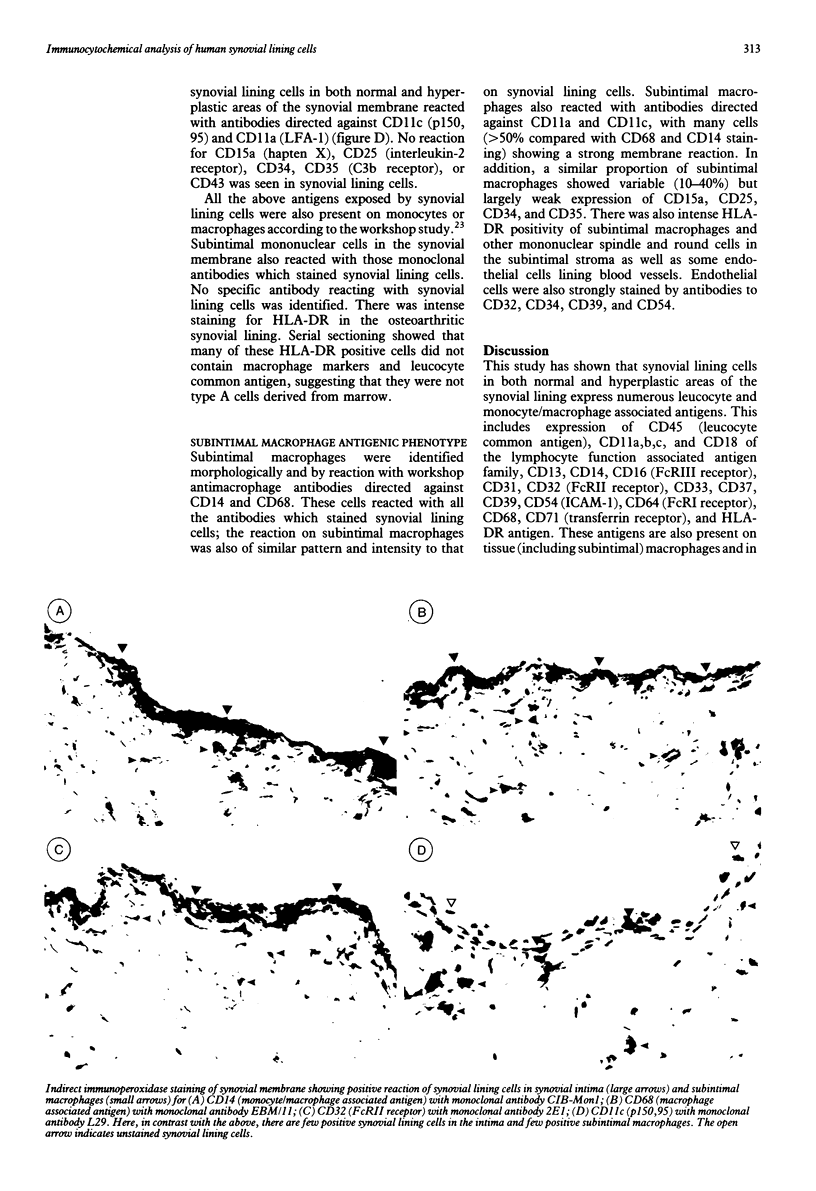
The antigenic phenotype of human synovial lining cells in normal and hyperplastic synovium intima was determined with a panel of monoclonal antibodies directed against a large number of well defined myeloid (macrophage/granulocyte associated) antigens. Synovial lining cells express numerous macrophage associated antigens, including CD11b (CR3), CD13, CD14, CD16 (FcRIII), CD18, CD32 (FcRII), CD45 (leucocyte common antigen), CD54 (ICAM-1), CD64 (FcRI), CD68, and CD71 (transferrin receptor). Few synovial lining cells expressed CD11a (LFA-1) and CD11c (p150,95). Subintimal macrophages expressed all the macrophage associated antigens which were present on synovial lining cells and, in addition, expressed CD15a, CD25 (interleukin-2 receptor), CD34, and CD35 (C3b receptor), none of which was present on synovial lining cells. Synovial lining cell expression of a wide range of macrophage antigens argues in favour of their marrow origin and membership of the mononuclear phagocyte system.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam W. S. Fine structure of synovial membrane: phagocytosis of colloidal carbon from the joint cavity. Lab Invest. 1966 Apr;15(4):680–691. [PubMed] [Google Scholar]
- Allen C. A., Highton J., Palmer D. G. Increased expression of p150,95 and CR3 leukocyte adhesion molecules by mononuclear phagocytes in rheumatoid synovial membranes. Comparison with osteoarthritic and normal synovial membranes. Arthritis Rheum. 1989 Aug;32(8):947–954. doi: 10.1002/anr.1780320803. [DOI] [PubMed] [Google Scholar]
- BALL J., CHAPMAN J. A., MUIRDEN K. D. THE UPTAKE OF IRON IN RABBIT SYNOVIAL TISSUE FOLLOWING INTRA-ARTICULAR INJECTION OF IRON DEXTRAN. A LIGHT AND ELECTRON MICROSCOPE STUDY. J Cell Biol. 1964 Aug;22:351–364. doi: 10.1083/jcb.22.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmester G. R., Dimitriu-Bona A., Waters S. J., Winchester R. J. Identification of three major synovial lining cell populations by monoclonal antibodies directed to Ia antigens and antigens associated with monocytes/macrophages and fibroblasts. Scand J Immunol. 1983 Jan;17(1):69–82. doi: 10.1111/j.1365-3083.1983.tb00767.x. [DOI] [PubMed] [Google Scholar]
- COCHRANE W., DAVIES D. V., PALFREY A. J. ABSORPTIVE FUNCTIONS OF THE SYNOVIAL MEMBRANE. Ann Rheum Dis. 1965 Jan;24:2–15. doi: 10.1136/ard.24.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey F. R., Cordell J. L., Erber W. N., Pulford K. A., Gatter K. C., Mason D. Y. Monoclonal antibody (Y1/82A) with specificity towards peripheral blood monocytes and tissue macrophages. J Clin Pathol. 1988 Jul;41(7):753–758. doi: 10.1136/jcp.41.7.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty G. J., McBride W. H. Macrophage heterogeneity. J Clin Lab Immunol. 1984 May;14(1):1–11. [PubMed] [Google Scholar]
- Duke O., Panayi G. S., Janossy G., Poulter L. W. An immunohistological analysis of lymphocyte subpopulations and their microenvironment in the synovial membranes of patients with rheumatoid arthritis using monoclonal antibodies. Clin Exp Immunol. 1982 Jul;49(1):22–30. [PMC free article] [PubMed] [Google Scholar]
- Edwards J. C. The origin of type A synovial lining cells. Immunobiology. 1982 Apr;161(3-4):227–231. doi: 10.1016/S0171-2985(82)80078-8. [DOI] [PubMed] [Google Scholar]
- Edwards J. C., Willoughby D. A. Demonstration of bone marrow derived cells in synovial lining by means of giant intracellular granules as genetic markers. Ann Rheum Dis. 1982 Apr;41(2):177–182. doi: 10.1136/ard.41.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fell H. B. Synoviocytes. J Clin Pathol Suppl (R Coll Pathol) 1978;12:14–24. [PMC free article] [PubMed] [Google Scholar]
- Gatter K. C., Brown G., Trowbridge I. S., Woolston R. E., Mason D. Y. Transferrin receptors in human tissues: their distribution and possible clinical relevance. J Clin Pathol. 1983 May;36(5):539–545. doi: 10.1136/jcp.36.5.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghadially F. N. Overview article: the articular territory of the reticuloendothelial system. Ultrastruct Pathol. 1980 Apr-Jun;1(2):249–264. doi: 10.3109/01913128009141422. [DOI] [PubMed] [Google Scholar]
- Ghadially F. N., Roy S. Ultrastructure of rabbit synovial membrane. Ann Rheum Dis. 1966 Jul;25(4):318–326. doi: 10.1136/ard.25.4.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graabaek P. M. Characteristics of the two types of synoviocytes in rat synovial membrane. An ultrastructural study. Lab Invest. 1984 Jun;50(6):690–702. [PubMed] [Google Scholar]
- Graabaek P. M. Ultrastructural evidence for two distinct types of synoviocytes in rat synovial membrane. J Ultrastruct Res. 1982 Mar;78(3):321–339. doi: 10.1016/s0022-5320(82)80006-3. [DOI] [PubMed] [Google Scholar]
- Hale L. P., Martin M. E., McCollum D. E., Nunley J. A., Springer T. A., Singer K. H., Haynes B. F. Immunohistologic analysis of the distribution of cell adhesion molecules within the inflammatory synovial microenvironment. Arthritis Rheum. 1989 Jan;32(1):22–30. doi: 10.1002/anr.1780320105. [DOI] [PubMed] [Google Scholar]
- Henderson B., Pettipher E. R. The synovial lining cell: biology and pathobiology. Semin Arthritis Rheum. 1985 Aug;15(1):1–32. doi: 10.1016/0049-0172(85)90007-1. [DOI] [PubMed] [Google Scholar]
- Henderson B., Revell P. A., Edwards J. C. Synovial lining cell hyperplasia in rheumatoid arthritis: dogma and fact. Ann Rheum Dis. 1988 Apr;47(4):348–349. doi: 10.1136/ard.47.4.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg N., Palmer D. G., Revell P. A. Mononuclear phagocytes of normal and rheumatoid synovial membrane identified by monoclonal antibodies. Immunology. 1985 Dec;56(4):673–681. [PMC free article] [PubMed] [Google Scholar]
- Kelly P. M., Bliss E., Morton J. A., Burns J., McGee J. O. Monoclonal antibody EBM/11: high cellular specificity for human macrophages. J Clin Pathol. 1988 May;41(5):510–515. doi: 10.1136/jcp.41.5.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klareskog L., Forsum U., Malmnäs Tjernlund U. K., Kabelitz D., Wigren A. Appearance of anti-HLA-DR-reactive cells in normal and rheumatoid synovial tissue. Scand J Immunol. 1981 Aug;14(2):183–192. doi: 10.1111/j.1365-3083.1981.tb00198.x. [DOI] [PubMed] [Google Scholar]
- Kurtin P. J., Pinkus G. S. Leukocyte common antigen--a diagnostic discriminant between hematopoietic and nonhematopoietic neoplasms in paraffin sections using monoclonal antibodies: correlation with immunologic studies and ultrastructural localization. Hum Pathol. 1985 Apr;16(4):353–365. doi: 10.1016/s0046-8177(85)80229-x. [DOI] [PubMed] [Google Scholar]
- Palmer D. G., Hogg N., Revell P. A. Lymphocytes, polymorphonuclear leukocytes, macrophages and platelets in synovium involved by rheumatoid arthritis. A study with monoclonal antibodies. Pathology. 1986 Oct;18(4):431–437. doi: 10.3109/00313028609087564. [DOI] [PubMed] [Google Scholar]
- Palmer D. G., Selvendran Y., Allen C., Revell P. A., Hogg N. Features of synovial membrane identified with monoclonal antibodies. Clin Exp Immunol. 1985 Mar;59(3):529–538. [PMC free article] [PubMed] [Google Scholar]
- Parwaresch M. R., Radzun H. J., Kreipe H., Hansmann M. L., Barth J. Monocyte/macrophage-reactive monoclonal antibody Ki-M6 recognizes an intracytoplasmic antigen. Am J Pathol. 1986 Oct;125(1):141–151. [PMC free article] [PubMed] [Google Scholar]
- Poulter L. W., Duke O., Hobbs S., Janossy G., Panayi G. Histochemical discrimination of HLA-DR positive cell populations in the normal and arthritic synovial lining. Clin Exp Immunol. 1982 May;48(2):381–388. [PMC free article] [PubMed] [Google Scholar]
- Revell P. A., Mayston V. Histopathology of the synovial membrane of peripheral joints in ankylosing spondylitis. Ann Rheum Dis. 1982 Dec;41(6):579–586. doi: 10.1136/ard.41.6.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothlein R., Dustin M. L., Marlin S. D., Springer T. A. A human intercellular adhesion molecule (ICAM-1) distinct from LFA-1. J Immunol. 1986 Aug 15;137(4):1270–1274. [PubMed] [Google Scholar]
- Sanchez-Madrid F., Nagy J. A., Robbins E., Simon P., Springer T. A. A human leukocyte differentiation antigen family with distinct alpha-subunits and a common beta-subunit: the lymphocyte function-associated antigen (LFA-1), the C3bi complement receptor (OKM1/Mac-1), and the p150,95 molecule. J Exp Med. 1983 Dec 1;158(6):1785–1803. doi: 10.1084/jem.158.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiozawa S., Shiozawa K., Fujita T. Presence of HLA-DR antigen on synovial type A and B cells: an immunoelectron microscopic study in rheumatoid arthritis, osteoarthritis and normal traumatic joints. Immunology. 1983 Dec;50(4):587–594. [PMC free article] [PubMed] [Google Scholar]
- Staunton D. E., Marlin S. D., Stratowa C., Dustin M. L., Springer T. A. Primary structure of ICAM-1 demonstrates interaction between members of the immunoglobulin and integrin supergene families. Cell. 1988 Mar 25;52(6):925–933. doi: 10.1016/0092-8674(88)90434-5. [DOI] [PubMed] [Google Scholar]
- Wright S. D., Detmers P. A. Adhesion-promoting receptors on phagocytes. J Cell Sci Suppl. 1988;9:99–120. doi: 10.1242/jcs.1988.supplement_9.5. [DOI] [PubMed] [Google Scholar]