Abstract
There has been limited research on patient-provider communication dynamics regarding Lyme disease (LD) diagnosis and treatment. Evidence suggests communication in the clinical encounter improves when both patient and healthcare provider (HCP) have concordant orientations (or beliefs) on discussed topics, resulting in higher patient satisfaction and care outcomes. The purpose of this scoping review was to characterize and summarize current research findings on patient and provider knowledge and experiences regarding LD - two factors that may influence the orientation of both patients and providers toward LD in the clinical setting. None of the articles included in the review specifically addressed patient-provider interaction and relationships as the main objective. However, the existing literature indicates notable HCP uncertainty regarding LD diagnosis, treatment, and applied practice patterns. Current research also describes limited knowledge of LD among patient populations and a high prevalence of negative perceptions of care received in mainstream healthcare settings among individuals with persistent symptoms. We identified a critical gap in research that seeks to understand the dynamic of patients and HCPs communicating on the topic of LD in the clinical setting. Future research may identify opportunities where the patient-provider communication dynamic can be improved.
Keywords: Illness experiences, Knowledge, Lyme disease, Patient-provider communication, Scoping review
1. Introduction
Lyme disease (LD), also referred to as Lyme borreliosis, is the most frequently reported tick-borne disease in the northern hemisphere (Stanek et al., 2012). The causative agents within the Borrelia burgdorferi sensu lato complex can be transferred to humans from infected nymph or adult stage Ixodes ticks (Hu, 2016). Although the Centers for Disease Control and Prevention (CDC) document approximately 30,000–40,000 reported cases of LD in the United States each year (Centers for Disease Control and Prevention, 2019a), recent CDC estimates indicate that LD incidence in the US may be closer to between roughly 340,000 and 470,000 cases per year (Hinckley et al., 2014; Kugeler et al., 2021; Nelson et al., 2015).
LD presents with varying signs and symptoms and it is sometimes difficult for physicians to recognize and diagnose (Nadelman and Wormser, 1998). Serologic diagnosis of LD is currently based on a two-step approach using an initial sensitive enzyme immunoassay (EIA) or immunofluorescence assay followed by IgM and IgM immunoblots or second EIA (Mead et al., 2019). According to clinical practice guidelines established by the Infectious Diseases Society of America (IDSA), the erythema migrans lesion (EM) is the only manifestation of LD in the US considered sufficiently distinctive to allow clinical diagnosis in the absence of laboratory confirmation (Lantos et al., 2021). The recommended treatment for early stages of LD includes oral antibiotics (doxycycline, amoxicillin, or cefuroxime axetil) for 10–21 days (Centers for Disease Control and Prevention, 2019b; Hu, 2016).
Diagnosis and treatment of LD has become a controversial topic in the US. The primary focus points of this controversy include reliability of two-tiered serologic testing to diagnose LD in early infection as compared with later stages of disease, efficacy of recommended antibiotic treatments, and the persistence of LD infection after treatment, which some groups refer to as chronic Lyme disease (CLD) (Aguero-Rosenfeld and Wormser, 2015; Cameron et al., 2014; Goodlet and Fairman, 2018; Maloney, 2016; Shapiro et al., 2017). The IDSA, American Academy of Neurology (AAN), and American College of Rheumatology (ACR) issued updated guideline recommendations for the prevention, diagnosis and treatment of LD in November 2020, marking the first update to guidelines on the diagnosis and treatment of LD by the IDSA since 2006 (Lantos et al., 2021; Wormser et al., 2006). These IDSA guidelines faced scrutiny by segments of the public and some congressional officials as an outdated resource prior to the 2020 update (Naktin, 2017). While the IDSA guideline recommendations remained largely consistent between 2006 and 2020, heightened social and conventional media attention on LD has yielded conflicting, and at times misleading, information for both physicians and patients on the diagnosis and treatment of LD signs and symptoms. This misinformation has contributed to confusion over the use of ‘chronic’ to describe late LD infection versus the imprecisely defined entity of CLD (Feder et al., 2007). Current literature has highlighted the consequences of this confusion and misinformation in the overdiagnosis of CLD and use of unconventional, and at times dangerous, treatment approaches for this condition (Auwaerter et al., 2011; Feder et al., 2007; Lantos et al., 2015; Maloney, 2016; Shapiro et al., 2017). In addition, the spread of misinformation may have negative consequences on the quality of patient-provider communication in the clinical setting (Boucher, 2010; Chaet, 2018; Merck Manuals, 2018).
Communication in the clinical encounter encompasses the means by which a patient’s symptoms are elicited, diagnosis is delivered, and treatment is recommended and monitored (McCabe and Healey, 2018). The perceived quality of patient-provider communication has been shown to influence health outcomes through adherence to clinical recommendations and patient satisfaction with their care, in addition to other factors (Belasen and Belasen, 2018; Quigley et al., 2014; Riedl and Schüßler, 2017; Stewart et al., 2000; Street, 2013; Underhill and Kiviniemi, 2011). Studies have revealed that a higher level of patient-provider satisfaction in communication and decision-making is achieved when patients and providers have matching orientations (or direction of thought) on specific topics (Krupat et al., 2001, 2000). Factors that can influence the level of congruence between patient and provider orientations include culture, education, knowledge, and social perception (Calo et al., 2014; Eddy, 1990; Howe et al., 2019; Langford and Loeb, 2019; Song et al., 2014).
Following McCabe and Healey (2018), a “shared understanding between doctor and patient about the nature of the problem and the treatment plan has been found to improve the therapeutic relationship, treatment satisfaction, and treatment adherence”. Given minimum insight on the current status of patient-provider communication regarding LD diagnosis and treatment, it is important to synthesize what is known about factors that may influence the orientation of both patients and providers toward LD in the clinical setting. We addressed this critical knowledge gap through a scoping review of the literature, with a goal of summarizing the current research approaches and findings regarding both patient and provider knowledge and experiences in clinical settings with respect to LD in the North American continent. Our results may guide future research to improve the patient-provider communication dynamic and, ultimately, patient care.
2. Material and methods
The aim of a scoping review is to rapidly map the key concepts underpinning a research area in terms of the volume, nature, and characteristics of available primary research sources. Scoping reviews are appropriate for exploring topics that have previously not undergone extensive review or are of a complex nature, and can often identify research gaps in the existing literature (Pham et al., 2014). We conducted a scoping review of the literature following the Arksey and O’Malley (2005) five-stage methodological framework. This included (i) identifying a research question, (ii) identifying relevant studies, (iii) performing study selection, (iv) charting and collating, and (v) summarizing and reporting results.
2.1. Literature search
A comprehensive search of the electronic databases PubMed, CINAHL, Scopus, and PsycINFO was conducted for articles published between January 2000 and July 2020. Searches were conducted using the general categories: Lyme disease (LD), patient, provider, knowledge, experience, clinical, and combinations of these categories utilizing Boolean operators. The complete search terms and PubMed strategy are outlined in Table 1. Reference lists of articles identified through the database search were reviewed for additional relevant publications. While review articles were not included in the scoping review, relevant primary sources identified from the reference lists of reviews were included.
Table 1.
Scoping Review Search Strategy for the PubMed Database.
| 1 | ((Lyme[mesh]) OR (lyme[tiab]) OR (chronic lyme disease[tiab]) OR (post treatment lyme disease syndrome[tiab])) |
| 2 | ((communication[mesh]) OR (communication[tiab]) OR (knowledge[tiab]) OR (experience*[tiab]) OR (belief*[tiab]) OR (attitude*[tiab]) OR (perspective*[tiab]) OR (understanding[tiab]) OR (practice*[tiab]) OR (education[tiab]) OR (perception[tiab]) OR (encounter*[tiab]) OR (learn*[tiab]) OR (aware*[tiab])) |
| 3 | ((patient*[mesh]) OR (patient*[tiab]) OR (public[tiab]) OR (community[tiab]) OR (resident*[tiab]) OR (client[tiab]) OR (individual*[tiab])) |
| 4 | ((provider[mesh]) OR (provider*[tiab]) OR (physician*[tiab]) OR (doctor[tiab]) OR (clinician[tiab]) OR (practitioner*[tiab]) OR (healthcare provider[tiab]) OR (healthcare professional[tiab])) |
| 5 | ((clinical[mesh]) OR (clinic*[tiab]) OR (practice*[tiab]) OR (family medicine[tiab]) OR (urgent care[tiab]) OR (primary care[tiab]) OR (healthcare[tiab]) OR (emergency room[tiab]) OR (general practice[tiab]) OR (health center*[tiab])) |
| 6 | 1 AND 2 AND 3 |
| 7 | 1 AND 2 AND 4 |
| 8 | 1 AND 2 AND 5 |
Searches were conducted using the general categories: Lyme disease, patient, provider, knowledge and experience, clinical, and combinations of these categories utilizing Boolean operators. Terms [mesh] and [tiab] are terms which direct the database to include search terms if they are included as a mesh term or are included in the title/abstract, respectively.
2.2. Selection criteria and review process
Research articles were included if they met the following criteria: (i) conducted in North America; (ii) empirical study; (iii) explicitly conducted with respect to or within a healthcare setting; (iv) examined LD, CLD, post-treatment Lyme disease syndrome (PTLDS), or any synonym; and (v) explicitly included an assessment of one or any combination of the following: experience of patient and/or provider, knowledge or understanding of patient and/or provider, perspective of patient and/or provider, or patient-provider communication. Articles were also limited to those with data collected no earlier than the year 2000, after the release of the initial IDSA guidelines, which were used as the reference for understanding recommended approaches to LD diagnosis and treatment associated with North American strains of Borrelia burgdorferi sensu stricto. Studies that did not meet any one of the above listed inclusion criteria were excluded from the scoping review.
The review process was conducted using Covidence systematic review software (Covidence, Veritas Health Information, Melbourne, Australia). After removing duplicates, each article was screened for inclusion by title and abstract by a single reviewing author (AN or EM). The remaining articles underwent a full text review, with each one screened by two reviewers independently (AN and EM). Conflicts about article inclusion or exclusion were resolved and finalized by the two reviewers.
2.3. Data extraction
Characteristics of the included literature were tagged using ATLAS.ti 8 (ATLAS.ti Scientific Software Development GmbH, Berlin, Germany). Document tags were used to categorize type of publication, study type, methods, and study population. A coding outline was developed based on a preliminary review of the full texts, and used to extract quotations that contributed to the understanding of provider knowledge and experience, patient knowledge and experience, or patient-provider relationship and interaction.
The distinction between provider knowledge and provider experience was based on the evaluation of knowledge and comprehension in didactic form versus the evaluation of implemented practice patterns. Although providers may demonstrate appropriate knowledge in a survey or interview setting, their actions and practice patterns may not reflect this during patient encounters; the distinction between the provider experience and provider knowledge categories was based on this difference.
3. Results
3.1. Summary of included articles
As shown in the PRISMA flow diagram (Fig. 1), 5,642 references were imported to Covidence for screening. After duplicate removal, 2,151 unique studies remained for title and abstract screening. Of these, 162 studies were screened for inclusion via full-text review. A total of 30 articles met the criteria for inclusion in this scoping review.
Fig. 1.
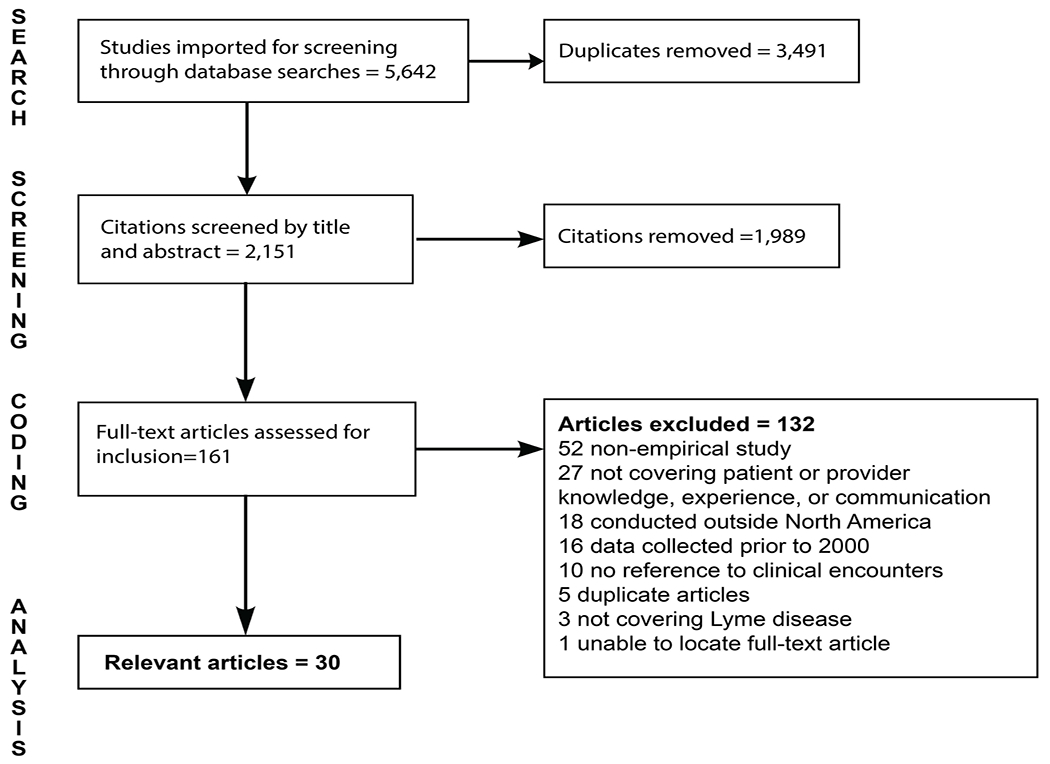
PRISMA Flow Diagram for Article Inclusion in the Scoping Review.
Of the 30 included articles, two were doctoral dissertations and the remaining 28 were articles published in peer-reviewed journals. These studies include 14 cross-sectional surveys, nine qualitative studies, four non-random experimental studies, two cohort studies, and one mixed methods study. Tables 2 and 3 summarize the 30 studies by the population of interest.
Table 2.
Articles Examining Lyme Disease Knowledge and Experiences of Healthcare Providers.
| Citation | Study Population (sample size) | Methods | Aims |
|---|---|---|---|
| Bakken (2002) | Physicians from multiple specialties in endemic and nonendemic areas for LD (9) | Semi-structured in-depth interviews | Describe the learning process of physicians for diagnosing LD |
| Brett et al. (2014) | Healthcare providers (2261) | Cross-sectional survey | Characterize frequency of TBDs in clinical practice and knowledge of their management |
| Butler et al. (2017) | Primary care providers from LD endemic states (76) | Non-randomized experimental study | Investigate providers’ ability to recognize common ticks and pathogens they transmit |
| Conant et al. (2018) | Clinicians at Vermont academic medical center (144) | Cross-sectional survey | Assess clinicians’ knowledge and practices regarding LD testing |
| Ferrouillet et al. (2015) | Family physicians in Quebec Province (201) | Cross-sectional survey | Describe the clinical experience, knowledge and practices of family physicians related to LD diagnosis and management |
| Gasmi et al. (2017) | Clinical data on patients with tick bite (254) | Retrospective cohort | Describe the knowledge and practices of GPs regarding recognition of early LD clinical signs and appropriateness of treatment and testing |
| Greseth (2017) | Healthcare providers who deliver care to individuals at risk for LD (305) | Non-randomized experimental study | Evaluate a CME module on participant knowledge and awareness of LD prevention, diagnosis, and treatment |
| Hill and Holmes (2015) | Arkansas primary care physicians (660) | Cross-sectional survey | Evaluate Arkansas providers’ knowledge and attitudes on the diagnosis and treatment of LD |
| Kobayashi et al. (2019) | Patient charts with presumptive diagnosis of LD and referral to rule out LD (1261) | Cohort study | Evaluate demographic characteristics, clinical history, laboratory test results, and antibiotic treatment history of patients referred for LD disease consultation. |
| Levesque and Klohn (2019) | Family physicians and nurse practitioners (40), government health officials (9) | Cross-sectional survey | Examine potential challenges facing LD patients in Canada’s Maritime provinces |
| Murray and Feder (2001) | Connecticut primary care physicians (267) | Cross-sectional survey | Determine how providers treat deer tick bites and EM lesion |
| Perea et al. (2015) | Primary care providers (1485) | Cross-sectional survey | Clarify provider practices regarding tick bite prophylaxis |
| Portman (2020) | Patients (61) and providers (62) in VA health center PharmD clinic | Non-randomized experimental | Describe implementation of pharmacist-run LD postexposure prophylaxis clinic augmented by academic detailing |
| Ramsey et al. (2004) | Physicians submitting specimens for LD serologic testing (356) | Cross-sectional survey | Assess factors contributing to appropriate and inappropriate use of LD serologic tests |
| Singh et al. (2016) | Physicians at West Virginia academic medical center (91) | Cross-sectional survey | LD distribution in West Virginia; providers’ knowledge of both disease and surveillance |
Table 3.
Articles Examining Lyme Disease Knowledge and Experiences of Patients and Public.
| Authors | Study Population (sample size) | Methods | Aims |
|---|---|---|---|
| Ali et al. (2014), | Patients diagnosed with or self-identifying as having CLD (12) | Semi-structured in-depth interviews | Gather insights about experiences of patients who carry a diagnosis of in US healthcare system |
| Boudreau et al. (2018) | Patients seeking care for LD outside of traditional Canadian healthcare system (45) | Interview questionnaire | Describe the experiences of Canadians who seek diagnosis and treatment for Lyme disease outside of the conventional Canadian health-care system |
| Butler et al. (2016) | Connecticut public (275) | Cross-sectional survey | Examine knowledge and beliefs about tick-borne diseases and personal prevention methods among Connecticut residents |
| Drew and Hewitt (2006) | Patients with a diagnosis of CLD identified from home infusion company database (10) | In-depth interviews | Explore lived experience of becoming diagnosed with LD |
| Gaudet et al. (2019) | Parents who self-identify as having a child with LD (23) | Narrative review | Investigate medical and psychological issues highlighted by parents describing their family’s LD experiences |
| Green (2015) | Patients with a diagnosis of CLD who consider themselves healed (6) | Semi-structured in-depth interviews | Examine subjective experiences of individuals who have healed from CLD |
| Hirsch et al. (2018) | Patients from an integrated health system with a diagnosis of LD (26) | In-depth interviews | Identify themes around belated diagnosis or treatment of LD using General Model of Total Patient Delay |
| Hu et al. (2019) | Individuals visiting outpatient clinics and community gathering places (306) | Cross-sectional survey | Characterize the knowledge, attitudes, and practices regarding TBDs in the Hispanic population |
| Jenks and Trapasso (2005) | South American immigrant patients visiting a community health center (80) | Non-randomized experimental study | Determine awareness of LD among recent immigrants and test effectiveness of educational intervention |
| Johnson et al. (2011) | Individuals who completed a web-based survey developed by the California Lyme Disease Association (2424) | Cross-sectional survey | Evaluate challenges faced by LD patients in obtaining care |
| Macauda et al. (2011) | General public in a LD endemic area (421) | Qual-quant mixed methods | Explore public perception of persistent symptoms following LD and need for long-term treatment |
| Rebman et al. (2017) | Patients meeting case definition for PTLDS (29) | Semi-structured in-depth interviews | Elicit patient illness narratives and identify emerging issues for patients with PTLDS/CLD |
| St. Pierre et al. (2020) | Members of professional and recreational organizations (137) | Cross-sectional survey | Explore extant knowledge and educational needs on LD among outdoor workers and recreational users |
| Valente et al. (2015) | Martha’s Vineyard, MA, public (946) | Cross-sectional survey | Assess how sociodemographic data and knowledge correlate with preventive behaviors in LD endemic area |
| Vassell et al. (2020) | Campaigners on medical crowdfunding sites seeking support for LD related care, treatment or diagnosis | Narrative review | Qualitatively explore narratives shared on crowdfunding campaigns to support LD treatment or diagnosis |
Table 4 provides a summary of the key findings and gaps in the current LD literature regarding patient-provider knowledge and communication.
Table 4.
Summary of Key Findings and Gaps in the Current Literature Regarding Patient-Physician Knowledge, Experience, and Communication Regarding Lyme Disease.
| Focus Area | Key Findings | Gaps in Literature / Areas for Future Research |
|---|---|---|
| LD Knowledge and Experience of HCPs | • Data predominantly collected for primary care physicians through cross-sectional surveys and chart reviews • Consistent knowledge gaps regarding EM lesion, patients with non-specific and long-standing symptoms, and appropriate use of testing and prophylaxis. • Misalignment between knowledge and practice patterns when LD diagnosis and treatment becomes contested |
• What resources do HCPs use to learn about LD diagnosis and management? • What are the best avenues to provide LD continuing education for HCPs? • What mediating factors influence HCP decision-making for LD in the clinical setting? • What are the LD knowledge and practice patterns for NPs, PAs, and emergency care providers? |
| LD Knowledge and Experience of Patients and the Public | • Data predominantly collected for CLD or chronic symptom patients through qualitative methods • Knowledge on LD gained through personal experience, peers, and family members rather than from medical community or public health • Patient study populations frequently report inaccurate beliefs on LD treatment, disease progression, and chronicity |
• What are the experiences of patients diagnosed with LD who are not categorized as chronic symptom/CLD in the clinical setting? • Why is information from the medical community on LD less salient than information gathered through word of mouth? • What areas of LD infection, diagnosis, and treatment are most frequently misunderstood by the general patient population? |
| Patient-Provider Communication Dynamic Regarding LD | • No articles specifically address patient-provider interaction as the main objective • Chronic symptom patients report negative experiences in mainstream healthcare settings and seek alternative care • HCPs report pressure from patients to prescribe testing and treatment for LD |
• What tools or resources can support constructive communication between patients and providers on LD in the clinical setting? |
3.2. Healthcare provider knowledge and experience
Fifteen of the 30 included articles evaluated healthcare provider (HCP) knowledge and experiences regarding LD (Bakken, 2002; Brett et al., 2014; Butler et al., 2017; Conant et al., 2018; Ferrouillet et al., 2015; Gasmi et al., 2017; Greseth, 2017; Hill and Holmes, 2015; Kobayashi et al., 2019; Levesque and Klohn, 2019; Murray and Feder, 2001; Perea et al., 2015; Portman, 2020; Ramsey et al., 2004; Singh et al., 2016). The majority of the studies (9/15) evaluated provider knowledge and experiences regarding LD through the use of cross-sectional surveys (Brett et al., 2014; Conant et al., 2018; Ferrouillet et al., 2015; Hill and Holmes, 2015; Levesque and Klohn, 2019; Murray and Feder, 2001; Perea et al., 2015; Ramsey et al., 2004; Singh et al., 2016). Seven of these nine cross-sectional surveys used patient vignettes to assess HCP testing and treatment approaches (Brett et al., 2014; Conant et al., 2018; Ferrouillet et al., 2015; Hill and Holmes, 2015; Murray and Feder, 2001; Perea et al., 2015; Singh et al., 2016). Three non-randomized experimental studies assessed HCP knowledge of LD diagnosis and approaches to treatment in conjunction with an educational intervention (Butler et al., 2017; Greseth, 2017; Portman, 2020). Two cohort studies attempted to understand HCP practice patterns regarding LD diagnosis and treatment through patient chart reviews, assessing appropriateness of testing and care against expert review and the IDSA guidelines (Gasmi et al., 2017; Kobayashi et al., 2019). One qualitative study assessed how physicians learn to diagnose LD using in-depth interviews (Bakken, 2002).
Four articles addressed knowledge regarding LD etiology and epidemiology, indicating that surveyed HCPs were moderately informed (48–55 %) regarding areas endemic for LD (Ferrouillet et al., 2015; Greseth, 2017; Levesque and Klohn, 2019), and less informed (38–48 %) on tick-pathogen associations and bacterial transmission (Butler et al., 2017; Greseth, 2017). Assessments of HCP knowledge regarding LD signs and symptoms concluded HCPs were moderately to highly knowledgeable (52–93 %) of early LD signs and symptoms (Conant et al., 2018; Ferrouillet et al., 2015; Gasmi et al., 2017; Hill and Holmes, 2015; Portman, 2020), but only moderately knowledgeable (42–51 %) on late LD signs and symptoms (Greseth, 2017; Singh et al., 2016). Five studies found low percentages of surveyed HCPs (18.4–44.7 %) correctly recognized EM and exposure history as sufficient for diagnosis and empirical treatment (Ferrouillet et al., 2015; Gasmi et al., 2017; Hill and Holmes, 2015; Murray and Feder, 2001; Singh et al., 2016). Surveyed HCPs appeared to struggle with clinical scenarios that assessed treatment of long-standing, non-specific symptoms (Conant et al., 2018; Singh et al., 2016), and those that assessed treatment for known recent tick bites with no symptoms (Brett et al., 2014; Murray and Feder, 2001; Perea et al., 2015).
Several studies assessed knowledge and experience regarding appropriate LD testing as supported by the IDSA. Approximately one-third of HCPs across five studies ordered inappropriate serologic testing for patients presenting with tick bite or EM (Ferrouillet et al., 2015; Hill and Holmes, 2015; Murray and Feder, 2001; Ramsey et al., 2004; Singh et al., 2016). Of note, Ramsey et al. (2004) reported that of all LD serology tests processed by two Wisconsin laboratories, 53 % were discretionary and 27 % were inappropriate (i.e., ordered when a patient was asymptomatic, EM present, or ordered as a test of cure). Four studies also indicated that ordering and interpreting the two-tiered serology for LD diagnosis can cause confusion for HCPs (Conant et al., 2018; Greseth, 2017; Hill and Holmes, 2015; Kobayashi et al., 2019).
Assessments of HCP treatment practices predominantly evaluated use of tick bite prophylaxis and the initiation of antibiotic treatment for EM or for nonspecific symptoms. Six studies identified inappropriate prescribing practices for tick bite prophylaxis, including use of amoxicillin or other antibiotics not evaluated for this purpose (Gasmi et al., 2017; Murray and Feder, 2001) and over-prescribing prophylaxis outside of the IDSA guideline criteria for initiation, dose, and duration of treatment (Brett et al., 2014; Gasmi et al., 2017; Greseth, 2017; Murray and Feder, 2001; Perea et al., 2015; Portman, 2020). Hill and Holmes (2015) and Perea et al. (2015) reported 39.6 % and 45.2 % of physicians, respectively, initiated antibiotic treatment despite not believing it was indicated. Kobayashi et al. (2019) reported that 84 % of patients treated with antibiotics for LD did not have active or recent LD, and over one-third of these patients had been treated with multiple courses of antibiotics.
Across the reviewed studies, HCPs practicing in endemic areas of LD were more knowledgeable and followed the IDSA treatment guidelines to a higher degree than HCPs from non-endemic areas of North America (Brett et al., 2014; Conant et al., 2018; Ferrouillet et al., 2015; Hill and Holmes, 2015; Levesque and Klohn, 2019; Murray and Feder, 2001; Perea et al., 2015; Singh et al., 2016). Articles that included multiple medical specialties indicated that HCPs working in emergency medicine had lower overall knowledge regarding LD compared to other specialties (Brett et al., 2014; Perea et al., 2015; Ramsey et al., 2004; Singh et al., 2016), and inappropriate testing was more likely to be ordered by emergency room or urgent care physicians (Ramsey et al., 2004). Only 12.5–34 % of HCPs studied in referenced articles represented non-physician disciplines, such as nurse practitioners (NPs), physician assistants (PAs), or pharmacists (PharmD) (Brett et al., 2014; Conant et al., 2018; Perea et al., 2015; Portman, 2020). A dissertation by Greseth (2017) was the only source that exclusively assessed nurses, NPs, or PAs. These studies demonstrated that NPs and PAs had among the lowest knowledge scores on LD diagnosis and treatment of all HCP knowledge assessments represented.
Data on where providers acquire information on LD is limited. Only four of the relevant studies included information on where providers acquire information on LD. Brett et al.(2014) reported that the majority of surveyed providers (80.9 %) prefer accessing online manuals and resources. Levesque and Klohn (2019) reported that surveyed HCPs in Canada access the academic literature (78 %), public health agency reports (51 %), and continuing medical education programs (59 %), while Ferrouillet et al. (2015) reported Canadian HCPs used public health newsletters (51 %), websites (46 %), and consults with professional colleagues (26 %) to learn about LD. Bakken (2002) found that physicians learn about diagnosis of LD through experience - specifically, through counter-cases, such as patients with similar signs and symptoms not caused by LD, as opposed to typical ‘textbook’ LD cases. Several studies addressed how the volume of LD clinical encounters completed by HCPs may influence approaches to diagnosis and treatment. Bakken (2002) found that physicians learn to diagnose problems through a learning loop, wherein HCPs with a higher number of clinical encounters (specifically with counter-cases) are able to improve their diagnostic ability for various presentations of LD. Three survey-based studies found that HCPs who saw more patients for LD in their practice were more likely to order appropriate LD serologic testing and had higher overall knowledge of the disease (Brett et al., 2014; Ferrouillet et al., 2015; Murray and Feder, 2001). Conversely, a separate study found that volume of LD clinical encounters was not associated with increased knowledge or appropriate practice (Singh et al., 2016).
3.3. Patient knowledge and experience
Fifteen of the 30 included articles contained information on the knowledge and experiences regarding LD for patients and the public (Ali et al., 2014; Boudreau et al., 2018; Butler et al., 2016; Drew and Hewitt, 2006; Green, 2015; Hirsch et al., 2018; Hu et al., 2019; Jenks and Trapasso, 2005; Johnson et al., 2011; Macauda et al., 2011; Rebman et al., 2017; St. Pierre et al., 2020; Valente et al., 2015; Vassell et al., 2020). The majority of studies used qualitative methods (in-depth interviews, narrative review) to explore patient knowledge and experiences with LD. Five qualitative studies assessed patient experiences living with long-term LD symptoms, CLD, or PTLDS (Ali et al., 2014; Gaudet et al., 2019; Green, 2015; Rebman et al., 2017; Vassell et al., 2020). Four qualitative studies documented patient experiences in the process of becoming diagnosed with LD (Boudreau et al., 2018; Drew and Hewitt, 2006; Hirsch et al., 2018; Vassell et al., 2020). Five studies explored knowledge and experiences using cross-sectional surveys. Four of these surveys used knowledge, attitudes, and practices (KAP) questionnaires with specific populations regarding ticks and LD (Butler et al., 2016; Hu et al., 2019; St. Pierre et al., 2020; Valente et al., 2015). The fifth cross-sectional survey study assessed patient challenges accessing care for LD (Johnson et al., 2011). One study used a qualitative and quantitative (qual-quant) mixed methods approach to explore perceptions of persistent symptoms following LD treatment (Macauda et al., 2011), and one non-randomized experimental study assessed patient knowledge of LD in conjunction with an educational intervention (Jenks and Trapasso, 2005).
General findings from these articles indicate that the public living in LD endemic areas is aware of the etiology of LD, including that blacklegged ticks (Ixodes scapularis and Ixodes pacificus) carry and transmit the causative agent of LD to people in North America, and can identify the early signs and symptoms of infection (Butler et al., 2016; Macauda et al., 2011; Valente et al., 2015). However, immigrant populations and individuals living in emerging areas for LD had lower overall knowledge levels in these areas (Hu et al., 2019; Jenks and Trapasso, 2005; St. Pierre et al., 2020). Surveys of the general population by Butler et al. (2016) and Valente et al. (2015) found that respondents understood antibiotics to be effective treatment for LD. However, the study by Macauda et al. (2011) reported that 78–89 % of respondents thought B. burgdorferi can persist in the body after antibiotic treatment.
Ten studies focused on the experiences of patients regarding diagnosis, disease course, and chronicity of LD symptoms (Ali et al., 2014; Boudreau et al., 2018; Drew and Hewitt, 2006; Gaudet et al., 2019; Green, 2015; Hirsch et al., 2018; Johnson et al., 2011; Macauda et al., 2011; Rebman et al., 2017; Vassell et al., 2020). It is important to note that patient populations included in almost all of these studies were selected based on self-reported diagnoses of PTLDS or CLD (Ali et al., 2014; Boudreau et al., 2018; Drew and Hewitt, 2006; Gaudet et al., 2019; Green, 2015; Macauda et al., 2011; Vassell et al., 2020). One study used the PTLDS case definition in their inclusion criteria (Rebman et al., 2017), and one study used positive two-tier serology in their inclusion criteria (Hirsch et al., 2018). The study conducted by Johnson et al. (2011) included both patients with positive two-tier serology as well as patients tested through methods not supported by IDSA or CDC guidance. Four of the these studies were funded by LD advocacy and support groups or foundations (Gaudet et al., 2019; Hirsch et al., 2018; Rebman et al., 2017; St. Pierre et al., 2020).
The majority of studies addressing patient experiences regarding LD diagnosis and duration defined chronic symptoms as including chronic fatigue, body and joint pain, cognitive impairment, and psychological complaints, but did not provide a summary of patient-reported symptoms from their study populations (Ali et al., 2014; Boudreau et al., 2018; Drew and Hewitt, 2006; Green, 2015; Johnson et al., 2011; Macauda et al., 2011; Vassell et al., 2020). Two studies provided a detailed list of specific patient-reported symptoms, which included those commonly attributed to CLD or PTLDS (fatigue, cognitive impairment, joint and body pain) as well as diverse variety of other physical and psychological symptoms (Gaudet et al., 2019; Hirsch et al., 2018).
Three studies reported that patients’ decision to consult a HCP was prompted by finding a tick or a rash, or to address symptoms associated with LD infection (Gaudet et al., 2019; Green, 2015; Hirsch et al., 2018). Several studies reported patients often visited multiple providers before obtaining a diagnosis, with many seeking out alternative medicine providers or Lyme specialists (Ali et al., 2014; Boudreau et al., 2018; Drew and Hewitt, 2006; Gaudet et al., 2019; Green, 2015; Hirsch et al., 2018; Johnson et al., 2011; Rebman et al., 2017; Vassell et al., 2020). Patients in these studies also reported undergoing multiple tests, including unconventional testing, and experiencing multiple misdiagnoses before arriving at a diagnosis of LD or CLD. Seven of the studies covered the emotional nature of LD diagnosis, with patients describing the road to diagnosis as long and frustrating, and experiencing relief and validation upon arriving at a LD or CLD diagnosis (Ali et al., 2014; Boudreau et al., 2018; Drew and Hewitt, 2006; Gaudet et al., 2019; Green, 2015; Rebman et al., 2017; Vassell et al., 2020).
Another common theme related to the patient experience centered on uncertainties regarding the LD disease course. Patients from four studies reported feeling unsure of both the severity of their illness or symptoms and their medical decisions (Ali et al., 2014; Hirsch et al., 2018; Macauda et al., 2011; Rebman et al., 2017). Patients in these studies also expressed uncertainty about the present and future, including doubts on recovery, the normalcy of their illness experience, and if their LD was cured. Patients also felt social isolation and lack of support due to their illness (Ali et al., 2014; Green, 2015; Rebman et al., 2017). Four articles noted that the social burden of disease included the effect of chronic symptoms on the ability to work and that chronic symptoms resulted in a negatively altered quality of life (Ali et al., 2014; Green, 2015; Hirsch et al., 2018; Johnson et al., 2011). Common emotions felt by patients throughout their disease experience included frustration, hurt, anger, and polarizing feelings of hope or optimism and despondence throughout the disease experience (Ali et al., 2014; Boudreau et al., 2018; Drew and Hewitt, 2006; Gaudet et al., 2019; Green, 2015; Rebman et al., 2017).
Articles assessing patient experience with treatment included a particular focus on antibiotic treatment, such as length of treatment, prolonged treatment, and re-treatment (Ali et al., 2014; Boudreau et al., 2018; Macauda et al., 2011; Vassell et al., 2020). Some patients with chronic symptoms reported use of unconventional therapies to treat LD, while others reported concerns over the safety of alternative therapies (Ali et al., 2014; Boudreau et al., 2018; Gaudet et al., 2019; Green, 2015). Studies conducted in Canada reported patients seeking care in the United States due to long-term antibiotics not being available to them in the traditional Canadian healthcare system (Boudreau et al., 2018; Gaudet et al., 2019; Vassell et al., 2020). The financial burden of disease included difficulty with insurance coverage, cost of care, and financial worry, and the travel burden to seek care (Ali et al., 2014; Drew and Hewitt, 2006; Green, 2015; Hirsch et al., 2018; Johnson et al., 2011; Vassell et al., 2020).
Eight of the ten articles included information on where patients and the public gather information on LD (Boudreau et al., 2018; Drew and Hewitt, 2006; Gaudet et al., 2019; Green, 2015; Jenks and Trapasso, 2005; Macauda et al., 2011; St. Pierre et al., 2020; Valente et al., 2015). Participants from several studies reported learning about LD from experience (e.g., from personally having LD or knowing someone who had it); these patients reported feeling responsible to educate themselves and others (Boudreau et al., 2018; Drew and Hewitt, 2006; Gaudet et al., 2019; Green, 2015; Macauda et al., 2011). Study participants also cited internet resources and educational pamphlets as sources of information (Drew and Hewitt, 2006; Macauda et al., 2011; St. Pierre et al., 2020). Macauda et al. (2011) and St. Pierre et al. (2020) reported 24 % and 37 % of their participants, respectively, learned about LD from public health or HCP sources.
3.4. Patient-provider interaction and relationships
None of the included articles specifically addressed patient-provider interaction and relationships as the main objective. However, fourteen articles contained themes relevant to this objective, including conflicted patient relationships with HCPs, patient self-advocacy, and HCP experiences of pressure from patients (Ali et al., 2014; Bakken, 2002; Boudreau et al., 2018; Conant et al., 2018; Drew and Hewitt, 2006; Gaudet et al., 2019; Green, 2015; Hill and Holmes, 2015; Hirsch et al., 2018; Levesque and Klohn, 2019; Perea et al., 2015; Ramsey et al., 2004; Rebman et al., 2017; Vassell et al., 2020). The role of the patient-provider relationship in the diagnosis and treatment process was predominantly covered through articles focused on patient experiences.
Positive interactions included those where HCPs were supportive, attentive, good listeners, and validated the patients’ experience, symptoms, and feelings (Ali et al., 2014; Boudreau et al., 2018; Gaudet et al., 2019; Green, 2015; Hirsch et al., 2018; Rebman et al., 2017). These experiences were often reported in alternative medicine or mental health care rather than mainstream primary or specialty care (Ali et al., 2014; Boudreau et al., 2018; Gaudet et al., 2019; Green, 2015). Studies in which patients reported negative interactions with providers showed that these experiences occurred when patients felt HCPs were dismissive, condescending, patronizing, left patients feeling invalidated, or delivered substandard care (Ali et al., 2014; Boudreau et al., 2018; Drew and Hewitt, 2006; Gaudet et al., 2019; Green, 2015; Hirsch et al., 2018; Johnson et al., 2011; Rebman et al., 2017; Vassell et al., 2020). Patient self-advocacy emerged as a theme in seven of the patient-focused studies; this included patients themselves suggesting LD as a diagnosis to their physician, seeking specialized labs to conduct testing, or directly seeking alternative forms of treatment outside mainstream healthcare services (Ali et al., 2014; Boudreau et al., 2018; Drew and Hewitt, 2006; Gaudet et al., 2019; Green, 2015; Rebman et al., 2017; Vassell et al., 2020). Participants in several of these studies reported a sense of betrayal from the mainstream healthcare system and that HCPs were uneducated or uninformed on LD (Ali et al., 2014; Boudreau et al., 2018; Drew and Hewitt, 2006; Gaudet et al., 2019; Green, 2015; Rebman et al., 2017; Vassell et al., 2020).
Comparatively, four articles reported that HCPs felt pressure or received suggestions from patients regarding LD diagnosis, testing, or treatment (Conant et al., 2018; Hill and Holmes, 2015; Perea et al., 2015; Ramsey et al., 2004). This included patients requesting LD serologic testing, nonstandard serology, tick bite prophylaxis or other treatment for LD, and patients introducing the idea of a LD diagnosis. Up to 40 % of providers in one study reported initiating tick bite prophylaxis upon patient request (Perea et al., 2015). In a separate study, patient-initiated testing was more likely to be inappropriate than testing initiated by clinicians (Ramsey et al., 2004). HCPs in two studies also reported that some of their patients sought nonstandard serology and treatment from clinicians who were self-labeled Lyme specialists (Conant et al., 2018; Levesque and Klohn, 2019). Bakken (2002) examined the provider perspective, and found that knowing a patient’s history and background was a significant factor in framing a diagnostic problem. This practice contributed to a positive interaction and likelihood of accurate diagnosis. Studies conducted in emerging areas for LD in Canada reported that HCPs rarely had LD conversations with patients in the clinical setting, and felt patients were overall poorly informed on LD (Ferrouillet et al., 2015; Levesque and Klohn, 2019).
4. Discussion
4.1. Characterization of the literature
The reviewed articles demonstrated notable differences in methodology based on their target research subjects. Articles focused on knowledge and experience of HCPs predominantly utilized survey research methods and clinical chart reviews, while a larger proportion of articles examining knowledge and experiences of patients and the public relied on qualitative research methods. The result is that the current body of evidence provides an unbalanced description of patient-provider interactions, wherein the perspective of the patient is described through nuanced, subjective data while the perspective of HCPs is largely described through discrete or statistical data. Future research addressing the qualitative perspective of HCPs and adding statistical measures for patient knowledge may identify opportunities for intervention.
The knowledge and practice patterns of HCPs summarized in the existing literature is predominantly focused on primary care physician providers. The knowledge and experiences of NPs, PAs, and emergency care providers are understudied. Prompt treatment in the acute phase of infection can prevent spread of B. burgdorferi to joints, the heart, and nervous system (Centers for Disease Control and Prevention, 2018). Acute LD presentation is well within the scope of practice for NPs and PAs. Understanding the knowledge and practice patterns of these disciplines and specialties is important for developing targeted educational interventions, preventing misdiagnosis, and avoiding preventable symptoms.
It is important to note that the majority of the qualitative studies addressing patient experiences in this review focused on patients identifying with CLD or with chronic symptoms. In addition, these studies frequently lacked specific descriptions of patient-reported symptoms. In absence of a clear definition of the clinical manifestations of CLD or PTLDS, inclusion of these details will help better define the parameters of the disease experience and may help lead to a more consistent characterization of the CLD or PTLDS entity.
The current literature does not adequately describe experiences of the general patient population who are diagnosed with LD but are not categorized as CLD or chronic symptom patients. The majority of patients who present with acute LD, and are diagnosed and treated appropriately, do not experience chronic or prolonged symptoms (Bechtold et al., 2017; Centers for Disease Control and Prevention, 2019b; Lantos et al., 2015). Future studies focusing on non-chronic symptom patients will help us more fully understand the patient-provider communication dynamic with respect to LD.
4.2. Patterns in LD knowledge and experience for HCPs
The reviewed studies demonstrated concerning LD knowledge patterns among HCPs. Areas of concern include recognition and diagnosis of EM, how to proceed with patients who have non-specific and long-standing symptoms, and appropriate use of testing and prophylaxis following known tick bite (Brett et al., 2014; Butler et al., 2017; Conant et al., 2018; Gasmi et al., 2017; Greseth, 2017; Hill and Holmes, 2015; Kobayashi et al., 2019; Murray and Feder, 2001; Perea et al., 2015; Portman, 2020; Ramsey et al., 2004; Singh et al., 2016). Several studies indicated HCPs across multiple specialties may have less robust knowledge of LD or may be more likely to order inappropriate testing (Brett et al., 2014; Kobayashi et al., 2019; Murray and Feder, 2001; Portman, 2020; Ramsey et al., 2004; Singh et al., 2016). The existing literature provides limited information on sources used by HCPs to acquire knowledge on LD. Additional investigation may provide insight on factors influencing this apparent range in accuracy and gaps in LD knowledge among HCPs, as well as the best avenues through which to provide continuing education on LD diagnosis and management.
The literature does not adequately address what factors influence how and why HCPs incorporate LD knowledge into clinical practice. As reported in four articles, providers often felt pressure from patients to test or treat for LD despite not believing that it was clinically appropriate (Conant et al., 2018; Hill and Holmes, 2015; Perea et al., 2015; Ramsey et al., 2004). Further, three studies reported notable percentages of physicians initiating testing and treatment against their own clinical judgement (Hill and Holmes, 2015; Murray and Feder, 2001; Perea et al., 2015). This apparent misalignment of knowledge to practice patterns mirrors physician experiences in other contested areas of clinical uncertainty, such as identifying and treating low vitamin D status (Rockwell et al., 2018). Further research investigating mediating factors for HCP decision-making in the clinical setting may aid our understanding of why this misalignment occurs. Additionally, studies focused on how new and existing tools in continuing medical education and clinical decision support can be used by providers to incorporate LD training into clinical practice will be essential to further reducing this misalignment (Middleton et al., 2016).
4.3. Patterns in LD knowledge and experiences for patients and the public
Patients and the public report gaining much of their information on LD from their own personal experiences and those of peers or family members who have had LD (Boudreau et al., 2018; Drew and Hewitt, 2006; Gaudet et al., 2019; Green, 2015; Macauda et al., 2011). However, patient study populations frequently reported inaccurate beliefs regarding LD treatment, disease progression, and chronicity (Ali et al., 2014; Boudreau et al., 2018; Butler et al., 2016; Drew and Hewitt, 2006; Gaudet et al., 2019; Macauda et al., 2011; St. Pierre et al., 2020; Valente et al., 2015). Patients, particularly those identifying with CLD, have a highly conceptualized and personal understanding of their disease and disease experience. Further, they feel responsible to educate and share their LD experience, knowledge, and beliefs with others (Ali et al., 2014; Boudreau et al., 2018; Drew and Hewitt, 2006; Gaudet et al., 2019; Green, 2015; Hirsch et al., 2018; Rebman et al., 2017; Vassell et al., 2020). Few survey respondents or patients with LD reported gathering information from medical providers or other messaging created specifically by HCPs or public health sources (Macauda et al., 2011; St. Pierre et al., 2020).
A study conducted by Basch et al.(2017) assessed the 100 most viewed YouTube videos related to LD in 2016, and identified only 16 that were created by HCPs; factors such as celebrity presence were correlated with increased number of views. A recent assessment by Langford and Loeb (2019) concluded that a higher perceived quality of patient-provider communication is associated with lower viewership of health-related videos on YouTube. Evaluating why information from the medical community is viewed as less trustworthy, or at least less salient, as that gathered by word of mouth and media platforms, and identifying the information gathering patterns of patients could be used to enhance health messaging on LD with accurate and evidence-based information from the public health and medical community.
4.4. Patient-provider communication on LD
Conspicuously lacking from the published literature was a study that specifically addressed the patient-provider communication dynamic with respect to LD. However, experiences reported by patients and HCPs allude to an important trend between LD patients with chronic symptoms and medical providers. Patients in this group reported feelings of frustration or invalidation from HCPs perceived as paternalistic and often opted to leave mainstream medicine for alternative, or ‘Lyme literate’, providers (Ali et al., 2014; Boudreau et al., 2018; Gaudet et al., 2019; Green, 2015; Hirsch et al., 2018; Rebman et al., 2017; Vassell et al., 2020). Providers felt pressure to accommodate patient wishes when counter to their professional opinion, and appeared to lack an effective way to discuss treatment or testing in these situations (Conant et al., 2018; Hill and Holmes, 2015; Perea et al., 2015; Ramsey et al., 2004).
The negative experiences driving patients to depart from mainstream healthcare are concerning. Lantos et al. (2015) investigated the 30 alternative therapies recommended for LD on the internet, finding that none are backed by evidence meeting the standards by which the scientific community accepts or rejects new treatments, and some are potentially harmful. This work highlights the importance of exploring the patient-provider communication dynamic and evaluating tools or point-of-care interventions to support constructive communication between patients and doctors. Clinical decision support, for example, is a process that supports enhancing health-related decision-making by providing both HCPs and patients with relevant, organized clinical knowledge at the point-of-care. Clinical decision support has been recognized for improving adherence to guidelines, treatment quality, and reducing variance in HCP practice (Beeler et al., 2014; Middleton et al., 2016; Tan et al., 2020). Additionally, incorporation of patient-centered approaches that promote patient engagement and voicing of patient concern, such as shared decision-making, present a promising avenue to explore with LD patients with chronic symptoms or identifying with a diagnosis of CLD (Austin et al., 2015; Schrager et al., 2017; Tamhane et al., 2015).
4.5. Limitations
Scoping reviews do not, by design, formally evaluate the quality of included research articles, nor do they provide a synthesized result or answer to a specific question, as seen in meta-analyses or systematic literature reviews (Arksey and O’Malley, 2005). While this approach has allowed for a more complete overview of the existing research on LD patient-provider communication, an assessment of the quality of articles in the current literature would aid in identification of gaps in the evidence base and will be necessary for any future systematic review (Pham et al., 2014). Our criteria for this scoping review excluded research articles conducted outside North America. A large volume of research has been conducted on patient and provider knowledge and experiences regarding LD in Europe. Inclusion of this evidence may have yielded additional insights into the patient-provider communication dynamic, albeit from notably diverse cultural and healthcare perspectives. Additionally, while the authors made every attempt to identify articles that meet the inclusion criteria of this scoping review, it is possible that relevant articles were not identified and included in the final overview.
5. Conclusions
The objective of this scoping review was to describe the current research literature on patient and provider knowledge and experiences in the clinical setting with respect to LD. This includes where providers obtain information, where patients obtain information, and if and how that information is understood and translated between the two groups. Despite the fact that understanding these dynamics is critical to improving LD patient care, major research gaps exist in all three areas. Negative and polarizing patient experiences, juxtaposed with uncertainty and limited knowledge among HCPs, may produce poor health outcomes. Further, understanding why patients often seek information from sources outside of the medical community may aid in developing and enhancing health messaging related to LD.
Ultimately, we lack research that seeks to comprehensively understand the patient-provider communication dynamic regarding LD. Healthcare consumer assessments and evaluations of patient-centered primary and preventive care have shown that improving patient-provider communication can lead to better health outcomes and patient satisfaction, as seen in the areas of (Belasen and Belasen, 2018; Quigley et al., 2014; Stewart et al., 2000; Underhill and Kiviniemi, 2011), and this may also be the case for LD. Future research specifically focusing on LD will be critical to identifying opportunities where this communication dynamic can be strengthened to improve LD care and outcomes.
Supplementary Material
Acknowledgements
The authors thank Kate Ghezzi-Kopel, previously the Systematic Review Librarian and now Health Sciences and Evidence Synthesis Librarian at Mann Library, Cornell University for her assistance with the scoping review methods.
Funding
This work was supported by the Cooperative Agreement Number 1U01CK000509-01 between the U.S. Centers for Disease Control and Prevention (CDC) and Cornell University. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention or the Department of Health and Human Services.
Abbreviations:
- CDC
Centers for Disease Control and Prevention
- CLD
chronic Lyme disease
- EM
erythema migrans
- HCP
healthcare provider
- IDSA
Infectious Diseases Society of America
- ILADS
International Lyme and Associated Diseases Society
- LD
Lyme disease
- NP
nurse practitioner
- PA
physician assistant
- PTLDS
post-treatment Lyme disease syndrome
Footnotes
Declaration of Competing Interest
None.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.ttbdis.2021.101714.
References
- Aguero-Rosenfeld ME, Wormser GP, 2015. Lyme disease: diagnostic issues and controversies. Expert Rev. Mol. Diagn 15, 1–4. 10.1586/14737159.2015.989837. [DOI] [PubMed] [Google Scholar]
- Ali A, Vitulano L, Weiss TR, Colson ER, Lee R, 2014. Experiences of patients identifying with chronic Lyme disease in the healthcare system: a qualitative study. BMC Fam. Pract 15, 1–8. 10.1186/1471-2296-15-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arksey H, O’Malley L, 2005. Scoping studies: towards a methodological framework. Int. J. Soc. Res. Methodol 8, 19–32. 10.1080/1364557032000119616. [DOI] [Google Scholar]
- Austin CA, Mohottige D, Sudore RL, Smith AK, Hanson LC, 2015. Tools to promote shared decision making in serious illness: a systematic review. JAMA Intern. Med 175, 1213–1221. 10.1001/jamainternmed.2015.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auwaerter PG, Bakken JS, Dattwyler RJ, Dumler JS, Halperin JJ, McSweegan E, Nadelman RB, O’Connell S, Sood SK, Weinstein A, Wormser GP, 2011. Scientific evidence and best patient care practices should guide the ethics of Lyme disease activism. J. Med. Ethics 37, 68–73. 10.1136/jme.2009.032896. [DOI] [PubMed] [Google Scholar]
- Bakken LL, 2002. Role of experience and context in learning to diagnose Lyme disease. J. Contin. Educ. Health Prof 22, 131–141. 10.1002/chp.1340220302. [DOI] [PubMed] [Google Scholar]
- Basch CH, Mullican LA, Boone KD, Yin J, Berdnik A, Eremeeva ME, Fung IC-H, Chun-Hai Fung I, 2017. Lyme disease and YouTube TM: a cross-sectional study of video contents. Osong Public Heal. Res. Perspect 8, 289–292. 10.24171/j.phrp.2017.8.4.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechtold KT, Rebman AW, Crowder LA, Johnson-Greene D, Aucott JN, 2017. Standardized symptom measurement of individuals with early Lyme disease over time. Arch. Clin. Neuropsychol 32, 129–141. 10.1093/arclin/acw098. [DOI] [PubMed] [Google Scholar]
- Beeler PE, Bates DW, Hug BL, 2014. Clinical decision support systems. Swiss Med. 144, w14073 10.4414/smw.2014.14073. [DOI] [PubMed] [Google Scholar]
- Belasen A, Belasen AT, 2018. Doctor-patient communication: a review and a rationale for using an assessment framework. J. Health Organ. Manag 32, 891–907. 10.1108/JHOM-10-2017-0262. [DOI] [PubMed] [Google Scholar]
- Boucher JL, 2010. Technology and patient-provider interactions: Improving quality of care, but is it improving communication and collaboration? Diabetes Spectr. 23, 142–144. 10.2337/diaspect.23.3.142. [DOI] [Google Scholar]
- Boudreau CR, Lloyd VK, Gould ON, 2018. Motivations and experiences of Canadians seeking treatment for Lyme disease outside the conventional Canadian health-care system. J. Patient Exp 5, 120–126. 10.1177/2374373517736385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett ME, Hinckley AF, Zielinski-Gutierrez EC, Mead PS, 2014. U.S. Healthcare providers’ experience with Lyme and other tick-borne diseases. Ticks Tick. Dis 5, 404–408. 10.1016/j.ttbdis.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler AD, Sedghi T, Petrini JR, Ahmadi R, 2016. Tick-borne disease preventive practices and perceptions in an endemic area. Ticks Tick. Dis 7, 331–337. 10.1016/j.ttbdis.2015.12.003. [DOI] [PubMed] [Google Scholar]
- Butler AD, Carlson ML, Nelson CA, 2017. Use of a tick-borne disease manual increases accuracy of tick identification among primary care providers in Lyme disease endemic areas. Ticks Tick. Dis 8, 262–265. 10.1016/j.ttbdis.2016.11.010. [DOI] [PubMed] [Google Scholar]
- Calo WA, Ortiz AP, Colon V, Krasny S, Tortolero-Luna G, 2014. Factors associated with perceived patient-provider communication quality among Puerto Ricans. J. Health Care Poor Underserved 25, 491–502. 10.1353/hpu.2014.0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron DJ, Johnson LB, Maloney EL, 2014. Evidence assessments and guideline recommendations in Lyme disease: the clinical management of known tick bites, erythema migrans rashes and persistent disease. Expert Rev. Anti. Ther 12, 1103–1135. 10.1586/14787210.2014.940900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, 2018. Treatment: Lyme Disease [WWW Document]. URL https://www.cdc.gov/lyme/treatment/index.html (accessed 3.8.19).
- Centers for Disease Control and Prevention, 2019a. Data and Surveillance: Lyme Disease [WWW Document]. URL https://www.cdc.gov/lyme/datasurveillance/index.html (accessed 3.8.19).
- Centers for Disease Control and Prevention, 2019b. Lyme Disease [WWW Document]. URL https://www.cdc.gov/lyme/index.html (accessed 10.11.19).
- Chaet DH, 2018. AMA Code of Medical Ethics’ opinions related to false beliefs in health care. AMA J. Ethics 20, E1049–51. 10.1001/amajethics.2018.1049. [DOI] [PubMed] [Google Scholar]
- Conant JL, Powers J, Sharp G, Mead PS, Nelson CA, 2018. Lyme disease testing in a high-incidence state. Am. J. Clin. Pathol 149, 234–240. 10.1093/ajcp/aqx153. [DOI] [PubMed] [Google Scholar]
- Drew D, Hewitt H, 2006. A qualitative approach to understanding patients’ diagnosis of Lyme disease. Public Health Nurs. 23, 20–26. 10.1111/j.0737-1209.2006.230104.x. [DOI] [PubMed] [Google Scholar]
- Eddy DM, 1990. Anatomy of a decision. JAMA 263, 441–443. 10.1001/jama.1990.03440030128037. [DOI] [PubMed] [Google Scholar]
- Feder HM, Johnson BJ, O’Connell S, Shapiro ED, Steere AC, Wormser GP, Ad Hoc International Lyme Disease Group, 2007. A critical appraisal of “Chronic Lyme Disease”. N. Engl. J. Med 357, 1422–1430. 10.1056/NEJMra072023. [DOI] [PubMed] [Google Scholar]
- Ferrouillet C, Milord F, Lambert L, Vibien A, Ravel A, 2015. Lyme disease: knowledge and practices of family practitioners in southern Quebec. Can. J. Infect. Dis. Med. Microbiol 26, 151–156. 10.1155/2015/846963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasmi S, Ogden NH, Leighton PA, Adam-Poupart A, Milord F, Lindsay LR, Barkati S, Thivierge K, 2017. Practices of Lyme disease diagnosis and treatment by general practitioners in Quebec, 2008–2015. BMC Fam. Pract 18 10.1186/s12875-017-0636-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudet E, Gould O, LLoyd V, 2019. Parenting when children have Lyme disease: fear, frustration, advocacy. Healthcare 7, 95. 10.3390/healthcare7030095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodlet KJ, Fairman KA, 2018. Adverse events associated with antibiotics and intravenous therapies for post–Lyme disease dyndrome in a commercially insured sample. Clin. Infect. Dis 67, 1568–1574. 10.1093/cid/ciy329. [DOI] [PubMed] [Google Scholar]
- Green F, 2015. Common Psychosocial and Spiritual Factors among Individuals Who Have Healed From Chronic Lyme Disease. Antioch University, New England. [Google Scholar]
- Greseth SR, 2017. Addressing Lyme Disease: an Educational Module for Healthcare Providers. North Dakota State University of Agriculture and Applied Science. [Google Scholar]
- Hill D, Holmes T, 2015. Provider knowledge, attitudes, and practices regarding Lyme disease in Arkansas. J. Community Health 40, 339–346. 10.1007/s10900-014-9940-9. [DOI] [PubMed] [Google Scholar]
- Hinckley AF, Connally NP, Meek JI, Johnson BJ, Kemperman MM, Feldman KA, White JL, Mead PS, 2014. Lyme disease testing by large commercial laboratories in the United States. Clin. Infect. Dis 59, 676–681. 10.1093/cid/ciu397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch AG, Herman RJ, Rebman A, Moon KA, Aucott J, Heaney C, Schwartz BS, 2018. Obstacles to diagnosis and treatment of Lyme disease in the USA: a qualitative study. BMJ Open 8, e021367. 10.1136/bmjopen-2017-021367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe LC, Leibowitz KA, Crum AJ, 2019. When your doctor “gets it” and “gets you”: the critical role of competence and warmth in the patient-provider interaction. Front. Psychiatry 10, 475. 10.3389/fpsyt.2019.00475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu LT, 2016. Lyme disease. Ann. Intern. Med 164, ITC65–ITC80. 10.7326/AITC201605030. [DOI] [PubMed] [Google Scholar]
- Hu YS, Starr JA, Gharpure R, Mehta SP, Feldman KA, Nelson CA, 2019. Knowledge and prevention of tickborne diseases among Hispanic and non-Hispanic residents of Maryland and Virginia. Zoonoses Public Health 66, 805–812. 10.1111/zph.12627. [DOI] [PubMed] [Google Scholar]
- Jenks NP, Trapasso J, 2005. Lyme risk for immigrants to the United States: the role of an educational tool. J. Travel Med 12, 157–160. 10.2310/7060.2005.12302. [DOI] [PubMed] [Google Scholar]
- Johnson L, Aylward A, Stricker RB, 2011. Healthcare access and burden of care for patients with Lyme disease: a large United States survey. Health Policy (New. York). 102, 64–71. 10.1016/j.healthpol.2011.05.007. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Higgins Y, Samuels R, Moaven A, Sanyal A, Yenokyan G, PM L, MT M, PG A, 2019. Misdiagnosis of Lyme disease with unnecessary antimicrobial treatment characterizes patients referred to an academic infectious diseases clinic. Open Forum Infect. Dis 6 10.1093/ofid/ofz299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupat E, Rosenkranz S, Yeager C, Barnards K, SM P, Inui T, 2000. The practice orientations of physicians and patients: the effect of doctor-patient congruence on satisfaction. Patient Educ. Couns 39, 49–59. 10.1016/s0738-3991(99)00090-7. [DOI] [PubMed] [Google Scholar]
- Krupat E, Bell R, Kravitz R, Thom D, Azari R, 2001. When physicians and patients think alike: patient-centered beliefs and their impact on satisfaction and trust. J. Fam. Pract 50, 1057–1062. [PubMed] [Google Scholar]
- Kugeler KJ, Schwartz AM, Delorey MJ, Mead PS, Hinckley AF, 2021. Estimating the frequency of Lyme disease diagnoses, United States, 2010–2018. Emerg. Infect. Dis 27, 616–619. 10.3201/eid2702.202731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford A, Loeb S, 2019. Perceived patient-provider communication quality and cociodemographic factors associated with watching health-related videos on YouTube: a cross-sectional analysis. J. Med. Internet Res 21, e13512 10.2196/13512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantos PM, Shapiro ED, Auwaerter PG, Baker PJ, Halperin JJ, Mcsweegan E, Wormser GP, 2015. Unorthodox alternative therapies marketed to treat Lyme disease. Clin. Infect. Dis 60, 1776–1782. 10.1093/cid/civ186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantos PM, Rumbaugh J, Bockenstedt LK, Falck-Ytter YT, Aguero-Rosenfeld ME, Auwaerter PG, Baldwin K, Bannuru RR, Belani KK, Bowie WR, 2021. Clinical practice guidelines by the Infectious Diseases Society of America (IDSA), American Academy of Neurology (AAN), and American College of Rheumatology (ACR): 2020 guidelines for the prevention, diagnosis and treatment of Lyme disease. Clin. Infect. Dis 72, e1–e48. 10.1093/cid/ciaa1215. [DOI] [PubMed] [Google Scholar]
- Levesque M, Klohn M, 2019. A multiple streams approach to understanding the issues and challenges of Lyme disease management in Canada’s Maritime Provinces. Int. J. Environ. Res. Public Health 16. 10.3390/ijerph16091531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macauda MM, Erickson P, Miller J, Mann P, Closter L, Krause PJ, 2011. Long-term Lyme disease antibiotic therapy beliefs among New England residents. Vector-Borne Zoonotic Dis. 11, 857–862. 10.1089/vbz.2010.0116. [DOI] [PubMed] [Google Scholar]
- Maloney EL, 2016. Controversies in persistent (Chronic) Lyme disease. J. Infus. Nurs 39, 369–375. 10.1097/NAN.0000000000000195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe R, Healey PG, 2018. Miscommunication in doctor-patient communication. Top. Cogn. Sci 10, 409–424. 10.1111/tops.12337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead P, Petersen J, Hinckley A, 2019. Updated CDC recommendation for serologic diagnosis of Lyme disease. Morb. Mortal. Rep. Surveill. Summ 68, 703. 10.15585/mmwr.mm6832a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merck Manuals, 2018. Physicians Weigh in: How Online Medical Info Has Changed Family Practice. Merck Manuals. [Google Scholar]
- Middleton B, Sittig D, Wright A, 2016. Clinical decision support: a 25 year retrospective and a 25 year vision. Yearb. Med. Inform (Suppl. 1), S103–S116. 10.15265/IYS-2016-s034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray T, Feder HM, 2001. Management of tick bites and early Lyme disease: a survey of Connecticut physicians. Pediatrics 108, 1367–1370. 10.1542/peds.108.6.1367. [DOI] [PubMed] [Google Scholar]
- Nadelman RB, Wormser GP, 1998. Lyme borreliosis. Lancet 352, 557–565. 10.1016/S0140-6736(98)01146-5. [DOI] [PubMed] [Google Scholar]
- Naktin JP, 2017. Late you come: legislation on Lyme treatment in an era of conflicting guidelines. Open Forum Infect. Dis 4, ofx152. 10.1093/ofid/ofx152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CA, Saha S, Kugeler KJ, Delorey MJ, Shankar MB, Hinckley AF, Mead PS, 2015. Incidence of clinician-diagnosed Lyme disease, United States, 2005–2010. Emerg. Infect. Dis 21, 1625–1631. 10.3201/eid2109.150417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perea AE, Hinckley AF, Mead PS, 2015. Tick bite prophylaxis: results from a 2012 survey of healthcare providers. Zoonoses Public Health 62, 388–392. 10.1111/zph.12159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham MT, Rajic A, Greig JD, Sargeant JM, Papadopoulos A, McEwan SA, 2014. A scoping review of scoping reviews: advancing the approach and enhancing the consistency. Res. Synth. Methods 5, 371–385. 10.1002/jrsm.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portman DB, 2020. Implementing a pharmacist-run Lyme disease postexposure prophylaxis clinic augmented by academic detailing within the Veterans Health Administration. J. Am. Pharm. Assoc 10.1016/j.japh.2020.01.002. [DOI] [PubMed] [Google Scholar]
- Quigley DD, Elliott MN, Farley DO, Burkhart Q, Skootsky SA, Hays RD, 2014. Specialties differ in which aspects of doctor communication predict overall physician ratings. J. Gen. Intern. Med 29, 447–454. 10.1007/s11606-013-2663-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey AH, Belongia EA, Chyou PH, Davis JP, 2004. Appropriateness of Lyme disease serologic testing. Ann. Fam. Med 2, 341–344. 10.1370/afm.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebman AW, Aucott JN, Weinstein ER, Bechtold KT, Smith KC, Leonard L, 2017. Living in limbo: contested narratives of patients with chronic symptoms following Lyme disease. Qual. Health Res 27, 534–546. 10.1177/1049732315619380. [DOI] [PubMed] [Google Scholar]
- Riedl D, Schüßler G, 2017. The influence of doctor-patient communication on health outcomes: a systematic review. Z. Psychosom. Med. Psychother 63, 131–150. 10.13109/zptm.2017.63.2.131. [DOI] [PubMed] [Google Scholar]
- Rockwell M, Kraak V, Hulver M, Epling J, 2018. Clinical management of low vitamin D: a scoping review of physicians’ practices. Nutrients 10, E493. 10.3390/nu10040493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrager SB, Phillips G, Burnside E, 2017. Shared decision making in cancer screening. Fam. Pract. Manag 24, 5–10. [PMC free article] [PubMed] [Google Scholar]
- Shapiro ED, Baker PJ, Wormser GP, 2017. False and misleading information about Lyme disease. Am. J. Med 130, 771–772. 10.1016/j.amjmed.2017.01.030. [DOI] [PubMed] [Google Scholar]
- Singh S, Parker D, Mark-Carew M, White R, Fisher M, 2016. Lyme disease in West Virginia: an assessment of distribution and clinicians’ knowledge of disease and surveillance. W. V. Med. J 112, 48–54. [PubMed] [Google Scholar]
- Song L, Weaver MA, Chen RC, Bensen JT, Fontham E, Mohler JL, Mishel M, Godley PA, Sleath B, 2014. Associations between patient-provider communication and socio-cultural factors in prostate cancer patients: a cross-sectional evaluation of racial differences. Patient Educ. Couns 97, 339–346. 10.1016/j.pec.2014.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. Pierre S, Gould O, Lloyd V, 2020. Knowledge and knowledge needs about Lyme disease among occupational and recreational users of the outdoors. Int. J. Environ. Res. Public Health 17. 10.3390/ijerph17010355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanek G, Wormser GP, Gray J, Strle F, 2012. Lyme borreliosis. Lancet 379, 461–473. 10.1016/S0140-6736(11)60103-7. [DOI] [PubMed] [Google Scholar]
- Stewart M, Brown J, Donner A, McWhinney I, Oates J, Weston W, Jordan J, 2000. The impact of patient-centered care on outcomes. J. Fam. Pract 49, 796–804. [PubMed] [Google Scholar]
- Street R, 2013. How clinician–patient communication contributes to health improvement: modeling pathways from talk to outcome. Patient Educ. Couns 92, 286–291. 10.1016/J.PEC.2013.05.004. [DOI] [PubMed] [Google Scholar]
- Tamhane S, Rodriguez-Gutierrez R, Hargraves I, Montori VM, 2015. Shared decision-making in diabetes care. Curr. Diab. Rep 15, 112. 10.1007/s11892-015-0688-0. [DOI] [PubMed] [Google Scholar]
- Tan A, Durbin M, Chung FR, Rubin AL, Cuthel AM, McQuilkin JA, Modrek AS, Jamin C, Gavin N, Mann D, Swartz JL, Austrian JS, Testa PA, Hill JD, Grudzen CR, 2020. Design and implementation of a clinical decision support tool for primary palliative care for emergency medicine (PRIM-ER). BMC Med. Inform. Decis. Mak 20 10.1186/s12911-020-1021-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underhill ML, Kiviniemi MT, 2011. The association of perceived provider–patient communication and relationship quality with colorectal cancer screening. Health Educ. Behav 39, 555–563. 10.1177/1090198111421800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente SL, Wemple D, Ramos S, Cashman SB, Savageau JA, 2015. Preventive behaviors and knowledge of tick-borne illnesses: results of a survey from an endemic area. J. Public Health Manag. Pract 21, E16–23. 10.1097/PHH.0000000000000098. [DOI] [PubMed] [Google Scholar]
- Vassell A, VA C, Snyder J, 2020. What was lost, missing, sought and hoped for: qualitatively exploring medical crowdfunding campaign narratives for Lyme disease. Health (Irvine. Calif). 10.1177/1363459320912808. [DOI] [PubMed] [Google Scholar]
- Wormser GP, Dattwyler RJ, Shapiro ED, Halperin JJ, Steere AC, Klempner MS, Krause PJ, Bakken JS, Strle F, Stanek G, Bockenstedt L, Fish D, Dumler JS, Nadelman RB, 2006. The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the infectious diseases society of America. Clin. Infect. Dis 43, 1089–1134. 10.1086/508667. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


