SUMMARY
The central dogma of the flow of genetic information is arguably the crowning achievement of 20th century molecular biology. Reversing the flow of information from RNA to DNA or chromatin has come to the fore in recent years, from the convergence of fundamental discoveries and synthetic biology. Inspired by the example of long noncoding RNAs (lncRNAs) in mammalian genomes that direct chromatin modifications and gene expression, synthetic biologists have repurposed prokaryotic RNA-guided genome defense systems such as CRISPR to edit eukaryotic genomes and epigenome. Here we explore the parallels of these two fields and highlight opportunities for synergy and future breakthroughs.
eTOC blurb
Here, Chang and Qi discuss reversing the flow of information in the Central Dogma, from RNA to DNA or chromatin, highlighting lncRNAs and prokaryotic RNA-guided genome defense systems such as CRISPR to edit eukaryotic genomes and epigenome. They explore the parallels between the two and the future of RNA-based technologies.
INTRODUCTION
The central dogma of gene expression is stunning in its explanatory power and ubiquity in biological systems. As a fundamental theory in molecular biology, the central dogma states that genetic information flows in one direction, from DNA, to RNA, to protein. From a historical context, the discovery of reverse transcriptase was one the first major revision to the idea of unidirectional information flow from DNA to RNA to protein. In the first part of the 21st century, discovery of small regulatory RNAs was initially focused on post-transcriptional regulation. Subsequent studies of PIWI-interacting RNAs (piRNAs) and long noncoding RNAs (lncRNAs) led to the recognition of the potential of RNA to influence DNA—specifically changing DNA methylation or chromatin in a heritable fashion1. In bacteria, retrons are genetic components composed of reverse transcriptase (RT) and non-coding RNA (ncRNA). The RT uses the ncRNA as a template to generate a chimeric RNA/DNA molecule with covalently bonded RNA and DNA components, which work as defenses against phages2. The discovery of CRISPR greatly simplified the task of targeting specific DNA sequences3 and led to a flowering of ideas and new strategies to expand the programmability of RNA, with the aid of protein factors, to control the information flow backwards to DNA (Figure 1). While proteins are considered major players on controlling gene expression, these mounting examples highlight RNA as the often-overlooked and active ingredient in reversing the central dogma.
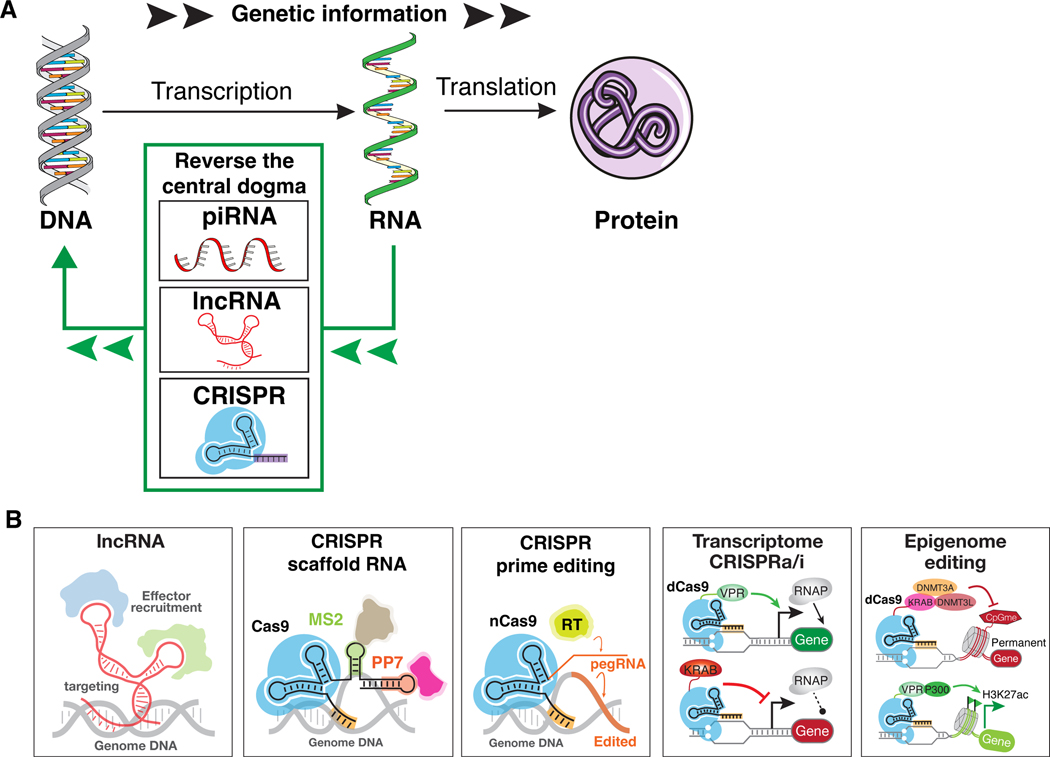
Figure 1.
Diagram showing scheme for reversing the Central Dogma. (A) Genetic information flows from DNA to RNA to protein. Natural and synthetic systems allow reversing this flow direction by modifying DNA based on information encoded in RNA, with examples including piRNA, lncRNA, and CRISPR. (B) Examples for lncRNA and CRISPR for targeting genome regions and interacting with host factors or synthetic regulators to modulate gene expression, chromatin status, etc.
LONG NONCODING RNAS: INFORMATION FLOW FROM RNA TO CHROMATIN
Eukaryotic genomes are pervasively transcribed to produce numerous lncRNAs. LncRNAs are defined as transcripts that are greater than 200 nucleotides, may be spliced or unspliced, and apparently do not function by encoding for a protein or peptide. This definition of exclusion arose during an era of a protein-centric view of the genome and genome assemblies, despite the fact that just 2% of the human genome is coding. The discovery, characterization, function of lncRNAs have been extensively reviewed elsewhere4 and we will focus on salient examples that facilitate our discussion. In brief, the human genome encodes over 60,000 lncRNAs, three times the number of protein coding genes. Inherited mutations in lncRNA genes cause Mendelian disorders 5 such as developmental malformations 6.Unbiased genome-wide association studies revealed that inherited variation of lncRNA loci in the human population underlies over one thousand trait associations, 800 of which were not explained by neighboring protein-coding genes7. For instance, lncRNA quantitative trait loci are linked to inflammatory bowel disease, type 1 and type 2 diabetes, and coronary artery disease, as well as rare variant associations to body mass index. Systematic functional screens in cells show that approximately 2% of lncRNA gene loci are required for cell growth or survival, the vast majority in a tissue or cell-type selective function8, which is concordant with more limited examples in genetically engineered animal models.
Many lncRNAs have their functions in the nucleus, recognized archetypes of signal guides, scaffolds, and decoys9. First, lncRNAs are produced by the act of transcription, and thus lncRNA marks its chromosomal allele in cis as transcriptionally active. Classic examples of imprinting, where paternal vs. maternally inherited alleles are differentially expressed, are often associated and regulated in part by allele-specific transcription of lncRNA 10,11. Second, lncRNAs can serve as guides to target chromatin modification machinery. Most if not all chromatin modifying enzymes lack intrinsic DNA binding specificity and rely on RNA or transcription factors to guide them to the target DNA loci. The mechanism of lncRNA targeting is diverse and may be limited to the local genome neighborhood or chromosome from which it is transcribed (in cis) or broadened to loci throughout the genome (in trans) 5. Reported target mechanisms include RNA: DNA: DNA triple formation12,13, lncRNA RNA duplex interaction with nascent RNA 14, and exploitation of 3D spatial proximity15,16. Third, lncRNAs are comprised of multiple modular domains that create a scaffold for the assembly of multiple chromatin modification activities. The lncRNA Xist beautifully illustrates each of these archetypes17. In each female cell in mammals, one of two X chromosomes is transcriptionally silenced by action of Xist. Xist is only transcribed from the inactive X chromosome (Xi), and Xist RNA spreads across the Xi to nucleate and spread dozens of RNA binding proteins and chromatin regulators to silence gene expression through the lifetime of that cell and all its progenies. This classical example of lncRNA and many other recent entries highlight Nature’s heretical invention and elaboration of using RNA to alter and dictate information flow from DNA.
RNA GUIDED DNA TARGETING
The discovery that prokaryotes possess a form of RNA-targeted immunity, termed clustered regularly interspaced short palindromic repeat (CRISPR), has dramatically expanded the view on the physiological role played by noncoding RNAs 18–20. Complexed with CRISPR-associated (Cas) proteins, these noncoding RNAs, termed CRISPR RNA (crRNA), serves as guides to direct Cas proteins to specific DNA sequences for DNA cleavage 21,22. This provides an unprecedented example for reversing the Central Dogma, -- how information encoded in RNA can alter information in DNA. In this review, we focus on how RNAs are leveraged for reversing the central dogma in eukaryotes.
Cas9 and Cas12
There are two distinct classes of CRISPR systems23, and Class II enzymes have their targeting and nuclease functions encoded in a single protein (including Cas9) which span across a large group of RNA-guided nucleases that have evolved in various prokaryotic species. The most used single Cas effectors are Cas9 and Cas12a (formerly known as Cpf1) 24,25.
Evolution in different host environments have endowed Cas enzymes with unique traits, but they all share a common feature: formation of a ribonucleoprotein (RNP) for DNA or RNA targeting. For example, all Cas9 enzymes require complexation with two non-coding RNAs: one CRISPR-associated RNA (crRNA) encoding the target DNA complementarity and one trans-acting crRNA (tracrRNA) 26. On the contrary, all Cas12a enzymes utilize a single crRNA without tracrRNA. This can be explained by the fact that Cas12a encodes RNase activity that processes the precursor crRNA array to individual crRNAs for RNP formation, while Cas9 requires tracrRNA and host factors (e.g., RNase III) for the same process 27. Nevertheless, the evolution of tracrRNA is an interesting event, which may endow unique features, such as offering a versatile evolutionary solution to process long pre-crRNA arrays to individual crRNAs, while maintaining the compactness of the crRNA array. To simplify the use of two RNAs, crRNA and tracrRNA are fused as a single guide RNA (sgRNA).
All DNA-targeting Cas nucleases require a protospacer adjacent motif (PAM), short DNA segments flanking the targeted genomic locus that are not encoded in the corresponding region flanking the crRNA 28. PAM sites provide an entry point for the Cas-guide RNA complex for DNA binding and melting. PAMs from different Cas9 or Cas12 species have varied stringency. Examples include that, Streptococcus pyogenes Cas9 requires a NGG PAM, Staphylococcus aureus Cas9 recognizes an NNGRRT PAM (N is any nucleotide and R is A or G), and some Cas12a molecules use a TTTV PAM (V is A or C or G) 25,29,30. Protein engineering of the PAMbinding domain of the Cas proteins can alter the PAM preference, which is an important factor for determining its targetable genomic space 31 32 33 34. Structural and mechanistic insights of the sgRNA have also improved CRISPR-mediated gene editing. For example, varying the full-length of tracrRNA may improve the editing activity35 36. Truncated sgRNAs that guide Cas9 binding but not cleavage can shield off target sites and prevent off-target cleavage without affecting on-target sites37. Similarly, introduction of RNA hairpins to stabilize sgRNA can increase the specificity of cleavage of Cas9 and Cas12a38.
Engineered guide RNA with aptamers for instructions
SgRNA can be universally engineered into a scaffold that recruits diverse enzymatic functions. In this case, an sgRNA sequence can be fused to a protein-interacting RNA aptamer that recruits specific RNA-binding proteins (RBPs), termed scaffold RNAs (scRNAs)39,40. RBPs fused with activators or repressors can then be used to bring additional instructions onto the scRNAs. When orthogonal aptamer–RBP pairs (e.g., MS2–MCP, PP7–PCP) are used to generate different scRNAs, distinct regulatory modules can be recruited to different DNA sites for distinct regulation. These scRNAs mimic the scaffolding natural function of lncRNAs that can encode both the target location and independent mode of regulatory action. Several systems for enhanced CRISPR-mediated gene regulation leverages this approach, including the broadly used SAM system that fuses MS2 aptamers to the sgRNA structure and recruits multiple activator domains to enhance target gene regulation41
Engineering guide RNA with additional RNA domains is a powerful approach to expand CRISPR functionality on the DNA. In addition to DNA regulation, the method of incorporating MS2 or PP7 RNA aptamers into the sgRNA has been utilized for multiple-color chromatin labeling, wherein RNA aptamers recruit MS2 or PP7 coat proteins fused with different fluorescent proteins to various target genomic loci 42. In a method termed CRISP-Display, large RNA motifs including natural lncRNAs was directly inserted into the CRISPR sgRNA, allowing for targeting functional RNAs and associated ribonucleoprotein (RNP) complexes to desired genomic loci43. Young and colleagues tethered a short RNA (60-nt RNA derived from the promoter sequence of Arid1a) to the sgRNA using CRISPR-Cas9 in the vicinity of YY1 binding sites and determined the tethered RNA specifically increased the occupancy of YY1 at nearby enhancers44. Thus, engineering guide RNA by coupling with other RNA domains offers a scaffolding system that enables multimodal functionality on target genes using a single Cas protein.
Compact Cas molecules
Recently identified CRISPRs revealed new compact Cas effectors. Specifically, Cas12f (also called Cas14, 400–700 amino acids) and Cas12j (also called CasΦ, 700–800 amino acids) have been characterized from Archaea and bacteriophages45,46 . In the case of Cas12f, two Cas12f molecules form a homodimer which binds to one crRNA-tracrRNA complex as a RNP 47. This reduces the ‘genetic cost’ of expression and delivery, as expressing Cas12f and its associated crRNA-tracrRNA takes a smaller fragment size. While the wildtype system showed limited performance in mammalian cells, protein engineering was used to create better performing Cas variants in mammalian systems. One example is our development of a modified Cas12f system (termed CasMINI), which is 1.6 kb and less than half the size of Cas9 48. Notably, sgRNA engineering by altering the scaffold sequence played an important role in enhancing the activity of engineered Cas12f systems, which is likely due to better RNP formation and stability.
The compact size of miniature Cas proteins offers useful solutions for better delivery, using adeno-associated virus (AAV) or lipid nanoparticles (LNP), and promise better in vivo applications. Interestingly, large Cas effectors such as Cas9 or Cas12a (3~4kb) often utilizes smaller crRNA (or crRNA-tracrRNA)29, and smaller Cas effectors such as Cas12f (1.2~1.8kb) uses larger crRNA complexes49. This makes sense structurally as smaller proteins require larger noncoding RNAs, serving as ‘ligand’, to form a sufficiently stable ribonucleoprotein for interaction with the stable double-stranded DNA.
Base editors
RNA-guided Cas nucleases induce double-strand break (DSBs) in DNA and DSB-mediated random indels (insertion/deletion), which is a ‘primary’ form of RNA->DNA information writing using CRISPR. Beyond this, Cas molecules can be fused to nucleotide modifiers to enable other forms of DNA editing. DNA base editing enzymes have been fused to the nuclease-dead dCas or the nickase variant of Cas that generates a single-strand break, to generate precise DNA mutations at a targeted location. Noncoding RNA transcripts guide activation-induced cytidine deaminase to mediate somatic hypermutation in B cells 50, providing a precedent for using RNA to guide base editing.
The cytidine deaminase enzyme APOBEC1, which converts cytosine (C) to uracil (U), was fused to nCas9. This base editor, termed cytosine base editors (CBEs), catalyzes a targeted C-to-U on the DNA strand not bound by sgRNA, which is read as a thymine (T) upon DNA replication and creates a precise C to T mutation 51–53. Fusing uracil DNA glycosylase (UGI) and mutating APOBEC1, enhances the C-to-T reaction. In a similar manner, nCas9 fused with an engineered Escherichia coli TadA, termed adenosine base editors (ABEs), created an adenosine (A) to guanine (G) conversion 54–56. ABE was also engineered into an efficient cytosine base editor 57. Fusing both cytosine and adenosine deaminases to nCas9 generates both C-to-T and A-to-G conversions at a single site 58,59. Base editors for nucleotide transversion, conversion between a purine and a pyrimidine, have been developed for C-to-G and C-to-A 60–62. In addition to nCas9, the nuclease-dead Cas12a can efficiently modulate base editing 63. Base editors are great examples for how RNAs are used as instructions to introduce specific mutations at DNA regions.
RNA AS GENOME TEMPLATE
Beyond RNA as a guide, RNA can directly template DNA sequence addition and connectivity. In yeast Saccharomyces cerevisiae, endogenous transcript RNA is used to mediate homologous recombination with chromosomal DNA 64 65. In eukaryotic cells, telomerase RNA is the scaffold for the assembly of the multiprotein telomerase enzyme complex that protects the ends of linear chromosomes (reviewed by 66). Moreover, telomerase RNA provides the template for the addition of hundreds to thousands of a precise repeat sequence to chromosome ends by the reverse transcriptase activity of TERT. Ciliates, like the unicellular organism Oxytrichia, undergo a highly unusual life cycle that involves fragmenting its germline genome into numerous small pieces, eliminating ~95% of its genome, and then assembling the remaining fragments into ~16,000 genesized chromosomes. The assembly into chromosomes is not random but has been shown to be guided by RNA 67,68—that is the order of genes on the RNA specifies the order of the DNA pieces that are stitched together 69. Current model suggests base pairing between the RNA and regions of DNA sequences. However, because point mutations in the RNA are not copied into the assembled chromosomal DNA, the Oxytrichia lncRNA is likely not a template that is reverse transcribed into the DNA. Nonetheless, these examples illustrate the possibility of RNA to program long sequences into DNA by either direct or indirect means.
Prime editors
To further expand the mode of DNA writing, Liu and colleagues developed a method termed prime editing to insert longer stretches of DNA 70–72 Click or tap here to enter text.. In this method, nCas9 is fused to a reverse transcriptase and the sgRNA is engineered with an elongated 3’ sequence that encodes the desired mutations and a priming region complementary to the DNA. This altered sgRNA is a critical development, serving as a template encoding multiple instructions (both target location and desired mutations), which is collectively called prime editing guide RNA (pegRNA). In this RNA-directed system, the pegRNA primes the nicked DNA strand and the reverse transcriptase converts the template sequence from RNA to DNA, creating a targeted insertion or deletion in place. It was utilized to make all 12 base-to-base conversions, as well as small-fragment insertions and deletions (<100 bp). Combining with Bxb1 recombinase, the pegRNA system enables larger insertion or deletions (up to several kb) 74,75.
Notably, several improvements in prime editing have taken place via iterative improvement of the pegRNA design. Nelson et al. found that degradation of the 3’ template end of pegRNA appears to be a major bottleneck 73. Such truncated pegRNAs cannot serve as template for RT, and moreover may block productive full length pegRNA-CasRT complexes from occupying target sites in a dominant negative manner. Enhancing greater stability of linear pegRNAs has improved the prime editing. Liu et al. further found that pegRNA can be split into a guide and a template RNA, and greatly stabilized by circularization, which makes the template RNA impervious to exonucleases 76. Circular template RNA appears to enable greater duration of productive enzyme occupancy of the target site DNA, because dCas9 and RT can be expressed as separate proteins and still support prime editing. This split design solves the size problem of the Cas9-RT fusion construct, a current limitation for AAV delivery of genetic medicines in vivo 77.
RNA-guided DNA transposition
More recently, diverse Cas proteins were discovered to exhibit targeted genomic transposition or insertions. For example, A Type I-F and a Type II-K CRISPR system can insert DNA fragments to a genomic site via RNA-guided CRISPR-mediated recruitment of transposition machinery 78,79. This ability for targeted transposition has been demonstrated in vitro and in prokaryotic hosts 78. While targeted transposon needs to be developed in human cells, these offer excellent examples on how CRISPR can use RNA instructions to enable diverse forms of DNAlevel information rewriting. Beyond natural Cas-transposase systems, Durrant et al. repurposed and fused bacterial serine integrase with dCas9 to enable programmable insertion with multikilobase DNA inserts 80 .
RNA-GUIDE GENE REGULATION AND EPIGENETIC EDITING
CRISPRa
What best illustrates the concept of reversed RNA->DNA information flow is probably the development of CRISPR activation (CRISPRa) 81,82. In this method, a nuclease-dead dCas (e.g., dCas9, dCas12a, dCasMINI) is fused to a transcriptional activator 83–85. Guided by its cognate sgRNA bearing a sequence indicating the target DNA locus, the RNP can specifically bind to an endogenous genomic locus to switch on the gene that is nearby. In mammalian cells, various transactivator domains such as VP64, tripartite VPR (VP64, p65, and RTA) are fused to dCas9 86, tandem repeats of peptides 87, or indirect recruitment via engineered RNA scaffold, have been adopted for gene activation 88. In bacterial cells, fusing synthetic transcriptional activators or the ω subunit of RNA polymerase to dCas9 enabled gene activation 89,90. Unlike ectopic transgene expression, CRISPRa can be used to precisely tune the magnitude of gene upregulation. Furthermore, CRISPRa can be utilized to upregulate multiple gene targets by simply using multiple sgRNAs. In some cases, via a dCas12a-activator system, a compact crRNA array that can encode multiple gene-site instructions to activate many genes (7~10) can be achieved 91,92.
CRISPRi and CRISPRoff
CRISPR interference (CRISPRi) allows programmable gene silencing and is the conceptual opposite of CRISPRa. In CRISPRi, dCas9 is fused to a protein domain, such as KRAB, that recruits the endogenous SETDB1 histone modification enzyme and additional factors that places the repressive chromatin mark histone H3 lysine 9 trimethylation (H3K9me3) 93. CRISPRi deposits H3K9me3 on ~1 kb window surrounding the target locus and can be targeted to promoters and distal enhancers in a multiplexed fashion to study gene or DNA element function 94,95. One fruitful application of CRISPRi was the systematic functional interrogation of human lncRNA genes 96, and the factors required for maintenance of XIST-mediated gene silencing in adult somatic cells 97.
CRISPRoff is a new generation of programmable gene silencing with epigenetic memory. CRISPRoff builds on CRISPRi and contains dCas9, KRAB domain, and is further fused to DNMT3A and DNMT3L, components of the de novo DNA methyltransferase complex 98. This combination of DNA element deactivation (KRAB) and maintenance (DNMT3A/3L) mimics the natural mechanism of epigenetic genetic silencing, such as X inactivation. CRISPRoff silences gene expression from the targeted promoters or enhancers, and further adds DNA methylation to the target locus. DNA methylation is copied and propagated by endogenous DNMT1 during cell division, thus ensuring memory of gene silencing over multiple somatic cell generations or even cell differentiation 99. CRISPRoff allows a “hit-and-run” approach to epigenetic editing. Unlike CRISPRa or CRISPRi that needs to be continually expressed to exert their effects, transient expression of CRISPRoff construct and sgRNA allows durable gene silencing in cultured cells. The application of CRISPRoff in vivo and primary cells is an active area of investigation.
RNA guided epigenetic editing
Fusion of dCas with epigenetic domains with different enzymatic activities can be used to write or erase a broad spectrum of chromatin chemical modifications. To erase DNA methylation, TET1 was fused with dCas9 to remove DNA methyl groups and upregulate gene expression at specific chromatin sites 99,100. In addition, to specifically add acetyl groups to local histones, the catalytic core of the acetyltransferase p300 domain was fused to dCas9 101–103. When directed to enhancers, this fusion adds an acetyl group to Lysine 27 of Histone H3 (H3K27ac), leading to activation of gene expression. Other modes of writing methylation at H3K4, H3K9, and H3K27 have been achieved by engineering the fusion effectors to dCas proteins 104. All of these expand the modes that RNA->DNA epigenetic changes.
IN THE HORIZON
Here we have discussed several parallel themes in lncRNA biology and CRISPR technologies. What are potential areas of cross pollination and advances in the future? We offer several suggestions that are by no means exhaustive. Indeed, we look forward to being surprised by the richness of biology and the ingenuity of investigators in the field.
Gene activation with memory
While CRISPRoff has achieved gene silencing with epigenetic memory, no comparable technology exists for durable gene activation. CRISPRa requires continual expression of the dCas9-VP64 construct. The ability to durably induce gene expression after a transient expression of a synthetic regulator will offer many advantages for experimental biology and medicine. For instance, genetic haploinsufficiency can be treated by durable gene activation. LncRNA biology offers case studies for how durable gene activation is achieved in nature. Dosage compensation in Drosophila involves the transcriptional activation of the single male X chromosome by two-fold via two lncRNAs named roX1 and roX2 38,105.The roX RNAs are also transcribed from the X chromosome and guide a protein complex that hyperacetylates histones and promotes transcriptional elongation on the X, creating a positive feedback loop. In the mammalian HOXA locus, the lncRNA HOTTIP is located on the 5’ end of the locus, associates with the MLL H3K4 methylase complex and promotes expression of the 5’ HOXA genes, including itself 106. Both dosage compensation and positional identity are both remarkably stable once established and can even persist despite extensive ex vivo culture 107,108. Accessing endogenous systems of positive feedback loops with cis-acting lncRNAs may unlock programmable gene activation with greater durability.
RNA control 3D genome and nuclear architecture
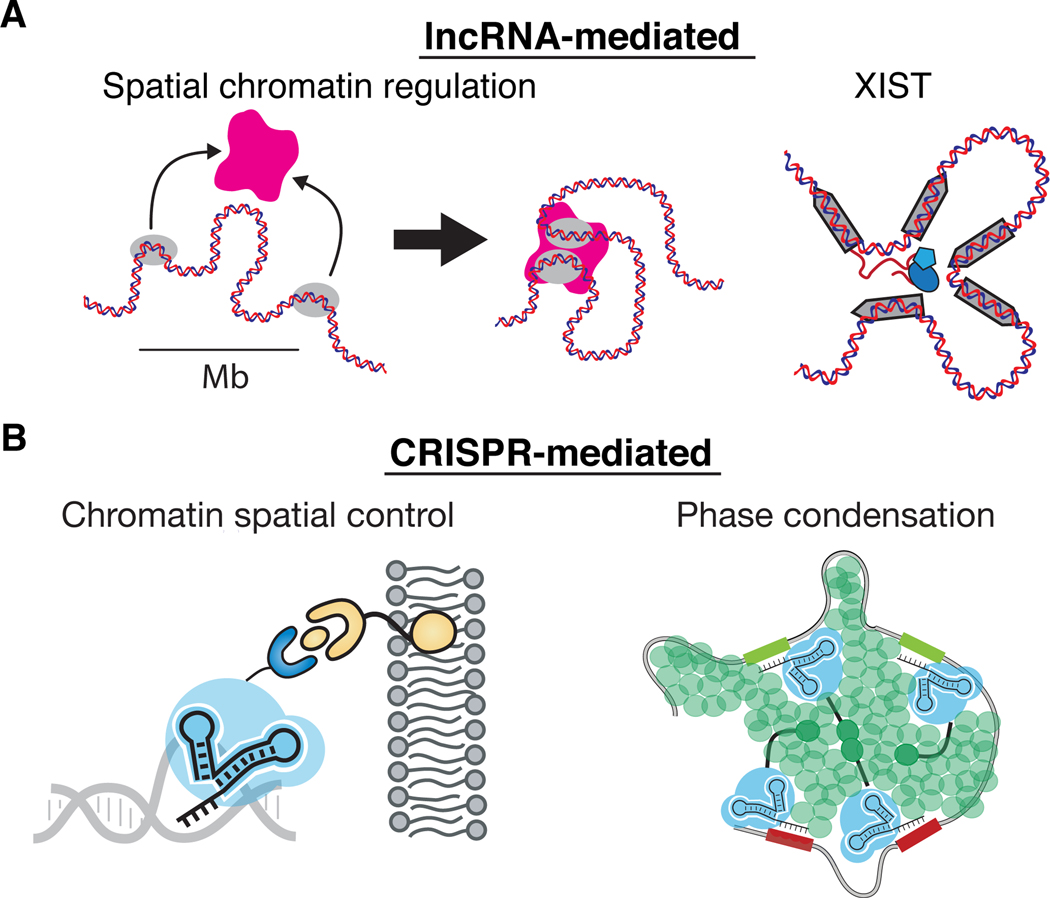
LncRNAs exert powerful roles in the compartmentalization of nuclear neighborhoods (reviewed by 109,110) (Figure 2). The nucleolus (rRNA repeats), nuclear paraspeckle (Neat1), and the Barr body (Xist) are several prominent examples. In X inactivation, Xist not only spreads across the inactive X chromosome (Xi), Xist also compacts and pulls in the Xi and localizes the Xi to the nuclear periphery 111, creating a chromosomal structure and nuclear territory that are distinct from that of the homologous active X chromosome. Synthetic biologists have started to use RNA to program the 3D genome. CLOUD9 is a system that targets multiple dCas9 proteins fused to inducible dimerization domains to DNA elements spaced kilobases apart 112. Upon sgRNA expression and dimerization, distal DNA elements such as enhancers and promoters can be pulled into spatial proximity, leading to gene activation 112. CRISPR-GO is a method to direct chromosomal loci position within the nucleus. Via a dimerization system that repositions the target DNA to desired nuclear compartment, CRISPR-GO mediates RNA-guided spatial relocalization of specific chromatin to a different location including nuclear periphery, Cajal bodies, or heterochromatin condensates 113–115. The apparent difference is the scale and complexity of the 3D genome architecture being programmed by endogenous lncRNAs. One potential mechanistic distinction is that endogenous lncRNAs have much more elaborate scaffold function, and each unit of the scaffold is repeated multiple times. Xist is an 81 kb lncRNA in the mouse and consists of multiple repeats, named A-F, which have a division of labor in spreading across the chromosome, gene deactivation, and epigenetic memory 116,117.
Figure 2.
Future direction for using RNA to modulate DNA 3D configuration and phase condensation for using lncRNA (A) or CRISPR (B).
RNA induced condensates
Nuclear RNAs are powerful nucleators and participants in multivalent RNA-protein interactions. Studies of condensates to create membraneless compartments have relied extensively on in vitro biochemistry of purified component proteins and imaging in cells. Tools to program condensate formation and dissolution are starting to be developed118. LncRNAs offer some potentially useful lessons. The lncRNA NORAD contains 18 copies of binding sites for the RNA binding protein Pumilio and titrates Pumilio activity in the cell. NORAD can compete Pumilio away from the multitude of Pum binding sites in the mRNA transcriptome, even though the latter collectively exists in ~40-fold excess 119. Mendell and colleagues showed that the repeated nature of Pum binding sites in NORAD creates multivalency that facilitates Pumilio condensate formation in a super-stochiometric manner 119. A synthetic circular RNA composed of Pum binding sites phenocopied NORAD function. These emerging studies suggest the possibility that elaborating and engineering scaffolds on sgRNAs targeting DNA or RNA may be a fruitful path to new technologies to dissect and manipulate condensates and complex multivalent molecular interactions.
The 21th century has been envisioned as the century of biology. Rewiring the the central dogma with RNA may play major roles in the next phase of exciting biological advances.
ACKNOWLEDGEMENT
We thank Dr. M.C. Tsai for assistance and comments. L.S.Q. acknowledges support from National Science Foundation CAREER award (Award #2046650), National Institutes of Health (Grant # 1R01CA266470), Stanford Innovative Medicines Accelerator. L.S.Q. is a Chan Zuckerberg Biohub investigator. H.Y.C. is supported by NIH RM1-HG007735, R35-CA209919 and is an Investigator of the Howard Hughes Medical Institute.
Footnotes
Declaration of Interests
L.S.Q. is a founder and scientific advisor of Epicrispr Biotechnologies. H.Y.C. is a co founder of Accent Therapeutics, Boundless Bio, Cartography Biosciences, Orbital Therapeutics, and is an advisor of 10x Genomics, Arsenal Biosciences, Chroma Medicine, and Spring Discovery.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sabin LR, Delás MJ & Hannon GJ Dogma derailed: the many influences of RNA on the genome. Mol Cell 49, 783–794 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Millman A. et al. Bacterial Retrons Function In Anti-Phage Defense. Cell 183, 1551–1561.e12 (2020). [DOI] [PubMed] [Google Scholar]
- 3.Hsu PD, Lander ES & Zhang F. Development and applications of CRISPR-Cas9 for genome engineering. Cell 157, 1262–1278 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herman AB, Tsitsipatis D. & Gorospe M. Integrated lncRNA function upon genomic and epigenomic regulation. Mol Cell 82, 2252–2266 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rinn JL, Chang HY & Chang HY Long Noncoding RNAs: Molecular Modalities to Organismal Functions. Annu Rev Biochem 89, 283–308 (2020). [DOI] [PubMed] [Google Scholar]
- 6.Allou L. et al. Non-coding deletions identify Maenli lncRNA as a limb-specific En1 regulator. Nature 592, 93–98 (2021). [DOI] [PubMed] [Google Scholar]
- 7.de Goede OM et al. Population-scale tissue transcriptomics maps long non-coding RNAs to complex disease. Cell 184, 2633–2648.e19 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu SJ et al. CRISPRi-based genome-scale identification of functional long noncoding RNA loci in human cells. Science 355, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang KC & Chang HY Molecular mechanisms of long noncoding RNAs. Mol Cell 43, 904–914 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sleutels F, Zwart R. & Barlow DP The non-coding Air RNA is required for silencing autosomal imprinted genes. Nature 415, 810–813 (2002). [DOI] [PubMed] [Google Scholar]
- 11.Latos PA et al. Airn transcriptional overlap, but not its lncRNA products, induces imprinted Igf2r silencing. Science 338, 1469–1472 (2012). [DOI] [PubMed] [Google Scholar]
- 12.Zhang X. et al. KCNQ1OT1 promotes genome-wide transposon repression by guiding RNA-DNA triplexes and HP1 binding. Nat Cell Biol (2022) doi: 10.1038/S41556-022-01008-5. [DOI] [PubMed] [Google Scholar]
- 13.Postepska-Igielska A. et al. LncRNA Khps1 Regulates Expression of the Proto-oncogene SPHK1 via Triplex-Mediated Changes in Chromatin Structure. Mol Cell 60, 626–636 (2015). [DOI] [PubMed] [Google Scholar]
- 14.Lee N, Moss WN, Yario TA & Steitz JA EBV noncoding RNA binds nascent RNA to drive host PAX5 to viral DNA. Cell 160, 607–618 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang KC et al. A long noncoding RNA maintains active chromatin to coordinate homeotic gene expression. Nature 472, 120–126 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Engreitz JM et al. The Xist lncRNA exploits three-dimensional genome architecture to spread across the X chromosome. Science 341, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loda A, Collombet S. & Heard E. Gene regulation in time and space during X-chromosome inactivation. Nat Rev Mol Cell Biol 23, 231–249 (2022). [DOI] [PubMed] [Google Scholar]
- 18.Barrangou R. et al. CRISPR provides acquired resistance against viruses in prokaryotes. Science 315, 1709–1712 (2007). [DOI] [PubMed] [Google Scholar]
- 19.Mojica FJM, Díez-Villaseñor C, Soria E. & Juez G. Biological significance of a family of regularly spaced repeats in the genomes of Archaea, Bacteria and mitochondria. Mol Microbiol 36, 244–246 (2000). [DOI] [PubMed] [Google Scholar]
- 20.Ishino Y, Shinagawa H, Makino K, Amemura M. & Nakatura A. Nucleotide sequence of the iap gene, responsible for alkaline phosphatase isozyme conversion in Escherichia coli, and identification of the gene product. J Bacteriol 169, 5429–5433 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jansen R, van Embden JDA, Gaastra W. & Schouls LM Identification of genes that are associated with DNA repeats in prokaryotes. Mol Microbiol 43, 1565–1575 (2002). [DOI] [PubMed] [Google Scholar]
- 22.Haft DH, Selengut J, Mongodin EF & Nelson KE A guild of 45 CRISPR-associated (Cas) protein families and multiple CRISPR/Cas subtypes exist in prokaryotic genomes. PLoS Comput Biol 1, 0474–0483 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Makarova KS et al. Evolutionary classification of CRISPR-Cas systems: a burst of class 2 and derived variants. Nat Rev Microbiol 18, 67–83 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zetsche B. et al. Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR-Cas system. Cell 163, 759–771 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jinek M. et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816–821 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deltcheva E. et al. CRISPR RNA maturation by trans-encoded small RNA and host factor RNase III. Nature 471, 602–607 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fonfara I, Richter H, BratoviÄ M, le Rhun A. & Charpentier E. The CRISPR-associated DNA-cleaving enzyme Cpf1 also processes precursor CRISPR RNA. Nature 532, 517–521 (2016). [DOI] [PubMed] [Google Scholar]
- 28.Marraffini LA & Sontheimer EJ Self versus non-self discrimination during CRISPR RNA-directed immunity. Nature 463, 568–571 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zetsche B. et al. Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR-Cas system. Cell 163, 759–771 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ran FA et al. In vivo genome editing using Staphylococcus aureus Cas9. Nature 520, 186–191 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu JH et al. Evolved Cas9 variants with broad PAM compatibility and high DNA specificity. Nature 556, 57–63 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao L. et al. Engineered Cpf1 variants with altered PAM specificities. Nat Biotechnol 35, 789–792 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kleinstiver BP et al. Engineered CRISPR-Cas9 nucleases with altered PAM specificities. Nature 523, 481–485 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nishimasu H. et al. Engineered CRISPR-Cas9 nuclease with expanded targeting space. Science 361, 1259–1262 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bikard D. et al. Exploiting CRISPR-Cas nucleases to produce sequence-specific antimicrobials. Nat Biotechnol 32, 1146–1150 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen B. et al. Dynamic imaging of genomic loci in living human cells by an optimized CRISPR/Cas system. Cell 155, 1479–1491 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rose JC et al. Suppression of unwanted CRISPR-Cas9 editing by co-administration of catalytically inactivating truncated guide RNAs. Nat Commun 11, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kocak DD et al. Increasing the specificity of CRISPR systems with engineered RNA secondary structures. Nat Biotechnol 37, 657–666 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dong C, Fontana J, Patel A, Carothers JM & Zalatan JG Synthetic CRISPR-Cas gene activators for transcriptional reprogramming in bacteria. Nat Commun 9, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zalatan JG et al. Engineering complex synthetic transcriptional programs with CRISPR RNA scaffolds. Cell 160, 339–350 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Konermann S. et al. Genome-scale transcriptional activation by an engineered CRISPRCas9 complex. Nature 517, 583–588 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang S, Su JH, Zhang F. & Zhuang X. An RNA-aptamer-based two-color CRISPR labeling system. Sci Rep 6, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shechner DM, Hacisuleyman E, Younger ST & Rinn JL Multiplexable, locus-specific targeting of long RNAs with CRISPR-Display. Nat Methods 12, 664–670 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sigova AA et al. Transcription factor trapping by RNA in gene regulatory elements. Science 350, 978–991 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pausch P. et al. CRISPR-CasΦ from huge phages is a hypercompact genome editor. Science 369, 333–337 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harrington LB et al. Programmed DNA destruction by miniature CRISPR-Cas14 enzymes. Science 362, 839–842 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takeda SN et al. Structure of the miniature type V-F CRISPR-Cas effector enzyme. Mol Cell 81, 558–570.e3 (2021). [DOI] [PubMed] [Google Scholar]
- 48.Xu X. et al. Engineered miniature CRISPR-Cas system for mammalian genome regulation and editing. Mol Cell 81, 4333–4345.e4 (2021). [DOI] [PubMed] [Google Scholar]
- 49.Harrington LB et al. Programmed DNA destruction by miniature CRISPR-Cas14 enzymes. Science 362, 839–842 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pefanis E. et al. Noncoding RNA transcription targets AID to divergently transcribed loci in B cells. Nature 514, 389–393 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arbab M. et al. Determinants of Base Editing Outcomes from Target Library Analysis and Machine Learning. Cell 182, 463–480.e30 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim YB et al. Increasing the genome-targeting scope and precision of base editing with engineered Cas9-cytidine deaminase fusions. Nat Biotechnol 35, 371–376 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Komor AC, Kim YB, Packer MS, Zuris JA & Liu DR Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature 533, 420–424 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gaudelli NM et al. Programmable base editing of A·T to G·C in genomic DNA without DNA cleavage. Nature 551, 464–471 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Richter MF et al. Phage-assisted evolution of an adenine base editor with improved Cas domain compatibility and activity. Nat Biotechnol 38, 883–891 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gaudelli NM et al. Directed evolution of adenine base editors with increased activity and therapeutic application. Nat Biotechnol 38, 892–900 (2020). [DOI] [PubMed] [Google Scholar]
- 57.Neugebauer ME et al. Evolution of an adenine base editor into a small, efficient cytosine base editor with low off-target activity. Nat Biotechnol (2022) doi: 10.1038/S41587-022-01533-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang X. et al. Dual base editor catalyzes both cytosine and adenine base conversions in human cells. Nat Biotechnol 38, 856–860 (2020). [DOI] [PubMed] [Google Scholar]
- 59.Grünewald J. et al. A dual-deaminase CRISPR base editor enables concurrent adenine and cytosine editing. Nat Biotechnol 38, 861–864 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhao D. et al. Glycosylase base editors enable C-to-A and C-to-G base changes. Nat Biotechnol 39, 35–40 (2021). [DOI] [PubMed] [Google Scholar]
- 61.Kurt IC et al. CRISPR C-to-G base editors for inducing targeted DNA transversions in human cells. Nat Biotechnol 39, 41–46 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Koblan LW et al. Efficient C·G-to-G·C base editors developed using CRISPRi screens, target-library analysis, and machine learning. Nat Biotechnol 39, 1414–1425 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kempton HR, Love KS, Guo LY & Qi LS Scalable biological signal recording in mammalian cells using Cas12a base editors. Nat Chem Biol 18, 742–750 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Keskin H. et al. Transcript-RNA-templated DNA recombination and repair. Nature 515, 436–439 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mazina OM, Keskin H, Hanamshet K, Storici F. & Mazin A. v. Rad52 Inverse Strand Exchange Drives RNA-Templated DNA Double-Strand Break Repair. Mol Cell 67, 19–29.e3 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Roake CM & Artandi SE Regulation of human telomerase in homeostasis and disease. Nat Rev Mol Cell Biol 21, 384–397 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lindblad KA, Bracht JR, Williams AE & Landweber LF Thousands of RNA-cached copies of whole chromosomes are present in the ciliate Oxytricha during development. RNA 23, 1200–1208 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nowacki M. et al. RNA-mediated epigenetic programming of a genome-rearrangement pathway. Nature 451, 153–158 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bracht JR et al. Chromosome fusions triggered by noncoding RNA. RNA Biol 14, 620–631 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Anzalone A. v. et al. Search-and-replace genome editing without double-strand breaks or donor DNA. Nature 576, 149–157 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen PJ et al. Enhanced prime editing systems by manipulating cellular determinants of editing outcomes. Cell 184, 5635–5652.e29 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nelson JW et al. Engineered pegRNAs improve prime editing efficiency. Nat Biotechnol 40, 402–410 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nelson JW et al. Engineered pegRNAs improve prime editing efficiency. Nat Biotechnol 40, 402–410 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Anzalone A. v. et al. Programmable deletion, replacement, integration and inversion of large DNA sequences with twin prime editing. Nat Biotechnol 40, 731–740 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yarnall MTN et al. Drag-and-drop genome insertion of large sequences without double-strand DNA cleavage using CRISPR-directed integrases. Nat Biotechnol (2022) doi: 10.1038/S41587-022-01527-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu B. et al. A split prime editor with untethered reverse transcriptase and circular RNA template. Nat Biotechnol 40, 1388–1393 (2022). [DOI] [PubMed] [Google Scholar]
- 77.Liu B. et al. A split prime editor with untethered reverse transcriptase and circular RNA template. Nat Biotechnol 40, 1388–1393 (2022). [DOI] [PubMed] [Google Scholar]
- 78.Strecker J. et al. RNA-guided DNA insertion with CRISPR-associated transposases. Science 365, 48–53 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Klompe SE, Vo PLH, Halpin-Healy TS & Sternberg SH Transposon-encoded CRISPR-Cas systems direct RNA-guided DNA integration. Nature 571, 219–225 (2019). [DOI] [PubMed] [Google Scholar]
- 80.Durrant MG et al. Systematic discovery of recombinases for efficient integration of large DNA sequences into the human genome. Nat Biotechnol (2022) doi: 10.1038/S41587-022-01494-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Guo LY et al. Multiplexed genome regulation in vivo with hyper-efficient Cas12a. Nat Cell Biol 24, 590–600 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gilbert LA et al. CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes. Cell 154, 442 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xu X. et al. Engineered miniature CRISPR-Cas system for mammalian genome regulation and editing. Mol Cell 81, 4333–4345.e4 (2021). [DOI] [PubMed] [Google Scholar]
- 84.Kempton HR, Goudy LE, Love KS & Qi LS Multiple Input Sensing and Signal Integration Using a Split Cas12a System. Mol Cell 78, 184–191.e3 (2020). [DOI] [PubMed] [Google Scholar]
- 85.Qi LS et al. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell 152, 1173–1183 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chavez A. et al. Comparison of Cas9 activators in multiple species. Nat Methods 13, 563–567 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu Y. et al. CRISPR Activation Screens Systematically Identify Factors that Drive Neuronal Fate and Reprogramming. Cell Stem Cell 23, 758–771.e8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Konermann S. et al. Genome-scale transcriptional activation by an engineered CRISPR-Cas9 complex. Nature 517, 583–588 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bikard D. et al. Programmable repression and activation of bacterial gene expression using an engineered CRISPR-Cas system. Nucleic Acids Res 41, 7429–7437 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dong C, Fontana J, Patel A, Carothers JM & Zalatan JG Synthetic CRISPR-Cas gene activators for transcriptional reprogramming in bacteria. Nat Commun 9, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Campa CC, Weisbach NR, Santinha AJ, Incarnato D. & Platt RJ Multiplexed genome engineering by Cas12a and CRISPR arrays encoded on single transcripts. Nat Methods 16, 887–893 (2019). [DOI] [PubMed] [Google Scholar]
- 92.Magnusson JP, Rios AR, Wu L. & Qi LS Enhanced Cas12a multi-gene regulation using a CRISPR array separator. Elife 10, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gilbert LA et al. CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes. Cell 154, 442 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Thakore PI et al. Highly specific epigenome editing by CRISPR-Cas9 repressors for silencing of distal regulatory elements. Nat Methods 12, 1143–1149 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lin X. et al. Nested epistasis enhancer networks for robust genome regulation. Science 377, 1077–1085 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu SJ et al. CRISPRi-based genome-scale identification of functional long noncoding RNA loci in human cells. Science 355, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yu B. et al. B cell-specific XIST complex enforces X-inactivation and restrains atypical B cells. Cell 184, 1790–1803.e17 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Amabile A. et al. Inheritable Silencing of Endogenous Genes by Hit-and-Run Targeted Epigenetic Editing. Cell 167, 219–232.e14 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nuñez JK et al. Genome-wide programmable transcriptional memory by CRISPR-based epigenome editing. Cell 184, 2503–2519.e17 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liu XS et al. Editing DNA Methylation in the Mammalian Genome. Cell 167, 233–247.e17 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dominguez AA et al. CRISPR-Mediated Synergistic Epigenetic and Transcriptional Control. CRISPR J 5, 264–275 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hilton IB et al. Epigenome editing by a CRISPR-Cas9-based acetyltransferase activates genes from promoters and enhancers. Nat Biotechnol 33, 510–517 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gemberling MP et al. Transgenic mice for in vivo epigenome editing with CRISPR-based systems. Nat Methods 18, 965–974 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nakamura M, Gao Y, Dominguez AA & Qi LS CRISPR technologies for precise epigenome editing. Nat Cell Biol 23, 11–22 (2021). [DOI] [PubMed] [Google Scholar]
- 105.Samata M. & Akhtar A. Dosage Compensation of the X Chromosome: A Complex Epigenetic Assignment Involving Chromatin Regulators and Long Noncoding RNAs. Annu Rev Biochem 87, 323–350 (2018). [DOI] [PubMed] [Google Scholar]
- 106.Wang KC et al. A long noncoding RNA maintains active chromatin to coordinate homeotic gene expression. Nature 472, 120–126 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rinn JL et al. A dermal HOX transcriptional program regulates site-specific epidermal fate. Genes Dev 22, 303–307 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Quinn JJ et al. Revealing long noncoding RNA architecture and functions using domain-specific chromatin isolation by RNA purification. Nat Biotechnol 32, 933–940 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Quinodoz SA & Guttman M. Essential Roles for RNA in Shaping Nuclear Organization. Cold Spring Harb Perspect Biol 14, (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Batista PJ & Chang HY Long noncoding RNAs: cellular address codes in development and disease. Cell 152, 1298–1307 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chaumeil J, le Baccon P, Wutz A. & Heard E. A novel role for Xist RNA in the formation of a repressive nuclear compartment into which genes are recruited when silenced. Genes Dev 20, 2223–2237 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Morgan SL et al. Manipulation of nuclear architecture through CRISPR-mediated chromosomal looping. Nat Commun 8, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang H, Han M. & Qi LS Engineering 3D genome organization. Nat Rev Genet 22, 343–360 (2021). [DOI] [PubMed] [Google Scholar]
- 114.Gao Y, Han M, Shang S, Wang H. & Qi LS Interrogation of the dynamic properties of higher-order heterochromatin using CRISPR-dCas9. Mol Cell 81, 4287–4299.e5 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang H. et al. CRISPR-Mediated Programmable 3D Genome Positioning and Nuclear Organization. Cell 175, 1405–1417.e14 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lu Z. et al. Structural modularity of the XIST ribonucleoprotein complex. Nat Commun 11, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chu C. et al. Systematic discovery of Xist RNA binding proteins. Cell 161, 404–416 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bracha D, Walls MT & Brangwynne CP Probing and engineering liquid-phase organelles. Nat Biotechnol 37, 1435–1445 (2019). [DOI] [PubMed] [Google Scholar]
- 119.Elguindy MM & Mendell JT NORAD-induced Pumilio phase separation is required for genome stability. Nature 595, 303–308 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]